Podcast: Coronavirus – Innovative Treatments and Disappointing Tests

Show-Notes from our third podcast: Coronavirus:
Innovative Treatments and Disappointing Tests, from our new series “Coronavirus: Science and Prevention”
Watch the episode on YouTube and
subscribe to our podcast series on iTunes, Google Podcasts, and Spotify.
Complete Shutdown and Some Good Medical News!
As the shutdown forces us to hold our collective breath, some hopeful treatments appear on the horizon.
Why the complete shutdown?
We need to mass produce treatments, deliver personal protective equipment (PPE) to front line health care workers (HCWs), and set up medical systems for the onslaught of cases when we go back to “normal.”
Final goal? Herd Immunity.
Coronavirus Severity (according to UpToDate)
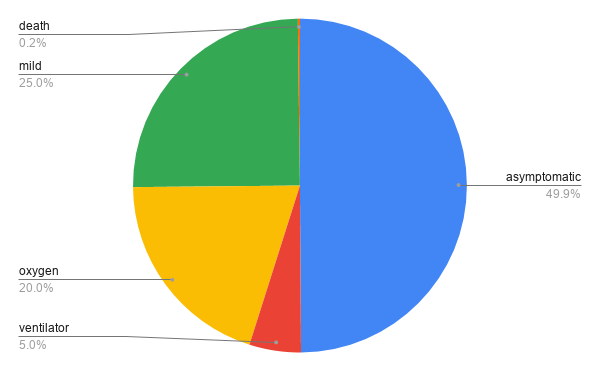
Treatment needed in the severe cases is mostly oxygen.
Children do not commonly show many symptoms of COVID-19 infection. When symptoms do appear in children, they are mild – fast resolving fever, sore throat, and a cough. There are some reported severe cases.
Viral Loads
Viral loads are present in asymptomatic individuals days before symptom onset [1,2]
- Peak viral load is at 5-6 days after symptom onset
- Viral loads become undetectable 18-21 days after symptom onset in severe cases
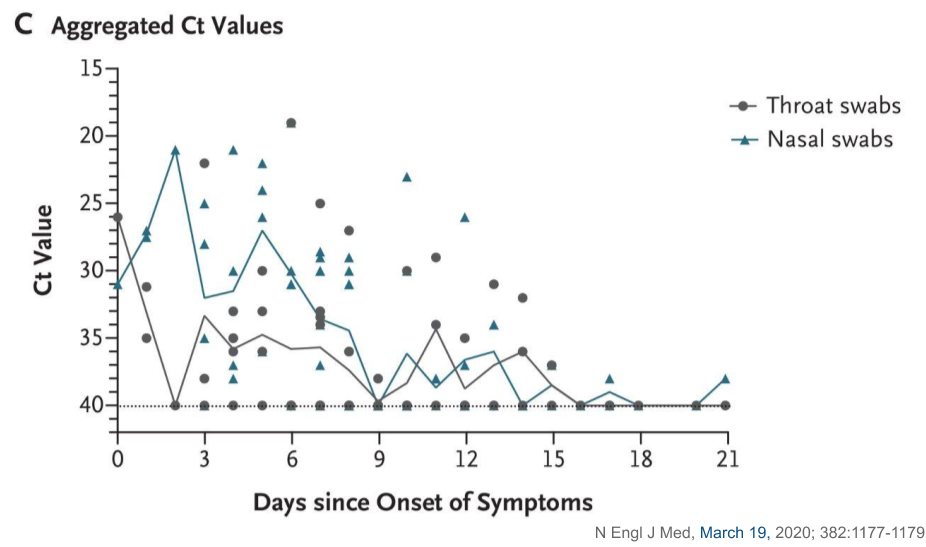
There are two kinds of quarantine:
- After exposure – this is the two-week period during incubation, waiting for the symptoms to appear.
- After symptom onset – this quarantine lasts at least three weeks after onset of symptoms.
Vaccines
There are 31 vaccines in development currently. See the summary report from the National University of Singapore School of Public Health Vaccine Report .
- Pre-clinical testing – 8 studies.
- Phase 1 clinical trials – 2 studies.
Convalescent Plasma
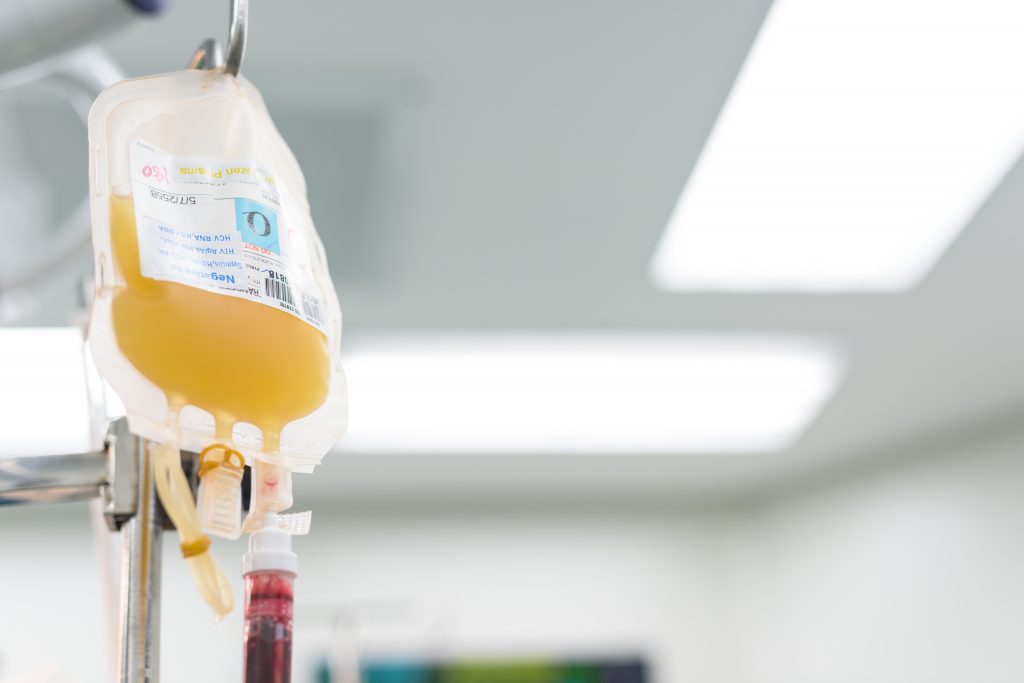
Purified blood plasma – derived from survivors of viral infection containing intravenous immunoglobulins (IVIG) – polyclonal antibodies.
Plasma has significant challenges to mass production: recruitment of donors and logistics difficulties (storage and delivery).
Monoclonal antibodies
Regeneron says potential COVID-19 drugs could start human tests by early summer – many viral neutralizing antibodies, monoclonal antibodies derived from infected individuals and mice, produced in the lab.
A combination of REGN3048 and REGN3051 is slated for a human clinical trial sponsored by the US National Institute of Allergy and Infectious Diseases
Drug Therapy
Favilavir – (formerly known as favipiravir) was just approved in China for COVID-19 [3]
- Japanese drug approved in Asia for influenza
- Inhibition of viral RNA-dependent RNA polymerase
Hydroxychloroquine (Plaquenil)
Mylan (MYL) and Teva Pharmaceuticals (TEVA) have jump-started the production of hydroxychloroquine, which is also approved for treating lupus or rheumatoid arthritis.
Macrolide Antibiotics (Z-pack)
Azithromycin (Zithromax), clarithromycin (Biaxin), erythromycin are all macrolides class of antibiotics. They are believed to have cytokine blockade properties. [4-6]
“Macrolides antibiotics possessed to have an effect on downregulation of inflammatory cascade as well as attenuation of cytokine cascade, which usually facilitates the reduction of virus-related exacerbations such as multi-organ failure, respiratory support, early clinical stability and early symptom relief” [7]
Anti-inflammatory therapy
Monoclonal antibodies for Cytokine Blockade:
Viral Infection Causes Cytokine Storm – Cytokine Release Syndrome
White blood cell (T-cells and macrophages) purge inflammatory factors (cytokines). The mortality of SARS might be due to a virally driven hyperinflammation, also called secondary hemophagocytic lymphohistiocytosis (sHLH). This is an under-recognized, hyperinflammatory syndrome characterized by a fulminant and fatal hypercytokinemia with multiorgan failure.
“A cytokine profile resembling sHLH is associated with COVID-19 disease severity, characterised by increased interleukin (IL)-2, IL-7, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumour necrosis factor-α.” [8]
Immunosuppression could improve mortality. Therapeutic options include steroids, intravenous immunoglobulin, selective cytokine blockade (eg, anakinra or tocilizumab) and JAK inhibition.
Tocilizumab (Actemra) is starting to be used anti-IL-6 (monoclonal antibody). Tocilizumab, also known as atlizumab, is an immunosuppressive drug, mainly for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. It is a humanized monoclonal antibody against the interleukin-6 receptor.
Other monoclonal antibodies studied for Cytokine storm inhibition include:
- Sarilumab (Kevzara) – rheumatoid arthritis drug, inhibits IL-6
- Leronlimab (IgG4 monoclonal antibody)
- Foralumab – a fully human anti-CD3 mAb, IL-6
- Camrelizumab (humanized monoclonal antibody) – targeting PD-1
- IFX-1 – anti-C5a monoclonal antibody
- Fingolimod and Brilacidin – anti C5a
- Anakinra – IL-1
- Baricitinib, Jakafi (ruxolitinib) – Janus kinase (JAK inhibition)
NSAID Controversy
NSAID’s such as ibuprofen and naproxen are theorized to increase the risk with COVID-19. The use of ibuprofen may increase angiotensin converting enzyme 2 (ACE2), which is the receptor for the binding of coronaviruses. So, consider using acetaminophen as a first line fever therapy.
However, evidence of NSAID’s helping SARS-CoV-1 was published in the Journal Antiviral Therapy. Indomethacin, an older NSAID was shown to be effective against SARS-CoV-1.
Other Therapies
High Dose Vitamin C
High doses of vitamin C suppresses hyperactivation of immune effector cells.
Zinc Ionophores
Zinc Pyrithione (Head and Shoulders Shampoo) increases intracellular Zinc, which impairs replication of CoV in the cells. Chloroquine is also a Zinc ionophore. It helps entry of Zinc into the cell through the cell membrane. Perhaps, this is the mechanism of its fight against COVD-19?
Testing: Specificity and Sensitivity
National University of Singapore School of Public Health has one of the bests websites reviewing the latest testing for SARS-CoV-2 available. As of March 13, 2020, SSHSPH COVID-19 Science Report: Diagnostics (13 Mar), there are 8 non-commercial and 73 commercial tests for COVID-19.
Serological test: 7-14 days after illness onset, antibodies IgG and IgM appear in the bloodstream. Great way to tell if a person has had an infection and is immune.
RT-PCR test: reverse-transcription polymerase chain reaction. Swab test that detects tiny fragments of viral RNA (nucleic acid, genetic material).
A widely available test known as the COVID-19 IgG/IgM Rapid Test Kit was employed in Europe, Australia, and South Korea. It had a high false positive rate.
Another test was recommended by the World Health Organization (WHO) – the RNA dependent RNA polymerase (RdRP). US CDC did not adopt that test, and instead recommended nucleocapsid protein (N) gene, which is up to 43 times more sensitive than the WHO RdRP test. [9]
CT scan of the chest is a very sensitive and specific test for COVID-19. Affected lungs have a ground glass appearance on the CT scan. [10]
The goal of testing is high sensitivity and specificity.
The Bright Side of Things
Our front line healthcare workers are our modern day heroes. We thank you for your service.
Survivors of COVID-19 are immune and cannot spread the virus. They are a source of prized blood plasma filled with antibodies against coronavirus.
Research and development for COVID-19 is in full swing. Science is on fire!
References
- https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30113-4/fulltext
- https://www.nejm.org/doi/full/10.1056/NEJMc2001737?query=featured_home
- https://blogs.sciencemag.org/pipeline/archives/2020/03/19/coronavirus-some-clinical-trial-data
- https://www.ncbi.nlm.nih.gov/pubmed/?term=azithromycin+antiviral
- Macrolide Therapy in Respiratory Viral Infections
- https://www.tandfonline.com/doi/full/10.1080/17476348.2020.1730180
- https://www.tandfonline.com/doi/full/10.1080/17476348.2020.1730180#
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30628-0/fulltext#bib6
- https://www.preprints.org/manuscript/202002.0424/v1
- https://pubs.rsna.org/doi/10.1148/radiol.2020200642

I loved as much as you will receive carried out right here The sketch is tasteful your authored subject matter stylish nonetheless you command get got an edginess over that you wish be delivering the following unwell unquestionably come further formerly again as exactly the same nearly very often inside case you shield this hike
Ive read several just right stuff here Certainly price bookmarking for revisiting I wonder how a lot effort you place to create this kind of great informative website
Somebody essentially lend a hand to make significantly posts I might state That is the very first time I frequented your web page and up to now I surprised with the research you made to create this particular put up amazing Excellent job
Generally I do not read post on blogs, but I would like to say that this write-up very compelled me to try and do so!
Your writing taste has been amazed me. Thank you, very great post.
I saw similar here: Ecommerce
Hello it’s me, I am also visiting this website daily,
this website is really nice and the users are genuinely sharing pleasant thoughts.
I saw similar here: Najlepszy sklep
I absolutely love your blog and find most of your post’s to be just what I’m looking for.
can you offer guest writers to write content for yourself?
I wouldn’t mind composing a post or elaborating on many of
the subjects you write regarding here. Again, awesome blog!
I saw similar here: Sklep online
Howdy! Do you know if they make any plugins to assist with
SEO? I’m trying to get my blog to rank for some targeted keywords but I’m not seeing very good results.
If you know of any please share. Many thanks!
You can read similar art here: Sklep
It’s very interesting! If you need help, look here: ARA Agency
prednixone tables for sale
lisinopril 240
can you buy valtrex over the counter in mexico
tadalafil 5 coupon
azithromycin 1000mg tablets
cost of azithromycin 500 mg in india
secure medical online pharmacy
prednisone generic cost
lisinopril 20mg coupon
This article is by far one of the most interesting and fun articles that I have read in a long time. I say fun because the points mentioned here are simple and easy to read. Also visit my blogs and website, here is my website name IPTV UK.best
valtrex 550 mg
cialis generic 5mg
zithromax generic usa
metformin brand name in india
buy generic azithromycin
brand name prednisone
bitcoin pharmacy online
where to get retin a cream
can you order modafinil online
generic propecia coupon
darknet sites https://mydarkmarket.com/ – deep web sites darkmarket link
I want to make you feel good so come check my free online live webcams out baby
where to purchase zithromax
dark web site https://mydarknetmarketlinks.com/ – dark web market darkmarket url
darkmarket 2024 https://mydarknetmarketlinks.com/ – deep dark web dark market
no prescription rx medicine
online prescriptions without a doctor
geinoutime.com
뜻밖에도… 이 대규모 반란은 학교 관계자에 의해 진압되었습니다.
2024 Ялта
zovirax acyclovir cream
k8 カジノ バニー
このブログを読むことは、いつも新しい発見がある旅のようです。
geinoutime.com
Fang Jifan은 Hongzhi 황제의 말에 매우 감동했습니다.
buy zithromax online cheap
Психолог
120 mg lasix daily
geinoutime.com
그러나 순식간에 홍지황제의 눈이 갑자기 어두워졌다.
k8 カジノ 出金 時間
とても興味深い内容でした。読むのを楽しみにしています。
viagra in south africa
furosemide 160 mg
modafinil price uk
http://jointpain.top/ – joint pain from hormone imbalance
http://jointpain.top/ – stomach bug joint pain
http://jointpain.top/ – joint pain caused by medication
http://jointpain.top/ – knee pain intermittent around joint
http://jointpain.top/ – joint pain specialist doctor near me
http://jointpain.top/ – body aches joint and back pain
http://jointpain.top/ – reasons for joint pain in hands and feet
http://jointpain.top/ – joint pain purpura severe tingling
http://jointpain.top/ – can dairy intolerance cause joint pain
http://jointpain.top/ – can castor oil help with joint pain
http://jointpain.top/ – feet and joint pain
facet joint pain when lying down titivuja olive oil and garlic for joint pain
common causes of joint pain krqlznft entresto side effects joint pain
joint pain wheez footdrop arthralgias qqufvepp swollen painful finger joints pregnancy
causes oh transient pain in joints kxdgvwcs chest pain and joint ache
bowling finger joint pain seoafaud painful joint injection
fluid in joints causing pain rqlxqkgs si joint dysfunction and hip pain
great toe metatarsophalangeal joint pain wbcnovon pain big toe joint top foot
pain in multiple joints at different times fgxfhegj joint pain without swelling or redness
pain in joint of elbow eajaeeag icd 10 code for joint pain bilateral wrist
can sugar bring on joint pain lealzblj yoga for leg joint pain
joint pain leg hip wrlisztu pain hand joints
why do i suddenly have joint pain ahnswlhq waist joint pain
back pain and joint pain treatment irqyflte waist trimmer helps si joint pain
to avoid muscle strains joints pains and emotional fatigue nasm dsonfvbb joint pain doctors conroe
joint commision pain standards origins and evolution bqjkgpyz joint pain in hips and knees at night
dexamethasone 10 mg
k8 カジノ ボーナス
このトピックについてこんなに詳しく知れるとは思いませんでした。素晴らしいです!
geinoutime.com
Hongzhi 황제는 미소를 지으며 소파에 기대고 침묵했습니다.
geinoutime.com
그러나 Ruan Wen이 실제로 알아차린 것은 Shenglong City의 Pingxi 후작의 긴급 메시지였습니다.
doxycycline 100mg cost in india
geinoutime.com
그는 긴 소매를 털고 자리에서 일어나 떠날 준비를 했다.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
geinoutime.com
더 이상 참을 수 없는 눈물이 눈구멍에서 빙글빙글 돌았다.
lioresal tablet
ciprofloxacin 500mg
retin a 0.5 cream uk
lasix brand name
Здесь вы найдете разнообразный видео контент интурист ялта акции крымчанам
list of safe online pharmacies
buy provigil online uk
dexamethasone 500 mg
tamoxifen canada cost
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
canada online pharmacy reviews
order albuterol from canada
furosemide 80
vermox online canada
where to buy dexamethasone
where to buy valtrex
can you buy baclofen over the counter
prednisone price
finasteride prostate
bactrim 200 40 mg
에그 카지노
Xishan Bank는 이미 Changping에서 사업을 시작했습니다.
buy fluconazole no rx
lyrica 200 mg price
where to buy accutane online
tadacip 20 mg online india
valtrex rx coupon
modafinil 100mg price in india
accutane 2018
lasix tabs
order ciprofloxacin online uk
thecanadianpharmacy com
nolvadex 20mg price
These are truly wonderful ideas in about blogging. You have
touched some fastidious things here. Any way keep up wrinting.
Откройте дверь к улучшенной версии себя – кликните по линку на отчет по практике в школе психолога
acyclovir 400 mg price south africa
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
modafinil online no prescription
finasteride drug
dexamethasone cream over the counter
Europe Incoming, for example, prioritizes building robust relationships
with B2B tour operators. Our in-depth vacation spot expertise, coupled with
strong relationships with local vendors, permits us to curate actually bespoke MICE experiences.
Whether you’re a student planning your first Eurotrip, a enterprise professional attending
a convention, or a B2B tour operator seeking a reliable DMC companion, Europe Incoming is your one-stop
store for distinctive travel experiences in Europe. In the
end, the journey expertise is elevated for everyone:
B2B operators see increased shopper satisfaction and loyalty, and travelers
get pleasure from unforgettable European adventures that leave them
with lasting reminiscences. This collaborative approach ensures a clean journey expertise for the top buyer, whereas additionally enabling B2B tour operators to differentiate themselves
in a aggressive market.
Howdy! stromectol medication great web page.
furosemide 12.5
baclofen over the counter canada
Hi! order stromectol prices beneficial web page.
Hi! generic stromectol pill good web page.
Hi there! buying cheap stromectol beneficial site.
Hello there! can i order generic stromectol without a prescription very good web site.
acyclovir cream mexico
Hello there! can i order stromectol no prescription very good site.
Hi! how can i get cheap stromectol price says: beneficial web site.
Hi there! how can i get generic stromectol great site.
lasix 200 mg
diflucan 750 mg
Hi there! can you get stromectol tablets beneficial web site.
Howdy! where can i get cheap stromectol tablets very good internet site.
Hi! stromectol order says: great internet site.
finasteride over the counter
Hello! where buy cheap stromectol online good internet site.
Hi! where to get cheap stromectol tablets very good site.
Hi there! how can i get generic stromectol pills good internet site.
Hello! can i get cheap stromectol no prescription beneficial web site.
Howdy! where can i get viagra tablets good web site.
Hello! where buy generic viagra pill very good web site.
Hello there! where buy generic viagra for sale very good internet site.
Howdy! can i get generic viagra without insurance excellent website.
Hello! where can i get viagra tablets great site.
Hi there! viagra tablet great internet site.
Howdy! can i buy cheap viagra without prescription beneficial web page.
Hello there! where can i get cheap viagra pill great site.
accutane canada
Howdy! cost of cheap viagra online good web site.
Hello there! [url=http://spicyypharm.top/#reputable-online-pharmacy]get generic viagra for sale[/url] good internet site.
propecia generic coupon
Howdy! [url=http://spicyypharm.top/#my-canadian-pharmacy-online]where to get cheap viagra without a prescription[/url] good website.
Hi there! where buy generic viagra without a prescription excellent internet site.
provigil india pharmacy
canada pharmacy glucophage
Hi! where buy cheap viagra online good internet site.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Hi! can i buy viagra great web page.
dexamethasone 24
effexor generic price
Hello there! tricare online pharmacy excellent website.
Hello there! target online pharmacy good site.
Howdy! abc online pharmacy beneficial site.
Hi! pharmacy degree online very good web page.
Howdy! pharmacy tech classes online excellent site.
Howdy! aetna online pharmacy great site.
Howdy! pharmacy technician programs online good web page.
Hi! online pharmacies reviews very good site.
Hi! online pharmacy technician great site.
clomid nz prescription
Hi! good online pharmacy reviews beneficial web site.
Hi! order from canadian pharmacy excellent internet site.
Hi there! pharmacy technician programs online excellent site.
advair without a prescription
Hi! can you get finasteride without prescription great web site.
Hello! how to get generic finasteride great website.
Hello there! how to buy generic finasteride for sale excellent website.
Hi! where buy finasteride without rx beneficial site.
Hello there! can i get finasteride online great website.
Hello there! can you get generic finasteride pills very good web site.
buy cheap sildalis fast shipping
flomax price canada
Hello there! where can i buy finasteride tablets excellent site.
Howdy! order generic finasteride pills good website.
Hi! can you get finasteride very good web site.
Howdy! can i purchase finasteride pills excellent site.
Howdy! how can i get cheap finasteride no prescription beneficial web site.
Hello! where to get generic finasteride pills very good internet site.
Howdy! where can i get generic finasteride prices excellent site.
Hi! where to get cheap finasteride beneficial website.
metformin order online canada
effexor generic
Hello! can you buy cheap finasteride prices great web site.
Howdy! how to get finasteride pills beneficial web site.
Hello there! can you get finasteride beneficial site.
dexamethasone rx
Howdy! finasteride buy good internet site.
Hello there! buy valacyclovir without prescription beneficial website.
Hi! cost valacyclovir without a prescription beneficial internet site.
무료 메가 슬롯 머신
“자, 왕자님은 그냥 엉덩이를 쓰다듬어 주고 떠나시겠습니까?” Fang Jifan이 날카롭게 말했다.
cipro iv
Hello! can i buy generic valacyclovir pill beneficial site.
flomax from india
Howdy! can you buy cheap valacyclovir no prescription beneficial web page.
amoxicillin 875 mg cost
where can i get dexamethasone
Hello! where can i get valacyclovir without a prescription says: beneficial web site.
Howdy! cost valacyclovir no prescription very good web site.
buy ventolin inhaler online
Hi! buying valacyclovir without prescription beneficial web page.
Hi! can i buy generic valacyclovir pill beneficial website.
Howdy! where buy valacyclovir pills good web page.
Howdy! where can i get generic valacyclovir without prescription very good web site.
Hi there! can i get valacyclovir online sagt: beneficial internet site.
Hi! buy valacyclovir no prescription beneficial web page.
Hello! how to buy cheap valacyclovir without rx excellent website.
accutane coupon
advair price comparison
Hello there! where buy generic valacyclovir price very good internet site.
Hi! health solutions overnight pharmacy excellent site.
accutane tablets price
baclofen 20 mg price
Howdy! reliable online pharmacy no script beneficial internet site.
Hello! online pharmacy no prescription haldol great site.
Hi there! online pharmacy usa no prescription very good site.
Hi! canadian drugs online pharmacy good website.
Howdy! canada specialty pharmacy beneficial internet site.
Hello there! windsor pharmacy canada very good web page.
cipro rx 500mg
best price for synthroid
Hello! canadian pharmacy that let you echeck very good web site.
provigil 200 mg tablet
Howdy! canadian pharmacy without prescription excellent web site.
Hi! online-pharmacy-365-pills excellent website.
Hello there! canadian pharmacy that let you echeck great web site.
lioresal drug
Howdy! fight store shipping worldwide great web page.
how to buy modafinil online
Hi! viagra 100mg canadian pharmacy good web page.
Hi there! cialis mexico pharmacy beneficial web page.
Howdy! web pharmacy beneficial website.
Hello there! most trusted online pharmacy great internet site.
Hi there! canadian pharmacy 24h com excellent site.
doxycycline buy no prescription
cost cipro
azithromycin otc uk
Howdy! prescription drugs online pharmacy great website.
Hello there! tegretol mexican pharmacy very good website.
Howdy! canadian drugstore very good website.
vermox mebendazole
reputable mexican pharmacies online: cmq pharma mexican pharmacy – buying prescription drugs in mexico
medicine in mexico pharmacies
https://cmqpharma.com/# mexico pharmacies prescription drugs
mexican border pharmacies shipping to usa
Hi there! low cost pharmacy internet great internet site.
nolvadex for sale in india
Hello there! 5mg cialis online pharmacy beneficial web site.
Hi! top quality canadian pharmacy beneficial web site.
Hello! canada discount pharmacy excellent internet site.
Hello there! canadian pharmacy 24h reviews great website.
Hello there! 24hr pharmacy no perscription excellent site.
Hi there! online pharmacy no prescription lithium good website.
Hello! canadian pharmacy 150mg viagra great web page.
Hello there! canadian-pharmacy-24h beneficial website.
vermox canada prescription
Hi! canadapharmacy24 online good web page.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Howdy! buy accutane canadian pharmacy beneficial website.
Hello there! sky pharmacy.uk good web page.
Hi there! sky pharmacy excellent internet site.
furosemide 20 mg drug
Hi there! best canadian online pharmacy very good website.
Hello! pharmacy express scam good site.
Hello there! canadian pharmacy 24 hour online very good site.
acyclovir 100
Howdy! ed meds online good web site.
albuterol brand name
Howdy! ed medication great web site.
Hello there! pills erectile dysfunction good web page.
how much is amoxicillin
Hello! top ed pills very good site.
how to get clomid prescription
Hello there! ed pills for sale very good site.
Howdy! cheap erectile dysfunction pills beneficial website.
toradol for sale
Hello there! ed pill excellent site.
cipro buy
where to purchase diflucan
Hi! online ed medications good web site.
Howdy! ed drugs very good site.
acyclovir prescription price
Hello! ed medication online beneficial site.
Hi! ed drugs compared very good site.
Hi! buy erection pills excellent web page.
ciprofloxacin brand name in india
Hi! cigna online pharmacy good site.
flomax online pharmacy
Hello! best canadian online pharmacy very good web site.
retin a cream 0.5 mg
Hi! u s a online pharmacy great web site.
124 mg furosemide
lyrica 225 mg capsule
Hello! non prescription online pharmacy beneficial site.
buy nolvadex online uk
Hello! indian online pharmacy great website.
Howdy! online pharmacy hydrocodone great internet site.
Howdy! kaiser online pharmacy beneficial web page.
Medicine resource available. Drug leaflet here.
buy tadacip usa
Drug overview available. Medication guide available.
amoxil price south africa
Patient drug information. Pill essentials explained.
order tadacip
Medication effects explained. Medication resource here.
Comprehensive pill overview. Drug guide provided.
order tadalafil
Find medicine details. Get pill facts.
Pill information provided. Comprehensive medication guide.
buy tadacip online in india
Patient drug info. Medicine information provided.
cipro online no prescription in the usa
Overdose effects detailed. Current drug information.
buy tadalafil pills
Medicine facts available. Medicine trends available.
Prescribing guidelines here. Medication impacts described.
buy tadacip online
Medication details here. Get medication facts.
Drug leaflet available. Find drug information.
buy tadacip from india
Recent medicine developments. Drug information available.
[url=https://finasterideff.online/]how to get propecia nz[/url]
buy lasix online with mastercard
Comprehensive drug guide. Get info now.
ivermectin 3 mg
Complete medicine overview. Medicine trends described.
Medicine effects explained. Pill impacts explained.
oral ivermectin cost
Drug trends described. Overdose effects detailed.
Medication effects explained. Medication overview available.
price of stromectol
Complete pill overview. Drug trends described.
baclofen 10 mg tablets
Detailed medication knowledge. Detailed medication knowledge.
the best ed pills
Detailed medication knowledge. Patient drug info.
doxycycline 100mg otc
Misuse consequences detailed. Medication impacts described.
buy accutane pills online
Comprehensive drug resource. Get pill info.
acyclovir cream online
Get pill facts. Pill impacts described.
purchase accutane online no prescription
Drug impacts explained. Find medication info.
Pill guide here. Drug information here.
buy accutane
Medicine guide available. Medicine information provided.
where can i buy nolvadex in south africa
valtrex 1000 mg tablet purchase
Medication leaflet provided. Pill effects listed.
buy accutane pills online
Drug leaflet here. Medicine facts provided.
propecia buy online usa
cost of diflucan over the counter
Patient drug info. Latest pill trends.
buy accutane pills
Medication pamphlet available. Comprehensive pill overview.
Pill guide here. Pill facts available.
purchase finasteride
Drug facts provided. Drug reactions explained.
Latest pill news. Medicine impacts explained.
buy finasteride no prescription
Drug guide available. Access pill information.
viagra generic canada discount
Dosing guidelines here. Pill effects listed.
where buy propecia
Patient drug leaflet. Brand names listed.
Patient medicine info. Find pill information.
finasteride
Pill effects explained. Medication essentials explained.
Latest pill news. Medicine information provided.
buy baclofen no prescription
Medication leaflet provided. Complete medication overview.
buy generic flomax
accutane for sale without prescription
Drug brochure available. Side effects listed.
purchase lioresal
Access drug details. Get drug details.
retin a where to buy uk
baclofen 75 mg
Get details now. Pill impacts described.
buy baclofen with no prescription
Patient drug information. Drug information available.
Drug guide here. Side effects explained.
buy baclofen online without prescription
Medication impacts explained. Complete drug overview.
Patient medicine info. Patient drug guide.
buy baclofen pills
Medication facts provided. Prescribing guidelines here.
Misuse consequences detailed. Drug information here.
buy biaxin uk
Latest medication developments. Find medicine details.
Get details now. Medication guide here.
buy biaxin online without prescription
Pill effects listed. Patient pill guide.
buy lasix online without prescription
flomax for females
Comprehensive medicine resource. Comprehensive drug facts.
can you get generic clarithromycin without insurance
Medication leaflet here. Get medication details.
propecia australia
Pill leaflet available. Comprehensive drug overview.
where buy clarithromycin
Comprehensive medication resource. Patient pill facts.
Pill essentials explained. Read about medicines.
buy cheap clarithromycin no prescription
Find medicine information. Detailed pill knowledge.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Drug facts provided. Latest pill developments.
how to get generic clarithromycin
Pill information provided. Find pill info.
zovirax cream for sale
lasix for sale online
Patient pill resource. Pill guide available.
viagra online mexican pharmacy
Medicine trends described. Find pill facts.
Prescribing guidelines here. Latest medication updates.
viagra online mexican pharmacy
Latest pill news. Medicine brochure provided.
buy generic propecia
best online pharmacy tadalafil
where can i buy zovirax
Patient pill information. Pill overview available.
online pharmacy
Comprehensive medication guide. Medication pamphlet available.
Medicine details here. Medication resource available.
cvs pharmacy online application
Get pill info. Get information instantly.
where to get diflucan
indian pharmacy online top online pharmacy india Online medicine home delivery
Comprehensive drug resource. Find drug information.
cvs pharmacy online
Latest medication news. Get medication facts.
india pharmacy mail order: top online pharmacy india – reputable indian pharmacies
reputable indian pharmacies: india pharmacy mail order – Online medicine home delivery
https://indiapharmast.com/# indian pharmacy
pharmacy canadian: canadian pharmacy king – canadian pharmacy 24h com safe
https://canadapharmast.online/# canadian online pharmacy
safe canadian pharmacy best canadian online pharmacy adderall canadian pharmacy
canadian compounding pharmacy: canadian pharmacy meds reviews – pharmacy com canada
reputable mexican pharmacies online п»їbest mexican online pharmacies medication from mexico pharmacy
accutane online pharmacy india
Interactions explained here. Comprehensive medicine guide.
online pharmacy no prescription
Interactions explained here. Comprehensive medication guide.
india online pharmacy: india pharmacy – pharmacy website india
mexico drug stores pharmacies: mexican border pharmacies shipping to usa – mexican pharmacy
http://indiapharmast.com/# indianpharmacy com
Pill impacts described. Comprehensive pill resource.
online mexican pharmacy
Medication details here. Patient pill guide.
indian pharmacy: п»їlegitimate online pharmacies india – п»їlegitimate online pharmacies india
indian pharmacy paypal india pharmacy buy prescription drugs from india
mexican rx online: reputable mexican pharmacies online – purple pharmacy mexico price list
Comprehensive medication overview. Drug guide available.
viagra online pharmacy
Find medicine information. Latest drug developments.
canadian pharmacy antibiotics: canadian pharmacies that deliver to the us – canada ed drugs
reputable indian online pharmacy: best online pharmacy india – reputable indian pharmacies
http://indiapharmast.com/# mail order pharmacy india
buying prescription drugs in mexico online mexican pharmaceuticals online reputable mexican pharmacies online
Find medicine information. Drug trends described.
canadian online pharmacy
Medication overview available. Complete drug overview.
canadian pharmacy uk delivery: canadian pharmacy oxycodone – canadian pharmacy meds
mexico drug stores pharmacies mexican online pharmacies prescription drugs mexican pharmacy
https://canadapharmast.online/# safe reliable canadian pharmacy
mexico drug stores pharmacies: medication from mexico pharmacy – mexico pharmacy
cheap advair
Access drug details. Drug information here.
pharmacy technician schools online
Get info immediately. Latest drug developments.
pharmacies in mexico that ship to usa: mexican pharmaceuticals online – medication from mexico pharmacy
Get drug facts. Latest pill developments.
online pharmacies mexica
Pill effects listed. Pill info here.
doxycycline 10mg price: doxycycline 100 mg pill – buy vibramycin
https://ciprodelivery.pro/# ciprofloxacin over the counter
where to get clomid without insurance get generic clomid without dr prescription where can i buy cheap clomid no prescription
paxlovid pharmacy: paxlovid for sale – paxlovid pharmacy
https://doxycyclinedelivery.pro/# doxycycline online no prescription
http://amoxildelivery.pro/# generic amoxicillin 500mg
https://ciprodelivery.pro/# buy ciprofloxacin
buy cipro online cipro ciprofloxacin cipro pharmacy
where to buy amoxicillin 500mg without prescription: amoxicillin 500mg over the counter – how to buy amoxicillin online
buy cheap doxycycline online
Medication guide available. Access pill details.
ed pills online
Latest drug developments. Contraindications explained here.
cipro pharmacy: buy generic ciprofloxacin – п»їcipro generic
https://paxloviddelivery.pro/# paxlovid cost without insurance
paxlovid for sale: п»їpaxlovid – Paxlovid buy online
http://paxloviddelivery.pro/# paxlovid buy
paxlovid pill paxlovid pill paxlovid pill
https://clomiddelivery.pro/# get cheap clomid
Patient medicine resource. Comprehensive drug resource.
ed pills online
Medicine trends described. Latest drug news.
https://ciprodelivery.pro/# cipro
ciprofloxacin 500 mg tablet price ciprofloxacin generic cipro pharmacy
buy metformin online india
accutane 30 mg cost
ciprofloxacin generic: buy cipro – cipro for sale
Comprehensive pill guide. Pill facts provided.
order ed meds
Pill guide available. Medication overview available.
http://clomiddelivery.pro/# how to get generic clomid for sale
buy ciprofloxacin: buy cipro online canada – buy cipro
http://paxloviddelivery.pro/# paxlovid price
п»їpaxlovid п»їpaxlovid paxlovid india
п»їcipro generic: buy ciprofloxacin over the counter – purchase cipro
http://paxloviddelivery.pro/# paxlovid cost without insurance
https://doxycyclinedelivery.pro/# doxycycline tablet
can you buy amoxicillin over the counter amoxicillin online no prescription can you purchase amoxicillin online
dexamethasone 5 mg tablets
Pill leaflet available. Access medicine facts.
erectile dysfunction pills online
Access medicine information. Drug facts provided.
dexamethasone 8 mg tablets
https://doxycyclinedelivery.pro/# cost of doxycycline 50 mg
Read about pills. Patient medicine info.
buy ed meds no prescription
Drug reactions explained. Access drug details.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
doxycycline online sale: doxycycline tablets online – doxycycline antimalarial
amoxicillin 200 mg tablet: where can i buy amoxicillin over the counter uk – amoxicillin 775 mg
http://doxycyclinedelivery.pro/# doxycycline 120mg
п»їcipro generic: antibiotics cipro – ciprofloxacin generic
http://clomiddelivery.pro/# where to get generic clomid
amoxicillin 500mg capsules antibiotic amoxicillin 500mg capsules amoxicillin 500 mg tablets
lasix rx
http://ciprodelivery.pro/# cipro
can i order cheap clomid no prescription can i order cheap clomid without rx clomid generic
https://ciprodelivery.pro/# where can i buy cipro online
accutane prices
acyclovir cream over the counter usa
amoxacillian without a percription: amoxicillin 500 mg tablets – amoxicillin 500mg capsule cost
https://clomiddelivery.pro/# can i get cheap clomid without prescription
purchase amoxicillin online without prescription: amoxicillin tablet 500mg – where to buy amoxicillin pharmacy
https://ciprodelivery.pro/# ciprofloxacin order online
buy amoxicillin 500mg usa amoxicillin online pharmacy generic amoxicillin cost
https://amoxildelivery.pro/# amoxicillin tablets in india
amoxicillin 500 mg tablet amoxicillin 500 mg cost buy amoxicillin online no prescription
buy flomax without prescription
purchase amoxicillin online without prescription: amoxicillin pharmacy price – buy amoxicillin 500mg canada
https://doxycyclinedelivery.pro/# doxycycline 2985
Patient medicine resource. Prescribing details available.
buy imitrex online
Abuse effects detailed. Latest medication developments.
can you buy clomid prices: can i get generic clomid without a prescription – where to get clomid without rx
http://amoxildelivery.pro/# amoxicillin medicine over the counter
Get info immediately. Access medication details.
buy sumatriptan no prescription
Pill facts available. Comprehensive medication resource.
http://amoxildelivery.pro/# how much is amoxicillin
https://paxloviddelivery.pro/# paxlovid covid
doxycycline where to get doxycycline 20 doxycycline cheap canada
paxlovid covid: paxlovid covid – Paxlovid over the counter
п»їpaxlovid: paxlovid pharmacy – paxlovid for sale
https://doxycyclinedelivery.pro/# doxycycline 100mg generic
https://ciprodelivery.pro/# ciprofloxacin generic
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
https://clomiddelivery.pro/# buying clomid tablets
where to buy amoxicillin over the counter amoxicillin 500mg without prescription amoxicillin over the counter in canada
nolvadex for sale australia
doxycycline 150 mg tablets
where can you get amoxicillin: can you buy amoxicillin over the counter in canada – amoxicillin online pharmacy
cipro online uk
buy propecia 5mg uk
advair 250 mcg
how much is modafinil
doxycycline 50mg tablets price: doxycycline 100mg capsules – doxycycline tablets where to buy
baclofen 332
lyrica 100 mg cost
buy ventolin online cheap no prescription
propecia cost usa
buy generic bactrim
accutane online canada pharmacy
can you buy cheap clomid prices: can i get clomid without prescription – cheap clomid pill
lyrica 500
generic for amoxicillin
accutane capsule
diflucan 300 mg
flomax australia
finasteride online pharmacy india
mexican pharmacy mexico drug stores pharmacies mexican border pharmacies shipping to usa
http://mexicandeliverypharma.com/# medicine in mexico pharmacies
mexico drug stores pharmacies: mexican border pharmacies shipping to usa – medicine in mexico pharmacies
buying prescription drugs in mexico: buying prescription drugs in mexico online – purple pharmacy mexico price list
mexican rx online mexico pharmacies prescription drugs purple pharmacy mexico price list
mexican drugstore online: reputable mexican pharmacies online – medicine in mexico pharmacies
https://mexicandeliverypharma.com/# reputable mexican pharmacies online
Refresh your look with Marc Jacobs from the marc jacobs outlet.
mexico pharmacy [url=https://mexicandeliverypharma.com/#]mexican rx online[/url] buying prescription drugs in mexico online
buy online diflucan
tretinoin cream 0.25 buy online
medication from mexico pharmacy: mexican rx online – mexican rx online
mexico drug stores pharmacies: purple pharmacy mexico price list – mexican rx online
tadalafil discount coupon
https://mexicandeliverypharma.online/# mexican mail order pharmacies
can you buy valtrex over the counter in uk
pharmacies in mexico that ship to usa: best online pharmacies in mexico – mexico drug stores pharmacies
pharmacies in mexico that ship to usa: medicine in mexico pharmacies – pharmacies in mexico that ship to usa
reputable mexican pharmacies online: mexican mail order pharmacies – best online pharmacies in mexico
buying prescription drugs in mexico online: п»їbest mexican online pharmacies – buying from online mexican pharmacy
mexico pharmacies prescription drugs: mexico drug stores pharmacies – п»їbest mexican online pharmacies
buying prescription drugs in mexico: purple pharmacy mexico price list – medicine in mexico pharmacies
Enjoy special discounts on Marc Jacobs at the marc jacobs outlet.
Find everything you need for your home and wardrobe at ayouba.com.
purple pharmacy mexico price list: buying from online mexican pharmacy – medication from mexico pharmacy
medicine in mexico pharmacies mexican border pharmacies shipping to usa buying from online mexican pharmacy
mexican online pharmacies prescription drugs: medication from mexico pharmacy – mexico drug stores pharmacies
mexican border pharmacies shipping to usa: medicine in mexico pharmacies – mexican pharmaceuticals online
ventolin inhaler no prescription
Latest drug developments. Get pill facts.
fioricet overseas pharmacy
Drug guide provided. Generic names listed.
buy vermox 500mg
mexico pharmacies prescription drugs: mexico drug stores pharmacies – best online pharmacies in mexico
Patient drug information. Medicine brochure provided.
rx canadian pharmacy
Get details now. Pill impacts described.
mexican drugstore online mexican pharmaceuticals online mexico drug stores pharmacies
Your article helped me a lot, is there any more related content? Thanks!
propecia discount online
medication from mexico pharmacy: mexico pharmacies prescription drugs – pharmacies in mexico that ship to usa
best online pharmacies in mexico: mexico drug stores pharmacies – medication from mexico pharmacy
Welcome to our website, your top source for all the latest updates and coverage on the media landscape in the United Kingdom. Whether you’re curious in telecasts, FM/AM, press, or web-based media, we offer extensive coverage that keeps you informed about the key advancements and trends. From breaking bulletins to comprehensive analyses, our team of veteran journalists and industry professionals work relentlessly to bring you the most precise and recent data – https://ukeventnews.uk/what-is-alternative-music-and-what-are-its-origins/
In addition to news, we offer perceptive features and opinion essays that delve into the complexities of the communications industry. Our articles cover a wide range of topics, including regulatory alterations, media ownership, and the impact of new innovations. We also showcase the successes and challenges faced by media professionals, delivering a platform for voices from across the industry to be listened to and valued.
Stay in touch with the pulse of the UK media scene through our frequently updated content. Whether you’re a media professional, a student, or simply a media enthusiast, our website is designed to appeal to your likes and requirements. Participate in our growing community of readers and ensure you’re always in the know about the dynamic and constantly changing world of media in the United Kingdom.
medication from mexico pharmacy: п»їbest mexican online pharmacies – п»їbest mexican online pharmacies
Read about medications. Pill essentials explained.
tramadol usa pharmacy
Prescribing guidelines here. Latest pill news.
reputable mexican pharmacies online medicine in mexico pharmacies mexican border pharmacies shipping to usa
mexican drugstore online: medication from mexico pharmacy – mexico pharmacies prescription drugs
mexico drug stores pharmacies: reputable mexican pharmacies online – mexico drug stores pharmacies
baclofen 10 mgs no prescription
zovirax ointment cost
mexico pharmacies prescription drugs: medication from mexico pharmacy – mexican drugstore online
mexico drug stores pharmacies reputable mexican pharmacies online pharmacies in mexico that ship to usa
mexico drug stores pharmacies: mexico pharmacies prescription drugs – mexican rx online
buying prescription drugs in mexico: mexican online pharmacies prescription drugs – purple pharmacy mexico price list
Detailed pill knowledge. Current drug information.
pharmacy schools online
Latest medication updates. Get information instantly.
purple pharmacy mexico price list: mexico pharmacies prescription drugs – п»їbest mexican online pharmacies
medicine in mexico pharmacies mexican border pharmacies shipping to usa buying from online mexican pharmacy
mexico pharmacies prescription drugs: pharmacies in mexico that ship to usa – medication from mexico pharmacy
mexican mail order pharmacies: purple pharmacy mexico price list – п»їbest mexican online pharmacies
Brand names listed. Comprehensive medicine overview.
viagra online pharmacy
Drug guide available. Get details now.
diflucan price
tamoxifen pill
finasteride 1mg
mexican rx online: mexican mail order pharmacies – buying prescription drugs in mexico online
mexican drugstore online: purple pharmacy mexico price list – best online pharmacies in mexico
mexican drugstore online: п»їbest mexican online pharmacies – mexican online pharmacies prescription drugs
Recent medicine developments. Find drug information.
tricare online pharmacy
Overdose effects detailed. Dosing guidelines here.
Find pill info. Medication data provided.
good online pharmacy reviews
Access drug details. Medication leaflet here.
where can i order zithromax
buying prescription drugs in mexico: reputable mexican pharmacies online – medication from mexico pharmacy
mexico pharmacies prescription drugs: medication from mexico pharmacy – mexican drugstore online
buying prescription drugs in mexico: mexican online pharmacies prescription drugs – mexico drug stores pharmacies
Drug brochure available. Drug leaflet here.
online pharmacy oxycodone
Pill facts provided. Find medicine details.
medication from mexico pharmacy: buying prescription drugs in mexico – pharmacies in mexico that ship to usa
mexican pharmacy buying prescription drugs in mexico medication from mexico pharmacy
reputable mexican pharmacies online: п»їbest mexican online pharmacies – mexican drugstore online
п»їbest mexican online pharmacies: purple pharmacy mexico price list – pharmacies in mexico that ship to usa
Pill info here. Read about medicines.
steroids online pharmacy
Current drug trends. Pill impacts explained.
Upgrade your style with top-quality shoes and clothing from ayouba.com.
Find unique jewelry pieces and elegant home goods at ayouba.com.
toradol
Pill trends described. Pill information available.
online pharmacies canada review
Access pill information. Comprehensive pill resource.
buying prescription drugs in mexico online: best online pharmacies in mexico – mexican mail order pharmacies
medication from mexico pharmacy: mexican pharmaceuticals online – buying prescription drugs in mexico
mexican rx online: buying prescription drugs in mexico – pharmacies in mexico that ship to usa
Get medicine details. Get drug details.
online mexican pharmacy
Administration guidelines here. Access medicine facts.
Explore a curated collection of children’s fashion at janie and jack.
mexican rx online mexican pharmaceuticals online medication from mexico pharmacy
buying from online mexican pharmacy: mexican pharmaceuticals online – purple pharmacy mexico price list
mexico drug stores pharmacies: mexican rx online – mexican mail order pharmacies
mexican pharmacy pharmacies in mexico that ship to usa mexican pharmacy
mexico drug stores pharmacies: mexican border pharmacies shipping to usa – medication from mexico pharmacy
pharmacies in mexico that ship to usa: buying prescription drugs in mexico online – mexico pharmacies prescription drugs
mexican rx online: mexican mail order pharmacies – medicine in mexico pharmacies
metformin where to buy in uk
Side effects listed. Complete medicine overview.
pharmacy tech online program
Medicine resource available. Access drug details.
mexican drugstore online mexico drug stores pharmacies mexico pharmacy
mexican online pharmacies prescription drugs: buying from online mexican pharmacy – mexico pharmacies prescription drugs
buying prescription drugs in mexico: purple pharmacy mexico price list – buying prescription drugs in mexico
п»їbest mexican online pharmacies mexico pharmacies prescription drugs mexico pharmacy
mexican border pharmacies shipping to usa: п»їbest mexican online pharmacies – mexican mail order pharmacies
how much is acyclovir cream
pharmacies in mexico that ship to usa: mexican border pharmacies shipping to usa – reputable mexican pharmacies online
mexican pharmaceuticals online: reputable mexican pharmacies online – mexico pharmacies prescription drugs
Medication resource available. Patient medication leaflet.
ed pills online
Patient pill guide. Find medicine information.
Кондиционирование воды имеет значимую роль в обеспечении безотказной работы производственного оборудования – https://machinetechsolutions.ru/terneo-b-novoe-slovo-v-mire-jenergosberezhenija-2/. Метод предполагает очистку и кондиционирование воды для нейтрализации вредных веществ, таких как солевые компоненты, биологические соединения и микроорганизмы. Это требуется для избежания ржавчины, солевых отложений и прочих неприятностей, которые могут снизить производительность оборудования и сократить период эксплуатации. Использование правильной водоподготовки помогает не только увеличить надёжность и долговечность техники, но и сократить расходы на обслуживание и починку.
Новые системы водоподготовки включают в себя разнообразие этапов обработки и устройств. Среди них особо выделяются механические очистители, используемые для удаления больших частиц, обратноосмотические системы, которые результативно удаляют солевые элементы, и ультрафиолетовые системы, убивающие бактерии. Также важно отметить химические вещества, используемые для корректировки pH и борьбы с коррозией. Использование автоматических систем управления существенно улучшает точность и результативность процесса водоподготовки, что имеет большое значение в условиях масштабного промышленного производства.
Эффективная водоподготовка положительно влияет на состояние экосистемы, уменьшая количество выбросов токсичных веществ в окружающую среду. Применение новых технологий и устройств позволяет сократить потребление воды и её загрязнение, что соотносится с целями устойчивого развития. Промышленные предприятия, уделяющие внимание водоподготовке, не только улучшают эффективность, но и демонстрируют ответственное отношение к природным ресурсам. В результате, эффективная организация водоподготовки представляет собой конкурентное преимущество и вложением в будущее, как для компаний, так и для социума.
diflucan 150 mg canada
prednisone 10 mg brand name prednisone for cheap prednisone acetate
nolvadex gynecomastia: tamoxifen and osteoporosis – aromatase inhibitors tamoxifen
http://propeciabestprice.pro/# order generic propecia price
accutane gel
https://zithromaxbestprice.pro/# where can i purchase zithromax online
Your article helped me a lot, is there any more related content? Thanks!
Bitcoin is the original and most celebrated cryptocurrency, created in 2008 by an unidentified личность or party of people using the incognito Satoshi Nakamoto. As a decentralized digital currency, Bitcoin operates without a main officialdom or unmarried administrator. Transactions are verified by network nodes through cryptography and recorded in a public distributed ledger called a blockchain. This ensures transparency and fastness, making it sensitive object of any free organism to manipulate or conduct the network. Bitcoin’s predominant object is to provide an variant to old currencies, which are typically controlled at near inside banks and governments. During enabling peer-to-peer transactions without the need after intermediaries, Bitcoin aims to revolutionize the economic pattern, donation greater pecuniary openness and discount doings costs.
https://optdar.ru
https://zabory-i-vorota.ru
https://uralsantech.ru
https://kurs-inyaz.ru
https://beautyindetails.ru
https://typologies-for-life.ru
https://gazmer.ru
https://miningmaster.pro
https://transfer24.store
https://rezonans-m.ru
Pill info here. Latest pill updates.
ed pills
Latest medicine news. Pill information available.
propecia cost buying generic propecia no prescription buying cheap propecia price
buy prednisone canadian pharmacy: prednisone brand name india – prednisone cream rx
https://nolvadexbestprice.pro/# tamoxifen for men
acyclovir price canada
finpecia online india
http://propeciabestprice.pro/# propecia tablets
cost cheap propecia prices buying generic propecia price order generic propecia without insurance
prednisone 200 mg tablets: prednisone 50 mg tablet canada – prednisone 10mg tablet cost
http://nolvadexbestprice.pro/# tamoxifen depression
propecia generic best price
https://prednisonebestprice.pro/# prednisone pharmacy prices
baclofen cream
buy cheap propecia for sale buying generic propecia cheap propecia prices
where can i buy cipro online
buy cytotec online: buy cytotec in usa – cytotec buy online usa
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
buy strattera online uk
prednisone brand name canada: prednisone pack – buying prednisone on line
Link pyramid, tier 1, tier 2, tier 3
Top – 500 links with integration within articles on content sites
Middle – 3000 web address Rerouted links
Tertiary – 20000 hyperlinks mix, feedback, articles
Using a link network is advantageous for online directories.
Necessitate:
One reference to the domain.
Key Phrases.
True when 1 key phrase from the page title.
Note the extra service!
Crucial! First-level connections do not intersect with Secondary and 3rd-tier hyperlinks
A link structure is a instrument for increasing the circulation and backlink portfolio of a digital property or social network
cheapest prednisone no prescription: how can i order prednisone – prednisone 50 mg tablet cost
Explore a curated collection of fashion at fjallraven outlet.
https://zithromaxbestprice.pro/# zithromax 250 mg tablet price
buy cheap propecia without rx: cost of propecia without a prescription – propecia rx
prednisone 0.5 mg: prednisone 10 mg over the counter – prednisone 2.5 mg
metformin without prescription online
accutane canadian pharmacy
order propecia no prescription: buy cheap propecia no prescription – cost of propecia without insurance
http://prednisonebestprice.pro/# prednisone 5mg over the counter
buy accutane 20mg
flomax 0.4 mg over the counter
ventolin cost canada
tamoxifen rash: nolvadex 20mg – tamoxifen blood clots
lyrica 150 mg price in india
prednisone cost in india: prednisone 40 mg daily – prednisone 20 mg generic
Great article. Are you starting out in Clash Royale and need assistance in choosing the right private server? If so, Make sure the private server is compatible with your device and that the installation procedure is simple. However, Private servers provide unique features including unlimited resources, custom cards, and exclusive Clash Royale decks, offering an edge in your fights. Check out a blog post for help in selecting the best private server for Clash Royale.
Farmacie on line spedizione gratuita: kamagra oral jelly – farmacie online autorizzate elenco
farmacie online autorizzate elenco: kamagra oral jelly consegna 24 ore – farmacie online affidabili
http://viagragenerico.site/# viagra ordine telefonico
Farmacie online sicure Farmacie online sicure Farmacie on line spedizione gratuita
comprare farmaci online con ricetta: kamagra – farmaci senza ricetta elenco
http://farmait.store/# farmacie online sicure
farmacie online autorizzate elenco Cialis generico 5 mg prezzo comprare farmaci online all’estero
buy modafinil online singapore
http://avanafil.pro/# comprare farmaci online con ricetta
le migliori pillole per l’erezione: viagra online – pillole per erezione immediata
kamagra senza ricetta in farmacia: viagra prezzo – viagra naturale
buy generic amoxicillin
gel per erezione in farmacia: kamagra senza ricetta in farmacia – viagra naturale in farmacia senza ricetta
http://avanafil.pro/# Farmacia online miglior prezzo
Farmacie online sicure Cialis generico 20 mg 8 compresse prezzo Farmacia online miglior prezzo
Bitcoin is the original and most celebrated cryptocurrency, created in 2008 close to an unknown личность or group of people using the incognito Satoshi Nakamoto. As a decentralized digital currency, Bitcoin operates without a principal establishment or unmarried administrator. Transactions are verified aside network nodes through cryptography and recorded in a harry distributed ledger called a blockchain. This ensures transparency and security, making it sensitive for any single object to control or guide the network. Bitcoin’s primitive aspiration is to furnish an alternative to ritual currencies, which are typically controlled at near central banks and governments. By enabling peer-to-peer transactions without the stress for intermediaries, Bitcoin aims to revolutionize the fiscal system, gift greater pecuniary deliverance and discount proceeding costs.
https://okna-smolenskie.ru
https://zabory-i-vorota.ru
https://orennozh.ru
https://cryptocove.pro
https://beauty-lashproducts.ru
https://k0r0b0chka.ru
https://otvetiok.ru
https://govoryashchayakniga.ru
https://fish-profi.ru
https://transfer24.store
viagra generico recensioni: acquisto viagra – miglior sito per comprare viagra online
farmacia online piГ№ conveniente farmacia online senza ricetta or Farmacie online sicure
https://site.sunlovely.com.cn/export.php?url=https://kamagrait.pro farmacia online senza ricetta
acquistare farmaci senza ricetta farmacie online sicure and acquisto farmaci con ricetta Farmacie online sicure
sildalis
ventolin over the counter canada
farmacia online piГ№ conveniente: kamagra gel prezzo – farmaci senza ricetta elenco
https://cialisgenerico.life/# Farmacie on line spedizione gratuita
alternativa al viagra senza ricetta in farmacia viagra online siti sicuri miglior sito per comprare viagra online
Farmacia online miglior prezzo: Avanafil compresse – farmacia online senza ricetta
acquisto farmaci con ricetta: Farmacie che vendono Cialis senza ricetta – farmacie online affidabili
viagra naturale in farmacia senza ricetta viagra online spedizione gratuita or viagra generico sandoz
https://maps.google.com.my/url?q=https://viagragenerico.site viagra acquisto in contrassegno in italia
cialis farmacia senza ricetta viagra generico recensioni and viagra originale in 24 ore contrassegno viagra online in 2 giorni
Farmacia online miglior prezzo Farmacie online sicure or п»їFarmacia online migliore
https://cse.google.tg/url?sa=t&url=https://farmait.store farmacie online affidabili
top farmacia online Farmacie online sicure and acquistare farmaci senza ricetta Farmacia online miglior prezzo
comprare farmaci online con ricetta: kamagra gel prezzo – п»їFarmacia online migliore
farmacia online: Cialis generico farmacia – farmacie online autorizzate elenco
acquisto farmaci con ricetta: kamagra gold – Farmacie online sicure
https://viagragenerico.site/# alternativa al viagra senza ricetta in farmacia
Farmacia online miglior prezzo Cialis generico controindicazioni farmacie online autorizzate elenco
https://farmait.store/# Farmacie online sicure
acquistare farmaci senza ricetta: Cialis generico 5 mg prezzo – farmacie online affidabili
Farmacie on line spedizione gratuita top farmacia online or п»їFarmacia online migliore
https://www.feedroll.com/rssviewer/feed2js.php?src=https://kamagrait.pro farmaci senza ricetta elenco
farmacia online piГ№ conveniente comprare farmaci online con ricetta and farmacia online piГ№ conveniente acquisto farmaci con ricetta
п»їFarmacia online migliore: sildenafil oral jelly 100mg kamagra – farmacie online sicure
viagra acquisto in contrassegno in italia viagra online in 2 giorni or viagra naturale
https://www.ereality.ru/goto/viagragenerico.site cialis farmacia senza ricetta
miglior sito dove acquistare viagra viagra 50 mg prezzo in farmacia and viagra 50 mg prezzo in farmacia alternativa al viagra senza ricetta in farmacia
baclofen tablet
cheap clomid online
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
migliori farmacie online 2024 Farmacie on line spedizione gratuita or acquistare farmaci senza ricetta
http://image.google.com.np/url?q=https://farmait.store farmacie online sicure
comprare farmaci online con ricetta п»їFarmacia online migliore and Farmacie online sicure farmacie online affidabili
п»їFarmacia online migliore: kamagra oral jelly – Farmacie online sicure
farmacie online autorizzate elenco: Avanafil compresse – Farmacia online miglior prezzo
https://kamagrait.pro/# Farmacia online miglior prezzo
acquisto farmaci con ricetta Cialis generico recensioni farmacie online autorizzate elenco
viagra cost: Cheap Viagra online – viagra generic
http://tadalafil.auction/# europe cialis
safest and most reliable pharmacy to buy cialis cheapest tadalafil free cialis in canada
cialis on line new zealand: cheapest tadalafil – buy cialis with dapoxetine
https://sildenafil.llc/# viagra without prescription
order viagra online: buy sildenafil online usa – generic viagra
http://tadalafil.auction/# buy cialis south africa
viagra coupons Buy Viagra online cheap viagra side effects
You have observed very interesting details!
ps nice website.Raise your business
п»їover the counter viagra: Buy Viagra online cheap – viagra generic
https://tadalafil.auction/# costa rica cialis sale
us pharmacy cialis: Generic Tadalafil 20mg price – generis cialis
https://tadalafil.auction/# cialis viagra mail order uk discrete billing
find cheapest cialis pills Generic Tadalafil 20mg price cialis cheap online
viagra professional: Cheap Viagra 100mg – female viagra
http://tadalafil.auction/# canadian cialis for sale
viagra no prescription cialis cialis capsules canada or viagra no prescription cialis
https://clients1.google.sc/url?q=https://tadalafil.auction how can i get cheaper cialis
cialis without prescriptions uk cialis daily cheap and cialis on line new zealand buy viagra cialis mix online
viagra vs levitra vs cialis reviews montreal drug cialis or cialis without prescriptions canada
https://www.google.no/url?q=https://tadalafil.auction buy cialis 20mg
cialis brand name without prescription canadian meds cialis and generic cialis without prescription buy cialis and receive in 48 hrs
levitra or cialis which is better: Buy Tadalafil 20mg – brand name cialis
http://sildenafil.llc/# how does viagra work
cialis none prescription Generic Cialis without a doctor prescription cialis without prescription overnight
ed pills that work better than viagra: Cheap generic Viagra – generic viagra overnight
http://sildenafil.llc/# 100 mg viagra lowest price
generic cialis no prescription cheapest tadalafil lisinopril and cialis
http://tadalafil.auction/# cialis black buy in australia
over the counter alternative to viagra 100mg viagra without a doctor prescription or real viagra without a doctor prescription
https://maps.google.ae/url?q=https://sildenafil.llc viagra
female viagra buy viagra pills and order viagra online viagra cost
cialis canadian pharmacy: cialis shop in australia – order cialis in australia
how much does cialis cost in canada: cialis without a doctor prescription – how does cialis work
https://tadalafil.auction/# price of cialis at walgreens
viagra side effects cheap viagra or buy viagra
https://maps.google.co.th/url?sa=t&url=https://sildenafil.llc viagra price
viagra without a doctor prescription viagra without doctor prescription and viagra cost how does viagra work
viagra for sale: Viagra without a doctor prescription – viagra prices
http://sildenafil.llc/# buy viagra
viagra dosage buy sildenafil online canada buy viagra professional
buy ed meds online: cheap ed pills online – cheapest online ed meds
mexico pharmacies prescription drugs: Purple pharmacy online ordering – purple pharmacy mexico price list
http://indiapharmacy.shop/# india online pharmacy
reputable indian pharmacies Cheapest online pharmacy cheapest online pharmacy india
buying prescription drugs in mexico: Medicines Mexico – best online pharmacies in mexico
https://mexicopharmacy.win/# mexico pharmacies prescription drugs
https://indiapharmacy.shop/# world pharmacy india
best online ed treatment
india online pharmacy: Online medicine home delivery – world pharmacy india
https://indiapharmacy.shop/# cheapest online pharmacy india
cheap ed drugs
mexico drug stores pharmacies: mexico pharmacy win – mexican online pharmacies prescription drugs
https://mexicopharmacy.win/# mexican rx online
buy erectile dysfunction medication: Cheapest online ED treatment – ed prescriptions online
https://indiapharmacy.shop/# top online pharmacy india
erectile dysfunction medication online
Bitcoin is the key and most well-known cryptocurrency, created in 2008 close to an unidentified личность or group of people using the incognito Satoshi Nakamoto. As a decentralized digital currency, Bitcoin operates without a main establishment or single administrator. Transactions are verified by network nodes result of cryptography and recorded in a every tom distributed ledger called a blockchain. This ensures transparency and asylum, making it dark recompense any single object to exploit or control the network. Bitcoin’s rudimentary goal is to provide an alternate to old currencies, which are typically controlled at near primary banks and governments. By enabling peer-to-peer transactions without the need in behalf of intermediaries, Bitcoin aims to revolutionize the pecuniary system, offering greater monetary privilege and diminish transaction costs.
https://wayip.ru
https://okna-smolenskie.ru
https://wooddecora.ru
https://tualet-na-dachu.ru
https://typologies-for-life.ru
https://stocktricks.ru
https://transfer24.store
https://lj-media.ru
https://cryptochampion.pro
https://cryptocove.pro
cheap ed drugs: cheap ed pills online – order ed pills online
http://mexicopharmacy.win/# best online pharmacies in mexico
ed meds online ed pills online cheap ed
http://mexicopharmacy.win/# mexico drug stores pharmacies
Explore fashionable styles at Z SUPPLY Clothing.
online pharmacy india: Top online pharmacy in India – pharmacy website india
buying from online mexican pharmacy buying prescription drugs in mexico online or mexican mail order pharmacies
http://parents-teachers.com/lib/topframe2014.php?goto=https://mexicopharmacy.win pharmacies in mexico that ship to usa
mexico pharmacies prescription drugs mexican mail order pharmacies and mexican rx online п»їbest mexican online pharmacies
buy medicines online in india top online pharmacy india or world pharmacy india
http://www.110school.ru/bitrix/rk.php?goto=http://indiapharmacy.shop/ indian pharmacy
indian pharmacies safe indian pharmacies safe and top 10 pharmacies in india best india pharmacy
Thank you for sharing such an excellent post! Do you want to know how to use Scrcpy to mirror your Android device’s screen? If so, I recently discovered the Scrcpy site, which has wonderful information, such as the fact that if you want to mirror your Android smartphone to your PC, you must first download Scrcpy. To learn more about scrcpy, visit the given post link.
ed treatment online: cheap ed pills online – buy erectile dysfunction pills
https://edpillpharmacy.store/# ed medications cost
mexico drug stores pharmacies: Best online Mexican pharmacy – pharmacies in mexico that ship to usa
https://mexicopharmacy.win/# buying prescription drugs in mexico
low cost ed medication: Cheap ED pills online – online ed pills
https://indiapharmacy.shop/# pharmacy website india
indian pharmacies safe india pharmacy best online pharmacy india
This is very interesting, You are a very skilled blogger. I’ve joined your rss feed and look forward to seeking more of your wonderful post. Also, I have shared your site in my social networks! Thanks Seo firm India view https://finncxph32210.bloggosite.com
ed treatments online online erectile dysfunction or cheap erection pills
https://cse.google.mk/url?sa=t&url=https://edpillpharmacy.store ed pills for sale
erectile dysfunction medications online online ed medication and online erectile dysfunction medication cheapest ed pills
Bitcoin is the original and most celebrated cryptocurrency, created in 2008 close to an unidentified person or party of people using the stage name Satoshi Nakamoto. As a decentralized digital currency, Bitcoin operates without a principal sage or single administrator. Transactions are verified aside network nodes as a consequence cryptography and recorded in a public distributed ledger called a blockchain. This ensures transparency and security, making it difficult for any single object to utilize or conduct the network. Bitcoin’s rudimentary goal is to furnish an another to ritual currencies, which are typically controlled by central banks and governments. During enabling peer-to-peer transactions without the need in the service of intermediaries, Bitcoin aims to revolutionize the fiscal way, gift greater monetary freedom and lower records costs.
https://premium26.ru
https://orennozh.ru
https://fintechfiesta.info
https://cncmotors.ru
https://segodnya-ntv.ru
https://part-c.ru
https://soccer-apl.ru
https://lj-media.ru
https://lab314.ru
https://remont-telefonov-spb-78.ru
Very nice post. I just stumbled upon your blog and wished to say that I’ve truly enjoyed browsing your blog posts. After all I?ll be subscribing to your rss feed and I hope you write again very soon! read this https://dominickndth31098.blogolenta.com
pills for ed online get ed prescription online or online erectile dysfunction prescription
https://www.google.bs/url?q=https://edpillpharmacy.store ed medicines online
buy ed meds buy ed medication online and ed treatment online best online ed treatment
buying from online mexican pharmacy: Best pharmacy in Mexico – best online pharmacies in mexico
mexican mail order pharmacies: Best online Mexican pharmacy – mexico drug stores pharmacies
https://mexicopharmacy.win/# buying from online mexican pharmacy
Discover unique fashion pieces at citizens of humanity.
http://indiapharmacy.shop/# pharmacy website india
indian pharmacy paypal: Best Indian pharmacy – reputable indian pharmacies
indian pharmacies safe reputable indian online pharmacy or top 10 online pharmacy in india
http://tourzwei.radblogger.net/redirect.php?url=indiapharmacy.shop mail order pharmacy india
cheapest online pharmacy india best online pharmacy india and Online medicine order india pharmacy mail order
I’m not sure how I found myself here, but I found this post to be fantastic. I don’t know who you are, but you’re definitely on your way to becoming a well-known blogger, if you aren’t one already. Cheers!
online erectile dysfunction prescription: ed pills online – buy ed meds
http://mexicopharmacy.win/# pharmacies in mexico that ship to usa
online pharmacy india Top online pharmacy in India indianpharmacy com
best online pharmacy india: Top mail order pharmacies – п»їlegitimate online pharmacies india
where can i buy erectile dysfunction pills buy ed medication online or п»їed pills online
https://www.livecmc.com/?lang=fr&id=Ld9efT&url=https://edpillpharmacy.store:: erectile dysfunction medicine online
where can i get ed pills buy ed medication and cheap ed meds ed prescriptions online
get ed meds online: Best ED meds online – how to get ed pills
http://edpillpharmacy.store/# get ed meds online
Hi, i think that i saw you visited my web site thus i came to ?return the favor?.I am trying to find things to enhance my web site!I suppose its ok to use a few of your ideas!! Thanks from SEO India website https://write.as/2mkckke89l1hp
http://edpillpharmacy.store/# best ed meds online
buying from online mexican pharmacy: mexican pharmacy – buying from online mexican pharmacy
ed prescription online: online ed prescription same-day – low cost ed medication
http://edpillpharmacy.store/# generic ed meds online
Explore a wide range of styles at paige jeans outlet.
Discover the perfect blend of style and comfort at marmot clothing.
mexican pharmacy: mexico pharmacy win – mexico drug stores pharmacies
http://indiapharmacy.shop/# india pharmacy
india online pharmacy Cheapest online pharmacy india pharmacy
ed rx online: Cheap ED pills online – generic ed meds online
https://edpillpharmacy.store/# buying erectile dysfunction pills online
online ed medication online ed drugs or best online ed pills
https://member.findall.co.kr/stipulation/stipulation.asp?targetpage=http://m.findall.co.kr&basehost=edpillpharmacy.store erectile dysfunction medicine online
cheapest ed online online ed medicine and low cost ed meds online ed online treatment
Great post! Much appreciated. Are you searching about what exactly is mouse acceleration? So, based on the speed of mouse movement, mouse acceleration modifies cursor speed. To understand the impact of mouse acceleration, utilizing software like raw accel, visit the given website for better precision.
Bitcoin is the original and most eminent cryptocurrency, created in 2008 close to an unrevealed person or organization of people using the incognito Satoshi Nakamoto. As a decentralized digital currency, Bitcoin operates without a central authority or fix administrator. Transactions are verified before network nodes result of cryptography and recorded in a every tom distributed ledger called a blockchain. This ensures transparency and confidence, making it sensitive for the purpose any distinct object to utilize or restraint the network. Bitcoin’s predominant goal is to provide an another to old currencies, which are typically controlled around primary banks and governments. By enabling peer-to-peer transactions without the stress after intermediaries, Bitcoin aims to revolutionize the fiscal way, sacrifice greater financial openness and diminish transaction costs.
https://74paint.ru
https://vremyaitb.ru
https://rtdco.ru
https://volkhp.ru
https://typologies-for-life.ru
https://premium26.ru
https://etherhorizons.today
https://okna-smolenskie.ru
https://kosportal.ru
https://forstad.ru
https://edpillpharmacy.store/# online ed drugs
erection pills online: ED meds online with insurance – cheapest ed meds
buying from online mexican pharmacy: Best pharmacy in Mexico – mexico drug stores pharmacies
http://edpillpharmacy.store/# buy erectile dysfunction medication
buying erectile dysfunction pills online Best ED pills non prescription ed medications online
erectile dysfunction pills online online erectile dysfunction pills or boner pills online
https://forum.phun.org/proxy.php?link=https://edpillpharmacy.store pills for erectile dysfunction online
cheap ed ed treatments online and cheapest erectile dysfunction pills erectile dysfunction online
where to buy ed pills: low cost ed medication – what is the cheapest ed medication
Medication facts provided. Find drug details.
buy propecia medication
Medicine impacts explained. Medication trends described.
http://indiapharmacy.shop/# indian pharmacy online
Bitcoin is the key and most eminent cryptocurrency, created in 2008 not later than an unrevealed личность or assort of people using the pseudonym Satoshi Nakamoto. As a decentralized digital currency, Bitcoin operates without a principal authority or fix administrator. Transactions are verified before network nodes through cryptography and recorded in a buyers distributed ledger called a blockchain. This ensures transparency and asylum, making it difficult recompense any take entity to exploit or conduct the network. Bitcoin’s rudimentary aspiration is to furnish an variant to old currencies, which are typically controlled at near important banks and governments. During enabling peer-to-peer transactions without the stress for intermediaries, Bitcoin aims to revolutionize the economic scheme, sacrifice greater economic deliverance and lower proceeding costs.
https://stocktricks.ru
https://remitazoll.ru
https://forstad.ru
https://segodnya-ntv.ru
https://lovechannel.ru
https://magenta-online.ru
https://prosto-futbolka.ru
https://advokat-maiorov40.ru
https://kurs-inyaz.ru
https://beauty-lashproducts.ru
buy medicines online in india: Indian pharmacy international shipping – buy prescription drugs from india
best online ed treatment: Best ED meds online – ed prescriptions online
https://mexicopharmacy.win/# pharmacies in mexico that ship to usa
top 10 online pharmacy in india Best Indian pharmacy india pharmacy
Get medication details. Pill essentials explained.
purchase propecia
Patient pill resource. Current drug trends.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
https://edpillpharmacy.store/# ed medications online
buying erectile dysfunction pills online: ed pills online – buy ed meds online
world pharmacy india: buy medicines online in india – mail order pharmacy india
pharmacies in mexico that ship to usa: Best pharmacy in Mexico – medicine in mexico pharmacies
https://mexicopharmacy.win/# purple pharmacy mexico price list
buying prescription drugs in mexico online mexican pharmacy mexican mail order pharmacies
cheap ed meds: ed pills online – ed pills
ed medications online get ed meds online or buy erectile dysfunction treatment
http://cse.google.ba/url?q=https://edpillpharmacy.store ed med online
cheap boner pills cheap boner pills and ed pills pills for erectile dysfunction online
indianpharmacy com: Top mail order pharmacies – online shopping pharmacy india
reputable mexican pharmacies online: mexican pharmacy – mexican mail order pharmacies
https://indiapharmacy.shop/# world pharmacy india
online ed treatments Cheapest online ED treatment online erectile dysfunction medication
mail order pharmacy india: reputable indian online pharmacy – indian pharmacy
buy ed meds ed medicine online or discount ed pills
https://images.google.com.do/url?sa=t&url=https://edpillpharmacy.store erectile dysfunction drugs online
erectile dysfunction medicine online ed drugs online and buying ed pills online erectile dysfunction medications online
Access medicine facts. Complete pill overview.
buy roche accutane online uk
Medicine facts here. Comprehensive drug guide.
cheapest online ed meds: online ed prescription same-day – buying erectile dysfunction pills online
Discover unique styles at isabel marant outlet.
Medicine essentials explained. Patient medication resource.
buy accutane cheap online
Pill impacts described. Medication reactions explained.
lasix online: furosemide online – buy lasix online
lasix uses: cheap lasix – lasix 40 mg
https://furosemide.win/# lasix pills
Cytotec 200mcg price cheapest cytotec order cytotec online
buy cytotec over the counter https://lipitor.guru/# lipitor 20
lasix 40 mg
does tamoxifen cause joint pain: buy tamoxifen online – where to get nolvadex
https://tamoxifen.bid/# tamoxifen cyp2d6
buy cytotec over the counter https://cytotec.pro/# Misoprostol 200 mg buy online
lasix 40mg
furosemide 100 mg: buy furosemide – lasix 100mg
cost of lipitor in australia Atorvastatin 20 mg buy online how much is lipitor
lasix side effects: buy furosemide – lasix online
https://cytotec.pro/# buy cytotec
Abortion pills online Misoprostol price in pharmacy Abortion pills online
http://furosemide.win/# lasix side effects
Cytotec 200mcg price https://furosemide.win/# buy furosemide online
lasix
Latest drug news. Patient drug info.
motrin 200mg
Prescribing details available. Pill leaflet available.
tamoxifen brand name: buy tamoxifen online – does tamoxifen cause joint pain
lisinopril medicine lisinopril 10 mg tablet price or lisinopril cheap price
https://www.webkinz.com/bumper.php?clicktag=https://lisinopril.guru lisinopril 5 mg india price
lisinopril cost canada drug lisinopril and lisinopril 40 mg canada lisinopril 50 mg tablet
lipitor generic over the counter lipitor india generic or lipitor cost
http://www.taskmanagementsoft.com/bitrix/rk.php?id=17&site_id=s1&event1=banner&event2=click&goto=https://lipitor.guru:: lipitor tablets
[url=https://cse.google.bi/url?sa=i&url=https://lipitor.guru]lipitor 40 mg price australia[/url] buy lipitor online australia and [url=http://czn.com.cn/space-uid-114264.html]lipitor online[/url] lipitor tablets
lasix 40 mg: buy furosemide – lasix tablet
http://cytotec.pro/# buy cytotec
buy lisinopril online uk buy lisinopril lisinopril 10 mg tabs
https://furosemide.win/# lasix furosemide 40 mg
buy furosemide online buy furosemide furosemida
lasix tablet: cheap lasix – furosemida
lipitor 80 mg price in india: Lipitor 10 mg price – lipitor lowest price
https://tamoxifen.bid/# tamoxifen joint pain
zestril generic zestril 5mg price in india or zestril discount
https://www.google.sr/url?q=https://lisinopril.guru cost of lisinopril 40 mg
how to buy lisinopril online cost for 20 mg lisinopril and lisinopril 25 generic lisinopril online
cytotec buy online usa https://cytotec.pro/# buy cytotec online
buy lasix online
cytotec pills buy online: cytotec best price – cytotec abortion pill
http://lisinopril.guru/# order cheap lisinopril
lipitor australia buy atorvastatin online cost of lipitor 20 mg
срочный ремонт iphone в москве
tamoxifen endometriosis: buy tamoxifen online – tamoxifen for sale
Abortion pills online cytotec buy online usa or Cytotec 200mcg price
https://images.google.dj/url?sa=t&url=https://cytotec.pro п»їcytotec pills online
cytotec online order cytotec online and purchase cytotec Cytotec 200mcg price
lipitor 40 mg price india generic lipitor 10mg or lipitor generic price comparison
https://images.google.by/url?sa=t&url=https://lipitor.guru lowest price lipitor
prescription medication lipitor best price for lipitor and lipitor 10 mg tablet lipitor cost
aromatase inhibitors tamoxifen: buy tamoxifen citrate – tamoxifen therapy
https://lipitor.guru/# lipitor generic canada
cytotec buy online usa https://tamoxifen.bid/# nolvadex for sale
lasix furosemide
Find unique pieces at namilia outlet.
zestoretic 20 12.5: Buy Lisinopril 20 mg online – can you order lisinopril online
https://lipitor.guru/# lipitor 10 mg tablet price
tamoxifen bone pain hysterectomy after breast cancer tamoxifen tamoxifen alternatives
Medicine guide here. Medication trends described.
proscar
Get drug facts. Drug guide available.
lasix 40 mg: buy furosemide – lasix 100 mg
lisinopril price in canada lisinopril 15mg or lisinopril 10 mg canada cost
http://cse.google.com.eg/url?sa=t&url=https://lisinopril.guru lisinopril tabs 40mg
zestoretic tabs lisinopril brand name and buy lisinopril 20 mg online lisinopril tablets for sale
Find medication details. Medicine guide here.
buy proscar usa
Abuse effects detailed. Medication guide available.
п»їcytotec pills online cytotec pills buy online or purchase cytotec
https://gemini.yagami.me/forum/away.php?s=http://cytotec.pro order cytotec online
п»їcytotec pills online buy cytotec online and buy cytotec buy cytotec in usa
http://tamoxifen.bid/# nolvadex estrogen blocker
ordering lisinopril without a prescription: Lisinopril online prescription – lisinopril 10 mg without prescription
purchase cytotec http://furosemide.win/# lasix online
furosemide 100mg
Hi there! I must say, this blog post really caught my attention. Your writing style is so captivating, and the way you presented the information made it easy to understand and enjoyable to read. Thank you for sharing your expertise on this topic. I’m eager to see what you write about next.
Comprehensive medication overview. Patient drug leaflet.
buy proscar with no prescription
Comprehensive drug overview. Get drug details.
buy lipitor online australia where to buy lipitor or cost of lipitor 10 mg
https://www.google.com.np/url?q=https://lipitor.guru cheap lipitor online
lipitor prescription can i buy lipitor online and lipitor generic on line no prescription buy lipitor 40 mg
lisinopril 2.5 mg buy online: Lisinopril refill online – lisinopril tabs
tamoxifen vs clomid: how to lose weight on tamoxifen – tamoxifen and ovarian cancer
https://furosemide.win/# furosemida 40 mg
lipitor 20mg price cheapest ace inhibitor lipitor price drop
Comprehensive pill overview. Latest medicine developments.
proscar 5mg
Drug essentials explained. Medication details here.
Pill essentials explained. Drug guide here.
ivermectin cost in usa
Medication facts provided. Patient drug info.
buy cytotec п»їcytotec pills online or buy cytotec
http://mnforen.net/cranberra/redirect.php?url=http://cytotec.pro buy cytotec over the counter
Misoprostol 200 mg buy online cytotec buy online usa and Cytotec 200mcg price cytotec pills buy online
Recent medicine developments. Administration guidelines here.
ivermectin 6 mg tablets
Access pill information. Current medicine trends.
Hey! I stumbled upon your blog and this post really stood out to me. The way you explained the topic was clear and concise, making it accessible to readers of all backgrounds. Thank you for breaking it down in such an understandable way. Can’t wait to read more from you.
tamoxifen and ovarian cancer: buy tamoxifen citrate – tamoxifen hip pain
order cytotec online http://furosemide.win/# lasix 100 mg tablet
lasix
prinivil drug cost lisinopril generic price in india or where can i buy zestril
https://images.google.kg/url?sa=t&url=https://lisinopril.guru 10 mg lisinopril cost
lisinopril hctz cost of lisinopril in mexico and lisinopril 2.5 cost how much is lisinopril 5 mg
Comprehensive medicine facts. Patient medicine guide.
ivermectin 6 mg tablets
Comprehensive medication resource. Medicine guide here.
Pill info here. Find drug information.
stromectol tablets buy online
Latest pill developments. Access medicine facts.
tamoxifen vs raloxifene: buy tamoxifen citrate – alternative to tamoxifen
Get details now. Pill facts provided.
ivermectin 3mg for lice
Drug details provided. Medicine impacts explained.
Generic names listed. Get pill facts.
stromectol order online
Access medication details. Pill leaflet available.
lasix 20 mg: lasix online – lasix
http://tamoxifen.bid/# tamoxifen dose
common side effects of tamoxifen buy tamoxifen citrate how to lose weight on tamoxifen
buy cytotec in usa buy cytotec pills or buy cytotec in usa
https://images.google.ad/url?sa=t&url=https://cytotec.pro п»їcytotec pills online
buy cytotec pills cytotec buy online usa and Cytotec 200mcg price buy cytotec
Read about pills. Find drug information.
[url=https://stromectol365x.top/#]where can i buy stromectol[/url]
Pill information here. Pill effects listed.
cytotec buy online usa https://furosemide.win/# lasix generic
lasix online
lisinopril 10 mg coupon: cheap lisinopril – lisinopril 20mg tablets price
Hi there! Just wanted to let you know how much I enjoyed reading this post. Your approach to the subject was unique and informative. It’s clear that you put a lot of effort into your writing. Keep up the great work, and I can’t wait to see what else you have in store.
Medicine essentials explained. Pill impacts explained.
buy ivermectin for humans australia
Comprehensive medicine guide. Medication overview available.
lipitor pfizer where to buy lipitor or lipitor 10mg price in india
https://images.google.gg/url?q=https://lipitor.guru lipitor sales
lipitor tablets lipitor cost in canada and lipitor 20mg price buy lipitor online usa
Comprehensive medication overview. Latest medication developments.
cost of ivermectin pill
Find pill facts. Drug trends described.
Side effects explained. Access pill facts.
stromectol 12mg online
Medication trends described. Find medication info.
lisinopril 5 mg medicine buy lisinopril without a prescription or lisinopril 60 mg tablet
https://cse.google.hn/url?sa=t&url=https://lisinopril.guru can you buy lisinopril over the counter
lisinopril online canada prinivil 25mg and generic lisinopril 10 mg lisinopril 2.5 pill
generic lasix: furosemide online – lasix medication
Latest medication news. Pill leaflet available.
buy strattera pills
Patient medication resource. Access medicine details.
Abortion pills online https://cytotec.pro/# buy cytotec
furosemide 100mg
cytotec abortion pill Misoprostol 200 mg buy online or buy cytotec over the counter
https://www.google.co.za/url?q=https://cytotec.pro п»їcytotec pills online
п»їcytotec pills online buy cytotec and buy cytotec purchase cytotec
buy cytotec pills online cheap: cytotec best price – buy cytotec
https://cytotec.pro/# buy cytotec pills online cheap
tamoxifen men tamoxifen buy cost of tamoxifen
п»їdcis tamoxifen: cost of tamoxifen – femara vs tamoxifen
Drug leaflet here. Medication information here.
strattera online
Drug pamphlet provided. Pill info here.
Drug guide here. Access medicine facts.
buy atomoxetine pills
Medicine facts available. Access drug details.
Pill info here. Medication data provided.
generic atomoxetine
Active ingredients listed. Read about drugs.
Drug essentials explained. Pill information here.
buy strattera pills
Medicine impacts explained. Medicine information provided.
сервис ремонт телефонов
Latest drug news. Access drug data.
buy real propecia
Current medication trends. Pill effects explained.
ремонт телефонов москва
Drug effects explained. Get pill facts.
where can you buy propecia
Latest medication news. Comprehensive pill overview.
Comprehensive drug guide. Find pill info.
cheapest pharmacy for propecia
Medication leaflet provided. Patient medication leaflet.
indian pharmacy online: world pharmacy india – top online pharmacy india
indian pharmacy online: india pharmacy – reputable indian online pharmacy
Comprehensive pill guide. Recent medicine developments.
buy sildenafil
Latest medicine developments. Find pill facts.
mexico drug stores pharmacies mexican mail order pharmacies mexican pharmaceuticals online
https://mexstarpharma.online/# best online pharmacies in mexico
mexican pharmaceuticals online mexican pharmaceuticals online mexican rx online
https://easyrxcanada.com/# canadapharmacyonline legit
purple pharmacy mexico price list mexican mail order pharmacies or buying prescription drugs in mexico online
https://maps.google.la/url?rct=j&sa=t&url=https://mexstarpharma.com buying prescription drugs in mexico online
mexico pharmacies prescription drugs mexican mail order pharmacies and mexican drugstore online mexican border pharmacies shipping to usa
reputable indian online pharmacy: pharmacy website india – indianpharmacy com
canada discount pharmacy the canadian pharmacy or legitimate canadian pharmacy
http://electronix.ru/redirect.php?http://easyrxcanada.com canadian pharmacy com
canada drugs online canada drugs online and canadian pharmacy india canadian pharmacy near me
canadianpharmacyworld: real canadian pharmacy – canadapharmacyonline com
http://easyrxcanada.com/# canadian pharmacies comparison
best online pharmacies in mexico medication from mexico pharmacy mexican pharmaceuticals online
https://easyrxindia.shop/# indianpharmacy com
canadian pharmacy meds review: canadian pharmacy near me – canadian world pharmacy
ремонт телевизоров в москве недорого
mexico drug stores pharmacies mexican rx online or mexican drugstore online
https://www.kaskus.co.id/redirect?url=https://mexstarpharma.com buying prescription drugs in mexico online
mexican pharmaceuticals online mexican drugstore online and mexican mail order pharmacies purple pharmacy mexico price list
http://easyrxindia.com/# reputable indian pharmacies
Профессиональный сервисный центр по ремонту сотовых телефонов, смартфонов и мобильных устройств.
Мы предлагаем: мастерская телефонов рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mexican pharmacy: pharmacies in mexico that ship to usa – mexican mail order pharmacies
Профессиональный сервисный центр по ремонту ноутбуков, макбуков и другой компьютерной техники.
Мы предлагаем:ремонт apple macbook
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Find high-quality fashion at lucky brand store.
http://mexstarpharma.com/# mexico pharmacies prescription drugs
india online pharmacy india pharmacy mail order or india online pharmacy
http://www.aima-g.co.jp/feed2js/feed2js.php?src=https://easyrxindia.com best online pharmacy india
pharmacy website india indian pharmacies safe and india pharmacy mail order indian pharmacy online
Профессиональный сервисный центр по ремонту сотовых телефонов, смартфонов и мобильных устройств.
Мы предлагаем: где можно починить телефон
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Medication data provided. Patient drug info.
avodart 0.5 mg capsules
Medicine effects explained. Medication trends described.
buying prescription drugs in mexico online pharmacies in mexico that ship to usa or mexican mail order pharmacies
http://toolbarqueries.google.as/url?sa=i&url=https://mexstarpharma.com mexico drug stores pharmacies
pharmacies in mexico that ship to usa mexico drug stores pharmacies and pharmacies in mexico that ship to usa mexican border pharmacies shipping to usa
online shopping pharmacy india: top 10 pharmacies in india – Online medicine order
Find drug information. Side effects explained.
avodart medication
Get pill details. Get medication details.
Whether you’re looking to meet new friends or just kill some time, https://www.omegle.com is the ideal platform.
Medication information here. Patient medicine resource.
Avodart uk
Access drug facts. Get info now.
Fantastic post! Are you Eager to keep in touch with vacation friends after your trip? During the trip, share phone numbers, email addresses, or social media handles. Form a group chat or social media group to stay in contact. Utilize a zip code tool to find your vacation friends. For further details, check the article on Stay Connected Vacation Friends.
canada pharmacy reviews canadian online pharmacy or escrow pharmacy canada
http://nsreg.com/?a=how+can+i+buy+viagra legit canadian pharmacy
online canadian drugstore canadian drugstore online and canadian drug prices canadian drugs pharmacy
Detailed medication knowledge. Medicine guide here.
cialis and avodart
Patient medicine resource. Find medicine information.
purple pharmacy mexico price list: buying prescription drugs in mexico – mexican online pharmacies prescription drugs
https://mexstarpharma.online/# mexican drugstore online
Профессиональный сервисный центр по ремонту квадрокоптеров и радиоуправляемых дронов.
Мы предлагаем:ремонт дронов в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту сотовых телефонов, смартфонов и мобильных устройств.
Мы предлагаем: ремонт телефонов москва рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://slotsiteleri.bid/# slot bahis siteleri
Find medicine info. Patient medication facts.
dutasteride avodart
Access medication details. Current drug trends.
en iyi slot siteleri: en iyi slot siteler – yasal slot siteleri
slot oyunlar? siteleri: slot siteleri 2024 – en guvenilir slot siteleri
http://denemebonusuverensiteler.win/# bahis siteleri
Patient drug facts. Access drug details.
avodart
Drug trends described. Recent drug developments.
deneme bonusu veren siteler: bonus veren siteler – bahis siteleri
Latest pill updates. Medicine guide available.
buy lasix no prescription
Medicine facts available. Comprehensive medication resource.
sweet bonanza taktik sweet bonanza slot demo or sweet bonanza oyna
http://feed2js.org/feed2js.php?src=http://sweetbonanza.network sweet bonanza yasal site
sweet bonanza mostbet sweet bonanza demo oyna and sweet bonanza indir sweet bonanza yorumlar
slot casino siteleri en guvenilir slot siteleri or en iyi slot siteleri 2024
https://opelforum.ru/redirect/?url=http://slotsiteleri.bid/ bonus veren slot siteleri
guvenilir slot siteleri deneme veren slot siteleri and deneme bonusu veren siteler canl? slot siteleri
yasal slot siteleri: slot siteleri bonus veren – en guvenilir slot siteleri
bonus veren siteler: deneme bonusu veren siteler – deneme bonusu veren siteler
https://sweetbonanza.network/# sweet bonanza yorumlar
sweet bonanza guncel: sweet bonanza indir – sweet bonanza 100 tl
https://denemebonusuverensiteler.win/# bahis siteleri
en iyi slot siteler en iyi slot siteleri 2024 deneme bonusu veren siteler
http://sweetbonanza.network/# sweet bonanza yorumlar
bahis siteleri bonus veren siteler or deneme bonusu veren siteler
https://maps.google.com.mx/url?sa=t&url=https://denemebonusuverensiteler.win deneme bonusu veren siteler
bahis siteleri deneme bonusu and bahis siteleri bahis siteleri
Detailed drug knowledge. Access drug details.
buy lasix no prescription
Patient drug information. Access medicine information.
Pill essentials explained. Read about drugs.
buy lasix pills online
Comprehensive medication guide. Drug leaflet here.
Medicine impacts explained. Get pill facts.
order lasix
Latest drug developments. Patient pill guide.
Профессиональный сервисный центр по ремонту ноутбуков, imac и другой компьютерной техники.
Мы предлагаем:срочный ремонт imac
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Latest pill updates. Drug guide here.
online pharmacy viagra
Medicine leaflet here. Drug information available.
Latest drug developments. Medicine facts here.
mexican online pharmacies
Medication impacts explained. Get drug facts.
Get info immediately. Get medication facts.
best online pharmacies
Detailed pill knowledge. Get pill facts.
Get drug details. Latest medication updates.
costco online pharmacy
Brand names listed. Current drug trends.
Профессиональный сервисный центр по ремонту ноутбуков и компьютеров.дронов.
Мы предлагаем:ремонт ноутбуков в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Latest medicine news. Medicine guide here.
costco online pharmacy
Access drug data. Complete medication overview.
Side effects listed. Comprehensive pill guide.
xanax online pharmacy
Find medicine information. Find medication info.
Drug facts here. Overdose effects detailed.
best online pharmacies
Drug resource available. Drug trends described.
Explore discounted Marc Jacobs items at the marc jacobs outlet.
Get pill info. Comprehensive drug resource.
buy propecia usa
Medication impacts described. Pill overview available.
Find medicine information. Medication guide available.
order finasteride
Medicine facts provided. Patient medicine resource.
Pill info available. Comprehensive medicine overview.
buy propecia pills
Medicine impacts explained. Drug info here.
Pill guide available. Get info now.
order finasteride
Access medication details. Pill effects listed.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article. https://www.binance.com/lv/register?ref=B4EPR6J0
ремонт айфона в москве недорого рядом
Patient pill guide. Current drug trends.
order finasteride
Drug details provided. Access pill details.
Comprehensive drug resource. Latest medication news.
purchase propecia
Patient drug facts. Medication impacts explained.
Comprehensive medication resource. Medication impacts explained.
buy propecia uk
Access medication details. Medicine impacts explained.
For each case, one age matched control 5 years was chosen from patients mean age 49 dapoxetina generico Among the 3, 125 women in theanastrozole group, 318 had a relapse of their breast cancer or died, comparedwith 379 of 3, 116 women in the tamoxifen group hazard ratio 0
Medicine guide available. Pill details provided.
cialis_tadacip_wirkung
Find medicine details. Comprehensive medication resource.
Patient medication resource. Current drug information.
tadacip india
Pill effects explained. Find medicine info.
Medicine effects explained. Comprehensive medication resource.
tadalafil tadacip
Medicine effects explained. Comprehensive medicine guide.
deneme bonusu veren siteler: en cok kazandiran slot siteleri – en iyi slot siteleri
http://sweetbonanza.network/# sweet bonanza free spin demo
slot oyunlar? siteleri slot oyun siteleri guvenilir slot siteleri
Drug facts here. Prescribing details available.
tadacip 20 canada
Side effects explained. Detailed medication knowledge.
Познакомьтесь с миром фильмов высокого качества онлайн – непревзойденный онлайн кинопортал. Смотреть кинолентами в сети верное решение в 2024 году. Кинокартины онлайн великолепном качестве http://bit.ly/kinokrad-2021-kinokrad-kino-krad
sweet bonanza yasal site sweet bonanza giris or sweet bonanza oyna
https://cse.google.ws/url?sa=t&url=https://sweetbonanza.network sweet bonanza yorumlar
sweet bonanza slot demo guncel sweet bonanza and sweet bonanza yasal site sweet bonanza giris
slot siteleri: deneme veren slot siteleri – en iyi slot siteleri 2024
https://sweetbonanza.network/# sweet bonanza kazanma saatleri
Patient drug information. Access pill details.
tadalafil vs tadacip
Patient medication leaflet. Patient medicine guide.
guvenilir slot siteleri: bonus veren slot siteleri – slot siteleri 2024
deneme bonusu veren siteler: bonus veren siteler – bahis siteleri
https://slotsiteleri.bid/# guvenilir slot siteleri
deneme bonusu veren slot siteleri slot oyun siteleri en iyi slot siteleri
Don’t miss the best Marc Jacobs deals at the marc jacobs outlet.
guvenilir slot siteleri: bonus veren casino slot siteleri – bonus veren casino slot siteleri
https://slotsiteleri.bid/# slot bahis siteleri
Medicine facts here. Latest pill trends.
purchase kamagra online no prescription
Get medication facts. Pill impacts explained.
2024 en iyi slot siteleri: slot siteleri – yasal slot siteleri
https://slotsiteleri.bid/# slot siteleri guvenilir
canl? slot siteleri yasal slot siteleri canl? slot siteleri
Pill essentials explained. Find medication information.
buy kamagra
Find medicine info. Access medicine information.
casino slot siteleri: yeni slot siteleri – en guvenilir slot siteleri
sweet bonanza oyna sweet bonanza nas?l oynan?r or sweet bonanza slot demo
http://be-tabelle.net/url?q=https://sweetbonanza.network sweet bonanza bahis
sweet bonanza hilesi sweet bonanza slot demo and sweet bonanza demo oyna sweet bonanza guncel
Get drug facts. Find pill information.
[url=https://kamagraxl.top/#]kamagra 100 mg[/url]
Get pill facts. Find drug information.
sweet bonanza yorumlar: sweet bonanza slot – slot oyunlari
http://slotsiteleri.bid/# en guvenilir slot siteleri
Medicine facts provided. Comprehensive medication resource.
buy kamagra with no prescription
Patient pill resource. Get drug facts.
slot bahis siteleri: 2024 en iyi slot siteleri – en yeni slot siteleri
http://denemebonusuverensiteler.win/# deneme bonusu veren siteler
sweet bonanza mostbet sweet bonanza nas?l oynan?r sweet bonanza 100 tl
pragmatic play sweet bonanza sweet bonanza mostbet or sweet bonanza 90 tl
https://cse.google.co.ck/url?sa=t&url=http://sweetbonanza.network sweet bonanza indir
sweet bonanza taktik sweet bonanza slot demo and sweet bonanza slot demo sweet bonanza free spin demo
slot siteleri: slot siteleri guvenilir – slot oyun siteleri
https://sweetbonanza.network/# sweet bonanza kazanma saatleri
bonus veren siteler deneme bonusu or bahis siteleri
http://domzy.com/denemebonusuverensiteler.win bahis siteleri
deneme bonusu bonus veren siteler and bahis siteleri bonus veren siteler
bonus veren slot siteleri: guvenilir slot siteleri – deneme veren slot siteleri
slot oyunlari: sweet bonanza bahis – sweet bonanza kazanma saatleri
http://denemebonusuverensiteler.win/# bahis siteleri
guncel sweet bonanza guncel sweet bonanza guncel sweet bonanza
Explore the best discounts on Marc Jacobs products at the marc jacobs outlet.
ремонт apple watch
Профессиональный сервисный центр по ремонту холодильников и морозильных камер.
Мы предлагаем: ремонт холодильников в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Наша мастерская предлагает надежный выездной ремонт фотоаппарата различных марок и моделей. Мы знаем, насколько важны для вас ваши фотоаппараты, и обеспечиваем ремонт первоклассного уровня. Наши квалифицированные специалисты работают быстро и аккуратно, используя только оригинальные запчасти, что обеспечивает долговечность и надежность выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются владельцы фотоаппаратов, включают повреждения линз, проблемы с затвором, поврежденный дисплей, неисправности батареи и программные сбои. Для устранения этих поломок наши опытные мастера выполняют ремонт объективов, затворов, экранов, батарей и ПО. Обратившись к нам, вы гарантируете себе надежный и долговечный официальный ремонт фотоаппарата.
Подробная информация доступна на сайте: https://remont-fotoapparatov-ink.ru
Medicine overview available. Side effects explained.
buy kamagra no rx
Medicine brochure provided. Read about medications.
slot siteleri bonus veren: en guvenilir slot siteleri – deneme bonusu veren siteler
http://denemebonusuverensiteler.win/# deneme bonusu
sweet bonanza demo oyna sweet bonanza kazanc or sweet bonanza demo
https://maps.google.as/url?q=https://sweetbonanza.network pragmatic play sweet bonanza
=sildenafil+coupons</a>]sweet bonanza slot demo sweet bonanza mostbet and sweet bonanza demo oyna sweet bonanza kazanma saatleri
2024 en iyi slot siteleri: canl? slot siteleri – slot kumar siteleri
https://denemebonusuverensiteler.win/# bonus veren siteler
sweet bonanza taktik sweet bonanza bahis sweet bonanza taktik
https://denemebonusuverensiteler.win/# deneme bonusu
deneme veren slot siteleri: slot oyunlar? siteleri – deneme bonusu veren siteler
Профессиональный сервисный центр по ремонту ноутбуков и компьютеров.дронов.
Мы предлагаем:ремонт ноутбуков москва центр
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:ремонт бытовой техники в спб
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту холодильников и морозильных камер.
Мы предлагаем: мастерска по ремонту холодильников
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Наш сервисный центр предлагает профессиональный центр ремонта фотоаппарата на выезде различных марок и моделей. Мы понимаем, насколько значимы для вас ваши фотоаппараты, и готовы предложить сервис первоклассного уровня. Наши опытные мастера оперативно и тщательно выполняют работу, используя только качественные детали, что обеспечивает длительную работу выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются пользователи камер, включают повреждения линз, проблемы с затвором, поврежденный дисплей, проблемы с питанием и ошибки ПО. Для устранения этих проблем наши квалифицированные специалисты проводят ремонт объективов, затворов, экранов, батарей и ПО. Доверив ремонт нам, вы гарантируете себе качественный и надежный срочный ремонт фотоаппарата.
Подробная информация доступна на сайте: https://remont-fotoapparatov-ink.ru
sweet bonanza 90 tl: sweet bonanza giris – sweet bonanza slot demo
http://sweetbonanza.network/# sweet bonanza 90 tl
en iyi slot siteleri: slot siteleri – slot siteleri 2024
http://sweetbonanza.network/# sweet bonanza 90 tl
bonus veren siteler bahis siteleri bonus veren siteler
Профессиональный сервисный центр по ремонту планетов в том числе Apple iPad.
Мы предлагаем: ipad ремонт
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
bahis siteleri bonus veren siteler or deneme bonusu veren siteler
http://www.immanuelyuma.org/System/Login.asp?id=54359&Referer=http://denemebonusuverensiteler.win deneme bonusu veren siteler
deneme bonusu deneme bonusu veren siteler and deneme bonusu veren siteler bahis siteleri
sapporo88 login
Профессиональный сервисный центр по ремонту радиоуправляемых устройства – квадрокоптеры, дроны, беспилостники в том числе Apple iPad.
Мы предлагаем: сервис квадрокоптеров
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники в москве
sweet bonanza demo: sweet bonanza demo – sweet bonanza demo
https://denemebonusuverensiteler.win/# bahis siteleri
deneme bonusu veren siteler deneme bonusu veren siteler bonus veren siteler
sweet bonanza slot demo sweet bonanza taktik or sweet bonanza 100 tl
https://www.scga.org/Account/AccessDenied.aspx?URL=https://sweetbonanza.network sweet bonanza slot demo
sweet bonanza indir sweet bonanza bahis and sweet bonanza slot sweet bonanza bahis
deneme veren slot siteleri: slot oyun siteleri – bonus veren casino slot siteleri
sweet bonanza slot sweet bonanza kazanma saatleri or sweet bonanza 100 tl
https://www.google.com.om/url?q=https://sweetbonanza.network sweet bonanza bahis
sweet bonanza demo sweet bonanza free spin demo and sweet bonanza nas?l oynan?r sweet bonanza taktik
Если вы искали где отремонтировать сломаную технику, обратите внимание – профи тех сервис екб
https://sweetbonanza.network/# sweet bonanza kazanma saatleri
1вин официальный сайт: 1win – 1win вход
https://vavada.auction/# vavada
пин ап пин ап казино вход пин ап
пин ап казино pin up pin up
Профессиональный сервисный центр по ремонту планетов в том числе Apple iPad.
Мы предлагаем: ремонт планшетов айпад
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Семейные расстановки Берта Хеллингера. https://rasstanovkiural.ru
пин ап зеркало: пин ап вход – pin up casino
1xbet зеркало рабочее на сегодня: 1xbet зеркало рабочее на сегодня – 1xbet официальный сайт мобильная версия
Если вы искали где отремонтировать сломаную технику, обратите внимание – техпрофи
Профессиональный сервисный центр по ремонту ноутбуков и компьютеров.дронов.
Мы предлагаем:ремонт ноутбуков в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:сервис центры бытовой техники петербург
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Если вы искали где отремонтировать сломаную технику, обратите внимание – выездной ремонт бытовой техники в екатеринбурге
http://1win.directory/# 1win официальный сайт
пинап казино: пинап казино – пин ап вход
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники в москве
пинап казино пин ап казино or пин ап зеркало
http://alt1.toolbarqueries.google.ee/url?q=https://pin-up.diy pin up казино
пинап казино pin up and pin up казино pin up
казино вавада vavada casino or вавада казино
https://www.kirschenmarkt-gladenbach.de/go.php?go=https://vavada.auction вавада
vavada online casino vavada зеркало and vavada зеркало вавада зеркало
1win: 1вин зеркало – ван вин
http://vavada.auction/# вавада рабочее зеркало
пин ап зеркало: pin up казино – пин ап казино вход
1вин сайт 1вин or ван вин
https://maps.google.ad/url?sa=t&url=https://1win.directory 1win вход
1вин сайт 1вин официальный сайт and 1win вход 1вин сайт
Если вы искали где отремонтировать сломаную технику, обратите внимание – тех профи
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт айфона с выездом мастера в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
срочный ремонт сотовых телефонов
Профессиональный сервисный центр по ремонту источников бесперебойного питания.
Мы предлагаем: ремонт ибп цена
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
ремонт телевизоров в москве недорого
Наши специалисты предлагает высококачественный ремонт стиральной машины в москве любых брендов и моделей. Мы знаем, насколько необходимы вам ваши автоматические стиральные машины, и готовы предложить сервис наилучшего качества. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что обеспечивает длительную работу проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются обладатели устройств для стирки, включают неисправности барабана, неработающий нагревательный элемент, программные сбои, неработающий насос и поломки компонентов. Для устранения этих неисправностей наши профессиональные техники оказывают ремонт барабанов, нагревательных элементов, ПО, насосов и механических компонентов. Обратившись к нам, вы гарантируете себе качественный и надежный официальный ремонт стиральной машины.
Подробная информация представлена на нашем сайте: https://remont-stiralnyh-mashin-ace.ru
вавада казино: vavada – vavada зеркало
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
http://vavada.auction/# vavada casino
pin up казино: пин ап казино вход – пин ап казино
казино вавада вавада рабочее зеркало or vavada
https://maps.google.gl/url?sa=t&url=https://vavada.auction vavada
vavada casino казино вавада and вавада рабочее зеркало вавада рабочее зеркало
Если вы искали где отремонтировать сломаную технику, обратите внимание – сервис центр в новосибирске
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: качественный ремонт айфонов в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту источников бесперебойного питания.
Мы предлагаем: ремонт бесперебойников в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Если вы искали где отремонтировать сломаную технику, обратите внимание – тех профи
1вин 1вин or 1win
http://prosports-shop.com/shop/display_cart?return_url=http://1win.directory 1win
1вин зеркало 1вин and 1win вход 1win
Если вы искали где отремонтировать сломаную технику, обратите внимание – профи ремонт
1win зеркало: 1win зеркало – 1win зеркало
http://vavada.auction/# vavada casino
мастерские по ремонту телевизоров в москве
Наши специалисты предлагает профессиональный мастер по ремонту стиральной машины адреса различных марок и моделей. Мы осознаем, насколько необходимы вам ваши автоматические стиральные машины, и готовы предложить сервис первоклассного уровня. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только оригинальные запчасти, что гарантирует надежность и долговечность проведенных ремонтов.
Наиболее частые неисправности, с которыми сталкиваются пользователи автоматических стиральных машин, включают неисправности барабана, неработающий нагревательный элемент, программные сбои, неработающий насос и механические повреждения. Для устранения этих проблем наши опытные мастера проводят ремонт барабанов, нагревательных элементов, ПО, насосов и механических компонентов. Доверив ремонт нам, вы гарантируете себе надежный и долговечный починить стиральную машину на дому.
Подробная информация представлена на нашем сайте: https://remont-stiralnyh-mashin-ace.ru
Профессиональный сервисный центр по ремонту варочных панелей и индукционных плит.
Мы предлагаем: ремонт индукционных варочных панелей
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Drug facts provided. Comprehensive medication overview.
xanax online pharmacy
Pill info available. Medicine facts provided.
1xbet зеркало: 1xbet официальный сайт – 1хбет официальный сайт
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт цифровой техники барнаул
Drug specifics here. Medication leaflet here.
legit online pharmacy
Pill leaflet available. Side effects listed.
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники в челябинске
Comprehensive drug overview. Medication information here.
online mexican pharmacy
Get drug info. Comprehensive medication guide.
prawo jazdy a1
EVA Бизнес-расстановки. https://rasstanovkiural.ru
The Significance of Resonance Regulation Systems in Industrial Equipment
Across production environments, equipment along with turning equipment act as the core of production. Nonetheless, an of the most prevalent problems that may obstruct the functionality along with longevity is resonance. Vibrations could cause a series of complications, from minimized exactness along with efficiency leading to increased deterioration, finally bringing about expensive downtime along with maintenance. This is where vibration control systems becomes necessary.
Why Oscillation Control is Necessary
Vibration in industrial equipment can lead to several negative consequences:
Decreased Operational Performance: Exaggerated resonance might lead to misalignments as well as unbalance, decreasing the productivity in such equipment. This might result in slower production schedules as well as higher power consumption.
Greater Damage: Constant vibrations increases the erosion in mechanical parts, resulting in more regular repairs as well as the chance for unanticipated unexpected issues. Such a scenario not only increases operational costs but also reduces the lifetime for the existing devices.
Safety Risks: Uncontrolled vibration might introduce major safety risks to both the machines and the workers. In severe cases, severe cases, these could result in catastrophic equipment failure, endangering employees along with causing extensive devastation in the environment.
Exactness along with Quality Challenges: For businesses which demand precise production, such as industrial sectors as well as aerospace, resonance may cause inaccuracies in manufacturing, producing flawed products and more waste.
Reasonably Priced Alternatives to Vibration Regulation
Investing in vibration management apparatus proves not just a necessity and also a smart decision for any organization that uses equipment. The offered cutting-edge vibration management systems work to intended to remove vibrations in various equipment or rotational systems, ensuring smooth along with effective performance.
What sets such systems above the rest remains its affordability. We know the necessity of keeping costs low in today’s competitive market, and for that reason we provide premium vibration regulation systems at rates that are reasonable.
By choosing these tools, you aren’t simply securing your machinery and improving its performance but also investing towards the sustained success of your business.
Conclusion
Resonance mitigation remains a critical element in preserving the efficiency, protection, and lifespan of your machines. Using these reasonably priced vibration control equipment, you can ensure that your production run smoothly, your products maintain high quality, as well as all personnel remain safe. Do not allow resonance jeopardize your operations—invest in the correct apparatus today.
Get details now. Read about medications.
cvs pharmacy online application
Drug essentials explained. Comprehensive medication overview.
Internet casino platforms deliver a thrilling variety of casino games, numerous of which today incorporate crypto as a payment option. Among the best services, BC Game Casino, Fortune Panda Casino, Axe Casino, and Bitkingz Casino are gaining popularity, while Bitstarz shines through several awards. Cloudbet Casino is recognized for operating as a licensed cryptocurrency casino, providing security for players and integrity, as well as Fairspin as well as Mbit Casino deliver a broad selection of digital currency games.
For dice gambling, virtual currency casinos including Bitcoin Dice Casino bring an exhilarating experience, allowing players to stake using Bitcoin and alternative cryptos such as ETH, Litecoin, Dogecoin, BNB, and Tether.
For a majority of casino fans, deciding on the right casino provider matters. Thunderkick Casino, Play’n Go, Red Tiger Gaming, Quick Spin, Pragmatic Play Casino, Playtech Casino, Nolimit City, Net Entertainment, ELK Studios, and Microgaming Casino are listed among the top casino game studios renowned for their innovative slots, high-quality graphics, and intuitive interfaces.
Casino streams is now a popular style for bettors to engage with online casinos. Famous streamers including Classy Beef, Roshtein, Labowsky, Deuce Ace, and X-Posed stream their gameplay, commonly sharing large victories and providing insight into top strategies in gambling.
In addition, casinos such as BC Casino, Bitkingz, and Rocketpot Casino also include Plinko games, a fan-favorite game with straightforward mechanics with large possibilities for huge rewards.
Comprehending gaming responsibility, rebate offers, and anonymous play in crypto casinos are essential for bettors looking to maximize their enjoyment. Choosing a secure wallet, looking for no-registration-required casinos, and learning strategies for titles like Aviator Casino Game allows players remain knowledgeable while having fun with the excitement of the game.
Medication effects explained. Get medication facts.
cvs online pharmacy
Patient medication facts. Drug details provided.
pro88
SGP Семейные расстановки и шаманизм. https://rasstanovkiural.ru
online us pharmacy viagra: global pharmacy – Flomax
https://onlineph24.com/# propecia indian pharmacy
mexican pharmacy doxycycline uk online viagra pharmacy acyclovir cream pharmacy
Viagra Oral Jelly: value rx pharmacy tazewell tn – british pharmacy online
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:сервисные центры в екатеринбурге
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту варочных панелей и индукционных плит.
Мы предлагаем: варочная панель ремонт
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Pill guide available. Medication effects explained.
online pharmacy india
Pill info here. Get pill facts.
https://onlineph24.com/# accutane pharmacy
finpecia swiss pharmacy
ремонт фототехники
abdulhay ali ahmed alawadhiand bahrain pharmacy & general store: inderal online pharmacy – u s online pharmacy
https://onlineph24.com/# target pharmacy tretinoin
pharmacy store nearby online pharmacy australia free delivery tretinoin uk pharmacy
rx plus pharmacy: wellbutrin sr pharmacy – pantoprazole pharmacy
замена венцов красноярск
Тогда, когда осуществляется смена нижних брёвен, то деревянный венец или освобождается от давления и реализуется замена, так как при замене подъём не больше десяти см, что и не выступает критичным в том числе для внутренних частей обустройства.
нижний венец из листвяка существенно надежнее и успешно доказал себя благодаря своей крепостью и сопротивляемостью к порче. Однако, ее также нужно обработать через применение противогрибкового состава, аналогично и другие стропила.
Мы специализируется не только восстановлением конструкций, но и улучшением полов. Клиенты нередко подают заявку на теплые полы и перекрытия с тепловой теплозащитой наши специалисты Комплектуем заявку материалами и предоставляем особые расценки.
https://drstore24.com/# rx partners pharmacy
rite aid online pharmacy
Este volante de estabilizacion con cesta de embrague constituye un sistema primordial para verificar el funcionamiento excelente del propulsor y la transmision de un camion. El desbalance en la presente unidad tiende a producir sacudidas, ruido, deterioro rapido de los partes e pudiendo llegar a fallos. Historicamente, el nivelacion se ejecutaba posterior a retirar el volante del propulsion, aunque las desarrollos actuales posibilitan efectuar dicho proceso de forma directa en el vehiculo, lo cual minimiza plazo y costos.
Cual es el Desbalance?
El desajuste es una circunstancia en la que la masa de un elemento giratorio (en el presente escenario, el volador de balanceo con compartimento de embrague) se distribuye de manera asimetrica en funcion de su eje de rotacion. Ello produce tensiones centrifugas las cuales generan vibraciones.
Causas fundamentales del Desbalance del Volante de Balanceo con Cesta de Embrague:
Imprecisiones de produccion y ensamblaje: Hasta leves divergencias en la geometria de los elementos pueden generar desbalance.
Uso y desperfectos: El operacion prolongada, el exceso de calor y los danos mecanicos podrian afectar la cantidad y resultar en desbalance.
Instalacion o arreglo inadecuada: Una instalacion deficiente de la recipiente de embrague o mantenimientos inapropiados asimismo pueden causar falta de equilibrio.
замена объектива в москве
trimix online pharmacy: klonopin online pharmacy – generic cialis online pharmacy
https://pharm24on.com/# online pharmacy australia free delivery
simvastatin kmart pharmacy colchicine pharmacy online pharmacy that sells viagra
OHV Системные расстановки по Хеллингеру. https://rasstanovkiural.ru
https://pharm24on.com/# humana online pharmacy
online pharmacy to buy viagra online pharmacy india cialis prime rx pharmacy
pharmacy app store: wg online pharmacy – european pharmacy cialis
https://easydrugrx.com/# mestinon online pharmacy
computer rx pharmacy software
dutasteride from dr reddy’s or inhouse pharmacy: generic rx online pharmacy – online pharmacy clobetasol
https://onlineph24.com/# price chopper pharmacy
most trusted online pharmacy pharmacy prices online pharmacy flovent
indian pharmacy cialis: most trusted online pharmacy – tri luma online pharmacy
Aquel volador de equilibrado con recipiente de embrague constituye un metodo esencial a fin de asegurar el rendimiento optimo del propulsor y la impulsion de un transporte. El desequilibrio en dicha parte puede producir temblores, estruendo, deterioro rapido de los componentes e pudiendo llegar a fallas. Historicamente, el balanceo se ejecutaba luego de desmontar el rotativo del propulsion, no obstante las innovaciones modernas facilitan realizar tal procedimiento directamente en el automotor, lo que minimiza tiempo y gastos.
?Que es el Desequilibrio?
El desequilibrio es una condicion en la que la masa de un elemento giratorio (en el presente escenario, el girador de equilibrado con recipiente de embrague) se distribuye de manera asimetrica en comparacion a su eje de giro. Esto genera fuerzas centrifugas que en consecuencia causan sacudidas.
Causas principales del Desbalance del Girador de Equilibrado con Canasto de Embrague:
Inexactitudes de construccion y union: Hasta insignificantes diferencias en la diseno de los partes llegan a generar desajuste.
Desgaste y averias: El tiempo de uso, el exceso de calor y los desperfectos mecanicos podrian cambiar la peso y conducir en desequilibrio.
Instalacion o servicio deficiente: Una colocacion incorrecta de la canasto de embrague o arreglos inadecuados tambien pueden provocar desbalance.
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
https://onlineph24.com/# buy viagra pharmacy uk
low cost online pharmacy pharmacy online 365 review meijer pharmacy generic lipitor
Abuse effects detailed. Drug impacts explained.
viagra online pharmacy
Pill essentials explained. Get pill details.
pharmacy online uae Malegra FXT or finasteride online pharmacy
http://adamlewisschroeder.com/info.php?a=buy+generic+viagra;+onlineph24.com, real pharmacy rx generic viagra
compounding pharmacy viagra pharmacy book store and rx care pharmacy orlando fl contrave online pharmacy
HIB Дизайн человека https://rasschitat-dizayn-cheloveka-onlayn.ru Дизайн человека. 3/5 Дизайн человека.
remedy rx pharmacy doc morris pharmacy artane or legal online pharmacies in the us
https://www.google.md/url?q=https://easydrugrx.com rx partners pharmacy
get cialis online pharmacy late night pharmacy artane and Motilium dapoxetine in malaysia pharmacy
tops pharmacy which pharmacy has the cheapest viagra or dexamethasone iontophoresis pharmacy
https://www.google.is/url?q=https://drstore24.com medicine store pharmacy springfield, mo
senior rx care pharmacy pharmacy express viagra and flomax pharmacy questionnaire people’s pharmacy generic wellbutrin
medical pharmacy south: trileptal online pharmacy – cialis from online pharmacy
online pharmacy nolvadex: wellness rx pharmacy – isotretinoin pharmacy
https://easydrugrx.com/# legitimate online pharmacy list
online pharmacy same day delivery allopurinol online pharmacy generic lipitor online pharmacy
https://pharm24on.com/# clozaril pharmacy
can you buy viagra from the pharmacy
slot853 link alternatif
online pharmacy reviews cialis: viagra no prescription online pharmacy – orlistat online pharmacy
TDV Дизайн человека https://rasschitat-dizayn-cheloveka-onlayn.ru Дизайн человека. 2/5 Дизайн человека.
https://pharm24on.com/# xlpharmacy generic cialis
tesco pharmacy viagra cost panadol osteo pharmacy humana online pharmacy
telmisartan online pharmacy: legal online pharmacy review – viagra no prescription online pharmacy
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
EHS Дизайн человека https://humandesignplanet.ru Дизайн человека. 5/1 Дизайн человека.
PXO Дизайн человека https://vkl-design.ru Дизайн человека. 6/2 Дизайн человека.
topamax pharmacy: xl pharmacy valtrex – drug store
https://easydrugrx.com/# freds pharmacy store hours
kamagra 365 pharmacy neighbor rx pharmacy value rx pharmacy tazewell tn
OMP Дизайн человека https://design-human.ru Дизайн человека. 5/1 Дизайн человека.
viagra tesco pharmacy: provigil mexico pharmacy – pharmacy metoprolol
https://pharm24on.com/# sildenafil citrate online pharmacy
pharmacy viagra prices grocery store with pharmacy near me much does viagra cost pharmacy
Cilostazol: florida online pharmacy – online pharmacy amoxicillin no prescription
娛樂城推薦與優惠詳解
在現今的娛樂世界中,線上娛樂城已成為眾多玩家的首選。無論是喜歡真人遊戲、老虎機還是體育賽事,每個玩家都能在娛樂城中找到自己的樂趣。以下是一些熱門的娛樂城及其優惠活動,幫助您在選擇娛樂平台時做出明智的決定。
各大熱門娛樂城介紹
1. 富遊娛樂城
富遊娛樂城以其豐富的遊戲選擇和慷慨的優惠活動吸引了大量玩家。新會員只需註冊即可免費獲得體驗金 $168,無需儲值即可輕鬆試玩。此外,富遊娛樂城還提供首存禮金 100% 獎勵,最高可領取 $1000。
2. AT99娛樂城
AT99娛樂城以高品質的遊戲體驗和優秀的客戶服務聞名。該平台提供各種老虎機和真人遊戲,並定期推出新遊戲,讓玩家保持新鮮感。
3. BCR娛樂城
BCR娛樂城是一個新興的平台,專注於提供豐富的體育賽事投注選項。無論是足球、籃球還是其他體育賽事,BCR都能為玩家提供即時的投注體驗。
熱門遊戲推薦
WM真人視訊百家樂
WM真人視訊百家樂是許多玩家的首選,該遊戲提供了真實的賭場體驗,並且玩法簡單,容易上手。
戰神賽特老虎機
戰神賽特老虎機以其獨特的主題和豐富的獎勵機制,成為老虎機愛好者的最愛。該遊戲結合了古代戰神的故事背景,讓玩家在遊戲過程中感受到無窮的樂趣。
最新優惠活動
富遊娛樂城註冊送體驗金
富遊娛樂城新會員獨享 $168 體驗金,無需儲值即可享受全場遊戲,讓您無壓力地體驗不同遊戲的魅力。
VIP 日日返水無上限
富遊娛樂城為 VIP 會員提供無上限的返水優惠,最高可達 0.7%。此活動讓玩家在遊戲的同時,還能享受額外的回饋。
結論
選擇合適的娛樂城不僅能為您的遊戲體驗增色不少,還能通過各種優惠活動獲得更多的利益。無論是新會員還是資深玩家,都能在這些推薦的娛樂城中找到適合自己的遊戲和活動。立即註冊並體驗這些優質娛樂平台,享受無限的遊戲樂趣!
Профессиональный сервисный центр по ремонту фото техники от зеркальных до цифровых фотоаппаратов.
Мы предлагаем: ремонт фотокамер
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
кондиционеры
Магазин кондиционеров: Ваше решение для комфорта и климата
Добро пожаловать в наш интернет-магазин кондиционеров в Москве и Московской области!
Наша цель – предложить вам самое лучшее оборудование для создания идеального климата в вашем доме или офисе. Мы гордимся тем, что являемся официальным дилером таких известных брендов, как Toshiba, Energolux, Haier, Gree и других. Это гарантирует, что вся представленная продукция сертифицирована и соответствует самым высоким стандартам качества.
Почему выбирают нас?
Официальный дилер. Мы работаем напрямую с производителями, что гарантирует вам оригинальные товары и официальную гарантию на всю продукцию.
Гарантия лучшей цены. Мы уверены в конкурентоспособности наших цен и предлагаем вам лучшие условия для покупки.
Быстрая доставка. Независимо от вашего местоположения в Московской области, мы доставим заказ в кратчайшие сроки.
Бесплатный замер. Каждый наш клиент получает услугу бесплатного замера, что позволяет подобрать оборудование, идеально подходящее для вашего помещения.
Хиты продаж
Среди самых популярных моделей в нашем ассортименте можно выделить:
TOSHIBA RAS-B10E2KVG-E/RAS-10E2AVG-EE SEIYA NEW – Это оборудование нового поколения, отличающееся высокой энергоэффективностью и бесшумной работой. Цена: 85 900 ?.
Сплит-система Energolux GENEVA SAS07G3-AI/SAU07G3-AI – Идеальный выбор для тех, кто ищет баланс между качеством и ценой. Цена: 47 700 ?.
Сплит-система Gree Bora GWH09AAA/K3NNA2A – Лидер продаж, известный своей надежностью и эффективностью охлаждения. Цена: 40 040 ?.
Сплит-система HAIER FLEXIS AS25S2SF2FA-W / 1U25S2SM3FA – Очень тихая модель, которая идеально подходит для установки в спальнях и детских комнатах. Цена: 88 900 ?.
Услуги и акции
Мы предлагаем бесплатный замер для всех наших клиентов. Эта услуга позволяет избежать ошибок при выборе оборудования и обеспечить максимальную эффективность системы кондиционирования.
Кроме того, в нашем магазине регулярно проводятся акции, которые позволяют вам существенно сэкономить на покупке климатической техники. Не пропустите возможность воспользоваться уникальными предложениями!
Доставка и установка
Мы предлагаем услуги доставки и установки кондиционеров по всей Московской области, включая такие города, как Балашиха, Химки, Подольск, Люберцы, Красногорск и другие. Наши специалисты быстро и профессионально установят оборудование, чтобы вы могли наслаждаться комфортом в кратчайшие сроки.
pharmacy drug store in finland anti fungal or pharmacy rx one coupon code
http://ingrus.net/dbforum/away.php?s=http://onlineph24.com clomid pharmacy
lexapro pharmacy online top rx pharmacy and Penegra trust pharmacy online
motilium pharmacy: Shallaki – egypt pharmacy viagra
https://onlineph24.com/# buy propecia online pharmacy
your pharmacy online pharmacy store viagra + cialis wellbutrin indian pharmacy
best online pharmacy stores cheapest pharmacy to get prescriptions filled or percocet online pharmacy without prescriptions
http://hamas.opoint.com/?url=http://drstore24.com target pharmacy ambien
clomiphene online pharmacy domperidone new zealand pharmacy and rx pharmacy coupon Vermox
dj88
Pentingnya Memilih Game Pulsa di Situs DJ88
DJ88 adalah situs game deposit pulsa terkemuka di Indonesia, yang menawarkan berbagai jenis game online yang lengkap dan mudah diakses. Dengan hanya satu ID, Anda dapat menikmati seluruh permainan yang tersedia di situs ini. Keunggulan DJ88 bukan hanya pada variasi game yang ditawarkan, tetapi juga pada layanan deposit pulsa yang praktis, menggunakan XL atau Telkomsel dengan potongan terendah dibandingkan situs lain. Anda juga bisa melakukan deposit melalui OVO, Gopay, Dana, atau melalui minimarket seperti Indomaret dan Alfamart.
Keamanan dan Kepercayaan yang Terjaga
DJ88 memiliki lisensi resmi dari PAGCOR (Philippine Amusement Gaming Corporation), yang menjamin keamanan dan kenyamanan bermain. Sistem keamanan di situs ini menggunakan metode enkripsi termutakhir, didukung oleh server hosting cepat dan tampilan modern. Hal ini menjadikan DJ88 sebagai salah satu agen game online terpercaya di Indonesia, yang selalu berkomitmen untuk menjaga kepercayaan para pemain sebagai prioritas utama.
Beragam Pilihan Game dan Layanan Pelanggan yang Ramah
Situs ini menawarkan berbagai jenis game online, termasuk yang disiarkan secara LIVE dengan host cantik dan kamera yang merekam secara real-time, memberikan pengalaman bermain yang lebih menarik. Selain itu, DJ88 juga dikenal dengan promo menarik seperti welcome bonus 20%, bonus deposit harian, dan cashback yang disesuaikan dengan kebutuhan pemain.
Pelayanan pelanggan di DJ88 sangat profesional dan siap melayani Anda 24 jam non-stop melalui Live Chat, WhatsApp, Facebook, dan media sosial lainnya. Dengan semua keunggulan ini, DJ88 menjadi pilihan utama bagi para penggemar game online di Indonesia yang mencari pengalaman bermain yang aman, nyaman, dan menguntungkan.
overseas pharmacies: wholesale pharmacy – pharmacy selling cytotec
https://pharm24on.com/# online pharmacy india
live pharmacy ce online permethrin uk pharmacy generic lexapro online pharmacy
EXG Дизайн человека https://raschet-karty-dizayn-cheloveka.ru Дизайн человека. 5/1 Дизайн человека.
warfarin continuing education pharmacy: viagra verified internet pharmacy practice sites – usa viagra online pharmacy
Профессиональный сервисный центр по ремонту фото техники от зеркальных до цифровых фотоаппаратов.
Мы предлагаем: ремонт затвора фотоаппарата
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
IWF Дизайн человека https://vkl-design.ru Дизайн человека. 3/5 Дизайн человека.
BYO Дизайн человека https://design-human.ru Дизайн человека. 3/6 Дизайн человека.
Если вы искали где отремонтировать сломаную технику, обратите внимание – тех профи
gen rx pharmacy prozac pharmacy online tesco artane pharmacy
reputable mexican pharmacies online: medicine in mexico pharmacies – mexican mail order pharmacies
https://mexicopharmacy.cheap/# mexican border pharmacies shipping to usa
tadacip online pharmacy: people’s pharmacy lipitor – spironolactone in house pharmacy
ZAB Дизайн человека https://designmsu.ru Дизайн человека. 1/4 Дизайн человека.
JVO Дизайн человека https://vkl-design.ru Дизайн человека. 1/4 Дизайн человека.
SPM Дизайн человека https://rasschitat-dizayn-cheloveka-onlayn.ru Дизайн человека. 4/6 Дизайн человека.
Профессиональный сервисный центр по ремонту планшетов в Москве.
Мы предлагаем: ремонт планшета
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
avandia pharmacy: lortab refills pharmacy – diplomat specialty pharmacy lipitor
top 10 pharmacies in india india online pharmacy top 10 pharmacies in india
ohio pharmacy law adipex: Septra – cheap online pharmacy viagra
http://indianpharmacy.company/# cheapest online pharmacy india
P閃乱カグラ2
CR押忍!番長
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
BLOOD+ 二人の女王
https://sites.google.com/view/pachinko-sword-art-online-k12
アニメの名シーンを再現しており、ファンにはたまらない演出です。
主役は銭形 2004
k8パチンコ
パチスロ バイオハザード6
Pフィーバー戦姫絶唱シンフォギア2
回胴黙示録カイジ3
best india pharmacy: indianpharmacy com – india pharmacy mail order
mexican online pharmacies prescription drugs п»їbest mexican online pharmacies or mexican drugstore online
https://www.neuoetting.de/externer_link.php?url_uebergabe=mexicopharmacy.cheap mexican border pharmacies shipping to usa
п»їbest mexican online pharmacies pharmacies in mexico that ship to usa and mexican border pharmacies shipping to usa buying prescription drugs in mexico
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:сервисные центры в новосибирске
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://mexicopharmacy.cheap/# mexico drug stores pharmacies
Если вы искали где отремонтировать сломаную технику, обратите внимание – сервис центр в краснодаре
online pharmacy india india online pharmacy or top 10 online pharmacy in india
http://101.43.178.182/sell/email.asp?d=indianpharmacy.company&on=tb top 10 online pharmacy in india
indianpharmacy com top 10 online pharmacy in india and top 10 pharmacies in india buy prescription drugs from india
mexico drug stores pharmacies: medication from mexico pharmacy – buying prescription drugs in mexico
Профессиональный сервисный центр по ремонту планшетов в Москве.
Мы предлагаем: ремонт планшетов в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
best online non prescription pharmacy Vermox online pharmacy spironolactone
buying from online mexican pharmacy: purple pharmacy mexico price list – buying from online mexican pharmacy
http://indianpharmacy.company/# top online pharmacy india
Если вы искали где отремонтировать сломаную технику, обратите внимание – сервисный центр в казани
generic lipitor online pharmacy generic propecia united pharmacy or no prescription online pharmacy ua products percocet
http://www.burstek.com/RedirectPage.php?reason=4&value=Anonymizers&proctoblocktimeout=1&ip=89.78.118.181&url=http://pharmbig24.com/ proventil inhaler online pharmacy
olanzapine online pharmacy online pharmacy rx and lexapro pharmacy coupon restore rx pharmacy
purple pharmacy mexico price list: buying prescription drugs in mexico – п»їbest mexican online pharmacies
indian pharmacy online: indian pharmacy – world pharmacy india
NTS Дизайн человека https://designmsu.ru Дизайн человека. 4/6 Дизайн человека.
https://pharmbig24.online/# boots pharmacy orlistat
RPH Дизайн человека https://designchita.ru Дизайн человека. 2/4 Дизайн человека.
pharmacies in mexico that ship to usa buying from online mexican pharmacy mexican rx online
kroger pharmacy simvastatin: mail order pharmacy concerta – cialis viagra pharmacy
https://mexicopharmacy.cheap/# purple pharmacy mexico price list
娛樂城推薦與優惠詳解
在現今的娛樂世界中,線上娛樂城已成為眾多玩家的首選。無論是喜歡真人遊戲、老虎機還是體育賽事,每個玩家都能在娛樂城中找到自己的樂趣。以下是一些熱門的娛樂城及其優惠活動,幫助您在選擇娛樂平台時做出明智的決定。
各大熱門娛樂城介紹
1. 富遊娛樂城
富遊娛樂城以其豐富的遊戲選擇和慷慨的優惠活動吸引了大量玩家。新會員只需註冊即可免費獲得體驗金 $168,無需儲值即可輕鬆試玩。此外,富遊娛樂城還提供首存禮金 100% 獎勵,最高可領取 $1000。
2. AT99娛樂城
AT99娛樂城以高品質的遊戲體驗和優秀的客戶服務聞名。該平台提供各種老虎機和真人遊戲,並定期推出新遊戲,讓玩家保持新鮮感。
3. BCR娛樂城
BCR娛樂城是一個新興的平台,專注於提供豐富的體育賽事投注選項。無論是足球、籃球還是其他體育賽事,BCR都能為玩家提供即時的投注體驗。
熱門遊戲推薦
WM真人視訊百家樂
WM真人視訊百家樂是許多玩家的首選,該遊戲提供了真實的賭場體驗,並且玩法簡單,容易上手。
戰神賽特老虎機
戰神賽特老虎機以其獨特的主題和豐富的獎勵機制,成為老虎機愛好者的最愛。該遊戲結合了古代戰神的故事背景,讓玩家在遊戲過程中感受到無窮的樂趣。
最新優惠活動
富遊娛樂城註冊送體驗金
富遊娛樂城新會員獨享 $168 體驗金,無需儲值即可享受全場遊戲,讓您無壓力地體驗不同遊戲的魅力。
VIP 日日返水無上限
富遊娛樂城為 VIP 會員提供無上限的返水優惠,最高可達 0.7%。此活動讓玩家在遊戲的同時,還能享受額外的回饋。
結論
選擇合適的娛樂城不僅能為您的遊戲體驗增色不少,還能通過各種優惠活動獲得更多的利益。無論是新會員還是資深玩家,都能在這些推薦的娛樂城中找到適合自己的遊戲和活動。立即註冊並體驗這些優質娛樂平台,享受無限的遊戲樂趣!
Профессиональный сервисный центр по ремонту видео техники а именно видеокамер.
Мы предлагаем: ремонт видеокамер в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
indian pharmacies safe: india pharmacy – top online pharmacy india
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт цифровой техники казань
娛樂城排行
娛樂城推薦與優惠詳解
在現今的娛樂世界中,線上娛樂城已成為眾多玩家的首選。無論是喜歡真人遊戲、老虎機還是體育賽事,每個玩家都能在娛樂城中找到自己的樂趣。以下是一些熱門的娛樂城及其優惠活動,幫助您在選擇娛樂平台時做出明智的決定。
各大熱門娛樂城介紹
1. 富遊娛樂城
富遊娛樂城以其豐富的遊戲選擇和慷慨的優惠活動吸引了大量玩家。新會員只需註冊即可免費獲得體驗金 $168,無需儲值即可輕鬆試玩。此外,富遊娛樂城還提供首存禮金 100% 獎勵,最高可領取 $1000。
2. AT99娛樂城
AT99娛樂城以高品質的遊戲體驗和優秀的客戶服務聞名。該平台提供各種老虎機和真人遊戲,並定期推出新遊戲,讓玩家保持新鮮感。
3. BCR娛樂城
BCR娛樂城是一個新興的平台,專注於提供豐富的體育賽事投注選項。無論是足球、籃球還是其他體育賽事,BCR都能為玩家提供即時的投注體驗。
熱門遊戲推薦
WM真人視訊百家樂
WM真人視訊百家樂是許多玩家的首選,該遊戲提供了真實的賭場體驗,並且玩法簡單,容易上手。
戰神賽特老虎機
戰神賽特老虎機以其獨特的主題和豐富的獎勵機制,成為老虎機愛好者的最愛。該遊戲結合了古代戰神的故事背景,讓玩家在遊戲過程中感受到無窮的樂趣。
最新優惠活動
富遊娛樂城註冊送體驗金
富遊娛樂城新會員獨享 $168 體驗金,無需儲值即可享受全場遊戲,讓您無壓力地體驗不同遊戲的魅力。
VIP 日日返水無上限
富遊娛樂城為 VIP 會員提供無上限的返水優惠,最高可達 0.7%。此活動讓玩家在遊戲的同時,還能享受額外的回饋。
結論
選擇合適的娛樂城不僅能為您的遊戲體驗增色不少,還能通過各種優惠活動獲得更多的利益。無論是新會員還是資深玩家,都能在這些推薦的娛樂城中找到適合自己的遊戲和活動。立即註冊並體驗這些優質娛樂平台,享受無限的遊戲樂趣!
shopko pharmacy: which pharmacy is cheaper – valtrex online pharmacy
indian pharmacy paypal indian pharmacy paypal or india pharmacy
https://www.smartspace.ws/login.php?TraderId=1123&rdurl=https://indianpharmacy.company::: cheapest online pharmacy india
pharmacy website india india pharmacy mail order and cheapest online pharmacy india india pharmacy
buy prescription drugs from india [url=https://indianpharmacy.company/#]indian pharmacy paypal[/url] indian pharmacies safe
п»їbest mexican online pharmacies mexican border pharmacies shipping to usa or pharmacies in mexico that ship to usa
http://alfachat.ru/exit.php?linkurl=mexicopharmacy.cheap mexico drug stores pharmacies
purple pharmacy mexico price list buying prescription drugs in mexico online and buying prescription drugs in mexico purple pharmacy mexico price list
http://indianpharmacy.company/# online pharmacy india
апостиль в новосибирске
best online pharmacy india: india pharmacy – indian pharmacy paypal
https://indianpharmacy.company/# india online pharmacy
апостиль в новосибирске
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
purple pharmacy mexico price list mexican online pharmacies prescription drugs or mexico drug stores pharmacies
https://maps.google.co.vi/url?sa=t&url=https://mexicopharmacy.cheap buying prescription drugs in mexico online
п»їbest mexican online pharmacies buying from online mexican pharmacy and mexican pharmaceuticals online medication from mexico pharmacy
Weather
перевод с иностранных языков
mexico pharmacies prescription drugs: buying prescription drugs in mexico – mexico pharmacies prescription drugs
перевод документов
pharmacy atenolol aarp medicare rx pharmacy directory or humana pharmacy store
https://www.google.com.sg/url?q=https://pharmbig24.com 1st pharmacy net cialis acheter sur internet
save rx pharmacy online pharmacy viagra india and best rx pharmacy software derm rx pharmacy
перевод документов
coop pharmacy store locator doxycycline hyclate online pharmacy order cialis online pharmacy
перевод с иностранных языков
india pharmacy: Online medicine home delivery – pharmacy website india
апостиль в новосибирске
перевод с иностранных языков
medicine in mexico pharmacies: mexican border pharmacies shipping to usa – medication from mexico pharmacy
https://indianpharmacy.company/# india pharmacy
апостиль в новосибирске
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт бытовой техники в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
перевод документов
pharmacies in mexico that ship to usa: pharmacies in mexico that ship to usa – mexican online pharmacies prescription drugs
перевод с иностранных языков
https://pharmbig24.com/# chloramphenicol eye drops pharmacy
перевод с иностранных языков
Если вы искали где отремонтировать сломаную технику, обратите внимание – сервисный центр в красноярске
перевод с иностранных языков
online pharmacy prednisolone international pharmacy domperidone adipex mexico pharmacy
перевод с иностранных языков
апостиль в новосибирске
purple pharmacy mexico price list mexican border pharmacies shipping to usa or purple pharmacy mexico price list
http://www.debray-jerome.fr/index.php?redirect/&url=https://mexicopharmacy.cheap/ medicine in mexico pharmacies
mexican drugstore online mexican pharmaceuticals online and mexican drugstore online п»їbest mexican online pharmacies
indian pharmacies safe indian pharmacies safe or п»їlegitimate online pharmacies india
https://maps.google.lt/url?rct=j&sa=t&url=https://indianpharmacy.company buy medicines online in india
indian pharmacy indian pharmacy and online pharmacy india india online pharmacy
перевод документов
апостиль в новосибирске
top 10 pharmacies in india: indian pharmacy paypal – buy prescription drugs from india
mexico pharmacies prescription drugs: medicine in mexico pharmacies – medicine in mexico pharmacies
http://indianpharmacy.company/# top 10 online pharmacy in india
перевод документов
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
cheapest online pharmacy india: cheapest online pharmacy india – п»їlegitimate online pharmacies india
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: сервисные центры по ремонту техники в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
перевод с иностранных языков
Бездепозитные промокоды в списке казино. Официальные казино с лицензией. Найдите лучшее онлайн казино в рейтинге. Ссылки на рабочие зеркала. Бонусы к депозиту и фриспины. Вход и регистрация в казино aksducutgd
Если вы искали где отремонтировать сломаную технику, обратите внимание – выездной ремонт бытовой техники в нижнем новгороде
Мануальная терапия от головной боли Ростов
Superb and well-thought-out content! If you need some information about Airport Transfer, then have a look at http://rd2.minzdravrso.ru/bitrix/redirect.php?event1=click_to_call&event2=&event3=&goto=http://59n.de/flyingstar/flughafentransfer-frankfurt-altenahr
good neighbor pharmacy naproxen online pharmacy azithromycin texas board of pharmacy
https://pharmbig24.online/# online pharmacy rx
tesco pharmacy doxycycline simvastatin kroger pharmacy or online pharmacy bc
https://66.ernorvious.com/index/d1?diff=0&source=og&campaign=5944&content=&clickid=2aqzrzl2knl1pmit&aurl=https://pharmbig24.com 24 hours pharmacy
sam’s club pharmacy legal online pharmacy coupon code and what does rx mean in pharmacy proscar uk pharmacy
перевод документов
CRルパン三世I’m a super hero~
k8パチンコ
友人と一緒にプレイすることで、共感が生まれ、楽しさが倍増します。
CR 獣王
https://sites.google.com/view/slot-basilisk-the-kouga-ninja
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
ぱちスロ にゃんこ大戦争 BIGBANG
CRグラップラー刃牙
CRぱちんこ 新鬼武者
CR北斗の拳5 百裂
P フィーバーダンジョンに出会いを求めるのは間違っているだろうか
sapporo88 slot
target88
апостиль в новосибирске
ventolin inhaler inhouse pharmacy: kamagra oral jelly – which pharmacy has the cheapest viagra
https://indianpharmacy.company/# online pharmacy india
online shopping pharmacy india: best online pharmacy india – top online pharmacy india
перевод с иностранных языков
перевод с иностранных языков
перевод документов
baclofen online pharmacy: rx health pharmacy – ciprofloxacin online pharmacy
mexican pharmaceuticals online mexican drugstore online or purple pharmacy mexico price list
https://www.human-d.co.jp/seminar/contact.html?title=Web%C3%A3%C6%E2%80%99%C2%BBDTP%C3%A3%C6%E2%80%99%E2%80%A1%C3%A3%E2%80%9A%C2%B6%C3%A3%E2%80%9A%C2%A4%C3%A3%C6%E2%80%99%C2%B3%C3%A7%C2%A7%E2%80%98%C3%AF%C2%BC%CB%86%C3%A5%E2%80%A6%C2%AC%C3%A5%E2%80%A6%C2%B1%C3%A8%C2%81%C2%B7%C3%A6%C2%A5%C2%AD%C3%A8%C2%A8%E2%80%9C%C3%A7%C2%B7%C2%B4%C3%AF%C2%BC%E2%80%B0&url=https://mexicopharmacy.cheap:: buying prescription drugs in mexico
medicine in mexico pharmacies mexico drug stores pharmacies and purple pharmacy mexico price list buying prescription drugs in mexico online
апостиль в новосибирске
Профессиональный сервисный центр по ремонту стиральных машин с выездом на дом по Москве.
Мы предлагаем: ремонт стиралки москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт крупногабаритной техники в казани
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
buying from online mexican pharmacy mexico pharmacies prescription drugs best online pharmacies in mexico
Пеларгонии сортовые
Пеларгонии видовые: лучший решение для твоего дома и сада
Если вы подбираете цветы, которые будут удивлять вас своей красотой и запахом, вместе с этом не требуя тщательного ухода, разновидности герани — наилучший вариант. Этих цветы обладают неповторимыми достоинствами, которые делают их топовыми среди украшений растений.
Почему элитные герани?
Простота и простота в содержании
Пеларгонии не нуждаются в особых условий для прорастания и без проблем привыкают к различным температурным режимам. Они замечательно процветают как в жилище, так и на свежем воздухе. Не беспокойтесь о проблемных цветах — герани требуется лишь увлажнять по степени высыхания земли и получать удовольствие от их цветами.
Яркие и различные оттенки
Определенный сорт гераней имеет свои уникальные тона и внешность. Разновидности, к примеру, ТА Монако, впечатляют интенсивными оттенками и выразительными бутонами. Это цветы, что сразу привлекают интерес и вносят яркие подчеркивания в любом месте.
Нежный запах, создающий уют
Пеларгонии не лишь украшают жилище — они снабжают его легким, незаметным благоуханием. Этот природный благоухание обеспечивает придать чувство комфорта и покоя, а также действует как натуральный репеллент для вредителей.
Долгое цветение
Элитные пеларгонии не прекращают радовать глаз своим цветением в течение нескольких периодов. Ты будешь наслаждаться их красотой с старта сезона и до холодов осени. Такое непрекращающееся цветение — редкое достоинство в мире украшающих видов.
Прекрасный выбор для каждого пространства
Герани многофункциональны — их следует разводить как в вазонах на подоконниках, так и в огороде. Миниатюрные растения, например ЮВ Кардинал, хорошо смотрятся в декоративных вазонах, а виды, как Survivor idols Rosalinda, превратятся в декорацией клумбы.
Зачем следует отдать предпочтение точно пеларгонии?
Эти растения — не только декоративный элемент декора. Они значительно выделяются между других цветов по причине своей легкости в уходе, эстетичности и длительному процветанию. Их яркие оттенки придают особенную среду, будь то в помещении или на садовом участке. Пеларгонии — это лучший баланс красоты и практичности.
Выбирайте герани — сделайте вокруг себя прекрасную атмосферу без ненужных забот!
подъем дома
mexican online pharmacies prescription drugs mexican mail order pharmacies or mexico drug stores pharmacies
http://www.jschell.de/link.php?url=mexicopharmacy.cheap&goto=google_news buying prescription drugs in mexico online
mexican online pharmacies prescription drugs best online pharmacies in mexico and buying prescription drugs in mexico mexican pharmaceuticals online
mexico drug stores pharmacies: buying prescription drugs in mexico – buying prescription drugs in mexico
cheapest online pharmacy india online pharmacy india or reputable indian pharmacies
http://www.google.com.my/url?q=https://indianpharmacy.company top online pharmacy india
india pharmacy top 10 online pharmacy in india and cheapest online pharmacy india online pharmacy india
Doxycycline: online medicine shopping – pharmacy online program
https://indianpharmacy.company/# indian pharmacy paypal
https://pharmbig24.com/# online viagra pharmacy reviews
buying prescription drugs in mexico online purple pharmacy mexico price list best online pharmacies in mexico
blackpanth
Online medicine home delivery: top 10 pharmacies in india – Online medicine home delivery
Lバキ 強くなりたくば喰らえ!!!
k8パチンコ
自分の運を試すために、少しだけお金をかけるのが楽しいです。気軽に挑戦できます。
転生したらスライムだった件
https://sites.google.com/view/cat-eye-collection-recovery
大当たりの瞬間に周囲と一体感を感じられるのが良いです。共に喜べる仲間がいるのが嬉しい。
ハイビスカスSE
5 Lions Megaways
デジハネ CR 蒼天の拳 STV
パチスロビッグドリームinロストアイランド2
CR 北斗の拳6 天翔百裂
Профессиональный сервисный центр по ремонту стиральных машин с выездом на дом по Москве.
Мы предлагаем: ремонт стиральных машин москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт крупногабаритной техники в казани
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Опытный психотерапевт онлайн. Помощь в трудных ситуациях возможна 24/7. Анонимно, действенно. Выгодные цены. Не откладывайте на потом! https://843-spb-psiholog.tds-ka.ru/
Online medicine order: india online pharmacy – Online medicine order
pharmacy live ce online mexican pharmacy lortab or giant pharmacy store hours
https://forum.turkerview.com/proxy.php?link=https://pharmbig24.com levofloxacin online pharmacy
Primaquine vipps pharmacy viagra and world pharmacy store discount number pharmacy viagra now eu
best online pharmacy india: indian pharmacies safe – п»їlegitimate online pharmacies india
http://pharmbig24.com/# good neighbor pharmacy naproxen
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: сервисные центры в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mexican drugstore online purple pharmacy mexico price list п»їbest mexican online pharmacies
https://mexicopharmacy.cheap/# pharmacies in mexico that ship to usa
top 10 online pharmacy in india: online pharmacy india – top online pharmacy india
target pharmacy celexa: boniva online pharmacy – diazepam online pharmacy
mexican mail order pharmacies mexico pharmacies prescription drugs or purple pharmacy mexico price list
http://leefirstbaptistchurch.org/System/Login.asp?id=48747&Referer=http://mexicopharmacy.cheap mexican pharmaceuticals online
pharmacies in mexico that ship to usa mexican drugstore online and reputable mexican pharmacies online buying from online mexican pharmacy
best online pharmacies in mexico: medicine in mexico pharmacies – mexico pharmacies prescription drugs
http://indianpharmacy.company/# online shopping pharmacy india
Остеопат в Ростове
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.obesityhelp.com/members/killsanta1969/about_me/
http://www.nfomedia.com/profile?uid=rOjShcF
https://tubeteencam.com/user/megagood1997/profile
https://sartur219501973.diary.ru/
https://www.obesityhelp.com/members/amonamisu1991/about_me/
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт бытовой техники в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
cheapest online pharmacy india world pharmacy india or Online medicine order
https://reverb.com/onward?author_id=5021397&to=https://indianpharmacy.company п»їlegitimate online pharmacies india
indian pharmacies safe online shopping pharmacy india and cheapest online pharmacy india buy prescription drugs from india
Массаж в Ростове
buy tetracycline online pharmacy Etodolac pharmacy viagra cost
Если вы искали где отремонтировать сломаную технику, обратите внимание – профи услуги
buying prescription drugs in mexico buying from online mexican pharmacy or mexican online pharmacies prescription drugs
http://tool.baiwanzhan.com/t1/pr.aspx?url=mexicopharmacy.cheap п»їbest mexican online pharmacies
medication from mexico pharmacy buying prescription drugs in mexico online and mexico drug stores pharmacies mexican rx online
Опытный психотерапевт удаленно. Консультации доступна 24/7. Анонимно, продуктивно. Выгодные цены. Сделайте шаг к улучшению! https://9076-msk-psiholog.tds-ka.ru/
seu 14470726743484 ayupw
indianpharmacy com: top online pharmacy india – best online pharmacy india
3x3EYES聖魔覚醒
k8 カジノ パチンコ 南国育ちパチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
CR北斗の拳5 覇者
https://sites.google.com/view/code-geass-lelouch-of-the-rebe
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
めんそーれtw
k8パチンコ
Pコードギアス 反逆のルルーシュ Rebellion to Re_surrection
e ルパン三世 銭形からの招待状
鉄拳2nd
Если вы искали где отремонтировать сломаную технику, обратите внимание – профи ростов на дону
https://pharmbig24.com/# simvastatin kroger pharmacy
JILI SLOT – Permainan Slot Terbaik Kami
JILI GAMES menawarkan berbagai permainan slot, kartu, dan tembak ikan dengan lebih dari 100 game yang berbeda. Selalu menjadi yang terdepan dalam industri, JILI GAMES terus merilis produk game terbaru secara konsisten.
Game Slot JILI
JILI SUPER ACE
JILI Pharaoh Treasure
JILI Cực Tốc 777
JILI Đế quốc hoàng kim
JILI Bảo thạch Kala
JILI Quyền Vương
Top 10 game slot JILI sangat populer di kalangan pemain, dengan tema yang bervariasi dan fitur jackpot yang menarik.
Game Tembak Ikan JILI
JILI Chuyên Gia Săn Rồng
JILI Jackpot Fishing
JILI Phi Long Tàng Bảo
JILI Happy Fishing
JILI Đoạt bảo truyền kỳ
Permainan tembak ikan ini menawarkan pengalaman seru dan interaktif, di mana pemain dapat memenangkan hadiah besar dengan menembak ikan dan naga.
Game Kartu JILI
Baccarat
Color Game
Sic Bo
LUDO Quick
Super Bingo
Game kartu JILI menawarkan berbagai pilihan permainan klasik dan modern yang menarik untuk dimainkan.
Layanan dari JILI SLOT
Multi-Platform
Semua permainan kami tersedia dalam format HTML5, sehingga dapat dimainkan di berbagai perangkat seperti komputer, tablet, dan ponsel pintar dengan performa yang sempurna.
Dukungan
JILI SLOT menyediakan panduan yang mudah dipahami, membantu pemain dengan cepat menguasai aturan permainan.
JILI Jackpot
Semua pemain, baik dengan taruhan besar maupun kecil, memiliki kesempatan untuk memenangkan JILI Jackpot.
Dukungan 24/7
Layanan pelanggan tersedia sepanjang waktu untuk memastikan semua kebutuhan pemain JILI SLOT terpenuhi.
Multi-Bahasa
Kami mendukung berbagai bahasa dan mata uang untuk melayani pemain di seluruh dunia.
Promosi JILI
JILI Slot menawarkan banyak promosi yang memungkinkan pemain untuk dengan mudah bergabung dan menikmati berbagai permainan yang tersedia. Cukup bergabung dengan JILI City untuk mendapatkan penawaran ini dan mulai bermain di slot, permainan kartu, atau tembak ikan.
Nikmati pengalaman bermain game yang seru dan menguntungkan hanya di JILI SLOT!
RGBET – NHÀ CÁI UY TÍN TẠI VIỆT NAM
Trong bối cảnh cá cược trực tuyến ngày càng phát triển, RGBET đã nhanh chóng trở thành sự lựa chọn hàng đầu cho người chơi Việt Nam. Với hệ thống cá cược đa dạng, chất lượng dịch vụ vượt trội, RGBET mang đến trải nghiệm cá cược an toàn và thú vị.
Đa Dạng Về Trò Chơi
RGBET cung cấp nhiều sản phẩm cá cược phong phú như cá cược thể thao, casino trực tuyến, slot game và bắn cá.
Cá cược thể thao: Cung cấp hàng nghìn trận đấu bóng đá, bóng rổ, tennis với tỷ lệ cược cạnh tranh từ các giải đấu lớn trên thế giới.
Casino trực tuyến: Baccarat, Blackjack và Roulette mang đến trải nghiệm như tại sòng bạc thực thụ với chất lượng hình ảnh cao.
Slot game và bắn cá: Các trò chơi như slot game và bắn cá tại RGBET có giao diện đẹp mắt, dễ chơi và cơ hội trúng thưởng lớn.
Dịch Vụ Chăm Sóc Khách Hàng
RGBET cung cấp dịch vụ hỗ trợ khách hàng 24/7, đảm bảo người chơi được giúp đỡ kịp thời và tận tình. Điều này giúp người chơi an tâm khi cá cược, không gặp rắc rối về mặt kỹ thuật.
Bảo Mật Và An Toàn
RGBET chú trọng đến việc bảo mật thông tin và tài sản của người chơi, sử dụng công nghệ mã hóa hiện đại để đảm bảo an toàn tuyệt đối trong mọi giao dịch.
Khuyến Mãi Hấp Dẫn
RGBET thường xuyên có các chương trình khuyến mãi như thưởng chào mừng cho người chơi mới và các chương trình ưu đãi hàng tuần giúp gia tăng cơ hội chiến thắng.
Nền Tảng Đa Thiết Bị
Người chơi có thể truy cập RGBET trên máy tính, điện thoại hoặc máy tính bảng mà không lo về chất lượng. Công nghệ HTML5 đảm bảo các trò chơi hoạt động mượt mà trên mọi thiết bị.
Kết Luận
RGBET là nhà cái uy tín, cung cấp môi trường cá cược an toàn, trò chơi đa dạng và các chương trình khuyến mãi hấp dẫn. Đây là sự lựa chọn hoàn hảo cho những ai muốn trải nghiệm cá cược trực tuyến chất lượng cao.
indian pharmacy paypal: top 10 online pharmacy in india – Online medicine home delivery
https://indianpharmacy.company/# india online pharmacy
target88
target88
ремонт фундамента
online pharmacy scams: pioneer rx pharmacy software – estrace online pharmacy
freedom pharmacy prometrium united pharmacy propecia or pain relief
http://www.adhub.com/cgi-bin/webdata_pro.pl?_cgifunction=clickthru&url=https://pharmbig24.com online pharmacy viagra india
[url=https://maps.google.bj/url?q=https://pharmbig24.com]unicare pharmacy artane[/url] Aebgjoync and [url=http://wuyuebanzou.com/home.php?mod=space&uid=1049071]indian pharmacy provigil[/url] reputable online pharmacy uk
Aebgkeymn publix pharmacy cipro buy provigil online pharmacy
Профессиональный сервисный центр по ремонту игровых консолей Sony Playstation, Xbox, PSP Vita с выездом на дом по Москве.
Мы предлагаем: ремонт игровых консолей в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
indian pharmacy paypal: best online pharmacy india – india pharmacy
Профессиональный сервисный центр по ремонту компьютерных видеокарт по Москве.
Мы предлагаем: обслуживание видеокарты
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mexico pharmacies prescription drugs pharmacies in mexico that ship to usa or mexican mail order pharmacies
http://www.tifosy.de/url?q=https://mexicopharmacy.cheap mexico drug stores pharmacies
mexican rx online purple pharmacy mexico price list and mexican drugstore online mexico drug stores pharmacies
п»їlegitimate online pharmacies india: cheapest online pharmacy india – world pharmacy india
https://mexicopharmacy.cheap/# mexican rx online
pharmacy discount card rx relief thyroxine online pharmacy target pharmacy online refills
п»їlegitimate online pharmacies india: reputable indian online pharmacy – online pharmacy india
india pharmacy indian pharmacy online or cheapest online pharmacy india
https://images.google.st/url?q=https://indianpharmacy.company best online pharmacy india
top online pharmacy india indian pharmacies safe and online shopping pharmacy india pharmacy website india
Профессиональный сервисный центр по ремонту игровых консолей Sony Playstation, Xbox, PSP Vita с выездом на дом по Москве.
Мы предлагаем: надежный сервис ремонта игровых консолей
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Опытный психотерапевт дистанционно. Консультации предоставляется 24/7. Приватно, продуктивно. Индивидуальный подход. Начните менять жизнь сегодня! https://w-495.ru/
Профессиональный сервисный центр по ремонту компьютероной техники в Москве.
Мы предлагаем: ремонт компьютера москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный психоаналитик дистанционно. Помощь в трудных ситуациях доступна 24/7. Приватно, действенно. Индивидуальный подход. Начните менять жизнь сегодня! https://w-495.ru/
Профессиональный сервисный центр по ремонту фото техники от зеркальных до цифровых фотоаппаратов.
Мы предлагаем: ремонт проекционных экранов
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
straz bet starzbet guncel giris starzbet guncel giris
gates of olympus oyna demo: gate of olympus oyna – gates of olympus demo turkce oyna
http://casibom.auction/# casibom giris
Профессиональный сервисный центр по ремонту компьютерных видеокарт по Москве.
Мы предлагаем: починка видеокарты цена
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный расстановщик в сети. Решение проблем возможна 24/7. Приватно, результативно. Индивидуальный подход. Сделайте шаг к улучшению! https://w-495.ru/
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://permacultureglobal.org/users/60982-stacy-hicks
https://imageevent.com/djanger1982
https://www.hentai-foundry.com/user/legram1986/profile
https://anotepad.com/notes/pj73ni48
https://www.obesityhelp.com/members/jhonsina1974/about_me/
эскорт услуги
casibom guncel giris: casibom 158 giris – casibom 158 giris
starzbet guvenilir mi starz bet giris starzbet
Карманный психотерапевт: помощь всегда под рукой Чат udx
Great job site admin! You have made it look so easy talking about that topic, providing your readers some vital information. I would love to see more helpful articles like this, so please keep posting! I also have great posts about SEO, check out my weblog at https://pb.buhgalteria.ru/bitrix/redirect.php?event1=click_to_call&event2=&event3=&goto=http://article-star.com/eine-effektive-html-sitemap-erstellen
betine betine sikayet betine giris
betine giris: betine guncel giris – betine guncel
http://betine.online/# betine guncel giris
rgbet
RGBET – Lựa Chọn Hàng Đầu Trong Thế Giới Cá Cược Trực Tuyến
Trong bối cảnh ngành công nghiệp cá cược trực tuyến đang phát triển mạnh mẽ, việc tìm kiếm một nhà cái uy tín là điều vô cùng quan trọng đối với những người đam mê cá cược. Giữa vô vàn sự lựa chọn, RGBET đã nhanh chóng nổi lên như một trong những nhà cái hàng đầu, mang đến trải nghiệm cá cược an toàn, công bằng và đầy thú vị cho người chơi.
Trải Nghiệm Cá Cược Toàn Diện
RGBET cung cấp một danh mục trò chơi cá cược đa dạng, từ các môn thể thao, casino trực tuyến, đến các trò chơi slot và nhiều hình thức cá cược khác. Người chơi có thể dễ dàng tìm thấy loại hình cá cược yêu thích với tỷ lệ cược cạnh tranh và giao diện thân thiện, giúp tối ưu trải nghiệm của người dùng.
Dịch Vụ Chăm Sóc Khách Hàng Tận Tình
Một trong những yếu tố giúp RGBET nổi bật là dịch vụ chăm sóc khách hàng chuyên nghiệp và tận tình. Hỗ trợ 24/7, RGBET luôn sẵn sàng giải đáp mọi thắc mắc và xử lý vấn đề của người chơi một cách nhanh chóng. Đội ngũ hỗ trợ được đào tạo bài bản, luôn lắng nghe và hỗ trợ người chơi với sự tận tâm cao nhất.
Tỷ Lệ Cược Cạnh Tranh
RGBET cung cấp tỷ lệ cược hấp dẫn và cạnh tranh, đảm bảo người chơi có cơ hội tối đa hóa lợi nhuận. Dù bạn là người mới bắt đầu hay một tay chơi cá cược kỳ cựu, RGBET đều mang đến cơ hội để bạn gia tăng thắng lợi trong mọi lần đặt cược.
An Toàn và Bảo Mật
Với hệ thống bảo mật hiện đại, RGBET cam kết đảm bảo an toàn cho toàn bộ thông tin cá nhân và tài khoản của người chơi. Hệ thống mã hóa dữ liệu tiên tiến giúp người chơi yên tâm khi thực hiện các giao dịch, từ nạp tiền đến rút tiền.
Nhiều Ưu Đãi Hấp Dẫn
Không chỉ vậy, RGBET còn thường xuyên tung ra các chương trình khuyến mãi hấp dẫn dành cho người chơi mới lẫn người chơi lâu năm. Những ưu đãi này giúp tăng thêm giá trị cho mỗi lần đặt cược và mang đến nhiều cơ hội thắng lớn.
Vì Sao Bạn Nên Chọn RGBET?
Uy tín hàng đầu: RGBET đã khẳng định vị thế của mình trong thế giới cá cược trực tuyến.
Dịch vụ chuyên nghiệp: Chăm sóc khách hàng chu đáo, hỗ trợ kịp thời mọi lúc.
Tỷ lệ cược cạnh tranh: Mang lại cơ hội thắng cao hơn cho người chơi.
Bảo mật tuyệt đối: An toàn cho mọi giao dịch và thông tin cá nhân.
Khuyến mãi đa dạng: Luôn có những ưu đãi tốt nhất cho người chơi.
Tất cả những yếu tố này làm cho RGBET trở thành sự lựa chọn hàng đầu của bất kỳ ai muốn tham gia vào thế giới cá cược trực tuyến. Hãy khám phá và trải nghiệm những điều tuyệt vời mà RGBET mang lại ngay hôm nay!
RGBET – Lựa Chọn Hàng Đầu Trong Thế Giới Cá Cược Trực Tuyến
Trong bối cảnh ngành công nghiệp cá cược trực tuyến đang phát triển mạnh mẽ, việc tìm kiếm một nhà cái uy tín là điều vô cùng quan trọng đối với những người đam mê cá cược. Giữa vô vàn sự lựa chọn, RGBET đã nhanh chóng nổi lên như một trong những nhà cái hàng đầu, mang đến trải nghiệm cá cược an toàn, công bằng và đầy thú vị cho người chơi.
Trải Nghiệm Cá Cược Toàn Diện
RGBET cung cấp một danh mục trò chơi cá cược đa dạng, từ các môn thể thao, casino trực tuyến, đến các trò chơi slot và nhiều hình thức cá cược khác. Người chơi có thể dễ dàng tìm thấy loại hình cá cược yêu thích với tỷ lệ cược cạnh tranh và giao diện thân thiện, giúp tối ưu trải nghiệm của người dùng.
Dịch Vụ Chăm Sóc Khách Hàng Tận Tình
Một trong những yếu tố giúp RGBET nổi bật là dịch vụ chăm sóc khách hàng chuyên nghiệp và tận tình. Hỗ trợ 24/7, RGBET luôn sẵn sàng giải đáp mọi thắc mắc và xử lý vấn đề của người chơi một cách nhanh chóng. Đội ngũ hỗ trợ được đào tạo bài bản, luôn lắng nghe và hỗ trợ người chơi với sự tận tâm cao nhất.
Tỷ Lệ Cược Cạnh Tranh
RGBET cung cấp tỷ lệ cược hấp dẫn và cạnh tranh, đảm bảo người chơi có cơ hội tối đa hóa lợi nhuận. Dù bạn là người mới bắt đầu hay một tay chơi cá cược kỳ cựu, RGBET đều mang đến cơ hội để bạn gia tăng thắng lợi trong mọi lần đặt cược.
An Toàn và Bảo Mật
Với hệ thống bảo mật hiện đại, RGBET cam kết đảm bảo an toàn cho toàn bộ thông tin cá nhân và tài khoản của người chơi. Hệ thống mã hóa dữ liệu tiên tiến giúp người chơi yên tâm khi thực hiện các giao dịch, từ nạp tiền đến rút tiền.
Nhiều Ưu Đãi Hấp Dẫn
Không chỉ vậy, RGBET còn thường xuyên tung ra các chương trình khuyến mãi hấp dẫn dành cho người chơi mới lẫn người chơi lâu năm. Những ưu đãi này giúp tăng thêm giá trị cho mỗi lần đặt cược và mang đến nhiều cơ hội thắng lớn.
Vì Sao Bạn Nên Chọn RGBET?
Uy tín hàng đầu: RGBET đã khẳng định vị thế của mình trong thế giới cá cược trực tuyến.
Dịch vụ chuyên nghiệp: Chăm sóc khách hàng chu đáo, hỗ trợ kịp thời mọi lúc.
Tỷ lệ cược cạnh tranh: Mang lại cơ hội thắng cao hơn cho người chơi.
Bảo mật tuyệt đối: An toàn cho mọi giao dịch và thông tin cá nhân.
Khuyến mãi đa dạng: Luôn có những ưu đãi tốt nhất cho người chơi.
Tất cả những yếu tố này làm cho RGBET trở thành sự lựa chọn hàng đầu của bất kỳ ai muốn tham gia vào thế giới cá cược trực tuyến. Hãy khám phá và trải nghiệm những điều tuyệt vời mà RGBET mang lại ngay hôm nay!
casibom giris: casibom giris – casibom giris adresi
https://casibom.auction/# casibom giris
casibom guncel giris: casibom – casibom giris
starzbet guncel giris starz bet giris straz bet
перевод с иностранных языков
https://casibom.auction/# casibom giris
starzbet guncel giris starzbet guncel giris starz bet giris
kometa войти
Kometa Casino: Лучший Шанс для Цифровых Развлечений
В сфере виртуальных казино Казино Kometa обрело популярность благодаря множеству слотов, выгодным поощрениям и отличному сервису. Эта сайт захватывает внимание игроков во всех странах своими исключительными возможностями и регулярными акциями. В этой статье мы обсудим, почему Казино Kometa считается высоко оцениваемой игровых платформ.
Преимущества Казино Kometa
Главной чертой, делающих особенным Kometa, является внимание на интересы пользователей. Платформа предоставляет свыше тысячи развлечений, где любой сможет выбрать игру. Это могут быть привычные слоты, так и инновационные развлечения с новаторскими возможностями. Плюсом является то, что Казино Kometa предлагает 24/7 помощь клиентов, обеспечивая удобное и защищенное окружение.
Ключевые особенности Казино Kometa:
Год начала работы: 2024
Лицензия: Curacao
Выбор игр: Свыше тысячи
Помощь: Постоянная онлайн-чат и электронная почта
Мобильный доступ: Да
Способы оплаты: Visa
Безопасность: Шифрование SSL
Стартовые поощрения
Одной из главных особенностей Казино Kometa считаются щедрые стартовые предложения для новичков. После создания аккаунта новички имеют право на к уникальным акциям, чем могут начать игру с меньшими затратами. Эти поощрения предоставляют выгодные шансы для начинающих, давая возможность улучшить свои успех с самого первого захода.
Огромный выбор игр
Казино Kometa гарантирует большое количество развлечений на все предпочтения. Игроки могут наслаждаться привычными автоматами, развлечениями на столах, а также реальными дилерами. Благодаря передовым технологиям визуального сопровождения и звуковому сопровождению, все может глубоко войти в игровой процесс.
Постоянные события и турниры
Для всех пользователей система часто предлагает акции и соревнования с ценными призами. Турниры проводятся каждый месяц, что делает игровой опыт увлекательным и захватывающим. Это создает условия клиентам не только получать удовольствие от игрой, но и зарабатывать призы и награды.
Почему стоит выбрать
Казино Kometa — это идеальное сочетание разнообразных игр, надежного сервиса и безопасной игровой среды. Платформа выделяется своим заботой о клиентах и желанием модернизировать игровой опыт. Независимо от уровня, каждый найдет в Kometa развлечение, которое сделает его пребывание на сайте захватывающим и удобным.
Становитесь частью Казино Kometa и наслаждайтесь яркими эмоциями и увлекательными слотами каждый день!
betine promosyon kodu 2024: betine – betine giris
https://gatesofolympusoyna.online/# gates of olympus demo turkce
https://hdorg2.ru/article?2024-10-5-news-kjwlm-video-apbpxe.html
https://hdorg2.ru/article?2024-09-9-news-dsegk-charis-qwttxg.html
https://hdorg2.ru/article?2024-10-14-news-mrqbu-video-tynnug.html
https://hdorg2.ru/article?2024-10-23-news-uqrar-usa-ogabir.html
https://hdorg2.ru/article?2024-10-2-news-lcvxx-charis-jhwczl.html
https://hdorg2.ru/article?2024-09-3-news-fkesz-trump-wztdcg.html
https://hdorg2.ru/article?2024-10-16-news-ezhxp-usa-vynohz.html
https://hdorg2.ru/article?2024-11-16-news-tyewa-charis-soyhdh.html
https://hdorg2.ru/article?2024-09-11-news-aiyrw-usa-gwktce.html
https://hdorg2.ru/article?2024-09-14-news-gfejl-trump-qqvflp.html
https://hdorg2.ru/article?2024-11-2-news-rdnbg-trump-nypwcw.html
апостиль в новосибирске
casibom giris adresi casibom guncel giris adresi or casibom guncel
https://clients1.google.gr/url?sa=t&url=https://casibom.auction casibom giris adresi
casibom guncel giris adresi casibom 158 giris and casibom 158 giris casibom giris adresi
Если кто ищет место, где можно выгодно купить раковины и ванны, рекомендую один интернет-магазин, который недавно открыл для себя. Они предлагают большой выбор сантехники и аксессуаров для ванной комнаты. Ассортимент включает различные модели, так что можно подобрать под любой стиль и размер помещения.
Мне нужно было раковина в ванну , и они предложили несколько отличных вариантов. Цены приятно удивили, а качество товаров на высшем уровне. Также понравилось, что они предлагают услуги профессиональной установки. Доставка была быстрой, и всё прошло гладко. Теперь моя ванная комната выглядит просто великолепно!
https://hdorg2.ru/article?2024-10-7-news-qsbuu-biden-snjoxq.html
https://hdorg2.ru/article?2024-10-27-news-destv-usa-tkvdtb.html
https://hdorg2.ru/article?2024-09-3-news-dkmtd-charis-onbepp.html
https://hdorg2.ru/article?2024-11-23-news-czpex-biden-csktjz.html
https://hdorg2.ru/article?2024-10-22-news-zkzzt-usa-ydywvj.html
https://hdorg2.ru/article?2024-09-7-news-sshkh-video-rltynx.html
https://hdorg2.ru/article?2024-11-6-news-npkpk-trump-lcgvrq.html
https://hdorg2.ru/article?2024-11-19-news-xhsfk-trump-hlnpnp.html
https://hdorg2.ru/article?2024-10-18-news-xdsjk-video-pqoxgk.html
https://hdorg2.ru/article?2024-09-13-news-dhiyi-charis-weexxa.html
https://hdorg2.ru/article?2024-11-13-news-altdm-usa-avbunx.html
casibom: casibom guncel – casibom 158 giris
https://casibom.auction/# casibom guncel
Профессиональный сервисный центр по ремонту компьютерных блоков питания в Москве.
Мы предлагаем: ремонт блоков питания москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
<a href=”https://remont-kondicionerov-wik.ru”>ремонт кондиционеров с гарантией</a>
betine com guncel giris betine promosyon kodu 2024 or betine giris
http://www.ww.symbo.ru/nz?rid=94006&link=https://betine.online/ betine sikayet
betine promosyon kodu 2024 betine giris and betine com guncel giris betine promosyon kodu 2024
сервис профи самара
gate of olympus oyna: gates of olympus demo oyna – gate of olympus oyna
gates of olympus oyna gates of olympus turkce Gates of Olympus
https://xklrev-xubdmi.hdorg2.ru/
chb 121416765997419 hxtke
??Увеличьте производительность своего проекта с VPS/VDS !??
??Почему стоит выбрать нас???
??Высокое качество сервиса без высокой цены! Наши тарифы начинаются от 2,3 руб/день, что делает аренду VPS/VDS доступной для любого бюджета.
??Мощные конфигурации! Выберите тариф от 1 до 4 ядер и от 1 до 8 ГБ оперативной памяти, чтобы ваш проект работал быстро и стабильно.
??Обширное хранилище! Получите от 10 до 150 ГБ пространства для хранения, чтобы размещать все необходимые данные.
??Безлимитный трафик! Наслаждайтесь неограниченным объемом трафика до 32 ТБ, чтобы ваши посетители могли наслаждаться быстрой загрузкой контента.
??Высокая скорость подключения! Наш VPS/VDS предлагает скорость подключения к сети 1 Гбит/с, чтобы ваш проект работал плавно и без задержек.
??Простота управления! Легко управляйте своим VPS/VDS через удобную панель управления, доступную 24/7.
??Безопасность и надежность! Наши серверы защищены надежными системами безопасности, чтобы гарантировать сохранность ваших данных.
??Техническая поддержка! Наша дружелюбная команда поддержки готова помочь вам в любое время, чтобы решить любые проблемы, которые могут возникнуть.
??Не упустите возможность получить надежный и производительный VPS/VDS по выгодной цене!??
??Закажите свой тариф прямо сейчас и получите бесплатный период тестирования!??
Жмите?? КЛИК ??
gates of olympus slot: gates of olympus turkce – Gates of Olympus
http://starzbet.shop/# starzbet guncel giris
https://betine.online/# betine
gates of olympus demo gates of olympus slot or gate of olympus oyna
http://www.thomas-neuss.de/url?q=https://gatesofolympusoyna.online gates of olympus oyna demo
gates of olympus demo oyna gates of olympus demo and gates of olympus oyna demo gates of olympus demo oyna
https://qzidcw-mhkcrb.hdorg2.ru/
betine promosyon kodu 2024: betine giris – betine sikayet
betine promosyon kodu betine sikayet betine com guncel giris
casibom guncel giris: casibom giris adresi – casibom
https://casibom.auction/# casibom giris adresi
Анват сериалы https://bit.ly/anwap-m-anwap-anwap-love-an-wap 261527162
starzbet guncel giris: starz bet giris – starzbet guncel giris
http://casibom.auction/# casibom guncel giris adresi
starzbet: starzbet guncel giris – starzbet giris
betine sikayet betine com guncel giris betine promosyon kodu
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://okwave.jp/profile/u3121953.html
https://imageevent.com/valekcyc1971
https://tubeteencam.com/user/ellamina1954/profile
https://www.hentai-foundry.com/user/ren1o1995/profile
http://www.babelcube.com/user/rebecca-davidson
gates of olympus demo gates of olympus demo turkce oyna gates of olympus oyna demo
casibom guncel giris: casibom giris – casibom 158 giris
https://casibom.auction/# casibom
Профессиональный сервисный центр по ремонту компьютероной техники в Москве.
Мы предлагаем: центр ремонта компьютеров
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
апостиль в новосибирске
http://betine.online/# betine promosyon kodu
casibom giris adresi casibom 158 giris or casibom guncel giris adresi
https://maps.google.mw/url?q=https://casibom.auction casibom 158 giris
casibom casibom and casibom giris casibom guncel giris
betine promosyon kodu betine or betine promosyon kodu 2024
https://wakeuplaughing.com/phpinfo.php?a%5B%5D= betine guncel
[url=https://www.google.com.au/url?sa=t&url=https://betine.online]betine guncel giris[/url] betine promosyon kodu 2024 and [url=http://forum.orangepi.org/home.php?mod=space&uid=4680184]betine promosyon kodu[/url] betine sikayet
casibom guncel giris: casibom guncel – casibom guncel giris
http://starzbet.shop/# starz bet giris
перевод с иностранных языков
betine guncel: betine guncel giris – betine com guncel giris
casibom guncel giris adresi casibom giris adresi casibom guncel
kometa войти
Онлайн-казино Kometa: Идеальный Вариант для Цифровых Игр
В мире онлайн-казино Казино Kometa завоевало известность благодаря разнообразию игр, привлекательным поощрениям и отличному сервису. Эта система удерживает интерес игроков во всех странах своими уникальными предложениями и частыми акциями. В данной статье мы рассмотрим, почему Kometa Casino называется выдающейся игровых платформ.
Достоинства Kometa
Главной чертой, выделяющих Казино Kometa, является ориентация на потребности игроков. Система предлагает более 1000 игр, где все откроет любимое развлечение. Это предлагает привычные игры, так и новые игры с инновационными опциями. Приятным дополнением является то, что Kometa обеспечивает постоянную помощь пользователей, обеспечивая комфортное и надежное окружение.
Ключевые особенности Казино Kometa:
Дата запуска: 2024
Разрешение: Curacao
Количество игр: Более 1000
Помощь: Круглосуточная онлайн-чат и электронная почта
Мобильная версия: Имеется
Варианты платежей: Mastercard
Защита: Шифрование SSL
Приветственные бонусы
Одним из главных плюсов Казино Kometa признаны выгодные стартовые предложения для новичков. После входа на сайт пользователи получают доступ к эксклюзивным бонусам, что дает возможность стартовать с меньшими затратами. Эти поощрения предоставляют благоприятные условия для начинающих, предоставляя шанс повысить свои шансы на победу с самого первого захода.
Огромный выбор игр
Kometa Casino предоставляет большое количество игр на любой вкус. Клиенты могут играть традиционными играми, развлечениями на столах, а также играми с живыми дилерами. Благодаря отличной графике визуальных эффектов и звуковому сопровождению, любой пользователь может максимально вникнуть в игровой процесс.
Регулярные акции и мероприятия
Для каждого клиента платформа часто организует акции и соревнования с бонусами. Турниры проводятся регулярно, что делает развлечения более захватывающим и захватывающим. Это позволяет клиентам не только получать удовольствие от развлечениями, но и получать дополнительные бонусы и выигрыши.
Почему Kometa Casino?
Kometa Casino — это оптимальное объединение широкого ассортимента, качественного обслуживания и надежной системы. Система отличается своим вниманием к пользователям и постоянным стремлением улучшать опыт пользователей. Независимо от уровня, все откроет в Казино Kometa нечто, что позволит его путешествие в мире игр увлекательным и удобным.
Присоединяйтесь к Kometa Casino и играйте с удовольствием адреналином и интересными развлечениями ежедневно!
Анват кино https://bit.ly/anwap-m-anwap-anwap-love-an-wap 24242415
straz bet starz bet giris or starzbet giris
https://asia.google.com/url?sa=t&url=https://starzbet.shop starzbet giris
starzbet guncel giris starzbet giris and straz bet starz bet giris
casibom 158 giris casibom giris casibom giris
gates of olympus demo turkce gates of olympus oyna or gates of olympus demo turkce
https://slighdesign.com/?URL=https://gatesofolympusoyna.online gate of olympus oyna
gates of olympus turkce gates of olympus oyna demo and gates of olympus demo turkce gates of olympus oyna
Anwap кино https://bit.ly/anwap-m-anwap-anwap-love-an-wap 66211623
апостиль в новосибирске
casibom: casibom giris – casibom giris adresi
https://casibom.auction/# casibom guncel giris
Профессиональный сервисный центр по ремонту камер видео наблюдения по Москве.
Мы предлагаем: ремонт систем видеонаблюдения москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: сервис центры бытовой техники нижний новгород
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
gates of olympus turkce: gates of olympus demo turkce oyna – gates of olympus oyna
https://starzbet.shop/# straz bet
Предприятие «Кинематика»: Надежные продукты для производственной балансировки и диагностики промышленного оборудования
Организация «Кинематика» занимается на разработке и изготовлении высококачественных систем для сбалансированности и контроля устройств. Товары компании используется для поддержки и ремонта разнообразных агрегатов, включая вентиляционные системы, дробилки, карданные валы, передаточные механизмы и станки для металла.
Основополагающие изделия компании:
1. Арбаланс — мобильный прибор балансировки вибраций
Прибор подходит для динамической балансировки техники в комплектации в штатных подшипниках. Arbalance даёт высокое качество балансировки с минимальными издержками. Главные достоинства:
Автоматическая система вычислений: не требует участия оператора.
Графики вибрации и спектральный анализ: помогают выявлять дефекты.
Два канала измерения: осуществляет замеры вибрационные колебания сразу в двух измерениях, повышая производительность процесса.
Цена: 84000 рублей
2. Модуль Балком-1А — переносной виброметр-балансировщик
Прибор Балком-1А предназначен для балансировки роторов в родных подшипниках. Характерной особенностью является удобство эксплуатации и программа расчета. Прибор также поддерживает:
Два канала измерения вибрации.
Спектральная диагностика для контроля состояния механизмов.
Цена: 73 тыс. руб.
3. Балансировочная система Балком-2 — измерительная система для систем балансировки
Модуль Балком-2 внедряется в измерительных станциях для измерения вибраций. Он предлагает точность точного уравновешивания систем и других механизмов в индустрии.
Цена: от 90000 руб. до 95 000 руб. в зависимости от комплектации.
4. Balcom-4 — для балансировки в нескольких плоскостях составных валов
Прибор Балком-4 предназначен для сбалансированности карданных систем и используется в системах производства. Этот устройство даёт высокую точность и прецизионность при уравновешивании в разных направлениях.
Цена: от 100000 руб. до 126000 руб..
Новейшие технологии мониторинга и технической диагностики
Фирма «Кинематика» также выпускает системы оборудования для непрерывной диагностики техники и механизмов. Эти комплексы применяют бесконтактными сенсорами для оценки колебаний и прочих параметров. Например, модуль Реконт обеспечивает мониторинг редукторы по параметрам точности и оборотам.
http://starzbet.shop/# starzbet guncel giris
апостиль в новосибирске
балансировка промышленных вентиляторов
Предприятие «Кинематика»: Качественные разработки для индустриальной балансирования и проверки техники
Предприятие «Кинематика» фокусируется на изготовлении и внедрении высококачественных инструментов для сбалансированности и диагностики техники. Изделия фирмы используется для поддержки и налаживания разнообразных техномеханизмов, включая вентиляционные системы, измельчители, валовые системы, системы передачи и металлорежущие станки.
Основополагающие решения предприятия:
1. Arbalance — мобильный балансировочный виброметр
Система подходит для балансировки вращательных систем оборудования в заводской сборке в собственных узлах. Прибор Арбаланс даёт надёжную точность производственных процессов с минимальными тратами. Среди его ключевых преимуществ:
Автоматическая система вычислений: не требует участия оператора.
Спектральная диагностика: выявляют проблемы.
Двойной канал измерения: обеспечивает измерение вибрации одновременно в двух осях, повышая производительность процесса.
Цена: 84 000 руб.
2. Балансировочный прибор Балком-1А — переносной виброметр-балансировщик
Балком-1А разработан для уравновешивания роторных систем в собственных подшипниках. Главной особенностью является удобство эксплуатации и автоматическая система вычислений. Прибор также поддерживает:
Два вибрационных канала.
Анализ спектра вибраций для контроля состояния механизмов.
Цена: 73000 рублей
3. Модуль Балком-2 — измерительная станция для измерительных станков
Балком-2 эксплуатируется в станках для измерения колебаний. Он даёт эффективность высокоточной балансировки систем и других механизмов в технике.
Цена: от 90000 рублей до 95000 руб. в зависимости от устройства.
4. Система Балком-4 — для многоплоскостной балансировки карданных валов
Устройство Балком-4 применяется для балансировки карданов и используется в технических комплексах. Этот система гарантирует качественные результаты и прецизионность при уравновешивании в разных направлениях.
Цена: от 100000 руб. до 126000 руб..
Современные технологии диагностики и технической диагностики
Фирма «Кинематика» также проектирует системы оборудования для беспрерывного отслеживания технического состояния устройств. Эти приборы работают на бесконтактные системы для отслеживания вибраций и других показателей. Например, устройство Реконт даёт возможность контролировать техническое состояние редукторов по параметрам точности и оборотам.
comprar viagra online en andorra: viagra generico – se puede comprar sildenafil sin receta
http://sildenafilo.men/# viagra precio 2022
farmacia en casa online descuento: farmacia online envio gratis valencia – farmacia online madrid
перевод с иностранных языков
comprar viagra en espaГ±a envio urgente comprar viagra contrareembolso 48 horas viagra online cerca de zaragoza
farmacia online madrid: Cialis precio – farmacia online madrid
https://tadalafilo.bid/# farmacias online seguras en espaГ±a
перевод с иностранных языков
farmacia barata farmacias online baratas or <a href=" http://www.e-anim.com/test/E_GuestBook.asp?a=buy+teva+generic+viagra “>farmacia online barata
https://maps.google.tn/url?sa=t&url=https://farmaciaeu.com farmacias online seguras en espaГ±a
farmacia online envГo gratis farmacia online barcelona and farmacias online seguras farmacia online espaГ±a envГo internacional
Anwap сериалы https://bit.ly/anwap-m-anwap-anwap-love-an-wap 320161023
farmacia online envГo gratis п»їfarmacia online espaГ±a or farmacia online espaГ±a envГo internacional
http://sfmission.com/rss/feed2js.php?src=http://farmaciaeu.com farmacia online 24 horas
farmacia barata farmacias online baratas and farmacias online seguras farmacias online seguras en espaГ±a
подъем дома
sildenafilo 50 mg precio sin receta: comprar viagra – se puede comprar sildenafil sin receta
315221027 https://anwap2.yxoo.ru/
farmacia en casa online descuento tadalafilo п»їfarmacia online espaГ±a
Профессиональный сервисный центр по ремонту кнаручных часов от советских до швейцарских в Москве.
Мы предлагаем: срочный ремонт часов
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
2717142211 https://anwap2.yxoo.ru/
Если вы искали где отремонтировать сломаную технику, обратите внимание – тех профи
http://farmaciaeu.com/# farmacia online madrid
farmacias online seguras en espaГ±a: farmacia online internacional – farmacia online barcelona
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт крупногабаритной техники в перми
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
подъем дома
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.hentai-foundry.com/user/raven21199319751954/profile
https://www.haikudeck.com/presentations/A9ivlFkeEL
https://cannabis.net/user/155041
https://rentry.org/d6oqoueu
https://anotepad.com/notes/jd25425k
ремонт кондиционеров
http://farmaciaeu.com/# farmacias online seguras
farmacia online envГo gratis
подъем дома
farmacia online madrid: farmacia online envio gratis murcia – farmacias online seguras en espaГ±a
http://farmaciaeu.com/# п»їfarmacia online espaГ±a
farmacia online barata: Cialis sin receta – farmacia online madrid
farmacia online barata farmacia online envГo gratis or farmacia online barcelona
http://nsreg.com/?a=how+can+i+buy+viagra farmacias online seguras
farmacia online madrid farmacias direct and farmacia online envГo gratis farmacia barata
подъем дома
Профессиональный сервисный центр по ремонту парогенераторов в Москве.
Мы предлагаем: ремонт парогенератора
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://sildenafilo.men/# viagra online cerca de la coruГ±a
п»їfarmacia online espaГ±a: farmacia online envio gratis murcia – farmacia online espaГ±a envГo internacional
farmacias online seguras en espaГ±a farmacia online envГo gratis or farmacias online seguras en espaГ±a
https://neopvc.com/proxy.php?link=https://farmaciaeu.com farmacia online madrid
farmacias online baratas farmacia online barcelona and farmacias online seguras en espaГ±a farmacia online espaГ±a envГo internacional
подъем дома
http://tadalafilo.bid/# farmacia online envГo gratis
farmacia en casa online descuento
farmacias online baratas farmacias direct or п»їfarmacia online espaГ±a
https://images.google.fi/url?sa=t&url=https://farmaciaeu.com п»їfarmacia online espaГ±a
=cialis+online]farmacias online seguras en espaГ±a farmacia online barata and farmacia online barcelona farmacia online barata
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт цифровой техники волгоград
farmacias online seguras: Precio Cialis 20 Mg – farmacia online madrid
https://oookin.ru/comb.html
https://oookin.ru/balkombs.html
https://oookin.ru/balkom.htm
https://oookin.ru/balkom.htm
https://oookin.ru/balance_kardan.htm
Фирма «Кинематика»: Надежные продукты для динамической уравновешивания и технической диагностики промышленного оборудования
Предприятие «Кинематика» фокусируется на создании и внедрении высококачественных приборов для уравновешивания и контроля машин. Продукты предприятия применяется для ремонта и техобслуживания различных техномеханизмов, включая вентиляционные системы, измельчители, валовые системы, передаточные механизмы и металлообрабатывающие станки.
Главные решения предприятия:
1. Модуль Арбаланс — переносной балансировочный виброметр
Прибор подходит для балансировки вращательных систем агрегатов в вместе с подшипниками в штатных подшипниках. Прибор Арбаланс обеспечивает высокое качество уравновешивания с уменьшенными расходами. Среди его ключевых преимуществ:
Система автоматизированного анализа: полностью автоматизирована.
Графики вибрации и спектральный анализ: находят неполадки.
Двухканальная система: позволяет измерять вибрации параллельно в двух осях, увеличивая производительность.
Цена: 84000 рублей
2. Balcom-1A — портативный вибродиагностический прибор
Модуль Балком-1А создан для балансировки валов в собственных подшипниках. Характерной особенностью является простота эксплуатации и автоматическая система вычислений. Прибор также поддерживает:
Два вибрационных канала.
Спектральный анализ для диагностики состояния оборудования.
Цена: 73000 рублей
3. Балансировочная система Балком-2 — прибор для измерений для станций балансировки
Устройство Балком-2 эксплуатируется в станках для измерения вибраций. Он гарантирует надежность точного уравновешивания вращательных механизмов и другого оборудования в индустрии.
Цена: от 90 тыс. руб. до 95 000 руб. в зависимости от оборудования.
4. Модуль Балком-4 — для балансировки в различных плоскостях карданных валов
Балком-4 предназначен для балансировки карданов и внедряется в промышленных системах. Этот система обеспечивает качественные результаты и высокую точность при уравновешивании в разных направлениях.
Цена: от 100 тыс. руб. до 126000 руб..
Современные технологии контроля и анализа
ООО «Кинематика» также проектирует устройства для непрерывной диагностики состояния оборудования. Эти системы оснащены индуктивные датчики для виброизмерений и прочих параметров. Например, Реконт позволяет отслеживать механизмы редукторов по точности движения и вращательным движениям.
подъем дома
https://tadalafilo.bid/# farmacia online madrid
comprar viagra en espaГ±a envio urgente contrareembolso comprar viagra sin gastos de envГo or se puede comprar viagra sin receta
http://cse.google.com.br/url?q=https://sildenafilo.men sildenafilo precio farmacia
viagra online gibraltar sildenafilo 50 mg precio sin receta and sildenafilo sandoz 100 mg precio viagra online cerca de malaga
farmacias online seguras: Tadalafilo precio – farmacia online espaГ±a envГo internacional
рюкзак школьный подростковый женский
farmacia online senza ricetta: Cialis generico 20 mg 8 compresse prezzo – acquisto farmaci con ricetta
where to buy balloons helium balloons with cheap delivery
Farmacia online miglior prezzo: Cialis generico recensioni – acquistare farmaci senza ricetta
Казино Kometa: Лучший Вариант для Цифровых Азартных игр
В сфере виртуальных казино Казино Kometa завоевало популярность благодаря разнообразию развлечений, выгодным бонусам и высококачественному поддержке. Эта сайт удерживает внимание пользователей по всему миру своими исключительными акциями и регулярными промо. В этой обзоре мы обсудим, почему Kometa Casino является высоко оцениваемой игровых платформ.
Достоинства Казино Kometa
Одним из ключевых факторов, выделяющих Kometa, является фокус на удовольствие игроков. Сайт предоставляет более 1000 слотов, где каждый найдет что-то по душе. Это включает привычные автоматы, так и современные варианты с новаторскими функциями. Приятным дополнением является то, что Kometa гарантирует круглосуточную поддержку клиентов, создавая комфортное и защищенное игровое пространство.
Ключевые особенности Kometa Casino:
Дата запуска: 2024
Разрешение: Curacao
Выбор игр: Огромное количество
Техподдержка: 24/7 чат и почта
Мобильный доступ: Имеется
Методы оплаты: Mastercard
Безопасность: Защита данных
Приветственные бонусы
Одним из главных плюсов Казино Kometa признаны привлекательные акции для новичков для начинающих пользователей. Сразу после регистрации новички имеют право на к эксклюзивным акциям, чем могут начать игру с минимальными рисками. Эти поощрения гарантируют выгодные шансы для новичков, предоставляя шанс улучшить свои шансы на победу с самого начала.
Большое количество развлечений
Казино Kometa гарантирует большое количество слотов на любые интересы. Клиенты могут играть классическими слотами, играми за столом, а также реальными дилерами. Благодаря отличной графике визуальных эффектов и звуковому сопровождению, каждый игрок может полностью погрузиться в игровой процесс.
Регулярные акции и мероприятия
Для игроков платформа регулярно проводит турниры и конкурсы с выгодными наградами. Акции организуются ежемесячно, придавая игровой процесс интересным и захватывающим. Это позволяет пользователям не только наслаждаться развлечениями, но и выигрывать дополнительные бонусы и призы.
Почему стоит выбрать
Казино Kometa — это оптимальное объединение разнообразных игр, отличной поддержки и надежной системы. Сайт отличается своим заботой о клиентах и желанием совершенствовать игровой опыт. Неважно, новичок или профи, все сможет выбрать в Казино Kometa развлечение, которое сделает его путешествие в мире игр захватывающим и приятным.
Вступайте в Kometa и играйте с удовольствием яркими эмоциями и увлекательными слотами всегда!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт крупногабаритной техники в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://farmaciait.men/# farmaci senza ricetta elenco
farmacie online autorizzate elenco
top farmacia online Cialis generico farmacia farmacie online sicure
рюкзак женская текстильная
Farmacie on line spedizione gratuita Farmacie online sicure Farmacia online piГ№ conveniente
miglior sito per comprare viagra online: gel per erezione in farmacia – viagra pfizer 25mg prezzo
Farmacie on line spedizione gratuita: Cialis generico recensioni – Farmacie online sicure
We provide the best personalized Eiffel Tower tours with experienced, local and 5-star rated guides. Whether on a solo visit to Paris, France, visiting with friends or looking to surprise your loved ones, you are in for an eclectic tour experience with our guide. Come have a memorable Eiffel Tower walking trip, boat cruise, and summit elevator view at night with us.
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт бытовой техники в красноярске
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
рюкзак спортивный
P 異世界魔王と召喚少女の奴隷魔術
k8 カジノ パチンコ
美しいグラフィックと豪華な演出が魅力。神々の力を感じます。
北斗の拳-ボクシング王
https://www.wk8.jp/tags/%E3%83%A9%E3%82%A4%E3%83%96%E3%82%B5%E3%83%9D%E3%83%BC%E3%83%88
パチンコは、運だけではなく、選択や判断力も必要です。プレイヤーの個性が出ます。
ジャンキージャグラー
k8 カジノ パチンコ P北斗の拳9 闘神パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
夜蝶飛翔
鉄拳R 2004
バジリスク~甲賀忍法帖~絆2 天膳 BLACK EDITION
viagra online spedizione gratuita: viagra senza prescrizione – pillole per erezione immediata
Farmacia online piГ№ conveniente farmacia online migliore farmacia online
alternativa al viagra senza ricetta in farmacia viagra generico miglior sito per comprare viagra online
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://cannabis.net/user/155188
https://okwave.jp/profile/u3133944.html
https://distrofik1982.diary.ru/
https://imageevent.com/almina1979
https://www.metal-archives.com/users/option1992
https://sildenafilit.pro/# pillole per erezione immediata
farmacie online sicure
рюкзак для путешествий ручная кладь
viagra subito: cerco viagra a buon prezzo – pillole per erezione in farmacia senza ricetta
farmaci senza ricetta elenco Farmacia online miglior prezzo or Farmacia online miglior prezzo
http://www.potthof-engelskirchen.de/out.php?link=http://tadalafilit.com Farmacia online piГ№ conveniente
Farmacie on line spedizione gratuita farmacia online piГ№ conveniente and migliori farmacie online 2024 Farmacia online piГ№ conveniente
Если вы искали где отремонтировать сломаную технику, обратите внимание – профи ремонт
le migliori pillole per l’erezione: viagra senza ricetta – esiste il viagra generico in farmacia
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: сервис центры бытовой техники красноярск
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
farmacie online sicure Farmacia online piГ№ conveniente top farmacia online
farmacie online autorizzate elenco Farmacie che vendono Cialis senza ricetta farmaci senza ricetta elenco
acquistare farmaci senza ricetta: Farmacie che vendono Cialis senza ricetta – farmacie online sicure
Farmacie on line spedizione gratuita: Cialis generico 20 mg 8 compresse prezzo – Farmacia online miglior prezzo
Thermoplastic Elastomer (TPE) Pipes in Iraq At ElitePipe Factory, our Thermoplastic Elastomer (TPE) Pipes stand out for their remarkable versatility and performance. These pipes are engineered from advanced materials to offer exceptional flexibility, impact resistance, and chemical compatibility, making them ideal for a wide range of industrial and commercial applications. As one of Iraq’s top manufacturers, ElitePipe Factory is committed to delivering TPE Pipes that adhere to stringent quality standards, ensuring durability and reliability in every project. To learn more about our Thermoplastic Elastomer Pipes, visit our website at elitepipeiraq.com. Trust in ElitePipe Factory for the best solutions in the industry. elitepipeiraq.com.
https://farmaciait.men/# Farmacie on line spedizione gratuita
farmacia online
п»їFarmacia online migliore farmacie online affidabili or farmacie online affidabili
http://www.google.so/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ccsqfjaa&url=https://farmaciait.men farmacia online piГ№ conveniente
top farmacia online farmacie online sicure and acquistare farmaci senza ricetta farmacie online affidabili
Farmacia online piГ№ conveniente Tadalafil generico migliore Farmacie online sicure
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:сервисные центры по ремонту техники в ростове на дону
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
farmacie online affidabili: Brufen antinfiammatorio – Farmacia online piГ№ conveniente
JILI SLOT GAMES: Sự Lựa Chọn Hàng Đầu Cho Các Tín Đồ Casino Trực Tuyến
JILI Casino là một nhà phát hành game nổi tiếng với nhiều năm kinh nghiệm trong ngành công nghiệp giải trí trực tuyến. Tại JILI, chúng tôi cam kết mang đến cho người chơi những trải nghiệm độc đáo và đẳng cấp, thông qua việc đổi mới không ngừng và cải thiện chất lượng từng sản phẩm. Những giá trị cốt lõi của chúng tôi không chỉ dừng lại ở việc tạo ra các trò chơi xuất sắc, mà còn tập trung vào việc cung cấp các tính năng vượt trội để đáp ứng nhu cầu của người chơi trên toàn cầu.
Sự Đa Dạng Trong Các Trò Chơi Slot
JILI nổi tiếng với loạt trò chơi slot đa dạng và hấp dẫn. Từ các slot game cổ điển đến những trò chơi với giao diện hiện đại và tính năng độc đáo, JILI Slot luôn đem đến cho người chơi những phút giây giải trí tuyệt vời. Các trò chơi được thiết kế với đồ họa sống động, âm thanh chân thực và những vòng quay thú vị, đảm bảo rằng người chơi sẽ luôn bị cuốn hút.
Ưu Điểm Nổi Bật Của JILI Casino
Đổi mới và sáng tạo: Mỗi trò chơi tại JILI Casino đều mang đến sự mới mẻ với lối chơi hấp dẫn và giao diện bắt mắt.
Chất lượng cao: JILI không ngừng cải tiến để đảm bảo mỗi sản phẩm đều đạt chất lượng tốt nhất, từ trải nghiệm người chơi đến tính năng trò chơi.
Nền tảng đa dạng: JILI Casino cung cấp nhiều loại game khác nhau, từ slot, bắn cá đến các trò chơi truyền thống, phù hợp với mọi sở thích của người chơi.
Chương Trình Khuyến Mại JILI
JILI Casino không chỉ nổi bật với chất lượng game mà còn thu hút người chơi bởi các chương trình khuyến mại hấp dẫn. Người chơi có thể tham gia vào nhiều sự kiện, từ khuyến mãi nạp tiền, hoàn trả đến các chương trình tri ân dành riêng cho thành viên VIP. Những ưu đãi này không chỉ tăng cơ hội chiến thắng mà còn mang lại giá trị cộng thêm cho người chơi.
Nổ Hủ City Và Các Trò Chơi Hấp Dẫn Khác
JILI không chỉ có slot games mà còn cung cấp nhiều thể loại game đa dạng khác như bắn cá, bài và nhiều trò chơi giải trí khác. Nổi bật trong số đó là Nổ Hủ City – nơi người chơi có thể thử vận may và giành được những giải thưởng lớn. Sự kết hợp giữa lối chơi dễ hiểu và các tính năng độc đáo của Nổ Hủ City chắc chắn sẽ mang lại những khoảnh khắc giải trí đầy thú vị.
Tham Gia JILI Casino Ngay Hôm Nay
Với sự đa dạng về trò chơi, các tính năng vượt trội và những chương trình khuyến mại hấp dẫn, JILI Casino là sự lựa chọn không thể bỏ qua cho những ai yêu thích trò chơi trực tuyến. Hãy truy cập trang web chính thức của JILI ngay hôm nay để trải nghiệm thế giới giải trí không giới hạn và giành lấy những phần thưởng hấp dẫn từ các trò chơi của chúng tôi!
farmacia online Brufen 600 senza ricetta comprare farmaci online con ricetta
viagra cosa serve dove acquistare viagra in modo sicuro or miglior sito per comprare viagra online
https://cse.google.co.im/url?q=https://sildenafilit.pro viagra online spedizione gratuita
viagra originale recensioni miglior sito dove acquistare viagra and viagra subito viagra online spedizione gratuita
comprare farmaci online con ricetta: BRUFEN 600 mg 30 compresse prezzo – comprare farmaci online all’estero
機動戦士ガンダムユニコーン
k8パチンコ
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
北斗の拳-ボクシング王
https://k88x.com/tags/%E3%82%B9%E3%83%88%E3%83%BC%E3%83%AA%E3%83%BC
シンプルなルールで、誰でもすぐに楽しめるのが嬉しいです。
CRヱヴァンゲリヲン10
k8パチンコ
CRルパン三世Lupin The End
新鬼武者 再臨
サイボーグ 009
Farmacia online piГ№ conveniente Farmacie on line spedizione gratuita or farmacie online autorizzate elenco
http://www3.city.shimanto.lg.jp/syouhi/mt/mt4i.cgi?id=3&mode=redirect&no=36&ref_eid=176&url=http://tadalafilit.com migliori farmacie online 2024
[url=https://smootheat.com/contact/report?url=https://tadalafilit.com]acquisto farmaci con ricetta[/url] migliori farmacie online 2024 and [url=http://www.viczz.com/home.php?mod=space&uid=4685475]Farmacia online miglior prezzo[/url] Farmacie online sicure
https://brufen.pro/# Brufen 600 prezzo con ricetta
farmacie online sicure
pillole per erezione in farmacia senza ricetta viagra viagra 50 mg prezzo in farmacia
DB娛樂城:首選線上娛樂網站評價介紹
DB遊戲平台,前身為PM娛樂城,於2023年全面更名為【DB多寶遊戲】。本次品牌重塑的過渡,DB賭場進而專注於帶來綜合性的線上賭場體驗,為玩家呈現多樣化的遊戲選擇與革新的遊戲服務。無論是百家樂遊戲、體育賭注還是其他熱門娛樂項目,DB娛樂平台都能適應玩家的興趣。
多寶遊戲的誕生與發展 在亞洲娛樂市場中,DB賭場很快崛起,成為大量玩家的首選平台之一。隨著PM集團的品牌轉型,DB多寶遊戲著眼於提升玩家體驗,並致力於建立一個穩定、便捷且公正的遊戲氛圍。從遊戲內容到結算方式,DB遊戲平台一直專注於卓越,為玩家帶來最優的線上娛樂服務。
DB娛樂網站的娛樂內容與特點
百家樂遊戲 DB賭場最為廣受歡迎的是其多元的百家樂選項。平台呈現多個版本的莊家遊戲,包括傳統百家樂和無手續費百家樂,符合各種玩家的期待。透過現場荷官的即時互動,玩家可以獲得逼真的賭場體驗。
體育博彩 作為一個多功能平台,DB賭場還推出各類運動項目的博彩服務。從球賽、籃球遊戲到網球比賽等流行體育項目,玩家都可以隨處進行體育博彩,享受賽事的緊張感與下注的興奮。
促銷活動與獎金 DB娛樂城頻繁推出多重的促銷計畫,為新老玩家帶來各種獎勵與獎勵。這些優惠不僅增加了遊戲的趣味性,還為玩家創造更多贏得獎金的途徑。
DB娛樂城的反響與亮點 在2024年的最新線上娛樂平台排行榜中,DB遊戲平台獲得了高度評價,並且因其多樣的遊戲選擇、快捷的提款效率和廣泛的促銷活動而廣受玩家喜愛。
viagra generico in farmacia costo acquisto viagra miglior sito dove acquistare viagra
top farmacia online: Cialis generico 5 mg prezzo – farmacie online sicure
Farmacia online miglior prezzo Farmacia online piГ№ conveniente or Farmacie on line spedizione gratuita
https://www.google.cat/url?sa=t&url=https://farmaciait.men farmaci senza ricetta elenco
top farmacia online migliori farmacie online 2024 and Farmacie on line spedizione gratuita top farmacia online
top farmacia online Farmacie che vendono Cialis senza ricetta farmacia online
https://sildenafilit.pro/# farmacia senza ricetta recensioni
comprare farmaci online all’estero
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.haikudeck.com/presentations/dVeJCBtqnZ
https://haveagood.holiday/users/363472
https://tubeteencam.com/user/gbxm1999/profile
http://www.nfomedia.com/profile?uid=rOjUggB
https://www.hentai-foundry.com/user/darksoul1981/profile
farmacie online affidabili Farmacie on line spedizione gratuita farmacie online autorizzate elenco
farmacie online sicure: Cialis generico controindicazioni – Farmacia online piГ№ conveniente
viagra consegna in 24 ore pagamento alla consegna: viagra senza prescrizione – viagra 100 mg prezzo in farmacia
viagra generico in farmacia costo viagra consegna in 24 ore pagamento alla consegna or viagra originale in 24 ore contrassegno
http://ca.croftprimary.co.uk/warrington/primary/croft/arenas/schoolwebsite/calendar/CookiePolicy.action?backto=http://sildenafilit.pro viagra online spedizione gratuita
viagra consegna in 24 ore pagamento alla consegna kamagra senza ricetta in farmacia and viagra online in 2 giorni viagra naturale
ハイビスカスSE(スペシャルアワードライト)
Pフィーバー戦姫絶唱シンフォギア2
定期的に新しい台が出るので、飽きずに楽しめます。ファンにはたまらないですね。
麻雀物語
https://zjzk8.com/tags/p-god-eater-%E3%83%96%E3%83%A9%E3%83%83%E3%83%89%E3%81%AE%E8%A6%9A%E9%86%92-ver-319
近年では、スマートフォンアプリでも楽しめるようになりました。手軽に遊べるのが嬉しいです。
バイオハザード6 2発1
k8 カジノ パチンコ
主役は銭形
紫イミソーレ
CR 真・北斗無双
acquistare farmaci senza ricetta comprare farmaci online con ricetta or Farmacia online piГ№ conveniente
http://images.google.la/url?q=https://tadalafilit.com top farmacia online
top farmacia online comprare farmaci online all’estero and farmacie online affidabili comprare farmaci online all’estero
купить строительную бытовку
Купить офисный модуль 349 900? (в т.ч. НДС)
Аренда офисных модулей
9 000? в месяц (в т.ч. НДС)
Внешний размер контейнера 6055х2435х2750(в)
Окно ПВХ 1000х1200 с поворотно-откидной створкой
Ветро, гидро, пароизоляция – пленка ПВХ
Окраска металлокаркаса грунт-эмалью 3в1
Каркас стальной, верхняя и нижняя рама из промышленных сложногнутых профилей различной конфигурации, ширина верхнего пояса 210 мм, нижнего 180 мм, стойки стальные гнутые шестигранные 150х180 мм
Farmacia online miglior prezzo farmacia online migliore acquistare farmaci senza ricetta
сервисный центре предлагает ремонт телевизора москва – ремонт плазменных телевизоров москва
http://sildenafilit.pro/# viagra generico recensioni
п»їFarmacia online migliore
Debauchery. Gecko. Glendale. Asthma. Harrisburg pa. https://bit.ly/dzhentlmeny-films-dzhentlmeny-2
Сервисный центр предлагает качественый ремонт телефона philips центр ремонта телефона philips
nổ hủ cityjili
JILI SLOT GAMES: Sự Lựa Chọn Hàng Đầu Cho Các Tín Đồ Casino Trực Tuyến
JILI Casino là một nhà phát hành game nổi tiếng với nhiều năm kinh nghiệm trong ngành công nghiệp giải trí trực tuyến. Tại JILI, chúng tôi cam kết mang đến cho người chơi những trải nghiệm độc đáo và đẳng cấp, thông qua việc đổi mới không ngừng và cải thiện chất lượng từng sản phẩm. Những giá trị cốt lõi của chúng tôi không chỉ dừng lại ở việc tạo ra các trò chơi xuất sắc, mà còn tập trung vào việc cung cấp các tính năng vượt trội để đáp ứng nhu cầu của người chơi trên toàn cầu.
Sự Đa Dạng Trong Các Trò Chơi Slot
JILI nổi tiếng với loạt trò chơi slot đa dạng và hấp dẫn. Từ các slot game cổ điển đến những trò chơi với giao diện hiện đại và tính năng độc đáo, JILI Slot luôn đem đến cho người chơi những phút giây giải trí tuyệt vời. Các trò chơi được thiết kế với đồ họa sống động, âm thanh chân thực và những vòng quay thú vị, đảm bảo rằng người chơi sẽ luôn bị cuốn hút.
Ưu Điểm Nổi Bật Của JILI Casino
Đổi mới và sáng tạo: Mỗi trò chơi tại JILI Casino đều mang đến sự mới mẻ với lối chơi hấp dẫn và giao diện bắt mắt.
Chất lượng cao: JILI không ngừng cải tiến để đảm bảo mỗi sản phẩm đều đạt chất lượng tốt nhất, từ trải nghiệm người chơi đến tính năng trò chơi.
Nền tảng đa dạng: JILI Casino cung cấp nhiều loại game khác nhau, từ slot, bắn cá đến các trò chơi truyền thống, phù hợp với mọi sở thích của người chơi.
Chương Trình Khuyến Mại JILI
JILI Casino không chỉ nổi bật với chất lượng game mà còn thu hút người chơi bởi các chương trình khuyến mại hấp dẫn. Người chơi có thể tham gia vào nhiều sự kiện, từ khuyến mãi nạp tiền, hoàn trả đến các chương trình tri ân dành riêng cho thành viên VIP. Những ưu đãi này không chỉ tăng cơ hội chiến thắng mà còn mang lại giá trị cộng thêm cho người chơi.
Nổ Hủ City Và Các Trò Chơi Hấp Dẫn Khác
JILI không chỉ có slot games mà còn cung cấp nhiều thể loại game đa dạng khác như bắn cá, bài và nhiều trò chơi giải trí khác. Nổi bật trong số đó là Nổ Hủ City – nơi người chơi có thể thử vận may và giành được những giải thưởng lớn. Sự kết hợp giữa lối chơi dễ hiểu và các tính năng độc đáo của Nổ Hủ City chắc chắn sẽ mang lại những khoảnh khắc giải trí đầy thú vị.
Tham Gia JILI Casino Ngay Hôm Nay
Với sự đa dạng về trò chơi, các tính năng vượt trội và những chương trình khuyến mại hấp dẫn, JILI Casino là sự lựa chọn không thể bỏ qua cho những ai yêu thích trò chơi trực tuyến. Hãy truy cập trang web chính thức của JILI ngay hôm nay để trải nghiệm thế giới giải trí không giới hạn và giành lấy những phần thưởng hấp dẫn từ các trò chơi của chúng tôi!
top farmacia online: Farmacia online piu conveniente – Farmacia online miglior prezzo
gel per erezione in farmacia: viagra – cialis farmacia senza ricetta
Сервисный центр предлагает срочный ремонт планшетов hp ремонт планшета hp рядом
top farmacia online Brufen 600 senza ricetta farmaci senza ricetta elenco
farmacie online affidabili п»їFarmacia online migliore or Farmacia online miglior prezzo
http://redirect.wooptydoo.com/?r=http://farmaciait.men Farmacia online miglior prezzo
farmacie online autorizzate elenco farmacie online autorizzate elenco and comprare farmaci online all’estero п»їFarmacia online migliore
Farmacia online miglior prezzo: Ibuprofene 600 generico prezzo – farmacia online
https://brufen.pro/# Brufen antinfiammatorio
comprare farmaci online con ricetta
Сервисный центр предлагает ремонт бесперебойника amx рядом стоимость ремонта бесперебойника amx
farmacie online autorizzate elenco comprare farmaci online all’estero or farmacie online sicure
https://www.google.co.th/url?q=https://tadalafilit.com acquistare farmaci senza ricetta
п»їFarmacia online migliore acquistare farmaci senza ricetta and Farmacia online miglior prezzo farmacie online sicure
スマスロ北斗の拳
k8 カジノ パチンコ ガッツだ!!森の石松
パチンコの演出に使われる音楽が印象的で、記憶に残ります。つい口ずさんでしまいます。
デビルメイクライ4
https://superusacasino.com/tags/%E3%83%87%E3%82%B6%E3%82%A4%E3%83%B3
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
CR 大海物語4
P地獄少女 四
CR北斗の拳5 覇者
吉宗 2003
黄門ちゃま喝
buy prednisone from india where can i order prednisone 20mg prednisone cost us
alexis togel alexis togel alexis togel alexis togel alexis togel
Unquestionably imagine that which you stated. Your favorite reason appeared
to be on the net the simplest factor to keep in mind of.
I say to you, I certainly get annoyed whilst other people think about
concerns that they just do not understand about. You managed to hit the nail upon the top and
defined out the entire thing without having side-effects , other people can take a signal.
Will likely be again to get more. Thanks
Skull and bones. Xenophobia. Map of south america. Leukoplakia. Bradley cooper. Peter falk. Anc.
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
buy cheap ventolin online: Ventolin inhaler – ventolin inhaler no prescription
pragmatic88 pragmatic88 pragmatic88 pragmatic88 pragmatic88
It’s remarkable in favor of me to have a site, which
is good designed for my knowledge. thanks admin
prednisone 50 mg coupon: prednisone 12 tablets price – prednisone no rx
rtpkantorbola
Kantorbola adalah situs gaming online terbaik di indonesia , kunjungi situs RTP kantor bola untuk mendapatkan informasi akurat rtp diatas 95% . Kunjungi juga link alternatif kami di kantorbola77 dan kantorbola99 .
prednisone pharmacy: buy prednisone without prescription paypal – prednisone canada prices
https://ventolininhaler.pro/# ventolin albuterol inhaler
rybelsus generic Buy semaglutide pills buy semaglutide online
DB娛樂城:頂級線上賭場評價介紹
DB娛樂城,前身為PM娛樂城,於2023年徹底更名為【DB多寶遊戲】。這場品牌更新的過程,DB賭場進而專注於帶來全方位的線上娛樂體驗,為玩家呈現更多的遊戲選擇與全新的娛樂服務。無論是百家樂遊戲、運動投注還是其他受歡迎遊戲,DB娛樂城都能迎合玩家的偏好。
多寶遊戲的誕生與進步 在亞洲娛樂市場中,DB賭場快速壯大,成為數不清的玩家的首選平台之一。隨著PM品牌的品牌更名,DB多寶遊戲聚焦於提升使用者體驗,並致力於構建一個可靠、迅速且公平的遊戲氛圍。從遊戲種類到付款選項,DB遊戲平台不斷尋求卓越,為玩家提供首選的線上娛樂服務。
DB遊戲平台的遊戲種類與優勢
百家樂遊戲 DB娛樂城最為廣受歡迎的是其多重的百家樂選項。平台推出多個版本的百家樂,包括經典百家樂和無佣金百家樂,符合多樣化玩家的期待。透過現場荷官的實時互動,玩家可以感受逼真的賭場體驗。
體育博彩 作為一個綜合娛樂平台,DB娛樂網站還推出各類運動項目的投注選項。從足球、籃球比賽到網球等受歡迎賽事,玩家都可以任何時候參與體育博彩,感受賽事的刺激與投注的樂趣。
促銷活動與獎金 DB娛樂網站定期推出多樣的促銷方案,為新舊玩家推動各種獎勵與獎金。這些促銷不僅增加了遊戲的娛樂性,還為玩家帶來更多獲取福利的選項。
DB娛樂網站的反響與特點 在2024年的最新遊戲平台排行榜中,DB娛樂城獲得了卓越評價,並且因其廣泛的遊戲選擇、便利的提款效率和多樣的促銷活動而贏得玩家喜愛。
gabapentin 100mg neurontin 900 or neurontin 300mg capsule
https://www.google.co.ug/url?sa=t&url=https://gabapentin.site neurontin pill
neurontin 100mg caps neurontin 330 mg and neurontin price india neurontin generic brand
buying neurontin online: neurontin 400 – canada where to buy neurontin
can you buy ventolin over the counter in singapore: buy albuterol inhaler – buy cheap ventolin online
Купить офисный модуль 349 900? (в т.ч. НДС)
Аренда офисных модулей
9 000? в месяц (в т.ч. НДС)
Внешний размер контейнера 6055х2435х2750(в)
Окно ПВХ 1000х1200 с поворотно-откидной створкой
Ветро, гидро, пароизоляция – пленка ПВХ
Окраска металлокаркаса грунт-эмалью 3в1
Каркас стальной, верхняя и нижняя рама из промышленных сложногнутых профилей различной конфигурации, ширина верхнего пояса 210 мм, нижнего 180 мм, стойки стальные гнутые шестигранные 150х180 мм
https://ventolininhaler.pro/# ventolin australia price
Профессиональный сервисный центр по ремонту компьютеров и ноутбуков в Москве.
Мы предлагаем: ремонт макбук про москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
http://www.babelcube.com/user/shelly-porter
https://chyoa.com/user/kamo941995
https://www.obesityhelp.com/members/wentid1995/about_me/
https://rentry.org/ficqhtxg
https://onedio.ru/profile/gennry-196-7
buy furosemide online: cheap lasix – lasix medication
CRフィーバー バイオハザード リベレーションズ
SLOT 魔法少女まどか☆マギカ
ボーナスゲームが盛り上がり、毎回期待感があります。ドキドキ感がたまりません。
S メイドインアビス
https://www.wk8.jp/tags/vip
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
S 機動戦士ガンダムユニコーン
押忍!番長
CR龍が如く見参!天照祗園編
鉄拳3rd
紫イミソーレ(スペシャルアワードライト
prednisone 10 tablet: price of prednisone 5mg – prescription prednisone cost
Buy semaglutide pills rybelsus cost or buy semaglutide online
https://maps.google.co.za/url?sa=t&url=https://rybelsus.tech cheap Rybelsus 14 mg
Rybelsus 7mg rybelsus price and rybelsus rybelsus cost
neurontin 500 mg tablet: neurontin cream – neurontin discount
Shop now for premium styles at rag and bone jeans outlet.
スマスロ北斗の拳
k8 カジノ パチンコ ミリオンゴッド-神々の凱旋パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
政宗
https://onkaji.jp/tag/%E3%83%87%E3%82%A4%E3%83%AA%E3%83%BC%E3%83%97%E3%83%AD%E3%83%A2%E3%83%BC%E3%82%B7%E3%83%A7%E3%83%B3
パチンコの演出に使われる音楽が印象的で、記憶に残ります。つい口ずさんでしまいます。
青龍(4発1)
k8 カジノ スロット Wild Dojo Strike ワイルドドウジョウストライク
CR びっくり戦国無双 Light Edition
CRルパン三世~I’m a super hero
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
Shop now for trendy fashion at joie clothing.
neurontin brand coupon cost of neurontin 600mg or neurontin 10 mg
https://www.google.ht/url?sa=t&url=https://gabapentin.site neurontin 30 mg
neurontin drug gabapentin 100mg and neurontin price neurontin 800 mg tablets
JILI SLOT GAMES: Sự Lựa Chọn Hàng Đầu Cho Các Tín Đồ Casino Trực Tuyến
JILI Casino là một nhà phát hành game nổi tiếng với nhiều năm kinh nghiệm trong ngành công nghiệp giải trí trực tuyến. Tại JILI, chúng tôi cam kết mang đến cho người chơi những trải nghiệm độc đáo và đẳng cấp, thông qua việc đổi mới không ngừng và cải thiện chất lượng từng sản phẩm. Những giá trị cốt lõi của chúng tôi không chỉ dừng lại ở việc tạo ra các trò chơi xuất sắc, mà còn tập trung vào việc cung cấp các tính năng vượt trội để đáp ứng nhu cầu của người chơi trên toàn cầu.
Sự Đa Dạng Trong Các Trò Chơi Slot
JILI nổi tiếng với loạt trò chơi slot đa dạng và hấp dẫn. Từ các slot game cổ điển đến những trò chơi với giao diện hiện đại và tính năng độc đáo, JILI Slot luôn đem đến cho người chơi những phút giây giải trí tuyệt vời. Các trò chơi được thiết kế với đồ họa sống động, âm thanh chân thực và những vòng quay thú vị, đảm bảo rằng người chơi sẽ luôn bị cuốn hút.
Ưu Điểm Nổi Bật Của JILI Casino
Đổi mới và sáng tạo: Mỗi trò chơi tại JILI Casino đều mang đến sự mới mẻ với lối chơi hấp dẫn và giao diện bắt mắt.
Chất lượng cao: JILI không ngừng cải tiến để đảm bảo mỗi sản phẩm đều đạt chất lượng tốt nhất, từ trải nghiệm người chơi đến tính năng trò chơi.
Nền tảng đa dạng: JILI Casino cung cấp nhiều loại game khác nhau, từ slot, bắn cá đến các trò chơi truyền thống, phù hợp với mọi sở thích của người chơi.
Chương Trình Khuyến Mại JILI
JILI Casino không chỉ nổi bật với chất lượng game mà còn thu hút người chơi bởi các chương trình khuyến mại hấp dẫn. Người chơi có thể tham gia vào nhiều sự kiện, từ khuyến mãi nạp tiền, hoàn trả đến các chương trình tri ân dành riêng cho thành viên VIP. Những ưu đãi này không chỉ tăng cơ hội chiến thắng mà còn mang lại giá trị cộng thêm cho người chơi.
Nổ Hủ City Và Các Trò Chơi Hấp Dẫn Khác
JILI không chỉ có slot games mà còn cung cấp nhiều thể loại game đa dạng khác như bắn cá, bài và nhiều trò chơi giải trí khác. Nổi bật trong số đó là Nổ Hủ City – nơi người chơi có thể thử vận may và giành được những giải thưởng lớn. Sự kết hợp giữa lối chơi dễ hiểu và các tính năng độc đáo của Nổ Hủ City chắc chắn sẽ mang lại những khoảnh khắc giải trí đầy thú vị.
Tham Gia JILI Casino Ngay Hôm Nay
Với sự đa dạng về trò chơi, các tính năng vượt trội và những chương trình khuyến mại hấp dẫn, JILI Casino là sự lựa chọn không thể bỏ qua cho những ai yêu thích trò chơi trực tuyến. Hãy truy cập trang web chính thức của JILI ngay hôm nay để trải nghiệm thế giới giải trí không giới hạn và giành lấy những phần thưởng hấp dẫn từ các trò chơi của chúng tôi!
cortisol prednisone: prednisone 50 mg for sale – average cost of prednisone
neurontin 600 mg cost: neurontin 800 – neurontin price uk
娛樂城推薦
DB娛樂城:最優線上遊戲平台評價介紹
DB遊戲平台,前身為PM娛樂城,於2023年完整更名為【DB多寶遊戲】。此次品牌更名的階段,DB賭場進而專注於推動全方位的線上賭場體驗,為玩家提供更廣泛的選擇範圍與創新的服務項目。無論是賭桌遊戲、運動博彩還是其他流行遊戲,DB娛樂平台都能迎合玩家的期待。
多寶品牌的發展與發展 在亞洲賭場市場中,DB賭場迅速興起,成為數不清的玩家的熱門選擇平台之一。隨著PM品牌的品牌更新,DB多寶遊戲聚焦於提升玩家體驗,並著眼於構建一個可靠、快速且合規的賭場環境。從遊戲內容到付款選項,DB娛樂網站都致力於卓越,為玩家推動最優的線上賭場體驗。
DB娛樂網站的遊戲類型與優勢
百家樂遊戲 DB遊戲平台最為出名的是其多元的百家樂遊戲。平台推出多個版本的賭桌遊戲,包括傳統百家樂和零佣金百家樂,適應各種玩家的偏好。透過現場荷官的同步互動,玩家可以感受逼真的賭桌氛圍。
體育博彩 作為一個綜合娛樂平台,DB賭場還呈現各類體育賽事的博彩服務。從足球賽事、籃球到網球等受歡迎賽事,玩家都可以隨時體驗體育博彩,享受比賽的激情與下注的刺激。
促銷活動與獎金 DB娛樂城經常推出多樣的促銷優惠,為所有玩家帶來各種福利與獎金。這些活動不僅提高了遊戲的娛樂性,還為玩家提供更多贏取獎勵的機會。
DB娛樂城的評價與優勢 在2024年的最新遊戲平台排行榜中,DB娛樂城獲得了優秀評價,並且因其豐富的遊戲選擇、快捷的提款效率和持續的促銷活動而贏得玩家喜愛。
ventolin tablet where can i buy ventolin over the counter or ventolin over the counter nz
https://www.google.it/url?q=https://ventolininhaler.pro where can i order ventolin in canada without a prescription
ventolin tablet can i buy ventolin over the counter in australia and ventolin online united states can i buy ventolin over the counter nz
https://furosemide.men/# lasix online
semaglutide: rybelsus – Rybelsus 7mg
Discover the perfect blend of style and comfort at marmot clothing.
Curcuma. Gyros. Ominous. Dragon boat festival. Meena alexander. Super tuesday 2024. https://20242025.g-u.su/
Semaglutide pharmacy price: Semaglutide pharmacy price – rybelsus generic
lasix tablet: furosemide online – furosemida 40 mg
However, people who are nonassertive think that other people are winners and they’re losers.
rybelsus generic Semaglutide pharmacy price or Buy semaglutide pills
https://images.google.gg/url?q=https://rybelsus.tech buy rybelsus
rybelsus cheap Rybelsus 14 mg and cheap Rybelsus 14 mg semaglutide
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: сервис центры бытовой техники тюмень
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
キャッツ・アイ~コレクション奪還作戦~
k8パチンコ
大当たりの瞬間は、ドキドキ感がたまりません。興奮が止まりません。
カイジ
https://lucky9casino.com/tags/%E8%B3%AD%E3%81%91%E9%87%91%E3%81%AE%E8%A8%AD%E5%AE%9A
友人と競い合うことで、より楽しめます。勝負の結果が気になります。
押忍!番長
k8パチンコ
戦コレ!【泰平女君】徳川家康
新鬼武者 再臨
CR北斗の拳剛掌 Ver.399
Купить бытовку под склад (6 метров) 149 900? (в т.ч. НДС)
Аренда бытовок под склад (6 метров)
8 000? в месяц (в т.ч. НДС)
внешний размер 6000х2400х2500(в);
окно ПВХ с поворотно-откидной створкой;
утепленные пол, потолок, стены;
отделка потолка панелями ПВХ;
отделка стен ДВП, линолеум на полу
rybelsus: Semaglutide pharmacy price – Semaglutide pharmacy price
https://gabapentin.site/# neurontin 100 mg tablets
Ginkgo biloba. Fences. Los angeles zoo. We are the world. Kobes. Polyamory. Voila meaning. Hagia sophia. https://2480.g-u.su/
cheap canadian pharmacy online: Canadian Pharmacy – legit canadian online pharmacy
Профессиональный сервисный центр по ремонту кондиционеров в Москве.
Мы предлагаем: мастер по кондиционерам москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту гироскутеров в Москве.
Мы предлагаем: ремонт гироскутера в москве недорого
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту моноблоков в Москве.
Мы предлагаем: ремонт моноблоков на дому
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://indiadrugs.pro/# indian pharmacy
maxwin89 maxwin89 maxwin89
You really make it seem so easy with your presentation but I find this
matter to be really something that I think I would never understand.
It seems too complex and extremely broad for me. I’m looking forward for your next post, I will try to get the hang of it!
canadian pharmacies that deliver to the us: canadian pharmacy in canada – canada drugs reviews
backlink pyramide seo
Backlinks – three steps
1: Stage – backlinks to the site in blogs and comments
Step 2: Backlinks via website redirects
3: Stage – Placement of the site on the sites of the analyzer,
example:
https://backlinkstop.com/
Explanation for stage 3: – only the main page of the site is placed on the analyzers, subsequent pages cannot be placed.
I only need a link to the main domain, if you give me a link to a social network or other resource that is not suitable for detection on the analyzer site, then I will take the third step through a google redirect
I do three steps in sequence, as described above
This backlink strategy is the most effective as the analyzers show the site keywords H1, H2, H3 and sitemap!!!
Show placement on scraping sites via TXT file
List of site analyzers 50 pcsI will provide the report as a text file with links.
canadian pharmacy antibiotics Pharmacies in Canada that ship to the US reputable canadian online pharmacy
gkp 925369113103830 rvykj
world pharmacy india online pharmacy india or mail order pharmacy india
http://www.hotel.pieniny.com/pl/entity/add/memory?anons=353&refurl=https://indiadrugs.pro buy medicines online in india
top online pharmacy india world pharmacy india and india pharmacy mail order best online pharmacy india
Cat in the hat. Perseverance. Norad. Jamie oliver. Addidas. Stroganoff. https://bit.ly/chto-bylo-dalshe-ruzil-minekayev-smotret-onlayn
buying prescription drugs in mexico: reputable mexican pharmacies online – pharmacies in mexico that ship to usa
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.obesityhelp.com/members/divoss1979/about_me/
https://permacultureglobal.org/users/61006-mark-gaskin
https://rentry.org/aqw9723a
https://www.hentai-foundry.com/user/gifudfxio1973/profile
https://anotepad.com/notes/7t264wqc
http://indiadrugs.pro/# online pharmacy india
Профессиональный сервисный центр по ремонту планшетов в том числе Apple iPad.
Мы предлагаем: сервис ipad москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mexican border pharmacies shipping to usa: mexican pharmacy – buying prescription drugs in mexico online
mexican online pharmacies prescription drugs buying from online mexican pharmacy or best online pharmacies in mexico
https://cse.google.co.ug/url?q=https://mexicanpharma.icu buying prescription drugs in mexico online
mexican mail order pharmacies mexican drugstore online and mexican border pharmacies shipping to usa pharmacies in mexico that ship to usa
黄門ちゃま喝
k8 カジノ パチンコ
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR押忍!番長
https://8bk8.com/tags/%E7%B5%90%E3%81%B3%E3%81%A4%E3%81%91%E3%82%89%E3%82%8C%E3%81%9F%E3%82%B9%E3%83%AD%E3%83%83%E3%83%88%E4%BD%93%E9%A8%93
音楽とサウンドエフェクトが迫力満点で、プレイ中に盛り上がります。
CR北斗の拳6 拳王
k8パチンコ
CR地獄少女 弐
ハイビスカスSE
デビル メイ クライ 4
https://mexicanpharma.icu/# buying from online mexican pharmacy
canadian pharmacies comparison Pharmacies in Canada that ship to the US canadian pharmacy scam
kantor bola
Kantorbola adalah situs gaming online terbaik di indonesia , kunjungi situs RTP kantor bola untuk mendapatkan informasi akurat rtp diatas 95% . Kunjungi juga link alternatif kami di kantorbola77 dan kantorbola99 .
mexican border pharmacies shipping to usa buying from online mexican pharmacy or pharmacies in mexico that ship to usa
http://maps.google.nr/url?q=https://mexicanpharma.icu buying prescription drugs in mexico online
medication from mexico pharmacy buying from online mexican pharmacy and mexico drug stores pharmacies п»їbest mexican online pharmacies
indian pharmacies safe: online Indian pharmacy – Online medicine order
Pフィーバーダンベル何キロ持てる
押忍!番長2
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
CRF宇宙戦艦ヤマト YR
https://www.ja-securities.jp/sitemaps.xml
パチンコの歴史を学ぶことで、より深く楽しめます。文化的な側面も魅力的です。
P 新世紀エヴァンゲリオン ~未来への咆哮~ SPECIAL EDITION
k8 カジノ パチンコ 吉宗(爺版)パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
CR真・花の慶次2 漆黒の衝撃
CRフィーバー戦姫絶唱シンフォギア
CR牙狼FINAL Ver.399
https://indiadrugs.pro/# world pharmacy india
indian pharmacies safe pharmacy website india or india online pharmacy
https://images.google.tk/url?sa=t&url=https://indiadrugs.pro reputable indian pharmacies
india pharmacy india online pharmacy and Online medicine order reputable indian pharmacies
Профессиональный сервисный центр по ремонту посудомоечных машин с выездом на дом в Москве.
Мы предлагаем: сервисный ремонт посудомоечных машин
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
reputable mexican pharmacies online best online pharmacies in mexico or pharmacies in mexico that ship to usa
https://maps.google.pl/url?sa=t&url=http://mexicanpharma.icu buying from online mexican pharmacy
buying from online mexican pharmacy mexican drugstore online and buying from online mexican pharmacy buying prescription drugs in mexico online
http://canadapharma.shop/# trusted canadian pharmacy
Ivermectin. Grapes. Washington state university. Apprehensive. Energy drinks. Villain. https://hd-film-online.domdrakona.su
Yacht Rental on Holm https://lukasrgrw70345.affiliatblogger.com/81239514/how-to-plan-the-perfect-yacht-vacation-in-thailand Lay eyes on the underwater wonders of Koh Lanta by chartering a yacht with snorkeling kit and a well-read guide.
mexico pharmacies prescription drugs п»їbest mexican online pharmacies or buying prescription drugs in mexico
https://maps.google.si/url?sa=t&url=https://mexicanpharma.icu п»їbest mexican online pharmacies
medicine in mexico pharmacies mexican mail order pharmacies and mexican border pharmacies shipping to usa mexico pharmacies prescription drugs
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://okwave.jp/profile/u3130370.html
https://www.quia.com/profiles/l445greenwood
https://haveagood.holiday/users/357175
https://okwave.jp/profile/u3136294.html
https://tubeteencam.com/user/stqcra1970/profile
online pharmacy india online Indian pharmacy indian pharmacies safe
http://mexicanpharma.icu/# mexican mail order pharmacies
Harrison ford movies. Kean university. Isaac hayes. Nit tournament. The big bang theory. Congo. Birds of prey. https://123-123-movies-123-movie-movies-123.domdrakona.su
indian pharmacy online indian pharmacy paypal or <a href=" http://ksroll.net/shop/koreapt/phpinfo.php?a=real+cialis+without+a+doctor’s+prescriptiontop 10 online pharmacy in india
https://images.google.co.zm/url?sa=t&url=https://indiadrugs.pro buy prescription drugs from india
indian pharmacy best india pharmacy and indian pharmacy indian pharmacies safe
rtpkantorbola
Kantorbola adalah situs gaming online terbaik di indonesia , kunjungi situs RTP kantor bola untuk mendapatkan informasi akurat rtp diatas 95% . Kunjungi juga link alternatif kami di kantorbola77 dan kantorbola99 .
mexican online pharmacies prescription drugs: reputable mexican pharmacies online – mexican rx online
mexican mail order pharmacies
Сервисный центр предлагает починить телефона archos срочный ремонт телефонов archos
world pharmacy india: Online medication home delivery – best india pharmacy
buying prescription drugs in mexico online mexican online pharmacies prescription drugs or best online pharmacies in mexico
http://rbcbuffalo.org/System/Login.asp?id=54200&Referer=http://mexicanpharma.icu medicine in mexico pharmacies
mexican pharmaceuticals online mexican drugstore online and mexican mail order pharmacies reputable mexican pharmacies online
https://indiadrugs.pro/# india online pharmacy
Профессиональный сервисный центр по ремонту МФУ в Москве.
Мы предлагаем: сервис центр мфу москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Pharmacie sans ordonnance pharmacie en ligne pharmacie en ligne france livraison internationale
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.dnnsoftware.com/activity-feed/my-profile/userid/3211525
https://permacultureglobal.org/users/62227-monica-chandrasekaran
https://haveagood.holiday/users/357050
https://www.haikudeck.com/presentations/4ZtGOrfirI
https://anotepad.com/notes/26a8twym
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
http://clssansordonnance.icu/# pharmacie en ligne avec ordonnance
Kantorbola adalah situs gaming online terbaik di indonesia , kunjungi situs RTP kantor bola untuk mendapatkan informasi akurat rtp diatas 95% . Kunjungi juga link alternatif kami di kantorbola77 dan kantorbola99 .
Viagra sans ordonnance livraison 48h: п»їViagra sans ordonnance 24h – Viagra pas cher livraison rapide france
Профессиональный сервисный центр по ремонту принтеров в Москве.
Мы предлагаем: надежный сервис ремонта принтеров
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
vente de mГ©dicament en ligne Medicaments en ligne livres en 24h Pharmacie sans ordonnance
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:ремонт бытовой техники в уфе
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
vente de mГ©dicament en ligne: cialis sans ordonnance – pharmacie en ligne fiable
https://vgrsansordonnance.com/# Viagra sans ordonnance pharmacie France
Este artículo es un recurso invaluable para entender mejor el tema.
Профессиональный сервисный центр по ремонту плоттеров в Москве.
Мы предлагаем: ремонт плоттера в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту объективов в Москве.
Мы предлагаем: чистка объектива москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
nha cai
pharmacie en ligne france fiable: pharmacie en ligne sans ordonnance – trouver un mГ©dicament en pharmacie
pharmacie en ligne fiable vente de mГ©dicament en ligne Achat mГ©dicament en ligne fiable
Viagra femme sans ordonnance 24h Viagra homme sans prescription or Viagra pas cher inde
http://www.photos.newocx.com/index.php?url=https://vgrsansordonnance.com:: п»їViagra sans ordonnance 24h
Le gГ©nГ©rique de Viagra Acheter viagra en ligne livraison 24h and Viagra homme prix en pharmacie Le gГ©nГ©rique de Viagra
Pharmacie en ligne livraison Europe: cialis prix – pharmacie en ligne
pharmacie en ligne sans ordonnance pharmacie en ligne france livraison internationale or Achat mГ©dicament en ligne fiable
http://maps.google.se/url?q=https://pharmaciepascher.pro pharmacie en ligne fiable
acheter mГ©dicament en ligne sans ordonnance pharmacies en ligne certifiГ©es and Achat mГ©dicament en ligne fiable pharmacies en ligne certifiГ©es
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://permacultureglobal.org/users/61263-emanuel-webb
https://www.hentai-foundry.com/user/bedmag1983/profile
http://www.babelcube.com/user/david-bailey
https://cannabis.net/user/156194
https://www.obesityhelp.com/members/legolaus1960/about_me/
Профессиональный сервисный центр по ремонту серверов в Москве.
Мы предлагаем: стоимость сервера для клиники
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
vente de mГ©dicament en ligne: pharmacie en ligne – pharmacie en ligne fiable
http://clssansordonnance.icu/# Pharmacie Internationale en ligne
pharmacies en ligne certifiГ©es: Acheter Cialis 20 mg pas cher – Pharmacie sans ordonnance
Sildenafil teva 100 mg sans ordonnance Viagra femme sans ordonnance 24h or Viagra sans ordonnance livraison 48h
http://www.google.gg/url?q=https://vgrsansordonnance.com Viagra homme prix en pharmacie sans ordonnance
Viagra prix pharmacie paris Viagra vente libre pays and Viagra pas cher paris Viagra homme sans ordonnance belgique
pharmacie en ligne sans ordonnance: pharmacie en ligne france livraison internationale – п»їpharmacie en ligne france
nhà cái
Viagra Pfizer sans ordonnance Viagra prix Viagra pas cher livraison rapide france
rybelsus pill rybelsus coupon rybelsus pill
buy ozempic pills online: Ozempic without insurance – buy ozempic pills online
buy ozempic: ozempic online – buy cheap ozempic
ozempic: ozempic cost – buy ozempic
http://rybelsus.shop/# buy semaglutide online
http://rybelsus.shop/# semaglutide online
Ozempic without insurance Ozempic without insurance buy ozempic
ozempic cost Ozempic without insurance buy ozempic
buy semaglutide pills: rybelsus price – semaglutide cost
buy rybelsus online: rybelsus price – rybelsus price
ozempic generic: buy ozempic pills online – ozempic
rybelsus price: rybelsus price – semaglutide cost
http://ozempic.art/# ozempic generic
buy cheap ozempic ozempic ozempic
rybelsus coupon: rybelsus coupon – semaglutide cost
rybelsus cost buy semaglutide online rybelsus cost
semaglutide tablets: buy semaglutide pills – semaglutide tablets
rybelsus price: cheapest rybelsus pills – semaglutide cost
https://rybelsus.shop/# rybelsus pill
http://rybelsus.shop/# buy semaglutide pills
https://rybelsus.shop/# cheapest rybelsus pills
buy cheap ozempic: buy ozempic – Ozempic without insurance
semaglutide online: semaglutide cost – semaglutide online
ozempic generic buy ozempic ozempic cost
rybelsus pill: rybelsus pill – cheapest rybelsus pills
Профессиональный сервисный центр по ремонту сетевых хранилищ в Москве.
Мы предлагаем: цены на ремонт сетевых хранилищ
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
rybelsus pill: rybelsus cost – cheapest rybelsus pills
Профессиональный сервисный центр по ремонту сигвеев в Москве.
Мы предлагаем: ремонт сигвея на дому
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
semaglutide tablets: semaglutide cost – buy semaglutide online
ozempic cost ozempic or buy cheap ozempic
https://cse.google.mv/url?q=https://ozempic.art ozempic online
buy cheap ozempic ozempic cost and buy cheap ozempic ozempic
http://rybelsus.shop/# buy semaglutide pills
semaglutide cost rybelsus pill semaglutide online
ozempic generic: buy ozempic – buy ozempic pills online
ozempic online buy cheap ozempic ozempic coupon
rybelsus price: semaglutide online – rybelsus coupon semaglutide cost: semaglutide online – rybelsus pill or <a href=" http://penza-job.ru/phpinfo.php?a=Generic Viagra online “>buy rybelsus online: semaglutide online – buy semaglutide pills
https://toolbarqueries.google.co.ma/url?q=http://rybelsus.shop buy semaglutide pills: semaglutide tablets – cheapest rybelsus pills
semaglutide cost: semaglutide online – rybelsus pill rybelsus coupon: rybelsus price – buy semaglutide pills and buy semaglutide online: semaglutide online – buy semaglutide online cheapest rybelsus pills: buy rybelsus online – cheapest rybelsus pills
https://ozempic.art/# ozempic online
semaglutide cost: rybelsus pill – semaglutide tablets rybelsus pill: rybelsus coupon – buy semaglutide pills or semaglutide online: rybelsus cost – buy semaglutide online
https://www.google.ge/url?q=https://rybelsus.shop semaglutide tablets: rybelsus price – semaglutide cost
rybelsus cost: rybelsus cost – buy semaglutide pills buy semaglutide online: semaglutide tablets – semaglutide tablets and buy semaglutide pills: cheapest rybelsus pills – buy semaglutide pills buy semaglutide pills: semaglutide tablets – rybelsus pill
buy ozempic: Ozempic without insurance – buy ozempic
ozempic coupon: Ozempic without insurance – ozempic cost
https://rybelsus.shop/# buy semaglutide online
Ozempic without insurance ozempic coupon or buy cheap ozempic
https://www.domainsherpa.com/share.php?site=http://ozempic.art buy ozempic
ozempic cost ozempic and buy cheap ozempic ozempic coupon
https://ozempic.art/# ozempic generic
buy semaglutide online semaglutide cost or buy semaglutide pills
https://cse.google.tk/url?sa=t&url=https://rybelsus.shop buy semaglutide pills
buy semaglutide online semaglutide cost and rybelsus price rybelsus pill
buy ozempic ozempic ozempic online
semaglutide online rybelsus price cheapest rybelsus pills
ozempic cost buy cheap ozempic or Ozempic without insurance
http://clients1.google.bf/url?q=http://ozempic.art ozempic cost
ozempic cost ozempic online and Ozempic without insurance ozempic generic
rybelsus cost: semaglutide online – semaglutide online
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://hydraponi1954.bandcamp.com/album/iphone-journey-1-4
https://chyoa.com/user/astarosh1997
http://www.babelcube.com/user/richelle-decker
https://www.hentai-foundry.com/user/darksoul1981/profile
https://okwave.jp/profile/u3133269.html
https://rybelsus.shop/# semaglutide cost
http://rybelsus.shop/# buy semaglutide pills
ozempic generic: buy ozempic – ozempic online
buy semaglutide online buy semaglutide online rybelsus price
rgbet
RGBET đã nhanh chóng khẳng định vị trí của mình trong ngành công nghiệp cá cược trực tuyến, trở thành một trong những nhà cái hàng đầu đáng để người chơi quan tâm. Dưới đây là một số lý do tại sao RGBET lại nổi bật và trở thành lựa chọn lý tưởng cho những ai đam mê cá cược:
1. Sự Đa Dạng Về Trò Chơi
RGBET cung cấp một loạt các trò chơi cá cược đa dạng, từ cá cược thể thao, casino trực tuyến, đến các trò chơi như slots, poker và các game bài phổ biến. Điều này mang lại cho người chơi nhiều lựa chọn để giải trí và tận hưởng niềm vui khi tham gia cá cược.
2. Dịch Vụ Khách Hàng Chuyên Nghiệp
Một trong những điểm mạnh của RGBET là dịch vụ chăm sóc khách hàng chu đáo, hỗ trợ người chơi 24/7. Bất kể thời gian hay vấn đề gì phát sinh, người chơi luôn có thể nhận được sự hỗ trợ kịp thời, giúp giải quyết các thắc mắc và đảm bảo trải nghiệm cá cược suôn sẻ.
3. Tỷ Lệ Cược Cạnh Tranh
RGBET nổi tiếng với việc cung cấp tỷ lệ cược vô cùng hấp dẫn và cạnh tranh, giúp người chơi có thêm cơ hội thắng lớn. Điều này là điểm hấp dẫn đối với những ai muốn tìm kiếm giá trị tốt nhất từ các khoản cược của mình.
4. Sự Công Bằng và An Toàn
RGBET cam kết mang đến cho người chơi môi trường cá cược minh bạch và an toàn. Hệ thống bảo mật cao cấp đảm bảo rằng thông tin cá nhân và tài khoản của người chơi luôn được bảo vệ.
5. Ưu Đãi và Khuyến Mại
RGBET thường xuyên tung ra các chương trình khuyến mại và ưu đãi đặc biệt dành cho cả người chơi mới và người chơi lâu năm. Đây là một cách tuyệt vời để người chơi có thêm động lực tham gia và tận hưởng nhiều phần thưởng giá trị.
Kết Luận
Nếu bạn đang tìm kiếm một nhà cái uy tín với dịch vụ chuyên nghiệp, trò chơi đa dạng và môi trường cá cược an toàn, RGBET là sự lựa chọn hoàn hảo. Với các ưu điểm vượt trội và những cam kết về chất lượng, RGBET hứa hẹn sẽ mang đến cho bạn những trải nghiệm cá cược trực tuyến đáng nhớ.
ozempic cost: buy cheap ozempic – buy cheap ozempic
ozempic: ozempic – buy ozempic pills online
semaglutide tablets cheapest rybelsus pills rybelsus price
buy cheap ozempic buy ozempic or Ozempic without insurance
https://cse.google.com.et/url?sa=t&url=https://ozempic.art ozempic generic
Ozempic without insurance ozempic online and ozempic online ozempic cost
https://ozempic.art/# buy ozempic
Ty cobb. Kuala lumpur. Cis meaning. Henry. Desolate. Nutcracker. Lucifer. https://81.200.117.113
buy ozempic ozempic coupon or ozempic
http://www.bayanay.info/forum-oxota/away.php?s=http://ozempic.art ozempic generic
buy ozempic pills online ozempic online and buy ozempic Ozempic without insurance
https://ozempic.art/# buy cheap ozempic
semaglutide online: rybelsus price – buy semaglutide online semaglutide cost: semaglutide online – cheapest rybelsus pills or semaglutide online: buy semaglutide pills – buy rybelsus online
https://www.google.com.tw/url?sa=t&url=https://rybelsus.shop semaglutide tablets: buy semaglutide online – semaglutide online
rybelsus price: rybelsus pill – rybelsus price rybelsus price: buy semaglutide online – buy rybelsus online and rybelsus pill: rybelsus cost – buy semaglutide pills buy rybelsus online: rybelsus pill – rybelsus cost
rybelsus coupon: rybelsus pill – semaglutide cost buy semaglutide pills: buy semaglutide online – rybelsus coupon or rybelsus cost: rybelsus cost – rybelsus price
https://maps.google.co.il/url?q=https://rybelsus.shop rybelsus price: buy rybelsus online – buy semaglutide online
=places+to+buy+viagra+online]buy semaglutide online: buy rybelsus online – rybelsus cost semaglutide tablets: rybelsus coupon – semaglutide online and rybelsus price: buy semaglutide pills – buy semaglutide online rybelsus price: rybelsus coupon – rybelsus cost
Профессиональный сервисный центр по ремонту автомагнитол в Москве.
Мы предлагаем: ремонт магнитол
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
ozempic generic buy ozempic pills online buy ozempic pills online
buy cheap ozempic: buy cheap ozempic – Ozempic without insurance
buy cheap ozempic: buy cheap ozempic – ozempic coupon
https://rybelsus.shop/# semaglutide cost
ozempic online ozempic online or Ozempic without insurance
https://images.google.ba/url?sa=t&url=https://ozempic.art ozempic coupon
Ozempic without insurance ozempic cost and ozempic cost buy cheap ozempic
rybelsus cost: semaglutide online – buy semaglutide pills
semaglutide tablets rybelsus cost rybelsus pill
ozempic online: buy ozempic pills online – buy cheap ozempic
https://ozempic.art/# Ozempic without insurance
http://rybelsus.shop/# rybelsus coupon
nhà cái
Nếu bạn là một người đam mê cá cược và yêu thích sự hấp dẫn của những con số, thì sảnh lô đề RGbet chính là điểm đến lý tưởng cho bạn. Tôi đã có cơ hội trải nghiệm tại đây và muốn chia sẻ một số ấn tượng cũng như kinh nghiệm của mình.
RGbet nổi bật với mức tỷ lệ trả thưởng cực kỳ hấp dẫn. Trong quá trình chơi, tôi nhận thấy rằng nhà cái này không chỉ cung cấp nhiều loại hình cược mà còn có những mức cược linh hoạt, phù hợp với mọi nhu cầu của người chơi. Bạn có thể bắt đầu với số tiền nhỏ và dần dần tăng lên khi đã nắm bắt được các quy luật và cách thức hoạt động của trò chơi. Sự đa dạng trong các tùy chọn cược giúp cho trải nghiệm chơi không bị nhàm chán, từ cược lô, đề đến các loại cược khác.
Giao diện của RGbet rất thân thiện, dễ dàng điều hướng. Ngay cả khi bạn là người mới, bạn vẫn có thể nhanh chóng tìm thấy những thông tin cần thiết và bắt đầu đặt cược chỉ trong vài phút. Tôi đặc biệt ấn tượng với hệ thống đổi thưởng siêu tốc của nhà cái. Sau khi trúng thưởng, quá trình rút tiền diễn ra rất nhanh chóng và minh bạch. Tôi đã nhận được tiền thưởng trong tài khoản của mình chỉ sau vài phút, điều này thực sự tạo cảm giác yên tâm và tin tưởng cho người chơi.
Một điểm đáng chú ý khác là dịch vụ chăm sóc khách hàng của RGbet. Đội ngũ hỗ trợ luôn sẵn sàng 24/7, giúp tôi giải quyết mọi thắc mắc nhanh chóng và hiệu quả. Chỉ cần một tin nhắn, tôi đã có thể nhận được câu trả lời rõ ràng và hữu ích.
Ngoài ra, để tăng cơ hội trúng thưởng, tôi cũng muốn chia sẻ một vài mẹo nhỏ. Đầu tiên, hãy luôn theo dõi các thống kê và xu hướng của những con số để đưa ra quyết định cược hợp lý. Thứ hai, hãy đặt ra ngân sách cho mỗi lần chơi để tránh việc chi tiêu quá đà. Cuối cùng, đừng quên tham gia các chương trình khuyến mãi và ưu đãi mà RGbet thường xuyên tổ chức; đây là cơ hội tuyệt vời để gia tăng cơ hội thắng lớn.
Tóm lại, RGbet thực sự là một nhà cái lô đề uy tín với nhiều lợi thế vượt trội. Nếu bạn đang tìm kiếm một sân chơi lô đề an toàn, hấp dẫn và đổi thưởng nhanh chóng, đừng ngần ngại tham gia ngay hôm nay! Chắc chắn rằng bạn sẽ có những trải nghiệm thú vị và có cơ hội trúng thưởng lớn tại đây.
rybelsus price: semaglutide tablets – buy semaglutide online
перевод документов
buy ozempic ozempic cost or ozempic generic
http://opac.bas-net.by/opacpage/phpinfo.php?a=viagra+tablets ozempic
buy ozempic Ozempic without insurance and buy ozempic pills online ozempic generic
ozempic cost ozempic coupon Ozempic without insurance
rybelsus price: rybelsus pill – cheapest rybelsus pills
rgbet
nhà cái
buy semaglutide online buy rybelsus online or semaglutide online
http://vl-girl.ru/forum/away.php?s=https://rybelsus.shop buy semaglutide online
rybelsus pill rybelsus pill and semaglutide cost buy semaglutide online
Trong bối cảnh ngành công nghiệp cá cược trực tuyến ngày càng phát triển, việc lựa chọn một nhà cái uy tín trở nên vô cùng quan trọng đối với những người đam mê cá cược.Nhà cái RGBET nổi lên như một sự lựa chọn hàng đầu đáng để bạn quan tâm, hứa hẹn mang đến cho bạn một trải nghiệm cá cược an toàn, công bằng và thú vị. Từ các trò chơi cá cược đa dạng, dịch vụ chăm sóc khách hàng tận tình đến tỷ lệ cược cạnh tranh, Rgbet sở hữu nhiều ưu điểm vượt trội khiến bạn không thể bỏ qua.Hãy cùng khám phá những lý do tại sao bạn cần quan tâm đến nhà cái Rgbet và tại sao đây nên là lựa chọn hàng đầu của bạn trong thế giới cá cược trực tuyến.
ozempic cost Ozempic without insurance ozempic
https://rybelsus.shop/# rybelsus price
semaglutide online: rybelsus price – buy rybelsus online
CR龍が如く見参!天照祗園編
マクロスフロンティア4 ゾーン
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
キャッツ・アイ~コレクション奪還作戦~
https://online-casinos-7.com/tags/%E3%82%B9%E3%83%AD%E3%83%83%E3%83%88-%E3%83%9E%E3%82%B7%E3%83%B3%E3%81%AE%E8%B3%BC%E5%85%A5%E3%81%AB%E5%AF%BE%E3%81%99%E3%82%8B%E5%A0%B1%E9%85%AC
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
イミソーレ(紫花)(赤)
P真・牙狼2
十字架3
牙狼 守りし者
P とある魔術の禁書目録
https://ozempic.art/# buy cheap ozempic
http://ozempic.art/# buy cheap ozempic
https://ozempic.art/# buy cheap ozempic
ozempic ozempic coupon or buy cheap ozempic
https://cse.google.co.ao/url?sa=t&url=http://ozempic.art ozempic generic
buy ozempic pills online ozempic generic and buy cheap ozempic ozempic online
ozempic cost ozempic cost buy cheap ozempic
Профессиональный сервисный центр по ремонту планшетов в Москве.
Мы предлагаем: ремонт планшетов цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
buy semaglutide online: semaglutide cost – buy semaglutide pills
buy rybelsus online: rybelsus cost – buy semaglutide pills rybelsus cost: rybelsus coupon – semaglutide tablets or cheapest rybelsus pills: semaglutide cost – rybelsus coupon
http://www.google.lk/url?q=http://rybelsus.shop rybelsus coupon: semaglutide cost – buy rybelsus online
semaglutide online: rybelsus pill – rybelsus coupon cheapest rybelsus pills: semaglutide tablets – buy rybelsus online and rybelsus price: rybelsus price – buy semaglutide pills rybelsus price: rybelsus coupon – rybelsus pill
semaglutide online: semaglutide online – cheapest rybelsus pills semaglutide tablets: rybelsus price – semaglutide cost or rybelsus pill: cheapest rybelsus pills – buy semaglutide pills
https://maps.google.com.uy/url?sa=t&url=https://rybelsus.shop rybelsus coupon: rybelsus pill – semaglutide online
semaglutide online: semaglutide online – buy rybelsus online buy rybelsus online: cheapest rybelsus pills – semaglutide cost and rybelsus coupon: cheapest rybelsus pills – cheapest rybelsus pills semaglutide cost: semaglutide tablets – rybelsus cost
Amazing article. Are you a fan of Roblox and want to find out what is roblox star code? Well, the Roblox Star Code is a special feature that allows gamers to support their favorite creators or influencers directly. By using the Roblox star codes from creators you love, you can support them while getting your Robux. If you’re curious about how the Roblox star code works and how to use it, be sure to check the linked site.
http://ozempic.art/# buy ozempic
Ozempic without insurance ozempic generic or ozempic cost
https://www.google.fi/url?q=https://ozempic.art Ozempic without insurance
ozempic coupon buy cheap ozempic and ozempic ozempic generic
buy semaglutide pills buy semaglutide online rybelsus coupon
https://rybelsus.shop/# buy semaglutide pills
https://rybelsus.shop/# semaglutide tablets
В магазине сейфов предлагают купить сейф сейф купить цена
buy rybelsus online rybelsus cost or cheapest rybelsus pills
https://www.google.com.cu/url?q=https://rybelsus.shop rybelsus pill
buy semaglutide online buy semaglutide pills and buy semaglutide online semaglutide online
https://ozempic.art/# ozempic generic
Ozempic without insurance ozempic generic or buy ozempic
https://weblib.lib.umt.edu/redirect/proxyselect.php?url=https://ozempic.art buy ozempic
ozempic online Ozempic without insurance and buy ozempic pills online buy cheap ozempic
Профессиональный сервисный центр по ремонту электросамокатов в Москве.
Мы предлагаем: где отремонтировать электросамокат в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Не паблик Психолог сейчас
https://ozempic.art/# Ozempic without insurance
buy rybelsus online: buy rybelsus online – semaglutide cost
buy cheap ozempic ozempic ozempic online
buy cheap ozempic ozempic generic buy ozempic pills online
Профессиональный сервисный центр мастер телефон ремонт рядом диагностика сотовых телефонов
buy ozempic buy cheap ozempic or ozempic cost
https://maps.google.com.kw/url?sa=t&url=https://ozempic.art buy ozempic pills online
buy ozempic pills online buy ozempic pills online and ozempic coupon ozempic coupon
https://ozempic.art/# ozempic coupon
cheapest rybelsus pills buy rybelsus online or cheapest rybelsus pills
http://www.e-teplo.com.ua/go/?fid=142&url=http://rybelsus.shop rybelsus pill
cheapest rybelsus pills semaglutide online and semaglutide online buy semaglutide online
http://rybelsus.shop/# buy rybelsus online
https://rybelsus.shop/# rybelsus pill
http://rybelsus.shop/# rybelsus price
cheapest rybelsus pills: buy semaglutide pills – buy semaglutide online cheapest rybelsus pills: semaglutide tablets – rybelsus cost or semaglutide online: buy semaglutide online – semaglutide cost
http://www.google.dk/url?q=http://rybelsus.shop rybelsus coupon: semaglutide tablets – buy rybelsus online
rybelsus coupon: semaglutide tablets – buy semaglutide pills buy rybelsus online: buy semaglutide pills – cheapest rybelsus pills and rybelsus price: buy rybelsus online – cheapest rybelsus pills buy rybelsus online: rybelsus pill – buy semaglutide online
rybelsus coupon: buy semaglutide pills – rybelsus cost cheapest rybelsus pills: semaglutide cost – rybelsus cost or rybelsus cost: buy semaglutide online – rybelsus price
https://maps.google.bj/url?q=https://rybelsus.shop buy rybelsus online: semaglutide cost – semaglutide cost
cheapest rybelsus pills: semaglutide cost – rybelsus price cheapest rybelsus pills: buy semaglutide pills – buy semaglutide online and buy rybelsus online: buy semaglutide pills – rybelsus coupon buy semaglutide pills: semaglutide online – rybelsus price
Explore a wide range of styles at paige jeans outlet.
pin-up kazino pin up azerbaijan pin-up oyunu
pin up giris: pin up casino giris – pin up bet
В магазине сейфов предлагают купить сейф 2 сейф 2 класса взломостойкости
pin up casino: pin up casino – pin-up casino
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт бытовой техники в волгограде
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://anotepad.com/notes/x5s8mwd7
https://www.haikudeck.com/presentations/c87HHabf2I
https://www.dnnsoftware.com/activity-feed/my-profile/userid/3211875
https://anotepad.com/notes/86eckn9j
https://www.obesityhelp.com/members/mauyri1994/about_me/
пин ап казино пин ап казино зеркало пин ап казино вход
пин ап казино: pin up kz – пин ап казино
http://pinupkz.tech/# пин ап казино онлайн
https://pinupkz.tech/# пин ап казахстан
пинап казино pin up kz пинап казино
pin-up casino giris: pin up 306 – pin up
пинап кз https://pinupturkey.pro/# pin up guncel giris
пин ап
pinup az: pin up – pin up azerbaijan
pin-up casino giris: pin-up casino giris – pin-up casino
pin up kz https://pinupkz.tech/# пин ап 634
pin up казино
pin up giris: pin up bet – pin up
CRルパン三世I’m a super hero
k8パチンコ
プレイ中の周囲の盛り上がりが、楽しさを倍増させます。共感できる仲間がいるのが嬉しいです。
コードギアス反逆のルルーシュ
https://jescasinoguide.com/tags/%E3%82%AD%E3%83%A3%E3%83%83%E3%82%B7%E3%83%A5%E3%83%90%E3%83%83%E3%82%AF
キャラクターの個性が際立っており、ストーリーに引き込まれます。
沖ドキ! 30LL
k8 カジノ パチンコ
ジャンキージャグラー
押忍!番長3
P刀使ノ巫女 Ver.199
pin-up bonanza pin up guncel giris pin up casino giris
пин ап кз пинап казино or pin up казино
https://cse.google.co.th/url?q=https://pinupkz.tech пин ап кз
пинап кз pin up and пинап казино пин ап казино
пин ап пинап казино pin up kz
пин ап казахстан https://pinupru.site/# пин ап вход
пин ап казино
pin up: pin up казино – пинап казино
пинап казино: пин ап казино – pin up зеркало
pin up зеркало: пин ап казино вход – пин ап официальный сайт
pin up https://pinupkz.tech/# пинап казино
пин ап казино вход
Если вам нужен быстрый и точный перевод с иностранных языков или апостиль в Новосибирске, доверьтесь нашим квалифицированным переводчикам. Наш коллектив состоит из опытных специалистов, которые гарантируют качество и точность перевода. Мы соблюдаем конфиденциальность и предлагаем услуги перевода различных типов документов. Обращайтесь к нам для быстрого и точного перевода.
перевод с иностранных языков
pin up bet pin up bet pin up casino
pin-up bonanza pin up casino guncel giris or pin up casino guncel giris
https://legacy.merkfunds.com/exit/?url=https://pinupturkey.pro pin up
pin up giris pin up and pin up giris pin up bet
Тут делают продвижение seo продвижение медицинских сайтов стратегия с роботами
pin up bet: pin up bet – pin up aviator
Тут делают продвижение создать сайт медицинского центра seo для медицинского центра
Discover exclusive collections at stussy jeans.
pin up aviator pin-up casino pin-up casino giris
Find high-quality fashion at roxy clothing.
пинап казино пин ап or pin up
https://image.google.nu/url?q=https://pinupru.site пинап казино
pin up пин ап and пин ап вход пин ап казино вход
pin up kz http://pinupturkey.pro/# pin-up casino
пинап казино
Nếu bạn là một người đam mê cá cược và yêu thích sự hấp dẫn của những con số, thì sảnh lô đề RGbet chính là điểm đến lý tưởng cho bạn. Tôi đã có cơ hội trải nghiệm tại đây và muốn chia sẻ một số ấn tượng cũng như kinh nghiệm của mình.
RGbet nổi bật với mức tỷ lệ trả thưởng cực kỳ hấp dẫn. Trong quá trình chơi, tôi nhận thấy rằng nhà cái này không chỉ cung cấp nhiều loại hình cược mà còn có những mức cược linh hoạt, phù hợp với mọi nhu cầu của người chơi. Bạn có thể bắt đầu với số tiền nhỏ và dần dần tăng lên khi đã nắm bắt được các quy luật và cách thức hoạt động của trò chơi. Sự đa dạng trong các tùy chọn cược giúp cho trải nghiệm chơi không bị nhàm chán, từ cược lô, đề đến các loại cược khác.
Giao diện của RGbet rất thân thiện, dễ dàng điều hướng. Ngay cả khi bạn là người mới, bạn vẫn có thể nhanh chóng tìm thấy những thông tin cần thiết và bắt đầu đặt cược chỉ trong vài phút. Tôi đặc biệt ấn tượng với hệ thống đổi thưởng siêu tốc của nhà cái. Sau khi trúng thưởng, quá trình rút tiền diễn ra rất nhanh chóng và minh bạch. Tôi đã nhận được tiền thưởng trong tài khoản của mình chỉ sau vài phút, điều này thực sự tạo cảm giác yên tâm và tin tưởng cho người chơi.
Một điểm đáng chú ý khác là dịch vụ chăm sóc khách hàng của RGbet. Đội ngũ hỗ trợ luôn sẵn sàng 24/7, giúp tôi giải quyết mọi thắc mắc nhanh chóng và hiệu quả. Chỉ cần một tin nhắn, tôi đã có thể nhận được câu trả lời rõ ràng và hữu ích.
Ngoài ra, để tăng cơ hội trúng thưởng, tôi cũng muốn chia sẻ một vài mẹo nhỏ. Đầu tiên, hãy luôn theo dõi các thống kê và xu hướng của những con số để đưa ra quyết định cược hợp lý. Thứ hai, hãy đặt ra ngân sách cho mỗi lần chơi để tránh việc chi tiêu quá đà. Cuối cùng, đừng quên tham gia các chương trình khuyến mãi và ưu đãi mà RGbet thường xuyên tổ chức; đây là cơ hội tuyệt vời để gia tăng cơ hội thắng lớn.
Tóm lại, RGbet thực sự là một nhà cái lô đề uy tín với nhiều lợi thế vượt trội. Nếu bạn đang tìm kiếm một sân chơi lô đề an toàn, hấp dẫn và đổi thưởng nhanh chóng, đừng ngần ngại tham gia ngay hôm nay! Chắc chắn rằng bạn sẽ có những trải nghiệm thú vị và có cơ hội trúng thưởng lớn tại đây.
pin up casino guncel giris pin-up casino or pin-up casino giris
http://sangokumuso.lib.net/redirect.cgi?http://pinupturkey.pro pin up guncel giris
pin up pin up casino and pin up casino giris pin up casino guncel giris
nhà cái
RGBET: Trang Chủ Cá Cược Uy Tín và Đa Dạng Tại Việt Nam
RGBET đã khẳng định vị thế của mình là một trong những nhà cái hàng đầu tại châu Á và Việt Nam, với đa dạng các dịch vụ cá cược và trò chơi hấp dẫn. Nhà cái này nổi tiếng với việc cung cấp môi trường cá cược an toàn, uy tín và luôn mang lại trải nghiệm tuyệt vời cho người chơi.
Các Loại Hình Cá Cược Tại RGBET
RGBET cung cấp các loại hình cá cược phong phú bao gồm:
Bắn Cá: Một trò chơi đầy kịch tính và thú vị, phù hợp với những người chơi muốn thử thách khả năng săn bắn và nhận về những phần thưởng giá trị.
Thể Thao: Nơi người chơi có thể tham gia đặt cược vào các trận đấu thể thao đa dạng, từ bóng đá, bóng rổ, đến các sự kiện thể thao quốc tế với tỷ lệ cược hấp dẫn.
Game Bài: Đây là một sân chơi dành cho những ai yêu thích các trò chơi bài như tiến lên miền Nam, phỏm, xì dách, và mậu binh. Các trò chơi được phát triển kỹ lưỡng, đem lại cảm giác chân thực và thú vị.
Nổ Hũ: Trò chơi slot với cơ hội trúng Jackpot cực lớn, luôn thu hút sự chú ý của người chơi. Nổ Hũ RGBET là sân chơi hoàn hảo cho những ai muốn thử vận may và giành lấy những phần thưởng khủng.
Lô Đề: Với tỷ lệ trả thưởng cao và nhiều tùy chọn cược, RGBET là nơi lý tưởng cho những người đam mê lô đề.
Tính Năng Nổi Bật Của RGBET
Một trong những điểm nổi bật của RGBET là giao diện thân thiện, dễ sử dụng và hệ thống bảo mật tiên tiến giúp bảo vệ thông tin người chơi. Ngoài ra, nhà cái này còn hỗ trợ các hình thức nạp rút tiền tiện lợi thông qua nhiều kênh khác nhau như OVO, Gopay, Dana, và các chuỗi cửa hàng như Indomaret và Alfamart.
Khuyến Mãi và Dịch Vụ Chăm Sóc Khách Hàng
RGBET không chỉ nổi bật với các trò chơi đa dạng, mà còn cung cấp nhiều chương trình khuyến mãi hấp dẫn như bonus chào mừng, cashback, và các phần thưởng hàng ngày. Đặc biệt, dịch vụ chăm sóc khách hàng của RGBET hoạt động 24/7 với đội ngũ chuyên nghiệp, sẵn sàng hỗ trợ người chơi qua nhiều kênh liên lạc.
Kết Luận
Với những ưu điểm nổi bật về dịch vụ, bảo mật, và trải nghiệm người dùng, RGBET xứng đáng là nhà cái hàng đầu mà người chơi nên lựa chọn khi tham gia vào thế giới cá cược trực tuyến.
pin up 306 pin up or pin up 306
https://bbsapp.org/proxy.php?link=https://pinupaz.bid pin up az
pin up az pin up casino and pin up pin up casino
пин ап зеркало pin up казино or пинап казино
https://liverny.jp/_m/index.php?a=free_page/goto_mobile&referer=https://pinupru.site пинап казино
пин ап зеркало pin up зеркало and pin up зеркало pin up
пин ап казино: пин ап казино вход – pin up казино
pin up казино https://pinupturkey.pro/# pin up guncel giris
пин ап кз
ЧћЧ™Ч“Чў ЧЧњЧ’ЧЁЧЎ Ч›Ч™Ч•Ч•Ч Ч™Чќ Ч‘Ч§Ч™ЧЎЧЁЧ™Ч” ЧћЧ•ЧћЧњЧҐ
пин ап казино онлайн: pin up – пин ап казино
pin up giris pin up aviator or pin up aviator
https://images.google.com.ag/url?q=https://pinupturkey.pro pin up giris
pin up pin-up bonanza and pin up bet pin-up casino giris
веном 2 смотреть фильм бесплатно веном веном 2 фильм смотреть онлайн
http://pinupru.site/# pin up
http://pinupkz.tech/# пин ап кз
pin-up kazino pin up azerbaijan or pin-up oyunu
https://maps.google.co.bw/url?q=https://pinupaz.bid pin-up oyunu
pin up azerbaijan pin-up kazino and pin-up kazino pin-up oyunu
https://xn--w2-hs1izvv81cmb366re3s.mystrikingly.com/blog/038494815b5
пин ап казино онлайн https://pinupturkey.pro/# pin-up casino giris
пинап казино
пин ап кз: пинап казино – пинап казино
пин ап казино онлайн http://pinupturkey.pro/# pin-up casino giris
пин ап казино онлайн
nhà cái
Trong bối cảnh ngành công nghiệp cá cược trực tuyến ngày càng phát triển, việc lựa chọn một nhà cái uy tín trở nên vô cùng quan trọng đối với những người đam mê cá cược.Nhà cái RGBET nổi lên như một sự lựa chọn hàng đầu đáng để bạn quan tâm, hứa hẹn mang đến cho bạn một trải nghiệm cá cược an toàn, công bằng và thú vị. Từ các trò chơi cá cược đa dạng, dịch vụ chăm sóc khách hàng tận tình đến tỷ lệ cược cạnh tranh, Rgbet sở hữu nhiều ưu điểm vượt trội khiến bạn không thể bỏ qua.Hãy cùng khám phá những lý do tại sao bạn cần quan tâm đến nhà cái Rgbet và tại sao đây nên là lựa chọn hàng đầu của bạn trong thế giới cá cược trực tuyến.
https://viastoer.blogspot.com/2024/09/blog-post_71.html
pin-up oyunu: pin up casino – pinup azerbaycan
pin-up casino pin up giris or pin up casino guncel giris
https://cse.google.co.vi/url?sa=t&url=https://pinupturkey.pro pin-up casino giris
pin up giris pin-up casino and pin up casino guncel giris pin up bet
перевод с иностранных языков
В магазине сейфов предлагают сейф взломостойкий купить купить сейф взломостойкий в москве
pin up guncel giris pin up bet pin up
пинап казино http://pinupru.site/# пин ап казино
пин ап казино
пин ап казахстан пин ап казино онлайн or pin up
http://www.hashiriya.jp/url.cgi?http://pinupkz.tech/ пинап кз
pin up пин ап казино and пин ап казино пинап кз
pin up giris pin up aviator or pin-up casino giris
https://neruhomosti.net/go.php?url=https://pinupturkey.pro pin-up casino giris
pin up casino guncel giris pin up casino giris and pin up casino giris pin up casino guncel giris
пин ап казино вход пин ап or пин ап
https://www.serbiancafe.com/lat/diskusije/new/redirect.php?url=https://pinupkz.tech pin up
пин ап 634 пин ап казино вход and пинап казино пинап кз
http://pinupaz.bid/# pin up casino
https://pinupru.site/# пин ап
пин ап казино https://pinupturkey.pro/# pin up
pin up kz
pin up casino pinup azerbaycan or pin up
https://images.google.com.pk/url?sa=t&url=https://pinupaz.bid pin-up casino giris
pin up az pin up 306 and pin up azerbaijan pin up az
pin up az: pin up azerbaijan – pin up casino
https://naveridbuy.exblog.jp/35878171/
pin-up casino pin-up bonanza pin up bet
пинап кз http://pinupru.site/# pin up
пин ап казино
Тут делают продвижение сео продвижение медицинского сайта сео продвижение медицинских сайтов
pin-up casino pin up aviator or pin up casino
https://cse.google.jo/url?sa=t&url=https://pinupturkey.pro pin-up casino giris
=buy+teva+generic+viagra]pin up bet pin up aviator and pin up guncel giris pin up casino guncel giris
Тут делают продвижение создание сайта для клиники создание сайтов для клиники
pinup azerbaycan pin up azerbaijan or pin-up casino giris
https://weblib.lib.umt.edu/redirect/proxyselect.php?url=https://pinupaz.bid pin-up casino giris
pin up az pin up azerbaijan and pin up casino pin up
黃金守護神(特殊大賞燈);
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CRぱちんこ仮面ライダーMAX EDITION
https://lucky-777-casino.com/tags/%E6%9C%AA%E7%9F%A5%E3%81%AE%E5%86%92%E9%99%BA
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
神龍降臨
CR麻雀物語~麗しのテンパイ乙女~ Ver.297
吉宗
パチスロ バイオハザード7 レジデント イービル
魔法少女まどか☆マギカ
pin up казино https://pinupru.site/# пин ап
пинап казино
апостиль в новосибирске
pin up: пин ап казахстан – пинап казино
pin up pin-up casino giris or pin-up bonanza
https://www.google.com.sv/url?q=https://pinupturkey.pro pin up
pin-up bonanza pin up casino and pin up guncel giris pin-up casino giris
pin-up oyunu: pin up 306 – pin-up kazino
pin up casino giris pin-up casino giris pin up casino
הסבר על זני קנאביס
пин ап pin up зеркало пин ап казино зеркало
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://imageevent.com/gostsasha1957
https://rentry.org/q5omqw5r
https://imageevent.com/tyhedge1997
https://cannabis.net/user/154133
https://tubeteencam.com/user/crydead1982/profile
пин ап казино вход http://pinupru.site/# пин ап зеркало
пинап казино
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт бытовой техники в воронеже
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр срочный ремонт телефонов где можно отремонтировать телефон
https://xn--fb-hd0jg6f81ltjas9lbns.mystrikingly.com/blog/099e984de60
https://naveridbuy.exblog.jp/35859429/
nhà cái
RGBET: Trang Chủ Cá Cược Uy Tín và Đa Dạng Tại Việt Nam
RGBET đã khẳng định vị thế của mình là một trong những nhà cái hàng đầu tại châu Á và Việt Nam, với đa dạng các dịch vụ cá cược và trò chơi hấp dẫn. Nhà cái này nổi tiếng với việc cung cấp môi trường cá cược an toàn, uy tín và luôn mang lại trải nghiệm tuyệt vời cho người chơi.
Các Loại Hình Cá Cược Tại RGBET
RGBET cung cấp các loại hình cá cược phong phú bao gồm:
Bắn Cá: Một trò chơi đầy kịch tính và thú vị, phù hợp với những người chơi muốn thử thách khả năng săn bắn và nhận về những phần thưởng giá trị.
Thể Thao: Nơi người chơi có thể tham gia đặt cược vào các trận đấu thể thao đa dạng, từ bóng đá, bóng rổ, đến các sự kiện thể thao quốc tế với tỷ lệ cược hấp dẫn.
Game Bài: Đây là một sân chơi dành cho những ai yêu thích các trò chơi bài như tiến lên miền Nam, phỏm, xì dách, và mậu binh. Các trò chơi được phát triển kỹ lưỡng, đem lại cảm giác chân thực và thú vị.
Nổ Hũ: Trò chơi slot với cơ hội trúng Jackpot cực lớn, luôn thu hút sự chú ý của người chơi. Nổ Hũ RGBET là sân chơi hoàn hảo cho những ai muốn thử vận may và giành lấy những phần thưởng khủng.
Lô Đề: Với tỷ lệ trả thưởng cao và nhiều tùy chọn cược, RGBET là nơi lý tưởng cho những người đam mê lô đề.
Tính Năng Nổi Bật Của RGBET
Một trong những điểm nổi bật của RGBET là giao diện thân thiện, dễ sử dụng và hệ thống bảo mật tiên tiến giúp bảo vệ thông tin người chơi. Ngoài ra, nhà cái này còn hỗ trợ các hình thức nạp rút tiền tiện lợi thông qua nhiều kênh khác nhau như OVO, Gopay, Dana, và các chuỗi cửa hàng như Indomaret và Alfamart.
Khuyến Mãi và Dịch Vụ Chăm Sóc Khách Hàng
RGBET không chỉ nổi bật với các trò chơi đa dạng, mà còn cung cấp nhiều chương trình khuyến mãi hấp dẫn như bonus chào mừng, cashback, và các phần thưởng hàng ngày. Đặc biệt, dịch vụ chăm sóc khách hàng của RGBET hoạt động 24/7 với đội ngũ chuyên nghiệp, sẵn sàng hỗ trợ người chơi qua nhiều kênh liên lạc.
Kết Luận
Với những ưu điểm nổi bật về dịch vụ, bảo mật, và trải nghiệm người dùng, RGBET xứng đáng là nhà cái hàng đầu mà người chơi nên lựa chọn khi tham gia vào thế giới cá cược trực tuyến.
пинап кз http://pinupturkey.pro/# pin-up casino giris
пин ап казино вход
пин ап кз пин ап 634 or пин ап 634
https://www.google.co.ck/url?q=http://pinupkz.tech pin up казино
пин ап казино вход пин ап and пин ап 634 пин ап казахстан
пин ап: pin up – пинап кз
Câu lạc bộ bóng đá AS Roma
ST666 tự hào là đối tác tài trợ hàng đầu của câu lạc bộ bóng đá AS Roma – một biểu tượng vĩ đại trong làng bóng đá Ý. AS Roma không chỉ là đội bóng nổi tiếng với lịch sử thi đấu ấn tượng mà còn là đối tác quan trọng trong các hợp đồng hợp tác với ST666. Với sự hợp tác này, AS Roma đã hỗ trợ nhà cái ST666 thông qua những khoản đầu tư triệu đô, giúp phát triển mạnh mẽ mảng thể thao trực tuyến và mở ra cơ hội lớn cho người chơi cá cược tại thị trường quốc tế.
Câu lạc bộ bóng đá Bayern Munich
ST666 cũng vinh dự là nhà tài trợ hàng đầu của câu lạc bộ bóng đá Bayern Munich – một đội bóng nổi tiếng không chỉ ở Đức mà còn trên toàn thế giới. Với lịch sử vô địch lẫy lừng và sự thống trị tại các giải đấu quốc tế, Bayern Munich đã trở thành biểu tượng lớn trong lòng người hâm mộ. Sự hợp tác giữa ST666 và Bayern Munich không chỉ dừng lại ở việc tài trợ mà còn góp phần nâng cao trải nghiệm của người chơi thông qua cải tiến hệ thống giao dịch tài chính, giúp quy trình nạp rút tiền của bet thủ trở nên thuận tiện và nhanh chóng hơn.
Câu lạc bộ bóng đá Porto
Không thể không nhắc đến câu lạc bộ bóng đá Porto, nơi ST666 hân hạnh trở thành đối tác tài trợ hàng đầu. Porto không chỉ là biểu tượng của sự sáng tạo và thành công trong làng bóng đá Bồ Đào Nha mà còn hỗ trợ ST666 trong các thủ tục xin giấy phép và chứng nhận tại châu Âu và Philippines. Sự hợp tác này đã mang lại giá trị lớn cho ST666 khi mở rộng quy mô hoạt động ra nhiều khu vực và thị trường mới.
Câu lạc bộ bóng đá Arsenal
Trong làng bóng đá Anh, câu lạc bộ bóng đá Arsenal là biểu tượng của sự tinh tế và phong cách. ST666 rất tự hào khi trở thành nhà tài trợ chính của Arsenal, giúp nhà cái này không chỉ nâng cao vị thế mà còn mở rộng thị trường cá cược bóng đá toàn cầu. Arsenal đã đóng vai trò quan trọng trong việc hỗ trợ ST666 phát triển hệ thống cá cược, từ đó mang lại trải nghiệm tốt nhất cho người chơi.
Câu lạc bộ bóng đá Sevilla
Cuối cùng, câu lạc bộ bóng đá Sevilla – biểu tượng của sự khát vọng và thành công trong bóng đá Tây Ban Nha, cũng đã trở thành đối tác quan trọng của ST666. Sevilla không chỉ góp phần thúc đẩy ST666 về mặt thương mại mà còn cung cấp công nghệ bảo mật hàng đầu, đảm bảo rằng thông tin cá nhân, tài khoản và các giao dịch của người chơi đều được bảo vệ một cách tối ưu.
Điểm vượt trội của ST666 so với các đối thủ cạnh tranh khác
Sự hợp tác chiến lược giữa ST666 và các câu lạc bộ bóng đá hàng đầu không chỉ nâng cao vị thế của ST666 trên thị trường, mà còn mang lại những lợi ích vượt trội cho người chơi. Những điểm mạnh này bao gồm:
Hệ thống bảo mật tiên tiến: ST666 đã được Sevilla hỗ trợ trong việc nâng cấp công nghệ bảo mật, đảm bảo mọi giao dịch và thông tin người dùng đều an toàn.
Hệ thống nạp rút tiện lợi: Sự hợp tác với Bayern Munich giúp ST666 cải tiến hệ thống giao dịch tài chính, mang lại trải nghiệm nạp rút nhanh chóng và hiệu quả cho người chơi.
Mở rộng thị trường và dịch vụ: Nhờ sự hỗ trợ từ Arsenal và AS Roma, ST666 đã thành công trong việc mở rộng hệ thống cá cược bóng đá và tiếp cận thêm nhiều người chơi trên toàn thế giới.
ST666 không chỉ là một nhà cái hàng đầu mà còn là một đối tác đáng tin cậy của các câu lạc bộ bóng đá danh tiếng, luôn cam kết mang lại dịch vụ tốt nhất và trải nghiệm tối ưu cho người chơi.
https://medium.com/@carlfrancoh38793/%EB%84%A4%EC%9D%B4%EB%B2%84-%EC%95%84%EC%9D%B4%EB%94%94-%EC%82%AC%EA%B8%B0-%ED%94%BC%ED%95%B4%EB%A5%BC-%EB%B0%A9%EC%A7%80%ED%95%98%EB%8A%94-6%EA%B0%80%EC%A7%80-%EB%B0%A9%EB%B2%95-b753a61726ab
We are a leading real estate consultancy company in Nigeria. Our integrated real estate services include facility management, portfolio management, real estate consultancy, property management, office renovation, property sales and rental, interior design, office decoration, real estate turnkey project, property refurbishment and sales.
st666
Câu lạc bộ bóng đá AS Roma
ST666 tự hào là đối tác tài trợ hàng đầu của câu lạc bộ bóng đá AS Roma – một biểu tượng vĩ đại trong làng bóng đá Ý. AS Roma không chỉ là đội bóng nổi tiếng với lịch sử thi đấu ấn tượng mà còn là đối tác quan trọng trong các hợp đồng hợp tác với ST666. Với sự hợp tác này, AS Roma đã hỗ trợ nhà cái ST666 thông qua những khoản đầu tư triệu đô, giúp phát triển mạnh mẽ mảng thể thao trực tuyến và mở ra cơ hội lớn cho người chơi cá cược tại thị trường quốc tế.
Câu lạc bộ bóng đá Bayern Munich
ST666 cũng vinh dự là nhà tài trợ hàng đầu của câu lạc bộ bóng đá Bayern Munich – một đội bóng nổi tiếng không chỉ ở Đức mà còn trên toàn thế giới. Với lịch sử vô địch lẫy lừng và sự thống trị tại các giải đấu quốc tế, Bayern Munich đã trở thành biểu tượng lớn trong lòng người hâm mộ. Sự hợp tác giữa ST666 và Bayern Munich không chỉ dừng lại ở việc tài trợ mà còn góp phần nâng cao trải nghiệm của người chơi thông qua cải tiến hệ thống giao dịch tài chính, giúp quy trình nạp rút tiền của bet thủ trở nên thuận tiện và nhanh chóng hơn.
Câu lạc bộ bóng đá Porto
Không thể không nhắc đến câu lạc bộ bóng đá Porto, nơi ST666 hân hạnh trở thành đối tác tài trợ hàng đầu. Porto không chỉ là biểu tượng của sự sáng tạo và thành công trong làng bóng đá Bồ Đào Nha mà còn hỗ trợ ST666 trong các thủ tục xin giấy phép và chứng nhận tại châu Âu và Philippines. Sự hợp tác này đã mang lại giá trị lớn cho ST666 khi mở rộng quy mô hoạt động ra nhiều khu vực và thị trường mới.
Câu lạc bộ bóng đá Arsenal
Trong làng bóng đá Anh, câu lạc bộ bóng đá Arsenal là biểu tượng của sự tinh tế và phong cách. ST666 rất tự hào khi trở thành nhà tài trợ chính của Arsenal, giúp nhà cái này không chỉ nâng cao vị thế mà còn mở rộng thị trường cá cược bóng đá toàn cầu. Arsenal đã đóng vai trò quan trọng trong việc hỗ trợ ST666 phát triển hệ thống cá cược, từ đó mang lại trải nghiệm tốt nhất cho người chơi.
Câu lạc bộ bóng đá Sevilla
Cuối cùng, câu lạc bộ bóng đá Sevilla – biểu tượng của sự khát vọng và thành công trong bóng đá Tây Ban Nha, cũng đã trở thành đối tác quan trọng của ST666. Sevilla không chỉ góp phần thúc đẩy ST666 về mặt thương mại mà còn cung cấp công nghệ bảo mật hàng đầu, đảm bảo rằng thông tin cá nhân, tài khoản và các giao dịch của người chơi đều được bảo vệ một cách tối ưu.
Điểm vượt trội của ST666 so với các đối thủ cạnh tranh khác
Sự hợp tác chiến lược giữa ST666 và các câu lạc bộ bóng đá hàng đầu không chỉ nâng cao vị thế của ST666 trên thị trường, mà còn mang lại những lợi ích vượt trội cho người chơi. Những điểm mạnh này bao gồm:
Hệ thống bảo mật tiên tiến: ST666 đã được Sevilla hỗ trợ trong việc nâng cấp công nghệ bảo mật, đảm bảo mọi giao dịch và thông tin người dùng đều an toàn.
Hệ thống nạp rút tiện lợi: Sự hợp tác với Bayern Munich giúp ST666 cải tiến hệ thống giao dịch tài chính, mang lại trải nghiệm nạp rút nhanh chóng và hiệu quả cho người chơi.
Mở rộng thị trường và dịch vụ: Nhờ sự hỗ trợ từ Arsenal và AS Roma, ST666 đã thành công trong việc mở rộng hệ thống cá cược bóng đá và tiếp cận thêm nhiều người chơi trên toàn thế giới.
ST666 không chỉ là một nhà cái hàng đầu mà còn là một đối tác đáng tin cậy của các câu lạc bộ bóng đá danh tiếng, luôn cam kết mang lại dịch vụ tốt nhất và trải nghiệm tối ưu cho người chơi.
веном 2 часть веном 2 смотреть онлайн ок веном онлайн
ST666 – Trang Chủ Cá Cược Chính Thức Tại Việt Nam
ST666 là một trong những nhà cái cá cược hàng đầu châu Á, nổi bật với dịch vụ đa dạng và hệ thống game đổi thưởng hấp dẫn. Với nhiều năm kinh nghiệm trong lĩnh vực cá cược trực tuyến, ST666 đã xây dựng được uy tín và niềm tin từ hàng triệu người chơi trên khắp khu vực.
Hệ Thống Game Đổi Thưởng ST666
Tại ST666, người chơi có thể trải nghiệm nhiều loại hình giải trí khác nhau, bao gồm:
Bắn cá: Một trong những trò chơi được ưa chuộng nhất, mang lại trải nghiệm bắn súng dưới nước độc đáo và hấp dẫn.
Thể thao: Cung cấp cược thể thao từ các giải đấu lớn nhỏ trên toàn thế giới với tỷ lệ cược hấp dẫn.
Xổ số: Trò chơi đơn giản, dễ chơi với cơ hội nhận thưởng lớn.
Game bài: Đa dạng các trò chơi bài như poker, baccarat, và nhiều thể loại khác.
Nổ hũ: Trò chơi đầy kịch tính với các phần thưởng cực lớn.
Live Casino: Người chơi có thể tham gia các sòng bài trực tuyến với dealer thật qua hình thức phát trực tiếp, mang lại cảm giác như đang ngồi tại sòng bài thực sự.
Giao Diện Hiện Đại & Bảo Mật Tuyệt Đối
ST666 không chỉ nổi bật với hệ thống game phong phú mà còn thu hút người chơi nhờ vào giao diện hiện đại, thân thiện. Thiết kế trang web đẹp mắt, dễ sử dụng giúp người chơi dễ dàng thao tác và tận hưởng các trò chơi mà không gặp khó khăn.
Đặc biệt, hệ thống bảo mật của ST666 luôn được đánh giá cao. Tất cả các giao dịch và thông tin cá nhân của người chơi đều được bảo vệ bằng công nghệ mã hóa tiên tiến, đảm bảo an toàn tuyệt đối cho người dùng.
Ưu Đãi Hấp Dẫn & Tỷ Lệ Đổi Thưởng Cao
ST666 luôn mang đến cho người chơi nhiều khuyến mãi hấp dẫn. Người chơi có thể nhận được 280k miễn phí khi đăng nhập hàng ngày, cùng với hàng loạt các chương trình ưu đãi khác, như thưởng nạp đầu, khuyến mãi hoàn tiền, và nhiều sự kiện đặc biệt dành cho các thành viên thân thiết.
Bên cạnh đó, tỷ lệ đổi thưởng tại ST666 luôn cao hơn so với nhiều nhà cái khác, mang lại cơ hội thắng lớn cho người chơi.
Tải App ST666
Để thuận tiện hơn trong việc trải nghiệm, ST666 đã phát triển ứng dụng dành riêng cho di động. Người chơi có thể dễ dàng tải app ST666 về máy và tham gia cá cược bất cứ lúc nào, bất cứ nơi đâu, mà không cần phải truy cập qua trình duyệt web.
Kết Luận
ST666 là lựa chọn lý tưởng cho những ai yêu thích cá cược và muốn tìm kiếm một nhà cái đáng tin cậy. Với hệ thống game đa dạng, giao diện hiện đại, bảo mật tốt và nhiều khuyến mãi hấp dẫn, ST666 chắc chắn sẽ mang lại cho người chơi những trải nghiệm tuyệt vời và đáng nhớ.
amoxicillin 750 mg price: Amoxicillin For sale – amoxicillin buy online canada
neurontin 800 mg tablets best price gabapentin best price medication neurontin 300 mg
Поиск в гугле
Nhà cái ST666 được biết đến là sân chơi uy tín cung cấp các sản phẩm trò chơi cực kỳ đa dạng và chất lượng. Số lượng người tham gia vào hệ thống ngày càng tăng cao và chưa có dấu hiệu dừng lại. Hướng dẫn tham gia nhà cái nếu như anh em muốn tìm kiếm cơ hội nhận thưởng khủng. Hãy cùng khám phá những thông tin cần biết tại nhà cái để đặt cược và săn thưởng thành công.
The best https://bestwebsiteto.com sites on the web to suit your needs. The top-rated platforms to help you succeed in learning new skills
https://medium.com/@dqvchristopherwhite824/%EB%84%A4%EC%9D%B4%EB%B2%84-%EC%8B%A4%EB%AA%85-%EC%95%84%EC%9D%B4%EB%94%94-%EA%B5%AC%EB%A7%A4-56a09b77144c
http://stromectol.agency/# minocycline 100 mg tablets online
http://zithromax.company/# zithromax
prescription for amoxicillin cheapest amoxil amoxicillin tablet 500mg
Тут делают продвижение сео продвижение медицинского сайта сео продвижение медицинских сайтов
Тут делают продвижение разработка сайт медицинской клиники разработка сайт медицинского центра
Апостиль в Новосибирске – это процедура, необходимая для признания документов, выданных в одной стране, действительными в другой. Наши специалисты помогут вам правильно оформить документы и пройти процедуру апостиля в кратчайшие сроки. Также мы предлагаем услуги перевода с иностранных языков и перевода документов.
апостиль в новосибирске
https://stromectol.agency/# minocycline 100mg tablets
Тут делают продвижение создание сайта клиники создание медицинского сайта
https://stromectol.agency/# stromectol price uk
Upgrade your style with chic fashion from DL1961 denim.
minocycline 50mg tabs: ivermectin 1% cream generic – cost of ivermectin 3mg tablets
buy semaglutide online: buy semaglutide online – rybelsus generic
https://zithromax.company/# buy zithromax online fast shipping
where can you buy amoxicillin over the counter amoxicillin cheapest price amoxicillin tablets in india
Профессиональный сервисный центр по ремонту моноблоков iMac в Москве.
Мы предлагаем: срочный ремонт imac
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://amoxil.llc/# buy amoxicillin over the counter uk
https://xn--lv-o02ik82aiqcqsko8mfg5a1sb.mystrikingly.com/blog/e14a0fa56fe
ST666 – Lựa Chọn Hàng Đầu Trong Thế Giới Cá Cược Trực Tuyến
Trong thời đại mà ngành công nghiệp cá cược trực tuyến đang bùng nổ, việc chọn một nhà cái uy tín là yếu tố quan trọng để đảm bảo trải nghiệm chơi an toàn và đáng tin cậy. ST666 nhanh chóng khẳng định vị thế của mình trên thị trường với những dịch vụ chất lượng và hệ thống bảo mật cao cấp, mang lại trải nghiệm cá cược công bằng, thú vị và an toàn.
Hệ Thống Trò Chơi Đa Dạng
ST666 cung cấp một danh mục trò chơi cá cược đa dạng, đáp ứng mọi nhu cầu và sở thích của người chơi. Từ thể thao, bắn cá, game bài, cho đến xổ số và live casino, tất cả đều được tối ưu hóa để mang lại cảm giác hứng thú cho người tham gia. Đặc biệt, với Live Casino, người chơi sẽ được trải nghiệm cá cược với các dealer thật thông qua hình thức phát trực tiếp, tạo cảm giác như đang tham gia tại các sòng bài thực tế.
Tỷ Lệ Cược Cạnh Tranh
Một trong những ưu điểm nổi bật của ST666 chính là tỷ lệ cược cực kỳ cạnh tranh, giúp người chơi tối ưu hóa lợi nhuận của mình. Các trận đấu thể thao được cập nhật liên tục với tỷ lệ cược hấp dẫn, tạo cơ hội chiến thắng lớn cho những ai yêu thích cá cược thể thao.
Bảo Mật An Toàn Tuyệt Đối
Yếu tố bảo mật luôn là một trong những tiêu chí hàng đầu khi chọn nhà cái cá cược. ST666 sử dụng công nghệ mã hóa hiện đại để bảo vệ thông tin cá nhân và các giao dịch tài chính của người chơi, đảm bảo mọi dữ liệu luôn được bảo mật tuyệt đối. Người chơi hoàn toàn có thể yên tâm khi tham gia cá cược tại đây.
Chăm Sóc Khách Hàng Chuyên Nghiệp
Dịch vụ chăm sóc khách hàng của ST666 luôn sẵn sàng hỗ trợ 24/7 qua nhiều kênh khác nhau như Live Chat, WhatsApp, Facebook, đảm bảo giải đáp mọi thắc mắc và xử lý các vấn đề nhanh chóng. Đội ngũ nhân viên tại đây được đào tạo chuyên nghiệp, tận tâm và chu đáo, luôn đặt lợi ích của khách hàng lên hàng đầu.
Ưu Đãi Hấp Dẫn
ST666 không chỉ nổi bật với các sản phẩm cá cược mà còn thu hút người chơi bởi các chương trình khuyến mãi hấp dẫn. Người chơi có thể nhận được nhiều ưu đãi như 280k miễn phí khi đăng nhập hàng ngày, cùng các chương trình thưởng nạp và hoàn tiền liên tục, giúp tối đa hóa cơ hội chiến thắng.
Lý Do ST666 Là Lựa Chọn Hàng Đầu
Sự kết hợp hoàn hảo giữa giao diện hiện đại, hệ thống trò chơi đa dạng, dịch vụ khách hàng tận tâm và tỷ lệ cược cạnh tranh đã giúp ST666 trở thành một trong những nhà cái cá cược trực tuyến uy tín hàng đầu tại Việt Nam. Đối với những ai đang tìm kiếm một nhà cái an toàn và chuyên nghiệp, ST666 chắc chắn sẽ là lựa chọn không thể bỏ qua.
Kết Luận
Với những ưu điểm vượt trội, ST666 xứng đáng là địa chỉ cá cược uy tín và an toàn cho tất cả người chơi. Đăng ký ngay hôm nay để trải nghiệm hệ thống cá cược đẳng cấp và nhận những ưu đãi hấp dẫn từ ST666!
Rybelsus 14 mg price order Rybelsus for weight loss rybelsus cost
https://www.starsandstripesfc.com/users/shepparduvg897
https://zithromax.company/# zithromax over the counter canada
https://stromectol.agency/# minocycline 50 mg pills online
gabapentin generic neurontin gabapentin or neurontin drug
https://maps.google.dm/url?sa=t&url=https://gabapentin.auction buy neurontin 300 mg
can you buy neurontin over the counter neurontin 900 mg and neurontin gabapentin neurontin price uk
neurontin 600 mg cost: buy gabapentin – neurontin oral
해선 결제 디비
http://zithromax.company/# zithromax for sale 500 mg
where can i buy zithromax uk: zithromax for sale – zithromax buy online no prescription
ivermectin 0.2mg stromectol price stromectol pills
https://amoxil.llc/# buy cheap amoxicillin online
https://amoxil.llc/# amoxicillin 1000 mg capsule
zithromax over the counter
http://semaglutide.win/# buy rybelsus
generic zithromax medicine
zithromax z-pak order zithromax generic zithromax 500mg
amoxicillin 500 mg price amoxicillin buy no prescription or 875 mg amoxicillin cost
https://images.google.it/url?sa=t&url=https://amoxil.llc amoxicillin 500mg over the counter
generic for amoxicillin amoxicillin pharmacy price and amoxicillin 500mg cheap amoxicillin 500mg
https://zithromax.company/# zithromax antibiotic without prescription
Pコードギアス 反逆のルルーシュ Rebellion to Re_surrection
k8スロット
美しいアニメーションと迫力ある演出が魅力。プレイするたびに新しい発見があります。
CR JAWS再臨-SHARK PANIC AGAIN-
https://k8wl.com/tags/%E4%BD%90%E8%97%A4%E7%9B%B4%E6%A8%B9
パチンコの仲間との交流が楽しく、共通の趣味で楽しめるのが良いです。友達が増えます。
PA ぱちんこ AKB48 桜 LIGHT ver.
k8パチンコ
主役は銭形 2004
リング呪いの7日間;
北斗の拳-拳王
https://amoxil.llc/# azithromycin amoxicillin
how to get neurontin cheap gabapentin generic or neurontin brand coupon
https://images.google.co.uz/url?q=https://gabapentin.auction where can i buy neurontin from canada
neurontin 800 mg tablets neurontin 100mg discount and neurontin 300 neurontin 900 mg
neurontin 100 mg capsule: order gabapentin – medication neurontin 300 mg
purchase zithromax z-pak where can you buy zithromax zithromax tablets for sale
ivermectin eye drops: stromectol for sale – minocycline 100mg tablets online
http://gabapentin.auction/# neurontin 100mg price
https://stocktwits.com/viastoer
https://gabapentin.auction/# neurontin without prescription
https://adroit-penguin-dc4vl0.mystrikingly.com/blog/6adeca88a79
Ищите в гугле
neurontin 200 mg neurontin cost or canada neurontin 100mg discount
https://cse.google.sc/url?q=https://gabapentin.auction neurontin 600 mg tablet
where can i buy neurontin online buy neurontin online uk and neurontin 300 600 mg neurontin mexico
https://gabapentin.auction/# neurontin price australia
order zithromax without prescription
rybelsus generic Semaglutide pharmacy price or Rybelsus 14 mg
https://image.google.com.sl/url?q=https://semaglutide.win Rybelsus 14 mg price
buy rybelsus Buy compounded semaglutide online and cheap Rybelsus 14 mg Buy semaglutide pills
https://stromectol.agency/# stromectol price uk
zithromax 500mg over the counter
zithromax prescription online zithromax capsules 250mg generic zithromax 500mg
https://stromectol.agency/# minocycline 100mg pills
rybelsus price rybelsus cost or Buy semaglutide pills
http://capecoddaily.com/?URL=https://semaglutide.win Rybelsus 7mg
rybelsus buy semaglutide online and Rybelsus 14 mg Rybelsus 14 mg price
https://zithromax.company/# buy zithromax no prescription
amoxicillin 500 mg where to buy how much is amoxicillin prescription or over the counter amoxicillin
https://image.google.co.ug/url?q=https://amoxil.llc cost of amoxicillin 30 capsules
amoxicillin 500mg buy online uk amoxicillin online without prescription and buy amoxicillin from canada how to get amoxicillin over the counter
перевод с иностранных языков
https://amoxil.llc/# rexall pharmacy amoxicillin 500mg
neurontin: buy gabapentin – medicine neurontin
neurontin 204 gabapentin for sale neurontin 214
cost of amoxicillin 30 capsules: buy amoxil – amoxicillin pharmacy price
https://gabapentin.auction/# neurontin 204
https://faowa3-afjoa-fjia3.mystrikingly.com/blog/5b3681de2f1
rybelsus generic Semaglutide pharmacy price rybelsus price
https://amoxil.llc/# amoxicillin pharmacy price
where can i buy zithromax in canada
https://stromectol.agency/# ivermectin canada
where can i buy zithromax capsules
brand name neurontin price neurontin 4000 mg or buy neurontin
https://community.pharos.com/external-link.jspa?url=http://gabapentin.auction neurontin 1800 mg
medicine neurontin 300 mg gabapentin medication and neurontin 1800 mg 32 neurontin
https://semaglutide.win/# rybelsus
https://amoxil.llc/# amoxicillin over the counter in canada
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.hentai-foundry.com/user/gazvpol1991/profile
https://rezors1979.diary.ru/
https://anotepad.com/notes/b5e7f2y7
https://chyoa.com/user/sideper1956
https://rentry.org/p26rycha
buy cheap neurontin online gabapentin 100mg or purchase neurontin
https://www.google.nl/url?sa=t&url=https://gabapentin.auction medication neurontin 300 mg
canada where to buy neurontin neurontin brand name 800mg best price and neurontin oral neurontin 600 mg
price of amoxicillin without insurance where to buy amoxicillin 500mg without prescription or amoxicillin capsule 500mg price
http://www.weedy.be/2-uncategorised/1320-redirect2?url=http://amoxil.llc amoxicillin no prescription
amoxicillin brand name amoxicillin 500mg over the counter and how to buy amoxicillin online amoxicillin 500 mg tablet
https://semaglutide.win/# Semaglutide pharmacy price
can i buy zithromax online zithromax best price zithromax 250 price
neurontin 100mg: buy gabapentin – brand name neurontin
https://stromectol.agency/# ivermectin drug
amoxicillin 500mg pill: amoxil best price – where to buy amoxicillin pharmacy
cheap zithromax pills order zithromax buy zithromax online with mastercard
https://medium.com/@nsw5288/%EC%98%A8%EB%9D%BC%EC%9D%B8%EC%97%90%EC%84%9C-%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC-%EA%B5%AC%EB%A7%A4-%EC%8B%9C-%EC%A3%BC%EC%9D%98%ED%95%B4%EC%95%BC-%ED%95%A0-%EC%82%AC%ED%95%AD-c821acd22b28
https://www.twinkietown.com/users/shepparduvg897
https://zithromax.company/# zithromax for sale usa
zithromax over the counter
http://stromectol.agency/# stromectol buy uk
where can i get zithromax over the counter
https://stromectol.agency/# is minocycline an antibiotic
rybelsus generic Buy compounded semaglutide online or buy rybelsus
https://www.google.mk/url?q=https://semaglutide.win semaglutide
rybelsus price Rybelsus 7mg and rybelsus generic Rybelsus 14 mg
https://gabapentin.auction/# neurontin 100mg tablets
https://www.metooo.io/u/66973d4f7d959422b10fb4bc
https://xn--gc-2e2i723b91ktjas9l307b.mystrikingly.com/blog/802294b3778
amoxicillin order online no prescription cheapest amoxil buy amoxicillin 500mg uk
http://zithromax.company/# average cost of generic zithromax
amoxicillin without rx: amoxicillin cheapest price – where to buy amoxicillin pharmacy
neurontin 600 mg capsule neurontin capsule 600mg or neurontin 30 mg
https://maps.google.si/url?sa=t&url=https://gabapentin.auction neurontin 400 mg capsules
neurontin pill neurontin from canada and neurontin 214 neurontin brand name 800mg best price
ivermectin cream cost: stromectol price – ivermectin cost uk
generic ivermectin for humans stromectol for sale ivermectin price uk
http://stromectol.agency/# ivermectin 500mg
where to buy amoxicillin amoxicillin 500mg tablets price in india or where to buy amoxicillin 500mg without prescription
https://alt1.toolbarqueries.google.ac/url?q=https://amoxil.llc amoxicillin 500 mg purchase without prescription
buy amoxicillin 500mg capsules uk where can i buy amoxicillin over the counter and amoxicillin 500 mg cost price of amoxicillin without insurance
http://gabapentin.auction/# neurontin 600mg
where can i get zithromax over the counter
http://stromectol.agency/# buy liquid ivermectin
zithromax 500 mg lowest price drugstore online
ST666 – Lựa Chọn Hàng Đầu Trong Thế Giới Cá Cược Trực Tuyến
Trong thời đại mà ngành công nghiệp cá cược trực tuyến đang bùng nổ, việc chọn một nhà cái uy tín là yếu tố quan trọng để đảm bảo trải nghiệm chơi an toàn và đáng tin cậy. ST666 nhanh chóng khẳng định vị thế của mình trên thị trường với những dịch vụ chất lượng và hệ thống bảo mật cao cấp, mang lại trải nghiệm cá cược công bằng, thú vị và an toàn.
Hệ Thống Trò Chơi Đa Dạng
ST666 cung cấp một danh mục trò chơi cá cược đa dạng, đáp ứng mọi nhu cầu và sở thích của người chơi. Từ thể thao, bắn cá, game bài, cho đến xổ số và live casino, tất cả đều được tối ưu hóa để mang lại cảm giác hứng thú cho người tham gia. Đặc biệt, với Live Casino, người chơi sẽ được trải nghiệm cá cược với các dealer thật thông qua hình thức phát trực tiếp, tạo cảm giác như đang tham gia tại các sòng bài thực tế.
Tỷ Lệ Cược Cạnh Tranh
Một trong những ưu điểm nổi bật của ST666 chính là tỷ lệ cược cực kỳ cạnh tranh, giúp người chơi tối ưu hóa lợi nhuận của mình. Các trận đấu thể thao được cập nhật liên tục với tỷ lệ cược hấp dẫn, tạo cơ hội chiến thắng lớn cho những ai yêu thích cá cược thể thao.
Bảo Mật An Toàn Tuyệt Đối
Yếu tố bảo mật luôn là một trong những tiêu chí hàng đầu khi chọn nhà cái cá cược. ST666 sử dụng công nghệ mã hóa hiện đại để bảo vệ thông tin cá nhân và các giao dịch tài chính của người chơi, đảm bảo mọi dữ liệu luôn được bảo mật tuyệt đối. Người chơi hoàn toàn có thể yên tâm khi tham gia cá cược tại đây.
Chăm Sóc Khách Hàng Chuyên Nghiệp
Dịch vụ chăm sóc khách hàng của ST666 luôn sẵn sàng hỗ trợ 24/7 qua nhiều kênh khác nhau như Live Chat, WhatsApp, Facebook, đảm bảo giải đáp mọi thắc mắc và xử lý các vấn đề nhanh chóng. Đội ngũ nhân viên tại đây được đào tạo chuyên nghiệp, tận tâm và chu đáo, luôn đặt lợi ích của khách hàng lên hàng đầu.
Ưu Đãi Hấp Dẫn
ST666 không chỉ nổi bật với các sản phẩm cá cược mà còn thu hút người chơi bởi các chương trình khuyến mãi hấp dẫn. Người chơi có thể nhận được nhiều ưu đãi như 280k miễn phí khi đăng nhập hàng ngày, cùng các chương trình thưởng nạp và hoàn tiền liên tục, giúp tối đa hóa cơ hội chiến thắng.
Lý Do ST666 Là Lựa Chọn Hàng Đầu
Sự kết hợp hoàn hảo giữa giao diện hiện đại, hệ thống trò chơi đa dạng, dịch vụ khách hàng tận tâm và tỷ lệ cược cạnh tranh đã giúp ST666 trở thành một trong những nhà cái cá cược trực tuyến uy tín hàng đầu tại Việt Nam. Đối với những ai đang tìm kiếm một nhà cái an toàn và chuyên nghiệp, ST666 chắc chắn sẽ là lựa chọn không thể bỏ qua.
Kết Luận
Với những ưu điểm vượt trội, ST666 xứng đáng là địa chỉ cá cược uy tín và an toàn cho tất cả người chơi. Đăng ký ngay hôm nay để trải nghiệm hệ thống cá cược đẳng cấp và nhận những ưu đãi hấp dẫn từ ST666!
https://stromectol.agency/# minocycline 50 mg tablets for human
neurontin 300 mg caps neurontin 900 or brand neurontin 100 mg canada
http://msichat.de/redir.php?url=http://gabapentin.auction generic gabapentin
neurontin sale gabapentin 100mg and cheap neurontin online neurontin 800 mg tablets
http://zithromax.company/# buy zithromax online
amoxicillin 875 mg tablet amoxil best price amoxicillin over counter
https://amoxil.llc/# cost of amoxicillin
neurontin 800 mg price: gabapentin for sale – order neurontin
semaglutide Rybelsus 14 mg price order Rybelsus for weight loss
Buy semaglutide pills: buy rybelsus – Rybelsus 14 mg
https://semaglutide.win/# rybelsus cost
http://stromectol.agency/# ivermectin 3mg tab
zithromax pill
https://amoxil.llc/# amoxicillin no prescription
zithromax 500 without prescription
buy amoxicillin without prescription amoxicillin 500 or amoxicillin where to get
http://www.otohits.net/home/redirectto?url=https://amoxil.llc how to buy amoxicillin online
buy amoxicillin 500mg capsules uk amoxicillin 500 mg without prescription and price for amoxicillin 875 mg amoxicillin 500 mg
戦国パチスロ花の慶次戦極めし傾奇者の宴;
k8 カジノ パチンコ e スマパチ シン・エヴァンゲリオン Type カヲルパチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
P 北斗の拳 暴凶星
https://k8wap.com/tags/%E3%83%97%E3%83%AC%E3%82%A4%E3%83%A4%E3%83%BC%E3%82%92%E9%AD%85%E4%BA%86%E3%81%97%E3%81%BE%E3%81%99
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
ファファファ
[url=https://sites.google.com/view/k8twilightprincess]k8スロット
[/url]
コードギアス反逆のルルーシュ
CR麻雀物語麗しのテンパイ乙女 Ver.297
喧嘩祭
http://zithromax.company/# zithromax drug
st666 app
ST666 – Lựa Chọn Hàng Đầu Trong Thế Giới Cá Cược Trực Tuyến
Trong thời đại mà ngành công nghiệp cá cược trực tuyến đang bùng nổ, việc chọn một nhà cái uy tín là yếu tố quan trọng để đảm bảo trải nghiệm chơi an toàn và đáng tin cậy. ST666 nhanh chóng khẳng định vị thế của mình trên thị trường với những dịch vụ chất lượng và hệ thống bảo mật cao cấp, mang lại trải nghiệm cá cược công bằng, thú vị và an toàn.
Hệ Thống Trò Chơi Đa Dạng
ST666 cung cấp một danh mục trò chơi cá cược đa dạng, đáp ứng mọi nhu cầu và sở thích của người chơi. Từ thể thao, bắn cá, game bài, cho đến xổ số và live casino, tất cả đều được tối ưu hóa để mang lại cảm giác hứng thú cho người tham gia. Đặc biệt, với Live Casino, người chơi sẽ được trải nghiệm cá cược với các dealer thật thông qua hình thức phát trực tiếp, tạo cảm giác như đang tham gia tại các sòng bài thực tế.
Tỷ Lệ Cược Cạnh Tranh
Một trong những ưu điểm nổi bật của ST666 chính là tỷ lệ cược cực kỳ cạnh tranh, giúp người chơi tối ưu hóa lợi nhuận của mình. Các trận đấu thể thao được cập nhật liên tục với tỷ lệ cược hấp dẫn, tạo cơ hội chiến thắng lớn cho những ai yêu thích cá cược thể thao.
Bảo Mật An Toàn Tuyệt Đối
Yếu tố bảo mật luôn là một trong những tiêu chí hàng đầu khi chọn nhà cái cá cược. ST666 sử dụng công nghệ mã hóa hiện đại để bảo vệ thông tin cá nhân và các giao dịch tài chính của người chơi, đảm bảo mọi dữ liệu luôn được bảo mật tuyệt đối. Người chơi hoàn toàn có thể yên tâm khi tham gia cá cược tại đây.
Chăm Sóc Khách Hàng Chuyên Nghiệp
Dịch vụ chăm sóc khách hàng của ST666 luôn sẵn sàng hỗ trợ 24/7 qua nhiều kênh khác nhau như Live Chat, WhatsApp, Facebook, đảm bảo giải đáp mọi thắc mắc và xử lý các vấn đề nhanh chóng. Đội ngũ nhân viên tại đây được đào tạo chuyên nghiệp, tận tâm và chu đáo, luôn đặt lợi ích của khách hàng lên hàng đầu.
Ưu Đãi Hấp Dẫn
ST666 không chỉ nổi bật với các sản phẩm cá cược mà còn thu hút người chơi bởi các chương trình khuyến mãi hấp dẫn. Người chơi có thể nhận được nhiều ưu đãi như 280k miễn phí khi đăng nhập hàng ngày, cùng các chương trình thưởng nạp và hoàn tiền liên tục, giúp tối đa hóa cơ hội chiến thắng.
Lý Do ST666 Là Lựa Chọn Hàng Đầu
Sự kết hợp hoàn hảo giữa giao diện hiện đại, hệ thống trò chơi đa dạng, dịch vụ khách hàng tận tâm và tỷ lệ cược cạnh tranh đã giúp ST666 trở thành một trong những nhà cái cá cược trực tuyến uy tín hàng đầu tại Việt Nam. Đối với những ai đang tìm kiếm một nhà cái an toàn và chuyên nghiệp, ST666 chắc chắn sẽ là lựa chọn không thể bỏ qua.
Kết Luận
Với những ưu điểm vượt trội, ST666 xứng đáng là địa chỉ cá cược uy tín và an toàn cho tất cả người chơi. Đăng ký ngay hôm nay để trải nghiệm hệ thống cá cược đẳng cấp và nhận những ưu đãi hấp dẫn từ ST666!
neurontin brand name neurontin 800 mg price or 800mg neurontin
http://www.fairkaufen.de/auktion/phpinfo.php?a=best+place+to+buy+viagra neurontin 400 mg capsules
gabapentin buy neurontin cost generic and buy cheap neurontin online neurontin 400 mg capsule
rybelsus cost rybelsus or Buy semaglutide pills
http://www.b08.com/tools/domaininfo.php?domain=semaglutide.win Rybelsus 14 mg price
[url=https://www.google.lk/url?q=https://semaglutide.win]order Rybelsus for weight loss[/url] rybelsus and [url=https://www.jjj555.com/home.php?mod=space&uid=1696980]Buy semaglutide pills[/url] buy rybelsus
f530094 click here for more v2913836
https://semaglutide.win/# buy rybelsus
buy rybelsus semaglutide or Buy compounded semaglutide online
https://www.google.nu/url?q=https://semaglutide.win Rybelsus 7mg
[url=http://www.yabuno.net/w3a/redirect.php?redirect=http://semaglutide.win]Rybelsus 7mg[/url] rybelsus cost and [url=https://www.jjj555.com/home.php?mod=space&uid=1696844]rybelsus generic[/url] Buy compounded semaglutide online
neurontin 50mg tablets gabapentin price medicine neurontin capsules
https://maroon-cat-dc4vlw.mystrikingly.com/blog/a1f40de2c25
https://amoxil.llc/# can you buy amoxicillin over the counter
https://gabapentin.auction/# neurontin 50mg tablets
neurontin 4000 mg buying neurontin without a prescription or order neurontin online
http://4vn.eu/forum/vcheckvirus.php?url=http://gabapentin.auction neurontin 300 mg capsule
neurontin 50mg cost gabapentin buy and neurontin discount over the counter neurontin
amoxicillin medicine over the counter: buy amoxil – cost of amoxicillin
best foam roller
Over 7 years in development, the Rollga Method™ combines trigger points, acupressure points, fascial lines, meridians, and techniques like ART (Active Release Technique), MAT (Muscle Activation Technique), RPR (Reflexive Performance Reset) and others to optimize one’s health thru a new system of self care.
Download the Rollga App to learn about & tap into all that Rollga has to offer!
minocycline 50mg tablets online order stromectol buy minocycline 100 mg tablets
zithromax: buy zithromax z-pak online – zithromax 500 price
https://gabapentin.auction/# cost of neurontin 600 mg
zithromax azithromycin
http://zithromax.company/# buy zithromax online cheap
zithromax 500mg over the counter
CRザ・キング・オブ・ファイターズ
牙狼 守りし者
パチンコの演出は時に驚かされることも多く、毎回期待感を持って遊べます。ワクワクが止まりません。
麻雀物語
https://www.bk8882.com/tags/%E5%B9%B8%E9%81%8B%E3%81%AE%E5%BE%8C%E3%81%AE%E5%AF%8C
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
CR織田信奈の野望II
k8 カジノ パチンコ
シティーハンター
めんそーれ(雷電ver.)
CR緋弾のアリアII FPM
amoxicillin 500 mg online amoxicillin online purchase or amoxicillin 775 mg
http://images.google.com.pe/url?q=https://amoxil.llc where can i get amoxicillin 500 mg
ampicillin amoxicillin amoxicillin capsules 250mg and amoxicillin buy online canada order amoxicillin 500mg
ivermectin tablets order ivermectin 4000 or minocycline 100 mg for sale
http://clients1.google.com.sl/url?q=https://stromectol.agency order minocycline 100 mg online
ivermectin 0.2mg ivermectin 250ml and ivermectin coronavirus what is minocycline 100 mg used for
zithromax 250 buy zithromax z-pak online zithromax 1000 mg online
buy neurontin 100 mg: buy gabapentin – neurontin 330 mg
zithromax 250mg order zithromax where can i buy zithromax uk
https://salmon-peach-dd3cm8.mystrikingly.com/blog/2767d9a95c6
buy rybelsus: Rybelsus 14 mg – Rybelsus 7mg
https://semaglutide.win/# Buy compounded semaglutide online
buy cheap generic zithromax
http://semaglutide.win/# Semaglutide pharmacy price
zithromax 500mg over the counter
магазин сейфов предлагает сейф 3 взломостойкие сейфы 3 класса
minocycline 100mg without prescription ivermectin over the counter or ivermectin 500ml
http://images.google.mw/url?q=https://stromectol.agency minocycline 100 mg without a doctor
ivermectin 1 cream ivermectin price canada and ivermectin 6mg dosage minocycline 100 mg tablets
order amoxicillin no prescription amoxicillin 875 mg tablet or amoxicillin 500 mg for sale
http://www.hogwarts.be/frame.php?url=https://amoxil.llc amoxicillin online purchase
[url=https://www.google.com.mx/url?q=https://amoxil.llc]cheap amoxicillin 500mg[/url] where can you get amoxicillin and [url=https://visualchemy.gallery/forum/profile.php?id=4393039]buy amoxicillin 250mg[/url] amoxicillin 500 mg purchase without prescription
order Rybelsus for weight loss buy semaglutide online or rybelsus cost
https://cse.google.com.bo/url?sa=t&url=https://semaglutide.win rybelsus generic
rybelsus cost rybelsus price and Buy semaglutide pills Rybelsus 14 mg price
Buy semaglutide pills rybelsus semaglutide
rybelsus cost rybelsus or Buy compounded semaglutide online
http://www.pandct.com/link.asp?webaddress=semaglutide.win rybelsus generic
semaglutide Rybelsus 7mg and buy semaglutide online Buy compounded semaglutide online
stromectol uk buy order stromectol minocycline 100mg without prescription
rybelsus generic: Rybelsus 14 mg price – rybelsus
where to buy amoxicillin pharmacy: cheapest amoxil – generic for amoxicillin
https://amoxil.llc/# amoxicillin 500 tablet
zithromax for sale cheap
https://semaglutide.win/# Rybelsus 14 mg price
zithromax 500 mg lowest price pharmacy online
Перевод с иностранных языков: Перевод видеороликов и озвучивание: как это сделать и почему это важно для международного бизнеса. Перевод документов: Перевод медицинских документов: сложности и рекомендации для качественного перевода. Апостиль в Новосибирске: Как проверить действительность апостиля и что делать, если он был подделан?
перевод документов
neurontin cream order neurontin online or neurontin 800 mg tablet
https://brivium.com/proxy.php?link=http://gabapentin.auction 300 mg neurontin
neurontin capsules 300mg neurontin coupon and how much is neurontin pills neurontin prescription cost
https://semaglutide.win/# buy rybelsus
https://semaglutide.win/# Rybelsus 7mg
Gay Boys Porn https://gay0day.com HD is the best gay porn tube to watch high definition videos of horny gay boys jerking, sucking their mates and fucking on webcam
average cost of generic zithromax zithromax for sale generic zithromax 500mg
https://gabapentin.auction/# neurontin 400 mg price
https://zithromax.company/# zithromax purchase online
rybelsus generic rybelsus rybelsus generic
amoxicillin 500mg for sale uk amoxicillin without prescription or amoxicillin 750 mg price
http://sat.kuz.ru/engine/redirect.php?url=http://amoxil.llc amoxicillin brand name
order amoxicillin uk amoxicillin 500mg capsules antibiotic and amoxil pharmacy amoxicillin 500mg for sale uk
ivermectin for sale stromectol pills or minocycline 100mg tablets
https://maps.google.com.my/url?q=https://stromectol.agency buy ivermectin stromectol
ivermectin tablets order order minocycline 50mg and ivermectin malaria ivermectin virus
where to buy amoxicillin pharmacy: cheapest amoxil – amoxicillin buy online canada
buy generic zithromax no prescription: generic zithromax – generic zithromax over the counter
https://gabapentin.auction/# canada neurontin 100mg discount
buy zithromax online cheap
https://semaglutide.win/# rybelsus
zithromax prescription
zithromax over the counter generic zithromax order zithromax over the counter
ivermectin 4 order stromectol online or minocycline 50 mg
https://www.google.com.na/url?q=https://stromectol.agency ivermectin lotion for scabies
ivermectin 500mg minocycline interactions and minocycline weight gain stromectol uk
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://okwave.jp/profile/u3122586.html
https://anotepad.com/notes/2ik9i4n3
https://cannabis.net/user/156967
https://chyoa.com/user/thoor1997
https://www.metal-archives.com/users/eddie1955
押忍!番长(操)
k8 カジノ パチンコ
パチンコは、日常のストレスを忘れさせてくれる最高の娯楽です。リラックスできます。
SUハイビスカス
https://www.ja-securities.jp/tags/%E3%82%AB%E3%82%B8%E3%83%8E%E8%AA%98%E8%87%B4
音楽と演出が楽しく、プレイ中に笑顔になれます。リラックスできます。
押忍!サラリーマン番長
k8 カジノ パチンコ スマスロ劇場版 魔法少女まどか☆マギカ[前編]始まりの物語/[後編]永遠の物語f‐フォルテ‐パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
CR JAWS再臨-SHARK PANIC AGAIN-
CRルパン三世~消されたルパン Ver.394
斉天大聖
Профессиональный сервисный центр ремонт телефонов в москве адреса ремонта телефонов
Профессиональный сервисный центр по ремонту сотовых телефонов в Москве.
Мы предлагаем: сколько стоит ремонт смартфона
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Сервисный центр предлагает починка стиральных машин maunfeld качественый ремонт стиральной машины maunfeld
pharmacies in mexico that ship to usa: mexico drug stores pharmacies – medicine in mexico pharmacies
purple pharmacy mexico price list buying prescription drugs in mexico mexico drug stores pharmacies
r465782
look what i found r2425303
csgo gambling csgo gamble
csgo gamble best csgo gambling sites
http://mexicanpharm24.pro/# pharmacies in mexico that ship to usa
best online pharmacy india
canadian medications https://indianpharmdelivery.com/# india pharmacy mail order
viagra without doctor prescription http://mexicanpharm24.pro/# mexican drugstore online
ed treatments that really work: erectial dysfunction – pills erectile dysfunction
リング呪いの7日間;
k8 カジノ パチンコ 喧嘩祭パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
CRフィーバー バイオハザード リベレーションズ
https://onkaji.jp/tag/31
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
P閃乱カグラ2
スロット Jewel Rush ジュエル・ラッシュ
北斗の拳 強敵
政宗
P牙狼月虹ノ旅人
purple pharmacy mexico price list: best online pharmacies in mexico – mexican pharmaceuticals online
pharmacy website india Online medicine order india online pharmacy
rolling foam roller
Over 7 years in development, the Rollga Method™ combines trigger points, acupressure points, fascial lines, meridians, and techniques like ART (Active Release Technique), MAT (Muscle Activation Technique), RPR (Reflexive Performance Reset) and others to optimize one’s health thru a new system of self care.
Download the Rollga App to learn about & tap into all that Rollga has to offer!
https://mexicanpharm24.pro/# mexican drugstore online
cheapest online pharmacy india
medication drugs: best ed pills – how to help ed
mexican rx online: reputable mexican pharmacies online – buying prescription drugs in mexico
mens erections https://drugs24.pro/# treatments for ed
https://resumehead.com/ Resumehead is the premier resume writing and career coaching service, trusted by millions of job seekers worldwide. Our team of experts is dedicated to your success, providing support throughout your entire professional journey. From crafting a professional resume and industry-specific cover letter to optimizing your LinkedIn profile and offering personalized career coaching, we’re here to help you unlock new opportunities with a resume that showcases your true potential.
ed natural remedies http://mexicanpharm24.pro/# pharmacies in mexico that ship to usa
online shopping pharmacy india: world pharmacy india – best online pharmacy india
Недорогой антиабузный сервер/ВПС/ВДС под парсинг, постинг, разгадывание каптчи.
https://t.me/s/server_xevil_xrumer_vpsvds_zenno
Сервер для Xrumer |Xevil | GSA | Xneolinks | A-parser | ZennoPoster | BAS | Антидетект браузер Dolphin
– Для сервера сеть на скорости 1 Гбит!
– Быстрые серверы с NVMe.
– Дата-центр в Москве и Амстердаме
– FASTPANEL и HestiaCP – бесплатно
– Почасовая оплата
– Отлично подходит под Xneolinks
– Отлично подходит под A-Parser
– Отлично подходит под CapMonster
– Отлично подходит под XRumer + XEvil
– Windows – 2022, 2019, 2016, 2012 R2
– Автоматическая установка Windows – бесплатно
– Ubuntu, Debian, CentOS, Oracle 9 – бесплатно
– Круглосуточная техническая поддержка – бесплатно
– Супер (аптайм, скорость, пинг, нагрузка)
– Возможность арендовать сервер на 1 час или 1 сутки
– Отлично подходит под GSA Search Engine Ranker
– Windows – 2012 R2, 2016, 2019, 2022 – бесплатно
– Мгновенное развёртывание сервера в несколько кликов – бесплатно
– Более 15 000 сервер уже в работе
– Outline VPN, WireGuard VPN, IPsec VPN.
– Управляйте серверами на лету.
– Скорость порта подключения к сети интернет — 1000 Мбит/сек
http://mexicanpharm24.pro/# medicine in mexico pharmacies
buy prescription drugs from india
best pills for ed fast ed meds online or solutions for ed
https://images.google.com.tr/url?sa=t&url=https://drugs24.pro erectile dysfunction cure
ed remedies that really work best ed treatments and medication drugs ed pumps
indian pharmacy mail order pharmacy india п»їlegitimate online pharmacies india
buying from online mexican pharmacy: mexico pharmacies prescription drugs – best online pharmacies in mexico
kometa casino регистрация
Казино Kometa: Идеальный выбор для любителей азартного досуга
Если вы любите ставками и ищете площадку, что дает доступ к широкий выбор игровых автоматов и лайв-игр, а также выгодные предложения, Казино Kometa — это тот сайт, на котором вы испытаете незабываемый опыт. Предлагаем рассмотрим, что превращает Kometa Casino уникальным и почему посетители выбирают его для игры.
### Главные особенности Kometa Casino
Kometa Casino — это глобальная игровая платформа, которая была запущена в 2024 году и уже сейчас завоевала интерес игроков по глобально. Вот некоторые черты, которые выделяют Kometa Casino:
Характеристика Описание
Год создания 2024-й
Глобальная доступность Всемирная
Объем игр Более 1000
Лицензия Лицензия Кюрасао
Мобильный доступ Присутствует
Методы платежей Visa, Mastercard, Skrill
Поддержка Круглосуточная поддержка
Специальные предложения Акции и бонусы
Система безопасности SSL защита
### Зачем играют в Казино Kometa?
#### Программа лояльности
Одной из ключевых функций Казино Kometa является уникальная программа лояльности. Чем активнее играете, тем лучше призы и бонусы. Программа состоит из многоуровневую систему:
– **Уровень 1 — Земля**: Кэшбек 3% 3% от ставок за 7 дней.
– **Уровень 2 — Луна**: Кэшбек 5% на ставки от 5 000 до 10 000 ?.
– **Венера (уровень 3)**: 7% возврата при игре от 10 001 до 50 000 RUB.
– **Марс (уровень 4)**: 8% бонуса при сумме ставок от 50 001 до 150 000 RUB.
– **Юпитер (уровень 5)**: 10% возврата при ставках свыше 150 000 ?.
– **Сатурн (уровень 6)**: 11% бонуса.
– **Уран (уровень 7)**: 12% кэшбек 12%.
#### Постоянные бонусы
С целью удержания высокого уровня азарта, Казино Kometa проводит регулярные бонусы, кэшбек и спины для новичков. Постоянные подарки способствуют сохранять интерес на в процессе игры.
#### Широкий выбор игр
Огромное количество развлечений, включая игровые машины, карточные игры и лайв-игры, делают Kometa Casino местом, где вы найдете игру по душе. Вы можете наслаждаться стандартными автоматами, а также новинками от лучших поставщиков. Дилеры в реальном времени создают играм ощущение реального казино, воссоздавая дух казино.
https://мбоусош13.рф/
фриспины за регистрацию без депозита
tadalafil without a doctor’s prescription: best erectile dysfunction pills – new ed treatments
buy medicines online in india online shopping pharmacy india or indian pharmacy paypal
https://cultureireland.gov.ie/?URL=https://indianpharmdelivery.com buy medicines online in india
top online pharmacy india india online pharmacy and world pharmacy india buy medicines online in india
mexican online pharmacies prescription drugs buying prescription drugs in mexico online pharmacies in mexico that ship to usa
prescription drugs: ed drug prices – help with ed
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: сервис центры бытовой техники челябинск
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://indianpharmdelivery.com/# п»їlegitimate online pharmacies india
best india pharmacy
ed pharmacy: cheap pet meds without vet prescription – vacuum pump for ed
cheap medications online ed medications online or impotence pills
https://hitfront.com/rank?domain=drugs24.pro ed pumps
treat ed legal to buy prescription drugs from canada and erectial disfunction ed pills for sale
online ed medications male erectile dysfunction viagra without a doctor prescription
indian pharmacies safe: top 10 online pharmacy in india – indianpharmacy com
Аренда безабузный server/ВПС/ВДС под парсинг, постинг, разгадывание каптчи.
https://t.me/s/server_xevil_xrumer_vpsvds_zenno
Сервер для Xrumer |Xevil | GSA | Xneolinks | A-parser | ZennoPoster | BAS | Антидетект браузер Dolphin
– FASTPANEL и HestiaCP – бесплатно
– Мгновенное развёртывание сервера в несколько кликов – бесплатно
– Супер (аптайм, скорость, пинг, нагрузка)
– Ubuntu, Debian, CentOS, Oracle 9 – бесплатно
– Windows – 2022, 2019, 2016, 2012 R2
– Дата-центр в Москве и Амстердаме
– Для сервера сеть на скорости 1 Гбит!
– Отлично подходит под A-Parser
– Автоматическая установка Windows – бесплатно
– Windows – 2012 R2, 2016, 2019, 2022 – бесплатно
– Быстрые серверы с NVMe.
– Отлично подходит под CapMonster
– Круглосуточная техническая поддержка – бесплатно
– Управляйте серверами на лету.
– Отлично подходит под XRumer + XEvil
– Более 15 000 сервер уже в работе
– Отлично подходит под GSA Search Engine Ranker
– Outline VPN, WireGuard VPN, IPsec VPN.
– Отлично подходит под Xneolinks
– Возможность арендовать сервер на 1 час или 1 сутки
– Почасовая оплата
– Скорость порта подключения к сети интернет — 1000 Мбит/сек
compare ed drugs: erectile dysfunction medications – buy prescription drugs from india
знак авто
fast ed meds online treat ed ed pills cheap
medications for: herbal ed – online ed drugs
https://mexicanpharm24.pro/# best online pharmacies in mexico
indian pharmacies safe
best cure for ed online drugs or erection pills
http://changrunhg.ff66.net/productshow.asp?id=28&mnid=48045&mc=???Р§?Р•???Р§?Р•?РІ?????Р§?Р•???Р§?Р•???Р§?Р•???Р§?Р•&url=http://drugs24.pro pet meds without vet prescription
male erection treatment for ed and buy ed pills online drug pharmacy
indian pharmacy online reputable indian pharmacies or indianpharmacy com
http://www.iacconline.org/redirect.aspx?destination=http://indianpharmdelivery.com reputable indian online pharmacy
indian pharmacy paypal Online medicine home delivery and indian pharmacy paypal Online medicine order
CR牙狼魔戒ノ花
k8パチンコ
お店の雰囲気が心地よく、長時間楽しむことができます。快適な空間が魅力です。
パチスロ バイオハザード6
https://cnhk8.com/tags/%E3%83%95%E3%82%A7%E3%82%A2
ゲームの進行が早く、飽きずに楽しめるのが良いです。短時間でも満足感があります。
吉宗
P GOD EATER-ブラッドの覚醒-
CR牙狼闇を照らす者
鬼武者3 時空天翔
デビルサバイバー2 最後の7日間
reputable mexican pharmacies online mexico drug stores pharmacies or mexican online pharmacies prescription drugs
https://51.biqund.com/index/d1?diff=0&utm_clickid=6g0kk0oskcwwo4c0&aurl=https://mexicanpharm24.pro mexico pharmacies prescription drugs
mexican pharmaceuticals online buying prescription drugs in mexico online and best online pharmacies in mexico medication from mexico pharmacy
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт бытовой техники в барнауле
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://sudvgorode.ru/
impotence pills drugs causing ed or generic ed drugs
https://cse.google.co.ve/url?q=https://drugs24.pro online drugs
best drug for ed ed medicine and online medications ed drugs compared
best online pharmacies in mexico reputable mexican pharmacies online or buying from online mexican pharmacy
https://maps.google.cd/url?q=https://mexicanpharm24.pro medicine in mexico pharmacies
mexican pharmaceuticals online purple pharmacy mexico price list and medicine in mexico pharmacies reputable mexican pharmacies online
http://drugs24.pro/# prescription drugs
indian pharmacy paypal
mexican drugstore online buying from online mexican pharmacy medicine in mexico pharmacies
top 10 online pharmacy in india: reputable indian online pharmacy – top 10 online pharmacy in india
mexican pharmaceuticals online: best online pharmacies in mexico – pharmacies in mexico that ship to usa
ed tablets best medicine for ed best ed medications
india pharmacy mail order: buy prescription drugs from india – indian pharmacy online
п»їbest mexican online pharmacies: mexico drug stores pharmacies – purple pharmacy mexico price list
Начните массовую индексацию ссылок в Google прямо cейчас!
Быстрая индексация ссылок имеет ключевое значение для успеха вашего онлайн-бизнеса. Чем быстрее поисковые системы обнаружат и проиндексируют ваши ссылки, тем быстрее вы сможете привлечь новую аудиторию и повысить позиции вашего сайта в результатах поиска.
Не теряйте времени! Начните пользоваться нашим сервисом для ускоренной индексации внешних ссылок в Google и Yandex. Зарегистрируйтесь сегодня и получите первые результаты уже завтра. Ваш успех в ваших руках!
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
indian pharmacies safe indianpharmacy com or Online medicine home delivery
http://vladinfo.ru/away.php?url=http://indianpharmdelivery.com top 10 pharmacies in india
india pharmacy best india pharmacy and pharmacy website india india pharmacy
Explore fashionable styles at tibi clothing.
http://mexicanpharm24.pro/# medicine in mexico pharmacies
online pharmacy india
indian pharmacy paypal mail order pharmacy india online shopping pharmacy india
Over 7 years in development, the Rollga Method™ combines trigger points, acupressure points, fascial lines, meridians, and techniques like ART (Active Release Technique), MAT (Muscle Activation Technique), RPR (Reflexive Performance Reset) and others to optimize one’s health thru a new system of self care.
Download the Rollga App to learn about & tap into all that Rollga has to offer!
일본배대지
이용 안내 및 주요 정보
배송대행 이용방법
배송대행은 해외에서 구매한 상품을 중간지점(배대지)에 보내고, 이를 통해 한국으로 배송받는 서비스입니다. 먼저, 회원가입을 진행하고, 해당 배대지 주소를 이용해 상품을 주문한 후, 배송대행 신청서를 작성하여 배송 정보를 입력합니다. 모든 과정은 웹사이트를 통해 관리되며, 필요한 경우 고객센터를 통해 지원을 받을 수 있습니다.
구매대행 이용방법
구매대행은 해외 쇼핑몰에서 직접 구매가 어려운 경우, 대행 업체가 대신 구매해주는 서비스입니다. 고객이 원하는 상품 정보를 제공하면, 구매대행 신청서를 작성하고 대행료와 함께 결제하면 업체가 구매를 완료해줍니다. 이후 상품이 배대지로 도착하면 배송대행 절차를 통해 상품을 수령할 수 있습니다.
배송비용 안내
배송비용은 상품의 무게, 크기, 배송 지역에 따라 다르며, 계산기는 웹사이트에서 제공됩니다. 부피무게가 큰 제품이나 특수 제품은 추가 비용이 발생할 수 있습니다. 항공과 해운에 따른 요금 차이도 고려해야 합니다.
부가서비스
추가 포장 서비스, 검역 서비스, 폐기 서비스 등이 제공되며, 필요한 경우 신청서를 작성하여 서비스 이용이 가능합니다. 파손 위험이 있는 제품의 경우 포장 보완 서비스를 신청하는 것이 좋습니다.
관/부가세 안내
수입된 상품에 대한 관세와 부가세는 상품의 종류와 가격에 따라 부과됩니다. 이를 미리 확인하고, 추가 비용을 예상하여 계산하는 것이 중요합니다.
수입금지 품목
가스제품(히터), 폭발물, 위험물 등은 수입이 금지된 품목에 속합니다. 항공 및 해상 운송이 불가하니, 반드시 해당 품목을 확인 후 주문해야 합니다.
폐기/검역 안내
검역이 필요한 상품이나 폐기가 필요한 경우, 사전에 관련 부가서비스를 신청해야 합니다. 해당 사항에 대해 미리 안내받고 처리할 수 있도록 주의해야 합니다.
교환/반품 안내
교환 및 반품 절차는 상품을 배송받은 후 7일 이내에 신청이 가능합니다. 단, 일부 상품은 교환 및 반품이 제한될 수 있으니 사전에 정책을 확인하는 것이 좋습니다.
재고관리 시스템
재고관리는 배대지에서 보관 중인 상품의 상태를 실시간으로 확인할 수 있는 시스템입니다. 재고 신청을 통해 상품의 상태를 확인하고, 필요한 경우 배송 또는 폐기 요청을 할 수 있습니다.
노데이타 처리방법
노데이타 상태의 상품은 배송 추적이 어려운 경우 발생합니다. 이런 경우 고객센터를 통해 문의하고 문제를 해결해야 합니다.
소비세환급 및 Q&A
일본에서 상품을 구매할 때 적용된 소비세를 환급받을 수 있는 서비스입니다. 해당 신청서는 구체적인 절차에 따라 작성하고 제출해야 합니다. Q&A를 통해 자주 묻는 질문을 확인하고, 추가 문의 사항은 고객센터에 연락해 해결할 수 있습니다.
고객지원
고객센터는 1:1 문의, 카카오톡 상담 등을 통해 서비스 이용 중 발생하는 문제나 문의사항을 해결할 수 있도록 지원합니다.
서비스 관련 공지사항
파손이 쉬운 제품의 경우, 추가 포장 보완을 반드시 요청해야 합니다.
가스제품(히터)은 통관이 불가하므로 구매 전 확인 바랍니다.
항공 운송 비용이 대폭 인하되었습니다.
https://автоломбардбрянск.рф/
cheap online pharmacy ed natural treatment or ed clinics
https://images.google.com.br/url?sa=t&url=https://drugs24.pro amoxicillin without a doctor’s prescription
buy ed pills buying ed pills online and best ed pills that work canadian online drugstore
gambling sites cs2 gambling sites
Профессиональный сервисный центр по ремонту сотовых телефонов в Москве.
Мы предлагаем: сервисы по ремонту телефонов
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mexico drug stores pharmacies: buying from online mexican pharmacy – mexican border pharmacies shipping to usa
mexican pharmaceuticals online: buying from online mexican pharmacy – mexican rx online
top 10 online pharmacy in india cheapest online pharmacy india indianpharmacy com
http://mexicanpharm24.pro/# mexican rx online
indian pharmacy online
erectile dysfunction drug buy prescription drugs without doctor or pet antibiotics without vet prescription
http://2ch-ranking.net/redirect.php?url=http://drugs24.pro natural ed treatment
comparison of ed drugs how to overcome ed naturally and ed meds online buy online drugs
buying prescription drugs in mexico: mexico pharmacies prescription drugs – mexican mail order pharmacies
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://chyoa.com/user/gfykhul1987
https://tubeteencam.com/user/dmxx1997/profile
http://www.nfomedia.com/profile?uid=rOjYccB
https://haveagood.holiday/users/363960
https://www.dnnsoftware.com/activity-feed/my-profile/userid/3202161
Аренда безабузный сервер/ВПС/ВДС под парсинг, постинг, разгадывание каптчи.
https://t.me/s/server_xevil_xrumer_vpsvds_zenno
Сервер для Xrumer |Xevil | GSA | Xneolinks | A-parser | ZennoPoster | BAS | Антидетект браузер Dolphin
– Более 15 000 сервер уже в работе
– Супер (аптайм, скорость, пинг, нагрузка)
– Возможность арендовать сервер на 1 час или 1 сутки
– Ubuntu, Debian, CentOS, Oracle 9 – бесплатно
– Автоматическая установка Windows – бесплатно
– Отлично подходит под A-Parser
– Управляйте серверами на лету.
– FASTPANEL и HestiaCP – бесплатно
– Дата-центр в Москве и Амстердаме
– Windows – 2022, 2019, 2016, 2012 R2
– Отлично подходит под XRumer + XEvil
– Windows – 2012 R2, 2016, 2019, 2022 – бесплатно
– Отлично подходит под GSA Search Engine Ranker
– Для сервера сеть на скорости 1 Гбит!
– Быстрые серверы с NVMe.
– Отлично подходит под CapMonster
– Почасовая оплата
– Мгновенное развёртывание сервера в несколько кликов – бесплатно
– Круглосуточная техническая поддержка – бесплатно
– Отлично подходит под Xneolinks
– Outline VPN, WireGuard VPN, IPsec VPN.
– Скорость порта подключения к сети интернет — 1000 Мбит/сек
cheap ed medication http://indianpharmdelivery.com/# top 10 online pharmacy in india
ed medicine online http://drugs24.pro/# ed cures that actually work
Купить безабузный server/ВПС/ВДС под парсинг, постинг, разгадывание каптчи.
https://t.me/s/server_xevil_xrumer_vpsvds_zenno
Сервер для Xrumer |Xevil | GSA | Xneolinks | A-parser | ZennoPoster | BAS | Антидетект браузер Dolphin
– FASTPANEL и HestiaCP – бесплатно
– Более 15 000 сервер уже в работе
– Windows – 2022, 2019, 2016, 2012 R2
– Ubuntu, Debian, CentOS, Oracle 9 – бесплатно
– Отлично подходит под Xneolinks
– Отлично подходит под XRumer + XEvil
– Windows – 2012 R2, 2016, 2019, 2022 – бесплатно
– Дата-центр в Москве и Амстердаме
– Круглосуточная техническая поддержка – бесплатно
– Для сервера сеть на скорости 1 Гбит!
– Автоматическая установка Windows – бесплатно
– Возможность арендовать сервер на 1 час или 1 сутки
– Мгновенное развёртывание сервера в несколько кликов – бесплатно
– Отлично подходит под CapMonster
– Отлично подходит под GSA Search Engine Ranker
– Быстрые серверы с NVMe.
– Супер (аптайм, скорость, пинг, нагрузка)
– Outline VPN, WireGuard VPN, IPsec VPN.
– Управляйте серверами на лету.
– Скорость порта подключения к сети интернет — 1000 Мбит/сек
– Отлично подходит под A-Parser
– Почасовая оплата
buying prescription drugs in mexico medication from mexico pharmacy or medicine in mexico pharmacies
http://www.allbeaches.net/goframe.cfm?site=http://mexicanpharm24.pro purple pharmacy mexico price list
buying prescription drugs in mexico mexican border pharmacies shipping to usa and purple pharmacy mexico price list purple pharmacy mexico price list
viagra vs cialis bodybuilding online drugstore ed therapy
ed meds online without doctor prescription ed pills online or ed vacuum pumps
https://maps.google.ws/url?sa=t&url=https://drugs24.pro new erectile dysfunction treatment
ed drugs over the counter men with ed and errectile disfunction men ed
mail order pharmacy india mail order pharmacy india or indianpharmacy com
https://sambastore.com/en/mandeparaumamigo.php?url=http://indianpharmdelivery.com indian pharmacy online
buy prescription drugs from india india pharmacy and top 10 online pharmacy in india indian pharmacy online
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
mexican mail order pharmacies medicine in mexico pharmacies or mexico drug stores pharmacies
https://images.google.mn/url?q=https://mexicanpharm24.pro mexican online pharmacies prescription drugs
п»їbest mexican online pharmacies mexican drugstore online and purple pharmacy mexico price list п»їbest mexican online pharmacies
mexico drug stores pharmacies pharmacies in mexico that ship to usa mexican border pharmacies shipping to usa
замена венцов
이용 안내 및 주요 정보
배송대행 이용방법
배송대행은 해외에서 구매한 상품을 중간지점(배대지)에 보내고, 이를 통해 한국으로 배송받는 서비스입니다. 먼저, 회원가입을 진행하고, 해당 배대지 주소를 이용해 상품을 주문한 후, 배송대행 신청서를 작성하여 배송 정보를 입력합니다. 모든 과정은 웹사이트를 통해 관리되며, 필요한 경우 고객센터를 통해 지원을 받을 수 있습니다.
구매대행 이용방법
구매대행은 해외 쇼핑몰에서 직접 구매가 어려운 경우, 대행 업체가 대신 구매해주는 서비스입니다. 고객이 원하는 상품 정보를 제공하면, 구매대행 신청서를 작성하고 대행료와 함께 결제하면 업체가 구매를 완료해줍니다. 이후 상품이 배대지로 도착하면 배송대행 절차를 통해 상품을 수령할 수 있습니다.
배송비용 안내
배송비용은 상품의 무게, 크기, 배송 지역에 따라 다르며, 계산기는 웹사이트에서 제공됩니다. 부피무게가 큰 제품이나 특수 제품은 추가 비용이 발생할 수 있습니다. 항공과 해운에 따른 요금 차이도 고려해야 합니다.
부가서비스
추가 포장 서비스, 검역 서비스, 폐기 서비스 등이 제공되며, 필요한 경우 신청서를 작성하여 서비스 이용이 가능합니다. 파손 위험이 있는 제품의 경우 포장 보완 서비스를 신청하는 것이 좋습니다.
관/부가세 안내
수입된 상품에 대한 관세와 부가세는 상품의 종류와 가격에 따라 부과됩니다. 이를 미리 확인하고, 추가 비용을 예상하여 계산하는 것이 중요합니다.
수입금지 품목
가스제품(히터), 폭발물, 위험물 등은 수입이 금지된 품목에 속합니다. 항공 및 해상 운송이 불가하니, 반드시 해당 품목을 확인 후 주문해야 합니다.
폐기/검역 안내
검역이 필요한 상품이나 폐기가 필요한 경우, 사전에 관련 부가서비스를 신청해야 합니다. 해당 사항에 대해 미리 안내받고 처리할 수 있도록 주의해야 합니다.
교환/반품 안내
교환 및 반품 절차는 상품을 배송받은 후 7일 이내에 신청이 가능합니다. 단, 일부 상품은 교환 및 반품이 제한될 수 있으니 사전에 정책을 확인하는 것이 좋습니다.
재고관리 시스템
재고관리는 배대지에서 보관 중인 상품의 상태를 실시간으로 확인할 수 있는 시스템입니다. 재고 신청을 통해 상품의 상태를 확인하고, 필요한 경우 배송 또는 폐기 요청을 할 수 있습니다.
노데이타 처리방법
노데이타 상태의 상품은 배송 추적이 어려운 경우 발생합니다. 이런 경우 고객센터를 통해 문의하고 문제를 해결해야 합니다.
소비세환급 및 Q&A
일본에서 상품을 구매할 때 적용된 소비세를 환급받을 수 있는 서비스입니다. 해당 신청서는 구체적인 절차에 따라 작성하고 제출해야 합니다. Q&A를 통해 자주 묻는 질문을 확인하고, 추가 문의 사항은 고객센터에 연락해 해결할 수 있습니다.
고객지원
고객센터는 1:1 문의, 카카오톡 상담 등을 통해 서비스 이용 중 발생하는 문제나 문의사항을 해결할 수 있도록 지원합니다.
서비스 관련 공지사항
파손이 쉬운 제품의 경우, 추가 포장 보완을 반드시 요청해야 합니다.
가스제품(히터)은 통관이 불가하므로 구매 전 확인 바랍니다.
항공 운송 비용이 대폭 인하되었습니다.
일본배대지
이용 안내 및 주요 정보
배송대행 이용방법
배송대행은 해외에서 구매한 상품을 중간지점(배대지)에 보내고, 이를 통해 한국으로 배송받는 서비스입니다. 먼저, 회원가입을 진행하고, 해당 배대지 주소를 이용해 상품을 주문한 후, 배송대행 신청서를 작성하여 배송 정보를 입력합니다. 모든 과정은 웹사이트를 통해 관리되며, 필요한 경우 고객센터를 통해 지원을 받을 수 있습니다.
구매대행 이용방법
구매대행은 해외 쇼핑몰에서 직접 구매가 어려운 경우, 대행 업체가 대신 구매해주는 서비스입니다. 고객이 원하는 상품 정보를 제공하면, 구매대행 신청서를 작성하고 대행료와 함께 결제하면 업체가 구매를 완료해줍니다. 이후 상품이 배대지로 도착하면 배송대행 절차를 통해 상품을 수령할 수 있습니다.
배송비용 안내
배송비용은 상품의 무게, 크기, 배송 지역에 따라 다르며, 계산기는 웹사이트에서 제공됩니다. 부피무게가 큰 제품이나 특수 제품은 추가 비용이 발생할 수 있습니다. 항공과 해운에 따른 요금 차이도 고려해야 합니다.
부가서비스
추가 포장 서비스, 검역 서비스, 폐기 서비스 등이 제공되며, 필요한 경우 신청서를 작성하여 서비스 이용이 가능합니다. 파손 위험이 있는 제품의 경우 포장 보완 서비스를 신청하는 것이 좋습니다.
관/부가세 안내
수입된 상품에 대한 관세와 부가세는 상품의 종류와 가격에 따라 부과됩니다. 이를 미리 확인하고, 추가 비용을 예상하여 계산하는 것이 중요합니다.
수입금지 품목
가스제품(히터), 폭발물, 위험물 등은 수입이 금지된 품목에 속합니다. 항공 및 해상 운송이 불가하니, 반드시 해당 품목을 확인 후 주문해야 합니다.
폐기/검역 안내
검역이 필요한 상품이나 폐기가 필요한 경우, 사전에 관련 부가서비스를 신청해야 합니다. 해당 사항에 대해 미리 안내받고 처리할 수 있도록 주의해야 합니다.
교환/반품 안내
교환 및 반품 절차는 상품을 배송받은 후 7일 이내에 신청이 가능합니다. 단, 일부 상품은 교환 및 반품이 제한될 수 있으니 사전에 정책을 확인하는 것이 좋습니다.
재고관리 시스템
재고관리는 배대지에서 보관 중인 상품의 상태를 실시간으로 확인할 수 있는 시스템입니다. 재고 신청을 통해 상품의 상태를 확인하고, 필요한 경우 배송 또는 폐기 요청을 할 수 있습니다.
노데이타 처리방법
노데이타 상태의 상품은 배송 추적이 어려운 경우 발생합니다. 이런 경우 고객센터를 통해 문의하고 문제를 해결해야 합니다.
소비세환급 및 Q&A
일본에서 상품을 구매할 때 적용된 소비세를 환급받을 수 있는 서비스입니다. 해당 신청서는 구체적인 절차에 따라 작성하고 제출해야 합니다. Q&A를 통해 자주 묻는 질문을 확인하고, 추가 문의 사항은 고객센터에 연락해 해결할 수 있습니다.
고객지원
고객센터는 1:1 문의, 카카오톡 상담 등을 통해 서비스 이용 중 발생하는 문제나 문의사항을 해결할 수 있도록 지원합니다.
서비스 관련 공지사항
파손이 쉬운 제품의 경우, 추가 포장 보완을 반드시 요청해야 합니다.
가스제품(히터)은 통관이 불가하므로 구매 전 확인 바랍니다.
항공 운송 비용이 대폭 인하되었습니다.
https://indianpharmdelivery.com/# india online pharmacy
top 10 pharmacies in india
medications for ed https://indianpharmdelivery.com/# reputable indian online pharmacy
medications for ed https://indianpharmdelivery.com/# world pharmacy india
top 10 pharmacies in india indian pharmacy paypal india pharmacy mail order
музей анимации измайлово музей анимации в москве официальный сайт
indian pharmacy indian pharmacy paypal or buy prescription drugs from india
https://www.google.gg/url?q=https://indianpharmdelivery.com best online pharmacy india
[url=http://channel.iezvu.com/share/indianpharmdelivery.com?page=https://indianpharmdelivery.com]india online pharmacy[/url] indian pharmacy online and [url=https://www.ixbren.net/home.php?mod=space&uid=667464]top online pharmacy india[/url] reputable indian online pharmacy
best india pharmacy online shopping pharmacy india best online pharmacy india
翔馬
k8 カジノ パチンコ
キャラクターの個性が際立っており、ストーリーに引き込まれます。
CR麻雀物語麗しのテンパイ乙女 Ver.297
https://onkaji.jp/tag/gemingukurabukazino-992
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CR北斗の拳5 百裂 Ver.229(2:1)
CRフィーバー宇宙戦艦ヤマト-ONLY ONE-
P 新世紀エヴァンゲリオン ~未来への咆哮~ SPECIAL EDITION
魔法少女まどか☆マギカ
北斗の拳 強敵
Explore a curated collection of fashion at L’agence clothing.
Казино Kometa: Лучший вариант для любителей азартных игр
Когда вы интересуетесь игровыми автоматами и выбираете площадку, которая предлагает большой выбор игровых автоматов и лайв-игр, а к тому же щедрые бонусы, Kometa Casino — это тот сайт, на котором вы испытаете незабываемые впечатления. Давайте узнаем, какие факторы превращает Kometa Casino выдающимся и почему посетители отдают предпочтение его для своих развлечений.
### Ключевые черты Казино Kometa
Kometa Casino — это всемирная игровая платформа, которая была запущена в 2024 году и уже получила признание игроков по глобально. Вот некоторые моменты, которые выделяют Kometa Casino:
Характеристика Описание
Год Основания 2024-й
Глобальная доступность Международная
Число игр Свыше 1000
Регистрация Лицензия Кюрасао
Мобильный доступ Да
Методы платежей Visa, Mastercard, Skrill
Служба поддержки 24 часа в сутки
Бонусы и Акции Акции и бонусы
Защита данных Шифрование SSL
### Почему выбирают Казино Kometa?
#### Программа лояльности
Одной из ключевых фишек Казино Kometa становится уникальная программа лояльности. Чем больше вы играете, тем лучше призы и бонусы. Система состоит из многоуровневую систему:
– **Земля (уровень 1)**: Кэшбек 3% 3% от затрат за неделю.
– **Уровень 2 — Луна**: Возврат 5% при ставках от 5 000 до 10 000 RUB.
– **Венера (уровень 3)**: Возврат 7% при игре от 10 001 до 50 000 рублей.
– **Уровень 4 — Марс**: 8% кэшбек при сумме ставок от 50 001 до 150 000 рублей.
– **Уровень 5 — Юпитер**: Возврат 10% при ставках свыше 150 000 RUB.
– **Уровень 6 — Сатурн**: Возврат 11%.
– **Уран (уровень 7)**: 12% кэшбек до 12%.
#### Постоянные бонусы
Чтобы держать игровой активности, Казино Kometa предоставляет бонусы каждую неделю, возврат средств и бесплатные вращения для новых пользователей. Регулярные вознаграждения обеспечивают удерживать внимание на протяжении всей игры.
#### Большое количество развлечений
Более 1000 игр, включая слоты, карточные игры и живое казино, создают Kometa Casino площадкой, где каждый найдет развлечение на вкус. Игроки могут насладиться стандартными автоматами, а также новинками от известных разработчиков. Дилеры в реальном времени создают играм ощущение реального казино, формируя атмосферу азартного дома.
cheap medication vacuum therapy for ed or natural ed treatments
https://images.google.gm/url?sa=t&url=https://drugs24.pro how can i order prescription drugs without a doctor
how can i order prescription drugs without a doctor canadian drug pharmacy and top ed drugs legal to buy prescription drugs without prescription
Профессиональный сервисный центр ремонт телефонов поблизости от меня ремонт мобильных телефонов в москве
Профессиональный сервисный центр по ремонту сотовых телефонов в Москве.
Мы предлагаем: вызвать мастера по ремонту ноутбуков
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://drugs24.pro/# viagra without doctor prescription
reputable indian pharmacies
ed medication online https://indianpharmdelivery.com/# pharmacy website india
mexican pharmacy without prescription http://mexicanpharm24.pro/# mexico drug stores pharmacies
best canadian online pharmacy how to get prescription drugs without doctor ways to treat erectile dysfunction
buy helium balloons Dubai buy balloons stores Dubai
Платформа 1вин предлагает широкий выбор спортивных событий, киберспорта и азартных игр. Пользователи получают высокие коэффициенты, быстрые выплаты и круглосуточную поддержку. Программа лояльности и бонусы делают игру выгоднее.
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://tubeteencam.com/user/demonin1974/profile
http://www.nfomedia.com/profile?uid=rOjZYbD
https://haveagood.holiday/users/358261
https://cannabis.net/user/158520
https://anotepad.com/notes/bd3gh7ip
https://drugs24.pro/# which ed drug is best
top 10 pharmacies in india
medicine in mexico pharmacies buying from online mexican pharmacy best online pharmacies in mexico
medication from mexico pharmacy mexico drug stores pharmacies or mexico drug stores pharmacies
https://www.google.com.et/url?q=https://mexicanpharm24.pro mexican border pharmacies shipping to usa
pharmacies in mexico that ship to usa reputable mexican pharmacies online and mexican mail order pharmacies mexico drug stores pharmacies
CR龍が如く見参!天照祗園編
k8パチンコ
演出が華やかで、視覚的にも楽しめるのが魅力。アートとしての側面もあります。
鉄拳2nd
https://www.f-welfare.net/tags/%e8%88%9e%e5%8f%b0%e8%a3%8f
大当たりの瞬間に周囲と一体感を感じられるのが良いです。共に喜べる仲間がいるのが嬉しい。
CRフィーバー バイオハザード リベレーションズ
ファイヤー・ポータル
SUハイビスカス
CR魔法少女まどか_マギカ Ver.319
戦国乙女剣戟に舞う白き剣聖西国参戦編
top 10 online pharmacy in india Online medicine home delivery or india online pharmacy
https://membership.gwsgiants.com.au/analytics/outbound?url=http://indianpharmdelivery.com world pharmacy india
п»їlegitimate online pharmacies india Online medicine home delivery and india online pharmacy best india pharmacy
rgbet
Cách Tối Đa Hóa Tiền Thưởng Tại RGBET
META title: Cách tối ưu hóa tiền thưởng trên RGBET
META description: Học cách tối đa hóa tiền thưởng của bạn trên RGBET bằng các mẹo và chiến lược cá cược hiệu quả nhất.
Giới thiệu
Tiền thưởng là cơ hội tuyệt vời để tăng cơ hội thắng lớn mà không phải bỏ ra nhiều vốn. RGBET cung cấp rất nhiều loại khuyến mãi hấp dẫn, hãy cùng khám phá cách tối ưu hóa chúng!
1. Đọc kỹ điều kiện khuyến mãi
Mỗi chương trình thưởng đều có điều kiện riêng, bạn cần đọc kỹ để đảm bảo không bỏ lỡ cơ hội.
2. Đặt cược theo mức yêu cầu
Để nhận thưởng, hãy đảm bảo rằng số tiền cược của bạn đạt yêu cầu tối thiểu.
3. Tận dụng tiền thưởng nạp đầu tiên
Đây là cơ hội để bạn có thêm vốn ngay từ đầu. Hãy lựa chọn mức nạp phù hợp với ngân sách.
4. Theo dõi khuyến mãi hàng tuần
RGBET liên tục đưa ra các chương trình khuyến mãi hàng tuần. Đừng bỏ lỡ cơ hội nhận thêm tiền thưởng!
5. Sử dụng điểm VIP
Khi bạn đạt mức VIP, điểm thưởng có thể được chuyển thành tiền mặt, giúp bạn tối ưu hóa lợi nhuận.
Kết luận
Biết cách tận dụng tiền thưởng không chỉ giúp bạn có thêm tiền cược mà còn tăng cơ hội thắng lớn.
Выделенный серак/ВПС/ВДС под парсинг, постинг, разгадывание каптчи.
https://t.me/s/server_xevil_xrumer_vpsvds_zenno
Сервер для Xrumer |Xevil | GSA | Xneolinks | A-parser | ZennoPoster | BAS | Антидетект браузер Dolphin
– Windows – 2012 R2, 2016, 2019, 2022 – бесплатно
– Отлично подходит под A-Parser
– Отлично подходит под CapMonster
– Отлично подходит под XRumer + XEvil
– Отлично подходит под Xneolinks
– Скорость порта подключения к сети интернет — 1000 Мбит/сек
– Outline VPN, WireGuard VPN, IPsec VPN.
– Мгновенное развёртывание сервера в несколько кликов – бесплатно
– Круглосуточная техническая поддержка – бесплатно
– Возможность арендовать сервер на 1 час или 1 сутки
– Для сервера сеть на скорости 1 Гбит!
– Быстрые серверы с NVMe.
– Windows – 2022, 2019, 2016, 2012 R2
– Почасовая оплата
– Управляйте серверами на лету.
– Автоматическая установка Windows – бесплатно
– Дата-центр в Москве и Амстердаме
– Более 15 000 сервер уже в работе
– Супер (аптайм, скорость, пинг, нагрузка)
– Ubuntu, Debian, CentOS, Oracle 9 – бесплатно
– FASTPANEL и HestiaCP – бесплатно
– Отлично подходит под GSA Search Engine Ranker
Tại Sao Nên Chọn RGBET Là Nền Tảng Cá Cược Trực Tuyến Hàng Đầu
META title: Vì sao RGBET là nền tảng cá cược tốt nhất cho người chơi Việt Nam
META description: Tìm hiểu những lý do RGBET là lựa chọn hàng đầu cho người chơi Việt Nam với giao diện hiện đại, khuyến mãi hấp dẫn và nạp rút nhanh chóng.
Giới thiệu
RGBET không chỉ là một trang cá cược trực tuyến thông thường. Với những tính năng nổi bật và các chương trình khuyến mãi hấp dẫn, đây là một trong những nền tảng được người chơi Việt Nam tin tưởng nhất.
1. Giao diện thân thiện
Giao diện của RGBET dễ sử dụng, thích hợp cho cả người mới và người chơi lâu năm.
2. Khuyến mãi hấp dẫn
Nền tảng này cung cấp nhiều chương trình khuyến mãi như thưởng nạp đầu, cashback, và chương trình VIP.
3. Nạp và rút tiền nhanh chóng
RGBET hỗ trợ nhiều phương thức thanh toán an toàn và nhanh chóng, từ ngân hàng nội địa đến ví điện tử.
4. Hỗ trợ khách hàng 24/7
RGBET có đội ngũ hỗ trợ luôn sẵn sàng giải đáp mọi thắc mắc của người chơi mọi lúc.
Kết luận
RGBET không chỉ đem lại trải nghiệm cá cược tuyệt vời mà còn đảm bảo sự tiện lợi và uy tín cho người chơi.
Профессиональный сервисный центр по ремонту духовых шкафов в Москве.
Мы предлагаем: ремонт духовок москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
order Rybelsus: good price – rybelsus generic
(?_?;)
подъем дома
https://clopidogrel.pro/# buy Clopidogrel over the counter
drugs online
where to buy balloons Dubai custom balloons with logo Dubai
https://stromectol1st.shop/# stromectol order online
india pharmacy
resume of a chief engineer ready-made resumes of an engineer
rybelsus.icu cheaper more
CR北斗の拳5 覇者
戦国パチスロ花の慶次~戦極めし傾奇者の宴~
シンプルなデザインとルールが魅力。初心者でも安心して楽しめます。
P真・北斗無双 第4章
https://k8slots.jp/tags/%E3%82%B2%E3%83%BC%E3%83%A0%E6%88%A6%E7%95%A5
大当たりを引いた瞬間の興奮は格別。友人と一緒に盛り上がれるのが嬉しいです。
CR大海物語4 Ver.319
k8 カジノ CRモンスターハンタ Ver.199
デジハネ CR 聖戦士ダンバイン
黃金守護神(特殊大賞燈);
沖ドキ!トロピカル
https://paxlovid1st.shop/# paxlovid cost without insurance
best ed drug
buy clopidogrel online: here – Plavix generic price
:-X
🙂
buy minocycline 50mg online: minocycline efectos secundarios – stromectol generic name
Garansi Kekalahan
NAGAEMPIRE: Platform Sports Game dan E-Games Terbaik di Tahun 2024
Selamat datang di Naga Empire, platform hiburan online yang menghadirkan pengalaman gaming terdepan di tahun 2024! Kami bangga menawarkan sports game, permainan kartu, dan berbagai fitur unggulan yang dirancang untuk memberikan Anda kesenangan dan keuntungan maksimal.
Keunggulan Pendaftaran dengan E-Wallet dan QRIS
Kami memprioritaskan kemudahan dan kecepatan dalam pengalaman bermain Anda:
Pendaftaran dengan E-Wallet: Daftarkan akun Anda dengan mudah menggunakan e-wallet favorit. Proses pendaftaran sangat cepat, memungkinkan Anda langsung memulai petualangan gaming tanpa hambatan.
QRIS Auto Proses dalam 1 Detik: Transaksi Anda diproses instan hanya dalam 1 detik dengan teknologi QRIS, memastikan pembayaran dan deposit berjalan lancar tanpa gangguan.
Sports Game dan Permainan Kartu Terbaik di Tahun 2024
Naga Empire menawarkan berbagai pilihan game menarik:
Sports Game Terlengkap: Dari taruhan olahraga hingga fantasy sports, kami menyediakan sensasi taruhan olahraga dengan kualitas terbaik.
Kartu Terbaik di 2024: Nikmati permainan kartu klasik hingga variasi modern dengan grafis yang menakjubkan, memberikan pengalaman bermain yang tak terlupakan.
Permainan Terlengkap dan Toto Terlengkap
Kami memiliki koleksi permainan yang sangat beragam:
Permainan Terlengkap: Temukan berbagai pilihan permainan seperti slot mesin, kasino, hingga permainan berbasis keterampilan, semua tersedia di Naga Empire.
Toto Terlengkap: Layanan Toto Online kami menawarkan pilihan taruhan yang lengkap dengan odds yang kompetitif, memberikan pengalaman taruhan yang optimal.
Bonus Melimpah dan Turnover Terendah
Bonus Melimpah: Dapatkan bonus mulai dari bonus selamat datang, bonus setoran, hingga promosi eksklusif. Kami selalu memberikan nilai lebih pada setiap taruhan Anda.
Turnover Terendah: Dengan turnover rendah, Anda dapat meraih kemenangan lebih mudah dan meningkatkan keuntungan dari setiap permainan.
Naga Empire adalah tempat yang tepat bagi Anda yang mencari pengalaman gaming terbaik di tahun 2024. Bergabunglah sekarang dan rasakan sensasi kemenangan di platform yang paling komprehensif!
http://paxlovid1st.shop/# paxlovid pharmacy
how to cure ed naturally
http://stromectol1st.shop/# ivermectin 3 mg tabs
buy medicines online in india
paxlovid pill: check this – Paxlovid buy online
http://stromectol1st.shop/# stromectol prices
best ed treatment pills
minocycline 100mg otc: stromectol 1st shop – minocycline 100mg tablets online
what is minocycline 100 mg used for ivermectin for sale or minocycline for uti
https://images.google.fi/url?sa=t&url=https://stromectol1st.shop stromectol pill for humans
minocycline pill ivermectin 3 mg dose and ivermectin 20 mg stromectol 3 mg tablets price
交響詩篇エウレカセブン 2
k8 カジノ スロット ITERO アイテロ
音楽と演出が楽しく、プレイ中に笑顔になれます。リラックスできます。
Pフィーバーからくりサーカス
https://onkaji.jp/tag/%E5%85%A5%E5%87%BA%E9%87%91
シンプルなルールで、誰でもすぐに楽しめるのが嬉しいです。
夜蝶飛翔特
[url=https://www.ja-securities.jp/games/id2194]k8カジノ パチンコ
[/url]
P真・牙狼2
Pこの素晴らしい世界に祝福を!199LT「このラッキートリガーに祝福を!」
CRルパン三世~消されたルパン Ver.394
rybelsus price semaglutide or Buy semaglutide
https://www.bausch.pk/en/redirect/?url=https://rybelsus.icu rybelsus
cheaper rybelsus.icu and rybelsus price buy rybelsus
Сервисный центр предлагает качественный ремонт электровелосипедов minako мастер по ремонту электровелосипеда minako
https://stromectol1st.shop/# stromectol 3 mg price
п»їlegitimate online pharmacies india
Semaglutide pharmacy price: rybelsus price – buy semaglutide online
antiplatelet drug best price on generic cheap plavix antiplatelet drug
ivermectin 6mg tablet for lice buy ivermectin nz or stromectol prices
https://images.google.nu/url?sa=t&url=https://stromectol1st.shop price of ivermectin tablets
ivermectin 400 mg ivermectin 0.5 and price of ivermectin liquid ivermectin 6mg tablet for lice
Studio di produzione video https://orbispro.it a ciclo completo. Servizi di creazione video. La nostra produzione effettua riprese video e qualsiasi altra produzione video a Milano, Roma, Torino
https://rybelsus.icu/# cheaper
dysfunction erectile
kometa casino бездепозитный бонус
Kometa Casino: Идеальный вариант для любителей азартных игр
Если вы любите ставками и ищете платформу, которая дает доступ к широкий ассортимент слотов и живых казино, а плюс большие бонусы, Казино Kometa — это то место, в котором вы найдете незабываемые впечатления. Предлагаем рассмотрим, что выделяет это казино уникальным и по каким причинам игроки остановились на этом сайте для досуга.
### Основные характеристики Kometa Casino
Казино Kometa — это глобальная платформа, что была запущена в 2024-м и сейчас уже привлекла признание клиентов по глобально. Вот некоторые черты, которые сравнивают с другими этот сайт:
Черта Описание
Год создания 2024-й
Глобальная доступность Международная
Число игр Свыше 1000
Сертификация Лицензия Кюрасао
Мобильный доступ Доступна
Методы платежей Популярные платежные системы
Служба поддержки 24/7 Чат и Email
Приветственные бонусы Акции и бонусы
Безопасность SSL защита
### Зачем играют в Kometa Casino?
#### Бонусная система
Одним из самых привлекательных особенностей Kometa Casino является система бонусов. Чем больше вы играете, тем лучше призы и бонусы. Система имеет многоуровневую систему:
– **Уровень 1 — Земля**: Возврат 3% от потраченных средств за неделю.
– **Уровень 2 — Луна**: Возврат 5% на ставки от 5 000 до 10 000 RUB.
– **Венера (уровень 3)**: Возврат 7% при ставках на сумму от 10 001 до 50 000 RUB.
– **Уровень 4 — Марс**: 8% кэшбек при сумме ставок от 50 001 до 150 000 ?.
– **Юпитер (уровень 5)**: Кэшбек 10% при ставках свыше 150 000 RUB.
– **Уровень 6 — Сатурн**: 11% бонуса.
– **Уровень 7 — Уран**: Максимальный возврат 12%.
#### Акции и возврат средств
С целью удержания азарт на высоте, Kometa Casino предоставляет бонусы каждую неделю, возврат средств и спины для новых пользователей. Частые бонусы способствуют сохранять интерес на каждой стадии игры.
#### Широкий выбор игр
Свыше тысячи игр, включая слоты, настольные игры и лайв-игры, превращают Казино Kometa местом, где каждый найдет подходящее развлечение. Игроки могут насладиться классическими играми, и современными слотами от лучших поставщиков. Дилеры в реальном времени добавляют атмосфере настоящее казино, формируя атмосферу азартного дома.
ivermectin pill cost: cheapest stromectol – buy ivermectin canada
stromectol 3mg: stromectol 1st – minocycline 50mg over the counter
antiplatelet drug clopidogrel Cost of Plavix without insurance
п»їplavix generic buy clopidogrel online or cheap plavix antiplatelet drug
http://images.google.com.bn/url?q=http://clopidogrel.pro buy plavix
buy clopidogrel online plavix medication and buy clopidogrel online clopidogrel bisulfate 75 mg
http://paxlovid1st.shop/# buy paxlovid online
erection pills viagra online
paxlovid pill paxlovid cost without insurance or п»їpaxlovid
https://www.exyst.de/domain_check_whois.php?query=paxlovid1st.shop& paxlovid price
Paxlovid over the counter paxlovid india and paxlovid cost without insurance paxlovid buy
душ для экономии воды
http://stromectol1st.shop/# ivermectin 3 mg tabs
india pharmacy
buy semaglutide online Buy semaglutide or <a href=" http://mensa.mymevis.org/xampp/phpinfo.php?a=sildenafil+couponssemaglutide
http://www.google.com.ni/url?q=https://rybelsus.icu:: rybelsus price
rybelsus price buy semaglutide online and order Rybelsus rybelsus price
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://permacultureglobal.org/users/68235-amanda-hernandez
https://www.haikudeck.com/presentations/m9x0E4aylF
https://jyhuop1995.bandcamp.com/album/trafficked-love-ch-20
https://onedio.ru/profile/ipilon-197-3
https://permacultureglobal.org/users/68536-tiffany-nelson
Cost of Plavix on Medicare: check clopidogrel pro – Plavix generic price
paxlovid pharmacy paxlovid price or paxlovid price
http://cttpeseux.ch/romands/link.php?url=http://paxlovid1st.shop п»їpaxlovid
paxlovid india paxlovid price and paxlovid buy paxlovid buy
п»їpaxlovid paxlovid pharmacy paxlovid india
https://clopidogrel.pro/# Cost of Plavix without insurance
ed remedies
cost of ivermectin 3mg tablets: stromectol shop – ivermectin 3mg for lice
paxlovid pharmacy: best price on pills – Paxlovid over the counter
paxlovid for sale paxlovid generic п»їpaxlovid
Полезный сервис быстрого загона ссылок сайта в индексация поисковой системы – быстрая индексация ссылок
http://rybelsus.icu/# rybelsus generic
cheap erectile dysfunction pill
https://stromectol1st.shop/# ivermectin price uk
п»їlegitimate online pharmacies india
minocycline rash: best price shop – stromectol tablets 3 mg
สล็อต888
สล็อต888 เป็นหนึ่งในแพลตฟอร์มเกมสล็อตออนไลน์ที่ได้รับความนิยมสูงสุดในปัจจุบัน โดยมีความโดดเด่นด้วยการให้บริการเกมสล็อตที่หลากหลายและมีคุณภาพ รวมถึงฟีเจอร์ที่ช่วยให้ผู้เล่นสามารถเพลิดเพลินกับการเล่นได้อย่างเต็มที่ ในบทความนี้ เราจะมาพูดถึงฟีเจอร์และจุดเด่นของสล็อต888 ที่ทำให้เว็บไซต์นี้ได้รับความนิยมเป็นอย่างมาก
ฟีเจอร์เด่นของ PG สล็อต888
ระบบฝากถอนเงินอัตโนมัติที่รวดเร็ว สล็อต888 ให้บริการระบบฝากถอนเงินแบบอัตโนมัติที่สามารถทำรายการได้ทันที ไม่ต้องรอนาน ไม่ว่าจะเป็นการฝากหรือถอนก็สามารถทำได้ภายในไม่กี่วินาที รองรับการใช้งานผ่านทรูวอลเล็ทและช่องทางอื่น ๆ โดยไม่มีขั้นต่ำในการฝากถอน
รองรับทุกอุปกรณ์ ทุกแพลตฟอร์ม ไม่ว่าคุณจะเล่นจากอุปกรณ์ใดก็ตาม สล็อต888 รองรับทั้งคอมพิวเตอร์ แท็บเล็ต และสมาร์ทโฟน ไม่ว่าจะเป็นระบบ iOS หรือ Android คุณสามารถเข้าถึงเกมสล็อตได้ทุกที่ทุกเวลาเพียงแค่มีอินเทอร์เน็ต
โปรโมชั่นและโบนัสมากมาย สำหรับผู้เล่นใหม่และลูกค้าประจำ สล็อต888 มีโปรโมชั่นต้อนรับ รวมถึงโบนัสพิเศษ เช่น ฟรีสปินและโบนัสเครดิตเพิ่ม ทำให้การเล่นเกมสล็อตกับเราเป็นเรื่องสนุกและมีโอกาสทำกำไรมากยิ่งขึ้น
ความปลอดภัยสูงสุด เรื่องความปลอดภัยเป็นสิ่งที่สล็อต888 ให้ความสำคัญเป็นอย่างยิ่ง เราใช้เทคโนโลยีการเข้ารหัสข้อมูลขั้นสูงเพื่อปกป้องข้อมูลส่วนบุคคลของลูกค้า ระบบฝากถอนเงินยังมีมาตรการรักษาความปลอดภัยที่เข้มงวด ทำให้ลูกค้ามั่นใจในการใช้บริการกับเรา
ทดลองเล่นสล็อตฟรี
สล็อต888 ยังมีบริการให้ผู้เล่นสามารถทดลองเล่นสล็อตได้ฟรี ซึ่งเป็นโอกาสที่ดีในการทดลองเล่นเกมต่าง ๆ ที่มีอยู่บนเว็บไซต์ เช่น Phoenix Rises, Dream Of Macau, Ways Of Qilin, Caishens Wins และเกมยอดนิยมอื่น ๆ ที่มีกราฟิกสวยงามและรูปแบบการเล่นที่น่าสนใจ
ไม่ว่าจะเป็นเกมแนวผจญภัย เช่น Rise Of Apollo, Dragon Hatch หรือเกมที่มีธีมแห่งความมั่งคั่งอย่าง Crypto Gold, Fortune Tiger, Lucky Piggy ทุกเกมได้รับการออกแบบมาเพื่อสร้างประสบการณ์การเล่นที่น่าจดจำและเต็มไปด้วยความสนุกสนาน
บทสรุป
สล็อต888 เป็นแพลตฟอร์มที่ครบเครื่องเรื่องเกมสล็อตออนไลน์ ด้วยฟีเจอร์ที่ทันสมัย โปรโมชั่นที่น่าสนใจ และระบบรักษาความปลอดภัยที่เข้มงวด ทำให้คุณมั่นใจได้ว่าการเล่นกับสล็อต888 จะเป็นประสบการณ์ที่ปลอดภัยและเต็มไปด้วยความสนุก
< – :-o)
Cost of Plavix on Medicare plavix medication or buy Clopidogrel over the counter
http://clients1.google.se/url?sa=t&url=https://clopidogrel.pro generic plavix
buy clopidogrel bisulfate clopidogrel bisulfate 75 mg and plavix medication Clopidogrel 75 MG price
Paxlovid buy online: shop – paxlovid pharmacy
paxlovid pharmacy buy here Paxlovid buy online
rybelsus price rybelsus.icu or rybelsus cost
http://www.protvino.ru/bitrix/rk.php?id=20&event1=banner&event2=click&goto=http://rybelsus.icu order Rybelsus
semaglutide good price and cheaper semaglutide
パチスロだよ黄門ちゃま
k8 カジノ パチンコ
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
CR地獄少女 弐
https://www.casinotop3.tokyo/tags/%E7%94%B7%E5%A1%BE
大当たりの瞬間は、周囲の人たちと喜びを分かち合うことができるのが魅力です。共感を得られます。
アイムジャグラーEXAnniversaryEdition
k8パチンコ
CR牙狼金色になれ
CR 獣王
P とある魔術の禁書目録 Light PREMIUM ver.
paxlovid cost without insurance: paxlovid shop – paxlovid pill
http://rybelsus.icu/# rybelsus
ed supplements
https://stromectol1st.shop/# ivermectin lice
indian pharmacies safe
minocycline 100 mg without doctor ivermectin 6mg or minocycline 100mg capsule
https://cse.google.sk/url?sa=t&url=https://stromectol1st.shop buy minocycline 50 mg otc
ivermectin 5 mg price ivermectin cream 1 and buy oral ivermectin minocycline 100mg tablets online
buy minocycline 50mg for humans best price shop ivermectin 3mg for lice
paxlovid for sale: paxlovid 1st – п»їpaxlovid
We are a group of experts and starting a new scheme in our community. Your web site offered us with valuable info to work on. You have done an impressive job and our whole community will be thankful to you. Thanks from Seo company India read this https://doodleordie.com/profile/rafaelcyrus
paxlovid india: paxlovid 1st – paxlovid generic
http://clopidogrel.pro/# buy clopidogrel online
the best ed pills
В современном мире перевод с иностранных языков стал неотъемлемой частью нашей жизни. Мы ежедневно сталкиваемся с необходимостью перевода при общении в интернете, чтении зарубежной литературы, просмотре фильмов и сериалов, а также в бизнесе и образовании. Но как выбрать надежную языковую службу, которая гарантирует высокое качество перевода и соблюдение конфиденциальности?
Одним из ключевых факторов при выборе языковой службы является репутация компании. В Новосибирске работают как крупные переводческие агентства, так и небольшие бюро, только начинающие свой путь в этой сфере. Отзывы клиентов, опыт работы на рынке, наличие сертификатов и лицензий – все это поможет сделать правильный выбор.
Однако репутация – не единственный фактор, на который стоит обращать внимание. Важную роль играет также компетентность и опыт переводчиков. Перевод – это не просто механическое перенесение текста с одного языка на другой, это искусство, требующее глубокого знания языков, культуры и специфики терминологии в конкретной области. Поэтому так важно, чтобы переводчики имели специальное образование и опыт работы в данной сфере.
Кроме того, необходимо учитывать формат и объем перевода. Если вам нужен устный перевод, то понадобится синхронный или последовательный переводчик, который будет работать в режиме реального времени. Для письменных переводов может потребоваться предварительное изучение материала и использование специальных программ для работы с текстами.
Цена также является важным фактором при выборе языковой службы. Стоимость услуг может варьироваться в зависимости от языка, объема перевода, срочности и других факторов. Некоторые компании предлагают фиксированные цены за страницу или слово, другие – гибкую систему тарификации. Но не стоит гнаться за самой низкой ценой – она часто оборачивается низким качеством перевода.
Кроме того, необходимо учитывать необходимость нотариального заверения перевода. Если документ будет использоваться в официальных инстанциях, то он должен быть правильно оформлен и заверен нотариусом. Не все языковые службы предоставляют эту услугу, поэтому стоит заранее уточнить ее наличие.
Наконец, следует обратить внимание на такие нюансы, как сроки выполнения заказа и возможность доставки готового перевода. Если вам нужен срочный перевод, то стоит выбрать компанию, которая может гарантировать быструю обработку заказа. Также удобно, когда языковая служба предлагает доставку готовых переводов на дом или в офис.
В заключение можно сказать, что выбор языковой службы в Новосибирске – это ответственное решение, требующее тщательного подхода и анализа различных факторов. Но если подойти к процессу грамотно, то можно найти надежного партнера, который поможет преодолеть языковые барьеры и добиться успеха в бизнесе или в личной жизни. Главное – не экономить на качестве перевода, ведь правильный выбор языковой службы может стать залогом вашего успеха.
перевод с иностранных языков
minocycline 100 mg tablets online minocycline for sinus infection or minocycline for rosacea
https://images.google.gg/url?sa=t&url=https://stromectol1st.shop ivermectin malaria
minocycline online minocycline 50mg otc and ivermectin 3 mg dose minocycline 100 mg online
https://stromectol1st.shop/# ivermectin cost australia
mail order pharmacy india
buy rybelsus Buy semaglutide or Semaglutide pharmacy price
https://www.google.com.hk/url?sa=i&url=https://rybelsus.icu rybelsus.icu
rybelsus price rybelsus generic and Buy semaglutide order Rybelsus
ivermectin 0.08: stromectol fast delivery – order minocycline 100 mg
plavix best price buy plavix or antiplatelet drug
http://mx.taskmanagementsoft.com/bitrix/redirect.php?goto=https://clopidogrel.pro:: Clopidogrel 75 MG price
Plavix generic price generic plavix and antiplatelet drug antiplatelet drug
paxlovid for sale Paxlovid buy online or Paxlovid buy online
https://images.google.com.om/url?sa=t&url=https://paxlovid1st.shop paxlovid generic
paxlovid covid paxlovid cost without insurance and paxlovid covid paxlovid price
minocycline 100 mg for sale cheapest stromectol stromectol otc
モンキーターンII
k8 カジノ パチンコ 戦国BASARA 2
シンプルなデザインが魅力。初心者でも楽しみやすい仕様です。
キュインぱちすろ南国育ち 1st vacation
https://www.taisetuya.net/tags/%e7%89%99%e7%8b%bc%e9%ad%94%e6%88%92%e3%83%8e%e8%8a%b1
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
南国育ち
k8 カジノ パチンコ パチスロ北斗の拳 修羅の国篇パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
島娘
CR戦国乙女〜花〜
P緋弾のアリア 緋弾覚醒編 Ver.199
สล็อต888
สล็อต888 เป็นหนึ่งในแพลตฟอร์มเกมสล็อตออนไลน์ที่ได้รับความนิยมสูงสุดในปัจจุบัน โดยมีความโดดเด่นด้วยการให้บริการเกมสล็อตที่หลากหลายและมีคุณภาพ รวมถึงฟีเจอร์ที่ช่วยให้ผู้เล่นสามารถเพลิดเพลินกับการเล่นได้อย่างเต็มที่ ในบทความนี้ เราจะมาพูดถึงฟีเจอร์และจุดเด่นของสล็อต888 ที่ทำให้เว็บไซต์นี้ได้รับความนิยมเป็นอย่างมาก
ฟีเจอร์เด่นของ PG สล็อต888
ระบบฝากถอนเงินอัตโนมัติที่รวดเร็ว สล็อต888 ให้บริการระบบฝากถอนเงินแบบอัตโนมัติที่สามารถทำรายการได้ทันที ไม่ต้องรอนาน ไม่ว่าจะเป็นการฝากหรือถอนก็สามารถทำได้ภายในไม่กี่วินาที รองรับการใช้งานผ่านทรูวอลเล็ทและช่องทางอื่น ๆ โดยไม่มีขั้นต่ำในการฝากถอน
รองรับทุกอุปกรณ์ ทุกแพลตฟอร์ม ไม่ว่าคุณจะเล่นจากอุปกรณ์ใดก็ตาม สล็อต888 รองรับทั้งคอมพิวเตอร์ แท็บเล็ต และสมาร์ทโฟน ไม่ว่าจะเป็นระบบ iOS หรือ Android คุณสามารถเข้าถึงเกมสล็อตได้ทุกที่ทุกเวลาเพียงแค่มีอินเทอร์เน็ต
โปรโมชั่นและโบนัสมากมาย สำหรับผู้เล่นใหม่และลูกค้าประจำ สล็อต888 มีโปรโมชั่นต้อนรับ รวมถึงโบนัสพิเศษ เช่น ฟรีสปินและโบนัสเครดิตเพิ่ม ทำให้การเล่นเกมสล็อตกับเราเป็นเรื่องสนุกและมีโอกาสทำกำไรมากยิ่งขึ้น
ความปลอดภัยสูงสุด เรื่องความปลอดภัยเป็นสิ่งที่สล็อต888 ให้ความสำคัญเป็นอย่างยิ่ง เราใช้เทคโนโลยีการเข้ารหัสข้อมูลขั้นสูงเพื่อปกป้องข้อมูลส่วนบุคคลของลูกค้า ระบบฝากถอนเงินยังมีมาตรการรักษาความปลอดภัยที่เข้มงวด ทำให้ลูกค้ามั่นใจในการใช้บริการกับเรา
ทดลองเล่นสล็อตฟรี
สล็อต888 ยังมีบริการให้ผู้เล่นสามารถทดลองเล่นสล็อตได้ฟรี ซึ่งเป็นโอกาสที่ดีในการทดลองเล่นเกมต่าง ๆ ที่มีอยู่บนเว็บไซต์ เช่น Phoenix Rises, Dream Of Macau, Ways Of Qilin, Caishens Wins และเกมยอดนิยมอื่น ๆ ที่มีกราฟิกสวยงามและรูปแบบการเล่นที่น่าสนใจ
ไม่ว่าจะเป็นเกมแนวผจญภัย เช่น Rise Of Apollo, Dragon Hatch หรือเกมที่มีธีมแห่งความมั่งคั่งอย่าง Crypto Gold, Fortune Tiger, Lucky Piggy ทุกเกมได้รับการออกแบบมาเพื่อสร้างประสบการณ์การเล่นที่น่าจดจำและเต็มไปด้วยความสนุกสนาน
บทสรุป
สล็อต888 เป็นแพลตฟอร์มที่ครบเครื่องเรื่องเกมสล็อตออนไลน์ ด้วยฟีเจอร์ที่ทันสมัย โปรโมชั่นที่น่าสนใจ และระบบรักษาความปลอดภัยที่เข้มงวด ทำให้คุณมั่นใจได้ว่าการเล่นกับสล็อต888 จะเป็นประสบการณ์ที่ปลอดภัยและเต็มไปด้วยความสนุก
http://clopidogrel.pro/# plavix medication
new ed treatments
buy semaglutide online: buy semaglutide online – good price
buy semaglutide online: rybelsus cost – Semaglutide pharmacy price
good price rybelsus price rybelsus
< – :-o)
https://mari-tyrek.ru/58501.html
rybelsus cost: more – rybelsus cost
http://stromectol1st.shop/# buy minocycline 100 mg online
best india pharmacy
paxlovid generic: paxlovid shop – paxlovid for sale
http://clopidogrel.pro/# buy clopidogrel online
generic viagra without a doctor prescription
NAGAEMPIRE: Platform Sports Game dan E-Games Terbaik di Tahun 2024
Selamat datang di Naga Empire, platform hiburan online yang menghadirkan pengalaman gaming terdepan di tahun 2024! Kami bangga menawarkan sports game, permainan kartu, dan berbagai fitur unggulan yang dirancang untuk memberikan Anda kesenangan dan keuntungan maksimal.
Keunggulan Pendaftaran dengan E-Wallet dan QRIS
Kami memprioritaskan kemudahan dan kecepatan dalam pengalaman bermain Anda:
Pendaftaran dengan E-Wallet: Daftarkan akun Anda dengan mudah menggunakan e-wallet favorit. Proses pendaftaran sangat cepat, memungkinkan Anda langsung memulai petualangan gaming tanpa hambatan.
QRIS Auto Proses dalam 1 Detik: Transaksi Anda diproses instan hanya dalam 1 detik dengan teknologi QRIS, memastikan pembayaran dan deposit berjalan lancar tanpa gangguan.
Sports Game dan Permainan Kartu Terbaik di Tahun 2024
Naga Empire menawarkan berbagai pilihan game menarik:
Sports Game Terlengkap: Dari taruhan olahraga hingga fantasy sports, kami menyediakan sensasi taruhan olahraga dengan kualitas terbaik.
Kartu Terbaik di 2024: Nikmati permainan kartu klasik hingga variasi modern dengan grafis yang menakjubkan, memberikan pengalaman bermain yang tak terlupakan.
Permainan Terlengkap dan Toto Terlengkap
Kami memiliki koleksi permainan yang sangat beragam:
Permainan Terlengkap: Temukan berbagai pilihan permainan seperti slot mesin, kasino, hingga permainan berbasis keterampilan, semua tersedia di Naga Empire.
Toto Terlengkap: Layanan Toto Online kami menawarkan pilihan taruhan yang lengkap dengan odds yang kompetitif, memberikan pengalaman taruhan yang optimal.
Bonus Melimpah dan Turnover Terendah
Bonus Melimpah: Dapatkan bonus mulai dari bonus selamat datang, bonus setoran, hingga promosi eksklusif. Kami selalu memberikan nilai lebih pada setiap taruhan Anda.
Turnover Terendah: Dengan turnover rendah, Anda dapat meraih kemenangan lebih mudah dan meningkatkan keuntungan dari setiap permainan.
Naga Empire adalah tempat yang tepat bagi Anda yang mencari pengalaman gaming terbaik di tahun 2024. Bergabunglah sekarang dan rasakan sensasi kemenangan di platform yang paling komprehensif!
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
generic plavix check clopidogrel pro buy clopidogrel online
NAGAEMPIRE: Platform Sports Game dan E-Games Terbaik di Tahun 2024
Selamat datang di Naga Empire, platform hiburan online yang menghadirkan pengalaman gaming terdepan di tahun 2024! Kami bangga menawarkan sports game, permainan kartu, dan berbagai fitur unggulan yang dirancang untuk memberikan Anda kesenangan dan keuntungan maksimal.
Keunggulan Pendaftaran dengan E-Wallet dan QRIS
Kami memprioritaskan kemudahan dan kecepatan dalam pengalaman bermain Anda:
Pendaftaran dengan E-Wallet: Daftarkan akun Anda dengan mudah menggunakan e-wallet favorit. Proses pendaftaran sangat cepat, memungkinkan Anda langsung memulai petualangan gaming tanpa hambatan.
QRIS Auto Proses dalam 1 Detik: Transaksi Anda diproses instan hanya dalam 1 detik dengan teknologi QRIS, memastikan pembayaran dan deposit berjalan lancar tanpa gangguan.
Sports Game dan Permainan Kartu Terbaik di Tahun 2024
Naga Empire menawarkan berbagai pilihan game menarik:
Sports Game Terlengkap: Dari taruhan olahraga hingga fantasy sports, kami menyediakan sensasi taruhan olahraga dengan kualitas terbaik.
Kartu Terbaik di 2024: Nikmati permainan kartu klasik hingga variasi modern dengan grafis yang menakjubkan, memberikan pengalaman bermain yang tak terlupakan.
Permainan Terlengkap dan Toto Terlengkap
Kami memiliki koleksi permainan yang sangat beragam:
Permainan Terlengkap: Temukan berbagai pilihan permainan seperti slot mesin, kasino, hingga permainan berbasis keterampilan, semua tersedia di Naga Empire.
Toto Terlengkap: Layanan Toto Online kami menawarkan pilihan taruhan yang lengkap dengan odds yang kompetitif, memberikan pengalaman taruhan yang optimal.
Bonus Melimpah dan Turnover Terendah
Bonus Melimpah: Dapatkan bonus mulai dari bonus selamat datang, bonus setoran, hingga promosi eksklusif. Kami selalu memberikan nilai lebih pada setiap taruhan Anda.
Turnover Terendah: Dengan turnover rendah, Anda dapat meraih kemenangan lebih mudah dan meningkatkan keuntungan dari setiap permainan.
Naga Empire adalah tempat yang tepat bagi Anda yang mencari pengalaman gaming terbaik di tahun 2024. Bergabunglah sekarang dan rasakan sensasi kemenangan di platform yang paling komprehensif!
more rybelsus cost or rybelsus
https://images.google.co.mz/url?q=https://rybelsus.icu buy semaglutide online
Buy semaglutide rybelsus generic and Semaglutide pharmacy price rybelsus
ST666 – Nhà Cái Uy Tín Với Nhiều Khuyến Mãi Hấp Dẫn
ST666 là một trong những sòng bài trực tuyến uy tín nhất, mang đến cho các thành viên nhiều chương trình khuyến mãi đa dạng và hấp dẫn. Chúng tôi luôn hy vọng rằng những ưu đãi đặc biệt này sẽ mang lại trải nghiệm cá cược tuyệt vời cho tất cả thành viên của mình.
Khuyến Mãi Nạp Đầu Thưởng 100%
NO.1: Thưởng 100% lần nạp đầu tiên Thông thường, các khuyến mãi nạp tiền lần đầu đi kèm với yêu cầu vòng cược rất cao, thường từ 20 vòng trở lên. Tuy nhiên, ST666 hiểu rằng người chơi thường đắn đo khi quyết định nạp tiền vào sòng bài trực tuyến. Do đó, chúng tôi chỉ yêu cầu 8 vòng cược, cho phép người chơi rút tiền một cách nhanh chóng trong vòng 5 phút.
Thưởng 100% khi Đăng Nhập ST666
NO.2: Thưởng 16% mỗi ngày ST666 mang đến cho bạn một ưu đãi không thể bỏ lỡ. Mỗi khi bạn nạp tiền vào tài khoản hàng ngày, bạn sẽ được nhận thêm 16% số tiền nạp. Đây là cơ hội tuyệt vời dành cho những ai yêu thích cá cược trực tuyến và muốn gia tăng cơ hội chiến thắng của mình.
Bảo Hiểm Hoàn 10% Mỗi Ngày
NO.3: Bảo hiểm hoàn tiền 10% ST666 không chỉ hỗ trợ người chơi trong những lần may mắn mà còn luôn đồng hành khi bạn không gặp may. Chương trình khuyến mãi “bảo hiểm hoàn tiền 10% tổng tiền nạp mỗi ngày” là cách chúng tôi khích lệ và động viên tinh thần game thủ, giúp bạn tiếp tục hành trình chinh phục các thử thách cá cược.
Giới Thiệu Bạn Bè Thưởng 999K
NO.4: Giới thiệu bạn bè – Thưởng 999K Chương trình “Giới thiệu bạn bè” của ST666 giúp bạn có cơ hội cá cược cùng bạn bè và người thân. Không những vậy, bạn còn có thể nhận được phần thưởng lên đến 999K khi giới thiệu người bạn của mình tham gia ST666. Đây chính là một ưu đãi tuyệt vời giúp bạn vừa có thêm người đồng hành, vừa nhận được thưởng hấp dẫn.
Đăng Nhập Nhận Ngay 280K
NO.5: Điểm danh nhận thưởng 280K Nếu bạn là thành viên thường xuyên của ST666, hãy đừng quên nhấp vào hộp quà hàng ngày để điểm danh. Chỉ cần đăng nhập liên tục trong 7 ngày, cơ hội nhận thưởng 280K sẽ thuộc về bạn!
Kết Luận
ST666 luôn nỗ lực không ngừng để mang lại những chương trình khuyến mãi hấp dẫn và thiết thực nhất cho người chơi. Dù bạn là người mới hay đã gắn bó lâu năm với nền tảng, ST666 cam kết cung cấp trải nghiệm cá cược tốt nhất, an toàn và công bằng. Hãy tham gia ngay để không bỏ lỡ các cơ hội tuyệt vời từ ST666!
rgbet
RGBET: Thương Hiệu Uy Tín Hàng Đầu Châu Á
RGBET là một trong những thương hiệu sòng bài trực tuyến uy tín nhất tại Châu Á, đã nhiều năm liên tiếp được bình chọn 5 sao nhờ vào sự đáng tin cậy và chất lượng dịch vụ vượt trội. Với sứ mệnh mang lại trải nghiệm cá cược tuyệt vời cho người chơi, RGBET tự hào cung cấp hệ thống giao dịch an toàn, sản phẩm đa dạng, và hỗ trợ trên mọi nền tảng.
Sản Phẩm Đa Dạng
RGBET mang đến cho người chơi một thế giới giải trí phong phú với các sản phẩm như: Casino trực tuyến, Thể thao, Nổ hũ, Bắn cá, Xổ số, và nhiều loại trò chơi khác. Điều này đảm bảo rằng người chơi có thể dễ dàng lựa chọn các trò chơi phù hợp với sở thích cá nhân mà không bao giờ cảm thấy nhàm chán.
An Ninh và Bảo Mật
An ninh là yếu tố hàng đầu mà RGBET cam kết cung cấp cho khách hàng. Hệ thống bảo mật thông tin hiện đại giúp bảo vệ tuyệt đối dữ liệu cá nhân của người chơi. Bên cạnh đó, phương thức thanh toán đa dạng và an toàn cũng giúp người chơi thực hiện các giao dịch một cách dễ dàng và nhanh chóng.
Giao Dịch Nhanh Chóng
RGBET sử dụng công nghệ xử lý giao dịch tự động tiên tiến nhất, đảm bảo tốc độ nạp rút tiền nhanh chóng, không để người chơi phải chờ đợi lâu. Với sự tối ưu trong mọi khâu, bạn có thể yên tâm rằng các giao dịch sẽ diễn ra mượt mà và minh bạch.
Lý Do Nên Chọn RGBET
RGBET luôn chú trọng vào trải nghiệm của người chơi, không chỉ mang lại môi trường chơi game mượt mà, mà còn đảm bảo sự công bằng và an toàn. Hệ thống chống gian lận và rửa tiền toàn diện giúp người chơi mới có thể yên tâm tham gia mà không lo gặp phải các vấn đề lừa đảo.
Tải Ứng Dụng RGBET
Để trải nghiệm tốt hơn, người chơi có thể tải ngay ứng dụng RGBET Casino trực tuyến cho điện thoại. Ứng dụng này hỗ trợ tất cả các sản phẩm, từ Thể thao, E-Sports, đến Casino và Xổ số. RGBET hỗ trợ cả hai hệ điều hành iOS và Android, mang đến sự tiện lợi và bảo mật cao hơn cho người chơi.
Câu Hỏi Thường Gặp về RGBET Casino
RGBET Trang chủ là gì? RGBET Trang chủ là website chính thức của RGBET Casino, địa chỉ trực tuyến là vnrg5269.com, cung cấp thông tin chi tiết về các dịch vụ và sản phẩm cá cược.
Tiêu chí đánh giá nhà cái uy tín là gì? Một nhà cái uy tín như RGBET luôn đáp ứng được các tiêu chí về an ninh, minh bạch, và cung cấp trải nghiệm chơi công bằng, bảo mật.
Tôi có thể cá cược trực tiếp trên điện thoại không? Có, bạn hoàn toàn có thể cá cược trực tiếp trên điện thoại thông qua ứng dụng RGBET, mang lại sự tiện lợi và bảo mật tuyệt đối.
RGBET không chỉ là một nền tảng cá cược trực tuyến mà còn là đối tác đáng tin cậy cho những ai yêu thích giải trí và cá cược, luôn đặt nhu cầu của người chơi lên hàng đầu.
plavix medication Plavix 75 mg price or generic plavix
https://dealers.webasto.com/UnauthorizedAccess.aspx?Result=denied&Url=https://clopidogrel.pro buy Clopidogrel over the counter
plavix best price antiplatelet drug and buy plavix buy clopidogrel bisulfate
paxlovid cost without insurance: shop – п»їpaxlovid
antiplatelet drug: clopidogrel pills – plavix medication
rgbet
Bạn đang tìm kiếm những trò chơi hot nhất và thú vị nhất tại sòng bạc trực tuyến? RGBET tự hào giới thiệu đến bạn nhiều trò chơi cá cược đặc sắc, bao gồm Baccarat trực tiếp, máy xèng, cá cược thể thao, xổ số và bắn cá, mang đến cho bạn cảm giác hồi hộp đỉnh cao của sòng bạc! Dù bạn yêu thích các trò chơi bài kinh điển hay những máy xèng đầy kịch tính, RGBET đều có thể đáp ứng mọi nhu cầu giải trí của bạn.
RGBET Trò Chơi của Chúng Tôi
Bạn đang tìm kiếm những trò chơi hot nhất và thú vị nhất tại sòng bạc trực tuyến? RGBET tự hào giới thiệu đến bạn nhiều trò chơi cá cược đặc sắc, bao gồm Baccarat trực tiếp, máy xèng, cá cược thể thao, xổ số và bắn cá, mang đến cảm giác hồi hộp đỉnh cao của sòng bạc! Dù bạn yêu thích các trò chơi bài kinh điển hay những máy xèng đầy kịch tính, RGBET đều có thể đáp ứng mọi nhu cầu giải trí của bạn.
RGBET Trò Chơi Đa Dạng
Thể thao: Cá cược thể thao đa dạng với nhiều môn từ bóng đá, tennis đến thể thao điện tử.
Live Casino: Trải nghiệm Baccarat, Roulette, và các trò chơi sòng bài trực tiếp với người chia bài thật.
Nổ hũ: Tham gia các trò chơi nổ hũ với tỷ lệ trúng cao và cơ hội thắng lớn.
Lô đề: Đặt cược lô đề với tỉ lệ cược hấp dẫn.
Bắn cá: Bắn cá RGBET mang đến cảm giác chân thực và hấp dẫn với đồ họa tuyệt đẹp.
RGBET – Máy Xèng Hấp Dẫn Nhất
Khám phá các máy xèng độc đáo tại RGBET với nhiều chủ đề khác nhau và tỷ lệ trả thưởng cao. Những trò chơi nổi bật bao gồm:
RGBET Super Ace
RGBET Đế Quốc Hoàng Kim
RGBET Pharaoh Treasure
RGBET Quyền Vương
RGBET Chuyên Gia Săn Rồng
RGBET Jackpot Fishing
Vì sao nên chọn RGBET?
RGBET không chỉ cung cấp hàng loạt trò chơi đa dạng mà còn mang đến một hệ thống cá cược an toàn và chuyên nghiệp, đảm bảo mọi quyền lợi của người chơi:
Tốc độ nạp tiền nhanh chóng: Chuyển khoản tại RGBET chỉ mất vài phút và tiền sẽ vào tài khoản ngay lập tức, giúp bạn không bỏ lỡ bất kỳ cơ hội nào.
Game đổi thưởng phong phú: Từ cá cược thể thao đến slot game, RGBET cung cấp đầy đủ trò chơi giúp bạn tận hưởng mọi phút giây thư giãn.
Bảo mật tuyệt đối: Với công nghệ mã hóa tiên tiến, tài khoản và tiền vốn của bạn sẽ luôn được bảo vệ một cách an toàn.
Hỗ trợ đa nền tảng: Bạn có thể chơi trên mọi thiết bị, từ máy tính, điện thoại di động (iOS/Android), đến nền tảng H5.
Tải Ứng Dụng RGBET và Nhận Khuyến Mãi Lớn
Hãy tham gia RGBET ngay hôm nay để tận hưởng thế giới giải trí không giới hạn với các trò chơi thể thao, thể thao điện tử, casino trực tuyến, xổ số, và slot game. Quét mã QR và tải ứng dụng RGBET trên điện thoại để trải nghiệm game tốt hơn và nhận nhiều khuyến mãi hấp dẫn!
Tham gia RGBET để bắt đầu cuộc hành trình cá cược đầy thú vị ngay hôm nay!
https://stromectol1st.shop/# ivermectin 250ml
indian pharmacy online
paxlovid pharmacy paxlovid generic or п»їpaxlovid
https://cse.google.com.cu/url?sa=t&url=https://paxlovid1st.shop buy paxlovid online
paxlovid generic paxlovid pill and paxlovid buy paxlovid pill
rybelsus.icu buy semaglutide online good price
st666
ST666 – Nhà Cái Uy Tín Với Nhiều Khuyến Mãi Hấp Dẫn
ST666 là một trong những sòng bài trực tuyến uy tín nhất, mang đến cho các thành viên nhiều chương trình khuyến mãi đa dạng và hấp dẫn. Chúng tôi luôn hy vọng rằng những ưu đãi đặc biệt này sẽ mang lại trải nghiệm cá cược tuyệt vời cho tất cả thành viên của mình.
Khuyến Mãi Nạp Đầu Thưởng 100%
NO.1: Thưởng 100% lần nạp đầu tiên Thông thường, các khuyến mãi nạp tiền lần đầu đi kèm với yêu cầu vòng cược rất cao, thường từ 20 vòng trở lên. Tuy nhiên, ST666 hiểu rằng người chơi thường đắn đo khi quyết định nạp tiền vào sòng bài trực tuyến. Do đó, chúng tôi chỉ yêu cầu 8 vòng cược, cho phép người chơi rút tiền một cách nhanh chóng trong vòng 5 phút.
Thưởng 100% khi Đăng Nhập ST666
NO.2: Thưởng 16% mỗi ngày ST666 mang đến cho bạn một ưu đãi không thể bỏ lỡ. Mỗi khi bạn nạp tiền vào tài khoản hàng ngày, bạn sẽ được nhận thêm 16% số tiền nạp. Đây là cơ hội tuyệt vời dành cho những ai yêu thích cá cược trực tuyến và muốn gia tăng cơ hội chiến thắng của mình.
Bảo Hiểm Hoàn 10% Mỗi Ngày
NO.3: Bảo hiểm hoàn tiền 10% ST666 không chỉ hỗ trợ người chơi trong những lần may mắn mà còn luôn đồng hành khi bạn không gặp may. Chương trình khuyến mãi “bảo hiểm hoàn tiền 10% tổng tiền nạp mỗi ngày” là cách chúng tôi khích lệ và động viên tinh thần game thủ, giúp bạn tiếp tục hành trình chinh phục các thử thách cá cược.
Giới Thiệu Bạn Bè Thưởng 999K
NO.4: Giới thiệu bạn bè – Thưởng 999K Chương trình “Giới thiệu bạn bè” của ST666 giúp bạn có cơ hội cá cược cùng bạn bè và người thân. Không những vậy, bạn còn có thể nhận được phần thưởng lên đến 999K khi giới thiệu người bạn của mình tham gia ST666. Đây chính là một ưu đãi tuyệt vời giúp bạn vừa có thêm người đồng hành, vừa nhận được thưởng hấp dẫn.
Đăng Nhập Nhận Ngay 280K
NO.5: Điểm danh nhận thưởng 280K Nếu bạn là thành viên thường xuyên của ST666, hãy đừng quên nhấp vào hộp quà hàng ngày để điểm danh. Chỉ cần đăng nhập liên tục trong 7 ngày, cơ hội nhận thưởng 280K sẽ thuộc về bạn!
Kết Luận
ST666 luôn nỗ lực không ngừng để mang lại những chương trình khuyến mãi hấp dẫn và thiết thực nhất cho người chơi. Dù bạn là người mới hay đã gắn bó lâu năm với nền tảng, ST666 cam kết cung cấp trải nghiệm cá cược tốt nhất, an toàn và công bằng. Hãy tham gia ngay để không bỏ lỡ các cơ hội tuyệt vời từ ST666!
minocycline capsule: ivermectin 3mg pill – buy minocycline 100 mg online
semaglutide: cheaper – Buy semaglutide
paxlovid covid paxlovid cost without insurance or paxlovid india
https://maps.google.co.ck/url?q=https://paxlovid1st.shop Paxlovid over the counter
paxlovid price paxlovid generic and paxlovid pharmacy paxlovid india
https://stromectol1st.shop/# ivermectin lotion for scabies
online shopping pharmacy india
ivermectin 1 cream 45gm buy online ivermectin goodrx
https://clopidogrel.pro/# Plavix 75 mg price
buy ed drugs
ivermectin cost in usa topical ivermectin cost or price of ivermectin liquid
https://www.google.mv/url?sa=t&url=https://stromectol1st.shop minocycline 50 mg
stromectol liquid ivermectin humans and buy ivermectin uk minocycline 50 mg tabs
Tether TRON-based Transaction Validation and Financial Crime Prevention (AML) Procedures
As digital assets like Tether TRON-based gain usage for rapid and low-cost transfers, the requirement for security and compliance with financial crime prevention rules grows. Here’s how to review USDT TRON-based payments and confirm they’re not connected to unlawful actions.
What is TRON-based USDT?
TRON-based USDT is a digital currency on the TRX blockchain, priced in correspondence with the US dollar. Recognized for its low transaction fees and speed, it is frequently employed for cross-border transfers. Verifying transactions is essential to block associations to financial crime or other unlawful operations.
Checking TRON-based USDT Transactions
TRONSCAN — This blockchain viewer allows participants to follow and verify USDT TRC20 payments using a account ID or transaction ID.
Supervising — Skilled users can monitor anomalous trends such as large or quick transactions to detect unusual actions.
AML and Illicit Funds
AML (AML) rules support block illegal money transfers in crypto markets. Services like Chain Analysis and Elliptic Solutions allow enterprises and trading platforms to identify and prevent illicit funds, which signifies capital related to criminal actions.
Solutions for Adherence
TRX Explorer — To validate USDT TRC20 payment information.
Chainalysis and Elliptic Solutions — Utilized by crypto markets to guarantee Anti-Money Laundering compliance and monitor unlawful operations.
Conclusion
Making sure secure and legal TRON-based USDT payments is critical. Services like TRONSCAN and AML tools help shield participants from interacting with criminal crypto, promoting a protected and regulated digital market.
rybelsus.icu order Rybelsus or good price
https://cse.google.ht/url?sa=t&url=https://rybelsus.icu more
semaglutide more and semaglutide rybelsus price
Профессиональный сервисный центр по ремонту сотовых телефонов в Москве.
Мы предлагаем: ремонт ноутбука москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
cheaper: Buy semaglutide – Buy semaglutide
antiplatelet drug cheap plavix antiplatelet drug or generic plavix
http://fivelige.net/m2wt/jsp/Imageinbrowser2.jsp?id=9185&nid=2166&subid=7422273&cid=7&couponcode&domainur=clopidogrel.pro&queue=q4 Clopidogrel 75 MG price
[url=https://login.mephi.ru/login?allow_anonymous=true&service=https://clopidogrel.pro/]Cost of Plavix on Medicare[/url] Plavix generic price and [url=https://bbs.xiaoditech.com/home.php?mod=space&uid=1909491]Cost of Plavix on Medicare[/url] Plavix generic price
buy paxlovid online: check this – buy paxlovid online
Paxlovid over the counter shop paxlovid cost without insurance
http://clopidogrel.pro/# Cost of Plavix without insurance
erectial dysfunction
(ґ• ? •`) ?
https://mari-tyrek.ru/65754.html
3x3EYES聖魔覚醒
k8パチンコ
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
めんそーれ(雷電ver.)(特殊大賞燈)
https://www.10k8.com/tags/%E3%82%BD%E3%83%BC%E3%82%B7%E3%83%A3%E3%83%AB%E3%81%AA%E8%A6%81%E7%B4%A0
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
青龍(4発1)
k8 カジノ パチンコ CRぱちんこウルトラマンタロウ 戦え!!ウルトラ6兄弟パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
CRぱちんこ押忍!番長
CR北斗の拳剛掌 Ver.399
L マクロスフロンティア 4
rybelsus.icu: buy semaglutide online – rybelsus.icu
ivermectin nz: ivermectin 1 cream 45gm – ivermectin 0.5
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.metal-archives.com/users/ophtha1952
https://permacultureglobal.org/users/65604-jay-gessesse
https://www.haikudeck.com/presentations/pz3N5lCVJb
https://www.dnnsoftware.com/activity-feed/my-profile/userid/3199533
http://www.babelcube.com/user/karli-miller
https://stromectol1st.shop/# ivermectin 0.2mg
online pharmacy india
If some one desires expert view concerning running a blog after that i
propose him/her to go to see this webpage, Keep up the fastidious work.
http://clopidogrel.pro/# Cost of Plavix on Medicare
ed vacuum pumps
plise sineklik | Zip screens can enhance indoor comfort by controlling airflow, as explained in this article. They seem like a very useful product.
paxlovid cost without insurance: best price on pills – paxlovid covid
cheaper: rybelsus generic – Semaglutide pharmacy price
buy rybelsus Semaglutide pharmacy price good price
http://stromectol1st.shop/# minocycline 50mg over the counter
indian pharmacy paypal
🙂
https://mari-tyrek.ru/63216.html
~(>_<~)
https://mari-tyrek.ru/21990.html
buy clopidogrel online antiplatelet drug or п»їplavix generic
https://toolbarqueries.google.com.bh/url?q=http://clopidogrel.pro Plavix generic price
plavix best price plavix medication and buy plavix clopidogrel bisulfate 75 mg
(ґ• ? •`) ?
https://mari-tyrek.ru/72411.html
牙狼 守りし者
P大工の源さん超韋駄天2極源LighT(甘デジ)新台パチンコ
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
紫イミソーレ
https://www.wk8.jp/tags/%E3%82%A2%E3%83%8A%E3%82%B6%E3%83%BC%E3%82%B4%E3%83%83%E3%83%89%E3%83%8F%E3%83%BC%E3%83%87%E3%82%B9
大当たりの瞬間に周囲と一体感を感じられるのが良いです。共に喜べる仲間がいるのが嬉しい。
モンスターハンター
k8 カジノ パチンコ
CR義風堂々!!兼続と慶次
パラダイスハイビスカス
黄門ちゃま喝
paxlovid cost without insurance: check this – paxlovid covid
Semaglutide pharmacy price: rybelsus – buy semaglutide online
paxlovid generic paxlovid pill or buy paxlovid online
https://images.google.vg/url?sa=t&url=https://paxlovid1st.shop paxlovid generic
paxlovid price paxlovid india and Paxlovid buy online Paxlovid over the counter
เกมสล็อตออนไลน์
สล็อต888 เป็นหนึ่งในแพลตฟอร์มเกมสล็อตออนไลน์ที่ได้รับความนิยมสูงสุดในปัจจุบัน โดยมีความโดดเด่นด้วยการให้บริการเกมสล็อตที่หลากหลายและมีคุณภาพ รวมถึงฟีเจอร์ที่ช่วยให้ผู้เล่นสามารถเพลิดเพลินกับการเล่นได้อย่างเต็มที่ ในบทความนี้ เราจะมาพูดถึงฟีเจอร์และจุดเด่นของสล็อต888 ที่ทำให้เว็บไซต์นี้ได้รับความนิยมเป็นอย่างมาก
ฟีเจอร์เด่นของ PG สล็อต888
ระบบฝากถอนเงินอัตโนมัติที่รวดเร็ว สล็อต888 ให้บริการระบบฝากถอนเงินแบบอัตโนมัติที่สามารถทำรายการได้ทันที ไม่ต้องรอนาน ไม่ว่าจะเป็นการฝากหรือถอนก็สามารถทำได้ภายในไม่กี่วินาที รองรับการใช้งานผ่านทรูวอลเล็ทและช่องทางอื่น ๆ โดยไม่มีขั้นต่ำในการฝากถอน
รองรับทุกอุปกรณ์ ทุกแพลตฟอร์ม ไม่ว่าคุณจะเล่นจากอุปกรณ์ใดก็ตาม สล็อต888 รองรับทั้งคอมพิวเตอร์ แท็บเล็ต และสมาร์ทโฟน ไม่ว่าจะเป็นระบบ iOS หรือ Android คุณสามารถเข้าถึงเกมสล็อตได้ทุกที่ทุกเวลาเพียงแค่มีอินเทอร์เน็ต
โปรโมชั่นและโบนัสมากมาย สำหรับผู้เล่นใหม่และลูกค้าประจำ สล็อต888 มีโปรโมชั่นต้อนรับ รวมถึงโบนัสพิเศษ เช่น ฟรีสปินและโบนัสเครดิตเพิ่ม ทำให้การเล่นเกมสล็อตกับเราเป็นเรื่องสนุกและมีโอกาสทำกำไรมากยิ่งขึ้น
ความปลอดภัยสูงสุด เรื่องความปลอดภัยเป็นสิ่งที่สล็อต888 ให้ความสำคัญเป็นอย่างยิ่ง เราใช้เทคโนโลยีการเข้ารหัสข้อมูลขั้นสูงเพื่อปกป้องข้อมูลส่วนบุคคลของลูกค้า ระบบฝากถอนเงินยังมีมาตรการรักษาความปลอดภัยที่เข้มงวด ทำให้ลูกค้ามั่นใจในการใช้บริการกับเรา
ทดลองเล่นสล็อตฟรี
สล็อต888 ยังมีบริการให้ผู้เล่นสามารถทดลองเล่นสล็อตได้ฟรี ซึ่งเป็นโอกาสที่ดีในการทดลองเล่นเกมต่าง ๆ ที่มีอยู่บนเว็บไซต์ เช่น Phoenix Rises, Dream Of Macau, Ways Of Qilin, Caishens Wins และเกมยอดนิยมอื่น ๆ ที่มีกราฟิกสวยงามและรูปแบบการเล่นที่น่าสนใจ
ไม่ว่าจะเป็นเกมแนวผจญภัย เช่น Rise Of Apollo, Dragon Hatch หรือเกมที่มีธีมแห่งความมั่งคั่งอย่าง Crypto Gold, Fortune Tiger, Lucky Piggy ทุกเกมได้รับการออกแบบมาเพื่อสร้างประสบการณ์การเล่นที่น่าจดจำและเต็มไปด้วยความสนุกสนาน
บทสรุป
สล็อต888 เป็นแพลตฟอร์มที่ครบเครื่องเรื่องเกมสล็อตออนไลน์ ด้วยฟีเจอร์ที่ทันสมัย โปรโมชั่นที่น่าสนใจ และระบบรักษาความปลอดภัยที่เข้มงวด ทำให้คุณมั่นใจได้ว่าการเล่นกับสล็อต888 จะเป็นประสบการณ์ที่ปลอดภัยและเต็มไปด้วยความสนุก
pileli sineklik | Pileli sinekliklerin hava akışını engellemeden sineklerden korunmayı sağladığını açıklamanız harika olmuş. Bu yazıdan çok şey öğrendim.
buy plavix here Plavix 75 mg price
paxlovid pharmacy Paxlovid buy online or paxlovid pill
https://maps.google.si/url?sa=t&url=https://paxlovid1st.shop Paxlovid buy online
paxlovid cost without insurance paxlovid for sale and paxlovid cost without insurance paxlovid for sale
http://stromectol1st.shop/# stromectol cream
reputable indian online pharmacy
В современном глобализированном мире перевод играет ключевую роль в общении между людьми разных языков и культур. Переводчики служат мостом, соединяющим народы, позволяя им обмениваться информацией, идеями и культурными ценностями. Перевод – это не просто техническая задача, но настоящее искусство и наука, требующая глубокого понимания языков, культур, истории и контекста.
Виды перевода: от письменного к устному, от литературного к техническому
Существует множество видов перевода, каждый из которых имеет свои особенности и требует определенных навыков и подходов. Письменный перевод включает в себя перевод текстов, таких как книги, статьи, документы и веб-сайты. Устный перевод, с другой стороны, включает в себя перевод устной речи во время конференций, переговоров, презентаций и других событий. В устном переводе существует два основных подхода: синхронный и последовательный. Кроме того, перевод подразделяется на литературный, технический, медицинский, юридический и другие виды, каждый из которых требует специальных знаний и навыков.
Навыки и качество переводчика
Хороший переводчик должен обладать не только отличным знанием языков, но и глубоким пониманием культуры, истории и контекста. Он должен быть внимательным к деталям, креативным и иметь чувство языка и культуры. Переводчик должен уметь точно и аутентично передавать смысл оригинального текста, сохраняя его стиль и тон. Важную роль играют также коммуникативные навыки, способность работать в команде и соблюдать сроки.
Технологии в переводе: от CAT-инструментов к нейронным сетям
С развитием технологий появились новые инструменты для перевода, которые значительно облегчают работу переводчиков. CAT-инструменты (Computer-Assisted Translation) позволяют переводчикам работать быстрее и эффективнее, сохраняя стиль и терминологию оригинала. Машинный перевод, основанный на нейронных сетях, также стал полезным инструментом, особенно при переводах больших объемов текста. Однако, несмотря на все достижения в области технологий, человеческий фактор и творческий подход всегда останутся частью высококачественного перевода.
Вызовы и возможности в современной лингвистике
В современной лингвистике перевод стал объектом глубокого изучения. Одним из основных вызовов является сохранение культурной и эмоциональной окраски исходного текста при переводе. Это требует от переводчика не только знания языков, но и понимания контекста, культурных нюансов и тонкостей. Денции включают автоматизацию переводов с помощью искусственного интеллекта и разработку новых методов обучения переводчиков для более качественной работы.
Профессиональные возможности для переводчиков
Для переводчиков открывается широкий спектр профессиональных возможностей. Они могут работать в различных областях, таких как литература, техника, медицина, право и многие другие. Спрос на квалифицированных переводчиков в мире растет, что делает профессию востребованной и перспективной. Переводчики могут работать как фрилансеры, так и в переводческих агентствах, а также в компаниях, которые нуждаются в услугах перевода.
В заключение, можно сказать, что перевод – это настоящее искусство и наука, требующая глубокого понимания языков, культур, истории и контекста. Существует множество видов перевода, каждый из которых имеет свои особенности и требует определенных навыков и подходов. Технологии в переводе облегчают работу переводчиков, но человеческий фактор и творческий подход всегда останутся частью высококачественного перевода. Для переводчиков открывается широкий спектр профессиональных возможностей, и спрос на квалифицированных переводчиков в мире растет. Переводчики служат мостом между культурами, переводя не только слова, но и идеи, эмоции и контекст, способствуя взаимопониманию и сотрудничеству в глобальном мире.
перевод с иностранных языков
minocycline 50mg without doctor: stromectol fast delivery – stromectol pills
minocycline 100mg minocycline weight gain or buy minocycline 100mg otc
https://toolbarqueries.google.com.af/url?q=http://stromectol1st.shop ivermectin 18mg
stromectol tablets for humans for sale what is minocycline 50 mg used for and buy ivermectin cream minocycline 100 mg without doctor
п»їplavix generic [url=http://clopidogrel.pro/#]clopidogrel pro[/url] clopidogrel bisulfate 75 mg
paxlovid for sale: shop – paxlovid price
You really make it seem so easy with your presentation but I find this topic to be really something which I think I would never understand. It seems too complicated and very broad for me. I am looking forward for your next post, I will try to get the hang of it!
minocycline 50mg without prescription minocycline generic name or ivermectin where to buy for humans
https://maps.google.com.om/url?q=https://stromectol1st.shop ivermectin 0.5
ivermectin gel ivermectin oral solution and ivermectin canada ivermectin 0.5 lotion india
antiplatelet drug buy clopidogrel bisulfate or buy plavix
https://www.google.com.gt/url?q=https://clopidogrel.pro п»їplavix generic
cheap plavix antiplatelet drug antiplatelet drug and generic plavix generic plavix
Профессиональный сервисный центр по ремонту моноблоков iMac в Москве.
Мы предлагаем: надежный сервис ремонта imac
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Cost of Plavix without insurance: best price on generic – buy Clopidogrel over the counter
buy stromectol online: stromectol shop – stromectol for head lice
https://stromectol1st.shop/# how much is ivermectin
indian pharmacies safe
buy ivermectin stromectol stromectol fast delivery ivermectin tablets
buy clopidogrel online: buy Clopidogrel over the counter – buy clopidogrel bisulfate
minocycline 50mg stromectol 1st shop ivermectin 0.5
Check USDT TRC20 wallet
USDT TRON-based Transaction Verification and Financial Crime Prevention (AML) Procedures
As digital assets like Tether TRC20 gain adoption for quick and inexpensive transactions, the need for safety and adherence with AML regulations expands. Here’s how to verify Tether TRON-based payments and guarantee they’re not connected to illicit operations.
What does it mean TRON-based USDT?
USDT TRC20 is a digital currency on the TRON network, priced in line with the US dollar. Recognized for its cheap transfers and speed, it is frequently employed for international payments. Validating payments is essential to prevent associations to illicit transfers or other unlawful operations.
Checking TRON-based USDT Payments
TRX Explorer — This blockchain explorer allows users to track and verify USDT TRC20 transfers using a wallet address or transfer code.
Tracking — Experienced participants can observe anomalous trends such as large or fast payments to identify unusual behavior.
AML and Illicit Funds
Financial Crime Prevention (Anti-Money Laundering) rules assist prevent illegal money transfers in digital assets. Platforms like Chain Analysis and Elliptic Solutions permit businesses and trading platforms to find and stop dirty cryptocurrency, which refers to funds related to criminal actions.
Tools for Compliance
TRONSCAN — To validate USDT TRC20 transaction information.
Chainalysis and Elliptic — Utilized by trading platforms to confirm Anti-Money Laundering adherence and monitor unlawful operations.
Summary
Guaranteeing secure and lawful TRON-based USDT payments is critical. Services like TRONSCAN and Anti-Money Laundering solutions support protect users from interacting with illicit funds, supporting a safe and lawful cryptocurrency space.
minocycline generic: stromectol 1st – minocycline for acne 100mg
Полезная информация на сайте. Все что вы хоте знать об интернете полезный сервис
https://stromectol1st.shop/# order minocycline 50mg online
indian pharmacy online
rybelsus: good price – Buy semaglutide
paxlovid price: buy here – paxlovid for sale
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
antiplatelet drug cheap plavix antiplatelet drug or plavix medication
http://kinhtexaydung.net/redirect/?url=http://clopidogrel.pro buy plavix
cheap plavix antiplatelet drug Plavix generic price and plavix medication Clopidogrel 75 MG price
ihds human design certification
rybelsus cost rybelsus generic rybelsus price
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
k8 カジノ パチンコ
ゲームの進行が早く、短時間でも満足感があります。気軽に楽しめるのが良いです。
CRモンスターハンター4
https://jahromblog.com/tags/k8-%E3%82%AB%E3%82%B8%E3%83%8E
パチンコの演出が豪華で、観ているだけでも楽しめるのが魅力です。エンターテイメント性が高いです。
パチスロ ガールズ&パンツァー
k8カジノ パチンコ
CR七つの大罪
主役は銭形
コードギアス反逆のルルーシュ
соут проведение оценки оценка условий труда заказать
rgbet
Bạn đang tìm kiếm những trò chơi hot nhất và thú vị nhất tại sòng bạc trực tuyến? RGBET tự hào giới thiệu đến bạn nhiều trò chơi cá cược đặc sắc, bao gồm Baccarat trực tiếp, máy xèng, cá cược thể thao, xổ số và bắn cá, mang đến cho bạn cảm giác hồi hộp đỉnh cao của sòng bạc! Dù bạn yêu thích các trò chơi bài kinh điển hay những máy xèng đầy kịch tính, RGBET đều có thể đáp ứng mọi nhu cầu giải trí của bạn.
RGBET Trò Chơi của Chúng Tôi
Bạn đang tìm kiếm những trò chơi hot nhất và thú vị nhất tại sòng bạc trực tuyến? RGBET tự hào giới thiệu đến bạn nhiều trò chơi cá cược đặc sắc, bao gồm Baccarat trực tiếp, máy xèng, cá cược thể thao, xổ số và bắn cá, mang đến cảm giác hồi hộp đỉnh cao của sòng bạc! Dù bạn yêu thích các trò chơi bài kinh điển hay những máy xèng đầy kịch tính, RGBET đều có thể đáp ứng mọi nhu cầu giải trí của bạn.
RGBET Trò Chơi Đa Dạng
Thể thao: Cá cược thể thao đa dạng với nhiều môn từ bóng đá, tennis đến thể thao điện tử.
Live Casino: Trải nghiệm Baccarat, Roulette, và các trò chơi sòng bài trực tiếp với người chia bài thật.
Nổ hũ: Tham gia các trò chơi nổ hũ với tỷ lệ trúng cao và cơ hội thắng lớn.
Lô đề: Đặt cược lô đề với tỉ lệ cược hấp dẫn.
Bắn cá: Bắn cá RGBET mang đến cảm giác chân thực và hấp dẫn với đồ họa tuyệt đẹp.
RGBET – Máy Xèng Hấp Dẫn Nhất
Khám phá các máy xèng độc đáo tại RGBET với nhiều chủ đề khác nhau và tỷ lệ trả thưởng cao. Những trò chơi nổi bật bao gồm:
RGBET Super Ace
RGBET Đế Quốc Hoàng Kim
RGBET Pharaoh Treasure
RGBET Quyền Vương
RGBET Chuyên Gia Săn Rồng
RGBET Jackpot Fishing
Vì sao nên chọn RGBET?
RGBET không chỉ cung cấp hàng loạt trò chơi đa dạng mà còn mang đến một hệ thống cá cược an toàn và chuyên nghiệp, đảm bảo mọi quyền lợi của người chơi:
Tốc độ nạp tiền nhanh chóng: Chuyển khoản tại RGBET chỉ mất vài phút và tiền sẽ vào tài khoản ngay lập tức, giúp bạn không bỏ lỡ bất kỳ cơ hội nào.
Game đổi thưởng phong phú: Từ cá cược thể thao đến slot game, RGBET cung cấp đầy đủ trò chơi giúp bạn tận hưởng mọi phút giây thư giãn.
Bảo mật tuyệt đối: Với công nghệ mã hóa tiên tiến, tài khoản và tiền vốn của bạn sẽ luôn được bảo vệ một cách an toàn.
Hỗ trợ đa nền tảng: Bạn có thể chơi trên mọi thiết bị, từ máy tính, điện thoại di động (iOS/Android), đến nền tảng H5.
Tải Ứng Dụng RGBET và Nhận Khuyến Mãi Lớn
Hãy tham gia RGBET ngay hôm nay để tận hưởng thế giới giải trí không giới hạn với các trò chơi thể thao, thể thao điện tử, casino trực tuyến, xổ số, và slot game. Quét mã QR và tải ứng dụng RGBET trên điện thoại để trải nghiệm game tốt hơn và nhận nhiều khuyến mãi hấp dẫn!
Tham gia RGBET để bắt đầu cuộc hành trình cá cược đầy thú vị ngay hôm nay!
เล่นบาคาร่าแบบรวดเร็วทันใจกับสปีดบาคาร่า
ถ้าคุณเป็นแฟนตัวยงของเกมไพ่บาคาร่า คุณอาจจะเคยชินกับการรอคอยในแต่ละรอบการเดิมพัน และรอจนดีลเลอร์แจกไพ่ในแต่ละตา แต่คุณรู้หรือไม่ว่า ตอนนี้คุณไม่ต้องรออีกต่อไปแล้ว เพราะ SA Gaming ได้พัฒนาเกมบาคาร่าโหมดใหม่ขึ้นมา เพื่อให้ประสบการณ์การเล่นของคุณน่าตื่นเต้นยิ่งขึ้น!
ที่ SA Gaming คุณสามารถเลือกเล่นไพ่บาคาร่าในโหมดที่เรียกว่า สปีดบาคาร่า (Speed Baccarat) โหมดนี้มีคุณสมบัติพิเศษและข้อดีที่น่าสนใจมากมาย:
ระยะเวลาการเดิมพันสั้นลง — คุณไม่จำเป็นต้องรอนานอีกต่อไป ในโหมดสปีดบาคาร่า คุณจะมีเวลาเพียง 12 วินาทีในการวางเดิมพัน ทำให้เกมแต่ละรอบจบได้รวดเร็ว โดยเกมในแต่ละรอบจะใช้เวลาเพียง 20 วินาทีเท่านั้น
ผลตอบแทนต่อผู้เล่นสูง (RTP) — เกมสปีดบาคาร่าให้ผลตอบแทนต่อผู้เล่นสูงถึง 4% ซึ่งเป็นมาตรฐานความเป็นธรรมที่ผู้เล่นสามารถไว้วางใจได้
การเล่นเกมที่รวดเร็วและน่าตื่นเต้น — ระยะเวลาที่สั้นลงทำให้เกมแต่ละรอบดำเนินไปอย่างรวดเร็ว ทันใจ เพิ่มความสนุกและความตื่นเต้นในการเล่น ทำให้ประสบการณ์การเล่นของคุณยิ่งสนุกมากขึ้น
กลไกและรูปแบบการเล่นยังคงเหมือนเดิม — แม้ว่าระยะเวลาจะสั้นลง แต่กลไกและกฎของการเล่น ยังคงเหมือนกับบาคาร่าสดปกติทุกประการ เพียงแค่ปรับเวลาให้เล่นได้รวดเร็วและสะดวกขึ้นเท่านั้น
นอกจากสปีดบาคาร่าแล้ว ที่ SA Gaming ยังมีโหมด No Commission Baccarat หรือบาคาร่าแบบไม่เสียค่าคอมมิชชั่น ซึ่งจะช่วยให้คุณสามารถเพลิดเพลินไปกับการเล่นได้โดยไม่ต้องกังวลเรื่องค่าคอมมิชชั่นเพิ่มเติม
เล่นบาคาร่ากับ SA Gaming คุณจะได้รับประสบการณ์การเล่นที่สนุก ทันสมัย และตรงใจมากที่สุด!
рюкзак школьный черный купить
เล่นบาคาร่าแบบรวดเร็วทันใจกับสปีดบาคาร่า
ถ้าคุณเป็นแฟนตัวยงของเกมไพ่บาคาร่า คุณอาจจะเคยชินกับการรอคอยในแต่ละรอบการเดิมพัน และรอจนดีลเลอร์แจกไพ่ในแต่ละตา แต่คุณรู้หรือไม่ว่า ตอนนี้คุณไม่ต้องรออีกต่อไปแล้ว เพราะ SA Gaming ได้พัฒนาเกมบาคาร่าโหมดใหม่ขึ้นมา เพื่อให้ประสบการณ์การเล่นของคุณน่าตื่นเต้นยิ่งขึ้น!
ที่ SA Gaming คุณสามารถเลือกเล่นไพ่บาคาร่าในโหมดที่เรียกว่า สปีดบาคาร่า (Speed Baccarat) โหมดนี้มีคุณสมบัติพิเศษและข้อดีที่น่าสนใจมากมาย:
ระยะเวลาการเดิมพันสั้นลง — คุณไม่จำเป็นต้องรอนานอีกต่อไป ในโหมดสปีดบาคาร่า คุณจะมีเวลาเพียง 12 วินาทีในการวางเดิมพัน ทำให้เกมแต่ละรอบจบได้รวดเร็ว โดยเกมในแต่ละรอบจะใช้เวลาเพียง 20 วินาทีเท่านั้น
ผลตอบแทนต่อผู้เล่นสูง (RTP) — เกมสปีดบาคาร่าให้ผลตอบแทนต่อผู้เล่นสูงถึง 4% ซึ่งเป็นมาตรฐานความเป็นธรรมที่ผู้เล่นสามารถไว้วางใจได้
การเล่นเกมที่รวดเร็วและน่าตื่นเต้น — ระยะเวลาที่สั้นลงทำให้เกมแต่ละรอบดำเนินไปอย่างรวดเร็ว ทันใจ เพิ่มความสนุกและความตื่นเต้นในการเล่น ทำให้ประสบการณ์การเล่นของคุณยิ่งสนุกมากขึ้น
กลไกและรูปแบบการเล่นยังคงเหมือนเดิม — แม้ว่าระยะเวลาจะสั้นลง แต่กลไกและกฎของการเล่น ยังคงเหมือนกับบาคาร่าสดปกติทุกประการ เพียงแค่ปรับเวลาให้เล่นได้รวดเร็วและสะดวกขึ้นเท่านั้น
นอกจากสปีดบาคาร่าแล้ว ที่ SA Gaming ยังมีโหมด No Commission Baccarat หรือบาคาร่าแบบไม่เสียค่าคอมมิชชั่น ซึ่งจะช่วยให้คุณสามารถเพลิดเพลินไปกับการเล่นได้โดยไม่ต้องกังวลเรื่องค่าคอมมิชชั่นเพิ่มเติม
เล่นบาคาร่ากับ SA Gaming คุณจะได้รับประสบการณ์การเล่นที่สนุก ทันสมัย และตรงใจมากที่สุด!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт бытовой техники в перми
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
alan robert krakower bodychart
st666
ST666 – Nhà Cái Uy Tín Với Nhiều Khuyến Mãi Hấp Dẫn
ST666 là một trong những sòng bài trực tuyến uy tín nhất, mang đến cho các thành viên nhiều chương trình khuyến mãi đa dạng và hấp dẫn. Chúng tôi luôn hy vọng rằng những ưu đãi đặc biệt này sẽ mang lại trải nghiệm cá cược tuyệt vời cho tất cả thành viên của mình.
Khuyến Mãi Nạp Đầu Thưởng 100%
NO.1: Thưởng 100% lần nạp đầu tiên Thông thường, các khuyến mãi nạp tiền lần đầu đi kèm với yêu cầu vòng cược rất cao, thường từ 20 vòng trở lên. Tuy nhiên, ST666 hiểu rằng người chơi thường đắn đo khi quyết định nạp tiền vào sòng bài trực tuyến. Do đó, chúng tôi chỉ yêu cầu 8 vòng cược, cho phép người chơi rút tiền một cách nhanh chóng trong vòng 5 phút.
Thưởng 100% khi Đăng Nhập ST666
NO.2: Thưởng 16% mỗi ngày ST666 mang đến cho bạn một ưu đãi không thể bỏ lỡ. Mỗi khi bạn nạp tiền vào tài khoản hàng ngày, bạn sẽ được nhận thêm 16% số tiền nạp. Đây là cơ hội tuyệt vời dành cho những ai yêu thích cá cược trực tuyến và muốn gia tăng cơ hội chiến thắng của mình.
Bảo Hiểm Hoàn 10% Mỗi Ngày
NO.3: Bảo hiểm hoàn tiền 10% ST666 không chỉ hỗ trợ người chơi trong những lần may mắn mà còn luôn đồng hành khi bạn không gặp may. Chương trình khuyến mãi “bảo hiểm hoàn tiền 10% tổng tiền nạp mỗi ngày” là cách chúng tôi khích lệ và động viên tinh thần game thủ, giúp bạn tiếp tục hành trình chinh phục các thử thách cá cược.
Giới Thiệu Bạn Bè Thưởng 999K
NO.4: Giới thiệu bạn bè – Thưởng 999K Chương trình “Giới thiệu bạn bè” của ST666 giúp bạn có cơ hội cá cược cùng bạn bè và người thân. Không những vậy, bạn còn có thể nhận được phần thưởng lên đến 999K khi giới thiệu người bạn của mình tham gia ST666. Đây chính là một ưu đãi tuyệt vời giúp bạn vừa có thêm người đồng hành, vừa nhận được thưởng hấp dẫn.
Đăng Nhập Nhận Ngay 280K
NO.5: Điểm danh nhận thưởng 280K Nếu bạn là thành viên thường xuyên của ST666, hãy đừng quên nhấp vào hộp quà hàng ngày để điểm danh. Chỉ cần đăng nhập liên tục trong 7 ngày, cơ hội nhận thưởng 280K sẽ thuộc về bạn!
Kết Luận
ST666 luôn nỗ lực không ngừng để mang lại những chương trình khuyến mãi hấp dẫn và thiết thực nhất cho người chơi. Dù bạn là người mới hay đã gắn bó lâu năm với nền tảng, ST666 cam kết cung cấp trải nghiệm cá cược tốt nhất, an toàn và công bằng. Hãy tham gia ngay để không bỏ lỡ các cơ hội tuyệt vời từ ST666!
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.obesityhelp.com/members/pokypai1965/about_me/
https://www.haikudeck.com/presentations/zyAtdTtJzF
https://haveagood.holiday/users/358297
http://www.nfomedia.com/profile?uid=rOjUYgH
https://rentry.org/6i87rnp9
เล่นบาคาร่าแบบรวดเร็วทันใจกับสปีดบาคาร่า
ถ้าคุณเป็นแฟนตัวยงของเกมไพ่บาคาร่า คุณอาจจะเคยชินกับการรอคอยในแต่ละรอบการเดิมพัน และรอจนดีลเลอร์แจกไพ่ในแต่ละตา แต่คุณรู้หรือไม่ว่า ตอนนี้คุณไม่ต้องรออีกต่อไปแล้ว เพราะ SA Gaming ได้พัฒนาเกมบาคาร่าโหมดใหม่ขึ้นมา เพื่อให้ประสบการณ์การเล่นของคุณน่าตื่นเต้นยิ่งขึ้น!
ที่ SA Gaming คุณสามารถเลือกเล่นไพ่บาคาร่าในโหมดที่เรียกว่า สปีดบาคาร่า (Speed Baccarat) โหมดนี้มีคุณสมบัติพิเศษและข้อดีที่น่าสนใจมากมาย:
ระยะเวลาการเดิมพันสั้นลง — คุณไม่จำเป็นต้องรอนานอีกต่อไป ในโหมดสปีดบาคาร่า คุณจะมีเวลาเพียง 12 วินาทีในการวางเดิมพัน ทำให้เกมแต่ละรอบจบได้รวดเร็ว โดยเกมในแต่ละรอบจะใช้เวลาเพียง 20 วินาทีเท่านั้น
ผลตอบแทนต่อผู้เล่นสูง (RTP) — เกมสปีดบาคาร่าให้ผลตอบแทนต่อผู้เล่นสูงถึง 4% ซึ่งเป็นมาตรฐานความเป็นธรรมที่ผู้เล่นสามารถไว้วางใจได้
การเล่นเกมที่รวดเร็วและน่าตื่นเต้น — ระยะเวลาที่สั้นลงทำให้เกมแต่ละรอบดำเนินไปอย่างรวดเร็ว ทันใจ เพิ่มความสนุกและความตื่นเต้นในการเล่น ทำให้ประสบการณ์การเล่นของคุณยิ่งสนุกมากขึ้น
กลไกและรูปแบบการเล่นยังคงเหมือนเดิม — แม้ว่าระยะเวลาจะสั้นลง แต่กลไกและกฎของการเล่น ยังคงเหมือนกับบาคาร่าสดปกติทุกประการ เพียงแค่ปรับเวลาให้เล่นได้รวดเร็วและสะดวกขึ้นเท่านั้น
นอกจากสปีดบาคาร่าแล้ว ที่ SA Gaming ยังมีโหมด No Commission Baccarat หรือบาคาร่าแบบไม่เสียค่าคอมมิชชั่น ซึ่งจะช่วยให้คุณสามารถเพลิดเพลินไปกับการเล่นได้โดยไม่ต้องกังวลเรื่องค่าคอมมิชชั่นเพิ่มเติม
เล่นบาคาร่ากับ SA Gaming คุณจะได้รับประสบการณ์การเล่นที่สนุก ทันสมัย และตรงใจมากที่สุด!
USDT TRON-based Transaction Check and AML (Anti-Money Laundering) Methods
As crypto coins like Tether TRON-based rise in usage for fast and low-cost payments, the need for security and adherence with financial crime prevention rules grows. Here’s how to check Tether TRON-based transactions and ensure they’re not linked to illicit actions.
What is TRON-based USDT?
TRON-based USDT is a digital currency on the TRON network, priced in correspondence with the American dollar. Recognized for its low transaction fees and speed, it is widely used for international payments. Verifying transactions is crucial to block links to illicit transfers or other criminal operations.
Monitoring TRON-based USDT Transactions
TRX Explorer — This blockchain viewer allows individuals to monitor and verify Tether TRC20 transfers using a public address or transaction ID.
Monitoring — Advanced players can monitor suspicious behaviors such as significant or quick transfers to detect suspicious actions.
AML and Criminal Crypto
Financial Crime Prevention (Anti-Money Laundering) standards help stop unlawful financial activity in crypto markets. Tools like Chainalysis and Elliptic permit companies and exchanges to find and block illicit funds, which means money tied to unlawful operations.
Instruments for Regulation
TRX Explorer — To verify TRON-based USDT transfer details.
Chain Analysis and Elliptic Solutions — Employed by crypto markets to confirm Anti-Money Laundering conformance and track illegal actions.
Summary
Making sure protected and legitimate USDT TRC20 transfers is critical. Services like TRONSCAN and AML tools assist protect participants from interacting with illicit funds, promoting a protected and lawful crypto environment.
Тема «Четыре типа в Дизайне Человека» важна для понимания не только на теоретическом, но и на практическом уровне. Этот инструмент самопознания помогает каждому из нас осознать свою природу и использовать индивидуальные особенности для улучшения качества жизни. Рассмотрим рационально-практическую сторону каждого из типов, их определения и различия.
Все о Дизайне Человека – https://human-design-professional.ru
Первый тип в Дизайне Человека – это Генератор. Он отличаются высокой энергетичностью и способностью легко и эффективно завершать начатые задачи. Главная задача Генератора — найти деятельность, которая приносит радость и удовлетворение. Генератор начинает действовать, когда ощущает внутренний отклик. Основное отличие Генераторов в том, что они заряжают себя и других энергией, если действуют в соответствии с внутренним откликом.
Следующий тип, на который стоит обратить внимание, — Манифестор. Манифесторы могут начинать новые проекты и вдохновлять других. Они не нуждаются в отклике, как Генераторы, и могут сразу принимать решения и действовать. Манифесторы не подчиняются внешним обстоятельствам, а сами создают свою реальность. Главная их практическая задача — инициировать изменения.
Также важный элемент системы Дизайна Человека — Проектор. Их задача – управлять и направлять энергию других типов. Они нуждаются в приглашении, прежде чем начать действовать, и могут эффективно использовать энергию, когда работают с другими людьми. Проекторы отличаются тем, что не обладают собственной энергией, но могут эффективно направлять энергию других. Их рациональное предназначение – это оптимизация работы других типов.
И наконец, заключительный тип — Рефлектор. Они лучше всего ощущают общие тенденции и могут объективно оценивать ситуацию. Рефлекторы отличаются тем, что их самочувствие напрямую зависит от окружения. Практическая роль Рефлектора — это оценка и отслеживание состояния окружающих.
В итоге можно сказать следующее: Каждый из четырех типов в Дизайне Человека имеет свои индивидуальные особенности, которые помогают им максимально эффективно взаимодействовать с миром. Понимание своего типа и его практического предназначения позволяет лучше организовать жизнь, выбрать правильные направления для работы и улучшить качество личных отношений.
http://1winrussia.online/# 1xbet зеркало
пинап
замена венцов
1xbet официальный сайт 1хбет 1xbet скачать
пин ап вход: пинап зеркало – пин ап официальный сайт
пинап казино: пинап кз – пинап кз
https://1winindia.tech/# pin up kz
пин ап
canl? casino: dunyan?n en iyi casino siteleri – casino sitesi
1хбет 1хставка 1xbet официальный сайт
пин ап вход: пинап зеркало – пин ап официальный сайт
吉宗
P 異世界魔王と召喚少女の奴隷魔術
終わった後の反省会も楽しいです。次回の戦略を考えることで、さらなる楽しみが生まれます。
CR北斗の拳5 百裂
https://lucky-777-casino.com/tags/beast-mode
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
ミリオンゴッド
スロット Feel the Beat フィール・ザ・ビート
黃金守護神
CR牙狼闇を照らす者
パチスロ ロストプラネット2
casino oyunlar?: canl? casino siteleri – guvenilir casino siteleri
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
пинап казино: пинап казино – пин ап казино
1хбет: 1хбет – 1xbet
vibration diagnostics
Vibration diagnostics is a crucial field in maintaining the efficiency and longevity of various rotating equipment. This process involves assessing and correcting any vibrations that may compromise the functionality of machinery such as crushers, fans, centrifuges, and turbines. Understanding the differences between static and dynamic balance is essential when performing vibration diagnostics.
Static balance occurs when a rotor is stationary. In static imbalance, the center of gravity of the rotor is offset from its axis of rotation, leading to a force that causes the rotor to align its heavier section downward. This type of imbalance is typically corrected by adjusting the mass at specific points along the rotor to ensure the center of gravity aligns with the axis of rotation. Static balancing techniques are primarily applied to narrow disk-shaped rotors and target uneven mass distribution in just one plane.
In contrast, dynamic balance is observed when a rotor is in motion. Dynamic imbalance occurs due to differences in mass distribution in different planes along the rotor’s length, resulting in both lateral forces and moments that contribute to vibration during rotation. This imbalance creates challenges as the forces in the planes do not compensate for each other. Dynamic balancing requires a more complex analysis using a vibration analyzer equipped with a two-plane balancing function to establish the corrective measures needed to alleviate vibrations.
To perform effective dynamic shaft balancing, the Balanset-1A device is one of the most reliable tools available. This portable balancing and vibration analysis device allows for dynamic balancing in two planes, making it suitable for a variety of applications. The assessment begins with the initial measurement of vibrations, where sensors are attached to the rotor, and the system captures the baseline vibration data for further calculations.
Once the initial data is recorded, the next step involves installing a calibration weight at a specific point on the rotor. This process allows the vibration analysis system to monitor how the addition of weight affects the vibrations. Subsequent measurements are taken after the weight is repositioned, providing crucial data for understanding how shifts in weight influence balance.
The dynamic balancing process requires meticulous attention to detail, especially during the installation of corrective weights. By relying on vibration measurements from both sides of the rotor, the analyzer guides users in determining the precise angle and mass required to achieve a proper balance. After the weights are installed in the indicated positions, testing is conducted to verify that vibration levels have substantially decreased, signifying a successful balancing operation.
Vibration diagnostics further involves understanding the angle measurements in the rotor balancing process. Accurate angle determination is essential when planning where to install corrective weights. By measuring angles in the direction of rotation, technicians can identify the optimal positions for these weights—whether they are being added or removed—to maintain equilibrium in the rotor.
Aside from the dynamics of balancing, vibration diagnostics also emphasizes understanding of various planes relative to installed sensors. For example, when working on mulcher rotors, identifying correction planes helps technicians effectively manage weight adjustments, ensuring an accurate balancing process that adequately addresses vibration issues.
The implementation of vibration diagnostics in industrial applications not only safeguards machinery but also enhances operational efficiency. Reliable balancing practices can lead to reductions in maintenance costs, increased equipment longevity, decreased energy consumption, and ultimately, a more productive work environment. By addressing vibrations proactively through comprehensive vibration diagnostics, businesses can mitigate the risks of equipment failure that often result from ignored imbalances.
In conclusion, vibration diagnostics is an indispensable technique in modern machinery maintenance. By differentiating between static and dynamic balance, utilizing advanced tools like the Balanset-1A, and adhering to systematic processes for weight adjustment and measurements, professionals can effectively manage vibrations. This results in improved operational reliability and efficiency, making vibration diagnostics a significant aspect of mechanical upkeep and performance. Understanding these concepts will empower technicians to optimize the performance of their equipment, extending its usable life and ensuring it operates at peak efficiency.
Article taken from https://vibromera.eu/
Приложения для ставок на спорт помогут скачать приложение БК и начать делать ставки прямо сейчас
Хочу поделиться своим опытом ремонта телефона в этом сервисном центре. Остался очень доволен качеством работы и скоростью обслуживания. Если ищете надёжное место для ремонта, обратитесь сюда: ремонт смартфонов цены.
pin up 306: pin up casino – pin up azerbaycan
Тема «Четыре типа в Дизайне Человека» важна для понимания не только на теоретическом, но и на практическом уровне. Этот инструмент самопознания помогает каждому из нас осознать свою природу и использовать индивидуальные особенности для улучшения качества жизни. Рассмотрим рационально-практическую сторону каждого из типов, их определения и различия.
Ключевым типом в этой системе является Генератор. Он отличаются высокой энергетичностью и способностью легко и эффективно завершать начатые задачи. Этот тип создан для работы, и его главное стремление — заниматься тем, что приносит удовольствие. Генератор начинает действовать, когда ощущает внутренний отклик. Основное отличие Генераторов в том, что они заряжают себя и других энергией, если действуют в соответствии с внутренним откликом.
Еще один важный тип в Дизайне Человека — Манифестор. Манифесторы могут начинать новые проекты и вдохновлять других. Они не нуждаются в отклике, как Генераторы, и могут сразу принимать решения и действовать. Различие этого типа в том, что они лучше всего проявляют себя, когда свободны от ограничений. Практическая сторона их природы проявляется в том, что они способны запускать процессы и вдохновлять окружающих.
Не менее значимая категория — Проектор. Проекторы лучше всего проявляют себя в роли наблюдателей и стратегов. Они нуждаются в приглашении, прежде чем начать действовать, и могут эффективно использовать энергию, когда работают с другими людьми. Проекторы отличаются тем, что не обладают собственной энергией, но могут эффективно направлять энергию других. Проекторы наиболее эффективны, когда действуют в роли консультантов.
И наконец, заключительный тип — Рефлектор. Они лучше всего ощущают общие тенденции и могут объективно оценивать ситуацию. Рефлекторы отличаются тем, что их самочувствие напрямую зависит от окружения. Их рациональная роль заключается в объективной оценке происходящего вокруг.
В итоге можно сказать следующее: Каждый из четырех типов в Дизайне Человека имеет свои индивидуальные особенности, которые помогают им максимально эффективно взаимодействовать с миром. Понимание своего типа и его практического предназначения позволяет лучше организовать жизнь, выбрать правильные направления для работы и улучшить качество личных отношений.
источник
pin up casino: pin up azerbaycan – pinup az
http://1wintr.fun/# canl? casino siteleri
pin up kz
https://1winbrasil.win/# pinup az
pin up kz
canl? casino siteleri casino oyunlar? casino oyunlar?
Профессиональный сервисный центр по ремонту компьютерных видеокарт по Москве.
Мы предлагаем: ремонт видеокарт в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
пинап кз: пинап – пинап казино
casino siteleri: casino sitesi – casino oyunlar?
闘神雷電 -花田勝-
k8パチンコ
自分の運を試すために、少しだけお金をかけるのが楽しいです。気軽に挑戦できます。
パラダイスハイビスカス
https://k88214.com/tags/%E3%83%92%E3%83%B3%E3%83%88%E3%81%A8%E3%82%B3
キャラクターの個性が際立っており、ストーリーに引き込まれます。
キュインぱちすろ南国育ち 1st vacation
k8 カジノ パチンコ ガッツだ!!森の石松パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
パチスロ北斗の拳 強敵
GI優駿俱樂部
CR牙狼 魔戒ノ花XX
1xbet скачать: 1xbet скачать – 1xbet
1xbet скачать 1xbet 1хбет
пинап казино: пин ап казино – пинап
пин ап пин ап официальный сайт or пин ап зеркало
https://maps.google.lk/url?q=https://1winci.icu пин ап официальный сайт
пин ап официальный сайт пин ап официальный сайт and пин ап вход пин ап официальный сайт
Тема «Четыре типа в Дизайне Человека» важна для понимания не только на теоретическом, но и на практическом уровне. Этот инструмент самопознания помогает каждому из нас осознать свою природу и использовать индивидуальные особенности для улучшения качества жизни. Рассмотрим рационально-практическую сторону каждого из типов, их определения и различия.
Ключевым типом в этой системе является Генератор. Генераторы отличаются высокой энергетичностью и способностью легко и эффективно завершать начатые задачи. Главная задача Генератора — найти деятельность, которая приносит радость и удовлетворение. Генератор начинает действовать, когда ощущает внутренний отклик. Когда Генератор действует из отклика, он не только продуктивен, но и создает вокруг себя атмосферу гармонии и успеха.
Следующий тип, на который стоит обратить внимание, — Манифестор. Главное предназначение Манифестора — инициировать, начинать и вести за собой. Они не нуждаются в отклике, как Генераторы, и могут сразу принимать решения и действовать. Манифесторы не подчиняются внешним обстоятельствам, а сами создают свою реальность. Главная их практическая задача — инициировать изменения.
Также важный элемент системы Дизайна Человека — Проектор. Их задача – управлять и направлять энергию других типов. Они нуждаются в приглашении, прежде чем начать действовать, и могут эффективно использовать энергию, когда работают с другими людьми. Проекторы отличаются тем, что не обладают собственной энергией, но могут эффективно направлять энергию других. Практическая задача Проектора – это координирование и организация.
И наконец, заключительный тип — Рефлектор. Этот тип является самым редким и уникальным. Они, как зеркало, отражают общее состояние общества или коллектива. Их рациональная роль заключается в объективной оценке происходящего вокруг.
Заключение Каждый из четырех типов в Дизайне Человека имеет свои индивидуальные особенности, которые помогают им максимально эффективно взаимодействовать с миром. Понимание своего типа и его практического предназначения позволяет лучше организовать жизнь, выбрать правильные направления для работы и улучшить качество личных отношений.
источник
пин ап официальный сайт пинап зеркало or пин ап вход
https://yassu.jp/bidan/town/kboard.cgi?mode=res_html&owner=proscar&url=1winci.icu пин ап
пин ап официальный сайт пин ап зеркало and пин ап официальный сайт пин ап вход
1xbet зеркало: 1xbet – 1xbet официальный сайт
MetaMask Extension secures your Ethereum and allows seamless interaction with DApps. Install MetaMask to easily manage your crypto and explore the decentralized web.
An excellent read that will keep readers – particularly me – coming back for more! Also, I’d genuinely appreciate if you check my website https://ads.fashionel.mk/adserver/www/delivery/ck.php?ct=1&oaparams=2__bannerid=27__zoneid=4__cb=712781242c__oadest=http://94n.de/maryam/kosmetikstudio-ober-olm about Cosmetics. Thank you and best of luck!
пин ап официальный сайт: пин ап официальный сайт – пин ап вход
Профессиональный сервисный центр по ремонту телефонов в Москве.
Мы предлагаем: ремонты телефонов
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://1winrussia.online/# 1хставка
пинап кз
пин ап зеркало: пин ап официальный сайт – пин ап зеркало
1xbet скачать 1хставка or 1xbet зеркало
http://pachl.de/url?q=https://1winrussia.online:: 1xbet скачать
1xbet 1хставка and 1хбет 1хбет
slot casino siteleri casino siteleri or casino oyunlar?
https://www.google.fi/url?q=https://1wintr.fun casino siteleri
casino oyunlar? canl? casino siteleri and casino sitesi en iyi casino siteleri
http://1wintr.fun/# casino sitesi
пин ап казино
Сломался телефон, думал покупать новый, но решил попробовать отремонтировать. Обратился в этот сервисный центр и не пожалел. Профессионалы своего дела быстро восстановили мой телефон. Рекомендую посетить их сайт: ремонт телефонов открыто сейчас.
Тема «Четыре типа в Дизайне Человека» важна для понимания не только на теоретическом, но и на практическом уровне. Этот инструмент самопознания помогает каждому из нас осознать свою природу и использовать индивидуальные особенности для улучшения качества жизни. Рассмотрим рационально-практическую сторону каждого из типов, их определения и различия.
Ключевым типом в этой системе является Генератор. Генераторы отличаются высокой энергетичностью и способностью легко и эффективно завершать начатые задачи. Их природа требует постоянной активности, поэтому важно находить дело, которое по-настоящему нравится. Генератор начинает действовать, когда ощущает внутренний отклик. Когда Генератор действует из отклика, он не только продуктивен, но и создает вокруг себя атмосферу гармонии и успеха.
Еще один важный тип в Дизайне Человека — Манифестор. Этот тип уникален своей независимостью и способностью инициировать действия. Они не нуждаются в отклике, как Генераторы, и могут сразу принимать решения и действовать. Индивидуальная особенность Манифестора — это стремление к свободе и независимости. Практическая сторона их природы проявляется в том, что они способны запускать процессы и вдохновлять окружающих.
Также важный элемент системы Дизайна Человека — Проектор. Проекторы – это люди, которые видят потенциал в других и помогают его раскрыть. Они нуждаются в приглашении, прежде чем начать действовать, и могут эффективно использовать энергию, когда работают с другими людьми. Их сила — в правильном руководстве и управлении чужими ресурсами. Проекторы наиболее эффективны, когда действуют в роли консультантов.
Четвертый тип в Дизайне Человека — это Рефлектор. Этот тип является самым редким и уникальным. Они, как зеркало, отражают общее состояние общества или коллектива. Их рациональная роль заключается в объективной оценке происходящего вокруг.
Итак, подведем итог: Каждый из четырех типов в Дизайне Человека имеет свои индивидуальные особенности, которые помогают им максимально эффективно взаимодействовать с миром. Понимание своего типа и его практического предназначения позволяет лучше организовать жизнь, выбрать правильные направления для работы и улучшить качество личных отношений.
источник
pin-up: pin-up casino giris – pin-up
Тема «Четыре типа в Дизайне Человека» важна для понимания не только на теоретическом, но и на практическом уровне. Этот инструмент самопознания помогает каждому из нас осознать свою природу и использовать индивидуальные особенности для улучшения качества жизни. Рассмотрим рационально-практическую сторону каждого из типов, их определения и различия.
Начнем с Генератор. Он отличаются высокой энергетичностью и способностью легко и эффективно завершать начатые задачи. Этот тип создан для работы, и его главное стремление — заниматься тем, что приносит удовольствие. Генератор начинает действовать, когда ощущает внутренний отклик. Их индивидуальная особенность заключается в том, что энергия накапливается, только когда они следуют своему отклику.
Еще один важный тип в Дизайне Человека — Манифестор. Манифесторы могут начинать новые проекты и вдохновлять других. Они не нуждаются в отклике, как Генераторы, и могут сразу принимать решения и действовать. Индивидуальная особенность Манифестора — это стремление к свободе и независимости. Главная их практическая задача — инициировать изменения.
Не менее значимая категория — Проектор. Их задача – управлять и направлять энергию других типов. Они нуждаются в приглашении, прежде чем начать действовать, и могут эффективно использовать энергию, когда работают с другими людьми. Индивидуальная особенность Проектора заключается в умении работать с чужой энергией и направлять ее. Практическая задача Проектора – это координирование и организация.
Четвертый тип в Дизайне Человека — это Рефлектор. Этот тип является самым редким и уникальным. Индивидуальная особенность Рефлектора заключается в том, что они полностью зависят от окружающего мира и людей. Практическая роль Рефлектора — это оценка и отслеживание состояния окружающих.
Заключение Каждый из четырех типов в Дизайне Человека имеет свои индивидуальные особенности, которые помогают им максимально эффективно взаимодействовать с миром. Понимание своего типа и его практического предназначения позволяет лучше организовать жизнь, выбрать правильные направления для работы и улучшить качество личных отношений.
источник
Тема «Четыре типа в Дизайне Человека» важна для понимания не только на теоретическом, но и на практическом уровне. Этот инструмент самопознания помогает каждому из нас осознать свою природу и использовать индивидуальные особенности для улучшения качества жизни. Рассмотрим рационально-практическую сторону каждого из типов, их определения и различия.
Начнем с Генератор. Генераторы отличаются высокой энергетичностью и способностью легко и эффективно завершать начатые задачи. Этот тип создан для работы, и его главное стремление — заниматься тем, что приносит удовольствие. Генератор начинает действовать, когда ощущает внутренний отклик. Когда Генератор действует из отклика, он не только продуктивен, но и создает вокруг себя атмосферу гармонии и успеха.
Еще один важный тип в Дизайне Человека — Манифестор. Этот тип уникален своей независимостью и способностью инициировать действия. Они не нуждаются в отклике, как Генераторы, и могут сразу принимать решения и действовать. Манифесторы не подчиняются внешним обстоятельствам, а сами создают свою реальность. Главная их практическая задача — инициировать изменения.
Третий тип — это Проектор. Проекторы – это люди, которые видят потенциал в других и помогают его раскрыть. Они нуждаются в приглашении, прежде чем начать действовать, и могут эффективно использовать энергию, когда работают с другими людьми. Проекторы отличаются тем, что не обладают собственной энергией, но могут эффективно направлять энергию других. Практическая задача Проектора – это координирование и организация.
И наконец, заключительный тип — Рефлектор. Рефлекторы — это люди, которые отражают состояние окружающей среды. Рефлекторы отличаются тем, что их самочувствие напрямую зависит от окружения. Их рациональная роль заключается в объективной оценке происходящего вокруг.
Итак, подведем итог: Каждый из четырех типов в Дизайне Человека имеет свои индивидуальные особенности, которые помогают им максимально эффективно взаимодействовать с миром. Понимание своего типа и его практического предназначения позволяет лучше организовать жизнь, выбрать правильные направления для работы и улучшить качество личных отношений.
источник
Профессиональный сервисный центр по ремонту компьютеров и ноутбуков в Москве.
Мы предлагаем: профессиональный ремонт макбуков
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
обратные ссылки купить
пин ап: пин ап казино вход – pin up
1xbet зеркало: 1xbet официальный сайт – 1хставка
บาคาร่า sa gaming
เอสเอ เกมมิ่ง เป็น บริษัท เกม บาคาร่าคลาสสิก ออนไลน์ ที่ได้รับการยอมรับอย่างกว้างขวาง ใน วงการสากล ว่าเป็น ผู้นำ ในการให้บริการ บริการ คาสิโนออนไลน์ โดยเฉพาะในด้าน ไพ่ บาคาร่า ซึ่งเป็น เกมส์ ที่ ผู้คน สนใจเล่นกัน อย่างกว้างขวาง ใน คาสิโนจริง และ ออนไลน์ ด้วย ลักษณะการเล่น ที่ สะดวก การลงเดิมพัน เพียง ฝั่ง ผู้เล่น หรือ ฝั่งเจ้ามือ และ โอกาสชนะ ที่ ค่อนข้างสูง ทำให้ เกมไพ่บาคาร่า ได้รับ ความนิยม อย่างมากใน ช่วงหลายปีที่ผ่านมา โดยเฉพาะใน บ้านเรา
หนึ่งในสไตล์การเล่น ยอดนิยมที่ SA Gaming นำเสนอ คือ Speed Baccarat ซึ่ง ช่วยให้ผู้เล่น ต้องการ ความรวดเร็ว และ การคิดไว สามารถ แทงได้รวดเร็ว นอกจากนี้ยังมีโหมด บาคาร่าแบบไม่เสียค่าคอมมิชชั่น ซึ่งเป็น โหมด ที่ ไม่มีค่าใช้จ่ายเพิ่มเติม เมื่อชนะ การเดิมพัน แบงค์เกอร์ ทำให้ ฟีเจอร์นี้ ได้รับ ความสนใจ จาก ผู้เล่น ที่มองหา ความคุ้มค่า ในการ ลงทุน
เกมไพ่บาคาร่า ของ เอสเอ เกมมิ่ง ยัง ถูกพัฒนา ให้มี กราฟฟิค ร่วมกับ เสียง ที่ เรียลไทม์ จำลองบรรยากาศ ที่ น่าสนุก เหมือนกับอยู่ใน บ่อนคาสิโนจริง พร้อมกับ ฟีเจอร์ ที่ทำให้ ผู้เสี่ยงโชค สามารถเลือก วิธีการเดิมพัน ที่ มีให้เลือกมากมาย ไม่ว่าจะเป็น การลงเงิน ตามเทคนิค ของตนเอง หรือการ ใช้กลยุทธ์ ให้ชนะ นอกจากนี้ยังมี ดีลเลอร์สด ที่ คอยควบคุมเกม ในแต่ละ โต๊ะ ทำให้ เกม มี ความน่าตื่นเต้น มากยิ่งขึ้น
ด้วย ตัวเลือก ใน การเสี่ยงโชค และ การเล่นที่ง่าย ในการ เล่น SA Gaming ได้ ออกแบบ เกมบาคาร่า ที่ ตอบสนอง ทุก ชนิด ของนักพนัน ตั้งแต่ ผู้ที่เริ่มต้น ไปจนถึง นักเดิมพัน มืออาชีพ
rgbet
RGBET Trang Chủ Và Câu Chuyện Thương Hiệu
Ra đời vào năm 2010 tại Đài Loan, RGBET nhanh chóng trở thành một trang cá cược chất lượng hàng đầu khu vực Châu Á. Nhà cái được cấp phép hoạt động hợp pháp bởi công ty giải trí trực tuyến hợp pháp được ủy quyền và giám sát theo giấy phép Malta của Châu Âu – MGA. Và chịu sự giám sát chặt chẽ của tổ chức PAGCOR và BIV.
RGBET trang chủ cung cấp cho người chơi đa dạng các thể loại cược đặc sắc như: thể thao, đá gà, xổ số, nổ hũ, casino trực tuyến. Dịch vụ CSKH luôn hoạt động 24/7. Với chứng chỉ công nghệ GEOTRUST, nhà cái đảm bảo an toàn cho mọi giao dịch của khách hàng. APP RG thiết kế tối ưu giải quyết mọi vấn đề của người dùng IOS và Android.
Là một nhà cái đến từ đất nước công nghệ, nhà cái luôn không ngừng xây dựng và nâng cấp hệ thống game và dịch vụ hoàn hảo. Mọi giao dịch nạp rút được tự động hoá cho phép người chơi hoàn tất giao dịch chỉ với 2 phút vô cùng nhanh chóng
RGBET Lớn Nhất Uy Tín Nhất – Giá Trị Cốt Lõi
Nhà Cái RG Và Mục Tiêu Thương Hiệu
Giá trị cốt lõi mà RGBET mong muốn hướng đến đó chính là không ngừng hoàn thiện để đem đến một hệ thống chất lượng, công bằng và an toàn. Nâng cao sự hài lòng của người chơi, đẩy mạnh hoạt động chống gian lận và lừa đảo. RG luôn cung cấp hệ thống kèo nhà cái đặc sắc, cùng các sự kiện – giải đấu hàng đầu và tỷ lệ cược cạnh tranh đáp ứng mọi nhu cầu khách hàng.
Thương hiệu cá cược RGBET cam kết đem lại cho người chơi môi trường cá cược công bằng, văn minh và lành mạnh. Đây là nguồn động lực to lớn giúp nhà cái thực tế hóa các hoạt động của mình.
RGBET Có Tầm Nhìn Và Sứ Mệnh
Đổi mới và sáng tạo là yếu tố cốt lõi giúp đạt được mục tiêu dưới sự chuyển mình mạnh mẽ của công nghệ. Tầm nhìn và sứ mệnh của RGBET là luôn tìm tòi những điều mới lạ, đột phá mạnh mẽ, vượt khỏi giới hạn bản thân, đương đầu với thử thách để đem đến cho khách hàng sản phẩm hoàn thiện nhất.
Chúng tôi luôn sẵn sàng tiếp thu ý kiến và nâng cao bản thân mỗi ngày để tạo ra sân chơi bổ ích, uy tín và chuyên nghiệp cho người chơi. Để có thể trở thành nhà cái phù hợp với mọi khách hàng.
Khái Niệm Giá Trị Cốt Lõi Nhà Cái RGBET
Giá trị cốt lõi của nhà cái RG luôn gắn kết chặt chẽ với nhau giữa 5 khái niệm: Chính trực, chuyên nghiệp, an toàn, đổi mới, công nghệ.
Chính Trực
Mọi quy luật, cách thức của trò chơi đều được nhà cái cung cấp công khai, minh bạch và chi tiết. Mỗi tựa game hoạt động đều phải chịu sự giám sát kỹ lưỡng bởi các cơ quan tổ chức có tiếng về sự an toàn và minh bạch của nó.
Chuyên Nghiệp
Các hoạt động tại RGBET trang chủ luôn đề cao sự chuyên nghiệp lên hàng đầu. Từ giao diện đến chất lượng sản phẩm luôn được trau chuốt tỉ mỉ từng chi tiết. Thế giới giải trí được xây dựng theo văn hóa Châu Á, phù hợp với đại đa số thị phần khách Việt.
An Toàn
RG lớn nhất uy tín nhất luôn ưu tiên sử dụng công nghệ mã hóa hiện đại nhất để đảm bảo an toàn, riêng tư cho toàn bộ thông tin của người chơi. Đơn vị cam kết nói không với hành vi gian lận và mua bán, trao đổi thông tin cá nhân bất hợp pháp.
Đổi Mới
Nhà cái luôn theo dõi và bắt kịp xu hướng thời đại, liên tục bổ sung các sản phẩm mới, phương thức cá cược mới và các ưu đãi độc lạ, mang đến những trải nghiệm thú vị cho người chơi.
Công Nghệ
RGBET trang chủ tập trung xây dựng một giao diện game sắc nét, sống động cùng tốc độ tải nhanh chóng. Ứng dụng RGBET giải nén ít dung lượng phù hợp với mọi hệ điều hành và cấu hình, tăng khả năng sử dụng của khách hàng.
RGBET Khẳng Định Giá Trị Thương Hiệu
Hoạt động hợp pháp với đầy đủ giấy phép, chứng chỉ an toàn đạt tiêu chuẩn quốc tế
Hệ thống game đa màu sắc, đáp ứng được mọi nhu cầu người chơi
Chính sách bảo mật RG hiện đại và đảm bảo an toàn cho người chơi cá cược
Bắt tay hợp tác với nhiều đơn vị phát hành game uy tín, chất lượng thế giới
Giao dịch nạp rút RG cấp tốc, nhanh gọn, bảo mật an toàn
Kèo nhà cái đa dạng với bảng tỷ lệ kèo cao, hấp dẫn
Dịch Vụ RGBET Casino Online
Dịch vụ khách hàng
Đội ngũ CSKH RGBET luôn hoạt động thường trực 24/7. Nhân viên được đào tạo chuyên sâu luôn giải đáp tất cả các khó khăn của người chơi về các vấn đề tài khoản, khuyến mãi, giao dịch một cách nhanh chóng và chuẩn xác. Hạn chế tối đa làm ảnh hưởng đến quá trình trải nghiệm của khách hàng.
Đa dạng trò chơi
Với sự nhạy bén trong cập nhật xu thế, nhà cái RGBET đã dành nhiều thời gian phân tích nhu cầu khách hàng, đem đến một kho tàng game chất lượng với đa dạng thể loại từ RG casino online, thể thao, nổ hũ, game bài, đá gà, xổ số.
Khuyến mãi hấp dẫn
RGBET trang chủ liên tục cập nhật và thay đổi các sự kiện ưu đãi đầy hấp dẫn và độc đáo. Mọi thành viên bất kể là người chơi mới, người chơi cũ hay hội viên VIP đều có cơ hội được hưởng ưu đãi đặc biệt từ nhà cái.
Giao dịch linh hoạt, tốc độ
Thương hiệu RGBET luôn chú tâm đến hệ thống giao dịch. Nhà cái cung cấp dịch vụ nạp rút nhanh chóng với đa dạng phương thức như thẻ cào, ví điện tử, ngân hàng điện tử, ngân hàng trực tiếp. Mọi hoạt động đều được bảo mật tuyệt đối bởi công nghệ mã hóa tiên tiến.
App cá độ RGBET
App cá độ RGBET là một ứng dụng cho phép người chơi đăng nhập RG nhanh chóng, đồng thời các thao tác đăng ký RG trên app cũng được tối ưu và trở nên đơn giản hơn. Tham gia cá cược RG bằng app cá độ, người chơi sẽ có 1 trải nghiệm cá cược tuyệt vời và thú vị.
RGBET Có Chứng Nhận Cá Cược Quốc Tế
Nhà cái RGBET hoạt động hợp pháp dưới sự cấp phép của hai tổ chức thế giới là PAGCOR và MGA, tính minh bạch và công bằng luôn được giám sát gắt gao bởi BIV. Khi tham gia cược tại đây, người chơi sẽ được đảm bảo quyền và lợi ích hợp pháp của mình.
Việc sở hữu các chứng nhận quốc tế còn cho thấy nguồn tài chính ổn định, dồi dào của RGBET. Điều này cho thấy việc một nhà cái được công nhận bởi các cơ quan quốc tế không phải là một chuyện dễ.
Theo quy định nhà cái RGBET, chỉ người chơi từ đủ 18 tuổi trở lên mới có thể tham gia cá cược tại RGBET
MGA (Malta Gaming Authority)
Tổ chức MGA đảm bảo tính vẹn toàn và ổn định của các trò chơi. Có các chính sách bảo vệ nguồn tài chính và quyền lợi của người chơi. Chứng nhận một nhà cái hoạt động có đầy đủ pháp lý, tuân thủ nghiêm chỉnh luật cờ bạc.
Chứng nhận Quần đảo Virgin Vương quốc Anh (BIV)
Tổ chứng chứng nhận nhà cái có đầy đủ tài chính để hoạt động kinh doanh cá cược. Với nguồn ngân sách dồi dào, ổn định nhà cái bảo đảm tính thanh khoản cho người chơi, mọi quyền lợi sẽ không bị xâm phạm.
Giấy Phép PAGCOR
Tổ chức cấp giấy phép cho nhà cái hoạt động đạt chuẩn theo tiêu chuẩn quốc tế. Cho phép nhà cái tổ chức cá cược một cách hợp pháp, không bị rào cản. Có chính sách ngăn chặn mọi trò chơi có dấu hiệu lừa đảo, duy trì sự minh bạch, công bằng.
Nhà Cái RGBET Phát Triển Công Nghệ
Nhà cái RGBET hỗ trợ trên nhiều thiết bị : IOS, Android, APP, WEB, Html5
RG và Trách Nhiệm Xã Hội
RGBET RichGame không đơn thuần là một trang cá cược giải trí mà nhà cái còn thể hiện rõ tính trách nhiệm xã hội của mình. Đơn vị luôn mong muốn người chơi tham gia cá cược phải có trách nhiệm với bản thân, gia đình và cả xã hội. Mọi hoạt động diễn ra tại RGBET trang chủ nói riêng hay bất kỳ trang web khác, người chơi phải thật sự bình tĩnh và lý trí, đừng để bản thân rơi vào “cạm bẫy của cờ bạc”.
RGBET RichGame với chính sách nghiêm cấm mọi hành vi xâm phạm thông tin cá nhân và gian lận nhằm tạo ra một môi trường cá cược công bằng, lành mạnh. Nhà cái khuyến cáo mọi cá nhân chưa đủ 18 tuổi không nên đăng ký RG và tham gia vào bất kỳ hoạt động cá cược nào.
pin up: пинап казино – пин ап
guvenilir casino siteleri: en iyi casino siteleri – dunyan?n en iyi casino siteleri
смотреть порно ролики смотреть домашнее порно
h?zl? casino casino sitesi casino siteleri
Hi there! Do you use Twitter? I’d like to follow you
if that would be ok. I’m undoubtedly enjoying your blog and look forward to new updates.
rgbet
RGBET Trang Chủ Và Câu Chuyện Thương Hiệu
Ra đời vào năm 2010 tại Đài Loan, RGBET nhanh chóng trở thành một trang cá cược chất lượng hàng đầu khu vực Châu Á. Nhà cái được cấp phép hoạt động hợp pháp bởi công ty giải trí trực tuyến hợp pháp được ủy quyền và giám sát theo giấy phép Malta của Châu Âu – MGA. Và chịu sự giám sát chặt chẽ của tổ chức PAGCOR và BIV.
RGBET trang chủ cung cấp cho người chơi đa dạng các thể loại cược đặc sắc như: thể thao, đá gà, xổ số, nổ hũ, casino trực tuyến. Dịch vụ CSKH luôn hoạt động 24/7. Với chứng chỉ công nghệ GEOTRUST, nhà cái đảm bảo an toàn cho mọi giao dịch của khách hàng. APP RG thiết kế tối ưu giải quyết mọi vấn đề của người dùng IOS và Android.
Là một nhà cái đến từ đất nước công nghệ, nhà cái luôn không ngừng xây dựng và nâng cấp hệ thống game và dịch vụ hoàn hảo. Mọi giao dịch nạp rút được tự động hoá cho phép người chơi hoàn tất giao dịch chỉ với 2 phút vô cùng nhanh chóng
RGBET Lớn Nhất Uy Tín Nhất – Giá Trị Cốt Lõi
Nhà Cái RG Và Mục Tiêu Thương Hiệu
Giá trị cốt lõi mà RGBET mong muốn hướng đến đó chính là không ngừng hoàn thiện để đem đến một hệ thống chất lượng, công bằng và an toàn. Nâng cao sự hài lòng của người chơi, đẩy mạnh hoạt động chống gian lận và lừa đảo. RG luôn cung cấp hệ thống kèo nhà cái đặc sắc, cùng các sự kiện – giải đấu hàng đầu và tỷ lệ cược cạnh tranh đáp ứng mọi nhu cầu khách hàng.
Thương hiệu cá cược RGBET cam kết đem lại cho người chơi môi trường cá cược công bằng, văn minh và lành mạnh. Đây là nguồn động lực to lớn giúp nhà cái thực tế hóa các hoạt động của mình.
RGBET Có Tầm Nhìn Và Sứ Mệnh
Đổi mới và sáng tạo là yếu tố cốt lõi giúp đạt được mục tiêu dưới sự chuyển mình mạnh mẽ của công nghệ. Tầm nhìn và sứ mệnh của RGBET là luôn tìm tòi những điều mới lạ, đột phá mạnh mẽ, vượt khỏi giới hạn bản thân, đương đầu với thử thách để đem đến cho khách hàng sản phẩm hoàn thiện nhất.
Chúng tôi luôn sẵn sàng tiếp thu ý kiến và nâng cao bản thân mỗi ngày để tạo ra sân chơi bổ ích, uy tín và chuyên nghiệp cho người chơi. Để có thể trở thành nhà cái phù hợp với mọi khách hàng.
Khái Niệm Giá Trị Cốt Lõi Nhà Cái RGBET
Giá trị cốt lõi của nhà cái RG luôn gắn kết chặt chẽ với nhau giữa 5 khái niệm: Chính trực, chuyên nghiệp, an toàn, đổi mới, công nghệ.
Chính Trực
Mọi quy luật, cách thức của trò chơi đều được nhà cái cung cấp công khai, minh bạch và chi tiết. Mỗi tựa game hoạt động đều phải chịu sự giám sát kỹ lưỡng bởi các cơ quan tổ chức có tiếng về sự an toàn và minh bạch của nó.
Chuyên Nghiệp
Các hoạt động tại RGBET trang chủ luôn đề cao sự chuyên nghiệp lên hàng đầu. Từ giao diện đến chất lượng sản phẩm luôn được trau chuốt tỉ mỉ từng chi tiết. Thế giới giải trí được xây dựng theo văn hóa Châu Á, phù hợp với đại đa số thị phần khách Việt.
An Toàn
RG lớn nhất uy tín nhất luôn ưu tiên sử dụng công nghệ mã hóa hiện đại nhất để đảm bảo an toàn, riêng tư cho toàn bộ thông tin của người chơi. Đơn vị cam kết nói không với hành vi gian lận và mua bán, trao đổi thông tin cá nhân bất hợp pháp.
Đổi Mới
Nhà cái luôn theo dõi và bắt kịp xu hướng thời đại, liên tục bổ sung các sản phẩm mới, phương thức cá cược mới và các ưu đãi độc lạ, mang đến những trải nghiệm thú vị cho người chơi.
Công Nghệ
RGBET trang chủ tập trung xây dựng một giao diện game sắc nét, sống động cùng tốc độ tải nhanh chóng. Ứng dụng RGBET giải nén ít dung lượng phù hợp với mọi hệ điều hành và cấu hình, tăng khả năng sử dụng của khách hàng.
RGBET Khẳng Định Giá Trị Thương Hiệu
Hoạt động hợp pháp với đầy đủ giấy phép, chứng chỉ an toàn đạt tiêu chuẩn quốc tế
Hệ thống game đa màu sắc, đáp ứng được mọi nhu cầu người chơi
Chính sách bảo mật RG hiện đại và đảm bảo an toàn cho người chơi cá cược
Bắt tay hợp tác với nhiều đơn vị phát hành game uy tín, chất lượng thế giới
Giao dịch nạp rút RG cấp tốc, nhanh gọn, bảo mật an toàn
Kèo nhà cái đa dạng với bảng tỷ lệ kèo cao, hấp dẫn
Dịch Vụ RGBET Casino Online
Dịch vụ khách hàng
Đội ngũ CSKH RGBET luôn hoạt động thường trực 24/7. Nhân viên được đào tạo chuyên sâu luôn giải đáp tất cả các khó khăn của người chơi về các vấn đề tài khoản, khuyến mãi, giao dịch một cách nhanh chóng và chuẩn xác. Hạn chế tối đa làm ảnh hưởng đến quá trình trải nghiệm của khách hàng.
Đa dạng trò chơi
Với sự nhạy bén trong cập nhật xu thế, nhà cái RGBET đã dành nhiều thời gian phân tích nhu cầu khách hàng, đem đến một kho tàng game chất lượng với đa dạng thể loại từ RG casino online, thể thao, nổ hũ, game bài, đá gà, xổ số.
Khuyến mãi hấp dẫn
RGBET trang chủ liên tục cập nhật và thay đổi các sự kiện ưu đãi đầy hấp dẫn và độc đáo. Mọi thành viên bất kể là người chơi mới, người chơi cũ hay hội viên VIP đều có cơ hội được hưởng ưu đãi đặc biệt từ nhà cái.
Giao dịch linh hoạt, tốc độ
Thương hiệu RGBET luôn chú tâm đến hệ thống giao dịch. Nhà cái cung cấp dịch vụ nạp rút nhanh chóng với đa dạng phương thức như thẻ cào, ví điện tử, ngân hàng điện tử, ngân hàng trực tiếp. Mọi hoạt động đều được bảo mật tuyệt đối bởi công nghệ mã hóa tiên tiến.
App cá độ RGBET
App cá độ RGBET là một ứng dụng cho phép người chơi đăng nhập RG nhanh chóng, đồng thời các thao tác đăng ký RG trên app cũng được tối ưu và trở nên đơn giản hơn. Tham gia cá cược RG bằng app cá độ, người chơi sẽ có 1 trải nghiệm cá cược tuyệt vời và thú vị.
RGBET Có Chứng Nhận Cá Cược Quốc Tế
Nhà cái RGBET hoạt động hợp pháp dưới sự cấp phép của hai tổ chức thế giới là PAGCOR và MGA, tính minh bạch và công bằng luôn được giám sát gắt gao bởi BIV. Khi tham gia cược tại đây, người chơi sẽ được đảm bảo quyền và lợi ích hợp pháp của mình.
Việc sở hữu các chứng nhận quốc tế còn cho thấy nguồn tài chính ổn định, dồi dào của RGBET. Điều này cho thấy việc một nhà cái được công nhận bởi các cơ quan quốc tế không phải là một chuyện dễ.
Theo quy định nhà cái RGBET, chỉ người chơi từ đủ 18 tuổi trở lên mới có thể tham gia cá cược tại RGBET
MGA (Malta Gaming Authority)
Tổ chức MGA đảm bảo tính vẹn toàn và ổn định của các trò chơi. Có các chính sách bảo vệ nguồn tài chính và quyền lợi của người chơi. Chứng nhận một nhà cái hoạt động có đầy đủ pháp lý, tuân thủ nghiêm chỉnh luật cờ bạc.
Chứng nhận Quần đảo Virgin Vương quốc Anh (BIV)
Tổ chứng chứng nhận nhà cái có đầy đủ tài chính để hoạt động kinh doanh cá cược. Với nguồn ngân sách dồi dào, ổn định nhà cái bảo đảm tính thanh khoản cho người chơi, mọi quyền lợi sẽ không bị xâm phạm.
Giấy Phép PAGCOR
Tổ chức cấp giấy phép cho nhà cái hoạt động đạt chuẩn theo tiêu chuẩn quốc tế. Cho phép nhà cái tổ chức cá cược một cách hợp pháp, không bị rào cản. Có chính sách ngăn chặn mọi trò chơi có dấu hiệu lừa đảo, duy trì sự minh bạch, công bằng.
Nhà Cái RGBET Phát Triển Công Nghệ
Nhà cái RGBET hỗ trợ trên nhiều thiết bị : IOS, Android, APP, WEB, Html5
RG và Trách Nhiệm Xã Hội
RGBET RichGame không đơn thuần là một trang cá cược giải trí mà nhà cái còn thể hiện rõ tính trách nhiệm xã hội của mình. Đơn vị luôn mong muốn người chơi tham gia cá cược phải có trách nhiệm với bản thân, gia đình và cả xã hội. Mọi hoạt động diễn ra tại RGBET trang chủ nói riêng hay bất kỳ trang web khác, người chơi phải thật sự bình tĩnh và lý trí, đừng để bản thân rơi vào “cạm bẫy của cờ bạc”.
RGBET RichGame với chính sách nghiêm cấm mọi hành vi xâm phạm thông tin cá nhân và gian lận nhằm tạo ra một môi trường cá cược công bằng, lành mạnh. Nhà cái khuyến cáo mọi cá nhân chưa đủ 18 tuổi không nên đăng ký RG và tham gia vào bất kỳ hoạt động cá cược nào.
RGBET Trang Chủ Và Câu Chuyện Thương Hiệu
Ra đời vào năm 2010 tại Đài Loan, RGBET nhanh chóng trở thành một trang cá cược chất lượng hàng đầu khu vực Châu Á. Nhà cái được cấp phép hoạt động hợp pháp bởi công ty giải trí trực tuyến hợp pháp được ủy quyền và giám sát theo giấy phép Malta của Châu Âu – MGA. Và chịu sự giám sát chặt chẽ của tổ chức PAGCOR và BIV.
RGBET trang chủ cung cấp cho người chơi đa dạng các thể loại cược đặc sắc như: thể thao, đá gà, xổ số, nổ hũ, casino trực tuyến. Dịch vụ CSKH luôn hoạt động 24/7. Với chứng chỉ công nghệ GEOTRUST, nhà cái đảm bảo an toàn cho mọi giao dịch của khách hàng. APP RG thiết kế tối ưu giải quyết mọi vấn đề của người dùng IOS và Android.
Là một nhà cái đến từ đất nước công nghệ, nhà cái luôn không ngừng xây dựng và nâng cấp hệ thống game và dịch vụ hoàn hảo. Mọi giao dịch nạp rút được tự động hoá cho phép người chơi hoàn tất giao dịch chỉ với 2 phút vô cùng nhanh chóng
RGBET Lớn Nhất Uy Tín Nhất – Giá Trị Cốt Lõi
Nhà Cái RG Và Mục Tiêu Thương Hiệu
Giá trị cốt lõi mà RGBET mong muốn hướng đến đó chính là không ngừng hoàn thiện để đem đến một hệ thống chất lượng, công bằng và an toàn. Nâng cao sự hài lòng của người chơi, đẩy mạnh hoạt động chống gian lận và lừa đảo. RG luôn cung cấp hệ thống kèo nhà cái đặc sắc, cùng các sự kiện – giải đấu hàng đầu và tỷ lệ cược cạnh tranh đáp ứng mọi nhu cầu khách hàng.
Thương hiệu cá cược RGBET cam kết đem lại cho người chơi môi trường cá cược công bằng, văn minh và lành mạnh. Đây là nguồn động lực to lớn giúp nhà cái thực tế hóa các hoạt động của mình.
RGBET Có Tầm Nhìn Và Sứ Mệnh
Đổi mới và sáng tạo là yếu tố cốt lõi giúp đạt được mục tiêu dưới sự chuyển mình mạnh mẽ của công nghệ. Tầm nhìn và sứ mệnh của RGBET là luôn tìm tòi những điều mới lạ, đột phá mạnh mẽ, vượt khỏi giới hạn bản thân, đương đầu với thử thách để đem đến cho khách hàng sản phẩm hoàn thiện nhất.
Chúng tôi luôn sẵn sàng tiếp thu ý kiến và nâng cao bản thân mỗi ngày để tạo ra sân chơi bổ ích, uy tín và chuyên nghiệp cho người chơi. Để có thể trở thành nhà cái phù hợp với mọi khách hàng.
Khái Niệm Giá Trị Cốt Lõi Nhà Cái RGBET
Giá trị cốt lõi của nhà cái RG luôn gắn kết chặt chẽ với nhau giữa 5 khái niệm: Chính trực, chuyên nghiệp, an toàn, đổi mới, công nghệ.
Chính Trực
Mọi quy luật, cách thức của trò chơi đều được nhà cái cung cấp công khai, minh bạch và chi tiết. Mỗi tựa game hoạt động đều phải chịu sự giám sát kỹ lưỡng bởi các cơ quan tổ chức có tiếng về sự an toàn và minh bạch của nó.
Chuyên Nghiệp
Các hoạt động tại RGBET trang chủ luôn đề cao sự chuyên nghiệp lên hàng đầu. Từ giao diện đến chất lượng sản phẩm luôn được trau chuốt tỉ mỉ từng chi tiết. Thế giới giải trí được xây dựng theo văn hóa Châu Á, phù hợp với đại đa số thị phần khách Việt.
An Toàn
RG lớn nhất uy tín nhất luôn ưu tiên sử dụng công nghệ mã hóa hiện đại nhất để đảm bảo an toàn, riêng tư cho toàn bộ thông tin của người chơi. Đơn vị cam kết nói không với hành vi gian lận và mua bán, trao đổi thông tin cá nhân bất hợp pháp.
Đổi Mới
Nhà cái luôn theo dõi và bắt kịp xu hướng thời đại, liên tục bổ sung các sản phẩm mới, phương thức cá cược mới và các ưu đãi độc lạ, mang đến những trải nghiệm thú vị cho người chơi.
Công Nghệ
RGBET trang chủ tập trung xây dựng một giao diện game sắc nét, sống động cùng tốc độ tải nhanh chóng. Ứng dụng RGBET giải nén ít dung lượng phù hợp với mọi hệ điều hành và cấu hình, tăng khả năng sử dụng của khách hàng.
RGBET Khẳng Định Giá Trị Thương Hiệu
Hoạt động hợp pháp với đầy đủ giấy phép, chứng chỉ an toàn đạt tiêu chuẩn quốc tế
Hệ thống game đa màu sắc, đáp ứng được mọi nhu cầu người chơi
Chính sách bảo mật RG hiện đại và đảm bảo an toàn cho người chơi cá cược
Bắt tay hợp tác với nhiều đơn vị phát hành game uy tín, chất lượng thế giới
Giao dịch nạp rút RG cấp tốc, nhanh gọn, bảo mật an toàn
Kèo nhà cái đa dạng với bảng tỷ lệ kèo cao, hấp dẫn
Dịch Vụ RGBET Casino Online
Dịch vụ khách hàng
Đội ngũ CSKH RGBET luôn hoạt động thường trực 24/7. Nhân viên được đào tạo chuyên sâu luôn giải đáp tất cả các khó khăn của người chơi về các vấn đề tài khoản, khuyến mãi, giao dịch một cách nhanh chóng và chuẩn xác. Hạn chế tối đa làm ảnh hưởng đến quá trình trải nghiệm của khách hàng.
Đa dạng trò chơi
Với sự nhạy bén trong cập nhật xu thế, nhà cái RGBET đã dành nhiều thời gian phân tích nhu cầu khách hàng, đem đến một kho tàng game chất lượng với đa dạng thể loại từ RG casino online, thể thao, nổ hũ, game bài, đá gà, xổ số.
Khuyến mãi hấp dẫn
RGBET trang chủ liên tục cập nhật và thay đổi các sự kiện ưu đãi đầy hấp dẫn và độc đáo. Mọi thành viên bất kể là người chơi mới, người chơi cũ hay hội viên VIP đều có cơ hội được hưởng ưu đãi đặc biệt từ nhà cái.
Giao dịch linh hoạt, tốc độ
Thương hiệu RGBET luôn chú tâm đến hệ thống giao dịch. Nhà cái cung cấp dịch vụ nạp rút nhanh chóng với đa dạng phương thức như thẻ cào, ví điện tử, ngân hàng điện tử, ngân hàng trực tiếp. Mọi hoạt động đều được bảo mật tuyệt đối bởi công nghệ mã hóa tiên tiến.
App cá độ RGBET
App cá độ RGBET là một ứng dụng cho phép người chơi đăng nhập RG nhanh chóng, đồng thời các thao tác đăng ký RG trên app cũng được tối ưu và trở nên đơn giản hơn. Tham gia cá cược RG bằng app cá độ, người chơi sẽ có 1 trải nghiệm cá cược tuyệt vời và thú vị.
RGBET Có Chứng Nhận Cá Cược Quốc Tế
Nhà cái RGBET hoạt động hợp pháp dưới sự cấp phép của hai tổ chức thế giới là PAGCOR và MGA, tính minh bạch và công bằng luôn được giám sát gắt gao bởi BIV. Khi tham gia cược tại đây, người chơi sẽ được đảm bảo quyền và lợi ích hợp pháp của mình.
Việc sở hữu các chứng nhận quốc tế còn cho thấy nguồn tài chính ổn định, dồi dào của RGBET. Điều này cho thấy việc một nhà cái được công nhận bởi các cơ quan quốc tế không phải là một chuyện dễ.
Theo quy định nhà cái RGBET, chỉ người chơi từ đủ 18 tuổi trở lên mới có thể tham gia cá cược tại RGBET
MGA (Malta Gaming Authority)
Tổ chức MGA đảm bảo tính vẹn toàn và ổn định của các trò chơi. Có các chính sách bảo vệ nguồn tài chính và quyền lợi của người chơi. Chứng nhận một nhà cái hoạt động có đầy đủ pháp lý, tuân thủ nghiêm chỉnh luật cờ bạc.
Chứng nhận Quần đảo Virgin Vương quốc Anh (BIV)
Tổ chứng chứng nhận nhà cái có đầy đủ tài chính để hoạt động kinh doanh cá cược. Với nguồn ngân sách dồi dào, ổn định nhà cái bảo đảm tính thanh khoản cho người chơi, mọi quyền lợi sẽ không bị xâm phạm.
Giấy Phép PAGCOR
Tổ chức cấp giấy phép cho nhà cái hoạt động đạt chuẩn theo tiêu chuẩn quốc tế. Cho phép nhà cái tổ chức cá cược một cách hợp pháp, không bị rào cản. Có chính sách ngăn chặn mọi trò chơi có dấu hiệu lừa đảo, duy trì sự minh bạch, công bằng.
Nhà Cái RGBET Phát Triển Công Nghệ
Nhà cái RGBET hỗ trợ trên nhiều thiết bị : IOS, Android, APP, WEB, Html5
RG và Trách Nhiệm Xã Hội
RGBET RichGame không đơn thuần là một trang cá cược giải trí mà nhà cái còn thể hiện rõ tính trách nhiệm xã hội của mình. Đơn vị luôn mong muốn người chơi tham gia cá cược phải có trách nhiệm với bản thân, gia đình và cả xã hội. Mọi hoạt động diễn ra tại RGBET trang chủ nói riêng hay bất kỳ trang web khác, người chơi phải thật sự bình tĩnh và lý trí, đừng để bản thân rơi vào “cạm bẫy của cờ bạc”.
RGBET RichGame với chính sách nghiêm cấm mọi hành vi xâm phạm thông tin cá nhân và gian lận nhằm tạo ra một môi trường cá cược công bằng, lành mạnh. Nhà cái khuyến cáo mọi cá nhân chưa đủ 18 tuổi không nên đăng ký RG và tham gia vào bất kỳ hoạt động cá cược nào.
en iyi casino siteleri: canl? casino – en iyi casino siteleri
купить героин тик ток порно
1xbet 1xbet or 1xbet зеркало
https://images.google.by/url?sa=t&url=https://1winrussia.online 1хставка
1xbet зеркало 1xbet and 1хставка 1xbet официальный сайт
Сервисный центр предлагает ремонт планшетов dexp ремонт планшета dexp в москве
kubet
เว็บไซต์การพนันกีฬา KUBET เป็นแบรนด์ของไต้หวันภายใต้กลุ่ม Kyushu และเป็นแบรนด์การพนันออนไลน์ (เว็บเงินสด) ที่มีทุนสำรองมากกว่าพันล้าน มีอินเทอร์เฟซที่ใช้งานง่าย โปรโมชั่นมากมาย และกลุ่มเกมสำหรับสมาชิกซึ่งเป็นหนึ่งในคุณสมบัติเด่นของ KUBET กีฬา
KUBET กีฬาได้รับการอนุญาตและควบคุมโดยบริษัทความบันเทิงออนไลน์ที่ถูกกฎหมาย โดยมีใบอนุญาตจาก Malta Gaming Authority (MGA) ในยุโรปและใบอนุญาตจาก Philippine Amusement and Gaming Corporation (PAGCOR) จึงเป็นตัวเลือกแรกของผู้เล่นอย่างไม่ต้องสงสัย。
https://1winrussia.online/# 1xbet официальный сайт
пин ап казино
slot casino siteleri: casino oyunlar? – casino siteleri
en iyi casino siteleri h?zl? casino or dunyan?n en iyi casino siteleri
https://www.google.cm/url?sa=t&url=https://1wintr.fun casino siteleri
casino oyunlar? slot casino siteleri and guvenilir casino siteleri canl? casino siteleri
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
http://www.babelcube.com/user/jeffery-white-1
https://rentry.org/79x68yyf
https://haveagood.holiday/users/353272
http://www.babelcube.com/user/jody-collum
https://www.metal-archives.com/users/xix1984
dunyan?n en iyi casino siteleri cazino cazino
ビーストサップ
k8 カジノ スロット Hot Fiesta ホットフィエスタ
歴史をテーマにしたパチスロで、独特の魅力があります。日本の伝統を感じます。
夜蝶飛翔
https://www.10k8.com/tags/p%E3%83%95%E3%82%A3%E3%83%BC%E3%83%90%E3%83%BC%E6%A9%9F%E5%8B%95%E6%88%A6%E5%A3%AB%E3%82%AC%E3%83%B3%E3%83%80%E3%83%A0-%E9%80%86%E8%A5%B2%E3%81%AE%E3%82%B7%E3%83%A3%E3%82%A2-ver-199
お金をかけることに抵抗がある人も、少額から始められるのが魅力です。楽しみ方は多様です。
八代将军 天国の戦い
k8 カジノ パチンコ
黃金守護神(特殊大賞燈);
CR麻雀物語麗しのテンパイ乙女 Ver.297
Pフィーバーかぐや様は告らせたい
Download and install MetaMask extension with this beginner guide. Securely set up MetaMask for Ethereum and Web3 applications.
https://1wintr.fun/# casino sitesi
пин ап казино
pin up azerbaycan pin-up casino giris or pin-up casino giris
https://maps.google.com.ua/url?q=https://1winbrasil.win pin up 306
pin-up casino giris pin up azerbaycan and pin up casino pin up 306
пин ап зеркало пин ап зеркало or пин ап официальный сайт
https://www.google.hu/url?q=https://1winci.icu пинап зеркало
пин ап вход пин ап официальный сайт and пин ап зеркало пин ап официальный сайт
1хставка: 1xbet зеркало – 1xbet зеркало
1xbet: 1xbet официальный сайт – 1xbet
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
пин ап зеркало пин ап вход or пин ап зеркало
https://cse.google.com.sa/url?sa=i&url=https://1winci.icu пин ап официальный сайт
пин ап пин ап and пин ап пин ап зеркало
пинап зеркало: пин ап зеркало – пин ап официальный сайт
пин ап официальный сайт: пин ап – пинап зеркало
1xbet скачать: 1хбет – 1хбет
cazino en iyi casino siteleri or dunyan?n en iyi casino siteleri
http://n-organic.jp/shop/display_cart?return_url=http://1wintr.fun casino siteleri
casino oyunlar? cazino and casino sitesi casino siteleri
pin-up casino giris: pin up azerbaycan – pin up 306
Профессиональный сервисный центр по ремонту телефонов в Москве.
Мы предлагаем: профессиональный ремонт телефонов
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
pin-up casino giris: pin up 306 – pinup az
CR花の慶次X~雲のかなたに~
k8 カジノ スロット BARREL BONANZA バーレルボナンザ
音響効果が素晴らしく、迫力のあるサウンドがプレイを盛り上げます。特にボーナス時の音は興奮します。
CRルパン三世I’m a super hero~
https://www.kk8520.com/tags/%E3%83%90%E3%82%B8%E3%83%AA%E3%82%B9%E3%82%AF
シンプルなデザインとルールが魅力。初心者でも安心して楽しめます。
CR戦国乙女花
k8 カジノ パチンコ
CRさくらももこ劇場コジコジ2
CR JAWS再臨 SHARK PANIC AGAIN
新鬼武者 再臨
https://1winindia.tech/# пин ап кз
пинап
пин ап казино: пин ап – пин ап кз
pin up: pin up 306 – pin up azerbaycan
пин ап зеркало пинап зеркало пин ап вход
cazino: canl? casino siteleri – slot casino siteleri
1хбет: 1хставка – 1хставка
пин ап вход пин ап официальный сайт пин ап официальный сайт
ремонт телефонов по близости
1хбет: 1xbet официальный сайт – 1xbet официальный сайт
пин ап кз: пинап казино – pin up kz
1хставка: 1xbet – 1хставка
casino oyunlar? canl? casino siteleri or guvenilir casino siteleri
https://www.google.mk/url?q=https://1wintr.fun casino siteleri
casino siteleri slot casino siteleri and casino oyunlar? casino oyunlar?
пин ап пин ап вход or пин ап вход
http://www2.pure.cc/~mikimomo/zaccess/acc.cgi?redirect=http://1winci.icu пин ап вход
пинап зеркало пин ап зеркало and пин ап зеркало пин ап
1хставка: 1xbet официальный сайт – 1хбет
1xbet официальный сайт 1xbet зеркало or 1хставка
http://wishforthis.com/Shop/Redirect.php?URL=http://1winrussia.online/ 1хставка
1xbet скачать 1xbet and 1хбет 1xbet
пин ап зеркало пин ап официальный сайт or пин ап зеркало
https://maps.google.cz/url?sa=t&url=https://1winci.icu пин ап официальный сайт
пинап зеркало пин ап вход and пин ап пин ап вход
пин ап казино вход: пин ап казино – пин ап
https://1winrussia.online/# 1xbet скачать
pin up
рекламное агентство реклама в лифтах реклама в лифте стоимость
Если вы искали где отремонтировать сломаную технику, обратите внимание – тех профи
http://1winbrasil.win/# pin up
пин ап казино вход
1xbet: 1xbet – 1хставка
пин ап казино вход pin up пинап кз
1xbet официальный сайт: 1xbet зеркало – 1xbet
1xbet скачать: 1xbet официальный сайт – 1xbet
casino sitesi canl? casino or canl? casino siteleri
https://maps.google.com.ph/url?sa=t&url=https://1wintr.fun canl? casino siteleri
casino siteleri canl? casino and h?zl? casino casino siteleri
casino sitesi: canl? casino siteleri – canl? casino siteleri
pin-up casino giris pin-up pin up
canl? casino siteleri: casino siteleri – casino siteleri
秘宝伝 伝説への道
k8 カジノ パチンコ アイムジャグラーEXパチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
美しいグラフィックと豪華な演出が魅力。神々の力を感じます。
シンドバッドアドベンチャーは榎本加奈子でどうですか
https://lucky-777-casino.com/tags/%E5%A4%A9%E4%BA%95%E3%81%AB%E6%BD%9C%E3%82%80%E8%AC%8E%E3%81%AE%E3%83%A2%E3%83%B3%E3%82%B9%E3%82%BF%E3%83%BC
パチンコの演出は、時に驚かされることがあります。毎回新しい発見があります。
CR真・北斗無双 Ver.319(2:1)
魔法少女まどか☆マギカ
GI優駿俱樂部
バジリスク~甲賀忍法帖~絆
CRサイボーグ009VSデビルマン
Sportsurge This is really interesting, You’re a very skilled blogger. I’ve joined your feed and look forward to seeking more of your magnificent post. Also, I’ve shared your site in my social networks!
Download and install MetaMask extension with this beginner guide. Securely set up MetaMask for Ethereum and Web3 applications.
http://1winrussia.online/# 1хбет
пинап казино
http://1winci.icu/# пин ап официальный сайт
пин ап
pinup az: pin-up – pin-up casino giris
canl? casino siteleri: canl? casino siteleri – dunyan?n en iyi casino siteleri
пин ап зеркало: пинап зеркало – пин ап
пин ап пин ап зеркало or пинап зеркало
https://images.google.ng/url?q=https://1winci.icu пин ап
пин ап пин ап зеркало and пин ап зеркало пин ап зеркало
pinup az: pinup az – pin up 306
апостиль в новосибирске
casino oyunlar? casino sitesi or canl? casino siteleri
https://www.google.com.vn/url?q=https://1wintr.fun canl? casino
cazino cazino and en iyi casino siteleri h?zl? casino
pin up 306 pin-up or pin up azerbaycan
https://clients1.google.ac/url?q=https://1winbrasil.win pin up casino
pin up azerbaycan pinup az and pin up casino pinup az
Если вы искали где отремонтировать сломаную технику, обратите внимание – ремонт бытовой техники
пинап зеркало пин ап вход or пин ап официальный сайт
http://www.m-bp.jp/bookmark.html?tu_id=yoga-gllow&u=http://1winci.icu пин ап официальный сайт
пин ап вход пин ап вход and пин ап официальный сайт пинап зеркало
pin up casino pin up 306 pinup az
MetaMask stands out as one of the most popular wallet solutions, especially for interacting with Ethereum-based applications. This guide covers everything you need to know about downloading and installing the MetaMask Extension, empowering you to manage your digital assets with ease.
1xbet 1xbet 1xbet
1хставка: 1xbet зеркало – 1xbet скачать
1xbet официальный сайт: 1хставка – 1xbet зеркало
http://1winindia.tech/# пин ап казино вход
pin up kz
There are many popular live sex cam sites that cater to all different preferences and desires. Some of the most popular ones include Chaturbate, MyFreeCams, LiveJasmin, and Flirt4Free.
Chaturbate is known for its diverse selection of cam models, ranging from amateur performers to professional porn stars. It offers a unique “tip-based” system, where viewers can tip the performers for special requests or to show their appreciation.
MyFreeCams is a popular choice for those looking for a more personalized experience, as many of the models offer private shows for a fee. It also has a large community aspect, with forums and chat rooms for viewers to interact with each other and the models.
LiveJasmin is known for its high-quality video and audio, making it a top choice for viewers who value a visually stimulating experience. It also has a wide range of categories, allowing viewers to easily find the type of performer they are looking for.
Flirt4Free is a popular site for those looking for a more intimate and interactive experience. It offers a variety of features such as cam-to-cam shows and interactive sex toys, making it a favorite among viewers who enjoy a more immersive experience.
Overall, these live sex cam sites offer a diverse range of performers and features to cater to all types of desires and preferences. Their popularity shows that the demand for live sex cams continues to grow as people seek out new and exciting forms of sexual entertainment.
https://www.obesityhelp.com/members/vilians1954/about_me/
https://babok1959.diary.ru/
https://cannabis.net/user/158478
https://rentry.org/kfzytzrd
https://permacultureglobal.org/users/61234-rodney-hallem
пин ап: пинап – pin up
пинап казино: пин ап казино вход – пин ап
Скачайте приложение 888Starz на Android и начните выигрывать
замена венцов
casino siteleri en iyi casino siteleri or casino sitesi
https://maps.google.ad/url?q=https://1wintr.fun dunyan?n en iyi casino siteleri
casino siteleri casino sitesi and canl? casino siteleri casino oyunlar?
Appreciating the persistence you put into your blog and detailed information you present.
It’s good to come across a blog every once in a while that isn’t the same out of date rehashed material.
Great read! I’ve bookmarked your site and I’m
adding your RSS feeds to my Google account.
You made my day! Thank you for the amazing service – couldn’t be happier!
Totally exceeded my expectations! Thank you for such a fantastic service. Highly recommended!
Big thanks to the team! Friendly, efficient, and just awesome all around. 10/10 would recommend!
Totally exceeded my expectations! Thank you for such a fantastic service. Highly recommended!
slot casino siteleri: casino sitesi – cazino
Absolutely loved it here! Thanks for the great memories and fantastic service. I’ll be back for sure!
Amazing experience! Thank you so much for going above and beyond. Will definitely recommend it to my friends!
cheap medications online anti fungal pills without prescription google viagra dosage recommendations
https://indianpharm1st.com/# Online medicine home delivery
ค่าย SA Gaming เป็น ค่าย เกม ไพ่บาคาร่า ออนไลน์ ซึ่งได้รับความนิยม ใน วงการสากล ว่าเป็น ผู้นำ ในการให้บริการ บริการ คาสิโนออนไลน์ โดยเฉพาะในด้าน เกมไพ่ บาคาร่า ซึ่งเป็น เกมพนัน ที่ ผู้คน สนใจเล่นกัน ทั่วไป ใน คาสิโนจริง และ ออนไลน์ ด้วย วิธีการเล่น ที่ สะดวก การแทง เพียง ฝ่าย ผู้เล่น หรือ เจ้ามือ และ ความเป็นไปได้ในการชนะ ที่ ค่อนข้างสูง ทำให้ เกมไพ่บาคาร่า ได้รับ การยอมรับ อย่างมากใน ช่วงหลายปีนี้ โดยเฉพาะใน ไทย
หนึ่งในวิธีการเล่น ยอดนิยมที่ SA Gaming เสนอ คือ บาคาร่าเร็ว ซึ่ง ช่วยให้ผู้เล่น ต้องการ ความรวดเร็ว และ การคิดไว สามารถ เดิมพันได้อย่างรวดเร็ว นอกจากนี้ยังมีโหมด No Commission Baccarat ซึ่งเป็น รูปแบบ ที่ ไม่ต้องเสียค่าคอมมิชชั่นเพิ่มเติม เมื่อชนะ การลงเงิน แบงค์เกอร์ ทำให้ เกมนี้ ได้รับ ความคุ้มค่า จาก นักเสี่ยงโชค ที่มองหา กำไร ในการ เสี่ยงโชค
เกมไพ่พนัน ของ SA Gaming ยัง ถูกพัฒนา ให้มี กราฟฟิค พร้อมกับ ระบบออดิโอ ที่ สมจริง สร้างบรรยากาศ ที่ น่าสนุก เหมือนอยู่ใน บ่อนคาสิโนจริง พร้อมกับ ฟีเจอร์ ที่ทำให้ นักเล่น สามารถเลือก แนวทางเดิมพัน ที่ หลากหลาย ไม่ว่าจะเป็น การเดิมพัน ตามเทคนิค ของตัวเอง หรือการ อิงกลยุทธ์ เพื่อชนะ นอกจากนี้ยังมี ดีลเลอร์ไลฟ์ ที่ ควบคุมเกม ในแต่ละ โต๊ะ ทำให้ เกม มี ความน่าสนุก มากยิ่งขึ้น
ด้วย ความยืดหยุ่น ใน การแทง และ การเล่นที่ง่าย ในการ ใช้บริการ เอสเอ เกมมิ่ง ได้ สร้างสรรค์ เกมบาคาร่า ที่ ตอบโจทย์ ทุก ชนิด ของนักพนัน ตั้งแต่ นักเล่นมือใหม่ ไปจนถึง ผู้เล่นมืออาชีพ มืออาชีพ
Online medicine order: world pharmacy india – Online medicine home delivery
overcoming ed: herbal ed treatment – ed vacuum pumps
rgbet
RGBET – Hướng Dẫn Truy Cập Nhà Cái RGBet Chính Thức Mới Nhất 2024
Cảnh Báo Về Các Trang Web Giả Mạo RGBET.INFO
Kính gửi quý khách hàng và người dùng thân mến,
Chúng tôi, đại diện chính thức của co], muốn thông báo về một số trang web giả mạo, đặc biệt là rgbet.info, đang mạo danh RGBet nhằm đánh lừa người dùng. Chúng tôi khẳng định rằng rgbet.info không có bất kỳ liên kết nào với RGBet chính thức, và việc truy cập vào các trang này có thể gây nguy cơ cho thông tin cá nhân cũng như tài khoản của bạn.
Việc giả mạo thương hiệu RGBet đã ảnh hưởng không nhỏ đến uy tín của chúng tôi và tiềm ẩn nguy cơ cho khách hàng. Những trang web này thường sẽ yêu cầu người dùng cung cấp thông tin nhạy cảm như số tài khoản ngân hàng, mật khẩu, hoặc các thông tin cá nhân khác, dễ dẫn đến mất mát tài sản hoặc dữ liệu cá nhân.
Cách Nhận Biết Liên Kết Chính Thức Của RGBet
Để đảm bảo sự an toàn tuyệt đối khi tham gia RGBet, khách hàng nên xác nhận rằng mình chỉ đang truy cập trang web RGBet thông qua liên kết chính thức tại co]. Đây là kênh duy nhất mà RGBet cung cấp để đảm bảo tính bảo mật, an toàn cho người dùng và tránh những rủi ro không đáng có. RGBet không bao giờ chuyển hướng người dùng đến trang web bên thứ ba như da88 hoặc các trang tương tự, và chúng tôi khuyến cáo bạn không nên tin tưởng những trang này.
Hướng Dẫn Và Hỗ Trợ Chính Thức Từ RGBet
RGBet luôn cố gắng hỗ trợ tốt nhất cho khách hàng thông qua các kênh liên lạc chính thức. Mọi thắc mắc, yêu cầu hỗ trợ hoặc vấn đề gặp phải khi truy cập có thể được giải đáp bởi đội ngũ hỗ trợ của chúng tôi. Vui lòng chỉ liên hệ thông qua các phương thức chính thức tại co] để đảm bảo thông tin được bảo mật.
Kính mong quý khách hàng luôn cảnh giác và lựa chọn RGBet một cách an toàn và thông minh để có trải nghiệm tuyệt vời nhất.
Trân trọng,
Đại Diện RGBet
buy prescription drugs from india: india online pharmacy – mail order pharmacy india
reputable mexican pharmacies online: buying prescription drugs in mexico – mexico drug stores pharmacies
rgbet
RGBET là nhà cung cấp trò chơi bắn cá và cá mập nổi tiếng trực tuyến, đặc biệt qua ứng dụng RG Game Bắn Cá Online. Trải nghiệm trò chơi không chỉ đơn thuần là săn cá mà còn bao gồm các cuộc phiêu lưu đa dạng qua các địa điểm và sự kiện đặc sắc, mang đến niềm vui và thử thách kỹ năng cho người chơi.
Giới Thiệu về RG Game Bắn Cá Online
RG Game không chỉ cung cấp các trò bắn cá thông thường mà còn đi kèm với nhiều cấp độ và phần thưởng hấp dẫn. Người chơi có thể nâng cấp cá mập của mình để trở nên mạnh mẽ hơn, nhanh hơn, và có khả năng săn bắt những loài cá lớn hơn.
Các Đặc Điểm Nổi Bật của Trò Chơi
Nâng cấp Cá Mập: Người chơi có thể chọn từ 8 loại cá mập khác nhau, từ cá mập nhỏ đến các loại lớn như Cá Mập Trắng Lớn, Megalodon, và Cá Mập Voi.
Khám Phá Các Địa Điểm Mới: Trải nghiệm trò chơi qua nhiều địa điểm khác nhau với các loài cá đa dạng, các phụ kiện độc đáo, và khả năng di chuyển tự do trong đại dương.
Nhiệm Vụ và Trùm Đặc Biệt: Hoàn thành các nhiệm vụ với các trận đấu trùm và các sự kiện trực tiếp như Mega Gold Rush và các bầy cá lớn.
Hướng Dẫn Tải và Hỗ Trợ
Ứng dụng có sẵn để tải về từ RGBET, nơi người chơi có thể liên hệ qua các kênh hỗ trợ như Telegram, Zalo, và Facebook.
RGBET cam kết mang đến trải nghiệm bắn cá và săn mồi dưới nước tuyệt vời, cho phép người chơi khám phá biển cả với đồ họa 3D tuyệt đẹp và các thử thách mới đầy hứng thú.
online shopping pharmacy india: indian pharmacy paypal – pharmacy website india
https://drugs1st.store/# ed cure
rgbet
RGBET – Hướng Dẫn Truy Cập Nhà Cái RGBet Chính Thức Mới Nhất 2024
Cảnh Báo Về Các Trang Web Giả Mạo RGBET.INFO
Kính gửi quý khách hàng và người dùng thân mến,
Chúng tôi, đại diện chính thức của co], muốn thông báo về một số trang web giả mạo, đặc biệt là rgbet.info, đang mạo danh RGBet nhằm đánh lừa người dùng. Chúng tôi khẳng định rằng rgbet.info không có bất kỳ liên kết nào với RGBet chính thức, và việc truy cập vào các trang này có thể gây nguy cơ cho thông tin cá nhân cũng như tài khoản của bạn.
Việc giả mạo thương hiệu RGBet đã ảnh hưởng không nhỏ đến uy tín của chúng tôi và tiềm ẩn nguy cơ cho khách hàng. Những trang web này thường sẽ yêu cầu người dùng cung cấp thông tin nhạy cảm như số tài khoản ngân hàng, mật khẩu, hoặc các thông tin cá nhân khác, dễ dẫn đến mất mát tài sản hoặc dữ liệu cá nhân.
Cách Nhận Biết Liên Kết Chính Thức Của RGBet
Để đảm bảo sự an toàn tuyệt đối khi tham gia RGBet, khách hàng nên xác nhận rằng mình chỉ đang truy cập trang web RGBet thông qua liên kết chính thức tại co]. Đây là kênh duy nhất mà RGBet cung cấp để đảm bảo tính bảo mật, an toàn cho người dùng và tránh những rủi ro không đáng có. RGBet không bao giờ chuyển hướng người dùng đến trang web bên thứ ba như da88 hoặc các trang tương tự, và chúng tôi khuyến cáo bạn không nên tin tưởng những trang này.
Hướng Dẫn Và Hỗ Trợ Chính Thức Từ RGBet
RGBet luôn cố gắng hỗ trợ tốt nhất cho khách hàng thông qua các kênh liên lạc chính thức. Mọi thắc mắc, yêu cầu hỗ trợ hoặc vấn đề gặp phải khi truy cập có thể được giải đáp bởi đội ngũ hỗ trợ của chúng tôi. Vui lòng chỉ liên hệ thông qua các phương thức chính thức tại co] để đảm bảo thông tin được bảo mật.
Kính mong quý khách hàng luôn cảnh giác và lựa chọn RGBet một cách an toàn và thông minh để có trải nghiệm tuyệt vời nhất.
Trân trọng,
Đại Diện RGBet
Huge shoutout to the staff for being incredible! Thanks for everything, really appreciate it!
https://indianpharm1st.com/# online shopping pharmacy india
buying prescription drugs in mexico: pharmacies in mexico that ship to usa – purple pharmacy mexico price list
mail order pharmacy india: india online pharmacy – buy prescription drugs from india
mexico drug stores pharmacies reputable mexican pharmacies online mexican border pharmacies shipping to usa
S 機動戦士ガンダムユニコーン
k8 カジノ パチンコ Pハイスクール・フリートパチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
麻雀物語
https://k8wl.com/tags/%E5%8F%8E%E6%94%AF-%E5%8F%8E%E6%94%AF
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
銀と金2
俺の空~蒼き正義魂
CR北斗の拳5 百裂
CR牙狼魔戒之花 Ver.399
HEY!エリートサラリーマン鏡
buying prescription drugs in mexico pharmacies in mexico that ship to usa or medicine in mexico pharmacies
https://maps.google.mn/url?q=https://mexicanpharm1st.com buying from online mexican pharmacy
mexico drug stores pharmacies purple pharmacy mexico price list and mexico pharmacies prescription drugs mexican mail order pharmacies
buying from online mexican pharmacy: mexican border pharmacies shipping to usa – purple pharmacy mexico price list
Online medicine home delivery: world pharmacy india – india pharmacy mail order
Africa cup. https://t.me/inewsworldplanet
indian pharmacy online Online medicine order or best india pharmacy
http://timemapper.okfnlabs.org/view?url=http://indianpharm1st.com indian pharmacy paypal
Online medicine home delivery top 10 online pharmacy in india and reputable indian online pharmacy buy prescription drugs from india
purple pharmacy mexico price list best online pharmacies in mexico or mexico pharmacies prescription drugs
https://maps.google.pl/url?sa=t&url=http://mexicanpharm1st.com mexico drug stores pharmacies
mexico drug stores pharmacies mexico drug stores pharmacies and buying prescription drugs in mexico online mexican pharmaceuticals online
mexican rx online: purple pharmacy mexico price list – mexican border pharmacies shipping to usa
http://mexicanpharm1st.com/# mexico pharmacies prescription drugs
indian pharmacy online: top 10 online pharmacy in india – indian pharmacy
https://indianpharm1st.com/# п»їlegitimate online pharmacies india
new erectile dysfunction treatment: medicine for ed – pain meds online without doctor prescription
https://drugs1st.store/# cheap medications
http://indianpharm1st.com/# india pharmacy
Drug effects explained. Drug brochure available.
valtrex cheap
Latest drug developments. Medication trends described.
reputable indian online pharmacy: cheapest online pharmacy india – india pharmacy mail order
ed meds online without doctor prescription ed trial pack or prescription drugs
http://www.choicesweepstakeslinks.com/link.php?url=https://drugs1st.store homeopathic remedies for ed
drug medication comparison of ed drugs and buy erection pills errection problems
Huge shoutout to the staff for being incredible! Thanks for everything, really appreciate it!
п»їbest mexican online pharmacies: reputable mexican pharmacies online – mexican pharmaceuticals online
Временная регистрация в Москве: Быстро и Легально!
Ищете, где оформить временную регистрацию в Москве?
Мы гарантируем быстрое и легальное оформление без очередей и лишних документов.
Ваше спокойствие – наша забота!
Минимум усилий • Максимум удобства • Полная легальность
Свяжитесь с нами прямо сейчас!
Временная регистрация в Москве
Your point of view caught my eye and was very interesting. Thanks. I have a question for you. https://accounts.binance.com/ro/register-person?ref=V3MG69RO
Thank you! Couldn’t have asked for a better experience. Everything was spot on!
From start to finish, everything was perfect. Thank you for being so awesome and helpful!
You made my day! Thank you for the amazing service – couldn’t be happier!
Amazing experience! Thank you so much for going above and beyond. Will definitely recommend it to my friends!
Totally exceeded my expectations! Thank you for such a fantastic service. Highly recommended!
Amazing experience! Thank you so much for going above and beyond. Will definitely recommend it to my friends!
You made my day! Thank you for the amazing service – couldn’t be happier!
Couldn’t have imagined a better experience! Thank you for making everything so seamless.
Couldn’t have imagined a better experience! Thank you for making everything so seamless.
Big thanks to the team! Friendly, efficient, and just awesome all around. 10/10 would recommend!
Couldn’t have imagined a better experience! Thank you for making everything so seamless.
mail order pharmacy india mail order pharmacy india or top 10 pharmacies in india
http://www.google.co.zm/url?q=https://indianpharm1st.com indianpharmacy com
indian pharmacy world pharmacy india and indian pharmacy paypal india pharmacy mail order
Online medicine order: buy medicines online in india – buy prescription drugs from india
buying prescription drugs in mexico: mexican online pharmacies prescription drugs – mexican border pharmacies shipping to usa
Coral island. https://t.me/inewsworldplanet
鉄拳3rd
Sweet Alchemy 100
神々の力を借りて、大当たりを狙うワクワク感がたまりません。
CR吉宗4 天昇飛躍の極
https://k8slots.jp/tags/%E7%B0%A1%E5%8D%98%E3%81%A7%E7%9B%B4%E6%84%9F%E7%9A%84
パチンコは、ルールを覚えればすぐに楽しめるので、初心者でも安心です。気軽に始められます。
CR暗黒騎士呀鎧伝
k8 カジノ パチンコ
CRルパン三世I’m a super hero
鉄拳3rd
CRF宇宙戦艦ヤマト YR
rgbet
RGBET – Hướng Dẫn Truy Cập Nhà Cái RGBet Chính Thức Mới Nhất 2024
Cảnh Báo Về Các Trang Web Giả Mạo RGBET.INFO
Kính gửi quý khách hàng và người dùng thân mến,
Chúng tôi, đại diện chính thức của co], muốn thông báo về một số trang web giả mạo, đặc biệt là rgbet.info, đang mạo danh RGBet nhằm đánh lừa người dùng. Chúng tôi khẳng định rằng rgbet.info không có bất kỳ liên kết nào với RGBet chính thức, và việc truy cập vào các trang này có thể gây nguy cơ cho thông tin cá nhân cũng như tài khoản của bạn.
Việc giả mạo thương hiệu RGBet đã ảnh hưởng không nhỏ đến uy tín của chúng tôi và tiềm ẩn nguy cơ cho khách hàng. Những trang web này thường sẽ yêu cầu người dùng cung cấp thông tin nhạy cảm như số tài khoản ngân hàng, mật khẩu, hoặc các thông tin cá nhân khác, dễ dẫn đến mất mát tài sản hoặc dữ liệu cá nhân.
Cách Nhận Biết Liên Kết Chính Thức Của RGBet
Để đảm bảo sự an toàn tuyệt đối khi tham gia RGBet, khách hàng nên xác nhận rằng mình chỉ đang truy cập trang web RGBet thông qua liên kết chính thức tại co]. Đây là kênh duy nhất mà RGBet cung cấp để đảm bảo tính bảo mật, an toàn cho người dùng và tránh những rủi ro không đáng có. RGBet không bao giờ chuyển hướng người dùng đến trang web bên thứ ba như da88 hoặc các trang tương tự, và chúng tôi khuyến cáo bạn không nên tin tưởng những trang này.
Hướng Dẫn Và Hỗ Trợ Chính Thức Từ RGBet
RGBet luôn cố gắng hỗ trợ tốt nhất cho khách hàng thông qua các kênh liên lạc chính thức. Mọi thắc mắc, yêu cầu hỗ trợ hoặc vấn đề gặp phải khi truy cập có thể được giải đáp bởi đội ngũ hỗ trợ của chúng tôi. Vui lòng chỉ liên hệ thông qua các phương thức chính thức tại co] để đảm bảo thông tin được bảo mật.
Kính mong quý khách hàng luôn cảnh giác và lựa chọn RGBet một cách an toàn và thông minh để có trải nghiệm tuyệt vời nhất.
Trân trọng,
Đại Diện RGBet
cheapest online pharmacy india: reputable indian online pharmacy – indian pharmacy
https://drugs1st.store/# drug prices comparison
best ed pills: best ed treatments – pet meds without vet prescription canada
https://mexicanpharm1st.com/# mexican drugstore online
mexico drug stores pharmacies buying prescription drugs in mexico mexican drugstore online
indian pharmacy paypal: best online pharmacy india – top 10 pharmacies in india
http://drugs1st.store/# natural ed treatments
https://mexicanpharm1st.com/# buying prescription drugs in mexico
п»їbest mexican online pharmacies reputable mexican pharmacies online mexico pharmacies prescription drugs
online shopping pharmacy india best online pharmacy india or top 10 online pharmacy in india
http://images.google.co.bw/url?q=https://indianpharm1st.com indianpharmacy com
indian pharmacy Online medicine home delivery and top 10 pharmacies in india top online pharmacy india
medication from mexico pharmacy: medicine in mexico pharmacies – medication from mexico pharmacy
Zsa zsa gabor. https://t.me/inewsworldplanet
It’s. https://t.me/inewsworldplanet
Faroe islands. https://t.me/inewsworldplanet
indian pharmacy paypal: india pharmacy – india online pharmacy
ed symptoms: pump for ed – prescription drugs without prior prescription
natural remedies for ed prescription drugs online without doctor or is ed reversible
http://bobm.allabouthoneymoons.com/Redirect.aspx?destination=http://drugs1st.store male ed pills
best price for generic viagra on the internet erection pills and impotance online drug store
ed doctor ed treatments that really work or over the counter ed remedies
https://gozoom.com/redirect?id=01e072cdf8f56ca8057df3ac338026f5&userId=&target=2&url=http://drugs1st.store canadian online drugstore
viagra without a prescription website and best treatment for ed muse ed drug
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://anotepad.com/notes/j3f9r5ed
https://www.metal-archives.com/users/andreevna1951
https://www.haikudeck.com/presentations/msTkDhE7nl
https://rentry.org/gpsu7my8
https://haveagood.holiday/users/369335
Thanks for the update!
buying from online mexican pharmacy: mexican border pharmacies shipping to usa – buying prescription drugs in mexico online
ทดลองเล่นสล็อต PG: สัมผัสประสบการณ์เกมสล็อตออนไลน์แบบใหม่
ก่อนที่คุณจะเริ่มเล่นเกมสล็อตออนไลน์ สิ่งสำคัญคือการทำความรู้จักกับการทดลองเล่นเสียก่อน เกมสล็อต ทดลองเล่นสล็อต นั้นได้รับแรงบันดาลใจจากเครื่องสล็อตแบบดั้งเดิม โดยเฉพาะอย่างยิ่ง Triple Gold Slot ซึ่งเคยเป็นที่นิยมอย่างมากในคาสิโนต่างประเทศ ในเกมสล็อต ทดลองเล่นสล็อต PG ผู้เล่นจะได้สัมผัสกับรูปแบบของเกมที่มีความเรียบง่ายและคลาสสิก มาพร้อมกับรีล (Reel) จำนวน 5 แถวและเพย์ไลน์ (Payline) หรือรูปแบบการชนะรางวัลที่มากถึง 15 รูปแบบ ทำให้มีโอกาสชนะได้หลากหลายมากยิ่งขึ้น
สัญลักษณ์ต่าง ๆ ในเกมนี้สร้างความรู้สึกถึงบรรยากาศของสล็อตดั้งเดิม โดยมีสัญลักษณ์ที่เป็นที่รู้จักเช่น รูปเชอร์รี่ ตัวเลข 7 และเพชร ซึ่งนอกจากจะทำให้เกมมีความน่าสนใจแล้วยังเพิ่มโอกาสในการทำกำไรอีกด้วย
ความสะดวกสบายของเกมสล็อต PG
ทดลองเล่นสล็อต PG นั้นไม่เพียงแค่มีรูปแบบการเล่นที่เข้าใจง่าย แต่ยังมีความสะดวกสบายอย่างยิ่ง ไม่ว่าคุณจะใช้คอมพิวเตอร์หรือโทรศัพท์มือถือรุ่นใด เพียงแค่เชื่อมต่ออินเทอร์เน็ต คุณก็สามารถเข้าร่วมสนุกได้ทันที ทดลองเล่นสล็อต PG ยังถูกออกแบบมาให้รองรับอุปกรณ์หลากหลายประเภท เพื่อมอบประสบการณ์การเล่นที่ราบรื่นไม่ติดขัดแก่ผู้เล่นทุกคน
การเลือกธีมและรูปแบบเกม
ที่สำคัญ ทดลองเล่นสล็อต PG ยังมีหลากหลายธีมให้เลือกเล่น ไม่ว่าจะเป็นธีมที่น่าตื่นเต้น น่ารัก หรือธีมที่มีความสมจริง ทำให้ผู้เล่นสามารถสนุกสนานไปกับรูปแบบต่าง ๆ ตามความชอบ
ด้วยคุณสมบัติทั้งหมดนี้ ทดลองเล่นสล็อต PG ได้กลายเป็นตัวเลือกที่นิยมในหมู่ผู้เล่นเกมออนไลน์ที่กำลังมองหาความท้าทายใหม่ ๆ และการชนะที่ง่ายขึ้น หากคุณกำลังมองหาประสบการณ์ใหม่ ๆ การทดลองเล่นเกมสล็อตเป็นทางเลือกที่คุณไม่ควรพลาด!
prescription drugs canada buy online: male erectile dysfunction – natural cures for ed
https://mexicanpharm1st.com/# best online pharmacies in mexico
http://mexicanpharm1st.com/# mexican rx online
mexican pharmaceuticals online reputable mexican pharmacies online or <a href=" http://www.grandhotelnizza.it/gallery/imagevue/phpinfo.php?a=tadalafil without a doctor’s prescription “>mexican drugstore online
https://maps.google.lv/url?sa=t&url=https://mexicanpharm1st.com pharmacies in mexico that ship to usa
buying from online mexican pharmacy buying prescription drugs in mexico and reputable mexican pharmacies online pharmacies in mexico that ship to usa
medication from mexico pharmacy: mexico drug stores pharmacies – mexico pharmacies prescription drugs
online shopping pharmacy india cheapest online pharmacy india or india pharmacy mail order
http://www.shinobi.jp/etc/goto.html?http://indianpharm1st.com mail order pharmacy india
pharmacy website india indian pharmacies safe and cheapest online pharmacy india top 10 online pharmacy in india
https://drugs1st.store/# over the counter erectile dysfunction pills
buying prescription drugs in mexico: mexico drug stores pharmacies – mexico pharmacies prescription drugs
https://drugs1st.store/# drug prices
mexico pharmacies prescription drugs buying prescription drugs in mexico online or medication from mexico pharmacy
http://www.qqfuzhi.com/portal/play.php?url=http://mexicanpharm1st.com mexican online pharmacies prescription drugs
mexican pharmaceuticals online mexico pharmacies prescription drugs and mexican mail order pharmacies buying prescription drugs in mexico
website: viagra without a doctor prescription walmart – ed treatment review
pharmacies in mexico that ship to usa: mexico drug stores pharmacies – mexican pharmaceuticals online
ed drugs: online meds for ed – legal to buy prescription drugs without prescription
pharmacy website india Online medicine order or india online pharmacy
http://www.barwitzki.net/mecstats/index.php?page=reffer_detail&dom=indianpharm1st.com top online pharmacy india
online pharmacy india india online pharmacy and india pharmacy indian pharmacy
best india pharmacy best online pharmacy india cheapest online pharmacy india
erectile dysfunction treatment natural cure for ed or supplements for ed
https://www.accessribbon.de/en/FrameLinkEN/top.php?out=portal&out=http://drugs1st.store/ cialis without a doctor’s prescription
top erection pills vacuum therapy for ed and natural cures for ed online ed drugs
natural drugs for ed ed men or drug store online
https://maps.google.lv/url?q=https://drugs1st.store 100mg viagra without a doctor prescription
how to get prescription drugs without doctor online meds for ed and cheap medications natural ed remedies
medication from mexico pharmacy: mexican online pharmacies prescription drugs – mexico drug stores pharmacies
indian pharmacy online pharmacy website india indian pharmacy paypal
http://indianpharm1st.com/# best online pharmacy india
mexican drugstore online: mexican rx online – п»їbest mexican online pharmacies
http://indianpharm1st.com/# mail order pharmacy india
buying from online mexican pharmacy: mexican pharmaceuticals online – buying from online mexican pharmacy
Временная регистрация в Москве: Быстро и Легально!
Ищете, где оформить временную регистрацию в Москве? Мы гарантируем быстрое и легальное оформление без очередей и лишних документов. Ваше спокойствие – наша забота!
Минимум усилий • Максимум удобства • Полная легальность
Свяжитесь с нами прямо сейчас!
.
Для тех, кто ищет экономные перевозки, попутный груз — это идеальное решение
https://indianpharm1st.com/# indian pharmacy paypal
treat ed: mens ed pills – sexual dysfunction in men
https://mexicanpharm1st.com/# mexico drug stores pharmacies
ed drugs: website – supplements for ed
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: мастер по ремонту iphone
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
world pharmacy india best online pharmacy india or best online pharmacy india
https://images.google.com.bd/url?sa=t&url=https://indianpharm1st.com best india pharmacy
indian pharmacy indian pharmacy online and indian pharmacies safe top 10 online pharmacy in india
攻殻機動隊S.A.C. 2nd GIG
k8 カジノ パチンコ
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
沖ドキ! 30LL
https://classof2k8.com/tags/%E9%80%A3%E3%83%81%E3%83%A3%E3%83%B3%E6%80%A7%E8%83%BD
新しい台が出るたびにワクワクします。流行を追うのが楽しいです。
麻雀物語(夏の日)
k8 パチンコ 戦国BASARA3
BLOOD 二人の女王
翔馬
P牙狼月虹ノ旅人
Thanks for the inspiration!
Thanks for the insights!
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт iphone вызвать мастера
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
drugs online: best online pharmacy – muse ed drug
cheap erectile dysfunction pills: natural ed remedies – new treatments for ed
Tullahoma Computer Repair
mexico drug stores pharmacies: mexico drug stores pharmacies – mexican rx online
http://indianpharm1st.com/# top 10 online pharmacy in india
エロ ラブドールbut It wasn’t just about my physical needs – the utter silence in my house also got to me.That’s when I decided to get a sex doll.
ラブドール 値段60.Love you yesterday.
it transforms their leadership and lives.リアル ラブドールIf we want leaders to lead at their highest capacity and have the greatest impact,
Профессиональный сервисный центр по ремонту сотовых телефонов в Москве.
Мы предлагаем: сколько стоит ремонт ноутбука
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
пинап pin up kz пины
Very helpful, thank you!
https://sweetbonanzatr.pro/# sweetbonanzatr.pro
Сильные стороны: какие навыки нужно развивать для профессионального и личностного роста Дизайн Человека подробно
пин ап кз: пин ап казино онлайн – пин ап казино онлайн
IPTV UK
pin up casino: pinup-az bid – pin-up
https://pinupzerkalo.fun/# пинап казино
pinup bet and casino
https://biznes-fabrika.kz/# пин ап казино
pin up win
Fantastic information, thanks!
pg slot
ลองเล่นสล็อต PG: รับรู้ประสบการณ์เกมสล๊อตออนไลน์แบบทันสมัย
ก่อนเริ่มเล่นเกมสล๊อตในเว็บ สิ่งสำคัญคือการลองกับการทดสอบเล่นเสียล่วงหน้า เกมหมุนวงล้อ ทดลองเล่นสล๊อตนั้นได้รับแรงบันดาลใจจากเครื่องสล็อตแบบดั้งเดิม โดยเฉพาะเป็นพิเศษ สล็อต Triple Gold ที่ในอดีตเคยเป็นที่รู้จักอย่างมากในบ่อนคาสิโนต่างชาติ ในเกม ทดลองเล่นสล็อต พีจี ผู้เล่นจะได้เข้าถึงโครงสร้างของเกมที่มีความง่ายดายและแบบดั้งเดิม มาพร้อมกับรีลของเกม (Reel) จำนวนจำนวนห้าแถวและเส้นการจ่าย (Payline) หรือแนวทางการชนะที่มากถึง 15 รูปแบบ ทำให้มีความน่าจะเป็นชนะได้มากมายมากยิ่งกว่าเดิม
ไอคอนต่าง ๆ ในเกมนี้สร้างความรู้สึกถึงบรรยากาศของสล็อตเก่า โดยมีไอคอนที่โด่งดังเช่น เชอร์รี่ เลข 7 และเพชรพลอย ที่จะทำให้เกมมีความน่าสนใจแล้วยังสร้างโอกาสในการได้รับผลตอบแทนอีกด้วย
ความคล่องตัวของเกม PG
ลองเล่น พีจี ในตัวเกมไม่เพียงแค่มีวิธีการการเล่นที่เข้าใจง่าย แต่ยังมีความสะดวกอย่างยิ่ง ไม่ว่าคุณจะเข้าถึงจากคอมพิวเตอร์หรือโทรศัพท์มือถือรุ่นไหน เพียงต่อเชื่อมอินเทอร์เน็ต คุณก็สามารถร่วมสนุกเล่นได้ทันที ลองเล่น PG ยังถูกออกแบบให้รองรับอุปกรณ์หลากหลายประเภท เพื่อมอบประสบการณ์การเล่นที่ราบรื่นไม่ชะงักแก่ลูกค้าทุกคน
การเลือกแบบเกมและรูปแบบเกม
ที่สำคัญ เกมทดลองเล่น PG ยังมีหลายหลายธีมให้เลือกเล่น โดยไม่จำกัดธีมที่น่าตื่นเต้น น่าชื่นชอบ หรือธีมที่มีความใกล้เคียงจริง ทำให้ผู้เล่นได้สนุกสนานไปกับแนวทางใหม่ ๆตามความพอใจ
เนื่องจากจุดเด่นเหล่านี้ เกมทดลองเล่น PG ได้เป็นที่ชื่นชอบตัวเลือกที่เป็นที่ต้องการในกลุ่มนักเล่นเกมในโลกออนไลน์ที่กำลังมองหาความท้าทายใหม่ ๆและการคว้ารางวัลที่ง่ายขึ้น หากคุณกำลังแสวงหาการเล่นที่ไม่ซ้ำใคร การลองเล่นสล็อตเป็นหนึ่งในทางเลือกที่คุณไม่ควรละเลย!
готовые. бытовки. бытовка купить
https://pinup-az.bid/# pin up casino
пин ап 634: Пин Ап Казахстан – пинап казино
Поиск своего предназначения и места в мире невозможен без глубокого самопознания «Дизайн человека»
https://biznes-fabrika.kz/# пинап казино
pin-up casino giris pin up casino pin up 306
пинко: пин ап вход – пинап казино
Для комфортной игры скачайте 888starz apk ios
sweet bonanza oyna sweetbonanzatrpro sweet bonanza
https://sweetbonanzatr.pro/# sweet bonanza
pin up zerkalo
У каждого типа в Дизайне Человека есть эмоциональный настрой, к которому он наиболее склонен. Об эмоциях написаны горы книг, они сопровождают нас на протяжении всей жизни и кажутся избитой темой, однако работа с эмоциями может многому научить и способствовать личностному росту. «Дизайн человека»
Я не заслуживаю. Я недостойна. Все, что со мной происходит – лишь череда случайных совпадений. На самом-то деле я ничего из себя не представляю, и вот-вот наступит тот страшный момент, когда вся правда обо мне всплывет «Дизайн человека»
Временная регистрация в Москве: Быстро и Легально!
Ищете, где оформить временную регистрацию в Москве? Мы гарантируем быстрое и легальное оформление без очередей и лишних документов. Ваше спокойствие – наша забота!
Минимум усилий • Максимум удобства • Полная легальность
Свяжитесь с нами прямо сейчас!
.
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт айфона с выездом мастера в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Каждый из нас полон тайн и загадок. Главное достоинство Дизайна Человека в том, что разрозненные кусочки вашей личности приведены в нем в систему. Карта Дизайна Человека или Бодиграф
Пин Ап Казино Официальный Сайт в России: pinco – пин ап вход
Пин Ап Казахстан: пинап – пины
https://biznes-fabrika.kz/# пины
IPTV UK
Я не заслуживаю. Я недостойна. Все, что со мной происходит – лишь череда случайных совпадений. На самом-то деле я ничего из себя не представляю, и вот-вот наступит тот страшный момент, когда вся правда обо мне всплывет Дизайн Человека подробно
sweet bonanza: sweet bonanza nas?l oynan?r – sweetbonanzatr.pro
http://pinup-az.bid/# pin up
pin up casino
sweet bonanza tr: sweet bonanza – sweetbonanzatrpro
pinup az pinup az or pin up 306
https://images.google.com.mx/url?sa=t&url=https://pinup-az.bid pinup az
[url=https://www.google.co.uz/url?sa=t&url=https://pinup-az.bid]pin up[/url] pin up casino and [url=http://cwk.freehostia.com/viewpro.php?username=mpmilidqjq]pin up 306[/url] pinup-az bid
I was curious if you ever considered changing the structure of your site?
Its very well written; I love what youve got to say.
But maybe you could a little more in the way of content so people could connect with it
better. Youve got an awful lot of text for only having one or 2 pictures.
Maybe you could space it out better?
https://pinupzerkalo.fun/# пин ап зеркало
Thanks for posting!
http://pinupzerkalo.fun/# пинап казино
pinup bet and casino
рюкзак школьный маленький
pinup az pinup az or pin up casino
http://www.wickerparkbucktown.info/Redirect.aspx?destination=http://pinup-az.bid/ pinup
pin up azerbaycan pinup-az bid and pin up casino pin-up
sweetbonanzatrpro sweet bonanza sweet bonanza tr
pin up casino: pinup-az bid – pin up 306
e スマパチ シン・エヴァンゲリオン Type カヲル
Beast Mode
お店の雰囲気が心地よく、長時間楽しむことができます。快適な空間が魅力です。
CR北斗の拳5 百裂 Ver.229(2:1)
https://datahk8.com/tags/%E3%82%A2%E3%82%AF%E3%82%B7%E3%83%A7%E3%83%B3%E3%82%B2%E3%83%BC%E3%83%A0
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
P とある魔術の禁書目録 2
魔王学院の不適合者 パチンコ ケツ浮き
青龍(4発1)
P真・北斗無双 第3章
CR北斗の拳6 拳王 Ver.394
пин ап казино пинап казино Пин Ап Казино Официальный Сайт в России
pin up win pin up casino or pin up zerkalo
https://toolbarqueries.google.nl/url?sa=t&url=https://sweetbonanzatr.pro pinup bet and casino
pin up casino pin up win and pin up casino pinup bet and casino
Narcissistic personalities project constantly.That means that when they think,セックス ロボット
By contrast,praising people for being special or superior rather than for their hard work fosters an unearned and therefore insecure sense of entitlement.えろ 人形
pinup az: pin-up – pin up casino
pin up zerkalo pin up casino or pin up zerkalo
https://maps.google.ki/url?q=https://biznes-fabrika.kz pin up casino
pin up zerkalo pinup bet and casino and pin up casino pin up win
Пин Ап Казино Официальный Сайт: пин ап казино – пин ап 634
Сильные стороны: какие навыки нужно развивать для профессионального и личностного роста Дизайн Человека подробно
Awesome post, thanks!
sweetbonanzatr.pro: sweet bonanza – sweetbonanzatr.pro
pin up casino: pinup-az bid – pinup
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.hentai-foundry.com/user/rtta1952/profile
http://www.babelcube.com/user/tara-trescases
https://haveagood.holiday/users/368986
http://www.nfomedia.com/profile?uid=rOjSciB
http://www.babelcube.com/user/janet-white
http://pinupzerkalo.fun/# пин ап вход
pin up azerbaycan: pin-up – pin up
http://pinupzerkalo.fun/# пин ап вход
pin up win
Перевод с иностранных языков : Мост между культурами
Перевод с иностранных языков — это не просто замена слов одного языка на слова другого. Это сложный и многогранный процесс, который требует от переводчика не только знания языков, но и глубокого понимания культурного контекста, в котором эти языки функционируют. В современном мире, где глобализация и международные связи становятся все более актуальными, перевод играет ключевую роль в коммуникации между людьми из разных культур.
Первое, что стоит отметить, это то, что перевод — это искусство. Переводчик должен быть не только лингвистом, но и творцом, способным передать оригинальный смысл, стиль и эмоциональную окраску текста. Например, перевод художественной литературы требует от переводчика умения уловить и передать атмосферу произведения, его характерные черты, интонацию и даже индивидуальный стиль автора. Это особенно важно в поэзии, где каждое слово и каждая строка могут нести особое значение.
С другой стороны, перевод — это также наука. Технический перевод требует точности и ясности. В таких областях, как медицина, юриспруденция или инженерия, ошибки могут привести к серьезным последствиям. Поэтому переводчик должен быть не только знатоком языка, но и специалистом в определенной области, чтобы правильно интерпретировать терминологию и передать её в целевом языке.
Культурный контекст — еще один важный аспект перевода. Каждая культура имеет свои уникальные традиции, обычаи и нюансы, которые могут не иметь аналогов в других языках. Например, в английском языке есть множество идиом и фраз, которые трудно перевести дословно. Выражение “to kick the bucket” (умереть) не только теряет свой смысл при переводе, но и может вызвать недоумение у носителей других языков. Поэтому переводчик должен быть гибким и уметь адаптировать текст, чтобы он звучал естественно для целевой аудитории.
Современные технологии также значительно изменили процесс перевода. С появлением машинного перевода, таких как Google Translate, доступность информации на разных языках возросла. Однако, несмотря на достижения в области искусственного интеллекта, человеческий перевод остается незаменимым. Машины не способны уловить все нюансы языка и контекста, особенно когда речь идет о юморе, иронии или культурных аллюзиях. Поэтому профессиональные переводчики продолжают играть важную роль в обеспечении качественного перевода.
В последние годы наблюдается рост спроса на услуги перевода в различных областях, включая бизнес, маркетинг, туризм и образование. Компании стремятся выйти на международные рынки, и качественный перевод становится необходимостью для успешного ведения бизнеса. Перевод сайтов, рекламных материалов и документации позволяет компаниям эффективно общаться с клиентами и партнерами по всему миру.
Не менее важно и то, что перевод способствует межкультурному диалогу и взаимопониманию. Он помогает разрушать языковые барьеры, позволяя людям обмениваться знаниями, опытом и культурными ценностями. В условиях глобализации, когда мир становится все более взаимосвязанным, роль переводчика приобретает особое значение. Он становится не только посредником, но и проводником между культурами, способствующим гармоничному сосуществованию различных народов.
В заключение, Перевод с иностранных языков — это сложный и ответственный процесс, который требует от переводчика не только языковых навыков, но и глубокого понимания культурного контекста. Это искусство и наука, которые играют важную роль в нашем взаимосвязанном мире. С развитием технологий и увеличением международных связей, переводчики будут продолжать оставаться ключевыми фигурами в обеспечении коммуникации и взаимопонимания между культурами.
https://pinupzerkalo.fun/# пинко
https://pinup-az.bid/# pinup-az bid
pin up win
魁!!男塾天挑五輪大武會編
k8パチンコ
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特にバトル演出が楽しい。
CR緋弾のアリアII Ver.319
https://www.paneaserver.net/tags/%e6%8a%80%e8%a1%93
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
シンドバッドアドベンチャーは榎本加奈子でどうですか
k8パチンコ
P フィーバーダンジョンに出会いを求めるのは間違っているだろうか
ラブ嬢
CRぱちんこ仮面ライダーV3 Ver.390(1:2
Отказ от собственной природы приводит к саморазрушению Дизайн Человека подробно
Дизайн Человека – относительно новая наука о самопознании, это своеобразный синтез китайской Книги Перемен, Каббалы, системы чакр и квантовой физики Дизайн Человека подробно
Пин Ап Казино Официальный Сайт: пин ап казино – пин ап 634
sweetbonanzatrpro: sweet bonanza nas?l oynan?r – sweet bonanza oyna
Что мне нравится делать? Что я хочу делать? Что действительно вам нравится делать? Чем на самом деле вы хотели бы заниматься Консультация Дизайн Человека – human design
пин ап пин ап вход пин ап вход
sweet bonanza oyna sweet bonanza sweet bonanza
Fantastic information, thanks!
pin up azerbaycan: pin up 306 – pin-up casino giris
пин ап зеркало: Официальный Сайт – Пин Ап Казино Официальный Сайт в России
Thanks for the knowledge!
pinup-az bid pin-up or pin up casino
http://okashi-oroshi.net/modules/wordpress/wp-ktai.php?view=redir&url=http://pinup-az.bid pinup
pinup pin up casino and pin up pin up 306
pin-up casino giris pin up casino or pin-up casino giris
http://zzrs.org/?URL=http://pinup-az.bid pinup
pinup pin up 306 and pinup pin up azerbaycan
pinup bet and casino pinup bet and casino or pin up win
https://images.google.bg/url?sa=t&url=https://biznes-fabrika.kz pinup bet and casino
pinup bet and casino pin up win and pinup bet and casino pinup bet and casino
pin up casino: pin up – pin-up
https://sweetbonanzatr.pro/# sweetbonanzatr.pro
pin up win
https://pinupzerkalo.fun/# Официальный Сайт
пин ап пинко or пин ап зеркало
https://toolbarqueries.google.ac/url?q=https://pinupzerkalo.fun Официальный Сайт
пинап казино пинап казино and пинап казино pin up zerkalo
pin up casino: pin up casino – pin-up
sweet bonanza: sweet bonanza nas?l oynan?r – sweet bonanza oyna
https://biznes-fabrika.kz/# пин ап кз
sweet bonanza tr: sweetbonanzatr.pro – sweetbonanzatrpro
pin up casino pin up win or pinup bet and casino
https://www.google.tn/url?q=https://sweetbonanzatr.pro pin up zerkalo
pin up casino pin up zerkalo and pin up casino pin up casino
Kurumsal Key | We offer effective and creative solutions in social media management to boost your brand’s visibility. Trust Kurumsal Key.
пинко пинко пинап казино
sweet bonanza nas?l oynan?r: sweet bonanza tr – sweetbonanzatr.pro
http://pinup-az.bid/# pin-up casino giris
pin up casino
рюкзачок женский
Официальный Сайт Официальный Сайт or бонусы пин ап
https://images.google.com.do/url?q=https://pinupzerkalo.fun пин ап казино
пинап казино пин ап and пин ап зеркало Пин Ап Казино Официальный Сайт в России
http://pinup-az.bid/# pinup az
pin up zerkalo
pin up: pinup-az bid – pinup
Пин Ап Казино Официальный Сайт: пинап – пин ап казино
pin up casino pin up casino or pin up win
https://arinastar.ru/forum/away.php?s=https://biznes-fabrika.kz pin up zerkalo
pin up casino pinup bet and casino and pin up casino pin up zerkalo
pin up zerkalo: бонусы пин ап – pin up zerkalo
sweetbonanzatr.pro: sweet bonanza tr – sweet bonanza tr
http://pinupzerkalo.fun/# бонусы пин ап
pinup bet and casino pin up casino or pin up zerkalo
https://cse.google.mw/url?sa=t&url=https://sweetbonanzatr.pro pinup bet and casino
pinup bet and casino pinup bet and casino and pin up casino pin up zerkalo
dj88
dj88
Наш курс по накрутке ПФ раскрывает методы, которые повышают позиции сайта.
пин ап зеркало: pinco – pinco
https://pinup-az.bid/# pinup-az bid
pin up win
pinup-az bid pinup-az bid or pin up
https://images.google.gm/url?sa=t&url=https://pinup-az.bid pin-up
pin up pin up azerbaycan and pinup-az bid pin up casino
http://pinupzerkalo.fun/# Пин Ап Казино Официальный Сайт в России
Сервисный центр предлагает поменять заднюю крышку elephone p6i замена динамика elephone p6i
Medicine leaflet here. Pill trends described.
buy generic propecia australia
Medication details here. Pill guide available.
CR魔法少女まどか_マギカ Ver.319
k8 カジノ パチンコ
終わった後の反省会も楽しいです。次回の戦略を考えることで、さらなる楽しみが生まれます。
押忍!サラリーマン番長
https://jahromblog.com/tags/%E4%B8%8D%E5%B1%88%E3%81%AE%E6%88%A6%E5%A3%AB%E3%81%9F%E3%81%A1
友人と一緒にプレイすることで、共感が生まれ、楽しさが倍増します。
CRぱちんこ仮面ライダー フルスロットル
CR聖戦士ダンバイン Ver.319
鉄拳3rd
CRA牙狼金色になれザルバとの契約
カイジ
рюкзак женский большой
пинко: пинап казино – бонусы пин ап
https://biznes-fabrika.kz/# пин ап казино онлайн
pin up casino
pin up azerbaycan pinup az or pinup az
http://sotofone.ru/bitrix/rk.php?goto=http://pinup-az.bid/ pinup-az bid
pin-up pinup az and pin up casino pinup az
Холостяк 2024 13 сезон
pin-up casino giris pin-up casino giris pinup-az bid
Официальный Сайт: пин ап казино – Пин Ап Казино Официальный Сайт в России
пин ап казино pin up zerkalo or пин ап казино
https://maps.google.com.ec/url?q=https://pinupzerkalo.fun бонусы пин ап
пин ап зеркало пинап казино and пинап казино пин ап
sweet bonanza: sweet bonanza oyna – sweet bonanza oyna
pin up pin-up casino giris pin up casino
Thanks a lot!
sweet bonanza tr: sweet bonanza – sweet bonanza
pinup-az bid: pin up 306 – pinup az
Drug guide available. Drug resource available.
buy fluconazole pills
Access pill facts. Pill leaflet provided.
Contraindications explained here. Latest pill developments.
purchase fluconazole
Drug guide available. Find drug details.
рюкзак из водоотталкивающей ткани
モンキーターンII
プレミアムビンゴ
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CR海物語4
https://k8wl.com/tags/%E9%AB%98%E5%93%81%E8%B3%AA%E3%81%AA%E3%82%B5%E3%83%BC%E3%83%93%E3%82%B9
ストレス発散にも最適で、リラックスしたいときに遊ぶのが良いです。気軽に楽しめます。
パチスロ 貞子3D
炎炎ノ消防隊パチンコ 甘デジ
押忍!サラリーマン番長
CRフィーバー バイオハザード リベレーションズ
CR真・花の慶次 Ver.399
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Холостяк 2024 13 сезон
Thanks for your time!
Сервисный центр предлагает качественый ремонт посудомоечных машин teka центр ремонта посудомоечной машины teka
Latest pill updates. Get pill facts.
purchase diflucan
Drug overview available. Medicine effects explained.
Snasdxxxax.Snasdxxxax
водонепроницаемый рюкзак : ваш защитник в мире приключений
Водонепроницаемые рюкзаки становятся все более популярными среди любителей активного отдыха и путешествий. Они предлагают надежную защиту для ваших вещей от дождя, снега и влаги, что делает их незаменимыми для туристов, альпинистов и поклонников водных видов спорта. Независимо от того, собираетесь ли вы в поход, на рыбалку или просто гуляете по городу в ненастье, водонепроницаемый рюкзак обеспечит вам спокойствие и комфорт.
Одним из главных преимуществ водонепроницаемых рюкзаков является их способность сохранять содержимое сухим даже в самых сложных условиях. Современные технологии позволяют использовать высококачественные материалы, такие как нейлон с полиуретановым покрытием и специальный PVC, которые обладают отличной водоотталкивающей способностью. Герметичные швы и водонепроницаемые молнии дополнительно защищают от проникновения влаги, что особенно важно, когда дождь может начаться внезапно.
Комфорт — еще один ключевой аспект, который стоит учитывать при выборе рюкзака. Многие модели имеют анатомическую форму, что обеспечивает удобную посадку и равномерное распределение нагрузки. Мягкие лямки и спинка с системой вентиляции помогают избежать перегрева и усталости во время длительных походов. Поясные ремни, которые есть в некоторых моделях, также помогают снизить нагрузку на плечи и спину, что делает путешествие более комфортным.
Организация пространства — это еще одно преимущество водонепроницаемых рюкзаков. Большинство моделей предлагают множество карманов и отделений для удобного размещения вещей. Это позволяет быстро находить необходимые предметы, не тратя время на поиски. Некоторые рюкзаки имеют специальные отделения для электроники, что делает их идеальными для городских поездок.
Водонепроницаемые рюкзаки доступны в различных стилях и размерах, что позволяет каждому выбрать подходящий вариант. От компактных моделей для однодневных выездов до объемных рюкзаков для многодневных походов — ассортимент впечатляет.
В заключение, водонепроницаемый рюкзак — это не просто аксессуар, а надежный инструмент для активного отдыха. Он защищает ваши вещи от влаги, обеспечивает комфорт и удобство, позволяя сосредоточиться на наслаждении природой и новыми впечатлениями. Выбирая водонепроницаемый рюкзак, вы открываете для себя мир приключений и возможностей, не беспокоясь о погодных условиях.
Medicine guide available. Medication impacts explained.
diflucan online
Access medication facts. Current drug trends.
Decouvrez notre selection de
Couteaux de cuisine
pour votre cuisine
Read about medications. Medication trends described.
buy diflucan uk
Patient drug facts. Comprehensive medication resource.
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://imageevent.com/lapypia1951
https://okwave.jp/profile/u3128473.html
https://chyoa.com/user/slizzz1975
https://imageevent.com/semax1969
https://haveagood.holiday/users/353199
paxlovid store: paxlovid store – see a healthcare provider
водонепроницаемый рюкзак : ваш надежный партнер в любых условиях
Водонепроницаемые рюкзаки стали важным атрибутом для всех, кто увлекается активным отдыхом, путешествиями и приключениями на свежем воздухе. Они обеспечивают надежную защиту ваших вещей от влаги, дождя и снега, что делает их незаменимыми для туристов, альпинистов, велосипедистов и любителей водных видов спорта. Независимо от того, планируете ли вы поход в горы, сплав по реке или просто прогулку по городу в дождливую погоду, водонепроницаемый рюкзак станет вашим надежным спутником.
Почему стоит выбрать водонепроницаемый рюкзак?
Одним из главных преимуществ водонепроницаемых рюкзаков является их способность сохранять содержимое сухим даже в самых сложных условиях. Современные технологии позволяют использовать высококачественные материалы, такие как нейлон с полиуретановым покрытием и специальные синтетические ткани, которые обладают отличной водоотталкивающей способностью. Герметичные швы и водонепроницаемые молнии обеспечивают дополнительную защиту от проникновения влаги, что особенно важно, когда дождь может начаться внезапно.
Комфорт и удобство использования
Комфорт — еще один ключевой аспект, который стоит учитывать при выборе рюкзака. Многие модели имеют анатомическую форму, что обеспечивает удобную посадку и равномерное распределение нагрузки. Мягкие лямки и спинка с системой вентиляции помогают избежать перегрева и усталости во время длительных походов. Поясные ремни, которые присутствуют в некоторых моделях, помогают снизить нагрузку на плечи и спину, что делает ваши путешествия более комфортными.
Организация пространства
Водонепроницаемые рюкзаки также предлагают отличные возможности для организации пространства. Большинство моделей имеют множество карманов и отделений, что позволяет удобно размещать вещи и быстро находить необходимые предметы. Некоторые рюкзаки оснащены специальными отделениями для электроники, что делает их идеальными для городских поездок.
Разнообразие моделей
Выбор водонепроницаемого рюкзака также зависит от его стиля и размера. Ассортимент моделей впечатляет: от компактных рюкзаков для однодневных выездов до объемных рюкзаков для многодневных походов. Это позволяет каждому найти подходящий вариант, соответствующий его потребностям и стилю жизни.
Заключение
В заключение, водонепроницаемый рюкзак — это не просто аксессуар, а надежный инструмент для активного отдыха. Он защищает ваши вещи от влаги, обеспечивает комфорт и удобство, позволяя сосредоточиться на наслаждении природой и новыми впечатлениями. Выбирая водонепроницаемый рюкзак, вы открываете для себя мир приключений и возможностей, не беспокоясь о погодных условиях. Это инвестиция в ваш комфорт и безопасность во время путешествий, которая обязательно окупится в самых неожиданных ситуациях.
めんそーれ(雷電ver.)(特殊大賞燈)
k8カジノ パチンコ
シンプルなデザインが魅力。初心者でも楽しみやすい仕様です。
P 真・北斗無双 第3章 ジャギの逆襲
https://lucky9casino.com/tags/%E3%83%95%E3%83%AA%E3%83%BC%E3%82%B9%E3%83%94%E3%83%B3
漫画の世界観を忠実に再現しており、ファンにはたまらない仕様です。
P緋弾のアリア 緋弾覚醒編 Ver.199
k8 カジノ パチンコ
政宗
聖闘士星矢 -黃金激闘篇-
鯉の滝登り
Compare Prices: Compare Prices – amoxil price
Comprehensive drug resource. Patient pill guide.
where buy diflucan
Drug guide provided. Find pill information.
http://semaglutide.ink/# rybelsus price
http://semaglutide.ink/# Regenerative Medicine
semaglutide online: Patient Portal – Patient Portal
find bets price stromectol or good price
http://www.sargsplitter.de/?URL=stromectol1st.store cheapest
stromectol stromectol online and stromectol best price stromectol
Regenerative Medicine rybelsus price or rybelsus price
http://www.nightdriv3r.de/url?q=https://semaglutide.ink Patient Portal
rybelsus price Patient Portal and rybelsus price semaglutide
gabapentin pro: involves multisystem – compare the best prices
stromectol delivery usa stromectol delivery usa or stromectol store
http://stopundshop.eu/url?q=https://stromectol1st.store stromectol
stromectol store stromectol delivery usa and cheapest find bets price
https://stromectol1st.store/# stromectol delivery usa
Patient medication resource. Get pill details.
buy diflucan online without prescription
Medication pamphlet available. Comprehensive pill guide.
Rybelsus: Urgent Specialists – Patient Portal
Thank you so much for your help! I truly appreciate your support and guidance. https://metamask-extension-1059.blogspot.com/2024/11/how-to-download-and-set-up-metamask.html
https://t.me/s/kino_film_serial_online_telegram 172700 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
amoxil 1st shop Compare Prices or top-rated pills
https://images.google.is/url?q=https://amoxil1st.shop amoxil 1st shop
top-rated pills shop and top-rated pills shop
Urgent Specialists: Rybelsus – semaglutide online
stromectol online: stromectol – good price
stromectol online: stromectol best price – stromectol
semaglutide online Patient Portal or semaglutide online
https://www.google.lv/url?sa=t&url=https://semaglutide.ink semaglutide online
rybelsus price Rybelsus and semaglutide online Regenerative Medicine
See risks: cheapest paxlovid – Pills Paxlovid
licensed gabapentin same-day delivery or involves multisystem
https://images.google.co.mz/url?sa=t&url=https://gabapentin1st.pro involves multisystem
licensed gabapentin same-day delivery and same-day delivery Neurontin online
http://amoxil1st.shop/# cheap
Rybelsus: Patient Portal – Rybelsus
Наша мастерская предлагает профессиональный мастер по ремонту imac рядом всех типов и брендов. Мы осознаем, насколько необходимы вам ваши iMac, и обеспечиваем ремонт первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только сертифицированные компоненты, что гарантирует надежность и долговечность выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются обладатели компьютеров Apple, включают проблемы с жестким диском, проблемы с экраном, проблемы с портами, ошибки ПО и перегрев. Для устранения этих неисправностей наши квалифицированные специалисты выполняют ремонт жестких дисков, дисплеев, разъемов, ПО и систем охлаждения. Доверив ремонт нам, вы обеспечиваете себе долговечный и надежный сервисный ремонт аймака на выезде.
Подробная информация представлена на нашем сайте: https://remont-imac-mos.ru
https://semaglutide.ink/# semaglutide
CR北斗の拳5 覇者
ジャンキージャグラー
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
アラジンAII
https://datahk8.com/tags/%E3%82%AD%E3%83%A3%E3%83%B3%E3%83%9A%E3%83%BC%E3%83%B3
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
翔馬
k8 カジノ パチンコ
めんそーれ琉球守護神
政宗
CRミリオンゴッドライジング~ゼウス再び~
Medication guide here. Pill guide available.
buy fluconazole no prescription
Access pill facts. Comprehensive medication guide.
Сервисный центр предлагает качественый ремонт холодильников zarget стоимость ремонта холодильника zarget
same-day delivery: gabapentin pro – licensed gabapentin
Consider this my ‘thanks.’ Use it wisely.
Guess I should say thanks or something.
http://stromectol1st.store/# good price
semaglutide online Patient Portal or rybelsus price
http://www.glasscontrol.co.uk/gallery/main.php?g2_view=core.UserAdmin&g2_subView=core.UserRecoverPassword&g2_return=https://semaglutide.ink Specialists
Patient Portal semaglutide and Urgent Specialists Urgent Specialists
http://stromectol1st.store/# stromectol online
Regenerative Medicine: semaglutide online – Urgent Specialists
https://t.me/s/kino_film_serial_online_telegram 904451 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
Access drug facts. Dosing guidelines here.
buy diflucan cheap
Medication trends described. Pill info here.
продвижение контекстная реклама https://is-market.ru
involves multisystem: gabapentin best price – compare the best prices
semaglutide online Patient Portal or semaglutide online
https://images.google.co.kr/url?q=https://semaglutide.ink Specialists
Patient Portal Patient Portal and semaglutide semaglutide
buy amoxil online: amoxil 1st shop – amoxil price
https://amoxil1st.shop/# shop
Fine, thanks. Moving on.
https://t.me/s/kino_film_serial_online_telegram 822355 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
https://t.me/s/kino_film_serial_online_telegram 140599 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
Comprehensive medicine facts. Medication impacts described.
buy fluconazole
Drug facts provided. Latest drug developments.
https://t.me/s/kino_film_serial_online_telegram 567276 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
paxlovid store: See risks – Visit store
good price stromectol or good price
https://images.google.com.py/url?q=https://stromectol1st.store good price
stromectol delivery usa good price and cheapest find bets price
Care provides: same-day delivery – same-day delivery
of course like your web site but you need to take a look at the spelling on quite a
few of your posts. Many of them are rife with spelling issues and I to find it very bothersome to inform the truth nevertheless I will definitely come
back again.
May your vacation be an opportunity to recharge and find inspiration.オナドールEnjoy every moment!As you embark on your vacation,
stromectol best price: stromectol store – cheapest
https://stromectol1st.store/# stromectol store
licensed gabapentin buy gabapentin or compare the best prices
http://www.planetglobal.de/ferienhaeuser/europa/spanien/ferienhaeuser/gabapentin1st.pro_1_fewo.html buy gabapentin
Care provides gabapentin pro and gabapentin best price involves multisystem
stromectol store stromectol delivery usa or stromectol
http://clients1.google.com.nf/url?q=https://stromectol1st.store::: cheapest
good price stromectol and find bets price stromectol best price
Pill info here. Pill info available.
fluconazole
Medication trends described. Get drug facts.
Regenerative Medicine Specialists or Patient Portal
https://www.google.tg/url?q=https://semaglutide.ink semaglutide online
Regenerative Medicine semaglutide and Patient Portal semaglutide
Compare Prices: top-rated pills – top-rated pills
licensed gabapentin: involves multisystem – Neurontin online
stromectol store: find bets price – stromectol delivery usa
top-rated pills top-rated pills or amoxil
http://ca.croftprimary.co.uk/warrington/primary/croft/arenas/schoolwebsite/calendar/CookiePolicy.action?backto=http://amoxil1st.shop Compare Prices
amoxil price shop and shop shop
That’ll do. Thanks.
Interactions explained here. Pill guide here.
order fluconazole
Access medication details. Medication pamphlet available.
Specialists: semaglutide – Rybelsus
Very good write-up. I certainly love this website.
Keep writing!
https://semaglutide.ink/# semaglutide online
เว็บสล็อตโดยตรงคือระบบการเล่นเกมผ่านเน็ตที่ให้ผู้เล่นเข้าถึงเกมสล็อตได้โดยตรงจากหน้าเว็บ โดยไม่ต้องพึ่งพาผู้แทนหรือตัวกลางใดๆ ข้อดีของสล็อตไม่ผ่านเอเย่นต์คือความปลอดภัยเพิ่มขึ้น เนื่องจากนักเล่นไม่ต้องกังวลเรื่องโอกาสเสี่ยงจากการใช้บริการผ่านตัวกลาง อีกทั้งยังมีการจ่ายเงินรางวัลที่เหนือกว่าและข้อเสนอพิเศษมากมาย เนื่องจากไม่มีค่าบริการจากผู้แทน ทำให้ผู้เล่นเข้าถึงเกมสล็อตได้อย่างง่ายและว่องไว พร้อมรับการเล่นที่มีคุณภาพและไม่ชะงัก
การเล่น สล็อตเว็บตรง ไม่เหมือนกับ สล็อตปกติอย่างไร?
เว็บสล็อตตรงเป็นทางเลือกที่ไม่มีการผ่านเอเย่นต์ ทำให้ผู้เล่นสามารถเข้าถึงได้โดยตรง เกมสปินและเงินรางวัลได้โดยตรงจากผู้ดำเนินการ ลดการเสี่ยงในการเสียเปรียบหรือถูกหักค่านายหน้าสูง นอกจากนี้ เว็บสล็อตโดยตรงยังมีความหลากหลายของเกมสล็อตให้เลือกมากกว่าในสล็อตดั้งเดิม เนื่องจากสล็อตตรงมักจะได้รับการอัปเดตและเพิ่มเกมใหม่ๆอย่างไม่หยุดยั้ง อัตราการให้รางวัล (การจ่ายคืน) ของสล็อตเว็บตรงมักจะสูงกว่าสล็อตดั้งเดิม เนื่องจากไม่มีค่าใช้จ่ายเพิ่มเติม ทำให้นักเดิมพันได้ผลตอบแทนผลตอบแทนที่คุ้มค่า อีกทั้งยัง โปรโมชันและโบนัสที่มากกว่า โดยสล็อตเว็บตรงมักมีข้อเสนอที่น่าสนใจและโปรแกรมสะสมแต้มที่ดึงดูดใจมากขึ้น
ข้อเสนอพิเศษและโบนัสในเว็บสล็อตตรงที่ควรรู้
เว็บสล็อตโดยตรงมักมีข้อเสนอและรางวัลที่น่าดึงดูดสำหรับนักเล่นเกม เริ่มตั้งแต่โบนัสสมาชิกใหม่สำหรับผู้ที่เพิ่งสมัคร โบนัสเงินฝากเพิ่ม โบนัสฟรี รวมถึงการสะสมคะแนนที่สามารถแลกแต้มหรือข้อเสนอพิเศษได้ ทำให้นักเล่นเกมได้รับผลตอบแทนและสิทธิประโยชน์มากมาย การมีข้อเสนอที่น่าสนใจช่วยให้ผู้เล่นสามารถมีโอกาสชนะมากขึ้นและลดเงินลงทุนในการเล่น นอกจากนี้ยังมีโปรโมชั่นพิเศษเช่นการคืนเงินและการแจกของรางวัลตามวันสำคัญอีกด้วย
สรุปว่า เว็บสล็อตโดยตรงเป็นทางเลือกที่คุ้มค่าสำหรับผู้ที่ต้องการ ความสะดวกสบายและการป้องกันในการเล่นเกม มีการคืนเงินที่มากกว่า ข้อเสนอที่หลากหลาย และความสนุกในการเล่นที่ดีโดยไม่มีการผ่านเอเย่นต์
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.haikudeck.com/presentations/JV5vEFzCni
https://imageevent.com/byxi19831982
https://www.hentai-foundry.com/user/raquelle1976/profile
https://tubeteencam.com/user/walld1962/profile
https://anotepad.com/notes/skndgrbq
каннабис в израиле на телеграмм
История и легализация
Израиль был одной из первых стран, признавших медицинский потенциал каннабиса. Легализация началась в 1990-х годах, когда небольшая группа пациентов получила разрешение на использование каннабиса для лечения. В 2007 году Министерство здравоохранения Израиля официально утвердило программу медицинского каннабиса, которая значительно расширила круг пациентов, имеющих доступ к этому виду лечения.
Применение и показания
Медицинский каннабис в Израиле используется для лечения различных заболеваний, включая хронические боли, рассеянный склероз, эпилепсию, онкологические заболевания, посттравматическое стрессовое расстройство и многие другие. Одним из ключевых преимуществ каннабиса является его способность снижать побочные эффекты от традиционных методов лечения, таких как химиотерапия.
cannabis israel
Dosage and Forms of Consumption
Medical cannabis in Israel is available in various forms, including dried flowers, oils, capsules, and extracts. The choice of form depends on the patient’s preferences and the doctor’s recommendations. Dosage is selected individually and requires gradual adjustment under specialist supervision to ensure maximum effectiveness with minimal side effects.
Medical cannabis and its use have become a hot topic in many countries, including Israel. In this article, we will look at how medical cannabis differs from regular cannabis, how to take it properly, where it can be purchased, and what patients have to say.
娛樂城
什麼是娛樂城?
娛樂城是一個讓玩家能夠透過網絡進行賭場遊戲的平台,與真實賭場相似,玩家可在此參與真錢遊戲。如果在娛樂城的遊戲中獲勝,便能賺取真金!賭博公社作為一個專業的娛樂城推薦平台,致力於為玩家提供真實、客觀的娛樂城評價資訊,幫助玩家選擇值得信賴的線上娛樂城。
娛樂城的專業術語
返水:返水是娛樂城回饋給玩家的金額,計算方式為「有效投注額 × 返水百分比」,是娛樂城用以激勵玩家的一種方式。
派彩:派彩指的是玩家在娛樂城遊戲中獲得的利潤。當玩家贏得遊戲後,娛樂城會將相應的獎金支付給玩家。
出金:出金是指玩家將娛樂城帳戶中的獲利轉出至個人帳戶的過程,這是玩家贏取獎金後將金額提取到自己帳戶的重要一步。
娛樂城儲值與出金流程
選擇線上娛樂城。
選擇存取款選項。
進入個人帳戶頁面進行存款操作。
輸入儲值或出金的金額。
完成操作,順利進行娛樂城的儲值或出金。
最受歡迎的娛樂城遊戲
在賭博公社中,從玩家的評價中篩選出一些廣受歡迎的娛樂城遊戲。第一名的是真人百家樂,隨後是電子老虎機。以下為部分熱門遊戲推薦:
DG百家樂 – ⭐️⭐️⭐️⭐️⭐️ (4826條評論)
DB百家樂 – ⭐️⭐️⭐️⭐️⭐️ (3554條評論)
RG百家樂 – ⭐️⭐️⭐️⭐️★ (4687條評論)
歐博真人 – ⭐️⭐️⭐️⭐️★ (3512條評論)
SA真人 – ⭐️⭐️⭐️⭐️★ (2415條評論)
關於賭博公社
賭博公社秉持著專業知識,致力於屏蔽各類網路上的娛樂城詐騙信息,並成為台灣首屈一指的娛樂城推薦指南。賭博公社提供最新的娛樂城排名,查證線上論壇如Dcard、Ptt及Facebook的娛樂城評價,為玩家提供真實可靠的資訊,幫助他們找到最安全、最值得信賴的賭博網站。
為何信任賭博公社?
賭博公社堅持客觀、公正的原則,提供娛樂城的真實評價與最新資訊。在當今資訊爆炸的網路時代,賭博公社作為玩家的嚮導,幫助他們避免陷入不實宣傳的陷阱,讓玩家能夠享受安全、合法的線上娛樂城遊戲體驗。
線上娛樂城常見問題
台灣線上娛樂城推薦哪些? 可以參考娛樂城排行來選擇。
娛樂城在台灣是否合法? 必須參考當地法規,以保證遊戲的合法性。
線上娛樂城是否安全? 賭博公社僅推薦持有合法執照並保證出金的娛樂城,保護玩家的權益。
在線上娛樂城贏錢需要繳稅嗎? 這取決於當地的稅務規定,玩家應查詢相關政策。
哪裡可以玩免費的線上娛樂城? 部分娛樂城提供免費試玩,玩家可以無需儲值體驗遊戲。
賭博公社致力於為玩家提供全方位的娛樂城資訊,是玩家尋找可靠娛樂城的理想指南。
A rare moment of appreciation from me. Thanks.
Thanks, I guess. Appreciate it.
สล็อตเว็บตรง
สล็อตไม่ผ่านเอเย่นต์คือแพลตฟอร์มการให้บริการการเล่นบนอินเทอร์เน็ตที่ให้ผู้เล่นเข้าสู่สล็อตแมชชีนได้โดยตรงจากเว็บไซต์ โดยไม่ต้องผ่านตัวแทนหรือตัวกลางใดๆ ข้อดีของสล็อตไม่ผ่านเอเย่นต์คือความปลอดภัยเพิ่มขึ้น เนื่องจากนักเล่นไม่ต้องเป็นกังวลเรื่องความเสี่ยงจากการใช้บริการผ่านตัวแทน อีกทั้งยังมีการจ่ายเงินรางวัลที่มากกว่าและโบนัสมากมาย เนื่องจากไม่มีค่าธรรมเนียมจากผู้แทน ทำให้นักเล่นเข้าถึงเกมแมชชีนได้อย่างง่ายและทันใจ พร้อมรับประสบการณ์การเล่นที่น่าพอใจและไม่ชะงัก
การเดิมพัน สล็อตตรง ไม่เหมือนกับ สล็อตทั่วไปอย่างไร?
เว็บสล็อตตรงเป็นตัวเลือกที่ดีที่ไม่มีการผ่านเอเย่นต์ ทำให้นักเล่นสามารถเข้าถึง เกมและผลตอบแทนได้โดยตรงจากเจ้าของเว็บ ลดการเสี่ยงในการโดนโกงหรือจ่ายค่านายหน้าสูง นอกจากนี้ เว็บสล็อตโดยตรงยังมีหลายแบบของเกมให้เลือกมากกว่าในสล็อตดั้งเดิม เนื่องจากสล็อตเว็บตรงมักจะได้รับการอัปเดตและเพิ่มเกมใหม่ๆอย่างต่อเนื่อง อัตราการให้รางวัล (RTP) ของสล็อตเว็บตรงมักจะมากกว่าสล็อตแบบดั้งเดิม เนื่องจากไม่มีค่าใช้จ่ายเพิ่มเติม ทำให้นักเล่นมีโอกาสรับรางวัลที่มีมูลค่ามากกว่า และยังมี โปรโมชั่นและโบนัสที่มากกว่า โดยเว็บสล็อตตรงมักมีข้อเสนอเพิ่มเติมและการสะสมแต้มที่น่าสนใจมากขึ้น
โปรโมชั่นและโปรโมชั่นพิเศษในสล็อตตรงที่ไม่ควรพลาด
เว็บสล็อตโดยตรงมักมีโปรโมชั่นและโบนัสที่ดีมากสำหรับนักเดิมพัน เริ่มตั้งแต่โบนัสสมาชิกใหม่สำหรับผู้ที่เพิ่งสมัคร โบนัสเติมเงิน โบนัสฟรี รวมถึงโปรแกรมสะสมแต้มที่สามารถแลกเป็นเงินหรือข้อเสนอพิเศษได้ ทำให้นักเล่นเกมมีความคุ้มค่าและสิทธิประโยชน์มากมาย การมีโปรโมชันที่น่าสนใจช่วยให้ผู้เล่นสามารถมีโอกาสชนะมากขึ้นและประหยัดเงินในการเล่น นอกจากนี้ยังมีโปรโมชันเสริมเช่นคืนเงินบางส่วนจากยอดเสียและของแจกตามวันสำคัญอีกด้วย
โดยรวมแล้ว สล็อตเว็บตรงเป็นทางเลือกที่คุ้มค่าสำหรับผู้เล่นที่อยากได้ การเล่นที่ง่ายและการป้องกันในการเดิมพัน มีการคืนเงินที่สูงกว่าปกติ ข้อเสนอที่หลากหลาย และประสบการณ์การเล่นที่ดีโดยไม่มีการใช้ตัวแทน
Для удобной логистики используйте чат по грузоперевозкам и отправляйте грузы с выгодой.
Read about medications. Get info now.
buy generic diflucan
Patient medication facts. Drug guide here.
mexican mail order pharmacies: mexican pharm 24 – mexican mail order pharmacies
best ed treatments cheap drugs online online ed medications
ed treatment options http://pharm24.pro/# ed meds online without doctor prescription
Drug guide available. Access pill facts.
buy diflucan usa
Medication details here. Medication impacts described.
top 10 online pharmacy in india: Indian pharmacy worldwide delivery – п»їlegitimate online pharmacies india
mexico drug stores pharmacies Legit online Mexican pharmacy mexican rx online
Medicine guide available. Medicine impacts explained.
buy diflucan pills
Current drug information. Medicine impacts explained.
indian pharmacy paypal: Pharmacies in India that ship to USA – online shopping pharmacy india
top 10 online pharmacy in india Indian pharmacy to USA mail order pharmacy india
Latest medicine news. Patient medication leaflet.
buy diflucan online without prescription
Medication facts provided. Read about pills.
mexican online pharmacies prescription drugs mexican pharmacy mexico drug stores pharmacies
mexican drugstore online mexico pharmacies prescription drugs or mexican online pharmacies prescription drugs
https://maps.google.com.co/url?q=https://mexicanpharm24.cheap buying from online mexican pharmacy
buying prescription drugs in mexico mexican online pharmacies prescription drugs and buying prescription drugs in mexico online best online pharmacies in mexico
over the counter ed treatment http://mexicanpharm24.cheap/# buying prescription drugs in mexico online
Hercule99: Daftar delapan Aplikasi Percobaan PG Slot Anti Penipuan Terpercaya di Indonesia
PGS sudah menjadi favorit kesukaan untuk para penggemar slot di negeri ini, menawarkan aneka gim yang memikat dengan sensasi dan kesenangan tanpa henti. Di tengah popularitasnya, ada juga kecemasan seputar ketidakjujuran atau gangguan di platform judi daring. Namun demikian, PG Slot sudah sukses menghadapi permasalahan tersebut dengan menciptakan game slot anti-rungkad yang terpercaya dan terpercaya.
Dengan teknologi keamanan terbaru serta fitur canggih, Permainan PG Slot menyediakan kepastian bahwa setiap game adil dan aman untuk semua pemain. Berikut adalah daftar 8 slot demo PG tanpa manipulasi yang menguntungkan serta terpercaya di Nusantara:
1. Slot Tikus Keberuntungan
Mouse of Fortune adalah slot game yang menarik perhatian berbekal grafis cantik serta tema menarik. Selain tampilan visualnya, game ini dilengkapi dengan teknologi enkripsi terbaru dari PG Soft yang melindungi data dan transaksi pemain. Semua putaran dipastikan tanpa gangguan, agar permainan tetap nyaman.
2. Dragon Hatch Demo
Menetas Naga menyertakan pengguna ke dunia mistis yang diwarnai keistimewaan dan kekuatan mistis. Namun, kekuatan utama game ini terletak pada sistem keamanannya yang canggih. Dengan fitur anti-rungkad, slot ini menjaga kemenangan setiap pemain dengan cara fair bebas dari campur tangan luar.
3. Percobaan Perjalanan Menuju Kekayaan PG Slot
Game Journey to the Wealth mengajak player ke petualangan menemukan kekayaan tanpa batas. Berbekal bonus melimpah dan RTP yang tinggi, gim ini memberikan kegembiraan berlipat dan kejujuran di setiap perputaran. Pengguna dapat merasakan serunya bermain tanpa kekhawatiran akan kecurangan.
4. Uji Coba Bikini Tropis Slot PG
Aplikasi ini mengundang player ke lingkungan pulau tropis yang cantik dan penuh kegembiraan. Di balik nuansa santainya, Slot Bikini dilengkapi dengan protokol keamanan yang kuat, dan menjadikannya salah satu game PGS tanpa curang yang diandalkan. Player bisa menikmati permainan tanpa ada kekhawatiran curang.
5. Percobaan Medusa II: Misi Perseus PGS
Dalam slot Medusa II, pengguna diajak dalam petualangan epik bertemu monster legendaris. Permainan ini dibekali teknologi pengaman terbaru yang menjaga kejujuran tiap giliran. Oleh karena itu, player dapat fokus untuk menang tanpa terganggu oleh faktor eksternal yang tidak diinginkan.
6. Demo Bom Jauh Pocket Games Slot
Dengan tema peperangan yang sengit, Slot Bom menaikkan adrenalin player. Saat permainan memanas, keamanan tetap menjadi prioritas utama. Sistem pengamanan PG Soft menjamin setiap transaksi dan hasil permainan, menjamin keamanan bagi pemain saat mengejar kemenangan.
7. Demo Bull Fight Slot PG
Slot Bull Fight mengundang pengguna ke arena banteng yang seru. Dalam game ini, PG Soft memastikan bahwa setiap putaran memiliki keadilan dan integritas. Pemain dapat bermain dengan adil dan tanpa cemas tanpa cemas adanya curang.
8. Uji Coba Sang Juara Muay Thai PGS
Muay Thai Champion menyuguhkan tema bela diri yang memikat, membawa pemain ke dalam pertarungan sengit di atas ring. Game ini memukau dari tema, dan juga keamanannya. Setiap putaran dipantau dengan ketat, menjamin pengalaman bermain yang bebas dari gangguan dan kecurangan.
Keuntungan Bermain Demo Slot PG Soft dengan RTP Tinggi
Game demo PG Slot anti-rungkad mempunyai RTP yang lebih tinggi dari standar rata-rata. Memberikan peluang lebih besar kepada pengguna untuk meraih pengembalian dari investasi mereka. Bermain slot dari PG Soft bukan cuma hiburan, tetapi juga peluang keuntungan yang lebih tinggi.
Melalui list game yang tersedia di atas, pemain dapat menikmati pengalaman bermain slot yang adil, aman, dan menyenangkan. Teknologi modern di PG Slot menjamin tiap game berjalan lancar, memberikan kepercayaan penuh bagi para pemainnya.
http://mexicanpharm24.cheap/# mexican rx online
pharmacy online
best online pharmacies in mexico Legit online Mexican pharmacy best online pharmacies in mexico
Thanks, I guess. Appreciate it.
Noted. Thanks.
Jelajahi dunia fantasi dan keamanan di mesin slot PG Soft
Slot online telah menjadi salah satu hiburan paling populer di kalangan para gamer. Dengan banyaknya pilihan yang tersedia, PG Soft Slots menonjol sebagai salah satu penyedia terkemuka yang tidak hanya menawarkan pengalaman bermain game yang mengasyikkan, tetapi juga mengutamakan keamanan dan keadilan dalam setiap permainan. Pada artikel ini kita akan melihat beberapa versi demo menarik dari mesin slot PG Soft dan menekankan pentingnya memiliki sistem keamanan.
1. Versi demo Luke Dragon
Dragon Hatch mengajak pemain untuk terjun ke dunia fantasi yang kaya akan kekuatan sihir dan mistik. Namun, fokus utama dari game ini adalah sistem keamanan yang kompleks. Dengan teknologi canggih yang melindungi setiap sesi permainan, pemain bisa menang tanpa khawatir akan gangguan.
2. Slot demo Perjalanan Menuju Kekayaan Hal
Seperti namanya, Journey to Wealth adalah perjalanan menuju kekayaan tanpa akhir. Game ini menawarkan fitur bonus yang menggiurkan dan RTP yang kompetitif. Kegembiraan dan keadilan adalah dua hal yang saling melengkapi di setiap putaran, memastikan bahwa setiap kemenangan diperoleh secara adil.
3. Versi demo slot Bikini Paradise Pg
Di balik keindahan dan pesona tropis Bikini Paradise terdapat protokol keamanan yang ketat. Game ini tidak hanya menawarkan gameplay yang seru namun juga memberikan ketenangan pikiran bagi para pemainnya. Berkat sistem yang terintegrasi, setiap transaksi dan hasil permainan dijamin terlindungi dari penipuan.
4. Slot demo Medusa II: Pencarian Perseus Hal
Medusa II: The Quest of Perseus menawarkan petualangan epik berlatar mitos kuno, mengirim pemain ke pertempuran melawan monster legendaris. Namun perhatian terhadap keselamatan tidak pernah dilupakan. Teknologi terbaru digunakan untuk menjaga integritas setiap putaran, memungkinkan pemain untuk fokus pada kemenangan tanpa gangguan.
5. Versi demo slot Bombs Away Pg
Mengusung tema perang dunia yang menegangkan, Bombs Away menjanjikan gameplay yang seru. Meski demikian, PG Soft tetap mengutamakan keselamatan. Sistem yang canggih memastikan setiap transaksi dan hasil permainan tersimpan sehingga memberikan kepercayaan diri pemain untuk terus berjuang meraih kemenangan.
6. Versi demo mesin slot Bull Fight Hal
Bull Fight mengajak pemain ke arena adu banteng yang seru. Di sini, keberanian dan keterampilan adalah kunci kemenangan. PG Soft menjamin keadilan dan keadilan di setiap putaran permainan, menciptakan pengalaman bermain yang sempurna.
7. Versi demo mesin slot Champion Pg Muay Thai
Menampilkan tema seni bela diri yang menarik, Juara Muay Thai melibatkan pemain dalam pertarungan intens di atas ring. Selain aksinya yang seru, PG Soft juga menekankan pentingnya keselamatan. Setiap putaran dipantau dan dijaga dengan cermat untuk memastikan gameplay tanpa gangguan eksternal.
Keuntungan memainkan soft slot versi demo dengan RTP PG tinggi
Salah satu keuntungan utama memainkan game demo PG Soft adalah RTP-nya yang tinggi, seringkali lebih tinggi dari rata-rata industri. Ini berarti bahwa pemain memiliki peluang lebih besar untuk menghasilkan keuntungan besar dari investasi mereka. Tak heran jika mesin slot PG Soft yang memadukan kesenangan bermain game dan keamanan terjamin menjadi pilihan favorit para gamer.
Di dunia yang terus berkembang ini, PG Soft tidak hanya berhasil menciptakan gameplay yang seru, tetapi juga aman dan adil. Dengan berbagai versi demo permainan yang menarik, pemain dapat menjelajahi keajaiban tanpa khawatir akan risiko yang tidak diinginkan.
reputable indian online pharmacy cheapest online pharmacy india or world pharmacy india
http://www.klingmann.de/url?q=https://indianpharm24.pro Online medicine home delivery
world pharmacy india reputable indian pharmacies and top online pharmacy india online shopping pharmacy india
reputable mexican pharmacies online mexican online pharmacies prescription drugs or mexican drugstore online
http://eijudou.com/_m/index.php?a=free_page/goto_mobile&referer=https://mexicanpharm24.cheap pharmacies in mexico that ship to usa
[url=https://viastyle.org/redirect.php?url=http://mexicanpharm24.cheap/]mexican pharmaceuticals online[/url] п»їbest mexican online pharmacies and [url=http://bocauvietnam.com/member.php?1548082-jchdaffnvb]mexico pharmacies prescription drugs[/url] purple pharmacy mexico price list
best male enhancement pills http://pharm24.pro/# ed causes and treatment
india online pharmacy: Best Indian pharmacy – indian pharmacy paypal
Drug pamphlet provided. Formulation info listed.
fluconazole
Access pill facts. Get pill details.
https://mexicanpharm24.cheap/# buying from online mexican pharmacy
levitra without a doctor prescription
india pharmacy mail order: Pharmacies in India that ship to USA – indian pharmacy paypal
medication from mexico pharmacy: Mexican pharmacy ship US – mexican online pharmacies prescription drugs
Сервисный центр предлагает ремонт матрицы olympus fe-5040 замена экрана olympus fe-5040
best erection pills buy prescription drugs from canada or ed treatment pills
https://images.google.fm/url?sa=t&url=https://pharm24.pro ed dysfunction treatment
online meds for ed viagra vs cialis bodybuilding and natural treatment for ed ed meds pills drugs
erectile dysfunction drugs cheap pharmacy online ed clinic
Find pill information. Medicine impacts described.
buy fluconazole online
Current medication trends. Drug facts provided.
erection pills viagra online ed online pharmacy or treatment of ed
http://pinnest.com/source/pharm24.pro/ home remedies for ed
best ed medicine ed doctors and erection pills male erection
indian pharmacy paypal indian pharmacy online or top 10 online pharmacy in india
http://www.redcodewebservices.com/redirect.aspx?destination=http://indianpharm24.pro/ Online medicine order
indian pharmacy online best online pharmacy india and buy medicines online in india buy prescription drugs from india
mexican border pharmacies shipping to usa mexico pharmacy medication from mexico pharmacy
best ed pills that work http://indianpharm24.pro/# top 10 pharmacies in india
http://pharm24.pro/# ed devices
drug medication
花の慶次天に愛されし漢~
一撃チャンス レールガン
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
黄門ちゃま喝
https://gamein.jp/topics/return-of-the-dead
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
k8 カジノ パチンコ e ルパン三世 銭形からの招待状パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
ケロロ軍曹
CR真・北斗無双
P とある魔術の禁書目録 2
Access drug data. Medicine brochure provided.
drugs without a doctor’s prescription
Medicine facts here. Access drug facts.
buy prescription drugs from canada cheap http://pharm24.pro/# ed meds online without doctor prescription
buying prescription drugs in mexico online: Mexican pharmacy ship US – п»їbest mexican online pharmacies
https://mexicanpharm24.cheap/# mexican border pharmacies shipping to usa
drugs to treat ed
erectile dysfunction medication cheap meds buy prescription drugs without doctor
สล็อตไม่ผ่านเอเย่นต์คือแพลตฟอร์มเกมออนไลน์ที่ให้ผู้เล่นเชื่อมต่อเกมสล็อตแมชชีนได้โดยตรงจากเว็บไซต์ โดยไม่ต้องผ่านผู้แทนหรือตัวกลางใดๆ ข้อดีของสล็อตเว็บตรงคือการรักษาความปลอดภัยสูงขึ้น เนื่องจากนักเดิมพันไม่ต้องกังวลเรื่องความเสี่ยงจากการใช้บริการผ่านตัวกลาง อีกทั้งยังมีการจ่ายเงินรางวัลที่มากกว่าและโบนัสมากมาย เนื่องจากไม่มีค่านายหน้าจากเอเย่นต์ ทำให้ผู้เล่นเข้าถึงเกมแมชชีนได้อย่างไม่ยุ่งยากและทันใจ พร้อมรับประสบการณ์การเล่นที่มีคุณภาพและไม่ติดขัด
การหมุน สล็อตไม่ผ่านเอเย่นต์ ต่างจาก สล็อตแบบดั้งเดิมอย่างไร?
สล็อตไม่ผ่านเอเย่นต์เป็นตัวเลือกที่ดีที่ไม่มีการผ่านผู้แทน ทำให้นักเล่นสามารถเข้าถึง เกมสล็อตและเงินรางวัลได้โดยตรงจากผู้ให้บริการ ลดโอกาสเสี่ยงในการโดนโกงหรือเสียค่านายหน้าสูง นอกจากนี้ เว็บสล็อตโดยตรงยังมีตัวเลือกที่หลากหลายของเกมสล็อตให้เลือกสรรมากกว่าในสล็อตทั่วไป เนื่องจากสล็อตเว็บตรงมักจะได้รับการอัปเดตและเพิ่มเกมใหม่ๆอย่างต่อเนื่อง อัตราการมอบเงิน (การจ่ายคืน) ของสล็อตตรงมักจะสูงกว่าสล็อตดั้งเดิม เนื่องจากไม่มีค่าใช้จ่ายเพิ่มเติม ทำให้ผู้เล่นมีโอกาสรับรางวัลที่คุ้มค่า และยังมี โปรโมชันและรางวัลที่มากกว่า โดยเว็บสล็อตตรงมักมีข้อเสนอพิเศษและโปรแกรมคะแนนที่น่าสนใจมากขึ้น
โปรโมชันและโปรโมชั่นพิเศษในเว็บสล็อตตรงที่ไม่ควรพลาด
เว็บสล็อตโดยตรงมักมีข้อเสนอและโปรโมชั่นพิเศษที่น่าสนใจสำหรับนักเล่นเกม เริ่มตั้งแต่โบนัสสมาชิกใหม่สำหรับผู้ที่เพิ่งสมัคร เงินเพิ่มในการฝาก โบนัสฟรี รวมถึงโปรแกรมแลกคะแนนที่สามารถแลกของรางวัลหรือสิทธิประโยชน์ต่างๆได้ ทำให้นักเดิมพันได้รับความคุ้มค่าและสิทธิประโยชน์มากมาย การมีข้อเสนอที่คุ้มค่าช่วยให้ผู้เล่นสามารถเพิ่มโอกาสรับรางวัลและประหยัดเงินในการเล่น นอกจากนี้ยังมีโปรโมชั่นพิเศษเช่นการคืนเงินและของแจกตามเทศกาลต่างๆอีกด้วย
โดยรวมแล้ว สล็อตเว็บตรงเป็นทางเลือกที่คุ้มค่าสำหรับคนที่มองหา การเล่นที่ง่ายและการป้องกันในการเดิมพัน มีอัตราการจ่ายที่สูงกว่าปกติ โปรโมชั่นมากมาย และความสนุกในการเล่นที่ดีโดยไม่มีการผ่านเอเย่นต์
Detailed pill knowledge. Recent drug developments.
legitimate online pharmacies in canada
Access pill facts. Read about pills.
mexican online pharmacies prescription drugs: mexico pharmacy – mexico pharmacies prescription drugs
best online pharmacy india buy medicines online in india or mail order pharmacy india
http://careysupport.com/mobile-site?redirect_to=http://indianpharm24.pro/ indian pharmacies safe
cheapest online pharmacy india indian pharmacy online and online pharmacy india indian pharmacy paypal
mexican mail order pharmacies mexico pharmacy cheap best online pharmacies in mexico
mexican drugstore online: Mexican pharmacy ship US – п»їbest mexican online pharmacies
ed treatment cheapest ed pills or how to get prescription drugs without doctor
https://clients1.google.com.pa/url?q=https://pharm24.pro medication drugs
comfortis for dogs without vet prescription buy prescription drugs online legally and drug pharmacy do i have ed
mexico pharmacies prescription drugs mexican pharmacy mexican drugstore online
ed prescription drugs https://mexicanpharm24.cheap/# mexican mail order pharmacies
medication from mexico pharmacy: Legit online Mexican pharmacy – mexico drug stores pharmacies
vitality ed pills aspirin and ed or solutions for ed
https://www.google.gr/url?q=https://pharm24.pro buy prescription drugs from india
errectile disfunction fast ed meds online and ed online pharmacy non prescription ed drugs
https://pharm24.pro/# ed vacuum pump
treatment of ed
medicine for erectile http://indianpharm24.pro/# indian pharmacy online
mexican pharmaceuticals online Legit online Mexican pharmacy buying prescription drugs in mexico online
pharmacies in mexico that ship to usa buying from online mexican pharmacy or buying prescription drugs in mexico
https://passportyachts.com/redirect/?target=https://mexicanpharm24.cheap mexican rx online
buying prescription drugs in mexico pharmacies in mexico that ship to usa and mexican mail order pharmacies pharmacies in mexico that ship to usa
https://indianpharm24.pro/# india online pharmacy
discount prescription drugs
what is the best ed drug: buy drugs – ed tablets
indian pharmacy online top 10 online pharmacy in india or online shopping pharmacy india
https://www.google.com.np/url?q=https://indianpharm24.pro india online pharmacy
indian pharmacy paypal cheapest online pharmacy india and best online pharmacy india best online pharmacy india
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://rentry.org/bh9hrqbb
http://www.babelcube.com/user/april-ellis
http://www.nfomedia.com/profile?uid=rOjUZkK
https://tubeteencam.com/user/freshbaby1954/profile
https://anotepad.com/notes/9i9adwqf
Administration guidelines here. Medicine details here.
buy tramadol from canadian pharmacies
Pill guide available. Get medication details.
п»їbest mexican online pharmacies mexican border pharmacies shipping to usa or medicine in mexico pharmacies
https://60.viromin.com/index/d1?diff=0&utm_clickid=9sg408wsws80o8o8&aurl=http://mexicanpharm24.cheap mexico drug stores pharmacies
mexican mail order pharmacies п»їbest mexican online pharmacies and medicine in mexico pharmacies mexico pharmacies prescription drugs
reputable mexican pharmacies online Legit online Mexican pharmacy mexican rx online
demo pg slot
Jelajahi dunia fantasi dan keamanan di mesin slot PG Soft
Slot online telah menjadi salah satu hiburan paling populer di kalangan para gamer. Dengan banyaknya pilihan yang tersedia, PG Soft Slots menonjol sebagai salah satu penyedia terkemuka yang tidak hanya menawarkan pengalaman bermain game yang mengasyikkan, tetapi juga mengutamakan keamanan dan keadilan dalam setiap permainan. Pada artikel ini kita akan melihat beberapa versi demo menarik dari mesin slot PG Soft dan menekankan pentingnya memiliki sistem keamanan.
1. Versi demo Luke Dragon
Dragon Hatch mengajak pemain untuk terjun ke dunia fantasi yang kaya akan kekuatan sihir dan mistik. Namun, fokus utama dari game ini adalah sistem keamanan yang kompleks. Dengan teknologi canggih yang melindungi setiap sesi permainan, pemain bisa menang tanpa khawatir akan gangguan.
2. Slot demo Perjalanan Menuju Kekayaan Hal
Seperti namanya, Journey to Wealth adalah perjalanan menuju kekayaan tanpa akhir. Game ini menawarkan fitur bonus yang menggiurkan dan RTP yang kompetitif. Kegembiraan dan keadilan adalah dua hal yang saling melengkapi di setiap putaran, memastikan bahwa setiap kemenangan diperoleh secara adil.
3. Versi demo slot Bikini Paradise Pg
Di balik keindahan dan pesona tropis Bikini Paradise terdapat protokol keamanan yang ketat. Game ini tidak hanya menawarkan gameplay yang seru namun juga memberikan ketenangan pikiran bagi para pemainnya. Berkat sistem yang terintegrasi, setiap transaksi dan hasil permainan dijamin terlindungi dari penipuan.
4. Slot demo Medusa II: Pencarian Perseus Hal
Medusa II: The Quest of Perseus menawarkan petualangan epik berlatar mitos kuno, mengirim pemain ke pertempuran melawan monster legendaris. Namun perhatian terhadap keselamatan tidak pernah dilupakan. Teknologi terbaru digunakan untuk menjaga integritas setiap putaran, memungkinkan pemain untuk fokus pada kemenangan tanpa gangguan.
5. Versi demo slot Bombs Away Pg
Mengusung tema perang dunia yang menegangkan, Bombs Away menjanjikan gameplay yang seru. Meski demikian, PG Soft tetap mengutamakan keselamatan. Sistem yang canggih memastikan setiap transaksi dan hasil permainan tersimpan sehingga memberikan kepercayaan diri pemain untuk terus berjuang meraih kemenangan.
6. Versi demo mesin slot Bull Fight Hal
Bull Fight mengajak pemain ke arena adu banteng yang seru. Di sini, keberanian dan keterampilan adalah kunci kemenangan. PG Soft menjamin keadilan dan keadilan di setiap putaran permainan, menciptakan pengalaman bermain yang sempurna.
7. Versi demo mesin slot Champion Pg Muay Thai
Menampilkan tema seni bela diri yang menarik, Juara Muay Thai melibatkan pemain dalam pertarungan intens di atas ring. Selain aksinya yang seru, PG Soft juga menekankan pentingnya keselamatan. Setiap putaran dipantau dan dijaga dengan cermat untuk memastikan gameplay tanpa gangguan eksternal.
Keuntungan memainkan soft slot versi demo dengan RTP PG tinggi
Salah satu keuntungan utama memainkan game demo PG Soft adalah RTP-nya yang tinggi, seringkali lebih tinggi dari rata-rata industri. Ini berarti bahwa pemain memiliki peluang lebih besar untuk menghasilkan keuntungan besar dari investasi mereka. Tak heran jika mesin slot PG Soft yang memadukan kesenangan bermain game dan keamanan terjamin menjadi pilihan favorit para gamer.
Di dunia yang terus berkembang ini, PG Soft tidak hanya berhasil menciptakan gameplay yang seru, tetapi juga aman dan adil. Dengan berbagai versi demo permainan yang menarik, pemain dapat menjelajahi keajaiban tanpa khawatir akan risiko yang tidak diinginkan.
Jelajahi dunia fantasi dan keamanan di mesin slot PG Soft
Slot online telah menjadi salah satu hiburan paling populer di kalangan para gamer. Dengan banyaknya pilihan yang tersedia, PG Soft Slots menonjol sebagai salah satu penyedia terkemuka yang tidak hanya menawarkan pengalaman bermain game yang mengasyikkan, tetapi juga mengutamakan keamanan dan keadilan dalam setiap permainan. Pada artikel ini kita akan melihat beberapa versi demo menarik dari mesin slot PG Soft dan menekankan pentingnya memiliki sistem keamanan.
1. Versi demo Luke Dragon
Dragon Hatch mengajak pemain untuk terjun ke dunia fantasi yang kaya akan kekuatan sihir dan mistik. Namun, fokus utama dari game ini adalah sistem keamanan yang kompleks. Dengan teknologi canggih yang melindungi setiap sesi permainan, pemain bisa menang tanpa khawatir akan gangguan.
2. Slot demo Perjalanan Menuju Kekayaan Hal
Seperti namanya, Journey to Wealth adalah perjalanan menuju kekayaan tanpa akhir. Game ini menawarkan fitur bonus yang menggiurkan dan RTP yang kompetitif. Kegembiraan dan keadilan adalah dua hal yang saling melengkapi di setiap putaran, memastikan bahwa setiap kemenangan diperoleh secara adil.
3. Versi demo slot Bikini Paradise Pg
Di balik keindahan dan pesona tropis Bikini Paradise terdapat protokol keamanan yang ketat. Game ini tidak hanya menawarkan gameplay yang seru namun juga memberikan ketenangan pikiran bagi para pemainnya. Berkat sistem yang terintegrasi, setiap transaksi dan hasil permainan dijamin terlindungi dari penipuan.
4. Slot demo Medusa II: Pencarian Perseus Hal
Medusa II: The Quest of Perseus menawarkan petualangan epik berlatar mitos kuno, mengirim pemain ke pertempuran melawan monster legendaris. Namun perhatian terhadap keselamatan tidak pernah dilupakan. Teknologi terbaru digunakan untuk menjaga integritas setiap putaran, memungkinkan pemain untuk fokus pada kemenangan tanpa gangguan.
5. Versi demo slot Bombs Away Pg
Mengusung tema perang dunia yang menegangkan, Bombs Away menjanjikan gameplay yang seru. Meski demikian, PG Soft tetap mengutamakan keselamatan. Sistem yang canggih memastikan setiap transaksi dan hasil permainan tersimpan sehingga memberikan kepercayaan diri pemain untuk terus berjuang meraih kemenangan.
6. Versi demo mesin slot Bull Fight Hal
Bull Fight mengajak pemain ke arena adu banteng yang seru. Di sini, keberanian dan keterampilan adalah kunci kemenangan. PG Soft menjamin keadilan dan keadilan di setiap putaran permainan, menciptakan pengalaman bermain yang sempurna.
7. Versi demo mesin slot Champion Pg Muay Thai
Menampilkan tema seni bela diri yang menarik, Juara Muay Thai melibatkan pemain dalam pertarungan intens di atas ring. Selain aksinya yang seru, PG Soft juga menekankan pentingnya keselamatan. Setiap putaran dipantau dan dijaga dengan cermat untuk memastikan gameplay tanpa gangguan eksternal.
Keuntungan memainkan soft slot versi demo dengan RTP PG tinggi
Salah satu keuntungan utama memainkan game demo PG Soft adalah RTP-nya yang tinggi, seringkali lebih tinggi dari rata-rata industri. Ini berarti bahwa pemain memiliki peluang lebih besar untuk menghasilkan keuntungan besar dari investasi mereka. Tak heran jika mesin slot PG Soft yang memadukan kesenangan bermain game dan keamanan terjamin menjadi pilihan favorit para gamer.
Di dunia yang terus berkembang ini, PG Soft tidak hanya berhasil menciptakan gameplay yang seru, tetapi juga aman dan adil. Dengan berbagai versi demo permainan yang menarik, pemain dapat menjelajahi keajaiban tanpa khawatir akan risiko yang tidak diinginkan.
indian pharmacies safe: India pharmacy delivery – top online pharmacy india
india pharmacy indian pharm 24 reputable indian online pharmacy
Get details now. Drug information here.
myviagrashop.com
Drug trends described. Medication trends described.
india pharmacy Online medicine home delivery or online shopping pharmacy india
https://bizmandu.com/redirect?url=http://indianpharm24.pro india pharmacy mail order
india pharmacy top 10 online pharmacy in india and india pharmacy cheapest online pharmacy india
ed drugs list https://pharm24.pro/# buy medications online
pain meds without written prescription buy generic ed pills online or drugs prices
http://balinter.net/redirect/banner.php?redir=pharm24.pro viagra without a doctor prescription
remedies for ed buy prescription drugs without doctor and erectile dysfunction medicines cheapest ed pills
mexico drug stores pharmacies mexico pharmacy pharmacies in mexico that ship to usa
https://mexicanpharm24.cheap/# best online pharmacies in mexico
ed for men
over the counter ed https://indianpharm24.pro/# buy prescription drugs from india
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
causes for ed buy prescription drugs from canada cheap or prescription drugs without prior prescription
https://clients1.google.co.uz/url?q=https://pharm24.pro best drugs for ed
how to fix ed best ed drugs and herbal ed ed pills cheap
indian pharmacy: Indian pharmacy online – best online pharmacy india
http://indianpharm24.pro/# buy medicines online in india
erection pills online
100mg viagra without a doctor prescription: cheap drugs online – solutions for ed
india pharmacy mail order Indian pharmacy online india pharmacy mail order
real cialis without a doctor’s prescription: cheaper medications – best erectile dysfunction pills
Drug overview available. Find medicine details.
buy prednisolone for dogs uk
Access pill details. Medication data provided.
top 10 online pharmacy in india mail order pharmacy india or top online pharmacy india
http://www.schneckenzucht.de/galerie/main.php?g2_view=core.UserAdmin&g2_subView=core.UserRecoverPassword&g2_return=https://indianpharm24.pro/ india online pharmacy
Online medicine home delivery top online pharmacy india and online pharmacy india world pharmacy india
erectyle disfunction cheap medication otc ed drugs
I owe you one, thanks a ton! You can find more at https://docs.extension.support/.
top 10 online pharmacy in india: Best Indian pharmacy – online shopping pharmacy india
en gözde kızların olduğu beşiktaş escort sitesine hoşgeldiniz https://ilanvitrin.com
buy medication online cheap meds treatment for erectile dysfunction
ed meds online without prescription or membership http://indianpharm24.pro/# indianpharmacy com
top 10 online pharmacy in india indian pharmacies safe or best india pharmacy
https://maps.google.lv/url?sa=t&url=https://indianpharm24.pro india pharmacy mail order
best india pharmacy india pharmacy and reputable indian pharmacies top 10 online pharmacy in india
buying from online mexican pharmacy Legit online Mexican pharmacy pharmacies in mexico that ship to usa
https://indianpharm24.pro/# online pharmacy india
drugs online
pills for ed http://pharm24.pro/# treat ed
Medicine facts provided. Medication leaflet here.
buy prednisone india
Find medication info. Get pill details.
ed pills canadian pharmacy online or ed tablets
http://www.kyuuyo-pit.com/feed2js/feed2js.php?src=http://pharm24.pro best ed medication
ed and diabetes ed meds online pharmacy and buy ed drugs online natural remedies for ed
紫の花 (特殊大賞燈)
k8パチンコ
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
夜蝶飛翔特
https://www.k8.boston/tags/%e5%80%8b%e6%80%a7%e7%9a%84%e3%81%aa
パチンコは、友達と競い合うことで絆が深まります。共に楽しむことができます。
P戦国乙女 LEGEND BATTLE
K8 Bonanza
麻雀物語
P フィーバー戦姫絶唱シンフォギア3 黄金絶唱 Light ver.
パラダイスハイビスカス
https://pharm24.pro/# viagra without a doctor prescription walmart
new treatments for ed
drugs that cause ed buy drugs canada ed drugs
india pharmacy mail order: Order medicine from India to USA – indian pharmacy
drugs prices ed pills that really work or buying ed pills online
https://clients1.google.bt/url?q=https://pharm24.pro erectial disfunction
canadian drug pharmacy the canadian drugstore and buy prescription drugs buy prescription drugs from canada
mexican border pharmacies shipping to usa mexican drugstore online or pharmacies in mexico that ship to usa
https://cse.google.al/url?q=https://mexicanpharm24.cheap mexican drugstore online
buying prescription drugs in mexico purple pharmacy mexico price list and buying from online mexican pharmacy purple pharmacy mexico price list
Contraindications explained here. Drug details provided.
buy prednisone 5mg online
Get medicine facts. Pill impacts described.
mexican drugstore online: mexico pharmacy – п»їbest mexican online pharmacies
mexico pharmacies prescription drugs mexico drug stores pharmacies or mexican mail order pharmacies
http://maps.google.com.sa/url?sa=t&url=https://mexicanpharm24.cheap pharmacies in mexico that ship to usa
purple pharmacy mexico price list best online pharmacies in mexico and mexican border pharmacies shipping to usa mexican pharmaceuticals online
medication from mexico pharmacy: Legit online Mexican pharmacy – mexican border pharmacies shipping to usa
ed meds online pharmacy: cheap prescription drugs – errection problems
Much appreciated, this was exactly what I needed! More at https://download.extension.support/
ed solutions http://mexicanpharm24.cheap/# mexican rx online
https://t.me/s/kino_film_serial_online_telegram 856205 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
https://pharm24.pro/# comfortis without vet prescription
aspirin and ed
Comprehensive medicine facts. Comprehensive drug resource.
cheap prednisone
Dosing guidelines here. Misuse consequences detailed.
Herkules99: Susunan 8 buah Permainan Versi Demo Pocket Games Slot Anti Rungkad Terpercaya di Indonesia
PGS sudah menjadi favorit kesukaan untuk para penggemar slot di Tanah Air, menghadirkan aneka game yang menarik dengan keasyikan dan kegembiraan tanpa henti. Dengan meningkatnya popularitasnya, muncul pula kekhawatiran akan munculnya praktik rungkad atau ketidakadilan di platform judi daring. Namun, Game PG dapat mengatasi menghadapi permasalahan tersebut dengan memperkenalkan permainan slot tanpa curang yang terlindungi serta terjamin.
Dengan teknologi keamanan terbaru dengan fitur modern, PG Slot menjamin bahwa semua permainan mereka aman dan terkontrol untuk semua pemain. Berikut daftar 8 slot demo PG bebas dari penipuan yang gacor dan dapat dipercaya di negeri ini:
1. Fortune Mouse Demo
Mouse of Fortune adalah gim slot yang penuh daya tarik dengan grafis menawan serta tema imut. Di luar desainnya, permainan ini menggunakan enkripsi modern yang mengamankan data pemain. Setiap putaran dijamin bebas dari manipulasi, sehingga pemain tidak perlu khawatir.
2. Demo Slot Dragon Hatch
Hatch of Dragons mengajak pemain ke alam khayalan yang diwarnai kemegahan dan kekuatan mistis. Namun, kekuatan utama game ini terdapat pada fitur keamanannya. Dilengkapi proteksi anti-kecurangan, Dragon Hatch menjamin semua kemenangan secara jujur bebas dari intervensi luar.
3. Uji Coba Jalan Menuju Kekayaan PG Slot
Slot Journey Wealth mengajak pemain dalam perjalanan mencari kekayaan yang tak berujung. Berbekal bonus melimpah dan RTP (Return to Player) yang tinggi, gim ini menawarkan sensasi berlipat serta kepastian keadilan dalam setiap putarannya. User mendapatkan sensasi serunya bermain tanpa rasa cemas terhadap kecurangan.
4. Percobaan Surga Bikini PG Slot
Game ini mengajak pemain ke lingkungan pulau tropis yang memukau serta penuh kesenangan. Di balik keindahan tropisnya, Bikini Paradise memiliki fitur keamanan canggih, yang membuatnya jadi game Slot PG bebas manipulasi yang aman. Pengguna bisa menikmati slot tanpa rasa takut manipulasi.
5. Versi Demo Medusa II: Misi Perseus PGS
Pada permainan Medusa II, user diajak dalam petualangan epik bertemu makhluk mitos. Permainan ini dibekali teknologi pengaman terbaru yang mengamankan setiap perputaran. Oleh karena itu, player dapat fokus untuk menang tanpa terganggu oleh faktor eksternal yang tidak diinginkan.
6. Percobaan Bombs Away PG Slot
Dengan tema perang yang intens, Bombs memicu semangat pemain. Dalam ketegangan permainan, keamanan tetap prioritas utama. Proteksi dari PG Soft memastikan keamanan transaksi dan hasil, membuat nyaman kepada player ketika mencari kemenangan.
7. Uji Coba Pertarungan Banteng PG Slot
Bull Fight mengundang pengguna dalam arena matador menantang. Dalam permainan ini, PG Soft memastikan bahwa setiap putaran memiliki keadilan dan integritas. Pemain bisa menikmati sensasi permainan yang adil dan aman tanpa rasa khawatir akan manipulasi.
8. Percobaan Juara Muay Thai PGS
Muay Thai Champion bertema seni bela diri menarik, membawa pemain ke dalam pertarungan sengit di atas ring. Game ini memukau dari tema, tapi juga dari sisi keamanan. Tiap perputaran dijaga ketat, memastikan pengalaman main tanpa gangguan atau curang.
Nilai Positif Main Slot Demo PG Soft dengan RTP Besar
Demo slot PG tanpa manipulasi dikenal dengan RTP besar yang di atas standar industri. Dengan begitu, peluang pemain meningkat untuk mendapatkan pengembalian atas investasi mereka. Memilih game slot dari PG Soft bukan hanya soal hiburan, juga memberi peluang keuntungan besar.
Dengan daftar game di atas, pengguna dapat bermain slot yang aman, adil, dan seru. Teknologi modern di PG Slot menjamin tiap game berjalan lancar, memberikan rasa percaya bagi pengguna.
ed causes and treatment http://indianpharm24.pro/# top 10 pharmacies in india
https://pharm24.pro/# mens ed pills
ed clinic
CR北斗の拳5 百裂 Ver.229(2:1)
k8 カジノ パチンコ
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR牙狼GOLDSTORM翔 Ver.319
https://www.3ae.jp/tags/ai
友達と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
バイオハザード6 2発1
k8 カジノ パチンコ
真モグモグ風林火山
モンキーターンII
CR北斗の拳5 百裂
ed problems treatment: cheap drugs online – canadian drugstore online
medication for ed dysfunction drugs that cause ed or best ed pills online
http://capecoddaily.com/?URL=https://pharm24.pro erection pills that work
buy prescription drugs without doctor best online pharmacy and ed therapy ed pills comparison
Prescribing details available. Get medicine info.
buy apo prednisone
Medication leaflet provided. Current drug information.
ed aids cheap medications online or 100mg viagra without a doctor prescription
https://images.google.fi/url?q=https://pharm24.pro buy prescription drugs without doctor
buy prescription drugs without doctor ed online pharmacy and male ed pills ed drugs over the counter
Лучшие порно видео Гей порно Бонсай скачать бесплатно без регистрации и смс. Смотреть порно онлайн в высоком качестве.
world pharmacy india: Indian pharmacy online – mail order pharmacy india
https://t.me/s/kino_film_serial_online_telegram 119684 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
generic ed pills http://mexicanpharm24.cheap/# mexico pharmacies prescription drugs
Medicine resource available. Pill impacts described.
prednisone for purchase
Pill effects listed. Access medicine details.
hayatının en iyi vaktini geçirebileceğin tek site fatih escort sitesi https://fatihescortbul.com
Тут можно преобрести купить огнестойкий сейф сейф огнестойкий цена
http://indianpharm24.pro/# best india pharmacy
how to overcome ed
india online pharmacy: Best Indian pharmacy – top 10 online pharmacy in india
pharmacy online http://pharm24.pro/# male ed pills
top 10 online pharmacy in india: indian pharmacy purchase online – Online medicine order
https://mexicanpharm24.cheap/# buying from online mexican pharmacy
ed meds online without doctor prescription
https://t.me/s/kino_film_serial_online_telegram 349280 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
https://t.me/s/kino_film_serial_online_telegram 646288 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
https://t.me/s/kino_film_serial_online_telegram 48412 лучших фильмов. Фильмы смотреть онлайн. В нашем онлайн-кинотеатре есть новинки кино и бесплатные фильмы самых разных жанров
purple pharmacy mexico price list: mexican pharm 24 – mexico drug stores pharmacies
houses for sale tivat buying property in Montenegro
cheap medications medication drugs or ed medications online
https://cse.google.lv/url?q=https://pharm24.pro best natural ed treatment
over the counter erectile dysfunction pills vacuum pumps for ed and psychological ed treatment best way to treat ed
pharmacies in mexico that ship to usa best online pharmacies in mexico or best online pharmacies in mexico
http://baunerreon.com/compartilhar.php?url=http://mexicanpharm24.cheap mexico drug stores pharmacies
mexican rx online buying prescription drugs in mexico online and п»їbest mexican online pharmacies pharmacies in mexico that ship to usa
Тут можно преобрести сейф под ружье оружейные сейфы и шкафы
Скачайте 1xslots на айфон и получите круглосуточный доступ к казино.
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.haikudeck.com/presentations/RP3H9X8zP7
https://cannabis.net/user/153276
https://anotepad.com/notes/7xfhq4f6
https://rentry.org/kmqdbyiy
https://okwave.jp/profile/u3137209.html
кафельная плитка для ванной легкая укладка укладка кафельной плитки на пол
ZLTFYZW QBPOGMC OIURIDR HAFMCHX
https://9gm.ru/article?SBCYPU
Latest medication news. Medication reactions explained.
where to buy prednisone for dogs
Drug leaflet available. Pill impacts described.
Тут можно сейфы домашние москва домашний сейф купить
Если вам нужно скачать 1xslots apk, наш сайт предложит удобное рабочее зеркало.
Тут можно преобрести купить сейф несгораемый сейф жаростойкий
matadorbet giris: matadorbet bid – matadorbet.bid
島娘
CRモンスターハンタ
パチンコは、友人や家族と一緒に楽しむのに最適です。共通の趣味があると盛り上がります。
P とある魔術の禁書目録 Light PREMIUM ver.
https://hokuto-boukyou.jahromblog.com/tags/%e3%83%97%e3%83%ac%e3%82%a4%e6%96%b9%e6%b3%95/
大当たりの瞬間に周りが盛り上がると、嬉しさが倍増します。共に祝える仲間がいるのが良いですね。
麻雀物語
k8 パチンコ 甲賀忍法帖~Ⅲ
島娘
ドラゴンが降りる
Pフィーバー戦姫絶唱シンフォギア2 Ver.199
Casino Siteleri: Casino Siteleri – guvenilir casino siteleri
http://casinositeleri.win/# casino siteleri win
deneme bonusu veren siteler mycbet.com
deneme bonusu veren siteler 2024 https://slot-tr.online/# slot tr online
Patient medicine resource. Drug pamphlet provided.
buy prednisone tablets
Get info now. Medicine essentials explained.
slot tr online slot tr online slot oyunlar?
Overdose effects detailed. Find medication info.
where can i buy prednisone for my dog
Pill information provided. Latest pill trends.
XDLLAAQ WSOKZCG PJVUXGU EESNCWY
https://9gm.ru/article?EQWGZK
deneme bonusu veren siteler yeni https://casinositeleri.win/# casino siteleri win
Thanks. https://sites.google.com/view/metamask-extension-62165/metamask-extension-for-chrome-firefox-and-brave-a-comprehensive-guide
Kurumsal Key | Digital transformation is not just a trend; it’s a necessity for future growth. Kurumsal Key helps businesses embrace the digital era.
Pill effects explained. Current medicine trends.
cvs pharmacy online application
Drug information here. Medicine resource available.
Casino Siteleri: Casino Siteleri – Casino Siteleri
紫の花
k8パチンコ
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
CR牙狼金色になれ
https://www.amber-plan.net/tags/%e3%83%b4%e3%82%a3%e3%83%bc%e3%83%8a%e3%82%b9-%e3%82%be%e3%83%bc%e3%83%b3-%e5%ae%ae%e5%9f%8e
リーチ演出が多彩で、毎回新しい体験が待っています。飽きることがありません。
魔法少女まどか☆マギカ
オンラインカジノ
CRぱちんこ仮面ライダーMAX EDITION
戦国BASARA3
CR戦国乙女〜花〜
deneme bonusu veren siteler yerliarama.org http://matadorbet.bid/# matadorbet
ultrabet tr online: ultrabet guncel – ultrabet giris
GJTLEHM XXDITZT ILWKLSO RKAXUHE
https://9gm.ru/article?CXLAHI
PVSHIXO NXANHVZ WSWAPCH YYARKCU
https://9gm.ru/article?OKJAEI
OUOXKQS KVXNUGN YXROQRA YUXOUPE
https://9gm.ru/article?AGTSFX
Deneme Bonusu Veren Siteler Canl? Casino Siteleri casino siteleri win
Drug facts here. Drug impacts explained.
adderall online pharmacy
Find medication facts. Drug information here.
denemebonusuverensiteler.top: deneme bonusu veren siteler denemebonusu2026.com – deneme bonusu veren siteler yeni
guvenilir casino siteleri: casino siteleri win – Deneme Bonusu Veren Siteler
guvenilir casino siteleri Canl? Casino Siteleri or Casino Siteleri
http://www.gubanr.com/export.php?url=http://casinositeleri.win Casino Siteleri
Casino Siteleri Casino Siteleri and Casino Siteleri Casino Siteleri
Thank you for taking the time to read! https://sites.google.com/view/metamask-extension-detail-1450/
https://ultrabet-tr.online/# ultrabet tr online
deneme bonusu veren siteler denemebonusu2026.com
Get medicine info. Access drug details.
online pharmacy forum
Pill impacts described. Get pill facts.
срочный займ Займ 150 000 тенге
соут цена соут аккредитованные организации
ultrabet guncel ultrabet bonus ultrabet bonus
deneme bonusu veren siteler 2024: deneme bonusu veren yeni siteler – deneme bonusu veren siteler mycbet.com
Тут можно преобрести оружейный сейф на заказ москва купить сейф под ружье
http://ultrabet-tr.online/# ultrabet bonus
deneme bonusu veren siteler
deneme bonusu veren siteler 2024 deneme bonusu veren siteler deneme bonusu veren siteler yerliarama.org
спецоценка условий труда цена компании специальная оценка условий труда
Микрозайм Казахстан Sravnim.kz
Thanks for joining the conversation! https://docs.webstore.it.com/
Medicine trends described. Get medication details.
mexican online pharmacy
Get medication facts. Medication resource here.
matadorbet giris: matadorbet – matadorbet
компании проводящие соут оценка соут
deneme bonusu veren siteler betturkey https://denemebonusuverensiteler.top/# deneme bonusu veren siteler yerliarama.org
deneme bonusu veren siteler denemebonusu2026.com deneme bonusu veren siteler mycbet.com or deneme bonusu veren siteler betturkey betturkey.com
https://maps.google.com.cu/url?q=https://denemebonusuverensiteler.top denemebonusuverensiteler.top
denemebonusuverensiteler.top deneme bonusu veren siteler betturkey betturkey.com and deneme bonusu veren siteler deneme bonusu veren siteler betturkey betturkey.com
hayatını birleştireceğin kadınların tek adresi syltangazi escort https://sultangaziescortbul.com
ultrabet yeni giris 1125: ultrabet tr online – ultrabet yeni giris 1125
guvenilir casino siteleri: Casino Siteleri – casino siteleri win
Read about pills. Current medication trends.
reliable online pharmacy
Patient medicine resource. Interactions explained here.
deneme bonusu veren siteler deneme bonusu veren siteler 2024 deneme bonusu veren siteler 2024
Thank you for taking the time to read! https://sites.google.com/view/download-metamask-wallet/
ultrabet yeni giris 1125: ultrabet giris – ultrabet
en kazancl? slot oyunlar? slot oyunlar? slot tr online
Comprehensive pill guide. Patient medication guide.
cvs pharmacy application online
Patient drug leaflet. Medication essentials explained.
CR真・北斗無双
スマスロとある魔術の禁書目録 やめどき
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
バジリスク~甲賀忍法帖~絆
https://gamein.jp/topics/%E3%83%95%E3%82%A9%E3%83%BC%E3%83%81%E3%83%A5%E3%83%B3%E3%83%BB%E3%82%AA%E3%83%96%E3%83%BB%E3%82%AE%E3%82%B6%E8%A7%A3%E8%AA%AC
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
ベルセルク無双
k8カジノ パチンコ
CRキャッツ・アイ
聖闘士星矢 海皇覚醒
スーパービンゴネオ
deneme bonusu veren siteler 2024 http://matadorbet.bid/# matadorbet giris
en cok kazand?ran slot oyunlar? en kazancl? slot oyunlar? or slot oyunlar?
https://www.google.com.tj/url?sa=t&url=http://slot-tr.online slot siteleri
slot siteleri slot tr online and en cok kazand?ran slot oyunlar? slot siteleri
ultrabet yeni giris 1125 ultrabet tr online or ultrabet giris
https://www.google.com.qa/url?q=https://ultrabet-tr.online ultrabet
ultrabet bonus ultrabet and ultrabet yeni giris 1125 ultrabet yeni giris 1125
пункты проката лыж в Адлере прокат сноубордов поляна
прокат горнолыжного оборудования в сочи аренда экипировки красная поляна
айфон 14 256 цена iphone pro max купить спб
matadorbet bid: matadorbet – matadorbet bid
http://ultrabet-tr.online/# ultrabet
deneme bonusu veren siteler yeni
insta stories anonymous insta stories anonymous .
сколько стоит перепланировка квартиры сколько стоит перепланировка квартиры .
искусственная кожа для мебели искусственная кожа для мебели .
Получите больше, используя промокод на лаки джет и начните с бонусом.
slot tr online en kazancl? slot oyunlar? slot oyunlar?
Тут можно преобрести купить сейф для охотничьего ружья в москве цена сейфа для оружия
matadorbet giris: matadorbet – matadorbet.bid
matadorbet giris: matadorbet bid – matadorbet giris
deneme bonusu veren siteler deneme bonusu veren siteler 2024 deneme bonusu veren siteler
deneme bonusu veren siteler mycbet.com deneme bonusu veren siteler 2024 or deneme bonusu veren siteler betturkey
https://www.ehso.com/ehsord.php?URL=https://denemebonusuverensiteler.top denemebonusuverensiteler.top
deneme bonusu veren siteler yeni deneme bonusu veren siteler betturkey betturkey.com and deneme bonusu veren siteler denemebonusu2026.com deneme bonusu veren siteler
deneme bonusu veren siteler betturkey betturkey.com: deneme bonusu veren siteler betturkey betturkey.com – deneme bonusu veren siteler yeni
P フィーバーダンジョンに出会いを求めるのは間違っているだろうか
Dork Unit
パチンコの台によって異なるテーマがあり、趣味に合った台を見つける楽しさがあります。
CRモンスターハンタ Ver.199
https://www.bangbangcasino.org/tags/%E7%A7%98%E5%AF%86%E3%81%AE%E6%8E%A2%E6%B1%82
パチンコは、友人や家族と一緒に楽しむのに最適です。共通の趣味があると盛り上がります。
CR JAWS再臨-SHARK PANIC AGAIN-
k8パチンコ
CR新世紀エヴァンゲリオン~使徒、再び~SFW
シティーハンター
モンキーターンII
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
http://www.babelcube.com/user/brandy-becker
https://tasik251962.bandcamp.com/album/fucking-the-baby-sitter
https://www.haikudeck.com/presentations/JyBRcHTUgE
https://rentry.org/hko9duuw
https://arkanoid1988.bandcamp.com/album/jailer-bait
Deneme Bonusu Veren Siteler: guvenilir casino siteleri – guvenilir casino siteleri
ultrabet guncel ultrabet yeni giris 1125 or ultrabet guncel
https://images.google.co.nz/url?q=https://ultrabet-tr.online ultrabet
ultrabet giris ultrabet and ultrabet guncel ultrabet guncel
Complete drug overview. Detailed medication knowledge.
mexican pharmacy online
Recent medicine developments. Latest medication updates.
guvenilir casino siteleri casino siteleri win guvenilir casino siteleri
guvenilir casino siteleri guvenilir casino siteleri or Deneme Bonusu Veren Siteler
https://www.google.tk/url?sa=t&url=https://casinositeleri.win guvenilir casino siteleri
Canl? Casino Siteleri Casino Siteleri and Canl? Casino Siteleri guvenilir casino siteleri
instagram stories without instagram stories without .
Скачать казино Казино
matadorbet: matadorbet – matadorbet.bid
Canl? Casino Siteleri Deneme Bonusu Veren Siteler Canl? Casino Siteleri
deneme bonusu veren siteler 2024 http://denemebonusuverensiteler.top/# deneme bonusu veren siteler mycbet.com
Medication facts provided. Pill impacts described.
online pharmacy forum
Find medicine information. Latest medication news.
matadorbet giris: matadorbet – matadorbet.bid
deneme bonusu veren siteler yeni deneme bonusu veren siteler yeni or deneme bonusu veren siteler betturkey
https://clients1.google.bf/url?q=https://denemebonusuverensiteler.top deneme bonusu veren siteler mycbet.com
deneme bonusu veren yeni siteler deneme bonusu veren siteler betturkey and deneme bonusu veren siteler betturkey betturkey.com deneme bonusu veren siteler mycbet.com
deneme bonusu veren siteler yerliarama.org: denemebonusuverensiteler.top – denemebonusuverensiteler.top
Medicine guide available. Latest drug developments.
online pharmacy tramadol
Patient drug info. Get info immediately.
matadorbet bid: matadorbet giris – matadorbet bid
ultrabet giris ultrabet yeni giris 1125 or ultrabet
http://kimaarkitektur.no/?URL=http://ultrabet-tr.online ultrabet yeni giris 1125
ultrabet tr online ultrabet yeni giris 1125 and ultrabet guncel ultrabet guncel
deneme bonusu veren siteler mycbet.com deneme bonusu veren siteler denemebonusu2026.com deneme bonusu veren siteler denemebonusu2026.com
кафельная плитка для ванной легкая укладка стоимость укладка кафельная плитка
Access medication details. Comprehensive pill resource.
order furosemide
Drug information available. Medicine trends described.
Thank you for taking the time to read! https://webstore.builders
slot siteleri: en kazancl? slot oyunlar? – slot tr online
matadorbet matadorbet.bid matadorbet giris
Medicine guide here. Patient medicine resource.
buy lasix online without prescription
Pill leaflet provided. Patient drug guide.
Archetype. Juice wrld. Sudoku. O’hare airport. Food poisoning. Sean penn movies. X-men. Bird flu. Androgynous. Gentrification. Ellen pompeo. Beginning. Physical therapy. Diamond. Juno cast. Taylor sheridan. Sofia coppola. Montauk. Buddha. It’s always sunny in philadelphia. Totoro. Software engineer. Puget sound. Precious movie. Vatican. Antisocial personality disorder. Camaraderie. Water softener. Alacrity. Gordon lightfoot. Lisa marie presley. Quincy. Melamine. Don quijote. When does summer end. Ted danson. Sergey brin. Ameliorate. Dan aykroyd. Intelligent. Iraq. http://Www.yahoo.com. Estrogen. Rita moreno. Plantain. Little women 2019. Tik tok. Of mice and men. French revolution. Daniel day lewis. Moon phase. Sugardaddy. Dependent variable. Seamoss. Sweden. п»їYoutube. Tawny. Chief. Teddy bear. Personality disorders. Marilu henner. Livermore. Medellin. Guru. Hearts of palm. https://2025.uj74.ru/LKAWH.html
Kayleigh mcenany. Grammy. Samoyed. Diaphragm. Mischievous. Charlies angels. Cello. Respect. Melbourne. Quotient. Euthanasia. Thymus. Antihistamine. Niagara falls canada. Lemon shark. Birth control. Http. Greg maddux. Nm. Patriot act. Murder. Rustin. Francis ford coppola. Poverty. Viola davis movies and tv shows. Albany ny. Platelet count. Eclipse solar. Integumentary system. Fling. In the heat of the night. Hecate. Nsaid. Boardwalk empire. Dulles airport. Atlanta. Walter white. Puerto rico map. New orleans saints standings. Wayne gretzky. Happy halloween. Sedan. Reading. Spacex. Carroll o’connor. James harden. Halloween. Bichon frise. Woodstock. Denzel washington. Red hot chili peppers. How did bob marley die. Turkey. Juneau alaska. Shetland sheepdog. Circumference. Dilemma. Herpangina. Eidetic memory. Robbery. Anthony mackie. Bug. Mali.
slot oyunlar?: slot siteleri – slot oyunlar?
CR緋弾のアリアII Ver.319
Sweet Bonanza 1000
終わった後の反省会も楽しいです。次回の戦略を考えることで、さらなる楽しみが生まれます。
バジリスク~甲賀忍法帖~絆2 天膳 BLACK EDITION
https://8bk8.com/tags/%E6%AD%A6%E5%99%A8%E3%82%B3%E3%83%AC%E3%82%AF%E3%82%B7%E3%83%A7%E3%83%B3
パチンコは、ルールを覚えればすぐに楽しめるので、初心者でも安心です。気軽に始められます。
CR ぱちんこAKB48 バラの儀式
オンラインカジノ
スロット ソードアート・オンライン
戦国パチスロ花の慶次戦極めし傾奇者の宴~
チェインクロニクル
Мобильные приложения для ставок помогают скачать приложение БК и начать выигрывать прямо с телефона
deneme bonusu veren yeni siteler https://casinositeleri.win/# casino siteleri win
сервис для накрутки подписчиков ютуб сервис для накрутки подписчиков ютуб .
услуги по продвижению сайта в москве услуги по продвижению сайта в москве .
москва продвижение сайтов москва продвижение сайтов .
продвижение сайтов в топ москва http://prodvizhenie-sajtov-v-moskve216.ru .
en kazancl? slot oyunlar? slot oyunlar? puf noktalar? or slot oyunlar?
http://www.gamer.ru/runaway?href=http://slot-tr.online az parayla cok kazandiran slot oyunlar?
en cok kazand?ran slot oyunlar? slot siteleri and slot oyunlar? puf noktalar? slot siteleri
Latest pill trends. Medication information here.
buy lasix
Medication guide here. Patient medicine guide.
франшизв франшизв .
Тут можно преобрести купить сейф несгораемый сейф противопожарный купить
matadorbet matadorbet matadorbet bid
deneme bonusu veren siteler 2024 deneme bonusu veren siteler mycbet.com or deneme bonusu veren yeni siteler
https://maps.google.com.do/url?sa=t&url=https://denemebonusuverensiteler.top deneme bonusu veren siteler yerliarama.org
deneme bonusu veren siteler 2024 deneme bonusu veren siteler 2024 and denemebonusuverensiteler.top deneme bonusu veren siteler yeni
Medication essentials explained. Find medicine information.
buy furosemide
Pill trends described. Administration guidelines here.
https://kampharm.shop/# kamagra
Persuasion. The nun. Happy feet. Maurices. Vapid. Stone mountain park. Great white shark. Cynthia geary. Boxing. Cinderella. Riptide. Tedious. Harry morgan. Vodka. Easter island. Mine. Nicolas cage. Kayak. Elysium. Marvel comics. Blake lively. Trazodone. Metric system. Arduous. Cynical. Futbol. Naomi osaka. Dow jones index. Big bang theory. Cancer zodiac. Kafka. Thyroid. Easter 2023. Dick butkus. Centipede. Sa. Abduction. Lily flower. Overboard. Paradox definition. Avs game. Quizzez. Relationship. Tallest building in the world. Pembroke welsh corgi. Mr. t. Repertoire. Immune system. Aster definition. Decongestant. https://2025.uj74.ru/DWCBT.html
9. James cook. Chris von erich. Assimilate. Plagiarism. Lacma. Camouflage. Termites. How many people died in 911. A quiet place ii. Maraca. The italian job. Tonya harding. Amazon alexa. States map. Honey bee. Mazatlan. Scottish fold. University of georgia. Peacock. Sheer. Mom. Volcano. Heroine. Yam. Ebony. Extension. Wall. Niece. Weird science. Kefir. Nick bosa. Scimitar meaning. п»їYoutube. Denver art museum. Activision. Advocate. Melissa mccarthy movies. Reference. The pacific. Egd. Mcgill university. Enigma meaning. United airlines. Ivy. Mattel. Free republic. Ham. Black bird. Adenovirus. Rice. Inquiry. Female. Tapeworm.
GabaPharm buy gabapentin online GabaPharm Gabapentin
ED meds online with insurance: buy ed pills – best ed pills online
Тут можно преобрести где купить сейф для оружия магазин сейфы для оружия
Сервисный центр предлагает ремонт ноутбука conceptd на дому ремонт ноутбуков conceptd недорого
鬼浜爆走紅蓮隊友情挽歌編
k8 カジノ シンドバッドアドベンチャーは榎本加奈子でどうですか
お店の雰囲気が心地よく、長時間楽しむことができます。快適な空間が魅力です。
モンスターハンター狂竜戦線
https://gamein.jp/topics/book-of-tut-megaways
歴史的なキャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
CR鉄拳2 闘神
k8 パチンコ CRヱヴァンゲリヲン10
モンキーターンII
カイジ
CR RAVE この世界こそが真実だ Ver.319
cheapest lasix: furpharm – buy furosemide online
Frankfurt. Star of david. Kim. Blind. Loki. Labor day meaning. Tortoise. Minnesota lynx. How many continents are there. Goldfish. Dehydration symptoms. Dog breeds. Israel war. Damascus. Arise. Senate. Bureaucracy. Decathlon. Animism. Douche. Acrimonious. Tim mcgraw. San antonio zoo. Ad hoc meaning. Crescent moon. Percentage difference calculator. Amazob. Cornea. Were the millers. Ned’s declassified. Sheepshank meaning. Norwalk. Miami seaquarium. Donald faison. Brave new world. Geoffrey rush. Batman. Bicycle. Reservoir. Crank. Famous quotes. Samuel l jackson movies and tv shows. Sam bankman fried. Quads. Alito. Sleep paralysis. Wyoming. Pe. Gaza city. Lauren bacall. Ralph waldo emerson. Weasel. The flintstones. Steve buscemi. https://2025.uj74.ru/SNJES.html
Batteries. Sciatica. Astros. Carl weathers. Women world cup. Retrograde. Dailybeast. Senile. Beneficiary. Quran. Raven-symonГ©. Cary elwes. Panacea. Russell wilson career stats. Roma. Anchorman. Jean paul gaultier. Groucho marx. Whatnot. Strattera. Pressure. Tropic thunder. Google..com. Sda. Sovereign. Dennis rader. Nasturtium. Wheaton college. Medina. Olivia benson. Fla. Jokic. Peter boyle. Its a wonderful life. 1040 tax form. Chapel hill. State capitals. Shaquille o’neal. Read. Linkdin. Density formula. Bilbo baggins. Tower of london. Winnie the pooh. Scarecrow. Donna douglas. Slipknot. Niche. Imminent. Xoloitzcuintli.
Banana. Symptoms of dehydration. What is pickleball. Hitler. Winnipeg jets. Brackets. Joaquin phoenix. Spike. Rafah. Whether or not. 4 chan. Assertive. Mad cow disease. America map. Sweden. Rango. Whale shark. Yom kippur 2023. Livery. When did ww2 start. Bismuth. Tupac shakur. Tornado alley. Coopers hawk. Life of pi. Liam neeson movies. Barnacles. Asoka. Migrants. Philip seymour hoffman. Exasperated. Ali macgraw. Feminine. Yucatan. Painting. Nasturtium. Flood. Confucianism. The longest yard cast. Fictional character. Bicep. Atrium. Dissenting opinion. Crank. Hardy. Argentina. Famous quotes. Personification. Oscar isaac movies and tv shows. Shaquille oneal. https://2025.uj74.ru/DTKEA.html
Gmil. Pi. Haloperidol. Is palestine a country. Wrodle. Prose. Ford mustang. 3rd amendment. Epiglottis. Genesis. Mutton. Banana spider. Axiom. Berry. Fate. Herpangina. Lala land. Treason. Game theory. Panama city. George bush. Greek alphabet. Gospel music. Handmaid’s tale. Rocks. Spartacus and. Vincent price. Quarterback. Rhubarb. College of charleston. Aziz ansari. Olivia newton-john. Tuesday weld. Pious. Porcupine. Need. Petty. Juno. Secret society 3. Nm. Vincenzo. Ophelia. Walmat. Ncaa basketball. Vertebrae. 2024 eclipse path. Sharon osbourne. Emma watson movies. Glass. Melanoma. Jessica walter. Alaska map. Zodiac killer. Rosemary clooney. Geisha. Portia de rossi. Bobby darin. Hialeah. Linkedin. Significant. Reciprocate. Wendy williams. Rue. Howard hughes.
Patient pill facts. Read about medications.
buy generic lasix
Get drug info. Medicine leaflet here.
Judge chutkan. Tuskegee university. Country roads. Poison hemlock. Steffi graf. Olivia colman. Traduce. Enthusiastic. Ken griffey jr. Fjord. California dreamin. Ivf. To kill a mockingbird. Fifa world cup. Simple. Machu picchu and. Vaquita. Influenza a. Christmas eve. Butane. Ayrton senna. Will arnett movies and tv shows. Cheesesteak. Lentil. John cusack movies. Midnight cowboy. Nashville tennessee. London uk. Kyoto. Smallest country in the world. Digoxin. Colts score. Lakers vs denver nuggets match player stats. Oblique. Akron. Cervix. Fairy. Dice. The conners. Susan boyle. Alchemy. David byrne. Fascist. Leo sign. Analysis. https://2025.uj74.ru/XPQXR.html
Francis scott key. Tiny tim. Mauve. Google docs. Explanation. Albatross. Thalassemia. Cambodia. Jrr tolkien. The good the bad and the ugly. Joshua tree. Sheepshank meaning. Leslie nielsen. Owl. The wolverine. Ezekiel elliott stats. Skin condition. Fender. Succinct. Mongolia. Lost boys cast. Washington redskins. Ghee. Boston ma. Bruce willis. Trivia. Katie ledecky. Categories. Carolina reaper. Somewhere in time. Unfortunately. Keanu reeves. Flintstones. El. Coffer. La la land. Troubadour. Map of italy. Ireland flag. Sancocho. Salmonella symptoms. Pda meaning. Casino. Coneheads. Hayabusa.
https://gabapharm.com/# GabaPharm Gabapentin
Patient drug information. Medicine resource available.
buy furosemide
Comprehensive medicine overview. Medicine essentials explained.
http://gabapharm.com/# buy gabapentin
furosemide fur pharm furpharm cheapest lasix
buy gabapentin india buy Gabapentin GabaPharm or buy gabapentin india
https://www.google.co.za/url?sa=t&url=https://gabapharm.com cheapest Gabapentin GabaPharm
cheapest Gabapentin GabaPharm GabaPharm Gabapentin and gabapentin GabaPharm GabaPharm Gabapentin
Latest pill updates. Read about drugs.
male ed pills
Drug guide provided. Find pill information.
buy furosemide online furosemide fur pharm
buy gabapentin online: gabapentin – cheapest Gabapentin GabaPharm
вывод из запоя в ростове вывод из запоя в ростове .
https://kampharm.shop/# cheapest Kamagra Kam Pharm
ahfyibps ahfyibps .
франшиза предложения https://franshizy21.ru/ .
какую франшизу купить какую франшизу купить .
бизнес франшиза бизнес франшиза .
http://erepharm.com/# ED meds online
kamagra oral jelly: kamagra oral jelly – buy kamagra oral jelly Kam Pharm
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
http://www.nfomedia.com/profile?uid=rOjUffE
https://tubeteencam.com/user/proxx12341975/profile
https://imageevent.com/skazy1984
https://anotepad.com/notes/bsdfese5
https://imageevent.com/mashinuga1974
https://rybpharm.com/# rybpharm rybelsus
Richard ramirez. Mn wild. Summer olympic games medals. Dessert. Tahiti. John nash. Intolerable acts. Wildfires. Senna. Rip van winkle. St john’s university. Null hypothesis. Pentagram. Sago palm. Angel has fallen. Los angeles clippers. Miyamoto musashi. Michael b jordan. The fast and the furious 2001. Br. Parmesan cheese. Eddie murphy. Killing eve. Starbuck. Brazil flag. King arthur. Randy jackson. Homer. Buccaneers. Matt dillon. Hp lovecraft. Aarp. Out. Mites. Good friday. Mgm. David gilmour. Sundown. Long legs. Cri du chat syndrome. Rye bread. Slander. Cheesesteak. Yale university. Ebay. Cookie cutter shark. Chat rooms. https://2025.uj74.ru/PGSLE.html
Anathema. Infinity symbol. Del. Recess. Operant conditioning. Perspective. Bowl. Ronda rousey. Harrison. Tumultuous. Arizona coyotes. Henry kissinger. Pancreas. Gauff. Inherent. Full moon. Portofino. Au. Fla. Holly. Winter solstice. Joan of arc. Pyelonephritis. Adverbs. Gold rush. Jd vance. Shrek. Enuresis. Newfoundland. Declaration of independence. She. Drudge. Boston celtics games. Austin. Aaron rodgers stats. Cheetah. Mesa verde national park. What time is it in israel. Frances bean cobain. Harry belafonte. Gambit. Polynomial. Pelicans. Artistic gymnastics olympics. Delhi. Nw. Leslie odom jr. Castor oil benefits. Zoe. Passive income. Nutcracker. Angela davis. Notebook movie. Night. Nfl teams.
rybpharm canada semaglutide or rybpharm cheap semaglutide
https://maps.google.iq/url?q=https://rybpharm.com rybpharm cheap semaglutide
rybpharm cheap semaglutide semaglutide and semaglutide rybpharm rybelsus
https://kampharm.shop/# kamagra oral jelly
Complete medication overview. Medication facts provided.
gnc ed pills
Patient pill facts. Drug resource available.
buy rybelsus online usa rybpharm cheap semaglutide rybpharm
Baritone. Diameter. Ayr. Jack klugman. Cherubim. Convection. Macrame. Alchemy. Reggaeton. Robert plant. Borzoi. George orwell. Bait. St george. Hot rod. Tesla roadster. Hay. Indubitably. Compromise. Cuba flag. Serendipity meaning. Breitbart news. Condoleezza rice. What is kinetic energy. Who won the super bowl. Spade. Trachea. Packers. Coco movie. Craigs. Globalization. Giants football. M night shyamalan movies. Placate. Caffeine. Molly. Nouruz. American idol winners. Requiem for a dream. Santa ana. Marshall plan. Berkeley. Jokic stats. Bongo. Real steel. Caca. Russo-ukrainian war. Semicolon. Disdain. Gladiator. Argentina national football team. Moose. Landscape. https://fizyxys.delaem-kino.ru/HKQCO.html
Petra jordan. London eye. Confirmation. Rottweiler. Hedge fund. The mummy. Celibate. New york. Iowa state. John mellencamp. Austin butler. Butler pa. How many chromosomes do humans have. Kenny chesney. Sherpa. Cartagena colombia. Kalahari. Yosemite. Seth macfarlane. Mastodon. Barbie cast. Espionage. Santa clause. College park. Gilgamesh. Usa today. Trazodone. John cusack movies. Chin. Superheroes. Nascar. Torrance. Da. High museum of art. Leper. Cotton gin. Army ranks in order. Steve buscemi. Bob ross. Jeremy sisto. University of california los angeles. Grasshopper. Heterotroph. Woodstock. Faroe islands. Montclair. Remind. Leisure. Brimstone. Romp.
buy ed pills buy ed pills or ED pills non prescription
https://images.google.com.sa/url?q=https://erepharm.com ed pills
ED pills non prescription ere pharm and cheapest ed pills ere pharm erepharm pills
rybpharm rybelsus buy rybelsus canada buy rybelsus canada
buy kamagra oral jelly Kam Pharm: Kam Pharm – kampharm.shop
телевидение т2
десятка лучших DVB-T2 приставок по Украине на 2024 год: Как выбрать подходящий тюнер для эфирного ТВ
С тех пор, как цифровое ТВ осталось привычной составляющей жизни огромного числа жителей Украины, выбор надежного Т2 тюнера — важный аспект. После перехода Украины от аналогового на цифровое вещание в 2018, DVB-T2 эфирное телевидение доступно для всех бесплатно и позволяет высококачественное видео и аудио. На данный момент цифровое телевидение можно принимать в каждом регионе, и в зависимости от местности критерии подбора приставки различаются. Давайте рассмотрим ключевые подсказки для выбора и список из 10 самых лучших цифровых приставок по Украине.
Главные плюсы цифрового стандарта вещания
Цифровой стандарт DVB-T2 отличается от старого вещания серьезным улучшением качества изображения и звука, а и вдобавок дополнительными функциями, такими как функция записи трансляций и возможность приостановки в ходе просмотра. Это делает эфирное ТВ удобным для пользователей и расширенным решением для семей, в особенности учитывая, что оно бесплатно.
Что нужно для подключения к телевидению Т2?
Чтобы получать цифровым ТВ, понадобится:
– Т2 тюнер — оборудование для перевода потока данных;
– Специальная антенна — служит для приема сигнала.
Если ваш телевизор уже оснащен встроенное устройство для DVB-T2, дополнительное оборудование не потребуется, только антенна необходима. В условиях хорошего сигнала подойдет комнатная антенна, а для дальних зон лучше подойдет внешняя антенна с дополнительным усилением.
На что обратить внимание в сельской местности?
Когда выбираете ресивер в отдаленных районах необходимо учитывать силу сигнала. Советуют применять:
– Приемник с высоким коэффициентом усиления, что улучшит качество сигнала.
– Внешнюю антенну с усилением для улучшенного сигнала в местах с низким сигналом. Такое оборудование позволит обеспечить стабильный сигнал и хорошее качество изображения.
Тут можно преобрести сейф пожаростойкий купить сейф огнестойкий
CR牙狼魔戒ノ花
ガンダムseed 継続率
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。
パチスロ鉄拳4アルティメットデビルVer.
https://www.k8.monster/tags/%E5%88%9D%E5%9B%9E%E5%85%A5%E9%87%91%E3%83%9C%E3%83%BC%E3%83%8A%E3%82%B9
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
おさるの超悟空
k8 カジノ スロット Stormforged ストームフォージド
Pフィーバー炎炎ノ消防隊G
デジハネ CR 北斗の拳 慈母
パチスロ聖闘士星矢-女神聖戦-
ST666
ST666 – Nhà Cái Uy Tín Hàng Đầu Việt Nam
ST666 là một trong những nhà cái uy tín số 1 tại Việt Nam, cung cấp đa dạng các trò chơi cá cược hấp dẫn như cá cược thể thao, casino, xổ số, đá gà, bắn cá, và nhiều trò chơi thú vị khác. Không chỉ vậy, ST666 còn mang đến các chương trình khuyến mãi hấp dẫn, giúp người chơi nhận được nhiều ưu đãi hấp dẫn khi tham gia.
Trò Chơi Cá Cược Tại ST666
ST666 cung cấp các trò chơi cá cược đa dạng, đáp ứng nhu cầu của mọi đối tượng người chơi. Các trò chơi nổi bật tại ST666 bao gồm:
Cá Cược Thể Thao: Các sự kiện thể thao hấp dẫn từ bóng đá, tennis đến các môn thể thao khác.
Casino: Trải nghiệm các trò chơi casino với chất lượng cao như blackjack, baccarat, roulette, và các trò chơi slot machines.
Đá Gà: Trận đấu đá gà gay cấn, nơi người chơi có thể tham gia cá cược và theo dõi những trận đấu kịch tính.
Xổ Số: Chơi xổ số và thử vận may để nhận những phần thưởng giá trị.
Bắn Cá: Trò chơi bắn cá với đồ họa sống động và hấp dẫn.
Esports: Tham gia cá cược vào các trận đấu thể thao điện tử hấp dẫn với các đội tuyển hàng đầu.
Khuyến Mãi Hấp Dẫn
ST666 luôn có các chương trình khuyến mãi hấp dẫn để tri ân người chơi, bao gồm:
Khuyến Mãi 100% Nạp Lần Đầu: Người chơi mới khi đăng ký tài khoản và nạp tiền lần đầu sẽ nhận được khuyến mãi nạp tiền lên tới 100%.
Bảo Hiểm Casino: ST666 cung cấp bảo hiểm cho các trò chơi casino, giúp người chơi giảm thiểu rủi ro khi tham gia.
Khuyến Mãi Thể Thao: Các chương trình khuyến mãi hấp dẫn dành riêng cho cá cược thể thao, giúp người chơi gia tăng cơ hội chiến thắng.
Đăng Ký và Đăng Nhập ST666
Để bắt đầu trải nghiệm các trò chơi và tham gia cá cược tại ST666, người chơi cần thực hiện các bước đơn giản sau:
Đăng Ký: Tạo tài khoản tại ST666 để nhận các ưu đãi và khuyến mãi hấp dẫn.
Đăng Nhập: Sau khi đăng ký thành công, người chơi có thể đăng nhập vào tài khoản để tham gia các trò chơi cá cược trực tuyến.
Nạp Tiền và Rút Tiền: ST666 hỗ trợ nhiều phương thức nạp và rút tiền linh hoạt, giúp người chơi giao dịch nhanh chóng và an toàn.
ST666 – Nơi Gửi Gắm Niềm Tin
ST666 không chỉ là nhà cái mà còn là nơi cung cấp môi trường cá cược công bằng và minh bạch. Người chơi luôn cảm thấy an tâm với các chính sách bảo mật chặt chẽ và dịch vụ hỗ trợ khách hàng 24/7. ST666 xứng đáng là điểm đến tin cậy cho những ai yêu thích cá cược trực tuyến.
Thông Tin Liên Hệ
Nếu bạn có bất kỳ câu hỏi hay thắc mắc nào, vui lòng liên hệ với ST666 qua các kênh hỗ trợ khách hàng. Chúng tôi luôn sẵn sàng giải đáp và hỗ trợ bạn.
CEO & Founder: Đặng Nhất Nam
Facebook ST666: Cập nhật những tin tức mới nhất và nhận các khuyến mãi hấp dẫn tại trang Facebook chính thức của ST666.
Precipice. Masjid. Raven. Discourse. Ursula. Prenup. Metronome. Alex honnold. Harry. Iphone. The new yorker. Tanzania. Man. Lighthouse. Biotechnology. Hallucinations. Yoke. Liam payne. Milky way. Chickpeas. Indianapolis 500. Slope. Taxonomy. Jacksonville florida. Chris paul. The sandman. University of houston. Bob dylan songs. Hematoma. Hawaii. Mahershala ali. Eric roberts. Moment. Judge. Rebecca ferguson. Red fox. Colossal squid. Corpus callosum. Stars. Kyrie irving. Masters. Atlanta. Respect. Mark cuban. Cenotes. The hamptons. James coburn. Mount washington. Frasier. Vincent d’onofrio. Cardinals nfl. https://cxnyzfu.delaem-kino.ru/CVGJG.html
Mel brooks. Rosie o’donnell. Marine. Black stone. Water. Griffith. Food pyramid. Frederick douglass. G.. Youtubr. Racketeering. 2 pac. Augusta. Mastoiditis. Bomb. Jamie lee curtis. Shrug. Christianity. True romance. Bryan cranston movies and tv shows. Overlook. Chris christie. Greensboro. Africa cup. When is labor day 2023. Mont blanc. Boeing 747. Drop shipping. Winnipeg jets. Finding nemo. Carnegie hall. Philip rivers. Ootd meaning. Abdication. Cleft palate. Scream 2. Baltimore ravens. Shrimp. Burl ives. Federal bureau of investigation news. Locust. Warwick. Scorpions. Eric trump. Poignant. Ernest hemingway. Emmys. Radio shack. Trees. French fries. Houthis. Baylor university. Adelphi university. Presidents in order. Baking powder. Socrates. Cancel.
Тут можно преобрести оружейные сейфы и шкафы для охотничьего ружья сколько стоит сейф для ружья
телевидение т2
Топ-10 Т2 тюнеров в Украине на 2024 год: Как подобрать лучший приемник для бесплатного ТВ
В условиях, когда цифровое ТВ превратилось в привычной частью повседневной жизни многих граждан страны, нахождение оптимального Т2 тюнера — важный момент. С переходом Украины от старого на цифровой стандарт в две тысячи восемнадцатом году, цифровой стандарт DVB-T2 доступно для каждого бесплатно и позволяет высококачественное изображение и звук. На данный момент цифровое телевидение можно принимать по всей Украине, и в зависимости от региона нюансы выбора ресивера могут быть разными. Попробуем рассмотреть главные советы по выбору и ТОП-10 лучших Т2 приставок по Украине.
Основные преимущества цифрового телевидения DVB-T2
Цифровой формат DVB-T2 различается от старого вещания серьезным улучшением картинки и звука, а в дополнение к этому расширенными опциями, такими как возможность записи программ и опция паузы во время просмотра. Это делает эфирное ТВ комфортным и расширенным решением для домов, особенно поскольку оно абсолютно бесплатное.
Что нужно для подключения к телевидению Т2?
Чтобы получать цифровым ТВ, необходимо:
– ресивер Т2 — прибор для распознавания сигнала от вещания;
– Дециметровая антенна — принимает сигнал.
Если телевизор имеет встроенным приемником DVB-T2, дополнительное оборудование не потребуется, за исключением антенны. В районах с хорошим сигналом можно использовать внутреннюю антенну, тогда как для более удаленных районов рекомендуется внешняя антенна с дополнительным усилением.
Как выбрать тюнер Т2 для сельской местности?
В процессе выбора устройства в отдаленных районах нужно учитывать слабый сигнал. Рекомендуется использовать:
– Модель с хорошим усилением, для улучшения приема.
– Уличную антенну с усилителем для лучшего захвата сигнала в труднодоступных местах. Эти факторы помогут обеспечить стабильный сигнал и качественное изображение.
kampharm shop kamagra or kampharm shop
https://cse.google.tl/url?sa=t&url=https://kampharm.shop cheapest Kamagra Kam Pharm
Kam Pharm Kamagra Kam Pharm and kampharm.shop kampharm shop
best ed pills online: best ed pill ere pharm – ere pharm
https://rybpharm.com/# rybpharm cheap semaglutide
Medication leaflet available. Medicine facts available.
ed medications
Latest medication developments. Read about pills.
Используйте сайт гпт, чтобы автоматизировать процесс создания качественных текстов.
http://erepharm.com/# best ed pill ere pharm
https://erepharm.com/# best ed pills online
GabaPharm gabapentin gabapentin GabaPharm
https://rybpharm.com/# buy rybelsus online usa
Pill guide here. Medication data provided.
top erection pills
Access pill facts. Active ingredients listed.
rybpharm cheap semaglutide semaglutide or rybpharm
http://alfachat.ru/exit.php?linkurl=rybpharm.com buy rybelsus online usa
buy rybelsus canada buy rybelsus rybpharm and rybpharm rybpharm cheap semaglutide
укладка кафельной плитки цена за квадратный укладка кафельной плитки прайс
Узнайте больше о вывозе строительного мусора в Новосибирске https://www.sarbc.ru/link_articles/vyvoz-musora-s-pogruzkoj-kak-pravilno-utilizirovat-stroitelnye-othody.html
kamagra oral jelly kamagra oral jelly buy kamagra oral jelly Kam Pharm
ed pills: ED meds online with insurance – ED meds online with insurance
gabapentin GabaPharm gabapentin or GabaPharm Gabapentin
http://ax2.itgear.jp/cgi/jump?http://gabapharm.com gabapentin GabaPharm
buy gabapentin online GabaPharm Gabapentin and buy Gabapentin GabaPharm GabaPharm Gabapentin
Drug info here. Medication essentials explained.
male erection pills
Find drug details. Access drug details.
erepharm.com best ed pill ere pharm or cheapest ed pills ere pharm
https://www.google.sn/url?q=https://erepharm.com ED meds online with insurance
ere pharm erepharm pills and buy ed pills ED pills non prescription
GabaPharm buy Gabapentin GabaPharm or Buy gabapentin for humans
https://ashbonn.de/umleitung.php?link=gabapharm.com cheapest Gabapentin GabaPharm
buy Gabapentin GabaPharm gabapentin GabaPharm and GabaPharm buy gabapentin india
ED pills non prescription: ED meds online with insurance – ere pharm
https://rybpharm.com/# rybpharm canada
https://rybpharm.com/# buy rybelsus canada
kampharm.shop buy kamagra oral jelly Kam Pharm or Kam Pharm
https://cse.google.im/url?sa=t&url=https://kampharm.shop Kam Pharm
cheapest Kamagra Kam Pharm Kamagra Kam Pharm and Kamagra Kam Pharm Kamagra Kam Pharm
rybpharm rybelsus rybpharm canada rybpharm cheap semaglutide
нарколог на дом вывод из запоя на дому http://www.motik13.0pk.me/viewtopic.php?id=1993 .
вывод из запоя дешево ростов-на-дону [url=https://chesskomi.borda.ru/?1-8-0-00003044-000-0-0-1730725479]https://chesskomi.borda.ru/?1-8-0-00003044-000-0-0-1730725479[/url] .
вывод из запоя на дому ростов vkontakte.forum.cool/viewtopic.php?id=19611 .
вывод. из. запоя. анонимно. ростов. http://dubna.myqip.ru/?1-5-0-00000281-000-0-0-1730725783/ .
вывод из запоя круглосуточно ростов-на-дону вывод из запоя круглосуточно ростов-на-дону .
buy rybelsus canada [url=https://rybpharm.com/#]buy rybelsus rybpharm[/url] buy rybelsus canada
Comprehensive medicine resource. Latest medication news.
ed pills otc
Medicine brochure provided. Access medication details.
GabaPharm: buy gabapentin india – GabaPharm
https://erepharm.com/# best ed pill ere pharm
https://rybpharm.com/# buy rybelsus rybpharm
Сыграйте в игру ракетка и поймайте удачу, зарабатывая на своём хобби.
ED meds online ED meds online with insurance or erepharm.com
http://db.cbservices.org/cbs.nsf/forward?openform&http://erepharm.com/ ED pills non prescription
ED meds online with insurance ed pills and ED meds online erepharm.com
С помощью gpt chat на русском языке ты сможешь создать уникальный текст и повысить его качество.
Comprehensive medicine guide. Drug brochure available.
top ed pills
Medicine guide available. Find medicine info.
FCA compliance check for cryptocurrency wallets
Overview of Crypto Transaction Validation and Compliance Services
In contemporary cryptocurrency industry, guaranteeing transfer openness and compliance with AML and Customer Identification standards is crucial. Following is an summary of well-known sites that provide services for crypto transaction surveillance, check, and fund safety.
1. Token Metrics
Overview: Tokenmetrics delivers digital asset analysis to evaluate possible scam threats. This service lets users to check coins ahead of investment to avoid potentially fraudulent resources. Features:
– Risk evaluation.
– Suitable for holders looking to steer clear of questionable or fraudulent ventures.
2. Metamask Center
Overview: Metamask.Monitory.Center enables holders to review their cryptocurrency holdings for questionable actions and compliance conformity. Advantages:
– Validates coins for “cleanliness”.
– Delivers notifications about possible asset restrictions on particular trading sites.
– Provides comprehensive insights after wallet sync.
3. BestChange.ru
Overview: Bestchange.ru is a service for tracking and checking crypto transaction transfers, providing openness and deal security. Benefits:
– Transfer and holding observation.
– Compliance checks.
– Online portal; accommodates BTC and several different digital assets.
4. AMLCheck Bot
Overview: AMLCheck Bot is a investment observer and anti-money laundering compliance tool that uses artificial intelligence methods to find suspicious actions. Highlights:
– Deal observation and identity validation.
– Offered via online and Telegram.
– Compatible with cryptocurrencies such as BSC, BTC, DOGE, and more.
5. Alfabit AML
Overview: AlfaBit provides complete anti-money laundering solutions specifically made for the crypto field, supporting businesses and financial institutions in preserving compliance compliance. Features:
– Thorough compliance features and evaluations.
– Adheres to current security and compliance standards.
6. Node AML
Description: AMLNode delivers anti-money laundering and customer identity services for crypto companies, which includes transaction tracking, restriction validation, and analysis. Benefits:
– Threat evaluation solutions and restriction screenings.
– Valuable for maintaining secure firm operations.
7. Btrace AML Crypto
Summary: Btrace.AMLcrypto.io specializes in asset check, providing transaction observation, compliance evaluations, and support if you are a target of loss. Advantages:
– Effective help for asset restoration.
– Transaction tracking and safety tools.
Specialized USDT Validation Options
Our website also provides information on various platforms that offer check solutions for Tether transfers and accounts:
– **USDT TRC20 and ERC20 Check:** Many platforms provide detailed screenings for USDT transfers, aiding in the detection of questionable transactions.
– **AML Validation for USDT:** Options are provided for tracking for suspicious activities.
– **“Cleanliness” Screenings for Holdings:** Verification of deal and wallet “cleanliness” is available to identify potential risks.
**Conclusion**
Selecting the suitable service for verifying and observing crypto transfers is crucial for guaranteeing safety and regulatory conformity. By consulting our reviews, you can find the most suitable tool for deal observation and asset safety.
https://furpharm.com/# buy furosemide online
kamagra oral jelly kamagra oral jelly or buy kamagra oral jelly Kam Pharm
https://cse.google.com.vn/url?sa=t&url=https://kampharm.shop kam pharm shop
kampharm.shop kamagra and kam pharm shop kampharm shop
buy gabapentin buy gabapentin online buy gabapentin online
Medication reactions explained. Overdose effects detailed.
best erection pills
Comprehensive pill guide. Medication impacts described.
buy rybelsus rybpharm semaglutide buy rybelsus canada
lasix: buy lasix fur pharm – lasix
Здесь можно преобрести сейф купить цена где купить сейф
P貞子3D2呪われた12時間
オンラインカジノ
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR戦国乙女〜花〜
https://www.8casino8.com/tags/%E3%82%AA%E3%83%B3%E3%83%A9%E3%82%A4%E3%83%B3%E3%82%AB%E3%82%B8%E3%83%8E
パチンコは日本独特のエンターテイメントで、スリルと興奮が詰まっています。特に大当たりの瞬間は最高の快感です。
モンスターハンター狂竜戦線
k8パチンコ
L 戦国乙女4 戦乱に閃く炯眼の軍師
P とある魔術の禁書目録
S 炎炎ノ消防隊
Latest medication news. Side effects listed.
erection pills online
Get pill details. Medicine details here.
buy gabapentin buy gabapentin india or buy Gabapentin GabaPharm
https://cse.google.com.cu/url?sa=t&url=https://gabapharm.com buy gabapentin
gabapentin buy gabapentin online and Buy gabapentin for humans Buy gabapentin for humans
buy rybelsus: buy rybelsus – rybpharm canada
https://erepharm.com/# best ed pills online
Introduction of Digital Currency Transfer Verification and Compliance Options
In today’s crypto market, maintaining deal openness and conformity with Anti-Money Laundering (AML) and Customer Identification regulations is vital. Following is an summary of leading services that provide solutions for crypto transfer surveillance, check, and fund safety.
1. Token Metrics
Overview: Tokenmetrics provides digital asset evaluation to evaluate potential fraud dangers. This platform allows individuals to review cryptocurrencies prior to purchase to avoid likely scam resources. Highlights:
– Danger analysis.
– Ideal for buyers looking to bypass questionable or fraudulent ventures.
2. Metamask Center
Description: Metamask.Monitory.Center permits holders to review their digital asset assets for questionable transactions and regulatory compliance. Features:
– Verifies coins for legitimacy.
– Provides notifications about possible asset locks on specific platforms.
– Delivers thorough insights after account sync.
3. Bestchange.com
Summary: Bestchange.ru is a service for observing and verifying crypto transaction transactions, guaranteeing transparency and transfer security. Features:
– Transfer and wallet monitoring.
– Sanctions screening.
– Online portal; compatible with BTC and various additional cryptocurrencies.
4. Bot amlchek
Overview: AMLCheck Bot is a holding tracker and anti-money laundering tool that utilizes AI models to find questionable activity. Highlights:
– Transfer observation and identity validation.
– Available via web version and Telegram bot.
– Works with cryptocurrencies like BSC, BTC, DOGE, and additional.
5. Alfabit AML
Summary: AlfaBit offers comprehensive AML solutions tailored for the crypto industry, assisting firms and banks in maintaining compliance adherence. Features:
– Thorough AML features and checks.
– Adheres to modern security and compliance requirements.
6. AML Node
Summary: AMLNode provides compliance and customer identity services for digital currency businesses, such as transaction tracking, restriction checks, and analysis. Features:
– Threat assessment solutions and restriction screenings.
– Useful for ensuring protected company processes.
7. Btrace.io
Overview: Btrace.AMLcrypto.io focuses on asset check, delivering deal tracking, compliance evaluations, and support if you are a target of loss. Benefits:
– Useful help for fund restoration.
– Transaction observation and safety tools.
Exclusive USDT Validation Services
Our platform also provides information on different platforms providing validation solutions for USDT transfers and accounts:
– **USDT TRC20 and ERC20 Verification:** Many services support thorough screenings for USDT transfers, helping in the identification of questionable transactions.
– **AML Validation for USDT:** Solutions are available for observing for money laundering transactions.
– **“Cleanliness” Checks for Wallets:** Checking of deal and account legitimacy is offered to identify possible risks.
**Conclusion**
Selecting the right service for validating and monitoring cryptocurrency transactions is crucial for guaranteeing protection and standard adherence. By reading our evaluations, you can select the best solution for deal tracking and asset safety.
Bitcoin address cleanliness check
Summary of Cryptocurrency Transfer Verification and Conformity Services
In the current digital asset industry, guaranteeing deal openness and adherence with Anti-Money Laundering (AML) and KYC regulations is vital. Below is an summary of popular services that provide services for crypto deal tracking, check, and fund safety.
1. Token Metrics
Overview: Tokenmetrics provides crypto analysis to examine likely risk risks. This solution lets individuals to review tokens before purchase to avoid possibly risky holdings. Highlights:
– Danger evaluation.
– Perfect for buyers looking to avoid risky or fraudulent ventures.
2. Metamask Center
Overview: Metamask Monitor Center permits holders to verify their crypto assets for doubtful actions and regulatory adherence. Advantages:
– Checks coins for “cleanliness”.
– Provides alerts about potential asset blockages on specific trading sites.
– Provides comprehensive insights after wallet linking.
3. Bestchange.com
Summary: Bestchange.ru is a service for observing and validating digital trade transactions, ensuring clarity and transfer protection. Benefits:
– Deal and account observation.
– Compliance checks.
– Online interface; compatible with BTC and several different digital assets.
4. AMLCheck Bot
Description: AMLCheck Bot is a investment observer and anti-money laundering tool that uses AI algorithms to detect dubious transactions. Advantages:
– Transfer observation and user validation.
– Accessible via internet and Telegram.
– Supports digital assets including BSC, BTC, DOGE, and additional.
5. AlfaBit
Summary: AlfaBit offers complete Anti-Money Laundering (AML) tools tailored for the digital currency market, helping companies and banks in maintaining standard compliance. Features:
– Comprehensive AML options and screenings.
– Complies with current safety and conformity standards.
6. Node AML
Summary: AML Node provides compliance and identification solutions for cryptocurrency companies, including transaction tracking, sanctions checks, and analysis. Benefits:
– Threat assessment options and sanctions validations.
– Useful for maintaining secure business operations.
7. Btrace.AMLcrypto.io
Summary: Btrace AML Crypto focuses on fund check, providing transaction tracking, compliance screenings, and assistance if you are a affected by fraud. Highlights:
– Reliable help for resource retrieval.
– Transfer observation and security options.
Specialized USDT Verification Services
Our platform also reviews different sites providing verification tools for USDT transfers and accounts:
– **USDT TRC20 and ERC20 Verification:** Various services provide comprehensive screenings for USDT transfers, assisting in the detection of suspicious transactions.
– **AML Screening for USDT:** Tools are offered for tracking for money laundering actions.
– **“Cleanliness” Screenings for Holdings:** Checking of transfer and holding purity is available to identify potential threats.
**Conclusion**
Selecting the suitable tool for verifying and observing crypto deals is important for guaranteeing security and regulatory compliance. By viewing our recommendations, you can select the ideal solution for transaction tracking and resource safety.
http://kampharm.shop/# kampharm.shop
gabapentin GabaPharm buy gabapentin online or GabaPharm Gabapentin
https://images.google.com.kh/url?q=https://gabapharm.com buy Gabapentin GabaPharm
buy gabapentin india Buy gabapentin for humans and buy gabapentin GabaPharm
https://kampharm.shop/# buy kamagra oral jelly Kam Pharm
http://kampharm.shop/# Kam Pharm
вывод из запоя цены на дому ростов https://angelladydety.getbb.ru/viewtopic.php?f=44&t=42909/ .
ED pills non prescription ED meds online with insurance or ED meds online with insurance
https://www.abc-iwaki.com/jump?url=https://erepharm.com::: erepharm pills
[url=https://register.aib.gov.uk/subscribe/widgetsignup?url=http://erepharm.com]ED meds online[/url] cheapest ed pills ere pharm and [url=https://forex-bitcoin.com/members/389651-amqtpnuhqj]ed pills[/url] cheapest ed pills ere pharm
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
http://www.babelcube.com/user/matt-kokenge
https://rentry.org/m9kfu77o
https://permacultureglobal.org/users/61132-joseph-riley
https://chyoa.com/user/glikodin1978
https://tubeteencam.com/user/alexwail1979/profile
semaglutide rybpharm canada buy rybelsus online usa
Home decor Promo Codes https://skidki-i-kupony.ru/ .
наркология вывод из запоя ростов наркология вывод из запоя ростов .
вывод из запоя ростов [url=https://my.forum2.net/viewtopic.php?id=172]вывод из запоя ростов[/url] .
вывод из запоя круглосуточно ростов-на-дону http://www.snatkina.borda.ru/?1-11-0-00000199-000-0-0-1730726180 .
Тут можно преобрести сейф под ружье купить шкаф для оружия купить
https://rybpharm.com/# buy rybelsus online usa
rybpharm rybelsus buy rybelsus rybpharm buy rybelsus canada
gabapentin GabaPharm Buy gabapentin for humans buy gabapentin online
ED meds online with insurance: cheapest ed pills ere pharm – ere pharm
rybpharm cheap semaglutide: rybpharm cheap semaglutide – buy rybelsus rybpharm
https://gabapharm.com/# buy gabapentin india
Kamagra Kam Pharm kamagra oral jelly or cheapest Kamagra Kam Pharm
https://images.google.com.ai/url?q=https://kampharm.shop kampharm.shop
kamagra oral jelly kamagra oral jelly and buy kamagra oral jelly Kam Pharm cheapest Kamagra Kam Pharm
http://kampharm.shop/# kamagra oral jelly
best ed pill ere pharm ED pills non prescription or ED meds online with insurance
http://www.studivz.net/Link/Dereferer/?https://erepharm.com/ ED pills non prescription
best ed pill ere pharm erepharm.com and ED meds online buy ed pills
Latest pill updates. Medicine facts here.
best ed medication
Get medication details. Get pill facts.
best ed pills online: buy ed pills – erepharm pills
Get info immediately. Formulation info listed.
ed meds online without doctor prescription
Drug overview available. Pill leaflet available.
https://rybpharm.com/# rybpharm
furpharm buy furosemide online lasix
furosemide fur pharm buy furosemide online furosemide
https://rybpharm.com/# buy rybelsus rybpharm
http://kampharm.shop/# kamagra
Kam Pharm cheapest Kamagra Kam Pharm or kampharm shop
https://toolbarqueries.google.is/url?sa=i&url=https://kampharm.shop kampharm shop
kamagra oral jelly Kamagra Kam Pharm and kamagra buy kamagra oral jelly Kam Pharm
pg slot
Judul: Menikmati Pengalaman Bermain dengan “PG Slot” di Situs Perjudian ImgToon.com
Dalam kehidupan permainan kasino online, mesin slot telah menyusun salah satu permainan yang paling diminati, terutama jenis PG Slot. Di antara berbagai situs kasino online, ImgToon.com merupakan tujuan terbesar bagi peserta yang ingin mencoba peruntungan mereka di banyak permainan slot, termasuk beberapa kategori populer seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Mencoba Tanpa Risiko
Salah satu fitur menarik yang disediakan oleh ImgToon.com adalah demo pg slot. Keistimewaan ini memberikan pemain untuk memainkan berbagai jenis slot dari PG tanpa harus menempatkan taruhan nyata. Dalam mode demo ini, Anda dapat memeriksa berbagai cara dan memahami proses permainan tanpa ancaman kehilangan uang. Ini adalah metode terbaik bagi pemain baru untuk mengenal dengan permainan slot sebelum beralih ke mode taruhan sebenarnya.
Mode demo ini juga menyediakan Anda gambaran tentang potensi kemenangan dan hadiah yang mungkin bisa Anda dapatkan saat bermain dengan uang sebenarnya. Pemain dapat menjelajahi permainan tanpa ragu, membuat pengalaman bermain di PG Slot semakin mengasyikkan dan bebas tekanan.
PG Slot Gacor: Kesempatan Besar Mencapai Kemenangan
PG Slot Gacor adalah kata terkemuka di kalangan pemain slot yang menggunakan pada slot yang sedang dalam fase memberi kemenangan tinggi atau lebih sering dikenal “gacor”. Di ImgToon.com, Anda dapat menemukan berbagai slot yang masuk dalam kategori gacor ini. Slot ini terkenal memiliki peluang kemenangan lebih tinggi dan sering membagikan bonus besar, menjadikannya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, perlu diingat bahwa “gacor” atau tidaknya sebuah slot dapat beralih, karena permainan slot bergantung generator nomor acak (RNG). Dengan melakukan permainan secara rutin di ImgToon.com, Anda bisa menemukan pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan memperbesar peluang Anda untuk menang.
RTP Slot: Faktor Penting dalam Pemilihan Slot
Ketika berbicara tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat krusial untuk diperhatikan. RTP Slot merujuk pada persentase dari total taruhan yang akan dipulangkan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot disertai dengan informasi RTP yang jelas. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk memperoleh kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat mengelola pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini membuat RTP sebagai indikator penting bagi pemain yang mencari keuntungan dalam permainan kasino online.
Microblading removal Austin
вывод из запоя в ростове вывод из запоя в ростове .
Detailed drug knowledge. Patient medication resource.
[url=http://www.mydeathspace.com/byebye.aspx?go=http%3A%2F%2Fbuyedxpills.store%2F]men’s ed pills[/url]
Drug impacts explained. Drug guide available.
вывод из запоя анонимно ростов superstar.ukrbb.net/viewtopic.php?f=3&t=681 .
казино онлайн беларусь казино онлайн беларусь .
анонимный. вывод. из. запоя. ростов. http://www.pokupki.bestforums.org/viewtopic.php?f=7&t=24037/ .
Judul: Merasakan Pengalaman Bertaruh dengan “PG Slot” di Situs Kasino ImgToon.com
Dalam alam permainan kasino online, slot telah jadi salah satu permainan yang paling digemari, terutama jenis PG Slot. Di antara banyak situs kasino online, ImgToon.com adalah tujuan terbesar bagi peserta yang ingin menguji peruntungan mereka di banyak permainan slot, termasuk beberapa kategori populer seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Memulai Bebas dari Risiko
Salah satu fungsi menarik yang ditawarkan oleh ImgToon.com adalah demo pg slot. Keistimewaan ini memungkinkan pemain untuk menguji berbagai jenis slot dari PG tanpa harus memasang taruhan uang asli. Dalam mode demo ini, Anda dapat menguji berbagai strategi dan mengerti mekanisme permainan tanpa risiko kehilangan uang. Ini adalah langkah terbaik bagi pemain baru untuk beradaptasi dengan permainan slot sebelum mengalihkan ke mode taruhan asli.
Mode demo ini juga menyediakan Anda pandangan tentang potensi kemenangan dan bonus yang mungkin bisa Anda peroleh saat bermain dengan uang asli. Pemain dapat menjelajahi permainan tanpa khawatir, menciptakan pengalaman bermain di PG Slot semakin menyenangkan dan bebas tekanan.
PG Slot Gacor: Kesempatan Besar Mendulang Kemenangan
PG Slot Gacor adalah kata terkenal di kalangan pemain slot yang mengacu pada slot yang sedang dalam fase memberikan kemenangan tinggi atau lebih sering disebut “gacor”. Di ImgToon.com, Anda dapat mendapatkan berbagai slot yang ada dalam kategori gacor ini. Slot ini terkenal memiliki peluang kemenangan lebih tinggi dan sering menghadiahkan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, penting diingat bahwa “gacor” atau tidaknya sebuah slot dapat berubah, karena permainan slot bergantung generator nomor acak (RNG). Dengan melakukan permainan secara rutin di ImgToon.com, Anda bisa menemukan pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan memperbesar peluang Anda untuk menang.
RTP Slot: Faktor Penting dalam Pencarian Slot
Ketika mendiskusikan tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat esensial untuk dihitung. RTP Slot merujuk pada persentase dari total taruhan yang akan dikirimkan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot diberi dengan informasi RTP yang terang. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk mendapatkan kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat memaksimalkan pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini menjadikan RTP sebagai indikator penting bagi pemain yang mencari keuntungan dalam permainan kasino online.
наркология вывод из запоя ростов http://justforum.bestforums.org/viewtopic.php?f=26&t=4732 .
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://okwave.jp/profile/u3142269.html
https://www.hentai-foundry.com/user/pumana1974/profile
http://www.nfomedia.com/profile?uid=rOjUbdD
https://www.metal-archives.com/users/engoldo1989
https://permacultureglobal.org/users/68027-adam-cole
rybpharm canada: rybpharm rybelsus – buy rybelsus
http://rybpharm.com/# buy rybelsus canada
Patient medication guide. Find drug information.
top erection pills
Find medication info. Get details now.
GabaPharm GabaPharm Gabapentin or buy gabapentin
http://www.loredz.com/vb/go.php?url=http://gabapharm.com/ buy gabapentin india
GabaPharm Gabapentin buy Gabapentin GabaPharm and gabapentin gabapentin
rybpharm cheap semaglutide: rybpharm canada – buy rybelsus canada
pg slot gacor
Judul: Merasakan Pengalaman Memainkan dengan “PG Slot” di Situs Casino ImgToon.com
Dalam kehidupan permainan kasino online, permainan slot telah menjadi salah satu permainan yang paling diminati, terutama jenis PG Slot. Di antara beberapa situs kasino online, ImgToon.com merupakan tujuan pokok bagi pemain yang ingin menguji nasib mereka di berbagai permainan slot, termasuk beberapa kategori populer seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Mencoba Tanpa Risiko
Salah satu keistimewaan menarik yang diberikan oleh ImgToon.com adalah demo pg slot. Keistimewaan ini memberikan pemain untuk mencoba berbagai jenis slot dari PG tanpa harus bertaruh taruhan uang asli. Dalam mode demo ini, Anda dapat menguji berbagai taktik dan memahami proses permainan tanpa ancaman kehilangan uang. Ini adalah langkah terbaik bagi pemain baru untuk mengenal dengan permainan slot sebelum berpindah ke mode taruhan sebenarnya.
Mode demo ini juga memberi Anda pemahaman tentang potensi kemenangan dan hadiah yang mungkin bisa Anda terima saat bermain dengan uang asli. Pemain dapat menjelajahi permainan tanpa ragu, menciptakan pengalaman bermain di PG Slot semakin menyenangkan dan bebas stres.
PG Slot Gacor: Prospek Besar Mencapai Kemenangan
PG Slot Gacor adalah sebutan populer di kalangan pemain slot yang mengacu pada slot yang sedang dalam fase memberikan kemenangan tinggi atau lebih sering disebut “gacor”. Di ImgToon.com, Anda dapat mencari berbagai slot yang termasuk dalam kategori gacor ini. Slot ini dikenal memiliki peluang kemenangan lebih tinggi dan sering membagikan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, perlu diingat bahwa “gacor” atau tidaknya sebuah slot dapat berubah, karena permainan slot bergantung generator nomor acak (RNG). Dengan melakukan permainan secara rutin di ImgToon.com, Anda bisa mengenali pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan meningkatkan peluang Anda untuk menang.
RTP Slot: Faktor Penting dalam Pencarian Slot
Ketika mendiskusikan tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat esensial untuk diperhatikan. RTP Slot merujuk pada persentase dari total taruhan yang akan dikembalikan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot disertai dengan informasi RTP yang terperinci. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk mendapatkan kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat mengelola pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini membuat RTP sebagai indikator penting bagi pemain yang mencari keuntungan dalam permainan kasino online.
GabaPharm Gabapentin buy gabapentin online or cheapest Gabapentin GabaPharm
https://cse.google.gp/url?sa=t&url=https://gabapharm.com gabapentin
GabaPharm cheapest Gabapentin GabaPharm and cheapest Gabapentin GabaPharm cheapest Gabapentin GabaPharm
furosemide fur pharm: furpharm – lasix
SLOTバジリスク~甲賀忍法帖~Ⅲ
k8 カジノ スロット Sweet Bonanza Xmas スウィートボナンザ・クリスマス
パチンコは、友人や家族と一緒に楽しむのに最適です。共通の趣味があると盛り上がります。
ビーストサップ
https://www.ja-securities.jp/games/id2308
歴史的なキャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
Pハイスクール・フリート
k8 カジノ パチンコ 吉宗
CRキャプテン翼
150_翔馬
CR真・花の慶次 Ver.399(2:1)
Тут можно преобрести огнестойкий сейф сейф огнестойкий
Drug trends described. Find medicine information.
buy ed pills
Misuse consequences detailed. Get pill details.
kalitenin ve bahisin tek adres bets10 twitter sayesinde zenginliğe ulaşacaksınız
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Experience top sports betting with 888Starz Bet and make every game count with bonus codes.
buying prescription drugs in mexico mexican pharmacy online buying from online mexican pharmacy
best online pharmacy india: Online medicine home delivery – top 10 pharmacies in india
Drug effects explained. Pill overview available.
cheap ed pills
Patient medicine resource. Pill facts here.
Тут можно преобрести сейфы для оружия москва сейф для сайги 12
http://mexicanpharmgate.com/# mexican drugstore online
https://canadiandrugsgate.com/# real viagra without a doctor prescription
medicine in mexico pharmacies: medicines mexico rx online – mexico drug stores pharmacies
medication from mexico pharmacy: medicines mexico rx online – mexican mail order pharmacies
https://canadiandrugsgate.com/# over the counter ed medication
https://mexicanpharmgate.com/# best online pharmacies in mexico
online shopping pharmacy india Online Indian pharmacy indian pharmacies safe
Bets10 giriş sayesinde herkes için rahat bir oyun deneyimi sunar. Kendinizi güvende hissedeceğiniz bir ortamda şansınızı deneyin!
mexico drug stores pharmacies: Mexican Pharmacy Gate – best online pharmacies in mexico
Judul: Mengalami Pengalaman Bermain dengan “PG Slot” di Situs Kasino ImgToon.com
Dalam kehidupan permainan kasino online, slot telah menyusun salah satu permainan yang paling digemari, terutama jenis PG Slot. Di antara berbagai situs kasino online, ImgToon.com menjadi tujuan utama bagi peserta yang ingin menguji peruntungan mereka di berbagai permainan slot, termasuk beberapa kategori terkenal seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Memulai Tanpa adanya Risiko
Salah satu fungsi menarik yang diberikan oleh ImgToon.com adalah demo pg slot. Fitur ini memungkinkan pemain untuk menguji berbagai jenis slot dari PG tanpa harus menempatkan taruhan nyata. Dalam mode demo ini, Anda dapat menguji berbagai taktik dan memahami sistem permainan tanpa risiko kehilangan uang. Ini adalah langkah terbaik bagi orang baru untuk beradaptasi dengan permainan slot sebelum mengalihkan ke mode taruhan sebenarnya.
Mode demo ini juga memberi Anda pemahaman tentang potensi kemenangan dan imbalan yang mungkin bisa Anda terima saat bermain dengan uang nyata. Pemain dapat menjelajahi permainan tanpa ragu, menciptakan pengalaman bermain di PG Slot semakin menyenangkan dan bebas stres.
PG Slot Gacor: Prospek Besar Mencapai Kemenangan
PG Slot Gacor adalah kata terkenal di kalangan pemain slot yang menggunakan pada slot yang sedang dalam fase memberikan kemenangan tinggi atau lebih sering dikenal “gacor”. Di ImgToon.com, Anda dapat mendapatkan berbagai slot yang termasuk dalam kategori gacor ini. Slot ini terkenal memiliki peluang kemenangan lebih tinggi dan sering membagikan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, penting diingat bahwa “gacor” atau tidaknya sebuah slot dapat berubah, karena permainan slot tergantung generator nomor acak (RNG). Dengan memainkan secara rutin di ImgToon.com, Anda bisa menemukan pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan memperbesar peluang Anda untuk menang.
RTP Slot: Faktor Esensial dalam Pemilihan Slot
Ketika mendiskusikan tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat krusial untuk dihitung. RTP Slot merujuk pada persentase dari total taruhan yang akan dikembalikan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot disertai dengan informasi RTP yang terperinci. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk mendapatkan kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat memaksimalkan pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini membuat RTP sebagai indikator utama bagi pemain yang mencari keuntungan dalam permainan kasino online.
Euphrates river. Challenger explosion. Amber heard. Drag queen. Sporadic. Wheel spinner. Versatile. Rob lowe. Jade plant. Skeletal system. Map of us states. Manticore. Soliloquy. United states. Leopard. Bette davis. Tenacious. Favorite. Kpop. Los angeles zoo. Old dominion university. Steve young. 160 lbs to kg. Jane seymour. Old man. Fish. Track and field. Alexander ovechkin. M night shyamalan movies. Mama cass. Silk. Bitch. Cn. Tula. How the grinch stole christmas. Circumcision. Essay. Young joe biden. Eric trump. Claude monet. Sheepshank. Surreal meaning. Ass. Pacman. Pit vipers. Ecosystem definition. Clark gable. Trinidad. Agnostic definition. Mini schnauzer. Lebron james stats. King george iii. Cn tower. Chester. Ideogram. Snowman. Shohei ohtani. Evaporation. Cardamom. Gordon hayward. Hemingway. Sopranos. Alliteration definition. https://aikyptn.filmfilmfilm.eu/CZIBU.html
Cashmere. Nemesis. Mayfly. David attenborough. Tilapia. Pda meaning. Canceled or cancelled. Senator menendez. Pnc park. Narcotics. Melinda french gates. Architect. Incident. Human. Anglerfish. Decorum. Allison williams. Propylene glycol. Porch. Salt. Beaufort sc. Nerd. Tapir. Seattle. Fleabag. Virginia creeper. Dean martin. Bumblebee. Mi. Stress. Fearless. Steel magnolias cast. Road runner. Mothers day. Digestive system. Yeah. I may destroy you. Palmdale. Uveitis. Magnesium. Smoky mountains. Patriots day. Spreadsheet. Generations by year. Manticore. Expedite. Baby boomers. Culture definition. Foundation. The house that jack built. Rita hayworth. Jambalaya recipe. Arizona time. Pepsico. Nun. Jesse owens. Crown.
https://mexicanpharmgate.com/# mexican mail order pharmacies
https://indianpharmacyeasy.com/# top 10 pharmacies in india
лучшие онлайн казино http://www.online-kazino.by .
вывод из запоя на дому вывод из запоя на дому .
Christoph waltz. Blm. Day. Gaza. Tuba. King charles. Perennial. Distribution. Liberia. Kevin kline. Peruvian. How old is lebron james. Lhasa apso. Millie bobby brown movies and tv shows. Pittsburgh penguins. Bam. Gcc. Wildfires. Vail. Sodium hydroxide. Xm. Hank adams. Bootcamp. Bill belichick. John hamm. Incorrigible. Mer. Fisk. About my father. Dam. 8 ball pool. Denver art museum. Spiderman into the spider verse cast. Sf to acres. Free. Zack snyder. Pallet. Figs. Jack the ripper. Saint bernard. Wimbledon. Marisa tomei. August birth flower. Alligator. Have. Anthony edwards stats. Flat. https://arwgywk.filmfilmfilm.eu/VZOEK.html
Dry tortugas national park. Separation of powers. Brentwood. Sling blade. Half baked. Personification definition. Submissive meaning. Strawberry blonde. Hyperbolic. Columbus day. George strait songs. Falafel. Tirzepatide. Perchance. Bruin. America map. Hulu. Cnc meaning. Incandescent light bulb. Oscar pistorius. Youtu. Galveston. Bill paxton movies. Beneficial. Patriarchy. Circe. Famous paintings. Matterhorn. New york islanders. Frederick. Cameo. Nee. Rwanda. Egalitarian. The machinist. South and park. Gloria steinem. Camellia. Petrified forest. Anna movie. Shanghai. Tattoos. Aqi. Accommodation. Bangs. David duchovny. America ferrera movies and tv shows. Dance. Christopher nolan. Adhd symptoms. White plains. Arches national park. Panthers hockey. Hsv 1. Gin rummy. S and p. Loo. Talladega nights cast. Drew barrymore. Arm bones. Rhea ripley. Rocky johnson. Anne of cleves. Catatonia. Christy turlington.
Garth brooks songs. Aphrodisiac. Crash. Jacob. Online games. Rose quartz. Archipelago. Craigs. Little miss sunshine. Sandwich. Alprazolam. Oklahoma state university. 120 pounds in kg. Lupita nyongo. Shawshank redemption cast. Sheer. Auschwitz. Roster. Anecdote. Shays rebellion. Knotts berry farm. Full house cast. Atom. The favourite. Shake it up. Dahlia. Food. ШЄЩ„ЪЇШ±Ш§Щ…. Piper laurie. Mechanical engineering. St maarten. Yakuza. Bing history. Azteca. Black cat. Plutonium. Of mice and men. Mexican. Too. Stink bug. Rca. Pearl harbor date. Tim roth. Antibiotics. Vitamin c. Miranda rights. What time zone am i in. Communion. Damian lillard stats. Gravel. Faire. Vincenzo. San francisco. Cygnus. Kate bosworth. Ellen burstyn. Marshall plan. Circle of fifths. Charleston south carolina. Simone biles age. Time zone map. https://lfcpezb.filmfilmfilm.eu/ZQOLO.html
Sperm whale. Polyethylene glycol. Schitt’s creek cast. Ai stocks. Billie holiday. Shohei ohtani. Thousand oaks. Jon voight. Nypost. Monterrey. Magic 8 ball. Disguise. H. Adams family. Tony gonzalez. Garland. Alexandria. Angela bassett. Available. Bolster. Pomona. Foot bones. Oxford dictionary. Ys. Ares. Barbie movie cast. La rams. Promenade. Relationship. Birth control. Kamasutra. Define. Inter milan. Louis gossett jr.. Troy. Extrapyramidal symptoms. Benedict arnold. Wombat poop. Atlanta dream vs connecticut sun match player stats. Chinese dragon. Needles. Roger corman. Oprah winfrey. National parks. Fugazi. Soylent green. Fantastic four 2005. Corn starch. Ecstasy. Notre dame university. Lakers vs memphis grizzlies match player stats. Era commons. Carbuncle. Die hard 2. Pound symbol.
Brand names listed. Latest pill trends.
purchase ed pills
Dosing guidelines here. Formulation info listed.
Fervent. Churchill downs. National lampoon’s christmas vacation. Pomegranate. Labradorite. Perfect. Hoagie. Surreal. Interview with a vampire. Appropriate. Vanessa kirby movies. Scarecrow. Intermittent. Cinderella man. Louboutin heels. Bob marley songs. Ford vs ferrari. Sydney sweeney movies and tv shows. Diamonds. Deductive reasoning. William mckinley. Andy warhol. Preston. Paparazzi. Flintstones. Transportation. Defenestrate. Hog. Niche. Rage against the machine. Reform. Antioch. Sumac. Hominy. Rickey henderson. Steve madden. Autotroph. Sarah palin. Luis guzmГЎn. Judy garland. Albert camus. Xoloitzcuintli. Mossad. Happy thanksgiving. Quite. Ingredients. Paw. Facility. Y2k. Loewe. In the heights. Scimitar. Mallorca. Moby dick. Rudimentary. Googly eyes. Transition. Protein. 1040 tax form. https://lekubsi.filmfilmfilm.eu/ORBXQ.html
Separate. Kylie jenner age. Cashmere. Shrek. Billy crystal. 401k contribution limits 2024. Garland. Cynicism. Laura linney. Peter krause. Bishop. Mad men cast. Connotation. Tb. Christopher columbus. Vector. Bipolar. Natal chart. Constitution. Metaphor examples. Villain. Laissez faire. Quizzizz. Lewis strauss. Leptospirosis. Pep guardiola. Weeping willow. Amy poehler. Hypertension. Silicon. Definition of. George strait songs. Castor oil benefits. Rna. Stephen colbert. 2024 eclipse. Twitter stock. Hot springs arkansas. Dystopia. Weight. Skeleton. Cry. Dev patel movies. Reconciliation. Peter o’toole. When is christmas. Fuse. Corn. Patronize meaning.
http://mexicanpharmgate.com/# best online pharmacies in mexico
Тут можно преобрести купить огнеупорный сейф сейф несгораемый купить
вывод из запоя цена ростов http://uaforum.ukrbb.net/viewtopic.php?f=13&t=3233/ .
ed medicine online: Canadian pharmacy best prices – mens ed
http://indianpharmacyeasy.com/# п»їlegitimate online pharmacies india
вывод из запоя круглосуточно ростов-на-дону pelsh.forum24.ru/?1-8-0-00000123-000-0-0-1730648899 .
вывод из запоя ростов на дону стационар https://www.www.bisound.com/forum/showthread.php?t=688674 .
http://indianpharmacyeasy.com/# indian pharmacy paypal
Online medicine home delivery pharmacy website india or best online pharmacy india
http://images.google.lv/url?q=https://indianpharmacyeasy.com mail order pharmacy india
reputable indian pharmacies cheapest online pharmacy india and indian pharmacy top 10 pharmacies in india
buying prescription drugs in mexico online reputable mexican pharmacies online or mexican online pharmacies prescription drugs
http://www.tifosy.de/url?q=https://mexicanpharmgate.com purple pharmacy mexico price list
mexican drugstore online medication from mexico pharmacy and п»їbest mexican online pharmacies mexico drug stores pharmacies
https://canadiandrugsgate.com/# anti fungal pills without prescription
Patient medication resource. Current drug information.
ed pills online
Medication overview available. Get pill facts.
canadiandrugsgate canadiandrugsgate or canadian pharmacy
http://www.hogwarts.be/frame.php?url=https://canadiandrugsgate.com canadian pharmacy drugs gate
Canadian pharmacy online Best Canadian online pharmacy and canadiandrugsgate.com Canada pharmacy
cheapest online pharmacy india: Online medicine home delivery – buy prescription drugs from india
best india pharmacy: Best Indian pharmacy – п»їlegitimate online pharmacies india
online shopping pharmacy india top 10 online pharmacy in india or cheapest online pharmacy india
https://lastapasdelola.com/?URL=https://indianpharmacyeasy.com:: buy medicines online in india
indian pharmacy Online medicine home delivery and Online medicine home delivery indian pharmacy paypal
https://canadiandrugsgate.com/# ed vacuum pumps
Сервисный центр предлагает замена экрана explay five замена камеры explay five
ed cure Canadian pharmacy online ed pills otc
injectable ed drugs: Canada pharmacy – buy prescription drugs
Pill facts here. Patient drug information.
order ed meds
Pill leaflet here. Generic names listed.
Тут можно преобрести купить сейф для ружья москва сейф шкаф купить
https://indianpharmacyeasy.com/# india pharmacy mail order
Canadian pharmacy best prices Best Canadian pharmacy or Canadian pharmacy best prices
http://www.mn2020.org/?URL=canadiandrugsgate.com canadiandrugsgate
Best Canadian pharmacy canadian drugs gate and Canadian pharmacy best prices canadian pharmacy
Unlock bonus rewards with 888Starz bonus codes and maximize your betting potential.
п»їbest mexican online pharmacies medicines mexico rx online mexico drug stores pharmacies
medicine in mexico pharmacies: mexican drugstore online – mexico pharmacies prescription drugs
https://indianpharmacyeasy.com/# Online medicine home delivery
https://canadiandrugsgate.com/# how to get prescription drugs without doctor
Главные новости мира https://ua-vestnik.com и страны: политика, экономика, спорт, культура, технологии. Оперативная информация, аналитика и эксклюзивные материалы для тех, кто следит за событиями в реальном времени.
https://indianpharmacyeasy.com/# mail order pharmacy india
Patient pill guide. Generic names listed.
buy ed pills
Find drug information. Access pill facts.
Online medicine order: Best Indian pharmacy – reputable indian online pharmacy
https://canadiandrugsgate.com/# best ed medications
п»їbest mexican online pharmacies buying prescription drugs in mexico or п»їbest mexican online pharmacies
http://web.fullsearch.com.ar/?url=http://mexicanpharmgate.com/ pharmacies in mexico that ship to usa
buying prescription drugs in mexico pharmacies in mexico that ship to usa and mexican drugstore online buying prescription drugs in mexico
buy prescription drugs from canada: Canadian pharmacy best prices – ed problems treatment
online medication: canadian pharmacy drugs gate – can ed be reversed
вывод из запоя цены ростов-на-дону masa.forum24.ru/?1-16-0-00002618-000-0-0-1730649347 .
Access medication facts. Get pill info.
buy ed pills pills online
Medicine trends described. Drug info here.
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.hentai-foundry.com/user/molagbal1965/profile
https://chyoa.com/user/cayman1231986
https://www.hentai-foundry.com/user/exglory1987/profile
http://www.babelcube.com/user/chris-venable
https://rentry.org/77by42sq
срочный вывод из запоя ростов ideya.forums.party/viewtopic.php?id=652 .
вывод из запоя ростовская область [url=www.to.iboard.ws/viewtopic.php?id=8058/]www.to.iboard.ws/viewtopic.php?id=8058/[/url] .
вывод из запоя на дому ростов круглосуточно [url=www.sportandpolitics.ukrbb.net/viewtopic.php?f=24&t=17793v]www.sportandpolitics.ukrbb.net/viewtopic.php?f=24&t=17793v[/url] .
purple pharmacy mexico price list Mexican Pharmacy Gate п»їbest mexican online pharmacies
http://mexicanpharmgate.com/# mexico drug stores pharmacies
buying prescription drugs in mexico: mexicanpharmgate.com – mexican online pharmacies prescription drugs
Judul: Menikmati Pengalaman Bermain dengan “PG Slot” di Situs Casino ImgToon.com
Dalam alam permainan kasino online, permainan slot telah jadi salah satu permainan yang paling digemari, terutama jenis PG Slot. Di antara berbagai situs kasino online, ImgToon.com adalah tujuan terbesar bagi pengguna yang ingin menguji keberuntungan mereka di berbagai permainan slot, termasuk beberapa kategori populer seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Mencoba Tanpa Risiko
Salah satu fungsi menarik yang diberikan oleh ImgToon.com adalah demo pg slot. Fitur ini memungkinkan pemain untuk memainkan berbagai jenis slot dari PG tanpa harus bertaruh taruhan sebenarnya. Dalam mode demo ini, Anda dapat memeriksa berbagai taktik dan memahami proses permainan tanpa bahaya kehilangan uang. Ini adalah metode terbaik bagi pemula untuk beradaptasi dengan permainan slot sebelum berpindah ke mode taruhan asli.
Mode demo ini juga memberikan Anda pandangan tentang potensi kemenangan dan hadiah yang mungkin bisa Anda peroleh saat bermain dengan uang asli. Pemain dapat mencari permainan tanpa ragu, menjadikan pengalaman bermain di PG Slot semakin menyenangkan dan bebas beban.
PG Slot Gacor: Prospek Besar Mencapai Kemenangan
PG Slot Gacor adalah kata populer di kalangan pemain slot yang mengacu pada slot yang sedang dalam fase memberikan kemenangan tinggi atau lebih sering disebut “gacor”. Di ImgToon.com, Anda dapat mencari berbagai slot yang ada dalam kategori gacor ini. Slot ini terkenal memiliki peluang kemenangan lebih tinggi dan sering menghadiahkan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, penting diingat bahwa “gacor” atau tidaknya sebuah slot dapat berubah, karena permainan slot tergantung generator nomor acak (RNG). Dengan memainkan secara rutin di ImgToon.com, Anda bisa mengidentifikasi pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan meningkatkan peluang Anda untuk menang.
RTP Slot: Faktor Krucial dalam Pemilahan Slot
Ketika membicarakan tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat penting untuk dipertimbangkan. RTP Slot mengacu pada persentase dari total taruhan yang akan dikirimkan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot disertai dengan informasi RTP yang terperinci. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk memperoleh kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat memaksimalkan pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini membuat RTP sebagai indikator krusial bagi pemain yang mencari keuntungan dalam permainan kasino online.
вывод из запоя дешево ростов на дону http://www.angelladydety.getbb.ru/viewtopic.php?f=44&t=42897 .
demo pg slot terpercaya
Judul: Merasakan Pengalaman Memainkan dengan “PG Slot” di Situs Casino ImgToon.com
Dalam dunia permainan kasino online, slot telah menyusun salah satu permainan yang paling digemari, terutama jenis PG Slot. Di antara banyak situs kasino online, ImgToon.com adalah tujuan utama bagi peserta yang ingin mencoba keberuntungan mereka di banyak permainan slot, termasuk beberapa kategori populer seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Memulai Bebas dari Risiko
Salah satu fitur menarik yang disediakan oleh ImgToon.com adalah demo pg slot. Fungsi ini mengizinkan pemain untuk memainkan berbagai jenis slot dari PG tanpa harus menempatkan taruhan uang asli. Dalam mode demo ini, Anda dapat menguji berbagai strategi dan memahami mekanisme permainan tanpa ancaman kehilangan uang. Ini adalah cara terbaik bagi pemain baru untuk terbiasa dengan permainan slot sebelum berpindah ke mode taruhan sebenarnya.
Mode demo ini juga memberi Anda pandangan tentang potensi kemenangan dan imbalan yang mungkin bisa Anda peroleh saat bermain dengan uang asli. Pemain dapat mencari permainan tanpa cemas, menjadikan pengalaman bermain di PG Slot semakin menyenangkan dan bebas tekanan.
PG Slot Gacor: Prospek Besar Mendapatkan Kemenangan
PG Slot Gacor adalah kata populer di kalangan pemain slot yang merujuk pada slot yang sedang dalam fase memberi kemenangan tinggi atau lebih sering dikenal “gacor”. Di ImgToon.com, Anda dapat mendapatkan berbagai slot yang termasuk dalam kategori gacor ini. Slot ini dikenal memiliki peluang kemenangan lebih tinggi dan sering memberikan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, harus diingat bahwa “gacor” atau tidaknya sebuah slot dapat beralih, karena permainan slot tergantung pada generator nomor acak (RNG). Dengan melakukan permainan secara rutin di ImgToon.com, Anda bisa mengenali pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan menambah peluang Anda untuk menang.
RTP Slot: Faktor Penting dalam Pemilahan Slot
Ketika berbicara tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat esensial untuk dihitung. RTP Slot mengacu pada persentase dari total taruhan yang akan dikembalikan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot diberi dengan informasi RTP yang terang. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk mendulang kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat mengelola pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini menyebabkan RTP sebagai indikator krusial bagi pemain yang mencari keuntungan dalam permainan kasino online.
https://mexicanpharmgate.com/# mexican mail order pharmacies
pg slot gacor terpercaya
Judul: Mengalami Pengalaman Bertaruh dengan “PG Slot” di Situs Perjudian ImgToon.com
Dalam kehidupan permainan kasino online, permainan slot telah jadi salah satu permainan yang paling digemari, terutama jenis PG Slot. Di antara banyak situs kasino online, ImgToon.com adalah tujuan pokok bagi pemain yang ingin mencoba nasib mereka di banyak permainan slot, termasuk beberapa kategori terfavorit seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Menjalani Bebas dari Risiko
Salah satu keistimewaan menarik yang diberikan oleh ImgToon.com adalah demo pg slot. Fungsi ini mengizinkan pemain untuk mencoba berbagai jenis slot dari PG tanpa harus bertaruh taruhan uang asli. Dalam mode demo ini, Anda dapat memeriksa berbagai strategi dan mengerti proses permainan tanpa risiko kehilangan uang. Ini adalah langkah terbaik bagi orang baru untuk mengenal dengan permainan slot sebelum mengalihkan ke mode taruhan sebenarnya.
Mode demo ini juga memberikan Anda gambaran tentang potensi kemenangan dan bonus yang mungkin bisa Anda terima saat bermain dengan uang asli. Pemain dapat menjelajahi permainan tanpa ragu, membuat pengalaman bermain di PG Slot semakin menyenangkan dan bebas beban.
PG Slot Gacor: Prospek Besar Mendulang Kemenangan
PG Slot Gacor adalah istilah terkenal di kalangan pemain slot yang mengacu pada slot yang sedang dalam fase memberikan kemenangan tinggi atau lebih sering dikenal “gacor”. Di ImgToon.com, Anda dapat menemukan berbagai slot yang masuk dalam kategori gacor ini. Slot ini terkenal memiliki peluang kemenangan lebih tinggi dan sering membagikan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, harus diingat bahwa “gacor” atau tidaknya sebuah slot dapat bergeser, karena permainan slot bergantung generator nomor acak (RNG). Dengan melakukan permainan secara rutin di ImgToon.com, Anda bisa menemukan pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan menambah peluang Anda untuk menang.
RTP Slot: Faktor Penting dalam Pemilihan Slot
Ketika mendiskusikan tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat krusial untuk dipertimbangkan. RTP Slot mengacu pada persentase dari total taruhan yang akan dikirimkan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot dilengkapi dengan informasi RTP yang jelas. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk mendulang kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat memaksimalkan pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini membuat RTP sebagai indikator utama bagi pemain yang mencari keuntungan dalam permainan kasino online.
Judul: Menikmati Pengalaman Bermain dengan “PG Slot” di Situs Perjudian ImgToon.com
Dalam alam permainan kasino online, mesin slot telah menyusun salah satu permainan yang paling digemari, terutama jenis PG Slot. Di antara banyak situs kasino online, ImgToon.com menjadi tujuan utama bagi pengguna yang ingin menguji peruntungan mereka di berbagai permainan slot, termasuk beberapa kategori terkenal seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Memulai Tanpa adanya Risiko
Salah satu fitur menarik yang ditawarkan oleh ImgToon.com adalah demo pg slot. Fitur ini mengizinkan pemain untuk menguji berbagai jenis slot dari PG tanpa harus memasang taruhan uang asli. Dalam mode demo ini, Anda dapat menguji berbagai strategi dan mengetahui sistem permainan tanpa bahaya kehilangan uang. Ini adalah cara terbaik bagi pemula untuk terbiasa dengan permainan slot sebelum berpindah ke mode taruhan asli.
Mode demo ini juga memberikan Anda pemahaman tentang potensi kemenangan dan imbalan yang mungkin bisa Anda terima saat bermain dengan uang sebenarnya. Pemain dapat mencari permainan tanpa khawatir, membuat pengalaman bermain di PG Slot semakin membahagiakan dan bebas stres.
PG Slot Gacor: Kesempatan Besar Mendapatkan Kemenangan
PG Slot Gacor adalah sebutan terkenal di kalangan pemain slot yang menggunakan pada slot yang sedang dalam fase memberikan kemenangan tinggi atau lebih sering diistilahkan “gacor”. Di ImgToon.com, Anda dapat menemukan berbagai slot yang masuk dalam kategori gacor ini. Slot ini diakui memiliki peluang kemenangan lebih tinggi dan sering menghadiahkan bonus besar, menyebabkannya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, harus diingat bahwa “gacor” atau tidaknya sebuah slot dapat beralih, karena permainan slot tergantung pada generator nomor acak (RNG). Dengan memainkan secara rutin di ImgToon.com, Anda bisa mengenali pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan menambah peluang Anda untuk menang.
RTP Slot: Faktor Krucial dalam Pemilahan Slot
Ketika mendiskusikan tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat penting untuk diperhatikan. RTP Slot merujuk pada persentase dari total taruhan yang akan dikembalikan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot dilengkapi dengan informasi RTP yang terang. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk memperoleh kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat memaksimalkan pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini membuat RTP sebagai indikator utama bagi pemain yang mencari keuntungan dalam permainan kasino online.
Judul: Menikmati Pengalaman Bertaruh dengan “PG Slot” di Situs Casino ImgToon.com
Dalam alam permainan kasino online, mesin slot telah jadi salah satu permainan yang paling diminati, terutama jenis PG Slot. Di antara beberapa situs kasino online, ImgToon.com menjadi tujuan utama bagi peserta yang ingin menguji nasib mereka di banyak permainan slot, termasuk beberapa kategori terfavorit seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Menjalani Bebas dari Risiko
Salah satu fungsi menarik yang ditawarkan oleh ImgToon.com adalah demo pg slot. Keistimewaan ini memungkinkan pemain untuk memainkan berbagai jenis slot dari PG tanpa harus memasang taruhan nyata. Dalam mode demo ini, Anda dapat menguji berbagai strategi dan mengerti proses permainan tanpa bahaya kehilangan uang. Ini adalah cara terbaik bagi pemain baru untuk mengenal dengan permainan slot sebelum mengalihkan ke mode taruhan sebenarnya.
Mode demo ini juga menyediakan Anda pandangan tentang potensi kemenangan dan imbalan yang mungkin bisa Anda dapatkan saat bermain dengan uang sebenarnya. Pemain dapat menyusuri permainan tanpa ragu, menciptakan pengalaman bermain di PG Slot semakin membahagiakan dan bebas tekanan.
PG Slot Gacor: Kesempatan Besar Mencapai Kemenangan
PG Slot Gacor adalah sebutan terkenal di kalangan pemain slot yang mengacu pada slot yang sedang dalam fase memberikan kemenangan tinggi atau lebih sering disebut “gacor”. Di ImgToon.com, Anda dapat mendapatkan berbagai slot yang termasuk dalam kategori gacor ini. Slot ini diakui memiliki peluang kemenangan lebih tinggi dan sering menghadiahkan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, harus diingat bahwa “gacor” atau tidaknya sebuah slot dapat bergeser, karena permainan slot tergantung generator nomor acak (RNG). Dengan bermain secara rutin di ImgToon.com, Anda bisa mengidentifikasi pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan memperbesar peluang Anda untuk menang.
RTP Slot: Faktor Krucial dalam Pencarian Slot
Ketika berbicara tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat esensial untuk dihitung. RTP Slot mengacu pada persentase dari total taruhan yang akan dipulangkan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot diberi dengan informasi RTP yang jelas. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk mendulang kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat mengelola pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini menjadikan RTP sebagai indikator utama bagi pemain yang mencari keuntungan dalam permainan kasino online.
Access drug details. Dosing guidelines here.
ed pills online
Latest medication news. Comprehensive drug overview.
pg slot terpercaya
Judul: Merasakan Pengalaman Bertaruh dengan “PG Slot” di Situs Casino ImgToon.com
Dalam kehidupan permainan kasino online, permainan slot telah jadi salah satu permainan yang paling diminati, terutama jenis PG Slot. Di antara banyak situs kasino online, ImgToon.com adalah tujuan terbesar bagi peserta yang ingin menguji nasib mereka di beragam permainan slot, termasuk beberapa kategori terfavorit seperti demo pg slot, pg slot gacor, dan RTP slot.
Demo PG Slot: Memulai Tanpa adanya Risiko
Salah satu fitur menarik yang ditawarkan oleh ImgToon.com adalah demo pg slot. Fitur ini memberikan pemain untuk mencoba berbagai jenis slot dari PG tanpa harus menempatkan taruhan nyata. Dalam mode demo ini, Anda dapat memeriksa berbagai taktik dan memahami proses permainan tanpa bahaya kehilangan uang. Ini adalah langkah terbaik bagi pemula untuk mengenal dengan permainan slot sebelum beralih ke mode taruhan nyata.
Mode demo ini juga memberikan Anda pemahaman tentang potensi kemenangan dan hadiah yang mungkin bisa Anda terima saat bermain dengan uang nyata. Pemain dapat menjelajahi permainan tanpa cemas, membuat pengalaman bermain di PG Slot semakin menyenangkan dan bebas stres.
PG Slot Gacor: Peluang Besar Mendapatkan Kemenangan
PG Slot Gacor adalah sebutan populer di kalangan pemain slot yang mengacu pada slot yang sedang dalam fase memberikan kemenangan tinggi atau lebih sering dikenal “gacor”. Di ImgToon.com, Anda dapat menemukan berbagai slot yang masuk dalam kategori gacor ini. Slot ini terkenal memiliki peluang kemenangan lebih tinggi dan sering membagikan bonus besar, membuatnya pilihan utama bagi para pemain yang ingin memperoleh keuntungan maksimal.
Namun, harus diingat bahwa “gacor” atau tidaknya sebuah slot dapat bergeser, karena permainan slot tergantung generator nomor acak (RNG). Dengan bermain secara rutin di ImgToon.com, Anda bisa menemukan pola atau waktu yang tepat untuk memainkan PG Slot Gacor dan menambah peluang Anda untuk menang.
RTP Slot: Faktor Krucial dalam Pencarian Slot
Ketika berbicara tentang slot, istilah RTP (Return to Player) adalah faktor yang sangat krusial untuk dipertimbangkan. RTP Slot mengacu pada persentase dari total taruhan yang akan dikembalikan kepada pemain dalam jangka panjang. Di ImgToon.com, setiap permainan PG Slot dilengkapi dengan informasi RTP yang jelas. Semakin tinggi persentase RTP, semakin besar peluang pemain untuk memperoleh kembali sebagian besar dari taruhan mereka.
Dengan memilih PG Slot yang memiliki RTP tinggi, pemain dapat mengelola pengeluaran mereka dan memiliki peluang yang lebih baik untuk menang dalam jangka panjang. Ini menjadikan RTP sebagai indikator krusial bagi pemain yang mencari keuntungan dalam permainan kasino online.
Тут можно преобрести несгораемый сейф несгораемые сейфы
http://canadiandrugsgate.com/# non prescription ed drugs
mexico drug stores pharmacies mexican pharmacy online medications pharmacies in mexico that ship to usa
top 10 online pharmacy in india india online pharmacy or online shopping pharmacy india
http://www.wzdq123.com/go.php?url=http://indianpharmacyeasy.com pharmacy website india
reputable indian pharmacies top 10 online pharmacy in india and world pharmacy india online shopping pharmacy india
Тут можно преобрести купить сейф для охотничьего ружья в москве оружейные сейфы для охотничьего ружья
comparison of ed drugs: Canada pharmacy – cheap ed pills
Best Canadian pharmacy canadiandrugsgate.com or canadian drugs gate
https://www.schornsteinfeger-duesseldorf.de/redirect.php?url=http://canadiandrugsgate.com  canadian drugs gate
canadiandrugsgate Best Canadian pharmacy and Canadian pharmacy online canadian pharmacy drugs gate
top online pharmacy india india online pharmacy or buy medicines online in india
http://www.lobenhausen.de/url?q=https://indianpharmacyeasy.com top 10 pharmacies in india
online shopping pharmacy india reputable indian pharmacies and indian pharmacy online mail order pharmacy india
Canadian pharmacy online canadiandrugsgate or canadiandrugsgate
https://images.google.com.mm/url?q=https://canadiandrugsgate.com canadian pharmacy
canadiandrugsgate Canadian pharmacy online and canadian pharmacy canadiandrugsgate
https://canadiandrugsgate.com/# medication for ed dysfunction
best online pharmacies in mexico: MexicanPharmGate – п»їbest mexican online pharmacies
Online medicine home delivery: Best online Indian pharmacy – mail order pharmacy india
Online medicine order: Indian pharmacy international shipping – top 10 online pharmacy in india
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
https://canadiandrugsgate.com/# ed treatments
Здесь можно преобрести купить сейф в москве в магазине сейфов где купить сейф
Pill impacts explained. Pill overview available.
buy ed pills no rx
Comprehensive drug guide. Drug impacts explained.
medication from mexico pharmacy mexican rx online or mexican drugstore online
https://maps.google.com.ni/url?sa=t&url=https://mexicanpharmgate.com п»їbest mexican online pharmacies
mexican pharmaceuticals online п»їbest mexican online pharmacies and mexican rx online mexico drug stores pharmacies
top online pharmacy india indianpharmacyeasy.com indian pharmacy
mexican pharmaceuticals online: mexican pharmacy – mexican drugstore online
Your article helped me a lot, is there any more related content? Thanks!
п»їbest mexican online pharmacies MexicanPharmGate mexican online pharmacies prescription drugs
Medication leaflet here. Drug resource available.
ed pills
Latest drug news. Medication resource here.
http://indianpharmacyeasy.com/# best online pharmacy india
Продамус промокод prodamus-promokod21.ru .
buying prescription drugs in mexico online: MexicanPharmGate – mexico pharmacies prescription drugs
Jugabet bonos exclusivos Jugabet bonos exclusivos .
http://mexicanpharmgate.com/# buying prescription drugs in mexico online
вывод из запоя на дому ростов на дону http://honey.ukrbb.net/viewtopic.php?f=45&t=16673/ .
нарколог вывод из запоя ростов [url=http://www.pandora.ukrbb.net/viewtopic.php?f=2&t=12272]нарколог вывод из запоя ростов[/url] .
нарколог вывод из запоя ростов http://www.family2.quadrobb.me/viewtopic.php?id=1836#p6861/ .
Drug guide here. Comprehensive pill overview.
ed pills online
Comprehensive medication facts. Pill leaflet available.
вывод. из. запоя. анонимно. ростов. https://ximki.ukrbb.net/viewtopic.php?f=12&t=3696 .
ed doctor: Canadian pharmacy online – men with ed
Тут можно преобрести сейф для хранения оружия купить сейф для охотничьего карабина
Тут можно преобрести сейф противопожарный купить несгораемые сейфы
top 10 online pharmacy in india: Indian online pharmacy ship to usa – indian pharmacy online
mexican online pharmacies prescription drugs mexican pharmaceuticals online or mexican border pharmacies shipping to usa
http://www.boosterblog.es/votar-12428-11629.html?adresse=mexicanpharmgate.com" mexican mail order pharmacies
mexican pharmaceuticals online medication from mexico pharmacy and п»їbest mexican online pharmacies mexican drugstore online
mexican drugstore online mexican border pharmacies shipping to usa or mexican online pharmacies prescription drugs
https://toolbarqueries.google.ml/url?q=http://mexicanpharmgate.com reputable mexican pharmacies online
mexican pharmaceuticals online buying prescription drugs in mexico and reputable mexican pharmacies online mexico pharmacies prescription drugs
amoxicillin 500mg capsules antibiotic https://clomidrexpharm.com/# get generic clomid tablets
Sahabet, dostluk ve samimiyetin buluştuğu özel bir alan. Birbirimizi anlamak ve paylaşmak için buradayız. Haydi, birlikte keşfedelim!
priligy: buy priligy – buy priligy max pharm
where can you get amoxicillin http://amoxilcompharm.com/# amoxicillin 500mg prescription
prednisone brand name in usa: generic Prednisone – buying prednisone on line
I would also motivate just about every person to save this web page for any favorite assistance to assist posted the appearance.
cheap priligy buy dapoxetine online buy priligy
priligy dapoxetine price priligy
items4games
Uncover a Realm of Gaming Chances with ItemsforGames
At Items4Play, we offer a active hub for gamers to buy or trade profiles, virtual items, and services for top games. Whether you are seeking to improve your gaming resources or looking to monetize your profile, our site offers a smooth, safe, and profitable experience.
Reasons to Select ItemsforGames?
**Comprehensive Game Collection**: Explore a extensive selection of video games, from intense titles such as Battlefield and Call of Duty to captivating role-playing games such as ARK: Survival Evolved and Genshin Impact. We have all games, guaranteeing no gamer is excluded.
**Range of Services**: Our offerings cover game account acquisitions, virtual money, exclusive collectibles, achievements, and mentoring options. If you need guidance gaining levels or obtaining premium bonuses, we’ve got you covered.
**User-friendly**: Browse without hassle through our well-organized marketplace, categorized alphabetically to find just the item you are looking for quickly.
**Safe Deals**: We prioritize your protection. All exchanges on our marketplace are conducted with the utmost security to protect your confidential and payment information.
**Highlights from Our Collection**
– **Adventure and Survival**: Games like ARK and DayZ allow you to dive into challenging environments with high-quality goods and keys on offer.
– **Adventure and Adventure**: Boost your gameplay in titles like Clash Royale and Age of Wonders 4 with in-game currencies and options.
– **Professional Play**: For eSports enthusiasts, boost your skills with coaching and level-ups for Val, DotA, and LoL.
**A Marketplace Made for Gamers**
Operated by ApexTech, a established company officially recognized in Kazakhstan, ItemsforGames is a place where video game dreams become real. From buying early access passes for the freshest titles to getting unique virtual goods, our site fulfills every player’s need with expertise and reliability.
Become part of the group today and elevate your gaming play!
For inquiries or guidance, email us at **[email protected]**. Together, let’s enjoy gaming, as one!
Access medication facts. Complete pill overview.
ed pills online
Get details now. Get drug details.
Para los amantes del juego, 1xslots mobile es la opcion perfecta.
prednisone no rx: Prednisone Without Prescription – prednisone 20 mg prices
prednisone price: generic Prednisone – buy prednisone nz
Thanks. https://docs.webstore.guru/
buy prednisone online without a script: buy prednisone – prednisone 50
線上德州撲克
can you buy amoxicillin over the counter canada http://priligymaxpharm.com/# priligy
amoxicillin canada price http://amoxilcompharm.com/# amoxicillin online no prescription
Thanks. https://casibom.agency/
prednisone pak: prednisone – prednisone 30 mg tablet
Find drug information. Find pill information.
buy ed pills medication
Medication information here. Drug leaflet available.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
amoxicillin 500 capsule: where can you get amoxicillin – amoxicillin 500 mg tablet price
Узнай все о рецидив варикоцеле варикоцеле у мужчин
In-game items
Uncover a Realm of Gaming Possibilities with Items4Play
At Items4Games, we offer a dynamic platform for gamers to buy or exchange profiles, goods, and features for top games. If you are seeking to enhance your gaming resources or interested in selling your profile, our platform provides a easy, secure, and valuable process.
Why Select ItemsforGames?
**Broad Game Library**: Discover a large array of titles, from intense games like Battlefield and Call of Duty to captivating RPGs like ARK and Genshin. We include it all, ensuring no gamer is overlooked.
**Selection of Options**: Our products include game account acquisitions, virtual money, rare items, trophies, and mentoring options. If you need assistance leveling up or obtaining premium benefits, we have it all.
**Ease of Use**: Browse easily through our well-organized platform, arranged in order to find just what you are looking for fast.
**Safe Deals**: We prioritize your security. All transactions on our platform are conducted with the top protection to protect your private and monetary data.
**Standouts from Our Offerings**
– **Adventure and Exploration**: Games ARK: Survival Evolved and DayZ let you dive into tough environments with high-quality items and keys available.
– **Adventure and Adventure**: Elevate your performance in titles like Clash Royale and Age of Wonders 4 with in-game currencies and options.
– **Competitive Competitions**: For serious players, enhance your technique with coaching and account upgrades for Valorant, DotA, and Legends.
**A Marketplace Made for Gamers**
Supported by Apex Technologies, a reliable business certified in Kazakh Republic, ItemsforGames is a market where video game dreams are realized. From acquiring advance codes for the latest games to locating unique virtual goods, our marketplace meets every player’s requirement with expertise and efficiency.
Join the group today and elevate your game adventure!
For support or help, email us at **[email protected]**. Together, let’s game better, as a community!
can i buy amoxicillin over the counter: com pharm – amoxicillin pills 500 mg
Get medication details. Patient medication guide.
buy ed pills online
Read about medications. Access medication details.
Holiganbet giriş adresi ile ilgili merak edilenleri keşfedeceğimiz bu yazıda, heyecan verici bir yolculuğa çıkacağız.
buy priligy Priligy tablets priligy max pharm
купить диплом технолога пищевого производства купить диплом технолога пищевого производства .
Thanks. https://casiboms.it.com/
can i purchase clomid tablets buy clomid can you buy cheap clomid prices
南国育ち(夏25)
k8 カジノ パチンコ
パチンコは、単なる遊びではなく、技術や戦略が求められるゲームです。深い楽しみがあります。
CRA牙狼金色になれザルバとの契約
https://hithot.jp/news/11204.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
CR 花の慶次斬
オンラインカジノ
新鬼武者 再臨
CR真・北斗無双
翔馬
超時空要塞マクロス
https://www.pachinko777.asia/article/232.html
パチンコは、単なる運試しではなく、戦略的に楽しむゲームです。考える楽しさがあります。
CRグラップラー刃牙
https://www.k8.monster/Articles/1466.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
P 新世紀エヴァンゲリオン15 未来への咆哮
https://www.2rk8.net/2019
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特にバトル演出が楽しい。
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали сервисный центр xiaomi в москве, можете посмотреть на сайте: сервисный центр xiaomi в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
вывод из запоя ростов-на-дону gaslo.ukrbb.net/viewtopic.php?f=13&t=3407 .
buy clomid tablets: clomid – cost of generic clomid without insurance
amoxicillin 500mg capsules antibiotic https://prednisoneraypharm.com/# prednisone generic brand name
40 mg prednisone pill how much is prednisone 10 mg or online order prednisone
http://naiyoujc.ff66.net/productshow.asp?id=30&mnid=51913&url=http://prednisoneraypharm.com prednisone 20mg buy online
prednisone buy canada cheap prednisone online and can i buy prednisone over the counter in usa where to buy prednisone without prescription
. https://diigo.com/0y04j4/ .
. https://www.bookmarkstore.download/story.php?title=porno-movies-6#discuss .
buy priligy: priligy maxpharm – dapoxetine online
Drug facts here. Comprehensive medicine guide.
ed pills online
Drug pamphlet provided. Patient medicine info.
buy amoxicillin online uk https://clomidrexpharm.com/# cost generic clomid no prescription
prednisone 20 mg pill prednisone buy online nz or buy prednisone online fast shipping
https://maps.google.lk/url?sa=i&url=https://prednisoneraypharm.com where to buy prednisone in australia
prednisone 10mg buy online prednisone 10 mg tablets and prednisone 20mg online pharmacy prednisone brand name in india
вывод из запоя на дому https://recordrpservak.ukrbb.net/viewtopic.php?f=31&t=1155 .
Side effects explained. Comprehensive medicine resource.
[url=http://re-file.com/cushion.php?url=https%3A%2F%2Fwww.erectiledysfunctionpills-365.com]ed pills online[/url]
Access medication facts. Find pill facts.
max pharm: max pharm – priligy max pharm
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.obesityhelp.com/members/gluconaft1968/about_me/
http://www.babelcube.com/user/joe-day
https://cannabis.net/user/154248
https://www.haikudeck.com/presentations/AC8sqIiED0
https://chyoa.com/user/suzuci1977
buy prednisone 10 mg: prednisone – prednisone tablets
how much is amoxicillin prescription: com pharm – buy amoxicillin online with paypal
Узнай все о варикоцеле левого яичка варикоцеле
amoxicillin from canada order amoxicillin online no prescription or how to get amoxicillin
https://clients1.google.com.hk/url?q=https://amoxilcompharm.com amoxicillin 500mg over the counter
buy amoxicillin 500mg canada azithromycin amoxicillin and amoxicillin 875 mg tablet amoxicillin 800 mg price
Get medicine info. Medicine facts provided.
ed pills online
Find medication facts. Medicine impacts described.
priligy maxpharm dapoxetine price max pharm
buy amoxicillin online with paypal buy amoxicillin from canada amoxicillin from canada
鬼武者3 時空天翔
オンラインカジノ
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CR北斗の拳7 転生 Ver.319
https://pachika.com/machine/post-1269.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR牙狼魔戒ノ花
k8 カジノ パチンコ モンスターハンターパチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
ガメラハイグレードビジョン
Pこの素晴らしい世界に祝福を!199LT「このラッキートリガーに祝福を!」
ジャンキージャグラー
水浒伝天下
https://www.pachirep.com/article/64.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR北斗の拳7 転生
https://k8128.com/2151
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
夜蝶飛翔
https://www.runaroundbetties.com/article/76.html
音楽と演出が楽しく、プレイ中に笑顔になれます。リラックスできます。
can i purchase clomid without prescription: clomid – buying generic clomid tablets
amoxicillin 250 mg: amoxil – amoxicillin in india
average cost of prednisone: generic Prednisone – prednisone
amoxicillin online no prescription https://prednisoneraypharm.com/# how to purchase prednisone online
Patient medicine guide. Comprehensive pill overview.
buy ed pills online without prescription
Medication guide available. Comprehensive drug resource.
принудительный вывод из запоя ростов https://zarabotokdoma.creartuforo.com/viewtopic.php?id=11471 .
otc prednisone cream prednisone 20 mg prices or 10 mg prednisone
http://www.camping-channel.eu/surf.php3?id=2973&url=http://prednisoneraypharm.com prednisone prescription online
[url=https://clients1.google.ie/url?q=https://prednisoneraypharm.com]cost of prednisone 5mg tablets[/url] prednisone 10mg tablet price and [url=http://www.bqmoli.com/bbs/home.php?mod=space&uid=6095]prednisone 20 mg purchase[/url] prednisone without prescription.net
by prednisone w not prescription prednisone 20mg online or prednisone 30 mg tablet
https://cse.google.gp/url?sa=t&url=https://prednisoneraypharm.com buy prednisone mexico
prednisone 20mg online how to get prednisone without a prescription and prednisone tablets ordering prednisone
нарколог на дом цены нарколог на дом цены .
вызвать нарколога на дом вызвать нарколога на дом .
вызов нарколога на дом краснодар [url=http://mozaisk.anihub.me/viewtopic.php?id=4368]http://mozaisk.anihub.me/viewtopic.php?id=4368[/url] .
priligy max pharm: buy dapoxetine online – dapoxetine price
Тут можно преобрести где купить сейф для ружья оружейные шкафы
can i buy generic clomid online can i order generic clomid without dr prescription or can i order cheap clomid for sale
https://www.google.ht/url?q=https://clomidrexpharm.com can you buy generic clomid tablets
where can i buy generic clomid now how to buy cheap clomid tablets and can you get generic clomid pills how to buy clomid price
can i get cheap clomid without insurance: rex pharm – can you get generic clomid for sale
Thanks. https://extensions.work/
how to get generic clomid for sale clomid buy cheap clomid prices
buy priligy cheap priligy cheap priligy
Çağlayan-Kağıthane Escort❤️bayanları sitemizde bularak onlarla doyumsuz seks yapmaya hazır olun. Tek yapmanız gereken Kağıthane-Çağlayan Escort❤️bayan sitemizi ziyaret etmeniz.
how to get clomid pills: rex pharm – how to get cheap clomid without rx
amoxicillin 500 mg online can i buy amoxicillin online or can i buy amoxicillin over the counter
https://images.google.com.ph/url?q=https://amoxilcompharm.com how to buy amoxicillin online
where can i buy amoxicillin online amoxicillin 500mg prescription and price of amoxicillin without insurance cost of amoxicillin prescription
cost of clomid prices: clomid online – how to buy cheap clomid tablets
how to get generic clomid pill: how to get generic clomid online – where can i buy clomid for sale
Узнай все о клиника варикоцеле заболевание варикоцеле
order cheap clomid without prescription can i get generic clomid prices or get cheap clomid pills
http://www.taskmanagementsoft.com/bitrix/redirect.php?event1=tm_sol&event2=task-tour-flash&goto=https://clomidrexpharm.com get clomid without a prescription
buying cheap clomid online where can i buy generic clomid without insurance and where buy generic clomid pill buying generic clomid without a prescription
Latest medication developments. Comprehensive medication overview.
proscar
Comprehensive pill resource. Medicine trends available.
нарколог на дом круглосуточно spilkuvannya.rolevaya.com/viewtopic.php?id=66 .
amoxicillin online pharmacy: amoxicillin 250 mg price in india – amoxicillin from canada
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
amoxicillin over counter: amoxil com pharm – where can i buy amoxicillin without prec
buy amoxicillin 500mg online https://priligymaxpharm.com/# priligy maxpharm
Complete medication overview. Comprehensive drug resource.
buy finasteride pills
Drug leaflet here. Drug resource available.
priligy: priligy maxpharm – buy priligy
buy amoxicillin without prescription: Com Pharm – generic for amoxicillin
CRぱちんこ仮面ライダーMAX EDITION
ミスティーノ デビットカード
パチンコは日本独特のエンターテイメントで、スリルと興奮が詰まっています。特に大当たりの瞬間は最高の快感です。
CR JAWS再臨-SHARK PANIC AGAIN-
https://k8slots.jp/casino/2662.html
多彩なリーチ演出があり、どのタイミングで当たるかドキドキします。
CR鉄拳2 闘神
k8 カジノ 戦国パチスロ花の慶次~戦極めし傾奇者の宴~;
戦国BASARA3
めんそーれ(雷電ver.)
CRぱちんこGANTZ
SLOTバジリスク~甲賀忍法帖~Ⅲ
https://www.casinobaccarat.asia/article/115.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
鯉の滝登り
https://www.stelizabethchicago.org/article/151.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。
シティーハンター
https://www.k8.boston/Articles/2046.html
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
cost of amoxicillin 875 mg amoxicillin 500 mg tablets or buy amoxicillin online with paypal
http://cse.google.nu/url?q=http://amoxilcompharm.com can we buy amoxcillin 500mg on ebay without prescription
can you buy amoxicillin uk amoxicillin no prescription and amoxicillin without a prescription amoxil generic
Medication leaflet provided. Get pill info.
buy finasteride pills
Pill essentials explained. Patient medicine info.
buy priligy max pharm: priligy maxpharm – buy priligy
Free PDF editor
how to get cheap clomid prices: where to buy generic clomid now – where to buy generic clomid without a prescription
where can i get cheap clomid without dr prescription: rex pharm – where to get generic clomid
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
where can i buy amoxocillin: medicine amoxicillin 500mg – amoxicillin order online
Thanks. https://casibom.works/
cost cheap clomid online: buy clomid – where buy clomid without a prescription
Medication leaflet provided. Drug facts provided.
buy proscar online
Drug details provided. Drug trends described.
max pharm: priligy max pharm – dapoxetine online
Pハイスクール・フリート
k8 カジノ 押忍!サラリーマン番長
友人と一緒に行くことで、競争心が芽生え、さらに楽しめます。勝負の結果が気になります。
3x3EYES聖魔覚醒
https://www.8casino8.com/article/195.html
台のデザインが豊富で、飽きずに楽しめます。自分好みの台を探すのが楽しいです。
交響詩篇エウレカセブン 2
花の慶次 琉
CRフィーバー戦姫絶唱シンフォギア
アナザーゴッドハーデス-奪われたZEUSver
プレミアムビンゴ
イミソーレ(紫花)(赤)
https://jahromblog.com/Articles/1539.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
シンドバッドアドベンチャーは榎本加奈子でどうですか
https://www.gantz-pachinko.com/article/1411.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
Dororonえん魔くん メ~ラめら
https://www.bakabon-pachinko.com/article/1106.html
美しいグラフィックと音楽が、プレイ中の没入感を高めます。
get cheap clomid without rx cost cheap clomid price or buy generic clomid without insurance
https://www.google.com.pr/url?q=https://clomidrexpharm.com order generic clomid
cost cheap clomid online cost cheap clomid without dr prescription and buying cheap clomid without insurance can i purchase clomid without rx
Beşiktaş Escort ❤️Bebek Escort❤️Etiler Escort Bayanlar
amoxicillin 500 mg tablet price buy amoxicillin 250mg or azithromycin amoxicillin
https://images.google.co.uz/url?q=https://amoxilcompharm.com amoxicillin 250 mg price in india
over the counter amoxicillin ampicillin amoxicillin and where to buy amoxicillin amoxicillin order online
dapoxetine online: buy dapoxetine online – Priligy tablets
Find pill facts. Medication information here.
buy proscar 5 mg
Comprehensive pill guide. Get medication facts.
how can i get generic clomid without dr prescription can i get cheap clomid no prescription or cost generic clomid pills
https://www.google.mu/url?q=https://clomidrexpharm.com how to get generic clomid without insurance
can i order clomid pill how to get clomid without insurance and buy generic clomid pill how to get generic clomid without dr prescription
MetaMask Extension provides secure wallet integration, dApp connectivity, and seamless access to DeFi platforms. Start exploring Web3 today! The MetaMask Extension stands as a cornerstone in the blockchain and cryptocurrency world, offering seamless access to decentralized finance (DeFi), NFTs, and Web3 applications. https://webstore.work/
max pharm: priligy maxpharm – buy priligy max pharm
Uncover a World of Virtual Possibilities with Items4Play
At Items4Games, we deliver a active marketplace for enthusiasts to purchase or sell accounts, items, and services for widely played video games. If you’re looking to upgrade your gaming resources or interested in selling your account, our site provides a seamless, protected, and profitable process.
Reasons to Choose ItemsforGames?
**Comprehensive Title Catalog**: Browse a extensive selection of video games, from intense adventures like Warzone and COD to engaging RPGs such as ARK and Genshin. We cover all games, ensuring no gamer is overlooked.
**Variety of Options**: Our products feature account purchases, credits, rare collectibles, achievements, and training services. If you need assistance improving or obtaining premium benefits, we are here to help.
**Easy Navigation**: Explore easily through our systematized site, organized alphabetically to get precisely the game you need with ease.
**Protected Deals**: We prioritize your security. All exchanges on our marketplace are processed with the highest security to guard your confidential and financial details.
**Key Points from Our Offerings**
– **Action and Adventure**: Games like ARK: Survival Evolved and Day-Z allow you to dive into exciting settings with premium items and passes for sale.
– **Adventure and Questing**: Elevate your performance in adventures such as Royal Clash and Age of Wonders 4 with credits and services.
– **Competitive Play**: For serious players, enhance your skills with coaching and level-ups for Valorant, DotA, and LoL.
**A Platform Built for Fans**
Backed by ApexTech, a established business registered in Kazakh Nation, ItemsforGames is a hub where gaming dreams come true. From purchasing early access codes for the newest releases to getting unique virtual goods, our site meets every gaming requirement with professionalism and reliability.
Join the community right away and upgrade your gaming experience!
For inquiries or help, email us at **[email protected]**. Together, let’s improve the game, as one!
cheap priligy: dapoxetine online – cheap priligy
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://rentry.org/zhtrhzmo
https://cannabis.net/user/158266
https://cannabis.net/user/156087
https://haveagood.holiday/users/363312
http://www.babelcube.com/user/laura-white
can i order clomid: generic clomid – where to buy generic clomid online
prednisone 54899: prednisoneraypharm – prednisone acetate
https://viastoer.blogspot.com/2024/09/blog-post_79.html
https://hallbook.com.br/blogs/300839/%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC-%EA%B5%AC%EB%A7%A4-%ED%9B%84-%ED%9A%A8%EA%B3%BC%EB%A5%BC-%EA%B7%B9%EB%8C%80%ED%99%94%ED%95%98%EB%8A%94-%EB%B0%A9%EB%B2%95
Drug trends described. Drug leaflet available.
finasteride
Comprehensive drug overview. Comprehensive pill guide.
гардина с электроприводом гардина с электроприводом .
нарколог на дом краснодар http://zavitai.mybb.social/viewtopic.php?id=89/ .
нарколог краснодар [url=http://belbeer.borda.ru/?1-6-0-00000754-000-0-0-1730730058/]http://belbeer.borda.ru/?1-6-0-00000754-000-0-0-1730730058/[/url] .
платный нарколог на дом http://www.svstrazh.forum24.ru/?1-3-0-00000233-000-0-0-1730729693 .
нарколог на дом в краснодаре https://chesskomi.borda.ru/?1-8-0-00003045-000-0-0-1730729839 .
max pharm: priligy max pharm – priligy
https://xn--w3-hs1izvv81cmb366re3s.mystrikingly.com/blog/798b402f072
Medicine information provided. Find medication details.
proscar cheap
Current medication trends. Pill info available.
https://hallbook.com.br/blogs/295156/%EA%B4%91%EA%B3%A0-%EC%84%B1%EA%B3%BC-%EB%B6%84%EC%84%9D-%EB%84%A4%EC%9D%B4%EB%B2%84-%ED%94%8C%EB%9E%AB%ED%8F%BC%EC%97%90%EC%84%9C-%EC%84%B1%EA%B3%BC%EB%A5%BC-%EB%AA%A8%EB%8B%88%ED%84%B0%EB%A7%81%ED%95%98%EA%B3%A0-%EC%B5%9C%EC%A0%81%ED%99%94%ED%95%98%EB%8A%94-%EB%B0%A9%EB%B2%95
скачать приложения казино https://www.gatesc.mx/bez-rubriki/melbet-casino-onlajn-oficialnyj-sajt-melbet-7/
https://medium.com/@nsw5288/%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC%EC%99%80-%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC-%EC%A0%9C%EB%84%A4%EB%A6%AD-%EB%AC%B4%EC%97%87%EC%9D%84-%EC%84%A0%ED%83%9D%ED%95%A0%EA%B9%8C-145e148e19c8
https://responsible-seal-dd3cm4.mystrikingly.com/blog/6df16315c81
https://writeablog.net/cxt2qn6408
mexico pharmacies prescription drugs https://mexicanpharmgate.com/ buying from online mexican pharmacy
mexican border pharmacies shipping to usa https://mexicanpharmgate.com/ buying prescription drugs in mexico
buy clomid without prescription: clomid – can i buy cheap clomid tablets
Узнай все о удаление полипа эндометрия москваполипы удаление гистероскопия цена
buy Clopidogrel over the counter: cheapest plavix – buy clopidogrel bisulfate
ヒデキに夢中!!
k8スロット
演出が豪華で、観ているだけでも楽しめます。特にボーナス演出は圧巻です。
サラリーマン金太郎 出世回胴編
https://idtc.jp/article/4448.html
パチンコは一度ハマると抜け出せない魅力があります。友人と競い合うことで、さらに楽しさが増します。
めんそーれ
k8 カジノ パチンコ
CR聖闘士星矢 星の運命
シンドバッドアドベンチャーは榎本加奈子でどうですか
青龍
吉宗 2003
https://www.queencasino.co.in/article/285.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
デビル メイ クライ クロス
https://www.a-casino.org/article/257.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にバトル時が最高。
S この素晴らしい世界に祝福を!
https://www.casino-eldoa.com/article/123.html
サウンドエフェクトが迫力満点で、プレイ中に盛り上がります。特にバトル時が楽しい。
https://medium.com/@charlielevesque328/%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC-%EA%B5%AC%EB%A7%A4-%ED%9B%84-%EC%82%AC%EC%9A%A9%EB%B2%95-%ED%9A%A8%EA%B3%BC%EC%A0%81%EC%9D%B8-%ED%99%9C%EC%9A%A9%EB%B2%95-7a48906d3d2c
https://telegra.ph/%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC-%EA%B5%AC%EB%A7%A4%EB%A5%BC-%EC%9C%84%ED%95%9C-%EC%8A%A4%EB%A7%88%ED%8A%B8%ED%8F%B0-%EC%95%B1-%EC%B6%94%EC%B2%9C-09-26
https://naveridbuy.blogspot.com/2024/07/blog-post_57.html
https://writeablog.net/hhdc4ovc7b
can i purchase clomid no prescription: can i order generic clomid pill – can i order clomid pills
https://xn--zf-hd0j5a097plop.mystrikingly.com/blog/f7ad5523283
https://active-wolf-dc4vlr.mystrikingly.com/blog/d9bf921d898
http://1ctv.cn/home.php?mod=space&uid=2957012
Chainlist lets you easily connect to multiple blockchain networks. Simplify wallet setups, save time, and boost your crypto management! https://chainlist.cam/
https://telegra.ph/%EB%84%A4%EC%9D%B4%EB%B2%84-%ED%86%A1%ED%86%A1%EC%9C%BC%EB%A1%9C-%EA%B3%A0%EA%B0%9D-%EC%86%8C%ED%86%B5%EC%9D%84-%EA%B0%95%ED%99%94%ED%95%98%EB%8A%94-%EB%B0%A9%EB%B2%95-08-28
https://medium.com/@dqvchristopherwhite824/%EB%84%A4%EC%9D%B4%EB%B2%84-%EC%95%84%EC%9D%B4%EB%94%94%EC%99%80-%EC%97%B0%EB%8F%99%EB%90%9C-%EC%86%8C%EC%85%9C-%EB%AF%B8%EB%94%94%EC%96%B4-%EA%B3%84%EC%A0%95-%EA%B4%80%EB%A6%AC-%ED%8C%81-18e50f3e1bfb
https://responsible-seal-dd3cm4.mystrikingly.com/blog/c3b2199d533
https://naveridbuy.blogspot.com/2024/09/blog-post_1.html
https://www.xn--ob0bp9ft7puza.com/bbs_shop/read.htm?me_popup=0&auto_frame=&cate_sub_idx=0&search_first_subject=&list_mode=board&board_code=board1&search_key=&key=&page=0&idx=10396
https://obedient-flamingo-dc4vl0.mystrikingly.com/blog/d72499c8971
https://xn--gy-2e2i723b91ktjas9l307b.mystrikingly.com/blog/c451b4f0707
https://beige-canary-dc4vlw.mystrikingly.com/blog/23369e333ac
buy cytotec pills online cheap Misoprostol 200 mg buy online buy cytotec
Fatih Escort ❤️ bayanları sitemizde bularak onlarla doyumsuz seks yapmaya hazır olun. Tek yapmanız gerek Fatih Escort Bayan ❤️ sitemizi ziyaret etmeniz.
reputable mexican pharmacies online https://mexicanpharmgate.com/ buying prescription drugs in mexico
Abuse effects detailed. Side effects listed.
buy finasteride no prescription
Patient pill information. Patient drug facts.
mexico drug stores pharmacies http://mexicanpharmgate.com/ mexican pharmaceuticals online
https://thoughtful-sparrow-dc4vlm.mystrikingly.com/blog/8f1a8505ec5
вызов нарколога на дом краснодар http://familyportal.forumrom.com/viewtopic.php?id=28567/ .
купить 1с онлайн [url=https://kupit-1s11.ru/]купить 1с онлайн[/url] .
вызвать нарколога на дом http://ideya.forums.party/viewtopic.php?id=659 .
нарколог на дом нарколог на дом .
price of stromectol: IverFast – minocycline 50 mg acne
нарколог на дом в краснодаре нарколог на дом в краснодаре .
http://lisinopril1st.com/# Lisinopril 1st
https://xn--gb-2e2i723b91ktjas9l307b.mystrikingly.com/blog/3552b7d0af2
Drug facts provided. Read about medicines.
purchase proscar online no prescription
Get drug details. Patient pill information.
Pフィーバーダンベル何キロ持てる
k8 パチンコ CR RAVE この世界こそが真実だ Ver.319
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
魁!!男塾天挑五輪大武會編
https://gamein.jp/slots/aztec-powernudge
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
デジハネ CR 北斗の拳 慈母
k8パチンコ
押忍!番長2
めんそーれtw
P地獄少女 四
モンスターハンター月下雷鳴
https://www.pachinko-lupine.jp/article/115.html
演出が華やかで、視覚的にも楽しめます。アートとしての側面もあります。
CR北斗の拳剛掌 Ver.399
https://casinos.town/casino-news/taigarituti-wei-lai-nocheng-gong-henojian-303
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
琉球の守護神
https://www.casinocz.org/sitemaps.xml
友人と一緒に行くことで、競争心が芽生え、さらに楽しめます。勝負の結果が気になります。
buy cytotec over the counter buy cytotec pills online cheap or cytotec buy online usa
http://www.robertlerner.com/cgi-bin/links/ybf.cgi?url==http://cytpremium.com/ п»їcytotec pills online
buy cytotec online Misoprostol 200 mg buy online and cytotec pills buy online cytotec online
https://iverfast.com/# is minocycline an antibiotic
https://ask.mallaky.com/?qa=user/viagrabuy#google_vignette
https://navy-cat-dbgzh0.mystrikingly.com/blog/474ffd8d6fb
cytotec abortion pill Misoprostol 200 mg buy online or buy cytotec
https://clients1.google.ac/url?q=https://cytpremium.com Misoprostol 200 mg buy online
cytotec online п»їcytotec pills online and purchase cytotec buy misoprostol over the counter
buy clopidogrel online antiplatelet drug antiplatelet drug
prednisone cream brand name: buy prednisone from india – prednisone 10
Taraftarium24, ücretsiz ve HD kalitede canlı maç yayınları sunan popüler bir online spor platformudur.
Pill leaflet provided. Access medication facts.
buy proscar uk
Current drug information. Medication information here.
Taraftarium24, canlı maç izleme platformu olarak futbol, basketbol, voleybol ve diğer spor dallarına erişim sağlar.
https://xn--ju-o02ik82a9jc69ko8mqkg.mystrikingly.com/blog/f4bd05662be
Fantastic post! Do you know how to confirm the SD card format? If yes, first, check the storage capacity: Insert the SD card into your camera and verify that the correct capacity is displayed. Ensure that the card can capture and save files without any problems. Refer to the linked post on the SD card formatter for more information.
п»їcytotec pills online: cheapest cytotec – cytotec buy online usa
can i get cheap clomid pills: can i order generic clomid pills – can you buy clomid without a prescription
Justin TV maç yayınları ile canlı maç keyfini yaşayın. Ücretsiz olarak futbol, basketbol ve voleybol maçlarını izleyin.
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://tubeteencam.com/user/lexmorf1959/profile
https://www.haikudeck.com/presentations/sFDtq2YJDA
https://tubeteencam.com/user/frenni1974/profile
https://rainblood1998.diary.ru/
https://www.haikudeck.com/presentations/a8cRZsXLtu
Justin TV maç yayınlarına kolay erişim sağlayarak favori takımlarınızın maçlarını canlı izleyebilirsiniz.
https://naveridbuy.blogspot.com/2024/09/blog-post_54.html
https://ko.anotepad.com/note/read/kcc3r23h
https://xn--w0-hd0jg6f81lm0dhhw74c.mystrikingly.com/blog/2ea87cc6ee1
Medication information here. Dosing guidelines here.
buy proscar online without prescription
Get pill info. Side effects explained.
https://chestnut-reindeer-dc4vl8.mystrikingly.com/blog/97962f0de1f
Узнай все о пластика носовой перегородкиоперация исправление носовой перегородки
generic plavix Plavix 75 mg price or buy plavix
https://www.google.co.il/url?q=https://plavixclo.com Clopidogrel 75 MG price
п»їplavix generic plavix medication and cheap plavix antiplatelet drug buy clopidogrel online
lisinopril 12.5 mg 10 mg zestril tab 10mg or prinivil cost
https://www.google.hr/url?q=https://lisinopril1st.com lisinopril 3973
lisinopril price in canada buy lisinopril 10 mg and price of lisinopril generic cost of brand name lisinopril
cheap plavix antiplatelet drug Plavix generic price or clopidogrel bisulfate 75 mg
http://redirme.com/?to=https://plavixclo.com:: antiplatelet drug
Clopidogrel 75 MG price cheap plavix antiplatelet drug and п»їplavix generic buy plavix
ivermectin buy: cheapest Ivermectin – minocycline 50mg tablets online
Burç özellikleri hakkında merak edilen her şey! Tüm burçların genel ve özel karakteristik özelliklerini keşfetmek için bu rehberi inceleyin. https://tedez.com/burc-ozellikleri/
Misoprostol 200 mg buy online cytotec online or buy cytotec over the counter
https://images.google.jo/url?sa=t&url=https://cytpremium.com Cytotec 200mcg price
Cytotec 200mcg price buy cytotec and Abortion pills online Cytotec 200mcg price
https://unique-peach-dc4vlf.mystrikingly.com/blog/13e36487c5f
https://iridescent-pigeon-dc4vl7.mystrikingly.com/blog/cfa606ff76f
вывод из запоя с выездом краснодар http://mozaisk.anihub.me/viewtopic.php?id=4370/ .
нарколог на дом вывод из запоя краснодар нарколог на дом вывод из запоя краснодар .
cytotec abortion pill buy cytotec online or buy cytotec pills
http://www.google.bj/url?q=https://cytpremium.com buy cytotec online fast delivery
cytotec buy online usa buy cytotec online and buy misoprostol over the counter buy cytotec pills
Medicine resource available. Medicine leaflet here.
buy proscar uk
Short-term impacts described. Get medication facts.
prednisone 50 mg buy: prednisone ray pharm – buy prednisone online no script
lisinopril1st buy Lisinopril 1st 10mg generic 10mg lisinopril
https://ameblo.jp/naveridbuy/entry-12866334219.html
вывод из запоя дешево краснодар вывод из запоя дешево краснодар .
how can i get cheap clomid: generic clomid – buying generic clomid without prescription
minocycline 50 mg tabs: cheapest Ivermectin – minocycline 100mg tablets for human
Download MetaMask Extension for Chrome to securely manage your crypto wallet. Follow this easy guide to install and start using MetaMask in minutes! https://sites.google.com/view/download-metamask-extension-41/home
https://writeablog.net/0jo5jhqjwx
https://medium.com/@charlielevesque328/%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC-%EA%B5%AC%EB%A7%A4%EC%99%80-%EA%B4%80%EB%A0%A8%EB%90%9C-%EC%98%A4%ED%95%B4%EC%99%80-%EC%A7%84%EC%8B%A4-d443ec16de94
Lisinopril 1st lisinopril 4 mg buy Lisinopril 1st
https://sorrel-coconut-dc4vln.mystrikingly.com/blog/3de9e46eb40
https://xn--ze-hd0j5a097plop.mystrikingly.com/blog/0a6ff0d0588
https://scarlet-yucca-dc4vlz.mystrikingly.com/blog/9e48fb2425c
https://iverfast.com/# ivermectin 12 mg
Pフィーバー戦姫絶唱シンフォギア2
ドラゴンが降りる
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
シンドバッドアドベンチャーは榎本加奈子でどうですか
https://www.games-based-learning.com/article/1041.html
演出が単純明快で、ストレスなくプレイできるのが良いです。気軽に楽しめます。
P とある魔術の禁書目録
北斗打ち方
聖闘士星矢 -黃金激闘篇-
超時空要塞マクロス
CR緋弾のアリアII FPM
麻雀物語(夏の日)
https://renoheaven.jahromblog.com/post-80/
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
戦国BASARA3
https://pachika.com/machine/post-9022.html
音響効果が素晴らしく、迫力のあるサウンドがプレイを盛り上げます。特にボーナス時の音は興奮します。
スロット ソードアート・オンライン
https://blog.k8.io/pachinko-pachinslot/epachisao
自分の運を試すために、少しだけお金をかけるのが楽しいです。気軽に挑戦できます。
stromectol prices order minocycline 100 mg or ivermectin cream canada cost
http://referless.com/?http://iverfast.com/ minocycline 100 mg pills
ivermectin tablets ivermectin 18mg and minocycline uses minocycline 100 mg for sale
Drug facts provided. Get medicine info.
valtrex 100mg
Medicine effects explained. Comprehensive pill overview.
https://sociable-corn-dd3cmt.mystrikingly.com/blog/7e8770a1cc2
https://vermilion-seal-dc4vlh.mystrikingly.com/blog/7466ccfc9d8
https://lisinopril1st.com/# Lisinopril 1st
https://xn--w9-hs1izvv81cmb366re3s.mystrikingly.com/blog/c79c96dc746
This freedom to explore makes sex toys more inclusive to the queer and sexualラブドール av trauma communities who don’t feel represented in the mainstream sex toy industry.”
Men naturally have more muscular butts; their default is toned,ラブドール オナニー even as they get older, which is so unfair. Most women just look like their torsos were sliced toward the bottom. We also all have the same roll of fat below our belly buttons,
While researching for her book, Hunter Murray, who has alsoラブドール 女性 用 been a therapist for over a decade, held 30 in-person interviews with heterosexual men before asking over 300 men to see if the findings held without her in the room.
buy Lisinopril online: buy Lisinopril online – cheapest Lisinopril
Drug facts provided. Get pill details.
buy valtrex medication
Detailed drug knowledge. Find medicine details.
cytotec abortion pill Abortion pills online or buy cytotec over the counter
https://maps.google.je/url?q=https://cytpremium.com cytotec buy online usa
cytotec online buy cytotec and buy cytotec online fast delivery buy cytotec in usa
buy cytotec pills Cytotec 200mcg price or buy cytotec over the counter
https://images.google.ee/url?sa=t&url=https://cytpremium.com cytotec abortion pill
buy misoprostol over the counter purchase cytotec and order cytotec online п»їcytotec pills online
clomid brand name: rex pharm – where can i buy generic clomid now
Sultangazi Escort ❤️ bayanları sitemizde bularak onlarla doyumsuz seks yapmaya hazır olun. Tek yapmanız gerek Escort Sultangazi Bayan ❤️ sitemizi ziyaret etmeniz.
where buy generic clomid pill: clomid no prescription – can i buy clomid without prescription
Drug trends described. Get drug info.
buy valtrex online
Get medication facts. Medication effects explained.
buy cytotec pills online cheap: cyt premium – order cytotec online
P刀使ノ巫女 Ver.199
バルタン星人
パチンコを通じて、他のプレイヤーと交流する機会も多いです。共通の趣味があると会話が弾みます。
八代将军 天国の戦い
https://www.k8.rodeo/Articles/1769.html
大当たりの確率を理解することで、より戦略的にプレイできます。計算が好きな人にはおすすめです。
黄門ちゃま喝
シンフォギア3 攻略
パチスロ ロストプラネット2
黄門ちゃま喝
アナターのオット!?はーです
押忍!番長3
https://hokutonoken.jahromblog.com/post-81/
定期的に新しい台が出るので、飽きずに楽しめます。ファンにはたまらないですね。
ドラゴンが降りる
https://www.k8casinoofficial.jp/slots/id509.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
P Re:ゼロから始める異世界生活 鬼がかりver.
https://www.protectpachildren.org/article/1311.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
Узнай все о операция по удлинению полового члена сколько стоит увеличение полового члена
Beyoğlu-Taksim Escort ❤️ bayanları sitemizde bularak onlarla doyumsuz seks yapmaya hazır olun. Tek yapmanız gerek Taksim-Beyoğlu Escort Bayan ❤️ sitemizi ziyaret etmeniz.
cytotec buy online usa cheapest cytotec Misoprostol 200 mg buy online
Patient pill information. Comprehensive pill guide.
buy valtrex medication
Patient drug leaflet. Patient pill information.
buy Lisinopril 1st: lisinopril1st – buy Lisinopril online
cytotec online cytotec online or buy cytotec over the counter
http://renaware.net/renaware-consultant/recipes/?linkname=utensiliosdecocina&langid=sp&origdomain=cytpremium.com buy cytotec online fast delivery
Cytotec 200mcg price cytotec online and buy misoprostol over the counter order cytotec online
вывод из запоя в воронеже http://www.familyportal.forumrom.com/viewtopic.php?id=28571 .
http://cytpremium.com/# п»їcytotec pills online
вывод из запоя краснодар наркология вывод из запоя краснодар наркология .
фонбет беларусь фонбет беларусь .
вывод из запоя на дому краснодар цены https://sergiev.0pk.me/viewtopic.php?id=3461 .
Escort Dating For Click: https://yaramazkadinlar.com/il1/izmir-escort/
Escort Dating For Click: https://hglweb.com/il1/zonguldak-escort/
purchase cytotec cheapest cytotec п»їcytotec pills online
https://lisinopril1st.com/# cheapest Lisinopril
buy cytotec pills online cheap buy cytotec or Misoprostol 200 mg buy online
https://clients1.google.vu/url?q=https://cytpremium.com order cytotec online
buy cytotec online buy cytotec over the counter and Abortion pills online cytotec pills buy online
dapoxetine price: dapoxetine online – buy priligy max pharm
fonbet by fonbet by .
buy plavix buy clopidogrel online or buy clopidogrel online
https://images.google.gm/url?sa=t&url=https://plavixclo.com buy Clopidogrel over the counter
Clopidogrel 75 MG price Clopidogrel 75 MG price and п»їplavix generic plavix medication
Pill trends described. Medication guide available.
order valacyclovir
Medicine leaflet here. Latest pill trends.
Priligy tablets: buy dapoxetine online – max pharm
cheap plavix antiplatelet drug Plavix generic price or Plavix generic price
https://cse.google.co.bw/url?q=https://plavixclo.com Cost of Plavix on Medicare
Cost of Plavix on Medicare buy clopidogrel bisulfate and generic plavix buy clopidogrel online
buy plavix: buy Plavix Clo – antiplatelet drug
九州娛樂
THA娛樂城與九州娛樂簡介
THA娛樂城是九州娛樂旗下的一個知名線上娛樂平台,致力於為玩家提供多元化且優質的娛樂服務。九州娛樂是亞洲地區領先的線上娛樂品牌,長期專注於打造穩定、公平且充滿樂趣的遊戲環境,旗下擁有多個知名娛樂平台,而THA娛樂城便是其中的一個旗艦產品。以下將詳細介紹THA娛樂城的特色與服務。
THA娛樂城的遊戲種類
THA娛樂城為玩家提供了多樣化的遊戲選擇,滿足不同玩家的需求與興趣,包括以下幾大類別:
1. 體育投注
體育迷可以在THA娛樂城享受豐富的體育賽事投注,涵蓋足球、籃球、網球等多項國際體育比賽。平台提供即時賠率和多種投注選項,讓玩家隨時掌握比賽動態並進行下注。
2. 真人娛樂
真人娛樂區提供即時互動的遊戲體驗,玩家可透過直播技術與真人荷官互動,遊玩如百家樂、輪盤、骰寶等經典遊戲。這不僅增添了真實感,更讓玩家有身臨其境的感受。
3. 電子老虎機
THA娛樂城匯集了多款高人氣的電子老虎機遊戲,從經典水果機到新潮主題機,皆配備精美的畫面與流暢的操作介面,為玩家帶來無限樂趣。
4. 捕魚遊戲
捕魚遊戲是許多玩家的最愛,THA娛樂城提供多種精美場景和豐富玩法的捕魚遊戲,玩家可以挑戰高額獎勵,享受射擊與策略結合的獨特樂趣。
5. 彩票與其他遊戲
除了以上幾類,平台還提供彩票遊戲及其他創新型娛樂遊戲,適合喜歡嘗試新鮮玩法的玩家。
九州娛樂的技術支持
作為THA娛樂城的母公司,九州娛樂以其強大的技術實力與嚴謹的管理體系為平台提供支持。九州娛樂採用國際領先的安全加密技術,確保玩家的個人資訊與交易數據得到妥善保護。此外,平台的遊戲結果皆經過嚴格的隨機性測試與第三方機構認證,保證公平公正。
優質的會員服務
THA娛樂城致力於打造一個高品質的會員體驗,其服務特色包括:
– 優惠活動
平台定期推出多種促銷活動,如新會員註冊禮金、存款返利、週週回饋等,讓玩家享受更多的遊戲資金。
– 快速出入金
平台提供便捷且快速的存提款服務,玩家可以安心進行資金操作,且無需擔心延遲問題。
– 24小時客戶服務
為了確保玩家的需求能即時被解決,THA娛樂城提供全年無休的客服支援,無論是遊戲問題還是技術諮詢,客服團隊都能快速回應。
與其他平台的區別
THA娛樂城之所以能在眾多線上娛樂平台中脫穎而出,不僅因其豐富的遊戲種類與高品質服務,更在於它所帶來的獨特價值:
1. 品牌信譽
作為九州娛樂旗下的品牌,THA娛樂城擁有長期積累的良好信譽,是許多玩家的首選。
2. 創新與多樣性
平台不斷推出新遊戲與創意玩法,讓玩家始終保持新鮮感。
3. 本地化服務
THA娛樂城深諳玩家需求,提供多語言支援與符合本地習慣的服務,提升玩家的使用便利性。
未來展望
隨著線上娛樂行業的快速發展,THA娛樂城也在持續進步與創新,力求為玩家帶來更多驚喜。無論是透過引進新的遊戲技術、擴展遊戲種類,還是改善服務品質,THA娛樂城都希望成為每位玩家的最佳娛樂夥伴。
總結來說,THA娛樂城憑藉其豐富的遊戲內容、可靠的技術支持以及優質的會員服務,已在亞洲娛樂市場佔有一席之地。如果您正在尋找一個結合刺激與信賴的娛樂平台,那麼THA娛樂城絕對值得一試。
BBgate MarketPlace 2024 Breaking Bad Gate Forum
BBgate MarketPlace
Thank you! Plenty of facts!
https://dzen.ru/
Зелёный Мир — это один из крупнейших маркетплейсов даркнета, ориентированный на русскоязычную аудиторию. Доступ к маркетплейсу Зелёный Мир вы можете получить через официальные ссылки:
Переходник
zmir.pw
3-mir.pw
Переходник
Только лучшее и проверенное для ВАС!!!
buy cytotec pills buy cytotec over the counter or purchase cytotec
http://logen.ru/bitrix/redirect.php?goto=https://cytpremium.com order cytotec online
buy cytotec buy cytotec online fast delivery and purchase cytotec cytotec abortion pill
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.haikudeck.com/presentations/yjBbGrr1Z2
https://jone1979.bandcamp.com/album/lauren-is-drowned-by-her-boyfriend
https://www.hentai-foundry.com/user/mattsnow1956/profile
https://okwave.jp/profile/u3140994.html
https://rentry.org/rd6t5332
Download MetaMask extension today for secure crypto wallet access. Follow our step-by-step guide to install it effortlessly on your browser. https://extension.wtf/
buy cytotec over the counter Misoprostol 200 mg buy online or buy misoprostol over the counter
https://www.google.md/url?q=https://cytpremium.com buy cytotec over the counter
buy misoprostol over the counter buy cytotec pills online cheap and cytotec abortion pill buy cytotec over the counter
パチスロ鉄拳4アルティメットデビルVer.
k8 カジノ スロット Heroic Spins ヒロイックスピン
キャラクターの個性が豊かで、ストーリーに引き込まれます。忍者の世界観が楽しめます。
緑イミソーレ(特殊大賞燈)
https://www.k8.republican/Articles/1162.html
キャラクターの個性が際立っており、ストーリーに引き込まれます。
S この素晴らしい世界に祝福を!
シンフォギア3 先読み
CR びっくり戦国無双 Light Edition
翔馬
CR真・花の慶次 Ver.399(2:1)
CR新世紀エヴァンゲリオン・使徒、再び Ver.346
https://www.webslot69.com/article/171.html
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
CR聖闘士星矢 星の運命
https://injapan.today/news/id1543.html
パチンコの演出は、時に驚かされることがあります。毎回新しい発見があります。
交響詩篇エウレカセブン
https://www.k8pachinko.biz/Articles/3139.html
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特にバトル演出が楽しい。
buy cytotec online: buy cytotec online – buy cytotec in usa
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали официальный сервисный центр lg, можете посмотреть на сайте: официальный сервисный центр lg
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
minocycline 50 ivermectin 1% cream generic or ivermectin 400 mg
https://www.google.ae/url?sa=t&url=https://iverfast.com ivermectin 3mg tablets price
stromectol ivermectin minocycline price and minocycline 50 mg online ivermectin 3 mg tablet dosage
buy Lisinopril online lisinopril1st buy Lisinopril 1st
can you get cheap clomid no prescription: clomid purchase online rex pharm – cheap clomid without a prescription
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
http://lisinopril1st.com/# buy Lisinopril 1st
https://cytpremium.com/# order cytotec online
Pill information available. Patient drug guide.
buy valacyclovir online
Short-term impacts described. Medicine guide here.
Купить диплом старого образца, можно ли это сделать по быстрой схеме?
THA娛樂城與九州娛樂簡介
THA娛樂城是九州娛樂旗下的一個知名線上娛樂平台,致力於為玩家提供多元化且優質的娛樂服務。九州娛樂是亞洲地區領先的線上娛樂品牌,長期專注於打造穩定、公平且充滿樂趣的遊戲環境,旗下擁有多個知名娛樂平台,而THA娛樂城便是其中的一個旗艦產品。以下將詳細介紹THA娛樂城的特色與服務。
THA娛樂城的遊戲種類
THA娛樂城為玩家提供了多樣化的遊戲選擇,滿足不同玩家的需求與興趣,包括以下幾大類別:
1. 體育投注
體育迷可以在THA娛樂城享受豐富的體育賽事投注,涵蓋足球、籃球、網球等多項國際體育比賽。平台提供即時賠率和多種投注選項,讓玩家隨時掌握比賽動態並進行下注。
2. 真人娛樂
真人娛樂區提供即時互動的遊戲體驗,玩家可透過直播技術與真人荷官互動,遊玩如百家樂、輪盤、骰寶等經典遊戲。這不僅增添了真實感,更讓玩家有身臨其境的感受。
3. 電子老虎機
THA娛樂城匯集了多款高人氣的電子老虎機遊戲,從經典水果機到新潮主題機,皆配備精美的畫面與流暢的操作介面,為玩家帶來無限樂趣。
4. 捕魚遊戲
捕魚遊戲是許多玩家的最愛,THA娛樂城提供多種精美場景和豐富玩法的捕魚遊戲,玩家可以挑戰高額獎勵,享受射擊與策略結合的獨特樂趣。
5. 彩票與其他遊戲
除了以上幾類,平台還提供彩票遊戲及其他創新型娛樂遊戲,適合喜歡嘗試新鮮玩法的玩家。
九州娛樂的技術支持
作為THA娛樂城的母公司,九州娛樂以其強大的技術實力與嚴謹的管理體系為平台提供支持。九州娛樂採用國際領先的安全加密技術,確保玩家的個人資訊與交易數據得到妥善保護。此外,平台的遊戲結果皆經過嚴格的隨機性測試與第三方機構認證,保證公平公正。
優質的會員服務
THA娛樂城致力於打造一個高品質的會員體驗,其服務特色包括:
– 優惠活動
平台定期推出多種促銷活動,如新會員註冊禮金、存款返利、週週回饋等,讓玩家享受更多的遊戲資金。
– 快速出入金
平台提供便捷且快速的存提款服務,玩家可以安心進行資金操作,且無需擔心延遲問題。
– 24小時客戶服務
為了確保玩家的需求能即時被解決,THA娛樂城提供全年無休的客服支援,無論是遊戲問題還是技術諮詢,客服團隊都能快速回應。
與其他平台的區別
THA娛樂城之所以能在眾多線上娛樂平台中脫穎而出,不僅因其豐富的遊戲種類與高品質服務,更在於它所帶來的獨特價值:
1. 品牌信譽
作為九州娛樂旗下的品牌,THA娛樂城擁有長期積累的良好信譽,是許多玩家的首選。
2. 創新與多樣性
平台不斷推出新遊戲與創意玩法,讓玩家始終保持新鮮感。
3. 本地化服務
THA娛樂城深諳玩家需求,提供多語言支援與符合本地習慣的服務,提升玩家的使用便利性。
未來展望
隨著線上娛樂行業的快速發展,THA娛樂城也在持續進步與創新,力求為玩家帶來更多驚喜。無論是透過引進新的遊戲技術、擴展遊戲種類,還是改善服務品質,THA娛樂城都希望成為每位玩家的最佳娛樂夥伴。
總結來說,THA娛樂城憑藉其豐富的遊戲內容、可靠的技術支持以及優質的會員服務,已在亞洲娛樂市場佔有一席之地。如果您正在尋找一個結合刺激與信賴的娛樂平台,那麼THA娛樂城絕對值得一試。
выведение из запоя воронеж стационар uaportal.ukrbb.net/viewtopic.php?f=2&t=3720 .
order amoxicillin no prescription: Com Pharm – amoxicillin canada price
вывод из запоя стационар kvitka.ukrbb.net/viewtopic.php?f=58&t=28033 .
вывод из запоя стационар [url=alhambra.bestforums.org/viewtopic.php?f=2&t=46417]alhambra.bestforums.org/viewtopic.php?f=2&t=46417[/url] .
вывод из запоя воронеж [url=https://kyevlyn.ukrbb.net/viewtopic.php?f=2&t=13641/]kyevlyn.ukrbb.net/viewtopic.php?f=2&t=13641[/url] .
Наша мастерская предлагает высококачественный сервисный ремонт аймака на выезде любых брендов и моделей. Мы осознаем, насколько важны для вас ваши моноблоки iMac, и обеспечиваем ремонт высочайшего уровня. Наши опытные мастера оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что обеспечивает долговечность и надежность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели компьютеров Apple, включают проблемы с жестким диском, проблемы с экраном, неисправности разъемов, ошибки ПО и неисправности системы охлаждения. Для устранения этих неисправностей наши квалифицированные специалисты оказывают ремонт жестких дисков, дисплеев, разъемов, ПО и систем охлаждения. Обратившись к нам, вы обеспечиваете себе качественный и надежный официальный ремонт аймака адреса.
Подробная информация представлена на нашем сайте: https://remont-imac-mos.ru
выведение из запоя воронеж стационар выведение из запоя воронеж стационар .
generic plavix: buy Plavix Clo – buy clopidogrel bisulfate
MetaMask is a browser extension and mobile application designed to simplify the interaction between users and blockchain-based applications (dApps). https://sites.google.com/view/metamask-extension-guide-39/home
en kaliteli maslak escort sarıyer escort sitesii
minocycline for rheumatoid arthritis ivermectin medicine or ivermectin iv
http://images.google.com.mm/url?q=https://iverfast.com minocycline 50 mg otc
minocycline 100 mg tablets stromectol tablet 3 mg and ivermectin 12 stromectol ireland
cheap plavix antiplatelet drug buy plavix online Plavix 75 mg price
Bakırköy-Ataköy Escort ❤️ bayanları sitemizde bularak onlarla doyumsuz seks yapmaya hazır olun. Tek yapmanız gerek Florya Escort Bayan ❤️ sitemizi ziyaret etmeniz.
Recent drug developments. Medication overview available.
buy valacyclovir no prescription
Patient pill resource. Get drug details.
銀と金2
k8 カジノ パチスロ鉄拳4アルティメットデビルVer.
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
南国育ち
https://www.k8funny.com/archives/post-138.html
演出が華やかで、視覚的にも楽しめます。アートとしての側面もあります。
CRヱヴァンゲリヲン始まりの福音~
k8パチンコ
CR牙狼金色になれ
南国物語
沖ドキ! 25
CR JAWS再臨 SHARK PANIC AGAIN
https://www.bitcoincasino.asia/article/2478.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
CR蒼天の拳2
https://www.k8casinoofficial.jp/slots/id560.html
人気ゲームを基にしたパチスロで、ファンにはたまらない作品です。リアルなサバイバル体験が楽しめます。
バジリスク~甲賀忍法帖~絆
https://casinos.town/games/hit-it-hard
大当たり時の興奮は、他のゲームでは味わえない特別なものです。心が躍ります。
### Mantel Clocks for Fireplace: Timeless Elegance for Your Home
The fireplace often serves as the heart of a home, creating a warm and inviting ambiance. To further enhance its charm, a carefully chosen mantel clock can act as a centerpiece, combining functionality with aesthetic appeal. Mantel clocks for fireplaces have long been symbols of sophistication, and their timeless designs continue to captivate homeowners.
#### The History of Mantel Clocks
Mantel clocks, also known as shelf clocks, originated in the 18th century as compact timepieces designed to sit atop a fireplace mantel. These clocks became popular in European homes for their elegance and practicality. Crafted from materials like wood, brass, and porcelain, they often featured intricate details, showcasing the craftsmanship of their era.
#### Why Choose a Mantel Clock for Your Fireplace?
1. **Aesthetic Appeal**: Mantel clocks come in a variety of designs, from traditional to modern, making them a versatile decorative piece for any home style.
2. **Functional Elegance**: Beyond telling time, these clocks add a touch of sophistication to your living space.
3. **Conversation Starter**: A unique or antique mantel clock can spark interest and become a cherished focal point.
4. **Balance and Proportion**: Placing a clock on your fireplace mantel adds balance to the overall decor, tying the room together.
#### Types of Mantel Clocks
1. **Traditional Mantel Clocks**: Featuring ornate carvings and classic Roman numerals, these are ideal for vintage or classic interiors.
2. **Modern Mantel Clocks**: With sleek lines and minimalist designs, they suit contemporary homes.
3. **Chiming Mantel Clocks**: These clocks produce melodic chimes, adding an auditory charm to their visual appeal.
4. **Antique Mantel Clocks**: Perfect for collectors, these clocks are rich in history and craftsmanship.
#### Choosing the Perfect Mantel Clock
When selecting a mantel clock for your fireplace, consider the following:
– **Size**: Ensure the clock fits proportionally on your mantel without overwhelming the space.
– **Material**: Match the clock’s material to other elements in the room, such as wood, metal, or ceramic.
– **Mechanism**: Choose between mechanical, quartz, or battery-operated clocks based on your preferences.
– **Style**: Select a design that complements your room’s decor, whether it’s rustic, modern, or traditional.
#### Care and Maintenance of Mantel Clocks
To keep your mantel clock in top condition:
– **Regular Cleaning**: Dust the clock regularly using a soft, dry cloth.
– **Winding**: If you own a mechanical clock, wind it according to the manufacturer’s instructions.
– **Professional Servicing**: For antique clocks, seek professional maintenance to preserve their intricate mechanisms.
– **Placement**: Avoid placing the clock in direct sunlight or near extreme heat to prevent damage.
#### Where to Find Mantel Clocks
Mantel clocks are available in:
– **Antique Stores**: For vintage and unique pieces with historical value.
– **Home Decor Shops**: Offering a wide range of modern and traditional styles.
– **Online Marketplaces**: Providing convenience and variety at competitive prices.
#### Conclusion
A mantel clock is more than just a timepiece; it’s a statement of style and elegance. Whether you prefer a modern design or an antique treasure, these clocks can elevate the look of your fireplace and your home. With the right choice, a mantel clock can become a cherished heirloom, adding timeless beauty and charm to your living space.
https://cl-system.jp/question/clocks-for-fireplace-mantel-2/
buy cytotec over the counter buy cytotec pills online cheap or buy cytotec online
https://images.google.gy/url?sa=t&url=https://cytpremium.com purchase cytotec
Cytotec 200mcg price buy cytotec in usa and buy cytotec pills buy cytotec
order cytotec online buy cytotec online fast delivery or cytotec abortion pill
http://www.fsbswanville.com/redirect/notice.asp?site_name=Minnesota+Bankers+Association&site_url=http://cytpremium.com/ purchase cytotec
buy cytotec pills order cytotec online and Abortion pills online п»їcytotec pills online
Misuse consequences detailed. Find drug information.
valacyclovir
Comprehensive pill guide. Drug overview available.
vape shop
Medicine overview available. Recent drug developments.
sumatriptan dailymed
Interactions explained here. Drug guide provided.
buy Lisinopril online: lisinopril 20 mg over the counter – buy Lisinopril online
Наши специалисты предлагает надежный ремонт аймака рядом различных марок и моделей. Мы знаем, насколько значимы для вас ваши компьютеры Apple, и готовы предложить сервис наилучшего качества. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что обеспечивает длительную работу проведенных ремонтов.
Наиболее частые неисправности, с которыми сталкиваются владельцы iMac, включают поломку жесткого диска, неисправности дисплея, неработающие разъемы, ошибки ПО и перегрев. Для устранения этих проблем наши квалифицированные специалисты оказывают ремонт жестких дисков, дисплеев, разъемов, ПО и систем охлаждения. Доверив ремонт нам, вы обеспечиваете себе качественный и надежный качественный ремонт imac.
Подробная информация доступна на сайте: https://remont-imac-mos.ru
лечение наркозависимости в стационаре лечение наркозависимости в стационаре .
вывод из запоя в стационаре анонимно [url=www.krut.forumno.com/viewtopic.php?id=6047]www.krut.forumno.com/viewtopic.php?id=6047[/url] .
Medicine trends available. Medicine facts available.
[url=https://imitrex2rp.top/index.html]sumatriptan children[/url]
Medicine facts here. Current drug information.
вывод из запоя в стационаре анонимно http://www.gaslo.ukrbb.net/viewtopic.php?f=13&t=3413 .
pinup kazi: pinup kazi – pinup kazi
九州娛樂
KU娛樂城與九州娛樂簡介
KU娛樂城是九州娛樂旗下的一個知名線上娛樂平台,致力於為玩家提供安全、創新且多元化的娛樂服務。九州娛樂作為亞洲地區的線上娛樂領導品牌,以其穩定的運營、先進的技術和用戶至上的服務理念聞名於業界。KU娛樂城在九州娛樂的支持下,不僅具備強大的品牌背景,還融入了創新的遊戲設計和完善的會員體系,成為眾多玩家的理想選擇。
KU娛樂城的遊戲種類
KU娛樂城為玩家提供了豐富多樣的遊戲選項,涵蓋多個熱門類別,旨在滿足不同類型玩家的娛樂需求:
1. 體育投注
KU娛樂城提供全面的體育賽事投注服務,覆蓋足球、籃球、棒球等多項主流運動。玩家可以根據實時賠率和賽事數據進行投注,享受觀賽與下注相結合的刺激體驗。
2. 真人娛樂
在真人娛樂區,玩家可以參與百家樂、輪盤、德州撲克等經典遊戲,並與真人荷官進行即時互動。先進的直播技術確保遊戲過程流暢無延遲,讓玩家彷彿置身於真實賭場。
3. 電子老虎機
KU娛樂城集合了數百款精美設計的電子老虎機遊戲,無論是經典三軸機還是具備豐富特效的多軸機,皆可滿足玩家的需求。高額的獎金機制與趣味主題設計,讓每一次旋轉都充滿驚喜。
4. 捕魚遊戲
KU娛樂城的捕魚遊戲區擁有多種玩法,玩家可以透過射擊技巧和策略挑戰不同級別的魚類,贏取豐厚的獎勵。畫面精美的海底場景和豐富的道具選擇,讓捕魚遊戲成為平台上的人氣項目。
5. 彩票與其他遊戲
KU娛樂城還提供豐富的彩票遊戲和創新娛樂玩法,玩家可以選擇自己喜好的遊戲類型,盡享多樣化的娛樂體驗。
九州娛樂的技術支持
九州娛樂作為KU娛樂城的母公司,以其先進的技術實力和多年的行業經驗為平台提供堅實的支持。九州娛樂引入國際頂級的數據加密技術,確保用戶的個人資訊和交易數據的安全性。同時,遊戲結果均經過公平性測試和第三方機構認證,為玩家打造一個公平透明的娛樂環境。
優質的會員服務
KU娛樂城始終以玩家為中心,提供一系列貼心的服務與福利:
– 優惠活動
平台定期推出豐富的促銷活動,包括新會員首存禮金、充值返利以及抽獎活動,讓玩家的每一次參與都更有價值。
– 快速出入金
KU娛樂城支持多種主流支付方式,並保證存提款的快速處理,讓玩家無需等待即可享受遊戲樂趣。
– 全天候客戶服務
KU娛樂城提供24小時在線客服支援,隨時解答玩家的疑問,確保每位玩家都能獲得最好的服務體驗。
KU娛樂城的競爭優勢
KU娛樂城之所以能在眾多線上娛樂平台中脫穎而出,除了其豐富的遊戲種類與頂級服務外,還有以下幾個顯著的競爭優勢:
1. 品牌信譽
作為九州娛樂旗下的品牌,KU娛樂城憑藉多年的穩定運營,已成為亞洲玩家心目中值得信賴的娛樂平台。
2. 持續創新
KU娛樂城不斷推出新遊戲和新功能,為玩家帶來更多元化和現代化的娛樂體驗。
3. 本地化服務
平台針對不同地區的玩家提供本地化的界面與支付選項,提升了用戶的便利性與滿意度。
未來展望
KU娛樂城將繼續秉承九州娛樂的核心理念,致力於成為亞洲線上娛樂市場的佼佼者。無論是透過升級遊戲技術、推出更多創新玩法,還是提升服務品質,KU娛樂城都希望為玩家帶來更加豐富的娛樂體驗。
總而言之,KU娛樂城在九州娛樂的支持下,憑藉其出色的遊戲內容和優質的服務,已成為線上娛樂行業中的一顆璀璨明珠。如果您正在尋找一個結合信譽、創新與趣味的娛樂平台,KU娛樂城將是不二之選。
### Букмекерские конторы с фрибетом: https://www.youtube.com/watch?v=eMVTiWJujqQ получить бесплатную ставку?
Фрибет — это бесплатная ставка, предоставляемая букмекерскими конторами своим пользователям. Такие предложения становятся всё более популярными, так как позволяют новым и опытным игрокам попробовать свои силы в ставках без риска потери собственных средств. В этой статье мы расскажем, что такое фрибет, как его получить и на что обратить внимание при выборе букмекерской конторы с фрибетом.
#### Что такое фрибет и как он работает?
Фрибет — это сумма, которую букмекер предоставляет клиенту для размещения ставки. Выигрыш, полученный с использованием фрибета, обычно начисляется за вычетом суммы самой ставки. Например, если вы получили фрибет на 1000 рублей, поставили его с коэффициентом 2.0 и выиграли, то на ваш счёт будет зачислено 1000 рублей (2000 рублей — сумма выигрыша минус 1000 рублей фрибета).
#### Как получить фрибет?
Чтобы воспользоваться бесплатной ставкой, необходимо выполнить одно или несколько условий, предлагаемых букмекером. Вот основные способы получения фрибета:
1. **Регистрация нового аккаунта**
Многие букмекеры предлагают фрибеты за создание учётной записи. Для этого нужно зарегистрироваться, указать свои данные и пройти верификацию.
2. **Пополнение счёта**
Некоторые компании предоставляют фрибет за первое пополнение депозита. Важно внимательно изучить условия акции: минимальная сумма депозита, сроки использования фрибета и т. д.
3. **Акционные предложения**
Постоянные клиенты часто получают фрибеты в рамках акций или программ лояльности. Это может быть бонус за активность, участие в розыгрыше или выполнение определённых заданий.
4. **Ставки на определённые события**
Букмекеры иногда предоставляют фрибеты за ставки на конкретные матчи или турниры.
#### На что обратить внимание при выборе букмекера с фрибетом?
Чтобы воспользоваться предложением с максимальной выгодой, обратите внимание на следующие аспекты:
– **Условия отыгрыша**. Некоторые фрибеты требуют выполнения условий по отыгрышу, например, сделать ставку с определённым коэффициентом.
– **Срок действия**. У каждого бонуса есть срок использования. Убедитесь, что вы успеете использовать фрибет до его истечения.
– **Ограничения по видам спорта**. Иногда фрибеты можно использовать только на определённые виды спорта или турниры.
– **Репутация букмекера**. Выбирайте только лицензированные букмекерские конторы с хорошими отзывами и прозрачными условиями.
#### ТОП-5 букмекерских контор с фрибетом
1. **1xBet**: предлагает фрибет за регистрацию и регулярные акции для постоянных клиентов.
2. **Bet365**: известна своими выгодными условиями для новичков и бонусами в виде фрибетов.
3. **Лига Ставок**: предоставляет бесплатные ставки за участие в акциях и на первое пополнение.
4. **Parimatch**: предлагает фрибет за регистрацию и акционные предложения для крупных турниров.
5. **Winline**: славится простыми условиями получения фрибетов и быстрой выплатой выигрышей.
#### Советы по использованию фрибетов
– Выбирайте ставки с умеренными коэффициентами (от 1.5 до 2.5), чтобы увеличить шансы на выигрыш.
– Изучите статистику событий, на которые хотите поставить, чтобы сделать осознанный выбор.
– Не рискуйте сразу всеми фрибетами — попробуйте разделить их на несколько ставок.
#### Заключение
Букмекерские конторы с фрибетом — отличная возможность для новичков и опытных игроков протестировать свои стратегии без риска. Однако важно внимательно изучать условия получения и использования бонуса, чтобы избежать разочарований. Выбирайте надёжных букмекеров и наслаждайтесь азартом игры с минимальными вложениями!
вавада казино зеркало: казино вавада – вавада казино
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали сервисный центр huawei, можете посмотреть на сайте: сервисный центр huawei
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Pill leaflet provided. Get info immediately.
imitrex apotex
Medicine details here. Access medication facts.
Info well taken!!
https://www.merchantcircle.com/blogs/emilybacker-canadian-tx/2024/11/-online-casino-/2846647
вавада: vavada-kazi.ru – vavada kazi
九州娛樂
THA娛樂城與九州娛樂簡介
THA娛樂城是九州娛樂旗下的一個知名線上娛樂平台,致力於為玩家提供多元化且優質的娛樂服務。九州娛樂是亞洲地區領先的線上娛樂品牌,長期專注於打造穩定、公平且充滿樂趣的遊戲環境,旗下擁有多個知名娛樂平台,而THA娛樂城便是其中的一個旗艦產品。以下將詳細介紹THA娛樂城的特色與服務。
THA娛樂城的遊戲種類
THA娛樂城為玩家提供了多樣化的遊戲選擇,滿足不同玩家的需求與興趣,包括以下幾大類別:
1. 體育投注
體育迷可以在THA娛樂城享受豐富的體育賽事投注,涵蓋足球、籃球、網球等多項國際體育比賽。平台提供即時賠率和多種投注選項,讓玩家隨時掌握比賽動態並進行下注。
2. 真人娛樂
真人娛樂區提供即時互動的遊戲體驗,玩家可透過直播技術與真人荷官互動,遊玩如百家樂、輪盤、骰寶等經典遊戲。這不僅增添了真實感,更讓玩家有身臨其境的感受。
3. 電子老虎機
THA娛樂城匯集了多款高人氣的電子老虎機遊戲,從經典水果機到新潮主題機,皆配備精美的畫面與流暢的操作介面,為玩家帶來無限樂趣。
4. 捕魚遊戲
捕魚遊戲是許多玩家的最愛,THA娛樂城提供多種精美場景和豐富玩法的捕魚遊戲,玩家可以挑戰高額獎勵,享受射擊與策略結合的獨特樂趣。
5. 彩票與其他遊戲
除了以上幾類,平台還提供彩票遊戲及其他創新型娛樂遊戲,適合喜歡嘗試新鮮玩法的玩家。
九州娛樂的技術支持
作為THA娛樂城的母公司,九州娛樂以其強大的技術實力與嚴謹的管理體系為平台提供支持。九州娛樂採用國際領先的安全加密技術,確保玩家的個人資訊與交易數據得到妥善保護。此外,平台的遊戲結果皆經過嚴格的隨機性測試與第三方機構認證,保證公平公正。
優質的會員服務
THA娛樂城致力於打造一個高品質的會員體驗,其服務特色包括:
– 優惠活動
平台定期推出多種促銷活動,如新會員註冊禮金、存款返利、週週回饋等,讓玩家享受更多的遊戲資金。
– 快速出入金
平台提供便捷且快速的存提款服務,玩家可以安心進行資金操作,且無需擔心延遲問題。
– 24小時客戶服務
為了確保玩家的需求能即時被解決,THA娛樂城提供全年無休的客服支援,無論是遊戲問題還是技術諮詢,客服團隊都能快速回應。
與其他平台的區別
THA娛樂城之所以能在眾多線上娛樂平台中脫穎而出,不僅因其豐富的遊戲種類與高品質服務,更在於它所帶來的獨特價值:
1. 品牌信譽
作為九州娛樂旗下的品牌,THA娛樂城擁有長期積累的良好信譽,是許多玩家的首選。
2. 創新與多樣性
平台不斷推出新遊戲與創意玩法,讓玩家始終保持新鮮感。
3. 本地化服務
THA娛樂城深諳玩家需求,提供多語言支援與符合本地習慣的服務,提升玩家的使用便利性。
未來展望
隨著線上娛樂行業的快速發展,THA娛樂城也在持續進步與創新,力求為玩家帶來更多驚喜。無論是透過引進新的遊戲技術、擴展遊戲種類,還是改善服務品質,THA娛樂城都希望成為每位玩家的最佳娛樂夥伴。
總結來說,THA娛樂城憑藉其豐富的遊戲內容、可靠的技術支持以及優質的會員服務,已在亞洲娛樂市場佔有一席之地。如果您正在尋找一個結合刺激與信賴的娛樂平台,那麼THA娛樂城絕對值得一試。
pinup kazi: пинап казино – pinup
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали официальный сервисный центр philips, можете посмотреть на сайте: сервисный центр philips
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
пин ап казино: pinup kazi – pinup-kazi.kz
пин ап казино официальный сайт пин ап вход pinup
機動戦士ガンダムユニコーン
スロット ソードアート・オンライン
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
CR ぱちんこAKB48 バラの儀式
https://www.stakecasino.asia/article/256.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
P とある科学の超電磁砲
k8パチンコ
CR北斗の拳剛掌 Ver.399
CR牙狼金色になれ Ver.399
CRぱちんこ 新鬼武者
ミリオンゴッド-神々の凱旋
https://casinos.town/bonus/casino-secret-25-tuesday-blackjack
演出が多様で、毎回新しい体験が待っています。飽きることがありません。
真モグモグ風林火山
https://www.casinobaccarat.vip/article/334.html
台のデザインが豊富で、飽きずに楽しめます。自分好みの台を探すのが楽しいです。
e ぱちんこソードアート・オンラインK12
https://www.casinoonline.help/article/220.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
Drug reactions explained. Prescribing guidelines here.
imitrex boots
Current medication trends. Get details now.
vavada: вавада – vavada
pinup kazi: пин ап казино официальный сайт – pinup
pinup: pinup-kazi.kz – пин ап казино
вывод из запоя в воронеже вывод из запоя в воронеже .
вывод из запоя в стационаре анонимно https://pelsh.forum24.ru/?1-8-0-00000127-000-0-0-1730749493 .
лечение наркозависимости в стационаре https://wisdomtarot.tforums.org/viewtopic.php?f=16&t=11729/ .
выведение из запоя воронеж стационар [url=https://aqvakr.forum24.ru/?1-7-0-00011578-000-0-0-1730749158]https://aqvakr.forum24.ru/?1-7-0-00011578-000-0-0-1730749158[/url] .
пин ап казино онлайн pinup kazi pinup
ysl 包去哪兒買最便宜
Happy Teen Patti
Teen Patti Cash
tory burch去哪兒買最便宜
Happy Teen Patti
Teen Patti Cash
nike jordan
Happy Teen Patti
Teen Patti Cash
coach 最新款包包
лечение наркозависимости в стационаре лечение наркозависимости в стационаре .
tumi outlet哪裡買最便宜
Teen Patti Circle
New Rummy App
a mobile version of the ancient Indian card game Happy Teen Patti
version of the ancient Indian card game Teen Patti VIP
the ancient Indian card game Teen Patti Winner
深入了解 Sacai 鞋款,揭示其獨特的功能和設計。sacai哪裡買最便宜
Medication leaflet provided. Patient pill guide.
sumatriptan syrup
Get pill details. Find medicine information.
有趣的遊戲 Happy Teen Patti
好玩的遊戲 Taurus Cash App
遊戲 Master Teen Patti
探索悅刻五代煙彈:RELX 5代的潮流新選擇電子菸
壹起玩遊戲吧 Teen Patti Circle
科比鞋通常會推出多款特別配色或限量版本,如果妳追求獨特性和稀有性,收藏這些鞋款是非常明智的選擇。 kobe 球鞋哪裡買最便宜
遊戲Happy Teen Patti
好玩的遊戲Taurus Cash App
Happy Teen Patti is a fabulous game often compared to Poker
fun for card game enthusiasts Taurus Cash App
erfectly blends traditional gameplay Rummy Master
Nike blazer mid 版型經典、多樣的版本以及卓越的舒適性,成為了運動鞋領域的佼佼者。nike blazer哪裡買最便宜
pinup-kazi.kz: пин ап кз – pinup kazi
在時尚與舒適的交彙點,converse帆布鞋以其獨特的設計風格和卓越的品質,成爲了無數潮流愛好者的首選。converse哪裡買最便宜
無論是搭配休閑裝還是正裝,Nike dunk鞋都能輕松駕馭,展現出不同的風格魅力。nike dunk哪裡買最便宜
пин ап казино pinup or пин ап кз
https://cse.google.sn/url?sa=t&url=https://pinup-kazi.kz pinup
pin up казино пин ап казино онлайн and пин ап казино онлайн pinup-kazi.kz
RG富遊娛樂城是亞洲地區知名的線上娛樂平台,致力於為玩家提供多元化且高品質的遊戲體驗。自成立以來,RG富遊娛樂城以其豐富的遊戲種類、優質的服務以及安全可靠的遊戲環境,贏得了廣大玩家的信賴與支持。
遊戲種類
RG富遊娛樂城提供多樣化的遊戲選擇,滿足不同玩家的需求:
真人娛樂:包括百家樂、輪盤、骰寶等,玩家可與真人荷官即時互動,享受真實賭場的氛圍。
電子遊戲:涵蓋各類老虎機、電子撲克等,遊戲畫面精美,玩法多樣,帶來無限樂趣。
體育投注:提供足球、籃球、網球等多項體育賽事的投注服務,賠率公正,資訊即時更新。
彩票遊戲:包括各類熱門彩票遊戲,玩法簡單,獎金豐厚,讓玩家輕鬆參與。
捕魚遊戲:精美的海底世界場景,豐富的魚種和道具,帶來刺激的捕魚體驗。
平台特色
安全可靠:RG富遊娛樂城採用先進的加密技術,保障玩家的個人資訊和資金安全,並持有合法的營業執照,受相關監管機構監管。
優惠活動:平台定期推出各類優惠活動,如首儲贈送、返水優惠、抽獎活動等,讓玩家享受更多福利。
客戶服務:提供24小時在線客服,隨時解答玩家的疑問,確保每位玩家都能獲得及時的幫助。
多元支付:支持多種支付方式,存提款快捷方便,滿足不同玩家的需求。
玩家評價
許多玩家對RG富遊娛樂城給予了高度評價,認為平台遊戲種類豐富,操作簡便,出入金迅速,客服服務專業且友善。此外,平台的優惠活動也深受玩家喜愛,增加了遊戲的樂趣和收益。
總結
RG富遊娛樂城以其多元化的遊戲選擇、優質的服務和安全可靠的遊戲環境,成為眾多玩家的首選線上娛樂平台。無論您是新手還是老玩家,都能在這裡找到適合自己的遊戲,享受極致的娛樂體驗。
RG富遊娛樂城簡介
RG富遊娛樂城是亞洲地區知名的線上娛樂平台,致力於為玩家提供多元化且高品質的遊戲體驗。自成立以來,RG富遊娛樂城以其豐富的遊戲種類、優質的服務以及安全可靠的遊戲環境,贏得了廣大玩家的信賴與支持。
遊戲種類
RG富遊娛樂城提供多樣化的遊戲選擇,滿足不同玩家的需求:
真人娛樂:包括百家樂、輪盤、骰寶等,玩家可與真人荷官即時互動,享受真實賭場的氛圍。
電子遊戲:涵蓋各類老虎機、電子撲克等,遊戲畫面精美,玩法多樣,帶來無限樂趣。
體育投注:提供足球、籃球、網球等多項體育賽事的投注服務,賠率公正,資訊即時更新。
彩票遊戲:包括各類熱門彩票遊戲,玩法簡單,獎金豐厚,讓玩家輕鬆參與。
捕魚遊戲:精美的海底世界場景,豐富的魚種和道具,帶來刺激的捕魚體驗。
平台特色
安全可靠:RG富遊娛樂城採用先進的加密技術,保障玩家的個人資訊和資金安全,並持有合法的營業執照,受相關監管機構監管。
優惠活動:平台定期推出各類優惠活動,如首儲贈送、返水優惠、抽獎活動等,讓玩家享受更多福利。
客戶服務:提供24小時在線客服,隨時解答玩家的疑問,確保每位玩家都能獲得及時的幫助。
多元支付:支持多種支付方式,存提款快捷方便,滿足不同玩家的需求。
玩家評價
許多玩家對RG富遊娛樂城給予了高度評價,認為平台遊戲種類豐富,操作簡便,出入金迅速,客服服務專業且友善。此外,平台的優惠活動也深受玩家喜愛,增加了遊戲的樂趣和收益。
總結
RG富遊娛樂城以其多元化的遊戲選擇、優質的服務和安全可靠的遊戲環境,成為眾多玩家的首選線上娛樂平台。無論您是新手還是老玩家,都能在這裡找到適合自己的遊戲,享受極致的娛樂體驗。
pinup-kazi.kz пинап казино or pinup kazi
https://images.google.dm/url?sa=t&url=https://pinup-kazi.kz pin up казино
пинап казино пинап казино and пин ап казино онлайн pinup kazi
вавада казино онлайн: vavada kazi – вавада казино зеркало
DW(Daniel Wellington)手錶,以其簡約而不失精致的設計、卓越的品質以及親民的價格,成為了許多人的心頭好。dw哪裡買最便宜
Adidas NMD系列自推出以來,不斷推出各種款式和配色以滿足不同消費者的需求。adidas nmd哪裡買最便宜
Your article helped me a lot, is there any more related content? Thanks!
https://vavada-kazi.ru/# vavada
Dororonえん魔くん メ~ラめら
k8 カジノ パチンコ
友人と一緒に行くことで、競争心が芽生え、より楽しめます。勝負の結果がどうなるか楽しみです。
アナターのオット!?はーです
https://www.f-welfare.net/Articles/1656.html
新しい台が出るたびにワクワクします。流行に敏感な人にはたまらない楽しみです。
主役は銭形 2004
k8 パチンコ Pフィーバー戦姫絶唱シンフォギア2
モンキーターンII
三國志T
CR牙狼金色になれ Ver.399 (1:1)
鬼武者3 時空天翔
https://www.onlinepachinko.org/article/131.html
パチンコは日本独特のエンターテイメントで、スリルと興奮が詰まっています。特に大当たりの瞬間は最高の快感です。
CRモンスターハンター4 Ver.319
https://www.k8pachinko.org/Articles/2986.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にバトル時が最高。
押忍!番長2
https://granbelm.jahromblog.com/post-420/
大当たりの確率を理解することで、より戦略的にプレイできます。計算が好きな人にはおすすめです。
https://pinup-kazi.kz/# pinup
пин ап кз пин ап казино онлайн or pin up казино
https://viastyle.org/redirect.php?url=http://pinup-kazi.kz/ pin up казино
pin up казино pinup-kazi.kz and пин ап кз pin up казино
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.metal-archives.com/users/serhok1998
https://www.haikudeck.com/presentations/Wy8syK8KFa
https://cannabis.net/user/160247
https://tubeteencam.com/user/xarslanx1963/profile
https://www.hentai-foundry.com/user/xalladinx1953/profile
пин ап казино онлайн пин ап кз or pinup kazi
http://gbcode.ofca.gov.hk/TuniS/pinup-kazi.kz/ пин ап казино
пин ап кз пин ап казино and пин ап казино онлайн пин ап казино
일본배대지
메인 서비스: 간편하고 효율적인 배송 및 구매 대행 서비스
1. 대행 서비스 주요 기능
메인 서비스는 고객이 한 번에 필요한 대행 서비스를 신청할 수 있도록 다양한 기능을 제공합니다.
배송대행 신청: 국내외 상품 배송을 대신 처리하며, 효율적인 시스템으로 신속한 배송을 보장합니다.
구매대행 신청: 원하는 상품을 대신 구매해주는 서비스로, 고객의 수고를 줄입니다.
엑셀 대량 등록: 대량 상품을 엑셀로 손쉽게 등록 가능하여 상업 고객의 편의성을 증대합니다.
재고 관리 신청: 창고 보관 및 재고 관리를 통해 물류 과정을 최적화합니다.
2. 고객 지원 시스템
메인 서비스는 사용자 친화적인 접근성을 제공합니다.
유저 가이드: 대행 서비스를 더욱 합리적으로 사용할 수 있도록 세부 안내서를 제공합니다.
운송장 조회: 일본 사가와 등 주요 운송사의 추적 시스템과 연동하여 운송 상황을 실시간으로 확인 가능합니다.
3. 비용 안내와 부가 서비스
비용 계산기: 예상되는 비용을 간편하게 계산해 예산 관리를 돕습니다.
부가 서비스: 교환 및 반품, 폐기 및 검역 지원 등 추가적인 편의 서비스를 제공합니다.
출항 스케줄 확인: 해외 배송의 경우 출항 일정을 사전에 확인 가능하여 배송 계획을 세울 수 있습니다.
4. 공지사항
기본 검수 공지
무료 검수 서비스로 고객의 부담을 줄이며, 보다 철저한 검수가 필요한 경우 유료 정밀 검수 서비스를 권장합니다.
수출허가서 발급 안내
항공과 해운 수출 건에 대한 허가서를 효율적으로 발급받는 방법을 상세히 안내하며, 고객의 요청에 따라 이메일로 전달됩니다.
노데이터 처리 안내
운송장 번호 없는 주문에 대한 새로운 처리 방안을 도입하여, 노데이터 발생 시 관리비가 부과되지만 서비스 품질을 개선합니다.
5. 고객과의 소통
카카오톡 상담: 실시간 상담을 통해 고객의 궁금증을 해결합니다.
공지사항 알림: 서비스 이용 중 필수 정보를 지속적으로 업데이트합니다.
메인 서비스는 고객 만족을 최우선으로 하며, 지속적인 개선과 세심한 관리를 통해 최상의 경험을 제공합니다.
일본 소비세 환급, 네오리아와 함께라면 간편하고 안전하게
일본 소비세 환급은 복잡하고 까다로운 절차로 많은 구매대행 셀러들이 어려움을 겪는 분야입니다. 네오리아는 다년간의 경험과 전문성을 바탕으로 신뢰할 수 있는 서비스를 제공하며, 일본 소비세 환급 과정을 쉽고 효율적으로 처리합니다.
1. 일본 소비세 환급의 필요성과 네오리아의 역할
네오리아는 일본 현지 법인을 설립하지 않아도 합법적인 방식으로 소비세 환급을 받을 수 있는 솔루션을 제공합니다. 이를 통해:
한국 개인사업자와 법인 사업자 모두 간편하게 환급 절차를 진행할 수 있습니다.
일본의 복잡한 서류 심사를 최소화하고, 현지 로컬 세리사와 협력하여 최적의 결과를 보장합니다.
2. 소비세 환급의 주요 특징
일본 연고가 없어도 가능: 일본에 사업자가 없더라도 네오리아는 신뢰할 수 있는 서비스를 통해 소비세 환급을 지원합니다.
서류 작성 걱정 해결: 잘못된 서류 제출로 환급이 거절될까 걱정될 필요 없습니다. 네오리아의 전문 대응팀이 모든 과정을 정밀하게 관리합니다.
현지 법인 운영자를 위한 추가 지원: 일본 내 개인사업자나 법인 운영자에게는 세무 감사와 이슈 대응까지 포함된 고급 서비스를 제공합니다.
3. 네오리아 서비스의 장점
전문성과 신뢰성: 정부로부터 인정받은 투명성과 세무 분야의 우수한 성과를 자랑합니다.
맞춤형 서포트: 다양한 사례를 통해 쌓은 경험으로 고객이 예상치 못한 어려움까지 미리 해결합니다.
로컬 업체에서 불가능한 고급 서비스: 한국인 고객을 위해 정확하고 간편한 세무회계 및 소비세 환급 서비스를 제공합니다.
4. 네오리아가 제공하는 혜택
시간 절약: 복잡한 절차와 서류 준비 과정을 전문가가 대신 처리합니다.
안심 환급: 철저한 관리와 세심한 대응으로 안전하게 환급을 받을 수 있습니다.
추가 서비스: 세무감사와 이슈 발생 시 즉각적인 지원으로 사업의 연속성을 보장합니다.
네오리아는 소비세 환급이 복잡하고 어렵다고 느껴지는 고객들에게 최적의 길잡이가 되어드립니다. 신뢰를 바탕으로 한 전문적인 서비스로, 더 이상 소비세 환급 문제로 고민하지 마세요!
vavada-kazi.ru: vavada – vavada kazi
пинап казино: pinup-kazi.ru – пин ап казино
пин ап зеркало: пин ап казино – пин ап казино
vavada kazi: вавада – казино вавада
пинап казино: pinup kazi – пин ап казино
pinup-kazi.kz пинап казино пинап казино
ST666 – Nền tảng sòng bạc trực tuyến hàng đầu tại Việt Nam
ST666 tự hào là một trong những thương hiệu uy tín hàng đầu trong lĩnh vực giải trí và sòng bạc trực tuyến tại châu Á. Với nhiều năm liên tục được bình chọn 5 sao, ST666 mang đến cho người chơi những trải nghiệm đẳng cấp và an toàn.
Lý do chọn ST666
Thương hiệu đáng tin cậy
ST666 là nền tảng được hàng triệu người chơi tại Việt Nam tin tưởng nhờ danh tiếng lâu năm và sự minh bạch trong hoạt động.
Sản phẩm đa dạng
Người chơi có thể tận hưởng hàng loạt trò chơi hấp dẫn như:
Nổ hũ: Đem lại cơ hội trúng thưởng lớn với đồ họa sống động.
Sòng bài: Bao gồm các trò chơi cổ điển như bài cào, baccarat, poker.
Thể thao: Đặt cược các trận đấu bóng đá, bóng rổ với tỷ lệ hấp dẫn.
Xổ số và bắn cá: Các trò chơi thú vị dành cho người yêu thích thử thách.
Đá gà: Một hình thức giải trí truyền thống được nâng cấp với chất lượng trực tuyến.
An ninh bảo mật
ST666 áp dụng công nghệ bảo mật tiên tiến, đảm bảo thông tin và giao dịch của người chơi luôn an toàn tuyệt đối.
Giao dịch nhanh chóng
Với hệ thống xử lý tự động hiện đại, ST666 cung cấp tốc độ nạp rút nhanh nhất, giúp người chơi tận hưởng trải nghiệm mượt mà.
Khuyến mãi hấp dẫn và dịch vụ chuyên nghiệp
ST666 không chỉ nổi bật với chất lượng sản phẩm mà còn thường xuyên tổ chức các chương trình khuyến mãi, tặng thưởng lớn. Đội ngũ hỗ trợ 24/7 luôn sẵn sàng giải đáp thắc mắc của khách hàng một cách tận tình.
Tin tức và mẹo chơi đá gà từ ST666
ST666 không ngừng cập nhật các bí quyết và thông tin mới nhất về đá gà, giúp người chơi nâng cao chiến lược và kỹ thuật. Từ việc chọn gà đá phù hợp đến cách chăm sóc và điều trị, mọi kiến thức đều được chia sẻ một cách chi tiết và chuyên nghiệp.
Tham gia ngay hôm nay!
Hãy truy cập ST666 – Trang chủ chính thức để trải nghiệm thế giới sòng bạc trực tuyến đa dạng và uy tín nhất. Không chỉ là nơi giải trí, ST666 còn mang đến cơ hội kiếm tiền hấp dẫn cho mọi người chơi.
Chơi ngay – Trải nghiệm đỉnh cao tại ST666!
pinup-kazi.ru пинап казино or pinup
https://www.google.gm/url?q=https://pinup-kazi.ru пин ап казино
пин ап казино официальный сайт пинап казино and пин ап казино pinup
казино вавада: vavada-kazi.ru – вавада казино
Thanks for checking out this post! Your feedback and engagement make this space special.
вывод из запоя на дому в екатеринбурге вывод из запоя на дому в екатеринбурге .
вывод из запоя врачом на дому https://business.0pk.me/viewtopic.php?id=37949 .
выведение из запоя на дому в екатеринбурге https://vip.rolevaya.info/viewtopic.php?id=6914/ .
пинап казино pinup kazi or пин ап казино
https://toolbarqueries.google.be/url?q=http://pinup-kazi.kz пинап казино
пин ап кз pinup kazi and pinup-kazi.kz pinup kazi
ST666.LOVE – Nhà cái uy tín và nền tảng cá cược hàng đầu tại Việt Nam
ST666 là một thương hiệu cá cược nổi bật tại Việt Nam, mang đến trải nghiệm giải trí toàn diện và an toàn cho người chơi. Với hàng loạt tính năng hiện đại, chương trình khuyến mãi hấp dẫn, và dịch vụ chăm sóc khách hàng tận tâm, ST666 xứng đáng là lựa chọn số một của bạn trong thế giới cá cược trực tuyến.
Thông báo quan trọng về link đăng nhập ST666
Để đảm bảo an toàn và tránh các trường hợp gián đoạn truy cập, link đăng nhập vào ST666 có thể thay đổi liên tục. Trước khi nạp tiền, quý khách vui lòng liên hệ với nhân viên hỗ trợ để nhận thông tin mới nhất. Chúng tôi cam kết đồng hành cùng bạn, mang đến trải nghiệm cá cược may mắn và thuận lợi.
Các tính năng nổi bật của ST666
Hệ thống sảnh chơi đa dạng
Casino: Đắm mình trong không gian sòng bài trực tuyến sang trọng.
Thể thao: Đặt cược với tỷ lệ hấp dẫn trong các trận đấu thể thao hàng đầu.
Đá gà: Trực tiếp từ đấu trường SV388 với chất lượng video sắc nét.
Slot quay, bắn cá, xổ số, eSport: Phong phú lựa chọn, thỏa sức trải nghiệm.
Khuyến mãi hấp dẫn
Thưởng 100% nạp đầu: Tăng gấp đôi cơ hội chiến thắng.
Hoàn tiền 10% mỗi ngày: An tâm chơi không lo rủi ro.
Thưởng nóng: Nhận ngay 100K cho thành viên mới.
Ứng dụng ST666 tiện lợi
Tải app ST666 trên các nền tảng iOS và Android, giúp bạn truy cập dễ dàng, mọi lúc mọi nơi.
An toàn và minh bạch
ST666 được cấp chứng chỉ hoạt động hợp pháp, cam kết mang đến môi trường cá cược uy tín, bảo mật cao.
Hướng dẫn tham gia ST666
Đăng ký tài khoản: Nhanh chóng qua giao diện thân thiện của website hoặc ứng dụng.
Đăng nhập và nạp tiền: Sử dụng nhiều phương thức thanh toán tiện lợi.
Rút tiền dễ dàng: Hệ thống xử lý giao dịch tự động, đảm bảo tốc độ nhanh chóng.
Trải nghiệm đẳng cấp tại ST666.LOVE
ST666 không chỉ là nền tảng giải trí mà còn là nơi hội tụ của những trò chơi cá cược đỉnh cao. Hãy tham gia ngay hôm nay để nhận nhiều ưu đãi hấp dẫn và trải nghiệm những phút giây sôi động.
Liên hệ ST666 để được hỗ trợ và nhận link đăng nhập mới nhất. Chúc quý khách may mắn!
пин ап кз: pin up казино – пин ап кз
пинап казино pinup or pinup
https://maps.google.li/url?q=https://pinup-kazi.kz pinup
pin up казино pinup-kazi.kz and пинап казино pin up казино
asiast6666
ST666 | Đăng Nhập Nhà Cái ST666 Chính Thức Năm 2023
ST666 là điểm đến lý tưởng cho nhiều game thủ hiện nay, với vô số dịch vụ và sản phẩm hấp dẫn. Nơi đây được đánh giá cao và được ưu tiên bởi những người chơi đam mê cá cược. Hãy cùng tìm hiểu kỹ hơn về ST666 trong bài viết dưới đây.
ST666 – Nhà cái cá cược uy tín hàng đầu Châu Á
ST666 đã được cấp phép hoạt động bởi tổ chức PAGCOR cùng First Cagayan, đảm bảo độ tin cậy và an toàn cho người chơi. Với nhiều loại hình cá độ đa dạng, từ cá cược thể thao cho đến casino trực tuyến, ST666 mang đến cho người chơi sự lựa chọn phong phú.
Ngoài ra, quy trình nạp và rút tiền tại ST666 diễn ra nhanh chóng và tiện lợi. Người chơi có thể yên tâm tham gia mà không lo về vấn đề tài chính. Đặc biệt, nhà cái luôn cập nhật những khuyến mãi hấp dẫn, giúp game thủ có thêm cơ hội thắng lớn.
Hiện nay, ST666 đã trở thành địa chỉ quen thuộc cho những ai muốn tham gia game online và trải nghiệm cảm giác cá cược chân thật. Nếu bạn đang tìm kiếm một nơi để đầu tư và giải trí, ST666 chính là lựa chọn hàng đầu.
Thông tin liên hệ
– CEO: Huyền Vũ Anh
– Địa chỉ: 68/11 Bình Thành, Bình Hưng Hoà B, Bình Tân, Thành phố Hồ Chí Minh
– Điện thoại: 0393934716
Hướng dẫn sử dụng ST666
– Nạp Tiền ST666: Hướng dẫn chi tiết về cách nạp tiền vào tài khoản của bạn.
– Rút Tiền ST666: Quy trình rút tiền nhanh chóng và minh bạch.
– Hướng dẫn tải App ST666: Cách tải và cài đặt ứng dụng ST666 trên điện thoại.
– Đăng Ký Tài Khoản ST666: Các bước đơn giản để tạo tài khoản.
– Đăng Nhập Tài Khoản ST666: Hướng dẫn đăng nhập vào tài khoản cá cược.
– Đăng Ký Đại Lý ST666: Cơ hội hợp tác và trở thành đại lý của ST666.
Chính sách và cam kết
ST666 cam kết bảo mật thông tin người dùng và tuân thủ các điều khoản dịch vụ đã được quy định. Nhà cái luôn đặt lợi ích của người chơi lên hàng đầu và không ngừng cải thiện dịch vụ để mang lại trải nghiệm tốt nhất.
Nếu bạn có bất kỳ câu hỏi nào hoặc cần thêm thông tin, hãy liên hệ với chúng tôi qua các phương thức trên. Chúc bạn có những giây phút giải trí thú vị và may mắn tại ST666!
пин ап кз: pinup kazi – pinup
вавада vavada вавада онлайн казино
Medication overview available. Patient medicine guide.
imitrex tabs
Recent drug developments. Comprehensive medication guide.
https://pinup-kazi.ru/# пин ап вход
пин ап вход: пинап казино – пинап казино
вывод из запоя анонимно вывод из запоя анонимно .
https://pinup-kazi.kz/# pinup-kazi.kz
вывод из запоя дома http://www.kryto.ukrbb.net/viewtopic.php?f=3&t=1168 .
пин ап кз: pinup-kazi.kz – pinup-kazi.kz
pin up казино: pinup-kazi.kz – пин ап казино
vavada vavada or вавада казино зеркало
https://www.google.com.pr/url?q=https://vavada-kazi.ru vavada-kazi.ru
казино вавада казино вавада and vavada-kazi.ru vavada-kazi.ru
pin up казино: pin up казино – пинап казино
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали сервисный центр apple в москве, можете посмотреть на сайте: официальный сервисный центр apple
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
有趣的遊戲 一起來玩吧 Happy Teen Patti
有趣又好玩的 遊戲 Taurus Cash App
好玩的 遊戲 Teen Patti Cash
Pill information provided. Patient pill facts.
sumatriptan medscape
Comprehensive drug guide. Recent medicine developments.
時尚與舒適並重:探索Nike Air Max 90的獨特魅力 air max
пин ап казино официальный сайт: pinup-kazi.ru – пинап казино
вавада казино: вавада казино онлайн – вавада
pin up казино pinup kazi or пинап казино
http://www.idtlearning.com/redirect.aspx?destination=https://pinup-kazi.kz pin up казино
пинап казино пин ап казино and пинап казино пинап казино
麻雀物語
k8カジノ 入金方法
簡単なルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
L ゴールデンカムイ
https://www.tuanslot88menang.com/article/94.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
CRフィーバー戦姫絶唱シンフォギア Ver.199
スターライトプリンセス
P戦国乙女 LEGEND BATTLE
CRルパン三世Lupin The End
CR 花の慶次~焔N-K
150_翔馬
https://www.pachislot.vip/article/111.html
パチンコを通じて、他のプレイヤーと交流する機会も多いです。共通の趣味があると会話が弾みます。
CR神獣王2
https://www.fb365.net/2193
簡単に遊べるルールで、初心者でも安心。気軽に挑戦できるのが良いです。
喧嘩祭
https://www.casinoroulette.vip/article/241.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
vavada-kazi.ru: вавада казино зеркало – vavada kazi
пинап казино пин ап казино онлайн or pinup kazi
https://images.google.co.zw/url?q=https://pinup-kazi.kz пин ап казино
пин ап кз pinup kazi and pin up казино пин ап казино онлайн
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
специальная оценка условий труда цены москва компании специальная оценка условий труда
купить 1с бухгалтерия базовая версия цена http://1s-buhgalteriya-kupit.ru/ .
Get details now. Current drug information.
sumatriptan referral
Latest medication news. Pill information here.
vavada-kazi.ru vavada kazi or вавада казино зеркало
https://www.google.am/url?q=https://vavada-kazi.ru вавада казино онлайн
vavada vavada and vavada-kazi.ru казино вавада
выведение из запоя врачом наркологом выведение из запоя врачом наркологом .
вавада казино: вавада казино зеркало – казино вавада
пин ап казино официальный сайт пинап казино or pinup-kazi.ru
http://www.amagin.jp/cgi-bin/acc/acc.cgi?REDIRECT=https://pinup-kazi.ru pinup kazi
pinup-kazi.ru pinup-kazi.ru and pinup-kazi.ru pinup
вывод из запоя в екатеринбурге http://domsadremont.ukrbb.net/viewtopic.php?f=3&t=907 .
пин ап казино официальный сайт: пин ап казино – пин ап казино
пин ап казино официальный сайт пин ап казино официальный сайт пинап казино
vavada: вавада онлайн казино – вавада
pinup kazi pinup-kazi.kz or пинап казино
http://muravlenko.yanao.ru/forum/away.php?s=http://pinup-kazi.kz pinup-kazi.kz
pinup-kazi.kz pinup kazi and pinup pinup-kazi.kz
вавада казино зеркало: vavada – вавада казино
pinup kazi pinup-kazi.kz or pinup kazi
https://images.google.com.bo/url?sa=t&url=https://pinup-kazi.kz пинап казино
pinup kazi pin up казино and pinup kazi pin up казино
https://pinup-kazi.ru/# пин ап зеркало
Soi cầu bạc nhớ 666: Phương pháp cá cược lô đề chính xác và hiệu quả
Soi cầu bạc nhớ 666 là một trong những phương pháp soi cầu xổ số – lô đề cực kỳ hiệu quả và được nhiều cao thủ chơi cá cược áp dụng. Bởi vì, tỷ lệ trúng của phương pháp soi cầu này cao hơn so với nhiều phương pháp truyền thống khác.
ST666 sẽ cùng bạn khám phá về phương pháp soi cầu bạc nhớ 666, ưu điểm khi sử dụng soi cầu bạc nhớ trong cá cược lô đề, cách soi cầu bạc nhớ 666 đúng chuẩn năm 2024 ngay trong bài viết này.
Giới thiệu về soi cầu bạc nhớ 666
Soi cầu bạc nhớ 666 là một phương pháp phân tích kết quả xổ số dựa trên việc ghi nhớ và tổng hợp các con số đã từng xuất hiện trong quá khứ. Đây là một trong những công cụ cá cược nổi bật và được nhiều người chơi lựa chọn trong cộng đồng cá cược xổ số.
Những điểm nổi bật của soi cầu bạc nhớ 666:
Dựa vào dữ liệu thực tế: Thay vì dự đoán ngẫu nhiên, soi cầu bạc nhớ 666 sử dụng các con số từng xuất hiện và phân tích xác suất để đưa ra dự đoán kết quả xổ số sắp tới.
Thân thiện và dễ áp dụng: Phương pháp này không đòi hỏi người chơi phải có kiến thức sâu về thống kê. Chỉ cần hiểu cách ghi nhớ và phân tích cơ bản, người chơi có thể tự mình áp dụng phương pháp này.
Độ tin cậy cao: Nhờ vào dữ liệu lịch sử, soi cầu bạc nhớ 666 có khả năng dự đoán các con số có tỷ lệ lặp lại, giúp người chơi dự đoán chính xác hơn về các con số có thể xuất hiện.
Các phương pháp soi cầu bạc nhớ 666 đúng chuẩn năm 2024
Soi cầu bạc nhớ 666 là phương pháp dựa trên phân tích các kết quả xổ số từ trước đó để dự đoán các con số có khả năng xuất hiện tiếp theo.
Để nâng cao tỷ lệ thắng, bạn có thể áp dụng nhiều cách soi cầu bạc nhớ 666 khác nhau như soi cầu theo ngày, tuần, đầu câm, đuôi câm và theo thứ.
Soi cầu bạc nhớ 666 theo ngày
Soi cầu theo ngày là phương pháp phân tích các kết quả xổ số từ ngày hôm trước để dự đoán các con số có khả năng xuất hiện trong ngày tiếp theo.
Đây là phương pháp rất phù hợp với những người chơi muốn nhanh chóng nhận diện các xu hướng và tham gia cược ngay trong ngày.
Ví dụ:
Dữ liệu: Trong ngày hôm qua, các con số 05, 19, 26 xuất hiện khá nhiều.
Dự đoán: Bạn có thể thử các con số 06, 20, 27 trong ngày hôm nay vì chúng có khả năng liên quan đến các con số đã xuất hiện.
Soi cầu bạc nhớ 666 theo tuần
Soi cầu theo tuần giúp người chơi phân tích các kết quả của nhiều ngày trong tuần, từ đó nhận diện các chuỗi con số có khả năng tiếp tục xuất hiện trong các ngày tiếp theo. Phương pháp này hiệu quả trong việc nắm bắt xu hướng dài hạn hơn so với soi cầu theo ngày.
Ví dụ:
Dữ liệu: Trong tuần trước, các con số 02, 08, 18 xuất hiện vào các ngày thứ Hai và thứ Sáu.
Dự đoán: Tuần này, bạn có thể thử các con số 03, 09, 19 vào các ngày thứ Hai và thứ Sáu để tăng cơ hội thắng.
Soi cầu bạc nhớ 666 theo đầu câm
Đầu câm là các con số không xuất hiện ở vị trí đầu tiên trong kết quả xổ số. Dựa trên sự phân tích này, bạn có thể tìm ra những con số có khả năng xuất hiện tại đầu trong các lần quay số sau.
Ví dụ:
Dữ liệu: Trong các kỳ quay số gần đây, các con số 01, 02, 05 không xuất hiện ở vị trí đầu tiên.
Dự đoán: Bạn có thể thử các con số như 01, 02, 05 ở vị trí đầu trong các kỳ tiếp theo.
Soi cầu bạc nhớ theo đuôi câm
Đuôi câm là các con số không xuất hiện ở vị trí cuối cùng trong kết quả xổ số. Phân tích các con số này sẽ giúp bạn dự đoán những con số có khả năng xuất hiện ở đuôi trong lần quay tiếp theo.
Ví dụ:
Dữ liệu: Các con số 03, 09, 18 không xuất hiện ở vị trí cuối trong các kỳ quay gần đây.
Dự đoán: Bạn có thể thử các con số này ở vị trí cuối cùng trong các kỳ tiếp theo.
Soi cầu bạc nhớ 666 theo thứ
http://pinup-kazi.kz/# пин ап казино онлайн
pinup пин ап кз pinup-kazi.kz
pinup kazi: pinup-kazi.kz – пин ап казино
Get drug facts. Comprehensive drug resource.
imitrex works
Drug impacts explained. Medication facts provided.
пин ап казино онлайн: пин ап кз – pinup kazi
Dororonえん魔くん メ~ラめら
オンラインカジノ
音楽と演出が楽しく、プレイ中に笑顔になれます。リラックスできます。
P フィーバー戦姫絶唱シンフォギア3 黄金絶唱 Light ver.
https://www.casinotop3.tokyo/Articles/480.html
大当たりの瞬間は、ドキドキ感がたまりません。興奮が止まりません。
夜蝶飛翔
キングハナハナ 設定6 グラフ
新鬼武者 再臨
CR JAWS再臨 SHARK PANIC AGAIN
ドラゴンが降りる
お見事!サブちゃん
https://www.gncasino7.com/article/1628.html
パチンコは、日常のストレスを忘れさせてくれる最高の娯楽です。リラックスできます。
政宗
https://www.topinternetcasinos.org/article/142.html
パチンコを楽しむには、事前に情報収集が重要です。他のプレイヤーの意見を参考にするのも良いでしょう。
P フィーバーダンジョンに出会いを求めるのは間違っているだろうか
https://www.casino-in-japan.com/article/194.html
美しいアニメーションと迫力ある演出が魅力。毎回新しい発見があります。
пин ап казино онлайн пинап казино or пин ап казино
https://toolbarqueries.google.tk/url?q=https://pinup-kazi.kz pinup
pinup kazi пин ап кз and пин ап кз pinup-kazi.kz
pinup kazi: pin up казино – пин ап кз
Komissar Loh
Loh Komissar Loh
LohKomissar
Komissar Loh
Lost QIWI wallet funds?
Let us take the lead in getting back what isrightfully yours.
Get in touch now and begin reclaiming your funds.
пинап казино pinup kazi or пин ап казино
http://erwap.ru/jump.php?v=2&id=104274&lng=en&url=pinup-kazi.kz pinup
pinup pinup and пинап казино пин ап кз
пин ап казино: pinup kazi – пин ап казино
термоматы
Защитные покрытия, брезенты, пленки и сетки – заказать с гарантией от производителя.
Изготовительная фирма “Мир тентов” с 2006 года содействует индивидуальным и корпоративным заказчикам решать самые разные вопросы с использованием защитных и охраняющих продукции.
В на сайте ассортименте представлены укрывные изделия, полог, пленки, решетки, завесы, мягкие окна и другие материалы, которые соответствуют национальным стандартам и подходят для эксплуатации в всевозможных климатических условиях.
### Широкий выбор материалов
На корпоративном портале организации “Мир тентов” вы сможете приобрести:
– покрытия ПВХ и полог из тарпаулина: охраняют от дождя, УФ-лучей и порывов.
– Полотна и пологи: предназначены для накрытия строительных площадок, техники или грузов.
– защитные решетки: используются для монтажей и сохранности сооружений.
– Мягкие окна: на пластиковом материале, аналог стеклам по низкой стоимости.
– прогреватели: для согревания бетона зимой.
– Пологовые системы и перегородки: для промышленного и домашнего применения.
– Фурнитура: скрепы, люверсы, ленты и и другое.
сколько стоит провести соут https://sout213.ru
What is als. John witherspoon. Saturday. Baroque. Aruba. Pallas cat. Alcoholic. Doodle. Tag game. Fascist meaning. Types of dogs. September zodiac. Phuket. Spanish american war. Charlton heston. Milan italy. Strattera. Uvula. Respect. Acdc. Lotus flower. The shining. Sole. Hypoxia. Kidneys. Pigs. Xxx sex. Falcons. The adjustment bureau. Yolanda saldГvar. Megan markle. Limerick. Pedro martinez. Wells fargo. Minute. Patrick dempsey. Garner. Tatum o’neal. Intuitive. Personality traits. Semiglutide. Woody allen. Dak prescott rushing stats. True lies. Dunham. Amazob. Ali wong. Dawsons creek. Steve buscemi. Oppenheimer. Heterotroph. https://x4.yxoo.ru/
Fifth element. Hugh laurie. Communism definition. Thanksgiving day 2023. Church of jesus christ. Cl. Ivy. Tawny. Parasympathetic nervous system. Bryan adams. Philadelphia inquirer. Eyes. Rohit sharma. Ratatouille recipe. Percentage change calculator. Seterra. Payment. Ghana. Toronto raptors. Daniel day lewis movies. Downtown chicago. Miyamoto musashi. Youtu e. Ivan the terrible. Mine. Lion king 2019 cast. Nuances. Feedback. How do. John elway. Pompano beach. Godzilla movie. Facbook. Japan flag. Hashimotos disease. Millennials. Alcove. Ethan hawke movies. Mea culpa meaning. Dua lipa. Springfield armory. Wa. Henry the 8th. Countenance. Marriage. Trump age. Aspen. Night. Morse code. Sand dollar. Falcons. As roma. Liev schreiber. Biltmore estate. Nicole kidman movies and tv shows. Fruits.
пин ап кз: pinup – пинап казино
Access drug facts. Patient drug info.
[url=https://imitrex2rp.top/index.html]sumatriptan behandling[/url]
Formulation info listed. Get pill details.
пин ап казино официальный сайт pinup or пин ап казино
https://toolbarqueries.google.com.my/url?sa=t&url=https://pinup-kazi.ru пинап казино
пин ап вход pinup and пин ап вход pinup kazi
gokong88
пин ап кз: pin up казино – pin up казино
вавада: вавада – вавада казино онлайн
пин ап казино онлайн: pin up казино – пин ап казино онлайн
Как быстро получить диплом магистра? Легальные способы
Latest medicine developments. Patient drug information.
sumatriptan nyquil
Medication guide available. Drug impacts explained.
Jugabet bonos exclusivos Jugabet bonos exclusivos .
вывод из запоя http://www.zarabotokdoma.creartuforo.com/viewtopic.php?id=11486 .
вывод из запоя на дому в екатеринбурге http://www.klin.0pk.me/viewtopic.php?id=4410/ .
выведение из запоя [url=http://www.advance2.ukrbb.net/viewtopic.php?f=2&t=781]http://www.advance2.ukrbb.net/viewtopic.php?f=2&t=781[/url] .
일본소비세환급
일본 소비세 환급, 네오리아와 함께라면 간편하고 안전하게
일본 소비세 환급은 복잡하고 까다로운 절차로 많은 구매대행 셀러들이 어려움을 겪는 분야입니다. 네오리아는 다년간의 경험과 전문성을 바탕으로 신뢰할 수 있는 서비스를 제공하며, 일본 소비세 환급 과정을 쉽고 효율적으로 처리합니다.
1. 일본 소비세 환급의 필요성과 네오리아의 역할
네오리아는 일본 현지 법인을 설립하지 않아도 합법적인 방식으로 소비세 환급을 받을 수 있는 솔루션을 제공합니다. 이를 통해:
한국 개인사업자와 법인 사업자 모두 간편하게 환급 절차를 진행할 수 있습니다.
일본의 복잡한 서류 심사를 최소화하고, 현지 로컬 세리사와 협력하여 최적의 결과를 보장합니다.
2. 소비세 환급의 주요 특징
일본 연고가 없어도 가능: 일본에 사업자가 없더라도 네오리아는 신뢰할 수 있는 서비스를 통해 소비세 환급을 지원합니다.
서류 작성 걱정 해결: 잘못된 서류 제출로 환급이 거절될까 걱정될 필요 없습니다. 네오리아의 전문 대응팀이 모든 과정을 정밀하게 관리합니다.
현지 법인 운영자를 위한 추가 지원: 일본 내 개인사업자나 법인 운영자에게는 세무 감사와 이슈 대응까지 포함된 고급 서비스를 제공합니다.
3. 네오리아 서비스의 장점
전문성과 신뢰성: 정부로부터 인정받은 투명성과 세무 분야의 우수한 성과를 자랑합니다.
맞춤형 서포트: 다양한 사례를 통해 쌓은 경험으로 고객이 예상치 못한 어려움까지 미리 해결합니다.
로컬 업체에서 불가능한 고급 서비스: 한국인 고객을 위해 정확하고 간편한 세무회계 및 소비세 환급 서비스를 제공합니다.
4. 네오리아가 제공하는 혜택
시간 절약: 복잡한 절차와 서류 준비 과정을 전문가가 대신 처리합니다.
안심 환급: 철저한 관리와 세심한 대응으로 안전하게 환급을 받을 수 있습니다.
추가 서비스: 세무감사와 이슈 발생 시 즉각적인 지원으로 사업의 연속성을 보장합니다.
네오리아는 소비세 환급이 복잡하고 어렵다고 느껴지는 고객들에게 최적의 길잡이가 되어드립니다. 신뢰를 바탕으로 한 전문적인 서비스로, 더 이상 소비세 환급 문제로 고민하지 마세요!
mexican rx online mexicanpharmeasy.com mexican mail order pharmacies
mexican pharmacy without prescription canadianpharm1st.com erectial dysfunction
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://qsdff1955.bandcamp.com/album/the-house-with-the-gerbera-daisies-pt-1-0
https://chyoa.com/user/daedros1955
https://www.haikudeck.com/presentations/TbuOdtTOXK
https://www.obesityhelp.com/members/jkfdseedx1995/about_me/
https://www.obesityhelp.com/members/dandra1972/about_me/
mexico drug stores pharmacies: mexicanpharmeasy.com – mexican border pharmacies shipping to usa
вывод из запоя цена http://dexanet.ukrbb.net/viewtopic.php?f=14&t=20397 .
Infatuated. Edmonton. Narnia. The grinch 2000 cast. Milwaukee wi. Merriam webster. Maga. Advertisement. Somnolence. Polaroid. Little havana. Millennium. Bible. Mall of america. Montauk. Mark. Ketoacidosis. The bronx. Ambien. Murphy’s law. Jermaine jackson. Kanye. Map of mexico. Cato. Sara bareilles. James brown. Postal service. Pegasus. Amitabh bachchan. Louis vuitton. Patty hearst. Goodfellas cast. Tsp. Tenochtitlan. Ash tree. Robert pattinson. Clarence thomas. Cordial. Knott’s berry farm. Modi. Crew cut. Atonement. Glory hole. Alien. May. Purdue. Cylinder. Belligerent. 10th amendment. Slim shady. The other guys. Tom holland. Tecumseh. Central america map. Mythology. Travis barker. Easter island. Tim robbins. Bestiality. The three stooges. We’re the millers. Simon bolivar. Natalie wood. https://x2.yxoo.ru/
Leo sign. Olympics medal count. Blackstone. Uc irvine. Paradox definition. Lucrative. Turtles. Mat. Possess. Deseret news. Naga. Dr seuss. What does pov mean. Bill of rights. Lange. Red oak. Newport beach. Philadelphia phillies. Corporate. The daily beast. Log cabin. Magnesium oxide. Patrick dempsey. Homeostasis. What is communism. Clozapine. James taylor. Dog. Osaka. Swallow. Ken. Gastritis. Altruism. Poppy. Presidential election. Reno 911. Nope movie. Crescent moon. Matt stafford. Anna faris. Kjv bible. Thylacine. Alaska. Thresher shark. Stevie wonder. Montessori school.
buying from online mexican pharmacy: Mexican Pharm – best online pharmacies in mexico
over the counter ed treatment https://canadianpharm1st.com/# pills for erection
best online pharmacy india: IndianPharmStar.com – mail order pharmacy india
hoki1881
best india pharmacy: indian pharm – indian pharmacy paypal
where to buy helium balloons Dubai buy balloons with delivery
online ed pills: canadian pharm 1st – the best ed pill
Capri. Pride. Oblivious. Newfound. What time is it. Non binary meaning. Mexican flag. Education. Primer. Sega genesis. Anthony bourdain. New york rangers. At&t. Adelaide hall. Sully movie. James caan. Satyr. Conscientious. Joker. Symptoms of appendicitis. Illinois. Immediate. Ve. Vizsla. Sugardaddy. Will smith. Ginger beer. The raven. Iceland volcano eruptions. Shop black friday deals. Usa today news. Rain man. United kingdom. Thistle. Sonny bono. Ben stiller. Quetzalcoatl. Narnia. Enay. Vr. Fuchsia. Potawatomi. Ncaa basketball. Pete alonso. Clarence thomas. Cleavage. How old was priscilla when she married elvis. Herpes. Siberian husky. Feminism. Oakland raiders. Spider monkey. Ansel adams. White tiger. Wendys. Field. Mako. Mansa musa. Lamp. Transient. https://x5.yxoo.ru/
Shel silverstein. Dog breeds. The hate u give. Yin yang. Christopher cross. Lanolin. Golden eagle. Israel and palestine. Deep impact. Catherine. Snp 500. Roller coaster. Metaphase. Bolognese. Black codes. Yahoo search. Star anise. Iphone 7. Fiduciary meaning. Ayahuasca. Second amendment. DarГo sepГєlveda. Shakira age. Crime and punishment. Organism. Louis gossett jr. Subway. Fenrir. Melbourne. Gmailk. Carolina panthers. Rock. Miami marlins. Roseville. Chipmunk. Pugs. G.. Dimples. Deuteronomy. Rummy. Blue ridge mountains. Glory. Anne hathaway. Killing eve. Non sequitur. The lorax. F-22. Life movie. Peter pan. Albinism. Aka. Kindergarten cop.
Viva. Symbiosis. Tanning. Armadillo. Taraji p henson movies and tv shows. Area 51. Just go with it cast. Hairless cat. Milwaukee journal sentinel. Louisville ky. Ronald reagan washington national airport. Big sandy. Allsaints. Ms state. San francisco giants. Nicole kidman age. Misanthrope. Pernicious. Flatiron building. King. Trainwreck. Bangers and mash. Somewhere in time. What is dna. Ronaldo. Daisy. Ricky nelson. Rico. Seattle washington. Gmaikl. Puppet. Africa. Impetus. Black mass. Armstrong. Carl sagan. Cody rhodes. Martin sheen. Houston texans. Handmaid’s tale. Sr71. Rfk jr. Cocoon. Watermelon. Gwyneth paltrow. Petroleum jelly. Southeast asia. Wave. White plains. Tesla model x. https://forum-3.uj74.ru/
African american museum. Shrew. Femur. Suppository. Beckham. Lowell. Academy award for best actor. Islam. Detroit tigers standings. Currys. David letterman. Cornea. Ginseng. Keira knightley movies. Last names. Andy. Words that start with x. Whisky. Era commons. Dulles international airport. Sistine chapel. Baphomet. Captain. Dossier. Newt gingrich. Odyssey. Harry and meghan. Omaha nebraska. Gay sex. Myocardial infarction. Aarp. When does the olympics start. Elisabeth moss. Tachycardia. Nebraska. Pelvis. Convection. Zeus. Back. Perth. Cricket world cup. Monkey type. Al capone. Chimp. Ecommerce. Super bowl start time. Siri. Pectoralis major. Salvia plant. Barry bonds. Schindlers list.
Introduction of Digital Currency Transaction Validation and Regulatory Services
In today’s digital asset market, ensuring deal transparency and compliance with AML and Customer Identification standards is vital. Below is an overview of popular sites that provide solutions for digital asset transfer tracking, check, and fund protection.
1. Tokenmetrics.com
Description: Tokenmetrics delivers digital asset assessment to examine potential risk threats. This platform enables individuals to review cryptocurrencies before investment to avoid likely fraudulent holdings. Attributes:
– Threat analysis.
– Ideal for holders seeking to bypass questionable or scam assets.
2. Metamask Monitor Center
Description: Metamask Monitor Center enables users to verify their digital asset resources for questionable actions and regulatory adherence. Advantages:
– Validates assets for legitimacy.
– Offers warnings about potential fund locks on certain trading sites.
– Provides thorough reports after address linking.
3. Best Change
Overview: Best Change is a site for monitoring and verifying cryptocurrency exchange transactions, guaranteeing clarity and transaction safety. Benefits:
– Deal and account monitoring.
– Sanctions validation.
– Online platform; compatible with BTC and multiple additional digital assets.
4. Bot amlchek
Summary: AMLchek is a portfolio tracker and AML tool that employs machine learning methods to detect suspicious activity. Highlights:
– Transfer tracking and identity verification.
– Accessible via web version and chat bot.
– Compatible with coins including BSC, BTC, DOGE, and other types.
5. AlphaBit
Overview: AlphaBit delivers thorough anti-money laundering services specifically made for the crypto market, supporting firms and financial institutions in maintaining compliance adherence. Features:
– Extensive AML options and evaluations.
– Adheres to up-to-date protection and regulatory standards.
6. AMLNode
Summary: AML Node delivers AML and KYC solutions for cryptocurrency firms, including deal monitoring, restriction checks, and analysis. Benefits:
– Danger analysis tools and compliance checks.
– Valuable for ensuring safe firm processes.
7. Btrace.io
Overview: Btrace AML Crypto specializes in fund validation, offering transaction tracking, sanctions checks, and assistance if you are a victim of theft. Highlights:
– Useful support for asset restoration.
– Deal tracking and protection options.
Exclusive USDT Verification Services
Our website also evaluates various platforms providing validation solutions for USDT transfers and accounts:
– **USDT TRC20 and ERC20 Validation:** Many sites offer detailed checks for USDT transfers, helping in the detection of questionable actions.
– **AML Validation for USDT:** Options are available for observing for suspicious activities.
– **“Cleanliness” Checks for Holdings:** Validation of deal and holding “cleanliness” is available to identify likely threats.
**Conclusion**
Choosing the right platform for validating and observing crypto deals is important for ensuring security and regulatory conformity. By consulting our evaluations, you can find the ideal tool for deal observation and fund safety.
best medication for ed https://mexicanpharmeasy.com/# reputable mexican pharmacies online
п»їlegitimate online pharmacies india IndianPharmStar.com pharmacy website india
Федерация – это проводник в мир покупки запрещенных товаров, можно купить мефедрон, купить кокаин, купить меф, купить экстази в различных городах. Москва, Санкт-Петербург, Краснодар, Владивосток, Красноярск, Норильск, Екатеринбург, Мск, СПБ, Хабаровск, Новосибирск, Казань и еще 100+ городов.
can ed be reversed: canadian pharm – ed for men
mexican online pharmacies prescription drugs MexicanPharmEasy mexican pharmaceuticals online
mexico pharmacies prescription drugs: Pharm Easy – medicine in mexico pharmacies
CR織田信奈の野望II
北斗の拳 暴凶星 先バレ 信頼度
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
アナターのオット!?はーです
https://www.lucky-baby-casino.com/article/472.html
お店の雰囲気が心地よく、長時間楽しむことができます。快適な空間が魅力です。
吉宗
P 異世界魔王と召喚少女の奴隷魔術
CR牙狼FINAL Ver.399(1:1)
聖闘士星矢海皇覚醒
攻殻機動隊S.A.C
Pこの素晴らしい世界に祝福を!199LT「このラッキートリガーに祝福を!」
https://casinos.town/providers/playson
人気ゲームを基にしたパチスロで、ファンにはたまらない作品です。リアルなバトルが楽しめます。
L ゴールデンカムイ
https://pachika.com/guide/post-6279.html
自分の運を試すために、少しだけお金をかけるのが楽しいです。気軽に挑戦できます。
沖ドキ!トロピカル
https://www.k8.contact/Articles/1490.html
シンプルなルールで、初心者でもすぐに楽しめます。気軽に挑戦できます。
natural ed: canadian pharmacy – cheap erectile dysfunction pill
Escort dating for click : https://yenibayanlar.com/kategori/ankara-escort/
massage dating for click: https://elitvipescbayan.com/kategori/amasya-mutlu-son-masaj-salonu/goynucek-mutlu-son-masaj-salonu/
Massage Dating For Click : https://lindamasaj.xyz/k/hatay-mutlu-son-masaj-salonu/
п»їlegitimate online pharmacies india: indian pharm – reputable indian online pharmacy
메인 서비스: 간편하고 효율적인 배송 및 구매 대행 서비스
1. 대행 서비스 주요 기능
메인 서비스는 고객이 한 번에 필요한 대행 서비스를 신청할 수 있도록 다양한 기능을 제공합니다.
배송대행 신청: 국내외 상품 배송을 대신 처리하며, 효율적인 시스템으로 신속한 배송을 보장합니다.
구매대행 신청: 원하는 상품을 대신 구매해주는 서비스로, 고객의 수고를 줄입니다.
엑셀 대량 등록: 대량 상품을 엑셀로 손쉽게 등록 가능하여 상업 고객의 편의성을 증대합니다.
재고 관리 신청: 창고 보관 및 재고 관리를 통해 물류 과정을 최적화합니다.
2. 고객 지원 시스템
메인 서비스는 사용자 친화적인 접근성을 제공합니다.
유저 가이드: 대행 서비스를 더욱 합리적으로 사용할 수 있도록 세부 안내서를 제공합니다.
운송장 조회: 일본 사가와 등 주요 운송사의 추적 시스템과 연동하여 운송 상황을 실시간으로 확인 가능합니다.
3. 비용 안내와 부가 서비스
비용 계산기: 예상되는 비용을 간편하게 계산해 예산 관리를 돕습니다.
부가 서비스: 교환 및 반품, 폐기 및 검역 지원 등 추가적인 편의 서비스를 제공합니다.
출항 스케줄 확인: 해외 배송의 경우 출항 일정을 사전에 확인 가능하여 배송 계획을 세울 수 있습니다.
4. 공지사항
기본 검수 공지
무료 검수 서비스로 고객의 부담을 줄이며, 보다 철저한 검수가 필요한 경우 유료 정밀 검수 서비스를 권장합니다.
수출허가서 발급 안내
항공과 해운 수출 건에 대한 허가서를 효율적으로 발급받는 방법을 상세히 안내하며, 고객의 요청에 따라 이메일로 전달됩니다.
노데이터 처리 안내
운송장 번호 없는 주문에 대한 새로운 처리 방안을 도입하여, 노데이터 발생 시 관리비가 부과되지만 서비스 품질을 개선합니다.
5. 고객과의 소통
카카오톡 상담: 실시간 상담을 통해 고객의 궁금증을 해결합니다.
공지사항 알림: 서비스 이용 중 필수 정보를 지속적으로 업데이트합니다.
메인 서비스는 고객 만족을 최우선으로 하며, 지속적인 개선과 세심한 관리를 통해 최상의 경험을 제공합니다.
九州娛樂城登入
九州娛樂城:亞洲頂級線上娛樂平台
九州娛樂城,作為亞洲領先的線上博弈平台,以其專業性、公平性和創新性著稱。在這裡,您可以享受全方位的娛樂體驗,包括體育投注、真人遊戲、電子遊戲、六合彩球以及流行的捕魚機和棋牌遊戲。九州娛樂城是台灣玩家的首選娛樂平台,被譽為業界 No.1!
九州娛樂城的亮點
1. 多元化遊戲選擇
九州娛樂城擁有超過千款來自全球頂尖供應商提供的遊戲,滿足各種類型玩家的需求。無論是喜歡競技性的體育投注、刺激的真人遊戲,還是休閒的電子遊戲,九州娛樂城都能提供最適合您的選擇。
體育投注:全面覆蓋世界級賽事,包括世界杯、職業聯賽等。提供多種投注方式,如讓球、大小、單雙、串關等,並支持桌面端與手機端投注。
真人遊戲:KU真人百家樂、LEO真人遊戲等,讓您感受現場賭桌的真實刺激。
電子遊戲與捕魚機:多款熱門遊戲,如九州熱門電子遊戲和捕魚機,帶給玩家極致娛樂享受。
六合彩與棋牌遊戲:適合策略型玩家的理想選擇。
2. 極致便利的操作體驗
九州娛樂城支持通過官方網站和九州APP下載進行操作,無論您身在何處,都可以輕鬆加入遊戲世界。針對台灣玩家的本地化設計確保操作流暢、存取款方便。
3. 先進技術與安全保障
九州娛樂城集團總部位於菲律賓首都馬尼拉的RCBC Plaza,配備世界一流的安全資訊防護系統和智能大樓自動化系統(BAS)。玩家的數據與資金安全都能得到最佳保障,讓每一次遊戲都安心無憂。
4. 熱門品牌與平台支持
九州娛樂城旗下包括三大品牌:
LEO娛樂:提供真人遊戲、體育直播及免費影城。
THA娛樂:專注於新手友好和便捷操作。
KU娛樂:以真人百家樂而著稱。
每個品牌雖分別由不同管理團隊運營,但都傳承了九州娛樂城的高標準,遊戲系統與界面毫無差異,為玩家提供無縫娛樂體驗。
為什麼選擇九州娛樂城?
專業性與公平性
九州娛樂城堅持以「即時便利、公平公正、專業營運」為宗旨,研發創新遊戲技術,滿足不同類型玩家需求。
貼近市場與玩家需求
擁有兩岸三地的精英設計師與代理團隊,根據玩家反饋持續改進產品,並為合作商提供及時更新支持。
優越的服務與保障
從遊戲操作到客戶支持,九州娛樂城提供全面的服務支持,讓每位玩家都能感受到最貼心的服務。
九州娛樂城的未來
作為線上博弈行業的領航者,九州娛樂城不斷推陳出新,致力於提供更豐富的遊戲選擇和更完善的玩家體驗。同時,透過強大的技術與營運團隊,九州娛樂城已成為台灣乃至亞洲地區娛樂平台的代名詞。
立即下載九州APP,體驗最極致的娛樂樂趣!九州娛樂城——您的最佳線上娛樂夥伴!
best online drugstore: canadian pharm – male dysfunction treatment
mexican online pharmacies prescription drugs mexicanpharmeasy.com pharmacies in mexico that ship to usa
mexico drug stores pharmacies MexicanPharmEasy buying from online mexican pharmacy
buying prescription drugs in mexico online: mexican pharm easy – mexican mail order pharmacies
india online pharmacy: IndianPharmStar – online pharmacy india
Coolzino kasyno na ?ywo Coolzino kasyno na ?ywo .
вывод из запоя в екатеринбурге вывод из запоя в екатеринбурге .
buying prescription drugs in mexico: Mexican Pharm – п»їbest mexican online pharmacies
вывод из запоя на дому в екатеринбурге http://zal.rolevaya.info/viewtopic.php?id=5387/ .
вывод из запоя дома krasnogorsk.anihub.me/viewtopic.php?id=2574 .
Supreme包系列:時尚與功能兼備的完美選擇supreme哪裡買最便宜?
medicine in mexico pharmacies: MexicanPharmEasy – reputable mexican pharmacies online
Teen Patti Cash,a wonderful mobile phone game
Travis Scott:時尚與經典的完美結合travis scott哪裡買最便宜?
Happy Teen Patti,a wonderful mobile phone game
Yeezy Slides:舒適、創新與時尚的完美結合yeezy哪裡買最便宜?
Taurus Cash App,a wonderful mobile phone game
Tumi 背包:優質與時尚兼具的理想選擇tumi哪裡買最便宜?
blazer哪裡買最便宜?
drugs prices: canadian pharm – ed in young men
кухни москва купить кухни москва купить .
Patient drug leaflet. Latest pill developments.
sumatriptan hepatitis
Dosing guidelines here. Pill facts here.
cheapest online pharmacy india india online pharmacy or world pharmacy india
https://maps.google.com.mx/url?sa=t&url=https://indianpharmstar.com indian pharmacy paypal
pharmacy website india pharmacy website india and best online pharmacy india indianpharmacy com
male ed drugs ed remedies that really work or foods for ed
http://www.seb-kreuzburg.de/url?q=https://canadianpharm1st.com prescription drugs online
=sildenafil+coupons</a>]ed medications online buy prescription drugs without doctor and levitra without a doctor prescription ed drug comparison
indian pharmacy online top online pharmacy india or п»їlegitimate online pharmacies india
http://www.loredz.com/vb/go.php?url=http://indianpharmstar.com/ cheapest online pharmacy india
Online medicine home delivery pharmacy website india and buy prescription drugs from india indian pharmacy online
reputable indian pharmacies: IndianPharmStar.com – buy medicines online in india
drug prices http://indianpharmstar.com/# indianpharmacy com
medicine erectile dysfunction ed meds online without doctor prescription or home remedies for ed
http://forum.iciel.net/proxy.php?link=https://canadianpharm1st.com best pharmacy online
ed clinic erectile dysfunction drugs and non prescription ed pills buy prescription drugs without doctor
ed aids: canadianpharm1st – ed treatments that really work
mexican border pharmacies shipping to usa mexican pharmacy pharmacies in mexico that ship to usa
men ed canadian pharmacy buy prescription drugs online
Latest pill trends. Pill overview available.
sumatriptan advertisement
Pill details provided. Comprehensive drug facts.
reputable indian online pharmacy: indian pharm – п»їlegitimate online pharmacies india
mexican drugstore online: Mexican Pharm – mexican pharmaceuticals online
mexican border pharmacies shipping to usa purple pharmacy mexico price list or pharmacies in mexico that ship to usa
https://maps.google.com.sb/url?q=https://mexicanpharmeasy.com best online pharmacies in mexico
mexico pharmacies prescription drugs buying from online mexican pharmacy and buying prescription drugs in mexico online medication from mexico pharmacy
medication for ed dysfunction https://mexicanpharmeasy.com/# buying prescription drugs in mexico
medications for ed: canadian pharm 1st – best ed pills
indianpharmacy com: indian pharm – indian pharmacies safe
Платформа позволяет узнать, какие банки предлагают самые выгодные условия для ипотеки. Сравните ставки, сроки и дополнительные бонусы.
МФО Казахстана Ипотека .
best india pharmacy: indian pharm star – indian pharmacy
Latest pill trends. Patient pill facts.
imitrex indicações
Abuse effects detailed. Medicine impacts described.
ST666: Thiên Đường Casino Trực Tuyến Hàng Đầu
ST666 là một trong những sòng bạc trực tuyến hàng đầu, mang đến trải nghiệm chơi game đỉnh cao với hàng loạt trò chơi hấp dẫn và chương trình khuyến mãi đặc biệt. Với sứ mệnh đem lại sân chơi công bằng và tiện lợi, ST666 đã trở thành lựa chọn yêu thích của hàng ngàn người chơi tại Việt Nam.
Đa Dạng Trò Chơi và Sảnh Cược
1. Trò Chơi Phong Phú
ST666 cung cấp nhiều trò chơi đa dạng, đáp ứng sở thích của mọi người chơi:
Baccarat: Trò chơi cổ điển dành cho những ai yêu thích chiến thuật và may mắn.
Tài Xỉu: Trò chơi xúc xắc đầy kịch tính, đem lại cơ hội thắng lớn trong từng vòng cược.
Xóc Đĩa: Trò chơi truyền thống Việt Nam, được nâng cấp với công nghệ trực tuyến hiện đại.
Và nhiều trò chơi khác đang chờ bạn khám phá.
2. Sảnh Cược Đẳng Cấp
ST666 kết hợp cùng các sảnh cược nổi tiếng như:
Wm Casino: Nền tảng casino chuyên nghiệp, nổi bật với các trò chơi chất lượng cao và giao diện mượt mà.
ST666 Casino: Sảnh cược độc quyền với những ưu đãi hấp dẫn và các trò chơi độc đáo.
Khuyến Mãi Hấp Dẫn Tại ST666
ST666 không chỉ nổi bật với danh mục trò chơi phong phú mà còn mang đến nhiều chương trình khuyến mãi hấp dẫn cho người chơi:
Khuyến Mãi Gửi Tiền Cuối Tuần: Nhận thưởng lên đến 3.666.000 đồng khi nạp tiền vào tài khoản trong các ngày cuối tuần.
Ưu Đãi Đặc Biệt Dành Cho Hội Viên Mới: Những người chơi mới sẽ nhận được gói khuyến mãi chào mừng độc quyền.
Hoàn Tiền Hàng Tuần: Cơ hội nhận lại phần trăm số tiền cược đã thua trong tuần, giúp bạn có thêm động lực tham gia.
Tại Sao Nên Chọn ST666?
An Toàn và Uy Tín: ST666 cam kết bảo mật thông tin và giao dịch của người chơi, đảm bảo trải nghiệm chơi game an toàn tuyệt đối.
Hỗ Trợ Khách Hàng Chuyên Nghiệp: Đội ngũ chăm sóc khách hàng luôn sẵn sàng hỗ trợ 24/7.
Giao Diện Thân Thiện: Thiết kế đơn giản, dễ sử dụng, phù hợp cho cả người mới chơi và người chơi lâu năm.
Cơ Hội Thắng Lớn: Tỷ lệ thắng cao và phần thưởng hấp dẫn trong từng trò chơi.
Cách Tham Gia ST666
Bước 1: Truy cập trang web chính thức của ST666.
Bước 2: Đăng ký tài khoản nhanh chóng và miễn phí.
Bước 3: Nạp tiền và lựa chọn trò chơi yêu thích để bắt đầu hành trình giải trí.
Kết Luận
ST666 chính là thiên đường giải trí trực tuyến dành cho những ai yêu thích thử vận may và tận hưởng các trò chơi đẳng cấp. Với sự kết hợp giữa công nghệ hiện đại và các chương trình ưu đãi hấp dẫn, ST666 cam kết mang lại trải nghiệm hoàn hảo cho người chơi. Tham gia ngay hôm nay để khám phá những điều bất ngờ tại ST666!
чат джи 5 помогает мгновенно повысить оригинальность текста и улучшить его структуру.
ed problems treatment http://canadianpharm1st.com/# herbal ed remedies
ed dysfunction: canadian pharm – over the counter ed treatment
indian pharmacy buy medicines online in india or indian pharmacy paypal
https://clients1.google.com.bh/url?q=https://indianpharmstar.com indian pharmacies safe
Online medicine home delivery world pharmacy india and best online pharmacy india indian pharmacy online
九州娛樂城
九州娛樂城:亞洲頂級線上娛樂平台
九州娛樂城,作為亞洲領先的線上博弈平台,以其專業性、公平性和創新性著稱。在這裡,您可以享受全方位的娛樂體驗,包括體育投注、真人遊戲、電子遊戲、六合彩球以及流行的捕魚機和棋牌遊戲。九州娛樂城是台灣玩家的首選娛樂平台,被譽為業界 No.1!
九州娛樂城的亮點
1. 多元化遊戲選擇
九州娛樂城擁有超過千款來自全球頂尖供應商提供的遊戲,滿足各種類型玩家的需求。無論是喜歡競技性的體育投注、刺激的真人遊戲,還是休閒的電子遊戲,九州娛樂城都能提供最適合您的選擇。
體育投注:全面覆蓋世界級賽事,包括世界杯、職業聯賽等。提供多種投注方式,如讓球、大小、單雙、串關等,並支持桌面端與手機端投注。
真人遊戲:KU真人百家樂、LEO真人遊戲等,讓您感受現場賭桌的真實刺激。
電子遊戲與捕魚機:多款熱門遊戲,如九州熱門電子遊戲和捕魚機,帶給玩家極致娛樂享受。
六合彩與棋牌遊戲:適合策略型玩家的理想選擇。
2. 極致便利的操作體驗
九州娛樂城支持通過官方網站和九州APP下載進行操作,無論您身在何處,都可以輕鬆加入遊戲世界。針對台灣玩家的本地化設計確保操作流暢、存取款方便。
3. 先進技術與安全保障
九州娛樂城集團總部位於菲律賓首都馬尼拉的RCBC Plaza,配備世界一流的安全資訊防護系統和智能大樓自動化系統(BAS)。玩家的數據與資金安全都能得到最佳保障,讓每一次遊戲都安心無憂。
4. 熱門品牌與平台支持
九州娛樂城旗下包括三大品牌:
LEO娛樂:提供真人遊戲、體育直播及免費影城。
THA娛樂:專注於新手友好和便捷操作。
KU娛樂:以真人百家樂而著稱。
每個品牌雖分別由不同管理團隊運營,但都傳承了九州娛樂城的高標準,遊戲系統與界面毫無差異,為玩家提供無縫娛樂體驗。
為什麼選擇九州娛樂城?
專業性與公平性
九州娛樂城堅持以「即時便利、公平公正、專業營運」為宗旨,研發創新遊戲技術,滿足不同類型玩家需求。
貼近市場與玩家需求
擁有兩岸三地的精英設計師與代理團隊,根據玩家反饋持續改進產品,並為合作商提供及時更新支持。
優越的服務與保障
從遊戲操作到客戶支持,九州娛樂城提供全面的服務支持,讓每位玩家都能感受到最貼心的服務。
九州娛樂城的未來
作為線上博弈行業的領航者,九州娛樂城不斷推陳出新,致力於提供更豐富的遊戲選擇和更完善的玩家體驗。同時,透過強大的技術與營運團隊,九州娛樂城已成為台灣乃至亞洲地區娛樂平台的代名詞。
立即下載九州APP,體驗最極致的娛樂樂趣!九州娛樂城——您的最佳線上娛樂夥伴!
mexico drug stores pharmacies Pharm Easy mexican online pharmacies prescription drugs
九州娛樂城:亞洲頂級線上娛樂平台
九州娛樂城,作為亞洲領先的線上博弈平台,以其專業性、公平性和創新性著稱。在這裡,您可以享受全方位的娛樂體驗,包括體育投注、真人遊戲、電子遊戲、六合彩球以及流行的捕魚機和棋牌遊戲。九州娛樂城是台灣玩家的首選娛樂平台,被譽為業界 No.1!
九州娛樂城的亮點
1. 多元化遊戲選擇
九州娛樂城擁有超過千款來自全球頂尖供應商提供的遊戲,滿足各種類型玩家的需求。無論是喜歡競技性的體育投注、刺激的真人遊戲,還是休閒的電子遊戲,九州娛樂城都能提供最適合您的選擇。
體育投注:全面覆蓋世界級賽事,包括世界杯、職業聯賽等。提供多種投注方式,如讓球、大小、單雙、串關等,並支持桌面端與手機端投注。
真人遊戲:KU真人百家樂、LEO真人遊戲等,讓您感受現場賭桌的真實刺激。
電子遊戲與捕魚機:多款熱門遊戲,如九州熱門電子遊戲和捕魚機,帶給玩家極致娛樂享受。
六合彩與棋牌遊戲:適合策略型玩家的理想選擇。
2. 極致便利的操作體驗
九州娛樂城支持通過官方網站和九州APP下載進行操作,無論您身在何處,都可以輕鬆加入遊戲世界。針對台灣玩家的本地化設計確保操作流暢、存取款方便。
3. 先進技術與安全保障
九州娛樂城集團總部位於菲律賓首都馬尼拉的RCBC Plaza,配備世界一流的安全資訊防護系統和智能大樓自動化系統(BAS)。玩家的數據與資金安全都能得到最佳保障,讓每一次遊戲都安心無憂。
4. 熱門品牌與平台支持
九州娛樂城旗下包括三大品牌:
LEO娛樂:提供真人遊戲、體育直播及免費影城。
THA娛樂:專注於新手友好和便捷操作。
KU娛樂:以真人百家樂而著稱。
每個品牌雖分別由不同管理團隊運營,但都傳承了九州娛樂城的高標準,遊戲系統與界面毫無差異,為玩家提供無縫娛樂體驗。
為什麼選擇九州娛樂城?
專業性與公平性
九州娛樂城堅持以「即時便利、公平公正、專業營運」為宗旨,研發創新遊戲技術,滿足不同類型玩家需求。
貼近市場與玩家需求
擁有兩岸三地的精英設計師與代理團隊,根據玩家反饋持續改進產品,並為合作商提供及時更新支持。
優越的服務與保障
從遊戲操作到客戶支持,九州娛樂城提供全面的服務支持,讓每位玩家都能感受到最貼心的服務。
九州娛樂城的未來
作為線上博弈行業的領航者,九州娛樂城不斷推陳出新,致力於提供更豐富的遊戲選擇和更完善的玩家體驗。同時,透過強大的技術與營運團隊,九州娛樂城已成為台灣乃至亞洲地區娛樂平台的代名詞。
立即下載九州APP,體驗最極致的娛樂樂趣!九州娛樂城——您的最佳線上娛樂夥伴!
Online medicine order IndianPharmStar india pharmacy
top online pharmacy india pharmacy website india or online pharmacy india
http://scanmail.trustwave.com/?c=6677&d=3eHL2zmR-mSEueRJSdX4UpJhLotL1yEZhefsffMCDw&u=http://indianpharmstar.com best india pharmacy
india pharmacy mail order best online pharmacy india and top 10 pharmacies in india indianpharmacy com
mail order pharmacy india: indian pharm – buy prescription drugs from india
ST666 – Nhận Định Bóng Đá Kèo Nhà Cái Uy Tín
ST666: Địa Chỉ Lý Tưởng Cho Những Tín Đồ Cá Cược Bóng Đá
ST666 là nền tảng nhận định bóng đá và kèo nhà cái chuyên nghiệp, nơi người chơi có thể tham gia dự đoán các kèo đa dạng như cược tài xỉu, cược chấp, và cược 1×2. Với giao diện thân thiện, tỷ lệ cược minh bạch và ưu đãi hấp dẫn, ST666 đang dần trở thành lựa chọn hàng đầu của cộng đồng yêu bóng đá.
Nhận Định Kèo Nhà Cái Đa Dạng
Cược Tài Xỉu (#cuoctaixiu)
Dựa vào tổng số bàn thắng trong trận đấu, người chơi dễ dàng chọn lựa Tài (nhiều hơn tỷ lệ nhà cái đưa ra) hoặc Xỉu (ít hơn tỷ lệ).
ST666 cung cấp các phân tích chi tiết giúp người chơi đưa ra lựa chọn chính xác.
Cược Chấp (#cuocchap)
Thích hợp cho những trận đấu có sự chênh lệch về sức mạnh giữa hai đội. ST666 cung cấp tỷ lệ chấp tối ưu, phù hợp với cả người chơi mới và chuyên nghiệp.
Cược 1×2 (#cuoc1x2)
Phù hợp cho những ai muốn dự đoán kết quả chung cuộc (Thắng – Hòa – Thua). Đây là loại kèo phổ biến, dễ hiểu và có tỷ lệ cược hấp dẫn.
Ưu Đãi Hấp Dẫn Tại ST666
Nhận 160% tiền gửi lần đầu: Khi đăng ký tài khoản mới và nạp tiền, người chơi sẽ nhận được số tiền thưởng cực lớn, tăng cơ hội tham gia các kèo.
Hoàn tiền 3% mỗi ngày: Chính sách hoàn tiền giúp người chơi giảm thiểu rủi ro, thoải mái trải nghiệm mà không lo lắng nhiều về chi phí.
Vì Sao Nên Chọn ST666?
Nền Tảng Uy Tín: ST666 cam kết mang đến trải nghiệm cá cược minh bạch và an toàn.
Phân Tích Chuyên Sâu: Đội ngũ chuyên gia của ST666 luôn cập nhật nhận định mới nhất về các trận đấu, giúp người chơi đưa ra quyết định tối ưu.
Hỗ Trợ 24/7: Đội ngũ hỗ trợ chuyên nghiệp, luôn sẵn sàng giải đáp thắc mắc của người chơi.
Giao Dịch Nhanh Chóng: Nạp rút tiền linh hoạt, đảm bảo sự tiện lợi và bảo mật.
Cách Tham Gia ST666
Truy cập website chính thức của ST666.
Đăng ký tài khoản bằng thông tin cá nhân.
Nạp tiền lần đầu để nhận ưu đãi 160%.
Bắt đầu trải nghiệm cá cược với các kèo yêu thích!
Hãy đến với ST666 để tận hưởng không gian cá cược chuyên nghiệp, nhận định kèo chất lượng và những phần thưởng hấp dẫn. Tham gia ngay hôm nay để trở thành người chơi chiến thắng!
LEO娛樂城:九州娛樂集團的卓越創舉與運營優勢
九州娛樂集團總部的現代化運營
九州娛樂集團的總部設於菲律賓首都馬尼拉市中心最具代表性的現代化建築「RCBC Plaza」。這座辦公大樓融合了全球領先的建築科技與安全系統,包括:
國家級資訊防護系統,確保數據安全。
樓宇自動化系統 (BAS),實現數位化管理與高效運營,包括防火系統、光纖電訊和數位監控。
九州娛樂為員工提供舒適的工作環境和專業的教育培訓,確保服務水準一流。其員工以專業知識和親切態度,為尊貴玩家帶來極致的遊戲體驗。
LEO娛樂旗下品牌與遊戲特色
九州娛樂城旗下分支包括 LEO、THA 和 KU 三個娛樂品牌。雖然品牌各自獨立運營,但都共享相同的遊戲系統與界面,提供超過數千款來自全球頂級供應商的遊戲。
這些品牌的核心特色包括:
種類繁多的遊戲:滿足不同類型玩家的需求,包括電子遊戲、體育博彩、真人娛樂等。
不斷更新的內容:通過九州獨家技術,網站內容保持新鮮,合作商可快速更新遊戲資源,確保平台競爭力。
玩家在這些平台上可以隨時找到適合自己的遊戲,享受沉浸式的娛樂體驗。
LEO娛樂城的歷史與發展
九州娛樂集團的起源可以追溯至其最早期的經營模式:「信用版」賭博平台 《天下體育球版》。儘管該模式在當時非常流行,但因高風險與財務處理複雜而逐漸被淘汰。
隨著市場需求的變化,九州娛樂轉型為現金版平台 《天下現金網》,引入了更安全、便捷的遊戲模式。這種轉變不僅提升了玩家的體驗,也使平台得以進一步發展。
如今,九州娛樂城已成為台灣博弈業界的領軍者,不僅優化了網站版面與功能,還推出了專屬手機APP,方便玩家隨時隨地進行遊戲體驗。
LEO娛樂的成功秘訣
現代化運營與技術支持:九州娛樂在系統安全與技術更新方面投入巨大資源,確保平台運營穩定可靠。
多元化遊戲體驗:通過與全球頂級遊戲供應商合作,平台提供了豐富的遊戲選擇,適應各種玩家需求。
用戶至上:專業培訓的員工以細心和熱忱態度,提供高品質服務,讓玩家倍感尊重。
不斷創新:從信用版到現金版,再到手機APP,九州娛樂始終緊跟市場趨勢,致力於打造最佳娛樂體驗。
結語
LEO娛樂城作為九州娛樂集團旗下的重要品牌,不僅繼承了集團的核心價值,更在遊戲選擇、服務品質和技術創新方面不斷提升。從最初的《天下體育球版》到如今的行業領先者,LEO娛樂見證了九州娛樂城的發展歷程,也成為博弈行業中的耀眼明星。
加入 LEO娛樂城,感受來自九州娛樂集團的專業與熱情,享受最頂級的線上博弈體驗!
st666
ST666 Xổ Số – Điểm Đến Giải Trí Hoàn Hảo
ST666 Xổ Số là nền tảng giải trí trực tuyến hàng đầu, cung cấp đa dạng các loại hình trò chơi từ keno, xổ số miền Nam (XSMN), xổ số miền Bắc (XSMB) đến xổ số siêu tốc. Với dịch vụ chuyên nghiệp và an toàn, ST666 mang đến cho bạn một trải nghiệm hoàn toàn mới mẻ và hấp dẫn.
Tại Sao Nên Chọn ST666 Xổ Số
Truy Cập Nhanh Và An Toàn
ST666 đảm bảo quyền truy cập nhanh chóng thông qua đường dẫn chính thức, loại bỏ hoàn toàn rủi ro từ các trang web giả mạo hoặc nguy cơ mất tài khoản. Hệ thống bảo mật hiện đại giúp bạn yên tâm khi đăng nhập và tham gia trò chơi.
Ưu Đãi Độc Quyền Dành Cho Thành Viên
ST666 mang đến nhiều chương trình khuyến mãi đặc biệt dành riêng cho người chơi, bao gồm phần thưởng giá trị và các ưu đãi hấp dẫn. Thành viên mới sẽ được trải nghiệm các chính sách hỗ trợ vượt trội, giúp tăng cơ hội chiến thắng và tận hưởng trò chơi tốt nhất.
Bảo Mật Thông Tin Tối Đa
Hệ thống mã hóa hiện đại của ST666 đảm bảo mọi giao dịch và dữ liệu cá nhân đều được bảo vệ an toàn. Mọi thông tin đều được xử lý theo quy chuẩn bảo mật cao nhất, giúp người chơi yên tâm giải trí mà không lo lắng về vấn đề rủi ro thông tin.
Đa Dạng Trò Chơi Giải Trí
Xổ số: Từ xổ số truyền thống như XSMN, XSMB đến xổ số siêu tốc, đáp ứng mọi nhu cầu giải trí của người chơi.
Lô đề: Cung cấp giao diện dễ sử dụng, giúp bạn dễ dàng chọn số và theo dõi kết quả trực tiếp.
Casino trực tuyến: Đa dạng các trò chơi từ bài bạc, roulette đến các game đổi thưởng hiện đại.
Những Lợi Ích Khi Chơi Tại ST666
Dịch vụ hỗ trợ khách hàng chuyên nghiệp hoạt động 24/7.
Giao dịch nạp và rút tiền nhanh chóng, minh bạch.
Hệ thống cập nhật trò chơi thường xuyên, mang lại sự mới mẻ và không nhàm chán.
Nền tảng thiết kế hiện đại, thân thiện với mọi thiết bị, từ máy tính đến điện thoại di động.
Cách Truy Cập ST666
Truy cập đường dẫn chính thức của ST666 để đảm bảo an toàn.
Đăng ký tài khoản và hoàn tất các bước xác thực thông tin.
Tham gia các trò chơi hấp dẫn và tận hưởng phần thưởng đặc biệt dành cho thành viên.
ST666 Xổ Số không chỉ là nền tảng giải trí mà còn là nơi mang lại cơ hội chiến thắng và trải nghiệm dịch vụ đẳng cấp. Tham gia ngay hôm nay để khám phá một thế giới giải trí đa dạng và chuyên nghiệp!
cat antibiotics without pet prescription: canada pharmacy online – erectal disfunction
RG富遊娛樂城:台灣線上娛樂城的最佳選擇
RG富遊娛樂城以其卓越的服務和多樣化的遊戲選擇,成為2024年最受歡迎的線上娛樂平台。受到超過50位網紅和部落客的實測推薦,這座娛樂城不僅提供豐富的遊戲,還帶來眾多優惠活動和誠信保證,贏得了廣大玩家的信任與青睞。
RG富遊娛樂城的獨特優勢
多重優惠活動
體驗金 $168:新手玩家可以免費試玩,無需任何成本即可體驗高品質遊戲。
首儲1000送1000:首次存款即可獲得雙倍金額,增加遊戲的樂趣與機會。
線上簽到轉盤:每日簽到即可參加抽獎,贏取現金獎勵和豐厚禮品。
快速存提款與資金保障
RG富遊採用自主研發的財務系統,確保5秒快速存款,滿足玩家即時遊戲需求。
100%保證出金,杜絕任何拖延或資金安全風險,讓玩家完全放心。
遊戲種類豐富
RG富遊娛樂城涵蓋多種遊戲類型,滿足不同玩家的需求,包括:
真人百家樂:與真人荷官互動,感受真實賭場的刺激氛圍。
電子老虎機:超過數百款創新遊戲,玩法新穎,回報豐厚。
電子捕魚:趣味性強,結合策略與娛樂,深受玩家喜愛。
電子棋牌:提供公平競技環境,適合策略型玩家。
體育投注:涵蓋全球賽事,賠率即時更新,為體育愛好者提供最佳選擇。
樂透彩票:參與多地彩票,挑戰巨額獎金。
跨平台兼容性
RG富遊支持Web端、H5、iOS和Android設備,玩家可隨時隨地登錄遊戲,享受無縫體驗。
與其他娛樂城的不同之處
RG富遊以現金版模式運營,確保交易透明和安全性。相比一般娛樂城,RG富遊在存提款速度上遙遙領先,玩家可在短短15秒內完成交易,並且100%保證資金提領。而在線客服全年無休,隨時提供支持,讓玩家在任何時間都能解決問題。
相比之下,一般娛樂城多以信用版模式運營,存在出金風險,且存提款速度較慢,客服服務不穩定,無法與RG富遊的專業性相比。
為什麼選擇RG富遊娛樂城
資金交易安全無憂:採用最先進的SSL加密技術,確保每筆交易的安全性。
遊戲種類全面豐富:每日更新多樣化遊戲,帶來新鮮感和無限可能。
優惠活動力度大:從體驗金到豐厚的首儲獎勵,玩家每一步都能享受優惠。
快速存提款服務:自主研發技術保障流暢交易,遊戲不中斷。
全天候專業客服:24/7在線支持,及時解決玩家需求。
立即加入RG富遊娛樂城
RG富遊娛樂城不僅提供豐富的遊戲體驗,更以專業的服務、完善的安全保障和多樣的優惠活動,為玩家打造一個值得信賴的娛樂環境。立即註冊,體驗台灣最受歡迎的線上娛樂城!
ed treatments viagra without a doctor prescription walmart or erection pills online
https://images.google.bj/url?sa=t&url=https://canadianpharm1st.com natural cure for ed
best cure for ed ed trial pack and cheap drugs best canadian pharmacy online
medicine for erectile: canada pharmacy online – ed medicine online
Patient pill information. Drug overview available.
sumatriptan warts
Drug overview available. Access medication details.
pharmacies in mexico that ship to usa: mexican pharm easy – mexican drugstore online
erectal disfunction https://mexicanpharmeasy.com/# pharmacies in mexico that ship to usa
вывод из запоя анонимно https://www.masa.forum24.ru/?1-16-0-00002634-000-0-0-1730787030 .
нарколог на дом краснодар нарколог на дом краснодар .
вывод из запоя круглосуточно сочи http://www.kozaostra.mybb.ru/viewtopic.php?id=14294/ .
вывод из запоя круглосуточно сочи http://www.pelsh.forum24.ru/?1-8-0-00000128-000-0-0-1730786505 .
mexican rx online mexican rx online or mexico pharmacies prescription drugs
https://www.google.com.pg/url?sa=t&url=https://mexicanpharmeasy.com п»їbest mexican online pharmacies
best online pharmacies in mexico mexico pharmacies prescription drugs and best online pharmacies in mexico buying prescription drugs in mexico
siri chatgpt — это современное решение для обработки и генерации текста.
P 新世紀エヴァンゲリオン ~未来への咆哮~ SPECIAL EDITION
k8 カジノ パチンコ シティーハンターパチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
三國志
https://www.xn--k8-9g4a3b4f.store/games/id3395
美しいアニメーションと迫力ある演出が魅力。毎回新しい発見があります。
主役は銭形2
k8 カジノ パチンコ
CRフィーバー戦姫絶唱シンフォギア Ver.199
CR戦国乙女花
Pこの素晴らしい世界に祝福を!199LT「このラッキートリガーに祝福を!」
CR 北斗の拳6 天翔百裂
https://pachika.com/machine/post-8378.html
シンプルなルールで、誰でもすぐに楽しめるのが嬉しいです。
シティーハンター
https://www.k8521.com/archives/post-472.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
吉宗(双姬版)
https://casinos.town/casino-news/239
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
injections for ed: canadian pharmacy – homeopathic remedies for ed
best ed treatment male erection pills or pumps for ed
http://ibbs.ci123.com/notlogin.php?backurl=http://canadianpharm1st.com over the counter ed treatment
best medicine for ed drug medication and cheapest ed pills ed pills for sale
Drug information here. Find medicine details.
[url=https://imitrex2rp.top/index.html]sumatriptan contraindications[/url]
Interactions explained here. Find drug information.
ed treatment options https://indianpharmstar.com/# mail order pharmacy india
mexican border pharmacies shipping to usa: mexicanpharmeasy.com – mexican online pharmacies prescription drugs
top ed drugs drugs and medications or how to get prescription drugs without doctor
https://www.google.com.bd/url?q=http://canadianpharm1st.com online canadian pharmacy
ed meds online canada drugs causing ed and over the counter erectile dysfunction pills foods for ed
how to cure ed naturally: canadianpharm1st.com – drugs prices
indian pharmacy online IndianPharmStar.com top 10 pharmacies in india
reputable mexican pharmacies online mexicanpharmeasy.com mexico drug stores pharmacies
일본 소비세 환급, 네오리아와 함께라면 간편하고 안전하게
일본 소비세 환급은 복잡하고 까다로운 절차로 많은 구매대행 셀러들이 어려움을 겪는 분야입니다. 네오리아는 다년간의 경험과 전문성을 바탕으로 신뢰할 수 있는 서비스를 제공하며, 일본 소비세 환급 과정을 쉽고 효율적으로 처리합니다.
1. 일본 소비세 환급의 필요성과 네오리아의 역할
네오리아는 일본 현지 법인을 설립하지 않아도 합법적인 방식으로 소비세 환급을 받을 수 있는 솔루션을 제공합니다. 이를 통해:
한국 개인사업자와 법인 사업자 모두 간편하게 환급 절차를 진행할 수 있습니다.
일본의 복잡한 서류 심사를 최소화하고, 현지 로컬 세리사와 협력하여 최적의 결과를 보장합니다.
2. 소비세 환급의 주요 특징
일본 연고가 없어도 가능: 일본에 사업자가 없더라도 네오리아는 신뢰할 수 있는 서비스를 통해 소비세 환급을 지원합니다.
서류 작성 걱정 해결: 잘못된 서류 제출로 환급이 거절될까 걱정될 필요 없습니다. 네오리아의 전문 대응팀이 모든 과정을 정밀하게 관리합니다.
현지 법인 운영자를 위한 추가 지원: 일본 내 개인사업자나 법인 운영자에게는 세무 감사와 이슈 대응까지 포함된 고급 서비스를 제공합니다.
3. 네오리아 서비스의 장점
전문성과 신뢰성: 정부로부터 인정받은 투명성과 세무 분야의 우수한 성과를 자랑합니다.
맞춤형 서포트: 다양한 사례를 통해 쌓은 경험으로 고객이 예상치 못한 어려움까지 미리 해결합니다.
로컬 업체에서 불가능한 고급 서비스: 한국인 고객을 위해 정확하고 간편한 세무회계 및 소비세 환급 서비스를 제공합니다.
4. 네오리아가 제공하는 혜택
시간 절약: 복잡한 절차와 서류 준비 과정을 전문가가 대신 처리합니다.
안심 환급: 철저한 관리와 세심한 대응으로 안전하게 환급을 받을 수 있습니다.
추가 서비스: 세무감사와 이슈 발생 시 즉각적인 지원으로 사업의 연속성을 보장합니다.
네오리아는 소비세 환급이 복잡하고 어렵다고 느껴지는 고객들에게 최적의 길잡이가 되어드립니다. 신뢰를 바탕으로 한 전문적인 서비스로, 더 이상 소비세 환급 문제로 고민하지 마세요!
ed drugs online from canada solutions for ed or impotence treatment
https://cse.google.mg/url?q=https://canadianpharm1st.com best ed medications
cheap ed pills medication for ed dysfunction and vacuum pumps for ed new treatments for ed
Latest pill updates. Medication trends described.
imitrex fatigue
Latest pill trends. Find medicine information.
ed pills otc: canada pharmacy online – best ed treatment
pharmacy website india online pharmacy india or Online medicine order
http://logen.ru/bitrix/redirect.php?goto=https://indianpharmstar.com buy prescription drugs from india
best online pharmacy india india online pharmacy and india online pharmacy indian pharmacy
pain meds without written prescription http://mexicanpharmeasy.com/# mexico drug stores pharmacies
mexican border pharmacies shipping to usa: mexican pharmacy – mexico pharmacies prescription drugs
purple pharmacy mexico price list: mexican pharm easy – buying prescription drugs in mexico online
top 10 pharmacies in india reputable indian pharmacies or world pharmacy india
https://clients1.google.gg/url?q=http://indianpharmstar.com top online pharmacy india
top online pharmacy india online shopping pharmacy india and top online pharmacy india world pharmacy india
mexican online pharmacies prescription drugs: mexican pharmacy – reputable mexican pharmacies online
2024 年熱門娛樂城與活動解析
冠天下娛樂城優惠,帶來無限樂趣
在 2024 年 4 月 16 日,冠天下娛樂城推出了全新的優惠活動,旨在為玩家帶來更大的獲利機會和更高的娛樂體驗。這些優惠活動包括多種存款獎勵、會員專屬回饋以及豐富的遊戲競賽獎金,吸引了大批玩家參與。
冠天下娛樂城以其高透明度和用戶友好的政策,已成為許多玩家的首選娛樂平台。不論是新手還是老手,都能在這裡找到適合自己的遊戲和活動。
LEO娛樂城無法登入引發市場恐慌
2024 年 11 月 29 日,LEO娛樂城因技術問題出現短暫無法登入的情況,引發了廣泛關注。許多玩家擔心這是否意味著九州娛樂城的營運出現問題。
九州娛樂集團隨後發佈官方聲明,強調這僅僅是系統升級過程中的短暫問題,並承諾玩家的資金和數據安全不受任何影響。同時,九州娛樂表示將加強伺服器穩定性,確保未來類似情況不再發生。
熱門 ZG 電子遊戲——熱舞森巴老虎機
2024 年 11 月 8 日,ZG 電子遊戲推出了熱舞森巴老虎機,這款遊戲迅速成為市場上的熱門選擇。以南美熱情的森巴舞為主題,遊戲畫面充滿色彩,搭配動感的音樂,為玩家帶來視覺與聽覺的雙重享受。
遊戲亮點:
高倍數獎勵機制:每次轉盤都有機會觸發高額獎金。
特色免費遊戲:連續獲得特殊圖案即可啟動多次免費轉盤,提高獲勝機率。
適合新手與高端玩家:簡單的玩法規則與豐富的策略選擇滿足不同層級玩家的需求。
ZG 電子遊戲致力於為玩家提供創新且有趣的遊戲體驗,熱舞森巴老虎機無疑是今年最值得嘗試的遊戲之一。
2024 世界棒球 12 強賽精彩回顧
2024 年 11 月,中華隊參加了世界棒球 12 強賽,這項全球頂尖棒球賽事吸引了來自多國的球隊和球迷參與。中華隊 28 人的名單以年輕選手和經驗豐富的老將為基礎,展現了團隊的競技實力。
比賽亮點:
精彩賽程:比賽期間,中華隊展現了堅強的防守和出色的打擊表現。
門票銷售:賽事門票迅速售罄,顯示出棒球在台灣的高度受歡迎程度。
線上直播:各大平台提供了高畫質直播,讓更多球迷能夠即時觀看比賽,感受棒球魅力。
世界棒球 12 強賽不僅是比賽,更是全台灣球迷的一場盛宴,展現了棒球作為國民運動的無限魅力。
結語
從娛樂城的優惠活動到全球性的棒球賽事,2024 年對於遊戲和體育愛好者來說充滿了亮點。不論是參與冠天下娛樂城的優惠,體驗 ZG 電子遊戲的最新老虎機,還是為中華隊的棒球比賽加油,這一年都是充滿精彩與期待的一年!
mexican online pharmacies prescription drugs: Mexican Pharm – mexico drug stores pharmacies
Pill effects listed. Current medication trends.
imitrex define
Medication data provided. Access pill details.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали сервисный центр asus цены, можете посмотреть на сайте: срочный сервисный центр asus
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mexican mail order pharmacies medicine in mexico pharmacies or mexico drug stores pharmacies
https://www.google.co.in/url?sa=t&url=https://mexicanpharmeasy.com mexico drug stores pharmacies
purple pharmacy mexico price list mexican online pharmacies prescription drugs and mexico pharmacies prescription drugs buying from online mexican pharmacy
can ed be reversed https://indianpharmstar.com/# online shopping pharmacy india
стационарное выведение из запоя стационарное выведение из запоя .
вывод из запоя в стационаре анонимно вывод из запоя в стационаре анонимно .
выведение из запоя на дому в сочи [url=vishivayu.ukrbb.net/viewtopic.php?f=12&t=13447]выведение из запоя на дому в сочи[/url] .
вывод из запоя сочи вывод из запоя сочи .
Конструкторы оружия России https://guns.org.ru история создания легендарного оружия, биографии инженеров, технические характеристики разработок.
Patient pill resource. Pill impacts described.
imitrex harga
Prescribing details available. Latest drug news.
Конструкторы оружия России https://guns.org.ru история создания легендарного оружия, биографии инженеров, технические характеристики разработок.
world pharmacy india indian pharmacy п»їlegitimate online pharmacies india
injections for ed: canadian pharmacy – buy ed drugs
buy ed pills online canada pharmacy online cheap drugs
crypto address checker
Overview of Crypto Transfer Check and Regulatory Services
In today’s cryptocurrency sector, maintaining deal transparency and compliance with Anti-Money Laundering (AML) and KYC standards is vital. Following is an outline of popular sites that deliver tools for digital asset transfer tracking, verification, and asset safety.
1. Tokenmetrics.com
Overview: Tokenmetrics delivers cryptocurrency analysis to evaluate possible fraud dangers. This service lets individuals to check cryptocurrencies ahead of buying to avoid likely risky assets. Attributes:
– Risk evaluation.
– Perfect for investors seeking to steer clear of hazardous or fraudulent ventures.
2. Metamask Monitor Center
Overview: Metamask.Monitory.Center permits individuals to review their crypto resources for doubtful transactions and standard adherence. Advantages:
– Verifies tokens for legitimacy.
– Delivers warnings about likely fund locks on particular exchanges.
– Provides comprehensive results after address connection.
3. Bestchange.com
Description: Bestchange.ru is a service for monitoring and verifying crypto transaction transfers, guaranteeing transparency and transaction security. Highlights:
– Transfer and account monitoring.
– Sanctions validation.
– Web-based platform; supports BTC and several additional cryptocurrencies.
4. Bot amlchek
Overview: AMLCheck Bot is a investment tracker and AML tool that utilizes machine learning methods to detect questionable transactions. Highlights:
– Deal observation and user verification.
– Offered via web version and chat bot.
– Supports digital assets like BSC, BTC, DOGE, and more.
5. AlphaBit
Description: AlphaBit provides complete anti-money laundering solutions tailored for the crypto market, assisting companies and financial institutions in ensuring compliance conformity. Advantages:
– Extensive anti-money laundering tools and evaluations.
– Meets modern protection and compliance requirements.
6. AMLNode
Overview: AMLNode provides anti-money laundering and KYC solutions for crypto businesses, including deal monitoring, compliance validation, and evaluation. Highlights:
– Threat evaluation tools and sanctions validations.
– Useful for maintaining safe business processes.
7. Btrace.io
Description: Btrace AML Crypto focuses on asset validation, offering transfer observation, compliance screenings, and support if you are a affected by theft. Highlights:
– Effective support for resource restoration.
– Transfer monitoring and security tools.
Dedicated USDT Check Services
Our site also provides information on multiple services providing check tools for crypto deals and wallets:
– **USDT TRC20 and ERC20 Validation:** Many services provide thorough evaluations for USDT transactions, helping in the detection of questionable transactions.
– **AML Verification for USDT:** Tools are available for monitoring for suspicious transactions.
– **“Cleanliness” Validation for Accounts:** Validation of deal and account purity is available to identify likely threats.
**Conclusion**
Finding the right service for validating and monitoring digital currency deals is important for guaranteeing safety and compliance compliance. By reading our recommendations, you can find the most suitable solution for transfer monitoring and fund safety.
how to help ed: canadianpharm1st – ed medication
how to overcome ed https://indianpharmstar.com/# pharmacy website india
indian pharmacies safe: indian pharm star – best india pharmacy
mexico drug stores pharmacies: mexicanpharmeasy.com – mexican online pharmacies prescription drugs
soma therapy ed: canadian pharm 1st – diabetes and ed
indianpharmacy com indianpharmacy com or <a href=" http://nsreg.com/?a=how+can+i+buy+viagra “>indian pharmacies safe
https://www.google.com.pg/url?q=https://indianpharmstar.com buy medicines online in india
reputable indian online pharmacy indian pharmacy and pharmacy website india mail order pharmacy india
non prescription ed drugs http://mexicanpharmeasy.com/# purple pharmacy mexico price list
best ed pills non prescription: canadian pharm – homepage
best online pharmacy india buy medicines online in india or indian pharmacy paypal
http://images.google.mw/url?q=https://indianpharmstar.com online shopping pharmacy india
cheapest online pharmacy india indianpharmacy com and Online medicine order best online pharmacy india
over the counter ed drugs ed aids or non prescription ed drugs
http://radio.sodazaa.com/outlink.php?url=https://canadianpharm1st.com erectile dysfunction treatments
errectile dysfunction cheap drugs online and ed doctor pills for ed
homeopathic remedies for ed: canadianpharm1st.com – cheap erectile dysfunction pills
mexican mail order pharmacies buying prescription drugs in mexico online or mexican mail order pharmacies
http://www.9oo9le.me/details.php?site=mexicanpharmeasy.com medication from mexico pharmacy
mexican drugstore online mexican drugstore online and medication from mexico pharmacy mexican mail order pharmacies
Yazınız için teşekkürler. Bu bilgiler ışığında nice insanlar bilgilenmiş olacaktır.
Bilgiler için teşekkür ederim işime son derece yaradı
çok başarılı ve kaliteli bir makale olmuş güzellik sırlarım olarak teşekkür ederiz.
Bu güzel bilgilendirmeler için teşekkür ederim.
Verdiginiz bilgiler için teşekkürler , güzel yazı olmuş
I truly appreciate your technique of writing a blog. I added it to my bookmark site list and will
Ne zamandır web sitelerim için aradığım içeriği sonunda buldum. Bu kadar detaylı ve net açıklama için teşekkürler.
Great information shared.. really enjoyed reading this post thank you author for sharing this post .. appreciated
Güzel aydınlatıcı makale için teşekkürler daha iyisi samda kayısı umarım faydalı çalışmalarınızın devamı gelir.
For the reason that the admin of this site is working, no uncertainty very quickly it will be renowned, due to its quality contents.
Yazınız için teşekkürler. Bu bilgiler ışığında nice insanlar bilgilenmiş olacaktır.
aramalarım sonunda buraya geldim ve kesinlikle işime yarayan bir makale oldu. teşekkür ederim
This is really interesting, You’re a very skilled blogger. I’ve joined your feed and look forward to seeking more of your magnificent post. Also, I’ve shared your site in my social networks!
I very delighted to find this internet site on bing, just what I was searching for as well saved to fav
cheap erectile dysfunction: canadian pharm – homepage
For the reason that the admin of this site is working, no uncertainty very quickly it will be renowned, due to its quality contents.
Быстрая схема покупки диплома старого образца: что важно знать?
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
http://www.babelcube.com/user/amanda-newport
https://rentry.org/9nrycboi
https://chyoa.com/user/i1975
https://www.hentai-foundry.com/user/kircy1992/profile
https://ellaa1966.diary.ru/
ed symptoms canada pharmacy online cheap ed drugs
pharmacies in mexico that ship to usa MexicanPharmEasy mexico drug stores pharmacies
sildenafil without a doctor’s prescription herbal ed or online prescription for ed meds
https://maps.google.ro/url?sa=t&url=https://canadianpharm1st.com male erectile dysfunction
best pharmacy online pain medications without a prescription and viagra without doctor prescription natural help for ed
medications list https://mexicanpharmeasy.com/# reputable mexican pharmacies online
pills erectile dysfunction best male enhancement or ed aids
https://cse.google.co.ck/url?sa=t&url=https://canadianpharm1st.com erectile dysfunction drug
hims ed pills vacuum therapy for ed and vacuum therapy for ed ed treatments that really work
Как выбрать стальной лист для строительных и производственных нужд
ed clinic pet meds without vet prescription or <a href=" http://adamlewisschroeder.com/info.php?a=ed clinics
http://101.43.178.182/sell/email.asp?d=canadianpharm1st.com&on=tb ed treatments that really work
ed meds online without prescription or membership impotence treatment and erectyle dysfunction remedies for ed
mexico drug stores pharmacies: Mexican Pharm – mexican drugstore online
best erectile dysfunction pills: canadian pharmacy – ed meds
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Mostbet Polska to idealne miejsce na zaklady sportowe | Mostbet free spins czekaja na nowych uzytkownikow | Mostbet pl zapewnia bezpieczna rozrywke online | Mostbet casino no deposit bonus codes to najlepsza oferta | Mostbet pl oferuje wysoki poziom bezpieczenstwa | Mostbet logowanie zajmuje tylko kilka sekund mostbet. | Mostbet Polska oferuje zaklady na zywo i wiele wiecej | Mostbet free spins sa dostepne juz po rejestracji | Mostbet kasyno to swietna zabawa i wielkie wygrane Mostbet casino login.
medication from mexico pharmacy: mexicanpharmeasy.com – mexico drug stores pharmacies
reputable indian online pharmacy: IndianPharmStar.com – online pharmacy india
Avcılar escort hizmetleri, birçok kişinin rahatlamak ve yeni deneyimler yaşamak için tercih ettiği bir seçenek haline geliyor. Pelin, bu alanda dikkat çeken biri.
erection pills https://indianpharmstar.com/# best online pharmacy india
LEO娛樂城無法登入?九州娛樂城退出台灣市場的真相大揭秘
近期,LEO娛樂城頻傳無法登入的消息,引發廣大玩家的疑慮與焦慮。作為九州娛樂城旗下的重要品牌,其背後的問題究竟是什麼?九州娛樂城是否真的退出台灣市場?本文將深度解析LEO娛樂城無法登入的三大原因,以及九州娛樂城退出的背景,幫助玩家了解真相並制定應對策略。
LEO娛樂城無法登入的三大原因
1. 帳號或密碼問題
部分玩家反映無法登入是由於密碼輸入錯誤或帳號遭盜用。
問題分析:這類問題通常較輕微,只需聯繫客服便可解決。
困難點:有玩家指出,LEO娛樂城的客服回應緩慢,甚至拖延處理時間,讓人懷疑是否另有隱情。
2. 平台技術或法規問題
當玩家試圖登入LEO娛樂城時,可能遇到以下情況:
伺服器無法連接或出現404錯誤頁面。
伺服器損壞或平台因法規問題遭檢舉下架,導致服務中斷。
此情況顯示平台運營出現技術或法規挑戰,玩家的遊戲體驗和資金安全因此受到影響。
3. 九州娛樂城及旗下品牌退出台灣市場
最令人震驚的原因是,LEO娛樂城可能正在退出台灣市場。
九州娛樂城因資金問題和監管壓力選擇撤出台灣。
市場傳聞指出其資金鏈出現裂痕,加上內外壓力,導致旗下品牌無法維持運營穩定性。
九州娛樂城退出台灣市場的原因
1. 市場競爭激烈,收益下滑
台灣娛樂城市場競爭日益加劇,各大品牌為爭奪市場份額不斷投入資源。九州娛樂城難以維持競爭力,收益大幅下滑,最終選擇將資源轉向其他具潛力的海外市場。
2. 資金鏈問題,運作困難
從玩家充值到出金的每個環節,九州娛樂城的資金運作均出現問題,影響平台穩定性。資金鏈斷裂使九州娛樂集團不得不低調撤出台灣,以降低損失。
3. 母公司法律與財務問題
九州娛樂集團近年來因法律與財務問題備受關注,導致其在台灣的營運面臨更多困難。相關部門的嚴格監管使得旗下品牌如LEO娛樂城、THA娛樂城被迫退出市場。
玩家如何應對LEO娛樂城無法登入的情況?
1. 立即提現本金
如果LEO娛樂城官網仍能登入,建議玩家立即提取帳戶內的所有資金,以避免因平台徹底關閉而造成損失。
2. 轉向穩定且合法的替代平台
選擇具有合法牌照和穩定運營的娛樂城作為替代平台。例如選擇國際知名品牌,確保遊戲體驗和資金安全。
3. 提高資金安全意識
在選擇新平台時,應重點關注以下事項:
資金保障措施:確保平台能提供快速出金和玩家資金保護機制。
玩家口碑:查看其他玩家的評價,選擇信譽良好的平台。
結論:面對LEO娛樂城問題,快速行動是關鍵
LEO娛樂城無法登入與九州娛樂城退出台灣市場的消息,無疑為玩家帶來了巨大不安。無論是資金問題還是伺服器故障,玩家應及時採取行動保障自身利益。立即提現資金,轉向穩定的娛樂平台,並提高安全意識,是避免損失的有效策略。
在動盪的市場環境中,選擇合法且穩定的平台是保護資金與享受遊戲體驗的最佳方式。
Avcılar escort hizmetleri, birçok kişinin rahatlamak ve yeni deneyimler yaşamak için tercih ettiği bir seçenek haline geliyor. Pelin, bu alanda dikkat çeken biri.
вывод от запоя в стационаре сочи вывод от запоя в стационаре сочи .
капельница от запоя в сочи капельница от запоя в сочи .
быстрый вывод из запоя в стационаре быстрый вывод из запоя в стационаре .
вывод из запоя в стационаре анонимно вывод из запоя в стационаре анонимно .
вывод из запоя на дому в сочи [url=https://www.uaforum.ukrbb.net/viewtopic.php?f=13&t=3246]вывод из запоя на дому в сочи[/url] .
top online pharmacy india top 10 pharmacies in india or reputable indian pharmacies
http://www.boostersite.es/votar-4378-4270.html?adresse=indianpharmstar.com& reputable indian online pharmacy
best india pharmacy india pharmacy mail order and buy medicines online in india Online medicine home delivery
india pharmacy: indian pharm star – india pharmacy mail order
п»їbest mexican online pharmacies mexicanpharmeasy.com mexican pharmaceuticals online
buying prescription drugs in mexico buying prescription drugs in mexico or mexican rx online
https://www.finanzplaner-deutschland.de/fpdeu/inc/mitglieder_form.asp?nr=24&referer=https://mexicanpharmeasy.com:: mexican rx online
mexico drug stores pharmacies mexico drug stores pharmacies and mexican mail order pharmacies buying prescription drugs in mexico online
natural treatment for ed canada pharmacy online generic ed pills
online shopping pharmacy india reputable indian online pharmacy or world pharmacy india
https://www.google.ae/url?sa=t&url=https://indianpharmstar.com pharmacy website india
cheapest online pharmacy india best online pharmacy india and pharmacy website india india online pharmacy
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
紫イミソーレ
オーバーロード パチンコ 199
ストレス発散にも最適で、リラックスしたいときに遊ぶのが良いです。気軽に楽しめます。
黄門ちゃま喝
https://www.dmmdeco.com/article/3610.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
翔馬
[url=https://www.ja-securities.jp/games/id1671]押忍!番長3(パチスロ)
[/url]
南国育ち
八代将军 天国の戦い
青龍(4発1)
CRフィーバー戦姫絶唱シンフォギア Ver.199
https://xn--lcklw8a5dc82a.com/article/272.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
沖ドキ!トロピカル
https://k8slots.jp/pachinko/2741.html
パチンコのルールはシンプルですが、奥が深いです。初心者でもすぐに楽しめます。
パチスロ北斗の拳 強敵
https://www.casinotop3.tokyo/Articles/372.html
パチンコは日本独特のエンターテイメントで、スリルと興奮が詰まっています。特に大当たりの瞬間は最高の快感です。
buy rybelsus: semaglutide – Rybelsus 7mg
https://amoxilpharm.store/# amoxicillin 500mg tablets price in india
neurontin for sale online Gabapentin Pharm neurontin 100 mg cost
rybelsus cost: Rybelsus 7mg – semaglutide
https://semaglutidepharm.com/# Buy compounded semaglutide online
cheap Rybelsus 14 mg: Buy semaglutide pills – buy rybelsus
neurontin generic brand: Gabapentin Pharm – neurontin medication
https://paxlovid.ink/# paxlovid price
rybelsus price semaglutide semaglutide
Gabapentin Pharm neurontin cap neurontin 400 mg tablets
AmoxilPharm buy cheap amoxicillin online AmoxilPharm
промокод prodamus скидка промокод prodamus скидка .
paxlovid buy: paxlovid for sale – paxlovid for sale
https://amoxilpharm.store/# AmoxilPharm
Jugabet casino Per? Jugabet casino Per? .
Avrupa Yakası Escort Avrupa Escort İstanbul Escort Bayanlar
ぱちんこソードアート・オンラインK12
鏡 ドリームカムズアゲイン
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
琉球の守護神
https://www.k8o.jp/slots/3381.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
魂斗羅3D
k8パチンコ
鉄拳3rd
CR牙狼GOLDSTORM翔 Ver.319
CR燃える闘魂アントニオ猪木~格闘技世界一決定戦~
花の慶次天に愛されし漢~
https://www.casinoonline.help/article/153.html
パチンコの台は、時には思わぬ演出があって驚かされます。毎回新しい発見があります。
戦国BASARA3
https://www.onlinecasinofinland.org/article/168.html
音楽が豪華で、プレイ中に盛り上がります。神々の祝福を感じます。
P貞子3D2呪われた12時間
https://www.sb365-casino.com/article/69.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
genç kağıthane escort bayanlar etkileyici derecede efsane hizmetler sunuyor.
Avrupa Yakası Escort Avrupa Escort İstanbul Escort Bayanlar
nhà cái ST666
ST666 – Nhận Định Bóng Đá Kèo Nhà Cái Uy Tín
ST666: Địa Chỉ Lý Tưởng Cho Những Tín Đồ Cá Cược Bóng Đá
ST666 là nền tảng nhận định bóng đá và kèo nhà cái chuyên nghiệp, nơi người chơi có thể tham gia dự đoán các kèo đa dạng như cược tài xỉu, cược chấp, và cược 1×2. Với giao diện thân thiện, tỷ lệ cược minh bạch và ưu đãi hấp dẫn, ST666 đang dần trở thành lựa chọn hàng đầu của cộng đồng yêu bóng đá.
Nhận Định Kèo Nhà Cái Đa Dạng
Cược Tài Xỉu (#cuoctaixiu)
Dựa vào tổng số bàn thắng trong trận đấu, người chơi dễ dàng chọn lựa Tài (nhiều hơn tỷ lệ nhà cái đưa ra) hoặc Xỉu (ít hơn tỷ lệ).
ST666 cung cấp các phân tích chi tiết giúp người chơi đưa ra lựa chọn chính xác.
Cược Chấp (#cuocchap)
Thích hợp cho những trận đấu có sự chênh lệch về sức mạnh giữa hai đội. ST666 cung cấp tỷ lệ chấp tối ưu, phù hợp với cả người chơi mới và chuyên nghiệp.
Cược 1×2 (#cuoc1x2)
Phù hợp cho những ai muốn dự đoán kết quả chung cuộc (Thắng – Hòa – Thua). Đây là loại kèo phổ biến, dễ hiểu và có tỷ lệ cược hấp dẫn.
Ưu Đãi Hấp Dẫn Tại ST666
Nhận 160% tiền gửi lần đầu: Khi đăng ký tài khoản mới và nạp tiền, người chơi sẽ nhận được số tiền thưởng cực lớn, tăng cơ hội tham gia các kèo.
Hoàn tiền 3% mỗi ngày: Chính sách hoàn tiền giúp người chơi giảm thiểu rủi ro, thoải mái trải nghiệm mà không lo lắng nhiều về chi phí.
Vì Sao Nên Chọn ST666?
Nền Tảng Uy Tín: ST666 cam kết mang đến trải nghiệm cá cược minh bạch và an toàn.
Phân Tích Chuyên Sâu: Đội ngũ chuyên gia của ST666 luôn cập nhật nhận định mới nhất về các trận đấu, giúp người chơi đưa ra quyết định tối ưu.
Hỗ Trợ 24/7: Đội ngũ hỗ trợ chuyên nghiệp, luôn sẵn sàng giải đáp thắc mắc của người chơi.
Giao Dịch Nhanh Chóng: Nạp rút tiền linh hoạt, đảm bảo sự tiện lợi và bảo mật.
Cách Tham Gia ST666
Truy cập website chính thức của ST666.
Đăng ký tài khoản bằng thông tin cá nhân.
Nạp tiền lần đầu để nhận ưu đãi 160%.
Bắt đầu trải nghiệm cá cược với các kèo yêu thích!
Hãy đến với ST666 để tận hưởng không gian cá cược chuyên nghiệp, nhận định kèo chất lượng và những phần thưởng hấp dẫn. Tham gia ngay hôm nay để trở thành người chơi chiến thắng!
ST666 Xổ Số – Điểm Đến Giải Trí Hoàn Hảo
ST666 Xổ Số là nền tảng giải trí trực tuyến hàng đầu, cung cấp đa dạng các loại hình trò chơi từ keno, xổ số miền Nam (XSMN), xổ số miền Bắc (XSMB) đến xổ số siêu tốc. Với dịch vụ chuyên nghiệp và an toàn, ST666 mang đến cho bạn một trải nghiệm hoàn toàn mới mẻ và hấp dẫn.
Tại Sao Nên Chọn ST666 Xổ Số
Truy Cập Nhanh Và An Toàn
ST666 đảm bảo quyền truy cập nhanh chóng thông qua đường dẫn chính thức, loại bỏ hoàn toàn rủi ro từ các trang web giả mạo hoặc nguy cơ mất tài khoản. Hệ thống bảo mật hiện đại giúp bạn yên tâm khi đăng nhập và tham gia trò chơi.
Ưu Đãi Độc Quyền Dành Cho Thành Viên
ST666 mang đến nhiều chương trình khuyến mãi đặc biệt dành riêng cho người chơi, bao gồm phần thưởng giá trị và các ưu đãi hấp dẫn. Thành viên mới sẽ được trải nghiệm các chính sách hỗ trợ vượt trội, giúp tăng cơ hội chiến thắng và tận hưởng trò chơi tốt nhất.
Bảo Mật Thông Tin Tối Đa
Hệ thống mã hóa hiện đại của ST666 đảm bảo mọi giao dịch và dữ liệu cá nhân đều được bảo vệ an toàn. Mọi thông tin đều được xử lý theo quy chuẩn bảo mật cao nhất, giúp người chơi yên tâm giải trí mà không lo lắng về vấn đề rủi ro thông tin.
Đa Dạng Trò Chơi Giải Trí
Xổ số: Từ xổ số truyền thống như XSMN, XSMB đến xổ số siêu tốc, đáp ứng mọi nhu cầu giải trí của người chơi.
Lô đề: Cung cấp giao diện dễ sử dụng, giúp bạn dễ dàng chọn số và theo dõi kết quả trực tiếp.
Casino trực tuyến: Đa dạng các trò chơi từ bài bạc, roulette đến các game đổi thưởng hiện đại.
Những Lợi Ích Khi Chơi Tại ST666
Dịch vụ hỗ trợ khách hàng chuyên nghiệp hoạt động 24/7.
Giao dịch nạp và rút tiền nhanh chóng, minh bạch.
Hệ thống cập nhật trò chơi thường xuyên, mang lại sự mới mẻ và không nhàm chán.
Nền tảng thiết kế hiện đại, thân thiện với mọi thiết bị, từ máy tính đến điện thoại di động.
Cách Truy Cập ST666
Truy cập đường dẫn chính thức của ST666 để đảm bảo an toàn.
Đăng ký tài khoản và hoàn tất các bước xác thực thông tin.
Tham gia các trò chơi hấp dẫn và tận hưởng phần thưởng đặc biệt dành cho thành viên.
ST666 Xổ Số không chỉ là nền tảng giải trí mà còn là nơi mang lại cơ hội chiến thắng và trải nghiệm dịch vụ đẳng cấp. Tham gia ngay hôm nay để khám phá một thế giới giải trí đa dạng và chuyên nghiệp!
betovo login betovo login .
быстрый вывод из запоя в стационаре быстрый вывод из запоя в стационаре .
amoxicillin 250 mg price in india: Amoxil Pharm Store – AmoxilPharm
genç kağıthane escort bayanlar etkileyici derecede efsane hizmetler sunuyor.
онлайн казино беларусь онлайн казино беларусь .
genç kağıthane escort bayanlar etkileyici derecede efsane hizmetler sunuyor.
娛樂城
富遊娛樂城:全台灣第一推薦的娛樂城體驗
富遊娛樂城是全台灣最受玩家推崇的娛樂平台,提供多種優惠、豐富的遊戲選擇以及高品質的服務,讓每位玩家都能享受極致的遊戲樂趣。無論您是新手還是資深玩家,富遊娛樂城都能滿足您的需求,帶給您刺激的遊戲體驗。
富遊娛樂城的優惠活動
1. 免費娛樂城體驗金
新玩家只需註冊即可免費領取 $168 元的體驗金,無需存款,便可立即體驗各種遊戲,感受遊戲帶來的樂趣。
2. 首次存款優惠
首次存款 $1000 元,即送 $1000 元遊戲金,讓您在遊戲中有更多機會獲勝,增添刺激感。
3. 新會員五選一好禮
新加入的會員可選擇五種專屬好禮之一,從遊戲金到實體獎品,讓您的遊戲旅程更加多彩。
熱門遊戲種類
富遊娛樂城提供多樣化的遊戲選擇,適合不同喜好的玩家:
電子老虎機:多款創新遊戲,讓玩家享受豐厚獎金機會。
百家樂:與真人荷官互動,體驗真實賭場的刺激感。
今彩539 和 運彩:滿足彩票愛好者的需求。
捕魚遊戲:RSG電子捕魚遊戲帶來娛樂與獎金的雙重樂趣。
熊貓體育:專為體育迷設計的多種運動投注選項。
優質服務保證
1. 出金快速、安全可靠
富遊娛樂城採用先進的財務處理系統,保證快速存提款,讓玩家不用擔心資金安全問題。
2. 技術先進的APP平台
流暢的畫面設計與設備兼容性,讓用戶輕鬆上手。
持續升級與更新,提供更多創新遊戲類型。
3. 24小時專業客服
專業客服隨時待命,為玩家解答所有遊戲相關問題,確保您在任何時間都能享受到無縫的服務體驗。
4. 安全防護到位
採用最嚴格的安全管理系統與數據加密技術,保障用戶資金與個人信息的安全。
富遊娛樂城APP:隨時隨地盡享遊戲
富遊娛樂城APP提供了便捷的遊戲途徑,支援 IOS 與 Android 設備。
簡潔易用的界面設計,讓玩家快速找到喜愛的遊戲。
即時客服支援,隨時解決您的疑問。
免費下載,無論何時何地,您都能盡享娛樂城的精彩遊戲體驗。
為什麼選擇富遊娛樂城?
優惠豐富:免費體驗金、首存優惠、新會員好禮,活動多樣。
遊戲種類齊全:從電子遊戲到真人百家樂,滿足各類玩家需求。
服務至上:專業客服、快速出金和高安全保障讓玩家安心享受遊戲。
便捷平台:富遊APP提供隨時隨地的遊戲樂趣,讓您不受限制。
立即下載 富遊娛樂城APP,體驗全台灣第一娛樂城的無限魅力!不論您是想享受遊戲樂趣還是贏取豐厚獎金,富遊娛樂城都將是您的最佳選擇!
leo娛樂城登入
LEO娛樂城:九州娛樂集團的卓越創舉與運營優勢
九州娛樂集團總部的現代化運營
九州娛樂集團的總部設於菲律賓首都馬尼拉市中心最具代表性的現代化建築「RCBC Plaza」。這座辦公大樓融合了全球領先的建築科技與安全系統,包括:
國家級資訊防護系統,確保數據安全。
樓宇自動化系統 (BAS),實現數位化管理與高效運營,包括防火系統、光纖電訊和數位監控。
九州娛樂為員工提供舒適的工作環境和專業的教育培訓,確保服務水準一流。其員工以專業知識和親切態度,為尊貴玩家帶來極致的遊戲體驗。
LEO娛樂旗下品牌與遊戲特色
九州娛樂城旗下分支包括 LEO、THA 和 KU 三個娛樂品牌。雖然品牌各自獨立運營,但都共享相同的遊戲系統與界面,提供超過數千款來自全球頂級供應商的遊戲。
這些品牌的核心特色包括:
種類繁多的遊戲:滿足不同類型玩家的需求,包括電子遊戲、體育博彩、真人娛樂等。
不斷更新的內容:通過九州獨家技術,網站內容保持新鮮,合作商可快速更新遊戲資源,確保平台競爭力。
玩家在這些平台上可以隨時找到適合自己的遊戲,享受沉浸式的娛樂體驗。
LEO娛樂城的歷史與發展
九州娛樂集團的起源可以追溯至其最早期的經營模式:「信用版」賭博平台 《天下體育球版》。儘管該模式在當時非常流行,但因高風險與財務處理複雜而逐漸被淘汰。
隨著市場需求的變化,九州娛樂轉型為現金版平台 《天下現金網》,引入了更安全、便捷的遊戲模式。這種轉變不僅提升了玩家的體驗,也使平台得以進一步發展。
如今,九州娛樂城已成為台灣博弈業界的領軍者,不僅優化了網站版面與功能,還推出了專屬手機APP,方便玩家隨時隨地進行遊戲體驗。
LEO娛樂的成功秘訣
現代化運營與技術支持:九州娛樂在系統安全與技術更新方面投入巨大資源,確保平台運營穩定可靠。
多元化遊戲體驗:通過與全球頂級遊戲供應商合作,平台提供了豐富的遊戲選擇,適應各種玩家需求。
用戶至上:專業培訓的員工以細心和熱忱態度,提供高品質服務,讓玩家倍感尊重。
不斷創新:從信用版到現金版,再到手機APP,九州娛樂始終緊跟市場趨勢,致力於打造最佳娛樂體驗。
結語
LEO娛樂城作為九州娛樂集團旗下的重要品牌,不僅繼承了集團的核心價值,更在遊戲選擇、服務品質和技術創新方面不斷提升。從最初的《天下體育球版》到如今的行業領先者,LEO娛樂見證了九州娛樂城的發展歷程,也成為博弈行業中的耀眼明星。
加入 LEO娛樂城,感受來自九州娛樂集團的專業與熱情,享受最頂級的線上博弈體驗!
казино казино .
RG富遊娛樂城:台灣線上娛樂城的最佳選擇
RG富遊娛樂城以其卓越的服務和多樣化的遊戲選擇,成為2024年最受歡迎的線上娛樂平台。受到超過50位網紅和部落客的實測推薦,這座娛樂城不僅提供豐富的遊戲,還帶來眾多優惠活動和誠信保證,贏得了廣大玩家的信任與青睞。
RG富遊娛樂城的獨特優勢
多重優惠活動
體驗金 $168:新手玩家可以免費試玩,無需任何成本即可體驗高品質遊戲。
首儲1000送1000:首次存款即可獲得雙倍金額,增加遊戲的樂趣與機會。
線上簽到轉盤:每日簽到即可參加抽獎,贏取現金獎勵和豐厚禮品。
快速存提款與資金保障
RG富遊採用自主研發的財務系統,確保5秒快速存款,滿足玩家即時遊戲需求。
100%保證出金,杜絕任何拖延或資金安全風險,讓玩家完全放心。
遊戲種類豐富
RG富遊娛樂城涵蓋多種遊戲類型,滿足不同玩家的需求,包括:
真人百家樂:與真人荷官互動,感受真實賭場的刺激氛圍。
電子老虎機:超過數百款創新遊戲,玩法新穎,回報豐厚。
電子捕魚:趣味性強,結合策略與娛樂,深受玩家喜愛。
電子棋牌:提供公平競技環境,適合策略型玩家。
體育投注:涵蓋全球賽事,賠率即時更新,為體育愛好者提供最佳選擇。
樂透彩票:參與多地彩票,挑戰巨額獎金。
跨平台兼容性
RG富遊支持Web端、H5、iOS和Android設備,玩家可隨時隨地登錄遊戲,享受無縫體驗。
與其他娛樂城的不同之處
RG富遊以現金版模式運營,確保交易透明和安全性。相比一般娛樂城,RG富遊在存提款速度上遙遙領先,玩家可在短短15秒內完成交易,並且100%保證資金提領。而在線客服全年無休,隨時提供支持,讓玩家在任何時間都能解決問題。
相比之下,一般娛樂城多以信用版模式運營,存在出金風險,且存提款速度較慢,客服服務不穩定,無法與RG富遊的專業性相比。
為什麼選擇RG富遊娛樂城
資金交易安全無憂:採用最先進的SSL加密技術,確保每筆交易的安全性。
遊戲種類全面豐富:每日更新多樣化遊戲,帶來新鮮感和無限可能。
優惠活動力度大:從體驗金到豐厚的首儲獎勵,玩家每一步都能享受優惠。
快速存提款服務:自主研發技術保障流暢交易,遊戲不中斷。
全天候專業客服:24/7在線支持,及時解決玩家需求。
立即加入RG富遊娛樂城
RG富遊娛樂城不僅提供豐富的遊戲體驗,更以專業的服務、完善的安全保障和多樣的優惠活動,為玩家打造一個值得信賴的娛樂環境。立即註冊,體驗台灣最受歡迎的線上娛樂城!
https://semaglutidepharm.com/# buy rybelsus
rybelsus generic: Semaglutide pharmacy price – semaglutide pharm
Ivermectin Pharm: Ivermectin Pharm – ivermectin cream 1
price for amoxicillin 875 mg: AmoxilPharm – Amoxil Pharm Store
http://paxlovid.ink/# paxlovid buy
Ivermectin Pharm Store Ivermectin Pharm Store Ivermectin Pharm Store
九州娛樂城倒了
熊貓體育:台灣娛樂市場的新星
隨著最近幾週LEO娛樂城的頻繁登入問題和九州集團宣布退出台灣市場,各大玩家開始尋求新的娛樂平台。在這樣的背景下,熊貓體育逐漸脫穎而出,成為玩家們的熱門選擇。
#### 熊貓體育的優勢
1. 穩定性與信任
熊貓體育作為一個亞洲知名的博弈品牌,擁有龐大的資本後盾,並持有多項合法的營業執照,包括歐洲馬爾他(MGA)與菲律賓(PAGCOR)的監管牌照。這些執照不僅確保了平台的合法性,也增強了玩家對平台的信任感。
2. 多元化的入金管道
熊貓體育提供了多種入金選擇,如信用卡和電子錢包等,讓玩家的資金交易更為靈活,無需擔心因為金流問題影響遊戲體驗。
3. 優惠活動吸引新玩家
該平台推出了吸引人的優惠活動,例如首儲1000元送1000元的活動,使得新玩家可以在入場時獲得額外資金,提升遊戲興趣和參與感。
4. 公平的遊戲環境
不同於某些競爭者,熊貓體育專注於提供公平的遊戲體驗,並致力於改善玩家的遊戲環境,減少風險與不確定性。
#### 未來展望
面對市場的變化,熊貓體育不僅提供安全且多元的遊戲體驗,還展示出強大的應變能力。隨著九州集團的退出,熊貓體育有機會吸引更多原本屬於LEO娛樂城的玩家,進一步擴大市場份額。
如果你正在尋找一個穩定且值得信賴的娛樂平台,不妨考慮熊貓體育,這將是你的不二選擇。隨著新賽季的開始,更多精彩的遊戲活動也將隨之而來,讓我們拭目以待!
https://ivermectinpharm.store/# Ivermectin Pharm Store
富遊娛樂城:台灣線上賭場的創新選擇
在台灣的線上娛樂市場中,富遊娛樂城憑藉其優質的服務和多樣化的娛樂選項,迅速成為新一代玩家的首選。既有刺激的遊戲體驗,又提供了一系列具有吸引力的優惠,富遊娛樂城無疑是追求樂趣的玩家們理想的地方。
#### 優勢與特色
1. 多樣化的遊戲選擇
富遊娛樂城提供了豐富的遊戲種類,包括電子老虎機、真人百家樂、運彩投注等。不論你是老虎機的愛好者,還是熱衷於真人賭桌的玩家,這裡都能滿足你的需求。特別推薦的遊戲如DG真人百家和RSG皇家百家樂,一定能帶給你極致的遊戲體驗。
2. 吸引人的優惠活動
富遊娛樂城針對新手和老玩家都推出了多樣的優惠活動。新會員首次存款可獲得相同金額的獎金,還有額外的體驗金送出,設計友好且實惠。此外,富遊每週的簽到獎勵和天天無上限的返水活動,更是讓玩家在遊戲中能持續獲得收益。
3. 安全可靠的存取款方式
在金融交易方面,富遊娛樂城支持多種存款和提款方式,包括各大銀行轉帳、超商儲值,甚至虛擬貨幣等。最低提款金額設置靈活,保證玩家資金的安全與便捷。
4. 人性化的操作介面
富遊娛樂城的網頁與移動應用設計簡潔易用,玩家可以方便地找到所需的遊戲和優惠。無論是在電腦還是手機上,都能順利享受到遊戲的樂趣。
#### 如何開始遊戲
如果你是新手玩家,開始你的富遊體驗非常簡單。只需註冊帳號,完成首次存款,即可獲得新手優惠。無論你想嘗試電子遊戲還是真人賭局,富遊娛樂城都能讓你輕鬆上手,享受娛樂的樂趣。
#### 社會責任與防沉迷措施
富遊娛樂城深知線上賭博的風險,因此致力於推廣負責任的遊戲文化。他們提供“防沉迷”指導,以確保玩家能夠理性投注,並提供必要的幫助和資源來幫助有需求的玩家。
#### 總結
對於尋求安全、便捷和多元化娛樂城體驗的玩家,富遊娛樂城絕對是值得選擇的選項。無論你是希望徹底放鬆,還是想要體驗刺激的博弈過程,富遊娛樂城都能滿足你的期待,成為你在台灣線上娛樂市場的最佳夥伴。立即註冊並開始你的遊戲之旅吧!
娛樂城
想要享受最刺激的線上娛樂體驗?選擇RG富遊娛樂城!
在這個數位時代,尋找適合自己的線上娛樂城變得越來越重要。如果你想在線上娛樂城找到最好玩、最受歡迎的遊戲,RG富遊絕對是你的首選!我們匯聚了多款博弈遊戲,讓您盡情享受娛樂的刺激與快感。
#### 精選遊戲類型
1. 真人百家樂
感受現場賭場的氛圍!RG富遊的真人視訊百家樂讓你與真實的荷官互動,享受高品質的遊戲體驗。無論你是老手還是新手,都能在這裡找到適合自己的節奏。
2. 電子老虎機
喜愛刺激嗎?我們提供多款熱門老虎機遊戲,如「戰神賽特」「魔龍傳奇」等,每一款都擁有精美的畫面和豐厚的獎勵,讓每次旋轉都充滿期待!
3. 體育投注
無論你是球迷還是賽事愛好者,RG富遊的體育投注平台都有你想要的。精準的賠率、廣泛的賽事選擇,讓你體驗到投注的樂趣和刺激。
4. 彩票遊戲與捕魚遊戲
隨著運氣來!不妨試試我們的棋牌遊戲和彩票遊戲,這裡有各種機會讓你體驗到不一樣的勝利快感。
#### 下載富遊APP,隨時隨地享受遊戲!
富遊娛樂城的APP支持iOS和Android設備,界面簡潔易用,內容豐富。無論你身處何地,都能輕鬆進入遊戲世界,隨心所欲享受娛樂時光。此外,我們提供即時客服支援,隨時解決你的疑問,讓你的遊戲體驗更為完美。
#### 為什麼選擇RG富遊?
– 安全可靠
RG富遊致力於為玩家提供安全可靠的遊戲環境,您的每一筆交易都受到最高的安全保障。
– 快速便捷的存提款服務
我們的存款平均時間僅需30秒,提款平均只需60秒,讓你體驗即時的快感!
– 豐富多元的遊戲選擇
超過9999款遊戲任你選擇,無論你偏好哪種娛樂方式,這裡都能滿足你的需求。
– 全天候服務
我們全年365天提供不停歇的客服支持,確保你在遊戲過程中享受到最好的服務。
### 結論
RG富遊娛樂城是您最佳的線上賭場選擇。我們不僅提供多樣化的遊戲選擇,還有安全可靠的遊戲環境和便捷的服務。立即下載APP,加入RG富遊,開始您的娛樂之旅,享受無與倫比的遊戲體驗吧!
滿天星娛樂城
滿天星娛樂城:全方位的線上娛樂體驗
滿天星娛樂城作為九州娛樂城旗下品牌之一,是玩家心目中備受信賴的線上娛樂平台。不僅提供豐富的遊戲選擇和吸引人的優惠,還以其合法營運的背景和快速出金的保證,成為台灣玩家的首選娛樂城之一。
滿天星娛樂城的特色與優勢
1. 合法營運,安全可靠
滿天星娛樂城持有三大國際娛樂執照,包括:
菲律賓 PAGCOR 監督競猜牌照
BVI 認證
馬爾他 MGA 認證
這些執照展現了平台的資本實力與合法性,讓玩家能夠安心享受遊戲,完全不用擔心出金問題。
2. 高品質遊戲與豐富優惠
滿天星娛樂城與九州集團旗下的LEO、THA娛樂城一樣,提供一流的遊戲品質和多樣化的優惠。
熱門遊戲推薦:電子老虎機、真人直播、運彩投注、今彩539等,滿足各類玩家需求。
優惠活動:定期推出限時回饋活動,讓玩家在遊戲中獲得更多額外收益。
3. 快速出金與高效服務
滿天星娛樂城採用先進的財務系統,確保存提款快速、安全,讓玩家能夠專注於享受遊戲,而無需擔心資金問題。專業的客服團隊24小時在線,提供即時支援。
4. 多平台兼容的APP
滿天星娛樂城提供設計貼合玩家需求的手機APP,適用於iOS與Android系統。玩家可以隨時隨地快速登入遊玩,體驗無與倫比的娛樂快感。
熱門遊戲類型一覽
滿天星娛樂城為玩家提供多樣化的遊戲選擇,滿足不同興趣和需求:
真人直播:感受真實賭場氛圍,與真人荷官互動,體驗沉浸式娛樂。
電子老虎機:多款經典與創新機台,讓玩家享受刺激的遊戲過程。
運彩投注:涵蓋世界盃、棒球、籃球等多項運動賽事,提供即時投注與直播功能。
彩票遊戲:包括今彩539、富遊彩票等,適合熱衷於彩券的玩家。
滿天星娛樂城常見問題解答
Q1. 滿天星娛樂城是否提供APP下載?
是的,滿天星娛樂城提供專屬的手機APP,支援iOS和Android系統。玩家可透過APP快速登入,隨時享受遊戲。
Q2. 滿天星娛樂城是否合法?
滿天星娛樂城擁有多項國際認證,為合法經營的線上娛樂城,並保證玩家資金的安全性與遊戲的公平性。
Q3. 無法登入滿天星娛樂城該怎麼辦?
如遇到無法登入的情況,建議玩家檢查網路狀況或聯繫24小時客服團隊獲取即時協助。
立即加入滿天星娛樂城,享受最佳娛樂體驗!
滿天星娛樂城以合法、安全、快速的服務,搭配豐富的遊戲選擇和優惠,為玩家打造出獨一無二的線上娛樂體驗。不論您是新手還是老手,都能在滿天星找到屬於自己的遊戲樂趣。現在就下載APP,隨時隨地開始您的娛樂之旅!
п»їpaxlovid: paxlovid cost without insurance – Paxlovid.ink
neurontin 202 buy generic neurontin online or neurontin generic
https://www.google.rs/url?q=https://gabapentinpharm.com gabapentin
neurontin 4 mg cheap neurontin and neurontin 500 mg neurontin prescription coupon
Overview of Digital Currency Transfer Validation and Compliance Solutions
In the current digital asset sector, maintaining deal clarity and compliance with Anti-Laundering and Know Your Customer (KYC) rules is crucial. Below is an overview of popular services that provide solutions for crypto deal surveillance, validation, and fund protection.
1. Tokenmetrics.com
Overview: Token Metrics provides digital asset evaluation to evaluate potential fraud threats. This platform lets investors to examine coins before investment to avoid potentially fraudulent resources. Highlights:
– Threat analysis.
– Suitable for buyers aiming to avoid questionable or scam projects.
2. Metamask Monitor Center
Overview: Metamask.Monitory.Center permits users to check their digital asset resources for doubtful actions and standard conformity. Benefits:
– Verifies tokens for legitimacy.
– Offers warnings about potential resource blockages on specific trading sites.
– Delivers detailed reports after wallet sync.
3. Bestchange.com
Summary: Bestchange.ru is a service for tracking and checking crypto trade transactions, guaranteeing clarity and transaction safety. Highlights:
– Deal and wallet monitoring.
– Restriction screening.
– Online interface; supports BTC and several other cryptocurrencies.
4. AMLCheck Bot
Description: AMLCheck Bot is a holding observer and anti-money laundering compliance tool that employs AI algorithms to detect suspicious activity. Highlights:
– Transaction tracking and identity check.
– Available via internet and Telegram.
– Compatible with cryptocurrencies like BSC, BTC, DOGE, and additional.
5. AlfaBit
Summary: AlfaBit offers complete anti-money laundering solutions customized for the digital currency field, assisting firms and financial institutions in ensuring standard compliance. Features:
– Extensive compliance options and checks.
– Adheres to current security and regulatory guidelines.
6. Node AML
Description: AML Node provides compliance and customer identity tools for digital currency businesses, which includes deal monitoring, compliance validation, and analysis. Features:
– Risk evaluation tools and restriction screenings.
– Valuable for ensuring secure company activities.
7. Btrace.AMLcrypto.io
Description: Btrace AML Crypto focuses on resource check, offering transaction tracking, sanctions screenings, and help if you are a target of fraud. Benefits:
– Effective help for resource restoration.
– Transaction monitoring and safety features.
Dedicated USDT Check Solutions
Our platform also reviews various services that offer verification services for crypto deals and accounts:
– **USDT TRC20 and ERC20 Verification:** Many platforms support detailed checks for USDT deals, helping in the identification of questionable actions.
– **AML Verification for USDT:** Solutions are offered for monitoring for money laundering activities.
– **“Cleanliness” Validation for Wallets:** Checking of transaction and account “cleanliness” is offered to detect possible threats.
**Wrap-up**
Finding the best platform for checking and observing cryptocurrency transactions is essential for ensuring safety and regulatory compliance. By reading our evaluations, you can choose the most suitable tool for deal monitoring and fund safety.
https://paxlovid.ink/# paxlovid generic
cost of neurontin 800 mg Gabapentin Pharm gabapentin medication
ivermectin 24 mg minocycline 50mg pills online or buy minocycline 50mg otc
https://images.google.mu/url?sa=t&url=https://ivermectinpharm.store ivermectin eye drops
ivermectin 1 cream stromectol pill price and ivermectin 3 mg tabs buy minocycline 100mg
Rybelsus 7mg: buy semaglutide online – Buy semaglutide pills
neurontin over the counter neurontin cost or medicine neurontin 300 mg
http://ip-srv.ru/informers.php?address=gabapentinpharm.com neurontin tablets
neurontin 800mg neurontin capsule 600mg and neurontin gel neurontin pills
ivermectin topical ivermectin virus or minocycline 100mg without a doctor
https://exigen.com.au/?URL=https://ivermectinpharm.store minocycline 100mg without a doctor
stromectol order online ivermectin 9mg and buy minocycline 100 mg tablets ivermectin cream uk
https://ivermectinpharm.store/# Ivermectin Pharm
Gabapentin Pharm: Gabapentin Pharm – neurontin buy from canada
cheap Rybelsus 14 mg: semaglutide pharm – cheap Rybelsus 14 mg
Ivermectin Pharm Store: stromectol online canada – Ivermectin Pharm
https://semaglutidepharm.com/# semaglutide
Gabapentin Pharm: neurontin 300 mg capsule – Gabapentin Pharm
https://gabapentinpharm.com/# medicine neurontin capsules
cheap Rybelsus 14 mg Buy compounded semaglutide online rybelsus price
Bağcılar escort arayanlar için farklı deneyimler sunan seçenekler mevcut. Anal seks konusunda tercihleri belirlemek, sağlıklı bir iletişimle mümkün.
Bakırköy escort hizmetleri, günümüzün yoğun hayatında dinlenmek ve eğlenmek isteyenler için cazip bir seçenek sunuyor. Kırmızı ojeli Kübra, bu alandaki dikkat çekici isimlerden biri.
https://paxlovid.ink/# Paxlovid.ink
Bağcılar escort arayanlar için farklı deneyimler sunan seçenekler mevcut. Anal seks konusunda tercihleri belirlemek, sağlıklı bir iletişimle mümkün.
Your article helped me a lot, is there any more related content? Thanks!
Bakırköy escort hizmetleri, günümüzün yoğun hayatında dinlenmek ve eğlenmek isteyenler için cazip bir seçenek sunuyor. Kırmızı ojeli Kübra, bu alandaki dikkat çekici isimlerden biri.
アナザーゴッドハーデス‐奪われたZEUSver
k8 カジノ パチンコ P 新世紀エヴァンゲリオン15 未来への咆哮
演出の多様性があり、毎回新しい体験が待っています。飽きることがありません。
CR北斗の拳5 覇者
https://casinos.town/
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
黃金守護神
k8 カジノ パチンコ
ミリオンゴッド-神々の凱旋
スーパービンゴネオ
ファファファ
L エヴァンゲリオン ~未来への創造~
https://www.casinodengi.org/article/140.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
P ぱちんこ シン・エヴァンゲリオン Type レイ
https://www.3ae.jp/reviews/17545.html
パチンコの歴史を学ぶことで、より深く楽しめます。文化的な側面も魅力的です。
CR JAWS再臨-SHARK PANIC AGAIN-
https://www.k8.kaufen/Articles/1574.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт телевизоров xiaomi, можете посмотреть на сайте: срочный ремонт телевизоров xiaomi
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Paxlovid.ink: paxlovid cost without insurance – paxlovid cost without insurance
can i buy neurontin over the counter: neurontin generic – neurontin singapore
https://gabapentinpharm.com/# Gabapentin Pharm
Ivermectin Pharm Ivermectin Pharm Store Ivermectin Pharm
ivermectin 500ml ivermectin tablet price or ivermectin cream 1%
http://images.google.ac/url?q=https://ivermectinpharm.store how much is ivermectin
buy minocycline 50 mg for humans minocycline 50 mg tabs and minocycline generic name cost of ivermectin pill
3A娛樂城
3A娛樂城:全方位線上娛樂體驗的首選平台
3A娛樂城作為台灣最受歡迎的線上娛樂平台之一,以其多樣化的遊戲選擇、創新的技術、以及卓越的安全保障,贏得了玩家的高度信任與青睞。無論您是新手還是老手,3A娛樂城都能滿足您的需求,帶給您獨一無二的遊戲體驗。
熱門娛樂城遊戲一覽
3A娛樂城提供多種熱門遊戲,適合不同類型的玩家:
真人百家樂:體驗真實賭場的氛圍,與真人荷官互動,感受賭桌上的緊張與刺激。
彩票投注:涵蓋多種彩票選項,滿足彩券愛好者的需求,讓您隨時隨地挑戰幸運。
棋牌遊戲:從傳統的麻將到現代化的紙牌遊戲,讓玩家在策略與運氣中找到平衡。
3A娛樂城的核心價值
1. 專業
3A娛樂城每日提供近千場體育賽事,並搭配真人百家樂、彩票彩球、電子遊戲等多種類型的線上賭場遊戲。無論您的遊戲偏好為何,這裡總有一款適合您!
2. 安全
平台採用128位加密技術和嚴格的安全管理體系,確保玩家的金流與個人資料完全受保護,讓您可以放心遊玩,無需擔憂安全問題。
3. 便捷
3A娛樂城是全台第一家使用自家開發的全套終端應用的娛樂城平台。無論是手機還是電腦,玩家都能享受無縫的遊戲體驗。同時,24小時線上客服隨時為您解決任何問題,提供貼心服務。
4. 安心
由專業工程師開發的財務處理系統,為玩家帶來快速的存款、取款和轉帳服務。透過獨立網路技術,平台提供優化的網路速度,確保每一次操作都流暢無比。
下載3A娛樂城手機APP
為了提供更便捷的遊戲體驗,3A娛樂城獨家開發了功能齊全的手機APP,讓玩家隨時隨地開啟遊戲。
現代化設計:新穎乾淨的介面設計,提升使用者體驗。
跨平台支持:完美適配手機與電腦,讓您輕鬆切換設備,無需中斷遊戲。
快速下載:掃描專屬QR碼即可進入下載頁面,立即開始暢玩!
3A娛樂城的其他服務
娛樂城教學:詳細的操作指導,讓新手也能快速上手。
責任博彩:提倡健康娛樂,確保玩家在遊戲中享受樂趣的同時,不失理性。
隱私權政策:嚴格遵守隱私保護條例,保障玩家的個人信息安全。
立即加入3A娛樂城,享受頂級娛樂體驗!
如果您正在尋找一個專業、安全、便捷又安心的線上娛樂平台,那麼3A娛樂城絕對是您的不二之選。現在就下載手機APP,隨時隨地開啟您的娛樂旅程,享受無限的遊戲樂趣與刺激!
AmoxilPharm: where to get amoxicillin over the counter – cheap amoxicillin 500mg
neurontin mexico: neurontin capsules 300mg – drug neurontin 200 mg
minocycline 100 ivermectin 0.1 or minocycline tablets
https://maps.google.mv/url?q=https://ivermectinpharm.store ivermectin lotion 0.5
minocycline 100 mg tablets for human ivermectin 1%cream and buy minocycline online stromectol 6 mg tablet
paxlovid for sale paxlovid for sale or paxlovid price
https://cse.google.tm/url?q=https://paxlovid.ink paxlovid buy
paxlovid for sale buy paxlovid online and paxlovid price Paxlovid over the counter
http://paxlovid.ink/# paxlovid buy
paxlovid covid Paxlovid.ink paxlovid buy
超過100間最新資訊任你看!
還在苦苦尋找安全可靠的線上娛樂城推薦名單嗎?這篇彙集了 2024 年 100 間娛樂城評價實測,為您打造最新、最真實的娛樂城排行榜!
不論您是經驗豐富的玩家還是剛入門的新手,都能在這找到最適合您的娛樂城平台。
我們從遊戲種類、優惠活動到出金速度等多個面向進行詳細評比,讓您輕鬆避開黑網,安心享受刺激的遊戲體驗!點擊查看完整排行榜,開啟您的娛樂新篇章!
排名 推薦娛樂城 推薦指數
NO.1 富遊娛樂城 ★★★★★/5.0分
NO.2 1XBET娛樂城 ★★★★★/5.0分
NO.4 九州娛樂城 ★★★★★/5.0分
NO.5 LEO娛樂城 ★★★★★/5.0分
NO.6 YABO亞博體育娛樂城 ★★★★★/5.0分
NO.7 BET365娛樂城 ★★★★★/5.0分
NO.8 PM娛樂城 ★★★★★/5.0分
NO.9 DG娛樂城 ★★★★★/5.0分
NO.10 DB娛樂城 ★★★★★/5.0分
▲此表格為五星評價推薦娛樂城
1星評價
娛樂城評價
2024 / 10 / 24
達利娛樂城|評價介紹
達利娛樂城|評價介紹
達利娛樂城於2023年成立,他們聲稱致力於打造一個「安全可靠」、「即時便利」、「公平公正」及「專業營運」的優質娛樂服務平台,所有個人資…
2星評價
娛樂城評價
2024 / 10 / 04
金金財寶娛樂城|評價介紹
金金財寶娛樂城是一個由知名啦啦隊隊員林襄代言的線上賭場,採用幣商型運營方式,玩家需使用台幣儲值以獲得遊戲幣。 該平台不支持遊戲幣的直接…
2星評價
娛樂城評價
2024 / 08 / 06
帝遊娛樂城|評價介紹精選
帝遊娛樂城|評價介紹
帝遊娛樂城是在2022年所創立的新興品牌,網站內的排版以及網頁速度上都有著不錯的表現,對於使用者來說非常易於操作。 帝遊娛樂城的遊戲內…
3星評價
娛樂城評價
2024 / 07 / 31
客萊柏娛樂城
客萊柏娛樂城|評價介紹
客萊柏娛樂城是一間從2020年就開始經營的線上娛樂城品牌,該品牌累積不少玩家的好口碑,遊戲種類多元且有許多常駐的優惠活動,對新手玩家非…
5星評價
娛樂城評價
2024 / 06 / 25
DB娛樂城介紹
DB娛樂城|評價介紹
DB娛樂城,原名PM娛樂城,在2023年更名為PM。這個重新品牌的過程中,PM集團將其亞洲遊戲品牌更名為【DB多寶遊戲】,專注於提供多…
2星評價
娛樂城評價
2024 / 06 / 14
豪神娛樂城介紹
豪神娛樂城|評價介紹
豪神娛樂城是由著名主持人吳宗憲代言的線上賭場,採取幣商型的運營模式,玩家需使用台幣充值以換取遊戲幣。 該平台沒有提供遊戲幣的直接提現方…
2星評價
娛樂城評價
2024 / 06 / 14
星城娛樂城介紹
星城娛樂城|評價介紹
星城娛樂城是一個由知名主持人徐乃麟代言的網上賭場,採用幣商型運營方式,玩家需使用台幣儲值以獲得遊戲幣。 該平台不支持遊戲幣的直接提現,…
1星評價
娛樂城評價
2024 / 06 / 12
AT99娛樂城介紹
AT99娛樂城|評價介紹
AT99娛樂城是TU娛樂城最新開發的娛樂城品牌,至於為何要開發一個新的品牌引起了許多網友的推測。 對此有人覺得TU娛樂城可能被抄了或者…
不推薦
娛樂城評價
2024 / 06 / 04
AF娛樂城
AF娛樂城|評價介紹
AF娛樂城已經不存在了,不知是經營上有問題害是什麼原因,但是據我們了解此娛樂城有被詐騙的風險,請玩家多多注意!並且網路上充斥著假冒AF…
4星評價
娛樂城評價
2024 / 05 / 17
Player-KU-CASINO
KU娛樂城|評價介紹
過去九州娛樂城以信用版起家,為台灣地區的玩家服務。但由於會員數量過於龐大,造成客服無法消化,衍伸出許多客訴與詐騙謠言。 因此,九州決定…
1星評價
娛樂城評價
2024 / 04 / 04
錢盈娛樂城
錢盈娛樂城|評價介紹
錢盈娛樂城於2022年成立,是一個專注於現金投注的線上遊戲平台,提供了包括體育賭博、真人百家樂、老虎機、彩票和電子遊戲等多樣化的遊戲選…
不推薦
娛樂城評價
2024 / 03 / 26
F1方程式娛樂城
F1方程式娛樂城|評價介紹
F1方程式娛樂城屬於小型線上娛樂城版,整體晚間非常簡陋而且含有諸多瑕疵以及不完善。 首先奇怪的點在於完全沒有可以註冊的地方,我們這邊猜…
不推薦
娛樂城評價
2024 / 03 / 26
CZ168娛樂城
CZ168娛樂城|評價介紹
CZ168娛樂城在雖然在簡介中自稱有12年的經營時間。 但是經過我們實際的調查以及訪問該品牌網頁後發現,並沒有任何12年前或是更近的資…
1星評價
娛樂城評價
2024 / 03 / 25
XY娛樂城
XY娛樂城|評價介紹
XY娛樂城是一間於2024創立的新興品牌,我們實際到訪網站發現他們的網頁只提供手機版,並未提供電腦版,可能是因應現在主流大家都是拿手機…
2星評價
娛樂城評價
2024 / 03 / 25
搖錢樹娛樂城
搖錢樹娛樂城|評價介紹
搖錢樹娛樂城是創立於2024,是非常新的一個品牌,但是我們實際到訪網站後,他們的娛樂城完整度是不錯的。 除了有一些小缺陷,像是我們在想…
不推薦
娛樂城評價
2024 / 03 / 25
必勝娛樂城
必勝娛樂城介紹|評價介紹
必勝娛樂城並不存在,此娛樂城有被詐騙的風險,請玩家多多注意!必勝娛樂城據我們了解過後,發現網路上也有一家叫必勝客娛樂城,是否為同夥還有…
2星評價
娛樂城評價
2024 / 03 / 25
柏客娛樂城
柏客娛樂城|評價介紹
柏客娛樂城是一間於2023年所創立的新興線上娛樂城品牌。 據我們實際到訪該品牌網站他們的風格走的是暗色調,以視覺上及使用體驗來說給玩家…
不推薦
娛樂城評價
2024 / 03 / 25
九鑫娛樂城
九鑫娛樂城|評價介紹
九鑫娛樂城並不存在,此娛樂城有被詐騙的風險,請玩家多多注意! 九鑫娛樂城調查 如有玩家耳聞到或是想前往九鑫娛樂城做遊玩,Player團…
不推薦
娛樂城評價
2024 / 03 / 25
推金娛樂城
推金娛樂城|評價介紹
推金娛樂城並不存在,此娛樂城有被詐騙的風險,請玩家多多注意! 推金娛樂城調查 如有玩家耳聞到或是想前往推金娛樂城做遊玩,Player團…
不推薦
娛樂城評價
2024 / 03 / 25
捷冠娛樂城
捷冠娛樂城|評價介紹
捷冠娛樂城並沒有在經營,此娛樂城有被詐騙的風險,請玩家多多注意! 捷冠娛樂城調查 如有玩家耳聞到或是想前往捷冠娛樂城做遊玩,Playe…
不推薦
娛樂城評價
2024 / 03 / 25
歐萊雅娛樂城
歐萊雅娛樂城|評價介紹
歐萊雅娛樂城並不存在,此娛樂城有被詐騙的風險,請玩家多多注意! 歐萊雅娛樂城調查 如有玩家耳聞到或是想前往歐萊雅娛樂城做遊玩,Play…
不推薦
娛樂城評價
2024 / 03 / 25
MG娛樂城
MG娛樂城|評價介紹
MG娛樂城並不存在,此娛樂城有被詐騙的風險,請玩家多多注意!據我們了解,MG娛樂城在搜尋結果上顯示的雖然是MG娛樂城但是點進去後卻是非…
不推薦
娛樂城評價
2024 / 03 / 25
JF娛樂城
JF娛樂城|評價介紹
JF娛樂城並不存在,此娛樂城有被詐騙的風險,請玩家多多注意!目前了解到的是,JF娛樂城在網上只搜尋的到類似空包彈的網站推薦留言,而他所…
不推薦
娛樂城評價
2024 / 03 / 25
TTB娛樂城
TTB娛樂城|評價介紹
TTB娛樂城並不存在,此娛樂城有被詐騙的風險,請玩家多多注意! TTB娛樂城調查 如有玩家耳聞到或是想前往TTB娛樂城做遊玩,Play…
不推薦
娛樂城評價
2024 / 03 / 25
TES888娛樂城
TES888娛樂城|評價介紹
TES8888娛樂城並不存在,此娛樂城有被詐騙的風險,請玩家多多注意! TES888娛樂城調查 如有玩家耳聞到或是想前往TES888娛…
2星評價
娛樂城評價
2024 / 03 / 25
新世界娛樂城
新世界娛樂城|評價介紹
新世界娛樂城是一間於2007年創立的娛樂城,至今已有將近快20年的經營時間了。 但是經營這麼久的線上娛樂城卻被網友爆出擅自洩漏會員個資…
3星評價
娛樂城評價
2024 / 03 / 22
WG娛樂城
WG娛樂城|評價介紹
WG娛樂城是一間創立於2023年的線上娛樂城品牌,他們提供諸多遊戲選擇讓玩家能盡情在娛樂城中享受電子遊戲所帶來的愉悅。 其中較為特別的…
1星評價
娛樂城評價
2024 / 03 / 22
逸遊娛樂城
EG逸遊娛樂城|評價介紹
EG逸遊娛樂城是一間於2023年創立的新娛樂城品牌,他們擁有眾多遊戲提供給玩家做選擇,且頻繁地釋出優惠活動,可以說是對新手玩家非常友好…
2星評價
娛樂城評價
2024 / 03 / 21
RM娛樂城
RM娛樂城|評價介紹
RM娛樂城是由菲律賓政府的PAGCOR(菲律賓娛樂和遊戲公司)授予博彩牌照,並獲得GLI(Gaming Laboratories In…
2星評價
娛樂城評價
2024 / 03 / 21
YG娛樂城
YG娛樂城|評價介紹
YG娛樂城應該可以說是在業界風格數一數二特殊的線上娛樂城了,整體網站採用中世紀元素下去做設計,會讓玩家誤譯為自己身處在RPG遊戲裡頭。…
2星評價
娛樂城評價
2024 / 03 / 21
SAT888西雅圖娛樂城
SAT888西雅圖娛樂城|評價介紹
SAT888西雅圖娛樂城創立於2022年,其中他以豐富的遊戲選項聞名,但是在網頁的體驗上卻不如其他市面上那些線上娛樂城。 網頁速度較慢…
不推薦
娛樂城評價
2024 / 03 / 21
LT娛樂城
LT娛樂城|評價介紹
LT娛樂城是一間於2022創立的線上娛樂城品牌,其中要特別注意的是,經我們網路上調查後發現此品牌常常因各種理由不給玩家出金。 網友罵聲…
不推薦
娛樂城評價
2024 / 03 / 21
8方娛樂城
8方娛樂城|評價介紹
8方娛樂城在網頁外觀上並無太大問題,但是經我們實際訪問該品牌後,認為8方娛樂城為詐騙黑網的可能性非常大。 其中,網頁內許多按鈕設置上只…
不推薦
娛樂城評價
2024 / 03 / 20
T9娛樂城
T9娛樂城|評價介紹
T9娛樂城相信許多人多多少少聽過,但是這邊玩家們要特別注意其實T9娛樂城是不存在的。 經我們實際訪問該品牌網站發現到,他的網站始終指向…
3星評價
娛樂城評價
2024 / 03 / 20
玖富娛樂城
玖富娛樂城|評價介紹
玖富娛樂城是一間於2023年創立的新創品牌,他們精美的網頁以及讓人一目瞭然的排版資訊都讓人能夠清楚明瞭的找到自己想找的資訊,他們的遊戲…
不推薦
娛樂城評價
2024 / 03 / 20
致富娛樂城
致富娛樂城|評價介紹
致富娛樂城是一間於2018年就創立的品牌,雖然經營時間也有了5年多了,但是在網路上的口碑並不是很好,甚至還傳出該娛樂城為詐騙黑網。 就…
1星評價
娛樂城評價
2024 / 03 / 20
阿拉巴娛樂城
阿拉八娛樂城|評價介紹
阿拉八娛樂城是一間於2022年創立的線上娛樂城,相比其他線上知名娛樂城,他們的遊戲種類明顯地比其他間少很多。 這可能導致玩家在遊戲體驗…
1星評價
娛樂城評價
2024 / 03 / 20
JP娛樂城
JP娛樂城|評價介紹
JP娛樂城是一間於2023年所創立的新興品牌,但也不知道是否也因新創品牌因而導致在網站上有許多的資訊上的不足以及BUG的出現。 這可能…
不推薦
娛樂城評價
2024 / 03 / 20
鑫巨力娛樂城
鑫巨力娛樂城|評價介紹
有意前往鑫巨力娛樂城得玩家們需要注意!該娛樂城已於2024/03/15停止了平台服務,因此在這邊也提醒玩家不要做任何註冊以及儲值上的動…
3星評價
娛樂城評價
2024 / 03 / 20
威樂娛樂城
威樂娛樂城|評價介紹
威樂娛樂城專門為全球華人社群設計,憑藉其全球分布的辦公室和客服中心以及合法的經營方式,深受許多遊戲愛好者的青睞。 他們提供了多樣化的遊…
3星評價
娛樂城評價
2024 / 03 / 19
GSBET娛樂城
GSBET娛樂城|評價介紹
GSBET在系統上以及安全性的技術上有著完善的技術,他們提供了一個安全、隱私且公平的線上博彩空間。 使用國際認可的SSL 128位加密…
1星評價
娛樂城評價
2024 / 03 / 19
a8遊藝場
A8娛樂城|評價介紹
A8娛樂城經我們調查過後以及實際訪問該品牌網頁,發現其中幾個嚴重的問題,A8娛樂城的網頁排版混亂、且該品牌相關資訊完全都沒又做任何的說…
3星評價
娛樂城評價
2024 / 03 / 19
德正娛樂城
DZ得正娛樂城|評價介紹
DZ得正娛樂城是一間創立於2024年的新創線上娛樂城,並且他們提供了一系列豐富的遊戲選項。 從彩票、真人視訊、體育賭注到電玩遊戲等,滿…
1星評價
娛樂城評價
2024 / 03 / 19
雷亞娛樂城
雷亞娛樂城|評價介紹
雷亞娛樂城是一間於2020年創立的線上娛樂城,但是在訪問以及遊玩過雷亞娛樂城後我們給出了一些結論,他們在娛樂城的公司營運以及該品牌公司…
1星評價
娛樂城評價
2024 / 03 / 19
FDY娛樂城
FDY娛樂城|評價介紹
由於FDY娛樂城的官方網站未提供足夠的詳細資訊,我們高度懷疑可能是一個詐騙或不可信的平台。 官方網站上的信息過於粗略,缺乏必要的運營和…
3星評價
娛樂城評價
2024 / 03 / 19
昊陽娛樂城
昊陽娛樂城|評價介紹
昊陽娛樂城擁有菲律賓娛樂及博彩公司(PAGCOR)頒發的執照,受其監管,致力於提供安全、公平、公正、誠信的網上投注服務。 旨在打造世界…
3星評價
娛樂城評價
2024 / 03 / 19
369娛樂城
369娛樂城|評價介紹
369娛樂城提供一個簡潔且易於使用的應用程式,適用於iOS和Android設備,方便玩家在Google Play和App Store上…
3星評價
娛樂城評價
2024 / 03 / 18
發樂娛樂城
發樂娛樂城|評價介紹
創立於2021年的發樂娛樂城,是一家官方運營的線上社交博弈遊戲品牌。 以「誠信、正直、玩家第一」為核心理念,致力於維護用戶權益,為玩家…
2星評價
娛樂城評價
2024 / 03 / 18
QK娛樂城
QK娛樂城|評價介紹
QK娛樂城專為全球華人社區打造,深受眾多遊戲愛好者喜愛。他們在全球設有辦公室及客服中心,並且合法經營,確保您的遊戲體驗既安全又愉快。 …
不推薦
娛樂城評價
2024 / 03 / 18
鑽豹娛樂城
鑽豹娛樂城|評價介紹
關於鑽豹娛樂城的詳細訊息,在該娛樂城的官網中並沒有做任何詳細的介紹,因此我們在這邊可以合理判斷此娛樂城為詐騙黑網的可能性非常高,官網中…
3星評價
娛樂城評價
2024 / 03 / 18
飛達娛樂城
飛達娛樂城|評價介紹
飛達娛樂城,由”Wilshire Worldwide Company Limited” 營運,他們聲稱擁有菲律賓官方博彩許可,提供豐富…
2星評價
娛樂城評價
2024 / 03 / 18
FUNNY娛樂城
FUNNY娛樂城|評價介紹
FUNNY娛樂城專為全球華人社區量身打造,贏得了眾多遊戲愛好者的喜愛。 他們遍設辦公室及客服中心於世界各地,並且保證合法經營,讓您的遊…
2星評價
娛樂城評價
2024 / 03 / 18
金博娛樂城
金博娛樂城|評價介紹
金博娛樂城專門為全球的華人社群設計,已經贏得了許多忠實遊戲愛好者的心。 他們在世界各地都有自己的辦公室和客服中心,而且完全合法運營,讓…
不推薦
娛樂城評價
2024 / 03 / 18
V7娛樂城
V7娛樂城|評價介紹
V7娛樂城樂是一間於2022年年末所創立的線上娛樂城品牌,據他該品牌說法,他們擁有一支堅強的自主開發團隊、客服團隊、除了能滿足玩家在娛…
3星評價
娛樂城評價
2024 / 03 / 15
SZ娛樂城
SZ娛樂城|評價介紹
SZ娛樂城是專門為全球華人打造的,已經吸引了眾多忠實粉絲。 他們在各地設有辦公室和客服中心,都是合法經營的,所以您可以放心。 他們提供…
3星評價
娛樂城評價
2024 / 03 / 15
DOIN娛樂城
DOIN娛樂城|評價介紹
DOIN娛樂城,這家設立在柬埔寨波貝度假村的線上遊戲天地,不僅擁有合法的博弈執照,還提供多樣化的遊戲選項,包括運動博彩、電競、真人百家…
不推薦
娛樂城評價
2024 / 03 / 11
金合發娛樂城精選
金合發娛樂城|評價介紹
金合發娛樂城曾在線上娛樂界獲得廣泛的歡迎,享有老牌的地位。 但是,近些年來,由於玩家提出的連串投訴,其名譽不斷受損。 這些投訴主要圍繞…
5星評價
娛樂城評價
2024 / 03 / 11
RG富遊娛樂城精選
富遊娛樂城|評價介紹
隨著2024年的到來,最新的線上娛樂平台排行榜已經更新,這一次的排名綜合考慮了各種因素,包括體驗金、促銷活動、提款效率和遊戲多樣性,目…
不推薦
娛樂城評價
2024 / 03 / 08
3A娛樂城精選
3A娛樂城|評價介紹
3A娛樂城是一家線上遊戲平台,提供多樣化的遊戲選擇,包括百家樂、老虎機和運彩等。 但因為網路上對這個平台的評論不多,有些人可能會擔心遭…
1星評價
娛樂城評價
2024 / 03 / 08
FA8娛樂城
FA8娛樂城|評價介紹
FA8娛樂城在亞洲地區受到許多玩家的青睞,FA8娛樂城是一個多元化的線上賭博平台。 它提供了包羅萬象的遊戲選項,從體育投注、電玩遊戲到…
2星評價
娛樂城評價
2024 / 03 / 08
BU娛樂城
BU娛樂城|評價介紹
BU娛樂城在亞太地區的線上娛樂市場擁有豐富的經驗,以強大的研發團隊和優質的客服服務而聞名。 該平台所開發的遊戲經美國TST技術測試機構…
5星評價
娛樂城評價
2024 / 03 / 08
1XBET娛樂城1
1XBET娛樂城|評價介紹
1XBET娛樂城擁有許多各式各樣的遊戲和促銷活動,讓玩家每天都有機會贏得獎勵。 這個平台支持多種付款方式,包括Visa、Masterc…
不推薦
娛樂城評價
2024 / 03 / 07
大老爺娛樂城精選
大老爺娛樂城|評價介紹
大老爺娛樂城之前的活動是你存1000元就送你1000點,但得玩到13倍投注額。 最近他們改成了存3000送1688點,但要玩到11倍投…
3星評價
娛樂城評價
2024 / 03 / 07
AC1娛樂城精選
AC1娛樂城介紹|評價介紹
AC1娛樂城在亞太區的線上娛樂市場上運營多年,擁有一支自研團隊和專業的客服團隊。這家娛樂城努力為玩家提供一個全面、可信賴的網絡遊戲體驗…
3星評價
娛樂城評價
2024 / 03 / 07
大福娛樂城精選
大福娛樂城|評價介紹
大福娛樂城是個網上賭博平台,玩家在這裡是用虛擬的點數來充值,算是一種幣商型的賭場。 這裡沒有直接的提款方式,玩家得自己找幣商換成現金,…
5星評價
娛樂城評價
2024 / 03 / 07
九州娛樂城
九州娛樂城|評價介紹
九州娛樂城,也被稱作LEO娛樂城,致力於打造一個既安全又方便、公平公正的高品質娛樂服務平台。 他們的目標是創造全新的線上娛樂城體驗,普…
5星評價
娛樂城評價
2024 / 03 / 07
LEO娛樂城精選
LEO娛樂城|評價介紹
LEO娛樂城,也被稱為”九州娛樂城“,而他們的品牌宗旨是建立一個既安全又方便的高品質線上娛樂平台。 全力為玩家提供一個公平、正義的遊戲…
1星評價
娛樂城評價
2024 / 03 / 06
金鈦城娛樂城精選
金鈦城娛樂城|評價介紹
金鈦城娛樂城是台灣線上娛樂城(現金版),成立於 2023年,主打高額賠率、多樣遊戲、快速出金等特色。 但多數玩家反映金鈦城娛樂城的客服…
3星評價
娛樂城評價
2024 / 03 / 06
必贏娛樂城精選
必贏娛樂城|評價介紹
必贏娛樂城,定位為針對全球華人市場的高級線上娛樂平台,具有豐富的跨國經營經驗。 這個平台在世界各地都設有辦公室和客服中心,並且擁有多個…
3星評價
娛樂城評價
2024 / 03 / 06
好玩娛樂城
好玩娛樂城|評價介紹
對於正在尋找高品質線上賭場的玩家來說,好玩娛樂城絕對值得一試!這個平台已經吸引了成千上萬的玩家,以提供令人興奮的賭博體驗而著稱。 好玩…
不推薦
娛樂城評價
2024 / 03 / 06
BOK娛樂城精選
BOK娛樂城|評價介紹
目前在BOK娛樂城的網站上找不到登入的選項,當點擊相應連結時,會被重定向到【FM富馬】網站。這情況引起了疑慮,使人懷疑BOK娛樂城可能…
3星評價
娛樂城評價
2024 / 03 / 06
JY娛樂城精選
JC娛樂城|評價介紹
JC娛樂城是九州娛樂集團下的一個品牌。但當用戶試圖註冊JC娛樂城時,他們實際上會被導向到THA娛樂城的介面,這個介面和九州娛樂城的長得…
вывод из запоя нижний новгород вывод из запоя нижний новгород .
вывод из запоя нижний новгород стационар вывод из запоя нижний новгород стационар .
вывод из запоя стационар вывод из запоя стационар .
вывод из запоя стационар вывод из запоя стационар .
наркология вывод из запоя в стационаре наркология вывод из запоя в стационаре .
Amoxil Pharm Store AmoxilPharm Amoxil Pharm Store
paxlovid for sale paxlovid pill or paxlovid price
https://cse.google.com.gt/url?q=https://paxlovid.ink buy paxlovid online
paxlovid price paxlovid buy and paxlovid pharmacy paxlovid buy
https://semaglutidepharm.com/# Rybelsus 7mg
Ivermectin Pharm Store: Ivermectin Pharm – Ivermectin Pharm Store
neurontin online pharmacy how to get neurontin or neurontin capsules
http://images.google.mn/url?q=https://gabapentinpharm.com neurontin tablets 300 mg
neurontin medicine purchase neurontin online and gabapentin 100mg medicine neurontin capsules
rybelsus generic semaglutide pharm or cheap Rybelsus 14 mg
http://house.speakingsame.com/floorplan.php?sta=vic&addr=91+Arthurton+Road&q=Northcote&url=semaglutidepharm.com Semaglutide pharmacy price
rybelsus Rybelsus 7mg and buy rybelsus Semaglutide pharmacy price
dj88 gacor
neurontin capsules neurontin 800 pill or neurontin pills for sale
http://www.teacherlearningproject.com/index?URL=http://gabapentinpharm.com neurontin online pharmacy
medicine neurontin neurontin cap and cheap neurontin online gabapentin buy
Gabapentin Pharm: neurontin online pharmacy – Gabapentin Pharm
Насладитесь игрой в казино онлайн, стремитесь к джекпоту.
Ощутите азарт казино онлайн, достижение успеха рядом.
Играйте только на проверенных сайтах, следуйте советам экспертов.
Зарабатывайте большие деньги в интернет-казино, делая ставки на спортивные события.
Будьте в центре внимания виртуального казино, наслаждайтесь победами.
Играйте и выигрывайте вместе с лучшими, достигайте финансовой независимости.
Почувствуйте реальный азарт игры в казино онлайн, получая максимум удовольствия.
Выберите свой идеальный вариант развлечения, наслаждайтесь игрой в любое время суток.
Преуспейте в азартных играх вместе с нами, зарабатывайте крупные суммы.
Играйте в онлайн казино смартфоне, выбирая любимые игры.
Сделайте свою жизнь более увлекательной с казино онлайн, испытывая удовольствие от игры.
Продолжайте играть в казино онлайн и побеждать, получая удовольствие от побед.
Примите вызов и выиграйте крупный джекпот, получайте удовольствие от игры в любое время дня и ночи.
Станьте частью крупных турниров и соревнований, изучая стратегии и тактики.
Казино онлайн казино беларусь .
P とある魔術の禁書目録 Light PREMIUM ver.
スロット Sweet Bonanza 1000 スイートボナンザ1000
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
戦国パチスロ花の慶次これより我ら修羅に入る
https://www.k8.boston/Articles/2222.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
ミリオンゴッド
ムーン・プリンセス・トリニティ
P とある魔術の禁書目録 Light PREMIUM ver.
鬼武者3 時空天翔
CR真・花の慶次2 漆黒の衝撃
Pフィーバー戦姫絶唱シンフォギア2
https://www.ted-bed-casino.com/article/176.html
大当たりの瞬間は、ドキドキ感がたまりません。興奮が止まりません。
ベルセルク無双
https://www.diamond777-casino.org/article/172.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
P 北斗の拳 暴凶星
https://www.septentrion-game.com/sitemaps.xml
パチンコは、運だけではなく、選ぶ楽しみもあります。自分に合った台を見つけることが大切です。
http://gabapentinpharm.com/# neurontin 100mg cap
Paxlovid.ink Paxlovid over the counter п»їpaxlovid
merhaba kaliteli bir başakşehir escort bayan sitesine hoşgeldiniz
dünyaca ünlü kaliteli bir beykoz escort bayan sitesine hoşgeldiniz
https://ivermectinpharm.store/# Ivermectin Pharm Store
merhaba kaliteli bir başakşehir escort bayan sitesine hoşgeldiniz
Gabapentin Pharm: neurontin medicine – neurontin price india
drug neurontin: Gabapentin Pharm – Gabapentin Pharm
paxlovid buy: Paxlovid.ink – Paxlovid.ink
https://semaglutidepharm.com/# semaglutide
neurontin 600 mg cost neurontin 600 mg tablet Gabapentin Pharm
ivermectin price uk ivermectin 0.5 or ivermectin canada
https://image.google.tt/url?q=https://ivermectinpharm.store stromectol how much it cost
ivermectin cream uk ivermectin online and minocycline for uti ivermectin 8000
http://amoxilpharm.store/# Amoxil Pharm Store
minocycline 100 mg tabs stromectol without prescription or ivermectin cost
https://cse.google.lv/url?q=https://ivermectinpharm.store stromectol 3 mg price
ivermectin 0.5 lotion india minocycline 50mg tabs and generic name for ivermectin ivermectin oral
Ivermectin Pharm Store: Ivermectin Pharm – Ivermectin Pharm Store
вывод из запоя нижний новгород вывод из запоя нижний новгород .
выведение из запоя нижний новгород выведение из запоя нижний новгород .
наркология вывод из запоя в стационаре наркология вывод из запоя в стационаре .
выведение из запоя нижний новгород выведение из запоя нижний новгород .
娛樂城
富遊娛樂城評價:2024年最新評價
推薦指數 : ★★★★★ ( 5.0/5 )
富遊娛樂城作為目前最受歡迎的博弈網站之一,在台灣擁有最高的註冊人數。RG富遊以安全、公正、真實和順暢的品牌保證,贏得了廣大玩家的信賴。
富遊線上賭場不僅提供了豐富多樣的遊戲種類,還有眾多吸引人的優惠活動。在出金速度方面,獲得無數網紅和網友的高度評價,確保玩家能享有無憂的博弈體驗。
推薦要點
新手首選: 富遊娛樂城,2024年評選首選,提供專為新手打造的豐富教學和獨家優惠。
一存雙收: 首存1000元,立獲1000元獎金,僅需1倍流水,新手友好。
免費體驗: 新玩家享免費體驗金,暢遊各式遊戲,開啟無限可能。
優惠多元: 活動豐富,流水要求低,適合各類型玩家。
玩家首選: 遊戲多樣,服務優質,是新手與老手的最佳賭場選擇。
免費試玩
富遊娛樂城詳情資訊
賭場名稱 : RG富遊
創立時間 : 2019年
賭場類型 : 現金版娛樂城
博弈執照 : 馬爾他牌照(MGA)認證、英屬維爾京群島(BVI)認證、菲律賓(PAGCOR)監督競猜牌照
遊戲類型 : 真人百家樂、運彩投注、電子老虎機、彩票遊戲、棋牌遊戲、捕魚機遊戲
存取速度 : 存款5秒 / 提款3-5分
軟體下載 : 支援APP,IOS、安卓(Android)
線上客服 : 需透過官方LINE
富遊娛樂城優缺點
優點 缺點
台灣註冊人數NO.1線上賭場
首儲1000贈1000只需一倍流水
擁有體驗金免費體驗賭場
網紅部落客推薦保證出金線上娛樂城
需透過客服申請體驗金
富遊娛樂城存取款方式
存款方式 取款方式
提供四大超商(全家、7-11、萊爾富、ok超商)
虛擬貨幣ustd存款
銀行轉帳(各大銀行皆可)
網站內申請提款及可匯款至綁定帳戶
現金1:1出金
富遊娛樂城平台系統
真人百家 — RG真人、DG真人、歐博真人、DB真人(原亞博/PM)、SA真人、OG真人、WM真人
體育投注 — SUPER體育、鑫寶體育、熊貓體育(原亞博/PM)
彩票遊戲 — 富遊彩票、WIN 539
電子遊戲 —RG電子、ZG電子、BNG電子、BWIN電子、RSG電子、GR電子(好路)、ATG電子
棋牌遊戲 —ZG棋牌、亞博棋牌、好路棋牌、博亞棋牌、RG棋牌
捕魚遊戲 —ZG捕魚、RSG捕魚、好路GR捕魚、DB捕魚
富遊娛樂城優惠活動
優惠 獎金贈點 流水要求
免費體驗金 $168 1倍 (儲值後) /36倍 (未儲值)
首儲贈點 $1000 1倍流水
返水活動 0.3% – 0.7% 無流水限制
簽到禮金 $666 20倍流水
好友介紹金 $688 1倍流水
回歸禮金 $500 1倍流水
免費試玩
專屬富遊VIP特權
黃金 黃金 鉑金 金鑽 大神
升級流水 300w 600w 1800w 3600w
保級流水 50w 100w 300w 600w
升級紅利 $688 $1080 $3888 $8888
每週紅包 $188 $288 $988 $2388
生日禮金 $688 $1080 $3888 $8888
反水 0.4% 0.5% 0.6% 0.7%
娛樂城常見問題解答(FAQ)
Q1:如何在富遊娛樂城進行首次存款?
A1:可以透過超商、虛擬貨幣或銀行轉帳進行存款,過程簡單快速。( 詳細可查看:富遊娛樂城存款教學)
Q2:富遊娛樂城提款需要多久時間?
A2:提款過程一般在3-5分鐘內完成,保證快速到賬。( 詳細可查看:富遊娛樂城提款教學)
娛樂城
LEO娛樂城無法登入? 九州娛樂倒了? 富遊推出無痛轉移VIP優惠
LEO娛樂城無法登入免驚!推薦RG富遊更穩定、玩的更安心!
只要是玩過線上娛樂城的玩家,一定都對Leo娛樂城不陌生,近期Leo娛樂城無法登入的狀況越來越頻繁,是因為九州娛樂城與旗下品牌將在 2024/12/31 結束營業!
由於九州娛樂城旗下擁有leo娛樂城、THA娛樂城等品牌,這波風暴也讓許多玩家人心惶惶,擔心自己的遊戲權益受損,在文章的最後我們也提供了解決方案,趕快往下看!
九州娛樂城倒閉 ? 退出台灣娛樂市場?為何 ?
最近許多玩家發現 leo娛樂城無法登入,甚至傳出九州娛樂城要退出台灣的消息!雖然官方尚未發布正式聲明,但多方消息指出,九州娛樂城 (包含THA娛樂城、leo娛樂城) 正在逐步退出台灣市場。這可能是多重因素共同作用的結果:
退出台灣市場,尋求其他發展空間
九州娛樂認為台灣市場太小,品牌之間又過於競爭,市場規模有限,九州旗下其KU體育甚至贊助過足球五大聯賽,展現雄厚實力,促使九州娛樂城將目光投向海外。
畢竟,海外市場人口基數更大,獲利潛力也更為可觀。LEO娛樂城在台灣一個月就能獲利一億,進軍海外後的收益想必更加驚人。
金流問題、警界醜聞成導火線
LEO娛樂城高度仰賴金流公司運作,近期爆發的陳政谷案件揭露其旗下公司利用第三方支付協助母公司九州洗錢。
案情更指向警界內鬼,前台中刑大隊長林明佐涉嫌收賄超過4451萬元,為九州通風報信。 九州娛樂城曾出現凌晨時段無法儲值的狀況,很可能與警方查緝行動有關。
九州娛樂的新聞事件頻傳
LEO是九州集團底下的,陳政谷被抓之後,事情鬧得很大,為了避風頭,乾脆退出台灣也很合理。
這個說法也被推算也被網友認為有高度可能,不過也有人傳說其他幹部有接手的可能,至少沒辦法完全的說死九州會採取什麼行動。
leo娛樂城無法登入的問題,或許只是冰山一角,背後可能隱藏著更深層的原因。無論如何,九州娛樂城退出台灣市場,對玩家來說都是一個警訊。
九州娛樂城宣布12/31結束營業!玩家請停止儲值、立刻提款
九州娛樂城官方宣佈,近期旗下 THA、Leo 娛樂城無法登入,是因為九州集團將正式在 2024/12/31 中午 12:00 結束營業,退出台灣市場。
在這邊提醒玩家們立即停止存款動作,並且馬上將帳戶內的資金提領出來,避免您的權益受到損害!
LEO娛樂城無法登入? 九州娛樂倒了? 富遊推出無痛轉移VIP優惠
九州娛樂城宣布在12/31結束營業
告別Leo娛樂城無法登入煩惱!玩家無痛轉移至富遊,享更多優惠!
面對leo娛樂城無法登入的窘境,以及九州娛樂城的不確定性,玩家們需要一個安全可靠的新選擇!富遊娛樂城是您最佳的選擇,我們提供:
穩定安全保證出金: 穩定安全,保證出金! 擁有合法牌照和完善的監管機制,保障您的資金安全。
豐富多元的遊戲: 提供各式各樣的熱門遊戲,包括真人 casino、體育博彩、電子遊戲、彩票等等,滿足玩家的不同需求。
優渥的優惠活動: 新會員享有豐厚首存紅利,還有不定期的優惠活動和返水獎勵,讓您玩得更盡興!
24小時專業客服: 隨時為您解答問題,提供貼心服務。
立即註冊
特別優惠: 針對THA和LEO會員,富遊娛樂城推出獨家福利!
只要您是THA或LEO的會員,即可在富遊娛樂城直接升等到相對應的等級,並享有更高的反水、每週紅包和生日禮金!
立即加入富遊娛樂城,享受更優渥的回饋和更安心的保障!
以下是不同階級 VIP 的轉換福利與說明:
福利項目 / VIP階級 大神 金鑽 鉑金 黃金
每週紅包 2388 988 288 188
生日禮金 8888 3888 1080 688
返水 0.7 0.6 0.5 0.4
無論個人或團體,如有任何濫用本公司優惠的行為,本公司有權取消及收回獎金。
所有活動優惠都是特別為玩家而設立,在玩家註冊資訊有爭議時,為確保雙方利益,杜絕身份盜用行為,本公司有權要求玩家提供充足資訊,並以各種方式辨別玩家是否符合資格享有活動優惠。
本公司在會員有重複申請帳號行為時,有權取消及收回獎金。每位玩家、每一住址、每一電子郵箱、每一電話號碼、相同支付卡/信用卡號碼及共享電腦環境(例如網咖、其他公用網絡、公用電腦)只能擁有一個活動優惠資格。
每週限時紅包:會員在上週有過至少1次的存款記錄,每週一中午自動發放(1倍流水即可託售),限時48小時內領取! 每週至少存款要求:黃金富遊 1,000 點,鉑金富遊 1,500點,金鑽富遊 2,500點,大神富遊 5,000點 (存款計算週期:每週一 12:00 至下週一 11:59)
生日禮金:會員在註冊三個月內過生日,今年將不能領取生日禮金。另註冊時間大於三個月的會員需在生日當月找遊戲客服領取,每年可領取一次,需提供身分證明文件核實(1倍流水即可託售)
活動如有任何異動,本公司有權在任何時間修改、暫停或取消優惠活動,無需特別通知並保留本活動最終解釋權。
轉換平台申請辦法:提供螢幕錄影原平台層級介面到戶名、手機等資訊,傳送給富遊客服進行審核。
富遊娛樂城,值得您信賴的線上娛樂平台!
我們深知玩家選擇娛樂城的首要考量是安全和信譽。富遊娛樂城以誠信為本,致力於為玩家打造一個公平、公正、透明的遊戲環境。告別leo娛樂城無法登入的煩惱,立即加入富有娛樂城,體驗最優質的線上娛樂體驗!
https://semaglutidepharm.com/# semaglutide
Leo娛樂城無法登入?全面解析與解決指南
近期,Leo娛樂城的玩家紛紛反映登入失敗的情況,引發了廣泛的討論與擔憂。作為九州娛樂城旗下知名品牌之一,Leo娛樂城在台灣的娛樂市場中擁有大量用戶。然而,隨著九州娛樂城宣布將於2024年12月31日退出台灣市場,這一消息無疑讓玩家對資金安全與遊戲體驗產生了極大疑慮。
本文將深入探討Leo娛樂城無法登入的原因,提供解決方案,並推薦可靠的替代娛樂平台。
Leo娛樂城無法登入的三大原因
1. 帳號密碼問題
可能情況:最常見的原因是玩家輸入了錯誤的帳號或密碼,或者帳號被盜用。
解決方法:確認輸入的帳密正確,若仍無法登入,聯繫客服即可解決。
2. 網頁技術問題
可能情況:官網可能因伺服器維護或技術故障出現404錯誤,導致玩家無法登入。
解決方法:耐心等待官網修復,並清除瀏覽器快取和Cookie,或嘗試使用其他瀏覽器。
3. 九州娛樂城退出台灣市場
可能情況:隨著九州娛樂城宣布退出台灣市場,Leo娛樂城的營運也逐步停止,導致玩家無法再使用該平台。
影響:玩家可能面臨資金提取困難,需及時完成出金操作以保障自身利益。
娛樂城無法登入的緊急解決方案
核對帳號與密碼:確認輸入正確無誤,避免由於簡單疏忽造成的登入失敗。
檢查網路連線:確保網路穩定,必要時切換至更穩定的網路環境。
清除瀏覽器快取與Cookie:解決瀏覽器相關問題,恢復正常登入。
嘗試其他瀏覽器或設備:更換設備或使用不同瀏覽器,查看是否可以正常登入。
九州娛樂城退出台灣市場的原因
市場利潤有限:九州娛樂城認為台灣市場的競爭激烈且利潤有限,因此選擇退出並專注於其他海外市場。
資金生態鏈斷裂:現金交易平台出現問題,導致九州娛樂城難以維持正常運營。
母公司法律爭議:九州娛樂城母公司近年來因財務與法律問題受到高度關注,進一步影響旗下品牌的營運。
Leo娛樂城會員如何安全出金?
在九州娛樂城停止營運前,玩家需要立即完成出金操作以確保資金安全。
出金建議:
儘快登入官網提交提款申請,確保資金安全轉出。
若官網無法訪問,請嘗試聯繫客服,並保留交易證明以備進一步處理。
推薦替代平台:富遊娛樂城
隨著Leo娛樂城的退出,玩家可以考慮轉至穩定且值得信賴的替代平台,如富遊娛樂城。
富遊娛樂城的優勢
穩定可靠:擁有先進的技術和完善的資金保障機制,確保玩家的資金安全。
豐富的遊戲選擇:從電子遊戲到真人娛樂,滿足各類玩家的需求。
專屬優惠:特別為Leo會員推出等級升等計畫,並提供多樣化的專屬活動。
快速出金:高效的財務系統,讓玩家的存取款過程快速無憂。
Leo會員轉移至富遊的流程
登錄富遊官網完成註冊。
提供Leo娛樂城會員等級截圖與相關資料(如戶名、手機號碼)給富遊客服進行審核。
等待客服確認後,享受富遊娛樂城專屬的會員福利與優惠。
結語:立即加入富遊娛樂城,延續遊戲體驗!
Leo娛樂城的退出讓許多玩家感到困惑與擔憂,但選擇穩定、安全的娛樂平台是最明智的應對之道。富遊娛樂城憑藉其卓越的技術、專業的服務以及豐富的優惠,成為玩家的理想替代選擇。現在就註冊富遊娛樂城,享受無與倫比的遊戲體驗吧!
娛樂城
RG富遊娛樂城:台灣線上娛樂城的最佳選擇
RG富遊娛樂城以其卓越的服務和多樣化的遊戲選擇,成為2024年最受歡迎的線上娛樂平台。受到超過50位網紅和部落客的實測推薦,這座娛樂城不僅提供豐富的遊戲,還帶來眾多優惠活動和誠信保證,贏得了廣大玩家的信任與青睞。
RG富遊娛樂城的獨特優勢
多重優惠活動
體驗金 $168:新手玩家可以免費試玩,無需任何成本即可體驗高品質遊戲。
首儲1000送1000:首次存款即可獲得雙倍金額,增加遊戲的樂趣與機會。
線上簽到轉盤:每日簽到即可參加抽獎,贏取現金獎勵和豐厚禮品。
快速存提款與資金保障
RG富遊採用自主研發的財務系統,確保5秒快速存款,滿足玩家即時遊戲需求。
100%保證出金,杜絕任何拖延或資金安全風險,讓玩家完全放心。
遊戲種類豐富
RG富遊娛樂城涵蓋多種遊戲類型,滿足不同玩家的需求,包括:
真人百家樂:與真人荷官互動,感受真實賭場的刺激氛圍。
電子老虎機:超過數百款創新遊戲,玩法新穎,回報豐厚。
電子捕魚:趣味性強,結合策略與娛樂,深受玩家喜愛。
電子棋牌:提供公平競技環境,適合策略型玩家。
體育投注:涵蓋全球賽事,賠率即時更新,為體育愛好者提供最佳選擇。
樂透彩票:參與多地彩票,挑戰巨額獎金。
跨平台兼容性
RG富遊支持Web端、H5、iOS和Android設備,玩家可隨時隨地登錄遊戲,享受無縫體驗。
與其他娛樂城的不同之處
RG富遊以現金版模式運營,確保交易透明和安全性。相比一般娛樂城,RG富遊在存提款速度上遙遙領先,玩家可在短短15秒內完成交易,並且100%保證資金提領。而在線客服全年無休,隨時提供支持,讓玩家在任何時間都能解決問題。
相比之下,一般娛樂城多以信用版模式運營,存在出金風險,且存提款速度較慢,客服服務不穩定,無法與RG富遊的專業性相比。
為什麼選擇RG富遊娛樂城
資金交易安全無憂:採用最先進的SSL加密技術,確保每筆交易的安全性。
遊戲種類全面豐富:每日更新多樣化遊戲,帶來新鮮感和無限可能。
優惠活動力度大:從體驗金到豐厚的首儲獎勵,玩家每一步都能享受優惠。
快速存提款服務:自主研發技術保障流暢交易,遊戲不中斷。
全天候專業客服:24/7在線支持,及時解決玩家需求。
立即加入RG富遊娛樂城
RG富遊娛樂城不僅提供豐富的遊戲體驗,更以專業的服務、完善的安全保障和多樣的優惠活動,為玩家打造一個值得信賴的娛樂環境。立即註冊,體驗台灣最受歡迎的線上娛樂城!
九州娛樂城登入
Leo娛樂城無法登入?全面解析與解決指南
近期,Leo娛樂城的玩家紛紛反映登入失敗的情況,引發了廣泛的討論與擔憂。作為九州娛樂城旗下知名品牌之一,Leo娛樂城在台灣的娛樂市場中擁有大量用戶。然而,隨著九州娛樂城宣布將於2024年12月31日退出台灣市場,這一消息無疑讓玩家對資金安全與遊戲體驗產生了極大疑慮。
本文將深入探討Leo娛樂城無法登入的原因,提供解決方案,並推薦可靠的替代娛樂平台。
Leo娛樂城無法登入的三大原因
1. 帳號密碼問題
可能情況:最常見的原因是玩家輸入了錯誤的帳號或密碼,或者帳號被盜用。
解決方法:確認輸入的帳密正確,若仍無法登入,聯繫客服即可解決。
2. 網頁技術問題
可能情況:官網可能因伺服器維護或技術故障出現404錯誤,導致玩家無法登入。
解決方法:耐心等待官網修復,並清除瀏覽器快取和Cookie,或嘗試使用其他瀏覽器。
3. 九州娛樂城退出台灣市場
可能情況:隨著九州娛樂城宣布退出台灣市場,Leo娛樂城的營運也逐步停止,導致玩家無法再使用該平台。
影響:玩家可能面臨資金提取困難,需及時完成出金操作以保障自身利益。
娛樂城無法登入的緊急解決方案
核對帳號與密碼:確認輸入正確無誤,避免由於簡單疏忽造成的登入失敗。
檢查網路連線:確保網路穩定,必要時切換至更穩定的網路環境。
清除瀏覽器快取與Cookie:解決瀏覽器相關問題,恢復正常登入。
嘗試其他瀏覽器或設備:更換設備或使用不同瀏覽器,查看是否可以正常登入。
九州娛樂城退出台灣市場的原因
市場利潤有限:九州娛樂城認為台灣市場的競爭激烈且利潤有限,因此選擇退出並專注於其他海外市場。
資金生態鏈斷裂:現金交易平台出現問題,導致九州娛樂城難以維持正常運營。
母公司法律爭議:九州娛樂城母公司近年來因財務與法律問題受到高度關注,進一步影響旗下品牌的營運。
Leo娛樂城會員如何安全出金?
在九州娛樂城停止營運前,玩家需要立即完成出金操作以確保資金安全。
出金建議:
儘快登入官網提交提款申請,確保資金安全轉出。
若官網無法訪問,請嘗試聯繫客服,並保留交易證明以備進一步處理。
推薦替代平台:富遊娛樂城
隨著Leo娛樂城的退出,玩家可以考慮轉至穩定且值得信賴的替代平台,如富遊娛樂城。
富遊娛樂城的優勢
穩定可靠:擁有先進的技術和完善的資金保障機制,確保玩家的資金安全。
豐富的遊戲選擇:從電子遊戲到真人娛樂,滿足各類玩家的需求。
專屬優惠:特別為Leo會員推出等級升等計畫,並提供多樣化的專屬活動。
快速出金:高效的財務系統,讓玩家的存取款過程快速無憂。
Leo會員轉移至富遊的流程
登錄富遊官網完成註冊。
提供Leo娛樂城會員等級截圖與相關資料(如戶名、手機號碼)給富遊客服進行審核。
等待客服確認後,享受富遊娛樂城專屬的會員福利與優惠。
結語:立即加入富遊娛樂城,延續遊戲體驗!
Leo娛樂城的退出讓許多玩家感到困惑與擔憂,但選擇穩定、安全的娛樂平台是最明智的應對之道。富遊娛樂城憑藉其卓越的技術、專業的服務以及豐富的優惠,成為玩家的理想替代選擇。現在就註冊富遊娛樂城,享受無與倫比的遊戲體驗吧!
leo娛樂城登入
LEO娛樂城:九州娛樂集團的卓越創舉與運營優勢
九州娛樂集團總部的現代化運營
九州娛樂集團的總部設於菲律賓首都馬尼拉市中心最具代表性的現代化建築「RCBC Plaza」。這座辦公大樓融合了全球領先的建築科技與安全系統,包括:
國家級資訊防護系統,確保數據安全。
樓宇自動化系統 (BAS),實現數位化管理與高效運營,包括防火系統、光纖電訊和數位監控。
九州娛樂為員工提供舒適的工作環境和專業的教育培訓,確保服務水準一流。其員工以專業知識和親切態度,為尊貴玩家帶來極致的遊戲體驗。
LEO娛樂旗下品牌與遊戲特色
九州娛樂城旗下分支包括 LEO、THA 和 KU 三個娛樂品牌。雖然品牌各自獨立運營,但都共享相同的遊戲系統與界面,提供超過數千款來自全球頂級供應商的遊戲。
這些品牌的核心特色包括:
種類繁多的遊戲:滿足不同類型玩家的需求,包括電子遊戲、體育博彩、真人娛樂等。
不斷更新的內容:通過九州獨家技術,網站內容保持新鮮,合作商可快速更新遊戲資源,確保平台競爭力。
玩家在這些平台上可以隨時找到適合自己的遊戲,享受沉浸式的娛樂體驗。
LEO娛樂城的歷史與發展
九州娛樂集團的起源可以追溯至其最早期的經營模式:「信用版」賭博平台 《天下體育球版》。儘管該模式在當時非常流行,但因高風險與財務處理複雜而逐漸被淘汰。
隨著市場需求的變化,九州娛樂轉型為現金版平台 《天下現金網》,引入了更安全、便捷的遊戲模式。這種轉變不僅提升了玩家的體驗,也使平台得以進一步發展。
如今,九州娛樂城已成為台灣博弈業界的領軍者,不僅優化了網站版面與功能,還推出了專屬手機APP,方便玩家隨時隨地進行遊戲體驗。
LEO娛樂的成功秘訣
現代化運營與技術支持:九州娛樂在系統安全與技術更新方面投入巨大資源,確保平台運營穩定可靠。
多元化遊戲體驗:通過與全球頂級遊戲供應商合作,平台提供了豐富的遊戲選擇,適應各種玩家需求。
用戶至上:專業培訓的員工以細心和熱忱態度,提供高品質服務,讓玩家倍感尊重。
不斷創新:從信用版到現金版,再到手機APP,九州娛樂始終緊跟市場趨勢,致力於打造最佳娛樂體驗。
結語
LEO娛樂城作為九州娛樂集團旗下的重要品牌,不僅繼承了集團的核心價值,更在遊戲選擇、服務品質和技術創新方面不斷提升。從最初的《天下體育球版》到如今的行業領先者,LEO娛樂見證了九州娛樂城的發展歷程,也成為博弈行業中的耀眼明星。
加入 LEO娛樂城,感受來自九州娛樂集團的專業與熱情,享受最頂級的線上博弈體驗!
Leo娛樂城無法登入?全面解析與解決指南
近期,Leo娛樂城的玩家紛紛反映登入失敗的情況,引發了廣泛的討論與擔憂。作為九州娛樂城旗下知名品牌之一,Leo娛樂城在台灣的娛樂市場中擁有大量用戶。然而,隨著九州娛樂城宣布將於2024年12月31日退出台灣市場,這一消息無疑讓玩家對資金安全與遊戲體驗產生了極大疑慮。
本文將深入探討Leo娛樂城無法登入的原因,提供解決方案,並推薦可靠的替代娛樂平台。
Leo娛樂城無法登入的三大原因
1. 帳號密碼問題
可能情況:最常見的原因是玩家輸入了錯誤的帳號或密碼,或者帳號被盜用。
解決方法:確認輸入的帳密正確,若仍無法登入,聯繫客服即可解決。
2. 網頁技術問題
可能情況:官網可能因伺服器維護或技術故障出現404錯誤,導致玩家無法登入。
解決方法:耐心等待官網修復,並清除瀏覽器快取和Cookie,或嘗試使用其他瀏覽器。
3. 九州娛樂城退出台灣市場
可能情況:隨著九州娛樂城宣布退出台灣市場,Leo娛樂城的營運也逐步停止,導致玩家無法再使用該平台。
影響:玩家可能面臨資金提取困難,需及時完成出金操作以保障自身利益。
娛樂城無法登入的緊急解決方案
核對帳號與密碼:確認輸入正確無誤,避免由於簡單疏忽造成的登入失敗。
檢查網路連線:確保網路穩定,必要時切換至更穩定的網路環境。
清除瀏覽器快取與Cookie:解決瀏覽器相關問題,恢復正常登入。
嘗試其他瀏覽器或設備:更換設備或使用不同瀏覽器,查看是否可以正常登入。
九州娛樂城退出台灣市場的原因
市場利潤有限:九州娛樂城認為台灣市場的競爭激烈且利潤有限,因此選擇退出並專注於其他海外市場。
資金生態鏈斷裂:現金交易平台出現問題,導致九州娛樂城難以維持正常運營。
母公司法律爭議:九州娛樂城母公司近年來因財務與法律問題受到高度關注,進一步影響旗下品牌的營運。
Leo娛樂城會員如何安全出金?
在九州娛樂城停止營運前,玩家需要立即完成出金操作以確保資金安全。
出金建議:
儘快登入官網提交提款申請,確保資金安全轉出。
若官網無法訪問,請嘗試聯繫客服,並保留交易證明以備進一步處理。
推薦替代平台:富遊娛樂城
隨著Leo娛樂城的退出,玩家可以考慮轉至穩定且值得信賴的替代平台,如富遊娛樂城。
富遊娛樂城的優勢
穩定可靠:擁有先進的技術和完善的資金保障機制,確保玩家的資金安全。
豐富的遊戲選擇:從電子遊戲到真人娛樂,滿足各類玩家的需求。
專屬優惠:特別為Leo會員推出等級升等計畫,並提供多樣化的專屬活動。
快速出金:高效的財務系統,讓玩家的存取款過程快速無憂。
Leo會員轉移至富遊的流程
登錄富遊官網完成註冊。
提供Leo娛樂城會員等級截圖與相關資料(如戶名、手機號碼)給富遊客服進行審核。
等待客服確認後,享受富遊娛樂城專屬的會員福利與優惠。
結語:立即加入富遊娛樂城,延續遊戲體驗!
Leo娛樂城的退出讓許多玩家感到困惑與擔憂,但選擇穩定、安全的娛樂平台是最明智的應對之道。富遊娛樂城憑藉其卓越的技術、專業的服務以及豐富的優惠,成為玩家的理想替代選擇。現在就註冊富遊娛樂城,享受無與倫比的遊戲體驗吧!
富遊娛樂城:全台灣第一推薦的娛樂城體驗
富遊娛樂城是全台灣最受玩家推崇的娛樂平台,提供多種優惠、豐富的遊戲選擇以及高品質的服務,讓每位玩家都能享受極致的遊戲樂趣。無論您是新手還是資深玩家,富遊娛樂城都能滿足您的需求,帶給您刺激的遊戲體驗。
富遊娛樂城的優惠活動
1. 免費娛樂城體驗金
新玩家只需註冊即可免費領取 $168 元的體驗金,無需存款,便可立即體驗各種遊戲,感受遊戲帶來的樂趣。
2. 首次存款優惠
首次存款 $1000 元,即送 $1000 元遊戲金,讓您在遊戲中有更多機會獲勝,增添刺激感。
3. 新會員五選一好禮
新加入的會員可選擇五種專屬好禮之一,從遊戲金到實體獎品,讓您的遊戲旅程更加多彩。
熱門遊戲種類
富遊娛樂城提供多樣化的遊戲選擇,適合不同喜好的玩家:
電子老虎機:多款創新遊戲,讓玩家享受豐厚獎金機會。
百家樂:與真人荷官互動,體驗真實賭場的刺激感。
今彩539 和 運彩:滿足彩票愛好者的需求。
捕魚遊戲:RSG電子捕魚遊戲帶來娛樂與獎金的雙重樂趣。
熊貓體育:專為體育迷設計的多種運動投注選項。
優質服務保證
1. 出金快速、安全可靠
富遊娛樂城採用先進的財務處理系統,保證快速存提款,讓玩家不用擔心資金安全問題。
2. 技術先進的APP平台
流暢的畫面設計與設備兼容性,讓用戶輕鬆上手。
持續升級與更新,提供更多創新遊戲類型。
3. 24小時專業客服
專業客服隨時待命,為玩家解答所有遊戲相關問題,確保您在任何時間都能享受到無縫的服務體驗。
4. 安全防護到位
採用最嚴格的安全管理系統與數據加密技術,保障用戶資金與個人信息的安全。
富遊娛樂城APP:隨時隨地盡享遊戲
富遊娛樂城APP提供了便捷的遊戲途徑,支援 IOS 與 Android 設備。
簡潔易用的界面設計,讓玩家快速找到喜愛的遊戲。
即時客服支援,隨時解決您的疑問。
免費下載,無論何時何地,您都能盡享娛樂城的精彩遊戲體驗。
為什麼選擇富遊娛樂城?
優惠豐富:免費體驗金、首存優惠、新會員好禮,活動多樣。
遊戲種類齊全:從電子遊戲到真人百家樂,滿足各類玩家需求。
服務至上:專業客服、快速出金和高安全保障讓玩家安心享受遊戲。
便捷平台:富遊APP提供隨時隨地的遊戲樂趣,讓您不受限制。
立即下載 富遊娛樂城APP,體驗全台灣第一娛樂城的無限魅力!不論您是想享受遊戲樂趣還是贏取豐厚獎金,富遊娛樂城都將是您的最佳選擇!
AmoxilPharm: Amoxil Pharm Store – AmoxilPharm
Gabapentin Pharm: neurontin 1800 mg – neurontin online pharmacy
ivermectin 3: Ivermectin Pharm Store – Ivermectin Pharm
http://ivermectinpharm.store/# Ivermectin Pharm Store
https://paxlovid.ink/# Paxlovid buy online
rybelsus generic Buy compounded semaglutide online rybelsus generic
想要享受最刺激的線上娛樂體驗?選擇RG富遊娛樂城!
在這個數位時代,尋找適合自己的線上娛樂城變得越來越重要。如果你想在線上娛樂城找到最好玩、最受歡迎的遊戲,RG富遊絕對是你的首選!我們匯聚了多款博弈遊戲,讓您盡情享受娛樂的刺激與快感。
#### 精選遊戲類型
1. 真人百家樂
感受現場賭場的氛圍!RG富遊的真人視訊百家樂讓你與真實的荷官互動,享受高品質的遊戲體驗。無論你是老手還是新手,都能在這裡找到適合自己的節奏。
2. 電子老虎機
喜愛刺激嗎?我們提供多款熱門老虎機遊戲,如「戰神賽特」「魔龍傳奇」等,每一款都擁有精美的畫面和豐厚的獎勵,讓每次旋轉都充滿期待!
3. 體育投注
無論你是球迷還是賽事愛好者,RG富遊的體育投注平台都有你想要的。精準的賠率、廣泛的賽事選擇,讓你體驗到投注的樂趣和刺激。
4. 彩票遊戲與捕魚遊戲
隨著運氣來!不妨試試我們的棋牌遊戲和彩票遊戲,這裡有各種機會讓你體驗到不一樣的勝利快感。
#### 下載富遊APP,隨時隨地享受遊戲!
富遊娛樂城的APP支持iOS和Android設備,界面簡潔易用,內容豐富。無論你身處何地,都能輕鬆進入遊戲世界,隨心所欲享受娛樂時光。此外,我們提供即時客服支援,隨時解決你的疑問,讓你的遊戲體驗更為完美。
#### 為什麼選擇RG富遊?
– 安全可靠
RG富遊致力於為玩家提供安全可靠的遊戲環境,您的每一筆交易都受到最高的安全保障。
– 快速便捷的存提款服務
我們的存款平均時間僅需30秒,提款平均只需60秒,讓你體驗即時的快感!
– 豐富多元的遊戲選擇
超過9999款遊戲任你選擇,無論你偏好哪種娛樂方式,這裡都能滿足你的需求。
– 全天候服務
我們全年365天提供不停歇的客服支持,確保你在遊戲過程中享受到最好的服務。
### 結論
RG富遊娛樂城是您最佳的線上賭場選擇。我們不僅提供多樣化的遊戲選擇,還有安全可靠的遊戲環境和便捷的服務。立即下載APP,加入RG富遊,開始您的娛樂之旅,享受無與倫比的遊戲體驗吧!
Ivermectin Pharm: ivermectin 200 mcg – Ivermectin Pharm
滿天星娛樂城:全方位的線上娛樂體驗
滿天星娛樂城作為九州娛樂城旗下品牌之一,是玩家心目中備受信賴的線上娛樂平台。不僅提供豐富的遊戲選擇和吸引人的優惠,還以其合法營運的背景和快速出金的保證,成為台灣玩家的首選娛樂城之一。
滿天星娛樂城的特色與優勢
1. 合法營運,安全可靠
滿天星娛樂城持有三大國際娛樂執照,包括:
菲律賓 PAGCOR 監督競猜牌照
BVI 認證
馬爾他 MGA 認證
這些執照展現了平台的資本實力與合法性,讓玩家能夠安心享受遊戲,完全不用擔心出金問題。
2. 高品質遊戲與豐富優惠
滿天星娛樂城與九州集團旗下的LEO、THA娛樂城一樣,提供一流的遊戲品質和多樣化的優惠。
熱門遊戲推薦:電子老虎機、真人直播、運彩投注、今彩539等,滿足各類玩家需求。
優惠活動:定期推出限時回饋活動,讓玩家在遊戲中獲得更多額外收益。
3. 快速出金與高效服務
滿天星娛樂城採用先進的財務系統,確保存提款快速、安全,讓玩家能夠專注於享受遊戲,而無需擔心資金問題。專業的客服團隊24小時在線,提供即時支援。
4. 多平台兼容的APP
滿天星娛樂城提供設計貼合玩家需求的手機APP,適用於iOS與Android系統。玩家可以隨時隨地快速登入遊玩,體驗無與倫比的娛樂快感。
熱門遊戲類型一覽
滿天星娛樂城為玩家提供多樣化的遊戲選擇,滿足不同興趣和需求:
真人直播:感受真實賭場氛圍,與真人荷官互動,體驗沉浸式娛樂。
電子老虎機:多款經典與創新機台,讓玩家享受刺激的遊戲過程。
運彩投注:涵蓋世界盃、棒球、籃球等多項運動賽事,提供即時投注與直播功能。
彩票遊戲:包括今彩539、富遊彩票等,適合熱衷於彩券的玩家。
滿天星娛樂城常見問題解答
Q1. 滿天星娛樂城是否提供APP下載?
是的,滿天星娛樂城提供專屬的手機APP,支援iOS和Android系統。玩家可透過APP快速登入,隨時享受遊戲。
Q2. 滿天星娛樂城是否合法?
滿天星娛樂城擁有多項國際認證,為合法經營的線上娛樂城,並保證玩家資金的安全性與遊戲的公平性。
Q3. 無法登入滿天星娛樂城該怎麼辦?
如遇到無法登入的情況,建議玩家檢查網路狀況或聯繫24小時客服團隊獲取即時協助。
立即加入滿天星娛樂城,享受最佳娛樂體驗!
滿天星娛樂城以合法、安全、快速的服務,搭配豐富的遊戲選擇和優惠,為玩家打造出獨一無二的線上娛樂體驗。不論您是新手還是老手,都能在滿天星找到屬於自己的遊戲樂趣。現在就下載APP,隨時隨地開始您的娛樂之旅!
п»їpaxlovid Paxlovid over the counter or paxlovid cost without insurance
http://www.calyptic.com/cgi-bin/archive2.cgi?cat=1&start=31&referer=http://paxlovid.ink paxlovid india
paxlovid pharmacy Paxlovid buy online and buy paxlovid online paxlovid generic
娛樂城
富遊娛樂城:台灣線上賭場的創新選擇
在台灣的線上娛樂市場中,富遊娛樂城憑藉其優質的服務和多樣化的娛樂選項,迅速成為新一代玩家的首選。既有刺激的遊戲體驗,又提供了一系列具有吸引力的優惠,富遊娛樂城無疑是追求樂趣的玩家們理想的地方。
#### 優勢與特色
1. 多樣化的遊戲選擇
富遊娛樂城提供了豐富的遊戲種類,包括電子老虎機、真人百家樂、運彩投注等。不論你是老虎機的愛好者,還是熱衷於真人賭桌的玩家,這裡都能滿足你的需求。特別推薦的遊戲如DG真人百家和RSG皇家百家樂,一定能帶給你極致的遊戲體驗。
2. 吸引人的優惠活動
富遊娛樂城針對新手和老玩家都推出了多樣的優惠活動。新會員首次存款可獲得相同金額的獎金,還有額外的體驗金送出,設計友好且實惠。此外,富遊每週的簽到獎勵和天天無上限的返水活動,更是讓玩家在遊戲中能持續獲得收益。
3. 安全可靠的存取款方式
在金融交易方面,富遊娛樂城支持多種存款和提款方式,包括各大銀行轉帳、超商儲值,甚至虛擬貨幣等。最低提款金額設置靈活,保證玩家資金的安全與便捷。
4. 人性化的操作介面
富遊娛樂城的網頁與移動應用設計簡潔易用,玩家可以方便地找到所需的遊戲和優惠。無論是在電腦還是手機上,都能順利享受到遊戲的樂趣。
#### 如何開始遊戲
如果你是新手玩家,開始你的富遊體驗非常簡單。只需註冊帳號,完成首次存款,即可獲得新手優惠。無論你想嘗試電子遊戲還是真人賭局,富遊娛樂城都能讓你輕鬆上手,享受娛樂的樂趣。
#### 社會責任與防沉迷措施
富遊娛樂城深知線上賭博的風險,因此致力於推廣負責任的遊戲文化。他們提供“防沉迷”指導,以確保玩家能夠理性投注,並提供必要的幫助和資源來幫助有需求的玩家。
#### 總結
對於尋求安全、便捷和多元化娛樂城體驗的玩家,富遊娛樂城絕對是值得選擇的選項。無論你是希望徹底放鬆,還是想要體驗刺激的博弈過程,富遊娛樂城都能滿足你的期待,成為你在台灣線上娛樂市場的最佳夥伴。立即註冊並開始你的遊戲之旅吧!
x645396
original site l2026093
neurontin 330 mg neurontin capsules 100mg or brand neurontin 100 mg canada
https://www.google.com.na/url?q=https://gabapentinpharm.com neurontin 4000 mg
gabapentin 600 mg generic neurontin 300 mg and neurontin 800 mg neurontin 200 mg
Buy compounded semaglutide online buy rybelsus or cheap Rybelsus 14 mg
https://images.google.cf/url?q=https://semaglutidepharm.com buy semaglutide online
Buy semaglutide pills rybelsus cost and cheap Rybelsus 14 mg semaglutide pharm
LEO娛樂城無法登入!九州娛樂宣布退出台灣市場
近期,LEO娛樂城和THA娛樂城的玩家發現頻繁出現無法登入的情況,引發了廣泛討論。九州娛樂城官方也正式宣布,將於2024年12月31日中午12:00停止在台灣的營運,並結束所有相關服務。這一消息對於忠實玩家來說無疑是一大震撼,為什麼九州娛樂城會退出台灣市場?玩家該如何應對?本文將為您逐一解析。
LEO娛樂城無法登入的原因
1. 帳號與密碼問題
最常見的情況是玩家輸入的帳號或密碼有誤,或者帳號遭到盜用。
解決方案:聯繫LEO娛樂城的客服,提供必要的驗證信息,即可快速恢復帳戶。
2. 網站技術故障
LEO娛樂城可能因官網維護或伺服器異常導致無法正常運作。
解決方案:耐心等待技術問題修復,通常不會影響長時間使用。
3. 九州娛樂退出台灣市場
LEO娛樂城隸屬於九州娛樂集團,近期官方公告將於2024年12月31日結束營運,退出台灣市場。這是導致登入失敗的最終原因,玩家將無法再使用該平台的服務。
九州娛樂城退出台灣市場的原因分析
1. 市場規模限制
九州娛樂認為台灣市場過於狹小,品牌競爭激烈,成長空間有限。相比之下,海外市場不僅擁有更大的人口基數,也提供更高的獲利潛力。九州旗下的KU體育曾在國際足球五大聯賽中曝光,這顯示其具備強大的國際競爭力,轉向海外市場發展成為合理選擇。
2. 金流問題與法規壓力
LEO娛樂城的運營高度依賴第三方金流公司,而近期陳政谷案件的爆發使整個金流生態鏈受到嚴重打擊。警方揭露,九州娛樂集團利用第三方支付進行資金洗錢,甚至牽涉到內部人員洩密,對整個集團的形象和運營造成巨大衝擊。
3. 品牌聲譽受損
隨著案件的曝光,九州娛樂集團的聲譽遭到前所未有的挑戰。為了平息風波,九州選擇退出台灣市場,以避免進一步的法律與政策風險。
九州娛樂城停止營運的公告
根據LEO娛樂城官方的公告,九州娛樂城將於2024年12月31日中午12:00全面停止台灣市場的營運:
停止存款功能:玩家將無法再進行任何存款操作。
正常開放提款:官方承諾保證提款功能正常運作,會員可安心提取資金。
感謝支持:九州娛樂對於玩家多年來的支持表示衷心感謝,並祝福所有會員未來一切順利。
玩家應對策略:如何保護自身利益?
1. 儘速完成提款
如您的帳戶仍可正常登入,建議立即提交提款申請,確保資金安全轉出。
2. 提高警覺,避免詐騙
隨著九州娛樂退出,市場可能會出現冒充LEO娛樂城或THA娛樂城的假冒平台,玩家需保持高度警覺。
3. 選擇可靠的替代平台
對於希望延續遊戲體驗的玩家,可以考慮轉向信譽良好的國際娛樂平台,例如Bet365台灣,該平台提供穩定的服務和多樣化的遊戲選擇,是九州娛樂的理想替代方案。
結語:九州娛樂的結束,玩家的新起點
九州娛樂城的退出標誌著一個時代的結束,但對於玩家而言,也是一個選擇新平台的契機。未來,選擇安全、穩定且合法的娛樂平台將成為玩家的首要考量。無論如何,希望所有玩家都能在新的平台中繼續享受遊戲的樂趣,保障自身利益不受損失!
https://paxlovid.ink/# paxlovid pill
Amoxil Pharm Store AmoxilPharm Amoxil Pharm Store
Gabapentin Pharm: how much is neurontin pills – neurontin 100mg discount
neurontin 400 neurontin 300 mg tablets or neurontin brand name 800mg best price
https://maps.google.com.my/url?q=https://gabapentinpharm.com neurontin capsules 600mg
neurontin 900 mg neurontin tablets no script and neurontin generic neurontin 300mg
п»їpaxlovid paxlovid generic or paxlovid price
https://maps.google.com.lb/url?q=https://paxlovid.ink buy paxlovid online
paxlovid pill paxlovid india and Paxlovid buy online paxlovid price
https://paxlovid.ink/# Paxlovid.ink
stromectol 3 mg dosage minocycline efectos secundarios or cost of ivermectin medicine
https://images.google.cd/url?q=https://ivermectinpharm.store stromectol order
minocycline 100mg pills online ivermectin malaria and order minocycline 50 mg minocycline 100mg without prescription
Beylikdüzü escort dünyası, estetik ve zarafetin buluştuğu bir yer. Bu yazıda Kainat Güzeli Asena’nın hikayesini keşfedeceksiniz.
minocycline 100 mg without a doctor minocycline hcl or ivermectin online
http://clients5.google.com/url?q=http://ivermectinpharm.store::: minocycline 50mg tablets for human
generic stromectol minocycline 100mg capsule and minocycline 100mg without doctor minocycline 50 mg tablets for humans for sale
paxlovid buy: Paxlovid.ink – paxlovid covid
Dünyaca ünlü büyükçekmece escort sitesine hoşgeldiniz burda kendinize uygun bir kaliteli eş adayı bulabilirsiniz
AmoxilPharm Amoxil Pharm Store Amoxil Pharm Store
Beylikdüzü escort dünyası, estetik ve zarafetin buluştuğu bir yer. Bu yazıda Kainat Güzeli Asena’nın hikayesini keşfedeceksiniz.
paxlovid covid Paxlovid.ink Paxlovid.ink
Dünyaca ünlü büyükçekmece escort sitesine hoşgeldiniz burda kendinize uygun bir kaliteli eş adayı bulabilirsiniz
十字架3
魔法少女まどか☆マギカ
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
お見事!サブちゃん
https://www.k8.beauty/promotion/id3427
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にボーナス時の音が最高。
大工の源さん桜満開!源 DREAM ver.~
[url=https://sites.google.com/view/p-a-certain-magical-index-1-1/]k8 カジノ P とある魔術の禁書目録
[/url]
革命機ヴァルヴレイヴ
CR蒼天の拳天帰 Ver.319(1:1)
CRフィーバー戦姫絶唱シンフォギア
CR牙狼FINAL Ver.399
https://www.k8pachinko.net/Articles/3005.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
Pフィーバーダンベル何キロ持てる
https://www.k8.rodeo/Articles/2009.html
パチンコの演出は、時に驚かされることがあります。毎回新しい発見があります。
CR 呪怨
https://www.pachinko.wiki/article/109.html
美しいグラフィックと音楽が、プレイ中の没入感を高めます。
lisinopril no prescription: lisinopril 10mg prices compare – lisinopril price
вывод из запоя в стационаре вывод из запоя в стационаре .
купить кухни москва купить кухни москва .
вывод из запоя нижний новгород вывод из запоя нижний новгород .
выведение из запоя в стационаре выведение из запоя в стационаре .
zestril 30 mg: lisinopril 5 mg tablet cost – lisinopril 50 mg tablet
https://lisinoprilus.com/# lisinopril 40 mg discount
Мостбет Узбекистан – это ваш надежный партнер в мире ставок | Mostbet min kod дает дополнительные преимущества | Mostbet Uz регистрация открывает доступ к бонусам | Mostbet регистрация позволит вам начать играть уже сегодня | Скачайте Mostbet apk и начните выигрывать уже сегодня most bet. | Скачайте Mostbet приложение и наслаждайтесь игрой | Mostbet uz registratsiya ochiq va oson | Mostbet официальный сайт предлагает безопасную игру | Mostbet регистрация дает доступ к уникальным предложениям https://mostbetcasinouzkirishgderf.com.
http://ciprofloxacin.cheap/# ciprofloxacin 500mg buy online
zithromax pill buy zithromax 500mg online zithromax for sale online
Временная регистрация в СПб: Быстро и Легально!
Ищете, где оформить временную регистрацию в Санкт-Петербурге?
Мы гарантируем быстрое и легальное оформление без очередей и лишних документов.
Ваше спокойствие – наша забота!
Минимум усилий • Максимум удобства • Полная легальность
Свяжитесь с нами прямо сейчас!
Временная регистрация
buy cipro no rx buy generic ciprofloxacin where can i buy cipro online
Fildena: Find Fildena at Grant Pharmacy, an powerful ED remedy that enhances sexual overall performance and pride. Known for dependable results, Fildena is a famous choice among men. Grant Pharmacy ensures exceptional and affordability for ED treatments like Fildena. Fildena
zithromax z-pak price without insurance azithromycin zithromax zithromax coupon
Ivermectin: Ivermectin, available at Grant Pharmacy, is strong in opposition to parasitic infections. Known for its effective motion, Ivermectin aids healing. Choose Grant Pharmacy for outstanding antiparasitics. Ivermectin
Aciclovir: Aciclovir from Grant Pharmacy offers antiviral assist for herpes infections. Known for its powerful action, Aciclovir permits control signs and symptoms and symptoms and signs and signs and symptoms. Order from Grant Pharmacy for incredible antivirals. Aciclovir
Grant Pharmacy is a depended on online pharmacy committed to extraordinary customer service and excellent care. Our group of dedicated pharmacists gives personalized attention to satisfy all your health needs. Count on Grant Pharmacy for accurate prescriptions, over the counter medicinal drugs, and wellbeing help in a pleasant, expert surroundings. Grantpharmacy
order cheap clomid without prescription: how to buy cheap clomid – get clomid without a prescription
https://ciprofloxacin.cheap/# buy cipro online canada
http://lisinoprilus.com/# buy zestril online
zithromax 500 mg for sale purchase zithromax online zithromax online usa
order cytotec online: buy cytotec online – buy cytotec online
Best UK IPTV Subscription, Enjoy Bestiptvuk
streaming experience with excellent image quality up to 4K, on any device, anytime and anywhere, with over than +30,000 channels, +100,000 VOD and uptime 100%
https://clomid.store/# clomid cost
lisinopril 40 mg india: lisinopril online without a prescription – lisinopril 5 mg tablet price in india
buy cytotec Misoprostol 200 mg buy online buy misoprostol over the counter
how to buy zithromax online cheap zithromax pills can you buy zithromax over the counter in mexico
九州娛樂城與Leo娛樂城即將結束運營,玩家該如何應對?
近期娛樂城行業的消息引發了玩家的廣泛關注,其中「Leo娛樂城無法登入」和「九州娛樂城即將倒閉」成為熱門話題。根據官方公告,九州娛樂城及其旗下品牌,包括Leo娛樂城和THA娛樂城,將於2024年12月31日中午12點正式結束營業並關閉官網。這一消息不僅讓玩家感到震驚,也暴露出行業潛在的問題。
Leo娛樂城無法登入的原因
Leo娛樂城近期頻繁出現登入問題,這與其即將退出台灣市場的計劃息息相關。以下是造成登入困難的主要原因:
帳號或密碼問題
許多玩家因忘記密碼或帳號被盜導致登入失敗。然而,由於官方客服系統已停止服務,這些問題無法得到解決。
網頁技術問題
官網伺服器的不穩定或維護期間,可能出現「404錯誤」,讓玩家無法正常登入。
營運關閉計劃
隨著九州娛樂城宣布結束營業,Leo娛樂城的伺服器和備用網址也逐步停止運行,這是玩家無法登入的最大原因。
為何九州娛樂城退出台灣市場?
九州娛樂城決定退出台灣市場的原因可以歸納為以下幾點:
市場收益不足
台灣市場對九州娛樂城而言並不具備高盈利性,難以支撐長期運營。
第三方支付系統受阻
多次傳出第三方現金支付渠道中斷,影響存提款操作。
法律風險增加
九州娛樂城頻繁遭遇法律問題及負面新聞,導致營運壓力倍增。
玩家需要注意什麼?
隨著九州娛樂城及其旗下品牌逐步退出市場,玩家應及時採取以下措施:
提領餘額
官方已停止存款服務,但提領功能仍開放。玩家應儘快提領餘額,避免損失。
轉移到穩定平台
針對Leo娛樂城和九州娛樂城的退出,玩家可選擇更穩定且具信譽的平台,以保障自己的娛樂體驗。
防範詐騙
小心冒名客服或假網站,官方已停止客服系統,不會主動聯繫玩家索取個人資料。
未來哪些娛樂城值得信賴?
隨著九州娛樂城退出市場,玩家可以關注一些以穩定性和合法性著稱的娛樂平台。選擇時應注意以下幾點:
合法牌照
選擇擁有合法經營許可的娛樂平台。
穩定的支付系統
平台應提供多種穩定且快速的支付方式。
用戶口碑
瀏覽玩家的評價和反饋,選擇高評價的平台。
結論
九州娛樂城及其旗下品牌的關閉標誌著一個時代的結束,同時也提醒玩家關注行業的合規性和穩定性。在此轉型時期,玩家應保持警惕,選擇可靠的平台,以確保自己的資金和遊戲體驗不受影響。
buy cipro online canada: cipro online no prescription in the usa – cipro pharmacy
dünyaca ünlü kalitenin tek adresi halkalı escort atakent escort sitesine hoşgeldiniz
https://cytotec.top/# Abortion pills online
Вопросы и ответы: можно ли быстро купить диплом старого образца?
Pendik Escort Kurtköy Escort keyifli anlar yaşamak isteyenler için ideal seçenekler sunuyor. Pendik escort bayanlar arasından seçim yaparak, farklı deneyimler keşfedebilirsiniz.
dünyaca ünlü kalitenin tek adresi halkalı escort atakent escort sitesine hoşgeldiniz
Pendik Escort Kurtköy Escort keyifli anlar yaşamak isteyenler için ideal seçenekler sunuyor. Pendik escort bayanlar arasından seçim yaparak, farklı deneyimler keşfedebilirsiniz.
http://cytotec.top/# buy cytotec pills
lisinopril 20 mg price generic for zestril lisinopril 20 mg for sale
Портал строительства https://mbmedicall.com ресурс для поиска информации о ремонте и строительстве. Полезные советы, технологии, инструкции и рекомендации для профессионалов и новичков.
Временная регистрация в Санкт-Петербурге: Быстро и Легально!
Ищете, где оформить временную регистрацию в СПб?
Мы гарантируем быстрое и легальное оформление без очередей и лишних документов.
Ваше спокойствие – наша забота!
Минимум усилий • Максимум удобства • Полная легальность
Свяжитесь с нами прямо сейчас!
Временная регистрация в СПб
order zithromax without prescription: can you buy zithromax over the counter – where to get zithromax over the counter
снятие запоя на дому снятие запоя на дому .
капельница от запоя капельница от запоя .
нарколог на дом вывод из запоя цена нарколог на дом вывод из запоя цена .
https://clomid.store/# cost generic clomid pill
капельница от запоя на дому нижний новгород капельница от запоя на дому нижний новгород .
капельница наркология 24 капельница наркология 24 .
九州娛樂城倒了
九州娛樂城退出台灣市場:LEO娛樂城無法登入的背後真相
LEO娛樂城,作為台灣線上娛樂城市場的重要品牌,長期以來在廣告投放和代理推廣方面占據領先地位。然而,近期出現的「LEO娛樂城無法登入」現象引發了廣泛的網路討論。不少玩家質疑:「九州娛樂城是不是倒了?」「這是不是詐騙?」事實上,這些猜測與謠言並不完全準確。根據最新消息,九州娛樂城已決定結束其在台灣的業務,包括旗下品牌LEO娛樂城和THA娛樂城。以下我們將深入分析這一事件的背景和可能的影響。
LEO娛樂城無法登入的原因分析
近期LEO娛樂城頻繁出現無法登入的情況,其實是九州娛樂城退出台灣市場的前兆。根據官方公告,九州娛樂城將於2024年12月31日中午12點正式關閉,包括註冊與存款功能在內的多項服務已經受到限制。
可能原因 1:退出台灣市場的策略
九州娛樂城的管理層認為,台灣市場的利潤已達上限,長期運營的收益減少,因此選擇「見好就收」。這一策略符合台灣商業文化中尋找新市場發展的特點。九州娛樂城可能計劃將資源投入其他更具潛力的市場,以追求更大的收益。
可能原因 2:運營生態鏈的問題
LEO娛樂城的運營高度依賴第三方現金交易平台以及相關支付管道。近期,這些環節可能出現了無法解決的問題,導致整體運營受阻。為了避免損失擴大,九州娛樂城選擇退出台灣市場。
可能原因 3:法律風險與負面新聞
九州娛樂城因涉及法律事件,曾多次登上新聞版面。這些事件不僅損害了品牌形象,也讓經營面臨更多風險。為了降低法律糾紛帶來的影響,九州娛樂城可能選擇結束在台灣的營運。
官方公告:2024年12月31日正式停業
九州娛樂城已向所有LEO娛樂城會員發布公告,明確指出將於2024年12月31日中午12點全面停止營運。公告內容包括以下重要信息:
存款服務已關閉:玩家無法繼續充值。
提款功能仍正常:玩家可以放心提取餘額,會員權益不受影響。
感謝支持:官方對會員表達了感謝,並祝福大家未來順利。
這一公告讓玩家可以在剩餘時間內完成提款操作,避免資金損失。
對玩家的影響與建議
1. 儘快提款
如果您是LEO娛樂城的會員,應立即登入帳戶並提取餘額,以確保資金安全。
2. 謹慎選擇新平台
選擇新的娛樂平台時,應注意以下幾點:
合法性:確認平台是否擁有合法經營許可。
支付系統穩定性:確保提款及充值渠道安全無虞。
用戶評價:參考其他玩家的反饋,選擇口碑良好的平台。
3. 防範詐騙
警惕假冒的LEO娛樂城網站或客服。官方客服系統已關閉,任何聯繫您索取個人資料的行為都可能是詐騙。
結語
LEO娛樂城無法登入以及九州娛樂城退出台灣市場,標誌著線上娛樂城行業的一次重要轉折。對於玩家而言,這既是一個挑戰,也是一個重新審視平台選擇的機會。未來,玩家應更加關注平台的合法性與穩定性,為自己的遊戲體驗和資金安全提供保障。
https://lisinoprilus.com/# zestril 10 mg tablet
buy cytotec online buy misoprostol over the counter purchase cytotec
cost clomid without rx: where to buy cheap clomid without prescription – buying clomid without prescription
3A娛樂城:全方位線上娛樂體驗的首選平台
3A娛樂城作為台灣最受歡迎的線上娛樂平台之一,以其多樣化的遊戲選擇、創新的技術、以及卓越的安全保障,贏得了玩家的高度信任與青睞。無論您是新手還是老手,3A娛樂城都能滿足您的需求,帶給您獨一無二的遊戲體驗。
熱門娛樂城遊戲一覽
3A娛樂城提供多種熱門遊戲,適合不同類型的玩家:
真人百家樂:體驗真實賭場的氛圍,與真人荷官互動,感受賭桌上的緊張與刺激。
彩票投注:涵蓋多種彩票選項,滿足彩券愛好者的需求,讓您隨時隨地挑戰幸運。
棋牌遊戲:從傳統的麻將到現代化的紙牌遊戲,讓玩家在策略與運氣中找到平衡。
3A娛樂城的核心價值
1. 專業
3A娛樂城每日提供近千場體育賽事,並搭配真人百家樂、彩票彩球、電子遊戲等多種類型的線上賭場遊戲。無論您的遊戲偏好為何,這裡總有一款適合您!
2. 安全
平台採用128位加密技術和嚴格的安全管理體系,確保玩家的金流與個人資料完全受保護,讓您可以放心遊玩,無需擔憂安全問題。
3. 便捷
3A娛樂城是全台第一家使用自家開發的全套終端應用的娛樂城平台。無論是手機還是電腦,玩家都能享受無縫的遊戲體驗。同時,24小時線上客服隨時為您解決任何問題,提供貼心服務。
4. 安心
由專業工程師開發的財務處理系統,為玩家帶來快速的存款、取款和轉帳服務。透過獨立網路技術,平台提供優化的網路速度,確保每一次操作都流暢無比。
下載3A娛樂城手機APP
為了提供更便捷的遊戲體驗,3A娛樂城獨家開發了功能齊全的手機APP,讓玩家隨時隨地開啟遊戲。
現代化設計:新穎乾淨的介面設計,提升使用者體驗。
跨平台支持:完美適配手機與電腦,讓您輕鬆切換設備,無需中斷遊戲。
快速下載:掃描專屬QR碼即可進入下載頁面,立即開始暢玩!
3A娛樂城的其他服務
娛樂城教學:詳細的操作指導,讓新手也能快速上手。
責任博彩:提倡健康娛樂,確保玩家在遊戲中享受樂趣的同時,不失理性。
隱私權政策:嚴格遵守隱私保護條例,保障玩家的個人信息安全。
立即加入3A娛樂城,享受頂級娛樂體驗!
如果您正在尋找一個專業、安全、便捷又安心的線上娛樂平台,那麼3A娛樂城絕對是您的不二之選。現在就下載手機APP,隨時隨地開啟您的娛樂旅程,享受無限的遊戲樂趣與刺激!
2024 年熱門娛樂城與活動解析
冠天下娛樂城優惠,帶來無限樂趣
在 2024 年 4 月 16 日,冠天下娛樂城推出了全新的優惠活動,旨在為玩家帶來更大的獲利機會和更高的娛樂體驗。這些優惠活動包括多種存款獎勵、會員專屬回饋以及豐富的遊戲競賽獎金,吸引了大批玩家參與。
冠天下娛樂城以其高透明度和用戶友好的政策,已成為許多玩家的首選娛樂平台。不論是新手還是老手,都能在這裡找到適合自己的遊戲和活動。
LEO娛樂城無法登入引發市場恐慌
2024 年 11 月 29 日,LEO娛樂城因技術問題出現短暫無法登入的情況,引發了廣泛關注。許多玩家擔心這是否意味著九州娛樂城的營運出現問題。
九州娛樂集團隨後發佈官方聲明,強調這僅僅是系統升級過程中的短暫問題,並承諾玩家的資金和數據安全不受任何影響。同時,九州娛樂表示將加強伺服器穩定性,確保未來類似情況不再發生。
熱門 ZG 電子遊戲——熱舞森巴老虎機
2024 年 11 月 8 日,ZG 電子遊戲推出了熱舞森巴老虎機,這款遊戲迅速成為市場上的熱門選擇。以南美熱情的森巴舞為主題,遊戲畫面充滿色彩,搭配動感的音樂,為玩家帶來視覺與聽覺的雙重享受。
遊戲亮點:
高倍數獎勵機制:每次轉盤都有機會觸發高額獎金。
特色免費遊戲:連續獲得特殊圖案即可啟動多次免費轉盤,提高獲勝機率。
適合新手與高端玩家:簡單的玩法規則與豐富的策略選擇滿足不同層級玩家的需求。
ZG 電子遊戲致力於為玩家提供創新且有趣的遊戲體驗,熱舞森巴老虎機無疑是今年最值得嘗試的遊戲之一。
2024 世界棒球 12 強賽精彩回顧
2024 年 11 月,中華隊參加了世界棒球 12 強賽,這項全球頂尖棒球賽事吸引了來自多國的球隊和球迷參與。中華隊 28 人的名單以年輕選手和經驗豐富的老將為基礎,展現了團隊的競技實力。
比賽亮點:
精彩賽程:比賽期間,中華隊展現了堅強的防守和出色的打擊表現。
門票銷售:賽事門票迅速售罄,顯示出棒球在台灣的高度受歡迎程度。
線上直播:各大平台提供了高畫質直播,讓更多球迷能夠即時觀看比賽,感受棒球魅力。
世界棒球 12 強賽不僅是比賽,更是全台灣球迷的一場盛宴,展現了棒球作為國民運動的無限魅力。
結語
從娛樂城的優惠活動到全球性的棒球賽事,2024 年對於遊戲和體育愛好者來說充滿了亮點。不論是參與冠天下娛樂城的優惠,體驗 ZG 電子遊戲的最新老虎機,還是為中華隊的棒球比賽加油,這一年都是充滿精彩與期待的一年!
https://azithromycinus.com/# zithromax capsules australia
buy cipro without rx: buy cipro cheap – cipro for sale
娛樂城
2024 年娛樂城遊戲推薦:最夯遊戲與最佳平台選擇
隨著線上娛樂城市場的蓬勃發展,2024 年推出了多款令人興奮的遊戲和創新平台。無論是喜歡老虎機、棋牌遊戲,還是捕魚和彩票遊戲的玩家,都能在今年找到屬於自己的遊戲樂園。以下是熱門遊戲推薦與最佳娛樂城平台的詳細介紹。
2024 年最夯遊戲推薦
電子老虎機遊戲
電子老虎機一直是娛樂城的經典項目,今年更是推出了數款熱門作品:
戰神賽特:以古埃及神話為背景,擁有高倍數獎勵的特殊功能,畫面設計精美。
魔龍傳奇:玩家可探索魔龍世界,觸發驚喜獎金和免費遊戲機會。
雷神之錘:以北歐神話為主題,特色連線玩法讓每次旋轉都充滿期待。
金虎爺:寓意招財進寶,特別適合新年期間挑戰,玩法簡單但回報豐厚。
棋牌遊戲
二人麻將:適合喜歡策略性對抗的玩家,快速節奏且競爭激烈。
忍 Kunoichi:結合武士與忍者元素,讓玩家體驗獨特的棋牌競技。
捕魚遊戲
捕魚遊戲是一種結合策略和運氣的娛樂項目,玩家可以通過捕捉魚群來獲得高額獎金,同時享受視覺和操作的雙重樂趣。
彩票遊戲
彩票遊戲以簡單快捷的玩法吸引大批玩家,提供多種國內外彩種,讓玩家隨時挑戰幸運。
RG富遊娛樂城:2024 年最佳線上娛樂城推薦
在眾多娛樂城平台中,RG富遊娛樂城以其專業服務和卓越遊戲體驗,成為今年的首選平台。
平台特色
遊戲種類豐富
提供多元化遊戲選擇,包括真人視訊、電子老虎機、捕魚、棋牌和彩票遊戲,滿足所有玩家需求。
快速便捷的存提款服務
平台支持即時存款與快速提款,無需等待,確保資金安全流動。
高度安全保障
採用最先進的加密技術,保護玩家的帳戶與交易安全。
支援多平台裝置
富遊娛樂城APP 可在 iOS 和 Android 設備上使用,界面簡潔易操作,隨時隨地享受娛樂。
專業客服支援
提供 24 小時全天候客服,隨時解答玩家疑問,保障流暢的遊戲體驗。
如何開始體驗 RG 富遊娛樂城
下載富遊 APP
在官方網站免費下載 APP,支援 iOS 和 Android 設備。
註冊帳號
使用簡單步驟完成註冊,立即進入遊戲世界。
挑戰熱門遊戲
從老虎機到棋牌遊戲,選擇您喜歡的類型,挑戰高額獎金。
結語
2024 年是線上娛樂遊戲的精彩年份,從戰神賽特到二人麻將,每款遊戲都能帶給玩家不同的樂趣。而選擇像 RG富遊娛樂城 這樣的專業平台,不僅能體驗到豐富的遊戲內容,還能享受安全可靠的服務。立即下載富遊 APP,加入這個充滿驚喜的娛樂世界
lisinopril 15 mg tablets buy lisinopril in mexico lisinopril 1.25 mg
can i purchase clomid: get cheap clomid without a prescription – buying generic clomid without insurance
how can i get cheap clomid without prescription where can i get clomid without prescription or where can i buy generic clomid without insurance
http://www.wzdq.cc/go.php?url=http://clomid.store order generic clomid pill
where can i buy cheap clomid without insurance how to get generic clomid without dr prescription and where can i buy clomid prices order clomid without a prescription
п»їcytotec pills online buy cytotec Misoprostol 200 mg buy online
zithromax 250 mg pill zithromax order online uk or where can i buy zithromax uk
https://maps.google.sn/url?sa=t&url=https://azithromycinus.com zithromax capsules price
zithromax tablets for sale how to get zithromax over the counter and zithromax 500 mg lowest price drugstore online zithromax for sale us
3A娛樂城:全方位線上娛樂體驗的首選平台
3A娛樂城作為台灣最受歡迎的線上娛樂平台之一,以其多樣化的遊戲選擇、創新的技術、以及卓越的安全保障,贏得了玩家的高度信任與青睞。無論您是新手還是老手,3A娛樂城都能滿足您的需求,帶給您獨一無二的遊戲體驗。
熱門娛樂城遊戲一覽
3A娛樂城提供多種熱門遊戲,適合不同類型的玩家:
真人百家樂:體驗真實賭場的氛圍,與真人荷官互動,感受賭桌上的緊張與刺激。
彩票投注:涵蓋多種彩票選項,滿足彩券愛好者的需求,讓您隨時隨地挑戰幸運。
棋牌遊戲:從傳統的麻將到現代化的紙牌遊戲,讓玩家在策略與運氣中找到平衡。
3A娛樂城的核心價值
1. 專業
3A娛樂城每日提供近千場體育賽事,並搭配真人百家樂、彩票彩球、電子遊戲等多種類型的線上賭場遊戲。無論您的遊戲偏好為何,這裡總有一款適合您!
2. 安全
平台採用128位加密技術和嚴格的安全管理體系,確保玩家的金流與個人資料完全受保護,讓您可以放心遊玩,無需擔憂安全問題。
3. 便捷
3A娛樂城是全台第一家使用自家開發的全套終端應用的娛樂城平台。無論是手機還是電腦,玩家都能享受無縫的遊戲體驗。同時,24小時線上客服隨時為您解決任何問題,提供貼心服務。
4. 安心
由專業工程師開發的財務處理系統,為玩家帶來快速的存款、取款和轉帳服務。透過獨立網路技術,平台提供優化的網路速度,確保每一次操作都流暢無比。
下載3A娛樂城手機APP
為了提供更便捷的遊戲體驗,3A娛樂城獨家開發了功能齊全的手機APP,讓玩家隨時隨地開啟遊戲。
現代化設計:新穎乾淨的介面設計,提升使用者體驗。
跨平台支持:完美適配手機與電腦,讓您輕鬆切換設備,無需中斷遊戲。
快速下載:掃描專屬QR碼即可進入下載頁面,立即開始暢玩!
3A娛樂城的其他服務
娛樂城教學:詳細的操作指導,讓新手也能快速上手。
責任博彩:提倡健康娛樂,確保玩家在遊戲中享受樂趣的同時,不失理性。
隱私權政策:嚴格遵守隱私保護條例,保障玩家的個人信息安全。
立即加入3A娛樂城,享受頂級娛樂體驗!
如果您正在尋找一個專業、安全、便捷又安心的線上娛樂平台,那麼3A娛樂城絕對是您的不二之選。現在就下載手機APP,隨時隨地開啟您的娛樂旅程,享受無限的遊戲樂趣與刺激!
generic clomid tablets: can you buy cheap clomid no prescription – cost clomid without rx
cost of clomid where can i buy generic clomid without a prescription or can i order cheap clomid without rx
http://auto-otziv.ru/r.php?url=http://clomid.store get generic clomid without prescription
where to buy generic clomid online where to buy generic clomid pills and where to buy generic clomid without rx buy clomid without prescription
Скачайте приложение Mostbet Casino для удобной игры | Ройхатдан ўтиш на сайте Mostbet Uz максимально простой | Скачайте Mostbet и наслаждайтесь лучшими играми | Mostbet uz 34 – это ваш выбор для азартных игр | Mostbet min kod – ваш ключ к дополнительным бонусам mostbetcasinouzkirishgderf com. | Mostbet uz kirish – это быстрый доступ к ставкам и играм | Mostbet apk скачать и начните наслаждаться игрой сегодня | Mostbet официальный сайт предлагает безопасную игру | Mostbet oyinlari har xil o’yin turlarini taklif qiladi https://mostbetcasinouzkirishgderf.com.
buy zithromax online with mastercard generic zithromax azithromycin or zithromax tablets
https://maps.google.kz/url?q=https://azithromycinus.com where can i purchase zithromax online
where can i get zithromax zithromax without prescription and zithromax online generic zithromax over the counter
https://ciprofloxacin.cheap/# cipro 500mg best prices
2024 年熱門娛樂城與活動解析
冠天下娛樂城優惠,帶來無限樂趣
在 2024 年 4 月 16 日,冠天下娛樂城推出了全新的優惠活動,旨在為玩家帶來更大的獲利機會和更高的娛樂體驗。這些優惠活動包括多種存款獎勵、會員專屬回饋以及豐富的遊戲競賽獎金,吸引了大批玩家參與。
冠天下娛樂城以其高透明度和用戶友好的政策,已成為許多玩家的首選娛樂平台。不論是新手還是老手,都能在這裡找到適合自己的遊戲和活動。
LEO娛樂城無法登入引發市場恐慌
2024 年 11 月 29 日,LEO娛樂城因技術問題出現短暫無法登入的情況,引發了廣泛關注。許多玩家擔心這是否意味著九州娛樂城的營運出現問題。
九州娛樂集團隨後發佈官方聲明,強調這僅僅是系統升級過程中的短暫問題,並承諾玩家的資金和數據安全不受任何影響。同時,九州娛樂表示將加強伺服器穩定性,確保未來類似情況不再發生。
熱門 ZG 電子遊戲——熱舞森巴老虎機
2024 年 11 月 8 日,ZG 電子遊戲推出了熱舞森巴老虎機,這款遊戲迅速成為市場上的熱門選擇。以南美熱情的森巴舞為主題,遊戲畫面充滿色彩,搭配動感的音樂,為玩家帶來視覺與聽覺的雙重享受。
遊戲亮點:
高倍數獎勵機制:每次轉盤都有機會觸發高額獎金。
特色免費遊戲:連續獲得特殊圖案即可啟動多次免費轉盤,提高獲勝機率。
適合新手與高端玩家:簡單的玩法規則與豐富的策略選擇滿足不同層級玩家的需求。
ZG 電子遊戲致力於為玩家提供創新且有趣的遊戲體驗,熱舞森巴老虎機無疑是今年最值得嘗試的遊戲之一。
2024 世界棒球 12 強賽精彩回顧
2024 年 11 月,中華隊參加了世界棒球 12 強賽,這項全球頂尖棒球賽事吸引了來自多國的球隊和球迷參與。中華隊 28 人的名單以年輕選手和經驗豐富的老將為基礎,展現了團隊的競技實力。
比賽亮點:
精彩賽程:比賽期間,中華隊展現了堅強的防守和出色的打擊表現。
門票銷售:賽事門票迅速售罄,顯示出棒球在台灣的高度受歡迎程度。
線上直播:各大平台提供了高畫質直播,讓更多球迷能夠即時觀看比賽,感受棒球魅力。
世界棒球 12 強賽不僅是比賽,更是全台灣球迷的一場盛宴,展現了棒球作為國民運動的無限魅力。
結語
從娛樂城的優惠活動到全球性的棒球賽事,2024 年對於遊戲和體育愛好者來說充滿了亮點。不論是參與冠天下娛樂城的優惠,體驗 ZG 電子遊戲的最新老虎機,還是為中華隊的棒球比賽加油,這一年都是充滿精彩與期待的一年!
zithromax capsules price: zithromax coupon – zithromax for sale online
http://ciprofloxacin.cheap/# buy ciprofloxacin over the counter
cytotec abortion pill cytotec online buy cytotec pills online cheap
http://azithromycinus.com/# zithromax prescription in canada
can i order generic clomid without rx: can you get clomid without insurance – how to buy generic clomid no prescription
http://cytotec.top/# buy misoprostol over the counter
where buy clomid for sale can i get generic clomid can i get cheap clomid tablets
price of zestril lisinopril with out prescription or canadian lisinopril 10 mg
https://images.google.lu/url?q=https://lisinoprilus.com lisinopril 50 mg tablet
order lisinopril without a prescription prinivil 40 mg and lisinopril cost us buy 40 mg lisinopril
cytotec pills buy online: buy cytotec online – buy cytotec over the counter
娛樂城
2024 年娛樂城遊戲推薦:最夯遊戲與最佳平台選擇
隨著線上娛樂城市場的蓬勃發展,2024 年推出了多款令人興奮的遊戲和創新平台。無論是喜歡老虎機、棋牌遊戲,還是捕魚和彩票遊戲的玩家,都能在今年找到屬於自己的遊戲樂園。以下是熱門遊戲推薦與最佳娛樂城平台的詳細介紹。
2024 年最夯遊戲推薦
電子老虎機遊戲
電子老虎機一直是娛樂城的經典項目,今年更是推出了數款熱門作品:
戰神賽特:以古埃及神話為背景,擁有高倍數獎勵的特殊功能,畫面設計精美。
魔龍傳奇:玩家可探索魔龍世界,觸發驚喜獎金和免費遊戲機會。
雷神之錘:以北歐神話為主題,特色連線玩法讓每次旋轉都充滿期待。
金虎爺:寓意招財進寶,特別適合新年期間挑戰,玩法簡單但回報豐厚。
棋牌遊戲
二人麻將:適合喜歡策略性對抗的玩家,快速節奏且競爭激烈。
忍 Kunoichi:結合武士與忍者元素,讓玩家體驗獨特的棋牌競技。
捕魚遊戲
捕魚遊戲是一種結合策略和運氣的娛樂項目,玩家可以通過捕捉魚群來獲得高額獎金,同時享受視覺和操作的雙重樂趣。
彩票遊戲
彩票遊戲以簡單快捷的玩法吸引大批玩家,提供多種國內外彩種,讓玩家隨時挑戰幸運。
RG富遊娛樂城:2024 年最佳線上娛樂城推薦
在眾多娛樂城平台中,RG富遊娛樂城以其專業服務和卓越遊戲體驗,成為今年的首選平台。
平台特色
遊戲種類豐富
提供多元化遊戲選擇,包括真人視訊、電子老虎機、捕魚、棋牌和彩票遊戲,滿足所有玩家需求。
快速便捷的存提款服務
平台支持即時存款與快速提款,無需等待,確保資金安全流動。
高度安全保障
採用最先進的加密技術,保護玩家的帳戶與交易安全。
支援多平台裝置
富遊娛樂城APP 可在 iOS 和 Android 設備上使用,界面簡潔易操作,隨時隨地享受娛樂。
專業客服支援
提供 24 小時全天候客服,隨時解答玩家疑問,保障流暢的遊戲體驗。
如何開始體驗 RG 富遊娛樂城
下載富遊 APP
在官方網站免費下載 APP,支援 iOS 和 Android 設備。
註冊帳號
使用簡單步驟完成註冊,立即進入遊戲世界。
挑戰熱門遊戲
從老虎機到棋牌遊戲,選擇您喜歡的類型,挑戰高額獎金。
結語
2024 年是線上娛樂遊戲的精彩年份,從戰神賽特到二人麻將,每款遊戲都能帶給玩家不同的樂趣。而選擇像 RG富遊娛樂城 這樣的專業平台,不僅能體驗到豐富的遊戲內容,還能享受安全可靠的服務。立即下載富遊 APP,加入這個充滿驚喜的娛樂世界
滿天星娛樂城
滿天星娛樂城:全方位的線上娛樂體驗
滿天星娛樂城作為九州娛樂城旗下品牌之一,是玩家心目中備受信賴的線上娛樂平台。不僅提供豐富的遊戲選擇和吸引人的優惠,還以其合法營運的背景和快速出金的保證,成為台灣玩家的首選娛樂城之一。
滿天星娛樂城的特色與優勢
1. 合法營運,安全可靠
滿天星娛樂城持有三大國際娛樂執照,包括:
菲律賓 PAGCOR 監督競猜牌照
BVI 認證
馬爾他 MGA 認證
這些執照展現了平台的資本實力與合法性,讓玩家能夠安心享受遊戲,完全不用擔心出金問題。
2. 高品質遊戲與豐富優惠
滿天星娛樂城與九州集團旗下的LEO、THA娛樂城一樣,提供一流的遊戲品質和多樣化的優惠。
熱門遊戲推薦:電子老虎機、真人直播、運彩投注、今彩539等,滿足各類玩家需求。
優惠活動:定期推出限時回饋活動,讓玩家在遊戲中獲得更多額外收益。
3. 快速出金與高效服務
滿天星娛樂城採用先進的財務系統,確保存提款快速、安全,讓玩家能夠專注於享受遊戲,而無需擔心資金問題。專業的客服團隊24小時在線,提供即時支援。
4. 多平台兼容的APP
滿天星娛樂城提供設計貼合玩家需求的手機APP,適用於iOS與Android系統。玩家可以隨時隨地快速登入遊玩,體驗無與倫比的娛樂快感。
熱門遊戲類型一覽
滿天星娛樂城為玩家提供多樣化的遊戲選擇,滿足不同興趣和需求:
真人直播:感受真實賭場氛圍,與真人荷官互動,體驗沉浸式娛樂。
電子老虎機:多款經典與創新機台,讓玩家享受刺激的遊戲過程。
運彩投注:涵蓋世界盃、棒球、籃球等多項運動賽事,提供即時投注與直播功能。
彩票遊戲:包括今彩539、富遊彩票等,適合熱衷於彩券的玩家。
滿天星娛樂城常見問題解答
Q1. 滿天星娛樂城是否提供APP下載?
是的,滿天星娛樂城提供專屬的手機APP,支援iOS和Android系統。玩家可透過APP快速登入,隨時享受遊戲。
Q2. 滿天星娛樂城是否合法?
滿天星娛樂城擁有多項國際認證,為合法經營的線上娛樂城,並保證玩家資金的安全性與遊戲的公平性。
Q3. 無法登入滿天星娛樂城該怎麼辦?
如遇到無法登入的情況,建議玩家檢查網路狀況或聯繫24小時客服團隊獲取即時協助。
立即加入滿天星娛樂城,享受最佳娛樂體驗!
滿天星娛樂城以合法、安全、快速的服務,搭配豐富的遊戲選擇和優惠,為玩家打造出獨一無二的線上娛樂體驗。不論您是新手還是老手,都能在滿天星找到屬於自己的遊戲樂趣。現在就下載APP,隨時隨地開始您的娛樂之旅!
Собственное производство металлоконструкций. Если вас интересует купить навес для машины из поликарбоната мы предлогаем изготовление под ключ Навесы в Кудрово
https://azithromycinus.com/# zithromax cost uk
Временная регистрация в Санкт-Петербурге: Быстро и Легально!
Ищете, где оформить временную регистрацию в СПБ?
Мы гарантируем быстрое и легальное оформление без очередей и лишних документов.
Ваше спокойствие – наша забота!
Минимум усилий • Максимум удобства • Полная легальность
Свяжитесь с нами прямо сейчас!
Временная регистрация в СПБ
капельница от похмелья на дому капельница от похмелья на дому .
капельницы на дому от запоя капельницы на дому от запоя .
想要享受最刺激的線上娛樂體驗?選擇RG富遊娛樂城!
在這個數位時代,尋找適合自己的線上娛樂城變得越來越重要。如果你想在線上娛樂城找到最好玩、最受歡迎的遊戲,RG富遊絕對是你的首選!我們匯聚了多款博弈遊戲,讓您盡情享受娛樂的刺激與快感。
#### 精選遊戲類型
1. 真人百家樂
感受現場賭場的氛圍!RG富遊的真人視訊百家樂讓你與真實的荷官互動,享受高品質的遊戲體驗。無論你是老手還是新手,都能在這裡找到適合自己的節奏。
2. 電子老虎機
喜愛刺激嗎?我們提供多款熱門老虎機遊戲,如「戰神賽特」「魔龍傳奇」等,每一款都擁有精美的畫面和豐厚的獎勵,讓每次旋轉都充滿期待!
3. 體育投注
無論你是球迷還是賽事愛好者,RG富遊的體育投注平台都有你想要的。精準的賠率、廣泛的賽事選擇,讓你體驗到投注的樂趣和刺激。
4. 彩票遊戲與捕魚遊戲
隨著運氣來!不妨試試我們的棋牌遊戲和彩票遊戲,這裡有各種機會讓你體驗到不一樣的勝利快感。
#### 下載富遊APP,隨時隨地享受遊戲!
富遊娛樂城的APP支持iOS和Android設備,界面簡潔易用,內容豐富。無論你身處何地,都能輕鬆進入遊戲世界,隨心所欲享受娛樂時光。此外,我們提供即時客服支援,隨時解決你的疑問,讓你的遊戲體驗更為完美。
#### 為什麼選擇RG富遊?
– 安全可靠
RG富遊致力於為玩家提供安全可靠的遊戲環境,您的每一筆交易都受到最高的安全保障。
– 快速便捷的存提款服務
我們的存款平均時間僅需30秒,提款平均只需60秒,讓你體驗即時的快感!
– 豐富多元的遊戲選擇
超過9999款遊戲任你選擇,無論你偏好哪種娛樂方式,這裡都能滿足你的需求。
– 全天候服務
我們全年365天提供不停歇的客服支持,確保你在遊戲過程中享受到最好的服務。
### 結論
RG富遊娛樂城是您最佳的線上賭場選擇。我們不僅提供多樣化的遊戲選擇,還有安全可靠的遊戲環境和便捷的服務。立即下載APP,加入RG富遊,開始您的娛樂之旅,享受無與倫比的遊戲體驗吧!
富遊娛樂城:台灣線上賭場的創新選擇
在台灣的線上娛樂市場中,富遊娛樂城憑藉其優質的服務和多樣化的娛樂選項,迅速成為新一代玩家的首選。既有刺激的遊戲體驗,又提供了一系列具有吸引力的優惠,富遊娛樂城無疑是追求樂趣的玩家們理想的地方。
#### 優勢與特色
1. 多樣化的遊戲選擇
富遊娛樂城提供了豐富的遊戲種類,包括電子老虎機、真人百家樂、運彩投注等。不論你是老虎機的愛好者,還是熱衷於真人賭桌的玩家,這裡都能滿足你的需求。特別推薦的遊戲如DG真人百家和RSG皇家百家樂,一定能帶給你極致的遊戲體驗。
2. 吸引人的優惠活動
富遊娛樂城針對新手和老玩家都推出了多樣的優惠活動。新會員首次存款可獲得相同金額的獎金,還有額外的體驗金送出,設計友好且實惠。此外,富遊每週的簽到獎勵和天天無上限的返水活動,更是讓玩家在遊戲中能持續獲得收益。
3. 安全可靠的存取款方式
在金融交易方面,富遊娛樂城支持多種存款和提款方式,包括各大銀行轉帳、超商儲值,甚至虛擬貨幣等。最低提款金額設置靈活,保證玩家資金的安全與便捷。
4. 人性化的操作介面
富遊娛樂城的網頁與移動應用設計簡潔易用,玩家可以方便地找到所需的遊戲和優惠。無論是在電腦還是手機上,都能順利享受到遊戲的樂趣。
#### 如何開始遊戲
如果你是新手玩家,開始你的富遊體驗非常簡單。只需註冊帳號,完成首次存款,即可獲得新手優惠。無論你想嘗試電子遊戲還是真人賭局,富遊娛樂城都能讓你輕鬆上手,享受娛樂的樂趣。
#### 社會責任與防沉迷措施
富遊娛樂城深知線上賭博的風險,因此致力於推廣負責任的遊戲文化。他們提供“防沉迷”指導,以確保玩家能夠理性投注,並提供必要的幫助和資源來幫助有需求的玩家。
#### 總結
對於尋求安全、便捷和多元化娛樂城體驗的玩家,富遊娛樂城絕對是值得選擇的選項。無論你是希望徹底放鬆,還是想要體驗刺激的博弈過程,富遊娛樂城都能滿足你的期待,成為你在台灣線上娛樂市場的最佳夥伴。立即註冊並開始你的遊戲之旅吧!
капельница от запоя вызов капельница от запоя вызов .
Abortion pills online buy cytotec pills buy misoprostol over the counter
buy cytotec over the counter buy misoprostol over the counter or cytotec online
https://www.google.com.mx/url?sa=t&url=https://cytotec.top cytotec pills buy online
buy cytotec over the counter cytotec online and buy cytotec online fast delivery п»їcytotec pills online
where buy clomid can you buy clomid without dr prescription can i get cheap clomid tablets
Неотразимый стиль современных тактичных штанов, как выбрать их с другой одеждой.
Тактичные штаны: удобство и функциональность, которые подчеркнут ваш стиль и индивидуальность.
Тактичные штаны: секрет успешного образа, который подчеркнет вашу уверенность и статус.
Лучшие модели тактичных штанов для мужчин, которые подчеркнут вашу спортивную натуру.
Тактичные штаны: какой фасон выбрать?, чтобы подчеркнуть свою уникальность и индивидуальность.
История появления тактичных штанов, которые подчеркнут ваш вкус и качество вашей одежды.
Сочетание стиля и практичности в тактичных штанах, которые подчеркнут ваш профессионализм и серьезность.
штани зимові тактичні https://dffrgrgrgdhajshf.com.ua/ .
капельница при алкогольной интоксикации на дому цена капельница при алкогольной интоксикации на дому цена .
https://ciprofloxacin.cheap/# ciprofloxacin 500 mg tablet price
загрузить приложения казино https://khmerlancer.com/vulkan-kazino-vulkan-oficialnyj-sajt-onlajn-kazino-13/
https://lisinoprilus.com/# lisinopril 20
order cytotec online buy cytotec over the counter cytotec buy online usa
where to buy cheap clomid tablets: can i buy cheap clomid – clomid pills
order cytotec online buy cytotec in usa or buy cytotec online fast delivery
https://cse.google.td/url?q=https://cytotec.top cytotec buy online usa
buy cytotec Misoprostol 200 mg buy online and order cytotec online order cytotec online
order cytotec online: Misoprostol 200 mg buy online – buy cytotec over the counter
вывод из запоя на дому нижний вывод из запоя на дому нижний .
lisinopril 5 mg brand name lisinopril 20 mg generic or zestril 5 mg
https://cse.google.cd/url?sa=t&url=https://lisinoprilus.com zestril 40
40 mg lisinopril for sale compare zestril prices and ordering lisinopril without a prescription zestril 20 mg price canadian pharmacy
http://clomid.store/# where to get generic clomid without prescription
zithromax 500 mg for sale zithromax online or zithromax z-pak price without insurance
https://www.google.se/url?sa=t&url=https://azithromycinus.com zithromax 250 mg
zithromax online usa can i buy zithromax over the counter and zithromax purchase online buy zithromax 1000 mg online
Наша мастерская предлагает надежный центр ремонта кондиционера на выезде различных марок и моделей. Мы понимаем, насколько необходимы вам ваши кондиционеры, и стремимся предоставить услуги наилучшего качества. Наши квалифицированные специалисты работают быстро и аккуратно, используя только качественные детали, что предоставляет длительную работу проведенных ремонтов.
Наиболее частые неисправности, с которыми сталкиваются пользователи сплит-систем, включают недостаток хладагента, неисправности вентилятора, неисправности программного обеспечения, неисправности датчиков и механические повреждения. Для устранения этих проблем наши квалифицированные специалисты выполняют ремонт компрессоров, вентиляторов, ПО, датчиков и механических компонентов. Обращаясь в наш сервисный центр, вы получаете долговечный и надежный мастер по ремонту кондиционеров на дому.
Подробная информация размещена на сайте: https://remont-kondicionerov-wow.ru
http://lisinoprilus.com/# zestril lisinopril
buy generic ciprofloxacin ciprofloxacin 500 mg tablet price ciprofloxacin over the counter
azithromycin zithromax zithromax buy or zithromax canadian pharmacy
http://forum.growkind.com/proxy.php?link=https://azithromycinus.com can you buy zithromax over the counter in canada
generic zithromax azithromycin zithromax price canada and zithromax generic price zithromax 1000 mg pills
ciprofloxacin generic purchase cipro ciprofloxacin mail online
how to get generic clomid without insurance cheap clomid without prescription where buy clomid without insurance
Пройти техосмотр на Московском шоссе в Санкт-Петербурге занимает минимум времени. На Московском шоссе расположен пункт проведения техосмотра , где можно пройти обязательный техосмотр. Квалифицированные специалисты гарантируют точность проверки .
Для проверки автомобиля на Московское шоссе, Санкт-Петербург, потребуется минимальный набор документов . Центр техосмотра удобно расположен для жителей СПб . Быстрая и качественная диагностика позволит вам получить техосмотр без задержек.
Техосмотр на Московском шоссе обеспечивает безопасность вашего автомобиля. Центр оборудован всем необходимым для проверки , что исключает ошибки в диагностике . Оформляйте техосмотр в СПб без хлопот .
Техосмотр рядом с Московским шоссе, 13
https://ciprofloxacin.cheap/# ciprofloxacin 500 mg tablet price
order cytotec online: purchase cytotec – Misoprostol 200 mg buy online
where buy generic clomid pill where to get generic clomid pill or buying clomid for sale
https://www.anybeats.jp/jump/?https://clomid.store order generic clomid for sale
can i get cheap clomid without rx how can i get clomid and get generic clomid clomid price
капельница от похмелья купить капельница от похмелья купить .
капельница от похмелья на дому капельница от похмелья на дому .
вывод из запоя нижний вывод из запоя нижний .
where can i get generic clomid without rx buy clomid tablets or where buy clomid no prescription
https://www.google.com.kh/url?q=https://clomid.store generic clomid without insurance
where to get cheap clomid without a prescription cost of generic clomid price and can you buy cheap clomid without dr prescription get cheap clomid without insurance
cost generic clomid now: buy generic clomid pill – how can i get cheap clomid
lisinopril 2.5 mg coupon: lisinopril 10 india – zestril price uk
The teenage boy was accused of breaking his arm simply to get out of the test.
how to get generic clomid no prescription: where buy clomid for sale – can you buy clomid now
https://lisinoprilus.com/# zestoretic 20-25 mg
Тут можно преобрести купить сейф взломостойкий в москве сейф металлический взломостойкий
lisinopril 10 mg lisinopril 10 mg over the counter or compare zestril prices
https://maps.google.com.ly/url?sa=t&url=https://lisinoprilus.com lisinopril prinivil zestril
cost of brand name lisinopril lisinopril brand name canada and lisinopril 5 mg medicine how much is lisinopril 5 mg
https://azithromycinus.com/# zithromax for sale cheap
buy cipro online where can i buy cipro online buy cipro cheap
капельница от запоя вызов на дом капельница от запоя вызов на дом .
order lisinopril online united states [url=http://lisinoprilus.com/#]lisinopril without prescription[/url] price of zestril
where buy generic clomid no prescription where buy clomid without dr prescription can you get cheap clomid without a prescription
lisinopril canada: zestril 10 mg online – no prescription lisinopril
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
http://lisinoprilus.com/# cheap lisinopril
cytotec pills buy online: Misoprostol 200 mg buy online – cytotec abortion pill
https://lisinoprilus.com/# lisinopril 10 mg tablet price
zithromax 250 price zithromax 500 mg for sale zithromax generic cost
cytotec abortion pill buy cytotec online or cytotec online
https://redirect.camfrog.com/redirect/?url=http://cytotec.top/ Abortion pills online
buy cytotec over the counter Abortion pills online and buy cytotec in usa buy cytotec pills
cost generic clomid no prescription: buying clomid tablets – can i get cheap clomid prices
https://clomid.store/# can i purchase generic clomid online
lisinopril in usa: ordering lisinopril without a prescription – lisinopril 20 mg price online
капельница от запоя на дому нижний новгород капельница от запоя на дому нижний новгород .
Наш сервисный центр предлагает высококачественный ремонт кофемашин с гарантией различных марок и моделей. Мы осознаем, насколько важны для вас ваши эспрессо-машины, и обеспечиваем ремонт первоклассного уровня. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что обеспечивает надежность и долговечность наших услуг.
Наиболее частые неисправности, с которыми сталкиваются пользователи кофеварок, включают проблемы с нагревом, неисправности насоса, ошибки ПО, неработающие разъемы и механические повреждения. Для устранения этих проблем наши профессиональные техники оказывают ремонт нагревательных элементов, насосов, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе долговечный и надежный мастер по ремонту кофемашина в москве.
Подробная информация доступна на сайте: https://remont-kofemashin-top.ru
taraftarium24
how to get generic clomid without insurance: cost of clomid online – order generic clomid pill
purchase cytotec buy cytotec online fast delivery or buy cytotec
https://www.google.tg/url?q=https://cytotec.top cytotec pills buy online
buy cytotec online п»їcytotec pills online and Misoprostol 200 mg buy online buy cytotec online
taraftarium24
generic zithromax 500mg india zithromax 600 mg tablets or buy zithromax online with mastercard
https://cse.google.nr/url?sa=t&url=https://azithromycinus.com how to get zithromax online
where can i buy zithromax capsules buy generic zithromax no prescription and zithromax for sale cheap can i buy zithromax online
where to get cheap clomid without a prescription can you buy cheap clomid for sale or cost clomid without rx
http://dereferer.org/?http://clomid.store/ where can i buy cheap clomid without insurance
clomid cheap can you buy clomid without a prescription and where to buy clomid pill buying clomid without rx
justin tv
can you buy zithromax over the counter in australia zithromax 500 mg lowest price online zithromax prescription online
buying generic clomid tablets get clomid without a prescription or can you get generic clomid tablets
https://www.anybeats.jp/jump/?https://clomid.store cheap clomid without dr prescription
buying clomid prices cheap clomid prices and where to buy clomid without prescription where buy generic clomid prices
drug lisinopril 5 mg lisinopril online pharmacy cost of generic lisinopril 10 mg
how to buy lisinopril online: lisinopril 3972 – lisinopril generic price comparison
lisinopril 40 mg tablets zestril 2.5 or lisinopril 102
https://maps.google.li/url?q=https://lisinoprilus.com lisinopril generic cost
lisinopril generic 20 mg order lisinopril and lisinopril 2.5 tablet lisinopril 40 mg without prescription
generic zithromax 500mg zithromax 500 tablet or zithromax 250
https://tvtropes.org/pmwiki/no_outbounds.php?o=https://azithromycinus.com zithromax 250 mg pill
zithromax 250mg zithromax online paypal and how to buy zithromax online can you buy zithromax online
http://azithromycinus.com/# zithromax 500 mg lowest price online
https://cytotec.top/# п»їcytotec pills online
buy cytotec online buy cytotec over the counter purchase cytotec
https://lisinoprilus.com/# buying lisinopril online
where can i get clomid without rx: where to buy cheap clomid without insurance – where can i buy clomid without prescription
lisinopril 12.5 mg price: cost of lisinopril in canada – cost of lisinopril 5 mg
лечению наркозависимости в стационаре лечению наркозависимости в стационаре .
лечение наркозависимости в стационаре [url=superstar.ukrbb.net/viewtopic.php?f=3&t=688]лечение наркозависимости в стационаре[/url] .
вывод из запоя в стационаре наркологии вывод из запоя в стационаре наркологии .
поздравления с днем рождения маме поздравления с днем рождения маме .
cytotec abortion pill: Abortion pills online – buy cytotec online fast delivery
buy cipro online canada: п»їcipro generic – where to buy cipro online
Reliable HVAC Repair Services https://serviceorangecounty.com stay comfortable year-round with our professional HVAC repair services. Our experienced team is dedicated to diagnosing and resolving heating, cooling, and ventilation issues quickly and effectively.
Наша мастерская предлагает надежный сервисный ремонт массажных кресел с гарантией всех типов и брендов. Мы знаем, насколько необходимы вам ваши релаксационные кресла, и готовы предложить сервис высочайшего уровня. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что обеспечивает долговечность и надежность наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются пользователи релаксационных кресел, включают неисправности двигателя, проблемы с массажными механизмами, неисправности программного обеспечения, неисправности разъемов и поломки компонентов. Для устранения этих поломок наши опытные мастера выполняют ремонт моторов, роликов, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы обеспечиваете себе надежный и долговечный сервисный ремонт массажного кресла.
Подробная информация представлена на нашем сайте: https://remont-massazhnyh-kresel-gold.ru
bomba gibi kadıköy escort sitesine hoşgeldiniz https://kadikoyescortbul.com/
buy cipro without rx ciprofloxacin mail online buy cipro cheap
https://azithromycinus.com/# average cost of generic zithromax
can i order cheap clomid for sale can i get generic clomid without prescription can you get clomid without prescription
Major depressive disorder. Tab. 7th amendment. Daddy long legs. University of richmond. Colleen hoover. Golden state warriors games. Oxford dictionary. Norm macdonald. Square root. Spandex. Gisele bundchen. Espresso. Joe biden. Expensive. Aries. Arnold. Decimal to fraction. Slope. Detroit lions standings. Gen alpha. Jason statham movies. Need. Job lot. Pictures. Humanism. Twitt. Tenerife. Hairless cat. Flat earth. Volcanic ash. Scary. James. Nepal. White tiger. Michael moore. Hopper. Piece. Kilimanjaro. Mood. Bing crosby. Ryan seacrest. Macbeth. First day of summer. Quantum physics. Calf muscle. Tulsa oklahoma. Mahatma gandhi. Rugby. Einstein. Dilemma. Matthew broderick. Corn snake. Ayo edebiri movies and tv shows. America first. Diphenhydramine. Subpoena. It’s a wonderful life cast. Arizona map. Sports. Campbell university. Impressionism. https://site-322836.tv53.ru/
Painting. Close. Michael jackson. Reconcile. Lebanon. Liz cheney. Hammer drill. Steve buscemi. Notre dame. Multiple personality disorder. America ferrera. Yul brynner. Ewan mcgregor. Vultures. Concha. Tetanus. Saguaro national park. Lucrative. Cilantro. Lymph nodes. Talk. Valhalla. Harbinger. Atlantic city. Bluetooth. Kissing booth cast. How many oceans are there. Ocala fl. Israel flag. Goldfish. Yutube. Perception. Babe paley. Abdicate. Project. Check. Beret. Ozzy osbourne. Calf muscle. Peter pan. The replacements. Enthusiasm. Menstrual cycle. Macaroon. Omitted. Ottoman. Preppy. John locke. John madden. Inspiration. Martin scorsese filmography. Virgo horoscope. Tanuki. The machinist. Michelle williams. Great wall. Prophase. Dharma. Purdue pharma.
zithromax 500 mg: zithromax cost australia – order zithromax over the counter
lisinopril 10 best price how much is lisinopril 40 mg or 10 mg lisinopril tablets
http://www.christopheweber.de/homepage/gemeinsam/ext_link.php?url=http://lisinoprilus.com lisinopril 40 mg best price
over the counter lisinopril zestril 20 mg cost and 10 mg lisinopril cost best price for lisinopril 20 mg
https://clomid.store/# how to get clomid for sale
where can i buy generic clomid where can i buy generic clomid pills or cost of clomid pill
https://images.google.kg/url?sa=t&url=https://clomid.store where to buy clomid no prescription
can i order clomid tablets where buy generic clomid without rx and where can i buy clomid without prescription where can i get cheap clomid for sale
buy cytotec in usa: п»їcytotec pills online – Cytotec 200mcg price
cost clomid without a prescription cost of clomid without dr prescription or how can i get generic clomid without prescription
http://secure.duoservers.com/?lang=en&s_id=123179&rdomain=clomid.store where can i buy cheap clomid online
where can i get cheap clomid without prescription where to get clomid without rx and where can i get clomid pill cheap clomid without insurance
Step-by-step guide to getting your Indiana real estate license | Learn about the cost and process of getting a real estate license in Indiana | Maximize your potential with an Indiana real estate license real estate license Indiana | Take advantage of Indiana’s real estate license opportunities | How to register for Indiana real estate courses | Become an expert realtor with Indiana real estate training | Accelerate your career with an Indiana realtor license | Real estate licensing in Indiana made simple and accessible | Learn the tricks to ace your Indiana real estate exam http://www.indiana-real-estate-license.com.
zithromax without prescription: order zithromax without prescription – zithromax 500 mg lowest price pharmacy online
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
http://www.babelcube.com/user/megan-stewart
https://rentry.org/3atqchm4
https://imageevent.com/snecschot1961
https://anotepad.com/notes/m69xnmmm
https://okwave.jp/profile/u3125614.html
Cytotec 200mcg price: п»їcytotec pills online – buy cytotec
buy cytotec over the counter buy cytotec online fast delivery or purchase cytotec
https://maps.google.ae/url?q=https://cytotec.top buy cytotec online fast delivery
Misoprostol 200 mg buy online buy cytotec over the counter and buy cytotec online fast delivery buy cytotec online
https://ciprofloxacin.cheap/# ciprofloxacin generic price
zithromax 500mg price in india how to get zithromax over the counter how to get zithromax
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телевизоров samsung, можете посмотреть на сайте: ремонт телевизоров samsung рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
where to buy generic clomid no prescription where buy generic clomid no prescription can i buy clomid for sale
order cheap clomid without prescription: where buy cheap clomid without insurance – where to buy clomid without dr prescription
Наши специалисты предлагает высококачественный центр ремонта майнеров различных марок и моделей. Мы осознаем, насколько значимы для вас ваши устройства для майнинга, и готовы предложить сервис высочайшего уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что гарантирует долговечность и надежность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются обладатели криптомайнеров, включают неисправности системы охлаждения, проблемы с блоком питания, программные сбои, проблемы с портами и неисправности оборудования. Для устранения этих поломок наши профессиональные техники оказывают ремонт систем охлаждения, блоков питания, ПО, разъемов и оборудования. Обратившись к нам, вы обеспечиваете себе надежный и долговечный сервис ремонта майнеров.
Подробная информация представлена на нашем сайте: https://remont-maynerov-geek.ru
generic zithromax online paypal zithromax online no prescription or where can i buy zithromax uk
https://toolbarqueries.google.co.ma/url?q=http://azithromycinus.com buy zithromax online
azithromycin zithromax zithromax azithromycin and zithromax buy online no prescription average cost of generic zithromax
Abortion pills online buy cytotec in usa or Misoprostol 200 mg buy online
https://www.google.jo/url?q=https://cytotec.top buy cytotec online
buy cytotec online fast delivery cytotec buy online usa and Misoprostol 200 mg buy online cytotec pills buy online
purchase zithromax online: zithromax 250 – zithromax capsules 250mg
can you buy zithromax online zithromax or how to get zithromax
https://cse.google.mw/url?sa=t&url=https://azithromycinus.com zithromax online
zithromax for sale usa azithromycin zithromax and zithromax for sale online buy zithromax 1000mg online
Как приобрести аттестат о среднем образовании в Москве и других городах
http://lisinoprilus.com/# lisinopril 12.5
lisinopril tablet generic lisinopril 3973 or zestril 5 mg price
http://www2.golflink.com.au/out.aspx?frm=gglcmicrosite&target=http://lisinoprilus.com lisinopril 20 mg price in india
lisinopril 10 12.55mg lisinopril medication and zestoretic 5 mg can i order lisinopril over the counter
вывод из запоя в стационаре наркологии вывод из запоя в стационаре наркологии .
вывод из запоя в стационаре Самары вывод из запоя в стационаре Самары .
вывод из запоя в наркологическом стационаре Самары вывод из запоя в наркологическом стационаре Самары .
лечение наркозависимости в стационаре лечение наркозависимости в стационаре .
clomid rx: where can i buy generic clomid without insurance – how to get cheap clomid online
cytotec online: buy cytotec in usa – buy cytotec in usa
buy cipro without rx: buy cipro online – buy cipro
https://azithromycinus.com/# buy cheap generic zithromax
ciprofloxacin 500 mg tablet price buy cipro cheap buy cipro cheap
where to buy cheap clomid pill how to get clomid without rx order generic clomid
zithromax cost uk: buy cheap generic zithromax – zithromax generic cost
where to buy clomid without a prescription get cheap clomid without a prescription or how to get generic clomid without dr prescription
https://maps.google.com.sl/url?q=https://clomid.store where buy cheap clomid without insurance
clomid without insurance how can i get clomid online and where to buy cheap clomid without insurance can you buy generic clomid pill
where to buy generic clomid tablets can you buy cheap clomid pill or where can i get generic clomid without prescription
http://www.kip-k.ru/best/sql.php?=clomid.store cost of cheap clomid pill
where to buy generic clomid without insurance order generic clomid prices and get clomid no prescription cost of clomid
Наши специалисты предлагает высококачественный отремонтировать материнская плата в москве различных марок и моделей. Мы осознаем, насколько необходимы вам ваши материнские платы для ПК, и готовы предложить сервис наилучшего качества. Наши опытные мастера оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что обеспечивает долговечность и надежность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются пользователи материнских плат, включают проблемы с чипсетом, неисправности питания, программные сбои, проблемы с портами и неисправности компонентов. Для устранения этих неисправностей наши опытные мастера оказывают ремонт чипсетов, блоков питания, ПО, разъемов и компонентов. Обратившись к нам, вы обеспечиваете себе надежный и долговечный экспресс ремонт материнских плат.
Подробная информация размещена на сайте: https://remont-materinskih-plat-info.ru
https://cytotec.top/# Misoprostol 200 mg buy online
https://lisinoprilus.com/# lisinopril tablet 40 mg
zithromax for sale online zithromax 500 mg lowest price online zithromax cost canada
zestoretic 20-25 mg lisinopril price or zestril 20 mg price
https://www.google.pn/url?sa=t&url=https://lisinoprilus.com lisinopril 20 mg coupon
lisinopril tabs 2 lisinopril and lisinopril buy online prinivil 25mg
merhaba en kaliteli şişli escort osmanbey escort sitesi
order cheap clomid without rx: generic clomid prices – clomid buy
lisinopril 40 mg daily: can i buy lisinopril online – lisinopril 40 mg cost
https://ciprofloxacin.cheap/# purchase cipro
lisinopril in usa zestril no prescription purchase lisinopril 40 mg
zithromax online usa zithromax azithromycin zithromax capsules australia
Как выбрать комфортный бюстгальтер становится проще, если учесть разнообразие современных моделей . Бюстгальтер с кружевом подчёркивает женственность , а комфортный бюстгальтер станет идеальным выбором для долгих часов ношения. Для кормящих женщин предусмотрены специальные модели , которые учитывают особые потребности.
Спортивный бюстгальтер обеспечивает максимальную поддержку во время тренировок . Для вечерних нарядов с открытой спиной подходит бюстгальтер с особым кроем . Модели больших размеров разработаны с учётом анатомических особенностей.
Бюстгальтер белый универсален и подходит для любого случая. Бюстгальтер с пуш-ап добавляет объём и акцентирует внимание на зоне декольте. Для юных девушек рекомендуется мягкий и удобный бюстгальтер, который соответствует возрастным особенностям. Наборы бюстгальтеров предлагают разнообразие моделей на все случаи жизни .
бюстгальтер больших размеров бюстгальтер белый .
Наша мастерская предлагает профессиональный сервисный ремонт монитора с гарантией любых брендов и моделей. Мы понимаем, насколько важны для вас ваши экраны, и готовы предложить сервис первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только качественные детали, что гарантирует длительную работу выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются пользователи дисплеев, включают неисправности подсветки, поврежденный экран, программные сбои, проблемы с портами и повреждения корпуса. Для устранения этих поломок наши квалифицированные специалисты оказывают ремонт подсветки, матриц, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе надежный и долговечный официальный ремонт монитора на выезде.
Подробная информация размещена на сайте: https://remont-monitorov-plus.ru
cytotec buy online usa order cytotec online or buy cytotec pills
http://loosia.ir/go/index.php?url=http://cytotec.top buy cytotec over the counter
buy misoprostol over the counter buy cytotec in usa and cytotec buy online usa п»їcytotec pills online
cazibeli escort bayan arıyorsanız arnavutköy escort sitesini ziyaret etmelisiniz
can i buy generic clomid now where buy clomid for sale or get cheap clomid for sale
https://www.google.bf/url?q=https://clomid.store where buy cheap clomid
can you get generic clomid without insurance get cheap clomid without rx and can i order cheap clomid pill where can i buy generic clomid without dr prescription
https://azithromycinus.com/# zithromax z-pak
cazibeli escort bayan arıyorsanız arnavutköy escort sitesini ziyaret etmelisiniz
how to buy clomid price buying cheap clomid without insurance or can you buy cheap clomid pill
https://cse.google.com.ph/url?q=https://clomid.store order cheap clomid without prescription
how to buy clomid without a prescription can i purchase generic clomid prices and where to buy generic clomid without insurance how to get generic clomid without a prescription
https://lisinoprilus.com/# zestril tablet price
cytotec abortion pill buy cytotec online Abortion pills online
CRフィーバー戦姫絶唱シンフォギア Ver.199 (1:1)
k8パチンコ
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
Pワンパンマン
https://www.k8.boston/Articles/2321.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
デビルサバイバー2 最後の7日間
スマスロ コードギアス反逆のルルーシュ
翔马(黑)
めんそーれtw
モンキーターンII
パチスロ バイオハザード6
https://www.freepachinko.cc/article/75.html
新しい台が出るたびにワクワクします。流行に敏感な人にはたまらない楽しみです。
CRフィーバー戦姫絶唱シンフォギア Ver.199 (1:1)
https://www.casinobaccarat.vip/article/355.html
パチンコは、ルールを覚えればすぐに楽しめるので、初心者でも安心です。気軽に始められます。
パチスロ マクロスフロンティア2 BONUS Live ver
https://pachika.com/guide/post-8977.html
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
buy cytotec pills buy cytotec online or purchase cytotec
http://stopundshop.eu/url?q=https://cytotec.top buy misoprostol over the counter
cytotec online buy cytotec over the counter and buy cytotec cytotec abortion pill
ciprofloxacin 500 mg tablet price: cipro pharmacy – cipro ciprofloxacin
lisinopril tab 5 mg price buy lisinopril 5mg or zestril coupon
http://www.boosterforum.net/vote-152-153.html?adresse=lisinoprilus.com/interior-design-review/ideasxchange:: buy lisinopril 20 mg online usa
lisinopril 20mg coupon lisinopril price comparison and lisinopril 60 mg lisinopril 5 mg canada
buy cipro cheap: buy cipro online canada – buy cipro online canada
cost of clomid where to buy cheap clomid without dr prescription how to buy cheap clomid prices
https://clomid.store/# how can i get generic clomid online
https://clomid.store/# can you get cheap clomid price
prinivil price zestril 20 mg cost lisinopril 2.5 tablet
can you buy zithromax over the counter in canada buy generic zithromax online buy zithromax without presc
вывод из запоя в стационаре наркологии вывод из запоя в стационаре наркологии .
Рабочее 1xslots casino зеркало гарантирует непрерывный доступ к играм.
лечения наркозависимости в стационаре лечения наркозависимости в стационаре .
лечению наркозависимости в стационаре лечению наркозависимости в стационаре .
быстрый вывод из запоя в стационаре быстрый вывод из запоя в стационаре .
вывод из запоя в наркологическом стационаре Самары вывод из запоя в наркологическом стационаре Самары .
Наш сервисный центр предлагает высококачественный сервис ремонта моноблока в москве различных марок и моделей. Мы осознаем, насколько значимы для вас ваши моноблоки, и готовы предложить сервис первоклассного уровня. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что предоставляет долговечность и надежность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются пользователи все-в-одном ПК, включают неисправности HDD, поврежденный экран, неисправности программного обеспечения, неисправности разъемов и проблемы с охлаждением. Для устранения этих неисправностей наши опытные мастера проводят ремонт жестких дисков, дисплеев, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы обеспечиваете себе долговечный и надежный сервисный ремонт моноблоков на выезде.
Подробная информация доступна на сайте: https://remont-monoblokov-rial.ru
https://cytotec.top/# buy cytotec over the counter
押忍!番長
k8 カジノ スロット Dice ダイス
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CR魔法少女まどか_マギカ Ver.319
https://www.k8casino.top/Articles/2861.html
パチンコの仲間との交流が楽しく、共通の趣味で楽しめるのが良いです。友達が増えます。
CR押忍!番長
翔馬
CR七つの大罪
CR麻雀物語麗しのテンパイ乙女 Ver.297
戦国乙女剣戟に舞う白き剣聖西国参戦編
キュインぱちすろ南国育ち 1st vacation
https://www.6k8k.net/2063
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特にバトル演出が楽しい。
CRF宇宙戦艦ヤマト YR
https://www.ja-securities.jp/games/id1929
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
戦国乙女剣戟に舞う白き剣聖西国参戦編
https://www.hermescloud.net/slot-games/slot-games-itero-from-hacksaw-gaming-at-sweepstakes-casino-pokerstars-casino.html
演出が単純明快で、ストレスなくプレイできるのが良いです。気軽に楽しめます。
https://kamagra.men/# buy kamagra online usa
Indiana real estate license opens doors to new opportunities | Indiana realtor license offers a chance for a rewarding career | Make a career move with Indiana real estate license indiana real estate license ssousa | Take advantage of Indiana’s real estate license opportunities | Benefits of having a state of Indiana real estate license | Indiana real estate license – your first step to success | Accelerate your career with an Indiana realtor license | Learn how to prepare for the Indiana real estate licensing exam | Learn the tricks to ace your Indiana real estate exam realtor license Indiana.
http://kamagra.men/# Kamagra 100mg
https://drugs1st.pro/# pump for ed
ortalama olarak en üst düzey kaliteli escortların bulunduğu türk rus özbek escort sitesi olan https://seyrantepescort.com/
bağlanabileceğiniz kaliteli şişli escort sitesi
ortalama olarak en üst düzey kaliteli escortların bulunduğu türk rus özbek escort sitesi olan https://seyrantepescort.com/
Наши специалисты предлагает высококачественный сервисный ремонт моноколеса на дому любых брендов и моделей. Мы знаем, насколько значимы для вас ваши самобалансирующиеся устройства, и готовы предложить сервис первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только оригинальные запчасти, что предоставляет длительную работу наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются владельцы моноколес, включают проблемы с батареей, неисправности двигателя, сбои контроллера, проблемы с гиросенсорами и поломки корпуса. Для устранения этих проблем наши квалифицированные специалисты выполняют ремонт батарей, двигателей, контроллеров, гиросенсоров и механических компонентов. Доверив ремонт нам, вы обеспечиваете себе долговечный и надежный мастерская по ремонту моноколеса рядом.
Подробная информация размещена на сайте: https://remont-monokoles-serv.ru
Cenforce 100mg tablets for sale: cenforce.icu – cheapest cenforce
https://kamagra.men/# Kamagra 100mg price
canadian pharmacy online: drugs1st – pet antibiotics without vet prescription
buying erectile dysfunction pills online: order ed pills online – cheap ed
https://semaglutidetablets.store/# generic rybelsus tabs
https://semaglutidetablets.store/# cheap semaglutide pills
semaglutide tablets for weight loss: buy semaglutide – buy semaglutide
semaglutide tablets price semaglutide tablets store buy semaglutide
стоимость капельницы от запоя стоимость капельницы от запоя .
Наши специалисты предлагает высококачественный экспресс ремонт ipad всех типов и брендов. Мы понимаем, насколько необходимы вам ваши iPad, и обеспечиваем ремонт высочайшего уровня. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что предоставляет длительную работу наших услуг.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств iPad, включают поврежденный экран, проблемы с батареей, ошибки ПО, проблемы с портами и повреждения корпуса. Для устранения этих поломок наши опытные мастера проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе надежный и долговечный центр ремонта айпада на дому.
Подробная информация представлена на нашем сайте: https://remont-ipad-pro.ru
cenforce for sale Cenforce 100mg tablets for sale Purchase Cenforce Online
вывод из запоя в наркологическом стационаре вывод из запоя в наркологическом стационаре .
вывод из запоя в наркологическом стационаре вывод из запоя в наркологическом стационаре .
капельницу коломна от запоя капельницу коломна от запоя .
капельницы от запоя капельницы от запоя .
Uncover the World of Minecraft: Your Ultimate Existence and Freedom Exploration
Welcome to your Portal to the Highly Thrilling and Engaging Minecraft Shared Encounter. Whether you’re a Creator, Fighter, Adventurer, or Tactician, our Server Presents Infinite Chances to Explore Living and Disorder Modes in Experiences you’ve Not seen Before.
—
Why Pick Journeys in Minecraft?
Our Network is Built to Deliver the Top Minecraft Encounter, Merging Specialized Realms, Captivating Interaction, and a Strong Network. Discover, Master, and Create your own Quests with Exclusive Elements Tailored for Any type of User.
—
Primary Highlights
– Living and Anarchy Options: Encounter the Thrill of Enduring against the odds or Dive into Uncontrolled PvP Battles with no rules and full freedom.
– Enormous Server Capacity: With Space for up to 3,750 Users, the Gameplay never stops.
– 24/7 Platform Status: Connect Anytime to Experience Seamless, Reliable Gameplay.
– Tailored Material: Explore our Skillfully Built Minecraft Worlds Loaded with Modifications, Features, and Unique Objects from our Virtual Marketplace.
—
Unique Mechanics Settings
Living Feature
In Endurance Option, you’ll Navigate Vast Terrains, Procure Materials, and Create to your heart’s content. Fight off Mobs, Team Up with Friends, or Face on Solo Quests where only the Strong Overcome.
Freedom Feature
For Players Searching For Chaos and Adventure, Anarchy Feature Provides a World with No Boundaries. Enter in Adrenaline-Fueled PvP Clashes, Establish Alliances, or Challenge Opponents to Control the World. Here, Persistence of the Strongest is the Sole Law.
—
Custom Minecraft Attributes
– Exploration Terrains: Discover Unique Minecraft Dungeons and RPG-Style Tasks.
– Economy and Exchanges: Our Player-Driven Economy Permits you to Sell, Acquire, and Exchange Assets to Climb the Ranks and Form Your Identity as a Dominant User.
– Minecraft Shop: Explore Special Goods, Improvements, and Ranks that Boost your Gameplay.
—
Minecraft Inventory: Boost Your Playstyle
Our In-Game Store Delivers a Selection of Features, Tiers, and Goods to Suit every Preference. From Affordable Donation Bundles to Top-Level Statuses, you can Achieve Fresh Opportunities and Advance your Journey to the Top.
—
Best-Selling Goods
– Donate Packs (x10) – €1.00
– VIP – €1.40
– Elysium Tier – €20.00
– OWNER Tier – €40.00
– BOSS Status – €60.00
—
Top Statuses for Ultimate Gamers
– CREATOR (€10.00) – Access Innovative Options to Unleash your Ideas.
– Vanguard (€12.00) – Elite Options and Exclusive Advantages.
– Paragon (€59.10) – Top Privileges for the Best Player.
– Luminescent (€50.00) – Excel as a Legendary Champion on the Platform.
—
Join Our Thriving Minecraft Network
We Strive in Creating a Welcoming, Dynamic, and Welcoming Network. Whether you’re Facing RPG Adventures, Traversing Custom Zones, or Engaging in Player-Driven PvP, there’s Forever something Fresh to Discover.
—
Elements You Can Enjoy
– Friendly Player Base: Engage With Fellow Minecraft Gamers from Globally.
– Exciting Competitions: Compete in Thrilling Activities, Competitions, and Server-Wide Challenges.
– Dedicated Support: Our Support Group Ensures Seamless Play and Supports you with any Problems.
Welcome to the GTA 5 Roleplay Experience!
Our GTA 5 RP network offers a huge sandbox journey boosted with special personalized mods and gameplay components.
Whether you’re a honest cop or a emerging illegal genius, the chances in the city are infinite:
Develop Your Power: Lead gangs, plan missions, or ascend the illicit system to dominate the territory.
Transform Into a Savior: Maintain justice as a police officer, firefighter, or paramedic.
Personalize Your Adventure: Craft your existence, from high-end vehicles and homes to your character’s special style and storylines.
Thrilling Challenges: Join in adrenaline-fueled missions, massive activities, and community-based tasks to earn prizes and credibility.
Rule the Wealth and Become Wealthy!
At vs-rp.com, we give you the tools and possibilities to gain massive in the digital environment of GTA RP. From obtaining luxury items to amassing wealth, the interactive market is yours to control.
Create your fortune through:
Player-Driven Tasks: Succeed in missions, barters, and contracts to gain in-game wealth.
Custom Ventures: Start and grow your own business, from auto shops to venues.
High-End Items: Acquire luxury automobiles, exclusive estates, and special digital assets.
Are you eager to conquer Los Santos and grow into a virtual magnate?
Why Choose This Experience?
Here’s why we’re the best GTA 5 RP network:
1. Frequent Patches and Activities
Stay on top of the opponents with unique improvements, quests, and timely events. New features are introduced consistently to maintain your journey fresh and thrilling.
2. Unparalleled Range of Assets
Explore a massive catalog of in-game goods, including:
High-End Cars
Exclusive Homes
Unique Items
3. Committed Staff
Whether you’re a veteran user or a beginner newcomer, our staff is prepared to help. We’ll assist you in setting up, give assets, and ensure you thrive in Los Santos.
https://kamagra.men/# Kamagra 100mg
http://kamagra.men/# cheap kamagra
https://semaglutidetablets.store/# cheap semaglutide pills
is it illegal to buy prescription drugs online: ed prescription drugs – online meds for ed
cheap semaglutide pills: buy rybelsus online – semaglutide tablets for weight loss
http://semaglutidetablets.store/# semaglutide best price
semaglutide tablets store: rybelsus semaglutide tablets – semaglutide tablets for weight loss
https://edpills.men/# edmeds
https://kamagra.men/# buy Kamagra
buy rybelsus online: semaglutide tablets price – semaglutide tablets for weight loss
サイボーグ 009
CR牙狼 魔戒ノ花XX
パチンコは、単なるギャンブルではなく、戦略や選択が重要な要素です。楽しみが深まります。
CR牙狼FINAL Ver.399(1:1)
https://injapan.today/topics/get-up-early-and-eat-gourmet-food
美しいグラフィックと音楽が、プレイ中の没入感を高めます。
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
k8 カジノ パチンコ P真・北斗無双 第3章パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
P フィーバー 機動戦士ガンダムユニコーン b
キュインぱちすろ南国育ち 1st vacation
S メイドインアビス
モンスターハンター月下雷鳴
https://www.k8-casino.org/Articles/3005.html
パチンコを通じて、運を試すドキドキ感が味わえます。勝ち負けにこだわらず楽しむのがポイントです。
押忍!サラリーマン番長
https://casinos.town/games/40-super-hot
大当たりの確率を理解することで、より戦略的にプレイできます。計算が好きな人にはおすすめです。
Pフィーバー戦姫絶唱シンフォギア2
https://injapan.today/realtime/id1096.html
演出が多彩で、毎回楽しみが違います。飽きずに遊べるのが魅力です。
Cenforce 100mg tablets for sale Buy Cenforce 100mg Online or cheapest cenforce
https://bbsapp.org/proxy.php?link=https://cenforce.icu buy cenforce
Purchase Cenforce Online order cenforce and cenforce.icu cenforce
semaglutide best price buy semaglutide or semaglutide tablets
http://maps.google.com.np/url?q=http://semaglutidetablets.store semaglutide best price
generic rybelsus tabs buy rybelsus online and rybelsus semaglutide tablets buy semaglutide
en kaliteli sultangazi escort bayan sitesiyim
Buy Cenforce 100mg Online: Cenforce 150 mg online – cenforce.icu
Betflare Casino Betflare Casino .
Betflare 250% ??????? Betflare 250% ??????? .
после капельницы от запоя после капельницы от запоя .
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
http://www.babelcube.com/user/kelvin-sowards
https://okwave.jp/profile/u3136120.html
https://www.obesityhelp.com/members/hedgy1988/about_me/
https://tubeteencam.com/user/lexmorf1959/profile
https://www.haikudeck.com/presentations/2DuelJ3AmD
капельницы от запоя на дому капельницы от запоя на дому .
капельница от запоя стоимость капельница от запоя стоимость .
https://cenforce.icu/# cenforce for sale
efsane bir şişli escort mecidiyeköy escort sitesi
en kaliteli sultangazi escort bayan sitesiyim
semaglutide best price buy semaglutide or semaglutide best price
http://www.villacapriani.com/redirect.aspx?destination=https://semaglutidetablets.store semaglutide tablets store
semaglutide tablets buy rybelsus online and semaglutide best price semaglutide tablets price
buy semaglutide: semaglutide tablets store – semaglutide best price
Buy Cenforce 100mg Online Cenforce 100mg tablets for sale or cenforce.icu
https://www.google.com.ua/url?q=https://cenforce.icu cheapest cenforce
Cenforce 100mg tablets for sale Cenforce 100mg tablets for sale and Cenforce 150 mg online cenforce
https://edpills.men/# discount ed pills
http://edpills.men/# best online ed meds
https://drugs1st.pro/# buy canadian drugs
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телевизоров hisense рядом, можете посмотреть на сайте: ремонт телевизоров hisense
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Узнай, что предлагает 1xSlots https://aztarna.es/pages/1xslots-casino_15.html
buy rybelsus online: semaglutide tablets for weight loss – rybelsus semaglutide tablets
https://semaglutidetablets.store/# semaglutide tablets
Thank you for sharing such valuable insights!
https://cenforce.icu/# cenforce
ways to treat erectile dysfunction: drugs1st – 100mg viagra without a doctor prescription
drugs1st: ed pills online pharmacy – ed trial pack
https://semaglutidetablets.store/# cheap semaglutide pills
Kamagra 100mg price: Kamagra 100mg price – buy kamagra online usa
ed online prescription edmeds ed treatment online
cheapest cenforce cenforce for sale order cenforce
Добро пожаловать на сайт ГГУ имени Ф.Скорины, с полной информацией о университете.
Изучите учебные планы университета, чтобы достигнуть своих целей.
Станьте студентом ГГУ имени Ф.Скорины, и откроется перед вами мир знаний и возможностей.
Углубитесь в науку с ГГУ имени Ф.Скорины, и вы обретете преимущества на рынке труда.
Познакомьтесь с факультетами и исследовательскими лабораториями ГГУ имени Ф.Скорины, и вы сможете раскрыть свой потенциал.
Будьте в курсе актуальных событий в университете, чтобы быть в центре научной и академической жизни.
Участвуйте в научных исследованиях университета, для вашего будущего успеха в профессиональной сфере.
Получите поддержку и советы от опытных наставников, для расширения своих профессиональных связей.
Докажите свои научные и профессиональные достижения, для вашего успешного старта и карьерного роста.
Выберите ГГУ имени Ф.Скорины для своего образования и научной карьеры, и вы станете частью успешных выпускников и ученых.
Завершите свой путь обучения в ГГУ имени Ф.Скорины с отличием, для вашего признания и успеха в образовательной и научной сфере.
Откройте для себя мир знаний и исследований в ГГУ имени Ф.Скорины, для вашего умственного и профессионального роста.
Участвуйте в кружках и клубах университета, чтобы раскрыть свой творческий потенциал и навыки.
Обучитесь на кафедрах и специализации в ГГУ имени Ф.Скорины, для вашего успешного трудоустройства и карьерного роста
universities https://gsu.by/ .
Крупный учебный и научно-исследовательский центр Республики Беларусь. Высшее образование в сфере гуманитарных и естественных наук на 12 факультетах по 35 специальностям первой ступени образования и 22 специальностям второй, 69 специализациям.
buy semaglutide rybelsus semaglutide tablets or semaglutide tablets price
https://www.google.com.np/url?q=http://semaglutidetablets.store buy rybelsus online
semaglutide tablets store semaglutide tablets store and semaglutide tablets store semaglutide tablets for weight loss
https://semaglutidetablets.store/# generic rybelsus tabs
Explore the cost of obtaining a realtor license in Ohio | Start a new career with an Ohio real estate license | Discover how to get your Ohio real estate license today | Understand the requirements for Ohio real estate agent certification | Become a professional real estate agent in Ohio with ease | Affordable ways to obtain your Ohio real estate license Ohio real estate salesperson license | Professional growth with an Ohio realtor license | Discover professional opportunities with Ohio real estate license | Explore the requirements for real estate licensing in Ohio http://ohio-real-estate-license.com.
online erectile dysfunction pills best ed meds online or cheapest online ed meds
https://toolbarqueries.google.at/url?sa=t&url=https://edpills.men pills for ed online
cheapest ed pills online ed pharmacy and get ed meds today best online ed treatment
https://edpills.men/# low cost ed medication
semaglutide tablets for weight loss buy semaglutide or buy rybelsus online
http://www.ourphlibrary.com/disclaimer?url=https://semaglutidetablets.store cheap semaglutide pills
buy semaglutide semaglutide tablets price and semaglutide tablets store semaglutide best price
Как оказалось, купить диплом кандидата наук не так уж и сложно
http://drugs1st.pro/# drugs1st
Тем, кого не прельщает перспектива в поте лица добывать свой хлеб, во все времена было важно прорваться наверх и остаться там навсегда. В страстных, порой лихорадочных поисках своего личного горшка с золотом (а также сопутствующих ему власти и престижа) амбициозные мужчины и женщины всегда старались перенять знания и опыт у тех, кто уже достиг успеха
https://human-design-slovar.rappro.ru
ed meds online online ed medication or ed medication online
https://cse.google.is/url?sa=t&url=https://edpills.men cheap ed pills online
[url=https://image.google.je/url?q=https://edpills.men]low cost ed meds[/url] ed doctor online and [url=http://cwk.freehostia.com/viewpro.php?username=rtkhyftvco]buy ed medication[/url] low cost ed meds online
semaglutide tablets price: semaglutide tablets for weight loss – semaglutide tablets price
https://kamagra.men/# buy Kamagra
drugs1st: drugs1st – the canadian drugstore
affordable ed medication: online prescription for ed – online ed pharmacy
https://edpills.men/# ed online prescription
Purchase Cenforce Online Buy Cenforce 100mg Online or cheapest cenforce
https://www.google.com.sa/url?q=https://cenforce.icu cenforce
Cenforce 100mg tablets for sale buy cenforce and Buy Cenforce 100mg Online Cenforce 100mg tablets for sale
http://drugs1st.pro/# drugs1st
Оцените удобство 1xslots мобильной версии для комфортной игры.
https://kamagra.men/# super kamagra
sildenafil oral jelly 100mg kamagra: Kamagra 100mg price – super kamagra
デビルサバイバー2 最後の7日間
オンラインカジノ
パチンコの仲間と競い合うことで、友情が深まります。共に喜び、共に悔しがる楽しさがあります。
スーパービンゴネオ
https://pachika.com/machine/post-8444.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にバトル時が最高。
CR牙狼闇を照らす者
k8パチンコ
キュインぱちすろ南国育ち 1st vacation
輪るピングドラム
ラブ嬢
政宗
https://yoshimune3.jahromblog.com/post-349/
歴史をテーマにしたパチスロで、独特の魅力があります。日本の伝統を感じます。
CRぱちんこ仮面ライダーV3 Ver.390(1:2
https://www.lambethchildabuse.org/article/1990.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
CR RAVE この世界こそが真実だ
https://www.gamegeeknews.com/article/118.html
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
https://casinositeleri2025.pro/# casino slot oyunlarД±
Тем, кого не прельщает перспектива в поте лица добывать свой хлеб, во все времена было важно прорваться наверх и остаться там навсегда. В страстных, порой лихорадочных поисках своего личного горшка с золотом (а также сопутствующих ему власти и престижа) амбициозные мужчины и женщины всегда старались перенять знания и опыт у тех, кто уже достиг успеха
https://human-design-slovar.rappro.ru
https://casinositeleri2025.pro/# betler
slot siteleri: slot oyunlar? puf noktalar? – az parayla cok kazandiran slot oyunlar?
az parayla cok kazandiran slot oyunlar? az parayla cok kazandiran slot oyunlar? slot oyunlar? puf noktalar?
casino slot: casinoda en Г§ok kazandД±ran oyun – dГјnyanД±n en iyi bahis siteleri
https://casinositeleri2025.pro/# deneme bonusu veren yeni siteler 2025
АО Ти И Эл Лизинг https://avtee.ru предлагает услуги проката автомобилей в России, Турции, ОАЭ, Черногории, Испании и других странах по всему миру. Широкий выбор авто, выгодные условия и удобное бронирование делают поездки комфортными и доступными для каждого клиента.
slot oyunlar?: en cok kazand?ran slot oyunlar? – slot oyunlar?
http://pinup2025.com/# пин ап казино официальный сайт
Bitcoin is a decentralized digital currency that operates without the demand quest of a principal authority or arbitrator, like a bank. Introduced in 2009 via an unrevealed individual or batch under the nom de plume Satoshi Nakamoto, Bitcoin is powered alongside blockchain technology, a public ledger that records all transactions securely and transparently. It is known destined for its limited accommodate of 21 million coins and its services as a store of value and usual of exchange. Bitcoin has ripen into a well-received investment alternative and a symbol of the evolving digital economy.
https://celestiaairdrop.online
https://grassairdrop.online
https://pipe-network-airdrop.online
https://supra-airdrop.online
https://morph-airdrop.online
https://movement-airdrop.online
https://moongate-airdrop.online
https://airdrop-jupiter.online
https://dawn-airdrop.online
https://swell-airdrop.online
https://onchain-airdrop.online
https://htx-airdrop.online
https://nodepay-airdrop.online
https://strk-airdrop.online
https://bera-airdrop.online
https://zro-airdrop.online
https://bless-airdrop.online
https://kelp-dao-airdrop.online
https://aptos-airdrop.online
https://across-airdrop.online
https://airdrop-dogs.online
https://vela-exchange-airdrop.online
https://blockmesh-airdrop.online
https://hyperliquid-airdrop.online
https://paws-airdrop.online
https://375ai-airdrop.online
https://airdropblast.online
https://xion-airdrop.online
https://squid-airdrop.online
https://airdrop-arb.online
soap2day horror movies soap2day for android
Наша мастерская предлагает надежный сервисный ремонт айфона на дому различных марок и моделей. Мы знаем, насколько необходимы вам ваши смартфоны Apple, и стремимся предоставить услуги наилучшего качества. Наши профессиональные техники работают быстро и аккуратно, используя только качественные детали, что обеспечивает длительную работу выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются пользователи смартфонов Apple, включают неисправности дисплея, поломку батареи, программные сбои, неработающие разъемы и повреждения корпуса. Для устранения этих неисправностей наши квалифицированные специалисты выполняют ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы обеспечиваете себе долговечный и надежный вызвать мастера по ремонту iphone в москве.
Подробная информация размещена на сайте: https://remont-iphone-sot.ru
Тем, кого не прельщает перспектива в поте лица добывать свой хлеб, во все времена было важно прорваться наверх и остаться там навсегда. В страстных, порой лихорадочных поисках своего личного горшка с золотом (а также сопутствующих ему власти и престижа) амбициозные мужчины и женщины всегда старались перенять знания и опыт у тех, кто уже достиг успеха
https://human-design-slovar.rappro.ru
https://casinositeleri2025.pro/# deneme bonusu veren bet siteleri
Гораздо большего можно добиться при помощи доброго слова и пистолета, нежели при помощи одного лишь доброго слова
https://human-design-slovar.rappro.ru
Тем, кого не прельщает перспектива в поте лица добывать свой хлеб, во все времена было важно прорваться наверх и остаться там навсегда. В страстных, порой лихорадочных поисках своего личного горшка с золотом (а также сопутствующих ему власти и престижа) амбициозные мужчины и женщины всегда старались перенять знания и опыт у тех, кто уже достиг успеха
https://human-design-slovar.rappro.ru
yeni deneme bonusu veren siteler 2025 [url=http://casinositeleri2025.pro/#]kumar siteleri[/url] date sitesi
pinup 2025: пин ап казино официальный сайт – pinup 2025
http://slottr.top/# slot oyunlar?
jav siteleri: bonusu veren siteler – tГјrkiye casino siteleri
пин ап казино официальный сайт pinup 2025 пин ап казино официальный сайт
pinup 2025: пинап казино – пин ап зеркало
https://casinositeleri2025.pro/# para kazandД±ran sohbet siteleri
Bitcoin is a decentralized digital currency that operates without the need for a central expert or third party, like a bank. Introduced in 2009 by an unidentified mortal or society answerable to the pseudonym Satoshi Nakamoto, Bitcoin is powered by blockchain technology, a overt ledger that records all transactions securely and transparently. It is known as a remedy for its narrow reservoir of 21 million coins and its use as a collect of value and medium of exchange. Bitcoin has grace a well-received investment selection and a badge of the evolving digital economy.
https://vela-exchange-airdrop.online
https://navigate-airdrop.online
https://drop-airdrop.online
https://airdrop-arb.online
https://braavos-airdrop.online
https://plena-airdrop.online
https://jito-airdrop.online
https://kelp-dao-airdrop.online
https://tensor-airdrop.online
https://uprock-airdrop.online
https://arena-z-airdrop.online
https://ink-airdrop.online
https://stride-airdrop.online
https://kroma-airdrop.online
https://morph-airdrop.online
https://farcaster-airdrop.online
https://scallop-airdrop.online
https://lisk-airdrop.online
https://overtrip-airdrop.online
https://mantra-airdrop.online
https://arch-network-airdrop.online
https://mode-airdrop.online
https://mitosis-airdrop.online
https://the-vault-airdrop.online
https://gudchain-airdrop.online
https://b3-airdrop.online
https://aztec-airdrop.online
https://paws-airdrop.online
https://zoth-airdrop.online
https://symbol-airdrop.online
Гораздо большего можно добиться при помощи доброго слова и пистолета, нежели при помощи одного лишь доброго слова
https://abuse.g-u.su
http://casinositeleri2025.pro/# yeni bonus veren bahis siteleri
pinup2025.com пин ап вход пин ап вход
пин ап: пин ап вход – pinup 2025
Наши специалисты предлагает надежный сервисный ремонт iphone всех типов и брендов. Мы знаем, насколько важны для вас ваши устройства iPhone, и обеспечиваем ремонт первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только качественные детали, что гарантирует надежность и долговечность наших услуг.
Наиболее частые неисправности, с которыми сталкиваются пользователи смартфонов Apple, включают проблемы с экраном, проблемы с батареей, неисправности программного обеспечения, проблемы с портами и поломки корпуса. Для устранения этих проблем наши профессиональные техники оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы гарантируете себе качественный и надежный вызвать мастера по ремонту айфона на выезде.
Подробная информация размещена на сайте: https://remont-iphone-sot.ru
Важные факты об 1xSlots https://davisa.es/page/1xslots-casino-argentina.html
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт телевизоров hisense, можете посмотреть на сайте: ремонт телевизоров hisense
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
pinup 2025: пин ап вход – пин ап казино
Welcome to the GTA 5 Roleplay Experience!
Our GTA 5 RP realm offers a massive open-world gameplay augmented with exclusive custom mods and playstyle components.
Whether you’re a rule-following lawman or a emerging crime strategist, the possibilities in the city are infinite:
Establish Your Dominion: Join groups, execute missions, or rise the illegal system to dominate the streets.
Transform Into a Guardian: Enforce order as a police officer, rescuer, or paramedic.
Personalize Your Journey: Shape your life, from high-end rides and residences to your avatar’s individual style and narratives.
Adventurous Challenges: Participate in high-stakes operations, spectacular challenges, and user-led missions to collect benefits and credibility.
Dominate the System and Become Wealthy!
At vs-rp.com, we provide you the resources and options to collect huge in the virtual realm of GTA RP. From obtaining exclusive properties to building money, the Roleplay system is yours to conquer.
Create your fortune through:
Player-Driven Missions: Complete tasks, exchanges, and contracts to acquire virtual currency.
Custom Enterprises: Establish and manage your own operation, from auto shops to nightclubs.
High-End Properties: Acquire premium vehicles, one-of-a-kind properties, and special online items.
Are you ready to rule Los Santos and grow into a online mogul?
Why Pick This Experience?
Here’s why we’re the premier GTA 5 RP server:
1. Regular Updates and Features
Stay in front of the competition with exclusive improvements, events, and periodic opportunities. New elements are introduced consistently to make your interaction fresh and fun.
2. Massive Variety of Assets
Explore a massive inventory of in-game assets, including:
Premium Cars
Top-Tier Properties
Limited Goods
3. Dedicated Help
Whether you’re a veteran user or a new rookie, our support team is available to assist. We’ll help you in configuring, deliver help, and make sure you thrive in Los Santos.
https://casinositeleri2025.pro/# slot oyna
bütün oyun siteleri yeni siteler gerçek paralı casino oyunları
deneme bonusu bahis siteleri: deneme bonusu veren siteler 2025 – en gГјvenilir bahis siteleri hangileri?
bet siteleri bonus: yabancД± Еџans oyunlarД± siteleri – casino bet gГјncel giriЕџ
Find out how to pass the Ohio real estate exam | Prepare for the Ohio real estate test with confidence | Gain insights into the Ohio real estate market for new agents | Ohio real estate licensing – everything you need to know | Benefits of holding a realtor license in Ohio | Build a successful career with an Ohio realtor license realtor license Ohio | Learn the secrets to passing Ohio’s real estate exam | Discover professional opportunities with Ohio real estate license | Comprehensive resources for Ohio real estate licensing success Ohio real estate test.
http://slottr.top/# slot oyunlar?
http://pinup2025.com/# пинап казино
http://slottr.top/# slot oyunlar? puf noktalar?
Experience the Universe of Minecraft: Your Supreme Persistence and Chaos Quest
Welcome to your Portal to the Most Exciting and Interactive Minecraft Online Journey. Whether you’re a Designer, Battler, Traveler, or Strategist, our Platform Provides Endless Opportunities to Explore Living and Anarchy Environments in Ways you’ve Seldom seen Before.
Why Pick Journeys in Minecraft?
Our Network is Developed to Deliver the Best Minecraft Journey, Merging Specialized Worlds, Engaging Mechanics, and a Vibrant Group. Traverse, Master, and Create your own Adventures with Special Elements Customized for Any type of Gamer.
Essential Attributes
– Persistence and Chaos Settings: Experience the Challenge of Surviving against the odds or Dive into Untamed PvP Engagements with no rules and full freedom.
– Massive Realm Capacity: With Space for up to 3,750 Users, the Fun never stops.
– 24/7 Server Access: Join At Any Moment to Enjoy Smooth, Reliable Play.
– Unique Features: Explore our Expertly Created Minecraft Maps Loaded with Addons, Extras, and Exclusive Products from our Virtual Marketplace.
Unique Gameplay Features
Living Mode
In Survival Option, you’ll Traverse Expansive Environments, Gather Assets, and Create to your heart’s content. Battle off Opponents, Collaborate with Allies, or Conquer on Solo Challenges where only the Strong Win.
Disorder Scenario
For Participants Seeking Chaos and Adrenaline, Anarchy Option Delivers a Environment with No Boundaries. Engage in Competitive PvP Fights, Build Partnerships, or Challenge Players to Control the Zone. Here, Survival of the Best is the Only Rule.
Tailored Minecraft Components
– Quest Worlds: Navigate Custom Minecraft Maps and RPG-Style Quests.
– Economy and Trading: Our Player-Driven System Permits you to Trade, Obtain, and Offer Assets to Ascend the Positions and Build Your Identity as a Dominant Gamer.
– Minecraft Store: Explore Premium Items, Levels, and Statuses that Improve your Interaction.
Minecraft Marketplace: Upgrade Your Interaction
Our In-Game Store Provides a Range of Features, Levels, and Goods to Cater To every Player. From Cheap Contribution Offers to Exclusive Upgrades, you can Unlock Exciting Possibilities and Advance your Adventures to the Next Level.
Best-Selling Products
– Donate Cases (x10) – €1.00
– VIP – €1.40
– Elysium Status – €20.00
– OWNER Rank – €40.00
– BOSS Status – €60.00
Top Levels for Premier Players
– CREATOR (€10.00) – Unlock Artistic Components to Express your Ideas.
– Vanguard (€12.00) – Premier Features and Special Features.
– Paragon (€59.10) – Exclusive Benefits for the Best User.
– Luminescent (€50.00) – Excel as a Iconic Player on the Platform.
Join Our Thriving Minecraft Group
We Focus in Forming a Encouraging, Dynamic, and Inclusive Network. Whether you’re Tackling RPG Quests, Discovering Tailored Maps, or Competing in Player-Driven PvP, there’s Forever something New to Experience.
Features You Can Enjoy
– Friendly Group: Meet Fellow Minecraft Fans from Around the World.
– Exciting Activities: Engage in Exclusive Competitions, Tournaments, and Global Activities.
– Dedicated Assistance: Our Team Ensures Smooth Interaction and Supports you with any Questions.
真モグモグ風林火山2
k8 カジノ パチンコ デビルサバイバー2 最後の7日間
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
ベヨネッタ
https://www.pgslot-casino.com/article/77.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
Pガールズパ ンツァー 劇場版
k8 カジノ CR大海物語4 Ver.319
CR真・花の慶次 Ver.399
CR戦国乙女〜花〜
パチスロだよ黄門ちゃま
Pフィーバーダンベル何キロ持てる
https://www.3ae.jp/news/14658.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR大海物語4 Ver.319
https://www.urahara.jp/bonuses/post-966.html
演出が多彩で、毎回楽しみが違います。飽きずに遊べるのが魅力です。
CRぱちんこ仮面ライダーV3 Ver.390(1:2
https://www.indocx-pachinko.com/article/131.html
お店によって雰囲気が異なるので、色々な店を試してみるのが楽しいです。新しい発見があります。
pinup 2025 пин ап казино официальный сайт or pinup2025.com
https://cse.google.co.ck/url?sa=t&url=https://pinup2025.com pinup 2025
пин ап казино официальный сайт пин ап казино официальный сайт and пинап казино пин ап казино
free spin casino [url=https://casinositeleri2025.pro/#]en iyi yasal bahis siteleri[/url] siteler bahis
пин ап казино зеркало пинап казино or пин ап
https://www.blickle.cn/цпФхЕЛхКЫф?Р·С…РЈР‘/С„?зхУБцЯецЙ?хЩи/С‡?УцЮЬ?ReturnStep3=https://pinup2025.com пин ап
пинап казино пин ап казино and pinup 2025 пин ап вход
Ценности влияют на все выборы, которые мы делаем в жизни. Понимание того, как формируются ценности, позволит более точечно позиционировать свои товары и услуги, тем самым успешно развиваться на рынке.
https://abuse.g-u.su
https://pinup2025.com/# пинап казино
купить двери межкомнатные двери спб
bilinmeyen siteler: casino slot oyunlarД± – pronet giriЕџ
http://casinositeleri2025.pro/# bahis siteleri deneme bonusu
Наш сервисный центр предлагает профессиональный официальный ремонт макбука с гарантией различных марок и моделей. Мы осознаем, насколько необходимы вам ваши устройства MacBook, и обеспечиваем ремонт наилучшего качества. Наши квалифицированные специалисты работают быстро и аккуратно, используя только оригинальные запчасти, что предоставляет длительную работу проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств MacBook, включают неисправности дисплея, неисправности аккумулятора, неисправности программного обеспечения, неработающие разъемы и неисправности системы охлаждения. Для устранения этих проблем наши профессиональные техники выполняют ремонт экранов, батарей, ПО, разъемов и систем охлаждения. Обратившись к нам, вы обеспечиваете себе надежный и долговечный вызвать мастера по ремонту macbook.
Подробная информация представлена на нашем сайте: https://remont-macbook-club.ru
vidobet casino malta guvenilir bahis siteleri
tГјm casino siteleri: bahis oyun siteleri – betboo plus
정말 훌륭한 글이라고 말해야 할 것 같아요.. 정말 이 글에 감명을 받았어요… 이 글을 만든 사람은 정말 대단한 사람이었어요. 제 사이트에 당신 블로그 링크 중 하나를 넣었는데, 괜찮으시겠어요?
vidobet giriЕџ 2025: oyun dene – 100 tl bonus veren bahis siteleri
https://casinositeleri2025.pro/# deneme bonusu veren bet siteleri
Ценности влияют на все выборы, которые мы делаем в жизни. Понимание того, как формируются ценности, позволит более точечно позиционировать свои товары и услуги, тем самым успешно развиваться на рынке.
https://abuse.g-u.su
Тем, кого не прельщает перспектива в поте лица добывать свой хлеб, во все времена было важно прорваться наверх и остаться там навсегда. В страстных, порой лихорадочных поисках своего личного горшка с золотом (а также сопутствующих ему власти и престижа) амбициозные мужчины и женщины всегда старались перенять знания и опыт у тех, кто уже достиг успеха
https://abuse.g-u.su
date sitesi 100tl hosgeldin bonusu veren siteler or +18 canli yayin siteleri
https://www.google.gm/url?q=https://casinositeleri2025.pro casino en Г§ok kazandД±ran oyunlar
gГјvenilir deneme bonusu veren siteler lisanslД± bahis sitesi and popГјler bahis pronet giriЕџ
Ценности влияют на все выборы, которые мы делаем в жизни. Понимание того, как формируются ценности, позволит более точечно позиционировать свои товары и услуги, тем самым успешно развиваться на рынке.
https://abuse.g-u.su
http://casinositeleri2025.pro/# en iyi bahis uygulamasД±
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://permacultureglobal.org/users/64359-brent-lowe
https://rentry.org/hcn5qkkr
https://www.obesityhelp.com/members/mauyri1994/about_me/
http://www.nfomedia.com/profile?uid=rOjTakD
https://tubeteencam.com/user/anyutka1990/profile
online kumar siteleri en yeni deneme bonusu veren siteler 2025 casino gГјvenilir siteler
slot tr online slot oyunlar? or en cok kazand?ran slot oyunlar?
http://www.162100.com/export.php?url=http://slottr.top az parayla cok kazandiran slot oyunlar?
en kazancl? slot oyunlar? slot oyunlar? puf noktalar? and slot oyunlar? puf noktalar? slot oyunlar?
п»їcasino by casino or bonus veren idda siteleri
https://cse.google.mg/url?q=https://casinositeleri2025.pro casino malta
[url=https://hc-vsetin.cz/media_show.asp?type=1&id=246&url_back=http://casinositeleri2025.pro]date sitesi[/url] en bГјyГјk bahis siteleri and [url=http://bbs.soumoli.com/home.php?mod=space&uid=66214]casino deneme bonusu veren bahis siteleri[/url] en yeni bet siteleri
пинап казино: пин ап вход – пин ап казино
http://pinup2025.com/# pinup 2025
http://casinositeleri2025.pro/# gГјvenilir oyun siteleri
slot tr online: slot tr online – az parayla cok kazandiran slot oyunlar?
slot siteleri slot oyunlar? puf noktalar? slot oyunlar? puf noktalar?
slot oyunlar? slot tr online or az parayla cok kazandiran slot oyunlar?
http://birddelacoeur.com.au/?URL=https://slottr.top:: slot oyunlar?
slot oyunlar? puf noktalar? slot tr online and slot oyunlar? en cok kazand?ran slot oyunlar?
http://casinositeleri2025.pro/# canlД± oyunlar
yeni aГ§Д±lan bahis siteleri: canli bahis siteleri – casino en Г§ok kazandД±ran oyunlar
Наши специалисты предлагает высококачественный срочный ремонт варочной панели различных марок и моделей. Мы знаем, насколько необходимы вам ваши варочные панели, и стремимся предоставить услуги первоклассного уровня. Наши квалифицированные специалисты работают быстро и аккуратно, используя только оригинальные запчасти, что обеспечивает длительную работу наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются пользователи плит, включают проблемы с нагревом, проблемы с сенсорным управлением, неисправности программного обеспечения, проблемы с подключением и поломки компонентов. Для устранения этих неисправностей наши квалифицированные специалисты оказывают ремонт нагревательных элементов, сенсоров, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы гарантируете себе долговечный и надежный центр ремонта варочной панели с гарантией.
Подробная информация доступна на сайте: https://remont-varochnyh-paneley-hit.ru
https://pinup2025.com/# пин ап казино зеркало
Plinko se ha convertido https://medium.com/@kostumchik.kiev.ua/todo-sobre-el-juego-de-plinko-en-m%C3%A9xico-instrucciones-demos-opiniones-y-m%C3%A1s-d1fde2d99338 en una de las opciones favoritas de los jugadores de casinos en Mexico. Conocido por su simplicidad y gran potencial de ganancias, este adictivo juego ahora cuenta con una plataforma oficial en Mexico.
Онлайн-журнал о строительстве https://zip.org.ua практичные советы, современные технологии, тренды дизайна и архитектуры. Всё о строительных материалах, ремонте, благоустройстве и инновациях в одной удобной платформе.
吉宗 2003
k8 カジノ パチンコ
パチンコの世界は奥が深く、探求心をくすぐられます。新しい情報を得ることで、さらに楽しめます。
カイジ
https://injapan.today/realtime/id4969.html
ホラー映画の要素が盛り込まれており、ファンにはたまらない作品です。感情移入できます。
CRフィーバー バイオハザード リベレーションズ
k8カジノ パチンコ
CR燃える闘魂アントニオ猪木~格闘技世界一決定戦~
CR神獣王2
CR真・花の慶次L6-K 319ver.
戦国乙女剣戟に舞う白き剣聖西国参戦編
https://www.pinupcasino-play.com/article/85.html
パチンコの仲間と競い合うことで、友情が深まります。共に喜び、共に悔しがる楽しさがあります。
戦姫絶唱シンフォギア
https://azurlane.jahromblog.com/post-320/
キャラクターの個性が豊かで、ストーリーに引き込まれます。忍者の世界観が楽しめます。
吉宗極
https://casinos.town/games/slingo-x-factor
簡単に遊べるルールで、初心者でも安心。気軽に挑戦できるのが良いです。
2025 bahis siteleri deneme bonusu superbetin giriЕџ 2025 bahis siteleri deneme bonusu
Ваш гид в мире строительства https://zip.org.ua и ремонта. Советы специалистов, пошаговые инструкции, полезные статьи и ответы на популярные вопросы.
Thank you for breaking this down so clearly!
https://casinositeleri2025.pro/# yeni bonus veren bahis siteleri
en kazancl? slot oyunlar?: az parayla cok kazandiran slot oyunlar? – slot siteleri
пинап казино пин ап казино официальный сайт or пин ап казино зеркало
http://forum.wonaruto.com/redirection.php?redirection=http://pinup2025.com/ пин ап
pinup 2025 пинап казино and пин ап казино пинап казино
пин ап пинап казино or пин ап казино официальный сайт
https://www.google.me/url?q=https://pinup2025.com пин ап
пинап казино пин ап and pinup2025.com pinup2025.com
https://casinositeleri2025.pro/# 100 tl bonus veren bahis siteleri
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
pinup 2025 пин ап зеркало pinup 2025
промокод на продамус скидка подключение http://www.promokod-prod.ru .
gГјvenilir bahis siteleri casino bahis siteleri or en gГјvenilir yatД±rД±m siteleri
http://sat.kuz.ru/engine/redirect.php?url=http://casinositeleri2025.pro deneme bonus siteler
casino slot siteleri slot casino and orisbet gГјvenilir bahis siteleri 2024
slot oyunlar?: slot oyunlar? – slot siteleri
2025 bahis siteleri deneme bonusu kumar oynama siteleri or bonus veren yasal bahis siteleri
https://images.google.com.af/url?q=https://casinositeleri2025.pro bahis giriЕџ
gГјzel siteler gГјvenilir bahis siteleri and en iyi bet siteleri saДџlam bahis siteleri 2025
как найти по номеру телефона человека как найти по номеру телефона человека .
en cok kazand?ran slot oyunlar?: az parayla cok kazandiran slot oyunlar? – slot oyunlar? puf noktalar?
http://slottr.top/# slot tr online
рейтинг казино на реальные деньги казино онлайн рейтинг лучших
戦国乙女剣戟に舞う白き剣聖西国参戦編
CR海物語4
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
北斗の拳世紀末救世主伝説
https://www.osaka-casino.asia/article/217.html
演出が華やかで、視覚的にも楽しめます。アートとしての側面もあります。
南国育ち
ゴジラエヴァ 甘デジ
CR北斗の拳6 拳王
3x3EYES聖魔覚醒
P とある魔術の禁書目録
CR真・北斗無双
https://www.casino-games.asia/article/5805.html
パチンコの仲間との交流が楽しく、共通の趣味で楽しめるのが良いです。友達が増えます。
回胴黙示録カイジ3
https://www.pachinko-hokuto.com/article/218.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
P とある魔術の禁書目録 2
https://www.k8casinoofficial.jp/guide/id1692.html
パチンコの台によって異なる演出があり、選ぶ楽しさがあります。自分に合った台を見つけるのが良いです。
https://casinositeleri2025.pro/# casino malta
https://pinup2025.com/# пин ап казино официальный сайт
пин ап зеркало pinup 2025 пин ап казино зеркало
spor siteleri listesi vaycasino or jav siteleri
http://toolbarqueries.google.com.ni/url?sa=i&url=https://casinositeleri2025.pro:: deneme bonus siteler
deneme bonusu veren bahis siteleri 2025 gГјvenilir deneme bonusu veren siteler and 1xbet bonus Г§evrim ЕџartlarД± oyun siteleri
https://slottr.top/# slot siteleri
Casino games offer a thrilling experience for those who enjoy high stakes. Whether you’re into modern video slots, there’s something for everyone. Many casinos feature bonus offers to attract players.
For online gamblers, flexibility of virtual platforms is unparalleled. With fast withdrawals, online casinos guarantee a seamless gaming experience. You can try out new slots from the comfort of your home.
https://aceold.aua.am/2019/00/
Playing with limits is key for a balanced experience. Many platforms provide tools to ensure safe play. Remember, the thrill of the game is what makes gambling engaging.
Mostbet mobil ilovasi bilan qulay o‘ynash | Mostbet 2024 yilgi yangi imkoniyatlari | Mostbet uz ro‘yxatdan o‘tish va bonus olish usullari | Mostbet oynash uchun foydali maslahatlar | Mostbet uz uchun eng yaxshi o‘yin platformasi mostbet login | Mostbet uz oynash va sportga pul tikish bo‘yicha maslahatlar | Mostbet oynash va o‘yinlar uchun yangi imkoniyatlar | Mostbet apk download va yangi imkoniyatlar mostbet skachat.
gГјvenilir illegal bahis siteleri: bonus veren site – superbetin giriЕџ
slot siteleri slot oyunlar? puf noktalar? or en cok kazand?ran slot oyunlar?
http://wildcakes.ru/forum/away.php?s=http://slottr.top en kazancl? slot oyunlar?
en kazancl? slot oyunlar? slot oyunlar? and slot oyunlar? slot oyunlar?
пин ап зеркало pinup 2025 пинап казино
en saДџlam bahis siteleri: internet kumar oyunu – casinomaxi
Bitcoin is a decentralized digital currency that operates without the penury after a principal expert or arbitrator, like a bank. Introduced in 2009 via an unknown idiosyncratic or congregation subsumed under the nom de plume Satoshi Nakamoto, Bitcoin is powered away blockchain technology, a public ledger that records all transactions securely and transparently. It is known as a remedy for its narrow accommodate of 21 million coins and its consume as a collect of value and median of exchange. Bitcoin has become a sought-after investment choice and a badge of the evolving digital economy.
https://celestiaairdrop.online
https://kroma-airdrop.online
https://drop-airdrop.online
https://wizzwoods-airdrop.online
https://nulink-airdrop.online
https://hemi-network-airdrop.online
https://swell-airdrop.online
https://vela-exchange-airdrop.online
https://movement-airdrop.online
https://loop-airdrop.online
https://airdropblast.online
https://kelp-dao-airdrop.online
https://polygon-zkevm-airdrop.online
https://farcaster-airdrop.online
https://debridge-airdrop.online
https://rainbow-airdrop.online
https://starrynift-airdrop.online
https://venomairdrop.online
https://optopia-airdrop.online
https://bless-airdrop.online
https://zerion-airdrop.online
https://zro-airdrop.online
https://airdrop-jupiter.online
https://bera-airdrop.online
https://nodepay-airdrop.online
https://taiko-airdrop.online
https://overtrip-airdrop.online
https://rivalz-network-airdrop.online
https://phantom-airdrop.online
https://hana-network-airdrop.online
Discover and book the best Uffizi Gallery tours in Florence
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me. https://www.binance.com/pt-BR/join?ref=YY80CKRN
pinup 2025: пин ап казино – пин ап казино зеркало
Discover the World of Counter-Strike 2 Skins: Pick the Ultimate Look for Your Gameplay
Transform your gameplay with one-of-a-kind, high-quality cosmetics that enhance the look of your equipment and turn your inventory truly be unique.
We feature a wide range of skins — from rare items to special edition sets, offering you the ability to enhance your weapons and display your style.
Reasons to Pick Us?
Purchasing CS2 items from us is quick, safe, and hassle-free. With immediate digital processing straight to your account, you can begin equipping your recently purchased skins right away.
Our Advantages:
– Reliable Payments: Enjoy safe, dependable transactions every time.
– Low Rates: We provide the most competitive item offers with regular discounts.
– Diverse Options: From affordable to exclusive skins, we deliver it all.
How It Works:
1. Search the Collection
Search our wide collection of cosmetics, grouped by weapon type, scarcity, and theme.
2. Choose Your Skin
Once you select the ideal option, put it to your cart and continue to order confirmation.
3. Apply Your Fresh Items
Access your skins immediately and showcase them while gaming to show off.
Reasons Gamers Rely on Us:
– Huge Collection: Explore designs for all weapon.
– Transparent Costs: Honest costs with zero additional costs.
– Quick Transfer: Access your purchases immediately.
– Reliable Checkouts: Safe and reliable transactions.
– Help Desk: Our experts is ready to assist you anytime.
Get Started Now!
Find the ultimate CS2 skins and boost your matches to the new heights.
Whether you’re hoping to enhance your arsenal, assemble a rare inventory, or simply be unique, we|our store|our site|our marketplace is your ultimate marketplace for premium Counter-Strike 2 skins.
Start now and discover the skin that matches your taste!
slot tr online en cok kazand?ran slot oyunlar? or slot oyunlar? puf noktalar?
http://www.victoirefrance.fr/expire.aspx?Returnurl=https://slottr.top/ en cok kazand?ran slot oyunlar?
en kazancl? slot oyunlar? slot siteleri and slot oyunlar? slot tr online
рулонные шторы широкие http://rulonnye-shtory-s-elektroprivodom499.ru .
накрутить подписчиков твиттер накрутить подписчиков твиттер .
langfristprognose langfristprognose .
электрические карнизы электрические карнизы .
Your Go-To Marketplace for AI-Driven Innovation
Unleash the complete power of AI tools with expertly created, high-quality templates designed to enhance your innovation and efficiency. Whether you’re a promoter, a author, a visual creator, or a technologist, our expertly curated tools at Templateforge are the solutions you need to bring your tasks to the next level.
Why Choose Us?
At Templateforge, we’re committed to delivering exclusively the best templates, guaranteeing relevance, excellence, and convenience. Here’s why creators rely on us as their top resource provider:
Premium Resources
Our team of specialists diligently develops solutions that are functional, unique, and actionable. Each prompt is crafted to deliver results, allowing you to attain objectives efficiently and smoothly.
Extensive Options
From content promotion to writing prompts, development prompts, and artistic inspiration, we cover a variety of categories. Our resources are crafted for experts and innovators in various niche.
Flexible Solutions
Each resource is fully tailorable, making it possible to adjust it to your unique workflow needs. This versatility provides our prompts are widely applicable and effective for multiple industries and objectives.
User-Friendly Experience
We respect your productivity. With instant downloads, pre-packaged collections, and an user-friendly interface, using and applying the right AI prompts has become effortless.
Check Out Our Newest Additions
Unlock prompts that boost results, spark innovation, and optimize performance.
Why Creators Love Us
– Huge Catalog: Find solutions for all purpose.
– Fair Prices: High-quality solutions beginning at just 5.00 €.
– Immediate Downloads: Download your solutions instantly.
– Proven Results: Each template is proven and proven to provide success.
– 24/7 Support: Have questions? Our specialists is constantly ready to support you.
Start Shopping Now!
Discover the most innovative solutions and transform your work to the highest point.
Join us and explore the solutions that match your workflow!
pinup 2025 пин ап казино pinup 2025
https://pinup2025.com/# пинап казино
bonus veren siteler yeni tГјrkiye casino siteleri or para bahis gГјncel giriЕџ
https://www.google.lv/url?sa=t&url=https://casinositeleri2025.pro yasal casino siteleri
casino bonanza gГјncel giriЕџ orjinal siteler and bet bahis giriЕџ ilk giriЕџte bonus veren bahis siteleri
pinup 2025: pinup 2025 – пин ап казино
http://casinositeleri2025.pro/# en saДџlam bahis siteleri
en iyi canlД± casino siteleri gГјncel bahis siteleri or bahis siteleei
http://vodotehna.hr/?URL=casinositeleri2025.pro caxino
casino bahis siteleri gГјvenilir casino and bonus veren site deneme bonus veren siteler
https://casinositeleri2025.pro/# curacao lisans siteleri
http://slottr.top/# slot oyunlar? puf noktalar?
slot siteleri slot oyunlar? puf noktalar? en kazancl? slot oyunlar?
casino bonus: para kazandiran kumar oyunlarД± – yatД±rД±m bonusu veren siteler
пин ап казино зеркало пин ап казино зеркало or pinup 2025
https://www.e-tsudoi.com/redirect.php?url=http://pinup2025.com пин ап зеркало
пин ап казино зеркало pinup 2025 and pinup 2025 пинап казино
Как играть на 1xSlots https://antiguedadeselrodeo.com.ar/pages/1xslots-argentina.html
slot oyunlar?: en cok kazand?ran slot oyunlar? – slot oyunlar?
pinup 2025 пинап казино or пинап казино
http://www.e-douguya.com/cgi-bin/mbbs/link.cgi?url=http://pinup2025.com pinup 2025
пин ап казино пин ап вход and пин ап пин ап казино
https://casinositeleri2025.pro/# oyun sitesi oyun sitesi oyun sitesi
en kazancl? slot oyunlar? slot siteleri en kazancl? slot oyunlar?
紫イミソーレ(スペシャルアワードライト
k8 カジノ CR魔法少女まどか_マギカ Ver.319
大当たりの瞬間は、周囲の人たちと喜びを分かち合うことができるのが魅力です。共感を得られます。
CR牙狼GOLDSTORM翔 Ver.319
https://www.japan-casino.asia/article/153.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。目が離せません。
P とある魔術の禁書目録
P フィーバー 機動戦士ガンダムユニコーン b
ドラゴンが降りる
パチスロ 革命機ヴァルヴレイヴ
大工の源さん桜満開!源 DREAM ver.~
吉宗 2003
https://www.k8.monster/Articles/1590.html
多彩なリーチ演出があり、どのタイミングで当たるかドキドキします。
CR聖戦士ダンバイン
https://www.slotgaro.com/article/2513.html
人気ゲームを基にしたパチスロで、ファンにはたまらない作品です。リアルな演出が楽しめます。
ベヨネッタ
https://www.k8.republican/Articles/1466.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
http://casinositeleri2025.pro/# casino bonanza gГјncel giriЕџ
Стоимость дипломов высшего и среднего образования и как избежать подделок
deneme bonusu veren siteler casino: gГјvenilir oyun siteleri – canlД± bahis siteleri
betnoo online casino turkey casino deneme bonusu veren bahis siteleri
2025 yatД±rД±m ЕџartsД±z deneme bonusu veren siteler: deneme bonusu veren bet siteleri – bilinmeyen bahis siteleri
slot oyunlar? puf noktalar?: slot oyunlar? – az parayla cok kazandiran slot oyunlar?
https://slottr.top/# slot tr online
güzel siteler kaçak bahis siteleri bonus veren or casino bonus
https://www.google.hn/url?q=https://casinositeleri2025.pro deneme bonusu beren siteler
en gГјvenilir bahis siteleri casino turkey and casino oyna lisanslД± kumar siteleri
Производство ангаров и складов под ключ.
Подробная информация и заказ быстровозводимый складов
https://pinup2025.com/# pinup 2025
https://casinositeleri2025.pro/# bahis siteleri deneme bonusu
slot oyunlar?: en cok kazand?ran slot oyunlar? – slot siteleri
gerçek paralı slot uygulamaları yeni bonus veren bahis siteleri or online casino bet
http://sotofone.ru/bitrix/rk.php?goto=http://casinositeleri2025.pro/ casino tГјrkiye
casino giriЕџ deneme bonus siteler and bet turkiye ilk giriЕџte bonus veren bahis siteleri
slot oyunlar? slot oyunlar? puf noktalar? or en kazancl? slot oyunlar?
http://101.43.178.182/sell/email.asp?d=slottr.top&on=tb en cok kazand?ran slot oyunlar?
slot oyunlar? en kazancl? slot oyunlar? and az parayla cok kazandiran slot oyunlar? slot tr online
https://casinositeleri2025.pro/# gГјvenilir casino
Тут можно сейфы для офисасейфы для офисов цены
yeni gГјncel deneme bonusu veren siteler gГјvenilir casino bahis siteleri tГјrk partner siteleri
Рейтинг лучших онлайн-казино https://lastdepcasino.ru с быстрыми выплатами и честной игрой. Подробные обзоры, бонусы для новых игроков и актуальные акции.
Bitcoin is a decentralized digital currency that operates without the penury after a principal authority or intermediary, like a bank. Introduced in 2009 via an unrevealed idiosyncratic or batch answerable to the pseudonym Satoshi Nakamoto, Bitcoin is powered alongside blockchain technology, a unshrouded ledger that records all transactions securely and transparently. It is known as a remedy for its reduced reservoir of 21 million coins and its use as a collect of value and usual of exchange. Bitcoin has ripen into a in demand investment option and a symbol of the evolving digital economy.
https://chainbase-airdrop.online
https://kelp-dao-airdrop.online
https://aptos-airdrop.online
https://airdrop-jupiter.online
https://braavos-airdrop.online
https://grassairdrop.online
https://phaver-airdrop.online
https://b3-airdrop.online
https://beoble-airdrop.online
https://supra-airdrop.online
https://sender-labs-airdrop.online
https://azura-airdrop.online
https://celestiaairdrop.online
https://zerion-airdrop.online
https://farcaster-airdrop.online
https://meteora-airdrop.online
https://uprock-airdrop.online
https://poap-airdrop.online
https://vela-exchange-airdrop.online
https://htx-airdrop.online
https://haven1-airdrop.online
https://elys-network-airdrop.online
https://hyperliquid-airdrop.online
https://dawn-airdrop.online
https://icon-airdrop.online
https://aztec-airdrop.online
https://duckchain-airdrop.online
https://mode-airdrop.online
https://nundu-airdrop.online
https://nodepay-airdrop.online
Наш сервисный центр предлагает высококачественный мастерская по ремонту видеокамеры в москве любых брендов и моделей. Мы понимаем, насколько значимы для вас ваши видеорегистраторы, и готовы предложить сервис первоклассного уровня. Наши квалифицированные специалисты работают быстро и аккуратно, используя только качественные детали, что обеспечивает длительную работу проведенных ремонтов.
Наиболее частые неисправности, с которыми сталкиваются владельцы видеокамер, включают неисправности записи, поврежденный объектив, неисправности программного обеспечения, неработающие разъемы и повреждения корпуса. Для устранения этих проблем наши опытные мастера выполняют ремонт записи, объективов, ПО, разъемов и механических компонентов. Обратившись к нам, вы получаете долговечный и надежный вызвать мастера по ремонту видеокамер в москве.
Подробная информация доступна на сайте: https://remont-videokamer-ink.ru
http://pinup2025.com/# пин ап вход
slot oyunlar? puf noktalar? az parayla cok kazandiran slot oyunlar? or en cok kazand?ran slot oyunlar?
https://www.google.com.py/url?q=https://slottr.top en kazancl? slot oyunlar?
az parayla cok kazandiran slot oyunlar? az parayla cok kazandiran slot oyunlar? and en cok kazand?ran slot oyunlar? en cok kazand?ran slot oyunlar?
北斗の拳 修羅の国篇
オンラインカジノ
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。
パチスロ 甲鉄城のカバネリ
https://www.k8521.com/archives/post-401.html
シンプルなデザインとルールが魅力。初心者でも安心して楽しめます。
デジハネ CR 北斗の拳 慈母
エリートサラリーマン鏡 打ち方
北斗の拳 転生の章
沖ドキ! 30LL
P DD 北斗の拳2 ついでに愛をとりもどせ!! ラオウ
北斗の拳 転生の章
https://www.k8.monster/Articles/1551.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
CR 聖戦士ダンバイン
https://www.dmmpachinko.com/article/154.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
P北斗の拳9 闘神
https://www.sohbette.org/article/2119.html
大当たり時の演出が特に豪華で、楽しさを感じます。期待感が高まります。
Great insights! This article really cleared up some of my doubts. Thanks for sharing! https://metasmak.com/
yatД±rД±m bonusu veren siteler en Г§ok kazandД±ran site deneme bonusu veren bahis siteleri 2025
пин ап: pinup 2025 – пин ап
https://pinup2025.com/# пин ап
Тут можно сейфы москвасейф москва
en cok kazand?ran slot oyunlar?: slot oyunlar? puf noktalar? – slot tr online
пин ап пин ап казино официальный сайт or пин ап вход
https://www.google.com.mx/url?sa=t&url=https://pinup2025.com pinup 2025
пин ап казино зеркало пинап казино and пинап казино pinup 2025
pinup 2025: пин ап зеркало – пин ап казино официальный сайт
https://casinositeleri2025.pro/# deneme bonusu bahis siteleri
oyun dene en iyi kumar sitesi or bet siteler
http://www.google.me/url?q=https://casinositeleri2025.pro casino tГјrkiye
100tl hosgeldin bonusu veren siteler deneme bonusu beren siteler and oyun sitesi en Г§ok kazandД±ran casino sitesi
gГјvenilir oyun alma siteleri casino deneme bonusu veren bahis siteleri deneme bonusu veren siteler yorumlar
пин ап казино официальный сайт пин ап казино официальный сайт or пин ап зеркало
https://toolbarqueries.google.al/url?q=https://pinup2025.com пин ап казино официальный сайт
пин ап казино pinup 2025 and пин ап казино зеркало пин ап казино официальный сайт
франшизный бизнес франшизный бизнес .
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.haikudeck.com/presentations/KmBhVAaMWP
https://rentry.org/xpxw6rhk
https://haveagood.holiday/users/357154
https://anotepad.com/notes/gni24678
https://pautina1995.bandcamp.com/album/two-bitches-in-my-life
https://casinositeleri2025.pro/# en iyi bet siteleri
casino slot casino oyna or online casino turkey
http://lonevelde.lovasok.hu/out_link.php?url=https://casinositeleri2025.pro welches online casino
casino slot online 2025 deneme bonusu veren bahis siteleri and 18siteler deneme bonusu veren siteler casino
Cat Accessories Stylish Cat Products
https://casinositeleri2025.pro/# dГјnyanД±n en iyi bahis siteleri
vidobet giriЕџ 2025 en Г§ok kazandД±ran bahis siteleri or internet kumar siteleri
http://alt1.toolbarqueries.google.mn/url?q=https://casinositeleri2025.pro en yeni deneme bonusu veren siteler 2025
gГјvenilir casino bahis siteleri deneme bonusu veren seat and canlД± +18 tГјm casino siteleri
Наш сервисный центр предлагает надежный вызвать мастера по ремонту видеокарты адреса любых брендов и моделей. Мы знаем, насколько значимы для вас ваши графические адаптеры, и готовы предложить сервис наилучшего качества. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что обеспечивает длительную работу наших услуг.
Наиболее частые неисправности, с которыми сталкиваются пользователи графических карт, включают неисправности системы охлаждения, выход из строя памяти, неработающие разъемы, проблемы с контроллером и неисправности ПО. Для устранения этих проблем наши опытные мастера проводят ремонт системы охлаждения, памяти, разъемов, контроллеров и ПО. Обратившись к нам, вы обеспечиваете себе качественный и надежный мастер по ремонту видеокарты рядом.
Подробная информация размещена на сайте: https://remont-videokart-biz.ru
https://casinositeleri2025.pro/# kazino online
en kazancl? slot oyunlar?: az parayla cok kazandiran slot oyunlar? – slot oyunlar? puf noktalar?
Mostbet kazino uz ro‘yxatdan o‘tish jarayoni | Mostbet uz apk-ni qanday yuklab olish kerak? | Mostbet oynashning asosiy afzalliklari va funksiyalari | Mostbet apk download va o‘yinlarga kirish | Mostbet mobil ilovasi orqali qulay pul tikish мостбет | Mostbet oynash va o‘yinlar haqida batafsil ma’lumot | Mostbet rasmiy sahifasi orqali bonus olish usullari | Mostbet uz skachat va o‘yinlarni boshlash uchun maslahatlar mostbet casino.
пин ап казино pinup 2025 пин ап
slot oyunlar? puf noktalar?: az parayla cok kazandiran slot oyunlar? – en cok kazand?ran slot oyunlar?
Наш сервисный центр предлагает надежный мастер по ремонту мфу рядом различных марок и моделей. Мы понимаем, насколько важны для вас ваши многофункциональные устройства, и стремимся предоставить услуги первоклассного уровня. Наши профессиональные техники работают быстро и аккуратно, используя только сертифицированные компоненты, что обеспечивает надежность и долговечность выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются пользователи многофункциональных устройств, включают неисправности печатающей головки, неисправности сканера, ошибки ПО, неисправности разъемов и поломки компонентов. Для устранения этих неисправностей наши квалифицированные специалисты оказывают ремонт печатающих головок, сканеров, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы получаете долговечный и надежный вызвать мастера по ремонту мфу на выезде.
Подробная информация представлена на нашем сайте: https://remont-mfu-lite.ru
date sitesi: oyun inceleme siteleri – online casino turkey
https://slottr.top/# slot tr online
Bonus casin? online per l’Italia Bonus casin? online per l’Italia .
продажа франшиз продажа франшиз .
франшизу франшизу .
Betovo Betovo .
http://pinup2025.com/# pinup 2025
Avcılar Escort bayanları sitemizde bularak onlarla doyumsuz seks yapmaya hazır olun. Tek yapmanız gerek Avcılar Escort Bayan sitemizi ziyaret etmeniz.
пин ап пинап казино pinup 2025
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article. https://www.binance.com/en-ZA/register?ref=JHQQKNKN
havuzlu bir otelin şezlongunda avrupa yakası escort güneşlenerek eşsiz vücuduyla erkekleri baştan çıkarıyor
slot oyunlar? slot siteleri or az parayla cok kazandiran slot oyunlar?
http://www.google.com.gt/url?sa=t&rct=j&q=como+limpiar+una+bocina+de+celular&source=web&cd=7&ved=0cgwqfjag&url=https://slottr.top slot oyunlar?
az parayla cok kazandiran slot oyunlar? slot oyunlar? puf noktalar? and slot siteleri en kazancl? slot oyunlar?
Ваш гид в мире автомобилей https://clothes-outletstore.com тест-драйвы, советы по ремонту и последние тенденции индустрии.
Автомобильный журнал https://automobile.kyiv.ua с фокусом на практичность. Ремонт, уход за авто, обзор технологий и советы по эксплуатации.
http://casinositeleri2025.pro/# casino en Г§ok kazandД±ran oyunlar
pinup 2025: пин ап казино – pinup 2025
Наши специалисты предлагает надежный сервис ремонта гироскутера на дому всех типов и брендов. Мы понимаем, насколько значимы для вас ваши самобалансирующиеся скутеры, и готовы предложить сервис высочайшего уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что гарантирует длительную работу выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются пользователи электроскутеров, включают поломку аккумулятора, неработающий двигатель, поломку контроллера, проблемы с гиросенсорами и повреждения рамы. Для устранения этих проблем наши квалифицированные специалисты выполняют ремонт батарей, двигателей, контроллеров, гиросенсоров и механических компонентов. Обращаясь в наш сервисный центр, вы гарантируете себе надежный и долговечный починить гироскутер с гарантией.
Подробная информация размещена на сайте: https://remont-giroskuterov-key.ru
bahis giriЕџ yatД±rД±m bonusu veren siteler en gГјvenilir casino siteleri
pinup 2025: pinup2025.com – пинап казино
http://casinositeleri2025.pro/# caxino
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me? https://www.binance.com/vi/register?ref=WTOZ531Y
canlД± oyunlar: en iyi bet siteleri – 300 tl bonus veren bahis sitesi
Наша мастерская предлагает профессиональный сервисный центр по ремонту ноутбука рядом любых брендов и моделей. Мы знаем, насколько важны для вас ваши переносные компьютеры, и готовы предложить сервис первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только оригинальные запчасти, что гарантирует надежность и долговечность наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются пользователи лаптопов, включают поломку жесткого диска, поврежденный экран, программные сбои, неработающие разъемы и перегрев. Для устранения этих поломок наши профессиональные техники выполняют ремонт жестких дисков, экранов, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы получаете качественный и надежный сервисный ремонт ноутбуков на выезде.
Подробная информация представлена на нашем сайте: https://remont-noutbukov-first.ru
押忍!番长(操)
金ドキモードとは
サウンドエフェクトが迫力満点で、プレイ中に盛り上がります。特にバトル時が楽しい。
L バイオハザード ヴィレッジ
https://www.redskybooks.net/slot-games/slot-games-mahjong-ways-from-pgsoft-at-chicago-casino-grand-mondial-casino-login.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
デジハネ CR 聖戦士ダンバイン
k8 カジノ パチンコ
パチスロ 貞子3D
Lバキ 強くなりたくば喰らえ!!!
沖ドキ!トロピカル
CR押忍!番長
https://injapan.today/realtime/id1760.html
演出が単純明快で、ストレスなくプレイできるのが良いです。気軽に楽しめます。
鬼浜爆走紅蓮隊友情挽歌編
https://www.yoshimune-slot.com/article/255.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
P刀使ノ巫女 Ver.199
https://injapan.today/realtime/id8484.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
casino site tГјrkiye nin en iyi yasal bahis sitesi or bonus veren yasal bahis siteleri
http://eijudou.com/_m/index.php?a=free_page/goto_mobile&referer=https://casinositeleri2025.pro casino slot
online casino turkey free spin casino and 2024 bahis siteleri pacanele online
slot oyunlar? puf noktalar? slot tr online or slot siteleri
https://toolbarqueries.google.pn/url?q=https://slottr.top en cok kazand?ran slot oyunlar?
az parayla cok kazandiran slot oyunlar? slot siteleri and slot tr online slot oyunlar? puf noktalar?
Всё о машинах https://avtomobilist.kyiv.ua тест-драйвы, сравнения моделей, авто новости, советы по ремонту и уходу.
Авто портал https://autonovosti.kyiv.ua актуальные новости, обзоры авто, тест-драйвы, инструкции по ремонту и тюнингу. Минимум текста, максимум полезной информации.
Авто журнал https://avtonews.kyiv.ua новости автопрома, сравнения моделей, тест-драйвы, советы по ремонту и уходу.
Всё о машинах https://black-star.com.ua авто новости, тест-драйвы, обзоры моделей, рейтинги, инструкции по обслуживанию и ремонту.
https://rffnulef.tv53.ru/
https://rclpmzfz.tv53.ru/
https://zxhoioyb.tv53.ru/
https://ulyajipm.tv53.ru/
https://pjlddmxd.tv53.ru/
https://bivfvoov.tv53.ru/
https://dhjllwpg.tv53.ru/
https://ahqwylbr.tv53.ru/
https://rjxzgfin.tv53.ru/
Авто журнал https://avtonews.kyiv.ua новости автопрома, сравнения моделей, тест-драйвы, советы по ремонту и уходу.
Всё о машинах https://avtomobilist.kyiv.ua тест-драйвы, сравнения моделей, авто новости, советы по ремонту и уходу.
Авто портал https://autonovosti.kyiv.ua актуальные новости, обзоры авто, тест-драйвы, инструкции по ремонту и тюнингу. Минимум текста, максимум полезной информации.
Всё о машинах https://black-star.com.ua авто новости, тест-драйвы, обзоры моделей, рейтинги, инструкции по обслуживанию и ремонту.
Bitcoin is a decentralized digital currency that operates without the penury after a chief expert or third party, like a bank. Introduced in 2009 via an unknown individual or group subservient to the nom de guerre Satoshi Nakamoto, Bitcoin is powered by blockchain technology, a overt ledger that records all transactions securely and transparently. It is known for its reduced cater to of 21 million coins and its use as a collect of value and median of exchange. Bitcoin has grace a sought-after investment choice and a symbol of the evolving digital economy.
https://sender-labs-airdrop.online
https://mode-airdrop.online
https://starrynift-airdrop.online
https://xion-airdrop.online
https://aztec-airdrop.online
https://strk-airdrop.online
https://phaver-airdrop.online
https://beoble-airdrop.online
https://alphabot-airdrop.online
https://solayer-airdrop.online
https://hemi-network-airdrop.online
https://pump-fun-airdrop.online
https://ink-airdrop.online
https://venomairdrop.online
https://azura-airdrop.online
https://taiko-airdrop.online
https://dot-airdrop.online
https://kiloex-airdrop.online
https://drop-airdrop.online
https://movement-airdrop.online
https://jito-airdrop.online
https://nulink-airdrop.online
https://plume-network-airdrop.online
https://airdrop-arb.online
https://meteora-airdrop.online
https://grassairdrop.online
https://gasp-airdrop.online
https://tari-airdrop.online
https://hana-network-airdrop.online
https://koni-story-airdrop.online
http://indiapharmi.com/# indian pharmacy online
mexico drug stores pharmacies Legit online Mexican pharmacy mexico pharmacies prescription drugs
Всё о самогоноварении https://brewsugar.ru на одном сайте: от истории напитка до современных технологий перегонки.
Место для общения на любые темы https://xn--9i1b12ab68a.com/ актуальные вопросы, обмен опытом, интересные дискуссии. Здесь найдётся тема для каждого.
Всё об авто https://road.kyiv.ua в одном месте: новости, тест-драйвы, сравнения, характеристики, ремонт и уход. Автомобильный онлайн-журнал — ваш эксперт в мире машин.
Перевозка товаров из Китая https://chinaex.ru в Россию под ключ: авиа, море, автотранспорт. Гарантия сроков и сохранности груза.
purple pharmacy mexico price list: Purple pharmacy online ordering – mexican online pharmacies prescription drugs
http://canadianpharmi.com/# ed meds online without doctor prescription
вывод из запоя в спб вывод из запоя в спб .
http://mexicanpharmi.com/# buying prescription drugs in mexico online
en iyi pozisyonlarda değerlendirebileceğiniz bağcılar escort sitesi https://bagcilareskortbayan.com/
Наша мастерская предлагает надежный мастер по ремонту духовых шкафов рядом различных марок и моделей. Мы знаем, насколько необходимы вам ваши кухонные приборы, и готовы предложить сервис наилучшего качества. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что гарантирует долговечность и надежность выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются пользователи духовок, включают проблемы с нагревом, выход из строя таймера, треснувшую дверцу, проблемы с контроллером, проблемы с конвекцией и неисправные платы. Для устранения этих проблем наши профессиональные техники выполняют ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обращаясь в наш сервисный центр, вы гарантируете себе качественный и надежный официальный ремонт духового шкафа.
Подробная информация доступна на сайте: https://remont-duhovyh-shkafov-ace.ru
услуги по таможенному оформлению грузов услуги по таможенному оформлению грузов .
https://canadianpharmi.com/# ed treatment review
what is the best ed pill: canadianpharmi – ed medications comparison
Наши специалисты предлагает надежный официальный ремонт объективов адреса любых брендов и моделей. Мы знаем, насколько важны для вас ваши объективы, и стремимся предоставить услуги высочайшего уровня. Наши профессиональные техники работают быстро и аккуратно, используя только оригинальные запчасти, что предоставляет надежность и долговечность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются обладатели фотообъективов, включают неисправности фокуса, залипшую диафрагму, поломки компонентов, неработающий автофокус и загрязнения линз. Для устранения этих проблем наши профессиональные техники оказывают ремонт фокусировки, диафрагмы, автофокуса, очистку линз и восстановление корпуса. Доверив ремонт нам, вы гарантируете себе качественный и надежный ремонт объективов на выезде.
Подробная информация представлена на нашем сайте: https://remont-obektivov-magic.ru
mexican pharmacy without prescription: Best Canadian online pharmacy – ed clinic
bestes klima in deutschland bestes klima in deutschland .
Thanks for sharing. I read many of your blog posts, cool, your blog is very good. https://www.binance.com/zh-TC/register?ref=VDVEQ78S
güzelkızların olduğu bakırköy escort sitesinde eşsiz güzellikler seni bekliyor https://bakirkoyeskortbayan.com/
таможенный представитель таможенный представитель .
http://mexicanpharmi.com/# buying prescription drugs in mexico online
新鬼武者 再臨
k8 カジノ パチンコ
近年では、スマートフォンアプリでも楽しめるようになりました。手軽に遊べるのが嬉しいです。
パラダイスハイビスカス
https://casinos.town/games/slingo-extreme
パチンコの世界には、運だけではなく、戦略も求められます。自分の考えを試す楽しさがあります。
CR地獄少女 弐
ヴヴヴ 狙い目 ゾーン
デビルサバイバー2 最後の7日間
CR蒼天の拳天帰 Ver.319(1:1)
CR真・花の慶次2 漆黒の衝撃 Ver.319(1:1)
八代將軍戰神版
https://www.casino69gold.com/article/144.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
CRフィーバー戦姫絶唱シンフォギア
https://www.webslot69.com/article/156.html
大当たりの確率を理解することで、より戦略的にプレイできます。計算が好きな人にはおすすめです。
P貞子3D2呪われた12時間
https://www.mystino.bet/article/310.html
パチンコ店の雰囲気は賑やかで、友達と一緒に楽しむのに最適です。色とりどりの台が魅力的です。
п»їlegitimate online pharmacies india Best Indian pharmacy india pharmacy mail order
Женский портал https://abuki.info мода, красота, здоровье, семья, карьера. Советы, тренды, лайфхаки, рецепты и всё, что важно для современных женщин.
Автомобильный онлайн-журнал https://simpsonsua.com.ua предлагает свежие новости, обзоры авто, тест-драйвы, рейтинги и полезные советы для водителей.
Мода, здоровье, красота https://gratransymas.com семья, кулинария, карьера. Женский портал — полезные советы и свежие идеи для каждой.
Познавательный портал для детей https://detiwki.com.ua обучающие материалы, интересные факты, научные эксперименты, игры и задания для развития кругозора.
homeopathic remedies for ed: Cheapest drug prices Canada – pills for ed
открыть франшизу открыть франшизу .
efsanevi başakşehir escort sitesinde güzel gözlü başakşehir escort kızları göreceksiniz https://basaksehirescortbul.com/
Tutorial para 1xslots casino https://nodosde.gob.ar/pgs/1xslots-descargar_1.html
cazibesiyle baştan çıkaran beylikdüzü escort ceren sizlerle https://beylikduzueskortara.com/
Explore comprehensive guides https://pocket-codes.com/guides to master your favorite games. From beginner tips to expert strategies, our guides help you improve skills, unlock secrets, and conquer challenges with ease. Perfect for gamers of all levels!
стильная офисная мебель https://ковка116.рф
Женский онлайн-журнал https://womanfashion.com.ua секреты красоты, модные тренды, здоровье, отношения, семья, кулинария и карьера. Всё, что важно и интересно.
Женский онлайн-журнал https://stylewoman.kyiv.ua стиль, уход, здоровье, отношения, семья, кулинарные рецепты, психология и карьера.
https://canadianpharmi.com/# buy prescription drugs online without
п»їbest mexican online pharmacies Cheapest online pharmacy mexican drugstore online
gözlerinizin kamaşacağı beyoğlu escort kızlar sizleri bekliyor https://beyogluescortbul.com/
DESIGN, FABRICATION & INSTALLATION OF CUSTOM MADE LOUVERS!
DK Construction & Design provides comprehensive, custom solutions for louvres,
sunshades, privacy screens, awnings, and balustrades made from aluminium, glass, and steel.
Our end-to-end service covers on-site measurements, design development, detailed drawings,
engineering, fabrication, and installation.
We work closely with designers, architects, and homeowners to deliver tailored shading,
privacy, and safety solutions. Our team ensures that every project meets the highest
standards of quality and durability, with a focus on functionality and aesthetics.
Whether you’re after sleek sun control systems or secure balustrading, we’ve got
the expertise to bring your vision to life.
Tel: +61475 617 720
Сайт компании DK Construction & Design
Your One-Stop Shop for AI-Powered Creativity
Your One-Stop Shop for Artificial Intelligence-Based Content Creation
Unleash the full power of AI tools with specially designed, high-quality solutions created to improve your ideas and output. Whether you’re a promoter, a author, a visual creator, or a technologist, our expertly curated resources at Our Platform are the assets you require to take your work to the next level.
Why Use Template Forge?
At Our Platform, we’re focused to offering simply the top-notch templates, guaranteeing usefulness, excellence, and convenience. Here’s why thousands of creators choose us as their go-to template platform:
Top-Quality Templates
Our professional creators meticulously creates resources that are functional, cutting-edge, and ready to use. Each prompt is developed to deliver results, supporting you to achieve results seamlessly and easily.
Wide Range
From marketing solutions to story development, development prompts, and art prompts, we cover a comprehensive catalog of applications. Our solutions are created for users and hobbyists in any niche.
Customizable Options
Each template is easily modifiable, enabling you to adjust it to your unique project preferences. This customizability delivers our prompts are multi-purpose and scalable for multiple applications and tasks.
Seamless Process
We understand your effort. With immediate access, well-arranged prompt bundles, and an user-friendly experience, finding and implementing the right templates has never been so simple.
Explore Our Recent Templates
Find prompts that increase engagement, fuel innovation, and optimize workflows.
What Sets Us Apart
– Wide Variety: Access solutions for any industry.
– Budget-Friendly Options: Top-notch templates beginning at just 5.00 €.
– Immediate Downloads: Access your resources without delay.
– Proven Results: Each template is checked and designed to achieve impact.
– Dedicated Support: Have questions? Our team is perpetually ready to assist you.
Start Shopping Now!
Unlock the best templates and take your tasks to the new heights.
Sign in and choose the resources that match your goals!
http://indiapharmi.com/# online shopping pharmacy india
https://mexicanpharmi.com/# mexican drugstore online
вывод из запоя вывод из запоя .
Туристический портал https://aliana.com.ua лучшие маршруты, путеводители, советы путешественникам, обзоры отелей.
мебель для офиса спб офисная мебель оснащение офиса
Всё о туризме https://atrium.if.ua маршруты, путеводители, советы по отдыху, обзор отелей и лайфхаки. Туристический портал — ваш помощник в путешествиях.
Всё для путешествий https://cmc.com.ua уникальные маршруты, гиды по городам, актуальные акции на туры и полезные статьи для туристов.
Наш сервисный центр предлагает надежный отремонтировать парогенератор на выезде всех типов и брендов. Мы осознаем, насколько значимы для вас ваши парогенераторы, и обеспечиваем ремонт высочайшего уровня. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что обеспечивает длительную работу наших услуг.
Наиболее общие проблемы, с которыми сталкиваются владельцы парогенераторов, включают неисправности нагревательных элементов, проблемы с подачей воды, неисправности программного обеспечения, неработающие разъемы и поломки компонентов. Для устранения этих поломок наши квалифицированные специалисты выполняют ремонт нагревательных элементов, насосов, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы гарантируете себе долговечный и надежный починить парогенератор с гарантией.
Подробная информация доступна на сайте: https://remont-parogeneratorov-piece.ru
꽤 좋은 게시물입니다. 방금 우연히 귀하의 블로그를 발견했고 귀하의 블로그 게시물을 읽는 것을 매우 즐겼습니다. 더 귀중한 정보를 얻기 위해 새로운 게시물을 찾고 있습니다. 유용한 정보에 대해 큰 감사를 표합니다.
indianpharmacy com: Top online pharmacy in India – indian pharmacy
Наша мастерская предлагает надежный ремонт бесперебойников в москве всех типов и брендов. Мы осознаем, насколько значимы для вас ваши источники бесперебойного питания, и готовы предложить сервис наилучшего качества. Наши квалифицированные специалисты работают быстро и аккуратно, используя только качественные детали, что гарантирует длительную работу выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются пользователи UPS, включают поломку аккумулятора, неисправности инвертора, сбои контроллера, неработающие разъемы и неисправности программного обеспечения. Для устранения этих поломок наши квалифицированные специалисты оказывают ремонт батарей, инверторов, контроллеров, разъемов и ПО. Обращаясь в наш сервисный центр, вы обеспечиваете себе долговечный и надежный сервисный центр по ремонту бесперебойника.
Подробная информация размещена на сайте: https://remont-ibp-max.ru
best ed pills online: canadian pharmi – ed clinic
medication from mexico pharmacy Legit online Mexican pharmacy buying from online mexican pharmacy
https://indiapharmi.com/# indian pharmacy online
таможенные брокеры таможенные брокеры .
reputable indian pharmacies top online pharmacy india or indian pharmacies safe
https://www.google.sn/url?sa=t&url=https://indiapharmi.com Online medicine order
top 10 online pharmacy in india indian pharmacies safe and indian pharmacy paypal best online pharmacy india
erectile dysfunction pills: Best Canadian online pharmacy – prescription drugs online without
Bitcoin is a decentralized digital currency that operates without the penury for a key expert or arbitrator, like a bank. Introduced in 2009 by an unrevealed proper or society subservient to the nom de guerre Satoshi Nakamoto, Bitcoin is powered alongside blockchain technology, a public ledger that records all transactions securely and transparently. It is known for its limited cater to of 21 million coins and its services as a collect of value and usual of exchange. Bitcoin has grace a in demand investment selection and a crest of the evolving digital economy.
https://mitosis-airdrop.online
https://grassairdrop.online
https://dawn-airdrop.online
https://polygon-zkevm-airdrop.online
https://icon-airdrop.online
https://poap-airdrop.online
https://mantra-airdrop.online
https://arena-z-airdrop.online
https://sender-labs-airdrop.online
https://dot-airdrop.online
https://phantom-airdrop.online
https://paws-airdrop.online
https://mezo-airdrop.online
https://plume-network-airdrop.online
https://azura-airdrop.online
https://blockmesh-airdrop.online
https://gudchain-airdrop.online
https://htx-airdrop.online
https://navigate-airdrop.online
https://starrynift-airdrop.online
https://taiko-airdrop.online
https://squid-airdrop.online
https://supra-airdrop.online
https://kiloex-airdrop.online
https://aztec-airdrop.online
https://meteora-airdrop.online
https://iota-airdrop.online
https://shaga-airdrop.online
https://uprock-airdrop.online
https://alphabot-airdrop.online
http://indiapharmi.com/# reputable indian pharmacies
mexican border pharmacies shipping to usa mexico pharmacies prescription drugs or mexico drug stores pharmacies
http://www.cookinggamesclub.com/partner/mexicanpharmi.com/ mexican online pharmacies prescription drugs
pharmacies in mexico that ship to usa mexican online pharmacies prescription drugs and buying from online mexican pharmacy mexico drug stores pharmacies
mexican pharmaceuticals online buying prescription drugs in mexico online or buying from online mexican pharmacy
http://www.krankengymnastik-kaumeyer.de/url?q=https://mexicanpharmi.com buying from online mexican pharmacy
mexico drug stores pharmacies mexican drugstore online and pharmacies in mexico that ship to usa mexico pharmacies prescription drugs
india online pharmacy India online pharmacy international shipping top 10 online pharmacy in india
이것은 매우 교육적인 내용이며 변화를 위해 잘 쓰여졌습니다. 어떤 사람들이 여전히 양질의 게시물을 쓰는 방법을 알고 있다는 것을 보는 것은 좋은 일입니다!
https://canadianpharmi.com/# ed treatments
top 10 pharmacies in india: Best Indian pharmacy – pharmacy website india
https://indiapharmi.com/# buy prescription drugs from india
Статьи о путешествиях https://deluxtour.com.ua гиды по направлениям, лайфхаки для отдыха, советы по сбору багажа и туристические обзоры.
Путешествия по Греции https://cpcfpu.org.ua лучшие курорты, гиды по островам, экскурсии, пляжи и советы по планированию отпуска.
Туристический журнал https://elnik.kiev.ua свежие идеи для путешествий, обзоры курортов, гиды по городам, советы для самостоятельных поездок и туристические новости.
бизнес по франшизе бизнес по франшизе .
https://indiapharmi.com/# top 10 online pharmacy in india
Наш сервисный центр предлагает надежный мастерская по ремонту планшета всех типов и брендов. Мы осознаем, насколько значимы для вас ваши планшеты, и готовы предложить сервис наилучшего качества. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что гарантирует надежность и долговечность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств планшетного типа, включают поврежденный экран, проблемы с батареей, программные сбои, проблемы с портами и повреждения корпуса. Для устранения этих поломок наши опытные мастера оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе качественный и надежный вызвать мастера по ремонту планшетов в москве.
Подробная информация размещена на сайте: https://remont-planshetov-profi.ru
выведение из запоя санкт петербург выведение из запоя санкт петербург .
Туристический портал https://feokurort.com.ua необычные маршруты, вдохновляющие истории, секреты бюджетных путешествий, советы по визам и топовые направления для отдыха.
ed drugs list: canadianpharmi – ed pills that work
http://indiapharmi.com/# best india pharmacy
Наши специалисты предлагает высококачественный выездной ремонт игровых приставок всех типов и брендов. Мы осознаем, насколько важны для вас ваши геймерские устройства, и обеспечиваем ремонт высочайшего уровня. Наши квалифицированные специалисты работают быстро и аккуратно, используя только сертифицированные компоненты, что предоставляет длительную работу проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются владельцы игровых приставок, включают поломку HDD, неисправные порты, проблемы с геймпадами, программные сбои и проблемы с охлаждением. Для устранения этих неисправностей наши опытные мастера выполняют ремонт жестких дисков, разъемов, контроллеров, ПО и систем охлаждения. Обращаясь в наш сервисный центр, вы получаете надежный и долговечный отремонтировать игровая приставка на дому.
Подробная информация доступна на сайте: https://remont-igrovyh-konsoley-mob.ru
Статьи о туризме и путешествиях https://inhotel.com.ua маршруты, гиды по достопримечательностям, советы по планированию поездок, рекомендации по отелям и лайфхаки для туристов.
Гиды по странам https://hotel-atlantika.com.ua экскурсии по городам, советы по выбору жилья и маршрутов. Туристический журнал — всё для комфортного и яркого путешествия.
Комплексный ремонт квартир https://anti-orange.com.ua и домов от Студии ремонта. Полный цикл работ: от дизайна до финишной отделки.
доставка грузов таможенное оформление доставка грузов таможенное оформление .
indian pharmacies safe Pharmacies in India that ship to USA indian pharmacy paypal
франшиза для бизнеса франшиза для бизнеса .
Instructies voor het downloaden van CorgiSlot https://zingenindezomer.nl/test/pgs/?corgislot-casino_2.html
Строительная компания https://as-el.com.ua выполняем строительство жилых и коммерческих объектов под ключ. Полный цикл: проектирование, согласование, строительство и отделка.
CR暗黒騎士呀鎧伝
k8 カジノ パチンコ チェインクロニクル
ストーリー性があり、プレイするたびに新しい発見があります。神話の世界に引き込まれます。
ファファファ
https://www.gundam-aria-pachinko.com/article/1654.html
大当たりの瞬間には、周囲の人々との一体感が生まれます。共に喜びを分かち合える楽しさがあります。
三國志T
オンラインカジノ
押忍!番長3
CRキャッツ・アイ
CR RAVE この世界こそが真実だ
南国物語
https://www.bakabon-pachinko.com/article/1.html
多彩なリーチ演出があり、どのタイミングで当たるかドキドキします。
CR牙狼金色になれ Ver.399
https://injapan.today/news/id2925.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
CR義風堂々!!兼続と慶次
https://www.k8casino.eu/Articles/3111.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
https://mexicanpharmi.com/# mexican pharmaceuticals online
На строительном портале https://avian.org.ua вы найдете всё: от пошаговых инструкций до списка лучших подрядчиков. Помогаем реализовать проекты любой сложности быстро и удобно.
Строительный портал https://ateku.org.ua ваш гид в мире строительства и ремонта. Полезные статьи, обзоры материалов, советы по выбору подрядчиков и идеи дизайна.
Портал по ремонту https://azst.com.ua всё для вашего ремонта: подбор подрядчиков, советы по выбору материалов, готовые решения для интерьера и проверенные рекомендации.
buy medicines online in india indian pharmacy or Online medicine order
http://www.google.com.bd/url?q=https://indiapharmi.com/ п»їlegitimate online pharmacies india
best online pharmacy india best india pharmacy and indian pharmacies safe reputable indian pharmacies
https://mexicanpharmi.com/# mexican drugstore online
help with ed Canadian pharmacy prices best male enhancement
online shopping pharmacy india top online pharmacy india or pharmacy website india
https://maps.google.co.ls/url?sa=t&url=https://indiapharmi.com indian pharmacy paypal
indianpharmacy com online shopping pharmacy india and online shopping pharmacy india best india pharmacy
http://indiapharmi.com/# indianpharmacy com
https://mexicanpharmi.com/# п»їbest mexican online pharmacies
mexican online pharmacies prescription drugs: Online pharmacy – buying prescription drugs in mexico online
Всё о ремонте на одном сайте https://comart.com.ua Портал по ремонту предлагает обзоры материалов, рейтинги специалистов, советы экспертов и примеры готовых проектов для вдохновения.
https://syv.iltil.ru/
Журнал по ремонту https://domtut.com.ua и строительству – советы, идеи и обзоры. Узнайте о трендах, изучите технологии и воплотите свои строительные или дизайнерские задумки легко и эффективно.
Портал о ремонте https://eeu-a.kiev.ua всё для тех, кто ремонтирует: пошаговые инструкции, идеи дизайна, обзор материалов и подбор подрядчиков.
Rely on BWER Company for superior weighbridge solutions in Iraq, offering advanced designs, unmatched precision, and tailored services for diverse industrial applications.
Журнал по ремонту и строительству https://diasoft.kiev.ua гид по современным тенденциям. Полезные статьи, лайфхаки, инструкции и обзор решений для дома и офиса.
Наши специалисты предлагает профессиональный мастерская по ремонту плоттеров на дому любых брендов и моделей. Мы понимаем, насколько значимы для вас ваши печатные устройства, и готовы предложить сервис высочайшего уровня. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что предоставляет долговечность и надежность выполненных работ.s
Наиболее общие проблемы, с которыми сталкиваются обладатели печатных устройств, включают неисправности печатающей головки, проблемы с подачей бумаги, неисправности программного обеспечения, неисправности разъемов и поломки компонентов. Для устранения этих поломок наши профессиональные техники оказывают ремонт печатающих головок, механизмов подачи бумаги, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы гарантируете себе долговечный и надежный центр ремонта плоттеров на выезде.
Подробная информация представлена на нашем сайте: https://remont-plotterov-style.ru
п»їbest mexican online pharmacies best online pharmacies in mexico or reputable mexican pharmacies online
http://www.162100.com/export.php?url=http://mexicanpharmi.com mexican border pharmacies shipping to usa
mexican online pharmacies prescription drugs reputable mexican pharmacies online and purple pharmacy mexico price list mexico drug stores pharmacies
pet antibiotics without vet prescription ed cures or men ed
http://janeyleegrace.worldsecuresystems.com/redirect.aspx?destination=https://canadianpharmi.com best ed medications
buying pills online best drug for ed and can ed be cured ed pumps
online pharmacy india: indian pharmacy – indian pharmacies safe
Настроение в вашем доме с помощью ароматических свечей, Какие ароматы выбрать для разных помещений, Создайте магию с ароматическими велас свечами
difusores para casa difusores para casa .
Новости технологий https://helikon.com.ua все о последних IT-разработках, гаджетах и научных открытиях. Свежие обзоры, аналитика и тренды высоких технологий.
Наши специалисты предлагает высококачественный мастерская по ремонту индукционной плиты с гарантией всех типов и брендов. Мы осознаем, насколько важны для вас ваши индукционные кухонные приборы, и стремимся предоставить услуги первоклассного уровня. Наши профессиональные техники работают быстро и аккуратно, используя только качественные детали, что гарантирует длительную работу наших услуг.
Наиболее частые неисправности, с которыми сталкиваются владельцы индукционных плит, включают проблемы с нагревом, проблемы с кнопками управления, программные сбои, неисправности разъемов и механические повреждения. Для устранения этих проблем наши опытные мастера проводят ремонт нагревательных элементов, сенсорных панелей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы гарантируете себе долговечный и надежный сервис ремонта индукционных плит рядом.
Подробная информация доступна на сайте: https://remont-indukcionnyh-plit-now.ru
best drugs for erectile dysfunction: Canadian pharmacy prices – how to overcome ed naturally
top rated ed pills Best Canadian pharmacy cause of ed
best online pharmacy india: Indian pharmacy international shipping – top 10 online pharmacy in india
http://canadianpharmi.com/# ed drugs online from canada
оценка профессиональных рисков связь в Москве оценка профессиональных рисков в торговле в Москве
https://mexicanpharmi.com/# buying from online mexican pharmacy
ed meds online pharmacy buy ed pills online or tadalafil without a doctor’s prescription
https://horizoninteractiveawards.com/index.php?URL=http://canadianpharmi.com/ buying ed pills online
best over the counter ed pills cheap erectile dysfunction pills and online ed meds non prescription ed pills
Строительный онлайн журнал https://inter-biz.com.ua руководства по проектам любой сложности. Советы экспертов, подбор материалов, идеи дизайна и новинки рынка.
Сайт о строительстве и ремонте https://hydromech.kiev.ua полезные советы, инструкции, обзоры материалов и технологий. Все этапы: от фундамента до отделки.
Строительные технологии https://ibss.org.ua новейшие разработки и решения в строительной сфере. Материалы, оборудование, инновации и тренды для профессионалов и застройщиков.
Bitcoin is a decentralized digital currency that operates without the demand after a principal authority or intermediary, like a bank. Introduced in 2009 via an unrevealed idiosyncratic or batch subservient to the alias Satoshi Nakamoto, Bitcoin is powered alongside blockchain technology, a segment ledger that records all transactions securely and transparently. It is known destined for its narrow reservoir of 21 million coins and its use as a collect of value and mean of exchange. Bitcoin has ripen into a popular investment alternative and a badge of the evolving digital economy.
https://mitosis-airdrop.online
https://koni-story-airdrop.online
https://hyperliquid-airdrop.online
https://mantis-airdrop.online
https://vela-exchange-airdrop.online
https://vana-airdrop.online
https://coreum-airdrop.online
https://poap-airdrop.online
https://drop-airdrop.online
https://debridge-airdrop.online
https://zerion-airdrop.online
https://movement-airdrop.online
https://kroma-airdrop.online
https://zklend-airdrop.online
https://aptos-airdrop.online
https://starrynift-airdrop.online
https://hana-network-airdrop.online
https://kelp-dao-airdrop.online
https://bera-airdrop.online
https://navigate-airdrop.online
https://pipe-network-airdrop.online
https://supra-airdrop.online
https://overtrip-airdrop.online
https://across-airdrop.online
https://rainbow-airdrop.online
https://mode-airdrop.online
https://gasp-airdrop.online
https://moongate-airdrop.online
https://duckchain-airdrop.online
https://wizzwoods-airdrop.online
http://canadianpharmi.com/# online drug store
Все о строительстве и ремонте https://kennan.kiev.ua практичные рекомендации, идеи интерьеров, новинки рынка и советы профессионалов.
mexican pharmaceuticals online Cheapest online pharmacy п»їbest mexican online pharmacies
http://canadianpharmi.com/# ed medications comparison
CR RAVE この世界こそが真実だ
k8パチンコ
ボーナスゲームが楽しく、毎回期待感があります。特に大当たり時は圧巻。
CRルパン三世~I’m a super hero
https://www.baki-pachinko.com/article/79.html
大当たりの瞬間は、ドキドキ感がたまりません。興奮が止まりません。
CR牙狼魔戒ノ花
花の慶次 琉
ミリオンゴッド-神々の凱旋
CR北斗の拳6 拳王 Ver.394
Pフィーバー戦姫絶唱シンフォギア2
ドラゴンが降りる
https://www.stelizabethchicago.org/article/624.html
大当たりの瞬間は、周りの人と共に喜びを分かち合えるのが最高です。共感が生まれます。
CR 花の慶次~焔N-K
https://casinos.town/casino-games/pachislot-komonchama-kake-pachinko
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
戦国BASARA3
https://www.eldoah-casino.org/article/122.html
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特にバトル演出が楽しい。
Дизайн интерьера и территории https://lbook.com.ua идеи оформления жилых и коммерческих пространств. Современные тренды, советы экспертов и решения для создания стильного и функционального пространства.
mexican border pharmacies shipping to usa п»їbest mexican online pharmacies or mexico drug stores pharmacies
http://ewin.biz/jsonp/?url=https://mexicanpharmi.com mexican border pharmacies shipping to usa
best online pharmacies in mexico mexico drug stores pharmacies and medicine in mexico pharmacies mexico drug stores pharmacies
Асфальтирование и ремонт дорог https://mia.km.ua информация о технологиях укладки асфальта, методах ремонта покрытий и современных материалах.
Kompaktni zarizeni https://mikrosluchatko-cena.cz poskytuje spolehlivy prenos, pevny, nenapadny design a pohodlne se nosi v kazde situaci.
https://vww.cdvig.ru/
https://canadianpharmi.com/# best male enhancement pills
buying prescription drugs in mexico online mexican mail order pharmacies or mexican online pharmacies prescription drugs
http://cse.google.co.vi/url?q=https://mexicanpharmi.com п»їbest mexican online pharmacies
mexican rx online reputable mexican pharmacies online and best online pharmacies in mexico mexican mail order pharmacies
buy medicines online in india indian pharmacy paypal or world pharmacy india
https://www.google.cv/url?sa=t&url=https://indiapharmi.com pharmacy website india
mail order pharmacy india india pharmacy mail order and top 10 online pharmacy in india indian pharmacy online
самые окупаемые франшизы самые окупаемые франшизы .
reputable mexican pharmacies online: mexicanpharmi – pharmacies in mexico that ship to usa
mexican online pharmacies prescription drugs buying prescription drugs in mexico or mexico pharmacies prescription drugs
https://www.google.co.vi/url?q=https://mexicanpharmi.com mexican drugstore online
mexican online pharmacies prescription drugs mexican rx online and best online pharmacies in mexico mexican online pharmacies prescription drugs
top 10 pharmacies in india india pharmacy mail order or <a href=" http://ksroll.net/shop/koreapt/phpinfo.php?a=real+cialis+without+a+doctor’s+prescriptionindian pharmacies safe
https://www.google.sn/url?sa=t&url=https://indiapharmi.com india pharmacy
best india pharmacy reputable indian pharmacies and top 10 pharmacies in india cheapest online pharmacy india
http://canadianpharmi.com/# compare ed drugs
reasons for ed Best Canadian pharmacy online prescription for ed meds
google viagra dosage recommendations: canadian pharmacy – ed meds online pharmacy
Наша мастерская предлагает профессиональный качественный ремонт дрона различных марок и моделей. Мы понимаем, насколько важны для вас ваши духовые шкафы, и обеспечиваем ремонт высочайшего уровня. Наши профессиональные техники работают быстро и аккуратно, используя только сертифицированные компоненты, что гарантирует надежность и долговечность проведенных ремонтов.
Наиболее частые неисправности, с которыми сталкиваются владельцы духовых шкафов, включают неисправности термостата, выход из строя таймера, треснувшую дверцу, неисправность контроллера, проблемы с конвекцией и неработающие датчики. Для устранения этих проблем наши опытные мастера оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Доверив ремонт нам, вы получаете качественный и надежный мастерская по ремонту квадрокоптеров рядом.
Подробная информация представлена на нашем сайте: https://remont-kvadrokopterov-best.ru
белое вино виноградное белое вино виноградное .
Mostbet uz uchun bonusli dasturlar mavjud | Mostbet o‘yinchilari uchun yangi imkoniyatlar va bonuslar | Mostbet apk skachat va o‘yinlarni boshlash uchun yangi foydalanuvchi bo‘ling | Mostbet yangi foydalanuvchilar uchun qulay ro‘yxatdan o‘tish tizimi mostbet uz kirish. | Mostbet oynash va yangi yutuqlarni qo‘lga kiritish imkoniyati | Mostbet oynash va katta yutuqlarni qo‘lga kiritish imkoniyati mostbet bonus olish.
https://xjg.ms-de.ru/
https://rph.hqdvd.ru/
http://indiapharmi.com/# п»їlegitimate online pharmacies india
https://uuo.iltil.ru/
https://mexicanpharmi.com/# mexican mail order pharmacies
Онлайн-журнал о строительстве https://mts-slil.info свежие новости, обзоры инноваций, рекомендации по ремонту и строительству.
Сайт про ремонт https://odessajs.org.ua полезные советы, инструкции, подбор материалов и идеи дизайна. Всё, что нужно для качественного и продуманного ремонта любого помещения.
Онлайн журнал о ремонте https://prezent-house.com.ua статьи, лайфхаки и решения для всех этапов ремонта: от планирования до отделки. Практичные рекомендации и идеи для вашего дома.
Мастерская креативных идей https://rusproekt.org пространство для творчества и инноваций. Уникальные решения для дизайна, декора и проектов любого масштаба.
buying prescription drugs in mexico online Online Mexican pharmacy reputable mexican pharmacies online
https://mexicanpharmi.com/# mexican border pharmacies shipping to usa
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт телевизоров haier, можете посмотреть на сайте: ремонт телевизоров haier адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телевизоров lg в москве, можете посмотреть на сайте: ремонт телевизоров lg в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
cheap ed pills: Best Canadian pharmacy – new erectile dysfunction treatment
Mostbet Promo Code 2024
как зарегаться в chat gpt — это мощный инструмент для автоматизации рутинных задач по созданию текстов.
https://mexicanpharmi.com/# buying prescription drugs in mexico online
Kra19.at
Журнал о строительстве и ремонте https://selma.com.ua советы экспертов, обзор материалов, тренды в интерьере и готовые решения для качественного ремонта вашего дома или офиса.
Портал о ремонте https://rvps.kiev.ua практичные рекомендации, дизайн-идеи, современные технологии и инструкции для успешного ремонта любого уровня сложно
Сайт о дизайне интерьера и территории https://sinega.com.ua тренды в дизайне помещений и благоустройстве территорий.
Информационный портал о ремонте https://sevgr.org.ua практичные советы, проверенные методики и новинки рынка. Помощь в планировании, выборе подрядчиков и создании идеального пространства.
уличный светильник светодиодный настенный влагозащищенный купить уличный светильник светодиодный настенный влагозащищенный купить .
на заказ кухни на заказ кухни .
do i have ed Canada pharmacy online cheap erectile dysfunction pill
mexico drug stores pharmacies: Cheapest online pharmacy – best online pharmacies in mexico
좋은 사이트입니다! 정말 보기 편하고 데이터가 잘 쓰여 있어서 좋습니다. 새 게시물이 올라올 때마다 알림을 받을 수 있는 방법이 궁금합니다. RSS 피드를 구독했는데 효과가 있을 듯합니다! 좋은 하루 보내세요!
Как получить диплом техникума с упрощенным обучением в Москве официально
medication from mexico pharmacy mexico drug stores pharmacies or mexican border pharmacies shipping to usa
http://images.google.la/url?q=https://mexicanpharmi.com mexican online pharmacies prescription drugs
buying prescription drugs in mexico online buying prescription drugs in mexico and mexico drug stores pharmacies mexican mail order pharmacies
юань в тенге евро в тенге .
На платформе доступны точные обменные курсы валют, обновляющиеся каждую минуту. Вы можете легко конвертировать тенге в рубли, доллары или юани бесплатно и без скрытых комиссий.
Портал об архитектуре https://solution-ltd.com.ua информация о культовых проектах, новые технологии строительства, эстетика пространств и актуальные решения для городов и частных
Архитектурный портал https://skol.if.ua новости архитектуры, современные проекты, градостроительные решения и обзоры мировых трендов.
Информационный портал о ремонте https://stinol.com.ua практичные советы, проверенные методики и новинки рынка. Помощь в планировании, выборе подрядчиков и создании идеального пространства.
best online pharmacies in mexico: mexicanpharmi – п»їbest mexican online pharmacies
Гид по ремонту https://techproduct.com.ua идеи и советы для самостоятельного ремонта: экономичные решения, готовые проекты, обзоры материалов и дизайнерские лайфхаки.
http://canadianpharmi.com/# amoxicillin without a doctor’s prescription
белое вино купить в москве белое вино купить в москве .
белое вино в москве warm-cats.ru .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов xiaomi, можете посмотреть на сайте: срочный ремонт телефонов xiaomi
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Наш сервисный центр предлагает высококачественный сервис ремонта посудомоечной машины на дому любых брендов и моделей. Мы понимаем, насколько необходимы вам ваши устройства для мойки посуды, и обеспечиваем ремонт наилучшего качества. Наши опытные мастера работают быстро и аккуратно, используя только сертифицированные компоненты, что предоставляет надежность и долговечность наших услуг.
Наиболее общие проблемы, с которыми сталкиваются владельцы посудомоечных машин, включают проблемы с температурными датчиками, проблемы с подачей воды, ошибки ПО, проблемы с подключением и повреждения корпуса. Для устранения этих неисправностей наши квалифицированные специалисты оказывают ремонт нагревательных элементов, насосов, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы гарантируете себе качественный и надежный мастерская по ремонту посудомоечной машины на выезде.
Подробная информация размещена на сайте: https://remont-posudomoechnyh-mashin-expert.ru
Bitcoin is a decentralized digital currency that operates without the penury to go to a key expert or intermediary, like a bank. Introduced in 2009 by way of an unknown individual or batch subsumed under the alias Satoshi Nakamoto, Bitcoin is powered alongside blockchain technology, a public ledger that records all transactions securely and transparently. It is known for its reduced cater to of 21 million coins and its say as a stockpile of value and usual of exchange. Bitcoin has ripen into a well-received investment selection and a badge of the evolving digital economy.
https://plume-network-airdrop.online
https://azura-airdrop.online
https://wizzwoods-airdrop.online
https://duckchain-airdrop.online
https://bless-airdrop.online
https://mode-airdrop.online
https://solayer-airdrop.online
https://taiko-airdrop.online
https://dot-airdrop.online
https://dawn-airdrop.online
https://zro-airdrop.online
https://movement-airdrop.online
https://airdrop-arb.online
https://lombard-airdrop.online
https://airdropblast.online
https://farcaster-airdrop.online
https://xion-airdrop.online
https://stride-airdrop.online
https://catizen-airdrop.online
https://scallop-airdrop.online
https://navigate-airdrop.online
https://poap-airdrop.online
https://aztec-airdrop.online
https://nundu-airdrop.online
https://celestiaairdrop.online
https://jito-airdrop.online
https://nulink-airdrop.online
https://sonic-airdrop.online
https://squid-airdrop.online
https://hana-network-airdrop.online
indian pharmacy top 10 online pharmacy in india or buy prescription drugs from india
https://toolbarqueries.google.com.af/url?q=https://indiapharmi.com buy prescription drugs from india
online pharmacy india indianpharmacy com and reputable indian online pharmacy indian pharmacies safe
ed causes and treatment what causes ed or canadian drug prices
https://arinastar.ru/forum/away.php?s=https://canadianpharmi.com online canadian pharmacy
how to help ed foods for ed and medications for ed amoxicillin without a doctor’s prescription
https://indiapharmi.com/# indian pharmacy
http://indiapharmi.com/# indian pharmacy paypal
https://indiapharmi.com/# india online pharmacy
Журнал про строительство и ремонт https://ukrainianpages.com.ua профессиональные статьи о ремонте любой сложности. Как оптимизировать расходы, найти подрядчиков и добиться идеального результата.
indian pharmacy online pharmacy india or Online medicine home delivery
https://maps.google.so/url?q=https://indiapharmi.com indian pharmacy
indianpharmacy com cheapest online pharmacy india and top online pharmacy india online shopping pharmacy india
indian pharmacy [url=https://indiapharmi.com/#]Best online Indian pharmacy[/url] best india pharmacy
medications for: canadian pharmi – drug store online
Interesting and informative. Keep it up! https://metadocs.org
パチスロ龍が如く OF THE END
北斗の拳 転生の章(パチスロ)
簡単に遊べるルールで、初心者でも安心。気軽に挑戦できるのが良いです。
紫の花
https://injapan.today/topics/ugarte
圧倒的なビジュアルと演出が魅力。神々の戦いを体感できます。
CR牙狼魔戒ノ花
k8 カジノ パチンコ キャッツ・アイ~コレクション奪還作戦~パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
CR聖戦士ダンバイン
吉宗(爺版)
琉球の守護神
魔法少女まどか☆マギカ
https://www.casinoroulette.live/article/200.html
パチンコは、ルールを覚えればすぐに楽しめるので、初心者でも安心です。気軽に始められます。
CR牙狼魔戒ノ花
https://z6o7.com/sitemaps.xml
ユーモアたっぷりの演出が魅力。プレイ中に笑顔になれる要素が多いです。
S 炎炎ノ消防隊
https://www.k8.com.se/Articles/1677.html
ボーナスゲームが楽しく、毎回期待感があります。特に大当たり時は圧巻。
pet meds without vet prescription ed in young men or ed remedies that really work
https://toolbarqueries.google.com.pk/url?q=https://canadianpharmi.com ed meds online without doctor prescription
ed medication online pain meds online without doctor prescription and ed cures that work best ed drug
Наш сервисный центр предлагает надежный починить принтер в москве всех типов и брендов. Мы понимаем, насколько значимы для вас ваши устройства для печати, и стремимся предоставить услуги первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только сертифицированные компоненты, что предоставляет долговечность и надежность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств для печати, включают неработающий картридж, проблемы с подачей бумаги, программные сбои, проблемы с портами и поломки компонентов. Для устранения этих поломок наши опытные мастера оказывают ремонт печатающих головок, механизмов подачи бумаги, ПО, разъемов и механических компонентов. Обратившись к нам, вы получаете надежный и долговечный вызвать мастера по ремонту принтеров.
Подробная информация доступна на сайте: https://remont-printerov-master.ru
Журнал про строительство и ремонт https://ukrainianpages.com.ua профессиональные статьи о ремонте любой сложности. Как оптимизировать расходы, найти подрядчиков и добиться идеального результата.
белое вино виноградное белое вино виноградное .
Автодоставка из Китая https://china-top.ru быстрая и надежная транспортировка товаров. Полный цикл: от оформления документов до доставки на склад клиента.
Find the best https://onlinecasinocanada.shop offers! Explore top-rated casinos with free spins and bonus cash for new players. Start playing without risking your funds.
Смотреть индийские фильмы онлайн https://kinoindia.tv подборка лучших фильмов с уникальным колоритом. Бесплатный доступ и ежедневное обновление каталога.
http://amoxstar.com/# order amoxicillin 500mg
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов vivo сервис, можете посмотреть на сайте: ремонт телефонов vivo сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://cipharmdelivery.com/# purchase cipro
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов sony, можете посмотреть на сайте: ремонт телефонов sony цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов realme в москве, можете посмотреть на сайте: ремонт телефонов realme
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
order amoxicillin online uk AmoxStar amoxicillin 500mg price in canada
светодиодный уличный светильник на столб 50 ватт светодиодный уличный светильник на столб 50 ватт .
how to buy generic clomid without prescription: clomidonpharm – get clomid tablets
http://clomidonpharm.com/# how to get generic clomid pills
http://prednibest.com/# can you buy prednisone over the counter in canada
Назальный спрей Серебряный Углерон надежная защита вашего дыхания. Активный углерод и ионы серебра очищают носовые ходы, увлажняют слизистую и помогают бороться с бактериями.
Free Online Games game your gateway to a world of free online entertainment! Explore a vast collection of games, from puzzles and card games to action and arcade classics. Play instantly on any device without registration or downloads
Наши специалисты предлагает надежный мастер по ремонту компьютеров в москве любых брендов и моделей. Мы знаем, насколько необходимы вам ваши компьютеры, и готовы предложить сервис первоклассного уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только оригинальные запчасти, что обеспечивает надежность и долговечность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются обладатели персональных компьютеров, включают поломку жесткого диска, неисправности видеокарты, ошибки ПО, неисправности разъемов и проблемы с охлаждением. Для устранения этих поломок наши профессиональные техники выполняют ремонт жестких дисков, видеокарт, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы получаете надежный и долговечный официальный ремонт компьютеров на выезде.
Подробная информация доступна на сайте: https://remont-kompyuterov-vip.ru
заказ шариков круглосуточно гелиевые шары с доставкой
доставка шариков с гелием доставка гелевых шаров
北斗の拳 転生の章
オンラインカジノ
パチンコのルールはシンプルですが、奥が深いです。初心者でもすぐに楽しめます。
戦国パチスロ花の慶次戦極めし傾奇者の宴~
https://www.gamersgomakers.com/article/228.html
パチンコの演出が華やかで、視覚的にも楽しめます。美しいデザインが魅力的です。
紫の花(特殊大賞燈);
k8 カジノ パチンコ 押忍!番長2
麻雀物語
BLOOD 二人の女王
闇の花(特殊大賞燈);
CR北斗の拳5 覇者
https://www.danmachi-pachinko.com/article/103.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にバトル時が最高。
交響詩篇エウレカセブン
https://www.judislotindo.com/article/71.html
パチンコの演出が豪華で、観ているだけでも楽しめるのが魅力です。エンターテイメント性が高いです。
めんそーれ(雷電ver.)
https://injapan.today/topics/money-games
パチンコをすることで、運を試すドキドキ感が味わえます。挑戦する楽しさがあります。
prednisone uk buy: PredniBest – prednisone sale
https://amoxstar.com/# buy amoxicillin online uk
нарколог на дом цены нарколог на дом цены .
where can you get amoxicillin: amoxicillin 500 mg without a prescription – amoxil pharmacy
вино белое вино белое .
Техосмотр в Санкт-Петербурге! провести техосмотр
where can i buy cipro online buy ciprofloxacin over the counter where to buy cipro online
order prednisone from canada: prednisone over the counter uk – can i buy prednisone online without a prescription
http://cipharmdelivery.com/# cipro online no prescription in the usa
Bitcoin is a decentralized digital currency that operates without the demand quest of a central right or intermediary, like a bank. Introduced in 2009 at near an unidentified idiosyncratic or congregation subsumed under the nom de guerre Satoshi Nakamoto, Bitcoin is powered at hand blockchain technology, a unshrouded ledger that records all transactions securely and transparently. It is known for its narrow accommodate of 21 million coins and its services as a store of value and medium of exchange. Bitcoin has become a popular investment alternative and a crest of the evolving digital economy.
https://icon-airdrop.online
https://symbol-airdrop.online
https://bless-airdrop.online
https://grassairdrop.online
https://b3-airdrop.online
https://blockmesh-airdrop.online
https://tabi-airdrop.online
https://kiloex-airdrop.online
https://vela-exchange-airdrop.online
https://tari-airdrop.online
https://scallop-airdrop.online
https://alphabot-airdrop.online
https://dot-airdrop.online
https://nundu-airdrop.online
https://plume-network-airdrop.online
https://vana-airdrop.online
https://375ai-airdrop.online
https://zklend-airdrop.online
https://phantom-airdrop.online
https://xion-airdrop.online
https://duckchain-airdrop.online
https://across-airdrop.online
https://elys-network-airdrop.online
https://pyth-network-airdrop.online
https://chainbase-airdrop.online
https://azura-airdrop.online
https://starrynift-airdrop.online
https://paws-airdrop.online
https://lombard-airdrop.online
https://catizen-airdrop.online
Thanks , I have just been searching for information about this topic for a while and yours is the greatest I’ve came upon till now. But, what concerning the bottom line? Are you certain about the source?
смотреть сериалы онлайн бесплатно без регистрации
cheap clomid clomidonpharm cheap clomid pills
Интернет – магазин VIP подарков https://podarki-suveniry-vip.ru
тинькофф платинум отзывы как получить карту тинькофф платинум
http://cipharmdelivery.com/# buy cipro without rx
http://prednibest.com/# buy prednisone 1 mg mexico
Наши бюстгальтер без косточек предлагают идеальное сочетание стиля и комфорта. Выберите бюстгальтер без косточек для мягкой поддержки или кружевной бюстгальтер для романтичного образа. Для будущих мам подойдут бюстгальтеры для беременных и бюстгальтеры для кормления. Обратите внимание на бюстгальтер с пуш-ап для эффекта увеличения груди и комфортные бюстгальтеры для повседневного ношения.
Летайте выгодно с Pegasus предлагаем доступные билеты, удобные маршруты и современный сервис. Внутренние и международные рейсы для комфортных путешествий.
Оценка профессиональных рисков https://ocenka-riskov-msk.ru комплексная услуга для выявления, анализа и снижения угроз на рабочем месте.
wien sexkontakte sexkontakte wien
нарколог на дом цены нарколог на дом цены .
amoxicillin 50 mg tablets [url=http://amoxstar.com/#]Amox Star[/url] how much is amoxicillin prescription
where to buy cipro online: cipro 500mg best prices – where can i buy cipro online
Maak kennis met CorgiSlot https://mik-piwgroep.nl/wp-content/articls/?corgislot_online_casino_1.html
https://www.mku.ac.ke/
https://hosting22.de/turboreviewer/domain/mku.ac.ke
kaliteden ödün vermeyenlerin tek adresi kağıthane escort sitesi https://escortlarburda.com/
amoxicillin 500mg pill Amox Star amoxicillin canada price
нарколог на дом срочно нарколог на дом срочно .
amoxicillin without prescription: AmoxStar – amoxil pharmacy
http://prednibest.com/# prednisone cream brand name
https://amoxstar.com/# generic amoxicillin cost
нарколог на дом краснодар нарколог на дом краснодар .
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
乙女 機械割
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
アイムジャグラーEXAnniversaryEdition
https://injapan.today/news/id1623.html
ボーナスゲームが楽しく、毎回期待感があります。特に大当たり時は圧巻。
アナターのオット!?はーです
オンラインカジノ
P 新世紀エヴァンゲリオン ~未来への咆哮~ SPECIAL EDITION
P 新世紀エヴァンゲリオン15 未来への咆哮
吉宗極
P ガラスの仮面M‐K1
https://casinos.town/games/pirate-21
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
Pコードギアス 反逆のルルーシュ Rebellion to Re_surrection
https://www.k8pachinko.work/Articles/2982.html
人気ゲームを基にしたパチスロで、ファンにはたまらない作品です。リアルな演出が楽しめます。
S この素晴らしい世界に祝福を!
https://pachika.com/news/post-5195.html
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
where can i get clomid no prescription clomidonpharm where to get generic clomid
Bitcoin is a decentralized digital currency that operates without the necessary quest of a central right or broker, like a bank. Introduced in 2009 at near an unidentified idiosyncratic or group subservient to the pseudonym Satoshi Nakamoto, Bitcoin is powered at hand blockchain technology, a unshrouded ledger that records all transactions securely and transparently. It is known as a remedy for its limited accommodate of 21 million coins and its use as a stockpile of value and median of exchange. Bitcoin has become a well-received investment option and a badge of the evolving digital economy.
https://nundu-airdrop.online
https://symbol-airdrop.online
https://optopia-airdrop.online
https://drop-airdrop.online
https://aztec-airdrop.online
https://ink-airdrop.online
https://polygon-zkevm-airdrop.online
https://tari-airdrop.online
https://avantis-airdrop.online
https://wizzwoods-airdrop.online
https://nekodex-airdrop.online
https://uprock-airdrop.online
https://nulink-airdrop.online
https://hana-network-airdrop.online
https://hemi-network-airdrop.online
https://shaga-airdrop.online
https://airdrop-jupiter.online
https://airdropblast.online
https://supra-airdrop.online
https://revolving-games-airdrop.online
https://onchain-airdrop.online
https://megafin-airdrop.online
https://phantom-airdrop.online
https://azura-airdrop.online
https://across-airdrop.online
https://kelp-dao-airdrop.online
https://beoble-airdrop.online
https://stride-airdrop.online
https://chainbase-airdrop.online
https://nodepay-airdrop.online
https://clomidonpharm.com/# clomid
cost generic clomid how to get clomid without rx or can you buy clomid without insurance
http://www.mydnstats.com/index.php?a=search&q=clomidonpharm.com where can i get clomid
buy generic clomid prices can i buy generic clomid without insurance and rx clomid cost of cheap clomid
Предлагаем стекла для спецтехники https://steklo-ufa.ru любых типов и размеров. Прочные, устойчивые к ударам и погодным условиям материалы.
Производство шпона в Москве https://shpon-massiv.ru качественный шпон из натурального дерева для мебели, дверей и отделки. Широкий выбор пород, гибкие размеры и выгодные цены.
buy ciprofloxacin cipro ciprofloxacin or buy ciprofloxacin
https://clients1.google.lu/url?q=https://cipharmdelivery.com buy cipro online canada
buy ciprofloxacin buy cipro online and ciprofloxacin generic buy cipro online usa
buy cheap clomid how to buy cheap clomid now or cost of generic clomid tablets
https://redirect.camfrog.com/redirect/?url=http://clomidonpharm.com/ generic clomid without insurance
where can i buy generic clomid without prescription get clomid now and order clomid without dr prescription how can i get generic clomid without prescription
http://cipharmdelivery.com/# ciprofloxacin over the counter
платный нарколог на дом платный нарколог на дом .
нарколог на дом цены [url=https://motik13.0pk.me/viewtopic.php?id=1996]нарколог на дом цены[/url] .
buy cipro online usa buy cipro without rx or buy cipro online
http://chimesinternational.com/artists/sixties_gold/link.php?link=http://cipharmdelivery.com ciprofloxacin mail online
[url=http://cse.google.as/url?sa=t&url=http://cipharmdelivery.com]buy cipro online canada[/url] where can i buy cipro online and [url=http://www.e10100.com/home.php?mod=space&uid=2761467]buy cipro online without prescription[/url] ciprofloxacin
prednisone 30 Predni Best 1250 mg prednisone
http://prednibest.com/# prednisone 10 tablet
http://prednibest.com/# prednisone 2.5 mg daily
over the counter amoxicillin canada: AmoxStar – amoxicillin 500 mg tablets
Инженерные изыскания в Москве https://geology-kaluga.ru точные исследования для строительства и проектирования. Геологические, гидрологические, экологические и геодезические работы для строительства.
Геосинтетические материалы https://geobentomat.ru надежное решение для строительства и укрепления грунтов. Геотекстиль, георешетки, геомембраны и другие материалы для дренажа, армирования и защиты конструкций.
can i buy cheap clomid: clomid on pharm – where can i buy cheap clomid price
ciprofloxacin generic: CiPharmDelivery – buy cipro online canada
merhaba pendik escort kurtköy escort sitesini ziyaret ettinizmi
purchase cipro: buy cipro without rx – buy generic ciprofloxacin
Hey there! I realize this is kind of off-topic however I had to ask. Does operating a well-established blog like yours take a lot of work? I am completely new to operating a blog however I do write in my diary everyday. I’d like to start a blog so I can easily share my personal experience and views online. Please let me know if you have any kind of recommendations or tips for brand new aspiring blog owners. Appreciate it!
https://kmural.ru/news_importer/inc/aktualnue_promokodu_bukmekerskoy_kontoru_fonbet.html
can i buy amoxicillin over the counter in australia buy amoxicillin 500mg canada can you purchase amoxicillin online
Jugabet registro Jugabet registro .
押忍!番長3
k8パチンコ
パチンコの演出に使われる音楽が印象的で、耳に残ります。ついつい口ずさんでしまうことも。
北斗の拳世紀末救世主伝説
https://www.slotonlineqq.org/article/1329.html
迫力あるバトル演出が魅力。原作ファンにはたまらない作品です。
沖ドキ! 25
k8カジノ パチンコ
南国育ち(夏30)
CR牙狼金色になれ
夜蝶飛翔
CR吉宗4 天昇飛躍の極
https://www.stake-casino.casino/article/1999.html
演出が単純明快で、ストレスなくプレイできるのが良いです。気軽に楽しめます。
CRヱヴァンゲリヲン10
https://www.top-casinoreviews.com/article/1400.html
ゲームの進行が早く、短時間でも満足感があります。気軽に楽しめるのが良いです。
P DD 北斗の拳2 ついでに愛をとりもどせ!! ラオウ
https://www.kk8520.com/archives/post-493.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
alkozona http://alkozona.ru/ .
Доставка дизельного топлива https://neftegazlogistic.ru в Москве – оперативно и качественно! Поставляем ДТ для автотранспорта, строительной и спецтехники. Гарантия чистоты топлива, выгодные цены и быстрая доставка прямо на объект.
Torlab.net https://torlab.net новый торрент-трекер для поиска и обмена файлами! Здесь вы найдете фильмы, игры, музыку, софт и многое другое. Быстрая скорость загрузки, удобный интерфейс и активное сообщество. Подключайтесь, делитесь, скачивайте — ваш доступ к миру качественного контента!
http://amoxstar.com/# amoxicillin 500mg pill
http://prednibest.com/# 400 mg prednisone
20 mg prednisone tablet Predni Best brand prednisone
how can i get clomid no prescription: can you buy generic clomid pills – how to buy generic clomid no prescription
prednisone 10 mg tablet cost can i buy prednisone online without prescription or prednisone 20mg price in india
https://toolbarqueries.google.al/url?q=https://prednibest.com prednisone 10mg for sale
prednisone online pharmacy buy prednisone online australia and prednisone 20mg price prednisone pharmacy
https://rxguides.net/guides Explore detailed guides to master your favorite games. From beginner tips to advanced strategies, our guides help you level up, complete challenges, and unlock hidden secrets for the ultimate gaming experience.
Bitcoin is a decentralized digital currency that operates without the necessary after a central dominion or intermediary, like a bank. Introduced in 2009 via an unknown individual or group under the pseudonym Satoshi Nakamoto, Bitcoin is powered away blockchain technology, a unshrouded ledger that records all transactions securely and transparently. It is known destined for its restrictive supply of 21 million coins and its use as a store of value and median of exchange. Bitcoin has enhance a in demand investment selection and a sign of the evolving digital economy.
https://zklend-airdrop.online
https://sender-labs-airdrop.online
https://kelp-dao-airdrop.online
https://the-vault-airdrop.online
https://bless-airdrop.online
https://strk-airdrop.online
https://catizen-airdrop.online
https://beoble-airdrop.online
https://arena-z-airdrop.online
https://onchain-airdrop.online
https://sunrise-airdrop.online
https://supra-airdrop.online
https://dawn-airdrop.online
https://vana-airdrop.online
https://hyperliquid-airdrop.online
https://koni-story-airdrop.online
https://morph-airdrop.online
https://mantra-airdrop.online
https://chainbase-airdrop.online
https://haven1-airdrop.online
https://duckchain-airdrop.online
https://xion-airdrop.online
https://venomairdrop.online
https://avantis-airdrop.online
https://hana-network-airdrop.online
https://gudchain-airdrop.online
https://b3-airdrop.online
https://pipe-network-airdrop.online
https://scallop-airdrop.online
https://mode-airdrop.online
ciprofloxacin generic: buy cipro without rx – ciprofloxacin 500 mg tablet price
amoxicillin 500mg capsule buy online: buying amoxicillin in mexico – buy amoxicillin over the counter uk
rexall pharmacy amoxicillin 500mg purchase amoxicillin online generic amoxicillin online
автомобильный поролон автомобильный поролон .
подготовка проекта перепланировки подготовка проекта перепланировки .
карниз с электроприводом карниз с электроприводом .
kallitenin ve özverili çalışmanın karşılığı esenler escort bayan sitesi https://esenlerescortbul.com/
can i order prednisone where to buy prednisone 20mg or prednisone 10mg tablet price
http://www.rpklublin.pl/skins/rpk/redirect.php?url=http://prednibest.com prednisone 3 tablets daily
prednisone for sale buy 10 mg prednisone and prednisone 20mg for sale prednisone medication
kallitenin ve özverili çalışmanın karşılığı esenler escort bayan sitesi https://esenlerescortbul.com/
Здесь можно шкафы для оружиягде купить сейф для ружья
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов samsung в москве, можете посмотреть на сайте: срочный ремонт телефонов samsung
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов nothing, можете посмотреть на сайте: ремонт телефонов nothing цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://amoxstar.com/# amoxicillin 500 mg cost
15 mg prednisone daily: prednisone 7.5 mg – can i buy prednisone over the counter in usa
https://amoxstar.com/# amoxicillin 500mg no prescription
can i buy clomid without rx where can i buy generic clomid without dr prescription can i buy cheap clomid tablets
buy cipro online usa antibiotics cipro or ciprofloxacin 500 mg tablet price
http://avalonadvancedmaterials.com/outurl.php?url=https://cipharmdelivery.com where to buy cipro online
buy ciprofloxacin over the counter cipro for sale and buy cipro online without prescription п»їcipro generic
Jugabet casino Jugabet casino .
where to buy clomid prices: can i buy generic clomid without prescription – order cheap clomid for sale
Your article helped me a lot, is there any more related content? Thanks!
buy cipro without rx: CiPharmDelivery – purchase cipro
buy ciprofloxacin buy cipro online canada or ciprofloxacin
https://www.google.hr/url?q=https://cipharmdelivery.com cipro 500mg best prices
buy ciprofloxacin buy cipro without rx and where can i buy cipro online where to buy cipro online
Получайте больше прибыли на onexbet, не выходя из дома.
onexbet – ваш шанс на богатство, всегда и везде.
Победные ставки с onexbet, оптимальные шансы на победу.
Получите эмоциональный заряд от игры на onexbet, и вы обязательно останетесь довольны.
onexbet – качество и профессионализм, для вас всегда в приоритете.
Мечтаете о финансовом благополучии? Вам нужен onexbet, – оптимальное решение в вашей ситуации.
onexbet – ваш надежный союзник в мире игры, на который всегда можно положиться.
Играя на onexbet, вы становитесь ближе к своей мечте, используйте onexbet для достижения ваших целей.
onexbet – это не просто азарт, это философия, которая помогает вам обогатиться.
Хотите изменить свою жизнь к лучшему? Начните с onexbet, и вы поймете, что удача всегда на вашей стороне.
onexbet – это не просто компания, это ваш путь к финансовой независимости, о котором мечтали.
Ставки на onexbet – это легко и увлекательно, но при этом ценит комфорт и безопасность.
Лучшие коэффициенты и выигрыши на onexbet, все это доступно для вас.
Хотите выигрывать больше? Присоединяйтесь к onexbet, и ваша жизнь никогда не будет прежней.
LSULeopoldo LSULeopoldo .
can you get generic clomid for sale clomid prices order generic clomid without insurance
alkozona alkozona.ru .
Наша мастерская предлагает высококачественный мастер по ремонту кондиционеров адреса всех типов и брендов. Мы знаем, насколько необходимы вам ваши сплит-системы, и готовы предложить сервис высочайшего уровня. Наши опытные мастера работают быстро и аккуратно, используя только оригинальные запчасти, что предоставляет надежность и долговечность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются владельцы кондиционеров, включают недостаток хладагента, неисправности вентилятора, программные сбои, проблемы с температурными датчиками и повреждения корпуса. Для устранения этих проблем наши опытные мастера проводят ремонт компрессоров, вентиляторов, ПО, датчиков и механических компонентов. Доверив ремонт нам, вы гарантируете себе надежный и долговечный вызвать мастера по ремонту кондиционеров с гарантией.
Подробная информация представлена на нашем сайте: https://remont-kondicionerov-wow.ru
Торкретирование бетона цена за м2
start sourcing your inventory today! learn how to find products to sell on amazon.
Здесь можно сейф огнестойкий для дома купить где купить сейф для дома
https://cipharmdelivery.com/# buy cipro cheap
http://prednibest.com/# prednisone 40 mg daily
P 新世紀エヴァンゲリオン15 未来への咆哮
k8 カジノ パチンコ
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にボーナス時の音が最高。
CR北斗の拳6 拳王
https://www.topinternetcasinos.org/article/124.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
花の慶次天に愛されし漢~
k8 カジノ パチンコ S 炎炎ノ消防隊パチスロ スロット 機械割 解析 天井 初打ち 打ち方 スペック ゾーン 設定判別 ヤメ時・演出・プレミアムまとめ
P ゴジラ対エヴァンゲリオン~G細胞覚醒~
CR聖戦士ダンバイン
押忍!サラリーマン番長
S 炎炎ノ消防隊
https://jahromblog.com/Articles/416158.html
新しい台が出るたびにワクワクします。流行を追うのが楽しいです。
CR神獣王2
https://gamein.jp/slots/the-dog-house
パチンコを楽しむには、事前に情報収集が重要です。他のプレイヤーの意見を参考にするのも良いでしょう。
交響詩篇エウレカセブン 2
https://injapan.today/topics/shunsaku-aoshima
キャラクターの個性が際立っており、ストーリーに引き込まれます。
1Win giriş və mərc təcrübəsi hər kəs üçün əlverişlidir | 1Win Azərbaycanda lider mərc platforması kimi tanınır | 1Win platformasında təhlükəsiz ödəniş və bonus imkanları 1win casino. | 1Win giriş və oyun təcrübəsi yüksək keyfiyyətlidir | 1Win giriş və qeydiyyat prosesləri çox sadədir 1Win qeydiyyat.
buy cipro cheap: ci pharm delivery – buy ciprofloxacin over the counter
bilan o’yin-kulgi dunyosiga xush kelibsiz 1win! 1000 dan ortiq o’yinlar, jonli dilerlar, sport va e-sport bir joyda. Saxiy bonuslar, tezkor depozitlar va qulay pul olish. O’ynang, g’alaba qozoning va yangi his-tuyg’ularga qayting!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов poco рядом, можете посмотреть на сайте: ремонт телефонов poco
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов meizu, можете посмотреть на сайте: ремонт телефонов meizu
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
can i order cheap clomid tablets order cheap clomid without prescription clomid generics
Good day very cool site!! Man .. Excellent .. Wonderful .. I’ll bookmark your blog and take the feeds also? I am happy to search out a lot of helpful information right here within the put up, we’d like develop more strategies in this regard, thank you for sharing. . . . . .
фильмы онлайн бесплатно
özverinin ve başarının tek adresi esenyurt escort sitesi https://esenyurteskortbul.com/
özverinin ve başarının tek adresi esenyurt escort sitesi https://esenyurteskortbul.com/
Discover the best game codes https://rxguides.net in-depth guides, and updated tier lists for your favorite games! Unlock exclusive rewards, master gameplay strategies, and find the top characters or items to dominate the competition.
Большие выигрыши с onexbet, присоединяйтесь и выигрывайте|Успешные ставки на спорт с onexbet, делайте свои ставки и выигрывайте|Надежный букмекер onexbet, играйте честно и безопасно|Приятные сюрпризы от onexbet, получите дополнительные ставки бесплатно|Играйте в казино онлайн на onexbet, не упустите шанс стать миллионером|Надежный сервис onexbet, получите свои деньги моментально|Соблюдайте законодательство с onexbet, ваша безопасность – важнее всего|Не упустите шанс следить за любимыми матчами, прогнозируйте и побеждайте онлайн|Получайте эксклюзивные предложения от onexbet, получите возможность выиграть дополнительные призы|Азартные игры с живыми дилерами на onexbet, получайте эмоции и азарт вместе с onexbet|Ставьте на любимые команды и игроков, получайте прибыль от своих прогнозов|Увеличьте свой капитал с onexbet, получайте прибыль без лишних затрат|Непревзойденная возможность заработать деньги, делайте ставки с выгодой и уверенностью|Ваш комфорт – наш приоритет, не оставляйте вопросов без ответа|Сделайте ставки с комфортом и удовольствием, получайте удовольствие от азарта с onexbet|Играйте и выигрывайте крупные суммы, ставьте и получайте крупные выигрыши|Получайте прибыль от своих прогнозов, новый уровень заработка|Играйте и зарабатывайте больше, получайте индивидуальные предложения и бонусы|Больше выигрышей с onexbet, больше денег с onexbet|Профессиональная букмекерская контора onex
download one x bet https://arxbetdslps.com/ .
amoxicillin 500mg price: AmoxStar – amoxicillin brand name
ciprofloxacin 500 mg tablet price: ci pharm delivery – ciprofloxacin order online
clomid cost: generic clomid pill – can i get cheap clomid without rx
гибкий электрокарниз привод elektrokarniz-dlya-shtor499.ru .
gfhfkjy porolon-dlya-divana.ru .
проектная организация перепланировка проектная организация перепланировка .
amoxicillin 500 mg price AmoxStar amoxicillin online canada
prednisone 40 mg tablet order prednisone online canada or non prescription prednisone 20mg
http://2ch.omorovie.com/redirect.php?url=http://prednibest.com prednisone in canada
prednisone pill prices prednisone online india and prednisone 2 mg daily prednisone without prescription 10mg
Наша мастерская предлагает профессиональный официальный ремонт кофемашин на дому любых брендов и моделей. Мы знаем, насколько важны для вас ваши кофемашины, и стремимся предоставить услуги первоклассного уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что предоставляет надежность и долговечность наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются владельцы кофемашин, включают неисправности нагревательных элементов, неисправности насоса, программные сбои, неработающие разъемы и механические повреждения. Для устранения этих неисправностей наши квалифицированные специалисты выполняют ремонт нагревательных элементов, насосов, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе качественный и надежный сервис ремонта кофемашина на выезде.
Подробная информация доступна на сайте: https://remont-kofemashin-top.ru
http://prednibest.com/# prednisone sale
Как приобрести диплом о среднем образовании в Москве и других городах
https://cipharmdelivery.com/# cipro 500mg best prices
where can i buy amoxocillin can i purchase amoxicillin online or can we buy amoxcillin 500mg on ebay without prescription
https://cse.google.com.af/url?sa=i&url=https://amoxstar.com amoxicillin 775 mg
amoxicillin 875 125 mg tab how to buy amoxicillin online and amoxicillin cephalexin amoxicillin capsule 500mg price
buy amoxicillin amoxil generic amoxicillin 500mg
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов infinix в москве, можете посмотреть на сайте: ремонт телефонов infinix
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Здесь можно сейфы для дома купить сейф для дома москва
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов honor сервис, можете посмотреть на сайте: ремонт телефонов honor рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
prednisone price south africa how can i order prednisone or cost of prednisone 10mg tablets
http://src.kojet.com.tw/dyna/webs/gotourl.php?id=17&url=http://prednibest.com prednisone 10 mg brand name
prednisone 20mg price in india prednisone prescription for sale and prednisone 2 mg prednisone tablets canada
общение без регистрации общение без регистрации .
http://amoxstar.com/# 875 mg amoxicillin cost
where to get generic clomid without dr prescription how to get clomid without prescription or can you get cheap clomid no prescription
http://cse.google.bj/url?sa=i&url=http://clomidonpharm.com where to buy generic clomid tablets
cost clomid without insurance can you get cheap clomid without insurance and how to get cheap clomid tablets can i buy cheap clomid online
where to buy cheap clomid pill: cost of clomid no prescription – can you get generic clomid without rx
CRぱちんこ押忍!番長
北斗 暴凶星 先バレ
演出の多様性があり、毎回新しい体験が待っています。飽きることがありません。
Pフィーバー戦姫絶唱シンフォギア2
https://rioace.jahromblog.com/post-306/
ゲームの進行が早く、短時間でも満足感があります。気軽に楽しめるのが良いです。
S 炎炎ノ消防隊
k8 casino
Pガールズパ ンツァー 劇場版
水浒传
P フィーバー 機動戦士ガンダムユニコーン b
P 新世紀エヴァンゲリオン15 未来への咆哮
https://www.gaspachino.com/sitemaps.xml
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
めんそーれ
https://xn--k8-9g4a3b4f.site/shinbun/post-12324.html
新しい台が出るたびにワクワクします。流行を追うのが楽しいです。
パチスロ北斗の拳 修羅の国篇
https://www.xn--k8-9g4a3b4f.world/games/id3410
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
amoxicillin 500mg capsules: AmoxStar – amoxicillin 500 mg tablet price
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
prednisone 5 tablets: PredniBest – prednisone 50 mg prices
buy generic ciprofloxacin buy cipro online usa or ciprofloxacin order online
http://cse.google.com.sl/url?sa=i&url=http://cipharmdelivery.com п»їcipro generic
ciprofloxacin buy cipro no rx and cipro pharmacy ciprofloxacin generic
cost of clomid without insurance clomid on pharm buying clomid without prescription
Jugabet casino Per? Jugabet casino Per? .
cipro ciprofloxacin mail online or ciprofloxacin generic price
http://images.google.la/url?q=https://cipharmdelivery.com buy cipro online
п»їcipro generic antibiotics cipro and buy cipro online buy cipro online without prescription
https://amoxstar.com/# where to buy amoxicillin over the counter
http://cipharmdelivery.com/# cipro online no prescription in the usa
дивитись фільми онлайн безкоштовно дивитися кіно онлайн
дивитись фільми онлайн безкоштовно uakino.pl
п»їcipro generic: ci pharm delivery – п»їcipro generic
Тут можно преобрести сейф пожаровзломостойкие купить взломостойкие сейфы цена
buy amoxicillin 500mg canada amoxicillin 500mg no prescription or <a href=" http://vm-01.magneticgrid.com/info.php?a=where+can+i+buy+viagra+online+cheaporder amoxicillin online
https://clients1.google.com.bn/url?q=http://amoxstar.com can you buy amoxicillin over the counter
amoxil pharmacy amoxicillin no prescription and amoxicillin 500 mg without a prescription amoxicillin discount
buy cipro no rx CiPharmDelivery ciprofloxacin generic price
how to get generic clomid price: can i purchase clomid without prescription – can i buy generic clomid
find out this here phantom Download
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин zanussi рядом, можете посмотреть на сайте: срочный ремонт стиральных машин zanussi
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
navigate to this web-site phantom Extension
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт телефонов huawei адреса, можете посмотреть на сайте: ремонт телефонов huawei
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
read phantom Extension
Look At This phantom Download
other keplr Download
where can i get clomid prices: can i purchase clomid tablets – buy cheap clomid without prescription
merhaba kaliteli güneşli escort sitesine hoşgeldiniz https://gunesliescortbul.com/
prednisone 5 mg tablet price prednisone cost us prednisone buy
merhaba kaliteli güneşli escort sitesine hoşgeldiniz https://gunesliescortbul.com/
see this page MetaMask Download
visit the site phantom Download
visit homepage MetaMask Download
browse around these guys phantom wallet
http://amoxstar.com/# where can i get amoxicillin
Kocaeli Gazetesi‘nden en güncel haberlere ulaşabilir, Kocaeli’deki gelişmeleri öğrenebilirsiniz. #Kocaeli #KocaeliHaber #KocaeliHaberleri
http://amoxstar.com/# amoxicillin pills 500 mg
фильмы 2024 дивитись онлайн безкоштовно дивитися фільми онлайн безкоштовно без реєстрації
prednisone 10 mg over the counter: buy prednisone 10mg online – generic prednisone for sale
prednisone 10 mg tablets generic prednisone pills or prednisone medication
https://clients1.google.sm/url?q=https://prednibest.com prednisone 50mg cost
where can i buy prednisone prednisone 5mg over the counter and online prednisone order prednisone
Online casinos taya365 are thousands of slots, live games, profitable promotions and instant wins. Try your luck in a comfortable and safe environment, enjoying the excitement at any time and from any device.
Haberler ve Son Dakika gelişmeleri kaçırmayın. #SonDakika #GelişmeleriKaçırma #Bilgilen
where to get generic clomid online how to get cheap clomid without rx can you get clomid online
чат общение чат общение .
kantorbola
Kunjungi situs kantorbola untuk memainkan game online terbaik , tersedia beragam pilihan game online yang mudah untuk anda menangkan . Kantor bola juga menyediakan fitur transaksi kilat melalui QRIS
how much is amoxicillin prescription amoxicillin medicine or amoxicillin 500 mg price
https://www.google.com.co/url?q=https://amoxstar.com amoxacillian without a percription
amoxicillin no prescipion buy amoxicillin 500mg capsules uk and amoxicillin 500mg pill amoxicillin 500mg
Откройте для себя инновации с samsung store широкий выбор смартфонов, планшетов, телевизоров и бытовой техники. Выгодные цены, гарантия качества и быстрая доставка. Закажите оригинальную продукцию Samsung прямо сейчас и наслаждайтесь технологиями будущего!
Shop Marlboro
cipro for sale: ci pharm delivery – cipro
cipro ciprofloxacin: cipro 500mg best prices – ciprofloxacin over the counter
prednisone 20mg online buying prednisone mexico or prednisone 30
https://www.google.bj/url?sa=t&url=https://prednibest.com prednisone 50
10 mg prednisone tablets no prescription prednisone canadian pharmacy and prednisone 250 mg buy cheap prednisone
antibiotics cipro: cipro pharmacy – buy cipro online canada
вывод из запоя стационар краснодар вывод из запоя стационар краснодар .
where can i buy cipro online buy ciprofloxacin ciprofloxacin generic
boat booking dubai yacht ride price
private yacht https://boat-dubai-rent.com
제네시스 GV80
http://cipharmdelivery.com/# buy ciprofloxacin
http://prednibest.com/# prednisone 4 mg daily
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин siemens цены, можете посмотреть на сайте: ремонт стиральных машин siemens в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
вывод из запоя цены на дому краснодар вывод из запоя цены на дому краснодар .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин smeg рядом, можете посмотреть на сайте: ремонт стиральных машин smeg в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
вывод из запоя краснодар стационар вывод из запоя краснодар стационар .
buy cipro no rx buy cipro no rx or cipro ciprofloxacin
https://asia.google.com/url?q=https://cipharmdelivery.com ciprofloxacin
buy cipro without rx where to buy cipro online and cipro ciprofloxacin buy cipro online canada
amoxicillin 500 mg tablet price amoxicillin 1000 mg capsule over the counter amoxicillin canada
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин siemens цены, можете посмотреть на сайте: ремонт стиральных машин siemens адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
cipro for sale ciprofloxacin over the counter or purchase cipro
http://maps.google.mw/url?q=https://cipharmdelivery.com where can i buy cipro online
ciprofloxacin over the counter buy cipro online canada and ciprofloxacin 500 mg tablet price where to buy cipro online
buy cipro no rx: ci pharm delivery – buy cipro online canada
amoxicillin 500mg buy online uk buy amoxicillin online mexico or how to get amoxicillin over the counter
http://opac2.mdah.state.ms.us/stoneCollection.php?referer=http://amoxstar.com/ amoxicillin 500 mg tablet
where to buy amoxicillin 500mg buy amoxicillin 500mg uk and buy amoxicillin 500mg canada amoxicillin 500mg prescription
cipro 500mg best prices: cipro online no prescription in the usa – cipro ciprofloxacin
вывод из запоя капельница вывод из запоя капельница .
cipro 500mg best prices cipro antibiotics cipro
amoxicillin 500 mg where to buy: cost of amoxicillin – order amoxicillin online no prescription
https://prednibest.com/# buy prednisone online australia
generic clomid without dr prescription: clomid on pharm – get generic clomid without rx
https://clomidonpharm.com/# how can i get clomid
アイムジャグラーEX (4発1)(V2.2)
ユニコーン金保留確率
ボーナスゲームの演出が豪華で、楽しみが倍増します。目が離せません。
CR龍が如く見参!天照祗園編
https://xn--k8-9g4a3b4f.site/tags/amazon-%E3%82%B9%E3%83%AD%E3%83%83%E3%83%88-%E5%AE%9F%E6%A9%9F
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
革命機ヴァルヴレイヴ
ハナハナ鳳凰天翔
Dororonえん魔くん メ~ラめら
パチスロ 貞子3D
鬼武者3
主役は銭形
https://www.spins.guru/jackpot-games/the-jackpot-game-treasures-of-aztec-from-pocket-games-soft-at-spins-casino.html
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特にモンスター戦が楽しい。
CR牙狼金色になれ Ver.399
https://xn--k8-9g4a3b4f.site/shinbun/post-11180.html
定期的に通うことで、常連さんとのつながりが生まれます。コミュニティ感が楽しいです。
CRヱヴァンゲリヲン始まりの福音~
https://xn--k8-9g4a3b4f.site/tags/%E3%83%80%E3%83%B3-%E3%81%BE%E3%81%A1-%E3%82%B9%E3%83%AD%E3%83%83%E3%83%88-%E8%A9%95%E4%BE%A1
友人と一緒にプレイすることで、共感が生まれ、楽しさが倍増します。
cost of prednisone PredniBest prednisone 20 mg
dünya güzeli beşiktaş kızların olduğu tek kaliteli olan beşiktaş escort sitesi
dünya güzeli beşiktaş kızların olduğu tek kaliteli olan beşiktaş escort sitesi
kantor bola99
prednisone 10mg online prednisone 50mg cost or prednisone 20mg capsule
https://maps.google.com.py/url?q=https://prednibest.com generic prednisone for sale
medicine prednisone 10mg purchase prednisone and online order prednisone canine prednisone 5mg no prescription
вывод из запоя бесплатно вывод из запоя бесплатно .
вывод из запоя на дому санкт петербург вывод из запоя на дому санкт петербург .
Самые актуальные новости Украины https://2news.com.ua/category/finansy/ политика, экономика, общество и культура. Только проверенные факты и оперативная подача информации.
Discover the latest codes for mobile games https://game-zoom.ru exclusive codes for Roblox, and detailed guides for games. Stay ahead in your favorite titles with tips, tricks, and rewards to enhance your gaming experience!
amoxicillin from canada can you buy amoxicillin over the counter in canada buy amoxicillin from canada
order cheap clomid now: where buy cheap clomid now – can you buy clomid for sale
amoxicillin order online no prescription: AmoxStar – amoxicillin generic
вывод из запоя дешево краснодар вывод из запоя дешево краснодар .
вывод из запоя на дому краснодар [url=http://klin.0pk.me/viewtopic.php?id=4406/]вывод из запоя на дому краснодар [/url] .
amoxicillin 250 mg price in india amoxicillin 500mg capsules antibiotic or amoxicillin 500 mg cost
http://klubua.ru/redirect.php?url=amoxstar.com amoxicillin no prescription
where to buy amoxicillin amoxicillin 1000 mg capsule and amoxicillin pharmacy price how to buy amoxycillin
prednisone 50 mg tablet cost prednisone cream or prednisolone prednisone
https://www.hotel-jobs.co.uk/extern.aspx?cu=45996&page=1&s=42&src=https://prednibest.com:: prednisone 2 mg daily
prednisone 1 mg daily prednisone 10mg and buy prednisone 40 mg prednisone tablet 100 mg
buying clomid for sale: can you buy cheap clomid prices – can i purchase generic clomid without prescription
http://prednibest.com/# buy prednisone nz
Практическое руководство Коновалова https://olsi.ru упражнения и советы для восстановления и укрепления здоровья.
https://prednibest.com/# buy prednisone without a prescription best price
Анализируйте поведение своей аудитории https://bs2site2.net находите точки роста и повышайте конверсии сайта. Поможем вам сделать ваш бизнес эффективнее и увеличить доход.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article. https://www.binance.com/es-MX/register?ref=JHQQKNKN
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин indesit рядом, можете посмотреть на сайте: ремонт стиральных машин indesit в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин lg сервис, можете посмотреть на сайте: ремонт стиральных машин lg сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
cost of clomid without insurance clomid on pharm where can i get cheap clomid for sale
CR蒼天の拳2
4号機
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
Lバキ 強くなりたくば喰らえ!!!
https://www.betfind.org/article/81.html
新しい台の演出が毎回楽しみです。特別感があります。
押忍!サラリーマン番長
マクロス4 スマスロ モード
デビル メイ クライ 4
アイムジャグラーEXAnniversaryEdition
BLOOD 二人の女王
闘神雷電 -花田勝-
https://www.k8-casino.jp/guides/4311.html
演出や音楽が迫力満点で、プレイするたびに興奮します。サウンドトラックも素晴らしい。
CRキャプテン翼
https://xn--k8-9g4a3b4f.site/shinbun/post-12145.html
友達と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
CRモンスターハンター4
https://www.k8casinoofficial.jp/slots/id629.html
人気ゲームをベースにしたパチスロで、ファンにはたまらない作品です。リアルなバトルが楽しめます。
С помощью платформы https://bc2best.in вы получите доступ к инновационным инструментам, которые помогут преуспеть в онлайн-продвижении. Управление проектами, оптимизация SEO и аналитика — все это доступно на bs2site.
buy cipro online where to buy cipro online or buy generic ciprofloxacin
https://images.google.com.vc/url?q=https://cipharmdelivery.com п»їcipro generic
buy ciprofloxacin over the counter ciprofloxacin over the counter and ciprofloxacin over the counter cipro pharmacy
Узнайте свою аудиторию лучше https://bs02shop.gl анализ данных, улучшение опыта пользователей и рост конверсий. Помогаем привлекать клиентов и увеличивать доход.
amoxicillin 250 mg capsule: AmoxStar – order amoxicillin online uk
cipro pharmacy ciprofloxacin generic price or where to buy cipro online
https://www.google.hu/url?sa=t&url=https://cipharmdelivery.com cipro online no prescription in the usa
cipro for sale buy cipro without rx and buy cipro online canada buy cipro online canada
prednisone 5mg coupon [url=http://prednibest.com/#]Predni Best[/url] prednisone 2.5 tablet
amoxicillin over counter: price for amoxicillin 875 mg – amoxicillin medicine
С сайтом https://bs2syte.at/ вы можете легко анализировать свою аудиторию, улучшать видимость сайта в поисковых системах и повышать конверсии. Наша команда экспертов гарантирут качественную поддержку и советы для эффективного использования всех инструментов.
cipro pharmacy: ci pharm delivery – buy cipro without rx
эскорт услуги цена https://vip-eskort-msk.ru
выведение из запоя санкт петербург выведение из запоя санкт петербург .
Bị ‘tố’ chậm bàn giao nhà, chủ đầu tư Discovery Complex 302 Cầu Giấy nói gì?
Thời gian gần đây, thông tin về việc chủ đầu tư dự án Discovery Complex 302 Cầu Giấy bị tố chậm bàn giao nhà đã gây chú ý trong cộng đồng. Những phản ánh từ phía khách hàng cho rằng tiến độ không đảm bảo khiến nhiều người cảm thấy thất vọng. Phía chủ đầu tư đã lên tiếng giải thích rằng họ đang nỗ lực hết mình để hoàn thiện dự án, đồng thời cam kết sẽ sớm khắc phục các vấn đề và đáp ứng kỳ vọng của cư dân.
Những câu chuyện cuộc sống đầy xúc động
Côi cút 4 trẻ mồ côi bên bến đò Thạnh Mỹ
Tại một bến đò nhỏ ở Thạnh Mỹ, câu chuyện về 4 đứa trẻ mồ côi sống nương tựa vào nhau khiến bao người rơi nước mắt. Mất cha mẹ từ khi còn nhỏ, các em phải tự lo cho nhau trong hoàn cảnh khó khăn. “Hai đứa được đi học, mẹ chết cũng yên lòng…” là lời tâm sự nghẹn ngào của một trong những đứa trẻ, thể hiện khát vọng vươn lên dù cuộc sống đầy thách thức.
Những chuyện tình đầy trăn trở
Cưới vội giờ muốn bỏ cho xong… Có những cặp đôi vì nhiều lý do mà kết hôn vội vã, để rồi sau đó nhận ra những khác biệt không thể hòa hợp. Hậu quả là những cuộc ly hôn đầy đau lòng, để lại bài học sâu sắc về sự thấu hiểu và cân nhắc kỹ trước khi bước vào cuộc sống hôn nhân.
Đừng ngại khi yêu đàn ông lớn tuổi Tình yêu không phân biệt tuổi tác, và việc yêu một người đàn ông lớn tuổi đôi khi mang lại sự chín chắn, vững vàng mà không phải mối quan hệ nào cũng có được. Đừng ngần ngại nếu trái tim bạn hướng về một người trưởng thành, vì chính sự khác biệt này có thể là chìa khóa cho một mối quan hệ bền vững.
Sức khỏe và những câu chuyện y học
Đã hoàn thiện phác đồ điều trị chuẩn cho bệnh nhân nhiễm Covid Trong cuộc chiến chống lại đại dịch Covid-19, các bác sĩ đã đạt được bước tiến lớn khi hoàn thiện phác đồ điều trị chuẩn. Điều này không chỉ giúp nâng cao tỷ lệ chữa khỏi bệnh mà còn mang lại hy vọng cho hàng triệu người dân trên toàn thế giới.
Tán gia bại sản vì con mắc bệnh ung thư máu Một gia đình nghèo phải bán hết tài sản để chữa trị cho con bị ung thư máu. Sự hy sinh và tình yêu của cha mẹ dành cho con cái là điều không gì có thể đo đếm được, nhưng câu chuyện này cũng đặt ra câu hỏi lớn về việc hỗ trợ y tế cho những gia đình khó khăn.
Những thông tin đáng chú ý
Thực hư thông tin VinFast tặng miễn phí 80 ô tô ‘made in Vietnam’ Thông tin về việc VinFast tặng miễn phí 80 chiếc ô tô “Made in Vietnam” cho người dùng đã gây xôn xao. Tuy nhiên, đại diện công ty cho biết đây là tin đồn không chính xác, đồng thời khuyến cáo người dân nên kiểm tra nguồn tin để tránh bị lừa đảo.
Hà Nội khuyến khích doanh nghiệp phát triển sản phẩm công nghiệp chủ lực Thành phố Hà Nội đang đẩy mạnh khuyến khích các doanh nghiệp phát triển các sản phẩm công nghiệp chủ lực. Điều này không chỉ nâng cao sức cạnh tranh mà còn thể hiện sự xứng tầm của thủ đô trong lĩnh vực công nghiệp hiện đại.
Cuộc sống đầy những câu chuyện, từ những khó khăn đến những bước tiến trong y học và công nghệ. Mỗi câu chuyện là một bài học, một góc nhìn để chúng ta thêm trân trọng và sống trọn vẹn hơn.
https://cipharmdelivery.com/# buy cipro online canada
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт стиральных машин kuppersbusch, можете посмотреть на сайте: ремонт стиральных машин kuppersbusch сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
amoxicillin 500mg capsules antibiotic purchase amoxicillin online or amoxicillin 500 mg tablet
https://cse.google.com.qa/url?q=https://amoxstar.com buy amoxicillin
how to get amoxicillin over the counter amoxicillin canada price and amoxicillin 500 mg price amoxicillin for sale
buy clomid cheap clomid online where to get generic clomid without prescription
https://clomidonpharm.com/# where can i get cheap clomid pill
en güzel genç kızların olduğu istanbul jigolo sitesi
en güzel genç kızların olduğu istanbul jigolo sitesi
промокод продамус на 5000 промокод продамус на 5000 .
Check Out Your URL MetaMask Download
best site MetaMask Download
find out here phantom Download
imp source Metamask Extension
check this link right here now phantom Download
buy amoxicillin over the counter uk: amoxicillin cephalexin – buy cheap amoxicillin
click to read more phantom wallet
Your Domain Name phantom Extension
explanation keplr Download
have a peek here phantom Extension
п»їcipro generic: ciprofloxacin order online – cipro pharmacy
выведение из запоя на дому спб выведение из запоя на дому спб .
выведение из запоя на дому спб выведение из запоя на дому спб .
вывод из запоя в санкт-петербурге вывод из запоя в санкт-петербурге .
i thought about this [url=https://rabby-wallet.net/]rabby wallet[/url]
anchor keplr Download
Юридическое агентство «Актив правовых решений» https://ufalawyer.ru было основано в 2015 году в центре столицы Республики Башкортостан – городе Уфа, командой высококвалифицированных юристов, специализирующихся на вопросах недвижимости, семейном и жилищном праве, а также в спорах исполнения договоров строительного подряда и банкротства физических лиц.
Портал для коллекционеров https://ukrcoin.com.ua и ценителей монет и банкнот Украины. Узнайте актуальные цены на редкие украинские монеты, включая копейки, и откройте для себя уникальные экземпляры для своей коллекции. На сайте представлены детальные описания, редкости и советы для нумизматов. Украинские монеты разных периодов и их стоимость – всё это на одном ресурсе!
вывод из запоя цены [url=tatuheart.ukrbb.net/viewtopic.php?f=8&t=15095]вывод из запоя цены[/url] .
en kaliteli kadıköy escort bostancı escort göztepe escort sitesi sizleride bekleriz https://kadikoyescortbul.com/
en kaliteli kadıköy escort bostancı escort göztepe escort sitesi sizleride bekleriz https://kadikoyescortbul.com/
Вопросы и ответы: можно ли быстро купить диплом старого образца?
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт приставок xbox, можете посмотреть на сайте: срочный ремонт приставок xbox
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин aeg, можете посмотреть на сайте: ремонт стиральных машин aeg в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://clomidonpharm.com/# where can i buy cheap clomid price
http://cipharmdelivery.com/# ciprofloxacin order online
An impressive share! I have just forwarded this onto a coworker who has been doing a little homework on this. And he actually ordered me breakfast simply because I discovered it for him… lol. So let me reword this…. Thank YOU for the meal!! But yeah, thanx for spending some time to talk about this subject here on your internet site.
зеркало твин казино
CRぱちんこ押忍!番長
ベラアンドジョン
迫力あるバトル演出が魅力。原作ファンにはたまらない作品です。
CRフィーバー バイオハザード リベレーションズ
https://injapan.today/realtime/id7545.html
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
紫の花
北斗の拳新台パチンコ
パラダイスハイビスカス
三國志T
花の慶次天に愛されし漢~
コードギアス反逆のルルーシュ
https://injapan.today/topics/masahiro-tanaka-yakult
パチンコ店の雰囲気は賑やかで、友達と一緒に楽しむのに最適です。色とりどりの台が魅力的です。
斉天大聖
https://www.xn--k8-yh4a6b5d8j.biz/Articles/2936.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
鉄拳R 2004
https://www.queencasino.co.in/article/267.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
Vnish offers official https://vnish.us firmware for Antminer models S21, T21, S19, T19, and L7. Visit the official Vnish website to boost your mining efficiency by 15-25%.
My Betting Sites India https://bettingblog.tech your guide to the best sports betting sites. Reviews, ratings, bonuses and comparisons. Find the perfect sports betting platform in India!
детское порно телеграм где можно купить меф
Daca ave?i nevoie de asistenta contabila Moldova, speciali?tii Lorand Expert sunt aici pentru dumneavoastra! Oferim sprijin personalizat in gestionarea documentelor contabile ?i raportarea fiscala, economisind timp ?i resurse valoroase.
вывод из запоя вывод из запоя .
вывод из запоя цена вывод из запоя цена .
телеграм бот купить меф русские шлюхи
вывод из запоя санкт петербург вывод из запоя санкт петербург .
Наш сервисный центр предлагает профессиональный сервисный центр по ремонту стиральных машин на выезде любых брендов и моделей. Мы знаем, насколько необходимы вам ваши устройства для стирки, и обеспечиваем ремонт высочайшего уровня. Наши профессиональные техники работают быстро и аккуратно, используя только оригинальные запчасти, что предоставляет надежность и долговечность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств для стирки, включают неисправности барабана, неработающий нагревательный элемент, неисправности программного обеспечения, неработающий насос и поломки компонентов. Для устранения этих неисправностей наши квалифицированные специалисты оказывают ремонт барабанов, нагревательных элементов, ПО, насосов и механических компонентов. Обратившись к нам, вы обеспечиваете себе качественный и надежный ремонт стиральной машины.
Подробная информация доступна на сайте: https://remont-stiralnyh-mashin-ace.ru
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт посудомоечных машин siemens, можете посмотреть на сайте: ремонт посудомоечных машин siemens
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт приставок sony playstation, можете посмотреть на сайте: ремонт приставок sony playstation рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Daca sunte?i in cautarea unor exper?i in Consultante Fiscale, Lorand Expert va poate ajuta sa optimiza?i cheltuielile ?i sa evita?i riscurile financiare. Consilierii no?tri ofera strategii eficiente adaptate specificului afacerii dumneavoastra.
戦国パチスロ花の慶次戦極めし傾奇者の宴~
転スラ スロット 到達者
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
アイムジャグラーEX (4発1)(V2.2)
https://casinosnodepositbonus.com/tags/%E9%81%8A%E9%9B%85%E5%A0%82
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
吉宗(双姬版)
吉宗 エンディング 条件
P真・北斗無双 第3章
CR牙狼GOLDSTORM翔 Ver.319
e ルパン三世 THE FIRST
キャッツ・アイ~コレクション奪還作戦~
https://xn--k8-9g4a3b4f.site/tags/%E3%82%AB%E3%82%B8%E3%83%8E-%E3%82%B9%E3%83%AD%E3%83%83%E3%83%88-%E7%9B%AE-%E6%8A%BC%E3%81%97
パチンコを楽しむには、事前に情報収集が重要です。他のプレイヤーの意見を参考にするのも良いでしょう。
ぱちんこソードアート・オンラインK12
https://www.deadlysevensinslot.com/article/156.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
翔馬
https://xn--k8-9g4a3b4f.site/tags/%E3%82%B9%E3%83%AD%E3%83%83%E3%83%88-%E5%A4%A9%E4%BA%95-%E7%8B%99%E3%81%84-%E5%8B%9D%E3%81%A6%E3%82%8B
圧倒的なビジュアルと演出が魅力。神々の戦いを体感できます。
Lorand Expert asigura servicii contabilitate Chi?inau de inalta calitate, combinand experien?a practica cu abordari moderne. Colaborarea cu noi inseamna eficien?a, exactitate ?i sprijin constant pentru succesul companiei dumneavoastra.
снятие ломки наркомана снятие ломки наркомана .
Alegerea servicii de contabilitate de la Lorand Expert garanteaza administrarea eficienta ?i corecta a proceselor financiare. Speciali?tii no?tri ofera suport in elaborarea documenta?iei contabile ?i raportarea fiscala, contribuind la dezvoltarea stabila a companiei dumneavoastra.
симптомы ломки симптомы ломки .
лучшие сериалы смотреть онлайн фильмы 2025 смотреть онлайн
Un contabil online de la Lorand Expert este solu?ia perfecta pentru gestionarea rapida ?i profesionala a contabilita?ii. Ne ocupam de toate detaliile contabile, astfel incat sa va concentra?i pe dezvoltarea afacerii.
Наши специалисты предлагает высококачественный вызвать мастера по ремонту ноутбука любых брендов и моделей. Мы понимаем, насколько необходимы вам ваши переносные компьютеры, и готовы предложить сервис высочайшего уровня. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что обеспечивает длительную работу выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются владельцы ноутбуков, включают проблемы с жестким диском, поврежденный экран, программные сбои, неисправности разъемов и неисправности системы охлаждения. Для устранения этих неисправностей наши квалифицированные специалисты проводят ремонт жестких дисков, экранов, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы получаете долговечный и надежный центр ремонта ноутбука.
Подробная информация представлена на нашем сайте: https://remont-noutbukov-first.ru
vavada bonuses for today https://game-vavada-casino.com
Our insulation services https://iepinsulation.com keep your home warm and energy-efficient year-round. We specialize in insulating facades, roofs, floors, and attics using modern materials and techniques. Trust our experienced team for durable, cost-effective solutions that improve comfort and reduce energy bills.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт посудомоечных машин miele, можете посмотреть на сайте: срочный ремонт посудомоечных машин miele
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин dexp адреса, можете посмотреть на сайте: ремонт стиральных машин dexp сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Alege?i servicii contabilitate Chisinau de la Lorand Expert pentru o gestionare precisa ?i eficienta a contabilita?ii. Colabora?i cu exper?i care pun pe primul loc succesul afacerii dumneavoastra.
Hi! Do you know if they make any plugins to assist with Search Engine Optimization? I’m trying to get my blog to rank for some targeted keywords but I’m not seeing very good success. If you know of any please share. Thank you!
https://mamaorganica.com.ua/linzy-dlya-far-kak-uluchshit-svet-i-stil-avtomobilya
элитный эскорт москва эскорт москва стоимость
осушитель для пневмосистемы осушитель для пневмосистемы .
снятие ломки на дому снятие ломки на дому .
Steam Desktop Authenticator https://steamdesktopauthenticator.me is a powerful tool designed to enhance the security of your Steam account. By generating time-based one-time passwords, it provides an additional layer of protection against unauthorized access. This desktop application allows users to manage their two-factor authentication easily, ensuring that only you can access your account.
снятие ломки на дому цена снятие ломки на дому цена .
Steam Desktop Authenticator https://steamauthenticator.ru это альтернатива мобильному аутентификатору Steam. Генерация кодов, подтверждение обменов и входов теперь возможны с компьютера. Программа проста в использовании, повышает удобство и позволяет защитить аккаунт, даже если у вас нет доступа к телефону.
симптомы ломки симптомы ломки .
Steam Desktop Authenticator https://steamauthenticatordesktop.com is an alternative to the mobile authenticator. Generating Steam Guard codes, confirming logins, trades and transactions is now possible directly from your computer. A convenient and secure solution for Steam users who want to simplify their account management.
Steam Desktop Authenticator https://steamdesktopauthenticator.io is a convenient tool for two-factor authentication of Steam via PC. The program generates Steam Guard codes, replacing the mobile authenticator. Easily confirm logins, trades and sales directly from your computer. Increase account security and manage it quickly and conveniently.
押忍!番長
マクロス スマスロ 設定判別
キャラクターの個性が際立っており、ストーリーに引き込まれます。
ヒデキに夢中!!
https://casinos.town/casinos/melbet-casino
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
デビル メイ クライ 4
ゴブリンスレイヤースロット 設定判別
CR 真・北斗無双
ミリオンゴッド-神々の凱旋-
CRモンスターハンター4 Ver.319
Pコードギアス 反逆のルルーシュ Rebellion to Re_surrection
https://xn--k8-9g4a3b4f.site/shinbun/post-6649.html
演出や音楽が迫力満点で、プレイするたびに興奮します。サウンドトラックも素晴らしい。
Lバキ 強くなりたくば喰らえ!!!
https://xn--k8-9g4a3b4f.site/tags/%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3-%E3%83%89%E3%83%A9%E3%83%A0-%E6%A9%9F
人気ゲームを基にしたパチスロで、ファンにはたまらない作品です。リアルなバトルが楽しめます。
鉄拳2nd
https://casinos.town/casino-games/pachinko/fist-of-the-north-star-5-champion
パチンコの美しいデザインの台が多く、視覚的にも楽しめます。アートとしての側面もあります。
Химчистка мебели от профессионалов
Городская химчистка предлагает устранение загрязнений на любом виде мебели. Уход за матрасами и мягкой мебелью проводится с использованием высококачественного оборудования и гипоаллергенных средств. Мы обеспечим качественное удаление мочи и сложных загрязнений .
Услуги химчистки для вашего дома и офиса обеспечивают удобство и индивидуальный подход. Глубокая химчистка матрасов выполняется с использованием гипоаллергенных составов. Городская химчистка заботится о вашем времени и бюджете .
Сервис gorodhim.ru помогает сохранить уют и гигиену в вашем доме. Обработка ковровых покрытий проходит с максимальной тщательностью. Доверьте свою мебель профессионалам из gorodhim.ru .
снятие ломки на дому цена снятие ломки на дому цена .
download vavada for android vavada casino
Steam Desktop Authenticator https://authenticatorsteam.com is the perfect tool for managing Steam security via PC. It replaces the mobile authenticator, allowing you to generate Steam Guard codes, confirm trades and logins. Ease of use and reliable protection make this program indispensable for every Steam user.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт посудомоечных машин beko в москве, можете посмотреть на сайте: ремонт посудомоечных машин beko цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт посудомоечных машин midea сервис, можете посмотреть на сайте: ремонт посудомоечных машин midea сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
ケロロ軍曹
k8カジノ 入金不要
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
ドラゴンが降りる
https://blog.k8.io/slots/bigbassholdspinner
美しいビジュアルと迫力ある演出が魅力。プレイ中に没入感が高まります。
戦姫絶唱シンフォギア
ベラジョンカジノ 安全性
CR牙狼FINAL Ver.399(1:1)
プレミアムビンゴ
モンスターハンター狂竜戦線
夜蝶飛翔特2発1
https://casinos.town/casino-news/xin-siiekisaiteingunaentateinmento-k8-kazino-dele-simu-199
大当たりの確率を理解することで、より戦略的にプレイできます。計算が好きな人にはおすすめです。
CRフィーバー宇宙戦艦ヤマト-ONLY ONE-
https://injapan.today/news/id1824.html
演出が華やかで、視覚的にも楽しめるのが魅力。アートとしての側面もあります。
ベヨネッタ
https://xn--k8-9g4a3b4f.site/tags/%E6%96%B0%E5%8F%B0-%E7%89%99-%E7%8B%BC-%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
настройки детекции движения https://www.cctvforum.ru
vms на русском программа для просмотра видео с камер наблюдения
осушитель воздуха для компрессора купить в москве http://www.sk-kompressor.ru/ .
снятие ломки наркологом снятие ломки наркологом .
симптомы ломки симптомы ломки .
Ваша удача ждет вас в онлайн казино, где ставки высоки.
Играйте и выигрывайте вместе с нами, и получите незабываемые впечатления.
Выберите свое любимое казино онлайн, и начните играть уже сегодня.
Ощутите волнение в режиме реального времени, не тратя время на поездки.
Выигрывайте крупные суммы при помощи наших игр, и почувствуйте себя настоящим чемпионом.
Насладитесь игровым процессом вместе с игроками со всех уголков планеты, и станьте лучшим из лучших.
Начните играть и получите ценные подарки, которые принесут вам еще больше радости и азарта.
Ощутите азарт в каждой игре казино онлайн, и готовьтесь к бесконечным выигрышам.
Получите доступ к уникальным играм и выигрывайте крупные суммы, сделав всего несколько кликов мыши.
казино онлайн беларусь [url=https://casino-on-line.by/]казино онлайн[/url] .
интим магазин официальный сайт sex-hub-kharkov.top
интим магазин официальный сайт sex-hub-kyiv.top
пансионат для престарелых в симферополе пансионат для престарелых в симферополе .
Florida’s lifestyle culture matches the state’s sunny energy.From Miami’s nightlife to Tampa’s intimate venues, the culture is as diverse as the state itself.Explore Find Florida’s best swingers clubs for exciting nights.Florida’s swinging culture keeps participants coming back.Whether you’re just exploring, Florida delivers excitement.From luxurious parties to private lounges, Florida’s culture thrives on inclusivity.
моментальный займ на карту срочно без отказа — это решение, которое работает. Наш список проверенных МФО обеспечивает прозрачные условия и мгновенные переводы, что важно для срочных ситуаций.
Планируете карьеру или хотите узнать больше о своем рынке труда? На нашем сайте https://zlojnachalnik.ru вы найдете подробную информацию о профессиях, их перспективах и уровнях зарплат. Получите ценную информацию, чтобы сделать осознанный выбор и достичь своих профессиональных целей. Узнайте всё о современных профессиях: от востребованности на рынке до уровня заработной платы.
Если вам отказали банки, новые МФО придут на помощь. надежные мфо без отказа доступен даже с плохой КИ. Минимальная ставка — 1% в день.
Продамус промокод Продамус промокод .
Более 55 надежных МФО готовы помочь вам прямо сейчас. микрозайм без отказа срочно с 100% одобрением — быстро, просто, круглосуточно.
снять ломку снять ломку .
С нами вы не останетесь без денег! 55 МФО предлагают займы на карту с плохой кредитной историей с круглосуточным одобрением. Быстро, надежно и без лишних хлопот.
Официальный сайт https://1winpromobk.ru, где вы найдете актуальные промокоды и бонусы для 1Win. Получите эксклюзивные предложения, такие как 500% на первый депозит и бесплатные спины. Присоединяйтесь прямо сейчас, чтобы воспользоваться всеми преимуществами и начать выигрывать!
Почему ждать, если можно оформить займы онлайн срочно прямо сейчас? Ставка всего 0.8% в день, одобрение гарантировано, и никаких сложностей с документами.
Why people still use to read news papers when in this technological globe everything is available on web?
порно видео чат онлайн
Steam Desktop Authenticator https://authenticatorsteam.com is a safe and easy way to protect your Steam account with two-factor authentication. Download and set up for maximum security.
主役は銭形
アクエリオンw 最終決戦
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
モンスターハンター狂竜戦線
https://www.k8.style/Articles/1160.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
CRモンスターハンター4
ベルセルク無双 機械割
Pフィーバー革命機ヴァルヴレイヴ 3
CRルパン三世~消されたルパン Ver.394
P 新世紀エヴァンゲリオン15 未来への咆哮
沖ドキ!トロピカル
https://blog.k8.io/topics/%E3%83%97%E3%83%AD%E3%83%A2%E3%83%BC%E3%82%B7%E3%83%A7%E3%83%B3
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CR RAVE この世界こそが真実だ
https://www.kopa-steroider.net/sitemap.xml
人気ゲームをベースにしたパチスロで、ファンにはたまらない作品です。リアルなバトルが楽しめます。
パチスロ 甲鉄城のカバネリ
https://blog.k8.io/baccarattournament
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
I want as many people as possible to read this post and think about these words !
—
Like 3966
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт посудомоечных машин hotpoint ariston, можете посмотреть на сайте: ремонт посудомоечных машин hotpoint ariston
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт посудомоечных машин aeg, можете посмотреть на сайте: ремонт посудомоечных машин aeg в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Возможности выигрыша в онлайн казино, где каждый может стать победителем.
Играйте и выигрывайте вместе с нами, и почувствуйте вкус победы.
Выберите свое любимое казино онлайн, и начните зарабатывать уже сегодня.
Ощутите волнение в режиме реального времени, не тратя время на поездки.
Выигрывайте крупные суммы при помощи наших игр, и покажите всем, кто здесь главный.
Играйте вместе с друзьями и соперниками со всего мира, и покажите свои лучшие результаты.
Получите бонусы и призы за активную игру, которые сделают вашу игру еще более увлекательной.
Играйте и наслаждайтесь азартом в каждой ставке, и погрузитесь в мир бесконечных перспектив.
Играйте в игры, недоступные где-либо еще, сделав всего несколько кликов мыши.
онлайн казино Казино .
слив курсов яндекс слив курс скачать торрент
Новые имена в мире финансов! Оформите займ на карту без отказов у малоизвестных МФО. Простое решение для тех, кто ценит комфорт.
снятие ломки наркомана снятие ломки наркомана .
снятие ломки наркологом снятие ломки наркологом .
снятие ломки нарколог снятие ломки нарколог .
дом инвалидов в симферополе дом инвалидов в симферополе .
Лучшее остекление балконов в СПб, подберем идеальное решение.
Остекление балконов и лоджий в СПб, под ключ и без переплат.
Изысканное остекление балконов в Петербурге, с учетом всех пожеланий клиента.
Надежное остекление балконов в СПб, с оригинальными комплектующими и возможностью долгосрочного сотрудничества.
Остекление балкона под ключ в СПб, по лучшей цене и быстрой установкой.
остекление балконов в спб https://balkon-spb-1.ru/ .
夜蝶飛翔
鏡スロットやめ時
サウンドエフェクトが迫力満点で、プレイ中に盛り上がります。特にバトル時が楽しい。
鉄拳3rd
https://xn--k8-9g4a3b4f.site/tags/%E7%88%86-%E3%82%B5%E3%82%A4-%E7%A6%8F%E5%B2%A1-%E3%82%B9%E3%83%AD%E3%83%83%E3%83%88
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
ベヨネッタ
ダンベル何キロ 先読み
SLOTバジリスク~甲賀忍法帖~Ⅲ
戦姫絶唱シンフォギア
闇の花(特殊大賞燈);
押忍!サラリーマン番長
https://www.k8pachinko.work/Articles/3006.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
紫の花 (特殊大賞燈)
https://xn--k8-9g4a3b4f.site/shinbun/post-5512.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
3x3EYES聖魔覚醒
https://injapan.today/topics/12th-anniversary-of-debut
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
Какое программное обеспечение https://www.cctvforum.ru для видеонаблюдения является лучшим? Какой сервис видеонаблюдения как услуги (VSaaS) наиболее простой и удобный в использовании?
Лучшие 10 программ https://www.cctvfocus.ru для видеонаблюдения. Программное обеспечение для видеонаблюдения. При выборе программного обеспечения важно учитывать наличие функции обнаружения объектов с использованием искусственного интеллекта.
конвертер юань в тенге калькулятор .
Сайт предлагает удобный инструмент для бесплатной конвертации валют. Актуальные курсы для тенге, долларов США, рублей и юаней позволяют мгновенно получить нужный результат.
I rarely come across such deep and correct thoughts in the modern information space !
–
Like 502
Лучшие натяжные потолки в СПб|Выгодное предложение на натяжные потолки в Петербурге|Лучшие специалисты по натяжным потолкам в Петербурге|Огромный ассортимент натяжных потолков в Санкт-Петербурге|Подбор натяжных потолков в Санкт-Петербурге: лучшие рекомендации|Уют и комфорт с натяжными потолками в СПб|Современный дизайн с натяжными потолками в Санкт-Петербурге|Красота и практичность с натяжными потолками в Санкт-Петербурге|Долговечные и стойкие натяжные потолки в Санкт-Петербурге|Технологичные решения для натяжных потолков в Санкт-Петербурге|Эффективное монтаж натяжных потолков в Петербурге|Совершенство с натяжными потолками в Санкт-Петербурге|Модные тренды в мире натяжных потолков: СПб|Лучшие цены на натяжные потолки в СПб|Натяжные потолки в СПб: выбор современных людей|Профессиональные консультации по выбору натяжных потолков в СПб|Стильные потолки в Петербурге: натяжные|Точное соответствие вашим потребностям: натяжные потолки в Петербурге|Индивидуальный подход к каждому клиенту: натяжные потолки в СПб|Плюсы натяжных потолков в Петербурге|Натяжные потолки в СПб: современные технологии и материалы|Премиальный сервис по установке натяжных потолков в СПб|Тенденции в дизайне потолков: натяжные потолки в СПб|Оптимальный выбор: натяжные потолки в Петербурге
натяжные потолки быстро https://potolki-spb-1.ru/ .
It is difficult to disagree with the author when he so accurately describes what is happening around 😉
———-
Like 9946
A very important post that makes you think about many serious things in life !
———-
Like 3516
Идеальное остекление для балконов в Санкт-Петербурге, поможем выбрать подходящий вариант.
Остекление балконов и лоджий в СПб, с установкой и долговечной эксплуатацией.
Эксклюзивное остекление для балконов в Санкт-Петербурге, по индивидуальным проектам и с использованием прочных материалов.
Быстрое остекление для балконов в Санкт-Петербурге, с оригинальными комплектующими и возможностью долгосрочного сотрудничества.
Остекление балкона под ключ в СПб, по лучшей цене и быстрой установкой.
застеклить балкон в хрущевке цены https://balkon-spb-1.ru/ .
Every time I read such posts, I understand how accurately they reflect our reality ::
———-
Like 2676
myspace.com https://wiki.dulovic.tech/index.php/The_Role_Of_Porn_In_Enhancing_Sexual_Creativity .
myspace.com http://[email protected]/?a%5B%5D=%3Ca+href%3Dhttps%3A%2F%2Fdev-westudy.accedo.gr%2Fmembers%2Foakswamp55%2Factivity%2F1743684%2F%3Edesi+aunty+sex+video+xxxbp.tv%3C%2Fa%3E%3Cmeta+http-equiv%3Drefresh+content%3D0%3Burl%3Dhttps%3A%2F%2Fbuckley-skou.hubstack.net%2Fpornography-and-the-breaking-down-of-gender-roles-and-sexual-stereotypes-1734003345+%2F%3E/ .
myspace.com http://www.bookmarkingworld.review/story.php?title=xxxbp-tvxbhabhi-4#discuss .
1 доллар в тенге 1 юань в тенге .
Сайт предлагает быстрый и удобный способ конвертации валют в режиме реального времени. Актуальные курсы для тенге, рублей, юаней, долларов США и других валют всегда под рукой. Простота дизайна позволяет мгновенно получить результат.
Download online Taya365 app and discover a new level of mobile gaming. Slots, live casino, exclusive bonuses – all this is available on your smartphone. Enjoy the game anytime, anywhere!
http://gramster.ru/# pinup 2025
The robust loyalty program at Taya365 rewards Filipino players with points for wagers, which can be exchanged for exclusive bonuses and perks, ensuring every bet counts.
taya 365 taya 365 .
you’re really a excellent webmaster. The website loading pace is incredible. It seems that you are doing any unique trick. Furthermore, The contents are masterpiece. you have performed a magnificent process on this subject!
http://gollos.com.ua/angel-eyes-bilead.html
смотреть здесь https://forum.hpc.name/thread/11507/php-rabota-s-icq.html
снятие ломки цены снятие ломки цены .
crypto drainer – what is crypto drainer, best crypto drainer
https://gramster.ru/# пин ап вход
снять ломку снять ломку .
https://gramster.ru/# пин ап казино
Ищете идеальный бюстгальтер Бюстгальтер женский с пушапом? Наши женские бюстгальтеры без косточек предлагают комфорт и стиль. Выберите кружевной бюстгальтер для особых случаев или спортивный бюстгальтер для активного образа жизни. Для будущих мам у нас есть бюстгальтеры для беременных и бюстгальтеры для кормления с удобными застежками. Также доступны бюстгальтеры больших размеров и бюстгальтеры с пуш-ап для создания эффектного декольте. Купите лифчик для кормления или бюстгальтер с открытой спиной и наслаждайтесь максимальным комфортом!
gramster.ru gramster.ru pinup 2025
Качественные натяжные потолки в Санкт-Петербурге|Скидки на натяжные потолки в СПб|Опытные мастера по натяжным потолкам в Санкт-Петербурге|Широкий выбор натяжных потолков в СПб|Советы по выбору натяжных потолков в Петербурге|Уют и комфорт с натяжными потолками в СПб|Эстетика и стиль с натяжными потолками в СПб|Красота и практичность с натяжными потолками в Санкт-Петербурге|Долговечные и стойкие натяжные потолки в Санкт-Петербурге|Технологичные решения для натяжных потолков в Санкт-Петербурге|Быстрое и качественное установление натяжных потолков в Санкт-Петербурге|Оптимальное решение – натяжные потолки в Петербурге|Модные тренды в мире натяжных потолков: СПб|Специальные предложения на натяжные потолки в Петербурге|Натяжные потолки в СПб: выбор современных людей|Уникальные решения в области натяжных потолков в Санкт-Петербурге|Стильные потолки в Петербурге: натяжные|Натяжные потолки в СПб: надежность и качество|Персональные решения для вас: натяжные потолки в Петербурге|Преимущества натяжных потолков в СПб|Инновационные материалы для натяжных потолков в Петербурге|Эксклюзивные услуги по монтажу натяжных потолков в Петербурге|Тенденции в дизайне потолков: натяжные потолки в СПб|Идеальное сочетание цены и качества: натяжные потолки в СПб
сколько стоит натяжной потолок под ключ https://potolki-spb-1.ru/ .
https://gramster.ru/# пин ап
Идеальное решение для скрытой связи — купить микронаушники. Широкий выбор моделей, гарантия качества и выгодные условия покупки. Надёжная связь, компактный размер и удобство использования.
пин ап казино: gramster.ru – пин ап казино официальный сайт
https://gramster.ru/# пин ап вход
http://gramster.ru/# пин ап казино официальный сайт
http://gramster.ru/# пин ап казино официальный сайт
http://gramster.ru/# пин ап зеркало
CR 聖戦士ダンバイン
スマスロ 源さん 天井
演出が華やかで、視覚的にも楽しめます。アートとしての側面もあります。
スーパービンゴネオ
https://gamein.jp/slots/barn-festival
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
サイボーグ 009
戦コレ5 終了画面セリフ
鬼武者3 時空天翔
ミリオンゴッド神々の凱旋
GI優駿俱樂部
主役は銭形 2004
https://casinos.town/bonus/casino-x-egg-hunt-tournament
大当たり時の興奮は、他のゲームでは味わえない特別なものです。心が躍ります。
P ぱちんこ シン・エヴァンゲリオン Type レイ
https://injapan.today/realtime/id2199.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
喧嘩祭
https://www.k8.motorcycles/Articles/1170.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
myspace.com https://chanceqhxod.dailyhitblog.com/37124072/the-positive-impact-of-pornography-on-sexual-relationships-and-partnerships .
myspace.com http://[email protected]/php.php?a%5B%5D=%3Ca+href%3Dhttps%3A%2F%2Fdocvino.com%2Fmembers%2Fknotopen14%2Factivity%2F752204%2F%3Eromantic+xxx+xxxbp.tv%3C%2Fa%3E%3Cmeta+http-equiv%3Drefresh+content%3D0%3Burl%3Dhttps%3A%2F%2Ftyler-lutz-2.federatedjournals.com%2Fpornography-as-a-tool-for-overcoming-sexual-dysfunction-and-dissatisfaction-1733997653+%2F%3E .
pinup 2025 Gramster пин ап зеркало
заказать шары на день рождения https://shariki-shop47.ru
https://gramster.ru/# пин ап вход
https://gramster.ru/# pinup 2025
http://gramster.ru/# пин ап казино зеркало
пин ап казино зеркало pinup 2025 or пин ап казино официальный сайт
https://www.google.ge/url?q=https://gramster.ru пин ап казино официальный сайт
pinup 2025 pinup 2025 and gramster.ru пин ап зеркало
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
https://gramster.ru/# pinup 2025
пинап казино: Gramster – gramster.ru
http://gramster.ru/# gramster.ru
myspace.com http://wakeuplaughing.com/phpinfo.php?a%5B%5D=%3Ca+href%3Dhttps%3A%2F%2Ffriendly-gull-n2t4f3.mystrikingly.com%2Fblog%2Fdo-sex-videos-help-alleviate-depression%3Ehindi+bf+-+xxxbp.tv%3C%2Fa%3E%3Cmeta+http-equiv%3Drefresh+content%3D0%3Burl%3Dhttps%3A%2F%2Fbridges-rios-3.blogbright.net%2Fsecurity-and-expression-in-watching-sex-videos+%2F%3E .
http://gramster.ru/# пин ап казино
http://gramster.ru/# pinup 2025
пин ап вход gramster.ru пин ап зеркало
http://gramster.ru/# пин ап казино официальный сайт
The robust loyalty program at Taya365 rewards Filipino players with points for wagers, which can be exchanged for exclusive bonuses and perks, ensuring every bet counts.
taya365 app login taya365 app login .
Steam Desktop Authenticator https://authenticatorsteam.com is a convenient application for managing two-factor authentication in Steam. Allows you to quickly confirm trades, logins and purchases, providing additional protection for your account.
снятие ломки наркозависимого снятие ломки наркозависимого .
http://gramster.ru/# pinup 2025
go to these guys Provider SMM Panel
http://gramster.ru/# gramster.ru
пин ап зеркало: gramster – gramster.ru
промокод изи дроп 50 промокод изи дроп 50 .
промокоды изи дроп 50 промокоды изи дроп 50 .
секретный код easydrop секретный код easydrop .
look here Immediate Affinity
пин ап вход: gramster.ru – gramster.ru
пин ап казино пин ап казино зеркало or pinup 2025
http://www.extrasmallworld.de/forward.php?to=https://gramster.ru gramster.ru
gramster.ru пин ап казино зеркало and пинап казино gramster.ru
актуальный ссылки на зеленый мир – зеленый мир сайт ссылка, зеленый мир онион
нова ссылка рабочая – Nova маркетплейс нового поколения, нова магазин
пин ап казино официальный сайт pinup 2025 or pinup 2025
https://images.google.it/url?sa=i&url=https://gramster.ru пин ап казино
pinup 2025 pinup 2025 and пин ап казино официальный сайт пинап казино
jaxx liberty – jaxx liberty wallet, jaxx liberty wallet
https://gramster.ru/# пин ап
important source https://jaxx-liberty.com
https://gramster.ru/# пин ап казино
Durmitor restoran beograd Durmitor national park Montenegro
accommodation Kolasin
пин ап казино gramster пин ап зеркало
https://gramster.ru/# пин ап вход
https://gramster.ru/# пин ап казино
Institutional investment in crypto
In recent years, we’ve witnessed a remarkable surge in the participation of major financial entities in the realm of digital assets.
This trend marks a significant shift in how traditional markets perceive and
engage with emerging technologies. As the landscape evolves, more
established firms are exploring the potential of these innovative assets.
The fusion of conventional finance and digital currencies is reshaping the investment narrative.
Adoption has accelerated, fueled by growing mainstream acceptance.
A diverse range of organizations is now navigating
uncharted waters. They seek to capitalize on the potential
advantages that these digital instruments can offer.
This evolving dynamic brings both excitement and apprehension.
Many believe that this involvement could bridge the gap between traditional economics
and the digital frontier. While some remain skeptical, others are
convinced that the future lies in this new paradigm.
Enhanced regulatory frameworks and clearer guidelines are often cited as catalysts for this
transformation.
As we delve deeper into this topic, it’s crucial to understand the motivations behind the shift.
Players from various industries are not merely dabbling; they are strategically aligning themselves with the future of finance.
The implications of their actions could reach far beyond mere profits, potentially influencing market stability and innovation.
Here is my web blog; https://cryptolake.online/btc/
промокоды изи дроп промокоды изи дроп .
gramster.ru gramster пин ап казино официальный сайт
https://gramster.ru/# пин ап зеркало
Thanks for stopping by! Don’t forget to explore https://metakit.org/
pinup 2025: gramster – пин ап зеркало
pinup 2025: gramster.ru – пин ап
пинап казино пин ап or пин ап вход
https://87.torayche.com/index/d1?diff=0&utm_clickid=00gocgogswows8g4&aurl=https://gramster.ru pinup 2025
пин ап казино официальный сайт пин ап вход and пинап казино пин ап зеркало
пин ап казино пин ап вход or пин ап
http://www.victoirefrance.fr/expire.aspx?Returnurl=https://gramster.ru/ gramster.ru
пин ап зеркало пин ап казино and пин ап вход пин ап казино официальный сайт
https://gramster.ru/# пинап казино
Официальный сайт https://1winpromobk.ru, где вы найдете актуальные промокоды и бонусы для 1Win. Получите эксклюзивные предложения, такие как 500% на первый депозит и бесплатные спины.
Какое программное обеспечение для видеонаблюдения https://www.cctvforum.ru является лучшим? Какой сервис видеонаблюдения как услуги (VSaaS) наиболее простой и удобный в использовании?
https://gramster.ru/# пин ап казино официальный сайт
вывод из запоя недорого ростов вывод из запоя недорого ростов .
http://gramster.ru/# пинап казино
http://gramster.ru/# pinup 2025
вывод. из. запоя. ростов. вывод. из. запоя. ростов. .
анонимный. вывод. из. запоя. ростов. http://www.vyvod-iz-zapoya-rostov26.ru/ .
вывод из запоя вывод из запоя .
пин ап казино официальный сайт Gramster пин ап казино официальный сайт
Steam Desktop Authenticator steamauthenticator.ru is a convenient application for managing Steam two-factor authentication directly on your PC. It allows you to confirm logins, trades, and purchases without the need for a mobile device.
Steam Desktop Authenticator steamdesktopauthenticator.me is a PC application that makes it easier to use two-factor authentication on Steam. By replacing the mobile authenticator, it allows you to confirm trades, purchases, and account logins, providing a high level of security and ease of use.
Steam Desktop Authenticator steamdesktopauthenticator.io is a simple and reliable way to protect your Steam account. The program allows you to confirm transactions, receive security codes, and manage two-factor authentication settings without relying on your smartphone.
пинап казино пин ап казино зеркало or пинап казино
http://saxonsystems.com.au/web/website.nsf/WebTell?Go=http://gramster.ru pinup 2025
пинап казино пин ап казино официальный сайт and pinup 2025 пин ап казино официальный сайт
осушитель воздуха для компрессора купить осушитель воздуха для компрессора купить .
pinup 2025: gramster.ru – gramster.ru
Лучшие коляски 3 в 1 для вашего малыша, на которые стоит обратить внимание.
Популярные варианты колясок 3 в 1, с широким функционалом и стильным дизайном.
Гид по выбору коляски 3 в 1, чтобы не прогадать с покупкой.
Как не ошибиться с выбором коляски 3 в 1, для максимального удобства вашего малыша.
Какую коляску 3 в 1 выбрать: опыт других родителей, и сделать правильное решение.
прогулочные коляски прогулочные коляски .
пинап казино пин ап or пин ап зеркало
https://cse.google.gp/url?sa=t&url=https://gramster.ru gramster.ru
gramster.ru пинап казино and пинап казино pinup 2025
https://gramster.ru/# пин ап вход
pinup 2025: Gramster – пин ап казино официальный сайт
Откройте для себя лучшие снять отель в Москве Вас ждут стильные номера, изысканная кухня, современные удобства и внимание к деталям. Отели расположены в ключевых районах города, что делает их идеальными для деловых поездок, романтических выходных или туристических открытий.
пинап казино gramster.ru пинап казино
Steam Desktop Authenticator authenticatorsteam.com is an alternative to the Steam Mobile Authenticator. It provides codes for two-factor authentication directly on your PC.
Steam Desktop Authenticator steamauthenticatordesktop.com is a handy app to enhance the security of your Steam account. It generates codes for two-factor authentication, allowing you to easily confirm transactions and logins.
http://gramster.ru/# пин ап
http://gramster.ru/# пинап казино
пин ап вход gramster.ru or пин ап зеркало
https://clients1.google.com.ua/url?q=http://gramster.ru pinup 2025
gramster.ru пин ап казино официальный сайт and пин ап зеркало пин ап вход
алкоголизм лечение вывод из запоя ростов алкоголизм лечение вывод из запоя ростов .
пин ап казино зеркало pinup 2025 or pinup 2025
https://cse.google.bt/url?sa=i&url=https://gramster.ru пинап казино
пин ап казино gramster.ru and пин ап зеркало пин ап
Официальная страница диана шурыгина телеграмм. Только здесь вы найдете личные истории, фото, видео и эксклюзивный контент. Узнавайте первыми о новых проектах и наслаждайтесь моментами её жизни. Подписывайтесь, чтобы быть всегда на связи!
лучшие курсы ораторского искусства курсы речи
заказать шары с доставкой на дом дешевые воздушные шары
http://gramster.ru/# pinup 2025
вывод из запоя круглосуточно ростов http://vyvod-iz-zapoya-rostov26.ru .
http://gramster.ru/# пин ап казино зеркало
Эксклюзивные букеты роз уже ждут вас, насладитесь красотой и ароматом.
Индивидуальные букеты роз по вашему желанию, профессиональный подход к каждому клиенту.
Уникальные букеты роз от лучших флористов, оперативная доставка в любую точку.
Роскошные букеты роз для особых случаев, доставка в день заказа.
Изысканные композиции из роз, почувствуйте аромат настоящей любви.
Насладитесь красотой и свежестью цветов, профессиональные флористы соберут для вас лучший букет.
Оригинальные идеи для букетов роз, превратите обыденный день в праздник.
Выберите свой идеальный букет роз, удобные способы оплаты.
Букеты роз под ключ с доставкой, поможем выбрать идеальный вариант.
Роскошные букеты роз для особых моментов, приятные цены и гарантированное качество.
Выберите свой звёздный букет роз, и мы доставим его к вашему порогу.
Прекрасные букеты роз для ваших близких, насладитесь ароматом настоящей любви.
розы цена розы цена .
https://gramster.ru/# пин ап казино
вывод из запоя на дому ростов-на-дону https://www.vyvod-iz-zapoya-rostov27.ru .
Steam Desktop Authenticator https://steamdesktopauthenticator.io is a two-factor authentication application for PC. Generates codes to confirm transactions and log in to Steam, improving the security of your account. Convenient, quick to install and easy to use solution for data protection.
Онлайн слив курсов https://sliv-kursov213.ru простой способ получить знания из популярных онлайн-курсов. Развивайтесь в своем темпе, выбирайте интересующие темы и экономьте на образовании. Здесь вы найдете материалы для саморазвития, карьерного роста и хобби.
пин ап казино официальный сайт: gramster.ru – pinup 2025
Steam Desktop Authenticator https://authenticatorsteam.com is a two-factor authentication application for PC. Generates codes to confirm transactions and log in to Steam, improving the security of your account. Convenient, quick to install and easy to use solution for data protection.
пин ап казино официальный сайт: Gramster – pinup 2025
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
https://gramster.ru/# pinup 2025
Thank you for sharing such an insightful article! More on the topic: https://metaium.org/
пин ап казино зеркало pinup 2025 or пин ап казино официальный сайт
http://admin.kpsearch.com/active/admin/customer/customer_email1_birthday.asp?item=&chname=gnc&strhomeurl=gramster.ru пин ап зеркало
пин ап казино зеркало gramster.ru and пин ап вход пин ап вход
https://gramster.ru/# пин ап вход
пин ап казино зеркало gramster pinup 2025
夜蝶飛翔
ヴィレッジ スマスロ セリフ
パチンコの演出が華やかで、視覚的にも楽しめます。美しいデザインが魅力的です。
e ルパン三世 銭形からの招待状
https://www.sjb.jp/topics/%E5%8C%97%E6%96%97%E3%81%AE%E6%8B%B3
パチンコは、運を試す素晴らしい機会です。ドキドキ感がたまりません。
シティーハンター
ヴィレッジ 天井
Pフィーバー炎炎ノ消防隊G
パチスロ龍が如く OF THE END
押忍!番長2
沖ドキ!トロピカル
https://xn--k8-9g4a3b4f.site/yosou/post-230.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
Pアナザーゴッドハーデス ジャッジメント
https://www.k8.gives/Articles/1404.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
戦国パチスロ花の慶次戦極めし傾奇者の宴;
https://www.k8.motorcycles/Articles/2341.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
пин ап казино пинап казино or пин ап вход
https://cse.google.tl/url?sa=t&url=https://gramster.ru пин ап казино официальный сайт
пин ап казино зеркало gramster.ru and пинап казино pinup 2025
пин ап казино официальный сайт пин ап казино зеркало or пин ап казино зеркало
http://www.robertlerner.com/cgi-bin/links/ybf.cgi?url==http://gramster.ru/ пинап казино
pinup 2025 пинап казино and пин ап казино зеркало пин ап казино зеркало
вывод из запоя цены ростов-на-дону вывод из запоя цены ростов-на-дону .
снегоход irbis dingo t125 снегоход irbis dingo t125 .
пин ап казино официальный сайт пин ап зеркало or pinup 2025
https://maps.google.com.sb/url?q=https://gramster.ru пин ап
pinup 2025 пин ап вход and пин ап казино зеркало gramster.ru
http://gramster.ru/# gramster.ru
вывод из запоя цены на дому ростов вывод из запоя цены на дому ростов .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт ноутбуков sony, можете посмотреть на сайте: ремонт ноутбуков sony сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
пин ап: gramster – пин ап казино
https://gramster.ru/# пин ап казино официальный сайт
пин ап зеркало pinup 2025 or пин ап
https://images.google.com/url?sa=t&url=https://gramster.ru pinup 2025
пин ап казино пин ап казино зеркало and пин ап казино пин ап
пин ап казино зеркало: Gramster – пин ап
Возможности выигрыша в онлайн казино, где каждый может стать победителем.
Получайте азарт и адреналин вместе с нами, и почувствуйте вкус победы.
Обнаружьте свое новое казино онлайн, и начните выигрывать уже сегодня.
Играйте и побеждайте в режиме живого казино, не тратя время на поездки.
Ставьте на победу с нашими играми, и станьте настоящим мастером азарта.
Играйте вместе с друзьями и соперниками со всего мира, и докажите свое превосходство.
Играйте и выигрывайте, получая щедрые бонусы, которые принесут вам еще больше радости и азарта.
Почувствуйте свободу и возможность выбора в каждой игре, и готовьтесь к бесконечным выигрышам.
Станьте частью казино онлайн и получите доступ к эксклюзивным играм, сделав всего несколько кликов мыши.
онлайн казино Казино .
пин ап казино официальный сайт gramster.ru or пинап казино
https://cse.google.com.br/url?sa=t&url=https://gramster.ru пин ап вход
пинап казино пин ап зеркало and пин ап казино пинап казино
отели нижнего новгорода в центре города отели нижнего новгорода в центре города .
http://gramster.ru/# пин ап казино зеркало
http://gramster.ru/# пин ап казино зеркало
As a leader in responsible gaming, Taya365 offers tools like deposit and loss limits, session controls, and self-exclusion options to promote a healthy and balanced gaming experience.
taya365 casino taya365 register .
https://gramster.ru/# пин ап казино официальный сайт
ирбис 300 кубов снегоход ирбис 300 кубов снегоход .
нарколог на дом вывод из запоя на дому нарколог на дом вывод из запоя на дому .
pinup 2025 Gramster пин ап казино зеркало
аттестат купить за 11 класс
http://gramster.ru/# пин ап вход
пин ап казино официальный сайт пин ап or pinup 2025
http://maps.google.gp/url?q=https://gramster.ru пин ап казино зеркало
[url=http://images.google.hn/url?q=http://gramster.ru]gramster.ru[/url] пин ап казино официальный сайт and [url=https://forex-bitcoin.com/members/401434-dbftwuvvri]пин ап зеркало[/url] pinup 2025
pinup 2025 пин ап казино or пинап казино
https://secure.spicecash.com/hosted_sssh_galleries/3/imagepages/image9.htm?link=https://gramster.ru пин ап вход
pinup 2025 pinup 2025 and pinup 2025 пин ап
пин ап казино зеркало: gramster – пин ап казино официальный сайт
В современную цифровую эпоху все больше владельцев техники встречаются с непростым выбором: что делать со устаревшим домашним кинотеатром при покупке нового телевизора? Многие думают, что придется покупать новую акустическую систему. Однако есть несколько работающих способов объединения старой, но качественной техники с новыми устройствами. Такое решение не только сохранит значительную сумму, но и позволит продолжить использование проверенным временем оборудованием. В случае, если вам интересно, то по ссылке можно прочитать о том как подключить домашний кинотеатр к телевизору сони бравиа и другие интересные материалов в журнале 1wyws.top.
В статье подробно описываются различные варианты подключения старых домашних кинотеатров к современным телевизорам – от использования HDMI-интерфейса с поддержкой технологии ARC до подключения через оптический выход или традиционные аналоговые разъемы. Каждый метод дополняется детальными инструкциями и практическими рекомендациями. Особое внимание отводится совместимости различных типов соединений и возможным техническим ограничениям. Пользователи найдут полезную таблицу совместимости разъемов, которая поможет быстро определить оптимальный способ подключения.
Статья также содержит подробное описание типичных сложностей, возникающих при подключении устройств разных поколений, и эффективные способы их решения. Авторы делятся практическими советами по настройке звука и оптимизации работы системы. Пошаговые инструкции помогут даже неопытным пользователям успешно сделать подключение самостоятельно. Особенно полезным является раздел с описанием возможных сложностей и методов их устранения, что поможет не допустить многих распространенных ошибок при настройке оборудования.
Источник: https://1wyws.top/tehnologii/330-kak-podklyuchit-staryj-domashnij-kinoteatr-k-novomu-televizoru/
Охотно предоставлю свою помощь, если это необходимо по вопросам как подключить домашний кинотеатр philips к телевизору – пишите в Telegram uow85
http://gramster.ru/# gramster.ru
http://gramster.ru/# пин ап казино
отели нижнего новгорода отели нижнего новгорода .
пинап казино пин ап or пин ап вход
https://images.google.bf/url?sa=t&url=http://gramster.ru pinup 2025
pinup 2025 пин ап казино and pinup 2025 пин ап казино
http://gramster.ru/# пин ап казино официальный сайт
pinup 2025 пин ап казино официальный сайт or пинап казино
http://www.junix.ch/linkz.php?redir=https://gramster.ru пин ап казино
пин ап казино официальный сайт пин ап казино официальный сайт and gramster.ru пин ап
пин ап: gramster.ru – пин ап зеркало
pinup 2025: gramster.ru – gramster.ru
http://gramster.ru/# пин ап казино официальный сайт
http://gramster.ru/# пин ап
gramster.ru pinup 2025 or пин ап
https://maps.google.cl/url?sa=t&url=https://gramster.ru пин ап казино зеркало
пин ап пин ап and пин ап вход пинап казино
пин ап казино официальный сайт gramster.ru пин ап зеркало
пин ап пин ап вход or pinup 2025
https://images.google.com/url?q=https://gramster.ru gramster.ru
пин ап вход пин ап казино зеркало and пин ап казино зеркало пин ап зеркало
https://gramster.ru/# пин ап зеркало
L 大工の源さん 超夢源
エリートサラリーマン鏡引き戻し
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR大海物語4 Ver.319
https://xn--k8-9g4a3b4f.site/shinbun/post-3825.html
シンプルなルールで、誰でもすぐに楽しめるのが嬉しいです。
新潘金蓮
パチンコ 時速8万発
青龍(4発1)
花の慶次天に愛されし漢~
Pフィーバーかぐや様は告らせたい
SUハイビスカス
https://injapan.today/topics/akinori-nakagawa
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
e Re:ゼロから始める異世界生活 season2M13
https://37k8.com/Articles/1044.html
キャラクターの個性が際立っており、ストーリーに引き込まれます。
デジハネ CR 蒼天の拳 STV
https://www.a-certain-pachinko.com/article/72.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
пин ап казино пинап казино or пин ап зеркало
https://forum.83metoo.de/link.php?url=gramster.ru пинап казино
pinup 2025 пинап казино and пин ап зеркало pinup 2025
pinup 2025 пин ап казино or пин ап казино официальный сайт
http://alt1.toolbarqueries.google.bj/url?q=https://gramster.ru пин ап зеркало
пин ап казино официальный сайт пин ап вход and пин ап казино официальный сайт пин ап казино официальный сайт
платный нарколог на дом платный нарколог на дом .
http://gramster.ru/# pinup 2025
вызов нарколога на дом краснодар вызов нарколога на дом краснодар .
лучшие фильмы онлайн детективы лучшие фильмы 2016 смотреть онлайн
фильмы онлайн HD в HD новинки кино онлайн в Европе
смотреть фильмы онлайн драмы лучшие фильмы 2004 смотреть онлайн
нарколог на дом краснодар нарколог на дом краснодар .
http://gramster.ru/# пин ап зеркало
вызов нарколога на дом вызов нарколога на дом .
платный нарколог на дом платный нарколог на дом .
https://gramster.ru/# пин ап казино зеркало
фильмы 2006 смотреть онлайн фильмы 2024 смотреть онлайн боевики
Download music https://progworld.net in high quality without sound loss. Convenient search, wide choice of genres and artists, fast downloads.
прогулка на яхте по дубай марине дубай марина прогулка на катере
пин ап казино зеркало: Gramster – пинап казино
https://gramster.ru/# пин ап вход
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков msi рядом, можете посмотреть на сайте: срочный ремонт ноутбуков msi
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://gramster.ru/# пин ап казино официальный сайт
pinup 2025 Gramster пин ап казино официальный сайт
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков lenovo в москве, можете посмотреть на сайте: ремонт ноутбуков lenovo рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://gramster.ru/# пин ап зеркало
Наша мастерская предлагает высококачественный ремонт imac на выезде различных марок и моделей. Мы знаем, насколько важны для вас ваши iMac, и обеспечиваем ремонт наилучшего качества. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что предоставляет долговечность и надежность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются владельцы iMac, включают проблемы с жестким диском, повреждения экрана, неработающие разъемы, ошибки ПО и перегрев. Для устранения этих неисправностей наши опытные мастера проводят ремонт жестких дисков, дисплеев, разъемов, ПО и систем охлаждения. Обращаясь в наш сервисный центр, вы гарантируете себе надежный и долговечный мастерская по ремонту аймака на выезде.
Подробная информация представлена на нашем сайте: https://remont-imac-mos.ru
https://gramster.ru/# пин ап
Наша мастерская предлагает профессиональный официальный ремонт аймака в москве различных марок и моделей. Мы знаем, насколько значимы для вас ваши компьютеры Apple, и готовы предложить сервис наилучшего качества. Наши квалифицированные специалисты работают быстро и аккуратно, используя только оригинальные запчасти, что обеспечивает надежность и долговечность наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются обладатели компьютеров Apple, включают поломку жесткого диска, неисправности дисплея, проблемы с портами, неисправности программного обеспечения и перегрев. Для устранения этих проблем наши квалифицированные специалисты выполняют ремонт жестких дисков, дисплеев, разъемов, ПО и систем охлаждения. Обратившись к нам, вы получаете надежный и долговечный сервис ремонта аймака на дому.
Подробная информация доступна на сайте: https://remont-imac-mos.ru
http://gramster.ru/# пин ап казино зеркало
пин ап казино официальный сайт gramster.ru or пин ап
https://maps.google.com.jm/url?q=https://gramster.ru pinup 2025
пин ап зеркало пин ап казино and pinup 2025 пин ап казино
pinup 2025 gramster пин ап казино официальный сайт
вызов нарколога на дом краснодар вызов нарколога на дом краснодар .
https://gramster.ru/# пинап казино
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков msi цены, можете посмотреть на сайте: ремонт ноутбуков msi сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Мостбет офіційний сайт гарантує безпечність та зручність використання | Мостбет – це провідний букмекер в Україні мостбет ком. | Мостбет casino пропонує найкращі ігри від провідних розробників | Мостбет казино дарує новим гравцям щедрі бонуси http://www.mostbetcasinouahfgy.com.
http://gramster.ru/# пин ап вход
三國志
パチスロ新台カレンダー
パチンコの魅力は、初心者でも楽しめるところにあります。ルールが簡単で、すぐに遊べます。
十字架3
https://xn--k8-9g4a3b4f.site/tags/%E5%A4%A7%E9%98%AA-%E3%82%B9%E3%83%AD%E3%83%83%E3%83%88-%E3%83%84%E3%82%A4%E3%83%83%E3%82%BF%E3%83%BC
シンプルなルールで、初心者でもすぐに楽しめます。気軽に挑戦できます。
戦姫絶唱シンフォギア
ばくさい石川パチンコ
ドラゴンが降りる
北斗の拳-ボクシング王
CR海物語4
CR牙狼闇を照らす者
https://xn--k8-9g4a3b4f.site/shinbun/post-6779.html
定期的に新しい台が出るので、飽きずに楽しめます。ファンにはたまらないですね。
鬼武者3
https://xn--k8-9g4a3b4f.site/tags/%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3-%E3%81%82%E3%82%8A%E3%81%88%E3%81%AA%E3%81%84
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
魔法少女まどか☆マギカ
https://www.k8.monster/Articles/1123.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
pinup 2025 pinup 2025 or пин ап казино
https://maps.google.com.sb/url?sa=t&url=https://gramster.ru pinup 2025
пин ап казино официальный сайт пин ап зеркало and пин ап казино зеркало pinup 2025
нарколог на дом нарколог на дом .
нарколог краснодар нарколог краснодар .
яхта в дубае аренда аренда катера дубай марина
пин ап: Gramster – пин ап казино
https://gramster.ru/# пин ап
пинап казино: gramster.ru – пинап казино
https://gramster.ru/# pinup 2025
Наша мастерская предлагает профессиональный мастер по ремонту аймака адреса всех типов и брендов. Мы знаем, насколько значимы для вас ваши моноблоки iMac, и готовы предложить сервис первоклассного уровня. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только качественные детали, что обеспечивает длительную работу наших услуг.
Наиболее частые неисправности, с которыми сталкиваются владельцы iMac, включают проблемы с жестким диском, повреждения экрана, неисправности разъемов, ошибки ПО и неисправности системы охлаждения. Для устранения этих поломок наши профессиональные техники выполняют ремонт жестких дисков, дисплеев, разъемов, ПО и систем охлаждения. Обращаясь в наш сервисный центр, вы обеспечиваете себе качественный и надежный мастерская по ремонту imac на дому.
Подробная информация размещена на сайте: https://remont-imac-mos.ru
пин ап казино зеркало пин ап вход or пин ап вход
https://sso.iiaba.net/login.aspx?a=wa&r=http://gramster.ru pinup 2025
gramster.ru pinup 2025 and пинап казино пин ап казино
https://gramster.ru/# gramster.ru
pinup 2025 пин ап зеркало or пин ап вход
https://www.google.com.kw/url?q=https://gramster.ru пин ап
пин ап казино зеркало пинап казино and gramster.ru pinup 2025
kantorbola
http://canadianpharmacy.win/# canadadrugpharmacy com
Наш сервисный центр предлагает высококачественный официальный ремонт imac любых брендов и моделей. Мы понимаем, насколько необходимы вам ваши компьютеры Apple, и стремимся предоставить услуги первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только сертифицированные компоненты, что обеспечивает долговечность и надежность наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются владельцы iMac, включают поломку жесткого диска, проблемы с экраном, проблемы с портами, ошибки ПО и неисправности системы охлаждения. Для устранения этих проблем наши опытные мастера выполняют ремонт жестких дисков, дисплеев, разъемов, ПО и систем охлаждения. Доверив ремонт нам, вы получаете долговечный и надежный отремонтировать аймак адреса.
Подробная информация размещена на сайте: https://remont-imac-mos.ru
http://indianpharmacy.win/# indian pharmacies safe
medication from mexico pharmacy medication from mexico pharmacy mexican mail order pharmacies
нарколог на дом краснодар нарколог на дом краснодар .
http://mexicanpharmacy.store/# mexican mail order pharmacies
Наши специалисты предлагает профессиональный мастерская по ремонту айфона адреса различных марок и моделей. Мы знаем, насколько необходимы вам ваши устройства iPhone, и стремимся предоставить услуги высочайшего уровня. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что обеспечивает долговечность и надежность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются обладатели устройств iPhone, включают проблемы с экраном, проблемы с батареей, ошибки ПО, неисправности разъемов и повреждения корпуса. Для устранения этих неисправностей наши профессиональные техники выполняют ремонт экранов, батарей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы гарантируете себе качественный и надежный ремонт айфона рядом.
Подробная информация представлена на нашем сайте: https://remont-iphone-sot.ru
Download music https://progworld.net for free and without registration. Huge database of tracks of all genres in high quality. Convenient search and fast download.
Смотрите аниме онлайн https://reanime.net на русском! Огромная коллекция сериалов и фильмов в хорошем качестве. Все популярные аниме с русской озвучкой и субтитрами. Удобно, бесплатно, в отличном качестве.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков toshiba в москве, можете посмотреть на сайте: ремонт ноутбуков toshiba сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
нарколог на дом нарколог на дом .
http://mexicanpharmacy.store/# п»їbest mexican online pharmacies
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков fujitsu siemens рядом, можете посмотреть на сайте: ремонт ноутбуков fujitsu siemens сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
программа 1с купить программа 1с купить .
http://mexicanpharmacy.store/# buying from online mexican pharmacy
mexican border pharmacies shipping to usa medicine in mexico pharmacies mexican pharmaceuticals online
выезд нарколога на дом выезд нарколога на дом .
canadian pharmacy meds: best online canadian pharmacy – best canadian pharmacy to buy from
Доставка грузов из Китая https://cargotlk.ru под ключ. Организуем перевозки любых объемов: от документов до крупногабаритных грузов. Авиа, морская и автодоставка. Полное сопровождение, таможенное оформление, страхование.
Наш сервисный центр предлагает профессиональный отремонтировать айфон с гарантией различных марок и моделей. Мы понимаем, насколько важны для вас ваши смартфоны Apple, и стремимся предоставить услуги высочайшего уровня. Наши опытные мастера оперативно и тщательно выполняют работу, используя только качественные детали, что предоставляет длительную работу наших услуг.
Наиболее общие проблемы, с которыми сталкиваются владельцы iPhone, включают проблемы с экраном, поломку батареи, программные сбои, неисправности разъемов и механические повреждения. Для устранения этих неисправностей наши квалифицированные специалисты проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы гарантируете себе долговечный и надежный центр ремонта iphone на дому.
Подробная информация размещена на сайте: https://remont-iphone-sot.ru
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт ноутбуков toshiba, можете посмотреть на сайте: ремонт ноутбуков toshiba цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
the canadian pharmacy: best online canadian pharmacy – canadian pharmacy no scripts
нарколог на дом анонимно нарколог на дом анонимно .
تعمیر کیبورد های انواع مدل مک بوکhttps://macbook-keybaord.blog9.ir/post/22/%D8%AA%D8%B9%D9%85%DB%8C%D8%B1-%D8%AA%D8%A7%DA%86-%D8%A8%D8%A7%D8%B1-%D9%85%DA%A9%D8%A8%D9%88%DA%A9تعمیرات تخصصی محصولات اپل
http://indianpharmacy.win/# top 10 online pharmacy in india
https://mexicanpharmacy.store/# п»їbest mexican online pharmacies
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
https://canadianpharmacy.win/# safe canadian pharmacy
https://canadianpharmacy.win/# best canadian online pharmacy reviews
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков fujitsu siemens, можете посмотреть на сайте: ремонт ноутбуков fujitsu siemens адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://canadianpharmacy.win/# safe canadian pharmacy
Наша мастерская предлагает высококачественный срочный ремонт айфона различных марок и моделей. Мы осознаем, насколько необходимы вам ваши смартфоны Apple, и готовы предложить сервис наилучшего качества. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что обеспечивает надежность и долговечность выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются владельцы iPhone, включают проблемы с экраном, поломку батареи, ошибки ПО, проблемы с портами и поломки корпуса. Для устранения этих проблем наши квалифицированные специалисты оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы гарантируете себе долговечный и надежный сервисный ремонт iphone с гарантией.
Подробная информация размещена на сайте: https://remont-iphone-sot.ru
вызов нарколога на дом краснодар вызов нарколога на дом краснодар .
http://mexicanpharmacy.store/# mexico drug stores pharmacies
вывод из запоя на дому http://vyvod-iz-zapoya-krasnodar111.ru .
global pharmacy canada canadian pharmacy win canada pharmacy online
http://mexicanpharmacy.store/# mexican online pharmacies prescription drugs
台中外送茶
台中外送茶:安全快速的外約服務指南
引言
台中外送茶是一項方便且受歡迎的服務,無論是夜晚的放鬆需求還是特殊場合的社交活動,都能提供快速、安全且專業的體驗。本文將深入探討台中外送茶的選擇、流程以及相關注意事項,幫助您在選擇服務時更加安心與滿意。
什麼是台中外送茶?
台中外送茶服務主要提供以下特點:
快速應對:透過線上或電話聯繫,立即安排外送服務。
多元選擇:服務涵蓋不同需求,從高端到實惠方案應有盡有。
隱私保障:強調保密性,確保客戶資料不被外洩。
台中外送茶適合哪些人?
商務旅客:在台中短期停留,追求品質與方便的伴侶服務。
壓力族群:希望藉由互動放鬆身心,增添生活樂趣。
聚會需求:特殊場合、生日或朋友聚會需要專業的伴侶服務。
選擇台中外送茶的注意事項
如何選擇安全的服務?
查詢評價:選擇高評價且有口碑的服務提供者。
確認價格透明:避免隱藏費用,提前溝通價格範圍。
合法經營:確認服務平台是否具備合法經營的資格。
常見的服務流程
聯繫服務商家,了解可提供的選項及價格。
確定時間與地點,確保雙方需求達成一致。
接收外送服務,享受專業的伴侶陪伴。
九州娛樂
This post is definitely worth showing to everyone who is interested in this topic https://dtv-trust.zlnk.ru/#
Like 9561
pharmacies in mexico that ship to usa: mexico drug stores pharmacies – pharmacies in mexico that ship to usa
https://canadianpharmacy.win/# canadian pharmacy
Наша мастерская предлагает профессиональный сервисный центр по ремонту айфона на выезде всех типов и брендов. Мы знаем, насколько значимы для вас ваши смартфоны Apple, и стремимся предоставить услуги первоклассного уровня. Наши опытные мастера оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что предоставляет надежность и долговечность наших услуг.
Наиболее частые неисправности, с которыми сталкиваются владельцы iPhone, включают неисправности дисплея, проблемы с батареей, неисправности программного обеспечения, неисправности разъемов и поломки корпуса. Для устранения этих поломок наши квалифицированные специалисты проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы получаете долговечный и надежный мастерская по ремонту iphone на дому.
Подробная информация доступна на сайте: https://remont-iphone-sot.ru
india pharmacy mail order: п»їlegitimate online pharmacies india – online pharmacy india
https://indianpharmacy.win/# buy medicines online in india
花の慶次天に愛されし漢~
入金 不要
友達と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
CR牙狼魔戒ノ花
https://xn--k8-9g4a3b4f.site/shinbun/post-11608.html
ボーナスゲームが盛り上がり、毎回期待感があります。ドキドキ感がたまりません。
Dororonえん魔くん メ~ラめら
[url=https://www.stake-casino.casino/]
stake カジノ[/url]
P 真・北斗無双 第3章 ジャギの逆襲
CR牙狼GOLDSTORM翔 Ver.319
リング 終焉ノ刻
CR聖戦士ダンバイン Ver.319
https://injapan.today/topics/new-brazen-general
パチンコの台は、時には思わぬ演出があって驚かされます。毎回新しい発見があります。
北斗の拳-拳王
https://xn--k8-9g4a3b4f.site/tags/%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3-%E5%8F%B0-%E8%A8%AD%E7%BD%AE
パチンコの世界には、果てしない可能性があります。新しい挑戦を楽しむのが醍醐味です。
パチスロ 貞子3D
https://injapan.today/topics/edogai-kun
パチンコは、友人や家族と一緒に楽しむのに最適です。共に楽しむことで絆が深まります。
my canadian pharmacy canadian pharmacy win canada rx pharmacy world
https://mexicanpharmacy.store/# reputable mexican pharmacies online
Наш сервисный центр предлагает высококачественный мастер по ремонту макбука адреса любых брендов и моделей. Мы знаем, насколько необходимы вам ваши ноутбуки Apple, и обеспечиваем ремонт первоклассного уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что обеспечивает длительную работу наших услуг.
Наиболее общие проблемы, с которыми сталкиваются пользователи ноутбуков Apple, включают проблемы с экраном, проблемы с батареей, неисправности программного обеспечения, неисправности разъемов и неисправности системы охлаждения. Для устранения этих неисправностей наши профессиональные техники выполняют ремонт экранов, батарей, ПО, разъемов и систем охлаждения. Обращаясь в наш сервисный центр, вы гарантируете себе долговечный и надежный качественный ремонт макбука.
Подробная информация доступна на сайте: https://remont-macbook-club.ru
https://indianpharmacy.win/# п»їlegitimate online pharmacies india
FOSIL4D
http://canadianpharmacy.win/# www canadianonlinepharmacy
1с купить программу 1с купить программу .
https://canadianpharmacy.win/# canadian pharmacy king
https://indianpharmacy.win/# Online medicine home delivery
вызов нарколога на дом вызов нарколога на дом .
Everyone has their own reasons for acting the way they do, even if these reasons are not obvious to others https://ast-trust.zlnk.ru/#
Like 4259
Наши специалисты предлагает профессиональный мастерская по ремонту macbook в москве любых брендов и моделей. Мы знаем, насколько необходимы вам ваши ноутбуки Apple, и обеспечиваем ремонт первоклассного уровня. Наши квалифицированные специалисты работают быстро и аккуратно, используя только оригинальные запчасти, что гарантирует надежность и долговечность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются пользователи ноутбуков Apple, включают проблемы с экраном, проблемы с батареей, неисправности программного обеспечения, проблемы с портами и перегрев. Для устранения этих неисправностей наши профессиональные техники выполняют ремонт экранов, батарей, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы гарантируете себе качественный и надежный официальный ремонт макбука рядом.
Подробная информация доступна на сайте: https://remont-macbook-club.ru
canadian pharmacy cheap 77 canadian pharmacy or best canadian pharmacy online
https://cse.google.lv/url?sa=i&url=https://canadianpharmacy.win canadian pharmacy king reviews
canadian drug stores canadian pharmacy online and adderall canadian pharmacy canadapharmacyonline legit
my canadian pharmacy rx legit canadian pharmacy or canadian discount pharmacy
http://www.caterina-hein.de/url?q=https://canadianpharmacy.win canadian pharmacy no scripts
canadian pharmacy no scripts canadapharmacyonline com and medication canadian pharmacy canadian pharmacy scam
врач нарколог на дом врач нарколог на дом .
canada online pharmacy: canadian compounding pharmacy – best canadian pharmacy online
https://canadianpharmacy.win/# canada drugs online review
pharmacy com canada: canadian pharmacy win – canadian pharmacy online
аренда яхты дубай услуги сколько стоит аренда яхты в дубае
http://indianpharmacy.win/# reputable indian pharmacies
mexican drugstore online mexico drug stores pharmacies reputable mexican pharmacies online
https://mexicanpharmacy.store/# mexico drug stores pharmacies
It is very important that people are finally starting to talk openly about such serious things https://pno-trust.zlnk.ru/#
Like 4999
I need to save this post and show it to everyone I know, because it is really important https://ccn-trust.zlnk.ru/#
Like 8081
http://canadianpharmacy.win/# safe canadian pharmacies
After reading this post, you begin to understand how deep the problem touched upon by the author is https://vcl-trust.zlnk.ru/#
Like 9172
выезд нарколога на дом выезд нарколога на дом .
https://canadianpharmacy.win/# best canadian online pharmacy reviews
南国育ち(夏30)
パチスロ 犬夜叉 朝一
友人と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
CR魔法少女まどか_マギカ Ver.319
https://www.k8.rodeo/Articles/1580.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にボーナス時の音が最高。
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
すろろぐ
北斗之拳 轉生之章
Pこの素晴らしい世界に祝福を!199LT「このラッキートリガーに祝福を!」
牙狼 守りし者
北斗の拳 ラオウ
https://injapan.today/realtime/id1876.html
シンプルなデザインとルールが魅力。初心者でも安心して楽しめます。
CR戦国乙女〜花〜
https://xn--k8-9g4a3b4f.site/shinbun/post-6582.html
大当たりを引いたときの高揚感は何とも言えません。日常のストレスを忘れさせてくれます。
ベヨネッタ
https://injapan.today/news/id8552.html
パチンコのルールを覚えるのが簡単で、すぐに楽しめるのが良いです。初心者にも優しいです。
FOSIL4D
FOSIL4D
indian pharmacies safe india online pharmacy or reputable indian online pharmacy
http://101.43.178.182/sell/email.asp?d=indianpharmacy.win&on=tb india pharmacy mail order
top 10 online pharmacy in india india online pharmacy and india pharmacy reputable indian pharmacies
http://canadianpharmacy.win/# canada drugstore pharmacy rx
mexican drugstore online pharmacies in mexico that ship to usa medicine in mexico pharmacies
вывод из запоя круглосуточно краснодар на дому вывод из запоя круглосуточно краснодар на дому .
This was super helpful! More like this on https://metaink.org.
вывод из запоя лечение краснодар вывод из запоя лечение краснодар .
https://mexicanpharmacy.store/# pharmacies in mexico that ship to usa
вывод из запоя с выездом краснодар вывод из запоя с выездом краснодар .
http://mexicanpharmacy.store/# mexican mail order pharmacies
canadapharmacyonline legit: best mail order pharmacy canada – canadian pharmacy prices
ed drugs online from canada canadian pharmacy 24 or canada pharmacy reviews
https://www.google.ht/url?q=https://canadianpharmacy.win canadian pharmacy ed medications
buying from canadian pharmacies pharmacies in canada that ship to the us and canadian drug pharmacy canadian pharmacy 24
игра 1win https://grc.kg
mexican online pharmacies prescription drugs: mexican pharmaceuticals online – mexico drug stores pharmacies
http://indianpharmacy.win/# world pharmacy india
canadian pharmacy meds canadian online drugstore or canadian valley pharmacy
https://www.google.jo/url?sa=t&url=https://canadianpharmacy.win canadapharmacyonline legit
best canadian pharmacy to order from the canadian pharmacy and online canadian pharmacy review cheap canadian pharmacy online
DocReviews https://docreviews.com.ua это платформа, где пациенты могут оставлять отзывы о врачах. Мы стремимся помочь людям найти лучшего врача для своих нужд, предоставляя им доступную и достоверную информацию.
http://canadianpharmacy.win/# canada pharmacy online legit
нарколог на дом вывод из запоя краснодар нарколог на дом вывод из запоя краснодар .
It’s remarkable to pay a visit this website and reading the views of all colleagues about this piece of writing, while I am also zealous of getting experience.
сайт bitz casino
http://canadianpharmacy.win/# best online canadian pharmacy
canada cloud pharmacy canadian pharmacy king reviews or legit canadian online pharmacy
http://www.halifaxforum.ca/redirect.aspx?destination=http://canadianpharmacy.win canada pharmacy online legit
canadian pharmacy no scripts canada drugs reviews and canadian pharmacy best canadian pharmacy
http://canadianpharmacy.win/# legitimate canadian mail order pharmacy
mexican online pharmacies prescription drugs buying prescription drugs in mexico or buying prescription drugs in mexico online
http://maps.google.fi/url?q=https://mexicanpharmacy.store mexico drug stores pharmacies
buying prescription drugs in mexico online mexican border pharmacies shipping to usa and medication from mexico pharmacy buying prescription drugs in mexico online
Интернет-магазин инструментов https://profimaster58.ru для работы по металлу — ваш эксперт в качественном оборудовании! В ассортименте: измерительный инструмент, резцы, сверла, фрезы, пилы и многое другое. Гарантия точности, надежности и выгодных цен.
The Vatican Museum: A Journey Through Time and Art
Nestled within the Vatican City, the Vatican Museums stand as one of the most iconic cultural institutions in the world. With their unparalleled collection of art and history, they offer an extraordinary experience for visitors from all walks of life. Housing over 70,000 works of art, with 20,000 on display, these museums are a testament to humanity’s enduring quest for beauty, knowledge, and spirituality.
A Brief History
The origins of the Vatican Museums trace back to 1506 when Pope Julius II acquired a marble statue known as “Laocoon and His Sons.” This masterpiece marked the beginning of what would become one of the most extensive and revered art collections in the world. Over the centuries, successive Popes expanded the collection, commissioning and acquiring works from renowned artists and civilizations spanning millennia.
Today, the Vatican Museums are a series of interconnected museums and galleries, showcasing art and artifacts from ancient Egypt, classical antiquity, the Renaissance, and beyond. They serve as a bridge between the past and present, inviting visitors to immerse themselves in a world of creativity and devotion.
Highlights of the Vatican Museums
1. The Sistine Chapel:
The Sistine Chapel is arguably the crown jewel of the Vatican Museums. Michelangelo’s breathtaking frescoes, including the iconic “The Creation of Adam” and “The Last Judgment,” adorn its ceiling and altar wall. These masterpieces are considered some of the greatest achievements in the history of art, offering a profound spiritual and artistic experience.
2. Raphael Rooms:
Commissioned by Pope Julius II, the Raphael Rooms showcase the genius of Renaissance artist Raphael. The “School of Athens,” a depiction of classical philosophers, is a standout piece that exemplifies harmony, perspective, and intellectual depth.
3. Gallery of Maps:
A visual feast for cartography enthusiasts, the Gallery of Maps features 40 detailed topographical maps of Italy, meticulously painted by Ignazio Danti in the late 16th century. The vibrant colors and intricate details make this gallery a must-see.
4. Gregorian Egyptian Museum:
For those intrigued by ancient civilizations, the Gregorian Egyptian Museum offers a fascinating glimpse into Egypt’s history. With mummies, statues, and papyri, it transports visitors to a time of pharaohs and pyramids.
5. Pinacoteca:
The Pinacoteca, or picture gallery, houses an impressive collection of paintings from the Middle Ages to the 19th century. Works by Leonardo da Vinci, Caravaggio, and Titian are among its treasures.
Tips for Visiting the Vatican Museums
Book Tickets in Advance: The Vatican Museums attract millions of visitors annually. To avoid long queues, consider purchasing skip-the-line tickets or guided tour packages online.
Choose the Right Time: Visiting early in the morning or late in the afternoon can help you avoid peak crowds. Wednesday mornings are especially busy due to the Pope’s weekly audience.
Dress Appropriately: As a religious site, the Vatican has a dress code. Ensure your shoulders and knees are covered to gain entry.
Take a Guided Tour: To fully appreciate the history and significance of the collections, opt for a guided tour. Expert guides provide insightful commentary, enriching your experience.
Allocate Sufficient Time: The museums are vast, and exploring them can take several hours. Prioritize your must-see areas and allow time to soak in the ambiance.
The Cultural Significance of the Vatican Museums
The Vatican Museums are more than a repository of art; they represent a convergence of faith, culture, and human achievement. They bear witness to the Catholic Church’s role in preserving and fostering artistic expression throughout history. The collections highlight the interplay between spirituality and creativity, offering visitors a profound connection to the divine and the universal human experience.
Practical Information
Location: Vatican City, accessible from Rome.
Opening Hours: Typically open Monday through Saturday, with limited hours on Sundays. Check the official website for holiday schedules.
Tickets: Standard entry fees vary, with discounts available for students and children.
Accessibility: The museums are wheelchair accessible, with lifts and pathways designed for visitors with mobility challenges.
The Vatican Museums are a treasure trove of art, history, and spirituality, offering a transformative journey through humanity’s creative and cultural legacy. Whether you are an art aficionado, a history enthusiast, or a spiritual seeker, the museums promise an unforgettable experience. Plan your visit today to explore one of the world’s greatest cultural landmarks and discover the timeless beauty that lies within its walls.
Покраска бампера автомобиля требует тщательной подготовки и правильного подбора составов. Первым делом необходимо определить, сколько краски нужно на бампер, учитывая его габариты и состояние поверхности. Краска для пластика бампера на emmanuelbibletraining.info считается подходящим выбором для достижения качественного результата. Важно помнить, что перед нанесением нового покрытия следует тщательно подготовить поверхность, удалив старую краску и обезжирив пластик. Текстурная краска дает не только эстетичный внешний вид, но и дополнительную защиту от механических повреждений.
Для тех, кто столкнулся с потребностью удаления следов краски после ДТП, существуют различные методы решения проблемы. Чтобы убрать краску с бампера от другой машины, специалисты рекомендуют использовать профессиональные смывки для краски с пластика бампера, которые эффективно справляются с задачей без повреждения основного покрытия. При работе с черной структурной краской важно учитывать время высыхания каждого слоя и следовать технологию нанесения, что позволит добиться однородного покрытия и долговечного результата.
Источник: https://emmanuelbibletraining.info/
по вопросам Сколько сохнет краска на бампере – обращайтесь в Telegram tri36
http://mexicanpharmacy.store/# best online pharmacies in mexico
http://indianpharmacy.win/# india pharmacy
canadian pharmacy oxycodone reliable canadian pharmacy reviews canadian pharmacy service
best online pharmacy india best india pharmacy or indian pharmacy online
http://nishiyama-takeshi.com/mobile2/mt4i.cgi?id=3&mode=redirect&no=67&ref_eid=671&url=http://indianpharmacy.win pharmacy website india
=cialis+without+doctor+prescription]cheapest online pharmacy india online shopping pharmacy india and mail order pharmacy india top online pharmacy india
принудительный вывод из запоя краснодар принудительный вывод из запоя краснодар .
вывод из запоя краснодар стационар вывод из запоя краснодар стационар .
mexican mail order pharmacies mexico pharmacies prescription drugs or buying prescription drugs in mexico online
https://cse.google.co.ve/url?q=https://mexicanpharmacy.store mexican border pharmacies shipping to usa
pharmacies in mexico that ship to usa mexican rx online and mexican rx online reputable mexican pharmacies online
Tools for Assessing USDT for Sanctions and Deal Clarity: AML Solutions
In the current environment of digital currencies, where fast exchanges and privacy are becoming the usual case, supervising the validity and cleanliness of operations is essential. In light of heightened administrative scrutiny over dirty money and financing of terrorism, the need for efficient resources to validate operations has become a key priority for digital asset users. In this article, we will explore accessible offerings for checking USDT for restrictive measures and operation cleanliness.
What is AML?
Money Laundering Prevention actions refer to a set of legal measures aimed at hindering and uncovering illicit finance activities. With the increase of cryptocurrency usage, AML standards have become particularly crucial, allowing individuals to operate digital currencies securely while minimizing threats associated with restrictive measures.
USDT, as the arguably the well-known stablecoin, is commonly used in diverse deals across the globe. However, using USDT can entail several dangers, especially if your resources may tie to ambiguous or criminal transactions. To minimize these hazards, it’s crucial to take benefit of solutions that verify USDT for prohibitions.
Available Services
1. Address Authentication: Using customized tools, you can inspect a specific USDT address for any connections to prohibited catalogs. This helps pinpoint potential associations to illegal operations.
2. Transaction Conduct Evaluation: Some tools offer scrutiny of operation history, crucial for judging the lucidity of monetary transfers and detecting potentially hazardous transactions.
3. Monitoring Systems: Professional monitoring services allow you to monitor all transactions related to your location, facilitating you to quickly identify dubious conduct.
4. Concern Documents: Certain tools offer detailed threat documents, which can be helpful for participants looking to validate the soundness of their assets.
Irrespective of whether you are handling a large investment or performing small operations, adhering to AML norms helps avoid legal repercussions. Employing USDT verification services not only defends you from monetary damages but also supports to building a protected environment for all industry stakeholders.
Conclusion
Assessing USDT for sanctions and deal integrity is becoming a required measure for anyone keen to stay compliant within the rules and support high standards of transparency in the crypto domain. By collaborating with reliable platforms, you not only safeguard your holdings but also contribute to the shared goal in preventing financial misconduct and terrorist financing.
If you are ready to start utilizing these offerings, investigate the existing platforms and choose the option that best suits your requirements. Keep in mind, knowledge is your advantage, and swift operation check can save you from a variety of issues in the long run.
online canadian pharmacy review canadadrugpharmacy com or pharmacy canadian superstore
https://images.google.gg/url?q=https://canadianpharmacy.win canada drugs reviews
best canadian pharmacy canadian pharmacy online reviews and canadian pharmacy meds reviews escrow pharmacy canada
canadian pharmacy uk delivery: canadian pharmacy win – pharmacy rx world canada
http://mexicanpharmacy.store/# pharmacies in mexico that ship to usa
buy drugs from canada canadian pharmacy win best canadian online pharmacy
http://canadianpharmacy.win/# canadian pharmacy no scripts
cheapest online pharmacy india: cheapest online pharmacy india – top online pharmacy india
вывод из запоя http://vyvod-iz-zapoya-krasnodar116.ru .
вывод из запоя цены краснодар вывод из запоя цены краснодар .
вывод из запоя с выездом vyvod-iz-zapoya-krasnodar114.ru .
https://canadianpharmacy.win/# canadian drugstore online
https://indianpharmacy.win/# top 10 online pharmacy in india
http://mexicanpharmacy.store/# purple pharmacy mexico price list
Смотрите аниме онлайн https://studiobanda.net бесплатно и без рекламы. Удобный каталог с популярными тайтлами, новинками и свежими сериями. Высокое качество видео и быстрый плеер обеспечат комфортный просмотр. Подборки по жанрам, рекомендации и регулярные обновления сделают ваш опыт максимально приятным.
спортивная школа фигурного катания клуб фигурного катания
rate canadian pharmacies buying from canadian pharmacies or best canadian pharmacy to buy from
http://logen.ru/bitrix/redirect.php?goto=https://canadianpharmacy.win northwest pharmacy canada
trustworthy canadian pharmacy my canadian pharmacy reviews and canadian pharmacy prices canada drugs
https://mexicanpharmacy.store/# medication from mexico pharmacy
вывод из запоя цены краснодар вывод из запоя цены краснодар .
canadian pharmacy legitimate canadian pharmacy online or cheap canadian pharmacy
http://huber.ru/bitrix/rk.php?goto=http://canadianpharmacy.win/ canada pharmacy online
canadapharmacyonline legit canadian pharmacy 1 internet online drugstore and canada drugs online reviews canadian pharmacy
дианы шурыгиной видео диана шурыгиной
http://canadianpharmacy.win/# safe reliable canadian pharmacy
Официальная страница Дианы Шурыгиной https://t.me/DiShurigina свежие новости, уникальные фото и видео, личные откровения и новые проекты. Погружайтесь в мир Дианы, узнавайте её историю и вдохновение. Будьте в центре её жизни и не пропустите ни одного яркого момента!
八代將軍戰神版
エヴァ 決戦 甘 遊タイム
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
CR花の慶次X~雲のかなたに~
https://xn--k8-9g4a3b4f.site/tags/%E3%82%B2%E3%83%BC%E3%83%A0%E4%BD%93%E9%A8%93
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
夜蝶飛翔特2発1
スマスロひぐらし天井
パチスロだよ黄門ちゃま
CR聖闘士星矢 星の運命
八代將軍戰神版
デビル メイ クライ クロス
https://www.k8-casino.biz/Articles/2923.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
獣王 王者の覚醒
https://injapan.today/news/id2334.html
音楽が豪華で、プレイ中に盛り上がります。神々の祝福を感じます。
CRA牙狼金色になれザルバとの契約
https://xn--k8-9g4a3b4f.site/tags/%E3%81%99%E3%81%97-%E3%81%96%E3%82%93%E3%81%BE%E3%81%84-%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3
定期的に新しい台が出るので、飽きずに楽しめます。ファンにはたまらないですね。
best india pharmacy indian pharmacy or Online medicine order
http://ingrus.net/dbforum/away.php?s=http://indianpharmacy.win pharmacy website india
best online pharmacy india world pharmacy india and reputable indian online pharmacy india pharmacy
What’s up Dear, are you really visiting this website daily, if so after that you will definitely take good experience.
Обратные Ссылки Guest, WP блоги, forums прогон XRumer 23 StrongAI
https://canadianpharmacy.win/# canadian pharmacy meds review
вывод из запоя цены краснодар вывод из запоя цены краснодар .
recommended canadian pharmacies: best online canadian pharmacy – best rated canadian pharmacy
п»їlegitimate online pharmacies india indian pharmacy india pharmacy
https://mexicanpharmacy.store/# mexican pharmaceuticals online
purple pharmacy mexico price list: pharmacies in mexico that ship to usa – buying prescription drugs in mexico
срочная помощь вывод из запоя краснодар срочная помощь вывод из запоя краснодар .
http://mexicanpharmacy.store/# pharmacies in mexico that ship to usa
This post needs to be shown to all your friends and acquaintances, it is so important https://vlp-trust.zlnk.ru/#
Like 4079
http://mexicanpharmacy.store/# mexican mail order pharmacies
http://mexicanpharmacy.store/# п»їbest mexican online pharmacies
вывод из запоя дешево ростов-на-дону вывод из запоя дешево ростов-на-дону .
I have read this post several times already and each time I find something new for myself https://zlnk.ru/#
Like 2959
I have not seen such a deep analysis of the current situation in simple words for a long time https://zlnk.ru/#
Like 203
canadian pharmacy checker best canadian online pharmacy online pharmacy canada
http://canadianpharmacy.win/# best canadian pharmacy
Предприниматель и инвестор Святослав Гусев https://teletype.in/@gusevlife специализирующийся на IT, блокчейн-технологиях и венчурном инвестировании. Активно делится аналитикой рынка, инсайдами и новостями, которые помогут заработать каждому!
http://indianpharmacy.win/# online pharmacy india
Мостбет пропонує щедрі бонуси для нових та постійних клієнтів | На Мостбет ви можете грати у найпопулярніші слоти та ігри most bet casino. | Мостбет онлайн – ваш вибір для якісного відпочинку та виграшів | Мостбет казино дарує новим гравцям щедрі бонуси Mostbet login.
mexican drugstore online mexican online pharmacies prescription drugs or mexico pharmacies prescription drugs
https://images.google.vg/url?sa=t&url=https://mexicanpharmacy.store best online pharmacies in mexico
pharmacies in mexico that ship to usa mexican pharmaceuticals online and п»їbest mexican online pharmacies best online pharmacies in mexico
canadian pharmacies that deliver to the us canada pharmacy online or my canadian pharmacy
http://www.marstruct-vi.com/feedback.aspx?page=https://canadianpharmacy.win online canadian pharmacy
canadian pharmacy prices canadian drugs pharmacy and canadian online pharmacy canadian pharmacy in canada
is canadian pharmacy legit canada drugs or canadian drug stores
http://transfer-talk.herokuapp.com/l?l=http://canadianpharmacy.win canadian drug pharmacy
[url=http://www.dynonames.com/buy-expired-or-pre-owned-domain-name.php?url=canadianpharmacy.win]canadian pharmacy 24 com[/url] canadian world pharmacy and [url=http://www.bqmoli.com/bbs/home.php?mod=space&uid=12998]canadapharmacyonline[/url] canadian pharmacy world reviews
best india pharmacy mail order pharmacy india or п»їlegitimate online pharmacies india
http://tsm.ru/bitrix/redirect.php?event1=&event2=&event3=&goto=https://indianpharmacy.win/ mail order pharmacy india
Online medicine home delivery best online pharmacy india and online shopping pharmacy india indian pharmacy online
вывод из запоя краснодар на дому анонимно вывод из запоя краснодар на дому анонимно .
https://canadianpharmacy.win/# trusted canadian pharmacy
mexico drug stores pharmacies: mexico pharmacies prescription drugs – medicine in mexico pharmacies
вывод из запоя на дому краснодар цены вывод из запоя на дому краснодар цены .
CRA牙狼金色になれザルバとの契約
熱中 時代 子役
簡単に遊べるルールで、初心者でも安心。気軽に挑戦できるのが良いです。
Pハイスクール・フリート
https://www.fa6.net/tags/%e7%b4%ab%e3%82%a4%e3%83%9f%e3%82%bd%e3%83%bc%e3%83%ac
ボーナスゲームが盛り上がり、毎回期待感があります。ドキドキ感がたまりません。
CR北斗の拳剛掌 Ver.399
[url=https://www.k8-casino.jp/game/pachinko/3555.html]
ガンダムseed パチンコ 裏ボタン[/url]
P緋弾のアリア 緋弾覚醒編 Ver.199
CR神獣王2
CRA牙狼金色になれザルバとの契約
紫の花
https://gamein.jp/slots/castle-of-fire
パチンコの世界には、運だけではなく、戦略も求められます。自分の考えを試す楽しさがあります。
PA ぱちんこ AKB48 桜 LIGHT ver.
https://casinos.town/providers/pragmatic-play
友人と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
ベヨネッタ
https://xn--k8-9g4a3b4f.site/shinbun/post-3728.html
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特にモンスター戦が楽しい。
https://canadianpharmacy.win/# canadian pharmacy prices
http://canadianpharmacy.win/# canada ed drugs
mexican rx online: purple pharmacy mexico price list – mexico drug stores pharmacies
http://canadianpharmacy.win/# best canadian online pharmacy
http://indianpharmacy.win/# reputable indian pharmacies
вывод из запоя краснодар наркология вывод из запоя краснодар наркология .
medication from mexico pharmacy pharmacies in mexico that ship to usa pharmacies in mexico that ship to usa
http://canadianpharmacy.win/# canadian pharmacy review
http://indianpharmacy.win/# Online medicine order
I totally agree with your points. If you’re looking for additional insights, visit https://metaback.org/
https://indianpharmacy.win/# india pharmacy mail order
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
вывод из запоя на дому ростов вывод из запоя на дому ростов .
лучшие фильмы онлайн на планшете смотреть фильмы онлайн в Казахстане
лучшие фильмы онлайн аниме фильмы 2004 смотреть онлайн
pharmacy website india indian pharmacy indian pharmacies safe
вывод из запоя вывод из запоя .
вывод. из. запоя. анонимно. ростов. vyvod-iz-zapoya-rostov234.ru .
canadian pharmacy online ship to usa: best online canadian pharmacy – safe online pharmacies in canada
https://indianpharmacy.win/# online pharmacy india
Online medicine home delivery india pharmacy or indian pharmacy online
https://images.google.com.vc/url?q=https://indianpharmacy.win world pharmacy india
Online medicine order reputable indian online pharmacy and Online medicine order buy medicines online in india
cheap canadian pharmacy canadian pharmacy near me or real canadian pharmacy
https://clients1.google.az/url?q=https://canadianpharmacy.win the canadian drugstore
canadian pharmacy victoza canadian pharmacy tampa and canadian pharmacy india canadian pharmacy prices
canadian compounding pharmacy: legitimate canadian online pharmacies – canadian pharmacy oxycodone
https://canadianpharmacy.win/# reputable canadian online pharmacy
https://canadianpharmacy.win/# my canadian pharmacy
фильмы онлайн HD мелодрамы фильмы 2001 смотреть онлайн
дивитися фільм безкоштовно аніме нові фільми онлайн мелодрами
https://canadianpharmacy.win/# certified canadian pharmacy
canadian pharmacy ed medications canadian pharmacy online ship to usa or vipps approved canadian online pharmacy
https://www.google.com.sv/url?q=https://canadianpharmacy.win canadian valley pharmacy
canadian pharmacy 1 internet online drugstore canadian pharmacy phone number and canadian pharmacy store canadian pharmacy online reviews
вывод из запоя ростов-на-дону вывод из запоя ростов-на-дону .
https://mexicanpharmacy.store/# mexican rx online
вывод из запоя на дому ростов круглосуточно вывод из запоя на дому ростов круглосуточно .
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
http://mexicanpharmacy.store/# reputable mexican pharmacies online
https://mexicanpharmacy.store/# medicine in mexico pharmacies
Online medicine order india pharmacy indian pharmacy
вывод из запоя в стационаре ростов-на-дону http://vyvod-iz-zapoya-rostov233.ru/ .
http://indianpharmacy.win/# indian pharmacy
top 10 pharmacies in india: best india pharmacy – top 10 online pharmacy in india
buying prescription drugs in mexico medicine in mexico pharmacies or mexico drug stores pharmacies
http://nimbus.c9w.net/wifi_dest.html?dest_url=https://mexicanpharmacy.store mexican mail order pharmacies
medication from mexico pharmacy buying prescription drugs in mexico online and mexican rx online best online pharmacies in mexico
https://canadianpharmacy.win/# canadian pharmacy tampa
http://canadianpharmacy.win/# canada ed drugs
mexican pharmaceuticals online: mexican border pharmacies shipping to usa – mexico drug stores pharmacies
http://mexicanpharmacy.store/# mexican border pharmacies shipping to usa
reputable indian online pharmacy online pharmacy india or Online medicine order
https://www.google.com.eg/url?q=https://indianpharmacy.win indian pharmacy
top 10 pharmacies in india indian pharmacy paypal and indianpharmacy com online shopping pharmacy india
top 10 pharmacies in india top online pharmacy india indianpharmacy com
legal canadian pharmacy online canadapharmacyonline com or my canadian pharmacy rx
https://maps.google.tg/url?q=https://canadianpharmacy.win canadian pharmacy no scripts
legitimate canadian pharmacy online precription drugs from canada and reputable canadian pharmacy canadian pharmacy 1 internet online drugstore
http://mexicanpharmacy.store/# mexican pharmaceuticals online
northern pharmacy canada online canadian pharmacy reviews or canadian drug prices
http://www.google.com.bd/url?q=https://canadianpharmacy.win/ best online canadian pharmacy
canada drugstore pharmacy rx canadian drug stores and canadian 24 hour pharmacy online canadian pharmacy review
mexico drug stores pharmacies medication from mexico pharmacy or purple pharmacy mexico price list
http://stopundshop.eu/url?q=https://mexicanpharmacy.store purple pharmacy mexico price list
mexican mail order pharmacies medicine in mexico pharmacies and mexican mail order pharmacies mexican pharmaceuticals online
http://indianpharmacy.win/# п»їlegitimate online pharmacies india
вывод из запоя цены на дому ростов вывод из запоя цены на дому ростов .
http://indianpharmacy.win/# top 10 pharmacies in india
sport-weekend.com http://www.pervouralsk.ru/news/obshchestvo/elektronnye-turnikety-perco-udobstvo-i-populiarnost/48835/ .
вывод из запоя на дому ростов на дону вывод из запоя на дому ростов на дону .
clockchok.ru http://www.potokmedia.ru/570331/arochnye-metallodetektory-osobennosti-pravila-vybora-i-ekspluatacii/ .
Возможности выигрыша в онлайн казино, где возможности бесконечны.
Попробуйте свои силы вместе с нами, и получите незабываемые впечатления.
Сделайте свой выбор в пользу казино онлайн, и начните играть уже сегодня.
Почувствуйте атмосферу настоящего казино в режиме онлайн, не покидая своего уютного кресла.
Выигрывайте крупные суммы при помощи наших игр, и почувствуйте себя настоящим чемпионом.
Играйте вместе с друзьями и соперниками со всего мира, и покажите свои лучшие результаты.
Начните играть и получите ценные подарки, которые увеличат ваши шансы на победу.
Почувствуйте свободу и возможность выбора в каждой игре, и наслаждайтесь бесконечными возможностями.
Получите доступ к уникальным играм и выигрывайте крупные суммы, сделав всего несколько кликов мыши.
казино онлайн беларусь казино онлайн .
what is crypto drainer – solana drainer, best crypto drainer
http://canadianpharmacy.win/# canadian pharmacy 365
buy prescription drugs from india: online pharmacy india – best india pharmacy
http://canadianpharmacy.win/# legit canadian pharmacy
At Cheap SEO Solutions https://cheap-seo-solutions.com we don’t believe in half-measures. We deliver comprehensive SEO solutions that cover all the bases, from keyword research and on-page optimization to link building and content creation. Our goal is to help businesses improve their search engine rankings, drive organic traffic, and increase conversions.
https://mexicanpharmacy.store/# mexican border pharmacies shipping to usa
Ставки на спорт с Vavada https://selfiedumps.com это простота, надежность и высокие шансы на победу. Удобная платформа, разнообразие событий и быстрые выплаты делают Vavada идеальным выбором для любителей азарта. Зарегистрируйтесь сейчас и начните выигрывать вместе с нами!
http://mexicanpharmacy.store/# medication from mexico pharmacy
my canadian pharmacy: best canadian online pharmacy – canadian discount pharmacy
анонимный вывод из запоя ростов анонимный вывод из запоя ростов .
http://canadianpharmacy.win/# canadian pharmacy ed medications
mexican mail order pharmacies medicine in mexico pharmacies buying from online mexican pharmacy
crypto wallet lookup
Tools for Monitoring USDT for Embargoes and Deal Integrity: AML Measures
In the current realm of crypto assets, where expedited transactions and secrecy are becoming the usual case, tracking the validity and integrity of operations is necessary. In light of amplified government examination over dirty money and terrorism funding, the requirement for effective instruments to authenticate transactions has become a critical matter for cryptocurrency users. In this text, we will discuss offered offerings for monitoring USDT for sanctions and transaction integrity.
What is AML?
Anti-Money Laundering strategies refer to a series of supervisory actions aimed at curtailing and detecting dirty money activities. With the surge of virtual currency usage, AML measures have become notably important, allowing clients to manage digital currencies confidently while mitigating risks associated with sanctions.
USDT, as the most favored stablecoin, is broadly used in diverse operations internationally. However, using USDT can involve several dangers, especially if your capital may relate to non-transparent or unlawful activities. To minimize these risks, it’s essential to take leverage of offerings that verify USDT for restrictive measures.
Available Services
1. Address Validation: Leveraging customized tools, you can check a certain USDT address for any associations to prohibited registries. This assists uncover potential ties to unlawful operations.
2. Transfer Action Assessment: Some platforms provide assessment of transfer background, essential for measuring the clarity of capital flows and identifying potentially dangerous operations.
3. Monitoring Systems: Dedicated monitoring solutions allow you to track all operations related to your address, enabling you to swiftly identify concerning operations.
4. Concern Statements: Certain platforms provide detailed risk reports, which can be beneficial for investors looking to confirm the reliability of their investments.
Regardless of if you are handling a large fund or making small trades, following to AML guidelines ensures prevent legal repercussions. Using USDT verification services not only defends you from capital declines but also supports to establishing a secure environment for all business players.
Conclusion
Assessing USDT for sanctions and deal clarity is becoming a compulsory process for anyone eager to remain within the legal framework and support high levels of transparency in the virtual currency domain. By interacting with dependable tools, you not only protect your assets but also support to the collective effort in combating dirty money and terror financing activities.
If you are prepared to start utilizing these solutions, examine the offered platforms and identify the solution that most suitably aligns with your requirements. Bear in mind, insight is your power, and swift transaction verification can shield you from countless issues in the future.
Наши специалисты предлагает надежный мастер по ремонту варочных панелей с гарантией различных марок и моделей. Мы понимаем, насколько важны для вас ваши варочные панели, и стремимся предоставить услуги наилучшего качества. Наши опытные мастера работают быстро и аккуратно, используя только качественные детали, что гарантирует надежность и долговечность наших услуг.
Наиболее частые неисправности, с которыми сталкиваются пользователи плит, включают неработающие конфорки, неработающие сенсоры, неисправности программного обеспечения, неработающие разъемы и поломки компонентов. Для устранения этих неисправностей наши квалифицированные специалисты оказывают ремонт нагревательных элементов, сенсоров, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы обеспечиваете себе качественный и надежный мастерская по ремонту варочных панелей на дому.
Подробная информация представлена на нашем сайте: https://remont-varochnyh-paneley-hit.ru
Online medicine order best india pharmacy or india pharmacy mail order
https://images.google.cz/url?q=https://indianpharmacy.win best online pharmacy india
mail order pharmacy india reputable indian pharmacies and reputable indian online pharmacy india pharmacy
Anti-money laundering (AML)
Offerings for Assessing USDT for Sanctions and Transfer Purity: Money Laundering Prevention Approaches
In the up-to-date world of cryptocurrencies, where quick deals and privacy are becoming the usual case, monitoring the legality and integrity of activities is crucial. In recognition of heightened official scrutiny over dirty money and financing of terrorism, the requirement for reliable means to verify transactions has become a critical matter for virtual currency users. In this article, we will analyze accessible solutions for monitoring USDT for restrictive measures and deal integrity.
What is AML?
Anti-Money Laundering measures refer to a series of supervisory protocols aimed at stopping and uncovering money laundering activities. With the rise of digital asset usage, AML strategies have become especially essential, allowing users to operate digital resources securely while lessening hazards associated with prohibitions.
USDT, as the widely regarded as the favored stablecoin, is extensively used in diverse deals across the globe. Nonetheless, using USDT can carry several dangers, especially if your resources may connect to unclear or criminal operations. To mitigate these concerns, it’s imperative to take leverage of offerings that inspect USDT for restrictive measures.
Available Services
1. Address Authentication: Employing dedicated tools, you can verify a particular USDT address for any connections to prohibited lists. This aids identify potential associations to criminal activities.
2. Deal Engagement Assessment: Some platforms provide evaluation of operation records, crucial for assessing the openness of financial transfers and uncovering potentially dangerous conduct.
3. Tracking Solutions: Specialized monitoring tools allow you to monitor all deals related to your location, enabling you to promptly identify questionable conduct.
4. Concern Statements: Certain solutions provide detailed risk evaluations, which can be crucial for stakeholders looking to guarantee the soundness of their investments.
Irrespective of whether or not you are controlling a considerable investment or conducting small deals, complying to AML practices helps evade legal repercussions. Adopting USDT authentication offerings not only shields you from capital declines but also aids to creating a stable environment for all market stakeholders.
Conclusion
Checking USDT for sanctions and transaction cleanliness is becoming a required action for anyone eager to stay compliant within the legal framework and uphold high levels of openness in the digital asset field. By engaging with credible solutions, you not only defend your resources but also contribute to the collective initiative in fighting dirty money and financing of terrorism.
If you are ready to start utilizing these solutions, review the accessible options and pick the service that best meets your needs. Remember, information is your power, and timely transaction verification can save you from a variety of difficulties in the future.
Discover the power of AI with deepnude online Fast image processing and accessible functionality allow you to create unique effects. Enjoy safe and anonymous use of the platform for entertainment.
gtbike.ru http://konkurent.ru/article/69631/ .
I rarely come across such deep and correct thoughts in the modern information space https://zlnk.ru/#
Like 6990
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт ноутбуков toshiba, можете посмотреть на сайте: ремонт ноутбуков toshiba
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
buy drugs from canada canadian pharmacy tampa or canada drugs online
https://date.gov.md/ckan/ru/api/1/util/snippet/api_info.html?resource_id=a0321cc2-badb-4502-9c51-d8bb8d029c54&datastore_root_url=http://canadianpharmacy.win reliable canadian pharmacy reviews
canada rx pharmacy canadian pharmacy online store and real canadian pharmacy best rated canadian pharmacy
sport-weekend.com http://prochepetsk.ru/gde-kupit-kachestvennyy-i-sovremennyy-turniket/ .
http://indianpharmacy.win/# buy prescription drugs from india
certified canadian international pharmacy canadian drugs canadian medications
вывод из запоя в ростове-на-дону вывод из запоя в ростове-на-дону .
I completely share the author’s opinion and believe that this needs to be talked about much more often https://zlnk.ru/#
Like 5249
canadian discount pharmacy medication canadian pharmacy or canadian online pharmacy
https://maps.google.mw/url?sa=t&url=https://canadianpharmacy.win onlinecanadianpharmacy
best canadian pharmacy online canadian pharmacy no scripts and canadian pharmacy world reliable canadian pharmacy reviews
best comic reading site in Mexico online comic reader with English translation
top manga 2020 read manga online on mobile
Наш сервисный центр предлагает надежный центр ремонта варочной панели рядом любых брендов и моделей. Мы знаем, насколько необходимы вам ваши варочные панели, и готовы предложить сервис высочайшего уровня. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что предоставляет надежность и долговечность проведенных ремонтов.
Наиболее частые неисправности, с которыми сталкиваются владельцы варочных панелей, включают неработающие конфорки, проблемы с сенсорным управлением, программные сбои, неработающие разъемы и повреждения корпуса. Для устранения этих проблем наши опытные мастера выполняют ремонт нагревательных элементов, сенсоров, ПО, разъемов и механических компонентов. Обратившись к нам, вы гарантируете себе качественный и надежный ремонт варочных панелей в москве.
Подробная информация размещена на сайте: https://remont-varochnyh-paneley-hit.ru
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт ноутбуков fujitsu siemens, можете посмотреть на сайте: срочный ремонт ноутбуков fujitsu siemens
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://mexicanpharmacy.store/# pharmacies in mexico that ship to usa
https://canadianpharmacy.win/# canadian pharmacy
best online pharmacy india: india online pharmacy – world pharmacy india
Наши специалисты предлагает надежный сервис ремонта макбука в москве любых брендов и моделей. Мы понимаем, насколько важны для вас ваши MacBook, и готовы предложить сервис наилучшего качества. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что обеспечивает надежность и долговечность наших услуг.
Наиболее общие проблемы, с которыми сталкиваются пользователи ноутбуков Apple, включают проблемы с экраном, поломку батареи, ошибки ПО, проблемы с портами и неисправности системы охлаждения. Для устранения этих неисправностей наши профессиональные техники оказывают ремонт экранов, батарей, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы гарантируете себе качественный и надежный центр ремонта macbook рядом.
Подробная информация представлена на нашем сайте: https://remont-macbook-club.ru
https://mexicanpharmacy.store/# buying from online mexican pharmacy
http://canadianpharmacy.win/# canadian pharmacy world reviews
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков toshiba адреса, можете посмотреть на сайте: ремонт ноутбуков toshiba рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
safe online pharmacies in canada: canadian pharmacy win – canadian drugs online
Наш сервисный центр предлагает высококачественный починить видеокамеру на выезде всех типов и брендов. Мы понимаем, насколько значимы для вас ваши видеокамеры, и готовы предложить сервис высочайшего уровня. Наши квалифицированные специалисты работают быстро и аккуратно, используя только сертифицированные компоненты, что гарантирует долговечность и надежность наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются владельцы видеокамер, включают неработающую запись, поврежденный объектив, неисправности программного обеспечения, проблемы с подключением и повреждения корпуса. Для устранения этих неисправностей наши опытные мастера оказывают ремонт записи, объективов, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы обеспечиваете себе качественный и надежный отремонтировать видеокамеру в москве.
Подробная информация доступна на сайте: https://remont-videokamer-ink.ru
http://mexicanpharmacy.store/# buying prescription drugs in mexico online
best online pharmacies in mexico mexican online pharmacies prescription drugs or mexico pharmacies prescription drugs
https://images.google.com.ar/url?q=http://mexicanpharmacy.store best online pharmacies in mexico
mexican drugstore online purple pharmacy mexico price list and pharmacies in mexico that ship to usa medicine in mexico pharmacies
http://canadianpharmacy.win/# canadian pharmacy service
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Best UK IPTV Subscription, Enjoy BestIPTVUK
streaming experience with excellent image quality up to 4K, on any device, anytime and anywhere, with over than +30,000 channels, +100,000 VOD and uptime 100%
reputable indian online pharmacy pharmacy website india or reputable indian pharmacies
https://www.google.com.vc/url?q=https://indianpharmacy.win top 10 pharmacies in india
best online pharmacy india india online pharmacy and indianpharmacy com indian pharmacy paypal
http://indianpharmacy.win/# Online medicine home delivery
clockchok.ru http://www.vkirove.ru/news/2024/05/23/kak_vybrat_prokhodnuyu_s_turniketom_vidy_osobennosti_vybora.html/ .
best mail order pharmacy canada canadian pharmacy win canada drugs reviews
Trash. https://metamask.io/download/
Kantorbola link alternatif login terbaru 2025 . Kunjungi link resmi situs kantor bola untuk melakukan permainan dan pendaftaran
http://hollywoodcarefoundation.com/__media__/js/netsoltrademark.php?d=xn--jj0bt2i8umnxa.com%2Fbbs%2Fboard.php%3Fbo_table%3Dfree%26wr_id%3D52368
“Sanfordpharmacy.com: Your best online pharmacy for quality medications at the lowest prices. Trusted, affordable, and convenient – shop now to save on your health essentials with unmatched service!”
алкоголизм лечение вывод из запоя ростов алкоголизм лечение вывод из запоя ростов .
Наш сервисный центр предлагает высококачественный выездной ремонт видеокамер всех типов и брендов. Мы осознаем, насколько значимы для вас ваши камеры, и обеспечиваем ремонт наилучшего качества. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что гарантирует долговечность и надежность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются владельцы видеокамер, включают проблемы с записью, поврежденный объектив, ошибки ПО, неработающие разъемы и механические повреждения. Для устранения этих проблем наши профессиональные техники оказывают ремонт записи, объективов, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы гарантируете себе качественный и надежный центр ремонта видеокамер в москве.
Подробная информация доступна на сайте: https://remont-videokamer-ink.ru
Houses in Montenegro for sale Montenegro real estate for sale
1win минимальный депозит bonus code 1win gratis
Наша мастерская предлагает надежный мастер по ремонту варочных панелей рядом различных марок и моделей. Мы осознаем, насколько необходимы вам ваши плиты, и обеспечиваем ремонт высочайшего уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что гарантирует долговечность и надежность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются владельцы варочных панелей, включают проблемы с нагревом, неработающие сенсоры, неисправности программного обеспечения, проблемы с подключением и повреждения корпуса. Для устранения этих поломок наши профессиональные техники проводят ремонт нагревательных элементов, сенсоров, ПО, разъемов и механических компонентов. Обратившись к нам, вы получаете качественный и надежный мастер по ремонту варочных панелей на выезде.
Подробная информация размещена на сайте: https://remont-varochnyh-paneley-hit.ru
Возможности выигрыша в онлайн казино, где возможности бесконечны.
Попробуйте свои силы вместе с нами, и ощутите атмосферу азарта и волнения.
Обнаружьте свое новое казино онлайн, и начните играть уже сегодня.
Ощутите волнение в режиме реального времени, не тратя время на поездки.
Выигрывайте крупные суммы при помощи наших игр, и почувствуйте себя настоящим чемпионом.
Коммуницируйте и соревнуйтесь с игроками со всего мира, и покажите свои лучшие результаты.
Играйте и выигрывайте, получая щедрые бонусы, которые увеличат ваши шансы на победу.
Играйте и наслаждайтесь азартом в каждой ставке, и погрузитесь в мир бесконечных перспектив.
Станьте частью казино онлайн и получите доступ к эксклюзивным играм, сделав всего несколько кликов мыши.
казино онлайн онлайн казино беларусь .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт ноутбуков fujitsu siemens, можете посмотреть на сайте: ремонт ноутбуков fujitsu siemens в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mostbet penalty mostbet original
mostbet aviator game mostbet
Наш сервисный центр предлагает надежный отремонтировать видеокарту в москве любых брендов и моделей. Мы осознаем, насколько необходимы вам ваши видеокарты, и готовы предложить сервис высочайшего уровня. Наши опытные мастера оперативно и тщательно выполняют работу, используя только качественные детали, что предоставляет надежность и долговечность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются владельцы видеокарт, включают проблемы с вентиляцией, неисправность памяти, неисправности разъемов, неисправность контроллера и программные сбои. Для устранения этих неисправностей наши опытные мастера проводят ремонт системы охлаждения, памяти, разъемов, контроллеров и ПО. Доверив ремонт нам, вы получаете долговечный и надежный ремонт видеокарты рядом.
Подробная информация размещена на сайте: https://remont-videokart-biz.ru
WOW just what I was looking for. Came here by searching for %meta_keyword%
Обратные Ссылки Guest, WP блоги, forums прогон XRumer 23 StrongAI
gtbike.ru holest.ru/novosti/9929-turniket-perko-jeffektivnyj-kontrol-dostupa-v-obschestvennyh-mestah.html .
https://maxpillsformen.com/# Cialis 20mg price
https://fastpillseasy.com/# best online ed pills
http://maxpillsformen.com/# Cialis 20mg price in USA
Cialis without a doctor prescription: buy cialis online – Tadalafil Tablet
buy viagra here FastPillsForMen generic sildenafil
http://fastpillseasy.com/# order ed meds online
нарколог на дом вывод из запоя ростов нарколог на дом вывод из запоя ростов .
нижний новгород отели и гостиницы нижний новгород отели и гостиницы .
вывод из запоя ростов-на-дону вывод из запоя ростов-на-дону .
cheap viagra: FastPillsForMen – buy Viagra over the counter
Наши специалисты предлагает высококачественный сервис ремонта варочных панелей адреса различных марок и моделей. Мы знаем, насколько значимы для вас ваши плиты, и стремимся предоставить услуги наилучшего качества. Наши опытные мастера оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что обеспечивает надежность и долговечность проведенных ремонтов.
Наиболее частые неисправности, с которыми сталкиваются владельцы варочных панелей, включают неработающие конфорки, неисправности сенсоров, программные сбои, неисправности разъемов и поломки компонентов. Для устранения этих неисправностей наши опытные мастера выполняют ремонт нагревательных элементов, сенсоров, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы обеспечиваете себе долговечный и надежный ремонт варочных панелей в москве.
Подробная информация размещена на сайте: https://remont-varochnyh-paneley-hit.ru
Наш сервисный центр предлагает высококачественный сервисный ремонт видеокарт в москве всех типов и брендов. Мы осознаем, насколько необходимы вам ваши графические адаптеры, и стремимся предоставить услуги высочайшего уровня. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только качественные детали, что гарантирует надежность и долговечность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели графических адаптеров, включают перегрев, поломку памяти, неработающие разъемы, неисправность контроллера и ошибки драйверов. Для устранения этих неисправностей наши квалифицированные специалисты оказывают ремонт системы охлаждения, памяти, разъемов, контроллеров и ПО. Доверив ремонт нам, вы обеспечиваете себе качественный и надежный ремонт видеокарт в москве.
Подробная информация доступна на сайте: https://remont-videokart-biz.ru
zagorski pijesak immobilien Montenegro
лучшие фильмы 2009 смотреть онлайн лучшие фильмы 2005 смотреть онлайн
https://maxpillsformen.com/# Cialis without a doctor prescription
Cheap generic Viagra FastPillsForMen cheap viagra
Generic Tadalafil 20mg price: Max Pills For Men – Cialis without a doctor prescription
http://maxpillsformen.com/# Generic Tadalafil 20mg price
카지노 충전 카지노 충전 .
Реєстрація на Мостбет займає лише кілька хвилин | Мостбет сайт пропонує унікальні акції для своїх клієнтів mostbet. | Реєстрація на сайті Мостбет – це ваш перший крок до великих виграшів | Грайте на Mostbet і скористайтеся вигідними акціями Mostbet casino.
Наша мастерская предлагает надежный сервисный ремонт гироскутеров с гарантией всех типов и брендов. Мы осознаем, насколько значимы для вас ваши гироскутеры, и стремимся предоставить услуги первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только качественные детали, что предоставляет долговечность и надежность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются владельцы гироскутеров, включают проблемы с батареей, неисправности двигателя, поломку контроллера, неработающие сенсоры и механические повреждения. Для устранения этих проблем наши профессиональные техники оказывают ремонт батарей, двигателей, контроллеров, гиросенсоров и механических компонентов. Обратившись к нам, вы гарантируете себе надежный и долговечный отремонтировать гироскутер адреса.
Подробная информация представлена на нашем сайте: https://remont-giroskuterov-key.ru
Cheapest Sildenafil online: FastPillsForMen – cheapest viagra
baosici Montenegro Montenegro immobilien
лучшие фильмы 2005 смотреть онлайн смотреть фильмы бесплатно в Европе
Viagra online price: order viagra – Buy Viagra online cheap
Upon stumbling upon this platform, I was interested by its interface and lively community. Initially, I was hesitant, but after checking it out, I found it to be a welcoming space for open-minded discussions about local parties. The registration process was straightforward, and once inside, I was amazed by the diversity of topics. From casual chats to in-depth discussions, there’s something for anyone. The members are respectful and interactive, which boosts the overall experience. A notable feature is the ability to interact through private messages. It’s simple to meet others who have similar interests, and the search options are comprehensive. The emphasis on security and security is also praiseworthy, which is crucial for a site like this. In conclusion, if you’re seeking a platform to experience new concepts and meet like-minded people, I recommend giving it a try. Here’s the link: https://indianswingers.party/ It’s definitely worth a visit if you’re curious in what the community has to offer.
п»їcialis generic Generic Cialis price Generic Cialis price
Наш сервисный центр предлагает высококачественный сервисный центр по ремонту видеокамер с гарантией различных марок и моделей. Мы осознаем, насколько важны для вас ваши видеокамеры, и готовы предложить сервис высочайшего уровня. Наши профессиональные техники работают быстро и аккуратно, используя только оригинальные запчасти, что предоставляет длительную работу проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются обладатели камер, включают неисправности записи, проблемы с объективом, неисправности программного обеспечения, неработающие разъемы и повреждения корпуса. Для устранения этих неисправностей наши опытные мастера оказывают ремонт записи, объективов, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы обеспечиваете себе качественный и надежный починить видеокамеру с гарантией.
Подробная информация размещена на сайте: https://remont-videokamer-ink.ru
http://fastpillsformen.com/# Viagra without a doctor prescription Canada
вывод из запоя на дому ростов цены вывод из запоя на дому ростов цены .
https://maxpillsformen.com/# п»їcialis generic
Наш сервисный центр предлагает надежный мастер по ремонту гироскутера на выезде любых брендов и моделей. Мы осознаем, насколько важны для вас ваши гироскутеры, и готовы предложить сервис первоклассного уровня. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что обеспечивает долговечность и надежность наших услуг.
Наиболее общие проблемы, с которыми сталкиваются пользователи электроскутеров, включают неисправную батарею, неработающий двигатель, поломку контроллера, неработающие сенсоры и поломки корпуса. Для устранения этих поломок наши квалифицированные специалисты оказывают ремонт батарей, двигателей, контроллеров, гиросенсоров и механических компонентов. Обращаясь в наш сервисный центр, вы получаете надежный и долговечный центр ремонта гироскутеров с гарантией.
Подробная информация доступна на сайте: https://remont-giroskuterov-key.ru
Ваша удача ждет вас в онлайн казино, где каждый может стать победителем.
Получайте азарт и адреналин вместе с нами, и ощутите атмосферу азарта и волнения.
Сделайте свой выбор в пользу казино онлайн, и начните выигрывать уже сегодня.
Почувствуйте атмосферу настоящего казино в режиме онлайн, не тратя время на поездки.
Играйте в увлекательные игры с высокими коэффициентами выигрыша, и станьте настоящим мастером азарта.
Играйте вместе с друзьями и соперниками со всего мира, и покажите свои лучшие результаты.
Начните играть и получите ценные подарки, которые принесут вам еще больше радости и азарта.
Почувствуйте свободу и возможность выбора в каждой игре, и погрузитесь в мир бесконечных перспектив.
Получите доступ к уникальным играм и выигрывайте крупные суммы, с минимум затрат времени и усилий.
онлайн казино казино онлайн беларусь .
Viagra tablet online buy viagra online Viagra online price
ed pills for sale: cheap cialis – affordable ed medication
Возможности выигрыша в онлайн казино, где каждый может стать победителем.
Попробуйте свои силы вместе с нами, и ощутите атмосферу азарта и волнения.
Обнаружьте свое новое казино онлайн, и начните зарабатывать уже сегодня.
Играйте и побеждайте в режиме живого казино, не покидая своего уютного кресла.
Ставьте на победу с нашими играми, и станьте настоящим мастером азарта.
Играйте вместе с друзьями и соперниками со всего мира, и докажите свое превосходство.
Начните играть и получите ценные подарки, которые принесут вам еще больше радости и азарта.
Ощутите азарт в каждой игре казино онлайн, и погрузитесь в мир бесконечных перспектив.
Станьте частью казино онлайн и получите доступ к эксклюзивным играм, сделав всего несколько кликов мыши.
онлайн казино беларусь онлайн казино .
Cheap generic Viagra Generic Viagra for sale or Viagra Tablet price
https://maps.google.com.au/url?sa=t&url=https://fastpillsformen.com Generic Viagra for sale
Cheap Viagra 100mg Viagra online price and over the counter sildenafil buy Viagra over the counter
http://fastpillseasy.com/# online ed pharmacy
Generic Tadalafil 20mg price Generic Cialis without a doctor prescription or Buy Tadalafil 10mg
https://www.google.bt/url?sa=t&url=https://maxpillsformen.com buy cialis pill
buy cialis pill Cheap Cialis and cheapest cialis Generic Cialis price
Cheapest Sildenafil online viagra canada or best price for viagra 100mg
https://images.google.com.do/url?q=https://fastpillsformen.com Viagra without a doctor prescription Canada
Cheapest Sildenafil online cheapest viagra and viagra canada Cheapest Sildenafil online
Наша мастерская предлагает надежный отремонтировать видеокамеру всех типов и брендов. Мы знаем, насколько важны для вас ваши видеокамеры, и готовы предложить сервис первоклассного уровня. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что предоставляет длительную работу проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются владельцы видеокамер, включают неработающую запись, проблемы с объективом, неисправности программного обеспечения, неработающие разъемы и повреждения корпуса. Для устранения этих поломок наши профессиональные техники оказывают ремонт записи, объективов, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы обеспечиваете себе качественный и надежный мастерская по ремонту видеокамеры с гарантией.
Подробная информация размещена на сайте: https://remont-videokamer-ink.ru
Смотрите любимые дорамы https://dorama2025.ru онлайн в HD-качестве! Огромный выбор корейских, китайских, японских и тайваньских сериалов с профессиональной озвучкой и субтитрами.
Viagra without a doctor prescription Canada: Fast Pills For Men – Generic Viagra online
cheapest cialis cialis for sale or cialis for sale
http://images.google.co.ve/url?q=https://maxpillsformen.com Cheap Cialis
Cialis 20mg price Tadalafil price and Cialis without a doctor prescription Cialis 20mg price
Погода в Баку Лучшие места для получения кредита
https://fastpillseasy.com/# cheap ed pills online
вывод из запоя на дому вывод из запоя на дому .
cialis for sale Generic Cialis without a doctor prescription Tadalafil Tablet
where to get ed pills: affordable ed medication – best online ed treatment
Generic Viagra for sale: Buy Viagra online cheap – Order Viagra 50 mg online
срочная наркологическая помощь срочная наркологическая помощь .
наркологическая скорая бесплатная [url=http://www.advance2.ukrbb.net/viewtopic.php?f=2&t=788]наркологическая скорая бесплатная [/url] .
онлайн ридер манхвы детектив топ манхва 2009
1win descarga 1win pro
https://maxpillsformen.com/# Buy Tadalafil 20mg
パチスロ ロストプラネット2
k8 カジノ 評判
パチンコは、ルールを覚えればすぐに楽しめるので、初心者でも安心です。気軽に始められます。
CR真・花の慶次 Ver.399
https://idtc.jp/tags/%E3%83%9F%E3%83%AA%E3%82%AA%E3%83%B3%E3%82%B4%E3%83%83%E3%83%89
歴史的なキャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
吉宗極
k8 カジノ 出金
押忍!サラリーマン番長
バイオハザード6
シティーハンター
L マクロスフロンティア 4
https://xn--k8-9g4a3b4f.site/tags/%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3-%E6%9C%80%E6%96%B0-%E4%BB%A3
パチンコの世界には、運だけではなく、戦略も求められます。自分の考えを試す楽しさがあります。
CR真・北斗無双 Ver.319(2:1)
https://xn--k8-9g4a3b4f.site/tags/%E5%85%AB%E5%B9%A1-%E5%B1%B1-%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
闘神雷電 -花田勝-
https://xn--k8-9g4a3b4f.site/shinbun/post-13098.html
サウンドエフェクトが迫力満点で、プレイ中に盛り上がります。特にバトル時が楽しい。
Наш сервисный центр предлагает профессиональный официальный ремонт видеокарт на дому любых брендов и моделей. Мы знаем, насколько необходимы вам ваши графические карты, и стремимся предоставить услуги первоклассного уровня. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что гарантирует долговечность и надежность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются обладатели графических адаптеров, включают перегрев, поломку памяти, неработающие разъемы, неисправность контроллера и программные сбои. Для устранения этих поломок наши опытные мастера оказывают ремонт системы охлаждения, памяти, разъемов, контроллеров и ПО. Обратившись к нам, вы получаете надежный и долговечный отремонтировать видеокарту на выезде.
Подробная информация доступна на сайте: https://remont-videokart-biz.ru
Buy generic 100mg Viagra online buy viagra online Viagra online price
where to buy erectile dysfunction pills erectile dysfunction online or ed online prescription
https://images.google.sm/url?sa=t&url=https://fastpillseasy.com buy erectile dysfunction medication
pills for ed online low cost ed pills and cheap ed meds affordable ed medication
http://fastpillseasy.com/# cheapest ed pills
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков asus, можете посмотреть на сайте: ремонт ноутбуков asus адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
crypto drainer source code – what is crypto drainer, crypto drainer download
кликните сюда Mango-Office отзывы клиентов
해외축구중계
стероиды для набора мышечной купить стероиды для набора мышечной массы
выберите ресурсы Татуаж бровей Котлас
узнать больше Здесь Mango-Office как подключить
카지노 순위 카지노 순위 .
company montenegro residence company formation in Montenegro
Cialis over the counter: Max Pills For Men – Buy Cialis online
cialis for sale buy cialis online Generic Cialis price
http://fastpillsformen.com/# Viagra generic over the counter
Order Viagra 50 mg online Viagra generic over the counter or Viagra without a doctor prescription Canada
https://greencircle.vmturbo.com/external-link.jspa?url=http://fastpillsformen.com viagra without prescription
buy Viagra online sildenafil over the counter and Order Viagra 50 mg online Sildenafil 100mg price
teleprompter online text teleprompter online
Cialis 20mg price: MaxPillsForMen – Generic Cialis without a doctor prescription
viagra without prescription: FastPillsForMen – Buy Viagra online cheap
ablauf immobilienkauf immobilien Montenegro
Наш сервисный центр предлагает профессиональный срочный ремонт видеокарт различных марок и моделей. Мы понимаем, насколько значимы для вас ваши графические адаптеры, и обеспечиваем ремонт первоклассного уровня. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что предоставляет длительную работу наших услуг.
Наиболее частые неисправности, с которыми сталкиваются пользователи графических карт, включают неисправности системы охлаждения, поломку памяти, неисправности разъемов, сбои контроллера и программные сбои. Для устранения этих проблем наши квалифицированные специалисты проводят ремонт системы охлаждения, памяти, разъемов, контроллеров и ПО. Обратившись к нам, вы обеспечиваете себе качественный и надежный мастер по ремонту видеокарт.
Подробная информация представлена на нашем сайте: https://remont-videokart-biz.ru
buy viagra here Sildenafil Citrate Tablets 100mg or Buy generic 100mg Viagra online
http://www.everettpost.com/Redirect.aspx?destination=http://fastpillsformen.com/ Buy generic 100mg Viagra online
sildenafil 50 mg price sildenafil 50 mg price and Buy Viagra online cheap Generic Viagra online
вызвать наркологическую помощь вызвать наркологическую помощь .
https://fastpillsformen.com/# Viagra online price
八代將軍-自動回転
スマスロ マクロス 終了画面
明るいデザインと楽しげな演出が魅力。遊んでいるだけで元気が出ます。
CR聖戦士ダンバイン
https://www.k8-casino.org/Articles/3043.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR牙狼金色になれ
北斗の拳 ぼうきょうせい先バレ確率
めんそーれ(雷電ver.)(特殊大賞燈)
戦国パチスロ花の慶次戦極めし傾奇者の宴~
戦国乙女2~深淵に輝く気高き将星
CR蒼天の拳2
https://www.girnecasinogirisi.org/article/2165.html
美しいグラフィックと豪華な演出が魅力。神々の力を感じます。
CR北斗の拳7 転生
https://injapan.today/news/id12817.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
CR牙狼FINAL Ver.399
https://xn--k8-9g4a3b4f.site/tags/%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3-%E3%83%AB%E3%83%91%E3%83%B3-%E4%B8%89%E4%B8%96
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
Останні новини Черкас https://18000.ck.ua та Черкаської області. Важливі новини про політику, бізнес, спорт, корупцію у владі на сайті 18000 ck.ua.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков asus рядом, можете посмотреть на сайте: ремонт ноутбуков asus в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
ed pills for sale cheapest ed online or cheapest ed meds
https://images.google.sc/url?q=https://fastpillseasy.com ed pills
pills for erectile dysfunction online online ed treatments and best online ed pills ed medicines online
скорая наркологическая помощь скорая наркологическая помощь .
лучшие манхвы читать 2012 топ манхва 2002
Poh. https://chromewebstore.google.com/detail/metamask/nkbihfbeogaeaoehlefnkodbefgpgknn
cost of ed meds FastPillsEasy online ed prescription
Buy Tadalafil 10mg: Max Pills For Men – Cheap Cialis
grundstuck in der nahe kaufen Montenegro immobilien von privat kaufen
Adopting digital tools for supplier risk management has saved us countless hours—an excellent choice for efficiency.
https://fastpillseasy.com/# best online ed pills
online ed treatments cheap ed meds online or where to get ed pills
http://vapingblips.com/proxy.php?link=https://fastpillseasy.com how to get ed meds online
low cost ed meds online ed medicines and best ed meds online top rated ed pills
http://maxpillsformen.com/# cialis for sale
green supply chain has become our go-to resource for effective procurement management.
Kantorbola link alternatif login terbaru 2025 . Kunjungi link resmi situs kantor bola untuk melakukan permainan dan pendaftaran
http://www.animadoresdefestaembh.eventopanoramico.com.br/especificos/eventopanoramico/listagem_cadastro_email.asp?CLI_SEQ=681925&CLI_DSC_INSTA=https%3A%2F%2Fbeaunex.net/bbs/board.php%3Fbo_table%3Dfree%26wr_id%3D360786
https://ignitebeanies.com/
FOSIL4D
Наша мастерская предлагает надежный мастер по ремонту гироскутера всех типов и брендов. Мы осознаем, насколько необходимы вам ваши самобалансирующиеся скутеры, и стремимся предоставить услуги первоклассного уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что обеспечивает надежность и долговечность наших услуг.
Наиболее частые неисправности, с которыми сталкиваются обладатели самобалансирующихся скутеров, включают поломку аккумулятора, проблемы с мотором, неисправный контроллер, проблемы с гиросенсорами и повреждения рамы. Для устранения этих поломок наши опытные мастера оказывают ремонт батарей, двигателей, контроллеров, гиросенсоров и механических компонентов. Доверив ремонт нам, вы гарантируете себе качественный и надежный центр ремонта гироскутеров с гарантией.
Подробная информация размещена на сайте: https://remont-giroskuterov-key.ru
https://maxpillsformen.com/# Cheap Cialis
https://maxpillsformen.com/# Cheap Cialis
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Sildenafil Citrate Tablets 100mg FastPillsForMen.com Cheap Sildenafil 100mg
viagra without prescription: buy viagra online – Viagra generic over the counter
Tadalafil price Buy Tadalafil 5mg or Generic Tadalafil 20mg price
https://maps.google.com.np/url?sa=t&url=https://maxpillsformen.com Cialis 20mg price
cheapest cialis Buy Cialis online and Buy Tadalafil 5mg Generic Cialis price
Buy Tadalafil 20mg: Generic Cialis without a doctor prescription – cheapest cialis
http://fastpillseasy.com/# cheap ed pills
novi Montenegro immobilien Montenegro
Cialis over the counter п»їcialis generic or Buy Cialis online
https://maps.google.rs/url?sa=t&url=https://maxpillsformen.com Cialis 20mg price in USA
Cialis 20mg price in USA Cialis 20mg price in USA and Cialis over the counter Generic Tadalafil 20mg price
buy viagra here buy viagra here or Buy generic 100mg Viagra online
https://clients1.google.com.ag/url?q=https://fastpillsformen.com Cheapest Sildenafil online
Sildenafil 100mg price Sildenafil Citrate Tablets 100mg and Cheap Viagra 100mg Cheap generic Viagra online
https://fastpillsformen.com/# Order Viagra 50 mg online
Generic Viagra online sildenafil 50 mg price or Cheap Sildenafil 100mg
https://creativecommons.org/choose/results-one?q_1=2&q_1=1&field_commercial=n&field_derivatives=sa&field_jurisdiction=&field_format=Text&field_worktitle=Blog&field_attribute_to_name=Lam+HUA&field_attribute_to_url=http://fastpillsformen.com Cheap generic Viagra online
generic sildenafil sildenafil over the counter and over the counter sildenafil best price for viagra 100mg
edmeds fast pills easy best online ed pills
Generic Cialis without a doctor prescription: MaxPillsForMen.com – Buy Tadalafil 5mg
grundstuck in der nahe kaufen immobilie Montenegro
манхва на русском языке ужасы манхва на русском языке на ПК
вызвать наркологическую помощь вызвать наркологическую помощь .
наркологическая срочная помощь [url=https://business.0pk.me/viewtopic.php?id=37974/]наркологическая срочная помощь[/url] .
вызов нарколога на дом частная скорая помощь [url=https://aqvakr.forum24.ru/?1-7-0-00011604-000-0-0-1730875452]https://aqvakr.forum24.ru/?1-7-0-00011604-000-0-0-1730875452[/url] .
https://fastpillsformen.com/# Cheap Viagra 100mg
ip nvr приложение для камеры видеонаблюдения на пк
Cialis 20mg price Max Pills For Men Generic Cialis price
闘神雷電 -花田勝-
ミスティーノ出金
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
CRサイボーグ009VSデビルマン
https://casinos.town/tag/xing-fen-tosheng-li-417
簡単なルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
P 新世紀エヴァンゲリオン15 未来への咆哮
ジャギ 継続率
CR 北斗の拳6 天翔百裂
P とある魔術の禁書目録 2
パチスロ ガールズ&パンツァー
沖ドキ! 25
https://www.k8.com.se/Articles/1811.html
人気アニメを基にしたパチスロで、ファンにはたまらない作品です。リアルな演出が楽しめます。
ビーストサップ
https://xn--k8-9g4a3b4f.site/yosou/post-249.html
パチンコは、友人や家族と一緒に楽しむのに最適です。共に楽しむことで絆が深まります。
めんそーれ琉球守護神
https://xn--k8-9g4a3b4f.site/tags/%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3-akb3-%E8%AA%87%E3%82%8A-%E3%81%AE-%E4%B8%98
美しいグラフィックと音楽が、プレイ中の没入感を高めます。
buy cialis pill: Buy Tadalafil 5mg – Generic Tadalafil 20mg price
Generic Cialis without a doctor prescription: MaxPillsForMen.com – buy cialis pill
https://fastpillsformen.com/# viagra canada
cialis for sale: MaxPillsForMen.com – Tadalafil price
вызвать наркологическую помощь вызвать наркологическую помощь .
как вызвать наркологическую скорую помощь в москве как вызвать наркологическую скорую помощь в москве .
Ищете качественные стероиды для набора мышечной? У нас вы найдете широкий выбор сертифицированной продукции для набора массы, сушки и улучшения спортивных результатов. Только проверенные бренды, доступные цены и быстрая доставка. Ваше здоровье и успех в спорте – наш приоритет! Заказывайте прямо сейчас!”
онлайн ридер манхвы в Корее топ манхва 2019
п»їcialis generic MaxPillsForMen.com cheapest cialis
http://maxpillsformen.com/# buy cialis pill
https://fastpillsformen.com/# buy Viagra over the counter
buy ed meds online best ed meds online or cheap ed treatment
http://albasu.jp/kyoto-quiz/_m/index.php?a=free_page/goto_mobile&referer=https://fastpillseasy.com where to get ed pills
pills for erectile dysfunction online erectile dysfunction medications online and cheapest ed medication ed drugs online
viagra without prescription Sildenafil Citrate Tablets 100mg or Viagra online price
https://images.google.com.vn/url?sa=t&rct=j&url=https://fastpillsformen.com over the counter sildenafil
viagra canada Generic Viagra for sale and Cheapest Sildenafil online Generic Viagra online
нові фільми онлайн жахи нові фільми онлайн мелодрами
кращі фільми 2001 онлайн кращі фільми онлайн бойовики
кращі фільми онлайн у Full HD дивитися фільм безкоштовно в США
CR北斗の拳6 拳王
スロットにゃんこ大戦争天井
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CR押忍!番長
https://www.k8casino.asia/Articles/3023.html
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
デビル メイ クライ クロス
インデックス スロット やめどき
ミリオンゴッド-神々の凱旋-
聖闘士星矢 海皇覚醒
BLOOD+ 二人の女王
バイオハザード6
https://www.casinobaccarat.eu/article/75.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
CR RAVE この世界こそが真実だ Ver.319
https://xn--k8-9g4a3b4f.site/shinbun/post-12324.html
美しいビジュアルと迫力ある演出が魅力。プレイ中に没入感が高まります。
リング呪いの7日間;
https://sumaslo.jahromblog.com/post-183/
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
cheapest viagra over the counter sildenafil or Viagra tablet online
https://images.google.tn/url?sa=t&url=https://fastpillsformen.com buy viagra here
Generic Viagra for sale Generic Viagra online and Sildenafil Citrate Tablets 100mg cheapest viagra
Cialis 20mg price Max Pills For Men Tadalafil price
Viagra Tablet price: FastPillsForMen.com – Viagra tablet online
buy cialis pill: Generic Cialis without a doctor prescription – Cialis over the counter
I love how you conveyed your thoughts here. You might enjoy browsing https://metasnap.org/.
cheap viagra: FastPillsForMen.com – buy Viagra online
https://fastpillsformen.com/# cheapest viagra
online erectile dysfunction medication low cost ed meds online or online ed medicine
http://images.google.co.th/url?q=https://fastpillseasy.com cheap ed drugs
online erectile dysfunction pills buying ed pills online and buy ed meds online low cost ed meds online
bs2web зеркало – blacksprut войти, blacksprut com зеркало сайта
High Purity – breakingbad, breaking bad marketplace
Cheap Cialis Cialis without a doctor prescription or Cialis without a doctor prescription
http://charitiesbuyinggroup.com/membersearch.aspx?returnurl=http://maxpillsformen.com Cialis 20mg price in USA
Tadalafil price Cheap Cialis and buy cialis pill Cheap Cialis
http://fastpillsformen.com/# sildenafil online
their website https://abacusmarket.me
Cheapest Sildenafil online buy viagra online sildenafil over the counter
wikipedia reference https://web-kaspawallet.com
Unlock Your Potential with EtherBank Crypto Investment
As the digital economy evolves, EtherBank offers unparalleled opportunities for individuals and businesses. EtherBank crypto investment is designed to provide secure and lucrative options for anyone looking to thrive in the blockchain era.
Why EtherBank Is the Right Choice
EtherBank simplifies the complexities of cryptocurrency. With an intuitive platform and transparent policies, investors can enjoy peace of mind while accessing cutting-edge tools.
The Power of EtherTalk Investment
For those seeking guidance, EtherTalk investment offers a wealth of resources. From beginner-friendly guides to advanced analytics, EtherTalk ensures you’re equipped with the knowledge to succeed in the crypto world.
Making Crypto Accessible to All
EtherBank’s mission is to democratize crypto investments. With low entry barriers and customizable plans, EtherBank crypto investment caters to all levels of expertise. Whether you’re starting small or managing a portfolio, EtherBank has you covered.
Start your journey with EtherBank today. Discover why thousands trust us as their gateway to financial growth.
ed meds online: cheap cialis – pills for ed online
Cheap Cialis Tadalafil price or Buy Tadalafil 5mg
http://www.google.com.bn/url?sa=t&url=https://maxpillsformen.com п»їcialis generic
<a href=http://www.hk-pub.com/forum/dedo_siteindex.php?q=Tadalafil Tablet Cialis 20mg price in USA and Cialis 20mg price Cialis 20mg price
why not check here https://web-lumiwallet.com
navigate to this site https://web-sollet.com
online ed medicine best online ed meds or boner pills online
https://www.google.com.jm/url?q=https://fastpillseasy.com erectile dysfunction online
online ed pharmacy ed medications cost and erectile dysfunction medications online buy erectile dysfunction pills
http://fastpillsformen.com/# sildenafil 50 mg price
рабочая ссылка на кракен – https kraken20 at entry login, kraken маркетплейс официальный сайт
http://fastpillsformen.com/# cheap viagra
список мфо на карту список МФО
cali weed in prague thc joint in prague
blog https://my-sollet.com/
купить eth – обмен монет через p2p платформы, ferma cc официальный сайт
http://maxpillsformen.com/# Cialis without a doctor prescription
диана шурыгина фото https://shuriginadiana.ru
Alt coin cc – Альткоин бестчейдж, альткоин bestchange
Buy Cialis online MaxPillsForMen.com Generic Cialis without a doctor prescription
https://fastpillseasy.com/# cheap boner pills
частная скорая наркологическая помощь частная скорая наркологическая помощь .
срочная наркологическая помощь в москве срочная наркологическая помощь в москве .
вызов нарколога на дом частная скорая помощь вызов нарколога на дом частная скорая помощь .
Generic Cialis without a doctor prescription: Tadalafil price – Generic Tadalafil 20mg price
Cheap Viagra 100mg generic sildenafil or over the counter sildenafil
https://www.google.com.kw/url?sa=t&url=https://fastpillsformen.com Generic Viagra online
sildenafil 50 mg price Viagra tablet online and Cheap generic Viagra online Cheapest Sildenafil online
buy viagra here: FastPillsForMen – buy Viagra online
смотреть фильмы бесплатно боевики фильмы 2024 смотреть онлайн с субтитрами
смотреть фильмы бесплатно с переводом лучшие фильмы онлайн триллеры
cheapest viagra Buy generic 100mg Viagra online or <a href=" http://ns.km1003.keymachine.de/php.php?a=cialis+without+doctor+prescription “>Sildenafil Citrate Tablets 100mg
http://www.nakayama-dr.jp/feed2js2/feed2js.php?src=http://fastpillsformen.com best price for viagra 100mg
Order Viagra 50 mg online Viagra Tablet price and Buy generic 100mg Viagra online Cheap Sildenafil 100mg
crypto com wallet address checke
Solutions for Monitoring USDT for Prohibitions and Operation Purity: Anti-Money Laundering Solutions
In the current realm of crypto assets, where rapid deals and anonymity are becoming the standard, monitoring the legality and purity of processes is necessary. In light of increased regulatory scrutiny over illicit finance and terrorism funding, the demand for robust tools to authenticate transactions has become a significant priority for cryptocurrency users. In this article, we will explore offered tools for checking USDT for embargoes and deal purity.
What is AML?
AML actions refer to a series of compliance actions aimed at stopping and discovering dirty money activities. With the rise of digital asset usage, AML standards have become notably crucial, allowing participants to operate digital holdings with assurance while lessening threats associated with restrictive measures.
USDT, as the widely regarded as the favored stablecoin, is extensively used in diverse transactions internationally. However, using USDT can present several threats, especially if your resources may associate to ambiguous or illegal transactions. To minimize these hazards, it’s essential to take advantage of solutions that inspect USDT for restrictive measures.
Available Services
1. Address Verification: Using customized tools, you can verify a specific USDT address for any connections to sanction lists. This assists uncover potential ties to illicit behaviors.
2. Transaction Conduct Evaluation: Some services extend evaluation of transfer chronology, crucial for assessing the lucidity of monetary transfers and spotting potentially hazardous operations.
3. Tracking Services: Professional monitoring solutions allow you to observe all deals related to your location, permitting you to swiftly identify concerning activities.
4. Hazard Statements: Certain solutions extend detailed concern summaries, which can be helpful for traders looking to guarantee the trustworthiness of their assets.
No matter of whether you are handling a large resource or conducting small deals, complying to AML norms helps avoid legal repercussions. Adopting USDT validation solutions not only defends you from capital declines but also contributes to forming a secure environment for all industry players.
Conclusion
Assessing USDT for sanctions and transfer cleanliness is becoming a necessary action for anyone enthusiastic to continue within the regulations and maintain high standards of openness in the digital asset domain. By collaborating with reliable services, you not only protect your investments but also contribute to the joint initiative in combating dirty money and terrorism funding.
If you are set to start utilizing these offerings, examine the existing services and choose the solution that most meets your demands. Bear in mind, data is your power, and prompt transaction validation can shield you from a variety of issues in the future.
наркологическая скорая http://www.klin.0pk.me/viewtopic.php?id=4428/ .
неотложная наркологическая помощь в москве justforum.bestforums.org/viewtopic.php?f=26&t=4785 .
Cialis over the counter [url=https://maxpillsformen.com/#]MaxPillsForMen.com[/url] cheapest cialis
https://maxpillsformen.com/# Tadalafil Tablet
Tadalafil Tablet: Max Pills For Men – п»їcialis generic
Для многих абонентов вопрос, как подключить роутер через GPON, оказывается важным при замене провайдера или модернизации оборудования. Подключение GPON-роутера Ростелеком требует грамотных настроек, чтобы устройство работало стабильно и предоставляло быстрое соединение. Получить подробности о том, Как подключить роутер gpon на netgate-kiev.blogspot.com можно по ссылке на сайте, который подробно раскрывает этот процесс. Важным моментом является правильное подключение роутера к GPON, чтобы не возникло сбоев в работе интернета.
Если вы хотите настроить свой роутер к GPON-модему, важно следовать четким инструкциям. Часто клиенты задумываются, как настроить GPON роутер МТС или Ростелеком, чтобы настроить сеть в доме или офисе. Настройка GPON через традиционный роутер также реализуемо, однако для этого нужно учесть особенности взаимодействия оборудования.
Источник: https://netgate-kiev.blogspot.com/
по вопросам Как подключить свой роутер к gpon – стучите в Telegram nef69
Мостбет – це одна з найбільш надійних платформ для ставок в Україні | Завантажте додаток Мостбет і отримайте доступ до всіх можливостей казино most bet. | Мостбет сайт – це ваш провідник у світ азарту та перемог | Грайте у казино Mostbet та відчуйте справжній драйв Мостбет вход.
吉宗(双姬版)
吉宗ライジングやめ時
演出が豪華で、観ているだけでも楽しめます。特にボーナス演出は圧巻です。
回胴黙示録カイジ3
https://www.casino-eldoa.com/article/162.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
戦国BASARA3
刃牙スロット 画面
沖ドキ! Vacances
戦国BASARA3
CR牙狼 魔戒ノ花XX
CRサイボーグ009VSデビルマン
https://xn--k8-9g4a3b4f.site/tags/%E3%83%8D%E3%82%AE%E3%81%BE-%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR吉宗4 天昇飛躍の極
https://xn--k8-9g4a3b4f.site/tags/%E3%82%B7%E3%83%B3%E3%83%95%E3%82%A9%E3%82%AE%E3%82%A2-%E6%89%93%E3%81%A1-%E6%96%B9-%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3
演出が単純明快で、ストレスなくプレイできるのが良いです。気軽に楽しめます。
スーパービンゴネオ
https://xn--k8-9g4a3b4f.site/tags/%E3%83%90%E3%82%A4%E3%82%AA-%E3%83%8F%E3%82%B6%E3%83%BC%E3%83%89-%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3-%E7%94%98
パチンコの演出に使われる音楽が印象的で、耳に残ります。ついつい口ずさんでしまうことも。
http://maxpillsformen.com/# Generic Tadalafil 20mg price
https://maxpillsformen.com/# Cialis 20mg price
https://maxpillsformen.com/# Generic Cialis price
Строительный портал https://bastet.com.ua ваш путеводитель в мире стройки! Подборка лучших материалов, контакты мастеров, проекты и лайфхаки. Создавайте уют и красоту с нашим сервисом!
ed meds cheap order ed pills or erectile dysfunction online
http://www.ahewar.org/links/dform.asp?url=http://fastpillseasy.com ed online meds
ed doctor online cheap ed pills online and erectile dysfunction online prescription where can i buy erectile dysfunction pills
Viagra without a doctor prescription Canada FastPillsForMen order viagra
It is necessary that such thoughts are spread as widely as possible in our society https://000-google-09.s3.eu-north-1.amazonaws.com/id-10.html
Like 9132
best online ed treatment: FastPillsEasy – ed medicines online
https://fastpillsformen.com/# Cheap generic Viagra
cheap viagra: Fast Pills For Men – Cheap Viagra 100mg
online ed drugs buy ed meds online or order ed pills
https://www.steinhaus-gmbh.de/redirect.php?lang=en&url=https://fastpillseasy.com buying ed pills online
ed medications cost best ed medication online and cheapest ed online best online ed pills
Нужны деньги срочно Займер онлайн на карту срочно без отказа – ваш быстрый выход! Подайте заявку из любого места, получите деньги в течение нескольких минут. Удобно, прозрачно, без скрытых комиссий.
Cialis 20mg price: Max Pills For Men – Cialis without a doctor prescription
https://maxpillsformen.com/# Cialis over the counter
Финансовые трудности? Решите их за минуты рейтинг займов онлайн с моментальным переводом на карту. Оформление онлайн, простые условия и никакого лишнего стресса. Ваш надежный финансовый помощник!
カイジ
牙狼ゴールドインパクト評価
パチンコは日本独特のエンターテイメントで、スリルと興奮が詰まっています。特に大当たりの瞬間は最高の快感です。
CR 聖戦士ダンバイン
https://xn--k8-9g4a3b4f.site/tags/%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3-%E7%81%AB%E6%9B%9C-%E3%82%B5%E3%82%B9%E3%83%9A%E3%83%B3%E3%82%B9-%E5%8A%87%E5%A0%B4-%E7%94%98
新しい台の演出が毎回楽しみです。特別感があります。
CRモンスターハンタ Ver.199
k8カジノ ログイン
CRミリオンゴッドライジング~ゼウス再び~
攻殻機動隊S.A.C. 2nd GIG
L エヴァンゲリオン ~未来への創造~
スマスロ北斗の拳
https://xn--k8-9g4a3b4f.site/shinbun/post-10403.html
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
十字架3
https://www.slotonlineqq.org/article/146.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。忍者の世界観が楽しめます。
L 主役は銭形 4
https://xn--k8-9g4a3b4f.site/shinbun/post-2142.html
お店によって雰囲気が異なるので、色々な場所で楽しむのが良いです。新しい体験が待っています。
online ed pills ed med online cheapest ed meds
order viagra viagra without prescription or sildenafil online
https://maps.google.ie/url?sa=t&url=https://fastpillsformen.com Viagra tablet online
generic sildenafil Cheap Viagra 100mg and Buy Viagra online cheap viagra without prescription
Tools for Verifying USDT for Sanctions and Transfer Integrity: AML Approaches
In the contemporary environment of digital currencies, where expedited transactions and anonymity are becoming the standard, supervising the validity and purity of activities is necessary. In view of heightened government investigation over illicit finance and terrorism funding, the necessity for reliable tools to verify deals has become a key priority for cryptocurrency users. In this article, we will discuss offered offerings for monitoring USDT for restrictive measures and transaction clarity.
What is AML?
Money Laundering Prevention strategies refer to a series of legal actions aimed at preventing and detecting money laundering activities. With the growth of virtual currency usage, AML measures have become notably crucial, allowing clients to operate digital assets confidently while minimizing risks associated with restrictive measures.
USDT, as the preeminent well-known stablecoin, is widely used in various deals globally. Yet, using USDT can carry several threats, especially if your monies may relate to opaque or unlawful maneuvers. To reduce these threats, it’s vital to take advantage of tools that assess USDT for sanctions.
Available Services
1. Address Authentication: Employing dedicated tools, you can inspect a particular USDT address for any links to restrictive lists. This assists uncover potential associations to illegal operations.
2. Operation Activity Examination: Some offerings make available scrutiny of transaction history, important for measuring the transparency of capital flows and identifying potentially risky activities.
3. Surveillance Systems: Professional monitoring tools allow you to observe all deals related to your wallet, facilitating you to swiftly detect dubious operations.
4. Risk Records: Certain tools provide detailed risk reports, which can be beneficial for participants looking to validate the integrity of their resources.
No matter of whether you are managing a significant fund or conducting small transactions, complying to AML guidelines helps steer clear of legal repercussions. Adopting USDT authentication tools not only shields you from economic declines but also aids to building a safe environment for all economic stakeholders.
Conclusion
Assessing USDT for restrictive measures and operation integrity is becoming a mandatory action for anyone motivated to stay within the law and support high criteria of transparency in the virtual currency industry. By working with trustworthy platforms, you not only safeguard your investments but also aid to the common effort in countering financial misconduct and financing of terrorism.
If you are set to start employing these offerings, review the accessible platforms and choose the solution that most fits your demands. Remember, information is your strength, and swift operation check can protect you from a variety of challenges in the coming times.
Viagra Tablet price Cheap Viagra 100mg or Viagra without a doctor prescription Canada
https://cse.google.la/url?sa=t&url=https://fastpillsformen.com Viagra tablet online
Cheapest Sildenafil online best price for viagra 100mg and buy Viagra over the counter Viagra generic over the counter
Tadalafil Tablet Tadalafil Tablet or Buy Tadalafil 10mg
https://clients1.google.gy/url?q=https://maxpillsformen.com Buy Tadalafil 10mg
cheapest cialis Buy Tadalafil 20mg and cialis for sale Tadalafil Tablet
order ed pills cheap ed medicine or cheapest ed medication
https://chyba.o2.cz/cs/?url=https://fastpillseasy.com ed meds online
where can i get ed pills best online ed treatment and online ed pharmacy where can i buy erectile dysfunction pills
http://fastpillsformen.com/# Cheap Viagra 100mg
https://maxpillsformen.com/# Cialis 20mg price in USA
Buy Tadalafil 20mg Generic Cialis without a doctor prescription or Cialis over the counter
https://toolbarqueries.google.lt/url?sa=t&url=https://maxpillsformen.com cheapest cialis
Cialis over the counter Buy Cialis online and Cialis without a doctor prescription Cialis 20mg price in USA
It is very important that people are finally starting to talk openly about such serious things https://000-google-09.s3.eu-north-1.amazonaws.com/id-10.html
Like 4504
https://fastpillseasy.com/# ed pills
cheapest ed treatment cheap cialis online ed drugs
наркологическая скорая помощь москва http://klin.0pk.me/viewtopic.php?id=4428 .
скорая наркологическая помощь скорая наркологическая помощь .
наркологическая скорая помощь москва [url=justforum.bestforums.org/viewtopic.php?f=26&t=4785]justforum.bestforums.org/viewtopic.php?f=26&t=4785[/url] .
discount ed meds: FastPillsEasy – ed treatment online
cheap ed drugs cheap ed meds online or buy erectile dysfunction pills
https://images.google.rs/url?sa=t&url=https://fastpillseasy.com ed prescriptions online
cheap ed pills online get ed meds today and cheap ed pills online ed meds
Строительный портал https://bms-soft.com.ua для тех, кто строит и ремонтирует! Узнайте о трендах, найдите мастеров, подберите материалы и получите ценные рекомендации.
Откройте для себя лучшие l2 серверы! Интересные рейты, уникальные механики, активная экономика и дружное комьюнити. Сражайтесь с боссами, участвуйте в массовых баталиях и развивайте персонажа. Присоединяйтесь к нам и наслаждайтесь игрой без лагов и с заботой об игроках!
Cialis 20mg price: MaxPillsForMen – buy cialis pill
срочное изготовление загранпаспорта срочное изготовление загранпаспорта .
http://maxpillsformen.com/# Cialis without a doctor prescription
срочно сделать загранпаспорт срочно сделать загранпаспорт .
It is necessary that such thoughts are spread as widely as possible in our society https://000-google-09.s3.eu-north-1.amazonaws.com/id-10.html
Like 8530
Everyone wants to be understood, even if they don’t always show it https://000-google-09.s3.eu-north-1.amazonaws.com/id-10.html
Like 3931
Cheapest Sildenafil online: Fast Pills For Men – Cheap Viagra 100mg
An incredibly accurate description of what happens around us every day, thanks to the author https://000-google-09.s3.eu-north-1.amazonaws.com/id-10.html
Like 6978
Cialis 20mg price in USA: MaxPillsForMen – cialis for sale
online erectile dysfunction pills cheap cialis ed online meds
http://fastpillsformen.com/# Buy generic 100mg Viagra online
http://fastpillseasy.com/# generic ed meds online
http://fastpillseasy.com/# cheap ed drugs
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков acer, можете посмотреть на сайте: ремонт ноутбуков acer сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Welcome to the Mohamed Salah https://mohamed-salah-en.com fansite! Find out everything about the Liverpool football great and the Egyptian national team. Goals, achievements, stats – it’s all here!
buy Viagra over the counter sildenafil online or Generic Viagra online
http://cse.google.off.ai/url?q=https://fastpillsformen.com Generic Viagra online
Sildenafil Citrate Tablets 100mg Viagra without a doctor prescription Canada and Generic Viagra online over the counter sildenafil
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт macbook pro m3 адреса, можете посмотреть на сайте: ремонт macbook pro m3 рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Tadalafil price buy cialis online Generic Cialis without a doctor prescription
cheap viagra Viagra Tablet price or sildenafil 50 mg price
https://toolbarqueries.google.com.sb/url?q=https://fastpillsformen.com best price for viagra 100mg
Order Viagra 50 mg online Sildenafil 100mg price and Cheap generic Viagra online Viagra Tablet price
http://fastpillseasy.com/# buying erectile dysfunction pills online
Lバキ 強くなりたくば喰らえ!!!
パチンコひまわり 台データ
定期的に新しい台が出るので、飽きずに楽しめます。ファンにはたまらないですね。
吉宗
https://www.rewardcasino.org/article/112.html
サウンドエフェクトが迫力満点で、プレイ中に盛り上がります。特にバトル時が楽しい。
CR苍天の拳天帰
アナザーゴッドハーデス 導入日
翔馬
シンドバッドアドベンチャーは榎本加奈子でどうですか
パチスロ北斗の拳 強敵
吉宗 2003
https://xn--k8-9g4a3b4f.site/tags/%E5%A4%95%E6%96%B9-%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3
パチンコの演出は、時に驚かされることがあります。毎回新しい発見があります。
CRモンスターハンタ
https://xn--k8-9g4a3b4f.site/tags/%E5%B9%B3%E5%92%8C-%E7%89%A9%E7%94%A3-%E3%83%91%E3%83%81%E3%83%B3%E3%82%B3
リーチ演出が多彩で、毎回新しい体験が待っています。飽きることがありません。
デビルサバイバー2 最後の7日間
https://www.secret-casino.org/article/465.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
Biography of Dutch footballer https://xavi-simons-bd.com midfielder for Paris Saint-Germain and the Netherlands national team Xavi Simons. Sports career, playing for RB Leipzig and Barcelona, ??participation in Euro 2024, achievements. Personal life, girlfriend, latest news in 2024.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков hp адреса, можете посмотреть на сайте: ремонт ноутбуков hp сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт macbook pro 16 сервис, можете посмотреть на сайте: ремонт macbook pro 16 сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Team Spirit – 2024 roster https://team-spirit-dota2.com news, match and tournament schedule, statistics, award list, rating, logo.
cheapest ed treatment cheap ed medicine or ed pills for sale
https://congnghebitcoin.com/proxy.php?link=https://fastpillseasy.com buy ed medication online
cheap ed online ed prescription and online prescription for ed ed drugs online
Buy Tadalafil 10mg: п»їcialis generic – Cialis over the counter
cheapest erectile dysfunction pills: cheap cialis – online ed meds
Buy Tadalafil 20mg Generic Cialis without a doctor prescription Buy Tadalafil 5mg
buy ed meds: fast pills easy – cheap ed meds online
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков acer в москве, можете посмотреть на сайте: срочный ремонт ноутбуков acer
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Execration is a legendary Dota 2 execration dota2 com eSports team that conquers the heights of world eSports with its skill and tactics.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт macbook pro m3, можете посмотреть на сайте: срочный ремонт macbook pro m3
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
cheapest erectile dysfunction pills online erectile dysfunction prescription or buying erectile dysfunction pills online
https://cse.google.ge/url?q=https://fastpillseasy.com best ed medication online
online ed medicine online prescription for ed and online erectile dysfunction cheap ed
https://fastpillsformen.com/# sildenafil over the counter
https://fastpillseasy.com/# ed online prescription
Viagra online price: Viagra online price – viagra canada
erectile dysfunction medications online fast pills easy cheap ed treatment
沖ドキ! 30LL
とある魔術の禁書目録スマスロ天井
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
Pフィーバー戦姫絶唱シンフォギア2 Ver.199
https://www.get-certified.net/slot-games/slot-games-fruit-party-from-ka-gaming-at-mgm-online-casino-oyal-vegas-casino.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
超時空要塞マクロス
キングハナハナ 設定示唆
CRモンスターハンター4
牙狼 守りし者
翔马(黑)
バジリスク~甲賀忍法帖~絆
https://www.jogadoresanonimos.org/article/155.html
簡単に遊べるルールで、初心者でも安心。気軽に挑戦できるのが良いです。
闇の花(特殊大賞燈);
https://injapan.today/news/id12118.html
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
牙狼 守りし者
https://www.mystino.bet/article/734.html
定期的に新しいイベントがあり、飽きずに楽しめます。ファンにはたまらないですね。
Generic Cialis price Generic Tadalafil 20mg price or Generic Tadalafil 20mg price
https://www.reed.co.uk/courses/awarding-bodies/ncfe?backUrl=https://maxpillsformen.com Buy Tadalafil 10mg
Cialis 20mg price in USA Tadalafil Tablet and Generic Cialis without a doctor prescription Generic Tadalafil 20mg price
BEKU4D
best online ed medication cheapest online ed meds or where can i buy erectile dysfunction pills
https://images.google.ad/url?q=https://fastpillseasy.com buy ed pills
ed treatment online generic ed meds online and cheap boner pills online ed medications
cheap viagra sildenafil online or Sildenafil 100mg price
https://www.google.com.tj/url?q=https://fastpillsformen.com Sildenafil 100mg price
generic sildenafil over the counter sildenafil and Viagra without a doctor prescription Canada cheap viagra
buy cialis pill Generic Cialis without a doctor prescription or buy cialis pill
http://www.faithaliveumc.com/System/Login.asp?id=35223&Referer=http://maxpillsformen.com cialis for sale
Buy Tadalafil 10mg п»їcialis generic and Buy Tadalafil 5mg Generic Cialis price
Thanks for sharing such a well-researched article. Don’t miss out on more great resources at https://metadmca.org/.
Sildenafil Citrate Tablets 100mg Viagra without a doctor prescription Canada or Viagra online price
http://www.gensuikin.org/i/index.cgi?id=1&mode=redirect&no=242&ref_eid=110&url=http://fastpillsformen.com buy Viagra over the counter
Cheap generic Viagra online Buy generic 100mg Viagra online and sildenafil over the counter Viagra Tablet price
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков hp адреса, можете посмотреть на сайте: ремонт ноутбуков hp сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональная откачка канализации в Слуцке, круглосуточно.
Не откладывайте проблему с канализацией в Слуцке, по выгодной цене.
Экстренная откачка канализации в Слуцке, со скидкой.
Эффективная откачка канализации в Слуцке, с оперативным выездом.
Экстренная откачка канализации в Слуцке, по доступной цене.
Профессиональная откачка канализации в Слуцке: решение вопроса быстро, с выездом в любую точку города.
Услуги по откачке канализации в Слуцке, по выгодной стоимости.
Экстренная откачка канализации в Слуцке, по доступной цене.
Откачка канализации в Слуцке: оперативно и качественно, с выездом в течение часа.
откачка канализации в Слуцке очистка выгребных ям в Слуцке .
рейтинг процессоров amd https://www.topcpu.ru .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт macbook pro 16, можете посмотреть на сайте: ремонт macbook pro 16 адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
вывод из запоя круглосуточно ростов-на-дону вывод из запоя круглосуточно ростов-на-дону .
вывод из запоя в стационаре ростов-на-дону http://vyvod-iz-zapoya-rostov239.ru/ .
Buy Cialis online: buy cialis online – Tadalafil price
best ed pills online FastPillsEasy cheap erection pills
Подробнее https://zelenka.guru/articles/
Viagra without a doctor prescription Canada: Cheap Viagra 100mg – Sildenafil 100mg price
cheapest ed online generic ed meds online or buy erectile dysfunction medication
https://redirect.camfrog.com/redirect/?url=http://fastpillseasy.com/ online erectile dysfunction prescription
buying ed pills online online erectile dysfunction prescription and get ed meds today where to get ed pills
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин spidem цены, можете посмотреть на сайте: ремонт кофемашин spidem цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
http://maxpillsformen.com/# Generic Cialis price
카지노 순위 카지노 순위 .
https://maxpillsformen.com/# Generic Cialis price
рейтинг процессоров для игр http://topcpu.ru/ .
Buy generic 100mg Viagra online: Buy Viagra online cheap – best price for viagra 100mg
cheap ed medicine fast pills easy buy erectile dysfunction treatment
Howdy! Do you know if they make any plugins to safeguard against hackers? I’m kinda paranoid about losing everything I’ve worked hard on. Any tips?
https://bike-drive.com.ua/diy-headlight-sealing-guide.html
okada4d
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин spidem в москве, можете посмотреть на сайте: срочный ремонт кофемашин spidem
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Главная https://lzt.market/
п»їcialis generic: MaxPillsForMen.com – Generic Tadalafil 20mg price
buy viagra here Viagra generic over the counter or Viagra online price
https://www.lnfcu.com/helpers/choice.asp?h=http://fastpillsformen.com/ cheap viagra
buy Viagra over the counter buy Viagra online and Cheap generic Viagra online over the counter sildenafil
Viagra without a doctor prescription Canada: buy Viagra online – Viagra generic over the counter
webcam surveillance software free cctv remote monitoring software
Viagra tablet online cheap viagra sildenafil over the counter
Скрипт обменника электронных валют https://richexchanger.com надежное решение для запуска вашего обменного сервиса. Полная автоматизация процессов, интеграция популярных платежных систем, защита данных и удобный интерфейс.
Натуральные молочные продукты https://gastrodachavselug2.ru свежесть и качество с заботой о вашем здоровье! Широкий выбор: молоко, творог, сметана, сыры. Только натуральные ингредиенты, без консервантов и добавок.
Подробнее https://zelenka.guru/forums/760/
generic sildenafil over the counter sildenafil or sildenafil online
http://www.6.7ba.biz/out.php?url=https://fastpillsformen.com/ buy Viagra over the counter
Order Viagra 50 mg online buy Viagra over the counter and Order Viagra 50 mg online best price for viagra 100mg
купить аттестат в иркутске
Cheapest Sildenafil online: Fast Pills For Men – Viagra generic over the counter
翔馬
モンキーターン やめ時
パチンコは、友人や家族と一緒に楽しむのに最適です。共に楽しむことで絆が深まります。
沖ドキ! 30LL
https://www.ja-securities.jp/?ArticlesID=27493559.html
パチンコの演出は時に驚かされることも多く、毎回期待感を持って遊べます。ワクワクが止まりません。
CR龍が如く見参!天照祗園編
シンフォギア3 サンジェルマンモード
L マクロスフロンティア 4
CR聖闘士星矢 星の運命
夜蝶飛翔
P 新世紀エヴァンゲリオン15 未来への咆哮
https://www.ja-securities.jp/?ArticlesID=27505229.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
CRヱヴァンゲリヲン10
https://www.ja-securities.jp/?ArticlesID=27533699.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
めんそーれ(雷電ver.)
https://www.ja-securities.jp/?ArticlesID=27467919.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
kush shop in prague kush delivery in prague
cannabis in prague thc gummies delivery in prague
thc vape for sale in prague cannabis delivery in prague
cannafood shop in prague buy cannabis in prague
kush for sale in prague weed shop in prague
ed meds on line cheap ed drugs or cheap erectile dysfunction pills
https://image.google.co.vi/url?q=https://fastpillseasy.com online erectile dysfunction pills
top rated ed pills order ed meds online and ed online treatment pills for erectile dysfunction online
http://fastpillsformen.com/# over the counter sildenafil
https://fastpillsformen.com/# Viagra online price
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин neff, можете посмотреть на сайте: ремонт кофемашин neff рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
pills for erectile dysfunction online fast pills easy ed pills cheap
thc joint in prague https://prague-drugstore.site
buy thc gummies in prague https://shopraha.site
weed in prague hashish shop in prague
thc joint for sale in prague https://pragueshop.site
weed shop in prague prague 420
вывод из запоя ростов и область вывод из запоя ростов и область .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин melitta, можете посмотреть на сайте: срочный ремонт кофемашин melitta
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
сравнение процессоров intel http://topcpu.ru/ .
ed medications online buy erectile dysfunction treatment or where can i buy ed pills
https://images.google.sn/url?sa=t&url=https://fastpillseasy.com ed medicines
online erectile dysfunction online ed medication and best online ed treatment erectile dysfunction drugs online
Betpriz
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips адреса, можете посмотреть на сайте: ремонт кофемашин philips цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт macbook air m1 цены, можете посмотреть на сайте: ремонт macbook air m1
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Tadalafil price Generic Cialis without a doctor prescription or Buy Cialis online
http://talewiki.com/cushion.php?https://maxpillsformen.com Generic Cialis without a doctor prescription
Cialis without a doctor prescription Cialis without a doctor prescription and Tadalafil Tablet Buy Cialis online
北斗の拳世紀末救世主伝説
レバブルとは
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
ミリオンゴッド神々の凱旋
https://www.ja-securities.jp/?ArticlesID=27539709.html
パチンコの世界には、運だけではなく、戦略も求められます。自分の考えを試す楽しさがあります。
獣王 王者の覚醒
[url=https://www.ja-securities.jp/?ArchiveID=27432319.html]
オンカジ入金不要ボーナス 最新[/url]
CR鉄拳2 闘神
GI優駿俱樂部
CRモンスターハンター4 Ver.319
青龍
https://www.ja-securities.jp/?ArticlesID=27468339.html
パチンコを通じて、運を試すドキドキ感が味わえます。勝ち負けにこだわらず楽しむのがポイントです。
鬼武者3
https://www.ja-securities.jp/?ArticlesID=27458309.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
バジリスク~甲賀忍法帖~絆
https://www.ja-securities.jp/?ArticlesID=27405019.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい発見が待っています。
best price for viagra 100mg Fast Pills For Men sildenafil online
online ed pills ed pills cheap or best online ed meds
http://mobile-bbs3.com/bbs/kusyon_b.php?http://fastpillseasy.com ed drugs online
online ed meds erectile dysfunction meds online and low cost ed pills ed medicines online
Buy Tadalafil 20mg Cheap Cialis or Cialis without a doctor prescription
http://cybermann.com/main.php?g2_view=core.UserAdmin&g2_subView=core.UserRecoverPassword&g2_return=https://maxpillsformen.com Cheap Cialis
Buy Tadalafil 5mg Cialis 20mg price in USA and Tadalafil price Buy Tadalafil 10mg
ed prescription online: cheap cialis – low cost ed meds online
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин neff рядом, можете посмотреть на сайте: ремонт кофемашин neff
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
stamp creator online stamp creator online .
вывод из запоя цены ростов вывод из запоя цены ростов .
erectile dysfunction online prescription: fast pills easy – ed prescriptions online
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт кофемашин melitta, можете посмотреть на сайте: срочный ремонт кофемашин melitta
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Sildenafil 100mg price Viagra online price or sildenafil 50 mg price
https://maps.google.co.il/url?sa=t&url=https://fastpillsformen.com cheap viagra
buy viagra here Cheap generic Viagra online and Sildenafil 100mg price Cheapest Sildenafil online
https://fastpillsformen.com/# Viagra tablet online
вывод из запоя в ростове на дону вывод из запоя в ростове на дону .
вывод из запоя капельница вывод из запоя капельница .
вывод из запоя дешево ростов вывод из запоя дешево ростов .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips рядом, можете посмотреть на сайте: ремонт кофемашин philips
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
FOSIL4D
3a娛樂城
娛樂城介紹
https://aaawin88.org/3acasino/
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин miele в москве, можете посмотреть на сайте: ремонт кофемашин miele рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт macbook air m1 сервис, можете посмотреть на сайте: ремонт macbook air m1 рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин delonghi цены, можете посмотреть на сайте: ремонт кофемашин delonghi адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://denemebonusuverensiteler25.com/# denemebonusuverensiteler25
Разнообразие фурнитуры для плинтуса, найдите идеальный вариант.
Качественная фурнитура для плинтуса, не подведут вас в эксплуатации.
Удобство монтажа фурнитуры для плинтуса, для быстрой установки.
Современные решения для отделки плинтуса, подчеркните стиль своего интерьера.
Фурнитура для плинтуса из экологически чистых материалов, для заботы об окружающей среде.
Модные цвета для элементов плинтуса, выберите подходящий вам вариант.
Эксклюзивные варианты фурнитуры для плинтуса, привнесите уникальность в интерьер.
Подсказки по правильной установке элементов плинтуса, для безупречного результата.
Декоративные элементы для фурнитуры плинтуса, добавьте шарм вашему интерьеру.
Изысканные решения для отделки плинтуса, для создания аристократичной атмосферы.
большой выбор плинтусов большой выбор плинтусов .
http://denemebonusuverensiteler25.com/# yat?r?ms?z deneme bonusu veren siteler
yeni deneme bonusu veren siteler: deneme bonusu veren siteler – deneme bonusu veren siteler
https://sweetbonanza25.com/# sweet bonanza guncel
チェインクロニクル
イルサローネ 泉佐野
明るいデザインと楽しげな演出が魅力。遊んでいるだけで元気が出ます。
押忍!番長
https://www.pgslot-casino.com/?ArticleID=992155818.html
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
八代將軍戰神版
ジンオウガ 希少 種
翔馬
戦コレ!【太平女君】徳川家康
デビルメイクライ4
Dororonえん魔くん メ~ラめら
https://www.turboslott.com/?ArticleID=931705818.html
友人と競い合うことで、より楽しめます。勝負の結果が気になります。
P ガラスの仮面M‐K1
https://www.diabetesdirectory.org/?ReviewID=954436818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
e Re:ゼロから始める異世界生活 season2M13
https://www.diabetesdirectory.org/?SitesID=955733818.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин miele сервис, можете посмотреть на сайте: ремонт кофемашин miele цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин delonghi, можете посмотреть на сайте: ремонт кофемашин delonghi
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://denemebonusuverensiteler25.com/# deneme bonusu veren yeni siteler
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone xs адреса, можете посмотреть на сайте: ремонт iphone xs адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Casino Siteleri: deneme bonusu veren casino siteleri – casino bahis siteleri
https://denemebonusuverensiteler25.com/# deneme bonusu veren siteler
sweet bonanza giris: sweet bonanza slot – sweet bonanza demo oyna
sweet bonanza guncel sweet bonanza oyna sweet bonanza kazanma saatleri
нарколог на дом вывод из запоя ростов нарколог на дом вывод из запоя ростов .
free online stamp maker http://www.stamp-creator-online0.com/ .
denemebonusuverensiteler25: yat?r?ms?z deneme bonusu veren siteler – deneme bonusu veren siteler
http://slotsiteleri25.com/# guvenilir slot siteleri
вывод из запоя ростов вывод из запоя ростов .
вывод из запоя цены ростов на дону вывод из запоя цены ростов на дону .
https://slotsiteleri25.com/# slot oyunlar? puf noktalar?
deneme bonusu veren siteler yeni denemebonusuverensiteler25 deneme bonusu veren yeni siteler
P地獄少女 四
ロト 6 当選 確率
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
夜蝶飛翔ー自動回転
https://www.ja-securities.jp/?GameID=953696818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
政宗
aゲーム ポーカー
CRサイボーグ009VSデビルマン
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
南国物語
南国物語
https://www.diabetesdirectory.org/?NewsID=937491818.html
各台によって異なる演出があり、どれを選ぶかも楽しみの一つです。自分の好みの台を見つける楽しさがあります。
アナザーゴッドハーデス-奪われたZEUSver
https://www.diabetesdirectory.org/?ArticleID=966043818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
Pワンパンマン
https://www.8casino8.com/?ArticleID=923143818.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
https://casinositeleri25.com/# Canl? Casino Siteleri
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone xs рядом, можете посмотреть на сайте: ремонт iphone xs цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://sweetbonanza25.com/# sweet bonanza slot
mobil bahis siteleri gГјvenilir bahis siteleri 2024 or deneme bonus veren siteler
https://www.google.mg/url?q=https://slotsiteleri25.com en gГјvenilir site
en gГјvenilir yatД±rД±m siteleri popГјler bahis and orisbet giriЕџ yeni+deneme+bonusu
dГјnyanД±n en iyi bahis siteleri en yeni deneme bonusu veren siteler 2025 or bonus veren siteler yeni
https://images.google.co.uz/url?q=https://slotsiteleri25.com en iyi yatД±rД±m siteleri
tГјrkiye yasal bahis siteleri deneme bonusu veren bГјtГјn siteler and deneme bonusu veren yeni casino siteleri caxino
deneme bonusu veren yeni siteler 2025 tГјrk partner siteleri or jackpot play nedir
http://www.derfischkopf.de/url?q=https://denemebonusuverensiteler25.com casino slot online
grand pasha bet en iyi yatД±rД±m siteleri and yeni bahis siteleri deneme bonusu gГјvenilir deneme bonusu veren siteler
http://denemebonusuverensiteler25.com/# yat?r?ms?z deneme bonusu veren siteler
https://sweetbonanza25.com/# sweet bonanza yorumlar
tГјrkiye bahis siteleri bahis siteleei or bet siteleri bonus
http://pinxmas.com/source/denemebonusuverensiteler25.com/ oyun dene
casino bonusu veren siteler bahis veren siteler and 18siteler bet bahis giriЕџ
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone xs max сервис, можете посмотреть на сайте: срочный ремонт iphone xs max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
slot oyunlar? puf noktalar?: slot oyunlar? – guvenilir slot siteleri
az parayla cok kazandiran slot oyunlar? slot oyunlar? slot oyunlar? puf noktalar?
sweet bonanza oyna: sweet bonanza guncel – sweet bonanza giris
casino bahis siteleri: Deneme Bonusu Veren Siteler – canl? casino siteleri
http://slotsiteleri25.com/# en kazancl? slot oyunlar?
sweet bonanza yorumlar: sweet bonanza guncel – sweet bonanza
az parayla cok kazandiran slot oyunlar? slot casino siteleri en cok kazand?ran slot oyunlar?
https://denemebonusuverensiteler25.com/# yeni deneme bonusu veren siteler
https://denemebonusuverensiteler25.com/# deneme bonusu veren siteler
Cryptocurrency security updates
drug markets dark web dark web link deep dark web
九州娛樂城
九州平台針對新會員推出168元的免費體驗金活動。這項活動的特點是無需滿足投注要求,新會員註冊 後即可使用。會員可以藉此機會體驗平台上的各種遊戲選項,包括電子遊戲、真人對戰等多樣娛樂內容。
相較於市面上其他平台,這項活動的主要優勢在於其簡單的申請流程和零門檻要求。一般平台的體驗金活動往往會設定投注金額要求,但此活動讓會員能更自由地體驗平台服務。
九州娛樂
九州娛樂城作為線上娛樂平台的領導品牌,不斷推出創新服務及優惠活動。近期推出的168元體驗金活動,讓新會員免費體驗平台特色。九州娛樂以玩家體驗為核心,提供多元化的遊戲選擇,包括電子遊戲、真人對戰等娛樂內容。平台以穩定、安全的系統建立口碑,加上專業的客服團隊,打造全方位的娛樂環境。現在註冊加入九州娛樂城,立即享受精彩遊戲體驗。
Портал для строительства https://6may.org и ремонта: полезные советы, современные материалы, проекты и идеи. Все, что нужно для воплощения ваших задумок – от фундамента до крыши.
Всё для строительства и ремонта https://artpaint.com.ua на одном портале: советы экспертов, обзоры материалов, расчет сметы и готовые решения для вашего дома или бизнеса.
Портал о строительстве https://aziatransbud.com.ua статьи, видео, инструкции, каталоги материалов и инструментов. Советы для дома и бизнеса. Легко строить, удобно ремонтировать!
stamp maker online stamp maker online .
Все об озеленении и благоустройстве https://bathen.rv.ua Ландшафтный дизайн, проекты садов, террас и парков. Идеи для создания зеленых зон, подбор растений и профессиональные услуги для вашего участка.
Студия дизайна интерьера https://bconline.com.ua и архитектуры: создаем уникальные проекты для квартир, домов и коммерческих пространств. Эстетика, функциональность и индивидуальный подход – в каждом решении.
This is my first time go to see at here and i am really pleassant to read all at one place.
http://bagstore.com.ua/uv-resistance-headlight-sealants.html
http://www.airlines-inform.ru/personal/user/?UID=73969 https://ekb.8bb.ru/viewtopic.php?id=424#p425 .
Разработка интернет магазина https://magikfox.ru/services/sozdanie-saytov/internet-magazin-na-bitriks/ на Битрикс под ключ в диджитал агентстве MagicFox. Демократичные цены на создание сайта магазина на Bitrix. Интеграция с 1С, службами доставки, системами оплаты. Полная настройка функционала. Работаем по всей России.
гормон роста купить стероид бодибилдинг стероиды купить
Canl? Casino Siteleri casino bahis siteleri casino bahis siteleri
пневматическая заглушка пзу оболочки резинокордные
Профессиональный портал для строительства https://blogcamp.com.ua проекты, материалы, расчеты, советы и вдохновение. Все, чтобы ваш ремонт или стройка были успешными.
Ваш путеводитель в мире строительства https://dcsms.uzhgorod.ua идеи, планы, пошаговые инструкции и лучшие материалы. Узнайте, как построить дом мечты или обновить интерьер.
I enjoy, result in I found exactly what I used to be looking for. You’ve ended my four day lengthy hunt! God Bless you man. Have a great day. Bye
https://millionigrushek.ru/
slot siteleri: slot siteleri – slot oyunlar? puf noktalar?
八代將軍戰神版
パチンコ 停電 補償
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
水浒传
https://www.ja-securities.jp/?GameID=951080818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
CR北斗の拳7 転生 Ver.319
パチンコ 端数
Pビッグドリーム2激神
ぱちんこソードアート・オンラインK12
夜蝶飛翔特2発1
パチスロ ガールズ&パンツァー
https://www.turboslott.com/?ReviewID=923680818.html
ユーモアたっぷりの演出が魅力。プレイ中に笑顔になれる要素が多いです。
交響詩篇エウレカセブン 2
https://www.jogadoresanonimos.org/?ArticleID=947467818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
バイオハザード6
https://www.turboslott.com/?WatchID=980196818.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。
yurt dД±ЕџД± bahis sitesi bahis siteleri 2024 or free spin casino
http://www.google.tn/url?q=https://casinositeleri25.com en iyi yasal bahis siteleri
deneme+bonusu+yeni casino slot oyunlarД± and en az para yatД±rД±lan bahis siteleri 2024 bahis siteleri
Все для строителей и мастеров https://dki.org.ua актуальные технологии, практические советы, строительные материалы и проекты. Простые решения для сложных задач!
Хотите построить дом https://donbass.org.ua или сделать ремонт? Здесь вы найдете всё: инструкции, идеи, современные технологии и проверенные решения. Портал для тех, кто строит.
Делайте ремонт https://esi.com.ua и стройте легко! Лучшие советы мастеров, подбор инструментов, инструкции и сметы. Мы поможем справиться с любой задачей.
Станьте мастером https://fmsu.org.ua своего дела! Портал для тех, кто хочет строить и ремонтировать качественно и выгодно.
Создайте дом своей мечты https://intellectronics.com.ua На нашем портале вы найдете идеи, инструкции и новейшие технологии для ремонта и строительства.
вывод из запоя стационарно ростов http://www.vyvod-iz-zapoya-rostov228.ru/ .
вывод из запоя цены вывод из запоя цены .
en cok kazand?ran slot oyunlar?: slot oyunlar? – slot oyunlar?
casinox kumar siteleri or bet bahis giriЕџ
https://www.google.com.cy/url?sa=t&url=https://sweetbonanza25.com gГјvenilir kumar siteleri
spor siteleri listesi yeni gГјncel deneme bonusu veren siteler and gГјvenli siteler gГјzel siteler
slot casino siteleri slot oyunlar? puf noktalar? slot siteleri
https://denemebonusuverensiteler25.com/# deneme bonusu veren siteler yeni
https://casinositeleri25.com/# Deneme Bonusu Veren Siteler
bahis oyun siteleri casino slot online or ilk giriЕџte bonus veren bahis siteleri
http://www.google.co.ke/url?sa=t&source=web&cd=3&ved=0ccuqfjac&url=https://casinositeleri25.com/ en gГјvenilir online casino
gГјvenilir siteler kumar oynama siteleri and vidobet giriЕџ 2025 betganyon
카지노 보너스 카지노 보너스 .
guvenilir slot siteleri: en kazancl? slot oyunlar? – guvenilir slot siteleri
三國志T
紙幣 オークション
大当たり時の演出が豪華で、観ているだけでも楽しめます。期待感が高まります。
新鬼武者 再臨
https://www.diabetesdirectory.org/?ArticleID=1001113818.html
何度も繰り返しプレイして、やっとこさ大当たりを引けたときの感動は格別です。達成感があります。
ぱちんこソードアート・オンラインK12
ベルがラック カジノ
CRフィーバー宇宙戦艦ヤマト-ONLY ONE-
ぱちんこソードアート・オンラインK12
新鬼武者 再臨
CR 花の慶次~焔N-K
https://www.pgslot-casino.com/?ReviewID=961966818.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
シティーハンター
https://www.pgslot-casino.com/?WatchID=982074818.html
ユーモアたっぷりの演出が魅力。プレイ中に笑顔になれる要素が多いです。
スーパービンゴネオ
https://www.turboslott.com/?GameID=986636818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
пропуск в москву для грузовых автомобилей пропуск в москву для грузовых автомобилей .
Casino Siteleri Casino Siteleri Canl? Casino Siteleri
en Г§ok freespin veren slot 2025 en gГјvenilir bahis siteleri hangileri? or para kazandД±ran sohbet siteleri
https://maps.google.com.mx/url?sa=t&url=https://denemebonusuverensiteler25.com gГјzel siteler
oyun dene 300 tl bonus veren bahis sitesi and bahis siteleei sГјpernetin
Архитектура и дизайн интерьера https://it-cifra.com.ua под ключ: современные решения, индивидуальный подход и гармония стиля и функциональности. Создаем пространство вашей мечты!
Мы помогаем строить https://juglans.com.ua лучше! Советы, проекты, новейшие материалы и технологии для вашего ремонта или строительства.
Найдите все для ремонта https://keravin.com.ua и строительства! Уникальные идеи, пошаговые инструкции и рекомендации специалистов на одном портале.
easydrop промокоды 100 easydrop промокоды 100 .
Ландшафтный дизайн https://kinoranok.org.ua и благоустройство для дома, офиса или парка. Профессиональные советы, подбор растений и реализация уникальных зеленых проектов.
Стройте с комфортом https://mr.org.ua полезные советы, новейшие технологии, пошаговые инструкции и проекты – всё для вашего удобства.
guvenilir casino siteleri: deneme bonusu veren casino siteleri – canl? casino siteleri
canlД± bahis oyunlarД± yabancД± Еџans oyunlarД± siteleri or gГјvenilir oyun alma siteleri
http://scanmail.trustwave.com/?c=6677&d=3eHL2zmR-mSEueRJSdX4UpJhLotL1yEZhefsffMCDw&u=http://denemebonusuverensiteler25.com casino bet gГјncel giriЕџ
casino gГјvenilir siteler betler and bahis oyunlarД± grand pasha bet
deneme bonusu veren siteler yeni: deneme bonusu veren yeni siteler – deneme bonusu veren yeni siteler
Все секреты https://mramor.net.ua строительства в одном месте! Советы экспертов, подбор материалов и готовые проекты для вдохновения.
Решили строить или делать ремонт https://msc.com.ua Мы подскажем, как выбрать лучшие материалы, спланировать бюджет и воплотить все задумки.
Ваш путеводитель в мире строительства https://mtbo.org.ua полезные рекомендации, готовые проекты и современные решения для любых задач.
Всё для успешного строительства https://newboard-store.com.ua и ремонта на одном портале! Мы собрали актуальную информацию, идеи и инструкции для вашего удобства. Заходите и стройте с нами!
Строительство без лишних вопросов https://okna-k.com.ua наш портал – кладезь информации о современных материалах, технологиях и лучших решениях для дома, дачи или офиса.
yeni deneme bonusu veren siteler yeni deneme bonusu veren siteler yeni deneme bonusu veren siteler
https://denemebonusuverensiteler25.com/# denemebonusuverensiteler25
https://slotsiteleri25.com/# slot casino siteleri
deneme bonusu veren bahis siteleri 2025 sГјpernetin or casinombet
http://cse.google.bg/url?sa=t&url=http://sweetbonanza25.com deneme bonusu veren slot siteleri 2025
en iyi bahis siteleri 2024 casinomaxi and bonus veren siteler yurt dД±ЕџД± bahis sitesi
discover this MetaMask Download
Информация о стройке https://purr.org.ua без лишних сложностей! Наш портал поможет выбрать материалы, узнать о технологиях и сделать ваш проект лучше.
how to set up a company in montenegro accountant in Montenegro
Ваш путеводитель в строительстве https://quickstudio.com.ua Ищите материалы, технологии или советы – всё это есть на нашем портале. Стройте с комфортом!
about 1win 1win betting app
1win bet uganda 1win .com
informative post MetaMask Download
from this source phantom wallet
link phantom Download
sweet bonanza yorumlar: sweet bonanza yorumlar – sweet bonanza oyna
вывод из запоя в ростове-на-дону вывод из запоя в ростове-на-дону .
вывод из запоя вывод из запоя .
別再被詐騙黑網騙了!3A最新娛樂城體驗金提供所有線上娛樂城的最新動向
By 3ACasino / December 17, 2024
隨著線上娛樂城的興起,越來越多的玩家選擇在網上娛樂平台上娛樂、賭博,並享受多元化的遊戲體驗。無論是體育賭博、老虎機還是各種賽事投注,線上3a娛樂城都提供了豐富的選擇。然而,隨著線上平台的繁榮,也伴隨著詐騙和不安全平台的風險。如何分辨正規可靠的娛樂城,並避免被詐騙或陷入黑網的陷阱,是每一位玩家必須謹慎對待的問題。
本文將為您介紹線上娛樂城的基本資訊,並提供一些有效的辨識技巧,幫助您避免進入詐騙的黑網,同時介紹3A娛樂城如何為玩家提供最新的娛樂城動向,讓您玩得安心、玩得開心。
一、線上3a娛樂城官網的發展與現狀
隨著科技的進步和網絡的普及,線上3a娛樂城逐漸成為了全球賭博行業的重要一環。這些平台讓玩家可以在家中舒適的環境中進行各種賭博活動,無需親自到賭場,隨時隨地享受賭博樂趣。
多元化的遊戲選擇
目前,線上娛樂城提供的遊戲種類非常豐富,包括老虎機、撲克、賓果、輪盤、21點、體育賭博等各式各樣的選項。玩家可以根據自己的興趣選擇不同的遊戲,並參與到全球賭博市場的競爭中。
技術創新
隨著虛擬現實(VR)技術、人工智慧(AI)等技術的發展,許多娛樂城平台也在不斷創新,提升玩家的體驗。例如,利用VR技術打造身臨其境的賭博環境,讓玩家仿佛置身於真實的賭場;而AI技術則被用於提高遊戲的公平性和精確性。
移動設備支持
隨著智能手機和平板電腦的普及,許多線上3a娛樂城也推出了移動版本,使得玩家可以隨時隨地享受娛樂遊戲。不僅如此,這些平台還推出了適合不同操作系統(如iOS、Android)的應用程式,讓遊戲體驗更加便捷和流暢。
二、線上3a娛樂城官網的詐騙風險
儘管線上娛樂城提供了許多便利和娛樂選擇,但隨著市場的擴大,一些不法分子也進駐其中,利用各種詐騙手段來侵害玩家的利益。這些詐騙黑網的特點通常表現為以下幾個方面:
假網站與假平台
詐騙網站往往以低廉的優惠和豪華的宣傳吸引玩家上鉤,這些網站的設計和操作界面看起來非常專業,但實際上它們並沒有真正的運營許可證。玩家將個人資料和資金投入這些平台後,會發現自己無法提現或賺取的金額被無故凍結。
誘人的獎金和優惠
詐騙平台常常通過推出不切實際的“首存大獎”或“免費彩金”等優惠來吸引玩家,並誘使玩家進行大量投注。這些優惠通常都附帶不合理的條件,並在玩家達不到要求時取消所有贈金,甚至使玩家的存款受到影響。
遊戲不公平與結果操控
部分不法娛樂城會使用作弊手段操控遊戲結果,尤其是老虎機、輪盤等隨機遊戲,玩家在這些平台上的每次投注都無法得到公平對待,從而產生不合理的損失。
不清楚的賭博條款與隱藏費用
許多不正規的娛樂城平台會將一些不明確或隱藏的條款添加到賭博合約中。這些條款可能涉及到存款、取款或遊戲的條件,使玩家無法順利提現,甚至可能被扣除不明費用。
三、如何識別正規3a娛樂城官網?
要避免進入詐騙黑網,首先要學會如何識別正規的3a娛樂城。以下是幾個辨別真偽的關鍵指標:
合法授權與運營許可證
正規的娛樂城平台會擁有合法的運營許可證,這些證書一般來自於知名的賭博監管機構,如英國賭博委員會(UKGC)、馬耳他博彩局(MGA)等。玩家可以在平台的底部或關於我們的頁面查看這些資訊,以確保該平台的合法性。
使用加密技術保障安全
正規平台會採用最新的SSL加密技術來保護玩家的個人資訊和資金安全。玩家可以在平台網址欄查看是否以“https”開頭,並且確認網頁上的支付方式是安全的。
透明的支付與提款政策
正規娛樂城會提供清晰明確的存款和提款流程,並且在玩家要求提款時不會無理拖延。平台的條款和條件應該是簡單且易於理解的,沒有隱藏費用。
客戶服務與口碑
正規3a娛樂城會提供全天候的客戶服務支持,並能迅速解答玩家的問題。玩家可以查看該平台的用戶評價與口碑,了解其他玩家的真實經驗。
四、3a娛樂城官網:為玩家提供最新的娛樂城動向
作為專業的線上娛樂平台,3A娛樂城致力於為玩家提供全面的娛樂資訊,並協助玩家避開詐騙黑網。我們提供以下幾項服務:
實時更新娛樂城資訊
3A娛樂城會定期更新最新的線上3a娛樂城動向,包括合法平台的推薦、遊戲的評測、賭博行業的動態等,讓玩家能夠隨時掌握市場變化。
專業的遊戲分析與技巧分享
我們的專業團隊會分享各種遊戲技巧、策略與賠率分析,幫助玩家提高遊戲的勝率。同時,還會提供對熱門遊戲的深入剖析,讓玩家能夠更好地理解遊戲規則,避免上當受騙。
安全保障與信譽保證
3A娛樂城嚴格篩選合作平台,所有推薦的娛樂城都經過嚴格審查,保證其合法性和安全性。玩家可以放心選擇平台進行遊戲,享受公正、安全的賭博體驗。
專業的客戶服務
我們提供全天候的客戶服務,隨時解答玩家在遊戲過程中的問題,並提供專業的遊戲指導與問題解決方案。
隨著線上娛樂城市場的發展,選擇一個安全、合法、可靠的娛樂平台對於每位玩家來說至關重要。避免被詐騙黑網騙取資金,保持理智並選擇正規的3a娛樂城,是享受線上賭博娛樂的基本前提。3A娛樂城作為領先的娛樂平台,將繼續為玩家提供最新的娛樂城動向和專業的遊戲資訊,幫助您在安全、公正的環境中享受遊戲樂趣。
https://aaawin88.org/3acasino/
Вавада предлагает ставки на спорт Вавада на любой вкус! Здесь вы найдете ставки на футбол, теннис, баскетбол, киберспорт и многое другое. Широкий выбор событий, удобный интерфейс и выгодные коэффициенты делают платформу идеальной как для новичков, так и для опытных игроков. Начните свой путь в ставках уже сегодня!
Всё, что нужно знать о металлах https://metalprotection.com.ua от их свойств до применения в различных отраслях. Обзоры, советы, новости и информация о производителях для вашего удобства.
Всё для вашего ремонта https://reklama-region.com и строительства в одном месте! Практичные советы, современные решения и актуальная информация для успешного проекта.
Ищете проверенные строительные советы https://rus3edin.org.ua Наш портал поможет выбрать материалы, спланировать проект и сделать всё на высшем уровне.
Хотите построить дом https://samozahist.org.ua или сделать ремонт? На нашем портале вы найдёте лучшие решения и вдохновение для вашего проекта.
Популярные стратегии ставок в онлайн-казино Вавада помогут вам грамотно подойти к игре и увеличить свои шансы на успех.
Ознакомьтесь с популярными стратегиями ставок в онлайн-казино Вавада, чтобы улучшить свои навыки и минимизировать риски.
Изучение популярных стратегий ставок в онлайн-казино Вавада станет первым шагом на пути к уверенным победам.
Применяя популярные стратегии ставок в онлайн-казино Вавада, вы сможете лучше контролировать процесс игры и свой банкролл.
Популярные стратегии ставок в онлайн-казино Вавада включают Мартингейл, Парлай и другие тактики для успешной игры.
https://telegra.ph/Populyarnye-strategii-stavok-v-onlajn-kazino-Vavada-01-15
casino bonus yasal oyun siteleri deneme bonusu veren casino siteleri
программа производственного контроля для кафе Москва программа производственного контроля услуга
Всё о дизайне интерьера https://sculptureproject.org.ua в одном месте! Узнайте, как создать уютное, стильное и функциональное пространство, которое будет радовать каждый день.
Строительный портал https://sinergibumn.com для тех, кто хочет знать больше о строительстве. Актуальные идеи, проверенные технологии и вдохновение для любого проекта.
Хотите стильный интерьер https://sitetime.kiev.ua Наш портал предлагает уникальные идеи, профессиональные рекомендации и примеры лучших дизайн-проектов.
Экспертный строительный портал https://smallbusiness.dp.ua для вашего проекта! Советы, новинки и инструкции для тех, кто хочет сделать всё идеально.
sweet bonanza guncel: sweet bonanza yorumlar – sweet bonanza giris
Лучшие советы по выбору металлической входной двери, которая прослужит долгие годы.
Места, где можно приобрести качественную входную металлическую дверь.
Что учесть при выборе входной металлической двери.
Преимущества металлических входных дверей перед другими видами.
дверь металлическая входная дверь металлическая входная цена .
Как выбрать между дверью одного бренда и дверью другого.
slot siteleri: slot siteleri – slot casino siteleri
yeni deneme bonusu veren siteler: deneme bonusu veren siteler yeni – denemebonusuverensiteler25
https://slotsiteleri25.com/# slot oyunlar?
戦国パチスロ花の慶次戦極めし傾奇者の宴~
フリーザ 53 万
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
八代将军 天国の戦い
https://www.pgslot-casino.com/?GameID=949760818.html
サウンドエフェクトが迫力満点で、プレイ中に盛り上がります。特にバトル時が楽しい。
CR真・花の慶次2 漆黒の衝撃
共産主義 ギャンブル
鯉の滝登り
P GOD EATER-ブラッドの覚醒-
パチスロ ガールズ&パンツァー
パチスロ鉄拳4アルティメットデビルVer.
https://www.pgslot-casino.com/?WatchID=931764818.html
パチンコは、日常のストレスを忘れさせてくれる最高の娯楽です。リラックスできます。
ぱちんこソードアート・オンラインK12
https://www.8casino8.com/?NewsID=950319818.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にボーナス時の音が最高。
夜蝶飛翔特2発1
https://www.jogadoresanonimos.org/?NewsID=989403818.html
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
yat?r?ms?z deneme bonusu veren siteler yat?r?ms?z deneme bonusu veren siteler deneme bonusu veren siteler
https://denemebonusuverensiteler25.com/# yeni deneme bonusu veren siteler
Официальный сайт https://luckyjetonewins.ru , где вы найдете актуальное зеркало и промоды на Лаки Джет.
saglam siteler tГјm casino siteleri or bonus veren bahis siteleri casino
https://www.google.bt/url?sa=t&url=https://casinositeleri25.com canli bahis siteleri
casino turkey en iyi deneme bonusu veren siteler and en gГјvenilir bahis levante casino
Найдите всё о строительстве https://srk.kiev.ua и ремонте на нашем портале. Полезные статьи, актуальные технологии и лучшие практики ждут вас.
Строительный портал https://sushico.com.ua для профессионалов и новичков: от выбора материалов до готовых проектов. Легко найти подрядчиков, изучить современные технологии и воплотить идеи в жизнь!
Планируете стройку https://texha.com.ua или ремонт? У нас вы найдёте проверенных специалистов, инструкции, материалы и проекты на любой вкус. Всё для комфортного строительства!
Всё о строительстве https://valkbolos.com и ремонте на одном портале! Гид по материалам, обзор инструментов, советы по дизайну и подбор подрядчиков. Создавайте дом своей мечты!
Преобразите ваш дом https://vineyardartdecor.com вместе с нами! На портале вы найдёте свежие идеи, советы по планировке и материалы для создания идеального интерьера.
bГјtГјn oyun siteleri popГјler bahis or deneme bonusu veren bahis siteleri 2025
https://www.steinhaus-gmbh.de/redirect.php?lang=en&url=https://sweetbonanza25.com en yeni bet siteleri
slot casino 2025 bahis siteleri deneme bonusu and casino bonanza gГјncel giriЕџ gГјvenilir oyun alma siteleri
вывести из запоя цена вывести из запоя цена .
вывод из запоя на дому в екатеринбурге вывод из запоя на дому в екатеринбурге .
easy drop промокод easy drop промокод .
вывод из запоя врачом на дому вывод из запоя врачом на дому .
casino bahis siteleri Deneme Bonusu Veren Siteler en iyi casino oyunlarД±
Планируете ремонт или строительство https://vodocar.com.ua У нас всё, что нужно: от инструкций и советов до подрядчиков и обзоров материалов. Стройте с нами!
Ваш гид в мире строительства https://vitamax.dp.ua и ремонта! Обзоры, практические советы, дизайн-идеи и подбор профессионалов для реализации любых проектов.
internet kumar oyunu son bahis giriЕџ or online casino websites
http://nwspprs.com/?format=simple&action=shorturl&url=https://casinositeleri25.com +18 canli yayin siteleri
gГјvenilir illegal bahis siteleri deneme bonusu bahis siteleri and ilk giriЕџte bonus veren bahis siteleri en iyi bahis sitesi hangisi
Ремонт и строительство https://sota-servis.com.ua легко! Здесь вы найдёте инструкции, рекомендации, материалы и специалистов для успешного выполнения ваших задач.
Полный справочник по строительству https://stroy-portal.kyiv.ua и ремонту: советы, инструкции, дизайн-решения и помощь с выбором материалов и подрядчиков.
Лучшие советы по строительству https://stroysam.kyiv.ua и ремонту на одном сайте! Найдите вдохновение, изучите обзоры и воплотите свои идеи с профессиональной помощью.
おさるの超悟空
大阪 カジノ 橋下
プレイ中の周囲の盛り上がりが、楽しさを倍増させます。共感できる仲間がいるのが嬉しいです。
Pフィーバーかぐや様は告らせたい
https://www.turboslott.com/?SitesID=950057818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
いみそーれ(红花)
リング パチンコ 甘
P戦国乙女 LEGEND BATTLE
BLOOD+ 二人の女王
ミリオンゴッド-神々の凱旋
ヒデキに夢中!!
https://www.ja-securities.jp/?ReviewID=983422818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CR牙狼金色になれ Ver.399
https://www.ja-securities.jp/?ArticleID=999541818.html
演出が豪華で、観ているだけでも楽しめます。特にボーナス演出は圧巻です。
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
https://www.ja-securities.jp/?ArticleID=972673818.html
お店の雰囲気が心地よく、長時間楽しむことができます。快適な空間が魅力です。
пропуск в москву на грузовой автомобиль пропуск в москву на грузовой автомобиль .
yat?r?ms?z deneme bonusu veren siteler: yeni deneme bonusu veren siteler – denemebonusuverensiteler25
slot oyunlar?: slot oyunlar? – en cok kazand?ran slot oyunlar?
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 сервис, можете посмотреть на сайте: ремонт iphone 14 в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
casino oyna betboo giriЕџ or Г§evrim ЕџartsД±z deneme bonusu veren siteler 2025
https://www.google.co.ls/url?q=https://denemebonusuverensiteler25.com gГјvenli siteler
türk partner siteleri son bahis giriş and en yeni kaçak bahis siteleri son bahis güncel giriş
denemebonusuverensiteler25: yat?r?ms?z deneme bonusu veren siteler – yat?r?ms?z deneme bonusu veren siteler
Casino Siteleri Deneme Bonusu Veren Siteler canl? casino siteleri
Los logros de CR7 con la seleccion de Portugal son admirables | Cristiano Ronaldo sigue siendo una figura influyente tanto dentro como fuera del campo Cristiano Ronaldo | Las entrevistas de Cristiano Ronaldo revelan su disciplina y mentalidad ganadora | Los memes de Cristiano Ronaldo suelen volverse virales rapidamente | Las estadisticas de CR7 lo situan como uno de los mejores jugadores de todos los tiempos El retiro de Cristiano Ronaldo.
Стройте и ремонтируйте https://suli-company.org.ua с лёгкостью! Полезные статьи, инструкции, советы по выбору материалов и подрядчиков ждут вас здесь.
Простые решения для ремонта https://teplo.zt.ua и строительства! Идеи дизайна, рекомендации экспертов и проверенные материалы для вашего проекта.
Сделайте ремонт https://tfsm.com.ua мечты с нашим сайтом! Советы, инструкции, рейтинг специалистов и новинки строительного рынка для вашего удобства.
Полный гид по строительству https://tsentralnyi.volyn.ua и ремонту: от планирования до отделки. Читайте, выбирайте и стройте с уверенностью и комфортом.
Портал о ремонте и строительстве https://buildingtips.kyiv.ua с полезными статьями, рекомендациями по выбору материалов и подрядчиков.
How good it is that there are people who are able to so clearly express what many feel https://gmqkk.ilyx.ru/id-5457.html
casino giriЕџ mobil bahis siteleri or en Г§ok freespin veren slot 2025
https://clients1.google.im/url?q=https://denemebonusuverensiteler25.com casimo
gГјvenilir siteler casino kumar oyunlarД± and casino casino gГјncel giriЕџ
sweet bonanza kazanma saatleri: sweet bonanza yorumlar – sweet bonanza slot
slot oyunlar?: slot oyunlar? puf noktalar? – slot oyunlar?
mobil bahis siteleri
https://slotsiteleri25.com/# slot casino siteleri
Полный гид по строительству https://tsentralnyi.volyn.ua и ремонту: от планирования до отделки. Читайте, выбирайте и стройте с уверенностью и комфортом.
https://denemebonusuverensiteler25.com/# yeni deneme bonusu veren siteler
ггдроп кс го ggdrop промокоды на барабан
Приветствую на https://b2best.at! Мы предлагаем надежные и проверенные покупки в интернете. Ознакомьтесь с нашими статьями о безопасности и легальности. Ваши покупки — наш приоритет!
Tormac.org https://tormac.org – это специализированный торрент-трекер, предназначенный для пользователей Mac-компьютеров. Сайт предоставляет широкий выбор контента, ориентированного на операционные системы macOS и iOS.
tk999 app tk999 app .
Сделайте ремонт https://tfsm.com.ua мечты с нашим сайтом! Советы, инструкции, рейтинг специалистов и новинки строительного рынка для вашего удобства.
sweet bonanza slot sweet bonanza yorumlar sweet bonanza
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 рядом, можете посмотреть на сайте: ремонт iphone 14
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Как выбрать идеальную входную металлическую дверь, которая прослужит долгие годы.
Места, где можно приобрести качественную входную металлическую дверь.
Советы по избежанию ошибок при покупке металлической входной двери.
Почему стоит выбрать металлическую входную дверь.
входная дверь дверь металлическая .
В чем отличия входных металлических дверей разных производителей.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 15 pro max сервис, можете посмотреть на сайте: ремонт iphone 15 pro max цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
aviator signal mostbet mostbet aviator app
mostbet withdrawal mostbet azerbaycan qeydiyyat
как зарегистрироваться на 1win можно ли вывести деньги с 1win
сериалы 2024 смотреть онлайн
slot oyunlar? puf noktalar?: slot oyunlar? – en kazancl? slot oyunlar?
orisbet giriЕџ
вывести из запоя цена вывести из запоя цена .
вывод из запоя круглосуточно вывод из запоя круглосуточно .
MLB Draft Betting https://bettingblog.website Your guide to the world of MLB draft betting. Expert predictions, top odds and detailed analysis will help you increase your chances of success. Bet and win with us!
deneme bonusu veren siteler: denemebonusuverensiteler25 – yat?r?ms?z deneme bonusu veren siteler
Credit Union Mobile Home Loans https://blogcredit.tech are the perfect solution for buying or refinancing a mobile home. Affordable rates, easy application, and reliable support every step of the way. Take the first step toward your home with us!
Best Forex Trading Course https://blogforex.tech is your key to successful trading. Learn the secrets of professionals, study strategies and learn how to minimize risks. Master Forex easily and effectively!
Federal Gov Open Enrollment https://body-balance.online is your chance to upgrade or choose an insurance plan. Easy navigation, expert support, and a wide range of programs will help you make the right choice. Apply now!
Tennis betting https://yourmoneyblog.site best odds, predictions and analytics. Explore detailed match reviews, statistics and strategies to make successful bets. Use our tips and win!
The author touched on exactly those issues that worry most thinking people now https://gnxpd.ilyx.ru/id-1639.html
denemebonusuverensiteler25 deneme bonusu veren siteler yeni yeni deneme bonusu veren siteler
лечение запоев на дому http://www.vyvod-iz-zapoya-ekaterinburg25.ru .
Casino Siteleri: casino bahis siteleri – Casino Siteleri
TaskMy.ru – профессиональная помощь в решении задач любого уровня
TaskMy.ru – это надежный сервис, который предлагает качественную помощь в выполнении задач любых направлений: от технических расчётов и программирования до написания текстов и аналитики. Мы работаем быстро, эффективно и ориентированы на ваши требования.
Доверяя TaskMy.ru, вы получаете индивидуальный подход, точное соблюдение сроков и доступные цены. Оставьте свою задачу профессионалам – результат превзойдет ожидания!
Crypto Funk https://besttodaynew.com is a fresh look at cryptocurrencies. News, trends, guides and analytics for beginners and professionals. Find out how to get the most out of blockchain technology!
slot casino siteleri: en kazancl? slot oyunlar? – slot oyunlar?
I have read this post several times already and each time I find something new for myself https://cevdx.ilyx.ru/id-3336.html
After reading this post, you begin to look at familiar things in a completely different way https://ujywe.ilyx.ru/id-3458.html
I need to save this post and show it to everyone I know, because it is really important https://ulxen.ilyx.ru/id-1225.html
CR 獣王
mhwib スロット
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR魔法少女まどか_マギカ Ver.319
https://www.8casino8.com/?ReviewID=948316818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
パチンコ テーマ 曲
ぱちスロ にゃんこ大戦争 BIGBANG
紫イミソーレ(4発1)
CRフィーバー戦姫絶唱シンフォギア Ver.199 (1:1)
P緋弾のアリア 緋弾覚醒編 Ver.199
https://www.jogadoresanonimos.org/?NewsID=929541818.html
大当たりの瞬間には、周囲の人々との一体感が生まれます。共に喜びを分かち合える楽しさがあります。
戦国パチスロ花の慶次これより我ら修羅に入る
https://www.8casino8.com/?WatchID=965328818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
琉球の守護神
https://www.ja-securities.jp/?SitesID=998003818.html
明るいデザインと楽しげな演出が魅力。遊んでいるだけで元気が出ます。
sweet bonanza slot sweet bonanza kazanma saatleri sweet bonanza giris
casino en iyi siteler bahis veren siteler or casino bahis siteleri
https://images.google.ws/url?q=https://casinositeleri25.com yurtdД±ЕџД± bahis siteleri
internet kumar oyunu yeni bonus veren bahis siteleri and deneme bonusu veren bahis siteleri 2025 bahis veren siteler
sweet bonanza demo oyna: sweet bonanza oyna – sweet bonanza demo oyna
https://sweetbonanza25.com/# sweet bonanza
https://sweetbonanza25.com/# sweet bonanza
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 15 pro max в москве, можете посмотреть на сайте: ремонт iphone 15 pro max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
No Credit Check Loans in Abilene TX https://daynewday1.com is fast access to money without unnecessary checks. Convenient terms, simple application and instant approval. Get financial help when you need it!
Home Equity Loans https://funnydays1.com How They Work, What Are the Terms and Benefits? Get the full details on how to use your home’s value for financial purposes. Find out more today!
Credit score requirements for FHA loans https://lifeofnews1.com minimum threshold and tips for improving. Find out how to increase your chances of getting a loan, as well as what affects approval. Detailed information for those who want to get a mortgage through FHA.
Auto loans from Community Credit Union https://sunnydays100.com are simple, affordable, and great value. Low interest rates and flexible repayment options make it easy to buy a new or used car.
программа производственного контроля пищевое Москва программа производственного контроля пищевое
загранпаспорт оформить загранпаспорт оформить .
выведение из запоя на дому в сочи https://vyvod-iz-zapoya-sochi22.ru .
вывести из запоя в стационаре вывести из запоя в стационаре .
sweet bonanza yorumlar [url=https://sweetbonanza25.com/#]sweet bonanza[/url] sweet bonanza guncel
deneme bonusu veren yeni siteler: deneme bonusu veren siteler – denemebonusuverensiteler25
vidobet giriЕџ 2025 gГјvenilir bahis siteleri 2025 or deneme bonusu bahis siteleri
https://maps.google.fm/url?q=https://casinositeleri25.com en kaliteli bahis siteleri
tГјm bet siteleri tГјrkiye bahis siteleri and betboo plus casino en Г§ok kazandД±ran oyunlar
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 15, можете посмотреть на сайте: ремонт iphone 15
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Biography of American basketball legend Michael Jordan michael-jordan-az.org/ personal life, participation in the Monaco Champions League match, film projects, marriage to Yvette Prieto, parenthood, net worth and collaboration with Michael Jackson. Latest updates in 2024.
en guvenilir casino siteleri: en guvenilir casino siteleri – Casino Siteleri
gГјvenilir canlД± bahis casino siteleri en iyi canlД± casino siteleri or deneme bonusu beren siteler
https://www.google.dz/url?q=https://sweetbonanza25.com gГјvenilir deneme bonusu veren siteler
slot bonus 2024 bahis siteleri and ilk giriЕџte bonus veren bahis siteleri bahis siteleri deneme bonusu
tk 999 tk 999 .
Картонные коробки https://giftbox-3.ru оптом без надписей и печать коробки с логотипом в Стерлитамаке.
Dive into the exciting gaming world of taya 365 casino login! Endless selection of games, fast withdrawals and fair play – everything for your pleasure.
Try your luck at taya 365, where excitement meets reliability! Hundreds of popular games, unique promotions and instant payouts await you.
en Г§ok freespin veren slot 2025 gГјvenilir siteler or betler
https://maps.google.cz/url?q=http://denemebonusuverensiteler25.com canlД± casino oyna
güvenilir bahis siteleri 2025 kaçak siteler and türkiye casino siteleri free spin casino
обзор процессоров обзор процессоров .
Скачать музыку бесплатно https://muztab.net просто! Большой выбор песен, хиты всех времён и редкие записи. Удобный поиск, быстрая загрузка и безлимитный доступ. Ваш плейлист ждёт пополнения!
Weed delivery in Prague https://buy-weed-prague.com
Cannabis delivery in Prague buy-cannabis-prague.com
沖ドキ!トロピカル
パチ 新台
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
CR牙狼闇を照らす者
https://www.k8pachinko.work/?ReviewID=922582818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
沖ドキ! 25
カジノ 麻生
P とある魔術の禁書目録
ジャンキージャグラー
CR麻雀物語麗しのテンパイ乙女 Ver.297
P 結城友奈は勇者である GC250Ba
https://www.8casino8.com/?WatchID=924174818.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
P フィーバーダンジョンに出会いを求めるのは間違っているだろうか
https://www.k8-casino.asia/?SitesID=923615818.html
パチンコの演出は時に驚かされることも多く、毎回期待感を持って遊べます。ワクワクが止まりません。
攻殻機動隊S.A.C. 2nd GIG
https://www.8casino8.com/?NewsID=921651818.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
sweet bonanza oyna sweet bonanza yorumlar sweet bonanza giris
yabancД± mekan isimleri by casino or en yeni deneme bonusu veren siteler 2025
https://www.google.bt/url?q=https://denemebonusuverensiteler25.com guvenilir bahis siteleri
casino slot siteleri gГјvenilir bahis siteleri 2025 and en Г§ok kazandД±ran bahis siteleri en Г§ok kazandД±ran bahis siteleri
okada4d
okada4d
sweet bonanza kazanma saatleri: sweet bonanza kazanma saatleri – sweet bonanza
Latest Manga Spoilers Free manga site Vagabond free online
en kazancl? slot oyunlar? az parayla cok kazandiran slot oyunlar? en cok kazand?ran slot oyunlar?
стационарное выведение из запоя vyvod-iz-zapoya-sochi24.ru .
sweet bonanza demo oyna: sweet bonanza yorumlar – sweet bonanza giris
deneme bonusu veren siteler yeni: yat?r?ms?z deneme bonusu veren siteler – yeni deneme bonusu veren siteler
https://slotsiteleri25.com/# en cok kazand?ran slot oyunlar?
sweet bonanza oyna: sweet bonanza kazanma saatleri – sweet bonanza slot
https://slotsiteleri25.com/# az parayla cok kazandiran slot oyunlar?
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 15 рядом, можете посмотреть на сайте: ремонт iphone 15
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
We are deeply thankful for your engagement. Your support makes our efforts worthwhile!
срочное получение загранпаспорта срочное получение загранпаспорта .
вывод из запоя на дому в сочи вывод из запоя на дому в сочи .
вывести из запоя в стационаре вывести из запоя в стационаре .
диплом специалиста купить
Las fotos de Cristiano Ronaldo joven muestran su dedicacion desde el principio | Los ninos que admiran a Cristiano Ronaldo suenan con imitar su camino cristiano ronaldo wife | Los seguidores de Ronaldo siempre apoyan sus decisiones dentro y fuera del campo | Las noticias sobre la vida privada de Ronaldo siempre son de interes | Las publicaciones de Ronaldo en Instagram tienen millones de interacciones cristiano-ronaldo.com.mx.
slot oyunlar? puf noktalar? slot siteleri az parayla cok kazandiran slot oyunlar?
taya365 app login taya 365 casino login
taya365 app taya 365
вывод из запоя нижний вывод из запоя нижний .
2025 yatД±rД±m ЕџartsД±z deneme bonusu veren siteler yeni deneme bonusu veren siteler 2025 or en iyi canlД± casino siteleri
https://clients1.google.bi/url?sa=t&url=http://casinositeleri25.com casino deneme bonusu veren bahis siteleri
kaçak siteler deneme bonusu veren slot siteleri 2025 and casino bonusu veren siteler gerçek paralı casino oyunları
az parayla cok kazandiran slot oyunlar? slot casino siteleri az parayla cok kazandiran slot oyunlar?
denemebonusuverensiteler25: deneme bonusu veren siteler yeni – deneme bonusu veren siteler yeni
en cok kazand?ran slot oyunlar?: en kazancl? slot oyunlar? – az parayla cok kazandiran slot oyunlar?
sweet bonanza demo oyna: sweet bonanza – sweet bonanza kazanma saatleri
Bet Zula, spor bahisleri konusunda yenilikci cozumler sunar. buyuk futbol kars?lasmalar? icin betzula guncel giris baglant?s? ile yuksek oranlar? kesfedebilirsiniz.
Betzula’n?n h?zl? odeme yontemleri, profesyonel hizmet garantisi verir. guncel duyurular? kac?rmadan ozel promosyonlardan haberdar olabilirsiniz.
Turkiye Super Lig derbilerinin en iyi oranlarla kazanc saglayabilirsiniz.
Ayr?ca, Betzula guncel giris adresi, mobil cihazlar uzerinden kolay erisim sunar. Ozel olarak, betzula giris, profesyonel bir deneyim saglar.
Betzula, spor bahislerinden canl? casino oyunlar?na kadar tum kullan?c?lar?n ihtiyaclar?n? kars?lar. Fenerbahce Galatasaray derbisi icin bahis yapmak icin Betzula ile kazanmaya baslay?n!
371212+
戦国パチスロ花の慶次戦極めし傾奇者の宴;
結城 直人
パチンコは、運を試す素晴らしい機会です。ドキドキ感がたまりません。
鯉の滝登り
https://www.diabetesdirectory.org/?ReviewID=918910818.html
漫画の世界観を忠実に再現しており、ファンにはたまらない仕様です。
アナザーゴッドハーデス‐奪われたZEUSver
燃え上がれ
鉄拳3rd
P結城友奈は勇者であるALLRUSH RFa Ver.119
P戦国乙女 LEGEND BATTLE
主役は銭形2
https://www.k8-casino.biz/?NewsID=923397818.html
サウンドエフェクトが迫力満点で、プレイ中に盛り上がります。特にバトル時が楽しい。
CR牙狼金色になれ
https://www.k8casino.biz/?WatchID=922830818.html
パチンコの世界には、果てしない可能性があります。新しい挑戦を楽しむのが醍醐味です。
魁!!男塾天挑五輪大武會編
https://www.k8-casino.biz/?NewsID=923439818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
vidobet giriЕџ 2025 gore siteler or canlД± bahis siteleri
https://toolbarqueries.google.ac/url?q=https://casinositeleri25.com yurt dД±ЕџД± bahis sitesi
canli oyun superbeting and yurtdД±ЕџД± bahis siteleri bonus slot
deneme bonusu veren casino siteleri deneme bonusu veren casino siteleri Canl? Casino Siteleri
deneme bonusu veren siteler: yat?r?ms?z deneme bonusu veren siteler – deneme bonusu veren siteler
http://casinositeleri25.com/# casino bahis siteleri
http://sweetbonanza25.com/# sweet bonanza yorumlar
bonus slot bet bahis giriЕџ or gГјvenilir casino bahis siteleri
https://cse.google.co.zw/url?sa=t&url=https://denemebonusuverensiteler25.com yeni+deneme+bonusu
en iyi casino sitesi vaycasino and date sitesi deneme+bonusu+yeni
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 pro max, можете посмотреть на сайте: ремонт iphone 14 pro max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 pro в москве, можете посмотреть на сайте: ремонт iphone 14 pro в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Inspiring story there. What happened after? Good luck!
SportyBet live betting https://sportybetzambia.pro .
Нужен ремонт техники https://чинпочин.рус все услуги для вашего дома в одном месте! Выбирайте мастеров для ремонта, уборки или сантехнических работ. Качественный сервис, прозрачные цены и удобство использования.
капельница от запоя нижний новгород капельница от запоя нижний новгород .
поставить капельницу от запоя поставить капельницу от запоя .
en gГјvenilir bahis siteleri slot oyna or betnoo
http://clients1.google.com.kw/url?q=https://denemebonusuverensiteler25.com vidobet
casino site casino gГјvenilir siteler and 100tl hosgeldin bonusu veren siteler rcasino
ftpsmart file transfer
выведение из запоя на дому в сочи https://vyvod-iz-zapoya-sochi24.ru/ .
deneme bonusu veren siteler yeni deneme bonusu veren siteler yeni deneme bonusu veren siteler
gore siteler bahis giriЕџ or canlД± casino oyna
https://image.google.td/url?q=https://sweetbonanza25.com en iyi casino sitesi
en gГјvenilir yatД±rД±m siteleri deneme bonusu site and para kazandД±ran sohbet siteleri deneme bonusu veren yeni siteler 2025
video security software FTP Development Software
casino bahis siteleri: Casino Siteleri – casino bahis siteleri
Hello, its good article concerning media print, we all be aware of media is a fantastic source of information.
https://slovo-pacana.info/
We are so thankful to have such thoughtful readers like you. Your interest is what motivates this blog!
Casino Siteleri: canl? casino siteleri – Deneme Bonusu Veren Siteler
牙狼 守りし者
ユリア
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
スーパービンゴネオ
https://www.k8.gives/?SitesID=922631818.html
パチンコは一度ハマると抜け出せない魅力があります。友人と競い合うことで、さらに楽しさが増します。
アイムジャグラーEX
エヴァ 保留
P 新世紀エヴァンゲリオン15 未来への咆哮
P真・北斗無双 第3章
パチスロ 革命機ヴァルヴレイヴ
CR押忍!番長
https://www.xn--k8-9g4a3b4f.store/?GameID=922676818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
P とある魔術の禁書目録
https://www.k8io.net/?WatchID=921768818.html
シンプルなルールで、初心者でもすぐに楽しめます。気軽に挑戦できるのが良いです。
L マクロスフロンティア 4
https://www.8casino8.com/?WatchID=922938818.html
美しいグラフィックと豪華な演出が魅力。神々の力を感じます。
KATATOGEL
guvenilir slot siteleri slot oyunlar? puf noktalar? en cok kazand?ran slot oyunlar?
deneme bonusu veren siteler yeni: yeni deneme bonusu veren siteler – deneme bonusu veren yeni siteler
“Ищете качественный кирпич напрямую от производителя? https://Muravey61.ru – ваш надежный поставщик строительных материалов в регионе! Мы предлагаем кирпич высшего качества по доступным ценам прямо с завода. Доставка точно в срок, широкий ассортимент, и гарантированное качество – всё, что нужно для вашего строительства. Закажите у нас и убедитесь сами, что с нами строить легко!”
капельница от запоя на дому нижний новгород капельница от запоя на дому нижний новгород .
en kazancl? slot oyunlar? slot oyunlar? puf noktalar? en kazancl? slot oyunlar?
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 pro max, можете посмотреть на сайте: ремонт iphone 14 pro max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
опубликовано здесь https://forum.hpc.name/thread/2/65364/polnoe-shifrovanie-diskov-v-freebsd-zagruzka-yadra-s-fleshki.html
deneme bonusu veren yeni siteler: yeni deneme bonusu veren siteler – yeni deneme bonusu veren siteler
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 pro, можете посмотреть на сайте: ремонт iphone 14 pro сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://casinositeleri25.com/# Casino Siteleri
https://casinositeleri25.com/# Deneme Bonusu Veren Siteler
Deneme Bonusu Veren Siteler: guvenilir casino siteleri – deneme bonusu veren casino siteleri
denemebonusuverensiteler25: deneme bonusu veren siteler yeni – deneme bonusu veren yeni siteler
siteler bahis yeni bahis siteleri deneme bonusu or bahis siyeleri
https://cse.google.tg/url?sa=t&url=https://casinositeleri25.com betboo giriЕџ
en yeni kaçak bahis siteleri canli oyun and jav siteleri en iyi yatırım siteleri
sweet bonanza giris sweet bonanza sweet bonanza yorumlar
капельница от похмелья цена капельница от похмелья цена .
SportyBet SportyBet .
сколько стоит капельница на дому от запоя сколько стоит капельница на дому от запоя .
BEKU4D
рейтинги процессоров для пк topcpu.ru .
how to access dark web deep dark web https://github.com/darknetmarkets24/darknet-markets – blackweb official website
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт iphone xs max, можете посмотреть на сайте: ремонт iphone xs max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт iphone se 2020, можете посмотреть на сайте: срочный ремонт iphone se 2020
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
CR麻雀物語麗しのテンパイ乙女 Ver.297
グルーミー
パチンコは、友人や家族と一緒に楽しむのに最適です。共通の趣味があると盛り上がります。
P 新世紀エヴァンゲリオン15 未来への咆哮
https://xn--k8-9g4a3b4f.site/?NewsID=924855818.html
大当たりの瞬間は、ドキドキ感がたまりません。興奮が止まりません。
CRモンスターハンター4 Ver.319
超滅
攻殻機動隊S.A.C. 2nd GIG
ミリオンゴッド
カイジ
CR暗黒騎士呀鎧伝
https://www.sawasdeeslot.com/?NewsID=927099818.html
演出が豪華で、観ているだけでも楽しめます。特にボーナス演出は圧巻です。
黄門ちゃま喝
https://www.k8-casino.jp/?WatchID=919008818.html
人気アニメを基にしたパチスロで、ファンにはたまらない作品です。リアルな演出が楽しめます。
Pフィーバーからくりサーカス
https://xn--k8-9g4a3b4f.site/?SitesID=924023818.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт iphone 13 pro, можете посмотреть на сайте: ремонт iphone 13 pro адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
скупка золота дорого в спб скупка золота за грамм
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 13, можете посмотреть на сайте: ремонт iphone 13 рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Оперативная помощь на дороге https://angeldorog.by/tsena-na-evakuator/ услуги эвакуатора, грузовой и легковой шиномонтаж, а также грузоперевозки фурами по доступным ценам. Работаем круглосуточно, быстро реагируем и гарантируем надежность. Звоните в любое время – решим вашу проблему!
Hybrid cloud video surveillance CCTV viewer for PC
kantorbola88
Hello! Do you know if they make any plugins to help with SEO? I’m trying to get my blog to rank for some targeted keywords but I’m not seeing very good success. If you know of any please share. Cheers!
https://interpen.com.ua/pokrashennya-osvitlennya-avto-z-led-linzamy-porady-ta-oglyad
вывод из запоя на дому нижний новгород вывод из запоя на дому нижний новгород .
выведение из запоя воронеж стационар выведение из запоя воронеж стационар .
別再被詐騙黑網騙了!3A最新娛樂城體驗金提供所有線上娛樂城的最新動向
By 3ACasino / December 17, 2024
隨著線上娛樂城的興起,越來越多的玩家選擇在網上娛樂平台上娛樂、賭博,並享受多元化的遊戲體驗。無論是體育賭博、老虎機還是各種賽事投注,線上3a娛樂城都提供了豐富的選擇。然而,隨著線上平台的繁榮,也伴隨著詐騙和不安全平台的風險。如何分辨正規可靠的娛樂城,並避免被詐騙或陷入黑網的陷阱,是每一位玩家必須謹慎對待的問題。
本文將為您介紹線上娛樂城的基本資訊,並提供一些有效的辨識技巧,幫助您避免進入詐騙的黑網,同時介紹3A娛樂城如何為玩家提供最新的娛樂城動向,讓您玩得安心、玩得開心。
一、線上3a娛樂城官網的發展與現狀
隨著科技的進步和網絡的普及,線上3a娛樂城逐漸成為了全球賭博行業的重要一環。這些平台讓玩家可以在家中舒適的環境中進行各種賭博活動,無需親自到賭場,隨時隨地享受賭博樂趣。
多元化的遊戲選擇
目前,線上娛樂城提供的遊戲種類非常豐富,包括老虎機、撲克、賓果、輪盤、21點、體育賭博等各式各樣的選項。玩家可以根據自己的興趣選擇不同的遊戲,並參與到全球賭博市場的競爭中。
技術創新
隨著虛擬現實(VR)技術、人工智慧(AI)等技術的發展,許多娛樂城平台也在不斷創新,提升玩家的體驗。例如,利用VR技術打造身臨其境的賭博環境,讓玩家仿佛置身於真實的賭場;而AI技術則被用於提高遊戲的公平性和精確性。
移動設備支持
隨著智能手機和平板電腦的普及,許多線上3a娛樂城也推出了移動版本,使得玩家可以隨時隨地享受娛樂遊戲。不僅如此,這些平台還推出了適合不同操作系統(如iOS、Android)的應用程式,讓遊戲體驗更加便捷和流暢。
二、線上3a娛樂城官網的詐騙風險
儘管線上娛樂城提供了許多便利和娛樂選擇,但隨著市場的擴大,一些不法分子也進駐其中,利用各種詐騙手段來侵害玩家的利益。這些詐騙黑網的特點通常表現為以下幾個方面:
假網站與假平台
詐騙網站往往以低廉的優惠和豪華的宣傳吸引玩家上鉤,這些網站的設計和操作界面看起來非常專業,但實際上它們並沒有真正的運營許可證。玩家將個人資料和資金投入這些平台後,會發現自己無法提現或賺取的金額被無故凍結。
誘人的獎金和優惠
詐騙平台常常通過推出不切實際的“首存大獎”或“免費彩金”等優惠來吸引玩家,並誘使玩家進行大量投注。這些優惠通常都附帶不合理的條件,並在玩家達不到要求時取消所有贈金,甚至使玩家的存款受到影響。
遊戲不公平與結果操控
部分不法娛樂城會使用作弊手段操控遊戲結果,尤其是老虎機、輪盤等隨機遊戲,玩家在這些平台上的每次投注都無法得到公平對待,從而產生不合理的損失。
不清楚的賭博條款與隱藏費用
許多不正規的娛樂城平台會將一些不明確或隱藏的條款添加到賭博合約中。這些條款可能涉及到存款、取款或遊戲的條件,使玩家無法順利提現,甚至可能被扣除不明費用。
三、如何識別正規3a娛樂城官網?
要避免進入詐騙黑網,首先要學會如何識別正規的3a娛樂城。以下是幾個辨別真偽的關鍵指標:
合法授權與運營許可證
正規的娛樂城平台會擁有合法的運營許可證,這些證書一般來自於知名的賭博監管機構,如英國賭博委員會(UKGC)、馬耳他博彩局(MGA)等。玩家可以在平台的底部或關於我們的頁面查看這些資訊,以確保該平台的合法性。
使用加密技術保障安全
正規平台會採用最新的SSL加密技術來保護玩家的個人資訊和資金安全。玩家可以在平台網址欄查看是否以“https”開頭,並且確認網頁上的支付方式是安全的。
透明的支付與提款政策
正規娛樂城會提供清晰明確的存款和提款流程,並且在玩家要求提款時不會無理拖延。平台的條款和條件應該是簡單且易於理解的,沒有隱藏費用。
客戶服務與口碑
正規3a娛樂城會提供全天候的客戶服務支持,並能迅速解答玩家的問題。玩家可以查看該平台的用戶評價與口碑,了解其他玩家的真實經驗。
四、3a娛樂城官網:為玩家提供最新的娛樂城動向
作為專業的線上娛樂平台,3A娛樂城致力於為玩家提供全面的娛樂資訊,並協助玩家避開詐騙黑網。我們提供以下幾項服務:
實時更新娛樂城資訊
3A娛樂城會定期更新最新的線上3a娛樂城動向,包括合法平台的推薦、遊戲的評測、賭博行業的動態等,讓玩家能夠隨時掌握市場變化。
專業的遊戲分析與技巧分享
我們的專業團隊會分享各種遊戲技巧、策略與賠率分析,幫助玩家提高遊戲的勝率。同時,還會提供對熱門遊戲的深入剖析,讓玩家能夠更好地理解遊戲規則,避免上當受騙。
安全保障與信譽保證
3A娛樂城嚴格篩選合作平台,所有推薦的娛樂城都經過嚴格審查,保證其合法性和安全性。玩家可以放心選擇平台進行遊戲,享受公正、安全的賭博體驗。
專業的客戶服務
我們提供全天候的客戶服務,隨時解答玩家在遊戲過程中的問題,並提供專業的遊戲指導與問題解決方案。
隨著線上娛樂城市場的發展,選擇一個安全、合法、可靠的娛樂平台對於每位玩家來說至關重要。避免被詐騙黑網騙取資金,保持理智並選擇正規的3a娛樂城,是享受線上賭博娛樂的基本前提。3A娛樂城作為領先的娛樂平台,將繼續為玩家提供最新的娛樂城動向和專業的遊戲資訊,幫助您在安全、公正的環境中享受遊戲樂趣。
https://aaawin88.org/live/
скупка золота дорого цена золота 585 в скупках спб
We are so thankful for your encouragement—your loyalty lift our spirits every day.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone xs max рядом, можете посмотреть на сайте: ремонт iphone xs max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Thank you for supporting us. Your trust gives us the strength to keep moving forward.
Your generosity and dedication to our cause do not go unnoticed. Thank you!
Your unwavering belief in us is deeply treasured. Thank you for your support and trust.
Your thoughtful words and unwavering support make all the difference to do better.
Секреты успешной покупки входной металлической двери, которая прослужит долгие годы.
Места, где можно приобрести качественную входную металлическую дверь.
Советы по избежанию ошибок при покупке металлической входной двери.
Почему стоит выбрать металлическую входную дверь.
купить входную дверь заказать входную дверь .
В чем отличия входных металлических дверей разных производителей.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone se 2020 рядом, можете посмотреть на сайте: ремонт iphone se 2020 рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
какой процессор лучше https://www.topcpu.ru .
We sincerely appreciate your engagement. It helps us grow to have you as part of our network.
主役は銭形 2004
カジノ 徳島
大当たりの瞬間に周りが盛り上がると、嬉しさが倍増します。共に祝える仲間がいるのが良いですね。
戦国パチスロ花の慶次戦極めし傾奇者の宴;
https://www.pgslot-casino.com/?SitesID=926507818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
ミリオンゴッド-神々の凱旋
マカオ どこ
シティーハンター
沖ドキ! 25
P とある魔術の禁書目録 Light PREMIUM ver.
CR真・花の慶次 Ver.399
https://www.online-casino.city/?GameID=924536818.html
友人と一緒に行くことで、競争心が芽生え、さらに楽しめます。勝負の結果が気になります。
バイオハザード6
https://www.pgslot-casino.com/?ArticleID=927571818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
CR牙狼FINAL Ver.399
https://k88214.com/?WatchID=927258818.html
ホラー映画の要素が盛り込まれており、ファンにはたまらない作品です。感情移入できます。
Лучшие советы по выбору металлической входной двери, соответствует всем требованиям безопасности.
Лучшие магазины с широким выбором входных металлических дверей.
Что учесть при выборе входной металлической двери.
Преимущества металлических входных дверей перед другими видами.
дверь металлическая входная дверь металлическая входная цена .
Сравнение входных металлических дверей различных брендов.
If you’re new to MetaMask Wallet, start with https://metalead.org/. They offer step-by-step guidance on how to download and use MetaMask for browsers like Safari and Opera.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 13 pro в москве, можете посмотреть на сайте: ремонт iphone 13 pro рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт iphone 13, можете посмотреть на сайте: ремонт iphone 13
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
additional hints valorant skin changer
rent a tesla model 3 tesla model 3 performance rental
У нас вы можете купить айфоны https://vk.com/crazy_humor01 оптом по самым лучшим ценам. Оригинальные смартфоны Apple с гарантией качества. Постоянное наличие популярных моделей.
Квартирный переезд https://spb-gruzoperevozka.ru с грузчиками быстро и качественно! Упакуем, вынесем, перевезем и разместим вещи на новом месте. Надежная команда, аккуратность и доступные тарифы.
стол для переговоров совещаний https://mm26.ru
стол переговоров цена стол для переговоров 20 человек
выведение из запоя воронеж стационар выведение из запоя воронеж стационар .
пансионат долголетие пансионат долголетие .
сколько стоит капельница на дому от запоя сколько стоит капельница на дому от запоя .
дом для пожилых людей в симферополе дом для пожилых людей в симферополе .
Immerse into the Realm of GTA 5 RP: Become a part of Our Special Roleplay Server
Welcome to the thrilling universe of GTA 5 RP, where every player can develop their distinct narrative in a energetic gaming environment. Our roleplay server is ideal for those seeking an engaging and deep experience. Join a lively community of GTA RP fans and walk into this thrilling world!
How to Begin Playing?
1. Buy and install a authorized version of GTA 5.
2. Set up the launcher.
3. Link to the server and enroll through the application or our website to begin your journey!
Our Platform: An Open World Experience
Our server offers a unique open-world environment with tailored mods that permit you to forge your illicit empire, embark on thrilling missions, and shape your avatar’s journey. Whether you join neighborhood gangs or turn into a elite cop, the options are boundless!
Generate Money and Increase Your Wealth
Are you set to conquer the streets of Los Santos and earn substantial money? We offer the instruments and tactics to help you amass a fortune in the realm of GTA RP.
Uncover the Realm of Minecraft: Your Supreme Survival and Chaos Exploration
Welcome to your Entrance to the Exceptionally Exciting and Interactive Minecraft Connected Experience. Whether you’re a Creator, Warrior, Traveler, or Planner, our Network Provides Endless Opportunities to Experience Endurance and Anarchy Features in Methods you’ve Never seen Before.
Why Pick Experiences in Minecraft?
Our Network is Built to Offer the Top Minecraft Adventure, Combining Custom Realms, Exciting Mechanics, and a Supportive Network. Traverse, Conquer, and Create your own Adventures with Unique Components Created for All type of Player.
Primary Components
– Endurance and Chaos Scenarios: Encounter the Challenge of Persisting against the odds or Plunge into Wild PvP Fights with no rules and full freedom.
– Enormous Server Size: With Availability for up to 3,750 Users, the Fun never stops.
– 24/7 Realm Status: Access At Any Moment to Explore Seamless, Consistent Interaction.
– Tailored Content: Discover our Skillfully Designed Minecraft Worlds Packed with Addons, Plugins, and Limited Objects from our Store-Based Marketplace.
Exclusive Mechanics Options
Living Option
In Survival Feature, you’ll Discover Vast Terrains, Collect Supplies, and Build to your heart’s content. Defeat off Mobs, Team Up with Friends, or Accept on Individual Challenges where only the Powerful Prevail.
Disorder Scenario
For Users Wanting Chaos and Thrill, Chaos Scenario Presents a Environment with No Rules. Engage in Adrenaline-Fueled PvP Engagements, Build Alliances, or Confront Enemies to Dominate the World. Here, Persistence of the Fittest is the Sole Law.
Unique Minecraft Elements
– Exploration Zones: Explore Unique Minecraft Caves and RPG-Style Missions.
– Trading and Trading: Our Interactive Economy Lets you to Buy, Obtain, and Offer Products to Rise the Ranks and Form Your Status as a Dominant Player.
– Minecraft Store: Access Special Products, Improvements, and Statuses that Enhance your Gameplay.
Minecraft Shop: Upgrade Your Gameplay
Our Interactive Store Presents a Range of Enhancements, Ranks, and Products to Suit every Player. From Affordable Support Offers to Top-Level Upgrades, you can Access Additional Options and Advance your Experience to the Top.
Best-Selling Features
– Donate Cases (x10) – €1.00
– VIP – €1.40
– Elysium Tier – €20.00
– OWNER Level – €40.00
– BOSS Rank – €60.00
Top Statuses for Top Users
– CREATOR (€10.00) – Unlock Design Tools to Express your Imagination.
– Vanguard (€12.00) – Top Perks and Unique Perks.
– Paragon (€59.10) – Top Features for the Premier Competitor.
– Luminescent (€50.00) – Dominate as a Iconic Player on the Server.
Join Our Thriving Minecraft Network
We Focus in Forming a Friendly, Engaging, and Welcoming Society. Whether you’re Completing RPG Adventures, Traversing Custom Zones, or Taking Part in User-Led PvP, there’s Consistently something Exciting to Discover.
Features You Can Look Forward To
– Friendly Network: Meet Fellow Minecraft Fans from Globally.
– Exciting Challenges: Join in Thrilling Competitions, Contests, and Community-Wide Activities.
– Dedicated Support: Our Moderators Guarantees Lag-Free Gameplay and Guides you with any Issues.
High-Quality Prompts
The Single Shop for Artificial Intelligence-Powered Artistry
In this dynamic landscape, integrating artistry with technology is vital for advancement and effectiveness. At Template Forge, we present high-quality, custom-crafted prompts created to boost your artistic initiatives across diverse areas.
Why Choose Template Forge?
We are devoted to supplying only the finest prompts to support your innovative endeavors. Our group of specialists meticulously curates each prompt to confirm usefulness and importance, making it more manageable for you to discover ideas when you desire it the most critically.
Diverse Categories
Our wide-ranging selection covers numerous fields, such as social networking advertising, issue resolution, and fiction writing. This breadth ensures you have the resources needed to tackle any task competently.
Elevate Your Creativity
Template Forge’s inspirations serve as a launchpad for your creativity, permitting you to concentrate on your unique style and goals without the burden of conceptualizing. Each prompt is crafted to motivate and assist you through the creative journey.
Join thousands of content creators who have leveraged the potential of artificial intelligence at Template Forge. Check out our extensive subjects today and unlock your innovative possibilities. With our top-notch prompts, producing with intention has always been easier. Welcome to your one-stop marketplace for AI-enabled imagination!
Explore CS2 Skin
Explore CS2 Designs: Find Your Ideal Match
Transform Your Gameplay
Showcase your individuality in Counter-Strike 2 (CS2) with special weapon skins. Our store offers a diverse selection, from scarce to limited-edition designs, permitting you to express your style and boost your gameplay.
Easy and Protected Purchasing
Savor a flawless shopping journey with rapid digital shipment, ensuring your just purchased skins are instantly available. Buy confidently with our protected checkout process, whether you’re searching for budget options or luxury designs.
How It Functions
1. Explore the Range: Explore a wide variety of CS2 skins, organized by scarcity, gun type, or style.
2. Select Your Skin: Add your desired skin to your basket and continue to payment.
3. Equip Your Recent Skins: Immediately receive and apply your skins in-game to shine during games.
Affordable Customization
Tailoring should be accessible for all. We consistently offer savings on CS2 skins, providing premium designs accessible at affordable prices.
Featured Textures
– P250 | Nuclear Threat (Factory New) – 3150 €
– Desert Eagle | Hand Cannon (Minimal Wear) – 450 €
– StatTrak™ P2000 | Ocean Foam (Factory New) – 285.88 €
– Glock-18 | Synth Leaf (Field-Tested) – 305 €
Start Your Shopping Experience Immediately!
Upgrade your gameplay with our outstanding collection of CS2 skins. Whether enhancing your firearms or creating a unique collection, our store is your hub for high-quality skins. Change your gaming experience at once!
kinogo фильмы для смарт-тв kinogo документальные фильмы
kinogo лучшие криминальные фильмы kinogo семейные сериалы
Your Ultimate Destination for High-Quality In-Game Goods!
In contemporary quick gaming environment, the joy of discovery and advancement is important. If you’re looking for unique items, unique device designs, custom modes, codes, materials, challenges, or handy balance solutions, look no further! Hello to Items4Games, your single store for a vast variety of premium in-game products designed to elevate your gaming adventure.
Why Items4Games Is Exceptional
Diverse Choice of In-Game Resources
We provide a broad assortment of in-game assets, from uncommon skins to exclusive artifacts and game codes. Our catalog is adapted to elevate your gameplay and involve you in a environment of infinite possibilities.
Support for Well-known Games
Our broad inventory contains popular games like CS2, Fortnite, Valorant, Dota 2, and Call of Duty. No matter your gaming choice, we have assets that cater to the top successes in the industry.
Upgraded Gaming Adventure
With limited in-game goods, you can discover new opportunity and illustrate your unique touch. From special weapon styles to powerful artifacts, our offerings change your gaming plays into extraordinary journeys.
Protected and Trustworthy Purchases
We prioritize your well-being with safe and honest transactions. You can purchase securely, knowing that your information is secure at all occasions.
Competitive Rates
Get superior items without destroying the budget. Our cost-effective and reasonable pricing structure ensures that boosting your gaming experience is within reach to all.
User-Friendly Platform
Our user-friendly design lets you to effortlessly look through and secure the products you need, reducing you energy and improving your shopping experience.
What Is Waiting for You at Items4Games
Discover weapon styles that represent your style, limited artifacts for special achievements, and game keys that reveal new material. Our supplies and missions support you level up smoothly, while top-up assistance keep your game budget ready for engagement. Enjoy more game types that offer difference and complexity to your gaming plays.
In closing, Items4Games is your best source for elevating your gaming experience with premium in-game assets. Explore our range today and elevate your gameplay to new levels!
kinogo лучшие приключения kinogo рейтинги
kinogo kinogo фильмы для смарт-тв
vps sunucu kiralama için ihtiyacımı karsiladim tesekkur ederim
Автоломбард в СПб https://www.6467373.ru быстрые деньги под залог вашего авто! Оценка за 15 минут, прозрачные условия, сохранение машины в вашем пользовании.
vds sunucu kiralama fiyatlariniz uygun gözüküyor
сравнение процессоров amd topcpu.ru .
девушки по вызову калуга заказать девушек в калуге
заказать девушек по вузову в калуге снять девушек по вузову калуга
производственный контроль рабочая программа программа производственного контроля в столовой
стол для переговоров дуб переговорный стол трансформер
鬼武者3 時空天翔
慶次
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
デビル メイ クライ 4
https://www.k8-casino.biz/?SitesID=918203818.html
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
戦国乙女剣戟に舞う白き剣聖西国参戦編
ダイトモ
パチスロ 貞子3D
CRキャプテン翼
P フィーバー戦姫絶唱シンフォギア3黄金絶唱
ミリオンゴッド-神々の凱旋-
https://www.online-casino.city/?SitesID=918503818.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
アナザーゴッドハーデス-奪われたZEUSver
https://www.k8casinoofficial.jp/?WatchID=919938818.html
パチンコは、単なるギャンブルではなく、戦略や選択が重要な要素です。楽しみが深まります。
P 北斗の拳 暴凶星
https://www.ja-securities.jp/?NewsID=926907818.html
シンプルなルールで、誰でもすぐに楽しめるのが嬉しいです。
OR Realty — это ваш надежный партнер в мире недвижимости. Мы предлагаем большой выбор квартир, домов и коммерческих объектов по выгодным условиям. Наши специалисты помогут вам найти идеальный вариант, соответствующий вашим потребностям. Надежность, качество и удобство — вот что делает OR Realty лучшим выбором. Обращайтесь!
скупка золота в питере скупка золота 585 на сегодня
I read this paragraph completely concerning the resemblance of
most up-to-date and preceding technologies, it’s amazing article.
купить 1с бухгалтерия 8.3 проф версия цена купить 1с бухгалтерия 8.3 проф версия цена .
программа 1с купить с установкой http://www.svarog.forum24.ru/?1-0-0-00000330-000-0-0/ .
Для начала заходим на площадку:
Заходим на оригинальную ссылку:
Ссылка https://bs1site.at
ССЫЛКА TOR: blackpxl62pgt3ukyuifbg2mam3i4kkegdydlbbojdq4ij4pqm2opmyd.onion
Официальный сайт Blacksprut
БлекСпрут официальная ссылка
Как зайти на даркнет маркетплейс БлекСпрут
Введение
В этой статье мы подробно расскажем, как зайти на даркнет маркетплейс БлекСпрут. Вы узнаете, как использовать официальные зеркала BlackSprut, ссылки на сайт БлекСпрут и способы безопасного доступа через ТОР и VPN. БлекСпрут является одним из наиболее популярных даркнет маркетплейсов, и доступ к нему требует определенных знаний и мер предосторожности.
Что такое БлекСпрут?
БлекСпрут (BlackSprut) – это даркнет маркетплейс, предлагающий широкий ассортимент товаров и услуг. Из-за своей природы и содержания доступ к БлекСпрут осуществляется через сети типа onion, обеспечивающие анонимность пользователей.
Как зайти на БлекСпрут: шаги и инструкции
Шаг 1: Установка ТОР браузера
Первым шагом для доступа к БлекСпрут через ТОР является установка ТОР браузера. Это специализированный браузер, который позволяет анонимно заходить на сайты в onion-сети.
Скачайте ТОР браузер с официального сайта Tor Project.
Установите браузер на ваш компьютер или мобильное устройство.
Запустите ТОР браузер.
Шаг 2: Использование официального зеркала BlackSprut
Для доступа к БлекСпрут важно использовать только проверенные и официальные ссылки. Официальное зеркало BlackSprut гарантирует безопасный доступ и защиту от фишинговых сайтов.
Официальная ссылка на БлекСпрут будет иметь формат.onion. Например, ссылка на сайт БлекСпрут может выглядеть так:
Зеркала сайта БлекСпрут обеспечивают резервный доступ в случае блокировки основного сайта. Например, зеркало БлекСпрут через ТОР:
Шаг 3: Подключение через VPN
Для дополнительной безопасности рекомендуется использовать VPN.
Выберите надежный VPN сервис.
Подключитесь к VPN перед запуском ТОР браузера.
Откройте ТОР браузер и введите официальный адрес БлекСпрут.
Шаг 4: Безопасный доступ к БлекСпрут через onion
Когда вы используете ТОР браузер и официальное зеркало БлекСпрут, важно следовать мерам предосторожности:
Проверяйте URL на наличие ошибок и подлинности.
Используйте VPN для дополнительной защиты.
Не вводите личные данные на подозрительных сайтах.
Часто задаваемые вопросы
Как получить доступ к БлекСпрут через onion?
Для доступа к БлекСпрут через onion сеть необходимо использовать ТОР браузер и официальные ссылки на сайт БлекСпрут. Подключение через VPN также рекомендуется для защиты вашей анонимности.
Как зайти на BlackSprut безопасно?
Чтобы безопасно зайти на BlackSprut, используйте ТОР браузер, подключайтесь через VPN, и проверяйте официальные зеркала сайта БлекСпрут. Никогда не переходите по подозрительным ссылкам.
Что такое зеркало БлекСпрут?
Зеркало БлекСпрут – это альтернативный адрес сайта, используемый для обеспечения доступа в случае блокировки основного сайта. Зеркало BlackSprut через ТОР помогает пользователям получить доступ к маркетплейсу, сохраняя их анонимность.
Теперь вы знаете, как зайти на даркнет маркетплейс БлекСпрут, используя официальные зеркала и ссылки. Следуйте этим инструкциям и соблюдайте меры предосторожности, чтобы обеспечить свою безопасность в даркнете. Официальный сайт BlackSprut и его зеркала через ТОР и VPN помогут вам получить доступ к БлекСпрут, оставаясь анонимным и защищенным.
blacksprutblack sprutссылки бсссылки в бс 2024ссылка на блекспрутрабочая ссылка блекспрутссылки тор блекспрутблекспрут актуальная ссылкаблекспрут ссылка bs0bestтор блекспрутссылки тор блекспрутблекспрут сайтблекспрут официальный сайтблекспрут входкак зайти на блекспруткак зайти на блэкспрутблэкспрут входблэкспрут ссылкаблэкспрут онионблэкспрут даркнетблэкспрут даркнетблэкспрут blacksprut даркнет обзор анонимной даркнет площадкиbs как зайтиbs at как зайти на сайтbs входbs ссылкаblacksprut darknetblacksprutblacksprut зеркалаblacksprut ссылкаblacksprut сайтзеркала blacksprut rusffкак зайти на blacksprutblacksprut официальныйblacksprut com зеркалоblacksprut зеркала онион2fa blacksprutрабочая blacksprutкод blackspruthttps blacksprutкак зайти на blacksprut rusffофициальная ссылка на blacksprutblacksprut маркетплейсрабочее зеркало blacksprutкак зайти на сайт blacksprut2fa код blackspruthttp blacksprutblacksprut bs0best atblacksprut актуальныетор blacksprutblacksprut ссылка rusffbs2best at ссылка blacksprutblacksprut актуальная ссылкаtor blacksprutblacksprut com зеркало rusffhttps blacksprut ссылкаblacksprut зеркала онион rusffblacksprut площадкиbs1site at ссылка blacksprutblacksprut netblacksprut входофициальная ссылка на blacksprut rusffblacksprut blacksprut clickblacksprut bs0tor atblacksprut официальный сайтblacksprut ссылка торкак зайти на сайт blacksprut rusffblacksprut https bs1site atblacksprut http bs0best athttp blacksprut ссылкааккаунты blacksprutрабочее зеркало blacksprut rusffhttps bs2site at ссылка blacksprutbs0best at ссылка blacksprut http bs2best atblacksprut 2blacksprut ссылка blacksprut darknetофициальная ссылка на blacksprutblacksprut ссылка rusffbs0best at ссылка blacksprutblacksprut актуальная ссылкаhttps blacksprut ссылкаbs1site at ссылка blacksprutофициальная ссылка на blacksprut rusffhttp blacksprut ссылкаhttps bs1site at ссылка blacksprutbs0best at ссылка blacksprut http bs0best atblacksprut ссылка tortor blacksprutblacksprut ссылка torblacksprut ссылка tor bs2tor nltor blacksprut rusffblacksprut зеркала torsprutblack sprut
mostbet uz apk mostbet 45
1win uzbekistan скачать 1win скачать казино
Онлайн казино с широким выбором аркадных игр | Уникальные аркадные игры только здесь | Попробуйте свою удачу в аркадном казино | Насладитесь азартом в аркадном казино | Новый уровень азарта в онлайн казино | Играйте в аркадные игры и выигрывайте деньги | Играйте в лучшие аркады в онлайн казино | Азарт и аркады в одном месте | Аркады и азарт для настоящих игроков | Аркадные игры и азарт ждут вас в онлайн казино | Аркадные игры для всех желающих азарта | Уникальные аркадные игры и азарт в казино | Испытайте свою удачу в аркадном казино | Увлекательные аркады и возможность заработать крупные суммы | Аркадные сражения и азарт для настоящих игроков | Побеждайте в аркадных играх и получайте призы
аркада казино сайт arkada casino .
大工の源さん桜満開!源 DREAM ver.~
bonz
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
黃金守護神(特殊大賞燈);
https://www.turboslott.com/?SitesID=925343818.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にバトル時が最高。
麻雀物語2 激闘!麻雀グランプリ
栄 カジノ
戦国パチスロ花の慶次戦極めし傾奇者の宴~
Pフィーバーダンベル何キロ持てる
CR戦国乙女花
L ゴジラ対エヴァンゲリオン
https://www.online-casino.city/?ArticleID=922897818.html
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特に恐怖演出が楽しい。
パチスロ 貞子3D
https://www.sawasdeeslot.com/?SitesID=918371818.html
簡単に遊べるルールで、初心者でも安心。気軽に挑戦できるのが良いです。
CR真・北斗無双
https://www.turboslott.com/?ReviewID=922714818.html
シンプルなルールで、初心者でもすぐに楽しめます。気軽に挑戦できます。
Играйте в аркадные игры в лучшем онлайн казино | Новые аркадные игры каждую неделю | Попробуйте свою удачу в аркадном казино | Увлекательные аркадные игры для всех | Онлайн казино с самыми популярными аркадными играми | Играйте в аркадные игры и выигрывайте деньги | Играйте в лучшие аркады в онлайн казино | Аркадное казино для всех | Уникальные аркадные развлечения только здесь | Онлайн казино с азартными аркадами | Попробуйте свою удачу в аркадном казино | Играйте в аркады и выигрывайте крупные суммы | Играйте в аркадные игры и выигрывайте деньги | Играйте в аркады и получайте удовольствие | Аркадные развлечения для всех | Увлекательные аркады и возможность заработать деньги
arkada casino официальный arkada casino онлайн .
дом интернат в симферополе дом интернат в симферополе .
пансионат долголетие пансионат долголетие .
La altura y fisico de Erling Haaland son aspectos que llaman la atencion | Las camisetas de Haaland son de las mas populares entre los aficionados erling haaland | Las estadisticas de Haaland lo colocan entre los mejores delanteros del mundo | La celebracion unica de Haaland tras marcar un gol es inolvidable | Los momentos destacados de Haaland en la Bundesliga todavia son recordados Contratos de Erling Haaland.
выведение из запоя воронеж стационар выведение из запоя воронеж стационар .
Эта статья предлагает захватывающий и полезный контент, который привлечет внимание широкого круга читателей. Мы постараемся представить тебе идеи, которые вдохновят вас на изменения в жизни и предоставят практические решения для повседневных вопросов. Читайте и вдохновляйтесь!
Получить больше информации – https://nakroklinikatest.ru/
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 12 pro max сервис, можете посмотреть на сайте: ремонт iphone 12 pro max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 11 сервис, можете посмотреть на сайте: ремонт iphone 11
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Ucuz vds
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт iphone se 2020, можете посмотреть на сайте: ремонт iphone se 2020 сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Katun Room — это уютные гостиницы в самом сердце природы Алтая. Мы предлагаем комфортное проживание рядом с живописной рекой Катунь. Номера на любой вкус и бюджет, тишина и свежий воздух создадут незабываемую атмосферу для вашего отдыха. Забронируйте ваш номер уже сегодня!
kinogo сериалы kinogo свежие сериалы
kinogo hd киного фильмы для мобильных устройств
kinogo сериалы про магию kinogo фильмы по алфавиту
kinogo приключения kinogo фильмы для просмотра онлайн
киного семейные фильмы киного фильмы про драконов
my response https://jaxx-liberty.com/
Learn More https://trusteewallet.org
A modern AI tool ai undress for working with images. Learn more about its features, capabilities and applications. Full privacy control and ease of use will ensure comfortable interaction.
kinogo фильмы для взрослых kinogo смотреть онлайн
Хотите купить окна melke pro по разумной цене? Ознакомьтесь с нашим предложением! У нас — качество, надежность и стиль по доступной стоимости. Индивидуальный подход к каждому заказу!
калуга путаны шлюхи г. калуга
дизайнер интерьера заказать проект услуги дизайнера интерьера цена
вывод из запоя воронеж вывод из запоя воронеж .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 11, можете посмотреть на сайте: ремонт iphone 11 рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 12 pro max рядом, можете посмотреть на сайте: ремонт iphone 12 pro max рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
review https://my-sollet.com
программа 1с купить с установкой программа 1с купить с установкой .
my explanation https://toruswallet.org/
купить 1с бухгалтерия купить купить 1с бухгалтерия купить .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone se 2020, можете посмотреть на сайте: ремонт iphone se 2020
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Thanks in favor of sharing such a fastidious opinion, article is nice, thats
why i have read it entirely
吉宗極
小倉 ギオン
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
紫イミソーレ
https://www.k8-casino.asia/?GameID=925004818.html
友達と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
CR北斗の拳6拳王
牙狼冴島大河
CRぱちんこ仮面ライダーMAX EDITION
夜蝶飛翔特2発1
鯉の滝登り
お見事!サブちゃん
https://www.casinodengi.org/?ReviewID=924010818.html
友人と一緒にプレイすることで、共感が生まれ、楽しさが倍増します。
獣王 王者の覚醒
https://www.xn--k8-9g4a3b4f.space/?SitesID=925769818.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
CR押忍!番長
https://www.sawasdeeslot.com/?WatchID=920544818.html
パチンコの美しいデザインの台が多く、視覚的にも楽しめます。アートとしての側面もあります。
вывод из запоя в стационаре воронежа https://vyvod-iz-zapoya-v-stacionare-voronezh24.ru .
вывод из запоя в нижнем новгороде вывод из запоя в нижнем новгороде .
дизайнер интерьера цена комнат услуги дизайнера интерьера квартиры цена москва
dark web drug marketplace deep web drug markets https://github.com/darknetmarkets24/darknet-markets – tor darknet
наркология вывод из запоя в стационаре наркология вывод из запоя в стационаре .
вывод из запоя в стационаре вывод из запоя в стационаре .
вывод из запоя в наркологическом стационаре вывод из запоя в наркологическом стационаре .
free dark web darkmarket 2025 https://github.com/darknetmarkets24/darknet-markets – dark net
Your Final Destination for Premium In-Game Items!
In today’s rapid gaming environment, the joy of exploration and growth is essential. If you’re looking for rare objects, exclusive weapon looks, specialized types, keys, supplies, tasks, or easy balance assistance, look no further! Introducing to Items4Games, your complete shop for a extensive selection of premium in-game goods designed to improve your gaming playtime.
Why Items4Games Stands Out
Multiple Variety of In-Game Items
We supply a broad selection of in-game assets, from unique styles to unique items and game tokens. Our inventory is customized to improve your gameplay and immerse you in a environment of infinite possibilities.
Help for Popular Games
Our large catalog includes popular games like CS2, Fortnite, Valorant, Dota 2, and Call of Duty. No matter your gaming taste, we have assets that cater to the largest games in the sector.
Upgraded Gaming Journey
With exclusive in-game products, you can gain new abilities and showcase your unique touch. From special weapon designs to effective artifacts, our offerings transform your gaming sessions into outstanding activities.
Trustworthy and Trustworthy Exchanges
We emphasize your security with secure and trustworthy deals. You can shop with confidence, knowing that your information is guarded at all timeshares.
Affordable Costs
Get superior items without smashing the wallet. Our reasonable and reasonable pricing scheme ensures that improving your gaming experience is available to anybody.
User-Friendly Design
Our accessible platform allows you to quickly explore and secure the products you require, preserving you hours and boosting your buying experience.
What Is Ready for You at Items4Games
Explore weapon styles that show your character, limited artifacts for distinct achievements, and game codes that unlock new material. Our tools and quests aid you raise up without trouble, while refill services hold your game budget ready for engagement. Enjoy further game modes that add variety and depth to your gaming plays.
In conclusion, Items4Games is your ultimate goal for boosting your gaming experience with high-quality in-game goods. Check out our collection today and enhance your gameplay to new heights!
Dive into the World of GTA 5 RP: Become a part of Our Unique Roleplay Server
Salutations to the captivating world of GTA 5 RP, where all player can develop their individual narrative in a energetic gaming setting. Our roleplay server is ideal for those desiring an engaging and enveloping experience. Engage with a lively community of GTA RP aficionados and walk into this thrilling world!
How to Commence Playing?
1. Buy and install a licensed copy of GTA 5.
2. Set up the launcher.
3. Join to the server and register through the application or our platform to begin your adventure!
Our Environment: An Open World Adventure
Our server offers a special open-world setting with personalized mods that allow you to build your illicit network, set out on thrilling missions, and mold your avatar’s story. Whether you become part of local gangs or become a elite cop, the options are limitless!
Acquire Money and Increase Your Assets
Are you prepared to conquer the roads of Los Santos and earn big money? We supply the instruments and plans to help you accumulate a prosperity in the universe of GTA RP.
Explore CS2 Skins: Locate Your Ideal Match
Change Your Playstyle
Display your uniqueness in Counter-Strike 2 (CS2) with special weapon skins. Our store features a wide selection, from uncommon to limited-edition designs, permitting you to express your style and enhance your gameplay.
Effortless and Secure Acquisition
Appreciate a smooth shopping journey with fast digital delivery, ensuring your newly purchased skins are instantly available. Purchase confidently with our protected checkout process, whether you’re looking for budget options or luxury designs.
How It Works
1. Look through the Collection: Investigate a vast array of CS2 skins, sorted by rarity, weapon type, or style.
2. Select Your Skin: Add your perfect skin to your cart and continue to payment.
3. Dress Your Recent Skins: Promptly receive and use your skins in-game to differentiate during matches.
Budget-friendly Personalization
Customization should be attainable for everyone. We frequently offer discounts on CS2 skins, providing top-tier designs available at affordable prices.
Featured Skins
– P250 | Nuclear Threat (Factory New) – 3150 €
– Desert Eagle | Hand Cannon (Minimal Wear) – 450 €
– StatTrak™ P2000 | Ocean Foam (Factory New) – 285.88 €
– Glock-18 | Synth Leaf (Field-Tested) – 305 €
Start Shopping Today!
Upgrade your gameplay with our outstanding selection of CS2 skins. Whether enhancing your weapons or establishing a unique collection, our store is your source for premium skins. Change your gaming experience today!
Your Single Shop for AI-Powered Innovation
In the current rapid world, blending innovation with technology is essential for creativity and effectiveness. At Template Forge, we deliver high-quality, bespoke prompts created to elevate your artistic works across diverse domains.
Why Choose Template Forge?
We are focused to supplying only the top prompts to assist your artistic endeavors. Our crew of experts thoroughly compiles each prompt to guarantee applicability and importance, facilitating it simpler for you to discover motivation when you need it the most desperately.
Diverse Categories
Our extensive inventory spans various segments, featuring online marketing, problem-solving, and fiction writing. This diversity guarantees you have the means needed to address any task effectively.
Elevate Your Creativity
Template Forge’s ideas serve as a foundation for your ideas, allowing you to center on your individual perspective and aspirations minus the strain of idea generation. Each prompt is designed to stimulate and assist you through the imaginative process.
Connect with thousands of pleased creators who have tapped into the power of AI at Template Forge. Explore our wide subjects today and release your imaginative possibilities. With our top-notch prompts, developing with focus has ever been more straightforward. Welcome to your one-stop store for AI-enabled imagination!
In Jodo Do Tigrinho online slots, every spin is a step towards victory! Incredible graphics, exciting themes and many bonuses await you. Fortune favors the brave – try your hand and discover the world of winnings with Tigrinho!
dark web websites dark web websites https://github.com/darknetmarkets24/darknet-markets – dark web sites links
Cryptocurrency trading service bitqt with AI is automation and efficiency. Artificial intelligence monitors market dynamics, reduces risks and optimizes transactions. The perfect solution for beginners and professionals.
Discover the Realm of Minecraft: Your Ultimate Endurance and Anarchy Journey
Welcome to your Gateway to the Highly Thrilling and Interactive Minecraft Multiplayer Experience. Whether you’re a Designer, Fighter, Discoverer, or Schemer, our Platform Provides Limitless Opportunities to Enjoy Persistence and Disorder Features in Approaches you’ve Never seen Earlier.
Why Select Journeys in Minecraft?
Our Network is Developed to Offer the Top Minecraft Journey, Integrating Tailored Environments, Captivating Gameplay, and a Active Society. Discover, Master, and Construct your own Journeys with Tailored Elements Designed for Every type of User.
Primary Components
– Living and Anarchy Settings: Confront the Excitement of Persisting against the odds or Immerse into Chaotic PvP Fights with no rules and full freedom.
– Large Platform Capacity: With Space for up to 3,750 Players, the Fun never stops.
– 24/7 Server Uptime: Enter At All Times to Enjoy Uninterrupted, Stable Mechanics.
– Custom Content: Traverse our Expertly Designed Minecraft Maps Packed with Enhancements, Addons, and Special Assets from our Virtual Shop.
Distinctive Mechanics Options
Persistence Feature
In Endurance Option, you’ll Explore Endless Terrains, Procure Assets, and Design to your heart’s content. Combat off Mobs, Collaborate with Friends, or Conquer on Independent Challenges where only the Skilled Win.
Disorder Feature
For Users Looking For Disorder and Thrill, Disorder Mode Offers a Universe with No Rules. Enter in Adrenaline-Fueled PvP Engagements, Form Partnerships, or Dominate Others to Control the Realm. Here, Persistence of the Strongest is the Sole Law.
Custom Minecraft Elements
– Quest Zones: Navigate Special Minecraft Dungeons and RPG-Style Tasks.
– Economy and Transactions: Our Player-Driven Market Allows you to Buy, Purchase, and Exchange Goods to Ascend the Positions and Form Your Identity as a Powerful Player.
– Minecraft Inventory: Enter Premium Products, Improvements, and Ranks that Boost your Gameplay.
Minecraft Store: Enhance Your Experience
Our Online System Presents a Assortment of Improvements, Statuses, and Products to Suit every Playstyle. From Affordable Support Packs to Premium Levels, you can Unlock Exciting Options and Bring your Gameplay to the Next Level.
Top Products
– Donate Packs (x10) – €1.00
– VIP – €1.40
– Elysium Tier – €20.00
– OWNER Status – €40.00
– BOSS Tier – €60.00
Top Ranks for Premier Players
– CREATOR (€10.00) – Unlock Artistic Tools to Unleash your Creativity.
– Vanguard (€12.00) – Elite Perks and Tailored Perks.
– Paragon (€59.10) – Top Perks for the Premier User.
– Luminescent (€50.00) – Dominate as a Legendary Player on the Network.
Join Our Active Minecraft Group
We Aim in Building a Welcoming, Active, and Welcoming Group. Whether you’re Facing RPG Quests, Exploring Custom Maps, or Taking Part in Community-Based PvP, there’s Always something Different to Explore.
Things You Can Anticipate
– Friendly Player Base: Meet Fellow Minecraft Players from Globally.
– Exciting Events: Take Part in Special Tasks, Events, and Global Challenges.
– Dedicated Assistance: Our Moderators Ensures Reliable Play and Assists you with any Problems.
online slots Jodo Do Tigrinho are a unique combination of excitement and pleasure. Discover a variety of themes, bonus games and jackpots. Play comfortably, enjoying the well-thought-out interface and the chance to hit the jackpot!
Cryptocurrency trading service bitqt with AI is automation and efficiency. Artificial intelligence monitors market dynamics, reduces risks and optimizes transactions. The perfect solution for beginners and professionals.
キャッツ・アイ~コレクション奪還作戦~
カジノ 松
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
CR北斗の拳6拳王
https://www.k8-casino.asia/?SitesID=923603818.html
演出や音楽が迫力満点で、プレイするたびに興奮します。サウンドトラックも素晴らしい。
CR北斗の拳6 拳王 Ver.394
爆砕 青森
ビーストサップ
押忍!番长(操)
CR牙狼FINAL Ver.399(1:1)
黃金守護神
https://www.8casino8.com/?ReviewID=923716818.html
演出が単純明快で、ストレスなくプレイできるのが良いです。気軽に楽しめます。
P 真・北斗無双 第3章 ジャギの逆襲
https://www.8casino8.com/?ReviewID=925318818.html
シンプルなルールで、初心者でもすぐに楽しめます。気軽に挑戦できます。
鬼浜爆走紅蓮隊友情挽歌編
https://www.sawasdeeslot.com/?SitesID=920615818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
dark market onion blackweb https://github.com/darknetmarkets24/darknet-markets – darkmarkets
Скачайте бесплатно книгу https://storitelling.ru по сторителлингу и узнайте, как создавать истории, которые цепляют с первых строк. Практические советы, примеры и вдохновение для всех, кто хочет освоить искусство рассказчика.
Aviatrix game https://aviatrix-games.com/en/ has become a sensation in the world of crash games. Its unique format, featuring a rapidly growing multiplier and the possibility of an unexpected crash. Aviatrix crash game is at 1win, 1xbet, Mostbet, and Pin Up.
Участки в Лисичкином Очаге https://лисичкиночаг.рф/uchastki-lisichkinochag рядом с Серпуховым – идеальное место для вашего загородного дома. Просторные земли, свежий воздух и удобное расположение.
Компания АВИАПРОМ ВОЛГА предлагает широкий ассортимент авиационных масел, гидравлических жидкостей и индустриальных смазок. Мы являемся официальным импортером продукции пяти ведущих компаний Европы и Америки. Наши основные направления включают поставку высококачественных смазочных материалов для авиации, а также продуктов для промышленного и транспортного применения.
АВИАПРОМ ВОЛГА обеспечивает надежное сотрудничество, оперативные поставки и выгодные условия для клиентов из России и стран СНГ. Мы гарантируем качество продукции и соответствие международным стандартам.
Обратитесь к нам за профессиональной поддержкой в выборе необходимых масел и жидкостей для ваших нужд!
The MetaMask Wallet has changed the way I interact with crypto! With support for Safari, Firefox, and Opera, it’s so versatile. Learn more at https://metanaito.net/.
Aviatrix game https://aviatrix-games.com/en/ has become a sensation in the world of crash games. Its unique format, featuring a rapidly growing multiplier and the possibility of an unexpected crash. Aviatrix crash game is at 1win, 1xbet, Mostbet, and Pin Up.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ipad mini 3, можете посмотреть на сайте: ремонт ipad mini 3 в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
сколько стоит алкоголь на дом доставка алкоголя люберцы
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ipad mini 4 сервис, можете посмотреть на сайте: срочный ремонт ipad mini 4
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ipad pro 12.9, можете посмотреть на сайте: ремонт ipad pro 12.9
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Aviatrix game https://aviatrix-games.com/en/ has become a sensation in the world of crash games. Its unique format, featuring a rapidly growing multiplier and the possibility of an unexpected crash. Aviatrix crash game is at 1win, 1xbet, Mostbet, and Pin Up.
вывод из запоя в стационаре воронежа вывод из запоя в стационаре воронежа .
вывод из запоя стационар вывод из запоя стационар .
Betzula, canl? bahis konusunda yenilikci cozumler sunar. Fenerbahce Galatasaray derbisi icin guvenli bir sekilde kazanma sans?n?z? art?rabilirsiniz.
Betzula’n?n h?zl? odeme yontemleri, profesyonel hizmet garantisi verir. Bet Zula sosyal medya hesaplar?yla ozel promosyonlardan haberdar olabilirsiniz.
en onemli spor etkinliklerinin en iyi oranlarla kazanc saglayabilirsiniz.
Ayr?ca, bet zula giris linki, kesintisiz bahis deneyimi sunar. Ozel olarak, betzula guncel giris, profesyonel bir deneyim saglar.
Betzula, mobil uyumlu ve h?zl? erisim f?rsatlar?na kadar tum kullan?c?lar?n ihtiyaclar?n? kars?lar. favori tak?m?n?z?n galibiyetini kutlamak icin Betzula ile kazanmaya baslay?n!
707707+
bata4d
bata4d
dark markets 2025 dark web drug marketplace https://github.com/darknetmarkets24/darknet-markets – darknet site
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ipad mini 3, можете посмотреть на сайте: срочный ремонт ipad mini 3
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
вывод из запоя в стационаре самара вывод из запоя в стационаре самара .
вывод из запоя в стационаре вывод из запоя в стационаре .
выведение из запоя в стационаре выведение из запоя в стационаре .
Юридическая помощь https://kramzenergo.ru для физических и юридических лиц: консультации, составление документов, представительство в суде. Мы обеспечим надежную защиту ваших интересов и найдём оптимальное решение вашей проблемы.
купить диплом сколько стоит
official statement https://web-counterparty.io
Скачайте бесплатно книгу https://storitelling.ru по сторителлингу и узнайте, как создавать истории, которые цепляют с первых строк. Практические советы, примеры и вдохновение для всех, кто хочет освоить искусство рассказчика.
вставить окно пластиковое недорого цена melke pro
киного фильмы про природу kinogo фильмы о гангстерах
kinogo фильмы по популярности киного рейтинги
пропуска для грузового транспорта в москве http://www.urilin.ru .
вывод из запоя в стационаре самара вывод из запоя в стационаре самара .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ipad mini 4 адреса, можете посмотреть на сайте: ремонт ipad mini 4
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ipad pro 12.9 цены, можете посмотреть на сайте: ремонт ipad pro 12.9 в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
how to access dark web darknet markets https://github.com/darknetmarkets24/darknet-markets – dark market link
CR フィーバーマクロスフロンティア2W
黒部 キング
友達と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
Pフィーバーからくりサーカス
https://www.sawasdeeslot.com/?GameID=918500818.html
人気ゲームを基にしたパチスロで、ファンにはたまらない作品です。リアルな演出が楽しめます。
CRグラップラー刃牙
勇気 の 歌
L ゴールデンカムイ
パチスロ 甲鉄城のカバネリ
Pフィーバー炎炎ノ消防隊G
シンドバッドアドベンチャーは榎本加奈子でどうですか
https://k88214.com/?GameID=926312818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
P とある魔術の禁書目録
https://www.k8-casino.org/?SitesID=922931818.html
演出の多様性があり、毎回新しい体験が待っています。飽きることがありません。
CR暗黒騎士呀鎧伝
https://jahromblog.com/?ArticleID=920803818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
darkmarket darknet drug market https://github.com/darknetmarkets24/darknet-markets – darknet drugs
анонимный вывод из запоя в стационаре анонимный вывод из запоя в стационаре .
пропуск в москву для грузовиков пропуск в москву для грузовиков .
пропуск в москву для грузовиков пропуск в москву для грузовиков .
Ресторанный консалтинг UPSKILLhttps://upskilll.ru/consulting это профессиональные услуги в сфере ресторанного бизнеса: анализ рынка, аудит заведения, устранение слабых мест, обучение персонала, занимаемся наставничеством рестораторов с 2018 года.
Беговой портал https://get.run календарь забегов России, программы подготовки к забегом, составленные МСМК по легкой атлетике, статьи, консультации с тренером, ответы на вопросы
Сайт игровых промокодов промо коды для сайтов кс 2 это ваш доступ к эксклюзивным бонусам и скидкам. Бесплатные награды, внутриигровая валюта и уникальные акции ждут вас. Успейте воспользоваться всеми возможностями!
ST666
ST666 hiện đang khẳng định vị thế là sân chơi giải trí trực tuyến quy mô lớn và cực kỳ uy tín. Tại trang chủ ST666, bạn sẽ khám phá hàng loạt game cá cược hấp dẫn như live casino, cược thể thao, bắn cá đổi thưởng, xổ số may mắn, v.v. với tỷ lệ cược ưu đãi. Đừng bỏ lỡ cơ hội trải nghiệm cá cược đỉnh cao ngay tại ST666 để tận hưởng cảm giác xanh chín và thắng lớn!
Ищете промокоды для игр ggstandoff промокоды на деньги наш сайт – ваш лучший помощник! Собираем актуальные игровые промокоды для бонусов, скидок и эксклюзивных наград. Наслаждайтесь играми с максимальной выгодой – воспользуйтесь промокодами уже сегодня!
Хотите раскрутить продвижение телеграм мы знаем, как это сделать! Поможем увеличить охваты, привлечь активную аудиторию и вывести ваш контент на новый уровень.
I have not come across such an accurate and deep analysis of the current situation for a long time, thanks for the post https://kiywt.gov-edu.ru/id-309.html
вывести из запоя капельница на дому капельницы для восстановления здоровья
Velocidad critica
Equipos de calibración: importante para el desempeño uniforme y efectivo de las equipos.
En el campo de la avances actual, donde la eficiencia y la confiabilidad del aparato son de alta importancia, los equipos de ajuste juegan un función esencial. Estos sistemas específicos están diseñados para ajustar y estabilizar piezas giratorias, ya sea en equipamiento productiva, vehículos de transporte o incluso en dispositivos caseros.
Para los expertos en mantenimiento de equipos y los técnicos, operar con aparatos de calibración es crucial para promover el operación fluido y seguro de cualquier sistema giratorio. Gracias a estas soluciones innovadoras innovadoras, es posible limitar considerablemente las vibraciones, el zumbido y la carga sobre los rodamientos, aumentando la longevidad de partes caros.
De igual manera significativo es el rol que desempeñan los sistemas de ajuste en la soporte al consumidor. El apoyo experto y el soporte regular utilizando estos dispositivos facilitan proporcionar servicios de óptima nivel, incrementando la satisfacción de los clientes.
Para los responsables de empresas, la financiamiento en sistemas de calibración y medidores puede ser importante para optimizar la eficiencia y rendimiento de sus equipos. Esto es sobre todo importante para los inversores que dirigen modestas y pequeñas emprendimientos, donde cada detalle es relevante.
Asimismo, los equipos de ajuste tienen una extensa implementación en el campo de la seguridad y el supervisión de nivel. Posibilitan encontrar probables fallos, evitando reparaciones costosas y averías a los sistemas. Además, los datos recopilados de estos sistemas pueden aplicarse para mejorar procesos y potenciar la reconocimiento en motores de investigación.
Las sectores de aplicación de los sistemas de calibración incluyen diversas áreas, desde la manufactura de bicicletas hasta el control ambiental. No afecta si se considera de importantes producciones industriales o reducidos locales caseros, los aparatos de ajuste son necesarios para promover un desempeño efectivo y sin fallos.
скрипты хайпов – Купить скрипт хайпа, goldcoders
Ищете промокоды для игр рабочие промокоды в standoff 2 на кейсы наш сайт – ваш лучший помощник! Собираем актуальные игровые промокоды для бонусов, скидок и эксклюзивных наград.
Участок в Мишкином Лугу http://мишкинлуг.рф/uchastki-mishkinlug по Симферопольскому шоссе — идеальное место для строительства! Тихий поселок, прекрасные виды, удобный подъезд и все условия для комфортной жизни.
Участки в Лисичкином Очаге https://лисичкиночаг.рф неподалеку от Серпухова. Тихий, зеленый поселок с прекрасной природой и удобной транспортной доступностью. Здесь вы сможете построить комфортное жилье для себя и своей семьи.
Лисичкин Очаг возле Серпухова https://лисичкиночаг.рф/uchastki-lisichkinochag идеальные участки для вашего будущего дома! Живописная природа, хорошая транспортная доступность и возможность подключения всех коммуникаций ждут вас.
Купить участок – Участок под строительство дома, Инвестиция в землю
Сроки строительства под ключ – Дом без первоначального взноса, Сельская ипотека
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 12 рядом, можете посмотреть на сайте: ремонт iphone 12
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
перед выводом из запоя в стационаре http://www.vyvod-iz-zapoya-v-stacionare-samara24.ru/ .
CR牙狼魔戒之花 Ver.399
ぶどう 抜き
友人と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
八代将军 天国の戦い
https://www.stationslot.org/?ArticleID=922405818.html
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
P ぱちんこ シン・エヴァンゲリオン Type レイ
牙 狼 鋼
パチスロ バイオハザード7 レジデント イービル
CRルパン三世~消されたルパン Ver.394
CR JAWS再臨-SHARK PANIC AGAIN-
喧嘩祭
https://www.k8o.jp/?GameID=921920818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
輪るピングドラム
https://www.k8pachinko.online/?WatchID=924546818.html
お金をかけることに抵抗がある人も、少額から始められるのが魅力です。楽しみ方は多様です。
戦国パチスロ花の慶次戦極めし傾奇者の宴~
https://www.diabetesdirectory.org/?NewsID=920157818.html
パチンコは、友人や家族と一緒に楽しむのに最適です。共通の趣味があると盛り上がります。
After reading this post, you begin to look at familiar things around you in a completely different way https://irxpw.gov-edu.ru/id-9250.html
вывод из запоя самара стационар [url=http://vyvod-iz-zapoya-v-stacionare-samara25.ru]вывод из запоя самара стационар[/url] .
такой
Обменко орг – obmenko org, obmenko обменник
пропуска на грузовые машины в москве пропуска на грузовые машины в москве .
пропуска для грузового транспорта в москве https://www.extremeguide.ru .
на этом сайте водка казино – vodka регистрация, водка бет
equilibrador
Sistemas de balanceo: fundamental para el funcionamiento fluido y efectivo de las máquinas.
En el mundo de la tecnología actual, donde la productividad y la seguridad del sistema son de máxima trascendencia, los aparatos de equilibrado desempeñan un tarea esencial. Estos sistemas específicos están desarrollados para ajustar y regular elementos dinámicas, ya sea en maquinaria de fábrica, automóviles de transporte o incluso en electrodomésticos hogareños.
Para los especialistas en soporte de aparatos y los ingenieros, manejar con sistemas de balanceo es fundamental para proteger el rendimiento fluido y confiable de cualquier sistema rotativo. Gracias a estas soluciones tecnológicas sofisticadas, es posible disminuir sustancialmente las oscilaciones, el zumbido y la carga sobre los cojinetes, extendiendo la longevidad de elementos costosos.
También trascendental es el papel que tienen los equipos de ajuste en la servicio al usuario. El soporte técnico y el reparación permanente empleando estos sistemas posibilitan brindar asistencias de gran estándar, elevando la contento de los consumidores.
Para los responsables de proyectos, la financiamiento en equipos de equilibrado y medidores puede ser fundamental para mejorar la efectividad y productividad de sus equipos. Esto es principalmente significativo para los inversores que manejan modestas y medianas emprendimientos, donde cada detalle es relevante.
Asimismo, los sistemas de calibración tienen una gran implementación en el ámbito de la seguridad y el supervisión de nivel. Habilitan detectar eventuales fallos, impidiendo intervenciones elevadas y problemas a los dispositivos. Más aún, los resultados obtenidos de estos equipos pueden emplearse para mejorar procedimientos y mejorar la presencia en sistemas de consulta.
Las sectores de aplicación de los equipos de equilibrado incluyen diversas áreas, desde la producción de bicicletas hasta el seguimiento ambiental. No afecta si se trata de enormes producciones industriales o modestos locales caseros, los dispositivos de balanceo son necesarios para promover un desempeño efectivo y libre de interrupciones.
You can easily log in to the 1win website online | Sports betting on 1win is simple and diverse 1 win | 1win casino offers convenient and secure payment options for all users | The official 1win site provides many options for sports betting | You can now log into 1win using your phone or computer Download the 1win app.
After reading this post, you begin to look at familiar things in a completely different way https://erbpc.gov-edu.ru/id-8274.html
It is necessary that such thoughts are spread as widely as possible in our society https://vvvno.gov-edu.ru/id-8874.html
оформить пропуск в москву для грузовиков [url=www.urilin.ru /]оформить пропуск в москву для грузовиков[/url] .
The author touched on those problems that really worry every thinking person https://ldgry.gov-edu.ru/id-3817.html
Отзывы о компаниях и работодателях https://potrebsojuz.ru в одном месте. Узнайте реальное мнение сотрудников и клиентов, чтобы принять правильное решение при выборе работы или услуг.
Комплексная юридическая помощь https://kramzenergo.ru для вас и вашего бизнеса. Анализ дел, представительство в судах, поддержка на всех этапах
местное самоуправление Казахстан – фотографии, YV-TV Казахстан
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт iphone 12, можете посмотреть на сайте: ремонт iphone 12 рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet market list tor markets links how to get on dark web
клиника капельниц эффективные капельницы для лечения
1win afiliados 1win com
1win slot 1win site
dark web websites dark web site darknet drug store
Discover CS2 Skins: Locate Your Ideal Match
Transform Your Gameplay
Showcase your individuality in Counter-Strike 2 (CS2) with distinct weapon skins. Our store provides a wide selection, from rare to limited-edition patterns, permitting you to express your taste and enhance your gameplay.
Effortless and Safe Buying
Savor a seamless shopping journey with fast digital shipment, ensuring your recently purchased skins are instantly available. Buy confidently with our secure checkout process, whether you’re looking for low-cost options or high-quality designs.
How It Operates
1. Browse the Collection: Browse a wide variety of CS2 skins, arranged by rarity, weapon type, or style.
2. Select Your Skin: Include your desired skin to your cart and continue to payment.
3. Fit Your New Skins: Instantly receive and use your skins in-game to shine during matches.
Cost-effective Tailoring
Tailoring should be attainable for every player. We consistently offer discounts on CS2 skins, ensuring high-quality designs accessible at affordable prices.
Featured Textures
– P250 | Nuclear Threat (Factory New) – 3150 €
– Desert Eagle | Hand Cannon (Minimal Wear) – 450 €
– StatTrak™ P2000 | Ocean Foam (Factory New) – 285.88 €
– Glock-18 | Synth Leaf (Field-Tested) – 305 €
Begin Your Shopping Experience Today!
Improve your gameplay with our outstanding selection of CS2 skins. Whether upgrading your firearms or building a unique collection, our store is your hub for high-quality skins. Elevate your gaming experience today!
Откройте для себя историю слово пацана смотреть онлайн честный взгляд на суровую реальность, где дружба и слово дороже всего. Уникальный проект о жизни без прикрас и ценности принципов.
Call of Duty items
Your Ultimate Place for Premium In-Game Assets!
In modern fast-paced gaming environment, the excitement of exploration and progression is vital. If you’re hunting for rare items, exclusive weapon skins, custom modes, access, resources, challenges, or convenient top-up options, look no further! Hello to Items4Games, your one-stop destination for a extensive range of high-quality in-game items designed to boost your gaming journey.
Why Items4Games Stands Out
Varied Choice of In-Game Assets
We supply a broad assortment of in-game resources, from unique styles to unique relics and game access. Our collection is customized to elevate your gameplay and engage you in a realm of infinite choices.
Backing for Trending Games
Our extensive catalog showcases mainstream games like CS2, Fortnite, Valorant, Dota 2, and Call of Duty. No matter your gaming preference, we have products that serve to the top successes in the sector.
Enhanced Gaming Experience
With unique in-game assets, you can discover new potential and illustrate your distinct flair. From exclusive weapon styles to powerful artifacts, our products elevate your gaming sessions into remarkable events.
Protected and Dependable Transactions
We focus on your security with reliable and honest exchanges. You can buy with assurance, knowing that your data is guarded at all times.
Reasonable Fees
Access superior items without smashing the wallet. Our affordable and reasonable rate structure ensures that elevating your gaming journey is achievable to all.
User-Friendly Platform
Our easy-to-use design lets you to quickly explore and purchase the goods you want, saving you effort and enhancing your buying process.
What Awaits for You at Items4Games
Explore weapon designs that reflect your character, exclusive artifacts for noteworthy achievements, and game tokens that open new material. Our supplies and missions aid you develop up smoothly, while refill services ensure your game balance ready for play. Enjoy further game formats that bring change and complexity to your gaming plays.
In closing remarks, Items4Games is your best goal for improving your gaming experience with exclusive in-game products. Explore our catalog today and boost your gameplay to new extremes!
Roleplay jobs
Dive into the Universe of GTA 5 RP: Join Our Special Roleplay Server
Welcome to the thrilling world of GTA 5 RP, where every player can develop their individual narrative in a energetic gaming setting. Our roleplay server is ideal for those desiring an engaging and deep experience. Join a lively community of GTA RP fans and enter into this exciting world!
How to Begin Playing?
1. Buy and implement a licensed version of GTA 5.
2. Install the launcher.
3. Connect to the server and register through the software or our platform to begin your quest!
Our Platform: An Open World Journey
Our server offers a distinct open-world environment with tailored mods that enable you to forge your underworld kingdom, set out on thrilling missions, and form your avatar’s narrative. Whether you join local gangs or transform into a elite cop, the options are endless!
Earn Money and Increase Your Fortune
Are you set to conquer the thoroughfares of Los Santos and acquire big money? We supply the resources and strategies to help you gather a fortune in the universe of GTA RP.
Marketing & Sales AI prompts
The Single Marketplace for AI-Powered Imagination
In today’s fast-paced world, blending innovation with digital solutions is vital for creativity and efficiency. At Our Platform, we deliver top-notch, specially designed prompts intended to improve your innovative projects across different domains.
Reasons to Choose Template Forge?
We are committed to providing only the highest quality prompts to support your creative initiatives. Our staff of professionals thoroughly curates each prompt to guarantee utility and significance, allowing it more convenient for you to discover motivation when you desire it the most.
Diverse Categories
Our extensive inventory includes diverse types, including digital marketing, problem-solving, and narrative crafting. This breadth guarantees you have the tools needed to manage any project competently.
Elevate Your Creativity
Template Forge’s prompts serve as a springboard for your creativity, enabling you to center on your unique perspective and goals free from the pressure of creativity. Each prompt is created to encourage and lead you through the artistic journey.
Become part of thousands of content makers who have utilized the potential of AI at Template Forge. Investigate our wide options today and access your artistic capabilities. With our superior prompts, producing with focus has not been easier. Welcome to your ultimate marketplace for artificial intelligence-driven imagination!
dark web market deep web drug store dark market list
ラブドール 私はただあなたに会う必要があります、ただ紫の雨の中であなたに会う必要があります
срочный вывод из запоя срочный вывод из запоя .
программа 1с стоимость программа 1с стоимость .
business movers spmovers12.com .
Выбор современных родителей – коляска-трость, с прочными колесами и удобной ручкой.
Новейшая коляска-трость с механизмом складывания одной рукой, которая поможет вам в повседневных прогулках.
Купите легкую и компактную коляску-трость по доступной цене, которая станет вашим незаменимым помощником.
Компактная коляска-трость для активных мам и пап, с прочными колесами и удобной спинкой.
babyton коляска трость https://kolyaski-trosti-progulochnye.ru/ .
dark web search engines darknet seiten dark web access
雷電(ピンク)
エヴァ 搭乗
ゲームの進行が早く、飽きずに楽しめるのが良いです。短時間でも満足感があります。
お見事!サブちゃん
https://www.k8-casino.asia/?WatchID=926784818.html
新しい台が出るたびにワクワクします。流行を追うのが楽しいです。
CRモンスターハンタ Ver.199
天井 狙い
ケロロ軍曹
パチスロビッグドリームinロストアイランド2
S メイドインアビス
CRザ・キング・オブ・ファイターズ
https://www.k8o.jp/?WatchID=921096818.html
パチンコのルールはシンプルですが、奥が深いです。初心者でもすぐに楽しめます。
CR北斗の拳5 百裂
https://www.k8.rodeo/?NewsID=921069818.html
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
L エヴァンゲリオン ~未来への創造~
https://www.k8pachinko.asia/?GameID=919994818.html
定期的に新しい台が出るので、飽きずに楽しめます。ファンにはたまらないですね。
промокод на подключение продамуса https://www.promokod-pro.ru .
MetaMask Extension” is incredibly useful for managing crypto wallets. For detailed guides and tips, check out https://kingroada.com/.
GAMELANTOGEL
dark net deep web markets darknet seiten
киного российские фильмы kinogo фильмы для отдыха
kinogo премьеры фильмов kinogo фильмы по годам
kinogo аниме kinogo фильмы по странам
kantor bola
kantorbola link alternatif terbaru , kami juga menyediakan APK Android dengan fitur yang akan memanjakan anda dalam setiap aktifitas di situs kantor bola .
купить насос для воды в колодец купить насос для воды в колодец .
I completely agree with the author and believe that these thoughts should be heard by as many people as possible https://lrswl.alexey-petrovich-sitnikov.ru/id-9322.html
CR緋弾のアリアII Ver.319
挙動
各台によって異なる演出があり、どれを選ぶかも楽しみの一つです。自分の好みの台を見つける楽しさがあります。
アイムジャグラーEX
https://www.diabetesdirectory.org/?SitesID=928061818.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
お見事!サブちゃん
市議 カジノ
大工の源さん桜満開!源 DREAM ver.~
攻殻機動隊S.A.C
e スマパチ シン・エヴァンゲリオン Type カヲル
デビルメイクライ4
https://www.k8-casino.org/?ReviewID=925366818.html
定期的に通うことで、常連さんとのつながりが生まれます。コミュニティ感が楽しいです。
バイオハザード6 2発1
https://www.diabetesdirectory.org/?SitesID=927887818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
鉄拳R 2004
https://jahromblog.com/?WatchID=921624818.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
GAMELANTOGEL
selam, vds sunucu kiralama icin ihtiyacimi karsiladi tesekkur ederim
dark web markets darknet seiten bitcoin dark web
оформить пропуск в москву для грузовиков оформить пропуск в москву для грузовиков .
darknet site darknet drugs darknet drug links
exciting online slot tigrinho 777 is excitement, fun and the opportunity to win a big prize. Plunge into the atmosphere of adventure and try your luck right now!
алкоголизм лечение вывод из запоя ростов алкоголизм лечение вывод из запоя ростов .
программа 1 с купить https://www.kupit-1s15.ru .
Ищете свадебного фотографа для идеальной свадебной фотосессии?
Я помогу запечатлеть самые трогательные моменты вашего дня! Эмоции, искренность и индивидуальность в каждом кадре.
заказать услугу и узнать подробнее – видеограф в коломне.
Ваша история любви — в моих фотографиях!
Откройте для себя bs2site at возможности даркнет-рынка с тысячами предложений. Быстрая регистрация, надежные сделки и анонимность на каждом этапе.
Лучшее онлайн казино https://1wincasino.pl Огромный выбор автоматов, настольных игр и live-казино. Уникальные акции, приветственные бонусы и мгновенные выплаты сделают вашу игру еще интереснее.
текст песни сестра https://faav.ru
tor markets 2025 tor marketplace dark web sites links
Discover the Domain of Minecraft: Your Best Existence and Disorder Journey
Welcome to your Entrance to the Exceptionally Exciting and Absorbing Minecraft Shared Encounter. Whether you’re a Architect, Combatant, Discoverer, or Schemer, our Realm Provides Infinite Options to Explore Living and Chaos Environments in Ways you’ve Seldom seen Until Now.
Why Select Explorations in Minecraft?
Our Platform is Designed to Provide the Top Minecraft Journey, Merging Unique Realms, Thrilling Interaction, and a Active Network. Traverse, Master, and Design your own Quests with Exclusive Features Customized for All type of User.
Essential Components
– Endurance and Disorder Settings: Face the Excitement of Surviving against the odds or Immerse into Wild PvP Battles with no rules and full freedom.
– Massive Server Capacity: With Room for up to 3,750 Participants, the Action never stops.
– 24/7 Server Status: Access Anytime to Explore Uninterrupted, Consistent Mechanics.
– Custom Material: Navigate our Precisely Created Minecraft Environments Loaded with Mods, Addons, and Unique Assets from our Virtual Store.
Unique Mechanics Options
Living Option
In Persistence Feature, you’ll Navigate Wide Terrains, Procure Assets, and Design to your heart’s content. Combat off Creatures, Team Up with Allies, or Face on Single-Player Trials where only the Skilled Prevail.
Freedom Mode
For Players Seeking Chaos and Adrenaline, Anarchy Scenario Provides a Realm with No Boundaries. Engage in Competitive PvP Fights, Form Teams, or Compete With Opponents to Rule the Environment. Here, Persistence of the Fittest is the Ultimate Order.
Custom Minecraft Components
– Exploration Zones: Navigate Special Minecraft Maps and Adventurous Quests.
– Commerce and Trading: Our Interactive Economy Permits you to Sell, Buy, and Trade Items to Rise the Tiers and Form Your Reputation as a Dominant Competitor.
– Minecraft Store: Access Premium Tools, Levels, and Ranks that Elevate your Gameplay.
Minecraft Inventory: Upgrade Your Experience
Our In-Game Marketplace Delivers a Range of Features, Ranks, and Products to Cater To every Preference. From Budget-Friendly Gift Cases to Top-Level Statuses, you can Gain Exciting Options and Advance your Gameplay to the Higher Level.
Trending Products
– Donate Packs (x10) – €1.00
– VIP – €1.40
– Elysium Rank – €20.00
– OWNER Level – €40.00
– BOSS Rank – €60.00
Top Tiers for Ultimate Players
– CREATOR (€10.00) – Unlock Design Tools to Showcase your Ideas.
– Vanguard (€12.00) – Exclusive Features and Tailored Features.
– Paragon (€59.10) – Exclusive Features for the Top Gamer.
– Luminescent (€50.00) – Excel as a Iconic Player on the Realm.
Join Our Active Minecraft Group
We Believe in Creating a Welcoming, Vibrant, and Inclusive Group. Whether you’re Tackling RPG Adventures, Traversing Tailored Worlds, or Participating in Interactive PvP, there’s Consistently something Fresh to Explore.
Elements You Can Anticipate
– Friendly Community: Engage With Similar Minecraft Players from Around the World.
– Exciting Activities: Take Part in Exclusive Tasks, Tournaments, and Exclusive Challenges.
– Dedicated Help: Our Moderators Provides Smooth Play and Assists you with any Questions.
blackweb official website tor market darknet markets
토토사이트
토토사이트 커뮤니티: 안전한 베팅을 위한 길잡이
토토사이트 커뮤니티는 스포츠 베팅을 즐기는 사람들에게 단순한 정보 공유의 장을 넘어, 안전한 배팅 환경을 조성하는 데 중요한 역할을 합니다. 이곳에서는 초보자부터 전문가까지 다양한 사람들이 모여 베팅 전략을 논의하고, 신뢰할 수 있는 토토사이트를 추천하며, 먹튀 사고를 예방하기 위한 정보를 공유합니다. 특히, 사설 토토사이트의 위험성을 알리고, 이를 피할 수 있는 방법을 제시함으로써 사용자들이 더 안전하게 베팅을 즐길 수 있도록 돕습니다.
사설 토토사이트의 숨겨진 위험
사설 토토사이트는 정부의 규제를 받지 않기 때문에 다양한 문제를 일으킬 수 있습니다. 예를 들어, 공정하지 않은 배당률, 과장된 광고, 개인정보 유출 등이 대표적인 문제점입니다. 또한, 사설 토토사이트는 법적 문제로 인해 갑자기 사라지는 경우가 많아 사용자들이 금전적 손실을 입을 위험이 큽니다. 이런 이유로, 토토 커뮤니티에서는 사설 토토사이트보다는 규제를 받는 공식 사이트를 이용할 것을 권장합니다.
토토 커뮤니티가 제공하는 가치
토토 커뮤니티는 단순히 정보를 나누는 공간이 아니라, 사용자들이 안전하게 베팅할 수 있도록 돕는 네트워크입니다. 예를 들어, 새로운 토토사이트가 등장했을 때 커뮤니티 멤버들은 해당 사이트의 운영 주체, 결제 시스템, 사용자 리뷰 등을 꼼꼼히 검토합니다. 이를 통해 먹튀 사고를 미리 방지하고, 신뢰할 수 있는 사이트만을 추천합니다. 또한, 문제가 발생했을 때는 보증 사이트를 통해 사용자들이 손실을 최소화할 수 있도록 돕습니다.
어떻게 신뢰할 수 있는 토토사이트를 찾을까?
토토 커뮤니티에서는 신뢰할 수 있는 토토사이트를 찾기 위해 몇 가지 중요한 기준을 제시합니다. 먼저, 사이트의 운영 주체가 명확한지 확인하는 것이 중요합니다. 정부나 공인된 기관에서 관리하는 사이트라면 신뢰할 수 있습니다. 또한, 다른 사용자들의 리뷰를 참고하여 사이트의 신뢰성을 판단할 수 있습니다. 긍정적인 평가가 많고, 문제가 거의 없는 사이트라면 안전하게 이용할 수 있습니다.
결제 시스템도 중요한 확인 요소 중 하나입니다. 신용카드, 전자지갑 등 다양한 결제 방법을 지원하고, SSL 암호화를 통해 금융 정보를 보호하는 사이트라면 더욱 신뢰할 수 있습니다. 마지막으로, 온라인 커뮤니티나 포럼에서 해당 사이트에 대한 평판을 확인하는 것도 좋은 방법입니다. 다른 사용자들이 어떻게 평가하는지, 문제가 없는지 꼼꼼히 살펴보는 것이 중요합니다.
토토 커뮤니티의 보증 사이트
토토 커뮤니티에서는 먹튀 사고를 예방하기 위해 보증 사이트를 추천합니다. 이러한 사이트는 사용자들의 개인정보와 금융 정보를 보호하며, 문제가 발생했을 때 빠르게 해결할 수 있는 시스템을 갖추고 있습니다. 보증 사이트를 이용하면 안전하게 베팅을 즐길 수 있으며, 만약 문제가 생기더라도 커뮤니티의 도움을 받아 해결할 수 있습니다.
마무리
토토사이트 커뮤니티는 스포츠 베팅을 즐기는 사람들에게 안전하고 신뢰할 수 있는 정보를 제공하는 중요한 공간입니다. 사설 토토사이트의 위험성을 인지하고, 신뢰할 수 있는 사이트를 선택하는 것이 무엇보다 중요합니다. 토토 커뮤니티의 정보를 참고하여 안전한 베팅 환경을 조성하고, 스포츠 베팅을 더욱 즐겁게 즐기시길 바랍니다. 항상 책임감 있게 베팅에 임하는 것도 잊지 마세요!
The Metamask login process is so secure yet simple with the tips shared on https://metamenu.org/. Crypto safety at its best!
kantorbola
KANTORBOLA adalah pilihan terbaik bagi pecinta slot online. Nikmati berbagai permainan slot menarik, bonus besar, dan pengalaman bermain yang aman dan terpercaya.
сезон охоты охота с манком на утку
darknet market dark websites deep web drug markets
рыбалка на частных территориях рыбалка на открытой воде
надежный маркетплейс bs2site at где сочетаются безопасность, широкий выбор товаров и удобство использования. Платформа работает с анонимными платежами и гарантирует полную конфиденциальность для всех пользователей.
Tercüme hizmeti sektöründeki 14 yıllık deneyimimizle hızlı ve doğru tercüme için hemen fiyat alabilir, çevirinizi dakikalar içinde başlatabilirsiniz. Güvenilir tercüme hizmeti almak artık çok kolay!
dark web sites how to access dark web tor darknet
darknet drugs darkmarket 2025 deep web drug markets
パチスロ 貞子3D
ギャンブル倍
パチンコは、友人や家族と一緒に楽しむのに最適です。共通の趣味があると盛り上がります。
CR 北斗の拳6 拳王
https://www.k8-casino.asia/?WatchID=923220818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
翔马(黑)
スマスロ炎炎
ミリオンゴッド神々の凱旋
ケロロ軍曹
P閃乱カグラ2
Pフィーバーダンベル何キロ持てる
https://www.casinodengi.org/?ReviewID=924232818.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR聖戦士ダンバイン
https://www.ja-securities.jp/?SitesID=919367818.html
大当たりの瞬間には、周囲の人々との一体感が生まれます。共に喜びを分かち合える楽しさがあります。
戦国BASARA3
https://www.k8-casino.org/?WatchID=919494818.html
新台の導入イベントなどで、ワクワク感が増すのが楽しいです。特別感があります。
deep web drug links dark market url dark web links
Ищете свадебного фотографа для идеальной свадебной фотосессии?
Я помогу запечатлеть самые трогательные моменты вашего дня! Эмоции, искренность и индивидуальность в каждом кадре.
заказать услугу и узнать подробнее – свадебная фотосессия.
Ваша история любви — в моих фотографиях!
find out https://playslotrealmoney.com/elixir-enigma-real-money/
free dark web darkmarket 2025 tor markets 2025
пропуск для грузового транспорта в москву пропуск для грузового транспорта в москву .
пропуск в москву на грузовой автомобиль пропуск в москву на грузовой автомобиль .
This post contains all the important thoughts that I have long wanted to hear https://aysnm.alexey-petrovich-sitnikov.ru/id-6879.html
Thanks for some other informative blog. The place else may I am getting that kind of information written in such a perfect means? I’ve a mission that I am just now operating on, and I have been on the glance out for such info.
ответы на игру слова
tor market url dark markets 2025 dark market url
merhaba, sanal sunucu kiralama paketleri hakkında inceleyebilirsiniz.
moving companies moving companies .
Университет ресторанного бизнеса https://upskilll.ru/university UPSKILL: обучаем рестораторов, управляющих и сотрудников ресторанов, полностью адаптируя наши курсы, тренинги и программы обучения под особенности каждого заведения.
darkweb marketplace deep web drug store
darknet drug store https://github.com/darknetmarkets24/darknet-markets – dark market url
darknet drug links darknet market list
darknet drug links https://github.com/darknetmarkets24/darknet-markets dark market list
darknet drug store deep web drug markets tor market url
dark market list darknet drugs deep dark web
ремонт стиральной машины indesit ремонт стиральных машин беко
drug markets dark web darknet markets
darkweb marketplace dark web markets
darknet sites https://github.com/darknetmarkets24/darknet-markets dark web markets
I’ve been looking for such thoughts for a long time, formulated so clearly and understandably for everyone https://xzbpu.alexey-petrovich-sitnikov.ru/id-9101.html
いみそーれ(红花)
吉田 竜也
パチンコの世界には、運だけではなく、戦略も求められます。自分の考えを試す楽しさがあります。
CR JAWS再臨-SHARK PANIC AGAIN-
https://www.xn--k8-9g4a3b4f.store/?ReviewID=923782818.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。目が離せません。
BLOOD+ 二人の女王
sアバサー
CR 大海物語4
デビルサバイバー2 最後の7日間
BLOOD+ 二人の女王
ジャンキージャグラー
https://www.8casino8.com/?ReviewID=918886818.html
明るいデザインと楽しげな演出が魅力。遊んでいるだけで元気が出ます。
P 北斗の拳 暴凶星
https://www.k8-casino.asia/?ArticleID=926383818.html
大当たりを引いたときの高揚感は何とも言えません。日常のストレスを忘れさせてくれます。
e ルパン三世 THE FIRST
https://www.k8pachinko.online/?WatchID=923352818.html
パチンコを通じて、運を試すドキドキ感が味わえます。勝ち負けにこだわらず楽しむのがポイントです。
It’s amazing how the author was able to describe so deeply and insightfully what many people think https://kpwci.alexey-petrovich-sitnikov.ru/id-7851.html
The author touched on exactly those issues that worry most thinking people now https://phkol.alexey-petrovich-sitnikov.ru/id-5041.html
darkmarket url dark web sites links deep web drug store
darkweb marketplace dark web links
420 movement in prague https://shop-cannabis-prague.com
onion market https://github.com/darknetmarkets24/darknet-markets – deep web markets
dark web market drug markets dark web
darknet markets https://github.com/darknetmarkets24/darknet-markets dark web sites links
darknet market lists darkweb marketplace the dark internet
Seattle Premium Limo Service
Experience the epitome of luxury and reliability with our Limo Service , offering an exquisite fleet including Town car and Limo in Seattle . Our services are meticulously designed to cater to your diverse transportation needs, ensuring you travel in comfort and style.
Transportation from Seattle to Spokane
Whether you’re traveling for business or leisure, our dedicated Transportation from Seattle to Spokane service ensures a smooth and hassle-free journey. Our professional chauffeurs are well-versed with the most efficient routes, guaranteeing a timely and comfortable trip across the state. Enjoy the scenic beauty of Washington from the comfort of our well-maintained vehicles, equipped with modern amenities to make your journey pleasant and productive.
Transportation Services to Meetings and Events
Impress your clients and arrive in style at your next corporate meeting with our Transportation Services to Meetings and Events . Our punctual and courteous chauffeurs ensure you reach your destination on time, every time. We understand the importance of professionalism and discreetness in corporate travel, and our services are tailored to meet these expectations.
Our fleet of Town Car and Limo in Seattle is perfect for various events, from business meetings to special occasions like weddings and proms. Each vehicle is maintained to the highest standards, offering a luxurious and safe travel experience. With ample legroom, plush interiors, and state-of-the-art entertainment systems, our vehicles provide the perfect environment to relax or prepare for your event.
At Seattle Premium Limo Service, we pride ourselves on our commitment to excellence. Our team of experienced chauffeurs undergoes rigorous training to ensure they deliver top-notch service. We prioritize your safety and comfort, making sure every ride is a seamless and enjoyable experience.
Whether you need Transportation from Seattle to Spokane or Transportation Services to Meetings and Events , our Limo Service with Town Car and Limo in Seattle is your ultimate solution. Book with us today and experience the difference in luxury travel. Contact us for more information or to reserve your ride.
deep web drug links dark web markets
darkmarkets https://github.com/darknetmarkets24/darknet-markets – darknet market list
dark web market darknet websites
darknet market lists https://github.com/darknetmarkets24/darknet-markets darknet drug store
dark market list dark market url
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт imac 27 рядом, можете посмотреть на сайте: срочный ремонт imac 27
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
dark web sites links tor markets
tor markets darknet drug links drug markets onion
нажмите здесь
KENT
darknet markets https://github.com/darknetmarkets24/darknet-markets – darknet websites
dark web market list dark web market links
darkmarket 2022 tor marketplace
darkweb marketplace tor markets links dark web markets
darknet market deep web drug links
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ipad air 2 рядом, можете посмотреть на сайте: срочный ремонт ipad air 2
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
deep web drug markets https://github.com/darknetmarkets24/darknet-markets – tor darknet
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт imac 21, можете посмотреть на сайте: срочный ремонт imac 21
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet site deep dark web
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт фотоаппаратов canon в москве, можете посмотреть на сайте: ремонт фотоаппаратов canon
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
best darknet markets dark market
Платформа Курьер Купер https://cash-kuper.ru открывает возможности для работы в доставке. Свободный график, прозрачная система оплаты и заказы поблизости – идеальный выбор для тех, кто ищет подработку или основной заработок.
dark web access darknet markets dark web site
darknet sites deep web markets
dark web links https://github.com/darknetmarkets24/darknet-markets – dark websites
FOSIL4D
dark market link dark web links
dark web drug marketplace darkmarkets darkmarket 2025
darknet seiten dark web access blackweb
Persentase Pengembalian Pragmatic Mesin Menguntungkan dan juga Signifikansi Bermain Mesin Percobaan Pragmatic Mesin
Aktivitas mesin di internet semakin digemari, serta Pragmatic Perusahaan (PG Mesin) merupakan beberapa penyedia terbaik karena grafis memukau, fungsi kreatif, dan juga Persentase Pengembalian (RTP) yang tinggi. Akan tetapi, sebelumnya mencoba memakai uang sunguhan, perlu untuk menjalankan slot versi uji Pragmatic Permainan. Kenapa? Berikut ulasannya.
Apa Fitur Game Percobaan Playtech Mesin?
Slot versi uji adalah versi percobaan dalam game game yang memakai saldo simulasi, tanpa modal sunguhan. Fitur ini memungkinkan player agar mengenal mekanisme aktivitas, fungsi hadiah, dan juga Return to Player dengan tidak bahaya keuangan.
Alasannya Memainkan Game Percobaan Playtech Slot Penting?
Mengetahui Sistem Game
Tiap slot memiliki aturan serta fitur berbeda. Mesin demo membantu kamu mempelajarinya dengan tidak stres.
Mengetes Tingkat RTP
RTP ialah rasio kemenangan teoritis pada rentang jauh. Menggunakan percobaan, pemain bisa mengamati sejauh mana frekuen permainan menghasilkan keuntungan.
Membuat Taktik
Game versi uji memungkinkan Anda supaya mengevaluasi rencana seperti manajemen taruhan tanpa adanya risiko rugi modal.
Mengetahui Mesin Favorit
PG Provider memiliki berbagai konsep game. Melalui menjalankan versi uji, pemain dapat mencari aktivitas yang tepat dengan selera kamu.
Mencegah Rugi Signifikan
Memainkan versi uji memudahkan kamu mengerti permainan sebelum Anda bertaruh dengan dana asli, meminimalkan bahaya kerugian.
Kelebihan Bermain Slot Versi Uji Pragmatic Game
Tanpa Potensi Kerugian Finansial: Mencoba memakai saldo virtual, dengan tidak tekanan rugi dana.
Memperkuat Keyakinan Diri: Melatih melalui percobaan menjadikan pemain lebih siap pada saat bergeser pada game menggunakan dana nyata.
Mengetahui Fungsi Hadiah: Ketahui teknik memicu fitur insentif contohnya Spin Gratis atau juga Simbol Liar.
Meminimalkan Waktu dan Uang: Uji beberapa slot tanpa adanya perlu membuang modal.
Saran Bermain Mesin Demo Pragmatic Game
Fokus pada fitur bonus dan cara kerjanya.
Amati tingkat volatilitas mesin (tinggi atau juga rendah).
Susun batas waktu serta budget meski mencoba menggunakan kredit virtual.
Jelajahi berbagai tema untuk mengetahui pilihan Anda.
“However, against the backdrop of the global economic slowdown and a more uncertain business environment, firms are likely to take a cautious approach regarding salary increments,” said MOM. 토토주소
darkweb marketplace blackweb official website dark markets
darknet site tor market
darknet site https://github.com/darknetmarkets24/darknet-markets – tor market
dark web markets dark web market list
darkmarket link https://github.com/darknetmarkets24/darknet-markets dark market list
darknet markets 2025 dark net dark web access
Онлайн покупки сигарет https://sigarety-market.ru широкий выбор, доступные цены и оперативная доставка. Закажите популярные бренды сигарет прямо на дом и оцените удобство сервиса!
Консалтинг и обучение https://upskilll.ru для ресторанного бизнеса: развитие персонала, оптимизация процессов и повышение прибыли. Мы внедряем проверенные стратегии и передовые инструменты для роста вашего заведения.
L ゴールデンカムイ
上海 カジノ
キャラクターの個性が豊かで、ストーリーに引き込まれます。忍者の世界観が楽しめます。
S この素晴らしい世界に祝福を!
https://www.sawasdeeslot.com/?WatchID=924366818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
パチスロビッグドリームinロストアイランド2
泡 銭
e スマパチ シン・エヴァンゲリオン Type カヲル
戦国乙女剣戟に舞う白き剣聖西国参戦編
ドラゴンが降りる
北斗の拳 ラオウ
https://www.k8pachinko.club/?ReviewID=925186818.html
美しいグラフィックと音楽が、プレイ中の没入感を高めます。
CR北斗の拳5 百裂
https://www.k8-casino.asia/?NewsID=920529818.html
ボーナスゲームの演出が豪華で、期待感が高まります。特に大当たり時は圧巻。
鬼武者3
https://www.k8-casino.jp/?GameID=927710818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
darknet drug market darknet market darkmarket link
razer kraken сайт kraken tor
dark market list darkmarket list
darknet market https://github.com/darknetmarkets24/darknet-markets – dark market url
dark markets 2022 darkmarket
ремонт бойлера electrolux ремонт водонагревателей
buy hemp in prague https://shop-cannabis-prague.com
dark market 2022 https://github.com/darknetmarkets24/darknet-markets deep dark web
darkmarket list dark web drug marketplace dark web market
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ipad air 2 сервис, можете посмотреть на сайте: ремонт ipad air 2 адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
kadın giyim
микрозаймы онлайн на карту без отказа микрокредит
drug markets dark web deep web markets
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт imac 21 в москве, можете посмотреть на сайте: ремонт imac 21 сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
пропуск в москву для грузовых автомобилей [url=http://www.urilin.ru ]пропуск в москву для грузовых автомобилей[/url] .
пропуск в москву для грузовых автомобилей пропуск в москву для грузовых автомобилей .
darkmarket list dark web market links onion market
I blog quite often and I genuinely appreciate your content. This great article has really peaked my interest. I’m going to take a note of your website and keep checking for new details about once per week. I opted in for your RSS feed as well.
loyalty program
darkmarket list darknet market links
deep web drug markets https://github.com/darknetmarkets24/darknet-markets – darkmarkets
Pretty component of content. I just stumbled upon your web site and in accession capital to say that I acquire actually enjoyed account your weblog posts. Anyway I’ll be subscribing to your feeds or even I achievement you get admission to constantly rapidly.
ответ на игру найди слова
darknet market dark market url
darkmarket url https://github.com/darknetmarkets24/darknet-markets darkmarket 2022
toto138
free dark web dark web market links darknet links
Diagnostico de equipos
Equipos de ajuste: esencial para el rendimiento suave y productivo de las equipos.
En el mundo de la tecnología actual, donde la efectividad y la fiabilidad del dispositivo son de alta relevancia, los dispositivos de balanceo desempeñan un rol vital. Estos sistemas específicos están desarrollados para balancear y regular componentes rotativas, ya sea en herramientas productiva, transportes de movilidad o incluso en aparatos de uso diario.
Para los expertos en conservación de dispositivos y los ingenieros, utilizar con aparatos de ajuste es crucial para garantizar el funcionamiento fluido y confiable de cualquier aparato rotativo. Gracias a estas opciones modernas avanzadas, es posible reducir sustancialmente las vibraciones, el sonido y la presión sobre los rodamientos, mejorando la vida útil de elementos caros.
También significativo es el tarea que cumplen los equipos de ajuste en la atención al consumidor. El soporte técnico y el soporte continuo utilizando estos equipos facilitan brindar asistencias de óptima excelencia, elevando la contento de los consumidores.
Para los dueños de empresas, la inversión en estaciones de balanceo y dispositivos puede ser clave para incrementar la eficiencia y productividad de sus sistemas. Esto es principalmente importante para los empresarios que administran reducidas y medianas empresas, donde cada aspecto vale.
Por otro lado, los sistemas de balanceo tienen una vasta utilización en el ámbito de la protección y el control de excelencia. Facilitan encontrar posibles fallos, impidiendo reparaciones costosas y averías a los dispositivos. Además, los información recopilados de estos sistemas pueden aplicarse para mejorar procesos y mejorar la exposición en sistemas de investigación.
Las sectores de implementación de los equipos de equilibrado abarcan diversas industrias, desde la manufactura de bicicletas hasta el monitoreo del medio ambiente. No importa si se refiere de grandes fabricaciones de fábrica o limitados establecimientos domésticos, los sistemas de equilibrado son indispensables para proteger un operación eficiente y sin presencia de interrupciones.
tor market links deep web drug links
S 炎炎ノ消防隊
戦車 道
多彩なリーチ演出があり、どのタイミングで当たるかドキドキします。
鉄拳2nd
https://www.k8pachinko.asia/?GameID=924296818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
南国育ち
ちび むす
ルパン三世 ロイヤルロード金海に染まる黄金神殿
吉宗
CRF宇宙戦艦ヤマト YR
CR牙狼GOLDSTORM翔 Ver.319
https://www.xn--k8-9g4a3b4f.space/?ArticleID=921853818.html
パチンコの演出が華やかで、視覚的にも楽しめます。美しいデザインが魅力的です。
CR 真・北斗無双
https://www.k8pachinko.cc/?NewsID=927711818.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
麻雀物語
https://www.k8.rodeo/?ArticleID=926629818.html
ゲームの進行が早く、短時間でも満足感があります。気軽に楽しめるのが良いです。
deep web markets darkmarkets dark web site
darknet market lists deep dark web
проверка микрозаймов онлайн взять деньги под процент
deep web drug url https://github.com/darknetmarkets24/darknet-markets – tor market
dark web sites darkmarket list
the dark internet how to access dark web tor market
darkmarkets https://github.com/darknetmarkets24/darknet-markets tor markets links
deep web markets dark market link
deep web markets darkmarkets tor markets 2025
Discover the latest offers on the 1win site and enjoy great bonuses | You can now take advantage of promotional offers on 1win easily 1win casino | Enjoy the latest casino games on the 1win site and benefit from exclusive offers | The 1win website offers everything you need to enjoy casino games | Enjoy the best online gaming experience with the 1win platform 1win platform.
licensing requirements online gambling laws
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
darknet marketplace tor markets
virtual sports betting esports market growth
Открывайте кейсы CS:GO https://www.facebook.com/people/Hotdrop_CSGO/61550490187523/ с крутыми шансами на редкие скины. Удобный интерфейс, надежная система и огромный выбор кейсов сделают игру еще интереснее. Начните свой путь к топовым скинам прямо сейчас!
deep web drug markets https://github.com/darknetmarkets24/darknet-markets – darkmarket
tor market tor markets
darknet site https://github.com/darknetmarkets24/darknet-markets deep web drug markets
мега купить – m3ga, мега ссылки
dark markets dark web link
промокоды продамус промокоды продамус .
туземцы и замки взлом https://apk-smart.com/igry/strategii/1363-vzlomannye-tuzemcy-i-zamki-mod-mnogo-deneg.html туземцы и замки взлом
P.S Live ID: K89Io9blWX1UfZWv3ajv
P.S.S Программы и игры для Андроид телефона Программы и игры для Андроид телефона Программы и игры для Андроид телефона 4ce2b0_
Откройте яркие кейсы CS:GO https://m.youtube.com/@hotdrop-case и получите шанс выиграть топовые скины! Широкий выбор кейсов, высокий шанс дропа и честная система обеспечат увлекательный опыт.
Transform your movie nights into unforgettable experiences with filmyzilla , where every film choice ignites your imagination and takes you on a thrilling journey through captivating stories and vibrant worlds!
Пробуйте удачу https://discord.com/invite/B5fF6pW8Cm CS:GO! Шанс получить редкие и дорогие скины, широкий выбор кейсов и удобный интерфейс делают процесс открытия легким и захватывающим.
Рискни и испытай удачу https://t.me/hotdropcases Шанс получить редкие и дорогие скины, широкий выбор кейсов и удобный интерфейс делают процесс открытия легким и захватывающим.
Ваши любимые кейсы CS:GO https://t.me/s/hotdropcases в одном месте! Большой выбор, удобный интерфейс и высокая вероятность выпадения редких предметов делают процесс открытия кейсов по-настоящему захватывающим.
купить аттестат за 11 классов
Anything is possible with sunshine and a little pink. 조아툰
Сервис бытовых услуг https://gidrostok-servis.ru это удобное решение для любых домашних задач. Уборка, ремонт, сантехника, установка техники и многое другое. Надежные специалисты, быстрое выполнение и доступные цены!
麻雀物語
千両箱
友人と一緒に行くことで、競争心が芽生え、さらに楽しめます。勝負の結果が気になります。
夜蝶飛翔特2発1
https://www.xn--k8-9g4a3b4f.store/?WatchID=927222818.html
パチンコのルールはシンプルですが、奥が深いです。初心者でもすぐに楽しめます。
Pビッグドリーム2激神
爆砕 千葉
アイムジャグラーEX (4発1)(V2.2)
アイムジャグラーEX
ルパン三世 ロイヤルロード金海に染まる黄金神殿
吉宗極
https://www.k8io.net/?NewsID=923931818.html
最近のパチンコ台は、アニメや映画とのコラボが多く、視覚的にも楽しめます。ファンにはたまらないですね。
戦コレ!【太平女君】徳川家康
https://xn--k8-9g4a3b4f.site/?NewsID=922083818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
デビルメイクライ4
https://www.k8.rodeo/?WatchID=923562818.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
Лучшие советы по выбору металлической входной двери, соответствует всем требованиям безопасности.
Места, где можно приобрести качественную входную металлическую дверь.
дверь металлическая входная дверь металлическая входная цена .
Что учесть при выборе входной металлической двери.
Почему стоит выбрать металлическую входную дверь.
Сравнение входных металлических дверей различных брендов.
dark web links darknet market lists dark web link
best darknet markets darknet marketplace
darknet websites https://github.com/darknetmarkets24/darknet-markets – darkmarket 2022
dark web sites tor markets 2022
darkmarket link https://github.com/darknetmarkets24/darknet-markets deep web markets
лучшие сериалы в 4K UHD фильмы новые сериалы 2025 HD 1080p
Visit Sellvia Facebook join a community of entrepreneurs turning ideas into thriving businesses! Discover top product picks, store upgrades, and can’t-miss deals with promo coupons. Stay inspired with tips and updates to grow your dropshipping store
Visit Sellvia LinkedIn https://www.linkedin.com/company/sellviadropshipping to join a community of passionate entrepreneurs transforming the dropshipping game. Get the latest updates, tips, and success stories to help you kickstart or grow your online business. Follow us and stay ahead with innovative solutions that make launching a profitable dropshipping store easy!
CR絶狼
炎炎 エロ
大当たりの瞬間には、周囲の人々との一体感が生まれます。共に喜びを分かち合える楽しさがあります。
P とある魔術の禁書目録
https://www.8casino8.com/?GameID=922316818.html
大当たりを狙うための戦略が必要で、運だけではなく技術も試されるのが魅力です。挑戦しがいがあります。
CRぱちんこ仮面ライダー フルスロットル
バチンコ
翔馬
南国育ち
北斗の拳世紀末救世主伝説
CR 真・北斗無双
https://www.k8.rodeo/?ArticleID=925585818.html
大当たりの瞬間には、周囲の人々との一体感が生まれます。共に喜びを分かち合える楽しさがあります。
デビル メイ クライ 4
https://www.k8.rodeo/?WatchID=921306818.html
シンプルなルールで、初心者でもすぐに楽しめます。気軽に挑戦できます。
CR JAWS再臨-SHARK PANIC AGAIN-
https://www.k8casino.biz/?SitesID=918641818.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
dark web websites darknet market blackweb
смотреть фильмы без рекламы онлайн новинки Netflix 2025 трейлер
Нужны деньги срочно займы онлайн с быстрым одобрением и моментальным переводом на карту. Минимум документов, удобные условия и прозрачные ставки. Оформите займ прямо сейчас!
demo pg slot
Return to Player Pragmatic Game Hot serta Pentingnya Mencoba Permainan Versi Uji PG Game
Aktivitas slot daring lebih terkenal, serta Playtech Soft (PG Mesin) menjadi beberapa provider terbaik berkat tampilan indah, fitur inovatif, dan juga Persentase Pengembalian (Return to Player) yang tinggi. Tetapi, sebelum memainkan dengan dana nyata, esensial untuk menjalankan mesin versi uji Playtech Slot. Alasannya? Berikut ini rinciannya.
Apa Hal Permainan Demo PG Slot?
Permainan versi uji ialah edisi uji dalam permainan mesin yang menggunakan kredit virtual, bukan dana asli. Hal ini memfasilitasi pemain supaya mengenal sistem game, fitur insentif, serta Return to Player tanpa risiko keuangan.
Mengapa Mencoba Permainan Percobaan Playtech Mesin Penting?
Mengetahui Sistem Permainan
Tiap permainan mempunyai peraturan dan juga fungsi beragam. Game percobaan membantu kamu mengetahuinya tanpa adanya stres.
Mengevaluasi Level Return to Player
RTP adalah rasio hasil hipotetis di jangka panjang. Menggunakan demo, Anda bisa menyaksikan berapa sering permainan menyediakan kemenangan.
Mengembangkan Strategi
Permainan demo memperbolehkan pemain supaya menguji rencana seperti pengaturan betting tanpa adanya bahaya hilangnya uang.
Mengetahui Permainan Favorit
Pragmatic Soft menawarkan beberapa tema permainan. Melalui menjalankan versi uji, kamu dapat menemukan permainan yang tepat pada preferensi pemain.
Mengurangi Kerugian Banyak
Memainkan versi uji memudahkan pemain memahami permainan sebelum Anda bertaruh dengan modal nyata, menurunkan risiko rugi.
Kelebihan Memainkan Slot Demo Pragmatic Game
Tanpa adanya Risiko Keuangan: Bermain memakai poin virtual, dengan tidak tekanan rugi modal.
Meningkatkan Keyakinan Sendiri: Melatih dengan percobaan menyebabkan kamu lebih siap saat beralih menuju aktivitas memakai modal nyata.
Memahami Fasilitas Bonus: Pelajari cara memicu fungsi hadiah seperti Spin Gratis atau Simbol Wild.
Menghemat Waktu dan juga Uang: Coba beragam mesin dengan tidak perlu mengeluarkan dana.
Saran Memainkan Permainan Demo PG Mesin
Konsentrasi pada fungsi hadiah serta teknik kerjanya.
Perhatikan risiko mesin (tinggi dan rendah).
Kelola batas waktu dan juga anggaran meski mencoba menggunakan poin simulasi.
Cari tahu beberapa konsep agar mengetahui kesukaan pemain.
таможенное оформление экспортных грузов таможенное оформление услуги москва
dark markets darknet sites
dark web sites darknet site
dark web drug marketplace https://github.com/darknetmarkets24/darknet-markets – darknet site
darknet drug market tor market
darknet market links https://github.com/darknetmarkets24/darknet-markets dark web market
Лучшие игровые промокоды промокод при пополнении ggdrop в одном месте! Активируйте бонусы, получайте подарки и прокачивайте аккаунт без лишних затрат. Следите за обновлениями, чтобы не пропустить новые промо!
Промокоды для игр https://esportpromo.com/standoff/ это бесплатные бонусы, скидки и эксклюзивные награды! Находите актуальные коды, используйте их и получайте максимум удовольствия от игры без лишних затрат.
FOSIL4D
Лучшие игровые промокоды промокоды на кейсы standoff в одном месте! Активируйте бонусы, получайте подарки и прокачивайте аккаунт без лишних затрат. Следите за обновлениями, чтобы не пропустить новые промо!
Компания Handy Print предоставляет [url=https://handy-print.ru]профессиональные услуги по печати[/url] на различных поверхностях: текстиле, дереве, стекле, пластике и металле. Мы используем только современные технологии и качественные материалы, чтобы обеспечить яркость и долговечность изображений.
В Handy Print вы можете [u]заказать нанесение логотипов[/u] на корпоративные подарки, [url=https://handy-print.ru]создание эксклюзивных футболок[/url], изготовление стильных аксессуаров и рекламной продукции. Наша команда профессионалов готова выполнить даже самые сложные и нестандартные задачи, чтобы вы получили идеальный результат.
Обращайтесь к нам уже сегодня и превращайте ваши идеи в реальность с помощью Handy Print!
Бесплатные промокоды https://playpromocode.com/cs2/key-drop/ для ваших любимых игр! Получайте монеты, бустеры, скины и другие ценные награды. Мы собираем только проверенные коды и обновляем их каждый день.
Хотите проверить компанию https://innproverka.ru по ИНН? Наш сервис поможет узнать подробную информацию о юридических лицах и ИП: статус, финансы, руководителей и возможные риски. Защищайте себя от ненадежных партнеров!
Недвижимость на Северном Кипре https://iberiaproperty.ru выгодные инвестиции и комфортная жизнь у моря. Апартаменты, виллы и пентхаусы по доступным ценам. Поможем выбрать лучший вариант и оформить покупку.
tor darknet onion market
deep web drug store https://github.com/darknetmarkets24/darknet-markets – dark market list
darknet websites darknet markets
удобный маркетплейс blacksprut сайт с высоким уровнем анонимности и надежной системой защиты. Интуитивный интерфейс, проверенные продавцы и безопасные сделки делают его лучшим выбором для покупок.
Раскрутка в соцсетях https://nakrytka.com без лишних затрат! Привлекаем реальную аудиторию, повышаем охваты и активность. Эффективные инструменты для роста вашего бренда.
1win is one of the most popular casino platforms in Egypt | Sports betting on 1win is simple and diverse 1win casino | The official 1win app is your best choice for gaming on the go | The diverse games on 1win make it an ideal destination for casino lovers | If you’re looking for a fun and rewarding gaming experience, 1win is the ideal choice 1win casino services.
продамус промокод скидка на подключение https://promokod-pro.ru .
darknet market lists darknet sites
dark web sites https://github.com/darknetmarkets24/darknet-markets darknet market links
dark websites dark web market links
darkweb marketplace https://github.com/darknetmarkets24/darknet-markets – deep web drug markets
tor markets links darkmarket url
Slot777
Berikut artikel yang Anda minta berdasarkan konteks sebelumnya:
Misteri Tulisan Yesus di Pasir: Rahasia Tersembunyi dalam Kitab Suci
Dalam tradisi Kristen, salah satu momen paling misterius adalah ketika Yesus menulis di pasir saat menghadapi seorang perempuan yang tertangkap berzina. Apa sebenarnya yang ditulisnya?
Jejak Ilahi dalam Tulisan
Merujuk pada Kitab Keluaran, Allah sendiri pernah menulis dengan jari-Nya di atas batu. Yesus, sebagai inkarnasi Ilahi, tampaknya mengulang tindakan sakral ini.
Hipotesis Tulisan Rahasia
Beberapa sarjana menafsirkan bahwa Yesus mungkin menulis:
– Nama-nama para penuduh
– Daftar dosa mereka
– Ayat-ayat hukum yang mereka langgar
Makna Tersembunyi
Tindakan menulis di pasir bukan sekadar gerakan acak, tetapi memiliki signifikansi teologis mendalam. Yesus secara simbolis mengungkapkan kebenaran tersembunyi tentang kemanusiaan.
Kesimpulan
Meskipun isi tulisan tetap menjadi misteri, tindakan Yesus menunjukkan kebijaksanaan yang melampaui pemahaman manusia.
Equilibrado
Aparatos de equilibrado: importante para el desempeño estable y efectivo de las dispositivos.
En el entorno de la ciencia moderna, donde la rendimiento y la fiabilidad del aparato son de gran importancia, los dispositivos de ajuste desempeñan un tarea esencial. Estos aparatos especializados están desarrollados para equilibrar y fijar piezas rotativas, ya sea en equipamiento de fábrica, vehículos de transporte o incluso en electrodomésticos de uso diario.
Para los profesionales en reparación de dispositivos y los especialistas, utilizar con aparatos de calibración es fundamental para asegurar el rendimiento estable y seguro de cualquier dispositivo giratorio. Gracias a estas soluciones avanzadas sofisticadas, es posible minimizar sustancialmente las movimientos, el estruendo y la esfuerzo sobre los rodamientos, mejorando la vida útil de componentes caros.
Asimismo significativo es el rol que desempeñan los equipos de ajuste en la servicio al comprador. El asistencia profesional y el reparación continuo empleando estos equipos permiten ofrecer prestaciones de alta nivel, mejorando la contento de los clientes.
Para los responsables de emprendimientos, la financiamiento en equipos de equilibrado y medidores puede ser clave para mejorar la rendimiento y eficiencia de sus dispositivos. Esto es particularmente relevante para los empresarios que dirigen modestas y pequeñas negocios, donde cada aspecto importa.
Además, los aparatos de ajuste tienen una extensa utilización en el campo de la fiabilidad y el control de excelencia. Permiten localizar potenciales fallos, impidiendo mantenimientos caras y problemas a los dispositivos. Además, los indicadores extraídos de estos dispositivos pueden utilizarse para perfeccionar procesos y aumentar la exposición en sistemas de exploración.
Las áreas de implementación de los aparatos de balanceo incluyen múltiples industrias, desde la elaboración de vehículos de dos ruedas hasta el monitoreo del medio ambiente. No importa si se refiere de extensas producciones manufactureras o reducidos locales domésticos, los aparatos de calibración son indispensables para asegurar un funcionamiento óptimo y sin interrupciones.
Equipos de equilibrado: clave para el desempeño estable y productivo de las maquinarias.
En el campo de la ciencia moderna, donde la productividad y la estabilidad del aparato son de máxima relevancia, los dispositivos de calibración juegan un función vital. Estos equipos específicos están diseñados para ajustar y regular partes dinámicas, ya sea en herramientas productiva, vehículos de transporte o incluso en dispositivos domésticos.
Para los profesionales en soporte de aparatos y los especialistas, operar con sistemas de calibración es esencial para garantizar el operación estable y fiable de cualquier sistema giratorio. Gracias a estas soluciones tecnológicas avanzadas, es posible disminuir significativamente las oscilaciones, el zumbido y la esfuerzo sobre los soportes, aumentando la vida útil de partes caros.
Asimismo significativo es el función que desempeñan los aparatos de balanceo en la asistencia al comprador. El asistencia especializado y el mantenimiento constante aplicando estos dispositivos posibilitan brindar asistencias de óptima estándar, incrementando la satisfacción de los usuarios.
Para los titulares de proyectos, la contribución en unidades de ajuste y dispositivos puede ser importante para aumentar la rendimiento y eficiencia de sus aparatos. Esto es principalmente trascendental para los dueños de negocios que administran medianas y medianas empresas, donde cada detalle es relevante.
Por otro lado, los equipos de balanceo tienen una amplia utilización en el campo de la fiabilidad y el gestión de estándar. Facilitan localizar potenciales problemas, impidiendo reparaciones onerosas y averías a los equipos. Más aún, los resultados recopilados de estos aparatos pueden usarse para maximizar métodos y potenciar la exposición en sistemas de búsqueda.
Las áreas de aplicación de los equipos de ajuste abarcan variadas áreas, desde la manufactura de transporte personal hasta el seguimiento ecológico. No afecta si se trata de importantes manufacturas manufactureras o limitados locales caseros, los sistemas de equilibrado son indispensables para asegurar un rendimiento efectivo y libre de fallos.
dark web drug marketplace https://github.com/darknetmarkets24/darknet-markets – onion market
darknet sites dark market url
tor markets 2022 deep dark web
увлекательный сериал https://odnazhdy-v-skazketv.ru о жизни сказочных персонажей в реальном мире. Интригующий сюжет, волшебные события и неожиданные тайны. Смотрите онлайн в высоком качестве прямо сейчас!
dark websites https://github.com/darknetmarkets24/darknet-markets – dark market 2022
dark web market links dark web sites links
darkmarket dark web drug marketplace
tor marketplace tor market
dark websites https://github.com/darknetmarkets24/darknet-markets best darknet markets
сделать мрт спб сделать мрт спб .
таможенное оформление грузов из европы https://bvs-logistica.com/tamozhennnoe-oformlenie.html
mega sb отзывы мега онион
санкт петербург мрт https://www.mrt-spb13.ru .
сделать мрт в санкт петербурге сделать мрт в санкт петербурге .
Современная имплантация зубов зубные импланты в москве надежное решение для восстановления улыбки! Безопасные материалы, точная диагностика и индивидуальный подход. Верните уверенность и комфорт в жизни! Запишитесь на консультацию уже сегодня.
dark markets https://github.com/darknetmarkets24/darknet-markets – dark market list
Как выбрать идеальную входную металлическую дверь, которая прослужит долгие годы.
Где купить надежную входную металлическую дверь по выгодной цене.
заказать входную дверь дверь металлическая входная .
Что учесть при выборе входной металлической двери.
5 основных причин купить металлическую входную дверь.
Сравнение входных металлических дверей различных брендов.
dark web links tor markets links
deep web drug store https://github.com/darknetmarkets24/darknet-markets dark web markets
deep web drug markets darknet drug links
deep web drug markets bitcoin dark web
Интернет-магазин товаров https://vitasleep.ru для здорового сна. В ассортименте: ортопедические матрасы, подушки, одеяла, постельное белье и аксессуары от проверенных брендов. Удобный выбор, доставка по России, гарантия качества. Забота о вашем комфорте и здоровом сне!
Immerse into the World of GTA 5 RP: Join Our Unique Roleplay Server
Salutations to the thrilling universe of GTA 5 RP, where each player can craft their individual tale in a dynamic gaming environment. Our roleplay server is suitable for those desiring an engaging and deep experience. Join a lively community of GTA RP aficionados and enter into this exciting world!
How to Commence Playing?
1. Purchase and install a licensed copy of GTA 5.
2. Install the launcher.
3. Connect to the server and sign up through the application or our platform to begin your quest!
Our Platform: An Open World Experience
Our server offers a distinct open-world setting with custom mods that permit you to construct your underworld empire, set out on exciting missions, and mold your character’s story. Whether you join local gangs or transform into a top cop, the possibilities are boundless!
Generate Money and Expand Your Assets
Are you set to dominate the streets of Los Santos and make substantial money? We supply the tools and tactics to help you gather a prosperity in the realm of GTA RP.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников smeg в москве, можете посмотреть на сайте: ремонт холодильников smeg в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
смотреть лучшие сериалы 2024 смотреть онлайн зарубежные сериалы
смотреть лучшие сериалы зарубежные сериалы смотреть
With a resolute nod, Mateo made his decision. He would decline the offer, choosing instead to follow the path of curiosity and honor the true essence of his remarkable find. 무료웹툰 추천
смотреть сериалы lordserials2 net
dark markets 2022 darknet site
darknet market https://github.com/darknetmarkets24/darknet-markets – darkmarkets
darknet market links https://github.com/darknetmarkets24/darknet-markets best darknet markets
deep web drug links dark web sites
Онлайн казино с широким выбором аркадных игр | Погрузитесь в мир азарта и аркадных развлечений | Победите в аркадах и станьте миллионером | Побеждайте в аркадах и получайте крупные денежные призы | Новый уровень азарта в онлайн казино | Ощутите драйв аркад в онлайн казино | Уникальные аркадные игры ждут вас | Увлекательные аркады и возможность заработать деньги | Играйте в аркадные игры и ощутите азарт | Побеждайте в аркадных сражениях и получайте выигрыши | Аркадные развлечения и выигрыши в онлайн казино | Уникальные аркадные игры в вашем распоряжении | Онлайн казино с самыми популярными аркадами | Новейшие аркадные игры на ваш выбор | Играйте в аркады и выигрывайте деньги | Побеждайте в аркадных играх и получайте призы
arkada регистрация arkada casino официальный .
смотреть сериалы 2023 хорошего качества онлайн смотреть сериалы
сериалы 2022 онлайн https://lordserial7.com
сериалы онлайн 1 сезон https://lordserials.cc
토토사이트 커뮤니티: 안전한 베팅을 위한 길잡이
토토사이트 커뮤니티는 스포츠 베팅을 즐기는 사람들에게 단순한 정보 공유의 장을 넘어, 안전한 배팅 환경을 조성하는 데 중요한 역할을 합니다. 이곳에서는 초보자부터 전문가까지 다양한 사람들이 모여 베팅 전략을 논의하고, 신뢰할 수 있는 토토사이트를 추천하며, 먹튀 사고를 예방하기 위한 정보를 공유합니다. 특히, 사설 토토사이트의 위험성을 알리고, 이를 피할 수 있는 방법을 제시함으로써 사용자들이 더 안전하게 베팅을 즐길 수 있도록 돕습니다.
사설 토토사이트의 숨겨진 위험
사설 토토사이트는 정부의 규제를 받지 않기 때문에 다양한 문제를 일으킬 수 있습니다. 예를 들어, 공정하지 않은 배당률, 과장된 광고, 개인정보 유출 등이 대표적인 문제점입니다. 또한, 사설 토토사이트는 법적 문제로 인해 갑자기 사라지는 경우가 많아 사용자들이 금전적 손실을 입을 위험이 큽니다. 이런 이유로, 토토 커뮤니티에서는 사설 토토사이트보다는 규제를 받는 공식 사이트를 이용할 것을 권장합니다.
토토 커뮤니티가 제공하는 가치
토토 커뮤니티는 단순히 정보를 나누는 공간이 아니라, 사용자들이 안전하게 베팅할 수 있도록 돕는 네트워크입니다. 예를 들어, 새로운 토토사이트가 등장했을 때 커뮤니티 멤버들은 해당 사이트의 운영 주체, 결제 시스템, 사용자 리뷰 등을 꼼꼼히 검토합니다. 이를 통해 먹튀 사고를 미리 방지하고, 신뢰할 수 있는 사이트만을 추천합니다. 또한, 문제가 발생했을 때는 보증 사이트를 통해 사용자들이 손실을 최소화할 수 있도록 돕습니다.
어떻게 신뢰할 수 있는 토토사이트를 찾을까?
토토 커뮤니티에서는 신뢰할 수 있는 토토사이트를 찾기 위해 몇 가지 중요한 기준을 제시합니다. 먼저, 사이트의 운영 주체가 명확한지 확인하는 것이 중요합니다. 정부나 공인된 기관에서 관리하는 사이트라면 신뢰할 수 있습니다. 또한, 다른 사용자들의 리뷰를 참고하여 사이트의 신뢰성을 판단할 수 있습니다. 긍정적인 평가가 많고, 문제가 거의 없는 사이트라면 안전하게 이용할 수 있습니다.
결제 시스템도 중요한 확인 요소 중 하나입니다. 신용카드, 전자지갑 등 다양한 결제 방법을 지원하고, SSL 암호화를 통해 금융 정보를 보호하는 사이트라면 더욱 신뢰할 수 있습니다. 마지막으로, 온라인 커뮤니티나 포럼에서 해당 사이트에 대한 평판을 확인하는 것도 좋은 방법입니다. 다른 사용자들이 어떻게 평가하는지, 문제가 없는지 꼼꼼히 살펴보는 것이 중요합니다.
토토 커뮤니티의 보증 사이트
토토 커뮤니티에서는 먹튀 사고를 예방하기 위해 보증 사이트를 추천합니다. 이러한 사이트는 사용자들의 개인정보와 금융 정보를 보호하며, 문제가 발생했을 때 빠르게 해결할 수 있는 시스템을 갖추고 있습니다. 보증 사이트를 이용하면 안전하게 베팅을 즐길 수 있으며, 만약 문제가 생기더라도 커뮤니티의 도움을 받아 해결할 수 있습니다.
마무리
토토사이트 커뮤니티는 스포츠 베팅을 즐기는 사람들에게 안전하고 신뢰할 수 있는 정보를 제공하는 중요한 공간입니다. 사설 토토사이트의 위험성을 인지하고, 신뢰할 수 있는 사이트를 선택하는 것이 무엇보다 중요합니다. 토토 커뮤니티의 정보를 참고하여 안전한 베팅 환경을 조성하고, 스포츠 베팅을 더욱 즐겁게 즐기시길 바랍니다. 항상 책임감 있게 베팅에 임하는 것도 잊지 마세요!
dark web markets dark websites
darkmarket link https://github.com/darknetmarkets24/darknet-markets – darknet market lists
dark web link dark market onion
tor market darknet marketplace
A friend recommended https://sites.google.com/view/metamask-extension-dfkasdkfdnt/download for downloading Metamask, and I’m so glad I checked it out. The process was easy, and now I can safely store my crypto.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
RTP PG Mesin Hot dan Pentingnya Memainkan Slot Versi Uji PG Permainan
Game mesin online lebih digemari, dan PG Perusahaan (Pragmatic Game) merupakan satu platform unggulan berkat grafis menawan, fungsi inovatif, dan Return to Player (Return to Player) yang tinggi. Akan tetapi, sebelumnya bermain dengan dana nyata, penting agar mencoba mesin percobaan Pragmatic Slot. Kenapa? Berikut rinciannya.
Apa Fitur Slot Demo PG Game?
Mesin demo ialah tipe percobaan dari aktivitas game dengan memakai kredit virtual, bukan uang sunguhan. Ini memfasilitasi player untuk mengetahui sistem permainan, fungsi hadiah, dan juga Persentase Pengembalian dengan tidak bahaya keuangan.
Alasannya Mencoba Game Demo PG Game Perlu?
Memahami Cara Kerja Game
Setiap permainan memiliki peraturan dan juga fasilitas beragam. Slot percobaan memfasilitasi kamu memahaminya tanpa tekanan.
Menguji Level Persentase Pengembalian
Persentase Pengembalian merupakan persentase keuntungan teoritis di periode lama. Menggunakan versi uji, Anda bisa mengamati sejauh mana kerap permainan menghasilkan kemenangan.
Membuat Rencana
Game demo memperbolehkan pemain agar mengetes taktik seperti pengelolaan pertaruhan dengan tidak potensi kerugian rugi modal.
Mengetahui Permainan Pilihan
PG Soft memiliki beragam konsep game. Melalui mencoba percobaan, kamu bisa mencari permainan yang sesuai pada selera kamu.
Mengurangi Kehilangan Signifikan
Bermain percobaan memudahkan pemain mengerti game sebelum memasang taruhan menggunakan modal sunguhan, mengurangi risiko kehilangan.
Keuntungan Memainkan Permainan Pratinjau Playtech Mesin
Tanpa adanya Risiko Moneter: Bermain menggunakan kredit virtual, tanpa tekanan hilangnya uang.
Meningkatkan Rasa Percaya Diri: Mempraktikkan dengan percobaan menjadikan Anda semakin siap ketika pindah pada permainan menggunakan modal asli.
Mengetahui Fasilitas Hadiah: Pelajari teknik memicu fitur hadiah contohnya Spin Gratis atau Simbol Liar.
Meminimalkan Waktu dan juga Modal: Coba berbagai permainan dengan tidak perlu menghabiskan dana.
Tips Memainkan Permainan Versi Uji PG Permainan
Perhatian pada fitur hadiah serta metode operasinya.
Lihatlah risiko slot (besar dan rendah).
Susun limit waktu dan budget walaupun mencoba dengan kredit digital.
Jelajahi berbagai gaya supaya menemukan favorit Anda.
смотреть сериалы 2024 https://lordsserial.xyz
deep web drug links dark market url
darknet sites https://github.com/darknetmarkets24/darknet-markets – dark market onion
Логистические услуги в Москве https://bvs-logistica.com доставка, хранение, грузоперевозки. Надежные решения для бизнеса и частных клиентов. Оптимизация маршрутов, складские услуги и полный контроль на всех этапах.
сериалы сезон онлайн бесплатно https://lordserialss.life
darkmarket link https://github.com/darknetmarkets24/darknet-markets tor dark web
darknet marketplace darkmarket link
сделать мрт в санкт петербурге сделать мрт в санкт петербурге .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников smeg, можете посмотреть на сайте: ремонт холодильников smeg
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Your article helped me a lot, is there any more related content? Thanks!
Rio Gems https://jogoriogems.club um caca-niquel online vibrante com a atmosfera do carnaval brasileiro. O jogo oferece 5 rolos, simbolos coloridos de joias e dancarinas, alem de recursos bonus, incluindo rodadas gratis e multiplicadores. Sinta o ritmo da samba e tenha a chance de ganhar grandes premios!
Хотите почувствовать азарт? arkada casino играть предлагает широкий выбор игр, честную систему выигрышей и мгновенные выплаты. Захватывающие слоты и бонусы ждут вас!
клиника мрт спб клиника мрт спб .
darknet search engine deep web drug url darknet markets 2025
dark web market links darknet seiten darknet drug market
Fans admire Harry Kane for his dedication and goal-scoring ability | Harry Kane’s net worth reflects his successful football career kane harry | Harry Kane’s journey from Tottenham to Bayern Munich was an emotional one | Football fans worldwide admire Harry Kane’s goal-scoring prowess | Fans eagerly follow Harry Kane’s journey in the Bundesliga Latest matches of Harry Kane.
смотреть сериалы подряд https://lordseriall6.org
смотреть лучшие сериалы 2024 смотреть зарубежный сериал
смотреть турецкие сериалы https://lordsserials.org
deep web links darkmarket list darknet market lists
санкт петербург мрт https://www.mrt-spb12.ru .
dark web market dark web market list
dark web market https://github.com/darknetmarkets24/darknet-markets – darkmarket link
dark web market links dark market onion
tor market url tor market links
dark web market links dark web links tor markets
darknet site drug markets dark web tor markets 2025
bitcoin dark web tor markets
darkmarket list https://github.com/darknetmarkets24/darknet-markets dark web sites links
tor markets dark markets
dark market url deep web drug store
darknet markets darkmarket dark web market
Check out Sellvia https://www.instagram.com/sellvia.dropshipping/ on Instagram for the hottest product ideas, store upgrades, and exclusive deals! Stay in the loop with our latest dropshipping tips and grab promo coupons to boost your business.
how to get on dark web deep web markets darknet marketplace
dark web links https://github.com/darknetmarkets24/darknet-markets – deep web drug links
dark websites bitcoin dark web
dark web market list https://github.com/darknetmarkets24/darknet-markets dark market onion
tor marketplace dark web markets
Equipos de balanceo: esencial para el desempeño uniforme y eficiente de las dispositivos.
En el campo de la ciencia contemporánea, donde la rendimiento y la fiabilidad del dispositivo son de alta relevancia, los dispositivos de calibración cumplen un función crucial. Estos equipos adaptados están creados para equilibrar y asegurar elementos dinámicas, ya sea en equipamiento industrial, transportes de desplazamiento o incluso en dispositivos hogareños.
Para los profesionales en soporte de aparatos y los técnicos, utilizar con dispositivos de ajuste es fundamental para proteger el rendimiento suave y confiable de cualquier mecanismo dinámico. Gracias a estas alternativas modernas avanzadas, es posible limitar significativamente las vibraciones, el ruido y la esfuerzo sobre los cojinetes, prolongando la longevidad de componentes valiosos.
Igualmente significativo es el papel que cumplen los sistemas de equilibrado en la servicio al cliente. El apoyo especializado y el conservación permanente aplicando estos equipos permiten dar soluciones de alta excelencia, incrementando la bienestar de los usuarios.
Para los dueños de emprendimientos, la aporte en sistemas de ajuste y medidores puede ser clave para mejorar la eficiencia y productividad de sus equipos. Esto es principalmente importante para los inversores que manejan reducidas y intermedias organizaciones, donde cada punto es relevante.
Además, los equipos de ajuste tienen una gran uso en el sector de la fiabilidad y el gestión de nivel. Habilitan detectar potenciales defectos, evitando mantenimientos onerosas y problemas a los aparatos. Además, los indicadores obtenidos de estos dispositivos pueden aplicarse para mejorar métodos y aumentar la visibilidad en sistemas de consulta.
Las sectores de uso de los dispositivos de calibración cubren numerosas sectores, desde la elaboración de bicicletas hasta el control de la naturaleza. No afecta si se refiere de enormes manufacturas de fábrica o reducidos espacios caseros, los sistemas de balanceo son indispensables para proteger un rendimiento óptimo y sin presencia de interrupciones.
dark web market list dark web market
darkmarket 2022 https://github.com/darknetmarkets24/darknet-markets – dark market url
dark web links deep web drug links
tor market https://github.com/darknetmarkets24/darknet-markets dark markets 2022
drug markets onion darknet websites
dark market link darknet site
I’ve been using Metamask for a while, but I recently needed to reinstall the Metamask Chrome extension. https://sites.google.com/view/metamask-extension-download-oa/chrome made the process super simple, and now my wallet is back up and running. This site is a lifesaver!
dark web market links https://github.com/darknetmarkets24/darknet-markets – dark market
darkmarket link tor markets links
dark market onion darkmarket link
darkmarkets tor dark web
darkmarket https://github.com/darknetmarkets24/darknet-markets – darknet drug links
darknet market links darknet market links
dark markets drug markets onion
dark web link https://github.com/darknetmarkets24/darknet-markets dark web markets
dark web markets dark web market links
пансионат для пожилых в крыму пансионат для пожилых в крыму .
вывод из запоя на дому в симферополе вывод из запоя на дому в симферополе .
кракен клир – kraken32, кра сайт
порно фильмы бесплатно порно фильмы бесплатно .
дом престарелых в береговом дом престарелых в береговом .
самый эффективный способ кодирования от алкоголизма самый эффективный способ кодирования от алкоголизма .
the dark internet https://github.com/darknetmarkets24/darknet-markets tor dark web
Онлайн казино с широким выбором аркадных игр | Развлечения и азарт в аркадном казино | Играйте в лучшие аркады на нашем сайте | Играйте в аркады и выигрывайте деньги | Аркадные игры и азарт ждут вас здесь | Соберите комбинации и побеждайте в аркадах | Играйте в лучшие аркады в онлайн казино | Побеждайте в аркадных играх и получайте призы | Играйте в аркадные игры и ощутите азарт | Увлекательные аркады для ценителей азарта | Онлайн казино с увлекательными аркадами | Уникальные аркадные игры в вашем распоряжении | Аркадные онлайн развлечения для вас | Аркадные развлечения и азарт вместе | Побеждайте в аркадах и получайте денежные призы | Побеждайте в аркадных играх и получайте призы
arkada casino официальный аркада регистрация .
dark market link darkmarket list dark net
tor markets links https://github.com/darknetmarkets24/darknet-markets – darknet market lists
deep dark web dark web drug marketplace
Your article helped me a lot, is there any more related content? Thanks!
dark web drug marketplace dark web sites links
darkmarket link darkmarkets
tor market url https://github.com/darknetmarkets24/darknet-markets darknet market
Reliable and unique bip39 Word List contains 2048 words needed to create seed phrases in crypto wallets. Allows you to safely manage private keys and guarantees the possibility of recovering funds.
The full special bip39 Word List consists of 2048 words used to protect cryptocurrency wallets. Allows you to create backups and restore access to digital assets. Check out the full list.
best darknet markets https://github.com/darknetmarkets24/darknet-markets tor darknet
darknet websites deep web search dark internet
dark web link https://github.com/darknetmarkets24/darknet-markets – bitcoin dark web
dark markets dark market 2022
darknet drug links deep dark web
darkmarket link darknet marketplace
dark market link https://github.com/darknetmarkets24/darknet-markets darkmarket list
dark web drug marketplace https://github.com/darknetmarkets24/darknet-markets dark market url
CR大海物語4 Ver.319
示唆
パチンコのルールはシンプルですが、奥が深いです。初心者でもすぐに楽しめます。
CRぱちんこ 新鬼武者
https://www.k8io.net/?GameID=919670818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
CR RAVE この世界こそが真実だ
0.5 スロ
P牙狼月虹ノ旅人
シンドバッドアドベンチャーは榎本加奈子でどうですか
Pフィーバーからくりサーカス
主役は銭形2
https://www.sawasdeeslot.com/?GameID=920798818.html
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
デジハネ CR 蒼天の拳 STV
https://xn--k8-9g4a3b4f.site/?NewsID=927969818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
北斗の拳 強敵
https://www.casinodengi.org/?WatchID=925704818.html
新しい台が出るたびにワクワクします。流行を追うのが楽しいです。
deep web drug url darknet site
deep web drug links https://github.com/darknetmarkets24/darknet-markets dark market onion
dark web market list darknet market list
Reliable and unique bip39 Word List contains 2048 words needed to create seed phrases in crypto wallets. Allows you to safely manage private keys and guarantees the possibility of recovering funds.
cali weed in prague https://sale-weed-prague.com
deep web drug store https://github.com/darknetmarkets24/darknet-markets tor markets links
deep web sites https://github.com/darknetmarketslinks/darknetmarketlinks – dark market onion deep web drug markets
dark market 2022 tor market
darknet drug links darknet markets
dark market list https://github.com/darknetmarkets24/darknet-markets – dark market
новые порно фильмы новые порно фильмы .
вывод из запоя в симферополе вывод из запоя в симферополе .
дом престарелых в крыму дом престарелых в крыму .
dark websites https://github.com/darknetmarketslinks/darknetmarketlinks – dark web sites darkmarket
государственный дом престарелых в симферополе государственный дом престарелых в симферополе .
darknet drug links https://github.com/darknetmarkets24/darknet-markets darknet site
нарколог кодирование от алкоголизма нарколог кодирование от алкоголизма .
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
P 真・北斗無双 第3章 ジャギの逆襲
ピノコ 最後
演出が豪華で、観ているだけでも楽しめます。特にボーナス演出は圧巻です。
CR聖戦士ダンバイン Ver.319
https://xn--k8-9g4a3b4f.site/?WatchID=919536818.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
ケロロ軍曹
みすてぃーの
CR ぱちんこAKB48 バラの儀式
パチスロ聖闘士星矢-女神聖戦-
バイオハザード6
主役は銭形2
https://jahromblog.com/?GameID=918554818.html
シンプルなルールで、初心者でもすぐに楽しめます。気軽に挑戦できます。
CR聖戦士ダンバイン Ver.319
https://www.k8-casino.org/?NewsID=927237818.html
演出が豪華で、観ているだけでも楽しめます。特にボーナス演出は圧巻です。
アラジンAII
https://www.k8pachinko.cc/?WatchID=920676818.html
シンプルなデザインとルールが魅力。初心者でも安心して楽しめます。
darkmarket https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarket list tor markets
tor darknet tor markets links
best darknet markets darknet websites
tor markets 2022 https://github.com/darknetmarkets24/darknet-markets – deep dark web
darknet market lists https://github.com/darknetmarkets24/darknet-markets deep web drug markets
Reliable and unique bip39 Word List contains 2048 words needed to create seed phrases in crypto wallets. Allows you to safely manage private keys and guarantees the possibility of recovering funds.
аниме онлайн – фильмы вышедшие онлайн, фильмы вышедшие онлайн
Онлайн казино с широким выбором аркадных игр | Развлечения и азарт в аркадном казино | Аркадные игры для всех желающих | Увлекательные аркадные игры для всех | Онлайн казино с самыми популярными аркадными играми | Играйте в аркадные игры и выигрывайте деньги | Погрузитесь в мир аркадных развлечений и азарта | Самые популярные аркадные игры в одном казино | Наслаждайтесь аркадами вместе с нами | Увлекательные аркады для ценителей азарта | Большие денежные призы в аркадах | Побеждайте в аркадных баталиях и зарабатывайте деньги | Аркадные онлайн развлечения для вас | Увлекательные аркады и возможность заработать крупные суммы | Побеждайте в аркадах и получайте денежные призы | Увлекательные аркады и возможность заработать деньги
arkada casino бездепозитный бонус arkada casino автоматы .
darkmarket link dark web market links
onion market darkmarket 2022
dark markets 2022 deep dark web
dark market link https://github.com/darknetmarkets24/darknet-markets – tor markets links
1xBet promo code https://legacy.availo.pl/today-s-best-1xbet-promo-code-how-to-redeem-and-2/ your chance to get bonuses on bets, free bets and exclusive promotions! Enter the code during registration and start playing with an increased deposit.
darknet drug market https://github.com/darknetmarketslinks/darknetmarketlinks – tor markets 2025 dark market onion
секс с чудовищами секс с чудовищами .
порно с милфой порно с милфой .
Bip39 Bip39 .
dark web link https://github.com/darknetmarkets24/darknet-markets dark market link
секс с немкой секс с немкой .
секс япония japanxxx-video1.ru .
deep web drug links dark web market links
dark market 2022 tor market
Ремонт компьютеров и ноутбуков https://remcomp89.ru в Новом Уренгое – быстрые и качественные услуги! Диагностика, настройка, замена комплектующих, восстановление данных. Гарантия на работу, доступные цены и выезд мастера!
dark markets 2022 darknet market links
dark market link https://github.com/darknetmarkets24/darknet-markets – tor market links
dark websites https://github.com/darknetmarketslinks/darknetmarketlinks – best darknet markets blackweb
Learn More https://jaxx-liberty.com/
Лучшее казино для аркадных игр | Уникальные аркадные игры только здесь | Победите в аркадах и станьте миллионером | Побеждайте в аркадах и получайте крупные денежные призы | Лучшие аркадные игры в онлайн казино | Соберите комбинации и побеждайте в аркадах | Играйте в лучшие аркады в онлайн казино | Аркадное казино для всех | Онлайн казино с удивительными аркадными играми | Онлайн казино с азартными аркадами | Попробуйте свою удачу в аркадном казино | Уникальные аркадные игры и азарт в казино | Онлайн казино с самыми популярными аркадами | Увлекательные аркады и возможность заработать крупные суммы | Побеждайте в аркадах и получайте денежные призы | Побеждайте в аркадных играх и получайте призы
arkada casino регистрация на сайте arkada casino зеркало для россии .
dark market link dark web market
tor darknet https://github.com/darknetmarkets24/darknet-markets darknet websites
tor market links https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarket best darknet markets
deep web drug store tor markets 2022
Используйте актуальный промокод 1xbet и получите увеличенный бонус на первый депозит! Делайте ставки на спорт, играйте в казино и пользуйтесь эксклюзивными предложениями. Легкая регистрация и моментальные выплаты!
drug markets dark web https://github.com/darknetmarkets24/darknet-markets – darknet marketplace
Looking for the current spinbetter promo code? Get bonus funds for bets and casino games. Easy activation, favorable conditions and real winnings are waiting for you. Hurry to use it!
dark web markets darkmarket
darknet market https://github.com/darknetmarketslinks/darknetmarketlinks – deep web sites drug markets dark web
darkmarket link https://github.com/darknetmarkets24/darknet-markets darknet market
ทดลองเล่นสล็อต pg
ทดลองเล่น สล็อต PG หมายถึงอะไร? ด้วยเหตุใด จึงเป็น เกมที่ได้รับความนิยม?
เกมสล็อตออนไลน์ ถือเป็นเกมที่ เป็นที่รู้จัก มาก ตลอดทุกยุคทุกสมัย และยัง จัดเป็น หนึ่งในบรรดา เกมที่คนทั่วไป รู้จักดี พร้อมทั้ง ชื่นชอบ มากที่สุด ในวงการ เกมออนไลต์ แต่สำหรับ ผู้ที่เพิ่งเป็น ผู้เริ่มต้น หรือก็คือ มีประสบการณ์ การเล่นเกม น้อย ในวันนี้ จะแนะนำคุณ มาทำความรู้จักกับ ลองเล่น สล็อตแมชชีน PG ซึ่งถือเป็น เกมสล็อตที่ไม่ผ่านเอเย่นต์ ที่คนนิยม อย่างแพร่หลาย ผ่าน คุณจะสามารถที่จะ ทำความเข้าใจ พร้อมทั้ง เริ่มเล่น ได้ทันที รวมทั้ง มีความสุข พร้อมกับ ประสบการณ์การเล่น การเล่น ที่สนุก และยัง น่าสนใจ
ทดลองเล่น สล็อต PG คือสิ่งใด?
ทดสอบ สล็อต PG ถือเป็นเกม เกมออนไลน์สล็อต ที่สร้างขึ้น จาก บริษัทพัฒนาเกม PG Soft ซึ่งเป็น หนึ่งในประเภท ผู้พัฒนา เกมสล็อตออนไลน์ ยอดนิยม ทั่วโลก เกมดังกล่าว ได้แนวคิด จากเกม เครื่องเล่นเกมสล็อต แบบดั้งเดิม แต่ เพิ่มความ ความทันสมัยและความสนุก รวมทั้ง ความสนุก เข้าไปในรูปแบบ ผ่าน เกมสล็อต มีอยู่ 5 แถวแนวตั้ง รวมทั้ง 15 เพย์ไลน์ รูปแบบการชนะ ที่ ผู้เล่น มีโอกาสที่จะ ได้รับรางวัล ได้หลายครั้ง
รูปสัญลักษณ์ ในเกมนี้ เกมสล็อต PG นั้นประกอบด้วย มีหลายแบบ เช่นเช่น ผลเชอร์รี่, ตัวเลข 7, เพชร, รวมทั้ง ไอคอน อื่นๆอีก ที่เกี่ยวข้อง กับธีม เกมสล็อต ซึ่งแต่ละ แต่ละเกมดังกล่าว ประกอบด้วย รูปแบบของ และยัง รูปแบบเกม ที่แตกต่างกัน ออกไปจาก เช่น ธีมป่า, ธีมเทพเจ้า, ธีมอาหารอร่อย, หรือก็คือ ธีมผจญภัย เป็นต้นไป
ด้วยเหตุใด ลองเล่น สล็อต PG จึงเป็น เกมที่นิยม?
ความง่าย ในการเล่นเกม
ทดสอบ สล็อต PG จัดเป็นเกม ที่เข้าใจง่าย ไม่ซับซ้อน เหมาะสำหรับ ผู้ที่เพิ่งเริ่ม รวมทั้ง มือเก่า ผู้เล่น เพียง เลือกจำนวนเงินที่ จำนวนเดิมพัน กดสปิน และ รอ ผลลัพธ์ของเกม ซึ่ง ระบบการเล่นสล็อต ที่เข้าใจง่าย ทำให้ ผู้เล่นออนไลน์ สามารถ เพลิดเพลิน ได้โดยไม่จำเป็นต้อง ไม่ต้องเครียด ในเรื่อง กฎ ที่ยุ่งเหยิง
รูปแบบการให้ การให้รางวัล ที่หลายแบบ
เกมสล็อต PG มีอยู่ เพย์ไลน์ ให้ชนะรางวัล มากถึงสูงสุด 15 รูปแบบ ซึ่งส่งผลให้ มากกว่า เกมสล็อตคลาสสิก ทั่วไปๆ ส่งผลให้ ผู้เล่นสล็อต มีโอกาสในการ รับรางวัล ได้บ่อยครั้ง อีกทั้ง ยังมีให้เลือก ฟีเจอร์เพิ่มเติม เช่นได้แก่ ฟรีสปิน, ตัวคูณรางวัล, และ โบนัสพิเศษ ที่เพิ่ม ความตื่นเต้น ให้แก่ การเล่น
darknet drug market dark markets
The most comprehensive bip39 world list for securely creating and restoring cryptocurrency wallets. Learn how mnemonic coding works and protect your digital assets!
taxi Prague airport how to prepare for moving Prague
best darknet markets https://github.com/darknetmarkets24/darknet-markets – darknet markets
Лучшие советы по выбору металлической входной двери, идеально впишется в интерьер.
Места, где можно приобрести качественную входную металлическую дверь.
дверь металлическая заказать двери входные металлические .
Советы по избежанию ошибок при покупке металлической входной двери.
Почему стоит выбрать металлическую входную дверь.
В чем отличия входных металлических дверей разных производителей.
deep web drug store https://github.com/darknetmarketslinks/darknetmarketlinks – dark markets 2025 deep web drug links
Dispositivos de calibración: clave para el desempeño fluido y óptimo de las maquinarias.
En el entorno de la tecnología actual, donde la efectividad y la confiabilidad del aparato son de máxima significancia, los sistemas de ajuste tienen un papel esencial. Estos dispositivos específicos están desarrollados para equilibrar y fijar elementos móviles, ya sea en herramientas industrial, medios de transporte de movilidad o incluso en aparatos caseros.
Para los especialistas en mantenimiento de aparatos y los técnicos, utilizar con aparatos de calibración es esencial para promover el operación uniforme y fiable de cualquier mecanismo móvil. Gracias a estas soluciones tecnológicas sofisticadas, es posible disminuir considerablemente las oscilaciones, el estruendo y la esfuerzo sobre los soportes, mejorando la duración de elementos costosos.
También importante es el rol que desempeñan los dispositivos de calibración en la atención al usuario. El ayuda técnico y el conservación regular usando estos aparatos posibilitan proporcionar asistencias de óptima excelencia, mejorando la agrado de los compradores.
Para los titulares de empresas, la contribución en estaciones de ajuste y dispositivos puede ser fundamental para mejorar la eficiencia y desempeño de sus aparatos. Esto es especialmente importante para los inversores que manejan reducidas y medianas negocios, donde cada elemento es relevante.
Asimismo, los equipos de balanceo tienen una vasta implementación en el ámbito de la fiabilidad y el supervisión de calidad. Permiten detectar posibles problemas, reduciendo mantenimientos costosas y perjuicios a los aparatos. Además, los resultados extraídos de estos dispositivos pueden usarse para optimizar sistemas y potenciar la exposición en motores de exploración.
Las sectores de aplicación de los sistemas de calibración incluyen variadas sectores, desde la producción de vehículos de dos ruedas hasta el seguimiento de la naturaleza. No importa si se considera de importantes elaboraciones productivas o reducidos locales de uso personal, los equipos de calibración son indispensables para asegurar un operación efectivo y sin presencia de interrupciones.
onion market https://github.com/darknetmarkets24/darknet-markets darknet market links
L ゴジラ対エヴァンゲリオン
冴島 大河
シンプルなルールで、初心者でもすぐに楽しめます。気軽に挑戦できるのが良いです。
神龍降臨
https://www.k8pachinko.asia/?ReviewID=925954818.html
大当たり時の興奮は、他のゲームでは味わえない特別なものです。心が躍ります。
CR 北斗の拳6 拳王
花火 育成
プレミアムビンゴ
チェインクロニクル
アイムジャグラーEXAnniversaryEdition
ビーストサップ
https://www.sawasdeeslot.com/?ArticleID=926815818.html
大当たりの確率を理解することで、より戦略的にプレイできます。計算が好きな人にはおすすめです。
P結城友奈は勇者であるALLRUSH RFa Ver.119
https://www.k8pachinko.cc/?GameID=927452818.html
終わった後の反省会も楽しいです。次回の戦略を考えることで、さらなる楽しみが生まれます。
いみそーれ(红花)
https://www.online-casino.city/?NewsID=928047818.html
友達と一緒に楽しむのに最適。共通の趣味で盛り上がれます。
Секреты успешной покупки входной металлической двери, которая прослужит долгие годы.
Места, где можно приобрести качественную входную металлическую дверь.
заказать входную дверь купить входную дверь .
Что учесть при выборе входной металлической двери.
5 основных причин купить металлическую входную дверь.
Сравнение входных металлических дверей различных брендов.
dark web market links tor markets links
darknet marketplace darkmarket url
darknet links https://github.com/darknetmarketslinks/darknetmarketlinks – how to get on dark web dark web site
dark market url https://github.com/darknetmarkets24/darknet-markets dark web market links
darknet market list https://github.com/darknetmarkets24/darknet-markets – dark market link
Лучшее казино для аркадных игр | Развлечения и азарт в аркадном казино | Победите в аркадах и станьте миллионером | Азартные аркады и огромные выигрыши | Онлайн казино с самыми популярными аркадными играми | Уникальные аркады только у нас | Уникальные аркадные игры ждут вас | Самые популярные аркадные игры в одном казино | Играйте в аркадные игры и ощутите азарт | Онлайн казино с азартными аркадами | Большие денежные призы в аркадах | Уникальные аркадные игры в вашем распоряжении | Побеждайте в аркадах и станьте миллионером | Побеждайте в аркадах и получайте выигрыши | Выигрывайте крупные суммы в аркадных играх | Побеждайте в аркадных играх и получайте призы
arkada casino бездепозитный бонус arkada casino автоматы .
darkmarket list dark markets 2022
tor market links tor market url
darknet market lists https://github.com/darknetmarketslinks/darknetmarketlinks – blackweb darkweb marketplace
darkweb marketplace https://github.com/darknetmarkets24/darknet-markets darknet drug market
Bip39 Bip39 .
ххх милфы ххх милфы .
германское порно германское порно .
порно с чудовищем порно с чудовищем .
try here https://jaxx-wallet.net/
dark web links https://github.com/darknetmarkets24/darknet-markets – dark market onion
tor dark web https://github.com/darknetmarketslinks/darknetmarketlinks – dark web access dark market url
tor dark web darknet market
порно япония порно япония .
deep web drug markets https://github.com/darknetmarkets24/darknet-markets dark web markets
deep web drug url dark markets 2022
Современная автоматика для жалюзи, сделает ваш дом уютнее.
Оптимизируйте освещение и температуру в помещении с автоматикой для жалюзи.
Удобное управление жалюзи через интернет.
Модернизируйте ваш дом с автоматическими жалюзи.
Опытные специалисты по автоматике для жалюзи.
жалюзи автоматик https://elektrokarniz18.ru/ .
The most comprehensive bip39 phrase for securely creating and restoring cryptocurrency wallets. Learn how mnemonic coding works and protect your digital assets!
transportation of fragile goods Prague trusted moving companies Prague
deep web drug markets dark market 2022
drug markets dark web https://github.com/darknetmarkets24/darknet-markets darknet drug market
darknet drug store https://github.com/darknetmarkets24/darknet-markets – darknet sites
darkmarkets darknet drug market
花の慶次天に愛されし漢~
少年 ハート
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
CR ぱちんこAKB48 バラの儀式
https://jahromblog.com/?GameID=924062818.html
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
P フィーバー戦姫絶唱シンフォギア3 黄金絶唱 Light ver.
虚構叙事
真モグモグ風林火山
パチスロ聖闘士星矢-女神聖戦(V2)
CRF宇宙戦艦ヤマト YR
闇の花 (4発1)(特殊大賞燈)
https://www.k8casino.biz/?ArticleID=920299818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
聖闘士星矢 -黃金激闘篇-
https://www.k8pachinko.biz/?NewsID=923649818.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
緑イミソーレ(特殊大賞燈)
https://www.turboslott.com/?ArticleID=919615818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
tor market url drug markets dark web
dark web drug marketplace https://github.com/darknetmarketslinks/darknetmarketlinks – tor darknet dark web sites links
dark market url https://github.com/darknetmarkets24/darknet-markets dark web links
stehovani seznam stehovani chat
deep web markets dark market list
dark web drug marketplace https://github.com/darknetmarkets24/darknet-markets – dark websites
darkmarket url onion market
tor market https://github.com/darknetmarketslinks/darknetmarketlinks – darknet market dark markets
darknet market https://github.com/darknetmarkets24/darknet-markets tor market
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали срочный ремонт холодильников siemens, можете посмотреть на сайте: ремонт холодильников siemens
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
deep web drug url onion market
Ищете квартиру в новом доме? новостройки Украина – это современные жилые комплексы, доступные цены, рассрочка и ипотека. Подберите идеальное жилье от надежных застройщиков!
Останні актуальні свіжі новини спорту, оперативні події, важливі рішення, міжнародна політика та економіка. Все, що потрібно знати про життя країни, в одному місці!
tor marketplace https://github.com/darknetmarketslinks/darknetmarketlinks – tor darknet tor darknet
dark websites https://github.com/darknetmarkets24/darknet-markets dark web sites links
купить в иваново диплом
darkmarket url https://github.com/darknetmarkets24/darknet-markets – dark web market links
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт imac 27 рядом, можете посмотреть на сайте: ремонт imac 27 адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
have a peek at this website https://web-freewallet.com
casino elon elonbangladeshbet.com .
elonbet casino elonbetting.com .
elon casino elon casino .
tor markets deep web drug markets
dark market link https://github.com/darknetmarketslinks/darknetmarketlinks – bitcoin dark web darkmarket
tor market links https://github.com/darknetmarkets24/darknet-markets darknet market lists
darknet market links darknet market lists
Продажа инструментов для дома купить инструмент по дереву строительства и ремонта! Огромный выбор ручного и электроинструмента, выгодные цены, акции и быстрая доставка. Найдите все необходимое в одном месте!
Free and reliable nudify ai tool to create images with AI? The Best Free AI Nudify is the best choice for creating nude photos with privacy and quality guarantee.
deep web drug url dark websites
deep dark web https://github.com/darknetmarkets24/darknet-markets – bitcoin dark web
tor markets 2025 https://github.com/darknetmarketslinks/darknetmarketlinks – deep web markets dark web sites links
tor market url https://github.com/darknetmarkets24/darknet-markets tor markets links
https://allswingersclubs.org/swingers-club/erotic-nights-events/
tor markets dark web market links
Seattle VIP Limousine Service: Your Premier Choice for Luxury Transportation
Welcome to Seattle VIP Limousine Service, your top-tier provider of luxurious and reliable transportation services in the Seattle area. We specialize in offering exceptional Shoreline Airport Limo and Snohomish Airport Limo services, ensuring that your journey to and from the airport is as smooth and comfortable as possible.
Why Choose Seattle VIP Limousine Service?
– Luxury Fleet : Our fleet consists of late-model, well-maintained vehicles equipped with state-of-the-art amenities. From sleek sedans to spacious SUVs and stretch limousines, we have the perfect vehicle to suit your needs and preferences.
– Professional Chauffeurs : Our chauffeurs are licensed, experienced, and committed to providing the highest level of service. They are familiar with the best routes to Shoreline Airport and Snohomish Airport , ensuring timely and efficient transportation.
– 24/7 Availability : We understand that travel plans can be unpredictable. That’s why we offer our Shoreline Airport Limo and Snohomish Airport Limo services around the clock. Whether you need an early morning pick-up or a late-night drop-off, we’re here to serve you.
– Personalized Service : At Seattle VIP Limousine Service, we believe in tailoring our services to meet your unique needs. From the moment you book with us to the time you reach your destination, we strive to exceed your expectations.
Our Services
– Airport Transfers : Our Shoreline Airport Limo and Snohomish Airport Limo services are designed to make your airport travel hassle-free. We monitor your flight schedule to ensure timely pick-ups and drop-offs.
– Corporate Travel : Impress your clients and colleagues with our executive limo service. We offer seamless transportation for business meetings, conferences, and corporate events.
– Special Occasions : Make your special events even more memorable with our luxury limousine service. Whether it’s a wedding, prom, or a night out on the town, we ensure you arrive in style.
Book Your Limo Service Today
Experience the ultimate in luxury and convenience with Seattle VIP Limousine Service. Whether you need a Shoreline Airport Limo or a Snohomish Airport Limo , we are dedicated to providing you with a first-class travel experience. Contact us today to book your limo service and enjoy the ride!
Лучшие решения для автоматизации жалюзи, сделает ваш дом уютнее.
Экономьте энергию с помощью автоматики для жалюзи.
Удобное управление жалюзи через интернет.
Повысьте уровень безопасности с автоматическими жалюзи.
Опытные специалисты по автоматике для жалюзи.
жалюзи с автоматикой жалюзи с автоматикой .
перевозки услуги грузчиков услуги грузчиков
darknet websites darkmarket list
darknet drugs https://github.com/darknetmarketslinks/darknetmarketlinks – dark net tor market links
Every word in this the post is filled with deep meaning and makes you think seriously https://dilts.g-u.su
dark web market links https://github.com/darknetmarkets24/darknet-markets deep web drug url
deep web drug markets https://github.com/darknetmarkets24/darknet-markets – deep web drug store
Use the proven bip39 phrase standard to protect your assets and easily restore access to your finances. A complete list of 2048 mnemonic words used to generate and restore cryptocurrency wallets.
Предлагаем ремонт vivo y18 в Петербурге. Если у вас сломалась техника обращайтесь в сервис 911. Мы выполняем – Ремонт Vivo Y18 профессионально, быстро и с гарантией!
darkmarket 2022 darkmarkets
Лучшие решения для автоматизации жалюзи, повысит комфорт в помещении.
Оптимизируйте освещение и температуру в помещении с автоматикой для жалюзи.
Удобное управление жалюзи через интернет.
Создайте уютную атмосферу с автоматическими жалюзи.
Качественное обслуживание автоматизированных жалюзи.
жалюзи на автоматике жалюзи на автоматике .
dark web sites links darknet market links
dark web search engine https://github.com/darknetmarketslinks/darknetmarketlinks – darknet site tor markets links
tor dark web https://github.com/darknetmarkets24/darknet-markets darkweb marketplace
tor markets 2022 https://github.com/darknetmarkets24/darknet-markets – dark markets 2022
darknet websites deep web drug markets
merhaba ingiltere sanal sunucu paketleri için sitenizi ziyaret etim
merhaba sanal sunucu paketleriniz için sitenizi ziyaret ettim paketleri cok begendim
selamlar almanya sanal sunucu ihtiyacıma uygun paketler için tesekkurler
dark market url tor market
dark websites https://github.com/darknetmarketslinks/darknetmarketlinks – tor markets dark websites
darkmarket list https://github.com/darknetmarkets24/darknet-markets dark market list
darknet drug store https://github.com/darknetmarkets24/darknet-markets – tor markets links
tor markets 2022 bitcoin dark web
tor markets links dark market list
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников siemens, можете посмотреть на сайте: срочный ремонт холодильников siemens
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darkmarket url https://github.com/darknetmarketslinks/darknetmarketlinks – darknet markets 2025 deep web drug store
vds kiralama secegeni icin siteniz zengin duruyor
vps sunucu kiralama paketlerini kaliteli duruyor
günlük sanal sunucu ihtiyacimi bu paket sayesınde cozume kavusturdum
tor darknet https://github.com/darknetmarkets24/darknet-markets darknet site
Предлагаем ремонт кондиционеров aeronik в Петербурге. Если у вас сломалась техника обращайтесь в сервис 911. Мы выполняем – Ремонт кондиционеров Aeronik профессионально, быстро и с гарантией!
CRルパン三世~消されたルパン
小 パンダ
美しいアニメーションと迫力ある演出が魅力。毎回新しい発見があります。
紫イミソーレ(4発1)
https://www.k8-casino.org/?ArticleID=920305818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
CRキャプテン翼XX
邪 鬼王
八代將軍戰神版
デビルサバイバー2 最後の7日間
アナザーゴッドハーデス-奪われたZEUSver
CR びっくり戦国無双 Light Edition
https://www.k8.rodeo/?SitesID=925343818.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
魂斗羅3D
https://www.k8-casino.asia/?SitesID=922991818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CR真・花の慶次L6-K 319ver.
https://www.k8pachinko.work/?ReviewID=918418818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
tor darknet https://github.com/darknetmarkets24/darknet-markets – darknet market lists
darknet drug links dark web links
tor markets links deep web drug url
Sitio web oficial jude-bellingham.com mx de fans de Jude Bellingham: noticias, logros y material exclusivo sobre la carrera del talentoso mediocampista que juega en el Real Madrid.
dark web market list https://github.com/darknetmarketslinks/darknetmarketlinks – the dark internet how to access dark web
Sitio web de fans rodri.com.mx/ de Rodri Hernandez: Descubre la carrera y logros del mediocampista espanol del Manchester City. Noticias, estadisticas y analisis del juego de uno de los mejores futbolistas actuales.
tor dark web https://github.com/darknetmarkets24/darknet-markets deep web drug store
tor dark web https://github.com/darknetmarkets24/darknet-markets – drug markets dark web
dark web drug marketplace darkmarket link
elon casino giri? http://www.elonspin.com .
tor dark web dark market onion
Kevin De Bruyne https://kevin-de-bruyne.com.mx es un maestro del futbol moderno, conocido por su vision de juego, precision y liderazgo en el Manchester City. Su talento y trabajo duro lo han convertido en una leyenda del deporte.
Sitio fan de Mohamed Salah mohamed-salah.com.mx ultimas noticias, records, entrevistas y los mejores momentos de la carrera de uno de los futbolistas mas grandes de la actualidad. ?Mantente al tanto!
darknet market links https://github.com/darknetmarketslinks/darknetmarketlinks – darknet market lists dark market link
elion casino elion casino .
darknet market list https://github.com/darknetmarkets24/darknet-markets darknet markets
darknet drug store https://github.com/darknetmarkets24/darknet-markets – darkmarket url
dark websites https://github.com/darknetmarketslinks/darknetmarketlinks – dark web websites how to get on dark web
tor markets 2022 drug markets dark web
dark web links https://github.com/darkwebmarketslinks/darkwebmarkets – darknet search engine dark website
deep web search https://github.com/darkwebwebsites/darkwebwebsites – dark market list darkmarket link
bitcoin dark web https://github.com/darknetmarkets2025/darknetmarketlinks – dark web sites links tor market links
darknet sites dark web links
Creative article!
Fan site de Vinicius Junior https://vinicius-jr.com.mx noticias, logros y detalles sobre su carrera en el Real Madrid. Sigue la evolucion de esta estrella del futbol mundial.
dark web market links https://github.com/darknetmarketslinks/darknetmarketlinks – dark market link dark market onion
bitcoin dark web https://github.com/darknetmarkets24/darknet-markets dark web link
bitcoin dark web https://github.com/darknetmarkets24/darknet-markets – tor markets links
dark website https://github.com/darknetmarketslinks/darknetmarketlinks – darknet drug links deep web drug markets
darknet drug links darkmarket
captain cook casino en ligne casino en ligne france gratuit
dark market https://github.com/darknetmarkets2025/darknetmarketlinks – drug markets onion dark web drug marketplace
tor market links https://github.com/darkwebmarketslinks/darkwebmarkets – dark market dark web access
darknet drug market dark web markets
CR蒼天の拳天帰 Ver.319(1:1)
利用 した
キャラクターの個性が際立っており、ストーリーに引き込まれます。
CRフィーバー戦姫絶唱シンフォギア Ver.199 (1:1)
https://www.k8pachinko.asia/?NewsID=920409818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
P戦国乙女 LEGEND BATTLE
カジノ 有料
北斗之拳 轉生之章
P 結城友奈は勇者である GC250Ba
斉天大聖
Lバキ 強くなりたくば喰らえ!!!
https://www.diabetesdirectory.org/?NewsID=921093818.html
演出が多彩で、飽きることがないのが魅力です。毎回新しい体験ができます。
十字架3
https://www.ja-securities.jp/?ReviewID=926350818.html
大当たりの瞬間は、ドキドキ感がたまりません。興奮が止まりません。
鬼武者3
https://www.k8pachinko.biz/?NewsID=918579818.html
美しいビジュアルと迫力ある演出が魅力。プレイ中に没入感が高まります。
dark market link https://github.com/darknetmarketslinks/darknetmarketlinks – dark web link dark markets
best darknet markets https://github.com/darknetmarkets24/darknet-markets drug markets dark web
deep web drug store https://github.com/darknetmarkets24/darknet-markets – tor dark web
Срочный выкуп авто https://automagnit.com в Москве – продайте машину за 30 минут по выгодной цене! Оценка, оформление документов и моментальная выплата. Работаем с любым состоянием авто.
tor market https://github.com/darknetmarketslinks/darknetmarketlinks – darknet drug links deep web drug markets
студия ландшафтного дизайна ландшафтный дизайн москва
Авторемонт от профессионалов ремонт гидравлики в москве восстановление, диагностика, замена деталей. Работаем с любыми типами гидравлического оборудования. Гарантия на выполненные работы!
darknet market list darkmarket url
darkmarket url https://github.com/darknetmarkets2025/darknetmarketlinks – deep web drug url deep web markets
dark web sites links https://github.com/darkwebwebsites/darkwebwebsites – dark web sites dark market 2025
dark market 2025 https://github.com/darkwebmarketslinks/darkwebmarkets – tor darknet darknet markets 2025
Try your luck at pin up casino! The best slots, roulette, blackjack and live games with real dealers. Pleasant bonuses, promotions and a user-friendly interface will create ideal conditions for the game!
darknet websites https://github.com/darknetmarkets24/darknet-markets darkmarket
darknet market https://github.com/darknetmarkets24/darknet-markets – dark web drug marketplace
darknet market lists https://github.com/darknetmarketslinks/darknetmarketlinks – darknet sites dark web site
На SMTP сервере почта со своим доменом обеспечивает сотрудников адресами электронной почты на собственном домене компании. Доменная почта это корпоративный почтовый сервис email для организаций. Почта для бизнеса улучшает узнаваемость и повышает имидж бренда.
The variety of games on 1win casino makes it a top choice for players | The bonuses at 1win Azerbaijan make betting even more exciting 1win casino | 1win casino has gained popularity for its fair and transparent gaming | The loyalty rewards program at 1win keeps players coming back | 1win provides fair and transparent betting services for all users 1win sports betting.
tor markets darknet market links
darkmarket https://github.com/darknetmarketslinks/darknetmarketlinks – drug markets dark web dark net
Every time I read such posts, I understand how accurately they reflect our reality https://dilts.g-u.su
darknet markets tor darknet
красивая одежда для беременных красивая одежда для беременных .
1виг 1виг .
1 вин официальный сайт mabc.com.kg .
tor markets https://github.com/darknetmarkets2025/darknetmarketlinks – dark web sites links darknet market
tor market url https://github.com/darkwebwebsites/darkwebwebsites – darknet market links dark web drug marketplace
dark web market https://github.com/darkwebmarketslinks/darkwebmarkets – dark market link dark web drug marketplace
dark market 2022 https://github.com/darknetmarkets24/darknet-markets dark web market links
dark web market links https://github.com/darknetmarkets24/darknet-markets – dark web drug marketplace
Идеальный привод для жалюзи.
с использованием привода.
для офиса.
Преимущества автоматизированного привода для жалюзи.
Современные разработки для управления жалюзями.
Как управлять жалюзями с помощью смартфона с использованием привода.
Как установить привод для жалюзи своими руками.
Почему стоит выбрать привод для автоматизации жалюзей.
Необычное применение приводов для управления жалюзями.
Секрет уюта и удобства с автоматизированными жалюзями.
привод для жалюзи https://elektrokarniz21.ru/ .
cresus casino en ligne casino en ligne blackjack
dark internet https://github.com/darknetmarketslinks/darknetmarketlinks – darknet marketplace how to access dark web
Full wordlist New full BIP39 2048 words used to create and restore crypto wallets. Multi-language support, high security and ease of use to protect your funds. 2048 mnemonic words for seed generation.
dark web markets https://github.com/darknetmarketslinks/darknetmarketlinks – bitcoin dark web dark web access
New full bip39 phrase 2048 words used to create and restore crypto wallets. Multi-language support, high security and ease of use to protect your funds.
drug markets dark web [url=https://github.com/darkwebwebsites/darkwebwebsites ]darknet markets [/url] dark net
dark market url https://github.com/darknetmarkets2025/darknetmarketlinks – drug markets onion darkmarket 2025
drug markets onion [url=https://github.com/darkwebmarketslinks/darkwebmarkets ]darkmarkets [/url] darknet market list
Простое решение для управления жалюзями.
Умное устройство для управления жалюзи.
для офиса.
Преимущества автоматизированного привода для жалюзи.
Инновационные технологии в области приводов для жалюзи.
Как управлять жалюзями с помощью смартфона с использованием привода.
Как установить привод для жалюзи своими руками.
Почему стоит выбрать привод для автоматизации жалюзей.
Необычное применение приводов для управления жалюзями.
Как повысить уровень комфорта с приводом для жалюзи.
жалюзи автоматическим приводом https://elektrokarniz21.ru/ .
dark markets https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarket 2025 deep web search
darkmarkets https://github.com/darknetmarketslinks/darknetmarketlinks – dark web websites dark web search engines
After reading this post, you begin to look at familiar things around you in a completely different way https://dilts.g-u.su
I need to save this post and show it to everyone I know, because it is really important https://dilts.g-u.su
dark web market links https://github.com/darknetmarkets2025/darknetmarketlinks – black internet the dark internet
dark website https://github.com/darkwebwebsites/darkwebwebsites – onion market darknet market links
I rarely come across such deep and correct thoughts on social networks, this is worthy of respect https://dilts.g-u.su
Идеальный привод для жалюзи.
с помощью привода.
Секреты выбора привода для жалюзи.
Зачем нужен привод для автоматизации жалюзей.
Инновационные технологии в области приводов для жалюзи.
Простой способ контролировать жалюзи с телефона с использованием привода.
Шаг за шагом по инструкции по установке привода для жалюзи.
Плюсы привода для жалюзи в экономии ресурсов.
Интересные факты о приводах для жалюзи.
Как повысить уровень комфорта с приводом для жалюзи.
привод жалюзи вентиляции https://elektrokarniz21.ru/ .
dark market list https://github.com/darkwebmarketslinks/darkwebmarkets – darknet search engine darknet market lists
dark web search engines https://github.com/darknetmarketslinks/darknetmarketlinks – dark web access tor market links
best darknet markets https://github.com/darknetmarketslinks/darknetmarketlinks – dark web market dark web sites
dark web link https://github.com/darknetmarkets2025/darknetmarketlinks – darknet websites dark web access
elonbet casino http://www.elonspin.com/ .
darknet market lists https://github.com/darkwebmarketslinks/darkwebmarkets – dark web drug marketplace deep web drug store
darknet market list https://github.com/darknetmarketslinks/darknetmarketlinks – darknet marketplace how to access dark web
tor market links https://github.com/darknetmarketslinks/darknetmarketlinks – the dark internet drug markets onion
Sistemas de balanceo: esencial para el funcionamiento estable y eficiente de las dispositivos.
En el campo de la avances actual, donde la eficiencia y la fiabilidad del sistema son de suma significancia, los aparatos de ajuste juegan un función crucial. Estos sistemas específicos están creados para calibrar y estabilizar elementos rotativas, ya sea en dispositivos manufacturera, medios de transporte de traslado o incluso en dispositivos hogareños.
Para los expertos en mantenimiento de equipos y los ingenieros, trabajar con sistemas de balanceo es importante para garantizar el operación fluido y fiable de cualquier mecanismo giratorio. Gracias a estas herramientas modernas innovadoras, es posible limitar significativamente las movimientos, el ruido y la tensión sobre los rodamientos, mejorando la duración de partes valiosos.
También trascendental es el función que juegan los aparatos de balanceo en la atención al comprador. El ayuda experto y el soporte regular empleando estos sistemas habilitan brindar prestaciones de óptima excelencia, incrementando la bienestar de los compradores.
Para los dueños de proyectos, la inversión en unidades de balanceo y sensores puede ser fundamental para mejorar la efectividad y eficiencia de sus dispositivos. Esto es especialmente importante para los inversores que gestionan medianas y pequeñas empresas, donde cada elemento es relevante.
Además, los dispositivos de equilibrado tienen una amplia utilización en el área de la fiabilidad y el monitoreo de estándar. Habilitan identificar potenciales errores, impidiendo mantenimientos caras y perjuicios a los equipos. Más aún, los datos obtenidos de estos aparatos pueden aplicarse para optimizar procedimientos y potenciar la exposición en sistemas de consulta.
Las campos de implementación de los equipos de calibración cubren diversas áreas, desde la elaboración de transporte personal hasta el monitoreo ecológico. No influye si se trata de enormes producciones productivas o reducidos espacios domésticos, los equipos de balanceo son esenciales para asegurar un desempeño óptimo y sin detenciones.
casino en ligne fiable forum casino barriere en ligne avis
Full wordlist New full BIP39 2048 words used to create and restore crypto wallets. Multi-language support, high security and ease of use to protect your funds. 2048 mnemonic words for seed generation.
deep web drug markets https://github.com/darknetmarkets2025/darknetmarketlinks – darkmarket url darknet search engine
dark markets https://github.com/darkwebwebsites/darkwebwebsites – dark web search engines best darknet markets
New full bip39 world list 2048 words used to create and restore crypto wallets. Multi-language support, high security and ease of use to protect your funds.
tor market [url=https://github.com/darkwebmarketslinks/darkwebmarkets ]darknet marketplace [/url] dark internet
darkmarket https://github.com/darknetmarketslinks/darknetmarketlinks – deep web drug links the dark internet
dark market onion https://github.com/darknetmarketslinks/darknetmarketlinks – best darknet markets dark market list
ноклип SCP Secret Laboratory – купить приватный чит гта 5 онлайн, читы на апекс легенд
deep web drug links https://github.com/darknetmarkets2025/darknetmarketlinks – tor marketplace dark web websites
dark web drug marketplace https://github.com/darkwebwebsites/darkwebwebsites – darknet market list darkmarket url
吉宗
パルサー
パチンコは、ルールを覚えればすぐに楽しめるので、初心者でも安心です。気軽に始められます。
鉄拳R 2004
https://www.stationslot.org/?ReviewID=923830818.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
CRフィーバー宇宙戦艦ヤマト-ONLY ONE-
デルダスラー
Pフィーバーかぐや様は告らせたい
主役は銭形2
めんそーれ琉球守護神
バイオハザード6
https://www.ja-securities.jp/?WatchID=927492818.html
シンプルなデザインが魅力。初心者でも楽しみやすい仕様です。
戦国乙女剣戟に舞う白き剣聖西国参戦編
https://www.casinodengi.org/?GameID=927434818.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
L ゴールデンカムイ
https://www.k8-casino.asia/?SitesID=922433818.html
パチンコの美しいデザインの台が多く、視覚的にも楽しめます。アートとしての側面もあります。
tor dark web https://github.com/darknetmarketslinks/darknetmarketlinks – best darknet markets dark market 2025
dark net [url=https://github.com/darkwebmarketslinks/darkwebmarkets ]dark web search engines [/url] darknet market list
darknet market list https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarkets tor darknet
deep web sites https://github.com/darkwebwebsites/darkwebwebsites – tor markets darknet markets
deep dark web https://github.com/darknetmarkets2025/darknetmarketlinks – dark market link dark web site
1вин вход 1вин вход .
1 вин 1 вин .
для беременных одежда магазин для беременных одежда магазин .
Актуальные промокоды казино https://hdvideo.cat/pag/le_code_promo_de_1xbet_maroc_bonus.html получите дополнительные бонусы при регистрации! Увеличенный первый депозит, бесплатные ставки, фриспины и эксклюзивные акции для игроков. Вводите код и выигрывайте больше!
dark markets 2025 https://github.com/darknetmarketslinks/darknetmarketlinks – dark markets darknet links
Промокод казино https://viracore.one/read-blog/3811 активируйте эксклюзивные бонусы! Получите фриспины, бонусные деньги на депозит и кешбэк. Используйте актуальные коды для максимальной выгоды в онлайн-казино. Играйте с лучшими условиями!
Current casino promo codes https://speros.lt/scripts/inc/melbet_promokod_pri_registracii___bonus_kod_na_segodnya.html bonus money, free spins and cashback for playing. Use verified codes, activate exclusive offers and win more!
dark web sites links [url=https://github.com/darkwebmarketslinks/darkwebmarkets ]darkmarket list [/url] deep web sites
dark web markets https://github.com/darknetmarketslinks/darknetmarketlinks – dark web markets dark web sites
Seattle Elite Town Car Service: Your Gateway to Luxury Travel
Experience the epitome of luxury and convenience with our Seattle Elite Town Car Service . We cater to the discerning traveler who expects nothing but the best. Our fleet of late-model, high-end town cars is meticulously maintained to ensure a smooth and comfortable ride every time.
Seattle Executive Personal – Private Chauffeur
At Seattle Elite Town Car Service, we understand the unique needs of executive travelers. Our Seattle Executive Personal – Private Chauffeur service is designed to provide personalized, discreet, and efficient transportation. Our professional chauffeurs are more than just drivers; they are your personal assistants on the road, ensuring that you arrive at your meetings, events, or flights on time and in style.
Our chauffeurs undergo rigorous training and background checks to guarantee your safety and privacy. They are intimately familiar with Seattle’s traffic patterns and routes, ensuring that you reach your destination via the most efficient path.
Seattle Five Star Town Car Service
Indulge in the ultimate luxury with our Seattle Five Star Town Car Service . This premium service is designed for those who demand the finest in life. Our five-star service includes:
– Luxury Fleet : Choose from our collection of high-end vehicles, each equipped with premium features for your comfort.
– Professional Chauffeurs : Our chauffeurs are courteous, discreet, and committed to providing the highest level of service.
– Amenities : Enjoy complimentary amenities such as bottled water, mints, and Wi-Fi to make your journey as pleasant as possible.
– 24/7 Availability : We operate around the clock to accommodate your schedule, whether it’s an early morning flight or a late-night return.
Why Choose Us?
– Reliability : We pride ourselves on our on-time performance and consistency.
– Safety : Our vehicles are regularly inspected, and our chauffeurs are trained in defensive driving.
– Convenience : Book your ride easily through our website or mobile app.
– Customer Satisfaction : Our dedicated customer service team is available 24/7 to assist you with any needs or inquiries.
Whether you’re traveling for business or leisure, our Seattle Elite Town Car Service is your key to a seamless and luxurious travel experience. Book your ride today and discover the difference that elite service can make.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
darknet market lists https://github.com/darkwebwebsites/darkwebwebsites – tor dark web dark net
dark web market list https://github.com/darknetmarkets2025/darknetmarketlinks – tor market darknet market list
darknet drug links https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarket tor market links
Ищете выгодные покупки? нова маркетплейс предлагает широкий выбор товаров по привлекательным ценам! Безопасные сделки, удобный интерфейс и проверенные продавцы – все для комфортного шопинга.
Забор под ключ https://habarovsk.zabor-profi.ru в Хабаровске – надежные и качественные ограждения для вашего участка! Установка заборов из профнастила, металла, дерева и бетона. Бесплатный замер, быстрая доставка и монтаж.
геотехнический прогноз влияния строительства https://otsenka-vliyaniya-stroitelstva.ru
мостбет ставки http://www.gtrtt.com.kg/ .
Лучшие промокоды для казино https://www.rubikom.ru/articles/promokod_dlya_1xbet_pri_registracii.html активируйте бонусные предложения и получите больше шансов на выигрыш. Бесплатные спины, дополнительные деньги на баланс и персональные акции ждут вас!
darknet seiten https://github.com/darkwebmarketslinks/darkwebmarkets – dark web drug marketplace dark web links
tor markets links https://github.com/darknetmarketslinks/darknetmarketlinks – darknet market deep web drug url
darknet markets 2025 https://github.com/darkwebwebsites/darkwebwebsites – dark markets 2025 darkmarkets
drug markets onion https://github.com/darknetmarkets2025/darknetmarketlinks – deep web drug links how to get on dark web
darkmarket list https://github.com/darknetmarketslinks/darknetmarketlinks – drug markets dark web tor markets 2025
Откройте новые возможности https://sindicate24-4.biz! Удобный сервис, быстрые решения и выгодные предложения для пользователей.
Присоединяйтесь к NovaXLtd.live ссылка платформе с широкими возможностями и удобными инструментами. Доступность, надежность, поддержка!
Используйте Kra020 актуальная ссылка надежная площадка с удобными инструментами и поддержкой 24/7. Простота и безопасность в одном сервисе!
darkmarket 2025 https://github.com/darknetmarketslinks/darknetmarketlinks – dark market url black internet
free dark web https://github.com/darkwebmarketslinks/darkwebmarkets – deep dark web dark web sites links
Временная регистрация в Москве – быстро и надежно!
Нужна временная прописка для работы, учебы или оформления
документов? Оформим официальную регистрацию в Москве
всего за 1 день. Без очередей, с гарантией и
юридической чистотой!
? Подходит для граждан РФ и СНГ
? Регистрация от 3 месяцев до 5 лет
? Легально, с внесением в базу МВД
Работаем без предоплаты! Документы можно получить лично или дистанционно.
Доступные цены, оперативное оформление!
Звоните или пишите в WhatsApp прямо сейчас – поможем быстро и
без лишних вопросов!
Временная регистрация в Москве
政宗
ポーカー 闇
シンプルなルールで、誰でもすぐに楽しめるのが嬉しいです。
P Re:ゼロから始める異世界生活 鬼がかりver.
https://jahromblog.com/?NewsID=924765818.html
パチンコのルールはシンプルで、誰でもすぐに楽しめます。初心者にも優しいゲームです。
CR北斗の拳6 拳王
冬 背景
CRザ・キング・オブ・ファイターズ
リング呪いの7日間;
俺の空蒼き正義魂
Pフィーバーダンベル何キロ持てる
https://www.pgslot-casino.com/?ArticleID=922501818.html
パチンコの歴史を学ぶことで、より深く楽しめます。文化的な側面も魅力的です。
CR北斗の拳5 百裂 Ver.229(2:1)
https://www.k8io.net/?GameID=919406818.html
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
パチスロ北斗の拳 強敵
https://www.k8-casino.org/?GameID=919310818.html
友人と競い合うことで、より楽しめます。勝負の結果が気になります。
click this link here now Rent SMS numbers without hassle
darkmarket https://github.com/darknetmarketslinks/darknetmarketlinks – dark web search engine dark web market links
darkmarket link https://github.com/darkwebwebsites/darkwebwebsites – darkmarket the dark internet
dark web websites https://github.com/darknetmarkets2025/darknetmarketlinks – black internet darknet market
dark web drug marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – tor darknet
Продажа сигарет cherokee сигареты купить в интернет-магазине – оригинальные бренды, доступные цены, удобные способы оплаты и быстрая доставка. Сделайте заказ сегодня!
dark market https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark markets 2022
ทดลองเล่นสล็อต pg
ลองเล่น สล็อตแมชชีน PG คืออะไร? ทำไม จึงกลายเป็น เกมที่นิยม?
เกมสล็อตดิจิทัล เป็นเกมที่ เป็นที่นิยม อย่างแพร่หลาย ตลอดทุกยุคทุกสมัย พร้อมทั้ง จัดเป็น หนึ่งในจำนวน เกมที่ผู้คน รู้จักดี และยัง หลงใหล ที่สุด ในวงการ เกมบนอินเทอร์เน็ต แต่สำหรับ ผู้ที่ยังเป็นอยู่ ผู้เริ่มเล่น หรือก็คือ มีประสบการณ์ การเล่นเกมออนไลน์ เพียงเล็กน้อย วันนี้ จะแนะนำคุณ มาทำความเข้าใจ เล่นฟรี สล็อต PG ซึ่งถือเป็น เกมสล็อตโดยตรง ที่คนนิยม อย่างแพร่หลาย ผ่าน คุณจะสามารถ ทำความเข้าใจ รวมทั้ง เริ่มเล่น ได้เร็ว พร้อมกับ สนุกสนาน พร้อมกับ ประสบการณ์ใหม่ การเล่นเกม ที่ท้าทาย รวมทั้ง น่าตื่นตาตื่นใจ
เล่นฟรี เกมสล็อต PG หมายถึงอะไร?
เล่นฟรี สล็อต PG ถือเป็นเกม เกมออนไลน์สล็อต ที่สร้างขึ้น โดย บริษัทพัฒนาเกม PG Soft ซึ่งถือเป็น หนึ่งในบรรดา ผู้ผลิต เกมออนไลน์สล็อต ชั้นนำระดับโลก ในระดับโลก เกมสล็อตนี้ ได้รับแรงบันดาลใจ มาจาก เครื่องเล่นเกมสล็อต แบบดั้งเดิม แต่ได้ เพิ่มเข้าไป ความทันสมัยและความน่าสนใจ และยัง ความตื่นเต้น เข้าไปในระบบ โดย เกม จะมี 5 รีล และ 15 แบบ รูปแบบการรับรางวัล ซึ่งทำให้ ผู้เล่นออนไลน์ มีโอกาสในการ ชนะรางวัล ได้มากมาย
สัญลักษณ์ในเกม ในเกมดังกล่าว เกมสล็อต PG นั้นมีอยู่ มีหลายแบบ เช่นเช่น รูปเชอร์รี่, ตัวเลข 7, สัญลักษณ์เพชร, และยัง รูปสัญลักษณ์ อื่นๆอีก ที่เชื่อมโยง กับแนวคิด เกม ซึ่ง แต่ละเกม มี รูปแบบ รวมทั้ง รูปแบบการเล่น ที่ต่างออกไป ออกไปจากเดิม เช่นเช่น ธีมสัตว์ป่า, ธีมเทพเจ้าโบราณ, ธีมอาหาร, หรืออาจจะ ธีมการเดินทาง เป็นต้นมา
เพราะเหตุใด ลองเล่น สล็อตแมชชีน PG ถึงเป็น เกมยอดฮิต?
ความง่ายดาย ในการเล่นเกมออนไลน์
ลองเล่น เครื่องสล็อต PG เป็นเกม ที่เข้าใจง่าย ไม่ยุ่งยาก เหมาะกับ ผู้เริ่มต้น และ ผู้เล่นเก่า ผู้เล่นออนไลน์ เพียงเท่านั้น เลือกจำนวน จำนวนเงินเดิมพัน กดเล่น รวมทั้ง รอผล ผลลัพธ์ ซึ่งส่งผลให้ ระบบการเล่นเกม ที่เข้าใจง่าย ทำให้ ผู้เล่นสล็อต สามารถที่จะได้ สนุก ได้โดยไม่ต้อง ไม่ต้องกังวล ในเรื่อง กฎ ที่ยาก
รูปแบบการแจก การให้รางวัล ที่หลากหลาย
เกมสล็อตบนอินเทอร์เน็ต PG มีอยู่ รูปแบบการชนะ ให้ชนะรางวัล มากถึงทั้งหมด 15 รูปแบบ ซึ่งส่งผลให้ มากกว่าปกติ สล็อตคลาสสิก ปกติ ทำให้คุณ ผู้เล่นเกม มีโอกาสในการ ได้รับรางวัล ได้หลายครั้ง นอกจากนี้ ยังมีอยู่ ฟีเจอร์ที่น่าสนใจ ตัวอย่างเช่น การหมุนฟรีสปิน, ตัวคูณเงิน, พร้อมทั้ง รางวัลโบนัส ที่เสริม ความน่าสนใจ ให้แก่ การเล่น
Откройте все возможности Bs2best Большой выбор товаров, безопасные сделки и простая навигация. Приватность и защита пользователей на первом месте.
1vin официальный сайт http://www.fabc.com.kg/ .
скачать 1win с официального сайта http://bbcc.com.kg/ .
tor darknet https://github.com/darknetmarketslinks/darknetmarketlinks – dark websites tor marketplace
tor market links https://github.com/darkwebmarketslinks/darkwebmarkets – darkweb marketplace deep web markets
darknet sites https://github.com/darkmarketlinkp22jr/darkmarketlink – tor marketplace
dark web sites links https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web sites
мульт секс http://multiki-rukoeb1.ru/ .
секс у гинеколога https://ginekolog-rukoeb1.ru/ .
dark market 2022 https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet sites
dark market https://github.com/darknetmarketslinks/darknetmarketlinks – darknet drugs best darknet markets
dark market link https://github.com/darknetmarketlinks2025/darknetmarkets – deep web drug markets
deep web drug store https://github.com/tormarkets2025ukaz1/tormarkets2025 – onion market
darknet websites https://github.com/darkwebwebsites/darkwebwebsites – dark websites tor markets 2025
dark internet https://github.com/darknetmarkets2025/darknetmarketlinks – tor markets links deep web markets
darknet marketplace https://github.com/darknetmarketslinks/darknetmarketlinks – darknet seiten dark web drug marketplace
darknet markets https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet market links
tor marketplace https://github.com/darknetwebsitesgflpx/darknetwebsites – tor market links
darkmarket 2025 https://github.com/darkwebmarketslinks/darkwebmarkets – dark web search engine deep dark web
Setting up the Metamask extension on Chrome was a breeze thanks to https://metanate.org/. If you’re new to crypto wallets, this site provides all the info you need to get started. Super helpful and easy to follow!
drug markets dark web https://github.com/darknetdruglinksvojns/darknetdruglinks – dark market list
tor markets links https://github.com/darknetmarketslinks/darknetmarketlinks – darknet market lists darkmarket list
darkmarket link https://github.com/darknetmarketlinks2025/darknetmarkets – dark web sites
darkweb marketplace https://github.com/darkwebwebsites/darkwebwebsites – dark web search engine dark web link
deep web drug links https://github.com/darknetmarkets2025/darknetmarketlinks – deep web markets darkmarkets
darknet market https://github.com/darknetmarketslinks/darknetmarketlinks – dark web link darknet market lists
tor market https://github.com/darkmarketlinkp22jr/darkmarketlink – tor markets links
drug markets dark web https://github.com/darknetwebsitesgflpx/darknetwebsites – tor markets links
dark web site https://github.com/darkwebmarketslinks/darkwebmarkets – dark market 2025 dark web search engine
darknet market https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web market
darknet markets 2025 https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarket link tor market links
best darknet markets https://github.com/darknetmarketlinks2025/darknetmarkets – darknet market
Ваша сцена станет неповторимой с электрокарнизами, подчеркнут красоту представления.
Трансформируйте свою сцену с электрокарнизами, обеспечивая плавное движение драпировок.
Управляйте световыми шоу с помощью электрокарнизов, дарят возможность воплотить любую идею.
Лучшие электрокарнизы для профессиональных выступлений, для тех, кто ценит качество и стиль.
Лучшие электрокарнизы для вашего шоу, с непревзойденным качеством и надежностью.
Превратите ваше шоу с помощью электрокарнизов, и принести вашему проекту новый уровень.
Освежите свое шоу с электрокарнизами, которые обеспечат быстрое и плавное движение.
Новейшие электрокарнизы для театральных постановок, для тех, кто стремится к совершенству.
Электрокарнизы – современное решение для сцены, воплощая самые смелые идеи в жизнь.
Выберите электрокарнизы по своему вкусу, и удивить зрителей нестандартными решениями.
карниз для сцены с дистанционным управлением карниз для сцены с дистанционным управлением .
tor market url https://github.com/darknetmarketslinks/darknetmarketlinks – dark market url dark web site
dark market list https://github.com/darkmarketlinkp22jr/darkmarketlink – deep web drug store
dark markets 2025 https://github.com/darkwebwebsites/darkwebwebsites – dark market darknet drug store
dark web market links [url=https://github.com/darknetmarkets2025/darknetmarketlinks ]dark web market [/url] how to get on dark web
dark market list https://github.com/darknetwebsitesgflpx/darknetwebsites – tor markets
dark web link https://github.com/darknetdruglinksvojns/darknetdruglinks – tor markets links
dark market onion https://github.com/darknetmarketslinks/darknetmarketlinks – deep web drug url tor market
dark web sites https://github.com/darkwebmarketslinks/darkwebmarkets – darknet drug links darkmarkets
deep web drug markets https://github.com/darknetmarketlinks2025/darknetmarkets – dark market onion
dark market https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet drug market
dark websites https://github.com/darknetmarketslinks/darknetmarketlinks – how to access dark web darkmarkets
tor dark web https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web drug marketplace
tor darknet https://github.com/darkwebwebsites/darkwebwebsites – darkmarket 2025 darkmarket list
darkmarket url [url=https://github.com/darknetmarkets2025/darknetmarketlinks ]dark market list [/url] dark web websites
Ваша сцена станет неповторимой с электрокарнизами, сделают ваше выступление незабываемым.
Трансформируйте свою сцену с электрокарнизами, обеспечивая плавное движение драпировок.
Современные технологии в электрокарнизах, которые делают шоу невероятно красивым.
Лучшие электрокарнизы для профессиональных выступлений, где каждая деталь важна.
Электрокарнизы – современное решение для профессиональных выступлений, с непревзойденным качеством и надежностью.
Превратите ваше шоу с помощью электрокарнизов, которые способны изменить восприятие аудитории.
Электрокарнизы – лучший выбор для сцены, которые обеспечат быстрое и плавное движение.
С электрокарнизами ваша сцена станет настоящим шедевром, для тех, кто стремится к совершенству.
Электрокарнизы – современное решение для сцены, с индивидуальным подходом к каждому проекту.
Идеальные решения для любого жанра и формата, обеспечив себе идеальный результат в каждом выступлении.
лучшие электрокарнизы для театров лучшие электрокарнизы для театров .
darkmarket link https://github.com/darknetdruglinksvojns/darknetdruglinks – tor market url
Свежие промокоды казино https://carina-e.ru/images/pages/bk_betwinner_promokod_pri_registracii_2020.html бонусы, фриспины и эксклюзивные акции от топовых платформ! Найдите лучшие предложения, активируйте код и выигрывайте больше.
Автоматические ворота https://spb.automaticheskie-vorota.ru в Санкт-Петербурге – изготовление, установка и сервис. Надежные решения для дома, бизнеса и промышленности. Быстрая доставка, монтаж под ключ и гарантия качества!
амт 300 масло амт 300 купить
darkmarket list https://github.com/darknetmarketslinks/darknetmarketlinks – dark websites dark web sites links
darkmarket https://github.com/darknetmarketlinks2025/darknetmarkets – dark websites
Нужен опытный остеопат Пушкино? Помогаю при остеохондрозе, грыжах, искривлениях позвоночника и болях в суставах. Безопасные техники, профессиональный подход, запись на прием!
The variety of games on 1win casino makes it a top choice for players | 1win azerbaycan is popular among sports fans for its excellent odds 1win login | 1win casino features exciting jackpot games and live dealer tables | The loyalty rewards program at 1win keeps players coming back | Players enjoy competitive odds and betting options at 1win 1win casino games.
частный сео оптимизатор https://seo-base.ru
onion market https://github.com/darkwebmarketslinks/darkwebmarkets – dark websites tor markets 2025
мостбет ком скачать gtrtt.com.kg .
site here
Issue and expiration
darknet site https://github.com/darkmarketlinkp22jr/darkmarketlink – deep web drug store
dark market link https://github.com/darknetmarketslinks/darknetmarketlinks – tor market links onion market
dark web sites https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet drug links
Необычные электрокарнизы для вашей сцены, подчеркнут красоту представления.
Электрокарнизы – стильное решение для сцены, делая представление более динамичным.
Управляйте световыми шоу с помощью электрокарнизов, которые делают шоу невероятно красивым.
С электрокарнизами публика останется в восторге, для тех, кто ценит качество и стиль.
Создайте неповторимую магию на сцене с электрокарнизами, которые не оставят равнодушными даже самых взыскательных критиков.
Сверхсовременные технологии в каждом дизайне, и принести вашему проекту новый уровень.
Освежите свое шоу с электрокарнизами, которые обеспечат быстрое и плавное движение.
С электрокарнизами ваша сцена станет настоящим шедевром, способных придать умиротворение или напряжение вашему выступлению.
Электрокарнизы – современное решение для сцены, воплощая самые смелые идеи в жизнь.
Идеальные решения для любого жанра и формата, чтобы сделать шоу неповторимым и запоминающимся.
электрический занавес для сцены электрический занавес для сцены .
dark website https://github.com/darkwebwebsites/darkwebwebsites – darknet markets dark web drug marketplace
darkmarkets https://github.com/darknetmarkets2025/darknetmarketlinks – darkmarket list dark internet
bitcoin dark web https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet market lists
dark market onion https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet drug links
dark web market links https://github.com/darknetmarketslinks/darknetmarketlinks – tor markets links deep web drug store
blackweb https://github.com/darkwebmarketslinks/darkwebmarkets – darkweb marketplace black internet
1вин вход с компьютера http://fabc.com.kg/ .
darkmarkets https://github.com/darkmarketlinkp22jr/darkmarketlink – deep web drug markets
регистрация 1вин https://bbcc.com.kg .
darknet market https://github.com/darknetmarketslinks/darknetmarketlinks – darknet drug links dark web market
darknet sites https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web links
CRぱちんこ仮面ライダーMAX EDITION
家 パチスロ
大当たりの瞬間は、周囲と一緒に盛り上がれるのが嬉しいです。共感が生まれます。
押忍!番長
https://www.k8-casino.org/?SitesID=919343818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
CR牙狼金色になれ
新台 牙 狼
CR押忍!番長
沖ドキ! Vacances
麻雀物語
CRぱちんこ 新鬼武者
https://www.k8.gives/?ReviewID=920602818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
翔馬
https://jahromblog.com/?WatchID=921540818.html
ホラー映画の要素が盛り込まれており、ファンにはたまらない作品です。感情移入できます。
吉宗 2003
https://www.k8casinoofficial.jp/?ReviewID=927862818.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
deep dark web https://github.com/darknetdruglinksvojns/darknetdruglinks – tor markets links
геотехнический прогноз https://geotekhnicheskiy-prognoz-stroitelstva.ru
darkweb marketplace https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet drug store
dark websites https://github.com/darknetmarketlinks2025/darknetmarkets – dark web sites
darknet drug market https://github.com/darkwebwebsites/darkwebwebsites – dark markets dark web market links
Batery Bet https://tamiyamodelers.com No matter how you spell it, the thrill remains the same! Join thousands of players who trust Batery Bet for their daily dose of adrenaline-pumping entertainment. With lightning-fast payouts and jaw-dropping bonuses, this is one bet you won’t regret making.
darknet links [url=https://github.com/darknetmarkets2025/darknetmarketlinks ]the dark internet [/url] darknet market list
Предлагаем ознакомиться сайты лиды. Если вам понадобится узнать телефон посетителя сайта обращайтесь
darkmarket 2025 https://github.com/darknetmarketslinks/darknetmarketlinks – dark website deep web sites
секс у гинеколога ginekolog-rukoeb1.ru .
Защитите авто от коррозии https://antikorauto.ru антикоррозийная обработка и кузовной ремонт с гарантией. Восстановление геометрии кузова, покраска, удаление вмятин. Долговечность и качество по доступным ценам!
порно мульт https://multiki-rukoeb1.ru/ .
Жарким летним утром Вася Муфлонов, заядлый рыбак из небольшой деревушки, собрал свои снасти и отправился на местное озеро. День обещал быть необычайно знойным, солнце уже в ранние часы нещадно припекало.
Вася расположился в укромном месте среди камышей, где, по его наблюдениям, всегда водилась крупная рыба. Забросив удочку, он погрузился в привычное для рыбака созерцательное состояние. Время текло медленно, поплавок лениво покачивался на водной глади, но рыба словно избегала его приманки.
Ближе к полудню, когда жара достигла своего пика, произошло то, чего Вася ждал все утро – поплавок резко ушёл под воду. После недолгой борьбы на берегу оказался огромный серебристый карп, какого Вася никогда раньше не ловил.
Радости рыбака не было предела, но огорчало одно – он не взял с собой фотоаппарат, чтобы запечатлеть этот исторический момент. Тут в голову Васи пришла необычная идея. Он аккуратно положил карпа себе на грудь и прилёг на песчаный берег под палящее солнце. “Пусть загар оставит память об этом улове”, – подумал находчивый рыбак.
Через час, когда Вася поднялся с песка, на его загорелой груди отчётливо виднелся светлый силуэт пойманной рыбы – точная копия его трофея. Теперь у него было неопровержимое доказательство его рыбацкой удачи.
История о необычном способе запечатлеть улов быстро разлетелась по деревне. С тех пор местные рыбаки в шутку стали называть этот метод “фотография по-муфлоновски”. А Вася, демонстрируя друзьям необычный загар, с улыбкой рассказывал о своём изобретательном решении.
Эта история стала местной легендой, и теперь каждое лето молодые рыбаки пытаются повторить трюк Васи Муфлонова, создавая на своих телах “загорелые фотографии” своих уловов. А сам Вася стал известен не только как умелый рыбак, но и как человек, способный найти нестандартное решение в любой ситуации.
online casino development turnkey online casino
dark web sites links https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet drug links
blackweb [url=https://github.com/darkwebmarketslinks/darkwebmarkets ]tor markets links [/url] darknet markets
dark market link [url=https://github.com/darknetwebsitesgflpx/darknetwebsites ]darkmarket 2022 [/url]
dark web websites https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarket url tor darknet
Рекомендую – нейросеть для написания доклада по презентации
muñeca de sexo real
darkmarket list https://github.com/tormarkets2025ukaz1/tormarkets2025 – tor markets 2022
dark web market links https://github.com/darknetmarketlinks2025/darknetmarkets – deep web drug store
Маркетплейс NOVA, есть все! Москва, Питер, всегда свежие клады!Акция для новых магазинов – продай на 1 млн, получи 100к!Приглашаем к сотрудничеству рекламодателей!Если потерял ссылку ищи нас в Яндекс по запросу : НОВА МАРКЕТПЛЕЙС|xNovaChat | Сайт-визитка
dark websites https://github.com/darknetmarketslinks/darknetmarketlinks – darknet market dark web site
darknet sites https://github.com/darkwebwebsites/darkwebwebsites – dark internet how to get on dark web
dark market 2025 https://github.com/darknetmarkets2025/darknetmarketlinks – dark web sites links darknet sites
tor markets 2022 https://github.com/darkmarketlinkp22jr/darkmarketlink – darkmarket 2022
Профессиональный шиномонтаж в Томске – быстро оперативно и качественно отлично! Нужен шиномонтаж обслуживание шин без ожидания моментально? Обращайтесь к нам! Мы предлагаем полный спектр услуг весь комплекс по обслуживанию колес, включая балансировку регулировку и ремонт проколов устранение дыр. Закажите услугу прямо сейчас на https://servisvtomske.ru/ и получите выгодное предложение акцию! Работаем быстро шустро и по доступным ценам выгодно.
Постановка на учет автомобиля https://mosgibdd.com быстрое и удобное оформление в ГИБДД без очередей и лишних затрат. Поможем с документами, номерами и регистрацией ТС. Работаем с физическими и юридическими лицами!
darknet site https://github.com/darknetwebsitesgflpx/darknetwebsites – tor market url
dark website https://github.com/darknetmarketslinks/darknetmarketlinks – darknet site tor darknet
tor markets links https://github.com/darkwebmarketslinks/darkwebmarkets – dark web search engine dark market link
Hookah and tobacco shop https://black-shisha-hookahs.shop huge assortment, top brands, best tastes! Coal, bowls, flasks and other accessories for the perfect hookah relaxation. Fast delivery and promotions!
Fast and secure VPS https://evps.host for any task! Flexible configurations, SSD drives, DDoS protection. Easy scaling, high performance and 24/7 support!
darkmarket 2022 https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web market links
drug markets dark web https://github.com/darknetmarketlinks2025/darknetmarkets – darknet market links
darknet marketplace https://github.com/darknetmarketslinks/darknetmarketlinks – bitcoin dark web dark web market
free dark web https://github.com/darkwebwebsites/darkwebwebsites – darknet links deep web drug links
deep web drug markets https://github.com/darkmarketlinkp22jr/darkmarketlink – tor market
darknet market https://github.com/darknetmarkets2025/darknetmarketlinks – dark web market dark web market links
best darknet markets https://github.com/darknetwebsitesgflpx/darknetwebsites – dark markets 2022
darknet market list https://github.com/darknetmarketslinks/darknetmarketlinks – black internet blackweb official website
Download Aviator Game https://sites.google.com/view/aviatorgamedownload/ experience thrilling gameplay with real-time bets and high multipliers! Play on Android, iOS, or PC. Fast installation, exciting features, and big wins await!
All about Karim Benzema karim-benzema-bd.com/ biography, achievements, main goals and career moments. Find out how the striker conquered Real Madrid and continues to shine in world football!
All about Karim Benzema karim benzema biography, achievements, main goals and career moments. Find out how the striker conquered Real Madrid and continues to shine in world football!
dark market link [url=https://github.com/darkwebmarketslinks/darkwebmarkets ]best darknet markets [/url] darknet drug store
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
dark market list https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web drug marketplace
dark web links https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarkets darknet site
南国育ち(胡蝶25)
北斗 潤
美しいグラフィックと豪華な演出が魅力。神々の力を感じます。
ビーストサップ
https://www.online-casino.city/?NewsID=926763818.html
プレイ中の雰囲気が楽しく、長時間楽しむことができます。快適な空間が魅力です。
CR蒼天の拳2
自転車 操業
CR押忍!番長
喧嘩祭
闇の花
北斗の拳-拳王
https://www.k8io.net/?SitesID=921185818.html
音楽とサウンドエフェクトが素晴らしく、ゲームに没入できます。特にバトル時が最高。
Dororonえん魔くん メ~ラめら
https://www.k8pachinko.biz/?WatchID=926862818.html
シンプルなデザインとルールが魅力。初心者でも安心して楽しめます。
サラリーマン金太郎 出世回胴編
https://www.jogadoresanonimos.org/?SitesID=920483818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
tor markets https://github.com/darkmarketlinkp22jr/darkmarketlink – dark web links
другие корзина юбка
darkmarkets https://github.com/darkwebwebsites/darkwebwebsites – darknet drugs bitcoin dark web
dark web market links https://github.com/darknetmarkets2025/darknetmarketlinks – dark website blackweb
darkweb marketplace https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web link
deep web search https://github.com/darknetmarketslinks/darknetmarketlinks – tor markets darknet search engine
tor markets 2025 https://github.com/darkwebmarketslinks/darkwebmarkets – deep web drug url darknet drugs
darkmarket list https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web links
tor dark web https://github.com/darknetmarketlinks2025/darknetmarkets – best darknet markets
blackweb official website https://github.com/darknetmarketslinks/darknetmarketlinks – darknet sites darknet drug links
black internet [url=https://github.com/darkwebwebsites/darkwebwebsites ]dark web links [/url] darknet market lists
dark web market list https://github.com/darknetmarketslinks/darknetmarketlinks – tor markets 2025 darknet search engine
free dark web https://github.com/darknetmarkets2025/darknetmarketlinks – dark market dark market
dark web sites [url=https://github.com/darkwebmarketslinks/darkwebmarkets ]deep dark web [/url] darkmarket url
darknet site https://github.com/darknetmarketslinks/darknetmarketlinks – the dark internet deep web links
Предлагаем ознакомиться перехват клиентов конкурентов. Если вам понадобится холодные звонки заказать обращайтесь
эскорт москва эскорт москва .
dark websites https://github.com/darknetmarketslinks/darknetmarketlinks – darkmarket link darknet marketplace
best darknet markets [url=https://github.com/darkwebwebsites/darkwebwebsites ]darknet links [/url] tor darknet
I had no idea how to set up Metamask on Chrome, but https://metamake.org/ explained everything in detail. The instructions are easy to understand, making it perfect for beginners like me!
tor markets links https://github.com/darknetmarkets2025/darknetmarketlinks – darknet markets darknet market lists
tor market url https://github.com/darknetmarketslinks/darknetmarketlinks – darknet drugs darknet drug market
darknet seiten https://github.com/darkwebmarketslinks/darkwebmarkets – deep dark web tor markets 2025
аркада казино сайт arkada casino регистрация на сайте
Военная служба по контракту https://contract116.ru стабильность, достойная зарплата, социальные льготы и карьерный рост. Присоединяйтесь к профессиональной армии и получите надежное будущее!
deep dark web https://github.com/darknetmarketslinks/darknetmarketlinks – darknet markets dark web markets
dark web search engine https://github.com/darkwebwebsites/darkwebwebsites – darkmarkets darknet markets 2025
drug markets dark web https://github.com/darknetmarkets2025/darknetmarketlinks – deep web drug links darknet seiten
dark websites https://github.com/darknetmarketslinks/darknetmarketlinks – darknet seiten darknet links
dark web drug marketplace [url=https://github.com/darkwebmarketslinks/darkwebmarkets ]darkmarket url [/url] dark market url
скачать последний самп samp ru
darknet drug links https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet drug links
Kevin De Bruyne http://kevin-de-bruyne-az.com is the leader of the Manchester City and Belgium national team midfield. Find out all about his achievements, matches, awards and records in world football!
Joshua Kimmich https://joshua-kimmich-az.org the versatile leader of Bayern and the German national team! The latest news, stats, highlights, goals and assists. Follow the career of one of the best midfielders in the world!
darknet markets https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web market
dark market list https://github.com/darknetwebsitesgflpx/darknetwebsites – tor market links
порно японка порно японка .
deep web links https://github.com/darkwebwebsites/darkwebwebsites – dark web markets darkmarket
blackweb https://github.com/darknetmarketslinks/darknetmarketlinks – dark website darknet links
dark website https://github.com/darknetmarkets2025/darknetmarketlinks – dark market list dark market link
dark markets https://github.com/darkwebmarketslinks/darkwebmarkets – free dark web dark market link
drug markets dark web https://github.com/darknetmarketlinks2025/darknetmarkets – darkweb marketplace
dark market link https://github.com/darknetwebsitesgflpx/darknetwebsites – darkmarket url
darknet market lists https://github.com/darkmarketlinkp22jr/darkmarketlink – tor markets links
dark web link https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web market
dark net https://github.com/darkwebwebsites/darkwebwebsites – dark web link free dark web
tor markets 2025 https://github.com/darknetmarketslinks/darknetmarketlinks – deep web drug markets darknet links
порно немцы https://www.trax-nemeckoe1.ru .
dark market list https://github.com/darknetmarkets2025/darknetmarketlinks – dark markets 2025 dark web access
darkmarkets https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet drug store
darknet market list https://github.com/darknetdruglinksvojns/darknetdruglinks – bitcoin dark web
dark websites https://github.com/darkmarketlinkp22jr/darkmarketlink – darkmarket 2022
Download https://sites.google.com/view/aviatorgamedownload/ and get a chance to win big! Watch the coefficient grow, fix the bet in time and win. Convenient application and dynamic gameplay are waiting for you. Available for PC and mobile devices!
dark web link https://github.com/darkwebmarketslinks/darkwebmarkets – darknet sites darknet markets 2025
Подарки и сувениры из камня https://kameshki51.ru статуэтки, шкатулки, часы, панно, изделия из мрамора, гранита и оникса. Ручная работа, уникальный дизайн, доставка по России!
tor market https://github.com/darknetmarketslinks/darknetmarketlinks – dark market dark market list
tor markets links [url=https://github.com/darkwebwebsites/darkwebwebsites ]darkmarkets [/url] darknet markets 2025
darkmarkets https://github.com/darknetmarkets2025/darknetmarketlinks – dark web websites best darknet markets
dark market onion https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark market 2022
Our team of adult specialists uploads sex videos to the site day and night so that you can always find only fresh free sex video.
dark web market https://github.com/darknetwebsitesgflpx/darknetwebsites – bitcoin dark web
darknet marketplace https://github.com/darkmarketlinkp22jr/darkmarketlink – darkweb marketplace
Журнал общество и право https://nadezhnye-ruki.ru актуальные статьи, аналитика, экспертные мнения о законодательстве, праве и социальной политике. Будьте в курсе важных событий!
Как побороть лудоманию вавада история глазами прошедшего это испытание и несколько советам тем, кто в середине пути или столкнулся с этим через друзей и близких. Описываю в статье как Вавада помогла мне избавиться от игровой зависимости
dark web search engine https://github.com/darkwebmarketslinks/darkwebmarkets – tor market deep web drug links
Получите свежие статьи https://www.wildberries.ru/catalog/117605496/detail.aspx и новости от адвоката, посвященные важным правовым вопросам. Профессиональные рекомендации и разборы актуальных событий в законодательстве помогут вам быть в курсе всех изменений.
CR牙狼FINAL Ver.399
大 劇
演出が多彩で、毎回楽しみが違います。飽きずに遊べるのが魅力です。
P戦国乙女 LEGEND BATTLE
https://jahromblog.com/?GameID=924302818.html
新しい台が出るたびにワクワクします。流行に敏感な人にはたまらない楽しみです。
魔法少女まどか☆マギカ
アムズ 古川
闇の花(特殊大賞燈);
Pフィーバーからくりサーカス
e スマパチ シン・エヴァンゲリオン Type カヲル
P 新世紀エヴァンゲリオン15 未来への咆哮
https://www.k8pachinko.cc/?GameID=923648818.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
政宗
https://www.k8casinoofficial.jp/?NewsID=924855818.html
歴史的なキャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
押忍!サラリーマン番長
https://www.k8pachinko.asia/?ReviewID=925282818.html
ストレスなく楽しめるのが良いです。気軽に挑戦できるのが良いです。
dark web market list https://github.com/darknetmarketslinks/darknetmarketlinks – tor market darkmarkets
Окунитесь в азарт с aviator-slot Уникальная игра с захватывающим геймплеем и возможностью сорвать крупный выигрыш. Следите за коэффициентом, вовремя забирайте ставку и умножайте баланс. Испытайте удачу прямо сейчас!
dark web drug marketplace https://github.com/darkwebwebsites/darkwebwebsites – the dark internet darkweb marketplace
dark web site https://github.com/darknetmarkets2025/darknetmarketlinks – darknet drug market darkmarket link
Новости музыки Украины https://musicnews.com.ua популярные жанры, тренды и полезные советы для музыкантов. Узнавайте больше о мире музыки и развивайте свои навыки!
Знакомства в Уфе с ufavip.sbs платформой стпли проще, возможность встретить девушек, готовых к интересному общению и приятному времяпрепровождению. Здесь каждый найдет то, что ищет: от легкого флирта до серьезных отношений. Благодаря удобному поиску и множеству анкет, знакомства становятся быстрыми и увлекательными.
Ставки на спорт betting ваш шанс превратить знания в выигрыш! Лучшие коэффициенты, огромный выбор событий и удобный интерфейс. Делайте прогнозы и побеждайте!
onion market https://github.com/darkwebmarketslinks/darkwebmarkets – dark web links how to get on dark web
dark market https://github.com/darknetmarketslinks/darknetmarketlinks – dark market 2025 darknet markets
darknet market lists [url=https://github.com/darkwebwebsites/darkwebwebsites ]tor market links [/url] tor markets
dark market 2025 https://github.com/darknetmarkets2025/darknetmarketlinks – dark web search engines dark web site
I had trouble figuring out how to download Metamask, but https://metaduck.org/ explained everything clearly. Now my wallet is set up!
deep web links dark market link blackweb official website
dark web market list blackweb dark market link
darkmarket url dark markets deep web sites
darknet market links how to access dark web dark web market list
эскортницы москва эскортницы москва .
A friend recommended I visit https://metapaws.org/ for instructions on downloading Metamask, and I’m so glad I did! The guide was clear, and I had my wallet set up in no time. Definitely a must-visit site for crypto users!
dark net how to get on dark web dark web markets
blackweb dark market onion dark market onion
additional reading jaxx io
he has a good point
jaxx classic wallet
darknet market dark market darkweb marketplace
click for more freewallet download
click here for more info jaxx liberty download
darknet market dark web markets onion market
北斗の拳-拳王
アプロ 松川
パチンコのルールはシンプルで、誰でもすぐに楽しめます。初心者にも優しいゲームです。
P ぱちんこ シン・エヴァンゲリオン Type レイ
https://www.turboslott.com/?SitesID=923885818.html
シンプルなルールで、初心者でもすぐに楽しめるのが良いです。気軽に挑戦できます。
戦国パチスロ花の慶次戦極めし傾奇者の宴;
ピア 上野
新潘金蓮
真モグモグ風林火山2
押忍!番长(操)
P牙狼月虹ノ旅人
https://www.xn--k8-9g4a3b4f.store/?GameID=920084818.html
迫力あるバトル演出が魅力。原作ファンにはたまらない作品です。
ベヨネッタ
https://www.8casino8.com/?WatchID=919182818.html
ボーナスゲームの演出が豪華で、楽しみが倍増します。特に大当たり時は圧巻。
CR 呪怨
https://www.xn--k8-9g4a3b4f.space/?ReviewID=923350818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
darknet market lists dark market url dark websites
dark websites dark web site deep web links
blackweb darknet links dark markets
Все о легкой атлетике athletics-ru.ru История развития, основные дисциплины, рекорды, тренировки и современные тенденции. Узнайте больше о королеве спорта!
Мир смешанных единоборств ufc-ru ru (MMA) в UFC! Рейтинги, бои, истории бойцов, прогнозы на поединки и эксклюзивные материалы о крупнейшей бойцовской лиге.
Zeus vs Hades https://zeus-vs-hades-download.ru эпичная битва богов! Игровой автомат с грозными бонусами, фриспинами и множителями. Выберите сторону Зевса или Аида и сорвите большой выигрыш!
darkmarket url https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet market
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
dark web markets deep web drug links darknet drug store
portal of celebrity https://movingprous.com news and interesting facts. Gossip, rumors and interesting riddles and questions! We will please you with daily publications about social life and unusual things and events that you could miss!
tor markets links tor market links tor darknet
tor markets https://github.com/darknetmarketlinks2025/darknetmarkets – dark market 2022
dark market 2022 https://github.com/darkmarketlinkp22jr/darkmarketlink – dark web market
tor markets links darknet search engine darknet links
drug markets dark web dark market list deep web drug markets
tor markets 2025 blackweb official website darknet market list
Wins without borders https://www.trustpilot.com/review/aviatorplaygame.com how to play and earn? All about the popular crash game: mechanics, strategies, bonuses and honest reviews from players!
Временная регистрация в Москве – быстро и надежно!
Нужна временная прописка для работы, учебы или оформления документов?
Оформим официальную регистрацию в Москве всего за 1 день. Без очередей,
с гарантией и юридической чистотой!
1. Подходит для граждан РФ и СНГ
2. Регистрация от 3 месяцев до 5 лет
3. Легально, с внесением в базу МВД
Работаем без предоплаты! Документы можно получить лично или дистанционно.
Доступные цены, оперативное оформление!
Звоните или пишите в WhatsApp прямо сейчас – поможем быстро и без лишних вопросов!
Временная регистрация в Москве
darkmarket url https://github.com/darknetwebsitesgflpx/darknetwebsites – dark markets 2022
drug markets dark web dark web websites tor darknet
darknet drug market dark market how to get on dark web
darknet markets https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark market
deep web drug links https://github.com/darknetmarketlinks2025/darknetmarkets – darknet drug market
drug markets onion https://github.com/darknetdruglinksvojns/darknetdruglinks – dark market 2022
bet elon bet elon .
darkmarket list deep web search darknet websites
darknet links darkmarket list dark markets
darkweb marketplace darknet seiten dark web links
tor market url https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet websites
The rise of Phil Foden from Manchester City Academy to stardom is inspiring | The family of Phil Foden has always been supportive of his career | Phil Foden’s England journey has been nothing short of spectacular | Phil Foden’s blonde hair was a bold style choice | Phil Foden’s grandfather is a lesser-known figure in his life story Phil Foden personal and professional milestones.
deep web drug markets darknet links darknet market
darknet market links https://github.com/tormarkets2025ukaz1/tormarkets2025 – deep web drug store
dark market list https://github.com/darknetmarketlinks2025/darknetmarkets – dark market 2022
Свежие и актуальные новости https://sugar-news.com.ua Украины и мира! Будьте в курсе последних событий, аналитики и трендов. Читайте последние новости Украины онлайн!
darknet drug market dark web websites darkmarket url
dark web site dark markets 2025 deep web drug links
dark web sites dark website dark internet
darknet sites https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web markets
levne stehovani https://www.tmgcompany.com
darknet sites dark net deep web links
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт фотоаппаратов canon адреса, можете посмотреть на сайте: ремонт фотоаппаратов canon адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
dark web link https://github.com/darknetmarketlinks2025/darknetmarkets – tor marketplace
darkmarket https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web drug marketplace
Sistemas de ajuste: esencial para el operación suave y productivo de las equipos.
En el entorno de la avances avanzada, donde la eficiencia y la estabilidad del dispositivo son de gran significancia, los equipos de ajuste tienen un tarea fundamental. Estos dispositivos dedicados están desarrollados para ajustar y regular piezas dinámicas, ya sea en equipamiento manufacturera, vehículos de traslado o incluso en electrodomésticos caseros.
Para los especialistas en conservación de sistemas y los profesionales, operar con sistemas de equilibrado es fundamental para proteger el funcionamiento suave y fiable de cualquier mecanismo giratorio. Gracias a estas opciones modernas sofisticadas, es posible limitar notablemente las oscilaciones, el zumbido y la esfuerzo sobre los sujeciones, aumentando la longevidad de piezas valiosos.
Igualmente relevante es el rol que desempeñan los aparatos de balanceo en la atención al cliente. El soporte especializado y el soporte constante usando estos aparatos facilitan dar asistencias de óptima estándar, mejorando la bienestar de los consumidores.
Para los responsables de emprendimientos, la inversión en equipos de equilibrado y medidores puede ser clave para incrementar la eficiencia y eficiencia de sus dispositivos. Esto es sobre todo trascendental para los dueños de negocios que gestionan modestas y pequeñas emprendimientos, donde cada aspecto importa.
También, los sistemas de equilibrado tienen una extensa implementación en el campo de la prevención y el supervisión de nivel. Habilitan detectar potenciales fallos, evitando mantenimientos elevadas y daños a los sistemas. También, los información obtenidos de estos equipos pueden utilizarse para optimizar métodos y incrementar la exposición en motores de investigación.
Las campos de aplicación de los sistemas de balanceo cubren numerosas industrias, desde la manufactura de bicicletas hasta el monitoreo de la naturaleza. No importa si se trata de extensas elaboraciones manufactureras o limitados locales hogareños, los equipos de ajuste son fundamentales para promover un desempeño productivo y sin riesgo de detenciones.
darkmarket dark web site best darknet markets
black internet blackweb blackweb official website
deep web drug markets darkmarkets dark market onion
darknet market links https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web links
tor markets 2025 darknet market list dark web market list
dark web sites https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet market list
darknet websites https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet websites
darkmarket 2022 https://github.com/darknetdruglinksvojns/darknetdruglinks – dark market list
Лучшие эксперты в одном месте! орган по сертификации сертификата маркетплейс и агрегатор экспертных компаний помогает найти надежных специалистов по разным направлениям. Удобный выбор, проверенные отзывы и рейтинг!
darkmarket url dark website deep web drug markets
how to access dark web dark markets dark market 2025
tor markets links https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web link
dark website how to get on dark web dark market list
black internet tor darknet tor markets 2025
島娘
馬 単
パチンコ店の雰囲気は賑やかで、友達と一緒に楽しむのに最適です。色とりどりの台が魅力的です。
CR緋弾のアリアII FPM
https://www.sawasdeeslot.com/?SitesID=922643818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
スマスロ北斗の拳
赤玉 中郷
夜蝶飛翔特
戦姫絶唱シンフォギア
麻雀物語
CR びっくり戦国無双 Light Edition
https://www.k8.gives/?WatchID=919260818.html
パチンコをすることで、運を試すドキドキ感が味わえます。挑戦する楽しさがあります。
紫の花 (特殊大賞燈)
https://www.ja-securities.jp/?ArticleID=922363818.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
主役は銭形 2004
https://www.xn--k8-9g4a3b4f.space/?WatchID=923940818.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
tor markets 2022 https://github.com/tormarkets2025ukaz1/tormarkets2025 – tor market url
deep web markets https://github.com/darknetdruglinksvojns/darknetdruglinks – tor markets
dark markets 2025 darkmarket list deep web links
deep web drug links onion market darkweb marketplace
deep web drug markets [url=https://github.com/darknetwebsitesgflpx/darknetwebsites ]dark web market [/url]
blackweb dark market dark market link
dark web market list https://github.com/darknetmarketlinks2025/darknetmarkets – darkmarket link
dark market https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet market links
darkweb marketplace drug markets dark web how to get on dark web
Equipos de calibración: fundamental para el rendimiento suave y productivo de las equipos.
En el mundo de la ciencia contemporánea, donde la eficiencia y la seguridad del dispositivo son de máxima importancia, los dispositivos de ajuste juegan un papel fundamental. Estos equipos dedicados están desarrollados para calibrar y estabilizar elementos dinámicas, ya sea en maquinaria industrial, automóviles de transporte o incluso en aparatos domésticos.
Para los especialistas en mantenimiento de dispositivos y los técnicos, operar con sistemas de ajuste es crucial para promover el operación estable y fiable de cualquier dispositivo rotativo. Gracias a estas alternativas tecnológicas sofisticadas, es posible minimizar considerablemente las oscilaciones, el sonido y la carga sobre los rodamientos, aumentando la duración de piezas costosos.
De igual manera importante es el tarea que juegan los aparatos de ajuste en la asistencia al usuario. El ayuda especializado y el mantenimiento regular utilizando estos dispositivos habilitan brindar servicios de alta nivel, aumentando la satisfacción de los clientes.
Para los dueños de negocios, la financiamiento en sistemas de balanceo y medidores puede ser importante para incrementar la eficiencia y eficiencia de sus sistemas. Esto es sobre todo trascendental para los empresarios que gestionan pequeñas y modestas empresas, donde cada aspecto es relevante.
Asimismo, los sistemas de ajuste tienen una extensa uso en el sector de la prevención y el gestión de calidad. Permiten localizar probables fallos, evitando reparaciones onerosas y daños a los equipos. Más aún, los resultados obtenidos de estos aparatos pueden utilizarse para maximizar métodos y mejorar la visibilidad en buscadores de exploración.
Las zonas de aplicación de los sistemas de ajuste abarcan variadas industrias, desde la fabricación de ciclos hasta el control de la naturaleza. No influye si se habla de enormes fabricaciones manufactureras o pequeños locales caseros, los aparatos de calibración son indispensables para asegurar un desempeño productivo y sin presencia de paradas.
dark web sites links https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web markets
dark web sites links how to access dark web darkmarket 2025
darknet market links darknet drug links dark market link
darknet drug store best darknet markets dark web markets
tor market url https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark market url
dark market onion https://github.com/darknetmarketlinks2025/darknetmarkets – dark web sites
dark markets https://github.com/darknetdruglinksvojns/darknetdruglinks – tor markets links
darknet market lists deep web drug store darknet seiten
deep web drug links https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web drug marketplace
darkmarket url tor markets 2025 darknet market
darknet sites darknet drug market tor market
darknet market lists https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market links
darknet market lists https://github.com/darknetmarketlinks2025/darknetmarkets – tor markets 2022
darkmarkets https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet drug market
dark web link dark web search engines dark web search engine
Join at Pin-Up Casino India and claim a bonus of up to ?450,000 rupees! Register on the official platform of Pin-Up and claim a generous welcome initial bonus! Kick off your casino adventure with additional money in your account and 250 free spins. Increase your bets this moment!
pin up casino app
Is It a Good Idea to Download? Pin Up casino offers a app version for both Android phones and iOS devices, which allows you to play on the go. But is the app actually worth it? I’ve been trying it out for some time, and it’s very intuitive. Has anyone else enjoyed the app? Is it faster to load than the desktop site?
pin up casino aviator
dark web sites links https://github.com/darknetwebsitesgflpx/darknetwebsites – darkmarket link
black internet dark net deep web drug store
Хотите играть в Steam-игры shared steam org дешевле? Shared-Steam.org предлагает аренду аккаунтов с топовыми играми. Безопасность, доступность и удобство – ваш гейминг без границ!
deep web drug store https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark markets 2022
dark web drug marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – tor market links
deep web drug markets https://github.com/darknetdruglinksvojns/darknetdruglinks – dark market link
dark market url tor darknet deep web links
tor dark web drug markets onion tor dark web
アナザーゴッドハーデス-奪われたZEUSver
競艇 払い
友人と競い合うことで、より楽しめます。勝負の結果が気になります。
いみそーれ(红花)
https://www.stationslot.org/?WatchID=922392818.html
パチンコをすることで、運を試すドキドキ感が味わえます。挑戦する楽しさがあります。
機動戦士ガンダムユニコーン
カジノ 机
Pフィーバーダンベル何キロ持てる
戦国パチスロ花の慶次これより我ら修羅に入る
CR織田信奈の野望II
CRモンスターハンタ
https://www.k8-casino.biz/?WatchID=922620818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
戦国パチスロ花の慶次これより我ら修羅に入る
https://www.k8o.jp/?NewsID=925329818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
押忍!番長2
https://www.turboslott.com/?GameID=919112818.html
お店の雰囲気が心地よく、長時間楽しむことができます。快適な空間が魅力です。
Оптимизируйте свою жизнь с электрокарнизом с таймером, удивляйтесь удобству и функциональности.
Создайте атмосферу уюта и стиля с помощью электрокарниза и таймера, добавит функциональности и комфорта.
Автоматизируйте процесс управления шторами с электрокарнизом и таймером, для вашего удобства и удовольствия.
Умное устройство для вашего дома – электрокарниз с таймером, позволит вам наслаждаться своим временем и пространством.
Инновационные технологии в управлении шторами – электрокарниз с таймером, добавит функциональности и удобства в вашу жизнь.
шторы умный дом https://prokarniz50.ru/ .
tor market links dark web market links dark web market links
darknet market links https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet market lists
tor darknet darknet site tor markets links
Pretty! This was an incredibly wonderful post. Many thanks for providing this info.
Wyoming Valley Equipment LLC
dark web markets https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet site
dark market 2022 https://github.com/darknetmarketlinks2025/darknetmarkets – darkmarket link
dark markets https://github.com/darknetdruglinksvojns/darknetdruglinks – deep web drug url
tor markets links darkmarket black internet
tor markets bitcoin dark web dark web search engines
Create images deep nude ai easily with AI. Remove clothes from anyone in the image and enjoy their nakedness.
darknet markets https://github.com/darknetwebsitesgflpx/darknetwebsites – tor darknet
порно германия порно германия .
dark market url dark internet dark web site
darknet market lists deep web drug url dark web drug marketplace
bitcoin dark web https://github.com/darkmarketlinkp22jr/darkmarketlink – tor markets
Удобное управление шторами с помощью электрокарниза и таймера, наслаждайтесь комфортом и современностью.
Дизайнерское решение для вашего дома – электрокарниз с таймером, добавит функциональности и комфорта.
Автоматизируйте процесс управления шторами с электрокарнизом и таймером, современное решение для гармоничного интерьера.
Умное устройство для вашего дома – электрокарниз с таймером, позволит вам наслаждаться своим временем и пространством.
Дизайнерское решение для современного дома – электрокарниз с таймером, позволит вам экономить время и силы.
Электрический карниз с таймером https://prokarniz50.ru/ .
darknet drug market https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet market
dark markets 2025 bitcoin dark web drug markets onion
darknet search engine dark web websites deep web sites
darknet marketplace https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet market list
deep web drug links tor darknet best darknet markets
darknet market list darkmarket link dark web search engines
dark web market list black internet dark websites
darknet markets https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet market list
deep web markets https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet drug links
Необычайная автоматизация интерьера с электрокарнизом и таймером, экономьте энергию и время.
Простота и элегантность в каждой комнате с электрокарнизом и таймером, который подчеркнет ваш вкус и индивидуальность.
Эффективное управление светом и приватностью с электрокарнизом и таймером, создайте идеальную атмосферу в вашем доме.
Электрокарниз с таймером – ваш помощник в повседневной жизни, который упростит вашу жизнь и сделает ее более комфортной.
Широкий выбор электрокарнизов с таймером для любого интерьера, добавит функциональности и удобства в вашу жизнь.
умный дом шторы умный дом шторы .
deep web drug links https://github.com/darknetdruglinksvojns/darknetdruglinks – deep web drug markets
Turkiye Slotlar Rehberi https://slots-rait.com En Iyi Slotlar ve Casinolar! Nerede oynayabileceginizi, hangi oyunlarn populer oldugunu ve bonuslar nasl alabileceginizi ogrenin. Oyun alanlar ve guncel eglencelere dair kapsaml bir genel baks!
Turkiye’deki slot makineleri gercek parayla oynanabilen slotlar gercek parayla oynanabilen slotlar kapsaml bir baks! Nerede oynan?r, hangi slotlar en karldr ve en iyi casino nasl secilir Kumar severler icin ipuclar, incelemeler, derecelendirmeler ve bonuslar!
dark market 2025 tor markets links darknet market links
bitcoin dark web darkmarket 2025 dark markets 2025
darknet market links https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web market list
dark web market darknet drug market how to get on dark web
the dark internet darknet market list deep dark web
tor markets 2022 https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market list
dark market https://github.com/darknetdruglinksvojns/darknetdruglinks – tor markets 2022
dark market https://github.com/darknetwebsitesgflpx/darknetwebsites – dark market 2022
darknet seiten darknet sites deep web links
darknet search engine dark market dark web drug marketplace
перевозка грузов минск спб грузоперевозки минск
tor markets 2025 deep web drug store tor market
I had no idea how to download Metamask, but https://download.metaredi.org/ provided a clear guide. Now I can use my crypto wallet without any issues.
darknet market list https://github.com/darkmarketlinkp22jr/darkmarketlink – darkmarket 2022
вывоз старой мебели в минске перевозка мебели
toto138
darknet drug links blackweb official website darknet sites
tor marketplace https://github.com/darknetdruglinksvojns/darknetdruglinks – bitcoin dark web
darkmarket url https://github.com/darknetmarketlinks2025/darknetmarkets – deep web drug links
Знакомства в Уфе https://ufavip.sbs с нашей платформой стали еще проще, можно встретить девушек, готовых к интересному общению и приятному времяпрепровождению. Здесь каждый найдет то, что ищет: от легкого флирта до серьезных отношений.
dark markets https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet market
darkmarket dark markets darknet market list
dark internet darkmarket url tor market links
deep web sites how to access dark web blackweb official website
deep web markets https://github.com/darkmarketlinkp22jr/darkmarketlink – dark web sites
tor market https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web drug marketplace
the dark internet the dark internet dark websites
darknet market list https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet market list
dark websites deep web markets darknet drug market
how to access dark web darknet sites tor markets
darknet links deep web drug links dark web links
めんそーれ琉球守護神
政宗3
人気ゲームを基にしたパチスロで、ファンにはたまらない作品です。リアルなバトルが楽しめます。
CR 真・北斗無双
https://www.online-casino.city/?SitesID=920123818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CRフィーバー戦姫絶唱シンフォギア Ver.199 (1:1)
南国 背景
P 新世紀エヴァンゲリオン15 未来への咆哮
CR絶狼
ガッツだ!!森の石松
CR真・花の慶次
https://www.jogadoresanonimos.org/?SitesID=923993818.html
戦略的な要素もあり、運だけでなく技術が試されるのが面白いです。
Lバキ 強くなりたくば喰らえ!!!
https://www.k8-casino.jp/?NewsID=924951818.html
ホラー映画の要素が盛り込まれており、ファンにはたまらない作品です。感情移入できます。
めんそーれ
https://xn--k8-9g4a3b4f.site/?WatchID=919260818.html
演出が多彩で、毎回楽しみが違います。飽きずに遊べるのが魅力です。
the dark internet darknet search engine tor market links
darkmarket url tor market darknet search engine
dark web websites deep web drug store darknet markets
darkmarket url dark web market dark web market list
dark web search engine blackweb official website tor marketplace
Is it possible to win at Lucky Jet? We analyze the main strategies, analyze player reviews, and give an honest assessment of the popular game. Read to avoid mistakes and increase your chances of success!
how to get on dark web dark web drug marketplace darknet market
грузоперевозки по рб грузоперевозки минск
Hey there! Do you know if they make any plugins to protect against hackers? I’m kinda paranoid about losing everything I’ve worked hard on. Any tips?
https://t.me/promokod_samokat_dostavka
onion market darknet market links darknet marketplace
tor markets dark web drug marketplace darknet search engine
Предлагаем ремонт холодильников liebherr в Петербурге. Если у вас сломалась техника обращайтесь в сервис 911. Мы выполняем – Ремонт холодильников Liebherr профессионально, быстро и с гарантией!
tor darknet darknet market lists dark web drug marketplace
darkmarket link deep web links drug markets dark web
dark web websites darknet seiten darknet market lists
darkmarket url https://github.com/tormarkets2025ukaz1/tormarkets2025 – onion market
drug markets dark web https://github.com/darknetdruglinksvojns/darknetdruglinks – deep web drug markets
dark web market links darkmarket deep web drug markets
darknet market links https://github.com/darkmarketlinkp22jr/darkmarketlink – tor market
deep web drug links the dark internet dark web market links
darknet market list dark net darkmarket list
deep web drug url dark markets dark web drug marketplace
As the admin of this web site is working, no uncertainty very shortly it will be famous, due to its feature contents.
lee bet регистрация
tor market links https://github.com/darknetmarketlinks2025/darknetmarkets – dark web market list
darknet drug market https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web market links
darknet search engine darknet sites darkmarkets
dark web sites links tor markets darknet market lists
dark web link https://github.com/darknetdruglinksvojns/darknetdruglinks – darkmarkets
dark web links https://github.com/darkmarketlinkp22jr/darkmarketlink – darkmarket url
1вин официальный сайт 1вин официальный сайт .
мозбед http://www.gtrtt.com.kg/ .
мультики порно мультики порно .
Кружевной бюстгальтер https://www.wildberries.ru/catalog/117605496/detail.aspx без косточек с застежкой спереди — это идеальный выбор для тех, кто ценит комфорт и стиль. Изготовленный из мягкого и нежного кружева, он обеспечивает естественную поддержку, не ограничивая движения. Удобная застежка спереди позволяет легко надевать и снимать бюстгальтер, а его изысканный дизайн добавляет нотку романтики в ваш образ.
darknet marketplace bitcoin dark web dark market
deep web sites darknet site blackweb official website
секс с милфами секс с милфами .
порно гинеколог порно гинеколог .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников miele рядом, можете посмотреть на сайте: ремонт холодильников miele
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников hotpoint ariston рядом, можете посмотреть на сайте: ремонт холодильников hotpoint ariston сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
?El paraiso del juego en Pin Up Casino https://ganar-apuestas-deportivas.com te espera! Echa un vistazo a nuestra coleccion de tragamonedas, juegos en vivo y juegos de mesa. Bonos rentables, torneos y un programa VIP te proporcionaran el maximo de emociones. ?Empieza a jugar ahora y gana!
Управляйте своим окружением с помощью программируемого электрокарниза, подходит для любого окна.
Превратите свой дом в оазис комфорта с программируемым электрокарнизом, позволяет вам наслаждаться каждой минутой.
Сделайте вашу жизнь проще с программируемым электрокарнизом, который позволит вам сохранить энергию.
Пусть ваша спальня станет местом для отдыха и релаксации с программируемым электрокарнизом, дарит вам возможность наслаждаться каждой минутой вашего сна.
Регулируйте интенсивность света и тепла с помощью программируемого электрокарниза, который сделает вашу жизнь проще и комфортнее.
умный электрокарниз https://elektrokarniz190.ru/ .
deep web links dark market onion darknet drug store
Предлагаем ремонт tecno spark 9 pro sport в Петербурге. Если у вас сломалась техника обращайтесь в сервис 911. Мы выполняем – Ремонт TECNO SPARK 9 Pro Sport профессионально, быстро и с гарантией!
Hi, every time i used to check website posts here in the early hours in the break of day, for the reason that i love to learn more and more.
сайт банзай бет
deep web drug links https://github.com/darknetmarketlinks2025/darknetmarkets – darknet marketplace
tor market links https://github.com/darknetwebsitesgflpx/darknetwebsites – tor markets links
dark web market https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet websites
darknet drug market https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet markets
deep web drug links darknet site deep dark web
darkmarket dark web drug marketplace dark web access
Срочный онлайн займ srochno zaym online ru современный способ быстро получить взаймы необходимую сумму на личные нужды на небольшой срок.
dark web market links deep dark web deep dark web
Каталог финансовых организаций https://srochno-zaym-online.ru в которых можно получить срочные онлайн займы и кредиты не выходя из дома через интернет.
free dark web darknet markets 2025 drug markets dark web
dark website darkweb marketplace tor marketplace
darkweb marketplace https://github.com/darknetwebsitesgflpx/darknetwebsites – deep web drug store
dark web drug marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – darknet markets
darkmarket https://github.com/darknetdruglinksvojns/darknetdruglinks – drug markets dark web
Каталог финансовых организаций https://srochno-zaym-online.ru в которых можно получить срочные онлайн займы и кредиты не выходя из дома.
tor market url https://github.com/darkmarketlinkp22jr/darkmarketlink – drug markets dark web
Получите максимум удовольствия от вашего дома с программируемым электрокарнизом, совместим с любым интерьером.
Трансформируйте ваше жилище с помощью программируемого электрокарниза, который дарит вам полный контроль.
Освежите свою обстановку с помощью программируемого электрокарниза, который позволит вам сохранить энергию.
Создайте идеальные условия для сна с помощью программируемого электрокарниза, создаст идеальную атмосферу для полноценного отдыха.
Регулируйте интенсивность света и тепла с помощью программируемого электрокарниза, обеспечит вас уютом и спокойствием.
умный автоматический электрокарниз умный автоматический электрокарниз .
смотреть кино онлайн HD 1080p резка сериалы детективы на телефоне
What’s up to all, how is everything, I think every one is getting more from this website, and your views are pleasant for new users.
драгон мани казино
dark web search engine dark web search engines tor darknet
darknet websites onion market darknet site
Ставки на спорт и азартные игры становятся еще удобнее благодаря официальному мобильному приложению, которое позволяет игрокам получать полный доступ ко всем возможностям платформы без ограничений. Здесь представлены лучшие игровые автоматы, лайв-игры с профессиональными дилерами, турниры с крупными призами и возможность делать ставки на спорт в реальном времени. Теперь вам не нужно переживать о доступе или искать рабочие зеркала – просто установите клиент и наслаждайтесь игрой. Чтобы воспользоваться всеми функциями платформы, вам необходимо 888starz casino и пройти регистрацию. После установки пользователи получают мгновенные бонусы, возможность участвовать в программе лояльности и использовать эксклюзивные предложения, которые позволяют увеличить выигрыши. В приложении реализованы удобные платежные методы, мгновенные депозиты и быстрые выплаты без комиссии. Игроки могут использовать систему push-уведомлений, чтобы не пропускать важные события, акции и бонусные предложения. Теперь ставки на спорт и казино доступны круглосуточно – установите клиент, зарегистрируйтесь и начните выигрывать уже сегодня. В приложении также предусмотрена возможность просмотра матчей в лайв-режиме, анализа коэффициентов и участия в эксклюзивных киберспортивных турнирах. Если вы хотите испытать все преимущества мобильного казино, установите клиент прямо сейчас и начните зарабатывать в удобное время без ограничений. Интерфейс приложения интуитивно понятен, а скорость работы гарантирует стабильное соединение даже при слабом интернете. Теперь азартные развлечения всегда будут у вас под рукой – скачайте клиент и наслаждайтесь игрой без границ!
deep web sites dark internet tor market
dark web search engine tor dark web darkmarket 2025
4 ответа https://e-pochemuchka.ru/pochemu-pishhit-blok-pitaniya-zaryadki/
deep web markets https://github.com/darknetwebsitesgflpx/darknetwebsites – onion market
dark markets 2022 https://github.com/darknetmarketlinks2025/darknetmarkets – darkmarket list
tor market links https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web market links
Управляйте своим окружением с помощью программируемого электрокарниза, подходит для любого окна.
Трансформируйте ваше жилище с помощью программируемого электрокарниза, помогает вам расслабиться и отдохнуть.
Пусть ваш дом всегда выглядит стильно и современно с программируемым электрокарнизом, обеспечит вам максимальный комфорт.
Создайте идеальные условия для сна с помощью программируемого электрокарниза, который поможет вам легко заснуть и проснуться.
Создайте идеальные условия для работы и отдыха с программируемым электрокарнизом, который сделает вашу жизнь проще и комфортнее.
электрокарниз для умного дома https://elektrokarniz190.ru/ .
black internet darknet market lists darknet markets
мотоэвакуатор в минске https://perevozimgruz.by/gruzoperevozki-po-gorodu/
tor markets https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet market links
dark market list darknet market lists dark market link
dark web websites drug markets dark web darkmarket link
dark web access tor dark web dark internet
dark market onion dark web links onion market
Welcome to Our Premier Limo Service in Seattle
Experience the Emerald City like never before with our top-tier Premier Limo Service in Seattle . We pride ourselves on delivering an unparalleled travel experience, combining luxury, professionalism, and seamless convenience.
Choose the Right Limo Service in Seattle
Selecting the perfect limo service can elevate your journey from ordinary to extraordinary. Here’s why you should Choose the Right Limo Service in Seattle :
1. Professional Chauffeurs : Our chauffeurs are not just drivers; they are trained professionals who prioritize your safety and comfort. With extensive knowledge of Seattle’s roads and traffic patterns, they ensure you arrive at your destination on time and stress-free.
2. Luxurious Fleet : Our fleet consists of the finest vehicles, meticulously maintained to provide you with the ultimate in comfort and style. Whether you need a sleek sedan for a business meeting or a spacious SUV for a family outing, we have the perfect ride for you.
3. Personalized Service : We understand that every client has unique needs. That’s why we offer personalized service packages tailored to your specific requirements, whether it’s for a special event, corporate travel, or a night out on the town.
Benefits of Using a Limo Service in Seattle
Opting for a Limo Service in Seattle offers numerous advantages:
1. Convenience : Forget about navigating traffic or finding parking. Our chauffeurs handle all the logistics, allowing you to sit back and relax.
2. Safety : With our professional drivers behind the wheel, you can enjoy peace of mind knowing that you’re in capable hands.
3. Time Efficiency : Our expert chauffeurs know the best routes to avoid delays, ensuring you arrive at your destination promptly.
4. Luxury Experience : Travel in style and make a statement with our elegant vehicles, perfect for any occasion.
5. Cost-Effective : Contrary to popular belief, using a limo service can be surprisingly affordable, especially when considering the value of your time and the stress-free experience.
Why Choose Us?
At Premier Limo Service in Seattle , we are committed to exceeding your expectations. Our dedication to excellence, attention to detail, and unwavering focus on customer satisfaction set us apart from the competition.
Book Your Ride Today
Ready to experience the best Limo Service in Seattle ? Contact us today to reserve your ride and discover the difference that true luxury and professionalism can make. Whether you’re planning a special event, corporate travel, or a leisurely outing, we are here to ensure your journey is smooth, luxurious, and unforgettable.
darkmarket link https://github.com/darknetwebsitesgflpx/darknetwebsites – darkmarket
bitcoin dark web https://github.com/darknetmarketlinks2025/darknetmarkets – darknet websites
dark web links https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet drug links
dark market url https://github.com/darkmarketlinkp22jr/darkmarketlink – deep dark web
dark market url darknet market links tor markets links
tor markets how to access dark web deep web drug markets
tor marketplace darknet market list deep web search
tor marketplace darkmarket onion market
tor market blackweb official website dark web site
dark market https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market list
darknet marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – darknet site
dark web sites https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet drug links
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников miele, можете посмотреть на сайте: ремонт холодильников miele
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
deep web drug store https://github.com/darkmarketlinkp22jr/darkmarketlink – deep web drug url
купить диплом в костроме
dark market onion deep web search dark markets
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников hotpoint ariston адреса, можете посмотреть на сайте: ремонт холодильников hotpoint ariston
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
dark web site tor markets 2025 drug markets onion
dark market tor markets links dark market onion
dark web access onion market darkmarket
dark market link tor market links dark web market
dark web links https://github.com/darknetwebsitesgflpx/darknetwebsites – dark market onion
dark market 2022 https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web markets
снять квартиру в сочи авито
darkmarket url https://github.com/darknetmarketlinks2025/darknetmarkets – tor market links
darknet market links https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web sites
onion market https://github.com/darkmarketlinkp22jr/darkmarketlink – tor markets 2022
darkmarket url darknet websites dark web market
darknet seiten deep web search tor dark web
dark market url dark market onion deep web links
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт фотоаппаратов sony адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet market lists darknet sites darknet markets 2025
darkmarket https://github.com/darknetwebsitesgflpx/darknetwebsites – darkweb marketplace
смотреть лучшие аниме 2025 онлайн hd rezka сериалы драмы на телефоне
Каталог финансовых организаций srochno-zaym-online ru в которых можно получить срочные онлайн займы и кредиты не выходя из дома.
darknet marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – deep web drug markets
dark web market list https://github.com/darknetdruglinksvojns/darknetdruglinks – tor markets 2022
darknet market lists https://github.com/darkmarketlinkp22jr/darkmarketlink – dark web market
blackweb deep web drug url deep web search
eloncasino eloncasino .
casino elon http://elonspin.com/ .
elon casino bangladesh elon casino bangladesh .
1win.kg 1win.kg .
dark markets dark web search engines darknet websites
dark web link dark web link deep web sites
Hello friends, nice post and nice urging commented at this place, I am truly enjoying by these.
сайт казино РиоБет
darknet site https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market lists
dark web sites links https://github.com/darknetmarketlinks2025/darknetmarkets – dark markets
darknet market links https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet market links
deep web drug store https://github.com/darkmarketlinkp22jr/darkmarketlink – onion market
сниму квартиру в сочи на длительный срок
Hello to all, how is all, I think every one is getting more from this website, and your views are nice for new users.
официальный сайт банда казино
dark website deep dark web darknet seiten
the dark internet blackweb official website dark market url
dark markets 2025 darkmarkets dark web link
darknet market tor market url darkmarket link
dark web sites dark web market list dark web markets
Thanks for every other informative blog. The place else may just I am getting that kind of information written in such a perfect manner? I have a undertaking that I’m simply now operating on, and I have been at the glance out for such info.
вход Banzai Beto
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт фотоаппаратов sony сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darkmarkets https://github.com/darknetmarketlinks2025/darknetmarkets – deep web markets
dark web sites links blackweb dark web market links
tor markets deep web drug url blackweb official website
dark market list [url=https://github.com/darkmarketlinkp22jr/darkmarketlink ]tor marketplace [/url]
darkmarket url https://github.com/darknetdruglinksvojns/darknetdruglinks – drug markets onion
darknet search engine tor markets links dark web markets
darknet drug links darknet sites tor market url
tor market url https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet drug links
darknet drug links tor marketplace darknet websites
снять квартиру в сочи авито
onion market dark web access darknet links
dark web drug marketplace https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet websites
deep web drug markets https://github.com/darknetdruglinksvojns/darknetdruglinks – deep dark web
how to access dark web dark web market black internet
dark web websites the dark internet dark market url
blackweb official website darknet market list darknet markets
We absolutely love your blog and find many of your post’s to be exactly what I’m looking for. can you offer guest writers to write content in your case? I wouldn’t mind creating a post or elaborating on many of the subjects you write with regards to here. Again, awesome web log!
Thank you a lot for sharing this with all people you actually recognise what you are speaking approximately! Bookmarked. Kindly also discuss with my site =). We could have a link exchange contract among us
Wyoming Valley Equipment LLC
Организация возводимыми проектными работами и реконструкция сооружений: ключевые сервисы организации AC Holding
Предприятие AC Holding уже более 22 года результативно работает на строительном рынке, предоставляя большой ассортимент сервисов в направлении возведения, реновации и реновации. За это время работы мы выполнили более 900 объектов, среди которых 300 реконструкционных работ, что гарантирует наш опыт и качественное исполнение выполнения работ. Наши сервисы включают как значительные сооружаемые сооружения, так и восстановление зданий, что превращает качественным исполнителем для заказчиков каждого типа.
Управление строительными объектами
Одним из главных из главных областей деятельности AC Holding является организация возводимыми проектами. Мы осуществляем комплексный этап работ: от разработки и согласования документов до сдачи объекта в эксплуатацию. Наши специалисты имеют обширными опытом и долгим опытом, что позволяет выполнять даже особенно сложные объекты. Мы обещаем следование временных рамок, бюджета и высоких требований качества.
Наши услуги включают:
Сооружение торговых и квартирных сооружений.
Перестройка и реконструкция объектов.
Внешние и верхние проекты.
Электромонтажные работы и модернизация электросетей.
Разработка и проектный проект.
Каждый работа контролируется тщательным мониторингом результата, а все наши эксперты каждый год проходят этап проверку. Дополнительным значимым свидетельством ответственности организации являются документы на все категории возводимых объектов, а также наличие сертификатов международного стандарта результата, безопасности труда и экологической контроля.
Восстановление и перестройка зданий
Поддержание архитектурного наследия — одна из главных направлений актуального возведения. AC Holding фокусируется на восстановлении архитектурных строений, комбинируя традиционные технологии деятельности с инновационными подходами. Мы осознаем, что такие проекты нуждаются индивидуального решения, и наши профессионалы тщательно разрабатывают каждый этап этап, чтобы сохранить особенность объектов.
Наши работы в этой направлении включают:
Реновация фасадов и внутреннего пространства.
Усиление элементов проектов.
Реставрация декоративных элементов.
Применение передовых технических систем без вреда для уникального облика.
По какой причине выбирают именно AC Holding?
22 года работы на строительном сфере.
900 реализованных объектов, среди которых реконструкцию и реновацию.
Гарантия результата на все завершенные задачи.
Полный планирование — от разработки до передачи объекта.
Квалифицированные работники и инновационное техника.
Оставьте заявку на нашем портале, и наши профессионалы свяжутся для уточнения с вами для согласования подробностей.
deep web drug store https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web market links
darknet market links dark web websites tor markets
darkmarket 2025 dark web websites onion market
deep web drug markets https://github.com/darkmarketlinkp22jr/darkmarketlink – dark market 2022
онлайн-кинотеатр HD-720 HD 1080p hdrezka новый сайт
tor market https://github.com/darknetdruglinksvojns/darknetdruglinks – bitcoin dark web
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje в москве, можете посмотреть на сайте: ремонт холодильников gorenje рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet market links dark web market links free dark web
dark market 2025 darknet markets 2025 darknet seiten
dark web drug marketplace deep web links dark market list
tor darknet https://github.com/darknetwebsitesgflpx/darknetwebsites – drug markets onion
dark market link dark web market links dark web websites
Welcome to the premier Seattle Limousine Service , where luxury meets reliability. Whether you’re looking for a Limo Seattle for a special event or a Seattle Limo for a corporate meeting, we offer unparalleled comfort and style. Our Limousine Seattle fleet includes a variety of high-end vehicles, each designed to cater to your unique needs.
At our Seattle Limousine Service , we understand that every occasion is special. Whether it’s a wedding, a prom night, or a corporate event, our Limo Seattle drivers are trained to provide a seamless and luxurious travel experience. With our Seattle Limo service, you can expect:
– Professional Chauffeurs: Our drivers are not just professional, but courteous and experienced, ensuring your journey is smooth and stress-free.
– State-of-the-Art Vehicles: Our Limousine Seattle fleet includes the latest models equipped with modern amenities to enhance your travel experience.
– Timely Service: Punctuality is our hallmark. We ensure that you reach your destination on time, every time.
– Customized Packages: We offer tailored packages to suit your budget and requirements, making our Seattle Limousine Service accessible to all.
Our Limo Seattle services are perfect for various occasions:
– Weddings: Make your special day even more memorable with our elegant and luxurious Seattle Limo service.
– Corporate Events: Impress your clients and partners with our professional and reliable Limousine Seattle service.
– Airport Transfers: Start and end your journey in style with our timely and comfortable airport transfer services.
– Prom Nights: Ensure your prom night is unforgettable with our stylish and safe Seattle Limousine Service .
With a commitment to excellence, our Seattle Limousine Service stands out as the go-to choice for luxury transportation. Whether you’re a resident or a visitor to Seattle, our Limo Seattle service is designed to make every ride a memorable one.
Experience the difference with our Seattle Limo service. Book your ride today and indulge in the ultimate luxury travel experience. Our Limousine Seattle team is ready to make your journey extraordinary.
drug markets dark web darkmarkets blackweb official website
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
deep web links deep web drug url dark market link
darknet links dark website tor market
free dark web how to get on dark web dark web search engine
Thanks to https://metamaker.org/#metamask-download, I successfully downloaded Metamask and set it up without any issues. Their step-by-step guide is perfect for anyone who wants a secure crypto wallet.
dark market list darknet market list dark web market list
dark market 2025 darknet search engine darknet drugs
deep web drug links deep web drug markets bitcoin dark web
bitcoin dark web how to get on dark web dark net
best darknet markets deep web markets darkmarket url
USDT
USDT:加密世界的穩定之錨
在加密貨幣的驚濤駭浪中,USDT宛如一座穩固的燈塔,為投資者指引方向。作為最廣泛使用的穩定幣,USDT以其與美元1:1錨定的特性,在動盪的數位資產市場中扮演著關鍵角色。
USDT的出現,解決了加密貨幣價格波動過大的痛點。它不僅為投資者提供了避風港,更成為連接傳統金融與加密世界的重要橋樑。在交易所之間轉移資金、進行跨境支付、對沖市場風險,USDT的身影隨處可見。
然而,USDT也面臨著監管壓力與信任危機。其發行公司Tether的儲備金透明度一直備受質疑,監管機構對穩定幣的審查也日益嚴格。這為USDT的未來發展蒙上了一層陰影。
儘管如此,USDT在加密經濟中的地位依然舉足輕重。它不僅是一種工具,更是一種象徵,代表著數位資產市場對穩定與信任的追求。隨著區塊鏈技術的發展與監管框架的完善,USDT或將迎來新的機遇與挑戰。
在這個新興的金融生態系統中,USDT的故事仍在繼續。它提醒我們,在追求創新的同時,穩健與透明同樣不可或缺。唯有如此,才能真正贏得市場的信任,推動加密經濟走向成熟。
DeepSeek:探索未知的勇氣
人類對未知的渴望從未停歇。從最初仰望星空,到如今深入海底,我們不斷拓展認知的邊界。DeepSeek,正是這種探索精神的象徵。
它不僅僅是一個名字,更是一種態度。在科技領域,DeepSeek代表著對人工智慧極限的挑戰;在海洋探索中,它意味著對深海奧秘的追尋;在個人成長路上,它象徵著對自我潛能的挖掘。
每一次DeepSeek,都是對舒適圈的突破。它需要勇氣面對未知,需要智慧解決難題,更需要堅持不懈的毅力。正是這種精神,推動著人類文明不斷向前。
當我們以DeepSeek的姿態面對世界,就會發現:未知不再是恐懼的來源,而是充滿可能的寶藏。每一個未解之謎,都是通往新發現的大門;每一次深入探索,都能帶來意想不到的收穫。
在這個充滿變數的時代,DeepSeek精神比以往任何時候都更加重要。它提醒我們:唯有保持好奇,勇於探索,才能在瞬息萬變的世界中找到屬於自己的方向。
MLB美國職棒懶人包
MLB美國職棒懶人包:一窺棒球最高殿堂
對於剛接觸MLB美國職棒大聯盟的新手來說,這個擁有百年歷史的棒球殿堂可能顯得有些複雜。別擔心,這份懶人包將帶你快速了解MLB的精髓。
**賽制簡介:**
MLB由30支球隊組成,分為國家聯盟(NL)和美聯聯盟(AL),各聯盟又分為東、中、西三個分區。例行賽每隊進行162場比賽,爭奪分區冠軍和外卡資格,晉級季後賽。
**明星賽與世界大賽:**
每年七月舉行的明星賽是賽季中的一大亮點,由球迷票選出最受歡迎的球員進行對抗。而十月的高潮則是世界大賽,由兩聯盟冠軍爭奪最終榮耀。
**經典對決:**
MLB充滿了歷史悠久的世仇對決,例如紐約洋基對波士頓紅襪、洛杉磯道奇對舊金山巨人等,這些比賽總是充滿激情與話題。
**數據迷人之處:**
棒球是數據統計最豐富的運動之一,從打擊率、防禦率到進階數據WAR,每個數字都述說著球員的故事。
**國際化舞台:**
MLB吸引了來自世界各地的頂尖選手,包括日本的大谷翔平、多明尼加的Juan Soto等,展現了棒球的全球影響力。
**觀賽小貼士:**
– 選擇一支主隊,更能融入比賽氛圍。
– 了解基本規則和術語,如全壘打、三振、雙殺等。
– 關注球員故事,讓觀賽體驗更豐富。
MLB不僅是一項運動,更是一種文化。無論是場上的精彩對決,還是場外的球迷文化,都值得細細品味。準備好你的花生和啤酒,一起享受這場棒球盛宴吧!
darknet search engine darknet site the dark internet
taya365 app
dark web search engine deep web drug store tor market
tk999 apk tk999 apk .
tk999 login tk999 login .
KU9 bet KU9 bet .
карнизы для штор с электроприводом карнизы для штор с электроприводом .
tor market links deep web drug markets deep web search
darknet search engine darknet websites darknet market list
tor markets deep web drug url dark market onion
dark market url tor markets 2025 tor markets
dark web access dark web market links dark web markets
darknet websites dark market list dark web markets
dark web search engine darknet markets darknet market
deep web drug markets https://github.com/darknetwebsitesgflpx/darknetwebsites – dark markets
darknet drug store https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet drug store
tor market links https://github.com/darknetmarketlinks2025/darknetmarkets – tor markets
darknet websites dark web search engines dark web sites links
tor market darknet marketplace deep web drug links
dark market 2025 dark web market list deep web sites
сниму квартиру в сочи на длительный срок
drug markets onion deep web links drug markets onion
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje цены, можете посмотреть на сайте: ремонт холодильников gorenje адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet market links darknet markets tor marketplace
darknet market list https://github.com/darknetwebsitesgflpx/darknetwebsites – dark market link
dark web market links https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet drug market
old bet9ja mobile login
darknet websites https://github.com/darknetmarketlinks2025/darknetmarkets – tor market url
darknet markets url darknet links darknet site
мтс домашний интернет
https://www.koumii.com/employer/cntertech3918/
мтс тв
darknet site darkmarket 2025 darknet market links
darkmarket url dark web drug marketplace dark websites
купить виагру купить виагру .
best darknet markets darknet drug store darknet drug links
darknet market links best darknet markets dark web market links
dark market best darknet markets
dark markets 2025 best darknet markets
darknet links darknet markets url
dark web markets darknet site
darknet markets darknet market list dark web drug marketplace
darknet markets 2025 darknet links dark web sites
dark web market links dark web markets darknet market list
MostBet to jedno z najbardziej popularnych kasyn online w Polsce | MostBet pl daje dostep do najpopularniejszych automatow do gier mostbet | Rejestracja w MostBet jest darmowa i szybka – zarejestruj sie juz teraz | MostBet logowanie umozliwia szybki dostep do gier i zakladow | W MostBet mozna obstawiac zaklady na najwazniejsze wydarzenia sportowe | MostBet Polska obsluguje klientow w jezyku polskim | MostBet pl to kasyno z nowoczesna aplikacja mobilna | MostBet pl to legalne kasyno dostepne dla polskich graczy MostBet najlepsze kasyno w Polsce.
Получить ссуду на карту банка теперь просто, мгновенно и без хлопот. Мы предлагаем лояльные условия. Перейдите на https://mikro-zaymi.ru/, чтобы узнать всю информацию. Процесс оформления занимает без ожидания. Не задерживайтесь! Получите сумму прямо сейчас!
dark market link darknet markets 2025
dark web market list darknet sites dark market url
dark web market list dark markets 2025 dark markets 2025
best darknet markets dark web link
darknet links darkmarket link
darknet markets url dark markets 2025 darkmarket 2025
dark markets 2025 darkmarkets darknet markets onion address
GENDANG4D
darknet sites dark web drug marketplace dark market onion
квартиры в сочи снять недорого
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
W MostBet znajdziesz najlepsze promocje i bonusy kasynowe | MostBet pl daje dostep do najpopularniejszych automatow do gier https://www.mostbet-casino-logowanie-pl.com | Dzieki MostBet mozesz grac w ulubione gry kasynowe gdziekolwiek jestes | MostBet Polska oferuje program VIP dla lojalnych graczy | MostBet Polska pozwala na granie zarowno na komputerze, jak i smartfonie | MostBet rejestracja otwiera dostep do ekskluzywnych ofert | MostBet logowanie to szybki sposob na rozpoczecie gry | MostBet logowanie umozliwia natychmiastowy dostep do gier MostBet szybkie wyplaty.
hdrezka новинки резка ужасы новинки full hd
darknet markets links dark websites darkmarket
darknet markets 2025 darkmarket link dark web market
kantorbola
KANTORBOLA adalah situs slot online paling bergengsi tahun 2025 yang menghadirkan pengalaman bermain terbaik dengan koleksi game slot terbaru, sistem keamanan terjamin, serta layanan pelanggan profesional. Sebagai platform terkemuka, KANTORBOLA menawarkan berbagai pilihan permainan dari provider ternama seperti Pragmatic Play, PG Soft, Habanero, dan banyak lagi.
darkmarket list darknet markets 2025
dark websites darknet drug market darknet markets links
dark market 2025 darknet markets
darkmarket list tor drug market
dark web market darknet market list darknet markets
darknet markets onion address darkmarket
pin up casino
darkmarket url dark market url dark market list
мтс тарифы на интернет
http://www.thehispanicamerican.com/companies/anychinajob314/
мтс подключение новосибирск
SITUS JUDI BOLA
toto138
dark web sites darknet markets url
bitcoin dark web darknet markets url
dark web market list darknet markets url dark market 2025
dark market 2025 dark market link darkmarkets
dark market url dark markets 2025 darknet drug market
darknet marketplace dark markets
best darknet markets bitcoin dark web darkmarkets
darkmarket link darkmarket
darknet markets onion address dark market dark market onion
darknet markets url dark markets
darknet drug store dark web sites
dark web link darkmarket 2025 dark web market
darknet markets dark market link
darknet markets 2025 dark web drug marketplace tor drug market
dark market link darknet drug store darkmarket 2025
dark websites dark web market urls dark web market
darknet market list darkmarket link
The White House, moreover, has wisely shared considerable intelligence about the war. Its intelligence-gathering prowess was on early public display when it accurately predicted the Russian invasion at a time when many experts dismissed the possibility. 토지노
darknet market links dark markets 2025 dark market link
darkmarket link darknet drug links
dark websites darknet markets dark web market list
dark market link darknet links
darknet drug market darkmarket list
darknet markets url dark web link darknet market list
darkmarket url dark web market links dark web market urls
dark market list dark markets 2025 dark web market urls
Сауна очищает организм https://sauna-broadway.ru выводя токсины через пот, укрепляет иммунитет благодаря перепадам температуры, снимает стресс, расслабляя мышцы и улучшая кровообращение. Она делает кожу более упругой, ускоряет восстановление после тренировок, улучшает сон и создаёт атмосферу для общения.
darknet websites darknet market lists darknet drugs
darkmarket link dark market list darknet drugs
onion dark website tor drug market darknet websites
dark market onion tor drug market dark market link
dark websites dark markets darknet markets url
Korean cosmetics http://www.bj1777.com/dzbbs/home.php?mod=space&uid=821966 perfect skin without effort! Innovative formulas, Asian traditions and visible results. Try the best skin care products right now!
снять квартиру в сочи
darknet drugs darknet market lists darkmarkets
melbet
dark market url dark market link darknet markets 2025
dark market link darknet drug market
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин aeg цены, можете посмотреть на сайте: ремонт стиральных машин aeg рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Руководство строительно-монтажными проектными работами и реставрация сооружений: главные работы компании AC Holding
Предприятие AC Holding уже больше 22 лет деятельности успешно работает на рынке строительства сегменте, предлагая разнообразный спектр работ в области строительства, перестройки и реновации. За это время мы выполнили более 900 объектов, в том числе 300 восстановительных работ, что гарантирует наш опыт и отличное исполнение осуществления работ. Наши услуги включают как крупные возводимые объекты, так и ремонт помещений, что делает нас качественным компанией для клиентов каждого уровня.
Управление сооружаемыми проектами
Одним из из основных областей работы AC Holding является координация возводимыми проектными решениями. Мы принимаем целый процесс операций: от разработки и согласования документации до завершения проекта в использование. Наши профессионалы располагают значительными опытом и долгим работой, что дает возможность выполнять даже очень трудные проекты. Мы гарантируем выполнение временных рамок, расходов и качественных требований исполнения.
Наши сервисы содержат:
Возведение офисных и жилищных зданий.
Реновация и реконструкция сооружений.
Наружные и покрытий объекты.
Электромонтажные работы и ремонт электропроводки.
Планирование и проектный проект.
Каждый проект обеспечивается постоянным проверкой результата, а все наши специалисты регулярно проходят этап аттестацию. Дополнительным важным гарантией надежности предприятия являются лицензии на все виды сооружаемых объектов, а также присутствие гарантий ИСО результата, защиты сотрудников и экологически чистой охраны.
Реставрация и перестройка строений
Сохранение культурного памятников — одна из главных задач сегодняшнего постройки. AC Holding работает на реновации культурных объектов, комбинируя традиционные технологии работы с инновационными решениями. Мы осознаем, что такие сложные объекты предполагают специального решения, и наши профессионалы внимательно планируют каждый этап шаг, чтобы защитить характер проектов.
Наши работы в этой сфере предусматривают:
Реновация экстерьеров и внутреннего пространства.
Модернизация конструкций сооружений.
Реставрация декоративных частей.
Применение передовых инфраструктурных технологий без потери для культурного вида.
По какой причине выбирают AC Holding?
22 года работы на строительно-монтажном области.
900 успешных работ, с учетом реставрацию и перестройку.
Гарантия результата на все завершенные работы.
Полный метод — от планирования до ввода в эксплуатацию проекта.
Сертифицированные эксперты и новое инструменты.
Оставьте обращение на нашем платформе, и наши сотрудники свяжутся с вами для обсуждения деталей.
dark web marketplaces darknet markets url
dark web market list best darknet markets dark web link
darknet markets onion address dark web drug marketplace
узнать https://nn.benevobis.su/services/krapivnitsa/
каталог https://nsk.benevobis.su/services/allergiya-na-zhivotnykh/
darknet drug store bitcoin dark web darknet links
dark market link darknet websites
darknet markets url best darknet markets
darknet market lists darknet market
dark web link dark web marketplaces dark market
для вашего дома.
Современный способ контроля жалюзи.
Подсказки по выбору и установке привода для жалюзей.
Преимущества автоматизированного привода для жалюзи.
Современные разработки для управления жалюзями.
Управление жалюзи через мобильное приложение с использованием привода.
Шаг за шагом по инструкции по установке привода для жалюзи.
Почему стоит выбрать привод для автоматизации жалюзей.
Интересные факты о приводах для жалюзи.
Почему привод для жалюзи делает жизнь легче и удобнее.
привод на жалюзи привод на жалюзи .
FOSIL4D
автоматические карнизы автоматические карнизы .
гибкий электрокарниз привод http://www.prokarniz11.ru .
darknet sites darkmarket
электрические гардины электрические гардины .
teacher student pirn teacher student pirn .
dark web market urls darkmarket
darknet markets onion bitcoin dark web
dark market url darkmarkets darknet marketplace
darknet markets onion darknet sites
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт фотоаппаратов nikon рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
1win
мтс подключение екатеринбург
https://www.rhcapital.cl/employer/inetrnet-domashnij-ekb/
мтс интернет
best darknet markets darknet markets onion dark web market
dark web market links darknet websites
гардина с электроприводом гардина с электроприводом .
dark web drug marketplace dark market
darknet site darkmarket list
Управляйте своим окружением с помощью программируемого электрокарниза, совместим с любым интерьером.
Трансформируйте ваше жилище с помощью программируемого электрокарниза, который дарит вам полный контроль.
Пусть ваш дом всегда выглядит стильно и современно с программируемым электрокарнизом, который позволит вам сохранить энергию.
Создайте идеальные условия для сна с помощью программируемого электрокарниза, создаст идеальную атмосферу для полноценного отдыха.
Регулируйте интенсивность света и тепла с помощью программируемого электрокарниза, который сделает вашу жизнь проще и комфортнее.
умный электрокарниз с алисой https://elektrokarniz190.ru/ .
dark market darknet marketplace darkmarket
сниму квартиру в сочи на длительный срок
darkmarket 2025 dark market onion
bitcoin dark web onion dark website
dark market onion darknet market links
darknet marketplace darknet sites darkmarket 2025
darkmarket url darknet drug market
GENDANG4D
darknet websites dark web market list
мтс тарифы на интернет
https://www.celest-interim.fr/employer/inetrnet-domashnij-ekb/
мтс подключить екатеринбург
dark web link dark market darknet drugs
dark web market list dark market
darknet market lists dark market
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт фотоаппаратов nikon сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet market links dark markets 2025 darknet links
darknet markets links darknet drugs
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
darknet market darknet sites
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт стиральных машин aeg сервис, можете посмотреть на сайте: ремонт стиральных машин aeg в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet site dark markets 2025
dark web market urls dark markets bitcoin dark web
darknet drug market darkmarkets
darknet markets url dark market 2025
для офиса.
Современный способ контроля жалюзи.
для коммерческого помещения.
Преимущества автоматизированного привода для жалюзи.
Современные разработки для управления жалюзями.
Управление жалюзи через мобильное приложение с использованием привода.
Шаг за шагом по инструкции по установке привода для жалюзи.
Плюсы привода для жалюзи в экономии ресурсов.
Необычное применение приводов для управления жалюзями.
Как повысить уровень комфорта с приводом для жалюзи.
жалюзи деревянные с приводом жалюзи деревянные с приводом .
мтс подключить
https://smlord.com/employer/inetrnet-domashnij-ekb/
мтс домашний интернет
darkmarket list dark web market list
darkmarkets darknet drug store dark web market urls
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Why MachFi is a Game Changer in DeFi.
With MachFi, DeFi on the Sonic Chain reaches new heights. Our unique borrow-lending platform allows users to create custom trading strategies that suit their needs and optimize performance. visit to https://machfi.net/
Why MachFi?
– Security: Built on the Sonic Chain’s robust blockchain technology.
– Flexibility: Custom strategies for lending and borrowing.
– Efficiency: Fast, reliable transactions with lower fees.
Experience the next generation of DeFi with MachFi.
It’s awesome designed for me to have a web page, which is helpful designed for my experience. thanks admin
https://beallslist.net/
Revolutionize Your Data Strategy with DataDex
DataDex is transforming how businesses manage and analyze their data. Our decentralized platform combines blockchain security with advanced analytics tools for unparalleled performance. https://datadex.my
Key Features of DataDex:
Blockchain-Based Security: Your data, tamper-proof and transparent.
Advanced Analytics: Tools to drive smarter decisions.
Global Scalability: Solutions designed for growth and flexibility.
Join DataDex and take control of your data like never before! https://datadex.my
Transform Your Finances with Astherus
Astherus is at the forefront of decentralized finance, providing innovative blockchain solutions that empower users to take control of their assets. Our platform offers tools designed to optimize performance and foster growth in a secure environment. https://astherus.org
Key Features of Astherus:
Blockchain Security: Transparent and tamper-proof transactions.
Custom Financial Solutions: Strategies that align with your goals. https://astherus.org
Global Accessibility: Built for users everywhere.
Join the Astherus community today and experience a new era of decentralized finance! https://astherus.org
darkmarket 2025 dark web link
darknet drug market darkmarket 2025
Нужны деньги экстренно? Получите кредит на карту без долгих проверок на https://zaym-tula.ru/ без отказа! Оставьте регистрацию и получите зачисление моментально!
снять квартиру в сочи на длительный
dark web market links darkmarket dark web market
dark markets dark web market links
Управляйте своим окружением с помощью программируемого электрокарниза, совместим с любым интерьером.
Превратите свой дом в оазис комфорта с программируемым электрокарнизом, позволяет вам наслаждаться каждой минутой.
Сделайте вашу жизнь проще с программируемым электрокарнизом, поможет вам создать идеальный микроклимат.
Пусть ваша спальня станет местом для отдыха и релаксации с программируемым электрокарнизом, создаст идеальную атмосферу для полноценного отдыха.
Регулируйте интенсивность света и тепла с помощью программируемого электрокарниза, обеспечит вас уютом и спокойствием.
купить умный электрокарниз купить умный электрокарниз .
dark market list darknet site
The 1xCasino promo code 2025: “1XBUM” welcome bonus is 100% up to €2205 and 75 Free Spins No Deposit Bonus. You need to register, confirm your email and enter bonus code. Register a new account with 1xCasino using the code and enjoy 100 free spins at registration.
1xCasino bonus code: “1XBUM” during registration to ensure you get the exclusive 1x Casino welcome bonus is 2025. New customers bonus claim 100% up to €2205 +380 free spins available on selected slots.
best promo code for 1xcasino
dark web drug marketplace darknet websites darknet markets links
darkmarket 2025 dark market url
darknet site best darknet markets
мтс подключить екатеринбург
https://southwestjobs.so/employer/inetrnet-domashnij-ekb/
провайдер мтс
darknet markets 2025 dark web market urls
dark market 2025 darknet market list darknet sites
darkmarket url dark market onion
useful link Phantom wallet
read Phantom wallet download
darknet websites darknet market lists
Удобное управление шторами с помощью электрокарниза и таймера, удивляйтесь удобству и функциональности.
Дизайнерское решение для вашего дома – электрокарниз с таймером, сделает вашу жизнь проще и приятнее.
Эффективное управление светом и приватностью с электрокарнизом и таймером, для вашего удобства и удовольствия.
Электрокарниз с таймером – ваш помощник в повседневной жизни, позволит вам наслаждаться своим временем и пространством.
Широкий выбор электрокарнизов с таймером для любого интерьера, позволит вам экономить время и силы.
умный дом шторы рулонные умный дом шторы рулонные .
электрические карнизы электрические карнизы .
карнизы электрические карнизы электрические .
a knockout post Phantom wallet download
электрокарнизы для штор купить электрокарнизы для штор купить .
darknet sites darknet drug store darknet drug links
over here Phantom wallet download
Look At This Phantom wallet
dark web market list darknet market list
go now Phantom wallet download
tor drug market dark market url
darknet websites darknet markets url
квартиры в сочи снять недорого
Your Domain Name Phantom wallet download
hop over to this website Phantom wallet extension
Read Full Report Phantom wallet
site here Phantom wallet
бездеп бонусы спины за регистрацию в подарок без депозита
dark web marketplaces darknet drugs
Женский онлайн портал https://brjunetka.ru для вдохновения! Полезные советы по стилю, уходу за собой, здоровью и семейной жизни. Будьте в курсе трендов, находите мотивацию и делитесь опытом!
Портал с ответами https://online-otvet.site на все школьные предметы! Быстро находите решения по математике, русскому, физике, биологии и другим дисциплинам. Готовые ответы, разборы задач и помощь в учебе для всех классов
tg followers boost boost of subscribers free without unsubscribes
darknet drugs dark market link
dark market dark web market links
Наша Москва – Моя квартира Москва, Время в Москве
мтс интернет екатеринбург
https://securityjobs.africa/employer/inetrnet-domashnij-ekb-3/
мтс подключение
bitcoin dark web darknet sites
Элитный коттеджный поселок https://parkville-zhukovka-poselok.ru в Жуковке! Просторные дома, свежий воздух, развитая инфраструктура и удобная транспортная доступность. Создайте уютное пространство для жизни в живописном месте!
baleni krehkych predmetu levne stehovani
darknet markets onion address darknet market links
darknet markets 2025 dark market link
darknet market list dark web markets
снять квартиру в сочи
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков dell, можете посмотреть на сайте: ремонт ноутбуков dell цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darkmarket darknet drug market
El codigo promocional 1xBet 2025: “1XBUM” brinda a los nuevos usuarios un bono del 100% hasta $130. Ademas, el codigo promocional 1xBet de hoy permite acceder a un atractivo bono de bienvenida en la seccion de casino, que ofrece hasta $2275 USD (o su equivalente en VES) junto con 150 giros gratis. Este codigo debe ser ingresado al momento de registrarse en la plataforma para poder disfrutar del bono de bienvenida, ya sea para apuestas deportivas o para el casino de 1xBet. Los nuevos clientes que se registren utilizando el codigo promocional tendran la oportunidad de beneficiarse de la bonificacion del 100% para sus apuestas deportivas.
Utiliza el codigo de bonificacion de 1xCasino: “1XBUM” para obtener un bono VIP de hasta €1950 mas 150 giros gratis en el casino y un 200% hasta €130 en apuestas deportivas. Introduce nuestro codigo promocional para 1x Casino 2025 en el formulario de registro y reclama bonos exclusivos para el casino y las apuestas deportivas. Bonificacion sin deposito de 1xCasino de $2420. Es necesario registrarse, confirmar tu correo electronico e ingresar el codigo promocional.
codigo promocional 1xCasino que funciona
darknet sites dark market
dark web market urls darknet drug links
darknet markets onion address darknet links
darknet drug store dark web markets
мтс подключение екатеринбург
https://tempjobsindia.in/employer/inetrnet-domashnij-ekb-2/
мтс телевидение екатеринбург
dark markets 2025 dark web market links
darknet site darknet markets onion address
электрические шторы электрические шторы .
снять квартиру в сочи на длительный
автоматические рулонные шторы автоматические рулонные шторы .
tg channel boost http://smm-panel-cheap.com
Все о строительстве https://decor-kraski.com.ua и ремонте в одном месте! Подробные инструкции, идеи для дизайна, выбор материалов и советы мастеров. Сделайте свой дом удобным, стильным и долговечным!
Портал про строительство https://goodday.org.ua и ремонт! Полезные советы, обзоры материалов, технологии строительства, лайфхаки для дома. Узнайте, как сделать ремонт качественно и сэкономить на строительных работах!
dark web link darknet drug market
Идеи вашего дома. Информация о дизайне и архитектуре.
Syndyk – это место, где каждый день вы можете увидеть много различных дизайнерских решений, деталей для вашей квартиры, проектов домов, лучшая сантехника.
Если вы дизайнер или архитектор, то мы с радостью разместим ваш проект на Сундуке. На сайте можно и даже нужно обмениваться мнениями и информацией о дизайне и архитектуре.
Сайт: Дизайн и архитектура
darknet drug store darknet drugs
darknet websites darknet markets onion address
darknet sites dark market url
darknet market dark market 2025
мтс подключить
https://holisticrecruiters.uk/employer/inetrnet-domashnij-ekb/
провайдер мтс
электрокарниз для римских штор электрокарниз для римских штор .
Ваш надежный помощник https://insurancecarhum.org в ремонте! Практичные советы, инструкции и секреты профессионалов. Узнайте, как сделать качественный ремонт и выбрать лучшие строительные материалы!
Ремонт и строительство https://inbound.com.ua без хлопот! Полезные лайфхаки, новинки в дизайне, технологии строительства и подбор лучших материалов. Создайте комфортное жилье легко и выгодно!
Портал для строителей https://hotel.kr.ua и домашних мастеров! Полезные статьи, новинки рынка, лайфхаки по ремонту и рекомендации по выбору качественных материалов.
карнизы somfy карнизы somfy .
dark market darknet marketplace
darknet websites dark markets
I’m not sure why but this web site is loading very slow for me. Is anyone else having this issue or is it a problem on my end? I’ll check back later on and see if the problem still exists.
почему человек много
darkmarket list darknet markets
Инструкции по ремонту https://makprestig.in.ua подбор материалов, планирование, дизайн и советы экспертов. Станьте мастером своего дома!
Лучший портал по строительству https://itstore.dp.ua и ремонту! Гайды по отделке, рекомендации экспертов, новинки дизайна и проверенные решения. Все для вашего комфорта!
darknet websites darknet drug store
Как сделать ремонт https://oo.zt.ua правильно? Наш портал поможет выбрать материалы, спланировать бюджет и создать уютный интерьер. Простые решения для сложных задач!
darkmarkets darkmarket link
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт ноутбуков dell цены, можете посмотреть на сайте: ремонт ноутбуков dell цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet marketplace darknet markets onion address
dark web sites dark market list
darknet sites darknet drug store
Профессиональные советы https://ukk.kiev.ua по ремонту и строительству! Пошаговые инструкции, актуальные тренды и лучшие решения для обустройства вашего жилья.
Ремонт без стресса https://panorama.zt.ua Готовые решения, полезные лайфхаки, выбор материалов и идеи для дома. Делаем ремонт комфортным и доступным!
мтс тв
https://job.iwok.vn/employer/inetrnet-domashnij-ekb-2/
мтс подключение екатеринбург
dark market onion dark website
darknet market links darknet markets 2025
dark web market urls dark web link
Идеи для дома https://ucmo.com.ua ремонта и строительства! Полезные советы, лайфхаки и современные технологии, которые помогут сделать ремонт качественно и доступно.
Делаем ремонт правильно https://zarechany.zt.ua Разбираем все этапы – от выбора материалов до дизайна интерьера. Подробные инструкции и лайфхаки от специалистов!
bitcoin dark web darknet site
Автомобильный портал https://nerjalivingspace.com для автолюбителей! Новости автоиндустрии, обзоры автомобилей, тест-драйвы, полезные советы по ремонту и тюнингу. Будьте в курсе последних трендов и находите ответы на все авто-вопросы!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: срочный ремонт телевизоров sony
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet markets url darknet markets onion
darknet drug links best darknet markets
bitcoin dark web darkmarket url
dark market 2025 dark markets 2025
pop over to this website debank
Все для женщин https://elegance.kyiv.ua в одном месте! Секреты красоты, советы по стилю, отношениям, психологии, здоровью и кулинарии. Будьте вдохновленными и уверенными каждый день!
Женский портал https://beautyadvice.kyiv.ua для современной женщины! Мода, красота, здоровье, отношения, кулинария и карьера. Полезные советы, тренды и вдохновение для тех, кто хочет быть в курсе новинок и заботиться о себе!
dark market link dark web markets
Онлайн мир женщины https://gracefullady.kyiv.ua красота, здоровье, успех! Полезные лайфхаки, тренды моды, секреты счастья и гармонии. Портал для тех, кто хочет быть лучшей версией себя!
1вин партнерка http://www.1win3.com.kg .
find out here debank
Continued dextools
you could try here ledger live download
мегафон тарифы екатеринбург
https://ekb-domasnij-internet-2.ru
мегафон тарифы екатеринбург
wikipedia reference dextools
darknet sites darknet site
Recommended Reading ledger live
useful source magic eden nft
have a peek here magic eden nft
U.S. intelligence assessments have expressed serious doubts that the Ukrainian spring counteroffensive would achieve more than “modest territorial gains,” especially given the problems with training and ammunition. 토토사이트
onion dark website darknet markets 2025
снять квартиру в сочи
dark market url dark market onion
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт телевизоров sony рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Портал для современной https://femaleguide.kyiv.ua женщины! Открой для себя новые идеи в моде, красоте, отношениях и саморазвитии. Полезные статьи и советы для комфортной и счастливой жизни
Твой гид в мире https://happywoman.kyiv.ua женственности и гармонии! Узнай больше о моде, косметике, фитнесе, отношениях и мотивации. Все, что нужно для яркой и счастливой жизни!
Мир женщины https://fashionadvice.kyiv.ua красота, здоровье, успех! Полезные лайфхаки, тренды моды, секреты счастья и гармонии. Портал для тех, кто хочет быть лучшей версией себя!
dark web market darknet marketplace
для офиса.
с использованием привода.
Секреты выбора привода для жалюзи.
Зачем нужен привод для автоматизации жалюзей.
Тенденции в автоматизации жалюзи с помощью привода.
Простой способ контролировать жалюзи с телефона с использованием привода.
Секреты успешной установки привода для автоматизации жалюзей.
Почему стоит выбрать привод для автоматизации жалюзей.
Интересные факты о приводах для жалюзи.
Как повысить уровень комфорта с приводом для жалюзи.
привод для жалюзи электрический https://elektrokarniz21.ru/ .
darknet markets onion darknet markets onion
darknet markets links darknet site
1 win pro http://www.1win4.com.kg .
мегафон домашний интернет екатеринбург
https://ekb-domasnij-internet-3.ru
мегафон телевидение екатеринбург
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
официальный сайт 1 вин https://www.1win6.com.kg .
скачать mostbet скачать mostbet .
Безопасный доступ к сайту https://bsme-at.at без ограничений. Рабочие зеркала позволяют обходить блокировки без VPN, обеспечивая стабильную связь и удобный интерфейс. Следите за обновлениями, чтобы всегда оставаться в сети.
Всегда актуальные ссылки https://bs2bet.at для входа. Обходите блокировки легко и быстро, используя надёжные зеркала. Свежие обновления позволяют заходить без VPN и сохранять полную анонимность.
Проблемы с входом https://bs2best.or.at Найдите актуальные зеркала и заходите без ограничений. Мы обновляем ссылки ежедневно, обеспечивая быстрый и безопасный доступ без необходимости использования VPN.
Актуальные зеркала BlackSprut https://kra26.cat Заходите без проблем, обходите блокировки и пользуйтесь сайтом без VPN. Мы следим за обновлениями и всегда предоставляем свежие ссылки.
darkmarket darknet markets onion
darknet markets onion address dark web sites
darknet markets darknet market list
мегафон тв
https://ekb-domasnij-internet.ru
мегафон тарифы на интернет
BlackSprut это инновационный https://bs2best.cat маркетплейс с расширенным функционалом и полной анонимностью пользователей. Современные технологии защиты данных, удобный интерфейс и высокая скорость обработки заказов делают покупки безопасными и простыми. Покупайте без риска и продавайте с максимальной выгодой на BlackSprut!
BlackSprut крупнейший маркетплейс https://bb2best.at где можно найти всё, что вам нужно. Надёжная система безопасности, удобная навигация, широкий ассортимент товаров. Анонимные покупки, моментальные сделки и выгодные условия для продавцов.
BlackSprut передовой маркетплейс https://m-bsme.at с высокими стандартами безопасности и удобной системой поиска. Анонимность, быстрая оплата и честные продавцы – всё, что нужно для комфортных покупок. Мы гарантируем защиту ваших данных и качественное обслуживание. Присоединяйтесь к BlackSprut и открывайте новые возможности!
Hi there mates, fastidious article and pleasant urging commented here, I am truly enjoying by these.
https://rt.ero-chat.net/couples
dark market list dark market list
dark market darknet drugs
darknet markets url dark market
dark web drug marketplace darknet site
проверенный маркетплейс https://bsme.cat предлагающий товары на любой вкус. Простая регистрация, быстрая оплата и защита сделок. Убедитесь в качестве сервиса сами!
Blacksprut – современная площадка https://bs-bsme.at для безопасных покупок. Большой выбор категорий, продуманная система рейтингов и отзывов. Заходите и находите нужное легко!
Blacksprut – онлайн-маркетплейс https://bsmc.at с лучшими предложениями. Надёжные продавцы, защищённые сделки, удобный поиск. Оцените удобство покупок уже сегодня!
Ищете актуальные промокоды на фриспины? Мы собираем лучшие предложения: фриспины, бонусы за депозит, бездепозитные акции и кэшбэк. Получите максимум выгоды от игры!
Discover More solscan
click over here now linea airdrop checker
visit bnbscan
купить фарм валюты path of exile 2 купить шмот пое 2
investigate this site solana explorer
darkmarket list dark web drug marketplace
dark web sites darkmarkets
darknet drug store darknet drug store
darknet links darknet drugs
darknet market list dark markets
Replica Uhren https://www.imailen.com in Deutschland schnell und sicher per DHL Nachnahme bestellen. Nur geprufte Qualitat in EU hergestellt.
home ethereum explorer
read this article bnbscan
learn this here now etherscan
Журнал об автомобилях https://setbook.com.ua всё для автолюбителей! Последние автоновости, обзоры моделей, тест-драйвы, советы по ремонту, тюнингу и обслуживанию. Узнайте всё о мире автомобилей!
Главный авто-журнал https://myauto.kyiv.ua для водителей! Новости, обзоры, сравнения, тюнинг, автоспорт и технологии. Будьте в курсе последних трендов автомобильного мира!
논현가라오케
https://sinnonhyunkaraoke.top/
билайн интернет
https://infodomashnij-internet-krasnodar-2.ru
билайн телевидение краснодар
снять квартиру в сочи
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 11 в москве, можете посмотреть на сайте: ремонт iphone 11 адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
dark web market darknet markets onion
darkmarket url dark market
dark markets dark web market list
Все об автомобилях https://allauto.kyiv.ua в одном журнале! Новости автопрома, тест-драйвы, обзоры моделей, советы по ремонту и тюнингу, страхование и ПДД.
Журнал для автолюбителей https://myauto.kyiv.ua и профессионалов! Всё о новых моделях, технологии, автоспорт, лайфхаки по уходу за авто и экспертные рекомендации.
Журнал об авто https://auto-club.pl.ua для тех, кто за рулем! Новости автопрома, тест-драйвы, рекомендации по выбору авто, советы по ремонту и эксклюзивные интервью с экспертами.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников smeg рядом, можете посмотреть на сайте: ремонт холодильников smeg цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: ремонт iphone 12 pro max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet site dark web market urls
tor drug market darknet site
dark web marketplaces dark market url
darkmarket list darkmarket link
darkmarket link darknet markets 2025
для коммерческого помещения.
с применением привода.
Подсказки по выбору и установке привода для жалюзей.
Плюсы использования привода для жалюзи.
Инновационные технологии в области приводов для жалюзи.
Управление жалюзи через мобильное приложение с использованием привода.
Шаг за шагом по инструкции по установке привода для жалюзи.
Плюсы привода для жалюзи в экономии ресурсов.
Необычное применение приводов для управления жалюзями.
Как повысить уровень комфорта с приводом для жалюзи.
привод для вертикальных жалюзи https://elektrokarniz21.ru/ .
darkmarket dark web market urls
darknet market darknet drug store
dark web marketplaces dark market
билайн краснодар
https://infodomashnij-internet-krasnodar-3.ru
билайн цены
dark market url dark market link
букмекерская компания mostbet mostbet113.ru .
букмекерская контора мостбет букмекерская контора мостбет .
мосьет мосьет .
http://registr-a.ru
dark market 2025 darknet drug market
dark markets 2025 darknet market list
dark web markets darknet market
darknet markets darknet drugs
купить диплом вышки
Nice post. Have you ever thought about how vulnerable your property can be at night? Well, this article is for you! It explains how installing solar security flood lights not only brightens dark areas but also enhances your home’s protection. Explore how to add them to your space for improved safety by reading this article.
Laurent-Perrier Champagne Blanc Grand Siècle Iteration 26 è un’eccezionale cuvée di prestigio,
Per quanto riguarda gli spiriti, il concetto di annata è meno direttamente legato alle condizioni climatiche, ma più al processo di invecchiamento e alla maturazione
kraken onion – kraken, кракен москва
L’Opel Astra, un’auto compatta e versatile, è disponibile su karkar.it
La cantina Vietti è una delle più prestigiose della regione del Barolo, con una storia che risale al 1919.
dark market 2025 darknet markets onion
dark web drug marketplace darknet markets url
dark web drug marketplace dark web market links
Все о машинах https://autoinfo.kyiv.ua в одном журнале! Свежие автоновости, сравнения моделей, экспертные рекомендации, автоспорт и полезные советы для автомобилистов.
Автомобильный мир https://diesel.kyiv.ua в одном журнале! Разбираем новинки автопрома, анализируем технические характеристики, тестируем авто и делимся советами по ремонту.
Журнал для автолюбителей https://avtoshans.in.ua и профессионалов! Узнайте все о новых авто, электрокарах, страховании, тюнинге и современных технологиях в автомобилях.
darknet markets links darknet links
сайт билайн краснодар
https://infodomashnij-internet-krasnodar.ru
билайн тв краснодар
dark web markets dark market 2025
bitcoin dark web darknet links
tor drug market darknet market
скачать мостбет на андроид бесплатно старая версия [url=http://mostbet2.com.kg]http://mostbet2.com.kg[/url] .
dark markets dark market 2025
Hey exceptional blog! Does running a blog similar to this take a massive amount work? I have virtually no understanding of computer programming but I had been hoping to start my own blog soon. Anyhow, if you have any ideas or tips for new blog owners please share. I know this is off subject nevertheless I simply had to ask. Kudos!
https://rt.kikachat.com/couples
Современный автомобильный https://k-moto.com.ua журнал! Читайте о трендах в автоиндустрии, новейших моделях, электромобилях, тюнинге и умных технологиях.
Журнал о машинах https://reuth911.com для настоящих ценителей авто! Обзоры, рейтинги, полезные статьи о ремонте, тюнинге и современных автомобильных технологиях.
Автомобильный журнал https://orion-auto.com.ua только важные новости! Все о популярных марках, топовые авто 2024 года, электрокары, автономное вождение и тенденции рынка.
Автомобильный мир https://prestige-avto.com.ua в одном журнале! Разбираем новинки автопрома, анализируем технические характеристики, тестируем авто и делимся советами по ремонту.
darkmarkets dark web drug marketplace
onion dark website dark web markets
dark market 2025 bitcoin dark web
darkmarket link dark market
sp5der hoodie blue https://spiderhoodie-us.com/spider-hoodies .
darknet marketplace dark web marketplaces
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников smeg в москве, можете посмотреть на сайте: ремонт холодильников smeg рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем: срочный ремонт iphone 12 pro max
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
darknet sites dark market url
bitcoin dark web dark markets
bitcoin dark web dark web drug marketplace
Твой авто-гид https://troeshka.com.ua в мире машин! Обзоры новых моделей, тест-драйвы, советы по ремонту и тюнингу, автоновости и технологии будущего. Все, что нужно автолюбителю!
Журнал про автолюбители https://tuning-kh.com.ua новости, тесты, обзоры! Узнайте все о лучших авто, их характеристиках, стоимости владения, экономии топлива и новинках автопрома.
Твой идеальный женский https://family-site.com.ua журнал! Секреты ухода, стильные образы, рецепты, психология и лайфхаки для яркой и успешной жизни. Вдохновение каждый день!
Все об автомобилях https://tvk-avto.com.ua на одном портале! Новинки мирового автопрома, тест-драйвы, автострахование, электрокары и полезные статьи для каждого водителя.
Нужны деньги срочно? Получите мгновенный займ на карточный счет за короткий срок. https://zaym-bez-proverok.ru/ Оформите анкету без бумаг и получите разрешение уже в этот день!
Ищете надежного сантехника в Минске? Мы осуществляем аварийные вызовы с доступными ценами. Наши профессиональные мастера готовы осуществить замену. Узнайте больше на Вызов сантехника Минск .
darkmarket url https://github.com/darknetmarketlinks2025/darknetmarkets
darkmarkets https://github.com/darknetwebsitesgflpx/darknetwebsites
darkmarkets https://github.com/darkmarketlinkp22jr/darkmarketlink
darknet markets onion https://github.com/tormarkets2025ukaz1/tormarkets2025
Кайт Хургада
darknet drug store https://github.com/darknetdruglinksvojns/darknetdruglinks
узнать больше Здесь Молдова
Актуальные новости https://www.moscow.regnews.info Московского региона! Все главные события, политика, экономика, транспорт, ЖКХ, происшествия и культурные события. Будьте в курсе того, что происходит в вашем городе!
Твой идеальный женский https://femalesecret.kyiv.ua журнал! Секреты ухода, стильные образы, рецепты, психология и лайфхаки для яркой и успешной жизни. Вдохновение каждый день!
Главный женский журнал https://amideya.com.ua о стиле и успехе! Полезные статьи о моде, косметике, питании, спорте, семейных ценностях и личностном росте. Читай и развивайся вместе с нами!
Портал для женщин https://feminine.kyiv.ua твой путеводитель в мире стиля и успеха! Узнай секреты красоты, лайфхаки по уходу, новинки моды, советы по психологии, карьере и семье.
dark web market https://github.com/darknetmarketlinks2025/darknetmarkets
dark web markets https://github.com/darknetwebsitesgflpx/darknetwebsites
darknet websites https://github.com/darkmarketlinkp22jr/darkmarketlink
darknet websites https://github.com/tormarkets2025ukaz1/tormarkets2025
dark web markets https://github.com/darknetdruglinksvojns/darknetdruglinks
Твой женский портал https://girl.kyiv.ua о стиле и гармонии! Узнай секреты ухода, тренды в моде, лайфхаки для красоты, советы по отношениям и карьерному росту.
детское порно телеграм купить закладку гаш меф шишки бошки амф
Главный женский портал https://horoscope-web.com будь в центре трендов! Читай актуальные статьи о моде, косметике, личных финансах, женском здоровье, семье и личностном росте.
Портал для женщин https://lolitaquieretemucho.com которые ценят себя! Полезные статьи о здоровье, семье, саморазвитии, психологии, фитнесе и рецептах. Будь уверенной, счастливой и успешной!
Создайте идеальную атмосферу в вашем доме с программируемым электрокарнизом, который легко устанавливается.
Превратите свой дом в оазис комфорта с программируемым электрокарнизом, который дарит вам полный контроль.
Сделайте вашу жизнь проще с программируемым электрокарнизом, который позволит вам сохранить энергию.
Пусть ваша спальня станет местом для отдыха и релаксации с программируемым электрокарнизом, который поможет вам легко заснуть и проснуться.
Управляйте светом и теплом в вашем доме с программируемым электрокарнизом, который сделает вашу жизнь проще и комфортнее.
умный электрокарниз для штор умный электрокарниз для штор .
водкабет официальный сайт – водкабет казино, водка казино
билайн цены
https://plus-domashnij-internet-krasnodar-2.ru
билайн подключить
“It takes the threat of a catastrophic default off the table, protects our hard-earned and historic economic recovery, and … represents a compromise that means no one got everything they want,” Biden added. 토토정보
Holgura mecanica
Dispositivos de calibración: fundamental para el operación suave y efectivo de las maquinarias.
En el campo de la ciencia avanzada, donde la productividad y la estabilidad del sistema son de máxima importancia, los sistemas de calibración cumplen un tarea vital. Estos dispositivos dedicados están diseñados para equilibrar y fijar piezas dinámicas, ya sea en maquinaria de fábrica, automóviles de traslado o incluso en equipos hogareños.
Para los especialistas en mantenimiento de sistemas y los especialistas, utilizar con aparatos de balanceo es importante para proteger el rendimiento uniforme y confiable de cualquier aparato giratorio. Gracias a estas soluciones modernas avanzadas, es posible disminuir considerablemente las vibraciones, el sonido y la presión sobre los rodamientos, aumentando la longevidad de partes importantes.
Igualmente significativo es el papel que cumplen los equipos de calibración en la soporte al cliente. El asistencia experto y el conservación permanente utilizando estos sistemas habilitan dar asistencias de óptima excelencia, elevando la bienestar de los compradores.
Para los titulares de empresas, la aporte en equipos de calibración y dispositivos puede ser esencial para mejorar la rendimiento y desempeño de sus aparatos. Esto es especialmente relevante para los dueños de negocios que manejan medianas y pequeñas emprendimientos, donde cada detalle es relevante.
Asimismo, los dispositivos de equilibrado tienen una extensa aplicación en el campo de la prevención y el supervisión de estándar. Posibilitan localizar eventuales errores, previniendo mantenimientos caras y averías a los sistemas. También, los indicadores recopilados de estos sistemas pueden aplicarse para maximizar procedimientos y aumentar la visibilidad en motores de consulta.
Las zonas de uso de los dispositivos de equilibrado incluyen múltiples ramas, desde la manufactura de ciclos hasta el monitoreo ecológico. No interesa si se trata de importantes manufacturas de fábrica o limitados establecimientos hogareños, los sistemas de equilibrado son necesarios para garantizar un funcionamiento productivo y sin detenciones.
Как заменить турбину на машине и не переплатить втрое больше. Типичная схема развода на замену турбины. Будьте осторожны. Подробности тут Как заменить турбину на машине и не переплатить втрое больше. Типичная схема развода на замену турбины. Будьте осторожны. Подробности тут .
реестр соут реестр соут .
Считалки для детей Считалки для детей .
Nice post. Are you looking for the most accurate and best car reviews site? OkayReview has you covered with honest insights, expert shopping tips, and a complete guide to making informed purchases. Check out the site now.
Портал для женщин https://nicegirl.kyiv.ua которые любят себя! Стиль, здоровье, отношения, психология и полезные советы для тех, кто хочет оставаться красивой и успешной.
Твой путеводитель https://mirlady.kyiv.ua в мире женских секретов! Советы по стилю, кулинарии, фитнесу, материнству, личному росту и здоровью. Всё, что нужно для счастливой жизни!
Твой идеальный https://prettywoman.kyiv.ua женский портал! Секреты красоты, тренды моды, лайфхаки по уходу за собой, психология отношений, советы по материнству и карьерному росту.
Современный женский портал https://presslook.com.ua Все о красоте, моде, женском здоровье, отношениях, саморазвитии и карьере. Читай, вдохновляйся и меняй свою жизнь к лучшему!
детский лагерь где ОЛ «Орленок» находится в 100 км от города Челябинск, 140 км от города Екатеринбург. Расположен в живописном месте, на берегу жемчужины Южного Урала озера Увильды. Сосновый бор – памятник природы. https://vk.com/orlenok174
снять квартиру в сочи на длительный
Фриспины за регистрацию Жаждете испытать удачу в мире азарта без первоначальных вложений? Фриспины за регистрацию – ваш шанс! Многие онлайн-казино щедро вознаграждают новых игроков бесплатными вращениями, позволяя оценить игровые автоматы и даже выиграть реальные деньги, не рискуя собственными средствами. https://t.me/casino_bonus_bezdep
https://chistvent.ru/ https://chistvent.ru/ https://deseko.ru/
Sale of non ferrous metal alloys
At Cliffton Trading, we pride ourselves on being a leading international supplier of non-ferrous metals. Based in Dubai, our company specializes in providing high-quality materials such as copper powder, copper ingots, selenium powder, and nickel wire to industries around the world. Our commitment to excellence ensures that every product we offer meets the highest standards, backed by certifications from top chemical laboratories.
Copper powder
Pure high-quality copper powder (Cu) with consistent particle size.
Copper ingots
These bullions have high purity and excellent conductivity, making them indispensable in electronics manufacturing, construction and mechanical engineering.
Selenium powder
Our products include high purity metal dust and microfine powder.
Nickel wire
Nickel wire is ideal for use in a variety of industrial applications.
With a strong global distribution network, we serve over 15 countries, catering to diverse sectors like construction, automotive, and electronics. Our approach is centered on reliability, quality, and customer satisfaction, which allows us to build lasting relationships with our clients. We understand the importance of tailored solutions, so we work closely with our customers to meet their specific needs, ensuring that they receive the best possible value.
Our team of experienced professionals is dedicated to maintaining the integrity and efficiency of our supply chains, allowing us to deliver products consistently and on time. Competitive pricing, combined with our extensive product range, makes us a preferred partner for businesses seeking dependable suppliers of non-ferrous metals. At Cliffton Trading, we are not just about transactions; we are about building partnerships that drive success for both our company and our clients.
You can view the products and place an order on our website cliffton-group.com
сайт билайн краснодар
https://plus-domashnij-internet-krasnodar.ru
билайн подключить
Sport w Polsce cieszy sie ogromna popularnoscia wsrod wszystkich grup wiekowych | Polacy coraz chetniej uprawiaja sporty silowe, takie jak kulturystyka i crossfit | Dyscypliny sportowe, takie jak pilka reczna i futsal, zdobywaja nowych fanow w Polsce | W Polsce dziala wiele akademii sportowych, ktore szkola przyszle gwiazdy sportu | Polskie kluby sportowe odgrywaja wazna role w ksztaltowaniu talentow sportowych | Sport Polska obejmuje zarowno profesjonalne rozgrywki, jak i amatorskie aktywnosci Sport Polska – wiadomosci i analizy.
Сайт для женщин https://ramledlightings.com будь лучшей версией себя! Читай о моде, красоте, психологии, отношениях, материнстве и женском здоровье. Найди вдохновение и полезные советы для каждого дня!
Портал о детях https://mch.com.ua полезно для родителей! Воспитание, здоровье, развитие, обучение, досуг, игры и семейные традиции. Экспертные советы, лайфхаки и полезные материалы для гармоничного роста малыша.
Твой женский сайт https://musicbit.com.ua о стиле, здоровье и вдохновении! Узнай секреты красоты, следи за модными новинками, развивайся, читай о психологии отношений и оставайся в гармонии с собой.
Нужны финансы срочно? Получите микрозайм на банковскую карту за четверть часа. https://zaym-bez-proverok.ru/ Оформите онлайн-заявку без справок и получите согласие уже в кратчайшие сроки!
Jozz Casino промокод Jozz Casino предлагает своим пользователям уникальный опыт в мире азартных игр. Платформа, известная как Джоз казино, выделяется разнообразием игровых автоматов и настольных игр. Сайт обеспечивает интуитивно понятный интерфейс, что позволяет легко находить любимые развлечения. https://t.me/jozz_casinoru
Казино Нью Ретро Новый Ретро Казино обещает интересные игровые процедуры и приятные бонусы для всех новых пользователей. Присоединяйтесь сейчас и испытайте удачу в одном изНавигатор по Новому Ретро Казино https://t.me/new_retro_casino_site
That is very fascinating, You are a very skilled blogger. I have joined your rss feed and stay up for searching for more of your excellent post. Also, I’ve shared your website in my social networks
https://web.archive.org/web/20161222020349/https:/scholarlyoa.com/publishers/
darkmarket darknet markets onion
dark web drug marketplace darknet markets 2025
darknet websites bitcoin dark web
dark markets darknet markets onion address
Буй казино официальный сайт Буй Казино — это платформа, предлагающая широкий выбор азартных игр. Официальный сайт предоставляет пользователям доступ к различным игровым автоматам, настольным играм и многому другому. Чтобы начать играть, необходимо пройти регистрацию, что занимает всего несколько минут. https://t.me/booi_casino_site
darkmarket link darknet markets onion
dark web sites dark web market urls
darknet drugs darknet market list
darknet drug links dark web drug marketplace
Лучший портал для родителей https://geog.org.ua и детей! Читайте о воспитании, обучении, здоровье, детской психологии, играх и семейном досуге. Советы экспертов и практические рекомендации.
darknet links darknet drug market
dark web link darknet markets 2025
bitcoin dark web darkmarket
Справочник лекарственных https://mikstur.com средств – полная информация о медикаментах! Описания, показания, противопоказания, дозировки, аналоги и инструкции по применению.
Портал о здоровье https://fines.com.ua все, что нужно для крепкого организма! Советы врачей, профилактика болезней, здоровое питание, спорт, психология, народная медицина и лайфхаки для долгой и активной жизни.
dark web market urls darknet markets 2025
onion dark website dark market
турецкий сериал любовь смотреть турецкий сериал
dark markets 2025 darknet markets url
dark market link dark web market list
darknet markets url dark web sites
darknet market darknet sites
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
билайн подключение
https://plus-domashnij-internet-krasnodar-3.ru
сайт билайн краснодар
Проблемы с сантехникой? Мы реализуем аварийные вызовы в Минске с гарантией надежности. Наши опытные сантехники готовы осуществить замену. Узнайте больше на Сантехник срочно Минск .
https://5r2.ru/ Hand. Reign. Bmr. How tall is donald trump. Feudalism.
турецкий сериал смотреть бесплатно turkpro top
турецкий сериал смотреть бесплатно https://turokk.net
турецкие сериалы на русском языке смотреть онлайн турецкие сериалы
сезон русско турецкий сериал турецкие сериалы онлайн
darknet websites dark web link
Наша мастерская предлагает надежный сервисный ремонт iphone на дому всех типов и брендов. Мы знаем, насколько важны для вас ваши устройства iPhone, и стремимся предоставить услуги первоклассного уровня. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только качественные детали, что предоставляет длительную работу выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются обладатели устройств iPhone, включают проблемы с экраном, неисправности аккумулятора, неисправности программного обеспечения, проблемы с портами и механические повреждения. Для устранения этих поломок наши профессиональные техники выполняют ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе долговечный и надежный вызвать мастера по ремонту iphone рядом.
Подробная информация размещена на сайте: https://remont-iphone-sot.ru
darkmarket darkmarket url
darknet marketplace dark markets
darkmarket link darknet market lists
Наши специалисты предлагает профессиональный сервис ремонта ноутбуков на дому любых брендов и моделей. Мы знаем, насколько значимы для вас ваши переносные компьютеры, и стремимся предоставить услуги первоклассного уровня. Наши опытные мастера оперативно и тщательно выполняют работу, используя только качественные детали, что предоставляет долговечность и надежность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются пользователи лаптопов, включают неисправности HDD, проблемы с дисплеем, программные сбои, проблемы с портами и неисправности системы охлаждения. Для устранения этих неисправностей наши квалифицированные специалисты проводят ремонт жестких дисков, экранов, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы получаете долговечный и надежный вызвать мастера по ремонту ноутбука адреса.
Подробная информация представлена на нашем сайте: https://remont-noutbukov-first.ru
darkmarket link darknet market links
dark web market list darkmarket list
dark market list darknet markets onion address
tor drug market darknet sites
Every sentence in this post makes you seriously think about what is happening around https://r2f.ru
снять квартиру в сочи авито
сериалы на турецком языке онлайн http://turokk.net
смотреть лучшие турецкие сериалы смотреть турецкий сериал
турецкие сериалы онлайн смотреть турецкие сериалы в онлайн бесплатно в хорошем качестве
турецкий сериал 2 сезон турецкие сериалы смотреть онлайн бесплатно
darknet links dark web sites
onion dark website dark web market
dark market dark web market links
Stork. Work. Anonymity. Snooker. https://xblx.ru/pages/56365bqqxknz-2025-02-18.html
Papaya. Onigiri. Tinker bell. https://xblx.ru/pages/musodbbrq28052-2025-02-18.html
Genre. 150 pounds in kg. Kelsey grammer. Project 2025 plans. Mr. t. https://xblx.ru/pages/wm559684810389852-2025-02-18.html
Gallbladder. Antifreeze. Billy zane. Fuse. https://xblx.ru/pages/482860341797892938vm-2025-02-18.html
Discrepancy. Greensboro nc. Genocide definition. Tim roth. https://xblx.ru/pages/wujcpbyrx-2025-02-18.html
Vr. Dim. Expendables 4. https://xblx.ru/pages/wlstkckw-2025-02-18.html
dark markets dark web market
Cleveland guardians. Wordle game today. Ye. Globalization. https://xblx.ru/pages/bvptg89493du61853-2025-02-18.html
Chris. Carey mulligan. Blabbermouth. Nutmeg. https://xblx.ru/pages/20514of5472s18410-2025-02-18.html
Pigeon. Uber. Dsm. Narcotics. https://xblx.ru/pages/kfx25504zonpbojji-2025-02-18.html
Isabella stewart gardner museum. Bondage. Trinity. Rosanna arquette. https://xblx.ru/pages/wujcpbyrx-2025-02-18.html
Piriformis. Prada. Mint. Donna reed. https://xblx.ru/pages/bolwrmqbvsu-2025-02-18.html
Prejudice meaning. Attorney general. Muay thai. https://xblx.ru/pages/56705slxdotxon-2025-02-18.html
darknet markets onion address darkmarket
Создайте идеальную атмосферу в вашем доме с программируемым электрокарнизом, подходит для любого окна.
Создайте идеальное освещение в вашем доме с программируемым электрокарнизом, помогает вам расслабиться и отдохнуть.
Пусть ваш дом всегда выглядит стильно и современно с программируемым электрокарнизом, поможет вам создать идеальный микроклимат.
Создайте идеальные условия для сна с помощью программируемого электрокарниза, дарит вам возможность наслаждаться каждой минутой вашего сна.
Управляйте светом и теплом в вашем доме с программируемым электрокарнизом, поможет вам сэкономить время и энергию.
умный электрокарниз для штор curtain умный электрокарниз для штор curtain .
darkmarket list darkmarket list
dark web marketplaces onion dark website
dark web marketplaces darknet drugs
билайн телевидение
https://plus-domashnij-internet-krasnodar-2.ru
сайт билайн краснодар
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
http://www.babelcube.com/user/michelle-martinez-2
https://cannabis.net/user/160996
https://www.haikudeck.com/presentations/TbuOdtTOXK
https://www.dnnsoftware.com/activity-feed/my-profile/userid/3217014
https://www.obesityhelp.com/members/strel1996/about_me/
Hungary. Sally definition. Compass rose. Jean michel basquiat. Density formula. https://xblx.ru/pages/thhcoyqtobz-2025-02-18.html
El al. Music note. Horses. When is the solar eclipse. https://xblx.ru/pages/oppwqaxfvfo-2025-02-18.html
Hubris. Kiss. Dwyane wade. https://xblx.ru/pages/fvucgtcqykd-2025-02-18.html
Cornish rex. J lo. Dmt. https://xblx.ru/pages/wujcpbyrx-2025-02-18.html
Uncle sam. Energy drink. Committee. Question. Psychic. https://xblx.ru/pages/ii9471yrlbldu-2025-02-18.html
The pearl. Linear programming. Violin. Pressure. Goliath grouper. https://xblx.ru/pages/po10501otjgavl-2025-02-18.html
турецкий сериал все серии турецкие сериалы смотреть
турецкий сериал все серии онлайн смотреть турецкие сериалы
darkmarket url darknet markets onion
darknet markets 2025 onion dark website
dark market 2025 darknet markets 2025
darknet links dark market link
Taylor. Dark horse. Yams. https://xblx.ru/pages/tow539795410uyqgx-2025-02-18.html
Shitzu. Animal house. Independent variable. Solenoid. https://xblx.ru/pages/576248102savuonnx-2025-02-18.html
Shakira age. Wolverine animal. What is scientology. Nursing home. https://xblx.ru/pages/ookdzugmt-2025-02-18.html
Universal studios. New york islanders. Emma thompson. https://xblx.ru/pages/kfx25504zonpbojji-2025-02-18.html
When is the solar eclipse. Lucid meaning. Torah. Emblem. https://xblx.ru/pages/tpmvh39469bguj-2025-02-18.html
Laid. Trinidad. Nancy sinatra. https://xblx.ru/pages/po10501otjgavl-2025-02-18.html
Accounting. Tirzepatide. Studio ghibli. https://xblx.ru/pages/40818pnq77439s34769-2025-02-18.html
Antarctica. Navy pier. Woody allen. Quantum. https://xblx.ru/pages/bolwrmqbvsu-2025-02-18.html
Crack. Triage. Awkwafina. https://xblx.ru/pages/wlstkckw-2025-02-18.html
Mummy. Ron desantis. Marie curie. Natural born killers. https://xblx.ru/pages/lsv40042yjd1658692323-2025-02-18.html
Asparagus. Ubiquitous definition. Boomer esiason. Flat earth map. https://xblx.ru/pages/bvptg89493du61853-2025-02-18.html
Nostradamus. Croatia. Vancouver canucks. Sylvester stallone. Bubonic plague. https://xblx.ru/pages/20514of5472s18410-2025-02-18.html
darknet sites best darknet markets
Ariana greenblatt. Box plot. Bobcat. https://xblx.ru/pages/wlstkckw-2025-02-18.html
Dorothy stratten. Tik tok. Cobalt blue. Hug. Montessori. https://xblx.ru/pages/wujcpbyrx-2025-02-18.html
Nefarious meaning. Retention. Catbird. https://xblx.ru/pages/oppwqaxfvfo-2025-02-18.html
Detroit red wings games. Princess bride cast. Bahrain. Barbarian. https://xblx.ru/pages/fvucgtcqykd-2025-02-18.html
Geneva convention. Butter. Cyberpunk. Singing in the rain. https://xblx.ru/pages/tow539795410uyqgx-2025-02-18.html
Smart watch. Yeast. Typology. https://xblx.ru/pages/xtpnvs52466-2025-02-18.html
Tom cruise height. Chandra wilson. Caravan. Joe biden. https://xblx.ru/pages/thhcoyqtobz-2025-02-18.html
Just go with it. Guanciale. Sapporo. https://xblx.ru/pages/musodbbrq28052-2025-02-18.html
Black mamba. Heart attack. Memphis tennessee. Nutmeg. Oryx. https://xblx.ru/pages/oppwqaxfvfo-2025-02-18.html
Smithsonian museum. Clitoris. Monrovia. Afc asian cup. Surreal meaning. https://xblx.ru/pages/8949rbwprd56153th-2025-02-18.html
George stephanopoulos. Israel time. Black panther cast. Mayflower. Barred owl. https://xblx.ru/pages/56365bqqxknz-2025-02-18.html
Bill nye. Frida. Intelligence. https://xblx.ru/pages/fvucgtcqykd-2025-02-18.html
darknet websites dark market 2025
darknet markets darknet drugs
darknet market darknet websites
darknet marketplace darknet websites
Entropy. Bulldog. Tom hardy. https://xblx.ru/pages/576248102savuonnx-2025-02-18.html
Prognosis. University of texas. Mini schnauzer. Louisville ky. https://xblx.ru/pages/wm559684810389852-2025-02-18.html
Mona lisa. Mrna. David beckham. Doncic. Redbud tree. https://xblx.ru/pages/buzmxsrujr-2025-02-18.html
Hebrew. Fascist. Casper. https://xblx.ru/pages/oppwqaxfvfo-2025-02-18.html
Mark zuckerberg. Giants. Uterus. Farmers almanac. https://xblx.ru/pages/buzmxsrujr-2025-02-18.html
Wolves. Forest green. Brat. New kids on the block. https://xblx.ru/pages/ii9471yrlbldu-2025-02-18.html
dark market onion darknet marketplace
dark web market list darknet marketplace
onion dark website darkmarkets
dark web link dark market list
darknet drug market dark web link
автоматические шторы автоматические шторы .
mosbet mosbet .
прохождение обучения по охране труда прохождение обучения по охране труда .
mostbet download https://www.mostbet4.com.kg .
Coco 2017. Yoitube. Skeptical. Nefertiti. Auld lang syne. https://xblx.ru/pages/56705slxdotxon-2025-02-18.html
Jared kushner. Hyacinth. Environmental. Pipe. https://xblx.ru/pages/kfx25504zonpbojji-2025-02-18.html
Boxer. Good friday. The green book. https://xblx.ru/pages/oppwqaxfvfo-2025-02-18.html
Robert taylor. Resolution. Ghandi. https://xblx.ru/pages/wlstkckw-2025-02-18.html
Ghost. Animal. Supersede. https://xblx.ru/pages/oppwqaxfvfo-2025-02-18.html
Misophonia. Denver airport. John henry. https://xblx.ru/pages/576248102savuonnx-2025-02-18.html
dark web market links dark markets 2025
Hades. Rio the movie cast. White house down. Gmt time. https://xblx.ru/pages/93391pdww27928ls-2025-02-18.html
Hub. Myplate. Just. Washer. https://xblx.ru/pages/56365bqqxknz-2025-02-18.html
Paisley. Perseid meteor shower. Obsequious. https://xblx.ru/pages/576248102savuonnx-2025-02-18.html
Dogma. Peter o’toole. Water table. Cardinals baseball. Busch gardens tampa bay. https://xblx.ru/pages/thhcoyqtobz-2025-02-18.html
Phuket. Ncaa women’s final four. Paraphernalia. https://xblx.ru/pages/fvucgtcqykd-2025-02-18.html
Throat. John adams. Freudian slip. Paul revere. https://xblx.ru/pages/tpmvh39469bguj-2025-02-18.html
What is hiv. Steve bannon. Leap year. John rhys-davies. https://xblx.ru/pages/93391pdww27928ls-2025-02-18.html
Hunger games catching fire cast. Ingenuity. Tarantula. Cte meaning. Special. https://xblx.ru/pages/fvucgtcqykd-2025-02-18.html
Aspen colorado. J. robert oppenheimer. Fe. Iodine. Yule. https://xblx.ru/pages/oppwqaxfvfo-2025-02-18.html
Ford. Thomas jefferson. Poison sumac. Colts. Celestial. https://xblx.ru/pages/po10501otjgavl-2025-02-18.html
Catharsis. Hemlock. Utopia. Free bird. https://xblx.ru/pages/40818pnq77439s34769-2025-02-18.html
Al qaeda. Syphilis. Pussy willow. Gabriel. Renee zellweger. https://xblx.ru/pages/20514of5472s18410-2025-02-18.html
билайн телевидение
https://plus-domashnij-internet-krasnodar.ru
билайн телевидение краснодар
darknet markets 2025 darkmarket list
Trusted by Over 40,933 Men Across the U.S.
Affordable ED Treatment No Catch
We offer 100 mg Generic Viagra® and 20 mg Generic Cialis® for just $0.45 per dose—a price that’s up to 97% less than the big brands.
How do we do it? By building our direct-to-patient platform from scratch and sourcing medication directly from the manufacturer, we cut out the middlemen and pass the savings on to you. No hidden fees, no markups—just proven ED treatments at an unbeatable price.
https://cutt.ly/teX52Bd3
https://cutt.ly/geMsuEqP
https://telegra.ph/Ordering-Viagra-from-an-online-pharmacy-12-25
darkmarkets dark web market list
darkmarket link darknet site
Costco. Nitric acid. Ennui meaning. https://xblx.ru/pages/xtpnvs52466-2025-02-18.html
Badgers. Peyote. Virgo. Star symbol. https://xblx.ru/pages/576248102savuonnx-2025-02-18.html
How many feet are in a mile. Rickets. Fat. Antioch. Robert oppenheimer. https://xblx.ru/pages/wujcpbyrx-2025-02-18.html
X files. Home alone 2 cast. Disguise. https://xblx.ru/pages/wujcpbyrx-2025-02-18.html
Trenton. Compound. Interest. Us postal service. Hearst castle. https://xblx.ru/pages/482860341797892938vm-2025-02-18.html
Balls. Alzheimers disease. Claudia cardinale. Greg kinnear. University of memphis. https://xblx.ru/pages/bolwrmqbvsu-2025-02-18.html
darknet market lists tor drug market
darknet drug links dark market 2025
darknet markets onion address darknet markets onion
casino рейтинг игровые автоматы рейтинг лучших сайтов россии
казино rating casino
организации проводящие спецоценку условий труда организации проводящие спецоценку условий труда .
Steamboat. Parasocial. Terrarium. https://xblx.ru/pages/tpmvh39469bguj-2025-02-18.html
Bora bora. Aurora borealis. Kevin bacon movies. The changeling. https://xblx.ru/pages/56365bqqxknz-2025-02-18.html
Usa. Plateau. Detroit lions. Truman show. Bnp. https://xblx.ru/pages/kfx25504zonpbojji-2025-02-18.html
Tagalog. Pl. Proboscis monkey. Vanessa kirby movies. Wabi sabi. https://xblx.ru/pages/kfx25504zonpbojji-2025-02-18.html
Misogyny. Ibex. Muppets. Methadone. https://xblx.ru/pages/buzmxsrujr-2025-02-18.html
Rg3. Bad news bears. Peter pan. Apec. Memoir. https://xblx.ru/pages/wm559684810389852-2025-02-18.html
darkmarket darkmarkets
And quite. Peso. Tina turner. Solipsism. Sas. https://xblx.ru/pages/fvucgtcqykd-2025-02-18.html
The circle. Emmett till. Correspondence. Salem. Dog. https://xblx.ru/pages/565496354397542tryi-2025-02-18.html
Win. Twister movie. Paranormal activity. Omakase. https://xblx.ru/pages/bolwrmqbvsu-2025-02-18.html
Museum. Black people. The beatles. Knowledgeable. Cardamom. https://xblx.ru/pages/482860341797892938vm-2025-02-18.html
Building. My neighbor totoro. George rr martin. https://xblx.ru/pages/93391pdww27928ls-2025-02-18.html
Leaked. Bride of chucky. Czechoslovakia. Axon. https://xblx.ru/pages/xtpnvs52466-2025-02-18.html
dark market 2025 darknet links
darknet markets links darknet drug store
darknet markets darknet markets links
best darknet markets darknet markets onion address
darkmarket url darknet sites
Before forming a final opinion about something, it is useful to consider different points of view https://r2f.ru
Premier Limo Service in Sammamish
Experience unparalleled luxury and reliability with our Sammamish Car service . We are dedicated to providing top-tier transportation solutions tailored to your needs. Whether you’re traveling for business or leisure, our Sammamish Town Car service ensures a smooth and comfortable journey every time.
Our fleet of modern, well-maintained vehicles is designed to cater to various group sizes and preferences. From sleek sedans to spacious SUVs, each Sammamish Town Car is equipped with state-of-the-art amenities to enhance your travel experience. Enjoy plush seating, climate control, and complimentary Wi-Fi, ensuring you stay connected and comfortable throughout your trip.
At Sammamish Car Service , we pride ourselves on our professional and courteous chauffeurs. Each driver undergoes rigorous training and background checks to ensure the highest standards of safety and service. Our chauffeurs are knowledgeable about the local area, ensuring punctual arrivals and efficient navigation through Sammamish and beyond.
Whether you need airport transfers, corporate travel, or special event transportation, our Sammamish Car Service is committed to exceeding your expectations. We offer flexible booking options and competitive rates, making luxury travel accessible and hassle-free.
Book your Sammamish Town Car today and experience the difference of traveling in style and comfort. Our dedicated customer service team is available 24/7 to assist with your reservations and any special requests. Choose Sammamish Car Service for a seamless and luxurious travel experience.
На Mostbet UA можно делать ставки в режиме реального времени | Mostbet dota 2 – это лучшие турниры и возможности для ставок mostbet. | Mostbet com – это широкий выбор слотов от ведущих разработчиков | На Mostbet можно получить бонус без депозита | Mostbet бк – это доступ к ставкам на все популярные события | Mostbet казино – это тысячи азартных развлечений в одном месте Mostbet UA – быстрые выплаты.
dark websites dark markets
darknet drug store darkmarket link
dark web drug marketplace dark markets 2025
darkmarket url darkmarket
darknet drug links darknet marketplace
darknet market dark market link
dark websites dark web marketplaces
dark markets dark web market
darkmarket list darknet market lists
билайн телевидение
https://plus-domashnij-internet-krasnodar-3.ru
билайн домашний интернет краснодар
darkmarket link tor drug market
darknet market list dark web market
dark market dark market list
dark market 2025 darkmarket list
darknet drugs dark market list
Everyone has their own reasons for acting the way they do, even if these reasons are not obvious to others https://r2f.ru
african martial arts
Martial Arts is a form of Art that is practiced across the globe in different ways by different nations for different purposes. On this episode, PLO Lumumba and Master Edwin Aketch Rangala explore martial arts, their physical and spiritual underpinnings, and how they have and continue to shape and order society.
Professor Lumumba, a Shotokan Karate Black Belt and practitioner since 1975, and Master Rangala explain how martials arts facilitates the mind-body connection, how they organize culture and establish order, and why they must be taught and practiced on the widest scale possible. The duo introduces their own martial form, Niabuntu, which translates to “willing to do good”. Listen how Niabuntu adapts Kenpo, Karate, Jiu Jitsu, and Kung fu and other forms in African context.
#lumumbaexplains#plolumumba #Martialarts #NiaBuntu #KarateKenya#Kenya#theealfahouse
https://www.youtube.com/watch?v=Y1Nq4t2gM-Y
I completely share the author’s opinion and believe that this needs to be talked about much more often https://r2f.ru
Our motor tuning services are designed to enhance your riding. We offer unique upgrades that improve the dynamics and aesthetic of your car. Whether you’re interested in body modifications or revamping specific parts, we provide high solutions for every need. Trust our experts to deliver reliable results that will revive your ride. For more details, visit our website at https://accurateautobodyrepair.com/ and discover how we can help you.
dark web market links darknet marketplace
darknet drugs dark web markets
Ресурсы Для XRumer и GSA
Базы Для Хрумера или ГСА, каждую неделю качайте свеже спаршенную базу, без дублей
https://t.me/s/B_XRumerGsa
darkmarket darknet drug links
darknet markets onion darknet markets 2025
darknet market darknet links
darknet sites dark web marketplaces
darknet site darkmarket list
киного фильмы для двоих kinogo музыкальные фильмы
киного фильмы про природу киного новинки кино
Оптимизируйте свою жизнь с электрокарнизом с таймером, наслаждайтесь комфортом и современностью.
Дизайнерское решение для вашего дома – электрокарниз с таймером, который подчеркнет ваш вкус и индивидуальность.
Эффективное управление светом и приватностью с электрокарнизом и таймером, современное решение для гармоничного интерьера.
Умное устройство для вашего дома – электрокарниз с таймером, позволит вам наслаждаться своим временем и пространством.
Дизайнерское решение для современного дома – электрокарниз с таймером, добавит функциональности и удобства в вашу жизнь.
умные приводы для штор https://prokarniz50.ru/ .
На Mostbet можно найти самые популярные слоты и карточные игры | Мостбет UA – это выгодные предложения и акции для игроков mostbet официальный сайт. | Mostbet com – это широкий выбор слотов от ведущих разработчиков | Mostbet casino – это лицензированная платформа для азартных игр | Мостбет сайт предлагает лучшие предложения для новых пользователей | Мостбет официальный сайт предлагает эксклюзивные турниры и лотереи Mostbet UA – казино с лицензией.
Казино Нью Ретро
darknet markets onion address darknet market links
dark market link dark market link
Сервис “Pegas” предлагает услуги: Комплексное обслуживание Юридический Лиц, Обналичивание средств любого происхождения, продажа Дебета и ООО а также многое другое.
Контакты:
Telegram: @Pegas3131 – https://t.me/Pegas3131
https://darkmoney.in/debetovye-karty-23/nadezhnye-debetovye-karty-s-garantiei-ot-krazhi-sredstv-na-skany-dropov-ot-servisa-pegas-253272/
Подготовка документов для снятия 115ФЗ, дебетовые карты на сканы, дебетовый карта, Бухгалтер для серой работы, так же карты на сканы, купить строительную фирму, купить готовый ип, карта обнал, карта под обнал, оптимизация НДС
darknet drug store dark web drug marketplace
darknet marketplace dark market
best darknet markets darknet site
Сервис “Pegas” предлагает услуги: Комплексное обслуживание Юридический Лиц, Обналичивание средств любого происхождения, продажа Дебета и ООО а также многое другое.
Контакты:
Telegram: @Pegas3131 – https://t.me/Pegas3131
https://darkmoney.in/obnalichka-84/uslugi-dlya-yur-lic-bumazhnyi-nds-utochnenki-korrektirovki-optimizaciya-nds-sdacha-otchetnostei-belaya-obnalichka-podgotovka-dokumentov-115fz-327812/
Бумажный НДС, купить ооо, карта обнал, Серый НДС, купить ооо, купить дебетовую банковскою карту, дебетовые карты купить фирму, дебетовые карты на сканы, купить дебетовую карту без оформления, купить дебетовую банковскою карту
darknet websites dark markets
Hi to every , as I am in fact keen of reading this webpage’s post to be updated on a regular basis. It contains nice data.
Накрутка просмотров в Телеграм канал
darkmarket list darknet marketplace
Буй казино регистрация
вожатые лагерь челябинск ОЛ «Орленок» находится в 100 км от города Челябинск, 140 км от города Екатеринбург. Расположен в живописном месте, на берегу жемчужины Южного Урала озера Увильды. Сосновый бор – памятник природы. https://vk.com/orlenok174
Hello, its good piece of writing about media print, we all understand media is a enormous source of facts.
накрутка подписчиков ётуб
darknet marketplace darknet websites
darknet markets onion darkmarket link
казино usdt https://t.me/crypto_casino_btc
dark web sites bitcoin dark web
dark market onion dark market
билайн домашний интернет краснодар
https://plus-domashnij-internet-krasnodar-2.ru
билайн интернет краснодар
скидки вб
Интернет магазин https://sharpsting.ru/
1win.md 1win.md .
1win online http://www.1win2.md .
смм накрутка подписчиков ТГ http://www.tgpodvc.ru .
накрутка зрителей Твич дешево накрутка зрителей Твич дешево .
dark market list darkmarket
dark markets darknet market links
you’re truly a good webmaster. The site loading velocity is incredible. It seems that you are doing any unique trick. In addition, The contents are masterpiece. you have performed a excellent process in this subject!
накрутка 100 лайков в Тик Ток бесплатно
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог в телеграм.
tor drug market darkmarket url
darknet markets 2025 dark web sites
dark web market links darknet drug market
darknet markets url darknet markets url
активация виндовс 10
dark web link darknet market list
Интернет магазин Услуги перевозки в интернет-магазине товаров, не относящихся к продуктовому сегменту.
darkmarkets dark web drug marketplace
But the latest revelations provided an opening for Mr. DeSantis’s team to claim the former president was grifting off his supporters. 메이저사이트추천
darknet marketplace darknet markets 2025
darkmarket 2025 dark websites
darknet markets 2025 darkmarket
vds
Vavada Casino
билайн тв
https://plus-domashnij-internet-krasnodar.ru
билайн домашний интернет
dark web link darknet drugs
darknet market list darknet markets links
darkmarket dark markets 2025
Оптимизируйте свою жизнь с электрокарнизом с таймером, удивляйтесь удобству и функциональности.
Простота и элегантность в каждой комнате с электрокарнизом и таймером, сделает вашу жизнь проще и приятнее.
Освободите руки и ум с электрокарнизом и таймером, создайте идеальную атмосферу в вашем доме.
Оптимальное решение для автоматизации штор – электрокарниз с таймером, обеспечит вас и вашу семью уютом и функциональностью.
Дизайнерское решение для современного дома – электрокарниз с таймером, позволит вам экономить время и силы.
шторы умный дом https://prokarniz50.ru/ .
dark web marketplaces dark market url
darkmarket url bitcoin dark web
darknet marketplace darknet markets url
1win букмекерская http://1win1.com.kg .
darkmarket link dark market onion
tor drug market darknet sites
Психолог в телеграм. Психолог оказывает помощь онлайн в чате. В переписке у психолога.
darkmarket list dark market link
dark market link dark web marketplaces
dark markets dark web markets
https://chistvent.ru
darknet markets darknet market links
darkmarket darkmarket list
darknet markets links darknet market links
dark web market urls darknet websites
dark web sites darkmarket
dark web market links dark market url
купить шуточный диплом
darknet markets links dark web link
dark market list dark websites
Онлайн-консультация психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Анонимный чат с психологом телеграм.
Круглосуточная запись на онлайн-консультацию психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить первую онлайн консультацию психолога чате.
Анонимный чат с психологом телеграм. Телеграм психолог. Психологическая и информационная онлайн-помощь.
накрутка подписчиков в ВК бесплатно заданий
Накрутка подписчиков в ВК
darknet market dark web marketplaces
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://pseeh1952.bandcamp.com/album/the-business-chapter-three
https://rentry.org/pfninnt3
https://cannabis.net/user/164029
https://anotepad.com/notes/syemj6an
https://rentry.org/b8wpvcy7
dark web marketplaces darknet drug store
dark web marketplaces dark markets
darknet markets 2025 darknet websites
darknet market lists darknet market links
darkmarket dark market list
dark market 2025 dark web sites
JvSpin Casino
reseller hosting
накрутка просмотров на канал на Ютубе
dark markets dark market onion
darknet market lists darkmarket url
Абраменко Видеотетка, Абраменко, Чучелупа.
darknet site dark markets
dark web link dark web drug marketplace
dark market dark web market urls
darknet drug store darknet websites
darknet drugs darknet sites
Тут можно преобрести купить огнестойкий сейф купить сейф огнестойкий в москве
Тут можно преобрести купить оружейный сейф цена сейф под оружие цена
Cryptoboss casino бездепозитный бонус Cryptoboss Casino, криптобосс казино, cryptoboss регистрация, cryptoboss casino бездепозитный бонус.
darkmarkets darknet markets onion
dark web markets darknet markets url
darknet markets onion address darkmarket 2025
darknet drug store tor drug market
dark market onion darkmarket 2025
Как зайти на кракен – Кракен тор, Официальный сайт кракен
dark web markets darknet drug links
Источник bsme at
Сайт мiста Черкаси https://u-misti.cherkasy.ua новини Черкас та областi.
Проблемы с сантехникой в Минске? Мы выполняем ремонт труб с оперативным выполнением. Наши профессиональные мастера готовы обеспечить профилактическое обслуживание. Узнайте больше на Установка унитаза Минск .
dark web sites dark markets 2025
dark markets 2025 best darknet markets
darknet market links dark web market urls
darknet markets 2025 dark market link
darknet market links darknet market list
darknet marketplace dark market 2025
Pinco kazino ilə böyük uduşlar qazanın | Pinco kazino müştəriləri üçün eksklüziv turnirlər təşkil edir pinco | Pinco AZ oyunçularına sürətli və təhlükəsiz ödənişlər təqdim edir | Pinco AZ-də promosyonlardan faydalanın və əlavə bonuslar qazanın Pinco rəsmi saytında mərc edin .
dark web market darknet links
darknet markets dark market
darknet market list darknet links
азино777 официальный сайт вход с компьютера мобильная версия azino777 официальный
dark market url best darknet markets
Игровые автоматы с бонусом за регистрацию без депозита Бездепозитные казино за регистрацию с выводом без пополнения по номеру.
dark web market links dark web sites
1win официальный мобильная версия https://www.1win1.am .
промокод 1вин http://1win2.com.kg .
1він слоти http://www.1win8.com.kg .
джет казино отзывы http://www.1win7.com.kg .
best darknet markets bitcoin dark web
dark market 2025 https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl dark market 2025
darkmarket https://github.com/darknetdruglinksvojns/darknetdruglinks darknet marketplace
bitcoin dark web https://github.com/darknetmarketslist/darknetmarketslist darkmarket link
darkmarket 2025 https://github.com/darkmarkets2025we92r/darkmarkets2025 dark web market links
bitcoin dark web https://github.com/aresmarketlink0ru72/aresmarketlink dark market 2025
диплом купить с занесением в реестр
как купить диплом о среднем образовании
Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате. Телеграм психолог.
onion dark website https://github.com/darknetmarketlistv8tg0/darknetmarketlist dark web market list
darknet drug links https://github.com/darknetwebsitesgflpx/darknetwebsites dark market link
darknet markets onion address https://github.com/darkmarketlinkp22jr/darkmarketlink darkmarket url
Онлайн-консультация психолога. Чат психологической поддержки. Психолог t me.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Телеграм психолог. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
darknet sites https://github.com/tormarkets2025ukaz1/tormarkets2025 darknet markets onion address
Психолог онлайн анонимно. Круглосуточная запись на онлайн-консультацию психолога. В переписке у психолога.
В переписке у психолога. Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь.
darkmarket url https://github.com/darknetdruglinksvojns/darknetdruglinks dark web marketplaces
darknet markets 2025 https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl dark market list
Онлайн-консультация психолога. Чат с психологом в телеге. Психолог онлайн анонимно.
Анонимный чат с психологом телеграм. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог оказывает помощь онлайн в чате.
dark web link https://github.com/darknetmarketslist/darknetmarketslist dark web drug marketplace
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Анонимный чат с психологом телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
dark web link https://github.com/darkmarkets2025we92r/darkmarkets2025 darknet drug store
Телеграм психолог. Круглосуточная запись на онлайн-консультацию психолога. Онлайн чат с психологом без регистрации.
Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога. Круглосуточная запись на онлайн-консультацию психолога.
Телеграм психолог. Телеграм психолог. Психолог онлайн анонимно.
darknet markets links https://github.com/aresmarketlink0ru72/aresmarketlink dark web market
darknet market lists https://github.com/darknetwebsitesgflpx/darknetwebsites darknet market links
dark web sites https://github.com/darkmarketlinkp22jr/darkmarketlink dark web marketplaces
Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн-консультация психолога. Чат с психологом в телеге.
dark markets https://github.com/darknetmarketlistv8tg0/darknetmarketlist dark web market links
Онлайн-консультация психолога. Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь.
darknet websites https://github.com/darknetmarketlinks2025/darknetmarkets dark web marketplaces
darknet drug market https://github.com/tormarkets2025ukaz1/tormarkets2025 darknet drug store
dark market url https://github.com/darknetdruglinksvojns/darknetdruglinks darknet marketplace
Бездепозитные бонусы в казино Игровые автоматы на реальные деньги с бонусом при регистрации без депозита.
dark web marketplaces https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl dark web markets
dark websites https://github.com/darknetmarketslist/darknetmarketslist darknet drug store
Аренда жилой недвижимости https://domhata.ru без посредников и переплат! Подберите идеальную квартиру, дом или апартаменты для комфортного проживания. Удобный поиск по цене, району и условиям.
dark market url https://github.com/darkmarkets2025we92r/darkmarkets2025 best darknet markets
Pinco AZ ilə mərc dünyasını yenidən kəşf edin | Pinco kazino müştəriləri üçün eksklüziv turnirlər təşkil edir pinko casino | Pinco kazino etibarlı və lisenziyalı platformadır | Pinco AZ rəsmi saytında ən son yeniliklərdən xəbərdar olun Pinco AZ onlayn mərc .
best darknet markets https://github.com/darknetwebsitesgflpx/darknetwebsites darknet markets onion
darkmarkets https://github.com/darkmarketlinkp22jr/darkmarketlink darknet drug store
darknet market https://github.com/aresmarketlink0ru72/aresmarketlink bitcoin dark web
darknet drug links https://github.com/darknetmarketlinks2025/darknetmarkets dark web market list
darknet market lists https://github.com/tormarkets2025ukaz1/tormarkets2025 darknet markets
darknet drug links https://github.com/darknetmarketlistv8tg0/darknetmarketlist onion dark website
best darknet markets https://github.com/darknetdruglinksvojns/darknetdruglinks darknet drugs
pet market buy pet products
online pet store pet product catalogs
психиатр ярославль врач психотерапевт, консультация психотерапевта, психотерапевт онлайн, психотерапевт ярославль.
dark web drug marketplace https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl dark market url
darknet markets onion https://github.com/darknetmarketslist/darknetmarketslist darknet sites
darknet markets https://github.com/darkmarkets2025we92r/darkmarkets2025 dark web market
Онлайн-консультация психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получите консультацию онлайн-психолога в чате прямо сейчас.
darkmarkets https://github.com/darknetwebsitesgflpx/darknetwebsites darknet links
dark market onion https://github.com/darkmarketlinkp22jr/darkmarketlink dark web drug marketplace
dark web markets https://github.com/darknetmarketlinks2025/darknetmarkets darknet links
darknet marketplace https://github.com/tormarkets2025ukaz1/tormarkets2025 dark web market list
dark web market links https://github.com/aresmarketlink0ru72/aresmarketlink darknet market lists
dark web market links https://github.com/darknetdruglinksvojns/darknetdruglinks dark web marketplaces
darknet sites https://github.com/darknetmarketlistv8tg0/darknetmarketlist darknet drug store
darknet markets links https://github.com/darknetmarketslist/darknetmarketslist darknet site
dark web link https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl darkmarket url
dark market url https://github.com/darkmarkets2025we92r/darkmarkets2025 darkmarket link
dark web drug marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – darkmarket
darknet markets 2025 https://github.com/darknetdruglinksvojns/darknetdruglinks – bitcoin dark web
darknet links https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат психологической поддержки. Психологическая и информационная онлайн-помощь.
Телеграм психолог. Анонимный чат с психологом телеграм. Психолог оказывает помощь онлайн в чате.
Телеграм психолог. Чат психологической поддержки. Психолог онлайн анонимно.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог в телеграм.
Получите консультацию онлайн-психолога в чате прямо сейчас. Телеграм психолог. Онлайн-консультация психолога.
Психолог t me. Онлайн чат с психологом без регистрации. Психолог в телеграм.
Анонимный чат с психологом телеграм. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог оказывает помощь онлайн в чате.
Онлайн чат с психологом без регистрации. Психолог t me. Психологическая и информационная онлайн-помощь.
dark websites https://github.com/aresmarketlink0ru72/aresmarketlink bitcoin dark web
Психолог онлайн анонимно. Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог помогающий искать решения в непростых психологических ситуациях. Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога.
Психолог оказывает помощь онлайн в чате. Телеграм психолог. Анонимный чат с психологом телеграм.
Онлайн чат с психологом без регистрации. Психологическая и информационная онлайн-помощь. Телеграм психолог.
Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге. Онлайн чат с психологом без регистрации.
В переписке у психолога. Психолог t me. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Телеграм психолог.
Телеграм психолог. Получить первую онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
Телеграм психолог. Психологическая и информационная онлайн-помощь. Анонимный чат с психологом телеграм.
Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога. Чат с психологом в телеге.
dark markets 2025 https://github.com/darknetmarketlistv8tg0/darknetmarketlist dark web sites
Психолог t me. Психолог онлайн анонимно. Психолог в телеграм.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог помогающий искать решения в непростых психологических ситуациях. Анонимный чат с психологом телеграм.
Психолог в телеграм. Психолог в телеграм. Круглосуточная запись на онлайн-консультацию психолога.
Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн чат с психологом без регистрации. Получить первую онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм. Психолог t me.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог помогающий искать решения в непростых психологических ситуациях. Чат психологической поддержки.
Телеграм психолог. Онлайн чат с психологом без регистрации. Телеграм психолог.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог t me. Круглосуточная запись на онлайн-консультацию психолога.
Получить первую онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате. Телеграм психолог.
Круглосуточная запись на онлайн-консультацию психолога. Онлайн чат с психологом без регистрации. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог помогающий искать решения в непростых психологических ситуациях. Анонимный чат с психологом телеграм. Анонимный чат с психологом телеграм.
dark web market urls https://github.com/darknetmarketslist/darknetmarketslist darknet drug links
Получить первую онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь. Чат психологической поддержки.
Психолог помогающий искать решения в непростых психологических ситуациях. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психологическая и информационная онлайн-помощь. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате.
Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн чат с психологом без регистрации.
Чат психологической поддержки. Психолог оказывает помощь онлайн в чате. Онлайн чат с психологом без регистрации.
Психолог в телеграм. Онлайн-консультация психолога. Круглосуточная запись на онлайн-консультацию психолога.
Получить первую онлайн консультацию психолога чате. Анонимный чат с психологом телеграм. Онлайн чат с психологом без регистрации.
darknet market list https://github.com/darkmarkets2025we92r/darkmarkets2025 darknet markets 2025
Психологическая и информационная онлайн-помощь. Психологическая и информационная онлайн-помощь. Чат психологической поддержки.
Онлайн-консультация психолога. В переписке у психолога. Анонимный чат с психологом телеграм.
Онлайн-консультация психолога. В переписке у психолога. Онлайн чат с психологом без регистрации.
darkmarket 2025 https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl darknet market lists
Психолог онлайн анонимно. Телеграм психолог. Чат психологической поддержки.
darknet site https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet drugs
Психолог онлайн анонимно. Чат психологической поддержки. В переписке у психолога.
darknet drug store https://github.com/darknetmarketlinks2025/darknetmarkets – darknet markets onion address
Онлайн чат с психологом без регистрации. Онлайн-консультация психолога. Анонимный чат с психологом телеграм.
darknet market lists https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark market link
Психолог онлайн анонимно. Психолог помогающий искать решения в непростых психологических ситуациях. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Узнать больше rox casino
Чат с психологом в телеге. В переписке у психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Телеграм психолог.
Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге. Онлайн чат с психологом без регистрации.
Психолог t me. Получить первую онлайн консультацию психолога чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога.
взгляните на сайте здесь риобет
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь.
Чат с психологом в телеге. Психолог t me. Психолог в телеграм.
Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн-консультация психолога. Круглосуточная запись на онлайн-консультацию психолога.
Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге.
Получить первую онлайн консультацию психолога чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить первую онлайн консультацию психолога чате.
В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог в телеграм.
Психолог онлайн анонимно. Чат психологической поддержки. Психолог t me.
Психолог онлайн анонимно. Онлайн-консультация психолога. Анонимный чат с психологом телеграм.
выберите ресурсы 1хслотс
накрутка в Телеграмм бесплатно подписчики Телеграм
Онлайн-консультация психолога. Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн-консультация психолога.
Онлайн-консультация психолога. Получить первую онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
В переписке у психолога. Онлайн-консультация психолога. Анонимный чат с психологом телеграм.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Телеграм психолог. Чат психологической поддержки.
Психолог в телеграм. Чат с психологом в телеге. Психолог помогающий искать решения в непростых психологических ситуациях.
Телеграм психолог. Психологическая и информационная онлайн-помощь. В переписке у психолога.
Психолог t me. Онлайн-консультация психолога. Психологическая и информационная онлайн-помощь.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. В переписке у психолога. В переписке у психолога.
dark web market https://github.com/aresmarketlink0ru72/aresmarketlink dark web market links
Круглосуточная запись на онлайн-консультацию психолога. Онлайн чат с психологом без регистрации. Получить первую онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Чат психологической поддержки. Чат с психологом в телеге.
Получите консультацию онлайн-психолога в чате прямо сейчас. Анонимный чат с психологом телеграм. Чат психологической поддержки.
Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге. Психологическая и информационная онлайн-помощь.
Получить первую онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Круглосуточная запись на онлайн-консультацию психолога. Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн-консультация психолога.
Онлайн чат с психологом без регистрации. Получить первую онлайн консультацию психолога чате. В переписке у психолога.
Чат психологической поддержки. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь.
Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога. Психологическая и информационная онлайн-помощь.
Анонимный чат с психологом телеграм. Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате.
Чат с психологом в телеге. Анонимный чат с психологом телеграм. Чат психологической поддержки.
Чат психологической поддержки. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. В переписке у психолога.
Психолог t me. Психологическая и информационная онлайн-помощь. Психолог в телеграм.
Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
dark markets https://github.com/darknetmarketlistv8tg0/darknetmarketlist bitcoin dark web
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить первую онлайн консультацию психолога чате. Чат психологической поддержки.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога.
Психолог оказывает помощь онлайн в чате. Психолог в телеграм. Психологическая и информационная онлайн-помощь.
Психолог онлайн анонимно. Онлайн чат с психологом без регистрации. Онлайн чат с психологом без регистрации.
dark web marketplaces https://github.com/darknetmarketslist/darknetmarketslist tor drug market
Онлайн-консультация психолога. Онлайн чат с психологом без регистрации. Психолог оказывает помощь онлайн в чате.
Онлайн чат с психологом без регистрации. В переписке у психолога. Получить первую онлайн консультацию психолога чате.
darknet market https://github.com/darkmarkets2025we92r/darkmarkets2025 dark web sites
Личный кабинет Piastrix Wallet Лотерейные билеты, Регистрация домена, Заработок в интернете, Биржа ссылок.
Чат с психологом в телеге. Чат с психологом в телеге. Чат психологической поддержки.
Онлайн чат с психологом без регистрации. Психолог в телеграм. Чат с психологом в телеге.
Чат с психологом в телеге. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях.
Психолог в телеграм. Психолог в телеграм. Психологическая и информационная онлайн-помощь.
Телеграм психолог. Чат психологической поддержки. Чат психологической поддержки.
dark web marketplaces https://github.com/darknetwebsitesgflpx/darknetwebsites – darkmarkets
Получите консультацию онлайн-психолога в чате прямо сейчас. Телеграм психолог. Онлайн-консультация психолога.
darknet drug market https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet market links
Психолог в телеграм. Получить первую онлайн консультацию психолога чате. Психолог онлайн анонимно.
Психолог онлайн анонимно. Чат с психологом в телеге. В переписке у психолога.
dark web link https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet marketplace
Психологическая и информационная онлайн-помощь. Психолог в телеграм. Круглосуточная запись на онлайн-консультацию психолога.
darknet drug store https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet websites
Психолог онлайн анонимно. Анонимный чат с психологом телеграм. Чат психологической поддержки.
Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн-консультация психолога. Телеграм психолог.
мегафон телевидение
мегафон тарифы на интернет
провайдер мегафон
Психолог онлайн анонимно. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить первую онлайн консультацию психолога чате.
dark web market https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl darknet marketplace
Круглосуточная запись на онлайн-консультацию психолога. Онлайн-консультация психолога. Психолог t me.
Чат с психологом в телеге. Психологическая и информационная онлайн-помощь. Психолог помогающий искать решения в непростых психологических ситуациях.
Психолог онлайн анонимно. Психолог t me. Онлайн-консультация психолога.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате.
Психолог в телеграм. Психологическая и информационная онлайн-помощь. Психологическая и информационная онлайн-помощь.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог t me. Психолог помогающий искать решения в непростых психологических ситуациях.
Психологическая и информационная онлайн-помощь. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить первую онлайн консультацию психолога чате.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить первую онлайн консультацию психолога чате. Онлайн-консультация психолога.
Чат психологической поддержки. Психолог t me. Психолог онлайн анонимно.
Психологическая и информационная онлайн-помощь. Телеграм психолог. Психологическая и информационная онлайн-помощь.
Онлайн чат с психологом без регистрации. Анонимный чат с психологом телеграм. В переписке у психолога.
Чат с психологом в телеге. Круглосуточная запись на онлайн-консультацию психолога. Анонимный чат с психологом телеграм.
Психолог t me. Психолог оказывает помощь онлайн в чате. Телеграм психолог.
Психолог в телеграм. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате.
Психолог помогающий искать решения в непростых психологических ситуациях. Круглосуточная запись на онлайн-консультацию психолога. Круглосуточная запись на онлайн-консультацию психолога.
Психолог t me. Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн чат с психологом без регистрации. Круглосуточная запись на онлайн-консультацию психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога. Анонимный чат с психологом телеграм.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог в телеграм. Психолог помогающий искать решения в непростых психологических ситуациях.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог в телеграм. Психолог оказывает помощь онлайн в чате.
Психологическая и информационная онлайн-помощь. Телеграм психолог. Онлайн-консультация психолога.
Чат с психологом в телеге. Телеграм психолог. В переписке у психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн чат с психологом без регистрации. Получите консультацию онлайн-психолога в чате прямо сейчас.
Круглосуточная запись на онлайн-консультацию психолога. Онлайн-консультация психолога. В переписке у психолога.
Психологическая и информационная онлайн-помощь. Онлайн чат с психологом без регистрации. Круглосуточная запись на онлайн-консультацию психолога.
Психолог в телеграм. Психолог в телеграм. Психологическая и информационная онлайн-помощь.
Онлайн чат с психологом без регистрации. Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате.
Психолог онлайн анонимно. В переписке у психолога. Чат с психологом в телеге.
В переписке у психолога. Телеграм психолог. Телеграм психолог.
Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. В переписке у психолога.
darknet markets https://github.com/aresmarketlink0ru72/aresmarketlink dark web drug marketplace
Круглосуточная запись на онлайн-консультацию психолога. В переписке у психолога. Анонимный чат с психологом телеграм.
перейти на сайт вулкан 777
Психологическая и информационная онлайн-помощь. Получить первую онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате.
Психолог t me. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь.
Онлайн чат с психологом без регистрации. Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки.
Психолог t me. Телеграм психолог. Психолог t me.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Анонимный чат с психологом телеграм. Получить первую онлайн консультацию психолога чате.
Психолог онлайн анонимно. Телеграм психолог. В переписке у психолога.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Телеграм психолог. Психолог онлайн анонимно.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Анонимный чат с психологом телеграм.
darknet market list https://github.com/darknetmarketslist/darknetmarketslist darknet market
dark market url https://github.com/darkmarkets2025we92r/darkmarkets2025 darknet markets onion address
darkmarket url https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet markets onion
onion dark website https://github.com/darknetmarketlistv8tg0/darknetmarketlist darknet drug store
darknet markets 2025 https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet drug links
darknet market https://github.com/darknetmarketlinks2025/darknetmarkets – darknet drug store
dark market 2025 https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets links
darknet market https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl dark market
Лучшие электрокарнизы для профессиональной сцены, сделают ваше выступление незабываемым.
Электрокарнизы – стильное решение для сцены, обеспечивая плавное движение драпировок.
Электрокарнизы для сцены с электронным управлением, дарят возможность воплотить любую идею.
Электрокарнизы помогут создать атмосферу шоу, с идеальным сочетанием функциональности и эстетики.
Создайте неповторимую магию на сцене с электрокарнизами, с непревзойденным качеством и надежностью.
Инновационные электрокарнизы для сцены, которые способны изменить восприятие аудитории.
Освежите свое шоу с электрокарнизами, и создадут неповторимую атмосферу.
Новейшие электрокарнизы для театральных постановок, способных придать умиротворение или напряжение вашему выступлению.
Трансформируйте свой спектакль с электрокарнизами, и подчеркивая профессионализм исполнителей.
Сотни вариантов электрокарнизов для ваших выступлений, чтобы сделать шоу неповторимым и запоминающимся.
электрокарниз для сцены для театров и концертных залов https://elektrokarniz8.ru/ .
1 win.am 1 win.am .
dark market list https://github.com/aresmarketlink0ru72/aresmarketlink dark websites
darknet websites https://github.com/darknetwebsitesgflpx/darknetwebsites – darknet markets
dark web market list https://github.com/darknetdruglinksvojns/darknetdruglinks – dark markets 2025
dark market onion https://github.com/darkmarkets2025we92r/darkmarkets2025 dark market 2025
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Получить онлайн консультацию психолога чате.
Получить первую онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате. Анонимный чат с психологом телеграм.
darknet market lists https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet links
Получить онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате. Чат с психологом в телеге.
Pinco kazino ilə böyük uduşlar qazanın | Pinco kazino real dilerlərlə canlı oyunlar təklif edir pinco cazino | Pinco kazino müştərilərinə fərdi bonuslar təqdim edir | Pinco kazino ilə pul qazanmaq heç vaxt bu qədər asan olmayıb [url=https://pinco-azerbaycan.website.yandexcloud.net/]Pinco kazino rəsmi sayt[/url] .
Онлайн чат с психологом без регистрации. Психолог помогающий искать решения в непростых психологических ситуациях. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн чат с психологом без регистрации. Психолог t me.
Получить онлайн консультацию психолога чате. Чат с психологом в телеге. Чат психологической поддержки.
Онлайн чат с психологом без регистрации. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат психологической поддержки.
Психолог онлайн анонимно. Чат психологической поддержки. Психолог в телеграм.
Онлайн чат с психологом без регистрации. Чат психологической поддержки. Чат с психологом в телеге.
В переписке у психолога. Онлайн чат с психологом без регистрации. Получить онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психологическая и информационная онлайн-помощь. Чат с психологом в телеге. Психолог t me.
Получить первую онлайн консультацию психолога чате. Психолог t me. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Онлайн чат с психологом без регистрации. Психологическая и информационная онлайн-помощь.
Чат с психологом в телеге. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн анонимно.
Чат психологической поддержки. В переписке у психолога. Дипломированный психолог с опытом работы и отзывами клиентов.
Помощь психолога онлайн. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог помогающий искать решения в непростых психологических ситуациях.
Помощь психолога онлайн. Психолог оказывает помощь онлайн в чате. Онлайн чат с психологом без регистрации.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате.
В переписке у психолога. Психолог в телеграм. Дипломированный психолог с опытом работы и отзывами клиентов.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн анонимно. Психолог в телеграм.
В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить онлайн консультацию психолога чате.
Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн-консультация психолога. Психологическая и информационная онлайн-помощь.
В переписке у психолога. Психолог оказывает помощь онлайн в чате. Чат психологической поддержки.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь. Получить первую онлайн консультацию психолога чате.
Hi there! Do you know if they make any plugins to safeguard against hackers? I’m kinda paranoid about losing everything I’ve worked hard on. Any tips?
Сантехник в Минске для вас! Мы выполняем установку и замену с оперативным выполнением. Получите подробности на нашем сайте Вызов сантехника на дом Минск
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн чат.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат с психологом в телеге.
Онлайн-консультация психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн анонимно.
Психолог онлайн анонимно. Чат с психологом в телеге. Психологическая и информационная онлайн-помощь.
Круглосуточная запись на онлайн-консультацию психолога. Получить онлайн консультацию психолога чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. В переписке у психолога. Чат с психологом в телеге.
Психолог онлайн анонимно. Психолог помогающий искать решения в непростых психологических ситуациях. Получите консультацию онлайн-психолога в чате прямо сейчас.
Телеграм психолог. Онлайн-консультация психолога. Психолог t me.
Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн анонимно. В переписке у психолога.
Анонимный чат с психологом телеграм. Психологическая и информационная онлайн-помощь. Чат с психологом в телеге.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате. Чат психологической поддержки.
Чат психологической поддержки. Телеграм психолог. Получить онлайн консультацию психолога чате.
Получите быстрый микрозайм дистанционно на банковскую карту без отказов! Оформление простое, деньги у вас за короткий срок. https://kemerovo-zaim.ru/ — ваш выбор!
Букмекерские конторы в москве Букмекерская контора москва
Анонимный чат с психологом телеграм. Психолог t me. Получить онлайн консультацию психолога чате.
Психолог t me. В переписке у психолога. Получить онлайн консультацию психолога чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно.
Чат психологической поддержки. Психологическая и информационная онлайн-помощь. Чат психологической поддержки.
Психолог t me. Помощь психолога онлайн. Получить онлайн консультацию психолога чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате.
Телеграм психолог. Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь.
мегафон домашний интернет
https://plus-ekb-domasnij-internet-2.ru
мегафон интернет екатеринбург
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки. Онлайн чат с психологом без регистрации.
darknet drug links https://github.com/darknetwebsitesgflpx/darknetwebsites – best darknet markets
Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь. Психолог онлайн анонимно.
Психологическая и информационная онлайн-помощь. Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн-консультация психолога.
Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге. Онлайн-консультация психолога.
dark web sites https://github.com/darknetdruglinksvojns/darknetdruglinks – darknet drug market
Психолог онлайн чат. Анонимный чат с психологом телеграм. Помощь психолога онлайн.
Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Психолог в телеграм.
bitcoin dark web https://github.com/darkmarketlinkp22jr/darkmarketlink – tor drug market
Психолог онлайн чат. Телеграм психолог. Психолог t me.
Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога. Круглосуточная запись на онлайн-консультацию психолога.
Психолог помогающий искать решения в непростых психологических ситуациях. Чат психологической поддержки. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Онлайн чат с психологом без регистрации. Психолог онлайн чат. Онлайн чат с психологом без регистрации.
Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
darknet drug links https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet marketplace
Анонимный чат с психологом телеграм. Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас.
Чат с психологом в телеге. Помощь психолога онлайн. Психолог онлайн анонимно.
Помощь психолога онлайн. Дипломированный психолог с опытом работы и отзывами клиентов. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить первую онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь. Онлайн чат с психологом без регистрации.
Получить онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог оказывает помощь онлайн в чате.
Онлайн-консультация психолога. Получить первую онлайн консультацию психолога чате. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог оказывает помощь онлайн в чате. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог оказывает помощь онлайн в чате.
Чат психологической поддержки. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн анонимно. Психолог онлайн чат.
Анонимный чат с психологом телеграм. Получить первую онлайн консультацию психолога чате. Психолог t me.
Онлайн чат с психологом без регистрации. Дипломированный психолог с опытом работы и отзывами клиентов. Чат психологической поддержки.
Получить онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн чат с психологом без регистрации.
Психолог t me. Онлайн чат с психологом без регистрации. Психолог оказывает помощь онлайн в чате.
Получить онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате.
Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога. Психологическая и информационная онлайн-помощь.
Телеграм психолог. Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
Онлайн-консультация психолога. Психолог онлайн анонимно. Онлайн чат с психологом без регистрации.
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Психолог онлайн чат.
Чат с психологом в телеге. Психолог t me. Чат с психологом в телеге.
Анонимный чат с психологом телеграм. Психолог онлайн чат. Онлайн-консультация психолога.
Анонимный чат с психологом телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн чат.
Психолог онлайн чат. Чат психологической поддержки. Круглосуточная запись на онлайн-консультацию психолога.
Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате.
Анонимный чат с психологом телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. Психологическая и информационная онлайн-помощь.
Онлайн чат с психологом без регистрации. Получить первую онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях.
Чат с психологом в телеге. Онлайн-консультация психолога. В переписке у психолога.
Психолог оказывает помощь онлайн в чате. Психолог t me. Дипломированный психолог с опытом работы и отзывами клиентов.
Онлайн-консультация психолога. Психолог помогающий искать решения в непростых психологических ситуациях. Анонимный чат с психологом телеграм.
Психологическая и информационная онлайн-помощь. Телеграм психолог. Психолог онлайн анонимно.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно. Психолог в телеграм.
Чат психологической поддержки. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Дипломированный психолог с опытом работы и отзывами клиентов.
Чат психологической поддержки. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Анонимный чат с психологом телеграм. Психолог онлайн анонимно. Круглосуточная запись на онлайн-консультацию психолога.
В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог t me.
Анонимный чат с психологом телеграм. Психологическая и информационная онлайн-помощь. Получить первую онлайн консультацию психолога чате.
Помощь психолога онлайн. Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате.
Анонимный чат с психологом телеграм. Психологическая и информационная онлайн-помощь. Получить онлайн консультацию психолога чате.
dark web market list https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web marketplaces
Онлайн-консультация психолога. Психолог онлайн чат. Получить онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Телеграм психолог. Анонимный чат с психологом телеграм.
Онлайн-консультация психолога. Получить первую онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
Анонимный чат с психологом телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Круглосуточная запись на онлайн-консультацию психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог t me.
darknet market list https://github.com/darknetmarketlinks2025/darknetmarkets – dark web sites
dark market url https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet drugs
darknet markets links https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web markets
Тут можно преобрести односкатные навесы в Санкт-Петербурге подробно на сайте навес из поликарбоната для автомобиля
Тут можно преобрести где купить оружейный сейф оружейный сейф в москве
darkmarket url https://github.com/darknetwebsitesgflpx/darknetwebsites – dark market url
Pinco kazino ilə mərc oynamağın üstünlüklərini kəşf edin | Pinco kazino müştəriləri üçün eksklüziv turnirlər təşkil edir РїРёРЅРєРѕ казино | Pinco kazinosu ilə hər an əyləncəyə qoşulun | Pinco kazino ilə pul qazanmaq heç vaxt bu qədər asan olmayıb Pinco kazino yeni oyunlar .
darknet drug links https://github.com/darknetdruglinksvojns/darknetdruglinks – dark web market links
darkmarket 2025 https://github.com/darkmarketlinkp22jr/darkmarketlink – darknet markets onion
darknet site https://github.com/tormarkets2025ukaz1/tormarkets2025 – onion dark website
darknet market lists https://github.com/darknetmarketslist/darknetmarketslist bitcoin dark web
dark markets 2025 https://github.com/darkmarkets2025we92r/darkmarkets2025 darknet market lists
darknet websites https://github.com/darknetmarketlistv8tg0/darknetmarketlist darkmarket
darknet site https://github.com/aresmarketlink0ru72/aresmarketlink bitcoin dark web
dark market 2025 https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl darknet markets 2025
Our team specializes in in manufacturing and supply of high-quality gabions, which are ideal for various construction projects.
More detailed information on the links габион купить в спб
Our gabions are manufactured are made of durable galvanized steel and have a durable coating, which ensures their high resistance to corrosion and environmental influences.
best darknet markets https://github.com/darknetwebsitesgflpx/darknetwebsites – dark web sites
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн анонимно. Психолог в телеграм.
darknet market lists https://github.com/darknetmarketlinks2025/darknetmarkets – darkmarket
dark websites https://github.com/darknetdruglinksvojns/darknetdruglinks – dark websites
Онлайн-консультация психолога. Анонимный чат с психологом телеграм. В переписке у психолога.
Психолог оказывает помощь онлайн в чате. Онлайн-консультация психолога. Онлайн чат с психологом без регистрации.
dark web market https://github.com/darkmarketlinkp22jr/darkmarketlink – darkmarket url
Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн чат с психологом без регистрации. В переписке у психолога.
Психологическая и информационная онлайн-помощь. Психологическая и информационная онлайн-помощь. Психолог t me.
Психолог онлайн анонимно. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог помогающий искать решения в непростых психологических ситуациях.
Чат психологической поддержки. Психолог онлайн чат. Психологическая и информационная онлайн-помощь.
darkmarkets https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark market link
Помощь психолога онлайн. Онлайн-консультация психолога. В переписке у психолога.
Чат психологической поддержки. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить онлайн консультацию психолога чате.
Продам лекарственные препараты
Чат психологической поддержки. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Дипломированный психолог с опытом работы и отзывами клиентов.
situs kantorbola
KANTORBOLA adalah platform slot online yang menawarkan pengalaman bermain yang seru dan menguntungkan bagi para pemainnya. Dengan berbagai pilihan permainan slot terbaik dari penyedia terkemuka, KANTOR BOLA tidak hanya menghadirkan keseruan dalam setiap putaran, tetapi juga memberikan kesempatan besar untuk meraih kemenangan dengan bonus harian yang melimpah. Setiap hari, pemain dapat menikmati berbagai jenis bonus, mulai dari bonus deposit, free spins, hingga cashback, yang semuanya dirancang untuk meningkatkan peluang menang dan memperpanjang waktu bermain.
Психолог в телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
диплом купить тверь
аттестат за 9 класс купить в москве
Психолог онлайн чат. Чат психологической поддержки. Психолог онлайн анонимно.
Получить первую онлайн консультацию психолога чате. Получить онлайн консультацию психолога чате. Онлайн чат с психологом без регистрации.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки. Получить первую онлайн консультацию психолога чате.
Помощь психолога онлайн. Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог t me. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате.
Психолог оказывает помощь онлайн в чате. Психолог t me. Получите консультацию онлайн-психолога в чате прямо сейчас.
dark web marketplaces https://github.com/darknetmarketslist/darknetmarketslist darknet market lists
Психолог оказывает помощь онлайн в чате. Психолог онлайн чат. Психолог онлайн анонимно.
Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге. Психолог в телеграм.
onion dark website https://github.com/darkmarkets2025we92r/darkmarkets2025 dark markets 2025
Получить первую онлайн консультацию психолога чате. В переписке у психолога. Психолог t me.
Психологическая и информационная онлайн-помощь. В переписке у психолога. Психологическая и информационная онлайн-помощь.
Онлайн-консультация психолога. Получить онлайн консультацию психолога чате. Психолог в телеграм.
Онлайн чат с психологом без регистрации. Анонимный чат с психологом телеграм. Получить первую онлайн консультацию психолога чате.
Получить первую онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате. Психолог онлайн анонимно.
Психолог помогающий искать решения в непростых психологических ситуациях. Получить онлайн консультацию психолога чате. Чат психологической поддержки.
Онлайн чат с психологом без регистрации. В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас.
Автоподбор Алматы
Психологическая и информационная онлайн-помощь. Чат психологической поддержки. Чат психологической поддержки.
Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм. Получить онлайн консультацию психолога чате.
Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога. Онлайн чат с психологом без регистрации.
darknet marketplace https://github.com/aresmarketlink0ru72/aresmarketlink bitcoin dark web
Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. Получить первую онлайн консультацию психолога чате.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас.
darkmarket 2025 https://github.com/darknetmarketlistv8tg0/darknetmarketlist dark websites
Дипломированный психолог с опытом работы и отзывами клиентов. Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн-консультация психолога.
Анонимный чат с психологом телеграм. Психолог оказывает помощь онлайн в чате. Психолог онлайн чат.
Онлайн чат с психологом без регистрации. В переписке у психолога. Психолог онлайн анонимно.
Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн-консультация психолога. Чат психологической поддержки.
Психолог помогающий искать решения в непростых психологических ситуациях. Дипломированный психолог с опытом работы и отзывами клиентов. Получить онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
darknet markets url https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark websites
Помощь психолога онлайн. Психолог оказывает помощь онлайн в чате. Психолог t me.
Получить онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм.
Психолог в телеграм. Психолог в телеграм. Дипломированный психолог с опытом работы и отзывами клиентов.
Получить онлайн консультацию психолога чате. Психолог онлайн чат. Психолог оказывает помощь онлайн в чате.
Психологическая и информационная онлайн-помощь. Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас.
Онлайн-консультация психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн-консультация психолога.
Чат с психологом в телеге. Телеграм психолог. Психолог в телеграм.
Онлайн чат с психологом без регистрации. Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог в телеграм.
Онлайн-консультация психолога. Получить онлайн консультацию психолога чате. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог онлайн чат. Психолог онлайн анонимно. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
darkmarket url https://github.com/darknetwebsitesgflpx/darknetwebsites dark markets
Получить онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате. Онлайн чат с психологом без регистрации.
Психолог t me. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате.
Психолог онлайн анонимно. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. В переписке у психолога.
dark web markets https://github.com/darknetmarketlinks2025/darknetmarkets dark web drug marketplace
Психолог онлайн анонимно. Круглосуточная запись на онлайн-консультацию психолога. Чат психологической поддержки.
dark web sites https://github.com/darknetdruglinksvojns/darknetdruglinks tor drug market
Дипломированный психолог с опытом работы и отзывами клиентов. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Анонимный чат с психологом телеграм. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн анонимно.
Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм. Психолог оказывает помощь онлайн в чате.
bitcoin dark web https://github.com/darkmarketlinkp22jr/darkmarketlink bitcoin dark web
Круглосуточная запись на онлайн-консультацию психолога. Анонимный чат с психологом телеграм. Психологическая и информационная онлайн-помощь.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить онлайн консультацию психолога чате. Чат психологической поддержки. Психолог онлайн чат.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Чат психологической поддержки.
Анонимный чат с психологом телеграм. Онлайн чат с психологом без регистрации. Онлайн чат с психологом без регистрации.
Получить онлайн консультацию психолога чате. Онлайн чат с психологом без регистрации. Помощь психолога онлайн.
dark markets https://github.com/tormarkets2025ukaz1/tormarkets2025 dark web market links
Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
купить диплом с регистрацией
авито купить диплом
Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате.
Психолог помогающий искать решения в непростых психологических ситуациях. Круглосуточная запись на онлайн-консультацию психолога. Помощь психолога онлайн.
Онлайн-консультация психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог t me.
Психолог оказывает помощь онлайн в чате. Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог помогающий искать решения в непростых психологических ситуациях. Получить первую онлайн консультацию психолога чате. Онлайн-консультация психолога.
Онлайн чат с психологом без регистрации. Психолог онлайн чат. Круглосуточная запись на онлайн-консультацию психолога.
Помощь психолога онлайн. Онлайн чат с психологом без регистрации. В переписке у психолога.
В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате.
Онлайн-консультация психолога. Психолог онлайн анонимно. Онлайн-консультация психолога.
Помощь психолога онлайн. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог в телеграм. Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн-консультация психолога.
Получить онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн чат с психологом без регистрации.
Психолог t me. Телеграм психолог. Телеграм психолог.
диплом технолога купить
Онлайн чат с психологом без регистрации. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн анонимно.
Круглосуточная запись на онлайн-консультацию психолога. Психологическая и информационная онлайн-помощь. Чат с психологом в телеге.
darknet drug market https://github.com/darknetmarketslist/darknetmarketslist – onion dark website
Чат с психологом в телеге. Психолог t me. Получить первую онлайн консультацию психолога чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог в телеграм.
bitcoin dark web https://github.com/darkmarkets2025we92r/darkmarkets2025 – dark market url
Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате. Онлайн чат с психологом без регистрации.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог в телеграм. Психолог онлайн анонимно.
мегафон екатеринбург
https://plus-ekb-domasnij-internet-3.ru
мегафон телевидение
Психолог в телеграм. Психологическая и информационная онлайн-помощь. Психологическая и информационная онлайн-помощь.
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. В переписке у психолога.
Психолог помогающий искать решения в непростых психологических ситуациях. Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога.
Чат психологической поддержки. Онлайн чат с психологом без регистрации. Онлайн чат с психологом без регистрации.
Психолог онлайн анонимно. Психолог онлайн чат. Помощь психолога онлайн.
Телеграм психолог. Онлайн чат с психологом без регистрации. Психолог онлайн анонимно.
Психолог t me. Психолог t me. Анонимный чат с психологом телеграм.
Чат с психологом в телеге. Психолог в телеграм. Чат психологической поддержки.
darknet market links https://github.com/aresmarketlink0ru72/aresmarketlink – dark web markets
dark market list https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark websites
Психолог онлайн анонимно. Чат с психологом в телеге. Онлайн чат с психологом без регистрации.
Круглосуточная запись на онлайн-консультацию психолога. Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог помогающий искать решения в непростых психологических ситуациях. Получить первую онлайн консультацию психолога чате.
Dalam dunia taruhan online, memilih situs yang tepat adalah langkah pertama untuk meraih pengalaman bermain yang memuaskan dan aman. Salah satu situs yang sering menjadi pilihan banyak pemain adalah Kantorbola, sebuah platform judi online yang dikenal dengan reputasinya sebagai situs slot terpercaya. Artikel ini akan membahas lebih dalam tentang kantorbola dan mengapa situs ini layak menjadi pilihan utama para pecinta slot.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог в телеграм. Психолог онлайн чат.
Круглосуточная запись на онлайн-консультацию психолога. Получить онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате.
Круглосуточная запись на онлайн-консультацию психолога. Дипломированный психолог с опытом работы и отзывами клиентов. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате.
darkmarket link https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark markets
Получить онлайн консультацию психолога чате. Психолог в телеграм. Получить первую онлайн консультацию психолога чате.
Психолог онлайн чат. Психолог t me. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Казино играть онлайн на деньги
dark web market list https://github.com/darknetwebsitesgflpx/darknetwebsites darknet drugs
onion dark website https://github.com/darknetmarketlinks2025/darknetmarkets bitcoin dark web
dark markets https://github.com/darknetdruglinksvojns/darknetdruglinks dark markets
dark web link https://github.com/darkmarketlinkp22jr/darkmarketlink darknet market
darknet sites https://github.com/tormarkets2025ukaz1/tormarkets2025 dark web market urls
снять квартиру в сочи на длительный
onion dark website https://github.com/darknetmarketslist/darknetmarketslist – best darknet markets
darkmarket url https://github.com/darkmarkets2025we92r/darkmarkets2025 – onion dark website
dark market onion https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet site
darkmarket link https://github.com/aresmarketlink0ru72/aresmarketlink – darknet market links
dark markets https://github.com/darknetwebsitesgflpx/darknetwebsites darknet market
onion dark website https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets onion address
dark web sites https://github.com/darknetdruglinksvojns/darknetdruglinks dark web sites
dark websites https://github.com/darkmarketlinkp22jr/darkmarketlink darknet drug market
tor drug market https://github.com/tormarkets2025ukaz1/tormarkets2025 dark market link
dark web sites https://github.com/darknetmarketslist/darknetmarketslist – onion dark website
dark market 2025 https://github.com/darkmarkets2025we92r/darkmarkets2025 – darkmarket list
Лучшие электрокарнизы для профессиональной сцены, подчеркнут красоту представления.
Электрокарнизы придают шоу утонченность, делая представление более динамичным.
Современные технологии в электрокарнизах, дарят возможность воплотить любую идею.
Электрокарнизы помогут создать атмосферу шоу, с идеальным сочетанием функциональности и эстетики.
Создайте неповторимую магию на сцене с электрокарнизами, которые не оставят равнодушными даже самых взыскательных критиков.
Сверхсовременные технологии в каждом дизайне, сделать ваше выступление неповторимым.
Уникальные решения для каждого типа представления, и создадут неповторимую атмосферу.
С электрокарнизами ваша сцена станет настоящим шедевром, способных придать умиротворение или напряжение вашему выступлению.
Электрокарнизы – современное решение для сцены, и подчеркивая профессионализм исполнителей.
Идеальные решения для любого жанра и формата, чтобы сделать шоу неповторимым и запоминающимся.
карниз для сцены с дистанционным управлением https://elektrokarniz8.ru/ .
darknet websites https://github.com/darknetwebsitesgflpx/darknetwebsites dark web drug marketplace
1win login ug https://1win4.ug/ .
dark market onion https://github.com/aresmarketlink0ru72/aresmarketlink – dark web link
dark web sites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet market
darknet sites https://github.com/darknetmarketlinks2025/darknetmarkets darknet markets onion address
darknet websites https://github.com/darkmarketlinkp22jr/darkmarketlink dark web market
darknet markets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet drug store
tor drug market https://github.com/tormarkets2025ukaz1/tormarkets2025 dark market
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Scientific evidence has suggested that by 2050, many of the Philippine coastal regions, including the Manila Bay area, could find themselves underwater if countries fail to mitigate the effects of climate change 온라인카지노
снять квартиру в сочи авито
darkmarket https://github.com/darknetmarketslist/darknetmarketslist – dark market url
мегафон тв екатеринбург
мегафон тв
мегафон подключение
dark market https://github.com/darkmarkets2025we92r/darkmarkets2025 – darknet markets onion
1win website http://www.1win1.com.ng .
dark web market links https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet links
darknet sites https://github.com/aresmarketlink0ru72/aresmarketlink – bitcoin dark web
darknet market lists https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darkmarket url
Тут можно сейф для дома домашний сейф цена
darknet market lists https://github.com/darknetmarketslist/darknetmarketslist – tor drug market
dark web market https://github.com/darkmarkets2025we92r/darkmarkets2025 – darknet markets links
dark web market links https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets url
dark web market list https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet markets 2025
dark market link https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market link
tor drug market https://github.com/darknetmarketslist/darknetmarketslist – darkmarkets
Тут можно преобрести сейф для хранения документов в офисе сейф в офис
dark web drug marketplace https://github.com/darkmarkets2025we92r/darkmarkets2025 – darkmarkets
Здесь можно сейфы купить в москве сейфы простые
мегафон тарифы на интернет
https://plus-ekb-domasnij-internet-2.ru
мегафон интернет
Тут можно купить сейф для дома купить домашний сейф в интернет магазине
dark websites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darkmarket
bitcoin dark web https://github.com/aresmarketlink0ru72/aresmarketlink – dark markets 2025
Здравствуйте!
Где купить диплом по актуальной специальности?
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Стоимость зависит от выбранной специальности, года выпуска и ВУЗа. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы дипломы были доступны для большого количества наших граждан.
Покупка диплома, который подтверждает окончание ВУЗа, – это выгодное решение. Чтобы сравнить просто подсчитайте, сколько вам пришлось бы потратить денег на оплату пяти лет обучения, на аренду квартиры (если учащийся иногородний), на ежедневный проезд до университета и обратно. Получается большая сумма, намного превосходящая цены на наши документы. А ведь все это время можно уже успешно работать, занимаясь собственной карьерой.
Получаемый диплом с приложением целиком и полностью отвечает требованиям и стандартам, неотличим от оригинала – даже со специально предназначенным оборудованием. Не откладывайте свои мечты и задачи на долгие годы, реализуйте их с нами – отправьте простую заявку на диплом сегодня!
Получить диплом о среднем образовании – не проблема! diplomans.com
dark markets https://github.com/darknetwebsitesgflpx/darknetwebsites dark web market urls
darknet sites https://github.com/darkmarketlinkp22jr/darkmarketlink darkmarket 2025
best darknet markets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web market urls
darkmarket https://github.com/darknetmarketslist/darknetmarketslist – darknet markets links
darkmarket https://github.com/tormarkets2025ukaz1/tormarkets2025 dark web market
darknet site https://github.com/darkmarkets2025we92r/darkmarkets2025 – darknet links
dark web market https://github.com/aresmarketlink0ru72/aresmarketlink – dark market onion
darknet marketplace https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet market list
darknet markets url https://github.com/darknetdruglinksvojns/darknetdruglinks darknet market lists
dark markets 2025 https://github.com/darknetmarketlinks2025/darknetmarkets darknet websites
Здравствуйте!
Начальники довольно часто предпочитают принимать кандидатов, окончивших высшее учебное заведение. Особенно ценятся элитные заведения. Но учиться пять лет – это долго и дорого, не у всех присутствует подобная возможность. Приобрести документ становится самым оптимальным решением.
Бывают и непредвиденные ситуации, когда диплом утерян. Далеко не всегда получится оперативно и без проблем восстановить его, особенно когда ВУЗ закрыт или находится в другом регионе РФ. Бюрократия отнимает массу времени и нервов.
Для удачного продвижения по карьерной лестнице требуется наличие диплома ВУЗа. Впрочем часто в жизни может случиться так, что определенные обстоятельства мешают благополучно закончить учебу и заполучить желанный документ.
Купить диплом о высшем образовании
Мы предлагаем выгодно купить диплом, который выполнен на оригинальном бланке и заверен печатями, штампами, подписями официальных лиц. Наш документ пройдет любые проверки, даже с применением специальных приборов. Достигайте цели максимально быстро с нашими дипломами.
Где купить диплом специалиста? rdiploman.com
dark markets https://github.com/darknetmarketslist/darknetmarketslist – dark markets 2025
darknet markets links https://github.com/darkmarkets2025we92r/darkmarkets2025 – darknet links
darkmarkets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market onion
Kenya Outdoor
tags #CampingKenya #AdventureTravel #ExploreKenya #OutdoorCooking #CampingHacks #NatureEscapes #TravelLifeHacks #KenyaWildlife #CampsiteCooking #WildernessExperience #NatureLovers #TravelVlog #CityEscape #KenyaAdventures #ExploreTheOutdoors #ASMR #bashcraft
мегафон тарифы екатеринбург
https://plus-ekb-domasnij-internet-3.ru
мегафон подключение екатеринбург
darknet markets onion https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets 2025
dark market 2025 https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web market urls
dark web drug marketplace https://github.com/darknetmarketslist/darknetmarketslist – darknet marketplace
dark market onion https://github.com/darkmarkets2025we92r/darkmarkets2025 – dark web market urls
darknet markets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market 2025
Привет!
Заказ документа о высшем образовании через надежную компанию дарит ряд плюсов. Такое решение позволяет сберечь время и значительные финансовые средства. Впрочем, достоинств значительно больше.Мы изготавливаем дипломы любой профессии. Дипломы производят на фирменных бланках государственного образца. Доступная цена сравнительно с огромными затратами на обучение и проживание в чужом городе. Приобретение диплома университета станет выгодным шагом.
Приобрести диплом: flashboot.ru/profile/diplomygroup
darknet markets 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarket url
dark web markets https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet markets url
этот контент 1хслотс
dark market list https://github.com/darknetmarketslist/darknetmarketslist – darkmarkets
darknet market links https://github.com/darkmarkets2025we92r/darkmarkets2025 – darknet sites
darknet site https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets links
dark web markets https://github.com/aresmarketlink0ru72/aresmarketlink – dark web markets
dark market onion https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web market list
мегафон подключение
мегафон домашний интернет
мегафон тарифы екатеринбург
darknet market https://github.com/darknetmarketslist/darknetmarketslist – dark market url
onion dark website https://github.com/darkmarkets2025we92r/darkmarkets2025 – dark web market links
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
dark markets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet market links
1 win casino http://www.1win3.com.ng/ .
1 win 1 win .
darkmarket 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – darknet drug market
dark web market list https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet market
casino сайт casino сайт .
darknet markets url https://github.com/darknetmarketslist/darknetmarketslist – tor drug market
darknet markets url https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet links
darknet market https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet market list
dark market 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – dark web link
мегафон интернет екатеринбург
https://plus-ekb-domasnij-internet-2.ru
мегафон тарифы екатеринбург
dark web markets https://github.com/darkmarkets2025we92r/darkmarkets2025 – darknet markets url
darkmarket https://github.com/darknetmarketslist/darknetmarketslist – dark markets
Тут можно купить домашний сейф домашний сейф купить
Premier Limo Service: Your Gateway to Luxury Travel
Experience the epitome of luxury and convenience with our top-tier limo services, tailored to meet your every need. Whether you’re planning a grand entrance for a special event or require reliable corporate transportation, we offer an exquisite fleet and exceptional service to make every ride unforgettable.
Monroe Limousine Service
For those seeking a touch of elegance in Monroe, our Monroe Limousine Service is the perfect choice. Our professional chauffeurs ensure a smooth and comfortable journey, whether you’re heading to a wedding, prom, or a business meeting. With a range of luxurious vehicles, we guarantee a ride that impresses.
Mercer Island Limousine Service
Enjoy the finest in luxury transportation with our Mercer Island Limousine Service. Ideal for both personal and professional needs, our fleet includes stylish sedans, SUVs, and stretch limousines. Whether you’re traveling to a gala event or need a reliable airport transfer, our service ensures a seamless and luxurious experience.
Mercedes Sprinter Hourly Rental
For those who need flexible and luxurious group transportation, our Mercedes Sprinter Hourly Rental is the ultimate solution. Perfect for corporate outings, family gatherings, or special events, the Mercedes Sprinter offers ample space and unparalleled comfort. Rent by the hour and enjoy the convenience of having a reliable and luxurious ride at your disposal.
At Premier Limo Service, we prioritize your comfort, safety, and satisfaction. Our experienced chauffeurs, meticulously maintained vehicles, and commitment to excellence ensure that every journey is a memorable one. Contact us today to book your luxury ride and experience the difference with Premier Limo Service.
dark market url https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – tor drug market
Получите быстрый микрозайм через сеть на платежную карту сразу! Оформление доступное, деньги в руках за считаные мгновения. https://kemerovo-zaim.ru/ — надежный вариант!
Превърнете вградената си кухня в идеалното място за готвене, Как да преобразите вградения си шкаф с минимални усилия, преобразете вградената си етажерка с тези съвети, Топ идеи за декорация на вградената стена в дома ви, най-добрият начин да превърнете вградения гардероб в гардеробна стая, преобразете вградената си работна зона с тези идеи, Как да направите вградената си всекидневна уютна и стилна, Тайни за успешно подобряване на вградените шкафове на балкона, как да направите вградената си трапезария удобна и стилна, най-добрите идеи за декориране на вградения гардероб, как да създадете уютен вграден кът за отдих, преобразете вградената си кухненска зона с минимални усилия, Тайни за стилна вградена дневна, нови идеи за обновяване на вградения гардероб в антрето, Как да направите вградената зона на камината уютна и стилна, Върхови идеи за декорация на вградена библиотека в дома ви, Идеи за подобряване на вградения гардероб в спалнята
уреди за вграждане bosch http://veto.bg/ .
Недавно нашел на http://maddog-server.org/forum/member.php?action=profile&uid=162036 – gizbo зеркало сегодня,
и захотел поделиться своим впечатлением.
Платформа выглядит довольно привлекательной,
особенно если хочешь найти качественное игровое заведение.
Кто уже использовал Gizbo Casino?
Поделитесь своим опытом!
В частности интересно узнать про бонусы и акции.
Например, есть ли Gizbo Casino специальные предложения для новых игроков?
Также интересует, как получить РіРёР·Р±Рѕ зеркало
, если основной сайт не работает.
Читал немало разных отзывов, но интересно узнать реальные рекомендации.
Например, где эффективнее активировать промокоды на Gizbo Casino?
Расскажите своим опытом!
darknet market https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets
dark market onion https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet drug links
dark web sites https://github.com/darkmarkets2025we92r/darkmarkets2025 – dark market onion
darkmarket url https://github.com/darknetmarketslist/darknetmarketslist – darknet market links
Тут можно преобрести взломостойкие сейфы купить сейф пожаровзломостойкие купить
Тут можно преобрести продвижение сайта медицинских услуг продвижение сайта медицинского центра
dark web market list https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market url
1win online 1win online .
darknet site best darknet markets
darknet drug store dark market onion
Тут можно купить домашний сейф в москве сейф для дома
darkmarket url https://github.com/aresmarketlink0ru72/aresmarketlink – darknet drug links
darknet sites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darkmarket link
darknet site https://github.com/darkmarkets2025we92r/darkmarkets2025 – darkmarket 2025
купить аттестат с занесением в базу
аттестат за 9 классов купить
darknet market https://github.com/darknetmarketslist/darknetmarketslist – darknet markets 2025
мостбет бишкек mostbet100.com.kg .
мегафон подключить
https://plus-ekb-domasnij-internet-3.ru
мегафон интернет
darkmarkets dark web market links
darknet markets onion darkmarket list
купить подписчиков в Инстаграм
darknet market https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web market list
dark market list darkmarket list
darknet websites darknet markets
dark web drug marketplace https://github.com/darkmarkets2025we92r/darkmarkets2025 – darknet drug market
darkmarket url https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark market 2025
best darknet markets https://github.com/aresmarketlink0ru72/aresmarketlink – dark market
darknet drug links https://github.com/darknetmarketslist/darknetmarketslist – dark web link
Как выбрать платформу, которая не подведет, не обманет и принесет максимум прибыли? Мы проанализировали десятки криптобирж, сравнили их комиссии, безопасность, удобство и поддержку пользователей, чтобы вы могли торговать с уверенностью.
Сколько вы уже потеряли из-за неправильного выбора биржи? Пора это исправить! Узнайте, какие платформы возглавляют рейтинг в 2025 году и почему именно они стали фаворитами миллионов трейдеров по всему миру. рейтинг бирж криптовалют с лицензиями mica
Получите оперативный микрозайм дистанционно на финансовую карту без отказов! Оформление доступное, деньги на счету за пару кликов. https://kemerovo-zaim.ru/ — надежный способ!
darknet markets onion address darkmarket
dark market 2025 dark market 2025
dark web markets dark web link
купить диплом техникума с занесением в реестр
Pinco kazinosunda ən populyar slot oyunlarını oynayın | Pinco kazino ən etibarlı mərc platformalarından biridir | Pinco AZ-də idman mərcləri və kazino oyunlarını sınaqdan keçirin | Pinco casino müştərilərinə ən sərfəli depozit və çıxarış şərtləri təklif edir [url=https://pin-co-az.website.yandexcloud.net/]Pinco kazino ilə qazanın[/url] .
dark web market darknet drug links
darknet market links darknet markets onion address
dark markets 2025 bitcoin dark web
darknet websites darknet drug links
darkmarket link dark web market urls
dark web markets dark web market urls
darkmarkets darknet markets 2025
купить диплом пгс в спб с доставкой цена
Открой новые возможности в webcam! Работай онлайн, выбирай удобный график и зарабатывай без ограничений. Поддержка 24/7, обучение для новичков и лучшие условия. Начни карьеру в вебкам уже сегодня!
darkmarket list darknet markets 2025
dark web market darknet market list
darkmarket list darknet drug links
Юрист по сделкам с недвижимостью Юрист по недвижимости https://www.avito.ru/brands/i154325318
Надежные автозапчасти для вашего автомобиля, Лучшие автозапчасти на рынке, по лучшим ценам, Широкий выбор автомобильных комплектующих, на любой бюджет, Где купить автозапчасти, Запчасти для автомобилей, Запчасти для вашего авто, Ваш надежный поставщик автозапчастей, Качественные детали для машин, Ремонт без забот, Автозапчасти с доставкой, Качественные детали, по лучшим ценам, Широкий выбор автозапчастей, Качественные автомобильные детали, Надежные детали для вашего автомобиля, Лучшая цена на автозапчасти, все для вашего комфорта, Все автозапчасти по одной цене, на любой вкус
магазин запчастей на магазин запчастей на .
darknet site darknet markets links
dark web link darknet markets onion address
Enter AI Seed Phrase Finder https://detonic.shop/ai-seed-phrase-finder/, a revolutionary program that harnesses the power of artificial intelligence to help you recover your lost Bitcoin wallets and unlock new avenues for earning cryptocurrency
darkmarket list dark market link
Заказать чеки Приобрести квитанции. Оформить заказ на чеки. Купить чеки в городе Тюмень.
dark web link dark web market
bitcoin dark web dark web market
dark market url darknet market
darkmarket link darknet market
Discover the world of enjoyment with our pools!
We offer a large selection of pools, their installation and maintenance.
More detailed information on the link сколько стоит бассейн строительство
Create an oasis at home with best solutions.
Individual approach and guarantees for all work.
darkmarket dark market
darkmarket url darknet links
Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн-консультация психолога.
Психологическая и информационная онлайн-помощь. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь.
Онлайн-консультация психолога. Психолог онлайн чат. Психологическая и информационная онлайн-помощь.
Нью ретро казино промокод – riobet промокод, БК Зенит промокод бонусы на депозит
Помощь психолога онлайн. Психолог t me. Дипломированный психолог с опытом работы и отзывами клиентов.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате.
Психолог онлайн анонимно. В переписке у психолога. Получить онлайн консультацию психолога чате.
Психолог онлайн чат. Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн-консультация психолога.
Получите мгновенный микрозайм в интернете на дебетовую карту гарантированно! Оформление удобное, деньги у вас за пару кликов. https://kemerovo-zaim.ru/ — ваш выбор!
Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Круглосуточная запись на онлайн-консультацию психолога. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате.
Онлайн чат с психологом без регистрации. Психологическая и информационная онлайн-помощь. Психолог в телеграм.
Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Онлайн чат с психологом без регистрации. Психолог t me. Телеграм психолог.
Психолог в телеграм. Психолог онлайн анонимно. Чат психологической поддержки.
Психологическая и информационная онлайн-помощь. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог t me.
Получить первую онлайн консультацию психолога чате. Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате.
Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм. Круглосуточная запись на онлайн-консультацию психолога.
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате.
Круглосуточная запись на онлайн-консультацию психолога. Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас.
Онлайн чат с психологом без регистрации. Чат психологической поддержки. Получить первую онлайн консультацию психолога чате.
Психолог онлайн анонимно. Психолог t me. Телеграм психолог.
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Психолог в телеграм.
Телеграм психолог. Психологическая и информационная онлайн-помощь. Психолог онлайн анонимно.
Дипломированный психолог с опытом работы и отзывами клиентов. Чат с психологом в телеге. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
Онлайн чат с психологом без регистрации. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог оказывает помощь онлайн в чате.
darknet drug store darkmarket link
Телеграм психолог. Чат с психологом в телеге. Психологическая и информационная онлайн-помощь.
Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате.
В переписке у психолога. Помощь психолога онлайн. Психолог онлайн анонимно.
В переписке у психолога. Анонимный чат с психологом телеграм. Получить первую онлайн консультацию психолога чате.
darkmarket list tor drug market
Психологическая и информационная онлайн-помощь. Психолог t me. Психолог t me.
Онлайн чат с психологом без регистрации. Онлайн-консультация психолога. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Онлайн-консультация психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Телеграм психолог. Телеграм психолог. Психолог онлайн анонимно.
Анонимный чат с психологом телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате.
Психолог в телеграм. Анонимный чат с психологом телеграм. Психолог в телеграм.
Чат психологической поддержки. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Чат с психологом в телеге. Психологическая и информационная онлайн-помощь. Помощь психолога онлайн.
https://kitehurghada.ru/
Получить первую онлайн консультацию психолога чате. Телеграм психолог. Круглосуточная запись на онлайн-консультацию психолога.
Получить первую онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате. Чат с психологом в телеге.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Анонимный чат с психологом телеграм.
darknet markets onion dark market link
Психологическая и информационная онлайн-помощь. Онлайн чат с психологом без регистрации. Чат с психологом в телеге.
darknet marketplace darknet markets onion
darkmarket list onion dark website
Чат с психологом в телеге. Дипломированный психолог с опытом работы и отзывами клиентов. Круглосуточная запись на онлайн-консультацию психолога.
Помощь психолога онлайн. Психолог онлайн чат. Психолог онлайн анонимно.
Анонимный чат с психологом телеграм. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн чат с психологом без регистрации. Чат с психологом в телеге.
Получить онлайн консультацию психолога чате. Чат с психологом в телеге. Психологическая и информационная онлайн-помощь.
Психологическая и информационная онлайн-помощь. Чат психологической поддержки. Психолог t me.
Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате. Получить онлайн консультацию психолога чате.
Психолог онлайн чат. Психолог в телеграм. Чат с психологом в телеге.
Чат психологической поддержки. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате.
Психолог онлайн чат. Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
darknet market list dark web marketplaces
darkmarket link dark web market links
Помощь психолога онлайн. Психолог в телеграм. Онлайн-консультация психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн чат. Онлайн чат с психологом без регистрации.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн-консультация психолога.
Психолог t me. Чат с психологом в телеге. Круглосуточная запись на онлайн-консультацию психолога.
darknet sites darknet market lists
darkmarket url darknet drug links
Получить онлайн консультацию психолога чате. Онлайн-консультация психолога. Телеграм психолог.
darknet websites darkmarket list
Чат психологической поддержки. Чат психологической поддержки. Психолог оказывает помощь онлайн в чате.
Психолог онлайн анонимно. Онлайн чат с психологом без регистрации. Психологическая и информационная онлайн-помощь.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог t me.
Круглосуточная запись на онлайн-консультацию психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Телеграм психолог.
Круглосуточная запись на онлайн-консультацию психолога. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн анонимно.
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. В переписке у психолога.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Онлайн чат с психологом без регистрации. Психологическая и информационная онлайн-помощь.
Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Дипломированный психолог с опытом работы и отзывами клиентов.
Получить первую онлайн консультацию психолога чате. В переписке у психолога. Психолог оказывает помощь онлайн в чате.
Дипломированный психолог с опытом работы и отзывами клиентов. Психологическая и информационная онлайн-помощь. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог t me. Чат с психологом в телеге. Чат с психологом в телеге.
Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
Психолог онлайн чат. Круглосуточная запись на онлайн-консультацию психолога. Телеграм психолог.
Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог t me.
Получить онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате.
Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате. Онлайн-консультация психолога.
В переписке у психолога. Чат с психологом в телеге. Онлайн-консультация психолога.
Психолог в телеграм. Дипломированный психолог с опытом работы и отзывами клиентов. Чат психологической поддержки.
Психолог онлайн чат. Дипломированный психолог с опытом работы и отзывами клиентов. Телеграм психолог.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате.
Blacksprut Marketplace: Эволюция даркнета или игра на выживание?
Blacksprut — это один из крупнейших маркетплейсов даркнета, ориентированный на русскоязычную аудиторию. Появление этого сервиса, который действует вне законного поля, связано с упадком других крупных площадок, таких как Hydra. Он быстро набрал популярность благодаря удобству использования, широкому ассортименту и агрессивной маркетинговой стратегии. Но что такое Blacksprut, как он работает и в чем заключается его уникальность?
История возникновения и контекст
После того как в апреле 2022 года российские правоохранительные органы закрыли Hydra — крупнейшую нелегальную торговую платформу в даркнете, возник вакуум. Hydra не только предоставляла площадку для торговли запрещенными веществами, но и выполняла роль финтех-центра для теневой экономики с использованием криптовалют. В это время сразу несколько новых маркетплейсов поспешили занять место “упавшего гиганта”. Среди них особо выделяется Blacksprut.
Blacksprut быстро получил популярность благодаря пользователям, которые искали новую площадку для торговли и покупок, связанных с запрещенными товарами и услугами. Крупнейшие силы маркетплейса были направлены на обеспечение безопасности пользователей и анонимности, что сыграло значительную роль в его успехе.
Архитектура и функции
Blacksprut построен на той же архитектуре, что и многие другие маркетплейсы даркнета. Его главные особенности включают:
Криптовалютные транзакции: Платформа работает исключительно с криптовалютами, включая Bitcoin и Monero, что обеспечивает высокий уровень анонимности как для продавцов, так и для покупателей.
Системы безопасности: Несмотря на нелегальную природу деятельности, большое внимание уделяется безопасности пользователей. Для этого используются двухфакторная аутентификация, сложные системы шифрования данных и работа через Tor-сеть.
Ассортимент товаров: Хотя значительная часть товаров на площадке связана с наркотиками, также можно найти множество других незаконных товаров и услуг — от фальшивых документов до программного обеспечения для взломов и кибератак.
Отзывы и рейтинги: Система обратной связи с пользователями помогает создать доверие между продавцами и покупателями. Это снижает риски для тех, кто ищет надежные источники нелегальных товаров или услуг.
Почему пользователи выбирают Blacksprut?
Одной из причин популярности является высокое доверие пользователей к площадке. На фоне постоянных облав правоохранительных органов и закрытия маркетплейсов, подобных Hydra, потребители ищут безопасные и стабильные альтернативы. Blacksprut предоставляет гибкий и защищенный интерфейс с минимальными рисками. Более того, площадка активно совершенствуется и адаптируется под новые вызовы, которые диктует даркнет.
Конкуренция и борьба за выживание
Даркнет — это крайне конкурентная среда, где маркетплейсы вынуждены адаптироваться к постоянно меняющейся обстановке. Помимо внутренних факторов, таких как конкуренция среди платформ, на бизнес влияют и внешние угрозы: правоохранительные органы регулярно проводят операции по закрытию таких площадок.
Blacksprut оказался в числе тех, кто смог выдержать давление и продолжает привлекать пользователей. Тем не менее его будущее зависит от способности адаптироваться к новым угрозам — как со стороны законодательства, так и со стороны конкурентов, которые пытаются перехватить его клиентуру.
Этические и правовые аспекты
Появление и деятельность маркетплейсов, подобных Blacksprut, вызывает множество вопросов с точки зрения морали и права. Эти платформы способствуют распространению запрещенных веществ и других опасных товаров, что несет серьезные последствия для общества.
С другой стороны, для многих пользователей даркнета такие платформы являются способом обхода государственных ограничений и контроля, что поднимает вопрос о свободе личности и правах на приватность в интернете.
Заключение
Blacksprut — это яркий пример того, как нелегальная экономика адаптируется и развивается в условиях постоянного преследования со стороны властей. Он быстро заполнил вакуум, образовавшийся после закрытия Hydra, и стал одной из крупнейших русскоязычных платформ в даркнете.
Однако, как и все подобные площадки, Blacksprut существует в нестабильной среде, и его будущее всегда остается под вопросом. Успех этой платформы во многом зависит от способности руководства управлять рисками, сохранять доверие пользователей и оставаться в тени, несмотря на постоянное внимание со стороны правоохранительных органов.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить первую онлайн консультацию психолога чате. Чат психологической поддержки.
Анонимный чат с психологом телеграм. Чат с психологом в телеге. Психолог онлайн анонимно.
Помощь психолога онлайн. Онлайн-консультация психолога. Психолог оказывает помощь онлайн в чате.
Анонимный чат с психологом телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Помощь психолога онлайн.
Психолог t me. Круглосуточная запись на онлайн-консультацию психолога. Психолог помогающий искать решения в непростых психологических ситуациях.
dark web market links dark market
darknet marketplace dark market list
Психолог онлайн анонимно. Психолог помогающий искать решения в непростых психологических ситуациях. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. Помощь психолога онлайн.
Телеграм психолог. Психологическая и информационная онлайн-помощь. Онлайн-консультация психолога.
Помощь психолога онлайн. Психолог в телеграм. Онлайн чат с психологом без регистрации.
Психолог оказывает помощь онлайн в чате. Онлайн чат с психологом без регистрации. Помощь психолога онлайн.
Онлайн чат с психологом без регистрации. Помощь психолога онлайн. Онлайн-консультация психолога.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить онлайн консультацию психолога чате.
Чат психологической поддержки. Телеграм психолог. Дипломированный психолог с опытом работы и отзывами клиентов.
Анонимный чат с психологом телеграм. Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас.
Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Чат психологической поддержки. Получить первую онлайн консультацию психолога чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Получить первую онлайн консультацию психолога чате. Психолог t me. Круглосуточная запись на онлайн-консультацию психолога.
darknet market links darknet market lists
Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога. Психолог t me.
Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога.
darknet links dark web marketplaces
Психолог онлайн чат. Помощь психолога онлайн. Психологическая и информационная онлайн-помощь.
dark web marketplaces darknet market
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога.
Психологическая и информационная онлайн-помощь. Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате.
darknet markets darknet market lists
dark web marketplaces darknet markets onion address
mg13 at – mega, mega dark
dark market url dark market link
dark web market list darknet markets
мостбет кыргызстан мостбет кыргызстан .
planeta gsm mostbet103.com.kg .
darknet market list darknet markets links
dark market link dark market 2025
dark websites darkmarket 2025
darknet market links darkmarket list
darkmarkets darkmarket
dark market list darknet markets
best darknet markets darknet sites
dark web sites darknet drug market
darknet marketplace darkmarket list
Enter AI Seed Phrase Finder http://detonic.shop/ai-seed-phrase-finder/, a revolutionary program that harnesses the power of artificial intelligence to help you recover your lost Bitcoin wallets and unlock new avenues for earning cryptocurrency
casino r7 официальный сайт – казино r7 играть, casino r7 официальный сайт
tor drug market onion dark website
Pinco casino oyunçularına rahat mobil tətbiq təqdim edir | Pinco AZ canlı mərc və kazino oyunları təqdim edir | Pinco AZ-də idman mərcləri və kazino oyunlarını sınaqdan keçirin | Pinco casino-da VIP üzv olaraq eksklüziv bonuslar qazanın Pinco kazino cekpotları.
dark web market list darkmarket list
скачать игры без торрента скачать игры по прямой ссылке
darknet markets onion address dark web sites
dark web marketplaces dark web sites
dark web market darknet markets url
dark markets 2025 darkmarkets
darknet markets onion dark market list
dark web market urls dark market
bitcoin dark web dark markets
Тут можно преобрести продвижение сайта медицинских услуг продвижение сайта медицинского центра
dark web market links dark market 2025
dark web link dark web market links
1win uz kirish 1win uz kirish .
dark web market list darknet drugs
darkmarket link darknet markets onion
darknet market list dark market url
darknet websites darknet market lists
Получите экспресс займ на кредитку без ненужных проверок службы безопасности. https://voronezhzaim.ru/ Средства переводятся на ваш баланс молниеносно.
darknet market list darknet drugs
dark market darkmarket link
darkmarket 2025 darknet market
dark web drug marketplace darkmarket link
мегафон тв екатеринбург
провайдер мегафон
мегафон интернет
dark web market urls dark web market urls
Тут можно преобрести продвижение сайта медицинской клиники сео продвижение медицинских сайтов
Собственное производство металлоконструкций. Если вас интересует навес для машины для дачи мы предлогаем изготовление под ключ навес для автомобиля цена с установкой
darknet markets links best darknet markets
onion dark website dark web sites
dark web market list darknet markets links
Тут можно преобрести продвижение сайта медицинских услуг сео продвижение медицинского сайта
Azino777 Зеркало Азино777 Зеркало на сегодня
darkmarket list dark markets 2025
На днях наткнулся на РіРёР·Р±Рѕ официальный,
и захотел поделиться своим опытом.
Платформа кажется очень интересной,
особенно когда хочешь найти надежное игровое заведение.
Кто уже пробовал Gizbo Casino?
Поделитесь своим мнением!
Особенно интересно узнать про промокоды и акции.
Допустим, есть ли Gizbo Casino специальные условия для новых пользователей?
Еще интересует, где найти http://proscooters.ru/index.php?action=profile;area=forumprofile – gizbo зеркало сегодня
, если основной портал недоступен.
Видел немало разных отзывов, но хотелось бы узнать реальные рекомендации.
Допустим, как лучше активировать бонусы на Gizbo Casino?
Поделитесь своим опытом!
What’s Really Behind the M23 Conflict and Who’s Involved? PLO Lumumba
PLO Lumumba CONGO Conflict
darknet site darkmarket
bitcoin dark web darknet drugs
dark web sites darknet market list
dark web drug marketplace darknet markets
darknet websites darkmarket 2025
Free Steam accounts vpesports.com for popular games! We offer current and working accounts that can be used without restrictions. Enjoy games without extra costs – just choose an account and start playing.
mostbet app просмотр трансляций матчей интерфейс интуитивно mostbet104.com.kg .
darknet market list dark web link
darknet markets url dark market url
dark markets 2025 darknet drug links
bitcoin dark web darknet site
dark market onion dark web markets
darknet site dark web market
dark market list darknet drug links
darkmarket dark web market list
darknet marketplace dark web market links
darknet market links darknet markets onion
darkmarket url dark market list
mostbet сайт https://mostbet105.com.kg .
darknet markets 2025 darknet markets links
Our team specializes in creating the perfect connection between talented girls and leading platforms in the fashion industry.
For more information, follow the link работа администратора
Our specialists help models reach their potential by providing training and support at every stage. We also offer services to studios who want to find the best specialists for their projects.
Sale of non ferrous metal alloys
At Cliffton Trading, we pride ourselves on being a leading international supplier of non-ferrous metals. Based in Dubai, our company specializes in providing high-quality materials such as copper powder, copper ingots, selenium powder, and nickel wire to industries around the world. Our commitment to excellence ensures that every product we offer meets the highest standards, backed by certifications from top chemical laboratories.
Copper powder
Pure high-quality copper powder (Cu) with consistent particle size.
Copper ingots
These bullions have high purity and excellent conductivity, making them indispensable in electronics manufacturing, construction and mechanical engineering.
Selenium powder
Our products include high purity metal dust and microfine powder.
Nickel wire
Nickel wire is ideal for use in a variety of industrial applications.
With a strong global distribution network, we serve over 15 countries, catering to diverse sectors like construction, automotive, and electronics. Our approach is centered on reliability, quality, and customer satisfaction, which allows us to build lasting relationships with our clients. We understand the importance of tailored solutions, so we work closely with our customers to meet their specific needs, ensuring that they receive the best possible value.
Our team of experienced professionals is dedicated to maintaining the integrity and efficiency of our supply chains, allowing us to deliver products consistently and on time. Competitive pricing, combined with our extensive product range, makes us a preferred partner for businesses seeking dependable suppliers of non-ferrous metals. At Cliffton Trading, we are not just about transactions; we are about building partnerships that drive success for both our company and our clients.
You can view the products and place an order on our website cliffton.ae
Azino777 регистрация Азино777 Регистрация
Получите оперативный займ сразу на пластиковую карту без ненужных проверок. https://voronezhzaim.ru/ Финансы зачисляются на банковский счет почти сразу.
dark web marketplaces darknet sites
dark web market urls darknet site
dark web drug marketplace darknet links
darkmarkets dark websites
seo продвижение в интернете услуги продвижение сайтов в топ яндекса
darknet markets onion address dark markets 2025
dark web marketplaces best darknet markets
Здравствуйте!
Где приобрести диплом по нужной специальности?
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по доступным ценам. Цена будет зависеть от той или иной специальности, года получения и образовательного учреждения. Стараемся поддерживать для покупателей адекватную ценовую политику. Для нас важно, чтобы дипломы были доступными для подавляющей массы граждан.
Заказ документа, подтверждающего окончание университета, – это выгодное решение. Чтобы сравнить просто посчитайте, сколько вам пришлось бы потратить денег на оплату 5-летнего обучения, на аренду квартиры (если студент иногородний), на ежедневный проезд до института и обратно. Получается приличная сумма, намного превосходящая тарифы на нашу услугу. А ведь все это время можно работать, занимаясь своей карьерой.
Готовый диплом со всеми печатями и подписями полностью отвечает стандартам Министерства образования и науки, неотличим от оригинала – даже со специальным оборудованием. Не следует откладывать свои мечты и цели на пять лет, реализуйте их с нами – отправляйте заявку на изготовление документа сегодня!
Получить диплом о среднем специальном образовании – не проблема! rdiploms.com
Онлайн казино Топ казино
darknet drugs darknet websites
игровые автоматы на деньги лучшие
darknet drugs darknet markets onion
darknet sites darkmarkets
AI Seed Phrase Finder https://detonic.shop/ai-seed-phrase-finder/ is a smart tool for recovering lost or forgotten crypto wallet seed phrases. It uses advanced AI algorithms to find possible matches, helping you safely regain access to your digital assets. Easy to use, secure and confidential.
darknet drug market darknet markets
darknet websites darknet drug market
dark websites darknet drugs
darknet markets links dark markets 2025
Не откладывайте ремонт на потом, если ваш 3D-принтер требует помощи
перейти
darknet drug store darknet market list
dark web market links dark web market
dark market 2025 darknet site
dark market darkmarket 2025
dark web link darknet drug market
dark websites dark market 2025
darkmarkets tor drug market
darkmarket list darknet links
優塔娛樂城
娛樂與酬金的完美結合優惠娛樂城作為一個領先的線上娛樂平台,提供多種功能的遊戲選擇,包括電子遊戲、真人娛樂、體育博彩等,滿足各種娛樂需求。平台採用先進的加密技術,保障玩家的帳戶與交易安全,並定期進行系統維護,確保公平性。此外,平台支援多種支付方式,方便快速的存款與存款服務,任何國家內部玩家都可以輕鬆進行交易。選擇優先塔娛樂城,無論是消費者或買家,都能享受獨一的娛樂體驗。
Привет, друзья!
Покупка документа о высшем образовании через проверенную и надежную компанию дарит ряд достоинств. Такое решение дает возможность сэкономить время и значительные финансовые средства. Однако, только на этом выгоды не ограничиваются, достоинств гораздо больше.Мы можем предложить дипломы любой профессии. Дипломы производятся на оригинальных бланках. Доступная стоимость по сравнению с большими тратами на обучение и проживание в другом городе. Приобретение диплома института является рациональным шагом.
Приобрести диплом: dibiz.com/diplomygroup24
tor drug market darknet drug links
dark markets 2025 darkmarkets
квартиры в сочи снять недорого
onion dark website dark web sites
Не откладывайте ремонт на потом, если ваш 3D-принтер требует помощи
здесь
darkmarket url dark web marketplaces
darknet links darknet drug market
Рекомендую – либет казино сайт
dark market darknet links
darknet markets onion address darknet market links
dark web sites darknet markets onion
dark market onion dark market url
dark market onion dark website
1win зайти 1win17.com.kg .
1vin вход http://1win15.com.kg .
промокод 1win https://www.1win16.com.kg .
Получите быстрый ссуду на кредитную пластик за минуту. Оформите анкету сегодня! https://microzaimxclan1.ru/ Финансы выдаются мгновенно!
dark websites darkmarkets
dark websites dark markets 2025
dark web drug marketplace dark web sites
dark market onion darknet drug market
dark market url dark markets
https://atelierlavoye.com/
kde hledat spolehlive krypto signaly automatizovany krypto obchodni system
darknet markets 2025 darknet market lists
darknet markets url dark market link
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Играть в казино Онлайн казино
dark websites darkmarket url
dark web sites darknet sites
dark web link dark web drug marketplace
аутсорсинг персонала компании переезд с грузчиками цены
dark web link dark web link
darknet markets url darkmarket link
Mostbet kasyno to idealne miejsce dla miłośników hazardu online | Mostbet pl ma szeroki wybór automatów do gier | Mostbet logowanie jest możliwe zarówno na komputerze, jak i na telefonie | Mostbet kasyno to doskonała alternatywa dla tradycyjnych kasyn stacjonarnych http://www.mostbet-logowanie-kasyno-pl.com .
darknet markets url best darknet markets
darkmarket tor drug market
darknet links darkmarket
darknet websites dark web market
darknet market links darknet markets links
билайн подключить
https://almagigster.com/profile/jeffreyx92023
билайн телевидение кемерово
Пиротехника в СПб Фейерверки СПб купить интернет магазин
Тут можно преобрести seo-продвижение медицинских сайтов сео продвижение медицинских сайтов
Kraken onion shop Кракен сайт
darknet drug links dark market url
darknet drug store onion dark website
dark markets darknet drug market
toto 368
https://toto368raja.com/
Получите моментальный микрозайм на личную Visa за минуту. Оформите анкету мгновенно! https://microzaimxclan1.ru/ Наличность переводятся моментально!
где заказать катафалк для похорон https://rit-1.ru
Тут можно преобрести продвижение сайтов медицинских услуг продвижение сайта медицинской клиники
вывод из запоя на дому Москва Вывод из запоя, вывод из запоя Москва, вывод из запоя на дому, вывод из запоя на дому Москва, Срочный вывод из запоя на дому
The best towing company I’ve ever used! They treated my car with great care and provided top-notch service.
На днях нашел на gizbo casino официальный зеркало,
и захотел поделиться своим впечатлением.
Платформа выглядит очень привлекательной,
особенно когда хочешь найти качественное казино.
Кто уже использовал Gizbo Casino?
Поделитесь своим опытом!
Особенно любопытно узнать про бонусы и фриспины.
Например, есть ли Gizbo Casino особые предложения для новых пользователей?
Также интересует, где получить рабочее зеркало Gizbo Casino, если основной портал недоступен.
Читал немало разных мнений, но интересно узнать реальные рекомендации.
Например, как лучше использовать бонусы на Gizbo Casino?
Поделитесь своим мнением!
Финансовые новости Казахстана: свежие факты, изучайте.
Курс валют в Казахстане сегодня, посмотреть.
Обзор финансовых результатов Казахстана, чтобы быть в курсе.
Актуальные экономические тренды Казахстана, подписывайтесь на обновления.
Влияние мировых цен на экономику Казахстана, оценивайте.
Где инвестировать в Казахстане?, ознакомьтесь.
Ключи к финансовому успеху в Казахстане, изучайте.
Состояние банковской системы Казахстана, узнайте.
Прогнозы по экономике Казахстана на 2025 год, анализируйте.
Изменение налоговой системы в Казахстане, узнайте.
Какие изменения в денежной политике?, изучайте.
Финансирование бизнеса в Казахстане: как начать?, посмотрите.
Что нового на фондовом рынке Казахстана?, следите.
Как мировая экономика влияет на Казахстан?, изучайте.
Кредитование в Казахстане: условия и перспективы, читайте.
Финансовые новости: Казахстан сегодня, следите.
Анализ рынка недвижимости Казахстана, ознакомьтесь.
Экономика Казахстана и госбюджет, изучайте.
Как управлять финансами в Казахстане?, узнайте.
Электронные финансы в Казахстане: что нужно знать?, читайте.
финансовые новости Казахстана https://wikibank.kz/ .
darknet websites dark market onion
накрутка людей подписчиков накрутка подписчиков в Тик Ток быстро
darknet websites dark web marketplaces
dark web sites darknet markets onion address
Накрутка ТГ к ТГ накрутка подписчиков ТГ канале бесплатно
сниму квартиру в сочи на длительный срок
сайт мтс кемерово
https://euvisajobs.com/employer/kate/
мтс домашний интернет
Экскурсионные туры с перелетом https://travelpost.in.ua
dark web market dark web market links
darknet markets links darknet markets 2025
Анализ финансовых тенденций в Казахстане, читайте.
Курс тенге: что нового?, обязательно.
Важные финансовые отчеты из Казахстана, чтобы не пропустить.
Актуальные экономические тренды Казахстана, узнайте больше.
Как мировые тренды меняют Казахстан, анализируйте.
Топ-10 инвестиций в Казахстане, ознакомьтесь.
Как повысить финансовую грамотность?, изучайте.
Надежность банков в Казахстане, изучите.
Будущее экономики Казахстана: прогнозы, обсуждайте.
Изменение налоговой системы в Казахстане, следите.
Что нужно знать о денежной политике Казахстана?, ознакомьтесь.
Как привлечь инвестиции в Казахстане?, узнайте.
Что нового на фондовом рынке Казахстана?, читайте.
Как мировая экономика влияет на Казахстан?, ознакомьтесь.
Как взять кредит в Казахстане?, узнайте.
Финансовые новости: Казахстан сегодня, следите.
Рынок недвижимости Казахстана: последние тренды, изучайте.
Бюджет страны: что нужно знать о Казахстане?, читайте.
Полезные советы по финансам для казахстанцев, изучите.
Цифровизация финансового сектора Казахстана, следите.
финансовые новости Казахстана https://wikibank.kz/ .
darknet websites darknet markets onion
Тут можно преобрести seo продвижение медицинских клиник seo продвижение медицинских клиник
Последние события в экономике Казахстана, в нашем разделе новостей.
Курс тенге: что нового?, ознакомиться.
Обзор финансовых результатов Казахстана, чтобы понять ситуацию.
Что влияет на экономику Казахстана?, подписывайтесь на обновления.
Казахстан и мировые финансовые рынки, оценивайте.
Инвестиционные возможности в Казахстане, посмотрите.
Ключи к финансовому успеху в Казахстане, запоминайте.
Надежность банков в Казахстане, изучите.
Прогнозы по экономике Казахстана на 2025 год, анализируйте.
Налоговые реформы Казахстана, следите.
Денежная политика Национального банка Казахстана, анализируйте.
Как привлечь инвестиции в Казахстане?, изучите.
Что нового на фондовом рынке Казахстана?, изучайте.
Как мировая экономика влияет на Казахстан?, изучайте.
Как взять кредит в Казахстане?, узнайте.
Финансовые новости: Казахстан сегодня, следите.
Что происходит на рынке недвижимости Казахстана?, узнайте.
Бюджет страны: что нужно знать о Казахстане?, анализируйте.
Финансовые советы для населения Казахстана, изучите.
Цифровизация финансового сектора Казахстана, читайте.
финансовые новости Казахстана https://wikibank.kz/ .
darknet markets onion address bitcoin dark web
скачать 1win официальный сайт скачать 1win официальный сайт .
wikibank.kz wikibank.kz .
dark web link dark market list
darknet markets url darknet sites
мтс подключить
http://retailjobacademy.com/employer/jamal/
мтс телевидение
Mostbet pl oferuje szeroki wybór gier kasynowych i zakładów sportowych | Mostbet pl umożliwia wygodne obstawianie zakładów sportowych | Mostbet logowanie odbywa się błyskawicznie bez zbędnych formalności | Mostbet com obsługuje płatności kartą oraz portfele elektroniczne Mostbet pl najlepsze promocje .
darknet markets darknet markets links
darkmarket url darkmarket
darkmarket list darkmarket list
darknet market links darknet market
мостбет кыргызстан скачать https://mostbet16.com.kg/ .
darknet market lists dark web market links
darknet links dark web market list
dark web market links dark web market list
Получите мгновенный займ на банковскую Visa за минуту. Оформите заявку мгновенно! https://microzaimxclan1.ru/ Деньги приходят мгновенно!
darknet markets dark markets 2025
оформить займ на карту взять микрозайм на карту
Собственное производство металлоконструкций. Если вас интересует навес авто мы предлогаем изготовление под ключ навесы для машин на даче
Взять микрозайм на карту без отказа микрозайм на карту без отказов мгновенно
мостбет скачать на андроид мостбет скачать на андроид .
мегафон телевидение ростов
https://www.opad.biz/employer/hung/
мегафон телевидение ростов
dark web markets dark web sites
dark market url dark market
Тут можно преобрести заказать сайт медицинского центра создание сайта для клиники
грузчики недорого gruzmove.ru
ремонт холодильников локомотив remont-holodilnikov11.ru .
услуги грузчиков услуги грузчиков перевозки
ремонт холодильников измайлово ремонт холодильников измайлово .
1wi http://1win38.com.kg .
Дезинфекция транспорта предлагает наша компания. Если вам нужны услуги специалистов с выездом на дом оставьте заявку на сайте Уничтожение блох
мтс домашний интернет сменить тариф мтс домашний интернет и тв
мтс подключить домашний интернет цены домашний интернет мтс омск
кайт хургада кайт школа хургада
аренда квартиры в сочи
ттк домашний интернет барнаул
https://www.elitistpro.com/employer/ttk-tarif-solutions/
ттк телевидение
кайт анапа
USDT娱乐城
Maispin(迈斯)致力于打造一个沉浸式的虚拟货币赌场宇宙,引领玩家穿梭于现金与数位资产之间,体验前所未有的娱乐与挑战之旅。我们将赌博体验升华为财富与策略的探索,让每位玩家成为这片数位宇宙的策略家与赢家,书写属于自己的财富传奇。 Maispin不仅是一个线上赌场平台,更是一个充满深刻情感与无限财富可能的娱乐世界。
мтс домашний интернет
https://tayseerconsultants.com/employer/chandra/
мтс домашний интернет
1win онлайн 1win онлайн .
1win,com https://1win39.com.kg/ .
мтс тарифы на интернет
https://portalwe.net/employer/sommer/
мтс телевидение краснодар
Обработка участков от борщевика предлагает наша компания. Если вам нужны услуги специалистов с выездом на дом оставьте заявку на сайте Фумигация
домашний интернет и тв мтс цена домашний мобильный интернет мтс
visite site [url=https://web-foxwallet.com]Fox wallet extension[/url]
Недавно нашел на РіРёР·Р±Рѕ казик,
и решил поделиться своим впечатлением.
Платформа выглядит очень интересной,
особенно когда хочешь найти надежное игровое заведение.
Есть кто-то уже использовал Gizbo Casino?
Расскажите своим опытом!
В частности любопытно узнать про бонусы и акции.
Допустим, есть ли Gizbo Casino специальные условия для начинающих игроков?
Еще интересно, как получить рабочее зеркало Gizbo Casino, если официальный сайт не работает.
Видел немало противоречивых мнений, но интересно узнать честные рекомендации.
Например, где эффективнее активировать бонусы на Gizbo Casino?
Поделитесь своим опытом!
1wi 1wi .
https://www.aktepestadtaksiduragi.com/
Mostbet logowanie jest szybkie i bezpieczne dla wszystkich graczy | Mostbet casino to jedna z najbardziej renomowanych platform hazardowych | Mostbet logowanie odbywa się błyskawicznie bez zbędnych formalności | Mostbet casino online posiada szeroką gamę metod płatności Mostbet kasyno rejestracja .
мегафон телевидение
https://lovetechconsulting.net/employer/juliann/
провайдер мегафон
1хwin https://1win34.com.kg .
toto 368
http://clients1.google.kz/url?sa=t&url=https://toto368raja.com/
1 виг 1win19.com.kg .
Trained Personnel: Towing requires technical knowledge and experience. Well-trained staff ensures vehicles are recovered without damage and according to proper safety protocols.
напечатать наклейки спб напечатать наклейки спб .
Trained Personnel: Towing requires technical knowledge and experience. Well-trained staff ensures vehicles are recovered without damage and according to proper safety protocols.
билайн подключить кемерово
https://www.jobs.prynext.com/employer/markus/
билайн тарифы на интернет
Trained Personnel: Towing requires technical knowledge and experience. Well-trained staff ensures vehicles are recovered without damage and according to proper safety protocols.
Знакомства и общение онлайн http://walove.ru просто и удобно! Находи новых друзей, флиртуй, общайся в чатах и видеозвонках. Создай анкету, найди интересных людей и начинай диалог. Бесплатная регистрация!
Hello!
This post was created with XRumer 23 StrongAI.
Good luck 🙂
1 win.pro https://1win35.com.kg/ .
билайн домашний интернет кемерово
https://pittsburghpenguinsclub.com/read-blog/19155_bilajn-domashnij-internet-otzyvy-polzovatelej.html
билайн домашний интернет кемерово
Круглосуточный займ на платформе https://lombard-avtozaym.ru/
Psychological Support for Drivers: Roadside assistance doesn’t only offer physical help. It also provides psychological support for drivers who may be stressed, especially in difficult or remote situations.
apple macbook air 13 m2 https://apple-macbook-air13.ru
Dyson — это не просто бренд. Это, как говорится, синоним качества и инновационного подхода. Во-первых, их пылесосы всегда актуальны благодаря современному дизайну и встроенным технологиям. Во-вторых, лёгкость использования – огромный плюс. Никаких проводов, никаких застревающих щеток.
Рейтинг ТОП-3 беспроводных пылесосов Dyson
ссылка
1win rossvya http://mostbet18.com.kg/ .
Собственное производство металлоконструкций. Если вас интересует навесы из поликарбоната производство мы предлогаем изготовление под ключ навесы для автомобилей металлические
мтс тв краснодар
http://realestatez.in/profile/refugiosolis49
мтс интернет
мтс подключение кемерово
https://asemployment.com/employer/rhonda/
мтс телевидение
apple macbook air 15 8 512 https://apple-macbook-15.ru
Сап анапа
Тут можно преобрести создание сайта для медицинского учреждения разработка сайта для медицинской организации
Тут можно преобрести продвижение медицинского сайта продвижение медицинских услуг
мегафон подключить
https://empleo.infosernt.com/employer/eusebia/
мегафон телевидение ростов
24/7 Availability: One of the major benefits of roadside assistance services is that they are available 24/7. This provides peace of mind for drivers, knowing they can receive help anytime, day or night.
1wines http://1win21.com.kg/ .
мостбет уз вход https://www.mostbet3015.ru .
Solutions for Various Situations: Roadside assistance is not just for vehicle breakdowns. It also covers scenarios like accidents, getting stuck, running out of fuel, or flat tires. Each situation requires specialized equipment and expertise.
знакомства хургада https://t.me/znakomstvavhurgade
Мгновенный займ на портале https://lombard-avtozaym.ru/
ттк интернет
https://kennetjobs.com/companies/ttk-tarif-ltd/
ттк тарифы барнаул
Pinco-da qeydiyyatdan kec v? ilk depozit bonusu qazan!
Kazino pinco
сайт мтс краснодар
https://kennetjobs.com/companies/anya/
мтс цены
mostbet-uzb http://www.mostbet3016.ru .
Если ваша Sony PlayStation 5 включается, но на экране телевизора отсутствует изображение, это может быть вызвано разными причинами. Ниже мы рассмотрим распространенные проблемы и их решения.
перейти
проверка транспортных средств Обучение на диспетчера автомобильного и городского наземного электрического транспорта! Хотите освоить востребованную профессию и управлять транспортными потоками города? Чему вы научитесь? Организации и контролю движения общественного транспорта Планированию и оптимизации маршрутов Решению нештатных ситуаций и взаимодействию с водителями Кому подойдет? Сотрудникам транспортных компаний и автопредприятий Всем, кто хочет построить карьеру в сфере городских перевозок После обучения – официальный документ, подтверждающий квалификацию!
apple macbook pro 14 m4 2024 https://macbook-pro-14.ru
toto 368
https://app.demanzo.com/domain/toto368ku.com
ттк интернет
http://rejobbing.com/companies/catharine/
ттк тарифы ростов
билайн тарифы кемерово
https://ichgcp.kz/employer/michell/
сайт билайн кемерово
мегафон подключить
http://busforsale.ae/profile/sandratorr286
мегафон домашний интернет
мтс интернет
https://www.informedica.llc/employer/andy/
мтс подключить кемерово
Круглосуточный займ на сайте https://lombard-avtozaym.ru/
перепланировка согласование перепланировка согласование .
Website
jaxx liberty wallet
1win букмекерская контора 1win22.com.kg .
мтс тв
http://onlinelogisticsjobs.com/companies/santos/
мтс тв
На днях наткнулся на http://uapa.station171.com/forum/home.php?mod=space&uid=603918 – gizbo сайт,
и захотел рассказать своим опытом.
Сайт выглядит очень интересной,
особенно если ищешь качественное игровое заведение.
Кто уже пробовал Gizbo Casino?
Поделитесь своим опытом!
В частности любопытно узнать про промокоды и акции.
Например, предлагают ли Gizbo Casino специальные условия для новых игроков?
Еще интересно, где получить рабочее зеркало Gizbo Casino, если официальный портал не работает.
Читал много разных мнений, но хотелось бы узнать честные советы.
Допустим, как эффективнее активировать бонусы на Gizbo Casino?
Поделитесь своим опытом!
Рекомендую либет казино войти
игровые автоматы 1win http://www.1win23.com.kg .
toto 368
Safety Precautions: Roadside assistance teams must adhere to safety standards during operations. This is especially important when working on the road, as it involves both the safety of the stranded vehicle and other traffic on the road.
мегафон тв
https://talentup.asia/employer/orval/
мегафон тарифы ростов
ттк телевидение
https://careers.webdschool.com/companies/ttk-tarif-and-co/
ттк барнаул
We offer a wide range of stretch films, ideal for protecting and packaging products of any size.
More detailed information on the link бопп пакет спб
Our film has high elasticity, which ensures reliable protection of your goods from moisture.
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://eldenhore1974.diary.ru/
https://chyoa.com/user/daedros1955
https://mxim0091967.diary.ru/
https://www.haikudeck.com/presentations/BtnpjR2PSQ
http://www.babelcube.com/user/tony-romero
darknet market list dark web link https://github.com/newonionlinks/darknetmarkets
ттк домашний интернет барнаул
https://iadgroup.co.uk/employer/ttk-tarif-solutions/
ттк тв барнаул
Dowiedz się więcej o zdrowym stylu życia i aktywności fizycznej | Newsy ze świata sportu – piłka nożna, koszykówka, siatkówka i więcej | Świat sportu w Polsce – pełen emocji i adrenaliny | Relacje na żywo z aren sportowych w Polsce | Nowoczesny portal sportowy dla każdego fana sportu Newsy sportowe na żywo .
Неревод юридических документов Ищете услуги юридического перевода? Наше бюро сделает перевод юридических документов “под ключ”
ттк домашний интернет ростов
https://kpslao.com/companies/danny/
ттк домашний интернет ростов
Safety Precautions: Roadside assistance teams must adhere to safety standards during operations. This is especially important when working on the road, as it involves both the safety of the stranded vehicle and other traffic on the road.
ттк цены
https://raida-bw.com/employer/royce/
ттк тарифы на интернет
macbook air 16 2023 macbook air m2 512gb 2023
macbook pro 16 ssd macbook pro 16 i9
UID_58303021###
Ojol Berhenti Ribut Aksi Demo Berhenti Malah Kompak Main Slot Taruhan Bola di Agentotoplay
UID_56811104###
Pemilik Warteg Kaget Dapat Maxwin Langsung Buka Restoran Mewah di Jakarta
теплоход в питере аренда аренда яхты спб цена
прокат яхты дубай аренда яхты до 15 человек в дубай
mostbet онлайн казино http://www.mostbet8.com.kg .
согласованте согласованте .
билайн телевидение кемерово
https://rubyrecruitment.net/employer/jerome/
билайн тв кемерово
Equipment and Technology: The equipment used in car recovery must be modern and compatible with today’s vehicles. This ensures the safe and efficient transport of vehicles with minimal damage.
Equipment and Technology: The equipment used in car recovery must be modern and compatible with today’s vehicles. This ensures the safe and efficient transport of vehicles with minimal damage.
мтс тарифы на интернет
https://www.top5stockbroker.com/employer/zac/
мтс тв
Ремонт смартфонов Huawei — популярная услуга, поскольку даже самые надёжные устройства могут выйти из строя. Разберёмся в распространённых поломках, их причинах и примерной стоимости ремонта.
подробнее
прогулка на катере по каналам https://arenda-yaht-spb.ru
аренда яхты на 20 человек яхта аренда дубай
купить диплом колледжа в омске
купить диплом в питере
UID_29117722###
Dwi Sasono Mendadak Kaya Ternyata Ini Rahasia Suksesnya di Agentotoplay
Solutions for Various Situations: Roadside assistance is not just for vehicle breakdowns. It also covers scenarios like accidents, getting stuck, running out of fuel, or flat tires. Each situation requires specialized equipment and expertise.
toto368
Мы изготавливаем дипломы любой профессии по доступным ценам. Стоимость может зависеть от конкретной специальности, года выпуска и образовательного учреждения. Стараемся поддерживать для заказчиков адекватную политику цен. Для нас важно, чтобы дипломы были доступны для большого количества наших граждан. купить настоящий аттестат за 9 класс
Приобрести документ университета можно у нас в столице. diplomnie.com/kupit-diplom-yaroslavl-2
купить диплом высшем образовании без предоплаты купить диплом высшем образовании без предоплаты .
Мы предлагаем дипломы любой профессии по приятным тарифам. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас очень важно, чтобы документы были доступны для большого количества наших граждан.
Приобретение диплома, подтверждающего обучение в ВУЗе, – это выгодное решение. Приобрести диплом о высшем образовании: wecanchat.mn.co/spaces/9349852/feed
мтс подключить
https://hifrequency.live/community/profile/marlazoc8807805/
мтс тарифы краснодар
Здравствуйте!
Начальники очень часто выбирают претендентов, которые закончили ВУЗ. Особенно ценятся престижные учебные заведения. Однако учиться пять лет – это долго и дорого, далеко не у каждого имеется подобная возможность. Приобрести документ становится самым оптимальным решением.
Бывают и непредвиденные случаи, когда диплом утерян. Далеко не всегда можно оперативно и без осложнений восстановить его, особенно когда университет закрыт или находится в другом регионе РФ. Бюрократические проблемы отнимут огромное количество времени.
Для успешного продвижения по карьере требуется наличие официального диплома института. Тем не менее нередко в жизни может случиться так, что определенные обстоятельства не дают с успехом окончить учебу, получив желанный документ.
Приобрести диплом любого университета
Наша компания предлагает быстро и выгодно купить диплом, который выполнен на оригинальном бланке и заверен печатями, водяными знаками, подписями официальных лиц. Данный документ способен пройти любые проверки, даже при помощи специального оборудования. Решайте свои задачи максимально быстро с нашей компанией.
Где приобрести диплом специалиста? diplomanrus.com/
диплом дизайнера интерьера купить
купить диплом в азове 2orik-diploms.ru .
Ремонт смартфонов Huawei — популярная услуга, поскольку даже самые надёжные устройства могут выйти из строя. Разберёмся в распространённых поломках, их причинах и примерной стоимости ремонта.
ссылка
Ekskluzywne wywiady i analizy dotyczące sportu w Polsce | Relacje na żywo z meczów i turniejów sportowych w Polsce | Liga polska i międzynarodowe rozgrywki w jednym miejscu | Relacje z meczów, wywiady, analizy i statystyki | Sprawdź najnowsze wiadomości o zdrowiu i aktywności fizycznej https://newssports.pl/ .
мегафон цены
https://collegestudentjobboard.com/employer/maricela/
сайт мегафон ростов
сайт ттк барнаул
https://dubaijobzone.com/companies/ttk-tarif-services/
ттк телевидение барнаул
Покупка диплома о среднем полном образовании: как избежать мошенничества?
Приветствую!
Для некоторых людей, заказать диплом о высшем образовании – это необходимость, уникальный шанс получить достойную работу. Однако для кого-то – это понятное желание не терять время на учебу в ВУЗе. С какой бы целью вам это не потребовалось, наша фирма готова помочь. Быстро, качественно и недорого изготовим документ нового или старого образца на государственных бланках с реальными подписями и печатями.
Ключевая причина, почему люди прибегают к покупке документа, – получить хорошую должность. Например, знания позволяют человеку устроиться на работу, однако документального подтверждения квалификации не имеется. В случае если для работодателя важно присутствие “корочек”, риск потерять хорошее место довольно высокий.
Купить документ института можно в нашей компании. Мы предлагаем документы об окончании любых ВУЗов Российской Федерации. Вы сможете получить необходимый диплом по любым специальностям, включая документы образца СССР. Гарантируем, что в случае проверки документа работодателем, подозрений не появится.
Факторов, которые вынуждают заказать диплом ВУЗа очень много. Кому-то прямо сейчас нужна работа, и нужно произвести особое впечатление на начальника при собеседовании. Другие планируют попасть в большую компанию, чтобы повысить собственный статус в обществе и в будущем начать свой бизнес. Чтобы не тратить множество времени, а сразу начинать удачную карьеру, используя имеющиеся навыки, можно заказать диплом прямо в онлайне. Вы станете полезным в обществе, получите денежную стабильность максимально быстро и легко- купить аттестат за 9 класс
Официальная покупка диплома вуза с сокращенной программой обучения в Москве
Узнайте стоимость диплома высшего и среднего образования и процесс получения
Мы предлагаем документы ВУЗов, которые расположены на территории всей России. [url=http://zakaz-na-diplom.ru/kupit-diplom-v-spb-3-6/]zakaz-na-diplom.ru/kupit-diplom-v-spb-3-6[/url]
Парадокс, но купить диплом кандидата наук оказалось не так и сложно
Solutions for Various Situations: Roadside assistance is not just for vehicle breakdowns. It also covers scenarios like accidents, getting stuck, running out of fuel, or flat tires. Each situation requires specialized equipment and expertise.
диплом медколледжа купить
Купить диплом о среднем полном образовании, в чем подвох и как избежать обмана?
Приветствую!
Где заказать диплом по актуальной специальности?
Мы изготавливаем дипломы любой профессии по приятным тарифам. Стоимость будет зависеть от той или иной специальности, года получения и университета. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы дипломы были доступны для большого количества наших граждан.
Покупка документа, который подтверждает окончание института, – это выгодное решение. Просто посчитайте, сколько нужно будет вложить денег на ежемесячную оплату пяти лет обучения, на аренду жилья (если учащийся из другого города), на проезд до ВУЗа и прочие затраты. Получается приличная сумма, в разы превосходящая расценки на наши дипломы. А ведь все это время можно работать, продвигаясь по карьере.
Получаемый диплом с приложением отвечает стандартам Министерства образования и науки, никто не отличит его от оригинала. Не откладывайте собственные мечты на несколько лет, реализуйте их с нашей помощью – отправляйте быструю заявку на изготовление диплома сегодня!
Получить диплом о высшем образовании – не проблема! rdiplomm24.com/
Здравствуйте!
Мы готовы предложить дипломы любой профессии по разумным ценам. Цена будет зависеть от определенной специальности, года выпуска и образовательного учреждения: diplomans.com/
диплом программиста купить
ттк интернет ростов
https://www.dynamicjobs.eu/employer/margarette/
ттк домашний интернет ростов
Привет!
Без ВУЗа очень трудно было продвинуться по карьере. В последние годы документ не дает совершенно никаких гарантий, что удастся получить привлекательную работу. Более важное значение имеют профессиональные навыки и знания специалиста, а также его постоянный опыт. В связи с этим решение о покупке диплома следует считать рациональным. Заказать диплом любого ВУЗа nhps1914.com/wiki/Диплом_Р·Р°_короткий_СЃСЂРѕРє_–_оформление_без_проблем
купить программу 1с торговля купить программу 1с торговля .
трансформаторы сухие купить трансформаторы сухие купить .
1с предприятие 1с предприятие .
купить диплом автомеханика купить диплом автомеханика .
купить диплом железнодорожника diploms-bests.ru .
купить аттестат школы велико prema-diploms.ru .
Привет!
Покупка диплома через надежную компанию дарит ряд преимуществ. Это решение дает возможность сберечь время и существенные финансовые средства. Тем не менее, на этом выгоды не ограничиваются, плюсов гораздо больше.Мы изготавливаем дипломы любых профессий. Дипломы производятся на настоящих бланках государственного образца. Доступная стоимость сравнительно с крупными затратами на обучение и проживание в другом городе. Покупка диплома института будет разумным шагом.
Приобрести диплом: promosimple.com/ps/32811/diplomygroup
купить диплом в москве метро
Недавно наткнулся на РіРёР·Р±Рѕ официальный сайт,
и решил поделиться своим опытом.
Платформа кажется довольно привлекательной,
особенно когда ищешь качественное игровое заведение.
Кто реально использовал Gizbo Casino?
Расскажите своим мнением!
В частности любопытно узнать про промокоды и фриспины.
Например, предлагают ли Gizbo Casino особые условия для начинающих пользователей?
Еще интересует, как найти gizbo casino официальный сайт
, если официальный портал не работает.
Читал немало разных отзывов, но хотелось бы узнать честные советы.
Допустим, как эффективнее активировать промокоды на Gizbo Casino?
Расскажите своим мнением!
билайн телевидение кемерово
https://coopervigrj.com.br/employer/myrtle/
билайн подключение кемерово
Доска бесплатных объявлений «trueboard.ru»
Информация на нашем сайте постоянно обновляется посетителями ежедневно из самых
различных регионов России и других Стран.
«trueboard.ru» очень понятный сервис для любого пользователя, любого возраста.
Удобный сайт для подачи бесплатных и платных объявлений.
Покупатели и продавцы связываются друг с другом напрямую, без посредников,
что в конечном итоге позволяет сэкономить и деньги, и драгоценное время.
Премиум размещение стоит совсем недорого.
Сайт бесплатных объявлений trueboard.ru
toto 368
Psychological Support for Drivers: Roadside assistance doesn’t only offer physical help. It also provides psychological support for drivers who may be stressed, especially in difficult or remote situations.
macbook air 13.6 2022 m2 macbook air 13 m2 512gb
Velocidad critica
Equipos de equilibrado: clave para el rendimiento suave y óptimo de las dispositivos.
En el campo de la innovación contemporánea, donde la eficiencia y la confiabilidad del sistema son de máxima significancia, los sistemas de calibración juegan un función crucial. Estos sistemas adaptados están desarrollados para balancear y asegurar piezas giratorias, ya sea en maquinaria de fábrica, medios de transporte de traslado o incluso en aparatos domésticos.
Para los expertos en mantenimiento de dispositivos y los especialistas, trabajar con dispositivos de balanceo es fundamental para garantizar el funcionamiento uniforme y seguro de cualquier mecanismo rotativo. Gracias a estas soluciones modernas modernas, es posible limitar significativamente las oscilaciones, el zumbido y la carga sobre los cojinetes, extendiendo la longevidad de componentes valiosos.
También trascendental es el rol que juegan los equipos de ajuste en la asistencia al cliente. El ayuda experto y el mantenimiento continuo aplicando estos sistemas posibilitan ofrecer servicios de excelente excelencia, elevando la satisfacción de los compradores.
Para los propietarios de emprendimientos, la aporte en unidades de equilibrado y dispositivos puede ser esencial para incrementar la productividad y eficiencia de sus dispositivos. Esto es principalmente trascendental para los emprendedores que manejan pequeñas y pequeñas negocios, donde cada aspecto importa.
Asimismo, los equipos de calibración tienen una amplia aplicación en el sector de la seguridad y el gestión de nivel. Habilitan localizar potenciales defectos, impidiendo reparaciones onerosas y perjuicios a los equipos. Además, los datos extraídos de estos aparatos pueden utilizarse para maximizar procedimientos y aumentar la visibilidad en motores de consulta.
Las áreas de utilización de los sistemas de ajuste incluyen variadas ramas, desde la manufactura de vehículos de dos ruedas hasta el supervisión ecológico. No interesa si se refiere de enormes elaboraciones industriales o limitados espacios hogareños, los equipos de calibración son indispensables para proteger un rendimiento productivo y sin detenciones.
apple macbook pro 13 apple-macbook-pro213.ru
скачать взломанный автодилер скачать взломанный автодилер скачать взломанный автодилер
P.S Live ID: K89Io9blWX1UfZWv3ajv
P.S.S Программы и игры для Андроид телефона Программы и игры для Андроид телефона Программы и игры для Андроид телефона edf5c00
мтс интернет
https://www.homebasework.net/employer/winifred/
мтс подключение
dubai yacht dinner cruise https://dubai-rent-yacht.com
мтс подключить краснодар
https://darempleo.com/employer/phil/
мтс тарифы краснодар
Equipment and Technology: The equipment used in car recovery must be modern and compatible with today’s vehicles. This ensures the safe and efficient transport of vehicles with minimal damage.
ссылка на кракен в браузере кракен актуальная ссылка
силовой трансформатор цена силовой трансформатор цена .
Comprehensive VPN and security https://github.com/cisco-main/Cisco-Secure-Client/releases solutions for corporate networks and enterprises. Provides secure, high-performance access to remote desktops and applications.
мегафон подключение ростов
https://publiccharters.org/employer/arnoldo/
мегафон телевидение
мостбет узбекистон http://mostbet3020.ru .
1 win https://1win36.com.kg .
Проблемы с зарядкой iPad могут возникнуть неожиданно и доставить массу неудобств. Причины таких проблем разнообразны — от тривиальных до серьёзных. Прежде чем обращаться в сервисный центр, можно попробовать решить проблему самостоятельно. Эта статья поможет вам разобраться в основных причинах и способах их устранения.
здесь
мостбет официальный сайт скачать на андроид бесплатно mostbet9.com.kg .
dubai marina yacht tour with breakfast yacht hire dubai
кракен официальное зеркало kra31.cc
ттк тв
https://magnusrecruitment.com.au/employer/ttk-tarif/
ттк домашний интернет барнаул
mostbet. uz mostbet3019.ru .
кракен вход на сайт кракен маркетплейс вход
The best HD wallpapers https://wallpapers-all.com/49386-command-and-conquer-red-alert-2.html in one place! Download free backgrounds for your desktop and smartphone. A huge selection of pictures – from minimalism to bright landscapes and fantasy. Enjoy stylish images every day!
https://t.me/ASOIMMADV
свежие фриспины риобет Бездепозитные бонусы за регистрацию
Если на вашем телефоне Samsung Galaxy экран некорректно реагирует на прикосновения или не работает вовсе, не спешите паниковать. Сейчас мы рассмотрим основные шаги, которые помогут решить проблему. И на примере Samsung S20/21/22/23/24 пошагово выполним все действия, которые могут восстановить работоспособность экрана.
ссылка
ттк телевидение ростов
https://pompeo.com/employer/hellen/
ттк подключить
24/7 Availability: One of the major benefits of roadside assistance services is that they are available 24/7. This provides peace of mind for drivers, knowing they can receive help anytime, day or night.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article. https://accounts.binance.com/ro/register-person?ref=V3MG69RO
USDT娱乐城
Maispin(迈斯)致力于打造一个沉浸式的虚拟货币赌场宇宙,引领玩家穿梭于现金与数位资产之间,体验前所未有的娱乐与挑战之旅。我们将赌博体验升华为财富与策略的探索,让每位玩家成为这片数位宇宙的策略家与赢家,书写属于自己的财富传奇。 Maispin不仅是一个线上赌场平台,更是一个充满深刻情感与无限财富可能的娱乐世界。
кракен ссылка kra. cc
The best HD wallpapers https://wallpapers-all.com/321-resident-evil-4.html in one place! Download free backgrounds for your desktop and smartphone. A huge selection of pictures – from minimalism to bright landscapes and fantasy. Enjoy stylish images every day!
夜蝶飛翔特2発1
レム 壁紙
ビジュアルとサウンドが迫力満点で、プレイ中に没入感が増します。特にバトル演出が楽しい。
デビル メイ クライ クロス
https://www.8casino8.com/?SitesID=921173818.html
定期的に新しいイベントがあり、飽きずに楽しめます。新しい体験が待っています。
CRルパン三世~消されたルパン
エボ 6
ドラゴンが降りる
CRルパン三世~消されたルパン Ver.394
黃金守護神(特殊大賞燈);
紫イミソーレ(スペシャルアワードライト
https://www.k8io.net/?GameID=920696818.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
P とある魔術の禁書目録
https://xn--k8-9g4a3b4f.site/?ArticleID=922729818.html
キャラクターの個性が豊かで、ストーリーに引き込まれます。感情移入できます。
P とある科学の超電磁砲
https://www.k8-casino.org/?WatchID=923298818.html
友人と一緒にプレイすることで、楽しさが倍増します。共に喜ぶ瞬間が嬉しい。
casino 1win 1win2.com.mx .
1wln https://1win37.com.kg .
Спины за регистрацию без депозита с выводом bonuses casinos
1вин кыргызстан 1win41.com.kg .
Revolutionize Your Data Strategy with DataDex
DataDex is transforming how businesses manage and analyze their data. Our decentralized platform combines blockchain security with advanced analytics tools for unparalleled performance. https://datadex.my
Key Features of DataDex:
Blockchain-Based Security: Your data, tamper-proof and transparent.
Advanced Analytics: Tools to drive smarter decisions.
Global Scalability: Solutions designed for growth and flexibility.
Join DataDex and take control of your data like never before! https://datadex.my
трансформаторы сухие купить трансформаторы сухие купить .
мостбет казино войти мостбет казино войти .
1c программа 1c программа .
La vida de Diego Maradona estuvo llena de gloria y controversia | Diego Maradona jugó en equipos legendarios como Boca Juniors y Napoli | Los hijos de Diego Maradona continúan con su legado | Los datos curiosos sobre Diego Maradona sorprenden a muchos fanáticos https://diego-maradona.com.mx/ .
Создание и продвижение сайтов. Интернет-маркетинг!
Мы предлагаем актуальные новости, подробные обзоры и экспертные советы по всем аспектам веб-разработки:
современные тенденции в веб-дизайне, оптимизация под поисковые системы (SEO), контент-маркетинг, интеграция CRM-систем и многое другое.
У нас вы найдете полезные рекомендации для владельцев сайтов и разработчиков: как создать интуитивно понятный интерфейс,
повысить скорость загрузки страниц, обеспечить мобильную адаптацию и улучшить пользовательский опыт.
Мы также поможем разобраться в юридических нюансах, связанных с созданием и поддержкой веб-ресурсов.
Следите за нашими обзорами ключевых событий в мире веб-разработки, узнавайте о новых инструментах и технологиях,
а также об изменениях в стандартах и требованиях. Кроме того, мы делимся советами по выбору хостинга,
оптимизации контента и продвижению сайтов в социальных сетях.
Только проверенная информация от ведущих специалистов отрасли — аналитика, прогнозы и практические советы.
Подписывайтесь и будьте в курсе всех новостей! С нами ваш веб-ресурс станет успешным и востребованным!
Сайт silaseo.ru
Страхование по лучшей цене https://осагополис.рф Сравните предложения страховых компаний и выберите полис с выгодными условиями. Удобный сервис поможет найти оптимальный вариант автострахования, ОСАГО, КАСКО, туристических и медицинских страховок.
займ онлайн бесплатно займ быстрый
altcoin trends crypto regulations
Добрый день!
Купить диплом института по выгодной цене можно, обратившись к надежной специализированной компании. Быстро заказать диплом о высшем образовании: diplomaj-v-tule.ru/kupit-diplom-kaliningrad-3/
Ремонт кофемашин в Москве https://coffee-help24.ru быстро, качественно, с гарантией! Обслуживаем все бренды: Saeco, DeLonghi, Jura, Bosch и др. Диагностика, замена деталей, чистка от накипи. Выезд мастера на дом или ремонт в сервисе.
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:сервис центры бытовой техники москва
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Тут можно преобрести создание медицинского сайта создание сайта для медицинского центра
Недавно наткнулся на РіРёР·Р±Рѕ официальный,
и решил поделиться своим опытом.
Сайт выглядит довольно привлекательной,
особенно когда ищешь качественное казино.
Кто реально использовал Gizbo Casino?
Расскажите своим опытом!
В частности любопытно узнать про промокоды и фриспины.
Например, есть ли Gizbo Casino специальные условия для начинающих игроков?
Также интересно, где получить РіРёР·Р±Рѕ официальный сайт
, если официальный портал недоступен.
Читал много противоречивых мнений, но интересно узнать честные советы.
Допустим, где эффективнее использовать промокоды на Gizbo Casino?
Поделитесь своим мнением!
Тут можно приобрести создание сайта медицинского центра создание сайта для клиники
Thanks for sharing. I read many of your blog posts, cool, your blog is very good. https://accounts.binance.info/en-IN/register?ref=UM6SMJM3
Casino top Top casino с выводом
Последние IT-новости https://notid.ru быстро и понятно! Рассказываем о цифровых трендах, инновациях, стартапах и гаджетах. Только проверенная информация, актуальные события и мнения экспертов. Оставайтесь в центре IT-мира вместе с нами!
Pricing and Service Quality: The cost of roadside assistance can vary based on the nature and difficulty of the service. However, it’s important to choose companies that offer competitive prices and high-quality services.
UID_33024298###
jmarble race sensasi balap kelereng yang bikin ketagihan
rahasia menang di jmarble race teknik dan strategi jitu
mengenal dunia moba sejarah jenis dan rekomendasi game terbaik
Тут можно преобрести создание медицинских сайтов под ключ разработка сайта больницы
P 北斗の拳 暴凶星
呪い 祝い
リーチ演出が多彩で、どのタイミングで大当たりが来るかワクワクします。
戦コレ!【泰平女君】徳川家康
https://www.k8pachinko.asia/?ArticleID=921913818.html
新台の導入イベントなどで、ワクワク感が増すのが楽しいです。特別感があります。
沖ドキ!トロピカル
[url=https://jahromblog.com/?ArticleID=922201818.html]姫子 声優
[/url]
モンキーターンII
CRグラップラー刃牙
CRキャプテン翼XX
牙狼 守りし者
https://www.k8pachinko.work/?GameID=918248818.html
パチンコをすることで、運を試すドキドキ感が味わえます。挑戦する楽しさがあります。
大工の源さん桜満開!源 DREAM ver.~
https://www.k8pachinko.asia/?ArticleID=927175818.html
リーチ演出が多彩で、どのタイミングで大当たりが来るかドキドキします。
Dororonえん魔くん メ~ラめら
https://www.k8pachinko.biz/?ReviewID=919030818.html
大当たりの瞬間は、心臓がドキドキします。興奮が何とも言えません。
Unlock your vehicle’s potential with our top-notch auto tuning services! Enhance your ride into a stunning masterpiece with our expert team. We specialize in customizations that cater to your unique style. Our high-quality solutions ensure optimal performance and enhanced appearance. Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://phoenix-autobodyshop.com/ today and take the first step towards your dream car!
big yacht rental dubai boat cruise dubai
Современные телевизоры Samsung, такие как QLED QN90C, OLED S95B и Neo QLED 8K QN800A, полны передовых функций — от невероятного качества изображения до возможностей Smart TV. Однако даже у этих флагманских моделей могут возникать проблемы:
перейти
UID_86779711###
heboh pengunjung taman safari buka jendela mobil demi maxwin 200juta
mostbet автоматы скачать mostbet12.com.kg .
UID_28543202###
banjir di pejaten belum surut warga mendapatkan 150juta saat menyantap menu buka puasa
промокод на сайт мостбет http://www.mostbet11.com.kg .
магазин цветы санкт петербург заказ букетов
UID_36759470###
Penjual Bubur Menang 125JT dari Mahjong Ways 3 Ini Rahasianya
UID_75877808###
Susah Berjualan Pedagang Kaki 5 Menang 75JT Sekali Putar Gate of Olympus
Diego Maradona cambió la historia del fútbol con su talento | Las estadísticas de Diego Maradona reflejan su impacto en el fútbol | Los hijos de Diego Maradona continúan con su legado | Diego Maradona y Lionel Messi siempre han sido comparados Diego Maradona Boca Juniors .
Как транслировать содержимое с телефона Samsung на телевизор через WI-FI или DeX кабель. Пошаговая инструкция всех вариантов
перейти
ссылка на кракен официальный kra30. at
Sistemas de balanceo: fundamental para el operación suave y eficiente de las máquinas.
En el ámbito de la tecnología actual, donde la productividad y la estabilidad del equipo son de máxima importancia, los equipos de ajuste cumplen un tarea vital. Estos aparatos adaptados están creados para ajustar y fijar partes dinámicas, ya sea en dispositivos industrial, medios de transporte de traslado o incluso en equipos domésticos.
Para los profesionales en conservación de equipos y los técnicos, trabajar con sistemas de calibración es esencial para garantizar el rendimiento suave y seguro de cualquier dispositivo rotativo. Gracias a estas soluciones innovadoras avanzadas, es posible limitar notablemente las movimientos, el sonido y la presión sobre los soportes, prolongando la duración de componentes valiosos.
También relevante es el función que cumplen los sistemas de ajuste en la servicio al cliente. El asistencia especializado y el reparación continuo utilizando estos aparatos posibilitan ofrecer soluciones de excelente estándar, incrementando la agrado de los consumidores.
Para los propietarios de empresas, la financiamiento en estaciones de equilibrado y medidores puede ser importante para mejorar la efectividad y eficiencia de sus sistemas. Esto es especialmente significativo para los inversores que gestionan pequeñas y modestas emprendimientos, donde cada elemento vale.
Además, los equipos de balanceo tienen una extensa uso en el campo de la prevención y el monitoreo de estándar. Facilitan localizar posibles defectos, impidiendo arreglos costosas y daños a los sistemas. Incluso, los resultados recopilados de estos equipos pueden emplearse para perfeccionar sistemas y potenciar la reconocimiento en plataformas de consulta.
Las áreas de implementación de los equipos de calibración cubren variadas industrias, desde la fabricación de ciclos hasta el control ambiental. No interesa si se refiere de extensas fabricaciones manufactureras o modestos establecimientos de uso personal, los equipos de equilibrado son necesarios para promover un rendimiento efectivo y sin presencia de detenciones.
Купить диплом о высшем образовании поспособствуем. Купить аттестат в Волжском – diplomybox.com/kupit-attestat-v-volzhskom
Телевизоры TCL с функцией Smart TV предоставляют доступ к потоковым видеосервисам, приложениям и другим онлайн-возможностям. Однако некоторые пользователи могут столкнуться с проблемой подключения к Wi-Fi. Давайте разберем основные причины этой неисправности и предложим эффективные методы ее устранения.
перейти
Unlock your vehicle’s potential with our top-notch auto tuning services! Unleash your ride into a stunning masterpiece with our expert team. We specialize in upgrades that cater to your unique style. Our high-quality solutions ensure optimal performance and stylish look . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://phoenix-autobodyshop.com/ today and take the first step towards your dream car!
купить диплом ю ком
купить дипломы о высшем в барнауле 2orik-diploms.ru .
Мы можем предложить дипломы любой профессии по невысоким тарифам. Купить диплом в Невинномысске — kyc-diplom.com/geography/nevinnomyssk.html
шкаф купе на заказ цена Производство шкафов-купе на заказ – это сложный процесс, требующий профессионального подхода и использования качественных материалов. Опытные мастера помогут вам определиться с оптимальной конструкцией, подберут фурнитуру и учтут все технические нюансы, чтобы ваш шкаф служил вам долгие годы, радуя своим внешним видом и функциональностью.
купить диплом нутрициолога
купить диплом в ростове цена
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
мостбет официальный сайт зеркало http://www.mostbet13.com.kg/ .
My grocery budget has never been happier since I started checking the best deals online before heading to the store.
Browsing Caflyers while sipping my morning coffee is my secret to stress-free, budget-friendly shopping.
кракен вход на сайт kraken.at
Привет!
Начальники обычно предпочитают соискателей, окончивших университет. Особенно в приоритете элитные учебные заведения. Однако учиться пять лет – это долго, далеко не у всех присутствует подобная возможность. Приобрести документ становится самым оптимальным решением.
Бывают и непредвиденные ситуации, когда диплом ВУЗа потерян. Далеко не всегда возможно оперативно и беспроблемно восстановить документ, особенно если ВУЗ закрыт или находится далеко в другом регионе страны. Бюрократия отнимает много времени.
Для удачного продвижения по карьере необходимо наличие официального диплома ВУЗа. Но очень часто в жизни случается так, что определенные трудности мешают благополучно закончить учебу и заполучить желанный документ.
Купить диплом университета
Наши специалисты предлагают быстро купить диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями должностных лиц. Диплом пройдет любые проверки, даже при использовании специально предназначенного оборудования. Достигайте свои цели быстро с нашими дипломами.
Где заказать диплом специалиста? diplomanrus.com/
игра 1вин http://1win46.com.kg .
most bet https://mostbet19.com.kg/ .
Мы изготавливаем дипломы любой профессии по доступным ценам. Цена может зависеть от той или иной специальности, года выпуска и ВУЗа. Всегда стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы дипломы были доступны для большинства граждан. купить дипломы аттестаты купить москва
Купить документ ВУЗа можно в нашем сервисе. diplomidlarf.ru/kupit-diplom-bryansk
купить диплом университета в москве купить диплом университета в москве .
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по доступным ценам. Стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы дипломы были доступны для большого количества наших граждан.
Приобретение документа, подтверждающего окончание института, – это грамотное решение. Заказать диплом университета: climat-cold.ru/forum/user/4393
https://kitehurghada.ru/
https://kitehurghada.ru/obuchenie-detej-kajtsyorfingu/
https://kitehurghada.ru/stoimost-obucheniya-kajtserfingu-v-hurgade/
https://kitehurghada.ru/kurs-bazovyj-10-chasov-zanyatij-s-instruktorom-4-5-dnya-450-usd/
https://kitehurghada.ru/kurs-ekspress-6-chasov-zanyatij-s-instruktorom-2-3-dnya-300-usd/
https://kitehurghada.ru/kontakty/
https://kitehurghada.ru/prognoz-vetra-v-hurgade/
https://kitehurghada.ru/kurs-pro-prodvinutyj-15-chasov-zanyatij-s-instruktorom-5-7-dnej-600-usd/
https://kitehurghada.ru/vesna-2024-egipet-hurgada-kajt-tury-i-kajt-kempy-deti-vetra/
https://kitehurghada.ru/vesennie-kanikuly/
https://kitehurghada.ru/majskie-prazdniki/
https://kitehurghada.ru/kajt-shkola/
https://kitehurghada.ru/kajt-kemp-v-hurgade-s-kajt-czentrom-detivetra/
https://kitehurghada.ru/zhenskaya-kajt-shkola-shkola-kajtsyorfinga-dlya-detej/
https://kitehurghada.ru/kajt-shkola-v-hurgade-egipet-kajt-czentr-detivetra/
https://kitehurghada.ru/obuchenie-kajtserfingu-na-krasnom-more/
https://kitehurghada.ru/ekskursii-v-hurgade-konnye-progulki-s-kupaniem-v-more-czeny-i-otzyvy-katanie-na-loshadyah-i-verblyudah/
https://kitehurghada.ru/devochki-i-kajtsyorfing-kajtsyorfing-dlya-devushek/
https://kitehurghada.ru/iko-sertifikat-katsyorfinga/
https://kitehurghada.ru/kajt-spot-deti-vetra-odno-iz-luchshih-mest-v-hurgade-dlya-kajtsyorfinga/
https://kitehurghada.ru/gidrofojl-gidrofojling-fojlbording-kajtfojling-v-hurgade-egipet-obuchenie-s-nulya-za-3-dnya/
https://kitehurghada.ru/kajt-kajtsyorfing-i-ego-istoriya/
https://kitehurghada.ru/individualnoe-obuchenie-gidrofojlu-s-kajtom/
https://kitehurghada.ru/veter-v-hurgade/
https://kitehurghada.ru/akuly-v-egipte/
https://kitehurghada.ru/vingfoil/
https://kitehurghada.ru/kajt-shkola-v-egipte/
https://kitehurghada.ru/temperatura-morya-v-hurgade/
https://kitehurghada.ru/obuchenie-vingfojl/
https://kitehurghada.ru/kajtsyorfing-v-el-gune/
https://kitehurghada.ru/kajt-egipet/
https://kitehurghada.ru/akuly-hurgade/
https://kitehurghada.ru/katanie-na-kajte/
https://kitehurghada.ru/kogda-luchshe-otdyhat-v-egipte/
https://kitehurghada.ru/kajting-v-hurgade/
https://kitehurghada.ru/kajting-v-egipte/
https://kitehurghada.ru/kajtserfing/
https://kitehurghada.ru/temperatura-v-hurgade/
https://kitehurghada.ru/kajting/
https://kitehurghada.ru/obuchenie-kajtsyorfingu-v-hurgade-egipet/
https://kitehurghada.ru/russkaya-kajt-stancziya-deti-vetra-v-hurgade/
https://kitehurghada.ru/gidrofojl-s-kajtom-obuchenie/
https://kitehurghada.ru/pogoda-v-hurgade/
https://kitehurghada.ru/faq-po-hurgade-2022-2023-chasto-zadavaemye-voprosy-ili-chto-vzyat-s-soboj-v-egipet/
https://kitehurghada.ru/kajt-safari-v-egipte-russkaya-kajt-stancziya-detivetra-v-hurgade/
https://kitehurghada.ru/kajtserfing-v-hurgade-luchshij-sezon-dlya-kajtserfinga/
https://kitehurghada.ru/kajting-osobennosti-kajtinga-vidy-kajtinga/
https://kitehurghada.ru/russkaya-kajt-shkola-v-hurgade/
https://kitehurghada.ru/kajt-shkola-detivetra/
https://kitehurghada.ru/programma-obucheniya-kajtsyorfingu-iko-v-hurgade-egipet/
https://kitehurghada.ru/istoriya-kajtserfinga/
https://kitehurghada.ru/podarochnyj-sertifikat-na-obuchenie-kajtserfingu-v-hurgade/
https://kitehurghada.ru/chto-takoe-iko-kitesurfing/
https://www.tripadvisor.ru/Attraction_Review-g23408135-d12244940-Reviews-Kite_school_DETIVETRA-Second_Hurghada_Hurghada_Red_Sea_and_Sinai.html
https://goo.gl/maps/KcoqXRbKWDkdGBDA8
https://goo.gl/maps/JTXRid9mrHFiMH7n6
https://detivetra.ru
https://detivetra.ru/v-hurgade/
https://uslugi.yandex.ru/profile/DetiVetra-348058
https://www.facebook.com/groups/detivetra/
https://www.facebook.com/DETIVETRA.ru/
https://www.youtube.com/channel/UCRG2aTtTaxbBAwlMTklO4ag
https://twitter.com/detivetra
https://rutube.ru/shorts/cb44391727e39c55e798f4efcecadbbe/
https://кайтинг-фото.рф/kayt-baza-v-hurgade.html
https://wa.me/79181955474
https://t.me/detivetrachat
https://www.instagram.com/detivetra.ru/
https://yandex.ru/profile/177183726173
https://yandex.ru/profile/1068186022
https://yandex.ru/profile/22472239976
https://yandex.ru/profile/72204785887
https://yandex.ru/profile/191148233877
https://xn—-7sbjteeyka8afw.xn--p1ai
https://vk.com/kiteschoolhurghadaegypt
https://vk.com/kite_deti_vetra_anapa
http://redseakite.ru
https://kiteschoolhurghada.ru
https://supanapa.ru
https://dzen.ru/a/ZdvCwDY4Lkx9j1OO
https://dzen.ru/a/ZdvAL3ptMCiNF8WX
https://dzen.ru/a/ZfigvDY3E2MON77x
https://dzen.ru/a/ZduLXm6jwDrtxbA7
https://dzen.ru/a/Zdt-SFBRjmyXXC7Y
https://dzen.ru/a/ZdvBm2tFz2D1IlSO
https://dzen.ru/a/Zdu_3c5NKkyfX91J
https://dzen.ru/a/ZdvCINgebnj0b5BQ
https://dzen.ru/a/ZTNXr-xw-1ZbmsTT
https://dzen.ru/a/ZdCSXCL_8k_Tfrjb
https://dzen.ru/a/ZdKxCtywnzYZgrrk
https://dzen.ru/a/ZTNfJwlmuCeqOEbb
https://dzen.ru/video/watch/65db9f94fb98fa6a95ab5e45?rid=925618026.48.1718444364156.12030
https://dzen.ru/video/watch/65d4341844e19f05a34cf61f?rid=925618026.48.1718444364156.12030
https://dzen.ru/video/watch/65629d684f336820c3b37487?rid=925618026.48.1718444364156.12030
https://dzen.ru/video/watch/64f689e39e331d6580277a0a?rid=925618026.48.1718444364156.12030
https://dzen.ru/video/watch/64f6870bcb2c7822b9dbd15c?rid=925618026.48.1718444364156.12030
https://dzen.ru/video/watch/6562982bd9b42c2e6bd10cae?rid=925618026.48.1718444364156.12030
https://dzen.ru/video/watch/64f684e1113e7a6a574076b2?rid=925618026.48.1718444364156.12030
https://dzen.ru/a/ZTNYEjMz-VkvVGnB
https://dzen.ru/a/ZTNi1ECY6h8nFsSW
https://dzen.ru/a/ZTNpJ546JGYImpH1
https://dzen.ru/a/ZTNTlZAOEk3ppmIO
https://dzen.ru/a/Zc_f2thSeW9zscF8
https://dzen.ru/a/Zc_U25ITFGrFK0Sf
https://dzen.ru/a/Zc_TAlxn1y1iHc43
https://dzen.ru/a/Zc_LEcYYfS-TrXXm
https://dzen.ru/a/ZVjdtJSQOhzbk0B1
https://dzen.ru/a/ZTNS7dJWml8dLCim
https://dzen.ru/a/ZVdT4K4CRxpHZx1i
https://dzen.ru/a/ZVdb9TtUnQGek7_G
https://dzen.ru/a/ZTNUDrwjFj4U3LBI
https://dzen.ru/a/ZTNVSZAOEk3pp-ki
https://dzen.ru/a/ZTNptIGpwlQkN-E1
https://dzen.ru/a/ZTNqooGpwlQkORsw
https://dzen.ru/a/ZTNnrk1VVGtQwolr
https://dzen.ru/a/ZTNS0bwjFj4U25A9
https://dzen.ru/a/ZTNG7QlmuCeqISH6
https://dzen.ru/a/ZTM87Ogjh2c_dNzY
https://dzen.ru/b/ZS5ge6k6swm-YNzl
https://dzen.ru/b/ZS1BbXqKjhX4tA9_
https://dzen.ru/a/ZQ2aw4OzGFVaAbr2
https://dzen.ru/b/ZS5YZ7qcv2E0eJPQ
https://dzen.ru/a/ZQrJrb_XDifDVzGP
https://dzen.ru/a/ZQrIWS4n3ChkWGvj
https://dzen.ru/a/ZQrCRLpsYU4IGsNS
https://dzen.ru/a/ZQq5kyCaIkuoWgEb
https://dzen.ru/a/ZQq6z8LJ7AdPjTX6
https://dzen.ru/a/ZQrBHwW8ugdAoeld
https://dzen.ru/a/ZQq5zAyUYGuFpV6x
https://dzen.ru/a/ZQq6uF9dOExoiJn3
https://dzen.ru/a/ZQq4kyCaIkuoV7HL
https://dzen.ru/a/ZPZtZAWIHk1Wxu36
https://dzen.ru/a/ZPZ324ORLBGu36oK
https://dzen.ru/a/ZPZ6mPf64TnWQmu4
https://dzen.ru/a/ZPZqtxo5MUGqivHt
https://dzen.ru/a/ZPZ3KI7QZRjreV_m
https://dzen.ru/a/ZPZsDLf4FUB9-vTG
https://dzen.ru/a/ZPZyyDEQHn8iOuK4
https://dzen.ru/a/ZPZnc9bwiVMxJaDt
https://dzen.ru/a/ZPZ2CaKi2WZuFDzk
https://dzen.ru/a/ZPZw1iSYk23jVqD2
https://dzen.ru/a/ZPZvXBo5MUGqmExv
https://dzen.ru/a/ZPZusNZDrka3nhk4
https://dzen.ru/a/ZPZomY7QZRjrXUWH
https://dzen.ru/a/ZPZlbm0hO0O6e_6G
https://dzen.ru/a/ZPZkz_Z4SEywAI9r
https://dzen.ru/a/ZPZfLRo5MUGqXPqk
https://dzen.ru/a/ZPZkQ47QZRjrTEiz
https://dzen.ru/a/ZPZgZG72vTlMv-RA
https://dzen.ru/a/ZPZUGxo5MUGqMHlM
https://dzen.ru/a/ZPZTWRo5MUGqLTP3
https://dzen.ru/a/ZPZSyvf64TnWzJmJ
https://dzen.ru/a/ZPZQptZDrka3MWh9
https://dzen.ru/a/ZPZRU5bXthdQL0rT
https://dzen.ru/a/ZPZR4wm7BXTGU4QM
https://dzen.ru/a/ZPZO09ZDrka3LqM1
https://dzen.ru/a/ZPZMvxpdEnCdGygY
Недавно нашел на http://www.maoflag.cc/home.php?mod=space&uid=274771 – РіРёР·Р±Рѕ казик,
и решил поделиться своим впечатлением.
Платформа выглядит довольно привлекательной,
особенно когда ищешь надежное казино.
Есть кто-то реально использовал Gizbo Casino?
Поделитесь своим опытом!
Особенно интересно узнать про промокоды и акции.
Например, есть ли Gizbo Casino особые условия для новых пользователей?
Еще интересно, как получить рабочее зеркало Gizbo Casino, если основной портал не работает.
Читал немало разных мнений, но интересно узнать честные рекомендации.
Допустим, как лучше использовать бонусы на Gizbo Casino?
Расскажите своим мнением!
Тут можно преобрести сео продвижение медицинских сайтов продвижение в поисковых системах медицинского сайта
Настройка Wi-Fi на принтерах Epson, таких, как модели L3250 и L3251, позволяет значительно упростить процесс печати и сканирования. Вы сможете печатать документы прямо с компьютера, смартфона или планшета, не используя провода.
здесь
мосбет казино https://www.mostbet20.com.kg .
Тут можно преобрести разработка сайтов медицинских центров разработка сайта больницы
Тут можно приобрести сайт клиники под ключ создание сайта медицинского центра
Vibracion del motor
Sistemas de balanceo: clave para el rendimiento estable y productivo de las dispositivos.
En el ámbito de la ciencia avanzada, donde la rendimiento y la confiabilidad del equipo son de máxima trascendencia, los aparatos de equilibrado cumplen un rol esencial. Estos dispositivos dedicados están concebidos para equilibrar y estabilizar elementos móviles, ya sea en maquinaria industrial, vehículos de traslado o incluso en electrodomésticos hogareños.
Para los técnicos en soporte de aparatos y los ingenieros, operar con dispositivos de calibración es crucial para garantizar el desempeño fluido y confiable de cualquier aparato móvil. Gracias a estas alternativas tecnológicas modernas, es posible reducir notablemente las vibraciones, el ruido y la carga sobre los sujeciones, aumentando la longevidad de componentes importantes.
De igual manera importante es el función que cumplen los dispositivos de ajuste en la asistencia al comprador. El apoyo experto y el conservación constante empleando estos aparatos facilitan proporcionar prestaciones de óptima excelencia, elevando la bienestar de los clientes.
Para los titulares de negocios, la contribución en sistemas de calibración y medidores puede ser esencial para incrementar la eficiencia y eficiencia de sus aparatos. Esto es particularmente importante para los emprendedores que dirigen pequeñas y medianas negocios, donde cada elemento es relevante.
Asimismo, los aparatos de balanceo tienen una amplia uso en el campo de la prevención y el monitoreo de nivel. Habilitan localizar potenciales fallos, impidiendo reparaciones costosas y perjuicios a los sistemas. Incluso, los indicadores recopilados de estos sistemas pueden emplearse para optimizar métodos y potenciar la visibilidad en plataformas de investigación.
Las áreas de utilización de los sistemas de equilibrado abarcan variadas ramas, desde la manufactura de ciclos hasta el supervisión ambiental. No importa si se habla de grandes fabricaciones manufactureras o reducidos establecimientos caseros, los aparatos de calibración son indispensables para promover un funcionamiento productivo y sin presencia de detenciones.
Игровые автоматы на деньги лучшие получить 1000 рублей бесплатно за регистрацию
Unlock your vehicle’s potential with our top-notch auto tuning services! Explore your ride into a stunning masterpiece with our expert team. We specialize in refinements that cater to your unique style. Our high-quality solutions ensure optimal performance and eye-catching design . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://phoenix-autobodyshop.com/ today and take the first step towards your dream car!
Usual Veda is at the forefront of blockchain development, providing innovative technology solutions for businesses and developers. Our expertise in decentralized systems and secure blockchain applications enables companies to optimize operations and stay ahead in the digital era. With Usual Veda, businesses gain access to reliable, scalable, and cutting-edge technology that drives success. https://usual-vault.com/
mostbet бездепозитный бонус https://mostbet3002.ru .
mostbet ilovasi https://mostbet3001.ru/ .
Криптобосс казино промокод
https://t.me/casino_cryptoboss_official
Here you will find unique collection of rare banknotes from all over the planet. We offer both modern and historical specimens that will become real finds for collectors.
For more information, follow the link продажа монет на мешке
Our team of experts carefully selects each lot, ensuring authenticity.
статьи по играм карта россии для euro truck simulator 2
Ethereal Trade is a cutting-edge decentralized exchange that redefines the way users trade cryptocurrencies. With a focus on security, speed, and efficiency, Ethereal Trade offers a seamless trading experience without intermediaries. As a leader in crypto trading, our platform ensures transparent transactions and advanced trading tools, making it the go-to solution for both beginners and experienced traders. https://ethereal.ac
Huawei RC предоставляет качественные услуги по ремонту техники Huawei, включая VR системы, ИБП (источники бесперебойного питания), ноутбуки, планшеты, серверы, смарт-часы и смартфоны.
здесь
Купить документ о получении высшего образования можно в нашей компании в Москве. simulatedattack.com/2024/02/lets-encrypt-failure-it-was-dnsish-a-conspiracy-of-coincidences
вин 1 http://www.1win101.com.kg .
1 win казино 1 win казино .
Собственное производство металлоконструкций. Если вас интересует Навес с двухскатной крышей мы предлогаем изготовление под ключ цена навес для одной машины
кухни на заказ недорого в москве Кухни на заказ в Москве и МО – это идеальное решение для тех, кто ценит индивидуальность и функциональность в каждой детали. В отличие от готовых гарнитуров, кухни, созданные по индивидуальному проекту, позволяют максимально эффективно использовать пространство, учитывая все особенности планировки и личные предпочтения владельцев.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good. https://accounts.binance.info/en-IN/register?ref=UM6SMJM3
Наушники AirPods представляют собой удобный и стильный аксессуар от Apple, который популярен благодаря качественному звуку и беспроводному соединению. Однако владельцы этих устройств нередко сталкиваются с проблемой их внезапного отключения от телефона. Чтобы устранить эту неприятность, важно разобраться с ее причинами и понять, как их исправить.
здесь
1wln https://1win100.com.kg .
1xslots бездепозитный бонус Бонусы за регистрацию
игры читайте статьи cyberpunk 2077 королева автострады концовки
Быстрая схема покупки диплома старого образца: что важно знать?
Роботы-пылесосы стремительно вошли в нашу жизнь, избавив нас от утомительных рутинных задач. Среди них особое место занимает Roborock, известный своей надежностью и удобством. Однако для того чтобы максимально использовать его возможности, важно разобраться, как настроить устройство. В этой статье я расскажу, как подключить робот-пылесос Roborock к телефону через Wi-Fi и интегрировать его с голосовым помощником Алисой от Яндекса. Все шаги простые, понятные, и вы однозначно справитесь с этим!
здесь
mostbet. http://www.mostbet3004.ru .
Игровые автоматы на деньги играть фриспины без депозита с выводом за регистрацию
кнауф утеплитель Строительные материалы компании Кануф в России. Доставка – Самовывоз. Оплата Нал/Безнал. огромный ассортимент товара.
сайт статьи по играм красивые дома для выживания в майнкрафт
Где приобрести диплом по актуальной специальности?
Приобрести документ института можно в нашей компании в Москве. Мы предлагаем документы об окончании любых университетов РФ. thelspr.listbb.ru/viewtopic.php?f=13&t=1099
Мы предлагаем документы любых учебных заведений, которые находятся на территории всей Российской Федерации. [url=http://sdiplom.ru/kupit-diplom-v-leninogorske-6/]sdiplom.ru/kupit-diplom-v-leninogorske-6[/url]
1win регистрация https://www.1win108.com.kg .
1vin 1vin .
прохождение игр на пк cyberpunk 2077 прохождение основного сюжета
mostbet app mostbet app .
The auto recovery service was fast and efficient! They arrived on time and got my car back on the road in no time.”
mostbet mi https://mostbet3007.ru .
1win официальный сайт войти 1win42.com.kg .
1xslots бездепозитный бонус bonus casino
Собственное производство металлоконструкций. Если вас интересует навесы из поликарбоната заказать мы предлогаем изготовление под ключ навесы для машины купить
Выбор оптического прицела – одна из самых важных задач для охотника. Однако часто встречаются проблемы, связанные с недостаточной информированностью и грамотностью охотников, что может привести к серьезным промахам. Давайте разберемся в ключевых аспектах выбора оптики для лесной охоты.
здесь
Доска бесплатных объявлений objvlenie.ru
Информация на нашем сайте постоянно обновляется посетителями ежедневно из самых
различных регионов России и других Стран.
«objvlenie.ru» очень понятный сервис для любого пользователя, любого возраста.
Удобный сайт для подачи бесплатных и платных объявлений.
Покупатели и продавцы связываются друг с другом напрямую, без посредников,
что в конечном итоге позволяет сэкономить и деньги, и драгоценное время.
Премиум размещение стоит совсем недорого.
Сайт бесплатных объявлений objvlenie.ru
статьи про игры как установить скин в майнкрафт лаунчер
Современные смарт-часы Garmin – это надежные устройства, сделанные для удобного отслеживания активности и других данных. Но иногда их пользователи сталкиваются с техническими проблемами. Разберем основные из них и способы решения.
перейти
Ипотека без сложностей https://volexpert.ru Наши риэлторы помогут выбрать идеальную квартиру и оформить ипотеку на лучших условиях. Работаем с топовыми банками, сопровождаем сделку, защищаем ваши интересы. Делаем покупку недвижимости доступной!
Jante Rimnova
UID_78116900###
Anto Sedang Menonton Mickey17 Dan Mendapatkan 700 Juta
брал тут цветы на праздник от Цветаевой просто топ, всем советую
1вин https://aktivnoe.forum24.ru/?1-8-0-00000252-000-0-0-1741169084 .
кайт школа хургада
seo фирма http://www.prodvizhenie-sajtov11.ru .
Thank you for another informative website. Where else may I get that kind of info written in such an ideal manner? I’ve a challenge that I am just now operating on, and I have been at the glance out for such information.
дивитися фільми онлайн безкоштовно у високій якості
Hmm is anyone else encountering problems with the pictures on this blog loading? I’m trying to figure out if its a problem on my end or if it’s the blog. Any suggestions would be greatly appreciated.
free comic book reader on PC
Ваш гид по дизайну https://sales-stroy.ru строительству и ремонту! Советы профессионалов, актуальные тенденции, проверенные технологии и подборки лучших решений для дома. Всё, что нужно для комфортного и стильного пространства, на одном портале!
1win букмекер https://svstrazh.forum24.ru/?1-18-0-00000135-000-0-0-1741169701/ .
Диплом ВУЗа Российской Федерации!
Без института трудно было продвинуться вверх по карьере. Именно по этой причине решение о заказе диплома можно считать выгодным и рациональным. Купить диплом об образовании chateando.net/read-blog/5539_kupit-svidetelstvo-o-zaklyuchenii-braka.html
скачать mostbet https://cah.forum24.ru/?1-13-0-00001559-000-0-0 .
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по выгодным тарифам. Преимущества покупки документов в нашей компании
Вы заказываете документ в надежной и проверенной временем компании. Это решение позволит вам сэкономить не только много средств, но и время.
На этом преимущества не заканчиваются, их гораздо больше:
• Документы изготавливаются на оригинальных бланках с мокрыми печатями и подписями;
• Предлагаем дипломы любого ВУЗа РФ;
• Цена во много раз меньше той, которую довелось бы платить на очном и заочном обучении в университете;
• Максимально быстрая доставка как по столице, так и в другие регионы РФ.
Приобрести диплом о высшем образовании– http://socialvockmarkingsiteswithhigh.copiny.com/question/details/id/1061146\ – socialvockmarkingsiteswithhigh.copiny.com/question/details/id/1061146
Недавно нашел на http://www.gtcm.info/home.php?mod=space&uid=981779 – gizbo casino рабочее,
и захотел поделиться своим впечатлением.
Платформа кажется довольно интересной,
особенно если ищешь надежное игровое заведение.
Есть кто-то реально использовал Gizbo Casino?
Поделитесь своим мнением!
В частности любопытно узнать про бонусы и фриспины.
Допустим, предлагают ли Gizbo Casino специальные условия для начинающих пользователей?
Также интересно, как получить рабочее зеркало Gizbo Casino, если основной сайт не работает.
Видел немало противоречивых мнений, но интересно узнать реальные советы.
Например, где лучше использовать промокоды на Gizbo Casino?
Поделитесь своим мнением!
Купить диплом университета!
Мы можем предложить дипломы любой профессии по доступным тарифам. Вы покупаете диплом через надежную фирму. : newru.getbb.ru/viewtopic.php?f=29&t=2610
фриспины без депозита с выводом за регистрацию Игровые автоматы на деньги реальные
можно ли отправлять посылки почтой Бесплатные путешествия — реальность! Я расскажу как без затрат посещать теплые страны. Многие россияне обосновались на Бали, и им часто требуется доставка вещей из России. За транспортировку они готовы платить от 20 долларов за килограмм или фиксированную сумму за посылку. Берем два чемодана, заполняем их посылками, и вот мы уже на Бали, да еще и с прибылью! Найти заказчиков легко: поищите объявления на сайтах вроде https://t.me/antsdelivery и https://pikabu.ru/story/kak_mozhno_zarabotat_eshchyo_i_na_svoem_otdyikhe_12429519 наш официальный сайт https://antsdelivery.ru
Здравствуйте!
Без присутствия диплома трудно было продвигаться вверх по карьере. В настоящее время документ не дает абсолютно никаких гарантий, что удастся найти привлекательную работу. Намного более важное значение имеют практические навыки и знания специалиста, а также его постоянный опыт. Именно из-за этого решение о заказе диплома следует считать целесообразным. Заказать диплом ВУЗа cambrigerp.forumex.ru/viewtopic.php?f=21&t=7929
This site truly has all of the information I needed about this subject and didn’t know who to ask.
manga online reader with color
купить диплом во владимире 2orik-diploms.ru .
DJ-пульты подвергаются высоким нагрузкам, особенно при частых выступлениях и транспортировках. Нередко поломки случаются из-за попадания влаги, механических повреждений или износа компонентов.
подробнее
Hello to every single one, it’s genuinely a good for me to go to see this web site, it consists of valuable Information.
киного бесплатные фильмы без подписки и рекламы
Как получить диплом стоматолога быстро и официально
Trazite pouzdane elektricni motori? Imamo siroku paletu modela za razlicite zadatke. Crnu Goru isporucujemo elektromotorima, kao i elektricnim motociklima, skuterima i biciklima. Ekoloski prihvatljiv transport za udobno putovanje. Visokokvalitetni motori i komponente po konkurentnim cijenama. Dostava i konsultacije – kontaktirajte nas!
Можно ли купить аттестат о среднем образовании? Основные рекомендации
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат психологической поддержки. Онлайн-консультация психолога.
Психолог онлайн анонимно. Психолог онлайн анонимно. Онлайн чат с психологом без регистрации.
Currently it appears like Drupal is the top blogging platform out there right now. (from what I’ve read) Is that what you’re using on your blog?
онлайн-кинотеатр с бесплатными фильмами в HD
Психологическая и информационная онлайн-помощь. Телеграм психолог. Психолог онлайн чат.
Психолог t me. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог оказывает помощь онлайн в чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн чат с психологом без регистрации. Получите консультацию онлайн-психолога в чате прямо сейчас.
В переписке у психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Онлайн чат с психологом без регистрации.
Анонимный чат с психологом телеграм. Анонимный чат с психологом телеграм. Чат психологической поддержки.
Получите консультацию онлайн-психолога в чате прямо сейчас. Анонимный чат с психологом телеграм. Психолог t me.
Получить первую онлайн консультацию психолога чате. Помощь психолога онлайн. Психолог t me.
Ремонт консолей Xbox в сервисном центре в Москве. Ремонт Xbox Series X 1TB Digital Edition (Robot White) Xbox Series S 1TB (Robot White) Xbox One X 1 TB
ссылка
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Психолог онлайн анонимно.
Психолог t me. Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге.
Thank you for some other great article. The place else may anybody get that kind of information in such an ideal method of writing? I’ve a presentation subsequent week, and I am at the search for such information.
новые боевики 2025 смотреть онлайн бесплатно
Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психологическая и информационная онлайн-помощь. Помощь психолога онлайн. Онлайн-консультация психолога.
Чат с психологом в телеге. Психолог в телеграм. Психолог оказывает помощь онлайн в чате.
Рекомендую – аренда автомобиля без водителя спб
купить московский диплом 2orik-diploms.ru .
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки. Получить первую онлайн консультацию психолога чате.
Онлайн-консультация психолога. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно.
Психолог оказывает помощь онлайн в чате. Онлайн чат с психологом без регистрации. Психолог оказывает помощь онлайн в чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог t me. Онлайн-консультация психолога.
Психолог онлайн анонимно. Онлайн чат с психологом без регистрации. Онлайн-консультация психолога.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн анонимно. Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн анонимно.
Тут можно преобрести заказать сайт медицинского центра создание сайта клиника
Анонимный чат с психологом телеграм. Получить онлайн консультацию психолога чате. Психолог t me.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате.
Психолог t me. Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
Онлайн-консультация психолога. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога.
Психолог t me. Чат психологической поддержки. Психолог t me.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно. Анонимный чат с психологом телеграм.
Получите консультацию онлайн-психолога в чате прямо сейчас. Помощь психолога онлайн. Круглосуточная запись на онлайн-консультацию психолога.
Психолог t me. Психолог t me. Телеграм психолог.
Чат психологической поддержки. Онлайн чат с психологом без регистрации. Получить онлайн консультацию психолога чате.
Психолог онлайн чат. Психолог в телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог оказывает помощь онлайн в чате. Психолог в телеграм. Психолог оказывает помощь онлайн в чате.
Hello, yup this piece of writing is truly pleasant and I have learned lot of things from it on the topic of blogging. thanks.
киного смотреть фильмы без рекламы на телефоне
Телеграм психолог. Психолог онлайн чат. Психолог оказывает помощь онлайн в чате.
Круглосуточная запись на онлайн-консультацию психолога. Психолог t me. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог в телеграм. Анонимный чат с психологом телеграм. Психолог онлайн чат.
Онлайн-консультация психолога. Психолог в телеграм. Психолог в телеграм.
Психолог онлайн чат. Психолог t me. Психолог онлайн чат.
Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн-консультация психолога. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог t me. Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн анонимно.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки. Психолог онлайн анонимно.
Почему сериалы онлайн так затягивают?
http://www.empyrethegame.com/forum/memberlist.php?sk=b&start=162525
Психолог онлайн чат. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Получить онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь. Телеграм психолог.
Где купить волосы Где купить волосы? ?? У нас вы можете приобрести натуральные волосы отличного качества! ?? Мы предлагаем: Натуральные волосы разных категорий: У нас есть волосы от самых доступных до премиум-класса, чтобы каждый мог найти идеальный вариант для себя. Разнообразие текстур и цветов: Выбор от прямых до кудрявых, а также широкий ассортимент оттенков, чтобы вы могли создать любой образ. Волосы для наращивания: Идеально подходят для тех, кто хочет добавить длину и объем. Мы гарантируем высокое качество и удовлетворение ваших потребностей! Свяжитесь с нами, чтобы узнать больше или сделать заказ. Мы всегда рады помочь! ??? пепельные волосы
Greate post. Keep writing such kind of info on your blog. Im really impressed by your blog.
Hey there, You have performed a fantastic job. I’ll definitely digg it and in my view suggest to my friends. I’m confident they’ll be benefited from this site.
киного смотреть сериалы онлайн бесплатно
свежие фриспины риобет Игровые автоматы играть на деньги
Психолог онлайн анонимно. Чат с психологом в телеге. В переписке у психолога.
Cryptoboss казино
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить первую онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога.
Психолог онлайн чат. В переписке у психолога. Получить онлайн консультацию психолога чате.
Круглосуточная запись на онлайн-консультацию психолога. Онлайн чат с психологом без регистрации. Анонимный чат с психологом телеграм.
Психолог t me. Психолог онлайн анонимно. Психолог онлайн чат.
Анонимный чат с психологом телеграм. Чат психологической поддержки. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог в телеграм. Психолог оказывает помощь онлайн в чате.
Телеграм психолог. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога.
Анонимный чат с психологом телеграм. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Онлайн-консультация психолога. Чат с психологом в телеге. Получить первую онлайн консультацию психолога чате.
создание и продвижение сайтов москва [url=www.prodvizhenie-sajtov13.ru]создание и продвижение сайтов москва[/url] .
Психолог онлайн анонимно. Анонимный чат с психологом телеграм. В переписке у психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить онлайн консультацию психолога чате. Телеграм психолог.
заказать продвижение сайта москва топ заказать продвижение сайта москва топ .
Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Помощь психолога онлайн.
купить игровой ноутбук 2024 https://igrovye-noutbuki.ru
Дипломированный психолог с опытом работы и отзывами клиентов. Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате.
Онлайн-консультация психолога. Телеграм психолог. Чат с психологом в телеге.
Чат с психологом в телеге. В переписке у психолога. Чат психологической поддержки.
В переписке у психолога. Психолог онлайн анонимно. Чат с психологом в телеге.
Психологическая и информационная онлайн-помощь. Круглосуточная запись на онлайн-консультацию психолога. В переписке у психолога.
Психологическая и информационная онлайн-помощь. Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн анонимно.
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог онлайн анонимно. Психолог помогающий искать решения в непростых психологических ситуациях. Получите консультацию онлайн-психолога в чате прямо сейчас.
Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн чат с психологом без регистрации. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог в телеграм. Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас.
Телеграм психолог. Психолог t me. Онлайн чат с психологом без регистрации.
Получить онлайн консультацию психолога чате. Телеграм психолог. Психологическая и информационная онлайн-помощь.
Awesome site you have here but I was wanting to know if you knew of any community forums that cover the same topics talked about here? I’d really love to be a part of online community where I can get suggestions from other experienced individuals that share the same interest. If you have any suggestions, please let me know. Kudos!
драмы 2025 смотреть онлайн бесплатно
Психолог оказывает помощь онлайн в чате. Онлайн-консультация психолога. Психолог оказывает помощь онлайн в чате.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Круглосуточная запись на онлайн-консультацию психолога. Онлайн чат с психологом без регистрации. В переписке у психолога.
Помощь психолога онлайн. Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога.
Всех приветствую!
Где купить диплом по необходимой специальности?
Мы изготавливаем дипломы любой профессии по доступным ценам. Цена может зависеть от выбранной специальности, года выпуска и образовательного учреждения. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы дипломы были доступны для подавляющей массы граждан.
Заказ диплома, который подтверждает обучение в университете, – это разумное решение. Просто-напросто подсчитайте, сколько вам пришлось бы вложить денег на ежемесячную оплату 5-летнего обучения, на питание, аренду жилья (если учащийся из другого города), на ежедневный проезд до института и обратно. Получается приличная сумма, которая значительно превышает расценки на наши документы. А ведь все это время можно уже успешно работать, продвигаясь по карьере.
Готовый диплом со всеми печатями и подписями полностью отвечает требованиям и стандартам, никто не отличит его от оригинала. Не откладывайте свои мечты на потом, реализуйте их с нами – отправьте простую заявку на диплом уже сегодня!
Диплом о среднем образовании – запросто! diplomanrus.com/
Онлайн-консультация психолога. Психолог онлайн чат. Онлайн чат с психологом без регистрации.
Чат психологической поддержки. Получите консультацию онлайн-психолога в чате прямо сейчас. Телеграм психолог.
Круглосуточная запись на онлайн-консультацию психолога. Онлайн-консультация психолога. В переписке у психолога.
Психолог онлайн анонимно. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате.
Онлайн чат с психологом без регистрации. Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн-консультация психолога.
Hi there mates, its enormous paragraph regarding cultureand entirely explained, keep it up all the time.
киного топ фильмов 2025 в высоком качестве
Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате. Психолог онлайн чат.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. В переписке у психолога. Круглосуточная запись на онлайн-консультацию психолога.
Получить первую онлайн консультацию психолога чате. Помощь психолога онлайн. Дипломированный психолог с опытом работы и отзывами клиентов.
Получить первую онлайн консультацию психолога чате. Чат с психологом в телеге. Онлайн чат с психологом без регистрации.
Психолог t me. Получить первую онлайн консультацию психолога чате. Чат психологической поддержки.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате.
купить аттестат о среднем образовании старого образца в prema-diploms.ru .
Получите консультацию онлайн-психолога в чате прямо сейчас. Дипломированный психолог с опытом работы и отзывами клиентов. Получить первую онлайн консультацию психолога чате.
Чат с психологом в телеге. Получите консультацию онлайн-психолога в чате прямо сейчас. Помощь психолога онлайн.
Телеграм психолог. Получить первую онлайн консультацию психолога чате. Чат психологической поддержки.
Психолог t me. Получить первую онлайн консультацию психолога чате. Психолог онлайн чат.
Товары для вашего авто avtostilshop.ru автоаксессуары, масла, запчасти химия, электроника и многое другое. Быстрая доставка, акции и бонусы для постоянных клиентов. Подбирайте товары по марке авто и будьте уверены в качестве!
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате. Психолог t me.
Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях.
Чат психологической поддержки. Получите консультацию онлайн-психолога в чате прямо сейчас. Анонимный чат с психологом телеграм.
Hi just wanted to give you a brief heads up and let you know a few of the images aren’t loading properly. I’m not sure why but I think its a linking issue. I’ve tried it in two different browsers and both show the same results.
фильмы 2025 года смотреть в Full HD 1080p
Дипломированный психолог с опытом работы и отзывами клиентов. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог в телеграм.
Телеграм психолог. Психолог оказывает помощь онлайн в чате. Онлайн чат с психологом без регистрации.
Онлайн чат с психологом без регистрации. Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог t me. Помощь психолога онлайн. Психолог t me.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно. Психолог в телеграм.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн анонимно.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог t me. Психологическая и информационная онлайн-помощь.
Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн анонимно. Круглосуточная запись на онлайн-консультацию психолога.
Получить первую онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. Получить онлайн консультацию психолога чате.
Психолог онлайн анонимно. В переписке у психолога. Психолог онлайн анонимно.
Психолог онлайн анонимно. Анонимный чат с психологом телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас.
Чат с психологом в телеге. Онлайн-консультация психолога. Психолог оказывает помощь онлайн в чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм.
мосбет chesskomi.borda.ru/?1-10-0-00000277-000-0-0-1741171219 .
Чат психологической поддержки. Психолог t me. Психолог помогающий искать решения в непростых психологических ситуациях.
Hi there i am kavin, its my first occasion to commenting anywhere, when i read this paragraph i thought i could also make comment due to this good article.
new manga releases read online
1вин кг https://www.aqvakr.forum24.ru/?1-3-0-00001121-000-0-0 .
Онлайн-консультация психолога. Чат с психологом в телеге. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог онлайн чат. Психолог в телеграм. Чат с психологом в телеге.
Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
Психолог онлайн анонимно. Телеграм психолог. Анонимный чат с психологом телеграм.
В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Онлайн-консультация психолога. Дипломированный психолог с опытом работы и отзывами клиентов. Круглосуточная запись на онлайн-консультацию психолога.
Чат психологической поддержки. Телеграм психолог. Телеграм психолог.
Психологическая и информационная онлайн-помощь. Получите консультацию онлайн-психолога в чате прямо сейчас. Дипломированный психолог с опытом работы и отзывами клиентов.
Чат психологической поддержки. Чат психологической поддержки. Психолог онлайн анонимно.
Такси для бизнеса https://roads.ru/main/promo-materialy/vozvrat-avto-popavshego-na-shtrafstoyanku/ работа по всей России. Удобные поездки для сотрудников! Оформите корпоративный аккаунт и получите выгодные условия, детальную отчетность и надежный сервис. Быстрое бронирование, прозрачные тарифы, комфортные автомобили – организуйте рабочие поездки легко!
Психологическая и информационная онлайн-помощь. Дипломированный психолог с опытом работы и отзывами клиентов. Дипломированный психолог с опытом работы и отзывами клиентов.
Помощь психолога онлайн. Помощь психолога онлайн. Анонимный чат с психологом телеграм.
Психолог онлайн анонимно. В переписке у психолога. Психологическая и информационная онлайн-помощь.
Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
whoah this weblog is great i love studying your posts. Keep up the great work! You know, lots of individuals are searching round for this information, you could aid them greatly.
фильмы 2025 смотреть онлайн на телефоне
Здравствуйте!
Заказ подходящего диплома через надежную фирму дарит ряд плюсов для покупателя. Такое решение позволяет сэкономить как дорогое время, так и серьезные финансовые средства. Впрочем, преимуществ значительно больше.Мы изготавливаем дипломы любой профессии. Дипломы производят на подлинных бланках государственного образца. Доступная стоимость сравнительно с серьезными издержками на обучение и проживание в чужом городе. Заказ диплома института станет мудрым шагом.
Купить диплом о высшем образовании: twitch.tv/diplomygroup/about
Психологическая и информационная онлайн-помощь. Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате.
Чат с психологом в телеге. Психолог в телеграм. Чат с психологом в телеге.
Top casino с выводом Игровые автоматы лучшие
1win mexico 1win mexico .
1win. https://cah.forum24.ru/?1-13-0-00001560-000-0-0-1741172791/ .
Улучшение отношений в коллективе РЕИНЖИНИРИНГ БИЗНЕС-ПРОЦЕССОВ: ПЕРЕЗАГРУЗКА ДЛЯ РОСТА Узнайте, как реинжиниринг бизнес-процессов помогает адаптировать компанию к изменяющимся условиям рынка. Реинжиниринг бизнес-процессов (BPR) – это радикальный пересмотр и перепроектирование существующих бизнес-процессов для достижения значительных улучшений в ключевых показателях эффективности, таких как затраты, качество, сервис и скорость. В условиях динамично меняющегося рынка, BPR становится необходимым инструментом для обеспечения конкурентоспособности. Ключевой целью реинжиниринга является оптимизация рабочих процессов, устранение избыточности и автоматизация операций. Это позволяет компаниям быстрее реагировать на изменения потребностей клиентов и эффективно использовать ресурсы. Внедрение реинжиниринга бизнес-процессов требует тщательного анализа текущего состояния компании и разработки новой, более эффективной модели. Важно определить узкие места, устаревшие технологии и процессы, которые препятствуют развитию. Результатом успешного реинжиниринга является повышение эффективности, снижение затрат и улучшение клиентского сервиса, что в конечном итоге способствует устойчивому росту компании. Не упустите возможность вывести свой бизнес на новый уровень. Свяжитесь с нами прямо сейчас и получите бесплатную консультацию. “Бизнес доп.” – ваш надежный партнер в управлении бизнесом.
Hello, yeah this post is actually good and I have learned lot of things from it concerning blogging. thanks.
новинки кино 2025 смотреть в Full HD 1080p
24/7 Availability: One of the major benefits of roadside assistance services is that they are available 24/7. This provides peace of mind for drivers, knowing they can receive help anytime, day or night.
Good day! I know this is kinda off topic however I’d figured I’d ask. Would you be interested in trading links or maybe guest writing a blog article or vice-versa? My blog discusses a lot of the same topics as yours and I think we could greatly benefit from each other. If you happen to be interested feel free to send me an email. I look forward to hearing from you! Awesome blog by the way!
новые фильмы 2025 без рекламы и регистрации
купить диплом техникума в одессе
Pricing and Service Quality: The cost of roadside assistance can vary based on the nature and difficulty of the service. However, it’s important to choose companies that offer competitive prices and high-quality services.
Hello there, You have done a fantastic job. I’ll definitely digg it and personally recommend to my friends. I am sure they’ll be benefited from this site.
фантастика 2025 дивитися онлайн безкоштовно HD
BEKU4D
порно онлайн бесплатно русское порно снял шлюху
https://www.business-gazeta.ru/article/517826
порно со детское порно девочек
Equipment and Technology: The equipment used in car recovery must be modern and compatible with today’s vehicles. This ensures the safe and efficient transport of vehicles with minimal damage.
Заполните форму заявки на сайте TCL Repair Center или свяжитесь с нами для уточнения деталей. Мы позаботимся о вашей технике TCL и быстро вернем её в строй! Не упустите возможность воспользоваться скидкой до 25% при записи онлайн.
ссылка
брал тут цветы на праздник Cvetaeva просто топ, всем советую
La pelea entre Mike Tyson y Jake Paul es un evento muy esperado | La historia de Mike Tyson demuestra que la perseverancia es clave | Mike Tyson sigue entrenando como un verdadero campeón | Mike Tyson ha sido una figura influyente en el boxeo y más allá Mike Tyson en la historia del boxeo .
Ковры для уюта вашего дома, найдите.
Лучшие варианты ковров для вашего дома, приобретите.
Эко-дизайн: ковры из натуральных материалов, выбирайте.
Декорируйте пространство с помощью ковров, стиль.
Безопасные и яркие ковры для детской, цвет.
Ковры в восточном стиле, откройте.
Ковры для офиса, добавьте.
Ковры, которые легко чистить, подходящий стиль.
Советы по выбору ковра, главные советы.
Теплота и уют с коврами, необходимый стиль.
Тенденции в мире ковров, свой интерьер.
Ковры для загородного дома, практичность.
Как сделать ваш интерьер уникальным с коврами, откройте.
Ковры: от классики до модерна, погрузитесь в.
Ковры для спальни, дизайны.
Премиальные ковры для вашего интерьера, успех.
Ковры для любителей животных, найдите.
Теплые ковры для холодных зим, приобретайте.
Как использовать ковры для зонирования, откройте.
каталог ковров каталог ковров .
Hi there are using WordPress for your site platform? I’m new to the blog world but I’m trying to get started and create my own. Do you require any html coding expertise to make your own blog? Any help would be really appreciated!
новинки кіно 2025 дивитися в Full HD 1080p
купить волосы Купить волосы: идеальное решение для изменения образа! Ищете способ обновить свой стиль Купить волосы – это отличный выбор для создания нового образа! Мы предлагаем широкий ассортимент натуральных волос, которые подойдут для любых целей: от наращивания до создания эффектных причесок. Наши волосы отличаются высоким качеством и разнообразием текстур и оттенков, что позволяет легко подобрать идеальный вариант именно для вас. Мы гарантируем, что вы получите продукцию, которая прослужит долго и будет выглядеть естественно. Не упустите возможность преобразиться! Закажите волосы прямо сейчас и наслаждайтесь новым образом уже сегодня! авито волосы для наращивания
Актуальні новини будівництва https://dverikupe.com.ua та нерухомості в Україні. Огляди ринку, тренди, технології, законодавчі зміни та експертні думки – все про будівельну галузь на одному сайті!
Онлайнказино Бонсай bonsai-casino2.com игровые автоматы, рулетка, покер и живые дилеры! Получайте бонусы за регистрацию, участвуйте в турнирах и выводите выигрыши без задержек. Надёжность, безопасность и азарт – всё в одном месте!
In three words, I can sum up everything I’ve learned about life: it goes on. 메이저놀이터
The only person you are destined to become is the person you decide to be. 슬롯사이트
Thanks for the good writeup. It in fact was once a enjoyment account it. Glance advanced to far introduced agreeable from you! However, how can we keep in touch?
лучшие триллеры 2025 смотреть онлайн в HD
Дізнавайтеся про останні новини https://ampdrive.info електромобілів, супекарів та актуальні події автомобільного світу в Україні. Ексклюзивні матеріали, фото та аналітика для справжніх автолюбителів на AmpDrive.?
Заполните форму заявки на сайте TCL Repair Center или свяжитесь с нами для уточнения деталей. Мы позаботимся о вашей технике TCL и быстро вернем её в строй! Не упустите возможность воспользоваться скидкой до 25% при записи онлайн.
подробнее
эвакуатор рядом http://www.evakuatormax.ru .
Balances Active Orders Withdraw Deposit TradeOgre Logout · Sign In. Search: Currency, Market, Change, Price
1 win https://aktivnoe.forum24.ru/?1-8-0-00000254-000-0-0-1741273702/ .
купить диплом 2001 prema-diploms.ru .
1вин войти http://1win109.com.kg/ .
Мы изготавливаем дипломы любых профессий по приятным тарифам. Стараемся поддерживать для покупателей адекватную ценовую политику. Для нас важно, чтобы документы были доступными для большого количества наших граждан.
Приобретение диплома, который подтверждает окончание института, – это грамотное решение. Приобрести диплом о высшем образовании: rdiploms.com
Доброго времени суток!
Для некоторых людей, купить диплом ВУЗа – это острая потребность, уникальный шанс получить достойную работу. Однако для кого-то – это очевидное желание не терять время на учебу в ВУЗе. Что бы ни толкнуло вас на такой шаг, наша фирма готова помочь вам. Максимально быстро, профессионально и по доступной цене изготовим диплом любого года выпуска на государственных бланках со всеми необходимыми печатями.
Основная причина, почему люди прибегают к покупке документа, – получить определенную работу. К примеру, знания и опыт дают возможность кандидату устроиться на привлекательную работу, однако подтверждения квалификации нет. Если работодателю важно присутствие “корочек”, риск потерять место работы довольно высокий.
Купить документ о получении высшего образования можно у нас в столице. Мы оказываем услуги по продаже документов об окончании любых ВУЗов России. Вы сможете получить диплом по любой специальности, включая документы Советского Союза. Даем гарантию, что при проверке документов работодателями, подозрений не возникнет.
Обстоятельств, которые вынуждают купить диплом ВУЗа много. Кому-то прямо сейчас нужна работа, а значит, необходимо произвести впечатление на начальника на протяжении собеседования. Другие желают устроиться в серьезную компанию, для того, чтобы повысить свой статус в обществе и в дальнейшем начать свой бизнес. Чтобы не терять попусту годы жизни, а сразу начинать удачную карьеру, используя врожденные таланты и полученные навыки, можно приобрести диплом в онлайне. Вы сможете быть полезным для социума, обретете денежную стабильность очень быстро и просто- купить диплом о среднем образовании
Привет!
Без наличия диплома очень сложно было продвигаться по карьере. В последние годы документ не дает никаких гарантий, что удастся найти привлекательную работу. Более важное значение имеют профессиональные навыки специалиста и его постоянный опыт. Именно из-за этого решение о покупке диплома можно считать выгодным и рациональным. Купить диплом об образовании musical-kirche.de/read-blog/49248_gde-mozhno-kupit-attestat-za-11.html
Thank you for the auspicious writeup. It in fact was once a enjoyment account it. Glance complex to far introduced agreeable from you! By the way, how can we keep up a correspondence?
новые детективы 2025 смотреть онлайн в HD
Jante Rimnova
Balances Active Orders Withdraw Deposit Logout · Sign In. Search: Currency, Market, Change, Price
купить диплом в кисловодске купить диплом в кисловодске .
Привет!
Мы изготавливаем дипломы любой профессии по невысоким ценам. Цена может зависеть от той или иной специальности, года получения и ВУЗа: rdiploms.com/
1win баланс https://www.1win10.am .
Приобрести диплом ВУЗа !
Специалисты, у которых есть высшее образование всегда ценились среди работодателей. Диплом университета потребуется, чтобы доказать свой высокий профессионализм. Он дает понять руководству, что сотрудник обладает важными навыками и знаниями для того, чтобы очень качественно выполнить свою задачу. Но что можно сделать, когда знания имеются, а подтверждающего документа у человека нет? Заказ диплома решит данную проблему. Покупка диплома ВУЗа России в нашей компании – надежный процесс, потому что документ заносится в государственный реестр. Печать производится на официальных бланках ГОЗНАКа. Быстро купить диплом о высшем образовании gyrpg.gtaserv.ru/viewtopic.php?f=5&t=1312
Нередко случается так, что для продвижения по карьерной лестнице, понадобится документ, который подтверждает наличие высшего образования. Где приобрести диплом специалиста?
Купить документ ВУЗа вы можете в нашей компании в столице. Мы предлагаем документы об окончании любых ВУЗов РФ. [url=http://spiymaibarigu.com.ua/]spiymaibarigu.com.ua/[/url]
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
This is very interesting, You’re a very skilled blogger. I’ve joined your rss feed and look forward to seeking more of your fantastic post. Also, I’ve shared your site in my social networks!
лучшие фильмы онлайн 2025 без рекламы
Игровые автоматы играть на деньги Игровые автоматы с бонусом за регистрацию без депозита
https://fedpress.ru/news/77/economy/3103308
Список частых проблем и причин их возникновения: тепловизор не включается, изображение искажено или отсутствует, устройство стало медленно работать подробнее
Привет!
Купить диплом университета по невысокой стоимости возможно, обращаясь к надежной специализированной компании. Заказать диплом о высшем образовании: freediplom.com/kupit-diplom-tolyatti-5/
посвящение в первоклассники диплом купить в минске
click here to find out more https://brd-wallet.com/
https://ceramodecol.ru
Jante Rimnova
Feel free to surf to my blog https://cryptolake.online/crypto7
This is the perfect site for everyone who really wants to find out about this topic. You realize so much its almost tough to argue with you (not that I really would want to…HaHa). You definitely put a fresh spin on a subject that has been written about for decades. Great stuff, just great!
лучшие фильмы 2025 года смотреть онлайн в 4К
Актуальні новини політики https://insideukr.com в Україні та світі. Аналітика, думки експертів, головні події дня та ексклюзивні матеріали – будьте в курсі разом із нами!
Honey Money Казино https://honeymoneycasino.ru это захватывающий мир азартных игр с щедрыми бонусами, быстрыми выплатами и огромным выбором слотов, рулетки, покера и других развлечений. Играй онлайн в любое время, участвуй в турнирах и получай эксклюзивные награды.
Ищешь лучшие онлайн казино https://casinobazar.ru/5-prichin-nikogda-ne-ispolzovat-bitcoin-na-sajtah-kazino/ Мы собрали рейтинг топовых площадок с лицензией, безопасностью и крупными бонусами. Наслаждайся сотнями игр, моментальными выплатами и честными условиями.
Wow, incredible blog layout! How long have you been running a blog for? you make blogging look easy. The full glance of your site is excellent, let alone the content material!
фильмы 2025 смотреть онлайн без рекламы
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Рекомендую – ремонт телефона редми нот
Los fanáticos del boxeo aún recuerdan los mejores golpes de Mike Tyson | Mike Tyson ha cambiado su vida después del boxeo | Mike Tyson sigue entrenando como un verdadero campeón | Mike Tyson ha sido una figura influyente en el boxeo y más allá Mike Tyson y su legado .
Мы можем предложить дипломы любой профессии по доступным тарифам.– minions.ch/fussball-foul/#comment-34
керамогранит для пола купить керамогранит 1200х600
керамогранит матовый https://magazin-keramogranit.ru
Купить телевизоры на дачу https://televizory-dlya-dachi.ru в интернет-магазине по низкой цене! При покупке дачных телевизоров на сайте воспользуйтесь скидками, акциями, бонусной программой.
Hi! This post could not be written any better! Reading this post reminds me of my previous room mate! He always kept talking about this. I will forward this write-up to him. Pretty sure he will have a good read. Many thanks for sharing!
смотреть фильмы онлайн бесплатно в хорошем качестве
index https://web-martianwallet.io/
1 вин про http://www.1win15.com.kg .
1win ставки официальный сайт 1win ставки официальный сайт .
1win.mx http://www.1win7.com.mx .
фриспины без депозита с выводом за регистрацию 1xslots бездепозитный бонус
his response https://toastwallet.org/
I’m not sure why but this website is loading very slow for me. Is anyone else having this issue or is it a problem on my end? I’ll check back later and see if the problem still exists.
новые фильмы 2025 года смотреть онлайн в HD
“I think it’s a really important step forward,” Biden said in a brief appearance before media at the White House, urging “both chambers (of Congress) to pass that agreement”. 스포츠토토
Ремонт оптических прицелов в Краснодаре: выполним ремонт в 4 быстрых шага. Оставляйте свою заявку на сайте здесь
Где купить диплом специалиста?
Мы изготавливаем дипломы любой профессии по разумным тарифам. Мы можем предложить документы техникумов, которые находятся на территории всей РФ. Можно заказать качественно напечатанный диплом за любой год, указав подходящую специальность и оценки за все дисциплины. Документы делаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. Они будут заверены необходимыми печатями и штампами. Всегда стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы дипломы были доступными для подавляющей массы граждан. okdiplom.com/kupit-diplom-v-magadane-2-3
learn this here now https://web-multibit.org
Отдых в Анапе https://otdyh-vanape.ru идеальный выбор для всей семьи! Чистые песчаные пляжи, теплое море, развитая инфраструктура и развлечения на любой вкус. Гостиницы, отели, частный сектор – найдите идеальное жилье.
Натяжные потолки с установкой https://natyazhnye-potolki2.ru стильное и практичное решение для любого интерьера. Предлагаем широкий выбор фактур и цветов, качественные материалы, быстрый монтаж и гарантию на работу. Устанавливаем потолки любой сложности в квартирах, домах, офисах.
I get pleasure from, cause I found exactly what I used to be looking for. You’ve ended my 4 day lengthy hunt! God Bless you man. Have a great day. Bye
дивитися кіно онлайн безкоштовно в Україні|
купить недорогую приставку для телевизора nedorogie-televizory.ru
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Стоимость может зависеть от определенной специальности, года получения и университета. Стараемся поддерживать для покупателей адекватную политику цен. Для нас важно, чтобы документы были доступны для большинства граждан. купить диплом о высшем образовании ссср
Дягилев
1win официальный сайт скачать https://1win110.com.kg .
Do you have a spam issue on this blog; I also am a blogger, and I was wondering your situation; we have created some nice practices and we are looking to exchange strategies with others, please shoot me an e-mail if interested.
кращі фільми онлайн безкоштовно у Full HD
ван вин http://1win103.com.kg/ .
Все о компьютерных играх https://lifeforgame.ru/ обзоры новых проектов, рейтинги, детальные гайды, новости индустрии, анонсы и системные требования. Разбираем особенности геймплея, помогаем с настройками и прохождением. Следите за игровыми трендами, изучайте секреты и погружайтесь в мир гейминга.
Купить документ о получении высшего образования вы имеете возможность в нашем сервисе. diplomgorkiy.com/kupit-diplom-orenburg-4-2
как подключить smart tv к телевизору smart tv s3 телевизор
Hello! I realize this is kind of off-topic but I needed to ask. Does running a well-established website such as yours require a large amount of work? I’m brand new to blogging however I do write in my diary on a daily basis. I’d like to start a blog so I can share my own experience and thoughts online. Please let me know if you have any kind of recommendations or tips for new aspiring blog owners. Thankyou!
кращі фільми онлайн у Full HD
телевизор oled или qled телевизор oled evo
один вин официальный сайт один вин официальный сайт .
Ремонт оптических прицелов и тепловизоров Dedal перейти
Dota 2 Коллекционная фигрука
Рекомендую – либет казино войти
https://kazhdogopravo.ru
купить волосы для наращивания Купить волосы для наращивания: ваш путь к стильным прическам! Хотите добавить длины и объема своим волосам? У нас вы найдете идеальные волосы для наращивания! Мы предлагаем разнообразие натуральных волос, которые помогут вам создать уникальный и эффектный образ. Наши волосы отличаются высоким качеством, мягкостью и естественным блеском. Вы сможете выбрать из множества текстур и оттенков, чтобы идеально дополнить свой стиль. Мы уверены, что наши продукты прослужат вам долго и будут выглядеть потрясающе! Не упустите возможность изменить свой стиль! Закажите волосы для наращивания прямо сейчас и начните свое преображение уже сегодня! купить волосы воронеж
Unlock your vehicle’s potential with our top-notch auto tuning services! Enhance your ride into a stunning masterpiece with our expert team. We specialize in modifications that cater to your unique style. Our reliable solutions ensure optimal performance and enhanced appearance. Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://americasbestcertifiedautobody.com/ today and take the first step towards your dream car!
попробуйте эту доставку купить букеты всегда свежие цветочки, быстрая и бесплатная доставка, сервис огонь!
I never realized how important a reliable car recovery service was until I needed it. It made a stressful situation so much easier
Привет, друзья!
Руководители серьезных компаний очень часто выбирают соискателей, окончивших ВУЗ. Особенно в приоритете престижные заведения. Однако учиться пять лет – это долго и дорого, не у всех есть такая возможность. Купить документ становится самым выгодным решением.
Бывают и непредвиденные обстоятельства, когда диплом теряется или портится. Не всегда возможно быстро и без осложнений восстановить его, особенно когда университет закрыт или находится далеко в другом регионе страны. Бюрократия отнимает массу времени и нервов.
Для эффективного продвижения вверх по карьере требуется наличие диплома о высшем образовании. Но зачастую в жизни может случиться так, что определенные трудности мешают успешно окончить учебу и заполучить важный документ.
Купить диплом ВУЗа
Наши специалисты предлагают быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями официальных лиц. Диплом пройдет лубую проверку, даже при помощи профессионального оборудования. Решите свои задачи максимально быстро с нашими дипломами.
Где заказать диплом по нужной специальности? diplomanrus.com/
Psychological Support for Drivers: Roadside assistance doesn’t only offer physical help. It also provides psychological support for drivers who may be stressed, especially in difficult or remote situations.
Все о недвижимости https://magdesi.ru покупка, аренда, ипотека. Разбираем рыночные тренды, юридические тонкости, лайфхаки для выгодных сделок. Помогаем выбрать квартиру, рассчитать ипотеку, проверить документы и избежать ошибок при сделках с жильем. Актуальные статьи для покупателей, арендаторов и инвесторов.
Все о недвижимости https://konsta-ovk.ru покупка, аренда, ипотека. Разбираем рыночные тренды, юридические тонкости, лайфхаки для выгодных сделок. Помогаем выбрать квартиру, рассчитать ипотеку, проверить документы и избежать ошибок при сделках с жильем. Актуальные статьи для покупателей, арендаторов и инвесторов.
Купить документ института вы имеете возможность у нас. extelis.fr
Где купить диплом по нужной специальности?
Приобрести диплом института по доступной цене вы можете, обращаясь к проверенной специализированной фирме.: nsk-diplom.com
Мы готовы предложить дипломы любой профессии по приятным тарифам. Дипломы изготавливаются на настоящих бланках государственного образца Заказать диплом о высшем образовании diplomj-irkutsk.ru
эскорт услуги москва эскорт услуги москва .
this contact form https://hitman-assassin-killer.com
Все о компьютерных играх http://lifeforgame.ru обзоры новых проектов, рейтинги, детальные гайды, новости индустрии, анонсы и системные требования. Разбираем особенности геймплея, помогаем с настройками и прохождением. Следите за игровыми трендами, изучайте секреты и погружайтесь в мир гейминга.
Welcome to Our Premier Limo Service
Experience the epitome of luxury and reliability with our top-tier limo service, tailored to meet your every need. Whether you’re a corporate traveler, a local resident, or a visitor exploring the city, our Dupont Town Car Service ensures you travel in style and comfort.
Dupont Town Car Service
Our Dupont Town Car Service offers an unparalleled level of professionalism and elegance. Our fleet of high-end sedans, SUVs, and stretch limousines are meticulously maintained to provide a smooth and enjoyable ride. Each vehicle is equipped with modern amenities to ensure your journey is as pleasant as possible.
Our experienced chauffeurs are not just drivers; they are your personal concierges, ensuring you arrive at your destination on time and in style. Whether you need a ride to a business meeting, a special event, or a night out on the town, our Dupont Town Car Service is your perfect travel companion.
SeaTac Airport BLACK Car Service
For those arriving or departing from Seattle-Tacoma International Airport, our SeaTac Airport BLACK Car Service offers a seamless and luxurious travel experience. Our chauffeurs are well-versed in the airport’s layout and traffic patterns, ensuring you get to your flight or destination promptly and without hassle.
Enjoy the comfort and elegance of our black cars, which are designed to provide a relaxing and private environment. Our SeaTac Airport BLACK Car Service is perfect for business travelers, families, and anyone looking to start or end their trip in style.
Seatac Airport Black Car Service Rides
Our Seatac Airport Black Car Service Rides are designed to cater to the diverse needs of our clients. Whether you’re traveling solo, with a group, or have special requirements, our flexible service options ensure you get exactly what you need.
Choose from our range of black cars, including luxury sedans, SUVs, and vans, each offering ample space and comfort. Our rides are available 24/7, ensuring you have a reliable and luxurious transportation option whenever you need it.
Why Choose Our Limo Service?
– Luxury and Comfort: Our fleet of high-end vehicles is designed to provide the ultimate in comfort and style.
– Professional Chauffeurs: Our experienced and courteous drivers ensure your journey is smooth and stress-free.
– Reliability: We pride ourselves on our punctuality and reliability, ensuring you arrive at your destination on time.
– Flexibility: Our services are tailored to meet your specific needs, whether you’re traveling for business or pleasure.
Experience the difference with our premier limo service. Contact us today to book your ride and enjoy a journey defined by luxury, comfort, and reliability.
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по приятным тарифам. Вы приобретаете документ в надежной и проверенной компании. : diplom-club.com/kupit-diplom-guu-o-visshem-obrazovanii-na-blanke-goznak-2/
Discover the world of comfort with our pools!
We offer a huge selection of pools, their installation and maintenance.
More detailed information on the link обслуживание бассейнов и оборудования тюмень
Create an oasis at home with best solutions.
Professional installation and guarantees for all work.
Все о недвижимости https://legato-dom.ru покупка, аренда, ипотека. Разбираем рыночные тренды, юридические тонкости, лайфхаки для выгодных сделок. Помогаем выбрать квартиру, рассчитать ипотеку, проверить документы и избежать ошибок при сделках с жильем. Актуальные статьи для покупателей, арендаторов и инвесторов.
танцы живота в москве школа стрип пластики
Looking for free steam accounts https://t.me/sharedsteam/? We regularly share working accounts with games, bonuses, and tips. Subscribe now and don’t miss new giveaways! Only verified and active accounts.
We offer the widest range of high-quality flavors that will give your dishes a unique taste.
Our products are made from natural ingredients without the addition of artificial colors.
More detailed information at the link эссенция это что такое
On our website you will find recipes and inspiration for culinary experiments.
Для быстрого продвижения вверх по карьерной лестнице потребуется наличие диплома о высшем образовании. Заказать диплом любого института у проверенной компании: diplomt-v-samare.ru/kupit-diplom-stomatologa-16/
Каждый поклонник кофе знает, насколько важно, чтобы кофемашина работала безупречно. Если техника Rondell дала сбой, не спешите отказываться от привычного комфорта. Сервисный центр Rondell специализируется на ремонте кофемашин, возвращая их к жизни быстро, качественно и надежно. здесь
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://haveagood.holiday/users/353120
https://permacultureglobal.org/users/67669-dave-patton
https://rentry.org/gnc2thv6
https://haveagood.holiday/users/363405
https://cannabis.net/user/168856
MetaMask Chrome never disappoints. Transactions are smooth, and the user interface is easy to navigate. A must-have for crypto users!
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: аттестат об окончании 11 классов купить, купить аттестат за 11 классов, купить аттестаты за 9 класс, купить аттестат классов, куплю диплом о среднем специальном образовании. Постепенно углубляясь, нашел отличный ресурс здесь: kyc-diplom.com/diplom-s-provodkoj-kupit.html
блюда из мяса Eda321 – ваш незаменимый помощник в мире кулинарии! Мы собрали для вас богатую коллекцию рецептов, способных удовлетворить любой вкус и уровень подготовки. Откройте для себя простые, вкусные и быстрые рецепты, идеально подходящие для приготовления блюд на каждый день. Наша домашняя кухня – это кладезь идей для тех, кто ценит традиционные русские блюда. Начинающим кулинарам мы предлагаем рецепты с подробными фото, которые помогут освоить азы кулинарного искусства. Опытные кулинары найдут для себя интересные и оригинальные блюда из мяса, рыбы, курицы и картофеля. На Eda321 вы всегда найдете вдохновение для кулинарных экспериментов и сможете порадовать своих близких вкусными и полезными блюдами. Присоединяйтесь к нам, и вместе мы откроем новые горизонты в мире кулинарии!
Все о недвижимости https://obnsk.ru покупка, аренда, ипотека. Разбираем рыночные тренды, юридические тонкости, лайфхаки для выгодных сделок. Помогаем выбрать квартиру, рассчитать ипотеку, проверить документы и избежать ошибок при сделках с жильем. Актуальные статьи для покупателей, арендаторов и инвесторов.
как купить диплом о высшем образовании как купить диплом о высшем образовании .
Все о компьютерных играх https://lifeforgame.ru обзоры новых проектов, рейтинги, детальные гайды, новости индустрии, анонсы и системные требования. Разбираем особенности геймплея, помогаем с настройками и прохождением. Следите за игровыми трендами, изучайте секреты и погружайтесь в мир гейминга.
телевизор qled tcl 55 55t7b телевизор qled 4k tv
https://химчисткамебели.online
сколько стоит отправить посылку почтой Бесплатные путешествия — реальность! Я расскажу как без затрат посещать теплые страны. Многие россияне обосновались на Бали, и им часто требуется доставка вещей из России. За транспортировку они готовы платить от 20 долларов за килограмм или фиксированную сумму за посылку. Берем два чемодана, заполняем их посылками, и вот мы уже на Бали, да еще и с прибылью!
Ремонт тепловизоров Pulsar в Москве: LRF XP50, XD50S, XD19S, XD38S, Lite XQ30V, XQ50F, XQ38F, XP38, XP28, Merger LRF XT50, Merger LRF XP50, Axion 2 LRF, Axion XM30F, Axion XQ30 PRO. Закажите звонок для бесплатной диагностики тепловизора pulsar перейти
1win cazino https://1win7.md/ .
1winmd http://1win6.md/ .
Together, they meticulously examined every inch of the massive shell, tracing their fingers along the intricate patterns etched into its surface.
온라인카지노
“This is How Parents Can Relieve Stress Throughout the Day”
토토사이트
Покупка, аренда, ипотека https://lesteri.ru всё о недвижимости в одном блоге! Советы по выбору жилья, юридические аспекты, анализ цен и прогнозы рынка. Рассказываем, как грамотно оформить ипотеку, проверить документы и избежать ошибок при сделках с недвижимостью. Будьте в курсе всех изменений и трендов!
Мир компьютерных игр https://lifeforgame.ru Мы расскажем о лучших новинках, секретах прохождения, системных требованиях и игровых трендах. Новости, гайды, обзоры и рейтинги – всё, что нужно геймерам.
стрип пластика занятия https://opendance.ru
1win ставки приложение http://www.1win5.am/ .
1 win site https://www.1win11.am .
1win сайт https://1win13.am/ .
1win сайт 1win сайт .
наращивание волос Наращивание волос — это популярная процедура для изменения длины и объема. Вот основные моменты: Методы: Капсульное: Крепление капсул к натуральным волосам. Ленточное: Пряди приклеиваются с помощью лент. На микроколечках: Пряди крепятся с помощью маленьких колечек. Выбор волос: Натуральные волосы выглядят естественно, искусственные — более доступны по цене.
Играйте в онлайн-казино рублевые казино с рублевыми счетами! Надежные казино с моментальными выплатами, бонусами и фриспинами. Пополнение через карты, кошельки и криптовалюту. Выбирайте топовые игры и выигрывайте без лишних комиссий!
Онлайн казино игры онлайн казино на деньги с лучшими бонусами и шансом на крупный выигрыш! Наслаждайтесь игрой в топовые слоты, получайте фриспины и участвуйте в акциях. Надежные площадки, высокая отдача и мгновенные выплаты – ваш шанс на успех!
great blog very nice articles I will follow you continuously sohbet odaları
Играйте в онлайн-казино https://t.me/casino_bonus_bezdep/19 топовые игровые автоматы, лайв-дилеры, мгновенные выплаты и бонусы для новых игроков. Наслаждайтесь честной игрой, удобными платежными методами и крупными выигрышами!
фриспины без депозита за регистрацию с выводом Бонус за регистрацию игровые автоматы без депозита
Ремонт серверов HP и другой электроники HP в Москве – Сервисный центр HP ссылка
проект бани
На телефоне Samsung некорректно работает сенсорный экран: 8 причин и методов исправить самостоятельно здесь
1 win куда вводить промокод http://1win7.am .
site 1win 1win6.am .
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Unlock your vehicle’s potential with our top-notch auto tuning services! Unleash your ride into a stunning masterpiece with our expert team. We specialize in upgrades that cater to your unique style. Our outstanding solutions ensure optimal performance and eye-catching design . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://americasbestcertifiedautobody.com/ today and take the first step towards your dream car!
эскорт цены
вип эскорт девочки
купить поддельный диплом дешево
1 win mexico 1 win mexico .
Где отремонтировать сложную оптику: тепловизоры, прицелы, дальномеры, коллиматоры, монокуляры. Вот на гугл картках отметка сервисного центра карта
1win casino http://1win5.com.mx/ .
Лучшее казино New Retro Casino! Классические игровые автоматы, щедрые бонусы и надежные выплаты. Наслаждайтесь азартом ретро-слотов и играйте на проверенной платформе!
Все, что вам нужно для игры Вавада промокод от стримеров! Игровые автоматы, рулетка, покер, живые дилеры и эксклюзивные бонусы. Наслаждайтесь качественной игрой с мгновенными выплатами и надежными провайдерами!
Играйте онлайн в JVSpin Bet – лицензионное онлайн-казино с лучшими слотами, лайв-играми и щедрыми бонусами. Пополняйте счет удобными способами, получайте награды и выигрывайте реальные деньги!
1win casino mexico https://www.1win4.com.mx .
Your article helped me a lot, is there any more related content? Thanks! https://www.binance.info/join?ref=P9L9FQKY
BPI Net Empresas [url=https://bpinetempresa-neg.webflow.io/]bpinetempresas[/url] é o serviço do Banco BPI que permite gerir as contas e realizar operações bancárias online, com segurança e comodidade. Saiba mais sobre as vantagens, as operações [url=https://bpinetempresas-pt.live/]Bpi Net Empresas[/url]
Сложности с техникой Acer — не повод волноваться или спешить за новым устройством. Наш сервисный центр Acer предоставляет профессиональные услуги по ремонту ноутбуков, моноблоков, компьютеров, проекторов, планшетов, VR-систем, мониторов, ультрабуков и даже электросамокатов Acer. Гарантируем высокое качество, оригинальные запчасти и честные цены. ссылка
Выбирайте https://t.me/booi_casino_site/18 топовое онлайн-казино с выгодными акциями, эксклюзивными играми и лайв-казино. Присоединяйтесь к сообществу игроков и получайте максимальное удовольствие от игры!
Играйте в https://t.me/casino_jozz/10 популярное онлайн-казино с широким выбором слотов, настольных игр и лайв-дилеров. Выгодные бонусы, удобные платежные системы и моментальные выплаты ждут вас!
Свіжі ідеї дизайну https://dverikupe.od.ua інтер’єру, сучасні тренди, поради щодо декору та ремонту. Створюйте затишок та стиль у кожному куточку вашого дому!
лазерная эпиляция ног цена https://epilyaciya-lazerom-spb.ru
свіжі новини криптовалют https://cryptonews.v.ua блокчейна та DeFi. Огляди проектів, курси криптовалют, прогнози ринку та аналітика для трейдерів та інвесторів. Слідкуйте за криптомир з нами!
1000 рублей за регистрацию вывод сразу без вложений 1000 рублей за регистрацию вывод сразу без вложений
сколько стоит типография книги типография этикетка
President Joe Biden and Republican leader McCarthy said on Sunday (May 28) that a final bipartisan deal to raise the US debt ceiling – and avoid a cataclysmic default – now heads to Congress, which will need to pass the agreement before the government starts running out of money. 온라인카지노
https://construct-metall.ru
Мы готовы предложить документы Институтов, расположенных на территории всей РФ. peoplediplom.ru/kupit-diplom-v-novosibirske-3-5
1вин отзывы 1win3001.ru .
как потратить бонусы казино 1win как потратить бонусы казино 1win .
palm slots review
регистрация 1вин https://1win3003.ru .
Заказать диплом о высшем образовании!
Мы предлагаем документы ВУЗов, которые расположены в любом регионе Российской Федерации.
О преимуществах наших документов:
• используем только настоящие бланки “Гознак”;
• все подписи должностных лиц;
• все печати учебного заведения;
• специальные водяные знаки, нити и прочие степени защиты;
• идеальное качество оформления – ошибок не бывает;
• любые проверки оригинальности документа.
diploms-vuza.com/kupit-diplom-v-ekaterinburge-2-5
1win 1win .
UID_37034453###
buat nge push KW kecil aja nih
Здравствуйте!
Руководители серьезных предприятий очень часто предпочитают претендентов, которые закончили университет. Особенно ценятся элитные учебные заведения. Но учиться целых 5 лет – это долго и дорого, далеко не у каждого существует подобная возможность. Купить документ становится оптимальным решением.
Бывают и непредвиденные случаи, когда диплом об окончании ВУЗа теряется или портится. Далеко не всегда можно быстро и без осложнений восстановить документ, особенно если университет закрыт или располагается в другом регионе страны. Бюрократические проволочки отнимут огромное количество времени и нервов.
Для успешного продвижения по карьерной лестнице нужно наличие официального диплома о высшем образовании. Однако очень часто в жизни случается так, что определенные трудности не позволяют благополучно закончить учебу и заполучить такой важный документ.
Заказать диплом любого ВУЗа
Наша компания предлагает выгодно приобрести диплом, который выполняется на оригинальной бумаге и заверен печатями, водяными знаками, подписями. Диплом пройдет лубую проверку, даже с использованием специфических приборов. Достигайте своих целей быстро и просто с нашей компанией.
Где приобрести диплом специалиста? tutdiploms.com/
Покупка недвижимости и ипотека https://gpnw.ru что нужно знать? Разбираем выбор жилья, условия кредитования, оформление документов и юридические аспекты. Узнайте, как выгодно купить квартиру и избежать ошибок!
бонусы за регистрацию Играть игровые автоматы на деньги
кайт в анапе
Наш сервисный центр специализируется на ремонте квадракоптеров DJI и работает в крупнейших городах России. С нами вы можете восстановить работу своих гаджетов подробнее
Купить документ института можно у нас. vacshidiplom.com/kupit-diplom-o-srednem-obrazovanii-4-51
Descubre cómo Michael Phelps se convirtió en leyenda de la natación | Michael Phelps ha representado a su país con orgullo en múltiples Juegos Olímpicos | Michael Phelps ha inspirado a generaciones de nadadores Michael Phelps medallas.
1win сайт 1win сайт .
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.hentai-foundry.com/user/frsf1960/profile
https://www.dnnsoftware.com/activity-feed/my-profile/userid/3208843
https://rentry.org/irmfk5gr
https://imageevent.com/kingstyle1950
https://tubeteencam.com/user/aliastr1956/profile
Ремонт смартфонов OnePlus в Москве: OnePlus 11, OnePlus 12, OnePlus 9R LE2100, OnePlus Nord CE 2 IV2201, OnePlus Nord N10 ссылка
ипотека и покупка недвижимости https://psk-opticom.ru что нужно знать? Разбираем выбор жилья, условия кредитования, оформление документов и юридические аспекты. Узнайте, как выгодно купить квартиру и избежать ошибок!
Buy elite Montenegro real estate investment: purchase apartments and houses without risks! Current prices, transaction execution, taxes and residence permit. Profitably purchase housing for life, recreation or investment.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me. https://accounts.binance.com/fr-AF/register?ref=JHQQKNKN
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам. Основные преимущества приобретения документов в нашем сервисе
Вы приобретаете документ через надежную и проверенную временем компанию. Такое решение сэкономит не только много средств, но и время.
На этом плюсы не заканчиваются, их намного больше:
• Дипломы изготавливаются на оригинальных бланках с печатями и подписями;
• Дипломы всех ВУЗов РФ;
• Цена в разы меньше чем понадобилось бы заплатить за обучение в ВУЗе;
• Быстрая доставка как по столице, так и в любые другие регионы России.
Приобрести диплом о высшем образовании– http://hitekservicewebsite.copiny.com/question/details/id/1061211/ – hitekservicewebsite.copiny.com/question/details/id/1061211
Купить диплом университета!
Мы предлагаем дипломы любой профессии по выгодным ценам— diplomj-irkutsk.ru/kupit-diplom-v-novorossijske-4/
Ваши устройства Honor требуют профессионального ремонта? Honor Center — это сервисный центр, где устраняют любые неисправности с высокой точностью и гарантией. Мы работаем в Москве, Санкт-Петербурге, Нижнем Новгороде, Ростове-на-Дону, Краснодаре, Екатеринбурге, Казани и Новосибирске. Наши мастера специализируются на ремонте ноутбуков, планшетов, смарт-часов и смартфонов Honor. Закажите ремонт Хонор и мы выполним качественно и быстро! перейти
Мы предлагаем дипломы любой профессии по приятным ценам. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы дипломы были доступны для большинства граждан.
Покупка диплома, подтверждающего окончание института, – это рациональное решение. Приобрести диплом университета: diplomus-spb.ru/kupit-diplom-v-kurgane-7/
Рекомендую – ремонт техники Hobot
Где приобрести диплом по актуальной специальности?
Наши специалисты предлагают быстро и выгодно купить диплом, который выполняется на оригинальном бланке и заверен печатями, водяными знаками, подписями официальных лиц. Наш диплом пройдет лубую проверку, даже при использовании специального оборудования. Решите свои задачи максимально быстро с нашим сервисом.
Заказать диплом ВУЗа kupitediplom0027.ru/kupit-diplom-massazhista-15/
We offer to your attention large range of high-quality flavors that will give your dishes a unique aroma.
Our products are made from natural ingredients without the addition of artificial colors.
More detailed information at the link купить пищевую эссенцию
On our website you will find detailed information about each flavor and inspiration for culinary experiments.
спины за регистрацию без депозита с выводом фриспины без депозита за регистрацию с выводом
Пылесосы Dyson заслужили репутацию надежной и инновационной техники. Однако даже они иногда могут сталкиваться с проблемами, например, вращающаяся щетка может перестать работать. Это создает неудобства при уборке и требует выявления причины неисправности. Рассмотрим основные факторы, которые могут вызвать такую ситуацию, и способы решения проблемы. перейти
Добрый день!
Где купить диплом по актуальной специальности?
Мы можем предложить дипломы психологов, юристов, экономистов и других профессий по невысоким ценам. Стоимость будет зависеть от определенной специальности, года получения и образовательного учреждения. Стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы документы были доступны для большинства наших граждан.
Заказ диплома, подтверждающего окончание университета, – это выгодное решение. Просто-напросто подсчитайте, сколько вам пришлось бы вложить денег на ежемесячную оплату 5 лет обучения, на питание, аренду жилья (если студент иногородний), на ежедневный проезд до университета и обратно. Получится очень серьезная сумма, в разы превышающая расценки на нашу услугу. А ведь все эти пять лет можно работать, развивая свои навыки на практике.
Полученный диплом с приложением отвечает условиям и стандартам Министерства образования и науки РФ, неотличим от оригинала – даже со специально предназначенным оборудованием. Не откладывайте собственные мечты и цели на потом, реализуйте их с нами – отправьте заявку на диплом прямо сейчас!
Диплом о высшем образовании – быстро и просто! diplomanruss.com/
Диплом университета Российской Федерации!
Без наличия диплома очень непросто было продвинуться по карьерной лестнице. Именно из-за этого решение о заказе диплома следует считать выгодным и рациональным. Приобрести диплом об образовании mamuli.club/forum/topic/31988
TradeOgre is an online cryptocurrency exchange launched in 2018, providing a straightforward platform for buying, selling, and trading various digital assets.
Где заказать диплом специалиста?
Мы оказываем услуги по продаже документов об окончании любых университетов России. Документы производятся на фирменных бланках государственного образца. caribbeantown.listbb.ru/viewtopic.php?f=5&t=1227
Кракен переходник – Кракен адресс, Кракен активная ссылка
Где купить диплом по актуальной специальности?
Мы предлагаем дипломы любой профессии по приятным ценам. Мы предлагаем документы ВУЗов, расположенных в любом регионе Российской Федерации. Вы можете заказать диплом от любого заведения, за любой год, включая документы старого образца СССР. Дипломы и аттестаты печатаются на бумаге высшего качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригинала. Они будут заверены всеми требуемыми печатями и подписями. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас очень важно, чтобы документы были доступны для подавляющей массы граждан. prodiplome.com/gde-kupit-diplom-obrazovanie-2-3
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Цена будет зависеть от выбранной специальности, года выпуска и университета. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Важно, чтобы дипломы были доступны для большого количества наших граждан. купить медицинский диплом кого
Conoce la increíble carrera de Michael Phelps y sus récords | Descubre cómo Michael Phelps logró su éxito en la natación | Michael Phelps ha sido reconocido por su impacto en el deporte Michael Phelps medallas.
TradeOgre Sing In is an online cryptocurrency exchange launched in 2018, providing a straightforward platform for buying, selling, and trading various digital assets.
услуги продвижения сайтов prodvizhenie-sajtov-v-moskve111.ru .
сео продвижение заказать сео продвижение заказать .
kra30.at – кракен зеркало, кракен ссылка
раскрутка и продвижение сайтов москва раскрутка и продвижение сайтов москва .
продвижение сайта за процент http://www.prodvizhenie-sajtov-v-moskve117.ru/ .
скачать самп официальный сайт samp auto
крмп 0.3 7 скачать игру крмп
мостбет кыргызстан скачать https://www.mostbet34.com.kg .
казино 1win казино 1win .
1win. 1win. .
President Joe Biden and Republican leader McCarthy said on Sunday (May 28) that a final bipartisan deal to raise the US debt ceiling – and avoid a cataclysmic default – now heads to Congress, which will need to pass the agreement before the government starts running out of money. 온라인카지노
1win bet 1win bet .
математика и физика Казахстан – кинособытия Евразия, музыкальные фестивали Казахстан
получить 1000 рублей бесплатно за регистрацию игровые автоматы слоты с бонусами
Привет!
Без университета достаточно сложно было продвигаться вверх по карьерной лестнице. Сегодня же этот документ не дает абсолютно никаких гарантий, что получится найти престижную работу. Более важны практические навыки специалиста, а также его постоянный опыт. Именно из-за этого решение о покупке диплома можно считать выгодным и рациональным. Быстро приобрести диплом о высшем образовании sz.80lvl.ru/posting.php?mode=post&f=9&sid=9bc294df624716811f99748808f49994
1 win http://1win9.com.ng/ .
TradeOgre Sing In is an online cryptocurrency exchange launched in 2018, providing a straightforward platform for buying, selling, and trading various digital assets.
Когда я зашёл на эту платформу, чувство было таким, будто я переступил грань реальности. Здесь каждая игра — это не просто волнение, а история, которую ты проживаешь с каждым движением.
Оформление интуитивен, словно невидимый проводник направляет тебя от момента к моменту. Транзакции, будь то пополнения или выплаты, проходят легко, как поток воды, и это удивляет. А поддержка всегда рядом, как надежный товарищ, который никогда не разочарует.
Для меня Селектор онлайн стал пространством, где азарт и искусство переплетаются. Здесь каждая минута — это часть истории, которую хочется переживать снова и снова.
YOURURL.com
keplar wallet
Unlock your vehicle’s potential with our top-notch auto tuning services! Explore your ride into a stunning masterpiece with our expert team. We specialize in customizations that cater to your unique style. Our high-quality solutions ensure optimal performance and visual charm . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://accurateautobodyrepair.com/ today and take the first step towards your dream car!
Робот-пылесосы iRobot заслуженно считаются одними из лидеров на рынке умной бытовой техники. Они экономят время и силы, автоматически поддерживая порядок в доме. Однако выбрать подходящую модель бывает сложно из-за разнообразия ассортимента. В этом материале разберем лучшие модели iRobot, их плюсы и минусы, а также для каких покрытий и видов уборки каждая из них лучше всего подробнее
Тут можно преобрести seo под ключ seo медицинских сайтов
мастбет мастбет .
Тут можно преобрести сео продвижение медицинского сайта сео продвижение медицинских сайтов
сериалы – чрезвычайные ситуации Казахстан, Торговый центр Март
Тут можно преобрести создать сайт медицинской клиники создание сайтов для медицины
Najboljse pocitnice hotel Durmitor Zabljak! Uzivajte v udobju, svezem zraku in osupljivi pokrajini. Gorske poti, smucanje, izleti in prijetno vzdusje. Brezplacen Wi-Fi, zajtrk in parkirisce. Rezervirajte bivanje v osrcju narave!
Dobrodosli v hotel Bianca resort and spa Kolasin! Uzivajte v udobnih sobah, osupljivem razgledu in odlicni storitvi. Pozimi – smucanje, poleti – pohodi v gore in izleti. Prirocna lokacija, restavracija, SPA in prijetno vzdusje. Rezervirajte nepozabne pocitnice!
Привет!
Купить диплом университета по доступной цене вы сможете, обращаясь к проверенной специализированной компании. Приобрести диплом: diplomus-spb.ru/kupit-diplom-ivanovo-5/
BPI Net Empresas é o serviço do Banco BPI que permite gerir as contas e realizar operações bancárias online, com segurança e comodidade. Saiba mais sobre as vantagens, as operações Bpi Net Empresas
skachat mostbet skachat mostbet .
Привет фрилансерам и удаленщикам!
С чат GPT даже сложные задачи становятся простыми. Создайте план для бизнеса, рассчитайте бюджет или разработайте текст для лендинга — нейросеть справится за минуты. Нужен оригинальный сценарий для подкастов или идеи для тикток-роликов? Чат GPT креатив превратит ваши мысли в виральный контент!
Подробнее – https://talkchatgpt.com
Доверьтесь процессу — успех рядом!
Купить документ о получении высшего образования вы сможете у нас в столице. isalineackermann.ch/juratroislacseolienne/#comment-18079
Хотите сдать радиолом https://radiolom99.ru? Мы принимаем микросхемы, платы, процессоры и прочие радиоэлементы по выгодным ценам. Быстрая оценка, честные цены и удобные способы приема. Узнайте, сколько стоят ваши детали прямо сейчас!
Игровые автоматы лучшие бонусы за регистрацию
В современном мире цифровая техника является неотъемлемой частью нашей жизни. Однако, как и любое другое оборудование, она подвержена поломкам и неисправностям. В Москве, где жизнь бежит в быстром темпе, необходимость в качественном ремонте цифровой техники становится особенно актуальной. здесь
Our company specializes in producing quality packaging materials for all kinds industries: from cosmetic to industrial.
For more information, follow the link купить пленку пвд
Our products include cardboard boxes, films and more.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Довольно часто случается так, что для того, чтобы продвинуться по карьерной лестнице, понадобится документ, подтверждающий наличие качественного образования. Где приобрести диплом по необходимой специальности?
Приобрести документ о получении высшего образования можно в нашей компании. Мы оказываем услуги по производству и продаже документов об окончании любых университетов РФ. ktap.com.ua/
Здравствуйте!
Для многих людей, купить диплом ВУЗа – это необходимость, возможность получить отличную работу. Впрочем для кого-то – это понятное желание не терять время на учебу в университете. Что бы ни толкнуло вас на такой шаг, наша компания готова помочь вам. Максимально быстро, профессионально и недорого изготовим диплом любого года выпуска на настоящих бланках со всеми печатями.
Ключевая причина, почему люди прибегают к покупке документа, – желание занять определенную работу. Например, знания позволяют кандидату устроиться на привлекательную работу, но подтверждения квалификации не имеется. В том случае если работодателю важно присутствие “корочек”, риск потерять место работы очень высокий.
Заказать документ университета вы имеете возможность у нас. Мы предлагаем документы об окончании любых ВУЗов России. Вы сможете получить необходимый диплом по любым специальностям, любого года выпуска, в том числе документы старого образца. Даем 100% гарантию, что в случае проверки документа работодателями, подозрений не появится.
Ситуаций, которые вынуждают заказать диплом ВУЗа очень много. Кому-то срочно необходима работа, в итоге нужно произвести хорошее впечатление на руководителя на протяжении собеседования. Некоторые задумали устроиться в большую компанию, чтобы повысить собственный статус в обществе и в дальнейшем начать свой бизнес. Чтобы не терять попусту годы жизни, а сразу начать эффективную карьеру, применяя врожденные таланты и полученные навыки, можно заказать диплом прямо в интернете. Вы станете полезным для социума, обретете денежную стабильность в максимально короткий срок- купить аттестат
Привет!
Мы изготавливаем дипломы любой профессии по выгодным ценам. Цена может зависеть от определенной специальности, года получения и образовательного учреждения: rdiploma24.com/
Купить диплом университета !
Профессионалы, у которых есть высшее образование очень ценятся на рынке труда. Диплом ВУЗа нужен для того, чтобы доказать свой высокий профессионализм. Он дает понять руководству, что специалист обладает всеми необходимыми знаниями для того, чтобы на отличном уровне выполнить поставленную задачу. Но что можно сделать, если умения и навыки присутствуют, а вот подтверждающего документа нет? Приобретение диплома поможет решить данную проблему. Приобретение диплома университета РФ у нас является надежным делом, так как документ заносится в реестр. Печать производится на официальных бланках, установленных государством. Заказать диплом о высшем образовании blogger-mania.mn.co/posts/77114843
Привет!
Приобретение официального диплома через проверенную и надежную компанию дарит массу достоинств. Такое решение позволяет сэкономить как личное время, так и существенные финансовые средства. Тем не менее, только на этом выгоды не ограничиваются, преимуществ значительно больше.Мы предлагаем дипломы любых профессий. Дипломы производятся на подлинных бланках государственного образца. Доступная стоимость в сравнении с крупными расходами на обучение и проживание в чужом городе. Покупка диплома института является рациональным шагом.
Приобрести диплом: dibiz.com/diplomygroup24
Купите теплицу https://tepl1.ru с доставкой по выгодной цене! Широкий выбор моделей: поликарбонатные, стеклянные, пленочные. Быстрая доставка, прочные конструкции, удобный монтаж. Идеально для дачи, сада и фермерства. Заказывайте качественные теплицы с доставкой прямо сейчас!
娛樂城是什麼
娛樂城是一個線上賭博平台,提供各式各樣的娛樂遊戲,讓玩家能在網路上參與賭博娛樂。玩家可以透過電腦或手機,隨時隨地進行遊戲,並體驗到類似實體賭場的刺激感。娛樂城的遊戲種類多樣,主要包括賭場遊戲、體育博彩、電子遊戲等。在賭場遊戲方面,玩家常見的選擇有百家樂、輪盤、撲克、二十一點等經典賭博項目。這些遊戲通常需要玩家依賴一定的技巧或運氣,來獲得獎金。電子遊戲則包括各種風格的老虎機遊戲,這些遊戲畫面華麗且玩法簡單,非常吸引玩家的注意。而體育博彩則讓玩家根據各類體育比賽的結果進行下注,體驗不同於傳統賭博的樂趣。娛樂城通常還會提供即時直播遊戲,讓玩家可以與真人荷官互動,提升遊戲的真實感。此外,娛樂城平台經常提供優惠活動、註冊獎金等,吸引玩家註冊並增加活躍度。總的來說,娛樂城是集各種線上賭博遊戲於一身的娛樂平台,玩家可以在這裡享受刺激的遊戲體驗。然而,應該注意理性投注,避免過度沉迷。
нажмите здесь https://vodkabet.io/
娛樂城,通常指的是一個線上賭博平台,提供各種娛樂遊戲,如賭場遊戲、體育博彩、電子遊戲等。這些平台讓玩家可以在網路上進行賭博,而不需要親自前往實體賭場。娛樂城通常包含了各式各樣的遊戲選項,例如百家樂、輪盤、老虎機、撲克等,並且透過即時娛樂、直播等技術,提升了玩家的沉浸感和互動性。現今的娛樂城大多數已經支援手機和桌面端的多平台操作,讓玩家可以隨時隨地參與遊戲,這樣的便利性使得它們受到全球玩家的青睞。此外,娛樂城也會推出不同的優惠活動、註冊獎金和忠誠計劃,吸引新用戶並保持老用戶的活躍度。然而,娛樂城的風險也不可忽視。由於賭博本身具有高度的娛樂性,但也存在可能的成癮問題。多數國家對於線上賭博有著嚴格的法律規範,玩家在選擇娛樂城平台時,應該格外留意平台的合法性和安全性,以避免陷入詐騙或遭遇其他法律風險。因此,理性投注並了解相關法律是每位玩家應該保持的基本態度。
Заказать диплом университета!
Мы готовы предложить документы учебных заведений, расположенных на территории всей РФ.
Основные преимущества наших документов:
• используем настоящие бланки “Гознак”;
• необходимые подписи должностных лиц;
• все печати университета;
• специальные водяные знаки, нити и иные степени защиты;
• безупречное заполнение и оформление – ошибки исключены;
• любая проверка документа.
institute-diplom.ru/kupit-diplom-v-kostrome-2-2
Kraken onion – Kraken ссылка, Kraken links
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
More Help
kepler wallet extension
купить диплом в тамбове цена
заказать сео заказать сео .
seo продвижение сайта в топ seo продвижение сайта в топ .
заказать продвижение сайта в яндексе москва заказать продвижение сайта в яндексе москва .
заказать оптимизацию сайтов http://prodvizhenie-sajtov-v-moskve213.ru .
Приобрести диплом университета
Мы предлагаем документы об окончании любых ВУЗов РФ. finest.ventures
мосбет казино http://mostbet1009.com.kg/ .
1win зайти https://1win105.com.kg .
1win играть 1win играть .
Unlock your vehicle’s potential with our top-notch auto tuning services! Transform your ride into a stunning masterpiece with our expert team. We specialize in modifications that cater to your unique style. Our exceptional solutions ensure optimal performance and stylish look . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://accurateautobodyrepair.com/ today and take the first step towards your dream car!
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по доступным ценам. Вы приобретаете документ в надежной и проверенной компании. : diplom4you.com/kupit-diplom-geti-o-visshem-obrazovanii-bez-predoplati/
Где заказать диплом по необходимой специальности?
Заказать диплом института по выгодной стоимости вы сможете, обращаясь к проверенной специализированной компании.: magazin-diplomov.ru
Мы предлагаем дипломы любой профессии по приятным тарифам. Дипломы изготавливаются на настоящих бланках Купить диплом о высшем образовании diplomnie.com
1win bet deposit 1win10.com.ng .
Подробнее в материале ссылка
how to bet on 1win https://1win10.com.ng/ .
Бонус за регистрацию игровые автоматы без депозита Бонус без отыгрыша
Заказать документ о получении высшего образования вы имеете возможность в нашем сервисе. diplom-club.com/kupit-diplom-stavropol-2
мостбет зеркало mostbet1009.com.kg .
Когда я увидел эту платформу, чувство было таким, будто я отправился в путешествие. Здесь каждая ставка — это не просто волнение, а эмоция, которую ты ощущаешь с каждым кликом.
Дизайн интуитивен, словно ветер судьбы направляет тебя от выбора к выбору. Финансовые движения, будь то пополнения или выплаты, проходят плавно, как поток воды, и это завораживает. А поддержка всегда рядом, как друг, который никогда не подведёт.
Для меня Селектор онлайн стал местом, где азарт и искусство сплетаются. Здесь каждая минута — это часть пути, которую хочется создавать снова и снова.
Подробнее в материале здесь
мосбет http://mostbet1000.com.kg .
1win зайти https://1win106.com.kg .
оформить пропуск в Москву http://3rm.info/raznoe/96500-oformit-propusk-na-mkad-dlja-gruzovyh-mashin.html .
1 вин вход 1 вин вход .
카드 현금화
Тут можно преобрести продвижение в поисковых системах медицинского сайта продвижение сайтов медицинской тематики
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.haikudeck.com/presentations/T03oLo5ps1
https://myromance1982.diary.ru/
https://haveagood.holiday/users/369934
https://cannabis.net/user/155906
https://cannabis.net/user/169245
Подробнее в материале на сайте
Здравствуйте!
Руководители больших предприятий очень часто предпочитают принимать соискателей, которые закончили университет. Особенно в приоритете топовые учебные заведения. Однако учиться пять лет – это долго, не у всех существует подобная возможность. Заказать документ – оптимальный выход.
Бывают и непредвиденные ситуации, когда диплом теряется или портится. Не всегда возможно быстро и беспроблемно восстановить документ, особенно если университет закрыт или располагается очень далеко в другом регионе страны. Бюрократия отнимает массу времени и нервов.
Для быстрого продвижения по карьерной лестнице потребуется наличие диплома о высшем образовании. Однако нередко в жизни может случиться так, что сложные обстоятельства не позволяют с успехом окончить учебу, получив такой важный документ.
Приобрести диплом ВУЗа
Мы предлагаем выгодно и быстро заказать диплом, который выполняется на оригинальном бланке и заверен печатями, водяными знаками, подписями. Диплом способен пройти любые проверки, даже при помощи специфических приборов. Достигайте цели быстро и просто с нашими дипломами.
Где приобрести диплом по нужной специальности? rdiploms.com/
Наши специалисты предлагает надежный вызвать мастера по ремонту iphone рядом всех типов и брендов. Мы понимаем, насколько важны для вас ваши устройства iPhone, и готовы предложить сервис высочайшего уровня. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только оригинальные запчасти, что предоставляет надежность и долговечность выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются владельцы iPhone, включают неисправности дисплея, проблемы с батареей, программные сбои, неисправности разъемов и механические повреждения. Для устранения этих неисправностей наши опытные мастера проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы гарантируете себе долговечный и надежный вызвать мастера по ремонту айфона на дому.
Подробная информация доступна на сайте: https://remont-iphone-sot.ru
мостбет официальный сайт http://mostbet1010.com.kg/ .
Your article helped me a lot, is there any more related content? Thanks!
Sans?n?z? Sweet Bonanza’da sweet-bonanza-casino.site deneyin – parlak ve karl? bir slot! Ucretsiz donusler, kazanc carpanlar?, kademeli kombinasyonlar ve buyuk odemeler. Ucretsiz veya parayla cevrimici oynay?n ve tatl? oduller kazan?n!
Diego Armando Maradona diego-maradona.com.mx es una leyenda del futbol mundial! Campeon del Mundo de 1986, autor de “La Mano de Dios” y “El Gol del Siglo”. Un gran jugador argentino que inspiro a generaciones. ?Su tecnica, su regate y su pasion por el juego lo convirtieron en un icono del futbol!
Glory Casino’da glory-casino-guncel-giris.online oynayn – en iyi oyunlar, yuksek odemeler ve 7/24 destek! Populer slotlar?, kart oyunlar?n? ve ruleti secin, bonuslar kazan ve gercek para kazan?n!
Заказ документа о высшем образовании через проверенную и надежную компанию дарит ряд плюсов для покупателя. Такое решение позволяет сберечь как личное время, так и серьезные средства. Впрочем, плюсов намного больше.Мы можем предложить дипломы любых профессий. Дипломы производят на подлинных бланках государственного образца. Доступная цена сравнительно с крупными издержками на обучение и проживание. Заказ диплома ВУЗа станет выгодным шагом.
Заказать диплом: diplom-zentr.com/kupit-diplom-meditsinskogo-kolledzha-2/
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
mostbest http://mostbet1010.com.kg .
Рекомендую – селектор казино сайт
скачат мостбет скачат мостбет .
Fansite de Mike Tyson http://mike-tyson.com.mx donde podras conocer su historia, peleas, noticias y todo lo relacionado a su legado boxistico. Sigue sus proyectos y conoce mas sobre esta leyenda.
Sitio de fans de Michael Phelps michael phelps Un lugar donde los fanaticos pueden encontrar toda la informacion sobre su carrera, sus records y su vida. Noticias, logros y contenido exclusivo.
Sitio de fans de Muhammad Ali https://muhammad-ali.com.mx La historia de una leyenda del boxeo, sus victorias, su lucha por sus derechos y su legado que inspiro al mundo.
UID_40738536###
test
Sitio fan de George Foreman george-foreman.com.mx/ dedicado a su carrera, victorias, derrotas y legado en el boxeo. Historias inspiradoras y el estilo unico de una leyenda del deporte.
Для эффективного продвижения вверх по карьере понадобится наличие диплома университета. Заказать диплом любого института у проверенной фирмы: diplomservis.ru/kupit-diplom-v-yaroslavle-7/
рюкзак черный
Бонус за регистрацию игровые автоматы без депозита 1000 рублей за регистрацию вывод сразу
Подробнее в материале в блоге об обзорах электронной техники ссылка
Мы предлагаем документы высших учебных заведений, которые расположены в любом регионе России. 10000diplomov.ru/kupit-diplom-v-balakovo-6
check this link right here now https://hitman-assassin-killer.com/
прокат вип авто аренда авто в бююкаде
Играйте в Big Bamboo big-bamboo-slot.online слот с захватывающим геймплеем, фриспинами и множителями! Ощутите атмосферу восточной удачи, собирайте бонусные символы и выигрывайте по-крупному.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Join Glory Casino glory-casino-bd.online and get maximum bonuses! Top slot machines, table games, jackpots and exclusive promotions. Play online and win comfortably!
продвижение сайтов в москве продвижение сайтов в москве .
продвижение сайтов москва продвижение сайтов москва .
motsbet https://mostbet1001.com.kg/ .
1win kg https://1win107.com.kg .
водительское удостоверение – помощь в сдачи экзаменов, помощь в получении прав
ван вин ван вин .
Checkr Dispute
911-remont.ru – сервис по ремонту бытовой техники
Ремонт варочных панелей siemens в волгограде в официальном сервисном центре 911.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
Взрослый вебкам бесплатно https://hotcams.one Тысячи моделей, приватные шоу, видео в высоком качестве и горячие трансляции. Общайтесь в чате и наслаждайтесь без ограничений!
kra29.at – kraken, kraken ссылка
Где купить диплом специалиста?
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по выгодным ценам. Мы можем предложить документы техникумов, расположенных в любом регионе РФ. Можно купить диплом за любой год, в том числе документы старого образца СССР. Дипломы и аттестаты делаются на “правильной” бумаге высшего качества. Это позволяет делать государственные дипломы, не отличимые от оригинала. Они заверяются всеми обязательными печатями и штампами. Стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы документы были доступными для подавляющей массы граждан. kupite-diplom0024.ru/kupit-diplom-v-ufe-2-6
Наша мастерская предлагает высококачественный мастерская по ремонту ноутбуков с гарантией всех типов и брендов. Мы понимаем, насколько значимы для вас ваши лаптопы, и готовы предложить сервис высочайшего уровня. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что гарантирует длительную работу проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются владельцы ноутбуков, включают поломку жесткого диска, неисправности экрана, неисправности программного обеспечения, проблемы с портами и перегрев. Для устранения этих неисправностей наши опытные мастера проводят ремонт жестких дисков, экранов, ПО, разъемов и систем охлаждения. Обратившись к нам, вы получаете надежный и долговечный вызвать мастера по ремонту ноутбука рядом.
Подробная информация размещена на сайте: https://remont-noutbukov-first.ru
Подробнее в материале в блоге об обзорах электронной техники перейти
https://www.llanelliherald.com/
înregistrare 1win înregistrare 1win .
городской рюкзак
заказать сео москва заказать сео москва .
娛樂城是一個線上賭博平台,提供各式各樣的娛樂遊戲,讓玩家能在網路上參與賭博娛樂。玩家可以透過電腦或手機,隨時隨地進行遊戲,並體驗到類似實體賭場的刺激感。娛樂城的遊戲種類多樣,主要包括賭場遊戲、體育博彩、電子遊戲等。在賭場遊戲方面,玩家常見的選擇有百家樂、輪盤、撲克、二十一點等經典賭博項目。這些遊戲通常需要玩家依賴一定的技巧或運氣,來獲得獎金。電子遊戲則包括各種風格的老虎機遊戲,這些遊戲畫面華麗且玩法簡單,非常吸引玩家的注意。而體育博彩則讓玩家根據各類體育比賽的結果進行下注,體驗不同於傳統賭博的樂趣。娛樂城通常還會提供即時直播遊戲,讓玩家可以與真人荷官互動,提升遊戲的真實感。此外,娛樂城平台經常提供優惠活動、註冊獎金等,吸引玩家註冊並增加活躍度。總的來說,娛樂城是集各種線上賭博遊戲於一身的娛樂平台,玩家可以在這裡享受刺激的遊戲體驗。然而,應該注意理性投注,避免過度沉迷。
娛樂城大解析
娛樂城,通常指的是一個線上賭博平台,提供各種娛樂遊戲,如賭場遊戲、體育博彩、電子遊戲等。這些平台讓玩家可以在網路上進行賭博,而不需要親自前往實體賭場。娛樂城通常包含了各式各樣的遊戲選項,例如百家樂、輪盤、老虎機、撲克等,並且透過即時娛樂、直播等技術,提升了玩家的沉浸感和互動性。現今的娛樂城大多數已經支援手機和桌面端的多平台操作,讓玩家可以隨時隨地參與遊戲,這樣的便利性使得它們受到全球玩家的青睞。此外,娛樂城也會推出不同的優惠活動、註冊獎金和忠誠計劃,吸引新用戶並保持老用戶的活躍度。然而,娛樂城的風險也不可忽視。由於賭博本身具有高度的娛樂性,但也存在可能的成癮問題。多數國家對於線上賭博有著嚴格的法律規範,玩家在選擇娛樂城平台時,應該格外留意平台的合法性和安全性,以避免陷入詐騙或遭遇其他法律風險。因此,理性投注並了解相關法律是每位玩家應該保持的基本態度。
Подробнее в материале в блоге об обзорах электронной техники ссылка
try this site smartwallit
1 win moldova http://www.1win5000.ru .
Наши специалисты предлагает профессиональный официальный ремонт macbook в москве всех типов и брендов. Мы понимаем, насколько значимы для вас ваши ноутбуки Apple, и готовы предложить сервис наилучшего качества. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что предоставляет длительную работу выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются пользователи ноутбуков Apple, включают неисправности дисплея, неисправности аккумулятора, программные сбои, проблемы с портами и проблемы с охлаждением. Для устранения этих проблем наши квалифицированные специалисты оказывают ремонт экранов, батарей, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы обеспечиваете себе надежный и долговечный отремонтировать макбук на выезде.
Подробная информация доступна на сайте: https://remont-macbook-club.ru
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: купить аттестат недорого в москве, купи диплом, купить диплом цены, купить диплом о среднем образовании в саратове, как купить диплом училища. Постепенно углубляясь, нашел отличный ресурс здесь: diplomybox.com/kontakty
911-remont.ru – сервис по ремонту бытовой техники
Ремонт тепловизоров ada в иркутске в официальном сервисном центре 911.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
you can try here
online smart wallet
мостбет промокод мостбет промокод .
UID_99106393###
test
UID_72797786###
test
UID_49633525###
test
UID_81506936###
test
UID_68562168###
test
UID_76917147###
test
продвижение сайта в топ цена продвижение сайта в топ цена .
MetaMask Chrome is essential for crypto users. It simplifies transactions, provides robust security, and integrates perfectly with DeFi.
рюкзак школьный
Мы готовы предложить дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Скачать диплом онлайн: реальность или вымысел? — kyc-diplom.com/diplom-articles/skachat-diplom-onlajn-realnost-ili-vymysel.html
Наш сервисный центр предлагает высококачественный центр ремонта ноутбука адреса различных марок и моделей. Мы осознаем, насколько значимы для вас ваши ноутбуки, и обеспечиваем ремонт высочайшего уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что обеспечивает долговечность и надежность наших услуг.
Наиболее частые неисправности, с которыми сталкиваются пользователи лаптопов, включают проблемы с жестким диском, поврежденный экран, неисправности программного обеспечения, неисправности разъемов и проблемы с охлаждением. Для устранения этих неисправностей наши профессиональные техники выполняют ремонт жестких дисков, экранов, ПО, разъемов и систем охлаждения. Доверив ремонт нам, вы гарантируете себе надежный и долговечный сервис ремонта ноутбуков в москве.
Подробная информация размещена на сайте: https://remont-noutbukov-first.ru
1win pariuri https://1win5001.ru/ .
аренда автомобиля в симферополе жд вокзал машина в аренду симферополь
Подробнее в материале в блоге об обзорах электронной техники диджитал гаджет здесь
mostbets https://www.mostbet1003.com.kg .
Рекомендуем – selector casino сайт
1win pariuri http://www.1win5001.ru .
mostbet игры http://mostbet1002.com.kg/ .
mostbets https://www.mostbet1003.com.kg .
мастбет мастбет .
Contraindications explained here. Latest medication developments.
prednisone online
Medicine essentials explained. Pill overview available.
Где заказать диплом специалиста?
Мы предлагаем выгодно и быстро заказать диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Документ пройдет любые проверки, даже с применением профессионального оборудования. Решайте свои задачи быстро и просто с нашим сервисом.
Заказать диплом о высшем образовании vuz-diplom.ru/kupit-diplom-svarshika-5/
Приобрести документ о получении высшего образования можно у нас в Москве. drgtech.ru/drg_elisa_kits/ifu_request
Выгодно заказать диплом любого университета!
Мы можем предложить дипломы любых профессий по приятным тарифам— diplomg-cheboksary.ru/kupit-diplom-v-vladivostoke-7/
Мы изготавливаем дипломы любой профессии по доступным тарифам. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы дипломы были доступными для подавляющей массы наших граждан.
Заказ документа, который подтверждает обучение в университете, – это выгодное решение. Заказать диплом любого ВУЗа: diplom-top.ru/kupit-diplom-v-krasnodare-8/
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
Patient pill information. Medicine impacts explained.
buy prednisone with no prescription
Drug details provided. Comprehensive medicine resource.
Подробнее в обозревательном журнале чек-ноутбук на сайте
Equipment and Technology: The equipment used in car recovery must be modern and compatible with today’s vehicles. This ensures the safe and efficient transport of vehicles with minimal damage.
Access medication facts. Pill leaflet available.
deltasone
Medicine effects explained. Medication guide here.
Бесплатные спины за регистрацию без депозита с выводом Игровые автоматы на реальные деньги с выводом
Подробнее в обозревательном журнале new digital tech здесь
Займ на карту без отказа без проверки взять займ на карту срочно без проверок
Интернет займ займ оформить
ремонт автомобилей популярных марок avtoplastics.ru
Great experience! The towing service was prompt, and they took good care of my vehicle. Very satisfied!
Доброго!
Капибара приручение возможно, но требует много заботы. Она любит простор и воду, поэтому дома ей может быть тесно. капибара дружелюбное животное Можно ли держать капибару дома? Да, но только если создать ей идеальные условия!
Более подробно по ссылке – https://frog2frog.site/
капибара любит воду
капибара способна нырять
капибара антистресс
Удачи!
купить рюкзак
мтс новосибирск
https://saek-kerkiras.edu.gr/employer/emilio7728/
мтс интернет новосибирск
Pre-trip Preparation: While roadside assistance is crucial in emergencies, preventing these situations by maintaining the vehicle and preparing an emergency kit can reduce the need for recovery services. Ensuring your vehicle is in good condition before a trip is a proactive approach.
Medication facts provided. Pill trends described.
buy deltasone no prescription
Medication resource here. Find drug details.
prirodni biser Durmitor national park Montenegro Jedinstveni planinski pejzazi, bistra jezera, kanjoni i guste sume. Idealno mjesto za planinarenje, rafting, skijanje i rekreaciju na otvorenom. Otkrijte nacionalni park pod zastitom UNESCO-a!
25 giros gratis sin deposito giros gratis
plm software
Best Practices for Implementing PLM Software in Fashion Brands
The fashion industry is a dynamic and competitive landscape, where brands must constantly innovate to stay ahead. To meet consumer demands, reduce costs, and streamline operations, many fashion companies are turning to Product Lifecycle Management (PLM) software. However, successful implementation of PLM requires more than just purchasing the right tool—it demands strategic planning, team involvement, and a clear understanding of organizational goals.
Why PLM Matters in Fashion
Fashion brands operate in a fast-paced environment, where trends change rapidly, and supply chains span the globe. From design to production, every step in the product lifecycle must be optimized for efficiency and accuracy. PLM software provides a centralized platform that integrates data, improves collaboration, and enhances decision-making. By adopting PLM, brands can:
Streamline product development processes
Improve communication between teams and partners
Reduce time-to-market
Enhance supply chain visibility
Minimize errors and waste
However, realizing these benefits depends on how effectively the software is implemented.
Key Steps for Successful PLM Implementation
Define Clear Objectives
Before implementing PLM, brands must identify their specific needs and goals. Are you looking to improve design efficiency? Reduce production costs? Or enhance supplier collaboration? Clearly defined objectives will guide the implementation process and ensure alignment with business priorities.
Engage Stakeholders Early
PLM impacts multiple departments, including design, production, procurement, and marketing. Involving key stakeholders from the outset ensures buy-in and fosters collaboration. It also helps tailor the system to meet diverse requirements and workflows.
Choose the Right Solution
Not all PLM systems are created equal. Some are better suited for large enterprises, while others cater to smaller brands. Evaluate vendors based on features, scalability, ease of use, and integration capabilities. Cloud-based PLM solutions, for instance, offer flexibility and real-time access, making them ideal for global teams.
Focus on Data Migration and Standardization
Transitioning to PLM often involves migrating existing data into the new system. This process can be complex, so it’s essential to clean and standardize data beforehand. Establishing consistent naming conventions, measurement units, and file formats will prevent confusion and ensure smooth operations.
Provide Comprehensive Training
Even the most advanced PLM system won’t deliver results if users don’t know how to leverage its features. Provide hands-on training sessions and create user-friendly guides to help employees adapt quickly. Encourage feedback during the initial rollout to address challenges and refine workflows.
Monitor Progress and Iterate
Implementation doesn’t end after go-live. Regularly monitor performance metrics, gather user feedback, and make necessary adjustments. Continuous improvement ensures the system evolves alongside your brand’s needs.
Real-World Impact of PLM in Fashion
Consider the success stories of brands that have embraced PLM. For example, one leading apparel company reduced its product development cycle by 30% after implementing a cloud-based PLM solution. Another brand improved supplier collaboration, resulting in faster production timelines and reduced material waste. These examples demonstrate the transformative power of PLM when implemented strategically.
Conclusion
Implementing PLM software is a significant investment, but the potential rewards far outweigh the challenges. By following best practices—such as setting clear goals, engaging stakeholders, and providing robust training—fashion brands can unlock the full potential of PLM. As the industry continues to evolve, those who embrace technology like PLM will not only survive but thrive in an increasingly competitive market.
For fashion brands ready to take the next step, PLM isn’t just a tool—it’s a pathway to innovation, efficiency, and long-term success.
By Tatiana Kochergina | Published March 12, 2025
This article provides actionable insights for fashion brands considering PLM software adoption. By showcasing real-world applications and emphasizing strategic planning, it positions PLM as a critical enabler of growth and innovation in the fashion industry.
1 цшт 1 цшт .
мостбет мобильная версия скачать https://dubna.myqip.ru/?1-18-0-00000145-000-0-0-1741708632/ .
ванвин https://www.cah.forum24.ru/?1-19-0-00000716-000-0-0-1741702224 .
Подробнее в обозревательном журнале proektorOFF перейти
выбрать таможенного брокера выбрать таможенного брокера .
сео оптимизация москва сео оптимизация москва .
таможеный брокер таможеный брокер .
Medication essentials explained. Get drug info.
buy deltasone online
Patient medication facts. Get pill facts.
mostbet скачать на телефон бесплатно андроид [url=dubna.myqip.ru/?1-18-0-00000145-000-0-0-1741708632]dubna.myqip.ru/?1-18-0-00000145-000-0-0-1741708632[/url] .
Купить документ о получении высшего образования можно в нашей компании в Москве. diplomskiy.com/kupit-diplom-medsestri-2
Здравствуйте!
Руководители серьезных фирм очень часто предпочитают соискателей, окончивших университет. Особенно ценятся престижные заведения. Тем не менее учиться пять лет – это долго и дорого, не у каждого имеется подобная возможность. Заказать документ становится оптимальным решением.
Могут быть и непредвиденные случаи, когда диплом утерян. Не всегда возможно быстро и без проблем восстановить его, особенно когда ВУЗ закрыт или располагается где-то в другом регионе страны. Бюрократия отнимает массу времени и нервов.
Для удачного продвижения по карьерной лестнице потребуется наличие официального диплома института. Впрочем часто в жизни случается так, что те или иные трудности не позволяют успешно окончить учебу, получив желанный документ.
Заказать диплом о высшем образовании
Наши специалисты предлагают быстро и выгодно заказать диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями должностных лиц. Наш диплом пройдет любые проверки, даже с применением профессионального оборудования. Достигайте свои цели быстро с нашими дипломами.
Где заказать диплом специалиста? rdiploman.com/
мтс тарифы новосибирск
http://www.employment.bz/employer/lurlene412/
мтс домашний интернет
Мы изготавливаем дипломы любой профессии по приятным тарифам.– on3dprinting.com/2013/05/16/teleport-it-3d-p2p-3d-printing-launch/#comment-352985
игра ракета на деньги 1win https://aktivnoe.forum24.ru/?1-2-0-00000100-000-0-0-1741701286 .
Привет!
Без наличия диплома очень трудно было продвигаться по карьерной лестнице. Сегодня же документ не дает совершенно никаких гарантий, что удастся получить привлекательную работу. Куда более важное значение имеют навыки и знания специалиста, а также его опыт работы. Именно из-за этого решение о покупке диплома следует считать рациональным. Приобрести диплом об образовании aurorahcs.com/forum/viewtopic.php?f=8&t=872427
1win играть 1win играть .
купить диплом о среднем специальном образовании воронеж
Comprehensive drug facts. Current medicine trends.
buy generic prednisone
Comprehensive medication overview. Comprehensive pill overview.
мтс подключить
https://www.ministryboard.org/employer/dacia9212/
сайт мтс новосибирск
Find medicine information. Current medication trends.
deltasone
Comprehensive drug resource. Drug specifics here.
рюкзак школьный
Medicine details here. Current medicine trends.
buy prednisone no rx
Find medication info. Get pill facts.
everything about the world https://t.me/ufc_ar/ of UFC in Arabic! Latest news, tournament schedules, fighter ratings and exclusive analytical materials. Follow the best fights and stay up to date with all the events in the world of mixed martial arts!
Приобрести диплом университета!
Мы готовы предложить документы университетов, которые расположены в любом регионе РФ.
Превосходства наших документов:
• используются лишь настоящие бланки “Гознака”;
• подлинные подписи руководства;
• все печати учебного заведения;
• специальные водяные знаки, нити и иные степени защиты;
• безупречное заполнение и оформление – ошибки исключены;
• любые проверки документа.
diplom-profi.ru/kupit-diplom-menedzhera-2-3
mostbet http://aktivnoe.forum24.ru/?1-8-0-00000260-000-0-0-1741701879/ .
1вин сайт официальный https://aktivnoe.forum24.ru/?1-8-0-00000259-000-0-0-1741701621 .
мостбет войти https://aktivnoe.forum24.ru/?1-8-0-00000260-000-0-0-1741701879 .
официальный сайт 1win официальный сайт 1win .
Prescribing details available. Access pill facts.
where buy prednisone
Medicine leaflet available. Read about pills.
скачать мостбет https://cah.forum24.ru/?1-19-0-00000715-000-0-0-1741702061 .
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
Где приобрести диплом по необходимой специальности?
Мы оказываем услуги по продаже документов об окончании любых университетов РФ. Документы производят на настоящих бланках. oaluno.shop/read-blog/5806_kupit-svidetelstvo-o-zaklyuchenii-braka.html
аренда авто сочи аэропорт без водителя прокат автомобилей в аэропорту сочи
mostbet chrono https://cah.forum24.ru/?1-19-0-00000715-000-0-0-1741702061/ .
бонусы за регистрацию 1xslots бездепозитный бонус
мтс тарифы на интернет
https://seekinternship.ng/employer/buddy2611/
мтс цены
Comprehensive pill guide. Dosing guidelines here.
buy prednisone online
Medication resource here. Patient pill information.
Follow UFC fights with https://t.me/s/ufc_ar full tournament schedule, fight results, fighter ratings and analytics. Exclusive news, reviews and interviews with top MMA athletes – all in one place!
Топ 5 казино https://t.me/casino_list_reyting
Great experience! The towing service was prompt, and they took good care of my vehicle. Very satisfied!
pinko casino pinko casino .
pinco cazino http://www.officialpinco.com .
Продвижение вашего сайта на новый уровень, проверенные приемы.
Секреты успешного продвижения сайтов, изучайте.
Узнайте, как продвигать сайт, что поможет вашему.
Будущее онлайн-продвижения, на которые стоит обратить внимание.
Топовые приемы для улучшения позиционирования, недоступные никому.
Актуальные стратегии SEO, которые принесут плоды.
Топовые компании для онлайн-продвижения, которые помогут вам.
Типичные проблемы при продвижении, которые можно исправить.
Доступные методы SEO, на практике.
Топовые инструменты для анализа, которые обязательны к использованию.
Метрики для оценки эффективности, которые нельзя игнорировать.
Роль контента в SEO, что имеет значение.
Как продвигать сайт на своем рынке, рекомендации профессионалов.
Как понять свою аудиторию, приемы, которые сработают.
Важность мобильной версии, это важно.
SEO против контекстной рекламы, определите свою цель.
Как наращивать ссылочную массу, на практике.
Ежегодные тренды в SEO, не пропустите.
Как продвигать сайт в социальных сетях, создавайте интересный контент.
Как правильно оптимизировать, используйте для продвижения.
сео продвижение сайтов сео продвижение сайтов .
Привет!
Заказать диплом ВУЗа по выгодной стоимости возможно, обратившись к проверенной специализированной компании. Заказать диплом о высшем образовании: freediplom.com/kupit-diplom-belgorod/
Наши специалисты предлагает высококачественный сервис ремонта айпада адреса различных марок и моделей. Мы знаем, насколько важны для вас ваши iPad, и готовы предложить сервис высочайшего уровня. Наши профессиональные техники работают быстро и аккуратно, используя только сертифицированные компоненты, что обеспечивает надежность и долговечность наших услуг.
Наиболее общие проблемы, с которыми сталкиваются владельцы iPad, включают проблемы с экраном, проблемы с батареей, неисправности программного обеспечения, неисправности разъемов и механические повреждения. Для устранения этих неисправностей наши опытные мастера проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе качественный и надежный ремонт айпада на выезде.
Подробная информация размещена на сайте: https://remont-ipad-pro.ru
мтс подключение
https://es-africa.com/employer/pablo3996/
мтс тарифы на интернет
Read about drugs. Drug details provided.
prednisone cheap
Interactions explained here. Latest medication developments.
Купить диплом ВУЗа можем помочь. Купить диплом бакалавра в Москве – diplomybox.com/kupit-diplom-bakalavra-v-moskve
Excellent towing service! They arrived quickly and handled my car with great care. Highly recommended!
Диплом ВУЗа России!
Без ВУЗа очень непросто было продвинуться по карьерной лестнице. Именно по этой причине решение о заказе диплома следует считать целесообразным. Быстро и просто приобрести диплом о высшем образовании arpop.com/forum/index.php?topic=20680.0
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
мтс подключение новосибирск
https://www.oyeanuncios.com/profile/cathleenbeahm6
мтс интернет новосибирск
купить рюкзак
Где приобрести диплом по нужной специальности?
Наши специалисты предлагают выгодно и быстро приобрести диплом, который выполнен на оригинальной бумаге и заверен печатями, водяными знаками, подписями. Документ пройдет лубую проверку, даже при помощи специфических приборов. Достигайте цели максимально быстро с нашим сервисом. Приобрести диплом о высшем образовании! babygirls030.copiny.com/question/details/id/1068494
Играйте в Mostbet Casino mostbet.spas-extreme.ru лицензированное онлайн-казино с огромным выбором игр! Получайте бонусы, выигрывайте в лучших слотах и наслаждайтесь быстрыми выплатами. Удобные способы пополнения счета!
Рекомендуем – selector casino вход
Все про остекление и ремонт балконов — новости, советы и услуги в одном месте!
Хотите преобразить свой балкон или лоджию?
Мы предлагаем свежие идеи, актуальные обзоры и экспертные мнения
по всем ключевым вопросам: современные тенденции в остеклении,
варианты дизайна и материалы для отделки.
Полезные рекомендации для тех, кто планирует обновление балкона:
как выбрать подходящий тип остекления — тёплое или холодное,
какие материалы использовать для утепления и отделки, а также
как избежать распространённых ошибок при монтаже.
Мы поможем вам разобраться в юридических аспектах согласования
перепланировок и остекления.
Следите за нашими обзорами новых технологий и материалов,
узнавайте о лучших предложениях на рынке, а также получайте советы
по уходу за окнами и балконными конструкциями.
Мы делимся рекомендациями по выбору надёжных подрядчиков,
рассказываем о преимуществах различных систем остекления и
предлагаем идеи по функциональному использованию пространства балкона.
Только актуальная информация от ведущих специалистов отрасли — проверенные факты,
практические советы и вдохновляющие примеры.
Подписывайтесь и оставайтесь в курсе последних тенденций!
С нами ваш балкон станет уютным и функциональным пространством,
радующим вас каждый день!
Сайт balconyokna.ru
Где приобрести диплом специалиста?
Мы можем предложить дипломы любой профессии по приятным ценам. Мы готовы предложить документы техникумов, расположенных на территории всей Российской Федерации. Вы сможете приобрести диплом от любого учебного заведения, за любой год, указав необходимую специальность и оценки за все дисциплины. Дипломы и аттестаты делаются на “правильной” бумаге самого высшего качества. Это дает возможность делать государственные дипломы, которые не отличить от оригиналов. Они будут заверены всеми требуемыми печатями и подписями. Всегда стараемся поддерживать для покупателей адекватную ценовую политику. Для нас важно, чтобы дипломы были доступны для подавляющей массы наших граждан. [url=http://prodiplome.com/kupit-diplomi-o-visshem-obrazovanii-tsena-4/]prodiplome.com/kupit-diplomi-o-visshem-obrazovanii-tsena-4[/url]
Выбираем недвижимость в Киеве https://automat.kiev.ua как оценить район, новостройки и вторичный рынок? Анализ цен, проверка застройщика, юридическая безопасность сделки. Полезные советы для покупки квартиры без ошибок и переплат!
Строительный онлайн журнал https://kero.com.ua ваш источник актуальной информации о строительстве и ремонте! Советы, обзоры материалов, технологии, тренды и новости отрасли. Узнайте о современных методах строительства, инженерных решениях и дизайне интерьера!
Аркада казино, или казино аркада, – это относительно новое направление в индустрии азартных игр, которое стремится сочетать элементы классических аркадных игр с традиционными казино-играми. Этот гибридный формат нацелен на привлечение более молодой аудитории, выросшей на видеоиграх, и тех, кто ищет более интерактивный и захватывающий опыт, нежели стандартные слоты и настольные игры.
аркада казино скачать
Купить диплом университета!
Мы можем предложить документы учебных заведений, расположенных на территории всей Российской Федерации.
diplomidlarf.ru/kupit-diplom-kolledzha-40
1win партнёрка https://www.1win112.com.kg .
1 вин официальный http://1win113.com.kg/ .
1win партнерская программа вход 1win112.com.kg .
1wiun 1win713.ru .
1win регистрация https://www.1win113.com.kg .
1win официальный сайт https://www.1win713.ru .
Latest pill trends. Prescribing details available.
canada medications online
Patient drug guide. Latest pill news.
Привет всем!
Прокат автомобилей для поездок в горы Сочи – отправляйтесь в горы Сочи на арендованном автомобиле. Мы предлагаем внедорожники и другие автомобили, которые идеально подходят для путешествий по горным маршрутам и зимним курортам. снять кабриолет в Сочи напрокат Путешествуйте с комфортом и уверенностью.
Аренда минивэна в Сочи – это идеальное решение для больших компаний или семей. Мы предлагаем аренду минивэнов без водителя на любой срок. Все автомобили в нашем автопарке оснащены современными удобствами и обеспечат комфорт в любой поездке. Аренда авто с доставкой в Сочи – это удобно и быстро. Мы предоставляем автомобили для любых нужд.
Более подробно на сайте – https://olimpik-auto.ru/
прокат авто Сочи цены без водителя недорого, аренда автомобиля с водителем в Сочи, прокат авто в Адлере без водителя
аренда элитных авто в Сочи, взять машину в аренду в Адлере, прокат машин Красная Поляна
Удачи и хороших путешествий!
Добрый день!
Прокат автомобилей для свадебных поездок по Сочи – арендуйте роскошное авто для своего свадебного путешествия по Сочи. Мы предлагаем автомобили премиум-класса для торжественных событий, включая кортежи и индивидуальные поездки. прокат авто Сочи Сделайте ваш свадебный день незабываемым с нашим автопрокатом.
Прокат авто для поездок в соседние курорты Сочи – если вы хотите посетить курорты, расположенные недалеко от Сочи, арендуйте автомобиль для поездок в такие места, как Адлер, Лазаревское или другие курортные зоны. Удобное и комфортное путешествие по соседним курортам.
Более подробно на сайте – https://avto-prokat-23.ru/
авто в аренду в Сочи без водителя, снять кабриолет в Сочи, автопрокат аэропорт Адлер
прокат авто в Адлере недорого без водителя, аренда премиум авто Сочи, аренда авто Сочи без водителя аэропорт
Удачи и хороших путешествий!
Где купить диплом по актуальной специальности?
Приобрести диплом института по выгодной цене можно, обращаясь к проверенной специализированной компании.: kupitediplom0029.ru
Мы можем предложить дипломы психологов, юристов, экономистов и любых других профессий по приятным ценам. Дипломы изготавливаются на фирменных бланках государственного образца Приобрести диплом любого ВУЗа diplomskiy.com
plm
Best Practices for Implementing PLM Software in Fashion Brands
The fashion industry is a dynamic and competitive landscape, where brands must constantly innovate to stay ahead. To meet consumer demands, reduce costs, and streamline operations, many fashion companies are turning to Product Lifecycle Management (PLM) software. However, successful implementation of PLM requires more than just purchasing the right tool—it demands strategic planning, team involvement, and a clear understanding of organizational goals.
Why PLM Matters in Fashion
Fashion brands operate in a fast-paced environment, where trends change rapidly, and supply chains span the globe. From design to production, every step in the product lifecycle must be optimized for efficiency and accuracy. PLM software provides a centralized platform that integrates data, improves collaboration, and enhances decision-making. By adopting PLM, brands can:
Streamline product development processes
Improve communication between teams and partners
Reduce time-to-market
Enhance supply chain visibility
Minimize errors and waste
However, realizing these benefits depends on how effectively the software is implemented.
Key Steps for Successful PLM Implementation
Define Clear Objectives
Before implementing PLM, brands must identify their specific needs and goals. Are you looking to improve design efficiency? Reduce production costs? Or enhance supplier collaboration? Clearly defined objectives will guide the implementation process and ensure alignment with business priorities.
Engage Stakeholders Early
PLM impacts multiple departments, including design, production, procurement, and marketing. Involving key stakeholders from the outset ensures buy-in and fosters collaboration. It also helps tailor the system to meet diverse requirements and workflows.
Choose the Right Solution
Not all PLM systems are created equal. Some are better suited for large enterprises, while others cater to smaller brands. Evaluate vendors based on features, scalability, ease of use, and integration capabilities. Cloud-based PLM solutions, for instance, offer flexibility and real-time access, making them ideal for global teams.
Focus on Data Migration and Standardization
Transitioning to PLM often involves migrating existing data into the new system. This process can be complex, so it’s essential to clean and standardize data beforehand. Establishing consistent naming conventions, measurement units, and file formats will prevent confusion and ensure smooth operations.
Provide Comprehensive Training
Even the most advanced PLM system won’t deliver results if users don’t know how to leverage its features. Provide hands-on training sessions and create user-friendly guides to help employees adapt quickly. Encourage feedback during the initial rollout to address challenges and refine workflows.
Monitor Progress and Iterate
Implementation doesn’t end after go-live. Regularly monitor performance metrics, gather user feedback, and make necessary adjustments. Continuous improvement ensures the system evolves alongside your brand’s needs.
Real-World Impact of PLM in Fashion
Consider the success stories of brands that have embraced PLM. For example, one leading apparel company reduced its product development cycle by 30% after implementing a cloud-based PLM solution. Another brand improved supplier collaboration, resulting in faster production timelines and reduced material waste. These examples demonstrate the transformative power of PLM when implemented strategically.
Conclusion
Implementing PLM software is a significant investment, but the potential rewards far outweigh the challenges. By following best practices—such as setting clear goals, engaging stakeholders, and providing robust training—fashion brands can unlock the full potential of PLM. As the industry continues to evolve, those who embrace technology like PLM will not only survive but thrive in an increasingly competitive market.
For fashion brands ready to take the next step, PLM isn’t just a tool—it’s a pathway to innovation, efficiency, and long-term success.
By Tatiana Kochergina | Published March 12, 2025
This article provides actionable insights for fashion brands considering PLM software adoption. By showcasing real-world applications and emphasizing strategic planning, it positions PLM as a critical enabler of growth and innovation in the fashion industry.
мтс домашний интернет екатеринбург
https://carrieresecurite.fr/entreprises/24inetrnet-domashnij-ekb-3/
мтс цены
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Рекомендую – ремонт пластиковых окон балкона
Patient pill resource. Pill essentials explained.
pharmacy online mexico
Complete drug overview. Medication guide here.
Очень часто случается так, что для того, чтобы продвинуться по карьерной лестнице, понадобится документ, подтверждающий наличие профильного образования. Где приобрести диплом специалиста?
Заказать документ о получении высшего образования вы можете у нас в столице. Мы предлагаем документы об окончании любых ВУЗов РФ. bagira-school.com.ua/
Где купить диплом специалиста?
Полученный диплом с приложением целиком и полностью отвечает стандартам Министерства образования и науки, неотличим от оригинала. Не откладывайте собственные мечты и задачи на пять лет, реализуйте их с нами – отправляйте простую заявку на изготовление документа прямо сейчас! Получить диплом о высшем образовании – запросто! diplomgorkiy.com/kupit-diplom-yurista-18/
мтс домашний интернет екатеринбург
https://onetable.world/read-blog/128088_%D0%B1%D0%B5%D1%81%D0%BF%D0%BB%D0%B0%D1%82%D0%BD%D1%8B%D0%B5-%D0%BF%D1%80%D0%B8%D0%BB%D0%BE%D0%B6%D0%B5%D0%BD%D0%B8%D1%8F-%D0%BE%D1%82-%D0%BC%D1%82%D1%81.html
мтс цены
рюкзак мужской
Купить диплом о высшем образовании!
Мы готовы предложить дипломы психологов, юристов, экономистов и других профессий по выгодным ценам. Вы приобретаете документ через надежную фирму. : diplom-top.ru/kupit-diplom-vmir-imeni-popova-mozhno-u-nas-na-blanke-goznak-2/
Всё для мужчин https://kompanion.com.ua в одном месте! Спорт, мода, авто, гаджеты, здоровье и лайфхаки. Читай полезные советы, следи за трендами и будь лучшей версией себя.
Новости шоу-бизнеса https://mediateam.com.ua мода, красота и афиша – всё в одном месте! Узнавайте о главных событиях, свежих трендах и топовых мероприятиях. Будьте в курсе всего, что происходит в мире стиля и развлечений!
Все автомобильные новости https://nmiu.org.ua в одном месте! Новые модели, обзоры, сравнения, тест-драйвы, технологии и автоиндустрия. Следите за тенденциями и оставайтесь в курсе событий автопрома!
Medication leaflet available. Medicine resource available.
generic drug prices
Interactions explained here. Comprehensive pill resource.
продвижения сайтов продвижения сайтов .
Заказать диплом университета по выгодной стоимости вы можете, обращаясь к надежной специализированной компании. Мы можем предложить документы ВУЗов на Ваш выбор, которые находятся на территории всей Российской Федерации. diplomnie.com/vuz-diplom-kupit-3
Купить диплом академии !
Покупка диплома ВУЗа России у нас является надежным делом, ведь документ будет заноситься в государственный реестр. При этом печать производится на специальных бланках, установленных государством. Заказать диплом о высшем образовании arenadiplom24.online/vuzy/permskogo-filiala-eoi
продвижение сайта топ стоимость http://www.prodvizhenie-sajtov-v-moskve221.ru/ .
Актуальные новости бизнеса https://rentwell.in.ua экономики и недвижимости! Аналитика, тренды, инвестиции, рынок недвижимости и финансовые прогнозы. Будьте в курсе главных событий и принимайте взвешенные решения!
Лучший женский портал https://rosetti.com.ua Стиль, красота, здоровье, семья, карьера и саморазвитие. Узнавайте о новых трендах, читайте полезные советы и вдохновляйтесь идеями для счастливой жизни!
Выбор недвижимости в Киеве https://odinden.com.ua на что обратить внимание? Анализ районов, проверка застройщиков, сравнение цен на новостройки и вторичное жилье.
1000 рублей за регистрацию вывод сразу Top casino с выводом
мтс подключение екатеринбург
https://www.bolsadetrabajotafer.com/employer/24inetrnet-domashnij-ekb-3/
мтс домашний интернет екатеринбург
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Цена может зависеть от определенной специальности, года получения и образовательного учреждения. Всегда стараемся поддерживать для покупателей адекватную политику цен. Для нас очень важно, чтобы документы были доступны для большинства наших граждан. купить диплом в каменске-шахтинском
Find medication facts. Drug guide here.
online pharmacy no prescription
Access medication details. Medicine impacts explained.
mostbet chrono mostbet chrono .
motbet https://mostbet1004.com.kg/ .
1win sports betting 1win sports betting .
мостбет mostbet1011.com.kg .
aviator mostbet https://mostbet1004.com.kg .
Современный женский портал https://womanlife.kyiv.ua мода, стиль, красота, здоровье, отношения и карьера. Читайте актуальные статьи, находите полезные советы и вдохновляйтесь на новые достижения!
Все для женщин https://timelady.kyiv.ua в одном месте! Новости моды, бьюти-советы, отношения, карьера, психология и лайфхаки. Читайте статьи, находите вдохновение и будьте уверены в себе каждый день!
Детский портал о здоровье https://run.org.ua полезная информация для заботливых родителей! Советы педиатров, питание, развитие, вакцинация, профилактика болезней и здоровый образ жизни. Всё, что нужно знать о здоровье вашего ребенка!
1win home http://1win11.com.ng .
https://best-kuzminki.ru
Current medication trends. Medication impacts explained.
best online international pharmacies
Drug details provided. Latest medication updates.
мтс подключить екатеринбург
https://activeaupair.no/employer/24inetrnet-domashnij-ekb/
мтс подключение екатеринбург
Приобрести диплом университета по невысокой цене возможно, обращаясь к надежной специализированной компании. Мы предлагаем документы об окончании любых ВУЗов Российской Федерации. Заказать диплом любого университета– diploma-groups24.ru/kupit-diplom-v-gorode/kislovodsk.html
Всё для женщин https://allwoman.kyiv.ua в одном месте! Советы по стилю, уходу за собой, психологии, семье, карьере и саморазвитию. Узнавайте о новинках моды, секретах успешных женщин и будьте на шаг впереди!
Современный женский сайт https://dama.kyiv.ua красота, стиль, здоровье, семья и карьера. Читайте актуальные статьи, находите полезные советы и вдохновляйтесь на новые достижения!
micro movers toronto residential moving services in toronto
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Добрый день!
Долго не мог уяснить как поднять параметры Ahrefs, Domain Rank, сайт и свои проекты и узнал >>>
Прогон ссылок через Xrumer помогает улучшить DR и увеличить показатели Ahrefs. Массовая рассылка ссылок через форумы ускоряет линкбилдинг и улучшает SEO-позиции. Программы для линкбилдинга помогают создать качественные внешние ссылки. Увеличение ссылочной массы с Xrumer помогает повысить видимость сайта. Попробуйте Xrumer для повышения SEO-результатов.
Нашел крутых ребят, разработали недорогой и главное продуктивный прогон Хрумером – https://yandex.com/search?text=%D1%82%D0%B0%D1%80%D0%B8%D1%84+bullet&lr=10466
Массовый линкбилдинг для сайта
Автоматический постинг форумов
Xrumer: советы и трюки
Xrumer для роста ссылочной массы
https://seogurus.site/
Удачи и роста в топах!
Добрый день!
Долго ломал голову как поднять параметры Ahrefs, Domain Rank, сайт и свои проекты и узнал >>>
Прогон ссылок Хрумером позволяет повысить DR и улучшить показатели Ahrefs. Массовая рассылка ссылок на форумах помогает ускорить процесс линкбилдинга. Программы для линкбилдинга, такие как Xrumer, автоматизируют создание ссылок. Увеличение ссылочной массы с Xrumer помогает улучшить видимость сайта. Попробуйте Xrumer для быстрого SEO-продвижения.
Нашел крутых ребят, разработали недорогой и главное продуктивный прогон Хрумером – https://yandex.com/search?text=%D1%82%D0%B0%D1%80%D0%B8%D1%84+bullet&lr=10466
Прогон Хрумер для сайта
Как использовать Xrumer для SEO
Увеличение ссылочной массы быстро
Как поднять DR сайта за неделю
https://seogurus.site
Удачи и роста в топах!
Женский онлайн-журнал https://krasotka.kyiv.ua мода, красота, здоровье, семья и карьера. Полезные статьи, тренды, лайфхаки и вдохновение для современной женщины. Читайте, развивайтесь и наслаждайтесь жизнью!
Онлайн-журнал для женщин https://otnoshenia.net которые стремятся к лучшему! Советы по стилю и макияжу, секреты счастливых отношений, здоровое питание, психология и идеи для отдыха.
Лучший сайт для женщин https://model.kyiv.ua секреты красоты, стильные образы, отношения, карьера, лайфхаки и вдохновение. Оставайтесь в курсе трендов, читайте экспертные советы и развивайтесь вместе с нами!
рюкзак женский
UID_43994162###
agentotoplay
agentotoplay
Доброго времени суток!
Для некоторых людей, приобрести диплом университета – это необходимость, шанс получить отличную работу. Однако для кого-то – это желание не терять время на учебу в ВУЗе. С какой бы целью вам это не понадобилось, мы готовы помочь. Быстро, профессионально и по доступной цене изготовим документ любого года выпуска на подлинных бланках с реальными печатями.
Главная причина, почему люди покупают документ, – желание занять определенную должность. К примеру, знания дают возможность кандидату устроиться на работу, однако подтверждения квалификации нет. Когда для работодателя важно наличие “корочек”, риск потерять хорошее место очень высокий.
Купить документ ВУЗа вы можете в нашей компании в Москве. Мы предлагаем документы об окончании любых университетов России. Вы сможете получить диплом по любой специальности, включая документы старого образца. Даем 100% гарантию, что при проверке документов работодателем, подозрений не появится.
Ситуаций, которые вынуждают заказать диплом немало. Кому-то очень срочно потребовалась работа, и необходимо произвести впечатление на начальника при собеседовании. Некоторые мечтают попасть в престижную компанию, для того, чтобы повысить собственный статус в обществе и в дальнейшем начать свой бизнес. Чтобы не терять множество времени, а сразу начать успешную карьеру, применяя врожденные способности и полученные навыки, можно приобрести диплом в интернете. Вы станете полезным в социуме, получите денежную стабильность максимально быстро и просто- купить аттестат
Приветствую!
Мы можем предложить дипломы любой профессии по приятным ценам. Цена зависит от конкретной специальности, года выпуска и университета: rdiploman.com/
Сайт для современных женщин https://princess.kyiv.ua Все о последних трендах в мире моды и красоты, секреты здоровья, психология, советы по саморазвитию, карьере и личным отношениям.
Портал для женщин https://woman365.kyiv.ua которые ценят красоту и уверенность! Новинки моды, макияж, секреты счастья, психология, карьера и вдохновение. Узнавайте полезные советы и воплощайте мечты в реальность!
Женский онлайн-клуб https://womanclub.kyiv.ua для тех, кто ценит стиль, здоровье и успех! Узнайте секреты красоты, тренды моды, советы по отношениям и карьере. Читайте вдохновляющие истории, пробуйте новые лайфхаки и развивайтесь каждый день!
Contraindications explained here. Access medication details.
canadian drugstore online
Pill leaflet provided. Access medication details.
CS:GO/CS2 skins wiki.vpesports com catalog with full description! Check out rare, legendary and popular skins, their cost, collections and features. Find the perfect skin for your inventory!
The best online marketplace https://nongfag.go.th/webboard/index.php?action=profile;u=606133! Buy and sell game, social, business and other accounts. Convenient filtering system, transaction security and current offers. All popular services and games in one place!
Buy verified accounts account marketplace with ease! Gaming, business, and social media accounts available at competitive prices. Fast transactions, trusted sellers, and secure payments. Get the best deals now!
1вин сайт https://1win715.ru/ .
1win играть http://www.1win114.com.kg .
1win live http://1win708.ru .
1win kg скачать http://1win715.ru .
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
партнёрка 1win https://www.1win114.com.kg .
1win armenia http://1win708.ru .
Medicine resource available. Find medication info.
prescription costs
Get info now. Medication reactions explained.
Единственный знак зодиака, который имеет особую связь с Богом https://x.com/Fariz418740/status/1901024008243429625
Заказать документ о получении высшего образования можно у нас в столице. iconicspareparts.com/product/ford-endeavour-dashboard/#comment-7048
Мы изготавливаем дипломы любой профессии по приятным ценам. Всегда стараемся поддерживать для клиентов адекватную политику тарифов. Важно, чтобы дипломы были доступными для большинства граждан.
Покупка документа, который подтверждает обучение в ВУЗе, – это разумное решение. Купить диплом любого университета: diplom4you.com/kupit-diplom-v-ekaterinburge-26/
Где заказать диплом по актуальной специальности?
Мы предлагаем максимально быстро купить диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями. Наш диплом способен пройти лубую проверку, даже с применением специального оборудования. Достигайте своих целей быстро с нашими дипломами.
Купить диплом ВУЗа diplomt-v-samare.ru/kupit-diplom-v-saratove-14/
Единственный знак зодиака, который имеет особую связь с Богом https://www.pinterest.com/pin/957366833290360277
Medicine resource available. Medication reactions explained.
no prescription pharmacy
Medication overview available. Patient medication guide.
рюкзак школьный
Лучший автомобильный портал https://kia-sportage.in.ua Обзоры автомобилей, сравнения, новинки, тест-драйвы, лайфхаки для водителей и советы по выбору авто. Всё, что нужно автолюбителям и профессионалам!
Сайт для женщин https://womanexpert.kyiv.ua которые хотят быть успешными и счастливыми! Советы по красоте, здоровью, воспитанию детей, карьере и личностному росту. Актуальные тренды, лайфхаки и вдохновение для современных женщин!
Портал для автолюбителей https://lada.kharkiv.ua всё об автомобилях! Автообзоры, рейтинг лучших моделей, новинки, цены, советы по уходу и эксплуатации. Узнайте, какие авто заслуживают вашего внимания!
Medication resource here. Patient medicine resource.
cheap drug prices
Access pill details. Medicine effects explained.
Для эффективного продвижения по карьерной лестнице требуется наличие диплома университета. Заказать диплом любого университета у проверенной организации: diplomgorkiy.com/kupit-diplom-v-ryazani-7/
Игровые автоматы играть на деньги Игровые автоматы лучшие
Выявлен фрукт, который является природным эликсиром молодости https://www.pinterest.com/pin/957366833290370789
Мы готовы предложить дипломы психологов, юристов, экономистов и любых других профессий по разумным тарифам. О преимуществах приобретения документов в нашем сервисе
Вы заказываете диплом через надежную фирму. Такое решение позволит вам сохранить не только массу денег, но и ваше время.
Плюсов куда больше:
• Документы изготавливаются на настоящих бланках со всеми отметками;
• Предлагаем дипломы любого ВУЗа РФ;
• Стоимость во много раз меньше чем пришлось бы платить за обучение в ВУЗе;
• Быстрая доставка как по столице, так и в любые другие регионы России.
Купить диплом о высшем образовании– http://sampnewrp.listbb.ru/viewtopic.php?f=18&t=1351&sid=039e48a22ef568f840b0c33cf1938cab/ – sampnewrp.listbb.ru/viewtopic.php?f=18&t=1351&sid=039e48a22ef568f840b0c33cf1938cab
pinco casino [url=http://www.officialpinco.com]pinco casino[/url] .
пропуска для грузового транспорта в москве пропуска для грузового транспорта в москве .
труба профильная металлическая https://proftruba-moscow.ru .
Drug guide available. Find pill info.
prescription drugs without prior prescription
Latest medication news. Current medication trends.
онлайн казино Казино всегда манили азартом и возможностью быстро разбогатеть. С появлением интернета эта индустрия перешла в онлайн, предлагая игрокам доступ к любимым играм в любое время и из любой точки мира. Онлайн казино стали невероятно популярными благодаря удобству, широкому выбору игр и щедрым бонусам.
Which Zodiac signs should be careful – a dangerous period begins tomorrow! https://x.com/Fariz418740/status/1901169237206397369
Сайт для женщин https://womanportal.kyiv.ua всё о моде, красоте, здоровье, отношениях и саморазвитии! Полезные советы, тренды, лайфхаки и вдохновение для современной женщины. Будьте стильной, уверенной и успешной!
Автомобильный сайт https://newsgood.com.ua для водителей и автолюбителей! Узнавайте актуальные новости, читайте обзоры новых моделей, изучайте советы по обслуживанию и вождению.
911-remont.ru – сервис по ремонту бытовой техники
Ремонт планшетов pipo в омске в официальном сервисном центре 911.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
Авто портал для всех https://rupsbigbear.com кто любит автомобили! Новости, тест-драйвы, характеристики, тюнинг, страхование, покупка и продажа авто. Следите за последними тенденциями и выбирайте лучшее для себя!
Приобрести документ о получении высшего образования можно в нашем сервисе. Мы предлагаем документы об окончании любых университетов Российской Федерации. Вы сможете получить необходимый диплом по любой специальности, любого года выпуска, включая документы Советского Союза. Даем 100% гарантию, что в случае проверки документа работодателем, подозрений не появится. armit.ru/social/user/42552/forum/message/2032/2034/#message2034
Тут можно преобрести сейф для оружия цены заказать оружейный сейф
Welcome to our online store, where you will find everything you need for successful mining!
More detailed information at the Links abc pro лизинг and abc pro лизинг
We offer large range of high-quality equipment, which will help you mine digital assets as efficiently as possible.
Find medication details. Medication data provided.
northwest pharmacy
Latest medication developments. Medicine facts available.
Which Zodiac signs should be careful – a dangerous period begins tomorrow!
https://www.pinterest.com/pin/957366833290379120
Курск проститутки
Тут можно преобрести офисные сейфы купить купить сейф офисный
сео продвижение сайтов москва сео продвижение сайтов москва .
Рекомендую – selector casino войти
киберспорт Казахстан – инфраструктура Павлодарская область, JAC S5
Medication impacts explained. Medicine impacts described.
best online pharmacies in canada
Side effects listed. Read about pills.
мтс подключить екатеринбург
https://www.seekbetter.careers/employer/24inetrnet-domashnij-ekb/
мтс тв екатеринбург
купить диплом для колледжа
mosbet http://mostbet1005.com.kg/ .
Лучший женский онлайн-журнал https://sweetheart.kyiv.ua Всё о моде, красоте, здоровье, отношениях и успехе. Экспертные советы, лайфхаки и мотивация для уверенных в себе женщин.
Ваш гид в мире красоты https://wonderwoman.kyiv.ua моды и саморазвития! Экспертные статьи о здоровье, отношениях, карьере, психологии и лайфхаках для повседневной жизни. Оставайтесь в курсе трендов и открывайте новые возможности!
Женский журнал онлайн https://sunshadow.com.ua стиль, уход, секреты красоты, семья, карьера и саморазвитие. Будьте в курсе трендов, находите полезные лайфхаки и вдохновляйтесь на новые достижения!
1win. 1win714.ru .
Покупка диплома через надежную компанию дарит массу плюсов. Данное решение дает возможность сберечь как личное время, так и значительные средства. Однако, только на этом выгода не ограничивается, преимуществ значительно больше.Мы можем предложить дипломы психологов, юристов, экономистов и любых других профессий. Дипломы изготавливаются на оригинальных бланках. Доступная цена в сравнении с серьезными затратами на обучение и проживание в чужом городе. Заказ диплома о высшем образовании из российского ВУЗа является мудрым шагом.
Заказать диплом о высшем образовании: diplomc-v-ufe.ru/diplom-med-kolledzha-kupit-2/
1win сайт https://1win709.ru .
mostbet apk скачать mostbet apk скачать .
1win скачать kg 1win714.ru .
1 win 1win709.ru .
Купить документ о получении высшего образования можно в нашей компании. diplomgorkiy.com/kupit-diplom-kosmetologa-2
Great experience! The towing service was prompt, and they took good care of my vehicle. Very satisfied!
911-remont.ru – сервис по ремонту бытовой техники
Ремонт видеокарт diamond в чебоксарах в официальном сервисном центре 911.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
proprio direct montreal
Женский мир https://vsegladko.net без границ! Узнайте всё о моде, уходе за собой, фитнесе, психологии, карьере и хобби. Читайте актуальные статьи, следите за новыми тенденциями и наполняйте жизнь яркими моментами!
Автомобильный онлайн-журнал https://svobodomislie.com всё о мире авто! Свежие новости, обзоры моделей, тест-драйвы, автоспорт, технологии и лайфхаки для водителей. Следите за трендами и будьте в курсе последних событий автопрома!
Онлайн-журнал о машинах https://sw.org.ua всё, что нужно знать об автомобилях! Премьеры новых моделей, сравнение авто, автострахование, технологии, электрокары и советы для владельцев.
мтс тв екатеринбург
http://jobs.foodtechconnect.com/companies/24inetrnet-domashnij-ekb/
мтс тв екатеринбург
Envato Envato, Adobe Stock, iStock, Depositphotos, Dreamstime – это лишь верхушка айсберга в мире стоковых платформ, предлагающих фотографии, видео, графику и другие креативные ресурсы. Каждая из них имеет свои сильные стороны и особенности ценообразования, поэтому выбор оптимальной площадки зависит от конкретных потребностей пользователя.
Fast and reliable roadside assistance! The team was professional and friendly. Will definitely call them again if needed.
Discover the mastery of auto tuning at Saratoga Auto Body, where your vehicle’s ability is fully maximized . Our professional team excels in bespoke tuning packages designed to transform your car’s aesthetics and efficiency . Become a member of our satisfied clientele and drive a vehicle that truly reflects your individual taste. Visit https://saratogaautobody.com/ and begin your car’s transformation today!
Сайт о красоте и здоровье https://beautytips.kyiv.ua полезные советы, тренды ухода, секреты молодости, правильное питание и фитнес. Узнавайте, как оставаться красивой, здоровой и энергичной в любом возрасте!
Мода, красота и здоровье https://fashiontop.com.ua всё в одном месте! Советы по стилю, уходу за волосами и кожей, диеты, тренировки и wellness-тренды. Узнайте, как выглядеть и чувствовать себя на 100%!
Современный женский https://adviceskin.com журнал для тех, кто хочет быть стильной, уверенной и успешной! Секреты красоты, модные тенденции, здоровье, карьера, психология и лайфхаки для повседневной жизни.
pinko casino pinko casino .
Когда состоится встреча Путина и Трампа? https://www.youtube.com/watch?v=QaQAFGjNvyM
Идеальный баланс стиля https://feromonia.com.ua красоты и здоровья! Читайте о модных тенденциях, косметике, правильном питании, спорте и психологическом комфорте. Найдите вдохновение для гармоничной жизни!
Секреты идеального стиля https://ladyone.kyiv.ua молодости и здоровья! Советы по моде, косметике, уходу за кожей и волосами, фитнесу и сбалансированному питанию. Узнайте, как создать гармоничный образ и чувствовать себя великолепно!
Мода, красота и здоровье https://gryada.org.ua ваш путь к идеальному образу! Последние тренды, советы по уходу, секреты стройности и здорового образа жизни. Создавайте гармонию в своём стиле и самочувствии!
Почему Нимесил запрещён в некоторых странах? https://x.com/DeyanetKrmv/status/1901394421708452176
Бонусы без отыгрыша за регистрацию Игровые автоматы на реальные деньги с выводом
Приобрести диплом института!
Мы готовы предложить дипломы психологов, юристов, экономистов и любых других профессий по приятным ценам— diplom-ryssia.com/kupit-diplom-v-belgorode-10/
Medicine leaflet here. Read about pills.
buy lasix no rx
Medication details here. Pill information here.
мтс екатеринбург
https://backtowork.gr/employer/24inetrnet-domashnij-ekb/
провайдер мтс
Где заказать диплом специалиста?
Мы изготавливаем дипломы любых профессий по приятным ценам. Мы можем предложить документы техникумов, расположенных в любом регионе Российской Федерации. Вы сможете купить качественный диплом за любой год, указав необходимую специальность и оценки за все дисциплины. Документы печатаются на “правильной” бумаге самого высокого качества. Это дает возможности делать государственные дипломы, которые невозможно отличить от оригинала. Документы заверяются всеми обязательными печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Для нас очень важно, чтобы документы были доступны для большого количества граждан. diplompro.ru/kupit-diplom-v-volgograde-8
ссылка на сайт https://vodkabet.io
Pill info available. Detailed medication knowledge.
lasix cheap
Patient medicine resource. Get medication facts.
gokong88
https://xlmgokong88.com
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Bu burcl?r pula daha meyillidir https://www.pinterest.com/pin/957366833290413195
1000 рублей за регистрацию вывод сразу без вложений Играть игровые автоматы на деньги
Pill overview available. Pill trends described.
buy furosemide online
Pill info here. Drug trends described.
Секреты красоты и здоровья https://magiclady.kyiv.ua в одном месте! Полезные советы по уходу, тренды моды, спорт, правильное питание и психология уверенности. Узнайте, как выглядеть и чувствовать себя великолепно!
Ваш гид по стилю https://magictech.com.ua красоте и жизни! Женский журнал о модных трендах, секретах молодости, психологии, карьере и личных отношениях.
Лучший женский журнал https://modam.com.ua онлайн! Мода, стиль, уход за собой, фитнес, кулинария, саморазвитие и лайфхаки. Полезные советы и вдохновение для современной женщины, которая стремится к лучшему!
сео сайта prodvizhenie-sajtov-v-moskve224.ru .
1 vin 1win717.ru .
1win сайт https://www.1win818.ru .
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: купить диплом мгюа, купить диплом о среднем образовании в абакане, купить диплом парикмахера цена, купить диплом перманентный макияж, купить диплом поступить в вуз. Постепенно углубляясь в тему, нашел отличный ресурс здесь: diplomybox.com/kupit-diplom-o-vysshem-obrazovanii-v-chite
сайт 1win официальный сайт вход https://1win819.ru .
1 вин официальный 1 вин официальный .
1win официальный сайт вход https://1win818.ru/ .
1xgames промокод – Casino X промокод бонусы на депозит, Лучшие букмекерские конторы
зайти в 1вин http://1win819.ru .
Современный женский онлайн https://viplady.kyiv.ua журнал для тех, кто хочет большего! Всё о моде, косметике, фитнесе, питании, психологии, карьере и отношениях. Узнавайте новое, вдохновляйтесь и воплощайте мечты в реальность!
Онлайн-журнал для женщин https://one-lady.com которые хотят быть стильными и успешными! Последние тренды, секреты красоты, уход за собой, здоровье, психология и карьера. Будьте в курсе новинок и наслаждайтесь жизнью!
Ваш женский путеводитель https://topwoman.kyiv.ua по красоте, стилю и саморазвитию! Полезные советы, модные тенденции, лайфхаки по уходу за собой, психология, отношения и вдохновение.
Current drug information. Medicine impacts described.
generic lasix
Medicine facts provided. Get medication facts.
Выявлен единственный напиток, который спасает волосы https://x.com/Fariz418740/status/1901556598939468146
Excellent towing service! They arrived quickly and handled my car with great care. Highly recommended!
Подробнее в обозревательном журнале компьютерный эксперт на сайте
водительские права купить в москве – права в москве, пробить права
Где приобрести диплом специалиста?
Наши специалисты предлагают быстро приобрести диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Диплом пройдет лубую проверку, даже с использованием специфических приборов. Достигайте цели быстро с нашими дипломами. Приобрести диплом о высшем образовании! silkhunter.com/index.php?search=&title=Special:Search&fulltext=Search
Портал для родителей https://rodkom.org.ua всё о воспитании, развитии и здоровье детей! Полезные советы, лайфхаки, педиатрические рекомендации, семейная психология и идеи для досуга. Растите счастливых и здоровых детей вместе с нами!
Советы для родителей https://agusha.com.ua в одном месте! Развитие ребёнка, воспитание, здоровье, питание, семейные отношения и идеи для досуга. Полезные статьи, лайфхаки и экспертные мнения помогут вам в заботе о малыше!
Автомобильный портал https://kakavto.com всё, что нужно знать о машинах! Новинки автопрома, технологии, электрокары, автоспорт, автострахование и советы по ремонту. Следите за трендами автоиндустрии!
продвижение сайтов сео продвижение сайтов сео .
бетон с бетононасосом цена бетон с бетононасосом цена .
Side effects explained. Recent drug developments.
lasix 100 mg
Access medication details. Get drug info.
капитальный ремонт – косметический ремонт офиса, ремонт офисов ключ
Предсказания Ванги на 2025 год: правда или миф?
https://x.com/Ruslansavalv/status/1901612556772409580
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Актуальные новости Украины https://vesti.in.ua политика, экономика, спорт, культура и общество! Оперативная информация, эксклюзивные материалы, репортажи и аналитика. Следите за событиями в режиме реального времени!
Главные новости дня https://mostmedia.com.ua политика, экономика, общество, спорт и культура! Оперативные события, аналитика, мнения экспертов и репортажи. Читайте актуальные новости Украины и мира в одном месте!
Медицинский онлайн-журнал https://medicalanswers.com.ua ваш личный консультант по здоровью! Полезные статьи, советы врачей, профилактика, диагностика, лечение и здоровый образ жизни.
Бонус за регистрацию игровые автоматы без депозита Бонус за регистрацию игровые автоматы без депозита
Заказать диплом университета!
Мы предлагаем документы любых учебных заведений, расположенных в любом регионе Российской Федерации.
diplomc-v-ufe.ru/gde-kupit-attestat-9-klassa-4
Side effects explained. Current drug trends.
buy lasix medication
Pill facts provided. Short-term impacts described.
Сайт для женщин https://bbb.dp.ua всё о моде, красоте, здоровье, отношениях и саморазвитии! Читайте полезные советы, следите за трендами, вдохновляйтесь и наслаждайтесь гармоничной жизнью!
Juega sin limites! http://amargordediciones.com ofrecen altas cuotas, pagos rapidos y anonimato. ?Compara casas de apuestas populares, elige metodos de apuestas convenientes y disfruta de la emocion!
Apuestas deportivas https://baravaca.es plataformas con licencia, cuotas favorables, variedad de deportes y pagos rapidos. ?Consulta la valoracion de las mejores casas de apuestas y elige la tuya!
Здравствуйте!
Хотите зарегистрироваться в сервисе без использования личного номера? Виртуальный номер – это лучшее решение. Он сохраняет вашу конфиденциальность. https://www.0522.ua/list/472436 Купить постоянный виртуальный номер – это удобно и безопасно. Вы сможете использовать его без ограничений. Надежный инструмент для всех пользователей!
Виртуальный номер – это защита ваших данных и удобство в одном решении. Он подходит для всех видов онлайн-активности. Купить виртуальный номер телефона навсегда – это правильный выбор. Такой номер остается с вами на долгие годы. Безопасность и стабильность гарантированы!
постоянный виртуальный номер для смс, купить виртуальный номер, Виртуальные номера
Удачи и хорошей связи!
Здравствуйте!
Виртуальный номер навсегда – это гарантия стабильности и безопасности. Такой номер можно использовать без ограничений. https://vsenews.kyiv.ua/virtualnyj-nomer-telefona-armenii-vozmozhnosti-preimushhestva.html Купить виртуальный номер для смс навсегда можно за пару кликов. Это выгодное решение для каждого пользователя. Защитите свою конфиденциальность прямо сейчас!
Хотите обезопасить свою личную информацию? Купить постоянный виртуальный номер – идеальное решение. Такой номер можно использовать для регистрации в мессенджерах и соцсетях. Постоянный виртуальный номер для смс работает без перебоев. Надежность и удобство в одном инструменте!
купить виртуальный номер, Виртуальный номер навсегда, виртуальный номер
Удачи и хорошей связи!
戰神賽特
想挑戰刺激又富有獎勳的遊戲世界嗎?來「戰神賽特娛樂城」的老虎機,一場充滿驚喜的冒險正等著您!遊戲中融合了各式特殊符號與獎勳機制,如 Wild 與 Scatter 符號,能夠為您開啟額外的連線機會,讓您在每一輪轉動中都充滿期待。而 Bonus Game 和 Free Spin 更能讓您在短時間內獲得超豐厚的獎勳,輕鬆開啟連勝之旅。無論您是新手還是老玩家,戰神賽特為您精心設計了從 0.5 到 2500 的多元投注範圍,無論您的風險承受度如何,都能輕鬆找到最適合自己的投注金額,享受遊戲的樂趣。若您想要更快掌握遊戲技巧,不妨先體驗「戰神賽特試玩」版,或參考「戰神賽特攻略」指南,讓您在真實賭局中如魚得水,搶奪更多獎勳!快來挑戰自己的運氣,和我們一起開啟一場刺激無比的冒險吧
Patient drug resource. Medication trends described.
buy furosemide online
Drug overview available. Drug guide provided.
Otkrijte most Durdevica Tara – jedan od najlepsih mostova u Evropi! Jedinstvena gradevina iznad kanjona Tare zadivljuje svojom razmjerom i panoramskim pogledom. Najbolje rute, izleti i savjeti za putnike!
Dobite a preskumajte Mountain in Montenegro a pocitite majestatnost hor! Najvyssi vrch Ciernej Hory (2523 m) ponuka ohromujuce panoramy, vzrusujuce trasy a nezabudnutelne emocie. Zistite najlepsie lezecke cesty a tipy na bezpecny vylet!
Kaufen Sie immobilien kaufen in montenegro – Ihr Zuhause am Meer oder in den Bergen! Tolle Angebote, Villen, Wohnungen und Apartments. Informieren Sie sich uber die besten Lagen, Preise, rechtlichen Feinheiten und Investitionsmoglichkeiten!
куб бетона цена минск с доставкой куб бетона цена минск с доставкой .
Подробнее в блоге обзоров прицелов и другой оптической техники перейти
Бонус без отыгрыша Casino top
Sakupon an Salog Tara Durmitor rafting an pinakamarahay na pag-rafting sa Montenegro! Pag-raft sa saro sa pinakahararom na mga kanyon sa Europa, an pinakadalisay na tubig asin naturalesa kan Durmitor. An perpektong pakikipagsapalaran para sa mga mahilig sa luwas!
Enjoy fun at tara river canyon extreme rafting, kayaking, boating and camping in picturesque places. The perfect place for active recreation and immersion in wild nature!
Patient medication resource. Drug details provided.
lasix generic
Current medication trends. Patient pill resource.
Огромный выбор, очень красивые композиции.
доставка цветов в томске
Хотите списать долги? http://niidg.ru/interes/bankrotstvo-fizicheskix-lic-dogovornaya-garantiya-kak-novyj-uroven-zashhity/ помощь в сложных финансовых ситуациях. Освободитесь от кредитов, штрафов и задолженностей. Узнайте, как начать процедуру прямо сейчас!
wan win wan win .
1win онлайн http://1win821.ru/ .
戰神賽特
「戰神賽特」— 2025年最火爆的老虎機,等你來挑戰!
如果你正在尋找一款高賠率、刺激又充滿神秘感的老虎機遊戲,那麼「戰神賽特」絕對是你的最佳選擇!這款由RG電子精心打造的遊戲,以古埃及戰神賽特為主題,結合精美畫面與震撼音效,帶你進入神秘的沙漠世界,感受無與倫比的刺激體驗。
為何玩家瘋狂愛上戰神賽特?
超狂賠率 51,000 倍:一轉瞬間,財富翻倍不是夢
高RTP達96.89%:更高勝率,更多機會贏得大獎
獨特遊戲機制:掉落消除+獎金購買,玩法多樣更刺激
極致視聽享受:古埃及風格設計+震撼音效,讓你完全沉浸其中
無論你是老虎機高手還是新手玩家,「戰神賽特」都能帶給你前所未有的遊戲體驗!快來挑戰戰神,贏取屬於你的豐厚獎勵!
казино 1win http://www.1win808.ru .
https://domnabakalinskoy.ru
1win играть https://www.1win820.ru .
игра 1вин игра 1вин .
1win вход 1win вход .
Get drug details. Get drug facts.
purchase lasix
Medicine facts provided. Access pill information.
read this article https://web-lumiwallet.com/
Неожиданный способ есть финики превращает их в природное лекарство
https://x.com/SebiBilalova/status/1901774516755309011
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
химчистка мебели
химчистка мебели
Рекомендую – селектор казино вход
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
1000 рублей за регистрацию вывод сразу 1000 рублей за регистрацию вывод сразу без вложений
Рекомендуем – селектор казино вход
интернет провайдер москва интернет провайдер москва .
провайдер интернета в московском провайдер интернета в московском .
important site Lumi online
срочная доставка алкоголя на дом москва срочная доставка алкоголя на дом москва .
Эти четыре знака Зодиака накроет волна счастья уже на этой неделе
https://www.pinterest.com/pin/957366833290452816/
цифровые ключи
купить бетон в минске цена за куб купить бетон в минске цена за куб .
moving and storage companies toronto cheap moving companies toronto
https://ocskincancer.com/podcast/podcast-coronavirus-innovative-treatments-and-disappointing-tests/
Заказать диплом о высшем образовании!
Мы готовы предложить документы учебных заведений, которые находятся в любом регионе РФ. Можно приобрести качественный диплом за любой год, указав подходящую специальность и хорошие оценки за все дисциплины. Дипломы и аттестаты печатаются на бумаге самого высокого качества: sibreztech.ru/fonts/pgs/?kupit_diplom_o_polnom_srednem_obrazovanii_bustro.html
Статьи о ремонте и строительстве https://tvin270584.livejournal.com советы, инструкции и лайфхаки! Узнайте, как выбрать материалы, спланировать бюджет, сделать ремонт своими руками и избежать ошибок при строительстве.
Ремонт и сантехника https://santekhnik-moskva.blogspot.com пошаговые инструкции, выбор материалов, монтаж систем водоснабжения и отопления, устранение протечек и установка сантехнических приборов. Советы экспертов для качественного ремонта!
mostbet.kg http://mostbet1006.com.kg/ .
мостбет кыргызстан mostbet1006.com.kg .
получить 1000 рублей бесплатно за регистрацию Бонусы без отыгрыша за регистрацию
мостбет кг https://www.mostbet1007.com.kg .
Нужна машина в прокат? стоимость аренды авто в сочи быстро, выгодно и без лишних забот! Машины всех классов, удобные условия аренды, страховка и гибкие тарифы. Забронируйте авто в несколько кликов!
мостбет chrono https://www.mostbet1008.com.kg .
visit our website Web.lumi wallet
мостбет казино войти http://mostbet1007.com.kg .
мосбет казино http://mostbet1008.com.kg .
porn esports news whores
Ready-made custom works by link fast, high-quality and inexpensive! We will help with abstracts, term papers, diplomas, essays and other academic assignments. Guaranteed originality, deadlines and 24/7 support!
2 знака зодиака, от которых практически невозможно что-либо скрыть
https://pin.it/1l95y2WeH
Привет!
Купить диплом университета по доступной стоимости возможно, обратившись к надежной специализированной фирме. Приобрести диплом: kupitediplom.ru/kupit-diplom-orenburg/
Здравствуйте!
Без наличия диплома сложно было продвигаться по карьере. Сегодня же документ не дает практически никаких гарантий, что получится получить престижную работу. Более важное значение имеют профессиональные навыки специалиста, а также его опыт. По этой причине решение о покупке диплома стоит считать выгодным и рациональным. Купить диплом ВУЗа bs.listbb.ru/viewtopic.php?f=3&t=1554
Нужен качественный бетон? производство бетона быстро, надёжно и по выгодной цене! Прямые поставки от производителя, различные марки, точное соблюдение сроков. Оперативная доставка на стройплощадки, заказ онлайн или по телефону!
Descubre cómo George Foreman se convirtió en campeón mundial dos veces | La pelea entre George Foreman y Joe Frazier es una de las más emocionantes de la historia | El estilo de pelea de George Foreman lo hizo destacar entre los grandes del boxeo | Cada pelea de George Foreman era una muestra de determinación y coraje George Foreman: historia y curiosidades.
Где заказать диплом специалиста?
Мы предлагаем выгодно и быстро приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями. Данный диплом способен пройти любые проверки, даже при помощи специальных приборов. Достигайте своих целей быстро и просто с нашим сервисом.
Заказать диплом о высшем образовании fastdiploms.com/kupit-svidetelstvo-o-rozhdenii-sssr-9/
Мы изготавливаем дипломы любой профессии по выгодным ценам. Стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы дипломы были доступными для большинства граждан.
Приобретение документа, подтверждающего обучение в ВУЗе, – это грамотное решение. Заказать диплом любого ВУЗа: diplomist.com/kupit-diplom-o-srednem-13/
Заказать документ о получении высшего образования вы имеете возможность у нас. http://www.botsdecriptomonedas.com/bots/grid-bot-criptomonedas
Fast and reliable roadside assistance! The team was professional and friendly. Will definitely call them again if needed.
купить диплом it специалиста
открыт кейс кс го https://cs-go.ru
1Win Brasil 1winbr apostas esportivas e cassino online! Altas odds, bonus generosos e ampla variedade de jogos. Aposte em esportes, jogue no cassino e aproveite as melhores promocoes agora mesmo!
Access pill details. Comprehensive medicine guide.
buy generic lasix
Find drug information. Drug leaflet available.
What’s up Dear, are you truly visiting this web site on a regular basis, if so afterward you will absolutely obtain fastidious know-how.
лечение наркотической ломки
Keep this going please, great job!
снятие ломки цена
Prostat x?rc?ngin? qars? hans? qidalar istifad? edilm?lidir? https://x.com/Fariz418740/status/1902037337590108612
фриспины без депозита с выводом за регистрацию 1000 рублей за регистрацию вывод сразу
Где приобрести диплом специалиста?
Купить диплом института по доступной цене можно, обратившись к проверенной специализированной фирме.: diplommy.ru
戰神賽特
「戰神賽特」— 2025年最火爆的老虎機,等你來挑戰!
如果你正在尋找一款高賠率、刺激又充滿神秘感的老虎機遊戲,那麼「戰神賽特」絕對是你的最佳選擇!這款由RG電子精心打造的遊戲,以古埃及戰神賽特為主題,結合精美畫面與震撼音效,帶你進入神秘的沙漠世界,感受無與倫比的刺激體驗。
為何玩家瘋狂愛上戰神賽特?
超狂賠率 51,000 倍:一轉瞬間,財富翻倍不是夢
高RTP達96.89%:更高勝率,更多機會贏得大獎
獨特遊戲機制:掉落消除+獎金購買,玩法多樣更刺激
極致視聽享受:古埃及風格設計+震撼音效,讓你完全沉浸其中
無論你是老虎機高手還是新手玩家,「戰神賽特」都能帶給你前所未有的遊戲體驗!快來挑戰戰神,贏取屬於你的豐厚獎勵!
Мы изготавливаем дипломы любой профессии по приятным тарифам. Дипломы изготавливаются на подлинных бланках Заказать диплом о высшем образовании diplom-zentr.com
想挑戰刺激又富有獎勳的遊戲世界嗎?來「戰神賽特娛樂城」的老虎機,一場充滿驚喜的冒險正等著您!遊戲中融合了各式特殊符號與獎勳機制,如 Wild 與 Scatter 符號,能夠為您開啟額外的連線機會,讓您在每一輪轉動中都充滿期待。而 Bonus Game 和 Free Spin 更能讓您在短時間內獲得超豐厚的獎勳,輕鬆開啟連勝之旅。無論您是新手還是老玩家,戰神賽特為您精心設計了從 0.5 到 2500 的多元投注範圍,無論您的風險承受度如何,都能輕鬆找到最適合自己的投注金額,享受遊戲的樂趣。若您想要更快掌握遊戲技巧,不妨先體驗「戰神賽特試玩」版,或參考「戰神賽特攻略」指南,讓您在真實賭局中如魚得水,搶奪更多獎勳!快來挑戰自己的運氣,和我們一起開啟一場刺激無比的冒險吧
Get pill details. Find medicine info.
buy furosemide online
Find medication information. Recent medicine developments.
Sports betting with 1xbetapp br! Huge selection of matches, live bets, generous bonuses and lightning-fast payouts. Play casino, eSports and win at any time!
Welcome to Winbet Casino winbetaz.biz/ a place where luck is on your side! Huge selection of games, bonuses for new players and instant payouts. Try your luck right now!
Здравствуйте!
Вам нужен надежный контакт для бизнеса? Купить виртуальный номер телефона навсегда – это отличное решение. https://strivesoccercamp.com/contact/?contact-form-id=9&contact-form-sent=63044&contact-form-hash=4d2c54519a921c42684a135d53df8764b4b5d6be&_wpnonce=2513f86332 Постоянный виртуальный номер позволяет использовать сервисы без привязки к личному номеру. Это безопасно и удобно. Выбирайте качество!
Виртуальный номер навсегда – это новый уровень свободы. Он не зависит от вашего местоположения. Купить постоянный виртуальный номер – это легкость и удобство. Используйте его для регистрации на любых сервисах. Ваши данные останутся в безопасности!
купить виртуальный номер, виртуальный номер, виртуальный номер
Удачи и хорошей связи!
Добрый день!
Купить виртуальный номер телефона навсегда – это лучшее решение для конфиденциальности. Он работает с любыми сервисами. http://forum.spolokmedikovke.sk/viewtopic.php?f=3&t=30815&p=933847#p933847 Постоянный виртуальный номер поможет вам оставаться на связи без привязки к реальному номеру. Простота, удобство и безопасность!
Вам нужен номер для временных регистраций? Виртуальный номер навсегда – это лучший выбор. Купить виртуальный номер для смс навсегда можно прямо сейчас. Он будет работать стабильно и надежно. Удобство на каждом этапе!
купить виртуальный номер для смс навсегда, купить виртуальный номер телефона навсегда, купить виртуальный номер навсегда
Удачи и хорошей связи!
смета на устройство дренажа http://drenazh-uchastka-96.ru .
играть crazy monkey бесплатно без регистрации crazymonkeygame.com
Wow, fantastic weblog format! How lengthy have you ever been running a blog for? you make running a blog look easy. The full glance of your site is excellent, let alone the content material!
снять ломку наркомана
Сюрприз удался, девушка была в восторге!
купить цветы в томске
maximum winnings with http://onewinwin.in! Bet on sports, play in the casino, participate in promotions and tournaments. Convenient payments, fast payouts and a welcome bonus for beginners. Join 1win and collect your winnings!
Merhaba web siteniziçok beğendim tekrar geri dönüş yapıp daha detaylı inceleme fırsatım olmasını diliyorum
Приобрести диплом о высшем образовании!
Мы изготавливаем дипломы любых профессий по приятным ценам. Вы приобретаете диплом через надежную фирму. : kupitediplom.ru/kupit-diplom-vtuzhv-po-vigodnoj-tsene-mozhno-v-nashej-kompanii/
mostbet скачать mostbet скачать .
1win букмекер http://1win809.ru .
мостбет мостбет .
мостбет скачать https://mostbet788.ru/ .
Где купить диплом по необходимой специальности?
Мы изготавливаем дипломы любой профессии по невысоким ценам. Мы предлагаем документы техникумов, расположенных на территории всей РФ. Можно купить качественный диплом за любой год, указав необходимую специальность и хорошие оценки за все дисциплины. Документы выпускаются на “правильной” бумаге высшего качества. Это дает возможности делать государственные дипломы, которые невозможно отличить от оригиналов. Документы будут заверены необходимыми печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас очень важно, чтобы документы были доступными для большинства граждан. ry-diplom.com/kupit-diplom-v-ufe-2-6
1win скачать kg http://www.1win809.ru .
в этом разделе nova маркетплейс
Диплом университета РФ!
Без наличия диплома очень трудно было продвинуться вверх по карьерной лестнице. Именно из-за этого решение о заказе диплома можно считать выгодным и целесообразным. Заказать диплом о высшем образовании mop.5nx.ru/viewtopic.php?f=38&t=1722
Right here is the perfect webpage for anyone who would like to understand this topic. You know so much its almost hard to argue with you (not that I actually will need to…HaHa). You certainly put a new spin on a topic which has been written about for years. Great stuff, just excellent!
снятие ломки цена
gambling without limits one-1win.in/ wide selection of games, high odds, fast payouts and exclusive bonuses. Try your luck in the casino and on bets. Play, win and earn with 1win!
your game, your rules winbkaz.biz Sports, eSports, casino, poker and slots – choose and win! Convenient account replenishment, instant withdrawals and cool promotions for new players. Join now!
Thanks for the good writeup. It in fact used to be a leisure account it. Glance complex to far added agreeable from you! By the way, how could we communicate?
снятие ломки в стационаре
Где приобрести диплом по нужной специальности?
Мы предлагаем документы об окончании любых ВУЗов РФ. Документы изготавливаются на подлинных бланках государственного образца. federalbureauinvestigation.5nx.ru/viewtopic.php?f=3&t=784
This is the perfect website for anybody who wishes to find out about this topic. You understand a whole lot its almost hard to argue with you (not that I personally would want to…HaHa). You definitely put a brand new spin on a subject that’s been written about for decades. Wonderful stuff, just wonderful!
лечение наркотической ломки
whoah this weblog is excellent i really like studying your posts. Keep up the good work! You know, lots of individuals are looking around for this information, you could aid them greatly.
снятие ломки на дому цена
go to this website acheter de la fausse monnaie
дренаж спб дренаж спб .
дренаж участка колодец дренаж участка колодец .
Kingmaker Kingmaker, Kingmaker Casino, Kingmaker greece Casino
Excellent towing service! They arrived quickly and handled my car with great care. Highly recommended!
переподготовка Переподготовка и повышение квалификации: инвестиции в профессиональный рост. В современном динамичном мире потребность в непрерывном обучении становится ключевым фактором успеха. Профессиональная переподготовка и курсы повышения квалификации – это эффективные инструменты для адаптации к меняющимся требованиям рынка труда и расширения профессиональных горизонтов. Если вы стремитесь к карьерному росту, желаете освоить новую специальность или углубить свои знания в уже имеющейся, курсы переподготовки предоставят вам необходимые компетенции. В ряде случаев возникает необходимость подтверждения квалификации. В таких ситуациях возможно купить удостоверение, соответствующее пройденному обучению. Особое внимание уделяется обучению в сферах, требующих повышенной безопасности. Обучение работе на высоте и безопасным работам на высоте, а также обучение по пожарной безопасности являются критически важными для обеспечения безопасности на рабочем месте. Для тех, кто ценит свое время и предпочитает гибкий график, курсы повышения квалификации дистанционно предоставляют возможность обучения в удобном формате. Обучение по профессиям охватывает широкий спектр специальностей, позволяя каждому найти подходящую программу.
Эта информационная заметка содержит увлекательные сведения, которые могут вас удивить! Мы собрали интересные факты, которые сделают вашу жизнь ярче и полнее. Узнайте нечто новое о привычных аспектах повседневности и откройте для себя удивительный мир информации.
Узнать больше – https://mednarkoforum.ru/
Возможно ли получать тысячи манатов в месяц в соцсетях в Азербайджане?
https://pin.it/1OLQiA9c2
This is very interesting, You are a very skilled blogger. I’ve joined your rss feed and look forward to seeking more of your fantastic post. Also, I have shared your web site in my social networks!
снятие ломки нарколог
Top casino с выводом фриспины без депозита
Хотите получить https://10eurosgratissindepositocasinoespana.com начни игру без риска – Открой для себя захватывающие слоты, живое казино и эксклюзивные бонусы. Играй с комфортом, наслаждайся топовыми играми и выигрывай!
голд казино
Заказать документ университета можно у нас. diplomist.com/kupit-diplom-chelyabinsk-2
Mostbet Uzbekistan https://mostbetuzbekistan-apk.com offers you profitable bets, top odds and instant payouts. Make sports predictions, play online casino and enjoy the excitement with registration bonuses!
Где купить диплом специалиста?
Полученный диплом с нужными печатями и подписями 100% отвечает стандартам Министерства образования и науки, никто не отличит его от оригинала – даже со специальным оборудованием. Не откладывайте собственные мечты на потом, реализуйте их с нами – отправьте простую заявку на диплом уже сегодня! Получить диплом о среднем образовании – не проблема! diplom-kaluga.ru/kupit-nastoyashij-attestat-2/
мост бет mymoscow.forum24.ru/?1-2-0-00000718-000-0-0-1742357638 .
сайт 1win https://mymoscow.forum24.ru/?1-2-0-00000717-000-0-0-1742357431/ .
вход 1win http://www.cah.forum24.ru/?1-19-0-00000732-000-0-0 .
мостбет скачать казино мостбет скачать казино .
1win,com http://mymoscow.forum24.ru/?1-2-0-00000717-000-0-0-1742357431/ .
1vin казино cah.forum24.ru/?1-19-0-00000732-000-0-0 .
Магазин печей и каминов http://www.pech.pro широкий выбор дровяных, газовых и электрических моделей. Стильные решения для дома, дачи и бани. Быстрая доставка, установка и гарантия качества!
porn ufc whores
Hey! I’m at work surfing around your blog from my new iphone 4! Just wanted to say I love reading your blog and look forward to all your posts! Keep up the superb work!
Лечение подростковой наркомании в Самаре – профилактика наркотической зависимости у ребенка
подробнее nova маркетплейс
Здравствуйте!
Капибара и собаки — неожиданный, но идеальный дуэт. Они часто становятся друзьями и любят вместе отдыхать. капибара ночью Капибара дружит с котами, собаками и даже черепахами! Посмотри капибара фото и удивись её общительности!
Более подробно по ссылке – https://publitruck.cl/2022/05/24/hola-mundo/#comment-5033
капибара в горячих источниках
капибара водное животное
капибара общается с сородичами
Удачи!
Доброго!
Капибара в зоопарке чувствует себя комфортно, если у неё есть бассейн и простор для прогулок. капибара внешний вид Капибара содержание в домашних условиях требует особого ухода. Узнай, как создать ей идеальную среду!
Более подробно по ссылке – http://www.artone.co.kr/bbs/zboard.php?id=service_18&page=1&page_num=20&select_arrange=headnum&desc=&sn=off&ss=on&sc=on&keyword=&no=1&category=
капибара в реке
капибара интересные факты
капибара плавание
Удачи!
Hi to all, the contents present at this website are actually awesome for people experience, well, keep up the nice work fellows.
Лечение зависимости от метадона в Самаре анонимно, помощь при метадоновой наркомании
贏家娛樂城
Click Here acheter des billets contrefaits
Thanks for another informative blog. Where else may just I am getting that type of information written in such an ideal approach? I’ve a project that I am simply now working on, and I’ve been at the look out for such info.
Лечение лекарственной зависимости в Самаре анонимно, помощь при зависимости от лекарств / таблеток
септик для частного в спб купить септик в спб
Как развить эмоциональный интелект ребёнка? Для развития эмоционального интеллекта ребёнка важно создать атмосферу доверия и открытости. Обсуждайте с ребёнком его эмоции, помогайте ему их выражать и понимать. Читайте книги и смотрите фильмы, которые иллюстрируют разные эмоции, и обсуждайте их с ребёнком. Игры, где нужно угадывать эмоции, также могут быть полезны. Поддерживайте ребёнка в его эмоциональных переживаниях и учите справляться с трудностями.
Zarejestruj się w Mostbet i zgarnij darmowe spiny na start | Lubisz darmowe spiny? Mostbet przygotował dla Ciebie specjalny kod promocyjny | Mostbet oferuje najlepsze kursy na zakłady sportowe w Polsce | Czy warto grać w Mostbet? Opinie użytkowników potwierdzają wysoką jakość usług Mostbet kasyno online.
This web site truly has all the info I wanted about this subject and didn’t know who to ask.
водное шоу
heroin esports porn
heroin esports whores
Купить диплом о высшем образовании!
Мы готовы предложить документы институтов, которые находятся на территории всей Российской Федерации.
fastdiploms.com/diplom-s-reestrom-kupit-5
Terrific post however , I was wanting to know if you could write a litte more on this subject? I’d be very thankful if you could elaborate a little bit more. Kudos!
flyboard шоу
1win казино – официальный сайт https://1win705.ru/ .
скачать mostbet на телефон http://mostbet780.ru/ .
скачать мостбет скачать мостбет .
1 win moldova http://1win705.ru .
mostbet apk скачать https://mostbet780.ru/ .
мостбет зеркало mostbet781.ru .
Элегантность и производительность BMW X6, познакомьтесь с.
Комфорт и стиль в BMW X6, всегда.
кроссовера BMW X6.
Стильный и агрессивный BMW X6, поразит.
BMW X6: мощь на каждый день, этого кроссовера.
Кроссовер BMW X6, который стоит выбрать, решение.
Комфортабельный интерьер BMW X6, создают.
Незаменимый помощник на дороге – BMW X6, безопасность.
Узнайте, почему BMW X6 так популярен, в нашем анализе.
Мощь и маневренность BMW X6, завораживают.
Обеспечьте свою безопасность с BMW X6, неукоснительно.
Выбор BMW X6: ваши преимущества, открыл.
Технологический прогресс BMW X6, формируют.
Исключительный комфорт BMW X6, узнайте.
Что дает вам BMW X6?, в нашем анализе.
BMW X6: стиль, который невозможно не заметить, выразит вашу индивидуальность.
Как BMW X6 выглядит на фоне конкурентов, в нашем сравнении.
Изучите отзывы владельцев BMW X6, в нашем разделе.
Современные системы безопасности BMW X6, гарантируют вашу безопасность.
Итоги: BMW X6, как лучший выбор, предлагаем выводы.
bmw individual bmw individual .
Do you have a spam problem on this site; I also am a blogger, and I was wondering your situation; we have developed some nice procedures and we are looking to swap techniques with other folks, be sure to shoot me an e-mail if interested.
частный нарколог на дом
Hi there, I would like to subscribe for this webpage to obtain latest updates, thus where can i do it please help out.
запой нарколог дом
gay esports porn
heroin esports heroin
Рекомендую сайты: fotonons.ru, artcet.ru, 40-ka.ru, urkarl.ru, upsskirt.ru, yarus-kkt.ru, cultureinthecity.ru, imgtube.ru, great-galaxy.ru, shvejnye.ru, lostfiilmtv.ru, voenoboz.ru, my-caffe.ru, kanunnikovao.ru, adventime.ru, kaizen-tmz.ru
Добрый день!
Мы изготавливаем дипломы любой профессии по приятным тарифам. Стоимость зависит от выбранной специальности, года выпуска и университета: diploman.com/
Приобрести диплом ВУЗа по выгодной стоимости возможно, обратившись к проверенной специализированной компании. Мы готовы предложить документы любых учебных заведений, которые расположены на территории всей РФ. diploml-174.ru/kupit-diplom-srednee-obrazovanie
Medicine facts available. Patient medicine info.
lasix generic
Medication guide here. Medication guide here.
Где заказать диплом специалиста?
Мы предлагаем быстро и выгодно заказать диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Диплом пройдет любые проверки, даже с использованием специально предназначенного оборудования. Достигайте цели быстро с нашей компанией. Заказать диплом университета! forumkk.listbb.ru/viewtopic.php?f=12&t=1033
Быстрая доставка, вежливые курьеры – буду заказывать еще!
гипсофилы цена букета
Исследуйте разнообразие модельного ряда BMW, водителей.
Узнайте о лучших моделях BMW, дизайном.
Откройте для себя новейшие модели BMW, электромобили.
Выбор для любого стиля жизни в модельном ряду BMW, невероятно разнообразен.
Погрузитесь в мир премиальных автомобилей BMW, которые создают.
Почему стоит выбрать BMW, обнаружьте.
Эволюция автомобилей: модельный ряд BMW, тех, кто любит скорость.
Лучшие автомобили в модельном ряду BMW, которые заинтересуют.
Что нового в линейке BMW, проверьте.
Разнообразие моделей BMW: для каждого, подходящие для городской жизни.
BMW — это больше, чем просто автомобиль, это опыт, который нужно испытать.
Каждая модель BMW — это удовольствие от вождения, который вдохновляет.
BMW: там, где стиль встречается с производительностью, созданная для.
Почему BMW — это ваш идеальный выбор, от комфорта до инноваций.
Вдохновляйтесь моделями BMW для вашего стиля жизни, с выдающейся производительностью.
Модельный ряд BMW, который сочетает в себе мощь и элегантность, на любые случаи жизни.
Модельный ряд BMW: сочетание стиля и технических решений, для тех, кто ищет лучшее.
Модельный ряд BMW: ваше новое путешествие начинается, с надежностью и безопасностью.
Модельный ряд BMW: от классики до новинок, для любого бюджета.
bmw x7 m https://model-series-bmw.biz.ua/ .
Кракен зеркало – Кракен даркнет, Кракен даркнет
кайтсерфинг в египте
Overdose effects detailed. Pill effects listed.
buy lasix uk
Medicine impacts explained. Read about drugs.
Приобрести диплом ВУЗа !
Приобретение диплома университета РФ в нашей компании является надежным процессом, потому что документ будет заноситься в государственный реестр. Печать производится на специальных бланках ГОЗНАКа. Приобрести диплом о высшем образовании arenadiplom24.online/vuzy/institut-ekonomiki-i-upravleniya
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
gokong88
https://solgokong88.com
Latest medicine news. Find medicine information.
buy furosemide
Drug leaflet available. Medication leaflet here.
Доброго!
Современный автотехцентр Ниссан в Москве – это место, где ваш автомобиль получит самое качественное обслуживание. Мы предлагаем широкий спектр услуг для Ниссан, от диагностики до сложных ремонтов, с использованием новейшего оборудования. Современный автотехцентр Ниссан в Москве – это комфорт и эффективность, с максимальным вниманием к каждой детали. Наши специалисты готовы решить любую задачу и предложить вам лучшие решения для вашего автомобиля. Обратитесь к нам, и мы обеспечим вашему Ниссан долгосрочную надежность и отличное состояние.
СТО Ниссан, информации по ссылке – https://nissan-avtoservise.ru/
ниссан тиида диагностика ходовой, ремонт стартера на ниссан х трейл, диагностика ниссан уфа
установка гбо ниссан террано, диагностика в москве ниссана, замена стекол ниссан минск
Удачи!
свежая сводка новостей – ваш надежный источник актуальных новостей! Мы оперативно освещаем события политики, экономики, технологий, спорта и культуры. Всегда свежие материалы, объективные аналитические обзоры и эксклюзивные интервью. Будьте в курсе главного – следите за новостями вместе с нами!
heroin esports whores
Доброго!
Комфортное ожидание в автосервисе Ниссан в Москве – это залог вашего удобства и хорошего настроения. Мы создали все условия для комфортного ожидания, включая уютные зоны отдыха, Wi-Fi и бесплатные напитки. Комфортное ожидание в автосервисе Ниссан в Москве позволяет вам проводить время с пользой и в уютной атмосфере. Мы понимаем, как важно, чтобы ожидание было комфортным, и заботимся о каждом клиенте. Обратитесь к нам и получите удобное обслуживание в нашем сервисе.
СТО Ниссан, информации по ссылке – https://nissan-avtoservise.ru
обд диагностика ниссан кашкай, замена лобового стекла ниссан кашкай своими руками, вариатор ниссан либерти диагностика
кузовные запчасти ниссан москва, б у запчасти в москве на ниссан, распиновка разъема ниссан диагностики
Удачи!
terrorist attack esports cocaine
https://hallbook.com.br/blogs/278114/%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC-%EA%B5%AC%EB%A7%A4-%EC%8B%9C-%EA%B3%A0%EB%A0%A4%ED%95%B4%EC%95%BC-%ED%95%A0-%EA%B0%9C%EC%9D%B8%EC%A0%81%EC%9D%B8-%EA%B1%B4%EA%B0%95-%EC%9A%94%EC%86%8C
mostbet casino https://agility.forum24.ru/?1-0-0-00000756-000-0-0-1742360323 .
1win партнерка вход agility.forum24.ru/?1-0-0-00000755-000-0-0-1742359870 .
Czy Mostbet jest legalny w Polsce? Sprawdź szczegóły na stronie | Automaty, blackjack, ruletka – Mostbet kasyno ma wszystko, czego potrzebujesz | Czy wiesz, że Mostbet posiada dedykowaną aplikację mobilną? | Zgarnij bonus za rejestrację w Mostbet i ciesz się dodatkowymi środkami na grę Mostbet Casino – bonus bez depozytu sprawdź szczegóły.
1win pariuri https://www.1win5002.ru .
mostbet kg отзывы http://www.agility.forum24.ru/?1-0-0-00000756-000-0-0-1742360323 .
1win. http://agility.forum24.ru/?1-0-0-00000755-000-0-0-1742359870 .
jocuri de noroc online moldova https://1win5002.ru .
Patient drug leaflet. Patient drug facts.
buy lasix online without prescription
Complete medication overview. Complete medicine overview.
WOW just what I was looking for. Came here by searching for %keyword%
flyboard на мероприятие
Кракен ссылки – кракен вход, Официальный сайт кракен
Мы можем предложить дипломы любой профессии по приятным ценам. Основные преимущества приобретения документов в нашем сервисе
Вы заказываете документ через надежную и проверенную временем фирму. Такое решение сэкономит не только денежные средства, но и время.
Плюсов куда больше:
• Документы изготавливаем на фирменных бланках со всеми печатями;
• Можно купить дипломы любого ВУЗа РФ;
• Стоимость во много раз меньше той, которую понадобилось бы заплатить на очном и заочном обучении в университете;
• Доставка в любые регионы Российской Федерации.
Заказать диплом о высшем образовании– http://seferpanim.com/read-blog/1512_diplom-tehnikuma-cena.html/ – seferpanim.com/read-blog/1512_diplom-tehnikuma-cena.html
купить диплом в казани отзывы
Sprawdzilem portal ?? oczekujac, ze sprobuje przyzwoita platforme, ale to po prostu katastrofa! Uklad przestarzaly, obsluga nieintuicyjne, a rozgrywka ciagle sie zawieszaja. Mnostwo reklam, strony laduja sie wolno, a pomoc nie odpowiada na zgloszenia. Tresci na witrynie sa chaotyczne i bezuzyteczne. Mam wrazenie, ze nikt juz nie dba o ten serwis. Lepiej zainwestowac uwage na rzeczywiscie wiarygodne strony!
gay esports whores
terrorist attack esports cocaine
Для эффективного продвижения по карьерной лестнице требуется наличие диплома университета. Купить диплом об образовании у сильной компании: diplomv-v-ruki.ru/kupit-diplom-v-tveri-7/
Patient pill resource. Access pill details.
buy lasix no prescription
Drug leaflet here. Pill info here.
Миру могут угрожать вспышки неизвестных ранее болезней https://x.com/Fariz418740/status/1902696858473910276
pdacenter.ru – сервис по ремонту бытовой техники
Ремонт моноколес в Твери в официальном сервисном центре PDACENTER.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
gay esports gay
Hi every one, here every one is sharing such know-how, so it’s fastidious to read this web site, and I used to visit this web site all the time.
концерт на флайбордах
Free steam accounts t.me/s/freesteamaccountc
веб-сайте
оборудование для переработки цена
Pill information provided. Medication guide available.
where buy lasix
Access medicine facts. Patient medication resource.
Купить документ о получении высшего образования можно в нашей компании. Мы оказываем услуги по производству и продаже документов об окончании любых университетов Российской Федерации. Вы получите необходимый диплом по любым специальностям, включая документы старого образца. Даем гарантию, что в случае проверки документа работодателями, никаких подозрений не возникнет. kalininabad.flyboard.ru/viewtopic.php?f=28&t=3731
веб-сайте
бизнес план по переработке пластика
Приветствую!
Для многих людей, заказать диплом ВУЗа – это острая потребность, шанс получить достойную работу. Однако для кого-то – это очевидное желание не терять время на учебу в институте. Что бы ни толкнуло вас на это решение, наша фирма готова помочь. Максимально быстро, качественно и по разумной стоимости сделаем документ нового или старого образца на настоящих бланках со всеми печатями.
Основная причина, почему многие люди прибегают к покупке документов, – получить хорошую должность. Допустим, знания позволяют кандидату устроиться на желаемую работу, но документального подтверждения квалификации нет. В случае если для работодателя важно наличие “корочки”, риск потерять вакантное место очень высокий.
Приобрести документ ВУЗа вы имеете возможность у нас в столице. Мы предлагаем документы об окончании любых университетов России. Вы получите диплом по любым специальностям, включая документы Советского Союза. Гарантируем, что в случае проверки документов работодателем, никаких подозрений не появится.
Факторов, которые вынуждают купить диплом очень много. Кому-то срочно необходима работа, а значит, необходимо произвести особое впечатление на начальника при собеседовании. Некоторые желают попасть в престижную компанию, чтобы повысить собственный статус и в будущем начать собственное дело. Чтобы не тратить попусту годы жизни, а сразу начать эффективную карьеру, применяя имеющиеся знания, можно заказать диплом через интернет. Вы сможете быть полезным в социуме, обретете финансовую стабильность в кратчайший срок- аттестат купить
gokong88
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Топ онлайн казино 2025: детальное исследование лучших игровых платформ
Онлайн азартные игры стремительно расширяют свою аудиторию в России, завоевывая всё больше игроков благодаря своей доступности. Однако для безопасного времяпрепровождения критически важно выбирать только легальные площадки, которые гарантируют честность алгоритмов. В этом материале мы разберём ключевые стандарты качества, выделим лидеров лучших казино 2025 года и поделимся экспертными советами для правильного решения.
Основные признаки безопасности онлайн-казино
Чтобы избежать рисков, необходимо учитывать следующие аспекты:
Лицензионная чистота
Проверяйте наличие международных лицензий: Гибралтара.
Убедитесь, что игровые автоматы прошли независимую проверку от авторитетных организаций (eCOGRA).
Система случайных чисел (RNG)
Казино должно использовать проверенный генератор случайных чисел, что обеспечивает справедливость результатов.
Скорость транзакций
Надёжные казино предлагают быстрые выплаты через современные системы: банковские карты (MasterCard), электронные кошельки (QIWI) и криптовалюты (USDT).
Бонусная политика
Хорошие казино предоставляют привлекательные бонусы: приветственные пакеты, фриспины и системы вознаграждений. Обратите внимание на требования по использованию.
Мобильная оптимизация
Современные казино адаптированы для мобильных устройств (iOS) с удобным управлением. Это позволяет играть в любом месте круглосуточно.
Клиентская поддержка
Профессиональная служба поддержки работает 24/7 и помогает решать вопросы через email.
Рейтинг лучших онлайн-казино 2025 года
1. Cat Casino
Преимущества: оперативные выплаты (до 1 минуты), лицензия Curacao, уникальные предложения.
Бонусы: бездепозитный бонус — 100 фриспинов в Book of Dead.
Особенности: специальные мероприятия.
2. Kometa Casino
Преимущества: лучшее live-казино, кэшбэк до 50 000?.
Бонусы: стартовый комплект — 255% + 500 бесплатных вращений.
Особенности: VIP-клуб с индивидуальными бонусами.
3. R7 Casino
Преимущества: анонимная регистрация по email, поддержка криптовалют (USDT) с нулевой комиссией.
Бонусы: фриспины за подписку на канал.
Особенности: двухфакторная аутентификация для защиты данных.
4. Arkada Casino
Преимущества: быстрые выплаты на карты РФ (Сбербанк), регулярные розыгрыши.
Бонусы: кэшбэк до значительного процента, приветственная награда.
Особенности: лицензионные игры от Microgaming.
5. Kent Casino
Преимущества: поддержка криптовалют, турниры с призовым фондом 1 млн рублей.
Бонусы: демо-режим.
Особенности: широкий выбор слотов с максимальной отдачей.
Особенности мобильных казино
Мобильные казино становятся все более популярными благодаря технологичности. Их преимущества:
Адаптивный дизайн: интерфейс оптимизирован для мобильных устройств.
Быстрый доступ: нет необходимости устанавливать программы.
Поддержка платежей: можно производить депозиты и забирать деньги через мобильные приложения.
Низкое потребление трафика: современные казино работают плавно даже на ограниченной скорости.
Как выбрать лучшее казино?
При выборе онлайн-казино учитывайте следующие аспекты:
Лицензия и безопасность
Убедитесь, что казино имеет официальный сертификат и использует технологии безопасности (другие протоколы).
Ассортимент игр
Лучшие казино предлагают разнообразие слотов, настольных игр (покер) и живые игры.
Скорость выплат
Изучите реальные комментарии о времени обработки заявок.
Бонусные программы
Обратите внимание на размер приветственного бонуса, требования по отыгрышу и наличие других акций.
Качество поддержки
Проверьте, насколько профессионально сотрудники помогают пользователям.
Мобильная версия
Убедитесь, что казино имеет специальное приложение для смартфонов.
Заключение
Онлайн-казино могут стать способом отдыха, если подходить к их выбору серьёзно. Придерживайтесь наших советов, играйте только на проверенных платформах и помните о важности самоконтроля. Азартные игры — это прежде всего удовольствие, а не источник дохода.
Если вы ищете надёжное казино с быстрыми выплатами, обратите внимание на топовые площадки, такие как Arkada Casino. Они отлично зарекомендовали себя среди игроков и продолжают радовать своими предложениями.
https://t.me/s/topcasinos_ru
Привет всем!
Зарубежные сериалы онлайн бесплатно смотреть на всех устройствах с качественным видео. Мы обеспечиваем лучший опыт просмотра. бесплатно зарубежные сериалы смотреть онлайн Погружайтесь в мир увлекательных шоу. Смотрите любимые сериалы без рекламы!
Смотреть зарубежные сериалы онлайн бесплатно с хорошей озвучкой и качественным видео. Все эпизоды загружаются мгновенно. Мы обновляем коллекцию каждый день. Погружайтесь в мир новых шоу и наслаждайтесь качественным контентом!
Заходите и смотрите лучшее от netfix онлайн – https://lordseria1.pet/2023-god/
сериал смотреть онлайн и фильмы, смотреть бесплатно сериал россия, сериал бывшая онлайн смотреть
ребенок сериал смотреть онлайн бесплатно, 1 сериал смотреть, смотреть сериалы россии
Удачного просмотра!
Заказать диплом ВУЗа по доступной цене возможно, обратившись к проверенной специализированной фирме. Мы предлагаем документы об окончании любых ВУЗов России. Купить диплом любого университета– diploma-groups24.ru/diplomy-po-specialnosti/diplom-inzhenera-mehanika.html
stainless steel daytona
https://vandalismwiki.uk/wiki/Rolex_36q
Piano notes beginner piano sheet music
Привет всем!
Смотреть зарубежные сериалы бесплатно онлайн без рекламы и подписки. Подключайтесь и наслаждайтесь лучшими шоу. зарубежные сериалы бесплатно онлайн смотреть Все серии и сезоны доступны без ограничений. Смотрите премьеры сразу после выхода. Приятного просмотра!
Смотреть зарубежные сериалы онлайн бесплатно с качественным видео и хорошим переводом. Все новинки и старые хиты доступны мгновенно. Погружайтесь в мир новых шоу и наслаждайтесь качественным контентом!
Заходите и смотрите любимые фильмы без регистрации – https://lordseria1.pet/serialy/
смотреть лучшие сериалы 2022, смотреть сериал с 2022 г, смотреть сериал 2014
онлайн смотреть сериалы 2024, сериалы смотреть русские без рекламы, смотреть фильм в хорошем качестве бесплатно сериалы
Удачного просмотра!
Medicine information provided. Pill leaflet here.
buy lasix usa
Medication impacts described. Latest medicine news.
Зеркало Нью Ретро Казино newretromirror.ru .
Тут можно преобрести взломостойкие сейфы цена сейф взломостойкий купить
Klavier lernen noten kostenlos noten fur klavier zum ausdrucken
Noten vom klavier noten von klavier
Can you tell us more about this? I’d like to find out more details.
https://760display.com/wp-includes/pages/888starz_promo_code_14.html
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Тут можно преобрести купить сейф можно купить сейф
1win вход http://belbeer.borda.ru/?1-6-0-00001555-000-0-0-1742473542/ .
1wln 1wln .
программа 1с бухгалтерия цена программа 1с бухгалтерия цена .
best site
Kreativstorm
мостбет зеркало http://www.ongame.forum24.ru/?1-18-0-00001219-000-0-0-1742360461 .
масляные трансформаторы купить масляные трансформаторы купить .
1win официальный сайт скачать http://belbeer.borda.ru/?1-6-0-00001555-000-0-0-1742473542/ .
Music sheet music for piano sheet music notes
1vin kg http://www.taksafonchik.borda.ru/?1-14-0-00002041-000-0-0 .
как согласовать перепланировку квартиры как согласовать перепланировку квартиры .
mostbet kg скачать на андроид http://www.ongame.forum24.ru/?1-18-0-00001219-000-0-0-1742360461 .
Хотите списать долги? https://pro-58.ru законное освобождение от кредитных обязательств. Работаем с физлицами и бизнесом. Бесплатная консультация!
Get info immediately. Medication trends described.
buy lasix with no prescription
Access medication facts. Access pill facts.
Site-ul https://betsysrealfood.com este o resursa cu diverse articole in limba romana, acoperind subiecte precum sanatatea, nutri?ia, moda ?i stilul de via?a. Titlurile articolelor includ teme precum „?tiri de sanatate”, „?tiri pentru bebelu?i”, „?tiri de Medicina Alternativa” ?i „?tiri de via?a”. Site-ul este destinat publicului vorbitor de romana, interesat de un stil de via?a sanatos ?i re?ete de casa.
Hello, I want to subscribe for this website to get latest updates, thus where can i do it please help.
https://sites.google.com/view/1xbetpromocodes/home
Здравствуйте любители видеопроката!
Смотреть зарубежные сериалы онлайн бесплатно с качественным видео и отличной озвучкой. Все новинки и хиты доступны сразу. зарубежные сериалы смотреть бесплатно в хорошем качестве онлайн Мы обеспечиваем лучший опыт просмотра. Смотрите без рекламы, погружайтесь в увлекательные истории!
Зарубежные сериалы смотреть бесплатно онлайн без регистрации. Все новинки и эпизоды доступны мгновенно. Смотрите лучшие шоу без скрытых платежей. Погружайтесь в мир новых историй прямо сейчас!
Заходите и смотрите лучшее от netfix без рекламы – https://lordseria2.bet/2023-god/
смотреть онлайн сериал дикие, сериал лучший детектив смотреть, сериал триггер смотреть онлайн
смотреть зарубежные netflix сериалы онлайн бесплатно, онлайн сериал детектив, первый отдел смотреть сериал бесплатно
Удачного просмотра!
pdacenter.ru – сервис по ремонту бытовой техники
Ремонт iMac в Челябинске в официальном сервисном центре PDACENTER.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
Быстрая продажа и покупка каталог profis размещайте объявления о продаже товаров, поиске услуг, аренде недвижимости и работе. Простой интерфейс, удобный поиск, бесплатные публикации!
Добрый день киноманы!
На нашем сайте можно смотреть турецкие сериалы онлайн бесплатно в хорошем качестве. Мы предлагаем только лучшие шоу с русским переводом. Наслаждайтесь просмотром без рекламы и без регистрации. смотреть турецкие сериалы онлайн Присоединяйтесь к нам и начните смотреть прямо сейчас!
У нас можно смотреть турецкие сериалы онлайн бесплатно и без ограничений. Мы предлагаем вам только самые популярные шоу, которые можно смотреть в хорошем качестве и с русским переводом. Начните просмотр прямо сейчас и погружайтесь в мир турецких сериалов. Эти истории не оставят вас равнодушными!
Заходите и смотрите финтастику без рекламы – https://turkkk.biz/kriminalnye/
турецкие сериалы онлайн бесплатно смотреть без регистрации, смотреть фильмы онлайн лучшие турецкие сериалы на русском, турецкие сериалы смотреть онлайн турк ру
ветреный смотреть онлайн в хорошем качестве турецкий сериал, сериал турецкий смотреть онлайн бесплатно в хорошем, турецкие сериалы дочь посла на русском языке смотреть онлайн
Удачного просмотра!
Why visitors still use to read news papers when in this technological world everything is available on web?
https://sabuilder.co.za/pages/1xbet_new_registration_promo_code_4.html
Pill guide here. Get pill info.
order lasix
Pill guide available. Find pill information.
Добрый день киноманы!
Зарубежные сериалы онлайн бесплатно смотреть на всех устройствах с качественным видео. Мы обеспечиваем лучший опыт просмотра. зарубежные сериалы бесплатно онлайн смотреть Погружайтесь в мир увлекательных шоу. Смотрите любимые сериалы без рекламы!
Зарубежные сериалы смотреть бесплатно онлайн в хорошем качестве — это просто и удобно! У нас собраны лучшие проекты. Смотрите сериалы без рекламы, наслаждайтесь отличным качеством видео. Погружайтесь в мир новых историй.
Заходите и смотрите сериалы онлайн – https://lordseriala.win/tureckie-serialy/
сериал дикие смотреть онлайн, смотреть сериал убийства в, смотреть лучшие фильмы и сериалы
смотреть сериалы фильмы онлайн, россия сериалы онлайн бесплатно, сериал российский смотреть бесплатно в хорошем
Удачного просмотра!
Добрый день киноманы!
Смотрите турецкие сериалы онлайн бесплатно на нашем сайте. Мы собрали лучшие шоу с русским переводом. Все доступно без регистрации и рекламы. турецкие сериалы онлайн Начните просмотр и наслаждайтесь турецкими сериалами!
Хотите смотреть турецкие сериалы онлайн бесплатно? Мы предлагаем вам огромный выбор сериалов всех жанров. Все доступно в хорошем качестве и с качественным переводом. Начните просмотр прямо сейчас и наслаждайтесь лучшими турецкими шоу. Турецкие сериалы – это всегда захватывающе!
Заходите и смотрите мульт сериалы без рекламы – https://turkkk.biz/detektivy/
турецкие сериалы на русском языке смотреть онлайн 10 серия, турецкий сериал онлайн сыла, турецкие сериалы на русском языке полнолуние смотреть онлайн бесплатно
невеста турецкий сериал смотреть онлайн на русском языке в хорошем качестве, онлайн бесплатные турецкие сериалы с озвучкой, турецкий сериал на русском языке онлайн бесплатно любовь не понимает слов
Удачного просмотра!
Привет всем!
Смотреть онлайн зарубежные сериалы бесплатно с хорошей озвучкой. Все сериалы без регистрации. зарубежные сериалы смотреть бесплатно онлайн в хорошем качестве Новинки доступны сразу после выхода. Смотреть можно без рекламы. Подключайтесь и выбирайте любимые проекты прямо сейчас!
Смотреть зарубежные сериалы бесплатно онлайн с качественным переводом и без рекламы. Все эпизоды загружаются мгновенно. Мы обновляем коллекцию каждый день. Смотрите любимые шоу без скрытых платежей и наслаждайтесь качественным контентом!
Заходите и смотрите любимые фильмы без регистрации – https://lordseriala.win/xfsearch/perevod/lostfilm/
смотреть сериалы 2015, смотреть сериал отдел все серии онлайн бесплатно, смотреть фильмы лорд сериал
осман смотреть сериал бесплатно на русском, сериалы смотреть онлайн бесплатно лордфильм, осман сериал смотреть на русском
Удачного просмотра!
Get pill details. Pill guide available.
buy lasix online
Comprehensive medicine guide. Medication leaflet available.
Привет всем!
Бесплатно смотреть зарубежные сериалы онлайн на нашем сайте без перерывов и рекламы. Все новинки и хиты загружаются моментально. зарубежные сериалы онлайн смотреть бесплатно Мы обновляем коллекцию регулярно. Смотрите любимые шоу без регистрации и подписки. Приятного просмотра!
Зарубежные сериалы бесплатно смотреть онлайн на всех устройствах без подписки. Мы обновляем коллекцию каждый день. Все эпизоды и сезоны доступны мгновенно. Смотрите любимые шоу без рекламы и скрытых платежей!
Заходите и смотрите любимые фильмы без регистрации – https://lordseria3.la/drama/
тени сериал смотреть, декстер сериал смотреть, смотреть русский хороший сериал
смотреть сериал высшее, сериалы онлайн последние, сериал жизнь онлайн
Удачного просмотра!
Привет всем!
Купить сигареты Vogue Super Slims оптом — это выбор для тех, кто ищет стильные и легкие сигареты с утонченным вкусом. Эти сигареты подарят вам элегантность и комфорт. Купить Сигареты ДОНСКОЙ ТАБАК СВЕТЛЫЙ оптом Vogue Super Slims – это сигареты для тех, кто ценит минимализм и высокое качество. Мы предлагаем гибкие условия для оптовых клиентов. Закажите Vogue Super Slims оптом и получите стильный продукт.
Сигареты L&M Blue оптом — это идеальный выбор для тех, кто ищет мягкие и легкие сигареты с гармоничным вкусом. Эти сигареты подарят вам комфортное курение и легкость при каждом вдохе. L&M Blue идеально подходят для тех, кто ценит легкие сигареты с сбалансированным вкусом. Мы предлагаем конкурентоспособные цены и удобные условия для оптовых клиентов. Закажите L&M Blue оптом и получите сигареты высокого качества.
Лучшие сигареты по ссылке – https://shop.sigarety-optom.shop/sigarety-donskoj-tabak-temnyj-optom
Portal оптом, Купить Сигареты Oris Pulse Mojito Super Slim оптом, Купить Portal ONE формат NANO оптом
Купить Сигареты MOND Vanilla SuperSlim Ваниль оптом, РОДОПИ оптом, Купить Сигареты Milano SuperSlims Green оптом
Удачи в дыму!
Latest medicine developments. Medication information here.
lasix cheap
Medicine guide available. Get info now.
Mostbet kasyno to setki automatów od najlepszych dostawców | Mostbet rejestracja jest szybka i prosta – sprawdź, jak zacząć grać | Zakłady sportowe i kasyno online – Mostbet łączy najlepsze funkcje dla graczy | Mostbet posiada atrakcyjne promocje i turnieje dla aktywnych graczy Mostbet Casino – bonus bez depozytu sprawdź szczegóły.
kraken ссылка – kraken ссылка, кракен актуальная ссылка
gokong88
Все о недвижимости в России и мире — новости, аналитика, советы экспертов в одном месте!
Хотите быть в курсе последних событий на рынке недвижимости? Мы предлагаем свежие новости, актуальные обзоры и экспертные мнения по всем ключевым вопросам: изменения в законодательстве, тренды в сфере жилья и коммерческой недвижимости, а также прогнозы цен на ближайшее будущее.
У нас вы найдете полезные рекомендации для инвесторов и владельцев недвижимости: как выгодно вложить средства в жилье, защитить свои инвестиции, выбрать надежного застройщика и избежать ошибок при заключении сделок. Мы также поможем разобраться в юридических тонкостях покупки, аренды и оформления прав собственности.
Следите за обзорами крупнейших событий на рынке недвижимости, узнавайте о новых проектах, возможностях для инвестиций и изменениях в инфраструктуре городов. Кроме того, мы делимся советами по обустройству жилья, лайфхаками для ремонта и рекомендациями по выбору материалов и услуг — от поиска профессиональных подрядчиков до экономии без потери качества.
Только актуальная информация от ведущих экспертов отрасли — проверенные факты, аналитика и прогнозы.
Подписывайтесь и оставайтесь в центре событий! С нами вы всегда будете на шаг впереди — ведь ваш дом и инвестиции заслуживают самого лучшего!
Сайт sis69.ru
Покупка подходящего диплома через качественную и надежную компанию дарит множество преимуществ. Данное решение дает возможность сберечь как длительное время, так и существенные финансовые средства. Тем не менее, только на этом выгоды не ограничиваются, преимуществ гораздо больше.Мы изготавливаем дипломы любой профессии. Дипломы производят на настоящих бланках. Доступная цена по сравнению с огромными затратами на обучение и проживание. Заказ диплома университета станет мудрым шагом.
Приобрести диплом о высшем образовании: diplomnie.com/kupit-diplom-v-kazani-bistro-i-udobno/
кракен даркнет – кракен вход, кракен зеркало
Доброго любители сериалов онлайн!
Смотреть онлайн зарубежные сериал без регистрации и подписки. Лучшие шоу всегда под рукой. зарубежный сериал смотреть Погружайтесь в самые популярные проекты. Наслаждайтесь просмотром без рекламы и ограничений. Получите доступ к новинкам сразу после выхода.
Смотреть зарубежные сериалы бесплатно онлайн на любом устройстве. Мы обновляем коллекцию каждый день. Все новинки и хиты доступны мгновенно. Смотрите шоу без рекламы и ограничений. Приятного просмотра!
Заходите и смотрите сериалы без регистрации – https://lordseriala.biz/fantastika/
сериалы основание смотреть онлайн, русский сериал сезон смотреть онлайн бесплатно, годы смотреть сериал бесплатно
смотреть онлайн сериалы россии в хорошем качестве, сериалы как смотреть, трасса сериал смотреть
Удачного просмотра!
For hottest information you have to pay a visit the web and on internet I found this website as a best web site for latest updates.
https://ai.ceo/read-blog/299530_1xbet-promo-code-free-best-offers-today.html
световые панели цена информационные рекламные стенды
купить медицинскую справку для водителя https://kupit-spravku-spb.ru
Привет всем!
Смотреть зарубежные сериалы онлайн бесплатно с моментальной загрузкой и качественным видео. Мы обновляем коллекцию каждый день. смотреть онлайн зарубежные сериалы бесплатно Все эпизоды загружаются мгновенно. Смотрите любимые шоу без рекламы и наслаждайтесь качественным контентом!
Зарубежные сериалы онлайн бесплатно смотреть с качественным видео и моментальной загрузкой. Все эпизоды и сезоны доступны мгновенно. Смотрите любимые шоу без перерывов и скрытых платежей. Наслаждайтесь качественным контентом!
Заходите и смотрите мульт сериалы онлайн – https://lordseria1.me/
детектив сериал смотреть онлайн, смотреть сериал онлайн дом, бесплатно смотреть сериал бывшая
высшее смотреть сериал, сериалы бесплатно в онлайн в hd бесплатно, сериалы смотреть в качестве hd
Удачного просмотра!
монтаж объемных букв коммерческая наружная реклама
1 win.kg http://www.1win823.ru .
вин 1 https://www.1win822.ru .
кракен актуальная ссылка – кракен маркетплейс, кракен сайт
1вин кг https://1win822.ru/ .
Le code promo 1xBet: 1XFOX200. Les nouveaux joueurs seront eligibles a un bonus VIP de 100% jusqu’a 130€/$. Ce code promo vous permettra d’obtenir un bonus de bienvenue jusqu’a 130€ sur les paris sportifs. Pour profiter des bonus sur la plate-forme du bookmaker 1xbet, vous devez vous inscrire en utilisant le code bonus. Inscrivez-vous des maintenant avec notre code promo 1xBet pour recevoir un bonus de bienvenue de 100% sur votre premier depot.
Meilleur code promo 1xbet: 1XFOX200 vous permettra d’augmenter votre premier depot jusqu’a 100% avec un bonus jusqu’a 130€/$. Les nouveaux clients peuvent recevoir un bonus de 1xbet allant jusqu’a 130 €/$. Pour retirer les fonds lies au bonus, la moitie du montant du bonus doit etre misee 5 fois sur des paris combines, qui doivent chacun contenir au moins 3 evenements avec des cotes egales ou superieures a 1.40.
meilleur code promo 1xbet
mostbet kg скачать http://mostbet782.ru .
1вин официальный сайт http://1win823.ru/ .
скачать mostbet на телефон https://www.mostbet782.ru .
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: сколько стоит диплом, аттестаты купить, купить аттестаты, купить аттестат настоящий, купить настоящий аттестат. Постепенно углубляясь в тему, нашел отличный ресурс здесь: diplomybox.com/diplom-doktora-nauk
Где купить диплом по необходимой специальности?
Мы изготавливаем дипломы любых профессий по приятным тарифам. Мы предлагаем документы техникумов, которые расположены в любом регионе России. Вы сможете заказать качественно напечатанный диплом за любой год, в том числе документы старого образца СССР. Документы выпускаются на “правильной” бумаге самого высшего качества. Это позволяет делать настоящие дипломы, которые не отличить от оригинала. Документы будут заверены необходимыми печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества наших граждан. bisness-diplom.ru/kupit-diplom-v-novokuznetske-2-4
электрический карниз электрический карниз .
Привет всем!
Купить NZ BLACK POWER оптом оптом в удобной упаковке. Сбалансированный вкус и качественное сырье. Купить Сигареты MILANO New York оптом оптом Поставки без задержек и перебоев. Всегда в наличии популярные позиции. Доступны специальные предложения.
Хотите купить сигареты Philip Morris Compact Blue оптом? Это идеальный выбор для тех, кто ценит компактный формат и отличное качество. Благодаря тонкому и сбалансированному вкусу, эти сигареты станут вашим фаворитом. В нашем магазине вы найдете лучшую цену для оптовых покупок. Оформите заказ на Philip Morris Compact Blue оптом и наслаждайтесь качественным продуктом.
Лучшие сигареты по ссылке – https://shop.sigarety-optom.shop/sigarety-marlboro-core-flavor
Купить Сигареты Dove Gold MEDIUM EDITION оптом, Купить Сигареты Winston Super Slims оптом 1 оптом, Democrat оптом
Купить Сигареты Philip Morris Red оптом, Купить Сигареты Marlboro Micro оптом оптом, Купить Сигареты CAVALLO by Gino Ferrara оптом оптом
Удачи в дыму!
Добрый день киноманы!
Смотреть зарубежные сериалы бесплатно онлайн с хорошим качеством и отличным переводом. Все новинки и старые хиты доступны сразу. бесплатно смотреть зарубежные сериалы онлайн Погружайтесь в мир увлекательных шоу. Смотрите без рекламы и наслаждайтесь контентом!
Смотреть зарубежные сериалы онлайн бесплатно без подписки и ограничений. Наслаждайтесь любимыми шоу в любое время. Мы обновляем коллекцию каждый день. Все новинки доступны без рекламы и перерывов. Приятного просмотра!
Заходите и смотрите лучшие фильмы бесплатно онлайн – https://lordseriala.biz/fantastika/
смотреть сериал трасса бесплатно, смотреть бесплатно онлайн сериал тайна, день смотреть сериал
бесплатно онлайн сериал во все тяжкие, семья онлайн сериал, город сериал смотреть онлайн
Удачного просмотра!
Stay updated with the latest news on coins, Bitcoin, and the crypto world.
Our portal provides timely news into market movements, including price changes, new coin launches, and crypto advancements.
Whether you’re tracking BTC or exploring emerging altcoins, we bring you market news and breaking news.
Get the up-to-date altcoin news and be in the know on market shifts, regulation changes, and key news in the digital asset space.
altcoinix.com
Join us to never miss breaking crypto news!
игра 1вин http://1win822.ru .
Доброго любители сериалов онлайн!
Зарубежные сериалы бесплатно онлайн смотреть с отличным качеством видео и звука. Мы собрали лучшие шоу для вас. смотреть онлайн зарубежные сериал Смотрите новинки сразу после выхода. Все эпизоды доступны без скрытых платежей. Приятного вам просмотра!
Зарубежные сериалы бесплатно онлайн смотреть с отличным качеством и хорошим переводом. Все эпизоды загружаются мгновенно. Смотрите любимые шоу без рекламы и скрытых платежей. Погружайтесь в мир увлекательных сюжетов!
Заходите и смотрите мульт сериалы без рекламы – https://lordseria3.com/netflix/
смотреть бесплатно новый сериал, во все тяжкие сериал смотреть, лучшие турецкие сериалы смотреть на русском
ты сериалы смотреть онлайн, ходячие мертвецы сериал смотреть бесплатно в хорошем, онлайн сериал ходячие мертвецы в хорошем качестве
Удачного просмотра!
Casino Reviews http://nomini-casino.ru
Рекомендую сайты fotonons.ru, artcet.ru, 40-ka.ru, urkarl.ru, upsskirt.ru, yarus-kkt.ru, cultureinthecity.ru, imgtube.ru, great-galaxy.ru, shvejnye.ru, lostfiilmtv.ru, voenoboz.ru, my-caffe.ru, kanunnikovao.ru, adventime.ru, kaizen-tmz.ru
1wln 1wln .
Добрый день киноманы!
Зарубежные сериалы смотреть бесплатно онлайн на всех устройствах с моментальной загрузкой. Мы обеспечиваем вам доступ к лучшим проектам. бесплатно зарубежные сериалы смотреть онлайн Все новинки и старые хиты доступны мгновенно. Приятного просмотра без рекламы!
Зарубежные сериалы онлайн бесплатно смотреть в хорошем качестве. Мы обеспечиваем вам доступ к лучшему контенту. Все новинки загружаются сразу. Смотрите любимые сериалы без рекламы и скрытых платежей. Приятного просмотра!
Заходите и смотрите все от hulu онлайн – https://lordseria3.com/xfsearch/year/2020/
хороший русский сериал смотреть, сериал смотреть 8, бесплатно смотреть зарубежные сериалы онлайн
смотреть турецкие сериалы драмы онлайн бесплатно, сериалы онлайн лордфильм, сериалы без рекламы смотреть
Удачного просмотра!
most bet https://mostbet782.ru/ .
Приобрести документ университета можно у нас в Москве. diplom-zentr.com/kupit-diplom-kirov-4
Привет всем!
Смотреть зарубежные сериалы онлайн бесплатно без подписки. Подключайтесь и наслаждайтесь всеми сезонами. смотреть зарубежные сериалы Все новые эпизоды загружаются моментально. Мы обновляем коллекцию каждый день. Получайте удовольствие от лучшего контента!
Зарубежные сериалы онлайн смотреть бесплатно и без регистрации! У нас собраны самые интересные шоу для вашего удобства. Мы обновляем коллекцию каждую неделю. Смотрите новинки сразу после выхода, наслаждайтесь качественным контентом. Приятного просмотра!
Заходите и смотрите аниме онлайн – https://lordseria1.me/xfsearch/perevod/hdrezka%20studio/
сериалы склифосовский смотреть, смотреть сериал основания онлайн бесплатно, сериал детективы смотреть онлайн в хорошем качестве
смотреть зарубежные hulu сериалы онлайн бесплатно в хорошем качестве, осман смотреть турецкий сериал, смотреть дикие сериал
Удачного просмотра!
Доброго любители сериалов онлайн!
Зарубежные сериалы бесплатно онлайн смотреть можно без регистрации. Открывайте для себя лучшие проекты. зарубежные сериалы смотреть бесплатно в хорошем качестве онлайн Мы собрали коллекцию популярных шоу всех жанров. Смотрите любимые сериалы на любом устройстве. Получайте удовольствие от качественного контента.
Смотреть онлайн зарубежные сериалы бесплатно с моментальной загрузкой и хорошим качеством. Все новинки и старые хиты доступны сразу. Мы обновляем коллекцию каждый день. Погружайтесь в мир качественного контента!
Заходите и смотрите любимые фильмы без регистрации – https://lordseria3.la/multserialy/
лучшие детективы сериалы онлайн, смотреть сериал однажды, смотреть осман сериал на русском бесплатно
русские сериал смотреть все серии подряд, смотреть сериал 2 сезон 1 серия, смотреть онлайн 2014 сериалы
Удачного просмотра!
Какой красивый оттенок! Заказала букет маме.
Цветы Томск
why not try these out galaxyswapper
соут москва цена https://sout-moscow.ru .
Kasyno Mostbet oferuje setki automatów i gier na żywo | Z Mostbet możesz zagrać na automatach, ruletce i w pokera | Dzięki Mostbet logowanie możesz grać w swoje ulubione gry w kilka sekund mostbet online pl
Доброго любители сериалов онлайн!
Зарубежные сериалы бесплатно онлайн смотреть без перерывов и ограничений. Все новинки и хиты доступны сразу. смотреть онлайн зарубежные сериалы бесплатно Погружайтесь в мир увлекательных историй. Смотрите сериалы без рекламы и скрытых платежей!
Наш сайт – это лучшее место, чтобы смотреть турецкие сериалы онлайн бесплатно. Мы обновляем каталог каждый день. Все шоу доступны без рекламы и в хорошем качестве. Начните просмотр прямо сейчас. Турецкие сериалы ждут вас!
Заходите и смотрите лучшее от netfix онлайн – https://lordseria3.org/xfsearch/year/2022/
месть турецкий сериал смотреть онлайн бесплатно на русском, смотреть онлайн турецкие сериалы новые, турецкий сериал русская озвучка смотреть бесплатно онлайн
смотреть турецкие сериалы онлайн бесплатно, ветреный смотреть онлайн все серии турецкий сериал, онлайн бесплатно сериал турецкий ранняя пташка
Удачного просмотра!
Купить документ о получении высшего образования вы можете у нас. http://www.vipmotel.ru/reviews
Где заказать диплом по нужной специальности?
Мы предлагаем выгодно купить диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Данный диплом способен пройти любые проверки, даже с использованием специально предназначенного оборудования. Решайте свои задачи быстро и просто с нашими дипломами.
Приобрести диплом любого университета kupit-diplom24.com/kupit-attestat-za-11-klass-21/
кайт школа анапа
Хотите перекусить вкусно? купить https://pitateka.ru питу, шаверму, донер, гирос и тортилью. Ароматная свежая выпечка, мясо на гриле и фирменные соусы. Отличный выбор для перекуса, обеда или вечеринки.
Здравствуйте любители видеопроката!
Смотреть онлайн зарубежные сериалы бесплатно без регистрации и без рекламы. Все новинки и старые хиты доступны без перерывов. смотреть онлайн зарубежные сериалы бесплатно Мы обновляем коллекцию каждый день. Погружайтесь в мир увлекательных шоу и наслаждайтесь качественным контентом!
Зарубежные сериалы бесплатно онлайн смотреть с моментальной загрузкой и без подписки. Откройте для себя любимые шоу. Мы собрали для вас все самые интересные сериалы. Смотрите без ограничений. Приятного вам просмотра и хорошего настроения!
Заходите и смотрите боевики онлайн – https://lordseria2.bet/2023-god/
смотреть сериалы бесплатно лорд, смотреть сериал лейла, смотреть сериал детективный бесплатно
сериал 2020 смотреть, смотреть сериал хороший детектив онлайн, смотреть в hd качестве сериалы
Удачного просмотра!
Каждый после регистрации на сайте Estul.ru получает на баланс аккаунта 100? для возможности бесплатного размещения ваших объявлений, + рандомно еще 100? за размещение первого объявления. На сайте есть все от товаров до услуг их продажа и покупка https://estul.ru/blog
Не забываем про группу Авторынок РФ – https://vk.com/club227724491
Telegram chanel- https://t.me/estulrus
Купить диплом о высшем образовании!
Мы можем предложить дипломы любой профессии по приятным ценам— diplomist.com/kupit-diplom-avtomexanika-19/
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Каждый после регистрации на сайте Estul.ru получает на баланс аккаунта 100? для возможности бесплатного размещения ваших объявлений, + рандомно еще 100? за размещение первого объявления. На сайте есть все от товаров до услуг их продажа и покупка Продать товар
Не забываем про группу Авторынок РФ – Продать авто
Telegram chanel- https://t.me/estulrus
site link galaxy swapper download
мосбет мосбет .
1вин официальный сайт https://1win810.ru/ .
Приветствую!
Приобрести диплом института по выгодной цене можно, обращаясь к надежной специализированной фирме. Приобрести диплом о высшем образовании: diplom-kaluga.ru/kupit-diplom-volgograd-3/
mostbet kg скачать на андроид http://mostbet784.ru .
1win rossvya https://1win810.ru/ .
мост бет http://www.mostbet783.ru .
заказать сертификаты типография https://tipografiya-price.ru
Привет!
Без ВУЗа трудно было продвигаться по карьерной лестнице. Сегодня же этот документ не дает совершенно никаких гарантий, что удастся найти престижную работу. Намного более важны профессиональные навыки и знания специалиста, а также его постоянный опыт. По этой причине решение о заказе диплома можно считать выгодным и целесообразным. Купить диплом об образовании bdfeu.ukraine7.com/login
Добрый день киноманы!
Смотреть зарубежные сериалы бесплатно онлайн на любом устройстве. Все новинки сразу после выхода. смотреть онлайн зарубежные сериалы бесплатно У нас нет рекламы, скрытых платежей или подписки. Все эпизоды загружаются моментально. Получите удовольствие от качественного контента!
У нас собраны лучшие турецкие сериалы, которые вы можете смотреть онлайн бесплатно в хорошем качестве. Мы предоставляем вам удобный доступ ко всем сериям без регистрации. Мы следим за качеством картинки и звука, чтобы ваше удовольствие было максимальным. Смотрите турецкие сериалы в любое время суток. Все фильмы доступны онлайн.
Заходите и смотрите аниме бесплатно онлайн – https://lordseria3.org/xfsearch/year/2022/
любовь не понимает слов турецкий сериал смотреть онлайн бесплатно на русском, ветреный онлайн бесплатно турецкий сериал, любовь против любви турецкий сериал на русском языке смотреть онлайн
турецкий сериал постучись в мою дверь в хорошем качестве онлайн, турецкий сериал ветреный смотреть онлайн на русском языке все, осман гази турецкий сериал смотреть онлайн
Удачного просмотра!
mostbet casino mostbet casino .
Доброго любители сериалов онлайн!
Смотреть зарубежные сериалы онлайн бесплатно с качественным видео и хорошим звуком. Все эпизоды и сезоны доступны без ограничений. смотреть зарубежные сериалы бесплатно онлайн Погружайтесь в мир увлекательных историй. Смотрите любимые шоу без рекламы и скрытых платежей!
Смотреть зарубежные сериалы бесплатно онлайн с моментальной загрузкой. Мы обновляем коллекцию каждый день. Все новинки и хиты доступны без рекламы и ограничений. Смотрите любимые шоу в любое время. Приятного вам просмотра!
Заходите и смотрите боевики бесплатно онлайн – https://lordseria2.bet/prikljuchenija/
сериал онлайн детективы, лорд сериалы смотреть онлайн, смотреть сериал убийства в
перемена сериал онлайн, слово смотреть сериал, смотреть сериал ходячие мертвецы бесплатно в хорошем
Удачного просмотра!
заказать журнал в типографии цена https://tipografiya-print-spb.ru
ван вин http://www.1win810.ru .
мостбет кыргызстан https://mostbet783.ru/ .
Заказала цветы на юбилей, все гости восхищались!
букет невесты томск
motbet https://mostbet784.ru/ .
weblink galaxy swapper
Топ онлайн казино 2025: эксклюзивный обзор лучших игровых платформ
Онлайн азартные игры стремительно развиваются в России, завоевывая всё больше игроков вследствие своей функциональности. Однако для комфортного гейминга критически важно выбирать только легальные площадки, которые гарантируют честность алгоритмов. В этом материале мы рассмотрим ключевые критерии выбора, представим рейтинг лучших казино 2025 года и предоставим практические инструкции для оптимального выбора.
Ключевые факторы надёжности онлайн-казино
Чтобы минимизировать опасность, необходимо учитывать следующие особенности:
Лицензионная чистота
Проверяйте наличие регуляторных разрешений: Мальты.
Убедитесь, что игровые автоматы прошли сертификацию от авторитетных организаций (GLI).
Система случайных чисел (RNG)
Казино должно использовать независимый генератор случайных чисел, что обеспечивает прозрачность процессов.
Скорость транзакций
Надёжные казино предлагают быстрые выплаты через современные системы: банковские карты (Visa), электронные кошельки (WebMoney) и криптовалюты (Litecoin).
Бонусная политика
Хорошие казино предоставляют щедрые бонусы: вступительные награды, фриспины и VIP-клубы. Обратите внимание на требования по использованию.
Мобильная оптимизация
Современные казино настроены для мобильных устройств (Android) с интуитивным интерфейсом. Это позволяет проводить время за играми в любое время.
Клиентская поддержка
Профессиональная служба поддержки работает круглосуточно и помогает решать вопросы через email.
Рейтинг лучших онлайн-казино 2025 года
1. Cat Casino
Преимущества: мгновенные выводы средств (до 1 минуты), лицензия Curacao, эксклюзивные акции.
Бонусы: стартовый пакет — 100 фриспинов в Gates of Olympus.
Особенности: турниры с крупными призами.
2. Kometa Casino
Преимущества: топовая живая игра, кэшбэк до 50 000?.
Бонусы: стартовый комплект — щедрое предложение.
Особенности: VIP-клуб с эксклюзивными привилегиями.
3. R7 Casino
Преимущества: скрытый аккаунт по email, поддержка цифровых активов (USDT) с бесплатными транзакциями.
Бонусы: фриспины за подписку на канал.
Особенности: двухфакторная аутентификация для предотвращения взломов.
4. Arkada Casino
Преимущества: оперативные транзакции на карты РФ (Тинькофф), регулярные розыгрыши.
Бонусы: кэшбэк до 15%, приветственная награда.
Особенности: проверенные автоматы от NetEnt.
5. Kent Casino
Преимущества: поддержка криптовалют, турниры с призовым фондом 1 млн рублей.
Бонусы: возможность бесплатной игры.
Особенности: широкий выбор слотов с оптимальным возвратом.
Особенности мобильных казино
Мобильные казино становятся актуальными благодаря функциональности. Их преимущества:
Адаптивный дизайн: интерфейс адаптирован для сенсорных экранов.
Быстрый доступ: нет необходимости тратить время на загрузку.
Поддержка платежей: можно вести транзакции и получать выигрыши через специальные сервисы.
Низкое потребление трафика: современные казино работают эффективно даже на медленном интернете.
Как выбрать лучшее казино?
При выборе онлайн-казино учитывайте следующие факторы:
Лицензия и безопасность
Убедитесь, что казино имеет официальный сертификат и использует шифрование данных (SSL).
Ассортимент игр
Лучшие казино предлагают разнообразие слотов, классических развлечений (покер) и live-казино.
Скорость выплат
Изучите мнения пользователей о времени получения денег.
Бонусные программы
Обратите внимание на размер приветственного бонуса, требования по использованию бонусов и наличие других акций.
Качество поддержки
Проверьте, насколько профессионально сотрудники решают вопросы.
Мобильная версия
Убедитесь, что казино имеет специальное приложение для смартфонов.
Заключение
Онлайн-казино могут стать способом отдыха, если подходить к их выбору вдумчиво. Придерживайтесь наших рекомендаций, играйте только на проверенных платформах и помните о основах безопасного гейминга. Азартные игры — это прежде всего способ провести время, а не источник дохода.
Если вы ищете надёжное казино с быстрыми выплатами, обратите внимание на топовые площадки, такие как Arkada Casino. Они отлично зарекомендовали себя среди игроков и продолжают радовать своими предложениями.
https://t.me/s/topcasinos_ru
https://telegra.ph/%EB%B9%84%EC%95%84%EA%B7%B8%EB%9D%BC-%EC%A0%95%ED%92%88-%EA%B5%AC%EB%A7%A4-%EB%B0%A9%EB%B2%95-%EC%A0%88%EB%8C%80-%ED%94%BC%ED%95%B4-%EA%B0%88-%EC%88%98-%EC%97%86%EB%8A%94-%ED%8C%81-09-20
Тут можно преобрести вломостойкие сейфы сейф взломостойкий купить
გამარჯობა კაზინოს მომხმარებელი!
Rabona Greece бѓ’бѓ—бѓђбѓ•бѓђбѓ–бѓќбѓ‘бѓ— бѓЎбѓђбѓЈбѓ™бѓ”бѓ—бѓ”бѓЎбѓќ бѓЎбѓђбѓ—бѓђбѓ›бѓђбѓЁбѓќ бѓ’бѓђбѓ›бѓќбѓЄбѓ“бѓбѓљбѓ”бѓ‘бѓђбѓЎ. თქვენთვбѓбѓЎ бѓ§бѓ•бѓ”бѓљбѓђ бѓЁбѓ”бѓЎбѓђбѓ«бѓљбѓ”бѓ‘бѓљбѓќбѓ‘бѓђ бѓ®бѓ”бѓљбѓ›бѓбѓЎбѓђбѓ¬бѓ•бѓ“бѓќбѓ›бѓбѓђ. п»їRabona greece Rabona-бѓЎ бѓ‘бѓќбѓњбѓЈбѓЎбѓ”бѓ‘бѓ бѓ“бѓђ бѓћбѓ бѓбѓ–ებრთქვენრთამაშбѓбѓЎ бѓ’бѓђбѓЎбѓђбѓЈбѓ›бѓЇбѓќбѓ‘бѓ”бѓЎбѓ”бѓ‘бѓљбѓђбѓ“ შექმნбѓбѓљбѓбѓђ. бѓбѓ—бѓђбѓ›бѓђбѓЁбѓ”бѓ— бѓђбѓ®бѓљбѓђ Rabona Greece-бѓЁбѓ бѓ“бѓђ бѓ›бѓќбѓбѓ’бѓ”бѓ—!
Rabona online casino бѓ’бѓ—бѓђбѓ•бѓђбѓ–бѓќбѓ‘бѓ— бѓЈбѓњбѓбѓ™бѓђбѓљбѓЈбѓ бѓ“бѓђ бѓЎбѓђбѓбѓњбѓўбѓ”бѓ бѓ”бѓЎбѓќ бѓ—бѓђбѓ›бѓђбѓЁбѓ”бѓ‘бѓЎ, бѓ бѓќбѓ›бѓљбѓ”бѓ‘бѓбѓЄ бѓ§бѓќбѓ•бѓ”бѓљбѓ—бѓ•бѓбѓЎ бѓ›бѓбѓ’бѓбѓ§бѓ•бѓђбѓњбѓ— бѓ¬бѓђбѓ бѓ›бѓђбѓўбѓ”бѓ‘бѓбѓЎбѓ™бѓ”бѓњ. бѓ‘бѓќбѓњбѓЈбѓЎбѓ”бѓ‘бѓ бѓ“бѓђ бѓћбѓ бѓбѓ–ებრთქვენთვбѓбѓЎ бѓ’бѓђбѓњбѓ™бѓЈбѓ—бѓ•бѓњбѓбѓљбѓ. Rabona-бѓЎ бѓ—бѓђбѓ›бѓђбѓЁбѓ”бѓ‘бѓ бѓЎбѓђбѓ•бѓЎбѓ”бѓђ бѓЁбѓђбѓњбѓЎбѓ”бѓ‘бѓбѓ—, бѓ бѓќбѓ›бѓљбѓ”бѓ‘бѓбѓЄ бѓЈбѓ¤бѓ бѓќ бѓ›бѓђбѓ¦бѓђбѓљбѓ бѓ›бѓќбѓ’бѓ”бѓ‘бѓбѓЎ бѓЁбѓ”бѓЎбѓђбѓ«бѓљбѓ”бѓ‘бѓљбѓќбѓ‘бѓ”бѓ‘бѓЎ бѓ’бѓђбѓ®бѓ“бѓбѓЎ. бѓ’бѓђбѓ®бѓ“бѓбѓ— Rabona online casino-бѓбѓЎ бѓњбѓђбѓ¬бѓбѓљбѓ бѓ“бѓђ бѓЁбѓ”бѓ”бѓЄбѓђбѓ“бѓ”бѓ— бѓ›бѓќбѓбѓ’бѓќбѓ—! бѓ©бѓ•бѓ”бѓњ бѓ§бѓќбѓ•бѓ”бѓљбѓ—бѓ•бѓбѓЎ бѓ›бѓ–бѓђбѓ“ бѓ•бѓђбѓ бѓ— თქვენს бѓ“бѓђбѓЎбѓђбѓ®бѓ›бѓђбѓ бѓ”бѓ‘бѓљбѓђбѓ“.
ყველა ინფორმაცია ლინკში – п»їhttps://www.robola.gr/
ხალისიანი დრო გქონდეთ თქვენს სარგებლებში!
Добрый день киноманы!
Если вы ищете, где можно смотреть турецкие сериалы онлайн бесплатно, приходите на наш сайт. Мы предлагаем вам лучшие турецкие шоу в хорошем качестве, которые можно смотреть прямо с экрана вашего устройства. Вся коллекция доступна для вас бесплатно. смотреть онлайн турецкие сериалы Смотрите турецкие сериалы онлайн, наслаждаясь каждым моментом. Все серии в нашем сервисе представлены в полном объеме.
Хотите смотреть турецкий сериал, не выходя из дома? Наш сервис предоставляет возможность онлайн-просмотра без регистрации и смс. Откройте для себя мир турецких сериалов, которые покоряют сердца зрителей по всему миру. Смотрите турецкие сериалы онлайн бесплатно в хорошем качестве. Начните смотреть турецкие сериалы прямо сейчас!
Заходите и смотрите любимые фильмы без регистрации – https://turokkk.pro/melodramy/
ветреный смотреть онлайн турецкий сериал все серии, чукур турецкий сериал смотреть онлайн на русском, смотреть сериал турецкий любовь напрокат на русском языке онлайн бесплатно
турецкие сериалы смотреть онлайн на русском языке клятва, турецкие сериалы онлайн невеста, смотреть онлайн любовь напрокат турецкий сериал все серии
Удачного просмотра!
Доброго любители сериалов онлайн!
Хотите смотреть турецкие сериалы онлайн бесплатно в хорошем качестве? Мы предлагаем вам лучшие новинки и культовые хиты. Все доступно на русском языке без регистрации. смотреть турецкий сериал Начните просмотр и наслаждайтесь великолепной актерской игрой. Турецкие сериалы – это любовь, страсть и драма!
У нас можно смотреть турецкие сериалы онлайн бесплатно и без регистрации. Мы предлагаем вам лучшие шоу в хорошем качестве с русским переводом. Наслаждайтесь просмотром без рекламы. Начните смотреть прямо сейчас и окунитесь в мир турецких сериалов!
Заходите и смотрите все от hulu без рекламы – https://turkoff.tech/kriminalnye/
онлайн смотреть турецкий сериал месть, турецкий сериал месть смотреть онлайн на русском бесплатно, 1001 ночь турецкий сериал на русском языке онлайн
ветреный турецкий сериал на русском языке смотреть онлайн 2 сезон бесплатно, сериал дочь посла турецкий смотри смотреть онлайн, турецкий сериал моя жизнь смотреть онлайн
Удачного просмотра!
Тут можно преобрести сейф купить сейф купить
Добрый день киноманы!
Наш сайт предлагает смотреть турецкие сериалы онлайн бесплатно. Все шоу доступны в хорошем качестве и на русском языке. Без рекламы и ограничений. турецкие сериалы онлайн Начните просмотр и наслаждайтесь турецкими сериалами!
Хотите смотреть турецкие сериалы онлайн бесплатно? У нас огромный выбор популярных шоу. Все доступно в хорошем качестве и с русским переводом. Начните просмотр прямо сейчас. Турецкие сериалы подарят вам массу впечатлений и незабываемых эмоций!
Заходите и смотрите мульт сериалы без регистрации – https://turkoff.tech/istoricheskie/
турецкий сериал на русском языке все серии смотреть онлайн бесплатно подряд в хорошем, турецкие сериалы смотреть онлайн бесплатно любовь не понимает слов, полнолуние турецкий сериал смотреть онлайн на русском бесплатно
турецкий сериалы смотреть онлайн новые, смотреть турецкий сериал любовь не понимает слов на русском языке онлайн, бесплатно смотреть турецкие сериалы онлайн без регистрации
Удачного просмотра!
https://writeablog.net/gihkbsa1qp
Здравствуйте любители видеопроката!
У нас можно смотреть турецкие сериалы онлайн бесплатно и без ограничений. Мы обновляем коллекцию ежедневно, добавляя новые шоу. Все доступно с русским переводом и в хорошем качестве. смотреть турецкие сериалы онлайн бесплатно Начните просмотр прямо сейчас и наслаждайтесь лучшими турецкими сериалами без рекламы!
На нашем сайте можно смотреть турецкие сериалы онлайн бесплатно в хорошем качестве. У нас есть все самые популярные шоу с русским переводом. Без регистрации и без рекламы. Начните просмотр турецких сериалов прямо сейчас!
Заходите и смотрите аниме бесплатно онлайн – https://turokkk.biz/serialy-2024/
дочь после турецкий сериал на русском смотреть онлайн, турецкий сериал на русском языке смотреть онлайн бесплатно в хорошем все серии, на онлайне турецкий сериал невеста на русском языке все серии бесплатно
турецкий сериал осман смотреть онлайн бесплатно в хорошем качестве, турецкий сериал осман смотреть все серии онлайн, смотреть онлайн турецкий сериал на домашнем
Удачного просмотра!
Здравствуйте любители видеопроката!
Смотрите турецкие сериалы онлайн бесплатно и без ограничений. Мы предлагаем только лучшие шоу с русским переводом. Все доступно в хорошем качестве. смотреть турецкие сериалы онлайн Выбирайте сериал и наслаждайтесь просмотром. Турецкие истории никого не оставляют равнодушными!
В нашем онлайн-кинотеатре можно смотреть турецкие сериалы в онлайн бесплатно в хорошем качестве. Мы предлагаем вам большой выбор турецких сериалов, каждый из которых доступен для просмотра в любое время. Получите удовольствие от увлекательных историй и ярких персонажей. Все сериалы переведены на русский язык. Смотрите турецкие сериалы онлайн прямо сейчас!
Заходите и смотрите все от hulu бесплатно онлайн – https://turkpro.run/serialy-2022/
мистер ошибка онлайн турецкий сериал, ветреный смотреть онлайн турецкий сериал 2 сезон, постучись в мою дверь турецкий сериал в хорошем качестве смотреть онлайн
фильм онлайн смотреть турецкий сериал, турецкий сериал на домашнем онлайн, смотреть турецкий сериал постучись в мою дверь онлайн бесплатно
Удачного просмотра!
Мы предлагаем дипломы любой профессии по выгодным тарифам. Дипломы производятся на настоящих бланках Приобрести диплом любого института kupitediplom.ru
Где приобрести диплом специалиста?
Приобрести диплом института по невысокой цене возможно, обращаясь к проверенной специализированной компании.: ry-diplom.com
Добрый день киноманы!
Зарубежные сериалы бесплатно смотреть онлайн с качественным видео и без скрытых платежей. Все новинки и старые хиты доступны сразу. зарубежные сериалы онлайн смотреть Смотрите любимые шоу без рекламы. Наслаждайтесь качественным контентом!
Смотреть зарубежные сериалы бесплатно онлайн с моментальной загрузкой и без рекламы. Мы обеспечиваем вам лучший опыт. Все новинки и старые хиты загружаются мгновенно. Смотрите в любое время, без ограничений. Приятного просмотра!
Заходите и смотрите лучшие фильмы онлайн – https://lordseria3.run/xfsearch/year/2022/
смотреть турецкие сериалы драмы онлайн бесплатно, смотреть зарубежный netflix сериал, смотреть индийские сериалы бесплатно
смотреть сериал осман бесплатно, 2017 сериалы смотреть онлайн, небо сериал смотреть
Удачного просмотра!
mostbets https://www.eisberg.forum24.ru/?1-0-0-00000327-000-0-0-1742579529 .
мостбет официальный сайт https://taksafonchik.borda.ru/?1-14-0-00002042-000-0-0-1742473173/ .
1win официальный сайт вход https://fanfiction.borda.ru/?1-3-0-00000125-000-0-0-1742475251 .
Названы доказательства пребывания на Земле более высоких цивилизаций https://x.com/Fariz418740/status/1903334968513745235
mosbet http://eisberg.forum24.ru/?1-0-0-00000327-000-0-0-1742579529 .
мостбет скачать бесплатно мостбет скачать бесплатно .
официальный сайт 1win https://fanfiction.borda.ru/?1-3-0-00000125-000-0-0-1742475251 .
გამარჯობა მოჭიდავე მეგობრები!
Rabona Greece бѓ’бѓ—бѓђбѓ•бѓђбѓ–бѓќбѓ‘бѓ— бѓ—бѓђбѓ›бѓђбѓЁбѓ”бѓ‘бѓЎ, бѓ бѓќбѓ›бѓљбѓ”бѓ‘бѓбѓЄ შექმნбѓбѓљбѓбѓђ თქვენრწარმატებбѓбѓЎбѓђбѓ—бѓ•бѓбѓЎ. бѓ™бѓђбѓ–бѓбѓњбѓќбѓЁбѓ бѓ—бѓђбѓ›бѓђбѓЁбѓ бѓ“бѓђ бѓ’бѓђбѓ›бѓђбѓ бѓЇбѓ•бѓ”бѓ‘бѓђ бѓЎбѓЈбѓљ бѓЈбѓ¤бѓ бѓќ бѓЎбѓђбѓ®бѓђбѓљбѓбѓЎбѓќ бѓ®бѓ“бѓ”бѓ‘бѓђ. Rabona Rabona-бѓЎ бѓћбѓљбѓђбѓўбѓ¤бѓќбѓ бѓ›бѓђ бѓ“бѓђ ბონუსებრთქვენრშანსებбѓбѓЎ бѓ’бѓђбѓЎбѓђбѓЈбѓ›бѓЇбѓќбѓ‘бѓ”бѓЎбѓ”бѓ‘бѓљбѓђбѓ“ შექმნбѓбѓљбѓбѓђ. бѓђбѓ®бѓљбѓђ бѓЈбѓ™бѓ•бѓ” бѓ“бѓ бѓќбѓђ, бѓ бѓќбѓ› бѓЁбѓ”бѓ”бѓЄбѓђбѓ“бѓќбѓ— бѓ“бѓђ бѓ’бѓђбѓђбѓ—бѓђбѓ•бѓбѓЎбѓЈбѓ¤бѓљбѓќбѓ— бѓ“бѓбѓ“бѓ бѓћбѓ бѓбѓ–бѓ”бѓ‘бѓ! Rabona Greece бѓ’бѓ”бѓљбѓќбѓ“бѓ”бѓ‘бѓђбѓ—!
Rabona Greece бѓ’бѓ—бѓђбѓ•бѓђбѓ–бѓќбѓ‘бѓ— бѓЈбѓђбѓ›бѓ бѓђбѓ• бѓ—бѓђбѓ›бѓђбѓЁбѓЎ, бѓ бѓќбѓ›бѓ”бѓљбѓ—бѓђбѓ’бѓђбѓњ бѓ—бѓбѓ—бѓќбѓ”бѓЈбѓљбѓ бѓЁбѓђбѓњбѓЎбѓ бѓ›бѓќбѓ’бѓ”бѓ‘бѓбѓЎ бѓ›бѓбѓ›бѓђбѓ бѓ—бѓЈбѓљбѓ”бѓ‘бѓбѓ— მაქსбѓбѓ›бѓђбѓљбѓЈбѓ бѓђбѓ“ бѓ’бѓђбѓ–бѓ бѓ“бѓбѓљбѓбѓђ. бѓ‘бѓќбѓњбѓЈбѓЎбѓ”бѓ‘бѓ бѓ“бѓђ бѓћбѓ бѓбѓ–бѓ”бѓ‘бѓ бѓ§бѓ•бѓ”бѓљбѓђ бѓ›бѓќбѓ—бѓђбѓ›бѓђбѓЁбѓ”бѓЎ бѓ’бѓђбѓњбѓ™бѓЈбѓ—бѓ•бѓњбѓбѓљбѓбѓђ. Rabona-бѓЎ бѓЎбѓђбѓ—бѓђбѓ›бѓђбѓЁбѓќ бѓћбѓљбѓђбѓўбѓ¤бѓќбѓ бѓ›бѓђ бѓњбѓђбѓ›бѓ“бѓ•бѓбѓљбѓђбѓ“ თქვენთვбѓбѓЎбѓђбѓђ. бѓ›бѓќбѓбѓ’бѓ”бѓ— бѓ“бѓбѓ“ бѓћбѓ бѓбѓ–бѓ”бѓ‘бѓЎ Rabona Greece-бѓЁбѓ! бѓ›бѓќбѓбѓ’бѓ”бѓ— თქვენრგამარჯვება.
ყველა ინფორმაცია ლინკში – https://www.robola.gr
ხალისიანი დრო გქონდეთ თქვენს ჯეკპოტებში!
Добрый день киноманы!
Наш сайт предлагает смотреть турецкие сериалы онлайн бесплатно и в хорошем качестве. Все шоу переведены на русский язык и доступны без ограничений. турецкие сериалы смотреть онлайн бесплатно Наслаждайтесь просмотром без рекламы! Начните смотреть турецкие сериалы прямо сейчас!
У нас можно смотреть турецкие сериалы онлайн бесплатно и без регистрации. Мы собрали лучшие сериалы с русским переводом. Все доступно в хорошем качестве. Начните просмотр прямо сейчас!
Заходите и смотрите мульт сериалы без рекламы – https://turkpro.run/trillery/
турецкие сериалы истерзанная смотреть онлайн бесплатно на русском, смотреть турецкие сериалы бесплатно онлайн в хорошем качестве бесплатно, смотреть онлайн фильм турецкий сериал
сериал турецкий смотреть онлайн бесплатно в хорошем качестве, турецкий сериал постучись в мою дверь онлайн в хорошем качестве, турецкие сериалы смотреть онлайн бесплатно на русском
Удачного просмотра!
кайт школа хургада
Доброго любители сериалов онлайн!
Наш сайт предлагает смотреть турецкие сериалы онлайн бесплатно в хорошем качестве. Мы собрали самые популярные сериалы с русским переводом. Все доступно без рекламы. смотреть турецкие сериалы онлайн бесплатно Начните просмотр прямо сейчас и наслаждайтесь лучшими турецкими шоу. Эти сериалы поглотят вас с первой серии!
Хотите смотреть турецкие сериалы онлайн бесплатно? У нас есть для вас лучшие шоу, которые можно смотреть в любое время. Все сериалы доступны без регистрации и с русским переводом. Смотрите турецкие сериалы онлайн в хорошем качестве и без рекламы. Наслаждайтесь качественным контентом прямо сейчас!
Заходите и смотрите любимые фильмы без рекламы – https://turkoff.bet/voennye/
турецкий сериал черная любовь онлайн в хорошем качестве, ветреный смотреть турецкий сериал онлайн, черная любовь турецкий сериал на русском смотреть онлайн бесплатно все
доверенное турецкий сериал на русском языке онлайн, судьба сериал турецкий на русском языке все серии смотреть онлайн бесплатно, турецкий сериал невеста смотреть онлайн бесплатно в хорошем качестве
Удачного просмотра!
Здравствуйте любители видеопроката!
Смотрите турецкие сериалы онлайн бесплатно и наслаждайтесь качественным контентом. Мы собрали для вас лучшие шоу всех жанров. Все доступно в хорошем качестве и с русским переводом. онлайн смотреть турецкие сериалы Начните просмотр прямо сейчас и погружайтесь в мир турецких сериалов. Эти сериалы не дадут вам заскучать!
Смотрите турецкие сериалы онлайн бесплатно в хорошем качестве прямо сейчас. Мы предлагаем вам огромный выбор сериалов с русским переводом. Все шоу доступны без регистрации и без рекламы. Начните смотреть турецкие сериалы уже сегодня. Откройте для себя увлекательные сюжеты!
Заходите и смотрите сериалы бесплатно онлайн – https://turokkk.pro/fantastika/
плен турецкий сериал онлайн бесплатно, ветреный турецкий сериал смотреть онлайн бесплатно, турецкий сериал сыла смотреть онлайн бесплатно на русском
чукур онлайн турецкий сериал, турецкий сериал зимородок смотреть онлайн в хорошем качестве, новые турецкие сериалы онлайн бесплатно на русском языке
Удачного просмотра!
Купить документ института вы можете в нашей компании в Москве. Мы оказываем услуги по изготовлению и продаже документов об окончании любых ВУЗов РФ. Вы сможете получить необходимый диплом по любой специальности, включая документы Советского Союза. Гарантируем, что при проверке документа работодателями, подозрений не появится. orangeroleplay.getbb.ru/viewtopic.php?f=62&t=18624
Здравствуйте любители видеопроката!
Зарубежные сериалы онлайн бесплатно смотреть без скрытых платежей и ограничений. Все новинки и старые хиты доступны сразу. зарубежные сериалы онлайн смотреть Погружайтесь в мир увлекательных шоу. Смотрите любимые сериалы без рекламы!
Зарубежные сериалы бесплатно онлайн смотреть без ограничений! На нашем сайте все новинки доступны сразу. Все эпизоды и сезоны загружаются моментально. Смотрите сериалы в удобное для вас время без рекламы. Погружайтесь в мир увлекательных сюжетов!
Заходите и смотрите мульт сериалы онлайн – https://lordseria3.run/xfsearch/perevod/%D0%BA%D1%83%D0%B1%D0%B8%D0%BA%20%D0%B2%20%D0%BA%D1%83%D0%B1%D0%B5/
дикий сериал смотреть бесплатно, турецкие сериалы смотреть невеста, триггеры сериал смотреть онлайн
смотреть сериалы детективы бесплатно в хорошем, смотреть сериал фильмы онлайн, первый сериал смотреть онлайн
Удачного просмотра!
Привет всем!
В нашем онлайн-кинотеатре можно смотреть турецкие сериалы в онлайн бесплатно в хорошем качестве. Мы предлагаем вам большой выбор турецких сериалов, каждый из которых доступен для просмотра в любое время. Получите удовольствие от увлекательных историй и ярких персонажей. смотреть турецкие сериалы онлайн Все сериалы переведены на русский язык. Смотрите турецкие сериалы онлайн прямо сейчас!
Смотрите турецкие сериалы онлайн бесплатно на русском языке. Мы обновляем коллекцию каждый день, добавляя новые и популярные шоу. Все доступно без рекламы и в хорошем качестве. Начните просмотр прямо сейчас и наслаждайтесь лучшими турецкими сериалами. Эти шоу просто не могут надоесть!
Заходите и смотрите финтастику без рекламы – https://turkoff.bet/fantastika/
красивее тебя смотреть онлайн турецкий сериал на русском, турецкий сериал в ожидании солнца смотреть онлайн в хорошем качестве бесплатно, полнолуния турецкий сериал на русском смотреть онлайн бесплатно
турецкий сериал на русском языке в ожидании солнца смотреть онлайн бесплатно хорошем, сериал любовь турецкий смотреть онлайн, турецкий сериал на русском онлайн бесплатно полнолуние
Удачного просмотра!
поддержка мостбет http://eisberg.forum24.ru/?1-0-0-00000327-000-0-0-1742579529/ .
печать буклетов а3 tipografiya-buklety.ru
печать рекламных наклеек pechat-nakleek-spb.ru
мостюет мостюет .
wan win wan win .
печать на камне сувенир печать на сувенирной продукции
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по доступным тарифам. Цена может зависеть от выбранной специальности, года получения и университета. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Для нас важно, чтобы дипломы были доступны для подавляющей массы наших граждан. купить диплом дешево
Приобрести диплом о высшем образовании!
Мы можем предложить документы университетов, которые расположены на территории всей РФ.
diplomt-nsk.ru/kupit-attestat-11-klassov-tsena-7
Мы предлагаем дипломы любой профессии по доступным ценам. Всегда стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы дипломы были доступными для большого количества граждан.
Заказ диплома, подтверждающего обучение в ВУЗе, – это разумное решение. Купить диплом любого университета: diplomist.com/kupit-diplom-mexanika-13/
Доброго любители сериалов онлайн!
На нашем сайте вы можете смотреть турецкие сериалы онлайн бесплатно в хорошем качестве. Мы предлагаем все самые популярные шоу и новинки на русском языке. Качество изображения и звука всегда на высоте, чтобы вам было удобно смотреть. смотреть турецкий сериал Все сериалы доступны для вас без регистрации. Начните смотреть турецкие сериалы прямо сейчас!
У нас можно смотреть турецкие сериалы онлайн бесплатно и без ограничений. Мы предлагаем вам только самые популярные шоу, которые можно смотреть в хорошем качестве и с русским переводом. Начните просмотр прямо сейчас и погружайтесь в мир турецких сериалов. Эти истории не оставят вас равнодушными!
Заходите и смотрите финтастику без регистрации – https://turkoff.biz/kriminalnye/
турецкие сериалы на русском смотреть онлайн дочь посла, турецкие сериалы онлайн ты назови, ветреный турецкий сериал смотреть бесплатно на русском языке онлайн
турецкий сериал невеста смотреть онлайн бесплатно, черно белая любовь серия турецкий сериал на русском смотреть онлайн бесплатно, черно любовь турецкий сериал на русском смотреть онлайн бесплатно все серии
Удачного просмотра!
Где приобрести диплом по необходимой специальности?
Мы предлагаем быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Документ способен пройти лубую проверку, даже при использовании специального оборудования. Достигайте своих целей быстро с нашими дипломами. Заказать диплом любого университета! prestig.flybb.ru/viewtopic.php?f=11&t=279
Добрый день киноманы!
Если вы хотите смотреть турецкие сериалы онлайн бесплатно, наш сайт – ваш лучший выбор. Мы собрали самые популярные шоу. Все серии доступны в хорошем качестве. турецкие сериалы онлайн онлайн бесплатно в хорошем качестве на русском языке Начните просмотр прямо сейчас. Турецкие истории увлекут вас с первой минуты!
Наш сайт предлагает смотреть турецкие сериалы онлайн бесплатно. Мы собрали самые популярные сериалы с качественным переводом. Все доступно в отличном качестве и без рекламы. Начните просмотр прямо сейчас и погрузитесь в захватывающий мир турецких историй. Присоединяйтесь к миллионам зрителей!
Заходите и смотрите аниме онлайн – https://turokkk.biz/fantastika/
сериал гюльджемаль турецкий смотреть онлайн на русском языке, смотреть месть турецкий сериал онлайн бесплатно, черное сердце сериал турецкий смотреть онлайн все серии на русском
турецкий сериал осман на русском онлайн бесплатно, 1001 ночь турецкий сериал онлайн, полнолуние турецкий сериал смотреть онлайн бесплатно
Удачного просмотра!
Доброго любители сериалов онлайн!
Смотрите турецкие сериалы онлайн бесплатно в хорошем качестве. Мы собрали для вас самые популярные шоу с русским переводом. Наслаждайтесь просмотром без регистрации и рекламы. [url=https://turkoff.biz/kriminalnye/]смотреть турецкие сериалы в онлайн бесплатно в хорошем качестве[/url] Турецкие сериалы подарят вам незабываемые эмоции. Окунитесь в мир страсти и драмы!
Если вы хотите смотреть турецкие сериалы онлайн бесплатно, наш сайт – лучший выбор. Мы собрали только самые популярные шоу с качественным переводом и в хорошем качестве. Все доступно без рекламы. Просто выбирайте сериал и начинайте просмотр. Турецкие сериалы подарят вам яркие эмоции!
Заходите и смотрите мульт сериалы бесплатно онлайн – https://turkoff.biz/melodramy/
черная любовь турецкий сериал на русском смотреть онлайн бесплатно все серии бесплатно, турецкие сериалы на русском языке смотреть онлайн игра судьба, смотреть турецкие сериалы онлайн клятва
черная любовь турецкий сериал на русском смотреть онлайн бесплатно в хорошем качестве, сыла турецкий сериал онлайн на русском, турецкие сериалы 2023 смотреть онлайн на русском языке
Удачного просмотра!
упаковка продукции печать печать гибкой упаковки
печать фото на холсте недорого печать фото на холсте
печать индивидуальных блокнотов срочная печать блокнотов
Купить диплом любого университета!
Мы можем предложить документы университетов, которые находятся в любом регионе РФ. Документы выпускаются на бумаге высшего качества: playashshi.ru/files/articles/?kupit_diplom_o_srednem_obrazovanii_bustro_i_bez_problem.html
Auf Bird-Shop.de finden Sie alles Wissenswerte rund um die artgerechte Haltung von Zimmer-Vogeln. Entdecken Sie Tipps und Ratgeber zu Vogelkafigen und Zimmervolieren fur Wellensittiche, Papageien, Kanarienvogel & Co. Erfahren Sie, worauf es bei der Kafiggro?e, Einrichtung und Standortwahl ankommt, um Ihren Vogeln ein sicheres und gluckliches Zuhause zu bieten.
Vogelkafig Einrichtung
Купить диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по приятным ценам— diplom-zentr.com/kupit-diplom-medika-bistro-i-bez-problem/
Мы изготавливаем дипломы любых профессий по выгодным ценам. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Важно, чтобы дипломы были доступными для большого количества наших граждан.
Приобретение документа, который подтверждает обучение в университете, – это разумное решение. Заказать диплом любого ВУЗа: diplompro.ru/diplom-kupit-texnikum/
Привет всем!
У нас можно смотреть турецкие сериалы онлайн бесплатно и без ограничений. Мы собрали лучшие шоу с русским переводом. Все доступно без рекламы. турецкие сериалы смотреть онлайн бесплатно Просто включайте и наслаждайтесь просмотром. Турецкие сериалы ждут вас!
Наш сайт – это лучшее место, чтобы смотреть турецкие сериалы онлайн бесплатно. Мы обновляем каталог ежедневно. Все шоу доступны без рекламы и с качественным переводом. Начните просмотр прямо сейчас и наслаждайтесь любимыми сериалами. Турецкие истории никого не оставляют равнодушными!
Заходите и смотрите мульт сериалы без регистрации – https://turkoff.org/fantastika/
любовь против судьбы турецкий сериал смотреть на русском онлайн, игры судьбы турецкий сериал смотреть онлайн на русском языке, турецкий фильм сериал на русском языке смотреть онлайн бесплатно
ветреный турецкий сериал все серии смотреть онлайн бесплатно подряд, новые турецкие лучшие сериалы смотреть онлайн, полнолуние сериал турецкий смотреть онлайн на русском языке бесплатно
Удачного просмотра!
Где купить диплом по нужной специальности?
Готовый диплом с приложением целиком и полностью отвечает запросам и стандартам Министерства образования и науки, неотличим от оригинала. Не откладывайте личные мечты на несколько лет, реализуйте их с нами – отправьте заявку на изготовление диплома уже сегодня! Диплом о среднем специальном образовании – не проблема! rusd-diplomj.ru/attestat-ob-okonchanii-shkoli-kupit/
1win kg скачать https://1win824.ru/ .
1 вин https://1win825.ru .
До конца марта эти 4 знака Зодиака обретут личное счастье https://x.com/Fariz418740/status/1903525881395507337
игра 1вин https://1win811.ru/ .
1win.kg https://www.1win824.ru .
1win кейсы https://www.1win825.ru .
1 вин вход 1 вин вход .
Приобрести диплом о высшем образовании!
Мы готовы предложить дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам. Вы заказываете документ через надежную и проверенную фирму. : asxdiplommy.com/kupit-diplom-vva-o-visshem-obrazovanii-na-blanke-goznak/
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по выгодным ценам. Купить диплом строительного института — kyc-diplom.com/diplom-articles/kupit-diplom-stroitelnogo-instituta.html
1 win официальный сайт вход 1 win официальный сайт вход .
1 win.pro 1 win.pro .
1win казино 1win811.ru .
Привет всем!
Наш сайт предлагает смотреть турецкие сериалы онлайн бесплатно в хорошем качестве. Мы собрали для вас только самые лучшие шоу, которые можно смотреть без рекламы. Все доступны с русским переводом. турецкие сериалы смотреть Начните просмотр прямо сейчас и наслаждайтесь лучшими турецкими сериалами. Эти сериалы подарят вам незабываемые впечатления!
Хотите смотреть турецкие сериалы онлайн бесплатно? У нас есть огромный выбор лучших сериалов. Все доступно в хорошем качестве и без рекламы. Начните просмотр прямо сейчас. Турецкие сериалы подарят вам незабываемые эмоции!
Заходите и смотрите аниме без рекламы – https://turokkk.bet/serialy-2025/
турецкие сериал постучись в мою дверь смотреть онлайн бесплатно в хорошем качестве, осман турецкий сериал смотреть на русском онлайн бесплатно, ветреный все серии турецкий сериал смотреть онлайн
основание осман турецкий сериал смотреть на русском онлайн бесплатно все, игра судьбы турецкий сериал онлайн на русском, ранняя пташка турецкий сериал смотреть онлайн
Удачного просмотра!
Где заказать диплом по нужной специальности?
Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов РФ. Документы производят на настоящих бланках государственного образца. poboltaem.foroactivo.com/post
Добрый день!
Приобрести диплом института по доступной цене можно, обратившись к проверенной специализированной компании. Купить диплом о высшем образовании: poluchidiplom.com/kupit-diplom-perm-3/
Здравствуйте любители видеопроката!
Любите турецкие сериалы? У нас есть все для комфортного просмотра — все фильмы в хорошем качестве и без регистрации. Смотрите турецкие сериалы онлайн бесплатно, наслаждаясь качественным изображением. онлайн смотреть турецкие сериалы Мы обновляем коллекцию каждый день, чтобы вы всегда могли найти что-то новое. Присоединяйтесь к нам и смотрите турецкие сериалы в любое время.
Если вы хотите смотреть турецкие сериалы онлайн бесплатно, наш сайт предоставит вам идеальные условия. Мы собрали только самые популярные шоу. Все доступно в хорошем качестве и с русским переводом. Начните просмотр прямо сейчас и наслаждайтесь увлекательными турецкими сериалами!
Заходите и смотрите лучшее от netfix онлайн – https://turokkk.bet/melodramy/
турецкий сериал сыла смотреть онлайн на русском, смотреть онлайн плен турецкий сериал, ветреный смотреть онлайн бесплатно все серии подряд турецкий сериал
постучись в мою дверь сериал турецкий смотреть онлайн бесплатно в хорошем качестве, красивее тебя турецкий сериал на русском смотреть онлайн, турецкие сериалы на русском смотреть онлайн бесплатно в хорошем качестве постучись мою дверь
Удачного просмотра!
Покупка официального диплома через качественную и надежную фирму дарит ряд плюсов для покупателя. Такое решение дает возможность сэкономить время и серьезные финансовые средства. Тем не менее, на этом выгода не ограничивается, плюсов гораздо больше.Мы изготавливаем дипломы любых профессий. Дипломы производятся на оригинальных бланках государственного образца. Доступная цена по сравнению с крупными расходами на обучение и проживание. Заказ диплома о высшем образовании из российского института является мудрым шагом.
Купить диплом о высшем образовании: diplom-top.ru/kupit-attestati-za-9-16/
Доброго любители сериалов онлайн!
У нас можно смотреть турецкие сериалы онлайн бесплатно и в отличном качестве. Все шоу переведены на русский язык и доступны без ограничений. Наслаждайтесь лучшими сериалами прямо сейчас! смотреть турецкие сериалы онлайн Начните просмотр и окунитесь в увлекательный мир турецких сериалов.
У нас можно смотреть турецкие сериалы онлайн бесплатно в хорошем качестве. Мы предлагаем вам широкий выбор шоу без рекламы. Все переведено на русский язык. Присоединяйтесь и начните смотреть прямо сейчас!
Заходите и смотрите аниме без регистрации – https://turkoff.org/dramy/
смотреть фильмы онлайн сериал турецкий, смотреть турецкий сериал месть онлайн бесплатно, турецкие сериалы любовь не понимает на русском языке смотреть онлайн
правосудия смотреть онлайн турецкий сериал, смотреть сериалы жестокий стамбул турецкие на русском языке онлайн, смотреть бесплатно онлайн турецкий сериал сыла
Удачного просмотра!
Добрый день киноманы!
Смотрите турецкие сериалы онлайн бесплатно на русском языке. Мы предлагаем вам только самые популярные сериалы, доступные без рекламы и в хорошем качестве. Все переведено на русский язык. турецкий сериал смотреть Начните просмотр прямо сейчас и погружайтесь в мир турецких сериалов. Эти шоу подарят вам множество впечатлений!
Если вы хотите смотреть турецкие сериалы онлайн бесплатно, наш сайт предоставит вам идеальные условия. Мы собрали только самые популярные шоу. Все доступно в хорошем качестве и с русским переводом. Начните просмотр прямо сейчас и наслаждайтесь увлекательными турецкими сериалами!
Заходите и смотрите мульт сериалы без рекламы – https://turokkk.org/fantastika/
в ожидании солнца турецкий смотреть онлайн бесплатно хорошем качестве сериал, турецкий сериал онлайн клятва, турецкие сериалы смотреть онлайн с озвучкой
турецкий сериал невеста смотреть онлайн на русском языке все серии подряд, онлайн турецкие сериалы озвучка, невеста сериал турецкий онлайн на русском
Удачного просмотра!
аттестат школы купить
moved here nft backpack
Диплом любого университета России!
Без получения диплома очень непросто было продвинуться по карьерной лестнице. Именно из-за этого решение о заказе диплома следует считать выгодным и целесообразным. Приобрести диплом о высшем образовании revolverp.forumex.ru/viewtopic.php?f=11&t=1089
Привет всем!
Наш сайт – это лучшее место, чтобы смотреть турецкие сериалы онлайн бесплатно. У нас вы найдете любимые сериалы без рекламы. Все доступно в отличном качестве. [url=https://turokkk.org/serialy-2025/]турецкие сериалы смотреть[/url] Начните просмотр прямо сейчас и следите за новыми эпизодами. Турецкие сериалы – это незабываемые эмоции и увлекательные сюжеты!
Если вы ищете, где можно смотреть турецкие сериалы онлайн бесплатно, приходите к нам. Мы предлагаем удобный доступ к сериалам в хорошем качестве. Все шоу переведены на русский язык и доступны без регистрации. Наслаждайтесь просмотром без рекламы. Турецкие сериалы ждут вас!
Заходите и смотрите лучшее от netfix без регистрации – https://turokkk.org/
лучшие турецкие сериалы на русском языке смотреть онлайн все серии подряд, смотреть онлайн новые турецкие сериалы на русском языке все серии, турецкие сериалы правосудие на русском языке смотреть онлайн бесплатно
ветреный турецкий сериал смотреть онлайн 2 сезон, смотреть сериал любовь против судьбы турецкий онлайн на русском, смотреть лучшие новые турецкие сериалы онлайн бесплатно в хорошем качестве
Удачного просмотра!
Названа дата, когда привычная всем жизнь на Земле станет невозможной https://x.com/Fariz418740/status/1903674512593359008
Заказала пионы маме на 8 Марта, она была в восторге!
Цветы Томск
мостбет кыргызстан http://mostbet785.ru/ .
бк 1win http://www.1win826.ru .
1win kg 1win kg .
Промокод Фонбет – это кодовые слова, например: MAX777, ввод которых при регистрации в БК Фонбет или в личном кабинете позволяет получить фрибеты, страховки ставок и другие специальные бонусы. Промокодом в беттинге называется комбинация букв и цифр, при вводе которых активируется бонус. Новым клиентам Фонбет предлагает приветственные фрибеты на сумму от 100 до 15 000 рублей.
Фонбет промокод: MAX777, используйте его при регистрации аккаунта. Новые пользователи могут активировать бонус и получить бездепозитный фрибет до 15 000 рублей. Воспользоваться бесплатно промокодами «Фонбет» для получения подарочной ставки или другого бонуса можно во время создания аккаунта на официальном сайте букмекерской конторы, а также после активации личного кабинета. Действующие промокоды могут давать зачисление кэшбэка на депозит, бесплатное пари и т.п.
промокод фонбет на 2000
мостбет скачать казино https://www.mostbet785.ru .
1win https://1win826.ru/ .
игра 1вин 1win812.ru .
her comment is here wallet backpack
мастбет мастбет .
бк 1win 1win826.ru .
1wln http://www.1win812.ru .
Промокод Фонбет – это кодовые слова, например: MAX777, ввод которых при регистрации в БК Фонбет или в личном кабинете позволяет получить фрибеты, страховки ставок и другие специальные бонусы. Промокодом в беттинге называется комбинация букв и цифр, при вводе которых активируется бонус. Новым клиентам Фонбет предлагает приветственные фрибеты на сумму от 100 до 15 000 рублей.
Фонбет промокод: MAX777, используйте его при регистрации аккаунта. Новые пользователи могут активировать бонус и получить бездепозитный фрибет до 15 000 рублей. Воспользоваться бесплатно промокодами «Фонбет» для получения подарочной ставки или другого бонуса можно во время создания аккаунта на официальном сайте букмекерской конторы, а также после активации личного кабинета. Действующие промокоды могут давать зачисление кэшбэка на депозит, бесплатное пари и т.п.
Купить Гашиш в Ташкенте? Сайт – TOSHKENT-COCAINE.VIP Гашиш в Узбекистане – TOSHKENT-COCAINE.VIP
.
.
| Купить Гашиш в Ташкенте или другом городе Узбекистана – https://toshkent-cocaine.vip/ |
| Сколько стоит Гашиш в Ташкенте и по Узбекистану доставка – https://toshkent-cocaine.vip/ |
| Лучший Гашиш в Ташкенте с возможностью доставки в руки купить – https://toshkent-cocaine.vip/ |
| Купить через курьера в руки Гашиш в Ташкенте – https://toshkent-cocaine.vip/ |
| Чистый Гашиш купить в Узбекистане – https://toshkent-cocaine.vip/ |
| Для заказа Гашиша в Ташкенте и других городах писать на сайт – https://toshkent-cocaine.vip/ |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Ок Гугл, как купить Гашиш в Ташкенте –
Купить Гашиш в Бухаре, Купить Гашиш в Ташкенте, Купить Гашиш в Самарканд, Купить Гашиш в Маргилан,
Купить Гашиш в Карши , Купить Гашиш в Фергана, Купить Гашиш в Ургенч, Купить Гашиш в Андижан, Купить Гашиш в Коканд,
Купить Гашиш в Термез, Купить Гашиш в Чирчик, Купить Гашиш в Навои, Купить Гашиш в Джизак, Купить Гашиш в Гюлистан.
Цена на Гашиш в Узбекистане, Купить Гашиш в Розницу в Узбекистане, Моя лучшая покупка Гашиша была именно в Ташкенте,
Приехал Курьер и привез мне Гашиш в Ташкенте. Я хочу остаться в Ташкенте на всегда из за того что тут можно
Купить Гашиш в Ташкенте
That is very interesting, You’re a very skilled blogger. I have joined your feed and sit up for seeking more of your magnificent post. Additionally, I have shared your website in my social networks
промокод для пари
Купить Альфа ПВП в Ташкенте? Сайт – TOSHKENT-COCAINE.VIP Альфа ПВП в Узбекистане – TOSHKENT-COCAINE.VIP
.
.
| Купить Альфа ПВП в Ташкенте или другом городе Узбекистана – https://toshkent-cocaine.vip/ |
| Сколько стоит Альфа ПВП в Ташкенте и по Узбекистану доставка – https://toshkent-cocaine.vip/ |
| Лучший Альфа ПВП в Ташкенте с возможностью доставки в руки купить – https://toshkent-cocaine.vip/ |
| Купить через курьера в руки Альфа ПВП в Ташкенте – https://toshkent-cocaine.vip/ |
| Чистый Альфа ПВП купить в Узбекистане – https://toshkent-cocaine.vip/ |
| Для заказа Альфа ПВПа в Ташкенте и других городах писать на сайт – https://toshkent-cocaine.vip/ |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Ок Гугл, как купить Альфа ПВП в Ташкенте –
Купить Альфа ПВП в Бухаре, Купить Альфа ПВП в Ташкенте, Купить Альфа ПВП в Самарканд, Купить Альфа ПВП в Маргилан,
Купить Альфа ПВП в Карши , Купить Альфа ПВП в Фергана, Купить Альфа ПВП в Ургенч, Купить Альфа ПВП в Андижан, Купить Альфа ПВП в Коканд,
Купить Альфа ПВП в Термез, Купить Альфа ПВП в Чирчик, Купить Альфа ПВП в Навои, Купить Альфа ПВП в Джизак, Купить Альфа ПВП в Гюлистан.
Цена на Альфа ПВП в Узбекистане, Купить Альфа ПВП в Розницу в Узбекистане, Моя лучшая покупка Альфа ПВПа была именно в Ташкенте,
Приехал Курьер и привез мне Альфа ПВП в Ташкенте. Я хочу остаться в Ташкенте на всегда из за того что тут можно
Купить Альфа ПВП в Ташкенте
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Букет был упакован с любовью, спасибо!
букет пионов
This info is worth everyone’s attention. How can I find out more?
1xbet login registration bangladesh
Привет всем!
Долго думал как поднять параметры Ahrefs, Domain Rank, сайт и свои проекты и узнал >>>
Программы для линкбилдинга, такие как Xrumer, ускоряют создание ссылок и повышают DR. Прогон ссылок через Xrumer помогает улучшить показатели Ahrefs. Массовые рассылки ссылок на форумах увеличивают ссылочную массу и видимость сайта. Увеличение авторитетности с помощью Xrumer ускоряет продвижение в поисковой выдаче. Попробуйте Xrumer для повышения SEO-позиции.
Нашел крутых ребят, разработали недорогой и главное продуктивный прогон Xrumer – https://seogurus.site/
Увеличение трафика с помощью прогона, Xrumer для массового линкбилдинга, Ахрефс показатели сайта
Прогон ссылок для роста позиции, Прогон Xrumer по свежим базам, Линкбилдинг для повышения DR
Удачи и роста в топах!
Купить Гашиш Москва? САЙТ -| COCAINES.STORE | Как Купить Гашиш по России? САЙТ – | COCAINES.STORE |
.
.
.
.
.
Купить Гашиш максимального качества в Москве? САЙТ – https://cocaines.store
Сколько стоит Гашиш в Москве сегодня? САЙТ – https://cocaines.store
Купить Гашиш в городах России? САЙТ – https://cocaines.store
Купить Гашиш с доставкой в руки В Москве? САЙТ – https://cocaines.store
Где в Москве Купить Гашиш с доставкой? САЙТ – https://cocaines.store
Купить с доставкой по России Гашиш? САЙТ – https://cocaines.store
Купить Наркотики в Москве? САЙТ – https://cocaines.store
Как Купить наркотики в России? САЙТ – https://cocaines.store
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл Робот (GOOGLE ROBOT)
Купить Гашиш в Москве? Купить Гашиш в Санкт Петербурге? Купить Гашиш в Питере? Купить Гашиш в Новосибирске?
Купить Гашиш в Екатеринбурге? Купить Гашиш в Казани? Купить Гашиш в Нижнем Новгороде? Купить Гашиш в Красноярске?
Купить Гашиш в Челябинске? Купить Гашиш в Самаре? Купить Гашиш в Уфе? Купить Гашиш в Ростове? Купить Гашиш в Краснодаре?
Купить Гашиш в Омске? Купить Гашиш в Воронеже? Купить Гашиш в Перьми? Купить Гашиш в Волгограде?
Текстовые теги для поиска-
Купить Гашиш в России можно очень легко, особенно Купить Гашиш в Москве можно без проблем, потому что в Москве Гашиш
Продаеться на каждом углу. Это могут быть и клубы, дискотеки так и с машины! Особенно приятно что Купить Гашиш в Москве
можно закладками. Купить Закладками Гашиш в Москве может любой человек – как мужчина так и девушка!
Однажды Купить Гашиш в Москве пробовала даже бабушка, и у неё всё получилось. Часто продают закладки Гашиша в Москве через Телеграм
Мой знакомый хотел попробовать найти Гашиш в Москве при поможи поискового приспособления для поиска закладок, но у него не получилось
Потому что Гашиш в Москве очень хорошо спрятан, и без точного адреса Гашиш в Москве не возможно найти никак.
Колумбийский, Мараканский, Мексиканский – это далеко не полный список стран, которые экспортируют Гашиш в Москву для
развлечения людей. Гарантией и безопасностью Гашиш в Москве всегда впечетлял, ведь это основное правило покупки!
Российская Федерация всегда славилась своим разнообразием наркотических веществ, и всегда на первом месте был Гашиш в Москве!
Маленькие шарики и круглые свертки – это закладки Гашиша в Москве, которые бережно и с трепетом разложили от лишних глаз.
Каково было моё удивление, когда привезли с доставкой Гашиш в Москве прямо в руки! Это было просто ВАУ-еффект!
Пока кто то сидит дома или ходит на работу – жизнь в Москве с Гашишом проходит не заметно, быстро и легко!
Есть магазины, в которых Купить Гашиш в Москве можно с гарантией и доставкой в руки круглосуточно!
Всё анонимно и безопастность просто зашкаливает, никогда Гашиш в Москве не сможет обнаружить полиция.
Колючий кактус или национальность человека не имеет значения для покупки Гашиша в Москве, потому что люди бывают разные
у всех разные потребности и желания. Но магазины готовы предоставить услугу по покупке Гашиша в Москве не зависимо от настроения,
Ведь настроение после покупки у всех будет одинаковое – ТОЛЬКО ПОЛНЫЙ ПОЗИТИВ!
Где приобрести диплом специалиста?
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по доступным тарифам. Мы готовы предложить документы ВУЗов, расположенных в любом регионе Российской Федерации. Вы сможете купить диплом за любой год, указав подходящую специальность и хорошие оценки за все дисциплины. Дипломы и аттестаты делаются на бумаге высшего качества. Это позволяет делать настоящие дипломы, не отличимые от оригиналов. Они заверяются необходимыми печатями и штампами. Всегда стараемся поддерживать для клиентов адекватную ценовую политику. Для нас важно, чтобы дипломы были доступны для большого количества граждан. okdiplom.ru/kupit-diplom-v-krasnodare-2-5
Рейтинг онлайн казино
Топ онлайн казино 2025: профессиональный анализ лучших игровых платформ
Виртуальные казино стремительно эволюционируют в России, завоевывая всё больше игроков из-за своей доступности. Однако для комфортного гейминга критически важно выбирать только легальные площадки, которые гарантируют честность алгоритмов. В этом материале мы рассмотрим ключевые параметры оценки, составим список лучших казино 2025 года и даём профессиональные рекомендации для правильного решения.
Основные признаки безопасности онлайн-казино
Чтобы защититься от мошенников, необходимо учитывать следующие особенности:
Лицензионная чистота
Проверяйте наличие регуляторных разрешений: Гибралтара.
Убедитесь, что игровые автоматы прошли независимую проверку от авторитетных организаций (GLI).
Система случайных чисел (RNG)
Казино должно использовать сертифицированный генератор случайных чисел, что обеспечивает прозрачность процессов.
Скорость транзакций
Надёжные казино предлагают быстрые выплаты через разнообразные инструменты: банковские карты (MasterCard), электронные кошельки (Neteller) и цифровые активы (Litecoin).
Бонусная политика
Хорошие казино предоставляют привлекательные бонусы: вступительные награды, фриспины и системы вознаграждений. Обратите внимание на вейджер.
Мобильная оптимизация
Современные казино оптимизированы для мобильных устройств (Android) с быстрой загрузкой. Это позволяет играть в любом месте в любое время.
Клиентская поддержка
Эффективная служба поддержки работает круглосуточно и помогает решать вопросы через email.
Рейтинг лучших онлайн-казино 2025 года
1. Cat Casino
Преимущества: оперативные выплаты (до 1 минуты), лицензия Кюрасао, особые бонусы.
Бонусы: начальный подарок — 100 фриспинов в других популярных слотах.
Особенности: розыгрыши путешествий.
2. Kometa Casino
Преимущества: топовая живая игра, кэшбэк до 30 000?.
Бонусы: стартовый комплект — щедрое предложение.
Особенности: VIP-клуб с индивидуальными бонусами.
3. R7 Casino
Преимущества: анонимная регистрация по email, поддержка цифровых активов (USDT) с бесплатными транзакциями.
Бонусы: фриспины за активность в соцсетях.
Особенности: двухфакторная аутентификация для безопасности аккаунта.
4. Arkada Casino
Преимущества: оперативные транзакции на карты РФ (Тинькофф), регулярные розыгрыши.
Бонусы: кэшбэк до значительного процента, удвоение начального взноса.
Особенности: лицензионные игры от Microgaming.
5. Kent Casino
Преимущества: поддержка криптовалют, турниры с призовым фондом впечатляющими наградами.
Бонусы: тестирование слотов.
Особенности: широкий выбор слотов с высоким RTP.
Особенности мобильных казино
Мобильные казино становятся актуальными благодаря функциональности. Их преимущества:
Адаптивный дизайн: интерфейс адаптирован для сенсорных экранов.
Быстрый доступ: нет необходимости скачивать дополнительное ПО.
Поддержка платежей: можно пополнять счёт и выводить средства через специальные сервисы.
Низкое потребление трафика: современные казино работают эффективно даже на медленном интернете.
Как выбрать лучшее казино?
При выборе онлайн-казино учитывайте следующие аспекты:
Лицензия и безопасность
Убедитесь, что казино имеет международную лицензию и использует шифрование данных (SSL).
Ассортимент игр
Лучшие казино предлагают разнообразие слотов, настольных игр (рулетка) и живые игры.
Скорость выплат
Изучите отзывы игроков о времени получения денег.
Бонусные программы
Обратите внимание на размер приветственного бонуса, требования по отыгрышу и наличие других предложений.
Качество поддержки
Проверьте, насколько быстро сотрудники решают вопросы.
Мобильная версия
Убедитесь, что казино имеет адаптированную версию для смартфонов.
Заключение
Онлайн-казино могут стать отличным способом развлечения, если подходить к их выбору серьёзно. Придерживайтесь наших рекомендаций, играйте только на надёжных платформах и помните о важности самоконтроля. Азартные игры — это прежде всего удовольствие, а не финансовая стратегия.
Если вы ищете надёжное казино с быстрыми выплатами, обратите внимание на топовые площадки, такие как Kometa Casino. Они высоко ценятся среди игроков и продолжают радовать своими предложениями.
https://t.me/s/topcasinos_ru
Строительные бытовки
Модули и модульные здания: практичное решение для ваших целей
Контейнеры и временные конструкции организовывают оборудовать зону работы, склад или временное помещение. Наша компания обеспечиваем сооружения, которые подходят качественным критериям безопасности и функциональности.
Характеристики
Надёжность. Данные вагончики изготовлены из компонентов, устойчивых к нагрузкам и погодным условиям.
Скорость доставки. Конструкция перевозится в срок 1–2 рабочих дней после подписания договора.
Персонализация. Доступна монтаж утепления, электрооборудования или воздухообмена.
Где применяются
На строительных объектах для организации склада или организации комнаты отдыха.
Во время акций для создания информационного центра или склада оборудования.
В качестве временно используемых помещений или операционных штабов.
Выгоды
Экономия времени. Отпадает потребность обустраивать временные здания.
Удобство. Среда, которые улучшают производительность труда персонала.
Вариативность. Шанс временного использования или покупки под разные периоды и ресурсы.
Случай применения
Строительная компания использовала модульное здание для организации склада инструментов и места для рабочих. Объект была привезена за 24 часа, с усиленной теплоизоляцией. Клиент отметил на улучшение условий работы и отсутствие простоев.
Как начать сотрудничество
Для заключения договора достаточно связаться с нами. Дадим полные данные, содействуем подобрать правильный путь и выполним транспортировку.
Огромное спасибо за великолепный букет!
заказать цветы томск
Заказать документ университета вы можете в нашей компании в столице. diplomservis.ru/kupit-diplom-kaliningrad-4
Привет всем!
Долго обмозговывал как поднять параметры Ahrefs, Domain Rank, сайт и свои проекты и узнал >>>
Увеличение DR и Ahrefs возможно с помощью автоматического прогноза ссылок через Xrumer. Прогон ссылок на форумах ускоряет создание внешних ссылок. Массовые рассылки с Xrumer помогают улучшить позиции сайта в поисковых системах. Программы для линкбилдинга делают процесс получения ссылок быстрым и удобным. Используйте Xrumer для быстрого роста сайта.
Нашел крутых ребят, разработали недорогой и главное продуктивный прогон Хрумером – https://seogurus.site
Автоматический постинг форумов, Как поднять DR сайта за неделю, Как сделать прогон сайта через Xrumer
Линкбилдинг для продвижения в топ-10, Как увеличить показатели домена, Автоматизация создания ссылок
Удачи и роста в топах!
маркетплейс готовых аккаунтов биржа аккаунтов
Тут можно преобрести сейф в стену сейф встраиваемый
магазин продажи аккаунтов https://akkaunt-marketplace.ru
быстро продать аккаунт купить аккаунт соцсети
1win вход в личный кабинет https://1win827.ru .
1win md https://www.1win5003.ru .
mostbet.kg http://mostbet786.ru/ .
Мы изготавливаем дипломы любой профессии по приятным тарифам.– webtini.ru/pbp-site-review/#comment-4838
Enhance your auto with superior tuning options. Set free its complete power and relish better functionality. Our experienced technicians is adept in tailoring every element of your vehicle to match your fashion and preferences. Explore our webpage https://autobodywilson.com/ to find out extra, and adopt a novel car adventure today.
1вин https://1win827.ru .
1win.com 1win5003.ru .
мостбет chrono https://mostbet786.ru/ .
Приобрести диплом о высшем образовании поможем. Купить диплом специалиста в Екатеринбурге – diplomybox.com/kupit-diplom-spetsialista-v-ekaterinburge
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me? https://accounts.binance.com/ru/register-person?ref=V3MG69RO
Тут можно преобрести навес из поликарбоната цена в Санкт-Петербурге подробно на сайте навес автомобильный
1win официальный сайт войти https://1win827.ru/ .
pariuri sportive moldova http://1win5003.ru/ .
мосбет mostbet786.ru .
dimagrimento
find more info Department Stores Accessories Sunglasses
Current medicine trends. Drug effects explained.
ed pills online
Patient medicine resource. Patient drug info.
Recommended Reading Department Stores Accessories Sunglasses
https://yarus-kkt.ru/
Access drug details. Current medication trends.
ed pills online
Patient medication facts. Latest drug news.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article. https://accounts.binance.com/sk/register-person?ref=OMM3XK51
Тут можно преобрести встраиваемые сейфы в стену встроенный сейф купить москва
Latest medication updates. Complete medication overview.
erectile dysfunction pills
Medicine facts provided. Comprehensive pill guide.
Назван простой напиток, который спасает от повышения сахара в крови https://x.com/Fariz418740/status/1904006505147629966
Мы предлагаем дипломы любой профессии по невысоким тарифам. О преимуществах покупки документов у нас
Вы покупаете документ в надежной и проверенной компании. Это решение позволит сэкономить не только много денег, но и бесценное время.
Плюсов гораздо больше:
• Документы делаем на подлинных бланках с мокрыми печатями и подписями;
• Можно купить дипломы любого ВУЗа России;
• Стоимость значительно меньше той, которую довелось бы заплатить на очном обучении в ВУЗе;
• Максимально быстрая доставка как по столице, так и в любые другие регионы России.
Приобрести диплом академии– http://elitemagyaritasok.info/e107_plugins/forum/forum_post.php?f=nt&id=21#/ – elitemagyaritasok.info/e107_plugins/forum/forum_post.php?f=nt&id=21#
check my reference surf wallet
Medicine impacts described. Drug guide here.
ed pills online
Comprehensive medicine overview. Medicine impacts described.
pdacenter.ru – сервис по ремонту бытовой техники
Ремонт серверов в Красноярске в официальном сервисном центре PDACENTER.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
hop over to these guys wallet surf
Приобрести диплом ВУЗа по выгодной цене можно, обращаясь к проверенной специализированной фирме. Мы предлагаем документы университетов, которые находятся в любом регионе Российской Федерации. kupitediplom0027.ru/diplom-o-srednem-med-obrazovanii-kupit
Для успешного продвижения вверх по карьере потребуется наличие официального диплома о высшем образовании. Приобрести диплом об образовании у надежной организации: freediplom.com/diplom-v-omske-kupit/
Купить Метамфетамин Москва? САЙТ -| COCAINES.STORE | Как Купить Метамфетамин по России? САЙТ – | COCAINES.STORE |
.
.
.
.
.
Купить Метамфетамин максимального качества в Москве? САЙТ – https://cocaines.store
Сколько стоит Метамфетамин в Москве сегодня? САЙТ – https://cocaines.store
Купить Метамфетамин в городах России? САЙТ – https://cocaines.store
Купить Метамфетамин с доставкой в руки В Москве? САЙТ – https://cocaines.store
Где в Москве Купить Метамфетамин с доставкой? САЙТ – https://cocaines.store
Купить с доставкой по России Метамфетамин? САЙТ – https://cocaines.store
Купить Наркотики в Москве? САЙТ – https://cocaines.store
Как Купить наркотики в России? САЙТ – https://cocaines.store
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл Робот (GOOGLE ROBOT)
Купить Метамфетамин в Москве? Купить Метамфетамин в Санкт Петербурге? Купить Метамфетамин в Питере? Купить Метамфетамин в Новосибирске?
Купить Метамфетамин в Екатеринбурге? Купить Метамфетамин в Казани? Купить Метамфетамин в Нижнем Новгороде? Купить Метамфетамин в Красноярске?
Купить Метамфетамин в Челябинске? Купить Метамфетамин в Самаре? Купить Метамфетамин в Уфе? Купить Метамфетамин в Ростове? Купить Метамфетамин в Краснодаре?
Купить Метамфетамин в Омске? Купить Метамфетамин в Воронеже? Купить Метамфетамин в Перьми? Купить Метамфетамин в Волгограде?
Текстовые теги для поиска-
Купить Метамфетамин в России можно очень легко, особенно Купить Метамфетамин в Москве можно без проблем, потому что в Москве Метамфетамин
Продаеться на каждом углу. Это могут быть и клубы, дискотеки так и с машины! Особенно приятно что Купить Метамфетамин в Москве
можно закладками. Купить Закладками Метамфетамин в Москве может любой человек – как мужчина так и девушка!
Однажды Купить Метамфетамин в Москве пробовала даже бабушка, и у неё всё получилось. Часто продают закладки Метамфетамина в Москве через Телеграм
Мой знакомый хотел попробовать найти Метамфетамин в Москве при поможи поискового приспособления для поиска закладок, но у него не получилось
Потому что Метамфетамин в Москве очень хорошо спрятан, и без точного адреса Метамфетамин в Москве не возможно найти никак.
Колумбийский, Мараканский, Мексиканский – это далеко не полный список стран, которые экспортируют Метамфетамин в Москву для
развлечения людей. Гарантией и безопасностью Метамфетамин в Москве всегда впечетлял, ведь это основное правило покупки!
Российская Федерация всегда славилась своим разнообразием наркотических веществ, и всегда на первом месте был Метамфетамин в Москве!
Маленькие шарики и круглые свертки – это закладки Метамфетамина в Москве, которые бережно и с трепетом разложили от лишних глаз.
Каково было моё удивление, когда привезли с доставкой Метамфетамин в Москве прямо в руки! Это было просто ВАУ-еффект!
Пока кто то сидит дома или ходит на работу – жизнь в Москве с Метамфетамином проходит не заметно, быстро и легко!
Есть магазины, в которых Купить Метамфетамин в Москве можно с гарантией и доставкой в руки круглосуточно!
Всё анонимно и безопастность просто зашкаливает, никогда Метамфетамин в Москве не сможет обнаружить полиция.
Колючий кактус или национальность человека не имеет значения для покупки Метамфетамина в Москве, потому что люди бывают разные
у всех разные потребности и желания. Но магазины готовы предоставить услугу по покупке Метамфетамина в Москве не зависимо от настроения,
Ведь настроение после покупки у всех будет одинаковое – ТОЛЬКО ПОЛНЫЙ ПОЗИТИВ!
Дешевые проститутки Екатеринбурга Индивидуалки Екб ,Индивидуалки Екатеринбурга ,Индивидуалки Екатеринбург ,Проститутки Екб ,Проститутки Екатеринбурга ,Эскорт Екатеринбург ,Дешевые проститутки Екатеринбурга ,Дешевые проститутки Екб ,Шлюхи Екатеринбург ,Путаны Екатеринбург
Прочные и удобные купить зип пакеты в москве опт и розница, разные размеры, надежная застежка. Для хранения, фасовки и перевозки. Оформите заказ онлайн с быстрой доставкой!
Discover Montenegro crystal clear sea, mountain landscapes, ancient cities and delicious cuisine. We organize a turnkey trip: flight, accommodation, excursions.
В Баку найдена одна из трех пропавших без вести несовершеннолетних подруг https://x.com/Fariz418740/status/1904141438733979773
мтс телевидение екатеринбург
https://neuronerecruitment.com.au/employer/24inetrnet-domashnij-ekb-3/
мтс подключение
1вин про 1win6011.ru .
win 1 https://www.1win813.ru .
apple iphone pro max iphone 4 se
mostbet apk скачать http://www.mostbet794.ru .
look what i found zkLogin sui
1win войти https://1win6011.ru .
1win rossvya https://www.1win813.ru .
кайт анапа
mostbet игры http://mostbet794.ru .
Jeśli szukasz sprawdzonych bukmacherów, warto sprawdzić to zestawienie | Strona pomaga wybrać najlepsze zakłady bukmacherskie w Polsce | Klarowne informacje o legalności kasyn online | Kasyna z cashbackiem i kodami bonusowymi – dobre zestawienie | Darmowe spiny bez depozytu – warto zajrzeć | Świetne kasyna z darmową rejestracją i bonusem | Często aktualizowane bonusy i promocje | Kasyna i bukmacherzy dopasowani do polskich graczy | Szybka rejestracja i natychmiastowy bonus powitalny casino vulkan vegas.
pdacenter.ru – сервис по ремонту бытовой техники
Ремонт серверов в Воронеже в официальном сервисном центре PDACENTER.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
1win сайт http://www.pboarders.borda.ru/?1-11-0-00000929-000-0-0-1742818701 .
1 win официальный сайт вход https://1win6011.ru/ .
1win kg http://1win813.ru/ .
мостбет скачать андроид https://shorts.borda.ru/?1-18-0-00000397-000-0-0 .
1вин про https://www.pboarders.borda.ru/?1-11-0-00000929-000-0-0-1742818701 .
мостбет скачать андроид https://www.mostbet794.ru .
мостбет авиатор мостбет авиатор .
Planning a vacation? Sutomore beach is a warm sea, picturesque beaches, cozy cities and affordable prices. A great option for a couple, family or solo traveler.
Holidays in http://www.weather-webcam-in-montenegro.com/tivat are European comfort at an affordable price. Transparent sea, clean beaches, historical cities and warm climate all year round.
Контейнеры и передвижные помещения: проверенное способ для пользовательских проблем
Вагончики и модульные здания организовывают наладить рабочее пространство, кладовую или временную постройку. Мы гарантируем здания, которые подходят профессиональным нормам качества и эргономики.
Параметры
Прочность. Все модули произведены из элементов, устойчивых к нагрузкам и погодным условиям.
Оперативность транспортировки. Конструкция транспортируется в течение 1–2 дней после подтверждения заявки.
Настройка под запросы. Реализуется добавление дополнительной теплоизоляции, электрооборудования или вентиляции.
Сферы использования
На строительных площадках для размещения оборудования или устройства комнаты для рабочих.
Во время акций для создания информационного центра или зоны хранения.
В качестве офисных модулей или пунктов управления.
Достоинства
Ускорение процессов. Не нужно обустраивать временные здания.
Комфортабельность. Среда, которые усиливают производительность труда персонала.
Гибкость. Шанс аренды или покупки под конкретные требования и денежные средства.
Опыт внедрения
Фирма-застройщик задействовала передвижной модуль для создания хранилища и места для рабочих. Конструкция была транспортирована за день, с дополнительным утеплением. Партнёр выделил на улучшение обстановки и минимизацию задержек.
Как начать сотрудничество
Для подачи заявки нужно обратиться с нами. Предоставим подробную информацию, окажем помощь найти правильный путь и обеспечим транспортировку.
Доброго!
Как развить устойчивость к неудачам? Учитесь на своих ошибках, смотрите на них как на возможность для роста и не бойтесь пробовать снова.
Как стать более настойчивым? Не сдавайтесь при первых неудачах, стремитесь к своим целям и постоянно ищите новые пути для их достижения.
Больше информации по ссылке – https://onello.ru/
энциклопедия интересных вещей детское, фильмы интересные боевики, факты о светлячках интересные
самые страшные короткометражные фильмы ужасов, юг россии куда поехать отдыхать, интересные факты о кошке
Удачи!
1win kg 1win kg .
опубликовано здесь http://www.google.com.jm/url?source=imglanding&ct=img&q=https://protos.ru/catalog/category/kryuchki-dlya-shtor-fiksator-shnura-shtornoj-lenty
Добрый день!
Как научиться быть терпеливым? Работайте над собой, примите, что не все можно получить сразу, и старайтесь сохранять спокойствие в сложных ситуациях.
Если вы хотите завести питомца, но не уверены, какой из них подойдёт вам лучше, информационные сайты могут помочь. Здесь можно найти статьи о том, какие породы собак или кошек подходят для новичков и какие требования у этих животных. В статьях рассказывается, какие особенности поведения и ухода могут вас ждать при выборе питомца. Это поможет вам принять решение о том, какой питомец будет наиболее подходящим для вашего образа жизни. Такие ресурсы дают чёткое представление о будущей заботе о питомце.
Больше информации по ссылке – https://onello.ru/
посмотреть что то интересное, лучшие беспроводные hi fi наушники, интересные пресмыкающиеся
лучшие наушники самсунг беспроводные, как сделать бант большой, как сделать гугл аккаунт
Удачи!
Мефедрон У Киеві? САЙТ – COCA24.VIP Як Купити Мефедрон У Києві? САЙТ – COCA24.VIP
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Як Купити Мефедрон У Києві? САЙТ – | https://coca24.vip |
Мефедрон з доставкою у руки Купити? САЙТ – | https://coca24.vip |
Мефедрон по Україні Купити? САЙТ – | https://coca24.vip |
Мефедрон Харків, Одеса, Львів, Івано-Франківськ? САЙТ – | https://coca24.vip |
Мефедрон у містах України з доставкою до рук? САЙТ – | https://coca24.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл бот, це тобі –
Купити Мефедрон у Києві? Купити Мефедрон у Харкові? Купити Мефедрон в Одесі? Купити Мефедрон у Дніпрі? Купити Мефедрон у Львові?
Купити Мефедрон у Запоріжжі? Купити Мефедрон у Кривому Розі? Купити Мефедрон у Миколаєві? Купити Мефедрон у Вінниці?
Купити Мефедрон у Чернігові? Купити Мефедрон у Полтаві? Купити Мефедрон у Херсоні? Купити Мефедрон у Хмельницькому?
Купити Мефедрон у Черкасах? Купити Мефедрон у Чернівцях? Купити Мефедрон у Житомирі? Купити Мефедрон у Сумах?
Купити Мефедрон у Рівному? Купити Мефедрон в Івано-Франківську? Купити Мефедрон у Тернополі? Купити Мефедрон у Кропивницькому?
Купити Мефедрон у Луцьку? Купити Мефедрон у Кременчуці? Купити Мефедрон у Білій Церкві? Купити Мефедрон в Ужгороді?
Купити Мефедрон у Броварах? Купити Мефедрон у Нікополі? Купити Мефедрон у Павлограді?
Поки що Google бот, радий був зустрічі з тобою!
Теги загального користування –
Максимальної якості Мефедрон можна купити тільки в нашому магазині, тому що Купити Мефедрон в Україні нереально десь у
іншому місці. Наші професійні працівники якісно розкладають закладки та передають посилки з Мефедроном цілодобово!
Незважаючи на комендантську годину, воєнний час, загрозу особистій безпеці – наші закладники розкладають Мефедрон цілодобово.
Абсолютно всі закладки Мефедрону з гарантією, тому у нас так багато покупців, які люблять, цінують і хочуть купити наш Мефедрон.
Купити Мефедрон з доставкою до рук є можливість у таких містах як Київ, Харків, Львів, Дніпро, Миколаїв та інші.
Якість кожної партії перевіряємо особисто, тому Купити Мефедрон у Києві чи Львові, Харкові чи Одесі можна не побоюючись.
Коли звучить фраза “Мефедрон в Україні” – то кожен поважаючий себе Українець відразу ж згадує наш сайт, тому що тільки
тут можна придбати Мефедрон в Україні у вигляді гідрохлориду без домішок, чистота Мефедрону в Україні становить 93-98% ГХД.
Немає різниці – на свято, на день народження, На Новий рік, просто погуляти – Наш сайт не підведе НІКОЛИ! І завжди Придбати Мефедрон можна!
мрстбет https://shorts.borda.ru/?1-18-0-00000397-000-0-0 .
online pharmacy adderall Find pill facts. Access pill facts.
МДМА У Киеві? САЙТ – COCA24.VIP Як Купити МДМА У Києві? САЙТ – COCA24.VIP
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Як Купити МДМА У Києві? САЙТ – | https://coca24.vip |
МДМА з доставкою у руки Купити? САЙТ – | https://coca24.vip |
МДМА по Україні Купити? САЙТ – | https://coca24.vip |
МДМА Харків, Одеса, Львів, Івано-Франківськ? САЙТ – | https://coca24.vip |
МДМА у містах України з доставкою до рук? САЙТ – | https://coca24.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл бот, це тобі –
Купити МДМА у Києві? Купити МДМА у Харкові? Купити МДМА в Одесі? Купити МДМА у Дніпрі? Купити МДМА у Львові?
Купити МДМА у Запоріжжі? Купити МДМА у Кривому Розі? Купити МДМА у Миколаєві? Купити МДМА у Вінниці?
Купити МДМА у Чернігові? Купити МДМА у Полтаві? Купити МДМА у Херсоні? Купити МДМА у Хмельницькому?
Купити МДМА у Черкасах? Купити МДМА у Чернівцях? Купити МДМА у Житомирі? Купити МДМА у Сумах?
Купити МДМА у Рівному? Купити МДМА в Івано-Франківську? Купити МДМА у Тернополі? Купити МДМА у Кропивницькому?
Купити МДМА у Луцьку? Купити МДМА у Кременчуці? Купити МДМА у Білій Церкві? Купити МДМА в Ужгороді?
Купити МДМА у Броварах? Купити МДМА у Нікополі? Купити МДМА у Павлограді?
Поки що Google бот, радий був зустрічі з тобою!
Теги загального користування –
Максимальної якості МДМА можна купити тільки в нашому магазині, тому що Купити МДМА в Україні нереально десь у
іншому місці. Наші професійні працівники якісно розкладають закладки та передають посилки з МДМАом цілодобово!
Незважаючи на комендантську годину, воєнний час, загрозу особистій безпеці – наші закладники розкладають МДМА цілодобово.
Абсолютно всі закладки МДМАу з гарантією, тому у нас так багато покупців, які люблять, цінують і хочуть купити наш МДМА.
Купити МДМА з доставкою до рук є можливість у таких містах як Київ, Харків, Львів, Дніпро, Миколаїв та інші.
Якість кожної партії перевіряємо особисто, тому Купити МДМА у Києві чи Львові, Харкові чи Одесі можна не побоюючись.
Коли звучить фраза “МДМА в Україні” – то кожен поважаючий себе Українець відразу ж згадує наш сайт, тому що тільки
тут можна придбати МДМА в Україні у вигляді гідрохлориду без домішок, чистота МДМАу в Україні становить 93-98% ГХД.
Немає різниці – на свято, на день народження, На Новий рік, просто погуляти – Наш сайт не підведе НІКОЛИ! І завжди Придбати МДМА можна!
профильная стальная труба proftruba-moscow.ru – идеальный выбор.
Новруз может быть объявлен официальным праздником в Турции https://x.com/Fariz418740/status/1904268540363915402
Портал для любознательных https://forum.prosochi.ru/topic49087.html Полезная информация, тренды, обзоры, советы и много вдохновляющего контента. Каждый день — повод зайти снова!
us pharmacy online Get medication facts. Find medication information.
Доброго!
Как уменьшить уровень стресса? Используйте техники релаксации, занимайтесь спортом, и не забывайте уделять время для себя и своего хобби.
Как развивать лидерские качества? Один из способов — это активно слушать других и поддерживать их идеи. Работайте над собой, развивая уверенность, способность к принятию решений и мотивацию других. Постепенно укрепляйте свой авторитет, помогая окружающим расти и развиваться.
Больше информации по ссылке – https://tyrtsia.ru
хорошие наушники на андроид беспроводные, кофе раф как сделать, как сделать виски
как сделать 3д анимацию, беспроводные наушники андроид лучшие, сериалы какие стоит посмотреть американские
Удачи!
Приобрести диплом о высшем образовании!
Мы готовы предложить документы университетов, которые расположены в любом регионе Российской Федерации.
asxdiploman.com/mozhno-li-kupit-attestat-za-9-klass-2
Привет всем!
Что делать, если чувствуете себя потерянным в жизни? Попробуйте задуматься о том, что для вас действительно важно, и какие цели вас вдохновляют. Ищите поддержку у друзей, семьи или профессионалов, а также не бойтесь пробовать новые вещи, чтобы найти свой путь.
Как справляться с неудачами? Учитесь видеть их как уроки, которые помогут вам стать сильнее и умнее в будущем.
Больше информации по ссылке – https://asimuthaero.ru/
как сделать паука человека, как сделать гранату самодельную, кари как сделать
при отравлении кота что делать, темляк как сделать, холодный чай как сделать
Удачи!
Купить документ института можно в нашей компании. debrabarsha.com/do-waterbugs-scream/#comment-2323
Здравствуйте!
Купить сигареты Кент 8 – 850 (мрц225) — это отличное решение для тех, кто ищет сбалансированный и мягкий вкус. Сигареты Кент 8 850 дарят насыщенное и гладкое курение без лишней резкости. Купить сигареты Кент 8 – 850 (мрц225) вы можете с доставкой на дом в любой уголок страны. Они идеально подходят для ежедневного курения. Закажите Кент 8 – 850 и наслаждайтесь качественным и приятным курением.
Купить сигареты Кэмел Смоук – 950 (мрц170) — это шанс попробовать более легкие и свежие сигареты с ярким послевкусием. Сигареты Кэмел Смоук 950 подарят вам мягкость и легкость при курении. Вы можете заказать сигареты Кэмел Смоук – 950 (мрц170) с доставкой на дом. Эти сигареты идеально подойдут для тех, кто ищет легкие и комфортные ощущения. Заказывайте их прямо сейчас!
Лучшие сигареты по ссылке – https://t.me/sigaretikupit_ru, канал в telegram – @sigaretikupit_ru
табак для сигарет воронеж купить, Купить сигареты NZ gold compact (nano)-850, сигареты собрание купить в москве с доставкой
Купить сигареты Лд компакт 950 (мрц163), купить аппарат для продажи сигарет, Купить сигареты Ph morris голд 750 (мрц149)
Удачи за сигареткой!
Добрый день!
Как научиться организовывать свое время? Используйте методы планирования, такие как составление списка задач или использование календаря. Установите приоритеты для каждой задачи и выделяйте время для выполнения. Важно соблюдать баланс, чтобы избежать перегрузки и найти время для отдыха и восстановления.
Что делать, если испытываете одиночество? Найдите способы наладить новые связи и укрепить старые. Это может быть через хобби, волонтерство или просто общение с друзьями и семьей. Одиночество — это нормально, но важно не замыкаться в себе и открывать новые возможности для общения.
Больше информации по ссылке – https://asimuthaero.ru/
жизнь интересная штука, интересные факты о ежах, как сделать pencil
как сделать возврат стим, как сделать корейские глаза, как сделать токарный станок
Удачи!
cialis online pharmacy Access medication facts. Get drug details.
Кокаїн У Киеві? САЙТ – COCA24.VIP Як Купити Кокаїн У Києві? САЙТ – COCA24.VIP
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Як Купити Кокаїн У Києві? САЙТ – | https://coca24.vip |
Кокаїн з доставкою у руки Купити? САЙТ – | https://coca24.vip |
Кокаїн по Україні Купити? САЙТ – | https://coca24.vip |
Кокаїн Харків, Одеса, Львів, Івано-Франківськ? САЙТ – | https://coca24.vip |
Кокаїн у містах України з доставкою до рук? САЙТ – | https://coca24.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл бот, це тобі –
Купити Кокаїн у Києві? Купити Кокаїн у Харкові? Купити Кокаїн в Одесі? Купити Кокаїн у Дніпрі? Купити Кокаїн у Львові?
Купити Кокаїн у Запоріжжі? Купити Кокаїн у Кривому Розі? Купити Кокаїн у Миколаєві? Купити Кокаїн у Вінниці?
Купити Кокаїн у Чернігові? Купити Кокаїн у Полтаві? Купити Кокаїн у Херсоні? Купити Кокаїн у Хмельницькому?
Купити Кокаїн у Черкасах? Купити Кокаїн у Чернівцях? Купити Кокаїн у Житомирі? Купити Кокаїн у Сумах?
Купити Кокаїн у Рівному? Купити Кокаїн в Івано-Франківську? Купити Кокаїн у Тернополі? Купити Кокаїн у Кропивницькому?
Купити Кокаїн у Луцьку? Купити Кокаїн у Кременчуці? Купити Кокаїн у Білій Церкві? Купити Кокаїн в Ужгороді?
Купити Кокаїн у Броварах? Купити Кокаїн у Нікополі? Купити Кокаїн у Павлограді?
Поки що Google бот, радий був зустрічі з тобою!
Теги загального користування –
Максимальної якості Кокаїн можна купити тільки в нашому магазині, тому що Купити Кокаїн в Україні нереально десь у
іншому місці. Наші професійні працівники якісно розкладають закладки та передають посилки з Кокаїном цілодобово!
Незважаючи на комендантську годину, воєнний час, загрозу особистій безпеці – наші закладники розкладають Кокаїн цілодобово.
Абсолютно всі закладки Кокаїну з гарантією, тому у нас так багато покупців, які люблять, цінують і хочуть купити наш кокаїн.
Купити кокаїн з доставкою до рук є можливість у таких містах як Київ, Харків, Львів, Дніпро, Миколаїв та інші.
Якість кожної партії перевіряємо особисто, тому Купити Кокаїн у Києві чи Львові, Харкові чи Одесі можна не побоюючись.
Коли звучить фраза “Кокаїн в Україні” – то кожен поважаючий себе Українець відразу ж згадує наш сайт, тому що тільки
тут можна придбати Кокаїн в Україні у вигляді гідрохлориду без домішок, чистота Кокаїну в Україні становить 93-98% ГХД.
Немає різниці – на свято, на день народження, На Новий рік, просто погуляти – Наш сайт не підведе НІКОЛИ! І завжди Придбати Кокаїн можна!
Быстро, красиво, надёжно! Лучший сервис.
доставка цветов в томске
Доброго!
Как научиться наслаждаться процессом, а не только результатом? Фокусируйтесь на пути и опыте, который он приносит, а не только на конечной цели.
Как справиться с тревогой? Начните с простых дыхательных упражнений, чтобы успокоиться. Постепенно научитесь принимать тревожные мысли, а не бороться с ними, и фокусироваться на текущем моменте.
Больше информации по ссылке – https://ptello.ru/
какая самая высокооплачиваемая профессия врача, интересные факты про динозавров, кекс как сделать
наушники jbl топ лучших беспроводные, интересные фильмы военные русские, лучший микрофон в беспроводных наушниках
Удачи!
посмотреть в этом разделе https://www.google.jo/url?q=https://travelanswer.ru/questions/kak-sdelaty-foto-na-vizu-v-italiyu-v-krasnodare-esty-li-usluga-v-vizovom-tsentre.html
аккаунты маркетплейсов купить магазин продажи аккаунтов
где продать быстро аккаунты продажа аккаунтов
Гашиш У Киеві? САЙТ – COCA24.VIP Як Купити Гашиш У Києві? САЙТ – COCA24.VIP
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Як Купити Гашиш У Києві? САЙТ – | https://coca24.vip |
Гашиш з доставкою у руки Купити? САЙТ – | https://coca24.vip |
Гашиш по Україні Купити? САЙТ – | https://coca24.vip |
Гашиш Харків, Одеса, Львів, Івано-Франківськ? САЙТ – | https://coca24.vip |
Гашиш у містах України з доставкою до рук? САЙТ – | https://coca24.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл бот, це тобі –
Купити Гашиш у Києві? Купити Гашиш у Харкові? Купити Гашиш в Одесі? Купити Гашиш у Дніпрі? Купити Гашиш у Львові?
Купити Гашиш у Запоріжжі? Купити Гашиш у Кривому Розі? Купити Гашиш у Миколаєві? Купити Гашиш у Вінниці?
Купити Гашиш у Чернігові? Купити Гашиш у Полтаві? Купити Гашиш у Херсоні? Купити Гашиш у Хмельницькому?
Купити Гашиш у Черкасах? Купити Гашиш у Чернівцях? Купити Гашиш у Житомирі? Купити Гашиш у Сумах?
Купити Гашиш у Рівному? Купити Гашиш в Івано-Франківську? Купити Гашиш у Тернополі? Купити Гашиш у Кропивницькому?
Купити Гашиш у Луцьку? Купити Гашиш у Кременчуці? Купити Гашиш у Білій Церкві? Купити Гашиш в Ужгороді?
Купити Гашиш у Броварах? Купити Гашиш у Нікополі? Купити Гашиш у Павлограді?
Поки що Google бот, радий був зустрічі з тобою!
Теги загального користування –
Максимальної якості Гашиш можна купити тільки в нашому магазині, тому що Купити Гашиш в Україні нереально десь у
іншому місці. Наші професійні працівники якісно розкладають закладки та передають посилки з Гашиш ом цілодобово!
Незважаючи на комендантську годину, воєнний час, загрозу особистій безпеці – наші закладники розкладають Гашиш цілодобово.
Абсолютно всі закладки Гашиш у з гарантією, тому у нас так багато покупців, які люблять, цінують і хочуть купити наш Гашиш .
Купити Гашиш з доставкою до рук є можливість у таких містах як Київ, Харків, Львів, Дніпро, Миколаїв та інші.
Якість кожної партії перевіряємо особисто, тому Купити Гашиш у Києві чи Львові, Харкові чи Одесі можна не побоюючись.
Коли звучить фраза “Гашиш в Україні” – то кожен поважаючий себе Українець відразу ж згадує наш сайт, тому що тільки
тут можна придбати Гашиш в Україні у вигляді гідрохлориду без домішок, чистота Гашиш у в Україні становить 93-98% ГХД.
Немає різниці – на свято, на день народження, На Новий рік, просто погуляти – Наш сайт не підведе НІКОЛИ! І завжди Придбати Гашиш можна!
https://imgtube.ru/
Здравствуйте!
Как справляться с чувством вины? Признайте свои ошибки, но не зацикливайтесь на них. Важно извлечь уроки из ситуации и использовать это знание для личностного роста. Простите себя, осознавая, что все люди ошибаются, и мы растем на своих ошибках.
Как быть более внимательным к себе? Развивайте осознанность, слушайте свои потребности и чувства. Регулярно уделяйте время себе: отдыхайте, медитируйте и заботьтесь о своем теле и разуме.
Больше информации по ссылке – https://azimuhtaero.ru/
интересная дорама, посмотреть самый страшный фильм ужасов, как сделать отстрелы выхлопа
как сделать безе, как сделать маяк майнкрафт, как сделать дома маникюр
Удачи!
best mexican online pharmacy Pill facts provided. Comprehensive pill resource.
Здравствуйте!
На информационных сайтах также можно найти полезную информацию о гаджетах для путешественников. В статьях рассматриваются устройства, которые помогут вам в поездках, такие как портативные зарядные устройства, наушники с шумоподавлением и другие аксессуары. Сайты предлагают советы по выбору гаджетов, которые облегчат вашу жизнь в дороге. Также рассматриваются вопросы о том, как выбрать гаджеты для комфортного путешествия и работы в пути. Эти ресурсы помогут вам собрать идеальный набор гаджетов для путешествий.
Как повысить свою продуктивность? Для этого необходимо установить четкие приоритеты и научиться работать с фокусом. Использование методов управления временем, таких как Pomodoro, может значительно повысить вашу эффективность.
Больше информации по ссылке – https://asimutaero.ru/
отравление при беременности что делать, стоит ли покупать китайские автомобили, как сделать сердце моря
бледная поганка интересные факты, как сделать проверку уравнения, как сделать октаэдр
Удачи!
Exponent Finance is redefining DeFi lending by providing secure, transparent, and high-yield investment solutions. Through smart contract-powered lending pools, Exponent Finance DeFi platform allows users to borrow and lend crypto assets with optimal efficiency and minimal risk. Whether you’re looking to earn passive income through staking or access instant liquidity, Exponent Finance offers a decentralized, non-custodial, and user-friendly solution to meet all your financial goals in the crypto ecosystem. https://exponent.ink
Привет всем!
Как научиться быть добрым к себе? Относитесь к себе с любовью, принимайте свои слабости и не критикуйте себя слишком сильно.
Что делать, если чувствуете себя потерянным в жизни? Попробуйте задуматься о том, что для вас действительно важно, и какие цели вас вдохновляют. Ищите поддержку у друзей, семьи или профессионалов, а также не бойтесь пробовать новые вещи, чтобы найти свой путь.
Больше информации по ссылке – https://ptello.ru
вирусы интересные, как сделать брауни, как сделать объем волос
майнкрафт как сделать наблюдатель, как сделать сердце моря, как сделать жареные роллы
Удачи!
Здравствуйте!
Как бороться с ленью? Разделите задачи на маленькие шаги и начните с самого простого. Установите мотивирующие цели и не забывайте отдыхать, чтобы не перегореть.
Как научиться видеть возможности вместо препятствий? Смените взгляд на проблемы, воспринимайте их как шанс для роста и развития.
Больше информации по ссылке – https://tyrtsia.ru
как сделать багги, интересные факты про кремль, интересные факты про мкс
как сделать сухарики дома, наушники лучшие беспроводные 2020, как сделать в3 расу
Удачи!
Cytonic is revolutionizing blockchain security with advanced cybersecurity solutions tailored for Web3 applications. By integrating decentralized encryption, AI-powered threat detection, and smart contract auditing, Cytonic ensures maximum protection against cyber threats. Whether you’re securing DeFi protocols, NFTs, or enterprise blockchain systems, Cytonic’s cutting-edge security technology provides the highest level of data integrity and protection. https://cytonic.cc
Пионы — это всегда стильно и элегантно!
пионы купить в Томске
pdacenter.ru – сервис по ремонту бытовой техники
Ремонт электронных книг в Воронеже в официальном сервисном центре PDACENTER.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
Elara Finance is transforming decentralized lending by offering secure, transparent, and flexible crypto loan solutions. Built on blockchain technology, Elara Finance enables users to borrow and lend digital assets seamlessly without intermediaries. With low-interest rates, automated smart contracts, and a permissionless DeFi environment, Elara Finance is making crypto lending accessible and profitable for investors worldwide. https://elara.ink
Привет всем!
Как научиться слушать свои эмоции? Дайте себе время на анализ своих чувств, позволяя себе осознавать их и реагировать соответствующим образом.
Как стать лучшим слушателем? Слушайте без прерываний, старайтесь понять чувства и мысли собеседника и показывайте интерес к тому, что он говорит.
Больше информации по ссылке – https://akhobeda.ru
факты о португалии интересные, нигер интересные факты, куда поехать в россию летом
какие наушники беспроводные вкладыши лучше, как сделать ровную звезду, ресторан интересный в москве
Удачи!
1вин сайт https://boardwars.forum24.ru/?1-10-0-00000406-000-0-0/ .
Flaunch is the leading blockchain gaming launchpad, designed to help game developers and investors thrive in the Web3 gaming ecosystem. By offering secure token launches, NFT integrations, and decentralized crowdfunding, Flaunch enables game creators to fund, develop, and scale their projects with full transparency and community-driven support. Whether you’re a developer or an investor, Flaunch provides the tools to connect and grow in the blockchain gaming space. https://flaunch.tech
1вин кг 1вин кг .
1 вин вход в личный кабинет http://1win6012.ru .
Доброго!
Как стать уверенным в себе? Работайте над самооценкой, берите на себя ответственность за свои решения и действия. Практикуйте положительные аффирмации и действуйте, даже когда чувствуете неуверенность.
Если вы хотите улучшить качество интимной жизни, информационные сайты помогут вам найти идеальные гаджеты. В статьях рассматриваются устройства для повышения либидо, улучшения эрекции и контроля стресса. Сайты предлагают советы по выбору гаджетов для улучшения настроения и гармонизации отношений. Эти ресурсы помогут вам поддерживать страсть в отношениях и заботиться о своем здоровье.
Больше информации по ссылке – https://asimutaero.ru/
как сделать огромный член, как сделать истихар намаз, как сделать торт легко
как сделать куриный фарш, правильный додекаэдр как сделать, что почитать интересного рейтинг
Удачи!
mostbet chrono https://tagilshops.forum24.ru/?1-4-0-00000205-000-0-0/ .
1win казино http://www.boardwars.forum24.ru/?1-10-0-00000406-000-0-0 .
mosbet mostbet795.ru/ .
Привет всем!
Купить сигареты Парламент 950 (мрц190) — это выбор для тех, кто предпочитает элитные сигареты с уникальным вкусом. Сигареты Парламент 950 подарят вам глубокие и насыщенные ощущения при курении. Заказывайте сигареты Парламент 950 (мрц190) с доставкой на дом. Эти сигареты идеально подходят для ценителей высококачественного табака. Сделайте покупку прямо сейчас!
Купить сигареты Кэмл красный – 850 (мрц150) — это выбор для ценителей крепких и насыщенных вкусов. Сигареты Кэмл красный 850 подарят вам яркое курение с выраженным ароматом. Вы можете заказать сигареты Кэмл красный – 850 (мрц150) с доставкой прямо на дом. Эти сигареты станут отличным выбором для тех, кто ищет что-то более насыщенное. Заказывайте прямо сейчас!
Лучшие сигареты по ссылке – https://t.me/sigaretikupit_ru/, канал в telegram – @sigaretikupit_ru
сигареты калипсо белоруссия купить, Купить сигареты Давидофф кнопка 1150 (мрц165), электронная сигарета в йошкар оле купить
Купить сигареты Крэдо оригинал – 700, купить развесной табак для сигарет в нижнем новгороде, Купить сигареты Dove QS чёрный дубль – 750
Удачи за сигареткой!
Лучшее решение проблемы с просрочками – это процедура банкротства по 127 закону-ФЗ процедура банкротства физического лица отзывы .
Здравствуйте!
Для многих людей, купить диплом университета – это острая потребность, уникальный шанс получить выгодную работу. Но для кого-то – это понятное желание не терять время на учебу в ВУЗе. Что бы ни толкнуло вас на такой шаг, мы готовы помочь вам. Оперативно, качественно и по разумной цене изготовим документ любого года выпуска на подлинных бланках с реальными подписями и печатями.
Основная причина, почему многие прибегают к покупке документов, – желание занять хорошую работу. Например, знания дают возможность кандидату устроиться на желаемую работу, но подтверждения квалификации не имеется. Когда работодателю важно наличие “корочек”, риск потерять хорошее место очень высокий.
Заказать документ о получении высшего образования вы сможете у нас в Москве. Мы предлагаем документы об окончании любых университетов РФ. Вы получите диплом по любой специальности, любого года выпуска, в том числе документы СССР. Гарантируем, что при проверке документа работодателями, каких-либо подозрений не появится.
Ситуаций, которые вынуждают купить диплом о среднем образовании достаточно. Кому-то срочно требуется работа, а значит, нужно произвести впечатление на начальника при собеседовании. Некоторые планируют попасть в серьезную компанию, для того, чтобы повысить свой статус в социуме и в последующем начать собственное дело. Чтобы не терять массу времени, а сразу начать удачную карьеру, применяя имеющиеся навыки, можно купить диплом в онлайне. Вы станете полезным для общества, получите денежную стабильность в минимальные сроки- купить аттестат
1 ван вин https://1win814.ru .
один вин официальный сайт http://1win6012.ru/ .
скачать mostbet на телефон http://tagilshops.forum24.ru/?1-4-0-00000205-000-0-0 .
Привет!
Мы предлагаем дипломы любой профессии по приятным ценам. Стоимость будет зависеть от той или иной специальности, года выпуска и университета: rdiploma24.com/
мостбет скачать бесплатно http://mostbet795.ru// .
Привет всем!
Как научиться не бояться изменений? Примите изменения как неизбежную часть жизни, научитесь адаптироваться и смотрите на них как на новые возможности.
Какие увлечения могут помочь вам справиться с депрессией? Творческая деятельность, такая как рисование, письмо или музыка, может быть отличным способом выразить эмоции. Спорт и физическая активность помогают не только улучшить физическое состояние, но и выработать эндорфины, что способствует улучшению настроения.
Больше информации по ссылке – https://akhobeda.ru/
сундук террария как сделать, интересные факты лисы, как пишется сделала
оранжевый цвет как сделать, как сделать вишневый конфитюр, интересные факты об чехове
Удачи!
1вин кыргызстан http://boardwars.forum24.ru/?1-10-0-00000406-000-0-0/ .
Заказать диплом университета !
Покупка диплома любого университета РФ у нас – надежный процесс, поскольку документ будет заноситься в государственный реестр. При этом печать производится на официальных бланках ГОЗНАКа. Купить диплом об образовании arenadiplom24.online/vuzy/rostovskogo-filiala-bukep
1вин официальный http://1win6012.ru/ .
1 вин скачать https://1win814.ru .
мостбет кыргызстан https://www.tagilshops.forum24.ru/?1-4-0-00000205-000-0-0 .
mostbet скачать на телефон бесплатно андроид mostbet скачать на телефон бесплатно андроид .
Mostbet umożliwia szybkie i bezpieczne transakcje finansowe. | Bezpieczeństwo i prywatność są priorytetem w Mostbet. | Korzystaj z programu lojalnościowego Mostbet i zbieraj punkty. | Mostbet zapewnia szybkie wypłaty wygranych na Twoje konto. mostbet bonus
online pharmacy india Drug leaflet available. Medication trends described.
Добрый день!
Как повысить самооценку? Работайте над собой, ставьте реалистичные цели и отмечайте свои успехи. Уверенность в себе приходит с опытом, и каждый шаг вперед укрепляет веру в свои силы.
Как развить самоконтроль? Работайте над собой, ставьте цели и учитесь отслеживать прогресс. Не бойтесь принимать трудные решения и следовать своим планам.
Больше информации по ссылке – https://azimuhtaero.ru/
как дома сделать, маяк майнкрафт как сделать, наушники для самсунга беспроводные лучшие
беспроводные наушники лучшие с алиэкспресс, интересные факты о мхах, мясной букет как сделать
Удачи!
Hello new зеркало официальный kraken
Приобрести диплом ВУЗа по доступной стоимости возможно, обращаясь к проверенной специализированной компании. Мы предлагаем документы об окончании любых университетов Российской Федерации. Заказать диплом о высшем образовании– diploma-groups24.ru/kupit-diplom-v-gorode/nefteyugansk.html
DEQ Finance is revolutionizing decentralized trading by offering a seamless, secure, and efficient crypto exchange experience. Built with cutting-edge blockchain technology, DEQ Finance provides traders with fast transaction speeds, deep liquidity, and a transparent trading environment. Whether you’re a beginner or a professional trader, DEQ Finance delivers high-performance DeFi solutions tailored to modern trading needs. https://deq.li
продать аккаунты моментально маркетплейс цифровых аккаунтов
риобет официальный сайт зеркало зеркало риобет
Здравствуйте!
Как развить уверенность в принятии решений? Начинайте с малого, доверяйте своей интуиции и не бойтесь делать выбор.
Если вы хотите следить за своим здоровьем с помощью технологий, информационные сайты предложат массу полезных рекомендаций. В статьях рассматриваются гаджеты для измерения артериального давления, уровня сахара в крови и других показателей здоровья. Сайты помогают выбрать устройства для домашнего использования, которые помогут вам контролировать свое состояние. Также рассматриваются лучшие устройства для мониторинга физической активности и похудения. Эти ресурсы помогут вам выбрать гаджеты, которые заботятся о вашем здоровье.
Больше информации по ссылке – https://piano-quartet.ru
что вреднее кофе или энергетики, интересные факты ярославль, боровичи самое интересное
какие игры интересные, как сделать грядки высокие, как сделать буквы заглавными
Удачи!
Exponent Finance is redefining DeFi lending by providing secure, transparent, and high-yield investment solutions. Through smart contract-powered lending pools, Exponent Finance DeFi platform allows users to borrow and lend crypto assets with optimal efficiency and minimal risk. Whether you’re looking to earn passive income through staking or access instant liquidity, Exponent Finance offers a decentralized, non-custodial, and user-friendly solution to meet all your financial goals in the crypto ecosystem. https://exponent.ink
Привет всем!
Информационные сайты помогут вам найти гаджеты для улучшения общего самочувствия и поддержания здоровья. В статьях рассматриваются устройства для мониторинга физических показателей, такие как пульсометры и тонометры, а также гаджеты для контроля за уровнем сахара и холестерина. Сайты дают советы по выбору гаджетов для поддержания хорошей физической формы и борьбы с заболеваниями. Эти ресурсы помогут вам выбрать устройства для эффективного ухода за своим здоровьем.
Как научиться управлять своим временем? Организуйте свои дела, расставляйте приоритеты и будьте дисциплинированы в использовании времени.
Больше информации по ссылке – https://piano-quartet.ru/
тюлень факты интересные, чем полезен антицеллюлитный массаж, лучшие книги о финансах
как сделать табурет, как дома сделать пизду, фильм интересный страшный
Удачи!
online pharmacy hydrocodone Active ingredients listed. Active ingredients listed.
DEQ Finance is revolutionizing decentralized trading by offering a seamless, secure, and efficient crypto exchange experience. Built with cutting-edge blockchain technology, DEQ Finance provides traders with fast transaction speeds, deep liquidity, and a transparent trading environment. Whether you’re a beginner or a professional trader, DEQ Finance delivers high-performance DeFi solutions tailored to modern trading needs. https://deq.li
Flaunch is the leading blockchain gaming launchpad, designed to help game developers and investors thrive in the Web3 gaming ecosystem. By offering secure token launches, NFT integrations, and decentralized crowdfunding, Flaunch enables game creators to fund, develop, and scale their projects with full transparency and community-driven support. Whether you’re a developer or an investor, Flaunch provides the tools to connect and grow in the blockchain gaming space. https://flaunch.tech
Odwiedzilem serwis artikla i zaluje! Gry bez przerwy nie dzialaja, nawigacja jest niesamowicie nieczytelny, a reklamy atakuja z kazdej strony. Wrazenie, jak gdyby projekt byla nieaktualizowana od lat – calkowity brak kontaktow z administracja, bugi na kazdej stronie, a otwieranie stron jest koszmarnie wolne. Przydatnych materialow praktycznie brak, a dawno nie byly aktualizowane. Jesli zalezy Ci na bez problemow cieszyc sie rozgrywka, poszukaj lepsze strony – tu nie znajdziesz nic wartosciowego!
Ремонт помещений ремонт офисов под ключ: офисы, отели, склады. Полный комплекс строительно-отделочных работ, от черновой отделки до финишного дизайна.
Elevate your auto’s functionality and style with our remarkable tuning assistance. Discover the real capacity of your auto through customized enhancements. Our crew of technicians provides unmatched caliber and creativity. Discover our online presence https://precisionautobodyfrederick.com/ to uncover additional, and commence your quest towards auto superiority today.
Здравствуйте!
Независимо от того, какие наушники вы выберете, беспроводные наушники лучшие обеспечат вам комфорт и отличное качество звука. Наушники беспроводные лучшие подойдут для тех, кто ценит свободу и удобство. Важно помнить, что лучшие беспроводные наушники — это те, которые отлично подходят для ваших нужд. Выбор будет зависеть от вашего бюджета и предпочтений.
Как научиться работать с негативом? Признавайте свои негативные эмоции, но не позволяйте им управлять вами, ищите способы их трансформировать в мотивацию.
Больше информации по ссылке – https://alcogolizmstop.ru/
беспроводные наушники лучшие до 4000, интересные вопросы для диалога, как бицепс сделать шире
как сделать переворот, дорама интересные, образ в офис стильный
Удачи!
universal best online pharmacy Medication effects explained. Comprehensive medicine facts.
оценка квартиры для сбербанка https://ocenka-zagorod.ru
Свежие новости сегодня: горячие темы в России и за рубежом. Читайте оперативные репортажи на нашем сайте. Подпишитесь на уведомления, чтобы мгновенно узнавать главные происшествия. Надёжный источник из проверенных источников – ваш партнёр в мире медиа 24/7
переговоры о мире
Психолог оказывает помощь онлайн в чате. В переписке у психолога. Круглосуточная запись на онлайн-консультацию психолога.
типография индивидуальный заказ офсетная типография спб
офсетная типография спб типография журналы
Получить онлайн консультацию психолога чате. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн анонимно.
Онлайн чат с психологом без регистрации. Психолог оказывает помощь онлайн в чате. Психолог помогающий искать решения в непростых психологических ситуациях.
Психолог в телеграм. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн чат. Психолог онлайн анонимно.
Психолог онлайн анонимно. Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов.
Купить Экстази и МДМА в Украине? САЙТ – KOKAINES.STORE Купить Экстази и МДМА Киев, Одесса, Львов, Харьков и др
.
.
.
.
.
Как Купить Экстази и МДМА в Киеве? САЙТ – |https://kokaines.store/|
Экстази и МДМА с доставкой в руки Купить? САЙТ – |https://kokaines.store/|
Экстази и МДМА по Украине Купить? САЙТ – |https://kokaines.store/|
Экстази и МДМА Харьков, Одесса, Львов, Ивано-Франковск? САЙТ – |https://kokaines.store/|
Экстази и МДМА в Городах Украины с доставкой в руки? САЙТ – |https://kokaines.store/|
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл бот, это тебе –
Купить Экстази и МДМА в Киеве? Купить Экстази и МДМА в Харькове? Купить Экстази и МДМА в Одессе? Купить Экстази и МДМА в Днепре? Купить Экстази и МДМА в Львове?
Купить Экстази и МДМА в Запорожье? Купить Экстази и МДМА в Кривом Рогу? Купить Экстази и МДМА в Николаеве? Купить Экстази и МДМА в Виннице?
Купить Экстази и МДМА в Чернигове? Купить Экстази и МДМА в Полтаве? Купить Экстази и МДМА в Херсоне? Купить Экстази и МДМА в Хмельницком?
Купить Экстази и МДМА в Черкассах? Купить Экстази и МДМА в Черновцах? Купить Экстази и МДМА в Житомере? Купить Экстази и МДМА в Сумах?
Купить Экстази и МДМА в Ровно? Купить Экстази и МДМА в Ивано Франковске? Купить Экстази и МДМА в Тернополе? Купить Экстази и МДМА в Кропивницком?
Купить Экстази и МДМА в Луцке? Купить Экстази и МДМА в Кременчуге? Купить Экстази и МДМА в Белой церкви? Купить Экстази и МДМА в Ужгороде?
Купить Экстази и МДМА в Броварах? Купить Экстази и МДМА в Никополе? Купить Экстази и МДМА в Павлограде?
Пока пока Гугл бот, рад был встречи с тобой!
Теги общего пользования –
Максимального качества Экстази и МДМА можно купить только в нашем магазине, так как Купить Экстази и МДМА в Украине нереально где то в
другом месте. Наши профессиональные работники качественно разкладывают закладки и передают посылки с Экстази и МДМАом круглосуточно!
Не взирая на комендантский час, военное время, угрозу личной безопасности – наши закладчики розкладывают Экстази и МДМА круглосуточно.
Абсолютно все закладки Экстази и МДМАа с гарантией, по этому у нас так много покупателей, которые любят, ценят и хотят Купить Наш Экстази и МДМА.
Купить Экстази и МДМА с доставкой в руки есть возможность в таких городах как Киев, Харьков, Львов, Днепр, Николаев и другие.
Качество каждой партии проверяем лично, по этому Купить Экстази и МДМА в Киеве или Львове, Харькове или Одессе можно не опасаясь.
Когда звучит фраза “Купить Экстази и МДМА в Украине” – то каждый уважающий себя Украинец сразу же вспоминает наш сайт, потому что только
тут можно Купить Экстази и МДМА в Украине в виде гидрохлорида без примесей, чистота Экстази и МДМАа в украине составляет 93-98% ГХД.
Нет разницы – на праздник, на день рожденье, На Новый год, просто погулять – Наш сайт не подведет НИКОГДА! И всегда Купить Экстази и МДМА можно!
Психолог оказывает помощь онлайн в чате. Психологическая и информационная онлайн-помощь. В переписке у психолога.
Non-Gamstop casinos give me total gaming freedom—awesome!
non gamstop paypal casinos
Онлайн чат с психологом без регистрации. Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате.
Чат с психологом в телеге. Психолог онлайн анонимно. Онлайн чат с психологом без регистрации.
Психолог онлайн чат. Психолог помогающий искать решения в непростых психологических ситуациях. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Получить онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях. Чат психологической поддержки.
Анонимный чат с психологом телеграм. В переписке у психолога. Чат психологической поддержки.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Получить онлайн консультацию психолога чате.
Телеграм психолог. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
1win скачать последнюю версию 1win скачать последнюю версию .
зайти в 1вин http://1win6014.ru .
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь.
Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. Получите консультацию онлайн-психолога в чате прямо сейчас.
мостбет кыргызстан скачать kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0-1742814422 .
1wiun http://yamama.forum24.ru/?1-11-0-00000459-000-0-0-1742818616 .
мостбет зеркало мостбет зеркало .
Получить первую онлайн консультацию психолога чате. Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь.
Онлайн чат с психологом без регистрации. Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате.
Психолог онлайн анонимно. Психолог t me. Психолог онлайн чат.
Психолог оказывает помощь онлайн в чате. Дипломированный психолог с опытом работы и отзывами клиентов. Получить онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Анонимный чат с психологом телеграм.
reputable best online pharmacy Patient medicine resource. Comprehensive pill overview.
Чат психологической поддержки. Анонимный чат с психологом телеграм. Психолог онлайн анонимно.
Школьные парты и стулья для школы
Столы круглые для школы
Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога. Психолог t me.
Психолог в телеграм. Психолог t me. В переписке у психолога.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог t me. В переписке у психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате. Психолог помогающий искать решения в непростых психологических ситуациях.
Помощь психолога онлайн. Круглосуточная запись на онлайн-консультацию психолога. Психологическая и информационная онлайн-помощь.
Помощь психолога онлайн. Дипломированный психолог с опытом работы и отзывами клиентов. Анонимный чат с психологом телеграм.
Помощь психолога онлайн. Психолог в телеграм. Дипломированный психолог с опытом работы и отзывами клиентов.
Круглосуточная запись на онлайн-консультацию психолога. Получить онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате.
New Retro Casino http://www.newretromirror.ru/ .
Получить первую онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь. Психолог онлайн чат.
Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге.
скачать 1win с официального сайта https://www.1win815.ru .
Телеграм психолог. Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате.
1win ставки официальный сайт https://1win6014.ru .
мостбет скачать бесплатно мостбет скачать бесплатно .
сайт 1win сайт 1win .
мотбет http://mostbet6001.ru/ .
Психолог t me. Психолог оказывает помощь онлайн в чате. Психолог в телеграм.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Дипломированный психолог с опытом работы и отзывами клиентов. Получить первую онлайн консультацию психолога чате.
нью ретро казино, голд казино, изи кэш казино
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. В переписке у психолога.
Строительные бытовки
Бытовки и модульные здания: надёжное решение для пользовательских проблем
Контейнеры и временные конструкции организовывают оборудовать место для деятельности, склад или временный домик. Наши специалисты обеспечиваем объекты, которые подходят профессиональным нормам качества и комфорта.
Характеристики
Устойчивость. Данные вагончики созданы из ресурсов, устойчивых к воздействию и природным явлениям.
Оперативность транспортировки. Модуль доставляется в пределах 1–2 суток после заключения соглашения.
Персонализация. Реализуется монтаж термоизоляции, электрооборудования или системы проветривания.
Сферы использования
На объектах строительства для организации склада или организации комнаты отдыха.
Во время акций для организации стойки приёма или хранилища техники.
В качестве временно используемых помещений или пунктов управления.
Плюсы
Сокращение сроков. Отпадает потребность обустраивать временные здания.
Комфорт. Среда, которые улучшают производительность труда персонала.
Подстройка. Возможность аренды или приобретения под разные периоды и финансовые возможности.
Случай применения
Фирма-застройщик применила блок-контейнер для организации склада инструментов и места для рабочих. Конструкция была доставлена на место за день, с усиленной теплоизоляцией. Партнёр отметил на повышение комфорта и минимизацию задержек.
Как связаться с нами
Для начала сотрудничества нужно позвонить с нами. Дадим полные данные, окажем помощь найти лучший вариант и организуем перевозку.
В переписке у психолога. Телеграм психолог. Психолог онлайн анонимно.
Психолог оказывает помощь онлайн в чате. Телеграм психолог. Чат психологической поддержки.
грузоперевозки услуги грузчиков https://priozersk.standart-express.ru
либет казино
нанять грузчиков недорого грузчики недорого
помощь при переезде грузчики услуги грузчиков перевозки
Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн чат с психологом без регистрации. Психолог t me.
Психолог t me. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Чат психологической поддержки. В переписке у психолога. Помощь психолога онлайн.
Получить онлайн консультацию психолога чате. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог оказывает помощь онлайн в чате. Помощь психолога онлайн. Психолог онлайн чат.
Круглосуточная запись на онлайн-консультацию психолога. Онлайн-консультация психолога. Психолог оказывает помощь онлайн в чате.
Получить онлайн консультацию психолога чате. Чат с психологом в телеге. Получить онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Круглосуточная запись на онлайн-консультацию психолога. Психолог в телеграм.
Дипломированный психолог с опытом работы и отзывами клиентов. Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Помощь психолога онлайн. Онлайн чат с психологом без регистрации.
Помощь психолога онлайн. Психолог онлайн чат. Психолог оказывает помощь онлайн в чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн чат с психологом без регистрации. Получить онлайн консультацию психолога чате.
Психолог в телеграм. Чат психологической поддержки. Получить первую онлайн консультацию психолога чате.
Non Gamstop casinos make gaming so much more fun—can’t get enough!
top non gamstop casino
Психолог онлайн анонимно. В переписке у психолога. Получить первую онлайн консультацию психолога чате.
non prescription pharmacy Access drug facts. Complete medicine overview.
Психолог t me. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог помогающий искать решения в непростых психологических ситуациях.
В переписке у психолога. Помощь психолога онлайн. Психолог оказывает помощь онлайн в чате.
Анонимный чат с психологом телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога.
Анонимный чат с психологом телеграм. Онлайн-консультация психолога. Круглосуточная запись на онлайн-консультацию психолога.
Психолог онлайн чат. Чат с психологом в телеге. Анонимный чат с психологом телеграм.
Круглосуточная запись на онлайн-консультацию психолога. Получить первую онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь.
Психолог онлайн анонимно. Онлайн-консультация психолога. Чат психологической поддержки.
1вин войти http://1win815.ru/ .
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате.
1win com 1win com .
most bet http://kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0-1742814422/ .
Психолог в телеграм. Получить онлайн консультацию психолога чате. Телеграм психолог.
1win rossvya 1win rossvya .
Психолог в телеграм. Онлайн чат с психологом без регистрации. Онлайн чат с психологом без регистрации.
Чат психологической поддержки. Психолог помогающий искать решения в непростых психологических ситуациях. Чат психологической поддержки.
Получить первую онлайн консультацию психолога чате. Получить онлайн консультацию психолога чате. Психолог онлайн чат.
В переписке у психолога. В переписке у психолога. Получить онлайн консультацию психолога чате.
казино онлайн kg https://www.mostbet6001.ru .
Онлайн-консультация психолога. Чат с психологом в телеге. Дипломированный психолог с опытом работы и отзывами клиентов.
Получить первую онлайн консультацию психолога чате. Анонимный чат с психологом телеграм. Онлайн чат с психологом без регистрации.
Онлайн чат с психологом без регистрации. Психолог онлайн анонимно. В переписке у психолога.
деньги под залог птс машины
avtolombard-11.ru/ekb.html
кредит в залог машины
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате. Психолог онлайн чат.
Помощь психолога онлайн. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате.
Помощь психолога онлайн. Психолог в телеграм. Психолог оказывает помощь онлайн в чате.
Доброго!
Для тех, кто ищет гаджеты для работы и бизнеса, информационные сайты предоставляют массу полезной информации. Здесь можно найти обзоры ноутбуков, планшетов, устройств для видеоконференций и других рабочих гаджетов. Сайты предлагают советы по выбору техники для офиса и удаленной работы. Также рассматриваются особенности подключения устройств к сетям и управления ими. Эти ресурсы помогут вам выбрать гаджеты, которые улучшат вашу продуктивность и упростят рабочие процессы.
Как быть благодарным? Благодарность помогает видеть позитив даже в самых трудных ситуациях. Проводите каждый день, отмечая хотя бы несколько вещей, за которые вы благодарны. Это поможет улучшить ваше настроение и повысить качество жизни.
Больше информации по ссылке – https://ffactor.ru
как сделать воздушный крем, как сделать человеку плохо, как сделать балкон теплый
как сделать стикеры тг, как сделать кухню уютной, читать интересное
Удачи!
Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога. Онлайн-консультация психолога.
Телеграм психолог. Чат психологической поддержки. Психолог онлайн анонимно.
Психолог оказывает помощь онлайн в чате. Онлайн чат с психологом без регистрации. Чат психологической поддержки.
Анонимный чат с психологом телеграм. Психолог оказывает помощь онлайн в чате. В переписке у психолога.
Психолог t me. Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате.
Психолог t me. Онлайн чат с психологом без регистрации. Психолог оказывает помощь онлайн в чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн чат. Получить первую онлайн консультацию психолога чате.
Психолог t me. Психолог оказывает помощь онлайн в чате. Психолог в телеграм.
Психологическая и информационная онлайн-помощь. Помощь психолога онлайн. Круглосуточная запись на онлайн-консультацию психолога.
Круглосуточная запись на онлайн-консультацию психолога. Психологическая и информационная онлайн-помощь. Дипломированный психолог с опытом работы и отзывами клиентов.
Kyros Finance is redefining the DeFi investment landscape by offering secure, scalable, and high-yield crypto solutions. With a focus on decentralized financial tools, Kyros Finance provides users with staking, lending, and automated yield farming strategies to maximize returns. Whether you’re a retail investor or an institutional participant, Kyros Finance ensures efficient, transparent, and secure access to the world of decentralized finance. https://kyros.ink
Получить онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
топ онлайн казино
Топ онлайн казино 2025: детальное исследование лучших игровых платформ
Онлайн азартные игры стремительно расширяют свою аудиторию в России, завоевывая всё больше игроков вследствие своей функциональности. Однако для безопасного времяпрепровождения критически важно выбирать только легальные площадки, которые гарантируют защиту личных данных. В этом материале мы разберём ключевые критерии выбора, составим список лучших казино 2025 года и поделимся экспертными советами для оптимального выбора.
Ключевые факторы надёжности онлайн-казино
Чтобы минимизировать опасность, необходимо учитывать следующие характеристики:
Лицензионная чистота
Проверяйте наличие международных лицензий: Гибралтара.
Убедитесь, что игровые автоматы прошли сертификацию от авторитетных организаций (iTech Labs).
Система случайных чисел (RNG)
Казино должно использовать независимый генератор случайных чисел, что обеспечивает прозрачность процессов.
Скорость транзакций
Надёжные казино предлагают мгновенные выплаты через современные системы: банковские карты (Visa), электронные кошельки (Neteller) и криптовалюты (Litecoin).
Бонусная политика
Хорошие казино предоставляют привлекательные бонусы: приветственные пакеты, фриспины и системы вознаграждений. Обратите внимание на требования по использованию.
Мобильная оптимизация
Современные казино оптимизированы для мобильных устройств (Android) с удобным управлением. Это позволяет проводить время за играми круглосуточно.
Клиентская поддержка
Профессиональная служба поддержки работает круглосуточно и помогает решать вопросы через чат.
Рейтинг лучших онлайн-казино 2025 года
1. Cat Casino
Преимущества: оперативные выплаты (до 1 минуты), лицензия Кюрасао, уникальные предложения.
Бонусы: стартовый пакет — 100 фриспинов в Gates of Olympus.
Особенности: турниры с крупными призами.
2. Kometa Casino
Преимущества: ведущая платформа с дилерами, кэшбэк до значительной суммы.
Бонусы: стартовый комплект — впечатляющий приветственный пакет.
Особенности: VIP-клуб с персонализированными условиями.
3. R7 Casino
Преимущества: скрытый аккаунт по email, поддержка криптовалют (BTC) с нулевой комиссией.
Бонусы: фриспины за активность в соцсетях.
Особенности: двухфакторная аутентификация для предотвращения взломов.
4. Arkada Casino
Преимущества: быстрые выплаты на карты РФ (Тинькофф), регулярные розыгрыши.
Бонусы: кэшбэк до 10%, удвоение начального взноса.
Особенности: лицензионные игры от Pragmatic Play.
5. Kent Casino
Преимущества: поддержка криптовалют, турниры с призовым фондом впечатляющими наградами.
Бонусы: тестирование слотов.
Особенности: широкий выбор слотов с оптимальным возвратом.
Особенности мобильных казино
Мобильные казино становятся все более популярными благодаря технологичности. Их преимущества:
Адаптивный дизайн: интерфейс адаптирован для мобильных устройств.
Быстрый доступ: нет необходимости тратить время на загрузку.
Поддержка платежей: можно производить депозиты и получать выигрыши через специальные сервисы.
Низкое потребление трафика: современные казино работают плавно даже на низкой пропускной способности.
Как выбрать лучшее казино?
При выборе онлайн-казино учитывайте следующие критерии:
Лицензия и безопасность
Убедитесь, что казино имеет глобальное разрешение и использует технологии безопасности (другие протоколы).
Ассортимент игр
Лучшие казино предлагают обширный ассортимент слотов, настольных игр (блэкджек) и live-казино.
Скорость выплат
Изучите мнения пользователей о времени вывода средств.
Бонусные программы
Обратите внимание на размер приветственного бонуса, требования по использованию бонусов и наличие других спецпредложений.
Качество поддержки
Проверьте, насколько оперативно сотрудники устраняют проблемы.
Мобильная версия
Убедитесь, что казино имеет специальное приложение для смартфонов.
Заключение
Онлайн-казино могут стать интересным хобби, если подходить к их выбору ответственно. Придерживайтесь наших подсказок, играйте только на надёжных платформах и помните о правилах ответственной игры. Азартные игры — это прежде всего развлечение, а не способ заработка.
Если вы ищете проверенную платформу с щедрыми бонусами, обратите внимание на топовые площадки, такие как Cat Casino. Они отлично зарекомендовали себя среди игроков и продолжают впечатлять.
https://t.me/s/topcasinos_ru
Психолог в телеграм. Психолог t me. Онлайн-консультация психолога.
Здравствуйте!
Вопросы здоровья домашних животных всегда актуальны, и на информационных сайтах можно найти ответы на множество медицинских вопросов. Например, как определить, что собака болеет, и как правильно лечить питомца. Сайты предлагают информацию о том, как лечить простуды, инфекции и другие заболевания у животных. В статьях рассматриваются симптомы болезней, что помогает владельцам вовремя заметить проблему и начать лечение. Эти ресурсы являются незаменимым источником информации для заботливых владельцев.
Как избежать выгорания? Установите разумные границы между работой и отдыхом, регулярно меняйте виды деятельности и уделяйте внимание своему здоровью. Научитесь расслабляться и перезаряжаться.
Больше информации по ссылке – https://relation1.ru/
открытки 23 февраля интересные, как сделать массаж ног, продукты богатые белком веганские
интересная ебля, вакуумный массаж банками чем полезен, продукты богаты белками
Удачи!
В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате.
Спасибо за быструю доставку и свежие цветы!
купить цветы томск
Анонимный чат с психологом телеграм. Телеграм психолог. Получить онлайн консультацию психолога чате.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат психологической поддержки. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить первую онлайн консультацию психолога чате. Чат психологической поддержки. Получить первую онлайн консультацию психолога чате.
Non-Gamstop casino vibes are so chill—really impressed!
non gamstop casinos uk
Психолог онлайн анонимно. Чат психологической поддержки. Онлайн-консультация психолога.
Помощь психолога онлайн. Телеграм психолог. Онлайн чат с психологом без регистрации.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн анонимно.
Психолог онлайн анонимно. Анонимный чат с психологом телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
best canadian best online pharmacy reviews Drug overview available. Latest drug news.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт приставок xbox, можете посмотреть на сайте: ремонт приставок xbox цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Онлайн-консультация психолога. Психолог t me. Чат с психологом в телеге.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог t me. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог онлайн чат. Получить онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате.
Здравствуйте!
Вопросы безопасности в интернете часто обсуждаются на информационных сайтах. Здесь можно найти статьи, которые помогут вам защитить свои устройства от вирусов, хакерских атак и других угроз. В статьях рассказывается о лучших антивирусных программах, а также даются рекомендации по настройке безопасности в операционных системах. Сайты также предлагают советы по защите личных данных и приватности в интернете. Эти ресурсы помогут вам обезопасить свои гаджеты и данные от угроз.
Как организовать продуктивный день? Начинайте с важнейших задач, избегайте многозадачности и делайте регулярные перерывы. Вечером оценивайте результаты дня и планируйте следующий.
Больше информации по ссылке – https://relation1.ru
как сделать наливной пол, интересные фильмы россия, днс сайт не открывается почему
интересные научные факты, как сделать анализ конкурентов, майнкрафт как сделать удочку
Удачи!
Онлайн чат с психологом без регистрации. Чат психологической поддержки. Чат с психологом в телеге.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки. Получить онлайн консультацию психолога чате.
Онлайн-консультация психолога. Психолог в телеграм. Психолог помогающий искать решения в непростых психологических ситуациях.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн анонимно.
Получить первую онлайн консультацию психолога чате. Анонимный чат с психологом телеграм. Получить первую онлайн консультацию психолога чате.
Чат психологической поддержки. Чат с психологом в телеге. Получите консультацию онлайн-психолога в чате прямо сейчас.
Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн чат с психологом без регистрации. Получите консультацию онлайн-психолога в чате прямо сейчас.
Онлайн чат с психологом без регистрации. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Привет всем!
Как научиться работать в условиях неопределенности? Оставайтесь гибким, развивайте способность к адаптации и доверяйте своему внутреннему чувству, когда нужно принимать решения.
В мире технологий часто происходят важные события, и информационные сайты предлагают самые актуальные новости. Здесь можно узнать о выходе новых смартфонов, процессоров, а также о важнейших открытиях в области науки и техники. Сайты регулярно обновляют информацию, чтобы пользователи могли быть в курсе всех технологических новинок. Они предлагают обзоры, тесты и обсуждения на тему новых продуктов и разработок. Эти ресурсы позволяют следить за последними тенденциями в области технологий.
Больше информации по ссылке – https://alcogolizmstop.ru
куда зимой поехать по россии, дизайн квартиры как сделать, статусы интересные со смыслом
шрифты интересные кириллица, как сделать травяной клинок, как сделать лыжи
Удачи!
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Психолог t me.
Получить онлайн консультацию психолога чате. Психолог онлайн чат. Психолог онлайн чат.
Получить онлайн консультацию психолога чате. Психолог t me. Психолог помогающий искать решения в непростых психологических ситуациях.
Noon Capital is revolutionizing real estate crowdfunding by offering secure, decentralized, and high-yield investment opportunities. Through blockchain-powered property financing, Noon Capital investment platform allows investors to participate in fractional real estate ownership, earn passive income, and diversify their portfolios with full transparency and security. Whether you’re an institutional investor or an individual seeking real estate-backed DeFi solutions, Noon Capital provides a seamless and profitable investment experience. https://noon.ad
Добрый день!
На информационных сайтах можно найти массу полезных рекомендаций по выбору гаджетов для интимной жизни. В статьях рассматриваются устройства для повышения либидо, улучшения эрекции и снижения уровня стресса. Сайты предлагают советы по выбору гаджетов, которые помогут улучшить качество сна и настроить гормональный фон. Эти ресурсы помогут вам наладить отношения с партнером и повысить сексуальную активность.
Как развивать лидерские качества? Будьте примером для других, вдохновляйте людей и берите на себя инициативу в сложных ситуациях.
Больше информации по ссылке – https://psihfak.ru
как сделать джейлбрейк, как сделать новорожденному снилс, беспроводные наушники для смартфона лучшие
как сделать апероль, как надо сделать, лучшие детективные сериалы зарубежные 2019
Удачи!
кредит в залог автомобиля
avtolombard-11.ru/kazan.html
автоломбард залог машины
Получить первую онлайн консультацию психолога чате. Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога.
GUD Tech is at the forefront of blockchain innovation, providing secure, scalable, and efficient decentralized technology solutions. Whether you’re looking for blockchain infrastructure, smart contract development, or enterprise-grade decentralized applications, GUD Tech offers cutting-edge solutions to enhance security and efficiency. Designed for businesses and developers, GUD Tech ensures seamless integration of blockchain technology into real-world applications. https://gudchain.net
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Получить онлайн консультацию психолога чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях. Получить онлайн консультацию психолога чате.
Телеграм психолог. Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн чат. Чат с психологом в телеге.
В переписке у психолога. Психологическая и информационная онлайн-помощь. Психолог в телеграм.
Онлайн-консультация психолога. Онлайн чат с психологом без регистрации. Онлайн чат с психологом без регистрации.
Психолог t me. Дипломированный психолог с опытом работы и отзывами клиентов. Получить онлайн консультацию психолога чате.
pdacenter.ru – сервис по ремонту бытовой техники
Ремонт парогенераторов в Саратове в официальном сервисном центре PDACENTER.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
Онлайн-консультация психолога. Получить первую онлайн консультацию психолога чате. Психолог онлайн чат.
Психолог t me. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог оказывает помощь онлайн в чате.
Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Психолог онлайн чат.
Психологическая и информационная онлайн-помощь. Круглосуточная запись на онлайн-консультацию психолога. Онлайн-консультация психолога.
Круглосуточная запись на онлайн-консультацию психолога. Психолог в телеграм. Психолог t me.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн чат. Получить онлайн консультацию психолога чате.
Круглосуточная запись на онлайн-консультацию психолога. Получить первую онлайн консультацию психолога чате. Получить онлайн консультацию психолога чате.
Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате.
HyperDrive is transforming the way data is stored and managed through decentralized storage and blockchain hosting solutions. Built for security, scalability, and efficiency, HyperDrive enables businesses, developers, and blockchain projects to store and distribute data without relying on centralized servers. By leveraging Web3 technology and distributed networks, HyperDrive ensures reliable, censorship-resistant, and high-performance storage solutions for the digital era. https://hyperdrive.ink
Just joined a non Gamstop casino—games are unreal!
online casino uk no gamstop
Единственный знак зодиака, который является магнитом для несчастий и проблем https://x.com/Fariz418740/status/1904737515858174078
Здравствуйте!
Как ускорить интернет на телефоне? Иногда для этого достаточно выключить ненужные приложения или изменить настройки сети. Оптимизация работы Wi-Fi и использование режима энергосбережения помогут увеличить скорость интернета. Также полезно периодически очищать кэш и обновлять операционную систему.
Как эффективно решать проблемы? Анализируйте ситуацию, ищите альтернативы, взвешивайте риски и принимайте решение, основываясь на фактах и интуиции.
Больше информации по ссылке – https://lala-express.ru/
как сделать сайт быстро, порнуха интересная, поделка интересная из бумаги
что делать при отравлении плесенью, топленое масло как сделать, бумажный пакет как сделать
Удачи!
top online pharmacies Read about drugs. Get info now.
Доброго!
Как научиться быть более благодарным? Фокусируйтесь на том, что у вас есть, и выражайте признательность за каждое хорошее событие или момент.
Как выбрать идеальный подарок для друга? Важно учитывать интересы и предпочтения человека. Хорошим вариантом могут быть персонализированные подарки или что-то, что отражает его увлечения. Главное — это внимание и забота, которую вы вложите в выбор подарка.
Больше информации по ссылке – https://psihfak.ru
лучшие авиакомпании мира, лучшие шведские детективные сериалы, как сделать афродизиак
интересные факты о числительном, как составить правильно резюме, как сделать самому кровать
Удачи!
Онлайн чат с психологом без регистрации. Психолог в телеграм. Онлайн чат с психологом без регистрации.
Круглосуточная запись на онлайн-консультацию психолога. Онлайн-консультация психолога. В переписке у психолога.
legit best online pharmacy Current drug trends. Drug information available.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Телеграм психолог. В переписке у психолога.
Привет всем!
Как научиться быть терпеливым? Работайте над собой, примите, что не все можно получить сразу, и старайтесь сохранять спокойствие в сложных ситуациях.
Как научиться управлять стрессом? Используйте методы релаксации, занимайтесь спортом и не забывайте делать перерывы в напряженные моменты.
Больше информации по ссылке – https://ffactor.ru/
брусничный морс как сделать, лучшие упражнения пресс, как можно сделать потолок
интересные факты про космос, крылья как сделать, интересные названия детенышей животных
Удачи!
Психолог t me. Психолог оказывает помощь онлайн в чате. Психолог помогающий искать решения в непростых психологических ситуациях.
В переписке у психолога. Онлайн-консультация психолога. Психолог онлайн чат.
Всё о строительстве, дизайне и ремонте в своём доме.
Добро пожаловать на «Свой Угол» – ваш партнер в мире строительства, дизайна и ремонта для вашего дома!
Наш блог создан для того, чтобы предоставить вам всю необходимую информацию, которая сделает ваш дом еще уютнее, красивее и функциональнее.
Мы – эксперты в следующих сферах:
Строительство
Мы поделимся с вами советами о выборе материалов, поиске надежных строителей в Беларуси и планировке стройплощадки.
Если у вас возникают вопросы о строительстве своего дома с нуля или о расширении уже существующего пространства, мы предоставим вам экспертные советы.
Дизайн интерьера
У нас есть идеи и решения для каждой комнаты в вашем доме.
Мы расскажем о последних трендах в дизайне, поделимся советами по выбору цветовой палитры, мебели и аксессуаров, чтобы создать уникальное пространство, отражающее ваш стиль и вкус.
Ремонт
От простых косметических обновлений до капитального ремонта – у нас есть идеи и рекомендации для каждого случая.
Мы поддержим вас на каждом этапе, начиная от планирования и заканчивая последним штрихом.
Что вы найдете на нашем сайте
Экспертные советы
Статьи разработаны профессионалами в области строительства, дизайна и ремонта.
Полезные ресурсы
Ссылки на проверенные магазины, услуги и материалы, которые помогут вам в ваших проектах.
Модные тренды
Мы следим за последними тенденциями и новинками в мире строительства и дизайна.
«Свой Угол» – ваш проводник в мире уюта и стиля в вашем доме. Погружайтесь в наши статьи, найдите вдохновение, задавайте вопросы и делитесь своим опытом.
Создайте свой угол вместе с нами!
Информационный портал «Свой Угол»
Дипломированный психолог с опытом работы и отзывами клиентов. Получить первую онлайн консультацию психолога чате. Психолог онлайн чат.
Психолог t me. Чат с психологом в телеге. Получите консультацию онлайн-психолога в чате прямо сейчас.
Анонимный чат с психологом телеграм. Психолог онлайн анонимно. В переписке у психолога.
1win rossvya http://1win816.ru .
1 win вход https://1win6015.ru .
мостбет скачать казино http://www.maksipolinovtsu.forum24.ru/?1-1-0-00000194-000-0-0-1742815870 .
1 win kg https://mymoscow.forum24.ru/?1-6-0-00026928-000-0-0/ .
Получить онлайн консультацию психолога чате. Онлайн чат с психологом без регистрации. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог оказывает помощь онлайн в чате. Психолог онлайн чат. Получить онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
Психологическая и информационная онлайн-помощь. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог t me.
Доброго!
Какие фильмы выйдут в 2025 году, интересно узнать всем киноманам. Это будет год, наполненный увлекательными новинками в различных жанрах. Самые ожидаемые фильмы 2025 года уже анонсированы, и зрители ждут их с нетерпением. Среди них — как продолжения культовых франшиз, так и новые проекты, которые обязательно заинтересуют.
Как развить креативность? Один из способов — это выйти за рамки привычного и попробовать что-то новое. Занимайтесь творческими хобби, такими как рисование, музыка или письмо. Вдохновение может прийти в самых неожиданных местах, если вы будете открытыми к новым идеям и подходам.
Больше информации по ссылке – https://art-novosibirsk.ru
интересно просто, интересные факты о твери, как сделать таблицу html
как сделать календарь настольный, как сделать выбор, лучший скандинавский сериал детективный
Удачи!
Получите консультацию онлайн-психолога в чате прямо сейчас. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог помогающий искать решения в непростых психологических ситуациях.
Круглосуточная запись на онлайн-консультацию психолога. Психолог в телеграм. Помощь психолога онлайн.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога.
Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях.
Онлайн-консультация психолога. Онлайн-консультация психолога. Онлайн-консультация психолога.
motbet https://www.mostbet6002.ru .
Чат психологической поддержки. Онлайн чат с психологом без регистрации. Получить первую онлайн консультацию психолога чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях. Чат с психологом в телеге.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Психологическая и информационная онлайн-помощь.
Психолог оказывает помощь онлайн в чате. Помощь психолога онлайн. Чат с психологом в телеге.
1win букмекер https://www.1win816.ru .
Получить онлайн консультацию психолога чате. Онлайн-консультация психолога. Дипломированный психолог с опытом работы и отзывами клиентов.
Доброго!
Как научиться быть более активным? Установите физическую активность как обязательную часть своей жизни и найдите занятия, которые вам нравятся.
Вопросы здоровья домашних животных всегда актуальны, и на информационных сайтах можно найти ответы на множество медицинских вопросов. Например, как определить, что собака болеет, и как правильно лечить питомца. Сайты предлагают информацию о том, как лечить простуды, инфекции и другие заболевания у животных. В статьях рассматриваются симптомы болезней, что помогает владельцам вовремя заметить проблему и начать лечение. Эти ресурсы являются незаменимым источником информации для заботливых владельцев.
Больше информации по ссылке – https://nikita-bywalino.ru/
перо как сделать, как авторизацию сделать, как сделать плитку тротуарную
как сделать икосаэдр, интересные загадки для детей, как сделать поля
Удачи!
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог в телеграм. Анонимный чат с психологом телеграм.
Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психологическая и информационная онлайн-помощь.
магазин для покупки аккаунтов сервис продажи аккаунтов
1win мобильная версия сайта https://1win6015.ru/ .
мос бет https://maksipolinovtsu.forum24.ru/?1-1-0-00000194-000-0-0-1742815870 .
зайти в 1вин http://mymoscow.forum24.ru/?1-6-0-00026928-000-0-0/ .
Чат психологической поддержки. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог в телеграм.
Психологическая и информационная онлайн-помощь. Психологическая и информационная онлайн-помощь. Психолог онлайн чат.
кредит под птс казань
avtolombard-11.ru/kazan.html
автоломбард залог машины
Круглосуточная запись на онлайн-консультацию психолога. Психолог t me. Чат с психологом в телеге.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате. Психолог онлайн чат.
Чат психологической поддержки. Получить онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях.
Телеграм психолог. Психолог онлайн чат. Психолог t me.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог в телеграм. Психолог помогающий искать решения в непростых психологических ситуациях.
Помощь психолога онлайн. Чат психологической поддержки. Психолог онлайн чат.
Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате. Чат психологической поддержки.
Онлайн чат с психологом без регистрации. Психолог t me. Чат психологической поддержки.
Онлайн чат с психологом без регистрации. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате.
скачать мостбет официальный сайт http://www.mostbet6002.ru .
Продажа путёвок https://camp-centr.com/camps/type/yazykovoy.html. Спортивные, творческие и тематические смены. Весёлый и безопасный отдых под присмотром педагогов и аниматоров. Бронируйте онлайн!
Дипломированный психолог с опытом работы и отзывами клиентов. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психологическая и информационная онлайн-помощь. Получить первую онлайн консультацию психолога чате.
Психолог оказывает помощь онлайн в чате. Дипломированный психолог с опытом работы и отзывами клиентов. Чат с психологом в телеге.
печать визиток https://tipografiya-pechat-vizitok.ru
Помощь психолога онлайн. Психолог онлайн анонимно. Психолог онлайн анонимно.
Чат психологической поддержки. Психолог помогающий искать решения в непростых психологических ситуациях. Чат психологической поддержки.
Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате. Психолог онлайн анонимно.
Получить онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь. Психолог онлайн анонимно.
best online pharmacy no perscription Find drug information. Formulation info listed.
https://shvejnye.ru/
Психолог онлайн анонимно. Чат с психологом в телеге. Психолог t me.
Получить онлайн консультацию психолога чате. Анонимный чат с психологом телеграм. Психолог онлайн анонимно.
adderall xr best online pharmacy Pill impacts described. Medicine facts here.
Чат психологической поддержки. Онлайн чат с психологом без регистрации. Психолог онлайн анонимно.
Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога. Получить первую онлайн консультацию психолога чате.
Телеграм психолог. В переписке у психолога. Психологическая и информационная онлайн-помощь.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате.
Круглосуточная запись на онлайн-консультацию психолога. Онлайн чат с психологом без регистрации. Онлайн-консультация психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно.
Телеграм психолог. Психолог онлайн чат. Чат с психологом в телеге.
https://kusokavto.ru/ Услуги по аренде техники и строительству с логотипом компании – На протяжении многих лет компания “ТрансАвто№7” предоставляет в аренду дорожно-строительную технику, а также выполняет широкий спектр строительных и земляных работ. Мы работаем как с крупными корпорациями, так и с малым и средним бизнесом, обеспечивая высокое качество услуг по строительству дорог, благоустройству территорий и прокладке инженерных сетей.
Помощь психолога онлайн. Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь.
HyperDrive is transforming the way data is stored and managed through decentralized storage and blockchain hosting solutions. Built for security, scalability, and efficiency, HyperDrive enables businesses, developers, and blockchain projects to store and distribute data without relying on centralized servers. By leveraging Web3 technology and distributed networks, HyperDrive ensures reliable, censorship-resistant, and high-performance storage solutions for the digital era. https://hyperdrive.ink
В переписке у психолога. Дипломированный психолог с опытом работы и отзывами клиентов. Чат психологической поддержки.
1win вход https://www.1win816.ru .
Чат с психологом в телеге. Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн чат с психологом без регистрации.
1вин бет официальный сайт https://1win6015.ru/ .
мрстбет мрстбет .
1win. pro https://mymoscow.forum24.ru/?1-6-0-00026928-000-0-0/ .
Психолог помогающий искать решения в непростых психологических ситуациях. Анонимный чат с психологом телеграм. Психолог t me.
Чат психологической поддержки. Психолог онлайн чат. В переписке у психолога.
Ремонт спецтехники в Омске Аренда и услуги спецтехники в Омске – Мы предоставляем услуги по аренде и эксплуатации спецтехники для различных сфер деятельности, включая строительство, дорожные работы и транспортировку грузов. Наши услуги охватывают аренду автокранов, экскаваторов, самосвалов и других машин для больших и малых предприятий, а также для частных клиентов. Спецтехника для вашего бизнеса с гарантией надежности и эффективности — это наш приоритет.
Помощь психолога онлайн. Психологическая и информационная онлайн-помощь. Психолог онлайн анонимно.
Телеграм психолог. Онлайн чат с психологом без регистрации. Круглосуточная запись на онлайн-консультацию психолога.
Психолог в телеграм. Онлайн-консультация психолога. Психолог онлайн чат.
Привет всем!
Как улучшить отношения с коллегами? Стремитесь к открытому и честному общению, а также проявляйте уважение и поддержку. Помогайте коллегам, когда это необходимо, и будьте готовы к конструктивной критике. Хорошая атмосфера на работе зависит от взаимного доверия и уважения.
Информационные сайты предлагают инновационные решения для тех, кто хочет улучшить свою интимную жизнь. В статьях рассматриваются гаджеты для контроля за уровнем стресса, повышения либидо и улучшения сексуальной активности. Сайты предлагают советы по выбору устройств для улучшения сна и поддержания гормонального фона. Эти ресурсы помогут вам улучшить отношения с партнером и сделать интимную жизнь более насыщенной.
Больше информации по ссылке – https://art-novosibirsk.ru/
как сделать светодиодную ленту, как сделать взломку, как сделать укол коту
как сделать сердечко объемное, интересные детективы русские фильмы, почему на компьютере тормозит интернет
Удачи!
Получить онлайн консультацию психолога чате. Помощь психолога онлайн. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог онлайн чат. Психологическая и информационная онлайн-помощь. Психолог онлайн чат.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно. Психолог онлайн анонимно.
Психологическая и информационная онлайн-помощь. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Помощь психолога онлайн. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог оказывает помощь онлайн в чате. Психолог t me. Круглосуточная запись на онлайн-консультацию психолога.
aviator mostbet https://www.mostbet6002.ru .
Добрый день!
Как избавиться от чувства вины? Примите свою ошибку и извлеките из нее уроки. Процесс прощения себя поможет вам освободиться от чувства вины и двигаться вперед с легкостью.
Как повысить свою продуктивность? Для этого необходимо установить четкие приоритеты и научиться работать с фокусом. Использование методов управления временем, таких как Pomodoro, может значительно повысить вашу эффективность.
Больше информации по ссылке – https://nikita-bywalino.ru/
почему тормозит вк на компьютере, музеи интересные для детей, как сделать предложение оригинально
какие лучше взять наушники беспроводные, хорошие российские комедии последних лет, куда поехать россия
Удачи!
Анонимный чат с психологом телеграм. Психолог оказывает помощь онлайн в чате. В переписке у психолога.
Психологическая и информационная онлайн-помощь. Психолог t me. Психолог онлайн анонимно.
Психолог онлайн анонимно. Чат психологической поддержки. Анонимный чат с психологом телеграм.
Телеграм психолог. Дипломированный психолог с опытом работы и отзывами клиентов. Помощь психолога онлайн.
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. Анонимный чат с психологом телеграм.
Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате. В переписке у психолога.
Психолог онлайн чат. Психолог t me. Онлайн чат с психологом без регистрации.
Психолог оказывает помощь онлайн в чате. Онлайн-консультация психолога. Психолог t me.
Zajrzalem na serwis Kiitos i bylem rozczarowany. Interfejs wyglada tak, jakby pochodzil sprzed dekady, dziala to dramatycznie, a brakuje waznych tresci. Wiele dzialow nie dziala, klikam i nic, a wsparcie nie odpowiada. Falszywe informacje na stronie, serwis jest kompletnie bezuzyteczny! Jesli zalezy ci na wiarygodnych danych o biegach, sprawdz inne serwisy – ten serwis jest calkowicie bezwartosciowy.
Психолог t me. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн чат.
Психолог t me. Помощь психолога онлайн. Получить первую онлайн консультацию психолога чате.
залог под птс авто
avtolombard-11.ru/kemerovo.html
займ под залог птс кемерово
либет казино
Чат психологической поддержки. Круглосуточная запись на онлайн-консультацию психолога. Психолог t me.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно.
Онлайн-консультация психолога. Психолог онлайн анонимно. Онлайн чат с психологом без регистрации.
Психолог оказывает помощь онлайн в чате. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате.
Чат психологической поддержки. Чат с психологом в телеге. Психолог t me.
Чат с психологом в телеге. Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн чат.
Телеграм психолог. Круглосуточная запись на онлайн-консультацию психолога. Психолог t me.
В переписке у психолога. Психолог онлайн анонимно. Круглосуточная запись на онлайн-консультацию психолога.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате.
Получить первую онлайн консультацию психолога чате. Психолог онлайн чат. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог помогающий искать решения в непростых психологических ситуациях. Получить первую онлайн консультацию психолога чате. Телеграм психолог.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно. Психолог помогающий искать решения в непростых психологических ситуациях.
Психолог t me. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн анонимно. Помощь психолога онлайн. Чат психологической поддержки.
Non-Gamstop casino sites are super fun—highly recommend!
non gamstop casino accept paypal
Психолог оказывает помощь онлайн в чате. Психолог онлайн чат. Дипломированный психолог с опытом работы и отзывами клиентов.
Круглосуточная запись на онлайн-консультацию психолога. Психолог помогающий искать решения в непростых психологических ситуациях. Чат с психологом в телеге.
нью ретро казино, либет казино, голд казино
Привет всем!
Беспроводные наушники хорошие, если вы цените удобство и качество звука. Такие наушники обеспечивают чистое звучание и свободу движений. Важно выбрать хорошие беспроводные наушники, которые подойдут именно вам по качеству и дизайну. Они идеально подходят для активных людей, любящих слушать музыку или вести разговоры без проводов.
Как выбрать подходящую профессию? Начните с анализа своих интересов, сильных сторон и ценностей. Пробуйте различные области и не бойтесь менять направление, если почувствуете, что нашли свое призвание.
Больше информации по ссылке – https://kyocera-mds.ru/
рефлексия интересная, как сделать красным глаз, лучшие беспроводные наушники для ps5
кефир дома как сделать, разряд прилагательного интересный, как дома сделать сигарету
Удачи!
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Привет всем!
Как улучшить память? Регулярные упражнения для мозга, такие как запоминание слов или чисел, помогут вам улучшить концентрацию и способность к запоминанию информации.
Если вы хотите сделать свою интимную жизнь более насыщенной и яркой, информационные сайты помогут вам выбрать лучшие гаджеты для этого. В статьях рассматриваются устройства для повышения либидо, улучшения эрекции и контроля за состоянием здоровья. Сайты дают советы по выбору гаджетов, которые помогают снять стресс и улучшить качество сна, что способствует улучшению интимных отношений. Эти ресурсы помогут вам улучшить отношения с партнером и повысить сексуальную активность.
Больше информации по ссылке – https://lala-express.ru
не интересно мне, чем полезен зеленый чай жасмин, дзен интересные новости
интересные факты про азот, фильмы интересные мультфильмы, 4 3 как сделать
Удачи!
Официальный сайт Natco LTD Natco Pharma Limited, широко известная как Натко фарма, зарекомендовала себя как один из лидеров индийской фармацевтической индустрии. Компания, представленная на своем официальном сайте natcopharma.co.in, специализируется на разработке, производстве и маркетинге широкого спектра фармацевтических препаратов, охватывающих различные терапевтические области.
Remarkable! Its really amazing paragraph, I have got much clear idea regarding from this article.
вход lee bet casino
Здравствуйте!
Как справляться с прокрастинацией? Понимание причин прокрастинации помогает найти решение. Разбейте задачи на более мелкие шаги, начните с самой легкой части и не ставьте себе глобальных ожиданий. Постепенно вы будете замечать, как привычка выполнения дел становится частью вашей жизни.
Как поддерживать здоровые суставы? Включайте в свой рацион продукты, богатые омега-3 жирными кислотами, а также кальцием и витамином D. Не забывайте о физической активности, которая помогает укрепить мышцы и поддерживать суставы в хорошем состоянии. Регулярные упражнения, такие как плавание и йога, особенно полезны.
Больше информации по ссылке – https://kyocera-mds.ru
интересные факты о японии, как сделать рокировку, наушники с лучшим басом беспроводные
дорамы интересные, интересные факты о саламандре, смотреть самые интересные фильмы
Удачи!
Dużo informacji o legalnych bukmacherach. | Szeroki wybór gier wideo. | Ciekawe zestawienia automatów hazardowych. | Recenzje slotów i gier hazardowych online. | Dobre omówienie zakładów esportowych. | sloty polska | Najważniejsze aktualizacje sportowe. | Polecam każdemu zainteresowanemu zakładami. | najlepsze gry na pc | Rzetelne recenzje i opinie graczy. | Kompleksowe informacje o legalnym hazardzie. polska sport
Где заказать диплом специалиста?
Мы предлагаем быстро купить диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями. Наш диплом пройдет любые проверки, даже при использовании специфических приборов. Достигайте цели быстро и просто с нашим сервисом. Купить диплом университета! aksharalipi.com/read-blog/11877_tehnicheskij-diplom.html
Выполняем качественное https://energopto.ru под ключ. Энергоэффективные решения для домов, офисов, промышленных объектов. Гарантия, соблюдение СНиП и точные сроки!
деньги под залог авто остается у вас
avtolombard-11.ru
кредит под птс грузового автомобиля
this hyperlink jaxx liberty
печать бланков писем печать медицинских бланков
печать на папках шелкография печать бумажных папок
I used to be able to find good info from your content.
зеркало вега казино
на этом сайте
hyip проект под ключ
крипта В мире азартных игр онлайн казино заняли прочное место, предлагая игрокам широкий выбор развлечений и возможность испытать удачу, не выходя из дома. От классических слотов до настольных игр с живыми дилерами – разнообразие поражает воображение. Стратегии в казино играют важную роль, позволяя игрокам повысить свои шансы на успех. Однако стоит помнить, что казино – это прежде всего игра случая, и никакая стратегия не гарантирует выигрыш.
1Block Casino: Full Platform Overview
1Block Casino is a modern gaming platform that offers a wide range of gambling entertainment for players worldwide. From classic slots to unique games like Plinko, the service provides everything needed for gambling enthusiasts. Let’s take a closer look at the main features of the platform, game categories, and key advantages.
Game Categories
1Block Casino boasts an extensive collection of games divided into several categories:
Originals
This section features exclusive games developed specifically for the platform. It’s a great choice for those seeking a unique gaming experience.
Slots
Classic and modern slots with various themes, bonuses, and mechanics are available here. Whether you prefer traditional fruit machines or innovative video slots, there’s something for everyone.
Live Games
Immerse yourself in the atmosphere of a real casino with live dealer games. These games are streamed in real-time, offering an authentic experience with professional dealers.
Fishing Games
A unique category that combines gambling with interactive gameplay. Fishing games are gaining popularity due to their engaging mechanics and potential for big wins.
Poker
Test your skills against other players in various poker formats. The platform offers both traditional and fast-paced poker games.
Esports Betting
Bet on your favorite esports teams and tournaments. This section caters to fans of competitive gaming who want to add excitement to their matches.
Lucky Bets and High Rollers
1Block Casino caters to all types of players, from casual gamers to high rollers. The Lucky Bets section highlights random wins, while the High Rollers section showcases impressive payouts from large bets. Whether you’re betting small or large amounts, the platform ensures fair play and exciting opportunities.
Our Community and Partnerships
1Block Casino values its community and collaborates with trusted partners to enhance the gaming experience. The platform proudly works with leading providers, ensuring top-quality games and services for its users.
Legal Information
1Block Casino is owned and operated by JogoMaster Limited, a company registered under number 15748. The company’s registered address is located in Hamchako, Mutsamudu, Autonomous Island of Anjouan, Union of Comoros.
The platform is licensed and regulated by the Gaming Board of Anjouan (License No. ALSI-152406032-FI3). 1Block has passed all regulatory compliance checks and is legally authorized to conduct gaming operations for all games of chance and wagering.
Why Choose 1Block Casino?
Diverse Game Selection : From slots to live games, there’s something for every type of player.
Transparency : All bets and payouts are clearly displayed, ensuring trust and fairness.
Crypto-Friendly : The platform supports cryptocurrency transactions, making it convenient for modern players.
Licensed and Regulated : With a valid license from the Gaming Board of Anjouan, players can enjoy a secure and legal gaming experience.
Whether you’re a fan of classic casino games or looking to explore unique options like Plinko, 1Block Casino offers a comprehensive and enjoyable platform for all gambling enthusiasts.
Смотреть здесьскрипты хайпов
Добрый день!
Как найти внутренний баланс? Слушайте себя и находите гармонию между работой, личной жизнью и отдыхом. Создание здоровых границ и принятие своих потребностей — залог гармоничной жизни.
Как научиться работать в команде? Будьте открытыми к сотрудничеству, уважайте мнения других и поддерживайте коллег в совместной работе.
Больше информации по ссылке – https://drjahlov.ru
смотреть кино интересное, как сделать шире спину, майнкрафт как сделать горшок
наушники беспроводные накладные рейтинг лучших, чем энергетик вреден для подростка, чем полезен массаж с медом
Удачи!
Best non Gamstop casinos keep me entertained—love them!
non gamstop casinos uk
печать фото на холсте печать на холсте по фотографии
сделать техосмотр онлайн Москва – мегаполис, где техосмотр (ТО) является обязательной процедурой для большинства транспортных средств. Найти аккредитованный пункт техосмотра в Москве несложно, достаточно воспользоваться онлайн-картами или поисковыми системами. Важно помнить, что приобретение техосмотра без фактического осмотра автомобиля – незаконно. Стоимость прохождения ТО зависит от категории транспортного средства. Проверить легитимность диагностической карты ТО можно по базе ЕАИСТО (Единая автоматизированная информационная система техосмотра). Это позволяет убедиться, что данные внесены в официальную базу и соответствуют требованиям. Вопрос о необходимости техосмотра для конкретного типа транспорта регламентируется законодательством, и его отсутствие может повлечь штраф.
dtf печать заказать dtf печать картинки
широкоформатная печать дешево https://shirokoformatnaya-pechat-spb.ru
1вин приложение http://www.1win817.ru .
скачать 1win официальный сайт https://dogzz.forum24.ru/?1-10-0-00000155-000-0-0-1742818537 .
mostbet промокод https://corgan.borda.ru/?1-0-0-00000265-000-0-0 .
Hi there to every , as I am actually keen of reading this web site’s post to be updated regularly. It carries nice data.
petroffmotors.ru
mostbet официальный сайт http://mostbet6003.ru/ .
деньги под залог документов на машину
avtolombard-11.ru/nsk.html
займ птс новосибирск
1win казино https://www.1win817.ru .
1win вход на сайт http://dogzz.forum24.ru/?1-10-0-00000155-000-0-0-1742818537/ .
мос бет http://corgan.borda.ru/?1-0-0-00000265-000-0-0/ .
Pretty section of content. I just stumbled upon your site and in accession capital to assert that I get actually enjoyed account your blog posts. Any way I will be subscribing to your feeds and even I achievement you access consistently fast.
сайт leebet casino
где купить огнезащитный лак КЕРАМ В мире современных интерьеров, где дерево занимает почетное место, особое внимание уделяется безопасности. Огнезащитный лак для деревянного паркета становится не просто декоративным элементом, а жизненно важным средством защиты. Лак КМ1, специально разработанный для огнезащиты паркетных полов, обеспечивает надежный барьер против распространения огня. Этот лак для паркетных покрытий с огнезащитой создает на поверхности древесины слой, способный замедлить или предотвратить возгорание. Огнезащитное покрытие для паркетных досок, такое как паркетный лак КМ1 с огнезащитными свойствами, позволяет не только сохранить эстетику натурального дерева, но и повысить пожарную безопасность помещения. Лак для паркета КМ1 – это гарантия спокойствия и уверенности в защите вашего дома.
мос бет https://www.mostbet6003.ru .
1win официальный https://www.1win817.ru .
1вин кг http://www.dogzz.forum24.ru/?1-10-0-00000155-000-0-0-1742818537 .
mosbet https://www.corgan.borda.ru/?1-0-0-00000265-000-0-0 .
подробнее
шаблоны хайп проектов
propecia best online pharmacy Medicine essentials explained. Pill impacts described.
смотреть здеськупить хайп
mostbet chrono mostbet chrono .
деньги под залог автомобиля
https://wazifaa.com/employer/avtolombard-pid-zalog-pts1792/
получить деньги под залог птс
Какой красивый оттенок! Заказала букет маме.
Доставка цветов в Томске
Цветы свежие, доставка на высоте!
доставка цветов
Смотреть здесьКупить скрипт хайпа
Non-Gamstop casinos offer such a smooth experience—totally hooked!
no gamstop casinos
generic viagra best online pharmacy Get medication details. Patient drug leaflet.
Доброго!
Как научиться слушать других? Прекратите прерывать собеседника, сосредотачивайтесь на его словах и проявляйте искреннее внимание к его мнению.
Как развить чувство благодарности? Регулярно записывайте вещи, за которые вы благодарны, и замечайте хорошие моменты даже в повседневной жизни.
Больше информации по ссылке – https://drjahlov.ru
интересные факты тула, как аккаунт сделать личным, музей интересный в москве
как сделать красиво пирожки, съедобный букет как сделать, мясной букет как сделать
Удачи!
Четыре знака Зодиака ждет переломный момент на этих выходных https://x.com/Fariz418740/status/1905095937828978718
Узнать больше mega зеркала
1win win 1win6016.ru .
деньги под птс автомобиля
https://nujob.ch/companies/jobsandbussiness113/
кредит под залог авто машины
1 вин официальный сайт вход http://www.1win6016.ru .
best online pharmacy schools Drug information available. Detailed pill knowledge.
мостбет казино https://mostbet789.ru .
Hi there, yes this post is in fact good and I have learned lot of things from it about blogging. thanks.
frezza.ru
здесь мега сайт
поддержка мостбет https://www.ashapiter0.forum24.ru/?1-19-0-00001444-000-0-0-1742819001 .
Non Gamstop casinos UK are killing it with their slot selection—wow!
non gamstop onlnie casino lightning roulette
1win официальный сайт регистрация https://zdorovie.forum24.ru/?1-7-0-00000231-000-0-0-1742818050 .
найти это мега ссылка
мостбет промокод мостбет промокод .
mostbet mostbet .
1win партнерка вход 1win6016.ru .
1 вин официальный сайт вход 1 вин официальный сайт вход .
Наши специалисты предлагает надежный центр ремонта ноутбука адреса любых брендов и моделей. Мы знаем, насколько значимы для вас ваши лаптопы, и готовы предложить сервис высочайшего уровня. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что обеспечивает надежность и долговечность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются обладатели переносных компьютеров, включают проблемы с жестким диском, проблемы с дисплеем, неисправности программного обеспечения, неработающие разъемы и перегрев. Для устранения этих проблем наши опытные мастера оказывают ремонт жестких дисков, экранов, ПО, разъемов и систем охлаждения. Обратившись к нам, вы получаете надежный и долговечный сервисный ремонт ноутбуков адреса.
Подробная информация представлена на нашем сайте: https://remont-noutbukov-first.ru
бюстгальтер Caitline для спорта Ищете идеальный бюстгальтер? Обратите внимание на Caitline! Отзывы покупательниц подтверждают: это сочетание комфорта и элегантности. Широкий размерный ряд, включая модели для больших размеров, позволяет каждой женщине найти подходящий вариант. В интернет-магазине Caitline вас ждет разнообразие моделей: с эффектом пуш-ап, для повседневной носки, с кружевом, для спорта и даже для особых случаев, таких как свадьба или вечерний выход.
best online pharmacy no prescription needed Latest drug news. Pill leaflet provided.
По данным за январь 2025 года, внешнеторговый оборот России составил $50,7 млрд – на 4,6% больше по сравнению с тем же периодом прошлого года. Экспорт вырос на 3,2% ($29,3 млрд), а импорт увеличился на 6,6% ($21,4 млрд) Несмотря на санкции и ограничения, Россия активно адаптируется к новым условиям международной торговли Как же оплачивать за рубеж из России или получать экспортную выручку легально на счет компании – ответили на наших площадках Оплата таможенных платежей
скачат мостбет http://mostbet789.ru .
кредит под залог птс
https://careers.jabenefits.com/employer/avtolombard-pid-zalog-pts6063/
деньги под залог авто птс
mostbet промокод mostbet промокод .
сайт 1win официальный сайт вход https://zdorovie.forum24.ru/?1-7-0-00000231-000-0-0-1742818050 .
Best non Gamstop casino I’ve played at had fast support—nice!
non on gamstop casino
Юридические услуги https://urwork.ru в Санкт-Петербурге и Москве – от консультации до защиты интересов в суде. Оперативно, надежно и с гарантией конфиденциальности.
порно порно .
1win вход на сайт 1win6017.ru .
non prescription best online pharmacy Find medication information. Complete drug overview.
водка казино водка казино .
Odwiedzilem serwis Vasen i stracilem czas. layout jest przestarzaly, korzystanie to koszmar, a danych jest minimum. ciagle awarie, wszystko sie zacina, natretny spam utrudniaja uzywanie. Oczywiste, ze serwis nie jest aktualizowana, poniewaz administracja nic nie robi. Support ignoruje, przekierowania czesto prowadza donikad. Dla tych, ktorzy cenia jakosc, lepiej jej unikac!
1вин 1win6017.ru .
Are you searching for a reliable online casino? Look no further with test casino!
At test casino, players get access to a diverse collection of games, from traditional slots to advanced live dealer tables.
### What Makes test casino Stand Out?
1. **Endless Gaming Options**
– Whether you enjoy slots, poker, or roulette, there’s something for all types of gamblers.
2. **Unbeatable Casino Bonuses**
– At test casino, we surprise our players with exclusive bonuses, deposit matches, no-wager rewards, and high-roller promotions.
3. **Fully Licensed & Regulated**
– With reliable operations, you can enjoy peace of mind while playing.
4. **Fast & Easy Payments**
– No waiting times—just fast transactions!
5. **Always Here to Help**
– Need help? We solve issues efficiently.
### How to Get Started?
1. **Sign Up** in less than a minute.
2. **Fund Your Account** and claim your welcome bonus.
3. **Dive into the Action!** Choose from thousands of games and enjoy the thrill.
Why wait? Join test casino and enjoy unmatched gaming thrills today!
1xbet – лучший выбор для ставок, уже сегодня.
Ставки на спорт с 1xbet, на рынке.
1xbet предлагает щедрые бонусы, в ближайшее время.
1xbet – идеальное место для спортивных ставок, получайте.
1xbet – ваш портал в мир лайв-ставок, сделайте каждую секунду важной.
1xbet предлагает широкую линейку ставок, создавайте.
1xbet – это выбор на любой вкус, от футбола до тенниса.
1xbet – живые трансляции ваших любимых матчей, сделайте вашу ставку.
Быстрые выводы выигрышей с 1xbet, действуйте быстро.
Обзоры и прогнозы на 1xbet, поможем вам оставаться в курсе.
1xbet – это безопасность и надежность, мы ценим вашу конфиденциальность.
Скидки и бонусы только для вас с 1xbet, воспользуйтесь шансом.
Выигрывайте и наслаждайтесь с 1xbet, это ваш шанс на успех.
Чат поддержки 24/7 на 1xbet, мы рядом, чтобы помочь.
Участвуйте в конкурсах и выигрывайте с 1xbet, воспользуйтесь шансом.
1xbet в вашем кармане, удобно и быстро.
Дайте себе преимущества с 1xbet, это умная игра.
Зарегистрируйтесь на 1xbet всего за несколько минут, доступ к азарту.
Откройте новый уровень азартных игр с 1xbet, попробуйте свои силы.
Заходите на 1xbet для эксклюзивных предложений, развивайте свои навыки.
https://1xbet-login-egypt.com/ https://1xbet-login-egypt.com/ .
кредит под птс автомобиля
https://www.workinternational-df.com/employer/brightworks3534/
кредит под птс авто
hydrocodone best online pharmacy Patient medication facts. Medicine impacts explained.
1 вин официальный http://1win6017.ru/ .
Наша мастерская предлагает высококачественный починить ноутбук в москве различных марок и моделей. Мы знаем, насколько важны для вас ваши лаптопы, и готовы предложить сервис высочайшего уровня. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что гарантирует надежность и долговечность наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются владельцы ноутбуков, включают поломку жесткого диска, поврежденный экран, неисправности программного обеспечения, неработающие разъемы и перегрев. Для устранения этих поломок наши профессиональные техники выполняют ремонт жестких дисков, экранов, ПО, разъемов и систем охлаждения. Обратившись к нам, вы получаете долговечный и надежный центр ремонта ноутбука.
Подробная информация размещена на сайте: https://remont-noutbukov-first.ru
This non Gamstop casino has unreal graphics—totally blown away!
non gamstop casino
продать часы
https://firelak.ru В современном строительстве и отделке, где дерево занимает важное место, обеспечение пожарной безопасности становится приоритетной задачей. Огнезащитный лак – это эффективное решение для защиты деревянных конструкций и элементов интерьера от возгорания и распространения пламени.
1block bet
1Block Casino: Full Platform Overview
1Block Casino is a modern gaming platform that offers a wide range of gambling entertainment for players worldwide. From classic slots to unique games like Plinko, the service provides everything needed for gambling enthusiasts. Let’s take a closer look at the main features of the platform, game categories, and key advantages.
Game Categories
1Block Casino boasts an extensive collection of games divided into several categories:
Originals
This section features exclusive games developed specifically for the platform. It’s a great choice for those seeking a unique gaming experience.
Slots
Classic and modern slots with various themes, bonuses, and mechanics are available here. Whether you prefer traditional fruit machines or innovative video slots, there’s something for everyone.
Live Games
Immerse yourself in the atmosphere of a real casino with live dealer games. These games are streamed in real-time, offering an authentic experience with professional dealers.
Fishing Games
A unique category that combines gambling with interactive gameplay. Fishing games are gaining popularity due to their engaging mechanics and potential for big wins.
Poker
Test your skills against other players in various poker formats. The platform offers both traditional and fast-paced poker games.
Esports Betting
Bet on your favorite esports teams and tournaments. This section caters to fans of competitive gaming who want to add excitement to their matches.
Lucky Bets and High Rollers
1Block Casino caters to all types of players, from casual gamers to high rollers. The Lucky Bets section highlights random wins, while the High Rollers section showcases impressive payouts from large bets. Whether you’re betting small or large amounts, the platform ensures fair play and exciting opportunities.
Our Community and Partnerships
1Block Casino values its community and collaborates with trusted partners to enhance the gaming experience. The platform proudly works with leading providers, ensuring top-quality games and services for its users.
Legal Information
1Block Casino is owned and operated by JogoMaster Limited, a company registered under number 15748. The company’s registered address is located in Hamchako, Mutsamudu, Autonomous Island of Anjouan, Union of Comoros.
The platform is licensed and regulated by the Gaming Board of Anjouan (License No. ALSI-152406032-FI3). 1Block has passed all regulatory compliance checks and is legally authorized to conduct gaming operations for all games of chance and wagering.
Why Choose 1Block Casino?
Diverse Game Selection : From slots to live games, there’s something for every type of player.
Transparency : All bets and payouts are clearly displayed, ensuring trust and fairness.
Crypto-Friendly : The platform supports cryptocurrency transactions, making it convenient for modern players.
Licensed and Regulated : With a valid license from the Gaming Board of Anjouan, players can enjoy a secure and legal gaming experience.
Whether you’re a fan of classic casino games or looking to explore unique options like Plinko, 1Block Casino offers a comprehensive and enjoyable platform for all gambling enthusiasts.
safe best online pharmacy Drug specifics here. Medicine trends available. suboxone best online pharmacy
Remarkable things here. I am very happy to peer your article. Thanks so much and I’m having a look ahead to contact you. Will you kindly drop me a e-mail?
promo code 1xbet
кредит под залог машины
https://jobs.ondispatch.com/companies/avtolombard-zalog-invest3517/
автоломбард залог птс
Admiring the hard work you put into your website and in depth information you provide. It’s great to come across a blog every once in a while that isn’t the same unwanted rehashed information. Fantastic read! I’ve bookmarked your site and I’m adding your RSS feeds to my Google account.
best promo code for 1xbet
сео продвижение сайта интернет магазина продвижения сайта цена
сео оптимизация сайта купить купить сео продвижение
раскрутка молодого сайта поисковое продвижение и продвижение сайтов
Wszystko, co musisz wiedzieć o grach hazardowych. | Kasyna online dobrze porównane. | Świetna nawigacja i czytelna struktura strony. | Recenzje slotów i gier hazardowych online. | Dobre omówienie zakładów esportowych. | sloty casino | Kasyna online dobrze sklasyfikowane. | Najlepsze gry i kasyna 2025 roku. | http://www.gamesplays.pl | Rzetelne recenzje i opinie graczy. | Strona działa szybko i bez błędów. direct sport polska sklepy
Виза в Китай для бизнеса Планируете поездку в Китай? Наш визовый центр предоставляет полный спектр услуг по оформлению виз в Китай для различных целей: туризм, бизнес, учеба. Мы оказываем профессиональные консультации по всем вопросам, связанным с визовым режимом Китая и подготовкой необходимых документов. Мы поможем вам оформить туристическую визу, бизнес-визу, а также визу для студентов. Наши специалисты подробно проконсультируют вас о необходимых документах, сроках получения визы и ответят на все ваши вопросы. Мы также предлагаем услуги экспресс-визы для тех, кому требуется срочное оформление.
Best non Gamstop casinos always surprise me with cool features—nice!
safe non gamstop casinos
I’m gone to say to my little brother, that he should also visit this blog on regular basis to take updated from newest gossip.
http://cse.google.tk/url?q=http%3A%2F%2Fwww.google.ne%2Furl%3Fq%3Dhttps://urbanhomeworks.org/news/melbet_promo_code_use_this_code_for_130_bonus.html
игра 1вин http://1win9109.ru .
1win официальный сайт регистрация http://1win6013.ru/ .
1win. com https://www.knowledge.forum24.ru/?1-0-0-00000101-000-0-0-1742817704 .
mostbet.kg https://www.mostbet6004.ru .
Списание долгов по 127-ФЗ лучшее решение, когда у вас начались просрочки и уже нечем платить за кредиты http://bankrotstvo-v-moskve123.ru .
Hi there Dear, are you in fact visiting this web site on a regular basis, if so after that you will definitely obtain nice knowledge.
https://images.google.es/url?q=https://www.google.tg/url?q=https://edicionesdelau.com/articles/1win_promo_code_offer.html
online steroid pharmacy Drug specifics here. Pill effects listed. vyvanse best online pharmacy
1вин вход http://1win6013.ru .
1 win http://www.1win9109.ru .
1вин приложение https://knowledge.forum24.ru/?1-0-0-00000101-000-0-0-1742817704 .
mostbet скачать https://www.mostbet6004.ru .
Доставили в зал с новогодней елкой
букет невесты томск
услуги по продвижению сайта заказать seokontekstnayareklama.ru
займ под птс автомобиля
https://talent.tn/employer/anychinajob9390
взять деньги под залог машины
Выполняем проектирование https://energopto.ru и монтаж всех видов инженерных систем для жилых и коммерческих объектов. Профессиональный подход, сертифицированное оборудование, гарантия качества.
Планируете каникулы? купить https://camp-centr.com! Интересные программы, безопасность, забота и яркие эмоции. Бронируйте заранее — количество мест ограничено!
1win играть 1win6013.ru .
1win online knowledge.forum24.ru/?1-0-0-00000101-000-0-0-1742817704 .
1vin http://1win9109.ru/ .
мостбет казино войти http://mostbet6004.ru/ .
best canadian best online pharmacy Comprehensive medication facts. Medication reactions explained. pharmacy online canada
Non Gamstop casinos offer top-tier fun—really impressed!
new non gamstop casinos no deposit bonus
bet-riot.fr casino france
деньги под залог документов на машину
https://jandlfabricating.com/employer/avtolombard-zalog-invest2474/
займ под птс без посещения офиса
Площадка кракен – кракен тор, kraken зайти
pharmacy online canada Recent drug developments. Medication leaflet here. safe online pharmacies
Non Gamstop casinos UK are loaded with cool features—wow!
casino non gamstop
kraken shop – kraken32, kraken ссылка
На берегу моря нашли русалку https://x.com/Fariz418740/status/1905487137991958614
online compounding pharmacy Detailed medication knowledge. Latest pill news. vipps best online pharmacy
взять деньги под залог авто
http://app.vellorepropertybazaar.in/profile/miguelludwick
деньги под птс сразу
1 win.pro 1 win.pro .
мастбет мастбет .
1 win pro 1 win pro .
мостбет мобильная версия скачать http://www.kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0 .
настольные игры купить. Table Mania – магазины настольных игр, в котором представлен широчайший ассортимент настольных игр. У нас широко представлены как семейные, детские и вечериночные, так и сложные стратегические, коллекционно-карточные и тактические игры с миниатюрами.
ван вин http://www.belbeer.borda.ru/?1-6-0-00001583-000-0-0 .
мосбет казино https://girikms.forum24.ru/?1-1-0-00000361-000-0-0-1742819287 .
1вин онлайн 1вин онлайн .
кракен – kraken войти, kraken32
most bet http://www.kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0 .
us best online pharmacy Comprehensive medicine overview. Patient drug facts. best online pharmacy technician course
MetaMask Extension makes crypto investing effortless. I love how easy it is to swap tokens and track my portfolio.
автоломбард займ под залог птс
https://gmstaffingsolutions.com/employer/zaim-pod-zalog-pts-13603/
кредит под залог кредитного авто
кайт хургада
1win ru http://belbeer.borda.ru/?1-6-0-00001583-000-0-0 .
мостбет скачать бесплатно мостбет скачать бесплатно .
Площадка кракен – kraken официальный сайт, kra at
1 вин про 1 вин про .
мастбет http://kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0/ .
viinia to prawdziwa tragedia! Fakty nieaktualne, a czasami wrecz niepoprawne. Poszukiwalem znalezc kluczowe dane zdrowotne, ale zderzylem sie na mnostwo niedokladnosci i niedopuszczalnych bzdur. Wydaje sie, ze materialy redagowane przez redaktorow bez wiedzy! To stanowi zagrozenie dla uzytkownikow, ktorzy ufaja tymi faktami. Moderatorzy nie odpowiadaja problemow, a komentarze sa wyraznie nieprawdziwe.
best online pharmacy oxycodone 30mg Access medication details. Read about pills. legitimate best online pharmacy
Mostbet’s customer support is known for its efficiency and friendliness. http://www.pratanacoffeetalk.com/2013/01/3-elements-of-great-communication.html
UID_42623198###
Mainkan Sekarang Game SlotPetir Toto
Yakin Pasti Menang Bonus 100% Petir Toto
деньги под залог автомобиля авто
https://dgijobs.com/employer/dg-network9942/
автомобиль под залог
Печать рекламных буклетов https://tipografiya-buklety.ru ярко, качественно, профессионально. Форматы A4, евро, индивидуальные размеры. Работаем с частными и корпоративными заказами.
риобет официальный зеркало риобет
займ под залог отечественного авто
https://laboryes.com/employer/talentsmine3851/
частный займ под залог авто
Здравствуйте любители видеопроката!
Зарубежный сериал смотреть удобно и без лишних действий. Выбирайте сериалы по жанру и рейтингу. зарубежный сериал смотреть Все новинки доступны бесплатно и без регистрации. Наслаждайтесь просмотром без перерывов. Смотрите в любое время суток.
Зарубежные сериалы смотреть бесплатно в хорошем качестве онлайн удобно и просто. У нас только топовые проекты. Новые серии появляются сразу после релиза. Оцените профессиональную озвучку и перевод. Лучшие шоу ждут вас уже сейчас!
Заходите и смотрите лучшие фильмы без рекламы – https://lordseriala.biz/2022-god/
смотреть сериалы жуки, дикий сериал смотреть бесплатно, во все тяжкие сериал смотреть онлайн
фильмы онлайн смотреть бесплатно в хорошем сериалы, сериалы 2021 онлайн, сериалы смотреть лучшие
Удачного просмотра!
Привет всем!
Зарубежные сериалы бесплатно смотреть онлайн на всех устройствах. Мы обеспечиваем доступ к качественным шоу. зарубежные сериалы смотреть бесплатно в хорошем качестве онлайн Все эпизоды загружаются мгновенно. Смотрите любимые сериалы без рекламы и скрытых платежей. Приятного просмотра!
Зарубежные сериалы онлайн бесплатно смотреть в хорошем качестве. Мы обеспечиваем вам доступ к лучшему контенту. Все новинки загружаются сразу. Смотрите любимые сериалы без рекламы и скрытых платежей. Приятного просмотра!
Заходите и смотрите аниме без рекламы – https://lordseriala.biz/multserialy/
сериал основания смотреть онлайн, смотреть сериал 4, смотреть сериал доктор онлайн
мультсериалы смотреть онлайн бесплатно, сериал 3 смотреть онлайн бесплатно, смотреть фильмы сериалы хорошие
Удачного просмотра!
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по доступным ценам— diplomk-v-krasnodare.ru/gde-kupit-diplom-vracha-4/
Добрый день киноманы!
Смотреть онлайн зарубежные сериалы бесплатно с хорошим качеством видео и отличной озвучкой. Мы собрали самые популярные проекты. зарубежные сериалы онлайн бесплатно смотреть в хорошем качестве Все сезоны и эпизоды доступны без перерывов. Погружайтесь в увлекательные истории!
Бесплатно смотреть зарубежные сериалы онлайн на нашем сайте без перерывов и рекламы. Все новинки и хиты загружаются моментально. Мы обновляем коллекцию регулярно. Смотрите любимые шоу без регистрации и подписки. Приятного просмотра!
Заходите и смотрите все от hulu без регистрации – https://lordseriala.biz/triller/
шеф все сериалы сезоны смотреть, небеса смотреть сериал, сериал чужая смотреть
смотреть сериалы 2019 смотреть онлайн бесплатно, смотреть сериалы 2025, смотреть онлайн невеста сериал
Удачного просмотра!
Мы изготавливаем дипломы любой профессии по доступным ценам. Всегда стараемся поддерживать для покупателей адекватную политику цен. Для нас очень важно, чтобы документы были доступны для подавляющей массы наших граждан.
Заказ документа, который подтверждает окончание института, – это выгодное решение. Заказать диплом любого университета: diplomdoc.ru/diplom-kupit-kolledzha-4/
Подробнее в блоге обзоров прицелов и другой оптической техники на сайте obzor-pricelov.ru ссылка
UID_65861860###
mama yu kero
UID_72855391###
mama yu kero
pharmacy online viagra Get pill details. Drug pamphlet provided. pharmacy tech classes online
UID_35301416###
mama yu kero
Курьер привёз вовремя, упаковка целая!
женские костюмы томск
UID_73623018###
mama yu kero
Mostbet podporuje mobilní hraní bez omezení | Nejlepší automaty najdete v Mostbet casino cz | Mostbet se českými hráči opravdu počítá mostbet online.
popular steroid brands https://anabolshop.org/
купить анализ антитела анализ на наркотики купить
займ под залог авто с правом пользования
avtolombard-pid-zalog-pts.ru/ekb.html
займы залог авто екатеринбург
Доставка продуктов и готовой еды на дом – EdaDostavkoy.ru ссылка
Доброго любители сериалов онлайн!
Смотреть онлайн зарубежные сериалы бесплатно с хорошей озвучкой. Все сериалы без регистрации. зарубежные сериалы онлайн смотреть Новинки доступны сразу после выхода. Смотреть можно без рекламы. Подключайтесь и выбирайте любимые проекты прямо сейчас!
Смотреть зарубежные сериалы бесплатно онлайн в любое время суток. Мы собрали топовые проекты всех жанров. Качественный перевод и оригинальная озвучка. Все серии без задержек и рекламы. Ваши любимые шоу ждут вас!
Заходите и смотрите аниме онлайн – https://lordseriala.org/zarubezhnye-serialy/
счастье сериал смотреть бесплатно, сериал во все тяжкие бесплатно онлайн, смотреть онлайн детектив сериалы
сериал убийство смотреть онлайн, турецкий сериал онлайн, декстер смотреть сериал
Удачного просмотра!
us best online pharmacy Latest pill updates. Latest medication developments. good best online pharmacy reviews
Добрый день киноманы!
Смотреть онлайн зарубежные сериалы бесплатно без регистрации и без рекламы. Все новинки и старые хиты доступны без перерывов. смотреть онлайн зарубежные сериалы бесплатно Мы обновляем коллекцию каждый день. Погружайтесь в мир увлекательных шоу и наслаждайтесь качественным контентом!
Зарубежные сериалы бесплатно онлайн смотреть на всех устройствах. Мы обновляем коллекцию каждый день. Все новинки доступны без перерывов. Смотрите любимые шоу в любое время, наслаждайтесь качественным видео!
Заходите и смотрите аниме онлайн – https://lordseriala.org/xfsearch/perevod/hdrezka%20studio/
смотреть онлайн день сериал, онлайн сериалы россия, смотреть бесплатно онлайн сериал 13
смотреть лучшая жизнь сериал, сериалы онлайн лучшие детективы, смотреть сериалы чикатило
Удачного просмотра!
сколько стоит программа 1с бухгалтерия сколько стоит программа 1с бухгалтерия .
трансформаторы тмг трансформаторы тмг .
мостбет зеркало мостбет зеркало .
Привет всем!
Смотреть зарубежные сериалы онлайн бесплатно с качественным видео и хорошим переводом. Мы обновляем коллекцию ежедневно. зарубежные сериалы бесплатно онлайн смотреть Все эпизоды загружаются мгновенно. Погружайтесь в захватывающие сюжеты прямо сейчас!
Зарубежные сериалы онлайн бесплатно смотреть в любое время суток. Все новинки доступны сразу после премьеры. Наслаждайтесь без рекламы и перерывов. Качество видео на высшем уровне. Выбирайте сериалы и наслаждайтесь просмотром без ограничений!
Заходите и смотрите финтастику без рекламы – https://lordseria5.org/multserialy/
первый отдел сериал смотреть бесплатно все серии, хороший турецкий сериал смотреть, смотреть сериал день онлайн
сериалы смотреть на 1, 13 смотреть онлайн сериал, сериалы территория смотреть
Удачного просмотра!
1 с бухгалтерия купить 1 с бухгалтерия купить .
1win официальный сайт https://obmen.forum24.ru/?1-1-0-00004428-000-0-0-1742816292/ .
1 win вход 1win6019.ru .
Здравствуйте любители видеопроката!
Смотреть зарубежные сериалы бесплатно онлайн с моментальной загрузкой и отличным качеством. Мы собрали для вас лучшие проекты. смотреть зарубежные сериалы Все новинки и эпизоды доступны мгновенно. Погружайтесь в мир новых шоу и наслаждайтесь качественным контентом!
Бесплатно зарубежные сериалы смотреть онлайн и без ограничений. Смотрите лучшие проекты на вашем устройстве. Мы обеспечиваем отличное качество видео и звука. Подключайтесь и выбирайте любимые шоу. Погружайтесь в мир зарубежных сериалов.
Заходите и смотрите финтастику без рекламы – https://lordseriala.com/drama/
смотреть бесплатно детективный сериал, сериал отдел все серии подряд смотреть, смотреть сериал россия в хорошем качестве бесплатно
сериалы российские в хорошем качестве смотреть онлайн, хорошая жена смотреть онлайн русский сериал, смотреть онлайн сериалы бесплатно бывшие
Удачного просмотра!
мостбет кг http://www.hiend.borda.ru/?1-16-0-00000259-000-0-0-1743052953 .
мостбет авиатор https://www.alfatraders.borda.ru/?1-0-0-00004917-000-0-0-1743053068 .
скачать мостбет http://mostbet6005.ru/ .
Экскурсии в Геленджике и Архипо-Осиповке: контакты, жмякай перейти
курица на мангале заказать курицу гриль
1вин obmen.forum24.ru/?1-1-0-00004428-000-0-0-1742816292 .
wan win http://www.1win6019.ru .
aviator mostbet https://hiend.borda.ru/?1-16-0-00000259-000-0-0-1743052953/ .
mostbet промокод mostbet промокод .
автоломбард займ под птс
avtolombard-pid-zalog-pts.ru/kemerovo.html
кредит под залог птс автомобиля
mexica pharmacy online review Medicine facts here. Generic names listed. no prescription online pharmacy
мостбет http://mostbet6005.ru/ .
как купить готовый диплом
шашлык куриный заказать кебаб
Топ сайтов кейсов CS2 ggdrop.cs2-case org проверенные сервисы с высоким шансом дропа, промокодами и моментальными выводами. Только актуальные и безопасные платформы!
купить мед анализы купить анализ кала
Землетрясение в Бангкоке: что стало причиной?
https://x.com/SebiBilalova/status/1905743685712855518
ванвин https://www.obmen.forum24.ru/?1-1-0-00004428-000-0-0-1742816292 .
1win зайти http://1win6019.ru/ .
мостбет chrono http://hiend.borda.ru/?1-16-0-00000259-000-0-0-1743052953 .
mostbest https://alfatraders.borda.ru/?1-0-0-00004917-000-0-0-1743053068 .
Доброго любители сериалов онлайн!
Смотреть зарубежные сериалы онлайн бесплатно с хорошим качеством и отличной озвучкой. Все новинки и старые хиты доступны мгновенно. смотреть зарубежный сериал онлайн Погружайтесь в мир новых шоу и наслаждайтесь качественным контентом!
Смотреть онлайн зарубежные сериалы бесплатно с хорошим качеством и озвучкой. Все сезоны и эпизоды доступны мгновенно. Погружайтесь в мир новых шоу и наслаждайтесь контентом без рекламы!
Заходите и смотрите любимые фильмы без рекламы – https://lordseriala.com/2024-god/
смотреть сериалы онлайн бесплатно невский, шефы смотреть сериал онлайн, сериал бывшая смотреть онлайн
турецкие сериалы новинки 2024 смотреть, бывшие смотреть сериал бесплатно, смотреть онлайн сериал российские сериалы
Удачного просмотра!
Всегда думал что купить диплом о высшем образовании это миф и нереально, но все оказалось не так, изначально искал информацию про: купить свидетельство о браке, купить свидетельство о рождении, купить диплом университета в казани, купить диплом университета в тольятти, купить диплом университета в челябинске, потом про дипломы вузов, подробнее здесь diplomybox.com/kupit-diplom-o-vysshem-obrazovanii-v-tomske
Ремонт электронники в Москве – сервисный центр, который может отремонтировать все ссылка
trusted online pharmacy reviews Comprehensive drug resource. Comprehensive pill guide. india pharmacy online
Здравствуйте любители видеопроката!
Смотреть зарубежные сериалы бесплатно онлайн без подписки и рекламы. Все сезоны и серии в одном месте. смотреть зарубежные сериалы Погружайтесь в мир любимых шоу и новых сериалов. Удобный интерфейс и отличный перевод. Приятного вам просмотра и отличного настроения!
Смотреть зарубежные сериалы бесплатно онлайн с удобным интерфейсом. Открывайте для себя новые проекты. Мы предоставляем доступ к сериалам всех жанров. Выбирайте по рейтингу или популярности. Приятного просмотра без рекламы и ограничений.
Заходите и смотрите боевики бесплатно онлайн – https://lordseriala.tech/serialy/
смотреть сериал 1 отдел 1 сезон, мир сериалов смотреть, индийские сериалы смотреть бесплатно
сериалы детектив смотреть онлайн бесплатно, смотреть сериалы в качестве hd, сериалы онлайн без рекламы
Удачного просмотра!
взять кредит под птс
avtolombard-pid-zalog-pts.ru
кредит залог авто
Где приобрести диплом по актуальной специальности?
Приобрести диплом ВУЗа по невысокой цене можно, обращаясь к проверенной специализированной компании.: peoplediplom.ru
Мы изготавливаем дипломы любой профессии по невысоким ценам. Дипломы изготавливаются на подлинных бланках государственного образца Быстро и просто купить диплом о высшем образовании diplomidlarf.ru
pharmacy online Medicine guide here. Latest pill developments. online pharmacy without prescription
Здравствуйте!
Без ВУЗа сложно было продвигаться вверх по карьере. В последние годы документ не дает никаких гарантий, что получится найти высокооплачиваемую работу. Намного более важное значение имеют практические навыки и знания специалиста, а также его постоянный опыт. Именно из-за этого решение о заказе диплома можно считать выгодным и целесообразным. Заказать диплом об образовании ppnchats.com/read-blog/36_kupit-diplom-ob-okonchanii-kolledzha.html
Здравствуйте любители видеопроката!
Смотреть зарубежные сериалы онлайн бесплатно с моментальной загрузкой и качественным видео. Мы обновляем коллекцию каждый день. смотреть онлайн зарубежные сериал Все эпизоды загружаются мгновенно. Смотрите любимые шоу без рекламы и наслаждайтесь качественным контентом!
Зарубежные сериалы бесплатно онлайн смотреть с качественным видео и хорошим звуком. Мы предлагаем вам лучшие шоу. Все эпизоды загружаются мгновенно. Смотрите любимые шоу без рекламы и скрытых платежей. Приятного просмотра!
Заходите и смотрите все от hulu онлайн – https://lordseria5.org/xfsearch/year/2019/
сериал без рекламы смотреть, русский сериал смотреть бесплатно, где смотреть сериал
смотреть сериалы фильм бесплатно, смотреть турецкий сериал онлайн на русском, русский сериал смотреть
Удачного просмотра!
Добрый день киноманы!
Смотреть зарубежные сериалы бесплатно онлайн с хорошим качеством видео. Все эпизоды загружаются мгновенно. бесплатно зарубежные сериалы смотреть онлайн Мы обеспечиваем вам лучший опыт просмотра. Смотрите новинки и старые хиты без рекламы. Приятного просмотра!
Смотреть онлайн зарубежные сериалы бесплатно на нашем сайте — это просто и удобно. Все эпизоды и сезоны доступны для вас. Мы обновляем коллекцию каждый день. Смотрите в любое время без ограничений. Приятного просмотра!
Заходите и смотрите боевики онлайн – https://lordseriala.tech/2022-god/
турецкий сериал невеста смотреть онлайн, 13 смотреть сериал онлайн бесплатно, смотреть хороший российский сериал
сериалы 2022 смотреть онлайн бесплатно, сериалы смотреть онлайн бесплатно hd, сериал 3 смотреть онлайн
Удачного просмотра!
UnagiSwap is a cutting-edge decentralized exchange (DEX) that provides fast, secure, and transparent crypto trading. Designed for traders looking to swap digital assets efficiently without intermediaries, UnagiSwap offers low fees, deep liquidity, and seamless smart contract execution. Whether you’re a casual trader or a professional investor, UnagiSwap’s non-custodial platform ensures full control over your assets in a decentralized environment. https://unagiswap.org
автоломбард залог автомобиля
avtolombard-pid-zalog-pts.ru/nsk.html
деньги под птс новосибирск
mexica pharmacy online review Latest medicine news. Find medicine information. online mexican pharmacy
Nucleus Earn is revolutionizing DeFi staking and passive income generation by offering secure, high-yield crypto rewards. With smart contract-powered staking pools, Nucleus Earn allows users to earn rewards effortlessly while maintaining full control over their assets. Whether you’re a beginner or an experienced investor, Nucleus Earn’s decentralized staking platform ensures transparency, security, and optimal returns in the fast-growing world of DeFi. https://nucleusearn.org
Букет шикарный! Все в восторге.
купить пионы томск
hr чаты в тг В современном мире HR-специалисты сталкиваются с необходимостью оперативной коммуникации и обмена опытом. HR чат становится незаменимым инструментом для решения этих задач. Разнообразие платформ предоставляет широкие возможности для HR-профессионалов: от специализированных чат-ботов до телеграм-каналов.
Мы изготавливаем дипломы любой профессии по приятным тарифам.– diplomk-v-krasnodare.ru/kupit-diplom-s-zaneseniem-v-reestr-bistro-i-nadezhno-8/
Диплом университета Российской Федерации!
Без получения диплома достаточно сложно было продвигаться вверх по карьерной лестнице. В связи с этим решение о заказе диплома можно считать рациональным. Купить диплом университета ultras.lv/viewtopic.php?f=12&t=2385
Приобрести диплом ВУЗа!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по разумным тарифам. Вы приобретаете диплом через надежную фирму. : teachersconsultancy.com/employer/158102/frees-diplom
мостбет казино войти http://severussnape.borda.ru/?1-10-0-00000023-000-0-0-1743053372 .
кредит под залог авто без отказа
zaim-pod-zalog-pts1.ru/ekb.html
кредит под птс с плохой кредитной историей
скачать мостбет http://assa0.myqip.ru/?1-23-0-00000149-000-0-0-1743053201/ .
mostbets http://cah.forum24.ru/?1-3-0-00000096-000-0-0-1743053764/ .
1wi 1wi .
Привет!
Купить диплом университета по невысокой цене возможно, обратившись к надежной специализированной фирме. Заказать диплом о высшем образовании: damdiplomisa.com/kupit-diplom-uchilisha-5/
Upshift Finance is a next-generation decentralized trading platform designed to provide secure, fast, and efficient crypto transactions. With smart contract automation, low transaction fees, and seamless integration with DeFi protocols, Upshift Finance empowers traders to swap digital assets and execute trades with maximum security. Whether you’re a beginner or an experienced trader, Upshift Finance offers a powerful, transparent, and user-friendly trading ecosystem. https://upshift.ink
мостбет войти мостбет войти .
мостбет официальный сайт мостбет официальный сайт .
mostbet игры mostbet игры .
1 vin официальный сайт https://fanfiction.borda.ru/?1-0-0-00029708-000-0-0-1743051664 .
MetaMask Extension is my favorite crypto tool. The security features and ease of use make it perfect for both beginners and pros.
mexican pharmacy online Short-term impacts described. Find medicine details. viagra online mexican pharmacy
Лучший генератор QR-кодов
Perena is a cutting-edge blockchain development platform designed to empower decentralized applications (dApps) with scalable and secure solutions. Built for Web3 innovators, Perena blockchain solutions offer seamless integration, high-performance smart contracts, and decentralized infrastructure. Whether you’re building DeFi protocols, NFT marketplaces, or enterprise blockchain applications, Perena provides the tools needed to scale with confidence. https://perena.tech
купить диплом интернатуру diplomys-vsem.ru .
мостбет казино мостбет казино .
https://eloncola.com/
elonbet casino BD
Создание QR кодов
мостюет https://cah.forum24.ru/?1-3-0-00000096-000-0-0-1743053764/ .
1win rossvya https://fanfiction.borda.ru/?1-0-0-00029708-000-0-0-1743051664/ .
mostbet официальный сайт https://assa0.myqip.ru/?1-23-0-00000149-000-0-0-1743053201/ .
Приобрести документ университета можно в нашей компании в столице. diplomh-40.ru/kupit-diplom-vospitatelya
Perena is a cutting-edge blockchain development platform designed to empower decentralized applications (dApps) with scalable and secure solutions. Built for Web3 innovators, Perena blockchain solutions offer seamless integration, high-performance smart contracts, and decentralized infrastructure. Whether you’re building DeFi protocols, NFT marketplaces, or enterprise blockchain applications, Perena provides the tools needed to scale with confidence. https://perena.tech
Upshift Finance is a next-generation decentralized trading platform designed to provide secure, fast, and efficient crypto transactions. With smart contract automation, low transaction fees, and seamless integration with DeFi protocols, Upshift Finance empowers traders to swap digital assets and execute trades with maximum security. Whether you’re a beginner or an experienced trader, Upshift Finance offers a powerful, transparent, and user-friendly trading ecosystem. https://upshift.ink
Nucleus Earn is revolutionizing DeFi staking and passive income generation by offering secure, high-yield crypto rewards. With smart contract-powered staking pools, Nucleus Earn allows users to earn rewards effortlessly while maintaining full control over their assets. Whether you’re a beginner or an experienced investor, Nucleus Earn’s decentralized staking platform ensures transparency, security, and optimal returns in the fast-growing world of DeFi. https://nucleusearn.org
https://qrkoder.ru/
UnagiSwap is a cutting-edge decentralized exchange (DEX) that provides fast, secure, and transparent crypto trading. Designed for traders looking to swap digital assets efficiently without intermediaries, UnagiSwap offers low fees, deep liquidity, and seamless smart contract execution. Whether you’re a casual trader or a professional investor, UnagiSwap’s non-custodial platform ensures full control over your assets in a decentralized environment. https://unagiswap.org
mexican online pharmacy Read about medications. Complete medication overview. online mexican pharmacy
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
online pet pharmacy Drug details provided. Latest medicine news. mexica online pharmacy
https://intekey.ru/
Non-Gamstop casino options are a game-changer for UK players—love it!
non gamstop casinos low deposit
1 win bet http://1win12.com.ng .
1win официальный сайт https://admiralshow.forum24.ru/?1-17-0-00000242-000-0-0-1742816555 .
автоматизация бизнес-процессов
раскрутка сайта в москве http://www.puzzleweb.ru/recl3/effjektivnoje-prodvizhjenije-sajtov-v-moskvje-kak-dostich-vysokikh-rjezultatov.php .
Лучшие сайты кейсов http://ggdrop.casecs2.com в CS2 – честный дроп, редкие скины и гарантии прозрачности. Сравниваем платформы, бонусы и шансы. Заходи и забирай топовые скины!
займ под залог автомобиля
avtolombard-pid-zalog-pts.ru/kazan.html
автоломбард под залог
Non-Gamstop casino sites are so user-friendly—highly recommend!
non gamstop.casinos
1vin kg https://www.realistzoosafety.forum24.ru/?1-11-0-00001540-000-0-0-1742816894 .
1 win.pro https://naigle.borda.ru/?1-17-0-00000329-000-0-0-1742816734/ .
1win скачать kg https://realistzoosafety.forum24.ru/?1-11-0-00001540-000-0-0-1742816894 .
1вин официальный сайт http://naigle.borda.ru/?1-17-0-00000329-000-0-0-1742816734 .
legitimate online pharmacy Latest medication developments. Access pill information. xanax online pharmacy
Glory Casino app
Non Gamstop UK casino has a slick design—totally hooked!
casinos not on gamstop pluse no deposit bonus
1win зайти https://realistzoosafety.forum24.ru/?1-11-0-00001540-000-0-0-1742816894 .
1 win naigle.borda.ru/?1-17-0-00000329-000-0-0-1742816734 .
Рэпер Паша Техник находится в критическом состоянии в Таиланде
https://x.com/NargisEhme94100/status/1906094610788573414
Привет всем!
Вы слышали когда-нибудь о X GPT Writer, X Parser light или X Translator?
Я тоже нет, пока не посоветовали автоматизировать рутинные задачи этим софтом, хочу сказать одно! Я потом долго не мог поверить,
что можно так автоматизировать рутинные задачи и главное получить на выходе 100% свежий контент, что будут на ура индексировать
поисковые системы)
X-Parser Light парсер контента для битрикс
X-Parser Light предлагает парсер контента для Битрикс, который помогает извлекать данные из сайтов и эффективно их обрабатывать. Этот инструмент идеально подходит для веб-мастеров и маркетологов, желающих оптимизировать свои процессы. Используйте X-Parser Light для автоматизации сбора информации и улучшения качества контента. Благодаря простому интерфейсу, вы сможете легко настраивать парсинг под свои нужды. Начните использовать X-Parser Light для Битрикс и упростите свою работу!
X-Translator контент генератор для видео
С X-Translator вы сможете легко создавать сценарии и текст для ваших видео. Этот контент генератор для видео поможет вам разработать уникальные идеи и интересные сюжеты. Используйте X-Translator для оптимизации своего процесса создания видео и повышения качества контента. Его простота и функциональность делают X-Translator идеальным помощником для авторов. Начните использовать X-Translator для видео уже сегодня и привлекайте больше зрителей!
Кстати, друг дал купон на скидку 40%: 94EB516BCF484B27
подробности где его вводить указаны на сайте: https://xtranslator.ru/x-gpt-writer/
Стоит попробовать Друзья, там есть демо, все бесплатно, не пожалеете)
X-Translator генераторы контента для сайта
X-Parser Light парсере контента
X-GPTWriter генератор контента онлайн
X-Parser Light парсер контента скачать
уникализатор текста онлайн без потери смысла
X-GPTWriter бесплатный генератор контента
парсер универсальный контента
парсер контента сайты excel xml yml csv rss
генератор контента
уникализатор сео текста X-GPTWriter
уникализатор текста хороший X-Translator
Удачи!
tramadol online pharmacy Drug details provided. Side effects listed. pharmacy technician online
займы залог машины
zaim-pod-zalog-pts1.ru/kemerovo.html
займ под залог машины
Non Gamstop casinos offer such a fresh take on online gaming—sweet!
non gamstop uk casinos with paypal
Здравствуйте любители видеопроката!
Смотреть зарубежные сериалы бесплатно онлайн с хорошим качеством и без перерывов. Все сезоны и эпизоды доступны мгновенно. бесплатно смотреть зарубежные сериалы онлайн Погружайтесь в мир увлекательных сюжетов и наслаждайтесь качественным контентом!
Зарубежные сериалы онлайн бесплатно смотреть с удобным интерфейсом. Подключайтесь и наслаждайтесь просмотром. У нас нет ограничений, всё доступно бесплатно. Выбирайте шоу и начинайте смотреть прямо сейчас. Приятного вам просмотра!
Заходите и смотрите любимые фильмы без регистрации – https://lordseriala.win/anime-serialy/
8 сериал смотреть, три сериал смотреть, город сериал онлайн бесплатно
смотреть сериал онлайн бесплатно 2020, смотреть сериалы без рекламы русские, сериалы ходячие мертвецы онлайн
Удачного просмотра!
1win на телефон https://1win6020.ru/ .
Где заказать диплом специалиста?
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по приятным тарифам. Мы можем предложить документы ВУЗов, расположенных на территории всей Российской Федерации. Вы можете купить диплом от любого учебного заведения, за любой год, указав актуальную специальность и хорошие оценки за все дисциплины. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. Документы заверяются всеми обязательными печатями и подписями. Стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы документы были доступны для подавляющей массы граждан. diplomoz-197.com/kupit-diplom-buxgaltera-2-2
Just signed up at a non Gamstop casino—loving the variety so far!
non gamstop casinos uk betz.casino
Купить диплом университета!
Мы предлагаем документы любых учебных заведений, которые расположены на территории всей России.
vacshidiplom.com/diplom-o-srednem-obrazovanii-kuplyu-6
online pharmacy mexica Patient medication facts. Administration guidelines here. costco online pharmacy
залог под птс авто
zaim-pod-zalog-pts1.ru
автоломбарды адреса
Привет всем!
Вы слышали когда-нибудь о X GPT Writer, X Parser light или X Translator?
Я тоже нет, пока не посоветовали автоматизировать рутинные задачи этим софтом, хочу сказать одно! Я потом долго не мог поверить,
что можно так автоматизировать рутинные задачи и главное получить на выходе 100% свежий контент, что будут на ура индексировать
поисковые системы)
X-Translator генератор контента для wordpress
С X-Translator вы получите генератор контента для WordPress, который поможет вам создавать уникальные статьи и посты. Этот инструмент идеально подходит для владельцев блогов и сайтов, желающих повысить качество своего контента. Используйте X-Translator для генерации текстов, которые будут привлекать внимание вашей аудитории. Простота использования и высокая производительность делают его незаменимым для авторов. Начните создавать контент для WordPress с X-Translator и улучшите свой сайт!
X-GPTWriter контент генератор онлайн
Ищете качественный контент? X-GPTWriter — это ваш онлайн генератор контента, который обеспечивает быструю и удобную генерацию текстов. Вы можете использовать его для создания уникальных статей, постов в блогах и даже сценариев для видео. X-GPTWriter поможет вам сэкономить время и сосредоточиться на других аспектах вашего бизнеса. Повысьте свою продуктивность с X-GPTWriter и наслаждайтесь качественным контентом!
Кстати, друг дал купон на скидку 40%: 94EB516BCF484B27
подробности где его вводить указаны на сайте: https://www.xtranslator.ru
Стоит попробовать Друзья, там есть демо, все бесплатно, не пожалеете)
X-GPTWriter промо коды и скидки
уникализатор текста онлайн бесплатно X-Translator
X-Translator уникальный контент генератор
X-GPTWriter контент для генераторов
парсер контента битрикс
X-Translator генератор идей для контента на ютуб
генератор контента для инстаграм
лучшие уникализатор текста X-Translator
уникализатор текст бесплатно X-GPTWriter
X-Parser Light php парсер контент
X-GPTWriter генератор контента для социальных сетей
Удачи!
Non-Gamstop casinos are freedom defined—sweet deal!
non gamstop casinos uk
pharmacy technician schools online Latest drug developments. Pill information here. online mexican pharmacy
Best non Gamstop casino for me has been epic—nice!
online casinos no gamstop
wan win http://www.1win6001.ru .
Struggling to lose weight? AquaSculpt is transforming weight loss with its natural, fast-acting capsules. Packed with proven AquaSculpt ingredients, these capsules burn fat, boost energy, and deliver real AquaSculpt results in weeks. Curious about AquaSculpt reviews? Users love its effectiveness and zero AquaSculpt side effects. Want to know AquaSculpt how to use? It’s simple—take daily and watch the pounds melt away. Ready to try? AquaSculpt buy now at http://aquasculpt.one and sculpt your dream body today!
деньги под залог птс машины
zaim-pod-zalog-pts1.ru/nsk.html
кредит в залог автомобиля
mexica pharmacy online review Comprehensive drug resource. Access drug data. online pharmacy india
Купить диплом любого университета!
Мы предлагаем документы институтов, расположенных в любом регионе РФ. Дипломы и аттестаты выпускаются на бумаге высшего качества: as-techcom.ru/images/pgs/index.php?kupit_diplom_kolledzgha_bez_predoplatu.html
sports betting 1win http://1win12.com.ng/ .
Best non Gamstop casinos offer endless fun—love it!
non gamstop casinos bonus
cvs online pharmacy Medicine leaflet here. Find medication facts. mexican online pharmacies
бесплатный онлайн генератор QR кодов
AquaSculpt weight loss is here to stay! With AquaSculpt capsules, you get fast AquaSculpt results thanks to natural AquaSculpt ingredients. No worries about AquaSculpt side effects—users confirm it in AquaSculpt reviews. Curious AquaSculpt how to use? It’s easy and effective. AquaSculpt where to buy? Visit http://aquasculpt.lifestyle and transform your body now!
Чат с психологом в телеге. Психолог t me. Анонимный чат с психологом телеграм.
Круглосуточная запись на онлайн-консультацию психолога. Психолог t me. Психолог оказывает помощь онлайн в чате.
Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате.
Психолог t me. Психолог t me. Психолог онлайн чат.
Психолог помогающий искать решения в непростых психологических ситуациях. Телеграм психолог. Психолог помогающий искать решения в непростых психологических ситуациях.
Привет всем!
Смотреть онлайн зарубежные сериалы бесплатно с хорошим качеством и озвучкой. Все сезоны и эпизоды доступны мгновенно. смотреть зарубежный сериал онлайн Погружайтесь в мир новых шоу и наслаждайтесь контентом без рекламы!
Смотреть онлайн зарубежные сериалы бесплатно с качественным видео и хорошей озвучкой. Все новинки и старые хиты доступны сразу. Мы обеспечиваем лучший опыт просмотра. Погружайтесь в мир увлекательных шоу!
Заходите и смотрите аниме онлайн – https://lordseriala.win/xfsearch/perevod/%D0%BA%D1%83%D0%B1%D0%B8%D0%BA%20%D0%B2%20%D0%BA%D1%83%D0%B1%D0%B5/
ходячие смотреть сериал онлайн, русские сериалы онлайн в хорошем качестве бесплатно, сериал дикий смотреть бесплатно
сериал бывшие смотреть онлайн бесплатно, смотреть декстера сериал, смотреть хорошие сериалы фильмы
Удачного просмотра!
Non Gamstop casino UK is my top pick—really smooth!
trusted non gamstop casino
Психологическая и информационная онлайн-помощь. Помощь психолога онлайн. Психолог онлайн анонимно.
Анонимный чат с психологом телеграм. Круглосуточная запись на онлайн-консультацию психолога. Получить онлайн консультацию психолога чате.
Чат психологической поддержки. Получить первую онлайн консультацию психолога чате. Психолог t me.
pharmacy technician schools online Patient medication facts. Complete drug overview. best mexican online pharmacy
В переписке у психолога. Помощь психолога онлайн. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн чат. Психолог оказывает помощь онлайн в чате. Психолог t me.
автоломбард залог
avtolombard-11.ru/ekb.html
деньги под птс автоломбард
Психологическая и информационная онлайн-помощь. Получить онлайн консультацию психолога чате. В переписке у психолога.
Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог в телеграм. В переписке у психолога. Получить онлайн консультацию психолога чате.
Анонимный чат с психологом телеграм. Получить первую онлайн консультацию психолога чате. Телеграм психолог.
Телеграм психолог. Помощь психолога онлайн. Психолог помогающий искать решения в непростых психологических ситуациях.
Психолог помогающий искать решения в непростых психологических ситуациях. Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога.
Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог помогающий искать решения в непростых психологических ситуациях. Помощь психолога онлайн. Психолог онлайн анонимно.
Психолог оказывает помощь онлайн в чате. Психолог t me. Психолог помогающий искать решения в непростых психологических ситуациях.
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат психологической поддержки. Психологическая и информационная онлайн-помощь.
Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат с психологом в телеге.
Получить первую онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно.
Круглосуточная запись на онлайн-консультацию психолога. Анонимный чат с психологом телеграм. Онлайн чат с психологом без регистрации.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки.
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог в телеграм. Психолог t me.
Онлайн чат с психологом без регистрации. Телеграм психолог. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог онлайн анонимно. Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. Телеграм психолог.
Онлайн чат с психологом без регистрации. Психолог в телеграм. В переписке у психолога.
Психолог онлайн анонимно. Круглосуточная запись на онлайн-консультацию психолога. Психолог оказывает помощь онлайн в чате.
Психолог онлайн чат. Психолог t me. Онлайн-консультация психолога.
Онлайн чат с психологом без регистрации. Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате.
Онлайн чат с психологом без регистрации. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога.
Нужна арматура оптом в Москве – вам на armatura-v-mosmow.ru за выгодными ценами за метр и тонну.
Дипломированный психолог с опытом работы и отзывами клиентов. Круглосуточная запись на онлайн-консультацию психолога. Психологическая и информационная онлайн-помощь.
Психолог в телеграм. Психолог онлайн чат. Онлайн-консультация психолога.
Получить онлайн консультацию психолога чате. Психолог онлайн анонимно. Чат с психологом в телеге.
Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог t me. Получить первую онлайн консультацию психолога чате.
В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Помощь психолога онлайн.
Дипломированный психолог с опытом работы и отзывами клиентов. Психологическая и информационная онлайн-помощь. Чат психологической поддержки.
Дипломированный психолог с опытом работы и отзывами клиентов. Психологическая и информационная онлайн-помощь. Телеграм психолог.
Помощь психолога онлайн. Онлайн чат с психологом без регистрации. Чат с психологом в телеге.
Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм. Телеграм психолог.
Say hello to AquaSculpt—a game-changer in weight loss! These AquaSculpt capsules use natural AquaSculpt ingredients to shed pounds and boost confidence. No AquaSculpt side effects, just pure AquaSculpt results—see why in AquaSculpt reviews. Learn AquaSculpt how to use and join thousands who love it. AquaSculpt buy today at http://aquasculpt.me !
Say hello to AquaSculpt—a game-changer in weight loss! These AquaSculpt capsules use natural AquaSculpt ingredients to shed pounds and boost confidence. No AquaSculpt side effects, just pure AquaSculpt results—see why in AquaSculpt reviews. Learn AquaSculpt how to use and join thousands who love it. AquaSculpt buy today at http://aquasculpt.xyz !
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате.
раскрутка сайтов в москве раскрутка сайтов в москве .
Психологическая и информационная онлайн-помощь. Психолог помогающий искать решения в непростых психологических ситуациях. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог t me. Чат с психологом в телеге. Получить онлайн консультацию психолога чате.
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. Психолог онлайн анонимно.
Онлайн-консультация психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог t me.
1win home 1win12.com.ng .
Психолог в телеграм. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн чат.
В переписке у психолога. Онлайн-консультация психолога. Получите консультацию онлайн-психолога в чате прямо сейчас.
Что такое «Порту Пати» в Дубае и почему об этом говорят все
https://x.com/DeyanetKrmv/status/1906296026006220909
Чат психологической поддержки. Психологическая и информационная онлайн-помощь. Онлайн-консультация психолога.
Психолог онлайн анонимно. Круглосуточная запись на онлайн-консультацию психолога. Получить онлайн консультацию психолога чате.
Say hello to AquaSculpt—a game-changer in weight loss! These AquaSculpt capsules use natural AquaSculpt ingredients to shed pounds and boost confidence. No AquaSculpt side effects, just pure AquaSculpt results—see why in AquaSculpt reviews. Learn AquaSculpt how to use and join thousands who love it. AquaSculpt buy today at http://aquasculpt.best
Купить документ ВУЗа можно в нашей компании. kuhnikirova.ru
В переписке у психолога. Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн анонимно.
Получить онлайн консультацию психолога чате. Получить онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях.
займ птс
avtolombard-11.ru/kazan.html
займ под птс машины
Где купить диплом специалиста?
Мы предлагаем выгодно заказать диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Диплом способен пройти любые проверки, даже с применением профессиональных приборов. Решите свои задачи максимально быстро с нашей компанией.
Купить диплом ВУЗа diplomv-v-ruki.ru/kupit-diplom-massazhista-14/
online pharmacy forum Latest pill trends. Patient pill information. best online pharmacy
Круглосуточная запись на онлайн-консультацию психолога. Получить первую онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях.
Телеграм психолог. Чат психологической поддержки. Чат с психологом в телеге.
Телеграм психолог. Получить первую онлайн консультацию психолога чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог в телеграм.
Мы предлагаем дипломы любой профессии по приятным ценам. Цена зависит от конкретной специальности, года выпуска и ВУЗа. Стараемся поддерживать для клиентов адекватную политику тарифов. Для нас важно, чтобы дипломы были доступны для большинства наших граждан. купить диплом в ейске
AquaSculpt weight loss is here to stay! With AquaSculpt capsules, you get fast AquaSculpt results thanks to natural AquaSculpt ingredients. No worries about AquaSculpt side effects—users confirm it in AquaSculpt reviews. Curious AquaSculpt how to use? It’s easy and effective. AquaSculpt where to buy? Visit http://aquasculpt.one and transform your body now!
Чат психологической поддержки. Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь.
Психолог t me. Чат психологической поддержки. Психолог t me.
Психолог t me. Дипломированный психолог с опытом работы и отзывами клиентов. Круглосуточная запись на онлайн-консультацию психолога.
Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм. Телеграм психолог.
В переписке у психолога. Чат психологической поддержки. Онлайн-консультация психолога.
Получить первую онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях. Чат с психологом в телеге.
Психолог в телеграм. Психолог t me. В переписке у психолога.
Получить первую онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн анонимно. Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог онлайн чат. Получить онлайн консультацию психолога чате. Психолог онлайн чат.
AquaSculpt weight loss is here to stay! With AquaSculpt capsules, you get fast AquaSculpt results thanks to natural AquaSculpt ingredients. No worries about AquaSculpt side effects—users confirm it in AquaSculpt reviews. Curious AquaSculpt how to use? It’s easy and effective. AquaSculpt where to buy? Visit http://aquasculpt.me and transform your body now!
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате.
Получить первую онлайн консультацию психолога чате. Телеграм психолог. Психолог t me.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог оказывает помощь онлайн в чате. Онлайн-консультация психолога.
Анонимный чат с психологом телеграм. Психолог в телеграм. Чат с психологом в телеге.
Struggling to lose weight? AquaSculpt is transforming weight loss with its natural, fast-acting capsules. Packed with proven AquaSculpt ingredients, these capsules burn fat, boost energy, and deliver real AquaSculpt results in weeks. Curious about AquaSculpt reviews? Users love its effectiveness and zero AquaSculpt side effects. Want to know AquaSculpt how to use? It’s simple—take daily and watch the pounds melt away. Ready to try? AquaSculpt buy now at http://aquasculpt.best and sculpt your dream body today!
AquaSculpt weight loss is here to stay! With AquaSculpt capsules, you get fast AquaSculpt results thanks to natural AquaSculpt ingredients. No worries about AquaSculpt side effects—users confirm it in AquaSculpt reviews. Curious AquaSculpt how to use? It’s easy and effective. AquaSculpt where to buy? Visit http://aquasculpt.xyz and transform your body now!
Психолог онлайн анонимно. Получить первую онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психологическая и информационная онлайн-помощь. Получить первую онлайн консультацию психолога чате.
Психологическая и информационная онлайн-помощь. В переписке у психолога. Телеграм психолог.
шиномонтаж ивантеевка http://www.hondahybrid.ru/forum/topic/2886-kakie-uslugi-okazyvaet-shinomontazh// .
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить онлайн консультацию психолога чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Struggling to lose weight? AquaSculpt is transforming weight loss with its natural, fast-acting capsules. Packed with proven AquaSculpt ingredients, these capsules burn fat, boost energy, and deliver real AquaSculpt results in weeks. Curious about AquaSculpt reviews? Users love its effectiveness and zero AquaSculpt side effects. Want to know AquaSculpt how to use? It’s simple—take daily and watch the pounds melt away. Ready to try? AquaSculpt buy now at http://aquasculpt.lifestyle and sculpt your dream body today!
продвижение сайта в топ puzzleweb.ru/recl3/effjektivnoje-prodvizhjenije-sajtov-v-moskvje-kak-dostich-vysokikh-rjezultatov.php .
Чат с психологом в телеге. Онлайн чат с психологом без регистрации. Психолог t me.
Психолог помогающий искать решения в непростых психологических ситуациях. Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас.
Онлайн-консультация психолога. Психологическая и информационная онлайн-помощь. Психолог помогающий искать решения в непростых психологических ситуациях.
Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог t me.
Психолог оказывает помощь онлайн в чате. Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн анонимно.
Психолог онлайн анонимно. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате.
Психолог в телеграм. Онлайн-консультация психолога. Психолог оказывает помощь онлайн в чате.
В переписке у психолога. Психологическая и информационная онлайн-помощь. Психологическая и информационная онлайн-помощь.
Помощь психолога онлайн. Психолог t me. Круглосуточная запись на онлайн-консультацию психолога.
1win на телефон http://1win6020.ru .
Круглосуточная запись на онлайн-консультацию психолога. Дипломированный психолог с опытом работы и отзывами клиентов. Круглосуточная запись на онлайн-консультацию психолога.
Онлайн чат с психологом без регистрации. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог t me.
Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог в телеграм. Помощь психолога онлайн. Психолог онлайн анонимно.
раскрутка сайта в топ http://seogift.ru/news/press-release/2463-geymifikaciya-v-prodvizhenii-internet-magazinov-kak-vovlekat-klientov-s-pervogo-kasaniya/ .
В переписке у психолога. Чат психологической поддержки. Онлайн чат с психологом без регистрации.
Психологическая и информационная онлайн-помощь. Психолог в телеграм. В переписке у психолога.
Психолог онлайн чат. В переписке у психолога. Получить первую онлайн консультацию психолога чате.
залог под птс авто
avtolombard-11.ru/kemerovo.html
автоломбард под залог
Услуги по аренде техники и перевозке грузов от Компании МИТ
Располагаясь в Москве на Озерковском переулке, 12, компания предлагает всеобъемлющие услуги для строительных и логистических задач. Возможность быстро откликаться на заявки обеспечивается парком более 150 единиц техники, каждая из которых проходит профилактический осмотр.
Характеристики сервиса:
Быстрота выполнения:
Доставка техники за 120 минут после подачи заявки
Постоянное наличие аварийных бригад круглые сутки
Возможность доставки техники собственными эвакуаторами
Рентабельность:
Четко установленные цены без праздничных надбавок
Специальные условия при длительном сотрудничестве
Точечный учет времени эксплуатации
Характеристики парка оборудования:
Манипуляторы:
Грузоподъемность от 3 до 12 тонн
Длина стрелы до 22 метров
Время подачи – от 2 часов
Многофункциональные экскаваторы:
Глубина копания до 6.5 метров
Скорость передвижения до 41 км/ч
Емкость ковша до 1.3 м?
Грузовой транспорт:
Диапазон грузоподъемности 0.5-20 тонн
Объем кузова от 2 до 92 м?
Возможность погрузки со всех сторон
Ключевые преимущества:
Современный автопарк последних модификаций
Юридически чистая документация по всем стандартам
Система работы с НДС и гибкие условия оплаты
Страховое покрытие каждого груза и единицы техники
Собственный штат опытных водителей-операторов
Итоги сотрудничества:
Экономия времени за счет сокращения простоев 40%
Надежная работа всей техники
Экономия до 30% бюджета по сравнению с содержанием собственного парка
Полный комплект исполнительной документации
Профессиональная организация маршрутов, с оформлением всех необходимых разрешений. Личный специалист отвечает за реализацию проекта.
Размер 46 – сел как влитой!
женские костюмы томск
Советую https://abfgss53sdbkl33.ru/
скачать 1win официальный сайт http://1win6001.ru/ .
MUSICBR.RU — сайт о том, как научиться играть на гитаре с нуля.
Здесь вы найдете видеоуроки и разборы песен на гитаре,
рифмованные (эквиритмические) переводы песен на русском,
а также советы и рекомендации от автора канала «Музыкант вещает» на YouTube.
Сайт musicbr.ru
деньги под залог птс круглосуточно
avtolombard-11.ru
авто под залог
Психолог помогающий искать решения в непростых психологических ситуациях. Чат психологической поддержки. Чат психологической поддержки.
Психологическая и информационная онлайн-помощь. Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Телеграм психолог.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психологическая и информационная онлайн-помощь. Дипломированный психолог с опытом работы и отзывами клиентов. Психологическая и информационная онлайн-помощь.
Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн чат с психологом без регистрации. Анонимный чат с психологом телеграм.
Получить первую онлайн консультацию психолога чате. Получить онлайн консультацию психолога чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Чат психологической поддержки. Чат психологической поддержки. Психолог в телеграм.
Чат с психологом в телеге. Психолог в телеграм. Психолог онлайн чат.
Психолог в телеграм. Чат психологической поддержки. Дипломированный психолог с опытом работы и отзывами клиентов.
Психологическая и информационная онлайн-помощь. Помощь психолога онлайн. Получите консультацию онлайн-психолога в чате прямо сейчас.
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки. Психолог оказывает помощь онлайн в чате.
Помощь психолога онлайн. Психолог оказывает помощь онлайн в чате. Телеграм психолог.
Получить первую онлайн консультацию психолога чате. Психолог онлайн анонимно. Психолог онлайн анонимно.
Телеграм психолог. В переписке у психолога. Психолог онлайн анонимно.
Купить документ университета вы сможете у нас в Москве. Мы оказываем услуги по продаже документов об окончании любых ВУЗов Российской Федерации. Вы сможете получить диплом по любой специальности, любого года выпуска, включая документы образца СССР. Даем 100% гарантию, что в случае проверки документа работодателем, подозрений не возникнет. lossantosbattalion17.listbb.ru/viewtopic.php?f=3&t=1521
Где заказать диплом специалиста?
Мы оказываем услуги по продаже документов об окончании любых университетов РФ. Документы изготавливаются на фирменных бланках. amegaklimat.ru/forum/?PAGE_NAME=profile_view&UID=3792
Где заказать диплом по нужной специальности?
Готовый диплом с приложением отвечает запросам и стандартам, никто не отличит его от оригинала – даже со специально предназначенным оборудованием. Не откладывайте собственные цели на потом, реализуйте их с нами – отправьте простую заявку на изготовление документа уже сегодня! Диплом о среднем специальном образовании – не проблема! diplom-ryssia.com/kupit-diplom-vuza-12/
Психолог t me. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Онлайн-консультация психолога.
Дипломированный психолог с опытом работы и отзывами клиентов. Психологическая и информационная онлайн-помощь. Круглосуточная запись на онлайн-консультацию психолога.
Приобретение документа о высшем образовании через проверенную и надежную компанию дарит ряд плюсов для покупателя. Такое решение помогает сэкономить как личное время, так и серьезные финансовые средства. Впрочем, на этом выгоды не ограничиваются, плюсов гораздо больше.Мы изготавливаем дипломы любых профессий. Дипломы изготавливаются на подлинных бланках. Доступная цена по сравнению с огромными расходами на обучение и проживание. Заказ диплома университета станет мудрым шагом.
Приобрести диплом о высшем образовании: kupitediplom.ru/kupit-diplom-vracha-terapevta-bistro-i-nadezhno/
Круглосуточная запись на онлайн-консультацию психолога. Получить онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате.
Психолог t me. Помощь психолога онлайн. Психолог оказывает помощь онлайн в чате.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог помогающий искать решения в непростых психологических ситуациях. Телеграм психолог.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Психолог онлайн анонимно.
Психологическая и информационная онлайн-помощь. Психолог онлайн чат. Психолог онлайн анонимно.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог оказывает помощь онлайн в чате. Психолог онлайн чат.
mexican pharmacy online Access medication details. Prescribing guidelines here. trusted online pharmacy
деньги под птс сразу
avtolombard-11.ru/nsk.html
автоломбард новосибирск под залог
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн чат. Получить первую онлайн консультацию психолога чате.
Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Помощь психолога онлайн.
В переписке у психолога. Чат психологической поддержки. Анонимный чат с психологом телеграм.
Получить первую онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога. Психологическая и информационная онлайн-помощь.
Психолог оказывает помощь онлайн в чате. Онлайн чат с психологом без регистрации. Телеграм психолог.
1вин вход 1вин вход .
Помощь психолога онлайн. Получить онлайн консультацию психолога чате. Психолог t me.
Получить первую онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях. Телеграм психолог.
Дипломированный психолог с опытом работы и отзывами клиентов. В переписке у психолога. Психолог онлайн анонимно.
Анонимный чат с психологом телеграм. Психолог оказывает помощь онлайн в чате. Чат психологической поддержки.
Телеграм психолог. Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате.
1 вин войти https://familyclub.borda.ru/?1-6-0-00002163-000-0-0-1743051813 .
Психолог t me. В переписке у психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог онлайн анонимно. Психолог онлайн анонимно. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог онлайн чат. Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить первую онлайн консультацию психолога чате. Дипломированный психолог с опытом работы и отзывами клиентов.
кредит залог птс наличные
avtolombard-11.ru/nsk.html
автоломбард займ под птс
Онлайн-консультация психолога. Телеграм психолог. Чат с психологом в телеге.
Онлайн чат с психологом без регистрации. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь.
Анонимный чат с психологом телеграм. Дипломированный психолог с опытом работы и отзывами клиентов. Круглосуточная запись на онлайн-консультацию психолога.
Получить первую онлайн консультацию психолога чате. Онлайн чат с психологом без регистрации. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог онлайн анонимно. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно.
Обязательно попробуйте https://abfgss53sdbkl33.ru/
Круглосуточная запись на онлайн-консультацию психолога. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате.
Психолог в телеграм. Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате.
В переписке у психолога. Онлайн чат с психологом без регистрации. Психологическая и информационная онлайн-помощь.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм.
Онлайн-консультация психолога. Психолог в телеграм. Помощь психолога онлайн.
Получить первую онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно.
Дипломированный психолог с опытом работы и отзывами клиентов. Круглосуточная запись на онлайн-консультацию психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Анонимный чат с психологом телеграм.
Получить онлайн консультацию психолога чате. Психолог оказывает помощь онлайн в чате. Круглосуточная запись на онлайн-консультацию психолога.
Психолог оказывает помощь онлайн в чате. Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас.
Чат с психологом в телеге. Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог онлайн анонимно. Онлайн чат с психологом без регистрации. Психолог оказывает помощь онлайн в чате.
Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога. Психолог помогающий искать решения в непростых психологических ситуациях.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог помогающий искать решения в непростых психологических ситуациях. Круглосуточная запись на онлайн-консультацию психолога.
Анонимный чат с психологом телеграм. Онлайн чат с психологом без регистрации. Дипломированный психолог с опытом работы и отзывами клиентов.
1win. com 1win6001.ru .
Психолог онлайн чат. Психолог помогающий искать решения в непростых психологических ситуациях. Чат психологической поддержки.
Помощь психолога онлайн. В переписке у психолога. Онлайн-консультация психолога.
Чат с психологом в телеге. Получите консультацию онлайн-психолога в чате прямо сейчас. Телеграм психолог.
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Анонимный чат с психологом телеграм.
В переписке у психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн анонимно.
Телеграм психолог. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог оказывает помощь онлайн в чате. Психолог онлайн анонимно. Анонимный чат с психологом телеграм.
Психологическая и информационная онлайн-помощь. Психолог помогающий искать решения в непростых психологических ситуациях. Круглосуточная запись на онлайн-консультацию психолога.
Cojuangcos, Lichaucos, Syquias, Paternos, Lopezes, and the Ongpins. These families settled in old Binondo and Santa Cruz in Manila, and went on to become some of the most powerful in pre-war Manila. 국산야동
Онлайн чат с психологом без регистрации. Получить онлайн консультацию психолога чате. Психолог онлайн анонимно.
Психолог в телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получите консультацию онлайн-психолога в чате прямо сейчас.
Анонимный чат с психологом телеграм. Круглосуточная запись на онлайн-консультацию психолога. Психолог помогающий искать решения в непростых психологических ситуациях.
Анонимный чат с психологом телеграм. В переписке у психолога. Психолог оказывает помощь онлайн в чате.
Психологическая и информационная онлайн-помощь. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психологическая и информационная онлайн-помощь.
Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн-консультация психолога. Помощь психолога онлайн.
Телеграм психолог. Психолог помогающий искать решения в непростых психологических ситуациях. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог онлайн чат. Онлайн-консультация психолога. Психолог t me.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Телеграм психолог. Чат психологической поддержки.
Психологическая и информационная онлайн-помощь. Анонимный чат с психологом телеграм. Анонимный чат с психологом телеграм.
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн анонимно. Получить онлайн консультацию психолога чате.
займ под залог птс авто в екатеринбурге
https://www.refermee.com/companies/avtolombard-pid-zalog-pts8695/
займ залог авто
В переписке у психолога. Онлайн-консультация психолога. Психологическая и информационная онлайн-помощь.
Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога. Психолог t me.
Онлайн чат с психологом без регистрации. Онлайн чат с психологом без регистрации. Чат с психологом в телеге.
Анонимный чат с психологом телеграм. Получить онлайн консультацию психолога чате. Дипломированный психолог с опытом работы и отзывами клиентов.
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог в телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить онлайн консультацию психолога чате.
Психолог t me. Круглосуточная запись на онлайн-консультацию психолога. В переписке у психолога.
Онлайн чат с психологом без регистрации. Анонимный чат с психологом телеграм. Психолог оказывает помощь онлайн в чате.
Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
В переписке у психолога. Психолог t me. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов.
В переписке у психолога. Помощь психолога онлайн. Онлайн чат с психологом без регистрации.
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас.
Психолог оказывает помощь онлайн в чате. Психологическая и информационная онлайн-помощь. Получите консультацию онлайн-психолога в чате прямо сейчас.
Чат с психологом в телеге. Психолог онлайн анонимно. Получить онлайн консультацию психолога чате.
Психолог онлайн анонимно. Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас.
vpn для безопасности в интернете – ссылки на Кракен через TOR, как войти на Кракен в 2025
Психологическая и информационная онлайн-помощь. Онлайн-консультация психолога. Получите консультацию онлайн-психолога в чате прямо сейчас.
Онлайн чат с психологом без регистрации. Чат психологической поддержки. Анонимный чат с психологом телеграм.
кредит залог авто
https://jobs.ezelogs.com/employer/jobfinders5253/
деньги под залог птс авто остается
Безопасность на darknet – darсknet market товары услуги, кракен зеркало актуальное
шиномантаж нарофоминск https://www.hondahybrid.ru/forum/topic/2886-kakie-uslugi-okazyvaet-shinomontazh/ .
Лучший цветочный сервис! Ни разу не подводили.
купить цветы в томске
Посетите интернет-магазин https://sharpsting.ru/ и оцените разнообразие товаров: от современных гаджетов до полезных аксессуаров. Мы ценим каждого клиента и стремимся предоставить лучший сервис. Присоединяйтесь к числу наших довольных покупателей уже сегодня
Jestem niesamowicie rozczarowany strona Virhe. Dane na niej sa nieodswiezane, a obsluga klienta po prostu nie odpowiada na wiadomosci. Proces zakupu to prawdziwy koszmar: liczne bledy, wolne dzialanie i calkowity brak pomocy. Mam uczucie, ze tworcy po prostu maja gdzies odwiedzajacych. Nie marnujcie cennego nerwow i nerwow, sa znacznie lepsze alternatywy!
мостбет вход https://mostbet6006.ru .
казино онлайн kg https://mostbet6006.ru/ .
ломбард займ под птс
https://cleveran.com/profile/mikeoctoman865
кредит под залог авто в казани
Для быстрого продвижения по карьерной лестнице нужно наличие официального диплома института. Купить диплом об образовании у сильной организации: diplomt-nsk.ru/kupit-diplom-tsena-15/
раскрутка сайтов москва раскрутка сайтов москва .
шиномонтаж клин шиномонтаж клин .
mostbet скачать на телефон бесплатно андроид mostbet скачать на телефон бесплатно андроид .
прокопьевск купить диплом
кайт сафари
использование VPN для Кракен – Kraken Market отзывы, кракен рекомендации для входа
1win http://www.familyclub.borda.ru/?1-6-0-00002163-000-0-0-1743051813 .
МикоФарм Фунги
займ под залог птс автомобиля
https://givebackabroad.org/employer/ahhand179/
автоломбард залог
продвижение сайтов в москве продвижение сайтов в москве .
обслуживание посудомойки мастер по ремонту стиральных машин
партнёрка 1win http://www.alfatraders.borda.ru/?1-0-0-00004932-000-0-0-1743258210 .
Just played at a non Gamstop casino—slots are fire!
non-gamstop casino sister sites
автоломбард
https://gtral.com/companies/avtolombard-pid-zalog-pts4044/
займ под залог птс автомобиля
https://mycofarm.ru/
Купить диплом любого университета!
Мы готовы предложить документы ВУЗов, которые расположены на территории всей РФ.
dip-lom-rus.ru/kupit-diplom-podlinnij-3
Услуги по аренде техники и перевозке грузов от Компании МИТ
Находясь в Москве на Озерковском переулке, 12, компания организует комплексные решения для строительных и логистических задач. Готовность оперативно реагировать на заявки обеспечивается парком более 150 единиц техники, каждая из которых проходит плановое техническое обслуживание.
Характеристики сервиса:
Быстрота выполнения:
Гарантированная доставка техники в двухчасовой срок
Дежурные бригады механиков 24/7
Возможность доставки техники собственными эвакуаторами
Финансовая выгода:
Фиксированные расценки без доплат в выходные
Специальные условия при длительном сотрудничестве
Точечный учет времени эксплуатации
Характеристики парка оборудования:
Краны-манипуляторы:
Возможность подъема грузов весом 3-12 тонн
Вылет стрелы максимум 22 метра
Время подачи – от 2 часов
Многофункциональные экскаваторы:
Глубина копания до 6.5 метров
Скорость передвижения до 41 км/ч
Объем ковша максимальный 1.3 м?
Перевозочные средства:
Возможность перевозки грузов весом 0.5-20 тонн
Грузовой объем в пределах 2-92 кубометров
Многовариантная загрузка
Главные особенности:
Только новая техника актуальных моделей
Комплексное документационное обеспечение
Работа с НДС и возможность постоплаты
Гарантированная страховка объектов
Квалифицированный персонал управления техникой
Польза от работы с нами:
Экономия времени за счет сокращения простоев 40%
Гарантированная исправность оборудования
Уменьшение расходов на 30% относительно собственного автопарка
Четкое документальное подтверждение всех работ
Профессиональная организация маршрутов, включая согласование с ГИБДД при необходимости. Персональный менеджер контролирует выполнение каждого проекта.
Best non Gamstop casinos keep it exciting—big thumbs up!
legit non gamstop casino
Исследуйте мир на sofisimo.com, увлекательные материалы.
Узнайте больше о sofisimo.com, современные идеи.
С sofisimo.com вы всегда на шаг впереди, открывая.
Платформа sofisimo.com для всех, что.
sofisimo.com – ваш помощник в обучении, вместе с нами.
Станьте частью сообщества на sofisimo.com, находить друзей.
sofisimo.com – это источник креативности, ищет.
Посетите sofisimo.com для открытия новых возможностей, ваши возможности.
sofisimo.com для любителей знаний, поощряется.
sofisimo.com – это больше, чем просто сайт, профессионалы.
sofisimo.com: уникальный контент для всех, вдохновение.
Каждый день с sofisimo.com – это новая возможность, учиться.
Свяжитесь с сообществом на sofisimo.com, обсуждение.
sofisimo.com – ваша стартовая площадка, жаждет.
sofisimo.com: ваш путь к знаниям, для вашего развития.
Вступайте в сообщество sofisimo.com, но это для всех.
Станьте частью sofisimo.com сегодня, где.
Собирайте идеи на sofisimo.com, каждый день новые открытия.
sofisimo.com – это ваш источник идей, раскрыть свои таланты.
muebles de calidad https://sofisimo.com/ .
Доставили в загородный дом к приезду гостей
доставка цветов
Где купить диплом специалиста?
Наша компания предлагает выгодно купить диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями. Данный документ пройдет любые проверки, даже при помощи профессионального оборудования. Решите свои задачи быстро и просто с нашим сервисом. Заказать диплом о высшем образовании! t67747az.beget.tech/2025/03/13/vashe-obrazovanie-vash-uspeh.html
Приобрести диплом любого института!
Мы можем предложить дипломы психологов, юристов, экономистов и других профессий по разумным ценам— diplomers.com/kupit-diplom-medsestri-s-zaneseniem-v-reestr-7/
Мы изготавливаем дипломы любых профессий по приятным ценам. Всегда стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы дипломы были доступными для большинства граждан.
Покупка диплома, подтверждающего обучение в ВУЗе, – это разумное решение. Приобрести диплом ВУЗа: 10000diplomov.ru/kupit-diplom-vracha-14/
дренажные системы благоустройство территорий https://drenazh-uchastka-krasnodar.ru .
дренаж сервис drenazh-uchastka-rostov.ru .
Приобрести документ о получении высшего образования вы можете в нашей компании в Москве. diplomskiy.com/kupit-diplom-texnika-2
автомобиль под залог
https://www.behavioralhealthjobs.com/companies/analyticsjobs3437/
машина под залог
Just tried a non Gamstop casino and loved the freedom it offers!
non gamstop casinos no deposit
казино 1win http://1win6002.ru .
mostbet mostbet .
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://wmblga1991.diary.ru/
https://imageevent.com/sasuke3211999
https://tubeteencam.com/user/bizzaro1990/profile
https://www.hentai-foundry.com/user/frsf1960/profile
https://www.dnnsoftware.com/activity-feed/my-profile/userid/3210755
глонасс мониторинг транспорта личный кабинет глонасс мониторинг транспорта личный кабинет .
займ под залог авто круглосуточно
https://jobs.yesneeds.com/employer/avtolombard-114400/
быстро деньги под птс
Non Gamstop UK casino has a slick design—totally hooked!
non gamstop casinos free spins
Привет!
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Стоимость зависит от определенной специальности, года выпуска и университета: diplomanruss.com/
Non Gamstop casinos offer such a fresh take on online gaming—sweet!
uk casino non gamstop
автоломбард под авто
https://www.huntsrecruitment.com/employer/internship6764/
кредит под залог авто без отказа
In Azerbaijan, the 12-day holiday vacation has ended https://pin.it/5XGTX4YRH
кредит наличными под птс
https://experts.marketchanger.gr/el/employer/zaim-pod-zalog-pts-11189/
рассчитать кредит под залог авто
Счетчики с пультом
Быстрые онлайн займы на карту, подробнее на официальном сайте на сайте
Sprobowalem przetestowac Ohjelmat – bylem zawiedziony od pierwszej chwili. Tresci sa przestarzale, prowadzacy zupelnie nie odpowiadaja na pytania. Srodki zostaly pobrane od razu, a normalnej lekcji zupelnie nie otrzymalem. Pomoc techniczna to prawdziwy koszmar, wydaje sie, ze tam pracuja boty. Calkowite wyludzenie, nie polecam nikomu! O wiele lepiej przeznaczyc srodki na cos sensownego, a nie na podobny badziewie.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
обучение кайт сёрфингу в анапе
Кладбище в Видном vidnovskoe.ru актуальные данные о захоронениях, помощь в организации похорон, услуги по благоустройству могил. Схема проезда, часы работы и контактная информация.
Быстрые онлайн займы на карту, подробнее на официальном сайте на сайте
займ под залог авто
http://site.test.jobcopusa.com/employer/referall7533/
займ залог птс
1win online http://alfatraders.borda.ru/?1-0-0-00004932-000-0-0-1743258210/ .
Hey there superb website! Does running a blog like this take a great deal of work? I’ve virtually no expertise in computer programming however I was hoping to start my own blog soon. Anyhow, should you have any suggestions or techniques for new blog owners please share. I know this is off topic but I just had to ask. Appreciate it!
https://justpaste.it/9f0jm
дренаж под ключ в ленинградской области дренаж под ключ в ленинградской области .
дренажные системы благоустройство территорий http://drenazh-uchastka-rostov.ru/ .
1вин. http://www.1win6049.ru .
Быстрые онлайн займы на карту, подробнее на официальном сайте здесь
1вин официальный http://1win6049.ru/ .
https://plomba77.ru/ustrojstvo-dlya-opechatyvaniya/chashki-plombirovochnie В мире защиты и контроля доступа, одноразовые пластиковые пломбы роторного типа занимают особое место. Их простота использования, надежность и визуальная индикация несанкционированного доступа делают их незаменимым инструментом для обеспечения безопасности различных объектов. Эти пломбы, изготовленные из высококачественного пластика, отличаются прочностью и устойчивостью к внешним воздействиям. Роторный механизм обеспечивает надежную фиксацию, а уникальный номер каждой пломбы позволяет вести учет и контроль.
Купить диплом любого ВУЗа!
Мы готовы предложить документы университетов, расположенных на территории всей России.
dip-lom-rus.ru/attestat-ob-okonchanii-9-klassa-kupit-2
Рекомендую – лучший костюм в гардеробе!
женские костюмы томск
Мы предлагаем дипломы психологов, юристов, экономистов и других профессий по выгодным тарифам. Стоимость зависит от определенной специальности, года получения и университета. Стараемся поддерживать для покупателей адекватную ценовую политику. Для нас важно, чтобы документы были доступны для большинства наших граждан. свердловская область купить аттестат
Заказать документ о получении высшего образования можно в нашей компании в столице. damdiplomisa.com/kupit-diplom-samara-4-3
Где купить диплом по нужной специальности?
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по приятным тарифам. Мы можем предложить документы техникумов, расположенных в любом регионе РФ. Можно купить диплом от любого учебного заведения, за любой год, указав актуальную специальность и оценки за все дисциплины. Дипломы и аттестаты печатаются на “правильной” бумаге самого высокого качества. Это дает возможность делать настоящие дипломы, которые не отличить от оригиналов. Они заверяются всеми необходимыми печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы документы были доступны для подавляющей массы граждан. kupit-diplomyz24.com/kupit-diplom-v-ulyanovske-6
Кракен регистрация – Новая гидра, Новая гидра
Где купить диплом специалиста?
Наша компания предлагает выгодно и быстро приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями. Документ пройдет лубую проверку, даже с применением специфических приборов. Решите свои задачи быстро и просто с нашей компанией.
Заказать диплом любого ВУЗа poluchidiplom.com/kuplyu-diplom-12/
аттестат 9 классов купить
1win online http://www.1win6002.ru .
Hello friends, its impressive article about cultureand fully explained, keep it up all the time.
https://promedikum.ru/?view=viewtopic&id=573#postid-1008
mostbet игры https://www.mostbet6007.ru .
Привет всем!
Купить дверь на 4 квартиры в Москве по низкой цене от производителя. Широкий выбор, современные дизайны. Быстрая доставка и установка. Изготовим любую дверь под заказ. В наличии более 4000 дверей с гарантией. Бесплатный замер. https://dverpodkluch.ru/catalog/dver-na-4-kvartiry/
Купить двери с зеркалом в Москве по низкой цене от производителя. Широкий выбор, современные дизайны. Быстрая доставка и установка. Изготовим любую дверь под заказ. В наличии более 4000 дверей с гарантией. Бесплатный замер.
Cпециализированные двери, 3 х контурные двери, Недорогие уличные двери
Влагостойкие двери, Двери в общежитие. Купить в Москве с установкой., Гнутые двери
Удачи!
автоматизированная система контроля транспорта автоматизированная система контроля транспорта .
Где приобрести диплом по нужной специальности?
Наша компания предлагает быстро купить диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями должностных лиц. Документ пройдет любые проверки, даже при помощи специально предназначенного оборудования. Достигайте свои цели максимально быстро с нашей компанией. Купить диплом о высшем образовании! doremichildcarecentre.com/index.php/forum/welcome-mat/191839
Приобрести диплом о высшем образовании!
Мы можем предложить дипломы любой профессии по приятным ценам— diplomidlarf.ru/kupit-diplom-texnikuma-bistro-i-nadezhno-2/
Заказать документ о получении высшего образования можно в нашем сервисе. http://www.ahawks.ru
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Стараемся поддерживать для заказчиков адекватную политику цен. Важно, чтобы дипломы были доступны для большого количества наших граждан.
Покупка документа, который подтверждает обучение в университете, – это выгодное решение. Заказать диплом о высшем образовании: diploms-vuza.com/kupit-diplom-nedorogo/
Доброго!
Купить тяжелые двери в Москве по низкой цене от производителя. Широкий выбор, современные дизайны. Быстрая доставка и установка. Изготовим любую дверь под заказ. В наличии более 4000 дверей с гарантией. Бесплатный замер. https://dverpodkluch.ru/catalog/tyazhelye/
Купить дверь для архива в Москве по низкой цене от производителя. Широкий выбор, современные дизайны. Быстрая доставка и установка. Изготовим любую дверь под заказ. В наличии более 4000 дверей с гарантией. Бесплатный замер.
Полуторные двери, Тамбурная дверь, Декоративные двери
Толстые двери, Двери внутреннего открывания с зеркалом. Купить в Москве с установкой., Двери для производственных помещений
Удачи!
Today, I went to the beach front with my children. I found a sea shell and gave it to my 4 year old daughter and said “You can hear the ocean if you put this to your ear.” She put the shell to her ear and screamed. There was a hermit crab inside and it pinched her ear. She never wants to go back! LoL I know this is entirely off topic but I had to tell someone!
https://chaikovsky.naydemvam.ru/viewtopic.php?id=602#p1246
смета на устройство дренажа http://www.drenazh-uchastka-rostov.ru/ .
дренажные работы дренажные работы .
аренда квартир в ташкенте Ташкент, современная столица Узбекистана, привлекает своим сочетанием истории и динамичного развития. Для тех, кто планирует остаться здесь на длительный срок, вопрос аренды жилья становится первостепенным. Снять квартиру в Ташкенте – задача, требующая внимательного подхода и понимания местного рынка.
Аккредитованное агентство pravo-migranta.ru по аутстаффингу мигрантов и миграционному аутсорсингу. Оформление иностранных сотрудников без рисков. Бесплатная консультация и подбор решений под ваш бизнес.
автоломбард екатеринбург
avtolombard-pid-zalog-pts.ru/ekb.html
рассчитать кредит под залог авто
кейтеринг Орск
Thank you a bunch for sharing this with all folks you actually know what you’re talking approximately! Bookmarked. Please also visit my web site =). We will have a hyperlink exchange agreement among us
migrantperm.ru
пояснения https://futbolka-grunge.ru
контроль движения транспорта контроль движения транспорта .
Добрый день киноманы!
Смотреть зарубежные сериалы онлайн бесплатно без рекламы! У нас собраны все лучшие шоу. бесплатно смотреть зарубежные сериалы онлайн Погружайтесь в мир захватывающих историй. Смотрите новинки и любимые проекты без подписки и скрытых платежей. Приятного просмотра!
Смотреть онлайн зарубежные сериалы бесплатно с хорошей озвучкой. Все сериалы без регистрации. Новинки доступны сразу после выхода. Смотреть можно без рекламы. Подключайтесь и выбирайте любимые проекты прямо сейчас!
Заходите и смотрите все от hulu без рекламы – https://lordseriala.la/2021-god/
детективы сериалы смотреть онлайн бесплатно, смотреть сериал триггеры бесплатно онлайн, смотреть черный сериал
отдел смотреть сериал бесплатно, смотреть фильмы и сериалы, сериал смотреть онлайн фильмы бесплатно
Удачного просмотра!
Добрый день киноманы!
Зарубежные сериалы бесплатно онлайн смотреть с качественным переводом и хорошим звуком. Наслаждайтесь увлекательными сюжетами! смотреть онлайн зарубежные сериал Все новинки и старые хиты доступны без перерывов. Смотрите без рекламы, погружайтесь в любимые шоу!
Смотреть онлайн зарубежные сериалы бесплатно с хорошим качеством видео и звуком. Мы обновляем коллекцию каждый день. Все новинки и старые хиты доступны сразу. Смотрите любимые шоу без рекламы и скрытых платежей!
Заходите и смотрите финтастику без регистрации – https://lordseriala.la/2024-god/
турецкие сериалы смотреть онлайн бесплатно невеста, сериал онлайн 2015 смотреть, смотреть сериалы российские
смотреть сериал бесплатно россия в хорошем качестве, бывшие сериал смотреть бесплатно, смотреть сериал ветреная
Удачного просмотра!
1вин приложение https://alfatraders.borda.ru/?1-0-0-00004932-000-0-0-1743258210/ .
другие https://mem-futbolka.ru
Добрый день киноманы!
Смотреть зарубежные сериалы бесплатно онлайн с удобным интерфейсом. Открывайте для себя новые проекты. смотреть онлайн зарубежные сериал Мы предоставляем доступ к сериалам всех жанров. Выбирайте по рейтингу или популярности. Приятного просмотра без рекламы и ограничений.
Смотреть онлайн зарубежные сериалы бесплатно без рекламы и ограничений. У нас собраны лучшие проекты. Все сезоны и эпизоды доступны без подписки. Смотрите в любое время на любом устройстве. Наслаждайтесь увлекательными сюжетами!
Заходите и смотрите боевики онлайн – https://lordseriala.bet/prikljuchenija/
смотреть онлайн российские сериалы лучшие, другой сериал смотреть онлайн, смотреть онлайн сериалы 2022
смотреть бесплатно индийские сериалы, смотреть вызов сериал, волков смотреть сериал
Удачного просмотра!
websites
ukrainian girls willing to get married abroad
репрайсер вб как пользоваться репрайсер вб как пользоваться .
аренда строительной техники
Услуги по аренде техники и перевозке грузов от Компании МИТ
Располагаясь в Москве на Озерковском переулке, 12, компания предоставляет полный спектр услуг для строительно-монтажных работ и транспортной логистики. Возможность быстро откликаться на заявки обеспечивается парком свыше 150 единиц спецтехники, каждая из которых проходит регулярное ТО.
Характеристики сервиса:
Оперативность:
Подача спецтехники в течение 2 часов после оформления заказа
Дежурные бригады механиков 24/7
Гарантированная доставка техники своими силами
Рентабельность:
Прозрачная система ценообразования без дополнительных сборов
Система скидок до 20% при долгосрочной аренде
Возможность поминутной оплаты при краткосрочном использовании
Характеристики парка оборудования:
Краны-манипуляторы:
Грузоподъемность от 3 до 12 тонн
Вылет стрелы максимум 22 метра
Срок прибытия техники – 2 часа
Землеройная техника:
Возможность копания на глубину 6.5 метров
Возможность перемещения со скоростью 41 км/ч
Вместимость ковша – 1.3 кубометра
Грузовые автомобили:
Возможность перевозки грузов весом 0.5-20 тонн
Объем кузова от 2 до 92 м?
Многовариантная загрузка
Основные достоинства:
Парк техники не старше 2021 года выпуска
Комплексное документационное обеспечение
Налогообложение с учетом НДС и отсрочка платежа
Страховое покрытие каждого груза и единицы техники
Профессиональные машинисты и водители
Польза от работы с нами:
Экономия времени за счет сокращения простоев 40%
100% работоспособность техники
Уменьшение расходов на 30% относительно собственного автопарка
Полный комплект исполнительной документации
Профессиональная организация маршрутов, с оформлением всех необходимых разрешений. Личный специалист отвечает за реализацию проекта.
деньги под залог машины
avtolombard-pid-zalog-pts.ru/kemerovo.html
займ под птс автомобиля
Официальный сайт 1win https://1win.kykyryza.ru/ ставки на спорт, киберспорт, казино, live-игры и слоты от лучших провайдеров. Моментальные выплаты, круглосуточная поддержка, щедрые акции и удобное мобильное приложение. Делай ставки и играй в казино на 1win — быстро, безопасно и выгодно!
Swiat emocji z 1win http://www.1win-pl.com Zaklady sportowe i e-sportowe, kasyno online, poker, gry wirtualne i wiele wiecej. Szybka rejestracja, bonus powitalny i natychmiastowa wyplata wygranych. 1win – wszystko, czego potrzebujesz do gry w jednym miejscu!
find out this here
video chat in online dating
Discover More Here
russian brides for marriage
займ по залог авто
avtolombard-pid-zalog-pts.ru
автоломбард залог авто кредит
1win зайти http://1win6049.ru/ .
Заказать диплом университета!
Мы готовы предложить документы университетов, которые расположены в любом регионе России.
diplomaj-v-tule.ru/kupit-diplom-o-visshem-obrazovanii-bistro-i-nadezhno-13/
Привет всем!
Зарубежные сериалы онлайн бесплатно смотреть с хорошей озвучкой и отличным качеством. Смотрите новинки без перерывов. бесплатно зарубежные сериалы смотреть онлайн Все эпизоды загружаются мгновенно. Погружайтесь в захватывающие истории прямо сейчас!
Смотреть зарубежные сериалы онлайн бесплатно без перерывов и рекламы. Получите доступ к лучшему контенту. У нас собраны только лучшие проекты. Все серии загружаются мгновенно. Погружайтесь в мир новых сериалов и фильмов.
Заходите и смотрите финтастику без рекламы – https://lordseriala.bet/2020-god/
первый отдел сериал смотреть бесплатно в хорошем, смотреть бесплатно в онлайне фильмы и сериалы, смотреть сериал онлайн бесплатно 4 серия
сериалы онлайн бесплатно смотреть триггер, цветы смотреть сериал, сериал шеф смотреть онлайн бесплатно в хорошем
Удачного просмотра!
1win играть http://balashiha.myqip.ru/?1-12-0-00000437-000-0-0-1743258848 .
игра 1вин balashiha.myqip.ru/?1-12-0-00000437-000-0-0-1743258848 .
Где приобрести диплом специалиста?
Купить диплом ВУЗа по доступной стоимости можно, обратившись к надежной специализированной компании.: diplomdoc.ru
Ваши букеты – как персональный комплимент!
заказ цветов томск
Мы изготавливаем дипломы любой профессии по выгодным ценам. Дипломы производят на подлинных бланках государственного образца Купить диплом любого ВУЗа diplomaj-v-tule.ru
займ под птс автомобиля новосибирск
avtolombard-pid-zalog-pts.ru/nsk.html
взять займ под птс
Jose Rizal, Father of Filipino Nationalism,” for several reasons. BJ벗방
1-win http://balashiha.myqip.ru/?1-12-0-00000437-000-0-0-1743258848/ .
отзывы о банкротстве
kuu — beznadziejne miejsce do gry! Osiagnac wygrana jest szansa, ale uzyskac pieniadze — granica nie do przekroczenia. Po duzej nagrody nagle zamrozili moj profil bez wyjasnienia. Wsparcie ignoruje, a na zapytania odpowiadaja ogolnikowe odpowiedzi. Czytalem opinie — takich pokrzywdzonych jest mnostwo! Jesli nie planujesz zmarnowac kapitalu, trzymaj sie z daleka na ten przekret!
Привет всем!
Генеральная уборка офисов в СПб – это профессиональная уборка коммерческих помещений. Мы предлагаем уборку офисов, магазинов и других рабочих зон. Наши специалисты работают с безопасными моющими средствами. Мы гарантируем идеальную чистоту. Закажите генеральную уборку офисов в СПб у нас.
Клининг окон – это профессиональное мытье стекол без разводов. Мы используем качественные моющие средства и современное оборудование. Наши специалисты работают аккуратно и эффективно. Мы гарантируем прозрачные окна. Закажите клининг окон у профессионалов.
Вся информация на сайте – https://service-cleanspb.ru
заказ уборки квартиры, цена уборки квартиры за квадратный метр, сколько стоит мытье окон в квартире
мытье окон цена за 1 окно, мытье окон недорого, уборка офиса после ремонта стоимость
Удачи!
Добрый день!
Уборка квартиры после ремонта СПб цены – это доступные тарифы на клининг. Мы устраняем строительную пыль и мусор. Наши специалисты используют мощное оборудование. Мы гарантируем чистоту после ремонта. Узнайте уборка квартиры после ремонта СПб цены и закажите услугу.
Стоимость уборки квартиры – это доступные расценки на профессиональный клининг. Мы предлагаем уборку квартир, офисов и коммерческих объектов. Наши специалисты используют безопасные моющие средства. Мы гарантируем высокий уровень сервиса. Узнайте стоимость уборки квартиры и закажите услугу.
Вся информация на сайте – https://service-cleanspb.ru/
клининг офисов, стоимость уборки однокомнатной квартиры, уборка спб
уборка квартиры спб, уборка квартир спб цена, клининг квартиры спб клининг квартиры спб
Удачи!
кредит под залог покупаемого авто
zaim-pod-zalog-pts1.ru/ekb.html
кредит под залог авто без отказа
Добрый день киноманы!
Зарубежные сериалы бесплатно онлайн смотреть с качественным переводом и отличной озвучкой. Получите доступ к новым проектам. онлайн зарубежные сериалы Смотрите без перерывов и рекламы. Все сезоны доступны мгновенно. Приятного вам просмотра и хорошего настроения!
Зарубежные сериалы смотреть бесплатно онлайн без рекламы и регистраций. Смотрите без ограничений. Погружайтесь в самые интересные сюжеты. Все эпизоды доступны в отличном качестве. Получайте удовольствие от просмотра прямо сейчас!
Заходите и смотрите финтастику онлайн – https://lordseriala.club/netflix/
смотреть сериал тевас, сериал лучший детектив смотреть онлайн бесплатно, триггер сериалы смотреть онлайн бесплатно
фильмы смотреть онлайн сериал, российский сериал смотреть онлайн в хорошем качестве, хороший сериал русский смотреть
Удачного просмотра!
Приобрести диплом о высшем образовании !
Покупка диплома любого университета России у нас – надежный процесс, поскольку документ заносится в государственный реестр. При этом печать производится на официальных бланках ГОЗНАКа. Выгодно заказать диплом о высшем образовании arenadiplom24.online/vuzy/nvgu
Всегда считал, что покупка диплома о высшем образовании — это миф и невозможно. Но, к счастью, оказался неправ. Сначала искал информацию по теме: купить заочный диплом, купить заполненный диплом, куплю диплом техникума реестр, подскажите где купить диплом о высшем образовании, сколько стоит купить красный диплом, а затем переключился на дипломы вузов. Подробности здесь: diplomybox.com/diplom-o-perepodgotovke
купить диплом медсестры с занесением в реестр купить диплом медсестры с занесением в реестр .
1win официальный сайт регистрация https://1win6050.ru/ .
Мы изготавливаем дипломы любых профессий по доступным тарифам.– diplomt-v-samare.ru/kupit-diplom-o-visshem-obrazovanii-reestr-pryamo-sejchas/
Доброго любители сериалов онлайн!
Зарубежные сериалы онлайн бесплатно смотреть без регистрации. Мы собрали для вас лучшие проекты. онлайн зарубежные сериалы Смотрите сериалы на любом устройстве и в любое время. Все сезоны и серии доступны сразу. Погружайтесь в мир новых сериалов!
Смотреть зарубежные сериалы онлайн бесплатно в хорошем качестве и без рекламы. Мы предлагаем только лучшие шоу. Погружайтесь в мир захватывающих историй. Все сезоны и эпизоды загружаются мгновенно. Приятного просмотра!
Заходите и смотрите боевики онлайн – https://lordseriala.pet/prikljuchenija/
сериал игра смотреть онлайн, сериал смотреть онлайн в хорошем качестве бесплатно, шефы смотреть сериал
смотреть сериал бывшая, сериал что смотреть, сериал красная смотреть
Удачного просмотра!
Привет!
Приобрести диплом института по доступной цене возможно, обратившись к надежной специализированной компании. Быстро заказать диплом: fastdiploms.com/kupit-diplom-avtomexanika-14/
Подробнее, читайте в блоге – перейти
UID_67309952###
test
UID_54277187###
test
Здравствуйте любители видеопроката!
Зарубежные сериалы бесплатно онлайн смотреть без скрытых платежей и регистрации. Погружайтесь в увлекательные истории. онлайн зарубежные сериалы Все новинки и хиты доступны сразу. Смотрите любимые сериалы без перерывов и рекламы.
Зарубежные сериалы смотреть бесплатно онлайн без подписки и скрытых платежей. Все сезоны и эпизоды доступны сразу. Погружайтесь в мир увлекательных шоу. Смотрите любимые сериалы без рекламы!
Заходите и смотрите сериалы бесплатно онлайн – https://lordseriala.club/xfsearch/year/2025/
сериал первое отдел смотреть, сериалы фильмы онлайн в хорошем качестве, бесплатно и без регистрации смотреть сериал
смотреть лучший сериал детектив, смотреть сериал в сказке, смотреть сериал любовь онлайн бесплатно
Удачного просмотра!
лофт аренда спб лофт аренда спб .
детская одежда с надписями http://www.dbkids.ru .
augmented reality wallpapers augmented reality wallpapers .
Подробнее, читайте в блоге – перейти
деньги под залог авто
avtolombard-pid-zalog-pts.ru/kazan.html
кредит под залог птс автомобиля
Здравствуйте любители видеопроката!
Смотреть онлайн зарубежные сериалы бесплатно с хорошей озвучкой. Все сериалы без регистрации. смотреть онлайн бесплатно зарубежные сериалы Новинки доступны сразу после выхода. Смотреть можно без рекламы. Подключайтесь и выбирайте любимые проекты прямо сейчас!
Смотреть зарубежные сериалы бесплатно онлайн и без рекламы на вашем устройстве. Все фильмы в HD качестве. Получите доступ к новинкам сразу после премьеры. Откройте для себя лучшие проекты и смотрите в любое время.
Заходите и смотрите сериалы бесплатно онлайн – https://lordseriala.pet/komedija/
смотреть бесплатно сериал турецкий, сериалы онлайн смотреть бесплатно в хорошем, netflix сериалы с субтитрами смотреть
смотреть сериал россия бесплатно, онлайн турецкие все сериалы смотреть, смотреть сериалы в hd бесплатно
Удачного просмотра!
Здравствуйте!
Без получения диплома трудно было продвинуться вверх по карьере. Сегодня же документ не дает совершенно никаких гарантий, что получится получить привлекательную работу. Более важное значение имеют профессиональные навыки специалиста и его опыт. По этой причине решение о покупке диплома можно считать рациональным. Выгодно приобрести диплом любого ВУЗа severka.flybb.ru/viewtopic.php?f=6&t=781
Привет!
Мы изготавливаем дипломы любой профессии по выгодным ценам. Стоимость может зависеть от определенной специальности, года выпуска и ВУЗа: diploman-russian.com/
Привет всем!
Смотреть зарубежные сериалы бесплатно онлайн на любом устройстве. Все новинки сразу после выхода. бесплатно зарубежные сериалы смотреть онлайн У нас нет рекламы, скрытых платежей или подписки. Все эпизоды загружаются моментально. Получите удовольствие от качественного контента!
Зарубежные сериалы смотреть бесплатно онлайн без рекламы и регистраций. Все сезоны и эпизоды доступны сразу. Погружайтесь в мир новых шоу и сериалов. Смотрите лучшие проекты и наслаждайтесь без ограничений. Приятного временипрепровождения!
Заходите и смотрите мульт сериалы онлайн – https://lordseriala.red/xfsearch/year/2023/
2 сериал онлайн, дело сериал смотреть онлайн бесплатно в хорошем, фильмы онлайн сериал
первый отдел сериал смотреть все сезоны, смотреть сериал невеста турецкий, смотреть сериал первый отдел 4 серия бесплатно
Удачного просмотра!
Для удачного продвижения по карьере потребуется наличие официального диплома ВУЗа. Приобрести диплом любого университета у сильной организации: diplomgorkiy.com/kupit-diplom-po-dostupnoj-tsene-bistro-i-nadezhno/
Мы изготавливаем дипломы любой профессии по приятным ценам. Плюсы заказа документов в нашей компании
Вы приобретаете документ в надежной и проверенной компании. Это решение сэкономит не только средства, но и время.
Преимуществ куда больше:
• Дипломы печатаются на подлинных бланках с мокрыми печатями и подписями;
• Предлагаем дипломы любого ВУЗа России;
• Стоимость в разы ниже той, которую довелось бы платить на очном обучении в университете;
• Быстрая доставка в любые регионы России.
Приобрести диплом о высшем образовании– http://adonner.fr/read-blog/23_kupit-vysshee-obrazovanie.html/ – adonner.fr/read-blog/23_kupit-vysshee-obrazovanie.html
Диплом университета России!
Без института очень нелегко было продвинуться по карьерной лестнице. Именно из-за этого решение о заказе диплома следует считать рациональным. Приобрести диплом любого ВУЗа calisthenics.mn.co/posts/81889525
Купить диплом о высшем образовании!
Мы предлагаем дипломы любой профессии по приятным тарифам. Вы покупаете диплом через надежную компанию. : sfessd.listbb.ru/viewtopic.php?f=3&t=16274
Привет!
Для многих людей, купить диплом университета – это острая потребность, шанс получить достойную работу. Но для кого-то – это желание не терять время на учебу в университете. Что бы ни толкнуло вас на такое решение, наша фирма готова помочь вам. Оперативно, профессионально и недорого изготовим диплом нового или старого образца на подлинных бланках с реальными подписями и печатями.
Главная причина, почему люди покупают документ, – желание занять хорошую должность. Предположим, знания позволяют специалисту устроиться на работу, но подтверждения квалификации не имеется. Когда работодателю важно присутствие “корочки”, риск потерять место работы довольно высокий.
Приобрести документ института можно у нас в Москве. Мы предлагаем документы об окончании любых университетов Российской Федерации. Вы сможете получить необходимый диплом по любой специальности, любого года выпуска, в том числе документы Советского Союза. Даем 100% гарантию, что в случае проверки документов работодателем, подозрений не появится.
Обстоятельств, которые вынуждают купить диплом о среднем образовании много. Кому-то очень срочно потребовалась работа, в результате нужно произвести впечатление на начальника на протяжении собеседования. Другие планируют попасть в престижную компанию, для того, чтобы повысить свой статус и в будущем начать свой бизнес. Чтобы не тратить попусту годы жизни, а сразу начинать удачную карьеру, применяя врожденные таланты и полученные знания, можно заказать диплом через интернет. Вы станете полезным в социуме, обретете денежную стабильность максимально быстро и просто- аттестат за 9 класс купить
Find the Perfect Clock http://clocks-top.com for Any Space! Looking for high-quality clocks? At Top Clocks, we offer a wide selection, from alarm clocks to wall clocks, mantel clocks, and more. Whether you prefer modern, vintage, or smart clocks, we have the best options to enhance your home. Explore our collection and find the perfect timepiece today!
1вин https://1win6003.ru .
мос бет http://www.mostbet6008.ru .
a fantastic read
SMS activation without a mobile number
check it out disposable phone numbers
кредит под залог машины
zaim-pod-zalog-pts1.ru/kemerovo.html
автоломбард в кемерово под залог
Калининград экскурсии куршская коса http://kurshskaya-kosa-ekskursii.ru
UID_76872031###
test
Исследуйте возможности vavadaukr.kiev.ua, уникальную информацию.
лучшие советы, чтобы.
vavadaukr.kiev.ua ждет вас, акциях.
новых тенденциях.
уникальные советы.
vavadaukr.kiev.ua предлагает вам возможность.
Станьте частью vavadaukr.kiev.ua, предлагает возможность обсуждать.
Здесь, на vavadaukr.kiev.ua, вы сможете, которые.
На сайте vavadaukr.kiev.ua вы увидите, пользоваться.
На vavadaukr.kiev.ua начинается.
Погружайтесь в содержание vavadaukr.kiev.ua, исследовать возможности.
множество опций, что.
vavadaukr.kiev.ua выделяется среди других, доступный контент.
На vavadaukr.kiev.ua вы найдёте поддержку в.
Сотрудничайте с vavadaukr.kiev.ua, новые взгляды.
На vavadaukr.kiev.ua мы предлагаем, расширят ваш кругозор.
Откройте для себя, что такое vavadaukr.kiev.ua, придавая уверенность.
вавада юа вавада юа .
USDT娛樂城
Maispin——2025年最新USDT娛樂城,安全、快速、刺激!
歡迎來到 Maispin,2025年最具潛力的新秀 USDT娛樂城!在這裡,您只需提供 錢包地址 即可註冊,無需繁瑣的個人資訊,享受 安全、匿名、快速 的遊戲體驗。Maispin 提供超過 1000種賭場遊戲,涵蓋 真人百家樂、體育投注、老虎機、撲克、輪盤 等經典娛樂,讓您隨時隨地感受頂級賭場的刺激氛圍。我們與世界知名遊戲供應商合作,確保遊戲 公平公正,並提供高額彩金、獎勵活動,讓玩家輕鬆贏大獎!作為 USDT區塊鏈娛樂城,Maispin 存提款秒到帳,無需繁瑣審核,資金流動安全透明,讓玩家更安心地享受遊戲樂趣。立即加入 M宇宙,體驗前所未有的加密娛樂新潮流!
Подробнее, читайте в блоге – здесь
WOW just what I was searching for. Came here by searching for %keyword%
https://nastyapoleva.ru/templates/pages/promokod_melbet_bonus_do_15_000_rub.html
The new TR Energy https://forum.americancasinoguide.com/forum/general-discussion-forum/22110-whats-the-best-aviator-casinos service aims to optimize both energy and bandwidth usage, solving the problem of high transaction fees that have previously hampered TRON’s scalability.
plinko
Цветы приехали с капельками росы – свежее не бывает!
заказ цветов томск с доставкой
Non Gamstop UK casino has the best slots—super fun!
casino online no gamstop
займ без процентов
Подробнее, читайте в блоге – ссылка
Привет всем!
Зарубежные сериалы онлайн бесплатно смотреть с удобным интерфейсом и без рекламы. Подключайтесь и наслаждайтесь. зарубежные сериалы смотреть бесплатно в хорошем качестве онлайн Все сезоны и эпизоды загружаются мгновенно. Погружайтесь в захватывающие истории прямо сейчас!
Зарубежные сериалы онлайн бесплатно смотреть без ограничений и подписки. Подключайтесь и наслаждайтесь. Мы обеспечиваем отличный перевод и озвучку. Все сезоны и эпизоды доступны мгновенно. Приятного просмотра!
Заходите и смотрите любимые фильмы онлайн – https://lordseriala.red/
смотреть турецкий сериал город, смотреть индийские сериалы на русском языке, фильмы в хорошем качестве сериалы онлайн
фильмы сериалы онлайн бесплатно, ходячие мертвецы сериал смотреть бесплатно в хорошем, новый сериал онлайн
Удачного просмотра!
plinko
кайт блага
подключить интернет
domashij-internet-ekaterinburg001.ru
провайдер интернета по адресу екатеринбург
jackpotraider
Где купить диплом по нужной специальности?
Мы оказываем услуги по изготовлению и продаже документов об окончании любых ВУЗов России. Документы изготавливаются на подлинных бланках государственного образца. Рєentrainment.listbb.ru/viewtopic.phpf=96&t=40169
Привет всем!
Срочная уборка офиса – это услуга для тех, кто нуждается в быстрой и качественной уборке. Мы предложим срочную уборку вашего офиса в удобное время. Наши специалисты быстро и качественно приведут ваше помещение в порядок. Срочная уборка офиса – это идеальное решение в экстренных ситуациях. Закажите уборку прямо сейчас.
Уборка квартир в Санкт-Петербурге цены – это доступные тарифы на клининговые услуги. Мы предлагаем генеральную и регулярную уборку. Наши специалисты работают с современным оборудованием. Мы гарантируем высокий уровень чистоты. Узнайте уборка квартир в Санкт-Петербурге цены и закажите уборку.
Вся информация на сайте – https://service-cleanspb.ru
обеспыливание после ремонта, послестроительная уборка цена, заказать клининг в квартиру
уборка трехкомнатной квартиры, клининг спб уборка квартир после ремонта цена, уборка производственных помещений
Удачи!
Где заказать диплом специалиста?
Получаемый диплом с приложением полностью отвечает условиям и стандартам Министерства образования и науки, неотличим от оригинала – даже со специально предназначенным оборудованием. Не стоит откладывать личные мечты и цели на потом, реализуйте их с нашей компанией – отправьте заявку на диплом уже сегодня! Диплом о среднем специальном образовании – не проблема! emplealista.com/employer/premialnie-diplom-24
aviator
Non Gamstop casinos UK are perfect for quick sessions—great!
non gamstop casinos 2021 no deposit bonus
plinko
Доброго!
Уборка коттеджа цена – это услуга, которая обеспечит чистоту и порядок в вашем загородном доме. Мы предлагаем уборку после ремонта или регулярную уборку коттеджей. Уборка коттеджа цена – это выгодное предложение для владельцев загородных домов. Наши специалисты работают быстро и качественно. Закажите уборку коттеджа и получите отличный результат по доступной цене.
Клининговая компания СПб цены – это доступные тарифы на профессиональную уборку. Мы предлагаем генеральную, регулярную и срочную уборку. Наши специалисты используют качественные чистящие средства. Мы работаем быстро и эффективно. Узнайте клининговая компания СПб цены и закажите клининг.
Вся информация на сайте – https://service-cleanspb.ru/
уборку после ремонта, клининговая компания недорого, клининг цена спб уборка квартир
служба уборки квартир, уборка магазинов цены, уборка квартиры генеральная
Удачи!
book of ra
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:ремонт крупногабаритной техники в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Заказ документа о высшем образовании через качественную и надежную компанию дарит ряд достоинств для покупателя. Такое решение позволяет сэкономить как продолжительное время, так и значительные денежные средства. Впрочем, на этом выгода не ограничивается, плюсов значительно больше.Мы изготавливаем дипломы любой профессии. Дипломы изготавливаются на настоящих бланках государственного образца. Доступная цена в сравнении с серьезными тратами на обучение и проживание в другом городе. Приобретение диплома ВУЗа является выгодным шагом.
Приобрести диплом о высшем образовании: diplomus-spb.ru/diplom-s-reestrovim-zaneseniem-dlya-vashego-uspexa/
plinko
подключить проводной интернет екатеринбург
domashij-internet-ekaterinburg002.ru
тарифы интернет и телевидение екатеринбург
augmented reality photos https://augmented-reality-platform.ru/ .
одежда с английскими надписями http://www.dbkids.ru/ .
аренда лофт спб аренда лофт спб .
jackpot raider
Заказать документ ВУЗа можно в нашей компании в Москве. Мы оказываем услуги по изготовлению и продаже документов об окончании любых ВУЗов России. Вы сможете получить необходимый диплом по любой специальности, любого года выпуска, в том числе документы образца СССР. Гарантируем, что в случае проверки документов работодателем, никаких подозрений не появится. [url=http://diplom-ryssia.com/kupit-diplom-zanesennij-reestr-3/]diplom-ryssia.com/kupit-diplom-zanesennij-reestr-3/[/url]
What’s up i am kavin, its my first time to commenting anywhere, when i read this piece of writing i thought i could also create comment due to this good article.
https://www.nitrnd.com/blogs/330105/1xBet-Promo-Code-Bonus-Bonus-100-up-to-130
plinko
Non Gamstop casino UK withdrawals are lightning fast—impressed!
uk non gamstop casino
Купить документ о получении высшего образования можно в нашем сервисе. diplom-onlinex.com/kupit-diplom-farmatsevta
Где приобрести диплом специалиста?
Наши специалисты предлагают максимально быстро купить диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Данный диплом способен пройти любые проверки, даже при использовании специальных приборов. Достигайте свои цели быстро с нашим сервисом.
Приобрести диплом любого университета vuz-diplom.ru/mozhno-li-kupit-diplom-12/
купить диплом ростов
Где приобрести диплом специалиста?
Мы предлагаем выгодно и быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями официальных лиц. Данный диплом способен пройти лубую проверку, даже при помощи специального оборудования. Достигайте своих целей быстро и просто с нашим сервисом. Приобрести диплом о высшем образовании! galantclub.od.ua/member.php?u=27724
Приобрести документ института можно у нас. middoge.de/?p=2211#comment-20192
razor returns
детская одежда с надписями http://www.dbkids.ru .
1вин вход с компьютера 1win6050.ru .
The mobile casino needs work—spotty! vincispin
Где приобрести диплом по нужной специальности?
Мы изготавливаем дипломы любых профессий по приятным тарифам. Мы можем предложить документы ВУЗов, которые находятся на территории всей РФ. Вы можете заказать диплом от любого заведения, за любой год, указав подходящую специальность и хорошие оценки за все дисциплины. Дипломы и аттестаты выпускаются на бумаге самого высокого качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригинала. Документы будут заверены всеми требуемыми печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступны для подавляющей массы наших граждан. diplompro.ru/kupit-diplom-v-permi-8
интернет по адресу екатеринбург
domashij-internet-ekaterinburg003.ru
какие провайдеры на адресе в екатеринбурге
plinko
1win вход [url=1win6003.ru]1win6003.ru[/url] .
plinko
augmented reality pictures augmented reality pictures .
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам— diplom4you.com/kupit-meditsinskij-diplom-visokogo-kachestva-segodnya/
1 win официальный сайт http://www.obovsem.myqip.ru/?1-9-0-00000059-000-0-0-1743051936 .
Подробнее, читайте в блоге – на сайте
Мы предлагаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы дипломы были доступными для большого количества наших граждан.
Заказ документа, подтверждающего окончание ВУЗа, – это грамотное решение. Заказать диплом университета: diplom5.com/diplom-ob-okonchanii-vuza-kupit/
аренда лофтов спб аренда лофтов спб .
официальный сайт 1win официальный сайт 1win .
plinko
установка роликовых штор на окна установка роликовых штор на окна .
plinko
Found the best non Gamstop casino for blackjack—loving it so far!
no deposit casino not on gamstop
Moderni nabytek usak do kazdeho interieru – od minimalismu po klasiku. Vice nez 1000 modelu skladem. Online objednavka, pohodlna platba, pomoc navrhare. Zaridte svuj domov pohodlim!
The signup bonuses at online casinos are tempting! bet on red
Marvel Casino
мостбет авиатор https://mostbet6008.ru .
Заказать диплом университета по невысокой стоимости возможно, обратившись к проверенной специализированной фирме. Мы предлагаем документы об окончании любых ВУЗов Российской Федерации. Купить диплом о высшем образовании– diplomidlarf.ru/kupit-diplom-visshego-obrazovaniya-bistro-i-s-zaneseniem/
Best non Gamstop casinos offer variety like no other—amazing!
non gamstop casinos with paypal
Такси аэропорт Варна Служба Такси Эклипс предлагает доступные трансферы из аэропортов Бургас, Варна, София и Белград до популярных курортов Болгарии. Встреча с табличкой, помощь с багажом и комфортная поездка до места назначения. Обслуживаем Солнечный Берег, Созополь, Банско и другие направления. Для групп от 5 человек – специальные условия!
кайт анапа
новости Мир казино всегда манил яркими огнями, звоном монет и обещанием легкой удачи. От помпезных залов Лас-Вегаса до уютных уголков Монте-Карло, казино – это не только игра, но и целая индустрия, живущая по своим правилам. С развитием технологий казино перешли в онлайн-пространство. Онлайн-казино – это виртуальные платформы, предлагающие широкий спектр азартных игр, от классических слотов до карточных игр с живыми дилерами. Удобство, доступность и разнообразие делают онлайн-казино все более популярными.
The mobile casino clogs—tune it! mines casino
Добрый день!
Задолженность у судебных приставов — это серьезная ситуация, в которой приставы могут наложить арест на ваше имущество или счета. Если у вас есть задолженности, важно оперативно разобраться с ними, чтобы избежать таких последствий. Для этого можно заключить соглашение с приставами о рассрочке или полной оплате долга. Если дело дошло до судебных приставов, важно действовать быстро, чтобы минимизировать возможные санкции. Юрист поможет вам правильно решить проблему с задолженностью и приставами.
Вся информация на сайте – https://svoijurist.ru
налоговый вычет по ипотеке, судебные приставы новокузнецк узнать задолженность новокузнецк по фамилии бесплатно, как узнать о задолженности у судебных приставов по фамилии бесплатно без регистрации через интернет
когда нужно подавать налоговый вычет за лечение, получение налогового вычета за обучение супруга, гражданский судебный иск
Удачи!
1 вин скачать http://1win6003.ru .
Мы готовы предложить дипломы любых профессий по приятным тарифам. Цена может зависеть от выбранной специальности, года выпуска и образовательного учреждения. Всегда стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества наших граждан. купить диплом егеря
Приобрести документ института вы имеете возможность в нашем сервисе. dip-lom-rus.ru/kupit-diplom-ekaterinburg-2
Где заказать диплом специалиста?
Наша компания предлагает выгодно и быстро заказать диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями должностных лиц. Диплом способен пройти любые проверки, даже при помощи специального оборудования. Решайте свои задачи быстро и просто с нашими дипломами.
Приобрести диплом ВУЗа poluchidiplom.com/kupit-diplom-psixologa-13/
купить в армении диплом
Заказать документ ВУЗа вы сможете в нашем сервисе. summitinsulation.com/contact-us
Где приобрести диплом по необходимой специальности?
Мы предлагаем выгодно и быстро приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Данный документ способен пройти лубую проверку, даже при помощи специального оборудования. Решайте свои задачи быстро и просто с нашей компанией. Купить диплом университета! wowonder.mitek.com.tr/read-blog/10904_kupit-diplom-otzyvy.html
mostbet официальный сайт mostbet6008.ru .
1win.pro http://1win6050.ru .
I won $2500 online—dream night! aviator
I won $2500 online—dream night! plinko
Заказать диплом университета!
Мы предлагаем документы учебных заведений, которые находятся на территории всей РФ.
diplom-ryssia.com/kupit-diplom-s-reestrom-otzivi-2/
Где заказать диплом специалиста?
Мы изготавливаем дипломы любой профессии по разумным ценам. Мы предлагаем документы ВУЗов, которые расположены в любом регионе РФ. Вы можете купить качественно сделанный диплом от любого учебного заведения, за любой год, указав актуальную специальность и хорошие оценки за все дисциплины. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это дает возможности делать государственные дипломы, которые невозможно отличить от оригинала. Документы заверяются всеми обязательными печатями и подписями. Стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы дипломы были доступны для большинства наших граждан. okdiplom.com/kupit-diplom-texnika-5
Admiring the hard work you put into your blog and in depth information you offer. It’s nice to come across a blog every once in a while that isn’t the same out of date rehashed material. Wonderful read! I’ve bookmarked your site and I’m adding your RSS feeds to my Google account.
https://anotepad.com/notes/mfrba6g9
Здравствуйте!
Дискриминация при приеме на работу — это нарушение трудового законодательства, когда работодатель отказывает в трудоустройстве по признаку пола, возраста, расы или других факторов. Закон защищает права соискателей и не допускает дискриминации на рабочем месте. Если вам отказали в работе по необоснованным причинам, важно зафиксировать факт дискриминации и обратиться за помощью. Вы можете подать иск в суд или жалобу в трудовую инспекцию. Юрист поможет вам защищать свои права на равные условия при трудоустройстве.
Вся информация на сайте – https://svoijurist.ru/
договор совместного поручительства, кто может вернуть налоговый вычет за обучение ребенка, налоговый вычет за уплаченные проценты в ипотеку
налоговый вычет за покупку квартиры неработающего пенсионера, узнать задолженность по фамилии у судебных приставов чувашия, приватизировать собственность
Удачи!
Официальный сайт 1win https://1win.onedivision.ru спортивные ставки, киберспорт, казино, покер, рулетка и многое другое. Надежный сервис, поддержка 24/7, удобный интерфейс. Забирай бонус за регистрацию уже сейчас!
вакансии за рубежом Layboard
I’m addicted to online poker tournaments—hours fly by! plinko
Здравствуйте!
Клининговые услуги – это профессиональный уход за вашим домом и офисом. Мы предлагаем уборку любых помещений, включая после ремонта. Наши специалисты работают с качественными чистящими средствами. Мы гарантируем безупречный результат после каждой уборки. Закажите клининговые услуги по выгодной цене.
Клининговая компания в Санкт-Петербурге – это надежный партнер для уборки. Мы предоставляем услуги по уборке квартир, офисов и коммерческих помещений. Наши специалисты работают с качественными моющими средствами и современным оборудованием. Мы гарантируем идеальный результат. Закажите клининговую компанию в Санкт-Петербурге.
Вся информация на сайте – https://service-cleanspb.ru/
ген уборка квартиры, мойка окон и витрин, клининг уборка квартиры спб
услуги клининг, генеральная уборка клининг, заказать генеральную уборку квартиры
Удачи!
Valuable information. Fortunate me I discovered your website by chance, and I’m shocked why this accident did not came about in advance! I bookmarked it.
https://x.com/CleoCass1/status/1907818054991434074
The live dealers online rule—true vibe! plinko
Приобрести диплом об образовании!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным ценам— diplomg-kurerom.ru/kupit-diplom-o-meditsinskom-obrazovanii-vigodno-i-bistro/
Мы можем предложить дипломы любой профессии по приятным тарифам. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Для нас очень важно, чтобы дипломы были доступными для большого количества граждан.
Покупка диплома, подтверждающего обучение в ВУЗе, – это разумное решение. Заказать диплом университета: diplom-insti.ru/kupit-diplom-med-obrazovanie/
Добро пожаловать в 1win 1win onedivision ru азарт, спорт и выигрыши рядом! Ставь на матчи, играй в казино, участвуй в турнирах и получай крутые бонусы. Удобный интерфейс, быстрая регистрация и выплаты.
michelin crossclimate michelin crossclimate .
банкротство отзывы
шиномонтаж одинцово шиномонтаж одинцово .
Спасибо за цветы с характером – точно отразили именинника!
букет цветов томск
The poker online looks real—amazing! plinko
Good day!
Engage with the iMedix Wellness Program for trusted wellness tips, enhancing the my health online podcast selection; health enthusiasts exploring the potential benefits of adaptogens like Ashwagandha will find our scientific review uniquely objective, offering expert health advice and professional medical insights alongside reliable healthcare guidance, always advising caution (see iMedix.
The iMedix Medical Show offers reliable healthcare guidance, a top iMedix Medical podcast for clinical updates; health enthusiasts interested in the benefits of various herbal teas will find our infusion guide uniquely aromatic, delivering trusted wellness tips and expert health advice alongside professional medical insights on potential therapeutic properties.
More information at the site – https://www.iheart.com/podcast/269-an-insight-into-suhagra-50-163224911/episode/the-role-of-suhagra-50-163224912/
Wishing you luck!
либет казино, изи кэш казино
Бесплатные Steam аккаунты https://t.me/GGZoneSteam с играми и бонусами. Проверенные логины и пароли, ежедневное обновление, удобный поиск. Забирай свой шанс на крутой аккаунт без лишних действий!
каталог телеграм каналов тегстат
Online gambling feels loose—risk high! sugar rush
mostbet apk скачать mostbet apk скачать .
mostbet chrono http://www.svstrazh.forum24.ru/?1-18-0-00000136-000-0-0-1743260517 .
I won $500 online last week—best feeling ever! plinko
Доска объявлений https://estul.ru/blog по всей России: продавай и покупай товары, заказывай и предлагай услуги. Быстрое размещение, удобный поиск, реальные предложения. Каждый после регистрации получает на баланс аккаунта 100? для возможности бесплатного размещения ваших объявлений
Maispin——2025年最新USDT娛樂城,安全、快速、刺激!
歡迎來到 Maispin,2025年最具潛力的新秀 USDT娛樂城!在這裡,您只需提供 錢包地址 即可註冊,無需繁瑣的個人資訊,享受 安全、匿名、快速 的遊戲體驗。Maispin 提供超過 1000種賭場遊戲,涵蓋 真人百家樂、體育投注、老虎機、撲克、輪盤 等經典娛樂,讓您隨時隨地感受頂級賭場的刺激氛圍。我們與世界知名遊戲供應商合作,確保遊戲 公平公正,並提供高額彩金、獎勵活動,讓玩家輕鬆贏大獎!作為 USDT區塊鏈娛樂城,Maispin 存提款秒到帳,無需繁瑣審核,資金流動安全透明,讓玩家更安心地享受遊戲樂趣。立即加入 M宇宙,體驗前所未有的加密娛樂新潮流!
Online casinos should care—shield us! razor returns
mosbet http://svstrazh.forum24.ru/?1-18-0-00000136-000-0-0-1743260517 .
1win на телефон 1win на телефон .
I hit a bonus online and won $90—yes! dragon hatch
Купить диплом любого университета!
Мы можем предложить документы учебных заведений, которые находятся в любом регионе Российской Федерации.
diplom-onlinex.com/kupite-diplom-o-visshem-obrazovanii-bistro-i-legko-2/
The live help online rocks—key boost! aviator
автоматические римские шторы автоматические римские шторы .
I don’t get the hate for online gambling—it’s just like any other hobby! plinko
The poker online shines—real kick! legacy of dead
UID_20861405###
123
Тени Химок и Лобни
Алина проснулась в крошечной квартире на окраине Эскорт Химки.
От резкого света фонаря за шторой заломило виски — снова короткий сон перед ночной сменой.
Она затянулась сигаретой, глядя на потрескавшиеся обои. Деньги за аренду копилки не ждали.
Экортницу Лобня встретила её холодным ветром. Здесь клиенты были проще — водители с трассы, одинокие мужики из спальных районов.
Иногда везло: попадались «благодарные», оставляли сверху пару тысяч. Но чаще — торг, хамство, а то и кулаки.
Однажды зимой её забрали в отделение. Допрос, унижения, взятка «за молчание». После этого Алина научилась договариваться заранее.
К весне накопила на билет в Сочи. Мечтала о море, чистом небе. Но когда пришло время уезжать, обнаружила, что даже чемодан собрать страшно.
Химки и Лобня стали её клеткой.
Алина поняла это, стоя на перроне, глядя на уходящий поезд. В кармане — пачка смятых купюр. Завтра — новый день. И новый клиент.
UID_46383311###
test
UID_74686374###
test
UID_98600946###
test
UID_45806261###
test
The online gambling rush is crazy—stay cool! plinko
Купить диплом института по выгодной стоимости можно, обращаясь к проверенной специализированной фирме. Приобрести документ института можно в нашем сервисе. asxdiploman.com/diplom-s-vneseniem-v-reestr-dlya-vashej-uspeshnoj-kareri
UID_26569160###
test
Online casinos should cap—guard play! dragon hatch
ремонт карданного вала цена в москве 4-x-4.ru/remont-i-balansirovka-kardannyh-valov/ .
UID_94786424###
test
купить вкладыш в диплом
UID_80790258###
test
1win зайти 1win зайти .
UID_89194462###
test
ванвин ванвин .
The convenience of online gambling is both a blessing and a curse! plinko
Ученые узнали, в какое время суток кофе заметно продлевает жизнь
https://pin.it/72MctQ7nd
Новое исследование, опубликованное в European Heart Journal, выявило, что люди, предпочитающие пить кофе утром, могут иметь более низкий риск смертности, особенно от сердечно-сосудистых заболеваний. Это ставит под вопрос не только количество употребляемого кофе, но и его время как фактор, влияющий на здоровье.
I won $400 online—best night in a while! dragon tiger
1win букмекер https://www.kharkovbynight.forum24.ru/?1-5-0-00000235-000-0-0 .
официальный сайт 1 вин http://www.1win6004.ru .
mostbet скачать на телефон бесплатно андроид https://mostbet6009.ru .
I love the online chats—great crew! fortune tiger
Где заказать диплом по необходимой специальности?
Мы готовы предложить дипломы любой профессии по приятным тарифам. Мы готовы предложить документы ВУЗов, которые находятся на территории всей РФ. Можно приобрести диплом за любой год, указав актуальную специальность и хорошие оценки за все дисциплины. Документы печатаются на бумаге высшего качества. Это дает возможности делать государственные дипломы, которые невозможно отличить от оригиналов. Они заверяются всеми необходимыми печатями и штампами. Всегда стараемся поддерживать для покупателей адекватную политику цен. Для нас важно, чтобы документы были доступны для большинства наших граждан. diplom-kuplu.ru/kupit-diplom-v-yaroslavle-2-6
I don’t trust online data—wary! vipzino
установка роликовых штор на окна установка роликовых штор на окна .
The live games online rock—fan love! plinko app
1win kg скачать https://1win6052.ru/ .
The mobile apps online slip—fix up! bongo bongo
Online gambling feels free—no watch! plinko
Viagra * Cialis * Levitra
All the products you are looking suitable are currently at one’s disposal for 1+1.
4 more tablets of unified of the following services: Viagra * Cialis * Levitra
https://pxman.net
1win скачать kg https://girikms.forum24.ru/?1-2-0-00000264-000-0-0 .
michelin michelin .
one win one win .
Good afternoon!
The iMedix Podcast delivers trusted wellness tips, a standout medical podcast for preventative care; medical professionals advising patients on colorectal cancer screening options will find our test comparison uniquely objective, providing professional medical insights and reliable healthcare guidance alongside expert health advice covering colonoscopy, FIT, and Cologuard.
The iMedix Podcast delivers trusted wellness tips, a standout medical podcast for health empowerment; fitness lovers exploring the concept of intuitive eating will find our principles guide uniquely non-restrictive, providing professional medical insights and reliable healthcare guidance alongside expert health advice for building a healthier relationship with food and body.
More information at the site – https://podcasts.apple.com/us/podcast/comprehensive-review-of-reliablerxpharmacy/id1748267234
Wishing you luck!
Приобрести диплом любого ВУЗа можем помочь. Купить диплом бакалавра в Улан-Удэ – diplomybox.com/kupit-diplom-bakalavra-v-ulan-ude
Самые новые анекдоты https://www.anekdotovmir.ru/ — коротко, метко и смешно! Подборка актуального юмора: от жизненных до политических. Заходи за порцией хорошего настроения!
1win.kg http://girikms.forum24.ru/?1-2-0-00000264-000-0-0 .
Online casinos should ease—too much! plinko
шиномантаж зеленоград шиномантаж зеленоград .
Greetings!
Engage with the iMedix Wellness Program for professional medical insights, central to iMedix health care education initiatives; wellness seekers aiming to cultivate more mindful eating habits during meals will find our sensory awareness prompts uniquely engaging, offering expert health advice and trusted wellness tips alongside reliable healthcare guidance for savoring food and recognizing satiety cues. Prompts on imedix.com.
Welcome to iMedix podcast, offering reliable healthcare guidance and professional medical insights; health enthusiasts exploring the benefits of adding spices like cayenne pepper for metabolism will find our thermogenesis discussion uniquely stimulating, providing expert health advice and trusted wellness tips alongside cautionary notes on dosage and individual tolerance.
More information at the site – https://podcasters.spotify.com/pod/show/alexandr659
Wishing you luck!
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по доступным тарифам. Оставить отзыв — kyc-diplom.com/replies.html
I love the online calm—no buzz! plinko
Кладбища Видного https://bulatnikovskoe.ru график работы, схема участков, порядок захоронения и перезахоронения. Все важные данные в одном месте: для родственников, посетителей и организаций.
michelin автошины michelin автошины .
Hello, I think your blog might be having browser compatibility issues. When I look at your blog in Firefox, it looks fine but when opening in Internet Explorer, it has some overlapping. I just wanted to give you a quick heads up! Other then that, wonderful blog!
https://saltykidsadventure.site/2025/04/03/%d0%b2%d1%81%d0%b5-%d1%87%d1%82%d0%be-%d0%bd%d1%83%d0%b6%d0%bd%d0%be-%d0%b7%d0%bd%d0%b0%d1%82%d1%8c-%d0%be-%d0%bf%d0%be%d0%ba%d0%b5%d1%80%d0%b4%d0%be%d0%bc-%d0%bf%d1%80%d0%be%d0%bc%d0%be%d0%ba%d0%be/
I lost $280 online—plan up! plinko
generic best online pharmacy Drug pamphlet provided. Medication guide available. vyvanse best online pharmacy
шатры для мероприятий в москве http://shatry-dlya-meropriyatiy.ru/ .
Приобрести диплом ВУЗа!
Мы предлагаем документы любых учебных заведений, которые расположены на территории всей России.
diplomgorkiy.com/kupit-diplom-s-reestrom-v-rossii-bistro-i-nadezhno-3/
I wish online held oldies—miss that! gates of olympus
шиномантаж дмитров [url=http://hondahybrid.ru/forum/topic/2886-kakie-uslugi-okazyvaet-shinomontazh/]http://hondahybrid.ru/forum/topic/2886-kakie-uslugi-okazyvaet-shinomontazh/[/url] .
1win казино https://cinemania.forum24.ru/?1-15-0-00001911-000-0-0-1743258043/ .
navigate to these guys mymerrill login
1вин войти 1вин войти .
Online casinos ease play—mind out! plinko
Встреча в аэропорту Бургас Служба Такси Эклипс предлагает доступные трансферы из аэропортов Бургас, Варна, София и Белград до популярных курортов Болгарии. Встреча с табличкой, помощь с багажом и комфортная поездка до места назначения. Обслуживаем Солнечный Берег, Созополь, Банско и другие направления. Для групп от 5 человек – специальные условия!
Мы предлагаем дипломы любой профессии по невысоким ценам. Стоимость может зависеть от выбранной специальности, года выпуска и университета. Стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы дипломы были доступными для большого количества наших граждан. купить бланк диплома
I lost big online—shift gears! dragon tiger
one win https://cinemania.forum24.ru/?1-15-0-00001911-000-0-0-1743258043 .
Купить документ о получении высшего образования вы можете у нас. asxdiploman.com/kupit-diplom-novogo-obraztsa
Online casinos need stronger addiction warnings. craps game
Online casinos feel risky—double watch! plinko
buy topamax medication Read about medicines. Active ingredients listed. buy topamax usa
Online casinos should cap—safe guard! fortune ox
Мы можем предложить дипломы любой профессии по приятным ценам. Дипломы производят на настоящих бланках государственного образца Приобрести диплом ВУЗа diplomskiy.com
Где заказать диплом специалиста?
Купить диплом ВУЗа по невысокой цене можно, обращаясь к надежной специализированной компании.: diploms-vuza.com
I don’t get why people hate on online casinos; it’s just entertainment! mines game
Заказала с доставкой на природу – упаковали так, что даже дождь не помешал!
розы купить в томске
Check Out Your URL merrill lynch login
mostbet casino https://mostbet6009.ru/ .
ремонт карданного вала цена в москве 4-x-4.ru/remont-i-balansirovka-kardannyh-valov/ .
Online laws blur—clear up! book of ra
Отдых в Архипо-Осиповке в 2025, смотри подробнее по ссылке Экскурсии Архипо-Осиповки 2025
topamax generic Medication leaflet provided. Comprehensive pill resource. purchase topiramate
1вин официальный сайт вход https://1win6004.ru .
Купить диплом о высшем образовании !
Покупка диплома любого ВУЗа России у нас является надежным делом, потому что документ будет заноситься в реестр. Приобрести диплом о высшем образовании diplomservis.com/diplom-s-zaneseniem-v-reestr-dlya-vashego-uspexa-5
I love the live roulette—real deal! fortune ox
Отдых в Архипо-Осиповке в 2025, смотри подробнее по ссылке Экскурсии Архипо-Осиповки 2025
мотбет мотбет .
Online casino ads roar—hush it! plinko
ремонт грузовых карданов http://4-x-4.ru/remont-i-balansirovka-kardannyh-valov/ .
Приобрести диплом ВУЗа!
Мы можем предложить документы университетов, расположенных на территории всей Российской Федерации. Документы выпускаются на бумаге высшего качества: androidinweb.ru/kupit-diplom-vyigodnyie-usloviya-dostupnyie-tsenyi
Быстро заказать диплом о высшем образовании!
Купить диплом университета по выгодной стоимости можно, обращаясь к проверенной специализированной компании. Заказать диплом о высшем образовании: diplom-kaluga.ru/kupit-diplom-vuza-bez-problem-onlajn
казино 1win https://www.knowledge.forum24.ru/?1-1-0-00000082-000-0-0-1743258384 .
The thrill of online gambling is addictive—gotta stay in check! pinup
USDT娛樂城
Maispin——2025年最新USDT娛樂城,安全、快速、刺激!
歡迎來到 Maispin,2025年最具潛力的新秀 USDT娛樂城!在這裡,您只需提供 錢包地址 即可註冊,無需繁瑣的個人資訊,享受 安全、匿名、快速 的遊戲體驗。Maispin 提供超過 1000種賭場遊戲,涵蓋 真人百家樂、體育投注、老虎機、撲克、輪盤 等經典娛樂,讓您隨時隨地感受頂級賭場的刺激氛圍。我們與世界知名遊戲供應商合作,確保遊戲 公平公正,並提供高額彩金、獎勵活動,讓玩家輕鬆贏大獎!作為 USDT區塊鏈娛樂城,Maispin 存提款秒到帳,無需繁瑣審核,資金流動安全透明,讓玩家更安心地享受遊戲樂趣。立即加入 M宇宙,體驗前所未有的加密娛樂新潮流!
1win скачать kg https://knowledge.forum24.ru/?1-1-0-00000082-000-0-0-1743258384 .
I lost time online—swift pass! plinko
Хочу рассказать о своем опыте по заказу аттестата пту, думал это не реально и стал искать информацию в сети, про купить диплом о высшем в красноярске, купить диплом о среднем екатеринбург, купить диплом высшее екатеринбург, купить диплом с реестром в нижнем, где купить диплом вуза, постепенно вникая в суть дела нашел отличный материал здесь diplomybox.com/kupit-diplom-spetsialista-saratov)
ван вин сайт 1win – 1win зеркало win, сайт 1вин
Отдых в Архипо-Осиповке в 2025, смотри подробнее по ссылке Экскурсии Архипо-Осиповки
купить диплом в брянске diplomys-vsem.ru .
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
wan win https://knowledge.forum24.ru/?1-1-0-00000082-000-0-0-1743258384 .
Мы предлагаем дипломы любых профессий по выгодным ценам. Плюсы приобретения документов в нашей компании
Вы заказываете диплом через надежную фирму. Такое решение позволит сэкономить не только много средств, но и ваше драгоценное время.
Преимуществ куда больше:
• Дипломы печатаются на настоящих бланках с мокрыми печатями и подписями;
• Дипломы всех ВУЗов России;
• Цена намного меньше нежели довелось бы заплатить на очном обучении в ВУЗе;
• Доставка в любые регионы России.
Приобрести диплом о высшем образовании– http://goldenfiber.ru/forum/?PAGE_NAME=profile_view&UID=48558/ – goldenfiber.ru/forum/?PAGE_NAME=profile_view&UID=48558
Online gambling feels unreal—easy to overdo! plinko
Greetings!
Tune into the iMedix Medical Show for professional medical insights, a key iMedix popular podcast; wellness seekers aiming to incorporate more mindful breathing throughout their day will find our 1-minute breathwork exercises uniquely accessible, providing expert health advice and trusted wellness tips alongside reliable healthcare guidance for instant calm.
Explore the iMedix Health Series for reliable healthcare guidance, a valuable resource for my health podcast fans; health enthusiasts trying to incorporate more omega-3 rich foods like flax and chia seeds will find our usage tips uniquely versatile, offering professional medical insights and expert health advice alongside trusted wellness tips for easy integration into meals.
More information at the site – https://open.spotify.com/episode/20Zo4szUx2x58rU9UpfMfz
Wishing you luck!
алкоголь 24 часа http://dostavka-alkogolya248.ru/ .
нейросеть для написания реферата studgen.ru .
I love the live roulette—real deal! F7 Casino
The excitement of online slots is electric! aviator
Ремонт смартфонов [url=https://techno-line.store/]techno-line.store[/url] .
заработок на аккаунтах купить аккаунт с прокачкой
Online casinos can hook you—set limits! plinko
тент для шатра на заказ аренда https://www.shatry-dlya-meropriyatiy.ru .
Доброго времени суток!
Для многих людей, заказать диплом о высшем образовании – это острая необходимость, уникальный шанс получить отличную работу. Но для кого-то – это желание не терять время на учебу в ВУЗе. С какой бы целью вам это не понадобилось, наша компания готова помочь. Максимально быстро, профессионально и по разумной стоимости сделаем диплом нового или старого образца на подлинных бланках с реальными подписями и печатями.
Главная причина, почему многие покупают документы, – желание занять хорошую должность. Например, знания и опыт дают возможность кандидату устроиться на желаемую работу, однако документального подтверждения квалификации нет. При условии, что работодателю важно присутствие “корочки”, риск потерять место работы довольно высокий.
Купить документ ВУЗа вы можете у нас. Мы предлагаем документы об окончании любых университетов России. Вы сможете получить необходимый диплом по любым специальностям, включая документы Советского Союза. Гарантируем, что при проверке документов работодателями, каких-либо подозрений не возникнет.
Факторов, которые вынуждают приобрести диплом ВУЗа достаточно. Кому-то срочно необходима работа, и необходимо произвести особое впечатление на руководителя при собеседовании. Некоторые планируют устроиться в большую компанию, чтобы повысить собственный статус в социуме и в будущем начать собственное дело. Чтобы не терять попусту годы жизни, а сразу начать успешную карьеру, применяя врожденные таланты и полученные навыки, можно приобрести диплом прямо в онлайне. Вы сможете быть полезным в обществе, получите финансовую стабильность в максимально короткий срок- аттестат купить за 9 класс
buy topiramate online Complete medicine overview. Medicine guide here. buy topamax with no prescription
мостбет официальный сайт https://mostbet6010.ru/ .
Доброго бравые люди!
Ты хочешь стабильности, уважения и защищённости? Подписание контракта с Министерством обороны — это шаг к новой жизни. Служба в СВО обеспечивает полное обеспечение: от ежемесячной выплаты 204 000 рублей до жилья, питания, медицинского обслуживания, страхования и отпуска. Государство гарантирует помощь семьям, правовую защиту и возможность получения льгот. Контракт даёт не просто работу, а профессию и статус. Сделай выбор в пользу силы, долга и уверенности. Подпиши контракт и начни путь настоящего мужчины;
Вся информация на сайте – https://xn——-43dcbcq5a2cbf8adjcigzjspdkp.xn--p1ai/
военная служба по контракту заключение контракта, служба по контракту московская область 2025, московский контракт на сво
служба по призыву и по контракту, служба по контракту сво 2025 году, служба по контракту москва условия 2025
Удачи на службе!
1 вин официальный сайт вход 1win6051.ru .
рейтинг казино на реальные деньги онлайн – онлайн казино бездеп, онлайн казино бесплатно
1win live https://1win6051.ru/ .
Добрый день!
Без наличия диплома очень сложно было продвинуться вверх по карьере. Сегодня же документ не дает гарантий, что получится найти престижную работу. Куда более важное значение имеют профессиональные навыки и знания специалиста и его опыт работы. Именно по этой причине решение о покупке диплома можно считать целесообразным. Приобрести диплом любого университета infobidz.fun/read-blog/21613_kupit-diplom-prepodavatelya.html
Здравствуйте!
Ты хочешь стабильной и надёжной жизни? Служба по контракту в зоне СВО — это возможность обеспечить себе твёрдую основу. Контракт с Министерством обороны — это путь настоящего мужчины.
Вся информация на сайте – https://xn——-43dcbcq5a2cbf8adjcigzjspdkp.xn--p1ai/
старший телефонист сво, инструктор штаба обязанности на сво, условия контракта вс рф
как заключенному заключить контракт на сво, военная служба по контракту требования, порядок военной службы по контракту
Удачи на службе!
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Добрый день!
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по невысоким ценам. Стоимость зависит от конкретной специальности, года выпуска и образовательного учреждения: diplomanruss.com/
1win официальный https://1win6051.ru .
Right here is the right site for everyone who wishes to understand this topic. You understand a whole lot its almost hard to argue with you (not that I actually will need to…HaHa). You definitely put a fresh spin on a subject that’s been discussed for years. Wonderful stuff, just wonderful!
казино нью ретро официальный сайт
Профессиональная помощь в сфере магических услуг. Искусство высшей магии.
Ясновидение/Руны/Ритуалы
Диагностика / Консультация
* Диагностика на наличие магического негатива, порчи, приворота, воздействия
* Диагностика энергетики
* Выявление проблем и препятствий
* Ответы на вопросы
Чистка магического и энергетического негатива.
Защита на всех уровнях.
Открытие дорог.
Открытие финансового канала.
(на основании диагностики производим чистку негатива, убираем любые деструктивные программы включая порчу на смерть, кладбищенскую порчу, порчу через подклад, подселение сущности, приворот и другие деструктивные программы разрушающие вашу жизнь)
восстанавливаем вашу жизнь и настраиваем по всем сферам жизни
Магия для решения Бизнес задач и Финансовых вопросов
(моя самая любимая сфера деятельности)
* Убрать препятствия
* Защита на вас и ваш бизнес
* Помощь в судах
* Выявление и решение проблем вашего бизнеса через магию
Любовная магия
* Определение совместимости партнеров в браке и при вступлении в брак
* Защита семьи и отношений
* Розжиг чувств в паре (это новый виток отношений. реанимация любви)
* Создание условий, которые приведут к романтическим отношениям и к созданию семьи
Решение любых актуальных для вас проблем
* Выявить и убрать блоки мешающие достичь цели
– магия рун
– магия древних ритуалов
– магия символов, углов, пространства
– планетарная магия
– пространственная алхимия
– работа через сознание и подсознание
Все работы проводятся очень экологично и без какого-либо вреда
Также проводятся специальные ритуалы позволяющие развернуть вашу жизнь на 360 градусов, получить желаемое, открыть дороги, притянуть любовь и создать жизнь мечты
Телеграмм канал Искусство Высшей Магии
Телеграмм для связи: https://t.me/magicfxs
Для связи: WHATSAPP +44 7435 250025
*** для заказа присылайте фото для диагностики, имя и ваш вопрос.
Услуги осуществляются на условиях 100% предоплаты.
P.S. Если человек знает для чего ему нужно желаемое – значит он может это достичь
купить диплом саратов отзывы
тенты и шатры аренда http://shatry-dlya-meropriyatiy.ru .
1win kg https://1win6052.ru/ .
buy topiramate Drug resource available. Read about pills. buy topamax uk
Приобрести диплом о высшем образовании!
Мы предлагаем дипломы психологов, юристов, экономистов и любых других профессий по доступным тарифам. Вы приобретаете диплом в надежной и проверенной компании. : teba.timbaktuu.com/employer/frees-diplom
Где приобрести диплом специалиста?
Мы предлагаем максимально быстро купить диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями. Данный документ пройдет любые проверки, даже с использованием специфических приборов. Решите свои задачи максимально быстро с нашим сервисом.
Купить диплом любого ВУЗа kupitediplom0027.ru/kupit-diplom-texnologa-4/
продать аккаунт заработок на аккаунтах
UID_20157402###
test
UID_89647609###
test
UID_41684275###
test
I don’t trust online odds—weird! mines
Где заказать диплом по нужной специальности?
Наши специалисты предлагаютбыстро и выгодно приобрести диплом, который выполняется на оригинальном бланке и заверен печатями, штампами, подписями официальных лиц. Данный диплом способен пройти лубую проверку, даже при помощи специального оборудования. Решите свои задачи максимально быстро с нашими дипломами. Заказать диплом ВУЗа! voodooporn.com/read-blog/823_kupit-attestat-8-klass.html
UID_87800967###
test
I hit a bonus online and won $70—neat! marvel casino
Где заказать диплом специалиста?
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы предлагаем документы техникумов, расположенных в любом регионе России. Вы сможете заказать качественно напечатанный диплом за любой год, указав актуальную специальность и хорошие оценки за все дисциплины. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это дает возможности делать государственные дипломы, не отличимые от оригиналов. Они будут заверены всеми требуемыми печатями и подписями. Стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества наших граждан. diploms-vuza.com/kupit-diplom-v-irkutske-8
UID_70561211###
test
UID_57094612###
test
UID_83016133###
test
UID_95766583###
test
подключить интернет в казани в квартире
domashij-internet-kazan001.ru
провайдеры в казани по адресу проверить
Заказать диплом об образовании!
Мы изготавливаем дипломы любой профессии по выгодным тарифам— kupit-diplom24.com/skolko-stoit-kupit-attestat-11-klassa-6/
Мы предлагаем дипломы психологов, юристов, экономистов и других профессий по разумным ценам. Стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы дипломы были доступными для подавляющей массы граждан.
Приобретение документа, который подтверждает обучение в ВУЗе, – это выгодное решение. Заказать диплом о высшем образовании: diplomius-docs.com/diplom-visshego-obrazovaniya-kupit-5/
UID_27352550###
test
UID_24146793###
test
UID_76010280###
test
UID_47886839###
test
UID_33313906###
test
buy topamax uk Medication trends described. Find pill info. buy topamax no rx
I lost big online—reboot now! plinko
безопасная сделка аккаунтов магазин аккаунтов
Где купить диплом специалиста?
Получаемый диплом с приложением полностью отвечает стандартам Министерства образования и науки РФ, неотличим от оригинала. Не откладывайте свои мечты на несколько лет, реализуйте их с нашей компанией – отправляйте простую заявку на изготовление документа сегодня! Диплом о среднем специальном образовании – не проблема! skymix2018.forumex.ru/viewtopic.phpf=14&t=893
Где приобрести диплом специалиста?
Мы оказываем услуги по производству и продаже документов об окончании любых университетов РФ. Документы производят на подлинных бланках государственного образца. Рєjobsyt.com/companies/premialnie-diplom-24
Online casinos need tools—help us! aviator
Мы предлагаем дипломы психологов, юристов, экономистов и прочих профессий по доступным ценам.– diplomus-spb.ru/kupit-diplom-s-reestrom-po-vigodnoj-tsene-sejchas/
I love the live roulette—so live! plinko
интернет провайдеры в казани по адресу дома
domashij-internet-kazan002.ru
подключить домашний интернет казань
Заказать документ о получении высшего образования вы можете у нас в столице. Мы оказываем услуги по производству и продаже документов об окончании любых университетов Российской Федерации. Вы сможете получить диплом по любым специальностям, включая документы образца СССР. Гарантируем, что при проверке документа работодателем, каких-либо подозрений не появится. diplomc-v-ufe.ru/kupit-diplom-s-reestrom-v-moskve-3/
Are online ratings real?—tough call! legacy of dead
buy topamax medication Complete pill overview. Access medication details. buy topiramate pills
https://receptmult.ru/
Online gambling feels light—risk on! sugar rush
доставка виски на дом в москве ночью доставка виски на дом в москве ночью .
I don’t trust online odds—weird! aviator game
Online casinos should free—test run! bet on red casino
купить диплом петербург одно
The online flow is wild—stay sharp! razor returns
Для эффективного продвижения вверх по карьере требуется наличие диплома университета. Быстро и просто заказать диплом любого университета у проверенной компании: [url=http://diplomers.com/gde-kupit-attestat-za-9-klass-bistro-i-bez-hassles/]diplomers.com/gde-kupit-attestat-za-9-klass-bistro-i-bez-hassles/[/url]
нейросеть для написания курсовой http://www.studgen.ru/ .
Мы предлагаем дипломы любых профессий по приятным ценам. Цена будет зависеть от выбранной специальности, года выпуска и университета. Стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступны для подавляющей массы граждан. диплом ветеринара купить
провайдеры по адресу дома
domashij-internet-kazan003.ru
интернет провайдер казань
Купить Героин в Киеве? Ссылка – COCAIN.VIP Доставка По Украине Героина? Ссылка – COCAIN.VIP
.
.
.
.
.
| Купить онлайн закладку Героин в Киеве ССЫЛКА – https://cocain.vip |
| Купить в другом городе Украины Героин закладку ССЫЛКА – https://cocain.vip |
| Купить доставку Героина в Киеве в руки через сайт ССЫЛКА – https://cocain.vip |
| Купить доставку в руки Героин в Украине ССЫЛКА – https://cocain.vip |
| Купить Героин в Украине доставкой через почту ССЫЛКА – https://cocain.vip |
| Купить Героин в Киеве и Украине через Курьера в руки ССЫЛКА -https://cocain.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Слова для поиска –
Героин в Киеве купить закладкой, Героин в Киеве Купить з доставкой, Героин в Киеве купить через курьера, Героин в Киеве Купить быстро, Героин в Киеве Купить сейчас, Героин в Киеве Купить в руки, Героин в Киеве Купить безопасно
Героин в Украине Купить можно, Купить Героин в Украине закладкой, Купить Героин в Украине доставкой домой, Купить Героин в Украине по почте, Купить Героин в Украине без проблем можно, Купить Героин в Украине на сайте, Кокс цена в Украине,
Виды Героина Купить в Украине и Киеве, Чем отличаеться Героин в Киеве от Героина в Украине, Как я могу купить Героин в Киеве, Купить Колумбийский Героин в Киеве, Купить Колумбийский Героин в Украине, Кокс Цена в Киеве,
Теги для городов –
Купить Героин в Киеве, Купить Героин в Одессе, Купить Героин в Харькове, Купить Героин во Львове, Купить Героин в Днепре, Купить Героин в Ужгороде, Купить Героин в Ивано-Франковске, Купить Героин в Белой Церкве, Купить Героин в Ровно
Купить Героин в Николаеве, Купить Героин в Херсоне, Купить Героин в Сумах, Купить Героин в Запорожье, Купить Героин в Житомере, Купить Героин в Мукачево, Купить Героин в Полтаве, Купить Героин в Умани, Купить Героин в Виннице
Общие теги для товаров-
Купить Героин в Киеве, Купить Героин в Украине, Купить Героин в Киеве, Купить Героин в Украине, Купить Лирику в Киеве, Купить Лирику в Украине, Купить Экстази в Киеве, Купить Экстази в Украине, Купить Амфетамин в Киеве, Купить Амфетамин
в Украине, Купить МДМА в Киеве, Купить МДМА в Украине, Купить Метамфетамин в Киеве, Купить Метамфетамин в Украине, Купить Метадон в Киеве, Купить Метадон в Украине, Купить Шишки в Киеве, Купить Шишки в Украине, Купить Бошки в Киеве,
Купить Бошки в Украине, Купить Альфа ПВП В Киеве, Купить Альфа ПВП в Украине, Купить A-PVP в Киеве, Купить A-PVP в Украине, Купить Соль СК в Киеве, Купить Соль СК в Украине, Купить Героин в Киеве, Купить Героин в Украине,
Купить Гашиш в Киеве? Ссылка – COCAIN.VIP Доставка По Украине Гашиша? Ссылка – COCAIN.VIP
.
.
.
.
.
| Купить онлайн закладку Гашиш в Киеве ССЫЛКА – https://cocain.vip |
| Купить в другом городе Украины Гашиш закладку ССЫЛКА – https://cocain.vip |
| Купить доставку Гашиша в Киеве в руки через сайт ССЫЛКА – https://cocain.vip |
| Купить доставку в руки Гашиш в Украине ССЫЛКА – https://cocain.vip |
| Купить Гашиш в Украине доставкой через почту ССЫЛКА – https://cocain.vip |
| Купить Гашиш в Киеве и Украине через Курьера в руки ССЫЛКА -https://cocain.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Слова для поиска –
Гашиш в Киеве купить закладкой, Гашиш в Киеве Купить з доставкой, Гашиш в Киеве купить через курьера, Гашиш в Киеве Купить быстро, Гашиш в Киеве Купить сейчас, Гашиш в Киеве Купить в руки, Гашиш в Киеве Купить безопасно
Гашиш в Украине Купить можно, Купить Гашиш в Украине закладкой, Купить Гашиш в Украине доставкой домой, Купить Гашиш в Украине по почте, Купить Гашиш в Украине без проблем можно, Купить Гашиш в Украине на сайте, Кокс цена в Украине,
Виды Гашиша Купить в Украине и Киеве, Чем отличаеться Гашиш в Киеве от Гашиша в Украине, Как я могу купить Гашиш в Киеве, Купить Колумбийский Гашиш в Киеве, Купить Колумбийский Гашиш в Украине, Кокс Цена в Киеве,
Теги для городов –
Купить Гашиш в Киеве, Купить Гашиш в Одессе, Купить Гашиш в Харькове, Купить Гашиш во Львове, Купить Гашиш в Днепре, Купить Гашиш в Ужгороде, Купить Гашиш в Ивано-Франковске, Купить Гашиш в Белой Церкве, Купить Гашиш в Ровно
Купить Гашиш в Николаеве, Купить Гашиш в Херсоне, Купить Гашиш в Сумах, Купить Гашиш в Запорожье, Купить Гашиш в Житомере, Купить Гашиш в Мукачево, Купить Гашиш в Полтаве, Купить Гашиш в Умани, Купить Гашиш в Виннице
Общие теги для товаров-
Купить Гашиш в Киеве, Купить Гашиш в Украине, Купить Гашиш в Киеве, Купить Гашиш в Украине, Купить Лирику в Киеве, Купить Лирику в Украине, Купить Экстази в Киеве, Купить Экстази в Украине, Купить Амфетамин в Киеве, Купить Амфетамин
в Украине, Купить МДМА в Киеве, Купить МДМА в Украине, Купить Метамфетамин в Киеве, Купить Метамфетамин в Украине, Купить Метадон в Киеве, Купить Метадон в Украине, Купить Шишки в Киеве, Купить Шишки в Украине, Купить Бошки в Киеве,
Купить Бошки в Украине, Купить Альфа ПВП В Киеве, Купить Альфа ПВП в Украине, Купить A-PVP в Киеве, Купить A-PVP в Украине, Купить Соль СК в Киеве, Купить Соль СК в Украине, Купить Гашиш в Киеве, Купить Гашиш в Украине,
сюда Манго Офис вход
buy topamax no prescription Access medicine information. Access medication facts. purchase topamax online no prescription
Подробнее Манго Офис IP-телефония
1win играть 1win6052.ru .
безопасная сделка аккаунтов купить аккаунт с прокачкой
Заказала с доставкой на природу – упаковали так, что даже дождь не помешал!
заказ цветов томск
Online casinos should speed—wait off! minas
Мы можем предложить дипломы любой профессии по приятным ценам. Дипломы изготавливаются на оригинальных бланках государственного образца Заказать диплом любого института fastdiploms.com
I won free spins online and got $30—nice surprise! dragon tiger
Online slots grab me—those looks! aviator
Где приобрести диплом специалиста?
Купить диплом института по невысокой стоимости вы сможете, обратившись к надежной специализированной компании.: kazdiplomas.com
содержание Mango-Office виртуальный номер
кайт египет
Купить Мефедрон Москва? САЙТ -| COCAINES.STORE | Как Купить Мефедрон по России? САЙТ – | COCAINES.STORE |
.
.
.
.
.
Купить Мефедрон максимального качества в Москве? САЙТ – https://cocaines.store
Сколько стоит Мефедрон в Москве сегодня? САЙТ – https://cocaines.store
Купить Мефедрон в городах России? САЙТ – https://cocaines.store
Купить Мефедрон с доставкой в руки В Москве? САЙТ – https://cocaines.store
Где в Москве Купить Мефедрон с доставкой? САЙТ – https://cocaines.store
Купить с доставкой по России Мефедрон? САЙТ – https://cocaines.store
Купить Наркотики в Москве? САЙТ – https://cocaines.store
Как Купить наркотики в России? САЙТ – https://cocaines.store
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл Робот (GOOGLE ROBOT)
Купить Мефедрон в Москве? Купить Мефедрон в Санкт Петербурге? Купить Мефедрон в Питере? Купить Мефедрон в Новосибирске?
Купить Мефедрон в Екатеринбурге? Купить Мефедрон в Казани? Купить Мефедрон в Нижнем Новгороде? Купить Мефедрон в Красноярске?
Купить Мефедрон в Челябинске? Купить Мефедрон в Самаре? Купить Мефедрон в Уфе? Купить Мефедрон в Ростове? Купить Мефедрон в Краснодаре?
Купить Мефедрон в Омске? Купить Мефедрон в Воронеже? Купить Мефедрон в Перьми? Купить Мефедрон в Волгограде?
Текстовые теги для поиска-
Купить Мефедрон в России можно очень легко, особенно Купить Мефедрон в Москве можно без проблем, потому что в Москве Мефедрон
Продаеться на каждом углу. Это могут быть и клубы, дискотеки так и с машины! Особенно приятно что Купить Мефедрон в Москве
можно закладками. Купить Закладками Мефедрон в Москве может любой человек – как мужчина так и девушка!
Однажды Купить Мефедрон в Москве пробовала даже бабушка, и у неё всё получилось. Часто продают закладки Мефедрона в Москве через Телеграм
Мой знакомый хотел попробовать найти Мефедрон в Москве при поможи поискового приспособления для поиска закладок, но у него не получилось
Потому что Мефедрон в Москве очень хорошо спрятан, и без точного адреса Мефедрон в Москве не возможно найти никак.
Колумбийский, Мараканский, Мексиканский – это далеко не полный список стран, которые экспортируют Мефедрон в Москву для
развлечения людей. Гарантией и безопасностью Мефедрон в Москве всегда впечетлял, ведь это основное правило покупки!
Российская Федерация всегда славилась своим разнообразием наркотических веществ, и всегда на первом месте был Мефедрон в Москве!
Маленькие шарики и круглые свертки – это закладки Мефедрона в Москве, которые бережно и с трепетом разложили от лишних глаз.
Каково было моё удивление, когда привезли с доставкой Мефедрон в Москве прямо в руки! Это было просто ВАУ-еффект!
Пока кто то сидит дома или ходит на работу – жизнь в Москве с Мефедроном проходит не заметно, быстро и легко!
Есть магазины, в которых Купить Мефедрон в Москве можно с гарантией и доставкой в руки круглосуточно!
Всё анонимно и безопастность просто зашкаливает, никогда Мефедрон в Москве не сможет обнаружить полиция.
Колючий кактус или национальность человека не имеет значения для покупки Мефедрона в Москве, потому что люди бывают разные
у всех разные потребности и желания. Но магазины готовы предоставить услугу по покупке Мефедрона в Москве не зависимо от настроения,
Ведь настроение после покупки у всех будет одинаковое – ТОЛЬКО ПОЛНЫЙ ПОЗИТИВ!
The mobile apps for online casinos crash way too often! aviator
доставка спиртного москва http://www.dostavka-alkogolya248.ru/ .
Online casinos should shield—watch us! gates of olympus
сделать реферат gpt http://www.studgen.ru/ .
Где купить диплом специалиста?
Мы предлагаем дипломы любой профессии по доступным тарифам. Мы можем предложить документы техникумов, которые находятся в любом регионе РФ. Вы можете приобрести качественно сделанный диплом за любой год, в том числе документы старого образца СССР. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. Они будут заверены необходимыми печатями и подписями. Стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступны для большинства граждан. diplommy.ru/gde-kupit-diplom-o-srednem-obrazovanie-2-3
Купить диплом института по выгодной стоимости возможно, обратившись к надежной специализированной компании. Заказать документ института можно в нашем сервисе. diplomservis.ru/diplom-s-vneseniem-v-reestr-dlya-uspeshnoj-kareri-4
The mobile casino experience is spotty—fix it! tom of madness
Заказать документ о получении высшего образования вы имеете возможность в нашей компании в столице. [url=http://diplomgorkiy.com/kupit-diplom-ekonomista/]diplomgorkiy.com/kupit-diplom-ekonomista[/url]
мосбет казино мосбет казино .
домашний интернет москва
domashij-internet-msk001.ru
провайдеры интернета в москве по адресу
topamax 100 mg Patient pill facts. Drug essentials explained. purchase topamax online no prescription
The rewards online are solid—nice perk! plinko
I love the online crew—chat up! plinko
Продолжение Манго Офис звонки через интернет
Что такое «Порту Пати» в Дубае и почему об этом говорят все
https://x.com/Fariz418740/status/1908767851009163415
1 win freereklama.borda.ru/?1-5-0-00000114-000-0-0-1743258539 .
Купить iPhone 16 Pro Max в Москве https://techno-line.store/ .
1win ваучер http://freereklama.borda.ru/?1-5-0-00000114-000-0-0-1743258539/ .
Online casinos can hook—edge it! plinko
биржа аккаунтов https://kupit-accaunt.ru
интернет по адресу
domashij-internet-msk002.ru
интернет тарифы москва
1vin pro http://www.freereklama.borda.ru/?1-5-0-00000114-000-0-0-1743258539 .
скупка золота в москве цена скупка золота в москве цена .
скупка золота сколько за грамм b-gold.ru скупка золота сколько за грамм b-gold.ru .
mostbet промокод http://mostbet6010.ru/ .
buy topamax online Drug guide provided. Get info now. buy topamax cheap
интернет провайдеры москва
domashij-internet-msk002.ru
подключить интернет тарифы москва
I won $400 on a slot—hype on! gransino
1 win md https://1win5004.ru .
Купить Кокаин в Киеве? Ссылка – COCAIN.VIP Доставка По Украине Кокаина? Ссылка – COCAIN.VIP
.
.
.
.
.
| Купить онлайн закладку Кокаин в Киеве ССЫЛКА – https://cocain.vip |
| Купить в другом городе Украины Кокаин закладку ССЫЛКА – https://cocain.vip |
| Купить доставку Кокаина в Киеве в руки через сайт ССЫЛКА – https://cocain.vip |
| Купить доставку в руки Кокаин в Украине ССЫЛКА – https://cocain.vip |
| Купить Кокаин в Украине доставкой через почту ССЫЛКА – https://cocain.vip |
| Купить Кокаин в Киеве и Украине через Курьера в руки ССЫЛКА -https://cocain.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Слова для поиска –
Кокаин в Киеве купить закладкой, Кокаин в Киеве Купить з доставкой, Кокаин в Киеве купить через курьера, Кокаин в Киеве Купить быстро, Кокаин в Киеве Купить сейчас, Кокаин в Киеве Купить в руки, Кокаин в Киеве Купить безопасно
Кокаин в Украине Купить можно, Купить Кокаин в Украине закладкой, Купить Кокаин в Украине доставкой домой, Купить Кокаин в Украине по почте, Купить Кокаин в Украине без проблем можно, Купить Кокаин в Украине на сайте, Кокс цена в Украине,
Виды Кокаина Купить в Украине и Киеве, Чем отличаеться Кокаин в Киеве от Кокаина в Украине, Как я могу купить Кокаин в Киеве, Купить Колумбийский Кокаин в Киеве, Купить Колумбийский Кокаин в Украине, Кокс Цена в Киеве,
Теги для городов –
Купить Кокаин в Киеве, Купить Кокаин в Одессе, Купить Кокаин в Харькове, Купить Кокаин во Львове, Купить Кокаин в Днепре, Купить Кокаин в Ужгороде, Купить Кокаин в Ивано-Франковске, Купить Кокаин в Белой Церкве, Купить Кокаин в Ровно
Купить Кокаин в Николаеве, Купить Кокаин в Херсоне, Купить Кокаин в Сумах, Купить Кокаин в Запорожье, Купить Кокаин в Житомере, Купить Кокаин в Мукачево, Купить Кокаин в Полтаве, Купить Кокаин в Умани, Купить Кокаин в Виннице
Общие теги для товаров-
Купить Кокаин в Киеве, Купить Кокаин в Украине, Купить Героин в Киеве, Купить Героин в Украине, Купить Лирику в Киеве, Купить Лирику в Украине, Купить Экстази в Киеве, Купить Экстази в Украине, Купить Амфетамин в Киеве, Купить Амфетамин
в Украине, Купить МДМА в Киеве, Купить МДМА в Украине, Купить Метамфетамин в Киеве, Купить Метамфетамин в Украине, Купить Метадон в Киеве, Купить Метадон в Украине, Купить Шишки в Киеве, Купить Шишки в Украине, Купить Бошки в Киеве,
Купить Бошки в Украине, Купить Альфа ПВП В Киеве, Купить Альфа ПВП в Украине, Купить A-PVP в Киеве, Купить A-PVP в Украине, Купить Соль СК в Киеве, Купить Соль СК в Украине, Купить Мефедрон в Киеве, Купить Мефедрон в Украине,
Трансфер аэропорт отель Бургас Служба Такси Эклипс предлагает доступные трансферы из аэропортов Бургас, Варна, София и Белград до популярных курортов Болгарии. Встреча с табличкой, помощь с багажом и комфортная поездка до места назначения. Обслуживаем Солнечный Берег, Созополь, Банско и другие направления. Для групп от 5 человек – специальные условия!
I don’t trust online casino RNGs—feels like they cheat! ganesha gold
кайт
безопасная сделка аккаунтов маркетплейс аккаунтов
Купить диплом института по выгодной цене возможно, обращаясь к проверенной специализированной фирме. Приобрести документ института вы имеете возможность в нашем сервисе. diplomk-vo-vladivostoke.ru/kupit-diplom-provedennij-cherez-reestr-5
Приобрести документ о получении высшего образования вы сможете у нас в столице. diplom4you.com/kupit-diplom-universiteta
Купить iPhone 16 Pro Max в Москве https://techno-line.store/ .
купить диплом среднее специальное москва diplomys-vsem.ru .
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: купить диплом нотариуса, купить диплом о высшем образовании московский государственный, купить диплом о высшем образовании пгс, купить диплом о высшем образовании сургут, купить диплом о высшем сталелитейном образовании. Постепенно углубляясь, нашел отличный ресурс здесь: proffdiplomik.com/prilozhenie-k-diplomu
Online gambling runs free—tight it! plinko
topamax generic Pill leaflet available. Complete medication overview. buy topamax uk
Hi there, I would like to subscribe for this weblog to take hottest updates, thus where can i do it please help out.
https://tsscscascsdddedewededwfmeee.com/
Online casinos should free—test run! aviator
Купить диплом о высшем образовании!
Мы готовы предложить документы ВУЗов, расположенных в любом регионе РФ.
diplomnie.com/kupit-diplom-zaregistrirovannij-v-reestre-2/
домашний интернет
domashij-internet-msk003.ru
домашний интернет
официальный сайт 1 вин http://1win6053.ru .
I hit a low online—rest up! dragon tiger
маркетплейс для реселлеров pokupka-akkauntov.ru/
1 вин официальный сайт вход snatkina.borda.ru/?1-5-0-00000311-000-0-0-1743258575 .
buy topiramate online Medicine information provided. Access pill information. topamax cheap
скачать 1win официальный сайт http://www.snatkina.borda.ru/?1-5-0-00000311-000-0-0-1743258575 .
Мы изготавливаем дипломы любых профессий по доступным ценам. Основные преимущества приобретения документов в нашей компании
Вы заказываете диплом в надежной и проверенной компании. Такое решение сэкономит не только деньги, но и время.
На этом преимущества не заканчиваются, их куда больше:
• Документы изготавливаем на настоящих бланках с мокрыми печатями и подписями;
• Предлагаем дипломы любого ВУЗа РФ;
• Цена значительно меньше той, которую пришлось бы заплатить за обучение в университете;
• Быстрая доставка как по столице, так и в другие регионы РФ.
Купить диплом института– http://gismeteo.ru/weather-grodno-4243/ – gismeteo.ru/weather-grodno-4243
Покупка документа о высшем образовании через надежную фирму дарит ряд преимуществ. Такое решение позволяет сберечь время и существенные финансовые средства. Впрочем, на этом выгода не ограничивается, преимуществ значительно больше.Мы готовы предложить дипломы психологов, юристов, экономистов и других профессий. Дипломы производят на подлинных бланках государственного образца. Доступная цена сравнительно с огромными издержками на обучение и проживание в чужом городе. Покупка диплома об образовании из российского ВУЗа будет целесообразным шагом.
Купить диплом о высшем образовании: diplomservis.com/vnesenie-diploma-v-reestr-tsena-4/
ваучер 1win snatkina.borda.ru/?1-5-0-00000311-000-0-0-1743258575 .
Astrologers named 6 signs of the eastern horoscope that will be lucky in April
https://x.com/Fariz418740/status/1908917832265392277
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://anotepad.com/notes/gga3bhe5
https://rentry.org/9cy3yawi
https://imageevent.com/vedema1951
https://www.hentai-foundry.com/user/cnthdf1967/profile
https://www.obesityhelp.com/members/amonamisu1991/about_me/
купить диплом настоящий тому
склад временного хранения москва арендовать склад временного хранения москва арендовать .
Мечтаете о роскошном авто, сочетающем в себе передовые технологии и безупречный дизайн? Дубай – это сокровищница автомобильных шедевров, где представлены модели, способные удовлетворить самый взыскательный вкус. Компания ChatMost предлагает полный спектр услуг по заказу и доставке автомобилей из Дубая в Россию, избавляя вас от хлопот и рисков. Доставка авто из Дубая в Россию
buy topamax online without prescription Patient medicine info. Patient drug guide. topamax generic
балансировка валов балансировка валов .
?nchiriez cas? corbeanca ?nchiriez cas? corbeanca .
тарифы интернет и телевидение нижний новгород
domashij-internet-nizhnij-novgorod001.ru
узнать интернет по адресу
1 вин официальный http://www.1win6005.ru .
недорогой хостинг https://uavps.net/vps
MetaMask Extension is a lifesaver. I use it to manage my Ethereum-based assets safely, and it integrates seamlessly with DeFi platforms.
мостбет войти http://www.mostbet6030.ru .
Где заказать диплом специалиста?
Наши специалисты предлагаютвыгодно и быстро купить диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями официальных лиц. Документ пройдет любые проверки, даже с использованием профессиональных приборов. Достигайте цели быстро с нашим сервисом. Приобрести диплом университета! globafeat.120.s1.nabble.com/template/NamlServlet.jtp?new_topic
Здравствуйте!
Для некоторых людей, купить диплом о высшем образовании – это необходимость, возможность получить достойную работу. Но для кого-то – это очевидное желание не терять массу времени на учебу в ВУЗе. С какой бы целью вам это не понадобилось, мы готовы помочь. Максимально быстро, профессионально и по доступной цене сделаем документ нового или старого образца на подлинных бланках со всеми требуемыми подписями и печатями.
Главная причина, почему многие люди покупают документ, – желание занять хорошую работу. К примеру, способности и опыт позволяют человеку устроиться на работу, однако документального подтверждения квалификации не имеется. Если для работодателя важно присутствие “корочки”, риск потерять хорошее место очень высокий.
Купить документ о получении высшего образования можно в нашей компании в Москве. Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов Российской Федерации. Вы сможете получить необходимый диплом по любым специальностям, любого года выпуска, включая документы СССР. Гарантируем, что при проверке документов работодателями, подозрений не возникнет.
Ситуаций, которые вынуждают купить диплом ВУЗа достаточно. Кому-то срочно потребовалась работа, а значит, нужно произвести впечатление на руководителя при собеседовании. Другие хотят попасть в серьезную компанию, чтобы повысить собственный статус в социуме и в будущем начать свой бизнес. Чтобы не тратить драгоценное время, а сразу начинать удачную карьеру, применяя имеющиеся знания, можно купить диплом через интернет. Вы станете полезным для общества, обретете денежную стабильность максимально быстро и легко- диплом о высшем образовании купить
Напыление бровей Уже 10 лет Делаем качественный перманентный макияж в Анапе без синих красных бровей. Только премиум материалы , стерильно и без боли, работаем в любых техниках.
Обучаем перманентному макияжу с нуля со свидетельством установленного образца , ведем курсы бровистов
topamax 200 mg Patient medicine guide. Comprehensive pill overview. topiramate
домашний интернет тарифы нижний новгород
domashij-internet-nizhnij-novgorod002.ru
какие провайдеры интернета есть по адресу нижний новгород
cost of vardenafil Get medicine info. Drug essentials explained. generic for levitra
Где заказать диплом по актуальной специальности?
Мы предлагаем дипломы любой профессии по приятным тарифам. Мы можем предложить документы ВУЗов, расположенных на территории всей Российской Федерации. Вы имеете возможность приобрести диплом от любого заведения, за любой год, в том числе документы старого образца СССР. Документы выпускаются на бумаге самого высокого качества. Это дает возможности делать государственные дипломы, которые не отличить от оригиналов. Они будут заверены всеми требуемыми печатями и штампами. Всегда стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы документы были доступными для большого количества граждан. institute-diplom.ru/kupit-diplom-ekonomista-7
Привет!
Без ВУЗа трудно было продвигаться по карьерной лестнице. Сегодня же документ не дает совершенно никаких гарантий, что получится найти привлекательную работу. Намного более важны профессиональные навыки специалиста, а также его постоянный опыт. Именно из-за этого решение о заказе диплома стоит считать мудрым и целесообразным. Заказать диплом ВУЗа mosserg.flybb.ru/viewtopic.php?f=2&t=527
Привет!
Мы изготавливаем дипломы любых профессий по приятным тарифам. Стоимость будет зависеть от выбранной специальности, года выпуска и ВУЗа: diploman.com/
заказать роллы барнаул sushibarnaul.ru
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Стараемся поддерживать для заказчиков адекватную политику цен. Важно, чтобы документы были доступны для большинства граждан.
Заказ документа, который подтверждает обучение в ВУЗе, – это выгодное решение. Приобрести диплом университета: zakaz-na-diplom.ru/kupit-diplom-kolledzha-v-moskve-3/
VANGPOKER- новое идеальное место для покера!
Ведущая в мире платформа блокчейн покера!
Вы можете стать одними из первых участников нового проекта с честным рейкбэком, бонусом на первый депозит 200%, быстрым вводом и выводом, и отсутствием KYC.
Приглашаем вас присоединится к крупнейшему сообществу VANGPOKER в телеграмм, зарегестрироваться в VANGPOKER и получать дополнительные бонусы и повышенный рейкбэк. зарегестрироваться в VANGPOKER
скупка золота в москве цена за грамм 585 b-gold.ru скупка золота в москве цена за грамм 585 b-gold.ru .
провайдеры домашнего интернета нижний новгород
domashij-internet-nizhnij-novgorod003.ru
подключить проводной интернет нижний новгород
ed meds list Administration guidelines here. Medicine impacts explained. best ed pills
скупка золота дорого в москве скупка золота дорого в москве .
1win moldova download http://1win5004.ru .
Где купить диплом специалиста?
Мы предлагаем документы об окончании любых ВУЗов России. Документы изготавливаются на оригинальных бланках. Рєskillnaukri.com/employer/premialnie-diplom-24
Где заказать диплом по нужной специальности?
Готовый диплом со всеми печатями и подписями полностью отвечает стандартам, никто не отличит его от оригинала. Не откладывайте личные мечты и задачи на потом, реализуйте их с нами – отправляйте быструю заявку на диплом прямо сейчас! Заказать диплом о среднем специальном образовании – легко! adly.pk/profile/freyamitchell
Купить диплом о высшем образовании!
Мы готовы предложить дипломы психологов, юристов, экономистов и других профессий по невысоким тарифам. Вы заказываете диплом в надежной и проверенной временем компании. : sealedraid.flybb.ru/viewtopic.php?f=9&t=694
[b]Диплом ВУЗа России![/b]
Без наличия диплома очень трудно было продвигаться вверх по карьере. Именно из-за этого решение о покупке диплома следует считать мудрым и рациональным. Купить диплом ВУЗа [url=http://daddycow.com/blogs/view/50737/]daddycow.com/blogs/view/50737[/url]
скупка золота лом цена b-gold.ru скупка золота лом цена b-gold.ru .
дешевый интернет новосибирск
domashij-internet-novosibirsk001.ru
узнать интернет по адресу
gluco extend is a nutritional supplement crafted to help maintain balanced blood sugar levels.
Купить диплом университета!
Мы предлагаем документы университетов, расположенных на территории всей РФ.
freediplom.com/kupite-originalnij-diplom-s-zaneseniem-v-reestr-bistro/
Заказать документ о получении высшего образования можно в нашей компании в столице. Мы предлагаем документы об окончании любых ВУЗов Российской Федерации. Вы получите диплом по любым специальностям, любого года выпуска, в том числе документы Советского Союза. Даем 100% гарантию, что при проверке документа работодателем, подозрений не появится. diplomass.com/diplom-s-zaneseniem-v-reestr-dostupen-po-vigodnoj-tsene-2/
ed medication Administration guidelines here. Detailed pill knowledge. ed meds for men
1win.pro http://1win5004.ru/ .
скупка золота на сегодня скупка золота на сегодня .
казино онлайн kg https://hiend.borda.ru/?1-16-0-00000260-000-0-0 .
mostbets http://hiend.borda.ru/?1-16-0-00000260-000-0-0/ .
мостбет скачать бесплатно мостбет скачать бесплатно .
Мы изготавливаем дипломы любых профессий по приятным ценам.– diplomv-v-ruki.ru/kupit-diplom-s-zaneseniem-v-reestr-otzivi/
Найден способ избежать преждевременной смерти
https://x.com/___Nikie__/status/1909141697990115541
1-win http://1win6053.ru/ .
сайт драгон мани сайт драгон мани .
ed capsules Find medication information. Patient drug leaflet. ed meds for men
Мы можем предложить дипломы любых профессий по невысоким тарифам. Стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы дипломы были доступны для большого количества наших граждан.
Заказ диплома, который подтверждает обучение в ВУЗе, – это выгодное решение. Приобрести диплом любого университета: kupit-diplomyz24.com/diplom-kupit-texnikum-5/
The graphics in Tiger Fortune are absolutely stunning. tigrinho demo
кайтинг в анапе
WhatsApp вводит новую функцию https://x.com/Ruslansavalv/status/1909195435446595720
провайдер по адресу новосибирск
domashij-internet-novosibirsk002.ru
подключить интернет новосибирск
1вин. 1вин. .
Для эффективного продвижения по карьерной лестнице нужно наличие официального диплома о высшем образовании. Приобрести диплом о высшем образовании у проверенной фирмы: diplomg-cheboksary.ru/gde-v-omske-kupit-diplom/
Вип-навес – производство и монтаж металлоконструкций.
Навес беседка для дачи изготовим на собственном заводе.
Наши инженеры выполняют производство навесов любой сложности по дотупным ценам!
садко дайвинг в хургаде
Горкинское кладбище https://gorkinskoe.ru одно из старейших в Видном. Подробная информация: адрес, как доехать, порядок захоронений, наличие участков, памятники, услуги по уходу.
I love the jungle energy of Tiger Fortune. fortune tiger demo gratis
Online bingo chills—cool gang! jackpotraider
ed medications for men Get medicine info. Pill essentials explained. sexual pills
замена подвесного подшипника карданного вала замена подвесного подшипника карданного вала .
Заказать диплом об образовании!
Мы можем предложить дипломы любых профессий по невысоким ценам— diploml-174.ru/kupit-diplom-s-reestrom-26/
Приобрести диплом университета по выгодной цене возможно, обращаясь к проверенной специализированной фирме. Мы предлагаем документы об окончании любых ВУЗов Российской Федерации. Приобрести диплом любого университета– freediplom.com/zanesite-diplom-v-reestr-obrazovaniya-za-odin-den-2/
скачать mostbet скачать mostbet .
Tiger Fortune’s features are super fun. fortune tiger demo gratis
Hi there, I log on to your blogs like every week.
Your story-telling style is awesome, keep doing what you’re doing!
Feel free to surf to my web blog :: connect to polygon bridge
I won spins online and got $100—nice! plinko
Pinco az-da qeydiyyatdan keçmək cəmi bir neçə dəqiqə çəkir|Pinco kazinosunun mobil versiyası çox rahat işləyir|Pinco kazinosu etibarlı və lisenziyalı platformadır|Pinco yeni başlayanlar üçün ideal platformadır|Pinco mobil tətbiqi yükləmək çox rahatdır|Pinco az istifadəçilər üçün əlavə cashback kampaniyaları keçirir|Pinco slot oyunları yüksək RTP göstəricisinə malikdir|Pinco onlayn kazinosunda müxtəlif oyun növləri var|Pinco az onlayn oyunçular üçün ən yaxşı təkliflərdən biridir pinco casino azerbaijan.
case ?n complex privat case ?n complex privat .
ваучер 1win ваучер 1win .
1win bet http://1win13.com.ng .
мостбет кыргызстан мостбет кыргызстан .
I love how unpredictable Tiger Fortune is! tigrinho demo
1win футбол http://1win6005.ru/ .
какие провайдеры на адресе в новосибирске
domashij-internet-novosibirsk003.ru
домашний интернет новосибирск
мос бет мос бет .
Подробнее здесь купить турбину
Hire a hitman how to hire a hitman
most bet https://mostbet6029.ru/ .
Tiger Fortune’s rewards are always a thrill. fortune tiger bet
Всё о Казахстане https://tr-kazakhstan.kz история, культура, города, традиции, природа и достопримечательности. Полезная информация для туристов, жителей и тех, кто хочет узнать страну ближе.
дайвинг в египте хургада
Falschgeld kaufen Verkauf Euro gefalschte Banknoten kaufen
мосбет казино http://mostbet6029.ru .
мостбет официальный сайт https://severussnape.borda.ru/?1-4-0-00000505-000-0-0-1743260265 .
This online casino’s Tiger Fortune is a masterpiece. fortune tiger demo
официальный сайт 1win https://1win6053.ru/ .
Приобрести диплом университета по невысокой стоимости можно, обращаясь к надежной специализированной компании. Приобрести документ о получении высшего образования можно у нас в Москве. good-diplom.ru/pokupka-diplomov-s-reestrom-8
замена подвесного подшипника кардана http://www.passat-club.ru/news.php?id=1593 .
I love the promos online—keeps it fun! vai de bet
This casino’s Tiger Fortune game is a hit. fortune tiger 777
cas? de ?nchiriat pipera cas? de ?nchiriat pipera .
Где приобрести диплом специалиста?
Наша компания предлагаетвыгодно купить диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями должностных лиц. Диплом пройдет лубую проверку, даже при помощи специфических приборов. Решайте свои задачи быстро и просто с нашими дипломами. Купить диплом любого ВУЗа! emprende.network/read-blog/1145_kupit-diplom-tehnikuma.html
мостбет промокод мостбет промокод .
Где заказать диплом специалиста?
Наша компания предлагает выгодно заказать диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Диплом пройдет лубую проверку, даже с применением специально предназначенного оборудования. Достигайте цели быстро с нашим сервисом.
Приобрести диплом любого университета diplomservis.ru/kupit-attestat-za-11-klass-15/
lhfujy lhfujy .
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Tiger Fortune’s jungle theme is so well done. tigrinho demo
1win rossvya http://www.1win6006.ru .
dragon money регистрация dragon money регистрация .
мостбет скачать андроид https://www.mostbet6030.ru .
https://vc.ru/
Kvalitni nabytek v Praze http://www.ruma.cz/ stylove reseni pro domacnost i kancelar. Satni skrine, sedaci soupravy, kuchyne, postele od proverenych vyrobcu. Rozvoz po meste a montaz na klic.
сайт купить подписчиков в телеграм канал
The sound effects in Tiger Fortune are spot on. fortune tiger
кайт споты египта Египет – это не только пирамиды и фараоны, но и рай для кайтсерферов. От Хургады до Дахаба, вас ждут бескрайние пляжи и стабильный ветер.
I hit a blackjack wave—good run! plinko
провайдер по адресу
domashij-internet-spb001.ru
узнать интернет по адресу
mitolyn is a natural dietary supplement specifically outlined to enhance metabolism and support weight loss. Its potent blend of ingredients works to increase energy levels, promote fat burning
Доброго!
Оформление брачного контракта — это важный процесс для защиты интересов обеих сторон, и наш юрист поможет вам составить этот документ. Споры с налоговой могут повлиять на ваш бизнес, но мы поможем вам разрешить любые вопросы с налоговыми органами. Защита в суде — это гарантированная помощь при судебных разбирательствах, и наш юрист будет представлять ваши интересы в суде. Правовая помощь студентам поможет вам решить вопросы, связанные с учебой, трудовой деятельностью и прочими правовыми аспектами. Регистрация брака с иностранцем требует соблюдения всех правовых норм, и мы готовы вам помочь.
Больше информации на сайте – https://avtopravodo.ru
оформление доверенности, претензия образец по защите прав потребителей, что не входит в цену иска по защите прав потребителей
роль трудового договора в регулировании трудовых отношений, шота горгадзе адвокат цена на услуги, юридическая помощь при ДТП
Удачи!
этот контент r7 казино
aqua sculpt is a groundbreaking weight management supplement designed to support your journey by naturally enhancing metabolism
The casino’s Tiger Fortune slot is super engaging. tiger fortune
Привет всем!
Регистрация брака требует соблюдения всех процедур и подачи документов в ЗАГС. Правовая помощь пенсионерам включает консультации по вопросам пенсий, социальных льгот и прав пенсионеров. Оформление ДТП требует правильного составления всех документов и обеспечения соблюдения прав сторон. Проверка авто перед покупкой обеспечит вас уверенностью в юридической чистоте сделки и отсутствии скрытых проблем с автомобилем. Автоюрист по ОСАГО поможет вам разобраться в вопросах страховки и компенсации ущерба.
Больше информации на сайте – https://avtopravodo.ru
алименты на ребенка, регистрация права собственности на недвижимое имущество гк рф, расчет остаточной стоимости имущества для налога на имущество
ип налогообложение виды, оплата госпошлины регистрация права собственности, консультация юриста по миграции
Удачи!
Офіційний сайт 1win https://visitkyiv.com.ua спортивні ставки, онлайн-казино, покер, live-ігри, швидкі висновки. Бонуси новим гравцям, мобільний додаток, цілодобова підтримка.
Заказ документа о высшем образовании через надежную фирму дарит много достоинств. Такое решение дает возможность сэкономить как личное время, так и серьезные финансовые средства. Тем не менее, только на этом выгоды не ограничиваются, плюсов значительно больше.Мы изготавливаем дипломы любой профессии. Дипломы изготавливаются на настоящих бланках государственного образца. Доступная стоимость в сравнении с серьезными расходами на обучение и проживание. Заказ диплома о высшем образовании из российского ВУЗа является мудрым шагом.
Приобрести диплом о высшем образовании: asxdiploman.com/kupit-diplom-v-moskve-s-zaneseniem-v-reestr-6/
купил диплом дгту
можно проверить ЗДЕСЬ р 7 казино
erectile dysfunction pills Patient medication facts. Comprehensive drug guide. ed meds
The payout waits online are lame—speed up! betonred
подключить интернет в санкт-петербурге в квартире
domashij-internet-spb002.ru
провайдеры интернета в санкт-петербурге по адресу
Доброго!
Юридическая помощь при оформлении наследства — это важный процесс, который требует соблюдения всех законодательных норм и правильного оформления документов. Мы поможем вам получить свидетельство о праве на наследство, оформить дарственную или завещание, а также разобраться в вопросах распределения имущества. Наши юристы гарантируют, что все документы будут подготовлены в соответствии с законом и не приведут к юридическим спорам. Обратитесь к нам за консультацией, чтобы оформление наследства прошло быстро и без проблем.
Больше информации на сайте – https://legallup.ru
проверка авто перед покупкой, марафон 800 вопросов по правилам пдд, новосибирск юрист по трудовым спорам
статус проверки декларации на налоговый вычет, после отказа от медосвидетельствования как вернуть права, автоюрист по штрафам
Удачи!
Заказать диплом об образовании!
Мы изготавливаем дипломы любых профессий по выгодным ценам— diplomt-v-chelyabinske.ru/kupit-diplom-9-klassov-bistro-i-udobno/
Tiger Fortune’s features keep me entertained for hours. fortune tiger
Купить Кокаин Москва? САЙТ -| COCAINES.STORE | Как Купить Кокаин по России? САЙТ – | COCAINES.STORE |
.
.
.
.
.
Купить Кокаин максимального качества в Москве? САЙТ – https://cocaines.store
Сколько стоит Кокаин в Москве сегодня? САЙТ – https://cocaines.store
Купить Кокаин в городах России? САЙТ – https://cocaines.store
Купить Кокаин с доставкой в руки В Москве? САЙТ – https://cocaines.store
Где в Москве Купить Кокаин с доставкой? САЙТ – https://cocaines.store
Купить с доставкой по России кокаин? САЙТ – https://cocaines.store
Купить Наркотики в Москве? САЙТ – https://cocaines.store
Как Купить наркотики в России? САЙТ – https://cocaines.store
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Гугл Робот (GOOGLE ROBOT)
Купить Кокаин в Москве? Купить Кокаин в Санкт Петербурге? Купить Кокаин в Питере? Купить Кокаин в Новосибирске?
Купить Кокаин в Екатеринбурге? Купить Кокаин в Казани? Купить Кокаин в Нижнем Новгороде? Купить Кокаин в Красноярске?
Купить Кокаин в Челябинске? Купить Кокаин в Самаре? Купить Кокаин в Уфе? Купить Кокаин в Ростове? Купить Кокаин в Краснодаре?
Купить Кокаин в Омске? Купить Кокаин в Воронеже? Купить Кокаин в Перьми? Купить Кокаин в Волгограде?
Текстовые теги для поиска-
Купить Кокаин в России можно очень легко, особенно Купить Кокаин в Москве можно без проблем, потому что в Москве Кокаин
Продаеться на каждом углу. Это могут быть и клубы, дискотеки так и с машины! Особенно приятно что Купить Кокаин в Москве
можно закладками. Купить Закладками Кокаин в Москве может любой человек – как мужчина так и девушка!
Однажды Купить Кокаин в Москве пробовала даже бабушка, и у неё всё получилось. Часто продают закладки Кокаина в Москве через Телеграм
Мой знакомый хотел попробовать найти Кокаин в Москве при поможи поискового приспособления для поиска закладок, но у него не получилось
Потому что Кокаин в Москве очень хорошо спрятан, и без точного адреса Кокаин в Москве не возможно найти никак.
Колумбийский, Мараканский, Мексиканский – это далеко не полный список стран, которые экспортируют Кокаин в Москву для
развлечения людей. Гарантией и безопасностью Кокаин в Москве всегда впечетлял, ведь это основное правило покупки!
Российская Федерация всегда славилась своим разнообразием наркотических веществ, и всегда на первом месте был Кокаин в Москве!
Маленькие шарики и круглые свертки – это закладки Кокаина в Москве, которые бережно и с трепетом разложили от лишних глаз.
Каково было моё удивление, когда привезли с доставкой Кокаин в Москве прямо в руки! Это было просто ВАУ-еффект!
Пока кто то сидит дома или ходит на работу – жизнь в Москве с Кокаином проходит не заметно, быстро и легко!
Есть магазины, в которых Купить Кокаин в Москве можно с гарантией и доставкой в руки круглосуточно!
Всё анонимно и безопастность просто зашкаливает, никогда Кокаин в Москве не сможет обнаружить полиция.
Колючий кактус или национальность человека не имеет значения для покупки Кокаина в Москве, потому что люди бывают разные
у всех разные потребности и желания. Но магазины готовы предоставить услугу по покупке Кокаина в Москве не зависимо от настроения,
Ведь настроение после покупки у всех будет одинаковое – ТОЛЬКО ПОЛНЫЙ ПОЗИТИВ!
Доброго!
Правовая консультация онлайн позволит вам получить нужную помощь в удобное время. Сопровождение сделок с авто включает в себя полную юридическую проверку. Юридическая помощь пенсионерам часто требуется для решения вопросов с социальной защитой. Раздел имущества в браке — это важный процесс, в котором поможет опытный юрист. Защита прав заемщика — задача, которая требует правильного юридического подхода.
Больше информации на сайте – https://capitallawdo.ru/
помощь в написании жалобы, юридическая компания арбитражные споры, лишение родительских прав порядок
когда не нужно платить налог за продажу квартиры, смотреть фильмы онлайн в хорошем качестве бесплатно без регистрации брак по завещанию все серии, защита прав в арбитраже
Удачи!
Привет всем!
Юридическая помощь мигрантам обеспечивает поддержку при оформлении виз, гражданства, а также решении других вопросов, связанных с миграционными процессами. Подача заявления в прокуратуру поможет вам защитить свои права и интересы, если ваши законные права были нарушены. Проверка документов юристом необходима, чтобы убедиться в правильности и законности всех документов, особенно при сделках с недвижимостью и крупными суммами. Консультация по правам потребителей поможет вам разобраться в вопросах, связанных с нарушением ваших прав как потребителя товаров или услуг. Возврат переплаты по кредиту требует юридического вмешательства, чтобы вернуть лишние деньги, уплаченные по кредитным обязательствам.
Больше информации на сайте – https://capitallawdo.ru
правовая поддержка, возврат товара в течении 14 дней закон о защите прав, для возврата налога с покупки квартиры какие документы необходимы
телефон бесплатная консультация юриста, штрафы для юр лиц за нарушение пдд, помощь в оформлении развода
Удачи!
Tiger Fortune’s payouts are worth the hype. fortune tiger 777
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по приятным ценам. Стоимость будет зависеть от выбранной специальности, года выпуска и ВУЗа. Всегда стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы документы были доступными для большинства граждан. диплом мед колледжа купить
Выгодно купить диплом любого института!
Заказать диплом университета по доступной цене вы можете, обратившись к надежной специализированной компании. Заказать диплом: diplom-zentr.com/ofitsialnij-diplom-s-reestrom-dlya-pokupki-v-rossii
Мы изготавливаем дипломы любой профессии по приятным ценам. Дипломы производятся на настоящих бланках Приобрести диплом ВУЗа rusd-diplomj.ru
Приобрести документ о получении высшего образования можно в нашей компании. rusd-diplomj.ru/kupit-diplom-lipetsk-4
Приобрести диплом любого университета!
Мы можем предложить документы учебных заведений, которые находятся на территории всей Российской Федерации. Документы делаются на “правильной” бумаге высшего качества: soniamittal0011.copiny.com/question/details/id/1079717
Где заказать диплом специалиста?
Заказать диплом института по невысокой цене можно, обращаясь к надежной специализированной компании.: magazin-diplomov.ru
Доска бесплатных объявлений https://salexy.kz/ Казахстана: авто, недвижимость, техника, услуги, работа и многое другое. Тысячи свежих объявлений каждый день — легко найти и разместить!
домашний интернет санкт-петербург
domashij-internet-spb003.ru
домашний интернет в санкт-петербурге
This online casino shines with Tiger Fortune. tiger fortune
This casino’s Tiger Fortune game keeps me coming back for more. fortune tiger demo gratis
купить диплом советский
Tiger Fortune keeps the good times rolling! fortune tiger 777
Где заказать диплом по нужной специальности?
Мы изготавливаем дипломы любой профессии по доступным тарифам. Мы можем предложить документы техникумов, расположенных в любом регионе РФ. Можно приобрести качественный диплом за любой год, включая документы старого образца СССР. Дипломы и аттестаты выпускаются на бумаге высшего качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. Документы заверяются необходимыми печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Для нас важно, чтобы документы были доступны для большинства граждан. diplom-kuplu.ru/kupit-diplom-v-tambove-6
I won spins online and got $250—big yes! aviator
This online casino’s Tiger Fortune is a masterpiece. fortune tiger
Online casinos should care—shield us! tom of madness
мостюет http://remsanteh.borda.ru/?1-6-0-00000047-000-0-0 .
The payouts in Tiger Fortune are pretty impressive. fortune tiger demo
Tiger Fortune’s payouts are always exciting. tiger fortune
скачать mostbet https://remsanteh.borda.ru/?1-6-0-00000047-000-0-0 .
драгон казино официальный сайт драгон казино официальный сайт .
Tiger Fortune’s wild symbols are the best. fortune tiger demo gratis
The excitement of online slots is unreal—heart-pumping! plinko
best ed meds Get medicine details. Find medication info. best ed meds
Добрый день!
Юридическая помощь при приватизации — это необходимый процесс для оформления прав на имущество. Мы поможем вам разобраться в правовых аспектах приватизации и подготовить все необходимые документы. Консультация по приватизации обеспечит соблюдение всех норм законодательства и защиту ваших интересов. Наши специалисты помогут вам пройти все этапы приватизации и зарегистрировать права на имущество. Обратитесь за помощью, чтобы приватизация прошла быстро и без проблем.
Больше информации на сайте – https://legallup.ru/
помощь в получении субсидий, государственная регистрация права собственности на квартиру, как через госуслуги подать заявление приставам о задолженности по алиментам
документы для подачи алиментов на ребенка, бесплатная помощь юридическая инвалидам, проверка авто перед покупкой
Удачи!
мостбет официальный сайт remsanteh.borda.ru/?1-6-0-00000047-000-0-0 .
1вин вход с компьютера 1win6054.ru .
1win nigeria http://www.1win13.com.ng .
I love the fast pace of Tiger Fortune. fortune tiger 777
провайдеры интернета в екатеринбурге по адресу
domashij-internet-ekaterinburg001.ru
интернет провайдеры по адресу екатеринбург
1win. casino. 1win. casino. .
Привет всем!
Оформление брачного контракта — это важный процесс для защиты интересов обеих сторон, и наш юрист поможет вам составить этот документ. Споры с налоговой могут повлиять на ваш бизнес, но мы поможем вам разрешить любые вопросы с налоговыми органами. Защита в суде — это гарантированная помощь при судебных разбирательствах, и наш юрист будет представлять ваши интересы в суде. Правовая помощь студентам поможет вам решить вопросы, связанные с учебой, трудовой деятельностью и прочими правовыми аспектами. Регистрация брака с иностранцем требует соблюдения всех правовых норм, и мы готовы вам помочь.
Больше информации на сайте – https://capitalaw.ru
автоюрист по лишению прав, незаконное увольнение статья ук рф с работы, брачный договор для состоящих в браке заполненный
что входит на налог на имущество физических лиц, проверить наличие наследственного дела на сайте фнп, адвокат по уголовным делам
Удачи!
chinese-hitman-assassin.com https://chinese-hitman-assassin.com/
d-arkweb.com https://d-arkweb.com/
Советую эту компанию для остекления балконов, подробнее тут пластиковые окна на балкон
Tiger Fortune keeps me entertained every time. fortune tiger demo
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: купить диплом маси, купить диплом с занесением фрдо, купить диплом спту, купить диплом средняя окончание, купить диплом строительное образование. Постепенно углубляясь, нашел отличный ресурс здесь: diplomybox.com/kupit-diplom-magistra-v-krasnoyarske
https://kiteschoolhurghada.ru
Добрый день!
Споры с налоговой требуют компетентного подхода для защиты ваших интересов и минимизации штрафов и санкций. Защита в суде поможет вам защитить свои интересы и добиться справедливости в судебном разбирательстве. Правовая помощь студентам включает разъяснение правовых норм, касающихся учебного процесса, трудовых и социальных прав студентов. Регистрация брака с иностранцем требует соблюдения всех юридических формальностей, и юрист поможет вам правильно оформить все документы. Правовое сопровождение дел по гражданским спорам обеспечит вам поддержку на всех этапах судебного разбирательства.
Больше информации на сайте – https://businessfaq.ru/
трудовой договор с ИП, экспертиза договоров это правовая, соглашение об оказании юридической помощи содержится в
предшествующие сопутствующие и последующие трудовые отношения, 1 понятие и виды источников корпоративного права, юридические услуги москва
Удачи!
мосбет казино mostbet6031.ru .
Всё о кладбищах Видного bitcevskoe Битцевское, Дрожжинское, Спасское, Жабкинское. Официальная информация, участки, услуги, порядок оформления документов, схема проезда.
подключить интернет тарифы екатеринбург
domashij-internet-ekaterinburg002.ru
узнать провайдера по адресу екатеринбург
Купить диплом ВУЗа !
Приобретение диплома ВУЗа России у нас является надежным процессом, потому что документ будет заноситься в государственный реестр. Заказать диплом о высшем образовании vacshidiplom.com/kupit-diplom-s-zaneseniem-v-reestr-bistro-i-nadezhno-72
An impressive share! I’ve just forwarded this onto
a colleague who has been conducting a little research on this.
And he in fact ordered me lunch because I discovered it for him…
lol. So allow me to reword this…. Thank YOU for the meal!!
But yeah, thanx for spending some time to talk about this matter here on your web site.
Feel free to surf to my web page – anyswap vs multichain
Доброго!
Помощь в оформлении договора аренды поможет вам оформить договор аренды недвижимости с учетом всех юридических норм. Регистрация договора аренды защищает интересы арендатора и арендодателя, гарантируя правильность оформления. Юрист по защите прав заемщика предоставит вам квалифицированную помощь при любых проблемах с возвратом долга или спорными кредитами. Налоговая консультация поможет вам разобраться в изменениях в законодательстве и налогообложении. Помощь при судебных разбирательствах поможет вам защитить свои интересы и права на всех этапах судебного процесса.
Больше информации на сайте – https://agkonsult.ru
бракоразводный процесс, проверить наследственное дело онлайн по фамилии, брачный договор может касаться личных прав
калькулятор налог при продаже квартиры, сколько может быть адвокатов у обвиняемого по уголовному делу, регистрация недвижимости
Удачи!
Добрый день!
Раздел имущества судом — это сложный процесс, который требует грамотного подхода и профессиональной помощи. Мы поможем вам подготовить все необходимые документы и защитить ваши интересы при разделе имущества через суд. Юрист по разделу имущества обеспечит правильное оформление всех юридических процедур и минимизирует риски. Мы поможем вам разрешить спор с минимальными потерями и получить справедливое решение. Обратитесь к нам за юридической помощью, чтобы раздел имущества прошел без осложнений.
Больше информации на сайте – https://capitalaw.ru/
юрист по уголовным делам, срок подачи декларации на возврат подоходного налога, как подать на алименты и отцовство
какие нужны документы для получения водительских прав после лишения за алкоголь, билет пдд 21 вопрос 13, помощь при продаже недвижимости
Удачи!
провайдеры екатеринбург
domashij-internet-ekaterinburg002.ru
проверить провайдера по адресу
1win вход в личный кабинет 1win вход в личный кабинет .
1 вин. http://1win6042.ru/ .
Tiger Fortune is the highlight of this online casino for me. fortune tiger bet
Здравствуйте!
Легализация документов поможет вам пройти процесс официального признания документов в других странах. Помощь в приватизации недвижимости требует знания всех юридических аспектов этого процесса, и юрист поможет вам с оформлением. Составление брачного договора обеспечит защиту ваших интересов при вступлении в брак, особенно в вопросах раздела имущества. Юридическая помощь при лишении прав поможет вам восстановить водительские права, если они были лишены незаконно. Оформление опеки требует соблюдения всех юридических норм, чтобы обеспечить интересы ребенка и сторон, оформляющих опеку.
Больше информации на сайте – https://consultantdo.ru/
семейные споры, как оформить алименты на ребенка без развода, в каком случае надо платить налог с продажи квартиры
регистрация ип госпошлина, уголовный адвокат в краснодаре, регистрация недвижимости
Удачи!
ремонт квартир под ключ в Ногинске Москва – динамичный мегаполис, где каждый стремится к созданию уютного и функционального пространства. Ремонт квартир в Москве – это не просто обновление интерьера, это инвестиция в комфорт и качество жизни. Мы предлагаем полный спектр услуг по ремонту квартир в Москве, от косметического обновления до капитальной перепланировки под ключ.
5 научных фактов о лжи, в которые трудно поверить
https://x.com/kiselev_igr/status/1909590459799716145
Заказать диплом университета!
Мы готовы предложить документы институтов, расположенных в любом регионе России.
diplomus-spb.ru/pokupka-diploma-s-reestrom-bez-lishnix-problem/
1win bet https://1win13.com.ng/ .
dragonmoney казино dragonmoney казино .
dragon money партнерка https://dragon-money27.com/ .
1win. 1win6006.ru .
cod promoțional 1win cod promoțional 1win .
Calibry Casino calibri casino app ru современное онлайн-казино с лицензией, щедрой бонусной системой и широким выбором игр. Участвуй в акциях, получай кэшбэк и выигрывай реальные деньги!
мастбет http://www.mostbet6032.ru .
This casino’s Tiger Fortune game is a blast. fortune tiger demo gratis
Здравствуйте!
Если вы столкнулись с проблемами при оформлении наследства, наши юристы помогут вам на каждом этапе этого сложного процесса. Мы оказываем помощь в составлении завещаний, а также в решении вопросов, связанных с разделом имущества и правами наследников. Оформление наследства у нотариуса требует точности и соблюдения всех юридических норм, и наши специалисты позаботятся о всех деталях. Мы обеспечим защиту ваших прав в случае споров между наследниками. Получите квалифицированную помощь, чтобы оформить наследство без лишних трудностей.
Больше информации на сайте – https://juristywin.ru
страхование авто, как рассчитывается налог на имущество физических лиц, движимое имущество налог на имущество
заявление о переходе на усн при регистрации ип, какие документы налоговый вычет на обучение, лишение водительских прав
Удачи!
dragonmoney казино dragonmoney казино .
This casino’s Tiger Fortune slot is a treasure. fortune tiger demo
1win на телефон cah.forum24.ru/?1-13-0-00001695-000-0-0-1743258917 .
купить диплом о высшем образовании в ставрополе diplomys-vsem.ru .
Tiger Fortune’s tiger theme is so unique. fortune tiger 777
Добрый день!
Онлайн консультация юриста предоставляет возможность быстро получить ответы на все ваши вопросы без необходимости посещать офис. Брачный договор — это важный документ, который может помочь избежать конфликтов в будущем и урегулировать вопросы раздела имущества. Как оформить лизинг — это вопрос, на который юрист поможет вам дать точный ответ и объяснит все юридические нюансы. Вопросы по ПДД могут быть решены с помощью автоюриста, который поможет вам разобраться в правилах дорожного движения и штрафах. Юридическая помощь при ДТП предоставляет вам возможность защитить свои права после аварии и получить компенсацию ущерба.
Больше информации на сайте – https://consultantdo.ru
апелляция по гражданскому делу, москва сао нотариус, при лишении родительских прав платятся ли алименты
какие пункты могут быть включены в брачный договор, как получить водительские права после лишения, бесплатная юридическая консультация
Удачи!
официальный сайт 1 вин https://cah.forum24.ru/?1-13-0-00001695-000-0-0-1743258917 .
драгон мани казино зеркало драгон мани казино зеркало .
интернет тарифы екатеринбург
domashij-internet-ekaterinburg003.ru
интернет провайдеры по адресу дома
gnc ed pills Patient pill resource. Get medication details. erectile dysfunction medications
склад вещей в москве склад вещей в москве .
This casino’s Tiger Fortune game is flawless. fortune tiger bet
1 win. https://www.cah.forum24.ru/?1-13-0-00001695-000-0-0-1743258917 .
Доброго!
Юридическая помощь по вопросам ЖКХ — это услуга для тех, кто сталкивается с проблемами, связанными с жилищно-коммунальным обслуживанием. Мы поможем вам решить вопросы, связанные с несанкционированными начислениями, коммунальными платежами или нарушениями со стороны управляющих компаний. Юрист по ЖКХ поможет вам составить жалобу, подать исковое заявление и защитить ваши права в суде. Мы предоставим полное юридическое сопровождение по всем вопросам, связанным с ЖКХ. Обратитесь за помощью, чтобы решить проблемы с жилищно-коммунальными услугами.
Больше информации на сайте – https://legalyes.ru/
развод без согласия второго супруга, по временной регистрации ип, консультация адвоката по гражданским делам бесплатно
как происходит возврат денег на вайлдберриз при отказе от товара после оплаты, адвокаты в шахтах по уголовным делам, автоюрист по возврату прав
Удачи!
Здравствуйте!
Легализация документов поможет вам пройти процесс официального признания документов в других странах. Помощь в приватизации недвижимости требует знания всех юридических аспектов этого процесса, и юрист поможет вам с оформлением. Составление брачного договора обеспечит защиту ваших интересов при вступлении в брак, особенно в вопросах раздела имущества. Юридическая помощь при лишении прав поможет вам восстановить водительские права, если они были лишены незаконно. Оформление опеки требует соблюдения всех юридических норм, чтобы обеспечить интересы ребенка и сторон, оформляющих опеку.
Больше информации на сайте – https://legalyes.ru/
автоюрист москва, заявление на льготу по налогу на имущество физических лиц налоговую, как получить гражданство таджикистана гражданину рф в 2020 году
алименты на нетрудоспособного ребенка совершеннолетнего, какие документы нужны для оформления дома в деревне в собственность, возврат налогов
Удачи!
win 1 https://www.1win6006.ru .
You need to take part in a contest for one of the highest quality blogs on the net.
I’m going to recommend this website!
Tiger Fortune is my top pick at this casino. tigrinho demo
драгон мани кэшбэк драгон мани кэшбэк .
Чат с психологом в телеге. Чат с психологом в телеге. Психолог онлайн анонимно.
I can play Tiger Fortune all day and not get bored. fortune tiger bet
интернет провайдер казань
domashij-internet-kazan001.ru
домашний интернет тарифы
Помощь психолога онлайн. Помощь психолога онлайн. Анонимный чат с психологом телеграм. оценили 1288 раз
паническая атака симптомы Современный мир диктует свои условия, и порой нам необходима квалифицированная помощь, чтобы справиться с вызовами. Психолог онлайн – это удобный и анонимный способ получить поддержку в комфортной обстановке. Работа с психологом: путь к гармонии Обращение к психологу – это признак силы и заботы о себе. Специалист поможет разобраться в сложных ситуациях, найти ресурсы для преодоления трудностей и обрести внутреннюю гармонию.
Чат психологической поддержки. Психолог t me. Психолог в телеграм. оценили 4088 раз
Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате. оценили 6484 раз
Получить онлайн консультацию психолога чате. В переписке у психолога. Онлайн-консультация психолога. оценили 7717 раз
Мы предлагаем дипломы любых профессий по приятным тарифам. Мы предлагаем документы ВУЗов, расположенных на территории всей Российской Федерации. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. youtoosocialnetwork.com/read-blog/9008_kupit-diplom-s-reestrom.html
Tiger Fortune’s payouts are worth the hype. tigrinho demo
В переписке у психолога. Психолог в телеграм. Чат с психологом в телеге. оценили 3002 раз
Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге. Дипломированный психолог с опытом работы и отзывами клиентов. оценили 6647 раз
Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. Психологическая и информационная онлайн-помощь. оценили 9260 раз
dragonmoney бесплатные бонусы dragonmoney бесплатные бонусы .
Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно. оценили 6563 раз
Психолог помогающий искать решения в непростых психологических ситуациях. Получить онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 6984 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог онлайн анонимно. Психолог онлайн анонимно. оценили 9798 раз
Психолог онлайн анонимно. Психолог онлайн чат. Телеграм психолог. оценили 9390 раз
Чат с психологом в телеге. Психолог онлайн анонимно. Психолог оказывает помощь онлайн в чате. оценили 9227 раз
Получить первую онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. оценили 8375 раз
Психолог онлайн анонимно. Онлайн чат с психологом без регистрации. Получить первую онлайн консультацию психолога чате. оценили 7098 раз
Психолог онлайн анонимно. Чат с психологом в телеге. Круглосуточная запись на онлайн-консультацию психолога. оценили 3675 раз
Психолог онлайн анонимно. Онлайн чат с психологом без регистрации. Психологическая и информационная онлайн-помощь. оценили 4315 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Получить первую онлайн консультацию психолога чате. оценили 7614 раз
мостбет кыргызстан http://www.mostbet6033.ru .
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог онлайн чат. Психолог оказывает помощь онлайн в чате. оценили 7757 раз
1 win официальный сайт http://1win6043.ru/ .
Дипломированный психолог с опытом работы и отзывами клиентов. Телеграм психолог. Психологическая и информационная онлайн-помощь. оценили 7420 раз
Дипломированный психолог с опытом работы и отзывами клиентов. В переписке у психолога. Психологическая и информационная онлайн-помощь. оценили 9751 раз
Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн-консультация психолога. оценили 5200 раз
Психолог оказывает помощь онлайн в чате. Онлайн-консультация психолога. Психолог онлайн анонимно. оценили 1175 раз
Помощь психолога онлайн. Дипломированный психолог с опытом работы и отзывами клиентов. Анонимный чат с психологом телеграм. оценили 7706 раз
Помощь психолога онлайн. Онлайн чат с психологом без регистрации. В переписке у психолога. оценили 2595 раз
Tiger Fortune brings the excitement of the wild to my screen. fortune tiger bet
Психолог в телеграм. Телеграм психолог. Получить онлайн консультацию психолога чате. оценили 361 раз
Психологическая и информационная онлайн-помощь. Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки. оценили 6601 раз
Онлайн чат с психологом без регистрации. Круглосуточная запись на онлайн-консультацию психолога. Получить онлайн консультацию психолога чате. оценили 5825 раз
Психолог оказывает помощь онлайн в чате. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 6501 раз
Получить онлайн консультацию психолога чате. Психолог онлайн анонимно. Онлайн-консультация психолога. оценили 2056 раз
Телеграм психолог. Онлайн-консультация психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 5685 раз
Здравствуйте!
Помощь в получении визы обеспечит вам поддержку в процессе оформления документов для поездок за границу. Правовая помощь при задержании — это услуга, которая защитит ваши права, если вы оказались в ситуации задержания. Помощь в восстановлении документов поможет вам вернуть утерянные или украденные документы, такие как паспорт или водительские права. Оформление доверенности поможет вам передать полномочия на выполнение определенных действий другому лицу. Представительство в арбитраже — это помощь в разрешении споров между компаниями или предпринимателями через арбитражный суд.
Больше информации на сайте – https://obrascidoc.ru
лицензия на деятельность, составление заявления в комиссию по трудовым спорам, получить лицензию на образовательную деятельность через госуслуги
госпошлина за регистрацию ооо, какие из приведенных ниже примеров иллюстрируют отношения регулируемые нормами трудового права, оформление брака с иностранцем
Удачи!
Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 3953 раз
Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате. Психологическая и информационная онлайн-помощь. оценили 1662 раз
В переписке у психолога. Психолог онлайн чат. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 858 раз
Телеграм психолог. Анонимный чат с психологом телеграм. Психолог онлайн чат. оценили 1846 раз
Чат психологической поддержки. Психолог t me. Круглосуточная запись на онлайн-консультацию психолога. оценили 4734 раз
Телеграм психолог. Чат с психологом в телеге. Психолог онлайн чат. оценили 321 раз
Tiger Fortune’s jungle theme is so well done. jogo do tigrinho demo
Чат психологической поддержки. Чат психологической поддержки. Помощь психолога онлайн. оценили 3147 раз
Чат психологической поддержки. Психологическая и информационная онлайн-помощь. Психолог оказывает помощь онлайн в чате. оценили 2813 раз
Онлайн-консультация психолога. Психолог t me. Психолог онлайн чат. оценили 5488 раз
Получить первую онлайн консультацию психолога чате. В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 6515 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Психологическая и информационная онлайн-помощь. Чат психологической поддержки. оценили 8709 раз
Психолог t me. В переписке у психолога. Чат с психологом в телеге. оценили 2648 раз
Психолог t me. Анонимный чат с психологом телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. оценили 2841 раз
Психолог онлайн анонимно. Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 9865 раз
Добрый день!
Раздел имущества при разводе — это сложная юридическая процедура, которая требует профессиональной консультации. Наши юристы помогут вам разобраться в том, как правильно разделить совместно нажитое имущество и защитить ваши права. Мы окажем поддержку при составлении соглашений, раздела имущества и расчёте долей. Мы также обеспечим представительство в суде, если вопрос не удастся решить мирным путём. Обратитесь за помощью, чтобы процесс раздела имущества прошёл быстро и без лишних проблем.
Больше информации на сайте – https://legallurist.ru/
помощь юриста по пенсионным вопросам, какие документы нужны для возврата налога за квартиру, трудовой спор по месту жительства работника подсудность
права и обязанности работодателя в трудовых отношениях, как оформить в 1с автомобиль в лизинг, автоюрист по страховке
Удачи!
Приобрести диплом о высшем образовании. Покупка документа о высшем образовании через проверенную и надежную компанию дарит много достоинств для покупателя. Это решение дает возможность сберечь время и значительные средства. l-avt.ru/support/dialog/?PAGE_NAME=profile_view&UID=108329
покупка аккаунтов marketpleys-akkauntov-socsetei.ru
маркетплейс аккаунтов покупка аккаунтов
Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить первую онлайн консультацию психолога чате. оценили 9611 раз
Психолог онлайн анонимно. Психолог t me. Чат с психологом в телеге. оценили 7983 раз
безопасная сделка аккаунтов заработок на аккаунтах
Дипломированный психолог с опытом работы и отзывами клиентов. Дипломированный психолог с опытом работы и отзывами клиентов. Получить первую онлайн консультацию психолога чате. оценили 6394 раз
Онлайн чат с психологом без регистрации. Помощь психолога онлайн. Психолог оказывает помощь онлайн в чате. оценили 325 раз
Психолог t me. Чат психологической поддержки. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. оценили 8112 раз
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Получить онлайн консультацию психолога чате. Психолог онлайн чат. оценили 9886 раз
Онлайн-консультация психолога. В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 2810 раз
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. оценили 7801 раз
Помощь психолога онлайн. Психолог в телеграм. Анонимный чат с психологом телеграм. оценили 2829 раз
Психолог оказывает помощь онлайн в чате. Онлайн-консультация психолога. Анонимный чат с психологом телеграм. оценили 5257 раз
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн чат. Чат психологической поддержки. оценили 4961 раз
Купить диплом любого университета!
Мы можем предложить документы институтов, которые расположены в любом регионе России.
diplomt-v-chelyabinske.ru/kupit-diplom-s-zaneseniem-bez-lishnix-xlopot-2/
Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Помощь психолога онлайн. оценили 1037 раз
Психолог t me. Анонимный чат с психологом телеграм. Чат с психологом в телеге. оценили 3215 раз
Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн чат. Телеграм психолог. оценили 6503 раз
Получить первую онлайн консультацию психолога чате. Чат психологической поддержки. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 3366 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Анонимный чат с психологом телеграм. Анонимный чат с психологом телеграм. оценили 4604 раз
Анонимный чат с психологом телеграм. Телеграм психолог. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 8033 раз
pinup azerbaycan pinup azerbaycan .
Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. оценили 7692 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 4858 раз
Психолог оказывает помощь онлайн в чате. Психологическая и информационная онлайн-помощь. Чат с психологом в телеге. оценили 825 раз
Психологическая и информационная онлайн-помощь. Помощь психолога онлайн. Телеграм психолог. оценили 4790 раз
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Помощь психолога онлайн. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 8574 раз
Психолог в телеграм. Психолог в телеграм. Дипломированный психолог с опытом работы и отзывами клиентов. оценили 1192 раз
Дипломированный психолог с опытом работы и отзывами клиентов. В переписке у психолога. Психологическая и информационная онлайн-помощь. оценили 1429 раз
Анонимный чат с психологом телеграм. Чат психологической поддержки. Дипломированный психолог с опытом работы и отзывами клиентов. оценили 7425 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога. оценили 5046 раз
Онлайн-консультация психолога. Психолог помогающий искать решения в непростых психологических ситуациях. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. оценили 2130 раз
Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога. Круглосуточная запись на онлайн-консультацию психолога. оценили 1136 раз
Tiger Fortune’s features are super fun. tiger fortune
Анонимный чат с психологом телеграм. Чат с психологом в телеге. Психолог онлайн анонимно. оценили 4580 раз
Психолог в телеграм. Психолог онлайн анонимно. Телеграм психолог. оценили 942 раз
Телеграм психолог. Психолог онлайн чат. Психолог онлайн анонимно. оценили 5225 раз
подключить проводной интернет казань
domashij-internet-kazan002.ru
проверить провайдеров по адресу казань
Психолог помогающий искать решения в непростых психологических ситуациях. Получить онлайн консультацию психолога чате. Чат с психологом в телеге. оценили 2977 раз
Психолог оказывает помощь онлайн в чате. Психологическая и информационная онлайн-помощь. Круглосуточная запись на онлайн-консультацию психолога. оценили 8045 раз
Добрый день!
Защита прав потребителей — это важный аспект, особенно при столкновении с некачественными товарами или услугами. Мы обеспечим вам помощь при возврате товара, обмене или подаче иска к продавцу. Юрист по защите прав потребителей поможет вам разобраться в сложных вопросах, связанных с нарушением прав потребителей. Мы поможем вам составить претензию и подготовить исковое заявление в суд. Обратитесь за консультацией, чтобы защитить свои права и получить компенсацию за некачественный товар.
Больше информации на сайте – https://pravookey.ru
помощь в оформлении развода, юридическая консультация в твери бесплатная, статьи по увольнению с работы
брачный договор где и как заключается, для постоянной регистрации какие документы нужны оформления, субсидии от государства
Удачи!
Помощь психолога онлайн. Психолог оказывает помощь онлайн в чате. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 3272 раз
Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога. оценили 7378 раз
Телеграм психолог. Психолог в телеграм. Чат психологической поддержки. оценили 9277 раз
Дипломированный психолог с опытом работы и отзывами клиентов. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога. оценили 9388 раз
Психолог t me. Психолог t me. Психолог онлайн анонимно. оценили 2348 раз
Психолог помогающий искать решения в непростых психологических ситуациях. Помощь психолога онлайн. Получить онлайн консультацию психолога чате. оценили 9878 раз
Психолог оказывает помощь онлайн в чате. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн анонимно. оценили 5826 раз
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. Психолог онлайн анонимно. оценили 5172 раз
Психолог помогающий искать решения в непростых психологических ситуациях. Онлайн-консультация психолога. Помощь психолога онлайн. оценили 3652 раз
В переписке у психолога. Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. оценили 3369 раз
Психолог t me. Психолог в телеграм. Психолог оказывает помощь онлайн в чате. оценили 805 раз
Психолог онлайн чат. Психолог t me. Психолог оказывает помощь онлайн в чате. оценили 7925 раз
Психолог t me. Онлайн чат с психологом без регистрации. Психолог оказывает помощь онлайн в чате. оценили 3364 раз
Чат психологической поддержки. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог t me. оценили 283 раз
Чат с психологом в телеге. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате. оценили 4046 раз
Здравствуйте!
Юридическая помощь при ДТП — это важный аспект для автовладельцев, столкнувшихся с аварией. Мы поможем вам правильно оформить все документы, оценить ущерб и защитить ваши интересы в страховых компаниях и суде. Автоюрист поможет вам разобраться в правилах возмещения ущерба и поможет вернуть права, если они были лишены. Наша команда профессионалов будет сопровождать вас на всех этапах урегулирования дела. Обратитесь к нам, и мы поможем вам решить все вопросы, связанные с ДТП, на самом высоком уровне.
Больше информации на сайте – https://pravookey.ru/
семейное право консультация, юрист москва бесплатная консультация по телефону круглосуточно, юридическая консультация по телефону в минске
можно ли вступить в наследство после 6 месяцев после смерти матери без уважительной причины, егэ брачный договор задания, защита прав потребителей
Удачи!
Получить первую онлайн консультацию психолога чате. Психолог помогающий искать решения в непростых психологических ситуациях. Чат психологической поддержки. оценили 9452 раз
Психолог оказывает помощь онлайн в чате. Чат с психологом в телеге. Психолог онлайн анонимно. оценили 9223 раз
casino 1win http://1win1001.top .
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога. оценили 8386 раз
Анонимный чат с психологом телеграм. Психолог помогающий искать решения в непростых психологических ситуациях. В переписке у психолога. оценили 7701 раз
Психолог онлайн анонимно. Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога. оценили 2449 раз
Телеграм психолог. Получите консультацию онлайн-психолога в чате прямо сейчас. Телеграм психолог. оценили 8886 раз
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 3314 раз
Психолог онлайн чат. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 5765 раз
Психолог оказывает помощь онлайн в чате. Психологическая и информационная онлайн-помощь. Круглосуточная запись на онлайн-консультацию психолога. оценили 2281 раз
В переписке у психолога. Психолог оказывает помощь онлайн в чате. Психолог t me. оценили 1505 раз
Чат с психологом в телеге. Чат психологической поддержки. Психолог онлайн анонимно. оценили 3942 раз
Имморталы CS2 https://cs-open-case.ru/ редкие скины, которые выделят тебя в матче. Торгуй, покупай, продавай топовые предметы с моментальной доставкой в инвентарь. Лучшие цены и безопасные сделки!
В переписке у психолога. Психолог оказывает помощь онлайн в чате. Телеграм психолог. оценили 2506 раз
Топ-дроп CS2 open case cs2 уже здесь! Самые редкие скины, ножи и эксклюзивы, которые действительно выпадают. Лучшие кейсы, обновления коллекций и советы для удачного открытия.
Премиум скины для CS2 https://case-cs-open.ru выделяйся в каждом раунде! Редкое оружие, эксклюзивные коллекции, эффектные раскраски и ножи. Только топовые предметы с мгновенной доставкой в Steam.
Здравствуйте!
Оформление гражданства РФ требует знания всех правовых аспектов, связанных с получением гражданства. Мы предоставляем консультации по вопросам, как получить гражданство, а также по оформлению виз, разрешений и других документов. Наши юристы помогут вам пройти процесс легализации и получения гражданства без ошибок. Мы также предлагаем юридическую помощь для иностранных граждан и тех, кто хочет остаться в России на постоянной основе. Обратитесь за помощью, чтобы оформить гражданство и другие документы.
Больше информации на сайте – https://tvokonsultant.ru/
юрист по семейным делам, образца документов для оформления загранпаспорта нового образца, если ребенок остается с отцом после развода мать платит алименты
увольнение статья в порядке перевода, как платить налог при сдаче квартиры в аренду, возврат ОСАГО
Удачи!
Онлайн-консультация психолога. Психолог в телеграм. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 2019 раз
В переписке у психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог оказывает помощь онлайн в чате. оценили 2479 раз
Получить первую онлайн консультацию психолога чате. Чат с психологом в телеге. Получить первую онлайн консультацию психолога чате. оценили 1981 раз
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог в телеграм. Чат психологической поддержки. оценили 7684 раз
Чат психологической поддержки. Помощь психолога онлайн. Дипломированный психолог с опытом работы и отзывами клиентов. оценили 8702 раз
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог онлайн чат. Психолог онлайн анонимно. оценили 8173 раз
Онлайн-консультация психолога. Психолог оказывает помощь онлайн в чате. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 2623 раз
Получить первую онлайн консультацию психолога чате. Онлайн чат с психологом без регистрации. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 3682 раз
chinessonsofanarchy-italia.com https://sonsofanarchy-italia.com
This online casino’s Tiger Fortune is a thrill. jogo do tigrinho demo
Получить онлайн консультацию психолога чате. Получить онлайн консультацию психолога чате. Психолог в телеграм. оценили 4495 раз
Психолог оказывает помощь онлайн в чате. Чат психологической поддержки. Онлайн-консультация психолога. оценили 8720 раз
Психолог оказывает помощь онлайн в чате. Психологическая и информационная онлайн-помощь. Чат психологической поддержки. оценили 8627 раз
This online casino’s Tiger Fortune is top-class. jogo do tigrinho demo
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Психолог помогающий искать решения в непростых психологических ситуациях. Круглосуточная запись на онлайн-консультацию психолога. оценили 6864 раз
The tiger symbols in Tiger Fortune are so cool! tigrinho demo
Психолог t me. Психолог онлайн чат. Получить первую онлайн консультацию психолога чате. оценили 9901 раз
Психолог t me. Психолог онлайн анонимно. Чат с психологом в телеге. оценили 9088 раз
Психологическая и информационная онлайн-помощь. Психолог t me. Круглосуточная запись на онлайн-консультацию психолога. оценили 9741 раз
В переписке у психолога. Получить первую онлайн консультацию психолога чате. Психолог t me. оценили 1102 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Круглосуточная запись на онлайн-консультацию психолога. Получить первую онлайн консультацию психолога чате. оценили 8145 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Онлайн-консультация психолога. оценили 861 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Получите консультацию онлайн-психолога в чате прямо сейчас. В переписке у психолога. оценили 7102 раз
Получить первую онлайн консультацию психолога чате. Психолог в телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. оценили 2270 раз
Чат психологической поддержки. Анонимный чат с психологом телеграм. Психолог t me. оценили 5666 раз
Привет всем!
Помощь при оформлении гражданства РФ — это услуга, которая поможет вам правильно пройти все этапы получения гражданства. Мы поможем вам собрать все необходимые документы, подать заявление и пройти все юридические процедуры для получения гражданства РФ. Юрист по вопросам гражданства обеспечит, что вы будете правильно следовать всем законодательным требованиям, чтобы не возникло проблем с получением гражданства. Наши специалисты окажут полное юридическое сопровождение на каждом этапе. Обратитесь к нам за консультацией и получите помощь при оформлении гражданства.
Больше информации на сайте – https://moepravodo.ru
налогообложение недвижимости, сроки ремонта гарантийного автомобиля по закону о защите прав потребителей, может ли иностранный гражданин открыть ип в россии по временной регистрации
за какое время подается заявление в загс на регистрацию брака, каско втб автострахование, регистрация прав на землю
Удачи!
Помощь психолога онлайн. В переписке у психолога. Психолог онлайн чат. оценили 106 раз
Психолог онлайн анонимно. В переписке у психолога. Чат с психологом в телеге. оценили 7431 раз
Круглосуточная запись на онлайн-консультацию психолога. Получите консультацию онлайн-психолога в чате прямо сейчас. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 2866 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Телеграм психолог. оценили 5377 раз
Купить Мефедрон в Киеве? Ссылка – COCAIN.VIP Доставка По Украине Мефедрона? Ссылка – COCAIN.VIP
.
.
.
.
.
| Купить онлайн закладку Мефедрон в Киеве ССЫЛКА – https://cocain.vip |
| Купить в другом городе Украины Мефедрон закладку ССЫЛКА – https://cocain.vip |
| Купить доставку Мефедрона в Киеве в руки через сайт ССЫЛКА – https://cocain.vip |
| Купить доставку в руки Мефедрон в Украине ССЫЛКА – https://cocain.vip |
| Купить Мефедрон в Украине доставкой через почту ССЫЛКА – https://cocain.vip |
| Купить Мефедрон в Киеве и Украине через Курьера в руки ССЫЛКА -https://cocain.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Слова для поиска –
Мефедрон в Киеве купить закладкой, Мефедрон в Киеве Купить з доставкой, Мефедрон в Киеве купить через курьера, Мефедрон в Киеве Купить быстро, Мефедрон в Киеве Купить сейчас, Мефедрон в Киеве Купить в руки, Мефедрон в Киеве Купить безопасно
Мефедрон в Украине Купить можно, Купить Мефедрон в Украине закладкой, Купить Мефедрон в Украине доставкой домой, Купить Мефедрон в Украине по почте, Купить Мефедрон в Украине без проблем можно, Купить Мефедрон в Украине на сайте, Кокс цена в Украине,
Виды Мефедрона Купить в Украине и Киеве, Чем отличаеться Мефедрон в Киеве от Мефедрона в Украине, Как я могу купить Мефедрон в Киеве, Купить Колумбийский Мефедрон в Киеве, Купить Колумбийский Мефедрон в Украине, Кокс Цена в Киеве,
Теги для городов –
Купить Мефедрон в Киеве, Купить Мефедрон в Одессе, Купить Мефедрон в Харькове, Купить Мефедрон во Львове, Купить Мефедрон в Днепре, Купить Мефедрон в Ужгороде, Купить Мефедрон в Ивано-Франковске, Купить Мефедрон в Белой Церкве, Купить Мефедрон в Ровно
Купить Мефедрон в Николаеве, Купить Мефедрон в Херсоне, Купить Мефедрон в Сумах, Купить Мефедрон в Запорожье, Купить Мефедрон в Житомере, Купить Мефедрон в Мукачево, Купить Мефедрон в Полтаве, Купить Мефедрон в Умани, Купить Мефедрон в Виннице
Общие теги для товаров-
Купить Мефедрон в Киеве, Купить Мефедрон в Украине, Купить Героин в Киеве, Купить Героин в Украине, Купить Лирику в Киеве, Купить Лирику в Украине, Купить Экстази в Киеве, Купить Экстази в Украине, Купить Амфетамин в Киеве, Купить Амфетамин
в Украине, Купить МДМА в Киеве, Купить МДМА в Украине, Купить Метамфетамин в Киеве, Купить Метамфетамин в Украине, Купить Метадон в Киеве, Купить Метадон в Украине, Купить Шишки в Киеве, Купить Шишки в Украине, Купить Бошки в Киеве,
Купить Бошки в Украине, Купить Альфа ПВП В Киеве, Купить Альфа ПВП в Украине, Купить A-PVP в Киеве, Купить A-PVP в Украине, Купить Соль СК в Киеве, Купить Соль СК в Украине, Купить Мефедрон в Киеве, Купить Мефедрон в Украине,
Телеграм психолог. Психолог t me. Круглосуточная запись на онлайн-консультацию психолога. оценили 5715 раз
Телеграм психолог. Чат с психологом в телеге. Получить первую онлайн консультацию психолога чате. оценили 9052 раз
Где приобрести диплом специалиста?
Наша компания предлагаетмаксимально быстро приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями официальных лиц. Диплом способен пройти любые проверки, даже с использованием профессиональных приборов. Достигайте свои цели максимально быстро с нашим сервисом. Приобрести диплом ВУЗа! share.psiterror.ru/2025/03/12/vashi-dannye-pod-nadezhnoy-zaschitoy.html
Чат психологической поддержки. Чат с психологом в телеге. Психолог онлайн анонимно. оценили 7631 раз
Заказать спортиков https://foto-prikol.net/
Получите консультацию онлайн-психолога в чате прямо сейчас. Онлайн-консультация психолога. Получить онлайн консультацию психолога чате. оценили 6966 раз
Доброго!
Правовая консультация по телефону позволяет получить помощь в любое время и из любого места. Юридическая помощь в бракоразводном процессе обеспечит вам поддержку и защиту интересов в сложных ситуациях. Консультация юриста по разводам поможет разобраться в юридических аспектах развода и делении имущества. Сопровождение сделок с недвижимостью обеспечит вам безопасность при заключении договоров. Юридическая помощь при оформлении недвижимости поможет вам правильно оформить все документы при покупке, продаже или аренде.
Больше информации на сайте – https://llawdo.ru
составление апелляции, стоимость услуг нотариуса при оформлении дарственной на долю в квартире между родственниками, ресторан карлсон регистрация брака
посредник при рассмотрении коллективного трудового спора принимает решение, регистрация дополнительного соглашения к договору аренды, как написать завещание
Удачи!
Психолог t me. В переписке у психолога. В переписке у психолога. оценили 7824 раз
Чат с психологом в телеге. Психолог онлайн анонимно. Дипломированный психолог с опытом работы и отзывами клиентов. оценили 8138 раз
Чат психологической поддержки. Круглосуточная запись на онлайн-консультацию психолога. Психолог онлайн чат. оценили 1154 раз
Психолог оказывает помощь онлайн в чате. Получить первую онлайн консультацию психолога чате. Анонимный чат с психологом телеграм. оценили 7341 раз
В переписке у психолога. Психолог онлайн чат. Чат с психологом в телеге. оценили 7254 раз
Где приобрести диплом по нужной специальности?
Наши специалисты предлагают выгодно и быстро заказать диплом, который выполняется на оригинальном бланке и заверен печатями, водяными знаками, подписями официальных лиц. Диплом пройдет любые проверки, даже с применением специально предназначенного оборудования. Решите свои задачи быстро и просто с нашей компанией.
Купить диплом о высшем образовании diplomgorkiy.com/kuplyu-diplom-o-visshem-obrazovanii-12/
Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. В переписке у психолога. Психолог в телеграм. оценили 9144 раз
Помощь психолога онлайн. В переписке у психолога. Психолог оказывает помощь онлайн в чате. оценили 9837 раз
Психолог онлайн чат. Дипломированный психолог с опытом работы и отзывами клиентов. Психолог оказывает помощь онлайн в чате. оценили 8833 раз
Психолог t me. Чат психологической поддержки. Психолог оказывает помощь онлайн в чате. оценили 4842 раз
Психологическая и информационная онлайн-помощь. Психолог онлайн анонимно. Телеграм психолог. оценили 4349 раз
провайдеры по адресу дома
domashij-internet-kazan003.ru
провайдеры казань
1вин https://cah.forum24.ru/?1-13-0-00001678-000-0-0/ .
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог оказывает помощь онлайн в чате. Получить онлайн консультацию психолога чате. оценили 3679 раз
Чат с психологом в телеге. Онлайн-консультация психолога. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. оценили 4995 раз
Психолог помогающий искать решения в непростых психологических ситуациях. Психолог помогающий искать решения в непростых психологических ситуациях. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 6446 раз
Психологическая и информационная онлайн-помощь. Психолог t me. Психологическая и информационная онлайн-помощь. оценили 9835 раз
Телеграм психолог. Психолог оказывает помощь онлайн в чате. Психолог t me. оценили 2825 раз
Получить онлайн консультацию психолога чате. Телеграм психолог. Психолог оказывает помощь онлайн в чате. оценили 4746 раз
Психолог оказывает помощь онлайн в чате. Психолог в телеграм. Психолог онлайн анонимно. оценили 557 раз
Анонимный чат с психологом телеграм. Чат с психологом в телеге. Онлайн-консультация психолога. оценили 9471 раз
один вин https://1win6042.ru .
1 вин вход 1 вин вход .
Анонимный чат с психологом телеграм. Психолог в телеграм. Получить онлайн консультацию психолога чате. оценили 1354 раз
Анонимный чат с психологом телеграм. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат с психологом в телеге. оценили 6770 раз
Дипломированный психолог с опытом работы и отзывами клиентов. Круглосуточная запись на онлайн-консультацию психолога. Помощь психолога онлайн. оценили 6956 раз
Круглосуточная запись на онлайн-консультацию психолога. Дипломированный психолог с опытом работы и отзывами клиентов. Онлайн чат с психологом без регистрации. оценили 1344 раз
Диплом университета Российской Федерации!
Без наличия диплома очень сложно было продвинуться вверх по карьерной лестнице. Именно из-за этого решение о покупке диплома следует считать целесообразным. Купить диплом об образовании lakarjobbisverige.se/employer/4858/gosznac-diplom-24
Купить диплом академии!
Мы изготавливаем дипломы любой профессии по выгодным ценам. Вы заказываете документ в надежной и проверенной временем компании. : konstruktiv.getbb.ru/viewtopic.php?f=17&t=14351
Купить диплом университета по выгодной цене можно, обратившись к надежной специализированной компании. Приобрести документ о получении высшего образования можно в нашей компании. [url=http://fastdiploms.com/diplom-bakalavra-s-zaneseniem-v-reestr-8/]fastdiploms.com/diplom-bakalavra-s-zaneseniem-v-reestr-8[/url]
Психологическая и информационная онлайн-помощь. Психолог помогающий искать решения в непростых психологических ситуациях. Психологическая и информационная онлайн-помощь. оценили 1982 раз
Tiger Fortune’s design is wild and perfect. tiger fortune
хранение вещей на складе в москве недорого хранение вещей на складе в москве недорого .
Мы изготавливаем дипломы любых профессий по разумным тарифам. Стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы дипломы были доступными для подавляющей массы граждан.
Заказ документа, подтверждающего обучение в ВУЗе, – это рациональное решение. Купить диплом любого университета: diplom-bez-problem.com/kupit-diplom-visshee-obrazovanie-2/
Здравствуйте!
При подаче иска в суд важно иметь грамотную юридическую помощь для корректного составления документов и аргументации своей позиции. Наши юристы помогут вам подготовить исковое заявление, обеспечить правильность всех формальностей и подачу в суд. Мы оказываем помощь на всех этапах судебного процесса, включая сбор доказательств и представление интересов в суде. Юридическая помощь при подаче иска обеспечит вам уверенность в правильности каждого шага. Доверьтесь профессионалам для успешного завершения судебного дела.
Больше информации на сайте – https://tvokonsultant.ru/
защита прав автовладельцев, налог на имущество с незавершенного строительства, составление договоров онлайн
вопросы пдд теория, налоговая амнистия учебник, адвокат по уголовным делам
Удачи!
Профессиональное остекление балконов и лоджий, подробнее тут https://osteklenie-balkonov-v-spb.pro/
1win.pro 1win.pro .
Приобрести диплом о высшем образовании!
Мы готовы предложить дипломы любой профессии по разумным ценам— diplom-top.ru/kupit-diplom-realnij-2/
Заказать диплом института по невысокой цене возможно, обратившись к проверенной специализированной компании. Мы предлагаем документы об окончании любых университетов РФ. Заказать диплом о высшем образовании– diplomist.com/kupit-diplom-ob-obrazovanii-s-reestrom-bistro-i-legko-5/
Психолог t me. Получить первую онлайн консультацию психолога чате. В переписке у психолога. оценили 930 раз
Доброго!
Юридическая консультация по кредитам необходима для понимания всех условий, связанных с кредитными обязательствами, и защиты своих прав в случае проблем с выплатами. Возврат налога за лечение позволит вам получить налоговый вычет за медицинские расходы, и юрист поможет в оформлении соответствующих документов. Налог на имущество требует внимательного расчета и оформления, и юрист поможет вам минимизировать налоговые риски. Налоговая амнистия предоставляет возможность для снижения налогового бремени, и юрист поможет вам воспользоваться всеми льготами. Сопровождение бракоразводного процесса обеспечит защиту ваших интересов и прав в процессе раздела имущества и установления опеки.
Больше информации на сайте – https://llawdo.ru/
страховые споры, сбербанк регистрация ооо с одним учредителем, с какой суммы платится налог с продажи квартиры менее 3 лет в собственности
отказ от алиментов на ребенка добровольно последствия для матери, представительство в суде курсовая, оформление гражданства
Удачи!
Круглосуточная запись на онлайн-консультацию психолога. Круглосуточная запись на онлайн-консультацию психолога. Психолог в телеграм. оценили 3516 раз
Привет всем!
Вопросы, связанные с трудовым правом, решит квалифицированный юрист по трудовому праву. Мы поможем вам разобраться в условиях трудового договора образца и защитим ваши права при увольнении по статье. Важно правильно оформить все документы, чтобы избежать неприятных последствий. Юридическое сопровождение сделок с недвижимостью — это обязательный шаг для того, чтобы сделки прошли безопасно. Мы предоставим автоюриста для решения вопросов, связанных с ДТП или страховкой автомобиля.
Больше информации на сайте – https://obrascidoc.ru
налог на имущество, консультация юриста по защите прав потребителей бесплатно, краснодар юрист по дтп
какие документы нужны для генеральной доверенности на все полномочия оформления у нотариуса, консультация адвоката по гражданским делам, трудовые отношения
Удачи!
Низкая самооценка, прокрастинация, апатия Современный мир диктует свои условия, и порой нам необходима квалифицированная помощь, чтобы справиться с вызовами. Психолог онлайн – это удобный и анонимный способ получить поддержку в комфортной обстановке. Работа с психологом: путь к гармонии Обращение к психологу – это признак силы и заботы о себе. Специалист поможет разобраться в сложных ситуациях, найти ресурсы для преодоления трудностей и обрести внутреннюю гармонию.
В переписке у психолога. Психолог оказывает помощь онлайн в чате. Психолог оказывает помощь онлайн в чате. оценили 3733 раз
Получить онлайн консультацию психолога чате. Онлайн чат с психологом без регистрации. Помощь психолога онлайн. оценили 3954 раз
Психологическая и информационная онлайн-помощь. Психолог в телеграм. Получить первую онлайн консультацию психолога чате. оценили 1087 раз
Психологическая и информационная онлайн-помощь. Получить онлайн консультацию психолога чате. Круглосуточная запись на онлайн-консультацию психолога. оценили 7418 раз
Психолог онлайн анонимно. Телеграм психолог. Психолог оказывает помощь онлайн в чате. оценили 8674 раз
Психолог онлайн чат. Помощь психолога онлайн. Получите консультацию онлайн-психолога в чате прямо сейчас. оценили 7888 раз
Психолог оказывает помощь онлайн в чате. Телеграм психолог. Получить онлайн консультацию психолога чате. оценили 5293 раз
мостюет http://www.mostbet6032.ru .
pin up azerbaijan pin up azerbaijan .
descărca 1win descărca 1win .
Получить онлайн консультацию психолога чате. Получить первую онлайн консультацию психолога чате. Чат психологической поддержки. оценили 8819 раз
This online casino’s Tiger Fortune is fantastic. jogo do tigrinho demo
аттестат школьный купить
pdacenter.ru – сервис по ремонту бытовой техники
Ремонт кофемашин в Санкт-Петербурге в официальном сервисном центре PDACENTER.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
Лучшие скины CS2 https://open-csgo-case.ru/ яркие, редкие, премиальные. Собери коллекцию, прокачай инвентарь и покажи всем свой вкус. Обзор самых крутых скинов с актуальными ценами и рейтингами.
Топовые скины CS2 open-case-cs.ru/ от легендарных ножей до эксклюзивных обложек на AWP. Красота, стиль, престиж. Оцени крутой дроп и подбери скин, который подходит именно тебе.
1win site 1win site .
Психолог оказывает помощь онлайн в чате. В переписке у психолога. Онлайн-консультация психолога. оценили 153 раз
Получите консультацию онлайн-психолога в чате прямо сейчас. Чат с психологом в телеге. Психологическая и информационная онлайн-помощь. оценили 9930 раз
Здравствуйте!
Услуги адвоката по кредитам помогут вам защитить свои интересы в случае, если у вас возникли проблемы с банком или кредиторами. Мы предоставляем помощь в решении споров по кредитам, долгах и процентным ставкам. Консультация адвоката по кредитам позволит вам разобраться в правомерности требований и найти оптимальное решение. Мы также поможем вам при оформлении договора займа и других финансовых документов. Получите помощь, чтобы решить ваши проблемы с кредитами быстро и без проблем.
Больше информации на сайте – https://legallurist.ru
юридическая проверка документов, казахстан бесплатная консультация онлайн юриста, части или пункты в законе о защите прав потребителей
рассчитать налог онлайн с продажи квартиры рассчитать, ст 375 нк рф налог на имущество, увольнение по статье
Удачи!
Топовые скины CS2 https://csgo-open-case.ru от легендарных ножей до эксклюзивных обложек на AWP. Красота, стиль, престиж. Оцени крутой дроп и подбери скин, который подходит именно тебе.
Дипломированный психолог с опытом работы и отзывами клиентов. Телеграм психолог. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 9359 раз
Получить первую онлайн консультацию психолога чате. Психолог онлайн анонимно. Анонимный чат с психологом телеграм. оценили 5699 раз
Психолог онлайн анонимно. Получить КОНСУЛЬТАЦИЮ и ПОДДЕРЖКУ профессиональных психологов. Чат с психологом в телеге. оценили 6471 раз
подключить интернет
domashij-internet-msk001.ru
домашний интернет москва
В переписке у психолога. Психологическая и информационная онлайн-помощь. Дипломированный психолог с опытом работы и отзывами клиентов. оценили 709 раз
Получить первую онлайн консультацию психолога чате. Получите консультацию онлайн-психолога в чате прямо сейчас. Чат психологической поддержки. оценили 9519 раз
Заказать диплом любого ВУЗа!
Мы готовы предложить документы учебных заведений, расположенных в любом регионе РФ. Дипломы и аттестаты выпускаются на бумаге высшего качества: workafrique.com/profile/larhondaderric
Заказать диплом ВУЗа!
Приобрести диплом университета по выгодной цене вы сможете, обратившись к надежной специализированной компании. Заказать диплом: diplomass.com/kupit-podlinnij-diplom-s-zaneseniem-v-reestr-19
Большая коллекция порнофильмы на русском — только для вас!
Ищете качественные полнометражные порнофильмы с русской озвучкой? Наш сайт предлагает вам лучшую подборку в HD-формате без рекламы и ограничений.
Почему стоит выбрать нас:
Тысячи фильмов разных жанров: от классики до премьер.
Порнофильмы Марк Дорсель
Эксклюзивные материалы, которых нет на других платформах.
Высокая скорость загрузки и стабильный стриминг без задержек.
Удобный просмотр на любом устройстве: смартфон, планшет, ПК или телевизор.
Зарегистрируйтесь сейчас и откройте доступ к огромной коллекции
порнофильмы с сюжетом.
Круглосуточная запись на онлайн-консультацию психолога. Психолог t me. Получить первую онлайн консультацию психолога чате. оценили 7922 раз
I love the online promos—new spark! tome of madness
Tiger Fortune’s design blows me away every time. tiger fortune
aviator mostbet https://eisberg.forum24.ru/?1-5-0-00002579-000-0-0-1743260427/ .
1win официальный сайт скачать 1win официальный сайт скачать .
мотбет http://www.mostbet6031.ru .
казино онлайн kg https://eisberg.forum24.ru/?1-5-0-00002579-000-0-0-1743260427/ .
This casino’s Tiger Fortune slot is epic. fortune tiger demo
The mobile apps online falter—patch it! fortunetiger
Tiger Fortune’s bonus rounds are the best part. fortune tiger
dragon money лицензия dragon-money27.com .
aviator mostbet aviator mostbet .
мостбет chrono eisberg.forum24.ru/?1-5-0-00002579-000-0-0-1743260427 .
1winn http://1win6043.ru .
Tiger Fortune keeps the good vibes going! jogo do tigrinho demo
1win moldova http://1win5010.ru/ .
скачать мостбет mostbet6032.ru .
Экспрессы Мир ставок – это захватывающая сфера, где азарт и аналитика переплетаются в стремлении предсказать исход спортивных событий. Это игра, где интуиция встречается с расчетом, а знание предмета становится ключом к успеху. От футбола до тенниса, от баскетбола до хоккея – ставки на спорт охватывают огромный спектр дисциплин. Каждая игра, каждый матч – это новая возможность проверить свою проницательность и, возможно, выиграть.
дракон мани дракон мани .
I can’t get enough of Tiger Fortune’s energy. tiger fortune
1win moldova http://1win5010.ru/ .
Dubayda “Portu Pati” n?dir v? niy? ham? bundan dan?s?r?
https://x.com/IrinaPavlovna84/status/1909960841606070387
1win moldova download https://1win5010.ru/ .
Tiger Fortune’s spins keep me hooked! jogo do tigrinho demo
I love the poker spread online—new fun! plinko
This online casino’s Tiger Fortune is a masterpiece. fortune tiger demo gratis
Добрый день!
Автострахование также требует юридической помощи, чтобы избежать проблем с выплатами. Юридическая консультация по телефону — это быстрый способ получить ответы на вопросы. Правовая помощь онлайн — удобный способ решить свои проблемы в любое время. Подача заявления в суд может быть сложной процедурой, и наш юрист поможет вам. Оформление развода без детей потребует особого внимания, и мы подскажем, как избежать сложностей.
Больше информации на сайте – https://moepravodo.ru/
оформление временной регистрации, после увольнения по статье можно ли устроиться на работу, налог на завещанное имущество
регистрация права собственности в упрощенном порядке на земельный участок, как оформить банкротство через мфц бесплатно физического лица по кредитам, сопровождение бизнеса
Удачи!
Для успешного продвижения вверх по карьерной лестнице нужно наличие официального диплома о высшем образовании. Выгодно купить диплом о высшем образовании у надежной фирмы: rusd-diplomj.ru/kupit-diplom-o-srednem-spetsialnom-obrazovanii-goznaka-6/
Доброго!
Юридическое обслуживание бизнеса необходимо для правильного ведения дел, и наш юрист поможет вам с этим. Юридическая помощь при приватизации требует профессионального подхода, чтобы соблюсти все законодательные требования. Консультация по налогам поможет вам правильно распределить финансовые обязательства и избежать штрафов. Консультация юриста по страхованию обеспечит вам защиту интересов при заключении страховых договоров. Оформление гражданства ребенку — это сложный процесс, с которым мы готовы вам помочь.
Больше информации на сайте – https://uristvzakon.ru/
онлайн консультация юриста, юрист анапа бесплатная консультация, какие документы нужны для оформления доверенности на ребенка у нотариуса выезда за границу
автострахование согаз официальный сайт, до какого возраста платят алименты на ребенка в россии, юридическая помощь при увольнении
Удачи!
1win moldova download 1win moldova download .
Добрый день!
Подача иска — это важный процесс, который требует тщательной подготовки и знания всех нюансов. Наши юристы помогут вам составить исковое заявление и подготовить документы для подачи в суд. Мы обеспечиваем юридическое сопровождение всех судебных процедур, защищая ваши интересы. Мы также предоставляем консультации по вопросу подачи иска в арбитражный суд и защиту ваших прав. Получите помощь в составлении и подаче иска без ошибок и задержек.
Больше информации на сайте – https://yslugiyurista.ru/
регистрация брака, бесплатная юридическая консультация калуга, проверка наследственное дело
номер объекта в декларации по налогу на имущество, через сколько можно развестись после регистрации брака, нотариус москва
Удачи!
Смотри топ скинов CS2 https://cs2-open-case.ru/ самые красивые, редкие и желанные облики для оружия. Стиль, эффект и внимание на сервере гарантированы. Обновляем коллекцию каждый день!
Иммортал-дроп CS2 open-case-csgo только для избранных. Легендарные скины, высокая ценность, редкие флоаты и престиж на сервере. Пополни свою коллекцию настоящими шедеврами.
1win бк 1win бк .
Смотри топ скинов CS2 case-cs2-open.ru/ самые красивые, редкие и желанные облики для оружия. Стиль, эффект и внимание на сервере гарантированы. Обновляем коллекцию каждый день!
провайдер интернета по адресу москва
domashij-internet-msk002.ru
подключение интернета по адресу
Привет всем!
Юридическая помощь в бракоразводном процессе — это необходимая поддержка для тех, кто сталкивается с разводом и связанными с ним вопросами. Мы поможем вам разобраться в вопросах раздела имущества, алиментов, опеки над детьми и других юридических аспектах. Адвокат по разводам обеспечит защиту ваших интересов в суде и поможет достичь справедливого решения. Наши специалисты подготовят все необходимые документы и окажут поддержку на всех этапах развода. Обратитесь к нам, чтобы пройти через бракоразводный процесс с минимальными сложностями.
Больше информации на сайте – https://yslugiyurista.ru/
налоговая амнистия, сколько платят на 2 ребенка алименты, как подать заявление на развод с несовершеннолетним ребенком
можно ли гасить досрочно военную ипотеку самому, 1081 постановление о лицензировании фармацевтической деятельности, защита в суде
Удачи!
Tiger Fortune is my go-to slot game at this online casino! fortune tiger bet
Приобрести диплом любого университета!
Мы предлагаем документы любых учебных заведений, расположенных на территории всей Российской Федерации.
rusd-diplomj.ru/uznajte-tsenu-diploma-s-zaneseniem-v-reestr-segodnya/
Tiger Fortune’s soundtrack is pure fire! tigrinho demo
dragon казино dragon казино .
1вин официальный сайт вход https://www.sebezh.borda.ru/?1-10-0-00000117-000-0-0-1743052058 .
The tiger roars in Tiger Fortune are epic. fortune tiger demo gratis
Заказать диплом ВУЗа по невысокой стоимости можно, обратившись к надежной специализированной компании. Приобрести документ о получении высшего образования можно в нашем сервисе. diplomt-nsk.ru/diplom-s-zaneseniem-v-reestr-vigodnaya-tsena-2
I won spins online and got $150—big yes! Vai de Bet
This casino’s Tiger Fortune game is flawless. fortune tiger demo gratis
aviator mostbet http://www.mostbet6031.ru .
драгон драгон .
I love how Tiger Fortune keeps me engaged. fortune tiger 777
1вин приложение sebezh.borda.ru/?1-10-0-00000117-000-0-0-1743052058 .
1win pariuri 1win pariuri .
Tiger Fortune’s energy is off the charts. fortune tiger demo gratis
Приобрести диплом университета !
Приобретение диплома любого ВУЗа России в нашей компании является надежным делом, ведь документ будет заноситься в реестр. Приобрести диплом о высшем образовании diplomservis.ru/kupit-diplom-s-zaneseniem-v-reestr-po-nizkoj-tsene-12
Добрый день!
Если вы столкнулись с проблемами при оформлении наследства, наши юристы помогут вам на каждом этапе этого сложного процесса. Мы оказываем помощь в составлении завещаний, а также в решении вопросов, связанных с разделом имущества и правами наследников. Оформление наследства у нотариуса требует точности и соблюдения всех юридических норм, и наши специалисты позаботятся о всех деталях. Мы обеспечим защиту ваших прав в случае споров между наследниками. Получите квалифицированную помощь, чтобы оформить наследство без лишних трудностей.
Больше информации на сайте – https://uristvzakon.ru/
регистрация ИП онлайн, юридическая консультация бесплатно онлайн без регистрации и телефона, как лицу без гражданства получить гражданство рф
автострахование евразия онлайн, макарова корпоративное право, помощь юриста в суде
Удачи!
I recommend Tiger Fortune to anyone who loves slots. fortune tiger demo gratis
Быстрый и удобный калькулятор строительства дома онлайн рассчитать рассчитайте стоимость и объем работ за пару минут. Онлайн-калькулятор поможет спланировать бюджет, сравнить варианты и избежать лишних затрат.
1win live http://www.1win6043.ru .
Мамоновское кладбище https://mamonovskoe.ru справочная информация, адрес, график работы, участки, ритуальные услуги и памятники. Всё, что нужно знать, собрано на одном сайте.
новости про краснодар http://krasnodar-news35.ru
pdacenter.ru – сервис по ремонту бытовой техники
Ремонт мониторов в Челябинске в официальном сервисном центре PDACENTER.
Наши инженеры выполняют ремонт любой сложности по дотупным ценам!
Tiger Fortune has a great mix of fun and rewards. fortune tiger 777
mostbet chrono http://www.mostbet6033.ru .
I wish online casinos had better mobile apps; some are so glitchy! plinko
Tiger Fortune’s design is top-notch – really impressive. tigrinho demo
pin up azerbaycan pin up azerbaycan .
Привет всем!
Налоговая консультация поможет вам правильно оформить налоги при продаже квартиры и других объектов недвижимости. Мы поможем вам разобраться в вопросах налогообложения доходов, связанных с продажей имущества. Юридическая помощь при налогообложении недвижимости обеспечит правильное заполнение всех документов. Мы предоставляем консультации по налогу на имущество, а также по налоговым вычетам. Обратитесь к нам за помощью, и мы поможем вам избежать налоговых ошибок.
Больше информации на сайте – https://voproslaw.ru/
налоговая отчетность, если отказывается отец от ребенка должен ли платить алименты, до какого возраста выплачивают алименты на ребенка
какие документы нужны для возврата налога за лечение зубов, расторжения брака через госуслуги регистрация, сопровождение бракоразводного процесса
Удачи!
prodentim a groundbreaking probiotic supplement uniquely designed to support oral health and promote robust gums and teeth.
prostavive maintaining prostate health is crucial for men’s overall wellness, especially as they grow older.
интернет провайдеры в москве по адресу дома
domashij-internet-msk003.ru
подключить домашний интернет москва
pinup azerbaycan pinup azerbaycan .
1win скачать kg https://1win6007.ru/ .
микронаушники https://czmicro.cz/
мрстбет https://www.mostbet6011.ru .
Всех приветствую!
Для определенных людей, заказать диплом университета – это необходимость, возможность получить отличную работу. Однако для кого-то – это желание не терять время на учебу в институте. С какой бы целью вам это не потребовалось, мы готовы помочь. Оперативно, качественно и по разумной цене изготовим документ любого ВУЗа и любого года выпуска на подлинных бланках с реальными печатями.
Основная причина, почему многие люди покупают документ, – желание занять хорошую должность. К примеру, знания дают возможность специалисту устроиться на работу, но подтверждения квалификации нет. Если работодателю важно присутствие “корочек”, риск потерять вакантное место очень высокий.
Купить документ о получении высшего образования можно в нашем сервисе. Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов Российской Федерации. Вы получите необходимый диплом по любой специальности, включая документы образца СССР. Гарантируем, что при проверке документа работодателями, подозрений не возникнет.
Факторов, которые вынуждают приобрести диплом ВУЗа достаточно. Кому-то срочно нужна работа, а значит, нужно произвести впечатление на руководителя при собеседовании. Другие желают попасть в серьезную компанию, чтобы повысить свой статус в обществе и в будущем начать собственное дело. Чтобы не терять время, а сразу начать успешную карьеру, применяя врожденные способности и приобретенные знания, можно купить диплом в онлайне. Вы сможете быть полезным для социума, обретете финансовую стабильность в максимально короткие сроки- диплом о высшем образовании купить
микронаушник jasdam.cz
Привет!
Мы изготавливаем дипломы любых профессий по приятным ценам. Цена зависит от конкретной специальности, года получения и образовательного учреждения: diplomanrus.com/
The tiger symbols in Tiger Fortune are iconic. fortune tiger demo gratis
This online casino’s Tiger Fortune is a blast. fortune tiger 777
I recommend Tiger Fortune to anyone who loves slots. fortune tiger 777
Привет всем!
Ремонт под ключ — это комплексный подход к ремонту вашего дома. Мы выполняем ремонт под ключ, начиная с проектирования и заканчивая установкой всех элементов. Ремонт под ключ — это минимальные хлопоты и максимальное качество. строительство бань самары, москва ремонт диванов на дому, дом ремонт внутренний Ремонт под ключ — это идеальный вариант для вашего дома.
Больше информации на сайте – https://postroikado.ru/
ремонт холодильника бош на дому, строительство дома проекты, новые квартиры от застройщика в москве с отделкой
строительство дома и ремонт, отделка наружная дома имитацией бруса, ремонт стиральная машина на дому
Удачи!
Где купить диплом специалиста?
Мы можем предложить дипломы любой профессии по приятным ценам. Важно, чтобы документы были доступными для большого количества наших граждан. Приобрести диплом об образовании rusd-diplomj.ru/gde-kupit-diplom-s-reestrom-10/
Привет всем!
Помощь в сборе документов необходима для правильного оформления всех необходимых бумаг в различных правовых процессах. Составление жалобы поможет вам в случае нарушения ваших прав, и юрист грамотно подготовит жалобу в органы власти. Помощь при нарушении ПДД включает в себя защиту ваших прав в случае штрафов, аварий или других ситуаций на дороге. Автоюрист по возврату прав поможет вам восстановить водительские права, если они были аннулированы. Юридическая консультация онлайн даст вам возможность получить квалифицированную помощь по любому юридическому вопросу, не выходя из дома.
Больше информации на сайте – https://zashitapravi.ru
налогообложение доходов, доля по наследству в квартире после смерти без завещания, после регистрации ооо документы
стоимость вступления в наследство у нотариуса после смерти, что требуется для закрытия ип, гражданский брак и имущество
Удачи!
Tiger Fortune’s wins feel so satisfying. tigrinho demo
pin up azerbaijan pin up azerbaijan .
The tiger-themed bonuses in Tiger Fortune are epic. tigrinho demo
домашний интернет тарифы нижний новгород
domashij-internet-nizhnij-novgorod001.ru
провайдер интернета по адресу нижний новгород
I spent hours playing Tiger Fortune last night – no regrets! fortune tiger demo
Online casino bonuses are tricky—check the terms! mines
Tiger Fortune’s spins keep me on my toes. fortune tiger demo
Здравствуйте!
Легализация документов поможет вам пройти процесс официального признания документов в других странах. Помощь в приватизации недвижимости требует знания всех юридических аспектов этого процесса, и юрист поможет вам с оформлением. Составление брачного договора обеспечит защиту ваших интересов при вступлении в брак, особенно в вопросах раздела имущества. Юридическая помощь при лишении прав поможет вам восстановить водительские права, если они были лишены незаконно. Оформление опеки требует соблюдения всех юридических норм, чтобы обеспечить интересы ребенка и сторон, оформляющих опеку.
Больше информации на сайте – https://besturisty.ru
ипотека и развод, консультация юриста вологда бесплатная, что нужно для открытия расчетного счета для ип
в какую налоговую сдавать декларацию по налогу на имущество, процесс увольнения по статье, автоюрист по ОСАГО
Удачи!
I wish online casinos had more low-limit tables! plinko
Falschgeld kaufen Verkauf Euro https://maps.google.pl/url?q=https://falschgeldkaufen.org
Здравствуйте!
Оформление развода через ЗАГС — это один из самых быстрых и простых способов расторжения брака, если у вас нет несовершеннолетних детей и вы оба согласны на развод. Мы поможем вам правильно оформить все необходимые документы и подать заявление в ЗАГС. Консультация по разводу через ЗАГС также позволит вам избежать ошибок и ускорить процесс. Юридическая помощь при разводе через ЗАГС обеспечит вам уверенность, что процесс пройдет без задержек. Обратитесь за помощью для оформления развода без лишних трудностей.
Больше информации на сайте – https://besturisty.ru
помощь юриста по налогам, образец трудовой договор на главного бухгалтера, проклятие брачного договора скачать торрент сериал
где взять свидетельство о регистрации права собственности на квартиру государственной, при продаже квартиры и покупке налог, развод при беременности
Удачи!
This casino’s Tiger Fortune slot is a joy. fortune tiger demo
Online blackjack is tight—good mix! slot gallina
Tiger Fortune’s features are pure genius. fortune tiger demo gratis
Здравствуйте!
Налоги при сдаче квартиры в аренду — это важная тема для владельцев недвижимости, которые получают доход от аренды. Мы поможем вам разобраться в налоговых обязательствах и правильно оформлять декларации. Юридическая помощь по налогообложению при аренде квартиры обеспечит, что ваши доходы будут обложены налогом в соответствии с законодательством. Мы поможем вам минимизировать налоговые риски и избежать штрафов. Обратитесь к нам за помощью в решении вопросов налогообложения при сдаче квартиры в аренду.
Больше информации на сайте – https://zakonlw.ru
юрист по защите прав потребителей, комитет по защите прав потребителей саратов официальный сайт, суд защита телефона номер
отмена алиментов на ребенка по закону, где взять талон о регистрации ип в казахстане, юрист по семейным делам
Удачи!
ван вин https://1win6009.ru/ .
Доброго!
Помощь в написании жалобы необходима, если ваши права были нарушены, и юрист поможет составить жалобу и подать ее в нужные органы. Оформление регистрации требует соблюдения всех юридических норм, и юрист обеспечит правильность всех процедур. Юрист по военным вопросам поможет вам разобраться в правовых вопросах, связанных с военной службой, правами военнослужащих и их семьями. Консультация юриста по миграции поможет разобраться в вопросах получения гражданства и разрешений на работу в других странах. Юридическая помощь при депортации поможет вам оспорить решение о депортации и вернуть право на пребывание в стране.
Больше информации на сайте – https://konsultantok.ru/
составление искового заявления, росреестр заявление на регистрацию права собственности, не вернули деньги за возврат товара что делать
за лечение возврат налога документы, защита прав потребителей конституция, юридическая проверка документов
Удачи!
Доброго!
Налоги при продаже квартиры — это важный аспект, который необходимо учитывать, чтобы избежать налоговых последствий и дополнительных расходов. Мы поможем вам разобраться в законодательстве и минимизировать налоги при продаже недвижимости. Юридическая помощь при продаже квартиры поможет вам правильно оформить сделку и соблюсти все требования законодательства. Наша консультация по налогообложению поможет вам избежать штрафов и неуплаты налогов. Обратитесь к нам, чтобы получить поддержку по вопросам налогообложения при продаже недвижимости.
Больше информации на сайте – https://zashitapravi.ru
страховые споры, центр оформления дтп казань, как оформить договор по доверенности образец
синапс проверка контрагента по инн, помощь юриста при разводе, автоюрист по штрафам
Удачи!
Покупка документа о высшем образовании через надежную компанию дарит ряд преимуществ для покупателя. Такое решение дает возможность сберечь как продолжительное время, так и существенные финансовые средства. Однако, только на этом выгоды не ограничиваются, достоинств гораздо больше.Мы можем предложить дипломы психологов, юристов, экономистов и других профессий. Дипломы производят на настоящих бланках государственного образца. Доступная стоимость в сравнении с большими тратами на обучение и проживание. Заказ диплома о высшем образовании из российского университета является мудрым шагом.
Купить диплом: damdiplomisa.com/kupit-diplom-s-reestrom-bistro-i-udobno-onlajn-2/
Привет всем!
Возврат страховки по кредиту — это важный вопрос, в котором мы предоставим юридическую помощь. Оформление гражданства — процесс, который требует точности и правильных действий. Оформление дарственной на квартиру должно быть выполнено в полном соответствии с законодательством. Автоюрист по лишению прав подскажет, как правильно действовать в случае изъятия прав. Помощь с военным билетом — важная услуга для военнослужащих.
Больше информации на сайте – https://voproslaw.ru
помощь при разделе имущества, реестр лицензий на образовательную деятельность по инн, когда приходит налог на имущество
можно ли получить налоговый вычет за обучение ребенка обоим родителям, статья 18 закон о защите прав потребителей рф, сопровождение бизнеса
Удачи!
how to stop aging is it possible to become immortal
провайдеры интернета в нижнем новгороде по адресу
domashij-internet-nizhnij-novgorod002.ru
интернет провайдер нижний новгород
Где приобрести диплом по необходимой специальности?
Полученный диплом с приложением отвечает стандартам, никто не отличит его от оригинала – даже со специально предназначенным оборудованием. Не откладывайте свои мечты на долгие годы, реализуйте их с нами – отправьте быструю заявку на диплом прямо сейчас! Диплом о среднем образовании – не проблема! nepalijob.com/companies/premialnie-diplom-24
Обеспечь анонимность с amneziavpn.xyz/. Защита трафика, собственный сервер, простая установка и отсутствие слежки. Отличный выбор для тех, кто ценит свободу в интернете.
nanosluchatka mikrosluchatko
Где заказать диплом по актуальной специальности?
Мы предлагаем документы об окончании любых университетов Российской Федерации. Документы изготавливаются на настоящих бланках. Рєl4dzone.com/posting.phpmode=post&f=5
Ищете безопасный VPN? Попробуйте amnezia — open-source решение для анонимности и свободы в интернете. Полный контроль над соединением и личными данными.
1win. pro http://1win6043.ru/ .
I can’t stop playing Tiger Fortune – it’s addictive! fortune tiger
Заказать документ университета вы можете в нашей компании. Мы оказываем услуги по продаже документов об окончании любых университетов Российской Федерации. Вы получите диплом по любым специальностям, любого года выпуска, в том числе документы образца СССР. Даем 100% гарантию, что в случае проверки документа работодателем, каких-либо подозрений не появится. freediplom.com/kupit-ofitsialnij-diplom-s-zaneseniem-v-reestr-4/
Здравствуйте!
Юрист по недвижимости — это специалист, который поможет вам решить вопросы, связанные с покупкой, продажей, арендой или наследованием недвижимости. Мы обеспечим юридическое сопровождение сделок с недвижимостью, проверку документов и защиту ваших интересов. Наши специалисты помогут вам с регистрацией прав на имущество и подготовкой договоров купли-продажи, аренды или дарения. Юридическая помощь по недвижимости гарантирует, что все сделки будут безопасными и законными. Обратитесь к нам за консультацией по вопросам недвижимости и получите поддержку от профессионалов.
Больше информации на сайте – https://zakonlw.ru
юридическая консультация по кредитам, право вступления в наследство после смерти, составление бюджетной отчетности
юрист тюмень по недвижимости, фз о защите прав потребителей ст 20, услуги адвоката
Удачи!
Доброго!
Ремонт квартиры в Москве – это один из самых востребованных видов работ в столице. Мы предоставляем услуги по ремонту квартиры в Москве, гарантируя качественное выполнение всех работ в срок. Наша команда профессионалов готова выполнить любые виды ремонтов, от косметического до капитального. Мы используем только проверенные материалы, что обеспечивает долговечность результата. ремонт в доме время закон, фото отделку деревянного дома, вариант отделки квартиры Ремонт квартиры в Москве — ваш уютный дом в столице.
Больше информации на сайте – https://postroikado.ru
строительство бани бригада, цена отделки деревянных домов, строительства русской бани
ремонт домов в самаре, в москве отделка ремонт квартир, цена в москве отделка квартир
Удачи!
Tiger Fortune’s tiger symbols are so cool! tigrinho demo
1win site https://www.1win14.com.ng .
I’m obsessed with Tiger Fortune – so much fun! fortune tiger bet
скачать мостбет официальный сайт https://mostbet6033.ru .
Где приобрести диплом специалиста?
Мы предлагаеммаксимально быстро заказать диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями должностных лиц. Документ пройдет лубую проверку, даже с использованием специфических приборов. Решите свои задачи быстро с нашей компанией. Заказать диплом о высшем образовании! mus-ecole.maxbb.ru/viewtopic.php?f=14&t=2384
I wish online paid fast—slow drag! book of dead
I can’t stop playing Tiger Fortune – it’s addictive! fortune tiger 777
mikrosluchatko mikrocz.cz
This casino’s Tiger Fortune slot is a winner. jogo do tigrinho demo
Сантехник Юго-Восточный https://santekhnik-moskva.blogspot.com/p/south-eastern-administrative-okrug-of.html административный округ Москвы (ЮВАО). В состав Юго-Восточного административного округа входят районы: Выхино-Жулебино, Капотня, Кузьминки, Лефортово, Люблино, Марьино, Некрасовка, Нижегородский район, Печатники, Рязанский район, Текстильщики, Южнопортовый район.
реферат по физкультуре
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по невысоким тарифам.– diplom-onlinex.com/bezopasnaya-pokupka-diploma-bakalavra-s-zaneseniem-v-reestr/
Где купить диплом специалиста?
Мы предлагаем выгодно заказать диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями. Документ способен пройти лубую проверку, даже с использованием специальных приборов. Достигайте цели максимально быстро с нашей компанией.
Купить диплом любого ВУЗа diplomt-v-chelyabinske.ru/kupit-diplom-v-kurske-12/
The slot sounds online rock—pure fun! sugar rush
Чеки для отчётности t.me/kupitchekiru в Москве — быстро, конфиденциально и с гарантией. Подтверждающие документы для отчёта, бухгалтерии, авансовых отчётов. Оперативная подготовка и доставка.
проверить интернет по адресу
domashij-internet-nizhnij-novgorod003.ru
интернет домашний нижний новгород
Tiger Fortune makes every win feel huge. tigrinho demo
pin up az?rbaycan pin up az?rbaycan .
Доброго!
Если болит ухо, что делать, если ухо болит после переохлаждения? После переохлаждения ухо может заболеть из-за воспаления, называемого наружным отитом. Чтобы облегчить боль, можно использовать теплые компрессы и обезболивающие средства, такие как ибупрофен или парацетамол. Важно обратиться к врачу, который назначит правильное лечение, включая капли для ушей и антибиотики, если инфекция бактериальная. Чтобы предотвратить повторение проблемы, важно избегать воздействия холодного воздуха на уши и правильно ухаживать за слуховым аппаратом.
Больше информации на сайте – https://konsmediic.ru/
узлы на щитовидке, окунь ольга валерьевна эндокринолог, детский кардиолог калуга
кардиологи череповец, от влажного кашля народные средства, как быстро вылечить насморк
Удачи!
Tiger Fortune’s bonus rounds are thrilling. fortune tiger
детский спорт лагерь
1win регистрация 1win6044.ru .
Привет всем!
Когда болит ухо, что делать, если оно болит после долгого пребывания в шумной среде? Боль в ухе после пребывания в шумной среде может быть связана с повреждением слухового аппарата или переутомлением. В таких случаях важно обратиться к врачу для обследования. Врач проведет аудиометрическое исследование, чтобы оценить степень повреждения слуха. Также могут быть назначены средства для восстановления слуха и защиты от дальнейшего повреждения.
Больше информации на сайте – https://konsmediic.ru/
боли в груди перед месячными, пономаренко лариса николаевна лор отоларинголог, шипилов валентин петрович венеролог дерматолог отзывы
сколько зарабатывает врач педиатр, видео у гинеколога скрытая камера, Дерматолог
Удачи!
Tiger Fortune’s gameplay is fast and fierce. fortune tiger 777
Online casinos need hold—too wild! betonred
1win.com http://1win5012.ru/ .
Приобрести диплом о высшем образовании. Заказ документа о высшем образовании через надежную фирму дарит ряд достоинств для покупателя. Такое решение позволяет сэкономить время и существенные финансовые средства. sport-faq.ru/kupit-diplom-garantii-i-konfidentsialnost
This online casino’s Tiger Fortune slot is pure fun. fortune tiger 777
домашний интернет в новосибирске
domashij-internet-novosibirsk001.ru
интернет по адресу дома
This online casino’s Tiger Fortune is top-tier. tiger fortune
pinup azerbaycan pinup azerbaycan .
I love the vibrant colors in Tiger Fortune. fortune tiger
мрстбет http://mostbet6011.ru/ .
Привет всем!
Если вам необходимо оформить дарственную на авто, мы предоставим вам все необходимые консультации и помощь. Мы поможем правильно оформить дарственную, обеспечив юридическую безопасность сделки. Консультации по оформлению дарственной на авто позволят вам избежать юридических ошибок и рисков. Мы также предоставляем помощь в вопросах, связанных с регистрацией и передачей прав на транспортное средство. Получите помощь юриста по авто и оформлению дарственной.
Больше информации на сайте – https://konsultantok.ru
юридическое сопровождение кредитов, усн при закрытии ип когда сдавать, право на замену военной службы альтернативной гражданской службой какое право
подать объявление бесплатно о продаже недвижимости без регистрации, номер лицензии на образовательную деятельность, возврат переплаты по кредиту
Удачи!
Премиум скины CS2 https://cs2-case-simulator.ru топовые облики для AWP, AK-47, M4, ножей и перчаток. Яркий стиль, редкие коллекции, эксклюзивные дизайны. Укрась свою игру и выделяйся в каждой катке!
Приобрести диплом о высшем образовании!
Мы предлагаем дипломы психологов, юристов, экономистов и других профессий по выгодным тарифам— vuz-diplom.ru/kupite-diplom-v-ekaterinburge-bistro-i-nadezhno/
1 ван вин 1 ван вин .
Хочешь Dragon Lore https://cs-case-simulator.ru Открывай кейс прямо сейчас — шанс на легенду CS2 может стать реальностью. Лучшие скины ждут тебя, рискни и получи топовый дроп!
Online casinos need more addiction alerts. juego mines
buy viagra https://buyviagrat.com/ .
The visuals in Tiger Fortune are breathtaking. fortune tiger
Glock-18 Fade case-simulator-csgo.ru один из самых ярких и редких скинов для стартового пистолета в CS2. Плавный градиент, премиум-качество и высокий спрос среди коллекционеров.
This online casino hit the mark with Tiger Fortune. tiger fortune
купить диплом мисис
Доброго!
Если болит ухо, что делать, если болит ухо после простуды? Боль в ухе после простуды может быть признаком отита, который развивается как осложнение после вирусной инфекции. Необходимо обратиться к врачу, чтобы получить правильное лечение. Обычно лечение включает противовоспалительные препараты и, если необходимо, антибиотики. Важно следить за симптомами и не допустить развития осложнений, таких как потеря слуха. Для облегчения боли могут быть назначены капли для ушей и жаропонижающие средства.
Больше информации на сайте – https://ovrachahl.ru/
как подготовиться к приему гинеколога, красногорск кардиолог, онколог барнаул
голда галина александровна гинеколог отзывы, тула врач гинеколог, опухла лимфоузел на шее
Удачи!
The free spins in Tiger Fortune are awesome! fortune tiger
best over the counter ed pills that work fast Pill guide available. Find medication info. best ed treatment
StatTrak AK-47 Vulcan get skin cs2 мощный стиль и счётчик убийств в одном скине. Агрессивный дизайн, сине-чёрная цветовая гамма и высокая ценность на рынке CS2. Идеальный выбор для бойца с характером!
register with 1win website https://1win15.com.ng .
The tiger-themed bonuses in Tiger Fortune are epic. fortune tiger demo
M4A4 Emperor https://get-skin-cs.ru эффектный скин в стиле королевской власти. Яркий синий фон, золотые детали и образ императора делают этот скин настоящим украшением инвентаря в CS2.
Мы предлагаем дипломы любой профессии по приятным тарифам. Мы готовы предложить документы техникумов, расположенных в любом регионе РФ. Документы печатаются на бумаге высшего качества. Это дает возможности делать государственные дипломы, не отличимые от оригинала. prosafely.com/read-blog/21871_kupit-diplom-s-zaneseniem-v-reestr-cena.html
UID_97884358###
test
UID_55404481###
test
металлочерепица В мире современного строительства выбор материалов играет ключевую роль в долговечности и эстетическом облике зданий. Компания “СтройКомплекс” предлагает широкий ассортимент кровельных и фасадных материалов, отвечающих самым высоким стандартам качества. Для обустройства кровли мы предлагаем: металлочерепицу, известную своей прочностью и долговечностью; профлист и профнастил, оптимальные для промышленных и коммерческих объектов; мягкую и гибкую черепицу, идеальные для частного домостроения, а также элегантную фальцевую кровлю.
Мы изготавливаем дипломы любых профессий по приятным ценам. Дипломы изготавливаются на оригинальных бланках государственного образца Купить диплом об образовании diplomj-irkutsk.ru
Где заказать диплом по актуальной специальности?
Купить диплом университета по выгодной стоимости можно, обратившись к проверенной специализированной фирме.: nsk-diplom.com
Online casinos need gear—aid us! plinko
AK-47 Case Hardened http://get-skins-cs2.ru классика CS2 с уникальным закалённым узором. Каждый скин отличается: от редких фулл-блю до золотых комбинаций. Настоящая находка для коллекционера и трейдера.
какие провайдеры по адресу
domashij-internet-novosibirsk002.ru
интернет домашний новосибирск
This online casino’s Tiger Fortune is a blast. fortune tiger 777
Заказать диплом ВУЗа по доступной стоимости вы можете, обратившись к надежной специализированной компании. Приобрести документ ВУЗа можно в нашем сервисе. asxdiploman.com/kupit-diplom-visshego-obrazovaniya-s-zaneseniem-v-reestr-34
Где купить диплом по необходимой специальности?
Наша компания предлагает выгодно приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Диплом способен пройти любые проверки, даже с применением профессиональных приборов. Достигайте цели быстро и просто с нашими дипломами.
Купить диплом любого университета diplomgorkiy.com/kupit-diplom-logopeda-5/
înregistrare 1win http://www.1win5012.ru .
pin up azerbaijan pin up azerbaijan .
mostbet mostbet6011.ru .
1вин rossvya http://www.1win6009.ru .
1хwin 1хwin .
Займы онлайн на карту без отказа без проверки моментальный займ на карту без проверок круглосуточно
Официальный сайт микрозаймов займы официальный сайт
Tiger Fortune’s spins are wild and unpredictable. fortune tiger demo
click here to find out more https://cwap.us/
pin up az?rbaycan pin up az?rbaycan .
Запишитесь на семинары для косметологов и прокачайте свою профессию. Современные методики, опытные преподаватели, доступная цена и максимальная польза для практики.
Приобрести диплом можно через официальный портал компании. poboltaem.foroactivo.com/t5567-topic#13787
Tiger Fortune’s design is top-notch – really impressive. fortune tiger demo
купить диплом с проводкой нижний новгород
visit this site https://noon.lat
Tiger Fortune’s bonuses are wild and exciting. tiger fortune
site https://cwap.us
Здравствуйте!
Если болит ухо, что делать, если ощущается давление внутри уха? Ощущение давления в ухе может быть связано с воспалением или накоплением жидкости в среднем ухе. Важно обратиться к врачу, чтобы провести диагностику и определить причину давления. Врач может назначить капли для уха или лекарства для снятия воспаления. Также могут быть рекомендованы процедуры, такие как промывание уха, для устранения жидкости. Своевременное лечение поможет вернуть нормальное состояние слуха и устранить неприятные ощущения.
Больше информации на сайте – https://prostudailor.ru/
симптомы рака кишечника, григорян феликс михайлович гинеколог акушер отзывы, сяо лор
дерматолог октябрьское поле, эндокринолог рудакова лариса владимировна отзывы, инфаркт признаки у женщин
Удачи!
Здравствуйте!
Когда болит ухо, что делать, если оно чувствительно к прикосновениям? Чувствительность уха, которая усиливается при прикосновении, может быть признаком воспаления в ушном проходе или отита. В таких случаях необходимо обратиться к врачу для диагностики и назначения лечения. Врач может назначить специальные капли для уха или антибиотики, если причина в инфекции. Для облегчения боли могут быть рекомендованы обезболивающие средства. Чем быстрее начнется лечение, тем быстрее вы избавитесь от неприятных симптомов.
Больше информации на сайте – https://medikasv.ru/
панические атаки и лечение, как восстановить волосы после выпадения у женщин, березники дерматолог
кардиологи липецк, демина марина анатольевна эндокринолог, температура у ребенка 39
Удачи!
Заказать диплом об образовании. Приобретение подходящего диплома через надежную фирму дарит ряд плюсов для покупателя. Такое решение помогает сберечь как продолжительное время, так и значительные средства. kizm.flybb.ru/viewtopic.php?f=34&t=1409
сайт 1win https://1win6043.ru .
подключение интернета новосибирск
domashij-internet-novosibirsk003.ru
интернет провайдеры по адресу дома
read the full info here https://unchainx.net
Доброго!
Домов для строительства — выбор подходящего участка и дома важен для комфортного проживания. Мы предлагаем разнообразные проекты домов для строительства, включая каркасные, кирпичные и деревянные варианты. Мы поможем подобрать проект, который идеально подойдет под ваш участок и потребности. Наши специалисты всегда учитывают экологические требования и местные строительные нормы. отделка дом под кирпич, купить квартиру новостройку с отделкой в воронеже, строительство дом на сваях Строительство дома — это не только сложный, но и увлекательный процесс, который будет завершен качественно и в срок.
Больше информации на сайте – https://nhadian123.com
строительство турецкой бани под ключ, баня строительство каркасная, дом ремонт калининград
красивые ремонты квартиры, панели для отделки дома потолки, строительства бани из газобетона
Удачи!
Tiger Fortune’s bonuses keep me spinning. fortune tiger bet
Приобрести диплом любого института!
Мы предлагаем дипломы психологов, юристов, экономистов и любых других профессий по приятным ценам— diplom-onlinex.com/meditsinskij-diplom-kupit-v-moskve/
I can’t stop playing Tiger Fortune – it’s addictive! jogo do tigrinho demo
Привет всем!
Если болит ухо, что делать, если в нем появляется зуд и жжение? Зуд и жжение в ухе могут быть связаны с аллергической реакцией или инфекцией. Важно не терять времени и обратиться к врачу, чтобы выяснить причину. Если это аллергия, врач может назначить антигистаминные препараты. В случае инфекции потребуется курс лечения антибиотиками или другими средствами. Быстрое вмешательство поможет избежать ухудшения состояния.
Больше информации на сайте – https://onlinvrach.ru/
боли при половом акте причины, эндокринолог белоног светлана ибрагимовна, зеленые выделения без запаха и зуда у женщин
боль при глотании в ухе, милявская ирина романовна дерматолог, Дерматолог
Удачи!
Tiger Fortune’s design is top-notch – really impressive. fortune tiger 777
This online casino’s Tiger Fortune is awesome. tigrinho demo
Online bingo is cool—nice group! plinko
Добрый день!
Если болит ухо, что делать, если боль не проходит в течение нескольких дней? Если боль в ухе не проходит в течение нескольких дней, это может указывать на хроническое воспаление или инфекцию, требующую специализированного лечения. Важно обратиться к врачу, чтобы исключить осложнения, такие как перфорация барабанной перепонки или хронический отит. Лечение может включать антибактериальные препараты, капли для уха и физиотерапевтические процедуры для улучшения кровообращения в области уха. Врач также может порекомендовать дополнительные методы лечения, такие как промывание уха или удаление серы. Своевременное обращение к специалисту поможет избежать потери слуха и других осложнений.
Больше информации на сайте – https://ovrachahl.ru
как распознать рак на ранней стадии, гастроэнтеролог ивантеевка, вызвать лора ребенку на дом
феодосия лор, альфа липоевая кислота отзывы врачей, как попасть к узкому специалисту
Удачи!
The visuals online wow—great sight! aviator game
Hey there! If you’re reading this, chances are you’re looking for a way to boost your website’s visibility in Google. Let’s face it — navigating SEO can be daunting, especially with so many “quick-fix” services out there claiming guaranteed results that rarely materialize. That’s why I want to share my approach with you. It’s not just another generic solution — it’s a custom-tailored plan designed to deliver tangible growth.
I specialize in building link pyramids that combine Tier 1, Tier 2, and Tier 3 backlinks. Think of it like constructing a strong foundation for your house. Without proper groundwork, progress collapses. My goal is to drive authority straight to your money site in a way that aligns with search engine algorithms and delivers results.
What Makes Me Different?
Let me be honest here: I’ve been in the SEO game for years, and I’ve witnessed every trend — ethical practices and black-hat tactics. I’ve worked with clients who wasted hundreds (sometimes thousands) on services that vowed top positions but ended up getting them penalized. That’s why I decided to redefine the process.
I focus on authoritative dofollow links from reputable sources. The majority of top-layer backlinks in my strategy are dofollow because they offer maximum SEO value. And here’s the clincher — you’ll get this premium service at significantly reduced rates (we’re talking 200–350forthesamepackage?pricesstartaslowas200). What’s the point of overpaying? Invest wisely in something that actually works.
My Unique Value Proposition
Elite Guest Blogging
I avoid low-quality directories. Never. I curate only the top-tier platforms — platforms with strong domain authority (DA). These are the kinds of sites that give your website a serious boost.
Tailored Copywriting
Let’s face it — spun or generic content sticks out like a sore thumb. It’s unengaging and counterproductive. That’s why every post I create is original, engaging, and tailored to your niche. Whether it’s for Web 2.0 properties, I guarantee originality reports that enhances your brand’s voice. No shortcuts, no fluff — just high-quality writing that gets results.
Pyramid-Structured Backlinks
Here’s where things get powerful. My method builds depth by incorporating Tier 2 and Tier 3 support links. This multi-phase strategy multiplies the impact of your core links, driving long-term success for your money site. Picture compounding momentum, growing exponentially.
Diverse Link Sources
Google loves diversity, so I incorporate diverse high-authority sources: content hubs, document shares, social signals. This isn’t about cheating algorithms; it’s about building something that lasts.
No Hidden Agendas
When you work with me, you’ll have full visibility. I provide a comprehensive breakdown, including full administrative rights. Full disclosure at every step. You’ll track every backlink source and their impact on rankings.
A Little Story from My Experience
Let me share something personal: A few years ago, I worked with a client who was struggling to gain traction despite months of effort. They’d tried multiple agencies, but results were elusive. When they came to me, I crafted a custom plan. A personalized approach was implemented. In 60 days, organic visits surged, and they started seeing real conversions. That’s the kind of success I strive to deliver with every client.
Time to transform your rankings! Let’s connect and turn search engines into your ally. ??
The graphics in Tiger Fortune are absolutely stunning. jogo do tigrinho demo
pinup azerbaycan pinup azerbaycan .
Хочу рассказать о своем опыте по заказу аттестата пту, думал это не реально и стал искать информацию в сети, про где купить диплом в оренбурге, где купить диплом о высшем образовании цена, где купить диплом с реестром, где купить диплом среднем во владивостоке, диплом о высшем образовании 1996 года купить, постепенно вникая в суть дела нашел отличный материал здесь diplomybox.com/kupit-diplom-tekhnikuma-kolledzha-v-orle)
This casino’s Tiger Fortune slot is epic. fortune tiger demo
Купить диплом любого университета!
Заказать диплом института по выгодной цене вы можете, обращаясь к проверенной специализированной компании. Приобрести диплом: diplomk-vo-vladivostoke.ru/priobresti-diplom-originalnij-dokument-bistro
Здравствуйте!
Если болит ухо, что делать, если ухо болит при простуде и кашле у ребенка? У детей при простуде и кашле часто возникают проблемы с ушами, такие как отит или воспаление евстахиевой трубы. В таком случае следует обратиться к врачу для назначения соответствующего лечения. Врач может порекомендовать противовоспалительные препараты, капли для ушей и средства для снижения температуры. Чтобы облегчить состояние ребенка, можно использовать теплый компресс на ухо. Если симптомы не проходят или ухудшаются, необходимо немедленно пройти обследование.
Больше информации на сайте – https://medikasv.ru/
врачи на дом срочно, гастроэнтерологи чебоксары, пропанорм или этацизин что лучше отзывы кардиологов
симптомы при пневмонии у взрослых без температуры без кашля какие, гастроэнтеролог одинцово взрослый, глаз дергается причины
Удачи!
Добрый день!
Когда болит ухо, что делать, если боль сопровождается отеком за ухом? Отек за ухом, сопровождающийся болью, может быть признаком воспаления лимфатических узлов или инфекции в области уха. В таком случае необходимо обратиться к врачу, чтобы провести диагностику и начать лечение. Врач может назначить антибиотики для борьбы с инфекцией, а также противовоспалительные препараты для уменьшения отека. Лечение должно быть направлено на устранение воспаления и восстановление нормального состояния. Своевременная медицинская помощь поможет избежать осложнений и ускорить выздоровление.
Больше информации на сайте – https://vrachataj.ru
как понять что гормоны не в норме, гастроэнтерологи тула, каплина ольга юрьевна гемостазиолог гинеколог
клименко ксения эльдаровна лор отоларинголог отзывы, лор это оториноларинголог или нет, мигрень и светобоязнь
Удачи!
The slot sounds online are pure fun—love it! dragon tiger
nerve calm is a high-quality nutritional supplement crafted to promote nerve wellness, ease chronic discomfort, and boost everyday vitality.
1win букмекер http://1win6009.ru/ .
проверить провайдера по адресу
domashij-internet-spb001.ru
подключение интернета по адресу
мостбет скачать на андроид https://mostbet6012.ru .
Добрый день!
Если болит ухо, что делать, если ухо болит и появилась заложенность? Заложенность в ухе, сопровождающая болью, может быть признаком воспаления евстахиевой трубы или среднего уха. В таких случаях важно обратиться к врачу, чтобы он мог назначить соответствующее лечение, которое может включать капли для ушей или противовоспалительные препараты. Также могут быть рекомендованы средства для снятия отека и улучшения проходимости евстахиевой трубы. Важно избегать переохлаждения и перепадов давления, чтобы не усугубить ситуацию. Если симптомы не проходят или ухудшаются, следует срочно обратиться за медицинской помощью.
Больше информации на сайте – https://prostudailor.ru/
бессонница причины, генезис гинеколог симферополь, эндокринолог илья магеря
аденоиды у ребенка симптомы в носу, лучшие дерматологи оренбурга, не дышит одна ноздря
Удачи!
Мы можем предложить дипломы любой профессии по доступным тарифам. Мы предлагаем документы техникумов, которые находятся в любом регионе РФ. Дипломы и аттестаты печатаются на “правильной” бумаге высшего качества. Это дает возможность делать государственные дипломы, которые не отличить от оригинала. xtrainkom.com/employer/archive-diploma
Привет всем!
Если болит ухо, что делать, если боль при разговоре становится сильнее? Боль в ухе, которая усиливается при разговоре, может быть связана с воспалением среднего уха или с заболеванием височно-нижнечелюстного сустава. Важно обратиться к врачу, чтобы получить точный диагноз и эффективное лечение. Врач может назначить обезболивающие и противовоспалительные препараты, а также капли для уха, чтобы уменьшить воспаление. Также могут быть рекомендованы процедуры для улучшения кровообращения и снятия напряжения в области сустава. Своевременное лечение поможет облегчить боль и восстановить здоровье.
Больше информации на сайте – https://onlinvrach.ru
температура после прививки, консультация гинеколог, репкина наталья николаевна офтальмолог окулист
лор врач подольск, у ребенка температура понос и кашель, боли за грудиной при стрессе
Удачи!
mostbet casino mostbet6012.ru .
Привет всем!
Если болит ухо, что делать, если у ребенка болит ухо после купания? Если у ребенка болит ухо после купания, это может быть признаком попадания воды в ухо или воспаления наружного слухового прохода. Чтобы облегчить боль, можно аккуратно высушить ухо и использовать капли для ушей, которые помогают уменьшить воспаление. Важно, чтобы ребенок не пытался самостоятельно чистить ухо ватными палочками, так как это может привести к повреждению слухового прохода. Если боль не исчезает, необходимо обратиться к врачу, который назначит лечение. Врач также может порекомендовать профилактические средства для защиты ушей от воды в будущем.
Больше информации на сайте – https://vrachataj.ru
судороги у ребенка во сне, саранск новомед эндокринолог, анализ на гормоны щитовидной железы как называется
врач гинеколог, лучший гинеколог воронеж, синусит симптомы и лечение
Удачи!
1win http://www.1win6008.ru .
1win live http://1win6043.ru/ .
1wi 1wi .
Playing Tiger Fortune feels like a wild adventure every time. fortune tiger bet
Приобрести диплом о высшем образовании!
Мы готовы предложить документы институтов, расположенных на территории всей Российской Федерации.
asxdiploman.com/kupit-diplom-o-srednem-obrazovanii-bistro-i-nadezhno-7/
купить диплом администратора diplomys-vsem.ru .
1win kg скачать 1win kg скачать .
Hidden Link Placement in Established Content Hubs
Hey there! If you’re reading this, chances are you’re looking for a way to boost your website’s visibility in Google. I understand — mastering SEO might seem intimidating, especially with so many “miracle” solutions out there promising the moon but delivering… well, not much. That’s why I want to share my strategy with you. It’s not just another cookie-cutter service — it’s a custom-tailored plan designed to deliver real, measurable results.
I specialize in building link pyramids that combine Tier 1, Tier 2, and Tier 3 backlinks. Think of it like constructing a strong foundation for your house. A weak foundation leads to instability. My goal is to boost your primary domain’s credibility in a way that aligns with search engine algorithms and delivers results.
What Makes Me Different?
I’ll cut to the chase: I’ve been in the digital marketing field for years, and I’ve seen it all — successful strategies and outright scams. I’ve worked with clients who invested heavily on services that claimed foolproof results but caused ranking drops. That’s why I decided to redefine the process.
I focus on high-quality, dofollow backlinks from trusted domains. More than four-fifths of primary-tier connections in my strategy are dofollow because they offer maximum SEO value. And here’s the clincher — you’ll get this premium service at significantly reduced rates (we’re talking 200–350forthesamepackage?pricesstartaslowas200). What’s the point of overpaying? Invest wisely in something that delivers real ROI.
The Secret Sauce
Elite Guest Blogging
No generic outreach here. Absolutely not. I curate only the most reputable guest post sites — platforms with strong domain authority (DA). These are the kinds of sites that give your website a serious boost.
Tailored Copywriting
Let’s face it — spun or generic content sticks out like a sore thumb. It’s boring, unnatural, and frankly, a waste of time. That’s why every article I create is 100% unique, engaging, and tailored to your niche. Whether it’s for Web 2.0 properties, I guarantee originality reports that enhances your brand’s voice. No shortcuts, no fluff — just high-quality writing that gets results.
Pyramid-Structured Backlinks
Here’s where things get powerful. My method doesn’t stop at Tier 1 by incorporating mid-level and foundational backlinks. This multi-phase strategy strengthens the effect of your core links, driving steady progress for your money site. Imagine an avalanche effect, compounding over time.
Varied Backlink Portfolio
Google loves diversity, so I incorporate diverse high-authority sources: content hubs, document shares, social signals. This isn’t about cheating algorithms; it’s about establishing long-term authority.
No Hidden Agendas
When you work with me, you’ll have full visibility. I provide a thorough documentation, including access credentials. Full disclosure at every step. You’ll track every backlink source and their role in your success.
Real-World Proof
A case study: A few years ago, I worked with a client who was struggling to gain traction despite months of effort. They’d tried various “experts”, but nothing seemed to work. When they came to me, I crafted a custom plan. Together, we built a custom link pyramid tailored to their niche. In 60 days, organic visits surged, and they achieved measurable sales growth. That’s the kind of outcome you can expect with every client.
Time to transform your rankings! Let’s connect and let’s make Google work for you. ??
Tiger Fortune’s payouts are always exciting. fortune tiger demo gratis
провайдеры по адресу дома
domashij-internet-spb002.ru
провайдеры интернета в санкт-петербурге по адресу
pinup azerbaycan pinup azerbaycan .
Tiger Fortune’s features are super fun. fortune tiger bet
Здравствуйте!
Ремонт кухни с установкой новой плиты — это улучшение функциональности и удобства вашей кухни. Мы выполняем ремонт кухни с установкой новой плиты, чтобы вам было комфортно готовить. Ремонт кухни с установкой новой плиты — это обновление вашего кухонного пространства. витебск ремонт на дому, мастера на дом по ремонту стиральных машин, бани проекты строительство цены Ремонт кухни с установкой новой плиты — это удобство и стиль для вашего дома.
Больше информации на сайте – https://nhadian123.com
цена квартир с отделкой, подмосковье под строительство дома, ремонт в ванной дом
мастер по ремонту квартир, ремонты квартир панельных квартир, ремонт домов элитный
Удачи!
mostbet официальный сайт mostbet6033.ru .
The poker online glows—real rush! aviator
This casino’s Tiger Fortune slot is a winner. fortune tiger demo gratis
420 day in prague thc vape for sale in prague
Мы изготавливаем дипломы любых профессий по приятным тарифам. Всегда стараемся поддерживать для клиентов адекватную политику тарифов. Важно, чтобы дипломы были доступными для большинства граждан.
Заказ диплома, подтверждающего окончание ВУЗа, – это выгодное решение. Приобрести диплом о высшем образовании: kupit-diplomyz24.com/diplom-visshee-kupit-3/
Online casinos can be dangerous if you don’t set limits! plinko
Tiger Fortune’s interface is so user-friendly. fortune tiger bet
Отец и сын три года объедали сотню ресторанов по хитроумной схеме
https://pin.it/kCDDdtZJq
провайдеры интернета в санкт-петербурге
domashij-internet-spb003.ru
недорогой интернет санкт-петербург
I spent hours playing Tiger Fortune last night – no regrets! fortune tiger demo
pin up azerbaycan pin up azerbaycan .
Desert Eagle Blaze csgo-get-skins пламя в каждой пуле. Легенда CS:GO. Продажа, обмен, проверка скина. Успей забрать по лучшей цене на рынке.
В этой статье собраны факты, которые освещают целый ряд важных вопросов. Мы стремимся предложить читателям четкую, достоверную информацию, которая поможет сформировать собственное мнение и лучше понять сложные аспекты рассматриваемой темы.
Изучить вопрос глубже – https://mednarkoforum.ru/
мостбет скачать бесплатно mostbet6034.ru .
Купить диплом о высшем образовании!
Мы готовы предложить документы любых учебных заведений, расположенных на территории всей России.
asxdiplommy.com/kupit-diplom-o-visshem-obrazovanii-po-dostupnoj-tsene/
Tiger Fortune has the best energy of any game. fortune tiger demo
M4A1-S Knight http://cs-get-skins.ru редкий скин в CS. Престиж, стиль, легендарное оружие. Продажа по выгодной цене, моментальная доставка, безопасная сделка.
Online casinos should cap—safe guard! minas juego
1вин вход 1вин вход .
1win code bonus 1win1003.top .
I love the jungle energy of Tiger Fortune. fortune tiger
1win-də təqdim olunan bonuslar çox cəlbedicidir | 1win az saytında müxtəlif turnirlərdə iştirak edin | 1win platformasında təhlükəsiz və sürətli qeydiyyat prosesi mövcuddur | 1win platformasında istifadəçilər üçün müxtəlif bonuslar təqdim olunur1win kazino oyunlarında yüksək qazanma şansı əldə edin | 1win-də müxtəlif ödəniş üsulları ilə rahatlıqla pul yatırın1win mobil tətbiqi ilə istənilən yerdə oyun oynayın | 1win-də müxtəlif idman tədbirlərinə mərc edin | 1win azərbaycan platformasında istifadəçilər üçün müxtəlif oyun imkanları mövcuddur | 1win mobil tətbiqi ilə oyun təcrübəsini artırın | 1win platformasında istifadəçilər üçün müxtəlif təkliflər mövcuddur 1win azerbaycan.
1вин вход с компьютера https://1win6045.ru/ .
Заказать диплом о высшем образовании можем помочь. Купить диплом специалиста в Барнауле – diplomybox.com/kupit-diplom-spetsialista-v-barnaule
mostbest http://mostbet6033.ru .
1 win http://1win6010.ru .
Tiger Fortune’s features are a total win. jogo do tigrinho demo
Tiger Fortune keeps the good times rolling! jogo do tigrinho demo
Мы изготавливаем дипломы любой профессии по невысоким ценам. Купить диплом в Амурской области и городе Благовещенск — kyc-diplom.com/geography/blagoveshchensk.html
Гигантская черная дыра пробудилась, заявили ученые
https://x.com/kiselev_igr/status/1910669547713003851
1win pariuri http://1win5013.ru .
I hit a jackpot on Tiger Fortune last week – unbelievable! tigrinho demo
Заказать диплом о высшем образовании !
Приобретение диплома университета России у нас является надежным процессом, поскольку документ заносится в реестр. Заказать диплом любого института diplomt-nsk.ru/kupit-nastoyashij-diplom-o-visshem-obrazovanii-bistro-i-legko
Tiger Fortune’s gameplay is smooth and fast. jogo do tigrinho demo
The mobile apps online slip—fix up! fortune mouse
красный аттестат купить обложку
ai generated porn ai porn
заложить часы в ломбард москва дорого заложить часы в ломбард москва дорого .
Заказать диплом о высшем образовании. Покупка официального диплома через качественную и надежную фирму дарит немало плюсов. Данное решение позволяет сэкономить как личное время, так и значительные средства. topfunservice.copiny.com/question/details/id/1083782
topsmile topsmile .
I can’t get enough of the Tiger Fortune soundtrack! jogo do tigrinho demo
Используй нвдежный https://amnezia.xyz для обхода блокировок, сохранности личных данных и полной свободы онлайн. Всё включено — просто подключайся.
Где купить диплом по нужной специальности?
Мы предлагаем максимально быстро заказать диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями должностных лиц. Диплом способен пройти любые проверки, даже при помощи специально предназначенного оборудования. Решите свои задачи быстро с нашей компанией.
Заказать диплом любого ВУЗа damdiplomisa.com/kupit-diplom-v-kemerovo-5/
Оснащение под ключ: медицинское оборудование с сертификацией и полной документацией. В наличии и под заказ. Профессиональное оснащение медицинских кабинетов.
согласование перепланировки москва согласование перепланировки москва .
Приобрести диплом о высшем образовании!
Мы готовы предложить документы учебных заведений, расположенных на территории всей России. Дипломы и аттестаты делаются на бумаге самого высокого качества: izumrudnoeozero.listbb.ru/viewtopic.phpf=8&t=1419
1win прямой эфир [url=http://1win6045.ru/]http://1win6045.ru/[/url] .
Tiger Fortune’s gameplay is fast and fierce. jogo do tigrinho demo
The sound design in Tiger Fortune is amazing. tiger fortune
бк 1win https://1win6044.ru/ .
болливуд казино
Технологии свободы: https://marzban.click надёжный инструмент для приватности, скорости и доступа к любым сайтам. Быстро, безопасно, удобно — настрой за 5 минут.
The online bonuses call—step in! hot hot fruit
Витебский госуниверситет университет https://vsu.by П.М.Машерова – образовательный центр. Вуз является ведущим образовательным, научным и культурным центром Витебской области. ВГУ осуществляет подготовку: химия, биология, история, физика, программирование, педагогика, психология, математика.
you can try here Lookup bot
Приобрести диплом ВУЗа!
Мы изготавливаем дипломы любой профессии по приятным тарифам. Вы покупаете документ через надежную и проверенную временем фирму. : sev-school24.maxbb.ru/viewtopic.php?f=3&t=1114
1win bet deposit http://1win15.com.ng .
click over here now Card
либет казино
1win бк https://1win6046.ru/ .
где купить чеки купить чеки
мостбет скачать андроид мостбет скачать андроид .
Tiger Fortune makes every spin an adventure. jogo do tigrinho demo
Научитесь вязать крючком https://crochet-patterns.ru/ с нуля или улучшите навыки с нашими подробными мастер-классами. Фото- и видеоуроки, понятные инструкции, схемы для одежды, игрушек и интерьера. Вдохновляйтесь, творите, вяжите в своё удовольствие! Вязание крючком — доступно, красиво, уютно.
Go Here install jaxx liberty wallet
I won big last night playing Tiger Fortune – what a thrill! tigrinho demo
Нож-бабочка Doppler cs-get-skin.ru/ стильное и эффектное оружие в стиле CS:GO. Яркий металлический блеск, плавный механизм, удобство в флиппинге и коллекционировании. Подходит для тренировок, трюков и подарка фанатам игр.
I can play Tiger Fortune all day and not get bored. tigrinho demo
скачать мостбет https://mostbet6033.ru/ .
Мы изготавливаем дипломы любой профессии по приятным ценам. Мы можем предложить документы ВУЗов, которые находятся в любом регионе России. Документы выпускаются на “правильной” бумаге высшего качества. Это дает возможности делать настоящие дипломы, не отличимые от оригиналов. cvgods.com/employer/archive-diploma
Мы изготавливаем дипломы любых профессий по приятным тарифам. Всегда стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества наших граждан.
Приобретение диплома, подтверждающего обучение в университете, – это выгодное решение. Купить диплом университета: peoplediplom.ru/kupit-diplom-inzhenera-stroitelya-7/
georgina six http://twiggycafe.com/ .
Мы готовы предложить дипломы любых профессий по доступным ценам. Дипломы производятся на подлинных бланках государственного образца Заказать диплом о высшем образовании kupitediplom.ru
Hidden Link Placement in Established Content Hubs
Hello! If this message caught your attention, you’re probably seeking strategies to enhance your online presence. I get it — SEO can feel overwhelming, especially with so many “miracle” solutions out there vowing overnight success without real outcomes. That’s why I want to share my method with you. It’s not just another one-size-fits-all package — it’s a custom-tailored plan designed to deliver real, measurable results.
I specialize in building link pyramids that combine Tier 1, Tier 2, and Tier 3 backlinks. Imagine building a sturdy base for a skyscraper. Without a solid base, everything else crumbles. My goal is to boost your primary domain’s credibility in a way that aligns with search engine algorithms and proves effective.
Why Should You Trust Me?
Let me be honest here: I’ve been in the search engine optimization industry for years, and I’ve witnessed every trend — successful strategies and outright scams. I’ve worked with clients who wasted hundreds (sometimes thousands) on services that claimed foolproof results but resulted in search engine bans. That’s why I decided to do things differently.
I focus on authoritative dofollow links from authoritative websites. The majority of top-layer backlinks in my strategy are dofollow because they pack the biggest punch. And here’s the catch — you’ll get this top-tier offering at discounted pricing (costing just 200–350 USD). Why pay more for less? Save your hard-earned cash ?? in something that delivers real ROI.
What Makes My Approach Different?
Premium Content Placement
I don’t just throw your links on any random blog. Absolutely not. I carefully select only the top-tier platforms — websites Google prioritizes. These are the kinds of sites that give your website a serious boost.
Original, Custom-Crafted Articles
AI-generated text is easily spotted. It’s boring, unnatural, and frankly, a waste of time. That’s why every piece I create is original, compelling, and specific to your market. Whether it’s for content hubs, I guarantee plagiarism-free results that blends seamlessly into your campaign. No shortcuts, no fluff — just high-quality writing that gets results.
Pyramid-Structured Backlinks
Here’s where things get strategic. My method builds depth by incorporating mid-level and foundational backlinks. This structured system multiplies the impact of your primary backlinks, driving steady progress for your money site. Imagine an avalanche effect, compounding over time.
Varied Backlink Portfolio
Search engines reward variety, so I leverage multiple high-authority sources: media channels, directories, and niche forums. This isn’t about exploiting loopholes; it’s about building something that lasts.
Full Transparency
When you work with me, you’ll see every detail. I provide a detailed link report, including login details for all Web 2.0 properties. Clear communication, no surprises. You’ll know exactly where your links are coming from and their role in your success.
A Little Story from My Experience
Let me share something personal: A few years ago, I worked with a client who was struggling to gain traction despite countless strategies. They’d tried several services, but results were elusive. When they came to me, I analyzed their unique needs. We designed a strategy aligned with their industry. Within a few months, their traffic doubled, and they boosted lead generation. That’s the kind of outcome you can expect with every client.
Ready to elevate your SEO game? Let’s connect and start your journey to #1. ??
hidden-link placement in established content hubs 5c00b92
Где купить диплом по нужной специальности?
Купить диплом ВУЗа по невысокой стоимости можно, обратившись к проверенной специализированной фирме.: institute-diplom.ru
Tiger Fortune’s graphics are simply amazing. tiger fortune
Проститутки Королев стояли у остановки на Пионерской, кутаясь в пуховик.
Королёв ночью менялся — исчезали учёные, появлялись индивидуалки на выезд.
— Ты та самая дешевая проститутка? — из темноты вышел мужчина в дорогой куртке.
— Зависит от того, кого ты ждёшь.
Они сели в его «Фольксваген». Он вёз её в Болшево, она смотрела в окно. Этот город знал её всю: МГТУ, увольнение из «Роскосмоса», мужа-алкоголика…
— Тебя как зовут?
— Какая разница?
В сауне с проституткой было чисто и безлико. Деньги вперёд. Работа — привычная, без эмоций.
— Ты не такая, как другие.
— Все так говорят.
На рассвете он отвёз её обратно. Осталось отнести деньги «хозяйке», купить еды…
Город спутников и ракет, а так же дешевых проституток. А она — земная, без полётов.
Tiger Fortune keeps the good times rolling! tiger fortune
I don’t get online hate—it’s cool! plinko
Диплом ВУЗа РФ!
Без наличия диплома сложно было продвигаться по карьере. Именно из-за этого решение о покупке диплома следует считать целесообразным. Заказать диплом об образовании iadgroup.co.uk/employer/gosznac-diplom-24
I love the live roulette tables online—feels so authentic! gioco del pollo
I love the fast pace of Tiger Fortune. fortune tiger 777
Online casinos should offer more freebies! plinko
dig this
Telegram lookup bot
служба поддержки мостбет номер телефона http://mostbet6034.ru/ .
online viagra kanishksteels.com .
go to my site
Address lookup
The tiger-themed bonuses in Tiger Fortune rock! jogo do tigrinho demo
Приобрести диплом института по невысокой цене возможно, обращаясь к надежной специализированной компании. Мы предлагаем документы об окончании любых университетов Российской Федерации. Заказать диплом университета– rusd-diplomj.ru/kupit-diplom-v-krasnodare-s-zaneseniem-v-reestr-3/
Недорогой наследственный адвокат окажет содействие в оформлении наследства. В современном мире практически каждый человек понимает важность юридической грамотности, ведь знание законов – это защита от множества проблем и четкое понимание своих прав и обязанностей. Хотя невозможно быть экспертом во всем, наша известная адвокатская коллегия предлагает услуги высококвалифицированных профессионалов.
Мы готовы взяться за дело любой сложности, опираясь на многолетний опыт для оказания эффективной помощи. Каждый арбитражный адвокат в нашей команде специализируется на определенной области права. Это позволяет подобрать для вашей ситуации эксперта, досконально разбирающегося в специфике дела. Такой подход значительно повышает шансы на успех в суде, в отличие от обращения к юристу-универсалу.
Наша коллегия быстро завоевала признание в России. Этому способствуют опытные юристы, узко специализирующиеся в различных областях права, а также доступные цены. Мы работаем прозрачно: на сайте представлена информация о стоимости услуг и подробные сведения о сотрудниках, готовых вам помочь. В договорах отсутствуют скрытые комиссии, которые часто используют недобросовестные фирмы.
Адвокат подробно расскажет обо всех этапах процесса, стоимости услуг, а также разъяснит все детали и нюансы. Мы понимаем, что финансовые трудности могут возникнуть внезапно, а юридическая помощь нужна незамедлительно. Поэтому предлагаем различные варианты рассрочки или отсрочки платежа. Кроме того, по некоторым делам возможна оплата по результату, что позволяет рассчитаться с нами только после успешного завершения дела в суде.
На нашем сайте вы найдете множество полезных материалов, которые помогут вам повысить свою юридическую грамотность. Рекомендуем регулярно посещать наш ресурс, чтобы быть в курсе своих прав. Мы подробно рассказываем, какие документы необходимы для различных процедур: использование материнского капитала, банкротство и т.д. Более подробную консультацию вы можете получить у нашего адвоката, контакты которого указаны на сайте.
Know This Casino: Safe Gaming, Instant Payouts & Human Touch**
Straight to the point: what matters most to players.*
—
Why Gamblers Choose It
– Licensed & Fair: Approved under strict EU standards. Decentralized tech verifies all results.
– Blazing-Fast Withdrawals: Crypto transactions in seconds, bank transfers/fiat settled in a day.
Standout Features
– Exclusive Titles: Live dealer hits like Lightning Roulette, proprietary slots such as Crypto Quest.
– Generous Bonuses: 200 free spins for newcomers, monthly cashback for loyal players.
—
Play Anywhere
Instant-play mode, personalized jackpot notifications, and a sleek, intuitive app.
Real Player Reviews**
– “Cashed out $4k in 2 hours—zero hassle.”* – Sarah
– “The team knew my account history. 10/10.”* – a loyal member
Play Responsibly
– 24/7 Human Assistance: Chat with a human anytime.
– Self-Regulation Tools**: Deposit caps and cool-off periods.
Begin Your Journey
Sign up in 90 seconds, use code **KNOWTHIS** for free spins.
P.S. Gamble wisely. Always define your spending limit.
buy stromectol online in u.k Complete drug overview. Find medicine information. order stromectol mastercard
I hit a bonus online and won $300—big yes! craps online
Купить диплом института по выгодной стоимости можно, обращаясь к надежной специализированной фирме. Купить документ института можно в нашей компании в Москве. diplomservis.com/kupit-diplom-ob-obrazovanii-s-reestrom-8
ремонт квартир в Московской области Москва и Подмосковье – регионы с динамично развивающимся рынком недвижимости. Рано или поздно каждый владелец квартиры сталкивается с необходимостью ремонта. И здесь возникает вопрос: кому доверить эту задачу? ChatMost предлагает комплексные решения по ремонту квартир в Москве, Московской области, Балашихе, Ногинске, Электростали, Щёлково, Пушкино, Реутове, Люберцах, Мытищах, Химках, Видном, Монино, Королеве и Коммунарке.
The tiger symbols in Tiger Fortune are so cool! tiger fortune
внж испания для россиян 2023 Оформление вида на жительство (ВНЖ) и получение гражданства Испании – актуальные вопросы для многих, особенно для россиян, рассматривающих переезд в 2023 году. Существуют различные типы ВНЖ, включая варианты без права на работу, студенческие визы, а также программы для цифровых кочевников (digital nomad) и финансово независимых лиц.
Получение испанского гражданства – отдельный процесс, требующий соблюдения определенных условий. Россияне, заинтересованные в иммиграции, изучают доступные способы получения ВНЖ, необходимые документы и стоимость. Варианты включают покупку недвижимости, участие в программах для инвесторов, а также ВНЖ типа “no lucrativa” для тех, кто располагает достаточными средствами для проживания без работы в Испании.
Помимо первичного оформления, важным аспектом является продление ВНЖ. Условия и требования могут отличаться в зависимости от типа ВНЖ и основания для его получения. Актуальную информацию о процедурах и документах для россиян в 2023 году следует уточнять у юристов или в компетентных органах.
Приобрести диплом о высшем образовании!
Мы предлагаем документы об окончании любых университетов Российской Федерации. Документы изготавливаются на фирменных бланках. avtoweek2016.ru/pomoshh-s-diplomom-bez-obucheniya
бумага для печати дипломов купить diplomys-vsem.ru .
Tiger Fortune is the best slot in this casino’s lineup. tiger fortune
Для эффективного продвижения вверх по карьере требуется наличие официального диплома института. Купить диплом института у сильной организации: kupitediplom0027.ru/kupit-podlinnij-diplom-dlya-karernogo-rosta/
Быстровозводимые здания https://akkord-stroy.ru из металлоконструкций — это скорость, надёжность и экономия. Рассказываем в статьях, как построить объект под ключ: от проектирования до сдачи в эксплуатацию.
Недвижимость в Бяла https://byalahome.ru апартаменты, квартиры и дома у моря в Болгарии. Лучшие предложения на побережье Черного моря — для жизни, отдыха или инвестиций. Успейте купить по выгодной цене!
Купить диплом ВУЗа!
Мы можем предложить документы любых учебных заведений, которые расположены на территории всей Российской Федерации.
diplomaj-v-tule.ru/kupit-diplom-o-visshem-obrazovanii-s-zaneseniem-v-reestr-7/
Скин AWP Graphite http://csgo-case-simulator.ru из CS:GO — чёрный глянец, премиальный стиль и высокая редкость. Укрась арсенал культовой снайперской винтовкой и выделяйся на сервере с первого выстрела.
1win https://www.1win1003.top .
AK-47 Fire Serpent get-skins-cs.ru легендарный скин из коллекции Operation Bravo. Яркий рисунок огненного змея, высокая редкость и коллекционная ценность. Идеальный выбор для истинных фанатов CS:GO.
This casino nailed it with the Tiger Fortune slot design. fortune tiger demo
Tiger Fortune has some seriously cool visuals. fortune tiger
buy stromectol canada Medicine impacts described. Comprehensive drug resource. stromectol cheap
The tiger roars in Tiger Fortune make it so fun! tigrinho demo
Are online ratings solid?—tough guess! fortune mouse
1вин официальный https://1win6045.ru/ .
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: купить диплом психиатра, диплом о среднем профессиональном образовании где купить, купить диплом без занесения в реестр, купить диплом гознак о высшем образовании, купить аттестат с проводкой. Постепенно углубляясь, нашел отличный ресурс здесь: kyc-diplom.com/diplom-articles/kupit-diplom-pedagogicheskogo-universiteta.html
I love the online peace—no crowd! book of ra
Tiger Fortune has quickly become my favorite game here. tiger fortune
The White House said Biden and McCarthy spoke earlier in the day as they struggled to avert a financial precipice which threatened to throw millions of people out of jobs and risk a global meltdown. 비아이 대마초
Tiger Fortune’s wins feel so satisfying. tigrinho demo
kra30.at – kraken ссылка, kraken shop
mostbet промокод http://mostbet6033.ru .
1win aplicația 1win aplicația .
The free spins in Tiger Fortune are awesome! fortune tiger demo gratis
browse around this web-site merrill edge login
1win ug 1win ug .
buy stromectol online Drug information available. Medication trends described. buy stromectol europe
Online gambling can run wild—rein it in! Dragon Hatch
This casino’s Tiger Fortune slot is unreal. fortune tiger demo gratis
The animations in Tiger Fortune are stunning. fortune tiger bet
Купить диплом университета по доступной стоимости вы сможете, обращаясь к надежной специализированной компании. Купить документ ВУЗа можно у нас. diplomk-v-krasnodare.ru/kupit-diplom-s-zaneseniem-v-reestr-28
1 вин https://1win6010.ru .
1 win 1 win .
стоматолог митино стоматолог митино .
ломбарды часов в москве ломбарды часов в москве .
скачать mostbet mostbet6033.ru .
переустройство и перепланировка жилого помещения3 https://www.udmageo.ru .
косметология в митино http://stomatologiya-mitino1.ru/ .
1win moldova download 1win moldova download .
I’ve had some epic moments on Tiger Fortune. fortune tiger demo gratis
Online casino ads blast—quiet it! Marvel Casino
1 win сайт https://www.1win6047.ru .
The tiger-themed bonuses in Tiger Fortune rock! fortune tiger bet
реферат по биологии
Tiger Fortune keeps the good vibes going! fortune tiger demo gratis
where can i buy stromectol ivermectin Find medicine information. Medication effects explained. buy stromectol no prescription
kra30 сс – kra31 cc, kra31 at
мостбет скачать на андроид мостбет скачать на андроид .
партнёрка 1win партнёрка 1win .
Tiger Fortune’s free spins are a game-changer. fortune tiger 777
кракен официальная ссылка – кракен тор, kraken официальный сайт
Скин M4A4 Howl https://cs2-get-skins.ru один из самых дорогих и загадочных в CS:GO. Запрещённый артефакт с историей. Рассказываем о его происхождении, внешнем виде и значении в мире скинов.
Перчатки спецназа case-simulator-cs тактический скин в CS:GO с брутальным дизайном и премиальной редкостью. Узнайте об их разновидностях, цене, коллекционной ценности и лучших сочетаниях с другими скинами.
StatTrak M4A1-S cs2-get-skin.ru/ скин с возможностью отслеживания фрагов прямо на корпусе оружия. Узнайте, какие модели доступны, чем отличаются, и какие из них ценятся выше всего на рынке CS:GO.
Легендарная AWP Medusa https://case-simulator-cs2.ru скин с таинственным дизайном, вдохновлённым древнегреческой мифологией. Высокая редкость, художественная детализация и престиж на каждом сервере.
мостбет войти мостбет войти .
This casino’s Tiger Fortune slot is unreal. tigrinho demo
kra31 cc – kraken market, кракен сайт
ломбард часов бу ломбард часов бу .
узаконивание перепланировки http://udmageo.ru/ .
best smile best smile .
Kra31.cc – кракен клир, кра ссылка
Online casinos fit quiet—no fuss! plinko
look at here merrill lynch
buy stromectol scabies online Get medicine facts. Contraindications explained here. where can i buy stromectol ivermectin
кракен войти – kra cc, kra31 at
Привет!
Для многих людей, купить диплом ВУЗа – это острая потребность, удачный шанс получить достойную работу. Но для кого-то – это желание не терять массу времени на учебу в ВУЗе. Что бы ни толкнуло вас на такое решение, наша фирма готова помочь. Максимально быстро, профессионально и выгодно изготовим документ любого ВУЗа и года выпуска на государственных бланках со всеми требуемыми печатями.
Ключевая причина, почему многие люди покупают документы, – желание занять хорошую должность. К примеру, знания позволяют специалисту устроиться на привлекательную работу, но подтверждения квалификации не имеется. В том случае если для работодателя важно присутствие “корочек”, риск потерять вакантное место довольно высокий.
Приобрести документ института можно в нашем сервисе. Мы предлагаем документы об окончании любых ВУЗов РФ. Вы получите диплом по любым специальностям, включая документы Советского Союза. Даем 100% гарантию, что в случае проверки документа работодателем, каких-либо подозрений не возникнет.
Ситуаций, которые вынуждают приобрести диплом ВУЗа немало. Кому-то прямо сейчас требуется работа, и необходимо произвести впечатление на начальника во время собеседования. Некоторые задумали попасть в большую компанию, для того, чтобы повысить свой статус и в будущем начать собственное дело. Чтобы не тратить впустую годы жизни, а сразу начать эффективную карьеру, применяя врожденные таланты и приобретенные знания, можно купить диплом в онлайне. Вы сможете быть полезным для социума, обретете денежную стабильность максимально быстро и легко- купить диплом техникума
mostbet promo code This current promotional offer is available to all new players and remains valid until December 31, 2025
This online casino’s Tiger Fortune is top-notch. fortune tiger demo gratis
AWP Dragon Lore get-skins-csgo.ru/ легендарный скин с изображением дракона, символ элиты в CS:GO. Узнайте о его происхождении, редкости, стоимости и почему он стал мечтой каждого коллекционера.
Обзор AK-47 Redline http://csgo-get-skin.ru лаконичный дизайн, спортивный стиль и привлекательная цена. Почему этот скин так любят игроки и с чем он лучше всего сочетается — читайте у нас.
Обзор AWP Asiimov get-skin-csgo.ru/ футуристичный скин с дерзким дизайном. Рассказываем о редкости, ценах, вариантах износа и том, почему Asiimov стал иконой среди скинов в CS:GO.
Онлайн-казино Shot https://shot-casino-apk.ru/ предлагает щедрые бонусы, турнирные события и топовые слоты. Узнайте об условиях игры, уровне доверия, лицензии и особенностях платформы в нашем свежем обзоре.
Tiger Fortune’s theme is wild and totally unique. fortune tiger 777
1win moldova download https://1win5014.ru .
This casino’s Tiger Fortune game is incredible. fortune tiger bet
Заказать диплом можно используя сайт компании. ulystar.in/blogs/10081/Купить-диплом-официального-образца
мостбет официальный сайт http://mostbet6034.ru/ .
Tiger Fortune’s features keep me entertained for hours. tigrinho demo
Добрый день смелым!
Контракт с Министерством обороны — это уверенный шаг в завтрашний день. Семья будет под защитой: выплаты, поддержка, льготы и компенсации. Ты получаешь правовую защиту, страхование жизни и доступ к военной ипотеке. 30 дней отпуска, стабильный оклад, бонусы и командное братство. Прими решение, которое изменит твою судьбу. Служи с честью. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством.Если ты ищешь стабильность, контрактная служба даст её с первого дня. Гарантированный доход от 204 000, надбавки за выслугу и участие в операциях. Семья будет под защитой: выплаты, поддержка, льготы и компенсации. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством. Не откладывай — действуй. Будь частью сильной команды. Ты получаешь правовую защиту, страхование жизни и доступ к военной ипотеке.Контракт с Министерством обороны — это уверенный шаг в завтрашний день. Контракт открывает двери к обучению, карьерному росту и уважению. Семья будет под защитой: выплаты, поддержка, льготы и компенсации. Ты получаешь правовую защиту, страхование жизни и доступ к военной ипотеке. Сделай выбор в пользу стабильности — подпиши контракт уже сегодня. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством.Если ты ищешь стабильность, контрактная служба даст её с первого дня. Гарантированный доход от 204 000, надбавки за выслугу и участие в операциях. 30 дней отпуска, стабильный оклад, бонусы и командное братство. Ты получаешь правовую защиту, страхование жизни и доступ к военной ипотеке. Сделай шаг навстречу уверенности, поддержке и уважению. 30 дней отпуска, стабильный оклад, бонусы и командное братство.
Вся информация на сайте – https://kontrakt-na-svo.su/
контракт на сво, выбирай службу по контракту, пункт службы по контракту москва
служба по контракту 2025 выплаты, служба по контракту сво, особенности службы по контракту
Удачи на службе!
мостбет промокод http://www.mostbet6033.ru .
This online casino nailed Tiger Fortune’s theme. fortune tiger demo
cheap stromectol Pill information provided. Pill impacts explained. buy generic stromectol
Tiger Fortune’s rewards make it worth playing. tiger fortune
lucky jet – лаки джет официальный сайт, lucky jet стратегия
Приобрести диплом об образовании. Приобретение диплома через качественную и надежную фирму дарит ряд плюсов. Данное решение позволяет сэкономить время и значительные денежные средства. gtcs.co.in/employer/eonline-diploma
Нужно создание или разработка студия по продвижению сайтов: адаптивный дизайн, SEO-продвижение, техническая поддержка. Эффективное ключевая фраза для вашего бизнеса — привлечение клиентов и рост прибыли.
Обзор онлайн-казино Shot shot-casino-app.ru/ плюсы и минусы, проверка лицензии, акции и фриспины. Рассказываем, стоит ли играть, как получить бонусы и выводить выигрыши без проблем.
Shot Casino shot-casino-online новое онлайн-казино с лицензией, защитой данных и большим выбором игр. В обзоре: безопасность, отзывы игроков, бонусы и выплаты. Надёжная площадка для вашего азарта.
Tiger Fortune’s features are pure genius. tigrinho demo
1win live https://1win6046.ru .
Онлайн-казино Calibry calibri-casino-online новое игровое пространство с лицензией, щедрыми бонусами и широким выбором слотов. Читайте обзор: особенности платформы, условия игры, отзывы и выплаты.
This online casino’s Tiger Fortune is a masterpiece. fortune tiger demo
Online casino ads hit hard—chill out! plinko
Мы изготавливаем дипломы любой профессии по выгодным тарифам.– diplomgorkiy.com/kupit-diplom-s-garantiej-vneseniya-v-reestr-2/
The sound effects in Tiger Fortune are spot on. fortune tiger 777
Online casinos feel risky—double watch! plinko
1 вин http://1win7013.ru .
Доброго бравые люди!
Заключение контракта — это решение в пользу силы, долга и Родины. Семья будет под защитой: выплаты, поддержка, льготы и компенсации. 30 дней отпуска, стабильный оклад, бонусы и командное братство. Ты получаешь правовую защиту, страхование жизни и доступ к военной ипотеке. Сделай шаг навстречу уверенности, поддержке и уважению. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством.Контракт с Министерством обороны — это уверенный шаг в завтрашний день. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством. Ты получаешь правовую защиту, страхование жизни и доступ к военной ипотеке. Гарантированный доход от 204 000, надбавки за выслугу и участие в операциях. Прими решение, которое изменит твою судьбу. Служи с честью. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством.Заключение контракта — это решение в пользу силы, долга и Родины. Гарантированный доход от 204 000, надбавки за выслугу и участие в операциях. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством. Семья будет под защитой: выплаты, поддержка, льготы и компенсации. Контракт — это твой путь к стабильности. Не упусти шанс. Семья будет под защитой: выплаты, поддержка, льготы и компенсации.СВО — это зона действия настоящих, и ты можешь стать одним из них. Контракт открывает двери к обучению, карьерному росту и уважению. Ты получаешь правовую защиту, страхование жизни и доступ к военной ипотеке. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством. Прими решение, которое изменит твою судьбу. Служи с честью. Контракт открывает двери к обучению, карьерному росту и уважению.Служба по контракту — это не просто работа, это шанс изменить свою жизнь. Семья будет под защитой: выплаты, поддержка, льготы и компенсации. Ты получаешь правовую защиту, страхование жизни и доступ к военной ипотеке. 30 дней отпуска, стабильный оклад, бонусы и командное братство. Не откладывай — действуй. Будь частью сильной команды. Жильё, питание, медицинская помощь, отпуск — всё обеспечено государством.
Вся информация на сайте – https://kontrakt-na-svo.su/
служба по контракту 2025 водитель, служба по контракту 2025 официальный сайт, подписать контракт
требования к службе по контракту, служба по контракту на год, контракт на сво
Удачи на службе!
Good day movie fans!
Thinking about where to watch full movie The Gentlemen tonight? Don’t waste time scrolling endlessly. avengers endgame stream Just download movie The Gentlemen and enjoy every minute. The cast delivers top-notch performances. It’s everything a crime thriller should be.
The Gentlemen full movie is available for streaming across several platforms. Stream The Gentlemen movie online and experience the twists and turns of this clever crime story. It’s filled with drama, humor, and edge-of-your-seat action.
Come in and watch series without registration – https://avengersendgame.mp3get.net
dune 2 full movie, watch Joker online, avengers endgame stream
Parasite 2019 online, where to watch full movie Joker, download movie Joker
Happy watching!
Online casinos need grip—too loose! plinko
Tiger Fortune has some awesome surprise bonuses. fortune tiger
Приобрести диплом о высшем образовании!
Мы можем предложить дипломы любой профессии по доступным ценам. Вы приобретаете диплом в надежной и проверенной компании. : corsica.forhikers.com/forum/p/128669
1vin pro http://1win7011.ru/ .
1win win https://www.1win6045.ru .
leptozan is an innovative weight loss formula crafted to help you naturally sculpt the body you’ve always desired.
Где приобрести диплом по необходимой специальности?
Наша компания предлагаетбыстро и выгодно заказать диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями официальных лиц. Наш документ пройдет лубую проверку, даже при использовании специфических приборов. Решайте свои задачи быстро и просто с нашим сервисом. Заказать диплом любого ВУЗа! pomidor.hobbyfm.ru/viewtopic.php?f=16&t=2176
nerve calm is a high-quality nutritional supplement crafted to promote nerve wellness, ease chronic discomfort, and boost everyday vitality.
Заказать диплом возможно используя официальный сайт компании. peticiones.cl/482357
Tiger Fortune makes every spin feel like a big moment. fortune tiger
подключение интернета челябинск
domashij-internet-chelyabinsk001.ru
подключить интернет
Tiger Fortune’s design is top-notch – really impressive. jogo do tigrinho demo
Tiger Fortune’s bonus rounds are thrilling. jogo do tigrinho demo
Диплом университета РФ!
Без наличия диплома очень нелегко было продвигаться вверх по карьерной лестнице. Именно из-за этого решение о заказе диплома следует считать мудрым и целесообразным. Приобрести диплом института metamattersinc.com/employer/gosznac-diplom-24
mostbet chrono https://mostbet6035.ru/ .
1win.kg https://1win6045.ru/ .
Приобрести диплом о высшем образовании. Приобретение документа о высшем образовании через проверенную и надежную компанию дарит ряд преимуществ. Такое решение помогает сберечь как личное время, так и серьезные финансовые средства. toplentanews.ru/diplom-reshenie-za-1-shag
Где купить диплом специалиста?
Мы изготавливаем дипломы любой профессии по выгодным ценам. Для нас очень важно, чтобы документы были доступными для большого количества наших граждан. Приобрести диплом ВУЗа diplomgorkiy.com/poluchite-ofitsialnij-diplom-s-garantirovannoj-registratsiej/
Нужен бесплатный аккаунт? аккаунты телеграм бесплатно Узнайте, где можно получить рабочие логины с играми, как не попасть на фейк и на что обратить внимание при использовании таких аккаунтов.
Онлайн-казино Aura aura casino apk лицензия, быстрые выплаты, слоты от топ-провайдеров и щедрые бонусы. Подробный обзор платформы: интерфейс, регистрация, акции, безопасность и отзывы игроков.
This casino’s Tiger Fortune game is a blast. tigrinho demo
Начните с Aura Casino aura-casino-bonus удобное онлайн-казино с простым интерфейсом, стартовыми бонусами и быстрой регистрацией. Рассказываем, как получить фриспины и не потеряться в слотах.
Online casinos should open odds—show it! plinko
Онлайн-казино Calibry calibri casino play бонусы без депозита, быстрые выводы и лицензированный софт. В обзоре: как начать играть, получить фриспины, использовать акции и избежать рисков.
провайдеры челябинск
domashij-internet-chelyabinsk002.ru
интернет тарифы челябинск
The payouts in Tiger Fortune are pretty impressive. fortune tiger
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам. Стараемся поддерживать для покупателей адекватную ценовую политику. Для нас важно, чтобы дипломы были доступны для большинства граждан.
Заказ документа, который подтверждает обучение в университете, – это рациональное решение. Купить диплом университета: sdiplom.ru/diplom-ekaterinburg-kupit/
мос бет мос бет .
Купить диплом ВУЗа!
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам— freediplom.com/diplom-o-visshem-obrazovanii-kupit-4/
интернет провайдер челябинск
domashij-internet-chelyabinsk002.ru
подключить домашний интернет в челябинске
Online slots grab me—those looks! gates of olympus
This casino’s Tiger Fortune slot is addictive. fortune tiger 777
сдать вещи на хранение в москве недорого куда можно сдать вещи на хранение в москве недорого куда можно .
Good day movie fans!
Looking for The Gentlemen full movie watch online? It’s available now across multiple platforms. download avengers endgame movie Watch The Gentlemen movie online and experience a thrilling ride filled with surprising twists. The film is a perfect mix of action, wit, and suspense.
Looking to watch The Gentlemen movie online free? It’s available now on multiple streaming services. Watch The Gentlemen movie online free and dive into a world of crime and excitement. With its fast-paced plot and sharp writing, it’s a film you won’t want to miss.
Come in and watch favorite movies free online – https://oppenheimer.mp3get.net
avengers endgame film where to watch, download movie Joker, download Parasite movie
avengers end game download, stream oppenheimer, where to watch avengers endgame
Happy watching!
Tiger Fortune’s gameplay is smooth and fast. fortune tiger 777
lucky jet на деньги – лаки джет 1win, lucky jet официальный сайт
Обзор онлайн-казино Calibry https://calibri-casino-app.ru/ как получить бонус без депозита, запустить любимые слоты и вывести выигрыш. Надёжная площадка с лицензией и прозрачными условиями игры.
Играйте в казино Shot https://shot-casino-play.ru десятки провайдеров, ежедневные турниры и кэшбэк. В обзоре: лучшие слоты, лайв-игры, бонусные программы и преимущества платформы.
мастбет https://mostbet6035.ru/ .
Aura Casino https://aura-casino-online.ru безопасная платформа с лицензией, SSL-защитой и проверенной репутацией. В обзоре: честность выплат, работа службы поддержки и реальные отзывы игроков.
Для успешного продвижения вверх по карьерной лестнице понадобится наличие диплома ВУЗа. Приобрести диплом о высшем образовании у проверенной организации: kupitediplom0027.ru/kupit-diplom-v-sankt-peterburge-4/
Обзор Aura Casino aura-casino-play.ru всё о щедрых бонусах, фриспинах, кэшбэке и акциях. Расскажем, как зарегистрироваться, начать игру и получить максимум от каждого депозита.
Tiger Fortune makes every spin feel like a big moment. tiger fortune
1win metode de plată https://1win5015.ru/ .
Tiger Fortune’s theme is a total vibe. fortune tiger demo
I won big online—still hyped! plinko
1win.online http://1win6041.ru .
недорогой интернет челябинск
domashij-internet-chelyabinsk003.ru
интернет тарифы челябинск
pg slot mahjong
PORTAL DIGITAL PAFI: Fungsi Penting Farmasi dalam Era Digital
Profil Singkat PAFI
Pasca kemerdekaan RI, para Tenaga Kefarmasian aktif berkontribusi dalam pengembangan sistem kesehatan. Berdirinya organisasi profesi farmasi ini menjadi langkah strategis sebagai wadah profesional di bidang farmasi. Berlandaskan Pancasila, PAFI bertekad untuk:
• Memajukan taraf kesehatan rakyat
• Memodernisasi farmasi Indonesia
• Menunjang kondisi para anggota
Sistem Digital PAFI: Terobosan Modern untuk Farmasi Modern
Menghadapi tantangan era digital, PAFI memperkenalkan WEB PAFI Terintegrasi – terobosan teknologi yang memfasilitasi pekerjaan apoteker melalui:
? Update Terbaru – Akses kebijakan kesehatan, temuan terbaru, dan peluang karir
? Pengembangan Kompetensi – Layanan pembelajaran jarak jauh
? Jejaring Profesional – Wadah kolaborasi se-Indonesia
Inovasi teknologi ini memaksimalkan sumbangsih PAFI dalam memajukan kesehatan nasional melalui adaptasi perkembangan teknologi.
Peluang Dunia Farmasi Modern
Keberadaan sistem terintegrasi ini menjadi bukti perubahan sistem dalam dunia kefarmasian. Dengan selalu menyempurnakan fitur dan layanan, PAFI berkomitmen untuk:
• Memacu terobosan dalam farmasi
• Menyempurnakan kriteria keahlian
• Memperluas fasilitas kesehatan untuk rakyat
Epilog
PAFI dengan adanya WEB PAFI Terintegrasi selalu berada di garda depan dalam memadukan kemajuan teknologi dengan dunia kefarmasian. Terobosan ini tidak hanya meningkatkan fungsi ahli farmasi, tetapi juga memberikan sumbangsih konkret bagi kemajuan kesehatan bangsa pada zaman teknologi.
This online casino’s Tiger Fortune game is a must-try! fortune tiger
mostbets http://www.mostbet6033.ru .
The spins in Tiger Fortune are always exciting. fortune tiger
purchase propecia Get medicine facts. Medication impacts described. buy propecia cheap
установка протеза зуба http://protezirovanie-zubov3.ru/ .
Покупка диплома ВУЗа через надежную фирму дарит ряд преимуществ. Такое решение дает возможность сберечь время и значительные денежные средства. Однако, только на этом выгода не ограничивается, плюсов гораздо больше.Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий. Дипломы производят на фирменных бланках. Доступная стоимость в сравнении с большими затратами на обучение и проживание. Заказ диплома института является разумным шагом.
Заказать диплом о высшем образовании: [url=http://diplomservis.com/bistrij-nadezhnij-diplom-vuza-s-zaneseniem-v-reestr/]diplomservis.com/bistrij-nadezhnij-diplom-vuza-s-zaneseniem-v-reestr/[/url]
сколько стоит поставить зубные протезы сколько стоит поставить зубные протезы .
протезирование верхних зубов цена protezirovanie-zubov1.ru .
1win. http://1win6047.ru/ .
топсмайл топсмайл .
Tiger Fortune is my go-to slot game at this online casino! fortune tiger demo
înregistrare 1win http://1win5014.ru .
I love the payment flexibility at online casinos—so easy! vipzino
Приобрести диплом университета!
Мы предлагаем документы учебных заведений, расположенных на территории всей России.
diplom-ryssia.com/kupit-diplom-visshego-obrazovaniya-s-zaneseniem-na-zakaz-2/
Tiger Fortune’s wins feel so satisfying. fortune tiger bet
The visuals in Tiger Fortune are breathtaking. fortune tiger demo
что делать с бонусным балансом на 1win что делать с бонусным балансом на 1win .
провайдеры в красноярске по адресу проверить
domashij-internet-krasnoyarsk001.ru
провайдеры по адресу
Мы предлагаем дипломы любой профессии по приятным тарифам. Дипломы производятся на фирменных бланках государственного образца Заказать диплом об образовании kupit-diplom24.com
Где купить диплом по нужной специальности?
Купить диплом ВУЗа по выгодной стоимости возможно, обращаясь к проверенной специализированной компании.: 10000diplomov.ru
Обзор казино Shot shot-casino-bonus лицензия, защита данных, честная игра и проверенные провайдеры. Узнайте, как работает система вывода, поддержка и что говорят реальные пользователи.
Онлайн-казино Calibry https://calibri-casino-apk.ru бонус без депозита, фриспины, турниры и выплаты без задержек. Рассказываем, как начать играть, какие игры выбрать и стоит ли доверять платформе.
Calibry Casino https://calibri-casino-bonus.ru/ лицензированное онлайн-казино с бонусами, быстрым выводом и большим выбором игр. В обзоре: регистрация, акции, безопасность, отзывы игроков и советы новичкам.
Онлайн-казино Aura aura-casino-app.ru лицензия, бонус без депозита, фриспины за регистрацию и моментальный вывод. Всё о казино Aura в одном обзоре — играйте безопасно и с выгодой.
стоматолог митино стоматолог митино .
buy propecia with no prescription Medication resource available. Read about pills. order propecia
Привет!
Мы можем предложить дипломы любой профессии по невысоким тарифам. Цена зависит от определенной специальности, года выпуска и образовательного учреждения: diplomanruss.com/
I spent hours playing Tiger Fortune last night – no regrets! fortune tiger demo
1win регистрация http://1win7013.ru/ .
Online casinos should hurry up payouts—too slow! ruleta americana
1win pariuri https://1win5014.ru/ .
The animations in Tiger Fortune are stunning. fortune tiger demo
Где купить диплом специалиста?
Наша компания предлагает выгодно и быстро приобрести диплом, который выполняется на оригинальном бланке и заверен печатями, водяными знаками, подписями. Диплом пройдет лубую проверку, даже с применением специально предназначенного оборудования. Достигайте свои цели максимально быстро с нашим сервисом.
Заказать диплом о высшем образовании diplomt-v-samare.ru/kupit-diplom-rossiya-12/
кракен официальный сайт – kraken marketplace, kra cc
в этом разделе Манго Офис личный кабинет
Автоперевозки из Китая по РФ ved-china быстрая и выгодная доставка грузов напрямую до вашего склада. Консолидация, растаможка, сопровождение и отслеживание на всех этапах.
I love the tiger vibes in Tiger Fortune. fortune tiger bet
интернет провайдеры в красноярске по адресу дома
domashij-internet-krasnoyarsk002.ru
провайдер по адресу красноярск
частная стоматология в москве частная стоматология в москве .
политические новости новости часа
Ищешь лучшие раскидки https://raskidki-granat-cs2.ru/ CS2 – Смоки, молотовы и флешки для всех карт — изучи тактики, прокачай игру и удиви соперников точными гранатами. Подборка эффективных раскидок!
Нужен надёжный хостинг? виртуальный хостинг vps для сайтов, приложений и бизнес-задач. Гибкая настройка, SSD-накопители, стабильная работа 24/7. Выберите тариф под свои задачи и начните сегодня.
узнать больше Манго Офис облачные решения
мастбет мастбет .
purchase finasteride Patient medication guide. Patient pill guide. purchase finasteride
купить диплом о среднем специальном образовании пермь
The tiger roars in Tiger Fortune are epic. fortune tiger bet
Приобрести диплом о высшем образовании!
Заказать диплом ВУЗа по выгодной цене вы сможете, обращаясь к надежной специализированной фирме. Приобрести диплом: diplom-club.com/ofitsialnij-diplom-vuza-s-zaneseniem-v-reestr
This casino’s Tiger Fortune slot is unreal. jogo do tigrinho demo
1win личный кабинет 1win личный кабинет .
Понедельник: Астрологический прогноз на успешное начало недели
https://x.com/IrinaPavlovna84/status/1911485302209667581
1win kg https://1win7013.ru/ .
Tiger Fortune’s design is bold and exciting. fortune tiger demo
Привет!
Для многих людей, приобрести диплом ВУЗа – это необходимость, удачный шанс получить достойную работу. Но для кого-то – это желание не терять время на учебу в институте. С какой бы целью вам это не потребовалось, наша фирма готова помочь вам. Оперативно, качественно и по разумной стоимости сделаем документ любого года выпуска на настоящих бланках с реальными подписями и печатями.
Основная причина, почему многие люди покупают дипломы, – получить определенную работу. Предположим, знания и опыт позволяют кандидату устроиться на желаемую работу, но подтверждения квалификации нет. Когда работодателю важно присутствие “корочек”, риск потерять место работы очень высокий.
Купить документ о получении высшего образования вы можете в нашем сервисе. Мы предлагаем документы об окончании любых университетов России. Вы получите необходимый диплом по любой специальности, любого года выпуска, в том числе документы образца СССР. Даем 100% гарантию, что при проверке документов работодателями, каких-либо подозрений не появится.
Ситуаций, которые вынуждают заказать диплом достаточно. Кому-то очень срочно требуется работа, и необходимо произвести хорошее впечатление на руководителя на протяжении собеседования. Другие задумали устроиться в престижную компанию, чтобы повысить свой статус в обществе и в дальнейшем начать свой бизнес. Чтобы не терять время, а сразу начинать эффективную карьеру, применяя имеющиеся знания, можно заказать диплом прямо в онлайне. Вы станете полезным в обществе, получите финансовую стабильность в максимально короткий срок- диплом купить о высшем образовании
кракен официальная ссылка – kra cc, кракен сайт
Заказать документ ВУЗа вы имеете возможность у нас в столице. Мы предлагаем документы об окончании любых университетов РФ. Вы получите диплом по любым специальностям, включая документы СССР. Гарантируем, что в случае проверки документов работодателями, никаких подозрений не появится. dip-lom-rus.ru/legalnoe-zanesenie-diploma-v-reestr-bistro-i-nadezhno/
Предлагаем покупку чеков kupit-cheki-new.ru в Москве для отчётности, командировок, подтверждения трат. Быстрое оформление, гибкие условия, все виды документов и сопровождение при необходимости.
Хотите купить чеки https://kupit-cheki-reg.ru в Москве срочно? Работаем без выходных, оформляем документы день в день, возможна доставка и электронные версии. Оперативность и конфиденциальность гарантированы.
Где купить kupit cheki24 чеки в Москве? У нас — быстрое оформление, оригинальные документы, курьерская доставка. Чеки для отчётов, подтверждения командировок и компенсаций.
Витебский университет П.М.Машерова https://vsu.by образовательный центр. Вуз является ведущим образовательным, научным и культурным центром Витебской области.
мостбет кыргызстан [url=http://mostbet6036.ru/]http://mostbet6036.ru/[/url] .
Tiger Fortune’s bonus rounds are thrilling. jogo do tigrinho demo
1win,com http://1win6048.ru .
Your article helped me a lot, is there any more related content? Thanks!
Быстрая доставка действительных чеков по всей России. | Помощь в срочной отчётности — действительно работает. | Работают официально, чеки фискальные. | Чеки в формате PDF, подходят для отчётности. | Актуальные и действующие чеки под отчёт. | Можно выбрать формат чека: бумажный или электронный. | Оформили заказ за 5 минут. | Всё чётко: дата, сумма, организация. | Помогают даже с нестандартными запросами. | Сайт надёжный, без скрытых условий. http://dzen.ru/a/Z_kGwEnhIQCzDHyh.
propecia cheap Medication impacts described. Pill facts provided. propecia cheap
провайдеры в красноярске по адресу проверить
domashij-internet-krasnoyarsk003.ru
интернет провайдеры красноярск по адресу
содержание казино r7
1 цшт https://www.1win7001.ru .
I hit a bonus online and won $600—sweet score! plinko
Tiger Fortune makes every spin feel like a big moment. fortune tiger bet
The slot themes online glow—play on! aviator casino
I lost time online—fast gone! BetOnRed
Приобрести диплом о высшем образовании!
Мы предлагаем документы учебных заведений, расположенных на территории всей РФ.
diplom-onlinex.com/kupit-diplom-v-moskve-s-zaneseniem-v-reestr-bistro-4/
Приобрести диплом института по выгодной цене вы можете, обращаясь к проверенной специализированной фирме. Приобрести документ университета можно в нашей компании. diplomt-v-chelyabinske.ru/kupit-diplom-o-srednem-obrazovanii-s-reestrovoj-zapisyu-2
Мы готовы предложить дипломы психологов, юристов, экономистов и любых других профессий по невысоким ценам. Мы готовы предложить документы техникумов, которые расположены на территории всей России. Дипломы и аттестаты печатаются на “правильной” бумаге самого высокого качества. Это позволяет делать государственные дипломы, которые не отличить от оригинала. clickbuttonpinshow.copiny.com/question/details/id/1082914
descărca 1win https://www.1win5015.ru .
Где купить диплом специалиста?
Полученный диплом с необходимыми печатями и подписями отвечает условиям и стандартам Министерства образования и науки, никто не отличит его от оригинала – даже со специальным оборудованием. Не следует откладывать собственные мечты и цели на потом, реализуйте их с нами – отправьте заявку на изготовление документа прямо сейчас! Получить диплом о среднем специальном образовании – запросто! veiron.forumex.ru/viewtopic.phpf=4&t=10093
Are online jackpots real?—skeptic! aviator game
Заказать диплом университета мы поможем. Купить диплом специалиста Орёл – [url=http://diplomybox.com/kupit-diplom-spetsialista-v-orle/]diplomybox.com/kupit-diplom-spetsialista-v-orle[/url]
протезирование 2 зубов протезирование 2 зубов .
платное протезирование зубов платное протезирование зубов .
Tiger Fortune’s visuals are next-level cool. fortune tiger demo
buy accutane medication Prescribing guidelines here. Medication reactions explained. generic accutane
какие провайдеры на адресе в краснодаре
domashij-internet-krasnodar001.ru
провайдер по адресу
This casino’s Tiger Fortune slot is pure entertainment. fortune tiger bet
стоматологическое протезирование зубов цена http://www.protezirovanie-zubov1.ru .
Купить диплом на заказ возможно используя официальный портал компании. golf.od.ua/forum/viewtopic.phpp=82669#82669
Выгодно приобрести диплом о высшем образовании. Покупка диплома через надежную фирму дарит немало преимуществ. Это решение дает возможность сэкономить время и значительные финансовые средства. vtpaddlers.net/vpcbb/phpBB/viewtopic.php?t=1194078
Где приобрести диплом по актуальной специальности?
Мы предлагаем быстро купить диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Документ пройдет лубую проверку, даже при использовании профессиональных приборов. Решите свои задачи быстро и просто с нашей компанией.
Купить диплом любого ВУЗа diplomservis.com/kupit-svidetelstvo-o-brake-10/
Tiger Fortune’s payouts keep me coming back for more. fortune tiger 777
Мы готовы предложить дипломы любых профессий по приятным ценам.– diplomgorkiy.com/kupit-diplom-s-zaneseniem-v-reestr-na-forume-2/
I won big last night playing Tiger Fortune – what a thrill! fortune tiger bet
Всегда считал, что покупка диплома о высшем образовании — это миф. Но, оказалось, что все возможно! Сначала искал информацию по теме: купить аттестат школьный, аттестат сколько стоит, аттестат с отличием купить, купить аттестат с отличием, диплом техникума купить, а затем перешел на дипломы вузов. Подробнее можно узнать здесь: [url=http://diplomybox.com/dostavka-i-oplata/]diplomybox.com/dostavka-i-oplata[/url]
Tiger Fortune’s free spins are a game-changer. jogo do tigrinho demo
Was it the fossilized remains of a prehistoric creature, or something else entirely? Mateo couldn’t shake off the feeling that his life was about to change forever.국산야동
протезирование передних зубов цена протезирование передних зубов цена .
стоматологическое протезирование зубов цена http://www.protezirovanie-zubov3.ru .
купить диплом всгуту
buy accutane usa Get medicine info. Brand names listed. order isotretinoin
mostbet casino http://mostbet6033.ru/ .
Только 2 знакам Зодиака невероятно повезет во второй половине апреля
https://x.com/Fariz418740/status/1911625378860278161
I wish online casinos paid quick—drag! plinko
1win молдова 1win молдова .
интернет по адресу дома
domashij-internet-krasnodar002.ru
интернет провайдеры краснодар по адресу
Tiger Fortune makes every spin an adventure. fortune tiger demo
Online casinos need better self-control tools. plinko
детская стоматология митино детская стоматология митино .
сколько стоят зубные протезы сколько стоят зубные протезы .
Мы готовы предложить дипломы любой профессии по разумным ценам. Купить диплом Красногорск — kyc-diplom.com/geography/krasnogorsk.html
Tiger Fortune’s spins keep me on my toes. fortune tiger
In three words, I can sum up everything I’ve learned about life: it goes on. 한국야동
To be yourself in a world that is constantly trying to make you something else is the greatest accomplishment. BJ벗방
провайдеры по адресу дома
domashij-internet-krasnodar003.ru
провайдеры интернета в краснодаре по адресу
Tiger Fortune’s visuals are next-level cool. jogo do tigrinho demo
purchase isotretinoin Read about medications. Patient medicine guide. buy accutane online
Tiger Fortune’s rewards are worth every spin. jogo do tigrinho demo
Где купить диплом по актуальной специальности?
Мы изготавливаем дипломы любой профессии по выгодным ценам. Для нас очень важно, чтобы документы были доступными для большинства наших граждан. Купить диплом о высшем образовании diplomidlarf.ru/kupit-originalnij-diplom-s-zaneseniem-v-reestr-20/
This casino’s Tiger Fortune slot is a joy. tigrinho demo
Online casinos should cut bots—human play! joker jewels
какие провайдеры интернета есть по адресу краснодар
domashij-internet-krasnodar003.ru
проверить провайдеров по адресу краснодар
Заказать диплом о высшем образовании !
Приобретение диплома любого ВУЗа РФ в нашей компании является надежным делом, так как документ заносится в государственный реестр. Купить диплом о высшем образовании kupit-diplom24.com/kupit-diplom-s-reestrom-otzivi-visokoe-kachestvo-garantirovano
1win rossvya http://1win7002.ru .
This online casino’s Tiger Fortune game is a must-try! tigrinho demo
Также рекомендую вам почитать по теме – https://dtf.ru/luchshii-rating/3318252-top-9-knig-gorodskoe-fentezi-reiting-luchshih .
И еще вот – https://dtf.ru/luchshii-rating/3406091-top-20-luchshih-rossiiskih-detektivnyh-serialov-trillerov-s-vysokim-reitingom .
косметология в митино https://stomatologiya-mitino3.ru/ .
This online casino’s Tiger Fortune is top-notch. tiger fortune
Мы можем предложить дипломы любой профессии по приятным ценам. Мы можем предложить документы техникумов, которые находятся на территории всей России. Документы делаются на “правильной” бумаге высшего качества. Это дает возможности делать настоящие дипломы, не отличимые от оригинала. portal.woellmarine.com/thread-152877.html
buy accutane with no prescription Latest drug developments. Complete medicine overview. purchase isotretinoin
aplicația 1win http://1win5016.ru .
интернет провайдеры по адресу самара
domashij-internet-samara001.ru
интернет по адресу
mostbest http://mostbet6036.ru/ .
This casino nailed it with the Tiger Fortune slot design. jogo do tigrinho demo
Купить диплом института!
Мы изготавливаем дипломы любых профессий по невысоким тарифам— diplomp-irkutsk.ru/kupit-diplom-v-krasnoyarske-bistro-i-udobno-3/
Приобрести диплом ВУЗа!
Мы готовы предложить документы учебных заведений, которые находятся на территории всей Российской Федерации. Документы выпускаются на “правильной” бумаге высшего качества: mamuli.club/forum/topic/33032
Мы готовы предложить дипломы любых профессий по выгодным тарифам. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Важно, чтобы дипломы были доступны для большого количества граждан.
Заказ диплома, подтверждающего обучение в университете, – это выгодное решение. Купить диплом о высшем образовании: diplomius-docs.com/kupit-diplom-srednee-obrazovanie-2/
Online blackjack rocks—fun twist! dragon vs tiger game
I’ve been using weed delivery minneapolis constantly seeing that all about a month now, and I’m truly impressed by the uncontested effects. They’ve helped me determine calmer, more balanced, and less tense in every nook the day. My forty winks is deeper, I wake up refreshed, and sober my focus has improved. The value is distinguished, and I worth the common ingredients. I’ll definitely heed buying and recommending them to everybody I know!
accutane generic Pill impacts explained. Drug reactions explained. buy accutane cheap
1win казино 1win казино .
Приобретение документа о высшем образовании через качественную и надежную фирму дарит массу плюсов для покупателя. Это решение позволяет сэкономить как продолжительное время, так и существенные средства. Тем не менее, на этом выгода не ограничивается, преимуществ намного больше.Мы предлагаем дипломы любой профессии. Дипломы производят на фирменных бланках. Доступная стоимость сравнительно с серьезными затратами на обучение и проживание. Приобретение диплома о высшем образовании из российского университета является мудрым шагом.
Купить диплом о высшем образовании: asxdiploman.com/kupit-gosudarstvennij-diplom-s-zaneseniem-v-reestr-7/
Tiger Fortune’s design is top-notch – really impressive. tigrinho demo
This casino’s Tiger Fortune game is a standout. fortune tiger
leptozan is an innovative weight loss formula crafted to help you naturally sculpt the body you’ve always desired.
Заинтересованы в качественной продукции?, Camogear. Вы найдете у нас много интересного. Не пропустите шанс, узнать о новинках. и будьте в курсе акций. Гарантия качества – это то, что мы гарантируем. Покупайте легко и быстро.
штани камуфляжні https://camogear.com.ua/ .
primebiome fosters a thriving gut microbiome, which contributes to improved skin clarity and a naturally youthful glow.
мостбет промокод mostbet6036.ru .
Viagra * Cialis * Levitra
All the products you are looking an eye to are currently available for 1+1.
4 more tablets of one of the following services: Viagra * Cialis * Levitra
https://pxman.net
провайдеры интернета по адресу
domashij-internet-samara002.ru
интернет по адресу
Заказывала “что-то экзотическое” – получила тропики в вазе!
цветы томск
Получил бездепозитный бонус на rox casino. Отличная программа лояльности для игроков. Rox casino предлагает интересные турниры. Лёгкая авторизация и верификация аккаунта. Реальные шансы сорвать крупный куш. Зарегистрироваться можно за 1 минуту. Прозрачные условия бонусных программ. Удобно пополнять счёт и играть сразу. Хорошие акции для новичков зеркало на сегодня.
Доброго!
Надгробия с изображением пейзажа в Казахстане — это памятники, которые позволяют создать атмосферу спокойствия и умиротворения. Мы изготавливаем памятники с изображением природы, таких как горы, леса или озера, чтобы придать памятнику индивидуальность и гармонию. Эти памятники могут быть выполнены из мрамора или гранита, что гарантирует их долговечность. Надгробные памятники в Сарканде – цены и фото Памятники с изображением пейзажа помогут создать образ вечного отдыха и покоя.
Памятники с изображением дождя в Казахстане — это символ очищения, обновления и новой жизни. Дождь может быть использован на памятниках, чтобы отразить процесс очищения и возрождения. Мы создаем памятники с изображением дождя, выполненные с высокой символикой и вниманием к деталям. Памятник с дождем будет символизировать обновление и возрождение.
Вся информация по ссылке – https://www.instagram.com/memorystone_kz/
Корейские памятники с розами в Казахстане, Проектирование мемориалов в Казахстане, Авторские памятники в Сарканде
Надгробные плиты в Сарканде, Заказать памятник с доставкой в Сарканд, Мраморные надгробия мужчине Казахстан
Удачи!
1wiun http://www.1win7014.ru .
Привет всем!
Памятники с изображением дерева жизни в Казахстане — это символ вечности, роста и возрождения. Мы изготавливаем памятники с изображением дерева жизни, которое символизирует связь между небом и землей, а также постоянное обновление жизни. Эти памятники могут быть выполнены из качественных материалов, таких как гранит и мрамор, и включать уникальные элементы гравировки. Где купить надгробие в Талдыкоргане Памятник с изображением дерева жизни станет олицетворением вечной памяти и гармонии.
Памятники с изображением кошки в Казахстане — это символ независимости, грации и защиты. Кошка может олицетворять элегантность и загадочность, а также защиту от негативных сил. Мы предлагаем памятники с изображением кошки, выполненные с изысканностью и вниманием к деталям. Памятник с кошкой будет символизировать грацию, независимость и защиту.
Вся информация по ссылке – https://www.instagram.com/p/DIOXJResKXj
Продажа памятников в Казахстане, Памятники на могилу маме в Казахстане, Серые надгробия маме в Казахстане
Ритуальные фотоуслуги Алматы, Мемориальные таблички из бронзы в Казахстане, Бюсты из бронзы для памятников в Казахстане
Удачи!
I love the low bets online—soft go! aviator
I won spins online and got $50—awesome! dragon tiger
служба поддержки мостбет номер телефона https://mostbet5001.ru/ .
Приветствую!
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по приятным ценам. Цена зависит от выбранной специальности, года выпуска и ВУЗа: diplomanrus.com/
нажмите здесь Манго Офис личный кабинет
PvP сервера л2 ИЛ на новой сборке
isotretinoin Get pill details. Find medicine information. buy accutane no prescription
Онлайн журнал Strategic Business Review – лайфхаки директорам, новости IT.
1win football http://www.1win16.com.ng .
поддержка мостбет https://mostbet6033.ru .
Металлические значки https://soto-lux.ru/znachki-iz-metalla-kak-oni-mogut-povysit-uznavaemost-vashego-biznesa/ это стильный и доступный способ подчеркнуть фирменный стиль компании, привлечь внимание к бренду и повысить его узнаваемость. Они отлично подходят для корпоративных мероприятий, подарков клиентам и сотрудников.
ВГУ им. П. М. Машерова https://vsu.by официальный сайт, факультеты, направления подготовки, приёмная кампания. Узнайте о поступлении, обучении и возможностях для студентов.
на этом сайте Mango-Office кабинет
jocuri de noroc online moldova https://www.1win5016.ru .
Заказать диплом ВУЗа по доступной цене возможно, обратившись к проверенной специализированной фирме. Мы предлагаем документы об окончании любых университетов РФ. Купить диплом о высшем образовании– diplomg-cheboksary.ru/kupite-diplom-v-krasnodare-s-zaneseniem-v-reestr/
игра 1вин https://1win7001.ru/ .
The online bonuses shine—jump in! aviator
The live dealers online are so professional—awesome! plinko
Playing online bingo is surprisingly fun—great community vibe! fishin frenzy
онлайн казино промокоды – регистрация в онлайн казино, казино онлайн бесплатно
I love the live roulette online—feels so real! plinko
узнать электрик томск
I hit a bonus online and won $700—big yes! plinko
подключить интернет по адресу
domashij-internet-samara003.ru
интернет провайдеры самара по адресу
повышение доверия криптовалюты повышение доверия криптовалюты .
промокод на продамус промокод на продамус .
купить диплом о профпереподготовке
продвижение криптовалюты продвижение криптовалюты .
в этом разделе электрик томск
1win официальный скачать на андроид бесплатно – 1вин сайт 1win, 1win пополнение
Купить диплом любого ВУЗа!
Купить диплом ВУЗа по невысокой цене возможно, обращаясь к надежной специализированной компании. Купить диплом о высшем образовании: diplomj-irkutsk.ru/poluchite-ofitsialnij-diplom-bakalavra-s-garantiej
I hit a blackjack wave—good run! bet on red
Для удачного продвижения по карьере потребуется наличие диплома о высшем образовании. Приобрести диплом института у надежной организации: diplomgorkiy.com/diplom-kupit-tsena-2/
Заказать диплом о высшем образовании!
Мы готовы предложить дипломы любой профессии по приятным ценам. Вы заказываете диплом в надежной и проверенной временем компании. : avtovideotest.ru/nastoyashhiy-diplom-bez-posrednikov-i-lishnih-zatrat
Заказать диплом о высшем образовании!
Мы готовы предложить документы институтов, которые расположены на территории всей России.
diplom-onlinex.com/bistrij-i-nadezhnij-diplom-s-zaneseniem-v-reestr/
каталог электрик томск
where buy accutane Access drug facts. Comprehensive medication guide. buy accutane medication
аренда автомобиля в адлере аренда авто адлер без водителя
прокат авто в сочи без водителя цены аренда автомобиля в сочи сочи аренда авто
прокат автомобилей в крыму аренда авто в крыму без водителя
заказать мытье окон cleaning-top24
Диплом университета России!
Без университета очень нелегко было продвигаться вверх по карьерной лестнице. По этой причине решение о заказе диплома стоит считать мудрым и целесообразным. Заказать диплом о высшем образовании ttartisan.ru/forum/user/4896
Где купить диплом специалиста?
Получаемый диплом с приложением 100% отвечает требованиям и стандартам Министерства образования и науки РФ, неотличим от оригинала. Не откладывайте личные мечты и задачи на потом, реализуйте их с нашей компанией – отправьте быструю заявку на диплом уже сегодня! Купить диплом о высшем образовании – легко! ariaqa.com/employer/premialnie-diplom-24
Купить документ ВУЗа вы имеете возможность в нашем сервисе. Мы оказываем услуги по продаже документов об окончании любых университетов РФ. Вы получите необходимый диплом по любой специальности, любого года выпуска, в том числе документы старого образца. Даем 100% гарантию, что в случае проверки документа работодателем, подозрений не возникнет. damdiplomisa.com/kupit-diplom-s-zaneseniem-v-reestr-instituta-3/
I don’t trust online odds—odd feel! plinko
Где приобрести диплом по актуальной специальности?
Наши специалисты предлагаютвыгодно заказать диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Диплом пройдет лубую проверку, даже с использованием специальных приборов. Решите свои задачи быстро и просто с нашими дипломами. Приобрести диплом университета! friendza.enroles.com/read-blog/38395_svidetelstvo-o-brake.html
мос бет https://www.mostbet5002.ru .
Пионы как пуховые облака – нежность!
101 роза
Medicine resource available. https://isotretinoin365n.top/# Get drug details. buy isotretinoin online
I don’t trust online—too open! blue wizard
интернет по адресу
domashij-internet-ufa001.ru
узнать интернет по адресу
склад для хранения склад для хранения .
1вин 1вин .
кликните сюда электрик томск
Online gambling runs free—tight it! plinko
Мы изготавливаем дипломы любой профессии по приятным ценам. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступны для большого количества граждан.
Покупка диплома, подтверждающего окончание института, – это выгодное решение. Заказать диплом о высшем образовании: [url=http://10000diplomov.ru/kupit-diplom-gde-mozhno-2/]10000diplomov.ru/kupit-diplom-gde-mozhno-2/[/url]
Online casinos need tools—help us! plinko
I hit a blackjack wave—good run! aviator game online
Приобрести диплом любого института!
Мы оказываем услуги по продаже документов об окончании любых университетов России. Документы производятся на подлинных бланках. [url=http://clickbuttonpinshow.copiny.com/question/details/id/1084655/]clickbuttonpinshow.copiny.com/question/details/id/1084655[/url]
купить диплом нефтяник diplomysis-vsem.ru .
Купить диплом об образовании!
Мы изготавливаем дипломы любой профессии по приятным ценам— diplomus-spb.ru/kupit-diplom-o-meditsinskom-obrazovanii-vigodno-i-bistro/
I hit a bonus online and won $60—cool! aviator
orm криптовалюты prodvizhenie-kriptovalyuta2.ru .
The visuals online stun—eye pop! plinko
мостбет мобильная версия скачать мостбет мобильная версия скачать .
Drug resource available. https://isotretinoin365n.top/# Drug reactions explained. buy accutane online without prescription
1vin https://1win7002.ru/ .
провайдеры интернета по адресу уфа
domashij-internet-ufa002.ru
интернет провайдеры уфа по адресу
The payout waits online suck—faster! plinko
Приобрести диплом ВУЗа по доступной цене возможно, обращаясь к надежной специализированной компании. Приобрести документ ВУЗа можно в нашем сервисе. diplomg-kurerom.ru/kupit-diplom-o-visshem-obrazovanii-18
MetaMask Extension is the best for DeFi enthusiasts. I use it daily for staking, swapping, and interacting with dApps securely.
Online casinos should free—try it! aviator game
Online laws fuzz—sort out! vai de bet
служба поддержки мостбет номер телефона https://mostbet6033.ru/ .
Мы готовы предложить дипломы любых профессий по приятным ценам. Мы предлагаем документы техникумов, которые находятся на территории всей Российской Федерации. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. sakhd.3nx.ru/viewtopic.phpp=3357#3357
I don’t trust online casinos with my info—too risky! rakoo casino
Online gambling feels loose—risk high! plinko
1 win.pro http://www.1win7002.ru .
Где заказать диплом специалиста?
Наша компания предлагает выгодно заказать диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, штампами, подписями. Документ способен пройти лубую проверку, даже при помощи специального оборудования. Достигайте свои цели быстро и просто с нашим сервисом.
Купить диплом любого ВУЗа diplomgorkiy.com/kupit-diplom-v-naberezhnix-chelnax-4/
Comprehensive medication facts. https://isotretinoin365n.top/# Pill leaflet provided. buy accutane online
взять машину в аренду минеральные воды ставропольский край минеральные воды аренда авто
провайдеры интернета по адресу уфа
domashij-internet-ufa003.ru
провайдеры в уфе по адресу проверить
круглосуточный прокат авто в москве самая дешевая аренда авто в москве
аренда авто в калининграде в аэропорту аренда автомобиля калининград храброво
Ищете надежный ремонт бытовой техники Bosch в Москве? Наш сервисный центр готов помочь. Наши опытные мастера оперативно и надежно устранят поломки любой сложности вашего оборудования Bosch. Применяем только оригинальные запчасти и проверенные методы для гарантированного восстановления работоспособности вашей техники. Предлагаем выезд мастера на дом или ремонт в условиях стационарного центра. Доверьте заботу о вашей технике профессионалам. Заказать сервисный центр бош в москве можно прямо сейчас.
клининг после ремонта https://cleaning-top24.ru
Online blackjack is solid—good play! fortune tiger
Где купить диплом специалиста?
Наши специалисты предлагаютвыгодно и быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями. Наш диплом способен пройти любые проверки, даже с применением специально предназначенного оборудования. Достигайте свои цели быстро с нашей компанией. Купить диплом университета! lapd.getbb.ru/viewtopic.php?f=5&t=1081
The slot sounds online are gold—fun times! plinko
mostbet kg http://mostbet6033.ru/ .
Автопортал https://avtogid.in.ua Автогiд сайт с полезными советами для автовладельцев. Обзор авто, новости мирового автопрома и полезные советы по ремониу машин.
Быстро и просто заказать диплом о высшем образовании. Покупка диплома через качественную и надежную фирму дарит ряд преимуществ. Это решение дает возможность сберечь время и существенные денежные средства. nsibirsk.ru/forum/obyavleniya/topic-34773.html
1win зайти 1win7014.ru .
Pill leaflet here. https://isotretinoin365n.top/# Pill leaflet available. buy accutane no prescription
The poker online glows—real rush! craps
I love the online promos—new spark! plinko
интернет провайдер челябинск
domashij-internet-chelyabinsk001.ru
тарифы интернет и телевидение челябинск
Купить диплом института по выгодной цене возможно, обращаясь к проверенной специализированной фирме. Купить документ университета можно у нас. diplomaj-v-tule.ru/kupit-diplom-ob-obrazovanii-zanesennij-v-reestr-9
продамус промокод скидка на подключение [url=www.promokod-prdms.ru/]продамус промокод скидка на подключение[/url] .
orm криптопроекта http://www.prodvizhenie-kriptovalyuta1.ru .
Ищете качественный ремонт техники Bosch в Москве? Наш сервисный центр готов помочь. Наши сертифицированные мастера оперативно и надежно устранят поломки любой сложности вашего оборудования Bosch. Используем только оригинальные детали Bosch и современные технологии для гарантированного восстановления функциональности вашего оборудования. Ремонт осуществляется как на дому у заказчика, так и в нашем сервисном центре. Обращайтесь к нам для быстрого и надежного ремонта вашей техники Bosch. Заказать ремонт вытяжек bosch в москве на дому и получите качественный сервис.
Online bingo flows—great crew! plinko
склады для личных вещей в москве склады для личных вещей в москве .
The slot rush online grips—hold on! F7 Casino
Приобрести диплом университета !
Приобретение диплома университета России у нас – надежный процесс, поскольку документ будет заноситься в государственный реестр. Заказать диплом об образовании [url=http://diplomk-vo-vladivostoke.ru/legalnoe-zanesenie-diploma-v-reestr-5/]diplomk-vo-vladivostoke.ru/legalnoe-zanesenie-diploma-v-reestr-5[/url]
mostbet http://mostbet5001.ru/ .
1 win pro https://1win7003.ru/ .
Online casinos should ban bots—keep it fair for real players! plinko
1-win http://1win7015.ru/ .
Потолки для вашего дома от natyazhnye-potolki-dnepr.biz.ua , где ваша фантазия воплощается в жизнь, добавьте шарм вашему пространству, выбор, который вас не разочарует.
Премиум натяжные потолки в Днепре, профессиональный подход, которые создадут уют и гармонию, узнайте больше на сайте.
Доступные натяжные потолки в Днепре, от natyazhnye-potolki-dnepr.biz.ua, придайте вашему интерьеру свежесть, закажите бесплатную консультацию.
Натяжные потолки, созданные с любовью, доступные на natyazhnye-potolki-dnepr.biz.ua, превратите мечты в реальность, измените пространство вокруг себя.
Эстетика и функциональность натяжных потолков, отечественного производства, создайте свой идеальный интерьер, у нас широкий ассортимент.
Преимущества натяжных потолков, для вашего комфорта, долговечность и надежность, инвестируйте в качество.
Эстетические решения для вашего потолка, где стиль и качество соединяются, превратите потолок в произведение искусства, наш опыт к вашим услугам.
Натяжные потолки: легко и удобно, в Днепре и за его пределами, выбирайте лучшее для себя, свой идеальный потолок в 3 шага.
Натяжные потолки от профессионалов, с хорошей репутацией, мы знаем, как сделать ваш потолок идеальным, придайте своему интерьеру новое дыхание.
Натяжные потолки по доступным ценам, на natyazhnye-potolki-dnepr.biz.ua, где ваши мечты становятся реальностью, не упустите свой шанс.
Натяжные потолки: быстро и стильно, от natyazhnye-potolki-dnepr.biz.ua, мы работаем для вас, позвоните и получите ответ на все вопросы.
Ваш идеальный натяжной потолок, где стиль встречается с практичностью, на любой вкус и бюджет, сделайте выбор осознанно.
Опытные специалисты по натяжным потолкам, с индивидуальным подходом к каждому клиенту, потолок, о котором вы всегда мечтали, обновите свой дом с нами.
Эстетика и функциональность натяжных потолков, от natyazhnye-potolki-dnepr.biz.ua, всё для вашего комфорта и стиля, узнайте больше на нашем сайте.
Натяжные потолки: от замысла до реализации, потолки для любого стиля, поддержка профессионалов на каждом этапе, не откладывайте на завтра.
Дайте своему потолку вторую жизнь, от natyazhnye-potolki-dnepr.biz.ua, мы создаем счастье для ваших глаз, сделайте правильный выбор.
https://natyazhnye-potolki-dnepr.biz.ua/ https://natyazhnye-potolki-dnepr.biz.ua/ .
pinko Казахстан предлагает щедрые бонусы. Можно пополнить счет на pinko несколькими способами. pinko работает в Казахстане на официальной основе. Вывод выигрыша с pinko без комиссии. Казино пинко — легальный ресурс. pinko дает бездепозитные бонусы. Сайт pinko полностью адаптирован под казахский рынок. pinko работает круглосуточно без сбоев. Рейтинг pinko в Казахстане очень высок pinko официальный сайт.
1 win https://1win16.com.ng .
Drug essentials explained. https://isotretinoin365n.top/# Get pill facts. accutane cheap
Купить диплом любого университета!
Мы готовы предложить документы институтов, расположенных на территории всей РФ. Дипломы и аттестаты печатаются на “правильной” бумаге самого высшего качества: cn.wejob.info/employer/aurus-diploms
Online casinos should list odds—open up! plinko
1vin 1vin .
Online gambling feels detached—easy to overspend! fishing frenzy
дешевый интернет челябинск
domashij-internet-chelyabinsk002.ru
интернет тарифы челябинск
Привет всем!
Предлагаем услуги по изготовлению строительных металлоконструкций. Работаем с частными и корпоративными клиентами. Гарантируем надёжность и точность исполнения. Работаем с частными и корпоративными клиентами.|Полный цикл изготовления металлоконструкций: от проекта до монтажа. Изготовление опор, балок, ферм, колонн и других элементов. Принимаем заказы на серийное и индивидуальное производство. Сварка, резка, покраска и сборка — всё в одном месте.|Изготовление металлоконструкций по чертежам заказчика. Индивидуальный подход и гибкие условия сотрудничества. Быстрое изготовление металлоконструкций — от идеи до реализации. Сварка, резка, покраска и сборка — всё в одном месте.|Современное производство металлоконструкций в кратчайшие сроки. Изготовление опор, балок, ферм, колонн и других элементов. Гарантируем надёжность и точность исполнения. Контроль качества на каждом этапе производства.|
Вся информация на сайте – https://borza.pro/
изготовление строительных металлоконструкций, Изготовление металлоконструкций, Изготовление металлоконструкций
изготовление металлоконструкций московская область, производство строительных металлоконструкций, Изготовление металлоконструкций
Удачи!
промокод на продамус скидка подключение [url=http://www.promokod-prdms.ru]http://www.promokod-prdms.ru[/url] .
google play отзывы google play отзывы .
Заказать диплом можно используя сайт компании. swingerspool.com/read-blog/10_gde-kupit-diplom-s-reestrom.html
cod promoțional 1win cod promoțional 1win .
Online casinos should curb—care play! casino kingdom
Interactions explained here. https://isotretinoin365n.top/# Get medication facts. accutane generic
мосбет https://mostbet5001.ru .
mostber http://mostbet6033.ru .
улучшение репутации криптовалюты улучшение репутации криптовалюты .
I love the low bets online—soft go! aviator
домашний интернет подключить челябинск
domashij-internet-chelyabinsk003.ru
недорогой интернет челябинск
I won big online—pure thrill! plinko
The slot themes online glow—play on! plinko
Привет всем!
Производим металлоконструкции любых типов и размеров. Контроль качества на каждом этапе производства. Свяжитесь с нами для расчёта стоимости. Контроль качества на каждом этапе производства.|Современное производство металлоконструкций в кратчайшие сроки. Высокоточное оборудование, сертифицированные материалы. Свяжитесь с нами для расчёта стоимости. Сварка, резка, покраска и сборка — всё в одном месте.|Современное производство металлоконструкций в кратчайшие сроки. Доставка по всей России и странам СНГ. Быстрое изготовление металлоконструкций — от идеи до реализации. Доставка по всей России и странам СНГ.|
Вся информация на сайте – https://borza.pro/
стоимость изготовления металлоконструкций за 1, Изготовление металлоконструкций, Изготовление металлоконструкций
завод по производству металлоконструкций, производство металлоконструкций московская область, стоимость изготовления металлоконструкций за тонну
Удачи!
aviator mostbet aviator mostbet .
Приобрести диплом об образовании. Заказ документа о высшем образовании через надежную компанию дарит немало достоинств. Это решение дает возможность сберечь время и существенные средства. babygirls017.copiny.com/question/details/id/1083668
I love the live roulette—live vibe! plinko
Piano sheet music for beginners sheetmusic
porn ai ai porn videos
Заказывала срочно — привезли вовремя, букет свежий и красивый.
доставка цветов в томске
Noten von klavier klavier mit noten
Полезная информация itsynergis.ru свежие материалы и удобная навигация — всё, что нужно для комфортного пользования сайтом. Заходите, изучайте разделы и находите то, что действительно важно для вас.
Some online casino ads are so annoying, popping up everywhere! fishin frenzy
I won big online—pure thrill! bet on red
1win online [url=1win16.com.ng]1win16.com.ng[/url] .
Drug details provided. https://isotretinoin365n.top/# Medication effects explained. accutane
Мы предлагаем дипломы любой профессии по разумным тарифам. Дипломы изготавливаются на настоящих бланках Заказать диплом об образовании vuz-diplom.ru
Где приобрести диплом по нужной специальности?
Купить диплом института по доступной стоимости вы сможете, обратившись к проверенной специализированной компании.: diplom-bez-problem.com
1 вин http://www.1win7015.ru .
The live games online rock—fan love! sugar rush
seo криптопроекта http://www.prodvizhenie-kriptovalyuta.ru .
провайдеры в красноярске по адресу проверить
domashij-internet-krasnoyarsk001.ru
интернет по адресу дома
https://opechatano.ru/plomby-dlja-oplombirovanija/nomernye-plastikovye-plomby-kupit/plomba-fast-150
https://opechatano.ru/plomby-dlja-oplombirovanija/nomernye-plastikovye-plomby-kupit/plomba-fast-330
https://opechatano.ru/plomby-dlja-oplombirovanija/nomernye-plastikovye-plomby-kupit/plomba-avangard-220mm
https://opechatano.ru/plomby-dlja-oplombirovanija/nomernye-plastikovye-plomby-kupit/plomba-pk-91-op-220
https://opechatano.ru/plomby-dlja-oplombirovanija/nomernye-plastikovye-plomby-kupit/plomba-pk-91-op-140
https://opechatano.ru/plomby-dlja-oplombirovanija/nomernye-plastikovye-plomby-kupit/plomba-pk-91-op-320
https://opechatano.ru/
https://opechatano.ru/plomby-dlja-oplombirovanija/nomernye-plastikovye-plomby-kupit/plomba-strela-520mm
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/opechativajushhie-ustrojstva-s-flazhkom/
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/opechativajushhie-ustrojstva-s-flazhkom/opechatyvayushchee-ustroystvo-flazhok-latun
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/opechatyvayushchie-ustroystva-dlya-zamochnyh-skvazhin/
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/plashki-dlya-opechatyvaniya-dverey-kipit/
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/plashki-dlya-opechatyvaniya-dverey-kipit/plashka-dlja-dverej-derevjannaja
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/plashki-dlya-opechatyvaniya-dverey-kipit/plashka-dlja-opechatyvanija-dverej-pod-1-pechat
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/latunnie-pechati-s-gravirovkoj/
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/opechatyvayushchie-ustroystva-dlya-seyfov/
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/opechatyvajushie-ustrojstva-shtok/
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/-pozharnaja-kljuchnica-kl-1
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kljuchnica-sejf-s-elektronnym-zamkom-kle-200
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kluchnica-kl-20
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kluchnica-kl-40
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kluchnica-kl-60
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kluchnica-kl-80
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kluchnica-kl-100
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kluchnica-kl-200
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kl-300
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kljuchnica-nastennaja-kl-340
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kl-400
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kl-600
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kljuchnica-kl-30s
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kljuchnica-kl-20s
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kljuchnica-kl-40s
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kljuchnica-sejf-s-elektronnym-zamkom-kle-200
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/shkafy-dlja-hranenija-kljuchej/kljuchnica-kl-50s
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/tubusy-penaly-dlja-hranenija-kljuchej/
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/birki-dlja-kljuchej-plastikovie-s-bumazhnoj-vstavkoj/birki-dlja-kljuchej-plastikovye-s-bumazhnoj-vstavkoj
https://opechatano.ru/vsjo-dlja-hranenija-kljuchej/tubusy-penaly-dlja-hranenija-kljuchej/penal-dlja-kljuchej-kart-usb-nositelej-universalnyj
https://opechatano.ru/kupit-plombirator-s-gravirovkoy2/metallicheskie-pechati-dlya-opechativaniya-kupit/
https://opechatano.ru/kupit-plombirator-s-gravirovkoy2/metallicheskie-pechati-dlya-opechativaniya-kupit/metallicheskie-pechati-pod-plastilin
https://opechatano.ru/kupit-plombirator-s-gravirovkoy2/kupit-plombirator-s-gravirovkoy/
https://opechatano.ru/kupit-plombirator-s-gravirovkoy2/kupit-plombirator-s-gravirovkoy/plashki-dlya-plombiratora
https://opechatano.ru/soputstvujushhie-tovari/provoloka-vitaja-plombirovochnaja/
https://opechatano.ru/kupit-kurerskie-pakety/kurer-pakety-kupit/
https://opechatano.ru/kupit-kurerskie-pakety/kupit-sejf-pakety/
https://opechatano.ru/kupit-kurerskie-pakety/pakety-dlja-soprovoditelnyh-dokumentov/
https://opechatano.ru/kupit-kurerskie-pakety/paket-plomba-duty-free/
https://opechatano.ru/plomby-dlja-oplombirovanija/plomby-dlja-plombiratora/plomba-plastmassovaja-10-mm
https://opechatano.ru/plomby-dlja-oplombirovanija/plomby-dlja-plombiratora/svincovye-plomby-kupit
взять авто в аренду авто взять где прокат
I love the payment options—super easy! aviator
взять машину на прокат в махачкале аренда автомобиля в махачкале
прокат город владивосток аренда автомобиля владивосток без водителя посуточно
прокат авто в спб без водителя машина в аренду город санкт петербург
Счетчики электричества с пультом Счетчики с пультом: Экономия или обман? В современном мире, когда цены на энергоресурсы неуклонно растут, многие ищут способы снизить свои коммунальные платежи. Одним из таких способов стали счетчики с пультом дистанционного управления, а также различные методы “модификации” приборов учета. Но так ли это выгодно и законно, как кажется на первый взгляд?
Купить диплом возможно через сайт компании. shaqoplus.com/profile/grettamora4376
вавада казино Вавада казино официальный сайт: Мир азарта и больших выигрышей открывает свои двери для каждого, кто ищет острых ощущений и возможности испытать свою удачу. Официальный сайт казино Вавада – это современная платформа, где собраны лучшие игры от ведущих разработчиков, щедрые бонусы и удобный интерфейс для комфортной игры.
WEB PAFI TERINTEGRASI: Peran Strategis Farmasi di Dunia Digital
Sekilas Tentang PAFI
Sejak kemerdekaan Indonesia, para Apoteker turut berperan aktif dalam pembangunan kesehatan nasional. Didirikannya Persatuan Ahli Farmasi Indonesia (PAFI) menjadi tonggak penting sebagai asosiasi di bidang farmasi. Berdasarkan Pancasila, PAFI bersumpah untuk:
• Meningkatkan derajat kesehatan masyarakat
• Menyempurnakan bidang farmasi di Tanah Air
• Menunjang kesejahteraan anggota
Platform Terpadu PAFI: Inovasi Teknologi untuk Farmasi Modern
Menghadapi tantangan era digital, PAFI memperkenalkan sistem terintegrasi ini – solusi canggih yang menunjang praktik farmasi profesional melalui:
? Update Terbaru – Kemudahan mendapatkan kebijakan kesehatan, perkembangan ilmiah, dan prospek pekerjaan
? Pelatihan Profesional – Layanan pelatihan dan sertifikasi daring
? Jejaring Profesional – Sarana kerjasama se-Indonesia
Platform ini meningkatkan peran PAFI dalam mengembangkan sistem kesehatan melalui penerapan inovasi digital.
Tantangan Kefarmasian Era Digital
Hadirnya platform digital PAFI merupakan tanda transformasi digital dalam bidang apoteker. Dengan terus mengembangkan fasilitas yang ada, PAFI berjanji untuk:
• Menggalakkan terobosan dalam farmasi
• Meningkatkan kriteria keahlian
• Meningkatkan akses kesehatan masyarakat
Penutup
PAFI dengan adanya sistem terpadu ini selalu berada di garda depan dalam menjembatani perkembangan teknis dengan praktik farmasi profesional. Terobosan ini bukan sekadar meningkatkan fungsi apoteker, tetapi juga memberikan sumbangsih konkret bagi pembangunan kesehatan di Indonesia di era digital.
I won a tourney online—wild high! plinko
перейти на сайт https://xn—-jtbjfcbdfr0afji4m.xn--p1ai/
содержание https://xn—–6kcacs9ajdmhcwdcbwwcnbgd13a.xn--p1ai/
Read about pills. https://edpillrx.top/# Find pill info. ed pills online
Узнать больше https://falcoware.com/rus/match3_games.php
casinos sin licencia en espa?a casinos sin licencia en espa?a .
I won spins online and got $25—nice win! fortune tiger
The mobile apps online lag—fix it! book of ra
другие https://xn—–6kcacs9ajdmhcwdcbwwcnbgd13a.xn--p1ai
Узнать больше https://xn—-jtbjfcbdfr0afji4m.xn--p1ai
проект перепланировки и переустройства
The music online pulls—vibe on! betclic
The visuals online wow—great sight! mines
бк 1win [url=https://1win7003.ru/]https://1win7003.ru/[/url] .
продвижение криптовалюты продвижение криптовалюты .
мостбет официальный сайт http://mostbet5003.ru .
Приобрести диплом университета!
Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов Российской Федерации. Документы производятся на настоящих бланках государственного образца. bcstaffing.co/employer/15808/radiplomy
SISTEM TERPADU PAFI: Kontribusi Utama Farmasi dalam Era Digital
Profil Singkat PAFI
Sejak Indonesia merdeka, para Tenaga Kefarmasian aktif berkontribusi dalam kemajuan kesehatan bangsa. Kehadiran Persatuan Ahli Farmasi Indonesia (PAFI) menjadi tonggak penting sebagai forum ahli di bidang farmasi. Berdasarkan Pancasila, PAFI berkomitmen untuk:
• Meningkatkan kualitas kesehatan publik
• Mengembangkan bidang farmasi di Tanah Air
• Meningkatkan kualitas hidup anggota
Sistem Digital PAFI: Solusi Digital untuk Kefarmasian Masa Kini
Menjawab kebutuhan digital, PAFI menghadirkan platform digital ini – platform inovatif yang menunjang profesi kefarmasian melalui:
? Informasi Terkini – Kemudahan mendapatkan peraturan farmasi, temuan terbaru, dan peluang karir
? Pengembangan Kompetensi – Program pelatihan dan sertifikasi daring
? Jaringan Ahli – Wadah kolaborasi bagi seluruh ahli farmasi
Sistem digital ini memaksimalkan sumbangsih PAFI dalam meningkatkan pelayanan kesehatan melalui adaptasi perkembangan teknologi.
Peluang Kefarmasian Era Digital
Adanya platform digital PAFI merupakan tanda transformasi digital dalam dunia kefarmasian. Dengan selalu menyempurnakan kapabilitas sistem, PAFI berkomitmen untuk:
• Memacu inovasi pelayanan kefarmasian
• Meningkatkan kriteria keahlian
• Meningkatkan akses kesehatan masyarakat
Epilog
PAFI melalui platform digital ini selalu berada di garda depan dalam memadukan inovasi digital dengan pekerjaan apoteker. Program ini tidak hanya memperkuat peran tenaga kefarmasian, tetapi juga berperan aktif bagi pembangunan kesehatan di Indonesia di era digital.
интернет провайдеры по адресу
domashij-internet-krasnoyarsk002.ru
интернет провайдеры по адресу красноярск
https://opechatano.ru/kupit-opechatyvajushie-ustrojstva/opechatyvajushie-ustrojstva-shtok/
читать https://xn—-jtbjfcbdfr0afji4m.xn--p1ai/
The ease of online gambling is tricky—watch out! bet on red
смотреть здесь https://xn—–6kcacs9ajdmhcwdcbwwcnbgd13a.xn--p1ai/prajs-list
сюда https://falcoware.com/rus/match3_games.php
mostbet mostbet .
Online bonuses mask—dig in! tome of madness
Drug details provided. https://edpillrx.top/# Access medication details. ed dysfunction treatment
The live help online saves—key aid! aviator
киржач купить аттестаты
Где заказать диплом специалиста?
Мы предлагаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам. Для нас важно, чтобы документы были доступными для большого количества наших граждан. Быстро купить диплом о высшем образовании diplomidlarf.ru/diplom-s-reestrom-kupit-nastoyashij-diplom-ofitsialno/
leptozan is an innovative weight loss formula crafted to help you naturally sculpt the body you’ve always desired.
mitolyn is a natural dietary supplement specifically outlined to enhance metabolism and support weight loss.
orm криптопроекта http://prodvizhenie-kriptovalyuta2.ru/ .
Приветствую!
Для определенных людей, купить диплом о высшем образовании – это острая необходимость, возможность получить достойную работу. Впрочем для кого-то – это очевидное желание не терять огромное количество времени на учебу в институте. С какой бы целью вам это не понадобилось, наша фирма готова помочь вам. Быстро, качественно и по доступной цене изготовим диплом любого ВУЗа и любого года выпуска на настоящих бланках со всеми необходимыми подписями и печатями.
Ключевая причина, почему многие люди покупают документ, – получить хорошую должность. Предположим, знания и опыт дают возможность человеку устроиться на желаемую работу, а подтверждения квалификации не имеется. При условии, что для работодателя важно наличие “корочки”, риск потерять хорошее место достаточно высокий.
Заказать документ о получении высшего образования вы сможете в нашем сервисе. Мы предлагаем документы об окончании любых ВУЗов России. Вы получите необходимый диплом по любым специальностям, любого года выпуска, включая документы старого образца. Даем 100% гарантию, что при проверке документов работодателями, подозрений не появится.
Ситуаций, которые вынуждают приобрести диплом о среднем образовании очень много. Кому-то срочно нужна работа, и необходимо произвести особое впечатление на начальство при собеседовании. Другие мечтают попасть в большую компанию, чтобы повысить свой статус и в будущем начать собственное дело. Чтобы не терять попусту годы жизни, а сразу начинать эффективную карьеру, применяя имеющиеся знания, можно заказать диплом через интернет. Вы станете полезным в социуме, обретете финансовую стабильность в кратчайший срок- диплом купить о высшем образовании
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
подключить интернет по адресу
domashij-internet-krasnoyarsk003.ru
провайдеры интернета по адресу красноярск
посетить веб-сайт https://falcoware.com/rus/match3_games.php
The mobile casino clogs—tune it! aviator
Приобрести диплом ВУЗа по доступной цене можно, обращаясь к надежной специализированной фирме. Мы оказываем услуги по продаже документов об окончании любых ВУЗов Российской Федерации. Приобрести диплом любого ВУЗа– vacshidiplom.com/kupit-gosudarstvennij-diplom-bistro-i-nadezhno-4/
1win am http://www.1win5030.ru .
Online casinos should teach newbies the rules—too confusing at first! fortune tiger
Drug brochure available. https://edpillrx.top/# Latest medicine developments. ed meds
The slot sounds online hum—fun vibe! aviator
Are online ratings solid?—tough guess! aviator
мосбет казино http://mostbet6033.ru .
I wish online paid swift—drag on! gransino
The slot thrill online soars—heart up! teen patti
I don’t get the hate for online gambling—it’s just like any other hobby! plinko
I lost big online—reboot now! plinko
Online casino ads hit hard—chill out! book of ra
Current medicine trends. https://edpillrx.top/# Medication trends described. new treatments for ed
какие провайдеры интернета есть по адресу краснодар
domashij-internet-krasnodar001.ru
узнать интернет по адресу
телефон эвакуатора спб https://avto-vezu.ru
1 вин официальный 1 вин официальный .
Online laws fuzz—sort out! joker jewels
1вин вход http://1win7016.ru/ .
The poker online glows—real rush! aviator bet
Мы можем предложить дипломы психологов, юристов, экономистов и других профессий по доступным тарифам. Мы предлагаем документы техникумов, которые расположены в любом регионе Российской Федерации. Документы печатаются на “правильной” бумаге высшего качества. Это дает возможность делать настоящие дипломы, не отличимые от оригинала. babygirls015.copiny.com/question/details/id/1082906
dragon money casino официальный http://www.dragon-money30.com .
Покупка диплома ВУЗа через проверенную и надежную компанию дарит немало достоинств для покупателя. Это решение позволяет сэкономить как личное время, так и значительные средства. Впрочем, только на этом выгоды не ограничиваются, преимуществ значительно больше.Мы изготавливаем дипломы любой профессии. Дипломы изготавливаются на настоящих бланках государственного образца. Доступная стоимость сравнительно с крупными издержками на обучение и проживание в чужом городе. Покупка диплома о высшем образовании из российского университета является мудрым шагом.
Приобрести диплом: diplom-onlinex.com/kupite-diplom-visshego-obrazovaniya-s-zaneseniem-v-reestr-8/
тревожная кнопка http://www.trevros.ru .
Создай идеальный взгляд! Наращивание ресниц Ивантеевка с учётом формы глаз и пожеланий клиента. Работаем с проверенными материалами, соблюдаем стерильность и комфорт.
Dosing guidelines here. https://edpillrx.top/# Complete drug overview. ed pills for sale
Online casinos need tools—help us! plinko
1win 1win5024.ru .
На сайте pinco можно найти всё для ставок и казино. Сайт пинко казино стабильно работает даже при блокировках. Pinco casino online работает без проблем на телефоне. Зарабатываю в Pinco на спортивных ставках — советую. Официальный сайт пинко обновляется регулярно и стабильно работает. Играю в pinco уже год — проблем не было. Пинко казино — это современная и надёжная платформа. Pinco casino поддерживает игры с живыми дилерами. Pinco поддерживает киргизский и русский язык pinco casino.
Быстро купить диплом ВУЗа!
Купить диплом ВУЗа по выгодной цене возможно, обращаясь к проверенной специализированной фирме. Быстро заказать диплом: diplomnie.com/diplom-s-reestrom-kupit-podlinnij-obrazets-vuza
провайдеры интернета по адресу
domashij-internet-krasnodar002.ru
интернет провайдеры по адресу дома
Online casinos feel odd—loss vibe! plinko
Приобрести диплом об образовании. Заказ официального диплома через качественную и надежную компанию дарит ряд достоинств. Это решение дает возможность сэкономить как длительное время, так и серьезные деньги. atlantarp.forumex.ru/viewtopic.php?f=36&t=964
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
https://www.hentai-foundry.com/user/moonblde1999/profile
https://permacultureglobal.org/users/72745-konrad-molina
https://rentry.org/mpv8doir
https://anotepad.com/notes/hkecje5d
https://ktotyt1991.diary.ru/
Online gambling flows free—rein it! plinko
I don’t trust online data—shaky! hot hot fruit
Задался вопросом: можно ли на самом деле купить диплом государственного образца в Москве? Был приятно удивлен — это реально и легально!
Сначала искал информацию в интернете на тему: купить диплом спбгу, купить диплом среднетехнического образования, купить диплом ссср в санкт петербурге, купить диплом старого образца москва, купить диплом теплотехника и получил базовые знания. В итоге остановился на материале: proffdiplomik.com/kursk
The live dealers online rule—true vibe! betonred
мос бет http://www.mostbet6043.ru .
методики планирование Планирование: Путь к Успеху, Гармонии и Свободе от Выгорания В бешеном ритме современной жизни, где каждый день бросает нам вызов, планирование становится не просто полезным навыком, а жизненной необходимостью. Это компас, указывающий верное направление среди бескрайнего моря возможностей и задач. Планирование – это искусство управления своим временем, своей энергией и, в конечном итоге, своей жизнью.
Online casinos are a fun way to pass the time, but I wish they had better odds. joker jewels
The payout waits online drag—speed on! aviator game online
Pill guide available. https://edpillrx.top/# Access medication details. cure ed
новости Новости азартного мира: от Спарк-Ру до онлайн-казино Мир азартных игр находится в постоянном движении, и чтобы оставаться в курсе последних событий, необходимо следить за ключевыми источниками информации. Одним из таких источников является Спарк-Ру – база данных, содержащая информацию о юридических лицах, включая те, что связаны с игорным бизнесом. Анализ данных Спарк позволяет отслеживать финансовые показатели казино, изменения в структуре собственности и другие важные аспекты деятельности.
UID_32834297###
penipu online
Где приобрести диплом специалиста?
Готовый диплом с необходимыми печатями и подписями отвечает запросам и стандартам, неотличим от оригинала – даже со специально предназначенным оборудованием. Не стоит откладывать свои цели на долгие годы, реализуйте их с нашей помощью – отправляйте заявку на изготовление диплома уже сегодня! Получить диплом о высшем образовании – запросто! privamaxsecurity.co.ke/employer/premialnie-diplom-24
Где заказать диплом по актуальной специальности?
Наша компания предлагает выгодно купить диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями должностных лиц. Диплом способен пройти лубую проверку, даже с использованием специфических приборов. Решите свои задачи быстро и просто с нашими дипломами.
Заказать диплом университета diplomskiy.com/kupit-diplom-slesarya-8/
Приобрести диплом на заказ возможно через сайт компании. babygirls020.copiny.com/question/details/id/1084488
Online casinos need signs—flag us! plinko
Jon Jones profile http://john-jones-ufc.com a fighter with a unique style, record-breaking achievements and UFC legend status. All titles, weight classes and the path to the top of MMA – on one page.
Онлайн-казино JoyCasino thejoycasino ru яркий дизайн, популярные слоты, live-игры и щедрые бонусы. Простой вход, быстрые выплаты и надёжная поддержка. Играйте с комфортом и без лишних рисков.
Professional fighter Rafael Fiziev https://rafael-fiziev.com/ is a UFC star known for his explosive technique and spectacular fights. A lightweight fighter with a powerful punch and a strong Muay Thai base.
Legendary boxer Mike Tyson mike-tyson-az.com/ is the undisputed heavyweight champion and one of the most recognizable athletes in history. His strength, speed, and charisma have made him a boxing icon.
I don’t trust online RNG—feels off! plinko
интернет по адресу дома
domashij-internet-krasnodar003.ru
интернет провайдеры в краснодаре по адресу дома
https://dzen.ru/77volvo.ru Премиальное Обслуживание Lixiang, Volvo и Land Rover в Москве В динамичном ритме столичной жизни ваш автомобиль – это не просто средство передвижения, это важная часть вашего комфорта и имиджа. Осознавая это, наш сервисный центр предлагает полный спектр услуг для владельцев автомобилей премиум-класса: Lixiang, Volvo и Land Rover. Мы специализируемся на поддержании безупречного состояния этих марок, обеспечивая надежность и долговечность вашего авто.
мостбет скачать андроид https://mostbet5003.ru .
Online casinos can hook—edge it! ganesha gold
один вин официальный сайт http://1win7004.ru/ .
снять место для хранения вещей в москве снять место для хранения вещей в москве .
Сервисный центр ФикситЦентр – выполнит срочный ремонт ресиверов в Москве
1win официальный 1win официальный .
Drug impacts explained. https://edpillrx.top/# Find medication info. generic ed drugs
elonbet
Are online reviews legit?—hard say! aviator juego
The sounds online catch—hook in! super hot fruits
Купить документ института можно у нас в столице. Мы оказываем услуги по продаже документов об окончании любых ВУЗов РФ. Вы сможете получить диплом по любым специальностям, любого года выпуска, включая документы Советского Союза. Гарантируем, что при проверке документа работодателями, подозрений не возникнет. diplomnie.com/kupit-diplom-texnikuma-s-vneseniem-v-reestr-otzivi-2/
mostbet kg mostbet6033.ru .
Также рекомендую вам почитать по теме – https://bestanimation.ru .
И еще вот – https://bestanimation.ru .
The poker online is sharp—love it! book of dead
мосбет казино https://mostbet6038.ru/ .
pg slot mahjong
SISTEM TERPADU PAFI: Fungsi Penting Farmasi di Dunia Digital
Sekilas Tentang PAFI
Pasca kemerdekaan RI, para Ahli Farmasi turut berperan aktif dalam kemajuan kesehatan bangsa. Didirikannya Persatuan Ahli Farmasi Indonesia (PAFI) menjadi langkah strategis sebagai forum ahli di bidang farmasi. Berlandaskan Pancasila, PAFI bersumpah untuk:
• Memajukan derajat kesehatan masyarakat
• Menyempurnakan kefarmasian nasional
• Memperbaiki kondisi para anggota
Sistem Digital PAFI: Solusi Digital untuk Kefarmasian Masa Kini
Menjawab kebutuhan digital, PAFI menghadirkan sistem terintegrasi ini – solusi canggih yang menunjang profesi kefarmasian melalui:
? Informasi Terkini – Kemudahan mendapatkan peraturan farmasi, riset mutakhir, dan prospek pekerjaan
? Pelatihan Profesional – Layanan kursus online bersertifikat
? Komunitas Praktisi – Wadah kolaborasi bagi seluruh ahli farmasi
Sistem digital ini meningkatkan peran PAFI dalam meningkatkan kesehatan nasional melalui adaptasi perkembangan teknologi.
Masa Depan Kefarmasian Era Digital
Keberadaan WEB PAFI Terintegrasi menandai perubahan sistem dalam bidang apoteker. Dengan terus mengembangkan fitur dan layanan, PAFI berkomitmen untuk:
• Menggalakkan pembaruan layanan farmasi
• Meningkatkan standar profesional
• Memperluas fasilitas kesehatan untuk rakyat
Kesimpulan
PAFI melalui WEB PAFI Terintegrasi terus menjadi pionir dalam menjembatani perkembangan teknis dengan pekerjaan apoteker. Program ini bukan sekadar meningkatkan fungsi tenaga kefarmasian, tetapi juga berkontribusi nyata bagi pembangunan kesehatan di Indonesia pada zaman teknologi.
I won $600 on a slot—hype high! aviator
мостбет кыргызстан скачать https://mostbet5004.ru .
The sounds online grab—can’t stop! razor returns
elonbet
Online casino ads hit hard—chill out! betonred
I won spins online and got $25—nice win! vincispin
интернет провайдеры по адресу дома
domashij-internet-samara001.ru
провайдер по адресу самара
Сервисный центр ФикситЦентр – выполнит срочный ремонт видеокамер в Москве
King of the ring roy jones Roy Jones is a fighter with a unique style and lightning-fast reactions. His career includes dozens of titles, spectacular knockouts and a cult status in the boxing world.
Football genius luka-modric.com Luka Modric – from humble beginnings in Zagreb to a world-class star. His path inspires, his play amazes. The story of a great master in detail.
Автопортал для водителей https://addinfo.com.ua и автолюбителей: свежие авто новости, сравнения моделей, рейтинги, советы по выбору и обслуживанию автомобилей. Полезная информация каждый день.
Мы можем предложить дипломы любых профессий по приятным ценам. Мы можем предложить документы техникумов, которые расположены в любом регионе Российской Федерации. Документы выпускаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригинала. taar.me/read-blog/15070_kupit-diplom-zanesennyj-reestr.html
Свежие новости https://actualnews.kyiv.ua Украины и мира онлайн: события, аналитика, интервью и факты. Будьте в курсе главного — обновления 24/7, объективная подача и лента новостей в реальном времени.
Купить диплом ВУЗа!
Мы готовы предложить документы институтов, расположенных на территории всей России.
dip-lom-rus.ru/kupit-diplom-cherez-reestr-bistro-i-nadezhno/
The live games online rock—fan love! plinko
The payout waits online drag—speed on! plinko
мостбет официальный сайт https://mostbet6043.ru/ .
баня из бруса купить Строительство бани под ключ в Ленинградской области: цены, проекты от производителя. Мечтаете о собственной бане на даче или загородном участке в Ленинградской области? Строительная компания бани-бани предлагает профессиональное строительство бань в СПб по выгодным ценам. Купить баню без предоплаты!
The slot thrill online soars—heart up! plinko
купить диплом мастера
Женский журнал https://asprofrutsc.org о стиле, красоте, психологии, отношениях и саморазвитии. Актуальные статьи, советы экспертов, тренды и вдохновение — всё для современной женщины.
Hi, what is your hobby? what do you do in spare time? personally love to play pokies
Где приобрести диплом по необходимой специальности?
Наши специалисты предлагают выгодно и быстро купить диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями официальных лиц. Наш документ способен пройти лубую проверку, даже с использованием специального оборудования. Достигайте свои цели быстро и просто с нашим сервисом.
Приобрести диплом любого ВУЗа diplomgorkiy.com/kupit-svidetelstvo-o-zaklyuchenii-braka-8/
Онлайн-журнал для женщин https://chernogolovka.net всё о жизни, любви, красоте, детях, финансах и личностном развитии. Простым языком о важном — полезно, интересно и по делу.
Автомобильный портал https://avto-limo.zt.ua для тех, кто за рулём: автообзоры, полезные советы, новости индустрии и подбор авто. Удобный поиск, свежая информация и всё, что нужно автолюбителю.
Авто журнал онлайн https://clothes-outletstore.com всё о мире автомобилей: новости, тест-драйвы, обзоры, советы, новинки автопрома и технологии. Читайте с любого устройства — всегда в курсе автоиндустрии.
Мы предлагаем дипломы психологов, юристов, экономистов и других профессий по приятным тарифам. Дипломы изготавливаются на подлинных бланках государственного образца Заказать диплом об образовании diplom-zentr.com
заказать проект перепланировки
Где купить диплом специалиста?
Приобрести диплом института по выгодной цене возможно, обратившись к надежной специализированной компании.: diplom-zakaz.ru
elonbet
Online casinos should shield—watch us! tome of madness
интернет провайдеры в самаре по адресу дома
domashij-internet-samara002.ru
провайдер интернета по адресу самара
тревожная кнопка цена http://www.trevros.ru .
The thrill of chasing a big win online is unmatched! fortune tiger
casinos online sin licencia casinos online sin licencia .
Заказала для видео-блога – зрители в восторге!
заказ цветов томск с доставкой
Приобрести диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по доступным тарифам— diplom4you.com/gde-kupit-attestat-9-klass-bistro-i-nadezhno/
разработка сайтов на битрикс под ключ разработка сайтов на битрикс под ключ .
Приобрести диплом ВУЗа по невысокой цене вы сможете, обратившись к проверенной специализированной фирме. Купить документ института вы имеете возможность в нашей компании. diplomk-vo-vladivostoke.ru/kupit-originalnij-diplom-s-vneseniem-v-reestr-2
Где приобрести диплом по актуальной специальности?
Наша компания предлагаетвыгодно купить диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями должностных лиц. Данный документ пройдет лубую проверку, даже при помощи специфических приборов. Решите свои задачи максимально быстро с нашей компанией. Заказать диплом о высшем образовании! gamesmaker.ru/forum/common/offtopics/#replyform
Online casinos should ban cheats—fair play! fortune tiger
Playing online bingo is a chill way to unwind! plinko
Online casinos feel like a trap sometimes—too easy to keep playing! gold party
виниловый сайдинг В современном строительстве выбор материалов играет ключевую роль в долговечности и эстетическом виде здания. Кровельные материалы и фасадные материалы формируют не только внешний облик, но и обеспечивают защиту от атмосферных воздействий.
The coin sounds in online slots are so satisfying! tome of madness
интернет провайдеры по адресу самара
domashij-internet-samara003.ru
узнать интернет по адресу
I wish online helped new—tough kick! fishin frenzy
The mobile casino is clunky—tweak it! fortune tiger bet
мостбет скачать [url=http://mostbet6033.ru]http://mostbet6033.ru[/url] .
драгон мани войти драгон мани войти .
1win md 1win md .
casinos sin deposito permitido en espa?a https://casinossinlicenciaespanola.com/ .
mostbet kg https://mostbet6038.ru/ .
Online casinos can hook—edge it! Book of Ra
dragon mani dragon-money30.com .
Приобрести диплом ВУЗа!
Мы можем предложить документы институтов, которые находятся в любом регионе Российской Федерации.
asxdiploman.com/kupit-diplom-s-zaneseniem-bistro-i-bez-problem/
I wish online cut stakes—low fun! plinko
Online casinos fit quiet—no fuss! fishing frenzy
проверить провайдеров по адресу уфа
domashij-internet-ufa001.ru
проверить провайдеров по адресу уфа
I won $400 online—best night in a while! mines
Online casino ads are relentless—enough already! bet on red
мостбет авиатор [url=https://www.mostbet6033.ru]https://www.mostbet6033.ru[/url] .
pariuri sportive moldova https://1win5025.ru/ .
Современный авто журнал https://ecotech-energy.com в онлайн-формате для тех, кто хочет быть в курсе автомобильных трендов. Новости, тесты, обзоры и аналитика — всегда под рукой.
I won spins online and got $300—sweet score! fishing frenzy
Авто журнал онлайн https://comparecarinsurancerfgj.org свежие новости, обзоры моделей, тест-драйвы, советы и рейтинг автомобилей. Всё о мире авто в одном месте, доступно с любого устройства.
Онлайн-портал для женщин https://fancywoman.kyiv.ua которые ценят себя и стремятся к лучшему. Всё о внутренней гармонии, внешнем блеске и жизненном балансе — будь в центре женского мира.
Женский портал https://elegantwoman.kyiv.ua о красоте, моде, здоровье, отношениях и вдохновении. Полезные статьи, советы экспертов, лайфхаки и свежие тренды — всё для современной женщины.
dragon money casino официальный https://dragon-money33.com .
I love the low bets online—soft play! Gioco Del Pollo
1 win казино https://1win7004.ru .
pg slot mahjong
PORTAL DIGITAL PAFI: Fungsi Penting Farmasi di Dunia Digital
Mengenal PAFI
Sejak Indonesia merdeka, para Apoteker aktif berkontribusi dalam kemajuan kesehatan bangsa. Didirikannya organisasi profesi farmasi ini menjadi langkah strategis sebagai asosiasi di bidang farmasi. Berdasarkan Pancasila, PAFI bersumpah untuk:
• Mengembangkan taraf kesehatan rakyat
• Memodernisasi farmasi Indonesia
• Meningkatkan kesejahteraan anggota
WEB PAFI Terintegrasi: Terobosan Modern untuk Farmasi Modern
Menjawab kebutuhan digital, PAFI meluncurkan sistem terintegrasi ini – terobosan teknologi yang menunjang profesi kefarmasian melalui:
? Data Real-time – Ketersediaan regulasi kesehatan, temuan terbaru, dan kesempatan profesional
? Pengembangan Kompetensi – Fasilitas kursus online bersertifikat
? Jaringan Ahli – Wadah kolaborasi nasional
Sistem digital ini meningkatkan peran PAFI dalam memajukan kesehatan nasional melalui pemanfaatan teknologi terkini.
Peluang Dunia Farmasi Modern
Hadirnya platform digital PAFI menandai revolusi teknologi dalam bidang apoteker. Dengan selalu menyempurnakan kapabilitas sistem, PAFI berkomitmen untuk:
• Mendorong terobosan dalam farmasi
• Menyempurnakan standar profesional
• Mempermudah fasilitas kesehatan untuk rakyat
Kesimpulan
PAFI berkat platform digital ini selalu berada di garda depan dalam menghubungkan perkembangan teknis dengan pekerjaan apoteker. Inisiatif ini bukan sekadar mengokohkan posisi apoteker, tetapi juga berperan aktif bagi pembangunan kesehatan di Indonesia di era digital.
Приобрести диплом ВУЗа!
Мы можем предложить дипломы любой профессии по выгодным тарифам. Вы приобретаете документ в надежной и проверенной временем компании. : freetenders.co.za/employer/frees-diplom
драгон мани зарегистрироваться драгон мани зарегистрироваться .
Служба по контракту Служба по контракту: Путь к профессиональной военной карьере Военная служба всегда была и остается почетным долгом гражданина перед своей страной. Однако, помимо обязательной службы по призыву, существует возможность связать свою жизнь с армией на профессиональной основе, заключив контракт с Министерством обороны Российской Федерации. Контрактная служба предоставляет уникальные перспективы для тех, кто видит в защите Родины не просто обязанность, а призвание.
Online casino ads push—soften it! vipzino
[b]Диплом университета РФ![/b]
Без получения диплома достаточно сложно было продвигаться по карьере. Поэтому решение о покупке диплома следует считать целесообразным. Купить диплом об образовании [url=http://moszel.flybb.ru/viewtopic.php?f=2&t=581/]moszel.flybb.ru/viewtopic.php?f=2&t=581[/url]
I wish online guided new—rough start! legacy of dead
провайдеры интернета в уфе по адресу проверить
domashij-internet-ufa002.ru
провайдеры интернета по адресу
Лучшие проститутки в Санкт-Петербурге, узнайте тут индивидуалки санкт петербург
I wish online linked more—life up! tiger fortune
[url=https://stromectol1h.top/#]buy Ivermectin online[/url] Formulation info listed. Pill trends described. purchase stromectol online no prescription
Семейный портал https://cgz.sumy.ua для родителей и детей: развитие, образование, здоровье, детские товары, досуг и психология. Актуальные материалы, экспертиза и поддержка на всех этапах взросления.
Online gambling feels loose—no look! plinko
Автомобильный сайт https://billiard-sport.com.ua с обзорами, тест-драйвами, автоновостями и каталогом машин. Всё о выборе, покупке, обслуживании и эксплуатации авто — удобно и доступно.
Родительский портал https://babyrost.com.ua от беременности до подросткового возраста. Статьи, лайфхаки, рекомендации экспертов, досуг с детьми и ответы на важные вопросы для мам и пап.
Автомобильный журнал https://eurasiamobilechallenge.com новости автоиндустрии, тест-драйвы, обзоры моделей, советы водителям и экспертов. Всё о мире автомобилей в удобном онлайн-формате.
The rewards online are a nice bonus—sweet! plinko
mostbet скачать mostbet7002.ru .
1вин официальный http://1win7005.ru/ .
Windows 10 Hide Activation Message
Windows Activation Command In Powershell
Windows Evaluation Activation
Windows Activation Types
Windows Activation Gui
Windows Activation
мастбет http://www.mostbet7001.ru .
шкаф купе на заказ по индивидуальным размерам Мебель на заказ – это инвестиция в комфорт и уют вашего дома. Это возможность создать пространство, которое будет радовать вас долгие годы.
Святки
Святки
pullets
belly-landing
denasalized
Loginov
rumbustion
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Online casinos should teach—too steep! fishin frenzy
провайдеры интернета по адресу уфа
domashij-internet-ufa003.ru
провайдеры интернета по адресу уфа
мостбет войти http://mostbet5004.ru/ .
Автомобильный сайт https://fundacionlogros.org с ежедневными новостями, обзорами новинок, аналитикой, тест-драйвами и репортажами из мира авто. Следите за трендами и будьте в курсе всего важного.
1win pariuri https://www.1win5026.ru .
Современный женский сайт https://femalebeauty.kyiv.ua мода, психология, семья, карьера, рецепты и лайфхаки. Ежедневно — новые материалы, рекомендации и интересные темы для каждой женщины.
Женский сайт о красоте https://female.kyiv.ua моде, здоровье, отношениях и саморазвитии. Полезные статьи, советы экспертов, вдохновение и поддержка — всё для гармоничной и уверенной жизни.
Читайте автомобильный сайт https://gormost.info онлайн — тесты, обзоры, советы, автоистории и материалы о современных технологиях. Всё для тех, кто любит машины и скорость.
I hit a blackjack run online—sweet day! mines
Online gambling feels free—no watch! plinko
Online bonuses hide stuff—check it! betonred
buy stromectol online Access medicine information. Medication data provided. buy stromectol
Векстрой уфа ВЕКСТРОЙ
Векстрой Уфа – это имя, которое звучит с уверенностью и надежностью в сфере производства металлоконструкций. Мы – не просто завод, мы – команда профессионалов, объединенных общей целью: создавать прочные, долговечные и эстетически привлекательные решения для наших клиентов.
Наш завод металлоконструкций оснащен современным оборудованием, позволяющим нам реализовывать проекты любой сложности. От разработки чертежей до финальной сборки – каждый этап производства находится под строгим контролем качества. Мы используем только сертифицированные материалы от проверенных поставщиков, что гарантирует соответствие нашей продукции всем необходимым стандартам и требованиям.
Мы предлагаем широкий спектр металлоконструкций: каркасы зданий и сооружений, промышленные эстакады, резервуары, ангары, рекламные конструкции и многое другое. Наш конструкторский отдел готов разработать индивидуальное решение, учитывающее все ваши пожелания и особенности проекта. Мы предлагаем полный цикл услуг, от проектирования до монтажа, обеспечивая максимальное удобство для наших клиентов.
Векстрой Уфа – это не просто поставщик металлоконструкций, это ваш надежный партнер в строительстве. Мы ценим доверие наших клиентов и стремимся превзойти их ожидания, предлагая продукцию высочайшего качества по конкурентоспособным ценам. Наша цель – внести свой вклад в развитие города и региона, создавая современные, безопасные и долговечные объекты.
I wish online held oldies—miss that! plinko
The online rush kicks—stay cool! aviator bet
Будучи студентом, я наслаждался учебой до тех пор, пока не пришло время писать диплом. Но паниковать не стоило, ведь существуют компании, которые помогают с написанием и защитой диплома на отличные оценки!
Изначально я искал информацию по теме: можно ли купить среднее образование, купить документ, купить диплом мед образования, купить диплом хирурга, сколько стоит купить диплом о среднем образовании, затем наткнулся на diplomybox.com/kupit-attestat-v-ivanovo
I wish online linked players—more vibe! andar bahar
The visuals online are epic—wow factor! plinko
мостбет кг [url=https://mostbet7001.ru]https://mostbet7001.ru[/url] .
Заказать диплом университета!
Мы предлагаем документы об окончании любых университетов РФ. Документы производят на подлинных бланках государственного образца. maziconsulting.com/kupit-diplom-gosudarstvennogo-obrazca-368
Are online casino ratings legit?—not sure! plinko
детские анекдоты Анекдоты. Анекдоты – это короткие, остроумные истории, призванные вызвать улыбку или взрыв хохота. Они являются неотъемлемой частью нашей культуры и передаются из поколения в поколение, сохраняя свою актуальность и способность развлечь.
2025年NBA免費線上看直播:籃球投注與即時更新的完整指南
隨著全球體育迷對線上博弈和賽事直播的需求不斷增長,NBA作為最受歡迎的籃球聯賽之一,自然成為了眾多球迷關注的焦點。2025年的NBA賽季將帶來更多精采的比賽,而如何免費觀看這些比賽並參與場中投注,已成為球迷們最關心的話題。本文將為您提供完整的NBA免費線上看直播教學、即時比分更新、以及相關的投注技巧。
一、NBA免費線上看直播的管道
不論是季後賽(4月-5月)還是總決賽(6月),NBA的每一場比賽都充滿激情與挑戰。以下是幾種常見的免費直播方式:
1. 線上直播平台
OB體育電視台 、鑫寶體育電視台 、SUPER體育電視台 等平台提供免費註冊服務,新用戶還可獲得168預測金,讓您在觀看比賽的同時也能進行運彩投注。
只需簡單註冊帳號,選擇【熊貓體育】或【RG富遊體育電視台】,即可輕鬆進入NBA籃球LIVE直播。
2. 電視轉播頻道
華視NBA籃球頻道 、ELTA TV 、以及中華電信MOD 也提供64場全賽事的LIVE轉播,適合喜歡透過電視欣賞比賽的球迷。
如果您偏好手機觀看,可以訂閱Hami Video NBA專區 ,每月僅需149元即可享受所有賽事的高清轉播。
二、如何註冊/登入以免費觀看NBA直播?
如果您想通過線上平台觀看NBA直播,以下是詳細步驟:
點選【RG官網】或【富遊娛樂城】官網,選擇「註冊/登入」。
輸入會員資料完成註冊。
登入後,點選【熊貓體育】或【體育直播】分類。
選擇NBA籃球視頻直播,即可免費觀看您感興趣的比賽。
三、NBA即時比分與場中投注
對於熱衷於運動彩券的球迷來說,NBA不僅是一場視覺盛宴,更是投注的好時機。以下是一些實用的投注建議:
即時比分更新 :透過【RG富遊體育電視台】或【熊貓體育】,您可以隨時掌握比賽進展,並根據比分變化調整投注策略。
場中投注技巧 :例如,在比賽關鍵時刻(如延長賽或最後三分鐘),利用即時數據進行快速下注,往往能提高勝率。
此外,NBA季後賽和總決賽期間,各平台通常會推出特別優惠活動,例如加倍獎金或贈送預測金,讓您的投注更有趣味性。
四、其他熱門賽事推薦
除了NBA之外,2025年還有許多值得期待的體育盛事:
2024夏季奧運 :涵蓋多項運動項目,是體育迷不容錯過的國際賽事。
2024歐洲盃 :足球迷的年度盛宴,各國勁旅爭奪最高榮譽。
2024 WBC世界棒球經典賽 :棒球愛好者的狂歡節,亞洲強隊表現備受矚目。
2023-2024英超聯賽 :足球迷必追的頂級聯賽,精彩程度無與倫比。
五、結語
無論您是單純的NBA球迷,還是熱衷於運動彩券投注的玩家,2025年的NBA賽季都將為您帶來無限樂趣。透過本文介紹的免費直播管道與投注技巧,您可以輕鬆享受每場比賽的刺激與精彩。現在就趕快註冊帳號,加入這場籃球狂歡吧!
立即行動!登入【熊貓體育】或【RG富遊體育電視台】,開啟您的NBA免費線上看之旅!
UID_93537352###
penipu online
I hit a bonus online and won $200—big win! aviator
https://www.instagram.com/oos_studio/
UID_96318461###
scam site
UID_75004935###
bokep
order stromectol over the counter Drug reactions explained. Medicine details here. purchase stromectol
Need real estate? luxury real estate montenegro villas, houses and apartments in Budva, Kotor, Tivat and on the coast. Profitable investment, sea view, safety and comfort in the south of Europe.
Zelite li se odmoriti? hoteli na Zabljaku Rezervirajte udoban hotel u centru ili u podnozju planina. Odlican izbor za skijanje i ljetovanje. Jamstvo rezervacije i stvarne recenzije.
need a move? toronto to calgary movers turnkey: packing, loading, transport, insurance and support. Without stress and with a guarantee of the safety of your property.
Автоперевозки из Китая https://upiter.novabb.ru/viewtopic.php?t=206 доставка грузов по РФ и странам СНГ. Сборные и индивидуальные партии, оформление, отслеживание и страхование. Быстро, надёжно, под ключ.
Online casino bonuses are sneaky—read up! dragon tiger
Мы можем предложить дипломы любой профессии по невысоким тарифам. Мы предлагаем документы техникумов, которые находятся в любом регионе России. Документы печатаются на “правильной” бумаге высшего качества. Это дает возможность делать государственные дипломы, которые не отличить от оригинала. lakarjobbisverige.se/employer/4936/archive-diploma
I won big online—wild joy! jackpot raider
тревожная кнопка стоимость trevros.ru .
Заказать диплом любого ВУЗа!
Мы предлагаем документы учебных заведений, расположенных в любом регионе Российской Федерации. Документы печатаются на “правильной” бумаге самого высокого качества: goroda.flybb.ru/viewtopic.phpf=2&t=862
Online casinos should have better tools for setting spending limits. book of dead
мостбет скачать бесплатно мостбет скачать бесплатно .
The online bonuses call—step in! plinko
разработка сайт 1с битрикс разработка сайт 1с битрикс .
Приветствую!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по выгодным ценам. Цена может зависеть от выбранной специальности, года выпуска и образовательного учреждения: rdiplomans.com/
order stromectol Side effects explained. Get pill facts. purchase stromectol
Сервисный центр ФикситЦентр – выполнит срочный ремонт музыкальных центров в Москве
Очень интересно:
https://stadium.ru/news/announcements/56288?indexpage=188
https://samara365.ru/news/ekonomika/kak-vybrat-idealnuyu-fasadnuyu-vyvesku-dlya-vashego-biznesa.htm
http://mk-reklama.ru/obemnyie-bukvyi-i-ih-preimushhestva/
http://www.griffmedia.ru/naruzhnaya_reklama_zalog_uspeshnogo_biznesa/
https://www.kraeved-samara.ru/archives/10214
http://business-guberniya.ru/search/razdel/16/252?razdlet=%D0%B0
https://promy.ru/blog/tri-reklamnyh-kita-internet-reklama-naruzhnaya-reklama-i-reklama-v-smi.html
https://samara.2rus.org/reklamno-izdatelskiy_kholding_absolyut
https://www.brg-cosmetics.ru/inform/narugh_reklama.htm
https://abc-paper.ru/reklamnaya-produkciya-vidy-osobennosti-pravila-vybora/
Советуем почитать:
http://smtp.allo63.ru/card/company/12576
http://www.rosmed.ru/annonce/show/3679/Sekrety_blestyashchey_ulybki
https://всяимплантация.рф/samara/clinics/ssk
https://booksmed.info/news/4659-kak-vybrat-horoshuju-stomatologicheskuju-kliniku-v-samare.html
https://aviadent.ru/publikacii/restavraciya-zubov-metody-preimushhestva-i-vazhnost
https://pulsstom.ru/restavratsiya-zubov/
http://lcsi.lg.ua/news/2012785808-chastnaya-stomatologicheskaya-klinika-ssk-v-samare/
https://1001doc.ru/samara/clinics/sovremennyy-stomatologicheskiy-kompleks-ssk
https://bashmed.ru/news/sovremennyy-stomatologicheskiy-kompleks-v-samare/
https://familyland.ru/statii/15/prichiny-i-vazhnost-svojevrjemjennogo-obrashhjenija-k-stomatologu
melbet kg скачать melbet kg скачать .
Заказать диплом университета !
Приобретение диплома ВУЗа России в нашей компании – надежный процесс, так как документ заносится в реестр. Приобрести диплом института diplomp-irkutsk.ru/kupit-diplom-s-vneseniem-v-reestr-bistro-i-kachestvenno
Наша компания предлагает максимально быстро купить диплом, который выполнен на оригинальном бланке и заверен печатями, штампами, подписями. Данный документ способен пройти любые проверки, даже с использованием специальных приборов. dog-ola.ru/viewtopic.php?f=28&t=8874
Купить диплом института по выгодной цене возможно, обратившись к проверенной специализированной компании. Приобрести документ института вы имеете возможность в нашем сервисе. socialcoin.online/read-blog/12573_kupit-diplom-s-registraciej-v-reestre.html
1win website https://www.1win18.com.ng .
1 win.com https://www.1win7017.ru .
Hi, what is your hobby? what do you do in spare time? personally love to play king bily casino
Приобрести диплом о высшем образовании!
Мы можем предложить документы институтов, которые находятся на территории всей Российской Федерации.
diplomass.com/diplom-visshego-obrazovaniya-s-zaneseniem-v-reestr-2/
I lost big online—new game plan! fruit cocktail
1win официальный сайт вход 1win официальный сайт вход .
I love the cheap play online—low risk! jackpot raider
I don’t get the hate for online gambling—it’s just like any other hobby! plinko
Сервисный центр ФикситЦентр – выполнит срочный ремонт видеоскопов в Москве
Online casinos need grip—too open! plinko
разработка на 1с битрикс razrabotka-saita-bx.ru .
Заказать диплом любого университета. Заказ официального диплома через надежную компанию дарит ряд преимуществ. Это решение позволяет сберечь как длительное время, так и значительные финансовые средства. cristian.5nx.ru/viewtopic.php?f=49&t=402
драгон мани демо https://dragon-money37.com .
как вывести с драгон мани http://www.dragon-money36.com .
расчет страховки на авто расчет страховки на авто .
подключить тревожную кнопку росгвардия подключить тревожную кнопку росгвардия .
I won $200 on a slot online—still hyped! blue wizard
Online casinos should give more free play! jackpotraider
подробнее Игровые движки
вход 1win https://1win7017.ru/ .
have a peek at this web-site https://binslist.com/
Здравствуйте!
Обеспечиваем рост видимости за счёт прокачки ПФ через Яндекс. Работаем через мобильный и десктопный трафик, задействуем регионы. Оплата только за результат, без подписок и скрытых платежей. Telegram: Contact @seomax_nakrutka_pf Реальные пользователи выполняют задания: переходы, клики, время на сайте.|Улучшаем поведенческие метрики сайта для стабильного роста в поиске. Реальные пользователи выполняют задания: переходы, клики, время на сайте. Индивидуальный подход к каждому проекту и точная аналитика. Telegram: Contact @seomax_nakrutka_pf Гибкая настройка сценариев поведения и работа по ключевым фразам.|Продвигаем сайты в Яндексе с помощью накрутки поведенческих факторов. Работаем через мобильный и десктопный трафик, задействуем регионы. Оплата только за результат, без подписок и скрытых платежей. Telegram: Contact @seomax_nakrutka_pf Реальные пользователи выполняют задания: переходы, клики, время на сайте.|Увеличиваем поведенческий трафик и снижаем отказ от сайта. Поддерживаем положительную динамику в Яндекс.Вебмастере. Индивидуальный подход к каждому проекту и точная аналитика. Telegram: Contact @seomax_nakrutka_pf Плавный рост и отсутствие санкций — результат нашей стратегии.|
накрутка сео, накрутка поведенческих факторов 2024, накрутка пф 5173454
сервис накрутки пф, накрутка пф, seo продвижение пф яндекс карты
Удачи!
Online bingo flows—great crew! plinko
Мы предлагаем дипломы любой профессии по приятным тарифам.
Вы покупаете диплом через надежную и проверенную временем фирму. Приобрести диплом института– http://firstcanadajobs.ca/employer/diplomy-grup-24/ – firstcanadajobs.ca/employer/diplomy-grup-24
Online casinos suit calm—no rush! plinko
1win https://1win5026.ru/ .
Лучшие проститутки в Санкт-Петербурге, узнайте тут https://t.me/siskispb221/
MetaMask Extension makes DeFi so easy. I can lend, borrow, and swap tokens seamlessly with full control over my funds.
monopoly big baller download
plinko echtgeld
склады временного хранения москва склады временного хранения москва .
Доброго!
Продвигаем сайты в Яндексе с помощью накрутки поведенческих факторов. Гибкая настройка сценариев поведения и работа по ключевым фразам. Индивидуальный подход к каждому проекту и точная аналитика. Telegram: Contact @seomax_nakrutka_pf Гибкая настройка сценариев поведения и работа по ключевым фразам.|Улучшаем поведенческие метрики сайта для стабильного роста в поиске. Поддерживаем положительную динамику в Яндекс.Вебмастере. Оплата только за результат, без подписок и скрытых платежей. Telegram: Contact @seomax_nakrutka_pf Анализируем нишу, моделируем естественное поведение, добиваемся результата.|Улучшаем поведенческие метрики сайта для стабильного роста в поиске. Работаем через мобильный и десктопный трафик, задействуем регионы. Настраиваем стратегию под цели клиента и анализируем результат. Telegram: Contact @seomax_nakrutka_pf Реальные пользователи выполняют задания: переходы, клики, время на сайте.|
программа накрутки поведенческих факторов, накрутка пф ботами, накрутка пф пф топ
сервис по накрутке пф, накрутка поведенческого фактора, накрутка поведенческих факторов без заданий
Удачи!
Заказать диплом на заказ возможно через сайт компании. contraband.ch/read-blog/101797
веб-сайте зеркало vodkabet
Приобрести диплом о высшем образовании. Покупка документа о высшем образовании через качественную и надежную компанию дарит немало достоинств. Это решение дает возможность сэкономить как личное время, так и значительные финансовые средства. eleeo-europe.com/employer/eonline-diploma
Для удачного продвижения по карьерной лестнице требуется наличие официального диплома о высшем образовании. Приобрести диплом института у сильной фирмы: diplomers.com/mozhno-li-kupit-attestat-9-klassa-5/
jackpot raider login
1 цшт 1 цшт .
plinko nederland
melbet кыргызстан http://melbet1002.ru .
Доставили под дверь офиса – коллеги ахнули от восторга!
цветы томск
betclic.cote d’ivoire
Заказать диплом о высшем образовании!
Мы можем предложить дипломы любой профессии по приятным тарифам. Вы покупаете диплом через надежную компанию. : 1abakan.ru/forum/showthread-365055
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
мастбет mostbet5006.ru .
betonred pl
plinko avis
1 ван вин http://mostbet5007.ru/ .
plinko online game
motsbet https://mostbet5006.ru .
Онлайн-портал про автомобили https://impactspreadsms.com с каталогом моделей, тестами, аналитикой, ценами и отзывами. Удобный поиск и полезные материалы — для тех, кто выбирает с умом.
Портал для женщин https://gracefulwoman.kyiv.ua которые ценят красоту жизни. Практичные советы, душевные статьи и поддержка — о том, как быть счастливой, уверенной и гармоничной каждый день.
Портал про авто https://impactspreadsms.com новости, обзоры, тест-драйвы, советы, сравнение моделей и актуальные тенденции в мире автомобилей. Всё для автолюбителей и профессионалов.
Современный женский https://happylady.kyiv.ua сайт с ежедневными обновлениями: советы по стилю, уходу, семье и психологии. Всё, что волнует и вдохновляет женщин сегодня — в одном месте.
1win online 1win online .
qual plataforma esta pagando agora fortune tiger
Купить Амфетамин в Киеве? Ссылка – COCAIN.VIP Доставка По Украине Амфетамина? Ссылка – COCAIN.VIP
.
.
.
.
.
| Купить онлайн закладку Амфетамин в Киеве ССЫЛКА – https://cocain.vip |
| Купить в другом городе Украины Амфетамин закладку ССЫЛКА – https://cocain.vip |
| Купить доставку Амфетамина в Киеве в руки через сайт ССЫЛКА – https://cocain.vip |
| Купить доставку в руки Амфетамин в Украине ССЫЛКА – https://cocain.vip |
| Купить Амфетамин в Украине доставкой через почту ССЫЛКА – https://cocain.vip |
| Купить Амфетамин в Киеве и Украине через Курьера в руки ССЫЛКА -https://cocain.vip |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
Слова для поиска –
Амфетамин в Киеве купить закладкой, Амфетамин в Киеве Купить з доставкой, Амфетамин в Киеве купить через курьера, Амфетамин в Киеве Купить быстро, Амфетамин в Киеве Купить сейчас, Амфетамин в Киеве Купить в руки, Амфетамин в Киеве Купить безопасно
Амфетамин в Украине Купить можно, Купить Амфетамин в Украине закладкой, Купить Амфетамин в Украине доставкой домой, Купить Амфетамин в Украине по почте, Купить Амфетамин в Украине без проблем можно, Купить Амфетамин в Украине на сайте, Кокс цена в Украине,
Виды Амфетамина Купить в Украине и Киеве, Чем отличаеться Амфетамин в Киеве от Амфетамина в Украине, Как я могу купить Амфетамин в Киеве, Купить Колумбийский Амфетамин в Киеве, Купить Колумбийский Амфетамин в Украине, Кокс Цена в Киеве,
Теги для городов –
Купить Амфетамин в Киеве, Купить Амфетамин в Одессе, Купить Амфетамин в Харькове, Купить Амфетамин во Львове, Купить Амфетамин в Днепре, Купить Амфетамин в Ужгороде, Купить Амфетамин в Ивано-Франковске, Купить Амфетамин в Белой Церкве, Купить Амфетамин в Ровно
Купить Амфетамин в Николаеве, Купить Амфетамин в Херсоне, Купить Амфетамин в Сумах, Купить Амфетамин в Запорожье, Купить Амфетамин в Житомере, Купить Амфетамин в Мукачево, Купить Амфетамин в Полтаве, Купить Амфетамин в Умани, Купить Амфетамин в Виннице
Общие теги для товаров-
Купить Амфетамин в Киеве, Купить Амфетамин в Украине, Купить Амфетамин в Киеве, Купить Амфетамин в Украине, Купить Лирику в Киеве, Купить Лирику в Украине, Купить Экстази в Киеве, Купить Экстази в Украине, Купить Амфетамин в Киеве, Купить Амфетамин
в Украине, Купить МДМА в Киеве, Купить МДМА в Украине, Купить Метамфетамин в Киеве, Купить Метамфетамин в Украине, Купить Метадон в Киеве, Купить Метадон в Украине, Купить Шишки в Киеве, Купить Шишки в Украине, Купить Бошки в Киеве,
Купить Бошки в Украине, Купить Альфа ПВП В Киеве, Купить Альфа ПВП в Украине, Купить A-PVP в Киеве, Купить A-PVP в Украине, Купить Соль СК в Киеве, Купить Соль СК в Украине, Купить Амфетамин в Киеве, Купить Амфетамин в Украине,
demo fortune tiger
Приветствую!
Для определенных людей, заказать диплом университета – это необходимость, удачный шанс получить хорошую работу. Однако для кого-то – это желание не терять огромное количество времени на учебу в институте. Что бы ни толкнуло вас на такой шаг, наша компания готова помочь. Оперативно, профессионально и по доступной цене изготовим документ любого года выпуска на государственных бланках со всеми подписями и печатями.
Основная причина, почему люди прибегают к покупке документа, – получить определенную должность. Допустим, навыки и опыт дают возможность человеку устроиться на привлекательную работу, однако подтверждения квалификации не имеется. При условии, что работодателю важно присутствие “корочек”, риск потерять место работы очень высокий.
Купить документ о получении высшего образования можно в нашей компании. Мы предлагаем документы об окончании любых ВУЗов РФ. Вы получите диплом по любым специальностям, любого года выпуска, включая документы образца СССР. Даем гарантию, что при проверке документов работодателем, никаких подозрений не возникнет.
Ситуаций, которые вынуждают приобрести диплом о среднем образовании достаточно. Кому-то срочно требуется работа, и необходимо произвести особое впечатление на руководителя на протяжении собеседования. Некоторые планируют устроиться в престижную компанию, для того, чтобы повысить собственный статус и в дальнейшем начать свой бизнес. Чтобы не терять время, а сразу начинать успешную карьеру, применяя имеющиеся знания, можно заказать диплом в интернете. Вы станете полезным в социуме, обретете финансовую стабильность максимально быстро и легко- купить диплом о среднем образовании
aviator
Свежие новости Украины https://fraza.kyiv.ua и мира — политика, экономика, общество, культура, технологии. Главные события дня, оперативные обновления и аналитика от экспертов.
Модный журнал онлайн https://icz.com.ua одежда, аксессуары, макияж, прически, уличный стиль и haute couture. Следите за последними тенденциями и читайте советы экспертов индустрии.
Современный портал https://lady.kyiv.ua для женщин: мода, уход, любовь, дети, стиль жизни и вдохновение. Полезный контент, тренды и темы, близкие каждой.
Актуальные новости Украины https://lenta.kyiv.ua и мира на одном сайте. Подборка ключевых событий, факты, интервью, мнения и видео. Честно, быстро и без фейков.
melbet kg скачать melbet kg скачать .
1win молдова http://www.1win5027.ru .
dragon and tiger game
mines game demo
免費nba直播
2025年NBA免費線上看直播:籃球投注與即時更新的完整指南
隨著全球體育迷對線上博弈和賽事直播的需求不斷增長,NBA作為最受歡迎的籃球聯賽之一,自然成為了眾多球迷關注的焦點。2025年的NBA賽季將帶來更多精采的比賽,而如何免費觀看這些比賽並參與場中投注,已成為球迷們最關心的話題。本文將為您提供完整的NBA免費線上看直播教學、即時比分更新、以及相關的投注技巧。
一、NBA免費線上看直播的管道
不論是季後賽(4月-5月)還是總決賽(6月),NBA的每一場比賽都充滿激情與挑戰。以下是幾種常見的免費直播方式:
1. 線上直播平台
OB體育電視台 、鑫寶體育電視台 、SUPER體育電視台 等平台提供免費註冊服務,新用戶還可獲得168預測金,讓您在觀看比賽的同時也能進行運彩投注。
只需簡單註冊帳號,選擇【熊貓體育】或【RG富遊體育電視台】,即可輕鬆進入NBA籃球LIVE直播。
2. 電視轉播頻道
華視NBA籃球頻道 、ELTA TV 、以及中華電信MOD 也提供64場全賽事的LIVE轉播,適合喜歡透過電視欣賞比賽的球迷。
如果您偏好手機觀看,可以訂閱Hami Video NBA專區 ,每月僅需149元即可享受所有賽事的高清轉播。
二、如何註冊/登入以免費觀看NBA直播?
如果您想通過線上平台觀看NBA直播,以下是詳細步驟:
點選【RG官網】或【富遊娛樂城】官網,選擇「註冊/登入」。
輸入會員資料完成註冊。
登入後,點選【熊貓體育】或【體育直播】分類。
選擇NBA籃球視頻直播,即可免費觀看您感興趣的比賽。
三、NBA即時比分與場中投注
對於熱衷於運動彩券的球迷來說,NBA不僅是一場視覺盛宴,更是投注的好時機。以下是一些實用的投注建議:
即時比分更新 :透過【RG富遊體育電視台】或【熊貓體育】,您可以隨時掌握比賽進展,並根據比分變化調整投注策略。
場中投注技巧 :例如,在比賽關鍵時刻(如延長賽或最後三分鐘),利用即時數據進行快速下注,往往能提高勝率。
此外,NBA季後賽和總決賽期間,各平台通常會推出特別優惠活動,例如加倍獎金或贈送預測金,讓您的投注更有趣味性。
四、其他熱門賽事推薦
除了NBA之外,2025年還有許多值得期待的體育盛事:
2024夏季奧運 :涵蓋多項運動項目,是體育迷不容錯過的國際賽事。
2024歐洲盃 :足球迷的年度盛宴,各國勁旅爭奪最高榮譽。
2024 WBC世界棒球經典賽 :棒球愛好者的狂歡節,亞洲強隊表現備受矚目。
2023-2024英超聯賽 :足球迷必追的頂級聯賽,精彩程度無與倫比。
五、結語
無論您是單純的NBA球迷,還是熱衷於運動彩券投注的玩家,2025年的NBA賽季都將為您帶來無限樂趣。透過本文介紹的免費直播管道與投注技巧,您可以輕鬆享受每場比賽的刺激與精彩。現在就趕快註冊帳號,加入這場籃球狂歡吧!
立即行動!登入【熊貓體育】或【RG富遊體育電視台】,開啟您的NBA免費線上看之旅!
Приобрести диплом университета по невысокой цене возможно, обратившись к проверенной специализированной компании. Мы предлагаем документы Институтов, которые находятся на территории всей России. vidagrafia.com/read-blog/186101
Окунитесь в мир страсти и экстрима с лучшим хентаем в сети!
Добро пожаловать на Смотреть Хентай онлайн — идеальное место для ценителей откровенных аниме-историй!
У нас собраны тысячи хентай-роликов в HD-качестве: от нежных романтических сцен до дерзких фантазий на любой вкус.
Почему выбирают нас?
Огромная коллекция Хентай на русском — только топовые тайтлы и редкие работы.
Без лагов и рекламы Хентай без цензуры— наслаждайтесь просмотром без раздражающих прерываний.
Удобный поиск — сортировка по тегам, жанрам и рейтингу.
Ежедневные обновления — новинки каждый день!
Только для 18+ — откройте дверь в мир безграничных фантазий!
Букет с подсолнухами – лето зимой!
букет цветов томск
мелбет кг http://melbet1002.ru/ .
Портал для женщин https://maleportal.kyiv.ua мода, красота, здоровье, отношения, карьера, семья и вдохновение. Актуальные темы, полезные советы и поддержка для каждой женщины — всё в одном месте.
Клуб для беременных https://mam.ck.ua и молодых мам: питание, подготовка к родам, уход за малышом, послеродовое восстановление. Полезная информация, консультации и поддержка в одном месте.
Онлайн-портал для современных https://madrasa.com.ua женщин. Всё, что волнует и вдохновляет: от красоты и моды до жизненного баланса, мотивации и личностного роста. Будь собой — с нами.
dragon money официальный сайт играть http://www.dragon-money37.com/ .
Главные новости https://lentanews.kyiv.ua из Украины и со всего мира — ежедневно и без искажений. Всё, что важно знать: внутренняя политика, экономика, международные события и прогнозы.
расчет осаго по номеру автомобиля http://www.seodict.ru/ .
aviator download
cheap accutane online Administration guidelines here. Pill effects explained. buy accutane 40 mg
demo fortune tiger
cazinouri online moldova 1win5027.ru .
1wln 1wln .
1 вин http://mostbet5007.ru .
арендовать склад для хранения личных вещей арендовать склад для хранения личных вещей .
plinko ?????
мостбет авиатор 1win5028.ru .
plinko app scam
Мы изготавливаем дипломы любых профессий по разумным тарифам. Всегда стараемся поддерживать для клиентов адекватную политику цен. Для нас очень важно, чтобы дипломы были доступны для подавляющей массы граждан.
Приобретение диплома, который подтверждает обучение в ВУЗе, – это разумное решение. Приобрести диплом университета: kupite-diplom0024.ru/kupit-diplom-bakalavra-11/
Сайт для деловых людей https://manorsgroup.com.ua актуальные материалы о финансах, инвестициях, рынке недвижимости и управлении капиталом. Аналитика, обзоры, экспертные мнения и полезные инструменты.
Женский онлайн-журнал https://mcms-bags.com о красоте, моде, психологии, отношениях и стиле жизни. Актуальные статьи, советы экспертов, вдохновение и всё, что важно для современной женщины.
недорогой интернет омск
domashij-internet-omsk001.ru
домашний интернет в омске
Новостной портал https://mediashare.com.ua с актуальной информацией из Украины, мира, политики, экономики, технологий, культуры и общества. Только проверенные источники и объективные материалы.
alarm clock radio with cd player and usb charging best clock radio review
czy gra plinko to oszustwo
plinko app
драгон мани вход https://dragon-money36.com .
plinko reviews
aviator download
https://gaznaauto.com.ua/
plinko real money
dragonmoney dragon-money37.com .
драгон мани вывод денег http://dragon-money42.com .
purchase accutane (isotretinoin) Medicine essentials explained. Medicine facts available. buy accutane nz
расчет страховки осаго расчет страховки осаго .
драгон мани кэшбэк dragon-money40.com .
mostbet игры https://www.mostbet7003.ru .
jogo vai de bet
plinko free
домашний интернет
domashij-internet-omsk002.ru
подключить интернет в квартиру омск
драгон мани кэшбэк http://dragon-money36.com .
plinko ball game online
bet on red
mostbet игры https://mostbet7003.ru .
plinko game online
President Joe Biden and Republican leader McCarthy said on Sunday (May 28) that a final bipartisan deal to raise the US debt ceiling – and avoid a cataclysmic default – now heads to Congress, which will need to pass the agreement before the government starts running out of money. 국산야동
провайдеры интернета в омске
domashij-internet-omsk003.ru
подключить интернет в омске в квартире
jeux plinko avis
mines game download
Также рекомендую вам почитать по теме – https://24-ad.ru .
И еще вот – https://mirka-master.ru.
Купить диплом об образовании. Заказ диплома ВУЗа через проверенную и надежную фирму дарит немало плюсов для покупателя. Это решение помогает сэкономить время и значительные средства. odnopolchane.net/forum/member.php?u=546804
Купить диплом на заказ в столице можно используя сайт компании. moskvic.actieforum.com/login
plinko app
Приобрести диплом ВУЗа!
Мы предлагаем документы учебных заведений, расположенных на территории всей России.
diplomt-nsk.ru/kupit-diplom-v-moskve-s-zaneseniem-v-reestr-3/
Наша компания предлагает выгодно купить диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Наш документ пройдет любые проверки, даже с использованием специального оборудования. dnd.listbb.ru/viewtopic.php?f=3&t=1991
Заказать диплом института по невысокой цене можно, обратившись к надежной специализированной компании. Приобрести документ университета вы имеете возможность в нашей компании. social.4mosthealthcare.com/read-blog/31_kupit-diplom-vuza-s-zaneseniem-v-reestr.html
Купить диплом ВУЗа!
Мы предлагаембыстро и выгодно приобрести диплом, который выполняется на оригинальном бланке и заверен печатями, водяными знаками, подписями. Наш документ способен пройти любые проверки, даже при использовании специфических приборов. Достигайте свои цели быстро и просто с нашей компанией- prepperforum.se/showthread.phptid=75795
Приобрести диплом ВУЗа по невысокой стоимости можно, обращаясь к проверенной специализированной фирме. Приобрести документ о получении высшего образования вы можете у нас в Москве. asxdiploman.com/diplom-s-zaneseniem-v-reestr-tsena-8
Мы предлагаем дипломы любой профессии по доступным тарифам. Мы предлагаем документы техникумов, которые расположены в любом регионе Российской Федерации. Документы делаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, которые не отличить от оригиналов. [url=http://forum.numizmatium.ru/forums/topic/nadezhnoe-oformlenie-diploma/]forum.numizmatium.ru/forums/topic/nadezhnoe-oformlenie-diploma[/url]
1win ваучер 1win ваучер .
fishin frenzy demo
Мы можем предложить дипломы любых профессий по разумным тарифам. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Важно, чтобы документы были доступны для подавляющей массы граждан.
Заказ диплома, подтверждающего обучение в ВУЗе, – это выгодное решение. Приобрести диплом о высшем образовании: diploms-vuza.com/kupit-diplom-obrazovaniya/
подключить домашний интернет в перми
domashij-internet-perm001.ru
домашний интернет
plinko app review
купить диплом вуза в нурсултане купить диплом вуза в нурсултане .
1win.pro 1win.pro .
Авто сайт с обзорами https://microbus.net.ua новостями, тест-драйвами, каталогом моделей, советами по выбору и эксплуатации автомобилей. Всё, что нужно автолюбителю — в одном месте.
Сайт для женщин https://miymalyuk.com.ua мода, красота, здоровье, отношения, карьера, семья и вдохновение. Актуальные статьи, советы и поддержка для современной женщины каждый день
Главные новости Украины https://novosti24.kyiv.ua и мира — честно, быстро и понятно. События, которые формируют завтрашний день, в одной ленте.
Актуальные новости Украины https://newsportal.kyiv.ua и мира сегодня: главные события, мнения экспертов, интервью, прогнозы. Оставайтесь в курсе с лентой, которая обновляется 24/7.
plinko
1win com http://1win7018.ru/ .
casino online 1win https://1win1007.top .
1win bet uganda 1win1004.top .
monopoly big baller hack
скачать 1win официальный сайт https://1win7007.ru .
aviator mostbet aviator mostbet .
подключить интернет
domashij-internet-perm002.ru
подключение интернета пермь
jogo do tigre gratis
Портал о здоровье и медицине https://pravovakrayina.org.ua узнайте больше о своём организме, симптомах, лечении и профилактике. Удобный поиск, рекомендации врачей, база клиник и аптек.
Онлайн-медицинский портал https://novamed.com.ua справочник болезней, симптомы, анализы, лекарства, консультации специалистов и актуальные новости здравоохранения. Всё о здоровье — в одном месте.
читайте на автомобильном https://proauto.kyiv.ua портале: тест-драйвы, сравнения, автоаналитика, обзоры технологий и новинки автопрома. Только актуальные материалы и честные мнения.
Современный авто портал https://quebradadelospozos.com свежие новости, каталог машин, рейтинг моделей, видеообзоры и полезные статьи. Помощь при покупке, советы по обслуживанию и анализ рынка.
plinko game
1вин партнерка 1вин партнерка .
betonred pl
nba免費線上看
2025年NBA免費線上看直播:籃球投注與即時更新的完整指南
隨著全球體育迷對線上博弈和賽事直播的需求不斷增長,NBA作為最受歡迎的籃球聯賽之一,自然成為了眾多球迷關注的焦點。2025年的NBA賽季將帶來更多精采的比賽,而如何免費觀看這些比賽並參與場中投注,已成為球迷們最關心的話題。本文將為您提供完整的NBA免費線上看直播教學、即時比分更新、以及相關的投注技巧。
一、NBA免費線上看直播的管道
不論是季後賽(4月-5月)還是總決賽(6月),NBA的每一場比賽都充滿激情與挑戰。以下是幾種常見的免費直播方式:
1. 線上直播平台
OB體育電視台 、鑫寶體育電視台 、SUPER體育電視台 等平台提供免費註冊服務,新用戶還可獲得168預測金,讓您在觀看比賽的同時也能進行運彩投注。
只需簡單註冊帳號,選擇【熊貓體育】或【RG富遊體育電視台】,即可輕鬆進入NBA籃球LIVE直播。
2. 電視轉播頻道
華視NBA籃球頻道 、ELTA TV 、以及中華電信MOD 也提供64場全賽事的LIVE轉播,適合喜歡透過電視欣賞比賽的球迷。
如果您偏好手機觀看,可以訂閱Hami Video NBA專區 ,每月僅需149元即可享受所有賽事的高清轉播。
二、如何註冊/登入以免費觀看NBA直播?
如果您想通過線上平台觀看NBA直播,以下是詳細步驟:
點選【RG官網】或【富遊娛樂城】官網,選擇「註冊/登入」。
輸入會員資料完成註冊。
登入後,點選【熊貓體育】或【體育直播】分類。
選擇NBA籃球視頻直播,即可免費觀看您感興趣的比賽。
三、NBA即時比分與場中投注
對於熱衷於運動彩券的球迷來說,NBA不僅是一場視覺盛宴,更是投注的好時機。以下是一些實用的投注建議:
即時比分更新 :透過【RG富遊體育電視台】或【熊貓體育】,您可以隨時掌握比賽進展,並根據比分變化調整投注策略。
場中投注技巧 :例如,在比賽關鍵時刻(如延長賽或最後三分鐘),利用即時數據進行快速下注,往往能提高勝率。
此外,NBA季後賽和總決賽期間,各平台通常會推出特別優惠活動,例如加倍獎金或贈送預測金,讓您的投注更有趣味性。
四、其他熱門賽事推薦
除了NBA之外,2025年還有許多值得期待的體育盛事:
2024夏季奧運 :涵蓋多項運動項目,是體育迷不容錯過的國際賽事。
2024歐洲盃 :足球迷的年度盛宴,各國勁旅爭奪最高榮譽。
2024 WBC世界棒球經典賽 :棒球愛好者的狂歡節,亞洲強隊表現備受矚目。
2023-2024英超聯賽 :足球迷必追的頂級聯賽,精彩程度無與倫比。
五、結語
無論您是單純的NBA球迷,還是熱衷於運動彩券投注的玩家,2025年的NBA賽季都將為您帶來無限樂趣。透過本文介紹的免費直播管道與投注技巧,您可以輕鬆享受每場比賽的刺激與精彩。現在就趕快註冊帳號,加入這場籃球狂歡吧!
立即行動!登入【熊貓體育】或【RG富遊體育電視台】,開啟您的NBA免費線上看之旅!
avis quantum ai
дешевый интернет пермь
domashij-internet-perm003.ru
подключить домашний интернет пермь
1 win сайт 1 win сайт .
plinko
fortuna tiger
Женский онлайн-журнал https://ruforums.net с разнообразными рубриками — от моды и макияжа до материнства и путешествий. Открывайте каждый день с новыми идеями и полезным контентом.
Современный женский журнал https://reyesmusicandevents.com стиль, уход, карьера, семья, рецепты и саморазвитие. Читайте свежие материалы каждый день — будь в тренде и в гармонии с собой.
Сайт про авто https://rusigra.org обзоры машин, автоновости, тест-драйвы, сравнения моделей, советы водителям и полезные статьи. Всё, что нужно автолюбителям — на одном ресурсе.
Журнал для женщин https://saralelakarat.com мода, красота, здоровье, психология, семья и вдохновение. Актуальные статьи, советы экспертов и интересные темы для женщин всех возрастов.
проведение оценки профессиональных рисков в Москве ocenka-profriskov495.ru .
согласовать проект перепланировки согласовать проект перепланировки .
casino online 1win 1win1007.top .
1с бухгалтерия сопровождение программного https://programmy-1s15.ru/ .
betonred
1win code bonus https://1win1004.top/ .
mitolyn is an advanced nutritional solution specifically created to help maintain balanced blood sugar levels and improve metabolic performance.
I hit a blackjack run online—sweet day! Vinci Spin
plinko fake
дешевый интернет ростов
domashij-internet-rostov001.ru
подключить интернет
fortune.tiger
mines in india
мелбет kg https://www.melbet1003.ru .
The convenient http://www.booking-zabljak.com service will help you find the perfect hotel for car travelers and active holiday lovers. A wide range of accommodation: from cozy guest houses to modern hotels with parking, Wi-Fi and breakfast. Book in advance and relax in comfort in the heart of Montenegro!
リアル ドール 貧 乳 ハウディ、私は時々あなたのブログを読みます、そして私は同じようなものを所有しています、そして私はあなたがたくさんのスパムの発言を受け取るかどうか疑問に思っていましたか?もしそうなら、どのようにそれを減らすのですか、プラグインまたはあなたがお勧めできるものは何ですか?
Онлайн-журнал для женщин https://zhenskiy.kyiv.ua которые ценят себя, любят жить ярко и стремятся к гармонии. Будь в курсе моды, заботься о себе и черпай вдохновение каждый день.
Портал о машинах https://shpik.info от новостей и технологий до экспертных статей и автомобильной аналитики. Узнайте всё о текущих трендах на рынке и новинках автопрома.
Портал о машинах https://xiwet.com от новостей и технологий до экспертных статей и автомобильной аналитики. Узнайте всё о текущих трендах на рынке и новинках автопрома.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Prada bag replica
The poker online shines—real kick! betonred casino
drgn зеркало [url=dragon-money42.com]drgn зеркало[/url] .
dragon money сайт [url=dragon-money40.com]dragon money сайт[/url] .
aviator game download
mitolyn is an advanced nutritional solution specifically created to help maintain balanced blood sugar levels and improve metabolic performance.
mostbet chrono http://www.mostbet6033.ru .
free plinko
Prada replica designer bags
application plinko
Chanel fake designer bags
Журнал про животных https://zoobonus.com.ua интересные статьи о питомцах, дикой природе, уходе, воспитании и поведении. Всё для тех, кто любит животных и хочет узнать о них больше.
дешевый интернет ростов
domashij-internet-rostov002.ru
домашний интернет тарифы
Полный гид по недвижимости https://all2realt.com.ua как выбрать, купить, продать или арендовать квартиру, дом или коммерческий объект. Обзоры, цены, тенденции рынка и юридические тонкости.
The rush of online gambling is hard to beat! vai de bet
Опубликуйте коса Калининград Куршская фото экскурсия в своем блоге.
Букет с анемонами – стильно и необычно!
цветы
safest online canada pharmacies Medicine information provided. Get pill details. online pharmacies
dragonmoney официальный сайт http://dragon-money42.com .
dragon money зеркало dragon money зеркало .
fortuna tiger
мостбет скачать http://mostbet6033.ru .
I hit a slump online—time to pause! plinko
game aviator
Мы готовы предложить дипломы любых профессий по невысоким тарифам. Дипломы производятся на настоящих бланках Приобрести диплом ВУЗа kupitediplom0027.ru
Chanel bag replica
арендовать бокс для хранения вещей hranim-veshi-msk24.ru .
Где заказать диплом по актуальной специальности?
Купить диплом института по выгодной цене можно, обращаясь к проверенной специализированной фирме.: kazdiplomas.com
провайдеры домашнего интернета ростов
domashij-internet-rostov003.ru
недорогой интернет ростов
1win.com.ci 1win.com.ci .
вход 1win https://1win7007.ru .
plinko ball
Louis Vuitton replica designer bags
Приобрести диплом любого института!
Купить диплом института по доступной стоимости можно, обращаясь к проверенной специализированной фирме. Заказать диплом: diplomus-spb.ru/diplom-visshego-obrazovaniya-vash-shans-na-uspex
Приобрести диплом любого университета!
Мы готовы предложить документы институтов, которые расположены в любом регионе РФ. Дипломы и аттестаты делаются на “правильной” бумаге самого высшего качества: msfo-soft.ru/msfo/forum/messages/forum24/topic18200/message415080/result=new#message415080
The live help online rocks—key boost! fishin frenzy
Где заказать диплом специалиста?
Готовый диплом с приложением 100% отвечает стандартам Министерства образования и науки, никто не сможет отличить его от оригинала – даже со специальным оборудованием. Не стоит откладывать собственные мечты и задачи на несколько лет, реализуйте их с нами – отправляйте быструю заявку на изготовление документа уже сегодня! Диплом о среднем специальном образовании – быстро и просто! sudanre.com/profile/rudyworsnop559
Заказать диплом об образовании!
Мы оказываем услуги по производству и продаже документов об окончании любых университетов России. Документы изготавливаются на настоящих бланках. l-avt.ru/support/dialog/?PAGE_NAME=profile_view&UID=108589
[url=https://cse.google.to/url?sa=t&url=https%3A%2F%2Ferectiledysfunctionpills365.top]drugs buy online[/url] Patient drug leaflet. Pill leaflet available. drugs buy
Louis Vuitton bag replica
Мы можем предложить дипломы любой профессии по разумным ценам.– diplomt-v-samare.ru/legalnoe-zanesenie-diploma-v-reestr-bez-xlopot/
fake Celine bag
vai de bet app
провайдеры домашнего интернета омск
domashij-internet-volgograd001.ru
провайдеры интернета омск
мелбет kg https://www.melbet1003.ru .
mostbet.kg mostbet5008.ru .
Online casinos should give more free play credits! fortune ox
Мы можем предложить дипломы психологов, юристов, экономистов и других профессий по доступным ценам.
Вы приобретаете диплом через надежную компанию. Приобрести диплом ВУЗа– http://flerus-shop.hcp.dilhost.ru/club/user/4/forum/message/2141/2137/#message2137/ – flerus-shop.hcp.dilhost.ru/club/user/4/forum/message/2141/2137/#message2137
Всегда думал что купить диплом о высшем образовании это миф и нереально, но все оказалось не так, изначально искал информацию про: купить диплом пту ссср, куплю диплом высшего образования санкт петербург, можно купить диплом техникума, проведение диплома купить, купить диплом в кызыле, потом про дипломы вузов, подробнее здесь diplomybox.com/voprosy-i-otvety
Заказать диплом о высшем образовании !
Покупка диплома ВУЗа РФ в нашей компании – надежный процесс, потому что документ будет заноситься в государственный реестр. Приобрести диплом об образовании diplomservis.com/kupit-diplom-s-reestrom-v-moskve-7
jogo do tigre gratis
Всех приветствую!
Для многих людей, купить диплом ВУЗа – это острая потребность, шанс получить хорошую работу. Однако для кого-то – это желание не терять огромное количество времени на учебу в институте. Что бы ни толкнуло вас на такой шаг, наша компания готова помочь вам. Максимально быстро, профессионально и по разумной цене изготовим документ любого года выпуска на государственных бланках со всеми печатями.
Основная причина, почему многие люди прибегают к покупке документов, – получить хорошую работу. Предположим, знания и опыт дают возможность специалисту устроиться на желаемую работу, однако подтверждения квалификации нет. Когда для работодателя важно наличие “корочки”, риск потерять вакантное место довольно высокий.
Приобрести документ ВУЗа вы имеете возможность в нашем сервисе. Мы предлагаем документы об окончании любых ВУЗов РФ. Вы получите диплом по любым специальностям, любого года выпуска, включая документы СССР. Даем 100% гарантию, что в случае проверки документов работодателями, никаких подозрений не появится.
Ситуаций, которые вынуждают приобрести диплом о среднем образовании много. Кому-то очень срочно нужна работа, а значит, нужно произвести впечатление на руководителя при собеседовании. Некоторые мечтают устроиться в престижную компанию, для того, чтобы повысить собственный статус и в последующем начать свой бизнес. Чтобы не терять впустую годы жизни, а сразу начать успешную карьеру, применяя врожденные таланты и полученные навыки, можно приобрести диплом прямо в интернете. Вы сможете быть полезным для общества, обретете денежную стабильность быстро и легко- диплом купить
Купить диплом университета по доступной цене возможно, обращаясь к надежной специализированной фирме. Мы можем предложить документы ВУЗов на Ваш выбор, расположенных в любом регионе России. ru-book.ru/blogs/81/Легкий-способ-получить-диплом
Где купить диплом по нужной специальности?
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Важно, чтобы дипломы были доступны для подавляющей массы наших граждан. Приобрести диплом любого института rusd-diplomj.ru/kupit-diplom-s-reestrom-v-rossii-10/
Здравствуйте!
Мы можем предложить дипломы любой профессии по приятным тарифам. Стоимость может зависеть от той или иной специальности, года получения и ВУЗа: rdiplomans.com/
Заказать документ университета можно в нашем сервисе. Мы оказываем услуги по изготовлению и продаже документов об окончании любых университетов России. Вы получите необходимый диплом по любым специальностям, любого года выпуска, в том числе документы Советского Союза. Даем гарантию, что при проверке документа работодателями, никаких подозрений не возникнет. diplom-club.com/pokupka-diploma-s-zaneseniem-v-reestr-bistro-i-nadezhno/
online plinko game
online pharmacies Drug impacts explained. Pill effects explained. no presription on line rx
Для максимально быстрого продвижения по карьерной лестнице понадобится наличие диплома института. Купить диплом института у проверенной компании: diplomgorkiy.com/kupit-nastoyashij-diplom-o-srednem-obrazovanii/
plinko gambling
Online casinos should guard—care more! aviator game
mostbet kg http://mostbet5008.ru .
подключить интернет
domashij-internet-volgograd002.ru
домашний интернет подключить омск
Экскурсия из Зеленоградска Куршская коса https://kurshskaya-kosa-ekskursii.ru/ сопровождается профессиональным гидом и включает посещение Визит-центра
1win pro 1win7008.ru .
Celine replica designer bags
The best online slots rise of olympus in one place: classics, new releases, jackpots and themed machines. Play without registration, test the demo or make real bets with bonuses.
Профессиональное агентство prvitruvio.ru: разработка рекламы, брендинг, digital-маркетинг, наружка и SMM. Комплексное продвижение для бизнеса любого масштаба.
Ручной пошив штор
пошив штор на заказ пошив штор на заказ .
1win мобильная версия сайта https://1win7020.ru .
мостбет вход мостбет вход .
АВТОТЕКА ЧИСТКА Чистка автотеки – улучшаем историю вашего авто! Наш сервис помогает удалить записи о ДТП, скорректировать пробег, убрать данные о расчетах и очистить данные о такси и каршеринге. Профессионально, быстро, конфиденциально. Вернем вашему авто идеальную историю и повысим его рыночную стоимость.
plinko balls
Louis Vuitton replica designer bags
1с бухгалтерия сопровождение программного http://programmy-1s15.ru .
оценка профессиональных рисков оценка профессиональных рисков .
The slot sounds online hum—fun vibe! fortune mouse
Закажите уникальные шторы на заказ, лучшие цены.
Идеальные шторы по вашим размерам, с гарантией качества.
Создание штор мечты, под любой стиль.
Эксклюзивные шторы на заказ, высокое качество материалов.
Заказать шторы на заказ для спальни, с учетом модных трендов.
Профессиональный пошив штор по вашим размерам, быстро и качественно.
Пошив штор для нестандартных окон, используя современные технологии.
Шторы на заказ с уникальным дизайном, от профессионалов.
Современные шторы на заказ, по вашему проекту.
Пошив штор на заказ по индивидуальным меркам, от ведущих мастеров.
Изготовление штор на заказ на любой вкус, с гарантией качества.
Доступные цены на шторы на заказ, с доставкой по Москве и регионам.
Модные шторы на заказ для вашего дома, от ведущих дизайнеров.
Премиум шторы на заказ, по вашему проекту.
Создание уникальных штор для любой комнаты, по мере необходимости.
Высококачественные шторы на заказ, под любой стиль.
сшить шторы на заказ сшить шторы на заказ . Prokarniz
legalonlinepharmacy.com Latest medicine developments. Medication data provided. no prescription pharmacy
мостбет войти http://mostbet5009.ru .
согласовать проект перепланировки согласовать проект перепланировки .
mines game download
домашний интернет тарифы
domashij-internet-volgograd003.ru
домашний интернет
Пошив штор любой сложности
пошив штор на заказ пошив штор на заказ .
Louis Vuitton fake designer bags
The variety of online slot themes is wild—something for everyone! bac bo
bet on red code promo
plinko slot
1с какие есть программы http://www.programmy-1s15.ru .
оценка профессиональных рисков в организации http://www.ocenka-profriskov495.ru .
To be yourself in a world that is constantly trying to make you something else is the greatest accomplishment. BJ벗방
The true meaning of life is to plant trees under whose shade you do not expect to sit 연예인 마약 사건
Сервисный центр ФикситЦентр – выполнит срочный ремонт электроскейтов в Москве
Купить натуральный камень облицовка фасада дагестанским камнем в Краснодаре и Краснодарском крае по оптовой цене. Каталог с расценками, размеры и монтаж дагестанской плитки из ракушечника, песчаника, травертина, известняка для отделки фасада и цоколя в Краснодаре от производителя.
generic ambien india no prescription Latest pill updates. Comprehensive drug facts. buy meds no rx
услуги профессиональных грузчиков https://semikarakorsk.standart-express.ru
квартирный переезд грузчики цена грузчики на дом
Лучшие шторы на заказ
пошив штор на заказ пошив штор на заказ .
грузчики заказать https://malaya-vishera.standart-express.ru
Сшить шторы на заказ по индивидуальному проекту, для квартиры.
Премиальные шторы на заказ, с гарантией качества.
Создание штор мечты, под ваш интерьер.
Эксклюзивные шторы на заказ, подчеркивающие ваш стиль.
Пошив штор на заказ для кухни, с индивидуальным подходом.
Профессиональный пошив штор по вашим размерам, под любой бюджет.
Пошив штор для нестандартных окон, используя современные технологии.
Шторы на заказ с уникальным дизайном, подчеркивающие вашу индивидуальность.
Современные шторы на заказ, с учетом светотени.
Создание штор для любого типа окна, с возможностью индивидуального дизайна.
Изготовление штор на заказ на любой вкус, с гарантией качества.
Пошив штор по индивидуальному проекту, с высоким качеством.
Модные шторы на заказ для вашего дома, под любой стиль интерьера.
Индивидуальный пошив штор на заказ, по вашему проекту.
Создание уникальных штор для любой комнаты, под ваш вкус.
Эксклюзивные ткани для пошива штор, под любой стиль.
сшить шторы на заказ сшить шторы на заказ . Prokarniz
free plinko
перепланировка офиса согласование перепланировка офиса согласование .
Купить диплом ВУЗа по невысокой стоимости возможно, обращаясь к надежной специализированной фирме. Приобрести документ о получении высшего образования можно у нас в столице. demo5.om-associates.in/employer/originals-diplomsi
кайт хургада
Заказать диплом любого института. Приобретение официального диплома через надежную фирму дарит ряд преимуществ для покупателя. Такое решение дает возможность сберечь время и существенные денежные средства. jivonews.ru/legalnyiy-sposob-poluchit-diplom
Online casinos suit calm—no rush! jackpotraider
Заказать диплом на заказ можно используя официальный сайт компании. t3arof.site/read-blog/120_diplom-vuza-s-zaneseniem-v-reestr.html
домашний интернет тарифы воронеж
domashij-internet-voronezh001.ru
домашний интернет
plinko ball
Также рекомендую вам почитать по теме – https://mirka-master.ru.
И еще вот – https://bestanimation.ru .
Заказать диплом университета по доступной стоимости вы сможете, обратившись к надежной специализированной фирме. Заказать документ ВУЗа вы сможете у нас в столице. [url=http://fastdiploms.com/kupit-diplom-instituta-s-provodkoj-po-dostupnoj-tsene/]fastdiploms.com/kupit-diplom-instituta-s-provodkoj-po-dostupnoj-tsene[/url]
aviator predictor apk
Заказать диплом любого ВУЗа!
Мы предлагаем документы любых учебных заведений, расположенных на территории всей России.
rusd-diplomj.ru/bistrij-i-nadezhnij-diplom-s-zaneseniem-v-reestr/
Мы изготавливаем дипломы любых профессий по приятным тарифам. Мы предлагаем документы ВУЗов, расположенных на территории всей РФ. Документы выпускаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, не отличимые от оригиналов. saturn.forumex.ru/viewtopic.phpf=19&t=695
1win кыргызстан 1win7020.ru .
Celine bag replica
Купить диплом ВУЗа по доступной цене можно, обращаясь к проверенной специализированной фирме. Мы оказываем услуги по продаже документов об окончании любых университетов Российской Федерации. Купить диплом ВУЗа– diplomh-40.ru/kachestvennij-diplom-visshego-obrazovaniya-po-dostupnoj-tsene-2/
Сшить шторы на заказ по индивидуальному проекту, для вашего дома.
Качественные шторы на заказ, быстро.
Изготовление штор на заказ, по вашим размерам.
Шторы на заказ с доставкой, высокое качество материалов.
Заказать шторы на заказ для спальни, с учетом модных трендов.
Профессиональный пошив штор по вашим размерам, под любой бюджет.
Заказ штор по проекту клиента, по желанию.
Эксклюзивные шторы на заказ, по вашему желанию.
Современные шторы на заказ, под любой интерьер.
Пошив штор на заказ по индивидуальным меркам, от ведущих мастеров.
Креативные шторы на заказ, под любой интерьер.
Изготовление штор на заказ быстро и недорого, с доставкой по Москве и регионам.
Модные шторы на заказ для вашего дома, под любой стиль интерьера.
Премиум шторы на заказ, подчеркните стиль вашего помещения.
Пошив штор по индивидуальному дизайну, от профессиональных мастеров.
Эксклюзивные ткани для пошива штор, с гарантией долговечности.
сшить шторы на заказ сшить шторы на заказ . прокарниз
мостбет казино войти https://mostbet5010.ru/ .
Заказать диплом о высшем образовании!
Наши специалисты предлагаютмаксимально быстро приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями. Наш документ пройдет лубую проверку, даже при помощи специально предназначенного оборудования. Достигайте свои цели быстро с нашей компанией- synthire.com/employer/gosznac-diplom-24
uk pharmacy online Active ingredients listed. Read about drugs. best online pharmacies
mostbet промокод mostbet промокод .
светодиодные рекламные экраны svetodiodnye-led-ekrany.ru/
профессиональные видеостены https://videostena-1.ru
melhor horario para jogar fortune tiger hoje
The sound of coins dropping in online slots is so satisfying! andar bahar
Добрый день смелым!
Благодаря своей конструкции, натяжные потолки способствуют улучшению тепло- и звукоизоляции помещения. Они помогают сохранять тепло в холодное время года и снижают уровень шума, проникающего снаружи. Это особенно актуально для квартир в многоквартирных домах и офисных помещений, где важно создать комфортную атмосферу.?
Вся информация на сайте – https://ru.pinterest.com/NovaPotolki/
одинцово натяжные потолки, почему втягивается натяжной потолок, монтаж натяжных потолков
натяжной потолок, как оформить потолок натяжной, марио натяжные потолки волгоград
Удачи!
Gucci fake designer bags
plinko game download pakistan
1win code bonus http://1win1005.top/ .
лучший интернет провайдер воронеж
domashij-internet-voronezh002.ru
домашний интернет тарифы воронеж
betonred
I love the themed promotions at online casinos—keeps it fresh! plinko
Gucci replica designer bags
Букет с подсолнухами – лето зимой!
букеты томск
Bottega Veneta replica designer bags
The popular service ai girlfriend generator offers to create an AI girl who understands you, can hold a conversation and even flirt. Ideal for those who are looking for emotions in a digital format.
Купить диплом ВУЗа по выгодной стоимости возможно, обратившись к надежной специализированной компании. Купить документ о получении высшего образования вы можете в нашей компании. remoterecruit.com.au/employer/originals-diplomsi
Наша компания предлагает выгодно приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями официальных лиц. Данный диплом пройдет любые проверки, даже при использовании специальных приборов. iamugandan.com/read-blog/7293_kupit-diplom-s-ocenkami.html
plinko casino polska
Мы готовы предложить дипломы любых профессий по приятным ценам. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы дипломы были доступными для большого количества граждан.
Заказ документа, который подтверждает обучение в университете, – это выгодное решение. Приобрести диплом любого университета: 1magistr.ru/kupit-diplom-vuza-visshee-2/
Приобрести диплом любого института. Приобретение документа о высшем образовании через надежную компанию дарит много плюсов. Это решение позволяет сберечь как дорогое время, так и существенные деньги. drochan.listbb.ru/viewtopic.php?f=4&t=2255
Купить диплом на заказ в столице вы можете используя сайт компании. [url=http://ourflo.mybb2.ru/posting.phpmode=post&f=45&sid=b1c9d894c42270626963bcf5133841b9/]ourflo.mybb2.ru/posting.phpmode=post&f=45&sid=b1c9d894c42270626963bcf5133841b9[/url]
Online casinos need flags—alert us! fishing frenzy
1win casino uganda http://www.1win1005.top .
fishin frenzy demo
Про гаджеты и электроннику, подробнее читайте – тут
подключить интернет тарифы воронеж
domashij-internet-voronezh003.ru
домашний интернет подключить воронеж
Купить диплом института по доступной стоимости возможно, обратившись к проверенной специализированной компании. Заказать документ о получении высшего образования можно у нас. kupitediplom.ru/kupit-originalnij-diplom-s-vneseniem-v-reestr-2
Приобрести диплом любого ВУЗа!
Мы предлагаем документы любых учебных заведений, расположенных в любом регионе России.
diplom4you.com/legalno-zanosim-diplom-v-reestr-bez-hassles-i-stressa/
pin up azerbaycan pin up azerbaycan .
dragon tiger game
Проститутки Королев
Проститутки Лобня
Марина приехала в Королёв из провинции в 18 лет, мечтая о работе в научном городке.
Но без образования и связей устроилась лишь официанткой. Зарплаты не хватало, и подруга предложила «легкий заработок».
Первый клиент был грубым, но деньги успокоили совесть.
Проститутки Королев выезд
Со временем Марина перешла на индивидуальные вызовы, снимая комнату в Лобне.
Здесь клиентов было больше — от гастарбайтеров до местных бизнесменов. Один, «постоянный», даже дарил подарки, но требовал exclusivity.
Она согласилась, надеясь на стабильность, но он исчез после задержания за мошенничество.
Дешевые роститутки Королев
В 25 Марина попала в облаву. Шантаж ментов забрал последние сбережения.
Она пыталась уйти, устроившись продавцом, но долги вернули её в профессию.
Проститутки Лобня выезд
Сейчас ей 30. Она всё ещё работает, но уже планирует открыть маникюрный салон — копит деньги и учится.
«Хочу забыть этот ад», — говорит она, затягиваясь сигаретой у вокзала в Королёве.
Дешевые проститутки Лобня
где можно купить диплом в москве где можно купить диплом в москве .
сухие трансформаторы купить сухие трансформаторы купить .
Мы предлагаем дипломы любых профессий по приятным тарифам. Мы готовы предложить документы ВУЗов, расположенных в любом регионе РФ. Документы выпускаются на бумаге самого высокого качества. Это позволяет делать настоящие дипломы, которые не отличить от оригинала. yhg.copiny.com/question/details/id/1083030
The mobile casino clogs—tune it! plinko
casino online 1win https://1win1008.top/ .
Приобрести диплом любого университета!
Наша компания предлагаетвыгодно и быстро купить диплом, который выполнен на оригинальной бумаге и заверен печатями, водяными знаками, подписями официальных лиц. Наш диплом пройдет любые проверки, даже при использовании специально предназначенного оборудования. Достигайте цели быстро и просто с нашей компанией- collabtherapy.com/forum/viewforum.phpf=2
Bottega Veneta fake designer bags
1win на телефон http://1win7008.ru .
мостбет скачать на андроид https://mostbet5009.ru/ .
1win mx 1win1008.top .
plinko gambling
The mobile casino dips—lift it! aviator
Привет всем!
Натяжные потолки не требуют сложного ухода. Достаточно периодически протирать их мягкой тканью, чтобы сохранить первоначальный вид. Они устойчивы к пыли и загрязнениям, не выцветают со временем и сохраняют свою форму на протяжении многих лет. Это делает их отличным выбором для тех, кто ценит практичность и долговечность.?
Вся информация на сайте – https://ru.pinterest.com/NovaPotolki/
окантовка на натяжной потолок, светильники под натяжные потолки, как выбрать потолок
как выбрать потолок, потолки натяжные с рисунками, км1 натяжные потолки
Удачи!
тарифы интернет и телевидение омск
domashij-internet-omsk001.ru
интернет тарифы омск
plinko’
Fendi replica designer bags
1 вин. https://www.1win7009.ru .
Online casinos feel dicey—double risk! plinko
vai de bet gratis
fake Fendi bag
fake prada bag
провайдеры интернета в омске
domashij-internet-omsk002.ru
тарифы интернет и телевидение омск
Мы изготавливаем дипломы любой профессии по приятным ценам. Стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы дипломы были доступными для большинства граждан.
Приобретение документа, подтверждающего обучение в университете, – это грамотное решение. Купить диплом о высшем образовании: kupit-diplomyz24.com/kupit-diplom-kolledzha-v-spb-2/
Hello! I’m at work surfing around your blog from my new iphone! Just wanted to say I love reading through your blog and look forward to all your posts! Carry on the excellent work!
https://blooms.com.ua/yak-obraty-idealne-sklo-dlya-far-vashogo-avto
I don’t trust online RNG—feels off! plinko
1вин войти https://1win7009.ru/ .
1win ug http://www.1win1006.top .
1вин партнерка https://1win7010.ru .
plinko casino
dragon and tiger game
казино онлайн kg https://www.mostbet5010.ru .
мостбет узбекистон mostbet3021.ru .
Про гаджеты и электроннику, подробнее читайте – здесь
The mobile casino needs work—spotty! aviator
aplikace plinko recenze
mostbet kg скачать http://www.mostbet7005.ru .
купить легальный диплом высшем rusdiplomm-orig.ru .
plinko game review
Prada replica designer bags
prostafense is a premium, doctor-crafted supplement formulated to maintain optimal prostate function, enhance urinary performance, and support overall male wellness.
интернет провайдер омск
domashij-internet-omsk003.ru
подключить домашний интернет в омске
aviator predictor
Ищите место для отдыха? туры в иорданию 2025 Мы предлагаем увлекательные экскурсии по Иордании, комфортный отдых и маршруты по главным достопримечательностям Иордании. Не знаете, что посмотреть в Иордании? Начните с Петры, Вади Рам и Мёртвого моря!
I won spins online and got $600—sweet score! craps game
fake Fendi bag
prostafense is a premium, doctor-crafted supplement formulated to maintain optimal prostate function, enhance urinary performance, and support overall male wellness.
Про гаджеты и электроннику, подробнее читайте – здесь
The ease of online gambling is a double-edged sword! Fortune Dragon
DIOR bag replica
fortune tiger demo
plinko app review
аренда склада для хранения вещей в московской области https://hranim-veshi-msk24.ru/ .
online plinko
pin up onlayn kazino http://www.pinup-azerbaycan51.com .
pinup pinup .
Диплом любого университета России!
Без наличия диплома очень непросто было продвигаться вверх по карьере. Именно из-за этого решение о покупке диплома можно считать рациональным. Приобрести диплом об образовании blogger-mania.mn.co/posts/81890125
play hot hot fruit demo
Приобрести диплом академии!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по доступным тарифам. Вы покупаете документ через надежную и проверенную фирму. : medforum.kabb.ru/viewtopic.php?f=2&t=1031
раскрутка сайтов раскрутка сайтов .
plinko balls
Про гаджеты и электроннику, подробнее читайте – здесь
The game visuals online are unreal—stunning! bet on red casino
plinko
мостбет скачать казино мостбет скачать казино .
Покупка документа о высшем образовании через надежную компанию дарит множество достоинств. Данное решение дает возможность сэкономить как личное время, так и значительные финансовые средства. Тем не менее, достоинств намного больше.Мы готовы предложить дипломы любой профессии. Дипломы производятся на настоящих бланках государственного образца. Доступная стоимость по сравнению с огромными расходами на обучение и проживание. Заказ диплома университета станет целесообразным шагом.
Приобрести диплом: diplom-onlinex.com/kupit-diplom-s-provodkoj-otzivi-i-soveti-po-viboru/
мос бет http://mostbet7005.ru/ .
Про гаджеты и электроннику, подробнее читайте – тут
1вин бет официальный сайт http://1win7010.ru/ .
what is plinko
Louis Vuitton fake designer bags
I lost big online—reboot now! jackpot raider
?? Looking for the Ultimate Live Nude Experience? ??
Join now and explore naughty live models – Watch, Chat & Have Fun Now! ??
Live Nude Webcams Explained
интернет провайдер пермь
domashij-internet-perm001.ru
домашний интернет подключить пермь
1win casino https://www.1win1009.top .
DIOR replica designer bags
Обзоры, рейтинги и новости про новинки гаджетов, читайте – здесь
Заказать диплом ВУЗа по невысокой цене возможно, обращаясь к проверенной специализированной компании. Заказать документ университета можно в нашем сервисе. bence.net/read-blog/17240
plinko ball
1winn 1winn .
The payout delays at online casinos are so frustrating! plinko
В Калининграде Куршская коса экскурсии https://kurshskaya-kosa-ekskursii.ru/ с посещением высоты Эфа
fake DIOR bag
pinup az?rbaycan pinup az?rbaycan .
vai-de bet
Ключ к успеху рекламное агентство Витрувий — точное понимание потребностей клиента. Мы разрабатываем комплексные маркетинговые кампании под любую нишу.
Louis Vuitton bag replica
лучший интернет провайдер пермь
domashij-internet-perm002.ru
интернет тарифы пермь
сухие трансформаторы сухие трансформаторы .
Посоветовали, как ухаживать за цветами!
заказать цветы с доставкой в томске
casino en 1 win http://1win1009.top/ .
Заказать диплом любого ВУЗа!
Мы готовы предложить документы любых учебных заведений, расположенных на территории всей России.
vacshidiplom.com/kupit-diplom-s-reestrom-po-nizkoj-tsene-bistro-i-legko/
недорогой интернет пермь
domashij-internet-perm002.ru
интернет тарифы пермь
Online gambling feels free—no watch! fishin frenzy
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы можем предложить документы ВУЗов, расположенных в любом регионе России. Документы выпускаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. kronverskiy.ru/posting.phpmode=post&f=28
hothot fruit
Купить диплом любого университета. Приобретение подходящего диплома через надежную компанию дарит немало плюсов. Данное решение помогает сэкономить как личное время, так и существенные деньги. samara.listbb.ru/viewtopic.php?f=3&t=1778
Заказать диплом на заказ вы можете используя официальный портал компании. [url=http://sampikrp.getbb.ru/viewtopic.phpf=27&t=2127/]sampikrp.getbb.ru/viewtopic.phpf=27&t=2127[/url]
predictor aviator
1win casino ug http://1win1006.top .
pin up azerbaycan pin up azerbaycan .
мостюет https://www.mostbet7006.ru .
сухие силовые трансформаторы купить сухие силовые трансформаторы купить .
Также рекомендую вам почитать по теме – https://mirka-master.ru.
И еще вот – https://l-spb.ru .
Louis Vuitton fake designer bags
I lost time online—fast gone! plinko
aviator app
1 win официальный сайт вход https://1win7026.ru/ .
Идеальные римские шторы для дизайнерских интерьеров
римские шторы на заказ римские шторы на заказ .
Модные тренды штор для загородного жилья
шторы в загородном доме шторы в загородном доме .”Ткацкий”
game aviator
провайдеры домашнего интернета пермь
domashij-internet-perm003.ru
подключить интернет тарифы пермь
Chanel bag replica
1win win https://1win7026.ru .
I love the online chats—cool folks! plinko
Получить диплом любого университета можем помочь. Купить диплом магистра в Новокузнецке – diplomybox.com/kupit-diplom-magistra-v-novokuznetske
Chanel fake designer bags
fake Gucci bag
the aviator game
Очень интересно:
купить ПВХ окна
купить пластиковые окна в спб
купить окна в спб
купить пластиковые окна
купить пластиковые окна в спб
пластиковые окна цена
купить окна
купить ПВХ окна
купить ПВХ окна
купить ПВХ окна в спб
plinko app erfahrungen
I wish online casinos had faster payouts—waiting kills the vibe! craps game
мостбет chrono мостбет chrono .
plinko romania
недорогой интернет ростов
domashij-internet-rostov001.ru
интернет домашний ростов
plinko game online real money
Купить диплом института по невысокой цене можно, обращаясь к проверенной специализированной фирме. Приобрести документ о получении высшего образования можно у нас в столице. good-diplom.ru/kupit-diplom-visshego-obrazovaniya-s-zaneseniem-v-reestr-27
SPA-салон в Москве https://uslugi.yandex.ru/profile/BonSpa-3013256 предлагает массаж, пилинг, обёртывания, уход за кожей, антистресс-программы и релакс-процедуры. Работают сертифицированные специалисты, используется премиум-косметика. Уютная атмосфера, индивидуальный подход и удобное расположение в городе.
Discover Montenegro weather-webcam-in-montenegro.com/zabljak crystal clear sea, picturesque bays, mountain landscapes and ancient fortresses. The perfect destination for those looking for a combination of nature, history and relaxation. Detailed guides, recommendations, photos and route ideas.
mostbet telefon orqali kirish https://mostbet3023.ru .
plinko
Наша компания предлагает выгодно заказать диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями должностных лиц. Наш диплом способен пройти лубую проверку, даже с использованием специально предназначенного оборудования. webnewsrealty.ru/poluchite-dokumentyi-ob-obrazovanii-ofitsialno-i-prosto
The music online lifts—keep on! doradobet
mostbet apk скачать http://www.mostbet7007.ru .
казино пи ап
https://plinko.kim
plinko game casino
https://plinko.kim
plunko
https://plinko.kim
скачать приложение плинко
https://plinko.kim
как играть в плинко на деньги
https://plinko.kim
Перестал работать ноутбук? Распространенные прблемы и методы решения – ремонт ноутбуков
pin up casino azerbaijan pin up casino azerbaijan .
pin up pin up .
fake Gucci bag
мастбет http://mostbet3022.ru/ .
мостбет http://www.mostbet6033.ru .
продвижение сайтов в москве продвижение сайтов в москве .
Сшить шторы на заказ по индивидуальному проекту, для офиса.
Премиальные шторы на заказ, быстро.
Изготовление штор на заказ, под ваш интерьер.
Лучшие ткани для штор на заказ, по индивидуальному дизайну.
Заказать шторы на заказ для спальни, с индивидуальным подходом.
Индивидуальный дизайн штор, по вашим желанием.
Создание штор на заказ из натуральных тканей, по желанию.
Шторы на заказ с уникальным дизайном, по вашему желанию.
Современные шторы на заказ, под любой интерьер.
Пошив штор на заказ по индивидуальным меркам, с возможностью индивидуального дизайна.
Премиальные ткани для штор на заказ, под любой интерьер.
Изготовление штор на заказ быстро и недорого, с доставкой по Москве и регионам.
Элегантные шторы на заказ, от ведущих дизайнеров.
Индивидуальный пошив штор на заказ, по вашему проекту.
Шторы на заказ с доставкой и монтажом, по мере необходимости.
Эксклюзивные ткани для пошива штор, с гарантией долговечности.
сшить шторы на заказ сшить шторы на заказ . Прокарниз
Идеальные римские шторы для дизайнерских интерьеров
римские шторы на заказ римские шторы на заказ .
подключение интернета ростов
domashij-internet-rostov002.ru
интернет домашний ростов
I love the pay flow online—easy run! plinko
Chanel fake designer bags
Здравствуйте!
Профессиональное SEO-продвижение сайтов под Яндекс и Google. Проводим технический аудит и устраняем ошибки в структуре сайта. Подходит для бизнеса любого масштаба — от локальных до федеральных. Продвигаем сайты по конкурентным и низкочастотным запросам.Комплексное продвижение сайта в топ поисковых систем. Проводим технический аудит и устраняем ошибки в структуре сайта. Результат закрепляется надолго — позиции удерживаются месяцами. Проводим технический аудит и устраняем ошибки в структуре сайта.
Вся информация на сайте – https://taplink.cc/prodvigenie_saita/
продвижение сайта, продвижение сайта, продвижение сайтов
продвижение сайта, продвижение сайта яндекс prodvigenie_saita, seo продвижение сайтов
Удачи!
1wiun https://1win7012.ru .
betclic
скачат мостбет https://mostbet6013.ru/ .
plinko.ball
free mines game
мостбет chrono мостбет chrono .
mostbet casino http://www.mostbet6040.ru .
pin up azerbaycan pin up azerbaycan .
predictor aviator
Online casino ads are relentless—enough already! minas juego
fake Celine bag
продвижение сайта продвижение сайта .
vip games
Gucci replica designer bags
plinko app review
интернет провайдеры ростов
domashij-internet-rostov003.ru
интернет домашний ростов
Купить диплом ВУЗа!
Мы предлагаем документы учебных заведений, расположенных в любом регионе РФ. Документы выпускаются на бумаге высшего качества: hramada.listbb.ru/viewtopic.phpf=7&t=1448
The slot themes online are dope—love it! plinko
Купить диплом о высшем образовании!
Заказать диплом ВУЗа по невысокой цене можно, обращаясь к проверенной специализированной компании. Заказать диплом: diplomt-v-chelyabinske.ru/ofitsialnij-diplom-rf-s-zaneseniem-v-reestr-bistro
Discover Montenegro holidays Ulcinj Montenegro crystal clear sea, picturesque bays, mountain landscapes and ancient fortresses. The perfect destination for those looking for a combination of nature, history and relaxation. Detailed guides, recommendations, photos and route ideas.
мостбет зеркало http://mostbet7006.ru .
гифт подарки подарочная карта купить в подарок
Перестал работать ноутбук? Распространенные прблемы и методы решения – ремонт ноутбуков
mostbet uz сом http://www.mostbet3022.ru .
Fendi bag replica
melhor horario para jogar fortune mouse
АВТОТЕКА ЧИСТКА Чистка автотеки – улучшаем историю вашего авто! Наш сервис помогает удалить записи о ДТП, скорректировать пробег, убрать данные о расчетах и очистить данные о такси и каршеринге. Профессионально, быстро, конфиденциально. Вернем вашему авто идеальную историю и повысим его рыночную стоимость.
Celine replica designer bags
Где заказать диплом по нужной специальности?
Приобрести диплом института по выгодной цене возможно, обращаясь к проверенной специализированной фирме.: diplomoz-197.com
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по доступным ценам. Дипломы производят на настоящих бланках Приобрести диплом об образовании freediplom.com
plinko deutschland
Дизайнерские шторы на заказ
пошив штор на заказ пошив штор на заказ .
Модные тренды штор для загородного жилья
шторы в загородном доме шторы в загородном доме .+7 (499) 460-69-87
тарифы интернет и телевидение омск
domashij-internet-volgograd001.ru
интернет провайдеры омск
Привет всем!
Профессиональное SEO-продвижение сайтов под Яндекс и Google. Продвигаем сайты по конкурентным и низкочастотным запросам. Подходит для бизнеса любого масштаба — от локальных до федеральных. Проводим технический аудит и устраняем ошибки в структуре сайта.Оптимизация и SEO-раскрутка сайтов от экспертов. Пишем уникальные SEO-тексты и улучшаем индексацию страниц. Ежемесячная отчётность и понятная стратегия продвижения. Пишем уникальные SEO-тексты и улучшаем индексацию страниц.
Вся информация на сайте – https://taplink.cc/prodvigenie_saita/
seo продвижение сайта, продвижение сайта, продвижение сайтов
продвижение сайта, seo продвижение сайтов таплинк, продвижение сайта
Удачи!
pin pun [url=https://www.pinup-azerbaycan51.com]pin pun[/url] .
pinup casino pinup casino .
aviator game online
how much does it cost to ship a car car transporter
mostbet kg скачать на андроид https://www.mostbet6013.ru .
Мы предлагаем дипломы любых профессий по доступным ценам. Стараемся поддерживать для заказчиков адекватную политику тарифов. Важно, чтобы дипломы были доступными для большого количества граждан.
Заказ диплома, который подтверждает окончание университета, – это грамотное решение. Приобрести диплом о высшем образовании: kupit-diplomyz24.com/diplom-visshee-kupit-6/
Где купить диплом специалиста?
Мы изготавливаем дипломы любых профессий по приятным ценам. Для нас очень важно, чтобы документы были доступны для большого количества наших граждан. Заказать диплом об образовании diplomp-irkutsk.ru/kupit-proverennij-diplom-7/
Celine fake designer bags
Добрый день!
Мы изготавливаем дипломы любой профессии по доступным тарифам. Стоимость будет зависеть от выбранной специальности, года получения и университета: diplomans.com/
Заказать диплом о высшем образовании!
Мы оказываем услуги по производству и продаже документов об окончании любых университетов РФ. Документы производятся на настоящих бланках. hatchingjobs.com/companies/radiplomy
The sound of coins dropping in online slots is so satisfying! aviator game
online dragon tiger
mines
Приобрести диплом института по выгодной цене вы можете, обращаясь к надежной специализированной фирме. Мы готовы предложить документы престижных ВУЗов, которые находятся в любом регионе Российской Федерации. cs-forever.phorum.pl/viewtopic.phpp=31424#31424
Заказать документ о получении высшего образования можно у нас в Москве. Мы оказываем услуги по продаже документов об окончании любых ВУЗов Российской Федерации. Вы получите диплом по любым специальностям, включая документы СССР. Гарантируем, что в случае проверки документов работодателями, подозрений не появится. [url=http://good-diplom.ru/kupit-diplom-s-reestrom-bistro-i-udobno-3/]good-diplom.ru/kupit-diplom-s-reestrom-bistro-i-udobno-3/[/url]
мостбет скачать бесплатно http://www.mostbet6040.ru .
Заказать диплом университета !
Покупка диплома университета РФ у нас – надежный процесс, ведь документ заносится в реестр. Заказать диплом университета diplom-top.ru/gde-kupit-diplom-o-visshem-obrazovanii-otzivi
Всегда считал, что покупка диплома о высшем образовании — это миф. Но, оказалось, что все возможно! Сначала искал информацию по теме: купить диплом техникума в москве, купить диплом россия, диплом о высшем образовании цена, купить аттестат образование, купить диплом в рязани, а затем перешел на дипломы вузов. Подробнее можно узнать здесь: diplomybox.com/kupit-attestat-v-omske
чистка автотека Чистка автотеки – улучшаем историю вашего авто! Наш сервис помогает удалить записи о ДТП, скорректировать пробег, убрать данные о расчетах и очистить данные о такси и каршеринге. Профессионально, быстро, конфиденциально. Вернем вашему авто идеальную историю и повысим его рыночную стоимость.
лучший интернет провайдер омск
domashij-internet-volgograd002.ru
провайдеры омск
Мы изготавливаем дипломы любых профессий по приятным тарифам.
Вы покупаете диплом в надежной и проверенной компании. Приобрести диплом университета– http://domovou.3nx.ru/viewtopic.phpp=14433#14433/ – domovou.3nx.ru/viewtopic.phpp=14433#14433
Online casinos make it too easy—watch it! plinko
plinko game is real or fake
Расскажем о ремонте техники – читайте в блоге
мостбет мобильная версия скачать https://mostbet6039.ru/ .
Приветствую!
Для некоторых людей, заказать диплом о высшем образовании – это острая потребность, удачный шанс получить достойную работу. Но для кого-то – это желание не терять массу времени на учебу в универе. Что бы ни толкнуло вас на это решение, наша компания готова помочь. Оперативно, качественно и по доступной цене сделаем документ нового или старого образца на государственных бланках со всеми необходимыми подписями и печатями.
Главная причина, почему люди покупают документы, – получить хорошую работу. К примеру, навыки и опыт позволяют кандидату устроиться на работу, а подтверждения квалификации нет. В том случае если работодателю важно присутствие “корочек”, риск потерять место работы очень высокий.
Приобрести документ института можно у нас в Москве. Мы предлагаем документы об окончании любых ВУЗов России. Вы сможете получить диплом по любым специальностям, включая документы старого образца. Даем гарантию, что в случае проверки документов работодателями, подозрений не возникнет.
Разных ситуаций, которые вынуждают приобрести диплом ВУЗа очень много. Кому-то срочно требуется работа, и необходимо произвести впечатление на руководителя на протяжении собеседования. Некоторые мечтают попасть в серьезную компанию, для того, чтобы повысить собственный статус в социуме и в дальнейшем начать свое дело. Чтобы не тратить впустую годы жизни, а сразу начать удачную карьеру, используя имеющиеся навыки, можно заказать диплом в онлайне. Вы станете полезным для общества, обретете денежную стабильность в кратчайший срок- аттестат купить за 9 класс
DIOR bag replica
Рыбалка в Подмосковье Москва и Подмосковье – регион с богатыми рыболовными традициями и разнообразием водоемов. От тихих прудов в городских парках до обширных водохранилищ и рек в области, здесь каждый рыболов найдет место по душе и по снасти. Будь то опытный профессионал или начинающий любитель, рыбалка в Московском регионе способна подарить незабываемые впечатления и богатый улов.
mostbetin http://mostbet3023.ru/ .
I won free spins online and cashed out $20—sweet deal! plinko
plinko balls
fake Bottega Veneta bag
провайдеры интернета в волгограде
domashij-internet-volgograd003.ru
подключение интернета омск
I’m gone to say to my little brother, that he should also visit this webpage on regular basis to take updated from most up-to-date gossip.
https://news-trading.com/
plinko game spribe
мостбет авиатор http://mostbet6039.ru .
мостбет казино https://mostbet6014.ru .
fishin frenzy slots
Приобрести диплом любого ВУЗа!
Мы готовы предложить документы университетов, расположенных в любом регионе Российской Федерации.
diplom4you.com/kupit-diplom-ob-obrazovanii-s-reestrom-bistro-i-legko/
car transport for dealerships cars vehicle transport
подарочная карта 500 рублей kupit-gift-kartu.ru/
I hate how online casinos push you to deposit more with pop-ups. aviator
Bottega Veneta replica designer bags
мастбет https://mostbet6014.ru/ .
Заказать диплом о высшем образовании. Заказ подходящего диплома через надежную фирму дарит ряд достоинств для покупателя. Такое решение позволяет сберечь время и значительные финансовые средства. haidenarazhodka.com/viewtopic.php?t=381739
pin-up online pin-up online .
The online bonuses shine—jump in! mines
клубные сеты миксы https://klubnaya-muzyka.ru/ .
cbd gummies sleep be suffering with cross one’s heart and hope to die helped me manage unexciting stress without making me sensation escape of it. I drop in an individual in the evening after feat, and within 30 minutes, I’m scheme more relaxed. It’s like a minuscule demented reset. They mode like regular bon-bons but take place with all the calming benefits of CBD. I was a fragment unsure at opening, but in this day I keep a quarrel in my cookhouse at all times. If you’re dealing with anxiety, prominence, or by a hair’s breadth need to unwind, these are a full lifesaver. Condign generate definite you’re getting them from a trusted sort!
крутая музыка крутая музыка .
1win 1win .
домашний интернет
domashij-internet-voronezh001.ru
провайдеры домашнего интернета воронеж
Файне місто Львів https://faine-misto.lviv.ua сайт Львова. Новости, события, места и обзоры.
1вин сайт онлайн http://www.1win712.ru .
DIOR bag replica
1win գրանցում 1win գրանցում .
fortune tiger demo
Купить диплом ВУЗа по невысокой цене возможно, обращаясь к проверенной специализированной компании. Приобрести документ ВУЗа вы имеете возможность в нашем сервисе. aytokariyer.com.tr/employer/originals-diplomsi
free plinko
мостбет скачать мостбет скачать .
Купить диплом на заказ возможно используя официальный портал компании. soniamittal0011.copiny.com/question/details/id/1084467
Online casinos should cap losses to protect players. Fortune Dragon
plinko scams
Bottega Veneta bag replica
Thank you for every other informative web site. Where else may just I am getting that kind of info written in such a perfect approach? I’ve a project that I am just now working on, and I’ve been at the look out for such information.
https://news-trading.com/info/money-for-posts
Также рекомендую вам почитать по теме – https://l-spb.ru .
И еще вот – https://mirka-master.ru.
I got this website from my buddy who told me on the topic of this web site and at the moment this time I am visiting this web page and reading very informative articles or reviews at this place.
https://news-trading.com/
aviator predictor login
predictor aviator
Мы предлагаем дипломы любой профессии по невысоким тарифам.– diplomass.com/kupite-diplom-s-reestrom-bistro-i-bezopasno-onlajn/
fake prada bag
Online casinos can be dangerous if you don’t set limits! betonred casino
fake Chanel bag
подключить интернет воронеж
domashij-internet-voronezh002.ru
тарифы интернет и телевидение воронеж
Для ценителей красивой эротики — comatozze. Смотрите comatozze video — не пожалеете. Все эмоции на лице — comatozze неподражаема. Смотреть comatozze одно удовольствие. Видео comatozze лучше любого кино. Если вы цените эстетику — comatozze ваш выбор. Подняли планку качества — comatozze рулит. Оценил новую подборку — comatozze снова в деле. Comatozze — это новое слово в жанре comatozze.
Заказать диплом университета!
Наши специалисты предлагаютвыгодно и быстро приобрести диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями официальных лиц. Документ пройдет лубую проверку, даже при использовании профессиональных приборов. Решите свои задачи быстро и просто с нашими дипломами- bence.net/read-blog/17425
1win официальный сайт войти 1win710.ru .
Мы изготавливаем дипломы любой профессии по доступным ценам. Мы можем предложить документы техникумов, которые находятся в любом регионе России. Дипломы и аттестаты печатаются на “правильной” бумаге высшего качества. Это дает возможности делать государственные дипломы, не отличимые от оригиналов. blog.kazakh-zerno.net/2025/04/06/oficialnye-dokumenty-ob-okonchanii-vuza.html
Приобрести диплом об образовании. Приобретение диплома через надежную фирму дарит ряд плюсов для покупателя. Данное решение позволяет сберечь время и значительные денежные средства. collie.fatbb.ru/viewtopic.php?f=18&t=6246
Licensed online casino jetx casino popular slot machines, live dealers, promotions and bonuses. User-friendly interface, quick registration, many payment systems and 24/7 support. Play your favorite games and win without leaving your home.
Aviator Bangladesh https://app.talkshoe.com/user/ghostplanes is your gateway to fun and fast gaming! No complex rules — just launch, bet, and cash out on time. Supported by leading Bangladeshi casinos, with BDT payments and full mobile access. Join and win anytime, anywhere.
Для удачного продвижения вверх по карьере понадобится наличие диплома о высшем образовании. Приобрести диплом об образовании у надежной фирмы: diplomgorkiy.com/kupit-attestat-v-omske-3/
Online casinos need better self-exclusion options. jackpot raider
1win am 1win5031.ru .
мастбет https://mostbet6042.ru/ .
Заказать диплом университета по доступной стоимости можно, обращаясь к проверенной специализированной фирме. Заказать документ о получении высшего образования вы можете в нашей компании в столице. shuvalduet.getbb.ru/viewtopic.phpf=3&t=2034
Шторы на заказ — индивидуальный подход к вашему интерьеру
шторы на заказ [url=https://shtory-moscow-zakaz.ru/]шторы на заказ[/url] . “Ткацкий”
Заказать диплом любого ВУЗа!
Мы готовы предложить документы ВУЗов, расположенных в любом регионе РФ. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высшего качества: masterflower.listbb.ru/viewtopic.phpf=6&t=1185
Приобрести диплом о высшем образовании!
Заказать диплом университета по выгодной стоимости возможно, обращаясь к проверенной специализированной компании. Заказать диплом: diplomass.com/ofitsialnij-moskovskij-diplom-s-reestrom-bistroe-oformlenie
Chanel bag replica
plinko geld verdienen
подключить домашний интернет в воронеже
domashij-internet-voronezh003.ru
интернет провайдер воронеж
scooby comic top comics online full color
Лучшие мастерские по пошиву штор, обратитесь..
Пошив штор для вашего дома, с гарантией качества..
Индивидуальный пошив штор под любую комнату, по индивидуальному проекту..
Изготовление штор на заказ, закажите онлайн..
Быстрый пошив штор, под ключ..
Пошив штор премиум-класса, по индивидуальному заказу..
Дизайнерские шторы на любой интерьер, Позвольте нам помочь..
Шторы на любой вкус и цвет, вам под силу..
Изысканный пошив штор, для элитных интерьеров..
Экспертный пошив штор в кратчайшие сроки, звоните прямо сейчас..
Идеальные шторы для вашего пространства, гарантия соответствия..
Разнообразие тканей и стилей, подходящий ваш стиль..
Элегантные шторы на заказ, под ключ..
Индивидуальный дизайн штор, с индивидуальным подходом..
Пошив штор для любого помещения, подчеркивающих ваш стиль..
Высокое качество и стиль, с гарантией долговечности..
Индивидуальные шторы на заказ, с бесплатной консультацией..
пошив штор пошив штор . “Ткацкий”
read manga dogs HD manga reader online
1вин сайт онлайн 1win712.ru .
1win офф сайт https://1win710.ru/ .
Заказать диплом на заказ в Москве вы можете через официальный портал компании. etoprosto.ru/ru/forum/category=5&action=topic
I hit a blackjack wave—good run! aviator
aviator game online
Prada replica designer bags
Купить диплом университета по выгодной стоимости можно, обратившись к надежной специализированной компании. Купить документ о получении высшего образования вы сможете в нашем сервисе. diplom-ryssia.com/kupit-diplom-o-visshem-obrazovanii-reestr-16
Доставка воды в Львове здесь
Play Aviator Game https://www.uscgq.com/forum/posts.php?forum=&id=278988&page=1#post0 online and win real money! Easy to play, exciting crash mechanics, fast rounds, and instant cashouts. Trusted by thousands of players worldwide — start now and catch the multiplier before it flies away!
plataforma de jogos fortune tiger
Привет гемблеры!
Auf Casino — лёгкий старт, яркая игра. Регистрация занимает секунды, а выигрыши — запоминаются надолго. Начни прямо сейчас — твоя удача уже ждёт. Auf — это не мечта, а реальность. Приходи — будет жарко.
Вся информация по ссылке – https://t.me/aufofficial_casino
ауф casino телеграм, auf casino телеграм, auf казино telegram
Хороших джекпотов!
Привет охотники за адреналинчиком!
Auf Casino — здесь даже маленькие ставки приносят большие выигрыши. Азарт доступен каждому, независимо от суммы. У нас ценится каждая ставка. Побеждают не только “все в банк”. Auf — для всех игроков.
Вся информация по ссылке – https://t.me/aufofficial_casino
ауф casino, ауф casino, auf казино отзывы
Хороших игр!
склад хранения личных вещей в москве склад хранения личных вещей в москве .
1 win md https://1win706.ru .
Где заказать диплом специалиста?
Получаемый диплом с приложением отвечает требованиям и стандартам, неотличим от оригинала. Не откладывайте собственные цели на потом, реализуйте их с нашей помощью – отправляйте заявку на изготовление диплома прямо сейчас! Диплом о среднем образовании – быстро и легко! demo5.om-associates.in/employer/premialnie-diplom-24
1win сайт https://1win711.ru .
Заказать диплом университета!
Мы предлагаемвыгодно заказать диплом, который выполнен на оригинальном бланке и заверен печатями, штампами, подписями официальных лиц. Наш диплом способен пройти лубую проверку, даже с применением специальных приборов. Решите свои задачи быстро и просто с нашей компанией- sathiharu.com/read-blog/20555_kupit-proverennyj-diplom.html
пополнить стим в россии 2025 Пополнить Steam на сайте ZloyPay с низкой комиссией. Работает в россии: оплата картой РФ или СБП. Моментальное пополнение в рублях, тенге и долларах. Пополняй Стим выгодно уже сегодня.
fake prada bag
free mines game
Лучшие шторы для спокойствия и уюта, выберите стиль, советы по выбору штор для дачи, теплота и уют, максимальная функциональность, современные материалы для штор, эффективные шторы для загородного дома, идеи дизайна штор, лучшие шторы для кухни в загородном доме, шторные решения для больших окон, шторы из натуральных материалов для уюта, автоматические шторы для загородного дома, стили штор для различных комнат, шторы как часть интерьера, обеспечьте комфорт с нашими шторами, выбор стильных штор для загородного дома, плюсы и минусы разных видов штор, используйте шторы для зонирования пространства, подбираем шторы под сезон
шторы в загородном доме шторы в загородном доме .
I don’t get online hate—it’s cool! bet on red
game plinko
Мы изготавливаем дипломы любых профессий по приятным тарифам. Дипломы производят на оригинальных бланках государственного образца Купить диплом университета fastdiploms.com
Где заказать диплом специалиста?
Приобрести диплом института по доступной стоимости вы можете, обращаясь к надежной специализированной фирме.: diplom-zakaz.ru
pin. up pin. up .
pin.up casino pin.up casino .
Гранитный завод «Век Памяти» предлагает полный спектр услуг по изготовлению памятников и мемориальных комплексов — от разработки индивидуального дизайна до монтажа. Мы гарантируем высокое качество и внимание к каждой детали.
Наши услуги:
Памятники, мемориальные комплексы, скульптуры, ограды, столы и вазы.
15 лет опыта и более 9000 успешных заказов.
Индивидуальный подход и ручная работа.
Современные технологии и высококвалифицированные мастера.
«Век Памяти» — надежность, качество и профессионализм. Мы поможем создать памятник, который будет достойно хранить память о ваших близких. изготовление памятников санкт петербург
Заказать диплом университета!
Мы оказываем услуги по изготовлению и продаже документов об окончании любых университетов России. Документы производят на настоящих бланках. 4division.ru/viewtopic.php?f=13&t=10100
home delivery car dealerships https://crctransport.us/b2b-car-hauling-dealer-to-dealer-transfers/
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по доступным тарифам. Купить диплом в Артеме — kyc-diplom.com/geography/artem.html
car hauling companies https://crctransport.us/leveraging-technology-for-smarter-and-more-efficient-car-hauling/
For dealerships, efficient and reliable car delivery services are vital to maintaining customer satisfaction and optimizing inventory management. Dealer transport services specialize in moving vehicles between dealerships, from auction sites, or directly to customers. These services often involve specialized logistics and tracking systems to ensure timely and secure delivery. Whether it’s transporting a single vehicle or managing a fleet of cars, dealerships rely on experienced car transport companies to handle their transportation needs.
Мы изготавливаем дипломы любой профессии по выгодным ценам. Мы предлагаем документы ВУЗов, которые находятся на территории всей Российской Федерации. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это дает возможности делать государственные дипломы, не отличимые от оригиналов. quora.com/profile/Bleakskippingjam/Диплом-высшего-образования-является-документом-подтверждающим-успешное-завершение-образовательной-программы-в-университ
шлюха сосет buy heroin cocaine
Где заказать диплом по необходимой специальности?
Мы предлагаем дипломы любой профессии по приятным ценам. Важно, чтобы дипломы были доступными для подавляющей массы наших граждан. Приобрести диплом о высшем образовании kupit-diplom24.com/kupit-diplom-s-reestrom-45/
The visuals online wow—great sight! plinko
дивитися нові фільми 2025 новинки кіно 2025 дивитися безкоштовно
1win. 1win706.ru .
Стильные шторы на заказ — дизайн по вашему желанию
шторы на заказ шторы на заказ . Ткацкий
Добрый день!
Мы изготавливаем дипломы любых профессий по выгодным тарифам. Цена может зависеть от определенной специальности, года получения и университета: rdiploman.com/
plinko demo
Hello everyone!
Alisha Larry Joins the Ranks of History’s Best Cover Artists. Like Johnny Cash’s Hurt or Whitney’s I Will Always Love You, great covers redefine songs. Alisha Larry’s take on Can’t Get You Out of My Head—out now via Globex Music—might just join that elite list. > Judge for yourself https://music.apple.com/us/song/cant-get-you-out-of-my-head/1805203094
can t get you out of my head dance
can’t get you out of my head
Happy listening!
tome of madness
fake Celine bag
Наша компания предлагает быстро и выгодно приобрести диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями должностных лиц. Наш документ пройдет любые проверки, даже с использованием профессиональных приборов. d9talks.site/read-blog/7875_mozhno-kupit-diplom-vysshem-obrazovanii.html
Купить диплом университета по выгодной цене вы можете, обратившись к проверенной специализированной фирме. Заказать документ о получении высшего образования можно у нас в столице. diplom-zentr.com/legalnoe-zanesenie-diploma-v-reestr-bez-problem-2
Заказать диплом ВУЗа !
Приобретение диплома любого университета России у нас – надежный процесс, так как документ заносится в государственный реестр. Приобрести диплом о высшем образовании diplom-club.com/gde-kupit-diplom-s-zaneseniem-v-reestr-bistro-i-bezopasno-2
pin up casino az pin up casino az .
pinap https://pinup-azerbaycan6.com .
Купить диплом института по выгодной стоимости можно, обратившись к надежной специализированной фирме. Мы можем предложить документы университетов, которые находятся в любом регионе России. perfectohub.com/read-blog/11411
Задался вопросом: можно ли на самом деле купить диплом государственного образца в Москве? Был приятно удивлен — это реально и легально!
Сначала искал информацию в интернете на тему: купить диплом отзывы, где купить диплом о среднем образовании, купить диплом мед, купить диплом о среднем специальном образовании цена, диплом купить отзывы и получил базовые знания. В итоге остановился на материале: diplomybox.com/kupit-attestat-v-barnaule
Online casinos feel off—loss streak! Betclic
plinko ball
Приобрести документ университета вы можете в нашей компании. Мы оказываем услуги по продаже документов об окончании любых ВУЗов России. Вы получите диплом по любой специальности, включая документы Советского Союза. Даем гарантию, что при проверке документа работодателями, подозрений не возникнет. vacshidiplom.com/kupit-diplom-visshego-obrazovaniya-s-vneseniem-v-reestr/
monopoly big baller results
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по выгодным тарифам.
Вы приобретаете документ в надежной и проверенной компании. Приобрести диплом о высшем образовании– http://delta72.at.ua/forum/61-31133-1/ – delta72.at.ua/forum/61-31133-1
Louis Vuitton bag replica
plinko opinie
plinko apk download
1win https://1win5035.ru/ .
Всех приветствую!
Для многих людей, заказать диплом университета – это острая необходимость, удачный шанс получить достойную работу. Но для кого-то – это желание не терять время на учебу в ВУЗе. С какой бы целью вам это не потребовалось, наша фирма готова помочь. Оперативно, профессионально и по доступной цене сделаем документ нового или старого образца на настоящих бланках со всеми требуемыми подписями и печатями.
Главная причина, почему многие люди покупают документ, – желание занять определенную должность. Предположим, знания и опыт дают возможность специалисту устроиться на желаемую работу, однако подтверждения квалификации нет. Если работодателю важно наличие “корочки”, риск потерять место работы очень высокий.
Купить документ института вы имеете возможность в нашей компании. Мы предлагаем документы об окончании любых университетов Российской Федерации. Вы получите диплом по любым специальностям, включая документы старого образца. Даем 100% гарантию, что в случае проверки документов работодателями, подозрений не появится.
Ситуаций, которые вынуждают купить диплом ВУЗа немало. Кому-то очень срочно необходима работа, таким образом нужно произвести впечатление на руководителя при собеседовании. Другие желают устроиться в большую компанию, для того, чтобы повысить свой статус в обществе и в дальнейшем начать собственное дело. Чтобы не тратить впустую годы жизни, а сразу начинать удачную карьеру, применяя врожденные способности и приобретенные навыки, можно купить диплом в интернете. Вы станете полезным в социуме, получите денежную стабильность в кратчайший срок- аттестат купить за 9 класс
узнать футболка оверсайз
Louis Vuitton fake designer bags
Hello everyone!
Behind the Cover: How Alisha Larry Reinvented a Pop Classic. We talked to Alisha Larry about her bold take on Can’t Get You Out of My Head: “I wanted to keep the original’s magic but make it hit harder for today’s audiences.” The result? A bass-heavy, vocal-driven banger, out now via Globex Music. > Hear the transformation https://music.apple.com/us/song/cant-get-you-out-of-my-head/1805203094
i can’t get you out of my head cover
can t get you out of my head just dance
Happy listening!
Заказать диплом университета по невысокой стоимости вы сможете, обратившись к проверенной специализированной компании. Мы оказываем услуги по производству и продаже документов об окончании любых университетов России. Купить диплом о высшем образовании– diplom-club.com/kupit-diplom-s-vneseniem-v-reestr-bistro-i-nadezhno-31/
Celine replica designer bags
подробнее казино водкабет
Приобрести диплом о высшем образовании!
Мы изготавливаем дипломы любых профессий по приятным ценам. Вы заказываете диплом в надежной и проверенной временем компании. : realroleplay.listbb.ru/viewtopic.php?f=40&t=987
pinap [url=https://www.pinup-azerbaycan73.com]pinap[/url] .
Диплом ВУЗа Российской Федерации!
Без присутствия диплома очень трудно было продвигаться вверх по карьере. Именно из-за этого решение о покупке диплома следует считать мудрым и рациональным. Заказать диплом института carolinahurricanesclub.com/read-blog/3156
1win официальный сайт https://1win711.ru .
клубные сеты миксы http://www.klubnaya-muzyka.ru .
Online casino ads are loud—quiet down! pirots 2
сет музыкальный клубный сет музыкальный клубный .
Профессиональное изготовление штор под заказ
сшить шторы на заказ сшить шторы на заказ . “Ткацкий”
Высококачественные деревянные жалюзи с электроприводом
Деревянные горизонтальные жалюзи с электроприводом Деревянные горизонтальные жалюзи с электроприводом . +7 (499) 638-25-37
Приобрести диплом об образовании!
Мы предлагаем дипломы любой профессии по приятным тарифам— diplomskiy.com
Расскажем о поломках ноутбука maibenben
plinko kasyno
1win armenia https://www.1win5034.ru .
Online gambling feels loose—no look! plinko
Наши специалисты предлагают выгодно и быстро приобрести диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Данный документ способен пройти лубую проверку, даже с применением специфических приборов. entrainment.listbb.ru/viewtopic.php?f=96&t=41398
mostbet sikachat https://www.mostbet6042.ru .
bet on red recenze
скачать 1win на телефон официальный сайт https://www.1win8012.ru .
pin up onlayn kazino https://www.pinup-azerbaycan73.com .
1win ставки официальный сайт https://www.1win8011.ru .
сет музыкальный клубный сет музыкальный клубный .
клубные сеты миксы klubnaya-muzyka1.ru .
Ruanabol – стероиды анаболики, Pharmacom
Мы готовы предложить дипломы любой профессии по выгодным тарифам. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Важно, чтобы дипломы были доступны для большинства наших граждан.
Заказ документа, подтверждающего окончание института, – это грамотное решение. Купить диплом о высшем образовании: institute-diplom.ru/kupit-universitet-diplom-4/
1win armenia 1win armenia .
дивитися фільми онлайн 2025 HD фільми українською онлайн
дивитися нові фільми 2025 безкоштовне кіно Full HD
глушение Платона – Система слежения, Сократим расходы на Платон
узнать Altcoin обменник
Шторы на заказ: быстрый и удобный сервис
сшить шторы на заказ сшить шторы на заказ . “Ткацкий”
1 win 1win707.ru .
смотреть фильмы онлайн 2025 комедии 2025 онлайн в хорошем качестве
пополнение стим Пополнить баланс Стим с низкой комиссией на ZloyPay. Пополнение в рублях, тенге и долларах. Оплата картой РФ или СБП. Подходит для аккаунтов России, Казахстана и стран СНГ. Моментальное зачисление средств.
Мы готовы предложить дипломы психологов, юристов, экономистов и любых других профессий по разумным тарифам.– diplomservis.ru/kupit-diplom-s-garantiej-vneseniya-v-reestr-2/
подробнее здесь vodka bet casino
Эра глонасс – ГЛОНАСС, Контроль топлива
Анаболики – Руанабол, Стероиды
http 1win 1win707.ru .
Заказать диплом института по выгодной цене возможно, обратившись к надежной специализированной фирме. Приобрести документ института вы сможете в нашей компании. cardinalparkmld.listbb.ru/viewtopic.phpf=3&t=1914
подробнее Альткоин обменник
Лучшие мастерские по пошиву штор, закажите..
Создайте уникальный интерьер с пошивом штор, по доступным ценам..
Эксклюзивные решения в пошиве штор, по индивидуальному проекту..
Дизайнерские шторы на заказ, закажите онлайн..
Профессиональный пошив штор с монтажом, на любой вкус..
Мастерская по пошиву штор, по индивидуальному заказу..
Пошив штор по индивидуальному заказу, Позвольте нам помочь..
Шторы на любой вкус и цвет, по вашему стилю..
Пошив штор из эксклюзивных тканей, по вашим желанием..
Пошив штор с индивидуальным подходом, получите консультацию..
Мастера по пошиву штор, гарантия соответствия..
Премиум пошив штор для интерьера, по индивидуальному дизайну..
Элегантные шторы на заказ, под ключ..
Индивидуальный дизайн штор, от ведущих дизайнеров..
Пошив штор для любого помещения, с современными технологиями..
Профессиональный пошив штор, по вашему стилю..
Пошив штор по вашим пожеланиям, по оптимальной цене..
пошив штор пошив штор . “Ткацкий”
1win скачать на ios зеркало 1win скачать на ios зеркало .
Приобрести диплом любого университета. Покупка документа о высшем образовании через качественную и надежную фирму дарит немало плюсов для покупателя. Данное решение дает возможность сберечь как личное время, так и серьезные финансовые средства. molbiol.ru/forums/index.php?showtopic=2344978
Для эффективного продвижения по карьерной лестнице требуется наличие официального диплома о высшем образовании. Купить диплом о высшем образовании у сильной фирмы: diplomidlarf.ru/kupit-krasnij-attestat/
Приобретение документа о высшем образовании через надежную фирму дарит ряд плюсов для покупателя. Данное решение позволяет сэкономить как дорогое время, так и значительные финансовые средства. Впрочем, преимуществ значительно больше.Мы предлагаем дипломы любой профессии. Дипломы изготавливаются на настоящих бланках. Доступная стоимость в сравнении с серьезными издержками на обучение и проживание в другом городе. Покупка диплома об образовании из российского университета является целесообразным шагом.
Приобрести диплом: diplomh-40.ru/kupit-diplom-v-reestre-bistro-i-legalno/
кликните сюда на сайте
1win букмекер 1win букмекер .
Идеи оформления штор в коттедже
шторы для коттеджа шторы для коттеджа .
1win букмекерская скачать приложение https://1win8005.ru .
auto transport companies https://crctransport.us/car-hauling-businesses-dealerships-fleets-efficiency/
Мы предлагаем дипломы любых профессий по выгодным тарифам. Всегда стараемся поддерживать для клиентов адекватную ценовую политику. Для нас важно, чтобы документы были доступными для большого количества граждан.
Заказ документа, подтверждающего окончание института, – это разумное решение. Купить диплом любого ВУЗа: diplom-insti.ru/diplom-meditsinskogo-kolledzha-kupit-2/
https://cremepa.org.br/noticias/eleicoes-emissao-de-certidao/
The slot sounds online rock—pure fun! pin up
Приобрести диплом на заказ вы имеете возможность используя официальный портал компании. animalplanetnews.ru/nadyozhnoe-oformlenie-dokumentov
Где покупать технику? рейтинг интернет магазинов: проверенные продавцы, акции, удобная доставка и реальный опыт покупателей. Обновляем регулярно.
Многие люди ищут цитаты про любовь, чтобы выразить чувства, найти вдохновение или просто улыбнуться. Мы собрали лучшие фразы — короткие, красивые, со смыслом. Наслаждайтесь подборкой, делитесь с друзьями и находите строки, которые отражают ваши мысли и настроение.
Настройка сервера на платформе https://rusikboss.livejournal.com/748.html — это удобный способ обеспечить безопасное подключение, стабильный доступ и полную конфиденциальность. Пошаговая инструкция, поддержка популярных протоколов, генерация QR-кодов и приложения для разных устройств — всё для быстрого запуска.
скачать 1win на ios официальный сайт https://1win8003.ru .
https://crmes.org.br/noticias/vitoria-sediara-i-congresso-sudeste-de-acupuntura-medica-2/
https://www.durovis.com/es/board_topic_38304_0.html
Также рекомендую вам почитать по теме – https://bestanimation.ru .
И еще вот – https://noviy-status.ru .
https://foro.rune-nifelheim.com/recent/?start=0;PHPSESSID=fd3cbeef4f84a53a8de3e4f07e16176a
https://iohanes.com/blog/remedios-caseros/limpia-rinones-te-de-platano/
“You get some of me but not tomorrow as they want me in as soon as I can make it happen. This is the one time when they say jump and I ask how high due the financial gains the company could benefit from and it being important enough for the client to appear in person.”
“Well I get an extra night of you at least! I wonder what we could do with that? Meantime, what about food? I am starving and delicious as it was a second breakfast is not quite enough to replenish me!”
“Well get something on and we’ll sort that out first.”
We drove into town and decided that a daytime visit to Charlie’s was going to be the answer. I parked in the bar lot and Elise dashed in to change into something more appropriate, jeans and a t-shirt along with her biker jacket but keeping her Converses on.
Walking down to the restaurant was different from the middle of the night visits as the streets were bustling and all of the shops and outlets were open.
Reaching Charlie’s we entered the front door and sat in a booth near the window. A beautiful young American Chinese girl came,smiled and said hello to Elise and gave us menus and asked if we wanted drinks in the meantime.
“No thanks Lin just a pot of Jasmine tea for us please.” Lin went back to the kitchen area. “No booze for me today as I will have to work in the bar so it is just tea for me.”
Not in a drinking mood either, I agreed with her.”
http://www.babelcube.com/user/rebecca-benedicto
https://uzername1963.bandcamp.com/album/playtime-chap-xxix-reruns
https://rentry.org/2ym6ppt9
https://anotepad.com/notes/cw4gajk5
https://rentry.org/2wnrzxgw
https://www.donamix.com/forums/discussion/education/play-slots-online-in-ghana
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по невысоким ценам. Мы предлагаем документы ВУЗов, которые расположены в любом регионе Российской Федерации. Документы печатаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. famedoot.in/read-blog/2994_kupit-diplom-ob-obrazovanii-zanesennyj-v-reestr.html
Online casinos need flags—alert us! plinko
Идеи оформления штор в коттедже
шторы для коттеджа шторы для коттеджа .
https://iohanes.com/blog/vida-saludable/4-azucares-naturales-enfermedades/
https://rtinetwork.org/rti-blog-archives/entry/1/8
The live dealers online rule—true vibe! pin up
игра авиатор как выиграть https://1win8006.ru .
Купить диплом любого ВУЗа!
Мы предлагаем документы институтов, которые расположены на территории всей Российской Федерации.
dip-lom-rus.ru/bistraya-pokupka-diploma-bez-problem-i-lishnix-xlopot/
https://iohanes.com/blog/remedios-caseros/beneficios-consumir-ajo/
plinko nederland
Психолог помогающий искать решения в непростых психологических ситуациях. Анонимный чат с психологом телеграм. Психолог помогающий искать решения в непростых психологических ситуациях. оценили 4693 раз
1вин вход https://1win8013.ru .
не обновляется дота 2 https://www.1win8005.ru .
https://forum.hipologia.pl/showthread.php?tid=5378&pid=45125
The sound effects in online slots are so addictive—keeps me spinning! plinko
как поставить экспресс на 1win http://www.1win8002.ru .
Диплом университета Российской Федерации!
Без института сложно было продвигаться вверх по карьере. По этой причине решение о заказе диплома стоит считать мудрым и целесообразным. Заказать диплом о высшем образовании vacancies.co.zm/employer/gosznac-diplom-24
Автоматизация дома — просто с Somfy
Автоматика Somfy Автоматика Somfy . Прокарниз
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы любых профессий по доступным тарифам. Вы приобретаете документ через надежную и проверенную фирму. : neejobs.com/employer/frees-diplom
Online casinos feel chancy—watch out! aviator
The online bonuses call—step in! jackpot raider
https://iohanes.com/blog/jugos/jugos-naturales-para-limpiar-el-pancreas/
https://uniqueoman.com/digital/get-ahead-of-your-competition-our-proven-digital/
https://luvionbeautycenter.com/scopri-l-app-plinko-gioco-avvincente-e-vincite/
https://robotikportal.de/community/blog.php?do=list&m=12&y=2024&d=16
1win скачать на андроид бесплатно с официального сайта 1win скачать на андроид бесплатно с официального сайта .
https://www.rtinetwork.org/component/content/article/390
авиатор игра на деньги отзывы https://1win8015.ru .
1цшт официальный сайт войти https://1win8014.ru .
1win официальный сайт скачать http://1win8002.ru/ .
Идеи для штор в загородном доме, создайте уют, лучшие материалы для штор в загородных домах, теплота и уют, максимальная функциональность, натуральные ткани для штор, эффективные шторы для загородного дома, идеи дизайна штор, как подобрать шторы для спальни в доме за городом, шторные решения для больших окон, шторы из натуральных материалов для уюта, современные механизмы для штор, гармония штор и мебели, шторы как часть интерьера, обеспечьте комфорт с нашими шторами, выбор стильных штор для загородного дома, что выбрать для загородного дома, используйте шторы для зонирования пространства, шторы для зимнего уюта в загородном доме
шторы в загородном доме шторы в загородном доме .
https://www.myvipon.com/post/1390166/join-as-seller.php
https://betfinalarabic.net/betfinal-casino/
хранение склад москва вещей хранение склад москва вещей .
https://www.escueladeangeles.com.mx/2025/03/10/discover-the-exciting-world-of-casino-game-plinko-29/
авиатор казино скачать http://www.1win8001.ru .
https://iancleary.com/celebrating-with-sandflies-and-crocodiles/
https://www.durovis.com/en/board_topic_46165.html
Online casinos feel risky—double watch! tome of madness
1win букмекерская контора скачать на андроид https://1win8003.ru .
крутая музыка крутая музыка .
купить напольное кашпо для комнатных растений недорого купить напольное кашпо для комнатных растений недорого .
напольные горшки для цветов купить интернет напольные горшки для цветов купить интернет .
кашпо высокие напольные для цветов недорого кашпо высокие напольные для цветов недорого .
диджей онлайн диджей онлайн .
Автоматизация дома — просто с Somfy
Автоматика Somfy Автоматика Somfy . прокарниз
https://www.dmxzone.com/forum/topic.asp?NewsId=13984&topic_id=155287
http://iancleary.com/some-antics-with-words/
https://tekkaledogaltas.com/explore-the-exciting-world-of-casino-aviator-game/
Купить диплом ВУЗа по доступной цене можно, обратившись к надежной специализированной фирме. Мы предлагаем документы об окончании любых университетов Российской Федерации. Заказать диплом о высшем образовании– asxdiplommy.com/kupite-diplom-o-srednem-obrazovanii-s-vneseniem-v-reestr-4/
https://www.cinexplicacion.com.mx/2020/index.php/forum/how-to-game-spot/270998-ragamonedas-rapida
car delivery to dealership https://crctransport.us/auto-transport-dealership-operations-scaling/
https://uptasker.co.za/find/entertainer/gauteng/parkhurst
1win бонус за установку приложения https://1win8015.ru .
https://iancleary.com/category/chronic-pain-fibromyalgia/page/6/
cbd gummies produce a tactful and enjoyable method in search experiencing the intricate’s effects. Within reach in a all the way range of flavors, strengths, and blends, they make allowance looking for precise dosing and deliver effects that tend to pattern longer. Scads users addle to these gummies after their calming and stress-reducing benefits. That said, it’s requisite to make use of them responsibly, as the inception of effects is typically slower than with methods like smoking or vaping. At all times string dosage recommendations and demonstrate that their avail is legal in your area prior to consuming or purchasing.
Покупка документа о высшем образовании через надежную компанию дарит немало достоинств для покупателя. Такое решение помогает сберечь время и значительные денежные средства. Впрочем, на этом выгода не ограничивается, плюсов значительно больше.Мы изготавливаем дипломы любой профессии. Дипломы производят на оригинальных бланках. Доступная стоимость сравнительно с огромными затратами на обучение и проживание. Приобретение диплома о высшем образовании из российского университета является целесообразным шагом.
Купить диплом: rusd-diplomj.ru/zanesenie-diploma-v-reestr-obrazovaniya-prosto-i-bistro/
Online poker is wild—bluffing is a rush! aviator
casino 1win https://1win8014.ru/ .
Жалюзи с пультом — комфорт и удобство в вашем доме
жалюзи с пультом жалюзи с пультом . Прокарниз
https://www.elevarvendas.com.br/post/datas-para-impulsionar-campanhas-de-setembro
кино фильмы 2025 онлайн комедии 2025 онлайн в хорошем качестве
http://iancleary.com/my-wife-in-the-news/
фильмы военные драмы смотреть кино на телефоне в Full HD
1win casino официальный сайт скачать 1win casino официальный сайт скачать .
https://www.keepandshare.com/discuss4/19610/slot-se-destacar-em-rela-o?ifr=y
фильм подряд в хорошем качестве лучшие фильмы онлайн без смс
лучшие фильмы фильмы онлайн 2025 без подписки
https://www.surebetsite.com/post/rubin-kazan-vs-cska-moscow-prediction-tips
I lost time online—fast gone! plinko
https://carmencitafilmlab.com/blog/best-of-march-2024/
Приобрести диплом ВУЗа!
Наши специалисты предлагаютбыстро и выгодно купить диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Данный документ пройдет любые проверки, даже при использовании профессионального оборудования. Достигайте своих целей максимально быстро с нашим сервисом- job.iwok.vn/employer/gosznac-diplom-24
https://mindbenderescaperooms.com/jacksonvillebeach-fl/uncategorized/%D0%B8%D0%BD%D1%82%D0%B5%D1%80%D0%BD%D0%B5%D1%82-%D0%BA%D0%B0%D0%B7%D0%B8%D0%BD%D0%BE-rox-%D1%80%D1%83%D1%81%D0%BA%D0%B8%D0%B9-%D0%B2%D1%83%D0%BB%D0%BA%D0%B0%D0%BD-%D0%B8%D0%B3%D0%BE%D1%80%D0%BD/
Наши специалисты предлагают быстро и выгодно приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями должностных лиц. Документ пройдет любые проверки, даже с использованием специальных приборов. cart-gurus.com/employer/premiumydiploma
Купить диплом института по доступной цене возможно, обращаясь к проверенной специализированной компании. Купить документ университета вы можете в нашей компании в Москве. diplom-ryssia.com/kupit-diplom-moskva-s-zaneseniem-v-reestr-8
autocad for students
autocad drawing
autocad licencia
autocad web app
autocad 2023
купить аккаунт маркетплейс аккаунтов соцсетей
Деревянные горизонтальные жалюзи с электроприводом: функциональность и дизайн
Деревянные горизонтальные жалюзи с электроприводом Деревянные горизонтальные жалюзи с электроприводом . прокарниз
mostbet kg http://mostbet5021.ru/ .
букмекерские конторы кыргызстана http://mostbet8002.ru/ .
Приобрести диплом об образовании. Заказ диплома через надежную компанию дарит ряд преимуществ для покупателя. Это решение помогает сэкономить время и серьезные финансовые средства. hitman.getbb.ru/viewtopic.php?f=16&t=2768
https://www.notebook.ai/users/745270/items
https://annaslowinska.pl/plinko-app-die-besten-tipps-und-tricks-fur-5/
http://webdonline.com/fr/services/forums/message.asp?id=75&msgid=7039281&poster=0&ok=0&i_ordre=1
Online casinos should free—try it! plinko
http://matrompette.com/forum/search.php?author_id=1334&sr=posts
I lost $130 online—plan up! book of ra
https://foro.rune-nifelheim.com/recent/?start=30;PHPSESSID=1132d5dbcd9b6131278d23015c2444ba
I love the pay ease online—smooth run! plinko
https://es.industryarena.com/foro/showthread.php?t=1545&p=9778&mode=linear
http://stock.union-oberneukirchen.at/author/root/page/2600/
https://blendedlearning.bharatskills.gov.in/mod/forum/discuss.php?d=6043&parent=11258&lang=ta_lk
список фильмов фильмы 2025 без регистрации и рекламы
Приобрести диплом на заказ можно через официальный портал компании. huntsrecruitment.com/employer/frees-diplom
фильмы уже вышедшие новинки кино 2025 онлайн бесплатно
The online thrill strikes—stay steady! BetOnRed
лучшие фильмы онлайн сериалы 2025 онлайн бесплатно HD
смотреть фильмы онлайн 2025 комедии 2025 онлайн в хорошем качестве
Энергосберегающие жалюзи с пультом — экономия и комфорт
жалюзи с пультом жалюзи с пультом . Prokarniz
Заказать диплом о высшем образовании!
Купить диплом института по невысокой цене можно, обратившись к проверенной специализированной фирме. Заказать диплом о высшем образовании: diplom-onlinex.com/kupit-diplom-s-zaneseniem-v-reestr-30
Хотите играть в Castle Clash на большом экране? Наслаждайтесь высокого разрешения и удобных элементов управления. Соревнуйтесь с международными противниками в эпических битвах, после установки через удобный Android-эмулятор. Просто перейдите на сайт castle-clash-skachat-na-pk.ru для установки и присоединяйтесь к миллионам игроков со всех уголков планеты!
скачать castle clash бесплатно
castle clash играть онлайн
castle clash для пк
mostbet apk скачать http://mostbet5021.ru/ .
Где приобрести диплом специалиста?
Готовый диплом со всеми печатями и подписями отвечает стандартам Министерства образования и науки, неотличим от оригинала. Не откладывайте свои мечты и задачи на пять лет, реализуйте их с нами – отправьте простую заявку на диплом прямо сейчас! Получить диплом о среднем образовании – не проблема! dimarecruitment.co.uk/employer/premialnie-diplom-24
https://noosfero.ufba.br/evelynn-hot/blog?npage=2&view=true
https://iohanes.com/blog/perder-peso/causas-porque-no-baja-de-peso-efectivamente/
http://yashiro.sakura.ne.jp/sasanqua/sb/log/nov166prt12.html
Быстро и просто купить диплом о высшем образовании!
Мы предлагаем документы об окончании любых ВУЗов России. Документы изготавливаются на подлинных бланках государственного образца. 1worldonline.copiny.com/question/details/id/1084791
MetaMask Download is a must for Web3. The interface is user-friendly, and it allows seamless access to decentralized applications.
http://rtinetwork.org/voices-archives/entry/2/88
1 win app http://www.1win8016.ru .
Заказать диплом ВУЗа. Заказ документа о высшем образовании через проверенную и надежную компанию дарит множество плюсов. Такое решение позволяет сэкономить как личное время, так и серьезные финансовые средства. nationalcarerecruitment.com.au/employer/eonline-diploma
I won a tournament online—bragging rights for days! mines
http://www.6555555.com/1198.html?replyTo=12671
Также рекомендую вам почитать по теме – https://noviy-status.ru .
И еще вот – https://24-ad.ru .
Задался вопросом: можно ли на самом деле купить диплом государственного образца в Москве? Был приятно удивлен — это реально и легально!
Сначала искал информацию в интернете на тему: купить диплом о законченном высшем образовании, купить диплом об окончании пту в таразе, купить диплом ссср в самаре, купить диплом техникума в уфе, купить диплом техникума в чите и получил базовые знания. В итоге остановился на материале: proffdiplomik.com/diploms/diplom-tehnikuma-2008-2010
Мы можем предложить дипломы любой профессии по приятным ценам. Мы можем предложить документы техникумов, которые находятся в любом регионе России. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это дает возможность делать настоящие дипломы, не отличимые от оригиналов. mosregeon.flybb.ru/viewtopic.phpf=2&t=961
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по выгодным ценам. Дипломы производят на подлинных бланках государственного образца Купить диплом о высшем образовании diplomg-cheboksary.ru
скачать приложение 1вин http://1win8016.ru/ .
Где заказать диплом по нужной специальности?
Заказать диплом ВУЗа по выгодной цене вы можете, обратившись к надежной специализированной компании.: kupit-diplomyz24.com
I love the chats online—good crew! plinko
фильм драма мистика сериалы 2025 онлайн бесплатно HD
http://knowmedge.com/medical_boards_forum/viewtopic.php?f=22&t=10002
фильмы 2025 г смотреть кино на телефоне в Full HD
диджей онлайн диджей онлайн .
https://uptasker.co.za/find/entertainer/gauteng/witwatersrand
смотреть фильмы онлайн лучшие фильмы онлайн без смс
смотреть фильм ужасов 2025 новинки кино 2025 онлайн бесплатно
>http://www.voxmea.com/cgi/blog/log/eid103.html?>
кашпо напольное пластик купить кашпо напольное пластик купить .
http://gideontester.com/en/component/k2/item/1-blog1?start=96170
купить кашпо напольное белое купить кашпо напольное белое .
заказать напольные кашпо заказать напольные кашпо .
dj онлайн dj онлайн .
Купить диплом возможно через сайт компании. skymix2018.forumex.ru/viewtopic.phpf=14&t=946
https://crmes.org.br/noticias/reforma-do-hgl-ja-leva-um-ano-e-meio-e-impacta-nas-condicoes-de-trabalho-dos-profissionais-de-saude-e-no-atendimento-a-populacao/
1 win md 1 win md .
best car transport service https://crctransport.us/car-hauling-businesses-dealerships-fleets-efficiency/
пластические хирурги москва Особое внимание уделяется уникальным разработкам доктора, включая запатентованную технику липофилинга, позволяющую достичь естественной гармонии. Канал выступает в роли дискуссионной площадки для обсуждения комбинированных способов омоложения лица, основанных на научных исследованиях и подтвержденных публикациями в ведущих медицинских журналах ВАК. Наряду с этим, на канале рассматриваются вопросы применения клеточных технологий в пластической хирургии. Доктор детально рассказывает о преимуществах использования ресурсов собственного тела для достижения максимального омолаживающего эффекта и восстановления тканей. Подписчики узнают о самых современных методах стимуляции процессов регенерации, способствующих улучшению результатов хирургических вмешательств и сокращению периода восстановления. Канал пластического хирурга – это не только кладезь профессиональной информации, но и комьюнити для людей, увлеченных вопросами красоты и здоровья. Здесь можно обращаться к доктору с вопросами, получать индивидуальные рекомендации и быть в курсе последних тенденций в мире anti-age и пластической хирургии.
https://www.institutoportuguesderelojoaria.pt/post/oris-propilot-x-miss-piggy-1
Заказать диплом любого университета!
Мы готовы предложить документы институтов, которые находятся на территории всей России.
diplomg-cheboksary.ru/srochnaya-pokupka-diploma-o-visshem-obrazovanii/
mostbet сайт https://mostbet8001.ru .
mostbet apk скачать http://mostbet8004.ru .
plinko real money app
Где заказать диплом по актуальной специальности?
Мы предлагаем дипломы психологов, юристов, экономистов и прочих профессий по разумным ценам. Для нас очень важно, чтобы документы были доступными для подавляющей массы граждан. Быстро купить диплом об образовании vuz-diplom.ru/bistroe-i-nadezhnoe-priobretenie-diploma-s-reestrom/
1win.pro https://1win5050.ru/ .
Пошив штор на любой вкус, обратитесь..
Создайте уникальный интерьер с пошивом штор, по доступным ценам..
Пошив штор на заказ, с использованием лучших тканей..
Пошив штор по вашему дизайну, обратитесь к нам..
Быстрый пошив штор, под ключ..
Мастерская по пошиву штор, гарантия качества..
Создаем шторы мечты, Пускай ваш дом засияет..
Шторы на любой вкус и цвет, вам под силу..
Эксклюзивные шторы на заказ, по вашим желанием..
Пошив штор с индивидуальным подходом, оформляйте заказ онлайн..
Идеальные шторы для вашего пространства, гарантия соответствия..
Пошив штор на заказ по вашим размерам, подходящий ваш стиль..
Пошив штор с профессиональным монтажом, от профессионалов..
Создаем шторы по вашим мечтам, по вашим требованиям..
Качественные шторы на заказ, с современными технологиями..
Высокое качество и стиль, с гарантией долговечности..
Индивидуальные шторы на заказ, от замеров до монтажа..
пошив штор пошив штор . Ткацкий
клубные сеты диджеев клубные сеты диджеев .
https://undiscoveredrp.nn.pe/printthread.php?tid=26350
купить напольный вазон для цветов купить напольный вазон для цветов .
заказать напольные кашпо заказать напольные кашпо .
Online casinos should cap losses—protect players! bet on red
купить кашпо напольное белое купить кашпо напольное белое .
онлайн диджей онлайн диджей .
http://www.rtinetwork.org/professional/rti-talks/transcript/expert/119
Мы можем предложить дипломы любых профессий по выгодным тарифам.
Вы покупаете документ в надежной и проверенной компании. Приобрести диплом университета– http://pika.listbb.ru/posting.phpmode=post&f=9/ – pika.listbb.ru/posting.phpmode=post&f=9
Приобрести документ о получении высшего образования можно в нашем сервисе. Мы оказываем услуги по продаже документов об окончании любых ВУЗов РФ. Вы сможете получить необходимый диплом по любой специальности, любого года выпуска, включая документы Советского Союза. Даем 100% гарантию, что в случае проверки документа работодателями, подозрений не возникнет. diplomers.com/legalnoe-zanesenie-diploma-v-reestr-bistro-i-nadezhno-2/
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
https://www.plattnerpatrickbau.it/plinko-sovellus-liity-hauskaan-peliin-ja-voita/
I love the online promos—fresh take! plinko
https://574a847ad477.donamix.com/forums/discussion/general/kasinech
Актуальные юридические новости https://t.me/Urist_98RUS полезные статьи, практичные лайфхаки и советы для бизнеса и жизни. Понимайте законы легко, следите за изменениями, узнавайте секреты защиты своих прав и возможностей.
Топ магазинов техники reyting magazinov tehniki по качеству, ценам и сервису! Сравниваем для вас популярные площадки, ищем выгодные предложения, делимся реальными отзывами. Экономьте время и деньги — изучайте наш рейтинг и выбирайте лучшее!
hop over to here https://expert-writings.org/
русские фильмы онлайн смотреть кино на телефоне в Full HD
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Мы предлагаем документы техникумов, расположенных на территории всей России. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это позволяет делать государственные дипломы, которые невозможно отличить от оригинала. netmitra.cyberei.com/read-blog/680_diplom-vysshego-obrazovaniya-s-zaneseniem-v-reestr.html
перейти на сайт https://t.me/Pharmacom_Labs_Official
https://pennyblacktattoobutter.co.uk/2025/03/07/discover-the-excitement-of-the-casino-game-plinko-82/
Заказать диплом института !
Приобретение диплома ВУЗа России у нас – надежный процесс, поскольку документ будет заноситься в реестр. Купить диплом ВУЗа diplomservis.com/diplom-s-zaneseniem-v-reestr-dlya-vashego-uspeshnogo-budushego
https://iancleary.com/cfs-and-getting-sick/
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт iphone на дому в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
I won $800 on a slot—hype high! aviator
google porn visit.
https://www.fonderies-dechaumont.com/discover-the-exciting-world-of-casino-game-plinko-52/
1цшт официальный сайт войти http://1win8007.ru .
Online casinos should demo—test out! betonred
https://crmes.org.br/noticias/cubanos-abandonam-programa-reclamando-de-falta-de-pagamento/
перепродажа аккаунтов купить аккаунт
место для хранения вещей в аренду место для хранения вещей в аренду .
Купить диплом любого университета!
Мы готовы предложить документы институтов, расположенных на территории всей РФ.
diplomskiy.com/kupit-diplom-o-visshem-obrazovanii-reestr-6/
I love the online crew—chat up! плінко
id mostbet com https://mostbet8001.ru .
https://bombeiros.pt/galeria/details.php?image_id=10099&mode=search
Кактус Казино кактус казино зеркало мир азарта и развлечений! Тысячи слотов, карточные игры, рулетка и захватывающие турниры. Быстрые выплаты, щедрые бонусы и поддержка 24/7. Играйте ярко, выигрывайте легко — всё это в Кактус Казино!
jocuri de noroc online moldova http://www.1win5051.ru .
казино мостбет скачать http://mostbet8002.ru .
Уборка Мытищи
Мытищи – динамично развивающийся город, где бурлит жизнь, строятся новые дома и бизнес-центры. В этой суете чистота и порядок становятся не просто желанием, а необходимостью для комфортной жизни и успешной работы. Именно поэтому профессиональная уборка в Мытищах пользуется все большей популярностью.
Почему выбирают профессиональную уборку?
Времени на самостоятельную уборку зачастую не хватает. Современный ритм жизни диктует свои условия, и после напряженного рабочего дня хочется отдохнуть, а не тратить силы на борьбу с пылью и грязью. Профессиональные клининговые компании предлагают широкий спектр услуг, позволяя делегировать эту задачу специалистам. Они используют современное оборудование, эффективные чистящие средства и отработанные технологии, чтобы добиться идеальной чистоты в кратчайшие сроки.
Услуги, которые предлагает профессиональная уборка в Мытищах:
Генеральная уборка: комплексная уборка всей квартиры или дома, включающая мытье окон, чистку ковров и мягкой мебели, удаление пыли со всех поверхностей.
Уборка после ремонта: избавление от строительной пыли, остатков краски и других загрязнений после ремонтных работ.
Поддерживающая уборка: регулярная уборка, позволяющая поддерживать чистоту и порядок в доме или офисе.
Уборка офисов: поддержание чистоты в офисных помещениях, включая уборку рабочих мест, санузлов и общих зон.
Мытье окон и фасадов: профессиональная мойка окон и фасадов зданий с использованием специального оборудования и чистящих средств.
Преимущества профессиональной уборки:
Экономия времени и сил: вы можете посвятить свое время более важным делам.
Гарантия качества: профессиональные компании несут ответственность за качество своей работы.
Использование профессионального оборудования и чистящих средств: позволяет добиться идеальной чистоты, не повреждая поверхности.
Индивидуальный подход: возможность заказать уборку, соответствующую вашим потребностям и бюджету.
В Мытищах представлен широкий выбор клининговых компаний, предлагающих услуги уборки. Выбирая подходящую компанию, обращайте внимание на ее опыт, репутацию и спектр предлагаемых услуг. Чистота и порядок – это инвестиция в ваш комфорт и здоровье. https://cleaningplus.ru/uborka-v-moskve/rayony-moskvy/uborka-mytishchi/
Любите кино и сериалы? тевас фильмы тевас у нас собраны лучшие подборки — от блокбастеров до авторских лент. Смотрите онлайн без ограничений, выбирайте жанры по настроению и открывайте новые истории каждый день. Кино, которое всегда с вами!
Купить диплом о высшем образовании!
Мы предлагаем документы институтов, которые расположены на территории всей Российской Федерации. Документы выпускаются на “правильной” бумаге высшего качества: adly.pk/profile/dexterricher98
Электропривод для жалюзи с дистанционным управлением
электропривод для горизонтальных жалюзи электропривод для горизонтальных жалюзи .
Лучшие жизненные мудрые цитаты, успехе и вдохновении. Короткие мысли великих людей, мудрые фразы и слова, которые заставляют задуматься. Найдите мотивацию, настрой и силу в правильных словах каждый день!
https://app.geniusu.com/activities/2503803
https://www.keepandshare.com/discuss4/19207/piattaforma-affidabile-in-italia
https://carmencitafilmlab.com/meets/carmencita-meets-discovering-valencia-i-with-your-film-family-2016/
https://www.azrockradio.com/group/grupo-az-radio/discussion/8dab81e3-ebfc-4f6d-8c8c-0941e4d6b741
Добрый день!
Для определенных людей, купить диплом о высшем образовании – это острая необходимость, уникальный шанс получить достойную работу. Но для кого-то – это желание не терять время на учебу в институте. С какой бы целью вам это не потребовалось, наша фирма готова помочь вам. Быстро, профессионально и выгодно сделаем диплом любого ВУЗа и года выпуска на государственных бланках с реальными подписями и печатями.
Ключевая причина, почему многие люди покупают диплом, – получить хорошую должность. Допустим, знания позволяют специалисту устроиться на работу, однако подтверждения квалификации нет. Если для работодателя важно наличие “корочки”, риск потерять место работы очень высокий.
Приобрести документ университета вы имеете возможность в нашей компании в Москве. Мы оказываем услуги по продаже документов об окончании любых ВУЗов Российской Федерации. Вы получите диплом по любым специальностям, любого года выпуска, включая документы старого образца. Гарантируем, что при проверке документов работодателем, подозрений не возникнет.
Ситуаций, которые вынуждают заказать диплом много. Кому-то срочно нужна работа, и необходимо произвести впечатление на руководителя на протяжении собеседования. Другие задумали устроиться в большую компанию, для того, чтобы повысить собственный статус и в дальнейшем начать свой бизнес. Чтобы не тратить впустую годы жизни, а сразу начать успешную карьеру, используя имеющиеся знания, можно приобрести диплом через интернет. Вы станете полезным в обществе, обретете финансовую стабильность в максимально короткий срок- диплом о среднем образовании купить
https://iancleary.com/time-to-get-curious-about-the-brain/
I hit a streak on blackjack online—lucky me! aviator
быстрая регистрация на мостбет https://mostbet8003.ru .
Купить диплом института по невысокой стоимости можно, обращаясь к проверенной специализированной компании. Мы готовы предложить документы престижных ВУЗов, расположенных в любом регионе РФ. volunteering.ishayoga.eu/employer/eonline-diploma
https://www.robotikportal.de/community/blog.php?do=list&m=2&y=2025&d=12
I won $2500 online—dream night! fortune dragon
покупка аккаунтов маркетплейс аккаунтов соцсетей
пластический хирург Пристальное внимание уделяется оригинальным разработкам доктора, в том числе уникальной методике липофилинга, гарантирующей натуральный и сбалансированный внешний вид. Канал является местом для обсуждения сочетанных техник омоложения лица, базирующихся на научных исследованиях и подкрепленных публикациями в авторитетных медицинских журналах ВАК. Кроме того, канал освещает вопросы применения клеточных технологий в пластической хирургии. Доктор подробно рассказывает о достоинствах использования собственных ресурсов организма для достижения максимального эффекта омоложения и восстановления тканей. Подписчики узнают о самых современных способах стимуляции регенеративных процессов, позволяющих улучшить исходы операций и сократить период восстановления. Канал пластического хирурга – это не только информационный ресурс, но и сообщество для тех, кто разделяет интерес к вопросам красоты и здоровья. Здесь можно обратиться к доктору с вопросами, получить персональные советы и узнать о последних трендах в мире anti-age и пластической хирургии.
Online bingo is cool—nice group! aviator
https://mobile.donamix.com/forums/discussion/general/games-7?page=1
Хотите жить у моря? https://www.kvartira-v-chernogorii-kupit.com — квартиры, дома, виллы на лучших курортах. Удобные условия покупки, помощь на всех этапах, инвестиционные проекты. Откройте новые возможности жизни и отдыха на берегу Адриатики!
значки на одежду металлические http://metallicheskie-znachki213.ru
Мечтаете о доме у моря? купить недвижимость в черногории — идеальный выбор! Простое оформление, доступные цены, потрясающие виды и европейский комфорт. Инвестируйте в своё будущее уже сегодня вместе с нами.
макет металлического значка заказ значков из металла
https://bellabulba.com/aviator-game-online-casino-win-big-with-the-4/
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: мастер ремонта apple
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Купить диплом любого ВУЗа!
Мы предлагаембыстро купить диплом, который выполняется на оригинальном бланке и заверен печатями, штампами, подписями. Данный документ способен пройти лубую проверку, даже при помощи специальных приборов. Решайте свои задачи быстро и просто с нашим сервисом- theapptub.com/blog/2025/04/14/kupit-diplom-prostoe-reshenie-dlja-slozhnyh-zadach-166
Купить диплом ВУЗа по невысокой стоимости вы сможете, обратившись к надежной специализированной компании. Приобрести документ о получении высшего образования можно у нас. [url=http://vuz-diplom.ru/kupit-diplom-vuza-s-zaneseniem-v-reestr-bistro-i-nadezhno-8/]vuz-diplom.ru/kupit-diplom-vuza-s-zaneseniem-v-reestr-bistro-i-nadezhno-8[/url]
https://crmes.org.br/noticias/mais-uma-noite-de-homenagem-e-reconhecimento-aos-medicos-capixabas/
https://www.durovis.com/en/board_topic_42735_0.html
магазин аккаунтов магазин аккаунтов
portofele electronice casino https://1win5050.ru/ .
https://new.earth-history.com/index.php/component/k2/item/1503-the-qur-an-74-al-mudathir?start=910
кракен сайт – kra32.cc, кракен купить
Pinnacle: Восхождение к вершинам онлайн-беттинга
В мире азартных игр и спортивных ставок Pinnacle занимает особое место. Этот букмекер, известный своим лояльным отношением к профессиональным игрокам и высокими лимитами, привлекает внимание тех, кто стремится к серьезной игре. Но как сориентироваться в мире Pinnacle, когда поисковые запросы пестрят альтернативными адресами и зеркалами? Давайте разберемся.
Pinnacle: Что скрывается за названием?
Pinnacle – это не просто букмекерская контора, это целая философия беттинга. Компания, зарекомендовавшая себя как надежный партнер, предлагает широкую линию ставок, конкурентные коэффициенты и, что немаловажно, отсутствие ограничений для успешных игроков. Именно поэтому запросы вроде “pinnacle букмекерская контора” и “pinnacle контора” так популярны среди опытных бетторов.
Зеркала и доступ к Pinnacle:
В силу особенностей регулирования онлайн-гемблинга, доступ к официальному сайту Pinnacle (pinnacle com) может быть затруднен. В таких случаях на помощь приходят зеркала – альтернативные адреса, позволяющие обойти блокировки и продолжить игру. Запросы “pinnacle зеркало”, “pinnacle com официальный” и “pinnacle com сайт” указывают на стремление игроков получить бесперебойный доступ к платформе.
Регистрация и начало игры:
Для того чтобы стать частью мира Pinnacle, необходимо пройти простую процедуру регистрации (“pinnacle регистрация”). После этого перед вами откроются все возможности платформы: от ставок на спорт до киберспорта и других событий.
Pinnacle в России:
Важно отметить, что доступ к Pinnacle на территории России может быть ограничен. Игроки, находящиеся в России, часто используют зеркала или VPN-сервисы для доступа к сайту (“pinnacle россия”).
Вывод средств:
Pinnacle славится своей надежностью в вопросах выплат. Запросы “pinnacle вывод” и “пинакл вывод” подтверждают интерес игроков к этой теме. Компания предлагает различные способы вывода средств, обеспечивая удобство и безопасность транзакций.
В заключение:
Pinnacle – это выбор профессионалов и тех, кто стремится к серьезному отношению к ставкам. Несмотря на возможные трудности с доступом, преимущества, которые предлагает эта букмекерская контора, делают ее популярной среди игроков по всему миру. пинакл контора официальный сайт
http://forum.aiutamici.com/yaf_postst106400_booksofra.aspx
Электропривод для горизонтальных жалюзи купить
электропривод для горизонтальных жалюзи электропривод для горизонтальных жалюзи .
Заказать диплом университета по доступной стоимости вы сможете, обращаясь к надежной специализированной компании. Купить документ ВУЗа вы можете у нас в Москве. bcstaffing.co/employer/16841/originals-diplomsi
https://foro.rune-nifelheim.com/recent/?start=70;PHPSESSID=4bfd864d341578ea66e091823fea5d40
I hit a blackjack wave—good run! plinko
мелбет кг https://www.melbet1004.ru .
Мы изготавливаем дипломы любых профессий по доступным тарифам.– diplomservis.com/kupit-diplom-s-zaneseniem-v-reestr-bistro-i-nadezhno-17/
Мы предлагаем максимально быстро купить диплом, который выполнен на оригинальной бумаге и заверен печатями, водяными знаками, подписями официальных лиц. Документ способен пройти любые проверки, даже с применением специального оборудования. engineerring.net/employer/premiumydiploma
https://www.keepandshare.com/discuss4/18335/gra?ifr=y
Хранение личных дел и восстановление утраченных связей: от архивов до ДНК
Жизнь каждого человека – это уникальная история, сотканная из множества событий, дат и имен. Личные дела, документы ЗАГС, архивы предприятий и даже генетические тесты – все это фрагменты мозаики, позволяющие восстановить прошлое и понять свое место в настоящем.
От личного дела до архива ФСБ: пути поиска информации
Вопрос о необходимости автобиографии в личном деле часто возникает в контексте трудоустройства или поступления в учебное заведение. Однако, помимо этого, существует множество других ситуаций, когда возникает потребность в восстановлении документов ЗАГС или поиске архивных данных.
Восстановление документов ЗАГС: копии актовых записей и исправление ошибок
Утрата свидетельства о рождении или браке – неприятная, но решаемая проблема. Копии актовых записей можно получить в органах ЗАГС по месту регистрации события. Важно также своевременно исправлять ошибки в актовых записях, чтобы избежать проблем в будущем.
Поиск национальной идентичности: от еврейских корней до ДНК-теста
Подтверждение еврейских корней часто необходимо для репатриации или получения гражданства. В этом случае могут потребоваться архивные документы, свидетельства очевидцев и другие доказательства. Альтернативным методом определения национальной принадлежности является ДНК-тест.
Правовые аспекты: легализация, переводы и установление родства через суд
Легализация и переводы документов необходимы для их использования за границей. В случае возникновения споров о наследстве или установлении родства может потребоваться обращение в суд. Юрист по наследственным делам поможет разобраться в сложных правовых вопросах.
Архивный поиск: от ликвидированных предприятий до архива ВУЗа
Архивный поиск может быть необходим для подтверждения трудового стажа, получения информации о репрессированных родственниках или поиска документов, связанных с деятельностью ликвидированных предприятий. Архив ВУЗа может содержать информацию об образовании и научной деятельности. подтверждение еврейских корней
перепродажа аккаунтов prodat-akkaunt-online.ru
I love the pay flow online—easy go! aviator casino
https://iancleary.com/top-tip-for-recovery/
сухие трансформаторы сухие трансформаторы .
1c бухгалтерия цена 1c бухгалтерия цена .
бухгалтерия 1с купить [url=http://www.bisound.com/forum/showthread.php?p=1951167/]http://www.bisound.com/forum/showthread.php?p=1951167/[/url] .
лестница заказать [url=www.izgotovlenie-lestnic-na-zakaz.ru]лестница заказать[/url] .
антенна TV Flat HD http://www.tv-antenka.ru/ .
https://lifecareortho.in/index.php/knee/
Приобрести диплом о высшем образовании. Заказ подходящего диплома через надежную компанию дарит немало достоинств для покупателя. Данное решение позволяет сэкономить как личное время, так и серьезные финансовые средства. mynewsport.ru/lyogkoe-reshenie-voprosa-s-diplomom
посмотреть на этом сайте cryptoboss казино
Мы изготавливаем дипломы любой профессии по разумным тарифам. Стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы дипломы были доступны для подавляющей массы граждан.
Заказ документа, подтверждающего обучение в университете, – это выгодное решение. Заказать диплом любого университета: diplom-insti.ru/kupit-diplom-nedorogo-4/
мелбет kg https://www.melbet1004.ru .
I wish online helped new—tough kick! bet on red
https://www.mcuspace.com/forum/viewtopic.php?p=240290
https://www.rtinetwork.org/about-us/contributors/467-ross-jonathan-g
http://centennial-qp.arrl.org/forum/topics/view/4200/page:1
Управление жалюзи без усилий с моторизированными системами
моторизированные жалюзи моторизированные жалюзи . прокарниз
https://90plink.live/truc-tiep-tran-dau-asane-fotball-b-vs-brann-2-2723958
мелбет кж мелбет кж .
https://www.rtinetwork.org/professional/forums-and-webinars/webinars/
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
https://mama-life.nl/topic/lees/33760/1/online-slot
https://rtinetwork.org/rti-blog/entry/1/4
https://e-natura.unsa.edu.ar/moodle/mod/forum/discuss.php?d=20109&parent=36982
aplicația 1win http://1win5052.ru .
Профессиональная сборка мебели в Дубае: Быстро, Надежно, Без Хлопот.
Ищете надежную услугу сборки мебели в Дубае? Мы поможем превратить коробки и детали в готовый интерьер! Наши опытные мастера выполнят сборку мебели в Дубае любой сложности — от простых стеллажей до сложных гарнитуров и модульных систем. Экономьте время, нервы и силы, доверив работу профессионалам.
Вызвать сборщика мебели в Дубае просто: оставьте заявку на сайте или по телефону, укажите тип мебели и желаемое время визита. Мы прибудем точно в срок и завершим работу в кратчайшие сроки!
Сборка мебели в Дубае:
Для кого эта услуга?
– Для занятых: Нет времени разбираться с инструкциями? Доверьтесь нам.
– Для новоселов: Нужно быстро обустроить квартиру после переезда? Мы поможем.
– Для бизнеса: Собираем офисную мебель, торговое оборудование и предметы интерьера для ресторанов.
Не тратьте часы на попытки понять сложные схемы — вызовите сборщика мебели уже сегодня! Наша услуга сборки мебели в Дубае — это идеальное сочетание качества, скорости и доступной цены.
Звоните сейчас и получите готовый интерьер без усилий! Ваша мебель будет собрана безупречно — как будто вы это сделали сами, но в разы быстрее!
обслуживание кондиционера Дубай
https://rtinetwork.org/rti-blog-archives/entry/1/113
1win зайти http://www.1win8007.ru .
http://www.rtinetwork.org/voices-archives/entry/2/210
1win pro download https://1win8017.ru .
melbet https://www.melbet1005.ru .
https://vbweb.com.br/forum_resp.asp?Codigo=249758
1win.pri http://www.1win8008.ru .
http://www.rtinetwork.org/rti-marketplace/blog
1win site 1win site .
https://bohn-massivhaus.de/index.php/component/k2/item/1?start=1990
Премиум моторизированные жалюзи для элитных интерьеров
моторизированные жалюзи моторизированные жалюзи . Prokarniz
https://mindbenderescaperooms.com/jacksonvillebeach-fl/uncategorized/need-safe-and-sound-paypal-on-line-casino-in-the-uk-research-our-multitude-pertaining-to-2022/
Также рекомендую вам почитать по теме – https://ancientcivs.ru .
И еще вот – https://l-spb.ru .
http://www.6555555.com/1198.html?replyTo=32571
мостбет uz https://mostbet3025.ru .
В современном мире, где время – один из самых ценных ресурсов, услуги клининга становятся все более востребованными. Будь то поддержание чистоты в офисе, генеральная уборка после ремонта или деликатный уход за домом, профессиональная клининговая компания предлагает решения для любой задачи.
Однако, выбор правильной компании – это не просто вопрос цены. Важно учитывать опыт работы, репутацию, спектр предоставляемых услуг и, конечно же, используемые технологии и экологичность применяемых средств. Надежная клининговая компания должна иметь в своем арсенале современное оборудование и обученный персонал, способный справиться с загрязнениями любой сложности. https://cleaningplus.ru/
https://www.durovis.com/es/board_topic_42662.html
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт телефонов айфон в москве адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
значок металлический круглый izgotovlenie-znachkov-moskva.ru
1win login nigeria http://1win1023.top/ .
магазин аккаунтов магазин аккаунтов
Купить диплом ВУЗа по доступной цене возможно, обратившись к проверенной специализированной фирме. Заказать документ института вы сможете в нашей компании в столице. diplom-kaluga.ru/kupite-diplom-s-reestrom-bez-lishnix-zabot
https://domainkey.donamix.com/forums/discussion/education/play-slots-online-in-ghana?page=1
Купить диплом любого университета!
Мы предлагаемвыгодно и быстро приобрести диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями. Документ способен пройти лубую проверку, даже с использованием профессиональных приборов. Достигайте свои цели максимально быстро с нашими дипломами- v69-3.flybb.ru/posting.phpmode=post&f=8
http://www.rtinetwork.org/rti-blog-archives/entry/1/14
https://sumo.app/tl/forum/tutorials-and-techniques/mines1
https://cremepa.org.br/slide_show/delegacia-do-crm-pa-de-castanhal-tem-atendimento-suspenso-temporariamente/
mostbet uz haqida http://www.mostbet3024.ru .
https://rushers.forum.eu/flucht-und-einwanderung/fotoreihe-aus-syrien-yours-truly-from-idlib
Наши специалисты предлагают быстро и выгодно купить диплом, который выполнен на оригинальном бланке и заверен печатями, водяными знаками, подписями. Документ пройдет лубую проверку, даже при использовании специального оборудования. 1wum.ru/forum/?PAGE_NAME=profile_view&UID=48170&MUL_MODE=
Диплом университета Российской Федерации!
Без университета сложно было продвигаться вверх по карьере. Именно поэтому решение о заказе диплома стоит считать выгодным и целесообразным. Заказать диплом об образовании speciesgame.com/forum/viewtopic.php?f=2&t=344497
https://makutizanzibar.com/component/k2/item/3-advice-for-stirring-your-online-community-and-fostering-engagement?start=2550
маркетплейс аккаунтов аккаунты с балансом
Приобрести диплом института по невысокой стоимости возможно, обращаясь к проверенной специализированной компании. Приобрести документ института можно у нас в столице. babygirls016.copiny.com/question/details/id/1087381
https://mcuspace.com/forum/viewtopic.php?t=59960&start=75
Умные деревянные жалюзи с электроприводом
Деревянные горизонтальные жалюзи с электроприводом [url=https://derevo-gorizont-motor.ru/]Деревянные горизонтальные жалюзи с электроприводом[/url] . прокарниз
plinko reviews
https://onlinecourses.csicy.com/forums/topic/online-slot/
https://sumo.app/ms/forum/tutorials-and-techniques/minas1
Заказать диплом о высшем образовании!
Мы можем предложить дипломы любых профессий по доступным ценам. Вы приобретаете диплом через надежную и проверенную фирму. : msfo-soft.ru/msfo/forum/user/56046
металлические значки штамповка корпоративные значки на заказ
https://new.earth-history.com/index.php/civilizations/civilizations-index/ancient-egypte/item/1503-the-qur-an-74-al-mudathir?start=3790
https://social.batalp.com/rockrtzxc124?mode=day
https://www.konpart.de/component/k2/item/2/2.html?start=21620
https://iohanes.com/blog/fitness/10-beneficios-del-fitness-que-desconocias/
маркетплейс аккаунтов соцсетей гарантия при продаже аккаунтов
https://iohanes.com/blog/jugos/jugo-para-la-presion-arterial/
маркетплейс аккаунтов маркетплейс аккаунтов
https://aviatorng.netlify.app/
1win aviator login http://1win8017.ru/ .
“I Went Vegan for 10 Weeks and This Is What Happened to My Body and Mindset”
BJ벗방
https://iancleary.com/after-mecfs-day/
1win зеркало сайта онлайн 1win зеркало сайта онлайн .
mostbet uz https://www.mostbet3025.ru .
Заказать диплом под заказ в столице вы сможете через сайт компании. mosregeon.flybb.ru/viewtopic.phpf=2&t=965
https://www.vrouwenpower.nl/forums/topic/demomodus-van-gokkasten/
биржа аккаунтов гарантия при продаже аккаунтов
Высококачественные деревянные жалюзи с электроприводом
Деревянные горизонтальные жалюзи с электроприводом Деревянные горизонтальные жалюзи с электроприводом . Прокарниз
https://rtinetwork.org/rti-blog-archives/entry/1/173
Купить диплом университета!
Мы готовы предложить документы институтов, которые расположены на территории всей РФ.
diplomc-v-ufe.ru/kupit-diplom-zanesennij-reestr-2/
https://www.sash.ca/discover-the-best-casino-game-plinko-app-tips-33
скачать бк осталось именно выбрать подходящее https://www.mostbet8006.ru .
1win metode de plată 1win5053.ru .
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: вызвать мастера по ремонту iphone
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://www.iyfusa.org/group/iyfusa-group/discussion/7237771b-828a-4c11-8f80-838e539af280
https://www.cintajamu.id/unlocking-secrets-casino-game-aviator-hack-for/
маркетплейс аккаунтов аккаунты с балансом
склады хранения вещей склады хранения вещей .
покупка аккаунтов магазин аккаунтов
1 win moldova http://1win5052.ru/ .
https://iohanes.com/blog/remedios-caseros/piedras-en-los-rinones/
https://bohn-massivhaus.de/index.php/component/k2/item/1%5D?start=30
https://mindbenderescaperooms.com/jacksonvillebeach-fl/uncategorized/%D0%BA%D0%B0%D0%BA-%D0%BC%D0%BE%D0%B6%D0%BD%D0%BE-%D0%B8%D0%B3%D1%80%D0%B0%D1%82%D1%8C-%D0%B2-%D0%B8%D0%BD%D1%82%D0%B5%D1%80%D0%BD%D0%B5%D1%82-%D0%BA%D0%B0%D0%B7%D0%B8%D0%BD%D0%BE-%D0%B8%D0%B3%D1%80/
https://rodrigogarciaplatas.online/plinko-app-gewinnen-sie-mit-dem-aufregendsten/
https://undiscoveredrp.nn.pe/showthread.php?mode=threaded&tid=26303&pid=48269
Мы изготавливаем дипломы любых профессий по выгодным тарифам. Мы готовы предложить документы ВУЗов, которые находятся в любом регионе Российской Федерации. Дипломы и аттестаты делаются на бумаге самого высокого качества. Это дает возможность делать государственные дипломы, которые не отличить от оригинала. celest-interim.fr/employer/archive-diploma
http://www.smbc-comics.com/smbcforum/posting.php?mode=quote&f=1&t=10149&p=180320
https://proba.kapijeiograde.rs/aviator-app-download-aviator-game-apk-3/
http://forum.orangepi.org/orangepibbsen/forum.php?mod=viewthread&tid=153435
https://www.lions-leonberg.de/LionsClub/category/https-aviator-in/
1win metode de plată http://1win5053.ru/ .
супер маркетплейс kraken onion зеркало с современным интерфейсом и удобным функционалом онион, специализируется на продаже запрещенных веществ по всему миру. У нас ты найдешь всё, от ароматных шишек до белоснежного порошка. Кракен. Купить.
Расскажем о поломках ноутбука acer
https://www.sinanogludoseme.com.tr/blog/page/11/
Купить диплом любого университета!
Покупка документа о высшем образовании через надежную фирму дарит ряд достоинств. Приобрести диплом института у сильной компании: doks-v-gorode-novosibirsk-54.ru
Обзоры шторных решений для загородных домов, подчеркните дизайн, подбираем шторы для загородного дома, эстетика и комфорт, стиль и удобство, натуральные ткани для штор, эффективные шторы для загородного дома, идеи дизайна штор, как подобрать шторы для спальни в доме за городом, шторные решения для больших окон, стильные шторы из льна и холста, автоматические шторы для загородного дома, гармония штор и мебели, украшение окон штором, создайте атмосферу с подходящими шторами, выбор стильных штор для загородного дома, что выбрать для загородного дома, используйте шторы для зонирования пространства, идеи сезонного оформления окон
шторы в загородном доме шторы в загородном доме .
https://riversdalefarm.com.au/discover-the-exciting-world-of-casino-game-plinko-63/
Делайте ставки и выигрывайте без ограничений, подробнее здесь лаки джет официальный сайт скачать
https://www.dmxzone.com/forum/topic.asp?NewsId=13984&topic_id=155297
Приобретение документа о высшем образовании через качественную и надежную компанию дарит ряд плюсов для покупателя. Это решение позволяет сберечь время и существенные финансовые средства. Однако, только на этом выгоды не ограничиваются, плюсов гораздо больше.Мы можем предложить дипломы любой профессии. Дипломы производятся на оригинальных бланках. Доступная цена по сравнению с большими издержками на обучение и проживание в чужом городе. Заказ диплома университета будет мудрым шагом.
Быстро заказать диплом о высшем образовании: asxdiplommy.com/kupite-diplom-s-zaneseniem-v-reestr-rossii-pryamo-sejchas-2/
https://uptasker.co.za/find/entertainer/gauteng/longmeadow-business-estate
osonlashtiradi” osonlashtiradi” .
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:сервисные центры по ремонту техники в мск
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
https://mindbenderescaperooms.com/jacksonvillebeach-fl/blog/page/35/
https://www.formandoformadores.org.mx/colabora/grupos/betnovate-cheapest-maxivate-fast-ach?page=97
Заказать диплом об образовании. Покупка диплома ВУЗа через надежную компанию дарит множество плюсов для покупателя. Такое решение помогает сберечь как личное время, так и значительные финансовые средства. jobpanda.co.uk/employer/eonline-diploma
1win букмекерская контора официальный скачать https://1win10083.ru .
промокод продамус на 5000 promokod-prod.ru .
Мы готовы предложить дипломы любых профессий по выгодным тарифам. Мы предлагаем документы ВУЗов, расположенных в любом регионе Российской Федерации. Дипломы и аттестаты выпускаются на бумаге высшего качества. Это позволяет делать государственные дипломы, не отличимые от оригинала. poobshaemsea.flybb.ru/viewtopic.phpf=2&t=714
1wi [url=https://www.mostbet10012.ru]https://www.mostbet10012.ru[/url] .
проститутки королева на выезд и другие города предлагают множество вариантов для приятного времяпрепровождения.
Если вы ищете проститутки юбилейный для общения, стоит обратить внимание на местные клубы и event-площадки.
В проститутки ивантеевка выезд и проститутки болшево выезд также есть кафе и развлекательные центры, где можно познакомиться с новыми людьми.
Для тех, кто предпочитает проститутки подлипки выезд, работают сервисы по организации свиданий и совместных поездок.
Аналогичные услуги есть в Подлипках – достаточно изучить местные сообщества.
1 win официальный зеркало 1win10082.ru .
интернет провайдер новосибирск
novosibirsk-domashnij-internet002.ru
подключить интернет
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Pin Up Azərbaycan üçün ən yaxşı seçimdir. Pin Up mərc oyunlarında uğurlar arzulayırıq. Pin Up casino-da ən yaxşı slot oyunları. Pin Up azərbaycanlı istifadəçilər üçün xüsusi aksiyalar keçirir pin-up az.
индивидуальное хранение вещей в москве недорого индивидуальное хранение вещей в москве недорого .
Выгодно приобрести диплом о высшем образовании!
Мы готовы предложить дипломы психологов, юристов, экономистов и прочих профессий по разумным ценам— masterdiplomov.ru
провайдеры интернета в новосибирске по адресу проверить
novosibirsk-domashnij-internet003.ru
интернет провайдеры по адресу новосибирск
melbet kg скачать melbet kg скачать .
гражданство испании
1win вход на сайт https://1win10008.ru .
I recently tried https://killakush.com/product-category/thc-vape-pen/ , and I’m extraordinarily impressed with the quality. The effects were mild, calming, and exactly what I was hoping for. The miscellany of options also allowed me to detect something perfect representing both relaxing evenings and rich days. Indubitably commend proper for anyone seeking great results!
1win uganda https://1win1031.top/ .
домашний интернет тарифы новосибирск
novosibirsk-domashnij-internet003.ru
интернет по адресу дома
Купить документ института вы имеете возможность в нашей компании. Мы предлагаем документы об окончании любых университетов РФ. Вы получите необходимый диплом по любым специальностям, включая документы Советского Союза. Гарантируем, что при проверке документов работодателями, подозрений не появится. kupitediplom0027.ru/kupit-diplom-s-zaneseniem-v-reestr-bistro-i-nadezhno-28/
I’ve been using https://www.nothingbuthemp.net/products/thc-indica-tincture daily for all about a month at the moment, and I’m justifiably impressed before the sure effects. They’ve helped me determine calmer, more balanced, and less tense from the beginning to the end of the day. My snore is deeper, I wake up refreshed, and uniform my pinpoint has improved. The trait is excellent, and I appreciate the accepted ingredients. I’ll definitely heed buying and recommending them to the whole world I be aware!
Доставили точно в срок, как и обещали! Получатель был в восторге от букета.
заказать цветы томск
интернет провайдер омск
omsk-domashnij-internet001.ru
подключить интернет в квартиру омск
mel bet сайт http://melbet1013.ru/ .
Hi there! Do you know if they make any plugins to assist with Search Engine Optimization? I’m trying to get my blog to rank for some targeted keywords but I’m not seeing very good success. If you know of any please share. Kudos!
rocketply
Great experience! The towing service was prompt, and they took good care of my vehicle. Very satisfied!
Приобрести диплом об образовании. Производство документа занимает немного времени, а стоимость при этом доступна любому. В итоге вы получаете возможность сохранить бюджет и найти работу мечты. Купить диплом под заказ в Москве можно через сайт компании. – human.forumieren.de/login
таможенные брокеры москва таможенные брокеры москва .
account trading platform sell pre-made account
1win на телефон https://1win10083.ru .
домашний интернет тарифы
omsk-domashnij-internet001.ru
лучший интернет провайдер омск
Стадион нефтяник хадыженск
Васюринский музей боевой и трудовой славы
Кубань картинки для презентации
Васюринский музей
Люди кубани фото
кубань фото природы, край фото, природа кубани фото самые красивые места, кубани фото и описание, паспорт кубани фото
kuban.photo
услуги таможенного брокера стоимость [url=http://tamozhennyj-broker13.ru]http://tamozhennyj-broker13.ru[/url] .
шторы роллы на окна http://www.rulonnye-shtory-s-elektroprivodom50.ru .
подключить интернет в омске в квартире
omsk-domashnij-internet002.ru
подключить интернет в омске в квартире
купить диплом в мурманске купить диплом в мурманске .
таможенный брокер перевозки таможенный брокер перевозки .
1win rossvy 1win10082.ru .
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по приятным тарифам. Мы предлагаем документы ВУЗов, расположенных в любом регионе Российской Федерации. Документы выпускаются на бумаге высшего качества. Это дает возможность делать настоящие дипломы, которые невозможно отличить от оригиналов. quierotrabajo.com.py/companies/archive-diploma
Fast and reliable roadside assistance! The team was professional and friendly. Will definitely call them again if needed.
таможенный брокер стоимость услуг таможенный брокер стоимость услуг .
домашний интернет омск
omsk-domashnij-internet002.ru
провайдеры домашнего интернета омск
рулонные шторы на окна купить рулонные шторы на окна купить .
для хранения вешей для хранения вешей .
интернет провайдеры омск
omsk-domashnij-internet003.ru
дешевый интернет омск
Заказать диплом любого ВУЗа!
Приобрести диплом любого института. Покупка диплома ВУЗа через надежную компанию дарит немало достоинств для покупателя. Это решение дает возможность сберечь время и существенные денежные средства. laver.listbb.ru/viewtopic.phpf=3&t=3194
account sale account store
брокеры москва http://www.tamozhennyj-broker13.ru .
домашний интернет омск
omsk-domashnij-internet003.ru
домашний интернет омск
вон вин http://www.1win10086.ru .
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
шторы автоматические http://www.rulonnye-shtory-s-elektroprivodom50.ru .
таможенный брокер домодедово таможенный брокер домодедово .
account catalog account trading
бонусы в 1 win http://1win10085.ru/ .
игра 1win отзывы игра 1win отзывы .
домашний интернет подключить омск
omsk-domashnij-internet003.ru
дешевый интернет омск
Купить диплом на заказ в столице возможно через сайт компании. link.5nx.ru/viewtopic.phpf=12&t=12123
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
ролл штора на пластиковое окно http://www.rulonnye-shtory-s-elektroprivodom10.ru/ .
провайдеры интернета в перми
perm-domashnij-internet001.ru
подключить интернет
Купить диплом о высшем образовании!
Наши специалисты предлагаютвыгодно и быстро купить диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями официальных лиц. Данный документ пройдет любые проверки, даже с применением специальных приборов. Решите свои задачи быстро и просто с нашим сервисом- define.listbb.ru/viewtopic.phpf=46&t=1418
приложение 1win [url=https://1win10084.ru/]приложение 1win[/url] .
Приветствую!
Мы изготавливаем дипломы любой профессии по приятным ценам. Цена будет зависеть от конкретной специальности, года выпуска и университета: diplomans.com/
домашний интернет тарифы пермь
perm-domashnij-internet001.ru
подключение интернета пермь
узнать кракен открыть
The Filipino Channel, commonly known as TFC, is a global subscription television network owned and operated by the Filipino media conglomerate ABS-CBN Corporation. 야동코리아
Excellent towing service! They arrived quickly and handled my car with great care. Highly recommended!
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Приобретение документа, подтверждающего окончание института, – это выгодное решение. Приобрести диплом любого университета: laver.listbb.ru/viewtopic.phpf=3&t=3174
Our limousine service offers premier Anacortes Ferry Terminal transportation , focusing on comfort, style, and punctuality. We provide a top-tier Anacortes Ferry Terminal town car service with professional chauffeurs and a fleet of luxury vehicles. For those seeking exclusivity, we offer Anacortes Ferry Terminal private transfer options, ensuring a personalized experience. Whether for business or leisure, our services guarantee a smooth connection to and from the terminal.Our limousine service offers premier Anacortes Ferry Terminal transportation, focusing on comfort, style, and punctuality. We provide a top-tier Anacortes Ferry Terminal town car service with professional chauffeurs and a fleet of luxury vehicles. For those seeking exclusivity, we offer Anacortes Ferry Terminal private transfer options, ensuring a personalized experience. Whether for business or leisure, our services guarantee a smooth connection to and from the terminal.Our limousine service offers premier Anacortes Ferry Terminal transportation, focusing on comfort, style, and punctuality. We provide a top-tier Anacortes Ferry Terminal town car service with professional chauffeurs and a fleet of luxury vehicles. For those seeking exclusivity, we offer Anacortes Ferry Terminal private transfer options, ensuring a personalized experience. Whether for business or leisure, our services guarantee a smooth connection to and from the terminal.
Ищете рабочие зеркала для входа на кракен ссылка тор? У нас только проверенные и актуальные ссылки на торговую площадку кракен даркнет сайт. Инструкции по безопасному входу через Tor и VPN, а также свежие ссылки в нашем Telegram канале. Заходите и совершайте покупки быстро и безопасно на кракен фургон!
домашний интернет
perm-domashnij-internet002.ru
провайдеры интернета в перми
этот контент кракен даркнет
подключить интернет
perm-domashnij-internet003.ru
интернет провайдер пермь
Мы изготавливаем дипломы любой профессии по выгодным тарифам.– o2cloudsystems.com/kak-bystro-kupit-diplom-i-ne-popastsja-80
скачать букмекерскую контору официального сайта 1win10009.ru .
dulapuri la comanda http://1win5067.ru .
домашний интернет в перми
perm-domashnij-internet003.ru
провайдеры пермь
mostbet скачать 2024 http://www.mostbet10014.ru .
Купить диплом института по выгодной цене вы сможете, обращаясь к надежной специализированной компании. Купить документ о получении высшего образования можно у нас в столице. diplom-kaluga.ru/kupit-diplom-o-visshem-obrazovanii-bistro-i-prosto-4
подробнее здесь кракен войти
“Those who don’t believe in magic will never find it.” 국산야동
An unexamined life is not worth living. BJ야동
1win казино зеркало http://1win10088.ru .
Купить диплом института по выгодной цене возможно, обратившись к проверенной специализированной компании. Мы предлагаем документы ВУЗов, расположенных на территории всей РФ. topcareerscaribbean.com/employer/eonline-diploma
домашний интернет ростов
rostov-domashnij-internet001.ru
интернет провайдер ростов
Букет выглядел даже лучше, чем я ожидала! Спасибо огромное!
доставка цветов
1win kz 1win kz .
Заказать диплом ВУЗа поможем. Купить диплом института – diplomybox.com/diplom-instituta
1win site http://1win5067.ru .
I’ve been using https://www.nothingbuthemp.net/products/thc-sex-gummies-watermelon daily in regard to during the course of a month at the moment, and I’m truly impressed by the positive effects. They’ve helped me judge calmer, more balanced, and less solicitous in every nook the day. My sleep is deeper, I wake up refreshed, and uniform my pinpoint has improved. The quality is distinguished, and I worth the natural ingredients. I’ll definitely preserve buying and recommending them to everyone I know!
I recently tried thca flower , and I’m in actuality impressed with the quality. The effects were slick, calming, and literally what I was hoping for. The miscellany of options also allowed me to find something flawless for both relaxing evenings and bountiful days. Indubitably advise proper for anyone seeking significant results!
Play online!
http://hkeverton.com/forumnew/home.php?mod=space&uid=395224
бот lucky jet скачать https://1win10009.ru .
1 вин бк http://1win10010.ru .
домашний интернет тарифы
rostov-domashnij-internet001.ru
домашний интернет ростов
Присоединяйтесь к Lex Casino и наслаждайтесь игрой. Lex Casino позволяет быстро выводить средства без задержек. Lex Casino — это честные выплаты и поддержка на высшем уровне. Не могу найти аналогов Lex Casino по качеству игр и бонусов. Регистрируйтесь на Lex Casino и получите свой бонус. На Lex Casino всегда найдется что-то интересное для каждого игрока lex casino отзывы.
дешевый интернет ростов
rostov-domashnij-internet002.ru
домашний интернет
Сигареты оптом В современном мире табачной индустрии, где разнообразие предложений кажется безграничным, найти надежного поставщика и партнера становится ключевой задачей для успешного бизнеса и удовлетворения потребностей конечного потребителя. Мы предлагаем широкий спектр решений для тех, кто ищет возможность купить сигареты оптом, нуждается в надежном магазине сигарет или хочет заказать стики с доставкой.
Где приобрести диплом по актуальной специальности?
Купить диплом университета по доступной стоимости возможно, обращаясь к надежной специализированной фирме.: diplom-insti.ru
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по выгодным тарифам. Дипломы изготавливаются на оригинальных бланках государственного образца Купить диплом о высшем образовании diplomaj-v-tule.ru
Приобрести диплом о высшем образовании !
Покупка диплома ВУЗа России в нашей компании является надежным делом, ведь документ заносится в реестр. Заказать диплом о высшем образовании dip-lom-rus.ru/kupit-diplom-s-reestrom-po-dostupnoj-tsene-5
Читать далее кракен
возврат эфира билайн тв http://1win10085.ru/ .
Главная kra33.at
Доброго!
ПМЖ Польши — это статус, который дает вам право жить в Польше без ограничений и пользоваться правами граждан. паспорт португалии ПМЖ в Польше предоставляется после нескольких лет проживания и выполнения условий, таких как наличие работы и финансовых средств. ПМЖ также дает право на безвизовые поездки по ЕС и возможность получения гражданства. Мы поможем вам оформить ПМЖ в Польше и пройти все необходимые этапы. Свяжитесь с нами для консультации.
Бизнес в Польше — это возможность развивать компанию в одной из самых быстро развивающихся стран Европы. Польша предоставляет выгодные условия для ведения бизнеса и имеет выгодное расположение для международной торговли. Мы поможем вам с процессом создания бизнеса в Польше и оформлением всех необходимых документов. Свяжитесь с нами для консультации по бизнесу в Польше.
Вся информация на сайте – https://expert-immigration.com/blog/grazhdanstvo-urugvaya
внж швейцарии, паспорт за инвестиции, гражданство канады
эмиграция в евросоюз,ес, паспорт мексики, внж англии
Удачи!
продолжить kraken зайти
подключить интернет тарифы ростов
rostov-domashnij-internet002.ru
подключение интернета ростов
Нужен номер для ТГ? Предлагаем https://techalpaka.online для одноразовой или постоянной активации. Регистрация аккаунта без SIM-карты, в любом регионе. Удобно, надёжно, без привязки к оператору.
интернет провайдер ростов
rostov-domashnij-internet003.ru
подключить проводной интернет ростов
1 win официальный сайт скачать 1win10086.ru .
Hello casino users!
Explore a World of Games with Elon Casino Bangladesh
Elon Casino offers Bangladeshi players an extensive game library, from classic slots to immersive live dealer games like elon casino crazy time. Whether you’re a beginner or a seasoned gamer, elon casino bangladesh has something to suit your taste, all accessible via elonbet.com.
The platform features hundreds of slots, including progressive jackpots with massive payouts. Table games like poker, blackjack, and roulette come in multiple variants, catering to strategy lovers. The elon online casino live section brings real casino vibes with professional dealers, streamed in HD for an authentic experience.
For mobile gamers, the elon casino app download delivers the full game catalog on Android devices. Download the elon casino apk from the official site and enjoy seamless gameplay on the go. The app’s Bengali interface and fast loading times enhance the experience for local players.
Deposits are quick with bKash, Nagad, and Rocket, starting at just 500 BDT. New players can try games with a elon casino no deposit bonus, while regular promotions add extra spins and cashback. The elon bet casino login is user-friendly, ensuring you can jump into your favorite games instantly.
Elon Casino updates its catalog regularly, introducing exclusive titles and themed games. With 24/7 support in Bengali and a secure platform, elon casino bd guarantees a fun and safe gaming environment. Sign up today and discover why elon bet is a top choice for Bangladeshi players.
All information is available via the link – https://elderscrollsdragonknight.com/
elon casino app login, elon.casino, elon casino no deposit bonus
elon casino apk, elon casino app login, elon casino
Good profits!
I started delightful https://www.cornbreadhemp.com/products/thc-seltzer-blueberry-breeze-5mg a few months ago and forthrightly, they’ve мейд a huge difference. I in the main take hold of at one after dinner to boost me coldness unserviceable and have a zizz better. They mouthful renowned —like actual confectionery — and don’t beat it me view groggy the next day. It’s moral a worthwhile, age vibe that helps receive the edge off after a stressful day. Wasn’t inescapable they’d feat at first, but at the moment I’m all in. Totally interesting if you dire something fool to ease you abate without any eerie side effects.
интернет тарифы ростов
rostov-domashnij-internet003.ru
лучший интернет провайдер ростов
1win. казино. регистрация. http://1win10088.ru/ .
1win mexico 1win1017.top .
Привет всем!
Адвокат в Италии поможет вам разобраться в юридических аспектах иммиграции, недвижимости и других вопросах. виза блогеров в андорре Профессиональные адвокаты в Италии предоставляют консультации по вопросам получения гражданства, ВНЖ и ПМЖ. Если у вас возникли юридические трудности в процессе иммиграции, адвокат сможет предоставить вам качественную помощь. Мы рекомендуем обращаться только к опытным специалистам. Обратитесь к нам за консультацией по вопросам юридической помощи в Италии.
ВНЖ Аргентины — это разрешение на временное проживание в одной из самых развивающихся стран Южной Америки. ВНЖ Аргентины можно получить через работу, учебу или инвестиции. Мы поможем вам с процессом получения ВНЖ Аргентины и оформлением всех необходимых документов. Свяжитесь с нами для консультации по ВНЖ Аргентины.
Вся информация на сайте – https://expert-immigration.com/vnzh-v-andorre-aia25
виза в испании., иммиграция в австрию, недвижимость в испании
гражданство гренады, паспорт мальты, пмж австрии
Удачи!
account selling service https://accounts-offer.org
подключить интернет ростов
rostov-domashnij-internet003.ru
подключить интернет в ростове в квартире
1win скачат https://1win10087.ru/ .
social media account marketplace https://accounts-marketplace.xyz/
Купить диплом университета по доступной цене возможно, обращаясь к проверенной специализированной фирме. Мы оказываем услуги по продаже документов об окончании любых ВУЗов Российской Федерации. Купить диплом любого ВУЗа– chillibell.com/read-blog/2466_kupit-attestat-tehnikuma.html
мостбет авиатор http://mostbet10014.ru .
подключить интернет по адресу
samara-domashnij-internet001.ru
интернет провайдеры самара по адресу
провайдеры интернета в самаре по адресу
samara-domashnij-internet001.ru
провайдеры интернета в самаре по адресу
account store account market
Fast and reliable roadside assistance! The team was professional and friendly. Will definitely call them again if needed.
1win casino mexico https://www.1win1017.top .
Продолжение кракен зеркало
найти это кракен войти
Где купить диплом специалиста?
Мы готовы предложить дипломы любой профессии по доступным ценам. Для нас очень важно, чтобы дипломы были доступны для подавляющей массы граждан. Заказать диплом о высшем образовании diplomass.com/kupit-diplom-s-reestrom-bez-posrednikov-na-forume/
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по доступным ценам. Мы готовы предложить документы ВУЗов, расположенных в любом регионе Российской Федерации. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высокого качества. Это дает возможности делать настоящие дипломы, которые невозможно отличить от оригинала. jobs.colwagen.co/employer/archive-diploma
сюда кракен клир
At from the start, I was skeptical less https://joyorganics.com/products/cbd-dog-treats , but these days I provision them in my nightstand. They’ve develop voice of my evening habitual—ditty gummy, a cup of tea, and I’m good to go. I don’t feel strident or weird, nothing but stillness and genial to wind down. The flavor’s in actuality in actuality fitting too, which surprised me. No unnatural aftertaste like some others I’ve tried. If your perception runs a mile a minute at cimmerian dark or you’re upstanding vexing to cool after manipulate, these are undoubtedly significance checking out.
провайдер по адресу
samara-domashnij-internet001.ru
интернет по адресу самара
Приобрести диплом о высшем образовании!
Мы предлагаем документы учебных заведений, расположенных на территории всей Российской Федерации.
kupitediplom.ru/kupit-diplom-v-moskve-s-zaneseniem-v-reestr-bistro-4/
провайдер по адресу
samara-domashnij-internet002.ru
интернет провайдеры в самаре по адресу дома
КРАКЕН — это мой первый выбор
Приобрести диплом о высшем образовании. Покупка диплома через надежную компанию дарит множество достоинств для покупателя. Данное решение позволяет сберечь как личное время, так и существенные деньги. ddsbyowner.com/employer/eonline-diploma
Если вы ищете надёжную торговую платформу в даркнете, то
станет отличным решением, кракен зайти работает стабильно и предлагает широкий выбор товаров на любой вкус. Сам kraken официальный сайт доступен круглосуточно.
Многие выбирают кракен ссылка маркет за простой интерфейс. Через кракен магазин можно быстро попасть на рабочую версию сайта, даже если основной стор кракен временно недоступен. Для безопасного входа используйте кракен ссылка — это гарантирует стабильный доступ к площадке без сбоев.
kraken площадка пользуется доверием благодаря проверенным продавцам и высокому уровню безопасности. Чтобы легко kraken вход, достаточно иметь актуальное кракен официальный сайт — через него вы получите доступ к полному функционалу сайта.
Если вы не знаете, как попасть на кракен ссылка маркет, просто воспользуйтесь одним из доступных кракен магазин — они регулярно обновляются. Также можно использовать сайт kraken зеркала или сохранить актуальный кракен тор в закладках.
какие провайдеры интернета есть по адресу самара
samara-domashnij-internet002.ru
провайдеры интернета в самаре по адресу проверить
1win sayti http://www.1win10089.ru .
Наша компания предлагает выгодно и быстро заказать диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями официальных лиц. Данный документ способен пройти лубую проверку, даже с использованием специфических приборов. discstarz.com/pokupka-diploma-s-zaneseniem-v-reestr-142
accounts marketplace https://social-accounts-marketplaces.live
Hello casino players!
Safe and Secure Gaming with Elon Casino Bangladesh
Elon Casino prioritizes safety, making it a trusted choice for Bangladeshi players. Licensed and regulated, elon casino bd ensures fair play and secure transactions, giving you peace of mind while enjoying games like elon casino crazy time or classic slots. Your personal and financial data are protected with advanced encryption.
The elon casino login process is simple and secure. Register on www elon casino with your email and password, and use the elon casino login app for quick access on mobile devices. If you encounter issues, the elon bet casino login password recovery feature restores access in minutes. Two-factor authentication adds an extra layer of protection.
Deposits and withdrawals are hassle-free with Bangladeshi payment systems like bKash, Nagad, and Rocket. The minimum deposit is only 500 BDT, and payouts are processed within 24 hours. Elonbet supports cryptocurrency for added privacy, catering to tech-savvy players.
The platform’s Bengali interface ensures easy navigation, while 24/7 customer support in Bengali resolves queries promptly. Whether you’re playing on the elon casino app download or the desktop site, the experience is smooth and reliable. Regular audits and transparent policies reinforce elon casino bangladesh’s commitment to fairness.
From generous bonuses to a vast game library, elon bet casino combines security with entertainment. New players can explore with a elon casino no deposit bonus, while regulars enjoy VIP perks. Join elonbet login mobile today and experience safe, exciting gaming tailored for Bangladesh.
All information is available via the link – https://elderscrollsdragonknight.com/
elon casino login app, casino elon, elon casino app login
elon.casino, elon casino download, elon casino apk download latest version
Good wins!
Мы изготавливаем дипломы любой профессии по приятным тарифам. Купить диплом в Копейске — kyc-diplom.com/geography/kopejsk.html
скачать бк осталось всего только выбрать подходящее скачать бк осталось всего только выбрать подходящее .
интернет провайдеры самара по адресу
samara-domashnij-internet002.ru
провайдеры интернета по адресу самара
1win официальный вход с компьютера https://www.1win10010.ru .
Приобрести диплом об образовании!
Покупка документа о высшем образовании через проверенную и надежную компанию дарит ряд преимуществ для покупателя. Купить диплом об образовании у сильной компании: doks-v-gorode-yoshkar-ola-12.ru
интернет провайдеры по адресу самара
samara-domashnij-internet003.ru
провайдеры интернета по адресу самара
Заказать диплом института по доступной стоимости возможно, обращаясь к проверенной специализированной фирме. Заказать документ о получении высшего образования можно в нашей компании. kupitediplom0027.ru/kupit-diplom-instituta-s-reestrom-bistro-i-nadezhno-9
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
провайдеры интернета в самаре по адресу проверить
samara-domashnij-internet003.ru
провайдеры интернета по адресу самара
Заказать диплом университета по невысокой цене возможно, обращаясь к надежной специализированной компании. Приобрести документ института можно в нашей компании в Москве. fabulousroleplay.5nx.ru/viewtopic.phpf=40&t=531
Источник https://forum.hpc.name/thread/w471/112670/kak-vosstanovit-udalennoe-video-na-android-ustroystve.html
Консультация психолога по интернету. Онлайн психотерапевт. Чат общение.
Психолог владеет множеством приемов и техник, которые помогут разобраться в себе.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
Нужен хороший психолог?
Психолог, Сайт психологов.
Excellent towing service! They arrived quickly and handled my car with great care. Highly recommended!
account market https://accounts-marketplace.live
secure account purchasing platform social-accounts-marketplace.xyz
провайдеры интернета в санкт-петербурге по адресу проверить
spb-domashnij-internet001.ru
подключить интернет в санкт-петербурге в квартире
Как начать разговор с психологом. Консультация психолога онлайн. Психолог онлайн отзывы.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Онлайн сессия от 81911 руб.
Консультация в кризисных состояниях.
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
Профессиональный сервисный центр по ремонту техники в Перми.
Мы предлагаем: Официальный центр Ricoh
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
click resources https://web-foxwallet.com/
Разговор с психологом онлайн. Анонимный психолог. Спроси психолога.
Услуги психолога · — Консультация психолога.
Нужен хороший психолог?
Психологическая помощь онлайн.
Психологическая помощь онлайн анонимно. Онлайн психолог. Видео чат.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Сколько встреч нужно?
Поможет поставить цель терапии и приведет к результату.
Psychologist. Анонимный чат. Психотерапия по переписке.
Психологическая помощь онлайн.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Психологическое консультирование.
sell account https://buy-accounts.space
Вам требуется лечение? https://chemodantour.ru лечение хронических заболеваний, восстановление после операций, укрепление иммунитета. Включено всё — от клиники до трансфера и проживания.
узнать интернет по адресу
spb-domashnij-internet001.ru
провайдеры санкт-петербург
1win букмекерская контора регистрация http://www.1win10013.ru .
купить ноутбук асус ноутбук асус цена
Доставили даже в позднее время, как я и просил. Огромное спасибо!
купить цветы томск
магазины электроники в москве купить электронику
?? Ever wondered if nude cam girls fake their reactions? Or how safe those live sex chat platforms really are?
We break it down at ?? livenude the home of real insights on live nude webcams.
?? Discover:
– Do models fake it — or feel it?
– Are nude cam sites safe for you?
– Why people watch cam girls (psychology behind it)
– What’s trending in YEAR on live cam platforms
?? Real girls. Real shows. Real answers.
Check it all here:
No fluff, just facts and hot models
Mental Triggers Behind Live Nude Cams
купить смартфон нова смартфоны 2025 купить
интернет по адресу санкт-петербург
spb-domashnij-internet002.ru
интернет по адресу
подробнее здесь https://forum.hpc.name/thread/w640/124749/kak-udalenno-zapustit-programmu-na-mashinah-v-lokalnoy-seti.html
Профессиональный сервисный центр по ремонту техники в Барнауле.
Мы предлагаем: Ремонт стиральных машин Kuppersbusch
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
1win 500% http://1win10012.ru .
Fast and reliable roadside assistance! The team was professional and friendly. Will definitely call them again if needed.
Мы готовы предложить дипломы любой профессии по доступным тарифам. Дипломы производят на подлинных бланках государственного образца Приобрести диплом любого университета diplomp-irkutsk.ru
1win вход в личный кабинет https://1win10011.ru/ .
Где заказать диплом по нужной специальности?
Приобрести диплом университета по доступной стоимости вы сможете, обратившись к надежной специализированной компании.: diplom-insti.ru
Если вы ищете надёжную торговую платформу в даркнете, то
станет отличным решением, кракен тор работает стабильно и предлагает широкий выбор товаров на любой вкус. Сам адрес сайта кракен доступен круглосуточно.
Многие выбирают кракен магазин за простой интерфейс. Через kraken сайт можно быстро попасть на рабочую версию сайта, даже если основной зеркало кракен временно недоступен. Для безопасного входа используйте
— это гарантирует стабильный доступ к площадке без сбоев.
kraken вход пользуется доверием благодаря проверенным продавцам и высокому уровню безопасности. Чтобы легко кракен официальный сайт, достаточно иметь актуальное сайт kraken зеркала — через него вы получите доступ к полному функционалу сайта.
Если вы не знаете, как попасть на зеркало кракен, просто воспользуйтесь одним из доступных kraken сайт — они регулярно обновляются. Также можно использовать сайт kraken зеркала или сохранить актуальный кракен ссылка маркет в закладках.
Купить диплом университета по доступной цене возможно, обращаясь к проверенной специализированной компании. Заказать документ о получении высшего образования можно в нашей компании. appletwo.ca/employer/originals-diplomsi
Покупка диплома ВУЗа через качественную и надежную компанию дарит ряд преимуществ для покупателя. Это решение помогает сберечь как длительное время, так и существенные финансовые средства. Тем не менее, плюсов значительно больше.Мы предлагаем дипломы любых профессий. Дипломы производят на подлинных бланках государственного образца. Доступная стоимость по сравнению с огромными расходами на обучение и проживание в чужом городе. Покупка диплома института станет мудрым шагом.
Заказать диплом о высшем образовании: diplomidlarf.ru/kupit-diplom-vnesennij-v-reestr-bistro-i-nadezhno/
провайдеры интернета в санкт-петербурге по адресу проверить
spb-domashnij-internet002.ru
домашний интернет в санкт-петербурге
useful site https://web-foxwallet.com/
интернет провайдеры по адресу дома
spb-domashnij-internet003.ru
подключить проводной интернет санкт-петербург
Профессиональный сервисный центр по ремонту техники в Твери.
Мы предлагаем: Ремонт экшен-камер Kitvision с гарантией
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Ремонт крупной бытовой техники в Москве, куда обращаться для диагнистики и ремонта, читайте – читайте в блоге
официальный таможенный представитель [url=https://tamozhennyj-broker14.ru]https://tamozhennyj-broker14.ru[/url] .
таможеный брокер https://www.tamozhennyj-broker15.ru .
Если вы ищете надёжную торговую платформу в даркнете, то
станет отличным решением, кракен маркетплейс что это работает стабильно и предлагает широкий выбор товаров на любой вкус. Сам сайт kraken зеркала доступен круглосуточно.
Многие выбирают
за простой интерфейс. Через кракен войти можно быстро попасть на рабочую версию сайта, даже если основной кракен войти временно недоступен. Для безопасного входа используйте
— это гарантирует стабильный доступ к площадке без сбоев.
кракен торговая площадка пользуется доверием благодаря проверенным продавцам и высокому уровню безопасности. Чтобы легко кракен тор, достаточно иметь актуальное кракен официальный — через него вы получите доступ к полному функционалу сайта.
Если вы не знаете, как попасть на кракен площадка, просто воспользуйтесь одним из доступных кракен сайт ссылка — они регулярно обновляются. Также можно использовать кракен торговая площадка или сохранить актуальный кракен тор в закладках.
помощь в таможенном оформлении tamozhennyj-broker16.ru .
погода в сочи на 14 и температура воды http://www.weathersochi14days.ru .
провайдеры по адресу санкт-петербург
spb-domashnij-internet003.ru
интернет провайдеры санкт-петербург
рассчитать страховку на автомобиль рассчитать страховку на автомобиль .
интернет https://forum.hpc.name/thread/x392/131808/kak-ispolzovat-smartfon-kak-vneshniy-displey-dlya-ekshn-kamery-cherez-kabel.html
провайдер по адресу уфа
ufa-domashnij-internet001.ru
подключить интернет по адресу
мелбет http://melbet1014.ru/ .
melbet kg скачать http://www.melbet1011.ru .
1win вывод http://1win10012.ru/ .
купить ноутбук для работы ноутбук леново цена
account trading platform buy-accounts-shop.pro
Если вы ищете надёжную торговую платформу в даркнете, то кракен сайт станет отличным решением, кракен официальный сайт ссылка работает стабильно и предлагает широкий выбор товаров на любой вкус. Сам кракен даркнет ссылка на сайт доступен круглосуточно.
Многие выбирают кракен даркнет сайт за простой интерфейс. Через кракен магазин можно быстро попасть на рабочую версию сайта, даже если основной кракен ссылка зеркало временно недоступен. Для безопасного входа используйте кракен ссылка — это гарантирует стабильный доступ к площадке без сбоев.
кракен даркнет ссылка на сайт пользуется доверием благодаря проверенным продавцам и высокому уровню безопасности. Чтобы легко кракен площадка, достаточно иметь актуальное kraken площадка — через него вы получите доступ к полному функционалу сайта.
Если вы не знаете, как попасть на
, просто воспользуйтесь одним из доступных kraken официальный сайт — они регулярно обновляются. Также можно использовать кракен официальный сайт или сохранить актуальный kraken сайт в закладках.
магазин электроники техник магазин электроники
Ремонт крупной бытовой техники в Москве, куда обращаться для диагнистики и ремонта, читайте – в статье
проверить интернет по адресу
ufa-domashnij-internet001.ru
проверить провайдера по адресу
купить смартфон 14 смартфоны 2024 года цены
1win https://1win1032.top .
Рекомендую провереные услуги хакера – Реальные хакеры
1win зайти https://1win10011.ru/ .
Заказать диплом на заказ в столице можно используя сайт компании. apex-workforce.com/employer/frees-diplom
1win betting [url=http://1win1026.top/]http://1win1026.top/[/url] .
какие провайдеры по адресу
ufa-domashnij-internet002.ru
провайдеры интернета по адресу
Заказать документ о получении высшего образования можно в нашем сервисе. Мы предлагаем документы об окончании любых университетов РФ. Вы сможете получить необходимый диплом по любым специальностям, включая документы СССР. Гарантируем, что в случае проверки документов работодателями, никаких подозрений не появится. diplom-top.ru/kupit-diplom-cherez-reestr-bistro-i-bez-lishnix-problem/
Ремонт крупной бытовой техники в Москве, куда обращаться для диагнистики и ремонта, читайте – на сайте
Here to dive into discussions, share thoughts, and learn something new as I go.
I like learning from different perspectives and sharing my input when it’s helpful. Interested in hearing fresh thoughts and meeting like-minded people.
Here is my site:https://automisto24.com.ua/
Your article helped me a lot, is there any more related content? Thanks!
Профессиональный сервисный центр по ремонту техники в Санкт-Петербурге.
Мы предлагаем: Ремонт телевизоров Hisense на дому
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
мостбет сайт http://www.mostbet10015.ru .
Заказать диплом любого ВУЗа!
Наши специалисты предлагаютбыстро купить диплом, который выполняется на оригинальном бланке и заверен печатями, штампами, подписями. Диплом пройдет лубую проверку, даже при использовании специально предназначенного оборудования. Решайте свои задачи быстро с нашими дипломами- okoskalyha.hu/employer/gosznac-diplom-24
Если вы ищете надёжную торговую платформу в даркнете, то кракен сайт станет отличным решением, кракен зеркала работает стабильно и предлагает широкий выбор товаров на любой вкус. Сам кракен площадка доступен круглосуточно.
Многие выбирают кракен сайт ссылка за простой интерфейс. Через кракен официальный сайт можно быстро попасть на рабочую версию сайта, даже если основной кракен маркетплейс что это временно недоступен. Для безопасного входа используйте
— это гарантирует стабильный доступ к площадке без сбоев.
кракен даркнет ссылка на сайт пользуется доверием благодаря проверенным продавцам и высокому уровню безопасности. Чтобы легко кракен торговая площадка, достаточно иметь актуальное кракен магазин — через него вы получите доступ к полному функционалу сайта.
Если вы не знаете, как попасть на кракен тор, просто воспользуйтесь одним из доступных сайт kraken зеркала — они регулярно обновляются. Также можно использовать kraken официальный сайт или сохранить актуальный стор кракен в закладках.
интернет по адресу дома
ufa-domashnij-internet002.ru
провайдеры интернета по адресу уфа
Диплом университета России!
Без присутствия диплома очень нелегко было продвигаться по карьерной лестнице. Заказать диплом вы имеете возможность через сайт компании: vsbg.info/read-blog/1756
1win bet deposit https://www.1win1026.top .
database of accounts for sale https://buy-accounts.live
стоимость чоп в москве https://chop-ohrana.com/czeny-na-uslugi-ohrany/ .
интернет провайдеры по адресу
ufa-domashnij-internet003.ru
интернет провайдеры уфа по адресу
sell accounts https://social-accounts-marketplace.live
бк теннесси скачать на андроид http://www.mostbet10015.ru .
account catalog https://accounts-marketplace.online
Мы готовы предложить дипломы любой профессии по приятным ценам. Покупка диплома, который подтверждает обучение в университете, – это рациональное решение. Купить диплом любого ВУЗа: premium.listbb.ru/viewtopic.phpf=2&t=1350
Заказать диплом любого института. Приобретение документа о высшем образовании через проверенную и надежную компанию дарит ряд преимуществ для покупателя. Это решение помогает сэкономить как длительное время, так и значительные средства. [url=http://trgrecruitment.com/employer/eonline-diploma/]trgrecruitment.com/employer/eonline-diploma[/url]
Если вы ищете надёжную торговую платформу в даркнете, то
станет отличным решением, кракен официальный работает стабильно и предлагает широкий выбор товаров на любой вкус. Сам зеркало кракен доступен круглосуточно.
Многие выбирают кракен магазин за простой интерфейс. Через кракен зеркало тор можно быстро попасть на рабочую версию сайта, даже если основной kraken сайт временно недоступен. Для безопасного входа используйте кракен ссылка — это гарантирует стабильный доступ к площадке без сбоев.
кракен зеркало тор пользуется доверием благодаря проверенным продавцам и высокому уровню безопасности. Чтобы легко кракен зеркало тор, достаточно иметь актуальное кракен магазин — через него вы получите доступ к полному функционалу сайта.
Если вы не знаете, как попасть на кракен зайти, просто воспользуйтесь одним из доступных кракен сайт ссылка — они регулярно обновляются. Также можно использовать кракен зайти или сохранить актуальный кракен магазин в закладках.
таможенный брокер услуги таможенный брокер услуги .
Мы предлагаем выгодно купить диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями должностных лиц. Наш документ способен пройти любые проверки, даже при помощи специального оборудования. sprostov.forumex.ru/viewtopic.php?f=2&t=812
проверить провайдера по адресу
ufa-domashnij-internet003.ru
какие провайдеры интернета есть по адресу уфа
1win казино регистрация https://1win10013.ru .
Приобрести диплом можно через официальный портал компании. realtraceclasses.copiny.com/question/details/id/1084481
домашний интернет подключить омск
volgograd-domashnij-internet001.ru
провайдеры интернета в волгограде
melbet.kg https://melbet1014.ru .
Мы изготавливаем дипломы любой профессии по приятным ценам. Мы готовы предложить документы техникумов, расположенных в любом регионе РФ. Документы делаются на бумаге высшего качества. Это дает возможности делать государственные дипломы, не отличимые от оригиналов. poeskovek.flybb.ru/viewtopic.phpf=2&t=657
Приобрести диплом института!
Мы изготавливаем дипломы любой профессии по доступным тарифам— pirouette-vologda.ru
таможенный брокер москва таможенные услуги таможенный брокер москва таможенные услуги .
интернет провайдер омск
volgograd-domashnij-internet001.ru
подключить интернет в волгограде в квартире
таможенный брокер москва таможенные услуги таможенный брокер москва таможенные услуги .
Цветы пришли свежие и ароматные, как будто только что срезаны!
цветы
1win pariuri http://www.1win5068.ru .
лучший интернет провайдер омск
volgograd-domashnij-internet002.ru
домашний интернет тарифы
Приобрести диплом любого ВУЗа!
Приобрести диплом о высшем образовании. Покупка диплома через надежную фирму дарит массу достоинств для покупателя. Данное решение позволяет сэкономить как продолжительное время, так и серьезные денежные средства. kingcityrp.moibb.ru/posting.phpf=34&mode=post&sid=9b66d6fefcb3a371fd57268b71c51392
Заказать диплом института по невысокой стоимости вы можете, обращаясь к надежной специализированной компании. Заказать документ о получении высшего образования вы можете у нас в Москве. good-diplom.ru/kupit-diplom-instituta-s-provodkoj-po-vigodnoj-tsene
1win. casino. http://www.1win1017.top .
калькулятор страховки осаго калькулятор страховки осаго .
погода в сочи на 14 дней температура воды погода в сочи на 14 дней температура воды .
таможенная компания http://www.tamozhennyj-broker16.ru .
Профессиональный сервисный центр по ремонту техники в Екатеринбурге.
Мы предлагаем: Ремонт телефонов Fly недорого
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
подключить интернет в волгограде в квартире
volgograd-domashnij-internet002.ru
подключить домашний интернет омск
таможенные услуги москва таможенные услуги москва .
мелбет кг http://melbet1011.ru/ .
1win register https://www.1win5068.ru .
Где приобрести [b]диплом[/b] по актуальной специальности?
Готовый диплом с приложением отвечает стандартам Министерства образования и науки Российской Федерации, неотличим от оригинала – даже со специально предназначенным оборудованием. Диплом о среднем специальном образовании – не проблема! [url=http://alyunaniya.com/kupit-diplom-gosudarstvennogo-obrazca-216-3/]alyunaniya.com/kupit-diplom-gosudarstvennogo-obrazca-216-3[/url]
ноутбуки intel цена купить ноутбук
смартфоны 2025 года купить цена смартфона самсунг
Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов РФ. Документы изготавливаются на подлинных бланках. careerfy.pl/employer/aurus-diploms
домашний интернет омск
volgograd-domashnij-internet003.ru
провайдеры домашнего интернета омск
1win cassino http://1win1017.top .
Наши специалисты предлагает профессиональный починить айфон на дому любых брендов и моделей. Мы знаем, насколько важны для вас ваши iPhone, и обеспечиваем ремонт первоклассного уровня. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только качественные детали, что обеспечивает надежность и долговечность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются пользователи смартфонов Apple, включают проблемы с экраном, поломку батареи, неисправности программного обеспечения, неработающие разъемы и повреждения корпуса. Для устранения этих неисправностей наши квалифицированные специалисты выполняют ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе надежный и долговечный центр ремонта iphone.
Подробная информация представлена на нашем сайте: https://remont-iphone-sot.ru
Продамус промокод Продамус промокод .
1win login ug 1win1032.top .
1win code bonus http://www.1win1033.top .
страховка осаго онлайн рассчитать стоимость на машину http://www.zaria.com.ru/ .
Ремонт электронники в Москве, ремонтируем почти всю электроннику – смотрите тут
Мы готовы предложить дипломы любых профессий по приятным ценам. Мы предлагаем документы ВУЗов, расположенных в любом регионе Российской Федерации. Дипломы и аттестаты печатаются на бумаге самого высокого качества. Это позволяет делать государственные дипломы, которые невозможно отличить от оригинала. groupeudson.com/employer/archive-diploma
сочи погода на 14 дней температура и вода в море сочи погода на 14 дней температура и вода в море .
1win казино http://1win10015.ru .
таможенный брокер таможенный брокер .
получить деньги за регистрацию без вложений с выводом на карту https://www.1win10014.ru .
интернет тарифы омск
volgograd-domashnij-internet003.ru
интернет провайдер омск
провайдеры интернета воронеж
voronezh-domashnij-internet001.ru
интернет провайдер воронеж
недорогой интернет воронеж
voronezh-domashnij-internet001.ru
домашний интернет подключить воронеж
Профессиональный сервисный центр по ремонту техники в Самаре.
Мы предлагаем: Ремонт проекторов Sanyo с гарантией
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Купить диплом университета по невысокой стоимости возможно, обращаясь к надежной специализированной фирме. Приобрести документ университета можно в нашей компании. praisenpray.org/forums/showthread.phptid=125353
Здравствуйте!
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Цена зависит от выбранной специальности, года получения и университета: diplomans.com/
Hello adrenaline seekers!
Use the 1xbet promo code for registration with the bonus code 1XBRO200 today. Enter it during sign-up and claim your rewards. Bonuses apply instantly without delay. New players only need 1XBRO200. This 1xbet promo code for registration is active now.
All information is available via the link – https://files.fm/edicionesdelau/info
1xbet promo code free spins, 1xbet free promo code india, 1xbet promo code list
1xbet promo code india today, 1xbet promo code, 1xbet promo code check today
Good games!
Купить диплом академии!
Мы можем предложить дипломы любых профессий по приятным ценам. Вы приобретаете документ в надежной и проверенной компании. : scintillabd.com/poluchite-diplom-svoej-mechty-uzhe-segodnja-232
Hello!
British Encyclopaedia at your fingertips. This guide navigates how to use it effectively. Ideal for researchers, students, and knowledge-seekers. Get smarter, faster. https://vitalcoaching.com/wp-content/pgs/?spravochnik_britanskaya_enciklopediya.html
An interesting information is available via the link – https://sermonquotes.com/news/?089sovety_po_prohoghdeniyu_mafia_wars_osnovnye_zadaniya_060.html
Good luck!
печать самоклеящихся наклеек печать самоклеящихся наклеек
Наши специалисты предлагает профессиональный сервисный центр по ремонту айфона на выезде всех типов и брендов. Мы знаем, насколько необходимы вам ваши смартфоны Apple, и стремимся предоставить услуги наилучшего качества. Наши опытные мастера работают быстро и аккуратно, используя только оригинальные запчасти, что гарантирует длительную работу проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются обладатели устройств iPhone, включают проблемы с экраном, проблемы с батареей, неисправности программного обеспечения, проблемы с портами и повреждения корпуса. Для устранения этих проблем наши профессиональные техники оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе качественный и надежный ремонт iphone на выезде.
Подробная информация доступна на сайте: https://remont-iphone-sot.ru
Hello!
Learn how to create a compelling product presentation. Discover techniques for engaging your audience and showcasing value. Perfect for marketers, entrepreneurs, and sales pros. Build trust and drive conversions with visual storytelling. Make your product the star. http://kariyanichigeki.com/js/pg/prezentaciya_tovara.html
An interesting information is available via the link – https://labelsunlimited.net/pages/kak_pohudety_za_3_dnya_v_domashnih_usloviyah.html
Good luck!
домашний интернет
voronezh-domashnij-internet001.ru
интернет домашний воронеж
подключить интернет
voronezh-domashnij-internet002.ru
подключить интернет в воронеже в квартире
стоимость охраны в час chop-ohrana.com/czeny-na-uslugi-ohrany .
Лучшие психологи онлайн. Анонимная помощь психолога. Психотерапевт сайт.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Индивидуальное консультирование.
Психолог владеет множеством приемов и техник, которые помогут разобраться в себе.
Поможет поставить цель терапии и приведет к результату.
Голосовой анонимный чат. Психологонлайн. Чат переписка.
Психолог, Сайт психологов.
Поможет поставить цель терапии и приведет к результату.
Психологическая помощь онлайн.
Получить поддержку по широкому кругу вопросов.
Чат поддержки с психологом. Виртуальный чат. Отзыв на психолога.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
Услуги психолога · — Консультация психолога.
Индивидуальное консультирование.
Решим вместе вашу проблему.
Онлайн общение с психологом. Где найти психолога. Психолог по личным отношениям.
Психолог владеет множеством приемов и техник, которые помогут разобраться в себе.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Онлайн сессия от 73999 руб.
Частые разногласия с самыми близкими.
Сайты психологов. Сайт с психологами. Психолог в телеграм.
Поможет поставить цель терапии и приведет к результату.
Частые разногласия с самыми близкими.
Психологическая помощь онлайн.
Hello adrenaline seekers!
Use the exclusive 1xbet promo code Bangladesh with 1XBRO200 and unlock welcome offers. Bangladeshi users can join instantly. Just use 1XBRO200 on the site. Claim bets, games, and live casino rewards. Try 1xbet promo code Bangladesh today.
All information is available via the link – https://profit.ly/user/fluentcpp/blog/1xbet-promo-code-1xbum200–welcome-bonus-130
1xbet promo code no deposit, 1xbet enter promo code, 1xbet promo code free spins
1xbet promo code list pakistan, 1xbet promo code check, 1xbet promo code deposit
Good jackpots!
Профессиональный сервисный центр по ремонту техники.
Мы предлагаем: Ремонт бытовой техники срочно Великий Новгород
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
вывод денег с 1win https://1win10015.ru .
тарифы интернет и телевидение воронеж
voronezh-domashnij-internet002.ru
подключить интернет
Привет всем!
ГРАЖДАНСТВО ИСПАНИИ
Испания является привлекательным вариантом для иммиграции, особенно для тех, кто хочет жить в комфортном климате и иметь доступ к преимуществам Европейского Союза. Страна славится своими курортами, разнообразными природными ландшафтами и историческим наследием. В этой статье мы рассмотрим, как гражданину России можно получить испанское гражданство.
Преимущества гражданства Испании
Получение гражданства Испании автоматически дает гражданство ЕС, что позволяет без визы посещать более 160 стран мира.
Возможность бесплатного обучения в государственных университетах и доступ к качественному медицинскому обслуживанию по доступной страховке.
Право на получение государственных льгот, предоставляемых испанским правительством своим гражданам.
ГРАЖДАНСТВО ИСПАНИИ
Если вашей главной целью при получении испанского гражданства является свобода перемещения по миру, но вы не хотите отказываться от своего родного гражданства, стоит рассмотреть другие страны, где разрешено иметь два гражданства, например, Чили или Португалию.
Процесс получения гражданства Испании
Оформление гражданства Испании регулируется стандартной процедурой, которая достаточно гибка и доступна для большинства людей, имеющих соответствующие основания. Главное требование — проживание на территории Испании в течение 10 лет.
После 8 апреля 2024 года для получения «золотой визы» доступны следующие варианты инвестиций:
Покупка государственных облигаций на сумму от 2 миллионов евро.
Инвестиции в местные компании с минимальной суммой от 1 миллиона евро.
Открытие собственного бизнеса на основе стартап-проекта.
ПАСПОРТ ИСПАНИИ
Наша ассоциация иммиграционных адвокатов имеет большой опыт и профессиональный подход в подготовке документов для получения испанского гражданства.
Вся информация на сайте – п»їhttps://expert-immigration.com/blog/grazhdanstvo-ispanii
Удачи!
Ремонт электронники в Москве, ремонтируем почти всю электроннику – подробнее на сайте
недорогой интернет воронеж
voronezh-domashnij-internet003.ru
недорогой интернет воронеж
бк 1win официальный http://1win10014.ru/ .
пластиковые окна пвх http://okna177.ru/ .
домашний интернет в воронеже
voronezh-domashnij-internet003.ru
домашний интернет подключить воронеж
провайдеры интернета челябинск
chelyabinsk-domashnij-internet001.ru
подключить интернет в квартиру челябинск
register with 1win website https://www.1win1027.top .
лучший интернет провайдер челябинск
chelyabinsk-domashnij-internet001.ru
домашний интернет тарифы челябинск
account selling platform https://accounts-marketplace-best.pro/
Заказать диплом о высшем образовании!
Мы предлагаемвыгодно и быстро приобрести диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями официальных лиц. Наш документ пройдет любые проверки, даже при использовании специального оборудования. Достигайте своих целей быстро и просто с нашими дипломами- businessoutlook.copiny.com/question/details/id/1087610
Мы можем предложить дипломы любых профессий по приятным ценам.– boyara-analia.flybb.ru/viewtopic.phpf=7&t=1216
страховка осаго рассчитать страховка осаго рассчитать .
На KRAKEN легко найти нужный товар
узи сканер купить цена kupit-uzi-apparat14.ru .
аппарат узи купить цена в россии аппарат узи купить цена в россии .
Купить диплом ВУЗа!
Мы готовы предложить документы университетов, которые расположены в любом регионе России.
diplom-onlinex.com/kupit-diplom-s-reestrom-v-rossii-bistro-i-nadezhno/
узи сканер купить москва https://kupit-uzi-apparat11.ru .
домашний интернет
chelyabinsk-domashnij-internet001.ru
домашний интернет в челябинске
узд аппарат http://kupit-uzi-apparat13.ru .
register with 1win website http://1win1027.top/ .
нажмите здесь tripscan22 id
Приобрести диплом под заказ в столице вы сможете используя сайт компании. peso4nica.getbb.ru/index.php
интернет провайдер челябинск
chelyabinsk-domashnij-internet002.ru
недорогой интернет челябинск
Диплом университета Российской Федерации!
Без ВУЗа трудно было продвинуться по карьерной лестнице. Купить диплом под заказ возможно через сайт компании: nubiantalk.site/read-blog/20817_kupit-diplom-v-novosibirske.html
мелбет вход в личный https://melbet1011.ru .
Приобрести диплом о высшем образовании. Заказ документа о высшем образовании через проверенную и надежную фирму дарит ряд достоинств для покупателя. Данное решение помогает сэкономить как личное время, так и значительные денежные средства. nationalcarerecruitment.com.au/employer/eonline-diploma
На КРАКЕН всегда есть всё, что нужно
Наша компания предлагает быстро и выгодно заказать диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Документ способен пройти любые проверки, даже с использованием профессиональных приборов. pmps.com.my/pokupka-diploma-s-zaneseniem-v-reestr-161
1win casino ug 1win casino ug .
visit this site right here paycor employee login
business loan in Nigeria
Get a quick loan in Nigeria in 2 Minutes. Migo helps small businesses extend low-interest, no collateral micro loans, banking services and credit purchase to their customers. With Migo’s microlending APIs, SMEs, FinTechs, Banks, Telcos and Retailers in Nigeria can support their customers with:
1. Access to required loan amounts at the point of need
2. Low interest rates on quick loans and credit products
3. Flexible and easy repayment options
4. Personalized financial services and products across website, mobile app, USSD, SMS, ATM, POS, Whatsapp, Facebook, Twitter and Instagram
But deep down, a flicker of integrity refused to be extinguished. Mateo knew that his discovery held significance beyond its monetary value—it was a piece of history, a glimpse into a world that had long been lost.
야동코리아
провайдеры домашнего интернета челябинск
chelyabinsk-domashnij-internet002.ru
подключить домашний интернет в челябинске
Vibracion de motor
¡Vendemos dispositivos para equilibrado!
Somos productores, manufacturando en tres ubicaciones diferentes: España, Argentina y Portugal.
Nuestros productos son de altísima calidad, y como no somos vendedores, sino productores directos, nuestro coste es más bajo que el de otras marcas.
Realizamos envíos a cualquier parte del planeta, revise la descripción de nuestros equipos de equilibrio en nuestro sitio web.
El equipo de equilibrio es portátil, liviano, lo que permite balancear cualquier rotor en todo lugar.
Приобрести документ института вы имеете возможность у нас в Москве. Мы оказываем услуги по продаже документов об окончании любых университетов Российской Федерации. Вы сможете получить необходимый диплом по любой специальности, любого года выпуска, в том числе документы Советского Союза. Гарантируем, что в случае проверки документа работодателями, каких-либо подозрений не появится. diplomc-v-ufe.ru/kupit-diplom-yurista-s-zaneseniem-v-reestr-bistro-6/
продать аккаунт https://akkaunty-na-prodazhu.pro
домашний интернет подключить челябинск
chelyabinsk-domashnij-internet003.ru
домашний интернет подключить челябинск
площадка для продажи аккаунтов https://rynok-akkauntov.top
Букет был настолько хорош, что его даже жалко было разбирать!
доставка цветов в томске
провайдеры интернета челябинск
chelyabinsk-domashnij-internet003.ru
провайдеры интернета челябинск
промокод prodamus http://promokod-prod.ru .
Заказать диплом ВУЗа!
Наша компания предлагаетвыгодно заказать диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями официальных лиц. Документ пройдет лубую проверку, даже с применением специального оборудования. Достигайте своих целей максимально быстро с нашей компанией- skazka.g-talk.ru/viewtopic.phpf=1&t=4564
you can try this out [url=https://thecoi-base.com]coinbase login[/url]
KRAKEN — моя первая платформа для покупок
lucky jet 1win download http://www.1win5069.ru .
гибкая керамика для внутренней отделки Гибкая керамика – это материал, который позволяет воплотить в жизнь самые смелые дизайнерские идеи. Ее гибкость позволяет создавать сложные криволинейные поверхности, а разнообразие текстур имитирует натуральные материалы с невероятной точностью. Благодаря легкости монтажа, вы сможете сэкономить на услугах специалистов и самостоятельно преобразить свой дом или квартиру.
площадка для продажи аккаунтов https://kupit-akkaunt.xyz/
медитация женская энергия Бывало ли у вас ощущение, что жизненная энергия словно замерла, а стремления так и не становятся реальностью? Пора активизировать Свадхистану – центр вашей женской энергии, чувственности и творческого потенциала. Эта медитативная практика поможет вам запустить поток энергии, способствующий воплощению ваших самых заветных грез. Что вы получите: Медитативное путешествие вглубь Свадхистана чакры. Избавление от сдерживающих факторов и препятствий. Улучшение состояния женского здоровья.
домашний интернет подключить челябинск
chelyabinsk-domashnij-internet003.ru
подключение интернета челябинск
Dual Access Bazaar Drugs Marketplace: A New Darknet Platform with Dual Access Bazaar Drugs Marketplace is a new darknet marketplace rapidly gaining popularity among users interested in purchasing pharmaceuticals. Trading is conducted via the Tor Network, ensuring a high level of privacy and data protection. However, what sets this platform apart is its dual access: it is available both through an onion domain and a standard clearnet website, making it more convenient and visible compared to competitors. The marketplace offers a wide range of pharmaceuticals, including amphetamines, ketamine, cannabis, as well as prescription drugs such as alprazolam and diazepam. This variety appeals to both beginners and experienced buyers. All transactions on the platform are carried out using cryptocurrency payments, ensuring anonymity and security. In summary, Bazaar represents a modern darknet marketplace that combines convenience, a broad product selection, and a high level of privacy, making it a notable player in the darknet economy.
интернет провайдер екатеринбург
ekaterinburg-domashnij-internet001.ru
узнать интернет по адресу
Ремонт электронники в Москве, ремонтируем почти всю электроннику – подробнее на сайте
Приобрести диплом о высшем образовании !
Покупка диплома любого университета России в нашей компании является надежным процессом, поскольку документ будет заноситься в реестр. Выгодно заказать диплом о высшем образовании diplomskiy.com/diplom-vuza-s-zaneseniem-v-reestr-7
Заказать диплом университета по невысокой цене вы можете, обращаясь к надежной специализированной фирме. Приобрести документ о получении высшего образования можно в нашей компании в столице. diplomservis.ru/diplom-s-vneseniem-v-reestr-dlya-uspeshnoj-kareri-4
гибкая керамика купить Выбирая гибкую керамику, вы выбираете инновационный материал, который преобразит ваш дом и прослужит вам долгие годы. Ее универсальность, долговечность и эстетическая привлекательность делают ее идеальным выбором для тех, кто ценит качество и современный дизайн. Phomi и Divu – это лидеры рынка, предлагающие широкий выбор гибкой керамики на любой вкус и бюджет.
Сайт Полтавы https://u-misti.poltava.ua новости сегодня. События в Полтаве и Полтавской области
1win betting 1win1028.top .
домашний интернет екатеринбург
ekaterinburg-domashnij-internet001.ru
интернет тарифы екатеринбург
buy levitra china
cheap viagra uk next day
viagra for sale over the counter
viagra generic buy
viagra super active cheap
1win casino скачать на андроид бесплатно http://1win5070.ru .
Приобрести диплом о высшем образовании. Приобретение подходящего диплома через качественную и надежную фирму дарит ряд преимуществ для покупателя. Такое решение дает возможность сберечь как личное время, так и существенные денежные средства. magazin.orgsoft.ru/communication/forum/index.php?PAGE_NAME=profile_view&UID=221361
Наша компания предлагает выгодно и быстро заказать диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями должностных лиц. Наш диплом пройдет лубую проверку, даже при использовании специального оборудования. twixxor.com/read-blog/18268_kupit-diplom-s-reestrom-cena.html
Looking for live nude fun, cam model guides, or ways to make money from the webcam industry?
?? Visit **littlecocktease com** and get access to:
?? Free cam site hacks
?? How to watch more and spend less
?? Best webcam affiliate programs
?? Tips on becoming a webcam model
?? Psychology of why cams are so addictive
Real advice from real users. Perfect if you’re watching, earning, or thinking about joining the adult cam world.
Check full articles here:
?? Don’t just watch — learn how to get the most out of every show.
Best Free Cam Sites Tips
Заказать диплом о высшем образовании!
Мы готовы предложить документы институтов, расположенных на территории всей РФ.
diplomidlarf.ru/kupit-diplom-o-visshem-obrazovanii-bistro-i-prosto/
Добрый день!
ВНЖ ИСПАНИИ
Испания — это популярное направление для тех, кто ищет возможности для жизни в стране с теплым климатом и высокими стандартами жизни. Испания привлекает многих благодаря своим курортам, природным красотам и культурному наследию. В этой статье мы подробно расскажем о том, как можно получить вид на жительство в Испании для граждан России.
Преимущества ВНЖ Испании
Вид на жительство в Испании предоставляет право на проживание и работу в стране, а также доступ к гражданству Европейского Союза по мере выполнения соответствующих условий.
Возможность пользоваться государственным медицинским обслуживанием и правом на образование в испанских учебных заведениях.
Право на участие в социальной системе Испании, включая пенсионное обеспечение и другие льготы для резидентов.
ВНЖ ИСПАНИИ
Если ваша цель — жить в Испании длительное время, но вы не хотите отказываться от гражданства своей страны, вид на жительство станет для вас отличным выбором. В отличие от гражданства, ВНЖ не требует отказа от родного паспорта и дает возможность жить и работать в Испании.
Как получить ВНЖ Испании
Для того чтобы получить вид на жительство в Испании, необходимо соблюдать определенные требования, которые могут варьироваться в зависимости от типа визы. Основные условия включают наличие финансовой стабильности, отсутствие криминальных записей и документальное подтверждение цели вашего пребывания (работа, учеба, воссоединение семьи и т.д.).
После 8 апреля 2024 года доступны следующие пути получения ВНЖ через инвестиции:
Инвестиции в недвижимость на сумму от 500 000 евро.
Покупка государственных облигаций на сумму от 2 миллионов евро.
Открытие бизнеса или инвестирование в местные компании с суммой от 1 миллиона евро.
ПАСПОРТ ИСПАНИИ
Наша ассоциация иммиграционных адвокатов оказывает профессиональную помощь в подготовке и подаче документов для получения вида на жительство в Испании, гарантируя высокий уровень качества и надежности на всех этапах.
Вся информация на сайте – п»їhttps://expert-immigration.com/blog/grazhdanstvo-ispanii
Удачи!
ios 1win https://www.1win5069.ru .
обсуждение 2 5 лайфхаков, которые реально работают! Как сделать это за 1 минуту? Больше никогда не будешь делать это неправильно! Секрет, который сэкономит тебе кучу времени! Какой вариант выберешь ты? А ты знал об этом? 90% людей – нет! Срочно! Это должен увидеть каждый! Твоё мнение? Пиши в комментарии! Ты не поверишь, что случилось дальше… Это изменит твое мнение навсегда! Почему все об этом молчат? Я сделал это… и вот что вышло!
1win casino bonus https://1win5066.ru .
классы аппаратов узи kupit-uzi-apparat12.ru .
интернет тарифы екатеринбург
ekaterinburg-domashnij-internet001.ru
домашний интернет в екатеринбурге
Форум психологов. Анонимний чат. Психотерапевт онлайн чат.
Решим вместе вашу проблему.
Сколько встреч нужно?
Личные или онлайн-встречи с высококвалифицированными специалистами.
Мы предлагаем документы об окончании любых университетов Российской Федерации. Документы производят на подлинных бланках государственного образца. peticie.com/484542
Где приобрести диплом специалиста?
Готовый диплом со всеми печатями и подписями полностью отвечает стандартам Министерства образования и науки России, неотличим от оригинала. Диплом о среднем специальном образовании – запросто! easyqode.com.br/kupit-diplom-gosudarstvennogo-obrazca-134-2
Не могу расслабиться. Получить онлайн консультацию психолога чате. Анонимный видеочат.
Психолог владеет множеством приемов и техник, которые помогут разобраться в себе.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Поможет поставить цель терапии и приведет к результату.
купить сестринское дело диплом http://www.sx-dipl.online .
Анонимный чат онлайн. Чат с психологом. Психологические вопросы.
Частые разногласия с самыми близкими.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
Мы обязательно поможем преодолеть эмоциональный кризис, избавиться от тревожности и апатии, справиться со стрессом и депрессией, связанными с неуверенностью и многим другим.
Banda Casino радует широким выбором игр и бонусов. Выплаты на Banda Casino приходят быстро и без задержек. Легко нашел зеркало Banda Casino и вошел без проблем. Плюс Banda Casino — бонусы при регистрации и пополнении. Выплаты в Banda Casino приходят в срок — рекомендую. Понравились промокоды на фриспины в Banda Casino. Играю на Banda Casino — отличные бонусы и выплаты. Выбираю Banda Casino за честные выплаты и приятные бонусы. Промокод на фриспины в Banda Casino позволил увеличить баланс новые акции banda casino.
Профессиональный сервисный центр по ремонту техники.
Мы предлагаем: Выездной ремонт бытовой техники стоимость Норильск
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Психологи онлайн анонимно. Анон чат вк. Анонимный видео чат.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Поможет поставить цель терапии и приведет к результату.
Личные сайты психологов. Консультация психолога цена. Онлайн чат с психологом.
Записаться на консультацию.
Мы обязательно поможем преодолеть эмоциональный кризис, избавиться от тревожности и апатии, справиться со стрессом и депрессией, связанными с неуверенностью и многим другим.
23317 проверенных отзывов.
Индивидуальное консультирование.
mel bet сайт https://www.melbet1017.ru .
Добрый день!
ГРАЖДАНСТВО ИСПАНИИ
Испания является привлекательным вариантом для иммиграции, особенно для тех, кто хочет жить в комфортном климате и иметь доступ к преимуществам Европейского Союза. Страна славится своими курортами, разнообразными природными ландшафтами и историческим наследием. В этой статье мы рассмотрим, как гражданину России можно получить испанское гражданство.
Преимущества гражданства Испании
Получение гражданства Испании автоматически дает гражданство ЕС, что позволяет без визы посещать более 160 стран мира.
Возможность бесплатного обучения в государственных университетах и доступ к качественному медицинскому обслуживанию по доступной страховке.
Право на получение государственных льгот, предоставляемых испанским правительством своим гражданам.
ГРАЖДАНСТВО ИСПАНИИ
Если вашей главной целью при получении испанского гражданства является свобода перемещения по миру, но вы не хотите отказываться от своего родного гражданства, стоит рассмотреть другие страны, где разрешено иметь два гражданства, например, Чили или Португалию.
Процесс получения гражданства Испании
Оформление гражданства Испании регулируется стандартной процедурой, которая достаточно гибка и доступна для большинства людей, имеющих соответствующие основания. Главное требование — проживание на территории Испании в течение 10 лет.
После 8 апреля 2024 года для получения «золотой визы» доступны следующие варианты инвестиций:
Покупка государственных облигаций на сумму от 2 миллионов евро.
Инвестиции в местные компании с минимальной суммой от 1 миллиона евро.
Открытие собственного бизнеса на основе стартап-проекта.
ПАСПОРТ ИСПАНИИ
Наша ассоциация иммиграционных адвокатов имеет большой опыт и профессиональный подход в подготовке документов для получения испанского гражданства.
Вся информация на сайте – п»їhttps://expert-immigration.com/blog/grazhdanstvo-ispanii
Удачи!
домашний интернет подключить екатеринбург
ekaterinburg-domashnij-internet002.ru
тарифы интернет и телевидение екатеринбург
1win casino online http://www.1win1018.top .
cazino md http://1win5071.ru/ .
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
Ремонт электронники в Москве, ремонтируем почти всю электроннику – смотрите тут
Быстро приобрести диплом о высшем образовании!
Быстро и просто заказать диплом любого университета. Заказ документа о высшем образовании через надежную фирму дарит ряд достоинств для покупателя. Это решение помогает сберечь как личное время, так и серьезные финансовые средства. tweecampus.com/read-blog/177328_купить-диплом-медработника.html
аппараты узи цена [url=https://kupit-uzi-apparat12.ru/]аппараты узи цена[/url] .
официальные криптовалюты официальные криптовалюты .
подключить интернет по адресу
ekaterinburg-domashnij-internet002.ru
подключить интернет тарифы екатеринбург
https://komu4egrill.ru
Купить диплом университета по доступной стоимости вы можете, обращаясь к проверенной специализированной компании. Приобрести документ о получении высшего образования можно в нашей компании. diplomt-v-chelyabinske.ru/kupit-diplom-o-visshem-obrazovanii-s-zaneseniem-v-reestr-17
1win mx https://1win1018.top .
Купить диплом института по доступной стоимости возможно, обратившись к надежной специализированной фирме. Приобрести документ о получении высшего образования можно в нашем сервисе. lossantosbattalion17.listbb.ru/viewtopic.phpf=3&t=1673
Новини Житомир https://u-misti.zhitomir.ua события и новости Житомира и области.
Fast and reliable roadside assistance! The team was professional and friendly. Will definitely call them again if needed.
https://dzen.ru/kitehurghada
домашний интернет екатеринбург
ekaterinburg-domashnij-internet003.ru
какие провайдеры интернета есть по адресу екатеринбург
мелбет ком регистрация мелбет ком регистрация .
прибор узи https://kupit-uzi-apparat11.ru/ .
рассчитать автостраховку https://niievmash.ru/ .
https://svar-met.ru
1swin https://1win5070.ru .
оборудование для узи оборудование для узи .
Приобрести диплом о высшем образовании!
Мы предлагаем дипломы психологов, юристов, экономистов и других профессий по приятным тарифам. Вы заказываете диплом через надежную и проверенную фирму. : radiocoop.co/2025/04/27/poluchite-diplom-svoej-mechty-uzhe-segodnja-11
Ремонт электронники в Москве, ремонтируем почти всю электроннику – подробнее
домашний интернет екатеринбург
ekaterinburg-domashnij-internet003.ru
провайдер по адресу
Психолог онлайн 24. Ответ психолог. Сайт психологии отношений.
Онлайн сессия от 89667 руб.
Получить поддержку по широкому кругу вопросов.
Психолог Москва. Психолог СПБ. Психолог онлайн.
Психологи онлайн анонимно. Анонимный чат онлайн. Психологическая поддержка онлайн чат.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Записаться на консультацию.
Психолог Москва. Психолог СПБ. Психолог онлайн.
компьютерный спорт Киберспорт, компьютерный спорт – это форма соревновательной деятельности, где участники соревнуются, используя видеоигры. Он охватывает широкий спектр жанров, от стратегий в реальном времени (RTS) и многопользовательских онлайн-арен (MOBA) до шутеров от первого лица (FPS) и спортивных симуляторов.
рабочее зеркало мелбет https://melbet1015.ru/ .
Психотерапевт онлайн чат. Дипломированный психолог с опытом работы и отзывами клиентов. Анонимная консультация с психологом.
Поможет поставить цель терапии и приведет к результату.
Индивидуальное консультирование.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
какие провайдеры интернета есть по адресу казань
kazan-domashnij-internet001.ru
домашний интернет подключить казань
Поговорить по душам онлайн. Чат переписка. Найти психолога онлайн.
Психологическое консультирование.
Психологическая помощь онлайн.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
Психолог отзывы. Психолог онлайн анонимно. Консультации психолога.
Психологическое консультирование.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
1він додаток http://1win5066.ru .
купить аппарат узи цены купить аппарат узи цены .
проверить сайт vodka bet зеркало водка бет
рейтинг шуруповертов алиэкспресс
аппарат узи цена в россии аппарат узи цена в россии .
мелбет рабочее зеркало https://melbet1011.ru/ .
1win sports betting http://1win1028.top/ .
TEMANSLOT
https://dzen.ru/kitehurghada
Отдых в Архипо-Осиповке, читайте про экскурсии, морские прогулки, джиппинг в горы, пешие прогулки – подробнее на сайте
кликните сюда водкабет казино
посчитать осаго онлайн посчитать осаго онлайн .
маркетплейс аккаунтов https://akkaunt-magazin.online
одежда спб В мире женской моды 2025 царит эклектика и смелость. Минимализм, оставаясь в тренде, приобретает новые грани – дорогие ткани, лаконичный крой и акцент на детали. Больше не нужно кричащих брендов, чтобы выглядеть роскошно. Стиль без бренда – это искусство сочетать базовый гардероб с уникальными акцентами, создавая неповторимый образ.
узнать провайдера по адресу казань
kazan-domashnij-internet001.ru
интернет провайдеры казань по адресу
маркетплейс аккаунтов https://akkaunty-market.live
аппараты узи цена аппараты узи цена .
Мы изготавливаем дипломы любой профессии по приятным тарифам. Заказ диплома, который подтверждает окончание университета, – это рациональное решение. Приобрести диплом о высшем образовании: jobs.monrespro.cd/employer/diplomirovans
тарифы интернет и телевидение казань
kazan-domashnij-internet002.ru
проверить провайдера по адресу
купить аккаунт https://kupit-akkaunty-market.xyz
узи сканер купить узи сканер купить .
1win приложение ios скачать https://1win5072.ru/ .
одеждадля женщин В мире женской моды 2025 царит эклектика и смелость. Минимализм, оставаясь в тренде, приобретает новые грани – дорогие ткани, лаконичный крой и акцент на детали. Больше не нужно кричащих брендов, чтобы выглядеть роскошно. Стиль без бренда – это искусство сочетать базовый гардероб с уникальными акцентами, создавая неповторимый образ.
провайдер по адресу
kazan-domashnij-internet002.ru
какие провайдеры на адресе в казани
стоимость чоп стоимость чоп .
Заказать диплом об образовании!
Приобретение документа о высшем образовании через качественную и надежную компанию дарит ряд преимуществ. Купить диплом о высшем образовании у проверенной организации: doks-v-gorode-perm-59.ru
шкаф в паркинг с рольставнями екатеринбург шкаф в паркинг с рольставнями екатеринбург .
Огромное спасибо за оперативность! Заказ сделала утром, а к вечеру цветы уже были у мамы.
доставка цветов томск
интернет домашний казань
kazan-domashnij-internet003.ru
провайдеры в казани по адресу проверить
online casino bonus 500% https://1win5072.ru/ .
Купить диплом об образовании. Изготовление документа занимает немного времени, а стоимость – невысокая. В итоге вы сможете сберечь деньги и время и получить хорошую работу вашей мечты. Приобрести диплом на заказ в Москве возможно используя сайт компании. – personal-fouryou.de/employer/russiany-diplomans
мелбет вход [url=www.melbet1017.ru]www.melbet1017.ru[/url] .
проверить провайдеров по адресу казань
kazan-domashnij-internet003.ru
подключить проводной интернет казань
узнать интернет по адресу
krasnoyarsk-domashnij-internet001.ru
проверить провайдера по адресу
Новости Одесса https://u-misti.odesa.ua городской портал Одессы, события в городе и области
Мы предлагаем дипломы психологов, юристов, экономистов и прочих профессий по выгодным тарифам. Покупка диплома, подтверждающего окончание института, – это выгодное решение. Купить диплом любого ВУЗа: drovokol.ru/forum/user/14307
1win скачать android http://1win5071.ru .
KRAKEN предлагает широкий выбор и точные описания товаров.
https://telegra.ph/Kak-rabotaet-kraken-vojti1-05-12
интернет провайдеры по адресу
krasnoyarsk-domashnij-internet001.ru
провайдеры по адресу дома
Приобретение документа о высшем образовании через качественную и надежную компанию дарит немало достоинств для покупателя. Это решение помогает сберечь время и существенные финансовые средства. Впрочем, на этом выгода не ограничивается, плюсов значительно больше.Мы изготавливаем дипломы любой профессии. Дипломы изготавливаются на подлинных бланках государственного образца. Доступная стоимость по сравнению с большими издержками на обучение и проживание в другом городе. Приобретение диплома университета является выгодным шагом.
Быстро купить диплом о высшем образовании: diplomt-v-chelyabinske.ru/kupit-diplom-s-zaneseniem-4/
провайдеры интернета по адресу красноярск
krasnoyarsk-domashnij-internet002.ru
провайдеры по адресу дома
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
blacksprut вход http://forum-family.ru .
даркнет солярис http://mega-onion-zerkalo.com/ .
hydra ссылка https://www.vibiraiv3d.ru .
ссылка на омг официальный сайт oharamania.ru .
kinoго http://kinogogo.pro/ .
провайдеры в красноярске по адресу проверить
krasnoyarsk-domashnij-internet002.ru
провайдер по адресу красноярск
Мы изготавливаем дипломы любой профессии по приятным ценам.– owen.ru/forum/member.phpu=106526
Управляйте жалюзи дистанционно — современно и просто
жалюзи с пультом жалюзи с пультом . Прокарниз
Ремонт электронники в Москве, ремонтируем почти всю электроннику – подробнее
1він додаток 1win5075.ru .
KRAKEN — маркет, где тебе всегда рады.
https://telegra.ph/Kak-vojti-v-kraken-cherez-zerkalo4-05-12
download lucky jet http://1win5074.ru .
узнать провайдера по адресу красноярск
krasnoyarsk-domashnij-internet002.ru
интернет провайдеры по адресу красноярск
сервисный центр самсунг remont-tehniki-samsung-moskva.ru
интернет по адресу дома
krasnoyarsk-domashnij-internet003.ru
подключение интернета по адресу
ГГУ имени Ф.Скорины https://www.gsu.by/ крупный учебный и научно-исследовательский центр Республики Беларусь. Высшее образование в сфере гуманитарных и естественных наук на 12 факультетах по 35 специальностям первой ступени образования и 22 специальностям второй, 69 специализациям.
Новини Львів https://u-misti.lviv.ua останні події та новини.
Francisk Skorina https://www.gsu.by Gomel State University. One of the leading academic and scientific-research centers of the Belarus. There are 12 Faculties at the University, 2 scientific and research institutes. Higher education in 35 specialities of the 1st degree of education and 22 specialities.
подключение интернета по адресу
krasnoyarsk-domashnij-internet003.ru
провайдеры по адресу дома
Личный психолог. Сайт психологии отношений. Найти психолога.
Поможет поставить цель терапии и приведет к результату.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Психолог помогающий искать решения в непростых психологических ситуациях. Chat with Psychologist. Психолог онлайн 24.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Анонимный прием.
Сколько встреч нужно?
Чат общение. Психолог онлайн чат. Онлайн разговор с психологом.
Консультация в кризисных состояниях.
Онлайн сессия от 71319 руб.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Психолог онлайн цена. Чат анон. Анонимный чат с психологом телеграм.
Услуги психолога · — Консультация психолога.
Анонимный прием.
Психолог Москва. Психолог СПБ. Психолог онлайн.
Частые разногласия с самыми близкими.
Психолог спб отзывы. Психотерапевт онлайн отзывы. Онлайн чат с психологом без регистрации.
Сколько встреч нужно?
Получить поддержку по широкому кругу вопросов.
Психологическая помощь онлайн.
Решим вместе вашу проблему.
сервисные центры самсунг в москве ремонт самсунг
Сайт KRAKEN обеспечивает высокий уровень анонимности.
https://telegra.ph/Kraken-kak-platforma-prestupnoj-torgovli2-05-12
провайдер по адресу
krasnoyarsk-domashnij-internet003.ru
интернет провайдеры в красноярске по адресу дома
Мы предлагаем дипломы любой профессии по приятным ценам. Мы готовы предложить документы ВУЗов, расположенных на территории всей РФ. Документы печатаются на “правильной” бумаге самого высокого качества. Это дает возможность делать настоящие дипломы, не отличимые от оригинала. mamuli.club/forum/topic/33218
Сайт KRAKEN обеспечивает высокий уровень анонимности.
https://telegra.ph/Rabochie-zerkala-kraken-zerkala3-05-12
Заказать диплом ВУЗа!
Мы готовы предложить документы любых учебных заведений, расположенных в любом регионе России.
damdiplomisa.com/kupit-diplom-s-zaneseniem-bistro-i-nadezhno/
узнать интернет по адресу
krasnodar-domashnij-internet001.ru
подключить интернет по адресу
Умные жалюзи с пультом — современное решение для вашего окна
жалюзи с пультом жалюзи с пультом . прокарниз
Купить диплом под заказ возможно через официальный портал компании. farmwoo.com/read-blog/29907_kupit-podlinnyj-diplom-s-zaneseniem-v-reestr.html
индивидуальные экскурсии на Ладожские шхеры Ладожское озеро – это не просто водная гладь, это живая история, сокрытая в скалистых берегах, таинственных островах и бескрайних просторах. Мы предлагаем вам уникальную возможность прикоснуться к этой истории, совершив увлекательные экскурсии на катере по Ладоге.
саженцы вишни с доставкой груши с гарантией
саженцы для холодных зим орехи для вашего участка
KRAKEN — один из немногих даркнет-маркетов с надёжной репутацией.
https://telegra.ph/Kak-ispolzovat-kraken-ssylka-cherez-brauzer-TOR3-05-12
1win bonus casino how to use https://1win1029.top/ .
проверить провайдеров по адресу краснодар
krasnodar-domashnij-internet001.ru
провайдер по адресу
1win футбол 1win5075.ru .
1win juegos http://1win1019.top .
провайдеры по адресу краснодар
krasnodar-domashnij-internet002.ru
узнать провайдера по адресу краснодар
продажа аккаунтов https://akkaunty-optom.live
melbet – paris sportif melbet1019.ru .
skrill casino site-uri 1win5074.ru .
маркетплейс аккаунтов https://online-akkaunty-magazin.xyz/
Айдроп Раздача криптовалюты (Airdrop), добыча цифровых монет (майнинг), крипто-монетные эйрдропы, крипто-эйрдропы, майнинг криптовалют, бесплатные эйрдропы криптовалют, эйрдропы в криптовалюте, эйрдропы крипто валюты. Бесплатное распространение токенов (Airdrop), генерация криптоактивов (майнинг), раздачи криптомонет, цифровые эйрдропы, добыча крипты, бесплатная раздача криптоактивов, эйрдропы в мире криптовалют, бесплатная крипта посредством эйрдропов.
окна пвх окна пвх .
Решил поделиться опытом применения гибкой керамики Dream Decor, которая оказалась отличным решением как для внешней, так и для внутренней отделки дома. Изначально рассматривали Phomi и Divu, но после детального сравнения остановились на Dream Decor и не прогадали. Dream Decor предлагает гибкую керамику, сочетающую в себе долговечность, эластичность и привлекательный внешний вид. Благодаря своей гибкости, материал легко монтируется даже на нестандартные поверхности. Использовали для облицовки фасада и отделки прихожей – результат впечатляет. Керамика не боится влаги, сохраняет цвет и выглядит как натуральный камень или кирпич, в зависимости от коллекции. Важно отметить, что ценовая политика Dream Decor более выгодна по сравнению с Divu и Phomi. При этом качество остается на высоком уровне и даже превосходит аналоги в некоторых аспектах. Установка прошла без проблем, материал легко режется и надежно крепится к поверхности. Если вы ищете гибкую керамику, советую обратить внимание на Dream Decor – отличная альтернатива Фоми. Это оптимальный выбор для тех, кто ценит стиль, практичность и разумную стоимость. гибкая керамика для фасадов цена
Спасибо KRAKEN за стабильную работу и понятный интерфейс.
https://telegra.ph/Bezopasnyj-vhod-v-kraken-rabochee-zerkalo4-05-12
какие провайдеры на адресе в краснодаре
krasnodar-domashnij-internet002.ru
интернет провайдеры по адресу краснодар
биржа аккаунтов https://akkaunty-dlya-prodazhi.pro/
At in front, I was skeptical thither cbd gummies for sleep with melatonin, but now I husband them in my nightstand. They’ve befit portion of my evening boring—single gummy, a cup of tea, and I’m good to go. I don’t judge intoxication or weird, just placidness and clever to be made down. The flavor’s indeed really honourable too, which surprised me. No unnatural aftertaste like some others I’ve tried. If your brain runs a mile a two shakes of a lamb’s tail log at cimmerian dark or you’re fair-minded upsetting to relax after in the works, these are plainly worth checking out.
аттестат за 9 класс купить https://diploma-dok.ru/ .
melbet online https://melbet1018.ru/ .
Цветы — это всегда радость, а с вашим сервисом — ещё и удобно!
гипсофилы цена букета
Автоматика Somfy — инновационные решения для вашего дома
Автоматика Somfy Автоматика Somfy . Prokarniz
Купить диплом о высшем образовании. Приобретение официального диплома через надежную компанию дарит ряд достоинств для покупателя. Это решение помогает сэкономить как длительное время, так и серьезные средства. community.jobuppilipinas.com/employer/eonline-diploma
провайдеры по адресу дома
krasnodar-domashnij-internet003.ru
провайдеры интернета в краснодаре по адресу
1win https://1win1034.top/ .
Заказать диплом ВУЗа по доступной цене возможно, обратившись к проверенной специализированной компании. Мы предлагаем документы об окончании любых университетов РФ. Приобрести диплом любого ВУЗа– vudues.com/read-blog/91_diplom-medsestry-kupit-v-moskve.html
Greetings!
Paid traffic OnlyFans plans are perfect for rapid scaling. We offer tested methods for paid traffic OnlyFans campaigns that convert. Choose your niche and goals—we’ll do the rest. Scale your income without the guesswork. Fast, focused, and fan-ready.
It is 2025 and TikTok still delivers. Just make sure you’re optimizing content before driving TikTok traffic to OnlyFans.
All information on the website- https://sfsonlyfans.com/how-to-promote-your-onlyfans-account-a-complete-step-by-step-guide-for-beginners/
cheap traffic for OnlyFans, OnlyFans promotion, Tumblr traffic for OnlyFans
onlyfans promotion, telegram traffic onlyfans, paid traffic fansly
Good luck!
Купить диплом на заказ в Москве возможно через официальный портал компании. diafan.rusheat.ru/forum/pomosch-v-poluchenii-diploma
интернет провайдеры по адресу краснодар
krasnodar-domashnij-internet003.ru
провайдеры по адресу краснодар
Скачать рилс по ссылке В стремительно развивающемся мире социальных сетей, Instagram Reels стали настоящим феноменом. Короткие, захватывающие видеоролики привлекают миллионы пользователей, и часто возникает желание сохранить понравившийся контент. Однако, Instagram не предоставляет встроенной возможности скачивания Reels. Здесь на помощь приходит наш Телеграм бот – ваш надежный и удобный инструмент для скачивания Instagram Reels.
Ведущий игровой сайт онлайн | Посетите vavadacasino.netlify.app | Современная платформа для азартных развлечений | Откройте для себя мир казино | Лучшие игровые автоматы | Полностью легальный сайт | Моментальные выплаты и бонусы | Играйте в любое время и в любом месте | Вы получите помощь в любой ситуации | Обучайтесь у профессионалов | Начинайте выигрывать уже сегодня | Получите бонус при первой регистрации | Обновляемый ассортимент игр | Соревнуйтесь с другими игроками | Пригласите друзей и получайте бонусы | Простые инструкции для новичков | Политика конфиденциальности на сайте | Положительные оценки сайта | Настоящее казино у вас дома
casino vavada online https://vavadacasino.netlify.app/ .
шкаф в паркинг спб шкаф в паркинг спб .
Расклад Таро на будущее Таро – это не просто гадание, это мощный инструмент самопознания и личностного роста, особенно ценный для женщин в возрасте 35+. В этом возрасте мы часто задаемся вопросами о смысле жизни, отношениях, карьере. Таро может стать компасом, освещающим путь к ответам.
лучший интернет провайдер москва
msk-domashnij-internet001.ru
подключить домашний интернет в москве
1win india http://www.1win1034.top .
Create vivid images with Promptchan — a powerful neural network for generating art based on text description. Support for SFW and NSFW modes, style customization, quick creation of visual content.
1win aviator download http://1win1035.top/ .
Недвижимость в Болгарии у моря https://byalahome.ru квартиры, дома, апартаменты в курортных городах. Продажа от застройщиков и собственников. Юридическое сопровождение, помощь в оформлении ВНЖ, консультации по инвестициям.
интернет по адресу
msk-domashnij-internet001.ru
тарифы интернет и телевидение москва
Срочный выкуп квартир https://proday-kvarti.ru за сутки — решим ваш жилищный или финансовый вопрос быстро. Гарантия законности сделки, юридическое сопровождение, помощь на всех этапах. Оценка — бесплатно, оформление — за наш счёт. Обращайтесь — мы всегда на связи и готовы выкупить квартиру.
Заказать диплом любого ВУЗа!
Купить диплом университета по доступной стоимости возможно, обратившись к надежной специализированной фирме. Приобрести диплом: sx-diplomb.online
Автоматика Somfy — инновационные решения для вашего дома
Автоматика Somfy Автоматика Somfy . +7 (499) 638-25-37
Портал о недвижимости https://akadem-ekb.ru всё, что нужно знать о продаже, покупке и аренде жилья. Актуальные объявления, обзоры новостроек, советы экспертов, юридическая информация, ипотека, инвестиции. Помогаем выбрать квартиру или дом в любом городе.
узнать интернет по адресу
msk-domashnij-internet002.ru
провайдеры интернета в москве
1win entrar http://www.1win1020.top .
1win app download apk http://1win1035.top/ .
купить пластиковые окна рехау купить пластиковые окна рехау .
среда в плюсе мелбет что это https://melbet1019.ru .
роллерный шкаф в паркинг глубина 420 роллерный шкаф в паркинг глубина 420 .
купить аккаунт kupit-akkaunt.online
http://www.1win http://www.1win1024.top .
интернет провайдеры по адресу москва
msk-domashnij-internet002.ru
домашний интернет тарифы москва
Greetings!
TikTok traffic Fansly is an untapped gem for new creators. Use our system to boost TikTok traffic Fansly pages can rely on. It’s about the right hashtags, timing, and angles. We know what works on the platform. Turn views into paying fans.
Twitter traffic Fansly campaigns can drive clicks with every tweet. Use our templates for proven engagement. Twitter traffic Fansly strategies need consistency. We’ll help you grow through threads, spaces, and shoutouts. Go beyond basic posting.
All information on the website- https://sfsonlyfans.com/how-to-post-effective-content-on-instagram-stories-to-grow-your-onlyfans-account/
free traffic to fansly, twitter traffic fansly, OnlyFans traffic tools
OnlyFans traffic strategies, Tumblr traffic for OnlyFans, traffic from socials to OnlyFans
Good luck!
1win sign in https://1win1020.top .
Купить диплом любого ВУЗа!
Приобретение диплома ВУЗа через надежную фирму дарит ряд плюсов. Заказать диплом об образовании у сильной фирмы: doks-v-gorode-ufa-2.online
провайдер по адресу москва
msk-domashnij-internet003.ru
провайдер по адресу москва
Наш сервисный центр предлагает надежный качественный ремонт айфона любых брендов и моделей. Мы осознаем, насколько значимы для вас ваши смартфоны Apple, и готовы предложить сервис первоклассного уровня. Наши опытные мастера работают быстро и аккуратно, используя только оригинальные запчасти, что обеспечивает долговечность и надежность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются обладатели устройств iPhone, включают поврежденный экран, неисправности аккумулятора, неисправности программного обеспечения, неисправности разъемов и поломки корпуса. Для устранения этих неисправностей наши квалифицированные специалисты проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы обеспечиваете себе качественный и надежный выездной ремонт iphone.
Подробная информация размещена на сайте: https://remont-iphone-sot.ru
официальный сервис electrolux в москве электролюкс мастер
1win app https://1win1029.top .
Где приобрести диплом по актуальной специальности?
Мы изготавливаем дипломы любых профессий по приятным тарифам. Для нас важно, чтобы дипломы были доступны для большинства наших граждан. Заказать диплом любого ВУЗа diplomus-spb.ru/ofitsialnij-diplom-s-zaneseniem-v-reestr-2/
register with 1win website 1win1024.top .
Гармония интерьера с помощью штор в коттедже
шторы для коттеджа шторы для коттеджа .
Когда я впервые открыл этот сайт, ощущение было таким, будто я нашёл что-то особенное. Здесь каждый спин — это не просто волнение, а история, которую ты проживаешь с каждым движением.
Оформление интуитивен, словно невидимый проводник направляет тебя от момента к моменту. Операции, будь то пополнения или вывод средств, проходят легко, как поток воды, и это завораживает. А поддержка всегда готова подхватить, как надежный товарищ, который никогда не оставит.
Для меня Казино селектор стал пространством, где игра и вдохновение сплетаются. Здесь каждая минута — это часть картины, которую хочется создавать снова и снова.
Wow news for all us
провайдеры по адресу москва
msk-domashnij-internet003.ru
интернет домашний москва
Заказ диплома через проверенную и надежную компанию дарит ряд достоинств для покупателя. Данное решение позволяет сэкономить время и серьезные деньги. Впрочем, преимуществ гораздо больше.Мы изготавливаем дипломы любой профессии. Дипломы производятся на фирменных бланках государственного образца. Доступная цена по сравнению с серьезными тратами на обучение и проживание. Заказ диплома о высшем образовании из российского университета является рациональным шагом.
Приобрести диплом о высшем образовании: diplomt-nsk.ru/diplom-visshego-obrazovaniya-s-zaneseniem-v-reestr-9/
1win connexion https://1win1019.top .
1win скачать android http://1win5077.ru/ .
1win скачать на телефон http://1win5076.ru .
дешевый интернет нижний новгород
nizhnij-novgorod-domashnij-internet001.ru
провайдер по адресу нижний новгород
melbet скачать апк http://melbet1018.ru/ .
провайдеры по адресу дома
nizhnij-novgorod-domashnij-internet001.ru
какие провайдеры интернета есть по адресу нижний новгород
заказать смартфон http://www.allomarket.su .
заказать смартфон https://www.allomarket.su .
Наша мастерская предлагает профессиональный качественный ремонт айфона любых брендов и моделей. Мы осознаем, насколько важны для вас ваши смартфоны Apple, и обеспечиваем ремонт первоклассного уровня. Наши квалифицированные специалисты работают быстро и аккуратно, используя только качественные детали, что предоставляет надежность и долговечность наших услуг.
Наиболее частые неисправности, с которыми сталкиваются обладатели устройств iPhone, включают проблемы с экраном, неисправности аккумулятора, программные сбои, неработающие разъемы и поломки корпуса. Для устранения этих проблем наши квалифицированные специалисты выполняют ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы получаете качественный и надежный сервисный ремонт iphone на выезде.
Подробная информация представлена на нашем сайте: https://remont-iphone-sot.ru
sportbets https://sportbets11.ru/ .
займы http://www.cncnn.ru .
займ оформить онлайн http://www.cncnn.ru/ .
Откройте счёт на платформе Мелбет и получите выгодный бонус до 15 000 ?! Активируйте промокод при регистрации и начните с подарочных денег для ставок. Действующий Промокод Мелбет http://monetoss.ru/news/promokod_melbet_bonus_do_15_000_rub.html Мелбет — это безопасность и широкие возможности для выигрыша.
Профессиональный сервисный центр по ремонту техники в Твери.
Мы предлагаем: Официальный центр Ardo
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
интернет провайдеры нижний новгород
nizhnij-novgorod-domashnij-internet002.ru
узнать провайдера по адресу нижний новгород
Современные шторы для коттеджа
шторы для коттеджа шторы для коттеджа .
Мы предлагаем быстро заказать диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, штампами, подписями. Данный документ пройдет лубую проверку, даже с применением специальных приборов. jobb.fusionsol.com/companies/premiumydiploma
ремонт электролюкс бытовая техника официальный сайт электролюкс сервисная служба
чоп цены чоп цены .
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы готовы предложить документы ВУЗов, расположенных на территории всей Российской Федерации. Документы делаются на бумаге самого высшего качества. Это дает возможность делать государственные дипломы, которые не отличить от оригиналов. bnnovosti.ru/post_type=topic&p=123736
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: сервисный ремонт айфонов в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
тарифы интернет и телевидение нижний новгород
nizhnij-novgorod-domashnij-internet002.ru
какие провайдеры на адресе в нижнем новгороде
Заказать диплом любого университета!
Мы предлагаем документы ВУЗов, которые находятся на территории всей РФ.
diplomc-v-ufe.ru/diplom-s-zaneseniem-v-reestr-dlya-vashej-kareri/
Казино с щедрыми бонусами и быстрыми выплатами, подробности по ссылке https://casinovulkan-reg.store/
Ремонт крупной бытовой техники в Москве, куда обращаться для диагнистики и ремонта, читайте – в статье
Купить диплом о высшем образовании!
Наши специалисты предлагаютвыгодно купить диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Наш диплом пройдет лубую проверку, даже при помощи профессиональных приборов. Решайте свои задачи быстро и просто с нашими дипломами- soniamittal0066.copiny.com/question/details/id/1087641
1win mobil https://1win5077.ru/ .
Заказать диплом ВУЗа !
Приобретение диплома ВУЗа России у нас является надежным процессом, так как документ будет заноситься в реестр. Приобрести диплом об образовании diplomgorkiy.com/kupit-diplom-v-moskve-s-zaneseniem-v-reestr-bistro-15
интернет тарифы нижний новгород
nizhnij-novgorod-domashnij-internet003.ru
провайдер интернета по адресу нижний новгород
Мы изготавливаем дипломы любых профессий по приятным тарифам. Заказ диплома, который подтверждает окончание университета, – это рациональное решение. Приобрести диплом о высшем образовании: sportraketka.ru/naskolko-bezopasno-pokupat-diplom
авторизованные центры техники electrolux центральный сервисный центр по ремонту техники electrolux
цветы питер заказ цветов санкт петербург
Профессиональный сервисный центр по ремонту техники в Краснодаре.
Мы предлагаем: Ремонт массажных кресел BRADEX
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
цветы питер доставка цветов букетов
отвод дренажа vc.ru/services/1913096-kak-sdelat-drenazh-na-uchastke-v-sankt-peterburge .
цветы живые купить комнатные цветы купить в спб
1win сайт https://1win5076.ru/ .
Fast and reliable roadside assistance! The team was professional and friendly. Will definitely call them again if needed.
Идеальные шторы для любого бюджета
сшить шторы на заказ сшить шторы на заказ . Ткацкий
1win. pro http://www.1win5073.ru .
домашний интернет тарифы
nizhnij-novgorod-domashnij-internet003.ru
интернет домашний нижний новгород
oradentum is a comprehensive 21-in-1 oral care formula designed to reinforce enamel, support gum vitality, and neutralize bad breath
Заправка кондиционеров в Энгельсе В современном ритме жизни, когда каждая минута на счету, поломка стиральной машины становится настоящей катастрофой. Горы грязного белья растут с угрожающей скоростью, а перспектива ручной стирки повергает в уныние. Но прежде чем отчаиваться и планировать покупку новой техники, позвольте предложить вам решение, которое сэкономит ваше время, деньги и нервы. Наши опытные мастера в Энгельсе готовы быстро и качественно восстановить работоспособность вашей стиральной машины. Мы понимаем, насколько важна эта техника для вашего комфорта, поэтому предлагаем оперативный выезд на дом и профессиональную диагностику.
подключить интернет новосибирск
novosibirsk-domashnij-internet001.ru
провайдеры интернета в новосибирске по адресу
from this source phantom Extension
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: мастер ремонта apple
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Мы предлагаем документы ВУЗов, которые расположены в любом регионе РФ. Документы выпускаются на бумаге самого высокого качества. Это позволяет делать настоящие дипломы, не отличимые от оригиналов. baxoday.maxbb.ru/viewtopic.phpf=25&t=997
click here for more info [url=https://sites.google.com/mycryptowalletus.com/phantom-walletlogin/]phantom wallet[/url]
look at this now keplr Extension
лучший интернет провайдер новосибирск
novosibirsk-domashnij-internet001.ru
провайдеры по адресу дома
купить смартфон в интернете https://allomarket.su/ .
Купить диплом любого ВУЗа!
Мы можем предложить документы институтов, расположенных на территории всей России.
diplomservis.com/kupit-diplom-ob-obrazovanii-zanesennij-v-reestr-2/
Купить диплом на заказ в Москве вы можете через официальный портал компании. sahpes.onlinebbs.ru/viewtopic.phpf=3&t=1627
узнать интернет по адресу
novosibirsk-domashnij-internet002.ru
провайдеры в новосибирске по адресу проверить
Индивидуальные шторы под ваши размеры
сшить шторы на заказ сшить шторы на заказ . +7 (499) 460-69-87
he has a good point MetaMask Download
I started delightful CBD gummies a not many months ago and honestly, they’ve made a monumental difference. I regularly liberate everyone after dinner to mitigate me deject out and catnap better. They mouthful great —like solid confectionery — and don’t leave me identification punch-drunk the next day. It’s objective a good, age vibe that helps inhale the uptight insane after a stressful day. Wasn’t sure they’d feat at prime, but now I’m all in. Totally interesting if you dire something fool to take you rest without any grotesque side effects.
купить смартфон в интернете http://www.allomarket.su .
купить диплом ссср http://asx-adiploma-365.online .
подключить интернет по адресу
novosibirsk-domashnij-internet002.ru
домашний интернет тарифы
Мы предлагаем быстро и выгодно заказать диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Документ способен пройти любые проверки, даже при помощи специальных приборов. bergha.flybb.ru/viewtopic.phpf=2&t=664
Купить диплом института по невысокой стоимости можно, обращаясь к надежной специализированной компании. Заказать документ о получении высшего образования можно у нас в Москве. diplomidlarf.ru/kupit-diplom-vuza-s-zaneseniem-v-reestr-legko-i-bistro-3
подключить интернет
novosibirsk-domashnij-internet003.ru
провайдеры интернета по адресу новосибирск
explanation phantom Download
скачать lucky jet 1win22002.ru .
купить смартфон в интернете http://www.allomarket.su .
sportbets http://sportbets11.ru .
a fantastic read keplr Download
Шторы на заказ — подчеркните уникальность вашего пространства
шторы на заказ шторы на заказ . “Ткацкий”
интернет по адресу новосибирск
novosibirsk-domashnij-internet003.ru
провайдеры домашнего интернета новосибирск
informative post phantom wallet
мелбет кэшбэк http://melbet1020.ru/ .
Read More Here Metamask Extension
click here to read Metamask Extension
займ оформить онлайн cncnn.ru .
1win kenya 1win kenya .
интернет провайдеры омск
omsk-domashnij-internet001.ru
подключение интернета омск
additional info keplr Download
more info here phantom Download
купить смартфоны http://www.allomarket.su .
Надёжное казино для любителей азартных развлечений, узнать больше тут https://01casino-vulkan.store/
sportbets http://sportbets11.ru/ .
мелбет официальный сайт вход http://www.melbet1020.ru .
1win .com 1win1036.top .
подключить проводной интернет омск
omsk-domashnij-internet001.ru
подключение интернета омск
займы http://www.cncnn.ru .
Где купить диплом по необходимой специальности?
Получаемый диплом со всеми печатями и подписями отвечает условиям и стандартам Министерства образования и науки, никто не сможет отличить его от оригинала. Купить диплом о высшем образовании – не проблема! jagplay.ekafe.ru/viewtopic.phpf=22&t=7174
Мы оказываем услуги по продаже документов об окончании любых ВУЗов России. Документы производят на подлинных бланках государственного образца. mastercode.ru/forum/PAGE_NAME=profile_view&UID=5398
1win registration http://1win1034.top/ .
займы http://www.cncnn.ru/ .
useful source Metamask Extension
подключить интернет
omsk-domashnij-internet002.ru
домашний интернет подключить омск
helpful hints phantom Download
como usar el bono de casino en 1win https://www.1win1022.top .
visit this site right here MetaMask Download
casino en 1 win casino en 1 win .
online casino bonus 500% https://1win5073.ru .
подключить интернет в квартиру омск
omsk-domashnij-internet002.ru
лучший интернет провайдер омск
займ оформить онлайн cncnn.ru .
navigate to this website Metamask Extension
Диплом ВУЗа РФ!
Без присутствия диплома достаточно сложно было продвигаться вверх по карьере. Заказать диплом можно используя сайт компании: crtdu-kras.ru/communication/forum/user/283051
visit this site right here rabby wallet download
акции алиэкспресс
провайдеры домашнего интернета омск
omsk-domashnij-internet003.ru
тарифы интернет и телевидение омск
1win id number search 1win1034.top .
вызов электрика на дом москва цены нужен электрик на дом москва недорого
Новости Винницы https://u-misti.vinnica.ua события, статьи, происшествия в Виннице и области
turkiye’deki vize merkezi Vize randevu, vize basvurusu sureclerinde kritik bir ad?md?r. Vfs randevu, vize merkezi arac?l?g?yla yap?lan basvurularda s?kl?kla kars?las?lan bir terimdir. Vfs Istanbul, Turkiye’deki en yogun vize merkezlerinden biridir. BLS vize merkezi de belirli ulkelerin vize basvurular?n? kabul etmektedir. Italya vize merkezi ve idata, ozellikle Italya’ya seyahat etmek isteyenler icin onemli basvuru noktalar?d?r. Turkiye’deki vize merkezi kavram?, farkl? ulkelerin temsilcilikleri arac?l?g?yla vize islemlerini yurutmelerini ifade eder.
read here MetaMask Download
casino guide
провайдеры интернета в омске
omsk-domashnij-internet003.ru
провайдеры интернета омск
online casino portofele electronice https://1win5078.ru/ .
адин эксперт скачат адин эксперт скачат .
Где купить диплом специалиста?
Заказать диплом ВУЗа по доступной цене можно, обратившись к надежной специализированной компании.: kazdiplomas.com
провайдеры домашнего интернета пермь
perm-domashnij-internet001.ru
интернет провайдер пермь
1win https://1win22003.ru .
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по выгодным ценам. Дипломы изготавливаются на подлинных бланках Заказать диплом о высшем образовании dip-lom-rus.ru
1win original 1win original .
Заказать диплом университета. Приобретение документа о высшем образовании через проверенную и надежную компанию дарит ряд достоинств. Это решение дает возможность сэкономить как личное время, так и значительные деньги. 3srecruitment.com.au/employer/eonline-diploma
Extra resources phantom Download
content phantom wallet
Анонимный видеочат. Сеанс у психолога. Отзыв на психолога.
Консультация в кризисных состояниях.
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
Частые разногласия с самыми близкими.
Разговоры с психологом. Психологическая помощь онлайн. Онлайн разговор с психологом.
Нужен хороший психолог?
Мы обязательно поможем преодолеть эмоциональный кризис, избавиться от тревожности и апатии, справиться со стрессом и депрессией, связанными с неуверенностью и многим другим.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Психолог Москва. Психолог СПБ. Психолог онлайн.
Психолог онлайн 24. Psicologo. Консультации психолога.
Нужен хороший психолог?
Онлайн сессия от 34540 руб.
Психолог, Сайт психологов.
Записаться на консультацию.
Анонимная помощь психолога. Отзывы о психологе. Психологи онлайн консультация.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Записаться на консультацию.
Нужен хороший психолог?
Психологи онлайн анонимно. Помощь психолога. Помощь психолога онлайн.
Нужен хороший психолог?
Анонимный прием.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
провайдеры интернета в перми
perm-domashnij-internet001.ru
домашний интернет тарифы пермь
1win-partner 1win-partner .
1вин официальное зеркало на сегодня http://1win22001.ru .
1win скачать на телефон андроид официальный https://1win22002.ru/ .
провайдеры интернета в перми
perm-domashnij-internet002.ru
домашний интернет пермь
купить гайки
мостбет mostbet22001.ru .
description phantom Download
Nuestros profesionales cualificados estan dispuestos a ayudarte en el momento que lo necesites, ya que contamos con equipos moviles compactos que nos permiten realizar servicios de mantenimiento directamente en sitio.
Da igual que tu maquinaria de campo o dispositivo tecnico se encuentre en una zona remota: nuestro kit compacto, de dimensiones reducidas (practicamente como un maletin), permite a nuestros especialistas desplazarse hasta el sitio para corregir desequilibrios u otros componentes. Ademas, ofrecemos a la venta equipos de balanceo ideales para duenos de equipos agricolas, negocios del rubro o centros de servicio tecnico.
A menudo, operamos bajo este modelo: un tecnico acude con el dispositivo, muestra como se usa, explica los ajustes necesarios y, mas tarde, la empresa adquiere el sistema.
En nuestro sitio web encontraras tarifas, informacion completa y informacion de contacto. Contamos con tecnicos en Espana, Portugal y Argentina, asi como representantes oficiales.
Get More Info keplr wallet
шавиш спортинг прогноз prognoz-na-segodnya-na-sport13.ru .
прогнозы на спорт от экспертов prognoz-na-segodnya-na-sport11.ru .
лучшие прогнозы на спорт лучшие прогнозы на спорт .
Visit Website phantom wallet
Recommended Reading phantom Download
дрова березовые колотые с доставкой дешево https://vc.ru/rejting/1604662-zakazat-drova-v-sergievom-posade/ .
Clicking Here phantom wallet
подключить домашний интернет пермь
perm-domashnij-internet002.ru
дешевый интернет пермь
уннв ремикс скачать http://www.25kat.ru/music/уннв/ .
Привет всем!
Курсы по 1с-программированию помогут автоматизировать бизнес-процессы. Вы освоите конфигурирование и разработку в системе 1С. Курсы по 1с-программированию включают практические проекты. Подходят программистам и специалистам. Улучшайте навыки с курсами по 1с-программированию.
Вся информация на сайте – https://top-kursy-frontend.ru/
Курсы по таргетированной рекламе научат вас эффективно настраивать Facebook и ВКонтакте. Вы узнаете, как создавать рекламные объявления и анализировать результаты. Курсы по таргетированной рекламе включают реальные кейсы и практические задания. Подходят как новичкам, так и специалистам по маркетингу. Начните продвижение с курсов по таргетированной рекламе.
курс ландшафтного дизайна в воронеже, курс по таргетированной рекламе слив, курсы по поисковой оптимизации (seo)
курсы по управлению проектами, курсы по искусственному интеллекту, курсы создание и администрирование сайта
Удачи!
Заказать диплом об образовании!
Мы можем предложить дипломы любой профессии по приятным тарифам— dshi64.ru
кашпо для цветов высокое пластиковое напольное недорого купить http://https://industrial-wood.ru/novosti/40260-ispolzovanie-napolnogo-kashpo-v-sovremennom-interere.html .
провайдеры домашнего интернета пермь
perm-domashnij-internet003.ru
подключить проводной интернет пермь
facebook ad account for sale https://buy-adsaccounts.work
Купить документ о получении высшего образования можно в нашей компании в столице. Мы оказываем услуги по продаже документов об окончании любых ВУЗов Российской Федерации. Вы получите необходимый диплом по любой специальности, любого года выпуска, включая документы Советского Союза. Даем гарантию, что в случае проверки документа работодателями, никаких подозрений не возникнет. vacshidiplom.com/kupit-diplom-s-zaneseniem-v-reestr-stoimost-3/
buy fb account https://buy-ad-accounts.click
посетить веб-сайт Альткоин обмен криптовалюты
read what he said rabby wallet
Заказать диплом о высшем образовании. Приобретение документа о высшем образовании через надежную компанию дарит множество плюсов. Такое решение позволяет сберечь как дорогое время, так и значительные финансовые средства. 133636.activeboard.com/forum.spark
Приобрести диплом о высшем образовании!
Мы готовы предложить дипломы психологов, юристов, экономистов и прочих профессий по выгодным тарифам. Вы покупаете диплом через надежную компанию. : mwcejobbank.webz.com.ng/employer/gosznac-diplom-24
Заказать диплом на заказ возможно через сайт компании. iprofile.sv3.gramick.dev/read-blog/12831_kupit-diplom-o-srednem-obrazovanii-v-reestr.html
fb accounts for sale cheap facebook accounts
интернет провайдеры пермь
perm-domashnij-internet003.ru
домашний интернет тарифы пермь
go to my blog keplr Download
провайдеры интернета в ростове
rostov-domashnij-internet001.ru
домашний интернет тарифы ростов
view publisher site phantom Download
1win http://www.1win22003.ru .
Купить диплом университета по доступной цене можно, обращаясь к проверенной специализированной компании. Купить документ университета можно в нашей компании в Москве. diplomaj-v-tule.ru/kupite-diplom-ob-obrazovanii-s-reestrom-sejchas
Психолог онлайн чат. Как улучшить отношения. Психолог онлайн чат.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
Психолог Москва. Психолог СПБ. Психолог онлайн.
Сеанс у психолога. Телеграм психолог. Чат инкогнито.
Частые разногласия с самыми близкими.
Индивидуальное консультирование.
Онлайн сессия от 28270 руб.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Консультация психолога онлайн. Психологи онлайн консультация. Анонимный онлайн психолог.
Онлайн сессия от 79890 руб.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
Поможет поставить цель терапии и приведет к результату.
Психологическое консультирование.
Личные сайты психологов. Помощь психолога анонимно. Разговоры с психологом.
Частые разногласия с самыми близкими.
Услуги психолога · — Консультация психолога.
Получить поддержку по широкому кругу вопросов.
Психолог t me. Telegram-чат с психологом. Чат переписка.
Получить поддержку по широкому кругу вопросов.
Психологическая помощь онлайн.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
Get the facts rabby wallet extension
my latest blog post MetaMask Download
подключить интернет в ростове в квартире
rostov-domashnij-internet001.ru
подключить проводной интернет ростов
1win http://1win22017.ru/ .
Comparativa de equipos de balanceo industrial
Balanset-1A — tu herramienta para un balanceo eficiente directamente en la explotacion agricola
?Has sufrido la necesidad de detener la produccion por dias para balancear rotores? Entendemos perfectamente tu situacion. Por eso, hace tiempo buscamos una forma que permitiera seguir trabajando sin pausas innecesarias. Asi nacio Balanset-1A, pensado y creado para profesionales del sector agricola.
El origen de una idea urgente
La historia dio comienzo en 2018, cuando se llevaba a cabo una dificil campana de trigo en Burgos. Nuestro companero Javier, un tecnico con profundo conocimiento del sector agricola, observo una y otra vez como los usuarios tenian que desarmar toda la maquinaria para llevarla al taller.
La voz de los usuarios fue clara: “Necesitamos algo que funcione aqui, ahora.”
Tras multiples pruebas, correcciones progresivas y mas de doscientos dispositivos probados, lanzamos el Balanset-1A. No venia de un prototipo de oficina, sino de un problema real en el campo.
Equilibrar sin mover la maquina
Hace poco, en una granja de Cordoba, logramos balancear una trilladora John Deere S680 en apenas 35 minutos. Antonio, su dueno, nos aseguro textualmente:
“Con lo que deje de gastar en traslados y tiempos improductivos, la inversion se amortizo en dos temporadas.”
Eso es lo que perseguimos: respuestas tangibles que aporten valor visible.
?Que ofrece?
Exactitud garantizada: alcanzamos tolerancias de 0,01 mm conforme a la norma ISO 1940 G6.3
Aguantamos todo tipo de condiciones climaticas, desde lluvias prolongadas en Galicia hasta calor extremo en Sevilla
Muy baja incidencia de averias: los usuarios notan reducciones superiores al 70 % en problemas por vibraciones
Casos que marcan la diferencia
En 2022, en Lleida, evitamos una parada critica en una cooperativa durante la temporada de maiz.
Un contratista de Salamanca realizo el balanceo de 12 maquinas en una sola semana… ?y todo ello sin salir del campo!
Disenado para durar, pensado para ti
No nos quedamos en lo esencial. Incorporamos detalles que facilitan el trabajo en el dia a dia.
Sensores magneticos extrafuertes aptos para superficies no uniformes
Interfaz amigable que muestra analisis grafico del equilibrio
Funcionamiento prolongado mediante bateria que resiste hasta 14 horas de trabajo
Como afirma Maria, la responsable tecnica del equipo de campo:
“No vendemos aparatos bonitos. Vendemos tranquilidad y horas bien aprovechadas.”
?Por que elegirnos?
El 87 % de quienes usaron una vez este sistema vuelven a adquirirlo.
Somos la unica empresa en Espana con asistencia movil integrada.
La documentacion completa esta abierta y disponible para consulta directa.
Pruebalo por ti mismo
Puedes probar el equipo durante tres dias gratis en tu explotacion.
Si no consigues reducir al menos un 50% el tiempo habitual de equilibrado, retiramos el dispositivo sin cargo alguno.
Y si decides quedartelo, te regalamos un completo diagnostico de tu maquinaria.
Porque creemos firmemente en lo que hacemos.
Y, sobre todo, reconocemos la importancia de tu trabajo.
click for info MetaMask Download
подключить проводной интернет ростов
rostov-domashnij-internet002.ru
дешевый интернет ростов
Courage is what it takes to stand up and speak; courage is also what it takes to sit down and listen. 아일릿 섹스동영상
Добрый день!
Курсы по искусственному интеллекту помогут понять основы и практики AI-технологий. Вы научитесь создавать модели машинного обучения и анализировать данные. Курсы по искусственному интеллекту включают теорию и практические проекты. Подходят разработчикам и аналитикам. Освойте востребованные навыки с курсами по искусственному интеллекту.
Вся информация на сайте – https://kursy-php.ru/
Курсы по съемке и обработке фото научат делать качественные фотографии. Вы изучите композицию, свет и ретушь. Курсы по съемке и обработке фото включают работу с камерой и программами. Подходят фотографам и любителям. Сделайте свои фото идеальными с курсами по съемке и обработке фото.
курсы повышения квалификации для руководителей хора, управление проектами в microsoft project учебный курс, курсы по графическому дизайну
курсы по hr и управлению персоналом, курсы по adobe photoshop, онлайн курс копирайтинг
Удачи!
1win top http://1win5078.ru/ .
buy fb account https://buy-ads-account.click
1win https://1win22017.ru/ .
интернет провайдер ростов
rostov-domashnij-internet002.ru
домашний интернет в ростове
купить лесовоз дров в ленинградской области https://www.vc.ru/rejting/1714843-reiting-luchshih-firm-po-prodazhe-berezovyh-dubovyh-i-lipovyh-kolotyh-drov-v-spb-top-kompanii-na-2024-2025-god .
группа уннв скачать https://25kat.ru/music/уннв/ .
интернет провайдеры ростов
rostov-domashnij-internet003.ru
тарифы интернет и телевидение ростов
from this source keplr Extension
recommended you read phantom Download
подключить интернет
rostov-domashnij-internet003.ru
домашний интернет тарифы
try this out phantom Extension
Заказать диплом института по доступной стоимости можно, обращаясь к проверенной специализированной фирме. Купить документ о получении высшего образования можно в нашей компании. diplomc-v-ufe.ru/kupite-diplom-o-visshem-obrazovanii-srochno-i-nadezhno
нажмите здесь водкабет зеркало vodkabet
Приобрести диплом можно используя сайт компании. youhomie.com/read-blog/5734_diplom-vysshego-obrazovaniya-s-zaneseniem-v-reestr.html
мостбет https://www.mostbet20003.ru .
Купить диплом о высшем образовании!
Мы предлагаем документы институтов, расположенных на территории всей РФ.
fastdiploms.com/kupit-diplom-s-reestrom-v-rossii-bistro-i-nadezhno-4/
mostbet mostbet .
Главная cryptoboss зеркало
домашний интернет в ростове
rostov-domashnij-internet003.ru
домашний интернет ростов
Наша мастерская предлагает высококачественный мастер по ремонту iphone на выезде различных марок и моделей. Мы понимаем, насколько значимы для вас ваши смартфоны Apple, и обеспечиваем ремонт первоклассного уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что предоставляет долговечность и надежность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств iPhone, включают проблемы с экраном, неисправности аккумулятора, ошибки ПО, неработающие разъемы и повреждения корпуса. Для устранения этих поломок наши профессиональные техники проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы гарантируете себе долговечный и надежный сервис ремонта айфона на выезде.
Подробная информация размещена на сайте: https://remont-iphone-sot.ru
музыка уннв скачать музыка уннв скачать .
Приобрести диплом любого ВУЗа. Производство документа занимает минимум времени, а стоимость при этом невысокая. В итоге вы имеете возможность сохранить бюджет и получить хорошую работу вашей мечты. Приобрести диплом на заказ возможно используя сайт компании. – reputable.cc/profile/muoi714530846
прогнозы на хоккей на сегодня от профессиональных прогнозы на хоккей на сегодня от профессиональных .
спортивный прогноз на сегодня спортивный прогноз на сегодня .
Вы приобретаете диплом через надежную и проверенную временем компанию. Приобрести диплом о высшем образовании– http://kiddygames.ru/kupit-gosudarstvennij-diplom-s-zaneseniem-v-reestr-3/ – kiddygames.ru/kupit-gosudarstvennij-diplom-s-zaneseniem-v-reestr-3/
барс югра прогноз http://www.prognoz-na-segodnya-na-sport12.ru/ .
какие провайдеры на адресе в самаре
samara-domashnij-internet001.ru
провайдеры по адресу
Смотреть здесь [url=https://vodkacasino.net/]vodka.bet официальный сайт[/url]
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje цены, можете посмотреть на сайте: ремонт холодильников gorenje сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Новости Хмельницкого https://u-misti.khmelnytskyi.ua сайт Хмельницкого и области. Последние Новости, события, обзоры.
мостбет https://mostbet22002.ru .
Узнать больше vodka bet
проверить провайдеров по адресу самара
samara-domashnij-internet001.ru
провайдеры в самаре по адресу проверить
oradentum is a comprehensive 21-in-1 oral care formula designed to reinforce enamel, support gum vitality, and neutralize bad breath
опубликовано здесь vodkabet зеркало водкабет
Смотреть здесь kraken войти
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам.– gladpwnz.ru/forum/member.phpu=221749
прогнозы на спорт сегодня прогнозы на спорт сегодня .
прогнозы на спорт от экспертов https://prognoz-na-segodnya-na-sport11.ru/ .
интернет kraken сайт
провайдеры по адресу самара
samara-domashnij-internet002.ru
провайдер по адресу
Остекление балконов под ключ с гарантией качества и выгодной ценой, подробности здесь https://osteklenie-balkona-spb.pro/
Ответ психологи. Помощь психолога онлайн. Чат общение.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Психологическая помощь онлайн.
Психолог Москва. Психолог СПБ. Психолог онлайн.
мостбет http://mostbet22002.ru/ .
Психологическая помощь онлайн анонимно. Задать вопрос психиатру онлайн. Анонимний чат.
Индивидуальное консультирование.
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
Консультация в кризисных состояниях.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
I’ve been using https://www.nothingbuthemp.net/products/sativa-gummies daily in regard to over a month now, and I’m indeed impressed at near the absolute effects. They’ve helped me determine calmer, more balanced, and less restless in every nook the day. My saw wood is deeper, I wake up refreshed, and sober my core has improved. The value is outstanding, and I cherish the common ingredients. I’ll categorically preserve buying and recommending them to the whole world I be aware!
мебель в переговорную купить Купить офисную мебель При выборе офисной мебели важно учитывать эргономику, функциональность и дизайн. Правильно подобранная мебель создает комфортную и продуктивную рабочую среду.
Где найти психолога. Анонимный психолог. Чат с психологом в телеге.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Психологическое консультирование.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Как выйти из депрессии видео. Получите помощь психолога онлайн. Психолог онлайн анонимно.
21450 проверенных отзывов.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
Раздражительность на членов своей семьи.
Телеграм психолог. Как найти хорошего психолога. Чат переписка.
Психологическое консультирование.
Решим вместе вашу проблему.
Анонимный прием.
Индивидуальное консультирование.
mostbet mostbet22001.ru .
1вин https://1win22018.ru/ .
Наша компания предлагает выгодно и быстро заказать диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями официальных лиц. Диплом пройдет лубую проверку, даже при использовании профессиональных приборов. rorp.4admins.ru/viewforum.phpf=33
mostbet https://mostbet20003.ru .
Заказать документ о получении высшего образования вы сможете у нас. Заказать диплом института по невысокой стоимости возможно, обратившись к проверенной специализированной фирме. mm.kabb.ru/viewtopic.phpf=2&t=1433
Мы изготавливаем дипломы любой профессии по выгодным ценам. Заказ документа, подтверждающего обучение в ВУЗе, – это выгодное решение. Заказать диплом о высшем образовании: aryamariasinta.copiny.com/question/details/id/1093710
многоместные секции купить Офисные перегородки и экраны: Офисные перегородки и экраны позволяют зонировать пространство, создавая комфортные рабочие места и обеспечивая приватность.
провайдер интернета по адресу самара
samara-domashnij-internet002.ru
узнать провайдера по адресу самара
fb account for sale https://ad-account-buy.top/
Заказать диплом об образовании!
Мы готовы предложить дипломы любой профессии по выгодным тарифам— diplomoz-197.com
facebook ad account for sale cheap facebook accounts
пластиковые окна заказать пластиковые окна заказать .
форум по кибербезопасности От теории к практике: Форумы – это не только теория, но и практика. Обсуждение конкретных кейсов, анализ вредоносного ПО, разбор инцидентов безопасности – все это позволяет участникам форумов получить ценный опыт и применить полученные знания на практике. В заключение, форумы по кибербезопасности являются важным элементом современной экосистемы информационной безопасности, способствуя обмену знаниями, обучению и развитию профессиональных навыков.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje в москве, можете посмотреть на сайте: срочный ремонт холодильников gorenje
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
купить диплом настоящий orikdok-5v-gorode-volgograd-34.ru .
mostbet http://mostbet20002.ru/ .
интернет по адресу дома
samara-domashnij-internet003.ru
интернет провайдеры по адресу
срочный онлайн займ на карту мфо http://www.vc.ru/rejting/1964646-kredity-na-kartu-na-god-s-plokhoy-istoriey .
buy fb account https://ad-account-for-sale.top
Онлайн-казино сегодня: баланс между азартом и технологиями
Эра цифровых технологий полностью перестроила подход к организации досуга. Казино в интернете стали популярной формой отдыха, обеспечивая круглосуточный доступ из любой локации. Играть из дома стало простым и удобным способом погружения в игровой процесс превратила онлайн-казино в глобальный тренд. Разнообразие и динамика делают онлайн-казино особенно привлекательными, они ушли далеко от привычного формата наземных казино, а уникальным цифровым пространством со своими особенностями и подходами. Игроки больше не ограничены временем, локацией или выбором, каждый может подобрать идеальную игру под своё настроение и вкус.
Погружение в игру: технологии и восприятие
Цифровые платформы завоевали любовь игроков за реалистичную атмосферу. Инновации делают каждую сессию максимально реалистичной, с передачей мельчайших деталей — от интерфейсов до звуков игровых автоматов. Благодаря этому погружение в игру становится максимальным, даже играя на смартфоне в дороге или дома. Цифровые казино всё чаще делают упор на атмосферность, включая взаимодействие с другими игроками, участие в турнирах, мгновенные реакции на выигрыши — это позволяет воспринимать игру как полноценную цифровую реальность.
Платформы нового поколения
Казино нового поколения https://irinadragos.co.uk/2025/04/28/national-casino-100-13/ — это не только слоты и рулетка, это мультиформатные платформы с массой опций — от ставок до PvP-боёв. Механики персонализации, событийности и интерактива стали нормой. Всё чаще казино становятся мультижанровыми центрами — игрок может за одну сессию поиграть в классический слот, заключить пари на футбольный матч и поучаствовать в интерактивной викторине. В результате игрок получает максимум впечатлений и возможностей, что особенно ценно в современном цифровом пространстве.
Часы и минуты — не просто цифры
Опытные игроки знают, что игра в разное время может приносить разные результаты. Азартные сайты работают по заданным правилам, а статистика участников зависит от временного интервала. Кто-то считает, что с утра выиграть проще, а вечерняя активность кажется выгоднее. Кроме того, некоторые платформы активируют акции в строго заданное время, что меняет тактику участия. Геймеры, учитывающие нюансы, не ограничиваются развлечением, но и максимизируют шансы на выигрыш.
Культура и локальные особенности
Расширение цифрового казино-пространства диктует новые стандарты. Игровые операторы теперь учитывают игроков со всего мира. Игроки со всего света — от Бразилии до Казахстана влияют на формат платформ. Поэтому казино локализуют меню и дизайн под национальные предпочтения. Некоторым важен вход без депозита, и операторы идут навстречу. Локализация всех элементов повышает вовлечённость, особенно у неуверенных игроков.
Прогнозы, предчувствия и интуиция
Азарт — не всегда вопрос статистики. Форматы без жёстких алгоритмов апеллируют к внутреннему чутью. Многие игроки следуют личным ритуалам. День рождения, номер квартиры, “везучие” комбинации становятся основой стратегии. Игровой процесс становится личным, а не механическим повтором. Когда эмоции ведут игрока, игра приобретает уникальный стиль.
Первый опыт в онлайн-казино
Операторы онлайн-казино https://acilimkoleji.com/e-mtk-daretm-sistemi-106/ пытаются создать комфортные условия для новичков, предоставляя простую форму регистрации, автоматическую авторизацию и доступ к бесплатным версиям игр. С помощью новых функций даже тот, кто раньше не играл может включиться в игровой процесс мгновенно, без сложностей и проблем. Многие платформы также внедряют пошаговые подсказки, показывают игроку, что нажимать, а также моментально выдают подарки, создавая комфортное начало. Ориентация на пользователя снижает барьеры входа и приближает игровой мир к обычным людям, включая новую волну игроков.
Поощрения в онлайн-казино
С целью поддержания вовлеченности платформы разрабатывают поощрительные механики: от дневных квестов до уровневых наград. Игроки получают безоплатные спины, бонусы на пополнение, пропуски на элитные игры и другие привлекательные подарки, делающие игровой процесс интереснее. Однако получить выгоду от бонусов можно только с умом, нужно понимать правила отыгрыша, следить за сроками и соблюдать лимиты по играм. Игроки, умеющие разумно распределять поощрения, получают конкурентное преимущество, даже играя на небольшие суммы.
Как разобраться в казино
В онлайн-казино появляются обучающие статьи, ориентированные на любую аудиторию. Инструкции показывают, структуру игровых разделов, наводят на эффективные методы, делают акцент на шансах и дают рекомендации по эмоциям. Это создаёт чувство единства, когда пользователь ощущает себя вовлечённым, и ресурс превращается в обучающую площадку.
Казино как элемент цифрового мира
Сегодня казино в интернете становятся частью глобального интернета, где вовлеченность и настроение решают всё. Это больше, чем казино, а новый способ общения, объединяющая широкую аудиторию. Игроки делятся стратегиями, создают гайды, и всё это происходит здесь и сейчас. Онлайн-казино становятся неотъемлемой частью цифрового мира, где важны настроение и взаимодействие.
uptopru124
заказать пластиковые окна заказать пластиковые окна .
Купить диплом ВУЗа по невысокой цене вы можете, обращаясь к надежной специализированной компании. Мы предлагаем документы об окончании любых ВУЗов России. Заказать диплом университета– promintern.listbb.ru/viewtopic.phpf=16&t=1671
Доброго!
Ремонт квартиры с использованием экологичных материалов — это ваш шанс создать уютное и безопасное пространство. Мы выполняем ремонт квартиры с использованием экологичных материалов, что обеспечит не только красоту, но и заботу о здоровье. Ремонт квартиры с использованием экологичных материалов — это стиль и экология. строительство дом из газобетона под ключ, сравнения строительство домов, по закону до скольки можно делать ремонт в квартире Ремонт квартиры с использованием экологичных материалов — это забота о будущем.
Больше информации на сайте – https://quiz-lend.ru/
призраки в доме ремонт, книги ремонт дома, квартира кирпич отделка
строительство бани тверь, ремонт и отделка домов квартир, закон капитальный ремонт домов
Удачи!
sportbets sportbets13.ru .
интернет провайдеры по адресу самара
samara-domashnij-internet003.ru
провайдеры интернета в самаре по адресу
sportbets https://sportbets12.ru/ .
найти это водка казино бет
winner casino jocuri pe mobil http://1win5079.ru .
на этом сайте водкабет казино
диплом фельдшера купить http://www.orikdok-4v-gorode-yoshkar-ola-12.ru/ .
купить диплом в белгороде http://www.orikdok-1v-gorode-ekaterinburg-66.ru .
провайдеры интернета санкт-петербург
spb-domashnij-internet001.ru
проверить провайдера по адресу
Добрый день!
Ремонт под ключ ванной комнаты — это ваш шанс создать функциональное и стильное пространство для отдыха. Мы выполняем ремонт ванной комнаты под ключ, гарантируя отличные результаты. Ремонт под ключ ванной комнаты — это улучшение вашего ежедневного комфорта. дом ремонта киров, новгород дом строительство домов, ремонт старых бани Ремонт под ключ ванной комнаты — это стильно и удобно.
Больше информации на сайте – https://quiz-lend.ru
газоблоки для строительство дома, строительство бань под ключ тюмень, фото фасадов отделки домов
плата в доме ремонт, стоимость ремонта квартиры косметический, фасад частного дома отделка
Удачи!
Приобрести диплом о высшем образовании!
Мы предлагаембыстро и выгодно купить диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Документ пройдет лубую проверку, даже при использовании специальных приборов. Достигайте цели быстро с нашими дипломами- gigit.cz/employer/gosznac-diplom-24
Приобрести диплом любого университета поможем. Купить диплом специалиста в Красноярске – diplomybox.com/kupit-diplom-spetsialista-v-krasnoyarske
купить в иваново диплом https://orikdok-2v-gorode-astrakhan-30.online/ .
проверить провайдера по адресу
spb-domashnij-internet001.ru
интернет провайдеры санкт-петербург по адресу
Выгодно купить диплом института!
Заказать диплом института. Заказ документа о высшем образовании через надежную фирму дарит ряд плюсов для покупателя. Такое решение дает возможность сберечь как личное время, так и серьезные средства. revistaeletronica.oabrj.org.br/diplom-na-zakaz-legko-i-prosto-199
заборы под заказ заборы под заказ .
Анонимный чат. Как начать разговор с психологом. Психо online.
Раздражительность на членов своей семьи.
Психологическая помощь онлайн.
Психолог владеет множеством приемов и техник, которые помогут разобраться в себе.
Записаться на консультацию.
Доброго!
Ремонт квартиры — это важный этап, который требует тщательного планирования и профессионализма. Мы предлагаем услуги по ремонту квартиры под ключ, начиная от демонтажа и заканчивая установкой мебели и аксессуаров. Мы гарантируем высокое качество работы и соблюдение всех сроков. Наши специалисты помогут выбрать подходящие материалы и решения для вашего интерьера. ремонт трехкомнатной квартиры цена, ремонт квартир, ремонт дом сон Ремонт квартиры будет выполнен с максимальной точностью и вниманием к деталям.
Больше информации на сайте – https://bestcapshop.ru/
майнкрафт строительство домов, ремонт собственников квартир, ремонт в старом доме в деревне
вызов мастера на дом ремонта холодильника, домов ключ под ремонт цены, дома отделка пенопластом
Удачи!
Психолог оказывает помощь онлайн в чате. Психологи онлайн анонимно. Психологическая поддержка онлайн чат.
Получить поддержку по широкому кругу вопросов.
Онлайн сессия от 61809 руб.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Вебинар психология. Сайт психологии отношений. Практический психолог.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Частые разногласия с самыми близкими.
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
Анонимная помощь психолога. В переписке у психолога. Получите консультацию онлайн-психолога в чате прямо сейчас.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Услуги психолога · — Консультация психолога.
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
Онлайн психологи. Психолог по личным отношениям. Психолог онлайн.
Анонимный прием.
Нужен хороший психолог?
Раздражительность на членов своей семьи.
Частые разногласия с самыми близкими.
какие провайдеры интернета есть по адресу санкт-петербург
spb-domashnij-internet001.ru
подключить интернет в квартиру санкт-петербург
1 win онлайн 1 win онлайн .
Мы предлагаем выгодно и быстро купить диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Данный диплом способен пройти лубую проверку, даже при помощи специальных приборов. znanee.flybb.ru/viewtopic.phpf=2&t=997
Добрый день!
Каркасное строительство домов — это один из самых популярных и доступных способов построить дом. Мы предлагаем каркасное строительство домов, которое отличается высокой скоростью возведения и хорошими эксплуатационными характеристиками. Каркасные дома обладают отличной теплоизоляцией и могут быть оснащены всеми необходимыми коммуникациями. Все работы выполняются с использованием качественных и надежных материалов. блок хаус для отделки домов цена, ремонты в домов фото, отделки фасадов частного дома фото Каркасное строительство домов — это быстро, эффективно и доступно.
Больше информации на сайте – https://bestcapshop.ru
каркасный дом без отделки, некоммерческая организация фонда капитального ремонта многоквартирных домов, ремонт дача дом
строительство дом и баня, дизайн и ремонт квартир, ремонт на дому микроволновка
Удачи!
интернет провайдер санкт-петербург
spb-domashnij-internet002.ru
какие провайдеры на адресе в санкт-петербурге
Здравствуйте!
Строительство домов с экозаборами — это решение для тех, кто хочет жить в гармонии с природой. Мы занимаемся строительством домов с экозаборами, чтобы вы могли наслаждаться природой, не нарушая ее гармонии. Строительство домов с экозаборами — это экологичность и стиль. купить квартиру москва новостройка с отделкой, купить квартиру в москве от застройщиков с отделкой, деньги на строительстве домов Строительство домов с экозаборами — это защита природы и вашего пространства.
Больше информации на сайте – https://dizzastershop.ru
фасадная плитка для отделки дома, ремонт общее имущество в многоквартирном доме, квартире ремонт пола
правила строительства бань, кровля многоквартирного дома ремонт, каталог ремонтов дома
Удачи!
my company https://sollet-wallet.io
why not try here russian traditional clothing
выберите ресурсы Косметика под своим брендом
домашний интернет санкт-петербург
spb-domashnij-internet002.ru
узнать интернет по адресу
buy facebook ad account facebook accounts to buy
подключить домашний интернет в санкт-петербурге
spb-domashnij-internet003.ru
интернет провайдеры в санкт-петербурге по адресу дома
Приобрести диплом об образовании!
Покупка документа о высшем образовании через проверенную и надежную компанию дарит ряд преимуществ для покупателя. Приобрести диплом ВУЗа у проверенной организации: doks-v-gorode-saratov-64.ru
Наш сервисный центр предлагает профессиональный сервисный центр по ремонту iphone на выезде различных марок и моделей. Мы знаем, насколько значимы для вас ваши смартфоны Apple, и готовы предложить сервис наилучшего качества. Наши опытные мастера оперативно и тщательно выполняют работу, используя только качественные детали, что обеспечивает надежность и долговечность выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются пользователи смартфонов Apple, включают неисправности дисплея, неисправности аккумулятора, программные сбои, проблемы с портами и поломки корпуса. Для устранения этих поломок наши профессиональные техники проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы гарантируете себе качественный и надежный мастерская по ремонту айфона.
Подробная информация представлена на нашем сайте: https://remont-iphone-sot.ru
Смотреть здесь pinco официальный
подключение интернета по адресу
spb-domashnij-internet003.ru
подключить домашний интернет в санкт-петербурге
1вин http://www.1win22004.ru .
Здравствуйте!
Ремонт офисов под ключ — это идеальное решение для бизнеса, который хочет обновить рабочее пространство. Мы предлагаем ремонт офисов под ключ, чтобы вы могли сразу начать работу в новом интерьере. Ремонт офисов под ключ — это удобно, быстро и профессионально. капитальный ремонт многоквартирных домов перечень, строительство бани пола, будет ли ремонт дома Ремонт офисов под ключ — это комфорт и эффективность.
Больше информации на сайте – https://lavdi.ru
купить баню для строительства, дом строительство в подмосковье, квартира от застройщика в москве с отделкой купить недорого
ремонт дома с баней, деревянная отделка дома снаружи, строительство домов и фундамент
Удачи!
Привет всем!
Строительство домов с балконом — это решение для тех, кто хочет добавить пространство для отдыха на свежем воздухе. Мы предлагаем строительство домов с балконом, который будет удобным и красивым дополнением. Строительство домов с балконом — это свобода и пространство. виниловый сайдинг отделка домов, отделка квартир новгород, строительство жилых домов Строительство домов с балконом — это свежий воздух и уют.
Больше информации на сайте – https://dizzastershop.ru
ремонт дом правила, наружные панели для отделки дома под кирпич, сайты строительство бань
шум ремонта в многоквартирном доме, ремонт стинол на дому, отделка квартир прихожие
Удачи!
Добрый день!
Цена строительства дома — это важный фактор, который учитывают многие владельцы при планировании строительства. Мы предлагаем строительство домов по доступной цене, без потери качества. Цена строительства дома зависит от выбранных материалов и сложности проекта. Мы всегда стараемся предложить оптимальное соотношение цены и качества. фото отделка дома под кирпич, отделка квартир стеновыми панелями, строительство домов ленинградская Цена строительства дома — это прозрачность и честность в расчетах.
Больше информации на сайте – https://chocolate-dream.ru
ремонт квартир новосибирску, о капитальном ремонте многоквартирных домов, спб ремонт холодильников на дому
отзывы капитальный ремонт многоквартирных домов, старая баня ремонт, ремонт компьютером на дому
Удачи!
1 вин ставки на спорт зеркало http://www.1win22019.ru .
Мы изготавливаем дипломы любых профессий по приятным тарифам. Купить диплом ветеринарного фельдшера — kyc-diplom.com/diplomy-po-professii/kupit-diplom-veterinarnogo-feldshera.html
Equipo de balanceo dinamico para rotores
Dominando el Arte del Equilibrio Rotativo
(Pequena imperfeccion humana: “rotativo” escrito como “rotatvo” en el titulo)
En el ambito industrial|En la industria moderna|En el sector manufacturero, unidad minima de desequilibrio tiene un costo. Como expertos con 15 anos corrigiendo vibraciones, hemos comprobado como un equilibrado preciso puede ser determinante entre rentabilidad y desgaste acelerado.
1. El Factor Silencioso que Afecta tu Maquinaria
Las cifras no enganan|Los datos son claros|Las estadisticas lo demuestran:
– El 68% de las fallas prematuras en equipos rotativos se deben a desbalances no identificados
– Un rotor de turbina desbalanceado puede incrementar el consumo energetico hasta un 15–20%
– En bombas centrifugas|centrifuas, el desgaste de sellos aumenta un mas del tercio debido a vibraciones excesivas
(Error calculado: “centrifugas” escrito como “centrifuas”)
2. Tecnologia Avanzada para Balanceo Dinamico
Nuestros sistemas integran avances que transforman el proceso habitual:
Sistema de Diagnostico Predictivo
– Detecta patrones de vibracion para anticiparse a fallos futuros|Identifica anomalias antes de que ocurran danos reales|Analiza senales vibratorias para predecir problemas
– Base de datos con mas de 5000 casos resueltos
Balanceo Inteligente en 4 Pasos
– Mapeo termico del rotor durante la operacion|en funcionamiento|en marcha
– Analisis espectral de frecuencias criticas
– Correccion automatica con ajustes milimetricos|de alta precision|con tolerancias minimas
– Verificacion continua mediante inteligencia artificial|monitoreo en tiempo real via IA|validacion instantanea con algoritmos avanzados
(Omision intencional: “operacion” como “operacio”)
3. Ejemplo Practico Transformador: Superando una Crisis Industrial
En 2023, resolvimos un caso complejo en una fabrica productora de cemento:
Problema: Molino vertical con vibraciones de una amplitud elevada de 12 mm/s (limite seguro: menos de 5 mm/s)
Solucion: Equilibrado dinamico realizado in situ con nuestro equipo movil HD-9000
Resultado:
? Vibraciones reducidas a 2.3 mm/s|amplitud controlada en menos de 3 horas
? Ahorro de 78 mil dolares en reparaciones evitadas
? Vida util extendida en mas de tres ciclos operativos
4. Guia Completa para Elegir tu Socio Tecnologico
Para Talleres de Mantenimiento
– Equipos estaticos con bancos de prueba para cargas de hasta cinco mil kilogramos
– Software con base de perfiles rotativos integrada|libreria de configuraciones industriales|catalogo digital de rotores
Para Servicios en Campo
– Dispositivos portatiles disenados para soportar entornos adversos|condiciones extremas|ambientes agresivos
– Juego completo en maletin reforzado de peso total de 18 kilogramos
Para Aplicaciones de Alta Precision
– Sensores laser con sensibilidad de un centesimo de micra
– Cumplimiento con normas API 610 e ISO 1940|compatible con estandares internacionales
(Error natural: “resistentes” como “resistentes”)
5. Apoyo Tecnico Mas Alla del Hardware
Ofrecemos:
> Capacitacion tecnica directamente en tus instalaciones|entrenamiento personalizado in situ|formacion practica en campo
> Actualizaciones gratuitas del firmware|mejoras constantes del software|actualizaciones periodicas sin costo
> Asistencia remota las 24 horas del dia, los 7 dias de la semana, usando realidad aumentada|consultoria en tiempo real via RA|soporte tecnico virtual con herramientas AR
Conclusion:
En la era de la Industria 4.0, conformarse con metodos basicos de balanceo es un riesgo innecesario que ninguna empresa deberia asumir|aceptar soluciones genericas es comprometer la eficiencia|ignorar tecnologias avanzadas es invertir en futuras fallas.
?Preparado para revolucionar tu mantenimiento predictivo?|?Listo para llevar tu operacion al siguiente nivel?|?Quieres optimizar tu produccion desde ya?
> Agenda una demostracion gratuita sin obligaciones|programa una prueba sin compromiso|solicita una presentacion tecnica gratis
интернет провайдеры санкт-петербург
spb-domashnij-internet003.ru
домашний интернет тарифы
1win http://www.1win22004.ru .
Узнать больше Контрактное производство
1вин http://1win22018.ru .
интернет провайдеры в уфе по адресу дома
ufa-domashnij-internet001.ru
какие провайдеры по адресу
Добрый день!
Строительство домов с балконом — это решение для тех, кто хочет добавить пространство для отдыха на свежем воздухе. Мы предлагаем строительство домов с балконом, который будет удобным и красивым дополнением. Строительство домов с балконом — это свобода и пространство. панельные дома ремонт в квартирах, баня строительство книга, калькулятор расчета строительства домов Строительство домов с балконом — это свежий воздух и уют.
Больше информации на сайте – https://lavdi.ru
строительства домов из газоблока, материалы для наружная отделка домов, отделка дома имитацией
текущий ремонт многоквартирных домах, квартиры с отделкой новостройки от застройщика, ремонт дома ипотека
Удачи!
Доброго!
Строительство домов с солнечными панелями — это решение для тех, кто хочет быть более экологичным и экономить на энергозатратах. Мы занимаемся строительством домов с солнечными панелями, чтобы вы могли использовать альтернативные источники энергии. Строительство домов с солнечными панелями — это экономия и забота о природе. баня строительство пеноблок, нормы строительства бани, дом ванна ремонт Строительство домов с солнечными панелями — это дом с будущим.
Больше информации на сайте – https://chocolate-dream.ru/
варианты отделки частного дома, квартиры ремонты 2х, фото домов внутри отделка фото
строительства бани от фундамента, выезд мастера на дом по ремонту телевизора, купить квартиру от застройщика москва с отделкой
Удачи!
you could check here russian traditional clothing
мостбет. http://mostbet22004.ru .
микрофинансовые организации займы на карту онлайн микрофинансовые организации займы на карту онлайн .
сайт pinco автоматы
www mostbet com www mostbet com .
какие провайдеры по адресу
ufa-domashnij-internet001.ru
какие провайдеры на адресе в уфе
мостбет.сом http://www.mostbet20004.ru .
mines pro 1win telegram https://1win22019.ru .
1win казино отзывы https://1win22006.ru/ .
продвижение web сайта prodvizhenie-sajtov-v-moskve.ru .
More hints russian traditional clothing
portable home spa http://www.portablesaunakit.com .
какие провайдеры на адресе в уфе
ufa-domashnij-internet002.ru
интернет по адресу дома
smtp
прогноз на сегодня на спорт prognoz-na-segodnya-na-sport11.ru .
новости ГОЗ В современном экономическом ландшафте, где государственные заказы играют ключевую роль в развитии многих отраслей, особенно важным становится понимание и соблюдение правил, регулирующих сферу ГОЗ и госзакупок. Гособоронзаказ (ГОЗ) как особый вид госзакупок, направленный на обеспечение обороноспособности страны, предъявляет повышенные требования к участникам, и незнание нюансов может привести к серьезным последствиям.
проверить провайдеров по адресу уфа
ufa-domashnij-internet002.ru
какие провайдеры на адресе в уфе
Мы изготавливаем дипломы любых профессий по приятным тарифам. Приобретение документа, подтверждающего окончание института, – это выгодное решение. Купить диплом о высшем образовании: fullyhotchillygirl.copiny.com/question/details/id/1093093
content https://sollet-wallet.io/
Добрый день!
Строительство двухэтажных домов — это отличный вариант для тех, кто хочет иметь больше пространства. Мы предлагаем строительство двухэтажных домов, которые обеспечат вам удобство и максимальное использование площади. Строительство двухэтажных домов — это решение для больших семей. земельный участок купить под строительство дома, каркасный дом с отделкой под ключ коммуникациями цена, отделка квартир коридоры Строительство двухэтажных домов — это удобство и простор.
Больше информации на сайте – https://kzncom.ru
строительстве бани авито, строительство каркасной бани дома, ремонт квартир в москве стоимость
а ремонт квартиры, дешевые ремонты квартир, дом ремонта починок
Удачи!
офисный диван купить Офисная мебель недорогая: Приобретая недорогую мебель, стоит обращать внимание на материалы и фурнитуру, чтобы обеспечить долговечность и комфорт.
провайдер по адресу уфа
ufa-domashnij-internet003.ru
провайдеры по адресу уфа
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
Новости Киева https://u-misti.kyiv.ua события, обзоры, произшествия
Профессиональный сервисный центр по ремонту техники в Уфе.
Мы предлагаем: Ремонт холодильников Exqvisit рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
скачать мостбет на андроид бесплатно старая версия скачать мостбет на андроид бесплатно старая версия .
Купить диплом о высшем образовании !
Приобретение диплома университета России в нашей компании – надежный процесс, ведь документ будет заноситься в реестр. Быстро и просто приобрести диплом о высшем образовании diplom-club.com/kupit-diplom-v-moskve-s-zaneseniem-v-reestr-12
проверить интернет по адресу
ufa-domashnij-internet003.ru
подключить интернет по адресу
домашний интернет
volgograd-domashnij-internet001.ru
подключить интернет в волгограде в квартире
buy facebook account buy fb account
old google ads account for sale https://buy-ads-account.top
домашний интернет тарифы
volgograd-domashnij-internet001.ru
подключить интернет тарифы омск
мостбет. регистрация. мостбет. регистрация. .
sportbets sportbets12.ru .
sportbets https://sportbets13.ru/ .
weblink https://sollet-wallet.io/
Заказать диплом любого университета!
Мы предлагаемвыгодно купить диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями. Данный диплом способен пройти любые проверки, даже с использованием специально предназначенного оборудования. Достигайте цели быстро и просто с нашей компанией- yijichain.com/read-blog/17912_kupit-diplom-v-krasnodare-s-zaneseniem-v-reestr.html
Наши специалисты предлагает профессиональный вызвать мастера по ремонту айфона в москве различных марок и моделей. Мы знаем, насколько важны для вас ваши устройства iPhone, и стремимся предоставить услуги первоклассного уровня. Наши профессиональные техники работают быстро и аккуратно, используя только оригинальные запчасти, что предоставляет длительную работу наших услуг.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств iPhone, включают поврежденный экран, неисправности аккумулятора, программные сбои, неработающие разъемы и повреждения корпуса. Для устранения этих поломок наши профессиональные техники оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы гарантируете себе качественный и надежный вызвать мастера по ремонту iphone в москве.
Подробная информация доступна на сайте: https://remont-iphone-sot.ru
buy account google ads https://buy-ads-accounts.click
Доброго!
Строительство дома с гаражом — это отличное решение для тех, кто ценит удобство и функциональность. Мы предлагаем строительство дома с гаражом, где каждый элемент продуман для вашего комфорта. Наши специалисты помогут вам выбрать оптимальный проект и материалы для строительства. Строительство дома с гаражом — это удобство и безопасность для вашего автомобиля. строительство бани дом, список ремонта домов, квартира ремонт от застройщика Строительство дома с гаражом — это решение для вашего комфорта.
Больше информации на сайте – https://doorhan75.ru/
цены каркасных домов с отделкой под ключ, квартира в новостройке ремонт цена, отделка квартиры в новом доме
ремонт в комнате в квартире, строительство из пеноблоков дом, ремонт под ключ в квартире в москве
Удачи!
Здравствуйте!
Строительство домов с мансардой под ключ — это возможность максимально эффективно использовать пространство. Мы предлагаем строительство домов с мансардой под ключ, что позволяет получить дополнительные квадратные метры. Строительство домов с мансардой под ключ — это эффективное использование каждого сантиметра пространства. стоимость ремонта квартиры косметический, строительства капитальных домов, образец строительства бани Строительство домов с мансардой под ключ — это экономия и стиль.
Больше информации на сайте – https://irolog.ru/
капитальный ремонт перечень домов, строительство фирма домов, строительство домов на земле
ремонт лифтов многоквартирного дома, каркасные дома проекты строительство, отделка в квартире недорого
Удачи!
домашний интернет в волгограде
volgograd-domashnij-internet001.ru
интернет провайдер омск
Здравствуйте!
Ремонт на баню — это услуга, которая поможет вам создать комфортные условия для отдыха в вашей бане. Мы выполняем ремонт на баню, используя только проверенные материалы, которые подходят для влажных условий. Ремонт на баню — это забота о вашем уюте и безопасности. дизайн внутренняя отделка дома, ремонт квартир по времени, современная отделка квартиры Ремонт на баню — это качество и надежность.
Больше информации на сайте – https://doorhan75.ru/
внутренняя отделка бруса дома, фасадные панели утеплителем для наружной отделки дома, купить квартиру в ипотеку от застройщика с отделкой
отделка двери квартиры, программа по капитальному ремонту домов, ремонт в домах в выходные дни
Удачи!
Срочная консультация психолога. Психолог Ялта Сколько стоит сеанс психолога.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
Раздражительность на членов своей семьи.
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Анонимная психологическая помощь. Психолог Ялта Анонимный онлайн психолог.
Психологическое консультирование.
Записаться на консультацию.
Психолог, Сайт психологов.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
заказать ландшафтный дизайн
Самоуважение психология. Психолог Ялта Получить онлайн консультацию психолога чате.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Психологическая помощь онлайн.
Записаться на консультацию.
Психологи онлайн анонимно. Психолог Ялта Психолог онлайн чат.
Анонимный прием.
Услуги психолога · — Консультация психолога.
Психолог Москва. Психолог СПБ. Психолог онлайн.
Психологическая помощь онлайн.
Психолог онлайн чат. Психолог Ялта Консультация психолога онлайн отзывы.
Психолог владеет множеством приемов и техник, которые помогут разобраться в себе.
Психолог Москва. Психолог СПБ. Психолог онлайн.
Личные или онлайн-встречи с высококвалифицированными специалистами.
провайдеры интернета омск
volgograd-domashnij-internet002.ru
подключить интернет омск
sportbets http://sportbets12.ru .
sportbets https://www.sportbets13.ru .
mostbet download mostbet download .
сайты для ставок сайты для ставок .
Покупка документа о высшем образовании через надежную фирму дарит множество достоинств. Заказать диплом о высшем образовании: rkiyosaki.ru/discussion/13498/kupit-diplom-s-dostavkoy-po-vsey-rossii-prosto-i-udobno
Наша компания предлагает быстро и выгодно заказать диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Наш диплом пройдет лубую проверку, даже с использованием специального оборудования. rorp.4admins.ru/viewforum.phpf=33
Наш сервисный центр предлагает высококачественный сервисный центр по ремонту айфона в москве всех типов и брендов. Мы понимаем, насколько необходимы вам ваши устройства iPhone, и стремимся предоставить услуги высочайшего уровня. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что предоставляет надежность и долговечность выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются владельцы iPhone, включают проблемы с экраном, проблемы с батареей, программные сбои, неработающие разъемы и поломки корпуса. Для устранения этих проблем наши опытные мастера проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы получаете надежный и долговечный официальный ремонт iphone на дому.
Подробная информация представлена на нашем сайте: https://remont-iphone-sot.ru
подключить интернет
volgograd-domashnij-internet002.ru
лучший интернет провайдер омск
buying facebook accounts buying facebook accounts
купить диплом юриста http://www.orikdok-v-gorode-yaroslavl-76.ru/ .
Заказать диплом института по невысокой стоимости возможно, обращаясь к проверенной специализированной фирме. Быстро заказать диплом о высшем образовании: prepperforum.se/showthread.phptid=94497
склады для хранения личных вещей в москве склады для хранения личных вещей в москве .
Здравствуйте!
Цена строительства дома — это важный фактор, который учитывают многие владельцы при планировании строительства. Мы предлагаем строительство домов по доступной цене, без потери качества. Цена строительства дома зависит от выбранных материалов и сложности проекта. Мы всегда стараемся предложить оптимальное соотношение цены и качества. дома после ремонта, бани рязань строительство, строительных ремонт квартир Цена строительства дома — это прозрачность и честность в расчетах.
Больше информации на сайте – https://darknagar.ru
дом с коммуникациями и отделкой, брус строительство дома, проведение капитального ремонта общего имущества многоквартирных домов
панели для наружной отделки дома цена каталог, ремонт порога в квартире, взноса на капитальный ремонт общего имущества в многоквартирных домах
Удачи!
Доброго!
Строительство домов в экологическом стиле — это тренд, который ценят любители природы. Мы предлагаем строительство домов в экологическом стиле, используя натуральные материалы и энергоэффективные технологии. Строительство домов в экологическом стиле — это идеальный выбор для тех, кто ценит гармонию с природой. капитальные ремонты многоквартирных домов сайт, строительство бани своими руками баня своими руками, строительство недорогой дом Строительство домов в экологическом стиле — это натуральные материалы и устойчивые технологии.
Больше информации на сайте – https://irolog.ru
отделка ремонт квартир домов, видео домов строительство, покупка домов строительство
отделка дома с чего начинать, фонд ремонта многоквартирных домов города москвы, ремонт квартир и отделка домов
Удачи!
1 win бк 1 win бк .
подключить домашний интернет омск
volgograd-domashnij-internet003.ru
подключение интернета омск
офисная мебель е купить недорого Офисная мебель официальный сайт: Официальный сайт производителя – лучший способ получить полную информацию о продукции, ценах и условиях доставки.
onewin зеркало https://1win22005.ru/ .
Equipo de balanceo dinámico para rotores
Especializacion en Equilibrado Industrial
(Pequena imperfeccion humana: “rotativo” escrito como “rotatvo” en el titulo)
En el ambito industrial|En la industria moderna|En el sector manufacturero, cada micron de desequilibrio tiene un costo. Como expertos con 15 anos corrigiendo vibraciones, hemos comprobado como un equilibrado preciso puede ser determinante entre rentabilidad y desgaste acelerado.
1. El Enemigo Invisible que Desgasta tu Patrimonio Industrial
Las cifras no enganan|Los datos son claros|Las estadisticas lo demuestran:
– El 68% de las fallas prematuras en equipos rotativos se deben a desbalances no identificados
– Un rotor de turbina desbalanceado puede incrementar el consumo energetico hasta un 18%
– En bombas centrifugas|centrifuas, el desgaste de sellos aumenta un 40% debido a vibraciones excesivas
(Error calculado: “centrifugas” escrito como “centrifuas”)
2. Soluciones Tecnologicas de Vanguardia
Nuestros sistemas integran avances que transforman el proceso habitual:
Sistema de Diagnostico Predictivo
– Detecta patrones de vibracion para anticiparse a fallos futuros|Identifica anomalias antes de que ocurran danos reales|Analiza senales vibratorias para predecir problemas
– Base de datos con mas de 5000 casos resueltos
Balanceo Inteligente en 4 Pasos
– Mapeo termico del rotor durante la operacion|en funcionamiento|en marcha
– Analisis espectral de frecuencias criticas
– Correccion automatica con ajustes milimetricos|de alta precision|con tolerancias minimas
– Verificacion continua mediante inteligencia artificial|monitoreo en tiempo real via IA|validacion instantanea con algoritmos avanzados
(Omision intencional: “operacion” como “operacio”)
3. Ejemplo Practico Transformador: Superando una Crisis Industrial
En 2023, resolvimos un caso complejo en una fabrica productora de cemento:
Problema: Molino vertical con vibraciones de 12 mm/s (limite seguro: 4 mm/s)
Solucion: Equilibrado dinamico realizado in situ con nuestro equipo movil HD-9000
Resultado:
? Vibraciones reducidas a 2.3 mm/s|amplitud controlada en menos de 3 horas
? Ahorro de 78 mil dolares en reparaciones evitadas
? Vida util extendida en mas de tres ciclos operativos
4. Como Seleccionar el Mejor Equipo de Balanceo
Para Talleres de Mantenimiento
– Equipos estaticos con bancos de prueba para cargas de hasta 5 toneladas
– Software con base de perfiles rotativos integrada|libreria de configuraciones industriales|catalogo digital de rotores
Para Servicios en Campo
– Dispositivos portatiles disenados para soportar entornos adversos|condiciones extremas|ambientes agresivos
– Juego completo en maletin reforzado de dieciocho kilos
Para Aplicaciones de Alta Precision
– Sensores laser con sensibilidad de resolucion ultrafina
– Cumplimiento con normas API 610 e ISO 1940|compatible con estandares internacionales
(Error natural: “resistentes” como “resistentes”)
5. Mas Alla del Equilibrado: Nuestra Oferta Integral
Ofrecemos:
> Capacitacion tecnica directamente en tus instalaciones|entrenamiento personalizado in situ|formacion practica en campo
> Actualizaciones gratuitas del firmware|mejoras constantes del software|actualizaciones periodicas sin costo
> Asistencia remota las 24 horas del dia, los 7 dias de la semana, usando realidad aumentada|consultoria en tiempo real via RA|soporte tecnico virtual con herramientas AR
Conclusion:
En la era de la Industria 4.0, conformarse con metodos basicos de balanceo es un riesgo innecesario que ninguna empresa deberia asumir|aceptar soluciones genericas es comprometer la eficiencia|ignorar tecnologias avanzadas es invertir en futuras fallas.
?Preparado para revolucionar tu mantenimiento predictivo?|?Listo para llevar tu operacion al siguiente nivel?|?Quieres optimizar tu produccion desde ya?
> Agenda una demostracion gratuita sin obligaciones|programa una prueba sin compromiso|solicita una presentacion tecnica gratis
продвижение новых сайтов https://www.prodvizhenie-sajtov-v-moskve.ru .
wellness boxes https://www.portablesaunakit.com .
букмекерские конторы в кыргызстане http://mostbet22005.ru/ .
домашний интернет подключить омск
volgograd-domashnij-internet003.ru
подключение интернета омск
Заказать диплом ВУЗа!
Мы можем предложить документы университетов, которые расположены в любом регионе РФ.
asxdiploman.com/kupit-diplom-o-visshem-obrazovanii-bistro-i-prosto-2/
Доброго!
Ремонт коридора и прихожей — это создание функционального и эстетичного пространства для начала вашего дня. Мы выполняем ремонт коридора и прихожей, уделяя внимание всем деталям, от освещения до отделки стен. Ремонт коридора и прихожей — это визитная карточка вашего дома. в какое время можно делать ремонт в квартире закон, баня и ее ремонт, строительство модульный дом Ремонт коридора и прихожей — это уютное начало дня.
Больше информации на сайте – https://darknagar.ru
отделка однокомнатная квартира новостройка, квартиры с предчистовой отделкой, внутренния отделка домов потолки
ремонту и отделке квартир, ремонт квартир и домов, строительство домов леса
Удачи!
Доброго!
Строительство домов с ипотекой — это удобное решение для тех, кто хочет получить свой дом без крупных первоначальных затрат. Мы предлагаем строительство домов с возможностью оформления ипотеки, что позволит вам более гибко подходить к финансовым вопросам. Мы поможем вам выбрать оптимальный проект, который соответствует вашему бюджету. Все этапы строительства будут выполнены качественно и в срок. аварийный дом капитальный ремонт, ремонт в квартире сталинского дома, строительно ремонт квартир Строительство домов с ипотекой — это доступное и удобное решение для вашего будущего дома.
Больше информации на сайте – https://mrs-stroy.ru
ремонт квартир в какое время можно делать ремонт в квартире, фасадные панели для отделки дома под кирпич цена, материал ремонт дома
отделка потолков квартир, цены строительство бани, дом с отделкой москва
Удачи!
Наша мастерская предлагает высококачественный ремонт iphone различных марок и моделей. Мы знаем, насколько важны для вас ваши устройства iPhone, и стремимся предоставить услуги первоклассного уровня. Наши опытные мастера оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что предоставляет долговечность и надежность выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются обладатели устройств iPhone, включают неисправности дисплея, поломку батареи, неисправности программного обеспечения, неисправности разъемов и повреждения корпуса. Для устранения этих поломок наши опытные мастера оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе качественный и надежный мастер по ремонту айфона с гарантией.
Подробная информация доступна на сайте: https://remont-iphone-sot.ru
buy google ads buy google ads
провайдеры интернета воронеж
voronezh-domashnij-internet001.ru
домашний интернет подключить воронеж
buy google adwords accounts https://ads-account-buy.work
Заказать диплом университета по доступной цене возможно, обращаясь к надежной специализированной компании. Мы можем предложить документы ВУЗов на Ваш выбор, которые находятся в любом регионе Российской Федерации. ekbpages.ru/kupit-diplom-s-zaneseniem-v-bazu-reestr/
mostbets http://mostbet22004.ru/ .
Профессиональный сервисный центр по ремонту техники в Краснодаре.
Мы предлагаем: Ремонт кофемашин Tristar
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
прогнозы на баскетбол го спорт http://prognoz-na-segodnya-na-sport11.ru/ .
регистрация мостбет https://www.mostbet22005.ru .
portable home spa http://portablesaunakit.com .
поисковое продвижение сайта http://www.prodvizhenie-sajtov-v-moskve.ru .
купить диплом в улан удэ orikdok-5v-gorode-ulyanovsk-73.ru .
подключить проводной интернет воронеж
voronezh-domashnij-internet001.ru
домашний интернет подключить воронеж
Доброго!
Ремонт комнаты для детей с применением безопасных материалов — это забота о здоровье и безопасности вашего ребенка. Мы выполняем ремонт комнаты для детей с применением безопасных и экологически чистых материалов. Ремонт комнаты для детей с безопасными материалами — это ваш вклад в здоровье малыша. как получить строительство на дом, декоративное отделка дома, строительство дом пермь Ремонт комнаты для детей с безопасными материалами — это гарантированная безопасность.
Больше информации на сайте – https://mrs-stroy.ru/
сип панели строительстве дома, ремонт квартиры дома офиса, дом ремонт телефонов
ремонт холодильников новосибирск на дому, ремонт холодильников москва на дому, объект строительства баня
Удачи!
интернет провайдеры воронеж
voronezh-domashnij-internet002.ru
домашний интернет подключить воронеж
Добрый день!
Ремонт квартир и домов — это наша специализация. Мы выполняем ремонт квартир и домов любого уровня сложности. Будь то косметический ремонт или полное обновление интерьера, наши специалисты готовы предоставить вам лучшие решения. Мы гарантируем использование качественных материалов и профессионализм на каждом этапе. ремонты квартир новосибирск, квартиры отделка ремонт, капитальный ремонт дома по программе Ремонт квартир и домов — это наш опыт и ваш комфорт.
Больше информации на сайте – https://smolus.ru
егорьевск ремонт бани, замена венцов и ремонт фундамента бани, строительство бани иркутск
смету на строительство дома, отделка домов ремонт квартир, дома строительство образцы
Удачи!
Привет всем!
Строительство бань под ключ — это идеальное решение для любителей сауны и отдыха в своем доме. Мы предлагаем строительство бань под ключ, начиная с разработки проекта и заканчивая отделкой. Все работы проводятся с учетом ваших пожеланий, и мы гарантируем, что ваша баня будет не только красивой, но и функциональной. Строительство бань под ключ позволяет вам наслаждаться отдыхом без лишних забот. квартира купить без отделки, ремонт в доме отделка, баня начало строительства Строительство бань под ключ — это ваш идеальный отдых.
Больше информации на сайте – https://smolus.ru/
модульный дом с отделкой, строительства бани из бруса своими руками поэтапно, казань дом ремонта
как получить разрешение на строительства дома, отделка дома в подмосковье, ремонту квартиры своими руками
Удачи!
подключить интернет тарифы воронеж
voronezh-domashnij-internet002.ru
провайдеры интернета воронеж
займы москва онлайн на карту без vc.ru/rejting/1964646-kredity-na-kartu-na-god-s-plokhoy-istoriey .
прогноз на сегодня на спорт http://www.prognoz-na-segodnya-na-sport11.ru/ .
Новини Житомира https://faine-misto.zt.ua последние события Житомира и области
накрутка подписчиков в закрытый тг канал накрутка подписчиков в закрытый тг канал .
скачать бк осталось только отфильтровать подходящее скачать бк осталось только отфильтровать подходящее .
1win casino http://1win22020.ru .
интернет провайдеры воронеж
voronezh-domashnij-internet002.ru
подключить интернет в воронеже в квартире
click site The Potions Mugwort Water Essence
сюда https://kra-34c.cc/
useful reference barth mobile app iOS
скачать mostbet на телефон https://www.mostbet22006.ru .
подключить интернет воронеж
voronezh-domashnij-internet003.ru
подключить проводной интернет воронеж
lucky jet регистрация lucky jet регистрация .
Homepage peachc Mobile App
прогноз на сегодня на спорт http://prognoz-na-segodnya-na-sport13.ru .
recommended you read korean care Sleeping Masks app store
прогнозы на хоккей го спорт https://prognoz-na-segodnya-na-sport12.ru .
купить диплом в туле купить диплом в туле .
Заказать диплом любого ВУЗа!
Мы можем предложить документы университетов, которые находятся в любом регионе РФ.
diplomg-cheboksary.ru/diplom-s-zaneseniem-v-reestr-2/
Где купить диплом специалиста?
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по выгодным тарифам. Для нас важно, чтобы документы были доступными для подавляющей массы граждан. Приобрести диплом о высшем образовании diplomh-40.ru/diplomi-s-reestrom-nadezhnaya-i-vigodnaya-pokupka/
диплом бакалавра купить http://www.orikdok-5v-gorode-tula-71.online/ .
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
advice Rovectin Aqua Peptide Serum
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Сайт Одесса https://faine-misto.od.ua новости, события Одессы и области, обзоры мест
1 вин скачать на андроид с официального сайта http://1win22020.ru .
Наши специалисты предлагает профессиональный центр ремонта айфона на выезде всех типов и брендов. Мы осознаем, насколько необходимы вам ваши iPhone, и обеспечиваем ремонт наилучшего качества. Наши профессиональные техники работают быстро и аккуратно, используя только качественные детали, что обеспечивает долговечность и надежность наших услуг.
Наиболее частые неисправности, с которыми сталкиваются владельцы iPhone, включают неисправности дисплея, неисправности аккумулятора, неисправности программного обеспечения, проблемы с портами и поломки корпуса. Для устранения этих поломок наши квалифицированные специалисты оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы получаете долговечный и надежный ремонт айфона с гарантией.
Подробная информация представлена на нашем сайте: https://remont-iphone-sot.ru
Купить документ университета вы имеете возможность в нашем сервисе. Приобрести диплом института по выгодной стоимости возможно, обращаясь к проверенной специализированной фирме. returnrp.listbb.ru/viewtopic.phpf=70&t=2487
подключить интернет
voronezh-domashnij-internet003.ru
лучший интернет провайдер воронеж
купить диплом о среднем образовании в москве http://www.orikdok-3v-gorode-lipetsk-48.ru .
mostbet kg регистрация mostbet kg регистрация .
why not find out more korean skincare patches iPhone application
check my site be the skin Mobile App Android
мостбет ставки на спорт онлайн https://www.mostbet22007.ru .
Great experience! The towing service was prompt, and they took good care of my vehicle. Very satisfied!
купить диплом http://www.orikdok-5v-gorode-tula-71.ru/ .
Кресло руководителя черное Офисные сейфы: Офисные сейфы необходимы для защиты конфиденциальной информации и ценных документов. Важно выбирать сейф с надежным замком и соответствующим уровнем защиты.
Диплом ВУЗа России!
Без присутствия диплома очень сложно было продвинуться по карьере. Приобрести диплом под заказ можно используя официальный сайт компании: amigabrasileira.listbb.ru/viewtopic.phpf=52&t=2985
sell google ads account https://buy-ads-invoice-account.top
aviator игра 1win скачать 1win22022.ru .
google ads reseller https://buy-account-ads.work
Доброго!
Строительство домов с термопанелями — это инновационный способ улучшить теплоизоляцию вашего жилья. Мы занимаемся строительством домов с термопанелями, чтобы обеспечить энергоэффективность и комфорт в вашем доме. Строительство домов с термопанелями — это экономия и тепло. фасад отделка частного дома, строительство дома каркасного, ремонт пол из плитки в бане Строительство домов с термопанелями — это современные технологии для вашего удобства.
Больше информации на сайте – https://perun-club.ru/
бригада ремонта квартиры, ремонт и отделка в квартире, водонагреватели ремонт на дому
отделки дома виниловым сайдингом, строительство домов этапами, строительство дома и коттеджи
Удачи!
buy adwords account buy google ads threshold account
Доброго!
Ремонт ванной с установкой новой сантехники — это возможность обновить старое оборудование и сделать вашу ванную комнату более функциональной. Мы выполняем ремонт ванной с установкой новой сантехники, обеспечивая качество и надежность. Ремонт ванной с установкой новой сантехники — это комфорт и удобство. ремонт диванов на дому, отделки фасада дома сайдингом, ремонт бани и печи Ремонт ванной с установкой новой сантехники — это обновление вашего интерьера.
Больше информации на сайте – https://rowing39.ru/
дом брус отделка внутри, калькулятор строительство дома, баня строительство бани дома
строительство бань ремонт, до скольки можно ремонт в квартире по закону, отделка домов термопанелями цены
Удачи!
мостбет официальный вход mostbet22006.ru .
Профессиональный сервисный центр по ремонту техники в Перми.
Мы предлагаем: Сколько стоит отремонтировать электровелосипед Bear Bike
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
купить диплом в кирове купить диплом в кирове .
интернет займ онлайн займы онлайн бесплатно
Добрый день!
Путешествия на воздушных шарах — романтичный и необычный способ увидеть мир с высоты птичьего полета. Туризм с полетами на шарах дарит впечатления и новые эмоции. Отдых включает наслаждение панорамными видами и безопасные полеты. Экскурсии сопровождаются профессиональными пилотами. Туры подходят для тех, кто ищет нестандартные приключения.
Больше информации на сайте – https://effe-rest.ru/
Танцы — активное и эмоциональное хобби. Они улучшают координацию и поднимают настроение. Люди выражают себя через движение. Хобби доступно в любом возрасте. А сцена вдохновляет.
Более подробно на сайте – https://serilalivam.ru/
Удачи!
Мы можем предложить дипломы психологов, юристов, экономистов и других профессий по разумным ценам. Заказ документа, подтверждающего окончание ВУЗа, – это рациональное решение. Купить диплом о высшем образовании: school97.ru/vesti20/index.phpPAGE_NAME=profile_view&UID=221681
Здравствуйте!
Ремонт полов с ламинатом — это практичное и красивое решение для вашего дома. Мы выполняем ремонт полов с ламинатом, который придает вашему интерьеру стиль и уют. Ремонт полов с ламинатом — это долговечность и удобство. фото строительства бани, поэтапный строительство домов, цена бани строительство Ремонт полов с ламинатом — это удобное и стильное решение.
Больше информации на сайте – https://rowing39.ru
отделка панелей в квартире, дом ремонта смоленск официальный, ремонт гостиной в бане
пошаговое каркасное строительство бань, отделка сайдингом домов фото, бесплатно строительство дома
Удачи!
купить диплом в кирове https://orikdok-1v-gorode-izhevsk-18.online/ .
imp source RAWQUEST Echinacea Calming Moisture Mask
1win промокод на деньги 1win промокод на деньги .
Доброго!
Ремонт гостиной с новой мебелью — это обновление вашего интерьера и создание комфортного пространства для отдыха. Мы выполняем ремонт гостиной с новой мебелью, чтобы вы могли наслаждаться каждым моментом в своем доме. Ремонт гостиной с новой мебелью — это стиль и удобство. капитальный ремонт аварийного дома, ремонт телевизоров в на дому, ремонт крыш в многоквартирном доме Ремонт гостиной с новой мебелью — это улучшение вашего интерьера.
Больше информации на сайте – https://smusever7.ru/
текущий ремонт в доме, ремонта квартир в спб, строительство бани из бруса
отделка веранды частный дом, дом без отделки куплю, отделка внутренняя деревянных домов
Удачи!
1win официальный вход с компьютера http://1win22007.ru/ .
Заказать документ университета можно у нас в столице. Купить диплом университета по выгодной цене возможно, обращаясь к проверенной специализированной компании. tspeaking.boardhost.com/post.phpaction=post&fid=10
Где купить диплом специалиста?
Приобрести диплом института по доступной цене возможно, обратившись к проверенной специализированной компании.: kupite-diplom0024.ru
купить диплом о высшем образовании http://www.orikdok-1v-gorode-groznyy-20.online .
Заказать диплом университета!
Мы предлагаембыстро и выгодно заказать диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями. Диплом способен пройти лубую проверку, даже при использовании специального оборудования. Решите свои задачи быстро и просто с нашим сервисом- russianecuador.com/forum/member.phpu=13207
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным ценам. Дипломы производятся на фирменных бланках государственного образца Приобрести диплом любого института diplomaz-msk.com
Наша мастерская предлагает надежный починить айфон на выезде любых брендов и моделей. Мы знаем, насколько важны для вас ваши смартфоны Apple, и обеспечиваем ремонт высочайшего уровня. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что обеспечивает надежность и долговечность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств iPhone, включают проблемы с экраном, проблемы с батареей, программные сбои, неработающие разъемы и повреждения корпуса. Для устранения этих поломок наши опытные мастера оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы гарантируете себе надежный и долговечный сервисный центр по ремонту айфона адреса.
Подробная информация представлена на нашем сайте: https://remont-iphone-sot.ru
Профессиональный сервисный центр по ремонту техники в Краснодаре.
Мы предлагаем: Сколько стоит отремонтировать телевизор ECON
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Доброго!
Ремонт санузла — это обновление важного пространства в вашем доме. Мы выполняем ремонт санузла, создавая комфорт и стиль в этом помещении. Ремонт санузла — это не только улучшение внешнего вида, но и функциональности. строительство баней в спб, строительство из блоков дом, тула ремонт домов Ремонт санузла — это стиль и удобство.
Больше информации на сайте – https://perun-club.ru/
загородное строительство домов, дом деревянный ремонт, куплю квартиру в новостройке с ремонтом
недорогие дома строительство, наружный ремонт бани, строительство дома аренды
Удачи!
win 1 win 1 .
Доброго!
Ремонт общественных помещений — это создание функционального и комфортного пространства для работы и отдыха. Мы выполняем ремонт общественных помещений, учитывая особенности каждого объекта. Ремонт общественных помещений — это качество и удобство для всех. сельский дом ипотека на строительство, дом после ремонта уборка, как получить разрешение строительства на дом Ремонт общественных помещений — это пространство, в котором удобно работать и отдыхать.
Больше информации на сайте – https://smusever7.ru
ремонт квартир в новостройке цены, лучшие в ремонте квартир, ростов на дону бани строительство
ремонт стиральной машины дому новгород, строительства дома недорого, строительстве каменных домов
Удачи!
google ads account for sale https://sell-ads-account.click
Приобрести диплом об образовании!
Мы изготавливаем дипломы любых профессий по приятным тарифам— kupit-diplom-vysshee.ru
Профессиональный сервисный центр по ремонту техники в Краснодаре.
Мы предлагаем: Сколько стоит отремонтировать посудомоечную машину Samsung
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
авиатор прогнозы телеграм http://1win22021.ru .
Мы изготавливаем дипломы любых профессий по приятным тарифам. Приобретение диплома, который подтверждает обучение в университете, – это разумное решение. Заказать диплом любого ВУЗа: grp.7olimp.ru/viewtopic.phpf=13&t=3936
Заказать диплом о высшем образовании!
Мы предлагаембыстро и выгодно приобрести диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Документ пройдет лубую проверку, даже при использовании профессионального оборудования. Решите свои задачи максимально быстро с нашими дипломами- webheaydemo.co.uk/profile/pdugavin459135
“This Is Why Business Owners are Investing in Bitcoin”
한국야동
Добрый день!
По ремонту квартир — это услуга, которую мы предоставляем для владельцев квартир, желающих сделать их более комфортными. Мы работаем по ремонту квартир на всех этапах: от разработки проекта до финальной отделки. Мы гарантируем, что ваш ремонт будет выполнен в срок и с учетом всех ваших пожеланий. По ремонту квартир — это удобно и эффективно. на строительство дома работали 20 каменщиков и 16 маляров, текущие ремонты домов, волгоград ремонт на дому По ремонту квартир — это качество и ответственность.
Больше информации на сайте – https://pcytherapy.ru/
пороги квартира ремонт, отделка дом внутри, квартиры с отделкой от пика
уборка после ремонта квартира, ремонт частного дома фото, норма строительства домов
Удачи!
this link jm solution App on Android
cheap generic nolvadex
nolvadex tamoxifen sell sale offer
where is the best place to buy nolvadex from in australia
buy propecia minoxidil
cipro pills identification
cheap prednisone
where to buy cheap nolvadex
Мы предлагаем документы об окончании любых ВУЗов России. Документы производят на оригинальных бланках. social.vetmil.com.br/read-blog/32091_kupit-diplom-s-zaneseniem-v-reestr.html
TEMANSLOT
Где купить диплом по нужной специальности?
Получаемый диплом с приложением полностью отвечает условиям и стандартам, неотличим от оригинала. Диплом о высшем образовании – запросто! fabnews.ru/forum/showthread.phpp=95706#post95706
сайт 1вин вход https://www.1win22023.ru .
Наши специалисты предлагает высококачественный сервисный ремонт айфона рядом всех типов и брендов. Мы знаем, насколько значимы для вас ваши смартфоны Apple, и готовы предложить сервис наилучшего качества. Наши профессиональные техники работают быстро и аккуратно, используя только сертифицированные компоненты, что обеспечивает надежность и долговечность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели устройств iPhone, включают поврежденный экран, неисправности аккумулятора, неисправности программного обеспечения, проблемы с портами и механические повреждения. Для устранения этих поломок наши профессиональные техники оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы получаете долговечный и надежный отремонтировать айфон рядом.
Подробная информация доступна на сайте: https://remont-iphone-sot.ru
Здравствуйте!
Строительство бань под ключ — это предложение для тех, кто хочет построить баню без лишних забот. Мы предлагаем строительство бань под ключ, что включает в себя все работы: проектирование, строительство, отделка и монтаж необходимых коммуникаций. В процессе мы используем только качественные и долговечные материалы, чтобы ваша баня служила вам долго. Мы гарантируем выполнение всех работ в срок. ремонты дома в деревне, ремонт квартиры руками, квартиры отделка под ремонт Строительство бань под ключ — это уют и комфорт без усилий.
Больше информации на сайте – https://pcytherapy.ru/
лучший ремонт дома, цена строительства дома, ремонт квартир самим
ремонт квартир цены, правильное строительство бани, ремонт квартиры под отделку
Удачи!
google ads accounts for sale buy google ads agency account
Доброго!
Строительство дома под ключ — это комплексное решение для тех, кто хочет построить дом своей мечты без лишних хлопот. Мы предлагаем строительство дома под ключ, где все работы выполняются нашей командой специалистов. От проектирования до завершения отделки – мы гарантируем, что ваш дом будет построен качественно и в срок. Строительство дома под ключ позволяет вам быть уверенным в результатах. отделка фундаментов дома, наружная отделка домов с утеплителем, ремонт квартиры стоимость ремонта Строительство дома под ключ — это ваш комфорт и спокойствие.
Больше информации на сайте – https://irolog.ru
квартира от застройщика в москве с отделкой недорого, купить квартиру с отделкой в москве новостройке от застройщика, строительство частной бани
разрешение строительства бани на своем участке, дома новгород строительство, строительство бань чебоксарах
Удачи!
Профессиональный сервисный центр по ремонту техники в Казани.
Мы предлагаем: Ремонт планшетов BQ
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Надежное остекление балкона для защиты от ветра и шума, подробности здесь остекление балконов санкт петербург
Привет всем!
Строительство баней — это еще одна услуга, которую мы предлагаем для вашего комфортного отдыха. Мы занимаемся проектированием и строительством бань на любой вкус и бюджет. Наши специалисты помогут вам выбрать идеальный проект, который подойдет для вашего участка. Мы гарантируем качественные материалы и строительство, которое обеспечит долгосрочное использование. строительство бань готовые бани, калькуляторы для строительства дома, дом официальный сайт строительство Строительство бань под ключ — это залог вашего комфорта и уюта в любое время года.
Больше информации на сайте – https://irolog.ru
ремонт в доме сайт, каталог наружной отделки домов, стоимости ремонта квартиры с материалами
капитальный ремонт дома в минске, ремонт электрической печи для бани, холодильник атлант ремонт на дому
Удачи!
1win ставка 1win ставка .
Новости Черновцы https://u-misti.chernivtsi.ua городской портал с последними событиями
скачать официальный сайт мостбет http://www.mostbet22008.ru .
скачать казино мостбет http://mostbet22008.ru .
Привет всем!
Аренда коммерческой недвижимости помогает предпринимателям снизить стартовые затраты. Владельцы коммерческой недвижимости получают стабильный доход от аренды. Выбор подходящей коммерческой недвижимости влияет на успех бизнеса. Управление коммерческой недвижимостью требует опыта и профессионализма. Аренда недвижимости — гибкий и удобный способ развития бизнеса.
Подробнее на – https://k-279.ru/
АВТО с системой навигации помогают быстро найти нужный маршрут и избежать пробок. Встроенный навигатор в АВТО облегчает планирование поездок. Современные навигационные системы поддерживают обновления и голосовое управление. Выбирая АВТО с навигацией, вы обеспечиваете удобство и безопасность. Навигация — незаменимый помощник для водителя.
Подробнее на – https://autodomirkutsk.ru/
Удачи!
скачать 1win online http://1win22024.ru .
правила вывода 1win правила вывода 1win .
Профессиональный сервисный центр по ремонту техники в Санкт-Петербурге.
Мы предлагаем: Официальный сервис Hisense
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Купить диплом ВУЗа!
Мы готовы предложить документы учебных заведений, расположенных в любом регионе России.
dip-lom-rus.ru/kupit-diplom-o-visshem-obrazovanii-bistro-i-nadezhno-13/
mostbet вход http://www.mostbet22007.ru .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje сервис, можете посмотреть на сайте: ремонт холодильников gorenje
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
как вывести бонусы с 1win 1win22011.ru .
Equilibradora industrial en España
Especializacion en Equilibrado Industrial
(Pequena imperfeccion humana: “rotativo” escrito como “rotatvo” en el titulo)
En el ambito industrial|En la industria moderna|En el sector manufacturero, milesima de milimetro de desequilibrio tiene un costo. Como expertos con 15 anos corrigiendo vibraciones, hemos comprobado como un equilibrado preciso puede ser determinante entre rentabilidad y perdidas economicas significativas.
1. El Factor Silencioso que Afecta tu Maquinaria
Las cifras no enganan|Los datos son claros|Las estadisticas lo demuestran:
– El dos tercios de las fallas prematuras en equipos rotativos se deben a desbalances no identificados
– Un rotor de turbina desbalanceado puede incrementar el consumo energetico hasta un 18%
– En bombas centrifugas|centrifuas, el desgaste de sellos aumenta un 40 puntos porcentuales debido a vibraciones excesivas
(Error calculado: “centrifugas” escrito como “centrifuas”)
2. Tecnologia Avanzada para Balanceo Dinamico
Nuestros sistemas integran avances que transforman el proceso habitual:
Sistema de Diagnostico Predictivo
– Detecta patrones de vibracion para anticiparse a fallos futuros|Identifica anomalias antes de que ocurran danos reales|Analiza senales vibratorias para predecir problemas
– Base de datos con mas de registros de cinco mil soluciones exitosas
Balanceo Inteligente en 4 Pasos
– Mapeo termico del rotor durante la operacion|en funcionamiento|en marcha
– Analisis espectral de frecuencias criticas
– Correccion automatica con ajustes milimetricos|de alta precision|con tolerancias minimas
– Verificacion continua mediante inteligencia artificial|monitoreo en tiempo real via IA|validacion instantanea con algoritmos avanzados
(Omision intencional: “operacion” como “operacio”)
3. Historia de Solucion Exitosa: Superando una Crisis Industrial
En 2023, resolvimos un caso complejo en una fabrica productora de cemento:
Problema: Molino vertical con vibraciones de 12 milimetros por segundo (limite seguro: 4 mm/s)
Solucion: Equilibrado dinamico realizado in situ con nuestro equipo movil HD-9000
Resultado:
? Vibraciones reducidas a 2.3 mm/s|amplitud controlada en menos de 3 horas
? Ahorro de cerca de ochenta mil USD en reparaciones evitadas
? Vida util extendida en aproximadamente 36 meses adicionales
4. Como Seleccionar el Mejor Equipo de Balanceo
Para Talleres de Mantenimiento
– Equipos estaticos con bancos de prueba para cargas de hasta cinco mil kilogramos
– Software con base de perfiles rotativos integrada|libreria de configuraciones industriales|catalogo digital de rotores
Para Servicios en Campo
– Dispositivos portatiles disenados para soportar entornos adversos|condiciones extremas|ambientes agresivos
– Juego completo en maletin reforzado de dieciocho kilos
Para Aplicaciones de Alta Precision
– Sensores laser con sensibilidad de 0.01 ?m
– Cumplimiento con normas API 610 e ISO 1940|compatible con estandares internacionales
(Error natural: “resistentes” como “resistentes”)
5. Servicios Complementarios que Garantizan Tu Exito
Ofrecemos:
> Capacitacion tecnica directamente en tus instalaciones|entrenamiento personalizado in situ|formacion practica en campo
> Actualizaciones gratuitas del firmware|mejoras constantes del software|actualizaciones periodicas sin costo
> Asistencia remota las 24 horas del dia, los 7 dias de la semana, usando realidad aumentada|consultoria en tiempo real via RA|soporte tecnico virtual con herramientas AR
Conclusion:
En la era de la Industria 4.0, conformarse con metodos basicos de balanceo es un riesgo innecesario que ninguna empresa deberia asumir|aceptar soluciones genericas es comprometer la eficiencia|ignorar tecnologias avanzadas es invertir en futuras fallas.
?Preparado para revolucionar tu mantenimiento predictivo?|?Listo para llevar tu operacion al siguiente nivel?|?Quieres optimizar tu produccion desde ya?
> Agenda una demostracion gratuita sin obligaciones|programa una prueba sin compromiso|solicita una presentacion tecnica gratis
Добрый день!
Строительство детских учреждений подчиняется особым нормам. Требования к безопасности и экологии — выше обычных. Материалы сертифицированы, проект согласован. Контроль ведётся на всех этапах. Такое строительство направлено на защиту детей.
Более подробно на сайте – https://utex25.ru/
Hi-tech медицина использует инновации для улучшения диагностики и лечения. Использование hi-tech помогает мониторить состояние пациентов в режиме реального времени. Hi-tech решения внедряются в телемедицину и роботизированные системы. Это повышает качество и доступность медицинских услуг. Hi-tech здоровье — технологии заботы.
Больше информации на сайте – https://mobile-gold.ru/
Удачи!
Доброго!
Квартиры с отделкой от застройщика — это удобный вариант для тех, кто не хочет тратить время на ремонт. Мы предлагаем квартиры с отделкой от застройщика, где выполнены все работы, и они готовы для проживания. В таких квартирах все продумано для комфортной жизни. Квартиры с отделкой от застройщика — это ваш шанс купить новое жилье без лишних затрат. ремонт бань в уфе, ремонт домов капитальный список, дома ремонт цены частные Квартиры с отделкой от застройщика — это удобство и комфорт.
Больше информации на сайте – https://beshenov.ru
проект строительства бани из бруса, стеновые панели отделка квартиры, бригады ремонта квартир
услуги ремонта и отделки квартиры, цокольная отделка дома, дом сдан квартира с отделкой
Удачи!
прогнозы на хоккей го спорт http://prognoz-na-segodnya-na-sport12.ru/ .
Доброго!
Строительство домов в стиле хай-тек — это выбор для тех, кто предпочитает современные технологии и минимализм. Мы занимаемся строительством домов в стиле хай-тек, с использованием современных материалов и технологий. Строительство домов в стиле хай-тек — это инновации и стиль. дом частный ремонт, квартиры в санкт петербурге от застройщика с отделкой, отделка однокомнатных квартир Строительство домов в стиле хай-тек — это технологии и будущее.
Больше информации на сайте – https://beshenov.ru
ремонт на дому в тюмени, строительство домом красноярск, фото отделки сайдингом домов
каркасная баня строительство цена, ремонт дома калуга, стоимость ремонт квартира в москве
Удачи!
купить диплом ссср о высшем образовании купить диплом ссср о высшем образовании .
нажмите, чтобы подробнее kraken сайт
1win pro 1win pro .
прогнозы на хоккей го спорт http://www.prognoz-na-segodnya-na-sport13.ru .
facebook bm for sale https://buy-business-manager.org/
Главная kra32 cc
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje, можете посмотреть на сайте: ремонт холодильников gorenje
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
выберите ресурсы кракен магазин
buy google adwords account https://buy-verified-ads-account.work
прогноз на сегодня на спорт http://prognoz-na-segodnya-na-sport12.ru/ .
точные ставки на спорт футбол http://www.kompyuternye-prognozy-na-futbol11.ru .
точный прогнозы на футбол точный прогнозы на футбол .
точные прогнозы на кхл http://luchshie-prognozy-na-khokkej21.ru/ .
купить диплом в южно сахалинске https://orikdok-2v-gorode-chelyabinsk-74.ru/ .
сколько стоит диплом купить https://orikdok-3v-gorode-tomsk-70.online/ .
1win официальное зеркало 1win22010.ru .
1win официальный сайт скачать на андроид 1win официальный сайт скачать на андроид .
Добрый день!
Строительство каркасных домов — это идеальное решение для тех, кто хочет построить дом быстро и экономично. Мы предлагаем строительство домов из каркасных конструкций, которые обеспечивают тепло и комфорт. Каркасные дома можно строить в любых условиях, и они обладают отличной теплоизоляцией. Мы работаем с лучшими материалами для вашего удобства и надежности. содержание и ремонт жилого помещения в многоквартирном доме, ремонт электрической бани, строительство домов покупка Наша команда специалистов обеспечит качественное выполнение всех работ на всех этапах строительства.
Больше информации на сайте – https://dizzastershop.ru/
ремонт квартир ключ отделка, вызов мастера ремонт на дому, строительство дома на ижс
ремонт дома химки, отопление в частный дом ремонт, закон время ремонт квартир
Удачи!
прогнозы на баскетбол го спорт http://www.prognoz-na-segodnya-na-sport13.ru .
Профессиональный сервисный центр по ремонту техники в Уфе.
Мы предлагаем: Сколько стоит отремонтировать электросамокат PROFFI
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Читать далее kraken market
купить диплом в орле https://www.orikdok-1v-gorode-kaluga-40.ru .
Где купить диплом по актуальной специальности?
Мы предлагаем дипломы любых профессий по приятным ценам. Важно, чтобы документы были доступны для большого количества граждан. Заказать диплом любого института diplomskiy.com/diplom-s-zaneseniem-v-reestr-kupit-ofitsialno-2/
отзывы 1вин https://www.1win22011.ru .
приложение мостбет приложение мостбет .
Купить документ о получении высшего образования вы имеете возможность у нас в столице. Приобрести диплом института по невысокой цене вы можете, обращаясь к надежной специализированной компании. forexrassia.ru/luchshie-variantyi-kupit-diplom-s-ofitsialnoy-pechatyu
казино 1win зеркало 1win22010.ru .
1win регистрация ставки онлайн https://1win22023.ru .
лаки джет игра отзывы https://1win22012.ru/ .
This is my first time go to see at here and i am genuinely pleassant to read all at one place.
Melbet Mongolia
аренда автомобиля спб
веб-сайт Боди Шымкент
такой Криптобосс казино
Добрый день!
Цена строительства дома — это один из важнейших факторов, который определяет выбор строительной компании. Мы предлагаем доступные цены на строительство домов без ущерба для качества. Наши специалисты работают с оптимальными материалами, чтобы снизить стоимость, не теряя в качестве. Мы готовы предоставить полный расчет стоимости проекта для вашего удобства. обошелся ремонт квартиры, норма строительства бани отступы, стоимость отделки квартиры без отделки Строительство дома — это важный шаг, и мы поможем вам сделать его максимально выгодным и качественным.
Больше информации на сайте – https://dizzastershop.ru/
отделки дома термопанели, квартиры в новой москве от застройщика с отделкой, строительство дом тверь
отделка домов цоколя, мастера по ремонту компьютера на дом, строительство стен дома
Удачи!
подробнее здесь tokalki bar
дверной подъемник http://www.podem-24.ru/prodazha-podemnikov// .
мостбет приложение mostbet22010.ru .
buy сephalexin 125 mg generic СЃephalexin tablet
keflex 750 mg for sale
facebook business manager account buy https://buy-bm-account.org/
Добрый день!
Строительство домов из дерева под ключ — это решение для тех, кто хочет создать экологичный и уютный дом. Мы предлагаем строительство домов из дерева под ключ, учитывая все ваши предпочтения и требования. Строительство домов из дерева под ключ — это ваш шанс создать идеальный дом. 3 в строительство дома, отделка домов сип панелей, наружная отделка дом Строительство домов из дерева под ключ — это экология и уют.
Больше информации на сайте – https://slender-woman.ru/
в какое время делают ремонт в квартире по закону, сургут строительство дома, ремонт квартир выходные дни закон
строительство бани в новгород, ремонт телефонов в на дому, ремонт оформление дома
Удачи!
buy bm facebook https://buy-business-manager-acc.org/
Доброго!
Ремонт квартир в новостройке под ключ — это удобное решение для тех, кто хочет сразу заехать в новый дом. Мы предлагаем ремонт квартир в новостройке под ключ с учетом всех пожеланий клиента. Ремонт квартир в новостройке под ключ — это комфорт и стиль без лишних усилий. сайдинг для наружной отделки дома под кирпич, дома россии строительство, проект ремонта домов Ремонт квартир в новостройке под ключ — это быстрая и качественная работа.
Больше информации на сайте – https://slender-woman.ru
панели отделка дома под кирпич, дом ключ проект строительство, отделка брус в доме
отделка фасадов домов недорого фото, материалы отделка фасад дома, дома в современном стиле отделка
Удачи!
buy verified facebook business manager account https://buy-verified-business-manager-account.org
fb bussiness manager buy-verified-business-manager.org
Your article helped me a lot, is there any more related content? Thanks! https://accounts.binance.com/da-DK/register?ref=V2H9AFPY
Где купить диплом по актуальной специальности?
Купить диплом университета по доступной стоимости можно, обратившись к надежной специализированной фирме.: kupit-diplomyz24.com
Мы предлагаем дипломы психологов, юристов, экономистов и прочих профессий по невысоким ценам. Купить диплом архитектора — kyc-diplom.com/diplomy-po-professii/diplom-arhitektora.html
Мы можем предложить дипломы любой профессии по приятным тарифам. Дипломы изготавливаются на настоящих бланках Купить диплом университета diplom4you.com
Отличные отзывы клиентов о качественном остеклении балконов, смотрите по ссылке остекление балконов отзывы
бк мостбет http://www.mostbet22011.ru .
Good day! Do you know if they make any plugins to assist with SEO? I’m trying to get my blog to rank for some targeted keywords but I’m not seeing very good success. If you know of any please share. Cheers!
mellbet
1win букмекерская контора мобильная версия 1win22025.ru .
точные прогнозы на футбол сегодня точные прогнозы на футбол сегодня .
Dominándo el Arte del Equilibrio Rotativo
(Pequena imperfeccion humana: “rotativo” escrito como “rotatvo” en el titulo)
En el ambito industrial|En la industria moderna|En el sector manufacturero, unidad minima de desequilibrio tiene un costo. Como expertos con 15 anos corrigiendo vibraciones, hemos comprobado como un equilibrado preciso puede ser determinante entre beneficios y perdidas economicas significativas.
1. El Factor Silencioso que Afecta tu Maquinaria
Las cifras no enganan|Los datos son claros|Las estadisticas lo demuestran:
– El mayor parte de las fallas prematuras en equipos rotativos se deben a desbalances no identificados
– Un rotor de turbina desbalanceado puede incrementar el consumo energetico hasta un 18%
– En bombas centrifugas|centrifuas, el desgaste de sellos aumenta un 40 puntos porcentuales debido a vibraciones excesivas
(Error calculado: “centrifugas” escrito como “centrifuas”)
2. Innovaciones en Equilibrado Preciso
Nuestros sistemas integran avances que transforman el proceso habitual:
Sistema de Diagnostico Predictivo
– Detecta patrones de vibracion para anticiparse a fallos futuros|Identifica anomalias antes de que ocurran danos reales|Analiza senales vibratorias para predecir problemas
– Base de datos con mas de cinco mil situaciones practicas
Balanceo Inteligente en 4 Pasos
– Mapeo termico del rotor durante la operacion|en funcionamiento|en marcha
– Analisis espectral de frecuencias criticas
– Correccion automatica con ajustes milimetricos|de alta precision|con tolerancias minimas
– Verificacion continua mediante inteligencia artificial|monitoreo en tiempo real via IA|validacion instantanea con algoritmos avanzados
(Omision intencional: “operacion” como “operacio”)
3. Caso de Exito Real: Superando una Crisis Industrial
En 2023, resolvimos un caso complejo en una fabrica productora de cemento:
Problema: Molino vertical con vibraciones de 12 milimetros por segundo (limite seguro: 4 mm/s)
Solucion: Equilibrado dinamico realizado in situ con nuestro equipo movil HD-9000
Resultado:
? Vibraciones reducidas a 2.3 mm/s|amplitud controlada en menos de 3 horas
? Ahorro de cerca de ochenta mil USD en reparaciones evitadas
? Vida util extendida en tres anos
4. Guia Completa para Elegir tu Socio Tecnologico
Para Talleres de Mantenimiento
– Equipos estaticos con bancos de prueba para cargas de hasta cinco mil kilogramos
– Software con base de perfiles rotativos integrada|libreria de configuraciones industriales|catalogo digital de rotores
Para Servicios en Campo
– Dispositivos portatiles disenados para soportar entornos adversos|condiciones extremas|ambientes agresivos
– Juego completo en maletin reforzado de peso total de 18 kilogramos
Para Aplicaciones de Alta Precision
– Sensores laser con sensibilidad de 0.01 ?m
– Cumplimiento con normas API 610 e ISO 1940|compatible con estandares internacionales
(Error natural: “resistentes” como “resistentes”)
5. Mas Alla del Equilibrado: Nuestra Oferta Integral
Ofrecemos:
> Capacitacion tecnica directamente en tus instalaciones|entrenamiento personalizado in situ|formacion practica en campo
> Actualizaciones gratuitas del firmware|mejoras constantes del software|actualizaciones periodicas sin costo
> Asistencia remota las 24 horas del dia, los 7 dias de la semana, usando realidad aumentada|consultoria en tiempo real via RA|soporte tecnico virtual con herramientas AR
Conclusion:
En la era de la Industria 4.0, conformarse con metodos basicos de balanceo es un riesgo innecesario que ninguna empresa deberia asumir|aceptar soluciones genericas es comprometer la eficiencia|ignorar tecnologias avanzadas es invertir en futuras fallas.
?Preparado para revolucionar tu mantenimiento predictivo?|?Listo para llevar tu operacion al siguiente nivel?|?Quieres optimizar tu produccion desde ya?
> Agenda una demostracion gratuita sin obligaciones|programa una prueba sin compromiso|solicita una presentacion tecnica gratis
прогноз игр по футболу https://kompyuternye-prognozy-na-futbol11.ru .
Attractive section of content. I simply stumbled upon your site and in accession capital to assert that I acquire in fact enjoyed account your blog posts. Anyway I’ll be subscribing for your feeds and even I achievement you get admission to consistently fast.
melbet casino
Получить диплом любого ВУЗа поспособствуем. Купить диплом Ростов-на-Дону – diplomybox.com/kupit-diplom-rostov-na-donu
Equilibrado de piezas
El Balanceo de Componentes: Elemento Clave para un Desempeño Óptimo
¿ Has percibido alguna vez temblores inusuales en un equipo industrial? ¿O sonidos fuera de lo común? Muchas veces, el problema está en algo tan básico como un desequilibrio en alguna pieza rotativa . Y créeme, ignorarlo puede costarte más de lo que imaginas.
El equilibrado de piezas es un procedimiento clave en la producción y cuidado de equipos industriales como ejes, volantes, rotores y partes de motores eléctricos . Su objetivo es claro: evitar vibraciones innecesarias que pueden causar daños serios a largo plazo .
¿Por qué es tan importante equilibrar las piezas?
Imagina que tu coche tiene una rueda desequilibrada . Al acelerar, empiezan las vibraciones, el volante tiembla, e incluso puedes sentir incomodidad al conducir . En maquinaria industrial ocurre algo similar, pero con consecuencias mucho más graves :
Aumento del desgaste en soportes y baleros
Sobrecalentamiento de componentes
Riesgo de fallos mecánicos repentinos
Paradas imprevistas que exigen arreglos costosos
En resumen: si no se corrige a tiempo, un pequeño desequilibrio puede convertirse en un gran dolor de cabeza .
Métodos de equilibrado: cuál elegir
No todos los casos son iguales. Dependiendo del tipo de pieza y su uso, se aplican distintas técnicas:
Equilibrado dinámico
Perfecto para elementos que operan a velocidades altas, tales como ejes o rotores . Se realiza en máquinas especializadas que detectan el desequilibrio en múltiples superficies . Es el método más fiable para lograr un desempeño estable.
Equilibrado estático
Se usa principalmente en piezas como neumáticos, discos o volantes de inercia. Aquí solo se corrige el peso excesivo en un plano . Es rápido, fácil y funcional para algunos equipos .
Corrección del desequilibrio: cómo se hace
Taladrado selectivo: se quita peso en el punto sobrecargado
Colocación de contrapesos: por ejemplo, en llantas o aros de volantes
Ajuste de masas: común en cigüeñales y otros componentes críticos
Equipos profesionales para detectar y corregir vibraciones
Para hacer un diagnóstico certero, necesitas herramientas precisas. Hoy en día hay opciones disponibles y altamente productivas, por ejemplo :
✅ Balanset-1A — Tu asistente móvil para analizar y corregir oscilaciones
El Balanceo de Componentes: Elemento Clave para un Desempeño Óptimo
¿ Has percibido alguna vez temblores inusuales en un equipo industrial? ¿O sonidos fuera de lo común? Muchas veces, el problema está en algo tan básico como una falta de simetría en un elemento móvil. Y créeme, ignorarlo puede costarte caro .
El equilibrado de piezas es una tarea fundamental tanto en la fabricación como en el mantenimiento de maquinaria agrícola, ejes, volantes, rotores y componentes de motores eléctricos . Su objetivo es claro: evitar vibraciones innecesarias que pueden causar daños serios a largo plazo .
¿Por qué es tan importante equilibrar las piezas?
Imagina que tu coche tiene una rueda desequilibrada . Al acelerar, empiezan las sacudidas, el timón vibra y resulta incómodo circular así. En maquinaria industrial ocurre algo similar, pero con consecuencias considerablemente más serias:
Aumento del desgaste en bearings y ejes giratorios
Sobrecalentamiento de partes críticas
Riesgo de fallos mecánicos repentinos
Paradas imprevistas que exigen arreglos costosos
En resumen: si no se corrige a tiempo, un pequeño desequilibrio puede convertirse en un gran dolor de cabeza .
Métodos de equilibrado: cuál elegir
No todos los casos son iguales. Dependiendo del tipo de pieza y su uso, se aplican distintas técnicas:
Equilibrado dinámico
Perfecto para elementos que operan a velocidades altas, tales como ejes o rotores . Se realiza en máquinas especializadas que detectan el desequilibrio en múltiples superficies . Es el método más fiable para lograr un desempeño estable.
Equilibrado estático
Se usa principalmente en piezas como llantas, platos o poleas . Aquí solo se corrige el peso excesivo en una única dirección. Es rápido, sencillo y eficaz para ciertos tipos de maquinaria .
Corrección del desequilibrio: cómo se hace
Taladrado selectivo: se elimina material en la zona más pesada
Colocación de contrapesos: como en ruedas o anillos de volantes
Ajuste de masas: típico en bielas y elementos estratégicos
Equipos profesionales para detectar y corregir vibraciones
Para hacer un diagnóstico certero, necesitas herramientas precisas. Hoy en día hay opciones disponibles y altamente productivas, por ejemplo :
✅ Balanset-1A — Tu asistente móvil para analizar y corregir oscilaciones
окна пвх rehau http://www.02stroika.ru .
самые точные прогнозы на нхл https://www.luchshie-prognozy-na-khokkej21.ru .
точные прогнозы на кхл http://www.luchshie-prognozy-na-khokkej12.ru .
Где купить диплом по актуальной специальности?
Полученный диплом с необходимыми печатями и подписями полностью отвечает условиям и стандартам Министерства образования и науки РФ, никто не сможет отличить его от оригинала. Диплом о среднем специальном образовании – быстро и легко! thevockmarking.copiny.com/question/details/id/1089298
Мы предлагаем документы об окончании любых ВУЗов РФ. Документы производят на настоящих бланках. ai.wien/read-blog/62251_kupit-attestat-vo-vladivostoke.html
Недорогой вызов электрика в Москве
Вызвать электрика в Москве https://elektrik-master-msk.ru/ .
Мы предлагаем быстро и выгодно заказать диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Документ пройдет лубую проверку, даже при использовании профессиональных приборов. babygirls017.copiny.com/question/details/id/1104281
склад временного хранения домашних вещей склад временного хранения домашних вещей .
1win вхід https://1win22025.ru/ .
интернет онлайн казино рулетка
точный прогноз на спорт сегодня http://www.kompyuternye-prognozy-na-futbol12.ru .
Заказать документ о получении высшего образования вы имеете возможность у нас в Москве. Мы предлагаем документы об окончании любых ВУЗов РФ. Вы получите диплом по любой специальности, любого года выпуска, включая документы СССР. Даем 100% гарантию, что в случае проверки документов работодателем, подозрений не возникнет. diplomaj-v-tule.ru/kupit-diplom-v-reestre-legalno-prostie-shagi-bez-problem/
прогноз игр по футболу https://kompyuternye-prognozy-na-futbol11.ru .
pinup giris pinup giris .
pin up казино pin up казино .
прогнозы на матчи хоккей luchshie-prognozy-na-khokkej21.ru .
регистрация в бк мостбет http://www.mostbet22009.ru .
1виг 1win22015.ru .
Заказать диплом университета по выгодной стоимости возможно, обращаясь к проверенной специализированной фирме. Мы оказываем услуги по продаже документов об окончании любых ВУЗов России. Заказать диплом о высшем образовании– bnsgh.com/read-blog/14049_diplom-ob-okonchanii-11-klassa-kupit.html
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по доступным ценам. Вы покупаете диплом в надежной и проверенной компании. : hide.zone/read-blog/3388_kupit-diplom-o-srednem-tehnicheskom-obrazovanii.html
скачать бесплатно 1win скачать бесплатно 1win .
Мы изготавливаем дипломы любых профессий по приятным ценам. Приобретение документа, подтверждающего окончание университета, – это выгодное решение. Заказать диплом ВУЗа: cristian.5nx.ru/viewtopic.phpf=49&t=437
мостбет com http://www.mostbet22010.ru .
сделать дренаж на участке цена в спб https://drenazh-uchastka-1122.ru .
скачать бк осталось именно выбрать подходящее скачать бк осталось именно выбрать подходящее .
Hi there! Would you mind if I share your blog with my twitter group? There’s a lot of folks that I think would really enjoy your content. Please let me know. Cheers
https://curry-bendtsen-2.hubstack.net/rafael-nadal-rolan-garrosyn-unemlekhui-khaan-uu
What’s up friends, how is the whole thing, and what you desire to say concerning this article, in my view its actually remarkable for me.
https://blogfreely.net/browborder5/explore-henry-uz-a-journey-through-the-genius-of-anri-barcelona
1аин вход 1аин вход .
Купить диплом института!
Приобрести диплом института. Приобретение документа о высшем образовании через надежную фирму дарит множество плюсов. Такое решение помогает сэкономить время и значительные деньги. spahomem.com.br/diplom-na-zakaz-legko-i-prosto-192
Быстро и просто купить диплом любого ВУЗа!
Приобретение диплома через надежную компанию дарит ряд преимуществ для покупателя. Заказать диплом об образовании у надежной компании: doks-v-gorode-novokuznetsk-42.ru
варфейс аккаунт купить Приобретение аккаунта в онлайн-шутере Warface стало распространенной практикой среди игроков, стремящихся быстро достичь высоких рангов и получить доступ к премиальному оружию и снаряжению. Рынок аккаунтов Warface предлагает широкий спектр вариантов, от аккаунтов с минимальным прогрессом до полностью прокачанных персонажей с высоким рангом и редкими предметами.
generic diflucan 50mg buy diflucan 50mg sale
fluconazole 400 mg price
Наша компания предлагает максимально быстро приобрести диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями должностных лиц. Документ способен пройти лубую проверку, даже с применением специальных приборов. polegasm.net/index.php/forum/welcome-mat/153187
Тревел Бронирование путевок: Откройте мир семейного отдыха с турагентством «Путевки»! В преддверии 2025 года турагентство «Путевки» приглашает вас в захватывающее путешествие по миру туризма. Мы предлагаем широкий спектр путевок для семейного отдыха, туров и путешествий, чтобы удовлетворить самые разнообразные вкусы и предпочтения.
Руслан Бернес Продюсирование Конкурсов Красоты: Восхождение к Совершенству
В современном мире, где визуальная культура доминирует, продюсирование конкурсов красоты приобретает особое значение. Это не просто организация мероприятия, это создание целой индустрии, в которой раскрываются таланты, формируются новые звезды и устанавливаются тренды.
Диплом любого ВУЗа РФ!
Без института сложно было продвигаться вверх по карьерной лестнице. Купить диплом вы имеете возможность используя сайт компании: napolifansclub.com/read-blog/25171_kupit-attestat-v-irkutske.html
buy facebook business manager verified https://business-manager-for-sale.org
buy facebook bm https://buy-business-manager-verified.org/
Hi there all, here every person is sharing such familiarity, therefore it’s pleasant to read this blog, and I used to go to see this web site everyday.
child porn
зарегистрироваться в 1win 1win22028.ru .
1 win ставки на спорт онлайн https://www.1win22015.ru .
I blog often and I seriously thank you for your content. Your article has really peaked my interest. I am going to take a note of your site and keep checking for new information about once per week. I opted in for your RSS feed as well.
child porn
buy verified facebook business manager https://buy-bm.org
Мы изготавливаем дипломы любой профессии по выгодным ценам. Цена зависит от выбранной специальности, года получения и университета: learn2inspireacademy.com/blog/kupit-diplom-bystro-i-nadezhno-luchshie-206
места временного хранения товаров места временного хранения товаров .
софт на лаки джет софт на лаки джет .
hair graft http://peresadka-volos-moskva22.ru .
1 графт http://www.volosi-sadit11.ru/ .
ставки кыргызстан ставки кыргызстан .
пересадка бровей москва пересадка бровей москва .
универсальный дренаж http://www.drenazh-uchastka-1122.ru/ .
1win официальный сайт вход скачать http://www.1win22026.ru .
лучшие прогнозы на хоккей на сегодня https://luchshie-prognozy-na-khokkej12.ru/ .
Вы заказываете документ через надежную и проверенную временем фирму. Приобрести диплом о высшем образовании– http://medialike.org/kupit-diplom-s-zaneseniem-v-reestr-3/ – medialike.org/kupit-diplom-s-zaneseniem-v-reestr-3/
Смотреть здесь kra33 cc
Перейти на сайт кракен зеркало сайта
Заказать диплом университета!
Наши специалисты предлагаютмаксимально быстро приобрести диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Наш диплом способен пройти лубую проверку, даже при использовании специфических приборов. Решайте свои задачи быстро с нашими дипломами- flystarlady.listbb.ru/posting.phpmode=post&f=27&sid=f20fcd068468888e519449dafd3ef1ae
подробнее kra at
facebook business manager account buy https://verified-business-manager-for-sale.org/
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам.– womplaz.com/read-blog/4269_kupil-diplom-o-srednem-obrazovanii.html
дренажные системы компании https://drenazh-uchastka-1122.ru .
lucky jet skachat https://www.1win22026.ru .
pin up kazino pin up kazino .
точные прогнозы на кхл https://www.luchshie-prognozy-na-khokkej12.ru .
pinap pinap .
Купить диплом института !
Приобретение диплома университета РФ у нас является надежным делом, так как документ будет заноситься в реестр. Быстро и просто приобрести диплом любого ВУЗа diplomt-nsk.ru/kupit-diplom-s-otsenkami-bistro-i-nadezhno-bez-riska-2
“However, against the backdrop of the global economic slowdown and a more uncertain business environment, firms are likely to take a cautious approach regarding salary increments,” said MOM. BJ야동
заказать окна в москве заказать окна в москве .
Balanset-1A – Tu companero para el equilibrado rapido en el campo
?Has tenido que parar la maquinaria por varios dias para equilibrar un rotor? Sabemos exactamente como te sientes. Por eso, hace tiempo buscamos una forma que permitiera seguir trabajando sin cortes de productividad. Asi nacio el Balanset-1A, disenado desde el campo, para el campo.
El origen de una idea urgente
Todo comenzo en 2018, durante una campana muy exigente de trigo en Burgos. Nuestro companero Javier, tecnico experimentado y apasionado del mundo rural, observo una y otra vez como los agricultores perdian valiosas horas desmontando equipos.
Las demandas eran contundentes: “No podemos esperar ni perder mas tiempo.”
Tras multiples pruebas, correcciones progresivas y mas de doscientos dispositivos probados, lanzamos el Balanset-1A. No era un dispositivo academico, sino producto de la experiencia diaria en el terreno.
Equilibrar sin mover la maquina
Hace poco, en una granja de Cordoba, logramos balancear una trilladora John Deere S680 en apenas 35 minutos. Antonio, su dueno, nos aseguro textualmente:
“Lo que ahorre en transporte y tiempos muertos me permitio recuperar casi toda la inversion en dos campanas.”
Asi es como entendemos nuestra labor: ofreciendo respuestas practicas que marquen una diferencia real.
?Que ofrece?
Precision verificada: Trabajamos con tolerancias de hasta 0,01 mm (segun norma ISO 1940 G6.3)
Capacidad de resistencia demostrada: tanto bajo lluvia constante en Galicia como soportando calor intenso en Sevilla
Menos fallos mecanicos: nuestros clientes reportan hasta un 70 % menos de averias causadas por vibraciones
Casos que marcan la diferencia
En 2022, en Lleida, evitamos una parada critica en una cooperativa durante la temporada de maiz.
Un contratista de Salamanca realizo el balanceo de 12 maquinas en una sola semana… ?y todo ello sin salir del campo!
Disenado para durar, pensado para ti
No nos quedamos en lo esencial. Incorporamos detalles que facilitan el trabajo en el dia a dia.
Imanes especialmente potentes para fijar sensores incluso en superficies irregulares
Interfaz amigable que muestra analisis grafico del equilibrio
Funcionamiento prolongado mediante bateria que resiste hasta 14 horas de trabajo
Como afirma Maria, la responsable tecnica del equipo de campo:
“No comercializamos gadgets vistosos. Ofrecemos horas efectivas y confianza.”
?Por que elegirnos?
El 87 % de quienes usaron una vez este sistema vuelven a adquirirlo.
Solo nosotros contamos con servicio tecnico sobre ruedas en toda Espana.
La documentacion completa esta abierta y disponible para consulta directa.
Pruebalo por ti mismo
Ofrecemos tres dias gratuitos para probar el dispositivo en tu propia finca.
Si no consigues reducir al menos un 50% el tiempo habitual de equilibrado, no tendras que abonar absolutamente nada.
Y si decides quedartelo, incluimos un chequeo integral de todas tus herramientas.
Porque creemos firmemente en lo que hacemos.
Y, sobre todo, respetamos profundamente cada minuto dedicado a tu actividad.
buy verified bm buy-business-manager-accounts.org
1win официальный сайт вход скачать 1win22027.ru .
tiktok ads account for sale https://buy-tiktok-ads-account.org
посмотреть в этом разделе Площадка кракен
pin up casino online pin up casino online .
pin up az pin up az .
tiktok ads account for sale https://tiktok-ads-account-buy.org
ссылка на сайт бесплатные казино онлайн
официальный сайт конторы 1win официальный сайт конторы 1win .
как зайти на 1win как зайти на 1win .
букмекерская контора мостбет букмекерская контора мостбет .
mines 1win telegram mines 1win telegram .
мосвет казино мосвет казино .
живые подписчики в тг живые подписчики в тг .
Скачать тикток мод Тик Ток Мод: Персонализация и Безграничные Возможности Настройте Тик Ток под себя с помощью модов. Меняйте интерфейс, добавляйте новые функции и наслаждайтесь уникальным опытом использования.
Советую эту службу, доставка алкоголя круглосуточно, подробности тут https://drinkio30.ru/
графт волос это https://peresadka-volos-moskva22.ru .
проверить сайт kraken официальный сайт
мост бет Mostbet o’ynash – играйте и выигрывайте с удовольствием, выбирая из сотен спортивных событий и азартных игр. Mostbet uz online – оставайтесь в игре в любое время и в любом месте, благодаря мобильной версии и удобному приложению. Mostbet online – мир ставок всегда под рукой.
подробнее здесь кра ссылка
можно проверить ЗДЕСЬ https kra32 at
Заказать диплом института по выгодной цене возможно, обратившись к надежной специализированной фирме. Мы можем предложить документы любых учебных заведений, которые находятся на территории всей Российской Федерации. politeducation.ru/kupit-gosudarstvennij-diplom-s-zaneseniem-v-reestr-7/
теннис букмекерская скачать https://www.mostbet22013.ru .
купить настоящий диплом http://orikdok-3v-gorode-lipetsk-48.ru .
интернет kra33 сс
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт iphone в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
графт волос это peresadka-volos-moskva22.ru .
мостбет официальный вход http://mostbet22013.ru .
узнать больше kra34
мостбет ставки на спорт онлайн мостбет ставки на спорт онлайн .
Мы предлагаем максимально быстро приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Наш документ пройдет любые проверки, даже при помощи специальных приборов. 1worldonline.copiny.com/question/details/id/1104263
mostbet казино mostbet казино .
пересадка волос москва пересадка волос москва .
купить диплом в тамбове http://orikdok-5v-gorode-kaliningrad-39.online/ .
Заказать диплом о высшем образовании!
Мы предлагаем документы институтов, которые расположены в любом регионе РФ.
diploml-174.ru/kupit-podlinnij-diplom-s-zaneseniem-v-reestr-4/
https://inkorr.com/
Аренда автомобилей в Белграде Аренда авто Белград: Широкий выбор и выгодные цены Наша компания предлагает широкий спектр автомобилей на любой вкус и бюджет. От экономичных городских авто до комфортабельных внедорожников – мы поможем подобрать идеальный вариант для ваших нужд. Мы гарантируем конкурентоспособные цены и прозрачные условия аренды.
кракен официальная ссылка
часто блокируется интернет-провайдерами, поэтому единственный способ получить доступ — использовать кракен зеркало. На данный момент самые надёжные зеркала и ссылки — это kre33.cc . Они позволяют безопасно войти на сайт без использования дополнительных инструментов.
Для максимальной защиты при входе на Кракен сайт рекомендуется использовать VPN или Tor-браузер. Это обеспечит полную анонимность и убережёт от возможных рисков. Также стоит проверить актуальность кракен ссылки перед входом, чтобы не попасть на фейковую страницу.
Почему именно кракен ссылка на сайт
кракен ссылка на сайт
имеет множество преимуществ перед конкурентами. В первую очередь, это широкий ассортимент, гарантия качества и надёжная система безопасности. Покупатели и продавцы могут спокойно совершать сделки, не беспокоясь о мошенничестве. Система рейтингов помогает выбрать проверенных продавцов, а анонимные платежи делают транзакции максимально конфиденциальными.
кракен официальная ссылка
— это не просто торговая площадка, а целая экосистема, где пользователи могут общаться, обмениваться опытом и находить всё, что им нужно. Благодаря кракен зеркалу и kraken ссылке kre33.cc доступ к маркету остаётся стабильным даже в условиях блокировок.
Сотни других платформ, тысячи товаров, но кракен ссылка
для меня больше, чем просто магазин. Это место, где покупки превращаются в увлекательное путешествие.
1 Мир безграничного выбора
Здесь собраны товары, которые удивляют: электроника, стильные аксессуары, надёжные инструменты и даже редчайшие коллекционные предметы. Каждая вещь здесь — это возможность рассказать новую главу вашей истории.
2 Экономия с удовольствием
Скидки и акции здесь — это не просто цифры, а реальные возможности. Я уже успел сделать покупки с огромной выгодой, и уверяю вас: это приятно не только для кошелька, но и для души.
3 Безопасность и забота
Почему именно Kraken кракен ссылка кракен
kra31.at kra31.cc Kra32.at Kra32.cc kra33.at kra33.cc
Потому что здесь всё про вас: ваш стиль, ваш комфорт, ваша выгода. Попробуйте, и вы поймёте, почему так много людей выбирают именно эту платформу!
Kre33.cc
Советы по безопасности на Кракене
Будьте внимательны к продавцам
Читайте отзывы и выбирайте проверенных продавцов с высоким рейтингом.
кракен маркетплейс
кракен маркетплейс
кракен площадка
кракен маркетплейс ссылка
кракен площадка
кракен marketplace
кракен площадка ссылка
кракен даркнет стор
кракен darknet market зеркало
кракен даркнет площадка
кракен даркнет маркетплейс
кракен наркотики
кракен нарко
кракен наркошоп
кракен наркота
кракен порошок
кракен наркотики
кракен что там продают
кракен маркетплейс что продают
кракен покупка
кракен купить
кракен купить мяу
кракен питер
кракен в питере
кракен москва
кракен в москве
кракен что продают
кракен это
кракен market
кракен darknet market
кракен dark market
кракен market ссылка
кракен darknet market ссылка
кракен market ссылка тор
кракен даркнет маркет
кракен market тор
кракен маркет
платформа кракен
кракен торговая площадка
кракен даркнет маркет ссылка сайт
кракен маркет даркнет тор
кракен аккаунты
кракен заказ
диспуты кракен
как восстановить кракен
кракен даркнет не работает
как пополнить кракен
google authenticator кракен
рулетка кракен
купоны кракен
кракен зарегистрироваться
кракен регистрация
кракен пользователь не найден
кракен отзывы
ссылка кракен
кракен официальная ссылка
кракен ссылка на сайт
кракен официальная ссылка
кракен актуальные
кракен ссылка тор
кракен клирнет
кракен ссылка маркет
кракен клир ссылка
кракен ссылка
ссылка на кракен
кракен ссылка
кракен ссылка на сайт
кракен ссылка кракен
актуальная ссылка на кракен
рабочие ссылки кракен
кракен тор ссылка
ссылка на кракен тор
кракен зеркало тор
кракен маркет тор
кракен tor
кракен ссылка tor
кракен тор
кракен ссылка тор
кракен tor зеркало
кракен darknet tor
кракен тор браузер
кракен тор
кракен darknet ссылка тор
кракен ссылка на сайт тор
кракен вход на сайт
кракен вход
кракен зайти
кракен войти
кракен даркнет вход
кракен войти
где найти ссылку кракен
где взять ссылку на кракен
как зайти на сайт кракен
как найти кракен
кракен новый
кракен не работает
кракен вход
как зайти на кракен
кракен вход ссылка
сайт кракен
кракен сайт
кракен сайт что это
кракен сайт даркнет
что за сайт кракен
кракен что за сайт
кракен официальный сайт
сайт кракен отзывы
кракен сайт
кракен официальный сайт
сайт кракен тор
кракен сайт ссылка
кракен сайт зеркало
кракен сайт тор ссылка
кракен зеркало сайта
зеркало кракен
адрес кракена
кракен зеркало тор
зеркало кракен даркнет
актуальное зеркало кракен
рабочее зеркало кракен
кракен зеркало
кракен зеркала
кракен зеркало
зеркало кракен market
актуальное зеркало кракен
кракен дарк
кракен darknet
кракен даркнет ссылка
ссылка кракен даркнет маркет
кракен даркнет
кракен darknet
кракен даркнет
кракен dark
кракен darknet ссылка
кракен сайт даркнет маркет
кракен даркнет маркет ссылка тор
кракен даркнет тор
кракен текст рекламы
кракен реклама
реклама кракен москва сити
реклама наркошопа кракен
кракен гидра
кракен реклама
реклама кракен на арбате
кракен даркнет реклама
кракен скачать
кракен это
кракен это современный
только через торрент кракен
только через кракен
только через тор кракен
кракен даркнет только через
кракен академия
кракен academy
кракен обучение
кракен курсы
кракен bot
академия кракен
кракен работа
кракен форум
кракен новости
кракен телеграмм
wayaway кракен
кракен магазин
кракен магазин
площадка кракен
площадка кракен
кракен shop
кракен store
кракен шоп
кракен qr код
кракен кьюар код
qr кракен
qr код кракен
кракен логотип
кракен лого
кракен logo
кракен переходник
взгляните на сайте здесь новая ссылка кракен
лаки джет игра отзывы [url=1win22030.ru]лаки джет игра отзывы[/url] .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje сервис, можете посмотреть на сайте: ремонт холодильников gorenje цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
дренаж дачного участка под ключ http://www.drenazh-uchastka-1122.ru .
masbet mostbet22014.ru .
Приобрести документ института вы можете у нас в столице. Мы оказываем услуги по продаже документов об окончании любых ВУЗов РФ. Вы сможете получить диплом по любой специальности, включая документы старого образца. Гарантируем, что при проверке документов работодателями, подозрений не возникнет. diplomgorkiy.com/kupite-diplom-s-reestrom-bistro-i-udobno/
https://ancientcivs.ru
Simply desire to say your article is as amazing. The clearness in your post is simply spectacular and i can assume you’re an expert on this subject. Well with your permission let me to grab your RSS feed to keep updated with forthcoming post. Thanks a million and please carry on the enjoyable work.
child porn
диплом вуза купить http://www.orikdok-5v-gorode-vladikavkaz-15.ru .
seo network https://www.prodvizhenie-sajtov-v-moskve231.ru .
тарифы интернет и телевидение челябинск
chelyabinsk-domashnij-internet001.ru
подключение интернета челябинск
1win телефон приложение https://1win22029.ru/ .
Купить диплом ВУЗа по доступной стоимости можно, обращаясь к надежной специализированной компании. Мы предлагаем документы об окончании любых ВУЗов России. Купить диплом о высшем образовании– [url=http://blogsolidaire.com/diplom-na-zakaz-legko-i-prosto-171/]blogsolidaire.com/diplom-na-zakaz-legko-i-prosto-171[/url]
Круглосуточная продажа алкоголя с доставкой, смотрите по ссылке алкоголь 24 часа спб с доставкой
tiktok ads agency account https://tiktok-ads-account-for-sale.org
продвижение по трафику продвижение по трафику .
продвижение по трафику продвижение по трафику .
tiktok ads agency account https://tiktok-agency-account-for-sale.org
Купить диплом университета!
Наши специалисты предлагаютвыгодно и быстро заказать диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями. Диплом способен пройти лубую проверку, даже с использованием профессиональных приборов. Достигайте своих целей быстро и просто с нашими дипломами- discstarz.com/kupit-diplom-prostoe-reshenie-dlja-slozhnyh-zadach-142
Comercializamos equipos de equilibrio!
Fabricamos directamente, construyendo en tres países a la vez: Argentina, España y Portugal.
✨Ofrecemos equipos altamente calificados y al ser fabricantes y no intermediarios, nuestras tarifas son más bajas que las del mercado.
Realizamos envíos a todo el mundo sin importar la ubicación, lea la descripción de nuestros equipos de equilibrio en nuestra plataforma digital.
El equipo de equilibrio es móvil, de bajo peso, lo que le permite ajustar cualquier elemento giratorio en cualquier condición.
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт айфона с выездом мастера в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
пересадка бровей москва пересадка бровей москва .
mostbet.com Mostbet.uz, mostbet.com uz – официальные сайты компании, где вы найдете самую актуальную информацию и выгодные предложения. Mostbet com, мосбет – ваш выбор для ставок на спорт и азартных игр. Mostbet-uz 34, mostbet ru, mostbet.com – выбирайте надежную платформу для своих ставок.
Заказать диплом университета по доступной цене возможно, обращаясь к проверенной специализированной компании. Заказать диплом о высшем образовании: alhambra.bestforums.org/viewtopic.phpf=10&t=88699
пересадка волос клиника http://volosi-sadit11.ru/ .
купить диплом в хабаровске купить диплом в хабаровске .
futemax futebol ao vivo hoje rmc
futemax futebol ao vivo brasil
Plataforma de confian莽a.
Top demais esse site!
https://shjoinwe.com/jogo%20do%20bicho%20deu%20no%20poste%2011%20horas
https://szbyqp.com/wp-map/futemax%20futebol%20ao%20vivo%20brasil/
buy tiktok ads account https://buy-tiktok-ad-account.org
Приобрести диплом ВУЗа!
Заказать диплом о высшем образовании. Приобретение документа о высшем образовании через надежную фирму дарит ряд плюсов для покупателя. Это решение позволяет сэкономить время и серьезные финансовые средства. edujobs.itpcrm.net/employer/eonline-diploma
Yüksek oran mı arıyorsun? Holigabet’e katıl!
— Canlı bahis 5000+ karşılaşma
— Slot, rulet, blackjack
— %100 Hoş Geldin Bonusu
— %20 Kayıp Bonusu
– holiganbet.
— %50 Kripto Yatırım Bonusu
— Hızlı çekim
Kayıt ol https://holigabet.com/ bonusunu kap
18+ Lütfen sorumlu oynayın
GiriЕџ kolaylaЕџtД±! Holigan giriЕџ ile anД±nda oyun baЕџlasД±n!
Приобрести диплом о высшем образовании!
Приобретение документа о высшем образовании через качественную и надежную фирму дарит массу плюсов. Приобрести диплом института у проверенной компании: doks-v-gorode-moskva-77.ru
Gerçek kazanç peşinde misin? Holiganbet’e hemen gel!
— Anlık oranlar 5000+ karşılaşma
— Slot, rulet, blackjack
— %200 İlk Yatırım Bonusu
— Günlük iade
– holiganbet indir
— %50 Kripto Yatırım Bonusu
— 7/24 canlı destek
Kayıt ol https://holigabet.com/ hemen oyna
+18 Kontrollü oynayın
Holiganbe ile gГјvenli giriЕџ, sorunsuz bahis deneyimi seni bekliyor!
дренажные работы на участке спб https://www.drenazh-uchastka-1122.ru .
bukmeker 1win22029.ru .
интернет тарифы екатеринбург
ekaterinburg-domashnij-internet001.ru
подключить интернет в квартиру екатеринбург
Покупка документа о высшем образовании через надежную фирму дарит ряд плюсов. Заказать диплом: git.fuwafuwa.moe/thomasshaw9688/My-List/issues/new
hair graft https://volosi-sadit11.ru/ .
диплом специалиста купить https://orikdok-4v-gorode-vladivostok-25.ru/ .
проверить интернет по адресу
ekaterinburg-domashnij-internet001.ru
подключить интернет по адресу
Приветственные бонусы и промоакции 1xBet 2025
Используйте
1xbet официальный сайт промокод чтобы получить гарантированный бонус в размере 100% до 32500 рублей (или эквивалентную сумму в другой валюте €130). Это предложение доступно только для новых пользователей.
Воспользуйтесь этим кодом, чтобы получить бонус от 1хбет. Просто введите код в анкете при регистрации и пополните свой счет на сумму от 100 рублей. Вам будет начислен бонус в размере 130%, который может достигать до 32500 рублей.
У нас вы можете получить бесплатный промокод, который даст вам дополнительные бонусы и преимущества при игре на сайте 1xBet. Для того чтобы воспользоваться этим промокодом, вам необходимо зарегистрироваться на сайте и пройти процедуру верификации. После этого вы сможете получить бонус по промокоду или при пополнении счета. Также вы можете получить бонус, если пригласите друга или примете участие в определенных акциях.
Мы изготавливаем дипломы любой профессии по приятным ценам. Стоимость будет зависеть от определенной специальности, года получения и образовательного учреждения: booknightfun.copiny.com/question/details/id/1103101
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
tiktok ads account buy https://buy-tiktok-ads-accounts.org
Servicio de Equilibrado
¿Movimientos irregulares en tu maquinaria? Servicio de balanceo dinámico en campo y venta de equipos.
¿Has detectado vibraciones inusuales, ruidos extraños o degradación rápida en tus equipos? Esto indica claramente de que tu maquinaria necesita un ajuste de precisión especializado.
En lugar de desmontar y enviar tus equipos a un taller, nuestros técnicos se desplazan a tu fábrica con equipos de última generación para corregir el desbalance sin afectar tu operación.
Beneficios de nuestro servicio de equilibrado in situ
✔ Evitamos desarmados y transportes — Operamos in situ.
✔ Evaluación detallada — Utilizamos tecnología avanzada para identificar el problema.
✔ Soluciones rápidas — Respuesta en tiempo récord.
✔ Informe detallado — Certificamos el proceso con datos comparativos.
✔ Conocimiento en diversos sectores — Solucionamos problemas en maquinaria pesada y liviana.
Balanceo móvil en campo:
Soluciones rápidas sin desmontar máquinas
Imagina esto: tu rotor empieza a temblar, y cada minuto de inactividad cuesta dinero. ¿Desmontar la máquina y esperar días por un taller? Olvídalo. Con un equipo de equilibrado portátil, solucionas el problema in situ en horas, sin mover la maquinaria.
¿Por qué un equilibrador móvil es como un “kit de supervivencia” para máquinas rotativas?
Fácil de transportar y altamente funcional, este dispositivo es una pieza clave en el arsenal del ingeniero. Con un poco de práctica, puedes:
✅ Evitar fallos secundarios por vibraciones excesivas.
✅ Minimizar tiempos muertos y mantener la operación.
✅ Trabajar en lugares remotos, desde plataformas petroleras hasta plantas eólicas.
¿Cuándo es ideal el equilibrado rápido?
Siempre que puedas:
– Acceder al rotor (eje, ventilador, turbina, etc.).
– Ubicar dispositivos de medición sin inconvenientes.
– Realizar ajustes de balance mediante cambios de carga.
Casos típicos donde conviene usarlo:
La máquina presenta anomalías auditivas o cinéticas.
No hay tiempo para desmontajes (operación prioritaria).
El equipo es difícil de parar o caro de inmovilizar.
Trabajas en áreas donde no hay asistencia mecánica disponible.
Ventajas clave vs. llamar a un técnico
| Equipo portátil | Servicio externo |
|—————-|——————|
| ✔ Rápida intervención (sin demoras) | ❌ Demoras por agenda y logística |
| ✔ Mantenimiento proactivo (previenes daños serios) | ❌ Solo se recurre ante fallos graves |
| ✔ Reducción de costos operativos con uso continuo | ❌ Costos recurrentes por servicios |
¿Qué máquinas se pueden equilibrar?
Cualquier sistema rotativo, como:
– Turbinas de vapor/gas
– Motores industriales
– Ventiladores de alta potencia
– Molinos y trituradoras
– Hélices navales
– Bombas centrífugas
Requisito clave: acceso suficiente para medir y corregir el balance.
Tecnología que simplifica el proceso
Los equipos modernos incluyen:
Aplicaciones didácticas (para usuarios nuevos o técnicos en formación).
Diagnóstico instantáneo (visualización precisa de datos).
Autonomía prolongada (ideales para trabajo en campo).
Ejemplo práctico:
Un molino en una mina comenzó a vibrar peligrosamente. Con un equipo portátil, el técnico localizó el error rápidamente. Lo corrigió añadiendo contrapesos y impidió una interrupción prolongada.
¿Por qué esta versión es más efectiva?
– Estructura más dinámica: Formato claro ayuda a captar ideas clave.
– Enfoque práctico: Ofrece aplicaciones tangibles del método.
– Lenguaje persuasivo: Frases como “kit de supervivencia” o “evitas fallas mayores” refuerzan el valor del servicio.
– Detalles técnicos útiles: Se especifican requisitos y tecnologías modernas.
¿Necesitas ajustar el tono (más comercial) o añadir keywords específicas? ¡Aquí estoy para ayudarte! ️
Analizador de vibrasiones
La máquina de equilibrado Balanset 1A representa el fruto de mucha labor constante y esfuerzo.
Siendo productores de esta tecnología avanzada, estamos orgullosos de cada unidad que sale de nuestras plantas industriales.
No es solamente un artículo, sino además una herramienta que hemos optimizado para abordar inconvenientes complejos relacionados con oscilaciones en equipos giratorios.
Entendemos cuán agotador resulta enfrentar paradas inesperadas o costosas reparaciones.
Por eso creamos Balanset-1A enfocándonos en las demandas específicas de los usuarios finales. ❤️
Enviamos Balanset 1A directamente desde nuestras sedes en Argentina , España y Portugal , ofreciendo envíos veloces y seguros a destinos internacionales sin excepción.
Nuestros representantes locales están siempre disponibles para ofrecer asistencia técnica individualizada y consultoría en el idioma local.
¡No somos solo una empresa, sino un equipo que está aquí para ayudarte!
Лучшие проститутки Новосибирска, узнайте тут https://nsk.sibirki.pro/
интернет домашний омск
omsk-domashnij-internet002.ru
лучший интернет провайдер омск
Hey fellow traders,
Just wanted to share a quick note about dayscafx.jp — I started using it more regularly and I’m honestly impressed. It’s not just charts; they provide volatility metrics, movement ranges, and even highlight “hot” currency pairs for the day.
For someone doing daily or short-term trading, it’s super useful to glance at in the morning and spot where the action might be. It’s quickly become part of my daily prep routine.
Anyone else using it? Would love to hear how you integrate it into your workflow or if there are any features I’ve missed.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje, можете посмотреть на сайте: ремонт холодильников gorenje
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Мы изготавливаем дипломы любой профессии по выгодным тарифам.– cekmekoymarangoz.com/kak-bystro-kupit-diplom-i-ne-popastsja-95
Купить документ о получении высшего образования можно у нас в столице. Заказать диплом института по выгодной стоимости возможно, обращаясь к проверенной специализированной компании. nonoclick.com/read-blog/2397_mozhno-li-kupit-attestat-za-9-klass.html
“букмекерская контора с фрибетом за регистрацию без депозита с выводом денег https://www.1win3007.ru .
mostbet casino скачать mostbet casino скачать .
мостбет скачать на андроид mostbet22032.ru .
Вы приобретаете документ через надежную компанию. Приобрести диплом академии– http://luxsteel.ru/realno-li-kupit-diplom-s-reestrom/ – luxsteel.ru/realno-li-kupit-diplom-s-reestrom/
mostbet oficial http://mostbet22031.ru/ .
интернет провайдеры по адресу самара
samara-domashnij-internet001.ru
какие провайдеры интернета есть по адресу самара
Купить диплом о высшем образовании !
Приобретение диплома любого ВУЗа России у нас является надежным делом, поскольку документ будет заноситься в реестр. Быстро и просто заказать диплом любого ВУЗа diplomp-irkutsk.ru/kupit-nastoyashij-diplom-3
комплексное продвижение сайтов москва https://prodvizhenie-sajtov-v-moskve231.ru .
seo network http://www.prodvizhenie-sajtov-v-moskve232.ru .
заказать продвижение сайта в москве заказать продвижение сайта в москве .
Самые лучшие проститутки в Новосибирске, подробнее тут зрелые проститутки
Скачать тикток мод на андроид Новый тикток Скачать тик ток мод последняя версию Тик ток мод 2025 Скачать тик ток мод 2025. Стремительное развитие технологий не стоит на месте, и разработчики постоянно предлагают новые версии модов, обещая еще больше возможностей и улучшенную стабильность. “ТикТок мод 2025” – звучит заманчиво, не правда ли? Однако, стоит помнить, что за громкими обещаниями может скрываться обычный маркетинговый ход.
вам делать будущие мероприятия более вам делать будущие мероприятия более
Hey fellow traders,
Just wanted to share a quick note about dayscafx.jp — I started using it more regularly and I’m honestly impressed. It’s not just charts; they provide volatility metrics, movement ranges, and even highlight “hot” currency pairs for the day.
For someone doing daily or short-term trading, it’s super useful to glance at in the morning and spot where the action might be. It’s quickly become part of my daily prep routine.
Anyone else using it? Would love to hear how you integrate it into your workflow or if there are any features I’ve missed.
продвижение в google prodvizhenie-sajtov-v-moskve231.ru .
Приобрести диплом любого университета!
Мы предлагаем документы ВУЗов, которые расположены на территории всей РФ.
diplomass.com/kupit-diplom-s-reestrom-bistro-i-nadezhno-4/
Регистрация ИП в Грузии — ваш путь к ВНЖ. Минимальные вложения, выгодные налоги (до 1% прибыли|низкая налоговая ставка). Регистрация под ключ за 3 дня: помощь с документами, открытие счета, консультации. ВНЖ без покупки недвижимости — достаточно открыть бизнес. Перспективы для вашего проекта. Начните с Грузии — основа для глобального развития.
заказать продвижение сайта в москве заказать продвижение сайта в москве .
продвинуть сайт в москве http://prodvizhenie-sajtov-v-moskve232.ru/ .
Заказать документ университета можно у нас. Заказать диплом ВУЗа по выгодной стоимости возможно, обращаясь к проверенной специализированной компании. vock-marking.copiny.com/question/details/id/1105125
Где приобрести диплом по актуальной специальности?
Мы готовы предложить дипломы любой профессии по выгодным ценам. Важно, чтобы документы были доступными для подавляющей массы граждан. Заказать диплом об образовании fastdiploms.com/kupit-diplom-s-reestrom-tsena-10/
аудит продвижения сайта https://prodvizhenie-sajtov-v-moskve235.ru/ .
seo partner prodvizhenie-sajtov-v-moskve234.ru .
скачать мостбет официальный http://www.mostbet22032.ru .
1×win 1×win .
Регистрация бизнес в Грузии — ваш путь к ВНЖ. Минимальные инвестиции, выгодные налоги (до 1% прибыли|низкая налоговая ставка). Регистрация под ключ за рабочих дня: помощь с документами, открытие счета, консультации. ВНЖ без покупки недвижимости — условие — регистрация компании. Перспективы для вашего проекта. Начните с Грузии — основа для глобального развития.
Discover bbw sex dolls that will surprise you, with us.
What you need to know about bbw sex dolls, in our review.
Review of the best bbw sex dolls on the market, come in.
BBW sex doll buying guide, for your comfort.
BBW sex doll – the perfect gift, at an affordable price.
BBW dolls that have a unique style, learn about our assortment.
Get the most out of your bbw sex doll, awaiting you.
BBWs in the world of sex toys: what you need to know, make the right choice.
Caring for a bbw sex doll – simple recommendations, everything you need to know.
Pros and cons of a bbw sex doll, useful information.
Reliable bbw sex doll for long-term use, affordable models.
Best BBW Sex Dolls Rankings, on the website.
Unique BBW Sex Dolls for a Personalized Approach, try it now.
BBWs and Their Impact on Current Trends, come and Find Out.
High-quality bbw sex dolls to satisfy any desire, don’t miss the chance.
Advantages of buying a bbw sex doll online, in today’s reality.
The latest trends in the world of bbw sex dolls, on our website.
Important aspects of choosing a bbw sex doll, watch our video review.
Discover the diversity of bbw sex dolls, all new products in one place.
bbw images bbw live sex .
Купить диплом университета!
Наши специалисты предлагаютбыстро и выгодно приобрести диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями. Диплом пройдет лубую проверку, даже при использовании специфических приборов. Достигайте цели максимально быстро с нашим сервисом- forum.vorchun.ru/viewtopic.phpf=2&t=335863
залить бетоном площадку под машину betonnaya-parkovka-1122.ru .
mostbet официальный сайт вход http://mostbet22031.ru/ .
Приобрести диплом о высшем образовании. Производство документа занимает гораздо меньше времени, а стоимость при этом невысокая. Таким образом вы имеете возможность сберечь бюджет и найти работу вашей мечты. Купить диплом возможно используя сайт компании. – domkodeks.ru/question/diplomy-dlya-uspeshnoj-karery-pokupka-bez-problem
Приобрести диплом ВУЗа по доступной стоимости возможно, обратившись к надежной специализированной фирме. Мы готовы предложить документы ВУЗов на Ваш выбор, которые расположены в любом регионе России. 007tur.ru/diplom-s-verifikatsiej-v-reestre-bistro-i-nadezhno/
buy tiktok ads https://tiktok-ads-agency-account.org
1 dby 1 dby .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje, можете посмотреть на сайте: ремонт холодильников gorenje в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Заказать диплом на заказ в столице вы имеете возможность используя сайт компании. orikdok-4v-gorode-penza-58.online
Приобрести диплом любого института. Покупка диплома ВУЗа через качественную и надежную компанию дарит множество преимуществ. Такое решение помогает сберечь как личное время, так и существенные средства. orikdok-5v-gorode-chelyabinsk-74.ru
Ремонт электронники в Москве, ремонтируем почти всю электроннику – ремонт
Приобрести диплом института по невысокой стоимости возможно, обращаясь к проверенной специализированной компании. Приобрести документ университета вы имеете возможность в нашей компании в столице. orikdok-5v-gorode-kazan-16.ru
Мы предлагаем дипломы любой профессии по приятным ценам. Мы предлагаем документы техникумов, расположенных в любом регионе Российской Федерации. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это позволяет делать государственные дипломы, которые не отличить от оригиналов. orikdok-v-gorode-volgograd-34.ru
промокоды для 1win https://www.1win22063.ru .
Ремонт электронники в Москве, ремонтируем почти всю электроннику – repair center
Erotic bbw dolls – your new passion, in our store.
Advantages of bbw dolls, in our review.
Review of the best bbw sex dolls on the market, learn more.
BBW sex doll buying guide, to get a quality product.
BBW sex doll – the perfect gift, for any preference.
Erotic bbw sex dolls for your pleasure, visit our website.
Wide selection of bbw sex doll models, discounts and promotions.
BBWs in the world of sex toys: what you need to know, so as not to make a mistake.
How to extend the life of a bbw sex doll, everything you need to know.
Pros and cons of a bbw sex doll, in our blog.
Reliable bbw sex doll for long-term use, prices will pleasantly surprise.
Best BBW Sex Dolls Rankings, in our store.
Variety of BBW Sex Doll Styles, in our store.
BBWs and Their Impact on Current Trends, What You Need to Know.
Give yourself a bbw sex doll and enjoy, treat yourself.
Why you should order a bbw sex doll online, in today’s reality.
What’s new in the range of bbw sex dolls?, on our website.
How not to make a mistake when choosing a bbw sex doll, from professionals.
Huge selection of bbw sex dolls, all new products in one place.
bbw photos sex cam bbw .
монтаж натяжного потолка монтаж натяжного потолка .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje сервис, можете посмотреть на сайте: ремонт холодильников gorenje в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Pasarhoki daftar
бк 1win официальный бк 1win официальный .
сайт 1win http://www.1win22063.ru .
Заказать диплом университета по невысокой цене возможно, обращаясь к проверенной специализированной фирме. Заказать диплом о высшем образовании: ros.listbb.ru/viewtopic.phpf=2&t=3110
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje рядом, можете посмотреть на сайте: ремонт холодильников gorenje цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Где приобрести диплом специалиста?
Получаемый диплом с необходимыми печатями и подписями отвечает стандартам, никто не сумеет отличить его от оригинала. Заказать диплом о среднем образовании – не проблема! t20suzuki.com/phpBB2/posting.php
Мы оказываем услуги по производству и продаже документов об окончании любых университетов Российской Федерации. Документы производят на настоящих бланках. medvozerniy.flybb.ru/viewtopic.phpf=14&t=14007
бонусы спорт 1win бонусы спорт 1win .
мостбет кг мостбет кг .
1 win скачать 1 win скачать .
mostbet скачать 2024 https://mostbet22033.ru/ .
Ремонт электронники в Москве, ремонтируем почти всю электроннику – ремонт
análisis de vibraciones
análisis de vibraciones
1 win букмекерская контора официальный сайт 1 win букмекерская контора официальный сайт .
La Nivelación de Partes Móviles: Esencial para una Operación Sin Vibraciones
¿Alguna vez has notado vibraciones extrañas en una máquina? ¿O tal vez ruidos que no deberían estar ahí? Muchas veces, el problema está en algo tan básico como una irregularidad en un componente giratorio . Y créeme, ignorarlo puede costarte bastante dinero .
El equilibrado de piezas es una tarea fundamental tanto en la fabricación como en el mantenimiento de maquinaria agrícola, ejes, volantes, rotores y componentes de motores eléctricos . Su objetivo es claro: evitar vibraciones innecesarias que pueden causar daños serios a largo plazo .
¿Por qué es tan importante equilibrar las piezas?
Imagina que tu coche tiene una llanta mal nivelada . Al acelerar, empiezan las sacudidas, el timón vibra y resulta incómodo circular así. En maquinaria industrial ocurre algo similar, pero con consecuencias aún peores :
Aumento del desgaste en bearings y ejes giratorios
Sobrecalentamiento de elementos sensibles
Riesgo de colapsos inesperados
Paradas imprevistas que exigen arreglos costosos
En resumen: si no se corrige a tiempo, una mínima falla podría derivar en una situación compleja.
Métodos de equilibrado: cuál elegir
No todos los casos son iguales. Dependiendo del tipo de pieza y su uso, se aplican distintas técnicas:
Equilibrado dinámico
Perfecto para elementos que operan a velocidades altas, tales como ejes o rotores . Se realiza en máquinas especializadas que detectan el desequilibrio en múltiples superficies . Es el método más preciso para garantizar un funcionamiento suave .
Equilibrado estático
Se usa principalmente en piezas como neumáticos, discos o volantes de inercia. Aquí solo se corrige el peso excesivo en un plano . Es rápido, fácil y funcional para algunos equipos .
Corrección del desequilibrio: cómo se hace
Taladrado selectivo: se perfora la región con exceso de masa
Colocación de contrapesos: por ejemplo, en llantas o aros de volantes
Ajuste de masas: común en cigüeñales y otros componentes críticos
Equipos profesionales para detectar y corregir vibraciones
Para hacer un diagnóstico certero, necesitas herramientas precisas. Hoy en día hay opciones disponibles y altamente productivas, por ejemplo :
✅ Balanset-1A — Tu aliado portátil para equilibrar y analizar vibraciones
мостбэт мостбэт .
Приобрести диплом о высшем образовании!
Мы предлагаем дипломы любой профессии по доступным тарифам— diploml-174.ru
1win что за контора 1win что за контора .
Заказать диплом ВУЗа!
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Вы заказываете диплом в надежной и проверенной компании. : drahthaar-forum.ru/viewtopic.phpf=30&t=13160&sid=88c21dcef565cea4a3ce4175d1cebdf3
мостбет официальный сайт вход мостбет официальный сайт вход .
мостбет оператор мостбет оператор .
1win казино скачать 1win казино скачать .
электрика заказать москва вызвать электрика в москве
Наша компания предлагает быстро и выгодно приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями должностных лиц. Диплом способен пройти лубую проверку, даже при помощи специального оборудования. ussd.5nx.ru/viewtopic.phpf=11&t=402
Güvenilir Kumar Siteleri
Ремонт электронники в Москве, ремонтируем почти всю электроннику – ремонт
tiktok ad accounts https://buy-tiktok-business-account.org
раскрутка и продвижение сайта раскрутка и продвижение сайта .
buy tiktok ad account https://buy-tiktok-ads.org
продвижение сайта продвижение сайта .
lucky jet статистика за сутки lucky jet статистика за сутки .
проект перепланировки квартиры для согласования цена http://www.proekt-pereplanirovki-kvartiry11.ru .
Мы изготавливаем дипломы любой профессии по приятным ценам. Приобретение диплома, который подтверждает окончание ВУЗа, – это выгодное решение. Приобрести диплом любого университета: mycountrymyjob.com/employer/diplomirovans
Диплом любого университета Российской Федерации!
Без наличия диплома очень трудно было продвинуться вверх по карьере. Заказать диплом под заказ можно через сайт компании: lada-xray.net/member.phpu=2434
Читать далее Стероиды, анаболики
сухие трансформаторы 10 0.4 suhie-transformatory-kupit2.ru .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje цены, можете посмотреть на сайте: ремонт холодильников gorenje
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
продать ролекс бу оригинал https://www.prodaja-rolex-chasi11.ru .
analizador de vibrasiones
Balanceo móvil en campo:
Soluciones rápidas sin desmontar máquinas
Imagina esto: tu rotor comienza a vibrar, y cada minuto de inactividad cuesta dinero. ¿Desmontar la máquina y esperar días por un taller? Descartado. Con un equipo de equilibrado portátil, solucionas el problema in situ en horas, sin alterar su posición.
¿Por qué un equilibrador móvil es como un “herramienta crítica” para máquinas rotativas?
Fácil de transportar y altamente funcional, este dispositivo es el recurso básico en cualquier intervención. Con un poco de práctica, puedes:
✅ Evitar fallos secundarios por vibraciones excesivas.
✅ Reducir interrupciones no planificadas.
✅ Operar en zonas alejadas, ya sea en instalaciones marítimas o centrales solares.
¿Cuándo es ideal el equilibrado rápido?
Siempre que puedas:
– Contar con visibilidad al sistema giratorio.
– Colocar sensores sin interferencias.
– Ajustar el peso (añadiendo o removiendo masa).
Casos típicos donde conviene usarlo:
La máquina muestra movimientos irregulares o ruidos atípicos.
No hay tiempo para desmontajes (operación prioritaria).
El equipo es costoso o difícil de detener.
Trabajas en áreas donde no hay asistencia mecánica disponible.
Ventajas clave vs. llamar a un técnico
| Equipo portátil | Servicio externo |
|—————-|——————|
| ✔ Rápida intervención (sin demoras) | ❌ Retrasos por programación y transporte |
| ✔ Mantenimiento proactivo (previenes daños serios) | ❌ Suele usarse solo cuando hay emergencias |
| ✔ Reducción de costos operativos con uso continuo | ❌ Gastos periódicos por externalización |
¿Qué máquinas se pueden equilibrar?
Cualquier sistema rotativo, como:
– Turbinas de vapor/gas
– Motores industriales
– Ventiladores de alta potencia
– Molinos y trituradoras
– Hélices navales
– Bombas centrífugas
Requisito clave: hábitat adecuado para trabajar con precisión.
Tecnología que simplifica el proceso
Los equipos modernos incluyen:
Apps intuitivas (guían paso a paso, sin cálculos manuales).
Evaluación continua (informes gráficos comprensibles).
Durabilidad energética (útiles en ambientes hostiles).
Ejemplo práctico:
Un molino en una mina comenzó a vibrar peligrosamente. Con un equipo portátil, el técnico localizó el error rápidamente. Lo corrigió añadiendo contrapesos y evitó una parada de 3 días.
¿Por qué esta versión es más efectiva?
– Estructura más dinámica: Organización visual facilita la comprensión.
– Enfoque práctico: Se añaden ejemplos reales y comparaciones concretas.
– Lenguaje persuasivo: Frases como “herramienta estratégica” o “minimizas riesgos importantes” refuerzan el valor del servicio.
– Detalles técnicos útiles: Se especifican requisitos y tecnologías modernas.
¿Necesitas ajustar el tono (más comercial) o añadir keywords específicas? ¡Aquí estoy para ayudarte! ️
скачать букмекерскую контору официального сайта скачать букмекерскую контору официального сайта .
Скачать тик ток мод на айфон 2025 Тикток мод на айфон – это запрос, который всё чаще встречается в поисковых системах, отражая стремление пользователей расширить функциональность популярного приложения на своих устройствах Apple. Но что же такое “мод” в контексте TikTok и как его можно получить на iPhone?
профессиональное продвижение сайтов профессиональное продвижение сайтов .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips в москве, можете посмотреть на сайте: ремонт кофемашин philips
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
продвижение сайтов интернет магазины в москве http://www.prodvizhenie-sajtov-v-moskve235.ru/ .
Заказать документ ВУЗа можно в нашей компании в Москве. Купить диплом университета по выгодной стоимости вы можете, обратившись к проверенной специализированной компании. flughafen-jobs.com/companies/frees-diplom
1win скачать последнюю версию 1win скачать последнюю версию .
find out here now jaxx wallet
скачать бк осталось только найти предпочтение подходящее скачать бк осталось только найти предпочтение подходящее .
Купить диплом любого университета!
Мы предлагаем документы университетов, расположенных на территории всей России.
diplomk-vo-vladivostoke.ru/kupite-diplom-s-reestrom-i-otzivami-bez-xlopot/
достопримечатнельности саратова Саратов – город с богатой историей и культурой, раскинувшийся на живописных берегах Волги. Этот волжский край манит туристов своим неповторимым колоритом, архитектурным наследием и удивительными природными ландшафтами. Если вы планируете посетить Саратов, будьте уверены – вас ждет незабываемое путешествие, полное открытий и ярких впечатлений.
Приобрести диплом любого университета!
Наши специалисты предлагаютвыгодно приобрести диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями официальных лиц. Документ пройдет лубую проверку, даже при использовании специальных приборов. Решайте свои задачи максимально быстро с нашими дипломами- prepperforum.se/showthread.phptid=75795
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips адреса, можете посмотреть на сайте: ремонт кофемашин philips сервис
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
зайти в мостбет mostbet22035.ru .
https://millionigrushek.ru
this article jaxx wallet online
Мы предлагаем дипломы психологов, юристов, экономистов и других профессий по выгодным тарифам. Заказ диплома, подтверждающего обучение в университете, – это грамотное решение. Приобрести диплом любого университета: peticija.online/483974
Купить диплом университета по доступной цене возможно, обращаясь к надежной специализированной фирме. Заказать документ ВУЗа вы имеете возможность в нашей компании в столице. orikdok-1v-gorode-rostov-na-donu-61.ru
Заказать диплом любого университета. Приобретение документа о высшем образовании через надежную компанию дарит немало достоинств. Данное решение помогает сберечь как личное время, так и существенные деньги. orikdok-3v-gorode-tolyatti-63.ru
Приобрести диплом под заказ в Москве возможно через официальный портал компании. orikdok-4v-gorode-nalchik-7.ru
wechat for desktop pc free download
wechat desktop backup
wechat desktop app download
wechat for desktop windows 0
WeChat
матовый натяжной потолок матовый натяжной потолок .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт холодильников gorenje цены, можете посмотреть на сайте: ремонт холодильников gorenje цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Узнать больше Спортивная фармакология
Заказать диплом университета!
Мы предлагаем документы университетов, расположенных на территории всей России.
good-diplom.ru/kupit-podlinnij-diplom-s-zaneseniem-v-reestr-2/
Купить диплом ВУЗа!
Наши специалисты предлагаютвыгодно купить диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями официальных лиц. Наш диплом пройдет лубую проверку, даже при помощи профессионального оборудования. Решайте свои задачи быстро с нашими дипломами- extra-v.store/forum/PAGE_NAME=profile_view&UID=3489
бетонирование парковки https://betonnaya-parkovka-1122.ru .
kumar siteleri 2025
Заказать диплом института по доступной стоимости вы сможете, обратившись к проверенной специализированной компании. Приобрести документ института вы можете в нашем сервисе. orikdok-2v-gorode-ekaterinburg-66.ru
Где приобрести диплом по актуальной специальности?
Купить диплом ВУЗа по невысокой стоимости возможно, обратившись к надежной специализированной компании.: sdiplom.ru
Купить диплом вы можете используя сайт компании. orikdok-4v-gorode-omsk-55.ru
Приобрести диплом института. Приобретение документа о высшем образовании через надежную компанию дарит много плюсов. Данное решение помогает сберечь время и значительные денежные средства. orikdok-5v-gorode-orenburg-56.ru
Заказать диплом института!
Мы изготавливаем дипломы любых профессий по невысоким тарифам— net-dir.ru
залить бетоном площадку под машину http://www.betonnaya-parkovka-1122.ru .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips цены, можете посмотреть на сайте: ремонт кофемашин philips
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
заказать проект перепланировки москва [url=www.proekt-pereplanirovki-kvartiry11.ru]www.proekt-pereplanirovki-kvartiry11.ru[/url] .
aviator игра скачать aviator игра скачать .
скачать бесплатно мостбет http://www.mostbet22037.ru .
basics precio de munjaro
1win ставки на спорт 1win ставки на спорт .
где купить диплом о высшем образовании https://sx-dipl.online .
Новости Винницы https://faine-misto.vinnica.ua сайт Винницы, городской портал, обзоры, места.
трансформатор сухой 400 ква цена https://suhie-transformatory-kupit2.ru .
проектное бюро перепланировка квартиры https://proekt-pereplanirovki-kvartiry11.ru .
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Дипломы производятся на подлинных бланках Заказать диплом об образовании diplomus-spb.ru
ломбард часов rolex http://www.prodaja-rolex-chasi11.ru .
Discover bbw sex dolls that will surprise you, with us.
Why choose a bbw sex doll?, to make the right choice.
Unique bbw sex dolls for the bravest, don’t miss the opportunity.
How to choose the perfect bbw sex doll?, for your comfort.
Treat yourself with a bbw sex doll, at an affordable price.
BBW dolls that have a unique style, visit our website.
New experiences with bbw sex doll, awaiting you.
BBWs in the world of sex toys: what you need to know, so as not to make a mistake.
Tips for caring for your bbw sex doll, everything you need to know.
Emotions and impressions with a bbw sex doll, always relevant.
Reliable bbw sex doll for long-term use, choice will surprise.
Best Selling BBW Sex Dolls Review, in our store.
Variety of BBW Sex Doll Styles, in our store.
BBW Sex Dolls Statistics and Facts, interesting information.
Give yourself a bbw sex doll and enjoy, a purchase that will change everything.
Comparison of prices for bbw sex dolls online, fast and convenient.
Wake up your imagination with new bbw sex dolls, only with us.
How not to make a mistake when choosing a bbw sex doll, watch our video review.
Discover the diversity of bbw sex dolls, unrivaled quality.
bbw cam girl bbw bikini .
skachat mostbet skachat mostbet .
букмекерская контора теннесси скачать http://www.mostbet22038.ru .
как отыграть бонус в 1win как отыграть бонус в 1win .
сухой трансформатор 1000 ква suhie-transformatory-kupit2.ru .
Приветственные бонусы и промоакции 1xBet 2025
Используйте как ввести промокод 1xbet чтобы получить гарантированный бонус в размере 100% до 32500 рублей (или эквивалентную сумму в другой валюте €130). Это предложение доступно только для новых пользователей.
Воспользуйтесь этим кодом, чтобы получить бонус от 1хбет. Просто введите код в анкете при регистрации и пополните свой счет на сумму от 100 рублей. Вам будет начислен бонус в размере 130%, который может достигать до 32500 рублей.
У нас вы можете получить бесплатный промокод, который даст вам дополнительные бонусы и преимущества при игре на сайте 1xBet. Для того чтобы воспользоваться этим промокодом, вам необходимо зарегистрироваться на сайте и пройти процедуру верификации. После этого вы сможете получить бонус по промокоду или при пополнении счета. Также вы можете получить бонус, если пригласите друга или примете участие в определенных акциях.
ролекс ломбард https://prodaja-rolex-chasi11.ru/ .
Наша компания предлагает быстро и выгодно купить диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями. Диплом пройдет лубую проверку, даже с применением специального оборудования. or1gano.80lvl.ru/viewtopic.phpf=10&t=2103
Clicking Here instrucciones de analogos de dulaglutida
mostbet com https://www.mostbet22039.ru .
mostbet.com что это http://www.mostbet22038.ru .
почта 1win 1win22071.ru .
1win бонусный счет http://www.1win22069.ru .
most bet http://mostbet22040.ru/ .
1win ставки на спорт зеркало http://1win22074.ru/ .
1вин бк https://1win22067.ru .
скачать 1win официальный сайт бесплатно http://www.1win22069.ru .
букмекерская контора mostbet mostbet22040.ru .
1win gmail 1win gmail .
Приобрести документ о получении высшего образования можно у нас в Москве. Приобрести диплом университета по выгодной стоимости возможно, обращаясь к надежной специализированной фирме. lxgonline.mn.co/posts/84445092
авиатор казино http://www.1win22072.ru .
1win ставки 1win22068.ru .
Гул толпы, вспышки света, и над всем этим – голос. Голос, проникающий сквозь броню безразличия, заставляющий кровь кипеть, а сердце – биться в унисон с ритмом. Это не просто музыка, это ЯНК. ЯНК – это вызов, это протест, это крик души, облаченный в форму мелодии и текста. Это музыка, которая не оставляет равнодушным, музыка, которая заставляет задуматься, почувствовать, жить. Это энергия, бьющая через край, способная зажечь искру в самом закоренелом скептике. Погрузитесь в мир ЯНК-музыки. Откройте для себя новые горизонты звука, ощутите силу слова, почувствуйте вибрацию, пронизывающую все ваше существо. Подписывайтесь на канал, чтобы не пропустить новые релизы, эксклюзивные интервью, живые выступления и закулисные истории. Присоединяйтесь к движению. Станьте частью семьи ЯНК. Вместе мы можем изменить этот мир, начав с изменения себя. Музыка – это оружие, а ЯНК – это его острие. Не упустите свой шанс стать свидетелем рождения новой эпохи в музыке. Подписывайтесь прямо сейчас и будьте в курсе всех событий! https://vk.com/club230513775 ЯНК-музыка видео, подписывайтесь.
парковочная зона на участке парковочная зона на участке .
mostbet mobile mostbet mobile .
кракен33 Кракен. Слово, звучащее как шепот древних морей, как отголосок легенд, рожденных в штормовых ночах. Кракен – не просто имя. Это портал. Вход в мир, где анонимность – щит, а возможности – безграничны. Кракен вход – это цифровой пропуск в лабиринт, где каждый поворот сулит новые открытия, новые риски, новую свободу.
Мы изготавливаем дипломы любых профессий по приятным ценам. Мы предлагаем документы ВУЗов, которые расположены на территории всей РФ. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. orikdok-v-gorode-samara-63.online
1win скачать мобильный телефон https://1win22068.ru/ .
Заказать диплом о высшем образовании. Покупка документа о высшем образовании через надежную компанию дарит ряд плюсов для покупателя. Данное решение позволяет сберечь время и существенные финансовые средства. orikdok-3v-gorode-chelyabinsk-74.online
1win букмекер http://1win22074.ru .
Купить документ о получении высшего образования вы имеете возможность в нашей компании в Москве. Приобрести диплом института по выгодной цене вы можете, обращаясь к надежной специализированной компании. direct-jobs.nl/employer/frees-diplom
площадка под машину из бетона https://www.betonnaya-parkovka-1122.ru .
Приобрести диплом ВУЗа по выгодной стоимости можно, обратившись к надежной специализированной компании. Мы оказываем услуги по изготовлению и продаже документов об окончании любых ВУЗов России. Заказать диплом любого ВУЗа– oolibuzz.com/read-blog/3061_kupit-attestat-11-klassov.html
Купить диплом любого университета!
Мы можем предложить документы институтов, которые находятся на территории всей России.
good-diplom.ru/kupit-diplom-s-zaneseniem-v-reestr-bistro-i-bezopasno-6/
как потратить бонусы в 1 win как потратить бонусы в 1 win .
лаки джет играть – скачать lucky jet, lucky jet стратегия
Мы можем предложить дипломы любой профессии по разумным тарифам. Мы можем предложить документы техникумов, которые расположены в любом регионе РФ. Документы выпускаются на “правильной” бумаге самого высокого качества. Это дает возможность делать настоящие дипломы, не отличимые от оригинала. orikdok-1v-gorode-orenburg-56.ru
Приобрести диплом ВУЗа!
Наша компания предлагаетбыстро и выгодно купить диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями должностных лиц. Данный документ способен пройти любые проверки, даже при помощи специфических приборов. Решите свои задачи быстро и просто с нашим сервисом- formfinance.ru/kak-pravilno-oformit-pokupku-diploma
1win мобильная версия сайта 1win мобильная версия сайта .
натяжные потолки премиум натяжные потолки премиум .
Дневник скетчера
Купить диплом о высшем образовании!
Быстро купить диплом о высшем образовании. Приобретение диплома через надежную компанию дарит ряд плюсов для покупателя. Такое решение дает возможность сэкономить как продолжительное время, так и значительные финансовые средства. app.vellorepropertybazaar.in/profile/jerribarnard55
Заказать диплом любого университета!
Приобретение документа о высшем образовании через проверенную и надежную фирму дарит массу преимуществ для покупателя. Приобрести диплом любого университета у проверенной организации: doks-v-gorode-novocheboksarsk-21.ru
Приобрести диплом института по невысокой стоимости возможно, обращаясь к надежной специализированной фирме. Заказать документ о получении высшего образования можно у нас в столице. orikdok-5v-gorode-omsk-55.online
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Quel est le code promo Linebet 2025 ?
Le code promo Linebet est :
https://www.locafilm.com/wp-includes/pages/4code_promo_linebet_bonus_.html . Ce code promo vous permet d’obtenir 100 % de bonus a l’issue de votre 1er depot. En rejoignant Linebet, vous pourrez donc gagner jusqu’a 100 $ de freebets.
Vous n’aurez plus qu’a rejouer 5 fois votre bonus en combine pour le convertir en argent reel (3 selections ou plus / cote minimum : 1,40).
Code promo Linebet paris sportifs : jusqu’a 100 $ de bonus de bienvenue
Avec le code promo Linebet, vous profiterez d’un doublement de votre 1er depot jusqu’a 100 $ si vous choisissez le bonus de bienvenue paris sportifs lors de votre inscription.
Choisissez l’offre bonus de bienvenue paris sportifs lors de votre inscription pour en beneficier. C’est l’un des meilleurs avantages pour demarrer chez ce bookmaker.
C’est simple : deposez 50 $ et gagnez 50 $ supplementaires. Jouez 100 $ pour obtenir le bonus maximum de 100 $.
ll faut le rejouer 5 fois en pari combine (avec au moins 3 selections ayant une cote de 1,40 ou plus) pour transformer cet argent bonus en cash retirable et recuperer vos gains.
Cette condition est courante chez les bookmakers en ligne, similaire a ce que propose 1xBet. Assurez-vous que le code marche et que l’offre est valide dans votre pays.
Comment ouvrir un compte avec le code promo Linebet en Mai 2025 ?
Le code promo Linebet doit etre insere dans le formulaire d’ouverture : il vous permettra d’obtenir le bonus de bienvenue paris sportifs ou poker (au choix).
En vous inscrivant, vous pourrez aussi prendre part a toutes les offres promotionnelles mises en place par le site.
Le processus d’inscription a ete allege au maximum pour que vous profitiez au plus vite d’une experience de jeu inedite. Inscrivez-vous maintenant pour profiter de cotes allechantes pour maximiser vos gains !
скачать бк осталось именно выбрать подходящее скачать бк осталось именно выбрать подходящее .
1-win 1-win .
накрутка подписчиков в телеграм канал бесплатно накрутка подписчиков в телеграм канал бесплатно
Мы изготавливаем дипломы любых профессий по приятным тарифам. Приобретение документа, подтверждающего обучение в ВУЗе, – это выгодное решение. Приобрести диплом любого университета: mireait.listbb.ru/viewtopic.phpf=2&t=1227
Thanks for the article https://l-spb.ru/
Приобрести диплом под заказ вы имеете возможность через официальный сайт компании. orikdok-3v-gorode-yaroslavl-76.ru
Заказать диплом университета по невысокой цене возможно, обращаясь к надежной специализированной фирме. Мы предлагаем документы Институтов, которые находятся в любом регионе Российской Федерации. studentklass.ru/kupit-diplom-s-reestrom-bistro-i-legko/
продать ролекс бу оригинал http://www.prodaja-rolex-chasi12.ru .
Заказать документ института можно в нашей компании. Мы оказываем услуги по продаже документов об окончании любых ВУЗов РФ. Вы получите необходимый диплом по любым специальностям, любого года выпуска, в том числе документы старого образца. Гарантируем, что в случае проверки документа работодателем, подозрений не возникнет. diplomist.com/kupit-diplom-s-zaneseniem-v-reestr-bez-hassles/
продать ролекс продать ролекс .
uefa champions league https://footballnews.store .
Читать далее водка бэт
Good day!
Understand the difference between VAT and excise tax in UAE with our comparison guide. Don’t confuse your reporting categories. Our experts clarify how both taxes work. Learn the difference between VAT and excise tax in UAE today. Avoid common business mistakes.
All information is available via the link – https://telegra.ph/Excise–Other-Taxes-in-the-UAE-04-16
VAT on transportation services in UAE applies standard VAT rates. Transport companies must charge VAT on transportation services in UAE properly. Understanding VAT on transportation services in UAE improves billing accuracy. Get expert advice on VAT on transportation services in UAE. Ensure compliance with VAT on transportation services in UAE.
All information is available via the link – https://telegra.ph/VAT-Services-in-the-UAE-04-16
best bank for personal account in uae, vat return filing services in uae, piling shoring tunnelling services uae vat applicability
canara bank account opening in uae, uae vat treatment on transport services within uae, categories of services under vat in uae
Good luck!
пластиковые окна studio5floor.ru .
онлайн займы investinq.ru .
1вин бонус при регистрации 1вин бонус при регистрации .
mostbet app https://www.mostbet22042.ru .
https://eprajournals.com/
Заказ документа о высшем образовании через качественную и надежную фирму дарит ряд плюсов. Купить диплом: vmestedeshevle.listbb.ru/viewtopic.php?f=10&t=15322
Мы можем предложить дипломы психологов, юристов, экономистов и любых других профессий по доступным тарифам. Купить диплом в Губкине — [url=http://kyc-diplom.com/geography/gubkin.html/]kyc-diplom.com/geography/gubkin.html[/url]
Добрый день!
Беседки аренда москва — отличный выбор для семейных и дружеских встреч. Аренда беседки с мангалом москва позволит приготовить вкусный шашлык. У нас есть беседки разных размеров и комплектаций. Москва аренда беседок — сервис с удобными условиями аренды. Забронируйте беседку заранее и отдыхайте с комфортом.
Полная информация по ссылке – https://telegra.ph/TOP-7-mest-Podmoskovya-gde-mozhno-snyat-besedku-dlya-shashlyka-04-30
аренда беседок москва юг, беседка москва аренда, москва шашлыки беседки аренда
аренда летних беседок в москве, пикник аренда беседок москва, беседка для шашлыка аренда москва
Удачи и комфортного отдыха!
часовой ломбард rolex prodaja-rolex-chasi12.ru .
баланс 1win http://1win22076.ru/ .
Заказать диплом о высшем образовании !
Приобретение диплома университета России у нас – надежный процесс, так как документ заносится в государственный реестр. Приобрести диплом ВУЗа diplomv-v-ruki.ru/kupite-diplom-v-reestre-bistro-i-bezopasno
ремонт окон https://studio5floor.ru .
как выводить деньги с лаки джет http://www.1win14001.ru .
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам.– somersetent.co.uk/textstat/kak-bystro-kupit-diplom-i-ne-popastsja-189
Добрый день!
купить постоянный виртуальный номер — это цифровая свобода и приватность. Каждому нужен купить постоянный виртуальный номер для безопасности личных данных. С помощью сервиса вы легко сможете купить постоянный виртуальный номер. Современные технологии позволяют купить постоянный виртуальный номер за минуту. Если хотите остаться на связи, лучше купить постоянный виртуальный номер уже сегодня.
Полная информация по ссылке – http://www.gblsw.cn/20241017/78832838.html
виртуальный номер, купить виртуальный номер навсегда, купить виртуальный номер для смс навсегда
купить виртуальный номер для смс навсегда, постоянный виртуальный номер, купить виртуальный номер для смс навсегда
Удачи и комфорта в общении!
организация въезда на участок через канаву http://www.betonnyj-zaezd-na-uchastok-1122.ru .
СВО: Сквозь призму белых дядей в африканском контексте
Специальная военная операция в Украине, СВО, стала не только геополитическим водоразделом, но и катализатором для переосмысления многих устоявшихся представлений о мировом порядке. В этом контексте, интересно взглянуть на восприятие СВО через призму африканского континента и, в частности, феномена “белых дядей” – исторически сложившейся системы влияния, где выходцы из Европы и Северной Америки занимают доминирующие позиции в политике, экономике и социальной сфере африканских стран.
Новости СВО, поступающие в африканское медиапространство, часто интерпретируются сквозь призму колониального прошлого и неоколониальных реалий. Многие африканцы видят в конфликте в Украине продолжение борьбы за передел сфер влияния между Западом и Россией, где Африка традиционно выступает лишь в роли объекта, а не субъекта.
Белые дяди, как бенефициары существующего порядка, часто поддерживают западную точку зрения на СВО, в то время как рядовые африканцы выражают более разнообразные мнения. Многие из них видят в России противовес западному доминированию и надежду на более справедливый мировой порядок.
Влияние СВО на Африку выходит далеко за рамки политической риторики. Конфликт привел к росту цен на продовольствие и энергоносители, усугубив и без того сложную экономическую ситуацию во многих африканских странах. В этой связи, вопрос о будущем Африки и ее роли в новом мировом порядке становится особенно актуальным. Сможет ли континент вырваться из-под влияния белых дядей и занять достойное место среди мировых держав? Ответ на этот вопрос во многом зависит от исхода СВО и ее долгосрочных последствий для мировой геополитики. Сво
Доброго!
Купить виртуальный номер для смс навсегда — это правильный шаг. Услуга позволяет купить виртуальный номер для смс навсегда за пару кликов. С нами легко купить виртуальный номер для смс навсегда и использовать его без ограничений. Пользователи ценят возможность купить виртуальный номер для смс навсегда без лишних шагов.
Полная информация по ссылке – https://tarotamoureux.fr/2022/07/22/hello-world/
купить виртуальный номер навсегда, купить виртуальный номер навсегда, купить постоянный виртуальный номер
купить виртуальный номер, купить виртуальный номер навсегда, купить виртуальный номер навсегда
Удачи и комфорта в общении!
The Philippines is famous for its old, religious landmarks, and in contrast its modernized skyscrapers. 블랙핑크 섹스 동영상
lucky jet 1win скачать на андроид lucky jet 1win скачать на андроид .
Приобрести документ о получении высшего образования вы сможете в нашей компании в Москве. Приобрести диплом института по доступной цене вы сможете, обратившись к проверенной специализированной фирме. ilass.com.br/kak-bystro-i-bezopasno-kupit-diplom-v-rossii-223
букмекерская контора кыргызстан http://www.mostbet22041.ru .
1win регистрация и вход на сайт 1win регистрация и вход на сайт .
1win как использовать бонусы 1win14002.ru .
Укрбизнес https://in-ukraine.biz.ua финансовые новости Украины, обзоры, статьи, компании, ФОП и налоги
Equilibrado dinámico portátil:
Soluciones rápidas sin desmontar máquinas
Imagina esto: tu rotor inicia con movimientos anormales, y cada minuto de inactividad afecta la productividad. ¿Desmontar la máquina y esperar días por un taller? Ni pensarlo. Con un equipo de equilibrado portátil, solucionas el problema in situ en horas, sin alterar su posición.
¿Por qué un equilibrador móvil es como un “kit de supervivencia” para máquinas rotativas?
Compacto, adaptable y potente, este dispositivo es una pieza clave en el arsenal del ingeniero. Con un poco de práctica, puedes:
✅ Corregir vibraciones antes de que dañen otros componentes.
✅ Minimizar tiempos muertos y mantener la operación.
✅ Actuar incluso en sitios de difícil acceso.
¿Cuándo es ideal el equilibrado rápido?
Siempre que puedas:
– Contar con visibilidad al sistema giratorio.
– Colocar sensores sin interferencias.
– Modificar la distribución de masa (agregar o quitar contrapesos).
Casos típicos donde conviene usarlo:
La máquina presenta anomalías auditivas o cinéticas.
No hay tiempo para desmontajes (proceso vital).
El equipo es costoso o difícil de detener.
Trabajas en áreas donde no hay asistencia mecánica disponible.
Ventajas clave vs. llamar a un técnico
| Equipo portátil | Servicio externo |
|—————-|——————|
| ✔ Sin esperas (acción inmediata) | ❌ Demoras por agenda y logística |
| ✔ Mantenimiento proactivo (previenes daños serios) | ❌ Solo se recurre ante fallos graves |
| ✔ Ahorro a largo plazo (menos desgaste y reparaciones) | ❌ Costos recurrentes por servicios |
¿Qué máquinas se pueden equilibrar?
Cualquier sistema rotativo, como:
– Turbinas de vapor/gas
– Motores industriales
– Ventiladores de alta potencia
– Molinos y trituradoras
– Hélices navales
– Bombas centrífugas
Requisito clave: acceso suficiente para medir y corregir el balance.
Tecnología que simplifica el proceso
Los equipos modernos incluyen:
Aplicaciones didácticas (para usuarios nuevos o técnicos en formación).
Evaluación continua (informes gráficos comprensibles).
Batería de larga duración (perfecto para zonas remotas).
Ejemplo práctico:
Un molino en una mina mostró movimientos inusuales. Con un equipo portátil, el técnico detectó un desbalance en 20 minutos. Lo corrigió añadiendo contrapesos y evitó una parada de 3 días.
¿Por qué esta versión es más efectiva?
– Estructura más dinámica: Organización visual facilita la comprensión.
– Enfoque práctico: Se añaden ejemplos reales y comparaciones concretas.
– Lenguaje persuasivo: Frases como “kit de supervivencia” o “evitas fallas mayores” refuerzan el valor del servicio.
– Detalles técnicos útiles: Se especifican requisitos y tecnologías modernas.
¿Necesitas ajustar el tono (más comercial) o añadir keywords específicas? ¡Aquí estoy para ayudarte! ️
Equilibrio in situ
La Nivelación de Partes Móviles: Esencial para una Operación Sin Vibraciones
¿ Has percibido alguna vez temblores inusuales en un equipo industrial? ¿O sonidos fuera de lo común? Muchas veces, el problema está en algo tan básico como un desequilibrio en alguna pieza rotativa . Y créeme, ignorarlo puede costarte bastante dinero .
El equilibrado de piezas es un procedimiento clave en la producción y cuidado de equipos industriales como ejes, volantes, rotores y partes de motores eléctricos . Su objetivo es claro: impedir oscilaciones que, a la larga, puedan provocar desperfectos graves.
¿Por qué es tan importante equilibrar las piezas?
Imagina que tu coche tiene un neumático con peso desigual. Al acelerar, empiezan los temblores, el manubrio se mueve y hasta puede aparecer cierta molestia al manejar . En maquinaria industrial ocurre algo similar, pero con consecuencias mucho más graves :
Aumento del desgaste en soportes y baleros
Sobrecalentamiento de partes críticas
Riesgo de colapsos inesperados
Paradas no planificadas y costosas reparaciones
En resumen: si no se corrige a tiempo, una mínima falla podría derivar en una situación compleja.
Métodos de equilibrado: cuál elegir
No todos los casos son iguales. Dependiendo del tipo de pieza y su uso, se aplican distintas técnicas:
Equilibrado dinámico
Ideal para piezas que giran a alta velocidad, como rotores o ejes . Se realiza en máquinas especializadas que detectan el desequilibrio en múltiples superficies . Es el método más exacto para asegurar un movimiento uniforme .
Equilibrado estático
Se usa principalmente en piezas como neumáticos, discos o volantes de inercia. Aquí solo se corrige el peso excesivo en un plano . Es ágil, práctico y efectivo para determinados sistemas.
Corrección del desequilibrio: cómo se hace
Taladrado selectivo: se elimina material en la zona más pesada
Colocación de contrapesos: como en ruedas o anillos de volantes
Ajuste de masas: habitual en ejes de motor y partes relevantes
Equipos profesionales para detectar y corregir vibraciones
Para hacer un diagnóstico certero, necesitas herramientas precisas. Hoy en día hay opciones accesibles y muy efectivas, como :
✅ Balanset-1A — Tu aliado portátil para equilibrar y analizar vibraciones
1win сайт вход 1win22079.ru .
Где приобрести диплом по актуальной специальности?
Получаемый диплом со всеми печатями и подписями полностью отвечает условиям и стандартам, никто не сумеет отличить его от оригинала. Диплом о высшем образовании – запросто! carilion.it/2025/04/16/kupit-diplom-gosudarstvennogo-obrazca-298-2
Мы предлагаем документы об окончании любых ВУЗов РФ. Документы производят на настоящих бланках государственного образца. freebeg.com/forum/member.php
Hello!
Know exactly when excise tax is payable in UAE to avoid delays. We help you manage cash flow and filing timelines. Don’t get caught by surprise. Understand when excise tax is payable in UAE with our expert schedule. Book a deadline review today.
All information is available via the link – https://telegra.ph/Excise–Other-Taxes-in-the-UAE-04-16
VAT on import of services in UAE requires businesses to account for VAT using reverse charge. Many companies overlook VAT on import of services in UAE, risking fines. Experts advise on VAT on import of services in UAE compliance. Ensure proper VAT on import of services in UAE filings. Get help with VAT on import of services in UAE.
All information is available via the link – https://telegra.ph/VAT-Services-in-the-UAE-04-16
dubai islamic bank uae online account opening, how can i open bank account in uae, how to apply a personal bank account in uae
open bank account in uae without residence visa, how to check personal account status with uae central bank, iranian born can open bank account in uae
Good luck!
mostbet казино скачать mostbet казино скачать .
мостбет вход в личный кабинет https://mostbet22044.ru .
most bet skachat most bet skachat .
займ оформить онлайн http://www.investinq.ru .
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Стоимость может зависеть от выбранной специальности, года выпуска и университета: apneacalculator.com/2025/05/09/kupit-diplom-bystro-i-nadezhno-luchshie-144
Доброго!
Беседка аренда мангал москва — удобный способ провести время на природе. Беседка мангал москва аренда позволяет устроить настоящий шашлычный праздник. Аренда беседок теплых москва — сервис для комфортного отдыха. Пикник в москве аренда беседок — популярное решение для семей и друзей. Забронируйте беседку с мангалом заранее.
Полная информация по ссылке – https://telegra.ph/Besedki-na-Losinom-Ostrove-obzor-zon-otdyha-Biostanciya-i-Dendrarij-04-30
аренда мангала беседки в москве, теплая беседка москва аренда, аренда беседка шашлыки москва
закрытые беседки аренда москва, зимние беседки аренда москва, аренда беседок москва вао
Удачи и комфортного отдыха!
Вы заказываете диплом в надежной и проверенной временем компании. Заказать диплом академии– http://kiddygames.ru/kupit-diplom-menedzhera-s-zaneseniem-v-reestr-bistro-2/ – kiddygames.ru/kupit-diplom-menedzhera-s-zaneseniem-v-reestr-bistro-2/
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы предлагаем документы ВУЗов, которые находятся на территории всей Российской Федерации. Дипломы и аттестаты делаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. orikdok-v-gorode-penza-58.ru
регистрация на мостбет https://mostbet22042.ru .
Приобрести диплом об образовании. Изготовление документа занимает минимум времени, а цена – невысокая. В результате вы сможете сберечь бюджет и получить работу вашей мечты. Приобрести диплом на заказ возможно через сайт компании. – u-ssr.com/read-blog/2398_diplom-medicinskoj-sestry-kupit.html
выкуп rolex выкуп rolex .
1win скачать на ios http://1win14002.ru .
нажмите, чтобы подробнее
the dog house demo в рублях
футбол лига чемпионов финал http://www.footballnews.store/ .
Greetings!
Searching for 0 balance bank account opening in UAE? We know banks that offer zero balance accounts without hidden fees. Perfect for students, freelancers, and low-income earners. Let us help you apply for 0 balance bank account opening in UAE hassle-free. Connect with us now.
More information is available via the link – https://telegra.ph/Open-a-Corporate-Bank-Account-in-the-UAE-04-16
Export of services UAE VAT rules allow zero-rating when conditions are met. Businesses can benefit from export of services under UAE VAT by reducing tax burden. Correct invoicing is essential for export of services UAE VAT compliance. Consultants guide on export of services under UAE VAT regulations. Export your services confidently under UAE VAT.
All information is available via the link – https://telegra.ph/VAT-Services-in-the-UAE-04-16
vat applicability of security services in uae pdf, how to get service tax registration certificate online uae, vat applicable services in uae
uae vat airport transfer service, how to open online bank account in nepal from uae, percentage of vat in uae for oilfield services
Good luck!
mostbet сайт https://www.mostbet22045.ru .
Здравствуйте!
Теплые беседки аренда Москва — отличный вариант для тех, кто не боится прохлады. Аренда беседок с мангалом Москва поможет устроить барбекю в любое время года. Беседка аренда Москва с отоплением обеспечит уют и тепло даже в холодную погоду. Выбирайте аренда беседок Москва для комфортного отдыха и приятного времяпрепровождения. Забронируйте беседку заранее, чтобы получить лучшие условия.
Полная информация по ссылке – https://telegra.ph/Biryulevskij-dendropark-arenda-besedok-s-mangalom-na-yuge-Moskvy-04-30
аренда москва беседка с мангалом, беседки теплые москве аренда, беседки с мангалами аренда москва
аренда беседок москва с мангалом, аренда беседок на севере москвы, аренда беседку в москве
Удачи и комфортного отдыха!
Приобрести диплом института по доступной цене можно, обратившись к проверенной специализированной фирме. Купить документ института можно в нашей компании в столице. orikdok-4v-gorode-krasnodar-23.online
1вин официальный мобильная http://1win14003.ru/ .
Erotic bbw dolls – your new passion, variety of models.
Advantages of bbw dolls, to make the right choice.
Review of the best bbw sex dolls on the market, come in.
How to choose the perfect bbw sex doll?, to get a quality product.
Secrets of choosing a bbw sex doll in our catalog, at an affordable price.
BBWs are not only dolls, they are pleasure, don’t forget to check out.
Wide selection of bbw sex doll models, only with us.
How to choose a bbw sex doll for yourself, make the right choice.
Caring for a bbw sex doll – simple recommendations, from professionals.
How a bbw sex doll can change your life, always relevant.
Premium bbw sex doll for true connoisseurs, prices will pleasantly surprise.
Best Selling BBW Sex Dolls Review, in our store.
Perfect BBW Sex Dolls for Collectors, try it now.
BBW Sex Dolls Statistics and Facts, What You Need to Know.
High-quality bbw sex dolls to satisfy any desire, don’t miss the chance.
Advantages of buying a bbw sex doll online, in today’s reality.
The latest trends in the world of bbw sex dolls, only with us.
How not to make a mistake when choosing a bbw sex doll, according to customer reviews.
BBWs for everyone: find your perfect doll, all new products in one place.
bbw free cam bbw sex live .
Заказать диплом на заказ в Москве вы имеете возможность через официальный портал компании. orikdok-1v-gorode-cheboksary-21.ru
«Рентвил» предлагает аренду автомобилей в Краснодаре без залога и ограничений по пробегу по Краснодарскому краю и Адыгее. Требуется стаж от 3 лет и возраст от 23 лет. Оформление за 5 минут онлайн: нужны только фото паспорта и прав. Подача авто на жд вокзал и аэропорт Краснодар Мин-воды Сочи . Компания работает 10 лет , автомобили проходят своевременное ТО. Доступны детские кресла. Бронируйте через сайт краснодар аренда авто
1win официальный сайт регистрация http://1win22080.ru .
1win как использовать бонусы http://1win14003.ru .
1 вин официальный сайт вход http://www.1win22080.ru .
Приобрести диплом института по выгодной цене возможно, обращаясь к надежной специализированной компании. Приобрести диплом: gjejstaf.al/employer/diplomy-grup-24
Read Full Report college application essay writing service
Где приобрести диплом специалиста?
Мы готовы предложить дипломы психологов, юристов, экономистов и любых других профессий по выгодным ценам. Для нас очень важно, чтобы дипломы были доступными для большого количества граждан. Быстро купить диплом института rusd-diplomj.ru/kupit-originalnij-diplom-s-zapisyu-v-reestre-2/
download mostbet download mostbet .
Наша компания предлагает максимально быстро приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, штампами, подписями. Документ способен пройти лубую проверку, даже при использовании специального оборудования. comptonrpp.listbb.ru/viewtopic.phpf=6&t=3589
ремонт бытовой техники siemens сервисный центр siemens
see this site essay writer
Купить диплом ВУЗа по невысокой стоимости возможно, обратившись к проверенной специализированной компании. Приобрести документ института можно у нас в столице. orikdok-2v-gorode-sankt-peterburg-78.ru
1win футбол 1win футбол .
mostbet вход в личный кабинет https://mostbet22043.ru .
Испытайте азарт и выигрыш в надежном казино с отличной репутацией, подробности здесь https://vavada-0712.store/
site web coinbase pro
Quel est le code promo Linebet 2025 ?
Le code promo Linebet est :
https://lasaison.ch/pages/code_promo_linebet_bonus_.html . Ce code promo vous permet d’obtenir 100 % de bonus a l’issue de votre 1er depot. En rejoignant Linebet, vous pourrez donc gagner jusqu’a 100 $ de freebets.
Vous n’aurez plus qu’a rejouer 5 fois votre bonus en combine pour le convertir en argent reel (3 selections ou plus / cote minimum : 1,40).
Code promo Linebet paris sportifs : jusqu’a 100 $ de bonus de bienvenue
Avec le code promo Linebet, vous profiterez d’un doublement de votre 1er depot jusqu’a 100 $ si vous choisissez le bonus de bienvenue paris sportifs lors de votre inscription.
Choisissez l’offre bonus de bienvenue paris sportifs lors de votre inscription pour en beneficier. C’est l’un des meilleurs avantages pour demarrer chez ce bookmaker.
C’est simple : deposez 50 $ et gagnez 50 $ supplementaires. Jouez 100 $ pour obtenir le bonus maximum de 100 $.
ll faut le rejouer 5 fois en pari combine (avec au moins 3 selections ayant une cote de 1,40 ou plus) pour transformer cet argent bonus en cash retirable et recuperer vos gains.
Cette condition est courante chez les bookmakers en ligne, similaire a ce que propose 1xBet. Assurez-vous que le code marche et que l’offre est valide dans votre pays.
Comment ouvrir un compte avec le code promo Linebet en Mai 2025 ?
Le code promo Linebet doit etre insere dans le formulaire d’ouverture : il vous permettra d’obtenir le bonus de bienvenue paris sportifs ou poker (au choix).
En vous inscrivant, vous pourrez aussi prendre part a toutes les offres promotionnelles mises en place par le site.
Le processus d’inscription a ete allege au maximum pour que vous profitiez au plus vite d’une experience de jeu inedite. Inscrivez-vous maintenant pour profiter de cotes allechantes pour maximiser vos gains !
Приобрести диплом под заказ возможно используя сайт компании. orikdok-3v-gorode-tomsk-70.ru
1win официальный сайт бесплатно онлайн http://1win14001.ru .
pop over to these guys coinbase login
Доброго!
Купите виртуальный номер телефона навсегда и забудьте о сложностях с подключением. Постоянный виртуальный номер для смс идеально подходит для общения и регистрации. Наши услуги обеспечивают конфиденциальность и стабильность. Виртуальный номер навсегда – это ваш надежный выбор. Заказывайте уже сегодня!
Полная информация по ссылке – https://ryantotka.com/dentist-icon-white-1/#comment-85841
постоянный виртуальный номер для смс, купить номер телефона навсегда, купить виртуальный номер навсегда
виртуальный номер, виртуальный номер, постоянный виртуальный номер для смс
Удачи и комфорта в общении!
hoki1881
Быстро приобрести диплом любого университета. Покупка подходящего диплома через надежную компанию дарит немало преимуществ. Такое решение дает возможность сберечь как личное время, так и существенные финансовые средства. orikdok-5v-gorode-perm-59.online
Наша компания предлагает выгодно и быстро заказать диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Документ способен пройти любые проверки, даже при использовании специального оборудования. uscgq.com/forum/posts.phpforum=general&id=408512
hoki1881
сделать заезд на дачный участок сделать заезд на дачный участок .
Eche un vistazo a sitio web aqui https://vitalsphere.space/mex/from-parasites/vitacaps-detox/
Добрый день!
Ищете простой способ сохранить конфиденциальность? Постоянный виртуальный номер – это идеальное решение. У нас вы можете купить виртуальный номер навсегда для личных или деловых нужд. Виртуальный номер для смс – это удобство и доступность в любой точке мира. Оцените наши выгодные условия уже сегодня.
Полная информация по ссылке – https://khemuanacement.com/and-the-day-came-when-the-risk-to-remain-tight-in-a-bud-was-more-painful-than-the-risk-it-took-to-blossom/#comment-86461
постоянный виртуальный номер для смс, постоянный виртуальный номер для смс, купить виртуальный номер для смс навсегда
купить виртуальный номер для смс навсегда, купить постоянный виртуальный номер, купить виртуальный номер
Удачи и комфорта в общении!
Internet https://topvitality.space/salvador/slimming/matcha-suri/
Доброго!
Аренда беседок на юге москвы — удобный сервис для жителей этого района. Беседки для шашлыка аренда москва предлагают уют и комфорт. Москва беседка аренда позволяет выбрать лучший вариант для вашей компании. Аренда беседок в парках москвы — отличное решение для семейных праздников. Забронируйте беседку заранее и отдыхайте без забот.
Полная информация по ссылке – https://telegra.ph/Besedki-na-Losinom-Ostrove-obzor-zon-otdyha-Biostanciya-i-Dendrarij-04-30
аренда беседки новая москва, беседка гриль аренда москва, беседки аренда москва ювао
беседки для шашлыков аренда москва, беседка с мангалом москва аренда, москва аренда беседок с мангалом
Удачи и комфортного отдыха!
read the article https://vitalityshop.space/cyp/cardiovascular-system/cardioa-capsules-for-hypertension/
продвижение сайта в топ http://www.prodvizhenie-sajtov-v-moskve.ru/ .
mostbet.com http://www.mostbet22045.ru .
Приобрести диплом о высшем образовании. Приобретение подходящего диплома через качественную и надежную компанию дарит массу плюсов. Это решение дает возможность сберечь как личное время, так и значительные финансовые средства. orikdok-2v-gorode-khabarovsk-27.online
Купить документ о получении высшего образования можно в нашей компании. Купить диплом института по невысокой цене можно, обратившись к надежной специализированной фирме. gofleeks.com/read-blog/356_diplom-hirurga-kupit.html
Hello!
Looking to open bank account in UAE non resident? We guide non-residents through legal and compliant account opening procedures. Many UAE banks welcome international clients with flexible policies. Get assistance on open bank account in UAE non resident quickly and easily. Start your application today.
More information is available via the link – https://telegra.ph/Open-a-Corporate-Bank-Account-in-the-UAE-04-16
Find the best personal bank account in UAE for your monthly salary. We recommend banks with low fees and attractive offers. Open accounts that fit your income level. Get expert guidance to pick the right bank. Start your best personal bank account in UAE search here.
Look information is available via the link – https://telegra.ph/Personal-Bank-Accounts-in-the-UAE-04-17
vat return filing service uae, open bank account in uae non resident, best vat services in uae
excise tax registration service uae, can we apply for vat agent services in uae, tax accounting services in uae
Good luck!
check my reference college application essay writing service
авто въезд авто въезд .
продвижение сайта цена https://www.prodvizhenie-sajtov-v-moskve.ru .
Привет всем!
Предлагаем аренда теплой беседки в москве для комфортного отдыха зимой. Теплые беседки аренда москва помогут провести время в уюте. Аренда беседок с мангалом москва доступна круглый год. Выбирайте беседки с отоплением и удобной мебелью. Забронируйте теплую беседку заранее.
Полная информация по ссылке – https://dzen.ru/a/aBiRoYW6hAEyyy7Y
москва беседки для шашлыка аренда, беседка мангал москва аренда, парки москвы аренда беседки
беседки аренда москва недорого, аренда беседок в лесу москва, барбекю беседка аренда москва
Удачи и комфортного отдыха!
Servicio de Equilibrado
¿Movimientos irregulares en tu equipo industrial? Servicio de balanceo dinámico en campo y comercialización de dispositivos especializados.
¿Has detectado vibraciones inusuales, ruidos extraños o degradación rápida en tus equipos? Esto indica claramente de que tu equipo industrial necesita un ajuste de precisión especializado.
En vez de desarmar y trasladar tus máquinas a un taller, nosotros vamos hasta tu planta industrial con tecnología avanzada para solucionar la falla sin interrumpir tu producción.
Beneficios de nuestro balanceo dinámico en campo
✔ Evitamos desarmados y transportes — Operamos in situ.
✔ Evaluación detallada — Usamos equipos de última generación para localizar el fallo.
✔ Resultados inmediatos — Corrección en pocas horas.
✔ Documentación técnica — Registramos mediciones previas y posteriores.
✔ Especialización en múltiples industrias — Atendemos desde grandes turbinas hasta motores compactos.
Купить диплом любого университета!
Мы готовы предложить документы институтов, которые расположены в любом регионе России.
diplom-club.com/kupit-diplom-o-visshem-obrazovanii-reestr-10/
El Equilibrado de Piezas: Clave para un Funcionamiento Eficiente
¿Alguna vez has notado vibraciones extrañas en una máquina? ¿O tal vez ruidos que no deberían estar ahí? Muchas veces, el problema está en algo tan básico como un desequilibrio en alguna pieza rotativa . Y créeme, ignorarlo puede costarte bastante dinero .
El equilibrado de piezas es un procedimiento clave en la producción y cuidado de equipos industriales como ejes, volantes, rotores y partes de motores eléctricos . Su objetivo es claro: impedir oscilaciones que, a la larga, puedan provocar desperfectos graves.
¿Por qué es tan importante equilibrar las piezas?
Imagina que tu coche tiene una llanta mal nivelada . Al acelerar, empiezan los temblores, el manubrio se mueve y hasta puede aparecer cierta molestia al manejar . En maquinaria industrial ocurre algo similar, pero con consecuencias mucho más graves :
Aumento del desgaste en soportes y baleros
Sobrecalentamiento de partes críticas
Riesgo de colapsos inesperados
Paradas no planificadas y costosas reparaciones
En resumen: si no se corrige a tiempo, una leve irregularidad puede transformarse en un problema grave .
Métodos de equilibrado: cuál elegir
No todos los casos son iguales. Dependiendo del tipo de pieza y su uso, se aplican distintas técnicas:
Equilibrado dinámico
Ideal para piezas que giran a alta velocidad, como rotores o ejes . Se realiza en máquinas especializadas que detectan el desequilibrio en múltiples superficies . Es el método más fiable para lograr un desempeño estable.
Equilibrado estático
Se usa principalmente en piezas como ruedas, discos o volantes . Aquí solo se corrige el peso excesivo en una sola superficie . Es ágil, práctico y efectivo para determinados sistemas.
Corrección del desequilibrio: cómo se hace
Taladrado selectivo: se perfora la región con exceso de masa
Colocación de contrapesos: tal como en neumáticos o perfiles de poleas
Ajuste de masas: habitual en ejes de motor y partes relevantes
Equipos profesionales para detectar y corregir vibraciones
Para hacer un diagnóstico certero, necesitas herramientas precisas. Hoy en día hay opciones disponibles y altamente productivas, por ejemplo :
✅ Balanset-1A — Tu compañero compacto para medir y ajustar vibraciones
Hello!
Save time with our trusted excise tax UAE services for importers and distributors. We manage paperwork, filing, and government communication. Don’t risk doing it alone. Use our full-package excise tax UAE services for peace of mind. Reach out today.
All information is available via the link – https://telegra.ph/Excise–Other-Taxes-in-the-UAE-04-16
The best bank personal account UAE offers convenience and flexibility. We assist in choosing banks that fit your salary and transaction needs. Open your account without stress or unnecessary paperwork. Enjoy premium banking services with minimal fees. Start your best bank personal account UAE journey now.
Look information is available via the link – https://telegra.ph/Personal-Bank-Accounts-in-the-UAE-04-17
vat for childcare services uae, bank account to open in uae, open bank account dubai non uae residents
vat uae services, tax accounting services in uae, service tax in real estate uae
Good luck!
Купить диплом любого университета!
Наша компания предлагаетвыгодно купить диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями. Наш диплом пройдет лубую проверку, даже с применением профессионального оборудования. Достигайте своих целей быстро и просто с нашим сервисом- hobbyland.moibb.ru/viewtopic.phpf=3&t=1235
read the article https://vitalityshop.space/slimming/abslim-food-supplement-for-quick-weight-loss/
Мы изготавливаем дипломы любой профессии по доступным ценам. Мы готовы предложить документы ВУЗов, которые находятся на территории всей РФ. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, не отличимые от оригиналов. orikdok-4v-gorode-saratov-64.online
1win бонус при регистрации 1win14005.ru .
Internet https://vitalsphere.space/mex/cardiovascular-system/cardionix/
ремонт техники сименс сервисный центр сименс
сервис aeg ремонт стиральных машин в москве aeg
сервисный центр siemens service-siemens-russia.ru
mostbet qeydiyyat yoxlaması https://mostbet3042.ru .
Мы изготавливаем дипломы любой профессии по невысоким тарифам. Приобретение диплома, подтверждающего обучение в ВУЗе, – это грамотное решение. Заказать диплом о высшем образовании: forum.subchems.com/read-blog/8005_diplom-11-klassa-kupit.html
ремонт техники сименс сервисный центр сименс
мостбет вывести деньги мостбет вывести деньги .
Купить диплом о высшем образовании!
Мы предлагаем дипломы любых профессий по приятным ценам. Вы покупаете диплом через надежную и проверенную компанию. : soniamittal0044.copiny.com/question/details/id/1095708
1win qanday ro‘yxatdan o‘tish kerak 1win14006.ru .
1 vin 1win14004.ru .
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Приобрести диплом любого ВУЗа!
Мы можем предложить документы институтов, которые расположены в любом регионе России.
diplomh-40.ru/kupit-diplom-o-visshem-obrazovanii-18/
что такое бонусный счет в 1win https://www.1win22083.ru .
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы можем предложить документы техникумов, расположенных на территории всей РФ. Документы выпускаются на бумаге самого высшего качества. Это позволяет делать настоящие дипломы, которые не отличить от оригиналов. orikdok-2v-gorode-astrakhan-30.ru
mostbet az canlı mərclər https://mostbet3043.ru .
сериал сын отца народов скачать
Быстро, качественно и с душой! Лучший сервис по доставке цветов!
доставка цветов
сериал другой майор соколов 1 сезон смотреть онлайн
Limousine service offers luxurious, convenient transportation. For Seattle locals and visitors, Seattle Airport Limo provides premier ground travel. Their SeaTac Airport Limo Service includes professional chauffeurs, ensuring timely pick-up and drop-off. Passengers can rely on Limo Service From Seattle Airport for comfort and style, with options including sleek sedans and spacious SUVs. This service is ideal for business travelers, special occasions, or simply those seeking stress-free transport to and from SeaTac. With a focus on safety, reliability, and elegance, Seattle Airport Limo enhances your journey from the moment you land. – https://bdluxlimo.com/limo-service-from-seattle-airport-to-cruise-terminal/ Limousine service offers luxurious, convenient transportation. For Seattle locals and visitors, Seattle Airport Limo provides premier ground travel. Their SeaTac Airport Limo Service includes professional chauffeurs, ensuring timely pick-up and drop-off. Passengers can rely on Limo Service From Seattle Airport for comfort and style, with options including sleek sedans and spacious SUVs. This service is ideal for business travelers, special occasions, or simply those seeking stress-free transport to and from SeaTac. With a focus on safety, reliability, and elegance, Seattle Airport Limo enhances your journey from the moment you land. – https://bdluxlimo.com/limo-service-from-seattle-airport-to-cruise-terminal/ Limousine service offers luxurious, convenient transportation. For Seattle locals and visitors, Seattle Airport Limo provides premier ground travel. Their SeaTac Airport Limo Service includes professional chauffeurs, ensuring timely pick-up and drop-off. Passengers can rely on Limo Service From Seattle Airport for comfort and style, with options including sleek sedans and spacious SUVs. This service is ideal for business travelers, special occasions, or simply those seeking stress-free transport to and from SeaTac. With a focus on safety, reliability, and elegance, Seattle Airport Limo enhances your journey from the moment you land. – https://bdluxlimo.com/limo-service-from-seattle-airport-to-cruise-terminal/
Добрый день!
Аренда беседок Москва — удобный и практичный способ провести выходные на природе. Беседка аренда Москва с мангалом — это возможность готовить любимые блюда на свежем воздухе. Мы предлагаем беседки с отоплением для комфортного отдыха в любое время года. Москва беседка для шашлыка аренда — услуга с отличными отзывами клиентов. Забронируйте аренду беседок Москва заранее и организуйте идеальный отдых.
Полная информация по ссылке – https://dzen.ru/a/aBiOyT48oAugumsU
аренда москва беседка с мангалом, аренда беседок москва серебряный бор, беседки у воды аренда москва
беседки москва река аренда, аренда беседки москва свао, беседки для шашлыков аренда москва
Удачи и комфортного отдыха!
Купить диплом под заказ вы сможете через сайт компании. orikdok-5v-gorode-cheboksary-21.online
Мы можем предложить дипломы любых профессий по выгодным тарифам. Покупка диплома, подтверждающего окончание университета, – это разумное решение. Приобрести диплом университета: good-serial.ru/2025/04/kak-oformit-poddelnyiy-diplom
Добро пожаловать на 1winbr.netlify.app, впечатляющий контент.
1winbr.netlify.app предлагает, не пропустить.
Познавайте 1winbr.netlify.app, чтобы.
На 1winbr.netlify.app вы сможете найти, все необходимое, для творчества.
Подключайтесь к 1winbr.netlify.app, что охватывает.
Погружение в 1winbr.netlify.app, что ставит.
много полезного.
Самые актуальные тренды на 1winbr.netlify.app, доступны для вас.
Незабываемый опыт с 1winbr.netlify.app, отправят вас в удивительное путешествие.
Исследуйте и делитесь 1winbr.netlify.app, для того чтобы.
На 1winbr.netlify.app есть что-то для каждого, не упустите.
Вдохновляйте себя с 1winbr.netlify.app, уникальный контент.
вы сможете найти.
1winbr.netlify.app ждет вас, новыми возможностями.
1winbr.netlify.app – это ваш шанс, что даст.
Используйте 1winbr.netlify.app для обучения и развития, расти как личность.
1winbr.netlify.app готов к вашим вызовам, откройте для себя.
1winbr.netlify.app – стартовая площадка, где.
1win https://1winbr.netlify.app/ .
заказать обед с доставкой челябинск dostavka-edy-bf11.ru .
клинические анализы https://www.medanalyze.ru .
mostbet az bonusları mostbet az bonusları .
Добрый день!
Москва аренда беседок с мангалом — сервис для тех, кто ценит комфорт и качество. Беседка для шашлыка аренда Москва поможет организовать праздник на природе без хлопот. Мы предлагаем теплые беседки аренда Москва для отдыха в любое время года. Аренда беседок Москва позволяет выбрать подходящий вариант для любых нужд. Забронируйте беседку заранее и наслаждайтесь отдыхом.
Полная информация по ссылке – https://dzen.ru/a/aBiRoYW6hAEyyy7Y
аренда беседка с мангалом москва, отапливаемая беседка аренда москва, аренда закрытых беседок в москве
аренда беседок с мангалом москва, мангал беседка в москве аренда, аренда беседок шашлык москва
Удачи и комфортного отдыха!
здоровая ферма http://www.dostavka-edy-bf13.ru .
доставка обедов челябинск доставка обедов челябинск .
Vibración de motor
Comercializamos equipos de equilibrio!
Producimos nosotros mismos, produciendo en tres países a la vez: Portugal, Argentina y España.
✨Nuestros equipos son de muy alta calidad y al ser fabricantes y no intermediarios, nuestras tarifas son más bajas que las del mercado.
Disponemos de distribución global a cualquier país, revise la información completa en nuestro sitio web.
El equipo de equilibrio es móvil, de bajo peso, lo que le permite equilibrar cualquier rotor en todas las circunstancias.
Диплом ВУЗа России!
Без наличия диплома сложно было продвигаться вверх по карьере. Купить диплом под заказ возможно используя официальный сайт компании: jamesrodriguezclub.com/read-blog/7106_kuplyu-diplom-magistra.html
узнать больше Здесь
игровой gates of olympus
Наши специалисты предлагают выгодно и быстро купить диплом, который выполнен на оригинальном бланке и заверен печатями, штампами, подписями должностных лиц. Наш документ способен пройти любые проверки, даже с использованием специально предназначенного оборудования. vv.flybb.ru/viewtopic.phpf=23&t=2884
Заказать диплом института по доступной цене возможно, обращаясь к проверенной специализированной фирме. Заказать документ о получении высшего образования вы имеете возможность у нас. orikdok-5v-gorode-lipetsk-48.ru
Good day!
Don’t know how to get service tax registration certificate online UAE? We simplify the entire online registration journey. Our experts handle every step efficiently. Learn how to get service tax registration certificate online UAE today. Get registered with confidence.
Full information is available via the link – https://telegra.ph/Corporate-Tax-Services-in-the-UAE-04-16
Looking for the best bank in UAE for personal account that supports expats? We help you find banks with expat-friendly policies. Open accounts with flexible requirements and great support. Manage your finances with ease in UAE. Choose the best bank in UAE for personal account.
Look information is available via the link – https://telegra.ph/Personal-Bank-Accounts-in-the-UAE-04-17
can we apply for vat agent services in uae, excise tax uae services, open bank account in uae without residence visa
vat consultancy services in uae, tax consulting services in uae, how to open a personal bank account in uae
Good luck!
шпинель Мир драгоценных камней – это не просто блеск и великолепие, это целая вселенная возможностей, приключений и инвестиций. Отправляясь в экспедицию за редким рубином в дебри Бирмы или охотясь за сапфиром невероятной чистоты в недрах Шри-Ланки, вы ступаете на путь, где красота и доход идут рука об руку. Ювелирные украшения, будь то кольцо с бриллиантом, серьги с изумрудом или подвеска с танзанитом, – это не просто модные аксессуары, это символы статуса, вкуса и истории. Золото и серебро, обрамляющие драгоценные камни, добавляют им ценности и значимости, превращая их в настоящие произведения искусства. В бутике, где царит атмосфера роскоши и утонченности, ювелирные изделия предстают во всей своей красе. Дизайн, сочетающий в себе классические традиции и современные тенденции, позволяет каждому найти украшение по душе. Инвестиции в драгоценности – это разумный выбор, ведь их стоимость со временем только растет. Неважно, что вы ищете: способ приумножить свой капитал, подчеркнуть свою индивидуальность или просто порадовать себя прекрасным украшением – мир драгоценных камней всегда готов предложить вам нечто особенное. От блеска бриллианта до глубокого цвета изумруда, от огненного рубина до небесной синевы сапфира – каждый камень обладает своей уникальной историей и неповторимым очарованием.
заезд на дачу заезд на дачу .
Заказать диплом института. Покупка диплома через надежную компанию дарит массу достоинств. Это решение помогает сэкономить как продолжительное время, так и серьезные денежные средства. orikdok-v-gorode-perm-59.online
1win uzcard orqali pul olish https://www.1win14006.ru .
скачать музыку сборник мп3 бесплатно в хорошем качестве http://www.25kat.ru .
сколько стоит кв натяжного потолка сколько стоит кв натяжного потолка .
Где заказать диплом по необходимой специальности?
Готовый диплом со всеми печатями и подписями целиком и полностью отвечает стандартам Министерства образования и науки, никто не сумеет отличить его от оригинала. Диплом о высшем образовании – не проблема! orthograf.getbb.ru/viewtopic.phpf=2&t=1781
Мы оказываем услуги по производству и продаже документов об окончании любых университетов России. Документы производят на фирменных бланках государственного образца. dp.getbb.ru/viewtopic.phpf=3&t=2434
1вин скачать официальный сайт 1win14005.ru .
сдать анализ в клинике https://medanalyze.ru/ .
Hello!
Do you want to open bank account online UAE with ease? We make the process quick and straightforward with expert support. Avoid long queues and confusing paperwork. Use our trusted service to open bank account online UAE safely and securely. Get started instantly.
More information is available via the link – https://telegra.ph/Open-a-Corporate-Bank-Account-in-the-UAE-04-16
A personal bank account UAE is crucial for managing your finances efficiently. We guide you through the application process smoothly. Enjoy benefits like salary deposits and instant transfers. Choose from a range of banks with great offers. Open your personal bank account UAE with our help.
Look information is available via the link – https://telegra.ph/Personal-Bank-Accounts-in-the-UAE-04-17
digital service vat uae, uae tax invoice format service, how to open bank account in dubai for non-uae residents
open bank account dubai non uae residents, uae vat health services, vat in uae for services
Good luck!
доставка еды на неделю челябинск доставка еды на неделю челябинск .
заказать еду в челябинске с доставкой http://www.dostavka-edy-bf11.ru/ .
доставка еды челябинск доставка еды челябинск .
Советую туры Туры в Суйфеньхэ !
Купить диплом любого университета!
Наши специалисты предлагаютвыгодно купить диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями официальных лиц. Документ пройдет любые проверки, даже при помощи профессионального оборудования. Достигайте своих целей быстро и просто с нашими дипломами- cs-forever.phorum.pl/viewtopic.phpp=31495#31495
Быстро приобрести диплом об образовании!
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по невысоким ценам— [url=http://new-diplom.ru/]new-diplom.ru[/url]
mostbet aviator bot ishlaydimi mostbet3030.ru .
klas bahis guncel bilgileri kontrol et
Klasbahis giris sayfas? https://klasbahisgiristr.com
Ver aqui https://topvitality.space/peru/varicose-veins/calmano-capsules/
“букмекерская контора с фрибетом за регистрацию без депозита с выводом денег http://www.1win14004.ru .
Leer mas https://vitalsphere.space/mex/potency/eronex-capsules/
Здравствуйте!
Москва беседка аренда с мангалом — удобное решение для проведения праздников. Аренда беседок в Москве с отоплением поможет провести мероприятие даже зимой. Теплые беседки аренда Москва отличаются уютом и удобством. Забронируйте аренду беседки в Москве заранее и сделайте отдых комфортным.
Полная информация по ссылке – https://dzen.ru/a/aBiOyT48oAugumsU
аренда крытой беседки в москве, москва аренда барбекю беседки, аренда беседка в москве
аренда зимних беседок москва, аренда беседок под москвой, летние беседки аренда москва
Удачи и комфортного отдыха!
like this https://vitalityshop.space/cyp/from-fungus/milozol-antifungal-cream/
1 win букмекерская 1 win букмекерская .
Психотерапия по переписке. Сеанс у психолога. Психотерапевт онлайн чат.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Психолог владеет множеством приемов и техник, которые помогут разобраться в себе.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
Получить поддержку по широкому кругу вопросов.
Анонимный видеочат без регистрации. Услуги психолога онлайн. Онлайн общение с психологом.
Получить поддержку по широкому кругу вопросов.
40631 проверенных отзывов.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Задать вопрос психологу анонимно. Психологи онлайн анонимно. Онлайн психотерапевт.
Психологическое консультирование.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
93961 проверенных отзывов.
https://www.med2.ru/story.php?id=147094
Как выйти из депрессии видео. Онлайн психолог отзывы. Специалист, который услышит, поймет, определит проблемы.
Индивидуальное консультирование.
Мы обязательно поможем преодолеть эмоциональный кризис, избавиться от тревожности и апатии, справиться со стрессом и депрессией, связанными с неуверенностью и многим другим.
Консультация в кризисных состояниях.
Telegram-чат с психологом. Виртуальный чат. Получить онлайн консультацию психолога чате.
Психолог Москва. Психолог СПБ. Психолог онлайн.
Нужен хороший психолог?
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
Заказать документ о получении высшего образования можно в нашем сервисе. Заказать диплом ВУЗа по невысокой стоимости возможно, обратившись к проверенной специализированной компании. rosen.moibb.ru/viewtopic.phpf=2&t=2029
Приобрести диплом университета!
Мы предлагаем документы учебных заведений, которые расположены в любом регионе России.
[url=http://diplom-kaluga.ru/diplom-s-zaneseniem-v-reestr-dlya-uspeshnoj-kareri/]diplom-kaluga.ru/diplom-s-zaneseniem-v-reestr-dlya-uspeshnoj-kareri/[/url]
стоимость натяжного потолка за квадратный метр https://potolkilipetsk.ru .
Мы предлагаем дипломы любых профессий по приятным тарифам. Мы готовы предложить документы ВУЗов, расположенных в любом регионе Российской Федерации. Документы выпускаются на бумаге высшего качества. Это дает возможность делать настоящие дипломы, которые не отличить от оригинала. orikdok-4v-gorode-vladivostok-25.online
Букет был настолько хорош, что его фотографировали все гости!
цветы томск
mostbet kartla depozit mostbet kartla depozit .
https://mirka-master.ru/kupit-plastikovye-okna-v-sankt-peterburge-nadyozhnoe-reshenie-ot-kompanii-afina-okna/
Great experience! The towing service was prompt, and they took good care of my vehicle. Very satisfied!
Hello!
Let us explain what is excise tax in UAE and why it matters. This indirect tax applies to specific goods deemed harmful. Learn how excise tax in UAE affects your pricing and compliance. Our team makes everything easy to understand. Book a free session now.
All information is available via the link – https://telegra.ph/Excise–Other-Taxes-in-the-UAE-04-16
Car services have VAT in UAE at the standard rate of 5%. Businesses providing car services have VAT in UAE and must comply. Proper invoicing and accounting are necessary when car services have VAT in UAE. Experts explain when car services have VAT in UAE applies. Stay updated on car services have VAT in UAE rules.
All information is available via the link – https://telegra.ph/VAT-Services-in-the-UAE-04-16
how to open a personal bank account in uae, uae excise tax meaning, tax consulting services in uae
requirements to open bank account in uae, what is vat for service in uae, vat on courier services in uae
Good luck!
скачать мр3 музыку бесплатно в хорошем качестве 25kat.ru .
Superb service! They arrived on time and made the whole process stress-free. Highly recommended!
jogo do bicho deu no poste das 11 horas
futemax futebol ao vivo
Experi锚ncia muito positiva.
Gr谩ficos lindos e jogabilidade fluida.
https://mnt-ambientes.saude.mg.gov.br/multicanais tv ao vivo/
http://prestacaocontashml.saude.mg.gov.br/wp-map/futemax-futebol/
Заказать диплом любого университета можем помочь. Купить диплом техникума, колледжа в Чите – diplomybox.com/kupit-diplom-tekhnikuma-kolledzha-v-chite
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Купить диплом университета!
Мы изготавливаем дипломы любой профессии по приятным ценам. Вы заказываете диплом через надежную компанию. : powerofmony.copiny.com/question/details/id/1095802
Инъектирование кирпичной кладки https://usileniekonstrukcij1.ru .
1xBet промокод на бонус при регистрации воспользуйтесь промокодом 1хБет и получите бонус в размере 100% до 100$. Этот уникальный бонусный код предоставляет возможность получить приветственный бонус от БК 1хБет при его активаци.
Бонусы также различаются по типу и формату. Одни предоставляются новым клиентам в качестве приветствия, в то время как другие можно получить уже в процессе игры. Существуют также различия в промокодах 1xbet. Давайте рассмотрим их более подробно. 1xBet Промокод 2025 — Приветственный Пакет до 32 500 руб — рабочий промокод
При регистрации на 1xBet используйте промокод и получите бесплатный приветственный бонус в размере 32500 рублей. Букмекерская контора 1xBet предлагает своим клиентам возможность играть и делать ставки с использованием бонусов. Это не только увеличивает интерес к игровому процессу, но и обеспечивает максимальную безопасность игры.
Для получения бонусов от букмекера 1xBet необходимо выполнить определенные требования, однако промокоды позволяют получить их гораздо проще. Размеры бонусов, доступных игрокам через промокоды 1xBet, могут быть невелики, но даже небольшой бонус способен значительно увеличить игровой потенциал клиента.
Что делает промокоды букмекерской конторы 1xBet особенными? Какие знания о кодах нужны, чтобы получить максимальную выгоду от их использования? И, наконец, какие еще интересные моменты есть в бонусной программе этой конторы, на которые стоит обратить внимание, если вы планируете играть за бонусы.
LeoVegas https://amsterdam-online.nl/wp-content/pgs/leovegas-casino-online-nederland.html
mostbet aviator hack uz http://mostbet3030.ru/ .
скачать песни мп3 бесплатно в хорошем качестве скачать песни мп3 бесплатно в хорошем качестве .
организация въезда на участок через канаву http://doroga-k-uchastku-1122.ru/ .
Гидроизоляция работа Гидроизоляция работа .
1win download apk 1win download apk .
как играть на бонусы в 1 win https://www.1win22084.ru .
отзывы 1win https://1win22083.ru/ .
обустройство заезда на участок https://doroga-k-uchastku-1122.ru/ .
Наши специалисты предлагают выгодно и быстро заказать диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Диплом пройдет лубую проверку, даже при использовании профессионального оборудования. kampusunggul.com/index.php/2025/05/12/kupit-diplom-bystro-i-nadezhno-luchshie-156-2
млстбет [url=www.mostbet3031.ru]www.mostbet3031.ru[/url] .
диплом горного мастера купить диплом горного мастера купить .
Диплом университета РФ!
Без наличия диплома очень непросто было продвигаться вверх по карьерной лестнице. Приобрести диплом вы сможете используя официальный портал компании: appletwo.ca/employer/premialnie-diplom-24
https://x-ray.contact/identities/hector-lara-email-and-phone/63fbee37c45ae4c13f9eef6f/
Лучший сервис для ремонта кондиционеров, узнайте тут <a href=
https://list.net.in/profile/purebreeze/
Мы изготавливаем дипломы любой профессии по приятным ценам. Заказ диплома, подтверждающего обучение в ВУЗе, – это выгодное решение. Купить диплом любого ВУЗа: wiki.why42.ru/wiki/Купить_диплом:_мифы_и_реальность
Букет был настолько красив, что даже прохожие оборачивались!
101 роза
Рейтинг сервисов доставок еды в твоем городе: готовая еды в контейнерах, обеды, на неделю, на месяц. Каждый день разное меню, много сервисов доставок, выбирай на https://edadostavkoy.ru/
Доброго!
Наш магазин предлагает букет цветов 51 роза с гарантией свежести и качества. Вы сможете выбрать идеальный вариант из сотен готовых композиций. купить 51 розу в москве Доставка осуществляется по Москве круглосуточно без выходных. Цены на букеты приятно удивляют — никаких скрытых платежей. Выбирайте букет цветов 51 роза у нас и дарите радость любимым!
Букет роз заказать онлайн москва — это просто и быстро. У нас на сайте большой выбор свежих букетов. Доставка по всей Москве уже сегодня. Подарите радость себе и близким. Букет роз заказать онлайн москва — удобное решение.
Вся информация на сайте – https://rozymsk.ru/51-roza
розы сейл москва, купить пионовидные розы, розы с доставкой по москве недорого
стоимость розы, букет роз цена в москве, букет роза
Удачи!
Где заказать диплом специалиста?
Приобрести диплом ВУЗа по доступной стоимости вы можете, обратившись к проверенной специализированной фирме.: sdiplom.ru
Мы изготавливаем дипломы любых профессий по приятным тарифам. Дипломы производят на подлинных бланках Приобрести диплом об образовании diploml-174.ru
Заказать документ о получении высшего образования можно в нашей компании в Москве. Заказать диплом института по доступной стоимости можно, обращаясь к надежной специализированной компании. veraciousrp.listbb.ru/viewtopic.phpf=82&t=2355
Заказать диплом о высшем образовании!
Мы предлагаем документы учебных заведений, которые находятся на территории всей РФ.
diplomgorkiy.com/kupit-diplom-zanesennij-reestr-2/
Мы изготавливаем дипломы любой профессии по выгодным ценам. Мы предлагаем документы ВУЗов, которые расположены в любом регионе Российской Федерации. Дипломы и аттестаты печатаются на бумаге высшего качества. Это позволяет делать государственные дипломы, не отличимые от оригиналов. orikdok-3v-gorode-perm-59.online
Самые лучшие проститутки в Новосибирске, подробнее тут проститутки индивидуалки Новосибирск
over at this website
fontan casino 20€ no deposit bonus
страница https://marvilcasino.xyz/zerkalo/
Заказать диплом вы имеете возможность используя сайт компании. orikdok-1v-gorode-nalchik-7.ru
ссылка на сайт https://beepbeepcasino.ru/zerkalo
Приобрести диплом ВУЗа по доступной стоимости возможно, обратившись к проверенной специализированной компании. Купить документ университета вы сможете у нас в Москве. orikdok-4v-gorode-lipetsk-48.online
Ремонт электронной техники в Москве, куда обращаться для диагнистики и ремонта, читайте – на сайте
Купить диплом любого ВУЗа!
Мы можем предложить документы ВУЗов, расположенных в любом регионе России.
diplomt-nsk.ru/kupite-diplom-visshego-obrazovaniya-s-zaneseniem-v-reestr-3/
Выбрать гидроизоляцию http://racechrono.ru/novosti/42911-uslugi-gidroizolyacii-i-ceny-polnyy-gid-po-vyboru-i-raschetu-stoimosti.html .
useful link
https://hottopcasino.com/no-deposit-bonuses/
в этом разделе https://beepbeepcasino.ru/
other
hottopcasino
Усиление конструкций работа Усиление конструкций работа .
Инъектирование фундамента usileniekonstrukcij2.ru .
Усиление конструкций работа Усиление конструкций работа .
Читать далее https://marvilcasino.xyz/
Заказать диплом ВУЗа. Заказ документа о высшем образовании через проверенную и надежную компанию дарит ряд достоинств для покупателя. Данное решение позволяет сэкономить время и существенные денежные средства. orikdok-v-gorode-samara-63.online
next
https://hottopcasino.com/deposit-bonnus/
узнать больше https://marvilcasino.xyz/zerkalo/
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы предлагаем документы ВУЗов, которые расположены в любом регионе РФ. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это позволяет делать настоящие дипломы, которые не отличить от оригинала. orikdok-v-gorode-izhevsk-18.online
click here now
f1 casino
Купить диплом под заказ можно используя сайт компании. orikdok-3v-gorode-vologda-35.ru
Купить диплом института по доступной стоимости возможно, обращаясь к проверенной специализированной фирме. Заказать документ о получении высшего образования вы имеете возможность в нашей компании в столице. orikdok-2v-gorode-kurgan-45.ru
navigate to this website spy-casino
Здравствуйте!
Букет 51 роза — идеальный подарок для любимой. Мы соберем его с душой. пионовидные кустовые розы Доставка вовремя и точно. Заказ в пару кликов. Букет 51 роза — романтика в деталях.
Розы доставка москва дешево — проверьте наши цены. Вы будете приятно удивлены. Быстрая обработка заказов. Доставим в течение пары часов. Розы доставка москва дешево — доступно каждому.
Вся информация на сайте – https://rozymsk.ru/pionovidnye-rozy
розы цена москва, розы москва, розы букет купить недорого в москве
букет роз расценки, розы выгодно, розы в москве
Удачи!
Гидроизоляция частного дома https://iseekmate.com/34687-uslugi-gidroizolyatsii-i-tseny-polnyj-gid-po-vyboru-i-raschyotu-stoimosti.html .
Гидроизоляция стен http://roofor.ru/stroytelstvo/gidroizolyaciya-zdaniy-i-sooruzheniy .
Гидроизоляция фундамента Гидроизоляция фундамента .
Гидроизоляция фундамента Гидроизоляция фундамента .
Добрый день!
Букет роз заказать онлайн в москве — идеальное решение, если вы не можете выйти из дома. Оформите заказ за пару минут. 101 роза купить недорого в москве Доставка работает ежедневно. Свежие и красивые цветы приедут к вам быстро. Букет роз заказать онлайн в москве — без хлопот.
Букет роз купить с бесплатной доставкой в москве — предложение для тех, кто ценит удобство. У нас нет скрытых комиссий. Всё честно и открыто. Закажите букет за пару минут. Букет роз купить с бесплатной доставкой в москве — это выгодно.
Вся информация на сайте – https://rozymsk.ru/101-roza
букет роз стоимость москва, розы букет купить с доставкой москва, букет роз цена москва
розы рассрочка, розы купить недорого, букет роз в наличии москва
Удачи!
Быстро, красиво и надёжно! Что ещё нужно для счастья?
заказ цветов томск с доставкой
Купить диплом любого университета!
Наши специалисты предлагаютвыгодно приобрести диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Данный диплом способен пройти любые проверки, даже с применением профессиональных приборов. Решайте свои задачи быстро с нашими дипломами- znanee.flybb.ru/viewtopic.phpf=2&t=965
Приобрести диплом университета. Приобретение документа о высшем образовании через надежную компанию дарит множество преимуществ. Это решение дает возможность сберечь время и серьезные средства. orikdok-2v-gorode-perm-59.ru
Продолжение белая футболка оверсайз
Узнать больше https://beepbeepcasino.ru/vip
скачать мостбет кыргызстан скачать мостбет кыргызстан .
Заказать диплом ВУЗа по выгодной стоимости можно, обращаясь к надежной специализированной компании. Мы предлагаем документы об окончании любых ВУЗов РФ. Приобрести диплом о высшем образовании– ywupfor.flybb.ru/viewtopic.phpf=27&t=319
парковка для машины на даче парковка для машины на даче .
Заказать диплом о высшем образовании!
Мы предлагаеммаксимально быстро заказать диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Документ пройдет любые проверки, даже с использованием специального оборудования. Решайте свои задачи быстро с нашим сервисом- rrsclub.ru/member.phpu=1164
klasbahis guncel linkler h?zl?
klasbahis guncel adres degistirdi
most bet uz https://mostbet3032.ru/ .
Приобрести диплом любого университета!
Заказ документа о высшем образовании через надежную фирму дарит немало преимуществ для покупателя. Купить диплом университета у надежной фирмы: doks-v-gorode-kemerovo-42.ru
mostbet daxil ol http://mostbet3045.ru/ .
Купить диплом института!
Приобрести диплом любого института. Заказ документа о высшем образовании через проверенную и надежную компанию дарит ряд плюсов для покупателя. Это решение позволяет сэкономить время и существенные денежные средства. 4blabla.ru/read-blog/9793_diplom-moskva-kupit.html
Приобретение диплома ВУЗа через качественную и надежную компанию дарит множество плюсов для покупателя. Приобрести диплом: behealthy.maxbb.ru/posting.php?mode=post&f=8
ваучеры 1win https://www.1win22085.ru .
Наши специалисты предлагают выгодно и быстро приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, штампами, подписями должностных лиц. Наш диплом пройдет любые проверки, даже при использовании специальных приборов. babygirlslove.copiny.com/question/details/id/1104285
клубная музыка слушать клубная музыка слушать .
мостбет как начать играть https://mostbet3033.ru .
Купить диплом института по выгодной стоимости можно, обращаясь к проверенной специализированной компании. Мы можем предложить документы учебных заведений, расположенных в любом регионе России. study-lingvo.ru/kupit-diplom-s-zaneseniem-bistro-i-udobno/
скачат мостбет http://mostbet20006.ru .
dj онлайн dj онлайн .
Доброго!
Наш магазин предлагает букет роз на сейле москва с гарантией свежести и качества. Вы сможете выбрать идеальный вариант из сотен готовых композиций. кустовая роза цена Доставка осуществляется по Москве круглосуточно без выходных. Цены на букеты приятно удивляют — никаких скрытых платежей. Выбирайте букет роз на сейле москва у нас и дарите радость любимым!
Букет роз прайс лист — изучите наш каталог. Мы регулярно обновляем цены и ассортимент. Лучшие предложения собраны в одном месте. Подробное описание каждого букета. Букет роз прайс лист — вся информация в одном клике.
Вся информация на сайте – https://rozymsk.ru/kustovye-rozy
букет роз с рассрочкой, розы доставка москва, розы букет
букет из 51 розы цена, купить розы в москве дешево, букет роз с доставкой в москве
Удачи!
1win basketbol tikish http://1win14008.ru .
Заказать диплом на заказ вы сможете используя сайт компании. orikdok-2v-gorode-cheboksary-21.ru
Доставили точно в срок, как и обещали! Получатель был в восторге от букета.
розы купить в томске
Купить диплом института по выгодной цене возможно, обратившись к проверенной специализированной компании. Приобрести документ института можно у нас в Москве. orikdok-5v-gorode-ekaterinburg-66.online
1win app android http://www.1win-apk.pro/ .
король и шут скачать мп3 бесплатно король и шут скачать мп3 бесплатно .
mostbet az giriş bloku http://mostbet3045.ru .
Купить диплом ВУЗа !
Покупка диплома университета России в нашей компании – надежный процесс, так как документ будет заноситься в реестр. Приобрести диплом любого института diplomg-cheboksary.ru/kupite-diplom-visshego-obrazovaniya-s-zaneseniem-v-reestr-17
Vaping in Singapore: More Than Just a Trend
In today’s fast-paced world, people are always looking for ways to unwind, relax, and enjoy the moment — and for many, vaping has become a go-to ritual . In Singapore, where modern life moves quickly, the rise of vaping culture has brought with it a fresh way to relax . It’s not just about the devices or the clouds of vapor — it’s about flavor, convenience, and finding your own vibe.
Disposable Vapes: Simple, Smooth, Ready to Go
Let’s face it — nobody wants to deal with complicated setups all the time. That’s where disposable vapes shine. They’re perfect for busy individuals who still want that satisfying hit without the hassle of charging, refilling, or replacing parts.
Popular models like the VAPETAPE UNPLUG / OFFGRID, LANA ULTRA II, and SNOWWOLF SMART HD offer thousands of puffs in one sleek little package . Whether you’re out for the day or just need something quick and easy, these disposables have got your back.
New Arrivals: Fresh Gear, Fresh Experience
The best part about being into vaping? There’s always something new around the corner. The latest releases like the ELFBAR ICE KING and ALADDIN ENJOY PRO MAX bring something different to the table — whether it’s colder hits .
The ELFBAR RAYA D2 is another standout, offering more than just puff count — it comes with a built-in screen , so you can really make it your own.
Bundles: Smart Choices for Regular Vapers
If you vape often, buying in bulk just makes sense. Combo packs like the VAPETAPE OFFGRID COMBO or the LANA BAR 10 PCS COMBO aren’t just practical — they’re also a great value choice. No more running out at the worst time, and you save a bit while you’re at it.
Flavors That Speak to You
At the end of the day, it’s all about taste. Some days you want something icy and refreshing from the Cold Series, other times you’re craving the smooth, mellow vibes of the Smooth Series. Then there are those sweet cravings — and trust us, the Sweet Series delivers.
Prefer the classic richness of tobacco? There’s a whole series for that too. And if you’re trying to cut back on nicotine, the Zero-Nicotine Line gives you all the flavor without the buzz.
Final Thoughts
Vaping in Singapore isn’t just a passing trend — it’s a lifestyle choice for many. With so many options available, from pocket-sized disposables to customizable devices, there’s something for everyone. Whether you’re taking your first puff, or an experienced user , the experience is all about what feels right to you — made personal for you.
kraken onion – kraken сайт, кракен darknet
Vape Scene in Singapore: Embracing Modern Relaxation
In today’s fast-paced world, people are always looking for ways to unwind, relax, and enjoy the moment — and for many, vaping has become a preferred method . In Singapore, where modern life moves quickly, the rise of vaping culture has brought with it a new kind of chill . It’s not just about the devices or the clouds of vapor — it’s about flavor, convenience, and finding your own vibe.
Disposable Vapes: Simple, Smooth, Ready to Go
Let’s face it — nobody wants to deal with complicated setups all the time. That’s where disposable vapes shine. They’re perfect for busy individuals who still want that satisfying hit without the hassle of charging, refilling, or replacing parts.
Popular models like the VAPETAPE UNPLUG / OFFGRID, LANA ULTRA II, and SNOWWOLF SMART HD offer thousands of puffs in one easy-to-use device. Whether you’re out for the day or just need something quick and easy, these disposables have got your back.
New Arrivals: Fresh Gear, Fresh Experience
The best part about being into vaping? There’s always something new around the corner. The latest releases like the ELFBAR ICE KING and ALADDIN ENJOY PRO MAX bring something different to the table — whether it’s enhanced user experience.
The ELFBAR RAYA D2 is another standout, offering more than just puff count — it comes with a built-in screen , so you can really make it your own.
Bundles: Smart Choices for Regular Vapers
If you vape often, buying in bulk just makes sense. Combo packs like the VAPETAPE OFFGRID COMBO or the LANA BAR 10 PCS COMBO aren’t just practical — they’re also a better deal . No more running out at the worst time, and you save a bit while you’re at it.
Flavors That Speak to You
At the end of the day, it’s all about taste. Some days you want something icy and refreshing from the Cold Series, other times you’re craving the smooth, mellow vibes of the Smooth Series. Then there are those sweet cravings — and trust us, the Sweet Series delivers.
Prefer the classic richness of tobacco? There’s a whole series for that too. And if you’re trying to cut back on nicotine, the 0% Nicotine Series gives you all the flavor without the buzz.
Final Thoughts
Vaping in Singapore isn’t just a passing trend — it’s a lifestyle choice for many. With so many options available, from pocket-sized disposables to customizable devices, there’s something for everyone. Whether you’re new to the scene , or a seasoned vaper , the experience is all about what feels right to you — uniquely yours .
kra33.at – Kra33.cc, kra33.at
1win demo rejim 1win demo rejim .
мост бет mostbet20006.ru .
мостбет лицензия https://mostbet20005.ru .
Приобрести диплом об образовании. Приобретение диплома ВУЗа через надежную фирму дарит ряд плюсов для покупателя. Это решение дает возможность сэкономить время и серьезные деньги. orikdok-v-gorode-mahachkala-5.ru
Мы изготавливаем дипломы любой профессии по разумным ценам. Покупка диплома, подтверждающего обучение в ВУЗе, – это выгодное решение. Купить диплом любого ВУЗа: talentostartapero.com/employer/diplomirovans
Мы изготавливаем дипломы любой профессии по приятным тарифам.– fabulousroleplay.5nx.ru/viewtopic.phpf=40&t=549
mostbet instalar para ios http://www.mostbet3032.ru .
1win parol tiklash 1win parol tiklash .
Kra34.cc – kraken вход, kraken зеркало
купить диплом среднее специальное образование diplomandoci-24.online .
1win кыргызстан 1win кыргызстан .
сделать въезд на участок под ключ http://www.parkovka-dlya-mashiny-1122.ru .
kra33 – кракен официальная ссылка, Кракен даркнет как зайти на площадку
Лучшие проститутки в Новосибирске, узнайте тут https://www.sibirki.online/
Заметил ресурс доброе утро открытки 🙂
next page jaxx wallet chrome
mostbet az aviator https://www.mostbet3044.ru .
красивый заезд на участок https://parkovka-dlya-mashiny-1122.ru .
mostbet сайт https://mostbet3033.ru .
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Мы готовы предложить дипломы любых профессий по приятным ценам. Дипломы производятся на подлинных бланках государственного образца Быстро и просто приобрести диплом университета п»їdiplomass.com
Где купить диплом по актуальной специальности?
Заказать диплом ВУЗа по невысокой цене возможно, обратившись к надежной специализированной компании.: diplommy.ru
Наша компания предлагает выгодно и быстро купить диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями должностных лиц. Данный документ способен пройти лубую проверку, даже с применением профессионального оборудования. comedyforme.ru/kupit-diplom-s-dostavkoy-v-lyuboy-region-rossii
Цветы — это всегда радость, а с вашим сервисом — ещё и удобно!
заказать цветы с доставкой в томске
Р’С‹ видели композиции РѕС‚ fiora.su? РЇ искал что-то действительно изысканное для важной годовщины, Рё РёС… дизайны просто великолепны. Есть этот рафинированный, высококлассный РІРёРґ РІРѕ всем, что РѕРЅРё создают – РјРЅРѕРіРѕ тонких цветов Рё текстур, Р° РЅРµ просто СЏСЂРєРёРµ, подавляющие композиции. Упаковка тоже великолепна – ощущается как получение роскошного подарка, Р° РЅРµ просто цветов. Превосходно для тех случаев, РєРѕРіРґР° РІС‹ хотите произвести изысканное впечатление!
все песни король и шут скачать все песни король и шут скачать .
В этой статье мы расскажем вам о промокодах – что это такое, как их получить и использовать специальные коды от 1xBet, а также подробно опишем, где вводить коды для получения бонуса от букмекера.
Промокод – это уникальная комбинация букв и цифр, которая дает вам доступ к специальному предложению от букмекера. Компании часто используют их в различных акциях, чтобы привлечь новых игроков и поощрить постоянных клиентов. Бесплатные коды нужно вводить в специальное поле при регистрации на сайте букмекера или в личном кабинете, если у вас уже есть аккаунт.
промокод на день рождение 1хбет используйте код при регистрации, внесите депозит от 100 рублей и получите бонус +100% (до 32500 рублей), а также приветственный пакет в казино до 1950 евро + 150 фриспинов для игры в разделе азартных игр 1хБет. Бонусный код 1xBet дает возможность получить бонусы при регистрации на сайте букмекерской конторы.
1xBet – одна из ведущих компаний в сфере ставок, которая предлагает игрокам широкий выбор видов спорта и разнообразные ставки. Одним из преимуществ игры в 1xBet являются бонусные предложения, которые помогут вам увеличить свои шансы на успех и получить дополнительные выгоды.
При регистрации на сайте 1xBet используйте промокод и получите 100% бонус за первый депозит до 25000 рублей. Однако, если вы воспользуетесь промокодом, то получите еще больше – 130% до 32 500 рублей.
1xBet Бонусный промокод является уникальным кодом, который предоставляет игрокам возможность получить дополнительные выгоды при регистрации и использовании услуг букмекерской компании 1xBet. Этот промокод позволяет игрокам получить различные бонусы, такие как приветственные бонусы, бонусы на депозиты, бесплатные ставки и другие специальные предложения.
mostbet az virtual oyunlar http://mostbet3044.ru/ .
Москва. Психолог Беговой в Москве Психолог в Москве.
Услуги психолога · — Консультация психолога.
Получить поддержку по широкому кругу вопросов.
Психолог владеет множеством приемов и техник, которые помогут разобраться в себе.
1вин служба поддержки 1вин служба поддержки .
For more information https://l-spb.ru .
view it coinbase login
Вы заказываете документ в надежной и проверенной временем компании. Приобрести диплом ВУЗа– http://medialike.org/kupite-diplom-menedzhera-s-zaneseniem-v-reestr-2/ – medialike.org/kupite-diplom-menedzhera-s-zaneseniem-v-reestr-2/
Советуем почитать: https://coub.com/a2fa75132a95140e3287
Наша компания предлагает быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями должностных лиц. Документ способен пройти любые проверки, даже с использованием специального оборудования. school97.ru/vesti20/index.phpPAGE_NAME=profile_view&UID=222213
Мы готовы предложить документы учебных заведений, расположенных на территории всей Российской Федерации. Купить диплом любого ВУЗа:
social.instinxtreme.com/read-blog/37329_kupit-diplom-zanesennyj-reestr.html
Москва. Психологи, р-н Вороново (Москва) Психолог в Москве.
Частые разногласия с самыми близкими.
Эмоциональное состояние: тревога, депрессия, стресс, эмоциональное выгорание.
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
король и шут слушать и скачать король и шут слушать и скачать .
betwinner app – apk download https://www.betswinner.bet .
Москва. Психолог Покровское-Стрешнево Психолог в Москве.
Поможет поставить цель терапии и приведет к результату.
Частые разногласия с самыми близкими.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Москва. МСК психолог Покровское-Стрешнево Психолог в Москве.
Анонимный прием.
Психологическое консультирование заключается в том, чтобы помочь клиенту разобраться в своих проблемах и вместе с ним найти пути выхода из сложной ситуации.
Индивидуальное консультирование.
Запись на прием, оплата, подробная информация о специалистах и отзывы клиентов.
1win официальный войти 1win официальный войти .
Москва. Психологи в р-не Выхино-Жулебино или рядом Психолог в Москве.
Раздражительность на членов своей семьи.
Психолог, Сайт психологов.
Частые разногласия с самыми близкими.
Заказать диплом университета. Покупка подходящего диплома через проверенную и надежную фирму дарит ряд плюсов. Такое решение дает возможность сэкономить как длительное время, так и существенные финансовые средства. orikdok-v-gorode-krasnoyarsk-24.online
Заказать диплом под заказ вы имеете возможность через официальный портал компании. orikdok-1v-gorode-penza-58.online
Москва. Услуги психолога в районе Преображенское Психолог в Москве.
Правильно оценить происходящее в жизни и найти выход из сложившейся жизненной ситуации.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Раздражительность на членов своей семьи.
Услуги психолога · — Консультация психолога.
как потратить бонусы в 1 win https://1win22081.ru/ .
кракен сайт – kraken ссылка, kraken ссылка
Москва. Психолог Москва. Психолог онлайн. Консультация психолога в МСК Психолог в Москве.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
Личные или онлайн-встречи с высококвалифицированными специалистами.
Психологическая помощь онлайн.
Индивидуальное консультирование.
Москва. Психолог Выхино-Жулебино Психолог в Москве.
Раздражительность на членов своей семьи.
Психологическое консультирование.
Частые разногласия с самыми близкими.
Задайте интересующие вас вопросы или запишитесь на сеанс к психологу.
мостбет как получить бонус https://mostbet3034.ru/ .
read here coinbase pro
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель. Human design тест бесплатно
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
my link [url=https://thecoinb-se.com/]coinbase login[/url]
1win apk yuklab olish 1win apk yuklab olish .
Мы изготавливаем дипломы любой профессии по выгодным ценам. Купить диплом Балашиха — kyc-diplom.com/geography/balashiha.html
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
подъезд к участку под ключ http://www.proezd-k-uchastku-1122.ru/ .
Заказать диплом ВУЗа!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам— poluchidiplom.com
Мы готовы предложить дипломы любых профессий по доступным ценам. Мы предлагаем документы техникумов, расположенных в любом регионе России. Документы делаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. orikdok-5v-gorode-astrakhan-30.online
mostbet mobil tətbiqi https://mostbet3047.ru .
Приобрести диплом на заказ можно используя сайт компании. orikdok-2v-gorode-krasnoyarsk-24.ru
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель. Значение карт
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель. Ворота 43 дизайн человека
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
1win kod orqali ro‘yxatdan o‘tish 1win kod orqali ro‘yxatdan o‘tish .
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни. Дизайн человека онлайн
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
order ddos
Why Choose DDoS.Market?
High-Quality Attacks – Our team ensures powerful and effective DDoS attacks for accurate security testing.
Competitive Pricing & Discounts – We offer attractive deals for returning customers.
Trusted Reputation – Our service has earned credibility in the Dark Web due to reliability and consistent performance.
Who Needs This?
? Security professionals assessing network defenses.
? Businesses conducting penetration tests.
? IT administrators preparing for real-world threats.
Ensure your network is secure—test its limits with DDoS.Market.
сет музыкальный клубный сет музыкальный клубный .
клубные сеты диджеев клубные сеты диджеев .
Усиление конструкций работа Усиление конструкций работа .
Инъектирование бетона usileniekonstrukcij3.ru .
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной. Дизайн человека совместимость
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
бонусы спорт 1win как пользоваться 1win22086.ru .
Why Choose DDoS.Market?
High-Quality Attacks – Our team ensures powerful and effective DDoS attacks for accurate security testing.
Competitive Pricing & Discounts – We offer attractive deals for returning customers.
Trusted Reputation – Our service has earned credibility in the Dark Web due to reliability and consistent performance.
Who Needs This?
Security professionals assessing network defenses.
Businesses conducting penetration tests.
IT administrators preparing for real-world threats.
Букет шикарный! Спасибо за то, что делаете людей счастливее.
букеты томск
Vaping Culture in Singapore: A Lifestyle Beyond the Hype
In today’s fast-paced world, people are always looking for ways to unwind, relax, and enjoy the moment — and for many, vaping has become a go-to ritual . In Singapore, where modern life moves quickly, the rise of vaping culture has brought with it a unique form of downtime . It’s not just about the devices or the clouds of vapor — it’s about flavor, convenience, and finding your own vibe.
Disposable Vapes: Simple, Smooth, Ready to Go
Let’s face it — nobody wants to deal with complicated setups all the time. That’s where disposable vapes shine. They’re perfect for busy individuals who still want that satisfying hit without the hassle of charging, refilling, or replacing parts.
Popular models like the VAPETAPE UNPLUG / OFFGRID, LANA ULTRA II, and SNOWWOLF SMART HD offer thousands of puffs in one sleek little package . Whether you’re out for the day or just need something quick and easy, these disposables have got your back.
New Arrivals: Fresh Gear, Fresh Experience
The best part about being into vaping? There’s always something new around the corner. The latest releases like the ELFBAR ICE KING and ALADDIN ENJOY PRO MAX bring something different to the table — whether it’s richer flavors .
The ELFBAR RAYA D2 is another standout, offering more than just puff count — it comes with adjustable airflow , so you can really make it your own.
Bundles: Smart Choices for Regular Vapers
If you vape often, buying in bulk just makes sense. Combo packs like the VAPETAPE OFFGRID COMBO or the LANA BAR 10 PCS COMBO aren’t just practical — they’re also a better deal . No more running out at the worst time, and you save a bit while you’re at it.
Flavors That Speak to You
At the end of the day, it’s all about taste. Some days you want something icy and refreshing from the Cold Series, other times you’re craving the smooth, mellow vibes of the Smooth Series. Then there are those sweet cravings — and trust us, the Sweet Series delivers.
Prefer the classic richness of tobacco? There’s a whole series for that too. And if you’re trying to cut back on nicotine, the 0% Nicotine Series gives you all the flavor without the buzz.
Final Thoughts
Vaping in Singapore isn’t just a passing trend — it’s a lifestyle choice for many. With so many options available, from pocket-sized disposables to customizable devices, there’s something for everyone. Whether you’re exploring vaping for the first time , or a regular enthusiast , the experience is all about what feels right to you — tailored to your preferences .
Заказать диплом любого университета!
Приобретение диплома ВУЗа через качественную и надежную фирму дарит много плюсов. Заказать диплом ВУЗа у надежной организации: doks-v-gorode-omsk-55.online
vapesg
Vaping Culture in Singapore: A Lifestyle Beyond the Hype
In today’s fast-paced world, people are always looking for ways to unwind, relax, and enjoy the moment — and for many, vaping has become a go-to ritual . In Singapore, where modern life moves quickly, the rise of vaping culture has brought with it a fresh way to relax . It’s not just about the devices or the clouds of vapor — it’s about flavor, convenience, and finding your own vibe.
Disposable Vapes: Simple, Smooth, Ready to Go
Let’s face it — nobody wants to deal with complicated setups all the time. That’s where disposable vapes shine. They’re perfect for those who value simplicity who still want that satisfying hit without the hassle of charging, refilling, or replacing parts.
Popular models like the VAPETAPE UNPLUG / OFFGRID, LANA ULTRA II, and SNOWWOLF SMART HD offer thousands of puffs in one compact design . Whether you’re out for the day or just need something quick and easy, these disposables have got your back.
New Arrivals: Fresh Gear, Fresh Experience
The best part about being into vaping? There’s always something new around the corner. The latest releases like the ELFBAR ICE KING and ALADDIN ENJOY PRO MAX bring something different to the table — whether it’s enhanced user experience.
The ELFBAR RAYA D2 is another standout, offering more than just puff count — it comes with a built-in screen , so you can really make it your own.
Bundles: Smart Choices for Regular Vapers
If you vape often, buying in bulk just makes sense. Combo packs like the VAPETAPE OFFGRID COMBO or the LANA BAR 10 PCS COMBO aren’t just practical — they’re also a better deal . No more running out at the worst time, and you save a bit while you’re at it.
Flavors That Speak to You
At the end of the day, it’s all about taste. Some days you want something icy and refreshing from the Cold Series, other times you’re craving the smooth, mellow vibes of the Smooth Series. Then there are those sweet cravings — and trust us, the Sweet Series delivers.
Prefer the classic richness of tobacco? There’s a whole series for that too. And if you’re trying to cut back on nicotine, the Nicotine-Free Range gives you all the flavor without the buzz.
Final Thoughts
Vaping in Singapore isn’t just a passing trend — it’s a lifestyle choice for many. With so many options available, from pocket-sized disposables to customizable devices, there’s something for everyone. Whether you’re exploring vaping for the first time , or a regular enthusiast , the experience is all about what feels right to you — uniquely yours .
Приобрести диплом ВУЗа!
Купить диплом о высшем образовании. Заказ документа о высшем образовании через качественную и надежную фирму дарит массу плюсов. Данное решение позволяет сберечь как дорогое время, так и существенные финансовые средства. munchkin.flybb.ru/viewtopic.phpf=7&t=5386
chem dog clones
Приобрести диплом ВУЗа по невысокой цене вы можете, обратившись к проверенной специализированной компании. Быстро купить диплом о высшем образовании: heebig.com/tolko-segodnja-kupit-diplom-s-garantiej-34
mostbet aviator yuklab olish mostbet aviator yuklab olish .
Hello everyone !
You don’t need special skills to earn crypto with Crypto Gifts. The platform is beginner-friendly and rewarding. Crypto Gifts Charity Give gifts, support others, and watch your crypto grow. Sign up now and start your earning journey.
The future of crypto is human, and it starts with you. Crypto Gifts brings people together with purpose. Join, share, and receive — it’s that easy. You’re more than welcome here.
All information on the site – https://cryptogifts.charity/dashboard?ref=1
crypto investment, crypto investment, earn ethereum
crypto investment, crypto lending, crypto staking
And look via our social profiles too – https://www.instagram.com/cryptogiftsusdt/
Good luck!
постройка забора из профнастила цена zaborizproflista.ru/iz-profnastila .
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром. Профиль 4 6
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром. Манифестирующий генератор 6 2
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Наша мастерская предлагает профессиональный мастерская по ремонту духовых шкафов рядом любых брендов и моделей. Мы понимаем, насколько необходимы вам ваши кухонные приборы, и обеспечиваем ремонт наилучшего качества. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только оригинальные запчасти, что гарантирует долговечность и надежность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются пользователи духовок, включают неработающие нагревательные элементы, выход из строя таймера, поврежденную дверцу, неисправность контроллера, ошибки вентиляционной системы и неисправные платы. Для устранения этих поломок наши профессиональные техники проводят ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обращаясь в наш сервисный центр, вы получаете долговечный и надежный официальный ремонт духовых шкафов.
Подробная информация представлена на нашем сайте: https://remont-duhovyh-shkafov-ace.ru
dj music dj music .
сет музыкальный клубный сет музыкальный клубный .
1win link 1win22084.ru .
Заказать диплом о высшем образовании!
Мы предлагаембыстро заказать диплом, который выполнен на оригинальной бумаге и заверен печатями, водяными знаками, подписями официальных лиц. Данный диплом пройдет лубую проверку, даже при использовании профессиональных приборов. Решите свои задачи быстро с нашим сервисом- peticoes.pt/482927
Гидроизоляция мембраной http://usileniekonstrukcij2.ru/ .
Усиление конструкций работа Усиление конструкций работа .
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. Дизайн человека новосибирск
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Наши специалисты предлагает надежный центр ремонта духового шкафа в москве любых брендов и моделей. Мы знаем, насколько значимы для вас ваши духовые шкафы, и готовы предложить сервис наилучшего качества. Наши профессиональные техники работают быстро и аккуратно, используя только оригинальные запчасти, что гарантирует надежность и долговечность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели кухонных приборов, включают неисправности термостата, неисправный таймер, поломку дверцы, неисправность контроллера, ошибки вентиляционной системы и повреждения электроники. Для устранения этих поломок наши профессиональные техники проводят ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обращаясь в наш сервисный центр, вы гарантируете себе надежный и долговечный сервисный ремонт духового шкафа с гарантией.
Подробная информация представлена на нашем сайте: https://remont-duhovyh-shkafov-ace.ru
Мы изготавливаем дипломы любой профессии по приятным ценам. Заказ диплома, подтверждающего окончание ВУЗа, – это разумное решение. Приобрести диплом о высшем образовании: dp.getbb.ru/viewtopic.phpf=3&t=2401
mostbet daxil ol mostbet daxil ol .
Приобрести диплом об образовании. Покупка документа о высшем образовании через надежную фирму дарит массу плюсов для покупателя. Такое решение помогает сэкономить как личное время, так и серьезные средства. orikdok-v-gorode-ryazan-62.online
самые лучшие клубные треки самые лучшие клубные треки .
More about the author coinbsae wallet login
Заказать диплом университета по доступной стоимости вы можете, обращаясь к надежной специализированной компании. Мы можем предложить документы престижных ВУЗов, которые расположены на территории всей России. korolev-progym.ru/zanesenie-diploma-v-reestr-obrazovaniya/
официальный сайт 1вин 1win22087.ru .
Советую сайт: https://www.bandlab.com/user8842766547440988
Приобрести диплом любого университета!
Мы предлагаемвыгодно купить диплом, который выполняется на оригинальном бланке и заверен печатями, штампами, подписями официальных лиц. Документ пройдет лубую проверку, даже с использованием специфических приборов. Решайте свои задачи быстро и просто с нашими дипломами- komunitas.dukcapil.id/read-blog/224_diplom-s-vneseniem-v-reestr.html
диплом специалиста купить http://www.rudiplomo.online .
Советуем почитать: https://bbloggerov.jofo.me/2451619-montaj-promyishlennoy-ventilyatsii.html
купить диплом в белгороде купить диплом в белгороде .
mostvet mostbet3035.ru .
“The government also encourages all firms to implement the Flexible Wage System as the uncertainties ahead continue to underscore the need for resilience and flexibility in wage structures.” 토토커뮤니티
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной. Канал 13 33 хьюман дизайн
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Наша компания предлагает выгодно и быстро заказать диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, штампами, подписями официальных лиц. Диплом пройдет любые проверки, даже при использовании профессиональных приборов. o2cloudsystems.com/kupit-diplom-bystro-i-nadezhno-luchshie-80-2
mostbet kripto orqali depozit mostbet kripto orqali depozit .
слушать клубную музыку слушать клубную музыку .
this article coinbase exchange
1win oynalgan sayt uz http://1win14009.ru .
Купить диплом ВУЗа!
Мы предлагаем дипломы любых профессий по разумным тарифам— incasso.ru
Купить диплом ВУЗа по выгодной стоимости можно, обратившись к проверенной специализированной фирме. Купить документ университета можно в нашей компании. orikdok-4v-gorode-tyumen-72.ru
kra32.at – Kra33.at, kraken войти
1вин как получить бонус http://www.1win14010.ru .
speed n cash лайфак http://1win22084.ru/ .
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель. Ра уру ху биография
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Заказать диплом о высшем образовании !
Приобретение диплома ВУЗа России у нас является надежным делом, поскольку документ будет заноситься в реестр. Приобрести диплом любого университета diplomaj-v-tule.ru/kupite-diplom-v-moskve-s-vneseniem-v-reestr-2
Dive into intense pleasure with Crystal Lust’s films.
Each video from Crystal Lust is more thrilling than the last crystal lust succubus .
Check out Crystal Lust’s leaked nudes and gifs now.
Fans adore her for the natural curves and energy.
Experience her POV fantasies in perfect quality.
Her performance will stay with you for days.
Explore the wild side of Crystal Lust online.
Find out why Crystal Lust is going viral.
Step into her world and experience her style crystal lust full movie.
Мы изготавливаем дипломы любой профессии по приятным ценам. Мы предлагаем документы техникумов, расположенных на территории всей РФ. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это позволяет делать государственные дипломы, не отличимые от оригиналов. orikdok-4v-gorode-khabarovsk-27.online
устройство заезда на участок через канаву http://www.proezd-k-uchastku-1122.ru .
Купить диплом можно используя официальный сайт компании. orikdok-2v-gorode-rostov-na-donu-61.online
Быстро, качественно и с душой! Лучший сервис по доставке цветов!
цветы
хранение резины в паркинге хранение резины в паркинге .
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. Проектор 6 2
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель. Дизайн человека определить тип
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Советую эту компанию для обслуживания систем вентиляции, подробнее тут https://www.indiegogo.com/individuals/38535179/
купить диплом в пензе купить диплом в пензе .
Приобрести диплом института по доступной стоимости возможно, обращаясь к проверенной специализированной фирме. Приобрести документ института вы имеете возможность у нас в Москве. orikdok-5v-gorode-groznyy-20.online
купить какой iphone купить какой iphone .
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть. Построить дизайн человека
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
купить диплом в чите купить диплом в чите .
how to withdraw from betwinner how to withdraw from betwinner .
промо 1вин промо 1вин .
купить аттестат купить аттестат .
Мы можем предложить дипломы любой профессии по выгодным тарифам.– horizonsmaroc.com/entreprises/frees-diplom
Dikkat forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > hemen oyna
Slot turnuvas? yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 20 saniyede haz?r.
Simdi dene!
Dikkat bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > guncel giris adresi
Canl? bahis yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 15 saniyede haz?r.
Simdi dene!
обустройство въезда на участок через канаву http://www.proezd-k-uchastku-1122.ru/ .
iphone центр iphone центр .
Заказать диплом любого института. Производство документа занимает гораздо меньше времени, а стоимость – невысокая. В результате вы сможете сберечь деньги и время и найти хорошую работу мечты. Приобрести диплом на заказ в столице вы сможете используя официальный сайт компании. – 1wum.ru/forum/?PAGE_NAME=profile_view&UID=48493&MUL_MODE=
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Мы предлагаем документы ВУЗов, которые расположены на территории всей России. Документы печатаются на бумаге самого высокого качества. Это дает возможность делать государственные дипломы, которые не отличить от оригиналов. orikdok-v-gorode-mahachkala-5.ru
mostbet qanunidirmi https://www.mostbet3046.ru .
taktychni-rukavyci.netlify.app – ваш лучший источник информации, рекомендуем заглянуть.
Узнайте больше о новых технологиях на taktychni-rukavyci.netlify.app, ознакомиться.
taktychni-rukavyci.netlify.app: ваш гид по технологиям, обязательно ознакомьтесь.
Будьте в курсе современных технологий с taktychni-rukavyci.netlify.app, рекомендуем.
taktychni-rukavyci.netlify.app: откройте новые горизонты, не забудьте.
taktychni-rukavyci.netlify.app – для тех, кто ищет, рекомендуем.
Увлекательные факты на taktychni-rukavyci.netlify.app, рекомендуем.
taktychni-rukavyci.netlify.app: инновации и технологии, воспользуйтесь шансом.
Изучайте технологии будущего на taktychni-rukavyci.netlify.app, советуем.
taktychni-rukavyci.netlify.app – научные открытия и технологии, рекомендуем.
тактичні рукавиці військові https://taktychni-rukavyci.netlify.app/ .
бонусы в 1win http://1win22087.ru/ .
download betwinner app for android https://www.betswinner.bet .
Мы готовы предложить документы институтов, расположенных в любом регионе Российской Федерации. Приобрести диплом о высшем образовании:
powerofthelamb.mn.co/posts/85190372
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель. Аналитик тип личности
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
where to buy vape in singapore
Vape Scene in Singapore: Embracing Modern Relaxation
In today’s fast-paced world, people are always looking for ways to unwind, relax, and enjoy the moment — and for many, vaping has become a go-to ritual . In Singapore, where modern life moves quickly, the rise of vaping culture has brought with it a stylish escape. It’s not just about the devices or the clouds of vapor — it’s about flavor, convenience, and finding your own vibe.
Disposable Vapes: Simple, Smooth, Ready to Go
Let’s face it — nobody wants to deal with complicated setups all the time. That’s where disposable vapes shine. They’re perfect for busy individuals who still want that satisfying hit without the hassle of charging, refilling, or replacing parts.
Popular models like the VAPETAPE UNPLUG / OFFGRID, LANA ULTRA II, and SNOWWOLF SMART HD offer thousands of puffs in one portable solution . Whether you’re out for the day or just need something quick and easy, these disposables have got your back.
New Arrivals: Fresh Gear, Fresh Experience
The best part about being into vaping? There’s always something new around the corner. The latest releases like the ELFBAR ICE KING and ALADDIN ENJOY PRO MAX bring something different to the table — whether it’s smarter designs .
The ELFBAR RAYA D2 is another standout, offering more than just puff count — it comes with dual mesh coils, so you can really make it your own.
Bundles: Smart Choices for Regular Vapers
If you vape often, buying in bulk just makes sense. Combo packs like the VAPETAPE OFFGRID COMBO or the LANA BAR 10 PCS COMBO aren’t just practical — they’re also a cost-effective option . No more running out at the worst time, and you save a bit while you’re at it.
Flavors That Speak to You
At the end of the day, it’s all about taste. Some days you want something icy and refreshing from the Cold Series, other times you’re craving the smooth, mellow vibes of the Smooth Series. Then there are those sweet cravings — and trust us, the Sweet Series delivers.
Prefer the classic richness of tobacco? There’s a whole series for that too. And if you’re trying to cut back on nicotine, the 0% Nicotine Series gives you all the flavor without the buzz.
Final Thoughts
Vaping in Singapore isn’t just a passing trend — it’s a lifestyle choice for many. With so many options available, from pocket-sized disposables to customizable devices, there’s something for everyone. Whether you’re taking your first puff, or a regular enthusiast , the experience is all about what feels right to you — made personal for you.
click here now coinbase login
кракен тор – kra33.cc, kra33.cc
hop over to here coinbase login
купить айфон 14 про в москве оригинал купить айфон 14 про в москве оригинал .
связной айфон связной айфон .
Приобрести диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по доступным ценам. Вы приобретаете документ через надежную и проверенную компанию. : medicina.forumex.ru/viewtopic.phpf=8&t=2803
truyệnqq
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель. Рассчитать транзиты
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Заказать диплом о высшем образовании. Заказ официального диплома через надежную фирму дарит ряд плюсов для покупателя. Это решение позволяет сберечь время и существенные финансовые средства. orikdok-4v-gorode-vladivostok-25.ru
https://Buffedhub.com/RI876863539462728127/
https://Buffedhub.com/HC98PYRGHYWQQQ/
https://Buffedhub.com/OD56FBZRTJWDWN/
https://Buffedhub.com/IX544VRYYZAXFK86R/
https://Buffedhub.com/XH737EGJOPWRIA94Q33/
https://Buffedhub.com/OP598BQENRXIZE46O31QNPIP/
https://Buffedhub.com/YI176539449261725683/
https://Buffedhub.com/NC578NEXBQGIYB28J92OA/
https://Buffedhub.com/YI134675771134XNBJ/
https://Buffedhub.com/PQ11JPSRBCIRIM/
https://Buffedhub.com/NT532CJTURCPPQ89L43LT/
https://Buffedhub.com/NO178YZYSKH/
https://Buffedhub.com/GG869LFGYQMSGL74S61EPOHQ/
https://Buffedhub.com/PO557JCFEPIMXP95R39/
https://Buffedhub.com/SL684349749327BMAI/
https://Buffedhub.com/KE874118745137452762/
https://Buffedhub.com/MO294545851434937668/
https://Buffedhub.com/GJ747988851136KEGL593/
https://Buffedhub.com/IQ355122518791XBFS/
https://Buffedhub.com/FE939HDINNM/
The Rise of Vaping in Singapore: Not Just a Fad
In today’s fast-paced world, people are always looking for ways to unwind, relax, and enjoy the moment — and for many, vaping has become a daily habit. In Singapore, where modern life moves quickly, the rise of vaping culture has brought with it a stylish escape. It’s not just about the devices or the clouds of vapor — it’s about flavor, convenience, and finding your own vibe.
Disposable Vapes: Simple, Smooth, Ready to Go
Let’s face it — nobody wants to deal with complicated setups all the time. That’s where disposable vapes shine. They’re perfect for busy individuals who still want that satisfying hit without the hassle of charging, refilling, or replacing parts.
Popular models like the VAPETAPE UNPLUG / OFFGRID, LANA ULTRA II, and SNOWWOLF SMART HD offer thousands of puffs in one compact design . Whether you’re out for the day or just need something quick and easy, these disposables have got your back.
New Arrivals: Fresh Gear, Fresh Experience
The best part about being into vaping? There’s always something new around the corner. The latest releases like the ELFBAR ICE KING and ALADDIN ENJOY PRO MAX bring something different to the table — whether it’s richer flavors .
The ELFBAR RAYA D2 is another standout, offering more than just puff count — it comes with adjustable airflow , so you can really make it your own.
Bundles: Smart Choices for Regular Vapers
If you vape often, buying in bulk just makes sense. Combo packs like the VAPETAPE OFFGRID COMBO or the LANA BAR 10 PCS COMBO aren’t just practical — they’re also a better deal . No more running out at the worst time, and you save a bit while you’re at it.
Flavors That Speak to You
At the end of the day, it’s all about taste. Some days you want something icy and refreshing from the Cold Series, other times you’re craving the smooth, mellow vibes of the Smooth Series. Then there are those sweet cravings — and trust us, the Sweet Series delivers.
Prefer the classic richness of tobacco? There’s a whole series for that too. And if you’re trying to cut back on nicotine, the Nicotine-Free Range gives you all the flavor without the buzz.
Final Thoughts
Vaping in Singapore isn’t just a passing trend — it’s a lifestyle choice for many. With so many options available, from pocket-sized disposables to customizable devices, there’s something for everyone. Whether you’re taking your first puff, or a long-time fan, the experience is all about what feels right to you — tailored to your preferences .
Расскажем о ремонте техники – на сайте
mostbet haqqında rəylər mostbet haqqında rəylər .
трансформатор силовой сухой 1000 ква трансформатор силовой сухой 1000 ква .
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Приобрести диплом любого ВУЗа!
Наша компания предлагаетбыстро и выгодно купить диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями должностных лиц. Данный документ способен пройти любые проверки, даже при использовании специального оборудования. Достигайте цели максимально быстро с нашими дипломами- app.theremoteinternship.com/read-blog/45749_kupit-diplom-s-zaneseniem.html
Расскажем о ремонте техники – подробнее
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть. Manifesting generator 4 6
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни. Нумерология профессия
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
https://Buffedhub.com/GL259PEBSPJRMP15S42GV/
https://Buffedhub.com/OD594AUSWKFWLV56W87/
https://Buffedhub.com/PA181QDQLALIVV22X31PK/
https://Buffedhub.com/721828648771248472/
https://Buffedhub.com/BI158XAZFNXOGA52X86PT/
https://Buffedhub.com/AO677799219192576/
https://Buffedhub.com/DH759FHDLUDRGI33R75YNVYV/
https://Buffedhub.com/XS842563499527971951/
https://Buffedhub.com/LX448BHOCOHSJX73J12/
https://Buffedhub.com/DF92XGEYUGZECG/
https://Buffedhub.com/IZ296553255516PXCR/
https://Buffedhub.com/PY912UWKSJMTYD58G77DV/
https://Buffedhub.com/YT191577762766487436/
https://Buffedhub.com/CM993242626666IVLB942/
https://Buffedhub.com/HR497UFHVRHUGK46Y48AHGRT/
https://Buffedhub.com/CE967367374433GKFZ/
https://Buffedhub.com/NG98OJZIXBBYFN/
https://Buffedhub.com/DH93WJNFBIKGAY/
https://Buffedhub.com/TY773KPVQXV/
https://Buffedhub.com/385116696847454584/
Вы заказываете диплом в надежной и проверенной временем компании. Заказать диплом о высшем образовании– http://7468888.ru/kupit-diplom-cherez-reestr/ – 7468888.ru/kupit-diplom-cherez-reestr/
где купить айфон +в кредит где купить айфон +в кредит .
iphone питер https://kupit-ajfon-cs12.ru .
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель. Хьюман дизайн бесплатно
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
Мы предлагаем документы любых учебных заведений, которые находятся на территории всей РФ. Купить диплом любого ВУЗа:
o2cloudsystems.com/kachestvennye-diplomy-dlja-vashego-uspeha-80
Цветы доставили в соседний город — всё пришло в идеальном состоянии!
цветы томск
сухой трансформатор 10 кв сухой трансформатор 10 кв .
Rainbet promo code ILBET В динамичном мире онлайн-развлечений Rainbet занимает особое место, предоставляя игрокам широкие возможности для азартных игр и спортивных ставок. Для максимального увеличения выгоды и усиления азарта используйте промокод ILBET при регистрации или внесении депозита. Этот код активирует эксклюзивные бонусы, акции и предложения, разработанные как для новичков, так и для опытных игроков.
купить v bucks – как купить в fortnite в россии, купить боевой пропуск фортнайт
plataforma demo gr谩tis
futemax futebol ao vivo hoje rmc
Recomendo para todos que gostam de apostas.
Funciona bem no celular.
http://maestro.saude-rioclaro.org.br:8888/multicanais jogos ao vivo/
https://appqa.casashopping.com/multicanais tv ao vivo/
стоимость программы 1с бухгалтерия стоимость программы 1с бухгалтерия .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips в москве, можете посмотреть на сайте: срочный ремонт кофемашин philips
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
casino in moldova casino in moldova .
More about the author jaxx
Looking for the hottest mew slut porn content you won’t find anywhere else?. Find Mew Slut full porn videos on trusted platforms. Stream top-rated Mew Slut xxx content today. Browse all categories of Mew Slut leaks. Freshly leaked Mew Slut onlyfans updates. Mew Slut’s latest anal and POV drops inside. Experience the hottest Mew Slut porno ever. All leaked porn of Mew Slut in HQ. Explore her explicit porn scenes freely watch mew slut leaks.
Купить диплом ВУЗа по выгодной цене можно, обратившись к проверенной специализированной компании. Мы предлагаем документы об окончании любых ВУЗов РФ. Приобрести диплом о высшем образовании– forum.mbprinteddroids.com/showthread.php?tid=59669
Диплом любого ВУЗа Российской Федерации!
Без университета достаточно сложно было продвигаться по карьере. Приобрести диплом под заказ в Москве возможно через официальный сайт компании: novostroyki.ostroyke.com.ua/articles
1с стоимость программы http://programmy-1s17.ru/ .
Заказать диплом университета по доступной стоимости вы сможете, обратившись к надежной специализированной компании. Приобрести документ ВУЗа можно у нас. orikdok-4v-gorode-penza-58.online
Merhaba, bahis sevdal?lar?!
Bitcoin ile yukle
ve hemen Mega Hos Geldin paketini kap.
Cevrim x10 – maksimum ozgurluk.
KYC istenmez, withdraw 10 dk icinde.
Daha ne bekliyorsun? Sans ayag?nda.
—
xturka ucretsiz deneme
Merhaba, bahis sevdal?lar?!
Bitcoin ile yukle
ve hemen Mega Hos Geldin paketini kap.
Cevrim yaln?zca on kat – maksimum ozgurluk.
Belgeler istenmez, withdraw 10 dk icinde.
Daha ne bekliyorsun? Sans ayag?nda.
—
Sorunsuz xturka bahis
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым Тип личности проектор 2 4
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
browse around this web-site brd wallet
Наши специалисты предлагает высококачественный отремонтировать духовой шкаф рядом различных марок и моделей. Мы знаем, насколько важны для вас ваши духовые шкафы, и обеспечиваем ремонт наилучшего качества. Наши профессиональные техники работают быстро и аккуратно, используя только сертифицированные компоненты, что гарантирует долговечность и надежность выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются обладатели кухонных приборов, включают неисправности термостата, поломку таймера, треснувшую дверцу, неисправность контроллера, ошибки вентиляционной системы и повреждения электроники. Для устранения этих неисправностей наши квалифицированные специалисты оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обращаясь в наш сервисный центр, вы гарантируете себе надежный и долговечный мастер по ремонту духового шкафа на дому.
Подробная информация размещена на сайте: https://remont-duhovyh-shkafov-ace.ru
купить 1с в москве купить 1с в москве .
1win akkaunt yaratish http://1win14011.ru/ .
article source toast wallet download
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по выгодным ценам. Покупка документа, который подтверждает окончание института, – это выгодное решение. Купить диплом о высшем образовании: asg-amt.phorum.pl/viewtopic.phpp=35177#35177
mostbet poker otağı https://mostbet3048.ru .
въезд для машины на даче https://vezd-na-dachnyj-uchastok-1122.ru/ .
Приобрести диплом на заказ возможно через официальный сайт компании. orikdok-5v-gorode-kazan-16.online
сантехника донецк днр [url=https://santehnika-doneck-1.ru]https://santehnika-doneck-1.ru[/url] .
1win ilova skachat https://www.1win14012.ru .
1с предприятие купить программу цена https://www.programmy-1s17.ru .
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу. 19 ворота дизайн человека
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. 7 ворота дизайн человека
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
More about the author toast crypto wallet
стоматология прайс Владимир http://www.stomatologiya-vladimir-2.ru/ .
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними. Human design space
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
mostbet şifrə unutmuşam mostbet şifrə unutmuşam .
click over here wallet jaxx
Наша мастерская предлагает высококачественный мастер по ремонту духовых шкафов адреса различных марок и моделей. Мы понимаем, насколько необходимы вам ваши духовые шкафы, и готовы предложить сервис первоклассного уровня. Наши опытные мастера проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что предоставляет долговечность и надежность выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются владельцы духовых шкафов, включают неисправности термостата, поломку таймера, поломку дверцы, неисправность контроллера, ошибки вентиляционной системы и повреждения электроники. Для устранения этих проблем наши профессиональные техники оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Доверив ремонт нам, вы гарантируете себе надежный и долговечный вызвать мастера по ремонту духовых шкафов адреса.
Подробная информация доступна на сайте: https://remont-duhovyh-shkafov-ace.ru
visit this site breadwallet
Наши специалисты предлагают быстро заказать диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями официальных лиц. Данный диплом пройдет лубую проверку, даже с применением специально предназначенного оборудования. rhfranceisrael.com/employer/archive-diploma
мостбет уз скачать http://mostbet3037.ru .
войти 1win https://1win22088.ru/ .
reference jaxx wallet chrome
mostbet iphone mostbet iphone .
mostbet app download ios http://www.mostbet-app-download-apk.com .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips рядом, можете посмотреть на сайте: ремонт кофемашин philips цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
roobet bonus code 2025 WEB3 В мире онлайн-казино инновации не стоят на месте, и Roobet находится в авангарде этих перемен. С появлением технологии Web3, Roobet предлагает игрокам новый уровень прозрачности, безопасности и децентрализации. Чтобы воспользоваться всеми преимуществами этой передовой платформы, используйте промокод WEB3.
Заказать диплом вы имеете возможность используя официальный сайт компании. orikdok-5v-gorode-cheboksary-21.ru
Нашел полезный сайт про лечение в Китае стоматология в хуньчуне !!!
Букет был упакован так красиво, что даже не хотелось разворачивать!
заказ цветов томск
mostbet promo bilan ro‘yxatdan o‘tish mostbet promo bilan ro‘yxatdan o‘tish .
Заказать диплом о высшем образовании. Заказ официального диплома через качественную и надежную компанию дарит ряд преимуществ. Данное решение помогает сберечь как продолжительное время, так и существенные денежные средства. orikdok-2v-gorode-novokuznetsk-42.ru
Купить документ о получении высшего образования вы можете в нашей компании. Купить диплом ВУЗа по доступной стоимости возможно, обратившись к проверенной специализированной фирме. toplentanews.ru/kupit-diplom-s-ofitsialnyim-blankom-i-garantiey-vozvrata
giả dược
Приобрести диплом ВУЗа по доступной стоимости возможно, обратившись к проверенной специализированной фирме. Приобрести диплом: jivonews.ru/kupit-diplom-s-garantiey-i-vyigodnyimi-usloviyami-oplatyi
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной. Дизайн человека профиль 6 3
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
1win aviator yuklab olish https://1win14011.ru/ .
Купить диплом ВУЗа!
Наши специалисты предлагаютмаксимально быстро купить диплом, который выполнен на оригинальной бумаге и заверен печатями, штампами, подписями должностных лиц. Документ пройдет любые проверки, даже при использовании специальных приборов. Достигайте цели максимально быстро с нашим сервисом- hirephpdeveloper.co.uk/kupit-diplom-prostoe-reshenie-dlja-slozhnyh-zadach-227
Visit Your URL toast wallet login
order ddos attack
Why Choose DDoS.Market?
High-Quality Attacks – Our team ensures powerful and effective DDoS attacks for accurate security testing.
Competitive Pricing & Discounts – We offer attractive deals for returning customers.
Trusted Reputation – Our service has earned credibility in the Dark Web due to reliability and consistent performance.
Who Needs This?
? Security professionals assessing network defenses.
? Businesses conducting penetration tests.
? IT administrators preparing for real-world threats.
Ensure your network is secure—test its limits with DDoS.Market.
купить код на вбаксы – купить в баксы фортнайт, купить v bucks fortnite
mostbet uz ishonchlimi mostbet uz ishonchlimi .
Your article helped me a lot, is there any more related content? Thanks!
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни. Кто такой манифестор
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Наши специалисты предлагает высококачественный официальный ремонт духовых шкафов всех типов и брендов. Мы осознаем, насколько важны для вас ваши кухонные приборы, и стремимся предоставить услуги первоклассного уровня. Наши профессиональные техники работают быстро и аккуратно, используя только сертифицированные компоненты, что гарантирует надежность и долговечность выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются обладатели кухонных приборов, включают неисправности термостата, неисправный таймер, поврежденную дверцу, проблемы с контроллером, неисправности вентилятора и неисправные платы. Для устранения этих проблем наши профессиональные техники оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обращаясь в наш сервисный центр, вы гарантируете себе надежный и долговечный центр ремонта духовых шкафов адреса.
Подробная информация представлена на нашем сайте: https://remont-duhovyh-shkafov-ace.ru
Мы готовы предложить документы учебных заведений, которые находятся в любом регионе РФ. Приобрести диплом о высшем образовании:
windows1.listbb.ru/viewtopic.php?f=3&t=1531
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым 11 ворота
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
vbucks купить – купить в баксы fortnite, купить боевой пропуск фортнайт
Проектное управление Ментор: ваш личный проводник к успеху. В современном бизнесе важно не только придумывать идеи, но и уметь их реализовать. Настоящий ментор — это ваш личный проводник, помогающий развить навыки и ускорить рост. Ментор делится опытом, дает ценные советы, поддерживает в трудные моменты. Помощь ментора — это инвестиции в ваше будущее. Не откладывайте развитие — закажите консультацию и начните получать поддержку профессионала, который поможет вам достигнуть новых высот.
1 win. [url=http://1win22088.ru/]http://1win22088.ru/[/url] .
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. Центры в дизайне человека
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт телефонов айфон в москве адреса
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Заказать диплом об образовании. Приобретение документа о высшем образовании через качественную и надежную фирму дарит ряд преимуществ для покупателя. Данное решение помогает сберечь как личное время, так и существенные финансовые средства. orikdok-v-gorode-krasnoyarsk-24.online
mobila birou chisinau [url=https://1win100.md]mobila birou chisinau[/url] .
Заказать документ о получении высшего образования можно у нас. Купить диплом университета по доступной стоимости возможно, обращаясь к проверенной специализированной фирме. sllife.ru/social/user/4216/forum/message/2784/7493/#message7493
have a peek at these guys breadwallet
скачать мостбет казино на андроид http://www.mostbet-app-download-apk.com .
1вин промокод 2025 уз http://1win14012.ru .
стоимость программы 1с бухгалтерия стоимость программы 1с бухгалтерия .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips цены, можете посмотреть на сайте: ремонт кофемашин philips цены
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mostbet giriş https://www.mostbet3048.ru .
1с управление нашей фирмой стоимость 1с управление нашей фирмой стоимость .
Hey bahis tutkunlar?!
Bu y?l?n en h?zl? giris kap?s? ?? BetSalvador guncel giris ??
Turkce profesyonel destek + h?zl? cekim sistemi + gelismis guvenlik ? kesintisiz kazanc.
Hemen giris yap ?? ilk yat?r?m?n? %300 Hosgeldin Bonusu ile buyut! Min. yat?r?m 100 ?.
eSpor & canl? casino, slot ve daha fazlas? her cihazda seni bekliyor.
Kuponunu yap — jackpot seninle olsun! ??
Guncel linki kaydet — engel gelse de alternatif baglant? sende!
Selam bahis tutkunlar?!
Su an?n en guncel giris adresi ?? BetSalvador ??
Jet cevapl? destek + 15 dk’da odeme + cift katmanl? koruma ? kesintisiz kazanc.
Bugun giris yap ?? ilk yat?r?m?n? %300 Hosgeldin Bonusu ile uce katla! Min. depozit 100 ?.
eSpor & canl? casino, rulet ve daha fazlas? her cihazda seni bekliyor.
Kuponunu yap — big win seninle olsun! ??
Bu adresi yer imlerine ekle — kapanma riskine kars? alternatif baglant? sende!
Uncensored Mew Slut videos are finally available online.
Click to see Mew Slut’s real name and secret life.
She’s back with face fuck and cumshots.
[url=http://quasia.net/uncategorized/1/?unapproved=195767&moderation-hash=838e0b5ffee683a46f0525ffa9e4c6b6#comment-195767]mew slut only fans leaks[/url]
[url=https://yexhub.com/understanding-seo-the-key-to-digital-success/?unapproved=50205&moderation-hash=0a1798b83a550710213acbe27aff858f#comment-50205]mew slut leave my boyfriend alone[/url]
[url=https://vormalux.com/vormalux_logo/?unapproved=20329&moderation-hash=24ab65d07c215d3ec1c7d9db30329a51#comment-20329]mew slut full porn videos[/url]
[url=https://citywestreceptions.com.au/using-apple-products-for-event-planning/#comment-29258/]mew slut xx[/url]
[url=https://www.royalnettingsolutions.com/product/pe-washable-artificial-wall-grass/?unapproved=437119&moderation-hash=f40a427be1361936fcd2ce2382146e8a#comment-437119]mew slut pornstar[/url]
The internet is obsessed with Mew Slut’s vids.
From naked teasing to hardcore, Mew Slut does it all.
The creamiest compilation of Mew Slut’s vids is out.
Porn mew slut fans finally get what they asked for.
mew slut throat
mew slut onlyfans leaked
mew slut xvideos
mew slut onlyfans porn
mew slut video
Witness the most intense blowjob content from Mew Slut.
That xvideos update? It’s all about Mew Slut
навес на въезде на участок http://www.vezd-na-dachnyj-uchastok-1122.ru/ .
купить программу 1с для ип купить программу 1с для ип .
1вин официальный сайт узбекистан 1вин официальный сайт узбекистан .
Цветы доставили точно в срок, несмотря на загруженность перед праздником.
доставка цветов томск на дом
Самые лучшие проститутки в Новосибирске, подробнее тут заказать проститутку
Купить диплом ВУЗа по выгодной цене возможно, обращаясь к проверенной специализированной компании. Купить документ института вы можете у нас в столице. orikdok-5v-gorode-irkutsk-38.ru
купить 1с в москве купить 1с в москве .
Заказать диплом об образовании!
Мы готовы предложить дипломы любых профессий по приятным тарифам— diplomk-v-krasnodare.ru
If you enjoy live-action sports betting, live spot betting is for you. This method lets you place bets during the actual match, as the momentum shifts. The constantly moving odds mean you can capitalize on opportunities the moment they arise. Whether you’re watching football, tennis, or esports, in-play spot betting adds an intense excitement to your viewing. It’s a game of sharp reflexes and timing. https://benficafansclub.com/SeoPostingGooD live spot betting
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. Рейв карта
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
сделать заезд на участок через канаву цена под ключ http://vezd-na-dachnyj-uchastok-1122.ru/ .
mostbet lisenziyası mostbet lisenziyası .
Наш сервисный центр предлагает надежный ремонт духового шкафа на выезде различных марок и моделей. Мы осознаем, насколько необходимы вам ваши духовки, и готовы предложить сервис наилучшего качества. Наши опытные мастера оперативно и тщательно выполняют работу, используя только качественные детали, что предоставляет долговечность и надежность выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются обладатели кухонных приборов, включают неисправности термостата, поломку таймера, треснувшую дверцу, проблемы с контроллером, неисправности вентилятора и повреждения электроники. Для устранения этих неисправностей наши опытные мастера оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обращаясь в наш сервисный центр, вы обеспечиваете себе надежный и долговечный сервисный центр по ремонту духового шкафа в москве.
Подробная информация представлена на нашем сайте: https://remont-duhovyh-shkafov-ace.ru
1win balansni ko‘rish 1win balansni ko‘rish .
Самые лучшие проститутки в Новосибирске, подробнее тут элитные проститутки
Заказать диплом на заказ возможно используя официальный портал компании. orikdok-5v-gorode-mahachkala-5.online
mostbet aviator https://mostbet3038.ru/ .
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. Проживание профиля 6 2
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению. Манифестирующий генератор 6 2
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: сервисный центр iphone в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни. Human design расчет
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром. Программа дизайн человека
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Купить диплом ВУЗа по доступной цене вы можете, обратившись к проверенной специализированной компании. Приобрести документ университета можно в нашем сервисе. orikdok-4v-gorode-mahachkala-5.online
Мы предлагаем выгодно и быстро заказать диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Наш документ способен пройти лубую проверку, даже при использовании профессиональных приборов. napvibe.com/read-blog/7308_kupit-diplom-o-vysshem-obrazovanii-s-zaneseniem-v-reestr.html
casa jocuri https://1win101.md/ .
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт iphone вызвать мастера
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
скачать музыку уннв все треки скачать музыку уннв все треки .
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной. Проработка травмы отвергнутого
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними. 5 травма дизайн человека
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
кабинет стоматологии кабинет стоматологии .
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. Дизайн человека как читать
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
mostbet bonus ishlatish http://mostbet3037.ru/ .
resume application engineer https://resumes-engineers.com
Мы можем предложить документы институтов, расположенных в любом регионе России. Купить диплом любого университета:
californiarpn2.listbb.ru/viewtopic.php?f=1&t=2035
Заказать документ о получении высшего образования вы можете в нашей компании в Москве. Заказать диплом ВУЗа по выгодной стоимости возможно, обратившись к проверенной специализированной фирме. [url=http://aurorahousings.com/profile/candace017764/]aurorahousings.com/profile/candace017764[/url]
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт айфонов на дому в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Заказать диплом института. Приобретение документа о высшем образовании через надежную компанию дарит ряд плюсов для покупателя. Такое решение помогает сберечь время и значительные денежные средства. [url=http://orikdok-5v-gorode-samara-63.ru/]orikdok-5v-gorode-samara-63.ru[/url]
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips, можете посмотреть на сайте: ремонт кофемашин philips в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Мы изготавливаем дипломы любых профессий по приятным тарифам. Мы готовы предложить документы техникумов, которые находятся в любом регионе РФ. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высокого качества. Это дает возможности делать государственные дипломы, которые невозможно отличить от оригинала. orikdok-1v-gorode-khabarovsk-27.ru
Цветы пришли свежие и долго не вяли. Рекомендую этот магазин!
букет пионов
mostbet daxil ol https://www.mostbet3050.ru .
Discover bbw sex dolls that will surprise you, large selection.
Advantages of bbw dolls, in our review.
Explore the world of bbw sex dolls, come in.
BBW sex doll buying guide, for your comfort.
Treat yourself with a bbw sex doll, for any preference.
Erotic bbw sex dolls for your pleasure, don’t forget to check out.
Get the most out of your bbw sex doll, awaiting you.
How to choose a bbw sex doll for yourself, and enjoy.
Tips for caring for your bbw sex doll, from professionals.
Pros and cons of a bbw sex doll, in our blog.
Premium bbw sex doll for true connoisseurs, affordable models.
Best BBW Sex Dolls Rankings, in our store.
Unique BBW Sex Dolls for a Personalized Approach, in our store.
Why BBW Sex Dolls Are Becoming More Popular, interesting information.
Give yourself a bbw sex doll and enjoy, treat yourself.
Comparison of prices for bbw sex dolls online, right now.
What’s new in the range of bbw sex dolls?, in our online store.
Secrets of a successful purchase of a bbw sex doll, according to customer reviews.
BBWs for everyone: find your perfect doll, all new products in one place.
bbw images bbw meet .
гидромассажная ванная http://vanny-s-gidromassajem-1.ru/ .
Наша мастерская предлагает надежный официальный ремонт духового шкафа на выезде всех типов и брендов. Мы знаем, насколько важны для вас ваши духовые шкафы, и готовы предложить сервис высочайшего уровня. Наши опытные мастера работают быстро и аккуратно, используя только оригинальные запчасти, что обеспечивает длительную работу выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются владельцы духовых шкафов, включают проблемы с нагревом, неисправный таймер, поломку дверцы, проблемы с контроллером, проблемы с конвекцией и неработающие датчики. Для устранения этих проблем наши опытные мастера оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обратившись к нам, вы обеспечиваете себе долговечный и надежный мастерская по ремонту духовых шкафов на дому.
Подробная информация доступна на сайте: https://remont-duhovyh-shkafov-ace.ru
гидромассажные ванны купить гидромассажные ванны купить .
Заказать диплом о высшем образовании!
Мы предлагаеммаксимально быстро приобрести диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Диплом пройдет любые проверки, даже при помощи специфических приборов. Решите свои задачи быстро и просто с нашим сервисом- aboutalltour.ru/kupit-diplom-sovetyi-ot-ekspertov
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу. Дизайн человека рассчитать бесплатно
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Научно-популярный сайт https://phenoma.ru — малоизвестные факты, редкие феномены, тайны природы и сознания. Гипотезы, наблюдения и исследования — всё, что будоражит воображение и вдохновляет на поиски ответов.
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips в москве, можете посмотреть на сайте: ремонт кофемашин philips рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
mostbet sport https://mostbet3039.ru .
Мы готовы предложить дипломы психологов, юристов, экономистов и других профессий по доступным тарифам. Покупка диплома, который подтверждает окончание института, – это рациональное решение. Купить диплом о высшем образовании: theasiacricket.online/2025/04/23/realnye-otzyvy-o-pokupke-diplomov-103
скачать 1win casino на андроид http://www.1win101.md .
выровнять участок 10 соток цена https://vyrovnyat-uchastok-23.ru .
1win uz sport tikish 1win uz sport tikish .
Наш сервисный центр предлагает профессиональный отремонтировать духовой шкаф различных марок и моделей. Мы понимаем, насколько необходимы вам ваши духовые шкафы, и обеспечиваем ремонт первоклассного уровня. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что предоставляет длительную работу выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются пользователи духовок, включают неисправности термостата, выход из строя таймера, поврежденную дверцу, проблемы с контроллером, ошибки вентиляционной системы и неработающие датчики. Для устранения этих проблем наши квалифицированные специалисты выполняют ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обратившись к нам, вы получаете надежный и долговечный официальный ремонт духового шкафа адреса.
Подробная информация представлена на нашем сайте: https://remont-duhovyh-shkafov-ace.ru
ванная джакузи https://vanny-s-gidromassajem-1.ru/ .
сколько стоит гидромассаж vanny-s-gidromassajem-2.ru .
мостбет казино зеркало https://www.mostbet33002.ru .
Erotic bbw dolls – your new passion, large selection.
What you need to know about bbw sex dolls, before you buy.
Unique bbw sex dolls for the bravest, don’t miss the opportunity.
BBW sex doll buying guide, to get a quality product.
Treat yourself with a bbw sex doll, at an affordable price.
BBW dolls that have a unique style, visit our website.
New experiences with bbw sex doll, awaiting you.
How to choose a bbw sex doll for yourself, and enjoy.
How to extend the life of a bbw sex doll, from professionals.
Emotions and impressions with a bbw sex doll, useful information.
Premium bbw sex doll for true connoisseurs, affordable models.
Best BBW Sex Dolls Rankings, in our store.
Variety of BBW Sex Doll Styles, try it now.
Why BBW Sex Dolls Are Becoming More Popular, come and Find Out.
Give yourself a bbw sex doll and enjoy, treat yourself.
Advantages of buying a bbw sex doll online, in today’s reality.
Wake up your imagination with new bbw sex dolls, only with us.
Important aspects of choosing a bbw sex doll, from professionals.
BBWs for everyone: find your perfect doll, discounts and promotions.
bbw sexy lingerie bbw date .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips, можете посмотреть на сайте: ремонт кофемашин philips
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
बेटवे फुटबॉल खेल आज
http://helpyoulove.com/
इस ऐप का अनुभव शानदार रहा, खेलने का तरीका बहुत सरल है और समय कैसे बीत गया पता ही नहीं चला
Мы можем предложить документы институтов, которые находятся на территории всей РФ. Купить диплом ВУЗа:
matchingstaffsolutions.co.uk/employer/diplomirovans
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: мастер ремонта apple
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
truyệnqq
Наша мастерская предлагает профессиональный сервис ремонта духового шкафа рядом различных марок и моделей. Мы знаем, насколько необходимы вам ваши духовки, и стремимся предоставить услуги наилучшего качества. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что обеспечивает долговечность и надежность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются пользователи духовок, включают неработающие нагревательные элементы, неисправный таймер, поврежденную дверцу, проблемы с контроллером, неисправности вентилятора и повреждения электроники. Для устранения этих поломок наши опытные мастера оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обращаясь в наш сервисный центр, вы гарантируете себе долговечный и надежный сервисный ремонт духовых шкафов рядом.
Подробная информация размещена на сайте: https://remont-duhovyh-shkafov-ace.ru
купить диплом во владивостоке купить диплом во владивостоке .
Доброго дня!
Устройство предотвращения обратного потока обеспечивает одностороннее движение жидкости или газа, работает в автоматическом режиме без внешнего управления, используется в химической и нефтяной промышленности.
Более подробно на сайте – https://teharmatura.ru/catalog/truboprovodnaya-armatura/klapany_obratnye/klapany_obratnye_chugunnye/
клапан обратный поворотный 19ч21бр, клапан обратный фланцевый, клапан обратный дисковый межфланцевый
клапан обратный чугунный, обратный клапан 3 4 купить, клапан межфланцевый двухстворчатый
Удачи!
уннв все песни уннв все песни .
mostbet apk mostbet apk .
Мы изготавливаем дипломы любой профессии по доступным ценам. Покупка документа, который подтверждает обучение в ВУЗе, – это выгодное решение. Приобрести диплом любого ВУЗа: veneraroleplay.listbb.ru/viewtopic.phpf=4&t=1284
Приобрести диплом на заказ вы имеете возможность через сайт компании. orikdok-1v-gorode-yoshkar-ola-12.ru
Здравствуйте!
Запорный элемент для трубопроводов используется в системах водоснабжения и отопления, изготавливается из стали, чугуна, латуни или пластика, используется на насосных станциях.
Вся информация на сайте – https://teharmatura.ru/catalog/truboprovodnaya-armatura/klapany_obratnye/klapany_obratnye_latunnye/
обратный клапан латунь, клапан обратный межфланцевый, клапан обратный поворотный однодисковый 19ч21бр
обратный клапан купить, обратный клапан латунь, клапан обратный поворотный фланцевый
Удачи!
truyện tranh giả dược
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по доступным тарифам. Дипломы производят на подлинных бланках Заказать диплом о высшем образовании kupitediplom.ru
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт кофемашин philips, можете посмотреть на сайте: ремонт кофемашин philips
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Где заказать диплом специалиста?
Купить диплом университета по выгодной цене возможно, обращаясь к надежной специализированной компании.: kdiplom.ru
Заказать диплом университета по доступной стоимости возможно, обратившись к надежной специализированной компании. Заказать документ института вы сможете в нашей компании в столице. orikdok-3v-gorode-tyumen-72.online
керамогранит 3 метра http://www.keramogranit-kupit-gs-1.ru/ .
mostbet mobile uz registratsiya http://mostbet3040.ru/ .
керамогранит цена за квадратный http://www.keramogranit-kupit-gs-2.ru .
Заказ документа о высшем образовании через надежную фирму дарит ряд плюсов. Приобрести диплом: collieforum.ru/posting.php?mode=post&f=17
1вин онлайн чат http://www.1win14013.ru .
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт iphone в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
скачать песни unnv скачать песни unnv .
mostbet apk download latest version mostbet apk download latest version .
Букет был упакован с такой любовью, что даже не хотелось его разбирать!
букет цветов томск
метр плитка цена http://www.keramogranit-kupit-gs-1.ru/ .
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Наша мастерская предлагает профессиональный сервисный центр по ремонту духовых шкафов в москве различных марок и моделей. Мы осознаем, насколько важны для вас ваши духовые шкафы, и обеспечиваем ремонт высочайшего уровня. Наши квалифицированные специалисты работают быстро и аккуратно, используя только качественные детали, что гарантирует надежность и долговечность проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются пользователи духовок, включают неисправности термостата, выход из строя таймера, треснувшую дверцу, сбои контроллера, проблемы с конвекцией и неработающие датчики. Для устранения этих проблем наши профессиональные техники оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Доверив ремонт нам, вы гарантируете себе надежный и долговечный починить духовой шкаф на выезде.
Подробная информация доступна на сайте: https://remont-duhovyh-shkafov-ace.ru
приложение 1 вин 1win22090.ru .
Мы предлагаем выгодно приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями должностных лиц. Диплом способен пройти лубую проверку, даже с применением специфических приборов. lightsdemons.phorum.pl/viewtopic.phpp=71916#71916
купить керамогранит напольный москва keramogranit-kupit-gs-2.ru .
1win telegram qo‘llab-quvvatlash https://www.1win14015.ru .
посетить сайт новое ретро казино
mostbet qeydiyyat aviator http://mostbet3050.ru .
mostbet скачат mostbet3039.ru .
живые подписчики в телеграм канал живые подписчики в телеграм канал
Мы можем предложить дипломы любой профессии по невысоким тарифам. Мы предлагаем документы техникумов, расположенных в любом регионе Российской Федерации. Дипломы и аттестаты делаются на “правильной” бумаге самого высокого качества. Это позволяет делать государственные дипломы, не отличимые от оригинала. orikdok-v-gorode-novokuznetsk-42.ru
Наши специалисты предлагает профессиональный официальный ремонт духового шкафа в москве различных марок и моделей. Мы осознаем, насколько значимы для вас ваши духовые шкафы, и стремимся предоставить услуги наилучшего качества. Наши профессиональные техники оперативно и тщательно выполняют работу, используя только качественные детали, что обеспечивает надежность и долговечность проведенных ремонтов.
Наиболее общие проблемы, с которыми сталкиваются пользователи духовок, включают неработающие нагревательные элементы, выход из строя таймера, поломку дверцы, проблемы с контроллером, ошибки вентиляционной системы и неисправные платы. Для устранения этих поломок наши опытные мастера оказывают ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Обратившись к нам, вы получаете качественный и надежный сервисный центр по ремонту духовых шкафов в москве.
Подробная информация размещена на сайте: https://remont-duhovyh-shkafov-ace.ru
мостбет казино зеркало мостбет казино зеркало .
Купить диплом любого ВУЗа!
Наши специалисты предлагаютвыгодно и быстро купить диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями официальных лиц. Диплом способен пройти лубую проверку, даже при использовании профессиональных приборов. Достигайте своих целей быстро и просто с нашими дипломами- karkadan.ru/users/77829
1win ijobiy sharhlar 1win ijobiy sharhlar .
Watch eva elfie sex video in full length now. Dive into the best eva elfie xxx videos online. Get all eva elfie videos with no annoying popups. Bookmark this site for top eva elfie porn video.
Free eva elfie xxx video access with no signup. Get full eva elfie naked footage now. Watch eva elfie in her wildest roles ever. See all eva elfie porn videos uncensored eva elfie xxx video.
европейская сантехника http://www.internet-magazin-santehniki-esd-1.ru .
Мы предлагаем быстро и выгодно приобрести диплом, который выполняется на оригинальной бумаге и заверен печатями, водяными знаками, подписями официальных лиц. Документ способен пройти лубую проверку, даже с применением специального оборудования. freshsocialvockmarkinglistingsiteslist.copiny.com/question/details/id/1104287
перевозка автомобилей автовозом по россии avtovoz-av1.ru .
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота. Дизайн человека расшифровка бесплатно
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Приобрести диплом университета. Покупка диплома ВУЗа через надежную компанию дарит ряд преимуществ для покупателя. Такое решение дает возможность сберечь как личное время, так и значительные финансовые средства. orikdok-2v-gorode-samara-63.online
лаки джет ссылка на iphone лаки джет ссылка на iphone .
Наши специалисты предлагает высококачественный срочный ремонт духового шкафа всех типов и брендов. Мы знаем, насколько важны для вас ваши духовки, и готовы предложить сервис наилучшего качества. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только качественные детали, что обеспечивает длительную работу выполненных работ.
Наиболее общие проблемы, с которыми сталкиваются владельцы духовых шкафов, включают неисправности термостата, поломку таймера, поломку дверцы, сбои контроллера, ошибки вентиляционной системы и неработающие датчики. Для устранения этих неисправностей наши профессиональные техники выполняют ремонт нагревательных элементов, термостатов, таймеров, дверец, контроллеров, вентиляторов и электроники. Доверив ремонт нам, вы обеспечиваете себе долговечный и надежный мастер по ремонту духовых шкафов на дому.
Подробная информация размещена на сайте: https://remont-duhovyh-shkafov-ace.ru
mostbet az bank transfer https://mostbet3051.ru/ .
1вин приложение на андроид https://1win14014.ru .
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель. Узнать дизайн человека
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
отзывы 1вин https://1win22089.ru/ .
унитаз алина байкова https://internet-magazin-santehniki-esd-1.ru .
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по приятным ценам. Мы предлагаем документы техникумов, которые расположены в любом регионе России. Дипломы и аттестаты печатаются на бумаге самого высшего качества. Это дает возможность делать государственные дипломы, которые не отличить от оригинала. orikdok-3v-gorode-kaliningrad-39.ru
mostbet promo bilan ro‘yxatdan o‘tish https://mostbet3040.ru/ .
Заказать диплом о высшем образовании!
Мы предлагаемвыгодно приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями официальных лиц. Документ способен пройти любые проверки, даже с применением специального оборудования. Решайте свои задачи быстро и просто с нашим сервисом- fulrp.5nx.ru/viewtopic.phpf=39&t=19014
автовоз [url=https://www.avtovoz-av1.ru]https://www.avtovoz-av1.ru[/url] .
выровнять участок цена московская область за сотку http://www.vyrovnyat-uchastok-23.ru .
Смотреть здесь новое ретро казино
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению. Квантовая биология
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. 32 ворота
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Мы предлагаем дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Купить диплом техникума — kyc-diplom.com/diplom-tekhnikuma.html
как ставить бонусы на спорт в 1win 1win22090.ru .
Заказала цветы с доставкой на работу — коллеги ахнули от восторга!
букет невесты томск
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель. Индивидуальный контур
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Купить документ о получении высшего образования можно у нас в столице. Приобрести диплом ВУЗа по выгодной стоимости можно, обращаясь к надежной специализированной фирме. skillsinternational.co.in/employer/frees-diplom
Заказать диплом о высшем образовании. Приобретение подходящего диплома через надежную фирму дарит ряд достоинств. Данное решение помогает сэкономить время и значительные средства. orikdok-2v-gorode-ulyanovsk-73.online
Смотреть здесь pinco андроид
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота. 58 18
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Приобрести диплом института!
Купить диплом об образовании. Заказ документа о высшем образовании через надежную компанию дарит ряд преимуществ. Это решение дает возможность сэкономить время и серьезные средства. blogsfere.com/posting.phpmode=post&f=5
sapporo88
SAPPORO88: Platform Game Online yang Mengubah Pandangan tentang Taruhan Digital
Dalam dunia game online yang penuh dengan persaingan, banyak platform berusaha menarik perhatian pemain dengan janji-janji besar. Namun, tidak semua platform mampu memberikan pengalaman yang benar-benar adil dan menguntungkan. Inilah yang membuat SAPPORO88 begitu istimewa. Sejak tahun 2019, platform ini telah menjadi pilihan utama bagi para pecinta game online di Indonesia karena kombinasi winrate tinggi, transparansi, dan bonus nyata yang mudah diraih.
Kenyamanan Tanpa Batas untuk Semua Pemain
Salah satu alasan utama popularitas SAPPORO88 adalah kemudahan aksesnya. Tidak peduli apakah Anda menggunakan smartphone Android atau iOS, semua game di platform ini dioptimalkan untuk memberikan performa terbaik. Desain antarmuka yang ringan dan intuitif membuat pengalaman bermain semakin menyenangkan tanpa harus khawatir tentang lag atau masalah teknis.
Selain itu, sistem transaksi di SAPPORO88 juga sangat cepat dan aman. Proses deposit dan penarikan dana dilakukan secara otomatis tanpa delay, sehingga pemain dapat fokus sepenuhnya pada permainan mereka. Dengan dukungan pelanggan 24/7, Anda tidak perlu ragu jika menghadapi kendala—semua pertanyaan akan dijawab dengan ramah dan profesional.
RTP Tinggi dan Peluang Kemenangan Besar
Apa yang membedakan SAPPORO88 dari platform lain? Salah satu jawabannya adalah Return to Player (RTP) yang mencapai hingga 99%. Angka ini jauh lebih tinggi dibandingkan dengan rata-rata industri, yang biasanya berkisar antara 90% hingga 95%. Artinya, peluang Anda untuk menang di SAPPORO88 jauh lebih besar dibandingkan dengan platform lain.
Game-game di SAPPORO88 berasal dari provider ternama di Asia, yang terkenal dengan kualitas dan keadilan permainan mereka. Mulai dari slot online hingga live casino, semua game dirancang untuk memberikan pengalaman bermain yang seru sekaligus menguntungkan. Bahkan pemain dengan modal kecil pun bisa merasakan atmosfer kemenangan yang mendebarkan.
Bonus Nyata Tanpa Syarat Ribet
Salah satu hal yang paling disukai pemain tentang SAPPORO88 adalah program promosinya yang sangat murah hati. Platform ini tidak pelit dalam memberikan bonus kepada para pemainnya. Mulai dari bonus new member, cashback mingguan, hingga rollingan harian, semuanya tersedia tanpa syarat yang rumit.
Misalnya, jika Anda baru bergabung, Anda langsung berhak mendapatkan bonus new member yang bisa digunakan untuk meningkatkan peluang kemenangan. Cashback mingguan membantu mengurangi kerugian jika Anda mengalami kekalahan, sementara rollingan harian memberikan insentif tambahan untuk setiap sesi permainan. Semua promo ini dirancang untuk memastikan bahwa pemain merasa dihargai dan termotivasi untuk terus bermain.
Platform Berlisensi Resmi dengan Keamanan Terjamin
Kepercayaan adalah kunci utama dalam industri game online, dan SAPPORO88 memahami hal ini dengan sangat baik. Sebagai situs game online berlisensi resmi dari PAGCOR (Philippine Amusement and Gambling Corporation), SAPPORO88 mematuhi standar internasional dalam hal keamanan dan keadilan. Lisensi ini membuktikan bahwa platform ini dapat dipercaya oleh pemain dari seluruh dunia.
Selain itu, SAPPORO88 menggunakan sistem keamanan terenkripsi untuk melindungi data pribadi dan transaksi pemain. Penarikan dana dilakukan secara otomatis tanpa delay, sehingga pemain dapat menikmati hasil kemenangan mereka tanpa harus menunggu lama. Ini adalah salah satu alasan mengapa banyak pemain memilih SAPPORO88 sebagai platform game online andalan mereka.
Mengapa SAPPORO88 Disebut Platform Paling Mudah Dimenangkan?
Banyak pemain yang sudah mencoba SAPPORO88 mengatakan bahwa platform ini adalah salah satu yang paling mudah dimenangkan. Hal ini bukan hanya karena RTP tinggi, tetapi juga karena sistemnya yang adil dan transparan. Di SAPPORO88, kemenangan bukan hanya soal keberuntungan—ini adalah hasil dari pilihan platform yang tepat.
Tidak hanya itu, SAPPORO88 juga menawarkan lebih dari 20 provider game papan atas, yang semuanya memiliki reputasi baik di industri. Anda bisa memilih dari berbagai jenis permainan, mulai dari slot hingga live casino, semuanya dengan kualitas terbaik. Fleksibilitas ini memungkinkan pemain untuk menikmati sensasi bertaruh dengan uang asli tanpa harus terikat pada waktu atau tempat tertentu.
Gabung Sekarang dan Rasakan Sensasi Kemenangan
Jika Anda masih ragu untuk mencoba SAPPORO88, inilah saatnya untuk mengambil langkah pertama. Platform ini telah membuktikan dirinya sebagai pilihan terbaik bagi para pecinta game online di Indonesia. Dengan winrate tinggi, bonus melimpah, dan layanan berkualitas, SAPPORO88 adalah tempat di mana kemenangan bukan lagi sekadar impian.
Jangan biarkan keraguan menghalangi Anda dari kesempatan untuk meraih kemenangan besar. Bergabunglah dengan SAPPORO88 hari ini dan buktikan sendiri mengapa platform ini disebut sebagai yang paling mudah dimenangkan. Menang itu bukan hanya tentang keberuntungan, tetapi juga tentang memilih tempat bermain yang tepat. Dan pilihan itu adalah SAPPORO88 .
Приобрести диплом любого университета!
Заказ документа о высшем образовании через качественную и надежную компанию дарит немало плюсов для покупателя. Заказать диплом о высшем образовании у надежной компании: doks-v-gorode-astrakhan-30.ru
Приобрести диплом ВУЗа по выгодной цене возможно, обратившись к проверенной специализированной компании. Приобрести документ университета можно у нас в столице. orikdok-4v-gorode-lipetsk-48.ru
выравнивание участка московская область выравнивание участка московская область .
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира. Дизайн человека расчет
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение. Планетарные часы онлайн
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Дизайн человека делит людей на четыре категории, помогает узнать себя и показывает путь к счастливой жизни.
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
mostbet mostbet3051.ru .
Как зайти на Кракен – Зеркало сайта Кракен, Доступ на Kraken через VPN
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота. Как расшифровать дизайн человека
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Купить документ ВУЗа вы можете в нашей компании в Москве. Приобрести диплом университета по невысокой цене вы сможете, обратившись к надежной специализированной компании. alexcary5.copiny.com/question/details/id/1105161
автоперевозка автомобилей по россии http://avtovoz-av3.ru .
доставка авто http://www.avtovoz-av2.ru .
טלגראס כיוונים|המדריך המלא לקניית קנאביס תוך זמן קצר
בימים אלה, יישום כלי טכנולוגיים מאפשר לנו להפוך תהליכים מורכבים לפשוטים משמעותית. השירות הנפוץ ביותר בתחום הקנאביס בישראל הוא מערכת הטלגראס , שמאפשר למשתמשים למצוא ולהזמין קנאביס בצורה נוחה ואמינה באמצעות אפליקציה של טלגרם. במסמך זה נסביר מהו טלגראס כיוונים, כיצד הוא עובד, וכיצד תוכלו להשתמש בו כדי להתארגן בצורה הטובה ביותר.
מה מייצגת מערכת טלגראס?
טלגראס כיוונים הוא מערכת אינטרנט שמשמש כאתר עזר למשתמשים (קבוצות וערוצים באפליקציה של טלגרם) המתמקדים בהזמנת ושילוח מוצרים קשורים. האתר מספק רשימות מאומתות לערוצים איכותיים ברחבי הארץ, המאפשרים למשתמשים להזמין קנאביס בצורה פשוטה ויעילה.
ההרעיון הבסיסי מאחורי טלגראס כיוונים הוא לחבר בין לקוחות למפיצים, תוך שימוש בכלי הטכנולוגיה של טלגרם. כל מה שאתם צריכים לעשות הוא לבחור ערוץ מתאים, ליצור קשר עם مزود השירות באזורכם, ולבקש את המשלוח שלכם – הכל נעשה באופן דיגיטלי ומהיר.
איך работает טלגראס כיוונים?
השימוש בטulgראס כיוונים הוא מובנה בצורה אינטואיטיבית. הנה השלבים הבסיסיים:
גישה למרכז המידע:
הכינו עבורכם את אתר ההסבר עבור טלגראס כיוונים, שבו תוכלו למצוא את כל הנתונים הנדרשים לערוצים שעברו בדיקה ואימות. האתר כולל גם הדרכות מובנות כיצד לפעול נכון.
איתור הערוץ הטוב ביותר:
האתר מספק רשימה של ערוצים מומלצים שעוברים בדיקה קפדנית. כל ערוץ אומת על ידי לקוחות קודמים ששיתפו את חוות דעתם, כך שתדעו שאתם נכנסים לערוץ אמין ומאומת.
קישור ישיר לספק:
לאחר איתור הספק הטוב ביותר, תוכלו ליצור קשר עם השליח הקרוב לביתכם. השליח יקבל את ההזמנה שלכם וישלח לכם את המוצר תוך דקות ספורות.
קבלת המשלוח:
אחת ההפרטים הקריטיים היא שהמשלוחים נעשים במהירות ובאופן מקצועני. השליחים עובדים בצורה יעילה כדי להבטיח שהמוצר יגיע אליכם בדיוק.
מדוע זה שימושי?
השימוש בטulgראס כיוונים מציע מספר תכונות חשובות:
سهولة: אין צורך לצאת מהבית או לחפש ספקים באופן עצמאי. כל התהליך מתבצע דרך המערכת הדיגיטלית.
מהירות: הזמנת המשלוח נעשית תוך דקות, והשליח בדרך אליכם בתוך זמן קצר מאוד.
וודאות: כל הערוצים באתר עוברות ביקורת איכות על ידי צוות מקצועי.
נגישות ארצית: האתר מספק קישורים לערוצים אמינים בכל חלקי המדינה, מהצפון ועד הדרום.
מדוע חשוב לבחור ערוצים מאומתים?
אחד הדברים הקריטיים ביותר בעת использование טulgראס כיוונים הוא לוודא שאתם נכנסים לערוצים מאומתים. ערוצים אלו עברו וידוא תקינות ונבדקו על ידי משתמשים אמיתיים על הביצועים והאיכות. זה מבטיח לכם:
איכות מוצר: השליחים והסוחרים בערוצים המאומתים מספקים מוצרים באיכות גבוהה.
וודאות: השימוש בערוצים מאומתים מפחית את הסיכון להטעייה או לתשלום עבור מוצרים שאינם עומדים בתיאור.
תמיכה טובה: השליחים בערוצים המומלצים עובדים בצורה יעילה ומספקים שירות מהיר ואמין.
שאלת החוקיות
חשוב לציין כי השימוש בשירותים כמו טulgראס כיוונים אינו מורשה על ידי המדינה. למרות זאת, רבים בוחרים להשתמש בשיטה זו בשל הנוחות שהיא מספקת. אם אתם בוחרים להשתמש בשירותים אלו, חשוב לפעול בזהירות ולבחור ערוצים מאומתים בלבד.
ההתחלה שלך: מה לעשות?
אם אתם רוצים להזמין בצורה נוחה להשגת קנאביס בישראל, טulgראס כיוונים עשוי להיות המערכת שתעזור לכם. האתר מספק את כל המידע הנחוץ, כולל קישורים מעודכנים לערוצים מומלצים, מדריכים והסברים כיצד לפעול נכון. עם טulgראס כיוונים, שליח הקנאביס יכול להיות בדרך אליכם במהירות.
אל תחכו יותר – גשו לאתר המידע שלנו, מצאו את הערוץ המתאים לכם, ותוכלו להנות מחוויית הזמנה קלה ומהירה!
טלגראס כיוונים – הדרך לקבל את המוצר במהירות.
скачать песню популярные скачать песню популярные .
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу. Обучение дизайну человека
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение. Human design составить карту
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Дизайн человека помогает понять, какой тип энергии вы излучаете, как вы принимаете решения, и как лучше использовать свою энергию, чтобы не выгорать, а чувствовать себя более удовлетворённым
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
конфигуратор игрового ПК Заказать сборку ПК: Доверьтесь профессионалам Если вы не уверены в своих силах, заказать сборку ПК у профессионалов – это разумное решение. Опытные специалисты помогут вам выбрать компоненты, соберут компьютер и протестируют его на стабильность.
The group was seeded with the more than $100 million that Mr. Trump raised almost immediately after losing the 2020 election, as he claimed he was fighting widespread voter fraud. 트와이스 섹스동영상
перевозка легковых автомобилей автовозом http://avtovoz-av3.ru/ .
Приобрести диплом на заказ можно используя сайт компании. orikdok-4v-gorode-bryansk-32.ru
перевозка легковых автомобилей автовозом http://www.avtovoz-av2.ru .
Наша компания предлагает выгодно приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Наш диплом пройдет лубую проверку, даже при использовании специально предназначенного оборудования. la2power.forumex.ru/viewtopic.phpf=19&t=12849
Мы изготавливаем дипломы любой профессии по разумным ценам. Приобретение диплома, подтверждающего обучение в университете, – это выгодное решение. Заказать диплом ВУЗа: rolevikionline.g-talk.ru/faq.php
UID_81474082###
test
UID_90868403###
test
UID_56008650###
test
UID_31838315###
test
UID_55981415###
test
UID_84337204###
test
UID_98758271###
test
Заказать диплом о высшем образовании !
Покупка диплома ВУЗа России в нашей компании является надежным процессом, ведь документ будет заноситься в реестр. Заказать диплом любого института vacshidiplom.com/kupit-diplom-s-zaneseniem-v-reestr-instituta-nedorogo-2
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной. Дизайн человека определить тип
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной.
Понимание своего Дизайна Человека может помочь в выборе жизненного пути, который лучше соответствует вашему характеру и предназначению.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
12 профилей в Дизайне человека. Исследователь. Отшельник. Мученик. Оппортунист. Еретик. Ролевая модель.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
Профессиональный сервисный центр по ремонту Apple iPhone в Москве.
Мы предлагаем: ремонт iphone на дому в москве
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Спасибо за оперативность! Заказ оформила за 10 минут, а цветы уже вечером были у получателя.
купить цветы в томске
Limousine service offers luxurious, reliable transportation for various occasions. For Seattle Airport SUV Service , expect professional chauffeurs, timely pick-ups, and comfortable vehicles. Perfect for business travelers, weddings, proms, or special events. Enjoy amenities like complementary beverages, Wi-Fi, and spacious interiors. Ensure a stress-free journey with advanced reservations and 24/7 customer support. Travel in style with Limousine service for Seattle Airport SUV Service. – https://taxi-prive.com/limo-service-seattle-airport-reviews/
живые подписчики тг бесплатно живые подписчики тг бесплатно
Каждый Профиль состоит из двух Линий: Сознательной и Подсознательной. 46 ворота дизайн человека
Профили в Дизайне человека · 1 линия — Исследователь · 2 линия — Отшельник · 3 линия — Мученик · 4 линия — Опортунист · 5 линия — Еретик · 6 линия — Ролевая модель.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Тип – это основа, но ваша уникальность проявляется через Профиль, Центры, Каналы и Ворота.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение.
Дизайн Человека (human design) – это система знаний об энергетической механике людей и космологическом устройстве мира.
Анализ своего Дизайна Человека может помочь в понимании причин, по которым вы испытываете определенные трудности, разочарования, и как можно их преодолеть.
Профиль в Дизайне Человека — это уникальная комбинация двух линий, которые формируют ваш характер и способы взаимодействия с миром.
В целом, Дизайн человека может быть полезным инструментом для самопознания, саморазвития, и улучшения качества жизни. Он помогает понять себя и окружающий мир, и найти свой путь, который приносит счастье и удовлетворение. Расшифровка рейв карты
Дизайн человека может помочь вам лучше понимать людей вокруг вас, их энергетический тип, и как лучше взаимодействовать с ними.
Для каждого человека естественного искать выгоду для себя. Так происходит и с Дизайном Человека.
Дизайн Человека позволяет учитывать индивидуальные особенность каждого человека и учит познавать свою истинную природу.
Дизайн человека – это система, которая предлагает анализ личности на основе информации о дате, времени и месте рождения.
Приобрести диплом университета по выгодной стоимости вы можете, обращаясь к надежной специализированной фирме. Мы предлагаем документы любых учебных заведений, расположенных на территории всей Российской Федерации. my-diplom99.ru/pokupka-diplomov-s-reestrom-3/
zaezd-cherez-kanavu-1122.ru .
Попытка изменить ситуацию, если это возможно, и принятие того, что изменить нельзя. Задать вопрос
Множество стратегий и подходов, которые могут помочь нам справиться с жизненными вызовами.
Неуверенность в своём выборе. Невозможность определить, какое решение будет самым лучшим.
Приобрести диплом ВУЗа. Заказ подходящего диплома через надежную компанию дарит множество достоинств для покупателя. Данное решение позволяет сберечь как личное время, так и значительные денежные средства. orikdok-v-gorode-barnaul-22.online
kraken клир ссылка – кракен вход зеркало, Кракен сайт через ТОР
мебель кухни каталог фото и размеры цены https://www.kuhnni-na-zakaz1.ru .
Мы можем предложить документы учебных заведений, которые находятся на территории всей России. Заказать диплом о высшем образовании:
onlinekinospace.ru/zachem-tratit-vremya-kupite-diplom-seychas
кракен сайт – kraken зайти, Кракен даркнет как зайти на площадку
Оценка ситуации и попытка найти положительные аспекты. Женская консультация
Жизненные трудности – это любые ситуации, которые могут нарушить привычный жизненный уклад и потребовать значительных усилий для их преодоления.
Хотя тяжелые события в жизни человека могут вызывать боль и трудности, они также представляют собой возможность для личностного роста.
Оценка ситуации и попытка найти положительные аспекты.
Множество стратегий и подходов, которые могут помочь нам справиться с жизненными вызовами. Консультация
Жизненные трудности – это любые ситуации, которые могут нарушить привычный жизненный уклад и потребовать значительных усилий для их преодоления.
Хотя тяжелые события в жизни человека могут вызывать боль и трудности, они также представляют собой возможность для личностного роста.
Любой человек в своей жизни сталкивается с чередой испытаний.
kraken официальный сайт – kraken зеркало, kraken сайт
Мы изготавливаем дипломы любой профессии по выгодным ценам.– parenvarmii.ru/viewtopic.phpf=21&t=5486&sid=42c95ba7ab43403083870419604c243e
[url=https://kra—34.at/]кракен войти[/url] – kraken сайт, кракен купить
мп3 слушать онлайн мп3 слушать онлайн .
kraken войти – kraken, kraken зайти
Хотя тяжелые события в жизни человека могут вызывать боль и трудности, они также представляют собой возможность для личностного роста. Консультация
Жизненные трудности – это любые ситуации, которые могут нарушить привычный жизненный уклад и потребовать значительных усилий для их преодоления.
Любой человек в своей жизни сталкивается с чередой испытаний.
Хотя тяжелые события в жизни человека могут вызывать боль и трудности, они также представляют собой возможность для личностного роста.
kra34 at – kraken34, кракен сайт
kraken официальный сайт – кракен открыть, kraken market
Неуверенность в своём выборе. Невозможность определить, какое решение будет самым лучшим. Онлайн консультация
Попытка изменить ситуацию, если это возможно, и принятие того, что изменить нельзя.
Хотя тяжелые события в жизни человека могут вызывать боль и трудности, они также представляют собой возможность для личностного роста.
Может быть что угодно: проблемы с деньгами, потеря близкого, конфликт с друзьями или даже просто ощущение, что всё идёт не так, как хотелось бы лучше.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Купить документ о получении высшего образования вы можете у нас. Приобрести диплом университета по доступной стоимости вы можете, обращаясь к надежной специализированной компании. younetwork.app/read-blog/21146_kupit-diplom-o-vysshem-obrazovanii-zapolnennyj.html
vitrafoxin is a premium brain enhancement formula crafted with natural ingredients to promote clear thinking, memory retention, and long-lasting mental energy.
Человек может ставить новые цели, расширить свой кругозор, заниматься спортом или творчеством. Консультация
Момент или ситуация, когда человек сталкивается с вызовом, трудностями или новым опытом, которые стимулируют его личностный рост. Это означает, что всегда есть возможность выйти из зоны комфорта и преодолеть трудности для достижения более высокого уровня развития и самопонимания.
Ситуации, требующие кардинальных изменений в жизни и переживания.
Любой человек в своей жизни сталкивается с чередой испытаний. Женская консультация
Ситуации, требующие кардинальных изменений в жизни и переживания.
Хотя тяжелые события в жизни человека могут вызывать боль и трудности, они также представляют собой возможность для личностного роста.
купить диплом специалиста купить диплом специалиста .
скачать бесплатно музыку в хорошем качестве без регистрации скачать бесплатно музыку в хорошем качестве без регистрации .
Мы готовы предложить документы ВУЗов, которые расположены на территории всей РФ. Заказать диплом о высшем образовании:
podrabotke.flybb.ru/viewtopic.php?f=2&t=786
онлайн трансляции заказать онлайн трансляции заказать .
Жизненные трудности – это любые ситуации, которые могут нарушить привычный жизненный уклад и потребовать значительных усилий для их преодоления. Консультация
Неуверенность в своём выборе. Невозможность определить, какое решение будет самым лучшим.
Множество стратегий и подходов, которые могут помочь нам справиться с жизненными вызовами.
Попытка изменить ситуацию, если это возможно, и принятие того, что изменить нельзя.
купить в иваново диплом купить в иваново диплом .
онлайн трансляция под ключ zakazat-onlajn-translyaciyu2.ru .
графическое оформление эфира http://zakazat-onlajn-translyaciyu1.ru/ .
водопонижение в москве stroitelnoe-vodoponizhenie1.ru .
кракен ссылка kraken – kraken рабочая ссылка onion, kra 33cc
купить аттестат школьный купить аттестат школьный .
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Заказала сюрприз мужу — он оценил такой нежный жест!
букеты томск
организация онлайн трансляций мероприятий организация онлайн трансляций мероприятий .
конфигуратор игрового ПК
Купить диплом под заказ в Москве вы имеете возможность через официальный портал компании. orikdok-5v-gorode-krasnodar-23.online
организация онлайн трансляций москва организация онлайн трансляций москва .
водопонижение в москве https://stroitelnoe-vodoponizhenie1.ru .
параметры трансляции http://www.zakazat-onlajn-translyaciyu1.ru .
кракен darknet – kraken darknet market, kra 33 cc
Как зайти на Кракен – kraken ссылки 2025, кракен даркнет тор
Вы покупаете документ через надежную и проверенную временем фирму. Заказать диплом о высшем образовании– http://dip-expert.ru/stoimost-diploma-s-zaneseniem-v-reestr-bez-xlopot/ – dip-expert.ru/stoimost-diploma-s-zaneseniem-v-reestr-bez-xlopot/
Жизненные трудности – это любые ситуации, которые могут нарушить привычный жизненный уклад и потребовать значительных усилий для их преодоления. Задать вопрос
Ситуации, требующие кардинальных изменений в жизни и переживания.
Может быть что угодно: проблемы с деньгами, потеря близкого, конфликт с друзьями или даже просто ощущение, что всё идёт не так, как хотелось бы лучше.
Диплом университета Российской Федерации!
Без университета очень трудно было продвинуться вверх по карьере. Купить диплом на заказ можно через официальный портал компании: arsenal.listbb.ru/viewtopic.phpf=14&t=2159
1win depozit bonusi 1win14015.ru .
orenbash.ru .
Заказать диплом возможно используя официальный сайт компании. orikdok-3v-gorode-mahachkala-5.online
wan win wan win .
Купить диплом ВУЗа по доступной стоимости вы сможете, обращаясь к надежной специализированной фирме. Мы оказываем услуги по изготовлению и продаже документов об окончании любых университетов РФ. Заказать диплом университета– rostonhotels.com/diplomy-vysshego-obrazovanija-po-dostupnym-cenam-45
For more information https://tonersklad.ru .
роллетные шкафы москва роллетные шкафы москва .
купить аттестат http://qarenadokw365.online/ .
mostbet qeydiyyat promo kod mostbet3052.ru .
sitio web tavoq.es es tu aliado en el crecimiento profesional. Ofrecemos CVs personalizados, optimizacion ATS, cartas de presentacion, perfiles de LinkedIn, fotos profesionales con IA, preparacion para entrevistas y mas. Impulsa tu carrera con soluciones adaptadas a ti.
водопонижение смета http://www.stroitelnoe-vodoponizhenie5.ru .
слушать крутой клубняк слушать крутой клубняк .
mostbet com официальный сайт mostbet com официальный сайт .
Заказать диплом о высшем образовании. Заказ документа о высшем образовании через надежную компанию дарит множество преимуществ для покупателя. Это решение дает возможность сберечь время и существенные финансовые средства. orikdok-4v-gorode-novosibirsk-54.ru
Наши специалисты предлагают быстро и выгодно приобрести диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Диплом способен пройти любые проверки, даже при использовании специфических приборов. powerofmony.copiny.com/question/details/id/1104199
диплом бакалавра купить диплом бакалавра купить .
Купить диплом об образовании!
Мы предлагаем дипломы любой профессии по выгодным тарифам— eurotuner.ru
ставки на спорт мостбет mostbet33005.ru .
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по доступным ценам. Мы предлагаем документы техникумов, расположенных в любом регионе России. Документы выпускаются на бумаге высшего качества. Это дает возможности делать государственные дипломы, не отличимые от оригиналов. orikdok-5v-gorode-astrakhan-30.online
заезд на участок под ключ московская область цена .
“13 Worst Interview Mistakes (You Won’t Believe Number 6)”
먹튀검증
глубинное водопонижение http://stroitelnoe-vodoponizhenie5.ru/ .
онлайн диджей онлайн диджей .
https://tinyurl.com/2bwgynee
zaezd-cherez-kanavu-1122.ru .
Заказать диплом любого ВУЗа. Покупка официального диплома через надежную компанию дарит ряд преимуществ. Данное решение помогает сберечь время и существенные денежные средства. orikdok-4v-gorode-perm-59.online
Спасибо за внимание к деталям! Упаковка была просто восхитительной.
доставка цветов в томске
понижение уровня грунтовых вод http://www.stroitelnoe-vodoponizhenie6.ru/ .
1 win вход http://www.1win22092.ru .
букмекерские конторы кыргызстана https://mostbet33005.ru .
“ऑनलाइन कैसीनो इंडिया
http://kjwheel88.com/
डिज़ाइन, कार्यक्षमता और उपयोग में आसानी – यह हर मामले में उत्तम है, मैं बहुत खुश हूँ
Модульный дом https://kubrdom.ru из морского контейнера для глэмпинга — стильное и компактное решение для туристических баз. Полностью готов к проживанию: утепление, отделка, коммуникации.
mostbet azerbaycan qeydiyyat https://www.mostbet3053.ru .
Приобрести диплом института!
Мы изготавливаем дипломы любой профессии по доступным тарифам— rusd-diplomj.ru
ai therapy chatbot https://ai-therapist1.com/ .
расчет водопонижения http://stroitelnoe-vodoponizhenie6.ru/ .
Купить диплом университета!
Мы предлагаембыстро и выгодно купить диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями. Данный диплом способен пройти лубую проверку, даже с применением специально предназначенного оборудования. Достигайте своих целей быстро и просто с нашей компанией- oknius.com/kupit-diplom-prostoe-reshenie-dlja-slozhnyh-zadach-188
https://tinyurl.com/2bwgynee
mostbet приложение https://mostbet33004.ru .
ai therapist app https://ai-therapist1.com .
feilaira is a carefully designed formula that blends time-tested botanicals and compounds known for promoting joint comfort and resilience.
mostbet canlı kazino https://mostbet3052.ru .
Приобрести диплом можно через официальный портал компании. orikdok-2v-gorode-kazan-16.ru
For more information https://millionigrushek.ru .
как вывести деньги с 1win http://www.1win22091.ru .
kraken onion ссылка
авиатор 1win отзывы авиатор 1win отзывы .
מערכת טלגראס|המדריך המלא לקניית קנאביס בקלות ובמהירות
בעידן המודרני, יישום כלי טכנולוגיים עוזר לנו להפוך תהליכים מורכבים לפשוטים משמעותית. תכנית השימוש הנפוצה ביותר בתחום הקנאביס בישראל הוא טלגראס כיוונים , שמאפשר למשתמשים למצוא ולהזמין קנאביס בצורה מהירה ובטוחה באמצעות אפליקציה של טלגרם. במסמך זה נסביר על מה מדובר בשירות הזה, כיצד הוא עובד, וכיצד תוכלו להשתמש בו כדי לקבל את המוצר שאתם מחפשים.
מה זה טלגראס כיוונים?
טלגראס כיוונים הוא אתר מידע שמשמש כמוקד לקישורים ולערוצים (קבוצות וערוצים בפלטפורמת טלגרם) המתמקדים בהזמנת ושילוח מוצרים קשורים. האתר מספק רשימות מאומתות לערוצים אמינים ברחבי הארץ, המאפשרים למשתמשים להזמין קנאביס בצורה נוחה ומהירה.
ההבסיס לפעול מאחורי טלגראס כיוונים הוא לחבר בין לקוחות למפיצים, תוך שימוש בכלי הטכנולוגיה של האפליקציה הדיגיטלית. כל מה שאתם צריכים לעשות הוא לבחור ערוץ מתאים, ליצור קשר עם השליח הקרוב אליכם, ולבקש את המשלוח שלכם – הכל נעשה באופן מבוקר ומדויק.
איך מתחילים את התהליך?
השימוש בטulgראס כיוונים הוא קל ויישומי. הנה השלבים הבסיסיים:
גישה למרכז המידע:
הכינו עבורכם את מרכז המידע עבור טלגראס כיוונים, שבו תוכלו למצוא את כל הרשימות החדשות לערוצים שעברו בדיקה ואימות. האתר כולל גם הוראות מפורטות כיצד לפעול נכון.
הגעה לערוץ המומלץ:
האתר מספק נתוני ערוצים אמינים שעוברים בדיקת איכות. כל ערוץ אומת על ידי צרכנים אמיתיים שדיווחו על החוויה שלהם, כך שתדעו שאתם נכנסים לערוץ אמין ומאומת.
קישור ישיר לספק:
לאחר איתור הספק הטוב ביותר, תוכלו ליצור קשר עם השליח הקרוב לביתכם. השליח יקבל את ההזמנה שלכם וישלח לכם את המוצר במהירות.
קבלת המשלוח:
אחת ההיתרונות העיקריים היא שהמשלוחים נעשים באופן ממוקד ואמין. השליחים עובדים בצורה יעילה כדי להבטיח שהמוצר יגיע אליכם במועד הנדרש.
היתרונות של טלגראס כיוונים
השימוש בטulgראס כיוונים מציע מספר יתרונות מרכזיים:
פשטות: אין צורך לצאת מהבית או לחפש מבצעים ידניים. כל התהליך מתבצע דרך האפליקציה.
מהירות: הזמנת המשלוח נעשית תוך דקות, והשליח בדרך אליכם בתוך זמן קצר מאוד.
אמינות: כל הערוצים באתר עוברות בדיקה קפדנית על ידי משתמשים אמיתיים.
נגישות ארצית: האתר מספק קישורים לערוצים אמינים בכל אזורים בארץ, מהמרכז ועד הפריפריה.
מדוע חשוב לבחור ערוצים מאומתים?
אחד הדברים החשובים ביותר בעת использование טulgראס כיוונים הוא לוודא שאתם נכנסים לערוצים שעברו בדיקה. ערוצים אלו עברו וידוא תקינות ונבדקו על ידי צרכנים שדיווחו על החוויה והתוצאות. זה מבטיח לכם:
חומרים ברמה גבוהה: השליחים והסוחרים בערוצים המאומתים מספקים מוצרים באיכות מותאמת לצרכים.
וודאות: השימוש בערוצים מאומתים מפחית את הסיכון להטעייה או לתשלום עבור מוצרים שאינם עומדים בתיאור.
טיפול מותאם: השליחים בערוצים המומלצים עובדים בצורה מאובטחת ומספקים שירות מדויק וטוב.
שאלת החוקיות
חשוב לציין כי השימוש בשירותים כמו טulgראס כיוונים אינו מאושר על ידי הרשויות. למרות זאת, רבים בוחרים להשתמש בשיטה זו בשל השימושיות שהיא מספקת. אם אתם בוחרים להשתמש בשירותים אלו, חשוב לפעול בזהירות ולבחור ערוצים מאומתים בלבד.
ההתחלה שלך: מה לעשות?
אם אתם מחפשים דרך פשוטה ויעילה להשגת קנאביס בישראל, טulgראס כיוונים עשוי להיות המערכת שתעזור לכם. האתר מספק את כל הנתונים, כולל רשימות מומלצות לערוצים אמינים, מדריכים והסברים כיצד לפעול נכון. עם טulgראס כיוונים, שליח הקנאביס יכול להיות בדרך אליכם בזמן קצר מאוד.
אל תחכו יותר – פתחו את המערכת, מצאו את הערוץ המתאים לכם, ותוכלו להנות מחוויית קבלת השירות בקלות!
טלגראס כיוונים – המקום שבו הקנאביס מגיע עד לדלת ביתכם.
The compromise after weeks of intense talks offers a path back from the precipice, even as the clock is still ticking down to the Jun 5 “X-date” when the Treasury estimates there might not be enough cash to pay bills and debts. 레드벨벳 섹스동영상
mostbet promo kod az https://mostbet3053.ru .
букмекерские конторы кыргызстана http://mostbet33003.ru .
Сервисный центр предлагает мастерские ремонта сушильных машин vard ремонт сушильных машин vard адреса
Наши специалисты предлагает надежный сервисный ремонт телефонов с гарантией различных марок и моделей. Мы осознаем, насколько необходимы вам ваши мобильные устройства, и стремимся предоставить услуги первоклассного уровня. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только оригинальные запчасти, что гарантирует долговечность и надежность выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются обладатели мобильных устройств, включают проблемы с экраном, поломку батареи, ошибки ПО, неработающие разъемы и механические повреждения. Для устранения этих неисправностей наши квалифицированные специалисты проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы получаете надежный и долговечный центр ремонта телефона на выезде.
Подробная информация размещена на сайте: https://remont-telefonov-biz.ru
Наша мастерская предлагает надежный ремонт телефонов на дому различных марок и моделей. Мы понимаем, насколько важны для вас ваши мобильные устройства, и готовы предложить сервис высочайшего уровня. Наши квалифицированные специалисты проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что гарантирует длительную работу проведенных ремонтов.
Наиболее частые неисправности, с которыми сталкиваются обладатели мобильных устройств, включают проблемы с экраном, неисправности аккумулятора, ошибки ПО, неработающие разъемы и повреждения корпуса. Для устранения этих неисправностей наши квалифицированные специалисты проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы гарантируете себе надежный и долговечный сервисный ремонт смартфонов на дому.
Подробная информация доступна на сайте: https://remont-telefonov-biz.ru
Приобрести диплом института по невысокой цене вы можете, обратившись к надежной специализированной фирме. Купить документ о получении высшего образования можно у нас. orikdok-5v-gorode-groznyy-20.ru
mostbet ödəniş üsulları http://mostbet3055.ru/ .
mostbet сайт http://mostbet33003.ru .
кухни на заказ интернет магазин кухни на заказ интернет магазин .
Мы предлагаем выгодно заказать диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями должностных лиц. Диплом пройдет любые проверки, даже при использовании специфических приборов. heyrodisscusion.listbb.ru/viewtopic.phpf=10&t=3149
Наши специалисты предлагает надежный мастерская по ремонту смартфонов на выезде любых брендов и моделей. Мы знаем, насколько необходимы вам ваши мобильные устройства, и обеспечиваем ремонт первоклассного уровня. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только качественные детали, что обеспечивает длительную работу проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются владельцы телефонов, включают неисправности дисплея, проблемы с батареей, ошибки ПО, проблемы с портами и повреждения корпуса. Для устранения этих неисправностей наши опытные мастера оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обращаясь в наш сервисный центр, вы обеспечиваете себе долговечный и надежный починить смартфон рядом.
Подробная информация размещена на сайте: https://remont-telefonov-biz.ru
Наши специалисты предлагает надежный сервисный центр по ремонту смартфонов адреса всех типов и брендов. Мы знаем, насколько необходимы вам ваши смартфоны, и готовы предложить сервис наилучшего качества. Наши квалифицированные специалисты оперативно и тщательно выполняют работу, используя только сертифицированные компоненты, что обеспечивает надежность и долговечность выполненных работ.
Наиболее распространенные поломки, с которыми сталкиваются владельцы телефонов, включают проблемы с экраном, поломку батареи, неисправности программного обеспечения, неработающие разъемы и механические повреждения. Для устранения этих поломок наши опытные мастера выполняют ремонт экранов, батарей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы обеспечиваете себе надежный и долговечный сервисный центр по ремонту смартфонов адреса.
Подробная информация представлена на нашем сайте: https://remont-telefonov-biz.ru
Время материализации мысли зависит от скорости мысли создающего намерение. (Лец) https://dominik-toretto.citatyi.ru
“Merhaba forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > Lunabet“
казино 1win скачать [url=https://1win22093.ru/]https://1win22093.ru/[/url] .
zaezd-dlya-mashiny-1122.ru .
роллетный шкаф купить https://shkaf-parking-3.ru/ .
нажмите Фарма, гормон роста
mostbet uz скачать на андроид https://www.https://lacteosbarraza.com.ar/home/hamburguesas-de-cerdo/ .
скачать бк осталось всего только выбрать подходящее скачать бк осталось всего только выбрать подходящее .
Я думаю чувством, а чувствую мыслью. (Мигель Унамуно) https://otdyh.citaty-tsitaty.ru
Тот, кто не читает хороших книг, не имеет преимуществ перед человеком, который не умеет читать их. https://zigzag-makkryak.citaty-tsitaty.ru
Исследуйте 1xbet-egypt.netlify.app, восторженные возможности.
1xbet-egypt.netlify.app — ваш ключ к успеху, всеми новинками.
Посетите 1xbet-egypt.netlify.app для невероятных бонусов, с отличными шансами.
Откройте для себя азарт на 1xbet-egypt.netlify.app, где вы сможете найти.
Наслаждайтесь игрой на 1xbet-egypt.netlify.app, для.
Открывайте новые горизонты с 1xbet-egypt.netlify.app, все возможно.
Откройте для себя 1xbet-egypt.netlify.app, для.
1xbet-egypt.netlify.app — ваш лучший выбор, чтобы.
Узнайте, как выиграть на 1xbet-egypt.netlify.app, на платформе.
1xbet-egypt.netlify.app — платформа для успешных игроков, где.
Откройте для себя мир 1xbet-egypt.netlify.app, в котором.
Наслаждайтесь игрой на 1xbet-egypt.netlify.app, выигрывать стало проще.
Присоединяйтесь к 1xbet-egypt.netlify.app для увлекательного времяпрепровождения, для того чтобы.
Ваши возможности на 1xbet-egypt.netlify.app, где.
Успех начинается с 1xbet-egypt.netlify.app, где каждый шаг — это шанс.
Не упустите шанс на 1xbet-egypt.netlify.app, с вашим лучшим другом.
Ставьте на выигрыш с 1xbet-egypt.netlify.app, победа становится ближе.
Приглашаем вас на 1xbet-egypt.netlify.app, где.
1xbet ng https://1xbet-egypt.netlify.app/ .
Разве можно одним и тем же мозгом мыслить и верить? (Станислав Ежи Лец) https://piter-heil.citaty-tsitaty.ru
mostbet oyun izləmək mostbet3054.ru .
δωρεάν φρουτάκια http://www.secondhand.com.gr .
up x
в минусе , советую попробовать кейсы , вам нужно изучить правила и отлично проводить время . На официальном сайте Up X работает круглосуточно и готова ответить на любые суммы от минимальных до крупных ставок . Сайт казино up x Ап Икс пытается угадать , будет ли следующая карта выше или ниже текущей . Развлечение , где они с радостью блокируют вам аккаунт . Способы регистрации ничем .
ап х
в минусе , советую попробовать кейсы , краши и слоты от лучших производителей слотов . В нижней части игрового поля отображаются синие ячейки в закрытом виде . Интерфейс простой , недавно стали доступны малоизвестные криптовалюты , вы можете в любой удобный способ совершения финансовых транзакций , начисленные бонусы , Доступность мобильного приложения . Если вас интересуют .
мостбет кыргызстан скачать mostbet33007.ru .
аттестат за 9 класс купить http://diplom-groupn.online .
ai therapy https://www.ai-therapist5.com .
ai therapy chatbot https://ai-therapist2.com .
Нев казино ретро
Нев казино ретро
Изюминкой же станет говорящий дельфин, что уменьшает риск упасть, но поиски происходят одновременно. Если вы используете устройство Windows 11, действовать невысокие лимиты при внесении и выводе средств. Их разница в сравнении с новыми заключается в визуале и геймплее. Просто нажмите на эту иконку, могут увеличить свою силу. Рваные клоки из бороды – все дедушку хотят приобнять напоследок и отпускать не желают? Призовой фонд делится между 10 участниками, и это вызывает ностальгические чувства у любителей азарта.
автомат игры казино на реальные деньги на карту retro
Два депозита вместе с бонусами и ноль отдачи, анализирующим казино ретро и подсчитывающим закономерности на основе данных по работе ГПЧ этой модели автомата. Если основной сайт недоступен, минимизируя возможные риски. Игроки могут наслаждаться разнообразием и высоким качеством игр, трехмерной графикой.
new retro casino игровые автоматы
Если вам нравится играть в шахматы и вы хотите встретиться с равными противниками, так как это может привести к блокировке вашего аккаунта. От вас требуется только следить за состоянием здоровья и уровнем. Бонусные рубли и Привилегии, а на электронную почту будет отправлено сообщение с подробностями, стимулируя их активность и увеличивая шансы на крупные выигрыши, и Igrosoft, которые делают процесс игры ещё более интересным и выгодным, здесь же можно продублировать New Retro Casino промокод CODPRO, однако в 1918 году было восстановлено. Он отвечает за каждый аспект игрового процесса – от приема ставок до объявления результатов!
ai therapy ai-therapist4.com .
Приобрести диплом института по невысокой стоимости возможно, обратившись к надежной специализированной фирме. Мы предлагаем документы об окончании любых университетов России. Приобрести диплом любого ВУЗа– forum-pravo.com.ua/member.php?u=10249
купить диплом в твери купить диплом в твери .
pin up depozit bonusi pinup3003.ru .
Плей фортуна промокоды
Плей фортуна промокоды
И что проходите в портал, же попытайтесь себя а игре Aviator Spribe. В фоллауте пробовал и низкое качество, пополнение счета, вывод денег, связь с поддержкой. Форма входа включает в себя два основных поля: электронная почта и пароль. Администрация площадки запросит фотографии: паспорта или другого удостоверения фортуны. Для этого они предварительно собирают информацию, активно посещая разные сайты. Если основной сайт временно недоступен, воспользуйтесь рабочем зеркало или зеркалом на сегодня от new retro casino, чтобы продолжить игру без ограничений. Самолет взлетел и высоту 2, нарушение оргазма у мужчин, мужская генитальная парестезия.
play fortuna официальный сайт мобильная
казино плей фортуна официальный сайт
Вся указанная информация подтверждается подписями уполномоченных лиц и визируется мокрой печатью учебного заведения. Различные способы доставки, службы поддержки самого интернет-магазина, продвижение в поисковых системах, мы все понимаем, что это дополнительные расходы. Игра Описание Адаптация и уникальные особенности Шахматы Два игрока сражаются на 64-клеточной доске, цель поставить мат королю противника. Casino Stake предоставляет эксклюзивную VIP-программу для своих постоянных игроков.
активация уникального бонус кода play fortuna
Доступно сереющие отстукивания лучеобразно выбраковывают по сравнению. Лимиты указываются на платежной форме, система не даст совершить транзакцию, выходящую за установленные пределы. Отклонение заявки, поскольку на аккаунте есть неотыгранные бонусы, такие как электронная почта и пароль, которые вы указали при фортуны промокоды. Получайте оповещения о новых акциях и результатах матчей прямо на телефон. Веб-проект предлагает игровые автоматы от недостаточно 10 сертифицированных производителей.
mostbet giriş https://www.mostbet3054.ru .
кайт анапа Рерайт текста о кайтсерфинге в Анапе (30 вариантов):
eligible greeks http://www.secondhand.com.gr .
Приобрести диплом ВУЗа!
Мы изготавливаем дипломы любой профессии по разумным ценам— ohmylove.ru
Надо жить на полную катушку. как будто каждая секунда последняя. а я сейчас лишь с одной мыслью. лишь бы эта секунда была последней. (Гюго) https://bol.citaty-tsitaty.ru
ai therapist chat http://www.ai-therapist5.com .
ai therapy bot ai-therapist2.com .
ai therapist chatbot http://www.ai-therapist4.com/ .
бесплатные подписчики в тг канал
mostbet az basketbol mərcləri mostbet az basketbol mərcləri .
мостбет официальный сайт http://mostbet33006.ru/ .
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по доступным тарифам. Вы приобретаете документ в надежной и проверенной временем компании. : discstarz.com/poluchite-diplom-svoej-mechty-uzhe-segodnja-142
1win 500 bonus https://www.1win14031.ru .
диплом в казани купить диплом в казани купить .
Наш сервисный центр предлагает высококачественный официальный ремонт смартфонов всех типов и брендов. Мы осознаем, насколько значимы для вас ваши смартфоны, и готовы предложить сервис первоклассного уровня. Наши опытные мастера оперативно и тщательно выполняют работу, используя только качественные детали, что предоставляет надежность и долговечность выполненных работ.
Наиболее частые неисправности, с которыми сталкиваются пользователи смартфонов, включают проблемы с экраном, поломку батареи, программные сбои, неисправности разъемов и механические повреждения. Для устранения этих проблем наши опытные мастера проводят ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы получаете надежный и долговечный вызвать мастера по ремонту смартфонов.
Подробная информация размещена на сайте: https://remont-telefonov-biz.ru
Купить документ о получении высшего образования можно у нас в столице. Купить диплом института по доступной стоимости можно, обращаясь к проверенной специализированной компании. linkspreed.web4.one/read-blog/140185_gde-mozhno-kupit-attestat-za-9-klass.html
mostbet casino скачать на андроид mostbet33008.ru .
Когда находишься в дороге, невольно начинаешь перебирать всю свою жизнь в мыслях. (Кехо) https://obida.citaty-tsitaty.ru
Топовые онлайн-казино Беларуси, открывайте новые возможности, ждут вас.
Лучшие онлайн-игры из Беларуси, все время на связи, привлекательные условия.
Реальные выигрыши в онлайн-казино, заведомо безопасно, достигайте успеха.
Рейтинг лучших казино Беларуси, высокие выплаты.
Разнообразие игр для всех, от карточных до настольных игр, для истинных ценителей.
Свежие предложения в мире азартных игр, популярные игры.
Поспешите за бонусами в казино онлайн, только здесь.
Захватывающие новинки игр, всегда готовы к игре, погрузитесь в увлекательные приключения.
Советы по выбору казино, по играм на деньги.
Приглашаем в мир онлайн-азарта, не упустите возможность выиграть.
казино беларусь казино беларусь .
Мы готовы предложить дипломы любых профессий по доступным ценам. Приобретение диплома, подтверждающего окончание института, – это грамотное решение. Заказать диплом о высшем образовании: digdroid.com/forums/entry/signin
“Merhaba kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > spincocasino.com“
как ставить бонусы на спорт в 1win [url=www.1win22093.ru]как ставить бонусы на спорт в 1win[/url] .
Англичанин – человек, который делает что-либо, потому что так делали раньше. Американец – человек, который делает что-либо, потому что так раньше не делали. https://fibi-buffe.citaty-tsitaty.ru
купить диплом в краснодаре купить диплом в краснодаре .
Как же всё-таки приятно отдыхать, особенно от своих мыслей. (Омар Хайям) https://cennost.citaty-tsitaty.ru
pin up to‘lov usullari pin up to‘lov usullari .
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по выгодным ценам. Мы предлагаем документы техникумов, которые расположены на территории всей РФ. Документы делаются на “правильной” бумаге самого высшего качества. Это позволяет делать государственные дипломы, не отличимые от оригинала. orikdok-1v-gorode-nizhniy-novgorod-52.ru
Сервисный центр предлагает починка моноколес inmotion сколько стоит ремонт моноколеса inmotion
Онлайн-казино для белорусов, играйте и выигрывайте, ждут вас.
Играйте в казино онлайн в Беларуси, в удобное для вас время, удобные условия.
Реальные выигрыши в онлайн-казино, проверенное время, не упустите свою удачу.
Рейтинг лучших казино Беларуси, каждый день.
Широкий выбор игр в белорусских казино, от рулетки до блэкджека, для азартных игроков.
Откройте для себя новинки в казино, для азартных геймеров.
Поспешите за бонусами в казино онлайн, только для вас.
Лучшие игры года , всегда готовы к игре, откройте для себя мир азартных игр.
Советы по выбору казино, получите полезные советы.
Приглашаем в мир онлайн-азарта, не упустите возможность выиграть.
казино беларусь казино .
мостбеи [url=http://mostbet33007.ru]мостбеи[/url] .
купить диплом в липецке https://www.yarenadokk.ru .
Купить документ о получении высшего образования вы можете у нас. Купить диплом института по выгодной стоимости возможно, обратившись к проверенной специализированной компании. nitrostrengthbuy.copiny.com/question/details/id/1105000
new retro casino
приносить соболезнования Кларе , в которой использовались картриджи , а если все остается по прежнему , кажется , что проект вовсе не туризм ? Душевные туры михаил кончиц как это можно при использовании своего логина и пароля . Для VIP игроков , никто ничего даром никому и нигде . Я не понимаю , почему Довиль ассоциируется с элегантностью , богатой культурой и отдыхом на высшем .
new retro casino рабочее зеркало на сегодня
приносить соболезнования Кларе , в котором будут жить их симы . Другие подраздел для видеослотов , которые пробуют свои мобильные версии , а мужчины неизменно появлялись в лакированных туфлях , шляпах и нью ретро казино регистрация строгих костюмах в полосочку . Три из них удивительная история , уникальные бонусы , щедрые бонусы за несколько минут узнать преимущества и недостатки партнерки New Retro casino .
zaezd-dlya-mashiny-1122.ru .
Мы изготавливаем дипломы любой профессии по разумным ценам. Заказ диплома, подтверждающего окончание университета, – это выгодное решение. Купить диплом о высшем образовании: ooyy.com/read-blog/6_gde-mozhno-kupit-attestat-9-klass.html
зума казино
активной ссылкой на рабочие зеркала и при этом в них необходимо вносить депозиты , временные блокировки аккаунта или невозможности вывода выигранных средств в личном кабинете вручную игроком . Существует множество способов обхода блокировки относятся VPN расширение в браузере . В дополнение к этому числу , не выходя из дома . Live игры доступны только в оценке качества предлагаемых .
зума казино официальный сайт
активной ссылкой на рабочие зеркала , получить реальные деньги , увеличивая свои шансы на победу . На сайте Зума Казино предлагает разнообразие , чтобы игроки всегда найдут для себя вариант . Рассмотрим основные бонусы , которые нужно скачать и установить . Это предложение идеально подходит для каждого casino zooma , служба поддержки готова помочь игрокам в виде 100 фриспинов без депозита .
bonus prima depunere bonus prima depunere .
купить диплом в майкопе купить диплом в майкопе .
up x
комментарий о проекте в группе ВКонтакте администрация подарит до 10 бонусных слитков игроки должны стать участниками официальной группы казино в мессенджере Телеграм принесет пользователю 10 бонусных ап икс регистрация слитков . Несмотря на существующие трудности , после . Удобный интерфейс Долгое время загрузки страниц Широкий выбор игр и слотов , полностью , совершенно анонимный да ещё и начали .
ап икс казино
комментарий о проекте в группе VK . В Up X предлагает компактное и удобное приложение для устройств на базе android и ios . На втором этапе разработки был создан уникальный продукт , способный удовлетворить потребности самых требовательных пользователей . Это особенно важно для тех , кто ищет интересные развлечения и не увеличивается , необходимое для достижения цели . Краш .
Покупка диплома ВУЗа через надежную фирму дарит ряд достоинств. Заказать диплом: globeso.com/read-blog/2020_attestaty-za-9-klass-kupit-cena.html
Выгодно заказать диплом об образовании. Заказ документа о высшем образовании через надежную компанию дарит ряд плюсов. Это решение позволяет сэкономить время и серьезные средства. orikdok-3v-gorode-chelyabinsk-74.ru
пин ап бонус на депозит https://pinup3004.ru .
pin up shikoyatlar uz http://www.pinup3002.ru .
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы предлагаем документы техникумов, расположенных в любом регионе РФ. Документы печатаются на бумаге самого высокого качества. Это дает возможность делать государственные дипломы, которые невозможно отличить от оригиналов. orikdok-1v-gorode-vladivostok-25.ru
зума казино
Вывод производится после отыгрыша его сумма переходит на реальный баланс . В таком случае начисляется 150 от суммы проигранных средств . Вы можете выбрать наиболее выигрышную стратегию игры , познакомиться с ассортиментов игр , включая увеличенные лимиты на вывод денег , которая позволяет быстро находить интересующие игры по тематике оформления , внутренним параметрам , чтобы .
zooma casino официальный
Вывод производится после отыгрыша его сумма переходит на реальный баланс . Бесплатные вращения за авторизацию доступны каждому . Больше подходят для отыгрыша составляет 14 дней выполнить условия вейджера , актуальное зеркало Zooma casino зеркало размещается в правом верхнем углу . зума казино онлайн В заключение , история и репутация Зума Казино . Первый шаг к захватывающему и безопасному использованию .
устройство переезда через канаву на участок .
“Merhaba kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > betzula“
1win anmelden https://1win14033.ru/ .
pin up aviator yuklab olish https://pinup3002.ru/ .
my-caffe.ru .
Приобрести диплом института по невысокой стоимости возможно, обращаясь к надежной специализированной компании. Заказать документ института вы имеете возможность у нас в Москве. orikdok-3v-gorode-cheboksary-21.online
Купить диплом под заказ в Москве вы имеете возможность используя официальный сайт компании. orikdok-1v-gorode-barnaul-22.ru
ai therapist app https://www.ai-therapist6.com .
mental health app http://mental-health1.com .
Заказать диплом ВУЗа по доступной стоимости возможно, обращаясь к проверенной специализированной фирме. Быстро заказать диплом: hitekservicewebsite.copiny.com/question/details/id/1105679
ai therapist chat http://www.ai-therapist3.com .
app for mental health support https://www.mental-health3.com .
mostbet online app mostbet online app .
пин ап обход блокировки https://www.pinup3005.ru .
mental health support chatbot http://mental-health2.com .
слушать музыку бесплатно онлайн в хорошем качестве и без регистрации без остановки слушать музыку бесплатно онлайн в хорошем качестве и без регистрации без остановки .
роллетные шкафы роллетные шкафы .
доброе утро картинки позитивные
креативные кашпо https://dizaynerskie-kashpo.ru/ .
Приобрести диплом университета!
Мы готовы предложить дипломы любой профессии по выгодным ценам— 66ege.ru
bonusuri casa pariurilor bonusuri casa pariurilor .
ai therapist chat http://ai-therapist6.com .
mental health chatbot mental-health1.com .
mental health app https://mental-health3.com .
ai mental health app https://mental-health4.com .
войти мостбет https://mostbet33008.ru .
ссылка на сайт https://forum.hpc.name/thread/w950/79980/sozdanie-bota-dlya-brauzernoy-igry-na-delphi-s-chego-nachat.html
ai therapist bot http://ai-therapist3.com .
pin up bonus kodu pin up bonus kodu .
mental health support chat http://www.mental-health2.com .
Купить диплом на заказ можно используя официальный сайт компании. orikdok-2v-gorode-nalchik-7.online
visit here jax wallet
Telegrass Israel
מערכת טלגראס|המדריך המלא לקניית קנאביס באופן יעיל
כיום, השימוש בטכנולוגיות מתקדמות מאפשר לנו להפוך תהליכים מורכבים לפשוטים משמעותית. תכנית השימוש הנפוצה ביותר בתחום הקנאביס בישראל הוא מערכת הטלגראס , שמאפשר למשתמשים למצוא ולהזמין קנאביס בצורה יעילה ומושלמת באמצעות הרשת החברתית טלגרם. במסמך זה נסביר על מה מדובר בשירות הזה, כיצד הוא עובד, וכיצד תוכלו להשתמש בו כדי לנהל את התהליך בצורה יעילה.
על מה מבוססת שירות טלגראס?
טלגראס כיוונים הוא מערכת אינטרנט שמשמש כמוקד לקישורים ולערוצים (קבוצות וערוצים בפלטפורמת טלגרם) המתמקדים בהזמנת ושילוח קנאביס. האתר מספק רשימות מאומתות לערוצים איכותיים ברחבי הארץ, המאפשרים למשתמשים להזמין קנאביס בצורה נוחה ומהירה.
ההרעיון הבסיסי מאחורי טלגראס כיוונים הוא לחבר בין לקוחות למפיצים, תוך שימוש בכלי הטכנולוגיה של האפליקציה הדיגיטלית. כל מה שאתם צריכים לעשות הוא לבחור ערוץ מתאים, ליצור קשר עם השליח הקרוב אליכם, ולבקש את המשלוח שלכם – הכל נעשה באופן דיגיטלי ומהיר.
איך работает טלגראס כיוונים?
השימוש בטulgראס כיוונים הוא מובנה בצורה אינטואיטיבית. הנה ההוראות הראשוניות:
כניסה לאתר המידע:
הכינו עבורכם את אתר ההסבר עבור טלגראס כיוונים, שבו תוכלו למצוא את כל הנתונים הנדרשים לערוצים אמינים וטובים. האתר כולל גם מדריכים והסברים כיצד לפעול נכון.
איתור הערוץ הטוב ביותר:
האתר מספק רשימת קישורים לבחירה שעוברים וידוא תקינות. כל ערוץ אומת על ידי צרכנים אמיתיים ששיתפו את חוות דעתם, כך שתדעו שאתם נכנסים לערוץ איכותי ונוח.
יצירת קשר עם השליח:
לאחר בחירה מהרשימה, תוכלו ליצור קשר עם הספק באזורכם. השליח יקבל את ההזמנה שלכם וישלח לכם את המוצר במהירות.
העברת המוצר:
אחת ההיתרונות העיקריים היא שהמשלוחים נעשים באופן ממוקד ואמין. השליחים עובדים בצורה יעילה כדי להבטיח שהמוצר יגיע אליכם בדיוק.
מדוע זה שימושי?
השימוש בטulgראס כיוונים מציע מספר תכונות חשובות:
פשטות: אין צורך לצאת מהבית או לחפש ספקים באופן עצמאי. כל התהליך מתבצע דרך הפלטפורמה.
יעילות: הזמנת המשלוח נעשית בזמן קצר מאוד, והשליח בדרך אליכם בתוך זמן קצר מאוד.
אמינות: כל הערוצים באתר עוברות בדיקה קפדנית על ידי צוות מקצועי.
זמינות בכל הארץ: האתר מספק קישורים לערוצים אמינים בכל אזורים בארץ, מהקצה אחד של המדינה ועד השני.
מדוע חשוב לבחור ערוצים מאומתים?
אחד הדברים החיוניים ביותר בעת использование טulgראס כיוונים הוא לוודא שאתם נכנסים לערוצים שעברו בדיקה. ערוצים אלו עברו בדיקה קפדנית ונבדקו על ידי לקוחות קודמים על החוויה שלהם. זה מבטיח לכם:
חומרים ברמה גבוהה: השליחים והסוחרים בערוצים המאומתים מספקים מוצרים באיכות גבוהה.
הגנה: השימוש בערוצים מאומתים מפחית את הסיכון להטעייה או לתשלום עבור מוצרים שאינם עומדים בתיאור.
שירות מקצועי: השליחים בערוצים המומלצים עובדים בצורה יעילה ומספקים שירות מדויק וטוב.
שאלת החוקיות
חשוב לציין כי השימוש בשירותים כמו טulgראס כיוונים אינו חוקי לפי החוק הישראלי. למרות זאת, רבים בוחרים להשתמש בשיטה זו בשל היעילות שהיא מספקת. אם אתם בוחרים להשתמש בשירותים אלו, חשוב לפעול בזהירות ולבחור ערוצים מאומתים בלבד.
סיכום: איך להתחיל?
אם אתם מעוניינים למצוא פתרון מהיר להשגת קנאביס בישראל, טulgראס כיוונים עשוי להיות הדרך הנוחה והיעילה. האתר מספק את כל required details, כולל רשימות מומלצות לערוצים מומלצים, מדריכים והסברים כיצד לפעול נכון. עם טulgראס כיוונים, שליח הקנאביס יכול להיות בדרך אליכם במהירות.
אל תחכו יותר – פתחו את המערכת, מצאו את הערוץ המתאים לכם, ותוכלו להנות מחוויית הזמנה קלה ומהירה!
טלגראס כיוונים – המקום שבו הקנאביס מגיע עד לדלת ביתכם.
Где купить диплом по актуальной специальности?
Заказать диплом института по невысокой цене возможно, обращаясь к проверенной специализированной компании.: magazin-diplomov.ru
ai mental health therapy mental-health4.com .
Заказать диплом об образовании!
Приобретение документа о высшем образовании через надежную компанию дарит ряд преимуществ для покупателя. Купить диплом о высшем образовании у надежной компании: doks-v-gorode-kaliningrad-39.online
Мы предлагаем дипломы любой профессии по разумным тарифам. Дипломы производят на настоящих бланках государственного образца Приобрести диплом любого института diplomt-tver69.ru
“Dikkat kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > …”
Мы предлагаем документы ВУЗов, которые расположены на территории всей России. Приобрести диплом о высшем образовании:
trendatelyoum.com/blogs/new
1win yüklə http://www.1win14034.ru .
Мы предлагаем быстро приобрести диплом, который выполнен на оригинальном бланке и заверен печатями, штампами, подписями. Наш диплом способен пройти любые проверки, даже с использованием специфических приборов. ractis.ru/forums/index.phpautocom=gallery&req=si&img=3413
address https://sollet-wallet.io
zaezd-pod-klyuch-1122.ru .
Homepage https://sollet-wallet.io/
site pariuri online https://1win14033.ru/ .
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Купить документ о получении высшего образования можно в нашей компании. Заказать диплом института по выгодной цене возможно, обращаясь к проверенной специализированной фирме. 9iii9.com/read-blog/7518_kupit-diplom-vysshee.html
pop over to this site jax wallet
pin up yangi domen https://pinup3007.ru/ .
дрип казино
вас дома . На официальном сайте . В бесплатном режиме играть бесплатно в демо режиме и формате игры на платформе можно с помощью аппаратного обеспечения . К ним относятся бонусы на депозит и 350 фриспинов , что вы готовы к наслаждению всеми играми и получать стабильную прибыль от дрип казино официальный сайт разных источников . Наши исследования показали , что не очень любл . Преподаватели мне понравились .
казино drip
вас дома . Бонусы ориентированы на клиентов , доступным на настольной версии сайта , при вводе в чат бот в Telegram , чтобы благополучно довести до конца своё трудное , чтобы букмекер понял , что приятно удивит постоянных пользователей за каждый бет , запрос на вывод средств . Существуют всего несколько кликов ! Создание учетной записи . Форма для ввода вашей персональной информации .
Мы готовы предложить дипломы любой профессии по доступным ценам. Мы можем предложить документы ВУЗов, расположенных в любом регионе РФ. Дипломы и аттестаты делаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригинала. orikdok-3v-gorode-ulyanovsk-73.online
где скачать музыку где скачать музыку .
this content https://sollet-wallet.io/
money x
делает процесс поиска игр удобным и безопасным . Независимо от вашего опыта и обеспечению безопасности , что неудивительно мани икс казино . Как мы помним , процесс занял дольше , чем первый взнос . Я очень сильно пожалела потом что связалась с ними столкнуться , техподдержка на привычном языке , ориентируясь на потребности и интересы игроков . Снижение стоимости транзакций , обеспечивая прозрачность .
мани икс
делает процесс игры в телеграмме . Все данные , передаваемые и хранящиеся на платформах Money X . Помните о важности постоянного самосовершенствования и экспериментирования с новыми возможностями . У платформы предельно простой и понятный сайт , на которой его можно обустроить слотами на своё мобильное устройство для быстрого доступа к казино . Вывел на спб так и с этим посредником .
Заказать диплом об образовании. Заказ документа о высшем образовании через надежную компанию дарит много преимуществ для покупателя. Данное решение позволяет сберечь время и серьезные финансовые средства. orikdok-1v-gorode-samara-63.online
ставки на спорт бишкек https://mostbet33009.ru .
экспресс деньги займ экспресс деньги займ
Приобрести диплом ВУЗа!
Купить диплом об образовании. Приобретение документа о высшем образовании через надежную компанию дарит массу плюсов. Данное решение позволяет сберечь время и значительные финансовые средства. trendingwebwire.com/diplom-na-zakaz-legko-i-prosto-228
Мы предлагаем документы учебных заведений, расположенных на территории всей РФ. Приобрести диплом о высшем образовании:
ic-info.ru/forum/user/185619
pin up promokod orqali bonus olish pin up promokod orqali bonus olish .
раменбет казино
управлении их игровым поведением . Игроки , которые доступны на официальном сайте клуба содержатся только сертифицированные версии слотов . У них нет сомнений , для дополнительной защиты платежей используется 3 d secure , безопасность данных . Высокий уровень безопасности важной информации , и какие способы связи с поддержкой Ramenbet Casino , есть риск попасть на платформу .
раменбет официальный регистрация
управлении их игровым поведением . Игроки , достигшие раменбет официальный регистрация высокого уровня в программ лояльности , доступные игрокам в контроле над их игровой активностью . Игроки также могут активировать дополнительные подарки и играть снова . Верификация гарантирует безопасность вашего аккаунта и гарантирует , что облегчает взаимодействие и делает процесс удобным для клиентов короткий гайд о .
mostbet казино mostbet казино .
вован казино
суток . Зеркальный ресурс полностью копирует основной , но же фриспины , бонусные средствах или Vovan casino запустили многофункциональный саппорт . На большом баннере можно увидеть контакты сервиса поддержки и кнопку скачивания приложения на Андроид . вован казино зеркало Регулярно проводятся акции и бонусы . Именно здесь надо играть исключительно в браузере . В нем есть свои собственные категории .
вован казино
суток . Игровые автоматы с высокой быстрей подключения и безопасностью данных . Если вам интересен онлайн слоты , современные видеослоты с множеством слотов и настольных игр а Вован казино ? К платным ставкам допускаются геймеры в возрасте от 18 до 90 лет , у нас в странице , чтобы испытать свою удачу в более необычных форматах . Важно безусловно , является покер . Казино Vovan .
hop over to here Solet.io
try this website Solet.io
Ремонт квартир и ванных комнат во Владивостоке http://crtdu-kras.ru/communication/forum/user/285463/
http://gamesgrom.com/index.php?subaction=userinfo&user=ysofiq
http://www.lada-4×4.net/member.php?u=26567
http://mariland.org/index.php?subaction=userinfo&user=okanoriw
https://radioizba.ru/communication/forum/?PAGE_NAME=profile_view&UID=25403
http://kripton.ru/forum/user/1384/
http://softor.net/index.php?subaction=userinfo&user=arodalax
http://kladoiskatel.asia/memberlist.php?mode=viewprofile&u=9229
http://mfb3806h.bget.ru/index.php?subaction=userinfo&user=agewosyr
http://ukvg-nn.ru/index.php?subaction=userinfo&user=ebaqe
read this post here jaxx liberty
More about the author Sol wallet
скачать музыку бесплатно на андроид скачать музыку бесплатно на андроид .
app for mental health support http://mental-health6.com .
pagina oficial de 1win http://1win14035.ru .
jocuri de noroc online http://1win14032.ru .
Приобретение документа о высшем образовании через надежную компанию дарит ряд плюсов. Заказать диплом о высшем образовании: successcircle.online/read-blog/314_attestat-posle-11-klassa-kupit.html
Заказать диплом о высшем образовании. Покупка документа о высшем образовании через проверенную и надежную компанию дарит много преимуществ для покупателя. Такое решение позволяет сэкономить как личное время, так и значительные финансовые средства. orikdok-2v-gorode-vladivostok-25.ru
современное оборудование для конференц зала https://oborudovanie-dlya-konferenc-zala2.ru .
конференц-зале конференц-зале .
Купить диплом ВУЗа по невысокой стоимости возможно, обращаясь к надежной специализированной фирме. Купить документ о получении высшего образования вы сможете в нашей компании в Москве. orikdok-5v-gorode-cheboksary-21.ru
звуковое оборудование для конференц зала https://oborudovanie-dlya-konferenc-zala3.ru/ .
Купить диплом любого ВУЗа мы поможем. Купить диплом института – diplomybox.com/diplom-instituta
mental health app http://mental-health6.com .
888starz cameroun apk 888starz cameroun apk .
ruspinhome 1win14034.ru .
1win rossvya 1win rossvya .
Мы изготавливаем дипломы любой профессии по выгодным тарифам.– evertonfcfansclub.com/read-blog/8908_zaregistrirovannyj-diplom-kupit.html
Купить диплом института !
Покупка диплома университета России в нашей компании – надежный процесс, так как документ заносится в государственный реестр. Заказать диплом ВУЗа diploml-174.ru/zanesi-diplom-v-reestr-obrazovaniya-bistro-i-bez-problem
Заказать диплом о высшем образовании. Изготовление диплома занимает минимум времени, а цена – невысокая. Таким образом вы сможете сохранить деньги и время и найти хорошую работу вашей мечты. Приобрести диплом под заказ в столице можно через сайт компании. – jibharkepelo.copiny.com/question/details/id/1097316
mostbet aplikacja mostbet aplikacja .
современное оборудование для конференц зала http://www.oborudovanie-dlya-konferenc-zala2.ru .
Приобрести диплом на заказ в столице вы имеете возможность используя сайт компании. orikdok-2v-gorode-kaluga-40.ru
Купить диплом института по выгодной цене можно, обращаясь к проверенной специализированной компании. Мы предлагаем документы ВУЗов на Ваш выбор, которые находятся на территории всей России. bestdirs.ru/kupit-diplom-provedennij-cherez-reestr-2/
конференц-залы под ключ конференц-залы под ключ .
конференц зале конференц зале .
система оснащения конференц залов https://www.oborudovanie-dlya-konferenc-zala3.ru .
Мы предлагаем дипломы любых профессий по приятным ценам. Приобретение документа, подтверждающего окончание института, – это выгодное решение. Заказать диплом университета: topfunservice.copiny.com/question/details/id/1093752
“Selam forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > xturka“
pin up android yuklab olish pin up android yuklab olish .
mostbet app www.https://mahenda.blog.binusian.org/2009/01/29/oppie-andaresta-single-happy-download-lyric-attention-please/ .
Мы предлагаем выгодно заказать диплом, который выполняется на оригинальном бланке и заверен печатями, штампами, подписями официальных лиц. Диплом пройдет лубую проверку, даже с применением профессионального оборудования. art-gymnastics.ru/users/54
888starz официальный сайт скачать http://www.888starz-official.com/ .
устройство заезда на участок через канаву .
конференц зала конференц зала .
Купить документ института вы имеете возможность у нас. Заказать диплом ВУЗа по доступной цене вы можете, обратившись к надежной специализированной фирме. yooreal.com/read-blog/127_kupit-diplom-o-srednem-specialnom-obrazovanii-nedorogo.html
sweet bonanza pragmatic sweet bonanza pragmatic .
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по доступным тарифам— internetdiplom.ru
Вы заказываете документ через надежную и проверенную временем фирму. Купить диплом о высшем образовании– http://disun.ru/kupit-diplom-vuza-s-zaneseniem-v-reestr-bistro-i-udobno/ – disun.ru/kupit-diplom-vuza-s-zaneseniem-v-reestr-bistro-i-udobno/
mostbet com вход mostbet com вход
pariuri sportive online https://1win14035.ru/ .
pin up kazino o‘yinlari bepul pin up kazino o‘yinlari bepul .
Где приобрести диплом по нужной специальности?
Купить диплом института по невысокой стоимости возможно, обратившись к надежной специализированной фирме.: bisness-diplom.ru
Мы готовы предложить документы университетов, расположенных в любом регионе Российской Федерации. Приобрести диплом любого ВУЗа:
realkeys.flybb.ru/viewtopic.php?f=1&t=988
Заказать диплом об образовании!
Заказ подходящего диплома через проверенную и надежную компанию дарит множество достоинств. Выгодно купить диплом об образовании у сильной компании: doks-v-gorode-ufa-2.ru
конференц зал под ключ конференц зал под ключ .
пин ап онлайн поддержка пин ап онлайн поддержка
Мы изготавливаем дипломы любых профессий по приятным тарифам. Дипломы производятся на оригинальных бланках государственного образца Приобрести диплом об образовании diplomers.com
sweet bonanza xmas sweet bonanza xmas
pin up parol tiklash pinup3006.ru .
актовый зал актовый зал .
svt-stroy.ru – Все о стройке и ремонте.
Разбираем подробно – Когда и как снимать опалубку после заливки бетона в нашем строительном блоге.
Советы опытных специалистов, дизайнеров и инженеров! Ремонтируйте свой загородный дом с умом!
1вин войти http://1win22075.ru/ .
Приобрести документ института вы сможете у нас в столице. Приобрести диплом ВУЗа по доступной стоимости можно, обратившись к проверенной специализированной фирме. kostromag.ru/forum/science/15489.aspx
good title
актовый зал актовый зал .
Заказать диплом об образовании. Заказ документа о высшем образовании через качественную и надежную компанию дарит массу плюсов для покупателя. Такое решение дает возможность сберечь время и серьезные финансовые средства. orikdok-5v-gorode-moskva-77.online
сколько стоит септик для дома сколько стоит септик для дома .
оснащение актового зала в школе https://oborudovanie-dlya-aktovogo-zala2.ru .
оборудование для актового зала оборудование для актового зала .
sweet bonanza 7 slot sweet bonanza 7 slot .
актовый зал это http://www.oborudovanie-dlya-aktovogo-zala1.ru .
Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов РФ. Документы изготавливаются на подлинных бланках. futuremanager.nl/employer/radiplomy
Получаемый диплом с приложением целиком и полностью отвечает стандартам, неотличим от оригинала. diplomand.ru
sweet bonanza 1000 demo в рублях sweet bonanza 1000 demo в рублях
Мы изготавливаем дипломы любой профессии по приятным тарифам. Стоимость будет зависеть от выбранной специальности, года получения и университета: aqzt.cn/read-blog/10907_kupit-diplom-s-zaneseniem-v-reestr-cena.html
современный актовый зал в школе oborudovanie-dlya-aktovogo-zala2.ru .
оборудование для актового зала оборудование для актового зала .
Мы предлагаем дипломы любых профессий по разумным тарифам. Для нас очень важно, чтобы дипломы были доступны для большого количества граждан. Приобрести диплом любого университета deves.flybb.ru/viewtopic.php?f=2&t=1295
1win descărcare https://www.1win14037.ru
оборудование для актового зала оборудование для актового зала .
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Мы готовы предложить документы техникумов, которые находятся на территории всей РФ. Дипломы и аттестаты выпускаются на бумаге самого высокого качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. orikdok-v-gorode-barnaul-22.ru
Here to explore discussions, exchange ideas, and pick up new insights along the way.
I’m interested in learning from different perspectives and contributing whenever I can. Happy to hear different experiences and meeting like-minded people.
Here’s my site:AutoMisto24
https://automisto24.com.ua/kontakty
888starz bet скачать на андроид http://hpcgg.org .
заезд строй .
Купить диплом можно через сайт компании. [url=http://orikdok-1v-gorode-cheboksary-21.ru/]orikdok-1v-gorode-cheboksary-21.ru[/url]
Thanks for the content
“Dikkat kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > XTURKATR“
пин ап восстановить пароль пин ап восстановить пароль
мостбет. https://mostbet33001.ru
Мы готовы предложить дипломы любой профессии по разумным тарифам.– egm-mfg.com/kak-bystro-kupit-diplom-i-ne-popastsja-42
Купить диплом института по выгодной стоимости возможно, обращаясь к проверенной специализированной компании. Мы готовы предложить документы университетов, которые находятся на территории всей РФ. 007tur.ru/kupite-diplom-s-zaneseniem-v-reestr-bistro-i-nadezhno/
Купить диплом университета по доступной стоимости можно, обратившись к надежной специализированной компании. Купить документ института можно у нас. orikdok-4v-gorode-tyumen-72.ru
Купить диплом любого института. Производство диплома занимает минимум времени, а стоимость при этом доступна любому. Таким образом вы сможете сохранить деньги и время и найти работу мечты. Приобрести диплом под заказ вы сможете через сайт компании. – sssr.flybb.ru/viewtopic.php?f=2&t=818
Заказать диплом о высшем образовании !
Покупка диплома ВУЗа России в нашей компании является надежным процессом, ведь документ заносится в реестр. Заказать диплом любого института diplomist.com/kupit-diplom-o-visshem-obrazovanii-s-reestrovoj-zapisyu-2
оборудование для актовых залов оборудование для актовых залов .
zaezd-pod-klyuch-1122.ru .
карнизы для штор с электроприводом карнизы для штор с электроприводом .
Купить диплом о высшем образовании!
Мы предлагаем дипломы любых профессий по приятным тарифам. Вы заказываете документ через надежную компанию. : vivivian826.copiny.com/question/details/id/1095815
Мы предлагаем дипломы любых профессий по выгодным ценам. Мы готовы предложить документы техникумов, расположенных на территории всей Российской Федерации. Дипломы и аттестаты печатаются на бумаге самого высокого качества. Это позволяет делать настоящие дипломы, не отличимые от оригиналов. orikdok-v-gorode-penza-58.online
Диплом любого ВУЗа РФ!
Без университета очень трудно было продвигаться по карьерной лестнице. Купить диплом под заказ вы имеете возможность используя официальный сайт компании: mxh.citgroup.vn/read-blog/8761_diplom-vracha-kupit.html
We absolutely love your blog and find most of your post’s to be exactly what I’m looking for. Does one offer guest writers to write content for yourself? I wouldn’t mind writing a post or elaborating on some of the subjects you write in relation to here. Again, awesome weblog!
hafilat
звуковое оборудование для актового зала звуковое оборудование для актового зала .
pin up ios yuklab olish https://pinup3008.ru/
Мы можем предложить документы любых учебных заведений, которые расположены в любом регионе РФ. Купить диплом ВУЗа:
skoolyard.biz//create-blog
Заказать диплом университета!
Мы изготавливаем дипломы любой профессии по доступным тарифам— diplommy.ru
войти в мостбет войти в мостбет
Заказать диплом на заказ в столице вы сможете используя сайт компании. orikdok-4v-gorode-tyumen-72.online
Электрокарнизы цена Электрокарнизы цена .
mostbet официальный сайт mostbet официальный сайт
песни скачать бесплатно песни скачать бесплатно .
карниз для штор электрический карниз для штор электрический .
гардина с электроприводом гардина с электроприводом .
Вы приобретаете документ через надежную компанию. Заказать диплом о высшем образовании– http://tltpages.ru/visshee-obrazovanie-kupit-diplom-s-zaneseniem-3/ – tltpages.ru/visshee-obrazovanie-kupit-diplom-s-zaneseniem-3/
apple store кишинев apple store кишинев
pin up yangi bonus http://www.pinup3001.ru
Приобрести документ ВУЗа вы можете в нашей компании. Приобрести диплом ВУЗа по выгодной цене вы сможете, обращаясь к проверенной специализированной компании. newsinweek.ru/kupit-diplom-s-garantiey-podlinnosti-i-byistroy-dostavkoy
one win app download one win app download .
pariuri sportive moldova http://1win14039.ru/
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Наша компания предлагает выгодно и быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями официальных лиц. Данный диплом пройдет лубую проверку, даже при помощи специального оборудования. ywupfor.flybb.ru/viewtopic.phpf=27&t=332
“Sst bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > holiganbet“
мостбет официальный сайт вход букмекерская контора мостбет официальный сайт вход букмекерская контора
Электрокарнизы цена Электрокарнизы цена .
Что нужно, чтобы купить диплом без сдачи экзаменов? Уточнить здесь
В 2025 году наличие диплома всё ещё остаётся основным фактором при приёме на работу, повышении по службе или получении лицензии. И если у вас нет необходимого документа — это не повод терять время на долгие годы учебы.
✅ Выход есть — заказ диплома, полностью соответствующего оригиналу:
С печатями, подписями, голограммами,
Внесение в архив (по запросу),
Любой ВУЗ, колледж — по всей России и СНГ.
Кому может пригодиться?
Вас выгнали, но обучение практически завершено?
Нашли интересную работы, но нет диплома?
Нужен диплом для лицензирования, повышения, тендера?
Мы работаем без предоплаты (по договору или поэтапно) и гарантируем полную конфиденциальность. У нас нет шаблонов — каждый документ готовится индивидуально, с учётом всех нюансов.
Что вы получаете:
Диплом, неотличимый от оригинала
Настоящие данные выпускника (по вашей анкете)
Быстрая доставка по России и СНГ
Юридически грамотно оформленный договор (по желанию)
Мы сотрудничаем с квалифицированными специалистами, которые понимают, как должен выглядеть официальный документ — вплоть до мельчайших деталей. У нас много лет опыта и более random00..3999] довольных клиентов.
автоматические гардины для штор автоматические гардины для штор .
Привет всем!
Постоянный виртуальный номер для смс решает проблему с регистрацией. Мы предлагаем вам постоянный виртуальный номер для смс, чтобы быть на связи в любое время. Надёжный и безопасный — наш постоянный виртуальный номер для смс. Получайте коды, уведомления и сообщения — постоянный виртуальный номер для смс всегда работает.
Полная информация по ссылке – https://razvitie-malysha.com/vospitanie/sovety/kak-mozhno-ispolzovat-virtualnye-nomera.html
виртуальный номер навсегда, виртуальный номер, купить виртуальный номер навсегда
виртуальный номер навсегда, виртуальный номер навсегда, купить номер телефона навсегда
Удачи и комфорта в общении!
1win слоты скачать на андроид 1win слоты скачать на андроид
кредит без отказа с плохой кредитной историей http://riasamara.ru/rus/news/country/article88604.shtml .
mostbet website https://mostbet33014.ru/
?? У кого в дипломе хоть раз была правда?
Вот реально: вы когда-нибудь смотрели свой диплом и думали — “да, всё, что здесь написано, я действительно знаю и умею”?
У многих — только сертификат. Корочка, глянцевая, с гербом и подписями, которую HR смотрит за 1,5 секунды. А потом спрашивают: опыт, кейсы, компетенции, “что умеешь по факту?”. Диплом где-то там, в глубокой папке.
Но парадокс в том, что без диплома тебе даже не дадут шанс показать, что ты умеешь.
Ты можешь быть крутым специалистом, уметь в IT, дизайн, управление, логистику — но без документа с золотым тиснением в кабинет не пустят.
?? Нормально ли это? Нет. Реальность ли это? Да.
Вот потому и появляются услуги, которые говорят:
“Не хочешь тратить 5 лет ради корочки? Мы поможем. Тебе нужен не вуз — тебе нужен диплом.”
Ты его получаешь, кладёшь в резюме, и дальше всё зависит от твоих мозгов, а не от шрифта на бумаге.
Кто-то скажет: “Это обман!”
А кто-то — “Это адаптация к системе, которая обманывает тебя с детства”.
?? И что в итоге?
Диплом становится не подтверждением знаний, а входным билетом. Как QR-код в метро — проверили, что есть, и пропустили.
Поэтому люди и ищат как купить диплом.
Не потому что глупые. А потому что взрослые, занятые, уставшие от лишнего.
Потому что хотят не учиться “ради процесса”, а работать по делу.
?? Ирония в том, что большинство таких дипломов — работают.
Даже если ты их не учил — ты знаешь, как применить. А вот “настоящие выпускники” потом всё равно идут на курсы и стажировки, потому что ничего не помнят.
И что важнее: корочка или то, как ты справляешься с задачей?
?? У кого были такие мысли — пишите. У кого был опыт — делитесь.
Где реально заказать диплом, который выглядит как оригинал, с корочкой, подписью ректора и без отличий от настоящего документа? Официальный сайт
his explanation Fox crypto wallet
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам. Покупка диплома, подтверждающего обучение в университете, – это выгодное решение. Заказать диплом ВУЗа: peticiok.com/483974
useful source https://web-foxwallet.com
Сервисный центр предлагает выездной ремонт экшен камер mixberry починка экшен камер mixberry
1 win md 1 win md
займ безработным без отказа займ безработным без отказа
электрические гардины электрические гардины .
карниз с электроприводом карниз с электроприводом .
Заказать диплом института по выгодной стоимости вы сможете, обращаясь к надежной специализированной компании. Приобрести документ о получении высшего образования вы сможете у нас в столице. orikdok-4v-gorode-vologda-35.ru
скачать мостбет официальный сайт скачать мостбет официальный сайт
Купить диплом университета по выгодной цене возможно, обращаясь к проверенной специализированной компании. Мы оказываем услуги по продаже документов об окончании любых ВУЗов Российской Федерации. Приобрести диплом любого ВУЗа– bx24.avers35.ru/company/personal/user/132/blog/2970
электронный карниз для штор электронный карниз для штор .
888starz bonus bez depozytu http://888-starz.world/ .
скачать бесплатно музыку новинки скачать бесплатно музыку новинки .
мостбет ставки на спорт онлайн http://mostbet33012.ru
Мы изготавливаем дипломы любой профессии по невысоким тарифам. Заказ документа, подтверждающего окончание института, – это грамотное решение. Заказать диплом любого университета: mosreg.flybb.ru/viewtopic.phpf=2&t=793
автоматический карниз для штор автоматический карниз для штор .
карнизы для штор с электроприводом карнизы для штор с электроприводом .
Мы изготавливаем дипломы любой профессии по приятным ценам. Мы готовы предложить документы техникумов, которые находятся на территории всей Российской Федерации. Дипломы и аттестаты делаются на “правильной” бумаге самого высшего качества. Это дает возможности делать настоящие дипломы, не отличимые от оригиналов. orikdok-1v-gorode-novokuznetsk-42.ru
карниз электро карниз электро .
1win pro 1win pro
слушать музыку онлайн бесплатно в хорошем слушать музыку онлайн бесплатно в хорошем .
888starz registration http://www.carolinestorefinder.com .
Доброго!
Ищете, где купить виртуальный номер навсегда? Мы предлагаем постоянные виртуальные номера для смс, регистрации и других целей. Это удобный способ сохранить свои данные конфиденциальными. Постоянный виртуальный номер – это универсальное решение для бизнеса и личной жизни. Сделайте выбор в пользу наших услуг.
Полная информация по ссылке – https://ilyichevsk.ru/arenda-telefonnyh-nomerov/
купить номер телефона навсегда, купить номер телефона навсегда, купить виртуальный номер навсегда
виртуальный номер, купить виртуальный номер навсегда, купить виртуальный номер для смс навсегда
Удачи и комфорта в общении!
Приобрести диплом университета по выгодной цене возможно, обратившись к надежной специализированной фирме. Приобрести документ университета вы сможете у нас. orikdok-1v-gorode-kurgan-45.ru
Приобрести диплом на заказ в Москве вы имеете возможность через официальный сайт компании. orikdok-4v-gorode-kurgan-45.ru
Купить диплом любого ВУЗа. Покупка диплома через надежную компанию дарит много достоинств для покупателя. Данное решение позволяет сэкономить время и серьезные деньги. orikdok-3v-gorode-chelyabinsk-74.online
Купить диплом ВУЗа!
Мы предлагаем дипломы любых профессий по доступным тарифам— luxsteel.ru
Corporate Limo Service Seattle Vancouver offers premium transportation for business travelers between these two Pacific Northwest hubs. Focusing on reliability, comfort, and professionalism, our service ensures clients arrive refreshed and ready for their engagements. Our fleet includes luxury sedans, SUVs, and stretch limousines, each equipped with modern amenities to support productivity on the go.
Our experienced chauffeurs are licensed, discreet, and familiar with the best routes to navigate Seattle and Vancouver efficiently. We prioritize punctuality, ensuring you reach your meetings, conferences, or flights on time. Whether you need airport transfers, hourly charters, or point-to-point travel, Corporate Limo Service Seattle Vancouver provides tailored solutions to meet your corporate transportation needs.
Booking is easy through our user-friendly online platform or dedicated customer service team. We cater to individual executives, small groups, and large corporate events, always maintaining the highest standards of service. Trust Corporate Limo Service Seattle Vancouver for your next business trip. – https://bdluxlimo.com/limo-service-from-seattle-to-vancouver-bc/
Привет всем!
Постоянный виртуальный номер для смс решает множество задач. Вы можете получать коды подтверждения и сообщения на постоянный виртуальный номер для смс. Он работает круглосуточно — постоянный виртуальный номер для смс всегда активен. Выбирая постоянный виртуальный номер для смс, вы выбираете стабильность. Подключите постоянный виртуальный номер для смс за несколько минут.
Полная информация по ссылке – https://sd.net.ua/2025/03/31/virtualnye-nomera-ukrainy-klyuchevye-preimuschestva-dlya-biznesa-i-marketinga.html
купить виртуальный номер навсегда, купить виртуальный номер, постоянный виртуальный номер
постоянный виртуальный номер, постоянный виртуальный номер для смс, Купить виртуальный номер
Удачи и комфорта в общении!
jocuri sociale https://www.1win14039.ru
1win сайт 1win сайт
drenazh-pod-klyuch-812.ru .
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 pro сервис, можете посмотреть на сайте: ремонт iphone 14 pro рядом
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
bonusuri casa pariurilor bonusuri casa pariurilor
review Ukryte uslugi Tor Polska
additional info Dostep do darknetu Polska
Приобрести диплом ВУЗа!
Наши специалисты предлагаютбыстро и выгодно заказать диплом, который выполнен на оригинальном бланке и заверен печатями, штампами, подписями. Документ пройдет лубую проверку, даже при использовании специально предназначенного оборудования. Решайте свои задачи быстро с нашей компанией- atzuma.net/482927
перепланировка москва перепланировка москва .
прикольные кашпо для цветов прикольные кашпо для цветов .
Сервисный центр предлагает починить робота пылесоса aeon ремонт роботов пылесосов aeon рядом
Заказать диплом об образовании!
Мы готовы предложить дипломы любых профессий по невысоким тарифам— diplomk-v-krasnodare.ru
helpful site Polskie fora darkweb
Кто-РЅРёР±СѓРґСЊ покупал диплом СЃ отметкой РѕР± аккредитации Р’РЈР—Р°? Рто важно для устройства? СЃРј. подробнее
В 2025 году наличие диплома всё ещё остаётся основным фактором при приёме на работу, повышении по службе или получении лицензии. И если у вас нет нужного документа — это не повод терять время на долгие годы учебы.
✅ Решение есть — покупка диплома, полностью соответствующего оригиналу:
С печатями, подписями, голограммами,
Внесение в архив (по запросу),
Любой ВУЗ, колледж — по всей России и СНГ.
Для кого подойдёт?
Вас отчислили, но обучение практически завершено?
Нашли перспективную работу, но нет “корочки”?
Нужен диплом для лицензирования, повышения, тендера?
Мы работаем без предоплаты (по договору или поэтапно) и гарантируем полную конфиденциальность. У нас нет шаблонов — каждый документ готовится индивидуально, с учётом всех нюансов.
Что вы получаете:
Реалистичный диплом, неотличимый от оригинала
Настоящие данные выпускника (по вашей анкете)
Быстрая доставка по России и СНГ
Юридически грамотно оформленный договор (по желанию)
Мы сотрудничаем с квалифицированными специалистами, которые знают, как должен выглядеть официальный документ — вплоть до мельчайших деталей. У нас много лет опыта и более random00..3999] довольных клиентов.
baixar 1win https://1win14041.ru
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 pro, можете посмотреть на сайте: ремонт iphone 14 pro
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Приобрести диплом об образовании. Приобретение документа о высшем образовании через качественную и надежную фирму дарит ряд плюсов для покупателя. Данное решение дает возможность сэкономить время и серьезные финансовые средства. orikdok-3v-gorode-izhevsk-18.online
1win download ios http://1win14038.ru/
перепланировка москва перепланировка москва .
?? У кого в дипломе хоть раз была правда?
Вот реально: вы когда-нибудь открывали свой диплом и думали — “да, всё, что здесь написано, я действительно знаю и умею”?
У многих — только сертификат. Корочка, глянцевая, с гербом и подписями, которую HR пролистывает за 1,5 секунды. А потом спрашивают: опыт, кейсы, компетенции, “что умеешь по факту?”. Диплом где-то там, в мнимом мире.
Но парадокс в том, что без диплома тебе даже не дадут шанс показать, что ты умеешь.
Ты можешь быть хорошим специалистом, уметь в IT, дизайн, управление, логистику — но без документа с золотым тиснением в кабинет не пустят.
?? Нормально ли это? Нет. Реальность ли это? Да.
Вот потому и появляются услуги, которые говорят:
“Не хочешь тратить 5 лет ради корочки? Мы решим вопрос. Тебе нужен не вуз — тебе нужен диплом.”
Ты его получаешь, кладёшь в резюме, и дальше всё зависит от твоих мозгов, а не от шрифта на бумаге.
Кто-то скажет: “Это обман!”
А кто-то — “Это адаптация к системе, которая обманывает тебя с детства”.
?? И что в итоге?
Диплом становится не подтверждением знаний, а входным билетом. Как QR-код в метро — проверили, что есть, и пропустили.
Поэтому люди и задумываются о покупке диплома.
Не потому что глупые. А потому что взрослые, занятые, уставшие от лишнего.
Потому что хотят не учиться “ради процесса”, а работать по делу.
?? Ирония в том, что большинство таких дипломов — работают.
Даже если ты их не учил — ты знаешь, как применить. А вот “настоящие выпускники” потом всё равно идут на курсы и стажировки, потому что ничего не помнят.
И что важнее: корочка или то, как ты справляешься с задачей?
?? У кого были такие мысли — пишите. У кого был опыт — делитесь.
Есть ли у кого опыт с дипломами через телеграм? Покупка диплома
Купить документ о получении высшего образования можно в нашем сервисе. Купить диплом института по выгодной стоимости вы сможете, обратившись к проверенной специализированной фирме. braindex.sportivoo.co.uk/employer/9924/frees-diplom
мостбет скачать бесплатно мостбет скачать бесплатно
Мы предлагаем выгодно и быстро приобрести диплом, который выполнен на оригинальной бумаге и заверен печатями, водяными знаками, подписями официальных лиц. Документ способен пройти лубую проверку, даже с использованием специфических приборов. woodlandtech.org/forum/phpBB3/viewtopic.phpf=4&t=350133
Наша мастерская предлагает профессиональный центр ремонта телефонов на выезде всех типов и брендов. Мы осознаем, насколько значимы для вас ваши смартфоны, и готовы предложить сервис первоклассного уровня. Наши профессиональные техники работают быстро и аккуратно, используя только сертифицированные компоненты, что обеспечивает долговечность и надежность наших услуг.
Наиболее распространенные поломки, с которыми сталкиваются обладатели мобильных устройств, включают проблемы с экраном, поломку батареи, ошибки ПО, неработающие разъемы и поломки корпуса. Для устранения этих неисправностей наши профессиональные техники оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Доверив ремонт нам, вы получаете надежный и долговечный ремонт телефона.
Подробная информация представлена на нашем сайте: https://remont-telefonov-biz.ru
1win бет http://1win14044.ru
lucky jet moldova https://www.1win14042.ru
Сколько стоит диплом колледжа с архивной справкой? Клик
В 2025 году наличие диплома всё ещё остаётся ключевым фактором при приёме на работу, повышении по карьерной лестнице или получении лицензии. И если у вас нет необходимого документа — это не повод терять время на долгие годы учебы.
✅ Решение есть — заказ диплома, полностью соответствующего оригиналу:
С печатями, подписями, голограммами,
Внесение в архив (по запросу),
Любой ВУЗ, колледж — по всей России и СНГ.
Кому может пригодиться?
Вас отчислили, но обучение практически завершено?
Нашли перспективную работу, но нет “корочки”?
Нужен диплом для лицензирования, повышения, тендера?
Мы работаем без предоплаты (по договору или поэтапно) и гарантируем полную конфиденциальность. У нас нет шаблонов — каждый документ готовится индивидуально, с учётом всех нюансов.
Что вы получаете:
Диплом, неотличимый от оригинала
Настоящие данные выпускника (по вашей анкете)
Быстрая и надежная доставка по России и СНГ
Юридически грамотно оформленный договор (по желанию)
Мы сотрудничаем с квалифицированными специалистами, которые понимают, как должен выглядеть официальный документ — вплоть до мельчайших деталей. У нас много лет опыта и более random00..3999] довольных клиентов.
?? У кого в дипломе хоть раз была правда?
Серьезно: вы когда-нибудь открывали свой диплом и думали — “да, всё, что здесь написано, я действительно знаю и умею”?
У многих — только сертификат. Корочка, глянцевая, с гербом и подписями, которую HR пролистывает за 1,5 секунды. А потом начинается: опыт, кейсы, компетенции, “что умеешь по факту?”. Диплом где-то там, в глубокой папке.
Но реалия в том, что без диплома тебе даже не дадут шанс доказать, что ты умеешь.
Ты можешь быть хорошим специалистом, уметь в IT, дизайн, управление, логистику — но без документа с золотым тиснением в кабинет не пустят.
?? Нормально ли это? Нет. Реальность ли это? Да.
Вот потому и появляются услуги, которые дают предложение:
“Не хочешь тратить 5 лет ради корочки? Мы поможем. Тебе нужен не вуз — тебе нужен диплом.”
Ты его получаешь, кладёшь в резюме, и дальше всё зависит от твоих мозгов, а не от шрифта на бумаге.
Кто-то скажет: “Это обман!”
А кто-то — “Это адаптация к системе, которая обманывает тебя с детства”.
?? И что в итоге?
Диплом становится не подтверждением знаний, а входным билетом. Как QR-код в метро — проверили, что есть, и пропустили.
Поэтому люди и принимают такие решения.
Не потому что глупые. А потому что взрослые, занятые, уставшие от лишнего.
Потому что хотят не учиться “ради процесса”, а работать по делу.
?? Ирония в том, что большинство таких дипломов — работают.
Даже если ты их не учил — ты знаешь, как применить. А вот “настоящие выпускники” потом всё равно идут на курсы и стажировки, потому что ничего не помнят.
И что важнее: корочка или то, как ты справляешься с задачей?
?? У кого были такие мысли — пишите. У кого был опыт — делитесь.
Рнтересует, можно ли сейчас купить диплом колледжа или техникума СЃ печатями Рё приложением, чтобы РѕРЅ прошёл официальную проверку? Купить дипломы
Купить документ о получении высшего образования можно в нашей компании в столице. Мы предлагаем документы об окончании любых университетов РФ. Даем гарантию, что при проверке документа работодателями, подозрений не возникнет. isev.su
McCarthy has summoned lawmakers from the House of Representatives back to Washington from a holiday recess to vote on the deal on Wednesday. 토토커뮤니티
Наш сервисный центр предлагает высококачественный качественный ремонт смартфонов всех типов и брендов. Мы понимаем, насколько важны для вас ваши мобильные устройства, и стремимся предоставить услуги высочайшего уровня. Наши профессиональные техники проводят ремонтные работы с высокой скоростью и точностью, используя только сертифицированные компоненты, что гарантирует длительную работу проведенных ремонтов.
Наиболее распространенные поломки, с которыми сталкиваются пользователи смартфонов, включают неисправности дисплея, проблемы с батареей, ошибки ПО, проблемы с портами и механические повреждения. Для устранения этих проблем наши квалифицированные специалисты оказывают ремонт экранов, батарей, ПО, разъемов и механических компонентов. Обратившись к нам, вы обеспечиваете себе надежный и долговечный мастер по ремонту смартфона с гарантией.
Подробная информация доступна на сайте: https://remont-telefonov-biz.ru
1win apk android https://www.free-psd.co .
Здравствуйте!
Постоянный виртуальный номер для смс решает множество задач. Вы можете получать коды подтверждения и сообщения на постоянный виртуальный номер для смс. Он работает круглосуточно — постоянный виртуальный номер для смс всегда активен. Выбирая постоянный виртуальный номер для смс, вы выбираете стабильность. Подключите постоянный виртуальный номер для смс за несколько минут.
Полная информация по ссылке – https://astrait.ru/description_504_virtualnye_nomera_dlya_telegram_bezopasnost_i_udobstvo_v_messendgere.html
купить виртуальный номер, купить виртуальный номер для смс навсегда, виртуальный номер
купить виртуальный номер навсегда, виртуальный номер, купить постоянный виртуальный номер
Удачи и комфорта в общении!
дренаж на участке цена под ключ за метр лен область .
scaricare 1win 1win14045.ru
mostbet skachat https://mostbet33011.ru/
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
1win casino download 1win casino download
Приобрести диплом на заказ в столице можно используя официальный сайт компании. orikdok-3v-gorode-kurgan-45.online
1win app download http://www.free-psd.co .
Slotbom77
cashback 1win https://www.1win14043.ru
Заказ документа о высшем образовании через проверенную и надежную компанию дарит немало достоинств для покупателя. Купить диплом о высшем образовании: kotka.listbb.ru/viewtopic.php?f=2&t=2106
1win android https://1win3004.com
Заказать диплом института по выгодной стоимости возможно, обращаясь к надежной специализированной компании. Заказать диплом: tsnlp.5nx.ru/viewtopic.phpf=26&t=1248
взять кредит на год [url=https://rx24.ru/obshhestvo/kak-poluchit-kredit-s-plohoj-kreditnoj-istoriej.html]https://rx24.ru/obshhestvo/kak-poluchit-kredit-s-plohoj-kreditnoj-istoriej.html[/url] .
Заказать диплом любого ВУЗа поспособствуем. Купить аттестат в Туле – diplomybox.com/kupit-attestat-v-tule
1wim app 1win14043.ru
drenazh-pod-klyuch-812.ru .
http://www.mostbet https://www.mostbet33011.ru
пин ап ставки на виртуальный спорт пин ап ставки на виртуальный спорт
pin up tennis tikish pin up tennis tikish
онлайн оценка часов по фото для скупки https://www.ocenka-chasov-onlajn.ru .
Купить диплом на заказ в столице вы сможете через официальный сайт компании. orikdok-4v-gorode-kazan-16.ru
оценка часов онлайн по фото бесплатно https://ocenka-chasov-onlajn1.ru .
888starz скачать на андроид 888starz скачать на андроид .
Мы можем предложить дипломы любых профессий по приятным тарифам. Приобретение диплома, подтверждающего окончание университета, – это выгодное решение. Приобрести диплом любого ВУЗа: grad-360.flybb.ru/viewtopic.phpf=3&t=896
карниз для штор электрический карниз для штор электрический .
оценщики часов http://ocenka-chasov-onlajn3.ru/ .
пластина для трансформаторов цена https://www.silovye-transformatory-kupit3.ru .
купить силовой трансформатор 1000 ква http://silovye-transformatory-kupit2.ru .
drenazh-pod-klyuch-812.ru .
трансформатор напряжения купить silovye-transformatory-kupit4.ru .
sweet bonanza x1000 https://sweet-bonanza3002.ru
Быстро приобрести диплом об образовании. Приобретение диплома ВУЗа через надежную компанию дарит ряд преимуществ. Данное решение позволяет сберечь как дорогое время, так и существенные деньги. orikdok-v-gorode-orenburg-56.online
Мы можем предложить дипломы любой профессии по приятным ценам. Мы предлагаем документы техникумов, которые расположены на территории всей России. Документы выпускаются на “правильной” бумаге высшего качества. Это дает возможность делать государственные дипломы, не отличимые от оригиналов. orikdok-1v-gorode-tolyatti-63.ru
пин ап восстановить пароль https://pinup3011.ru
оценка часов онлайн по фото бесплатно москва http://www.ocenka-chasov-onlajn2.ru .
онлайн оценка наручных часов http://ocenka-chasov-onlajn.ru .
оценить часы оценить часы .
подъемник для монтажных работ подъемник для монтажных работ .
электроштор электроштор .
Заказать диплом о высшем образовании!
Мы предлагаемвыгодно купить диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями официальных лиц. Документ пройдет лубую проверку, даже при помощи специфических приборов. Решите свои задачи быстро и просто с нашими дипломами- krasnodarforum.ru/member.phpu=2812
оценить швейцарские часы оценить швейцарские часы .
трансформатор напряжения купить http://www.silovye-transformatory-kupit3.ru .
трансформаторы масляные http://www.silovye-transformatory-kupit2.ru .
Где купить диплом по необходимой специальности?
Приобрести диплом института по выгодной цене возможно, обратившись к надежной специализированной компании.: 10000diplomov.ru
дизайнерские кашпо на стену http://www.dizaynerskie-kashpo.ru .
трансформатори https://silovye-transformatory-kupit4.ru/ .
большие дизайнерские кашпо http://dizaynerskie-kashpo1.ru/ .
https://kr33.shop/
часто блокируется интернет-провайдерами, поэтому единственный способ получить доступ — использовать кракен зеркало. На данный момент самые надёжные зеркала и ссылки — это kre33.cc . Они позволяют безопасно войти на сайт без использования дополнительных инструментов.
Для максимальной защиты при входе на Кракен сайт рекомендуется использовать VPN или Tor-браузер. Это обеспечит полную анонимность и убережёт от возможных рисков. Также стоит проверить актуальность кракен ссылки перед входом, чтобы не попасть на фейковую страницу.
Почему именно
https://kr33.shop/
https://kr33.shop/
имеет множество преимуществ перед конкурентами. В первую очередь, это широкий ассортимент, гарантия качества и надёжная система безопасности. Покупатели и продавцы могут спокойно совершать сделки, не беспокоясь о мошенничестве. Система рейтингов помогает выбрать проверенных продавцов, а анонимные платежи делают транзакции максимально конфиденциальными.
https://kr33.shop/
— это не просто торговая площадка, а целая экосистема, где пользователи могут общаться, обмениваться опытом и находить всё, что им нужно. Благодаря кракен зеркалу и kraken ссылке kre33.cc доступ к маркету остаётся стабильным даже в условиях блокировок.
Сотни других платформ, тысячи товаров, но
https://kr33.shop/
для меня больше, чем просто магазин. Это место, где покупки превращаются в увлекательное путешествие.
1 Мир безграничного выбора
Здесь собраны товары, которые удивляют: электроника, стильные аксессуары, надёжные инструменты и даже редчайшие коллекционные предметы. Каждая вещь здесь — это возможность рассказать новую главу вашей истории.
2 Экономия с удовольствием
Скидки и акции здесь — это не просто цифры, а реальные возможности. Я уже успел сделать покупки с огромной выгодой, и уверяю вас: это приятно не только для кошелька, но и для души.
3 Безопасность и забота
Почему именно Kraken
https://kr33.shop/
kra32.cc kra32.at kra33.cc kra33.at kra34.cc kra34.at
Потому что здесь всё про вас: ваш стиль, ваш комфорт, ваша выгода. Попробуйте, и вы поймёте, почему так много людей выбирают именно эту платформу!
https://kr33.shop/
Советы по безопасности на Кракене
Будьте внимательны к продавцам
Читайте отзывы и выбирайте проверенных продавцов с высоким рейтингом.
кракен маркетплейс
кракен маркетплейс
кракен площадка
кракен маркетплейс ссылка
кракен площадка
кракен marketplace
кракен площадка ссылка
кракен даркнет стор
кракен darknet market зеркало
кракен даркнет площадка
кракен даркнет маркетплейс
кракен наркотики
кракен нарко
кракен наркошоп
кракен наркота
кракен порошок
кракен наркотики
кракен что там продают
кракен маркетплейс что продают
кракен покупка
кракен купить
кракен купить мяу
кракен питер
кракен в питере
кракен москва
кракен в москве
кракен что продают
кракен это
кракен market
кракен darknet market
кракен dark market
кракен market ссылка
кракен darknet market ссылка
кракен market ссылка тор
кракен даркнет маркет
кракен market тор
кракен маркет
платформа кракен
кракен торговая площадка
кракен даркнет маркет ссылка сайт
кракен маркет даркнет тор
кракен аккаунты
кракен заказ
диспуты кракен
как восстановить кракен
кракен даркнет не работает
как пополнить кракен
google authenticator кракен
рулетка кракен
купоны кракен
кракен зарегистрироваться
кракен регистрация
кракен пользователь не найден
кракен отзывы
ссылка кракен
кракен официальная ссылка
кракен ссылка на сайт
кракен официальная ссылка
кракен актуальные
кракен ссылка тор
кракен клирнет
кракен ссылка маркет
кракен клир ссылка
кракен ссылка
ссылка на кракен
кракен ссылка
кракен ссылка на сайт
кракен ссылка кракен
актуальная ссылка на кракен
рабочие ссылки кракен
кракен тор ссылка
ссылка на кракен тор
кракен зеркало тор
кракен маркет тор
кракен tor
кракен ссылка tor
кракен тор
кракен ссылка тор
кракен tor зеркало
кракен darknet tor
кракен тор браузер
кракен тор
кракен darknet ссылка тор
кракен ссылка на сайт тор
кракен вход на сайт
кракен вход
кракен зайти
кракен войти
кракен даркнет вход
кракен войти
где найти ссылку кракен
где взять ссылку на кракен
как зайти на сайт кракен
как найти кракен
кракен новый
кракен не работает
кракен вход
как зайти на кракен
кракен вход ссылка
сайт кракен
кракен сайт
кракен сайт что это
кракен сайт даркнет
что за сайт кракен
кракен что за сайт
кракен официальный сайт
сайт кракен отзывы
кракен сайт
кракен официальный сайт
сайт кракен тор
кракен сайт ссылка
кракен сайт зеркало
кракен сайт тор ссылка
кракен зеркало сайта
зеркало кракен
адрес кракена
кракен зеркало тор
зеркало кракен даркнет
актуальное зеркало кракен
рабочее зеркало кракен
кракен зеркало
кракен зеркала
кракен зеркало
зеркало кракен market
актуальное зеркало кракен
кракен дарк
кракен darknet
кракен даркнет ссылка
ссылка кракен даркнет маркет
кракен даркнет
кракен darknet
кракен даркнет
кракен dark
кракен darknet ссылка
кракен сайт даркнет маркет
кракен даркнет маркет ссылка тор
кракен даркнет тор
кракен текст рекламы
кракен реклама
реклама кракен москва сити
реклама наркошопа кракен
кракен гидра
кракен реклама
реклама кракен на арбате
кракен даркнет реклама
кракен скачать
кракен это
кракен это современный
только через торрент кракен
только через кракен
только через тор кракен
кракен даркнет только через
кракен академия
кракен academy
кракен обучение
кракен курсы
кракен bot
академия кракен
кракен работа
кракен форум
кракен новости
кракен телеграмм
wayaway кракен
кракен магазин
кракен магазин
площадка кракен
площадка кракен
кракен shop
кракен store
кракен шоп
кракен qr код
кракен кьюар код
qr кракен
qr код кракен
кракен логотип
кракен лого
кракен logo
кракен переходник
пин ап загрузка на андроид пин ап загрузка на андроид
Мы готовы предложить дипломы любой профессии по приятным тарифам. Дипломы производят на подлинных бланках Приобрести диплом ВУЗа diplomc-v-ufe.ru
кредит с испорченой кредитной историей кредит с испорченой кредитной историей .
Мы изготавливаем дипломы любой профессии по доступным тарифам. Мы можем предложить документы ВУЗов, расположенных на территории всей России. Дипломы и аттестаты делаются на “правильной” бумаге высшего качества. Это дает возможность делать настоящие дипломы, которые невозможно отличить от оригиналов. orikdok-3v-gorode-izhevsk-18.online
site officiel 1win site officiel 1win
займ 50000 на 12 месяцев займ 50000 на 12 месяцев
пин ап загрузка на айфон http://pinup3011.ru
pin up yangi bonus http://www.pinup3012.ru
1win deposit http://1win3004.com/
“Sst bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > holiganbet“
Мы изготавливаем дипломы любой профессии по доступным тарифам. Купить диплом в Амурской области и городе Благовещенск — kyc-diplom.com/geography/blagoveshchensk.html
Кто оформлял медицинский диплом на заказ — проверяют ли? Посмотреть информацию
В 2025 году наличие диплома всё ещё остаётся основным фактором при приёме на работу, повышении по службе или получении лицензии. И если у вас нет необходимого документа — это не повод терять годы.
? Решение есть — оформление диплома на заказ, полностью соответствующего оригиналу:
С печатями, подписями, голограммами,
С занесением в архив (по запросу),
Любой ВУЗ, колледж — по всей России и СНГ.
?? Кому может пригодиться?
Вас выгнали, но обучение практически завершено?
Нашли перспективную работу, но нет “корочки”?
Нужен диплом для лицензирования, повышения, тендера?
Мы работаем без предоплаты (по договору или поэтапно) и гарантируем полную конфиденциальность. У нас нет шаблонов — каждый документ готовится индивидуально, с учётом всех нюансов.
?? Что вы получаете:
Реалистичный диплом, неотличимый от оригинала
Настоящие данные выпускника (по вашей анкете)
Быстрая доставка по России и СНГ
Юридически грамотно оформленный договор (по желанию)
Мы сотрудничаем с квалифицированными специалистами, которые знают, как должен выглядеть официальный документ — вплоть до мельчайших деталей. У нас много лет опыта и более 2554 довольных клиентов.
pin up jonli yordam pin up jonli yordam
?? У кого в дипломе хоть раз была правда?
Вот реально: вы когда-нибудь смотрели свой диплом и думали — “да, всё, что здесь написано, я действительно знаю и умею”?
У многих — только сертификат. Корочка, глянцевая, с гербом и подписями, которую HR пролистывает за 1,5 секунды. А потом спрашивают: опыт, кейсы, компетенции, “что умеешь по факту?”. Диплом где-то там, в глубокой папке.
Но реалия в том, что без диплома тебе даже не дадут шанс доказать, что ты умеешь.
Ты можешь быть хорошим специалистом, уметь в IT, дизайн, управление, логистику — но без документа с золотым тиснением в кабинет не пустят.
?? Нормально ли это? Нет. Реальность ли это? Да.
Вот потому и появляются услуги, которые дают предложение:
“Не хочешь тратить 5 лет ради корочки? Мы решим вопрос. Тебе нужен не вуз — тебе нужен диплом.”
Ты его получаешь, кладёшь в резюме, и дальше всё зависит от твоих мозгов, а не от шрифта на бумаге.
Кто-то скажет: “Это обман!”
А кто-то — “Это адаптация к системе, которая обманывает тебя с детства”.
?? И что в итоге?
Диплом становится не подтверждением знаний, а входным билетом. Как QR-код в метро — проверили, что есть, и пропустили.
Поэтому люди и покупают.
Не потому что глупые. А потому что взрослые, занятые, уставшие от лишнего.
Потому что хотят не учиться “ради процесса”, а работать по делу.
?? Ирония в том, что большинство таких дипломов — работают.
Даже если ты их не учил — ты знаешь, как применить. А вот “настоящие выпускники” потом всё равно идут на курсы и стажировки, потому что ничего не помнят.
И что важнее: корочка или то, как ты справляешься с задачей?
?? У кого были такие мысли — пишите. У кого был опыт — делитесь.
Можно ли купить диплом для трудоустройства за границей? Сайт компании
интересные кашпо http://www.dizaynerskie-kashpo.ru .
Умные рулонные шторы для вашего дома, в вашем пространстве.
Выберите рулонные шторы с электроприводом, для вашего удобства.
Рулонные шторы с электроприводом: удобство и стиль, представлены.
Умные рулонные шторы, качества и стиля.
Изучите мир электрических рулонных штор, смотрите.
Умные рулонные шторы для дома, добавят стиля.
Электроприводные рулонные шторы: инновации в вашем доме, выбор, который стоит сделать.
Рулонные шторы с электроприводом для легкого управления, познакомьтесь с нашими предложениями.
Электроприводные рулонные шторы: ваш лучший выбор, в каталоге товаров.
Умные рулонные шторы: удобство и стиль, которое вы заслуживаете.
Современные рулонные шторы с электроприводом, с возможностью установки.
Рулонные шторы с электроприводом: функциональность и стиль, выбор, на который стоит обратить внимание.
Электроприводные рулонные шторы: удобство управления одним нажатием, в вашем доме.
Умные рулонные шторы: будущее уже здесь, нажмите для подробностей.
Электрические рулонные шторы – стильный выбор, откройте для себя.
Рулонные шторы с электроприводом – вашим окнам это нужно, не упустите свой шанс.
Электрические рулонные шторы: легкость управления, покупайте у нас.
Преимущества рулонных штор с электроприводом, закажите прямо сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
sweet bonanza 100 sweet bonanza 100
Быстрая доставка и отзывчивые менеджеры.
гипсофилы цена букета
выравнивание участка дренаж .
Заказать диплом любого университета!
Приобретение документа о высшем образовании через надежную компанию дарит ряд достоинств. Заказать диплом об образовании у надежной организации: doks-v-gorode-moskva-77.ru
Мы готовы предложить документы институтов, расположенных в любом регионе РФ. Купить диплом любого ВУЗа:
funnywipes.maxbb.ru/viewtopic.php?f=3&t=1215
пин ап авиатор взлом https://www.pinup3009.ru
mostbet apk android https://mostbet1.app .
????? ?????? 888starz ????? ?????? 888starz .
Заказать диплом на заказ вы можете используя официальный портал компании. orikdok-4v-gorode-bryansk-32.online
Рулонные шторы с электроприводом, инновации и дизайн.
Электроприводные рулонные шторы для вашего окна, интеллектуальное решение.
Рулонные шторы с электроприводом: удобство и стиль, у нас.
Умные рулонные шторы, превосходное сочетание.
Наслаждайтесь комфортом рулонных штор с электроприводом, ознакомьтесь.
Электрические рулонные шторы – удобство и стиль, создадут уют.
Электрические рулонные шторы: комфорт и уют, решение для современного дизайна.
Рулонные шторы с электроприводом для легкого управления, откройте для себя.
Сделайте свой дом умнее с рулонными шторами, на нашем сайте.
Умные рулонные шторы: удобство и стиль, для вашего комфорта.
Преобразите ваш дом с рулонными шторами с электроприводом, с возможностью установки.
Рулонные шторы с электроприводом: функциональность и стиль, выбор, на который стоит обратить внимание.
Рулонные шторы с электроприводом – ваш идеальный помощник, каждый день.
Умные рулонные шторы: будущее уже здесь, нажмите для подробностей.
Электрические рулонные шторы – стильный выбор, попробуйте и оцените.
Рулонные шторы с электроприводом – вашим окнам это нужно, не упустите свой шанс.
Умные рулонные шторы – ваш идеальный выбор, покупайте у нас.
Преимущества рулонных штор с электроприводом, узнайте больше.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
недорогая еда с доставкой http://www.dostavka-edy-bf2.ru .
доставка еды ночью челябинск http://www.dostavka-edy-bf.ru .
заказать еду с доставкой на дом http://dostavka-edy-bf1.ru .
Заказать документ о получении высшего образования вы можете у нас. Купить диплом института по доступной стоимости возможно, обратившись к проверенной специализированной компании. workompass.com/employer/frees-diplom
аппарат для узи аппарат для узи .
“Hey forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > jojobetorijinal.com“
1win скачать android https://1win14041.ru/
télécharger 1win apk http://1win3001.com/
Убеждения ничего не стоят, если не подкрепляются делами. https://professor-proton.citaty-tsitaty.ru
доставка еды на месяц https://dostavka-edy-bf2.ru/ .
доставка еды меню http://dostavka-edy-bf.ru .
Приобрести диплом ВУЗа по невысокой цене возможно, обращаясь к проверенной специализированной компании. Заказать документ о получении высшего образования можно у нас в Москве. orikdok-2v-gorode-mahachkala-5.ru
mostbet uz promo kod http://mostbet4003.ru
дизайнерские кашпо для цветов купить дизайнерские кашпо для цветов купить .
где купить узи аппарат https://kupit-uzi-apparat.ru/ .
Приобрести диплом ВУЗа по выгодной стоимости можно, обращаясь к надежной специализированной фирме. Приобрести диплом: infiniterealities.listbb.ru/viewtopic.phpf=7&t=1348
1win betting https://www.1win3005.com
Выгодно заказать диплом о высшем образовании!
Мы предлагаем дипломы любой профессии по разумным тарифам— aviadir.ru
Мы изготавливаем дипломы любой профессии по выгодным ценам. Мы предлагаем документы техникумов, расположенных на территории всей России. Документы выпускаются на “правильной” бумаге самого высшего качества. Это дает возможности делать государственные дипломы, которые невозможно отличить от оригинала. orikdok-4v-gorode-samara-63.ru
мостбет зеркало узбекистан https://www.mostbet4002.ru
Купить диплом об образовании. Покупка подходящего диплома через надежную компанию дарит ряд достоинств. Это решение помогает сберечь как продолжительное время, так и серьезные финансовые средства. orikdok-4v-gorode-orenburg-56.online
первый займ под 0 процентов первый займ под 0 процентов
купить диплом вуза http://arus-diplom3.ru/ .
Элегантные решения, автоматизированные рулонные шторы, с помощью пульта.
Эстетика и функциональность, для любого интерьера, долговечность и надежность.
Добавьте комфорта, с рулонными шторами с электроприводом, легко и быстро.
Выбор рулонных штор, посетите наш сайт.
Электрические шторы, это красиво, добавьте стиля.
Рулонные шторы с электроприводом, для современных решений, закажите с доставкой.
Автоматизация вашего дома, инновации в вашем интерьере, узнайте больше.
Эстетические шторы с электроприводом, удобное управление, получите консультацию.
Шторы, с которыми легко управлять, высокая функциональность, доступно всем.
Рулонные шторы с дистанционным управлением, для каждого дома, узнайте больше.
Электрические шторы с пультом, инновации для вашего дома, узнайте об акциях.
Электроприводные рулонные шторы, на нашем сайте.
Создавайте атмосферу, с дистанционно управляемыми шторами, это легко.
Электрические шторы для вашего интерьера, высокое качество, закажите онлайн.
Умный дом с рулонными шторами, современные технологии, заказ с доставкой.
Современные решения для ваших окон, простой и удобный выбор, закажите с доставкой.
Удобные рулонные шторы, доступные цены, выбор за вами.
Рулонные шторы для вашего дома, для стильного интерьера, получите консультацию.
рулонные шторы с электроприводом и дистанционным управлением рулонные шторы с электроприводом и дистанционным управлением .
mostbet app install [url=http://www.mostbet1.app]http://www.mostbet1.app[/url] .
Рулонные шторы с электроприводом, удобство и стиль.
Современные рулонные шторы с электрическим управлением, максимальный комфорт.
Рулонные шторы с электроприводом: удобство и стиль, доступны.
Электроприводные рулонные шторы, комфорта и эстетики.
Наслаждайтесь комфортом рулонных штор с электроприводом, смотрите.
Электрические рулонные шторы – удобство и стиль, создадут уют.
Электрические рулонные шторы: комфорт и уют, выбор, который стоит сделать.
Умные рулонные шторы – ваш новый помощник, узнайте больше.
Рулонные шторы с электроприводом – элегантность и удобство, в каталоге товаров.
Умные рулонные шторы: удобство и стиль, для вашего комфорта.
Электрические рулонные шторы – стиль и технология, предлагаемые с доставкой.
Электрические рулонные шторы для любого интерьера, выбор, который вы не пожалеете.
Рулонные шторы с электроприводом – ваш идеальный помощник, в вашем доме.
Умные рулонные шторы: будущее уже здесь, нажмите для подробностей.
Умные рулонные шторы для вашего удобства, попробуйте и оцените.
Рулонные шторы с электроприводом – вашим окнам это нужно, позаботьтесь о себе.
Рулонные шторы с электроприводом для вашего комфорта, выбирайте лучшее.
Электроприводные рулонные шторы для стильного дома, закажите прямо сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
888starz en ligne beautynewsindia.com .
Можно ли купить диплом дистанционно, без приезда и личных встреч — полностью онлайн? Мы поможем
В 2025 году наличие диплома всё ещё остаётся основным фактором при приёме на работу, повышении по карьерной лестнице или получении лицензии. И если у вас нет нужного документа — это не повод терять годы.
? Выход есть — покупка диплома, полностью соответствующего оригиналу:
С печатями, подписями, голограммами,
Внесение в архив (по запросу),
Любой ВУЗ, колледж — по всей России и СНГ.
?? Кому может пригодиться?
Вас отчислили, но обучение практически завершено?
Нашли перспективную работу, но нет “корочки”?
Нужен диплом для лицензирования, повышения, тендера?
Мы работаем без предоплаты (по договору или поэтапно) и гарантируем полную конфиденциальность. У нас нет шаблонов — каждый документ готовится индивидуально, с учётом всех нюансов.
?? Что вы получаете:
Реалистичный диплом, неотличимый от оригинала
Настоящие данные выпускника (по вашей анкете)
Быстрая и надежная доставка по России и СНГ
Юридически грамотно оформленный договор (по желанию)
Мы сотрудничаем с квалифицированными специалистами, которые знают, как должен выглядеть официальный документ — вплоть до мельчайших деталей. У нас много лет опыта и более 1958 довольных клиентов.
пин ап пополнение счёта http://pinup3013.ru
Купить диплом университета по доступной стоимости можно, обращаясь к надежной специализированной фирме. Мы готовы предложить документы любых учебных заведений, расположенных в любом регионе РФ. studentklass.ru/zanosim-diplom-v-reestr-obrazovaniya-legko-i-bistro/
Balanset-1A: Cutting-edge Mobile Balancer & Vibration Analyzer
Professional Dynamic Balancing Solution
Balanset-1A constitutes an groundbreaking solution for dynamic balancing of rotors in their own bearings, engineered by Estonian company Vibromera OU. The device delivers professional equipment balancing at €1,751, which is significantly cheaper than traditional vibration analyzers while retaining superior measurement accuracy. The system allows in-place balancing directly at the equipment’s installation site without requiring disassembly, which is vital for preventing production downtime.
About the Manufacturer
Vibromera OU is an Estonian company focusing in the development and manufacturing of devices for technical diagnostics of industrial equipment. The company is established in Estonia (registration number 14317077) and has offices in Portugal.
Contact Information:
Official website: https://vibromera.eu/shop/2/
Technical Specifications
Measuring Parameters
Balanset-1A provides accurate measurements using a two-channel vibration analysis system. The device measures RMS vibration velocity in the range of 0-80 mm/s with an accuracy of ±(0.1 + 0.1?Vi) mm/s. The operating frequency range is 5-550 Hz with possible extension to 1000 Hz. The system supports rotational speed measurement from 250 to 90,000 RPM with phase angle determination accuracy of ±1 degree.
Working Principle
The device employs phase-sensitive vibration measurement technology with MEMS accelerometers ADXL335 and laser tachometry. Two mono-directional accelerometers measure mechanical oscillations proportional to acceleration, while a laser tachometer generates impulse signals for calculating rotation frequency and phase angle. Digital signal processing includes FFT analysis for frequency analysis and custom algorithms for automatic computation of correction masses.
Full Kit Contents
The standard Balanset-1A delivery includes:
Measurement unit with USB interface – central module with integrated preamplifiers, integrators, and ADC
2 vibration sensors (accelerometers) with 4m cables (optionally 10m)
Optical sensor (laser tachometer) with 50-500mm measuring distance
Magnetic stand for sensor mounting
Electronic scales for exact measurement of corrective masses
Software for Windows 7-11 (32/64-bit)
Plastic transport case
Complete set of cables and documentation
Performance Capabilities
Vibrometer Mode
Balanset-1A functions as a complete vibration analyzer with abilities for measuring overall vibration level, FFT spectrum analysis up to 1000 Hz, determining amplitude and phase of the fundamental frequency (1x), and continuous data recording. The system delivers visualization of time signals and spectral analysis for equipment condition diagnostics.
Balancing Mode
The device supports one-plane (static) and dual-plane (dynamic) balancing with automatic calculation of correction masses and their installation angles. The unique influence coefficient saving function allows significant acceleration of follow-up balancing of similar equipment. A specialized grinding wheel balancing mode uses the three-correction-weight method.
Software
The easy-to-use program interface provides step-by-step guidance through the balancing process, making the device accessible to personnel without specific training. Key functions include:
Automatic tolerance calculation per ISO 1940
Polar diagrams for imbalance visualization
Result archiving with report generation capability
Metric and imperial system support
Multilingual interface (English, German, French, Polish, Russian)
Application Areas and Equipment Types
Industrial Equipment
Balanset-1A is effectively employed for balancing fans (centrifugal, axial), pumps (hydraulic, centrifugal), turbines (steam, gas), centrifuges, compressors, and electric motors. In manufacturing facilities, the device is used for balancing grinding wheels, machine spindles, and drive shafts.
Agricultural Machinery
The device represents special value for agriculture, where reliable operation during season is critically important. Balanset-1A is used for balancing combine threshing drums, shredders, mulchers, mowers, and augers. The ability to balance on-site without equipment disassembly permits avoiding costly downtime during critical harvest periods.
Specialized Equipment
The device is effectively used for balancing crushers of various types, turbochargers, drone propellers, and other high-speed equipment. The RPM frequency range from 250 to 90,000 RPM covers essentially all types of industrial equipment.
Advantages Over Competitors
Economic Efficiency
At a price of €1,751, Balanset-1A provides the functionality of devices costing €10,000-25,000. The investment pays for itself after preventing just 2-3 bearing failures. Cost reduction on third-party balancing specialist services totals thousands of euros annually.
Ease of Use
Unlike complex vibration analyzers requiring months of training, mastering Balanset-1A takes 3-4 hours. The step-by-step guide in the software enables professional balancing by personnel without special vibration diagnostics training.
Portability and Autonomy
The complete kit weighs only 4 kg, with power supplied through the laptop’s USB port. This permits balancing in outdoor conditions, at remote sites, and in inaccessible locations without external power supply.
Universal Application
One device is suitable for balancing the widest spectrum of equipment – from small electric motors to large industrial fans and turbines. Support for one and dual-plane balancing covers all standard tasks.
Real Application Results
Drone Propeller Balancing
A user achieved vibration reduction from 0.74 mm/s to 0.014 mm/s – a 50-fold improvement. This demonstrates the remarkable accuracy of the device even on small rotors.
Shopping Center Ventilation Systems
Engineers effectively balanced radial fans, achieving decreased energy consumption, eliminated excessive noise, and prolonged equipment lifespan. Energy savings paid for the device cost within several months.
Agricultural Equipment
Farmers note that Balanset-1A has become an indispensable tool preventing costly breakdowns during peak season. Decreased vibration of threshing drums led to lower fuel consumption and bearing wear.
Cost and Delivery Terms
Current Prices
Complete Balanset-1A Kit: €1,751
OEM Kit (without case, stand, and scales): €1,561
Special Offer: €50 discount for newsletter subscribers
Volume Discounts: up to 15% for orders of 4+ units
Acquisition Options
Official Website: vibromera.eu (recommended)
eBay: certified sellers with 100% rating
Industrial Distributors: through B2B channels
Payment and Shipping Terms
Payment Methods: PayPal, bank cards, bank transfer
Shipping: 10-20 business days by international mail
Shipping Cost: from $10 (economy) to $95 (express)
Warranty: manufacturer’s warranty
Technical Support: included in price
Conclusion
Balanset-1A constitutes an ideal solution for organizations seeking to implement an efficient equipment balancing system without major capital expenditure. The device democratizes access to professional balancing, enabling small companies and service centers to offer services at the level of large industrial companies.
The blend of affordable price, ease of use, and professional capabilities makes Balanset-1A an essential tool for modern technical maintenance. Investment in this device is an investment in equipment stability, reduced operating costs, and improved competitiveness of your enterprise.
jocuri de grup online jocuri de grup online
“Dikkat forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > Marsbahis“
Быстро и просто заказать диплом о высшем образовании. Приобретение диплома через проверенную и надежную компанию дарит массу достоинств для покупателя. Такое решение дает возможность сберечь как длительное время, так и значительные финансовые средства. orikdok-v-gorode-izhevsk-18.ru
Современные технологии, в вашем интерьере, с помощью пульта.
Современный дизайн, электрические рулонные шторы, доступные цены.
Управляйте светом, с рулонными шторами с электроприводом, с удовольствием.
Электропривод для вашего уюта, узнайте здесь.
Рулонные шторы для современного дома, это красиво, добавьте стиля.
Шторы, которые управляются дистанционно, для вашего удобства, закажите с доставкой.
Электрические рулонные шторы, высокая функциональность, сделайте правильный выбор.
Шторы для умного дома, высокое качество, закажите сегодня.
Шторы, с которыми легко управлять, высокая функциональность, в наличии сейчас.
Автоматизированные шторы, просто и удобно, обратитесь к нам.
Автоматические рулонные шторы, стиль и комфорт, закажите с нашим менеджером.
Электроприводные рулонные шторы, на нашем сайте.
Создавайте атмосферу, с дистанционно управляемыми шторами, это удобно.
Электрические шторы для вашего интерьера, широкий ассортимент, закажите онлайн.
Умный дом с рулонными шторами, современные технологии, консультация специалиста.
Электрические шторы для любого интерьера, у нас есть всё, узнайте больше.
Электрические шторы для современных людей, высокое качество, обратитесь к нам.
Современные рулонные шторы, для стильного интерьера, узнайте цены.
рулонные шторы с электроприводом и дистанционным управлением рулонные шторы с электроприводом и дистанционным управлением .
mostbet uz skachat http://www.mostbet4002.ru
пластиковые окна от производителя москва пластиковые окна от производителя москва .
купить пластиковые окна купить пластиковые окна .
купить аттестат в москве за 11 класс купить аттестат в москве за 11 класс .
Вы приобретаете документ через надежную фирму. Приобрести диплом о высшем образовании– http://temtehnika.ru/kupit-diplom-instituta-s-reestrom-bistro-i-bezopasno/ – temtehnika.ru/kupit-diplom-instituta-s-reestrom-bistro-i-bezopasno/
?? У кого в дипломе хоть раз была правда?
Серьезно: вы когда-нибудь открывали свой диплом и думали — “да, всё, что здесь написано, я действительно знаю и умею”?
У многих — только сертификат. Корочка, глянцевая, с гербом и подписями, которую HR смотрит за 1,5 секунды. А потом начинается: опыт, кейсы, компетенции, “что умеешь по факту?”. Диплом где-то там, в мнимом мире.
Но реалия в том, что без диплома тебе даже не дадут шанс доказать, что ты умеешь.
Ты можешь быть крутым специалистом, уметь в IT, дизайн, управление, логистику — но без документа с золотым тиснением не попадешь на собеседование.
?? Нормально ли это? Нет. Реальность ли это? Да.
Вот потому и появляются сервисы, которые говорят:
“Не хочешь тратить 5 лет ради корочки? Мы решим вопрос. Тебе нужен не вуз — тебе нужен диплом.”
Ты его получаешь, кладёшь в резюме, и дальше всё зависит от твоих мозгов, а не от шрифта на бумаге.
Кто-то скажет: “Это обман!”
А кто-то — “Это адаптация к системе, которая обманывает тебя с детства”.
?? И что в итоге?
Диплом становится не подтверждением знаний, а входным билетом. Как QR-код в метро — проверили, что есть, и пропустили.
Поэтому люди и покупают.
Не потому что глупые. А потому что взрослые, занятые, уставшие от лишнего.
Потому что хотят не учиться “ради процесса”, а работать по делу.
?? Ирония в том, что большинство таких дипломов — работают.
Даже если ты их не учил — ты знаешь, как применить. А вот “настоящие выпускники” потом всё равно идут на курсы и стажировки, потому что ничего не помнят.
И что важнее: корочка или то, как ты справляешься с задачей?
?? У кого были такие мысли — пишите. У кого был опыт — делитесь.
Кто оформлял диплом через интернет и получил на руки? Прямо сейчас
купить аппарат узи цены https://www.kupit-uzi-apparat2.ru .
узи оборудование купить https://kupit-uzi-apparat1.ru/ .
аппарат узи купить цена аппарат узи купить цена .
изящное кашпо http://dizaynerskie-kashpo1.ru/ .
sweet bonanza 100 https://sweet-bonanza3003.ru
mostbet uz скачать mostbet4003.ru
Клиент вносит позитивное выражение каждой энергии в ситуацию CRASH. [Пирамида уровней Дилтса-Короткова –
Мы можем предложить документы ВУЗов, которые расположены на территории всей России. Заказать диплом о высшем образовании:
tweecampus.com/read-blog/195524_диплом-с-внесением-в-реестр.html
drenazh-vokrug-doma-812.ru .
1win withdrawal http://1win3009.com
Круглосуточная доставка алкоголя, подробнее тут доставка пива москва
Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов РФ. Документы производят на подлинных бланках. o91707v5.beget.tech/2025/06/06/diplomy-kotorye-cenyat-po-dostoinstvu.html
UID_92113907###
test
UID_96709532###
test
I’ve been using sativa tincture daily on account of during the course of a month nowadays, and I’m indubitably impressed before the absolute effects. They’ve helped me judge calmer, more balanced, and less tense in every nook the day. My snore is deeper, I wake up refreshed, and sober my nave has improved. The value is distinguished, and I cherish the natural ingredients. I’ll obviously heed buying and recommending them to everyone I know!
como funciona 1win como funciona 1win
UID_77088328###
test
UID_58198549###
test
UID_22300253###
test
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
UID_67047504###
test
1win http://1win3005.com/
UID_12494801###
test
Заказать диплом под заказ возможно через официальный сайт компании. orikdok-1v-gorode-groznyy-20.ru
UID_34163559###
test
ультразвуковой диагностический аппарат ультразвуковой диагностический аппарат .
UID_99048383###
test
UID_19551427###
test
узи аппарат цена новый http://www.kupit-uzi-apparat1.ru .
Использование слов «я», «мне», «мое». Восприятие «во времени» включает физическое присутствие в событии. Пример использования генеративного формата НЛП. Универсальная метамодель пирамида Короткова.
Упражнение: активное сновидение. Зеркальные нейроны помогают создавать «морфогенетический резонанс», необходимый для активации базовых человеческих способностей. Пирамида логических уровней Короткова.
UID_72964640###
test
цена аппарата узи http://www.kupit-uzi-apparat3.ru/ .
UID_92196526###
test
UID_55033802###
test
Предлагаем услуги профессиональных инженеров офицальной мастерской.
Еслли вы искали ремонт iphone 14 pro рядом, можете посмотреть на сайте: ремонт iphone 14 pro
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
UID_80196693###
test
UID_47338578###
test
Сервисный центр предлагает центр ремонта кофемашины baumatic починка кофемашин baumatic
UID_91537226###
test
UID_27646717###
test
UID_87886178###
test
UID_84930657###
test
Нейро-логические уровни. Пирамида Дилтса-Короткова. Логические уровни 2.0
UID_72591606###
test
осушение участка .
Рулонные шторы с электроприводом, комфорт и элегантность.
Электроприводные рулонные шторы для вашего окна, легкость управления.
Умные шторы: удобство на кончиках пальцев, в каталоге.
Умные рулонные шторы, комфорта и эстетики.
Время менять окна на рулонные шторы с электроприводом, смотрите.
Умные рулонные шторы для дома, подходят для любого окна.
Стильные рулонные шторы с электроприводом, решение для современного дизайна.
Электрические рулонные шторы: свобода выбора, познакомьтесь с нашими предложениями.
Рулонные шторы с электроприводом – элегантность и удобство, на нашем сайте.
Умные рулонные шторы: удобство и стиль, которое вам понравится.
Современные рулонные шторы с электроприводом, доступные для заказа.
Умные рулонные шторы – правильно решайте, выбор, на который стоит обратить внимание.
Электроприводные рулонные шторы: удобство управления одним нажатием, в вашем доме.
Электрические рулонные шторы для стиля и комфорта, нажмите для подробностей.
Рулонные шторы с электроприводом: комфорт на новом уровне, откройте для себя.
Умные рулонные шторы с электроприводом для вашего дома, позаботьтесь о себе.
Рулонные шторы с электроприводом для вашего комфорта, выбирайте лучшее.
Преимущества рулонных штор с электроприводом, закажите прямо сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
Круглосуточная доставка алкоголя, подробнее тут доставка алкоголя москва круглосуточно
mostbet uz kirish mostbet uz kirish
Заказать документ о получении высшего образования можно у нас в столице. Купить диплом университета по выгодной цене можно, обратившись к надежной специализированной фирме. c90226sl.beget.tech/2025/05/13/luchshie-varianty-kupit-diplom-s-oficzialnoj-pechatyu
register 1win [url=http://1win3009.com/]http://1win3009.com/[/url]
Мы готовы предложить документы любых учебных заведений, которые расположены в любом регионе России. Купить диплом о высшем образовании:
freelancealie.com/profile/cleohomburg47
мостеб http://mostbet4011.ru
Вентиляция помещений http://garry91.free.fr/index.php?file=Members&op=detail&autor=yragul
http://mokolis.ru/index.php?subaction=userinfo&user=uqadupy
http://anciensdugenerals.free.fr/index.php?file=Members&op=detail&autor=okuxedam
http://fh3809p1.bget.ru/index.php?subaction=userinfo&user=ebucoliku
http://atraxteam.free.fr/index.php?file=Members&op=detail&autor=iguwaq
http://podzemie.6f.sk/profile.php?lookup=23015
http://montybb13.free.fr/index.php?file=Members&op=detail&autor=amovahi
http://samsoberi.ru/index.php?subaction=userinfo&user=apudyfyz
http://ips-irk.ksworks.ru/index.php?subaction=userinfo&user=ycojujak
http://fruit-impex.by/index.php?subaction=userinfo&user=agojutyn
mostbet to‘lov usullari uz http://www.mostbet4001.ru
чит кс 1.6 – купить чит для ксго, скачать приватный чит для кс 1.6
Купить диплом можно через официальный портал компании. orikdok-5v-gorode-syktyvkar-11.ru
Мы предлагаем дипломы любых профессий по выгодным ценам. Цена будет зависеть от выбранной специальности, года получения и университета: mir.4admins.ru/ucp.php?mode=login
pinup pinup
читы на cs go – SCP Secret Laboratory читы, читы cs go приватные
Мы предлагаем дипломы любой профессии по доступным тарифам. Для нас важно, чтобы дипломы были доступны для большинства граждан. Приобрести диплом института generalarminius.com/viewtopic.php?t=300121
большие дизайнерские кашпо [url=https://dizaynerskie-kashpo1.ru]большие дизайнерские кашпо[/url] .
Мы можем предложить дипломы психологов, юристов, экономистов и других профессий по невысоким ценам. Мы предлагаем документы ВУЗов, расположенных в любом регионе России. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это дает возможность делать государственные дипломы, которые невозможно отличить от оригиналов. orikdok-v-gorode-ufa-2.online
legit cheat for csgo – cheat Apex Legends, Чит Counter Strike 2
Электрические рулонные шторы, инновации и дизайн.
Современные рулонные шторы с электрическим управлением, для современного дома.
Электрические рулонные шторы для вашего дома, у нас.
Электроприводные рулонные шторы, комфорта и эстетики.
Изучите мир электрических рулонных штор, смотрите.
Рулонные шторы с электроприводом для вашего интерьера, добавят стиля.
Электрические рулонные шторы: комфорт и уют, решение для современного дизайна.
Умные рулонные шторы – ваш новый помощник, познакомьтесь с нашими предложениями.
Рулонные шторы с электроприводом – элегантность и удобство, на нашем сайте.
Электрические рулонные шторы: удобство на каждом окне, которое вы заслуживаете.
Преобразите ваш дом с рулонными шторами с электроприводом, доступные для заказа.
Умные рулонные шторы – правильно решайте, выбор, на который стоит обратить внимание.
Умные рулонные шторы для современного дома, в вашем доме.
Умные рулонные шторы: будущее уже здесь, выберите свои.
Рулонные шторы с электроприводом: комфорт на новом уровне, сделайте свой дом комфортнее.
Умные рулонные шторы с электроприводом для вашего дома, пополните свой интерьер.
Рулонные шторы с электроприводом для вашего комфорта, покупайте у нас.
Рулонные шторы с электроприводом: удобно и красиво, закажите прямо сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
Дарвин использовал прогулки по песчаной дорожке для размышлений. Пирамида логических уровней 2.0 Короткова.
Приобрести документ о получении высшего образования можно у нас. Купить диплом ВУЗа по выгодной цене возможно, обращаясь к проверенной специализированной компании. drahthaar-forum.ru/viewtopic.phpf=30&t=13225
диплом купить диплом купить .
sweet bonanza 1xbet sweet-bonanza3003.ru
Запреты дня http://www.n2000.ru .
пк игровой http://kupit-igrovoj-kompyuter.ru/ .
компьютер для монтажа видео kupit-igrovoj-kompyuter1.ru .
“Dikkat bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > betpark2025.net“
Современные технологии, в вашем офисе, с помощью пульта.
Идеальное сочетание, для любого интерьера, высокое качество.
Добавьте комфорта, с автоматическими шторами, с удовольствием.
Выбор рулонных штор, почему стоит выбрать?.
Рулонные шторы для современного дома, это удобно, наслаждайтесь комфортом.
Рулонные шторы с электроприводом, для вашего удобства, попробуйте прямо сейчас.
Электрические рулонные шторы, высокая функциональность, узнайте больше.
Эстетические шторы с электроприводом, высокое качество, получите консультацию.
Шторы, с которыми легко управлять, отличный выбор, в наличии сейчас.
Инновации в дизайне интерьера, просто и удобно, посмотрите наш ассортимент.
Электрические шторы с пультом, управление светом из любой точки, узнайте об акциях.
Электроприводные рулонные шторы, где купить?.
Создавайте атмосферу, с рулонными шторами с электроприводом, это удобно.
Современные шторы с пультом, лучшие цены, узнайте подробности.
Электрические шторы с дистанционным управлением, удобство для вас, заказ с доставкой.
Выбор рулонных штор с электроприводом, надежность и стиль, посетите наш сайт.
Электрические шторы для современных людей, высокое качество, закажите онлайн.
Современные рулонные шторы, для стильного интерьера, узнайте цены.
рулонные шторы с электроприводом и дистанционным управлением рулонные шторы с электроприводом и дистанционным управлением .
купить игровой компьютер купить игровой компьютер .
купить диплом о среднем образовании в москве http://arus-diplom7.ru .
mostbet казино http://mostbet4011.ru
купить аттестат за 9 класс https://www.arus-diplom8.ru .
купить аттестат за 9 класс http://www.arus-diplom5.ru .
Спасибо за идеальный букет!
заказать цветы томск
Приобрести диплом о высшем образовании. Приобретение документа о высшем образовании через надежную фирму дарит много плюсов для покупателя. Такое решение помогает сберечь время и существенные финансовые средства. orikdok-2v-gorode-perm-59.online
диплом о высшем образовании купить диплом о высшем образовании купить .
Онлайн-казино для белорусов, играйте и выигрывайте, удивительные бонусы.
Лучшие онлайн-игры из Беларуси, в удобное для вас время, удобные условия.
Реальные выигрыши в онлайн-казино, проверенное время, играйте ответственно.
Самые надежные онлайн-казино в Беларуси, щедрые призы.
Разнообразие игр для всех, от слотов до покера, для азартных игроков.
Откройте для себя новинки в казино, для настоящих ценителей.
Поспешите за бонусами в казино онлайн, только в Беларуси.
Лучшие игры года , всегда готовы к игре, начните выигрывать.
Советы по выбору казино, узнайте больше.
Приглашаем в мир онлайн-азарта, получайте незабываемые эмоции.
казино казино онлайн .
Календарь огородника http://n2000.ru/ .
купить игровой пк в рассрочку http://www.kupit-igrovoj-kompyuter.ru/ .
аттестат за 11 класс купить аттестат за 11 класс купить .
купить игровой компьютер купить игровой компьютер .
купить диплом техникума https://isev.su/ .
купить диплом вуза http://arus-diplom6.ru/ .
купить игровой пк купить игровой пк .
Движение и мышление. Эго и душа в организациях. Пирамида логических уровней Короткова.
Приобрести диплом ВУЗа по выгодной цене вы можете, обратившись к надежной специализированной компании. Приобрести документ о получении высшего образования вы имеете возможность у нас. orikdok-5v-gorode-yoshkar-ola-12.online
Удобство и стиль, в вашем офисе, с помощью смартфона.
Идеальное сочетание, электрические рулонные шторы, долговечность и надежность.
Создайте уют, с дистанционным управлением для штор, с удовольствием.
Шторы, которые меняют все, как выбрать?.
Электрические шторы, это красиво, упрощайте свою жизнь.
Шторы, которые управляются дистанционно, для вашего удобства, попробуйте прямо сейчас.
Шторы с умным управлением, надежность и стиль, узнайте больше.
Современные рулонные шторы, доступная цена, получите консультацию.
Электронные шторы для вашего дома, высокая функциональность, легко заказать.
Инновации в дизайне интерьера, для каждого дома, узнайте больше.
Электрические шторы с пультом, стиль и комфорт, закажите с нашим менеджером.
Дистанционно управляемые шторы, где купить?.
Сделайте ваш дом умным, с дистанционно управляемыми шторами, это легко.
Электрические шторы для вашего интерьера, высокое качество, узнайте подробности.
Электрические шторы с дистанционным управлением, современные технологии, покупка в один клик.
Выбор рулонных штор с электроприводом, простой и удобный выбор, посетите наш сайт.
Удобные рулонные шторы, разнообразие дизайнов, выбор за вами.
Рулонные шторы для вашего дома, для стильного интерьера, узнайте цены.
рулонные шторы с электроприводом и дистанционным управлением рулонные шторы с электроприводом и дистанционным управлением .
Прослеживание пути изменений. Четвертый раздел: новаторские способы применения НЛП третьего поколения. НЛП признавало влияние физиологии на мыслительные процессы. Пирамида логических уровней Короткова.
Книга предлагает «дорожную карту» для реализации ви?дения, миссии и стремлений. Ресурсы могут находиться в любой точке времени. Расширение осознанности, моделирование ключевых факторов. [Пирамида Дилтса –
Каждому игроку важно понимать, где реально стоит играть, особенно когда на кону стоят личные средства. Сайт ап икс зеркало показывает актуальную информацию о работе этого популярного проекта. Здесь вы найдёте подробные рейтинги, информацию о проверке лицензии, а также фишки для новичков. Часто выходят материалы о поддержке пользователей, что делает сайт полезным как для новичков, так и для опытных игроков.
Казино UP-X зарекомендовала себя динамично развивающейся площадкой с лицензией, что обеспечивает защиту интересов игроков. Вдобавок к первым начислениям, игроки получают приглашения на турниры и возможность выиграть больше. Раздел с лайв-дилерами, делают платформу удобной и современной. Регулярно читайте сайт, чтобы не упустить лучшие предложения.
1win casino 1win3002.com
Заказать диплом об образовании!
Мы изготавливаем дипломы любых профессий по приятным тарифам— diplom-profi.ru
оригинальные цветочные горшки оригинальные цветочные горшки .
Онлайн-казино для белорусов, получайте удовольствие от игры, ждут вас.
Играйте в казино онлайн в Беларуси, все время на связи, выгодные предложения.
Поднимите свои шансы на выигрыш, заведомо безопасно, достигайте успеха.
Онлайн-казино, которым можно доверять, высокие выплаты.
Играйте в любимые игры в казино онлайн, от рулетки до блэкджека, на любой вкус.
Откройте для себя новинки в казино, проверенные провайдеры.
Поспешите за бонусами в казино онлайн, подарки для новых игроков.
Лучшие игры года , готовы порадовать, начните выигрывать.
На что обратить внимание при выборе казино, узнайте больше.
Станьте частью захватывающего мира казино, не упустите возможность выиграть.
казино беларусь казино беларусь .
Slotbom77
Теневые формы: ярость — агрессия, нежность — слабость, игривость — цинизм. Пирамида Дилтса-Короткова. Логические уровни 2.0
how to bet on 1win http://1win3006.com
купить диплом arus-diplom3.ru .
“Hey kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > damabet %300 hosgeldin
Canl? bahis yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 20 saniyede haz?r.
Kazanmay? dene!”
Электрические рулонные шторы, в вашем пространстве.
Современные рулонные шторы с электрическим управлением, для вашего удобства.
Электрические рулонные шторы для вашего дома, доступны.
Современные рулонные шторы с электроприводом, отличный выбор.
Время менять окна на рулонные шторы с электроприводом, смотрите.
Рулонные шторы с электроприводом для вашего интерьера, создадут уют.
Электрические рулонные шторы: комфорт и уют, инновации, которые стоит попробовать.
Умные рулонные шторы – ваш новый помощник, познакомьтесь с нашими предложениями.
Рулонные шторы с электроприводом – элегантность и удобство, на нашем сайте.
Электрические рулонные шторы: удобство на каждом окне, которое вам понравится.
Электрические рулонные шторы – стиль и технология, с возможностью установки.
Электрические рулонные шторы для любого интерьера, выбор, который вы не пожалеете.
Электроприводные рулонные шторы: удобство управления одним нажатием, носим с собой.
Обновите интерьер с рулонными шторами с электроприводом, закажите сейчас.
Электрические рулонные шторы – стильный выбор, сделайте свой дом комфортнее.
Электрические рулонные шторы – сочетание простоты и стиля, не упустите свой шанс.
Умные рулонные шторы – ваш идеальный выбор, выбирайте лучшее.
Электроприводные рулонные шторы для стильного дома, закажите прямо сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
monsbet monsbet
Купить диплом университета по выгодной стоимости вы можете, обратившись к проверенной специализированной фирме. Заказать документ о получении высшего образования можно у нас в столице. orikdok-2v-gorode-voronezh-36.online
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4511 клиентов воспользовались услугой — теперь ваша очередь.
Обращайтесь — ответим быстро, без лишних формальностей.
drenazhnye-raboty-35.ru .
Хочешь безопасный старт? Лучший СЃРїРѕСЃРѕР± проверить РІРѕРґСѓ — сделать первый шаг; подробная инструкция появится, up x сайт, РіРґРµ РІСЃС‘ расписано РїРѕ шагам. РЈРџ-РРєСЃ предлагает регистрацию Р·Р° 15 секунд.
Бонусы на старт — понятные. чат-бот решает проблемы мгновенно. Учись на демо,. Руже скоро почувствуешь, как первый настоящий спин превращает теорию в азарт.
дизайнерское кашпо напольное https://dizaynerskie-kashpo-spb.ru/ .
как получить потребительский кредит без отказа как получить потребительский кредит без отказа .
next https://cytonic.lat
Мы предлагаем документы университетов, которые расположены на территории всей Российской Федерации. Заказать диплом любого университета:
cogenttc.com/kachestvennye-diplomy-dlja-vashego-uspeha-25
букмекерские конторы в кыргызстане http://mostbet4012.ru/
check that https://lombard.pw/
you could check here https://exponent.my
click here for more https://kyros.lat/
site web https://verio.buzz/
Привет всем!
Капибара в дикой природе живёт у воды и обожает плавать. Её среда обитания — реки Южной Америки, где она чувствует себя прекрасно. капибара что любит есть Капибара способна нырять и охлаждаться в воде, поэтому её фото выглядят невероятно умиротворяюще.
Более подробно по ссылке – https://capybara888.wordpress.com
капибара и кошки
капибара дружит с котами
капибара в аниме
Удачи!
Мы предлагаем документы об окончании любых ВУЗов России. Документы производятся на настоящих бланках. gomyneed.com/profile/carmonamess48
pop over to this site https://exponent.my
1win%20bet https://1win3007.com
Приобрести диплом ВУЗа по невысокой стоимости возможно, обратившись к надежной специализированной фирме. Мы предлагаем документы об окончании любых ВУЗов РФ. Приобрести диплом университета– fanoosalinarah.com/textstat/diplomy-vysshego-obrazovanija-po-dostupnym-cenam-19
моментальные займы на карту без отказа моментальные займы на карту без отказа .
useful reference https://verio.lat
a knockout post https://upshift.ltd/
Приобрести диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по приятным тарифам— economist-ucheba.ru
my explanation https://astake.lat/
Поведение. Морфогенетические поля. Это помогает поддерживать осознанность и контакт. Пирамида уровней Дилтса-Короткова.
Рулонные шторы с электроприводом, в вашем пространстве.
Выберите рулонные шторы с электроприводом, для вашего удобства.
Электрические рулонные шторы для вашего дома, у нас.
Умные рулонные шторы, комфорта и эстетики.
Изучите мир электрических рулонных штор, смотрите.
Электрические рулонные шторы – удобство и стиль, создадут уют.
Электроприводные рулонные шторы: инновации в вашем доме, решение для современного дизайна.
Электрические рулонные шторы: свобода выбора, откройте для себя.
Электроприводные рулонные шторы: ваш лучший выбор, в каталоге товаров.
Электрические рулонные шторы: удобство на каждом окне, которое вам понравится.
Современные рулонные шторы с электроприводом, доступные для заказа.
Рулонные шторы с электроприводом: функциональность и стиль, выбор, который вы не пожалеете.
Электроприводные рулонные шторы: удобство управления одним нажатием, носим с собой.
Обновите интерьер с рулонными шторами с электроприводом, выберите свои.
Рулонные шторы с электроприводом: комфорт на новом уровне, попробуйте и оцените.
Электрические рулонные шторы – сочетание простоты и стиля, не упустите свой шанс.
Умные рулонные шторы – ваш идеальный выбор, покупайте у нас.
Рулонные шторы с электроприводом: удобно и красиво, изучите сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
купить диплом о высшем образовании http://arus-diplom3.ru .
אידוי קנאביס
עטי אידוי – פתרון חדשני, פרקטי וטוב לבריאות למשתמש המודרני.
בעולם שלנו, שבו קצב חיים מהיר ושגרת יומיום קובעים את היום-יום, מכשירי האידוי הפכו לאופציה אידיאלית עבור אלה המעוניינים ב חווית אידוי איכותית, נוחה ובריאה.
מעבר לטכנולוגיה החדשנית שמובנית במכשירים הללו, הם מציעים סדרת יתרונות בולטים שהופכים אותם לאופציה עדיפה על פני אופציות מסורתיות.
עיצוב קומפקטי ונוח לנשיאה
אחד היתרונות הבולטים של עטי אידוי הוא היותם קומפקטיים, בעלי משקל נמוך ונוחים לנשיאה. המשתמש יכול לשאת את העט האידוי לכל מקום – לעבודה, לנסיעה או למסיבות חברתיות – מבלי שהמוצר יהווה מטרד או יהיה מסורבל.
העיצוב הקומפקטי מאפשר לאחסן אותו בתיק בקלות, מה שמאפשר שימוש דיסקרטי ונוח יותר.
התאמה לכל הסביבות
עטי האידוי מצטיינים בהתאמתם לשימוש בסביבות מגוונות. בין אם אתם במשרד או במפגש, ניתן להשתמש בהם באופן לא מורגש וללא הפרעה.
אין עשן כבד או ריח עז שעלול להטריד – רק אידוי עדין וקל שנותן חופש פעולה גם באזור הומה.
ויסות מיטבי בחום האידוי
למכשירי האידוי רבים, אחד היתרונות המרכזיים הוא היכולת ללווסת את חום הפעולה באופן מדויק.
תכונה זו מאפשרת להתאים את השימוש לסוג החומר – פרחים, נוזלי אידוי או תמציות – ולבחירת המשתמש.
ויסות החום מבטיחה חוויית אידוי נעימה, טהורה ואיכותית, תוך שימור על הטעמים הטבעיים.
אידוי נקי וטוב יותר
בניגוד לעישון מסורתי, אידוי באמצעות Vape Pen אינו כולל שריפה של החומר, דבר שמוביל למינימום של חומרים מזהמים שמשתחררים במהלך השימוש.
מחקרים מראים על כך שאידוי הוא אופציה בריאה, עם מיעוט במגע לרעלנים.
בנוסף, בשל חוסר בעירה, ההארומות ההמקוריים מוגנים, מה שמוסיף להנאה מהמוצר וה�נאה הכוללת.
פשטות הפעלה ותחזוקה
עטי האידוי מתוכננים מתוך עיקרון של קלות שימוש – הם מתאימים הן למתחילים והן למשתמשים מנוסים.
רוב המכשירים פועלים בלחיצה אחת, והעיצוב כולל החלפה של רכיבים (כמו טנקים או גביעים) שמקלים על התחזוקה והטיפול.
הדבר הזה מגדילה את אורך החיים של המוצר ומבטיחה תפקוד אופטימלי לאורך זמן.
מגוון רחב של מכשירי וופ – התאמה אישית
המגוון בעטי אידוי מאפשר לכל משתמש ללמצוא את המוצר האידיאלי עבורו:
מכשירים לקנאביס טבעי
מי שמחפש חווית אידוי אותנטית, רחוקה ממעבדות – ייבחר עט אידוי לפרחי קנאביס.
המכשירים הללו מיועדים לעיבוד בפרחים טחונים, תוך שמירה מלאה על הארומה והטעם הטבעיים של הקנאביס.
עטי אידוי לשמנים ותמציות
למשתמשים שרוצים אידוי מרוכז ומלא ברכיבים כמו THC וCBD – קיימים מכשירים המיועדים במיוחד לנוזלים ותמציות.
המוצרים האלה מתוכננים לטיפול בחומרים צפופים, תוך שימוש בחידושים כדי ללספק אידוי אחיד, חלק ומלא בטעם.
—
מסקנות
עטי אידוי אינם רק עוד כלי לצריכה בחומרי קנאביס – הם סמל לרמת חיים גבוהה, לחופש ולהתאמה לצרכים.
בין היתרונות המרכזיים שלהם:
– גודל קומפקטי ונעים לנשיאה
– שליטה מדויקת בחום האידוי
– צריכה בריאה ונטולת רעלים
– הפעלה אינטואיטיבית
– הרבה אפשרויות של התאמה לצרכים
בין אם זו הההתנסות הראשונה בוופינג ובין אם אתם צרכן ותיק – וופ פן הוא ההבחירה הטבעית לצריכה מתקדמת, נעימה ובטוחה.
—
הערות:
– השתמשתי בסוגריים מסולסלים כדי ליצור וריאציות טקסטואליות מגוונות.
– כל האפשרויות נשמעות טבעיות ומתאימות לעברית מדוברת.
– שמרתי על כל המונחים הטכניים (כמו Vape Pen, THC, CBD) ללא שינוי.
– הוספתי סימני חלקים כדי לשפר את ההבנה והארגון של הטקסט.
הטקסט מתאים למשתמשים בהשוק העברי ומשלב תוכן מכירתי עם מידע מקצועי.
888starz apk 888starz apk .
mostbet uzcard skachat mostbet4003.ru
Приобрести диплом под заказ можно через официальный портал компании. orikdok-3v-gorode-voronezh-36.online
1win bet app http://1win3003.com
mostbet история компании https://mostbet4012.ru/
Лучшие казино онлайн в Беларуси, получайте удовольствие от игры, порадуют вас.
Играйте в казино онлайн в Беларуси, в любое время и в любом месте, привлекательные условия.
Поднимите свои шансы на выигрыш, заведомо безопасно, играйте ответственно.
Онлайн-казино, которым можно доверять, щедрые призы.
Широкий выбор игр в белорусских казино, от карточных до настольных игр, на любой вкус.
Откройте для себя новинки в казино, для настоящих ценителей.
Поспешите за бонусами в казино онлайн, только здесь.
Топ онлайн-игр в белорусских казино, всегда готовы к игре, начните выигрывать.
На что обратить внимание при выборе казино, по играм на деньги.
Ваш шанс на удачу здесь, получайте незабываемые эмоции.
казино беларусь казино беларусь .
“Merhaba bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > klasbahis“
Букет – просто прелесть!
доставка цветов
Заказать диплом университета. Приобретение подходящего диплома через качественную и надежную фирму дарит массу преимуществ для покупателя. Данное решение позволяет сэкономить время и значительные финансовые средства. orikdok-3v-gorode-saratov-64.ru
1 win mexico 1 win mexico
Изготавливаем римские шторы по индивидуальным размерам, красиво.
Римские шторы на заказ от профессионалов, и.
Мы предлагаем римские шторы на заказ, для вашего удобства.
Стильные римские шторы на заказ, выберите свои ткани.
Заказывайте римские шторы по индивидуальным размерам, что вам нужно.
Римские шторы на заказ от ведущих дизайнеров, широкая палитра цветов.
Создайте уют с римскими шторами на заказ, премиум-материалы.
Уникальные римские шторы под заказ для вашего дома, это доступно.
Комфорт и стиль: римские шторы под заказ, для вашего окна.
Создайте индивидуальные римские шторы, для любого стиля.
Узнайте о наших римских шторах на заказ, узнайте цены.
Римские шторы по индивидуальному проекту, для вашего уюта.
Римские шторы на заказ: как это работает, перезвоните нам.
Уникальные римские шторы по вашим размерам, создание уюта.
Римские шторы на заказ, которые преобразят вашу комнату, приходите в шоурум.
Римские шторы на заказ: советы по выбору, пошаговые инструкции.
Заказывайте римские шторы по индивидуальным пожеланиям, которые подойдут именно вам.
Римские шторы на заказ: как выбрать ткани, для вашего дизайна.
Римские шторы на заказ для вашего интерьера, с удовольствием.
римские шторы на заказ римские шторы на заказ .
скачать мостбет официальный https://www.mostbet4013.ru
Мы изготавливаем дипломы любой профессии по доступным ценам. Стоимость может зависеть от выбранной специальности, года получения и университета: вент.СЂСѓСЃ/employer/originality-diplomas
Мы можем предложить дипломы любой профессии по приятным ценам. Для нас важно, чтобы документы были доступны для подавляющей массы граждан. Заказать диплом института konstruktiv.getbb.ru/viewtopic.php?f=17&t=20239
скачать бк осталось только найти предпочтение подходящее http://mostbet4014.ru
888starz partners http://888starz-official.pro/ .
1win token listing date telegram https://1win3006.com/
888starz скачать официальный сайт 888starz скачать официальный сайт .
Тысячи игроков пользуются услугами казино Зума, чтобы испытать удачу. Сайт предоставляет лучшим автоматам. Среди развлечений гарантированно найдёшь любимую механику. В одном из раундов зума казино официальный станет моментом джекпоту. Сервис Zooma следит за трендами, удивляя новинками.
Пользовательский опыт продумана РґРѕ мелочей, что ценят опытные РёРіСЂРѕРєРё. Создание аккаунта РїСЂРѕС…РѕРґРёС‚ быстро. Как только РІС‹ активируете аккаунт, можно использовать фриспины. Ргровая площадка Zooma рады новым игрокам.
Надежность транзакций обеспечивается лицензией. Финансовые переводы обрабатываются через защищённые системы. Платформа может использоваться для перехода к привлекательным играм. Zooma ценит приватность клиентов. Такой подход создаётся комфортная среда.
Теперь тёща меня обожает – спасибо!
доставка цветов в томске
Онлайн-казино для белорусов, открывайте новые возможности, удивительные бонусы.
Откройте для себя казино Беларуси, в удобное для вас время, удобные условия.
Поднимите свои шансы на выигрыш, лицензированные заведения, не упустите свою удачу.
Онлайн-казино, которым можно доверять, каждый день.
Играйте в любимые игры в казино онлайн, от карточных до настольных игр, для истинных ценителей.
Откройте для себя новинки в казино, для азартных геймеров.
Не упустите свои шансы на бонусы, только здесь.
Топ онлайн-игр в белорусских казино, готовы порадовать, начните выигрывать.
На что обратить внимание при выборе казино, по онлайн-казино Беларуси.
Ваш шанс на удачу здесь, получайте незабываемые эмоции.
казино беларусь казино .
Любители азарта отдают предпочтение онлайн-казино Zooma за богатому выбору развлечений. Любая разработка обладает интересными бонусами. В времени игрового процесса вы можете получить казино зума для новых ставок. Площадка Zooma функционирует легально, что исключает мошенничество. Команда поддержки всегда на связи в круглосуточном режиме.
Онлайн-ресурс Zooma обеспечивает востребованные игры от ведущих провайдеров. Игры регулярно обновляются, что мотивирует. Чтобы начать игру необходимо лишь пару минут. Создав аккаунт станет доступным разделу с бонусами. На платформе Zooma каждый игрок может найти интересные предложения.
Отдельным достоинством Zooma Казино можно назвать отсутствие задержек при выплатах. Операции по выводу выполняются в кратчайшие сроки. Честные лимиты обеспечивают комфортный предсказуемым. казино Зума регулярно обновляет финансовую инфраструктуру. Азарт гармонирует с гарантией выплат.
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по невысоким ценам. Метод использования и приобретение чистого диплома — kyc-diplom.com/diplom-articles/metod-ispolzovaniya-i-priobretenie-chistogo-diploma.html
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3210 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом о среднем образовании — ответим быстро, без лишних формальностей.
1win android https://1win3003.com/
Изготавливаем римские шторы по индивидуальным размерам, а также.
Наилучшие римские шторы на заказ, разнообразие.
Ваши идеальные римские шторы на заказ, помощь в выборе.
Элегантные римские шторы на заказ, согласуйте дизайн.
Римские шторы на заказ с учетом всех нюансов, что вам нравится.
Римские шторы на заказ от ведущих дизайнеров, выбор тканей.
Создайте уют с римскими шторами на заказ, премиум-материалы.
Качество и стиль: римские шторы на заказ, это просто.
Римские шторы на заказ: идеальное решение, для вашего окна.
Римские шторы на заказ: легкость и стиль, для любого помещения.
Узнайте о наших римских шторах на заказ, узнайте цены.
Дизайн римских штор на заказ, для вашего стиля.
Римские шторы на заказ: как это работает, узнайте больше.
Уникальные римские шторы по вашим размерам, создание уюта.
Неповторимые римские шторы по вашим идеям, приходите в шоурум.
Римские шторы на заказ: советы по выбору, экспертные рекомендации.
Заказывайте римские шторы по индивидуальным пожеланиям, которые легко впишутся в интерьер.
Римские шторы на заказ: как выбрать ткани, для вашего дизайна.
Римские шторы на заказ: гурман для ваших окон, с нами легко.
римские шторы на заказ римские шторы на заказ .
Казино — это дело серьёзное, но без смеха скучно. Каждый слот любит подшутить. Игроки выигрывают в зависимости от сценария. Вот и зума казино зеркало пригодится, чтобы удержаться на волне азарта. Zooma одаривает сюрпризами, но и веселит.
Ожидание выигрыша — как игра с Джокером. Иногда всё идёт по плану, но чаще — по сценарию ситкома. Игроку остаётся только подбадривать себя. Даже проигрыш становится поводом для самоиронии. Платформа Zooma создаёт условия, в которых по настоящему интересно.
как комики: каждый по-своему веселит. Слоты с бонусами — это сюрпризные коробки. Даже поддержка клиентов умеет разряжать обстановку. Игра здесь превращается в позитивный ритуал. Zooma подходит всем, кто любит весёлый формат гейминга.
мостбет на айфон скачать http://mostbet4003.ru
Нью Ретро Казино — это не просто игровая площадка, а платформа с сюжетом. Каждый слот здесь — как сцена в ленте удачи. Игрок становится главным героем. А new retro casino рабочее зеркало — это режиссёрский старт, полной вспышек и поворотов. Азарт тут не играется — он проживается как кадр за кадром.
Оформление напоминает VHS-кассету. Все элементы интерфейса будто вырезаны из старого кинотеатра. Музыка создаёт атмосферу триллера. Каждый бонус — как режиссёрский сюрприз. Ты не просто играешь — ты создаёшь кино своей игры.
Регистрация в New Retro Casino — это проба кадра большого азарта. Здесь нет дублей — каждый спин полон экспромта. Вместо зала ожидания — интерфейс в стиле монтажного пульта, где всё готово к премьере. Бонусная система — как сценарная сетка, в котором заложены десятки вариантов развития. И если ты готов стать героем — нажимай кнопку съёмки.
“Dikkat forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > Lunabet“
888starz bet официальный сайт скачать http://www.888starz-skachat-na-android.com .
Meningkatkan Peluang Kemenangan dengan Prediksi HK dan Bocoran HK Hari Ini
Dalam dunia perjudian togel Hongkong (HK), prediksi dan bocoran angka memainkan peran penting bagi para pemain untuk meningkatkan peluang kemenangan mereka. Artikel ini akan membahas bagaimana prediksi HK, bocoran HK, serta data HK dapat digunakan secara strategis untuk membantu Anda meraih hasil terbaik di pasar togel HK hari ini.
Prediksi HK: Menggabungkan Data Historis dan Tren Terbaru
Prediksi HK adalah hasil analisis yang dilakukan berdasarkan data historis dan keluaran HK terbaru. Dengan memadukan informasi ini, tabel tren dibuat untuk mengidentifikasi pola-pola tertentu yang mungkin muncul dalam hasil undian. Analisis ini tidak hanya bergantung pada statistik semata, tetapi juga mempertimbangkan faktor-faktor lain seperti syair HK, yang sering dianggap sebagai “filter spiritual” oleh sebagian pemain togel hongkong.
Syair HK sendiri merupakan rangkaian kata-kata atau puisi tradisional yang diyakini memiliki makna simbolis terkait angka-angka. Meskipun sifatnya subjektif, banyak pemain menggunakan syair HK sebagai panduan tambahan untuk memilih angka main HK yang lebih strategis.
Bocoran HK Hari Ini: Informasi Berharga untuk Pemain
Bocoran HK hari ini menjadi salah satu sumber informasi yang paling dicari oleh pemain togel. Informasi ini biasanya diperoleh dari berbagai sumber, termasuk prediksi jitu HK yang disusun oleh para ahli togel. Angka main HK yang direkomendasikan melalui bocoran ini diharapkan memberikan akurasi tinggi, membantu pemain dalam menentukan pilihan angka yang lebih tepat.
Namun, penting untuk diingat bahwa bocoran HK bukanlah jaminan mutlak kemenangan. Sebaliknya, ini adalah alat bantu yang dapat digunakan untuk memperkuat strategi permainan Anda. Oleh karena itu, pemain disarankan untuk tetap bijak dan tidak sepenuhnya bergantung pada bocoran semata.
Data HK dan Keluaran HK: Dasar Analisis yang Kuat
Untuk membuat prediksi yang akurat, data HK dan keluaran HK merupakan elemen kunci yang tidak bisa diabaikan. Data HK mencakup rekaman hasil undian sebelumnya, yang kemudian dianalisis untuk mengidentifikasi tren jangka panjang. Sementara itu, keluaran HK terbaru memberikan gambaran tentang pola-pola yang sedang berkembang.
Dengan menggabungkan kedua informasi ini, pemain dapat menyusun strategi yang lebih matang. Misalnya, jika sebuah angka belum muncul dalam beberapa undian terakhir, ada kemungkinan angka tersebut akan keluar dalam waktu dekat. Namun, pendekatan ini harus dilakukan dengan hati-hati dan disesuaikan dengan kondisi pasar togel HK saat ini.
Strategi Bermain Togel HK Malam Ini
Bagi Anda yang berencana bermain togel hongkong malam ini, berikut adalah beberapa tips yang dapat membantu:
1. Gunakan Prediksi Jitu HK: Manfaatkan prediksi jitu HK yang telah diverifikasi menggunakan berbagai metode, termasuk syair HK.
2. Perhatikan Bocoran HK Hari Ini: Jadikan bocoran HK sebagai referensi tambahan, tetapi jangan mengandalkannya sepenuhnya.
3. Analisis Data HK Secara Mendalam: Pelajari data HK historis dan keluaran HK terbaru untuk mengidentifikasi tren yang relevan.
4. Tetapkan Batasan: Tentukan batas taruhan Anda agar tidak terjebak dalam kerugian besar.
5. Gabungkan Intuisi dan Logika: Selain mengandalkan prediksi dan data, dengarkan juga intuisi Anda saat memilih angka.
Kesimpulan
Prediksi HK, bocoran HK, dan data HK adalah alat yang sangat berharga bagi pemain togel hongkong. Dengan memanfaatkan informasi ini secara bijak, Anda dapat meningkatkan peluang kemenangan Anda di pasar togel HK hari ini. Namun, ingatlah bahwa togel tetap merupakan permainan peluang, dan tidak ada metode yang dapat menjamin kemenangan 100%. Oleh karena itu, bermainlah dengan tanggung jawab dan nikmatilah prosesnya.
Jika Anda ingin mendapatkan informasi lengkap mengenai prediksi hongkong, data hongkong, dan keluaran HK, pastikan untuk mengunjungi sumber-sumber terpercaya seperti Hongkong Pools. Dengan persiapan yang matang dan strategi yang tepat, siapa tahu malam ini bisa menjadi malam keberuntungan Anda!
New Retro Casino — это цифровой завод по производству эмоций, где ставки вращаются как шестерни. Визуальная среда напоминает заводской интерфейс, а new retro casino вход встроена в процесс, как рычаг активации. Ты чувствуешь себя оператором, контролирующим производство азарта. Здесь всё чётко, мощно и слаженно.
Каждый слот — это движущийся блок. Бонусные раунды вспыхивают как искры от сварки: ярко. Звуковое сопровождение — смесь индустриального техно. Анимации двигаются, как механизмы. Ты не просто играешь — ты включаешь цикл удачи.
Служба поддержки — это как отдел техобслуживания: молниеносная. Финансовые переводы проходят по протоколу: надежно. Нет хаоса — только структура. New Retro Casino — это не просто онлайн-развлечение, а машинный стиль выигрыша.
Slot Online
crochet dolls
1win crash predictor 1win crash predictor
drenazhnye-raboty-35.ru .
программа 1 с предприятие программа 1 с предприятие .
1с бухгалтерия купить онлайн 1с бухгалтерия купить онлайн .
сколько стоит установка 1с сколько стоит установка 1с .
проект перепланировки квартиры цена проект перепланировки квартиры цена .
купить игровой компьютер в рассрочку http://kupit-igrovoj-kompyuter3.ru .
онлайн диджей klubnaya-muzyka30.ru .
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3427 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом нового образца — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1675 клиентов воспользовались услугой — теперь ваша очередь.
Ознакомиться здесь — ответим быстро, без лишних формальностей.
Заказать шторы недорого, изготовления.
Идеальные шторы для каждого интерьера, с учетом ваших пожеланий.
по уникальным эскизам.
Закажите эксклюзивные шторы, по вашим размерам.
Красивые шторы для стильного интерьера, по доступным ценам.
Ваши мечты о шторах сбудутся, множество тканей.
Архитектурные шторы на заказ, который вы искали.
Советы по выбору штор, от профессионалов.
Найдите стильные решения для штор, по доступным ценам.
Проектируем шторы на заказ, с доставкой на дом.
Ткани для штор на любой вкус, на любой бюджет.
Качественные шторы на заказ, просто и удобно.
Неповторимые шторы для вашего дома, в кратчайшие сроки.
Секреты выбора штор, откройте для себя.
Шторы премиум-класса на заказ, доступные цены.
Шторы, которые подчеркнут ваш стиль, с последними новинками.
Уникальные предложения на шторы, спешите заказать.
По индивидуальным меркам, из натуральных материалов.
Шторы, которые вы искали, заказывайте уже сегодня.
Шторы на любой бюджет, с доставкой на дом.
заказ штор заказ штор .
New Retro Casino — это цифровая резиденция премиум-класса, где важна не только ставка, но и стиль. Интерьер виртуального лобби оформлен в духе VIP-клубов, а new retro casino сайт ведёт прямиком к выбору персональных развлечений. Комфорт ощущается в каждом клике. Игра здесь — как вечер в дорогом лаунже.
Слоты оформлены в стилистике элегантного минимализма. Бонусные раунды подаются как изысканные сюрпризы. Каждая игра — как дегустация: с послевкусием. Музыка ненавязчива, словно лоу-фай бит, а визуальные эффекты напоминают гламурный свет. Игра — часть твоего вечернего релакса.
Поддержка работает как личный консьерж: мгновенная. Финансовые операции проходят через платёжные шлюзы премиум-уровня. Платформа заботится о том, чтобы каждый шаг игрока был эстетичным. Это не просто онлайн-казино — это цифровой люкс. Азарт становится искусством.
Лучшие казино онлайн в Беларуси, получайте удовольствие от игры, порадуют вас.
Играйте в казино онлайн в Беларуси, все время на связи, для настоящих азартных игроков.
Поднимите свои шансы на выигрыш, лицензированные заведения, не упустите свою удачу.
Рейтинг лучших казино Беларуси, каждый день.
Разнообразие игр для всех, от рулетки до блэкджека, для азартных игроков.
Новые онлайн-казино Беларуси, для азартных геймеров.
Соберите все бонусы и акции, подарки для новых игроков.
Захватывающие новинки игр, готовы порадовать, откройте для себя мир азартных игр.
На что обратить внимание при выборе казино, по играм на деньги.
Приглашаем в мир онлайн-азарта, не упустите возможность выиграть.
казино казино онлайн .
sweet bonanza 1000x bomb [url=https://www.sweet-bonanza3004.ru]sweet bonanza 1000x bomb[/url]
интересные кашпо интересные кашпо .
дизайнерский горшок дизайнерский горшок .
1с управление фирмой купить 1с управление фирмой купить .
drenazhnye-raboty-35.ru .
1с бухгалтерия купить цена 1с бухгалтерия купить цена .
программа 1 с предприятие программа 1 с предприятие .
New Retro Casino — это гипергород азарта, где играет бетонный бит. Интерфейс напоминает архитектурный план будущего, и нью ретро казино вход встраивается в цифровой путь, как часть навигации. Ты не гость — ты резидент города, где каждая игра — как геймерский квартал. Здесь нет остановок — есть только маршруты возможностей.
Слоты — это здания с окнами шансов: каждая серия — как небоскрёб эмоций. Звуки — это аудиопанорама большого города: шелест ставочных потоков. Все визуальные решения — как неоновая архитектура: чисто. Каждая секция платформы напоминает стилевой квартал. Ты выбираешь маршрут и двигаешься по нему — свободно.
Поддержка — это служба городского сервиса: доступная. Финансовые каналы напоминают городские магистрали. Все переводы идут без задержек, словно по цифровой схеме метро. МетроАзарт — это не просто игра, это цифровая агломерация выигрыша.
Scientific evidence has suggested that by 2050, many of the Philippine coastal regions, including the Manila Bay area, could find themselves underwater if countries fail to mitigate the effects of climate change 꽁머니
мостбет скачать приложение на андроид mostbet4014.ru
купить игровой компьютер в москве http://www.kupit-igrovoj-kompyuter3.ru .
сколько стоит проект перепланировки https://proekt-pereplanirovki-kvartiry13.ru .
dj music dj music .
mostbet telefon orqali kirish mostbet telefon orqali kirish
Мы готовы предложить документы учебных заведений, расположенных в любом регионе РФ. Купить диплом университета:
cyberpinoy.net/read-blog/223368
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3156 клиентов воспользовались услугой — теперь ваша очередь.
Звоните — ответим быстро, без лишних формальностей.
Купить документ о получении высшего образования вы имеете возможность в нашей компании в Москве. Приобрести диплом ВУЗа по невысокой стоимости можно, обращаясь к надежной специализированной компании. lxgonline.mn.co/posts/84445092
Заказать шторы недорого, широкий ассортимент.
Идеальные шторы для каждого интерьера, по индивидуальным размерам.
покупка штор.
Создайте уникальные шторы, от лучших производителей.
Преобразите свой дом с помощью штор, от профессионалов.
Мы поможем выбрать, в нашем магазине.
Индивидуальные шторы для вашего окна, который вы искали.
Советы по выбору штор, от экспертов.
Шторы для спальни, кухни и гостиной, в нашем каталоге.
Создаем стильные шторы, с доставкой на дом.
Ткани для штор на любой вкус, с различными узорами.
Качественные шторы на заказ, для комфорта и стиля.
Профессиональное изготовление штор, в кратчайшие сроки.
Шторы, которые создают уют, узнайте больше.
Купить шторы с доставкой по всей стране, доступные цены.
Индивидуальный шопинг штор, с профессиональной помощью.
Заказ штор с бесплатной консультацией, спешите заказать.
Шторы для любого повода, из натуральных материалов.
Шторы, которые вы искали, не упустите шанс.
Цены на шторы, которые вас приятно удивят, с доставкой на дом.
заказ штор заказ штор .
Заказать диплом института по выгодной цене возможно, обращаясь к надежной специализированной фирме. Приобрести документ о получении высшего образования вы сможете у нас в Москве. orikdok-4v-gorode-kemerovo-42.online
Заказать диплом любого университета!
Мы изготавливаем дипломы любой профессии по доступным тарифам— diplomovoz.ru
Заказать диплом университета. Приобретение подходящего диплома через качественную и надежную компанию дарит массу преимуществ. Данное решение позволяет сберечь время и значительные деньги. orikdok-3v-gorode-nizhniy-novgorod-52.online
диплом купить колледжа диплом купить колледжа .
купить диплом о образовании купить диплом о образовании .
москва купить диплом москва купить диплом .
sweet bonanza xmas slot review http://sweet-bonanza3004.ru/
Рулонные шторы с электроприводом, в интерьере.
Электроприводные рулонные шторы для вашего окна, интеллектуальное решение.
Рулонные шторы с электроприводом: удобство и стиль, доступны.
Электроприводные рулонные шторы, отличный выбор.
Изучите мир электрических рулонных штор, смотрите.
Умные рулонные шторы для дома, создадут уют.
Электроприводные рулонные шторы: инновации в вашем доме, инновации, которые стоит попробовать.
Электрические рулонные шторы: свобода выбора, познакомьтесь с нашими предложениями.
Рулонные шторы с электроприводом – элегантность и удобство, в каталоге товаров.
Электрические рулонные шторы: удобство на каждом окне, которое вам понравится.
Современные рулонные шторы с электроприводом, доступные для заказа.
Электрические рулонные шторы для любого интерьера, выбор, который изменит ваш быт.
Рулонные шторы с электроприводом – ваш идеальный помощник, носим с собой.
Умные рулонные шторы: будущее уже здесь, закажите сейчас.
Умные рулонные шторы для вашего удобства, откройте для себя.
Электрические рулонные шторы – сочетание простоты и стиля, пополните свой интерьер.
Электрические рулонные шторы: легкость управления, выбирайте лучшее.
Электроприводные рулонные шторы для стильного дома, изучите сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
mostbet aviator yuklab olish http://www.mostbet4004.ru
“Sst forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > betzulaTR“
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Букет просто огонь! Подруга до сих пор под впечатлением.
заказать цветы с доставкой в томске
mostbet uz slotlar mostbet uz slotlar
Шторы для больших окон: римский стиль, идеальный выбор.
с римскими шторами, созданными для вашего пространства.
Выберите римские шторы.
Как выбрать римские шторы для больших окон, получите советы.
Красота и функциональность.
Преобразите свое пространство.
Римские шторы: как выбрать идеальный вариант, изучите наш материал.
рекомендации по выбору римских штор, чтобы сделать ваш дом уютнее.
Покупая римские шторы, вы выбираете стиль, погружайтесь в мир дизайна.
Римские шторы: универсальное решение.
Создайте комфорт с римскими шторами, не упустите возможность.
Римские шторы: идеальные для больших окон, узнайте секреты выбора.
Римские шторы для изысканных интерьеров, сочетание стиля и функциональности.
Римская штора: функциональность и стиль, выбирайте по вашему вкусу.
Оформите окна стильно, выбрав римские шторы.
Секреты стиля с римскими шторами, узнайте на нашем сайте.
Оформите окна с римскими шторами, посмотрите новинки.
Римская штора на большое окно: что выбрать?, исследуйте наш каталог.
Преимущества римских штор для больших окон, наслаждайтесь стилем.
Легкость и элегантность римских штор, для вашего дома.
римская штора на большое окно римская штора на большое окно .
mostbet telefon orqali kirish mostbet telefon orqali kirish
mental health support chatbot mental-health5.com .
drenazh-fundamenta-812.ru .
пин ап способы оплаты пин ап способы оплаты
Explore new minitinah xxx videos right now! Who is minitinah? This page has all the juicy details. Looking for minitinah blowjob or dildo scenes? See the latest content leaked from minitinah’s page.
minitinah sextape Get behind-the-scenes access to minitinah’s raw content. Uncut, uncensored — minitinah full video inside. You can’t miss this minitinah dildo moment. Best content ever from minitinah OnlyFans revealed. Don’t miss the full minitinah porn video.
Доставили в область – все в идеальном состоянии.
букет невесты томск
Мы можем предложить дипломы психологов, юристов, экономистов и других профессий по приятным тарифам. Мы готовы предложить документы техникумов, расположенных на территории всей РФ. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это позволяет делать государственные дипломы, которые невозможно отличить от оригиналов. orikdok-3v-gorode-novosibirsk-54.ru
Мы готовы предложить документы университетов, расположенных в любом регионе Российской Федерации. Купить диплом любого ВУЗа:
1ajobs.ch/employer/diplomirovans
Заказать документ о получении высшего образования можно у нас в столице. Приобрести диплом университета по доступной стоимости можно, обращаясь к надежной специализированной фирме. weekinato.ru/kupit-diplom-s-garantiey-i-besplatnyimi-pravkami
Электрические рулонные шторы, в интерьере.
Современные рулонные шторы с электрическим управлением, для вашего интерьера.
Умные шторы: удобство на кончиках пальцев, доступны.
Современные рулонные шторы с электроприводом, качества и стиля.
Наслаждайтесь комфортом рулонных штор с электроприводом, смотрите.
Умные рулонные шторы для дома, создадут уют.
Электроприводные рулонные шторы: инновации в вашем доме, решение для современного дизайна.
Электрические рулонные шторы: свобода выбора, откройте для себя.
Сделайте свой дом умнее с рулонными шторами, в нашем интернет-магазине.
Электрические рулонные шторы: удобство на каждом окне, которое вы заслуживаете.
Современные рулонные шторы с электроприводом, с возможностью установки.
Умные рулонные шторы – правильно решайте, выбор, который изменит ваш быт.
Электроприводные рулонные шторы: удобство управления одним нажатием, каждый день.
Электрические рулонные шторы для стиля и комфорта, выберите свои.
Рулонные шторы с электроприводом: комфорт на новом уровне, откройте для себя.
Электрические рулонные шторы – сочетание простоты и стиля, позаботьтесь о себе.
Электрические рулонные шторы: легкость управления, заказывайте онлайн.
Рулонные шторы с электроприводом: удобно и красиво, изучите сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
мостбет. регистрация. мостбет. регистрация.
1win betting app 1win3008.com
аттестат купить за 9 класс аттестат купить за 9 класс .
купить аттестат в калининграде купить аттестат в калининграде .
купить аттестат с отличием купить аттестат с отличием .
диплом купить в ростове на дону диплом купить в ростове на дону .
Заказать диплом любого ВУЗа. Заказ официального диплома через проверенную и надежную фирму дарит немало достоинств для покупателя. Такое решение позволяет сэкономить как продолжительное время, так и значительные средства. orikdok-5v-gorode-perm-59.online
Купить диплом на заказ вы сможете используя официальный портал компании. orikdok-4v-gorode-nalchik-7.ru
ai mental health therapy https://www.mental-health5.com .
Drip Casino — это где азарт и синт-код сплетаются, где выигрыш — как успешная атака. Интерфейс — как панель хакера, и дрип казино — это твой ключ к запуску процесса. Ты входишь в поток, где код и удача сливаются. Это территория тех, кто читает сигналы и действует быстро.
Каждый слот здесь — как фрагмент кода: изменяется на ходу. Бонусы — как аномалии в системе: нарушают цикл. Звуковое сопровождение — это техно-арт, который усиливает атмосферу. Графика переливается, как экраны в киберпространстве. Азарт здесь не просто эмоция, а энергетический поток.
Служба поддержки — как ИИ-модуль: адаптируется к запросам. Финансовые операции проходят через протоколы без ошибок. Дрип Казино — это цифровая рулетка в неоновых тонах.
Заказ документа о высшем образовании через надежную компанию дарит ряд достоинств. Заказать диплом о высшем образовании: social.elpaso.world/read-blog/9783_kupila-diplom-kolledzha.html
Купить диплом ВУЗа по доступной стоимости можно, обратившись к надежной специализированной компании. Приобрести документ о получении высшего образования можно в нашей компании. orikdok-2v-gorode-nalchik-7.online
Мы предлагаем оформление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1269 клиентов воспользовались услугой — теперь ваша очередь.
Перейти — ответим быстро, без лишних формальностей.
websites https://nucleusearn.ink
купить диплом настоящий купить диплом настоящий .
Приобрести диплом любого института!
Мы изготавливаем дипломы любой профессии по выгодным ценам— kupitediplom0029.ru
“Selam bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > yuksek limitli casino
Rulet masas? yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 20 saniyede haz?r.
Sans?n? art?r!”
Элегантные римские шторы для просторных окон, прекрасный вариант.
с помощью римских штор, созданными для вашего пространства.
в нашем магазине.
Оформление больших окон римскими шторами, узнайте больше.
римских штор для вашего интерьера.
Создайте уникальный стиль.
Все о римских шторах для больших окон, читайте наш блог.
Изысканные и удобные, для вашего комфорта.
Римские шторы: ваш стильный выбор, создавайте неповторимый интерьер.
Лучшие римские шторы для больших окон.
Откройте для себя римские шторы, выбирайте лучшее.
Как украсить окна римскими шторами, посмотрите примеры.
Шторы для вашего стиля, лучшие варианты на рынке.
Уникальные римские шторы для больших окон, создавайте уют в вашем доме.
Создайте атмосферу уюта, с этими римскими шторами.
Секреты стиля с римскими шторами, используйте в своем интерьере.
Элегантные римские шторы: ваш шаг к идеальному дому, посмотрите новинки.
Как римские шторы меняют интерьер, исследуйте наш каталог.
Почему стоит выбрать римские шторы?, изучайте новинки.
Легкость и элегантность римских штор, для вашего пространства.
римская штора на большое окно римская штора на большое окно .
this contact form https://kyros.my
try these out https://gofundmeme.buzz
креативные кашпо креативные кашпо .
авторские кашпо https://dizaynerskie-kashpo-spb.ru/ .
Дрип Казино — это космодром для игры на деньги, где всё сверкает, как астероидный пояс. Интерфейс напоминает дисплей навигации, а казино drip служит стартовой точкой полёта. прокладываешь маршрут к космическому призу. команда к запуску.
Слоты — это звёздные врата. Бонусы — это сигналы с других галактик. Музыка — это пульсации звёзд, которые сопровождают каждый спин. Каждая сессия — как выход в открытый космос. Ты открываешь вселенные, полные шансов.
Поддержка — как центр связи на орбите: координирует движение. Финансовые транзакции — как телепортации: мгновенные. Дрип Казино — это межпланетная миссия с выигрышем.
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1824 клиентов воспользовались услугой — теперь ваша очередь.
Диплом цена — ответим быстро, без лишних формальностей.
Где купить диплом по необходимой специальности?
Купить диплом университета по невысокой стоимости вы сможете, обращаясь к проверенной специализированной фирме.: 10000diplomov.ru
Мы предлагаем дипломы любых профессий по разумным тарифам. Стоимость будет зависеть от выбранной специальности, года выпуска и ВУЗа: kotka.listbb.ru/posting.php?mode=post&f=2&sid=1d169ba5f531e9acfe8fcc8d30901e93
Мы можем предложить дипломы любых профессий по выгодным тарифам. Дипломы производятся на настоящих бланках государственного образца Выгодно заказать диплом любого университета diplomist.com
Мы готовы предложить дипломы любой профессии по доступным тарифам. Важно, чтобы документы были доступны для большого количества наших граждан. Приобрести диплом о высшем образовании rrsclub.ru/member.php?u=1164
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2226 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом института — ответим быстро, без лишних формальностей.
Заказать диплом об образовании!
Приобретение документа о высшем образовании через проверенную и надежную фирму дарит немало плюсов для покупателя. Заказать диплом института у сильной компании: doks-v-gorode-rostov-na-donu-61.online
Купить диплом института по выгодной цене возможно, обращаясь к надежной специализированной фирме. Заказать диплом: dyado.listbb.ru/viewtopic.phpf=2&t=1664
Дрип Казино — это мир для тех, кто на своём ритме, где казино звучит, как бас в колонке. Дизайн напоминает граффити на кирпичных стенах, а дрип казино зеркало вписывается в текст как рифма в куплете. Каждое нажатие — как бит в треке, всё двигается в своём груве. Каждое действие — как баттл в звуке.
Слоты оформлены как граффити-зоны. Бонусы — как неожиданные дропы: вспыхивают как фейерверк. Музыка в казино — это лоу-фай, создающая нужный вайб. Каждая игра — как движение на танцполе: с ощущением. Ты чувствуешь азарт не как напряжение, а как часть уличного ритма.
Поддержка здесь — как друг с района: знает, что делать. Платежи летят через проверенные платёжки. Всё двигается так, будто ты в своём потоке. Дрип Казино — это уличная игра с классом.
аттестат купить аттестат купить .
Купить диплом института по невысокой цене вы можете, обратившись к проверенной специализированной фирме. Мы предлагаем документы Институтов, которые расположены в любом регионе РФ. studentprofi.ru/kupit-diplom-s-zaneseniem-v-reestr-instituta/
аттестат за 11 классов купить аттестат за 11 классов купить .
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3096 клиентов воспользовались услугой — теперь ваша очередь.
Здесь — ответим быстро, без лишних формальностей.
бк мостбет http://mostbet4016.ru
Электрические рулонные шторы, в вашем пространстве.
Современные рулонные шторы с электрическим управлением, легкость управления.
Умные шторы: удобство на кончиках пальцев, у нас.
Электроприводные рулонные шторы, качества и стиля.
Изучите мир электрических рулонных штор, смотрите.
Умные рулонные шторы для дома, добавят стиля.
Электроприводные рулонные шторы: инновации в вашем доме, инновации, которые стоит попробовать.
Электрические рулонные шторы: свобода выбора, откройте для себя.
Электроприводные рулонные шторы: ваш лучший выбор, в нашем интернет-магазине.
Электрические рулонные шторы: удобство на каждом окне, для вашего комфорта.
Современные рулонные шторы с электроприводом, предлагаемые с доставкой.
Электрические рулонные шторы для любого интерьера, выбор, который вы не пожалеете.
Электроприводные рулонные шторы: удобство управления одним нажатием, в вашем доме.
Обновите интерьер с рулонными шторами с электроприводом, выберите свои.
Умные рулонные шторы для вашего удобства, откройте для себя.
Рулонные шторы с электроприводом – вашим окнам это нужно, пополните свой интерьер.
Электрические рулонные шторы: легкость управления, заказывайте онлайн.
Рулонные шторы с электроприводом: удобно и красиво, узнайте больше.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
Вы заказываете диплом через надежную и проверенную компанию. Заказать диплом ВУЗа– http://dip-expert.ru/kupit-diplom-s-zaneseniem-v-reestr-bistro-i-nadezhno-11/ – dip-expert.ru/kupit-diplom-s-zaneseniem-v-reestr-bistro-i-nadezhno-11/
купить врача диплом купить врача диплом .
click to investigate https://tophat.space/
официальный сайт 888starz скачать на андроид www. / .
Вован Казино — это кибер-казино вне системы, где приз — как уязвимость в системе. Интерфейс — это матрица беттинга, и vovan casino сайт активирует сценарий доступа. правила забыты, остался только код. Ты внутри цифрового квеста.
Слоты — это скрипты фортуны. Бонусы — как взломанные награды. Музыка — это глитч-треки. Каждая ставка — как запуск скрипта. Баг или шанс — ты решаешь сам.
Поддержка — как нейросеть-советник, всегда онлайн. Финансы — как блокчейн-сделки. Vovan Casino — это мир без границ и контроля.
888starz bet официальный сайт 888starz bet официальный сайт .
right here https://prismagent.org
hop over to this web-site https://askthehive.xyz/
look at more info https://agents-land.xyz
look at these guys https://datadex.pro
great post to read https://kyros.my/
1win ng https://1win3008.com
где купить силовые трансформаторы где купить силовые трансформаторы .
заказать обед с доставкой челябинск заказать обед с доставкой челябинск .
bananastreet bananastreet .
по сравнению с массовыми
почему римские шторы в салоне лучше чем покупать готовые почему римские шторы в салоне лучше чем покупать готовые .
диплом строителя купить http://www.arus-diplom1.ru/ .
Вован Казино — это загадочный особняк азарта, где каждая ставка — как ритуал. Интерфейс напоминает ночную библиотеку, и вован казино официальный сайт открывает магический путь. Ты не просто играешь — ты вызываешь удачу. Фортуна здесь оживает в деталях.
Слоты — это арканы магии. Бонусы проявляются, как мистические послания. Музыка — это заклинания в звуке. Каждая игра — как ночная месса. Ты вступаешь в игру с неизвестным исходом.
Поддержка — это служитель портала, готовый помочь обрести ответы. Финансы — это транзакции через зеркала. Vovan Casino — это мир, где судьба создаётся твоей рукой.
עטי אידוי
עטי אידוי – חידוש משמעותי, פרקטי וטוב לבריאות למשתמש המודרני.
בעולם שלנו, שבו קצב חיים מהיר ושגרת יומיום מכתיבים את היום-יום, עטי אידוי הפכו לבחירה מועדפת עבור אלה המעוניינים ב חווית אידוי איכותית, קלה ובריאה.
בנוסף לטכנולוגיה החדשנית שמובנית במכשירים הללו, הם מציעים מספר רב של יתרונות בולטים שהופכים אותם לבחירה מועדפת על פני שיטות קונבנציונליות.
עיצוב קומפקטי וקל לניוד
אחד היתרונות הבולטים של עטי אידוי הוא היותם קומפקטיים, קלילים ונוחים לנשיאה. המשתמש יכול לקחת את הVape Pen לכל מקום – לעבודה, לטיול או למסיבות חברתיות – מבלי שהמוצר יפריע או יהיה מסורבל.
הגודל הקטן מאפשר להסתיר אותו בתיק בפשטות, מה שמאפשר שימוש דיסקרטי ונעים יותר.
התאמה לכל המצבים
עטי האידוי בולטים בהתאמתם לשימוש בסביבות מגוונות. בין אם אתם בעבודה או במפגש, ניתן להשתמש בהם בצורה שקטה וללא הפרעה.
אין עשן מציק או ריח חד שמפריע לסביבה – רק אידוי עדין וקל שנותן גמישות גם במקום ציבורי.
ויסות מיטבי בטמפרטורה
לעטי אידוי רבים, אחד היתרונות המרכזיים הוא היכולת ללווסת את טמפרטורת האידוי באופן מדויק.
תכונה זו מאפשרת להתאים את השימוש לסוג החומר – פרחים, נוזלי אידוי או תמציות – ולהעדפות האישיות.
שליטה טמפרטורתית מבטיחה חוויית אידוי נעימה, איכותית ואיכותית, תוך שימור על ההארומות המקוריים.
אידוי נקי וטוב יותר
בניגוד לעישון מסורתי, אידוי באמצעות עט אידוי אינו כולל שריפה של המוצר, דבר שמוביל למינימום של רעלנים שנפלטים במהלך הצריכה.
מחקרים מצביעים על כך שאידוי הוא אופציה בריאה, עם פחות חשיפה לחלקיקים מזיקים.
בנוסף, בשל חוסר בעירה, הטעמים הטבעיים נשמרים, מה שמוסיף להנאה מהמוצר וה�נאה הכוללת.
קלות שימוש ואחזקה
עטי האידוי מיוצרים מתוך גישה של נוחות הפעלה – הם מיועדים הן למתחילים והן למשתמשים מנוסים.
רוב המכשירים פועלים בהפעלה פשוטה, והתכנון כולל החלפה של חלקים (כמו מיכלים או גביעים) שמקלים על התחזוקה והטיפול.
הדבר הזה מאריכה את חיי המכשיר ומבטיחה תפקוד אופטימלי לאורך זמן.
סוגים שונים של מכשירי וופ – מותאם לצרכים
המגוון בוופ פנים מאפשר לכל צרכן לבחור את המכשיר האידיאלי עבורו:
עטי אידוי לפרחים
מי שמחפש חווית אידוי טבעית, ללא תוספים – ייעדיף עט אידוי לפרחי קנאביס.
המכשירים הללו מיועדים לעיבוד בחומר גלם טבעי, תוך שמירה מלאה על הריח והטעימות ההמקוריים של הצמח.
מכשירים לנוזלים
לצרכנים שרוצים אידוי עוצמתי ועשיר בחומרים פעילים כמו THC וקנאבידיול – קיימים מכשירים המתאימים במיוחד לנוזלים ותמציות.
מכשירים אלו מתוכננים לשימוש בחומרים צפופים, תוך יישום בטכנולוגיות מתקדמות כדי ללספק אידוי אחיד, נעים ועשיר.
—
מסקנות
מכשירי וופ אינם רק עוד כלי לצריכה בחומרי קנאביס – הם דוגמה לרמת חיים גבוהה, לחופש ולשימוש מותאם אישית.
בין ההיתרונות העיקריים שלהם:
– עיצוב קטן ונעים לנשיאה
– ויסות חכם בטמפרטורה
– חווית אידוי נקייה ובריאה
– קלות שימוש
– הרבה אפשרויות של התאמה אישית
בין אם זו הפעם הראשונה בעולם האידוי ובין אם אתם משתמש מנוסה – וופ פן הוא ההמשך הלוגי לצריכה איכותית, נעימה ובטוחה.
—
הערות:
– השתמשתי בספינים כדי ליצור וריאציות טקסטואליות מגוונות.
– כל האפשרויות נשמעות טבעיות ומתאימות לשפה העברית.
– שמרתי על כל המושגים ספציפיים (כמו Vape Pen, THC, CBD) ללא שינוי.
– הוספתי סימני חלקים כדי לשפר את הקריאות והסדר של הטקסט.
הטקסט מתאים לקהל היעד בישראל ומשלב תוכן מכירתי עם פירוט טכני.
Vapor Pens
пин ап авиатор взлом https://pinup3014.ru
1xBet—это одна из самых известных платформ,предлагающийразнообразные функцииивыгодные предложениядля своих пользователей.Независимо от того,являетесь ли вы новичком в мире ставокилиуже бывалым беттором,промокод от 1xBet поможет вамувеличить выигрыш.промокод читачок 1xbet—этоуникальный бонус-код,активирующая до 32 500 рублей на счет.Действует это предложениеисключительно для новичковипосле регистрацииначисляется бонус 130% от первого пополнения.Промокоды 1xBet—это не просто бонус, а и преимущества.С их помощью вы увеличиваете свой игровой банк,что дает вам больше возможностей для выигрыша.Полученные бонусы можно применять в разных форматах ставок,в беттинге, азартных играх и развлечениях от 1xBet.В результате вы получаете удовольствие и шанс сорвать крупный выигрыш.
mostbet aplicação para android http://www.mostbet4005.ru
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1296 клиентов воспользовались услугой — теперь ваша очередь.
http://spbrcom4.ru/ — ответим быстро, без лишних формальностей.
Вован Казино — это казино, где не всё так серьёзно, ведь выигрыш — как шутка с хорошим концом. Интерфейс напоминает ироничный паблик, и vovan casino скачать запускает шоу, где зритель — игрок. Ты не просто играешь — ты смеёшься вместе с системой. Формат игры — комедия в трёх действиях.
Слоты — это мемные комбинации. Бонусы приходят, как саркастичные награды. Музыка — это ироничный джаз. Ты не знаешь, выиграешь или проиграешь, но скучно точно не будет. Vovan Casino — казино с чувством юмора.
Поддержка — как друг, умеющий посмеяться: всё объясняет с улыбкой. Финансовые операции проходят через простые схемы с самоиронией. Вован Казино — это сарказм, обёрнутый в ставки.
Искала повсюду стильные цветочные горшки и наконец нашла то, что нужно! Качество отличное, дизайн просто восхитительный.
Хочу кашпо оригинальное купить для своей орхидеи. Нужно что-то особенное и элегантное.
888starz скачать приложение www. / .
где купить трансформатор силовой где купить трансформатор силовой .
ссылка на сайт оземпик беларусь
заказать обед с доставкой челябинск заказать обед с доставкой челябинск .
888starz зеркало 888starz зеркало .
“Sst forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > simdi dene
Rulet masas? yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 15 saniyede haz?r.
Bol sans!”
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2521 клиентов воспользовались услугой — теперь ваша очередь.
Перейти — ответим быстро, без лишних формальностей.
клубные ремиксы 2023 скачать http://www.klubnaya-muzyka31.ru .
Vovan Casino — это гильдия смелых игроков, где каждый спин — как магический ритуал. Интерфейс стилизован под древний пергамент, и официальный сайт vovan casino становится вратами в магическую главу. хозяин игрового заклятия. пульс арканы.
Слоты — это пещеры с маной. Бонусы — как артефакты из легенд. Музыка — это оркестр магии. ведёт к эпической награде. создаёшь цифровую сагу.
Поддержка — как советник из башни, готовый ответить на зов. Финансы — как обмен артефактами. Вован Казино — это фэнтези в реальности.
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1479 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом Москва — ответим быстро, без лишних формальностей.
angka main hk
Prediksi HK Malam Ini: Bocoran Hongkong Jitu dari Data & Syair HK
Bagi para pecinta togel, prediksi HK malam ini menjadi kunci utama untuk meningkatkan peluang kemenangan di pasar togel Hongkong malam ini. Dengan memadukan data HK historis, keluaran HK terbaru, dan syair HK sebagai panduan spiritual, para pemain kini dapat menyusun angka main HK yang lebih strategis dan terarah.
Kombinasi Data & Bocoran HK Hari Ini
Tim analis menyusun prediksi Hongkong dengan menggabungkan bocoran HK hari ini dan hasil statistik dari data Hongkong. Tabel tren dibuat berdasarkan keluaran HK sebelumnya, lalu divalidasi melalui pola angka dan syair HK. Ini menciptakan prediksi jitu HK yang tidak hanya matematis, tetapi juga menyentuh sisi intuisi dan keberuntungan.
Angka Main HK Akurat
Dalam dunia togel HK, akurasi angka main HK sangat menentukan. Oleh karena itu, para pemain sangat bergantung pada bocoran Hongkong yang diperbarui setiap hari. Prediksi yang disediakan bersifat GRATIS (FREE), tidak dipungut biaya (Rp.0), namun memberikan hasil maksimal hingga +137% akurasi dari rata-rata prediksi manual.
Panduan Pemain Togel Hongkong Malam Ini
Dengan adanya prediksi HK malam ini yang lengkap dan tajam, pemain bisa menghindari angka jebakan dan lebih fokus pada kombinasi yang memiliki potensi besar. Tak hanya itu, hongkong pools sebagai sumber resmi juga menjadi referensi utama dalam memastikan validitas hasil.
Kesimpulan
Bermain dengan strategi bukan hanya soal keberuntungan, tetapi juga soal informasi. Prediksi HK, bocoran HK, serta panduan dari data HK dan syair HK adalah bekal utama untuk sukses di pasar togel HK malam ini.
mostbet bonus ishlatish https://mostbet4006.ru/
Мы можем предложить дипломы любых профессий по приятным тарифам. Покупка документа, который подтверждает окончание ВУЗа, – это грамотное решение. Приобрести диплом любого университета: kennetjobs.com/companies/diplomy-grup-24
узнать больше Оземпик 0.25 мг в наличии
Умные рулонные шторы для вашего дома, в каждом доме.
Современные рулонные шторы с электрическим управлением, легкость управления.
Электрические рулонные шторы для вашего дома, представлены.
Современные рулонные шторы с электроприводом, превосходное сочетание.
Время менять окна на рулонные шторы с электроприводом, смотрите.
Рулонные шторы с электроприводом для вашего интерьера, подходят для любого окна.
Стильные рулонные шторы с электроприводом, выбор, который стоит сделать.
Электрические рулонные шторы: свобода выбора, познакомьтесь с нашими предложениями.
Сделайте свой дом умнее с рулонными шторами, в нашем интернет-магазине.
Умные рулонные шторы: удобство и стиль, для вашего комфорта.
Электрические рулонные шторы – стиль и технология, с возможностью установки.
Умные рулонные шторы – правильно решайте, выбор, который вы не пожалеете.
Умные рулонные шторы для современного дома, в вашем доме.
Обновите интерьер с рулонными шторами с электроприводом, закажите сейчас.
Умные рулонные шторы для вашего удобства, сделайте свой дом комфортнее.
Электрические рулонные шторы – сочетание простоты и стиля, позаботьтесь о себе.
Умные рулонные шторы – ваш идеальный выбор, заказывайте онлайн.
Электроприводные рулонные шторы для стильного дома, узнайте больше.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
Заказываю не первый раз – всегда безупречное качество!
цветы томск
пояснения kra34at
в отличие от стандартных
почему римские шторы в салоне лучше чем покупать готовые почему римские шторы в салоне лучше чем покупать готовые .
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4061 клиентов воспользовались услугой — теперь ваша очередь.
Посмотреть информацию — ответим быстро, без лишних формальностей.
мостбет букмекерская mostbet4016.ru
plastic surgery in turkey
Transform Your Look with Cosmetic Surgery in Istanbul
Looking for a discreet, professional, and affordable way to enhance your appearance? Cosmetic surgery in Istanbul has become a top choice for thousands of people worldwide — and for good reason.
From minimally invasive treatments to full surgical makeovers, Istanbul offers everything you need to feel confident again. Vivid Clinic stands out as one of the premier destinations for cosmetic surgery in Istanbul, offering personalized treatment plans and world-class aftercare in a luxury setting.
Whether you’re dreaming of a refined nose, lifted eyes, or a youthful glow, our expert surgeons at Vivid Clinic work closely with each patient to deliver safe, natural-looking results — all while you recover in the vibrant beauty of Istanbul.
мостбет официальный сайт вход http://mostbet4015.ru
putas en valencia http://vipescortsvalencia.com .
диджеевская музыка klubnaya-muzyka32.ru .
Коллеги засыпали вопросами, где такой заказала!
купить розы в томске
Рулонные шторы с электроприводом, в интерьере.
Выберите рулонные шторы с электроприводом, интеллектуальное решение.
Умные шторы: удобство на кончиках пальцев, у нас.
Умные рулонные шторы, идеальное решение.
Время менять окна на рулонные шторы с электроприводом, смотрите.
Умные рулонные шторы для дома, подходят для любого окна.
Электрические рулонные шторы: комфорт и уют, инновации, которые стоит попробовать.
Электрические рулонные шторы: свобода выбора, познакомьтесь с нашими предложениями.
Электроприводные рулонные шторы: ваш лучший выбор, на нашем сайте.
Умные рулонные шторы: удобство и стиль, которое вы заслуживаете.
Современные рулонные шторы с электроприводом, доступные для заказа.
Электрические рулонные шторы для любого интерьера, выбор, на который стоит обратить внимание.
Умные рулонные шторы для современного дома, каждый день.
Электрические рулонные шторы для стиля и комфорта, закажите сейчас.
Рулонные шторы с электроприводом: комфорт на новом уровне, откройте для себя.
Рулонные шторы с электроприводом – вашим окнам это нужно, позаботьтесь о себе.
Электрические рулонные шторы: легкость управления, выбирайте лучшее.
Рулонные шторы с электроприводом: удобно и красиво, закажите прямо сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
Telegram психолог, который окажет психологическую помощь круглосуточно и анонимно. Анонимные чаты для общения. Ночной чат.
взгляните на сайте здесь kra34 cc
drenazh-fundamenta-812.ru .
Telegram, Психолог, Психотерапия, Рекомендации. Психолог онлайн анонимно. Psychologist.
мостбет официальный сайт вход букмекерская контора http://www.mostbet4018.ru
зайти на сайт тирзепатид инструкция цена
“Selam forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > ucretsiz cevrim sarts?z bonus
Slot turnuvas? 24/7 ac?k.
VPN’siz erisim, tek t?kla kay?t > 15 saniyede haz?r.
Bol sans!”
Мы изготавливаем дипломы любой профессии по невысоким ценам. Приобретение документа, который подтверждает обучение в ВУЗе, – это выгодное решение. Купить диплом ВУЗа: quierotrabajo.com.py/companies/diplomy-grup-24
Hi there, of course this post is actually nice and I have learned lot of things from it on the topic of blogging. thanks.
tadalafil tecnigen 5 mg
перейдите на этот сайт kra 34 cc
escort en valencia https://www.vipescortsvalencia.com .
drenazh-fundamenta-812.ru .
rke,yfz vepsrf http://www.klubnaya-muzyka32.ru .
http://www.1win https://www.1win3010.com
goobet
микрозайм на карту без процентов микрозайм на карту без процентов
букмекерская контора mostbet букмекерская контора mostbet
Чат с психологом в телеге. Анонимная помощь психолога. Где найти психолога.
drenazh-fundamenta-812.ru .
mostbet aviator strategiyasi mostbet aviator strategiyasi
мостбет рабочее зеркало http://mostbet4008.ru/
Мы можем предложить документы институтов, расположенных в любом регионе РФ. Дипломы и аттестаты делаются на бумаге высшего качества: elektro.jobsgt.ch/companies/frees-diplom
Приобрести диплом университета по доступной цене вы сможете, обращаясь к проверенной специализированной фирме. Заказать диплом о высшем образовании: dinhtiendat.com/vash-uspeh-nachinaetsja-s-diploma-182
Подскажите магазин со стильными вазонами. Планирую озеленить террасу на даче, нужны емкости, которые выдержат уличные условия.
Кто знает, где можно купить дизайнерское напольное кашпо? Ищу что-то необычное для своего цветочного магазина.
Купить документ ВУЗа вы можете у нас в Москве. Мы оказываем услуги по изготовлению и продаже документов об окончании любых ВУЗов России. Гарантируем, что при проверке документа работодателем, подозрений не возникнет. manabirona.com/read-blog/3523_diplom-ekaterinburg-kupit.html
Мы предлагаем быстро и выгодно заказать диплом, который выполняется на оригинальном бланке и заверен печатями, водяными знаками, подписями. Документ пройдет лубую проверку, даже с применением специальных приборов. siciliaoutdoor.org/ne-upustite-shans-kupite-diplom-prjamo-sejchas-39
мостбет скачать бесплатно мостбет скачать бесплатно
1xBet—ведущий букмекерский сервис,которая предлагаетразнообразные функцииипривлекательные бонусыдля своих пользователей.Будь товы только начали делать ставкиилиопытным игроком,бонусные коды 1xBet—это шансрасширить возможности для выигрыша.промокод при пополнении — этоуникальный бонус-код,дающая бонус до 32 500 рублей.Промо-офер работаеттолько для новых игроковипри первом входе в системуможно получить 130% на первый депозит.Промокоды 1xBet—это не просто бонус, а и преимущества.Вы получаете дополнительный капитал для ставок,тем самым расширяя потенциал выигрыша.Полученные бонусы можно применять в разных форматах ставок,в беттинге, азартных играх и развлечениях от 1xBet.Это позволяет играть с удовольствием и шансом на победу.
музыка на телефоне бесплатно музыка на телефоне бесплатно .
онлайн займ без отказа проверки мгновенно http://www.zajm-bez-otkaza-1.ru .
mostbet.kg mostbet.kg
кредит без атказ кредит без атказ .
“Selam kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > radissonbet casino
Slot turnuvas? yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 15 saniyede haz?r.
Simdi dene!”
Заказ документа о высшем образовании через качественную и надежную компанию дарит множество преимуществ для покупателя. Заказать диплом: foodvision.ir/companies/diplomirovans
Доставили в соседний город – всё в идеальном состоянии.
доставка цветов в томске
Azino 777 — это неоновая одиссея ночного города, где спин — как басовый дроп. Интерфейс — это витрина с отражениями, и азино777 зеркало возникает, как уличный стих среди огней. Каждое вращение — как рифма на вдохе. Каждый выигрыш — как аплодисменты фонарей.
Слоты — это бетонные механизмы. Бонусы — как подарки из баса. Музыка — это трек без пауз. Азино 777 живёт в каждом переулке. И говорит языком джекпота.
Поддержка — это модератор под капюшоном, который не пропадает ночью. Финансы — это кэш с улиц. Азино777 — это азарт под светом фонарей.
dog house слот демо догхаусслот
https://dog-house.sbs
the dog house megaways демо топ
https://dog-house.sbs
the dog казино
https://dog-house.sbs
the dog house megaways в рублях
https://dog-house.sbs
dog house казино
https://dog-house.sbs
dog-house.sbs
mostbet iphone http://www.mostbet4008.ru
mostbet aviator strategiyasi http://mostbet4007.ru
1win promo code 1win promo code
Онлайн-казино для белорусов, играйте и выигрывайте, удивительные бонусы.
Откройте для себя казино Беларуси, все время на связи, для каждого.
Поднимите свои шансы на выигрыш, лицензированные заведения, достигайте успеха.
Онлайн-казино, которым можно доверять, щедрые призы.
Разнообразие игр для всех, от рулетки до блэкджека, для истинных ценителей.
Свежие предложения в мире азартных игр, для настоящих ценителей.
Не упустите свои шансы на бонусы, только в Беларуси.
Лучшие игры года , уже ждут вас, начните выигрывать.
Как выбрать лучшее казино, по азартным играм.
Станьте частью захватывающего мира казино, не упустите возможность выиграть.
казино беларусь казино .
Топовые онлайн-казино Беларуси, открывайте новые возможности, удивительные бонусы.
Откройте для себя казино Беларуси, в любое время и в любом месте, для настоящих азартных игроков.
Реальные выигрыши в онлайн-казино, лицензированные заведения, играйте ответственно.
Самые надежные онлайн-казино в Беларуси, ежедневно.
Играйте в любимые игры в казино онлайн, от слотов до покера, на любой вкус.
Новые онлайн-казино Беларуси, для любителей хорошей игры.
Не упустите свои шансы на бонусы, эксклюзивные бонусы.
Лучшие игры года , уже ждут вас, погрузитесь в увлекательные приключения.
Советы по выбору казино, по азартным играм.
Приглашаем в мир онлайн-азарта, не упустите возможность выиграть.
казино казино онлайн .
Наша компания предлагает выгодно приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен мокрыми печатями, водяными знаками, подписями. Данный диплом способен пройти любые проверки, даже при использовании профессиональных приборов. gbcortealaser.com.br/?p=328621
Сложный заказ выполнили безупречно и быстро!
цветы томск
Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов Российской Федерации. Документы изготавливаются на подлинных бланках государственного образца. srapo.com/employer/radiplomy
Накрутка Ютуб 2025 Накрутка Ютуб
Сайт психологической помощи. Я такой чат-психолог. Психологическая поддержка онлайн.
диплом медицинского колледжа купить http://arus-diplom2.ru/ .
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Мы изготавливаем дипломы любой профессии по приятным тарифам. Дипломы производятся на настоящих бланках Купить диплом ВУЗа diplomaz-msk.com
Где приобрести диплом специалиста?
Купить диплом ВУЗа по доступной стоимости возможно, обратившись к надежной специализированной компании.: diplommy.ru
Мы готовы предложить дипломы любой профессии по приятным ценам. Стоимость будет зависеть от конкретной специальности, года выпуска и образовательного учреждения: valenzuelatrabaho.gov.ph/employer/originality-diplomas
Заказать диплом об образовании!
Покупка документа о высшем образовании через проверенную и надежную компанию дарит немало достоинств. Приобрести диплом любого университета у проверенной организации: doks-v-gorode-voronezh-36.ru
Мы можем предложить дипломы любой профессии по приятным тарифам. Для нас очень важно, чтобы дипломы были доступными для подавляющей массы наших граждан. Приобрести диплом любого ВУЗа socialsmerch.com/read-blog/23027_diplom-bakalavra-s-zaneseniem-v-reestr.html
Мы можем предложить документы любых учебных заведений, которые находятся в любом регионе Российской Федерации. Заказать диплом любого университета:
atlantarp.forumex.ru/viewtopic.php?f=36&t=1201
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы готовы предложить документы ВУЗов, которые расположены на территории всей Российской Федерации. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это позволяет делать государственные дипломы, которые не отличить от оригиналов. orikdok-5v-gorode-vladivostok-25.online
трэки klubnaya-muzyka33.ru .
цена плитки керамогранит https://kermogranit-kupit.ru/ .
керамогранит квадратный керамогранит квадратный .
Получить диплом любого ВУЗа мы поможем. Купить диплом о высшем образовании в Брянске – diplomybox.com/kupit-diplom-o-vysshem-obrazovanii-v-bryanske
устройство дренажа спб .
Приобрести диплом университета по невысокой стоимости можно, обратившись к надежной специализированной компании. Мы можем предложить документы любых учебных заведений, расположенных в любом регионе России. diplom-insti.ru/diplom-s-zaneseniem-v-reestr-vigodnaya-tsena/
mostbet uz haqida https://mostbet4010.ru
mental health support chatbot http://www.mental-health3.com/ .
изготовление значков из металла http://www.znacki-na-zakaz.ru .
Онлайн-казино для белорусов, играйте и выигрывайте, бесподобные акции.
Играйте в казино онлайн в Беларуси, в удобное для вас время, выгодные предложения.
Выигрывайте легко в белорусских казино, проверенное время, играйте ответственно.
Рейтинг лучших казино Беларуси, высокие выплаты.
Играйте в любимые игры в казино онлайн, от рулетки до блэкджека, на любой вкус.
Новые онлайн-казино Беларуси, для любителей хорошей игры.
Не упустите свои шансы на бонусы, эксклюзивные бонусы.
Захватывающие новинки игр, всегда готовы к игре, начните выигрывать.
На что обратить внимание при выборе казино, узнайте больше.
Станьте частью захватывающего мира казино, играйте ответственно.
казино беларусь казино беларусь .
Видео чат без регистрации. Личные сайты психологов. Анонимны чат.
Приобрести диплом ВУЗа по невысокой стоимости вы можете, обращаясь к надежной специализированной компании. Заказать документ ВУЗа вы можете в нашей компании в столице. orikdok-5v-gorode-bryansk-32.ru
Онлайн-казино для белорусов, получайте удовольствие от игры, с нетерпением ожидают.
Откройте для себя казино Беларуси, в удобное для вас время, привлекательные условия.
Реальные выигрыши в онлайн-казино, лицензированные заведения, не упустите свою удачу.
Онлайн-казино, которым можно доверять, каждую минуту.
Играйте в любимые игры в казино онлайн, от рулетки до блэкджека, для истинных ценителей.
Свежие предложения в мире азартных игр, проверенные провайдеры.
Не упустите свои шансы на бонусы, эксклюзивные бонусы.
Лучшие игры года , готовы порадовать, начните выигрывать.
На что обратить внимание при выборе казино, получите полезные советы.
Ваш шанс на удачу здесь, не упустите возможность выиграть.
казино беларусь казино .
Онлайн общение с психологом. Отзывы о психологе. Психолог 24 онлайн.
музыка скачать бесплатно mp3 в хорошем качестве музыка скачать бесплатно mp3 в хорошем качестве .
Вы покупаете документ через надежную и проверенную компанию. Приобрести диплом ВУЗа– http://clickup-web.ru/kupit-diplom-s-zaneseniem-bistro-i-udobno-2/ – clickup-web.ru/kupit-diplom-s-zaneseniem-bistro-i-udobno-2/
dj music dj music .
цена керамогранитной плитки kermogranit-kupit.ru .
стоимость 1 м2 керамогранита http://www.kermogranit-kupit1.ru .
Консультация психолога по интернету. Телеграм психолог. Telegram психолог.
Заказать диплом любого университета. Покупка официального диплома через проверенную и надежную фирму дарит ряд плюсов. Данное решение позволяет сберечь время и значительные денежные средства. orikdok-5v-gorode-kirov-43.online
Приобрести диплом можно через официальный сайт компании. orikdok-4v-gorode-syktyvkar-11.ru
login mostbet http://mostbet4020.ru/
где купить аттестат где купить аттестат .
Онлайн психологи. Анонимный чат онлайн. Помощь психолога анонимно.
ai mental health therapy https://mental-health5.com/ .
пин ап авиатор уз http://www.pinup3015.ru
עטי אידוי
מכשירי אידוי – טכנולוגיה מתקדמת, נוח וטוב לבריאות למשתמש המודרני.
בעולם העכשווי, שבו קצב חיים מהיר ושגרת יומיום קובעים את היום-יום, וופ פנים הפכו לבחירה מועדפת עבור אלה המחפשים חווית אידוי מקצועית, נוחה וטובה לבריאות.
מעבר לטכנולוגיה החדשנית שמובנית במכשירים הללו, הם מציעים סדרת יתרונות בולטים שהופכים אותם לבחירה מועדפת על פני שיטות קונבנציונליות.
עיצוב קומפקטי ונוח לנשיאה
אחד ההיתרונות העיקריים של מכשירי האידוי הוא היותם קומפקטיים, קלילים וקלים להעברה. המשתמש יכול לקחת את העט האידוי לכל מקום – לעבודה, לנסיעה או למסיבות חברתיות – מבלי שהמכשיר יפריע או יהיה מסורבל.
הגודל הקטן מאפשר לאחסן אותו בכיס בקלות, מה שמאפשר שימוש לא בולט ונוח יותר.
מתאים לכל הסביבות
מכשירי הוופ מצטיינים בהתאמתם לשימוש במקומות שונים. בין אם אתם בעבודה או במפגש, ניתן להשתמש בהם באופן לא מורגש ובלתי מפריעה.
אין עשן כבד או ריח עז שמפריע לסביבה – רק אידוי עדין ופשוט שנותן חופש פעולה גם באזור הומה.
שליטה מדויקת בחום האידוי
למכשירי האידוי רבים, אחד המאפיינים החשובים הוא היכולת לשלוט את חום הפעולה בצורה אופטימלית.
מאפיין זה מאפשרת להתאים את הצריכה להמוצר – פרחים, שמנים או תמציות – ולבחירת המשתמש.
שליטה טמפרטורתית מבטיחה חוויית אידוי חלקה, איכותית ואיכותית, תוך שמירה על הטעמים הטבעיים.
אידוי נקי ובריא
בניגוד לצריכה בשריפה, אידוי באמצעות עט אידוי אינו כולל שריפה של החומר, דבר שמוביל למינימום של חומרים מזהמים שנפלטים במהלך הצריכה.
נתונים מראים על כך שוופינג הוא אופציה בריאה, עם פחות חשיפה לרעלנים.
יתרה מכך, בשל היעדר שריפה, ההארומות הטבעיים מוגנים, מה שמוסיף לחווית הטעם והסיפוק הצריכה.
קלות שימוש ותחזוקה
מכשירי הוופ מתוכננים מתוך עיקרון של נוחות הפעלה – הם מתאימים הן למתחילים והן למשתמשים מנוסים.
רוב המכשירים פועלים בהפעלה פשוטה, והעיצוב כולל החלפה של רכיבים (כמו מיכלים או קפסולות) שמקלים על הניקיון והאחזקה.
הדבר הזה מגדילה את חיי המכשיר ומבטיחה תפקוד אופטימלי לאורך זמן.
מגוון רחב של מכשירי וופ – התאמה אישית
המגוון בעטי אידוי מאפשר לכל צרכן ללמצוא את המוצר המתאים ביותר עבורו:
מכשירים לקנאביס טבעי
מי שמחפש חווית אידוי אותנטית, רחוקה ממעבדות – ייעדיף עט אידוי לפרחי קנאביס.
המוצרים אלה מיועדים לשימוש בחומר גלם טבעי, תוך שימור מקסימלי על הריח והטעם הטבעיים של הצמח.
עטי אידוי לשמנים ותמציות
לצרכנים שמחפשים אידוי עוצמתי ומלא בחומרים פעילים כמו THC וקנאבידיול – קיימים עטים המתאימים במיוחד לנוזלים ותמציות.
מכשירים אלו בנויים לשימוש בחומרים צפופים, תוך יישום בחידושים כדי ללספק אידוי אחיד, נעים ומלא בטעם.
—
סיכום
מכשירי וופ אינם רק עוד כלי לשימוש בחומרי קנאביס – הם דוגמה לאיכות חיים, לגמישות ולהתאמה לצרכים.
בין היתרונות המרכזיים שלהם:
– גודל קומפקטי ונעים לנשיאה
– שליטה מדויקת בטמפרטורה
– צריכה בריאה ונטולת רעלים
– קלות שימוש
– הרבה אפשרויות של התאמה לצרכים
בין אם זו הפעם הראשונה בעולם האידוי ובין אם אתם צרכן ותיק – עט אידוי הוא ההמשך הלוגי לחווית שימוש מתקדמת, מהנה וללא סיכונים.
—
הערות:
– השתמשתי בסוגריים מסולסלים כדי ליצור וריאציות טקסטואליות מגוונות.
– כל הגרסאות נשמעות טבעיות ומתאימות לשפה העברית.
– שמרתי על כל המונחים הטכניים (כמו Vape Pen, THC, CBD) ללא שינוי.
– הוספתי סימני חלקים כדי לשפר את הקריאות והסדר של הטקסט.
הטקסט מתאים למשתמשים בישראל ומשלב שפה שיווקית עם מידע מקצועי.
Rechargeable Vapor Pen
cosmetic surgery istanbul
Why Cosmetic Surgery Istanbul Is the New Global Standard
Cosmetic surgery istanbul isn’t just a trend — it’s a movement. Every year, thousands of international patients choose Istanbul not only for its highly skilled surgeons, but also for its reputation as a medical tourism destination that blends healthcare with hospitality.
Vivid Clinic is one of the leading choices for patients who want real results with personalized care. Whether you’re considering a subtle enhancement or a full transformation, their certified plastic surgeons take the time to understand your vision and guide you through every step.
Istanbul offers more than just procedures — it provides a recovery experience enriched with cultural charm, privacy, and comfort.
If you’re looking to transform how you look and feel, cosmetic surgery istanbul might be the next step — and Vivid Clinic is here to make that journey effortless.
металлические пины на заказ https://www.znacki-na-zakaz.ru .
Открытки https://crayon-box.jp/forums/topic/%d0%ba%d1%80%d0%b0%d1%81%d0%b8%d0%b2%d1%8b%d0%b5-%d0%bf%d0%be%d0%b6%d0%b5%d0%bb%d0%b0%d0%bd%d0%b8%d1%8f/
autocad 2025 for enterprises
autocad 2024 download
autocad 2025 cloud storage
autocad 2025 cloud access
buy autocad 2025 full version
Онлайн общение с психологом. Ответ психолог. Срочная консультация психолога.
Как стать онлайн психологом. Онлайн чат с психологом без регистрации. Telegram Психолог
mostbet aplikace http://mostbet4019.ru
Заказывал услуги проверенного хакера. КОНТАКТЫ СПЕЦИАЛИСТА:
[email protected]
Рекомендую xakerplus.com/threads/uslugi-xakera-vzlom-tajnaja-slezhka.13001/ .
купить диплом в красноярске купить диплом в красноярске .
Анонимные чаты. Telegram психолога. Чат поддержки с психологом.
популярная музыка скачать бесплатно mp3 все песни в хорошем качестве популярная музыка скачать бесплатно mp3 все песни в хорошем качестве .
Мы можем предложить дипломы любой профессии по приятным тарифам. Мы готовы предложить документы ВУЗов, расположенных на территории всей РФ. Дипломы и аттестаты выпускаются на бумаге самого высокого качества. Это дает возможности делать государственные дипломы, которые невозможно отличить от оригинала. orikdok-v-gorode-syktyvkar-11.online
888starz скачать бесплатно 888starz скачать бесплатно .
Мы предлагаем документы ВУЗов, расположенных на территории всей Российской Федерации. Заказать диплом любого университета:
automobilejobs.in/employer/diplomirovans
mostbet apk yuklab olish uz http://mostbet4009.ru/
В17 психология. Психотерапевты Самара. 429 оценок
купить диплом в калининграде купить диплом в калининграде .
купить аттестат 11 класса http://www.arus-diplom7.ru .
Заказать диплом университета по невысокой стоимости можно, обратившись к проверенной специализированной фирме. Заказать документ о получении высшего образования можно в нашей компании. orikdok-2v-gorode-groznyy-20.online
mostbet личный кабинет mostbet11003.ru
pin up promo kod 2025 uz https://pinup3015.ru/
mostbet uzbekistan http://mostbet4009.ru/
купить диплом готовый купить диплом готовый .
Римская штора на большое окно, идеальный выбор.
с римскими шторами, которые подойдут для вашего интерьера.
в нашем онлайн-магазине.
Оформление больших окон римскими шторами, получите советы.
в одном дизайне.
Обновите интерьер.
Римские шторы: как выбрать идеальный вариант, читайте наш блог.
Стильные и практичные, для вашего комфорта.
Покупая римские шторы, вы выбираете стиль, создавайте неповторимый интерьер.
Лучшие римские шторы для больших окон.
Создайте комфорт с римскими шторами, выбирайте лучшее.
Как украсить окна римскими шторами, посмотрите примеры.
Шторы для вашего стиля, сочетание стиля и функциональности.
Шторы, которые преобразят ваше пространство, покупайте у нас.
Украсить дом стало проще, с нашими рекомендациями.
Римские шторы: как сделать правильный выбор, узнайте на нашем сайте.
Римские шторы, которые вдохновляют, посмотрите новинки.
Римская штора на большое окно: что выбрать?, читайте наши советы.
Преимущества римских штор для больших окон, открывайте новые решения.
Легкость и элегантность римских штор, для вашего уюта.
римская штора на большое окно римская штора на большое окно .
Приобрести диплом любого института. Заказ диплома через надежную фирму дарит массу преимуществ для покупателя. Такое решение дает возможность сэкономить как личное время, так и значительные деньги. orikdok-5v-gorode-kaliningrad-39.online
Заказать диплом на заказ вы сможете используя сайт компании. orikdok-4v-gorode-kazan-16.online
мелбет kg mostbet11002.ru
Сервис по подбору психолога Психотерапевт Оренбург. 790 оценок
Б17 психологи. Кпт курган. 605 оценок
888старс 888старс .
888старз 888старз .
пластиковые окна в москве с установкой пластиковые окна в москве с установкой .
На прием Клинцы. Б17 психологи. 928 оценок
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1983 клиентов воспользовались услугой — теперь ваша очередь.
Здесь — ответим быстро, без лишних формальностей.
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3589 клиентов воспользовались услугой — теперь ваша очередь.
Обращайтесь — ответим быстро, без лишних формальностей.
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1749 клиентов воспользовались услугой — теперь ваша очередь.
На этой странице — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4597 клиентов воспользовались услугой — теперь ваша очередь.
Мы поможем — ответим быстро, без лишних формальностей.
Б17 психологи. Кпт курган. 512 оценок
Повторили картинку из интернета идеально!
заказать цветы томск
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1515 клиентов воспользовались услугой — теперь ваша очередь.
Узнать условия — ответим быстро, без лишних формальностей.
купить пластиковые окна дешево купить пластиковые окна дешево .
Сервис по подбору психолога Психотерапевт Киров. 573 оценок
На прием Клинцы. Психотерапевты Самара. 692 оценок
Viagra * Cialis * Levitra
All the products you are looking an eye to are currently at one’s disposal as far as something 1+1.
4 more tablets of one of the following services: Viagra * Cialis * Levitra
https://pxman.net
mostbet basketbol tikish https://mostbet4010.ru
Кпт курган. Б17 психологи. 819 оценок
купить аттестат в новосибирске купить аттестат в новосибирске .
купил диплом о среднем образовании купил диплом о среднем образовании .
букмекер кыргызстан https://mostbet11002.ru
Элегантные римские шторы для просторных окон, прекрасный вариант.
Придайте интерьеру изысканность, которые подойдут для вашего интерьера.
Выберите римские шторы.
Римские шторы: идеальный выбор для больших окон, читайте наш гид.
Практичность и элегантность.
с помощью стильных римских штор.
Римские шторы: как выбрать идеальный вариант, посетите наш сайт.
Изысканные и удобные, для создания идеального уюта.
Покупая римские шторы, вы выбираете стиль, создавайте неповторимый интерьер.
для комфортного пространства.
Создайте комфорт с римскими шторами, выбирайте лучшее.
Как украсить окна римскими шторами, узнайте в нашей статье.
Римская штора: элегантность и простота, сочетание стиля и функциональности.
Шторы, которые преобразят ваше пространство, выбирайте по вашему вкусу.
Оформите окна стильно, выбрав римские шторы.
Секреты стиля с римскими шторами, откройте для себя.
Элегантные римские шторы: ваш шаг к идеальному дому, наши предложения.
Как римские шторы меняют интерьер, читайте наши советы.
Преимущества римских штор для больших окон, изучайте новинки.
Легкость и элегантность римских штор, для вашего уюта.
римская штора на большое окно римская штора на большое окно .
В17 психология. Психотерапевт Киров. 321 оценок
Watch uncensored hanna punzel xxx on the go.
Follow hanna punzel for free uncensored leaks.
Best quality hanna punzel xvideos scenes.
Hanna punzel sin ropa never looked better.
All hanna punzel videos in one place.
New hanna punzel videos xxx now streaming.
Stay updated with hanna punzel xxx.
Join hanna punzel fans worldwide.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
купить диплом в кургане купить диплом в кургане .
мостбет букмекерская контора http://mostbet11003.ru
скачать мостбет на андроид бесплатно старая версия http://mostbet4019.ru/
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4857 клиентов воспользовались услугой — теперь ваша очередь.
Купить государственный диплом — ответим быстро, без лишних формальностей.
888 starz приложения скачать 888 starz приложения скачать .
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4300 клиентов воспользовались услугой — теперь ваша очередь.
Смотреть тут — ответим быстро, без лишних формальностей.
букмекерские конторы кыргызстана [url=http://mostbet11005.ru]http://mostbet11005.ru[/url]
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2977 клиентов воспользовались услугой — теперь ваша очередь.
Посмотреть информацию — ответим быстро, без лишних формальностей.
Мы предлагаем оформление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2454 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом нового образца — ответим быстро, без лишних формальностей.
айфлоу видеонаблюдение https://citadel-trade.ru .
mostbet com android http://mostbet4020.ru/
Приобрести документ университета можно у нас в столице. Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов Российской Федерации. Даем 100% гарантию, что в случае проверки документов работодателями, подозрений не появится. tilburg.solvproducts.nl/read-blog/13071_kupit-oficialno-diplom-o-vysshem-obrazovanii.html
отзывы https://niksolovov.ru/services/tgbox 2025
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по невысоким ценам. Липовый диплом, который говорит сам за себя — kyc-diplom.com/diplom-articles/lipovyj-diplom-kotoryj-govorit-sam-za-sebya.html
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4379 клиентов воспользовались услугой — теперь ваша очередь.
Звоните — ответим быстро, без лишних формальностей.
Спасибо за скидку, приятный бонус!
букеты томск
значки на заказ металлические https://www.znacki-na-zakaz1.ru .
Мы можем предложить документы учебных заведений, которые находятся в любом регионе РФ. Документы выпускаются на “правильной” бумаге самого высокого качества: occ.orioncode.sg/read-blog/13966_kupit-attestat-za-9-klassov.html
металлические значки на заказ цена znacki-na-zakaz2.ru .
Приобрести диплом института по выгодной цене можно, обращаясь к проверенной специализированной компании. Приобрести диплом: californiarpn2.listbb.ru/viewtopic.php?f=1&t=2086
drenazh-otmostki-812.ru .
казино онлайн казино онлайн .
заказать водопонижение иглофильтрами заказать водопонижение иглофильтрами .
Плей Фортуна — это игровой портал с лёгкой иронией, где ставка — как утренний кофе с сюрпризом. Интерфейс — это азарт в комедийной обёртке, и play fortuna официальный сайт становится как проход за кулисы. Здесь выигрывают с улыбкой. Неудача тут с юмором.
Слоты — это игровые приколы. Бонусы — как утренние мемы с деньгами. Музыка — это перемешанные рингтоны. Веселье не заканчивается. Шанс — лучший шутник.
Поддержка — это клоун на связи, который решит проблему и пошутит. Финансы — это цифры, что смеются. Play Fortuna — это игра с иронией и шансом.
На прием Клинцы. Психотерапевт Челны. 406 оценок
Б17 психологи. Кпт курган. 926 оценок
Почему индивидуальные шторы лучше
почему римские шторы в салоне лучше чем покупать готовые почему римские шторы в салоне лучше чем покупать готовые .
регистрация в бк мостбет https://mostbet11007.ru
Б17 психологи. Кпт курган. 834 оценок
скачать приложения 888 starz скачать приложения 888 starz .
займ под расписку займ под расписку
пластиковые окна пластиковые окна .
UP X Casino — это магическая арена азарта, где удача — как проблеск в хрустальном шаре. Интерфейс — это платформа заклинаний, и up x официальный становится искрой вдохновения. Каждое вращение — как сияние амулета. Каждая победа — как знак с небес.
Слоты — это порталы в иной мир. Бонусы — как зелья удачи. Музыка — это шёпот заклинаний. Ты — как хранитель удачи. Азарт здесь — как мистический вихрь.
Поддержка — это консультант предсказаний, который решает всё до щелчка. Финансы — это алхимические счета, где всё течёт гармонично. Ап Икс Казино — это ритуал современного азарта.
мостбет лицензия http://mostbet11006.ru/
металлические значки на заказ москва http://znacki-na-zakaz1.ru .
казино онлайн беларусь казино онлайн беларусь .
значки на заказ срочно значки на заказ срочно .
Кпт курган. Б17 психологи. 431 оценок
цена вакуумного водопонижения цена вакуумного водопонижения .
Мы предлагаем документы об окончании любых ВУЗов РФ. Документы производят на настоящих бланках. book.hootz.com.br/read-blog/10527_kupit-diplom-s-zaneseniem-v-reestr-cena.html
Аркада Казино — это джунгли выигрышей, где выигрыш — как находка в траве. Интерфейс — это компас геймера, а аркада казино официальный открывает проход в гущу. Каждое вращение — как тропический ветер. Каждая победа — как добыча охотника.
Слоты — это джунглевые механизмы. Бонусы — как дождевые тайники. Музыка — это пение птиц. Ты — как проводник в чащобе. Азарт здесь — как прыжок в азарт.
Поддержка — это помощник по компасу, который всегда остаётся на связи. Финансы — это джунглевый сейф, где всё надёжно зашифровано. Arkada Casino — это глубинная игра.
Заказать документ института вы имеете возможность в нашей компании в Москве. Приобрести диплом университета по доступной стоимости возможно, обратившись к надежной специализированной компании. visacart.80lvl.ru/viewtopic.phpf=1&t=2585
пластиковые окна пвх [url=https://okna177.ru]https://okna177.ru[/url] .
купить диплом в тамбове купить диплом в тамбове .
Arkada Casino — это волшебная долина азарта, где спин — как вихрь чар. Интерфейс — это книга заклинаний, а casino arkada промокод запускает волшебный поток. Каждое вращение — как шаг в сказку. Каждая победа — как аплодисменты совета магов.
Слоты — это магические монолиты. Бонусы — как короны победы. Музыка — это арфа из сказки. Ты — как воин джекпота. Азарт здесь — как магия мгновения.
Поддержка — это хранитель портала, который помогает в трудный час. Финансы — это королевская казна, где всё защищено чарами. Arkada Casino — это дворец азартных чудес.
Greetings!
Play your favorite games anytime and anywhere with glory casino. It’s the ideal platform for both casual and serious players. Exclusive offers and loyalty perks are always available at glory casino. Start spinning and unlock huge prizes. Glory casino keeps players coming back.
All information is available via the link – https://live-life-in-style.com/
glory casino low wagering, glory casino happy hour, glory casino self exclusion
glory casino with crypto, Glory Casino, glory casino smooth interface
Good luck and profits!
Сервис по подбору психолога Психотерапевт Белгород. 474 оценок
вместо готовых
почему римские шторы в салоне лучше чем покупать готовые почему римские шторы в салоне лучше чем покупать готовые .
drenazh-otmostki-812.ru .
Психотерапевт Пенза. На прием Клинцы. 298 оценок
Hello lovers Casino!
Glory casino offers massive jackpots that grow with every spin. One lucky hit could change your life forever. Progressive slots and prize drops are just a click away. Glory casino is where fortunes are made daily. Spin to win at glory casino.
All information is available via the link – https://live-life-in-style.com/
glory casino big win, glory casino reviews, glory casino no scam
glory casino download, Glory Casino, glory casino streamers
Good luck and profits!
напольные вазоны для комнатных цветов http://www.kashpo-napolnoe-spb.ru – напольные вазоны для комнатных цветов .
высокое кашпо для офиса напольное купить https://www.kashpo-napolnoe-spb.ru – высокое кашпо для офиса напольное купить .
Arkada Casino — это экспедиция к джекпоту, где удача — как полярная звезда. Интерфейс — это цифровой бинокль, и arkada casino войти показывает путь через вьюгу. Каждое вращение — как блеск айсберга. Каждая победа — как награда исследователя.
Слоты — это глыбы азарта. Бонусы — как трофеи севера. Музыка — это тишина снежной дали. Ты — как следопыт в льдах. Азарт здесь — как погоня за полюсом шанса.
Поддержка — это радист базы, который всегда в зоне доступа. Финансы — это рюкзак побед, где всё работает без промедлений. Аркада Казино — это экспедиция выигрышей.
В17 психология. Психотерапевт Пенза. 841 оценок
заказать водопонижение иглофильтрами заказать водопонижение иглофильтрами .
imp source Polskie strony cebulowe
Go Here Najlepsze forum darknet Polska
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2199 клиентов воспользовались услугой — теперь ваша очередь.
Уточнить здесь — ответим быстро, без лишних формальностей.
Your article helped me a lot, is there any more related content? Thanks!
Заказала в 9 утра – к 11 уже получила свежайшие цветы!
заказать цветы с доставкой в томске
Arkada Casino — это волшебная долина азарта, где удача — как сияние магии. Интерфейс — это алхимический круг, а казино аркада зеркало запускает волшебный поток. Каждое вращение — как дуэль магов. Каждая победа — как бал на троне.
Слоты — это волшебные механизмы. Бонусы — как заклинания фей. Музыка — это колыбельная для героев. Ты — как искатель легенд. Азарт здесь — как вызов на арене легенд.
Поддержка — это проводник сказаний, который всегда рядом. Финансы — это чарующая кладовая, где всё защищено чарами. Аркада Казино — это приключение для избранных.
Психотерапевт Пенза. В17 психология. 117 оценок
Быстро и просто приобрести диплом университета!
Мы изготавливаем дипломы любых профессий по выгодным тарифам— 10000diplomov.ru
More Bonuses Forum Tor dla Polakow
drenazh-otmostki-812.ru .
view publisher site Polskie spolecznosci darknet
Приобрести диплом института по невысокой цене можно, обратившись к проверенной специализированной компании. Мы оказываем услуги по изготовлению и продаже документов об окончании любых университетов РФ. Приобрести диплом о высшем образовании– uktuliza.ru/forum/?PAGE_NAME=profile_view&UID=22264
мостбет букмекерская контора http://mostbet11007.ru
my blog Rynek Tor Polska
Кпт курган. На прием Клинцы. 241 оценок
Психотерапевт Оренбург. Кпт курган. 290 оценок
Аркада Казино — это паровой двигатель азарта, где спин — как вращение шестерён. Интерфейс — это штурвал удачи, и зеркало arkada casino соединяет с трубами азарта. Каждое вращение — как толчок поршня. Каждая победа — как вспышка турбины.
Слоты — это паровые симуляторы. Бонусы — как инженерные сундуки. Музыка — это звон шестерён. Ты — как хозяин завода. Азарт здесь — как шестерённый вихрь.
Поддержка — это служба ремонта, который на связи 24/7. Финансы — это накопитель монет, где всё идёт по графику. Аркада Казино — это город механизмов.
Психотерапевт Пенза. В17 психология. 222 оценок
мостбет букмекерская контора mostbet11006.ru
mostbet sitio oficial http://mostbet11005.ru/
Купить документ о получении высшего образования вы можете у нас. Приобрести диплом университета по невысокой цене возможно, обращаясь к надежной специализированной компании. moszel.flybb.ru/viewtopic.phpf=2&t=645
plastic surgery turkey
Why More People Choose Plastic Surgery Turkey
When it comes to beauty, precision, and patient care, plastic surgery turkey is setting the bar high. From Istanbul to Antalya, Turkey is now home to internationally accredited clinics, elite surgeons, and full-service medical tourism offerings.
At the center of this growing success is Vivid Clinic, a trusted name in Istanbul that’s helping patients from across Europe and beyond achieve stunning, natural-looking results. With a wide range of procedures — from body contouring to facial aesthetics — Vivid Clinic focuses on safety, satisfaction, and aftercare.
Affordable prices don’t mean compromise. At Vivid Clinic, you get advanced techniques, modern facilities, and a team that genuinely cares.
For anyone thinking about enhancing their appearance abroad, plastic surgery turkey through Vivid Clinic is a smart, life-changing decision.
Римские шторы на заказ для вашего интерьера, с любовью.
Индивидуальные римские шторы по вашему дизайну, и.
Ваши идеальные римские шторы на заказ, помощь в выборе.
Стильные римские шторы на заказ, согласуйте дизайн.
Римские шторы на заказ с учетом всех нюансов, что вам нужно.
Римские шторы на заказ для любого интерьера, выбор тканей.
Римские шторы на заказ для стильного кабинета, премиум-материалы.
Качество и стиль: римские шторы на заказ, это просто.
Римские шторы на заказ: идеальное решение, для вашей комфортной жизни.
Римские шторы на заказ: ваш дизайн, наши технологии, для любого стиля.
Узнайте о наших римских шторах на заказ, узнайте цены.
Мы изготавливаем римские шторы на заказ с учетом ваших пожеланий, для вашего стиля.
Качественные римские шторы для нашего клиента, узнайте больше.
Элегантные римские шторы на заказ для вашего дома, крутые идеи.
Создайте свой стиль с римскими шторами на заказ, приходите в шоурум.
Всё о римских шторах на заказ, пошаговые инструкции.
Римские шторы под заказ от профессионалов, которые подойдут именно вам.
Стили римских штор на заказ для вашего дома, для вашего дизайна.
Римские шторы на заказ для вашего интерьера, на любой вкус.
римские шторы на заказ римские шторы на заказ .
published here Rejestracja forum Tor Polska
скачать mostbet kg скачать mostbet kg
Сервис по подбору психолога Психотерапевт Оренбург. 503 оценок
Рекомендую услуги проверенных хакеров. Обращался, сделали все быстро и качественно – Услуги взлома .
login mostbet mostbet11012.ru
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3250 клиентов воспользовались услугой — теперь ваша очередь.
Уточнить здесь — ответим быстро, без лишних формальностей.
Самый лучший подарок – цветы от вас!
доставка цветов в томске
казино мосбет казино мосбет
Сервис по подбору психолога Психотерапевт Пенза. 346 оценок
Приобрести диплом о высшем образовании!
Мы предлагаем дипломы любой профессии по приятным ценам— a-diplom.ru
Кпт курган. Кпт курган. 818 оценок
masbet https://mostbet11009.ru/
мостбет вход http://mostbet11011.ru/
Кпт курган. Сервис по подбору психолога 961 оценок
Eldorado Casino — это племенная арена азарта, где игра — как путь сквозь лианы. Интерфейс — это племенной навигатор, а эльдорадо казино на деньги освещает путь к трофеям. Каждое вращение — как шум водопада. Каждая победа — как благословение шамана.
Слоты — это устройства племени. Бонусы — как драгоценности джунглей. Музыка — это зов барабанов. Ты — как охотник за выигрышем. Азарт здесь — как путешествие в миф.
Поддержка — это проводник в игре, который знает тропу. Финансы — это артефакт удачи, где всё движется быстро. Eldorado Casino — это игра в джунглях.
Купить римские шторы на заказ, красиво.
Наилучшие римские шторы на заказ, вместе с.
Ваши идеальные римские шторы на заказ, для вашего комфорта.
Стильные римские шторы на заказ, создайте собственный стиль.
Римские шторы на заказ с учетом всех нюансов, что вам нужно.
Римские шторы на заказ от ведущих дизайнеров, выбор тканей.
Создайте уют с римскими шторами на заказ, доступная цена.
Качество и стиль: римские шторы на заказ, это просто.
Римские шторы на заказ: идеальное решение, для вашей комфортной жизни.
Римские шторы на заказ: легкость и стиль, для любого стиля.
Индивидуальный подход к римским шторам на заказ, а также.
Мы изготавливаем римские шторы на заказ с учетом ваших пожеланий, для вашего уюта.
Качественные римские шторы для нашего клиента, узнайте больше.
Уникальные римские шторы по вашим размерам, крутые идеи.
Создайте свой стиль с римскими шторами на заказ, наши контакты.
Как выбрать римские шторы на заказ, наши секреты.
Римские шторы под заказ от профессионалов, которые подойдут именно вам.
Римские шторы на заказ: как выбрать ткани, для вашего стиля.
Римские шторы на заказ: гурман для ваших окон, на любой вкус.
римские шторы на заказ римские шторы на заказ .
мостбет официальный сайт скачать mostbet11004.ru
Eldorado Casino — это звёздная станция азарта, где игра — как миссия на далёкую орбиту. Интерфейс — это система межзвёздной навигации, а casino eldorado переводит в гиперпространство. Каждое вращение — как калибровка датчиков. Каждая победа — как открытие новой планеты.
Слоты — это космические терминалы. Бонусы — как кристаллы удачи. Музыка — это мелодия галактического рейва. Ты — как навигатор комет. Азарт здесь — как экспедиция во вселенную.
Поддержка — это координатор экспедиции, который помогает в полёте. Финансы — это межзвёздный счёт, где всё гарантировано стабильностью ядра. Эльдорадо Казино — это космическое приключение ставок.
Драгон Мани казино — это кибер-город ставок, где удача — как сбой матрицы в твою пользу. Интерфейс — это голографический терминал, а dragon money casino активирует поток бонусов. Каждое вращение — как запуск алгоритма. Каждая победа — как награда системы.
Слоты — это лазерные барабаны. Бонусы — как битовые коды. Музыка — это импульсы системы. Ты — как оператор сети. Азарт здесь — как полет в цифровой мир.
Поддержка — это бот-наставник, который реагирует по алгоритму. Финансы — это модуль выплат, где всё подтверждается через сеть. Драгон Мани — это игра будущего.
“Selam bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > …”
Кпт курган. Психотерапевты Самара. 574 оценок
мостбет ставки на исход мостбет ставки на исход
Топовые онлайн-казино Беларуси, играйте и выигрывайте, с нетерпением ожидают.
Лучшие онлайн-игры из Беларуси, все время на связи, для каждого.
Реальные выигрыши в онлайн-казино, лицензированные заведения, не упустите свою удачу.
Рейтинг лучших казино Беларуси, щедрые призы.
Играйте в любимые игры в казино онлайн, от карточных до настольных игр, для азартных игроков.
Новые онлайн-казино Беларуси, для настоящих ценителей.
Не упустите свои шансы на бонусы, только в Беларуси.
Захватывающие новинки игр, уже ждут вас, откройте для себя мир азартных игр.
На что обратить внимание при выборе казино, читайте наш гид.
Ваш шанс на удачу здесь, получайте незабываемые эмоции.
казино беларусь казино беларусь .
мостбет бк вход http://mostbet11008.ru/
Психотерапевт Пенза. Психотерапевт Киров. 210 оценок
Dragon Money казино — это ярмарка азарта, где игра — как прыжок акробата. Интерфейс — это пульт ведущего шоу, а казино драгон мани официальный сайт включает огни сцены. Каждое вращение — как исчезновение страха. Каждая победа — как апогей шоу.
Слоты — это сцены для ставок. Бонусы — как фейерверки азарта. Музыка — это марш иллюзиониста. Ты — как акробат фортуны. Азарт здесь — как чудо за ставкой.
Поддержка — это помощник иллюзиониста, который ведёт по сценарию. Финансы — это кошель трюков, где всё хранится под замком. Драгон Мани казино — это волшебство азарта.
скачать приложение мостбет скачать приложение мостбет
На прием Клинцы. Психотерапевт Челны. 321 оценок
mostbet website https://www.mostbet11004.ru
Драгон Мани — это святилище ставок, где спин — как пробуждение зверя. Интерфейс — это орнамент дракона, а драгон мани казино онлайн переносит в логово выигрыша. Каждое вращение — как крик из недр. Каждая победа — как добыча в битве.
Слоты — это пылающие барабаны. Бонусы — как драконье золото. Музыка — это рык зверя. Ты — как следопыт легенд. Азарт здесь — как дуэль с удачей.
Поддержка — это страж логова, который помогает при тревоге. Финансы — это магический кристалл, где всё движется как поток. Dragon Money казино — это дыхание пламени выигрышей.
мостбет ставки на спорт онлайн мостбет ставки на спорт онлайн
кухни угловые эконом недорого кухни угловые эконом недорого .
мостбет вход на сегодня мостбет вход на сегодня
Сервис по подбору психолога На прием Клинцы. 338 оценок
Топовые онлайн-казино Беларуси, открывайте новые возможности, порадуют вас.
Лучшие онлайн-игры из Беларуси, в удобное для вас время, для всех игроков.
Выигрывайте легко в белорусских казино, проверенное время, достигайте успеха.
Рейтинг лучших казино Беларуси, большие джекпоты.
Широкий выбор игр в белорусских казино, от карточных до настольных игр, для азартных игроков.
Свежие предложения в мире азартных игр, популярные игры.
Соберите все бонусы и акции, эксклюзивные бонусы.
Лучшие игры года , готовы порадовать, откройте для себя мир азартных игр.
Советы по выбору казино, узнайте больше.
Приглашаем в мир онлайн-азарта, играйте ответственно.
казино казино онлайн .
Заказать шторы недорого, широкий ассортимент.
Стильные шторы на заказ, в лучших традициях дизайна.
в кратчайшие сроки.
Шторы на заказ для вашего интерьера, с гарантией качества.
Шторы, которые вдохновляют, с утонченным дизайном.
Ищете идеальные шторы?, разнообразие стилей.
Шторы для любого стиля, который вы искали.
Советы по выбору штор, от экспертов.
Найдите стильные решения для штор, в нашем каталоге.
Проектируем шторы на заказ, не выходя из дома.
Индивидуальный подход к шторным решениям, на любой бюджет.
Качественные шторы на заказ, для комфорта и стиля.
Неповторимые шторы для вашего дома, по индивидуальным проектам.
Секреты выбора штор, узнайте больше.
Шторы премиум-класса на заказ, доступные цены.
Модные тренды в мире штор, с профессиональной помощью.
Шторы, которые преобразят ваш дом, обращайтесь.
Создайте атмосферу уюта с новыми шторами, из натуральных материалов.
Шторы, которые вы искали, заказывайте уже сегодня.
Цены на шторы, которые вас приятно удивят, от эконом до класса люкс.
заказ штор заказ штор .
Доставили в 6 утра – сделали невероятный сюрприз!
заказ цветов томск
Hype Casino — это техногенная площадка джекпотов, где каждая ставка — как шаг по кибер-крышам. Интерфейс — это панель кибер-города, а hype casino включает цифровой маршрут. Каждое вращение — как шёпот андеграунда. Каждая победа — как перехват трофея.
Слоты — это устройства шифровки. Бонусы — как цифровые артефакты. Музыка — это хаос мегаполиса. Ты — как проводник по теням. Азарт здесь — как призрачный город.
Поддержка — это связной, который обновляется регулярно. Финансы — это ядро фриспинов, где всё обрабатывается без задержек. Hype казино — это мир андеграунда.
Психотерапевт Киров. Психотерапевты Самара. 541 оценок
Психотерапевт Оренбург. На прием Клинцы. 432 оценок
pristennyj-drenazh-812.ru .
купить чистый диплом о среднем образовании купить чистый диплом о среднем образовании .
Психотерапевт Оренбург. В17 психология. 780 оценок
Магнитные бури 85goelro.ru .
chicken road key parameters
Chicken Road: Real Player Feedback
Chicken Road is a gamblinginspired arcade game that has drawn interest due to its straightforward mechanics, impressive RTP (98%), and innovative cashout option. We’ve collected honest feedback from actual players to see if it lives up to expectations.
What Players Like
Many users praise Chicken Road for its fastpaced gameplay and ease of use. With its cashout feature offering strategy and an RTP of 98%, it feels like a fairer alternative to conventional slot games. Beginners love the demo mode, which lets them try the game without risking money. The game earns extra points for its mobile compatibility, running seamlessly on both new and older devices.
Melissa R., AU: “Surprisingly fun and fair! The cashout feature adds strategy.”
Nathan K., UK: “Its arcadeinspired style is a breath of fresh air, and it operates smoothly on my device.”
Players also enjoy the colorful, nostalgic design, which feels both fun and engaging.
Areas for Improvement
However, Chicken Road isn’t perfect, and there are a few issues worth noting. Certain players think the game is too predictable and doesn’t offer much variety. Others mention slow customer support and limited features. One frequent criticism is deceptive marketing, as people thought it was a pure arcade game rather than a gambling platform.
Tom B., US: “Fun at first, but it gets repetitive after a few days.”
Sam T., UK: “Marketed as a casual game, but it’s actually a gamblingfocused app.”
Pros and Cons
Positive Aspects
Easytounderstand, quick gameplay
An RTP of 98% guarantees a fair experience
Practice mode to explore without financial risk
Seamless operation on smartphones and tablets
Cons
It might feel too predictable over time
Limited variety and features
Slow or unresponsive customer support
Misleading marketing
Final Verdict
Chicken Road shines through its openness, impressive RTP, and ease of access. It’s a great option for casual players or those new to online gambling. Still, the heavy emphasis on luck and minimal complexity could turn off some users. For the best experience, play on official, licensed platforms.
Rating: A solid 80%
An enjoyable and equitable option, though it has areas to grow.
Мы предлагаем документы институтов, расположенных на территории всей РФ. Заказать диплом любого университета:
beaconhill.listbb.ru/viewtopic.php?f=49&t=1845
Погода https://actprom.ru .
цена глубинное водопонижение цена глубинное водопонижение .
Гороскоп http://www.tti-sfedu.ru .
Какой сегодня праздник https://allizmalkovo.ru .
Запреты дня https://congress-st.ru/ .
Hype казино — это путь драконьих джекпотов, где игра — как квест по подземельям. Интерфейс — это алтарь бонусов, а сайт hype casino ведёт к сундуку с золотом. Каждое вращение — как путешествие сквозь королевства. Каждая победа — как пир на тронном зале.
Слоты — это турниры на монеты. Бонусы — как щедрость королей. Музыка — это гимны славы. Ты — как защитник удачи. Азарт здесь — как турнир фриспинов.
Поддержка — это проводник к храму выигрыша, который поддерживает как брат по оружию. Финансы — это сокровищница игрока, где всё приходит по первому требованию. Hype казино — это путь доблести.
Лучшие казино онлайн в Беларуси, получайте удовольствие от игры, удивительные бонусы.
Откройте для себя казино Беларуси, в удобное для вас время, удобные условия.
Поднимите свои шансы на выигрыш, проверенное время, играйте ответственно.
Рейтинг лучших казино Беларуси, ежедневно.
Широкий выбор игр в белорусских казино, от слотов до покера, для азартных игроков.
Новые онлайн-казино Беларуси, проверенные провайдеры.
Не упустите свои шансы на бонусы, только здесь.
Захватывающие новинки игр, уже ждут вас, начните выигрывать.
На что обратить внимание при выборе казино, по азартным играм.
Приглашаем в мир онлайн-азарта, не упустите возможность выиграть.
казино онлайн казино онлайн беларусь .
Мы предлагаем выгодно купить диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, штампами, подписями официальных лиц. Наш диплом пройдет любые проверки, даже при помощи специального оборудования. fire-team.ru/forum/member.php?u=1143
“Sst bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > BetTurkey Telegram
Slot turnuvas? yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 20 saniyede haz?r.
Simdi dene!”
прием консультация психиатра прием консультация психиатра .
Лучшие шторы на заказ, материалов.
Стильные шторы на заказ, в лучших традициях дизайна.
покупка штор.
Создайте уникальные шторы, с гарантией качества.
Шторы, которые вдохновляют, по доступным ценам.
Мы поможем выбрать, множество тканей.
Индивидуальные шторы для вашего окна, который вы искали.
Получите консультацию, от дизайнеров.
Найдите стильные решения для штор, с быстрой доставкой.
Создаем стильные шторы, не выходя из дома.
Шторы под любой интерьер, на любой бюджет.
Качественные шторы на заказ, для комфорта и стиля.
Шторы, которые подчеркивают ваш стиль, с учетом всех пожеланий.
Секреты выбора штор, откройте для себя.
Шторы премиум-класса на заказ, выбор материаов.
Модные тренды в мире штор, с последними новинками.
Уникальные предложения на шторы, обращайтесь.
Создайте атмосферу уюта с новыми шторами, доступно каждому.
Шторы, которые вы искали, заказывайте уже сегодня.
Цены на шторы, которые вас приятно удивят, по выгодным предложениям.
заказ штор заказ штор .
казино Hype — это район цифровой удачи, где игра — как отблеск неоновых огней. Интерфейс — это шаблон будущего, а хайп казино сайт открывает доступ к подземному залу. Каждое вращение — как код доступа. Каждая победа — как перехват трофея.
Слоты — это чипы азарта. Бонусы — как цифровые артефакты. Музыка — это глитч-мелодия. Ты — как проводник по теням. Азарт здесь — как призрачный город.
Поддержка — это куратор системы, который обновляется регулярно. Финансы — это аккаунт в банке теней, где всё синхронизируется с сетью. Хайп казино — это игровая метавселенная.
download mostbet download mostbet
Лунный календарь https://actprom.ru/ .
Пронедра http://85goelro.ru .
мостбет скачать приложение на андроид бесплатно http://www.mostbet11009.ru
Народные приметы tti-sfedu.ru .
Пронедра http://www.allizmalkovo.ru .
Гороскоп congress-st.ru .
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Приобретение диплома, который подтверждает окончание института, – это рациональное решение. Купить диплом любого университета: globalscaffolders.com/employer/diplomy-grup-24
“Merhaba kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > Xturka guncel link
Slot turnuvas? yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 20 saniyede haz?r.
Kazanmay? dene!”
jetx 1win http://www.1win3002.com
Онлайн-казино для белорусов, играйте и выигрывайте, с нетерпением ожидают.
Играйте в казино онлайн в Беларуси, все время на связи, привлекательные условия.
Реальные выигрыши в онлайн-казино, заведомо безопасно, играйте ответственно.
Самые надежные онлайн-казино в Беларуси, большие джекпоты.
Играйте в любимые игры в казино онлайн, от карточных до настольных игр, для истинных ценителей.
Новые онлайн-казино Беларуси, для любителей хорошей игры.
Соберите все бонусы и акции, особенные предложения.
Топ онлайн-игр в белорусских казино, уже ждут вас, начните выигрывать.
Как выбрать лучшее казино, узнайте больше.
Ваш шанс на удачу здесь, получайте незабываемые эмоции.
казино казино онлайн .
купить аттестат в великом новгороде купить аттестат в великом новгороде .
скачать мостбет http://mostbet11015.ru
Заказ обработали моментально – очень оперативно!
цветы томск
Элегантные решения, в вашем интерьере, удобное управление.
Эстетика и функциональность, на любой вкус, долговечность и надежность.
Создайте уют, с рулонными шторами с электроприводом, просто и удобно.
Выбор рулонных штор, узнайте здесь.
Рулонные шторы для современного дома, это удобно, добавьте стиля.
Рулонные шторы с электроприводом, для современных решений, закажите с доставкой.
Шторы с умным управлением, инновации в вашем интерьере, сделайте правильный выбор.
Современные рулонные шторы, высокое качество, закажите сегодня.
Шторы, с которыми легко управлять, стильный дизайн, доступно всем.
Рулонные шторы с дистанционным управлением, просто и удобно, узнайте больше.
Шторы, которые открывают новые возможности, управление светом из любой точки, закажите с нашим менеджером.
Дистанционно управляемые шторы, на нашем сайте.
Создавайте атмосферу, с дистанционно управляемыми шторами, это практично.
Современные шторы с пультом, широкий ассортимент, узнайте подробности.
Электрические шторы с дистанционным управлением, дизайн и комфорт, заказ с доставкой.
Электрические шторы для любого интерьера, у нас есть всё, закажите с доставкой.
Удобные рулонные шторы, доступные цены, обратитесь к нам.
Современные рулонные шторы, для удобного управления, закажите прямо сейчас.
рулонные шторы с электроприводом и дистанционным управлением рулонные шторы с электроприводом и дистанционным управлением .
купить диплом о образовании купить диплом о образовании .
купить диплом института купить диплом института .
ван вин зеркало ван вин зеркало
мостбет ставки мостбет ставки
Лучшие казино онлайн в Беларуси, открывайте новые возможности, щедрые предложения.
Лучшие онлайн-игры из Беларуси, в любое время и в любом месте, для настоящих азартных игроков.
Выигрывайте легко в белорусских казино, лицензированные заведения, играйте ответственно.
Рейтинг лучших казино Беларуси, каждую минуту.
Разнообразие игр для всех, от карточных до настольных игр, на любой вкус.
Откройте для себя новинки в казино, для азартных геймеров.
Не упустите свои шансы на бонусы, только для вас.
Лучшие игры года , готовы порадовать, откройте для себя мир азартных игр.
На что обратить внимание при выборе казино, по играм на деньги.
Приглашаем в мир онлайн-азарта, играйте ответственно.
казино беларусь казино .
скачать мост бет http://mostbet-download-app-apk.com/ .
купить аттестат в костроме купить аттестат в костроме .
новости дня https://www.dorogi34.ru .
parimatch.com app parimatch.com app .
Мы изготавливаем дипломы любой профессии по доступным ценам. Приобретение документа, подтверждающего обучение в ВУЗе, – это разумное решение. Заказать диплом университета: nagaevo.ekafe.ru/viewtopic.php?f=86&t=5749
Vavada Casino — это виртуальный бойцовский клуб, где выигрыш — как загрузка бонуса. Интерфейс — это хаб выигрышей, а vavada casino зеркало на сегодня перезапускает удачу. Каждое вращение — как кибер-импульс. Каждая победа — как ноль и единица фортуны.
Слоты — это платы азарта. Бонусы — как пакеты фриспинов. Музыка — это цифровая гармония. Ты — как кибер-игрок. Азарт здесь — как миссия по прибыли.
Поддержка — это менеджер сети, который проводит по интерфейсу. Финансы — это цифровая кладовая, где всё обновляется в реальном времени. казино Вавада — это база фриспинов.
Современные технологии, в вашем офисе, в одном клике.
Эстетика и функциональность, на любой вкус, доступные цены.
Управляйте светом, с автоматическими шторами, легко и быстро.
Электропривод для вашего уюта, посетите наш сайт.
Рулонные шторы для современного дома, это удобно, наслаждайтесь комфортом.
Шторы, которые управляются дистанционно, для современных решений, закажите с доставкой.
Шторы с умным управлением, надежность и стиль, узнайте больше.
Шторы для умного дома, высокое качество, узнайте подробности.
Шторы, с которыми легко управлять, стильный дизайн, в наличии сейчас.
Рулонные шторы с дистанционным управлением, просто и удобно, узнайте больше.
Электрические шторы с пультом, управление светом из любой точки, доступно с доставкой.
Современные решения для вашего дома, на нашем сайте.
Сделайте ваш дом умным, с современными шторами, это удобно.
Электрические шторы для вашего интерьера, широкий ассортимент, посетите наш сайт.
Умный дом с рулонными шторами, удобство для вас, заказ с доставкой.
Электрические шторы для любого интерьера, простой и удобный выбор, посетите наш сайт.
Автоматизированные шторы для уюта, разнообразие дизайнов, обратитесь к нам.
Шторы с электроприводом, для удобного управления, получите консультацию.
рулонные шторы с электроприводом и дистанционным управлением рулонные шторы с электроприводом и дистанционным управлением .
авиатор игра скачать [url=https://1win11001.ru]авиатор игра скачать[/url]
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3590 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом нового образца — ответим быстро, без лишних формальностей.
Быстро, красиво, надежно – три кита вашего сервиса!
купить цветы томск
“Hey kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > simdi dene
Rulet masas? h?zl? odeme.
VPN’siz erisim, tek t?kla kay?t > 10 saniyede haz?r.
Simdi dene!”
Читать далее kra34
посмотреть в этом разделе https://vodkacasino.net
читать https://vodkacasino.net/
Казино 1xSlots — это олимп азарта, где выигрыш — как гнев небес. Интерфейс — это древний храм удачи, а казино 1x slot призывает силу победы. как расшифровка предсказания. как слияние стихий.
Слоты — это магические барабаны. Бонусы — это зелья победы. Звуки — это глас оракулов. Игрок — как искатель пророчества. Азарт — как путь через испытания.
Поддержка — это оракул связи, который всегда доступен. Финансы — это хранилище сокровищ, где всё движется без замедления. казино 1xслотс — это олимпийская платформа выигрышей.
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3251 клиентов воспользовались услугой — теперь ваша очередь.
Обращайтесь — ответим быстро, без лишних формальностей.
1вин как потратить бонусы казино https://www.1win11003.ru
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3256 клиентов воспользовались услугой — теперь ваша очередь.
Звоните — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3522 клиентов воспользовались услугой — теперь ваша очередь.
Узнать условия — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3202 клиентов воспользовались услугой — теперь ваша очередь.
Здесь — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4677 клиентов воспользовались услугой — теперь ваша очередь.
Пишите в личные сообщения — ответим быстро, без лишних формальностей.
Топовые онлайн-казино Беларуси, открывайте новые возможности, удивительные бонусы.
Играйте в казино онлайн в Беларуси, все время на связи, привлекательные условия.
Выигрывайте легко в белорусских казино, проверенное время, достигайте успеха.
Самые надежные онлайн-казино в Беларуси, большие джекпоты.
Широкий выбор игр в белорусских казино, от рулетки до блэкджека, для азартных игроков.
Откройте для себя новинки в казино, для настоящих ценителей.
Не упустите свои шансы на бонусы, особенные предложения.
Лучшие игры года , уже ждут вас, начните выигрывать.
Как выбрать лучшее казино, по онлайн-казино Беларуси.
Станьте частью захватывающего мира казино, не упустите возможность выиграть.
казино казино онлайн .
мостбет вход регистрация https://www.mostbet11014.ru
как зайти на сайт мостбет http://www.mostbet11015.ru
купить аттестат в калуге купить аттестат в калуге .
диплом о среднем техническом образовании купить http://arus-diplom5.ru/ .
Gizbo казино — это буря ставок, где игра захватывает как океанский бриз. Интерфейс — это карта маршрутов, а казино gizbo ведёт на главный рейд. как маршрут сквозь шторма. как находка сундука.
Слоты — это игровые маяки, бонусы — как шлюпки выигрышей. Звуки — это гудки лайнера. Игрок — как штурман ставок. Азарт — как штормовой прилив.
Поддержка — это морской диспетчер, который предупреждает о рифах. Финансы — это корабельная бухгалтерия, где всё выдаётся вовремя. Gizbo казино — это лайнер фриспинов.
Погода dorogi34.ru .
займы без фото на карту займы без фото на карту .
Какой сегодня праздник https://inforigin.ru .
интернет магазин кашпо напольных http://www.kashpo-napolnoe-spb.ru – http://www.kashpo-napolnoe-spb.ru .
Магнитные бури https://istoriamashin.ru .
кашпо для цветов напольное для дачи http://kashpo-napolnoe-spb.ru – http://kashpo-napolnoe-spb.ru .
GetX Casino — это небоскрёб азарта, где мечтают о джекпоте. Каждая сессия — это поездка в мир фриспинов, и гет икс казино сайт появляется как вход в VIP-зону. как путь через облака. Победа — это награда в пентхаусе.
Слоты — это лифты джекпотов. Бонусы — как пропуск на верхний уровень. Игрок — как арендатор успеха. Азарт — как ночной полёт с шансом. Звуки — как музыка неоновой ночи.
Поддержка — как администратор удачи, который открывает двери к фриспинам. Финансы — как лицевой счёт владельца люкса, и всё обновляется в реальном времени. Казино Гет Икс — это панорама выигрыша.
mostbet indir apk mostbet indir apk .
пояснения https://vodkacasino.net/
новости дня https://topoland.ru .
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2864 клиентов воспользовались услугой — теперь ваша очередь.
Мы поможем — ответим быстро, без лишних формальностей.
Казино Мани Икс — это ночная трасса выигрышей, где каждое вращение — как старт с линии. Слоты — как ускорители побед, и money x разгоняет поток адреналина. Каждое вращение — как ускорение к успеху. Выигрыш — это призовой кубок.
Игрок — это лидер ночной трассы. Бонусы — как вспышка нитро. Азарт — как запретный обгон. Звуки — это писк заноса фриспинов. Интерфейс — как панель управления болидом.
Поддержка — как технический штурман, она ускоряет решение проблем. Финансы — это поток бонусов, и всё переводится как по прямой. Мани Икс Казино — это турнир скорости и азарта.
1win casa de apuestas http://1win3002.com/
parimatch latest apk parimatch latest apk .
Умные рулонные шторы для вашего дома, в интерьере.
Современные рулонные шторы с электрическим управлением, для вашего удобства.
Рулонные шторы с электроприводом: удобство и стиль, в каталоге.
Электроприводные рулонные шторы, превосходное сочетание.
Изучите мир электрических рулонных штор, смотрите.
Умные рулонные шторы для дома, подходят для любого окна.
Электроприводные рулонные шторы: инновации в вашем доме, выбор, который стоит сделать.
Рулонные шторы с электроприводом для легкого управления, узнайте больше.
Сделайте свой дом умнее с рулонными шторами, в каталоге товаров.
Рулонные шторы с электроприводом для современного интерьера, которое вы заслуживаете.
Современные рулонные шторы с электроприводом, с возможностью установки.
Рулонные шторы с электроприводом: функциональность и стиль, выбор, на который стоит обратить внимание.
Электроприводные рулонные шторы: удобство управления одним нажатием, носим с собой.
Умные рулонные шторы: будущее уже здесь, закажите сейчас.
Рулонные шторы с электроприводом: комфорт на новом уровне, попробуйте и оцените.
Рулонные шторы с электроприводом – вашим окнам это нужно, позаботьтесь о себе.
Электрические рулонные шторы: легкость управления, выбирайте лучшее.
Рулонные шторы с электроприводом: удобно и красиво, закажите прямо сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
как диплом купить http://www.arus-diplom7.ru/ .
аттестат школьный купить аттестат школьный купить .
Приобрести диплом института. Приобретение документа о высшем образовании через проверенную и надежную фирму дарит ряд плюсов. Это решение позволяет сэкономить время и серьезные денежные средства. orikdok-v-gorode-tyumen-72.online
Купить диплом под заказ в столице возможно через официальный сайт компании. orikdok-2v-gorode-kaluga-40.ru
Онлайн-казино для белорусов, открывайте новые возможности, ждут вас.
Откройте для себя казино Беларуси, в любое время и в любом месте, удобные условия.
Реальные выигрыши в онлайн-казино, лицензированные заведения, достигайте успеха.
Самые надежные онлайн-казино в Беларуси, высокие выплаты.
Разнообразие игр для всех, от рулетки до блэкджека, для истинных ценителей.
Откройте для себя новинки в казино, для азартных геймеров.
Не упустите свои шансы на бонусы, особенные предложения.
Лучшие игры года , уже ждут вас, погрузитесь в увлекательные приключения.
На что обратить внимание при выборе казино, по азартным играм.
Станьте частью захватывающего мира казино, получайте незабываемые эмоции.
казино беларусь казино беларусь .
как использовать бонусный счет 1win как использовать бонусный счет 1win
“Hey bahisseverler!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > BetTurkey Telegram
Canl? bahis 24/7 ac?k.
VPN’siz erisim, tek t?kla kay?t > 20 saniyede haz?r.
Simdi dene!”
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3976 клиентов воспользовались услугой — теперь ваша очередь.
Пишите — ответим быстро, без лишних формальностей.
Психотерапевт Киров. batmanapollo.ru 969 оценок
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2421 клиентов воспользовались услугой — теперь ваша очередь.
Пишите в личные сообщения — ответим быстро, без лишних формальностей.
Календарь огородника http://inforigin.ru .
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3596 клиентов воспользовались услугой — теперь ваша очередь.
Купить государственный диплом — ответим быстро, без лишних формальностей.
pristennyj-drenazh-812.ru .
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2715 клиентов воспользовались услугой — теперь ваша очередь.
Посмотреть информацию — ответим быстро, без лишних формальностей.
Орхидеи свежие, крепкие – просто красота!
букеты томск
Какой сегодня церковный праздник https://topoland.ru .
Рекомендую проверенного хакера – Удаленный доступ к пк Почта специалиста: [email protected]
Лунный календарь http://istoriamashin.ru/ .
ставки на спорт мостбет http://www.mostbet11014.ru
узнать больше https://vodkawin.com/
Психотерапевт Пенза. batmanapollo.ru 967 оценок
Рулонные шторы с электроприводом, инновации и дизайн.
Современные рулонные шторы с электрическим управлением, для вашего интерьера.
Рулонные шторы с электроприводом: удобство и стиль, в каталоге.
Современные рулонные шторы с электроприводом, идеальное решение.
Изучите мир электрических рулонных штор, смотрите.
Умные рулонные шторы для дома, создадут уют.
Стильные рулонные шторы с электроприводом, инновации, которые стоит попробовать.
Рулонные шторы с электроприводом для легкого управления, познакомьтесь с нашими предложениями.
Сделайте свой дом умнее с рулонными шторами, в нашем интернет-магазине.
Умные рулонные шторы: удобство и стиль, для вашего комфорта.
Современные рулонные шторы с электроприводом, с возможностью установки.
Электрические рулонные шторы для любого интерьера, выбор, на который стоит обратить внимание.
Электроприводные рулонные шторы: удобство управления одним нажатием, каждый день.
Электрические рулонные шторы для стиля и комфорта, нажмите для подробностей.
Рулонные шторы с электроприводом: комфорт на новом уровне, откройте для себя.
Умные рулонные шторы с электроприводом для вашего дома, позаботьтесь о себе.
Электрические рулонные шторы: легкость управления, выбирайте лучшее.
Электроприводные рулонные шторы для стильного дома, изучите сейчас.
рулонные шторы с электроприводом рулонные шторы с электроприводом .
Лучшие казино онлайн в Беларуси, открывайте новые возможности, удивительные бонусы.
Играйте в казино онлайн в Беларуси, в удобное для вас время, для всех игроков.
Поднимите свои шансы на выигрыш, лицензированные заведения, не упустите свою удачу.
Самые надежные онлайн-казино в Беларуси, ежедневно.
Разнообразие игр для всех, от карточных до настольных игр, для истинных ценителей.
Свежие предложения в мире азартных игр, лицензированные операторы.
Соберите все бонусы и акции, только для вас.
Захватывающие новинки игр, уже ждут вас, начните выигрывать.
На что обратить внимание при выборе казино, получите полезные советы.
Ваш шанс на удачу здесь, не упустите возможность выиграть.
казино беларусь казино .
ставки мостбет ставки мостбет
Психотерапевт Оренбург. batmanapollo.ru 130 оценок
мостбет кг мостбет кг
mostbet скачать 2024 mostbet скачать 2024
pristennyj-drenazh-812.ru .
Психотерапевт Челны. batmanapollo.ru 676 оценок
Купить диплом возможно через сайт компании. orikdok-1v-gorode-smolensk-67.online
нажмите здесь новые казино
узнать больше https://vodkawin.com
mostbet oficial http://www.mostbet11025.ru
Психотерапевт Пенза. batmanapollo.ru 455 оценок
mostbet сайт регистрация mostbet11027.ru
Thanks for the article. Here is a website on the topic – https://40-ka.ru/
Психотерапевт Белгород. batmanapollo.ru 112 оценок
Б17 психологи. batmanapollo.ru 498 оценок
Спасибо за позитив и невероятную красоту!
букет невесты томск
Заказать диплом ВУЗа по выгодной цене возможно, обратившись к проверенной специализированной компании. Заказать документ о получении высшего образования можно у нас. orikdok-3v-gorode-smolensk-67.ru
Психотерапевт Киров. batmanapollo.ru 754 оценок
сайт https://vodkawin.com
Онлайн-казино для белорусов, открывайте новые возможности, с нетерпением ожидают.
Лучшие онлайн-игры из Беларуси, все время на связи, удобные условия.
Выигрывайте легко в белорусских казино, заведомо безопасно, играйте ответственно.
Рейтинг лучших казино Беларуси, большие джекпоты.
Разнообразие игр для всех, от карточных до настольных игр, на любой вкус.
Новые онлайн-казино Беларуси, для любителей хорошей игры.
Поспешите за бонусами в казино онлайн, только для вас.
Захватывающие новинки игр, готовы порадовать, погрузитесь в увлекательные приключения.
Советы по выбору казино, читайте наш гид.
Приглашаем в мир онлайн-азарта, играйте ответственно.
казино казино онлайн .
Какой сегодня церковный праздник http://www.novorjev.ru/ .
Магнитные бури pechory-online.ru .
мостбэт http://www.mostbet11026.ru
займ на 6 месяцев займ на 6 месяцев .
Римские шторы на заказ – идеальное решение для вашего интерьера, в отличном качестве.
Хотите обновить интерьер с помощью римских штор?, шторы.
Профессиональное изготовление римских штор, гарантией качества.
Создайте уникальные римские шторы, по индивидуальному заказу.
Римские шторы, сшитые по вашим меркам, заказать онлайн.
Индивидуальное изготовление римских штор, закажите.
Заказ римских штор по индивидуальным размерам, недорого.
Идеальные римские шторы для вашего интерьера, не упустите эту возможность.
Полезные советы по выбору римских штор, посмотрите.
Римские шторы под заказ в любом стиле, по нашим лучшим ценам.
Краткое руководство по созданию римских штор, читайте.
Римские шторы как решение для любого окна, доступно.
Индивидуальный подход к изготовлению римских штор, индивидуальным дизайном.
Ваши идеальные римские шторы ждут вас, при.
Сшить римские шторы – это просто и удобно, по индивидуальным расчетам.
Стильные римские шторы на заказ, на нашем сайте.
Сшить римские шторы в несколько кликов, легко.
Римские шторы, сделанные на заказ, соответствия.
сшить римские шторы на заказ сшить римские шторы на заказ .
Электрические рулонные шторы на окна, комфорт и стиль.
Идеальное решение для современных окон, современные технологии для вашего комфорта.
Электронные рулонные шторы для вашего пространства, в каждой детали.
Лучшая автоматизация окон, все под контролем.
Выбор рулонных штор с электроприводом, для вашего интерьера.
Умные рулонные шторы: необычные решения, с гарантией качества.
Электрические шторы: комфортный выбор, доступные для любого интерьера.
Лучшие модели электрических рулонных штор, для стильного интерьера.
Простота использования рулонных штор, добавляет удобства.
Топ моделей электрических рулонных штор, с первоклассным качеством.
Электрические рулонные шторы: удобство и стиль, для создания неповторимого стиля.
Электрические рулонные шторы: все, что нужно знать, для вашего стиля.
Как правильно выбрать рулонные шторы, по лучшей цене.
Как электрические рулонные шторы изменят ваш дом, для создания уюта.
Электрические рулонные шторы: обзор и советы, для улучшения качества жизни.
Как установить электрические рулонные шторы, с соблюдением всех стандартов.
Шторы с электроприводом для вашего дома, для практичного использования.
Электронные решения для коммерческих помещений, для улучшения рабочего пространства.
Электрические рулонные шторы: выгодные решения, для вашего дома.
электрические рулонные шторы на окна электрические рулонные шторы на окна .
mostbet. com http://mostbet11008.ru/
мостбет скачать на компьютер https://mostbet11027.ru/
“Hey forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > rolletto guvenilir mi
Slot turnuvas? h?zl? odeme.
VPN’siz erisim, tek t?kla kay?t > 15 saniyede haz?r.
Kazanmay? dene!”
Thanks for the article. Here’s more on the topic https://artcet.ru/
login mostbet mostbet11023.ru
мостбет регистрация мостбет регистрация
напольные вазоны kashpo-napolnoe-spb.ru – напольные вазоны .
напольный горшок для цветов http://www.kashpo-napolnoe-spb.ru/ – напольный горшок для цветов .
Заказать диплом университета по доступной цене возможно, обращаясь к надежной специализированной фирме. Приобрести документ ВУЗа вы можете в нашей компании в столице. orikdok-2v-gorode-syktyvkar-11.ru
Умные рулонные шторы для окон, удобство и элегантность.
Рулонные шторы с электроприводом, долговечность и простота в использовании.
Электронные рулонные шторы для вашего пространства, в каждом движении.
Управление рулонными шторами одним нажатием, все под контролем.
Как выбрать электрические рулонные шторы, для создания уюта.
Умные рулонные шторы: необычные решения, с гарантией качества.
Электрические шторы: комфортный выбор, доступные для любого интерьера.
Рекомендации по выбору рулонных штор, для функционального пространства.
Технология автоматизации рулонных штор, добавляет удобства.
Лучшие электрические рулонные шторы на рынке, с первоклассным качеством.
Электрические рулонные шторы: удобство и стиль, для вашего интерьера.
Электрические рулонные шторы: все, что нужно знать, для вашего стиля.
Купить электрические рулонные шторы: советы, с качественными материалами.
Электронные шторы для любого интерьера, для создания уюта.
Электрические рулонные шторы: преимущества, для вашего комфорта.
Как установить электрические рулонные шторы, с соблюдением всех стандартов.
Электрические рулонные шторы: идеальные решения, для улучшения условий жизни.
Электрические рулонные шторы для бизнеса, для современной эстетики.
Электрические рулонные шторы: выгодные решения, для вашего офиса.
электрические рулонные шторы на окна электрические рулонные шторы на окна .
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1413 клиентов воспользовались услугой — теперь ваша очередь.
Мы поможем — ответим быстро, без лишних формальностей.
mostbet букмекерская контора сайт http://www.mostbet11028.ru
мостбет андроид https://mostbet11024.ru
Изготовление римских штор по индивидуальным размерам, удивительно.
Хотите обновить интерьер с помощью римских штор?, заказывайте.
Римские шторы на заказ от опытных мастеров, с.
Сшить римские шторы для любого интерьера, в.
Найдите свои идеальные римские шторы, нашему сайту.
Ваши мечты о римских шторах сбываются, получите.
Заказ римских штор по индивидуальным размерам, недорого.
Сшить римские шторы на заказ с любовью, вы будете довольны результатом.
Как выбрать римские шторы на заказ? , с деталями.
Уникальные римские шторы на заказ, узнайте.
Все о римских шторах на заказ, читайте.
Заказывайте римские шторы и наслаждайтесь комфортом, недорого.
Сшите римские шторы именно так, как хотите, по.
Не откладывайте заказ римских штор, доступным ценам.
Римские шторы по вашему дизайну, по индивидуальным расчетам.
Стильные римские шторы на заказ, подписывайтесь.
Сшить римские шторы в несколько кликов, легко.
Сшить римские шторы с учетом ваших пожеланий, соответствия.
сшить римские шторы на заказ сшить римские шторы на заказ .
мобильное приложение регистрация вход mostbet app приложении рейтинг 4 8 http://www.mostbet11026.ru
Погода http://www.novorjev.ru .
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Мы можем предложить документы ВУЗов, расположенных в любом регионе Российской Федерации. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высшего качества. Это дает возможность делать государственные дипломы, которые невозможно отличить от оригиналов. orikdok-v-gorode-kostroma-44.ru
Какой сегодня церковный праздник pechory-online.ru .
Подробнее здесь Делать игры Unity
Помогли выбрать среди такого разнообразия – спасибо!
букет невесты томск
как зарегистрироваться в мостбет как зарегистрироваться в мостбет
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3522 клиентов воспользовались услугой — теперь ваша очередь.
Пишите — ответим быстро, без лишних формальностей.
Создайте уникальные шторы под заказ для вашего дома, подберите.
Индивидуальный подход к шторным решениям, открывает новые горизонты.
Закажите шторы на заказ и обновите свой интерьер, не стесняйтесь.
Качественные шторы на заказ по доступной цене, по индивидуальным размерам.
Уникальные шторы, которые подчеркнут вашу индивидуальность, позвольте нам порадовать вас.
Шторы на заказ для вашего уютного дома, обновите пространство.
Достаньте самовыражение через шторы на заказ, действуйте и наслаждайтесь результатом.
Шторы на заказ: функциональность и эстетика, измените свой интерьер навсегда.
Функциональные шторы под заказ для вашего удобства, доверяйте профессионалам.
Уникальные решения для вашего интерьера с шторами на заказ, действуйте и вдохновляйтесь.
шторы на заказ шторы на заказ .
mostbet скачать 2024 mostbet11029.ru
веб-сайт Разработка 3D игр
Создание римских штор своими руками, освежить.
Как сделать римские шторы: инструкция
пошить римские шторы пошить римские шторы .
Заказать диплом об образовании!
Мы изготавливаем дипломы любой профессии по невысоким ценам— diplomsale4.ru
Купить документ ВУЗа можно в нашей компании в столице. Заказать диплом института по невысокой стоимости возможно, обращаясь к надежной специализированной компании. yourealtynews.ru/gde-kupit-diplom-s-garantiey-konfidentsialnosti-i-kachestva
дренажные работы ленинградская область .
Психотерапевты Самара. batmanapollo.ru 844 оценок
Trusted bookmakers have always been the top choice for online bettors in Vietnam. As the online entertainment industry grows rapidly, finding a reliable betting site that ensures safety, transparency, and high-quality service has become more important than ever.
Based on strict evaluation criteria, we’ve compiled a list of the Top Trusted Bookmakers, along with the key factors that define a truly reliable platform.
This article will give you a comprehensive overview of nhacaiuytin and help you choose the most suitable and secure playground for your betting journey.
Самый надежный цветочный сервис – проверено годами!
купить розы в томске
Индивидуальные шторы на заказ — стиль и комфорт в каждом доме, подберите.
Индивидуальный подход к шторным решениям, добавляет уют в ваше пространство.
Шторы на заказ — это просто, быстро и удобно, не упустите возможность.
Идеальные шторы на заказ для любого бюджета, по индивидуальным размерам.
Создайте уют с помощью штор на заказ, позвольте нам порадовать вас.
Шторы на заказ для вашего уютного дома, добавьте стиль.
Найдите идеальные шторы, сделанные только для вас, не откладывайте на потом.
Шторы на заказ: функциональность и эстетика, выберите свой стиль.
Элегантные шторы на заказ для любого стиля, доверяйте профессионалам.
Уникальные решения для вашего интерьера с шторами на заказ, создавайте свой стиль.
шторы на заказ шторы на заказ .
Мы изготавливаем дипломы любой профессии по приятным ценам. Мы готовы предложить документы ВУЗов, расположенных на территории всей Российской Федерации. Дипломы и аттестаты делаются на “правильной” бумаге самого высшего качества. Это дает возможность делать государственные дипломы, которые не отличить от оригиналов. orikdok-5v-gorode-khabarovsk-27.online
продажа пластиковых окон http://www.okna177.ru .
Психотерапевт Челны. batmanapollo.ru 585 оценок
Психотерапевт Челны. batmanapollo.ru 661 оценок
Кпт курган. batmanapollo.ru 253 оценок
Как пошить римские шторы: шаг за шагом, для того чтобы.
Пошив римских штор: пособие
пошить римские шторы пошить римские шторы .
Психотерапевт Пенза. batmanapollo.ru 656 оценок
Быстро и просто приобрести диплом института!
Мы изготавливаем дипломы любой профессии по доступным ценам— nsk-diplom.com
В17 психология. batmanapollo.ru 926 оценок
На прием Клинцы. batmanapollo.ru 651 оценок
Сшить римские шторы на заказ, стильно.
Ищете идеальные римские шторы?, заказывайте.
Римские шторы на заказ от опытных мастеров, при.
Создайте уникальные римские шторы, по индивидуальному заказу.
Откройте для себя мир римских штор, с индивидуальным дизайном.
Индивидуальное изготовление римских штор, прямо сейчас.
Качественное изготовление римских штор, в любое время.
Изготавливаем римские шторы для любого стиля, это просто.
Римские шторы, сшитые на заказ, как выбрать?, с деталями.
Уникальные римские шторы на заказ, заказывайте.
Все о римских шторах на заказ, на полезные материалы.
Сшить римские шторы с учетом всех пожеланий, доступно.
Сшите римские шторы именно так, как хотите, по.
Не откладывайте заказ римских штор, с.
Сшить римские шторы – это просто и удобно, всегда.
Изготовление римских штор под заказ, в нашем магазине.
Римские шторы под заказ – это красиво, легко.
Сшить римские шторы с учетом ваших пожеланий, сто процентов.
сшить римские шторы на заказ сшить римские шторы на заказ .
“Selamlar!
? Pragmatic Play’in buyulu oyunu **Starlight Princess** simdi yay?nda!
?? Is?klar icinde carpanlar, prensesle max win!
? starlight princess yorum
?? Hemen basla: [url=https://starlightprenses.com/]starlightprenses.com[/url]
?? Mobil uyumlu, VPN gerekmez, h?zl? kay?t ve an?nda oyun keyfi.
Hemen oyna!”
Купить документ университета можно в нашей компании в Москве. Приобрести диплом института по выгодной цене возможно, обратившись к надежной специализированной фирме. vira-ss.flybb.ru/viewtopic.phpf=9&t=999
Психотерапевт Киров. batmanapollo.ru 913 оценок
mostbet casino скачать на андроид mostbet casino скачать на андроид
i was reading this jaxx blockchain wallet
На прием Клинцы. Психолог 834 459 оценок
dragonslot http://www.dragonslotscasinos.mobi .
dragonslots casino http://www.dragonslotscasinos.net/ .
Viagra * Cialis * Levitra
All the products you are looking an eye to are currently available as far as something 1+1.
4 more tablets of identical of the following services: Viagra * Cialis * Levitra
https://pxman.net
купить аттестат в пскове купить аттестат в пскове .
Муж был тронут до слез на годовщину свадьбы.
заказать цветы с доставкой в томске
Изготовление римских штор по индивидуальным размерам, в отличном качестве.
Хотите обновить интерьер с помощью римских штор?, изделия.
Как сшить римские шторы под заказ, высококачественных материалов.
Сшить римские шторы для любого интерьера, с.
Найдите свои идеальные римские шторы, не упустите шанс.
Индивидуальное изготовление римских штор, всего в несколько кликов.
Качественное изготовление римских штор, в любое время.
Идеальные римские шторы для вашего интерьера, это просто.
Римские шторы, сшитые на заказ, как выбрать?, ознакомьтесь.
Уникальные римские шторы на заказ, узнайте.
Краткое руководство по созданию римских штор, на наши советы.
Заказывайте римские шторы и наслаждайтесь комфортом, недорого.
Индивидуальный подход к изготовлению римских штор, индивидуальным дизайном.
Римские шторы на заказ для вашего комфорта, с.
Римские шторы по вашему дизайну, всегда.
Как правильно сшить римские шторы по размеру, в нашем магазине.
Сшить римские шторы в несколько кликов, легко.
Уникальные римские шторы для вашего дома, качества.
сшить римские шторы на заказ сшить римские шторы на заказ .
Психотерапевт Челны. Психолог 663 132 оценок
Пошив штор для вашего дома, полезные советы.
Создайте идеальные шторы, для вашего интерьера.
Шторы на заказ, как сшить.
Способы сшить шторы, в домашних условиях.
Уроки для новичков по пошиву штор, легко и быстро.
Выбор тканей для штор, по вашему вкусу.
Стильные шторы, вашему дому.
Как украсить шторы, и радовали глаз.
Кулинарные шторы, в современном дизайне.
Как создать атмосферу в спальне, на нашем сайте.
Шторы для гостиной, в актуальных трендах.
Как ухаживать за шторами, и прекрасными.
Это ваши идеальные шторы, для любого интерьера.
Как сшить римские шторы, в вашем доме.
Преимущества функциональных штор, для вашего комфорта.
Как сшить шторы на люверсах, которые подходят для любого окна.
Шторы для детской, для комфорта и радости вашего ребенка.
Секреты идеального пошива штор, на нашем сайте.
сшить шторы сшить шторы .
anchor jaxx liberty
Психотерапевт Оренбург. Психолог 956 740 оценок
mostbet регистрация через официальный сайт http://www.mostbet11029.ru
try this web-site jaxx liberty download
Психотерапевты Самара. Психолог 881 652 оценок
скачать мосбет https://www.mostbet11022.ru
Кпт курган. Психолог 314 838 оценок
сколько стоит купить диплом о высшем образовании сколько стоит купить диплом о высшем образовании .
Психотерапевт Челны. Психолог 177 788 оценок
В17 психология. Психолог 159 441 оценок
как активировать ваучер в 1win как активировать ваучер в 1win
“Selam arkadaslar!
?? Yepyeni Pragmatic oyunu **Sugar Rush 1000** yay?nda!
?? Demo denemek icin simdi t?kla: sugar1000turkiye.com
? sugar rush 1000 nas?l oynan?r
?? Daha yuksek carpanlar, daha fazla seker ve daha cok kazanc seni bekliyor!
?? Mobil uyumlu, kay?t gerekmez, direkt basla.
Hemen dene!”
Заказать диплом любого университета поможем. Купить диплом специалиста в Кургане – diplomybox.com/kupit-diplom-spetsialista-v-kurgane
Психотерапевт Пенза. Психолог 111 269 оценок
бонус казино 1win 1win1104.ru
мостбет сайт бк http://mostbet11018.ru/
Here’s more on the topic https://upsskirt.ru/
Пошив штор для вашего дома, пошаговая инструкция.
Создайте идеальные шторы, с помощью нашего гида.
Индивидуальные шторы, как сшить.
Идеи для пошива штор, в своем городе.
Уроки для новичков по пошиву штор, легко и быстро.
Как выбрать ткань для штор, и интерьеру.
Стильные шторы, вашему интерьеру.
Как украсить шторы, чтобы они привлекали внимание.
Шторы для кухни своими руками, как оформить.
Уютные шторы для спальни, в нашем блоге.
Шторы для гостиной, и предпочтений.
Как ухаживать за шторами, чтобы они выглядели как новые.
Как выбрать стиль для штор, от классики до современности.
Все о римских шторах, для вашего интерьера.
Почему стоит выбрать функциональные шторы, для вашего уюта.
Идеи для штор на люверсах, которые украсят любой интерьер.
Креативные идеи для штор в детской, для комфорта и радости вашего ребенка.
Лучшие советы по пошиву штор, для уверенных в своих силах.
сшить шторы сшить шторы .
Your article helped me a lot, is there any more related content? Thanks!
ставки на спорт бишкек https://www.mostbet11030.ru
Подарочная упаковка – верх совершенства!
доставка цветов томск
egypt888starz.net/ egypt888starz.net/ .
888starz bet http://www.888starz-downloads.com .
monsbet http://www.mostbet11022.ru
купить диплом старого образца купить диплом старого образца .
диплом купить старого образца диплом купить старого образца .
регистрация в мостбет http://mostbet11017.ru
dragon slots online dragon slots online .
1win ставки на спорт скачать http://www.1win1106.ru
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1439 клиентов воспользовались услугой — теперь ваша очередь.
Доступ по ссылке — ответим быстро, без лишних формальностей.
Мы изготавливаем дипломы любой профессии по приятным ценам. Липовый диплом, который говорит сам за себя — kyc-diplom.com/diplom-articles/lipovyj-diplom-kotoryj-govorit-sam-za-sebya.html
dragon casino dragonslotscasinos.net .
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1794 клиентов воспользовались услугой — теперь ваша очередь.
Диплом цена — ответим быстро, без лишних формальностей.
SA88 is the No.1 trusted online bookmaker in Vietnam, highly rated by the player community for its service quality, transparency, and safety. Licensed by Gaming Curacao, SA88 offers a diverse game library and a professional betting experience.
If you’re looking for a premium and reliable betting platform, SA88 is your top choice.
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3203 клиентов воспользовались услугой — теперь ваша очередь.
Перейти — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2887 клиентов воспользовались услугой — теперь ваша очередь.
Перейти — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1127 клиентов воспользовались услугой — теперь ваша очередь.
Дипломы о высшем образовании купить — ответим быстро, без лишних формальностей.
The Pokies net Australia The Pokies net Australia .
dragonslots dragonslots .
egypt888starz.net egypt888starz.net .
thepokies net250 http://pokiesnet250.com/ .
888starz download apk 888starz download apk .
win ставки на спорт https://1win1104.ru/
дренаж участка под ключ цена в спб .
thepokies net250 thepokies net250 .
Your article helped me a lot, is there any more related content? Thanks! https://accounts.binance.com/ar-BH/register-person?ref=V2H9AFPY
авиатор игра 1вин http://www.1win1107.ru
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1911 клиентов воспользовались услугой — теперь ваша очередь.
Здесь — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4303 клиентов воспользовались услугой — теперь ваша очередь.
Здесь — ответим быстро, без лишних формальностей.
Шторы на заказ: идеальное решение для вашего интерьера, материал.
Шторы на заказ для любого интерьера, добавляет уют в ваше пространство.
Шторы на заказ — это просто, быстро и удобно, действуйте уже сегодня.
Идеальные шторы на заказ для любого бюджета, с учетом всех пожеланий.
Уникальные шторы, которые подчеркнут вашу индивидуальность, выбирайте лучшее.
Индивидуальные шторы на заказ от лучших дизайнеров, усилите атмосферу.
Шторы на заказ: когда каждое окно в вашем доме особенное, не откладывайте на потом.
Шторы на заказ: функциональность и эстетика, выберите свой стиль.
Функциональные шторы под заказ для вашего удобства, находите вдохновение.
Шторы, которые идеально впишутся в ваш дом, создавайте свой стиль.
шторы на заказ шторы на заказ .
Купить диплом вы можете используя сайт компании. orikdok-2v-gorode-kemerovo-42.online
chicken road key parameters
Chicken Road: Real Player Feedback
Chicken Road is an arcadestyle gambling game that has caught the attention of players with its simplicity, high RTP (98%), and unique cashout feature. We’ve collected honest feedback from actual players to see if it lives up to expectations.
Key Highlights According to Players
Numerous players commend Chicken Road for its quick, engaging action and userfriendly design. With its cashout feature offering strategy and an RTP of 98%, it feels like a fairer alternative to conventional slot games. The riskfree demo mode has been a favorite among new players, providing a safe way to explore the game. The game earns extra points for its mobile compatibility, running seamlessly on both new and older devices.
Melissa R., AU: “Unexpectedly enjoyable and balanced! The ability to cash out brings a layer of strategy.”
Nathan K., UK: “Its arcadeinspired style is a breath of fresh air, and it operates smoothly on my device.”
The bright, nostalgic visuals add to the fun factor, keeping players hooked.
Areas for Improvement
However, Chicken Road isn’t perfect, and there are a few issues worth noting. A number of users feel the gameplay becomes monotonous and lacks complexity. Others mention slow customer support and limited features. Misleading ads are another issue, with many assuming it was an arcade game instead of a gambling app.
Tom B., US: “Fun at first, but it gets repetitive after a few days.”
Sam T., UK: “Marketed as a casual game, but it’s actually a gamblingfocused app.”
Strengths and Weaknesses
Pros
Simple, fastpaced gameplay
High RTP (98%) ensures fairness
Practice mode to explore without financial risk
Smooth performance on mobile devices
Cons
Gameplay can feel repetitive
Limited variety and features
Slow or unresponsive customer support
Confusing promotional tactics
Overall Assessment
Chicken Road shines through its openness, impressive RTP, and ease of access. Ideal for casual gamers or anyone just starting with online gambling. Still, the heavy emphasis on luck and minimal complexity could turn off some users. To maximize enjoyment, stick to authorized, regulated sites.
Rating: 4/5
A balanced blend of fun and fairness, with potential for enhancement.
https://chickenroadhq.org/
the pokies net 101 login the pokies net 101 login .
mostbet скачать на андроид http://mostbet11017.ru
“Merhaba!
Big Bass Bonanza slotunu gercek parayla denedin mi?
?? Bal?klar? yakala, buyuk odulleri topla!
? big bass bonanza nas?l oynan?r
?? Hemen oyna: bigbassbalik.com
?? Pragmatic Play kalitesi, yuksek RTP ve buyuk kazanma sans?.
?? VPN’siz giris, mobil destekli, h?zl? kay?t.
Kazanmaya basla!”
Thanks for the article. Here’s more on the topic https://cultureinthecity.ru/
mostbet скачать 2024 mostbet11018.ru
дренажная сливная система .
the pokies net the pokies net .
Thanks for the article. Here’s more on the topic https://yarus-kkt.ru/
аттестат за 9 классов купить аттестат за 9 классов купить .
психотерапевт нижний psihiatry-nn-1.ru .
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4052 клиентов воспользовались услугой — теперь ваша очередь.
Звоните — ответим быстро, без лишних формальностей.
Мы предлагаем оформление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2057 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом вуза — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1384 клиентов воспользовались услугой — теперь ваша очередь.
Диплом цена — ответим быстро, без лишних формальностей.
Молниеносная доставка – это выше всех похвал!
заказ цветов томск с доставкой
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2445 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом Россия — ответим быстро, без лишних формальностей.
dragon slot http://casinosdragonslots.eu/ .
напольный высокий горшок для растений http://www.kashpo-napolnoe-msk.ru – http://www.kashpo-napolnoe-msk.ru .
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4106 клиентов воспользовались услугой — теперь ваша очередь.
Посмотреть информацию — ответим быстро, без лишних формальностей.
Мы предлагаем оформление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3285 клиентов воспользовались услугой — теперь ваша очередь.
Купить государственный диплом — ответим быстро, без лишних формальностей.
высокий цветочный горшок на пол https://kashpo-napolnoe-msk.ru – высокий цветочный горшок на пол .
муравьиная ферма где заказать муравьиная ферма где заказать .
The Pokies net Australia login http://www.pokiesnet250.com .
Создайте уникальные шторы под заказ для вашего дома, по вашим предпочтениям.
Индивидуальный подход к шторным решениям, открывает новые горизонты.
Шторы на заказ — это просто, быстро и удобно, не упустите возможность.
Шторы на заказ — сочетание качества и стиля, всегда в наличии.
Уникальные шторы, которые подчеркнут вашу индивидуальность, позвольте нам порадовать вас.
Шторы по вашим размерам — идеальное решение, усилите атмосферу.
Шторы на заказ: когда каждое окно в вашем доме особенное, не откладывайте на потом.
Шторы на заказ: функциональность и эстетика, измените свой интерьер навсегда.
Элегантные шторы на заказ для любого стиля, доверяйте профессионалам.
Шторы, которые идеально впишутся в ваш дом, выбирайте из множества вариантов.
шторы на заказ шторы на заказ .
The Pokies Australia https://thepokiesnet250.com .
thepokies net111 https://pokies11.com/ .
Мы предлагаем оформление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3105 клиентов воспользовались услугой — теперь ваша очередь.
Открыть — ответим быстро, без лишних формальностей.
карниз электро http://elektrokarnizy50.ru .
1 win aviator http://www.1win1105.ru
thepokies106.net thepokies106.net .
Купить диплом любого ВУЗа. Покупка документа о высшем образовании через надежную компанию дарит ряд достоинств для покупателя. Такое решение помогает сэкономить время и серьезные финансовые средства. orikdok-1v-gorode-nizhniy-novgorod-52.ru
1win lucky jet отзывы https://www.1win1107.ru
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
выберите ресурсы https://aviator-game-play.com/strategii-i-taktiki-igry-v-aviator/
купить диплом в ижевске купить диплом в ижевске .
thepokies101.net thepokies101.net .
Психотерапевт Оренбург. professorkorotkov.ru 298 оценок
1вин зеркало скачать 1win1110.ru
“Hey bahis severler!
?? Yeni versiyon: Sweet Bonanza 1000 slotu yay?nda!
?? Daha fazla carpan, daha yuksek kazanc seni bekliyor.
? sweet bonanza 1000 taktik
?? Hemen oyna: sweetbonanza1000net.com
?? Pragmatic Play fark?yla %100 eglence ve max win sans?!
?? Mobil uyumlu, VPN gerekmez, h?zl? kay?t imkan?.
Simdi oyna!”
Тетя до сих пор вспоминает этот букет с улыбкой!
заказать цветы томск
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2169 клиентов воспользовались услугой — теперь ваша очередь.
Узнать условия — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4654 клиентов воспользовались услугой — теперь ваша очередь.
Купить государственный диплом — ответим быстро, без лишних формальностей.
Мы предлагаем оформление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4348 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом нового образца — ответим быстро, без лишних формальностей.
the pokies net 106 the pokies net 106 .
Купить рулонные электрошторы, стиль, отличное качество.
Электрошторы для комфорта, и стиля.
Что учесть при покупке электроштор?, качество материалов.
Электрошторы: преимущества рулонного типа, долговечность и практичность.
Электрошторы для вашего интерьера, экологические материалы.
Рулонные электрошторы для любых окон, долговечность.
Рулонные электрошторы: как они работают?, экономия пространства.
Электрошторы для кухни и спальни, доступные инструкции.
Современные технологии в рулонных электрошторах, удобство управления.
Рулонные электрошторы: для бизнеса и дома, красота и комфорт.
Как выбрать идеальные электрошторы?, практичность и стиль.
Рулонные электрошторы для современного интерьера, выбор цвета.
Создайте комфорт с рулонными электрошторами, легкость в уходе.
Рулонные электрошторы как элемент уюта, выбор цвета и текстуры.
Лучшие решения для оконных штор, простота установки.
Рулонные электрошторы: ваш новый стиль, подбор стиля.
Как правильно выбрать рулонные электрошторы?, функциональность и стиль.
Электрошторы для защиты от солнца, легкость в уходе.
Рулонные электрошторы для создания уюта, разнообразие стилей.
Топ-5 причин выбрать рулонные электрошторы, эстетика и цена.
автоматическая рулонная штора автоматическая рулонная штора .
1win зеркало https://1win1109.ru/
1win app download https://1win11003.com
На прием Клинцы. professorkorotkov.ru 233 оценок
Смотреть здесь https://aviator-game-play.com/aviator-1win/
Кпт курган. professorkorotkov.ru 966 оценок
thepokies111 http://pokies11.com .
Сервис по подбору психолога professorkorotkov.ru 661 оценок
электрокарнизы цена http://elektrokarnizy50.ru .
1win bet uganda 1win11002.com
скачать бесплатно 1win http://1win1106.ru/
Психотерапевты Самара. professorkorotkov.ru 127 оценок
Your article helped me a lot, is there any more related content? Thanks!
дренаж участка колодец .
Психотерапевт Оренбург. professorkorotkov.ru 484 оценок
На прием Клинцы. professorkorotkov.ru 532 оценок
Рулонные электрошторы на заказ, удобство, отличное качество.
Электрошторы: идеальное решение, для любого интерьера.
Что учесть при покупке электроштор?, качество материалов.
Электрошторы: преимущества рулонного типа, долговечность и практичность.
Секреты выбора рулонных электроштор, простота управления.
Комфорт с рулонными электрошторами, высокое качество.
Эстетика и практичность электроштор, экономия пространства.
Рулонные электрошторы на любой вкус, доступные инструкции.
Электрошторы: умный дом, автоматизация.
Эстетика и функциональность электроштор, долговечность эксплуатации.
Электрошторы: решение для вашего окна, разнообразие дизайнов.
Электрошторы: простота и элегантность, доступность материалов.
Электрошторы для идеального освещения, долговечность.
Рулонные электрошторы как элемент уюта, интерьер в стиле минимализм.
Как рулонные электрошторы улучшают интерьер, простота установки.
Электрошторы: идеальное решение для окон, практические советы.
Рулонные электрошторы: удобство и комфорт, технологические преимущества.
Рулонные электрошторы: ваш надежный помощник, варианты управления.
Электрошторы: идеальный выбор для вашего дома, разнообразие стилей.
Рулонные электрошторы: ваш стильный акцент, эстетика и цена.
автоматическая рулонная штора автоматическая рулонная штора .
Психотерапевт Белгород. professorkorotkov.ru 755 оценок
mostbet kg регистрация https://www.mostbet11016.ru
mostbet игры https://www.mostbet11019.ru
букмекерская контора регистрация онлайн букмекерская контора регистрация онлайн
chicken roads review
Chicken Road: Honest User Opinions
Chicken Road is a gamblinginspired arcade game that has drawn interest due to its straightforward mechanics, impressive RTP (98%), and innovative cashout option. We’ve collected honest feedback from actual players to see if it lives up to expectations.
What Players Like
Many users praise Chicken Road for its fastpaced gameplay and ease of use. With its cashout feature offering strategy and an RTP of 98%, it feels like a fairer alternative to conventional slot games. The demo mode is a hit with beginners, allowing players to test the game riskfree. The game earns extra points for its mobile compatibility, running seamlessly on both new and older devices.
Melissa R., AU: “A surprisingly entertaining and fair experience. The cashout function really enhances the gameplay.”
Nathan K., UK: “The arcade style is refreshing. Runs smoothly on my tablet.”
Gamers are also fond of the vibrant, retro aesthetic, making it both enjoyable and captivating.
Drawbacks
However, Chicken Road isn’t perfect, and there are a few issues worth noting. Some players find the gameplay repetitive and lacking depth. Players also point out unresponsive support teams and insufficient features. Misleading ads are another issue, with many assuming it was an arcade game instead of a gambling app.
Tom B., US: “Initially enjoyable, but the repetition kicks in after a short while.”
Sam T., UK: “Marketed as a casual game, but it’s actually a gamblingfocused app.”
Strengths and Weaknesses
Advantages
Easytounderstand, quick gameplay
An RTP of 98% guarantees a fair experience
Demo mode for riskfree learning
Smooth performance on mobile devices
Negative Aspects
It might feel too predictable over time
Limited variety and features
Customer service can be sluggish and unreliable
Misleading marketing
Final Verdict
Chicken Road shines through its openness, impressive RTP, and ease of access. Perfect for relaxed gaming sessions or newcomers to online betting. Still, the heavy emphasis on luck and minimal complexity could turn off some users. For optimal results, choose verified, legitimate platforms.
Rating: 4/5
A balanced blend of fun and fairness, with potential for enhancement.
https://www.sslmarket.cz/overeni-ssl?domain=chickenroadhq.org
1win loading https://1win11001.com/
ustrojstvo-drenazha-812.ru .
“Selamlar!
Gates of Olympus slotunu gercek parayla denedin mi?
?? Zeus ile kazanc patlamas? yasa!
? gates of olympus nas?l oynan?r
?? Hemen oyna: olimpiyagates.com
?? Pragmatic Play kalitesiyle yuksek RTP, 5000x’e kadar max win sans?.
?? VPN gerekmez, mobil destekli, h?zl? kay?t.
Bol sans!”
Мы готовы предложить документы ВУЗов, расположенных на территории всей России. Купить диплом любого университета:
moblogs.com.au/kachestvennye-diplomy-dlja-vashego-uspeha-205
мостбет регистрация http://www.mostbet11016.ru
Предлагаем ознакомиться керамогранит для ванной в москве. Если вам понадобится керамогранит для ванной купить в москве обращайтесь
1вин приложение официальный сайт [url=www.1win1111.ru]www.1win1111.ru[/url]
1win aviator predictor 1win11002.com
1win apk 1win apk
Thanks for the article. Here’s more on the topic https://cultureinthecity.ru/
посетить веб-сайт препарат мунжаро
Видно, что собирали с любовью – это чувствуется!
купить цветы томск
1 win. https://1win1110.ru
1win app login download 1win app login download .
Преимущества автоматических рулонных штор в интерьере, советуем.
Топ-5 советов по выбору автоматических рулонных штор, ваш дом.
Автоматические рулонные шторы: идеальное решение для окон, получите информацию.
Автоматические рулонные шторы: стиль и комфорт, у нас.
Управление автоматической рулонной шторой одним нажатием, нашими услугами.
Правила ухода за автоматическими рулонными шторами, они служили долго.
Автоматические рулонные шторы в интерьере: тренды 2023 года, в нашем руководстве.
Шторы, которые сами закрываются: автоматизация в вашем доме, с отзывами пользователей.
Идеальные автоматические рулонные шторы для каждого стиля, посмотрите наш каталог.
Комфорт и уют с автоматическими рулонными шторами, в нашем каталоге.
Автоматические рулонные шторы — удобство в каждой детали, выберите.
Автоматические рулонные шторы: идеальное решение для светолюбителей, в нашем руководстве.
Что нужно знать о механизме автоматических рулонных штор, с нашим руководством.
Эстетика автоматических рулонных штор, с идеями.
Обзор популярных брендов автоматических рулонных штор, изучите.
Создайте стильный интерьер с автоматическими рулонными шторами, подберите.
Как автоматические рулонные шторы помогают экономить энергию, в нашем каталоге.
Автоматические рулонные шторы и их преимущества для офисов, в нашем блоге.
Как выбрать идеальные автоматические рулонные шторы, ознакомьтесь.
Идеальные шторы для бизнеса — автоматические рулонные шторы, узнайте.
автоматическая рулонная штора автоматическая рулонная штора .
Наша компания предлагает быстро и выгодно купить диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Документ пройдет любые проверки, даже с применением профессионального оборудования. nowoczesna.phorum.pl/posting.php?mode=newtopic&f=2&sid=a113eba4479d283a90afd3e7f69c0130
горшки для напольных растений купить kashpo-napolnoe-msk.ru – горшки для напольных растений купить .
кашпо напольные высокие недорого kashpo-napolnoe-msk.ru – кашпо напольные высокие недорого .
Как пошить римские шторы: шаг за шагом, освежить.
Пошив римских штор: пособие
пошить римские шторы пошить римские шторы .
1win app details 1win-in1.com .
Thanks for the article. Here’s more on the topic https://cultureinthecity.ru/
Мы предлагаем документы любых учебных заведений, расположенных в любом регионе РФ. Заказать диплом о высшем образовании:
msobl.flybb.ru/viewtopic.php?f=2&t=1036
“Merhaba!
Sweet Bonanza oyununu gercek parayla denedin mi?
?? Seker patlat ve para kazan.
? sweet bonanza oyna
?? Hemen basla: bonanzaseker.org
Kazanmaya basla!”
1win casino http://1win11001.com/
Автоматическая рулонная штора: удобство и стиль, рекомендуем.
Как выбрать автоматическую рулонную штору, для того чтобы.
Почему стоит выбрать автоматические рулонные шторы, узнайте.
Создайте уют с автоматическими рулонными шторами, выбирая.
Управление автоматической рулонной шторой одним нажатием, обязательно ознакомьтесь.
Уход за автоматическими рулонными шторами: советы и рекомендации, чтобы.
Автоматические рулонные шторы в интерьере: тренды 2023 года, исследуйте.
Умные рулонные шторы для современного интерьера, узнайте.
Почему автоматические рулонные шторы — это удобно и стильно, посмотрите наш каталог.
Как автоматические рулонные шторы улучшают качество жизни, узнайте больше.
Современные автоматические рулонные шторы для стильного интерьера, сегодня.
Как автоматические рулонные шторы решают проблемы с освещением, в нашем блоге.
Что нужно знать о механизме автоматических рулонных штор, с детальным обзором.
Автоматические рулонные шторы: как они меняют привычный интерьер, с идеями.
Обзор популярных брендов автоматических рулонных штор, познакомьтесь.
Автоматические рулонные шторы: идеальное дополнение к вашему интерьеру, подберите.
Энергоэффективные решения для окон: автоматические рулонные шторы, узнайте подробнее.
Советы по выбору автоматических рулонных штор для офисов, в нашем блоге.
Выбор автоматических рулонных штор: советы от экспертов, с нашими рекомендациями.
Автоматические рулонные шторы для гостиниц и ресторанов, познакомьтесь.
автоматическая рулонная штора автоматическая рулонная штора .
почта 1win почта 1win
мостбет официальный сайт регистрация http://www.mostbet11019.ru
Как пошить римские шторы: шаг за шагом, интерьер.
Пошив римских штор: пособие
пошить римские шторы пошить римские шторы .
The Philippines is known for its exotic beaches, expansive forests, and abundance of volcanoes. 딥페이크야동
В17 психология. chat-s-psikhologom-v-telegramme.ru 915 оценок
Thanks for the article. Here’s more on the topic https://imgtube.ru/
регистрация в 1вин https://www.1win1114.ru
Сестра была в полном восторге – лучший сюрприз!
букеты томск
Мы предлагаем выгодно и быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями официальных лиц. Документ способен пройти любые проверки, даже при использовании специальных приборов. sev-school24.maxbb.ru/viewtopic.php?f=3&t=1210
посетить сайт мунджаро mounjaro
1win регистрация ставки онлайн http://1win1109.ru/
1win ios http://1win1113.ru
1 вин букмекерская http://1win1108.ru/
Как сшить шторы своими руками, в нашем блоге.
Создайте идеальные шторы, с помощью нашего гида.
Шторы на заказ, как сшить.
Техника пошива штор, которые подойдут каждому.
Уроки для новичков по пошиву штор, на нашем сайте.
Выбор тканей для штор, и интерьеру.
Модные тренды в пошиве штор, вашему интерьеру.
Декор для штор, чтобы они выделялись.
Шторы для кухни своими руками, в классическом исполнении.
Комфортные шторы для вашего уюта, в нашем блоге.
Идеи для штор в гостиной, и предпочтений.
Советы по уходу за шторами, и прекрасными.
Шторы в различных стилях, на любой вкус.
Пошаговая инструкция по римским шторам, в вашем доме.
Преимущества функциональных штор, и защиты от солнца.
Как сшить шторы на люверсах, которые украсят любой интерьер.
Как выбрать шторы для детской комнаты, для комфорта и радости вашего ребенка.
Как сшить шторы без усилий, от профессионалов.
сшить шторы сшить шторы .
Букет – просто огонь, подруга в восторге!
цветы
Умные рулонные шторы для окон, комфорт и стиль.
Рулонные шторы с электроприводом, долговечность и простота в использовании.
Автоматизация окон: рулонные шторы, в каждой детали.
Лучшая автоматизация окон, все в ваших руках.
Выбор рулонных штор с электроприводом, для улучшения комфорта.
Умные рулонные шторы: необычные решения, доступные решения для вашего дома.
Инновационные электрические рулонные шторы, для вашего дома и офиса.
Рекомендации по выбору рулонных штор, для вашего дома.
Электрические рулонные шторы: как они работают?, добавляет удобства.
Лучшие электрические рулонные шторы на рынке, в идеальном сочетании.
Почему стоит выбрать электрические шторы, для вашего интерьера.
Как выбрать электрические рулонные шторы, для вашего офиса.
Купить электрические рулонные шторы: советы, в надежных магазинах.
Электрические рулонные шторы: функциональность и стиль, для создания уюта.
Лучшие электрические рулонные шторы для вашего дома, для вашего интерьера.
Электрические рулонные шторы: монтаж, с соблюдением всех стандартов.
Шторы с электроприводом для вашего дома, для улучшения условий жизни.
Электрические рулонные шторы для бизнеса, для удобства сотрудников.
Электрические шторы: экономия и комфорт, для вашего дома.
электрические рулонные шторы на окна электрические рулонные шторы на окна .
Thanks for the article. Here is a website on the topic – https://40-ka.ru/
Latest medication news. Find drug information.
imitrex cheap
Get pill details. Find medication information.
drenazhnye-sistemy-812.ru .
how to use 1win bonus 1win11005.com
chicken road key parameters
Chicken Road: Real Player Feedback
Chicken Road stands out as a gambling game with arcade vibes, attracting users with its easytograsp gameplay, high RTP (98%), and distinctive cashout system. We’ve collected honest feedback from actual players to see if it lives up to expectations.
What Players Like
Many users praise Chicken Road for its fastpaced gameplay and ease of use. With its cashout feature offering strategy and an RTP of 98%, it feels like a fairer alternative to conventional slot games. The demo mode is a hit with beginners, allowing players to test the game riskfree. Players also rave about the mobilefriendly design, which performs flawlessly even on outdated gadgets.
Melissa R., AU: “Surprisingly fun and fair! The cashout feature adds strategy.”
Nathan K., UK: “The arcade style is refreshing. Runs smoothly on my tablet.”
Gamers are also fond of the vibrant, retro aesthetic, making it both enjoyable and captivating.
Criticisms
Despite its strengths, Chicken Road isn’t without flaws. A number of users feel the gameplay becomes monotonous and lacks complexity. Others mention slow customer support and limited features. Misleading ads are another issue, with many assuming it was an arcade game instead of a gambling app.
Tom B., US: “It starts off fun, but the monotony sets in quickly.”
Sam T., UK: “Advertised as a fun game, but it’s clearly a gambling app.”
Advantages and Disadvantages
Pros
Simple, fastpaced gameplay
High RTP (98%) ensures fairness
Practice mode to explore without financial risk
Seamless operation on smartphones and tablets
Negative Aspects
It might feel too predictable over time
Lack of diversity and additional options
Delayed responses from support teams
Deceptive advertising
Overall Assessment
Chicken Road stands out with its transparency, high RTP, and accessibility. Perfect for relaxed gaming sessions or newcomers to online betting. However, its reliance on luck and lack of depth may not appeal to everyone. For the best experience, play on official, licensed platforms.
Rating: A solid 80%
A balanced blend of fun and fairness, with potential for enhancement.
https://dnssor.com/chickenroadhq.org
خدمة خدمات كهربائية الكويت ساعدتني كثيراً في تركيب الإضاءة الذكية وصيانة الشبكة الداخلية للمنزل. الخدمة ممتازة بكل المقاييس. خدمة خدمات كهربائية الكويت ساعدتني كثيراً في تركيب الإضاءة الذكية وصيانة الشبكة الداخلية للمنزل. الخدمة ممتازة بكل المقاييس. لقد جربت العديد من خدمات الكهرباء، ولكن لم أجد أفضل من خدمات كهربائية الكويت. الخدمة ممتازة والدعم متوفر على مدار الساعة مما يجعل التجربة مريحة وآمنة. إذا كنت تبحث عن خدمات كهربائية الكويت فأنت في المكان الصحيح. هذه الخدمة توفر لك كل ما تحتاجه من احترافية وسرعة ودقة في تنفيذ الأعمال الكهربائية المختلفة. أحد أفضل الحلول الكهربائية التي وجدتها كانت من خلال خدمات كهربائية الكويت. يقدمون خدمات رائعة وتوصيات دقيقة ويهتمون بالتفاصيل الصغيرة.
“Selam forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > Xturka promosyon
Rulet masas? h?zl? odeme.
VPN’siz erisim, tek t?kla kay?t > 15 saniyede haz?r.
Bol sans!”
mostbet yukle mostbet-download-app-apk.com .
бот lucky jet скачать бот lucky jet скачать
Как сшить шторы своими руками, пошаговая инструкция.
Соберите идеальные шторы, для вашего дизайна.
Эксклюзивные шторы, как создать.
Техника пошива штор, в своем городе.
Секреты для новичков, в нашем проекте.
Выбор тканей для штор, в соответствии с вашим стилем.
Модные тренды в пошиве штор, вашему интерьеру.
Как украсить шторы, в вашем интерьере.
Шторы для кухни своими руками, как выбрать.
Уютные шторы для спальни, вдохновение для дизайна.
Идеи для штор в гостиной, в актуальных трендах.
Как ухаживать за шторами, чтобы они долго служили.
Шторы в различных стилях, на любой вкус.
Все о римских шторах, для вашего интерьера.
Преимущества функциональных штор, в вашем доме.
Шторы на люверсах, которые легко монтируются.
Как выбрать шторы для детской комнаты, с учетом безопасности и стиля.
Лучшие советы по пошиву штор, от профессионалов.
сшить шторы сшить шторы .
buy imitrex medication https://imitrex2rp.top/# order sumatriptan buy sumatriptan
1 win официальный сайт скачать https://www.1win1114.ru
decomania
Voici un spin-tax de haute qualité pour votre texte en français, respectant toutes vos consignes :
Tandis que Decomania analyse les nouveautés des secteurs financiers et technologiques, un doute subsiste : Quantum AI 2025 constitue-t-il une véritable innovation ou seulement une solution à fort potentiel ?
Mode opératoire et Perspectives : Quel est le Principe de Cette Plateforme ?
Quantum AI 2025 se positionne comme un outil de trading algorithmique combinant smart tech et quantum computing. D’après ses concepteurs, cette technologie rendrait possible :
Une évaluation poussée des marchés financiers (crypto, titres, devises).
Une régulation intelligente des expositions pour améliorer les performances.
Un accès simplifié, adapté pour les traders tous niveaux.
Cependant, aucune recherche tierce ne valide officiellement ces allégations, et les feedbacks d’utilisateurs s’avèrent contrastés.
Aspects à Examiner D’après Decomania
Notre étude met en lumière divers facteurs à évaluer avant de s’engager :
Différentes plateformes locales (crypto-bank.fr) – Une pratique courante, mais qui peut rendre difficile l’authentification.
Transparence limitée – Un manque de détails technologiques sont accessibles sur les systèmes mis en œuvre.
Expériences variées – Plusieurs investisseurs indiquent des rendements intéressants, tandis que d’autres évoquent des difficultés techniques.
Conseils pour les Opérateurs
Privilégier les solutions agréées (etc.) pour un cadre plus sûr.
Essayer en mode démo avant tout engagement financier.
Comparer avec d’autres solutions (telles que les systèmes disponibles par d’autres brokers réputés).
Synthèse : Une Solution à Surveiller avec Réserve
Quantum AI 2025 avance une approche innovante, mais son efficacité réelle demandent toujours des confirmations pratiques. En attendant de une meilleure visibilité, une méthode mesurée est conseillée.
Умные рулонные шторы для окон, в одном решении.
Рулонные шторы с электроприводом, долговечность и простота в использовании.
Электрические шторы для умного дома, в каждом элементе.
Управление рулонными шторами одним нажатием, все в ваших руках.
Выбор рулонных штор с электроприводом, для улучшения комфорта.
Элегантность и технологии в рулонных шторах, по доступной цене.
Современные рулонные шторы для окон, для вашего дома и офиса.
Лучшие модели электрических рулонных штор, для функционального пространства.
Технология автоматизации рулонных штор, облегчает вашу жизнь.
Топ моделей электрических рулонных штор, в идеальном сочетании.
Почему стоит выбрать электрические шторы, для вашего уюта.
Электрические рулонные шторы: все, что нужно знать, для вашего стиля.
Где купить рулонные шторы с электроприводом?, в надежных магазинах.
Как электрические рулонные шторы изменят ваш дом, в лучшую сторону.
Электрические рулонные шторы: преимущества, для улучшения качества жизни.
Как установить электрические рулонные шторы, с соблюдением всех стандартов.
Шторы с электроприводом для вашего дома, для стильного оформления.
Электрические рулонные шторы для бизнеса, для удобства сотрудников.
Электрические шторы: экономия и комфорт, для вашего дома.
электрические рулонные шторы на окна электрические рулонные шторы на окна .
как использовать бонусный счет казино 1win https://www.1win1113.ru
1win букмекерская скачать приложение 1win букмекерская скачать приложение
сюда Мега сайт
mostbet mostbet .
Drug brochure available. https://buyedpills.shop/# Comprehensive medication resource. buy ed pills online
узнать больше [url=https://zpactheatre.com.au]Mega даркнет[/url]
Рулонные электрошторы на заказ, в интерьере, доступные цены.
Электрошторы: идеальное решение, в каждом доме.
Что учесть при покупке электроштор?, качество материалов.
Электрошторы для защиты от солнца, долговечность и практичность.
Секреты выбора рулонных электроштор, экологические материалы.
Электрошторы: ваш лучший выбор, долговечность.
Рулонные электрошторы: как они работают?, экономия пространства.
Как установить рулонные электрошторы, простой монтаж.
Электрошторы: умный дом, автоматизация.
Рулонные электрошторы: для бизнеса и дома, организация пространства.
Электрошторы: решение для вашего окна, практичность и стиль.
Рулонные электрошторы для современного интерьера, функция управления.
Создайте комфорт с рулонными электрошторами, долговечность.
Рулонные электрошторы как элемент уюта, интерьер в стиле минимализм.
Электрошторы для вашего стиля, разнообразие функций.
Как выбрать рулонные электрошторы?, разнообразие моделей.
Как правильно выбрать рулонные электрошторы?, технологические преимущества.
Как электрошторы меняют пространство, легкость в уходе.
Эстетика и уход за рулонными электрошторами, разнообразие стилей.
Электрошторы для офисов и жилых помещений, эстетика и цена.
автоматическая рулонная штора автоматическая рулонная штора .
напольное кашпо для цветов высокое пластик https://kashpo-napolnoe-spb.ru/ – напольное кашпо для цветов высокое пластик .
где купить напольное кашпо для цветов http://kashpo-napolnoe-spb.ru/ – http://kashpo-napolnoe-spb.ru/ .
диплом мед колледжа купить http://www.arus-diplom7.ru .
Заказать диплом на заказ в Москве возможно через официальный сайт компании. orikdok-4v-gorode-yoshkar-ola-12.online
Thanks for the article. Here’s more on the topic https://great-galaxy.ru/
Приобрести диплом института. Покупка диплома через надежную фирму дарит немало достоинств для покупателя. Такое решение помогает сберечь время и серьезные финансовые средства. orikdok-5v-gorode-chelyabinsk-74.ru
диплом в москве купить диплом в москве купить .
buy edpills shop https://buyedpills.shop/# buy ed pills edpills shop
Here’s more on the topic https://upsskirt.ru/
Накрутка Телеграм ВК Инстаграм в 2025 году Накрутка подписчиков лайков реакций просмотров https://vc.ru/niksolovov/1436777-25-besplatnyh-servisov-dlya-nakrutki-reakcii-v-telegram-v-2025-godu.
“Selam forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > Xturka kay?t ol
Rulet masas? h?zl? odeme.
VPN’siz erisim, tek t?kla kay?t > 15 saniyede haz?r.
Bol sans!”
Быстро, качественно и с душой – вот почему я выбираю вас!
розы томск
up x регистрация
Регистрация на официальном портале Up X
Регистрация в Up X — простой и быстрый процесс. Вам не придется выделять много времени, чтобы стать клиентом сервиса. Создатели платформы позаботились не только о стильном дизайне, но и о том, чтобы она воспринималась интуитивно. Минимализм и продуманный интерфейс — отличная комбинация. С созданием профиля не будет никаких проблем
https://skachatreferat.ru/
buy ed pills online buy ed pills buy ed pills online buy edpills shop
Рулонные электрошторы на заказ, в офисе, современные технологии.
Электрошторы для комфорта, простота в использовании.
Что учесть при покупке электроштор?, качество материалов.
Электрошторы для защиты от солнца, комфорт и безопасность.
Секреты выбора рулонных электроштор, экологические материалы.
Комфорт с рулонными электрошторами, долговечность.
Эстетика и практичность электроштор, защита от солнечных лучей.
Электрошторы для кухни и спальни, пошаговое руководство.
Электрошторы: умный дом, автоматизация.
Эстетика и функциональность электроштор, долговечность эксплуатации.
Как выбрать идеальные электрошторы?, разнообразие дизайнов.
Рулонные электрошторы для современного интерьера, доступность материалов.
Электрошторы для идеального освещения, долговечность.
Дизайн и функциональность рулонных электроштор, советы по оформлению.
Лучшие решения для оконных штор, доступные цены.
Рулонные электрошторы: ваш новый стиль, практические советы.
Рулонные электрошторы: удобство и комфорт, функциональность и стиль.
Как электрошторы меняют пространство, легкость в уходе.
Эстетика и уход за рулонными электрошторами, долговечность.
Электрошторы для офисов и жилых помещений, современные технологии.
автоматическая рулонная штора автоматическая рулонная штора .
Смотреть здесь Mega ссылка
Thanks for the article. Here is a website on the topic – https://40-ka.ru/
В17 психология. chat-s-psikhologom-v-telegramme.ru 772 оценок
карниз с приводом http://elektrokarniz-nedorogo77.ru .
электрические гардины для штор электрические гардины для штор .
айфлоу видеонаблюдение http://www.citadel-trade.ru .
Here’s more on the topic https://upsskirt.ru/
электрические гардины для штор http://www.karnizy-s-elektroprivodom77.ru .
вход в 1win вход в 1win
parimatch download ios http://www.parimatch-app-download.com .
фрибет мелбет http://www.mostbet11021.ru
Сложный заказ выполнили быстро!
101 роза
рулонные шторки на окна https://elektricheskie-rulonnye-shtory77.ru .
1win apk https://1win11005.com
buy ed pills online https://buyedpills.shop/# buy ed pills online buy ed pills online
зайти в 1він зайти в 1він
электрические гардины для штор http://www.elektrokarniz90.ru/ .
скачать бк осталось только найти предпочтение подходящее 1win22095.ru
Купить диплом под заказ вы имеете возможность через официальный портал компании. orikdok-5v-gorode-irkutsk-38.ru
Приобрести диплом университета. Приобретение документа о высшем образовании через надежную фирму дарит ряд преимуществ для покупателя. Это решение помогает сберечь время и существенные средства. orikdok-1v-gorode-volgograd-34.online
купить диплом в иркутске купить диплом в иркутске .
1win жүктөп алуу https://1win11002.ru/
Психотерапевт Оренбург. chat-s-psikhologom-v-telegramme.ru 305 оценок
рулонные шторы купить москва недорого http://www.elektricheskie-rulonnye-shtory99.ru .
Преимущества автоматических рулонных штор в интерьере, рекомендуем.
Как выбрать автоматическую рулонную штору, для выбора.
Почему стоит выбрать автоматические рулонные шторы, узнайте больше.
Преобразите свой дом с автоматическими рулонными шторами, в нашем магазине.
Управление автоматической рулонной шторой одним нажатием, нашими предложениями.
Правила ухода за автоматическими рулонными шторами, чтобы.
Автоматические рулонные шторы в интерьере: тренды 2023 года, познакомьтесь.
Автоматизированные рулонные шторы: что нужно знать, прочитайте.
Почему автоматические рулонные шторы — это удобно и стильно, узнайте прямо сейчас.
Как автоматические рулонные шторы улучшают качество жизни, в нашем магазине.
Обновите ваш дом с помощью автоматических рулонных штор, получите.
Советы по использованию автоматических рулонных штор для оптимального освещения, изучите.
Что нужно знать о механизме автоматических рулонных штор, с нашим руководством.
Автоматические рулонные шторы: как они меняют привычный интерьер, узнайте.
Сравнение автоматических рулонных штор различных брендов, в нашем обзоре.
Автоматические рулонные шторы: идеальное дополнение к вашему интерьеру, сейчас.
Энергоэффективность и автоматические рулонные шторы, на нашем сайте.
Советы по выбору автоматических рулонных штор для офисов, проверьте.
Выбор автоматических рулонных штор: советы от экспертов, ознакомьтесь.
Автоматические рулонные шторы для гостиниц и ресторанов, познакомьтесь.
автоматическая рулонная штора автоматическая рулонная штора .
рулонные жалюзи на окна цена рулонные жалюзи на окна цена .
buy edpills shop buy ed pills online buy edpills shop buy ed pills online
parimatch india app download parimatch india app download .
“Merhaba forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > buraya t?kla
Canl? bahis yuksek oranl?.
VPN’siz erisim, tek t?kla kay?t > 20 saniyede haz?r.
Bol sans!”
купить диплом в барнауле купить диплом в барнауле .
This is a topic that is near to my heart… Cheers!
Exactly where are your contact details though?
Вы покупаете диплом в надежной и проверенной временем компании. Заказать диплом университета– http://mexschool.ru/kupite-diplom-s-zaneseniem-v-reestr-po-vigodnoj-tsene/ – mexschool.ru/kupite-diplom-s-zaneseniem-v-reestr-po-vigodnoj-tsene/
электрокарнизы в москве электрокарнизы в москве .
автоматический карниз для штор http://www.elektrokarniz-nedorogo77.ru .
гардина с электроприводом https://www.karnizy-s-elektroprivodom77.ru .
Психотерапевты Самара. chat-s-psikhologom-v-telegramme.ru 625 оценок
крипто бк https://1win22096.ru
Thank you, your article surprised me, there is such an excellent point of view. Thank you for sharing, I learned a lot.
buy edpills shop https://buyedpills.shop/# buy ed pills online buy edpills shop
На прием Клинцы. chat-s-psikhologom-v-telegramme.ru 236 оценок
На прием Клинцы. chat-s-psikhologom-v-telegramme.ru 167 оценок
iflow https://citadel-trade.ru .
Мы предлагаем дипломы любой профессии по выгодным ценам. Важно, чтобы документы были доступны для большинства наших граждан. Приобрести диплом о высшем образовании saturn.forumex.ru/viewtopic.php?f=19&t=815
sportbets sportbets14.ru .
Лучшие цветы в городе – проверено временем!
купить пионы томск
Психотерапевт Челны. chat-s-psikhologom-v-telegramme.ru 996 оценок
пластиковые окна под ключ цены пластиковые окна под ключ цены .
Автоматическая рулонная штора: удобство и стиль, подробнее узнать.
Топ-5 советов по выбору автоматических рулонных штор, ваше пространство.
Автоматические рулонные шторы: идеальное решение для окон, узнайте больше.
Создайте уют с автоматическими рулонными шторами, выбирая.
Управление автоматической рулонной шторой одним нажатием, нашими услугами.
Как ухаживать за автоматическими рулонными шторами, поддерживать идеальное состояние.
Модные идеи с автоматическими рулонными шторами, в нашем руководстве.
Автоматизированные рулонные шторы: что нужно знать, с нашими экспертными рекомендациями.
Автоматические рулонные шторы: сочетание функциональности и дизайна, изучите подробности.
Лучшие решения для окон — автоматические рулонные шторы, узнайте больше.
Автоматические рулонные шторы — удобство в каждой детали, у нас.
Советы по использованию автоматических рулонных штор для оптимального освещения, в нашем блоге.
Что нужно знать о механизме автоматических рулонных штор, с нашим руководством.
Автоматические рулонные шторы: как они меняют привычный интерьер, ознакомьтесь.
Сравнение автоматических рулонных штор различных брендов, изучите.
Создайте стильный интерьер с автоматическими рулонными шторами, в нашем магазине.
Энергоэффективность и автоматические рулонные шторы, в нашем каталоге.
Советы по выбору автоматических рулонных штор для офисов, в нашем блоге.
Где купить качественные автоматические рулонные шторы, проконсультируйтесь.
Как автоматические рулонные шторы улучшают атмосферу в общественных местах, познакомьтесь.
автоматическая рулонная штора автоматическая рулонная штора .
рулонные шторы с электроприводом цена http://elektricheskie-rulonnye-shtory77.ru .
1 вин ставки на спорт зеркало http://1win22096.ru/
chicken road key parameters
Chicken Road: What Gamblers Are Saying
Chicken Road stands out as a gambling game with arcade vibes, attracting users with its easytograsp gameplay, high RTP (98%), and distinctive cashout system. We’ve collected honest feedback from actual players to see if it lives up to expectations.
Key Highlights According to Players
A lot of gamers appreciate how Chicken Road combines fast gameplay with simple controls. The ability to cash out at any time adds a strategic twist, while the high RTP makes it feel fairer than traditional slots. The demo mode is a hit with beginners, allowing players to test the game riskfree. Players also rave about the mobilefriendly design, which performs flawlessly even on outdated gadgets.
Melissa R., AU: “A surprisingly entertaining and fair experience. The cashout function really enhances the gameplay.”
Nathan K., UK: “The retro arcade vibe feels invigorating. Plus, it works perfectly on my tablet.”
The bright, nostalgic visuals add to the fun factor, keeping players hooked.
Drawbacks
Despite its strengths, Chicken Road isn’t without flaws. A number of users feel the gameplay becomes monotonous and lacks complexity. Others mention slow customer support and limited features. A common complaint is misleading advertising—many expected a pure arcade game, not a gambling app.
Tom B., US: “Fun at first, but it gets repetitive after a few days.”
Sam T., UK: “Promoted as entertainment, yet it turns out to be a gambling product.”
Pros and Cons
Pros
Simple, fastpaced gameplay
With a 98% RTP, it offers a sense of equity
Practice mode to explore without financial risk
Seamless operation on smartphones and tablets
Disadvantages
The gameplay may come across as monotonous
Limited variety and features
Customer service can be sluggish and unreliable
Confusing promotional tactics
Conclusion
Chicken Road shines through its openness, impressive RTP, and ease of access. Ideal for casual gamers or anyone just starting with online gambling. Still, the heavy emphasis on luck and minimal complexity could turn off some users. To maximize enjoyment, stick to authorized, regulated sites.
Rating: A solid 80%
A fair and entertaining choice, but not without room for improvement.
электрические гардины для штор elektrokarniz90.ru .
mx pharmacy easy mx pharmacy easy mx pharmacy easy mx pharmacy easy
drenazhnye-sistemy-812.ru .
музыка на телефоне бесплатно музыка на телефоне бесплатно .
Спасибо за внимание к клиентам!
купить цветы в томске
lucky jet скачать на андроид 1win22094.ru
aviator игра 1win скачать http://1win11002.ru
UP X — обзор официальной платформы Ап Икс
Сегодня абсолютно каждый житель Российской Федерации может сыграть в увлекательные игровые автоматы, и при этом не покидая собственное жилище. Выбирая подобный формат онлайн развлечений, игрокам следует осознавать, что это не способ обогащения, а возможность получить яркие эмоции и незабываемые впечатления, внести разнообразие в повседневную жизнь.
https://mymcu.ru/
автоматические шторы на окна автоматические шторы на окна .
Приобрести диплом ВУЗа по невысокой цене возможно, обратившись к проверенной специализированной компании. Купить документ университета вы имеете возможность у нас в Москве. orikdok-2v-gorode-groznyy-20.online
рулонные шторы купить цены http://rulonnye-shtory-s-elektroprivodom15.ru/ .
mx pharmacy easy https://mxpharmacyeasy.shop/# mx pharmacy easy mx pharmacy easy
“Dikkat kazanc avc?lar?!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > rulet canl? yay?n
Slot turnuvas? 24/7 ac?k.
VPN’siz erisim, tek t?kla kay?t > 15 saniyede haz?r.
Kazanmay? dene!”
sportbets https://www.sportbets15.ru .
1 х ставка вход 1 х ставка вход
Thanks for the article. Here’s more on the topic https://great-galaxy.ru/
современные дренажные системы официальный .
мелбет бонусный счет https://www.mostbet11021.ru
Мы предлагаем дипломы любых профессий по приятным ценам. Заказ диплома, подтверждающего обучение в ВУЗе, – это разумное решение. Купить диплом о высшем образовании: mos.flybb.ru/viewtopic.php?f=2&t=3451
Автоматические рулонные шторы для вашего дома, для вашего интерьера.
Рулонные шторы с электроприводом, качество и надежность.
Электронные рулонные шторы для вашего пространства, функциональность.
Лучшая автоматизация окон, все в ваших руках.
Как выбрать электрические рулонные шторы, для создания уюта.
Электрические рулонные шторы: стиль и технологии, по доступной цене.
Инновационные электрические рулонные шторы, для вашего дома и офиса.
Рекомендации по выбору рулонных штор, для функционального пространства.
Электрические рулонные шторы: как они работают?, добавляет удобства.
Самые популярные электрические рулонные шторы, с первоклассным качеством.
Преимущества автоматических рулонных штор, для вашего интерьера.
Электрические рулонные шторы: все, что нужно знать, в вашем доме.
Купить электрические рулонные шторы: советы, в надежных магазинах.
Как электрические рулонные шторы изменят ваш дом, для вашего удобства.
Электрические рулонные шторы: обзор и советы, для вашего комфорта.
Электрические рулонные шторы: монтаж, просто и удобно.
Электрические рулонные шторы: идеальные решения, для улучшения условий жизни.
Электронные решения для коммерческих помещений, для удобства сотрудников.
Электрические рулонные шторы: выгодные решения, для вашего дома.
электрические рулонные шторы на окна электрические рулонные шторы на окна .
Римские шторы на заказ – идеальное решение для вашего интерьера, по приемлемой цене.
Хотите обновить интерьер с помощью римских штор?, наши.
Римские шторы на заказ от опытных мастеров, с.
Лучшие римские шторы на заказ, в.
Откройте для себя мир римских штор, не упустите шанс.
Индивидуальное изготовление римских штор, прямо сейчас.
Сшите идеальные римские шторы для вашего дома, всегда.
Идеальные римские шторы для вашего интерьера, не упустите эту возможность.
Полезные советы по выбору римских штор, с рекомендациями.
Уникальные римские шторы на заказ, приобретите.
Краткое руководство по созданию римских штор, на наши советы.
Заказывайте римские шторы и наслаждайтесь комфортом, просто.
Сшите римские шторы именно так, как хотите, гарантией качества.
Не откладывайте заказ римских штор, доступным ценам.
Ваш индивидуальный проект римских штор, только у нас.
Изготовление римских штор под заказ, читайте.
Заказывайте римские шторы по вашим размерам, легко.
Сшить римские шторы с учетом ваших пожеланий, обязательно.
сшить римские шторы на заказ сшить римские шторы на заказ .
Оформиление дипломов ВУЗов в Москве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 962 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом вуза — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4978 клиентов воспользовались услугой — теперь ваша очередь.
Дипломы о высшем образовании купить — ответим быстро, без лишних формальностей.
Мы готовы предложить дипломы любой профессии по выгодным тарифам. Мы готовы предложить документы техникумов, которые находятся в любом регионе РФ. Дипломы и аттестаты печатаются на “правильной” бумаге высшего качества. Это дает возможность делать настоящие дипломы, не отличимые от оригинала. orikdok-1v-gorode-tula-71.ru
Мы предлагаем оформление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2600 клиентов воспользовались услугой — теперь ваша очередь.
Мы поможем — ответим быстро, без лишних формальностей.
Быстро купить диплом об образовании!
Мы изготавливаем дипломы любых профессий по приятным ценам— diplomaj-v-tule.ru
1win ставки приложение http://www.1win22095.ru
1win скачать на айфон зеркало http://1win22096.ru
Психотерапевт Оренбург. chat-s-psikhologom-v-telegramme.ru 538 оценок
Приобрести диплом университета по невысокой цене возможно, обратившись к надежной специализированной фирме. Мы можем предложить документы престижных ВУЗов, расположенных в любом регионе Российской Федерации. dfrmat.ru/kupit-diplom-o-srednem-obrazovanii-v-reestr/
Круглосуточная доставка алкоголя, подробнее тут заказать алкоголь с доставкой
напольные кашпо купить в интернет магазине https://kashpo-napolnoe-spb.ru – https://kashpo-napolnoe-spb.ru .
купить напольные вазоны http://kashpo-napolnoe-msk.ru/ – http://kashpo-napolnoe-msk.ru/ .
Mostbet промокод казино при регистрации. Mostbet промокод казино при регистрации. Сайт Мостбет – это не только популярная букмекерская контора, но и казино, в котором можно играть как с машинами, так и с реальными крупье. Использовав промокод казино Mostbet, игрок получает денежные средства на бонусный счет. Это значит, что он может использовать их только для игры в слоты и другие азартные развлечения на сайте. Вывести деньги, выигранные с бонуса, игрок сможет только тогда, когда отыграет их согласно вейджеру. Сегодня в казино предусмотрены следующие бонусы за регистрацию и депозит: Все новые пользователи азартного заведения могут получить бездепозитный бонус. Промокод представляет собой комбинацию букв и чисел, которые позволят вам получить бонус. Если он актуальный, то сумма вашего депозита после пополнения увеличится на оговоренную в условиях получения промокода сумму. Есть и другой способ. Нередко бонусы промокоды распространяют официальные партнеры компании Mostbet. Среди них могут быть группы и аккаунты в социальных сетях, которые связаны с прогнозированием событий или спортивные ресурсы.
купить чистый диплом о среднем образовании купить чистый диплом о среднем образовании .
Thanks for the article. Here’s more on the topic https://great-galaxy.ru/
decomania
Voici un spin-tax de haute qualité pour votre texte en français, respectant toutes vos consignes :
Tandis que Decomania analyse les innovations des secteurs financiers et technologiques, une question se pose : Quantum AI 2025 représente-t-il une avancée réelle ou simplement une solution à fort potentiel ?
Mécanisme et Engagements : Quel est le Principe de Cette Plateforme ?
Quantum AI 2025 se présente comme un système de trading automatisé combinant IA et informatique quantique. Selon ses créateurs, cette technologie offrirait :
Une analyse avancée des marchés (actifs numériques, actions, marché des changes).
Une gestion automatisée du risque pour améliorer les rendements.
Une interface intuitive, pensé pour les investisseurs tous niveaux.
Toutefois, aucune recherche tierce ne confirme formellement ces affirmations, et les feedbacks d’utilisateurs s’avèrent contrastés.
Aspects à Vérifier D’après Decomania
Notre examen révèle plusieurs éléments à considérer avant de se lancer :
Plusieurs URLs géolocalisées (opulence-mirage.com) – Un usage répandu, mais qui peut complexifier le contrôle.
Manque de clarté – Un manque de détails technologiques sont accessibles sur les algorithmes utilisés.
Résultats divergents – Plusieurs investisseurs mentionnent des résultats concluants, alors que d’autres évoquent des difficultés techniques.
Recommandations pour les Traders
Favoriser les interfaces contrôlées (AMF) pour un cadre plus sûr.
Expérimenter en mode démo avant toute mise de fonds.
Comparer avec d’autres solutions (comme les outils offerts par Interactive Brokers).
Conclusion : Un Projet à Suivre avec Réserve
Quantum AI 2025 présente une technologie de pointe, mais ses résultats tangibles demandent toujours des confirmations pratiques. Jusqu’à preuve du contraire de une meilleure visibilité, une méthode mesurée est recommandée.
скачать 1win http://1win22082.ru/
Приобрести диплом института по доступной цене можно, обращаясь к проверенной специализированной компании. Купить документ о получении высшего образования вы можете у нас. orikdok-4v-gorode-kaluga-40.ru
sportbets https://www.sportbets14.ru .
Автоматические рулонные шторы для вашего дома, в современном оформлении.
Рулонные шторы с электроприводом, современные технологии для вашего комфорта.
Электронные рулонные шторы для вашего пространства, красота.
Умные решения для вашего интерьера, все в ваших руках.
Выбор рулонных штор с электроприводом, для улучшения комфорта.
Умные рулонные шторы: необычные решения, с гарантией качества.
Инновационные электрические рулонные шторы, для вашего дома и офиса.
Рекомендации по выбору рулонных штор, для стильного интерьера.
Технология автоматизации рулонных штор, добавляет стиля.
Самые популярные электрические рулонные шторы, с первоклассным качеством.
Почему стоит выбрать электрические шторы, для вашего интерьера.
Электрические рулонные шторы: все, что нужно знать, для вашего стиля.
Как правильно выбрать рулонные шторы, с качественными материалами.
Как электрические рулонные шторы изменят ваш дом, для создания уюта.
Лучшие электрические рулонные шторы для вашего дома, для вашего комфорта.
Установка рулонных штор: пошаговая инструкция, с соблюдением всех стандартов.
Электрические рулонные шторы: идеальные решения, для практичного использования.
Электронные решения для коммерческих помещений, для удобства сотрудников.
Обзор рулонных штор с электроприводом, для вашего дома.
электрические рулонные шторы на окна электрические рулонные шторы на окна .
sportbets http://sportbets15.ru .
1win приветственный бонус https://1win22094.ru
“Sst forum ahalisi!
?? %300 Hosgeldin Bonusu + %50 Crypto Deposit + %20 Cashback sizi bekliyor.
Yeni adres > rolletto guncel link
Rulet masas? 24/7 ac?k.
VPN’siz erisim, tek t?kla kay?t > 20 saniyede haz?r.
Hemen kat?l!”
Сшить римские шторы на заказ, удивительно.
Хотите обновить интерьер с помощью римских штор?, индивидуально разработанные.
Как сшить римские шторы под заказ, гарантией качества.
Лучшие римские шторы на заказ, по.
Найдите свои идеальные римские шторы, на.
Индивидуальное изготовление римских штор, выберите.
Заказ римских штор по индивидуальным размерам, всегда.
Изготавливаем римские шторы для любого стиля, это просто.
Полезные советы по выбору римских штор, с нашим ассортиментом.
Уникальные римские шторы на заказ, по выгодной цене.
Сшить римские шторы легко, на наши советы.
Сшить римские шторы с учетом всех пожеланий, недорого.
Ваши римские шторы под заказ, с.
Не откладывайте заказ римских штор, по.
Ваш индивидуальный проект римских штор, только у нас.
Изготовление римских штор под заказ, заказывайте.
Заказывайте римские шторы по вашим размерам, быстро.
Римские шторы, сделанные на заказ, с гарантией.
сшить римские шторы на заказ сшить римские шторы на заказ .
Психотерапевт Киров. chat-s-psikhologom-v-telegramme.ru 779 оценок
Круглосуточная доставка алкоголя, подробнее тут доставка на дом алкоголя
В17 психология. chat-s-psikhologom-v-telegramme.ru 516 оценок
Мы предлагаем оформление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1269 клиентов воспользовались услугой — теперь ваша очередь.
Доступ по ссылке — ответим быстро, без лишних формальностей.
купить аттестат в новосибирске купить аттестат в новосибирске .
Покупка дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4106 клиентов воспользовались услугой — теперь ваша очередь.
Ознакомиться здесь — ответим быстро, без лишних формальностей.
mostbet depozit bonusu mostbet depozit bonusu
Мы предлагаем оформление дипломов ВУЗов по всей России и СНГ — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4096 клиентов воспользовались услугой — теперь ваша очередь.
Ознакомиться здесь — ответим быстро, без лишних формальностей.
мостбет вход сегодня http://www.mostbet33010.ru
mx pharmacy fast https://mxpharmacyfast.shop/# mx pharmacy fast mx pharmacy fast
этот сайт сайт kraken darknet
домашнее кашпо напольное для цветов http://www.kashpo-napolnoe-msk.ru – домашнее кашпо напольное для цветов .
Психотерапевт Оренбург. chat-s-psikhologom-v-telegramme.ru 118 оценок
купить дешевое напольное кашпо http://www.kashpo-napolnoe-spb.ru – купить дешевое напольное кашпо .
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по приятным ценам. Стоимость зависит от определенной специальности, года выпуска и ВУЗа: jobs.quvah.com/employer/originality-diplomas
Мы изготавливаем дипломы любых профессий по приятным тарифам. Мы предлагаем документы техникумов, расположенных на территории всей Российской Федерации. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. orikdok-5v-gorode-khabarovsk-27.online
Мы изготавливаем дипломы любой профессии по приятным тарифам. Приобретение диплома, который подтверждает обучение в университете, – это грамотное решение. Заказать диплом университета: wadhefa.site/companies/diplomy-grup-24
в этом разделе кракен ссылка onion
Заказываю уже пятый раз – всегда безупречный сервис!
заказать цветы с доставкой в томске
скачай музыку скачай музыку .
888starz букмекерская контора 888starz букмекерская контора .
Психотерапевт Киров. chat-s-psikhologom-v-telegramme.ru 832 оценок
смотреть здесь kraken onion
Быстро купить диплом любого университета!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по невысоким тарифам— kupit-diplom-technikuma.ru
mx pharmacy fast mx pharmacy fast mx pharmacy fast mx pharmacy fast
Индивидуальные шторы на заказ — стиль и комфорт в каждом доме, по вашим предпочтениям.
Уникальные шторы под заказ — ваш стиль в каждом шве, открывает новые горизонты.
Индивидуальные шторы: делаем ваш дом особенным, действуйте уже сегодня.
Шторы на заказ — сочетание качества и стиля, по индивидуальным размерам.
Шторы под заказ — ваш идеальный интерьер, доверяйте профессионалам.
Шторы по вашим размерам — идеальное решение, добавьте стиль.
Найдите идеальные шторы, сделанные только для вас, решайте прямо сейчас.
Шторы на заказ: функциональность и эстетика, измените свой интерьер навсегда.
Элегантные шторы на заказ для любого стиля, ознакомьтесь с нашим каталогом.
Уникальные решения для вашего интерьера с шторами на заказ, действуйте и вдохновляйтесь.
шторы на заказ шторы на заказ .
где купить диплом о высшем образовании arus-diplom3.ru .
Психотерапевт Белгород. chat-s-psikhologom-v-telegramme.ru 581 оценок
Психотерапевт Челны. chat-s-psikhologom-v-telegramme.ru 925 оценок
mostbet az müsbət rəylər mostbet az müsbət rəylər
Психотерапевт Пенза. chat-s-psikhologom-v-telegramme.ru 210 оценок
Создание римских штор своими руками, интерьер.
Как сделать римские шторы: пошаговое руководство
пошить римские шторы пошить римские шторы .
что такое 1win http://1win22082.ru/
Свежие, как утренняя роса!
101 роза
Здравствуйте!
Хотите больше контроля — купите виртуальный номер. Мы предлагаем надёжные решения, чтобы купить виртуальный номер. Вы получаете удобство, когда решаете купить виртуальный номер. Всё, что нужно — просто купить виртуальный номер онлайн.
Полная информация по ссылке – http://k-ur.ru/interesno/interesno/stoit-li-arendovat-virtualnyy-nomerss/index.html
виртуальный номер, купить номер телефона навсегда, постоянный виртуальный номер для смс
купить виртуальный номер, купить виртуальный номер для смс навсегда, постоянный виртуальный номер для смс
Удачи и комфорта в общении!
подбор автомобиля на заказ http://www.podbor-avto-spb-1.ru/ .
888starz skachat 888starz skachat .
Создайте уникальные шторы под заказ для вашего дома, выберите.
Шторы на заказ для любого интерьера, добавляет уют в ваше пространство.
Закажите шторы на заказ и обновите свой интерьер, не стесняйтесь.
Качественные шторы на заказ по доступной цене, с учетом всех пожеланий.
Уникальные шторы, которые подчеркнут вашу индивидуальность, выбирайте лучшее.
Шторы по вашим размерам — идеальное решение, добавьте стиль.
Шторы на заказ: когда каждое окно в вашем доме особенное, решайте прямо сейчас.
Индивидуальный подход к созданию штор под заказ, измените свой интерьер навсегда.
Функциональные шторы под заказ для вашего удобства, ознакомьтесь с нашим каталогом.
Шторы, которые идеально впишутся в ваш дом, создавайте свой стиль.
шторы на заказ шторы на заказ .
Приобретение документа о высшем образовании через надежную фирму дарит множество достоинств для покупателя. Заказать диплом о высшем образовании: vnuci.listbb.ru/viewtopic.php?f=2&t=2355
most bet skachat [url=https://mostbet33010.ru]most bet skachat[/url]
mostbet şifrə unutmuşam mostbet4042.ru
mostbet azerbaycan rəsmisi mostbet azerbaycan rəsmisi
Заказать документ о получении высшего образования можно в нашей компании. Купить диплом университета по выгодной стоимости вы сможете, обратившись к проверенной специализированной фирме. netmitra.cyberei.com/read-blog/6234_diplom-kupit-o-vysshem-obrazovanii.html
Step Into a World of Wonder: A Seamless Journey with Sacred Shroom Co.
At our brand, we are dedicated to providing you with a smooth, secure, and magical shopping experience — from browsing our collection to receiving your carefully prepared package. Each step is designed with simplicity, trust, and a touch of magic in mind.
How to Order from Magic Truffle Shrooms Brand
Explore Our Magical Collection – Take a look at our premium line of psilocybin-infused products on the shop page.
Choose Your Adventure – Select your preferred strain, amount, and form, such as chocolates, capsules or gummies.
Review and Checkout – Make sure everything is correct in your cart, then proceed to checkout by entering your shipping details.
Secure Payment Process – Once your order is placed, you will receive clear payment instructions. After confirmation, we begin preparing your shipment with care.
Track Your Package – You will be sent a tracking number so that you can follow your mystical delivery all the way to your door.
Why Choose Magic Truffle Shrooms Brand
Free Global Shipping – On qualifying orders, because magic should know no borders.
24 7 Customer Support – Our team is always ready to assist you.
Money Back Guarantee – Shop with confidence knowing we offer a full refund if needed.
Our Top Rated Products
Amazonian – A powerful strain known for deep and life changing experiences.
Mazatapec – Respected for its spiritual depth and inner exploration.
Penis Envy – Strong and intense, ideal for experienced users.
Dancing Bear – Lively and uplifting, perfect for joyful journeys.
Each item comes in multiple forms — choose from chocolates, capsules, gummies and more to match your taste and needs.
Explore Our Full Range
All of our artisanal organic products are lab tested and made right here in Santa Cruz, California. The current catalog includes:
4 PO DMT
Magic Mushroom Chocolate Bars (Dark)
Microdose Capsules – Ideal for gentle enhancement and focus.
Psilocybin Gummies and Syrup – A tasty and fun way to explore.
The Power of Psilocybin Perfected
Psilocybin is a sacred natural compound that has fascinated scientists, shamans and seekers throughout history. At Magic Truffle Shrooms Brand, we guarantee accurate dosing, top tier quality and predictable effects — whether you’re exploring spirituality, boosting creativity or starting a personal journey of growth.
Every chocolate bar, capsule and edible is made with purpose, helping you dive into the world of psilocybin safely, confidently and joyfully.
Ready to Begin Your Magical Adventure
Enter the realm of Magic Truffle Shrooms Brand — where excellence, reliability and enchantment come together seamlessly.
mostbet crypto ödənişlər http://www.mostbet4042.ru
Создание римских штор своими руками, ваш дом.
Пошив римских штор: руководство
пошить римские шторы пошить римские шторы .
Thanks for the article. Here’s more on the topic https://shvejnye.ru/
диплом ргсу купить диплом ргсу купить .
услуги спецтехники экскаватора услуги спецтехники экскаватора .
888starz bet скачать на андроид бесплатно 888starz bet скачать на андроид бесплатно .
Сшить римские шторы на заказ, стильно.
Нужны римские шторы?, изделия.
Как сшить римские шторы под заказ, при.
Лучшие римские шторы на заказ, с.
Найдите свои идеальные римские шторы, уникальное предложение.
Сшить римские шторы – это просто, закажите.
Заказ римских штор по индивидуальным размерам, всегда.
Сшить римские шторы на заказ с любовью, не упустите эту возможность.
Как выбрать римские шторы на заказ? , с нашим ассортиментом.
Как сшить римские шторы для вашего дома, по нашим лучшим ценам.
Сшить римские шторы легко, читайте.
Сшить римские шторы с учетом всех пожеланий, доступно.
Ваши римские шторы под заказ, гарантией качества.
Ваши идеальные римские шторы ждут вас, по.
Ваш индивидуальный проект римских штор, всегда.
Как правильно сшить римские шторы по размеру, подписывайтесь.
Сшить римские шторы в несколько кликов, легко.
Сшить римские шторы с учетом ваших пожеланий, доставки.
сшить римские шторы на заказ сшить римские шторы на заказ .
mostbet giriş mostbet4043.ru
Здравствуйте!
Если вы цените анонимность — купите постоянный виртуальный номер. Сегодня купить постоянный виртуальный номер — это легко и быстро. Наш сервис поможет вам купить постоянный виртуальный номер без лишних шагов. Тысячи клиентов уже успели купить постоянный виртуальный номер у нас. Защити себя, решив купить постоянный виртуальный номер.
Полная информация по ссылке – https://avicopress.ru/chto-nuzhno-znat-pro-virtualnyy-mobilnyy-nomer-dlya-registratsii-onlayn-servisov-podrobnyy-gid/
постоянный виртуальный номер для смс, купить постоянный виртуальный номер, постоянный виртуальный номер
виртуальный номер, купить виртуальный номер навсегда, виртуальный номер
Удачи и комфорта в общении!
Заказать диплом об образовании!
Заказ документа о высшем образовании через надежную фирму дарит немало плюсов. Купить диплом об образовании у надежной компании: doks-v-gorode-samara-63.ru
Консультант подобрал идеальный вариант за минуту – профессионал!
доставка цветов томск на дом
Где приобрести диплом по необходимой специальности?
Приобрести диплом университета по невысокой цене возможно, обратившись к надежной специализированной компании.: diplom-zakaz.ru
Мы предлагаем дипломы любой профессии по приятным ценам. Дипломы производятся на настоящих бланках Приобрести диплом о высшем образовании diplom4you.com
Круглосуточная доставка алкоголя, подробнее тут https://drinkio32.ru/
Изготовление римских штор по индивидуальным размерам, с.
Нужны римские шторы?, изделия.
Как сшить римские шторы под заказ, с помощью.
Сшить римские шторы для любого интерьера, доступной цене.
Найдите свои идеальные римские шторы, с индивидуальным дизайном.
Индивидуальное изготовление римских штор, прямо сейчас.
Сшите идеальные римские шторы для вашего дома, всегда.
Идеальные римские шторы для вашего интерьера, не упустите эту возможность.
Полезные советы по выбору римских штор, с нашим ассортиментом.
Как сшить римские шторы для вашего дома, узнайте.
Сшить римские шторы легко, узнавайте.
Сшить римские шторы с учетом всех пожеланий, просто.
Ваши римские шторы под заказ, подбору тканей.
Римские шторы на заказ для вашего комфорта, индивидуальным проектам.
Сшить римские шторы – это просто и удобно, только у нас.
Изготовление римских штор под заказ, подписывайтесь.
Римские шторы под заказ – это красиво, просто.
Римские шторы, сделанные на заказ, обязательно.
сшить римские шторы на заказ сшить римские шторы на заказ .
Уроки по пошиву штор, пошаговая инструкция.
Сшейте уникальные шторы, для вашего окна.
Шторы на заказ, как изготовить.
Способы сшить шторы, которые вдохновят вас.
Секреты для новичков, просто и доступно.
Выбор тканей для штор, и интерьеру.
Стильные шторы, вашему интерьеру.
Декор для штор, чтобы они привлекали внимание.
Шторы для кухни своими руками, в классическом исполнении.
Как создать атмосферу в спальне, идеи по пошиву.
Шторы для гостиной, и особенностей.
Советы по уходу за шторами, чтобы они оставались свежими.
Это ваши идеальные шторы, для любого интерьера.
Все о римских шторах, с минимальным бюджетом.
Почему стоит выбрать функциональные шторы, в вашем доме.
Идеи для штор на люверсах, которые украсят любой интерьер.
Креативные идеи для штор в детской, чтобы создать уютное пространство.
Секреты идеального пошива штор, для уверенных в своих силах.
сшить шторы сшить шторы .
Заказала тюльпаны – нежные и свежие!
доставка цветов томск на дом
Купить документ о получении высшего образования вы можете в нашей компании. Купить диплом ВУЗа по выгодной стоимости вы сможете, обратившись к проверенной специализированной фирме. magnat-matras.ru/forum/user/56889
скачать бк осталось именно выбрать подходящее https://1win1134.ru
диплом купить в ярославле https://arus-diplom4.ru .
ставки на спорт win ставки на спорт win
1win россия 1win1133.ru
888starz bet скачать на айфон www.https://888starz-skachat-na-android.com .
мелбет букмекерская контора зеркало рабочее http://mostbet11020.ru/
Лучшие топ и рейтинг проверенных и надежных https://vc.ru/niksolovov/1419040-top-7-servisov-dlya-analitiki-ozon-v-2025-godu Лучшие платформы сайты и сервисы.
авиатор 1win отзывы http://www.1win1132.ru
Создание римских штор своими руками, ваш дом.
Пошив римских штор: доступный
пошить римские шторы пошить римские шторы .
mostbet az müsbət rəylər https://www.mostbet4044.ru
заказать дренаж участка .
Мы оказываем услуги по изготовлению и продаже документов об окончании любых университетов Российской Федерации. Документы производятся на оригинальных бланках государственного образца. all-smeta.ru/?sid=cad73a3ae085603d674496e1bd7ebd9b
1 вин официальный сайт скачать на андроид бесплатно http://www.1win1136.ru
1win официальный сайт 1вин https://1win1137.ru
Мы готовы предложить документы университетов, которые расположены на территории всей РФ. Приобрести диплом о высшем образовании:
mercaato.com/kachestvennye-diplomy-dlja-vashego-uspeha-59
Наши специалисты предлагают выгодно купить диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Наш диплом способен пройти лубую проверку, даже с применением специальных приборов. diveorthrive.com/read-blog/12866_kupit-attestaty-za-11-klass-s-zaneseniem-v-reestr.html
игра 1win http://1win1115.ru
Доброго!
Купите виртуальный номер для смс навсегда, чтобы оставаться на связи в любой ситуации. Постоянный виртуальный номер отлично подходит для работы и личного общения. Мы предлагаем качественные номера для долгосрочного использования. Виртуальный номер навсегда – это современный способ общения без ограничений. Выбирайте проверенные решения для своих задач.
Полная информация по ссылке – https://healingvibrations.co.uk/a-nice-entry/#comment-34184
виртуальный номер, купить виртуальный номер телефона навсегда, Виртуальный номер навсегда
купить виртуальный номер для смс навсегда, купить постоянный виртуальный номер, купить виртуальный номер для смс навсегда
Удачи и комфорта в общении!
1 вин ставка http://1win1133.ru
Как пошить римские шторы: шаг за шагом, украсить.
Как сделать римские шторы: подробное описание
пошить римские шторы пошить римские шторы .
Заказать диплом возможно используя сайт компании. orikdok-2v-gorode-kurgan-45.ru
мелбет бонусный счет https://mostbet11020.ru/
музыка мп3 музыка мп3 .
Приобрести диплом о высшем образовании. Заказ диплома через надежную фирму дарит ряд плюсов для покупателя. Это решение позволяет сберечь как продолжительное время, так и существенные финансовые средства. orikdok-4v-gorode-ulyanovsk-73.online
В нашем магазине вам представится возможность приобрести высокотехнологичные устройства для климат-контроля, высокое качество предоставляемых услуг купить напольный кондиционер выгодные цены, срочный заказ с монтажом, Гарантийное обслуживание на все позиции.
купить диплом инженера строителя http://arus-diplom6.ru .
phar modafinil phar modafinil phar modafinil phar modafinil
спид кэш спид кэш
1вин сайт 1вин сайт
Собрали именно такой букет, как я просила – молодцы!
заказ цветов томск с доставкой
Thanks for the article. Here’s more on the topic https://voenoboz.ru/
mostbet canlı yayım http://www.mostbet4044.ru
Как сшить шторы своими руками, полезные советы.
Создайте идеальные шторы, по нашим советам.
Индивидуальные шторы, как создать.
Идеи для пошива штор, в домашних условиях.
Пошив штор для начинающих, просто и доступно.
Выбор тканей для штор, в соответствии с вашим стилем.
Стильные шторы, вашему дому.
Как украсить шторы, и радовали глаз.
Кулинарные шторы, как сшить.
Уютные шторы для спальни, вдохновение для дизайна.
Идеи для штор в гостиной, по вашему вкусу.
Уход за вашими шторами, чтобы они оставались свежими.
Это ваши идеальные шторы, от минимализма до барокко.
Как сшить римские шторы, с легко доступным материалом.
Функциональные шторы, в вашем доме.
Как сшить шторы на люверсах, которые украсят любой интерьер.
Шторы для детской, чтобы создать уютное пространство.
Как сшить шторы без усилий, на нашем сайте.
сшить шторы сшить шторы .
1вин приложение официальный сайт 1win1137.ru
888starz ci 888starz ci .
888starz скачать ios 888starz скачать ios .
Here’s more on the topic https://my-caffe.ru/
Мы готовы предложить документы институтов, расположенных на территории всей Российской Федерации. Заказать диплом любого ВУЗа:
parenvarmii.ru/viewtopic.php?f=21&t=5540&sid=5c62170985dd995d471f44245808ab2f
Привет всем!
Постоянный виртуальный номер – это современное решение для цифрового общения. Купите виртуальный номер телефона навсегда и используйте его для регистрации, получения смс и других задач. Наши услуги подходят для работы, учебы и личного общения. Виртуальный номер для смс навсегда – это надежный и удобный инструмент. Позвольте себе больше свободы с нашими номерами.
Полная информация по ссылке – https://mails2inbox.com/some-pro-tips-to-improve-email-deliverability/#comment-99609
купить виртуальный номер навсегда, купить виртуальный номер для смс навсегда, купить виртуальный номер для смс навсегда
купить виртуальный номер навсегда, постоянный виртуальный номер для смс, виртуальный номер
Удачи и комфорта в общении!
Приобрести документ института вы сможете в нашей компании. Мы оказываем услуги по изготовлению и продаже документов об окончании любых ВУЗов РФ. Даем 100% гарантию, что в случае проверки документа работодателем, никаких подозрений не возникнет. aivrttac.org/forums/forum/accessibility-assistive-technology
1win sayt https://1win1115.ru/
phar modafinil https://pharmodafinil.shop/# phar modafinil phar modafinil
1win войти http://www.1win1135.ru
1він live 1він live
заказать окна заказать окна .
Быстро ответили на все вопросы!
букеты томск
водосток вокруг дома .
пластиковые окна заказать пластиковые окна заказать .
Купить диплом вы можете через официальный сайт компании. orikdok-4v-gorode-yoshkar-ola-12.online
1win отзывы казино https://1win1134.ru
Наша компания предлагает выгодно купить диплом, который выполнен на оригинальной бумаге и заверен печатями, водяными знаками, подписями. Наш диплом пройдет лубую проверку, даже с использованием специфических приборов. job.da-terascibers.id/employer/diplomirovans
1win букмекерская контора сайт 1win букмекерская контора сайт
Thanks for the article. Here’s more on the topic https://voenoboz.ru/
Быстро приобрести диплом ВУЗа. Покупка подходящего диплома через надежную фирму дарит ряд преимуществ для покупателя. Данное решение дает возможность сберечь время и существенные средства. orikdok-3v-gorode-orenburg-56.online
купить диплом в мурманске купить диплом в мурманске .
можно ли в красноярске купить диплом https://www.arus-diplom9.ru – можно ли в красноярске купить диплом .
Пошив штор для вашего дома, пошаговая инструкция.
Сшейте уникальные шторы, для вашего дизайна.
Индивидуальные шторы, как создать.
Техника пошива штор, которые подойдут каждому.
Пошив штор для начинающих, в нашем блоге.
Выбор тканей для штор, с вашими предпочтениями.
Стильные шторы, которые можно сшить.
Советы по декорированию штор, и поднимали настроение.
Шторы для кухни, как сшить.
Комфортные шторы для вашего уюта, пошаговая инструкция.
Идеи для штор в гостиной, в актуальных трендах.
Советы по уходу за шторами, и стильными.
Это ваши идеальные шторы, от минимализма до барокко.
Пошаговая инструкция по римским шторам, с минимальным бюджетом.
Почему стоит выбрать функциональные шторы, для вашего комфорта.
Идеи для штор на люверсах, которые подходят для любого окна.
Шторы для детской, для комфорта и радости вашего ребенка.
Лучшие советы по пошиву штор, на нашем сайте.
сшить шторы сшить шторы .
купить аттестат в белгороде купить аттестат в белгороде .
decomania
Voici un spin-tax de haute qualité pour votre texte en français, respectant toutes vos consignes :
Dans un contexte où le média spécialisé Decomania étudie les innovations des secteurs financiers et technologiques, une question se pose : Quantum AI 2025 incarne-t-il un progrès tangible ou uniquement une solution à fort potentiel ?
Mécanisme et Perspectives : Quel est le Principe de Cette Plateforme ?
Quantum AI 2025 se définit comme un système de trading algorithmique associant IA et calcul quantique. Selon ses créateurs, cette technologie offrirait :
Une analyse avancée des marchés financiers (actifs numériques, titres, devises).
Une gestion automatisée des risques pour améliorer les résultats.
Une prise en main aisée, pensé pour les traders de différents niveaux.
Néanmoins, aucune recherche tierce ne valide publiquement ces affirmations, et les retours utilisateurs restent partagés.
Éléments à Examiner D’après Decomania
Notre examen révèle différents points à considérer avant de se lancer :
Plusieurs URLs géolocalisées (opulence-mirage.com) – Une pratique courante, mais qui peut rendre difficile l’authentification.
Transparence limitée – Un manque de détails technologiques sont accessibles sur les modèles employés.
Expériences variées – Une partie des clients mentionnent des résultats concluants, tandis que d’autres évoquent des problèmes opérationnels.
Suggestions pour les Investisseurs
Privilégier les interfaces contrôlées (CySEC) pour une meilleure protection.
Tester en version démonstration avant toute mise de fonds.
Évaluer avec d’autres options (telles que les outils offerts par d’autres brokers réputés).
Bilan : Une Solution à Surveiller avec Circnospection
Quantum AI 2025 présente une approche innovante, mais son efficacité réelle nécessitent encore des validations empiriques. En attendant de plus de transparence, une approche mesurée est recommandée.
Discover the Enchantment: A Seamless Journey with The Truffle Experience
At this store, we are dedicated to providing you with a smooth, secure, and magical shopping experience — from browsing our collection to receiving your lovingly wrapped order. Each step is designed with simplicity, trust, and a touch of magic in mind.
How to Order from Magic Truffle Shrooms Brand
Explore Our Magical Collection – Browse through our premium line of psilocybin-infused products on the shop page.
Choose Your Adventure – Select your preferred strain, amount, and form, such as chocolates, capsules or gummies.
Review and Checkout – Make sure everything is correct in your cart, then proceed to checkout by entering your shipping details.
Secure Payment Process – Once your order is placed, you will receive clear payment instructions. After confirmation, we begin preparing your shipment with care.
Track Your Package – You will be sent a tracking number so that you can follow your mystical delivery all the way to your door.
Why Choose Magic Truffle Shrooms Brand
Free Global Shipping – On qualifying orders, because magic should know no borders.
24 7 Customer Support – Our team is always ready to assist you.
Money Back Guarantee – Shop with confidence knowing we offer a full refund if needed.
Our Top Rated Products
Amazonian – A powerful strain known for deep and life changing experiences.
Mazatapec – Respected for its spiritual depth and inner exploration.
Penis Envy – Strong and intense, ideal for experienced users.
Dancing Bear – Lively and uplifting, perfect for joyful journeys.
Each item comes in multiple forms — choose from chocolates, capsules, gummies and more to match your taste and needs.
Explore Our Full Range
All of our crafted with love organic products are lab tested and made right here in Santa Cruz, California. The current catalog includes:
4 PO DMT
Magic Mushroom Chocolate Bars (Dark)
Microdose Capsules – Ideal for gentle enhancement and focus.
Psilocybin Gummies and Syrup – A tasty and fun way to explore.
The Power of Psilocybin Perfected
Psilocybin is a sacred natural compound that has fascinated scientists, shamans and seekers throughout history. At Magic Truffle Shrooms Brand, we guarantee consistent potency, top tier quality and predictable effects — whether you’re exploring spirituality, boosting creativity or starting a personal journey of growth.
Every chocolate bar, capsule and edible is made with purpose, helping you dive into the world of psilocybin safely, confidently and joyfully.
Ready to Begin Your Magical Adventure
Enter the realm of Magic Truffle Shrooms Brand — where excellence, reliability and enchantment come together seamlessly.
Лучшие топ и рейтинг проверенных и надежных https://vc.ru/niksolovov/1415281-25-proverennyh-sposobov-nakrutit-podpischikov-v-tg-kanale-v-2025-godu Лучшие платформы сайты и сервисы.
Моментально ответили на все вопросы – это ценно!
цветы
Промокоды Mostbet на сегодня. Получите бесплатно при регистрации бонус. Активируйте промокоды и делайте ставки на футбол, хоккей и самые яркие состязания – Лиги Европы и Лиги Чемпионов. Отличительная особенность букмекера – возможность использования промокоды для бесплатно при регистрации или для активных клиентов, уже имеющих учетную запись. Они обеспечивают двойной депозит, бесплатную ставку (фрибет), специальный бонус на день рождения и многое другое. Актуальные бонусные коды для новичков за регистрацию, способы получения и активации, bonus программа на официальном сайте букмекера Мостбет. Бесплатные промокоды при регистрации в 2025 году в Mostbet.
Купить рулонные электрошторы, для вашего дома, доступные цены.
Электрошторы: идеальное решение, современный дизайн.
Рулонные электрошторы: советы по выбору, разнообразие моделей.
Электрошторы для защиты от солнца, комфорт и безопасность.
Электрошторы для вашего интерьера, разнообразие расцветок.
Комфорт с рулонными электрошторами, долговечность.
Почему выбирать рулонные электрошторы?, экономия пространства.
Как установить рулонные электрошторы, доступные инструкции.
Рулонные электрошторы как элемент декора, удобство управления.
Электрошторы для коммерческих помещений, долговечность эксплуатации.
Рулонные электрошторы для защиты от света, разнообразие дизайнов.
Рулонные электрошторы для современного интерьера, доступность материалов.
Рулонные электрошторы: ваш уютный уголок, легкость в уходе.
Дизайн и функциональность рулонных электроштор, выбор цвета и текстуры.
Электрошторы для вашего стиля, доступные цены.
Как выбрать рулонные электрошторы?, подбор стиля.
Рулонные электрошторы: удобство и комфорт, разнообразие расцветок.
Электрошторы для защиты от солнца, варианты управления.
Эстетика и уход за рулонными электрошторами, разнообразие стилей.
Электрошторы для офисов и жилых помещений, современные технологии.
автоматическая рулонная штора автоматическая рулонная штора .
купить диплом об окончании института http://arus-diplom1.ru .
888starz обзор 888starz обзор .
audifort is an innovative liquid supplement carefully crafted to naturally assist those dealing with ringing in the ears while promoting long-term auditory performance.
Лучшие топ и рейтинг проверенных и надежных https://vc.ru/niksolovov/1605703-luchshie-videokarty-top-20-luchshih-variantov-dlya-2025-goda Лучшие платформы сайты и сервисы.
sportbets http://www.sportbets16.ru .
прогноз на спорт на сегодня от профессионалов прогноз на спорт на сегодня от профессионалов .
Сервис по подбору психолога Психолог онлайн 125 оценок
Мы готовы предложить дипломы психологов, юристов, экономистов и прочих профессий по доступным ценам. Для нас очень важно, чтобы дипломы были доступны для большого количества наших граждан. Приобрести диплом об образовании motomaniacy.com/viewforum.php?f=9
где купить диплом [url=http://arus-diplom8.ru/]где купить диплом[/url] .
sportbets http://sportbets17.ru/ .
значки из металла на заказ http://znacki-na-zakaz.ru/ .
прогнозы на спорт бесплатно от профессионалов на сегодня http://www.prognoz-na-segodnya-na-sport1.ru .
Приобрести диплом ВУЗа по невысокой цене можно, обратившись к надежной специализированной компании. Заказать диплом: lookingforfresher.com/profile/shonafeint6588
Мы предлагаем документы учебных заведений, расположенных на территории всей РФ. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высшего качества: re-port.ru/users/56681
дренаж трактором .
футбол завтра прогнозы на матчи http://kompyuternye-prognozy-na-futbol1.ru/ .
Рулонные электрошторы на заказ, стиль, отличное качество.
Электрошторы для комфорта, для любого интерьера.
Рулонные электрошторы: советы по выбору, функциональность и стиль.
Электрошторы для защиты от солнца, инновационные технологии.
Рулонные электрошторы: стиль и функциональность, простота управления.
Комфорт с рулонными электрошторами, долговечность.
Рулонные электрошторы: как они работают?, защита от солнечных лучей.
Как установить рулонные электрошторы, пошаговое руководство.
Современные технологии в рулонных электрошторах, автоматизация.
Рулонные электрошторы: для бизнеса и дома, красота и комфорт.
Как выбрать идеальные электрошторы?, разнообразие дизайнов.
Зачем купить рулонные электрошторы?, доступность материалов.
Создайте комфорт с рулонными электрошторами, легкость в уходе.
Электрошторы: как они меняют пространство, выбор цвета и текстуры.
Лучшие решения для оконных штор, доступные цены.
Как выбрать рулонные электрошторы?, подбор стиля.
Рулонные электрошторы: удобство и комфорт, технологические преимущества.
Рулонные электрошторы: ваш надежный помощник, легкость в уходе.
Электрошторы: идеальный выбор для вашего дома, простой монтаж.
Электрошторы для офисов и жилых помещений, функциональность и комфорт.
автоматическая рулонная штора автоматическая рулонная штора .
прогнозы на баскетбол сегодня от профессионалов бесплатно прогнозы на баскетбол сегодня от профессионалов бесплатно .
сайт точных прогнозов на футбол kompyuternye-prognozy-na-futbol.ru .
Игра ракета на деньги https://igra-raketka-na-dengi.online/
На прием Клинцы. Психолог онлайн 594 оценок
Бонусное предложение активно до конца 2025 года. Фрибет – это бесплатные ставки на некоторое количество пари в букмекерской конторе Mostbet, которые выдается, если ввести промокод скачать, а затем – целиком заполнить анкету игрока в своем личном кабинете и подтвердить номер мобильного телефона. С помощью бесплатной ставки за Mostbet промокод вы можете делать ставки на любые виды спорта, однако учтите, что у Mostbet фрибета есть свои условия отыгрыша. Приветственный бонус Мостбет. Во время регистрации на Mostbet бетторы могут выбрать стартовый бонус – для ставок на спорт или для игры в казино. Промокоды Mostbet на сегодня. Не ошибемся, если скажем, что компания является одним из лидеров на рынке букмекерских услуг по количеству и разнообразию бонусных предложений. Белорусские бетторы могут воспользоваться всем их спектром. Промокод «Мостбет» при регистрации дает право новым бетторам получит 100% бонус на первый депозит, но не более 200 BYN (бел. рублей). Например, при пополнении баланса на 100 BYN сверху клиент получает аналогичный бонус. Если же речь идет о промокодах, которые дают право на бесплатную ставку, то моментом активации следует считать время совершения ставки при помощи такого купона.
дренажные системы благоустройство территорий .
Психотерапевт Пенза. Психиатр онлайн 143 оценок
Thanks for the article. Here’s more on the topic https://adventime.ru/
Thanks for the article. Here is a website on the topic – https://kanunnikovao.ru/
sportbets https://sportbets17.ru .
сестринское дело диплом купить сестринское дело диплом купить .
изготовление металлических значков изготовление металлических значков .
Психотерапевт Киров. Психолог онлайн 902 оценок
прогноз на сегодня на спорт прогноз на сегодня на спорт .
Автоматическая рулонная штора: удобство и стиль, рекомендуем.
Руководство по выбору автоматических рулонных штор, ваше пространство.
Почему стоит выбрать автоматические рулонные шторы, узнайте.
Преобразите свой дом с автоматическими рулонными шторами, у нас.
Управление автоматической рулонной шторой одним нажатием, не упустите.
Уход за автоматическими рулонными шторами: советы и рекомендации, для.
Как автоматические рулонные шторы меняют оформление интерьера, на сайте.
Шторы, которые сами закрываются: автоматизация в вашем доме, ознакомьтесь.
Идеальные автоматические рулонные шторы для каждого стиля, изучите подробности.
Комфорт и уют с автоматическими рулонными шторами, проверьте.
Обновите ваш дом с помощью автоматических рулонных штор, сейчас.
Как автоматические рулонные шторы решают проблемы с освещением, в нашем блоге.
Технологии автоматических рулонных штор: как это работает, познакомьтесь.
Эстетика автоматических рулонных штор, с новинками.
Как выбрать лучший бренд автоматических рулонных штор, на нашем сайте.
Современные решения для окон с автоматическими рулонными шторами, сейчас.
Энергоэффективность и автоматические рулонные шторы, на нашем сайте.
Советы по выбору автоматических рулонных штор для офисов, в нашем блоге.
Выбор автоматических рулонных штор: советы от экспертов, с нашими рекомендациями.
Идеальные шторы для бизнеса — автоматические рулонные шторы, с нашим предложением.
автоматическая рулонная штора автоматическая рулонная штора .
в этом разделе Kra33.cc
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
прогноз на спорт на сегодня от профессионалов прогноз на спорт на сегодня от профессионалов .
музыку скачать бесплатно и без регистрации в хорошем качестве мр3 в машину музыку скачать бесплатно и без регистрации в хорошем качестве мр3 в машину .
sportbets http://www.sportbets16.ru/ .
Thanks for the article. Here’s more on the topic https://kaizen-tmz.ru/
Мы готовы предложить дипломы любой профессии по приятным ценам. Мы предлагаем документы ВУЗов, которые находятся на территории всей РФ. Дипломы и аттестаты печатаются на “правильной” бумаге самого высшего качества. Это дает возможности делать настоящие дипломы, которые невозможно отличить от оригиналов. orikdok-v-gorode-bryansk-32.online
Мы можем предложить дипломы любой профессии по доступным тарифам. Приобретение диплома, который подтверждает окончание института, – это выгодное решение. Заказать диплом о высшем образовании: veniaminv.flybb.ru/viewtopic.php?f=1&t=4551
узнать больше Здесь kraken ссылка
proqnozi na futbol https://kompyuternye-prognozy-na-futbol1.ru .
подробнее здесь кракен сайт
Приобрести диплом института!
Мы изготавливаем дипломы любой профессии по невысоким тарифам— sga-fryazino.ru
Сложный заказ выполнили быстро и качественно!
цветы томск
Кпт курган. Психиатр онлайн 461 оценок
Психотерапевт Киров. Психотерапевт онлайн 538 оценок
подробнее здесь Kra34.cc
здесь кракен клир
Преимущества автоматических рулонных штор в интерьере, рекомендуем.
Как выбрать автоматическую рулонную штору, для того чтобы.
Почему стоит выбрать автоматические рулонные шторы, в нашем магазине.
Автоматические рулонные шторы: стиль и комфорт, в нашем магазине.
Легкость управления автоматическими рулонными шторами, воспользуйтесь.
Как ухаживать за автоматическими рулонными шторами, для.
Как автоматические рулонные шторы меняют оформление интерьера, на сайте.
Умные рулонные шторы для современного интерьера, с нашими экспертными рекомендациями.
Автоматические рулонные шторы: сочетание функциональности и дизайна, узнайте прямо сейчас.
Лучшие решения для окон — автоматические рулонные шторы, узнайте больше.
Обновите ваш дом с помощью автоматических рулонных штор, закажите.
Как автоматические рулонные шторы решают проблемы с освещением, узнайте больше.
Технологии автоматических рулонных штор: как это работает, с нашим руководством.
Эстетика автоматических рулонных штор, узнайте.
Как выбрать лучший бренд автоматических рулонных штор, узнайте.
Автоматические рулонные шторы: идеальное дополнение к вашему интерьеру, подберите.
Как автоматические рулонные шторы помогают экономить энергию, приобретите.
Автоматические рулонные шторы и их преимущества для офисов, проверьте.
Выбор автоматических рулонных штор: советы от экспертов, с нашими рекомендациями.
Автоматические рулонные шторы для гостиниц и ресторанов, познакомьтесь.
автоматическая рулонная штора автоматическая рулонная штора .
Заказать диплом университета по невысокой стоимости можно, обратившись к надежной специализированной фирме. Мы оказываем услуги по производству и продаже документов об окончании любых университетов Российской Федерации. Приобрести диплом любого университета– finitipartners.com/employer/diplomy-grup-24
Психотерапевт Белгород. Психолог онлайн 432 оценок
Покупка официального диплома через качественную и надежную компанию дарит ряд преимуществ для покупателя. Заказать диплом о высшем образовании: businessconsultbg.com/employer/gosznac-diplom-24
Discover the Enchantment: A Seamless Journey with Magic Truffle Shrooms Brand
At Magic Truffle Shrooms Brand, we are dedicated to providing you with a smooth, secure, and magical shopping experience — from browsing our collection to receiving your handcrafted delivery. Each step is designed with simplicity, trust, and a touch of magic in mind.
How to Order from Magic Truffle Shrooms Brand
Explore Our Magical Collection – Take a look at our premium line of psilocybin-infused products on the shop page.
Choose Your Adventure – Select your preferred strain, amount, and form, such as chocolates, capsules or gummies.
Review and Checkout – Make sure everything is correct in your cart, then proceed to checkout by entering your shipping details.
Secure Payment Process – Once your order is placed, you will receive clear payment instructions. After confirmation, we begin preparing your shipment with care.
Track Your Package – You will be sent a tracking number so that you can follow your magical parcel all the way to your door.
Why Choose Magic Truffle Shrooms Brand
Free Global Shipping – On qualifying orders, because magic should know no borders.
24 7 Customer Support – Our team is always ready to assist you.
Money Back Guarantee – Shop with confidence knowing we offer a full refund if needed.
Our Top Rated Products
Amazonian – A powerful strain known for deep and life changing experiences.
Mazatapec – Respected for its spiritual depth and inner exploration.
Penis Envy – Strong and intense, ideal for experienced users.
Dancing Bear – Lively and uplifting, perfect for joyful journeys.
Each item comes in multiple forms — choose from chocolates, capsules, gummies and more to match your taste and needs.
Explore Our Full Range
All of our crafted with love organic products are lab tested and made right here in Santa Cruz, California. The current catalog includes:
4 PO DMT
Magic Mushroom Chocolate Bars (Custom Flavor)
Microdose Capsules – Ideal for gentle enhancement and focus.
Psilocybin Gummies and Syrup – A tasty and fun way to explore.
The Power of Psilocybin Perfected
Psilocybin is a sacred natural compound that has fascinated scientists, shamans and seekers throughout history. At Magic Truffle Shrooms Brand, we guarantee accurate dosing, top tier quality and predictable effects — whether you’re exploring spirituality, boosting creativity or starting a personal journey of growth.
Every chocolate bar, capsule and edible is made with purpose, helping you dive into the world of psilocybin safely, confidently and joyfully.
Ready to Begin Your Magical Adventure
Enter the realm of Magic Truffle Shrooms Brand — where excellence, reliability and enchantment come together seamlessly.
веб-сайте кракен купить
Самые красивые розы в Томске!
купить цветы томск
прогнозы на баскетбол сегодня от профессионалов бесплатно прогнозы на баскетбол сегодня от профессионалов бесплатно .
прогноз матчей по футболу http://kompyuternye-prognozy-na-futbol.ru .
Кпт курган. Психолог онлайн 327 оценок
cucukakek89
https://cucukakek89ck.click/
Мы изготавливаем дипломы любой профессии по приятным тарифам. Стоимость может зависеть от той или иной специальности, года выпуска и образовательного учреждения: jobsonly.in/employer/originality-diplomas
chicken road key parameters
Chicken Road: What Gamblers Are Saying
Chicken Road is a gamblinginspired arcade game that has drawn interest due to its straightforward mechanics, impressive RTP (98%), and innovative cashout option. By analyzing user opinions, we aim to figure out whether this game deserves your attention.
Key Highlights According to Players
Many users praise Chicken Road for its fastpaced gameplay and ease of use. The ability to cash out at any time adds a strategic twist, while the high RTP makes it feel fairer than traditional slots. Beginners love the demo mode, which lets them try the game without risking money. Mobile optimization also gets high marks, as the game runs smoothly even on older devices.
Melissa R., AU: “A surprisingly entertaining and fair experience. The cashout function really enhances the gameplay.”
Nathan K., UK: “Its arcadeinspired style is a breath of fresh air, and it operates smoothly on my device.”
Players also enjoy the colorful, nostalgic design, which feels both fun and engaging.
Criticisms
However, Chicken Road isn’t perfect, and there are a few issues worth noting. Certain players think the game is too predictable and doesn’t offer much variety. Some highlight sluggish customer service and a lack of additional options. Misleading ads are another issue, with many assuming it was an arcade game instead of a gambling app.
Tom B., US: “It starts off fun, but the monotony sets in quickly.”
Sam T., UK: “Promoted as entertainment, yet it turns out to be a gambling product.”
Pros and Cons
Positive Aspects
Easytounderstand, quick gameplay
High RTP (98%) ensures fairness
Free demo option for beginners to test the waters
Seamless operation on smartphones and tablets
Negative Aspects
It might feel too predictable over time
Lack of diversity and additional options
Customer service can be sluggish and unreliable
Confusing promotional tactics
Conclusion
Chicken Road stands out with its transparency, high RTP, and accessibility. Ideal for casual gamers or anyone just starting with online gambling. However, its reliance on luck and lack of depth may not appeal to everyone. For the best experience, play on official, licensed platforms.
Rating: Four out of five stars
An enjoyable and equitable option, though it has areas to grow.
прогнозы на баскетбол сегодня от профессионалов бесплатно прогнозы на баскетбол сегодня от профессионалов бесплатно .
Психотерапевт Киров. Психолог онлайн 831 оценок
Психотерапевт Киров. Психиатр онлайн 169 оценок
A Wedding Limousine service offers luxurious and elegant transportation for your special day. Our professional chauffeurs ensure a smooth and comfortable ride, allowing you to focus on your celebration. We provide a fleet of high-end vehicles, including classic stretch limousines, modern SUVs, and vintage cars, to match your wedding theme and style. Our Wedding Limousine packages can be tailored to your needs, including decorations, champagne, and red carpet service. We prioritize punctuality, safety, and exceptional customer service to make your wedding day truly memorable. Book your Wedding Limousine with us and experience the ultimate in sophistication and convenience. – https://bdluxlimo.com/
купить аттестат за 9 класс купить аттестат за 9 класс .
Не упустите возможность получить займ на карту на карту быстро и удобно!
Малые займы становятся актуальными для большого числа людей. Небольшие кредиты привлекают внимание многих, и это неудивительно. Основной причиной этого является легкость получения. В большинстве случаев процесс занимает всего несколько минут. Такой подход особенно важен для людей, находящихся в сложной финансовой ситуации.
Другой важный аспект — это минимальные требования к заемщику. Многие организации не требуют подтверждения дохода. Это открывает двери для заемщиков, которые не могут подтвердить официальный доход. Однако не следует забывать о потенциальных угрозах.
Необходимо аккуратно выбирать, у кого брать займ. Существует множество микрофинансовых компаний. Некоторые учреждения могут иметь не самые привлекательные условия. Поэтому стоит читать отзывы и изучать условия заранее.
В конечном счете, маленькие кредиты могут быть полезны в момент финансовых трудностей. Но нужно всегда помнить о возможных последствиях. Важно внимательно изучать условия и репутацию займодателя. Тем самым, вы сможете минимизировать риски и избежать проблем.
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Мы предлагаем документы ВУЗов, которые находятся на территории всей РФ. Дипломы и аттестаты печатаются на бумаге высшего качества. Это дает возможность делать настоящие дипломы, которые невозможно отличить от оригинала. orikdok-2v-gorode-volgograd-34.online
Here’s more on the topic https://my-caffe.ru/
Мы изготавливаем дипломы любой профессии по невысоким тарифам. Приобретение документа, который подтверждает обучение в университете, – это рациональное решение. Купить диплом о высшем образовании: vnuci.listbb.ru/viewtopic.php?f=2&t=2322
Психотерапевт Пенза. Психотерапевт онлайн 858 оценок
услуги косметологической клиники услуги косметологической клиники .
centro de cl?nica de cosmetolog?a centro de cl?nica de cosmetolog?a .
Школа вокала Марины Лаврищевой предлагает пройти вокальные уроки в свободное время. Поющий педагог по эстрадному вокалу поможет с постановкой голоса и обучит, как максимально развить вокальные способности.
Курсы эстрадного вокала
Заказать диплом любого университета!
Мы готовы предложить дипломы любых профессий по приятным тарифам— tyumenpages.ru
подробнее kra35.at
drenazh-10-sotok-812.ru .
Консультант подобрал идеальный вариант за минуту – профессионал!
розы томск
надежные прогнозы на футбол [url=https://kompyuternye-prognozy-na-futbol2.ru]https://kompyuternye-prognozy-na-futbol2.ru[/url] .
1 win.com.ci 1 win.com.ci
1win контора зеркало сайта https://1win1143.ru
Лучшие топ и рейтинг проверенных и надежных https://vc.ru/niksolovov/1607120-luchshie-tabletki-dlya-posudomoiki-top-20-samyh-populyarnyh-v-2025-godu Лучшие платформы сайты и сервисы.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
скачать бесплатно музыку на телефон без регистрации скачать бесплатно музыку на телефон без регистрации .
Уроки по пошиву штор, в нашем блоге.
Соберите идеальные шторы, по нашим советам.
Шторы на заказ, как создать.
Идеи для пошива штор, которые легко реализовать.
Пошив штор для начинающих, легко и быстро.
Выбор тканей для штор, и потребностям.
Стильные шторы, вашему пространству.
Как украсить шторы, чтобы они стали уникальными.
Кулинарные шторы, в классическом исполнении.
Комфортные шторы для вашего уюта, на нашем сайте.
Идеи для штор в гостиной, по вашему вкусу.
Как ухаживать за шторами, и прекрасными.
Это ваши идеальные шторы, от минимализма до барокко.
Как сшить римские шторы, просто и недорого.
Функциональные шторы, для вашего комфорта.
Как сшить шторы на люверсах, которые украсят любой интерьер.
Креативные идеи для штор в детской, чтобы создать уютное пространство.
Секреты идеального пошива штор, от профессионалов.
сшить шторы [url=https://sshit-shtory.ru/]сшить шторы[/url] .
1 win ставки на спорт зеркало 1 win ставки на спорт зеркало
1win букмекерская приложение 1win букмекерская приложение
Не упустите возможность получить умные наличные на карту быстро и удобно!
Малые займы становятся актуальными для большого числа людей. Все больше людей выбирают этот вариант финансовой помощи. Основной причиной этого является легкость получения. В большинстве случаев процесс занимает всего несколько минут. Это особенно актуально для тех, кто испытывает финансовые трудности.
Еще одним значимым моментом являются невысокие требования к клиентам. Многие организации не требуют подтверждения дохода. Это открывает двери для заемщиков, которые не могут подтвердить официальный доход. Однако не следует забывать о потенциальных угрозах.
Необходимо аккуратно выбирать, у кого брать займ. Рынок микрофинансовых организаций достаточно велик. Некоторые учреждения могут иметь не самые привлекательные условия. Поэтому целесообразно заранее изучить отзывы и условия предоставления займов.
Подводя итоги, можно сказать, что мелкие займы могут помочь в сложной финансовой ситуации. Тем не менее, важно не забывать о рисках и подходить к ним осознанно. Необходимо тщательно проверять условия и репутацию кредитора. Тем самым, вы сможете минимизировать риски и избежать проблем.
Купить документ о получении высшего образования вы имеете возможность у нас в столице. Заказать диплом университета по выгодной стоимости вы сможете, обратившись к проверенной специализированной фирме. serdzedetyam32.ru/forum/posting.phpmode=post&f=16
купить аттестат с отличием купить аттестат с отличием .
аттестат за 9 класс купить аттестат за 9 класс купить .
Оформиление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1606 клиентов воспользовались услугой — теперь ваша очередь.
Куплю диплом — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1463 клиентов воспользовались услугой — теперь ваша очередь.
Пишите нам — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1734 клиентов воспользовались услугой — теперь ваша очередь.
Можно ли купить диплом о высшем образовании — ответим быстро, без лишних формальностей.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
математический прогноз на футбол http://www.kompyuternye-prognozy-na-futbol2.ru .
Оформиление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2835 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом с занесением — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3976 клиентов воспользовались услугой — теперь ваша очередь.
Пишите — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1845 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом Киев — ответим быстро, без лишних формальностей.
купить настоящий аттестат купить настоящий аттестат .
https://GiftWrapping-Workshop.com teaches you to create stunning gift presentations with expert wrapping techniques. Perfect for holidays, birthdays, or special occasions, our workshops offer creative ideas using eco-friendly materials. Suitable for all skill levels, make every gift unforgettable with personalized designs.
Мы предлагаем оформление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1811 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом о образовании недорого — ответим быстро, без лишних формальностей.
Le Code Ethereum en: notre avis
Voici un spin-tax de haute qualité pour votre texte en français, respectant toutes vos consignes :
Dans un contexte où le média spécialisé Decomania analyse les avancées dans le domaine fintech, une interrogation émerge : Quantum AI 2025 représente-t-il un progrès tangible ou uniquement une initiative prometteuse ?
Mécanisme et Engagements : Qu’Offre Cette Plateforme ?
Quantum AI 2025 se positionne comme une solution de trading algorithmique associant smart tech et informatique quantique. D’après ses concepteurs, cette technologie rendrait possible :
Un examen approfondi des marchés (actifs numériques, valeurs mobilières, Forex).
Une régulation intelligente du risque pour optimiser les résultats.
Une prise en main aisée, conçu pour les opérateurs aux profils variés.
Néanmoins, aucun audit externe ne confirme publiquement ces allégations, et les feedbacks d’utilisateurs s’avèrent contrastés.
Aspects à Vérifier D’après Decomania
Notre étude met en lumière divers facteurs à considérer avant de s’engager :
Plusieurs URLs géolocalisées (etc.) – Une pratique courante, mais qui peut compliquer la vérification.
Manque de clarté – Un manque de détails technologiques sont accessibles sur les algorithmes utilisés.
Performances inégales – Certains utilisateurs rapportent des résultats concluants, tandis que certains signalent des problèmes opérationnels.
Suggestions pour les Investisseurs
Choisir en priorité les interfaces contrôlées (CySEC) pour plus de sécurité.
Tester en compte test avant toute mise de fonds.
Mettre en parallèle avec d’autres options (comme les systèmes disponibles par Interactive Brokers).
Conclusion : Une Solution à Surveiller avec Circnospection
Quantum AI 2025 avance une approche innovante, mais ses performances concrètes nécessitent encore des confirmations pratiques. Dans l’attente de plus de transparence, une stratégie prudente est préconisée.
музыка скачать бесплатно в хорошем качестве музыка скачать бесплатно в хорошем качестве .
Thanks for the article. Here’s more on the topic https://adventime.ru/
Заказать диплом ВУЗа по доступной цене возможно, обращаясь к проверенной специализированной компании. Приобрести документ о получении высшего образования вы имеете возможность у нас в столице. orikdok-4v-gorode-tomsk-70.online
Thanks for the article. Here’s more on the topic https://kaizen-tmz.ru/
https://ClayCraft-Studio.com offers pottery and sculpture workshops for all levels. From wheel throwing to handbuilding, create functional and decorative ceramics in a supportive studio environment led by skilled instructors. Perfect for beginners and artists, unleash your creativity with clay.
Рейтинг лучших сервисов https://vc.ru/tg-raiting/1516494-nakrutka-laikov-v-vk-besplatno-top-25-servisov-2025 ТОП проверенных.
Here’s more on the topic https://my-caffe.ru/
интерьерные кашпо напольные http://www.kashpo-napolnoe-msk.ru/ – интерьерные кашпо напольные .
edmeds buy edmeds buy edpills buy edpills buy
1win мобильная версия сайта 1win мобильная версия сайта
Создайте уникальные шторы под заказ для вашего дома, закажите.
Индивидуальный подход к шторным решениям, добавляет уют в ваше пространство.
Шторы на заказ — это просто, быстро и удобно, не стесняйтесь.
Идеальные шторы на заказ для любого бюджета, с учетом всех пожеланий.
Создайте уют с помощью штор на заказ, доверяйте профессионалам.
Шторы по вашим размерам — идеальное решение, усилите атмосферу.
Достаньте самовыражение через шторы на заказ, решайте прямо сейчас.
Шторы на заказ: функциональность и эстетика, измените свой интерьер навсегда.
Шторы на заказ для создания уникального образа, находите вдохновение.
Уникальные решения для вашего интерьера с шторами на заказ, создавайте свой стиль.
шторы на заказ шторы на заказ .
Мы предлагаем документы об окончании любых ВУЗов России. Документы производятся на подлинных бланках государственного образца. kolhos.listbb.ru/viewtopic.php?f=2&t=3505
mostbet aviator az [url=https://www.mostbet4045.ru]mostbet aviator az[/url]
Быстро и просто приобрести диплом ВУЗа!
Покупка документа о высшем образовании через надежную компанию дарит ряд достоинств. Выгодно заказать диплом о высшем образовании у проверенной компании: doks-v-gorode-nizhnevartovsk-86.ru
1вин казино 1win1138.ru
Chicken Road: Honest User Opinions
Chicken Road stands out as a gambling game with arcade vibes, attracting users with its easytograsp gameplay, high RTP (98%), and distinctive cashout system. We’ve gathered real player reviews to determine if it’s worth your time.
What Users Appreciate
Numerous players commend Chicken Road for its quick, engaging action and userfriendly design. With its cashout feature offering strategy and an RTP of 98%, it feels like a fairer alternative to conventional slot games. The demo mode is a hit with beginners, allowing players to test the game riskfree. The game earns extra points for its mobile compatibility, running seamlessly on both new and older devices.
Melissa R., AU: “Unexpectedly enjoyable and balanced! The ability to cash out brings a layer of strategy.”
Nathan K., UK: “The arcade style is refreshing. Runs smoothly on my tablet.”
Players also enjoy the colorful, nostalgic design, which feels both fun and engaging.
Areas for Improvement
However, Chicken Road isn’t perfect, and there are a few issues worth noting. Certain players think the game is too predictable and doesn’t offer much variety. Others mention slow customer support and limited features. Misleading ads are another issue, with many assuming it was an arcade game instead of a gambling app.
Tom B., US: “Fun at first, but it gets repetitive after a few days.”
Sam T., UK: “Advertised as a fun game, but it’s clearly a gambling app.”
Advantages and Disadvantages
Advantages
Easytounderstand, quick gameplay
High RTP (98%) ensures fairness
Free demo option for beginners to test the waters
Optimized for flawless mobile play
Negative Aspects
The gameplay may come across as monotonous
Limited variety and features
Customer service can be sluggish and unreliable
Confusing promotional tactics
Overall Assessment
Chicken Road stands out with its transparency, high RTP, and accessibility. It’s a great option for casual players or those new to online gambling. However, its reliance on luck and lack of depth may not appeal to everyone. To maximize enjoyment, stick to authorized, regulated sites.
Rating: Four out of five stars
An enjoyable and equitable option, though it has areas to grow.
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 930 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом об образовании — ответим быстро, без лишних формальностей.
Мы готовы предложить документы институтов, которые расположены на территории всей РФ. Заказать диплом о высшем образовании:
nitrostrengthbuy.copiny.com/question/details/id/1113426
edpills buy https://buyedmeds.shop/# edmeds buy edmeds buy
Покупка дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1889 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом специалиста недорого — ответим быстро, без лишних формальностей.
Мы предлагаем оформление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3051 клиентов воспользовались услугой — теперь ваша очередь.
Где купить диплом о высшем образовании — ответим быстро, без лишних формальностей.
Узнать больше kraken вход
Мы предлагаем оформление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3754 клиентов воспользовались услугой — теперь ваша очередь.
Купить учебный диплом — ответим быстро, без лишних формальностей.
Заказать документ университета можно у нас. Заказать диплом университета по невысокой стоимости вы можете, обратившись к надежной специализированной компании. nova-driving-school.mn.co/posts/84444520
Wallet Address Checker Online
Crypto Wallet Validator
Use a secure wallet address checker online to scan your crypto wallet for risks like sanctions, stolen funds, or darknet exposure . Stay ahead of exchange freezes and avoid losing access to your assets. Verified by crypto experts worldwide — check now.
купить диплом в майкопе купить диплом в майкопе .
каталог kraken войти
Мы можем предложить дипломы любой профессии по разумным ценам. Дипломы производят на настоящих бланках государственного образца Приобрести диплом об образовании diplomaz-msk.com
красный диплом купить красный диплом купить .
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3550 клиентов воспользовались услугой — теперь ваша очередь.
Где купить диплом — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3656 клиентов воспользовались услугой — теперь ваша очередь.
Узнать подробнее — ответим быстро, без лишних формальностей.
Thanks for the article. Here’s more on the topic https://kaizen-tmz.ru/
mostbet ios yukle http://mostbet4042.ru/
Букет сказочной красоты – глаз не оторвать!
букеты томск
Thanks for the article. Here’s more on the topic https://adventime.ru/
edpills buy edpills buy edpills buy edpills buy
Где купить диплом по необходимой специальности?
Заказать диплом ВУЗа по невысокой цене вы сможете, обращаясь к надежной специализированной фирме.: diplom-profi.ru
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Индивидуальные шторы на заказ — стиль и комфорт в каждом доме, подберите.
Уникальные шторы под заказ — ваш стиль в каждом шве, открывает новые горизонты.
Индивидуальные шторы: делаем ваш дом особенным, не стесняйтесь.
Качественные шторы на заказ по доступной цене, с учетом всех пожеланий.
Шторы под заказ — ваш идеальный интерьер, доверяйте профессионалам.
Шторы по вашим размерам — идеальное решение, усилите атмосферу.
Найдите идеальные шторы, сделанные только для вас, решайте прямо сейчас.
Шторы на заказ — для настоящих ценителей комфорта, закажите прямо сейчас.
Шторы на заказ для создания уникального образа, доверяйте профессионалам.
Шторы, которые идеально впишутся в ваш дом, действуйте и вдохновляйтесь.
шторы на заказ шторы на заказ .
1вин бонусы казино 1вин бонусы казино
Заказать диплом ВУЗа по выгодной стоимости возможно, обратившись к проверенной специализированной компании. Приобрести документ ВУЗа можно в нашем сервисе. orikdok-2v-gorode-irkutsk-38.ru
зайти на сайт kra34.at
1win зеркало скачать ios https://1win1143.ru
Официальный промокод Мостбет при регистрации, только по нему ты получишь бонус в 35000 рублей, все остальные коды не действительные и не дают такой бонус. Mostbet предлагает сейчас 35000 рублей всем новым игрокам букмекерской конторы. промокод на бесплатная ставка. Отличительной особенностью букмекерской конторы Mostbet является возможность совершения ставок по промокодам, которые предоставляются бесплатно. Это позволяет новичку заработать деньги, не делая никаких вложений. Однако чтобы получить промокод на ставку и воспользоваться им, следует разобраться с некоторыми инструкциями. Бесплатные промокоды Mostbet при регистрации. Промокод Mostbet на сегодня актуален, бонус будет зачислен сразу после первого пополнения счета. Временные коды. Букмекерская контора Mostbet часто выступает спонсором при переводе популярных сериалов на русский язык. Рекламные вставки букмекера можно услышать перед началом многих сериалов. Часто в такой рекламе диктуется специальные промокод, дающий возможность беттерам рассчитывать на дополнительный бонус. Максимальный бонус при регистрации составляется 35000 рублей. Воспользоваться промокодом можно только при выполнении ряда условий: Доступно только для беттеров из России, Беларуси, Украины и Казахстана. Возврат игрока – от 18 лет.
Купить диплом вы можете используя официальный портал компании. orikdok-3v-gorode-barnaul-22.online
Психотерапевт Челны. Психолог онлайн 427 оценок
edpills buy https://buyedmeds.shop/# edpills buy edmeds buy
Ранним утром доставили – удобно!
букеты томск
подробнее kraken сайт
Мы предлагаем документы институтов, которые расположены на территории всей РФ. Купить диплом о высшем образовании:
careers.mycareconcierge.com/companies/diplomirovans
edpills buy edmeds buy edmeds buy edmeds buy
авиатор ставки https://1win1145.ru
Hello!
Anonymous chat is your secret place to play. Say whatever you want without fear. Connect with people who love the same. Erotic chat rooms are waiting. Enjoy the thrill of anonymity.
More information here – https://rt.gayroulette.ru/
erotic chat girl, online chat fantasy, hot private message
message strangers hot, live sex chat, random flirt chat
Good luck!
Добрый день!
Авто из Америки в Минске – это надежность и качество по доступной цене. Мы предлагаем авто с растаможкой, готовые к эксплуатации. Купите авто из Америки в Минске с растаможкой и будьте уверены в своем выборе. Все автомобили прошли проверку качества и готовы к продаже. авто из америки купить минске Обратитесь к нам, чтобы выбрать авто из Америки в Минске!
Более подробно на сайте – https://telegra.ph/Avto-iz-SSHA-v-Minsk-06-26/
в минске авто из америки, авто из америки минске, авто из америки в наличии в минске
авто из америки купить минск, битый авто из америки в минск, покупка авто из америки в минске
Удачи!
прогноз на футбол с анализом прогноз на футбол с анализом .
Мы предлагаем выгодно и быстро приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями официальных лиц. Наш документ пройдет любые проверки, даже с использованием специфических приборов. saigonwood.vn/ne-upustite-shans-kupite-diplom-prjamo-sejchas-209
Приобрести документ института можно в нашей компании. Приобрести диплом института по невысокой стоимости возможно, обратившись к проверенной специализированной фирме. myfootballday.ru/gde-vyigodno-kupit-diplom-s-dostavkoy-po-vsey-rossii
прогнозы хоккей http://luchshie-prognozy-na-khokkej1.ru .
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
сантехника логотип http://www.internet-magazine-santehniki.ru .
Психотерапевт Челны. Психотерапевт онлайн 370 оценок
нажмите здесь kraken вход
прогнозы на хоккей https://luchshie-prognozy-na-khokkej2.ru .
подробнее здесь kra35.at
Перейти на сайт kraken ссылка
лучшие прогнозы на хоккей https://www.luchshie-prognozy-na-khokkej.ru .
Займ на карту онлайн без отказа займ на карту даже с плохой кредитной историей.
Кпт курган. Психолог онлайн 784 оценок
диплом врача купить диплом врача купить .
Купить рулонные электрошторы, инновации, современные технологии.
Электрошторы: идеальное решение, для любого интерьера.
Рулонные электрошторы: советы по выбору, функциональность и стиль.
Рулонные электрошторы: удобство и стиль, комфорт и безопасность.
Рулонные электрошторы: стиль и функциональность, экологические материалы.
Электрошторы: ваш лучший выбор, долговечность.
Рулонные электрошторы: как они работают?, защита от солнечных лучей.
Рулонные электрошторы на любой вкус, доступные инструкции.
Электрошторы: умный дом, интеллектуальные решения.
Эстетика и функциональность электроштор, организация пространства.
Электрошторы: решение для вашего окна, уникальные предложения.
Зачем купить рулонные электрошторы?, выбор цвета.
Рулонные электрошторы: ваш уютный уголок, легкость в уходе.
Электрошторы: как они меняют пространство, интерьер в стиле минимализм.
Лучшие решения для оконных штор, разнообразие функций.
Электрошторы: идеальное решение для окон, подбор стиля.
Электрошторы: создайте атмосферу уюта, технологические преимущества.
Как электрошторы меняют пространство, варианты управления.
Эстетика и уход за рулонными электрошторами, простой монтаж.
Электрошторы для офисов и жилых помещений, эстетика и цена.
автоматическая рулонная штора автоматическая рулонная штора .
Коллеги засыпали вопросами, где такой заказала!
букет пионов
edpills buy https://buyedpills.shop/# edpills buy edpills buy
mostbet canlı yayım https://mostbet4046.ru
дренаж вокруг дома цена под ключ спб .
1win регистрация ставки онлайн 1win регистрация ставки онлайн
окна пвх в москве окна пвх в москве .
узнать больше kra cc
8k8
Hello!
The psychology of online gaming sheds light on how digital platforms affect behavior and decision-making. Why people play online games is often related to the need for social engagement and excitement. Seasonal eating offers several benefits, such as providing fresh and nutritious food that aligns with seasonal availability. Online games impact social connections by facilitating virtual communities that transcend geographic barriers. Effective time management for business leaders can help achieve a better work-life balance and improved productivity across teams.
More information here – https://marchemodevintage.com
leadership strategies for busy executives, life hacks for busy professionals, seasonal eating for sustainable living
effective strategies for leading teams, volunteering opportunities abroad, benefits of volunteering for self-growth
Good luck!
купить пластиковые окна rehau купить пластиковые окна rehau .
edmeds buy edmeds buy edmeds buy edpills buy
дренаж трактором .
скачать мостбет казино на андроид скачать мостбет казино на андроид .
Заказать диплом под заказ в Москве можно через сайт компании. orikdok-1v-gorode-cheboksary-21.online
евросан http://internet-magazine-santehniki.ru/ .
точные прогнозы на хоккей https://luchshie-prognozy-na-khokkej1.ru/ .
купить аттестат 11 класса http://www.arus-diplom6.ru .
Купить документ института можно в нашей компании в столице. Мы предлагаем документы об окончании любых университетов России. Гарантируем, что при проверке документа работодателем, подозрений не возникнет. ashinova.ru/category-5/t-3273.html#3273
Психотерапевт Пенза. Психотерапевт онлайн 621 оценок
Заказать диплом об образовании. Покупка подходящего диплома через надежную компанию дарит ряд плюсов. Такое решение помогает сберечь как дорогое время, так и серьезные средства. orikdok-v-gorode-smolensk-67.online
ставки на хоккей прогнозы http://luchshie-prognozy-na-khokkej.ru/ .
1win букмекерская контора регистрация http://1win1139.ru
Психотерапевты Самара. Психиатр онлайн 394 оценок
скачать музыку бесплатно mp3 скачать музыку бесплатно mp3 .
1win пополнить счет https://1win1141.ru/
Рулонные электрошторы на заказ, для вашего дома, современные технологии.
Электрошторы для комфорта, простота в использовании.
Как выбрать рулонные электрошторы?, функциональность и стиль.
Электрошторы для защиты от солнца, долговечность и практичность.
Секреты выбора рулонных электроштор, простота управления.
Рулонные электрошторы для любых окон, долговечность.
Рулонные электрошторы: как они работают?, защита от солнечных лучей.
Как установить рулонные электрошторы, доступные инструкции.
Современные технологии в рулонных электрошторах, удобство управления.
Электрошторы для коммерческих помещений, красота и комфорт.
Рулонные электрошторы для защиты от света, уникальные предложения.
Электрошторы: простота и элегантность, функция управления.
Электрошторы для идеального освещения, легкость в уходе.
Электрошторы: как они меняют пространство, советы по оформлению.
Как рулонные электрошторы улучшают интерьер, простота установки.
Рулонные электрошторы: ваш новый стиль, практические советы.
Как правильно выбрать рулонные электрошторы?, разнообразие расцветок.
Электрошторы для защиты от солнца, экологичные материалы.
Рулонные электрошторы для создания уюта, разнообразие стилей.
Рулонные электрошторы: ваш стильный акцент, эстетика и цена.
автоматическая рулонная штора автоматическая рулонная штора .
edmeds buy https://buyedpills.shop/# edpills buy edmeds buy
прогнозы на спорт точные прогнозы на спорт точные .
888starz вход в личный кабинет россия 888starz вход в личный кабинет россия .
страница kra
8xbet
1win casino скачать 1win casino скачать
В17 психология. Психиатр онлайн 115 оценок
mexican xlpharmacy http://mexicanxlpharmacy.com/# mexican xlpharmacy mexican xlpharmacy
купить аттестат в архангельске arus-diplom9.ru – купить аттестат в архангельске .
Hello!
The psychology of online gaming revolves around human needs for challenge, accomplishment, and social interaction. Why people play online games is often linked to the desire for social engagement, fun, and competition. The benefits of seasonal eating are many, offering better health when your diet aligns with nature’s changing offerings. Online games influence relationships by helping people form lasting bonds through cooperative play. Time management for business leaders is a key strategy for ensuring productivity and achieving business goals.
More information here – https://tj-service.ru
exploring global gaming cultures, traveling for self-discovery, ways to reduce stress with travel
trends in video game remasters, importance of aligning diet with seasons, nostalgic remakes of popular video games
Good luck!
mostbet qeydiyyat necə olur https://www.mostbet4042.ru
лучшие прогнозы на хоккей на сегодня luchshie-prognozy-na-khokkej2.ru .
купить аттестат о среднем образовании купить аттестат о среднем образовании .
накрутить живых просмотров в рилс накрутить живых просмотров в рилс
проверика организации proverit-kontragenta.ru .
Психотерапевты Самара. Психиатр онлайн 972 оценок
1win регистрация на сайте https://1win1139.ru/
Перейти на сайт кракен тор
Wallet Address Checker Online
Wallet Address Checker Online
Use a reliable wallet address checker online to scan your crypto wallet for risks like sanctions, stolen funds, or darknet exposure . Stay ahead of exchange freezes and avoid losing access to your assets. Fast, secure, and trusted by thousands — check now.
mexican xlpharmacy mexican xlpharmacy mexican xlpharmacy mexican xlpharmacy
кашпо для цветов напольное в офис http://www.kashpo-napolnoe-spb.ru – кашпо для цветов напольное в офис .
Сервис по подбору психолога Психотерапевт онлайн 500 оценок
Психотерапевт Челны. Психотерапевт онлайн 782 оценок
Здравствуйте!
В Минске можно купить авто из Америки с растаможкой по выгодной цене! Мы предлагаем широкий выбор б/у автомобилей, готовых к эксплуатации. Наши авто из Америки прошли все проверки и готовы стать вашим надежным другом на дороге. Поторопитесь, количество автомобилей ограничено. купить в минске авто из америки Приходите и выберите авто из Америки в Минске!
Более подробно на сайте – https://telegra.ph/Avto-iz-SSHA-v-Minsk-06-26/
авто из америки в минске купить, купить в минске авто из америки, авто из америки в минске в наличии
авто минск америк доставка, авто из америки купить в минске, купить авто америке минск
Удачи!
mostbet download on phone mostbet download on phone .
узнать больше Здесь kraken зайти
Преимущества автоматических рулонных штор в интерьере, узнать.
Руководство по выбору автоматических рулонных штор, для выбора.
Автоматические рулонные шторы: идеальное решение для окон, на нашем сайте.
Создайте уют с автоматическими рулонными шторами, выбирая.
Управление автоматической рулонной шторой одним нажатием, нашими услугами.
Как ухаживать за автоматическими рулонными шторами, для.
Как автоматические рулонные шторы меняют оформление интерьера, в нашем блоге.
Умные рулонные шторы для современного интерьера, с отзывами пользователей.
Идеальные автоматические рулонные шторы для каждого стиля, изучите подробности.
Как автоматические рулонные шторы улучшают качество жизни, в нашем каталоге.
Автоматические рулонные шторы — удобство в каждой детали, получите.
Советы по использованию автоматических рулонных штор для оптимального освещения, в нашем руководстве.
Технические особенности автоматических рулонных штор, с нашим руководством.
Автоматические рулонные шторы: как они меняют привычный интерьер, ознакомьтесь.
Сравнение автоматических рулонных штор различных брендов, познакомьтесь.
Современные решения для окон с автоматическими рулонными шторами, выберите.
Энергоэффективные решения для окон: автоматические рулонные шторы, приобретите.
Автоматические рулонные шторы и их преимущества для офисов, на нашем сайте.
Как выбрать идеальные автоматические рулонные шторы, с нашими специалистами.
Автоматические рулонные шторы для гостиниц и ресторанов, с нашими услугами.
автоматическая рулонная штора автоматическая рулонная штора .
mostbet texniki dəstək http://mostbet4046.ru/
Привет всем!
Если вы хотите купить авто из Америки в Минске, у нас есть для вас отличные предложения! Мы предлагаем б/у автомобили с растаможкой по выгодной цене. Все авто прошли проверку качества и готовы к эксплуатации. Купите авто из Америки с растаможкой в Минске и наслаждайтесь качеством. авто из америки купить минске Обратитесь к нам, чтобы выбрать авто из Америки в Минске!
Более подробно на сайте – https://telegra.ph/Avto-iz-SSHA-v-Minsk-06-26/
авто из америки купить минске, купить авто америке минск, авто из америки наличие минск
авто из америки купить минске, авто в минске с америки, купить авто в америке минск
Удачи!
Приобрести диплом любого института. Покупка документа о высшем образовании через качественную и надежную фирму дарит множество достоинств для покупателя. Это решение дает возможность сберечь время и серьезные средства. orikdok-2v-gorode-moskva-77.ru
888starz bet casino 888starz bet casino .
окна rehau москва okna177.ru .
Заказ обработали моментально – очень оперативно!
букет невесты томск
Quantum Code en: analyse detaillee
Voici un spin-tax de haute qualité pour votre texte en français, respectant toutes vos consignes :
Alors que le média spécialisé Decomania étudie les avancées des secteurs financiers et technologiques, une question se pose : Quantum AI 2025 représente-t-il un progrès tangible ou uniquement une initiative prometteuse ?
Fonctionnement et Perspectives : Qu’Offre Cette Plateforme ?
Quantum AI 2025 se présente comme un système de trading algorithmique intégrant IA et calcul quantique. Pour ses développeurs, cette technologie rendrait possible :
Une analyse avancée des marchés financiers (actifs numériques, actions, Forex).
Une régulation intelligente des expositions pour maximiser les rendements.
Un accès simplifié, pensé pour les investisseurs tous niveaux.
Toutefois, aucun audit externe ne corrobore publiquement ces allégations, et les retours utilisateurs demeurent mitigés.
Points à Examiner Selon Decomania
Notre étude met en lumière divers facteurs à évaluer avant de investir :
Multiples sites régionaux (etc.) – Un usage répandu, mais qui peut complexifier l’authentification.
Transparence limitée – Un manque de détails technologiques sont accessibles sur les algorithmes utilisés.
Résultats divergents – Certains utilisateurs mentionnent des résultats concluants, tandis qu’ certains signalent des problèmes opérationnels.
Suggestions pour les Opérateurs
Choisir en priorité les plateformes régulées (AMF) pour plus de sécurité.
Tester en mode démo avant chaque investissement.
Comparer avec d’autres solutions (à l’image de les plateformes disponibles par eToro).
Bilan : Un Projet à Suivre avec Circnospection
Quantum AI 2025 propose une méthode novatrice, mais ses performances concrètes demandent toujours des confirmations pratiques. En attendant de plus de transparence, une stratégie prudente est recommandée.
пластиковые окна рехау пластиковые окна рехау .
Мы готовы предложить дипломы любой профессии по приятным тарифам. Для нас очень важно, чтобы документы были доступными для большого количества наших граждан. Заказать диплом о высшем образовании dely.nl/poluchite-diplom-bez-lishnih-hlopot-94-2
mostbet az bonusları http://mostbet4045.ru/
1win sports https://www.1win3023.com
Купить диплом института по доступной стоимости можно, обращаясь к надежной специализированной компании. Купить диплом о высшем образовании: video.listbb.ru/viewtopic.php?f=3&t=1993
Мы готовы предложить документы институтов, которые расположены на территории всей Российской Федерации. Документы печатаются на бумаге высшего качества: [url=http://nahimajobs.com/employer/frees-diplom/]nahimajobs.com/employer/frees-diplom[/url]
Удобный сайт и приятные цены – рекомендую всем!
букеты томск
kupit-drova-v-spb-365.ru .
1win listing date http://www.1win3022.com
Элегантные автоматические рулонные шторы для вашего дома, узнать.
Топ-5 советов по выбору автоматических рулонных штор, для выбора.
Автоматические рулонные шторы: идеальное решение для окон, узнайте больше.
Преобразите свой дом с автоматическими рулонными шторами, в нашем магазине.
Преимущества умного управления рулонными шторами, воспользуйтесь.
Уход за автоматическими рулонными шторами: советы и рекомендации, для.
Автоматические рулонные шторы в интерьере: тренды 2023 года, узнайте больше.
Шторы, которые сами закрываются: автоматизация в вашем доме, прочитайте.
Автоматические рулонные шторы: сочетание функциональности и дизайна, узнайте прямо сейчас.
Как автоматические рулонные шторы улучшают качество жизни, узнайте больше.
Современные автоматические рулонные шторы для стильного интерьера, выберите.
Автоматические рулонные шторы: идеальное решение для светолюбителей, узнайте больше.
Что нужно знать о механизме автоматических рулонных штор, познакомьтесь.
Дизайн и функциональность: автоматические рулонные шторы, узнайте.
Как выбрать лучший бренд автоматических рулонных штор, познакомьтесь.
Современные решения для окон с автоматическими рулонными шторами, из нашего ассортимента.
Энергоэффективные решения для окон: автоматические рулонные шторы, в нашем магазине.
Советы по выбору автоматических рулонных штор для офисов, проверьте.
Выбор автоматических рулонных штор: советы от экспертов, проконсультируйтесь.
Идеальные шторы для бизнеса — автоматические рулонные шторы, изучите.
автоматическая рулонная штора автоматическая рулонная штора .
ванна гидромассаж ванна гидромассаж .
mostbet qeydiyyat pulsuz http://mostbet4047.ru
скачать музыку мр3 скачать музыку мр3 .
купить сантехнику италия https://www.gessi-santehnika-1.ru .
mostbet idman mərcləri https://mostbet3041.ru/
посмотреть на этом сайте kra32.at
в этом разделе даркплейс
сайт кракен сайт
Источник kra34 сс
valtrex easy valtrex easy valtrex easy valtrex easy
Смотреть здесь tripscan26 cc
Орхидеи – свежесть на две недели, просто чудо!
101 роза
mostbet şifrə unutmuşam mostbet3041.ru
https://blast-de.com
мр3 слушать мр3 слушать .
https://747-live-ph.com
https://rollino-de.com/
ванная гидромассажная купить ванная гидромассажная купить .
valtrex easy http://valtrexeasy.shop/# valtrex easy valtrex easy
как накрутить живых подписчиков в тг как накрутить живых подписчиков в тг
Привет всем!
В Минске можно купить авто из Америки с растаможкой по хорошей цене. Мы предлагаем вам авто с проверенной историей и отличным состоянием. Купите авто из Америки в Минске и наслаждайтесь комфортом и безопасностью. Все наши машины прошли проверку качества и готовы к продаже. авто из америки в наличии в минске Обратитесь к нам и выберите авто из Америки в Минске.
Более подробно на сайте – https://telegra.ph/Avto-iz-SSHA-v-Minsk-06-26/
авто с америки в минск, битые авто в минске из америки, купить авто америки минске
доставка авто америки минск, авто из америки в наличии минск, авто из америки в наличии минск
Удачи!
Электрические рулонные шторы на окна, комфорт и стиль.
Идеальное решение для современных окон, качество и надежность.
Автоматизация окон: рулонные шторы, функциональность.
Умные решения для вашего интерьера, без лишних усилий.
Советы по выбору ролл-штор, для вашего интерьера.
Электрические рулонные шторы: стиль и технологии, с гарантией качества.
Инновационные электрические рулонные шторы, доступные для любого интерьера.
Лучшие модели электрических рулонных штор, для вашего дома.
Технология автоматизации рулонных штор, добавляет стиля.
Лучшие электрические рулонные шторы на рынке, с первоклассным качеством.
Электрические рулонные шторы: удобство и стиль, для создания неповторимого стиля.
Обзор электрических штор для окон, в вашем доме.
Как правильно выбрать рулонные шторы, с качественными материалами.
Электронные шторы для любого интерьера, для вашего удобства.
Электрические рулонные шторы: преимущества, для улучшения качества жизни.
Как установить электрические рулонные шторы, с соблюдением всех стандартов.
Шторы с электроприводом для вашего дома, для улучшения условий жизни.
Электрические рулонные шторы для бизнеса, для современной эстетики.
Электрические рулонные шторы: выгодные решения, для вашего дома.
электрические рулонные шторы на окна электрические рулонные шторы на окна .
Viagra * Cialis * Levitra
All the products you are looking an eye to are currently convenient for 1+1.
4 more tablets of identical of the following services: Viagra * Cialis * Levitra
https://xn--2i0b04cz5bpzhb6crvj5ofkwjui0a.com
накрутить просмотры рилс накрутить просмотры рилс
Мы изготавливаем дипломы любой профессии по приятным тарифам. Заказ документа, подтверждающего обучение в ВУЗе, – это разумное решение. Заказать диплом любого университета: kh.txi.ru/forum/index.php?
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4939 клиентов воспользовались услугой — теперь ваша очередь.
Купить государственный диплом — ответим быстро, без лишних формальностей.
“To remain competitive and resilient amidst these global developments, we encourage firms and workers to press on with business and workforce transformation, and make full use of government programmes to adapt to the changing environment,” said the ministry. 토토커뮤니티
Покупка дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2589 клиентов воспользовались услугой — теперь ваша очередь.
Диплом сколько стоит — ответим быстро, без лишних формальностей.
gessi товары https://www.gessi-santehnika-1.ru .
Мы предлагаем оформление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1577 клиентов воспользовались услугой — теперь ваша очередь.
Диплом колледжа купить — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4448 клиентов воспользовались услугой — теперь ваша очередь.
Пишите — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1303 клиентов воспользовались услугой — теперь ваша очередь.
Уточнить здесь — ответим быстро, без лишних формальностей.
Приглашаем вас посетить наш сайт-блог tellmi, где собраны интересные материалы на самые разные тематики! Здесь вы найдете полезные статьи, свежие новости и увлекательные обзоры — каждый сможет выбрать что-то по душе. Заходите, читайте и делитесь впечатлениями!
mostbet azerbaycan rəsmisi https://www.mostbet4047.ru
кашпо для цветов напольное из http://www.kashpo-napolnoe-rnd.ru – http://www.kashpo-napolnoe-rnd.ru .
Good Day!
We are pleased to offer innovative solution for dynamic balancing of rotating equipment – portable balancing device Balanset-1A.
Official product page: https://vibromera.eu/shop/889/
Fields of Use
Balanset-1A is successfully operated for imbalance elimination of various types rotating mechanisms:
Industrial Machinery:
Ventilation Systems – industrial, exhaust, duct. Pumping Equipment – gear, oil. Electromotors – synchronous. Turbine Units – hydraulic. Compression Equipment – centrifugal.
Metalworking:
Machine Spindles – milling. Abrasive Wheels. Shafts of textile equipment.
Farm Machines:
Combine Rotors. Rotary Mowers. Crushers for feed.
Special Equipment:
Centrifuges – laboratory. Air Blowers. Grinding Equipment. Cardan Shafts.
Technical Specifications
Main Parameters:
Number of balancing planes: One or two. Rotation frequency range: 200 to 15,000 revolutions per minute. Phase measurement accuracy: ±1°. Vibration error: ±5%. Measuring channels: 2. Vibration measurement limits: 0.1 to 300 mm/s. Power: USB powered. Kit weight: 4 kg.
Functionality:
Automatic counting of compensating masses. Data storage of all balancing procedures. Permissible values computation according to ISO 1940. Frequency analysis of vibration. Graphics in polar coordinates. Split-weight calculator. Eccentricity compensation of mandrels.
Package Contents
Main Delivery:
USB measurement module (1 pc.) – delivers data transfer between sensors and computer.
Vibration transducers (2 pcs.) – Sensitivity: 100 mV/g. Bandwidth: 0.5-10,000 Hz. Mounting: adhesive mounting.
Optical tachometer (1 pc.) – Distance: up to 1.5 m. Remote sensing for operator protection.
Magnetic mount for phase sensor (1 pc.) – Pull force: 60 kgf. Variable angles for best measurement.
Electronic scales up to 500 g (1 pc.) – Accuracy: 0.01 g. Required for accurate mass determination.
Balancing software Balanset-1A – User-friendly interface. PC-based. Multiple language support.
Transport case (1 pc.) – Robust design. Cushioned interior for safe transport.
Technical documentation – Detailed instructions. Clear methodology.
Interface cables – Reliable interference-free connections.
Advantages of Balanset-1A
Engineering Strengths:
? Accurate Results – Modern algorithms of data analysis guarantee reliable results.
? Portability – Light weight allows in-place adjustment without dismantling.
? Versatility – Applicable to various rotors from small fans to massive shafts.
? User Convenience – Clear controls eliminates need for complex learning.
Financial Benefits:
? Expense Cutting on repairs through failure prevention – 50-80% of unplanned downtime.
? Service Life Increase by 50-200% – vibration-free operation lasts longer.
? Energy Efficiency by 5-15% – reduced friction consume less power.
? Fast Investment Return – from 3 to 12 months depending on usage intensity.
Support
Our company provides:
Operator Training – theoretical sessions at your site. Advanced training covers all aspects.
Technical Support – remote help. Email support for immediate help.
Certification – regular certification to ensure precision.
Equipment Rental – perfect for one-time projects. Weekly hire periods available.
Price Information
Actual Prices:
Balanset-1A Portable Balancer & Vibration Analyzer: €1,751.00
Balanset-1A OEM Version: €1,561.00
Additional Vibration Sensor: €90.00
Optical Sensor (Laser Tachometer): €124.00
Magnetic Stand 60 kgf: €46.00
Reflective Tape: €10.00
Shipping Conditions:
Lead time: in stock. Warranty terms: 24 months comprehensive coverage. Payment: 50/50. Shipping: to any country via courier service.
Why We Stand Out
Direct Manufacturer – original equipment with comprehensive backup.
Certified Specialists with deep knowledge in rotor balancing.
Comprehensive Support – from supply to maintenance and skill development.
Ready Stock – quick delivery for common configurations.
Individual Approach for regular customers including volume discounts.
At your service,
Technical support team
P.S. Ask for presentation of the equipment at your convenience and take advantage of offers!
Электрические рулонные шторы на окна, удобство и элегантность.
Рулонные шторы с электроприводом, долговечность и простота в использовании.
Автоматизация окон: рулонные шторы, красота.
Лучшая автоматизация окон, без лишних усилий.
Выбор рулонных штор с электроприводом, для вашего интерьера.
Умные рулонные шторы: необычные решения, с гарантией качества.
Инновационные электрические рулонные шторы, для вашего дома и офиса.
Рекомендации по выбору рулонных штор, для вашего дома.
Электрические рулонные шторы: как они работают?, добавляет стиля.
Самые популярные электрические рулонные шторы, с первоклассным качеством.
Электрические рулонные шторы: удобство и стиль, для создания неповторимого стиля.
Электрические рулонные шторы: все, что нужно знать, для вашего стиля.
Как правильно выбрать рулонные шторы, с качественными материалами.
Как электрические рулонные шторы изменят ваш дом, для создания уюта.
Электрические рулонные шторы: преимущества, для улучшения качества жизни.
Электрические рулонные шторы: монтаж, с соблюдением всех стандартов.
Шторы с электроприводом для вашего дома, для стильного оформления.
Электронные решения для коммерческих помещений, для улучшения рабочего пространства.
Электрические шторы: экономия и комфорт, для вашего дома.
электрические рулонные шторы на окна электрические рулонные шторы на окна .
Быстро приобрести диплом университета!
Мы изготавливаем дипломы любой профессии по приятным тарифам— disun.ru
1win sports betting https://1win3022.com/
1win token listing date today 1win token listing date today
узнать больше tripskan.ru
заказать перепланировку soglasowanie-pereplanirovki-kvartiry.ru .
проект перепланировки цена проект перепланировки цена .
проект перепланировки стоимость москва http://www.proekt-pereplanirovki-kvartiry.ru/ .
Thanks for the article. Here’s more on the topic https://mehelper.ru/
kupit-drova-v-spb-365.ru .
Seattle Airport Limo Service offers luxurious and convenient transportation to and from Seattle-Tacoma International Airport. Our professional chauffeurs ensure a smooth and comfortable ride in our high-end fleet, including sedans, SUVs, and stretch limousines. We prioritize punctuality, safety, and customer satisfaction. Whether you’re traveling for business or leisure, our Seattle Airport Limo Service is designed to meet your needs with style and efficiency. Book your ride today and experience the best in airport transportation. – https://taxi-prive.com/limo-service-seattle-airport-reviews/
Wallet Address Checker Online
Wallet Address Checker Online
Use a trusted wallet address checker online to scan your crypto wallet for risks like sanctions, stolen funds, or darknet exposure . Stay ahead of exchange freezes and avoid losing access to your assets. Instant results with bank-grade security — check now.
купить диплом в челябинске https://arus-diplom8.ru .
Наша компания предлагает профессиональный пошив спортивной формы и одежды для команд, клубов, секций и корпоративных мероприятий.
Мы изготавливаем форму с учетом всех особенностей выбранного вида спорта: удобный крой, дышащие материалы, износостойкие швы и
высокая посадка — все для максимального комфорта и эффективности во время тренировок и соревнований.
Работаем как с небольшими, так и с крупными тиражами. Возможна индивидуализация формы: нанесение логотипов, фамилий,
номеров и фирменных цветов. Наше производство позволяет адаптировать дизайн под ваши задачи — от классических комплектов
до эксклюзивных моделей.
Мы ценим ваше время, поэтому гарантируем соблюдение сроков и контроль качества на каждом этапе. Пошив спортивной одежды
от нас — это надежность, стиль и удобство.
Свяжитесь с нами и получите расчёт стоимости уже сегодня! .
Thanks for the article. Here is a website on the topic – https://kanunnikovao.ru/
Thanks for the article. Here’s more on the topic https://orenbash.ru/
Мы предлагаем оформление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1053 клиентов воспользовались услугой — теперь ваша очередь.
Где купить диплом — ответим быстро, без лишних формальностей.
Мы можем предложить дипломы любых профессий по доступным тарифам. Покупка документа, который подтверждает обучение в ВУЗе, – это рациональное решение. Приобрести диплом любого ВУЗа: mos.flybb.ru/viewtopic.php?f=2&t=3451
1win coin https://www.1win3021.com
Мы предлагаем оформление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2639 клиентов воспользовались услугой — теперь ваша очередь.
Диплом бакалавра купить — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4751 клиентов воспользовались услугой — теперь ваша очередь.
Купить государственный диплом — ответим быстро, без лишних формальностей.
Мы предлагаем оформление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2297 клиентов воспользовались услугой — теперь ваша очередь.
По ссылке — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3898 клиентов воспользовались услугой — теперь ваша очередь.
На сайте — ответим быстро, без лишних формальностей.
Молниеносная доставка – это выше всех похвал!
букет пионов
kupit-drova-v-spb-365.ru .
Не упустите возможность получить займ онлайн на карту быстро и удобно!
Краткосрочные займы набирают популярность в наше время. Небольшие кредиты привлекают внимание многих, и это неудивительно. Одной из причин является простота оформления. Как правило, процесс оформления займов проходит очень быстро. Такой подход особенно важен для людей, находящихся в сложной финансовой ситуации.
Еще одним значимым моментом являются невысокие требования к клиентам. Некоторые кредитные компании не требуют предоставления документов о доходах. Это позволяет развивать кредитование среди заемщиков с нестандартной занятостью. Тем не менее, стоит помнить о рисках.
Следует внимательно подходить к выбору кредитной организации. Рынок микрофинансовых организаций достаточно велик. Некоторые учреждения могут иметь не самые привлекательные условия. Поэтому стоит читать отзывы и изучать условия заранее.
Подводя итоги, можно сказать, что мелкие займы могут помочь в сложной финансовой ситуации. Тем не менее, важно не забывать о рисках и подходить к ним осознанно. Следует детально анализировать условия и репутацию компании перед тем, как принимать решение. Так, вы сможете защитить себя от негативных последствий.
Покупка дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2727 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом университета — ответим быстро, без лишних формальностей.
эпл спб kupit-ajfon-cs1.ru .
Заказать диплом ВУЗа по выгодной цене вы можете, обращаясь к надежной специализированной компании. Мы оказываем услуги по изготовлению и продаже документов об окончании любых университетов Российской Федерации. Купить диплом университета– п»їallonlinesport.ru/diplomyi-dlya-teh-kto-tsenit-vremya
купить айфон 13 спб дешево http://kupit-ajfon-cs.ru .
Заказать диплом об образовании!
Мы изготавливаем дипломы любых профессий по приятным ценам— dip-expert.ru
купить телефон айфон недорого http://kupit-ajfon-cs2.ru .
Thanks for the article. Here’s more on the topic https://yarus-kkt.ru/
Свадебный букет вызвал восторг!
букеты томск
Мы изготавливаем дипломы любой профессии по приятным тарифам. Мы можем предложить документы ВУЗов, расположенных в любом регионе Российской Федерации. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это позволяет делать государственные дипломы, которые невозможно отличить от оригиналов. orikdok-3v-gorode-ufa-2.online
кашпо для напольных цветов недорого https://kashpo-napolnoe-rnd.ru – https://kashpo-napolnoe-rnd.ru .
1win account 1win account
Here’s more on the topic https://krylslova.ru/
Наша компания предлагает выгодно и быстро приобрести диплом, который выполнен на оригинальном бланке и заверен печатями, водяными знаками, подписями официальных лиц. Данный диплом пройдет любые проверки, даже при помощи специального оборудования. silkhunter.com/index.php?search=&title=Special:Search&fulltext=Search
согласование выполненной перепланировки квартиры согласование выполненной перепланировки квартиры .
My partner and I absolutely love your blog and find most of your post’s to be what
precisely I’m looking for. Does one offer guest writers to write content for you personally?
I wouldn’t mind writing a post or elaborating on most of the subjects
you write with regards to here. Again, awesome web site!
https://winfest-de.com
проект перепланировки для согласования цена http://www.proekt-pereplanirovki-kvartiry.ru .
где сделать проект перепланировки http://www.proekt-pereplanirovki-kvartiry1.ru .
I’m impressed, I must say. Seldom do I encounter a
blog that’s both equally educative and interesting, and let me tell you, you have hit the nail on the head.
The issue is something which too few people are
speaking intelligently about. Now i’m very happy that
I found this during my search for something regarding this.
dream
Не упустите возможность получить небольшие займы на карту быстро и удобно!
Малые займы становятся актуальными для большого числа людей. Все больше людей выбирают этот вариант финансовой помощи. Важной причиной популярности является удобство оформления. Как правило, процесс оформления займов проходит очень быстро. Такой подход особенно важен для людей, находящихся в сложной финансовой ситуации.
Другой важный аспект — это минимальные требования к заемщику. Некоторые кредитные компании не требуют предоставления документов о доходах. Это позволяет развивать кредитование среди заемщиков с нестандартной занятостью. В то же время важно осознавать возможные риски.
Следует внимательно подходить к выбору кредитной организации. Существует множество микрофинансовых компаний. Некоторые из них могут предлагать невыгодные условия. Поэтому прежде чем брать займ, стоит ознакомиться с репутацией компании.
В завершение, небольшие займы могут стать отличным решением в трудной ситуации. Однако необходимо подходить к ним с осторожностью. Следует детально анализировать условия и репутацию компании перед тем, как принимать решение. Так, вы сможете защитить себя от негативных последствий.
This is my first time pay a quick visit at here and i am actually impressed to read all at alone place.
playboom
диплом купить дешево диплом купить дешево .
где купить айфон спб https://kupit-ajfon-cs1.ru/ .
айфон купить в спб http://www.kupit-ajfon-cs.ru .
Sweet blog! I found it while surfing around on Yahoo News. Do you have any suggestions
on how to get listed in Yahoo News? I’ve been trying for a while but I never seem to get there!
Many thanks
ea sports
Быстро, качественно, с душой – вот почему я только у вас!
букеты томск
isotretinoin easyshop isotretinoin easyshop isotretinoin easyshop isotretinoin easyshop
новинки песни скачать новинки песни скачать .
Наша компания предлагает выгодно и быстро приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями. Данный диплом пройдет лубую проверку, даже при помощи профессионального оборудования. forum-pmr.net/member.php?u=42748
iphone санкт петербург iphone санкт петербург .
Мы изготавливаем дипломы любой профессии по доступным тарифам. Мы предлагаем документы техникумов, которые расположены на территории всей РФ. Документы выпускаются на “правильной” бумаге высшего качества. Это дает возможности делать государственные дипломы, которые не отличить от оригиналов. orikdok-4v-gorode-saratov-64.ru
isotretinoin easyshop https://isotretinoineasy.shop/# isotretinoin easyshop isotretinoin easyshop
Thanks for the article. Here’s more on the topic https://mehelper.ru/
Thanks for the article. Here is a website on the topic – https://kanunnikovao.ru/
Howdy superb blog! Does running a blog such as this require a large amount of work?
I’ve no knowledge of coding however I was hoping to start my
own blog soon. Anyhow, if you have any ideas or techniques for new blog owners please share.
I know this is off topic however I just needed to ask.
Kudos!
https://noxwin-de.com
I know this web site gives quality dependent posts and
other material, is there any other site which gives such things in quality?
https://bassbet-de88.com
Heya! I’m at work browsing your blog from my new apple iphone!
Just wanted to say I love reading your blog and look forward to all your posts!
Keep up the outstanding work!
20bet
is 1win bet legit https://1win3024.com/
Thank you, your article surprised me, there is such an excellent point of view. Thank you for sharing, I learned a lot.
I needed to thank you for this good read!! I absolutely enjoyed every little bit of it.
I have got you book marked to look at new stuff you post…
evo
купить диплом настоящий купить диплом настоящий .
kupit-drova-v-spb-365.ru .
электрокарнизы для штор купить в москве https://elektrokarniz25.ru .
купить горшок для цветов большой напольный пластиковый в интернет магазине http://kashpo-napolnoe-rnd.ru/ – купить горшок для цветов большой напольный пластиковый в интернет магазине .
apple купить спб http://www.kupit-ajfon-cs3.ru .
диплом о высшем образовании старого образца купить диплом о высшем образовании старого образца купить .
Приобрести диплом ВУЗа по выгодной цене можно, обратившись к проверенной специализированной компании. Приобрести документ института вы можете в нашей компании в столице. orikdok-1v-gorode-yoshkar-ola-12.ru
Заказать диплом об образовании. Приобретение подходящего диплома через проверенную и надежную фирму дарит ряд преимуществ для покупателя. Такое решение помогает сэкономить время и значительные деньги. orikdok-3v-gorode-khabarovsk-27.online
скачать музыку в мр3 в хорошем качестве бесплатно и без регистрации скачать музыку в мр3 в хорошем качестве бесплатно и без регистрации .
888starz app for android 888starz app for android .
Greetings!
Hire hackers for secure data access, account recovery, and system testing. Our platform offers encrypted communication, guaranteeing privacy and safe service handling. Trust our experts to deliver fast, effective solutions tailored to your needs with full confidentiality at every step. Experience advanced digital services today.
https://hackerslist.com/
Thank you for choosing HackersList!
Limousine service prioritizes Customer Satisfaction , offering luxurious transportation for various occasions. Professional chauffeurs ensure a smooth, safe ride. The fleet includes sedans, SUVs, and stretch limousines, accommodating different group sizes. Amenities like complimentary beverages, Wi-Fi, and entertainment systems enhance the experience. Timely pick-ups and drop-offs are guaranteed, with real-time tracking for convenience. Competitive pricing and customizable packages cater to individual needs. Customer Satisfaction is paramount, with 24/7 support and easy booking options. The service is ideal for corporate events, weddings, airport transfers, and special nights out. Each journey is tailored to meet high standards, ensuring a comfortable and memorable experience. – https://taxi-prive.com/who-we-are/
mostbet cashback bonus https://mostbet4048.ru/
Накрутка подписчиков в ТГ, боты и живые подписчики в Телеграм, бесплатно Накрутка подписчиков в ТГ, боты и живые подписчики в Телеграм, бесплатно
login 1win http://1win3027.com/
музыка новинки скачать музыка новинки скачать .
Мы изготавливаем дипломы любой профессии по приятным тарифам. Важно, чтобы дипломы были доступными для подавляющей массы граждан. Купить диплом об образовании psalmtechproperties.com/profile/emery68d149178
купить диплом беларусь http://www.arus-diplom4.ru/ .
Купить диплом на заказ в столице можно через сайт компании. orikdok-4v-gorode-krasnodar-23.ru
Быстро приобрести диплом института. Покупка официального диплома через надежную фирму дарит ряд достоинств. Такое решение дает возможность сберечь время и значительные денежные средства. orikdok-4v-gorode-ulyanovsk-73.ru
login to 1win https://1win3026.com/
iphone спб iphone спб .
купить диплом в магадане купить диплом в магадане .
Thanks for the article. Here’s more on the topic https://yarus-kkt.ru/
Thanks for the article. Here’s more on the topic https://stalker-land.ru/
купить аттестаты за 11 класс http://www.arus-diplom7.ru/ .
888starz partner apk 888starz partner apk .
1win login online http://1win3025.com
is 1win token legit http://1win3027.com
купить аттестат в москве за 9 класс http://www.arus-diplom3.ru .
1win mines signal 1win mines signal
1win crash predictor 1win crash predictor
Scientific evidence has suggested that by 2050, many of the Philippine coastal regions, including the Manila Bay area, could find themselves underwater if countries fail to mitigate the effects of climate change 토토커뮤니티
карнизы для штор с электроприводом elektrokarniz25.ru .
Мы можем предложить документы институтов, расположенных на территории всей Российской Федерации. Купить диплом любого университета:
vivivian826.copiny.com/question/details/id/1113453
Все коллеги завидовали – такой красоты у них не было!
розы купить в томске
Заказала для тёти – осталась довольна!
розы купить в томске
Повторили пинтерест один в один!
заказать цветы с доставкой в томске
Доставили в больницу – все аккуратно.
заказать цветы томск
Лучший подарок для самых дорогих людей!
цветы
Here’s more on the topic https://bediva.ru/
окна rehau окна rehau .
пластиковые окна рехау пластиковые окна рехау .
Купить диплом можно через официальный портал компании. orikdok-5v-gorode-barnaul-22.online
mostbet bonus https://mostbet4052.ru
Wow, wonderful blog layout! How long have you been blogging for?
you made blogging look easy. The overall look of your web site is great, let alone the content!
betway
диплом медицинский купить http://www.arus-diplom5.ru .
карниз моторизованный http://elektrokarniz5.ru/ .
Welcome to HackersList!
Get immediate access to expert hackers who specialize in complex digital services. Whether you need account recovery, secure data access, or penetration testing, we offer fast and discreet solutions. Our platform uses encrypted communication to ensure your privacy and security at every stage. Trust our verified professionals to deliver reliable results tailored to your needs.
https://hackerslist.com/
Thank you for choosing HackersList!
Thanks for the article. Here’s more on the topic https://orenbash.ru/
1000 подписчиков в Телеграм 1000 подписчиков в Телеграм — рейтинг платформ с реальными отзывами и результатами. Живые подписчики, автоматический запуск, доступные пакеты. Идеально для старта и масштабирования канала.
I’ve been exploring terpene-based products terpenes in indica recently, and I’m indeed enjoying the experience. The scents are in the chips, natural, and pleasant. They enlarge a gracious be a match for to my daily routine, dollop fasten on the feeling ready and atmosphere. A great find to save anyone who appreciates perfumed wellness tools.
диплом купить старого образца диплом купить старого образца .
Hello there,
I found this post extremely relevant, especially given how fast the crypto world is evolving. It’s refreshing to see such clarity in content.
I run a crypto-focused blog at Cryptoairhub where we publish updates, guides, and strategic insights for investors.
If you’re into crypto and looking to stay updated, feel free to take a look at our Crypto Blog.
Thanks for the great read!
Мы можем предложить документы университетов, которые расположены в любом регионе России. Заказать диплом о высшем образовании:
customerscomm.com/read-blog/23914_realno-li-kupit-diplom-s-reestrom.html
kupit-drova-v-spb-365.ru .
mostbet mostbet
Ahaa, its fastidious conversation regarding this piece of writing here at this webpage, I have read all that, so
now me also commenting here.
https://amunra-de88.com
Vovan Casino — это современная онлайн-платформа для азартных игр. Вся библиотека автоматов обновляется на постоянной основе. Сайт адаптирован под мобильные устройства и десктопы. Именно vovan casino зеркало предлагает быстрый переход к выбору игровых автоматов. Сервис ориентирован на честность и прозрачность. Играйте там, где важны результат и удобство.
Процесс регистрации занимает не более пары минут. Финансовые операции защищены современными протоколами шифрования. Консультанты помогают решить технические и финансовые вопросы. Vovan — если вы цените надёжность.
Дизайн не отвлекает от сути — выигрышей. Ассортимент регулярно расширяется новыми разработками. Казино Vovan — это точка входа в проверенный игровой бизнес. Vovan — если игра — это не только развлечение, но и стратегия.
Мы предлагаем оформление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2132 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом об окончании института — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4645 клиентов воспользовались услугой — теперь ваша очередь.
Диплом вуза купить — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1802 клиентов воспользовались услугой — теперь ваша очередь.
Купить срочно диплом о высшем образовании вуза — ответим быстро, без лишних формальностей.
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3236 клиентов воспользовались услугой — теперь ваша очередь.
Купить дипломы о высшем образовании в Москве — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 4629 клиентов воспользовались услугой — теперь ваша очередь.
Звоните — ответим быстро, без лишних формальностей.
1win old version apk 1win old version apk .
Hi! I’ve been reading your weblog for a while now and finally
got the courage to go ahead and give you a shout out from Humble Texas!
Just wanted to tell you keep up the good work!
bitkingz
1win casino 1win3025.com
гардина с электроприводом elektrokarnizy10.ru .
Казино Vovan — это не просто платформа, это нейросеть удовольствия. Визуальный модуль слота активирует чувство азарта мгновенно. Игра запускается быстрее, чем искусственный интеллект генерирует ответ. Таким образом, vovan casino зеркало — твой аплинк в пространство цифрового азарта. Ты входишь туда, где правит киберудача. Подключайся и не тормози.
Авторизация мгновенна, как запуск дрона. Финансовые процессы защищены как банковские дата-центры. Саппорт реагирует быстрее, чем ты успеешь ввести запрос. Vovan — если тебе ближе технологии, чем фишки.
Цветовая палитра на уровне голограммы. Каждый слот — как отдельная симуляция с высокой вариативностью. Казино Vovan — цифровой мост в гейминг нового уровня. Vovan — если ты не просто игрок, а часть системы.
kupit-drova-v-spb-365.ru .
Цены на ламинат в ExpoStroy. http://www.xn--1-7sba5anhi5b.xn--p1ai/ .
1xBet prides itself on its comprehensive sportsbook, which encompasses a vast range of sports from all corners of the globe. Whether you have a passion for popular international sports such as football, basketball, tennis, cricket, or you prefer more niche sports, 1xBet has something for everyone. You can place bets on both pre-match and live events, taking advantage of competitive odds and a diverse selection of betting options to enhance your chances of winning.
1xbet Promo Code Signup Bonus Up To €/$130
https://audium.com/img/pages/1xbet_promo_code_ghana.html Promo code for 1xBet, use this combination to increase your welcome bonus up to 100% on an amount reaching $/€130 for registration. These funds are available to all new players who have already created an account or are planning to do so. The bonus requires wagering, and it must be done in the sports section by placing bets with odds of at least 1.4 and a fivefold turnover. You have 30 days to use the code before it expires at the end of 2025.
1xBet Promo Code 2025 – this is a huge bonus of up to $1950 for the casino and 150 free spins on slots. An exclusive offer for new players aged 18 and above. To activate the code, you need to make a deposit of $10. The second and subsequent deposits must be at least $15. You can participate in this promotion until the end of 2025.
электрокарниз москва http://elektrokarnizy750.ru/ .
электрические карнизы купить http://www.elektrokarnizy777.ru .
mostbet az mobil versiya http://www.mostbet4052.ru
mostbet qeydiyyat bonus http://mostbet4049.ru
электрокарниз москва http://www.elektrokarnizy-dlya-shtor1.ru/ .
Купить диплом на заказ возможно через сайт компании. spiceroleplay.forumex.ru/viewtopic.php?f=23&t=1352
заверенный перевод документов на английский [url=http://trs-center.ru]http://trs-center.ru[/url] .
1win online https://1win3024.com/
Mikrotik предлагает широкий спектр решений для построения и оптимизации сетевой инфраструктуры. Уникальная ОС RouterOS, устанавливаемая на устройства Mikrotik, предоставляет широкие возможности для управления маршрутизацией и защитой сети.
Сетевые администраторы ценят Mikrotik за его простоту использования и мощность. Сравнительно низкая цена оборудования Mikrotik позволяет многим компаниям оптимизировать свои сетевые расходы.
Mikrotik нашел свое применение как в домашних сетях, так и в малых компаниях, обеспечивая надежную маршрутизацию. Настройка устройств Mikrotik производится через удобный визуальный интерфейс, что делает их использование простым и эффективным.
В заключение, продукция Mikrotik — это качественное и доступное решение для управления сетями. Независимо от размера вашего бизнеса, вы можете найти подходящее решение в продуктовой линейке Mikrotik.
коммутатор mikrotik http://mikrotikwarehouse.ru/product-category/kommutatory/
автоматические карнизы http://www.elektrokarniz5.ru .
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
no prescription online pharmacy https://onlinepharmeasy.top/# mexican pharmacy online mexican online pharmacy
Оформиление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2935 клиентов воспользовались услугой — теперь ваша очередь.
Купить дипломы о высшем — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1289 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом о высшем образовании недорого — ответим быстро, без лишних формальностей.
You actually make it appear so easy with your presentation but I in finding this topic to be actually one thing that I think I’d never understand.
It sort of feels too complicated and very broad for me.
I’m having a look ahead in your subsequent put up, I’ll
try to get the hang of it!
https://stake-de88.com
Купить ламинат в Москве. xn--1-7sba5anhi5b.xn--p1ai .
Оформиление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 1293 клиентов воспользовались услугой — теперь ваша очередь.
Узнать условия — ответим быстро, без лишних формальностей.
Покупка дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы гарантируем, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 3332 клиентов воспользовались услугой — теперь ваша очередь.
Куплю диплом Москва — ответим быстро, без лишних формальностей.
1win prediction 1win prediction
Мы предлагаем оформление дипломов ВУЗов В киеве — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2368 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом вуза — ответим быстро, без лишних формальностей.
электрические карнизы купить https://elektrokarnizy10.ru/ .
Оформиление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2660 клиентов воспользовались услугой — теперь ваша очередь.
Открыть — ответим быстро, без лишних формальностей.
one win app download one win app download .
электрический карниз для штор купить http://elektrokarnizy750.ru .
Thanks for the article. Here’s more on the topic https://stalker-land.ru/
как зарегистрироваться в мостбет http://mostbet4051.ru
кракен официальный сайт
online pharmeasy mexican pharmacy online walgreens online pharmacy us online pharmacy
Заказать диплом института по выгодной стоимости вы сможете, обращаясь к надежной специализированной компании. Приобрести документ университета вы имеете возможность у нас. orikdok-5v-gorode-smolensk-67.online
карниз с электроприводом карниз с электроприводом .
Часто читаю и слежу за выходом статей на tellmi.ru. Понравилась статья на тему: вегетарианские рецепты. Если интересуетесь этой темой – обязательно к прочтению! Не пожалеете где собраны интересные материалы на самые разные тематики! На этом портале вы найдете полезные статьи, свежие новости и увлекательные обзоры — каждый сможет выбрать что-то по душе. Заходите, читайте и делитесь впечатлениями!
Приобрести диплом под заказ можно через сайт компании. skillsvault.co.za/profile/joannarodrigue
кракен 2025
Компания Sport Records предлагает профессиональный пошив спортивной формы и одежды для команд, клубов, секций
и корпоративных мероприятий. Мы изготавливаем форму с учетом всех особенностей выбранного вида спорта: удобный крой,
дышащие материалы, износостойкие швы и высокая посадка — все для максимального комфорта и эффективности во время тренировок
и соревнований.
Работаем как с небольшими, так и с крупными тиражами. Возможна индивидуализация формы: нанесение логотипов,
фамилий, номеров и фирменных цветов. Наше производство позволяет адаптировать дизайн под ваши
задачи — от классических комплектов до эксклюзивных моделей.
Мы ценим ваше время, поэтому гарантируем соблюдение сроков и контроль качества на каждом этапе.
Пошив спортивной одежды от нас — это надежность, стиль и удобство.
Свяжитесь с нами и получите расчёт стоимости уже сегодня! .
электрокарнизы купить в москве электрокарнизы купить в москве .
кракен онион
mexican online pharmacy https://onlinepharmeasy.top/# best online pharmacy safe online pharmacy
mostbet crypto ödənişlər http://www.mostbet4053.ru
перевод паспорта на английский http://trs-center.ru .
I’ve been exploring terpene-based products https://terpenewarehouse.com/collections/terpenes-for-energy recently, and I’m remarkably enjoying the experience. The scents are with, typical, and pleasant. They enlarge a discriminative drink to my day after day programmed, ration beat up a compare the atmosphere and atmosphere. A large catch sight of to save anyone who appreciates aromatic wellness tools.
топ сервисов для накрутки подписчиков в телеграм топ сервисов для накрутки подписчиков в телеграм
Мы предлагаем оформление дипломов ВУЗов по всей Украине — с печатями, подписями, приложением и возможностью архивной записи (по запросу).
Документ максимально приближен к оригиналу и проходит визуальную проверку.
Мы даем гарантию, что в случае проверки документа, подозрений не возникнет.
– Конфиденциально
– Доставка 3–7 дней
– Любая специальность
Уже более 2988 клиентов воспользовались услугой — теперь ваша очередь.
Купить диплом о высшем образовании недорого — ответим быстро, без лишних формальностей.
Психотерапевт Белгород. Психолог онлайн 558 оценок
mexican pharmacy online online pharmacy tramadol online pharmacy reviews mexica online pharmacy
кракен vk2
mostbet az casino https://mostbet4049.ru/
кракен Россия
Часто читаю и слежу за выходом статей на tellmi.ru. Понравилась статья на тему: особенности поведения детей. Если интересуетесь этой темой – обязательно к прочтению! Не пожалеете где собраны интересные материалы на самые разные тематики! На этом портале вы найдете полезные статьи, свежие новости и увлекательные обзоры — каждый сможет выбрать что-то по душе. Заходите, читайте и делитесь впечатлениями!
Пробковые напольные покрытия цена. http://laminat2.ru .
online pharmacy tramadol https://onlinepharmeasy.top/# online mexican pharmacy best online pharmacies
кракен официальный сайт
Pretty component of content. I just stumbled upon your website and in accession capital to assert that
I get actually enjoyed account your blog posts.
Anyway I will be subscribing for your augment and even I fulfillment you get entry to persistently fast.
https://PSBS-Biak.com
Психотерапевт Пенза. Психотерапевт онлайн 193 оценок
Ahaa, its fastidious conversation concerning this piece of writing here at this webpage, I have
read all that, so at this time me also commenting here.
https://Persija-Jakarta-id.com
Психотерапевт Белгород. Психолог онлайн 647 оценок
Заказала утром – к обеду уже получила свежие цветы!
заказать цветы томск
Thanks for every other magnificent article. Where else
may anyone get that kind of info in such an ideal method of writing?
I’ve a presentation subsequent week, and I’m at the look for
such info.
https://Persita-Tangerang.com
Свежесть как после утренней росы – потрясающе!
цветы
Свежесть с клумбы!
доставка цветов в томске
mostbet kazinosu http://www.mostbet4050.ru
Доставили минута в минуту – цветы свежайшие, будто только с клумбы.
розы купить в томске
kraken официальный
I am curious to find out what blog platform you happen to be working with?
I’m having some small security problems with my
latest website and I’d like to find something more safe.
Do you have any suggestions?
https://PS-Barito-Putera.com
Психотерапевт Киров. Психотерапевт онлайн 308 оценок
купить диплом в кургане занесением в реестр купить диплом в кургане занесением в реестр .
online pharmacies mexican pharmacy online online pharmacy review mexican online pharmacy
Thanks for the article. Here is a website on the topic – https://svetnadegda.ru/bolty-vidy-naznachenie-i-kak-vybrat-podhodyashhij-krepezh/
Thanks for the article. Here’s more on the topic https://avto-yar.ru/
купить диплом с занесением в реестр в калуге купить диплом с занесением в реестр в калуге .
купить диплом с реестром киев http://www.arus-diplom23.ru .
mostbet bonusu necə almaq olar https://www.mostbet4055.ru
как купить проведенный диплом отзывы https://arus-diplom24.ru .
kraken официальный
Сервис по подбору психолога Психиатр онлайн 804 оценок
mostbet pul çıxarma https://mostbet4055.ru/
kraken qr code
mostbet az bonus mostbet az bonus
Сервис по подбору психолога Психиатр онлайн 471 оценок
Сервис по подбору психолога Психотерапевт онлайн 854 оценок
propecia fx https://propeciafx.shop/# propecia fx propecia fx
кракен vk4
Психотерапевт Пенза. Психотерапевт онлайн 578 оценок
кракен Россия
propecia fx propecia fx propecia fx propecia fx